
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 20 March 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1460900
Objective: Preventing colorectal adenoma (CRA) recurrence after polypectomy is essential. However, the current evidence of Chinese herbal medicine (CHM) for CRA recurrence is still limited. This study aims to synthesize the effects of CHM as a prevention method for CRA recurrence.
Methods: Nine databases were searched up to May 2024. Randomised controlled trials identifying the preventive effects of CHM among people with CRA post-polypectomy were included. spreadsheets were used to collect and extract data. RevMan and STATA were used for data analysis. We performed subgroup and sensitivity analyses to explore potentially influencing variables.
Results: Twenty trials (2,325 participants) were included. The commonly used botanical drugs belonged to the categories of strengthening the spleen and anti-tumour metabolites. Compared to routine care (RC) alone, oral CHM plus RC significantly reduced the CRA recurrence rate at 12 months (RR 0.51, 95% CI [0.39, 0.67], I2 = 42%), 6 months (RR 0.44, 95% CI [0.36, 0.55], I2 = 0%), and 3 months (RR 0.46, 95% CI [0.22, 0.96], I2 = 0%) post-polypectomy. Compared to CHM placebo plus RC, San zi granule combined with RC significantly reduced CRA recurrence at 12 months post-polypectomy (RR 0.39, 95% CI [0.16, 0.93], I2 = 0%) and during the 2-year follow-up (RR 0.73, 95% CI [0.58, 0.90]). There were no significant differences between groups for treatment duration and syndromes. Additional analysis showed that oral CHM containing the botanical drugs of Si jun zi decoction plus RC reduced CRA recurrence at 12 months post-polypectomy with a low heterogeneity, compared to RC alone (RR 0.26, 95% CI [0.13, 0.54], I2 = 0%). Adverse events were similar in the above two comparisons.
Conclusion: Oral CHM combined with RC may reduce CRA recurrence and be well-tolerated. San zi granule and Si jun zi decoction may be representative prescriptions Experimental studies of the frequent botanical drugs have found anti-cancer effects that may account for the clinical findings. Future rigorous clinical trials are needed due to low-to-moderate certainty of evidence.
Systematic Review Registration: PROSPERO (CRD42023324197), https://www.crd.york.ac.uk/PROSPERO/view/CRD42023324197.
Colorectal cancer (CRC) is regarded as the third most common malignancy worldwide, based on estimates from the World Health Organization in 2020 (Morgan et al., 2023). Approximately 70%–80% of CRCs develop from colorectal adenomas (CRA) through the adenoma–carcinoma pathway (Sung et al., 2022). CRA is a benign tumour in the large intestine and includes tubular, tubulovillous, villous, and serrated adenomas (Tanaka et al., 2020).
Since CRA plays an essential role in the development of CRC, polypectomy is the recommended primary treatment (Lieberman et al., 2012; Ferlitsch et al., 2017). However, CRA recurrence is common, varying from 19.3% to 59.46% at 1-year post-polypectomy (Yamaji et al., 2004; Gao et al., 2010; Shi et al., 2017). This rises to 87% at later follow-up times (>5 years) (Gao et al., 2010). Recurrence is associated with the number, size, and pathological results of adenomas diagnosed in the initial colonoscopy (Neugut et al., 1995; Huang et al., 2010). After polypectomy, people are treated with routine care (RC), consisting of fasting with energy support, liquid diet, or semi-liquid diet, as well as prevention for delayed bleeding (Association and Association, 2014). The duration of RC depends on the postoperative condition and usually lasts three to 7 days. The recommended follow-up interval times of repeated colonoscopy for people with adenoma range from one to 3 years, according to the condition of the lesion (number, size, and pathological type) (Colorectal Group, 2023). Currently, there is no established strategy for preventing CRA. Available pharmacotherapies have shown safety issues in clinical practice. Nonsteroidal anti-inflammatory drugs may cause gastrointestinal bleeding, especially in older people (Mahady et al., 2021), while non-selective cyclooxygenase-2 inhibitors may increase cardiovascular disease risk (Ribeiro et al., 2022). Therefore, new medications are being explored for CRA recurrence prevention. A meta-analysis with three trials and 1,076 participants revealed that berberine reduced the CRA recurrence rate, although there were more adverse events compared to the placebo (Fang et al., 2022). Another medication, metformin, was safe and effective for reducing the recurrence of colorectal polyps (including adenomas) at a low dose for 1 year of administration (Higurashi et al., 2016).
The effects of Chinese medicine (CM) on CRA recurrence have also been investigated. Two meta-analyses showed that CHM reduced the recurrence rate of colorectal polyps (including CRA) but did not focus on adenomas (Chen S. et al., 2020; Zhou et al., 2020). Another meta-analysis provided evidence on CHM for CRA and colorectal polyp recurrence. The primary outcome in this study was defined as the proportion of recurrent colorectal polyps instead of recurrent adenomas, which cannot reflect adenoma recurrence (Lin et al., 2020). Therefore, a systematic evaluation of the role of CHM on CRA recurrence is needed.
To address the above limitations, we conducted a systematic review to identify the clinical effects of CHM in preventing CRA recurrence and determine which botanical drugs and/or formulae are effective and safe.
We registered a protocol with PROSPERO (CRD42023324197) that was published (Cheng et al., 2023). This review was conducted with reference to the Cochrane Handbook (Higgins et al., 2022) and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021).
We conducted a comprehensive search until May 2024 through five English databases (MEDLINE [via PubMed], Cochrane Library, EMBASE [via Embase.com], The Allied and Complementary Medicine Database [AMED], and Cumulative Index to Nursing and Allied Health Literature [CINAHL]) and four Chinese databases (Chinese Biology Medicine disc, China National Knowledge Infrastructure, China Science and Technology Journal Database, and Wanfang Data). No restrictions were applied. The search strategy combined medical terms and keywords relating to CRA, CHM, and randomised controlled trial (RCT) (Supplementary Appendix S10).
a. Study type: RCTs.
b. Participants: people aged 18 years or older with a pathological diagnosis of CRA (tubular, villous or tubulovillous (Tanaka et al., 2020)) and who had received a polypectomy to remove the colorectal lesions.
c. Intervention: use of CHM alone or combined with other therapies.
d. Comparators: RC (defined as the usual care after polypectomy to prevent bleeding and other postoperative complications (Association and Association, 2014), including fasting with energy support, liquid diet, or semi-liquid diet), CHM placebo, no additional treatment, or conventional medications.
e. Primary outcome: CRA recurrence rate in the repeated colonoscopy at any time point (Chen Y.-X. et al., 2020).
f. Secondary outcomes: a. number of people who had one or two CRAs, b. number of people who had three or more than three CRAs, c. number of people who had CRA with 1 cm size or larger, d. number of people who had advanced CRAs, e., number of people who were newly diagnosed with CRC, and f. adverse events (AEs).
We excluded studies that used the purified metabolites of CHM as interventions and/or did not provide specific data on the CRA recurrence rate. Trials were also excluded if CHM was used in the control group.
Search results were checked, and duplicates were removed. Two reviewers (YC and YMD) conducted an initial selection of titles and abstracts, followed by an assessment of full texts for eligibility independently. The study data related to participants, interventions, comparators, and outcomes were extracted independently and double-checked by YC and YMD using a standardised spreadsheet. Any differences in selection and extraction were discussed with a third reviewer (ALZ). Authors were contacted by email if there were critical missing data.
Included studies were assessed by two reviewers (YC and YMD) independently using the Cochrane Collaboration Risk of Bias 2 (RoB 2) tool (Sterne et al., 2019). The following aspects were evaluated: randomisation procedure, intended interventions, missing outcome data, outcome measurement, and selection of the reported result (Sterne et al., 2019). Any disagreements were resolved by a senior reviewer (ALZ).
A narrative description was given when only one study was included in a comparison group. For comparison groups with more than one study, analyses were performed in Review Manager 5.4 (RevMan 5.4) and STATA (version 17.0) and presented with forest plots.
We used a risk ratio (RR) with a 95% confidence interval (CI) to assess dichotomous outcomes (CRA recurrence rate and the secondary outcomes) and risk difference (RD) to assess the absolute effect.
Considering the likely heterogeneity between the included studies, we selected the random-effects model for meta-analysis. Higgin’s I2 statistics were reported to assess heterogeneity between studies. We regarded an I2 value of more than 50% as substantial heterogeneity.
We conducted additional analyses based on the same CM formulae, treatment duration, comparator, and CM syndrome to explore the heterogeneity source and intervention effects.. We also performed a sensitivity analysis to evaluate the stability of results by omitting any one included trial and grouping the included studies based on different characteristics. Publication bias was measured by a funnel plot and Egger’s test if we included 10 or more studies.
The overall certainty of evidence was evaluated by Grading of Recommendations, Assessment, Development and Evaluations (GRADE) (Guyatt et al., 2008). We rated the certainty of evidence on clinically significant outcomes (CRA recurrence rate and AEs).
A total of 6,035 citations were identified from the database search, and 4,429 titles and abstracts were screened after removing duplicate records. We assessed 72 full-text citations for eligibility, and 20 RCTs were included in the systematic review (Figure 1).
Five theses (Yuan, 2017; Xv, 2019; Zhao, 2020; Ren, 2021; Wang, 2022), one conference paper (Yan et al., 2018), and 14 published articles (Huang et al., 2018; Li L. et al., 2020; Xiong and Gu, 2020; Zhang et al., 2020; Li Y. et al., 2021; Pan, 2021; Xv et al., 2021; Li et al., 2022; Chen et al., 2023; Jia et al., 2023; Li C. et al., 2023; Li M. et al., 2023; Yao et al., 2023; Ni et al., 2024) were included. Study characteristics of included trials are summarised in Table 1.
RCTs were conducted in China and included 2,325 participants (1,165 in treatment groups and 1,160 in control groups) aged 18–79. All participants were diagnosed with CRA and underwent a polypectomy to remove the CRA completely.
All included RCTs used oral CHM combined with RC as the intervention. Treatment duration ranged from 6 weeks to 9 months, except that one study did not clarify the exact treatment duration (Huang et al., 2018). Thirteen out of 20 studies reported CM syndrome differentiation. Eleven studies treated participants depending on syndrome differentiation.
Sixteen studies compared oral CHM plus RC with RC (Yuan, 2017; Huang et al., 2018; Yan et al., 2018; Xv, 2019; Li L. et al., 2020; Xiong and Gu, 2020; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Pan, 2021; Ren, 2021; Xv et al., 2021; Wang, 2022; Jia et al., 2023; Li M. et al., 2023; Yao et al., 2023), three compared oral CHM plus RC with CHM placebo plus RC (Li et al., 2022; Chen et al., 2023; Ni et al., 2024), and one compared oral CHM plus RC with the probiotic (Clostridium butyricum capsule) plus RC (Li C. et al., 2023). All RCTs reported on the CRA recurrence rate diagnosed by colonoscopy and biopsy, nine studies evaluated CRA recurrence rate at 6 months (Yuan, 2017; Yan et al., 2018; Li L. et al., 2020; Xiong and Gu, 2020; Pan, 2021; Xv et al., 2021; Li et al., 2022; Jia et al., 2023; Li M. et al., 2023), 13 studies had an assessment of CRA recurrence at 12 months (Huang et al., 2018; Xv, 2019; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Xv et al., 2021; Li et al., 2022; Wang, 2022; Chen et al., 2023; Li C. et al., 2023; Li M. et al., 2023; Yao et al., 2023), and one assessed recurrence rate during a 2-year follow-up (proportion of participants with at least one recurrent adenoma in any repeated colonoscopies during the follow-up). Twelve trials assessed and reported on AEs (Yuan, 2017; Xv, 2019; Li L. et al., 2020; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Xv et al., 2021; Wang, 2022; Chen et al., 2023; Jia et al., 2023; Ni et al., 2024), and two studies included a new diagnosis of CRC as an outcome (Xv, 2019; Chen et al., 2023). Two included studies (Zhang et al., 2020; Ni et al., 2024) assessed the number of recurrent advanced CRAs, and one study (Yao et al., 2023) reported recurrent adenomas sized 1 cm or larger.
Modified Li qi liu jun zi decoction (Huang et al., 2018; Pan, 2021), San zi granule (Li et al., 2022; Chen et al., 2023), Tiao chang xiao liu formula (Zhang et al., 2020; Li Y. et al., 2021), and modified Wu mei pill (Yuan, 2017; Li C. et al., 2023) were each used as an intervention in two studies. The remaining 12 studies used different formulae. The formula names in Chinese Pin yin, sources, botanical drugs, and quality control information are provided in Table 2.
The top 10 botanical drugs are Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae radix et rhizoma] (Gan cao) (n = 14), Atractylodes macrocephala Koidz. [Asteraceae; Atractylodis macrocephalae rhizoma] (Bai zhu) (n = 14), Codonopsis pilosula (Franch.) Nannf. [Campanulaceae; Codonopsis radix] (Dang shen) (n = 13), Astragalus mongholicus Bunge [Fabaceae; Astragali radix] (Huang qi) (n = 12), Citrus reticulata Blanco [Rutaceae; Citri reticulatae pericarpium] (Chen pi) (n = 10), Prunus mume (Sieb.) Sieb.et Zucc. [Rosaceae; Mume fructus] (Wu mei) (n = 10), Poria cocos (Schw.) Wolf [Polyporaceae; Poria] (Fu ling) (n = 9), Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf [Poaceae; Coicis semen] (Yi yi ren) (n = 9), Scleromitrion diffusum (Willd.) R.J.Wang [Rubiaceae; Hedyotis diffusae herba] (Bai hua she she cao) (n = 6), Curcuma phaeocaulis Valeton [Zingiberaceae; Curcumae rhizoma] (E zhu) (n = 6), and Panax notoginseng (Burkill) F.H.Chen [Araliaceae; Notoginseng radix et rhizome] (San qi) (n = 5) (Supplementary Table S1). These botanical drugs were given within the recommended dosages, according to the Chinese pharmacopoeia (Commission, 2020), and are mainly divided into two types: strengthening spleen and anti-tumour capability. The top seven commonly used botanical drugs contained Si jun zi decoction (Dang shen, Bai zhu, Fu ling, and Gan cao)—a typical formula for gastrointestinal disorders. Six studies used these four botanical drugs. Si jun zi decoction originated from “Prescriptions of the People’s Welfare Pharmacy (Tai ping hui min he ji ju fang) (1151 AD) and is used for spleen deficiency syndrome. Modern experiments showed that Si jun zi decoction had anti-tumour effects on colon and lung cancer (Zhou et al., 2019; Shao et al., 2022). One ongoing multicentre, placebo-controlled trial is testing Si jun zi decoction granule as an intervention to prevent CRA recurrence (Ni et al., 2023). The names of botanical drugs were sourced from Kew Science and the Chinese pharmacopoeia (Commission, 2020; Trustees of the Royal Botanic Gardens, 2024). We followed the guidelines to report the botanical drugs and formulae and used the ChnPhYMO tool for assessment (Supplementary ConPhyMP checklists Supplementary Tables S1, S2) (Heinrich et al., 2022).
The risk of bias for the CRA recurrence rate and AEs was assessed. For the CRA recurrence rate (Figure 2), we have no information on the data analysis after the pre-specified statistical plan for 19 studies because these included studies did not provide protocols. One trial supplied the protocol (Ni et al., 2024). Therefore, we rated the selective reporting domain of 19 studies as ‘some concern.’ We also judged the randomisation process domain as some concern in 15 studies due to insufficient detail about randomisation. Three RCTs that used a placebo were assessed. Two mentioned the blinding method, while another one did not. The drop-out rate for participants was acceptable, with the highest rate being 17.1%. CRA recurrence was measured by colonoscopy and pathological results, which were unlikely to have been affected by the awareness of the received treatment; therefore, we rated the studies as low risk on the domains of bias from intervention, missing data, and outcome measurement.
Twelve studies reported on AEs (Supplementary Figure S2). We judged the measurement outcome domain to be high risk because the studies did not indicate whether outcome assessors were aware of the allocation. In addition, AE was a patient-reported outcome, which may be influenced by knowledge of the intervention. Overall, the 12 studies were considered at high risk of bias for AEs.
All the included studies (n = 20) reported on recurrence rate. Two of these studies reported at 3 months, nine studies at 6 months, 13 studies at 12 months after polypectomy, and one for cumulative recurrence rate during the 2-year follow-up.
Compared to RC alone, RC plus CHM had a 22% decreased risk of developing recurrence of CRA at 12 months after polypectomy (RR 95%CI 0.51, [0.39, 0.67], p < 0.00001, I2 = 42%, 10 studies, 1,017 participants, certainty of evidence: low) (Figure 3) (Huang et al., 2018; Xv, 2019; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Xv et al., 2021; Wang, 2022; Li M. et al., 2023; Yao et al., 2023).
Compared to RC plus placebo, CHM plus RC had a 16% decreased risk of developing recurrence of CRA at 12 months after polypectomy (2 studies of San zi granule, 127 participants, (RR 95%CI 0.39, [0.16, 0.93], p < 0.03, I2 = 0%, 2 studies of San zi granule, 127 participants, certainty of evidence: low) (Figure 3) (Li et al., 2022; Chen et al., 2023).
Compared to probiotic plus RC, CHM plus probiotic plus RC had a 45% decreased risk of developing recurrence of CRA at 12 months after polypectomy (RR 95%CI 0.24, [0.11, 0.53], 1 study, 81 participants, certainty of evidence: low) (Figure 3) (Li et al., 2023a).
Subgroup analysis was performed based on treatment duration in nine studies (Xv, 2019; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Xv et al., 2021; Wang, 2022; Li M. et al., 2023; Yao et al., 2023) because one study did not mention the treatment duration (Huang et al., 2018). After 2 months of treatment or less, compared to RC alone, CHM plus RC had a 27% decreased risk of recurrence at 12 months after polypectomy (RR 0.49, [0.31, 0.78] I2 = 0%, 2 studies, 138 participants). After 3 months of treatment or more, there was a 21% decreased risk of recurrence (RR 0.53, [0.39, 0.74], I2 = 47%, 7 studies, 791 participants). There was no significant difference between these groups (Table 3; Supplementary Figure S3.1).
For the use of CM syndrome differentiation or no mention of syndrome differentiation, there was a decreased risk of recurrence at 12 months for CHM plus RC in each group with no significant difference between these groups (Table 3; Supplementary Figure S3.2).
For Tiao chang xiao liu formula, there was no significant difference between Tiao chang xiao liu formula plus RC and RC alone (RR 0.53, [0.23, 1.24], p = 0.06, I2 = 72%, 2 studies, 440 participants), but the heterogeneity was considerable (Supplementary Figure S3.3) (Zhang et al., 2020; Li Y. et al., 2021).
According to the commonly used botanical drugs in the interventions, we performed analysis based on including Si jun zi decoction, whose ingredients were listed in the top 10 used botanical drugs. Compared to RC alone, CHMs that included Si jun zi decoction plus RC had a 23% lower CRA recurrence rate (RR 0.26, [0.13, 0.54] I2 = 0%, 3 studies, 203 participants) (Supplementary Figure S3.5).
Sensitivity analyses showed that after omitting any one study of CRA recurrence rate at 12 months after polypectomy, the results were similar to the overall pooled analysis, indicating no strong effect on the overall result from any single study was observed (Table 3; Supplementary Figure S4.1; Supplementary Supplementary Table S4.1).
At 6 months after polypectomy, compared to RC alone, CHM plus RC had a 23% reduction in CRA recurrence (RR 0.44, [0.36, 0.55], p < 0.00001, I2 = 0%, 8 studies, 764 participants, certainty of evidence: moderate) (Figure 4) (Yuan, 2017; Yan et al., 2018; Li et al., 2020a; Xiong and Gu, 2020; Pan, 2021; Xv et al., 2021; Jia et al., 2023; Li et al., 2023b).
There was no significant difference between groups that received oral CHM plus RC or CHM placebo plus RC at 6 months after polypectomy (RR 0.50, [0.10, 2.53], p = 0.40, 1 study, 60 participants, certainty of evidence: low) (Figure 4) (Li et al., 2022).
The results of subgroup analyses for treatment duration and use of CM syndrome differentiation favoured the oral CHM plus RC groups compared with RC alone with no significant differences between the subgroups. CHMs that included Si jun zi decoction plus RC showed significant reductions in risk compared to RC alone at 6 months following polypectomy, but this group was not significantly different to the formulae that did not include Si jun zi decoction. (Table 3; Supplementary Figures S3.6, S3.7, S3.8).
Sensitivity analyses showed a slight difference in CRA recurrence at 6 months after polypectomy between the combined RR after omitting any given study and the total combined RR with all included studies, suggesting the pooled result was stable (Table 3; Supplementary Figure S4.2; Supplementary Table S4.2). At 3 months after polypectomy, compared to RC alone, CHM plus RC had a 9% reduction in recurrence rate (RR 0.46, [0.22, 0.96], p = 0.04, I2 = 0%, 2 studies, 201 participants, certainty of evidence: low) (Supplementary Figure S5) (Xv et al., 2021; Jia et al., 2023).
One trial of Shen bai granule reported the adenoma recurrence rate by calculating the proportion of participants with at least one recurrent adenoma at any repeated colonoscopies (two in total) during a 2-year follow-up (Ni et al., 2024). Compared to RC plus CHM placebo, CHM plus RC had a 16% reduction in the recurrence rate (RR 0.73, [0.58, 0.90], p = 0.004, 1 study, 336 participants, certainty of evidence: moderate) (Supplementary Figure S6).
Numbers of people who had one or two CRAs, or three and more than three CRAs, were not reported by the included studies. Two studies reported the number of advanced CRAs, showing that there was no significant difference between the integrative medicine groups and the control groups (Zhang et al., 2020; Ni et al., 2024). One RCT, comparing oral CHM plus RC with RC showed no significant difference in recurrence of CRA with 1 cm size or larger between the two groups (Yao et al., 2023). Two studies reported on the diagnosis of CRC. None of the participants were newly diagnosed with cancer (Xv et al., 2021; Chen et al., 2023).
Twelve studies (n = 1,546) assessed AEs as a safety outcome during the study period (3 months to 2 years) (Yuan, 2017; Xv, 2019; Li L. et al., 2020; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Xv et al., 2021; Wang, 2022; Chen et al., 2023; Jia et al., 2023; Ni et al., 2024). Among them, nine studies showed no AEs occurred in treatment and control groups : during the trials (3-12 months) (Yuan, 2017; Xv, 2019; Li L. et al., 2020; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Wang, 2022; Chen et al., 2023), with one study comparing oral CHM plus RC to CHM placebo plus RC (Chen et al., 2023) and eight studies comparing oral CHM plus RC to RC (Yuan, 2017; Xv, 2019; Li L. et al., 2020; Zhang et al., 2020; Zhao, 2020; Li Y. et al., 2021; Ren, 2021; Wang, 2022). Two studies comparing oral CHM plus RC to RC reported 16 minor and transient AEs (Xv et al., 2021; Jia et al., 2023), with seven in the oral CHM plus RC group and nine in the RC group. This was not a significant difference (RR 0.78, [0.30, 1.99], p = 0.60, I2 = 0%) (Supplementary Table S11.1). One trial (oral CHM plus RC versus CHM placebo plus RC) reported 17 AEs (during 2 years) with no significant difference between groups (RR 1.13, [0.44, 2.86], p = 0.80) (Ni et al., 2024) (Supplementary Table S11.2). These AEs included vomiting, skin rash, nausea, abdominal distension, diarrhoea, fatigue, and dizziness (Supplementary Figure S7).
The funnel plot of CRA recurrence rate in comparing oral CHM plus RC and RC at 12 months after polypectomy showed an obvious asymmetry, suggesting potential publication bias [(Supplementary Figure S8) and Egger’s test (t 11.34 [2.30, -1.52], (p < 0.000)] (Supplementary Figure S9.1; Supplementary Table S9.1). As a result of less than 10 included trials, other outcome measures were not assessed for publication bias.
Our systematic review presents an up-to-date and comprehensive review of oral CHM treatment for CRA recurrence after polypectomy. We identified 20 RCTs through nine English and Chinese databases and conducted meta-analysis to explore the effects of oral CHMs for CRA recurrence. Our findings suggest that compared to RC alone, oral CHM plus RC was associated with a lower CRA recurrence rate at 3 months, 6 months, and 12 months after polypectomy with low to moderate levels of certainty. Compared to CHM placebo plus RC, oral San zi granule plus RC was associated with lower risk of CRA recurrence at 12 months and at 2 years follow up. For AEs, there was no significant difference between oral CHM plus RC versus RC, and oral CHM plus RC versus CHM placebo plus RC, suggesting that oral CHMs were well tolerated in people following surgery for CRA.
CRA management is an integral part of cancer prevention. CHM shows potential effects on CRC (Chen et al., 2016) and adenoma management (Zhang et al., 2021). In addition, a meta-analysis reported that combining traditional oriental herbal medicine and conventional medications improved the level of natural killer cells compared to conventional medications, suggesting that the herbal medicines enhanced the immune function of cancer patients (Bae et al., 2017). Similar systematic reviews comparing oral CHM plus RC with RC revealed that oral CHM lowered the risk for colorectal polyps’ recurrence (Chen S. et al., 2020; Lin et al., 2020; Zhou et al., 2020).
Our findings further add to the published literature. The primary outcome (CRA recurrence rate) is defined as the number of participants with recurrent adenoma divided by the number of participants with adenoma at baseline colonoscopy, according to the current Chinese consensus (Fang et al., 2021). Previous meta-analyses assessing oral CHM for CRA recurrence did not use this precise definition of CRA recurrence rate (Lin et al., 2020). Therefore, our study is the first review to use this definition of recurrence rate to analyse the current evidence on oral CHM for CRA recurrence prevention.
In our study, only 3 out of 19 included studies used a CHM placebo in the control group (Li et al., 2022; Chen et al., 2023; Ni et al., 2024), suggesting that more placebo-controlled trials can be conducted to explore the efficacy of oral CHM for CRA recurrence. Placebo control may provide better scientific quality and reliability of results (Krol et al., 2020). The two placebo-controlled studies that used San zi granule combined with RC showed a significantly greater reduction in risk of CRA recurrence at 12 months in the pooled result (Figure 4). San zi granule is composed of nine botanical drugs, such as Wu mei, Huang qi, and other botanical drugs. A recent experiment reported that San zi granule altered the gut microbiome, including an increase in probiotics and a decrease in pernicious bacteria, and changed the levels of metabolites related to primary bile acid biosynthesis and taurine metabolism (Shang et al., 2022). The gut microbiome and relevant metabolites lead to gene expression disorders, changes in intestinal barrier, and activation of inflammation and lipopolysaccharide metabolism, which play essential roles in carcinogenesis (Ağagündüz et al., 2023). Furthermore, research into roles of micro-RNAs in regulating genes associated with cancer suppression has shown that bioactive substances in foods and particular dietary models may regulate the immune system, reduce pro-inflammatory signalling, and modulate the gut microbiome (Şahin et al., 2023). Future studies of CHMs could investigate effects on the microbiome and micro-RNAs.
We analysed subgroups to explore the effects of oral CHM plus RC in included studies with similar characteristics. For treatment duration, our meta-analysis showed that oral CHM plus RC may reduce the risk of CRA recurrence, irrespective of shorter (two months or less) or longer (three months or more) treatment duration. Expert consensus suggested that three to 6 months of CM treatment is recommended to reduce CRA recurrence (Zhang et al., 2021). Based on our results, more evidence is needed to identify the best treatment duration to reduce CRA recurrence. Similar results were observed for subgroup analysis based on the use of CM syndrome differentiation or no mention of its use. CM theory emphasises individual treatment based on different CM syndromes (Peng et al., 2022). A previous meta-analysis showed that strengthening the spleen method prevented the recurrence of colorectal polyps (including adenomas) (Zhou et al., 2020). However, in this review, CM syndrome differentiation did not affect the risk of CRA recurrence at 6 and 12 months after polypectomy. This result may be attributed to the limited number of included studies and the predominance of spleen deficiency syndrome among people with CRA, leading to less pronounced effects of syndrome differentiation (Liu and Chen, 2010). In addition, further studies may be needed to identify the full range of CM syndromes in people with CRA in different regions in China. For the analysis based on the specific formula, we found no difference between Tiao chang xiao liu formula plus RC and RC alone with a high heterogeneity (I2 = 72%), even though each of the included studies showed a significant difference. This result was due to differing effect sizes between the small and large studies. The result favoured the intervention when we changed to the fixed-effect model (Supplementary Figure S3.4). So, it calls for more clinical studies on the Tiao chang xiao liu formula.
We calculated the frequency of botanical drugs in the intervention and found that botanical drugs to strengthen the spleen and qi and suppress tumours were commonly used for CRA recurrence. The top six botanical drugs included the ingredients of Si jun zi decoction, which is a typical formula to strengthen the spleen and qi. Its effects have been associated with intestinal mucosal restoration (Shi et al., 2019). In its original version, Si jun zi decoction contained Panax ginseng C.A.Mey. [Araliaceae; Ginseng radix et rhizoma] (Ren shen), Fu ling, Bai zhu, and Gan cao, but in clinical practice and research, Dang shen is usually selected as a substitute for Ren shen. Previous research demonstrated that Si jun zi decoction or its modified form accelerated the apoptosis and autophagy of colon cells (Shang et al., 2023) and blocked the liver metastasis of colon cancer (Zhou et al., 2019). As for other anti-tumour activities, the original or modified Si jun zi decoction influenced oxidative phosphorylation to reduce gastric tumour growth (Zhu et al., 2024) and prevented epithelial–mesenchymal transition in a mouse model of non-small cell lung cancer (Shao et al., 2022) (Figure 5). Modification of Si jun zi decoction includes addition of Atractylodes lancea (Thunb.) DC. [Asteraceae; Atractylodis rhizoma] (Cang zhu), Citrus reticulata Blanco [Rutaceae; Citri reticulatae pericarpium] (Chen pi), and Citrus × aurantium L. [Rutaceae; Aurantii fructus] (Zhi ke) based on the individualized treatment. A major compound in Cang zhu is atractylodin, which was identified as an inhibitor of N-acylethanolamine-hydrolysing acid amidase (NAAA) that can upregulate palmitoylethanolamide (PEA), reduce microglial activation, and suppress inflammatory responses (Yang et al., 2020). A recent study showed that PEA, a fatty acid amide, restricted the proliferation, differentiation, and migration of colorectal tumour cells and induced cell cycle arrest in the G2/M phase (Pagano et al., 2021). NAAA, the main PEA hydrolytic enzyme, plays an important role in human colon cancer. In mice, an NAAA inhibitor reduced tumour growth and Ki-67 expression, a marker of cell proliferation (Romano et al., 2022). Chen pi, which is in the list of commonly used herbs, and Zhi ke are sources of bergamottin (Gao et al., 2022), a type of furanocoumarin (Ahmed et al., 2020). Furanocoumarins from natural products are reported to exert anti-tumour effects via apoptosis of malignant tumour cells, autophagy, and cell cycle arrest (Ahmed et al., 2020). Downregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, the phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein Kinase (PI3K/Akt) pathway and G2/M phase cell cycle arrest were potential mechanisms of bergamottin to inhibit carcinogenesis (Ahmed et al., 2020). Overall, formulae including Si jun zi decoction reduced CRA recurrence with low heterogeneity in our review. More research about Si jun zi decoction and its variants for CRA recurrence could be conducted.
Bai zhu and Dang shen are mainly used to strengthen the spleen and qi and remove dampness in CM theory. In modern research, they have been shown to affect intestinal health. Bai zhu has been reported to improve intestinal flora and immune function, reduce inflammation, and promote apoptotic tumour cell death (Zhu et al., 2018). Active metabolites of Bai zhu, the atractylenolides, were reported to inhibit CRA formation (Li et al., 2018) and cell proliferation in colon cancer (Li Y. et al., 2020), as well as regulate apoptosis of colon adenocarcinoma cells via mitochondria-related signalling (Chan et al., 2020). Recent studies found that metabolites derived from Dang shen attenuated inflammation and oxidative injury (Zou et al., 2023), enhanced the immune system in mice (Bai et al., 2020), and inhibited the proliferation and metastasis of tumour cells (Xin et al., 2012; Yu et al., 2023). A review of research showed that Bai zhu contained the flavonoids apigenin and luteolin (Zhu et al., 2018). Luteolin was obtained from Dang shen as well (Yu et al., 2023). Apigenin had inhibitory effects on p38 phosphorylation and expression of NF-κB, blocking epithelial to mesenchymal transition in the HCT116 CRC cell line (Fernández et al., 2021). Luteolin activated antioxidant enzymes, increased Bax expression, and induced apoptosis via caspase-9 and caspase-3 in the H29 human CRC cell line (Fernández et al., 2021). Overall, Bai zhu and Dang shen are possible botanical drugs to prevent CRA recurrence due to their anti-tumour effects in vivo and in vitro (Figure 6). Few studies related to Bai zhu or Dang shen for adenoma recurrence have been conducted; further research on these issues is necessary.
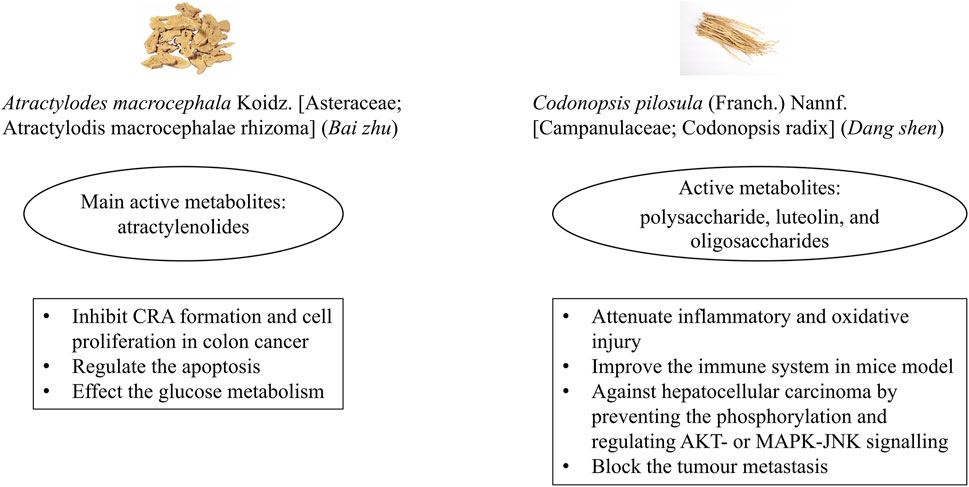
Figure 6. Potential mechanisms of Bai zhu and Dang shen to contribute to colorectal adenoma recurrence. Note: CRA, colorectal adenoma.
In addition, some anti-tumour botanical drugs are listed in the commonly used ingredients, including Yi yi ren, E zhu, Bai hua she she cao, and San qi. Each has received research attention. In a model of pre-CRC in rats, feeding with extracts of Yi yi ren inhibited formation of colonic preneoplastic lesions (Li et al., 2011). Coix seed oil has been shown to promote apoptosis of HT-29 colon cells (Ni et al., 2021). Modern pharmacological research revealed that the combined use of Huang qi and E zhu prevented the growth of CRC cells (Bian et al., 2022). Bai hua she she cao also has anti-tumour effects through regulating multi-pathways, including activation of apoptosis (Zhoufan et al., 2018), downregulation of Wnt signalling (Reyes-Farias and Carrasco-Pozo, 2019), inhibition of tumour cell invasion and migration (Wu et al., 2017), anti-inflammatory effects (He et al., 2018), and enhancement of immune response (Son et al., 2017). Overexpression of the Wnt/β-catenin pathway contributed to colorectal cancer development, with a positive correlation with the level of the transient receptor potential (TRP) cation channel, subfamily melastatin (M), member 8 (TRPM8) in primary colon tumours. Inhibition of TRPM8 led to downmodulation of Wnt pathway transcription and increased expression of oncogenes (C-Myc and Cylin D1) and β-catenin, suggesting a potential treatment target in CRC (Pagano et al., 2023).
We also found some lower-frequency botanical drugs with anti-cancer effects, such as Scutellaria barbata D.Don [Lamiaceae; Scutellariae barbatae herba] (Ban zhi lian), Lobelia chinensis Lour. [Campanulaceae; Lobeliae chinensis herba] (Ban bian lian), and Sparganium stoloniferum (Buch.-Ham. ex Graebn.) Buch.-Ham. ex Juz. [Typhaceae; Sparganii rhizoma] (San leng) (Suh et al., 2007; Jia et al., 2021; Luo et al., 2024). The intervention characteristics, many of which contained botanical drugs to strengthen the spleen plus ones with anti-tumour actions, may explain how the CHMs work to prevent CRA recurrence, even though the anti-tumour botanical drugs are not the same in each formula.
Comparing the recurrence rate (49.81%) of the control group (RC alone) at 12 months to epidemiological data in China, which showed 59.46% at follow-ups of less than and including 1 year (Gao et al., 2010), we found the results were similar. Therefore, the data in our study may reflect the real-world situation to some degree. In our review, oral CHM plus RC reduced the risk of recurrence by 22% (RD 95% CI -0.22 [-0.27, −0.16]) compared to RC alone, showing an acceptable effect of oral CHM.
We analysed sensitivity using the leave-one-out method and examined studies with different characteristics. The effects of omitting any one study were similar to the total effect of all the included studies for CRA recurrence at 12 months post-polypectomy. This suggests our results were not greatly influenced by any single study.
Specific CRA characteristics increase the risk of developing cancer, including adenoma size equal to or exceeding 10 mm, pathological examination showing villous or tubulovillous, high-grade dysplasia, and three or more adenomas (Gupta et al., 2020). However, few included studies assessed CRA characteristics in the follow-up colonoscopy (Zhang et al., 2020; Yao et al., 2023). Future trials should consider the assessment of CRA characteristics as an outcome when exploring CHM effects.
Potential publication bias was observed in studies comparing oral CHM plus RC to RC on CRA recurrence at 12 months after polypectomy. This may be due to the limited number of included studies or the higher chance of publication of studies with positive results.
This paper has limitations. First, we did not search RCT registries or ongoing studies, although we included other types of studies (published articles, theses, and conference papers) to collect a broad range of evidence. Second, the included studies presented “some concerns” on the risk of bias for CRA recurrence, mainly due to insufficient details for randomisation and allocation concealment, protocol registration, and protocol publication. In addition, it is challenging to implement the blinding process when using RC as a control. Participants may know their assigned group if they received oral CHM decoction. Consequently, judgement of risk of bias led to the downgrade of certainty of evidence in our meta-analysis. Finally, we could not assess the pooled result for the effectiveness of oral CHM on advanced CRA because few studies reported this outcome. CRA progression is an essential clinical feature for CRC development and could be investigated in future clinical trials. The growth of colorectal tumours is related to a series of complex pathways. Although consumption of CHMs may modulate some mechanisms in CRC development and become potential botanical drugs, dietary factors and the condition of the microbiome also play important roles.
Our systematic review and meta-analysis suggest there is low-to-moderate-level evidence that oral CHM plus RC is beneficial in reducing CRA recurrence and is well-tolerated in people with CRA. Based on previous pharmacological research, our results suggest that San zi granule and Si jun zi decoction potentially serve as the representative formulae for CRA recurrence prevention. Experimental studies on the frequent botanical drugs have found anti-cancer effects that may explain their mechanisms. However, due to the limited number of clinical studies and insufficient evidence level, it is recommended that large-scale, rigorously designed RCTs could be conducted in the future to provide higher-level clinical evidence.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YC: conceptualization, data curation, formal analysis, methodology, writing–original draft, and writing–review and editing. YD: conceptualization, data curation, formal analysis, methodology, and writing–review and editing. BM: supervision and writing–review and editing. AZ: conceptualization, data curation, formal analysis, methodology, supervision, and writing–review and editing. CX: conceptualization, methodology, resources, supervision, and writing–review and editing. BZ: conceptualization, funding acquisition, methodology, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by a PhD scholarship from the China-Australia International Research Centre for Chinese Medicine, RMIT University. It was also supported by the State Key Laboratory of Wet Syndrome of Traditional Chinese Medicine, co-established by Guangdong Provincial and Ministry of China, research on Artificial Intelligence Image Acquisition and Recognition of Tongue Coating (grant number SZ2021ZZ2701/B67016 and B67017), the Chinese Medicine Science and Technology Research Project of Guangdong Provincial Hospital of Traditional Chinese Medicine, the Establishment of Stepwise screening for early colorectal Cancer by Integrated Traditional Chinese and Western Medicine (YN2020ZWB08), the Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Diseases, the National Science Foundation of Guangdong Province (2022A1515010520), the Planned Science Technology Project of Guangzhou (2023A03J0758), and the Characteristic Innovation Project of Guangdong Province Ordinary University (2022KTSCX028). The funding would not affect the study design and data synthesis for this systematic review or writing of the manuscript.
The authors acknowledge Ms Louise Pobjoy for editing the manuscript and the contributions from the China-Australia International Research Centre for Chinese Medicine and the Department of Gastroenterology at Guangdong Provincial Hospital of Chinese Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1460900/full#supplementary-material
AE, adverse event; AMED, The Allied and Complementary Medicine Database; CHM, Chinese herbal medicine; CI, confidence interval; CRA, colorectal adenoma; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CM, Chinese medicine; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; MeSH, medical subject headings; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RC, routine care; RCT, randomised controlled trial; RoB 2, risk of bias 2; RR, risk ratio.
Ağagündüz, D., Cocozza, E., Cemali, Ö., Bayazıt, A. D., Nanì, M. F., Cerqua, I., et al. (2023). Understanding the role of the gut microbiome in gastrointestinal cancer: a review. Front. Pharmacol. 14, 1130562. doi:10.3389/fphar.2023.1130562
Ahmed, S., Khan, H., Aschner, M., Mirzae, H., Küpeli Akkol, E., and Capasso, R. (2020). Anticancer potential of furanocoumarins: mechanistic and therapeutic aspects. Int. J. Mol. Sci. 21 (16), 5622. doi:10.3390/ijms21165622
Bai, Y, Yang, F., Ma, D., and Zou, W.B. (2014). Guidelines on screening and endoscopic diagnosis and treatment for early colorectal cancer in China (2014, Beijing). Natl. Med. J. China 95 (28), 18. doi:10.3760/cma.j.issn.0376-2491.2015.28.002
Bae, K., Park, J. H., Kim, J., Cho, C. K., Oh, B., Costa, D., et al. (2017). Traditional oriental herbal medicine and natural killer cells for cancer patients: a systematic review and meta-analysis. Phytotherapy Res. 31, 519–532. doi:10.1002/ptr.5781
Bai, R. B., Zhang, Y. J., Fan, J. M., Jia, X. S., Li, D., Wang, Y. P., et al. (2020). Immune-enhancement effects of oligosaccharides from: Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food & Funct. 11 (4), 3306–3315. doi:10.1039/c9fo02969a
Bian, Y., Wang, G., Zhou, J., Yin, G., Liu, T., Liang, L., et al. (2022). Astragalus membranaceus (Huangqi) and Rhizoma curcumae (Ezhu) decoction suppresses colorectal cancer via downregulation of Wnt5/β-Catenin signal. Chin. Med. 17 (1), 11. doi:10.1186/s13020-021-00564-6
Chan, K. W., Chung, H. Y., and Ho, W. S. (2020). Anti-tumor activity of Atractylenolide I in human colon adenocarcinoma in vitro. Molecules 25 (1), 212. doi:10.3390/molecules25010212
Chen, M., May, B. H., Zhou, I. W., Xue, C. C. L., and Zhang, A. L. (2016). Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integr. cancer Ther. 15 (1), 40–59. doi:10.1177/1534735415596424
Chen, S., Yin, T., Pan, Y., Zeng, J., Gu, B., and Bao, Y. (2020a). Meta-analysis of Chinese Medicine for prevention and treatment of recurrence of colorectal polyps Clinical Journal of Traditional Chinese Medicine 32(1), 94–97.
Chen, Y., Tian, Y., Lin, L., Wei, Y., and Zhang, Q. (2023). Effect of Sanzi Prescription on postoperative efficacy, immune function and quality of life of patients treated by endoscopy for colorectal adenoma. Liaoning J. Traditional Chin. Med. 09 (1-10), 4. doi:10.13192/j.issn.1000-1719.2023.09.032
Chen, Y.-X., Gao, Q.-Y., Zou, T.-H., Wang, B.-M., Liu, S.-D., Sheng, J.-Q., et al. (2020b). Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterology & Hepatology 5 (3), 267–275. doi:10.1016/s2468-1253(19)30409-1
Cheng, Y., Di, Y. M., Zhang, A. L., Zhang, B., and Xue, C. C. (2023). Oral Chinese herbal medicine in reducing the recurrence of colorectal adenoma after polypectomy: a protocol for the systematic review and meta-analysis. PLoS One 18 (10), e0293244. doi:10.1371/journal.pone.0293244
Expert Group on Early Diagnosis and Treatment of Cancer, Chinese Society of Oncology, Chinese Medical Association. (2023). Expert consensus on endoscopic diagnosis and treatment for colorectal cancer and precancerous lesions in China (2023, Guangzhou). Chin. J. Dig. Endosc. 40 (7), 505–520. doi:10.3760/cma.j.cn321463-20230607-00229
Chinese, Pharmacopoeia Commission (2020). Chinese pharmacopoeia. Beijing, China: China Medical Science and Technology Press.
Fang, J., Li, Y., Chen, Y., Liu, S., Li, P., Shi, Y., et al. (2021). Chinese consensus on prevention of colorectal neoplasia (2021,Shanghai). Chin. J. Gastroenterology 26 (05), 33.
Fang, S., Guo, S., Du, S., Cao, Z., Yang, Y., Su, X., et al. (2022). Efficacy and safety of berberine in preventing recurrence of colorectal adenomas: a systematic review and meta-analysis. J. Ethnopharmacol. 282, 114617. doi:10.1016/j.jep.2021.114617
Ferlitsch, M., Moss, A., Hassan, C., Bhandari, P., Dumonceau, J. M., Paspatis, G., et al. (2017). Colorectal polypectomy and endoscopic mucosal resection (EMR): European society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy 49 (3), 270–297. doi:10.1055/s-0043-102569
Fernández, J., Silván, B., Entrialgo-Cadierno, R., Villar, C. J., Capasso, R., Uranga, J. A., et al. (2021). Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. & Pharmacother. 143, 112241. doi:10.1016/j.biopha.2021.112241
Gao, L., Zhang, H., Yuan, C.-H., Zeng, L.-H., Xiang, Z., Song, J.-F., et al. (2022). Citrus aurantium ‘Changshan-huyou’—an ethnopharmacological and phytochemical review. Front. Pharmacol. 13, 983470. doi:10.3389/fphar.2022.983470
Gao, Q. Y., Chen, H. M., Sheng, J. Q., Zheng, P., Yu, C. G., Jiang, B., et al. (2010). The first year follow-up after colorectal adenoma polypectomy is important: a multiple-center study in symptomatic hospital-based individuals in China. Front. Med. China 4 (4), 436–442. doi:10.1007/s11684-010-0200-9
Gupta, S., Lieberman, D., Anderson, J. C., Burke, C. A., Dominitz, J. A., Kaltenbach, T., et al. (2020). Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology 158 (4), 1131–1153.e5. doi:10.1053/j.gastro.2019.10.026
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
He, J., Lu, X., Wei, T., Dong, Y., Cai, Z., Tang, L., et al. (2018). Asperuloside and Asperulosidic acid exert an anti-inflammatory effect via suppression of the NF-κB and MAPK signaling pathways in LPS-Induced RAW 264.7 macrophages. Int. J. Mol. Sci. 19 (7), 2027. doi:10.3390/ijms19072027
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front. Pharmacol. 13, 953205–959812. doi:10.3389/fphar.2022.953205
Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, and Page, MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane, 2024. Available from www.training.cochrane.org/handbook.
Higurashi, T., Hosono, K., Takahashi, H., Komiya, Y., Umezawa, S., Sakai, E., et al. (2016). Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet. Oncol. 17, 475–483. doi:10.1016/S1470-2045(15)00565-3
Huang, G., Zhou, Z., and Li, L. (2018). Observation on curative effect of the method of replenishing qi, invigorating spleen, promoting blood circulation and removing blood stasis on the recurrence of adenomatous colorectal polyps after endoscopic resection. Shenzhen J. Intergrated Traditional Chin. West. Med. 28 (9), 42–44. doi:10.16458/j.cnki.1007-0893.2018.09.019
Huang, Y., Gong, W., Su, B., Zhi, F., Liu, S., Bai, Y., et al. (2010). Recurrence and surveillance of colorectal adenoma after polypectomy in a southern Chinese population. J. Gastroenterol. 45 (8), 838–845. doi:10.1007/s00535-010-0227-3
Jia, J., Li, X., Ren, X., Liu, X., Wang, Y., Dong, Y., et al. (2021). Sparganii Rhizoma: a review of traditional clinical application, processing, phytochemistry, pharmacology, and toxicity. J. Ethnopharmacol. 268, 113571. doi:10.1016/j.jep.2020.113571
Jia, Y., Ji, C., Zhang, L., Liu, H., and Xi, Z. (2023). Clinical study on Yiqi Sanjie Prescription combined with routine therapy for patient after surgery of colorectal adenomas. New Chin. Med. 55 (06), 121–126. doi:10.13457/j.cnki.jncm.2023.06.026
Krol, F. J., Hagin, M., Vieta, E., Harazi, R., Lotan, A., Strous, R. D., et al. (2020). Placebo—to be or not to be? Are there really alternatives to placebo-controlled trials? Eur. Neuropsychopharmacol. 32, 1–11. doi:10.1016/j.euroneuro.2019.12.117
Li, L, Jing, L, Wang, J, Hu, W, Gong, X, Zhao, Y, Ma, Y, Yao, X, and Sun, X (2018). Autophagic flux is essential for the downregulation of D-dopachrome tautomerase by atractylenolide I to ameliorate intestinal adenoma formation. J Cell Commun Signal. 2018 Dec; 12 (4): 689–698. doi:10.1007/s12079-018-0454-6
Li, C., Li, Y., and Gong, Y. (2023a). Clinical study of Wumei Pill for preventing recurrence of large intestine polyps after endoscopic resection. Med. Hyg. (9), 76–79.
Li, L., Feng, W., Jiang, Y., Wang, T., Xiao, G., and Zhao, L. (2020a). Effect of Jianpi Xingqi decoction on quality of life and prognosis of patients with colorectal adenoma after endoscopic resection. Hebei J. Traditional Chin. Med. 42 (6), 832–836.
Li, M., Ding, N., Yao, Y., Zhang, Y., and He, Y. (2023b). Clinical effect of Tianma Granules on preventing postoperative recurrence of adenomatous colorectal polyps. China J. Pharm. Econ. 18 (04), 69–71. doi:10.12010/j.issn.1673-5846.2023.04.015
Li, S.-C., Chen, C.-M., Lin, S.-H., Chiang, W., and Shih, C.-K. (2011). Effects of adlay bran and its ethanolic extract and residue on preneoplastic lesions of the colon in rats. Journal of the Science of Food and Agriculture 91 (3), 547–552. doi:10.1002/jsfa.4219
Li, Y, Wang, Y, Liu, Z, Guo, X, Miao, Z, and Ma, S. (2020). Atractylenolide I induces apoptosis and suppresses glycolysis by blocking the JAK2/STAT3 signaling pathway in colorectal cancer cells. Front Pharmacol. Mar 26;11:273. doi:10.3389/fphar.2020.00273
Li, Y., Zhong, C., Yang, S., and Zhang, B. (2021b). Effect of Tiaochang Xiaoliu Formula on the recurrence rate of patients with colorectal adenoma and the expression of Beclin1, p53 and Cox-2 in colon tissue. J. Traditional Chin. Med. 62 (05), 4. doi:10.13288/j.11-2166/r.2021.05.012
Li, Z., Zhu, C., Shang, J., Tian, Y., Wei, L., Hua, Y., et al. (2022). Clinical observation on the prevention and treatment of postoperative recurrence of adenomatous colonic polyp by ethnic medicine Sanzifang. Chin. J. Integr. Traditional West. Med. Dig. 30 (07), 500–504. doi:10.3969/j.issn.1671-039X.2022.07.07
Lieberman, D. A., Rex, D. K., Winawer, S. J., Giardiello, F. M., Johnson, D. A., and Levin, T. R. (2012). Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 143 (3), 844–857. doi:10.1053/j.gastro.2012.06.001
Lin, Y. D., Deng, X. M., and Fu, S. S. (2020). Meta analysis of the prevention and treatment of colorectal adenoma by oral administration of traditional Chinese medicine after endoscopic removal. World Chin. Med. 15 (16), 2408–2413.
Liu, T. W., and Chen, Y. (2010). Study of TCM syndrome features of colorectal polyps in patients. Chin. Archives Traditional Chin. Med. 28 (7), 3. doi:10.13193/j.archtcm.2010.07.220.liutw.041
Luo, J., Chen, Q.-x., Li, P., Yu, H., Yu, L., Lu, J.-l., et al. (2024). Lobelia chinensis Lour inhibits the progression of hepatocellular carcinoma via the regulation of the PTEN/AKT signaling pathway in vivo and in vitro. J. Ethnopharmacol. 318 (Pt A), 116886. doi:10.1016/j.jep.2023.116886
Mahady, S. E., Margolis, K. L., Chan, A., Polekhina, G., Woods, R. L., Wolfe, R., et al. (2021). Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut 70 (4), 717–724. doi:10.1136/gutjnl-2020-321585
Morgan, E., Arnold, M., Gini, A., Lorenzoni, V., Cabasag, C. J., Laversanne, M., et al. (2023). Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72 (2), 338–344. doi:10.1136/gutjnl-2022-327736
Neugut, A. I., Jacobson, J. S., Ahsan, H., Santos, J., Garbowski, G. C., Forde, K. A., et al. (1995). Incidence and recurrence rates of colorectal adenomas: a prospective study. Gastroenterology 108 (2), 402–408. doi:10.1016/0016-5085(95)90066-7
Ni, C., Li, B., Ding, Y., Wu, Y., Wang, Q., Wang, J., et al. (2021). Anti-cancer properties of Coix Seed oil against HT-29 colon cells through regulation of the PI3K/AKT signaling pathway. Foods 10 (11), 2833. doi:10.3390/foods10112833
Ni, M., Zhang, Y., Sun, Z., Zhou, Q., Xiao, J., Zhang, B., et al. (2024). Efficacy and safety of Shenbai Granules for recurrent colorectal adenoma: a multicenter randomized controlled trial. Phytomedicine 127, 155496. doi:10.1016/j.phymed.2024.155496
Ni, W., Liu, T., Liu, Y., Lu, L., Zhou, B., Dai, Y., et al. (2023). Sijunzi decoction granules in the prevention and treatment of recurrence of colorectal adenoma: study protocol for a multicenter, randomized, double-blind, placebo-controlled trial. Front. Pharmacol. 14, 1175811. doi:10.3389/fphar.2023.1175811
Pagano, E., Romano, B., Cicia, D., Iannotti, F. A., Venneri, T., Lucariello, G., et al. (2023). TRPM8 indicates poor prognosis in colorectal cancer patients and its pharmacological targeting reduces tumour growth in mice by inhibiting Wnt/β-catenin signalling. Br. J. Pharmacol. 180 (2), 235–251. doi:10.1111/bph.15960
Pagano, E., Venneri, T., Lucariello, G., Cicia, D., Brancaleone, V., Nanì, M. F., et al. (2021). Palmitoylethanolamide reduces colon cancer cell proliferation and migration, influences tumor cell cycle and exerts in vivo chemopreventive effects. Cancers 13 (8), 1923. doi:10.3390/cancers13081923
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pan, Z. (2021). Effect of Liqi Liujunzi Decoction on symptom improvement and recurrence rate in patients with adenomatous colorectal polyps after endoscopic resection. Pract. Clin. J. Integr. Traditional Chin. West. Med. 21 (6), 84–86. doi:10.13638/j.issn.1671-4040.2021.06.042
Peng, L., Zhang, K., Li, Y., Chen, L., Gao, H., and Chen, H. (2022). Real-world evidence of traditional Chinese medicine (TCM) treatment on cancer: a literature-based review. Evid. Based Complement. Altern. Med. 2022, 7770380. doi:10.1155/2022/7770380
Ren, J. (2021). “Clinical effect of Chu tan-jiedu decoction to prevent recurrence of colorectal adenoma after operation,” Chongqing Medical University. Master thesis.
Reyes-Farias, M., and Carrasco-Pozo, C. (2019). The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 20 (13), 3177. doi:10.3390/ijms20133177
Ribeiro, H., Rodrigues, I., Napoleão, L., Lira, L., Marques, D., Veríssimo, M., et al. (2022). Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: adjusting prescription to patient features. Biomed. & Pharmacother. 150, 112958–116007. doi:10.1016/j.biopha.2022.112958
Romano, B., Pagano, E., Iannotti, F. A., Piscitelli, F., Brancaleone, V., Lucariello, G., et al. (2022). N-Acylethanolamine acid amidase (NAAA) is dysregulated in colorectal cancer patients and its inhibition reduces experimental cancer growth. Br. J. Pharmacol. 179 (8), 1679–1694. doi:10.1111/bph.15737
Şahin, T. Ö., Yılmaz, B., Yeşilyurt, N., Cicia, D., Szymanowska, A., Amero, P., et al. (2023). Recent insights into the nutritional immunomodulation of cancer-related microRNAs. Phytotherapy Res. 37 (10), 4375–4397. doi:10.1002/ptr.7937
Shang, J., Guo, H., Li, J., Li, Z., Yan, Z., Wei, L., et al. (2022). Exploring the mechanism of action of Sanzi formula in intervening colorectal adenoma by targeting intestinal flora and intestinal metabolism. Front. Microbiol. 13, 1001372. doi:10.3389/fmicb.2022.1001372
Shang, L., Wang, Y., Li, J., Zhou, F., Xiao, K., Liu, Y., et al. (2023). Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 302, 115876–117573. doi:10.1016/j.jep.2022.115876
Shao, N., Xiao, Y., Zhang, J., Zhu, Y., Wang, S., and Bao, S. (2022). Modified Sijunzi Decoction inhibits epithelial-mesenchymal transition of non-small cell lung cancer by attenuating AKT/GSK3β pathway in vitro and in vivo. Front. Pharmacol. 12, 821567–829812. doi:10.3389/fphar.2021.821567
Shi, X., Yang, Z., Wu, Q., and Fan, D. (2017). Colorectal adenoma recurrence rates among post-polypectomy patients in the placebo-controlled groups of randomized clinical trials: a meta-analysis. Oncotarget 8 (37), 62371–62381. doi:10.18632/oncotarget.18181
Shi, Y., Zhu, H., Li, R., Wang, D., Zhu, Y., Hu, L., et al. (2019). Effect of polysaccharides from Sijunzi decoction on Ca(2+) related regulators during intestinal mucosal restitution. Phytomedicine 58, 152880. doi:10.1016/j.phymed.2019.152880
Son, Y.-O., Kook, S.-H., and Lee, J.-C. (2017). Glycoproteins and Polysaccharides are the main class of active constituents required for lymphocyte stimulation and antigen-specific immune response induction by traditional medicinal herbal plants. J. Med. Food 20 (10), 1011–1021. doi:10.1089/jmf.2017.3943
Sterne, J. A. C., Savovic, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Suh, S. J., Yoon Jw Fau - Lee, T.-K., Lee Tk Fau - Jin, U.-H., Jin Uh Fau - Kim, S.-L., Kim Sl Fau - Kim, M.-S., Kim Ms Fau - Kwon, D. Y., et al. (2007). Chemoprevention of Scutellaria bardata on human cancer cells and tumorigenesis in skin cancer. Phytotherapy Res. 21, 135–141. doi:10.1002/ptr.2010
Sung, J. J. Y., Chiu, H. M., Lieberman, D., Kuipers, E. J., Rutter, M. D., Macrae, F., et al. (2022). Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut 71 (11), 2152–2166. doi:10.1136/gutjnl-2022-327377
Tanaka, S., Kashida, H., Saito, Y., Yahagi, N., Yamano, H., Saito, S., et al. (2020). Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 32 (2), 219–239. doi:10.1111/den.13545
Trustees of the Royal Botanic Gardens, K. (2024). Kew science medical plant name services. Available at: http://mpns.kew.org/mpns-portal/ (Accessed July 03, 2024).
Wang, Y. (2022). Clinical efficacy of a traditional Chinese medicine Shenqi Herbal Paste for preventing postoperative recurrence of colorectal adenomatous polyps. Shandong University of Traditional Chinese Medicine. Master thesis.
Wu, C., Luo, H., Ma, W., Ren, X., Lu, C., Li, N., et al. (2017). Polysaccharides isolated from Hedyotis diffusa inhibits the aggressive phenotypes of laryngeal squamous carcinoma cells via inhibition of Bcl-2, MMP-2, and μPA. Gene 637, 124–129. doi:10.1016/j.gene.2017.09.041
Xin, T., Zhang, F., Jiang, Q., Chen, C., Huang, D., Li, Y., et al. (2012). The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int. J. Biol. Macromol. 51 (5), 788–793. doi:10.1016/j.ijbiomac.2012.07.019
Xiong, X., and Gu, Y. (2020). Clinical observation after the treatment of colorectal adenoma by JianPi QuShi Recipe under endoscope. Chin. J. Surg. Integr. Traditional West. Med. 26 (5), 964–967.
Xv, H. (2019). “Clinical study of postoperative recurrence of Shen Shao Cream prevents adenomatous polyposis of large intestine,”. Shandong University of Traditional Chinese Medicine. Master thesis.
Xv, Y., Long, R., Yang, J., Yang, J., Lv, W., Wang, X., et al. (2021). Study on the effect of Jianpi Huazhuo Prescription on serum inflammatory factors, gastrointestinal function recovery and polyp recurrence after endoscopic colorectal adenomatous polypectomy. Jiangsu J. Traditional Chin. Med. 53 (7), 30–33.
Yamaji, Y., Mitsushima, T., Ikuma, H., Watabe, H., Okamoto, M., Kawabe, T., et al. (2004). Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut 53 (4), 568–572. doi:10.1136/gut.2003.026112
Yan, X., Chen, F., and Kao, Y. (2018). “Clinical study on Shushao Granules in preventing postoperative recurrence of adenomatous colorectal polyps,” in The fourth spleen and stomach disease professional committee of shandong association of traditional Chinese medicine inaugural conference and the first academic exchange meeting (Jinan, China).
Yang, L., Ji, C., Li, Y., Hu, F., Zhang, F., Zhang, H., et al. (2020). Natural potent NAAA inhibitor atractylodin counteracts LPS-induced microglial activation. Front. Pharmacol. 11, 577319. doi:10.3389/fphar.2020.577319
Yao, T., Lin, L., Tian, Y., Zhu, C., Wei, L., and Fei, Z. (2023). Clinical study on treatment of colorectal adenoma with dampness and stasis block by Yiqi Huashi Xiaoyu Recipe. Beijing J. Traditional Chin. Med. 5.
Yu, Y., Ding, S., Xu, X., Yan, D., Fan, Y., Ruan, B., et al. (2023). Integrating network pharmacology and bioinformatics to explore the effects of Dangshen (Codonopsis pilosula) against hepatocellular carcinoma: validation based on the active compound luteolin. Drug Des. Dev. Ther. 17, 659–673. doi:10.2147/DDDT.S386941
Yuan, G. (2017). The clinical study of Wumei pill on preventing the recurrence of colorectal adenoma after endocospic resection. Nanjing University of Chinese Medicine. Master.
Zhang, B., Wei, W., Li, A., Zhong, C., Yan, Q., Yang, Y., et al. (2021). Expert consensus on diagnosis and treatment of colorectal adenoma and early colorectal cancer by integrated Chinese and Western medicine (2021). J. Traditional Chin. Med., 989–997.
Zhang, B., Zhong, C., Liang, B., Li, J., Zhao, X., and Wei, W. (2020). 1-year recurrence rate of colorectal adenoma treated by Tiaochang Xiaoliu Formula: a randomized controlled clinical trial of 176 cases. J. Traditoanl Chin. Med. 61 (22), 1971–1976.
Zhao, H. (2020). “Clinical study on Changxihuaji decoction in preventing recurrence of colorectal adenomatous polyps (Spleen and Stomach deficiency),” Inner Mongolia Medical University. Master thesis.
Zhou, J. Y., Chen, M., Wu, C. E., Zhuang, Y. W., Chen, Y. G., and Liu, S.A.-O. (2019). The modified Si-Jun-Zi Decoction attenuates colon cancer liver metastasis by increasing macrophage cells. BMC complementary Altern. Med. 19, 86–6882. doi:10.1186/s12906-019-2498-4
Zhou, M. L., Sun, J.W., Tan, J., and Chen, C.H. (2020). Meta-analysis on prevention and treatment of recurrence of colon polyp by spleen-strengthening method. China’s Naturopathy 28(20), 68–72.
Zhoufan, X., Ziyan, G., Yun, W., Jiachuan, L., and Jianqing, Y. (2018). Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy: protocatechuic acid inhibits growth of ovarian cancer cells. Phytotherapy Res. 32, 2256–2263. doi:10.1002/ptr.6163
Zhu, B., Zhang, Q.L., Hua, J.W., Cheng, W.L., and Qin, L.P. (2018). The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: a review. J. Ethnopharmacol. 226, 143–167. doi:10.1016/j.jep.2018.08.023
Zhu, Y., Ma, R., Cheng, W., Qin, M., Guo, W., Qi, Y., et al. (2024). Sijunzi decoction ameliorates gastric precancerous lesions via regulating oxidative phosphorylation based on proteomics and metabolomics. J. Ethnopharmacol. 318, 116925–117573. doi:10.1016/j.jep.2023.116925
Zou, Y., Yan, H., Li, C., Wen, F., Jize, X., Zhang, C., et al. (2023). A Pectic Polysaccharide from Codonopsis pilosula alleviates inflammatory response and Oxidative stress of aging mice via modulating intestinal microbiota-related gut–liver axis. Antioxidants 12 (9), 1781. doi:10.3390/antiox12091781
Keywords: Chinese herbal medicine, colorectal adenoma recurrence, San zi granule, Si jun zi decoction, systematic review, meta-analysis, colorectal cancer, colorectal polyp
Citation: Cheng Y, Di YM, May B, Zhang AL, Xue CC and Zhang B (2025) Effects of Chinese herbal medicine on colorectal adenoma recurrence following polypectomy: a systematic review and meta-analysis. Front. Pharmacol. 16:1460900. doi: 10.3389/fphar.2025.1460900
Received: 07 July 2024; Accepted: 05 February 2025;
Published: 20 March 2025.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2025 Cheng, Di, May, Zhang, Xue and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlie Changli Xue, Y2hhcmxpZS54dWVAcm1pdC5lZHUuYXU=; Beiping Zhang, ZG9jdG9yemJwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.