
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 31 January 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1449639
This article is part of the Research Topic Multi-omics Technology: Revealing the Pathogenesis of Diseases and the Mechanism of Drug Efficacy of Major Diseases such as Nutritional Metabolism Disorders and Mental Disorders View all 10 articles
 Chaofang Lei1
Chaofang Lei1 Jiaxu Chen2
Jiaxu Chen2 Zhigang Chen3
Zhigang Chen3 Chongyang Ma4
Chongyang Ma4 Xudong Chen5
Xudong Chen5 Xiongxing Sun1
Xiongxing Sun1 Xukun Tang1
Xukun Tang1 Jun Deng1
Jun Deng1 Shiliang Wang1
Shiliang Wang1 Junlin Jiang1
Junlin Jiang1 Dahua Wu1*
Dahua Wu1* Le Xie1*
Le Xie1*Spatial metabolomics is an emerging technology that integrates mass spectrometry imaging (MSI) with metabolomics, offering a novel visual perspective for traditional metabolomics analysis. This technology enables in-depth analysis in three dimensions: qualitative, quantitative, and localization of metabolites. Spatial metabolomics precisely reflects the characteristics of metabolic network changes in metabolites within entire tissues or specific micro-regions. It provides a detailed understanding of the pharmacodynamic material basis and mechanisms of action. These capabilities suggest that spatial metabolomics can offer significant technical support for studying the complex pathophysiology of mental disorders. Although the mechanisms underlying mental disorders have been reviewed multiple times, this paper provides a comprehensive comparison between traditional metabolomics and spatial metabolomics. It also summarizes the latest progress and challenges of applying spatial metabolomics to the study of mental disorders and traditional Chinese medicine.
Mental disorders are common and burdensome. Among people with severe mental illness, deaths from unnatural causes have increased significantly, occurring 13 times more frequently compared to the general population, with suicide being the leading cause (20 times higher) (Revier et al., 2015). Antidepressants and antipsychotics remain the primary strategies for treating psychiatric symptoms. However, the long-term efficacy of drug treatment has been questioned. For many individuals with mental illness, antipsychotics do not result in clinically meaningful long-term improvements and often cause significant side effects (Malhi and Mann, 2018), such as weight gain, elevated blood sugar levels, elevated blood lipids, and loss of sexual interest. These side effects frequently lead to withdrawal and discomfort (De Hert et al., 2011; Vancampfort et al., 2015). Over the past few decades, the use of traditional Chinese medicine (TCM) to treat various mental disorders, including depression, has grown significantly. Studies suggest that TCM is a safer alternative to drug therapy, with a lower risk of side effects (Yeung K. S. et al., 2018).
Traditional Chinese medicine operates under the guidance of traditional Chinese medicine theory. It exhibits characteristics such as multi-metabolite, multi-target, multi-approach, and holistic concept (Ma et al., 2023; Wang and Zhang, 2017; Zheng et al., 2024; Song et al., 2023). While traditional Chinese medicine demonstrates remarkable clinical efficacy, its modern development faces constraints due to unclear efficacy substances and mechanisms of action. In recent years, there has been rapid development in metabolomics technology (Misra, 2018; Bingol and Brüschweiler, 2017). Its research strategy, which is based on detecting the dynamic changes of global metabolites, aligns with traditional Chinese medicine theory (Zhang et al., 2010; Wei et al., 2024). This alignment presents new opportunities to address the developmental challenges faced by traditional Chinese medicine. Metabolomics technology has been extensively employed in researching the material basis and pharmacodynamic mechanisms of traditional Chinese medicine, yielding promising results (Wang et al., 2017).
However, the metabolism and distribution of traditional Chinese medicine metabolites in organisms often exhibit precise spatial positioning (Bai et al., 2022). The efficacy of the medication is closely linked to the spatial distribution of biological tissue or micro-regions. Nevertheless, traditional metabolomics methods have limitations in sample pre-processing, resulting in the absence of spatial distribution information of metabolites in tissues (Liu G. X. et al., 2023). This absence poses challenges in fully and objectively interpreting the sites of action and pharmacodynamic mechanisms of traditional Chinese medicine. Spatial metabolomics enables the correlation of metabolites and their biological functions with the anatomical characteristics of biological tissues (Sun et al., 2019; Nakabayashi et al., 2021). This approach allows for a more accurate and scientific analysis of the pharmacodynamic metabolites of traditional Chinese medicines and the regulatory mechanisms of diseases within organisms.
Indeed, these methods also facilitate the comprehensive characterization of metabolic functions at physiological and pathological time scales with high spatial resolution. Mass spectrometry imaging (MSI) is a powerful method to perform in situ analysis of the molecular composition of biological tissues while retaining spatial information (Parrot et al., 2018). Furthermore, spatial metabolic characterization holds significant relevance to our comprehension of normal physiological processes and the neuropathological manifestations of neurological disorders (Wang et al., 2022).
Hence, spatial metabolomics technology was utilized to establish the relationship of “molecular structure-spatial distribution-content change-metabolic pathway,” offering novel insights into the search for medicinal metabolites, therapeutic targets, and mechanisms of action of traditional Chinese medicine (Zhao et al., 2023a).
This paper provides a comprehensive overview of the research progress in spatial metabolomics technology concerning the quality control, metabolic distribution, pharmacodynamic mechanisms, and toxicity mechanisms of Chinese medicine. Additionally, it critically examines the limitations and future development directions of spatial metabolomics in the study of Chinese medicine for treating mental diseases. These insights aim to furnish a theoretical basis for advancing the modernization and internationalization of Chinese medicine in the treatment of mental diseases.
Metabolomics involves the systematic study of small and medium molecules in biological fluids. The term “metabolomics” was first coined by Dr. Nicholson of Imperial College London in 1999 (Yu et al., 2017). While metabolomic analysis shares similarities with other high-throughput methods like genome sequencing, its rapid response to both exogenous and endogenous stimuli renders it particularly sensitive to changes in health status (Dona et al., 2016). Spatial metabolomics has been developed based on mass spectrometry imaging technology, characterized by its lack of labeling, matrix, and short analysis cycle (McDonnell and Heeren, 2007; Zang et al., 2021). Serving as a novel molecular imaging technology, spatial metabolomics can directly provide spatial distribution information of numerous known or unknown endogenous metabolites and exogenous drugs from biological tissues (Wang Z. et al., 2021). By employing mass spectrometry imaging technology, spatial metabolomics enables the analysis of metabolites in different tissues and organs in three dimensions, including qualitative, quantitative, and localization aspects. This breakthrough overcomes the limitations of traditional metabolomics research, which often loses spatial information. The comparative analysis of traditional and spatial metabolomics platforms and their respective application conditions are summarized in Table 1.
DESI-MS, introduced in 2004, is an atmospheric pressure environmental ionization method that directly ionizes solid-phase samples (Takáts et al., 2004). DESI-MSI employs the fundamental principle of electrospray ionization, wherein solvent droplets are rasterized and desorbed directly onto the sample surface (Parrot et al., 2018; Eberlin et al., 2011). DESI operates at room temperature, eliminating the need for freeze-drying prior to analysis. This method of tissue imaging minimizes sample damage through environmental ionization mass spectrometry, enabling repeated measurements of samples from diverse biological sources (Soudah et al., 2023). Ambient MSI offers a user-friendly interface and facilitates the rapid analysis of larger samples, thereby facilitating real-time diagnostic capabilities (Luo et al., 2013; Keller et al., 2018).
However, enhancing the sensitivity of DESI-MSI presents substantial challenges (Wang et al., 2017). Recent studies have demonstrated that the sensitivity and specificity of DESI-MSI nanoparticles can be enhanced by incorporating silver ions into the solvents (Lillja and Lanekoff, 2022). Researchers have developed a compact post-photoionization module integrated with DESI, enabling the detection of enhanced signal strength for non-polar compounds. This advancement significantly enhances the sensitivity of DESI-MSI to non-polar compounds (Liu C. et al., 2019). Furthermore, there are emerging indications that the spatial resolution of DESI will pose a substantial impediment in numerous other applications where it could be potentially beneficial (Qi et al., 2021). Consequently, scientists are currently engaged in a concerted effort to significantly enhance the spatial resolution of DESI. Subsequent research has demonstrated that nano DESI-MSI possesses the potential to attain even finer spatial resolution, potentially reaching a resolution of 10 microns (Yin et al., 2018; Yin et al., 2019; Yang et al., 2023).
The concept of MALDI-MSI was initially introduced in the early 2000s (Morisasa et al., 2019). MALDI is a soft ionization technique that involves the co-crystallization of a sample molecule or analyte with a matrix to form a sample matrix crystal. The matrix functions as a proton donor or acceptor, ionizing the analyte (Yalcin and de la Monte, 2015). MALDI-MSI operates by directing a laser beam at the surface of a specimen, typically a frozen section of tissue. This laser action induces the desorption of ions from the tissue, which are subsequently analyzed through a mass spectrometer (Basu and Agar, 2021; Kuik et al., 2024). Despite ongoing technological advancements, the low detection sensitivity of certain compounds poses a significant challenge that requires effective solutions. Research has indicated that poor sensitivity is often associated with reduced ionization efficiency, low analyte and matrix ion abundance, or endogenous interference. Histochemical derivatization has emerged as a crucial strategy to address these challenges, preserve tissue integrity, and mitigate potential dislocations (Merdas et al., 2021).
AFADESI-MSI employs DESI technology to directly ionize the sample using an electrospray plume. Subsequently, a gas stream propels the ions over extended distances, enabling mass spectrometry imaging (Luo et al., 2013). In addition to inheriting the advantages of DESI-MSI, AFADESI-MSI can also attain exceptionally high metabolite coverage. It is an environmental molecular imaging technology characterized by its high sensitivity, extensive coverage, and exceptional chemical specificity (He M. J. et al., 2022). A significant advantage of this approach is its direct predictive applicability to a substantial number of candidate metabolites and metabolic enzymes, eliminating the necessity to define a specific target of interest beforehand (Sun et al., 2019). Although this novel technique yields drug signal strength, it cannot objectively reflect the absolute drug content in various tissues due to sample heterogeneity, ion inhibition, analyte extraction efficiency, and ionization efficiency (Zhang et al., 2020).
The SIMS instrument bombards the sample surface with a finely focused primary ion beam (an analysis gun) to generate characteristic secondary ions from the sample surface. These secondary ions are subsequently detected using a mass analyzer. By rasterizing the primary ion beam on the surface of a solid sample, mass-resolved secondary ion images can be obtained, thereby providing chemical mapping of each component of the surface (Huang et al., 2017). The primary advantage of SIMS lies in its capability to measure the spatial localization of molecules with exceptional spatial resolution. It is particularly effective in targeting inorganic compounds or biomolecules with relatively low molecular weights (Wu et al., 2013). Although samples for SIMS do not necessitate any special surface treatment, it is important to note that SIMS can be a destructive analysis technique, which may lead to sample loss. Furthermore, quantifying the composition of SIMS samples can be challenging due to matrix effects and fragmentation processes that occur during SIMS analysis (Huang et al., 2017).
In summary, mass spectrometry imaging (MSI), a tool capable of in situ quantitative qualitative and two-dimensional imaging, is characterized by high stability, high throughput, and label-free. The above MSI techniques have their characteristics. MALDI is suitable for detecting various small and large molecules, and it is the most used technique in multiple fields, but the preparation process is relatively complicated. DESI is more accurate for the in situ localization of small molecules in tissue slices, but the spatial resolution is relatively low compared with the other techniques. DESI has a broader range of application scenarios than the different techniques, and it can be used for detection at room temperature. SIMS can measure the spatial localization of molecules with high spatial resolution, but the sample components used for SIMS may be lost, generating fragment ions that can severely interfere with the detection signals of small chemical molecules. AFADESI directly inherits the advantages of DESI but also optimizes the technology based on it.
Chinese medicine quality control plays a vital role in ensuring the clinical efficacy of Chinese medicine (Li X. R. et al., 2021). The medicinal parts, metabolites, and distribution of traditional Chinese medicine directly reflect its quality, but traditional analysis methods often face challenges in achieving comprehensive assessments. MSI emerges as a novel analytical method that overcomes the technical limitations of traditional approaches. MSI technology encompasses secondary ionization (SI), matrix-assisted laser desorption ionization (MALDI), and desorption electrospray ionization (DESI) methods based on ionization techniques (Ganesana et al., 2017). Notably, MSI eliminates the need for intricate sample pretreatment steps and offers the capability to detect known or unknown metabolites with high throughput, sensitivity, and resolution (Zheng et al., 2023). It serves as a straightforward and swift approach for identifying quality markers in Chinese medicine, enabling the direct characterization of chemical features and spatial distribution across various samples. Consequently, MSI holds promising applications in Chinese medicine quality control (Jiang H. et al., 2022; Dong and Aharoni, 2022). Table 2 presents an overview of spatial metabolomics studies in Chinese medicine quality control.
The distribution and metabolism of TCM active metabolites in tissues are crucial for identifying target organs, understanding the pharmacodynamic material basis, and evaluating potential adverse reactions of TCM. However, traditional analysis techniques often destroy tissue structure during sample preparation, making it difficult to clearly characterize the regional distribution of active ingredients and metabolites of TCM. MSI can extract extensive data and provide information about the spatial distribution of these data by analyzing tissue slices (Xu et al., 2022). Spatial metabolomics can simultaneously characterize the spatial metabolic distribution of TCM active metabolites and their metabolites in the whole or micro-regions of different tissues and organs. This approach presents a more complete metabolic process and is a significant analytical technique in neuroscience research (Liang et al., 2022). Table 3 shows studies on the metabolic distribution of TCM in organisms.
Drug safety poses a significant threat to human health. Toxicological analysis and safety evaluation are crucial aspects of drug development. Conventional analysis methods cannot provide spatial distribution information. However, spatial dimension analysis can supplement safety evaluations, enabling better prediction and assessment of drug toxicity (Chen et al., 2023b). Spatial metabolomics allows us to study the distribution of toxic Chinese medicine components and their metabolites in tissues and organs. This technique provides a scientific basis for identifying toxic target organs and revealing toxic molecular mechanisms (Table 4).
Schizophrenia, a major mental illness, involves lipids playing a crucial role. The authors (Matsumoto et al., 2011) have demonstrated the association between lipid analysis and brain functional mapping in postmortem human brains. They identified the types of lipids in normal human brains using LC/ESI-MS/MS. Subsequently, MALDI-MSI analysis of brain tissue was conducted to screen for differentially expressed lipid types between the control group and two schizophrenia patients. In this study, the authors report the abnormal distribution of a molecular species of phosphatidylcholine (PC), specifically in the cortical layer of the frontal cortex region, postmortem in patients with schizophrenia. Additionally, PC (diacyl-16:0/20:4) containing arachidonic acid showed an increase in the frontal cortex of patients with schizophrenia. MALDI-MSI holds a specific advantage in revealing abnormalities in local lipid metabolism in the human brain after death. Moreover, it complements previous findings indicating abnormal brain lipid composition in schizophrenia patients (McNamara et al., 2007; Taha et al., 2013).
The corpus callosum (CC) serves to connect the brain’s hemispheres, yet individuals with schizophrenia exhibit impaired interhemispheric communication, potentially contributing to brain disconnection (Guo et al., 2013). Researchers (Vendramini et al., 2016) utilized DESI-MSI to compare lipid content in postmortem CC samples from two schizophrenia patients and two controls in a label-free manner. The findings reveal a noteworthy reduction in the distribution of phosphatidylcholine in patients with schizophrenia. Interestingly, the 760 Da ions show a much lower abundance of phosphatidylcholine compared to the 788 Da ions. This study marks the first investigation into CC white matter in schizophrenia patients and strongly supports the hypothesis that phospholipid dysfunction is prevalent in schizophrenia (Ross et al., 1997). Despite limitations in sample size, these studies contribute to the molecular understanding of the disease, as well as the identification of biomarkers and drug targets. Phospholipids are bioactive substances crucial for brain function. To analyze differences in the amount and type of phospholipids present in the brain tissue of schizophrenic patients, the authors (Matsumoto et al., 2017) conducted a comprehensive analysis of phospholipids in the postmortem brains of elderly schizophrenic patients. In LC-ESI/MS/MS, the authors found significantly reduced levels of 16:0/20:4-phosphatidylinositol (PI) in the prefrontal cortex of the brain in patients with schizophrenia, while 16:0/20:4-PI was most notably reduced in the gray matter in MALDI-MSI.
Stress represents a risk factor for the development and exacerbation of various diseases, including neuropsychiatric disorders and depression (Sanacora et al., 2022; Park et al., 2019). The endocannabinoid 2-arachidonoylglycerol (2AG) serves as a vital regulator of stress response, with its brain levels increasing in response to heightened stress. Researchers (Islam et al., 2022) investigated the impact of stress on 2AG levels in specific brain regions of senescence-accelerated mouse prone (SAMP8). Utilizing DESI-MSI, they observed a significant increase in 2AG levels in the hypothalamus, midbrain, and hindbrain of SAMP8 mice following 3 days of water immersion stress. Previous reports (Zhai et al., 2023) utilizing DESI-MSI analysis of coronal brain sections from stressed mice indicated that 2-AG levels were highest in the hypothalamus region and lowest in the hippocampus, spanning from forebrain to cerebellum. Furthermore, this study demonstrated elevated levels of endocannabinoid 2-AG in the Anterior Cingulate Cortex, Caudate Putamen, Nucleus Accumbens, and Piriform Cortex in individuals experiencing chronic stress. postpartum depression (PPD) presents a severe mental disorder with significant adverse effects on maternal health. Researchers (Sheng et al., 2024) employed MSI and targeted metabolomics analysis to investigate metabolic changes in the brains of postpartum mice with GABAAR Delta-subunit defects (Gabrd−/−), serving as a specific preclinical model of PPD. This study identified the downregulation of prostaglandin D2 (PGD2) in the central amygdala (CeA) as the most notable change in PPD.
Drug addiction remains a significant global health concern. Researchers (Uys et al., 2010) employed a combination of MALDI-MSI tissue mapping, MALDI-MSI tissue imaging, and bioinformatics analysis to discern differences in protein expression and localization in the nucleus accumbens (NAc) of cocaine-sensitized rats. Through additional sequencing experiments via MALDI tandem mass spectrometry and a database search of measurement quality, they identified an increase in expression of secretoneurin (m/z 3653). Moreover, the distribution of secretoneurin in the NAc was determined through MALDI tissue imaging, and the heightened expression of its precursor protein, secreted granuloprotein II, was verified via Western blotting. This spatial localization aligns with previous immunolocalization studies of secreted neurotin (Marksteiner et al., 1993). Prolonged exposure to morphine can lead to the development of addictive behaviors, and early diagnosis may mitigate the adverse effects of these behaviors on individuals and society. The authors (Bodzon-Kulakowska et al., 2016) utilized the brains of morphine-addicted rats for DESI analysis. Following morphine administration, the substance exhibited marked overexpression in the medial forebrain bundle, hypothalamic nuclei, and fornix region. Furthermore, two systems (BioMap, Datacube) were utilized to analyze images of rat brain tissue under morphine and compare their ease of use and the quality of results obtained. The ST (22:0) ratio of morphine to control rat brain peak intensity was 3.44 for BioMap and 3.55 for Datacube. Although the results were similar, the authors posit that BioMap proves more beneficial for DESI IMS analysis. The application of spatial metabolomics to mental disorders is summarized in Figure 1.
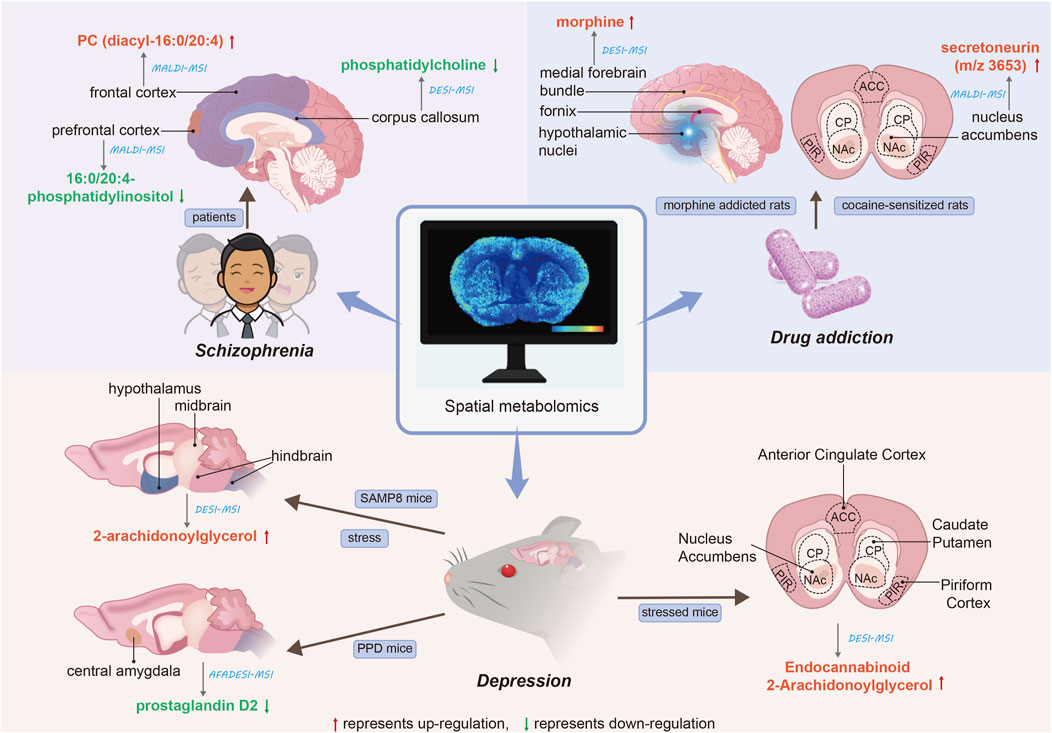
Figure 1. Applications of spatial metabolomics to mental disorders. The red arrows denote the upregulation of the corresponding metabolite, while the green arrows indicate the downregulation of the corresponding metabolite. The straight lines illustrate the spatial distribution of metabolites within brain tissue. The blue italics represent the analytical techniques employed, and the blue text boxes indicate the research subjects. The black italics denote the type of mental disorder.
Chinese medicine has unique advantages in psychiatric disorders and the adverse effects of antipsychotic drugs. Therefore, Chinese medicine’s efficacy in treating psychiatric disorders has been gradually emphasized in clinical practice. The application of antipsychotic drugs is still the primary treatment for mental disorders at this stage. However, poor patient compliance, a sense of discrimination, adverse drug reactions, and complex interactions between different drugs often adversely affect clinical efficacy during treatment. Moreover, almost all the essential principles of drug action established in Western psychopharmacology in the 20th century were discovered empirically in TCM during the 2000 years of evolution (Shorter and Segesser, 2013). In recent years, researchers have made significant progress in basic research and clinical treatment of mental disorders based on TCM characteristics. In today’s clinical therapeutic practice, TCM therapy combined with Western medicine is mainly used for treatment, which TCM treatment is diverse, including but not limited to decoction, Chinese patent drug, acupuncture, TCM gongfu (Baduanjin, Qigong, and Tai-Chi) and Five-Element Music (Xu et al., 2011; Lin et al., 2012; Chan et al., 2015; Zhao et al., 2019; Li et al., 2022; Lam et al., 2024; Zhou, 2020; Wu et al., 2024; Chen et al., 2015; Yeung A. et al., 2018; Zou et al., 2018).
TCM has a complex and diffuse composition, and its formulation is a complex combination of several natural medicines. The study of TCM on the etiology and pathogenesis of mental disorders is still at an exploratory stage. In recent years, accumulated studies have revealed the application of spatial metabolomics approaches to study the etiology and pathophysiology of complex systemic disorders, including depression and other psychiatric disorders, as well as the mechanisms of TCM effects. However, a single “metabonomics” technique may not fully reflect the mechanisms by which TCM treats mental disorders. Therefore, in the study of mental disorders, data from spatial metabolomics, spatial proteomics, and spatial transcriptomics should be integrated to decipher the biological significance and spatial correlation from differential metabolites, proteins, and genes further to explore the mechanism of TCM for mental disorders. So far, most of the studies on TCM for mental disorders first started with untargeted metabolomics. Then, a series of different endogenous metabolites were obtained from standard controls to infer disease-related metabolic pathways, which provided clues for further mechanistic studies but, at the same time, lacked specificity (Gu et al., 2021). Based on this phenomenon, we propose that future studies should not be limited to full-spectrum metabolites but should also focus on targeted metabolomics for further validation. In addition, each metabolomics platform has its advantages and limitations, and multiple platforms should be clustered to apply for targeted metabolomics studies to obtain different spatial metabolomics data to discover and characterize common biomarkers when conditions allow, which in turn will collectively provide new ideas for the development of antidepressant natural products for psychiatric disorders. Despite the rapid growth in the application of metabolomics for treating psychiatric disorders under TCM interventions (Liu, 2020; Zhu et al., 2024; Lv et al., 2022; Zhou et al., 2020), the application of spatial metabolomics is still in the preliminary research stage. We can foresee that shortly, researchers will vigorously carry out corresponding animal models and even clinical studies in spatial metabolomics research of TCM for the treatment of mental disorders. Meanwhile, under the extensive guidance of spatial metabolomics, TCM is expected to become a more acceptable therapeutic option for treating mental disorders.
Today, the unique position of spatial metabolomics in the field of nervous system research is widely acknowledged, and it has begun to find application in studying the metabolic mechanisms of human mental diseases and in the development of new drugs. Serving as a breakthrough technology, spatial metabolomics has opened up numerous new opportunities for the molecular diagnosis of mental diseases treated with traditional Chinese medicine. Nevertheless, it also encounters various challenges, such as metabolite identification and chromatographic separation, as well as issues related to mass spectrometry databases and data sharing (Collins et al., 2021).
Fortunately, advancements in instrumentation, experimental techniques, and analytical software have helped alleviate many of these challenges. For instance, researchers can overlay MS images with optical or HE scans and focus on tissue microregions or lesions of interest to accurately extract mass spectrometry data for the target region in metabolic studies. This approach mitigates the challenges associated with the difficult isolation of study specimens (Jiang H. et al., 2022). In future studies, we can explore three-dimensional MSI, construct multiple slices of two-dimensional MSI data, and visualize another dimension of drug distribution. Furthermore, the biological computing challenges associated with increased spatial resolution also necessitate the development of more efficient data mining tools (Angel and Caprioli, 2013).
It can be predicted that multi-omics joint analysis will become a key research strategy in the future. This approach not only mitigates the data deficiencies stemming from data noise and missingness in single omics analysis, but also reduces the false positive outcomes generated by single omics analysis through the mutual verification of multiple omics data resources. Consequently, multi-omics joint analysis is more conducive to systematically analyzing the multifaceted mechanisms or phenotypic connections of biological models at various levels and perspectives. Moreover, it facilitates the collaborative exploration of potential regulatory network mechanisms within organisms (Zheng et al., 2023).
Specifically, in-depth studies on the spatial distribution of active/toxic ingredients and their metabolites about different metabolites in vivo will be carried out to clarify the active/poisonous ingredients and their target areas and to elucidate the mechanisms of the efficacy or toxicity of traditional Chinese medicines more accurately. We can combine spatial metabolomics with spatial proteomics and spatial transcriptomics to realize multi-dimensional studies on quality control, metabolic distribution, and pharmacodynamic or toxicity mechanisms of TCM at metabolic, protein, and gene levels. The blood-brain barrier maintains the relative stability of the intracerebral environment and blocks drug molecules outside the barrier. The combination of MSI and 3D imaging is also strategically important in studying the intracerebral distribution of drugs and neurological side effects.
Currently, spatial metabolomics has shown vigorous development in exploring the metabolic mechanisms of the nervous system. However, the nascent application of traditional Chinese medicine in the treatment of mental diseases remains underdeveloped. It is worthwhile to expect that MSI technology will provide a new vision for treating mental disorders in Chinese medicine, and the application of spatial metabolomics in treating mental disorders in Chinese medicine will become a key research direction.
Therefore, further research is imperative, as it holds significant guiding implications for studying the metabolic mechanisms underlying TCM treatment of mental diseases. In summary, there exists substantial room for the development of spatial metabolomics in the realm of traditional Chinese medicine and mental illness. Through continual refinement and innovation, it can significantly contribute to the modernization of traditional Chinese medicine.
CL: Writing–original draft, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization. JC: Conceptualization, Methodology, Writing–review and editing. ZC: Conceptualization, Methodology, Writing–review and editing. CM: Data curation, Investigation, Writing–review and editing, Methodology. XC: Investigation, Methodology, Writing–review and editing. XT: Investigation, Writing–review and editing. XS: Investigation, Writing–review and editing. JD: Investigation, Software, Writing–review and editing. SW: Data curation, Investigation, Writing–review and editing. JJ: Data curation, Software, Writing–review and editing. LX: Methodology, Supervision, Writing–review and editing. DW: Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82104831), Furong Laboratory Science and Technology Project (No. 2023SK2113-2), Hunan Science and Technology Innovation Project (No. 2021SK51005), Hunan Science and Technology Innovation Project (No. 2023RC3215).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Angel, P. M., and Caprioli, R. M. (2013). Matrix-assisted laser desorption ionization imaging mass spectrometry: in situ molecular mapping. Biochemistry 52 (22), 3818–3828. doi:10.1021/bi301519p
Asampille, G., Cheredath, A., Joseph, D., Adiga, S. K., and Atreya, H. S. (2020). The utility of nuclear magnetic resonance spectroscopy in assisted reproduction. Open Biol. 10 (11), 200092. doi:10.1098/rsob.200092
Bagatela, B. S., Lopes, A. P., Cabral, E. C., Perazzo, F. F., and Ifa, D. R. (2015). High-performance thin-layer chromatography/desorption electrospray ionization mass spectrometry imaging of the crude extract from the peels of Citrus aurantium L. (Rutaceae). Rapid Commun. Mass Spectrom. 29 (16), 1530–1534. doi:10.1002/rcm.7246
Bai, H., Wang, S., Liu, J., Gao, D., Jiang, Y., Liu, H., et al. (2016). Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1026, 263–271. doi:10.1016/j.jchromb.2015.09.024
Bai, R. H. (2016). Analysis of saponins in panax species using MALDI-TOF mass spectrometry imaging. Tsinghua University.
Bai, X., Zhu, C., Chen, J., Jiang, X., Jin, Y., Shen, R., et al. (2022). Recent progress on mass spectrum based approaches for absorption, distribution, metabolism, and excretion characterization of traditional Chinese medicine. Curr. Drug Metab. 23 (2), 99–112. doi:10.2174/1389200223666220211093548
Ban, W., Jiang, X., Lv, L., Jiao, Y., Huang, J., Yang, Z., et al. (2024). Illustrate the distribution and metabolic regulatory effects of pterostilbene in cerebral ischemia-reperfusion rat brain by mass spectrometry imaging and spatial metabolomics. Talanta 266 (Pt 2), 125060. doi:10.1016/j.talanta.2023.125060
Basu, S. S., and Agar, N. Y. R. (2021). Bringing matrix-assisted laser desorption/ionization mass spectrometry imaging to the clinics. Clin. Lab. Med. 41 (2), 309–324. doi:10.1016/j.cll.2021.03.009
Beck, S., and Stengel, J. (2016). Mass spectrometric imaging of flavonoid glycosides and biflavonoids in Ginkgo biloba L. Phytochemistry 130, 201–206. doi:10.1016/j.phytochem.2016.05.005
Bingol, K., and Brüschweiler, R. (2017). Knowns and unknowns in metabolomics identified by multidimensional NMR and hybrid MS/NMR methods. Curr. Opin. Biotechnol. 43, 17–24. doi:10.1016/j.copbio.2016.07.006
Bodzon-Kulakowska, A., Marszalek-Grabska, M., Antolak, A., Drabik, A., Kotlinska, J. H., and Suder, P. (2016). “Comparison of two freely available software packages for mass spectrometry imaging data analysis using brains from morphine addicted rats,”Eur. J. Mass Spectrom., 22. 229–233. doi:10.1255/ejms.1445
Cai, M. T., Zhou, Y., Ding, W. L., Huang, Y. H., Ren, Y. S., Yang, Z. Y., et al. (2023). Identification and localization of morphological feature-specific metabolites in Reynoutria multiflora roots. Phytochemistry 206, 113527. doi:10.1016/j.phytochem.2022.113527
Calligaris, D., Feldman, D. R., Norton, I., Brastianos, P. K., Dunn, I. F., Santagata, S., et al. (2015). Molecular typing of meningiomas by desorption electrospray ionization mass spectrometry imaging for surgical decision-making. Int. J. Mass Spectrom. 377, 690–698. doi:10.1016/j.ijms.2014.06.024
Cao, M., Wu, J., Zhu, X., Jia, Z., Zhou, Y., Yu, L., et al. (2024). Tissue distribution of metabolites in Cordyceps cicadae determined by DESI-MSI analysis. Anal. Bioanal. Chem. 416 (8), 1883–1906. doi:10.1007/s00216-024-05188-x
Chan, Y. Y., Lo, W. Y., Yang, S. N., Chen, Y. H., and Lin, J. G. (2015). The benefit of combined acupuncture and antidepressant medication for depression: a systematic review and meta-analysis. J. Affect Disord. 176, 106–117. doi:10.1016/j.jad.2015.01.048
Chen, C. J., Sung, H. C., Lee, M. S., and Chang, C. Y. (2015). The effects of Chinese five-element music therapy on nursing students with depressed mood. Int. J. Nurs. Pract. 21 (2), 192–199. doi:10.1111/ijn.12236
Chen, W. J., Zheng, Y. N., Zhao, L., Song, S. H., Long, F., Pei, Z. Q., et al. (2022a). Distribution of bioactive compounds in different tissues of Paeonia lactiflora roots by DESI-MSI and UPLC. 47(16): p. 4333–4340. doi:10.19540/j.cnki.cjcmm.20220514.105
Chen, W. J., Zheng, Y. N., Zhao, L., Song, S. H., Long, F., Pei, Z. Q., et al. (2022b). Distribution of bioactive compounds in different tissues of Paeonia lactiflora roots by DESI-MSI and UPLC. China J. Chin. Materia Medica. 47 (16): p. 4333–4340. doi:10.19540/j.cnki.cjcmm.20220514.105
Chen, Y., Liu, Y., Li, X., He, Y., Li, W., Peng, Y., et al. (2023a). Recent advances in mass spectrometry-based spatially resolved molecular imaging of drug disposition and metabolomics. Drug Metab. Dispos. 51 (10), 1273–1283. doi:10.1124/dmd.122.001069
Chen, Y., Xie, Y., Li, L., Wang, Z., and Yang, L. (2023b). Advances in mass spectrometry imaging for toxicological analysis and safety evaluation of pharmaceuticals. Mass Spectrom. Rev. 42 (5), 2207–2233. doi:10.1002/mas.21807
Cheng, Y., Lan, Q., Wu, B. Y., Wang, J. Y., Liu, D. W., and Tong, Y. (2024). Analysis of brain absorption components and their distribution of tianyuan zhitong prescription based on UPLC-Q-TOF-MS and DESI-MSI. Chin. J. Exp. Traditional Med. Formulae. 30(12): p. 166–172. doi:10.13422/j.cnki.syfjx.20240661
Collins, S. L., Koo, I., Peters, J. M., Smith, P. B., and Patterson, A. D. (2021). Current challenges and recent developments in mass spectrometry-based metabolomics, Annu. Rev. Anal. Chem. 14(1): p. 467–487. doi:10.1146/annurev-anchem-091620-015205
Dai, S. Y., Jiang, S. H., Dong, J., Lian, C. J., Qiao, F., Zheng, J., et al. (2022). Visualization analysis of spatial distribution of alkaloid s in aconiti radix cocta during processing process by matrix assisted laser desorption ionization mass spectrometry imaging. Chin. Pharm. J. 57 (10), 834–839.
Dai, X., and Shen, L. (2022) “Advances and trends in omics technology development,” Front. Med., 9. 911861. doi:10.3389/fmed.2022.911861
De Hert, M., Detraux, J., van Winkel, R., Yu, W., and Correll, C. U. (2011). Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 8 (2), 114–126. doi:10.1038/nrendo.2011.156
Dona, A. C., Coffey, S., and Figtree, G. (2016). Translational and emerging clinical applications of metabolomics in cardiovascular disease diagnosis and treatment. Eur. J. Prev. Cardiol. 23 (15), 1578–1589. doi:10.1177/2047487316645469
Dong, Y., and Aharoni, A. (2022). Image to insight: exploring natural products through mass spectrometry imaging. Nat. Prod. Rep. 39 (7), 1510–1530. doi:10.1039/d2np00011c
Eberlin, L. S., Ferreira, C. R., Dill, A. L., Ifa, D. R., and Cooks, R. G. (2011). Desorption electrospray ionization mass spectrometry for lipid characterization and biological tissue imaging. Biochim. Biophys. Acta 1811 (11), 946–960. doi:10.1016/j.bbalip.2011.05.006
Eckelmann, D., Kusari, S., and Spiteller, M. (2016). Occurrence and spatial distribution of maytansinoids in Putterlickia pyracantha, an unexplored resource of anticancer compounds. Fitoterapia 113, 175–181. doi:10.1016/j.fitote.2016.08.006
Emwas, A. H., Roy, R., McKay, R. T., Tenori, L., Saccenti, E., Gowda, G. N., et al. (2019). NMR Spectroscopy for metabolomics research. Metabolites. 9(7). doi:10.3390/metabo9070123
Fan, W., Yang, Y., Li, L., Fan, L., Wang, Z., and Yang, L. (2022). Mass spectrometry-based profiling and imaging strategy, a fit-for-purpose tool for unveiling the transformations of ginsenosides in Panax notoginseng during processing. Phytomedicine 103, 154223. doi:10.1016/j.phymed.2022.154223
Fan, Y., Wang, A., Liu, Z., Xing, J., Zheng, Z., Song, F., et al. (2024). Integrated spatial metabolomics and network pharmacology to explore the pharmacodynamic substances and mechanism of Radix ginseng-Schisandra chinensis Herb Couple on Alzheimer's disease. Anal. Bioanal. Chem. 416 (19), 4275–4288. doi:10.1007/s00216-024-05364-z
Feng, B., Zhang, J., Chang, C., Li, L., Li, M., Xiong, X., et al. (2014). Ambient mass spectrometry imaging: plasma assisted laser desorption ionization mass spectrometry imaging and its applications. Anal. Chem. 86 (9), 4164–4169. doi:10.1021/ac403310k
Ganesana, M., Lee, S. T., Wang, Y., and Venton, B. J. (2017). Analytical techniques in neuroscience: recent advances in imaging, separation, and electrochemical methods. Anal. Chem. 89 (1), 314–341. doi:10.1021/acs.analchem.6b04278
Gao, H., and Li, Q. (2023). Study on the spatial distribution of coumarins in Angelica dahurica root by MALDI-TOF-MSI. Phytochem. Anal. 34 (1), 139–148. doi:10.1002/pca.3186
Gao, L., Zhang, Z., Wu, W., Deng, Y., Zhi, H., Long, H., et al. (2022). Quantitative imaging of natural products in fine brain regions using desorption electrospray ionization mass spectrometry imaging (DESI-MSI): uncaria alkaloids as a case study. Anal. Bioanal. Chem. 414 (17), 4999–5007. doi:10.1007/s00216-022-04130-3
Gu, X., Gao, X., Cheng, J., Xia, C., Xu, Y., Yang, L., et al. (2021). Emerging application of metabolomics on Chinese herbal medicine for depressive disorder. Biomed. Pharmacother. 141, 111866. doi:10.1016/j.biopha.2021.111866
Guo, N., Fang, Z., Zang, Q., Yang, Y., Nan, T., Zhao, Y., et al. (2023). Spatially resolved metabolomics combined with bioactivity analyses to evaluate the pharmacological properties of two Radix Puerariae species. J. Ethnopharmacol. 313, 116546. doi:10.1016/j.jep.2023.116546
Guo, S., Kendrick, K. M., Zhang, J., Broome, M., Yu, R., Liu, Z., et al. (2013). Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. Neuroimage Clin. 2, 818–826. doi:10.1016/j.nicl.2013.06.008
He, F., Huang, Y. F., Dai, W., Qu, X. Y., Lu, J. G., Lao, C. C., et al. (2022b). The localization of the alkaloids in Coptis chinensis rhizome by time-of-flight secondary ion mass spectrometry. Front. Plant Sci. 13, 1092643. doi:10.3389/fpls.2022.1092643
He, M. J., Pu, W., Wang, X., Zhang, W., Tang, D., and Dai, Y. (2022a). Comparing DESI-MSI and MALDI-MSI mediated spatial metabolomics and their applications in cancer studies. Front. Oncol. 12, 891018. doi:10.3389/fonc.2022.891018
Hou, Y., Fan, F., Xie, N., Zhang, Y., Wang, X., and Meng, X. (2024). Rhodiola crenulata alleviates hypobaric hypoxia-induced brain injury by maintaining BBB integrity and balancing energy metabolism dysfunction. Phytomedicine 128, 155529. doi:10.1016/j.phymed.2024.155529
Hou, Y. S., Pei, Z. Q., Xu, B. J., and Tang, C. (2023). Real-time monitoring of the paclobutrazol variations in different parts of Aconitum carmichaeli during its growth based on UPLC⁃Q⁃TOF-MS and DESI⁃MSI methods. Chin. Tradit. Pat. Med. 45(08): p. 2603–2608. doi:10.3969/j.issn.1001-1528.2023.08.026
Huang, D., Hua, X., Xiu, G. L., Zheng, Y. J., Yu, X. Y., and Long, Y. T. (2017). Secondary ion mass spectrometry: the application in the analysis of atmospheric particulate matter. Anal. Chim. Acta 989, 1–14. doi:10.1016/j.aca.2017.07.042
Huang, X., Wang, R., Wang, Y., Chen, C., and Liu, S. (2023). Investigation on property differences of ginseng and American ginseng by spatial metabolomics of neurochemicals with desorption electrospray ionization mass spectrometry imaging. J. Ethnopharmacol. 303, 116006. doi:10.1016/j.jep.2022.116006
Islam, A., Takeyama, E., Nabi, M. M., Zhai, Q., Fukushima, M., Watanabe, N., et al. (2022). Stress upregulates 2-arachidonoylglycerol levels in the hypothalamus, midbrain, and hindbrain, and it is sustained by green nut oil supplementation in SAMP8 mice revealed by DESI-MSI. Biochem. Biophys. Res. Commun. 609, 9–14. doi:10.1016/j.bbrc.2022.04.004
Jiang, H., Zhang, Y., Liu, Z., Wang, X., He, J., and Jin, H. (2022a). Advanced applications of mass spectrometry imaging technology in quality control and safety assessments of traditional Chinese medicines. J. Ethnopharmacol. 284, 114760. doi:10.1016/j.jep.2021.114760
Jiang, H. Y., Gao, H. Y., Li, J., Zhou, T. Y., Wang, S. T., Yang, J. B., et al. (2022b). Integrated spatially resolved metabolomics and network toxicology to investigate the hepatotoxicity mechanisms of component D of Polygonum multiflorum Thunb. J. Ethnopharmacol. 298, 115630. doi:10.1016/j.jep.2022.115630
Jiang, M., Li, X., Zhao, Y., Zou, Y., Bai, M., Yang, Z., et al. (2023). Characterization of ginsenosides from Panax japonicus var. major (Zhu-Zi-Shen) based on ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry and desorption electrospray ionization-mass spectrometry imaging. Chin. Med. 18 (1), 115. doi:10.1186/s13020-023-00830-9
Jing, F., Wang, L., Yang, M., Wu, C., Li, J., Shi, L., et al. (2022). Visualizing the spatial distribution of functional metabolites in Forsythia suspensa at different harvest stages by MALDI mass spectrometry imaging. Fitoterapia 162, 105285. doi:10.1016/j.fitote.2022.105285
Ke, Q. J., Luo, L. P., and Sun, X. O. (2021). Investigation on the delivery of isosteviol compounds across the blood-brain barrier in zebrafish by DESI-MSI. J. Jinan Univ. Nat. Sci. and Med. Ed. 42(06): p. 653–659. doi:10.11778/j.jdxb.2021.06.012
Keller, C., Maeda, J., Jayaraman, D., Chakraborty, S., Sussman, M. R., Harris, J. M., et al. (2018). Comparison of vacuum MALDI and AP-MALDI platforms for the mass spectrometry imaging of metabolites involved in salt stress in medicago truncatula. Front. Plant Sci. 9, 1238. doi:10.3389/fpls.2018.01238
Kennedy, J. H., and Wiseman, J. M. (2010). Direct analysis of Salvia divinorum leaves for salvinorin A by thin layer chromatography and desorption electrospray ionization multi-stage tandem mass spectrometry. Rapid Commun. Mass Spectrom. 24 (9), 1305–1311. doi:10.1002/rcm.4514
Krone, N., Hughes, B. A., Lavery, G. G., Stewart, P. M., Arlt, W., and Shackleton, C. H. L. (2010). Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 121 (3-5), 496–504. doi:10.1016/j.jsbmb.2010.04.010
Kuik, C., van Hoogstraten, S. W. G., Arts, J. J. C., Honing, M., and Cillero-Pastor, B. (2024). Matrix-assisted laser desorption/ionization mass spectrometry imaging for quorum sensing. Amb. Express 14 (1), 45. doi:10.1186/s13568-024-01703-6
Lam, L. K., Poon, L. Y., Xu, P. L., Xie, C. P., Xie, T., Xiao, Y., et al. (2024). Efficacy and safety of a Chinese medicine formula Diankuang Mengxing Decoction combined with antipsychotics in the treatment of schizophrenia: a meta-analysis of randomized controlled trials. Baltimore 103. doi:10.1097/md.0000000000039489
Lange, B. M., Fischedick, J. T., Lange, M. F., Srividya, N., Šamec, D., and Poirier, B. C. (2017). Integrative approaches for the identification and localization of specialized metabolites in tripterygium roots. Plant Physiol. 173 (1), 456–469. doi:10.1104/pp.15.01593
Lee, J. W., Ji, S. H., Lee, Y. S., Choi, D. J., Choi, B. R., Kim, G. S., et al. (2017). Mass spectrometry based profiling and imaging of various ginsenosides from panax ginseng roots at different ages. Int. J. Mol. Sci. 18 (6), 1114. doi:10.3390/ijms18061114
Li, B., Bhandari, D. R., Janfelt, C., Römpp, A., and Spengler, B. (2014). Natural products in Glycyrrhiza glabra (licorice) rhizome imaged at the cellular level by atmospheric pressure matrix-assisted laser desorption/ionization tandem mass spectrometry imaging. Plant J. 80 (1), 161–171. doi:10.1111/tpj.12608
Li, B., Bhandari, D. R., Römpp, A., and Spengler, B. (2016). High-resolution MALDI mass spectrometry imaging of gallotannins and monoterpene glucosides in the root of Paeonia lactiflora. Sci. Rep. 6, 36074. doi:10.1038/srep36074
Li, B., Ge, J., Liu, W., Hu, D., and Li, P. (2021b). Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 231 (2), 892–902. doi:10.1111/nph.17393
Li, B., Neumann, E. K., Ge, J., Gao, W., Yang, H., Li, P., et al. (2018). Interrogation of spatial metabolome of Ginkgo biloba with high-resolution matrix-assisted laser desorption/ionization and laser desorption/ionization mass spectrometry imaging. Plant Cell. Environ. 41 (11), 2693–2703. doi:10.1111/pce.13395
Li, H. Z., Zhao, Y. F., Wang, D. J., He, J. X., and Chen, X. F. (2024a). Identification and visual analysis of ginsenosides in multi-steamed roots of Panax quinquefolium based on UPLC-Q-TOF-MS/MS and MALDI-MSI. 49(6): p. 1526–1539. doi:10.19540/j.cnki.cjcmm.20231211.301
Li, H. Z., Zhao, Y. F., Wang, D. J., He, J. X., and Chen, X. F. (2024b). Identification and visual analysis of ginsenosides in multiple-steamed roots of Panax quinquefolium based on UPLC⁃Q⁃TOF⁃MS/MS and MALDI⁃MSI. China J. Chin. Materia Medica. 49(06): p. 1526–1539. doi:10.19540/j.cnki.cjcmm.20231211.301
Li, L., Cao, C., Guo, F., Wang, A., Lin, L., Lin, Z., et al. (2024c). Investigation of the active ingredients of Shuangshen Ningxin Fomula and the mechanism underlying their protective effects against myocardial ischemia-reperfusion injury by mass spectrometric imaging. Phytomedicine 123, 155184. doi:10.1016/j.phymed.2023.155184
Li, M., Wang, X., Han, L., Jia, L., Liu, E., Li, Z., et al. (2020a). Integration of multicomponent characterization, untargeted metabolomics and mass spectrometry imaging to unveil the holistic chemical transformations and key markers associated with wine steaming of Ligustri Lucidi Fructus. J. Chromatogr. A 1624, 461228. doi:10.1016/j.chroma.2020.461228
Li, P., Tian, Y., Du, M., Xie, Q., Chen, Y., Ma, L., et al. (2023b). Mechanism of rotenone toxicity against plutella xylostella: new perspective from a spatial metabolomics and lipidomics study. J. Agric. Food Chem. 71 (1), 211–222. doi:10.1021/acs.jafc.2c06292
Li, Q., D. T., Ji, and H., Gao (2023a). Spatial distribution of coumarins in Angelica pubescens fresh roots by MALDI-MSI. Chin. Traditional Herb. Drugs. 54(11): p. 3438–3445. doi:10.7501/j.issn.0253-2670.2023.11.006
Li, S., Zhu, N., Tang, C., Duan, H., Wang, Y., Zhao, G., et al. (2020b). Differential distribution of characteristic constituents in root, stem and leaf tissues of Salvia miltiorrhiza using MALDI mass spectrometry imaging. Fitoterapia 146, 104679. doi:10.1016/j.fitote.2020.104679
Li, X., Gong, M., Li, C., Li, J., Zhou, C., He, T., et al. (2022). Modified xiaochaihu decoction combined with mirtazapine in the treatment of persistent depression: a pilot randomized controlled trial. Contrast Media Mol. Imaging 2022, 8682612. doi:10.1155/2022/8682612
Li, X. R., Wei, F., and Cao, H. (2021a). Urgent need for medical institutions to develop traditional Chinese medicine decoction pieces prescription-based processing and related suggestions. 46(17): p. 4585–4590. doi:10.19540/j.cnki.cjcmm.20210121.301
Li, Y., Wu, Q., Hu, E., Wang, Y., and Lu, H. (2021c). Quantitative mass spectrometry imaging of metabolomes and lipidomes for tracking changes and therapeutic response in traumatic brain injury surrounding injured area at chronic phase. ACS Chem. Neurosci. 12 (8), 1363–1375. doi:10.1021/acschemneuro.1c00002
Li, Z., and Li, Q. (2024). Study on the anti-inflammatory mechanism of coumarins in peucedanum decursivum based on spatial metabolomics combined with network pharmacology. Molecules 29 (14), 3346. doi:10.3390/molecules29143346
Liang, Y., Feng, Q., and Wang, Z. (2022). Mass spectrometry imaging as a new method: to reveal the pathogenesis and the mechanism of traditional medicine in cerebral ischemia. Front. Pharmacol. 13, 887050. doi:10.3389/fphar.2022.887050
Lillja, J., and Lanekoff, I. (2022). Silver-doped nano-DESI MSI for increased specificity and sensitivity of alkenes. Methods Mol. Biol. 2437, 241–249. doi:10.1007/978-1-0716-2030-4_17
Lin, J., Pei, Z., Zhang, Y., Yu, Q., Zhong, J., Han, L., et al. (2024). Study on the white frost formation mechanism during storage of Phyllanthus emblica Linn. fruit based on component analysis and spatial metabolomics. J. Pharm. Biomed. Anal. 241, 115960. doi:10.1016/j.jpba.2023.115960
Lin, W. R., Huang, Y., Chen, J. Q., and Wang, S. X. (2012). Global improvement in agitated depression treated with the alliance therapy of acupuncture and seroxat and the observation of the quality of life. Zhongguo Zhen Jiu. 32(12): p. 1063–1069.
Liu, C., Qi, K., Yao, L., Xiong, Y., Zhang, X., Zang, J., et al. (2019a). Imaging of polar and nonpolar species using compact desorption electrospray ionization/postphotoionization mass spectrometry. Anal. Chem. 91 (10), 6616–6623. doi:10.1021/acs.analchem.9b00520
Liu, F. (2020). Spatial distribution of Panax ginseng saponins visualized by matrix-assisted laser resolving mass spectrometry imaging. Chin. J. Anal. Chem. 48(07): p. 881–888. doi:10.19756/j.issn.0253-3820.201066
Liu, G. X., Li, Z. L., Lin, S. Y., Wang, Q., Luo, Z. Y., Wu, K., et al. (2023a). Mapping metabolite change in the mouse brain after esketamine injection by ambient mass spectrometry imaging and metabolomics. Front. Psychiatry 14, 1109344. doi:10.3389/fpsyt.2023.1109344
Liu, Q., Huang, Y., Linghu, C., Xiao, J., and Gu, R. (2022b). Metabolic profiling, in-situ spatial distribution, and biosynthetic pathway of functional metabolites in Dendrobium nobile stem revealed by combining UPLC-QTOF-MS with MALDI-TOF-MSI. Front. Plant Sci. 13, 1125872. doi:10.3389/fpls.2022.1125872
Liu, Q. B., Lu, J. G., Jiang, Z. H., Zhang, W., Li, W. J., Qian, Z. M., et al. (2022a). In situ chemical profiling and imaging of cultured and natural cordyceps sinensis by TOF-SIMS. Front. Chem. 10, 862007. doi:10.3389/fchem.2022.862007
Liu, X., Liu, C., Tian, J., Gao, X., Li, K., Du, G., et al. (2020). Plasma metabolomics of depressed patients and treatment with Xiaoyaosan based on mass spectrometry technique. J. Ethnopharmacol. 246, 112219. doi:10.1016/j.jep.2019.112219
Liu, X., Liu, R., Dai, Z., Wu, H., Lin, M., Tian, F., et al. (2019b). Effect of Shenfu injection on lipopolysaccharide (LPS)-induced septic shock in rabbits. J. Ethnopharmacol. 234, 36–43. doi:10.1016/j.jep.2019.01.008
Liu, Y., Yang, X., Zhou, C., Wang, Z., Kuang, T., Sun, J., et al. (2022c). Unveiling dynamic changes of chemical constituents in raw and processed fuzi with different steaming time points using desorption electrospray ionization mass spectrometry imaging combined with metabolomics. Front. Pharmacol. 13, 842890. doi:10.3389/fphar.2022.842890
Liu, Y., Zhang, X., Yang, S., Zhou, Z., Tian, L., Li, W., et al. (2023b). Integrated mass spectrometry imaging reveals spatial-metabolic alteration in diabetic cardiomyopathy and the intervention effects of ferulic acid. J. Pharm. Anal. 13 (12), 1496–1509. doi:10.1016/j.jpha.2023.08.011
Lorensen, M., Bjarnholt, N., St-Pierre, B., Heinicke, S., Courdavault, V., O'Connor, S., et al. (2023b). Spatial localization of monoterpenoid indole alkaloids in Rauvolfia tetraphylla by high resolution mass spectrometry imaging. Phytochemistry 209, 113620. doi:10.1016/j.phytochem.2023.113620
Lorensen, M., Hayat, S. Y., Wellner, N., Bjarnholt, N., and Janfelt, C. (2023a). Leaves of Cannabis sativa and their trichomes studied by DESI and MALDI mass spectrometry imaging for their contents of cannabinoids and flavonoids. Phytochem. Anal. 34 (3), 269–279. doi:10.1002/pca.3202
Lu, Y., Cao, Y., Chen, D., Zhou, Y., Zhang, L., Su, Y., et al. (2023). An online derivatization strategy targeting carbon-carbon double bonds by laser-ablation carbon fiber ionization mass spectrometry imaging: unraveling the spatial characteristic in mountain-cultivated ginseng and garden-cultivated ginseng with different ages. Food Chem. 410, 135365. doi:10.1016/j.foodchem.2022.135365
Luo, S., Yang, X., Zhang, Y., Kuang, T., and Tang, C. (2024). Spatial metabolomics method to reveal differential metabolomes in microregions of Panax quinquefolius roots by using ultra-performance liquid chromatography quadrupole/time of flight-mass spectrometry and desorption electrospray ionization mass spectrometry imaging. Food Chem. 435, 137504. doi:10.1016/j.foodchem.2023.137504
Luo, Z., He, J., Chen, Y., He, J., Gong, T., Tang, F., et al. (2013). Air flow-assisted ionization imaging mass spectrometry method for easy whole-body molecular imaging under ambient conditions. Anal. Chem. 85 (5), 2977–2982. doi:10.1021/ac400009s
Luo, Z., He, J., He, J., Huang, L., Song, X., Li, X., et al. (2018). Quantitative analysis of drug distribution by ambient mass spectrometry imaging method with signal extinction normalization strategy and inkjet-printing technology. Talanta 179, 230–237. doi:10.1016/j.talanta.2017.11.005
Lv, S., Dai, W., Zheng, Y., Dong, P., Yu, Y., Zhao, Y., et al. (2022). Anxiolytic effect of YangshenDingzhi granules: integrated network pharmacology and hippocampal metabolomics. Front. Pharmacol. 13, 966218. doi:10.3389/fphar.2022.966218
Ma, T., Sun, C., Han, Y., Guo, L., Huang, L., and Wang, X. (2022). Matrix-assisted laser desorption/ionization mass spectrometry imaging reveals Spatial-Temporal-Content changes of parishins in Gastrodiae Rhizoma during the steaming process. Food Res. Int. 162 (Pt B), 112092. doi:10.1016/j.foodres.2022.112092
Ma, X., Ma, J., Leng, T., Yuan, Z., Hu, T., Liu, Q., et al. (2023). Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail 45 (1), 2146512. doi:10.1080/0886022x.2022.2146512
Malhi, G. S., and Mann, J. J., Depression (2018). 392(10161): p. 2299–2312. doi:10.1016/s0140-6736(18)31948-2
Mamun, M. A., Rahman, M. M., Sakamoto, T., Islam, A., Oyama, S., Nabi, M. M., et al. (2023). Detection of distinct distributions of acetaminophen and acetaminophen-cysteine in kidneys up to 10 μm resolution and identification of a novel acetaminophen metabolite using an AP-MALDI imaging mass microscope. J. Am. Soc. Mass Spectrom. 34 (7), 1491–1500. doi:10.1021/jasms.3c00149
Mandal, V., Ajabiya, J., Khan, N., Tekade, R. K., and Sengupta, P. (2024). Advances and challenges in non-targeted analysis: an insight into sample preparation and detection by liquid chromatography-mass spectrometry. J. Chromatogr. A 1737, 465459. doi:10.1016/j.chroma.2024.465459
Marksteiner, J., Kirchmair, R., Mahata, S. K., Mahata, M., Fischer-Colbrie, R., Hogue-Angeletti, R., et al. (1993). Distribution of secretoneurin, a peptide derived from secretogranin II, in rat brain: an immunocytochemical and radioimmunological study. 54(4): p. 923–944. doi:10.1016/0306-4522(93)90585-4
Matsumoto, J., Nakanishi, H., Kunii, Y., Sugiura, Y., Yuki, D., Wada, A., et al. (2017). Decreased 16:0/20:4-phosphatidylinositol level in the post-mortem prefrontal cortex of elderly patients with schizophrenia. Sci. Rep. 7, 45050. doi:10.1038/srep45050
Matsumoto, J., Sugiura, Y., Yuki, D., Hayasaka, T., Goto-Inoue, N., Zaima, N., et al. (2011). Abnormal phospholipids distribution in the prefrontal cortex from a patient with schizophrenia revealed by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal. Bioanal. Chem. 400 (7), 1933–1943. doi:10.1007/s00216-011-4909-3
McDonnell, L. A., and Heeren, R. M. (2007). Imaging mass spectrometry. Mass Spectrom. Rev. 26 (4), 606–643. doi:10.1002/mas.20124
McNamara, R. K., Jandacek, R., Rider, T., Tso, P., Hahn, C. G., Richtand, N. M., et al. (2007). Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr. Res. 91 (1-3), 37–50. doi:10.1016/j.schres.2006.11.027
Merdas, M., Lagarrigue, M., Vanbellingen, Q., Umbdenstock, T., Da Violante, G., and Pineau, C. (2021). On-tissue chemical derivatization reagents for matrix-assisted laser desorption/ionization mass spectrometry imaging. J. Mass Spectrom. 56 (10), 56. doi:10.1002/jms.4731
Mi, Y. L., Sun, W., Li, M. L., Zhao, H. Y., Bian, B. L., and Zhou, Y. Y (2020). Application of MALDI-mass spectrometry imaging in spatial distribution of secondary metabolites in medicinal plants – a case study of Lepidium meyenii root. 45(3): p. 596–601. doi:10.19540/j.cnki.cjcmm.20191209.201
Misra, B. B. (2018). New tools and resources in metabolomics: 2016-2017. Electrophoresis 39 (7), 909–923. doi:10.1002/elps.201700441
Mohana Kumara, P., Uma Shaanker, R., and Pradeep, T. (2019). UPLC and ESI-MS analysis of metabolites of Rauvolfia tetraphylla L. and their spatial localization using desorption electrospray ionization (DESI) mass spectrometric imaging, Phytochemistry. 159: p. 20–29. doi:10.1016/j.phytochem.2018.11.009
Morisasa, M., Sato, T., Kimura, K., Mori, T., and Goto-Inoue, N. (2019). Application of matrix-assisted laser desorption/ionization mass spectrometry imaging for food analysis. Foods 8(12). doi:10.3390/foods8120633
Nakabayashi, R., Hashimoto, K., Mori, T., Toyooka, K., Sudo, H., and Saito, K. (2021) “Spatial metabolomics using imaging mass spectrometry to identify the localization of asparaptine A in Asparagus officinalis,”Plant Biotechnol 38. 311–315. doi:10.5511/plantbiotechnology.21.0504b
Nie, L. X., Dong, J., Huang, L. Y., Qian, X. Y., Lian, C. J., Kang, S., et al. (2021). Microscopic mass spectrometry imaging reveals the distribution of phytochemicals in the dried root of isatis tinctoria. Front. Pharmacol. 12, 685575. doi:10.3389/fphar.2021.685575
Nie, L. X., Huang, Y. L., Wang, P. X., and Lv, F. L. (2023). Complementary mass spectrometry imaging and discovery of quality characters-related Markers of Isatidis Radix Based on AP-MALDI-IT-TOF/MS and DESI-Q-TOF/MS. Chin. Pharm. J. 58 (09), 823–830.
Nie, L. X., Huang, L. Y., Wang, X. P., Lv, L. F., Yang, X. X., Jia, X. F., et al. (2022a). Desorption electrospray ionization mass spectrometry imaging illustrates the quality characters of isatidis radix. Front. Plant Sci. 13, 897528. doi:10.3389/fpls.2022.897528
Nie, W., Lu, Q., Hu, T., Xie, M., and Hu, Y. (2022b). Visualizing the distribution of curcumin in the root of Curcuma longa via VUV-postionization mass spectrometric imaging. Analyst 148 (1), 175–181. doi:10.1039/d2an01516a
Park, C., Rosenblat, J. D., Brietzke, E., Pan, Z., Lee, Y., Cao, B., et al. (2019). Stress, epigenetics and depression: a systematic review. Neurosci. Biobehav. Rev. 102, 139–152. doi:10.1016/j.neubiorev.2019.04.010
Parrot, D., Papazian, S., Foil, D., and Tasdemir, D. (2018). Imaging the unimaginable: desorption electrospray ionization - imaging mass spectrometry (DESI-IMS) in natural product research. Planta Med. 84 (9-10), 584–593. doi:10.1055/s-0044-100188
Pérez-Fernández, V., Mainero Rocca, L., Tomai, P., Fanali, S., and Gentili, A. (2017). Recent advancements and future trends in environmental analysis: sample preparation, liquid chromatography and mass spectrometry. Anal. Chim. Acta 983, 9–41. doi:10.1016/j.aca.2017.06.029
Qi, K., Wu, L., Liu, C., and Pan, Y. (2021). Recent advances of ambient mass spectrometry imaging and its applications in lipid and metabolite analysis. Metabolites 11 (11), 780. doi:10.3390/metabo11110780
Qiao, F., Yun, S. D., Jie, L. C., Jie, L., Jing, D., Jian, Z., et al. (2022). Visualization analysis of spatial distribution of chemical compositions in Morindae Officinalis Radix processed product by matrix-assisted laser desorption ionization mass spectrometry imaging. Chin. J. Pharm. Analysis. 42(08): p. 1312–1318. doi:10.16155/j.0254-1793.2022.08.03
Qin, S. B., Tan, P., Hao, L., Xie, J., Lin, J., Zhang, L., et al. (2024). Investigating mechanism of fritillariae cirrhosae bulbus against pulmonary fibrosis based on spatial metabolomics. Chin. J. Exp. Traditional Med. Formulae. 30(13): p. 150–159. doi:10.13422/j.cnki.syfjx.20240565
Qu, Y. Z., Sun, B., Zhu, G. W., Ma, S. J., Wan, L. C., Li, Y. J., et al. (2020). Study on application of DESI-MSI in quality control of classical famous prescription Shaoyao Gancao Decoction. Chin. Traditional Herb. Drugs. 51(13): p. 3433–3443. doi:10.7501/j.issn.0253-2670.2020.13.010
Ren, Z., Zhang, H., Yang, L., Chen, X., Zhang, S., Chen, S., et al. (2023). Spatial distribution and comparative analysis of Aconitum alkaloids in Fuzi using DESI-MSI and UHPLC-QTOF-MS. Analyst 148 (7), 1603–1610. doi:10.1039/d2an02051c
Revier, C. J., Reininghaus, U., Dutta, R., Fearon, P., Murray, R. M., Doody, G. A., et al. (2015). Ten-year outcomes of first-episode psychoses in the MRC æsop-10 study. J. Nerv. Ment. Dis. 203 (5), 379–386. doi:10.1097/nmd.0000000000000295
Ross, B. M., Hudson, C., Erlich, J., Warsh, J. J., and Kish, S. J. (1997). Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium-independent phospholipase A2. Evid. Involv. a calcium-independent phospholipase A2. 54(5): p. 487–494. doi:10.1001/archpsyc.1997.01830170113015
Sanacora, G., Yan, Z., and Popoli, M. (2022). The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat. Rev. Neurosci. 23 (2), 86–103. doi:10.1038/s41583-021-00540-x
Serkova, N. J., and Brown, M. S. (2012). Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling to in vivo biomarkers. Bioanalysis 4 (3), 321–341. doi:10.4155/bio.11.320
Shen, Y., Song, W., Lin, D., Zhang, X., Wang, M., Li, Y., et al. (2023). VG161 activates systemic antitumor immunity in pancreatic cancer models as a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. J Med Virol. 95 (1). doi:10.1002/jmv.28108
Shen, Y., Howard, L., and Yu, X. Y. (2024) “Secondary ion mass spectral imaging of metals and alloys. Materials,”, 17. doi:10.2.3390/ma17020528
Sheng, Z., Liu, Q., Song, Y., Ye, B., Li, Y., Song, Y., et al. (2024). Astrocyte atrophy induced by L-PGDS/PGD2/Src signaling dysfunction in the central amygdala mediates postpartum depression. J. Affect Disord. 359, 241–252. doi:10.1016/j.jad.2024.05.083
Shimma, S., and Sagawa, T. (2019). Microscopy and mass spectrometry imaging reveals the distributions of curcumin species in dried turmeric root. J. Agric. Food Chem. 67 (34), 9652–9657. doi:10.1021/acs.jafc.9b02768
Shorter, E., and Segesser, K. (2013). Traditional Chinese medicine and Western psychopharmacology: building bridges. Phytother. Res. 27 (12), 1739–1744. doi:10.1002/ptr.4940
Song, Z., Yang, Z., Tian, L., Liu, Y., Guo, Z., Zhang, Q., et al. (2023). Targeting mitochondrial circadian rhythms: the potential intervention strategies of Traditional Chinese medicine for myocardial ischaemia‒reperfusion injury. Biomed. Pharmacother. 166, 115432. doi:10.1016/j.biopha.2023.115432
Soudah, T., Zoabi, A., and Margulis, K. (2023). Desorption electrospray ionization mass spectrometry imaging in discovery and development of novel therapies. Mass Spectrom. Rev. 42 (2), 751–778. doi:10.1002/mas.21736
Sun, C., Cui, L., Zhou, B., Wang, X., Guo, L., and Liu, W. (2022). Visualizing the spatial distribution and alteration of metabolites in continuously cropped Salvia miltiorrhiza Bge using MALDI-MSI. J. Pharm. Anal. 12 (5), 719–724. doi:10.1016/j.jpha.2021.09.011
Sun, C., Li, L., Wang, D., Liu, W., Liu, F., Guo, L., et al. (2021). Visualizing the distributions and spatiotemporal changes of metabolites in Panax notoginseng by MALDI mass spectrometry imaging. J. Ginseng Res. 45 (6), 726–733. doi:10.1016/j.jgr.2021.04.001
Sun, C., Li, T., Song, X., Huang, L., Zang, Q., Xu, J., et al. (2019). Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. U. S. A. 116 (1), 52–57. doi:10.1073/pnas.1808950116
Sun, C., Liu, W., Zhang, M., Geng, Y., and Wang, X. (2020). Development of a high-coverage matrix-assisted laser desorption/ionization mass spectrometry imaging method for visualizing the spatial dynamics of functional metabolites in Salvia miltiorrhiza Bge. J. Chromatogr. A 1614, 460704. doi:10.1016/j.chroma.2019.460704
Sun, C., Liu, W., Guo, L., and Wang, X. (2021). Analysis on tissue distribution of metabolites in Lotus seed by MALDI mass spectrometry imaging technique. J. Instrum. Analysis. 40 (1): 86–91.
Susniak, K., Krysa, M., Gieroba, B., Komaniecka, I., and Sroka-Bartnicka, A. (2020). Recent developments of MALDI MSI application in plant tissues analysis. Acta Biochim. Pol. 67 (3), 277–281. doi:10.18388/abp.2020_5394
Taha, A. Y., Cheon, Y., Ma, K., Rapoport, S. I., and Rao, J. S. (2013). Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J. Psychiatr. Res. 47 (5), 636–643. doi:10.1016/j.jpsychires.2013.01.016
Taira, S., Ikeda, R., Yokota, N., Osaka, I., Sakamoto, M., Kato, M., et al. (2010). Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am. J. Chin. Med. 38 (3), 485–493. doi:10.1142/s0192415x10008007
Takáts, Z., Wiseman, J. M., Gologan, B., and Cooks, R. G. (2004). Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306 (5695), 471–473. doi:10.1126/science.1104404
Tan, X., He, Q., Pei, Z., Liu, Y., Feng, Z., Li, C., et al. (2023). Rapid visual characterization of alkaloid changes in traditional processing of Tibetan medicine Aconitum pendulum by high-performance thin-layer chromatography coupled with desorption electrospray ionization mass spectrometry imaging. Front. Pharmacol. 14, 1104473. doi:10.3389/fphar.2023.1104473
Tang, X., Zhao, M., Chen, Z., Huang, J., Chen, Y., Wang, F., et al. (2021). Visualizing the spatial distribution of metabolites in Clausena lansium (Lour.) skeels using matrix-assisted laser desorption/ionization mass spectrometry imaging. Phytochemistry 192, 112930. doi:10.1016/j.phytochem.2021.112930
Tian, F., Liu, R., Fan, C., Sun, Y., Huang, X., Nie, Z., et al. (2020). Effects of thymoquinone on small-molecule metabolites in a rat model of cerebral ischemia reperfusion injury assessed using MALDI-MSI. Metabolites 10 (1), 27. doi:10.3390/metabo10010027
Tomalty, D., Giovannetti, O., Velikonja, L., Munday, J., Kaufmann, M., Iaboni, N., et al. (2023). Molecular characterization of human peripheral nerves using desorption electrospray ionization mass spectrometry imaging. J. Anat. 243 (5), 758–769. doi:10.1111/joa.13909
Tong, Q., Zhang, C., Tu, Y., Chen, J., Li, Q., Zeng, Z., et al. (2022). Biosynthesis-based spatial metabolome of Salvia miltiorrhiza Bunge by combining metabolomics approaches with mass spectrometry-imaging. Talanta 238 (Pt 2), 123045. doi:10.1016/j.talanta.2021.123045
Uys, J. D., Grey, A. C., Wiggins, A., Schwacke, J. H., Schey, K. L., and Kalivas, P. W. (2010). Matrix-assisted laser desorption/ionization tissue profiling of secretoneurin in the nucleus accumbens shell from cocaine-sensitized rats. J. Mass Spectrom. 45 (1), 97–103. doi:10.1002/jms.1697
Vancampfort, D., Stubbs, B., Mitchell, A. J., De Hert, M., Wampers, M., Ward, P. B., et al. (2015). Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 14 (3), 339–347. doi:10.1002/wps.20252
Veerasammy, K., Chen, Y. X., Sauma, S., Pruvost, M., Dansu, D. K., Choetso, T., et al. (2020). Sample preparation for metabolic profiling using MALDI mass spectrometry imaging. J. Vis. Exp. (166). doi:10.3791/62008
Vendramini, P. H., Gattaz, W. F., Schmitt, A., Falkai, P., Eberlin, M. N., and Martins-de-Souza, D. (2016). Pioneering ambient mass spectrometry imaging in psychiatry: potential for new insights into schizophrenia. Schizophr. Res. 177 (1-3), 67–69. doi:10.1016/j.schres.2015.10.019
Wang, H., Hong, L., Yang, F., Zhao, Y., Jing, Q., Wang, W., et al. (2024a). Desorption electrospray ionization-mass spectrometry imaging-based spatial metabolomics for visualizing and comparing ginsenosides and lipids among multiple parts and positions of the panax ginseng root. J. Agric. Food Chem. 72 (49), 27549–27560. doi:10.1021/acs.jafc.4c07461
Wang, H. M. (2022). Studies on the conversion of various classes of chemical components before and after the steaming of panax ginseng, panax quinquefolius and panax notoginseng based on highresolution liquid chromatography-mass spectrometry and mass spectrometry imaging. Tianjin University of Traditional Chinese Medicine.
Wang, J., van der Heijden, R., Spijksma, G., Reijmers, T., Wang, M., Xu, G., et al. (2009). Alkaloid profiling of the Chinese herbal medicine Fuzi by combination of matrix-assisted laser desorption ionization mass spectrometry with liquid chromatography-mass spectrometry. J. Chromatogr. A 1216 (11), 2169–2178. doi:10.1016/j.chroma.2008.11.077
Wang, L., Chaudhari, K., Winters, A., Sun, Y., Liu, R., and Yang, S. H. (2022). Characterizing region-specific glucose metabolic profile of the rodent brain using Seahorse XFe96 analyzer. J. Cereb. Blood Flow. Metab. 42 (7), 1259–1271. doi:10.1177/0271678x221077341
Wang, L., Zhu, T., Xu, H. B., Pu, X. P., Zhao, X., Tian, F., et al. (2021c). Effects of notoginseng leaf triterpenes on small molecule metabolism after cerebral ischemia/reperfusion injury assessed using MALDI-MS imaging. Ann. Transl. Med. 9 (3), 246. doi:10.21037/atm-20-4898
Wang, M., Chen, L., Liu, D., Chen, H., Tang, D. D., and Zhao, Y. Y. (2017). Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem. Biol. Interact. 273, 133–141. doi:10.1016/j.cbi.2017.06.011
Wang, S., Bai, H., Cai, Z., Gao, D., Jiang, Y., Liu, J., et al. (2016). MALDI imaging for the localization of saponins in root tissues and rapid differentiation of three Panax herbs. Electrophoresis 37 (13), 1956–1966. doi:10.1002/elps.201600027
Wang, T., Lee, H. K., Yue, G. G. L., Chung, A. C. K., Lau, C. B. S., and Cai, Z. (2021b). A novel binary matrix consisting of graphene oxide and caffeic acid for the analysis of scutellarin and its metabolites in mouse kidney by MALDI imaging. Analyst 146 (1), 289–295. doi:10.1039/d0an01539c
Wang, W. J., and Zhang, T. (2017). Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J. Integr. Med. 15 (1), 1–7. doi:10.1016/s2095-4964(17)60314-5
Wang, X., Zhang, X., Zhang, J., Yang, H., Liu, Z., Peng, D., et al. (2024b). Illustrate the metabolic regulatory mechanism of Taohong Siwu decoction in ischemic stroke by mass spectrometry imaging. Anal. Bioanal. Chem. 416 (29), 6931–6944. doi:10.1007/s00216-024-05591-4
Wang, Z., Fu, W., Huo, M., He, B., Liu, Y., Tian, L., et al. (2021a). Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm. Sin. B 11 (11), 3665–3677. doi:10.1016/j.apsb.2021.05.013
Wang, Z., He, B., Liu, Y., Huo, M., Fu, W., Yang, C., et al. (2020). In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta Pharm. Sin. B 10 (6), 1083–1093. doi:10.1016/j.apsb.2019.12.004
Wei, L., Chen, S., Deng, X., Liu, Y., Wang, H., Gao, X., et al. (2024). Metabolomic discoveries for early diagnosis and traditional Chinese medicine efficacy in ischemic stroke. Biomark. Res. 12 (1), 63. doi:10.1186/s40364-024-00608-7
Wei, W., Li, Z., Li, H., An, Y., Qu, H., Yao, C., et al. (2021). Exploration of tissue distribution of ginsenoside Rg1 by LC-MS/MS and nanospray desorption electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 198, 113999. doi:10.1016/j.jpba.2021.113999
Wu, C., Dill, A. L., Eberlin, L. S., Cooks, R. G., and Ifa, D. R. (2013). Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 32 (3), 218–243. doi:10.1002/mas.21360
Wu, H., Liu, X., Gao, Y., Dai, F., Lin, M., Tian, H., et al. (2019). Anti-Myocardial infarction effects of radix aconiti lateralis preparata extracts and their influence on small molecules in the heart using matrix-assisted laser desorption/ionization-mass spectrometry imaging. Int. J. Mol. Sci. 20(19). doi:10.3390/ijms20194837
Wu, Q., Jiang, L., Yan, Y., Yan, Q., Zhu, X., Zhang, J., et al. (2023). Geographical distribution-based differentiation of cultivated Angelica dahurica, exploring the relationship between the secretory tract and the quality. Sci. Rep. 13 (1), 21733. doi:10.1038/s41598-023-48497-4
Wu, Y., Li, J., Qiao, J., Jia, D., Pang, K., Yang, F., et al. (2024). Effects of traditional Chinese medicine five-element music therapy combined with mirtazapine on depression and limb function recovery after ischemic stroke. Altern. Ther. Health Med. 30 (9), 210–213.
Xia, J., He, X., Yang, W., Song, H., Yang, J., Zhang, G., et al. (2024). Unveiling the distribution of chemical constituents at different body parts and maturity stages of Ganoderma lingzhi by combining metabolomics with desorption electrospray ionization mass spectrometry imaging (DESI). Food Chem. 436, 137737. doi:10.1016/j.foodchem.2023.137737
Xia, J., Lou, G., Zhang, L., Huang, Y., Yang, J., Guo, J., et al. (2023). Unveiling the spatial distribution and molecular mechanisms of terpenoid biosynthesis in Salvia miltiorrhiza and S. grandifolia using multi-omics and DESI-MSI. Hortic. Res. 10. doi:10.1093/hr/uhad109
Xiao, X., Guan, X. K., Xu, Z. Y., and Lu, Q. (2024). Spatial distribution of the main metabolites in Radix Scutellariae slices based on mass spectrometry imaging. J. Hubei Univ. Med. 43(04): p. 385–389. doi:10.13819/j.issn.2096-708X.2024.04.009
Xu, B., Chen, L., Lv, F., Pan, Y., Fu, X., and Pei, Z. (2022). Visualization of metabolites identified in the spatial metabolome of traditional Chinese medicine using DESI-MSI. J. Vis. Exp. doi:10.3791/64912
Xu, L., and Wang, L. L. (2011). Clinical observation on depression treated by electroacupuncture combined with western medicine. Zhongguo Zhen Jiu. 31(9): p. 779–782.
Yalcin, E. B., and de la Monte, S. M. (2015). Review of matrix-assisted laser desorption ionization-imaging mass spectrometry for lipid biochemical histopathology. J. Histochem Cytochem 63 (10), 762–771. doi:10.1369/0022155415596202
Yang, M., Hu, H., Su, P., Thomas, J., Camarillo, J., Greer, B., et al. (2022). Proteoform-selective Imaging of tissues using mass spectrometry. Angew chem int. Ed. Engl., 61. (29). doi:10.1002/anie.202200721
Yang, M., Unsihuay, D., Hu, H., Nguele Meke, F., Qu, Z., Zhang, Z. Y., et al. (2023). Nano-DESI mass spectrometry imaging of proteoforms in biological tissues with high spatial resolution. Anal. Chem. 95 (12), 5214–5222. doi:10.1021/acs.analchem.2c04795
Yang, Y., Qiu, H., Ju, Z., Shi, Y., Wang, Z., Yang, L., et al. (2021). Localization of constituents for determining the age and parts of ginseng through ultraperfomance liquid chromatography quadrupole/time of flight-mass spectrometry combined with desorption electrospray ionization mass spectrometry imaging. J. Pharm. Biomed. Anal. 193, 113722. doi:10.1016/j.jpba.2020.113722
Yeung, A., Chan, J. S. M., Cheung, J. C., and Zou, L. (2018b). Qigong and tai-chi for mood regulation. Focus Am. Psychiatr. Publ. 16 (1), 40–47. doi:10.1176/appi.focus.20170042
Yeung, K. S., Hernandez, M., Mao, J. J., Haviland, I., and Gubili, J. (2018a). Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 32 (5), 865–891. doi:10.1002/ptr.6033
Yin, R., Burnum-Johnson, K. E., Sun, X., Dey, S. K., and Laskin, J. (2019). High spatial resolution imaging of biological tissues using nanospray desorption electrospray ionization mass spectrometry. Nat. Protoc. 14 (12), 3445–3470. doi:10.1038/s41596-019-0237-4
Yin, R., Kyle, J., Burnum-Johnson, K., Bloodsworth, K. J., Sussel, L., Ansong, C., et al. (2018). High spatial resolution imaging of mouse pancreatic islets using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 90 (11), 6548–6555. doi:10.1021/acs.analchem.8b00161
Yu, M., Ma, C., Tai, B., Fu, X., Liu, Q., Zhang, G., et al. (2024). Unveiling the regulatory mechanisms of nodules development and quality formation in Panax notoginseng using multi-omics and MALDI-MSI. J. Adv. Res., doi:10.1016/j.jare.2024.04.003
Yu, M., Zhu, Y., Cong, Q., and Wu, C. (2017). Metabonomics research progress on liver diseases. Can. J. Gastroenterol. Hepatol. 2017, 8467192. doi:10.1155/2017/8467192
Zang, Q., Sun, C., Chu, X., Li, L., Gan, W., Zhao, Z., et al. (2021). Spatially resolved metabolomics combined with multicellular tumor spheroids to discover cancer tissue relevant metabolic signatures. Anal. Chim. Acta 1155, 338342. doi:10.1016/j.aca.2021.338342
Zeki Ö, C., Eylem, C. C., Reçber, T., Kır, S., and Nemutlu, E. (2020). Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 190, 113509. doi:10.1016/j.jpba.2020.113509
Zhai, Q., Islam, A., Chen, B., Zhang, H., Chi, H. D., Mamun, A., et al. (2023). Endocannabinoid 2-arachidonoylglycerol levels in the anterior cingulate cortex, caudate putamen, nucleus accumbens, and Piriform cortex were upregulated by chronic restraint stress. Cells. 12(3). doi:10.3390/cells12030393
Zhang, A., Sun, H., Wang, Z., Sun, W., Wang, P., and Wang, X. (2010). Metabolomics: towards understanding traditional Chinese medicine. Planta Med. 76 (17), 2026–2035. doi:10.1055/s-0030-1250542
Zhang, G. H., Liu, X. L., Ma, C. X., Li, W. H., and Wang, X. (2022a). Spatial distribution characteristics of metabolities in rhizome of Paris polyphylla var. yunnanensis: based on MALDI-MSI. Zhongguo Zhong Yao Za Zhi. 47(5): p. 1222–1229. doi:10.19540/j.cnki.cjcmm.20211223.101
Zhang, G. H., Liu, X. L., Ma, C. X., Li, W. H., and Wang, X. (2022b). Spatial distribution characteristics of metabolities in rhizome of Paris polyphylla var. yunnanensis: based on MALDI-MSI. China J. Chin. Materia Medica. 47(05): p. 1222–1229. doi:10.19540/j.cnki.cjcmm.20211223.101
Zhang, J., Du, Q., Song, X., Gao, S., Pang, X., Li, Y., et al. (2020). Evaluation of the tumor-targeting efficiency and intratumor heterogeneity of anticancer drugs using quantitative mass spectrometry imaging. Theranostics 10 (6), 2621–2630. doi:10.7150/thno.41763
Zhao, B., Li, Z., Wang, Y., Ma, X., Wang, X., Wang, X., et al. (2019). Can acupuncture combined with SSRIs improve clinical symptoms and quality of life in patients with depression? Secondary outcomes of a pragmatic randomized controlled trial. Complement. Ther. Med. 45, 295–302. doi:10.1016/j.ctim.2019.03.015
Zhao, H., Shi, C., Han, W., Luo, G., Huang, Y., Fu, Y., et al. (2024). Advanced progress of spatial metabolomics in head and neck cancer research. Neoplasia 47, 100958. doi:10.1016/j.neo.2023.100958
Zhao, J., and Feng, S. S. (2023a). Application of spatial metabolomics in traditional Chinese medicine research. Chinese Traditional and Herbal Drugs. 54(20): p. 6569–6579. doi:10.7501/j.issn.0253-2670.2023.20.001
Zhao, W. H., Zhang, Y. D., and Shi, Y. P. (2021). Visualizing the spatial distribution of endogenous molecules in wolfberry fruit at different development stages by matrix-assisted laser desorption/ionization mass spectrometry imaging. Talanta 234, 122687. doi:10.1016/j.talanta.2021.122687
Zhao, Y., Jiang, M., Liu, M., Wang, H., Wang, W., Zhang, T., et al. (2023b). Spatial distribution and characterization of the small-molecule metabolites and in situ hydrolyzed oligosaccharides in the rhizome of Glycyrrhiza uralensis by desorption electrospray ionization-mass spectrometry imaging and high-resolution liquid chromatography-mass spectrometry. J. Agric. Food Chem. 71 (50), 20372–20385. doi:10.1021/acs.jafc.3c04996
Zheng, S., Liang, Y., Xue, T., Wang, W., Li, S., Zhang, P., et al. (2024). Application of network pharmacology in traditional Chinese medicine for the treatment of digestive system diseases. Front. Pharmacol. 15, 1412997. doi:10.3389/fphar.2024.1412997
Zheng, Y., Lin, C., Chu, Y., Gu, S., Deng, H., and Shen, Z. (2023). Spatial metabolomics in head and neck tumors: a review. Front. Oncol. 13, 1213273. doi:10.3389/fonc.2023.1213273
Zhou, P., Zuo, L., Liu, C., Xiong, B., Li, Z., Zhou, X., et al. (2024b). Unraveling spatial metabolome of the aerial and underground parts of Scutellaria baicalensis by matrix-assisted laser desorption/ionization mass spectrometry imaging. Phytomedicine 123, 155259. doi:10.1016/j.phymed.2023.155259
Zhou, Q., Fülöp, A., and Hopf, C. (2021). Recent developments of novel matrices and on-tissue chemical derivatization reagents for MALDI-MSI. Anal. Bioanal. Chem. 413 (10), 2599–2617. doi:10.1007/s00216-020-03023-7
Zhou, X., Wang, J., Lu, Y., Chen, C., Hu, Y., Liu, P., et al. (2020). Anti-depressive effects of Kai-Xin-San on lipid metabolism in depressed patients and CUMS rats using metabolomic analysis. J. Ethnopharmacol. 252, 112615. doi:10.1016/j.jep.2020.112615
Zhou, Y. J., Zhang, L., Wang, W., Wang, B., Xu, X. L., and Chen, Y. G. (2024). Spatial distribution characteristics of hording in rat tissues analyzed by DESIMSI. Chin. J. Hosp. Pharm. p. 1–8.
Zhou, Z. (2020) “Study of efficacy and safety of Jiaotai pill in the treatment of depression. Medicine”. Baltimore, 99. doi:10.1097/md.0000000000019999
Zhou, Z., Sun, Y., Yang, J., and Abliz, Z. (2024a). Mapping the metabolic characteristics and perturbation of adult casper zebrafish by ambient mass spectrometry imaging. Metabolites 14 (4), 204. doi:10.3390/metabo14040204
Zhu, C., Jiang, X., Tian, J., Chen, J., Lin, C., Wang, C., et al. (2022). Integrated approach toward absorption, distribution, metabolism, and excretion of Xiaoke pills in zebrafish based on UPLC-HRMS and DESI-MS techniques. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1200, 123276. doi:10.1016/j.jchromb.2022.123276
Zhu, T., Wang, L., Tian, F., Zhao, X., Pu, X. P., Sun, G. B., et al. (2020). Anti-ischemia/reperfusion injury effects of notoginsenoside R1 on small molecule metabolism in rat brain after ischemic stroke as visualized by MALDI-MS imaging. 129: p. 110470. doi:10.1016/j.biopha.2020.110470
Zhu, X., Wu, S., Zhou, Y., Xiao, T., Xia, L., Wang, Y., et al. (2024). The pharmacological actions of Danzhi-xiaoyao-San on depression involve lysophosphatidic acid and microbiota-gut-brain axis: novel insights from a systems pharmacology analysis of a double-blind, randomized, placebo-controlled clinical trial. J. Biomol. Struct. Dyn. 42 (18), 9309–9324. doi:10.1080/07391102.2023.2251067
Zinniel, D. K., Fenton, R. J., Halouska, S., Powers, R., and Barletta, R. G. (2012). Sample preparation of Mycobacterium tuberculosis extracts for nuclear magnetic resonance metabolomic studies. J. Vis. Exp., e3673 (67). doi:10.3791/3673
Keywords: mental disorders, traditional Chinese medicine, spatial metabolomics, mass spectrometry imaging, DESI-MSI, MALDI-MSI, SIMS
Citation: Lei C, Chen J, Chen Z, Ma C, Chen X, Sun X, Tang X, Deng J, Wang S, Jiang J, Wu D and Xie L (2025) Spatial metabolomics in mental disorders and traditional Chinese medicine: a review. Front. Pharmacol. 16:1449639. doi: 10.3389/fphar.2025.1449639
Received: 15 June 2024; Accepted: 15 January 2025;
Published: 31 January 2025.
Edited by:
Xiliang Du, Jilin University, ChinaReviewed by:
Zili Xie, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2025 Lei, Chen, Chen, Ma, Chen, Sun, Tang, Deng, Wang, Jiang, Wu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Xie, MTI4OTM5ODcwNkBxcS5jb20=; Dahua Wu, ODkzMDQ5MzUyQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.