
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 28 February 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1449062
 Dominic Chi-Chung Foo†
Dominic Chi-Chung Foo† Jiaxi Li†
Jiaxi Li† Zheng Huang†
Zheng Huang† Siming Sui
Siming Sui Ryan Wai-Yan Sin
Ryan Wai-Yan Sin Abraham Tak Ka Man
Abraham Tak Ka Man Wai-Lun Law
Wai-Lun Law Lui Ng*
Lui Ng*Background: Anti-hypertensive drugs have been reported to demonstrate anti-inflammatory and anti-angiogenic effects. This study aims to investigate the association between anti-hypertensive drugs and the prognosis of colorectal cancer (CRC) patients.
Methods: Clinical data of 1134 CRC patients with hypertensions and the prescription of anti-hypertensive drugs who had undergone curative surgery in our hospital between 2005 and 2015 were retrieved. Their survival data and immune cell population in circulatory blood were compared among different types of anti-hypertensive drugs and overall CRC patients.
Results: The 5-year overall survival for the antihypertensives-treated patients (65.2%) was higher than the CRC patients in Hong Kong (58.2%). Hydrochlorothiazide (HCTZ) group showed the best prognosis (79.1%) among different antihypertensive drug, particularly for advance stage or elderly patients, which are poor prognostic factors for overall CRC patients, demonstrated an obviously improved prognosis upon HCTZ treatment. Moreover, our data showed the recurrence rate was significantly lower for HCTZ group (18.3%) compared to non-HCTZ group (26.8%) and the reported rate (31%) of CRC patients in Hong Kong. Finally, patients with a lower pre-operative basophil level showed better overall and disease-free survival following HCTZ treatment.
Conclusion: This study demonstrated the association of HCTZ treatment with a better prognosis of CRC patients.
According to World Health Organization, colorectal cancer (CRC) is the third most common cancer worldwide, accounting for approximately 10% of all cancer cases and is the second leading cause of cancer-related deaths globally (Marcellinaro et al., 2023). The survival rates and quality of life for CRC patients are significantly low due to factors such as high mortality rates, late-stage diagnoses, metastasis to distant locations, and the limited effectiveness and adverse effects of standard treatments. Typically, CRC is treated using a single method or a combination of methods, including surgery, chemotherapy, and radiation therapy. While there has been some recent evidence of improved overall survival and disease-free survival in CRC patients, the 5-year survival rates remain below 65% in developed countries and below 50% in developing countries (Allemani et al., 2015). Furthermore, the 5-year relative survival rate is even poorer for CRC patients with distant metastases, ranging from 14% to 15% (Siegel et al., 2020). The bleak prognosis, especially for advanced-stage patients, underscores the urgent need for more effective treatments. In recent years, significant progress has been made in achieving long-term responses using immune checkpoint inhibitors (ICIs) for solid tumors like melanoma, non-small-cell lung cancer (NSCLC), and renal cell cancer (Webb et al., 2018). However, the majority of CRCs and CRC metastases do not exhibit deficient MisMatch Repair (dMMR), making most CRC patients ineligible for immunotherapy (Kamal et al., 2020). Therefore, there is an urgent need to identify better therapeutic targets for CRC patients. However, the process of developing new drugs involves lengthy preclinical and clinical trials before they are approved for use in CRC patients. As a result, drug repurposing has emerged as an attractive approach, utilizing already approved drugs to improve patient prognosis (Xia et al., 2024).
Anti-hypertensive drugs have been reported to demonstrate anti-inflammatory and anti-angiogenic effects (Nemati et al., 2011; Ferroni et al., 2012), whereas both inflammation and angiogenesis are closely related to development and progression of cancers (Aguilar-Cazares et al., 2019). Therefore we are interested to investigate the potential impacts of anti-hypertensive drugs on the prognosis of CRC patients. In fact, hypertension is one of the risk factors for the development of CRC, and many CRC patients have comorbidity of hypertension and are taking anti-hypertensive drugs (Kaneko et al., 2021). Hence there is a large patient population available for evaluating the potential therapeutic effect of different anti-hypertensive drugs. An American research group reported in June 2021 that anti-hypertensive drugs may improve survival in CRC (Balkrishnan et al., 2021), which is in line with our hypothesis. Their study used the Surveillance, Epidemiology, and End-Results (SEER) Medicare database to review outcomes of 13,982 patients aged 65 and older diagnosed with stage I-III colorectal cancer between 2007 and 2012, showing that patients treated with Angiotensin Converting Enzyme (ACE) inhibitors, beta blockers and thiazide diuretics were all associated with decreased cancer-specific mortality (Balkrishnan et al., 2021). The authors suggested that antihypertensive drugs could improve prognosis of CRC patients through functionally interacting with the tumor vasculature and microenvironment (Balkrishnan et al., 2021); however more clinical and experimental evidence is needed to support their repurposing for treating CRC patients.
The objective of this study is to examine the therapeutic impact of various anti-hypertensive medications on enhancing the prognosis of CRC patients. The flowchart of this study is shown in Figure 1.
CRC patients who underwent curative surgery and had a history of prescription of anti-hypertensive drugsat Queen Mary Hospital (Hong Kong) during 2005–2015 were included in the study. These patients were treated with different types of antihypertensive medications. For this study, we selected 7 common drugs from 5 different classes, including (A) ACE inhibitor-Captopril, (B) Beta-blockers- Metoprolol and Propranolol; (C) Calcium channel blockers- nifedipine; (D) Diuretics-Hydrochlorthiazide and Indapamide; (E) Alpha-2 agonist-Methyldopa. Patients who were not followed up for at least 5 years were excluded, except for those who exhibited signs of recurrence or death during the follow-up period. The data collection protocol has been approved (UW 21-114) by the Institutional Review Board (IRB) of the University of Hong Kong.
A population-based retrospective cohort was compiled from the Hong Kong Hospital Authority (HA) administrative database in the Hong Kong adult diabetes population from 1 January 2006 to 31 December 2015. CDARS has been extensively utilized for conducting high-quality large population-based studies (Ng et al., 2021). The database encompasses comprehensive demographic and clinical characteristics of CRC patient (ICD-9-CM Diagnosis Code: 153.0–153.9; 154.0–154.3; 154.8), including the year of diagnosis, age, sex, race/ethnicity, tumor location, tumor size, tumor stage, receipt of cancer treatment (such as surgery, chemotherapy, and radiation therapy); patient’s immune cell profile including lymphocyte count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), platelet count, platelet-to-lymphocyte ratio (PLR), monocyte count, lymphocyte-to-monocyte ratio (LMR), albumin level and prognostic nutritional index (PNI); HA drug prescription database containing information, including the date of drug dispensing, dosage unit, and quantity of drug dispensed, which was recorded. The baseline date for eligible patients was defined as the date of curative resection of primary CRC. Patients were monitored from the baseline date until the incidence of the event outcome, death from any cause, and censored at the last healthcare service utilization date, whichever came first.
Univariate analysis of the association between recurrence or no recurrence and the treatment received was performed using Fisher’s exact test. Survival curves were generated using the Kaplan–Meier test and compared by the log-rank test. The difference in immune cell profile between treatment and control group was compared by t-test, paired-test or Mann–Whitney U test. The criterion for statistical significance was a p < 0.05. All statistical analyses are conducted using SigmaPlot version 10.0 (Systat Software Inc., San Jose, CA, United States) and SPSS version 10.0 (SPSS Inc., Chicago, IL, United States).
Mendelian Randomization (MR) is a causal inference method that utilizes germline genetic variants (specifically single nucleotide polymorphisms (SNPs)) as genetic instruments to estimate and test the causal effect of an exposure variable on an outcome (Burgess and Thompson, 2017). In this study, the R package TwoSampleMR was utilized to implement various MR methods for analyzing the association between phenotypes and survival, as described in a previous study (Xin et al., 2023). The methods employed included inverse variance weighted (IVW), weighted median, penalized weighted median, and MR Egger methods. Additionally, a heterogeneity test was conducted to evaluate whether a genetic variant’s effect on the outcome was proportional to its effect on the exposure, and the MR-Egger intercept test was performed to assess the presence of horizontal pleiotropy (Burgess and Thompson, 2017). Suggestive evidence of an association between phenotypes and cancer survival was identified when specific criteria were met, including a P-value ≤0.05 for IVW analysis, a P-value >0.05 for the Egger intercept, and a P-value >0.05 for heterogeneity. A two-sample MR analysis was carried out using the selected SNPs to identify potential therapeutic targets for tumors. In this particular investigation, antihypertensive drug usage (ebi-a-GCST90018984 - Medication use (antihypertensives) obtained from MRanalysis (https://mranalysis.cn/index.html)) was considered as the exposure factor, while cancer-specific survival (CSS) served as the outcome factor (Xin et al., 2023).
We compared the 5-year overall survival of CRC patients who underwent surgery at our study site (Queen Mary Hospital) between 2005 and 2015 and were treated with different types of antihypertensive medications. In this study, we selected 7 common drugs from 5 different classes, including (A) ACE inhibitor-Lisinopril; (B) Beta-blockers- Metoprolol and Propranolol; (C) Calcium channel blockers-Nifedipine; (D) Diuretics-Hydrochlorothiazide and Indapamide; (E) Alpha-2 agonist-Methyldopa. Table 1 shows the age, gender and tumor location information for the CRC patients in different antihypertensive drug groups. A total of 1,134 patients were found to be treated with these drugs either individually or in combination. The distribution of CRC patients in each anti-hypertensive drug group (single or combined drug) is shown in Figure 2.Our findings revealed that the 5-year overall survival for patients treated with antihypertensives-treated patients (65.2%) was higher than that of CRC patients in Hong Kong (58.2%) (Registry, 2019).
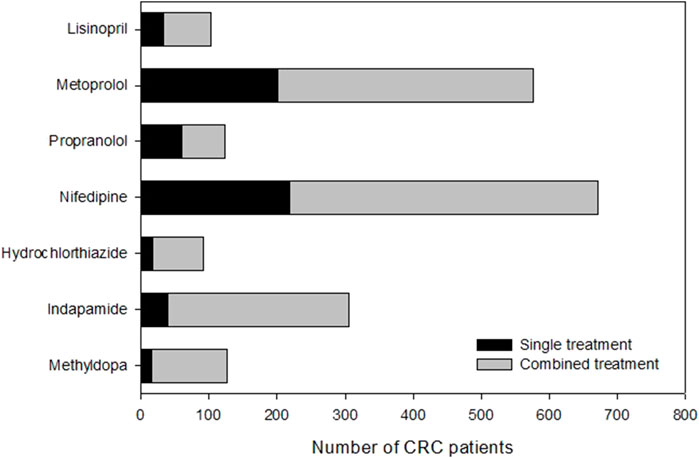
Figure 2. Number of CRC patients underwent surgery during 2005–2015 in Queen Mary Hospital and treated with antihypertensive drugs.
Among the 2,621 colorectal cancer (CRC) patients included in the MR analysis, 569 cancer-specific deaths were recorded (Xin et al., 2023). As shown in Figure 3, the analysis revealed a significant effect of antihypertensive drugs on CRC CSS (beta = −0.5561, P = 0.007) using IVW with 14 SNPs. No substantial directional pleiotropy was observed based on the MR-Egger test results (MR-Egger intercept 0.638, P > 0.05). Furthermore, no significant heterogeneity was detected when comparing the effect sizes of instrumental variables (IVs) on exposure to their effect sizes on CRC CSS survival (Pheterogeneity > 0.05). The MR estimates indicated that antihypertensive drug usage was associated with improved CSS in CRC, consistent with the findings of the clinical study.
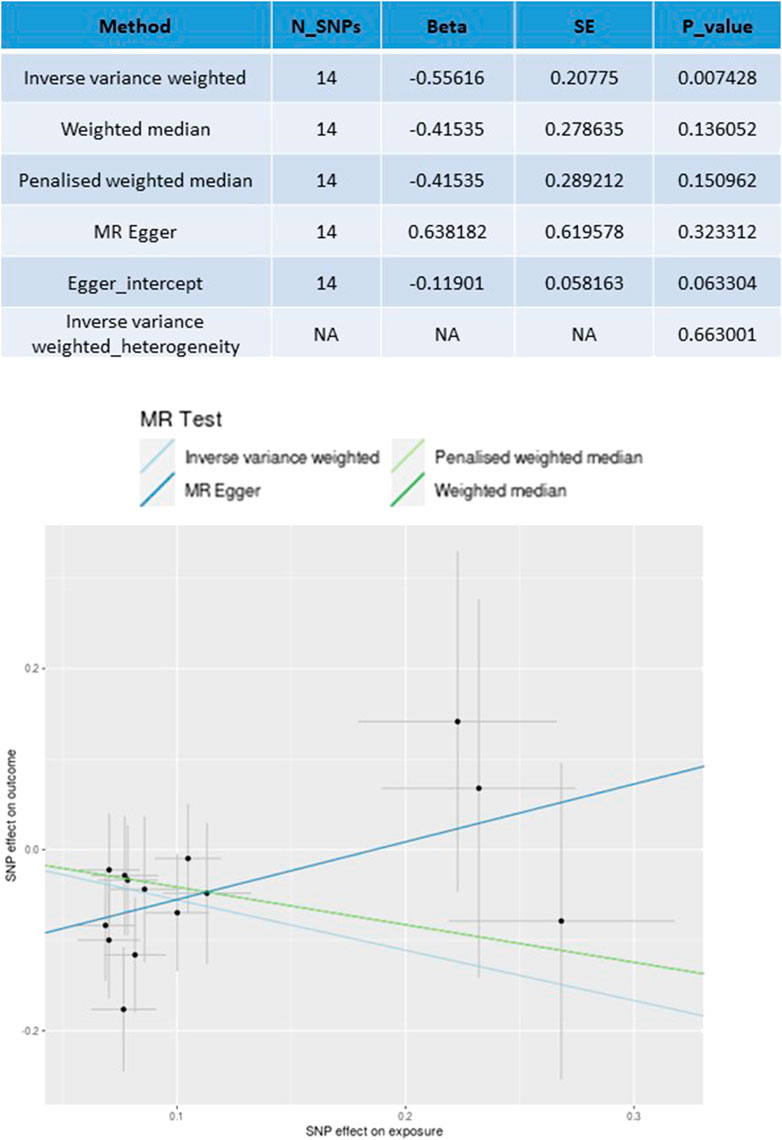
Figure 3. Mendelian randomization analyses for antihypertensive drugs use (exposure) on cancer-specific survival (CSS) outcome.
Hydrochlorothiazide (HCTZ) group exhibited the best prognosis (79.1%) among the different antihypertensive drug groups, and the results were statistically significant when compared to Lisinopril, Metoprolol, nifedipine, Indapamide and Methyldopa (Figures 4A, B).
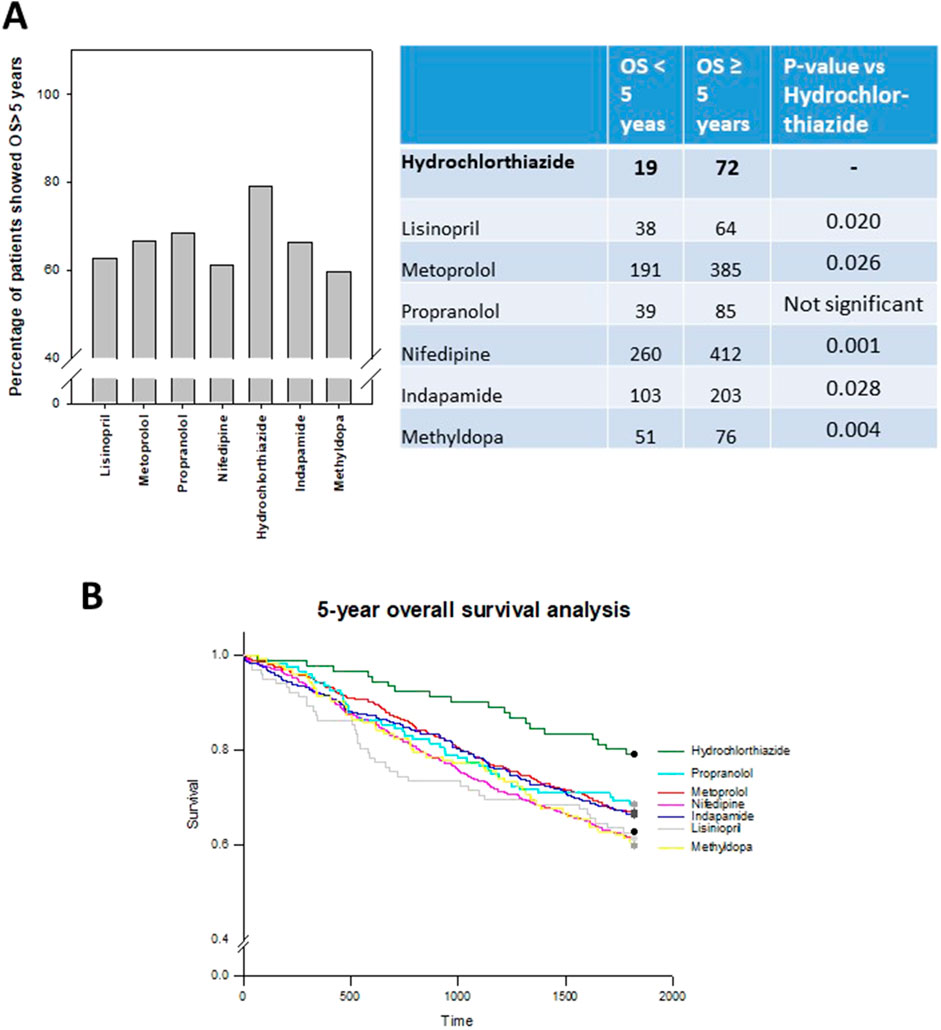
Figure 4. 5-year overall survival (OS) analysis of different antihypertensive drugs treated CRC patients (A) Percentage of patients who can live more than 5 years post-surgery. Hydrochlorthiazide (HCTZ) group showed better prognosis when compared to other antihypertensive drugs, and the difference was significant for some of the comparison as shown in the table. (B) Log-rank analysis showing the overall survival of patients in different drug groups. Hydrochlorthiazide group was obviously better than the remaining drug groups.
The 5-year overall survival rates for different stages of the HCTZ group were as follows: stage I: 15 out of 19, 78.9%; stage II: 25 out of 30, 83.3%; stage III: 25 out of 30, 83.3%; and stage IV: 2 out of 7, 28.6%, whereas the rates for stage III and IV were notably higher than those of overall CRC patients in Hong Kong (68.7% and 9.3%, respectively, as per the “Overview of Hong Kong Cancer Statistics of 2018” published by the Hospital authority) (Figure 5A). The relatively lower 5-year overall survival rates for stage I and II patients in the HCTZ group were likely due to the higher average age of patients in these groups (74.8 for stage I and 75.4 for stage II). In addition, the 5-year overall survival rate for elderly patients (age 75 or above) was the worst among different age groups for overall CRC patients in Hong Kong. Our data indicated that the rate for elderly patients in HCTZ group was 75.5%, which was much higher than the overall rate for elderly CRC patients (45.2%) (Figure 5B). These findings suggest that advanced stage or elderly CRC patients, which are poor prognostic factors for overall CRC patients in Hong Kong, showed a noably improved prognosis with HCTZ treatment.
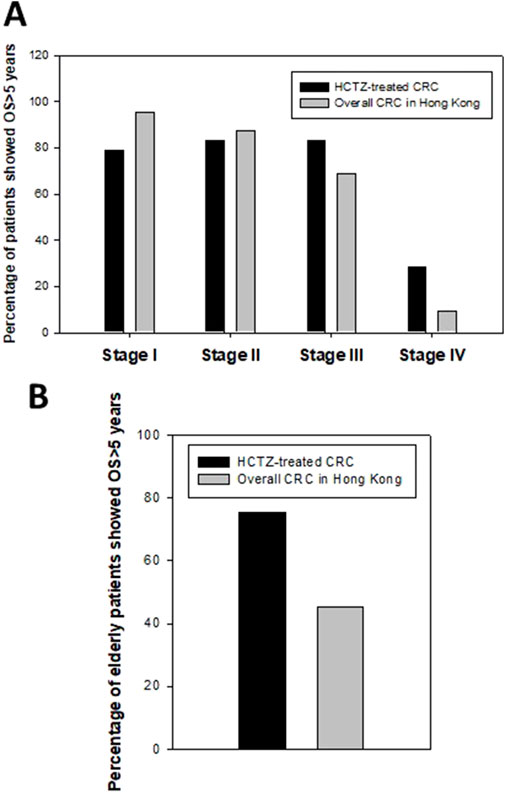
Figure 5. Effect of Hydrochlorthiazide (HCTZ) on prognosis of advance stage or elderly CRC patients (A) Stage-specific 5-year overall survival (OS) data of HCTZ-treated CRC patients and overall CRC patients in Hong Kong. The prognosis of advance stage group (Stage III and IV) in HCTZ group was better than the overall CRC patients in Hong Kong. (B) Comparison of 5-year overall survival (OS) of elderly patients between HCTZ-treated group and overall data in Hong Kong.
Furthermore, we investigated the association between HCTZ treatment and the recurrence of CRC patients who underwent curative surgery. Among our study cohort, we identified 82 CRC patients treated with HCTZ who underwent curative surgery, and we included 190 age-, gender- and tumor stage-matched non-HCTZ antihypertensive drug-treated patients for comparison. Table 2 shows the age, gender and tumor stage information for the CRC patients in the HCTZ and non-HCTZ groups. Our data revealed that 15 out of the 82 patients (18.3%) in the HCTZ group developed recurrent disease, which was lower than the rate in non-HCTZ patients (51 out of 190 patients (26.8%)). This recurrence rate was also significantly lower (p = 0.024) than the data reported from another local hospital (Cheng et al., 2008), which mentioned that 31% of 650 CRC patients who underwent curative-intent resection between 2000 and 2006 developed recurrent disease (Figure 6A).
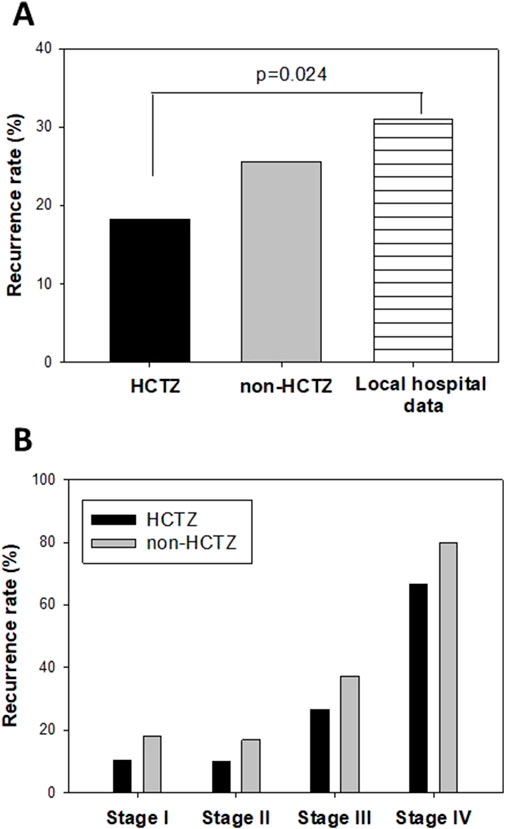
Figure 6. Effect of Hydrochlorthiazide (HCTZ) on disease recurrence of CRC patients who performed curative surgery (A) Comparison of overall recurrence rate among HCTZ-treated patients, non-HCTZ antihypertensive drug treated patients and data reported from a local hospital which included 650 CRC patients who underwent curative-intent resection between 2000 and 2006 (KC Cheng et al., 2008). (B) Comparison of stage-specific recurrence rate among HCTZ-treated and non-HCTZ antihypertensive drug treated patients in this study.
We also compared the recurrence rate of CRC patients at each stage between the HCTZ and non-HCTZ groups. As shown in Figure 6B, the recurrence rates in HCTZ at different stages of CRC (stage I: 2 out of 19, 10.5%; stage II: 3 out of 30, 10%; stage III: 8 out of 30, 26.7%; stage IV: 2 out of 3, 66.7%) were lower for all stages compared to the non-HCTZ group (stage I: 6 out of 33, 18.2%; stage II: 13 out of 77, 16.9%; stage III: 28 out of 75, 37.3%; stage IV: 4 out of 5, 80.0%).
Anti-hypertensive drugs, including HCTZ, have been reported to exhibit anti-inflammatory and anti-angiogenic effects (Nemati et al., 2011; Ferroni et al., 2012). We investigated whether pre-operative blood parameters could serve as potential biomarkers for predicting CRC patients with a good prognosis following HCTZ treatment. Among all circulatory blood parameters, we found that the pre-operative basophil level was significantly associated with overall survival and disease-free survival (Figure 6). Figure 7A shows that patients with a good prognosis in terms of overall survival had a significantly lower pre-operative basophil level (0.0209 × 109/L) compared to the poor prognosis group (0.0347 × 109/L, p = 0.05). The difference was particularly significant for stage III CRC, whereas the basophil level in the good prognosis group (0.0188 × 109/L) was significantly lower than that in the poor prognosis group (0.0480, p = 0.01). For disease-free survival analysis (Figure 7B), patients with a good prognosis also exhibited a significantly lower pre-operative basophil level (0.0197 × 109/L) compared to the poor prognosis group (0.0389 × 109/L, p = 0.0099). Once again the difference was particularly significant for stage III CRC (0.0168 × 109/L vs. 0.0457 × 109/L, p = 0.0034). These findings, if validated using a larger patient population, could provide a potential biomarker for identifying CRC patients who may have a better prognosis following HCTZ treatment.
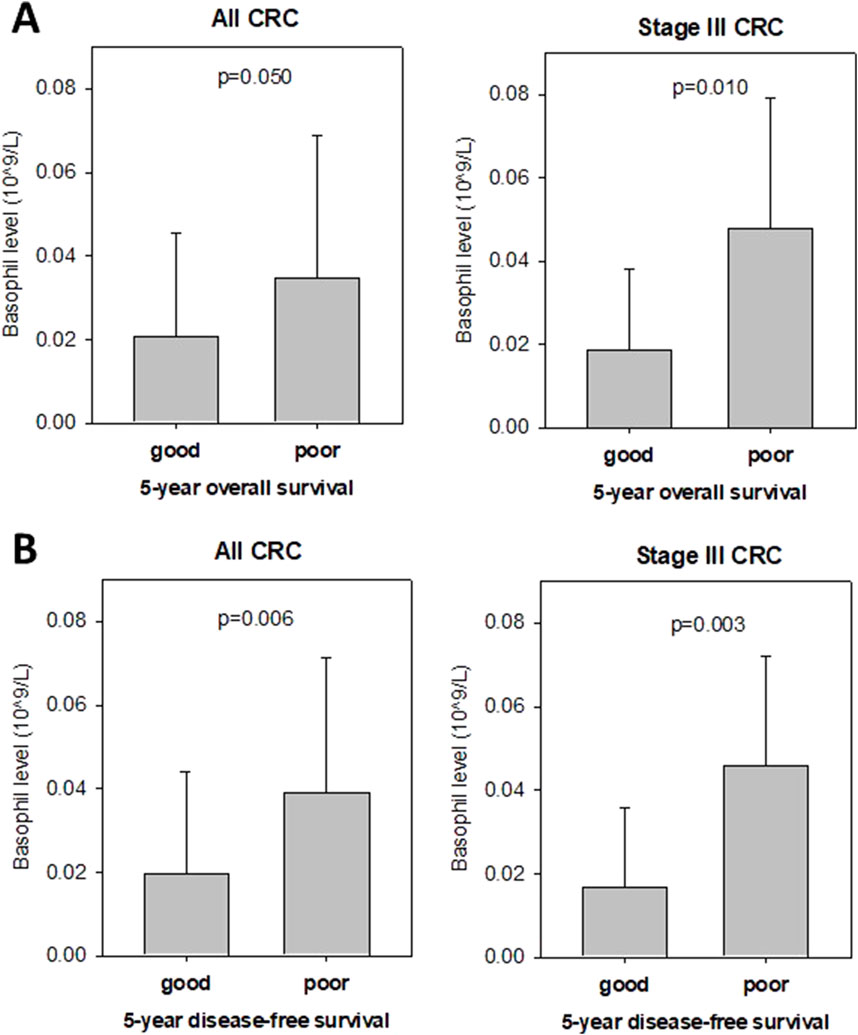
Figure 7. Pre-operative basophil level is associated with prognosis of Hydrochlorthiazide (HCTZ) –treated CRC patients (A) Level of pre-operative basophil in good or poor prognosis overall CRC patients (left) and stage III CRC patients (right) based on their 5-year overall survival status. It was shown that low basophil level was associated with good prognosis. (B) Level of pre-operative basophil in good or poor prognosis overall CRC patients (left) and stage III CRC patients (right) based on their 5-year disease-free survival status after curative surgery. It was shown that low basophil level was associated with good prognosis.
Colorectal cancer (CRC) is a prevalent malignant cancer worldwide, with increasing incidence and mortality rates (Marcellinaro et al., 2023). While targeted drugs and therapeutic regimens have been developed and proven effective in treating CRC, there is still a significant number of patients who lack effective targeted drugs or develop drug resistance during treatment (Kumar et al., 2023). Therefore, there is an urgent need to develop new therapeutic agents. Drug repurposing for cancer therapy is a promising strategy for discovering new drugs. Compared to developing new drugs from scratch, drug repurposing significantly reduces the time, investment, and improves the success rates of preclinical drug discovery (Carlos-Escalante et al., 2021). Antihypertensive drugs, widely prescribed and available at an affordable price with a well-known safety profile, are particularly interesting for repurposing (Carlos-Escalante et al., 2021). Additionally, the large number of cancer patients undergoing antihypertensive drug treatment allows for the analysis of their potential impact on cancer treatment.
In June 2021, an American research group reported that antihypertensive drugs may improve survival in CRC (Balkrishnan et al., 2021). Their study, using the Surveillance, Epidemiology, and End-Results (SEER) Medicare database, reviewed outcomes of 13,982 patients aged 65 and older diagnosed with stage I-III colorectal cancer between 2007 and 2012. The study found that patients treated with Angiotensin Converting Enzyme (ACE) inhibitors, beta blockers, and thiazide diuretics had decreased cancer-specific mortality (Balkrishnan et al., 2021). Specifically, anti-hypertensive drugs were associated with decreased cancer-specific mortality, with ACE inhibitors, beta-blockers and thiazide diuretics showing positive results (Balkrishnan et al., 2021). The authors further suggested that hypoxia and irregular tumor vascularization can contribute to treatment failure by promoting metastasis, complicating surgery, and limiting the efficacy of known cancer therapies. Strategies that normalize tumor vasculature function and hypoxia through anti-hypertensive drugs to improve the tumor microenvironment could be effective in optimizing cancer patient management (Balkrishnan et al., 2021). However, more clinical and experimental evidence is required to support the repurposing of antihypertensive drugs for treating CRC patients. So far, β-blockers, ACE inhibitors, and ARBs have been the most commonly evaluated antihypertensive agents in the context of cancer, while thiazide and thiazide-like diuretics have been less studied (Carlos-Escalante et al., 2021). CRC is a heterogeneous disease, and this diversity can have important implications for CRC prognosis and clinical management (Kocarnik et al., 2015). Therefore, it is uncertain whether the same effect of antihypertensive drugs observed in CRC patients in the United States will be seen in the Asian population. This study offers additional clinical evidence that antihypertensive drugs, particularly HCTZ, could enhance the prognosis of CRC patients. Additionally, the study examined the effects of commonly used specific antihypertensive drugs. It was found that within the diuretic drug group, the HCTZ group demonstrated a better prognosis (79.1%) compared to the indapamide group (66.3%).
This study analyzed CRC patients with concurrent hypertension in a single hospital in Hong Kong and compared the prognosis among CRC patients treated with various types of antihypertensive drugs. Our findings showed that the overall 5-year survival rate for antihypertensive-treated patients (64.4%) was higher than that reported for CRC patients in Hong Kong (58.2%). Specifically, the hydrochlorothiazide (HCTZ) group had the best 5-year overall survival rate (79.1%) among different antihypertensive drug groups. Additionally, the recurrence rate for CRC patients in the HCTZ group was lower than that of non-HCTZ patients, while both groups had lower recurrence rates than those reported from another local hospital for overall CRC patients (Cheng et al., 2008). These findings suggest that antihypertensive drugs, particularly HCTZ, have the potential to improve the prognosis of post-operative CRC patients. In the future, we plan to validate these findings further in a larger cohort of patients in multiple hospitals in Hong Kong and nearby regions. Additionally, we will investigate the best drug in combination with HCTZ for CRC patients in terms of both disease-free and overall survival. It is also necessary to investigate the functional effect of HCTZ in vitro, in vivo, or ex vivo.
It is important to note that HCTZ has known photosensitizing properties (Haisma et al., 2023), and some studies have reported an association between the cumulative use of HCTZ and cancer development (Rahamimov et al., 2024; Shin et al., 2019; VanWormer et al., 2022). The risk of any skin cancer, keratinocyte carcinoma, basal cell carcinoma, and squamous cell carcinoma was not significantly increased in general HCTZ users (Haisma et al., 2023). However, a clear association was observed between high cumulative HCTZ use (≥5,000 defined daily dose; ≥125,000 mg) and the risk of any skin cancer, keratinocyte carcinoma, basal cell carcinoma, and squamous cell carcinoma (Haisma et al., 2023; Park et al., 2020). Nevertheless, some studies have questioned this correlation, and whether HCTZ increases the risk of skin cancer remains controversial (Park et al., 2020). A nationwide case-control study reported that the use of HCTZ was associated with a substantially increased risk of lip cancer, and the risk increased with the increasing cumulative amount, duration, and intensity of HCTZ use (Pottegard et al., 2017). More than 100,000 mg of HCTZ, corresponding to more than 10 years of cumulative use, was associated with a seven-fold increased risk for squamous cell carcinoma lip cancer (Pedersen et al., 2018). On the other hand, a recent study investigated the association between HCTZ usage and the risk of melanoma (Park et al., 2020). The risk of melanoma was significantly lower in HCTZ-users compared to non-HCTZ users. High cumulative doses (≥50,000 mg) of HCTZ were associated with a decreased risk of both non-melanoma skin cancer and melanoma (Park et al., 2020).
This study possesses several notable strengths. Firstly, we conducted a thorough examination of the clinical effects of various common antihypertensive drugs across different categories on CRC patients. This analysis was based on comprehensive follow-up data obtained from CRC patients treated at our hospital, providing valuable insights into the impact of these medications on CRC outcomes. Secondly, a significant strength of this study lies in our identification of a blood biomarker capable of distinguishing CRC patients who are likely to experience improved prognoses when treated with HCTZ. This discovery not only contributes to a more personalized approach to treatment but also underscores the potential for utilizing biomarkers to tailor therapeutic strategies for individual patients. However, there are also limitations to this study. Firstly, it is a single-center study, and multicenter data is necessary to increase the sample size for each type of antihypertensive drug and validate our findings. Secondly, the functional therapeutic effects of HCTZ on CRC have not been explored, which could offer insight into how it enhances the prognosis of CRC patients and potentially improve its clinical applicability. For example, angiogenesis plays a pivotal role in tumor growth, invasion, and metastasis (Liu et al., 2023). Research indicates that HCTZ inhibits RhoA and Rho-associated protein kinase (ROCK) transcription and activation in mouse models (Mondaca-Ruff et al., 2021; Araos et al., 2016), and the RhoA/ROCK pathway is known to be crucial in angiogenesis (Chen et al., 2014). Furthermore, studies have shown that ROCK inhibitors reduce VEGF-induced angiogenesis and tumor cell growth, leading us to hypothesize that HCTZ exerts an anti-angiogenic effect by inhibiting the ROCK pathway.
This study demonstrated the association between HCTZ treatment and a better prognosis for CRC patients. Post-operative patients treated with HCTZ exhibited significantly enhanced 5-year overall survival and a lower recurrence rate compared to those treated with non-HCTZ antihypertensive drugs or the overall CRC patient population. Furthermore, our results demonstrated for the first time that the pre-operative basophil level was significantly associated with overall survival and disease-free survival following HCTZ treatment, suggesting that the pre-operative basophil level could be a potential biomarker for identifying patients who are likely to respond better to HCTZ treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board (or Ethics Committee) of the University of Hong Kong. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’; legal guardians/next of kin because Patient consent was waived because this is a retrospective review of medical records, and the patient management will not be affected.
D-CF: Investigation, Methodology, Resources, Supervision, Writing–review and editing. JL: Formal Analysis, Investigation, Methodology, Writing–review and editing. ZH: Formal Analysis, Investigation, Methodology, Writing–review and editing. SS: Investigation, Methodology, Writing–review and editing. R-YS: Investigation, Methodology, Writing–review and editing. AM: Methodology, Writing–review and editing. W-LL: Conceptualization, Resources, Supervision, Writing–review and editing. LN: Conceptualization, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguilar-Cazares, D., Chavez-Dominguez, R., Carlos-Reyes, A., Lopez-Camarillo, C., Hernadez de la Cruz, O. N., and Lopez-Gonzalez, J. S. (2019). Contribution of angiogenesis to inflammation and cancer. Front. Oncol. 9, 1399. doi:10.3389/fonc.2019.01399
Allemani, C., Weir, H. K., Carreira, H., Harewood, R., Spika, D., Wang, X. S., et al. (2015). Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385 (9972), 977–1010. doi:10.1016/S0140-6736(14)62038-9
Araos, P., Mondaca, D., Jalil, J. E., Yañez, C., Novoa, U., Mora, I., et al. (2016). Diuretics prevent Rho-kinase activation and expression of profibrotic/oxidative genes in the hypertensive aortic wall. Ther. Adv. Cardiovasc Dis. 10 (6), 338–347. doi:10.1177/1753944716666208
Balkrishnan, R., Desai, R. P., Narayan, A., Camacho, F. T., Flausino, L. E., and Chammas, R. (2021). Associations between initiating antihypertensive regimens on stage I-III colorectal cancer outcomes: a Medicare SEER cohort analysis. Cancer Med. 10 (15), 5347–5357. doi:10.1002/cam4.4088
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5), 377–389. doi:10.1007/s10654-017-0255-x
Carlos-Escalante, J. A., de Jesús-Sánchez, M., Rivas-Castro, A., Pichardo-Rojas, P. S., Arce, C., and Wegman-Ostrosky, T. (2021). The use of antihypertensive drugs as coadjuvant therapy in cancer. Front. Oncol. 11, 660943. doi:10.3389/fonc.2021.660943
Chen, W., Mao, K., Liu, Z., and Dinh-Xuan, A. T. (2014). The role of the RhoA/Rho kinase pathway in angiogenesis and its potential value in prostate cancer (Review). Oncol. Lett. 8 (5), 1907–1911. doi:10.3892/ol.2014.2471
Cheng, K. C., Yeung, Y. P., Lau, P. Y., and Meng, W. C. (2008). Surveillance and outcome of liver metastasis in patients with colorectal cancer who had undergone curative-intent operation. Hong Kong Med. J. 14 (6), 432–436.
Ferroni, P., Della-Morte, D., Palmirotta, R., Rundek, T., Guadagni, F., and Roselli, M. (2012). Angiogenesis and hypertension: the dual role of anti-hypertensive and anti-angiogenic therapies. Curr. Vasc. Pharmacol. 10 (4), 479–493. doi:10.2174/157016112800812836
Haisma, M. S., Greven, N., Logendran, M., Bos, J., van der Vegt, B., Horváth, B., et al. (2023). Chronic use of hydrochlorothiazide and risk of skin cancer in caucasian adults: a PharmLines initiative inception cohort study. Acta Derm. Venereol. 103, adv3933. doi:10.2340/actadv.v103.3933
Kamal, Y., Schmit, S. L., Frost, H. R., and Amos, C. I. (2020). The tumor microenvironment of colorectal cancer metastases: opportunities in cancer immunotherapy. Immunotherapy 12, 1083–1100. doi:10.2217/imt-2020-0026
Kaneko, H., Yano, Y., Itoh, H., Morita, K., Kiriyama, H., Kamon, T., et al. (2021). Untreated hypertension and subsequent incidence of colorectal cancer: analysis of a nationwide epidemiological database. J. Am. Heart Assoc. 10 (22), e022479. doi:10.1161/JAHA.121.022479
Kocarnik, J. M., Shiovitz, S., and Phipps, A. I. (2015). Molecular phenotypes of colorectal cancer and potential clinical applications. Gastroenterol. Rep. (Oxf) 3 (4), 269–276. doi:10.1093/gastro/gov046
Kumar, A., Gautam, V., Sandhu, A., Rawat, K., Sharma, A., and Saha, L. (2023). Current and emerging therapeutic approaches for colorectal cancer: a comprehensive review. World J. Gastrointest. Surg. 15 (4), 495–519. doi:10.4240/wjgs.v15.i4.495
Liu, Z. L., Chen, H. H., Zheng, L. L., Sun, L. P., and Shi, L. (2023). Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target Ther. 8 (1), 198. doi:10.1038/s41392-023-01460-1
Marcellinaro, R., Spoletini, D., Grieco, M., Avella, P., Cappuccio, M., Troiano, R., et al. (2023). Colorectal cancer: current updates and future perspectives. J. Clin. Med. 13 (1), 40. doi:10.3390/jcm13010040
Mondaca-Ruff, D., Araos, P., Yañez, C. E., Novoa, U. F., Mora, I. G., Ocaranza, M. P., et al. (2021). Hydrochlorothiazide reduces cardiac hypertrophy, fibrosis and rho-kinase activation in DOCA-salt induced hypertension. J. Cardiovasc Pharmacol. Ther. 26 (6), 724–735. doi:10.1177/10742484211053109
Nemati, F., Rahbar-Roshandel, N., Hosseini, F., Mahmoudian, M., and Shafiei, M. (2011). Anti-inflammatory effects of anti-hypertensive agents: influence on interleukin-1β secretion by peripheral blood polymorphonuclear leukocytes from patients with essential hypertension. Clin. Exp. Hypertens. 33 (2), 66–76. doi:10.3109/10641963.2010.496521
Ng, L., Foo, D. C. C., Wong, C. K. H., Man, A. T. K., and Law, W. L. (2021). Repurposing DPP-4 inhibitors for colorectal cancer: a retrospective and single center study. Cancers (Basel) 13 (14), 3588. doi:10.3390/cancers13143588
Park, E., Lee, Y., and Jue, M. S. (2020). Hydrochlorothiazide use and the risk of skin cancer in patients with hypertensive disorder: a nationwide retrospective cohort study from Korea. Korean J. Intern Med. 35 (4), 917–928. doi:10.3904/kjim.2019.218
Pedersen, S. A., Gaist, D., Schmidt, S. A. J., Hölmich, L. R., Friis, S., and Pottegård, A. (2018). Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J. Am. Acad. Dermatol 78 (4), 673–681. doi:10.1016/j.jaad.2017.11.042
Pottegard, A., Hallas, J., Olesen, M., Svendsen, M. T., Habel, L. A., Friedman, G. D., et al. (2017). Hydrochlorothiazide use is strongly associated with risk of lip cancer. J. Intern Med. 282 (4), 322–331. doi:10.1111/joim.12629
Rahamimov, R., Telem, S., Davidovichi, B., Bielopolski, D., Steinmetz, T., Nesher, E., et al. (2024). The association between hydrochlorothiazide use and non-melanoma skin cancer in kidney transplant recipients. Clin. Kidney J. 17 (5), sfae126. doi:10.1093/ckj/sfae126
Registry, H. K. C. (2019). Overview of Hong Kong cancer statistics of 2018. Available at: https://www3.ha.org.hk/cancereg/.
Shin, D., Lee, E. S., Kim, J., Guerra, L., Naik, D., and Prida, X. (2019). Association between the use of thiazide diuretics and the risk of skin cancers: a meta-analysis of observational studies. J. Clin. Med. Res. 11 (4), 247–255. doi:10.14740/jocmr3744
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics. CA Cancer J. Clin. 70 (1), 7–30. doi:10.3322/caac.21590
VanWormer, J. J., Abokede, E. B., and Berg, R. L. (2022). Hydrochlorothiazide use, sun exposure, and risk of keratinocyte cancer. BMC Public Health 22 (1), 1282. doi:10.1186/s12889-022-13705-9
Webb, E. S., Liu, P., Baleeiro, R., Lemoine, N. R., Yuan, M., and Wang, Y. H. (2018). Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 32 (5), 317–326. doi:10.7555/JBR.31.20160168
Xia, Y., Sun, M., Huang, H., and Jin, W. L. (2024). Drug repurposing for cancer therapy. Signal Transduct. Target Ther. 9 (1), 92. doi:10.1038/s41392-024-01808-1
Xin, J., Gu, D., Chen, S., Ben, S., Li, H., Zhang, Z., et al. (2023). SUMMER: a Mendelian randomization interactive server to systematically evaluate the causal effects of risk factors and circulating biomarkers on pan-cancer survival. Nucleic Acids Res. 51 (D1), D1160–D1167. doi:10.1093/nar/gkac677
Keywords: colorectal cancer, drug repurposing, antihypertensive drugs, hydrochlorothiazide, basophil, prognosis
Citation: Foo DC-C, Li J, Huang Z, Sui S, Sin RW-Y, Man ATK, Law W-L and Ng L (2025) Repurposing hydrochlorothiazide (HCTZ) for colorectal cancer: a retrospective and single center study. Front. Pharmacol. 16:1449062. doi: 10.3389/fphar.2025.1449062
Received: 14 June 2024; Accepted: 30 January 2025;
Published: 28 February 2025.
Edited by:
Sheema Khan, The University of Texas Rio Grande Valley, United StatesReviewed by:
Feng Yang, Weifang Traditional Chinese Hospital, ChinaCopyright © 2025 Foo, Li, Huang, Sui, Sin, Man, Law and Ng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lui Ng, bHVpbmdAaGt1Lmhr
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.