- 1Clinic of Hematology, University Clinical Centre of Serbia, Belgrade, Serbia
- 2Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 3Clinic for Otorhinolaryngology and Maxillofacial Surgery, University Clinical Centre of Serbia, Belgrade, Serbia
- 4Clinic for Obstetrics and Gynecology, University Clinical Centre of Serbia, Belgrade, Serbia
The treatment of chronic lymphocytic leukemia (CLL) consists of the continuous use of Bruton tyrosine kinase inhibitors (BTKis) such as ibrutinib, acalabrutinib, zanubrutinib and pirtobrutinib, or Bcl-2 inhibitors, such as venetoclax. Overall survival (OS) and progression-free survival (PFS) of CLL patients are significantly improved with the use of these therapies. Adverse effects (AEs) that can occur during treatment and the presence of pre-existing comorbidities in patients can influence subsequent treatment outcomes and, consequently, OS and PFS. Managing these AEs, including cardiologic toxicity and infections (including fungal infections), as well as treating cardiovascular and other comorbidities, can be challenging due to potential drug interactions with the medications used for the management of AEs and comorbidities. Therefore, this review examined the key challenges associated with the concomitant use of novel CLL therapies and medications for managing comorbidities and AEs. This review aims to enhance and facilitate the management of patients with CLL.
1 Introduction
The treatment of chronic lymphocytic leukemia (CLL), most prevalent adult leukemia, both initial and relapse management, consists of the continuous use of Bruton tyrosine kinase (BTK) inhibitors (BTKis) or the time-limited use of a Bcl-2 inhibitor (venetoclax), either used alone or in combination with CD20+ monoclonal antibodies (mAb). Covalent BTKis in current use include ibrutinib (a first-generation BTKi), and second-generation BTKis - acalabrutinib and zanubrutinib (Kutsch et al., 2022; Wierda et al., 2022; Eichhorst et al., 2021; Eichhorst et al., 2024; Byrd et al., 2015; Barr et al., 2022; Byrd et al., 2021; Sharman et al., 2020; Ghia et al., 2022). Pirtobrutinib is a highly selective, non-covalent, reversible BTKi, that is indicated for treatment of relapsed/refractory CLL (Wierda et al., 2022; Eichhorst et al., 2024; Thompson and Tam, 2023). In the relapsed/refractory setting, the combination of the phosphoinositide 3-kinase (PI3K) inhibitor idelalisib and rituximab may be considered as a treatment option when other alternatives are unavailable or unsuitable for the patient (Wierda et al., 2022; Eichhorst et al., 2021; Sharman et al., 2019; Nair and Cheson, 2016; Gopal and Graf, 2016).
The use of these therapies has greatly enhanced overall survival (OS) and progression-free survival (PFS) in patients with CLL, even in those with high-risk features such as unmutated immunoglobulin heavy chain variable, TP53 aberrations, or complex karyotype. Clinical trials have shown 5-year progression-free survival rates exceeding 60% for patients treated with these agents, and OS continues to improve for most patients (Barr et al., 2022; Ghia et al., 2022; Seymour et al., 2022; Sharman et al., 2022; Tam et al., 2022a). However, adverse effects (AEs) during treatment, along with pre-existing comorbidities, can compromise future treatment, OS, and PFS (Burger et al., 2015; Awan et al., 2019; Tam et al., 2022b; Itsara et al., 2023; Al-Sawaf et al., 2024; Fischer et al., 2019; Fresa et al., 2024). Along with BTK inhibition, BTKis can express off-target activity by binding other cysteine-containing kinases, leading to AEs (McMullen et al., 2014; Awan et al., 2020a; Bond and Woyach, 2019; Xiao et al., 2020). Most common AEs are fatigue, bruising, infection, neutropenia, thrombocytopenia, arthralgia, bleeding, hypertension, and heart rhythm abnormalities. A few patients develop AEs with higher grades (Eichhorst et al., 2021; Eichhorst et al., 2024) when additional medical treatment is indicated or following temporary or permanent drug discontinuation (Tam et al., 2022b; Bond and Woyach, 2019; Honigberg et al., 2010; Paydas, 2019; Coutre et al., 2019). The frequently observed AEs of venetoclax when combined with CD20+ mAb included cytopenia, infections, diarrhea, nausea, fatigue, with neutropenia and infections with the most frequent being grade 3 or 4 AEs (Al-Sawaf et al., 2024; Fischer et al., 2019; Eichhorst et al., 2023). Idelalisib most common AEs include diarrhea, pyrexia, and fatigue, with grade ≥3 diarrhea occurring exclusively in the idelalisib group in clinical trials. As serious AEs were reported pneumonia and febrile neutropenia, at comparable rates between idelalisib and placebo groups, leading to similar treatment discontinuation rates (Sharman et al., 2019; Coutré et al., 2015; Furman et al., 2014).
Due to potential drug-drug interactions (DDIs), treatment decisions (use of BTKi, venetoclax and idelalisib) require the thorough assessment of comorbidities and reassessment of concomitant treatment, which makes management of AEs challenging (Fresa et al., 2024; Paydas, 2019; de Jong et al., 2018; de Zwart et al., 2016). Therefore, this review examined the key challenges associated with the concomitant use of novel CLL therapies and treatments for managing comorbidities and AEs. This review aimed to enhance and facilitate the management of patients of CLL.
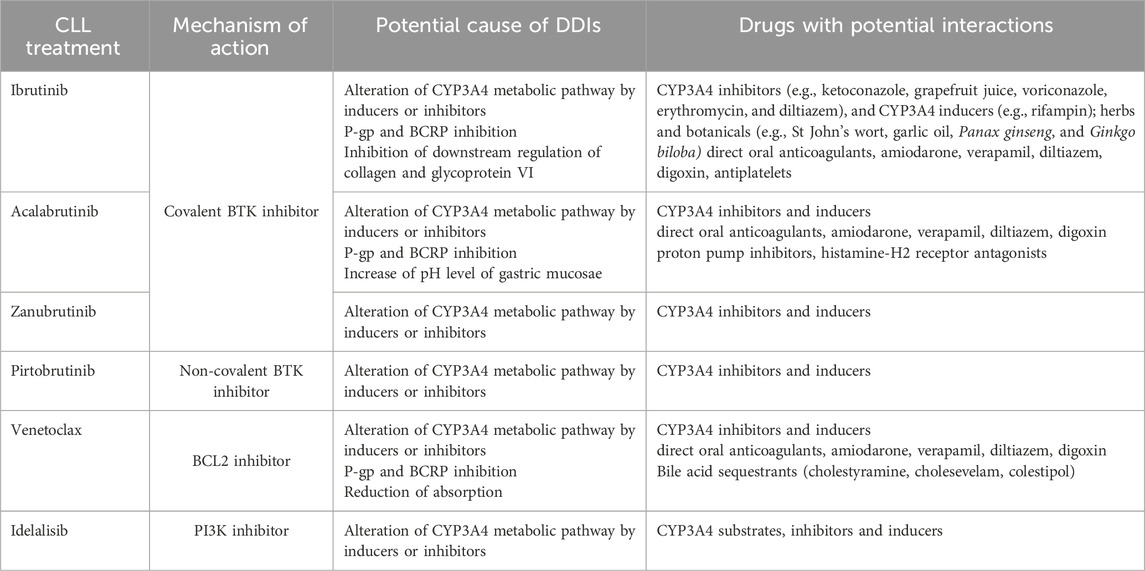
2 Mechanisms of drug-drug interactions in BTKis, Bcl-2 and PI3K inhibitors
DDIs refer to the ability of one drug to alter the pharmacological action or effect of another drug when administered simultaneously or consecutively, that can lead to enhanced, reduced, or modified therapeutic effects, posing potential risks to patient safety and treatment efficacy (Marengoni et al., 2014; Magro et al., 2021; Johnell and Klarin, 2007).
The primary metabolic and elimination pathways of ibrutinib are mediated by cytochrome P450 (CYP) 3A (CYP3A), an enzyme responsible for the metabolism of several other medications (Finnes et al., 2017; Fancher and Pappacena, 2020; Scheers et al., 2015). Although CYP2D6 contributes to ibrutinib metabolism, its role is clinically negligible, with no significant impact observed across CYP2D6 genotype variations (e.g., poor vs. extensive metabolizers) (Finnes et al., 2017; Scheers et al., 2015).
Given the critical role of CYP3A in ibrutinib metabolism, DDIs involving potent CYP3A inhibitors or inducers can significantly alter exposure to ibrutinib and the levels of its metabolites (de Jong et al., 2018; de Zwart et al., 2016). Based on the observation that ibrutinib concentrations can increase to 20-fold when co-administered with ketoconazole, a physiologically based pharmacokinetic model was developed to predict the DDI potential of mild/moderate CYP3A4 inhibitors and strong/moderate CYP3A4 inducers on ibrutinib levels (de Zwart et al., 2016). Ibrutinib exposure increases when administered with strong and moderate CYP3A4 inhibitors (e.g., ketoconazole, grapefruit juice, voriconazole, erythromycin, and diltiazem), and decreases in the presence of strong CYP3A4 inducers (e.g., rifampin) (de Jong et al., 2018; de Zwart et al., 2016; de Jong et al., 2015). The study concluded that strong CYP3A4 inhibitors and inducers should be avoided during treatment with ibrutinib because their significant impact on its pharmacokinetics could lead to AEs from increased drug exposure or reduced therapeutic efficacy due to decreased exposure (de Jong et al., 2018; de Zwart et al., 2016).
P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) contribute to the distribution of ibrutinib and its metabolites. Ibrutinib has the potential to inhibit P-gp and BCRP when administered at recommended doses, resulting in increased concentrations of oral P-gp and BCRP substrates (Fromm, 2004; Imbruvica. European Medicines Agency, 2024).
CYP3A4 and P-gp are both involved in the metabolism of anticoagulants, a potential for their interaction with ibrutinib exists, which may increase their plasma levels and, consequently, the risk of bleeding (Ganatra et al., 2018; Di Minno et al., 2017). Herbs and botanicals, e.g., St John’s wort, garlic oil, Panax ginseng, and Ginkgo biloba, as well as grapefruit juice alterate CYP3A4 enzyme activity and when taken with certain medication (e.g., CLL therapy) may lead to herb-drug interactions. Usage of products and suplements based on these herbs are contraindicated with BTKis and venetoclax (de Jong et al., 2018; Finnes et al., 2017; Gurley et al., 2005; Nicolussi et al., 2020).
Acalabrutinib significantly interacts with both CYP3A inducers and inhibitors. Acalabrutinib interacts with P-gp and BCRP, serving as a substrate for these transport proteins, and can potentially increase exposure to BCRP substrates by inhibiting BCRP activity in the intestine (Fancher and Pappacena, 2020). The solubility of acalabrutinib decreases at higher pH levels. Proton-pump inhibitors (PPIs) increase the pH level of the gastric mucosa, leading to a significant decrease in acalabrutinib bioavailability. Histamine-H2 receptor antagonists inhibit stomach acid production and increase gastric pH, thereby reducing the bioavailability of acalabrutinib. Staggered doses of histamine-H2 receptors and acalabrutinib was required to reduce DDIs and optimize therapeutic outcomes (Sharma et al., 2022; Pepin et al., 2019). However, newer formulation with pH independent release, acalabrutinib maleate tablet, reduces the impact of pH on acalabrutinib systemic exposure, enabling a wider ranges of patients to benefit from this treatment (Sharma et al., 2022).
Zanubrutinib metabolism is primarily mediated by the CYP3A isoforms. In vitro studies have indicated that zanubrutinib can reversibly inhibit CYP2C8, CYP2C9, and CYP2C19 in human liver microsomes, and can induce a two-fold or greater increase in the mRNA expression of CYP2B6, CYP2C8, CYP2C9, and CYP3A in human hepatocytes. According to a DDI study at clinically relevant concentrations, zanubrutinib exerted minimal to no impact on the activity of CYP2C9, BCRP, and P-gp. A decrease of less than 50% in systemic exposure of substrates sensitive to CYP3A and CYP2C19 was observed (Ou et al., 2021).
Pirtobrutinib is a CYP3A4 substrate, so it is recommended to avoid strong CYP3A4 inhibitors and inducers during pirtobrutinib therapy whenever possible (Pharmacist’s Application to Practice, 2023).
Venetoclax exhibits similar DDIs as BTKis owing to its predominant metabolism by CYP3A enzymes and as a substrate and inhibitor of both P-gp and BCRP (Fan et al., 2024). The total clearance of venetoclax from the plasma decreased by 19% when co-administered with moderate CYP3A inhibitors, and by up to 84% when co-administered with potent CYP3A inhibitors (Jones et al., 2016).
Strong CYP3A inducers, significantly reduce venetoclax plasma exposure (Fan et al., 2024). Population pharmacokinetics analysis has suggested that venetoclax can be administered without dose adjustment when combined with P-gp inhibitors, although a 50% dose reduction is generally recommended (Fan et al., 2024). During the venetoclax ramp-up period, DDIs with strong CYP3A4 and P-gp inhibitors can result in life-threatening complications by significantly increasing the risk of tumor lysis syndrome (TLS) and severe myelosuppression (e.g., neutropenia, thrombocytopenia, or anemia). To mitigate these risks, the use of strong CYP3A4 inhibitors should be avoided whenever possible. If their use is unavoidable, careful venetoclax dose adjustment, along with proactive TLS prevention measures and close monitoring, is essential to ensure patient safety and treatment efficacy (Fan et al., 2024; Tambaro and Wierda, 2020). Bile acid sequestrants (cholestyramine, cholesevelam, colestipol) can reduce the absorption of venetoclax, which may diminish its effectiveness, and their concurrent use with venetoclax should be avoided. If co-administration is necessary, venetoclax should be taken at least 6 h after the bile acid sequestrant to minimize this interaction (Janssens, 2024; AbbVie News Center, 2024).
Idelalisib is metabolized mostly by aldehyde oxidase and CYP3A. Idelalisib is not a sensitive substrate and can be given with CYP3A and P-gp inhibitors with caution for signs of idelalisib toxicity, but strong CYP3A and P-gp inducers should be avoided due to their potential to reduce drug concentrations. Use of CYP3A substrates and idelalisib should be avoided because its major metabolite (GS-563117) acts as moderate inhibitor of CYP3A (Nair and Cheson, 2016; Gopal and Graf, 2016; Jin et al., 2015a; Jin et al., 2015b; Ramanathan et al., 2016). Mechanism of DDIs and CLL treatment are summarized in Table 1.
3 BTKis and PI3K inhibitors and anticoagulants
CLL is associated with reduced platelet aggregation and often thrombocytopenia. Most bleeding events in patients with CLL are mild (grade 1–2) and commonly present as spontaneous bruising, petechiae, or hematomas. Approximately 5% of patients may experience more severe bleeding events (grade 3 or higher) (Byrd et al., 2016; Rushworth et al., 2013).
Bleeding complications from BTKis stem from off-target kinase inhibition (e.g., inhibition of Tec protein tyrosin kinase), disrupted platelet activation, and weakened glycoprotein VI signaling. Ibrutinib can impair platelet activation, reduce the collagen response, and decrease adhesion to the von Willebrand Factor, which may contribute to bleeding. The exact mechanisms of ibrutinib-associated bleeding are unclear, but both the disease and treatment likely contribute to platelet dysfunction (Lipsky et al., 2015; Lee et al., 2017; Awan et al., 2020b). Acalabrutinib, zanubrutinib and pirtobrutinib clinical trials have shown lower rates of major bleeding than ibrutinib, as they are more selective BTKis (Sharman et al., 2020; Brown et al., 2022; Nicolson et al., 2018; Bye et al., 2022). Approximately 10%–12% of patients treated with ibrutinib develop atrial fibrillation (AF) and require anticoagulant therapy, which heightens the risk of grade 3–4 bleeding events (Galitzia et al., 2024). The decision to begin anticoagulant therapy for AF should be tailored for each patient, considering the evaluation of risk scores for bleeding and stroke risk (Hindricks et al., 2021). Data regarding the safety of combining targeted therapy for CLL with anticoagulants are limited. Most BTKi trials have prohibited the simultaneous use of vitamin K antagonists due to the high bleeding incidence observed in earlier studies (Burger et al., 2015; Chanan-Khan et al., 2016).
The recommended anticoagulant treatment for patients receiving ibrutinib is direct oral anticoagulants, which are easier to administer and have fewer drug interactions. Rivaroxaban, apixaban and edoxaban (Xa inhibitors) are preferred over dabigatran because they have fewer drug interactions (Paydas, 2019; Quartermaine et al., 2023; Tang et al., 2018; Thorp and Badoux, 2018).
Idelalisib requires caution when co-administered with direct oral anticoagulants that are sensitive to strong P-gp or CYP3A4 modulators (Di Minno et al., 2017; Ramanathan et al., 2016; Mar et al., 2022).
4 BTKis, Bcl-2 and PI3K inhibitors and antimicrobial therapies
4.1 Immune dysfunction and infection risk in CLL
Patients with CLL have severe dysfunctions of both the innate and adaptive immune systems, exposing them to a higher risk of infections. In CLL, dendritic cells remain immature, resulting in suboptimal T-cell activation due to insufficient interleukin (IL)-12 production. The monocyte-phagocytic system is compromised, leading to impaired production of reactive oxygen species and increasing susceptibility to bacterial infections. Natural killer (NK) cells have altered expression of the NKG2D co-receptor with reduced cytotoxic capabilities. Reduced levels of complement components impair the activity of both the classical and alternative pathways, compromising the ability to opsonize bacteria using the C3b complement component (Rivera and Ferrajoli, 2022; Maffei et al., 2013; Orsini et al., 2003; Naseraldeen et al., 2021). CLL B-cells exhibit a phenotype similar to that of regulatory B-cells, leading to the suppression of immune responses primarily through the secretion of the IL-10. T-cells are dysfunctional, accompanied by elevated levels of regulatory T-cells. Hypogammaglobulinemia, which is observed in 85% of patients, is one of the most important causes of immunodeficiency in CLL and typically involves a reduction in IgG and IgA levels. In early-stage CLL, only a single immunoglobulin class is usually diminished, whereas in advanced CLL, all immunoglobulin classes are affected (Forconi and Moss, 2015; Crassini et al., 2018).
Beyond its effects on CLL cells, BTK inhibition can impair NK cell function and reduce the production of pro-inflammatory cytokines, such as tumor necrosis factor-α and IL-1β, leading to diminished activity of macrophages and neutrophils. Treatment with ibrutinib lowers the levels of both CD4+ and CD8+ T-cells, with cell counts recovering after 6 months of therapy and reducing in tumor burden. However, effector memory CD4+ and CD8+ T-cells may continue to decline, even after 12 months of treatment (Bojarczuk et al., 2014; Kohrt et al., 2014; Mulder et al., 2021). In the clinical trials examining ibrutinib, the incidence of infections was as high as 80%, with upper respiratory tract and urinary infections being the most common (Coutre et al., 2019). Grade ≥3 infections occurred in 40% of the patients, with pneumonia being the most frequent (12%–17%). The most common fungal infections were caused by Aspergillus, Cryptococcus and P. jirovecii (Byrd et al., 2015; Coutre et al., 2019; Tey et al., 2023; Ruchlemer et al., 2019). Considering the lack of the data, CLL guidelines do not suggest administering Pneumocystis jirovecii pneumonia prophylaxis for every patients, but only in the patients at high-risk (i.e., patients with relapsed/refractory disease). The incidence of Aspergillus fumigatus infection is higher in the early phase of treatment and among patients who were receiving corticosteroids. In a recent cross-trial comparison, similar changes during BTK inhibition were observed with ibrutinib and acalabrutinib (Hallek et al., 2018; Frei et al., 2020).
Venetoclax may increase the risk of bacterial and fungal infections owing to neutropenia and a reduction in T, B and NK cell levels; however other studies have shown that it may partially counteract the immunosuppressive environment by restoring the function of NK cells (de Weerdt et al., 2019; Roberts et al., 2016; Stilgenbauer et al., 2016). Meta-analysis showed that the probability of an increased infection risk during venetoclax treatment was 71.2% (Prosty et al., 2023).
PI3K inhibition by idelalisib increases the risk of infections by impairing T and NK cell functions, including reduced T-cell-mediated cytotoxicity, diminished granzyme B and cytokine secretion, and decreased proliferation and cytotoxicity of NK cells (Rohrbacher et al., 2021; Martinelli et al., 2018).
4.2 Antimicrobial therapy and BTKis, Bcl-2 and PI3K inhibitors dose modifications
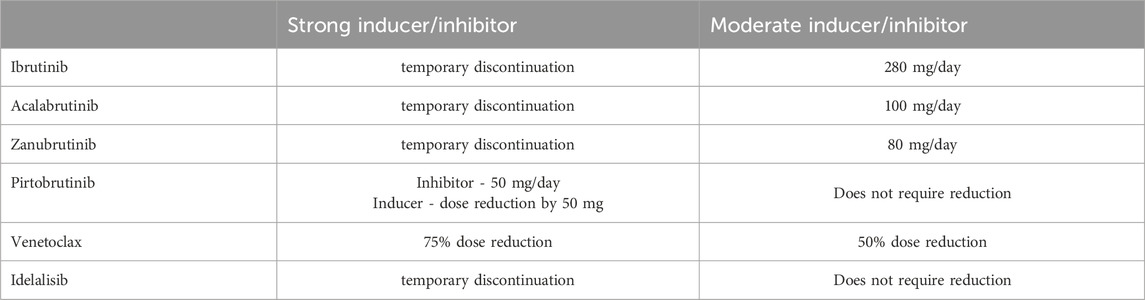
For short-time use (up to 7 days) of antibacterial (e.g., azithromycin, erythromycin and clarithromycin) or antifungal agents (e.g., ketoconazole, itraconazole and voriconazole) that are strong CYP3A inhibitors, temporarily discontinuation of ibrutinib may be necessary. If combined with moderate CYP3A4 inhibitors, the ibrutinib dose should be reduced to 280 mg/day, with further reductions in cases of strong inhibitors like voriconazole or posaconazole (de Zwart et al., 2016; Fancher and Pappacena, 2020; Bose et al., 2016). Similarly, acalabrutinib, zanubrutinib, and pirtobrutinib, which share similar metabolic pathways, require dose adjustments when co-administred with CYP3A inducers or inhibitors. (Fancher and Pappacena, 2020; Ou et al., 2021; Pharmacist’s Application to Practice, 2023).
Azole antifungal drugs should be avoided during the ramp-up phase of venetoclax treatment. Once a therapeutic dose of venetoclax is established, it should be reduced by 75% if concomitant drugs are indicated for infection. Certain weak inhibitors of CYP3A4 (ciprofloxacin and fluconazole), may significantly interact with Bcl-2 inhibitors; thus, a 50% dose reduction is recommended (Fan et al., 2024; Freise et al., 2017; Bhatnagar et al., 2021). Dose reduction of venetoclax by 50% is suggested when used concurrently with P-gp inhibitors (e.g., azithromycin). Therefore, the best approach would be to monitor venetoclax concentrations during concurrent treatment, as therapeutic efficacy significantly depends on serum drug levels (Fan et al., 2024; Freise et al., 2017; Agarwal et al., 2018).
Use of antimictrobial agents, such as erythromicin, that are CYP3A substrates should be avoided with idelalisib (Ramanathan et al., 2016; Cuneo et al., 2019).
5 BTKis, Bcl-2 and PI3K inhibitors and treatment of cardiovascular diseases
BTKis and venetoclax have been associated with an increased risk of cardiovascular events such as arrhythmias and hypertension, underscoring the importance of regular cardiovascular monitoring. Idelalisib is associated with cardiovascular AEs, though these are not common. Patients often present with additional cardiovascular risk factors, including older age, smoking, diabetes, and pre-existing hypertension. Effective management of these patients requires hematologist-cardiologist collaboration to adjust treatments and address cardiovascular risks (Sharman et al., 2019; Coutré et al., 2015; Quartermaine et al., 2023; Grewal et al., 2021; Fedele and Opat, 2024; Larsson et al., 2022).
5.1 BTKis and Bcl-2 inhibitors and antiarrhythmics
BTKis are associated with an increased risk of arrhythmias, particularly AF and ventricular arrhythmias. Although the precise mechanism remains unclear, potential factors include their effects on ion channels and inflammatory pathways via off-target activities (Xiao et al., 2020; Seymour et al., 2023; Dickerson et al., 2019; Archibald et al., 2021; Lampson et al., 2017). In contrast, venetoclax does not directly cause arrhythmias, but it can contribute indirectly through conditions like tumor lysis syndrome, which may lead to electrolyte imbalances that trigger arrhythmias (Anderson et al., 2024).
Drug interactions between novel CLL therapies and antiarrhythmic medications can be significant because both classes of drugs may influence the other’s metabolism and therapeutic efficacy. Drugs used to treat arrhythmias that inhibit the CYP3A4 enzyme (e.g., dronedarone, verapamil, and digoxin) can increase the concentrations of BTKis and BCL-2 inhibitors, potentially increasing the risk of AE (Mar et al., 2022; Asnani et al., 2017). Although amiodarone may not interact with ibrutinib as directly as dronedarone, it should still be used cautiously because of its complex interactions, which could potentially influence ibrutinib metabolism (Ganatra et al., 2018; Mar et al., 2022; Asnani et al., 2017). Sotalol, amiodarone and flecainide prolong the QT interval and, when used in conjunction with drugs that affect cardiovascular function, such as BTKis, can exacerbate this effect (Galitzia et al., 2024; Mar et al., 2022; Asnani et al., 2017).
Effective management of these risks requires close cardiovascular monitoring, seamless coordination between hematology and cardiology departments, and thorough patient education.
5.2 BTKis, Bcl-2 and PI3K inhibitors and antiplatelet drugs
Low-dose aspirin is widely used for cardiovascular disease (CVD) prevention in high-risk population. Studies have raised concerns about trend of overuse, particularly in those at low CVD risk, where the potential benefits of aspirin are outweighed by the risk of bleeding. In CLL patients, many may have been using aspirin without clear justification prior to their diagnosis. It is essential to reassess aspirin use before starting treatment. This evaluation should carefully balance the patient’s cardiovascular risk factors against the heightened risk of bleeding, ensuring aspirin is used only when the benefits clearly outweigh the risks (Luepker et al., 2021; Kim et al., 2019; Yoo et al., 2024).
Studies have shown that BTKis may impair platelet function by inhibiting the downstream regulation of collagen and glycoprotein VI. Concomitant use of antiplatelet agents (aspirin and clopidogrel) with BTKis increases the risk of major bleeding events (Mendez-Ruiz et al., 2023; Jones et al., 2017; Jiang et al., 2022). The frequency of major bleeding increases in BTKi-treated patients who receive antiplatelet therapy, with an incidence of 2.5% (Jones et al., 2017). Similarly, acalabrutinib has the potential to enhance bleeding risk when combined with antiplatelet medications, such as dipyridamole. Acalabrutinib and zanubrutinib has been associated with a lower bleeding risk than the other BTKis (Galitzia et al., 2024; Mendez-Ruiz et al., 2023; Jiang et al., 2022). In case that double antiplatelet therapy or combination of anticoagulation and antiplatelet therapy is required, alternative treatment to BTKis should be provided (Quartermaine et al., 2023). Venetoclax can lower platelet counts, significantly increasing the risk of bleeding and amplifying the effects of antiplatelet agents, such as ticagrelor (Galitzia et al., 2024). Idelalisib may exhibit antiplatelet effects and cause minor bleeding, given the role of PI3K in platelet activation, adhesion, and thrombus development (Barrachina et al., 2021).
5.3 BTKis and Bcl-2 inhibitors and antihypertensives
Hypertension is a recognized AE of BTKis that can emerge as a new condition during treatment, or BTKis can aggravate pre-existing hypertension (Quartermaine et al., 2023). Managing pre-existing hypertension during BTKi treatment presents a challenge because of the risk of developing severe hypertension (grade 3 or 4) as an AE, as well as potential DDIs with antihypertensive medications (Galitzia et al., 2024; Dickerson et al., 2019). DDIs between antihypertensive medications and CLL therapy (BTKis and venetoclax) mainly involve CYP3A4 metabolism, potentially affecting the effectiveness or increasing the side effects of CLL agents (Galitzia et al., 2024; Dickerson et al., 2019; Fernandez Turizo et al., 2024; Gordon et al., 2023). The use of non-dihydropyridine calcium channel blockers (diltiazem and nifedipine) should be avoided because of their major DDIs with BTKis (Quartermaine et al., 2023). Regular monitoring and potential dose adjustments are crucial for managing these interactions and ensuring effective treatment while minimizing side effect.
6 Recommendations on concurrent use of BTKis, Bcl-2 and PI3K inhibitors and concomitant medication
Given the potential for DDIs during the concurrent use of CLL treatments and other medications, it is essential to assess comorbidities and all concurrent medications before initiating CLL therapy. This evaluation helps to identify potential interactions and determine whether alternative treatments should be considered (Fresa et al., 2024; Paydas, 2019).
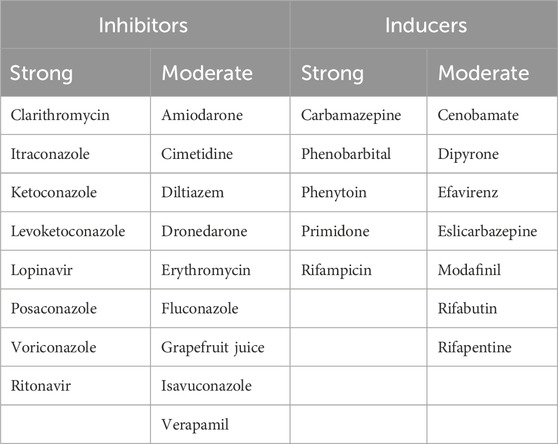
Attention should be focused on CYP3A4 inducers and inhibitors, as they can reduce efficacy and increase the incidence of AEs (Fresa et al., 2024; Paydas, 2019). Table 2 provides a list of CYP3A4 inducers and inhibitors (Liu et al., 2007).
Strong CYP3A4 inhibitors should be avoided during treatment with ibrutinib, acalabrutinib and zanubrutinib. If a strong interacting medication is required for a short duration (up to 7 days), temporary discontinuation of ibrutinib and acalabrutinib may be necessary. Combined with strong CYP3A inhibitors, zanubrutinib should be administered at 80 mg/day and pirtobrutinib requires dose reduction to 50 mg/day (Fancher and Pappacena, 2020; Ou et al., 2021; Pharmacist’s Application to Practice, 2023). When used in combination with moderate CYP3A4 inhibitors, the dose of ibrutinib should be reduced to 280 mg/day, with further reductions of up to 140 mg/day and 70 mg/day if necessary, and the dose of acalabrutinib should be reduced to 100 mg/day. No dose adjustment is required when ibrutinib and acalabrutinib are used alongside mild CYP3A4 inhibitors (de Zwart et al., 2016; Fancher and Pappacena, 2020; Bose et al., 2016). If a CYP3A4 inducer is unavoidable, pirtobrutinib dose should be increased to 300 mg daily for patients on 200 mg daily, or increased by 50 mg for those on 50 mg or 100 mg daily (Pharmacist’s Application to Practice, 2023). During venetoclax treatment, strong CYP3A4 inducers should be avoided or administered with a 75% venetoclax dose reduction, whereas moderate CYP3A4 inducers require a 50% dose reduction of venetoclax (Freise et al., 2017; Bhatnagar et al., 2021).
Table 3 provides dose adjustments for CLL therapy when co-administered with CYP3A4 inhibitors/inducers. Figure 1 present suggested algorithm for assessment CLL treatment in case of use of CYP3A4 inhibitors/inducers (Tam et al., 2022b; Al-Sawaf et al., 2024; Ramanathan et al., 2016; Awan et al., 2020b; Brown et al., 2022).
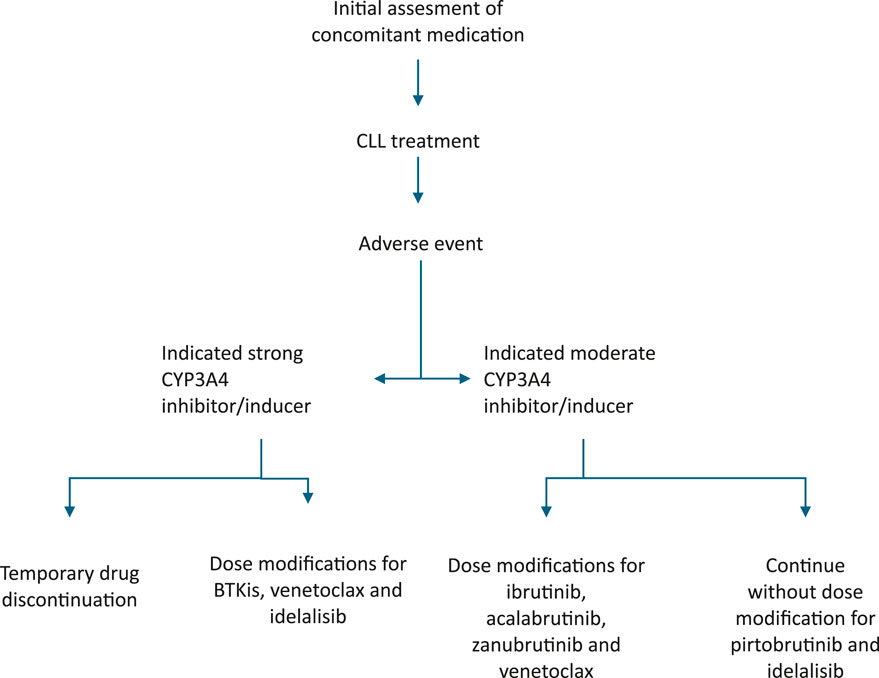
Figure 1. Suggested algorithm for assessment CLL treatment in case of CYP3A4 inhibitors/inducers treatment.
Owing to the increased risk of bleeding before and during ibrutinib treatment, the use of antiplatelet drugs should be reevaluated (Mendez-Ruiz et al., 2023; Jones et al., 2017). The preferred anticoagulant treatment for patients receiving ibrutinib is a direct oral anticoagulant (rivaroxaban, apixaban or edoxaban) (Paydas, 2019; Quartermaine et al., 2023).
7 Conclusion
To mitigate the risk of DDIs during the concurrent use of CLL treatment and other medications, it is crucial to thoroughly assess comorbidities and review all medications before initiating therapy. During treatment, any concomitant therapies used for managing AEs or comorbidities should be continuously reevaluated to ensure ongoing safety and effectiveness and to identify any emerging interactions that may necessitate dose adjustments in the treatment plan. Further studies are needed to provide more insight into the management of CLL in the context of concurrent medication use to offer a clearer comparison of the treatment options and their potential advantages and/or disadvantages. A multidisciplinary approach to treatment decisions when managing patients with CLL is crucial to ensuring optimal outcomes.
Author contributions
SK: Conceptualization, Writing–original draft, Writing–review and editing. JI: Writing–original draft, Writing–review and editing. MaM: Writing–original draft, Methodology. KT: Writing–original draft, Writing–review and editing. IA: Writing–original draft, Writing–review and editing. NS: Supervision, Writing–review and editing. AB: Supervision, Writing–review and editing. AV: Supervision, Writing–review and editing. MT: Supervision, Writing–review and editing. JB: Supervision, Writing–review and editing. MiM: Writing–review and editing, Supervision. DL: Supervision, Writing–review and editing. ID: Supervision, Writing–review and editing. MV: Supervision, Writing–review and editing. AT: Supervision, Writing–review and editing. JM: Supervision, Writing–review and editing. DA: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This article was supported by Project 451-03-65/2024-03/200110 - Ministry of Science, Technological Development and Innovation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbbVie News Center (2024). AbbVie’s VENCLYXTO®/VENCLEXTA® (venetoclax) continues to show sustained progression-free survival (PFS) in chronic lymphocytic leukemia (CLL) patients. Available at: https://news.abbvie.com/2023-06-09-AbbVies-VENCLYXTO-R-VENCLEXTA-R-venetoclax-Continues-to-Show-Sustained-Progression-Free-Survival-PFS-in-Chronic-Lymphocytic-Leukemia-CLL-Patients.
Agarwal, S. K., Tong, B., Bueno, O. F., Menon, R. M., and Salem, A. H. (2018). Effect of azithromycin on venetoclax pharmacokinetics in healthy volunteers: implications for dosing venetoclax with P-gp inhibitors. Adv. Ther. 35 (11), 2015–2023. doi:10.1007/s12325-018-0793-y
Al-Sawaf, O., Robrecht, S., Zhang, C., Olivieri, S., Chang, Y. M., Fink, A. M., et al. (2024). Venetoclax-Obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the phase 3 CLL14 study. Blood J. 2024024631. doi:10.1182/blood.2024024631
Anderson, M. A., Walewska, R., Hackett, F., Kater, A. P., Montegaard, J., O’Brien, S., et al. (2024). Venetoclax initiation in chronic lymphocytic leukemia: international insights and innovative approaches for optimal patient care. Cancers (Basel) 16 (5), 980. doi:10.3390/cancers16050980
Archibald, W. J., Rabe, K. G., Kabat, B. F., Herrmann, J., Ding, W., Kay, N. E., et al. (2021). Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes. Ann. Hematol. 100 (1), 143–155. doi:10.1007/s00277-020-04094-3
Asnani, A., Manning, A., Mansour, M., Ruskin, J., Hochberg, E. P., and Ptaszek, L. M. (2017). Management of atrial fibrillation in patients taking targeted cancer therapies. Cardiooncology 3, 2. doi:10.1186/s40959-017-0021-y
Awan, F. T., Al-Sawaf, O., Fischer, K., and Woyach, J. A. (2020b). Current perspectives on therapy for chronic lymphocytic leukemia. Am. Soc. Clin. Oncol. Educ. Book (40), 320–329. doi:10.1200/edbk_279099
Awan, F. T., Schuh, A., Brown, J. R., Furman, R. R., Pagel, J. M., Hillmen, P., et al. (2019). Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 3 (9), 1553–1562. doi:10.1182/bloodadvances.2018030007
Awan, F. T., Tong, D., and Zaha, V. G. (2020a). Cardio-oncology: a win-win situation: how solving the mystery of an ibrutinib off-target effect reveals new insights into atrial fibrillation mechanisms. Circulation 142 (25), 2456–2458. doi:10.1161/CIRCULATIONAHA.120.052047
Barr, P. M., Owen, C., Robak, T., Tedeschi, A., Bairey, O., Burger, J. A., et al. (2022). Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 6 (11), 3440–3450. doi:10.1182/bloodadvances.2021006434
Barrachina, M. N., Izquierdo, I., Hermida-Nogueira, L., Morán, L. A., Pérez, A., Arroyo, A. B., et al. (2021). The PI3Kδ inhibitor idelalisib diminishes platelet function and shows antithrombotic potential. Int. J. Mol. Sci. 22 (7), 3304. doi:10.3390/ijms22073304
Bhatnagar, S., Mukherjee, D., Salem, A. H., Miles, D., Menon, R. M., and Gibbs, J. P. (2021). Dose adjustment of venetoclax when co-administered with posaconazole: clinical drug-drug interaction predictions using a PBPK approach. Cancer Chemother. Pharmacol. 87 (4), 465–474. doi:10.1007/s00280-020-04179-w
Bojarczuk, K., Siernicka, M., Dwojak, M., Bobrowicz, M., Pyrzynska, B., Gaj, P., et al. (2014). B-cell receptor pathway inhibitors affect CD20 levels and impair antitumor activity of anti-CD20 monoclonal antibodies. Leukemia 28 (5), 1163–1167. doi:10.1038/leu.2014.12
Bond, D. A., and Woyach, J. A. (2019). Targeting BTK in CLL: beyond ibrutinib. Curr. Hematol. Malig. Rep. 14 (3), 197–205. doi:10.1007/s11899-019-00512-0
Bose, P., Gandhi, V. V., and Keating, M. J. (2016). Pharmacokinetic and pharmacodynamic evaluation of ibrutinib for the treatment of chronic lymphocytic leukemia: rationale for lower doses. Expert Opin. Drug Metab. Toxicol. 12 (11), 1381–1392. doi:10.1080/17425255.2016.1239717
Brown, J. R., Hillmen, P., Eichhorst, B., Lamanna, N., O’Brien, S., Tam, C. S., et al. (2022). CLL-115 first interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Clin. Lymphoma Myeloma Leukemia 22, S266. doi:10.1016/s2152-2650(22)01324-6
Burger, J. A., Tedeschi, A., Barr, P. M., Robak, T., Owen, C., Ghia, P., et al. (2015). Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 373 (25), 2425–2437. doi:10.1056/NEJMoa1509388
Bye, A. P., Kriek, N., Sage, T., Rawlings, S. J., Prodger, C., Kesavan, M., et al. (2022). Pirtobrutinib results in reversible platelet dysfunction compared to ibrutinib and acalabrutinib. Haematologica 108 (5), 1429–1435. doi:10.3324/haematol.2022.281402
Byrd, J. C., Furman, R. R., Coutre, S. E., Burger, J. A., Blum, K. A., Coleman, M., et al. (2015). Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125 (16), 2497–2506. doi:10.1182/blood-2014-10-606038
Byrd, J. C., Harrington, B., O’Brien, S., Jones, J. A., Schuh, A., Devereux, S., et al. (2016). Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374 (4), 323–332. doi:10.1056/NEJMoa1509981
Byrd, J. C., Hillmen, P., Ghia, P., Kater, A. P., Chanan-Khan, A., Furman, R. R., et al. (2021). Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J. Clin. Oncol. 39 (31), 3441–3452. doi:10.1200/JCO.21.01210
Chanan-Khan, A., Cramer, P., Demirkan, F., Fraser, G., Silva, R. S., Grosicki, S., et al. (2016). Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 17 (2), 200–211. doi:10.1016/S1470-2045(15)00465-9
Coutré, S. E., Barrientos, J. C., Brown, J. R., de Vos, S., Furman, R. R., Keating, M. J., et al. (2015). Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk. Lymphoma 56 (10), 2779–2786. doi:10.3109/10428194.2015.1022770
Coutre, S. E., Byrd, J. C., Hillmen, P., Barrientos, J. C., Barr, P. M., Devereux, S., et al. (2019). Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 3 (12), 1799–1807. doi:10.1182/bloodadvances.2018028761
Crassini, K. R., Zhang, E., Balendran, S., Freeman, J. A., Best, O. G., Forsyth, C. J., et al. (2018). Humoral immune failure defined by immunoglobulin class and immunoglobulin G subclass deficiency is associated with shorter treatment-free and overall survival in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 181 (1), 97–101. doi:10.1111/bjh.15146
Cuneo, A., Barosi, G., Danesi, R., Fagiuoli, S., Ghia, P., Marzano, A., et al. (2019). Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol. Oncol. 37 (1), 3–14. doi:10.1002/hon.2540
de Jong, J., Hellemans, P., De Wilde, S., Patricia, D., Masterson, T., Manikhas, G., et al. (2018). A drug-drug interaction study of ibrutinib with moderate/strong CYP3A inhibitors in patients with B-cell malignancies. Leuk. Lymphoma 59 (12), 2888–2895. doi:10.1080/10428194.2018.1460474
de Jong, J., Skee, D., Murphy, J., Sukbuntherng, J., Hellemans, P., Smit, J., et al. (2015). Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol. Res. Perspect. 3 (4), e00156. doi:10.1002/prp2.156
de Weerdt, I., Hofland, T., de Boer, R., Dobber, J. A., Dubois, J., van Nieuwenhuize, D., et al. (2019). Distinct immune composition in lymph node and peripheral blood of CLL patients is reshaped during venetoclax treatment. Blood Adv. 3 (17), 2642–2652. doi:10.1182/bloodadvances.2019000360
de Zwart, L., Snoeys, J., De Jong, J., Sukbuntherng, J., Mannaert, E., and Monshouwer, M. (2016). Ibrutinib dosing strategies based on interaction potential of CYP3A4 perpetrators using physiologically based pharmacokinetic modeling. Clin. Pharmacol. Ther. 100 (5), 548–557. doi:10.1002/cpt.419
Dickerson, T., Wiczer, T., Waller, A., Philippon, J., Porter, K., Haddad, D., et al. (2019). Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 134 (22), 1919–1928. doi:10.1182/blood.2019000840
Di Minno, A., Frigerio, B., Spadarella, G., Ravani, A., Sansaro, D., Amato, M., et al. (2017). Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 31 (4), 193–203. doi:10.1016/j.blre.2017.02.001
Eichhorst, B., Ghia, P., Niemann, C. U., Kater, A. P., Gregor, M., Hallek, M., et al. (2024). ESMO Clinical Practice Guideline interim update on new targeted therapies in the first line and at relapse of chronic lymphocytic leukaemia. Ann. Oncol. 35, 762–768. doi:10.1016/j.annonc.2024.06.016
Eichhorst, B., Niemann, C. U., Kater, A. P., Fürstenau, M., von Tresckow, J., Zhang, C., et al. (2023). First-Line venetoclax combinations in chronic lymphocytic leukemia. N. Engl. J. Med. 388 (19), 1739–1754. doi:10.1056/NEJMoa2213093
Eichhorst, B., Robak, T., Montserrat, E., Ghia, P., Niemann, C. U., Kater, A. P., et al. (2021). Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32 (1), 23–33. doi:10.1016/j.annonc.2020.09.019
Fan, W., Guo, J., Zhang, Y., Zhang, R., and Lin, B. (2024). Venetoclax dose adjustment due to drug-drug interactions: a case report and literature review. Anticancer Drugs 35 (1), 70–75. doi:10.1097/CAD.0000000000001541
Fancher, K. M., and Pappacena, J. J. (2020). Drug interactions with Bruton’s tyrosine kinase inhibitors: clinical implications and management. Cancer Chemother. Pharmacol. 86 (4), 507–515. doi:10.1007/s00280-020-04137-6
Fedele, P. L., and Opat, S. (2024). Chronic lymphocytic leukemia: time to care for the survivors. JCO 42 (17), 2005–2011. doi:10.1200/JCO.23.02738
Fernandez Turizo, M. J., Kim, E., Zhang, C., Yankama, T., Von Keudell, G., Sermer, D. J., et al. (2024). Pre-existing cardiovascular disease is associated with an increased risk of cardiovascular events during Bruton tyrosine kinase inhibitor therapy. Oncologist, oyae229. doi:10.1093/oncolo/oyae229
Finnes, H. D., Chaffee, K. G., Call, T. G., Ding, W., Kenderian, S. S., Bowen, D. A., et al. (2017). Pharmacovigilance during ibrutinib therapy for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in routine clinical Practice. Leuk. Lymphoma 58 (6), 1376–1383. doi:10.1080/10428194.2016.1251592
Fischer, K., Al-Sawaf, O., Bahlo, J., Fink, A. M., Tandon, M., Dixon, M., et al. (2019). Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 380 (23), 2225–2236. doi:10.1056/NEJMoa1815281
Forconi, F., and Moss, P. (2015). Perturbation of the normal immune system in patients with CLL. Blood 126 (5), 573–581. doi:10.1182/blood-2015-03-567388
Frei, M., Aitken, S. L., Jain, N., Thompson, P., Wierda, W., Kontoyiannis, D. P., et al. (2020). Incidence and characterization of fungal infections in chronic lymphocytic leukemia patients receiving ibrutinib. Leuk. Lymphoma 61 (10), 2488–2491. doi:10.1080/10428194.2020.1775215
Freise, K. J., Shebley, M., and Salem, A. H. (2017). Quantitative prediction of the effect of CYP3A inhibitors and inducers on venetoclax pharmacokinetics using a physiologically based pharmacokinetic model. J. Clin. Pharmacol. 57 (6), 796–804. doi:10.1002/jcph.858
Fresa, A., Innocenti, I., Tomasso, A., Stirparo, L., Mosca, A., Iadevaia, F., et al. (2024). Treatment sequencing in chronic lymphocytic leukemia in 2024: where we are and where we are headed. Cancers (Basel) 16 (11), 2011. doi:10.3390/cancers16112011
Fromm, M. F. (2004). Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 25 (8), 423–429. doi:10.1016/j.tips.2004.06.002
Furman, R. R., Sharman, J. P., Coutre, S. E., Cheson, B. D., Pagel, J. M., Hillmen, P., et al. (2014). Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 370 (11), 997–1007. doi:10.1056/NEJMoa1315226
Galitzia, A., Maccaferri, M., Mauro, F. R., Murru, R., and Marasca, R. (2024). Chronic lymphocytic leukemia: management of adverse events in the era of targeted agents. Cancers (Basel) 16 (11), 1996. doi:10.3390/cancers16111996
Ganatra, S., Sharma, A., Shah, S., Chaudhry, G. M., Martin, D. T., Neilan, T. G., et al. (2018). Ibrutinib-associated atrial fibrillation. JACC Clin. Electrophysiol. 4 (12), 1491–1500. doi:10.1016/j.jacep.2018.06.004
Ghia, P., Pluta, A., Wach, M., Lysak, D., Šimkovič, M., Kriachok, I., et al. (2022). Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere 6 (12), e801. doi:10.1097/HS9.0000000000000801
Gopal, A., and Graf, S. (2016). Idelalisib for the treatment of non-Hodgkin lymphoma. Expert Opin. Pharmacother. 17 (2), 265–274. doi:10.1517/14656566.2016.1135130
Gordon, M. J., Jones, J. E., George, B., Peterson, C., Burger, J. A., Jain, N., et al. (2023). Long-term outcomes in patients with chronic lymphocytic leukemia treated with ibrutinib: focus on hypertension and cardiovascular toxicity. Cancer 129 (14), 2192–2200. doi:10.1002/cncr.34787
Grewal, U. S., Thotamgari, S. R., Sheth, A. R., Gaddam, S. J., Ahmad, J., Beedupalli, K., et al. (2021). Cardiovascular complications associated with novel agents in the chronic lymphocytic leukemia armamentarium: a pharmacovigilance analysis. Int. J. Cardiol. 344, 186–189. doi:10.1016/j.ijcard.2021.10.011
Gurley, B. J., Gardner, S. F., Hubbard, M. A., Williams, D. K., Gentry, W. B., Cui, Y., et al. (2005). Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: st John's wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging 22 (6), 525–539. doi:10.2165/00002512-200522060-00006
Hallek, M., Cheson, B. D., Catovsky, D., Caligaris-Cappio, F., Dighiero, G., Döhner, H., et al. (2018). iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 131 (25), 2745–2760. doi:10.1182/blood-2017-09-806398
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., et al. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42 (5), 373–498. doi:10.1093/eurheartj/ehaa612
Honigberg, L. A., Smith, A. M., Sirisawad, M., Verner, E., Loury, D., Chang, B., et al. (2010). The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 107 (29), 13075–13080. doi:10.1073/pnas.1004594107
Imbruvica. Amsterdam, Netherlands: European Medicines Agency. 2024. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/imbruvica
Itsara, A., Sun, C., Bryer, E., Ahn, I. E., Soto, S., Wang, H. W., et al. (2023). Long-Term outcomes in chronic lymphocytic leukemia treated with ibrutinib: 10-year follow-up of a phase 2 study. Blood 142 (Suppl. 1), 201. doi:10.1182/blood-2023-182397
Janssens, A. (2024). Venetoclax, the first available bcl-2 antagonist for chronic lymphocytic leukaemia. Beerse, Belgium: V O LU M E.
Jiang, D., Song, Z., Hu, Y., Dong, F., and Zhao, R. (2022). Risk of bleeding associated with BTK inhibitor monotherapy: a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Clin. Pharmacol. 15 (8), 987–996. doi:10.1080/17512433.2022.2106968
Jin, F., Robeson, M., Zhou, H., Hisoire, G., and Ramanathan, S. (2015a). The pharmacokinetics and safety of idelalisib in subjects with moderate or severe hepatic impairment. J. Clin. Pharmacol. 55 (8), 944–952. doi:10.1002/jcph.504
Jin, F., Robeson, M., Zhou, H., Moyer, C., Wilbert, S., Murray, B., et al. (2015b). Clinical drug interaction profile of idelalisib in healthy subjects. J. Clin. Pharmacol. 55 (8), 909–919. doi:10.1002/jcph.495
Johnell, K., and Klarin, I. (2007). The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 30 (10), 911–918. doi:10.2165/00002018-200730100-00009
Jones, A. K., Freise, K. J., Agarwal, S. K., Humerickhouse, R. A., Wong, S. L., and Salem, A. H. (2016). Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and non-hodgkin’s lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J. 18 (5), 1192–1202. doi:10.1208/s12248-016-9927-9
Jones, J. A., Hillmen, P., Coutre, S., Tam, C., Furman, R. R., Barr, P. M., et al. (2017). Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br. J. Haematol. 178 (2), 286–291. doi:10.1111/bjh.14660
Kim, J. H., Shim, M. J., Lee, S. Y., Oh, J., and Kim, S. H. (2019). Aspirin for primary prevention of cardiovascular disease. J. Lipid Atheroscler. 8 (2), 162–172. doi:10.12997/jla.2019.8.2.162
Kohrt, H. E., Sagiv-Barfi, I., Rafiq, S., Herman, S. E. M., Butchar, J. P., Cheney, C., et al. (2014). Ibrutinib antagonizes rituximab-dependent NK cell–mediated cytotoxicity. Blood 123 (12), 1957–1960. doi:10.1182/blood-2014-01-547869
Kutsch, N., Fink, A. M., and Fischer, K. (2022). Management of front line chronic lymphocytic leukemia. Am. J. Hematol. 97 (S2), S3–S10. doi:10.1002/ajh.26677
Lampson, B. L., Yu, L., Glynn, R. J., Barrientos, J. C., Jacobsen, E. D., Banerji, V., et al. (2017). Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood 129 (18), 2581–2584. doi:10.1182/blood-2016-10-742437
Larsson, K., Söderling, J., Höglund, M., Glimelius, I., and Mattsson, M. (2022). Cardiovascular disease in patients with chronic lymphocytic leukemia: a Swedish nationwide register study with matched comparators. Am. J. Hematol. 97 (7), E255–E257. doi:10.1002/ajh.26558
Lee, R. H., Piatt, R., Conley, P. B., and Bergmeier, W. (2017). Effects of ibrutinib treatment on murine platelet function during inflammation and in primary hemostasis. Haematologica 102 (3), e89–e92. doi:10.3324/haematol.2016.155978
Lipsky, A. H., Farooqui, M. Z. H., Tian, X., Martyr, S., Cullinane, A. M., Nghiem, K., et al. (2015). Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica 100 (12), 1571–1578. doi:10.3324/haematol.2015.126672
Liu, Y. T., Hao, H. P., Liu, C. X., Wang, G. J., and Xie, H. G. (2007). Drugs as CYP3A probes, inducers, and inhibitors. Drug Metab. Rev. 39 (4), 699–721. doi:10.1080/03602530701690374
Luepker, R. V., Oldenburg, N., Misialek, J. R., Hof, J. R. V., Finnegan, J. R., Eder, M., et al. (2021). Aspirin use and misuse for the primary prevention of cardiovascular diseases. Am. J. Prev. Med. 60 (4), 513–519. doi:10.1016/j.amepre.2020.10.025
Maffei, R., Bulgarelli, J., Fiorcari, S., Bertoncelli, L., Martinelli, S., Guarnotta, C., et al. (2013). The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica 98 (7), 1115–1123. doi:10.3324/haematol.2012.073080
Magro, L., Arzenton, E., Leone, R., Stano, M. G., Vezzaro, M., Rudolph, A., et al. (2021). Identifying and characterizing serious adverse drug reactions associated with drug-drug interactions in a spontaneous reporting database. Front. Pharmacol. 11, 622862. doi:10.3389/fphar.2020.622862
Mar, P. L., Horbal, P., Chung, M. K., Dukes, J. W., Ezekowitz, M., Lakkireddy, D., et al. (2022). Drug interactions affecting antiarrhythmic drug use. Circ. Arrhythm. Electrophysiol. 15 (5), e007955. doi:10.1161/CIRCEP.121.007955
Marengoni, A., Pasina, L., Concoreggi, C., Martini, G., Brognoli, F., Nobili, A., et al. (2014). Understanding adverse drug reactions in older adults through drug-drug interactions. Eur. J. Intern Med. 25 (9), 843–846. doi:10.1016/j.ejim.2014.10.001
Martinelli, S., Maffei, R., Fiorcari, S., Quadrelli, C., Zucchini, P., Benatti, S., et al. (2018). Idelalisib impairs T-cell-mediated immunity in chronic lymphocytic leukemia. Haematologica 103 (12), e598–e601. doi:10.3324/haematol.2017.187070
McMullen, J. R., Boey, E. J. H., Ooi, J. Y. Y., Seymour, J. F., Keating, M. J., and Tam, C. S. (2014). Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 124 (25), 3829–3830. doi:10.1182/blood-2014-10-604272
Mendez-Ruiz, A., Lossos, I. S., and Cohen, M. G. (2023). Bleeding risk with antiplatelets and bruton’s tyrosine kinase inhibitors in patients with percutaneous coronary intervention. J. Soc. Cardiovasc Angiogr. Interv. 2 (3), 100608. doi:10.1016/j.jscai.2023.100608
Mulder, T. A., Peña-Pérez, L., Berglöf, A., Meinke, S., Estupiñán, H. Y., Heimersson, K., et al. (2021). Ibrutinib has time-dependent on- and off-target effects on plasma biomarkers and immune cells in chronic lymphocytic leukemia. Hemasphere 5 (5), e564. doi:10.1097/HS9.0000000000000564
Nair, K. S., and Cheson, B. (2016). The role of idelalisib in the treatment of relapsed and refractory chronic lymphocytic leukemia. Ther. Adv. Hematol. 7 (2), 69–84. doi:10.1177/2040620715625966
Naseraldeen, N., Michelis, R., Barhoum, M., Chezar, J., Tadmor, T., Aviv, A., et al. (2021). The role of alpha 2 macroglobulin in IgG-aggregation and chronic activation of the complement system in patients with chronic lymphocytic leukemia. Front. Immunol. 11, 603569. doi:10.3389/fimmu.2020.603569
Nicolson, P. L. R., Hughes, C. E., Watson, S., Nock, S. H., Hardy, A. T., Watson, C. N., et al. (2018). Inhibition of Btk by Btk-specific concentrations of ibrutinib and acalabrutinib delays but does not block platelet aggregation mediated by glycoprotein VI. Haematologica 103 (12), 2097–2108. doi:10.3324/haematol.2018.193391
Nicolussi, S., Drewe, J., Butterweck, V., and Meyer zu Schwabedissen, H. E. (2020). Clinical relevance of St. John’s wort drug interactions revisited. Br. J. Pharmacol. 177 (6), 1212–1226. doi:10.1111/bph.14936
Orsini, E., Guarini, A., Chiaretti, S., Mauro, F. R., and Foa, R. (2003). The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 63 (15), 4497–4506.
Ou, Y. C., Tang, Z., Novotny, W., Tawashi, M., Li, T. K., Coleman, H. A., et al. (2021). Evaluation of drug interaction potential of zanubrutinib with cocktail probes representative of CYP3A4, CYP2C9, CYP2C19, P-gp and BCRP. Br. J. Clin. Pharmacol. 87 (7), 2926–2936. doi:10.1111/bcp.14707
Paydas, S. (2019). Management of adverse effects/toxicity of ibrutinib. Crit. Rev. Oncol. Hematol. 136, 56–63. doi:10.1016/j.critrevonc.2019.02.001
Pepin, X. J. H., Sanderson, N. J., Blanazs, A., Grover, S., Ingallinera, T. G., and Mann, J. C. (2019). Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part I. Mechanistic modelling of drug product dissolution to derive a P-PSD for PBPK model input. Eur. J. Pharm. Biopharm. 142, 421–434. doi:10.1016/j.ejpb.2019.07.014
Pharmacist’s application to Practice: pirtobrutinib | HOPA. 2023 Available at: https://www.hoparx.org/latest-news/pharmacists-application-to-practice-pirtobrutinib/
Prosty, C., Katergi, K., Nguyen, A., Luo, O. D., Sorin, M., Cherniak, V., et al. (2023). Risk of infectious adverse events of venetoclax therapy for hematologic malignancies: a systematic review and meta-analysis of RCTs. Blood Adv. 8 (4), 857–866. doi:10.1182/bloodadvances.2023011964
Quartermaine, C., Ghazi, S. M., Yasin, A., Awan, F. T., Fradley, M., Wiczer, T., et al. (2023). Cardiovascular toxicities of BTK inhibitors in chronic lymphocytic leukemia: JACC: CardioOncology state-of-the-art review. JACC CardioOncology 5 (5), 570–590. doi:10.1016/j.jaccao.2023.09.002
Ramanathan, S., Jin, F., Sharma, S., and Kearney, B. P. (2016). Clinical pharmacokinetic and pharmacodynamic profile of idelalisib. Clin. Pharmacokinet. 55 (1), 33–45. doi:10.1007/s40262-015-0304-0
Rivera, D., and Ferrajoli, A. (2022). Managing the risk of infection in chronic lymphocytic leukemia in the era of new therapies. Curr. Oncol. Rep. 24 (8), 1003–1014. doi:10.1007/s11912-022-01261-9
Roberts, A. W., Davids, M. S., Pagel, J. M., Kahl, B. S., Puvvada, S. D., Gerecitano, J. F., et al. (2016). Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374 (4), 311–322. doi:10.1056/NEJMoa1513257
Rohrbacher, L., Brauchle, B., Ogrinc Wagner, A., von Bergwelt-Baildon, M., Bücklein, V. L., and Subklewe, M. (2021). The PI3K∂-Selective inhibitor idelalisib induces T- and NK-cell dysfunction independently of B-cell malignancy-associated immunosuppression. Front. Immunol. 12, 608625. doi:10.3389/fimmu.2021.608625
Ruchlemer, R., Ben-Ami, R., Bar-Meir, M., Brown, J. R., Malphettes, M., Mous, R., et al. (2019). Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: an observational study. Mycoses 62 (12), 1140–1147. doi:10.1111/myc.13001
Rushworth, S. A., MacEwan, D. J., and Bowles, K. M. (2013). Ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369 (13), 1277–1278. doi:10.1056/NEJMc1309710
Scheers, E., Leclercq, L., de Jong, J., Bode, N., Bockx, M., Laenen, A., et al. (2015). Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab. Dispos. 43 (2), 289–297. doi:10.1124/dmd.114.060061
Seymour, J. F., Byrd, J. C., Ghia, P., Kater, A. P., Chanan-Khan, A., Furman, R. R., et al. (2023). Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood 142 (8), 687–699. doi:10.1182/blood.2022018818
Seymour, J. F., Kipps, T. J., Eichhorst, B. F., D’Rozario, J., Owen, C. J., Assouline, S., et al. (2022). Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 140 (8), 839–850. doi:10.1182/blood.2021015014
Sharma, S., Pepin, X., Burri, H., Zheng, L., Kuptsova-Clarkson, N., de Jong, A., et al. (2022). Bioequivalence and relative bioavailability studies to assess a new acalabrutinib formulation that enables coadministration with proton-pump inhibitors. Clin. Pharmacol. Drug Dev. 11 (11), 1294–1307. doi:10.1002/cpdd.1153
Sharman, J. P., Coutre, S. E., Furman, R. R., Cheson, B. D., Pagel, J. M., Hillmen, P., et al. (2019). Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 37 (16), 1391–1402. doi:10.1200/JCO.18.01460
Sharman, J. P., Egyed, M., Jurczak, W., Skarbnik, A., Pagel, J. M., Flinn, I. W., et al. (2020). Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 395 (10232), 1278–1291. doi:10.1016/S0140-6736(20)30262-2
Sharman, J. P., Egyed, M., Jurczak, W., Skarbnik, A., Pagel, J. M., Flinn, I. W., et al. (2022). Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 36 (4), 1171–1175. doi:10.1038/s41375-021-01485-x
Stilgenbauer, S., Eichhorst, B., Schetelig, J., Coutre, S., Seymour, J. F., Munir, T., et al. (2016). Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 17 (6), 768–778. doi:10.1016/S1470-2045(16)30019-5
Tam, C. S., Allan, J. N., Siddiqi, T., Kipps, T. J., Jacobs, R., Opat, S., et al. (2022a). Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood 139 (22), 3278–3289. doi:10.1182/blood.2021014488
Tam, C. S., Brown, J. R., Kahl, B. S., Ghia, P., Giannopoulos, K., Jurczak, W., et al. (2022b). Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 23 (8), 1031–1043. doi:10.1016/S1470-2045(22)00293-5
Tambaro, F. P., and Wierda, W. G. (2020). Tumour lysis syndrome in patients with chronic lymphocytic leukaemia treated with BCL-2 inhibitors: risk factors, prophylaxis, and treatment recommendations. Lancet Haematol. 7 (2), e168–e176. doi:10.1016/S2352-3026(19)30253-4
Tang, C. P. S., McMullen, J., and Tam, C. (2018). Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leukemia & Lymphoma 59 (7), 1554–1564. doi:10.1080/10428194.2017.1375110
Tey, A., Schwarer, J., Raffa, R., Shi, E., Paul, E., Opat, S., et al. (2023). High risk of infection in ‘real-world’ patients receiving ibrutinib, idelalisib or venetoclax for mature B-cell leukaemia/lymphoma. Eur. J. Haematol. 110 (5), 540–547. doi:10.1111/ejh.13928
Thompson, P. A., and Tam, C. S. (2023). Pirtobrutinib: a new hope for patients with BTK inhibitor–refractory lymphoproliferative disorders. Blood 141 (26), 3137–3142. doi:10.1182/blood.2023020240
Thorp, B. C., and Badoux, X. (2018). Atrial fibrillation as a complication of ibrutinib therapy: clinical features and challenges of management. Leukemia & Lymphoma 59 (2), 311–320. doi:10.1080/10428194.2017.1339874
Wierda, W. G., Brown, J., Abramson, J. S., Awan, F., Bilgrami, S. F., Bociek, G., et al. (2022). NCCN Guidelines® insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, version 3.2022. J. Natl. Compr. Cancer Netw. 20 (6), 622–634. doi:10.6004/jnccn.2022.0031
Xiao, L., Salem, J. E., Clauss, S., Hanley, A., Bapat, A., Hulsmans, M., et al. (2020). Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal src kinase. Circulation 142 (25), 2443–2455. doi:10.1161/CIRCULATIONAHA.120.049210
Yoo, S. G. K., Chung, G. S., Bahendeka, S. K., Sibai, A. M., Damasceno, A., Farzadfar, F., et al. (2024). Global prevalence of aspirin use for primary prevention of cardiovascular disease: a cross-sectional study of nationally representative, individual-level data. Glob. Heart 19 (1), 44. doi:10.5334/gh.1323
Keywords: chronic lymphocytic leukemia, Bruton tyrosine kinase inhibitors, venetoclax, drug-drug interactions, comorbidities
Citation: Kozarac S, Ivanovic J, Mitrovic M, Tomic Vujovic K, Arsenovic I, Suvajdzic-Vukovic N, Bogdanovic A, Vidovic A, Todorovic-Balint M, Bila J, Mitrovic M, Lekovic D, Djunic I, Virijevic M, Trivic A, Micic J and Antic D (2025) Managing novel therapies and concomitant medications in chronic lymphocytic leukemia: key challenges. Front. Pharmacol. 15:1517972. doi: 10.3389/fphar.2024.1517972
Received: 27 October 2024; Accepted: 10 December 2024;
Published: 03 January 2025.
Edited by:
Xuelin Zhou, Capital Medical University, ChinaReviewed by:
Jose Angel Hernandez Rivas, Hospital Universitario Infanta Leonor, SpainNi Yan, Massachusetts General Hospital and Harvard Medical School, United States
Benyam Muluneh, University of North Carolina at Chapel Hill, United States
Copyright © 2025 Kozarac, Ivanovic, Mitrovic, Tomic Vujovic, Arsenovic, Suvajdzic-Vukovic, Bogdanovic, Vidovic, Todorovic-Balint, Bila, Mitrovic, Lekovic, Djunic, Virijevic, Trivic, Micic and Antic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darko Antic, ZGFya28uYW50aWMxNTEwOTc2QGdtYWlsLmNvbQ==
 Sofija Kozarac
Sofija Kozarac Jelena Ivanovic1
Jelena Ivanovic1 Marko Mitrovic
Marko Mitrovic Isidora Arsenovic
Isidora Arsenovic Jelena Bila
Jelena Bila Mirjana Mitrovic
Mirjana Mitrovic Danijela Lekovic
Danijela Lekovic Darko Antic
Darko Antic