- 1Department of Biotechnology, Faculty of Chemical and Life Sciences, Abdul Wali Khan University Mardan, Mardan, Khyber Pakhtunkhwa, Pakistan
- 2Clinical Research Center, Shantou Central Hospital, Shantou, China
- 3Government Girls Degree College Rustam, Higher Education Department, Mardan, Khyber Pakhtunkhwa, Pakistan
- 4Department of Zoology, Faculty of Chemical and Life Sciences, Abdul Wali Khan University Mardan, Mardan, Khyber Pakhtunkhwa, Pakistan
Editorial on the Research Topic
The roles of pathogens in gut-related diseases and the strategies for inhibiting their growth
Introduction
Healthy life is inconceivable without balanced microbial communities in the gut, known as the gut microbiome (GM), which play a critical role in digestion, metabolism, immune function, and overall health (Zhang et al., 2023). These commensals are sensitive to both internal and external factors that cause dysbiosis—a condition in which the diversity and composition of GM are disturbed. This imbalance negatively affects the complex relationship between the host and its microbiome (Wu et al.).
During dysbiosis, opportunistic pathogens dominate the gut ecosystem, individually or collectively, triggering serious health problems such as gastrointestinal infections, ulcers, inflammation, cancer, diarrhea, obesity, and other diseases (Wu et al.; Alam et al.). Factors that induce dysbiosis in GM include environment, imbalanced diet, chronic diseases, infection, and medication (Figure 1).
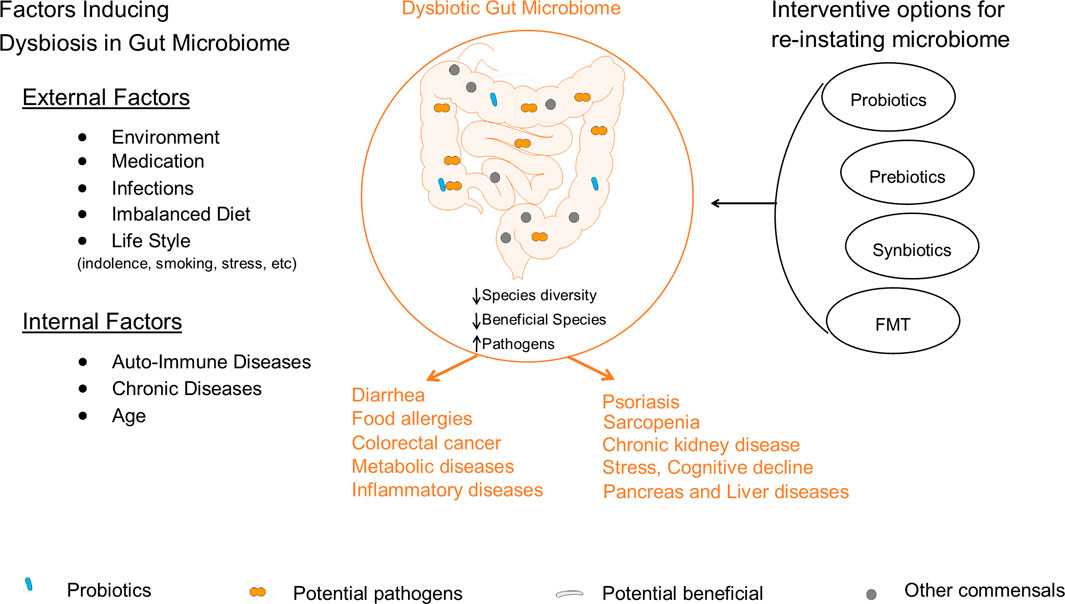
Figure 1. Illustration of internal and external factors that induce dysbiosis in gut microbiome and the associated consequences on health. These factors induce dysbiosis in GM by lowering the abundance of beneficial bacteria and elevating the growth of potential pathogens. The disturbed GM composition initiates/participates in the development of various diseases such as diarrhea, food allergies, cancer, metabolic diseases, stress, sarcopenia and other diseases. So far, several strategies are developed for reinstating the balance state of GM that include probiotic, prebiotics, synbiotics, and FMT interventions.
Among the medicinal treatment, antimicrobial agents induce drastic dysbiosis in the GM that trigger the emergence of opportunistic pathogens. There is growing evidence that has reported opportunistic infections supported by gut-dwelling bacteria that are commonly considered beneficial. One such bacterium is Bacteroides fragilis (normally a harmless gut inhabitant) that proliferates and cause infections after the antimicrobial therapies. Certain strains of this bacterium are linked to peritonitis and intra-abdominal abscesses (Jasemi et al., 2021). Similarly, the overgrowth of Clostridium difficile following antibiotic treatment can lead to antibiotic-associated diarrhea and, and in severe cases, can cause pseudomembranous colitis (López-Ureña et al., 2016).
The overabundance of opportunistic pathogens further worsens GM dysbiosis and cause serious complications that reach beyond the gastrointestinal tract, impacting various parts of the body. For example, Streptococcus gallolyticus, a gut-resident commensal, can breach a compromised intestinal barrier, enter the bloodstream, and cause infective endocarditis in individuals with damaged heart valves (Lourenc et al., 2010). Additionally, an increased prevalence of Prevotella species, typically gut commensals, has been associated with the onset of rheumatoid arthritis (Abdelsalam et al., 2023). Likewise, Helicobacter pylori, which infects more than half of the global population and, if left untreated, can lead to chronic gastritis, cap polyposis (Lu et al.), and peptic ulcer disease (Cheng et al.).
Realizing the importance of this growing enigma, we believed that the role of pathogens and opportunistic microbes in the onset, progression, and control of various diseases demand comprehensive attention. To gain deeper insight into the mechanisms underlying the pathogen-induced dysbiosis and its effects on host’ health, we initiated a Research Topic to provide a collaborative platform for researchers at the forefront of this field. In response to our call for submissions, we received 25 articles, of which only nine met publication standards. In the following section of this editorial, we provide a comprehensive summary of these manuscripts, focusing on the role of pathogens in gut microbiota dysbiosis, their impact on the host, and potential strategies for pathogen control. This section also highlights the current state of knowledge and identifies key areas for future research.
The impact of dysbiosis extends beyond the gastrointestinal tract
Numerous studies have demonstrated that dysbiotic microbial communities disrupt the gut barrier, lead to immune imbalances, and contribute to the development of chronic metabolic diseases. Even the intake of an imbalanced diet can have profound effects on the gut microbiome. In a recent study, Lu et al. fed rats an adenine-rich diet, which resulted in reduced diversity and compositional variation of GM compared to control rats. Pathophysiological analysis of the test group revealed calcification in the kidneys and thoracic aorta. Notably, vascular calcification is a recognized risk factor for cardiovascular disease, particularly in patients with chronic kidney disease (CKD). Furthermore, an increase level of Acinetobacter was noticed in the blood of the test group which was positively associated with increase in calcification factors (such as BSP, FGF-23, and SOST), lipopolysaccharide, phosphorous, and genes involve in the mineral absorption pathway. The authors suggest that elevated level of Acinetobacter in CKD holds significant potential as both a diagnostic marker and a therapeutic target. In addition, a cluster of five bacterial species has already been proposed as non-invasive diagnostic markers for CKD (Lu et al.).
Similarly, during nonalcoholic steatohepatitis (a severe form of nonalcoholic fatty liver disease), dysbiotic gut microbiota compromise the intestinal barrier, leading to increased translocation of toxic metabolites into the circulatory system and triggering liver inflammation (Huang et al.; Zhu et al., 2022). Bacteria that have been found abundant in the gut of non-alcoholic liver diseases include Proteobacteria, Enterobacteria, Escherichia. Whereas, bacterial metabolites that participate in the development of non-alcoholic liver disease include amino acids, bile acids, choline and butyrate (Alam et al.).
Fixing dysbiosis is a new target for treating disorders
Fixing disturbed GM composition has emerged as a new strategy for treatment of diseases. Interventions focused at reinstating the GM—such as the use of probiotics, prebiotics, dietary modifications, fecal microbiota transplants, and microbial-targeted therapies—have exhibited potential beneficial role in lowering the disease symptoms, improving immune responses, and uplifting the overall health.
In a recent study, Tang et al. demonstrated that balancing dysbiotic GM during psoriasis, a skin condition which is characterized by inflammation, could fix systemic inflammation and alleviate psoriasis symptoms. They successfully treated psoriasis mouse model with Shenling Baizhu Powder and concluded that fixing GM could impact lipid metabolism through reducing inflammatory mediators that conferring therapeutic abilities during psoriasis management.
Similarly, Zhang et al. fixed dysbiosis through aconite, a form of anti-diarrhea Chinese medicine. Aconite promoted the growth of bacteria belonging to Clostridia, Lachnospiraceae, Muribaculaceae, Prevotellaceae, and Ruminococcus whereas, inhibited the growth of Escherichia-Shigella and Parasutterella. Another study has reported disturbed microbiome composition during sarcopenia, a state of pathological decline of muscle mass and strength—associated with aging. They reported 29 genera with altered abundance in sarcopenia and proposed that Blautia, Lachnospiraceae, and Subdoligranulum could be developed as diagnostic markers for sarcopenia (Zhou et al.).
Another interventive strategy to restore GM composition and restrict potential pathogen is the use of herbal products (Dong et al., 2024). In a recent study, Litsea cubeba essential oil was found to positively affect gut microbial communities, leading to improved digestion, enhanced growth performance, increased antioxidant levels, and more efficient nutrient absorption (Cheng et al.). Similarly, Artemisia argyi essential oil has demonstrated weight management effects, which may be partly explained by its ability to modulate gut microbiota composition (Wang et al., 2022). Conversely, gut bacteria also play a role in improving the absorption and effectiveness of herbal products. A recent study utilized a biotransformation-integrated network pharmacology approach to explore how gut microbiota enhances the efficacy of Astragaloside IV in treating intracerebral hemorrhage (ICH). The researchers found that GM and liver metabolism converted Astragaloside IV into metabolites with improved bioavailability and better permeability across the blood-brain barrier, thus enhancing its therapeutic potential (Hu et al., 2023).
As medicine moves toward more personalized approaches, profiling an individual’s gut microbiome before administering herbal products becomes increasingly important. For instance, a recent study showed that podophyllotoxin, a naturally occurring aryltetralin lignan, induces neurotoxicity via the microbiota-gut-brain axis. Podophyllotoxin exposure altered the composition of gut microbiota, increasing Escherichia-Shigella populations while reducing Lactobacillus, which elevated pro-inflammatory factors and disrupted kynurenine metabolism in the hippocampus. These changes may contribute to cognitive dysfunction and neurotoxicity (Duan et al., 2024).
In conclusion, herbal products hold greater abilities for reinstating gut microbiome balance, which in turn can positively affect host health and alleviate disease symptoms. Herbal compounds not only stop pathogenic growth but also improve microbial diversity, improving functions such as digestion, nutrient absorption, antioxidant levels, and weight management.
Conclusion
GM dysbiosis has been linked to the development of various illnesses that results mainly from the overgrowth of opportunistic pathogens, which modulate mucosal homeostasis and affect distant organs such as the kidneys, heart, liver, and pancreas. Various therapeutic strategies are being developed to restore GM diversity and composition for alleviating disease symptoms and improving overall health. Among these strategies, prebiotics and probiotics target the promotion of beneficial bacteria and improve resilience against pathogenic colonization. These strategies fix dysbiotic GM which consequently treat systemic diseases that are associated with dysbiosis, thus creating the possibility for a novel field in preventative and therapeutic medicine.
Author contributions
IK: Conceptualization, Data curation, Investigation, Supervision, Validation, Writing–original draft, Writing–review and editing. HG: Conceptualization, Data curation, Methodology, Writing–review and editing. SK: Data curation, Formal Analysis, Methodology, Writing–review and editing. AA: Conceptualization, Writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelsalam, N. A., Hegazy, S. M., and Aziz, R. K. (2023). The curious case of Prevotella copri. Gut Microbes 15, 2249152. doi:10.1080/19490976.2023.2249152
Dong, X., Deng, P., Wang, X., Peng, C., and Peng, L., (2024). Structural characteristics and immunomodulatory effects of polysaccharides extracted from plant seeds: a review. Trends Food Sci. Technol. 153, 104747. doi:10.1016/j.tifs.2024.104747
Duan, J., Sun, J., Jiang, T., Ma, X., Li, X., Wang, Y., et al. (2024). Podophyllotoxin-mediated neurotoxicity via the microbiota-gut-brain axis in SD rats based on the toxicological evidence chain (TEC) concept. Sci. Total Environ. 907, 168106. doi:10.1016/j.scitotenv.2023.168106
Hu, E., Li, Z., Li, T., Yang, X., Ding, R., Jiang, H., et al. (2023). A novel microbial and hepatic biotransformation-integrated network pharmacology strategy explores the therapeutic mechanisms of bioactive herbal products in neurological diseases: the effects of Astragaloside IV on intracerebral hemorrhage as an example. Chin. Med. 18, 40. doi:10.1186/s13020-023-00745-5
Jasemi, S., Emaneini, M., Ahmadinejad, Z., Fazeli, M. S., Sechi, L. A., Sadeghpour Heravi, F., et al. (2021). Antibiotic resistance pattern of Bacteroides fragilis isolated from clinical and colorectal specimens. Ann. Clin. Microbiol. Antimicrob. 20, 27. doi:10.1186/s12941-021-00435-w
López-Ureña, D., Quesada-Gómez, C., Rodríguez, C., and Chaves-Olarte, E. (2016). in Role of Clostridium difficile toxins in antibiotic-associated diarrhea and pseudomembranous colitis BT - microbial toxins. Editors P. Gopalakrishnakone, B. Stiles, A. Alape-Girón, J. D. Dubreuil, and M. Mandal (Netherlands, Dordrecht: Springer), 1–18.
Lourenc, S., Caeiro, F., Ramos, A., Pacheco, M., and Malhado, J. A. (2010). Aortic and tricuspid endocarditis due to Streptococcus gallolyticus in an immunocompetent patient with a normal heart. J. Cardiol. Cases 1, e95–e97.
Wang, K., Ma, J., Li, Y., Han, Q., Yin, Z., Zhou, M., et al. (2022). Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front. Nutr. 9, 1024722. doi:10.3389/fnut.2022.1024722
Zhang, H., Wang, Z., Wang, G., Song, X., Qian, Y., Liao, Z., et al. (2023). Understanding the connection between gut homeostasis and psychological stress. J. Nutr. 153, 924–939. doi:10.1016/j.tjnut.2023.01.026
Keywords: gut microbiota, microbiome, bacteria, pathogen, prebiotics, dysbiosis, probiotics
Citation: Khan I, Gouxin H, Khan S and Ali A (2024) Editorial: The roles of pathogens in gut-related diseases and the strategies for inhibiting their growth. Front. Pharmacol. 15:1513694. doi: 10.3389/fphar.2024.1513694
Received: 18 October 2024; Accepted: 25 November 2024;
Published: 04 December 2024.
Edited and reviewed by:
Angelo A. Izzo, University of Naples Federico II, ItalyCopyright © 2024 Khan, Gouxin, Khan and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imran Khan, aW1yYW5raGFuMzFAYXdrdW0uZWR1LnBr, cnVzdGFta2hhbjMxQHlhaG9vLmNvbQ==
 Imran Khan
Imran Khan Huang Gouxin
Huang Gouxin Shafia Khan
Shafia Khan Abid Ali
Abid Ali