- 1State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy and Tianjin Key Laboratory of Molecular Drug Research, Nankai University, Tianjin, China
- 2The National Institutes of Pharmaceutical R&D Co., Ltd., Beijing, China
- 3Tianjin Key Laboratory of Molecular Drug Research, Tianjin International Joint Academy of Biomedicine, Tianjin, China
Introduction: Deglycosylated azithromycin (Deg-AZM), a new transgelin agonist with positive therapeutic effects on slow transit constipation, has been approved for clinical trials in 2024. This work investigated the drug metabolism and transport of Deg-AZM to provide research data for further development of Deg-AZM.
Methods: A combination of UPLC-QTOF-MS was used to obtain metabolite spectra of Deg-AZM in plasma, urine, feces and bile. Caco-2 cells was used to investigate the permeability of Deg-AZM and whether it is a potential substrate of the efflux transporter P-glycoprotein. Human liver microsome phenotyping assays with chemical inhibition and recombinant CYPs phenotyping assays were used to investigate the CYP450 enzyme phenotype involved in Deg-AZM metabolism in vitro. A HLM inhibition reaction system was established to evaluate the inhibitory effect of Deg-AZM on CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. The mRNA expression of human primary hepatocytes incubated with Deg-AZM or not was evaluate the induction of Deg-AZM on CYP1A2, CYP2B6, and CYP3A4.
Results: 44 metabolites of Deg-AZM were identified in rat urine, feces, bile, and plasma, the metabolic pathways included demethylation, monohydroxylation, dihydroxylation, dehydroxidation, hydroreduction, hydrolysis, methylation, glucuronidation and the combination of different metabolic pathways. Deg-AZM was a low permeability drug in the intestine and a potential substrate of the efflux transporter P-glycoprotein. CYP3A4 was the major CYP isoform responsible for Deg-AZM metabolism. Deg-AZM showed moderate inhibition with CYP2B6 and CYP2D6. Data in three batches of human primary hepatocytes disclosed induction potential of Deg-AZM on CYP2B6 and CYP3A4.
Conclusion: The in vivo metabolic pathway of Deg-AZM and in vitro possibility of drug interaction for Deg-AZM with CYP enzymes and drug transporter were fully investigated. It was suggested that dose adjustments may be warranted depending on the potency of the corresponding modulators in clinical.
1 Introduction
Chronic constipation manifests as prolonged and repeated obstruction of bowel movements, prolonged defecation time, or difficulty in bowel movement (Costilla and Foxx-Orenstein, 2014; Bharucha and Wald, 2019). It has become a common disease and seriously affects the normal life of patients (Oh et al., 2020). Large statistical analysis showed that the global prevalence of chronic constipation is approximately 15% (Bharucha and Lacy, 2020). A survey in China showed that the incidence rate of chronic constipation is 7.3%–20.39% (Zhi et al., 2021). The current treatment methods for chronic constipation are mainly divided into basic treatment and medication treatment (Rao et al., 2016; Włodarczyk et al., 2021). Basic treatment includes lifestyle adjustment and cognitive therapy (Nunan et al., 2022). The current clinical treatment drugs for chronic constipation include laxatives, volumetric laxatives, osmotic laxatives, irritants, prokinetic laxatives, secretagogues, probiotics/prebiotics, enemas, suppositories, and so on (Tack et al., 2011; Müller-Lissner et al., 2013; El-Salhy et al., 2014; Alsalimy et al., 2018). However, the current treatment methods cannot meet treatment expectations generally, it is still necessary to develop new drugs to treat chronic constipation with high efficiency and low toxicity.
Deglycosylated azithromycin (Deg-AZM) is a new small molecule transgelin agonist independently developed by Nankai University, which has been approved for clinical trials for the treatment of chronic constipation in 2024. Transgelin is the actin-binding protein in the Calponin family, with a relative molecular weight of 22 kDa, mainly distributed in smooth muscle cells (Lees-Miller et al., 1987; Jo et al., 2018; Yin et al., 2018). Deg-AZM can stimulate transgelin on intestinal smooth muscle cells, promote the aggregation of G-actin to F-actin, increase the formation of stress fiber bundles in intestinal smooth muscle cells, promote intestinal peristalsis (Zhong et al., 2020). Deg-AZM has a clear mechanism of action, significant efficacy and high safety. Notably, there are no similar drugs targeting transgelin to treat constipation on the clinical. Deg-AZM is expected to provide a new choice for treating chronic constipation.
More and more patients are using two or more drugs simultaneously, which greatly increases the risk of the potential drug-drug interactions (DDIs) and poses increasing challenges to risk management (Kalyanasundaram et al., 2011; van Leeuwen et al., 2014). Constipation increases with age and often coexists with many diseases, include bowel cancer, cardiovascular diseases, neurological disorders (such as Parkinson’s disease, spinal cord injury, stroke, or multiple sclerosis), and so on (Winge et al., 2003; Ishiyama et al., 2019; De Giorgio et al., 2021). Constipation treatment drugs are often taken together with other drugs and cause DDIs. Exploring DDIs of the new drug candidates is of great significance for the safe use in clinical practice. The major mechanisms of DDIs involve metabolic enzymes and transporters. Therefore, the metabolism and transport-mediated DDIs potential of Deg-AZM need to be thoroughly investigated during development.
The aim of the present study is to investigate the potential mechanisms of metabolic enzymes and transporter associated with Deg-AZM disposition and DDIs. A combination of UPLC-QTOF-MS was used to obtain metabolite spectra of plasma, urine, feces and bile, the structure of the metabolites was inferred, and the proportion of each metabolite of Deg-AZM in the biological sample was calculated according to the peak area. Caco-2 cells was used to investigate the permeability of Deg-AZM and whether it is a potential substrate of the efflux transporter P-glycoprotein (P-gp). Investigation of CYP450 enzyme phenotype involved in Deg-AZM metabolism used human liver microsome (HLM) phenotyping assays with chemical inhibition and Recombinant CYPs phenotyping assays. Establish a HLM inhibition reaction system, measure the concentration of metabolites of seven tool drugs in the system using LC-MS/MS method, IC50 was calculated and used to evaluate the inhibitory effect of Deg-AZM on CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. The mRNA expression of human primary hepatocytes incubated with Deg-AZM or not was evaluate the induction of Deg-AZM on CYP1A2, CYP2B6, and CYP3A4. This is the first report investigate the potential metabolic enzymes and transporter associated with Deg-AZM.
2 Experimental
2.1 Chemicals and materials
Deg-AZM was provided by Nankai University (Tianjin, China). Quinidine was obtained from International Laboratories Corp., Ltd. (San Francisco, CA, USA). Rhodamine 123 was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). CMC-Na was provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). [3H] Propranolol and [14C] PEG4000 were provided by Perkin Elmer Co. Ltd. (Waltham, MA, USA). Roxithromycin (IS) was provided by the National Institutes for food and drug control (Beijing, China). All reference substances were 98% purity. Methanol, formic acid and acetonitrile with HPLC grade were provided by Thermo Fisher Scientific India Pvt. Ltd. (Waltham, MA, USA). HPLC grade Ammonium acetate was provided by Tianjin Guangfu Technology Development Co., Ltd. (Tianjin, China). Deionized water used throughout the study was provided by Wahaha Corporation (Hangzhou, China).
2.2 Metabolism study in vivo
2.2.1 Biological sample collection
The plasma samples at 0.167 h, 0.5 h, 3 h and 8 h after administration were obtained from the study of tissue distribution in rats (Gu et al., 2024). Urine, feces and bile samples at 0–24 h after administration were obtained from the excretion study of a single gavage administration of 25 mg/kg Deg-AZM in rats (Gu et al., 2024).
2.2.2 Biological sample preparation
Plasma samples were precipitated with methanol at a ratio of 1:3. Urine samples were diluted with 50% methanol at a ratio of 1:80. The feces was thoroughly ground and sonicated with a 50% methanol at a ratio of 1 g/10 mL to obtain the homogenate, then the homogenate were precipitated with methanol at a ratio of 1:1. Bile samples were precipitated with 50% methanol at a ratio of 1:1. The above obtained samples were vortexed for 1 min and centrifugated at 4°C, 12,000 rpm for 10 min. Finally, the upper layer was analyzed using the UPLC-QTOF-MS system.
2.2.3 UPLC-QTOF-MS conditions
The metabolites of Deg-AZM in the plasma, urine, feces and bile samples were identified by UPLC-QTOF-MS system. Liquid chromatography was performed on an ACQUITY UPLCTMI-Class UPLC unit with a Waters ACQUITY UPLC HSS T3 (100 × 2.1 mm, 1.8 µm). A gradient program was employed with the mobile phase combining solvent A (0.02% formic acid in 5% acetonitrile) and solvent B (acetonitrile) as follows: 100% A (0–0.5 min), 100%–85% A (0.5–7 min), 85%–5% A (7–9 min), 5% A (9–12 min), 5%–100% A (12–15 min). The flow rate was 0.40 mL/min. The column temperature was maintained at 40°C.
A Waters Q-TOF Xevo G2-XS Spectrometer equipped with ESI source under positive ion mode was utilized to conduct mass spectrometry detection, for which the MSE mode was set at the mass range of 100–1,000 Da. The cone voltage was 40 V. The desolvation temperature was set at 500°C and source temperature was 100°C. The desolvation gas flow rate was 800 L/h and the cone gas flow rate was 50 L/h. In MSE centroid mode, the MS data were acquired with the low energy function in the trap collision energy (6 eV), and the tandem mass data were acquired with the high energy function in the ramp trap collision energy (35–45 eV).
2.3 P-gp transportation and inhibition
2.3.1 Caco-2 cell culture
The human colon adenocarcinoma Caco-2 cell line was provided by Institute of Basic Medicine, Chinese Academy of Medical Sciences (Beijing, China). Caco-2 cells were cultured in a high sugar DMEM medium containing 10% (v/v) fetal bovine serum at 37°C and 5% CO2 incubator. Caco-2 cells (2.5 ×105/mL) was inoculated into Transwell polycarbonate membrane 12 well plate. The culture medium was changed every 2 days for the first 14 days, and then every day. After 21 consecutive days, fully differentiated cell monolayers were obtained for drug transport assays.
2.3.2 Validation of Caco-2 cell model
Transepithelial electrical resistance (TEER) was used to indicate monolayer integrity. The electrode of the Millcell ERS-2 voltammeter was placed in HBSS solution for 20 min to pre-balance. At the same time, the culture medium was removed from the Transwell culture plate, 0.5 mL of pre-heated HBSS solution was added into the Apical (AP) of transwell, 1.5 mL of pre-heated HBSS solution was added into the Basolateral (BL) of transwell. After that, the transwell was balanced at 37°C for 20 min and then replaced the HBSS solution with the pre-heated HBSS solution, the TEER value was measured finally. Caco-2 cell monolayers with TEER >350 Ω cm2 which means that the cells have formed a tight monolayer, were used for the experiment. The TEER value was calculated according to the following formula:
where TEERT represented the TEER value (Ω) of the measured Transwell chamber with Caco-2 cells, TEERC was the TEER value (Ω) of the transwell chamber in the control group, and A was the membrane area of the transwell cavity (1.12 cm2 in this experiment).
In order to evaluate the usability of the Transwell model research, [3H] Propranolol (0.5 μCi/mL) and [14C] PEG4000 (1 μCi/mL) served as high-permeability and impermeability controls to verify the uptake direction on Caco-2 cell monolayer, respectively. Rhodamine 123 was used as a positive control for efflux mechanism in bidirectional transport assay on Caco-2 cell monolayer.
2.3.3 Drug transport assay
The transmembrane permeability of Deg-AZM (2, 10 or 50 µM) and the potential to inhibit P-gp by Deg-AZM (2, 10 or 50 µM) were evaluated using Caco-2 cell monolayer both apical-to-basolateral (A-B) and basolateral-to-apical (B-A) directions at 37°C for 1 h, the experiments were conducted using quinidine (50 μM) as the inhibitor for P-gp. At the end of incubation, receiver and donor samples were both diluted by assay buffer and analyzed by LC-MS/MS. Bidirectional apparent permeability (Papp) of A-B and B-A and the efflux ratios (Papp[B-A]/Papp[A-B]) were then calculated according to the following formula:
where V represented the volume of the solution in the receiving chamber (0.5 cm3 at the AP transwell chamber and 1.5 cm3 at the BL transwell chamber), dc was the concentration of the drug in the receiving chamber, dt was the transport time, A was the membrane area of the transwell cavity (1.12 cm2 at the Transwell membrane used in this experiment), and C0 was the initial concentration.
2.4 CYP phenotyping
2.4.1 HLM phenotyping assays
Deg-AZM (1 μM) was treated with pooled HLM (0.5 mg/mL) and NADPH (1 mM) for 90 min with or without seven specific inhibitors (10 µM furafylline for CYP1A2, 10 µM ticlopidine for CYP2B6, 5 µM montelukast for CYP2C8, 10 µM sulfaphenazole for CYP2C9, 10 µM ticlopidine for CYP2C19, 2.5 µM quinidine for CYP2D6, 1 µM ketoconazole for CYP3A). Isoform-selective probe substrates, 10 µM phenacetin for CYP1A2, 20 µM bupropion for CYP2B6, 2 µM amodiaquine for CYP2C8, 5 µM diclofenac for CYP2C9, 20 µM S-mephenytoin for CYP2C19, 5 µM bufuralol for CYP2D6, 2 µM midazolam for CYP3A4, were used as positive controls.
2.4.2 Recombinant CYPs phenotyping assays
Deg-AZM (1 μM) was treated with pooled NADPH (1 mM) and recombinant CYPs (rCYP1A2, rCYP2B6, rCYP2C8, rCYP2C9, rCYP2C19, rCYP2D6, and rCYP3A4, purchased from Research Institute for Liver Diseases Co. Ltd., Shanghai) at 25 pmol/mL for 90 min. The isoform-selective probe substrates served as positive controls (same as the positive controls in HLM phenotyping assays).
2.5 CYP inhibition
CYP inhibition potential of Deg-AZM was assessed in NADPH-fortified HLMs (0.5 mg/mL for CYP1A2, CYP2B6, CYP2C19 and CYP2D6 system, 0.035 mg/mL for CYP2C9 system, 0.05 mg/mL for CYP3A4 system, and 0.025 mg/mL for CYP2C8 system) using CYP probe substrates (same as the positive controls in HLM phenotyping assays). Deg-AZM in triplicates (0, 0.2, 0.6, 2.0, 6.0, 20, and 60 μM) was treated with each probe of CYP1A2 (20 min), CYP2B6 (20 min), CYP2C8 (10 min), CYP2C9 (60 min), CYP2C19 (45 min), CYP2D6 (30 min) and CYP3A4 (15 min). Probe substrate metabolites were analyzed by LC-MS/MS and the peak area ratios of analytes to internal standard (Indapamide) were calculated. The activity of the CYP450 enzyme subtype is directly proportional to the concentration of the corresponding probe substrate metabolites. The average of the measured values of each group was calculated, the control tube value was set as 100%, the residual probe substrate metabolites was calculated in the presence of positive inhibitors and Deg-AZM at series concentration. IC50 values were calculated by the dose-response model (nonlinear regression) in GraphPad Prism software.
2.6 CYP induction
Induction of Deg-AZM on CYP1A2, CYP2B6 and CYP3A4 in three batches of human hepatocytes (MWB, LHuf 17905B, HUB) was assessed by qPCR detection technology. Human hepatocytes were incubated individually with Deg-AZM (5, 25, or 100 μM), a negative control (erythromycin at 10 μM), a positive control (omeprazole at 50 μM for CYP1A2, phenobarbital at 1,000 μM for CYP2B6, or rifampin at 20 μM for CYP3A4) for 24 h, after that, the culture medium was replaced with new one, and hepatocytes was induced for 48 h. After culture and complete induction, total mRNA was extracted by TRNzol RNA Extraction Kit (purchased from Tiangen Biochemical Technology Co., Ltd., Beijing). The RNA was reverse transcribed into cDNA according to the instructions of the Transcriptor First Strand cDNA Synthesis Kit (purchased from Roche, Switzerland). Using reverse transcribed cDNA as a template, amplifying the internal reference genes of β-Actin of the sample and target genes of CYP1A2, CYP2B6, and CYP3A4 respectively to compare the expression differences.
3 Results and discussion
3.1 Metabolic pathway analysis in vivo
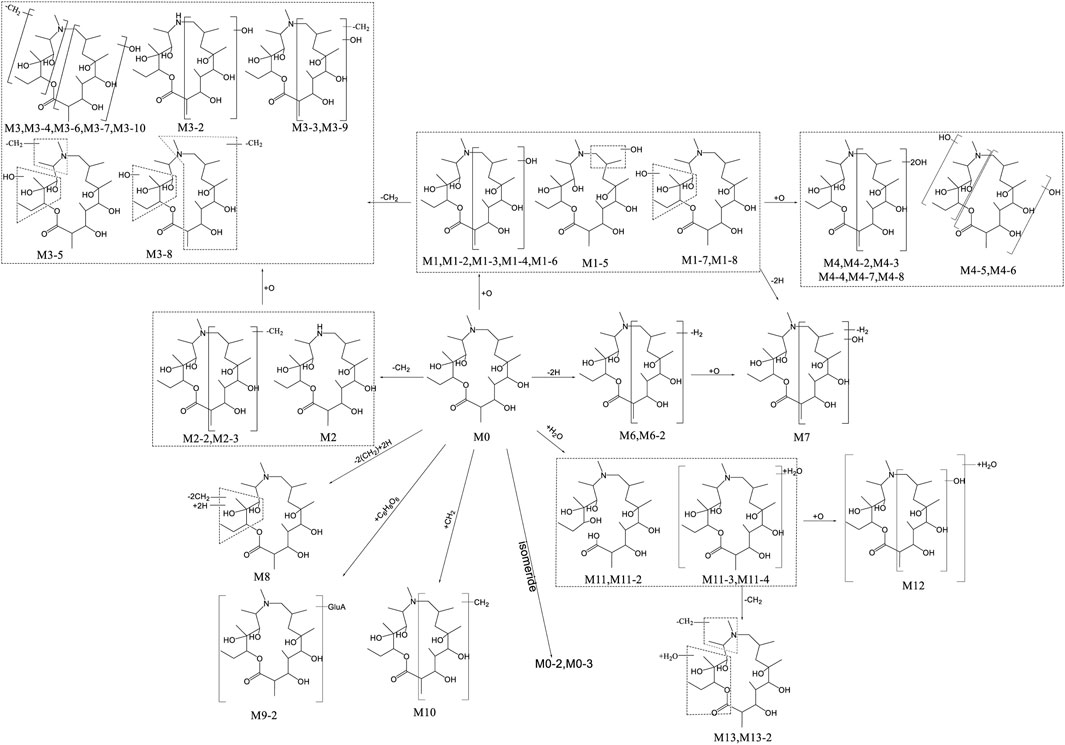
UPLC-QTOF-MS was applied to analyze blank and post administration urine, feces, bile, and plasma samples of rats. Combined with conventional phase I and II metabolic reaction patterns, a total of 44 metabolites were found in rat urine, feces, bile, and plasma, except for the original form (M0, Deg-AZM). Based on the precise molecular weight and elemental composition of each metabolite, and comparing the fragment ions of the original form and metabolites, the metabolite structure was analyzed. The summary of each metabolite information was shown in Table 1. The main metabolic pathways of Deg-AZM include demethylation, monohydroxylation, dihydroxylation, dehydroxidation, hydroreduction, hydrolysis, methylation, glucuronidation and the combination of different metabolic pathways, as shown in Figure 1.

Table 1. Metabolites identified in rat plasma and excretion samples after oral administration of Deg-AZM.
Our previous study (Gu et al., 2024) has shown that 8, 8, 9, 13 and 10 metabolites of Deg-AZM were identified from the liver microsomes of mice, rats, dogs, monkeys and humans respectively, the metabolic pathways in vitro include hydroxylation, methylation, and combinations of different metabolic pathways. It can be found that all in vitro metabolites can be detected in rats. Deg-AZM exhibited more abundant metabolic reactions in rats due to the more complex metabolic enzyme system in vivo compared to liver microsomes in vitro. The proportion of metabolites in vivo and in vitro played a crucial role in evaluating the efficacy and toxicity of Deg-AZM, and further research was needed to clarify this.
3.2 Exposure profiles of Deg-AZM and metabolites
The extraction ion chromatogram of plasma samples at 0.167 h, 0.5 h, 3 h and 8 h after administration of Deg-AZM in rats was shown in Figure 2. The extraction ion chromatogram of urine, feces and bile samples collected from 0 to 24 h after oral administration of Deg-AZM in rats was shown in Figure 3. The AUC0-t of Deg-AZM and its metabolites in plasma sample were calculated by trapezoidal rule based on the chromatographic peak area. The relative proportions of Deg-AZM and its metabolites in rat plasma were evaluated by AUC0-t, as shown in Figure 4. 21 metabolites were found in rat plasma, with M0 (Deg-AZM) and metabolite M2 (demethylated) being the main exposure forms in rat plasma. M0 and M2 accounted for 61.6% and 23.0% of the total related substances in rat plasma, respectively. The relative proportions of Deg-AZM and its metabolites in urine, feces and bile samples were evaluated by the chromatographic peak area, as shown in Figure 4. The detailed proportion of Deg-AZM and its metabolites could be found in Supplementary Table S1. M0 and 28 metabolites were found in the urine of rats. M0 (62.9%) and metabolite M2 (22.5%) were the main forms of urinary excretion of Deg-AZM in rats after oral administration. M0 and 42 metabolites were found in the feces of rats. Metabolite M1-2 (monohydroxylation, 18.1%) and metabolite M2 (16.6%) were the main forms of fecal excretion of Deg-AZM in rats after oral administration. M0 and 38 metabolites were found in the bile samples of rats. Metabolite M1-2 (monohydroxylation, 19.2%) and metabolite M2 (16.5%) were the main forms of biliary excretion of Deg-AZM in rats after oral administration.
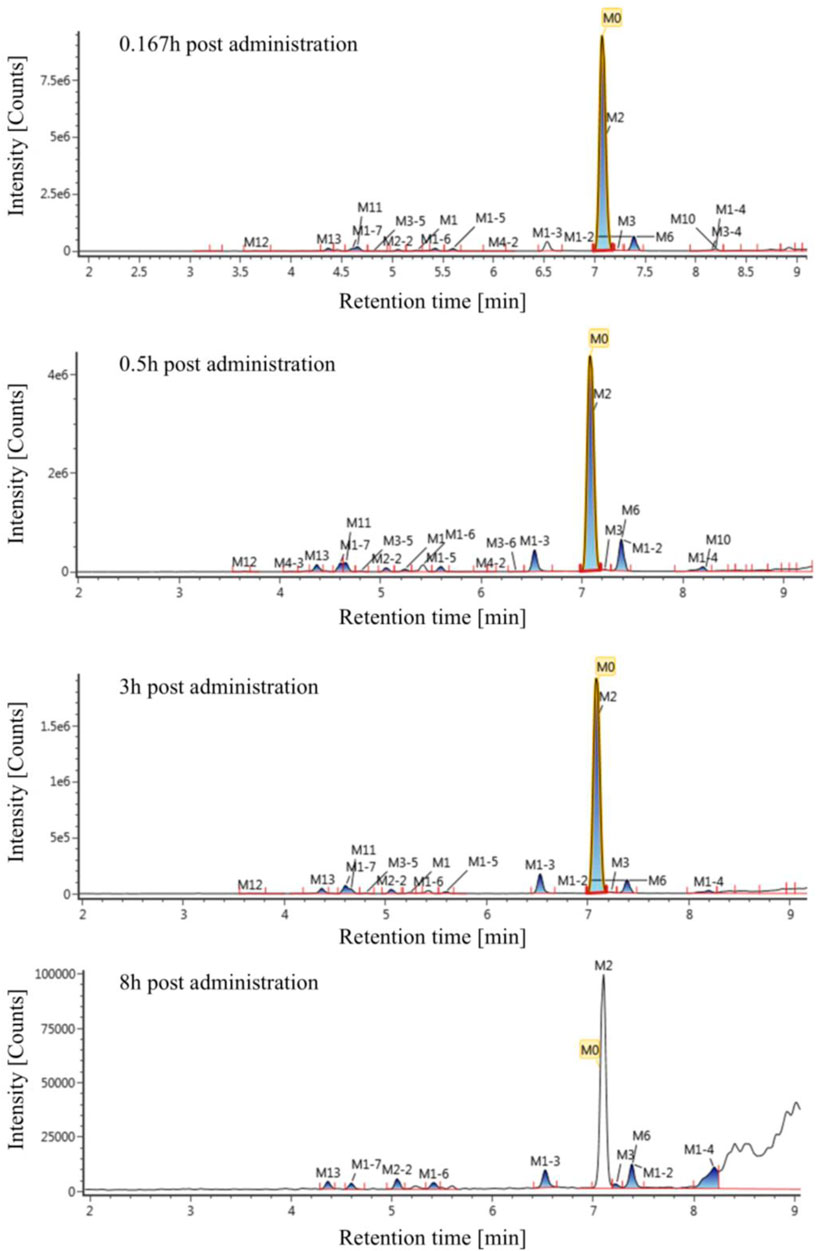
Figure 2. Extraction ion chromatogram of plasma samples at different time points after oral administration of Deg-AZM in rats.
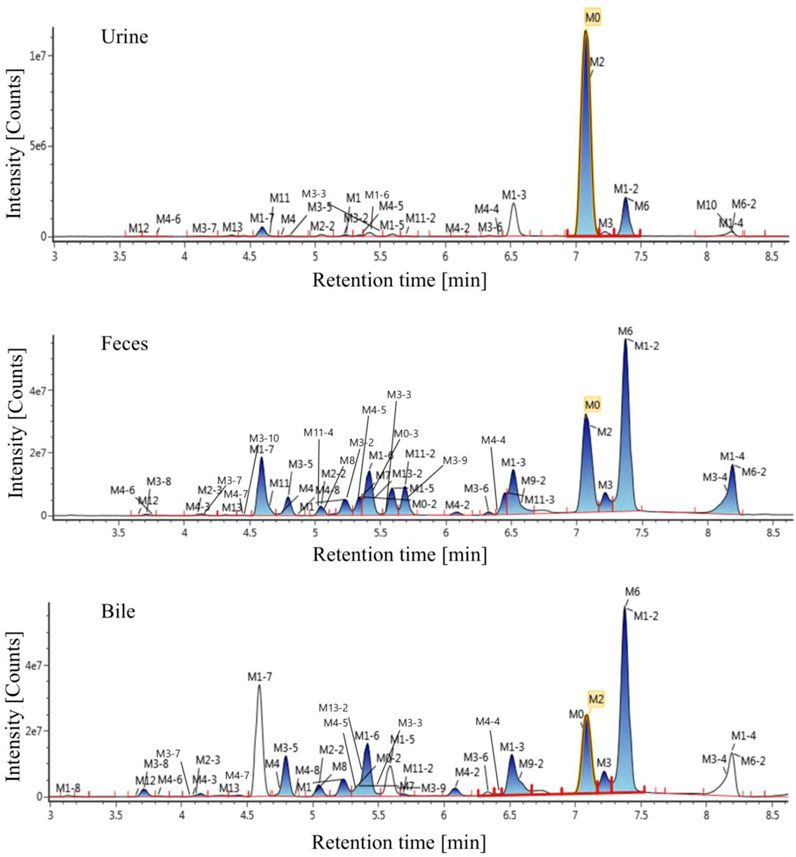
Figure 3. Extraction ion chromatogram of excretion samples after oral administration of Deg-AZM in rats.
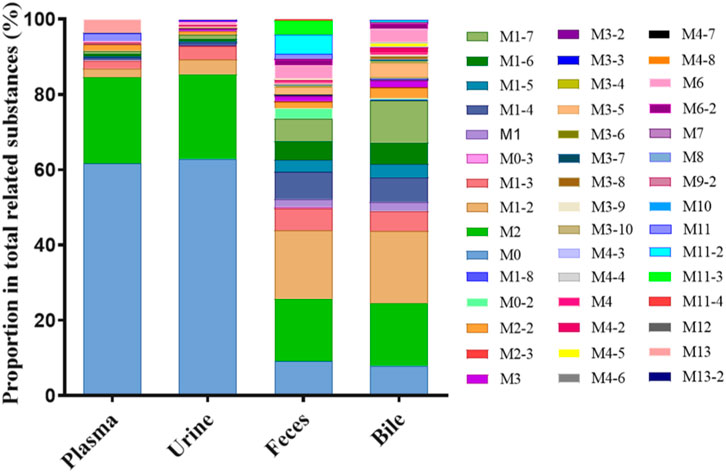
Figure 4. Comparison of exposure profiles of Deg-AZM and its metabolites in plasma, urine, feces, and bile of rats.
In our previous study (Gu et al., 2024), the excretion experiment in rats (0–96 h) showed that Deg-AZM was mainly excreted and through urine and widely metabolized in vivo. The types and proportions of metabolites in the excretion pathway were elucidated in this study. The excretion profile of Deg-AZM in rats (0–24 h) was extracted out showed that the amount of Deg-AZM excreted in its original form from urine and feces was 14.9% and 1.81% of the administered dose, respectively (Gu et al., 2024). Based on the peak area in this study, the total amount of metabolites in urine and feces of rats was 0.589 times and 9.96 times that of the original form of Deg-AZM, respectively. The total amount of Deg-AZM excreted in its original form and various metabolites in urine and feces was 23.7% and 19.8% of the administered dose, respectively, with a material balance coefficient of 43.5%. Deg-AZM was mainly excreted in the form of its original form M0 (cumulative excretion fraction of 14.9%) and metabolite M2 (cumulative excretion fraction of 5.33%) through the kidneys and urine, and in the form of metabolite M1-2 (cumulative excretion fraction of 4.29%) and M2 (cumulative excretion fraction of 3.93%) through the intestines and feces. This study applied high-resolution mass spectrometry to analyze the structure and relative content of the metabolites of Deg-AZM in rats. Due to the differences in mass spectrometry responses between the prototype and metabolites, the peak area normalization method mentioned above was not accurate for the assessment of the excretion ratio of the prototype and metabolites in biological samples. It is necessary to obtain standard samples of the main metabolites for accurate quantification or use isotope tracing techniques to further improve material balance research in the clinical stage.
3.3 P-gp substrate and inhibition
3.3.1 Validation of Caco-2 cell model
The TEER value is related to the degree of tight junctions between cells significantly. The larger the value, the tighter the connection between cells. The TEER values of the Caco-2 cell monolayer before administration were all greater than 350 Ω cm2 in this study, indicating that the compactness of the Caco-2 cell monolayer meets the experimental requirements.
[3H] Propranolol, [14C] PEG4000 and Rhodamine 123 were used as tool drugs to test the compactness and efflux function of the Caco-2 cell model. The Papp of [3H] Propranolol needed to be less than 27 × 10−6 cm/s, and the Papp of [14C] PEG4000 needed to be less than 1 × 10−6 cm/s. The ER value of Rhodamine 123 without inhibitor of P-gp needed to be greater than 4. As shown in Table 2, The results indicate that the density, integrity, and functionality of the Caco-2 cell monolayer meet the experimental requirements.
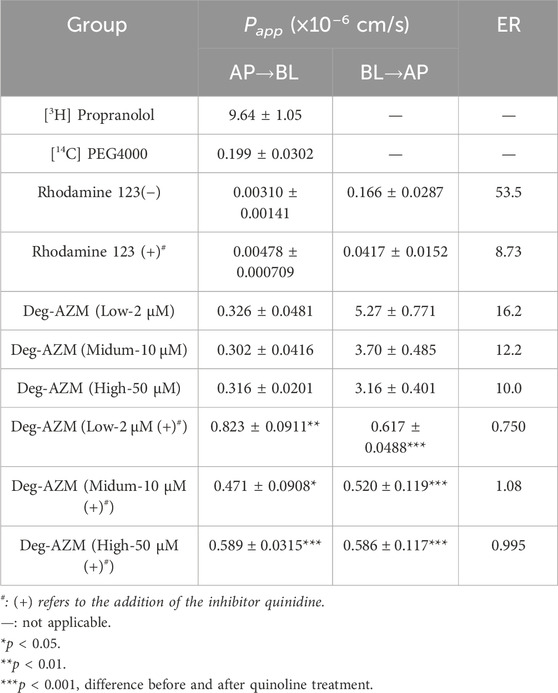
Table 2. Papp values and ER values of bidirectional transport for [3H] Propranolol, [14C] PEG4000, Rhodamine 123 and Deg-AZM in Caco-2 cell monolayer (n = 3).
3.3.2 Drug transport assay
The bidirectional transport results of the Caco-2 cell monolayer for Deg-AZM at different concentrations were shown in Table 2. At 2, 10 and 50 μM concentration level, the Papp values of Deg-AZM in the intake direction of Caco-2 model are 0.326 × 10−6, 0.302 × 10−6 and 0.316 × 10−6 cm/s respectively, which imply Deg-AZM was a low permeability drug with poor transmembrane ability. At 2, 10 and 50 μM concentration level, the efflux rates (ER) of Deg-AZM were 16.2, 12.2 and 10.0 respectively, all greater than 2. After the addition of quinidine, the inhibitor of P-gp, the ERs significantly reduced to 0.750, 1.08 and 0.995 respectively. It was inferred that Deg-AZM might be the substrate of intestinal efflux transporter P-gp.
P-gp is an efflux transporter with influencing the pharmacokinetic profiles of many drugs, which expressed on various cells, such as enterocytes, hepatocytes, brain capillary endothelial cells, renal proximal tubular cells (Elmeliegy et al., 2020). In this study, Deg-AZM was inferred to be a substrate of P-gp, which suggested that we should pay attention on the systemic exposure of Deg-AZM when combined with the inhibitors or inducers of P-gp in the clinical trials. A significant change in system exposure of the drug may lead to toxicity or insufficient efficacy. This study did not clarify whether Deg-AZM is an inducer or inhibitor of P-gp, and we need to continue researching to provide more guidance for the management of DDIs study in clinical trials.
3.4 CYP phenotyping
3.4.1 HLM phenotyping assays
The inhibition rates of the generation of corresponding metabolites in seven positive control reaction systems were investigated. Compared with the control groups without inhibitors, specific inhibitors significantly inhibited the generation of corresponding products of each probe substrate, with inhibition rates ranging from 83.1% to 94.9%, confirming the effectiveness of the experimental system.
Deg-AZM phenotyping of CYPs was evaluated by HLMs with specific inhibitors. After 90 min incubation, 45.7% Deg-AZM remained in the control group without inhibitors, indicating that Deg-AZM undergoes metabolic with CYPs. The addition of the CYP3A inhibitor ketoconazole resulted in 93.4% of Deg-AZM remaining, the addition of the CYP2C9 inhibitor sulfaphenazole resulted in 67.5% of Deg-AZM remaining, while the addition of all the other CYP inhibitors produced nearly no effect (Figure 5). It indicated that CYP3A4 mainly involved in the metabolic of Deg-AZM, CYP2C9 may be involved in the metabolic of some Deg-AZM.
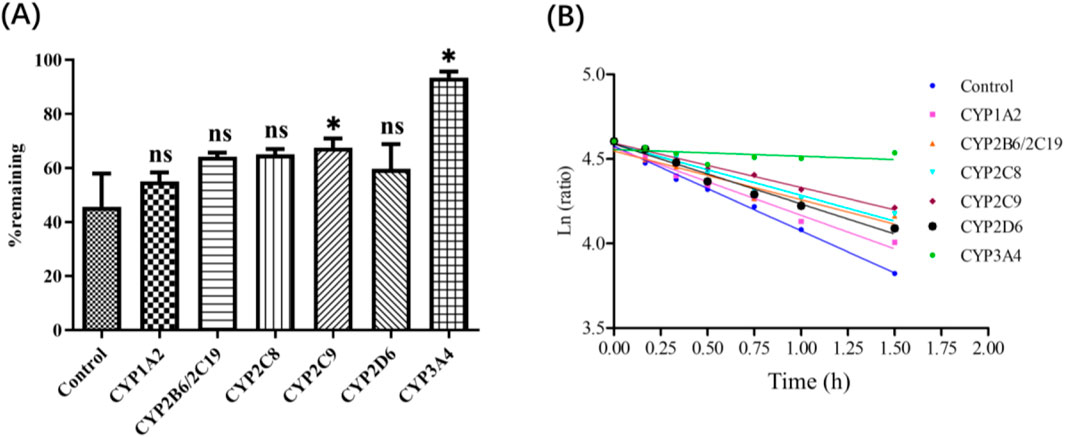
Figure 5. (A): Deg-AZM remaining after incubation in HLM with chemical inhibitors. (B): Half log fitting plot of Deg-AZM remaining at different time after incubation in HLM with chemical inhibitors. Data were presented as the mean ± SD, n = 3. ns: no significance; *p < 0.05, compared to the control group.
The above results showed that 45.7% Deg-AZM remained in the control group without inhibitors, but the addition of the CYP3A inhibitor ketoconazole resulted in 93.4% of Deg-AZM remaining, this contribution rate was already significant enough, so we did not verify the consumption of the inhibitors in the incubation system further. However, the disappearance of the inhibitors may affect their ability to block CYP-specific metabolism, which will lead to underestimation the contribution of the corresponding metabolic enzyme. In addition, the sufficient specificity of inhibitors also affects the accuracy of the results. Therefore, “Recombinant CYPs phenotyping assay” needed to be employed to verified the results of CYPs phenotyping of Deg-AZM.
3.4.2 Recombinant CYPs phenotyping assays
Deg-AZM phenotyping of CYPs was evaluated by rCYPs ulteriorly. The remaining proportion of Deg-AZM in each recombinant CYP monomer enzyme after incubation with Deg-AZM was shown in Figure 6A, and the average remaining proportion time semi logarithmic fitting curve was shown in Figure 6B. The results showed that the residual proportion of Deg-AZM in the control group with inactivated monomeric enzyme after 1.5 h of incubation was 107%, indicating that Deg-AZM was stable in the reaction system without significant non enzymatic metabolic elimination. Among the seven active experimental groups, after incubation for 1.5 h, the average residual proportion of Deg-AZM in the CYP3A4 monomeric enzyme system was 75.9%, the elimination proportion was greater than 15%, and the ke value was higher than that of the blank control group and other monomeric enzyme groups. There was no significant difference for the elimination proportion and ke value of Deg-AZM in other monomeric enzyme systems compared with the blank control group. In summary, only rCYP3A4 led to a remarkable disappearance of Deg-AZM.
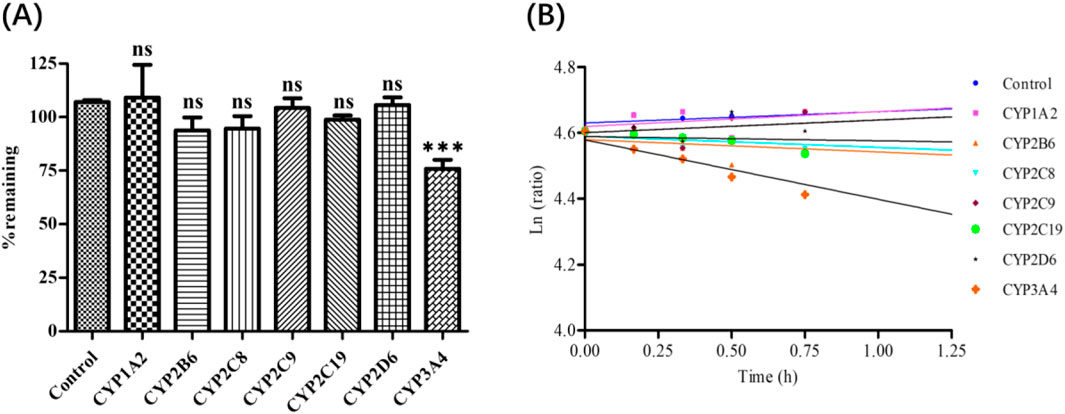
Figure 6. (A): Deg-AZM remaining after incubation in recombinant enzymes. (B): Half log fitting plot of Deg-AZM remaining at different time after incubation in recombinant enzymes. Data were presented as the mean ± SD, n = 3. ns: no significance; ***p < 0.001, compared to the control group.
Overall, the results of “HLM phenotyping assays” and “Recombinant CYPs phenotyping assays” showed that CYP3A4 is the main enzyme subtype mediating Deg-AZM liver metabolism, CYP2C8, CYP2D6, CYP1A2, CYP2B6, CYP2C9 and CYP2C19 did not participate in the metabolism of Deg-AZM.
3.5 CYP inhibition
The IC50 values of Deg-AZM against CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in HLMs was shown in Table 3. Deg-AZM has no inhibitory effect on CYP1A2, CYP2C8, CYP2C9, CYP2C19 and CYP3A4 with IC50 values greater than the maximum concentration set in the experiment of 60 μM, but has a moderate inhibitory effect on CYP2B6 and CYP2D6 with IC50 of 8.97 μM and 7.89 μM respectively.
The single probe substrate combined with LC-MS/MS method is the “gold standard” for in vitro evaluation of CYP inhibition. However, this method still has some limitations. In this study, Deg-AZM was used for pre-incubation up to 60 min with liver microsomes in some group to inhibit the activity of CYP enzymes. Our previous study (Gu et al., 2024) has shown that Deg-AZM was not stable in human liver microsomes (T1/2 = 46.5 min), which indicated that the content of Deg-AZM decreased in the system with incubation time increasing, which may lead to an underestimation of the inhibitory strength of Deg-AZM on some CYP enzymes. Further exploration is needed in subsequent clinical studies.
3.6 CYP induction
Induction results of positive control, negative control and Deg-AZM on CYP1A2, CYP2B6 and CYP3A4 in three batches of human hepatocytes were listed in Table 4. Positive inducers omeprazole, phenobarbital and rifampicin significantly induced mRNA expression of CYP1A2, CYP2B6 and CYP3A4 in human hepatocytes, respectively. The negative control group (Erythromycin) showed no significant effect on the mRNA expression levels of CYP1A2, CYP2B6, and CYP3A4 in human hepatocytes. Deg-AZM at 5, 25, and 100 µM showed no induction effect on CYP1A2. At 5, 25, and 100 µM concentration levels, the induction of Deg-AZM on CYP2B6 in MWB batch of human hepatocyte were 1.60, 1.83, and 4.33 times higher than those of the control group, and 4.39%, 6.09% and 24.3% (over 20%) of the positive control, respectively. The induction of Deg-AZM on CYP2B6 in LHuf17905B batch of human hepatocyte were 1.61, 1.46, and 2.84 times higher than that of the control group, and 6.61%, 5.00% and 19.8% of the positive control, respectively. Overall, Deg-AZM has an induction effect on CYP2B6 enzyme. At 5, 25, and 100 µM concentration levels, the induction of Deg-AZM on CYP3A4 in three batches of human hepatocytes showed a significant concentration dependent trend. The induction of Deg-AZM on CYP3A4 in MWB batch of human hepatocyte were 1.96, 4.05, and 15.2 times higher than those of the control group, and 1.99%, 6.32% and 29.3% (over 20%) of the positive control, respectively. The induction of Deg-AZM on CYP3A4 in LHuf17905B batch of human hepatocyte were 2.30, 3.84, and 13.7 times higher than those of the control group, and 5.55%, 12.1% and 54.2% (over 20%) of the positive control, respectively. The induction of Deg-AZM on CYP3A4 in HUB batch of human hepatocyte were 1.80, 2.13 and 6.01 times higher than those of the control group, and 2.15%, 3.06% and 13.5% of the positive control, respectively. Based on the above results, Deg-AZM has an inducing effect on the CYP3A4 enzyme in primary human hepatocytes.
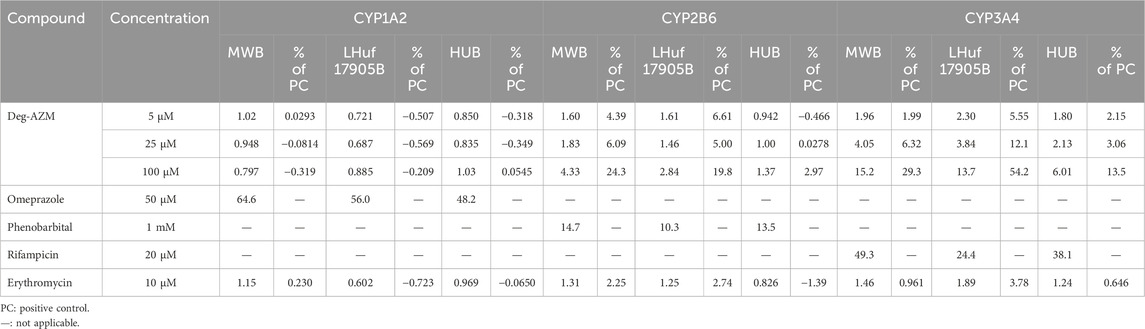
Table 4. Induction of Deg-AZM on CYP1A2, CYP2B6 and CYP3A4 in three batches of human hepatocytes (n = 3).
According to FDA guidelines (Huang et al., 2007), CYP2D6 has not been shown to be inducible, while CYP2C and CYP2B have synergistic inducing effects with CYP3A, which appears to be sensitive to all known synergistic inducers. Therefore, in order to evaluate whether the investigational drug has an inducing effect on CYP1A2, CYP2C8, CYP2C9, CYP2C19 or CYP3A, the initial in vitro induction evaluation may only include CYP1A2 and CYP3A. If the results of in vitro studies indicate that the investigational drug does not induce CYP3A metabolism, then there is no need to conduct in vivo interaction studies between the investigational drug and co-administered drugs eliminated by CYP2C/CYP2B and CYP3A at these enzyme induction levels. It is important to investigate the DDIs mediated by CYP2B6 in both preclinical and clinical stages. Other CYP enzymes including CYP2A6 and CYP2E1 are considered to be less involved in clinically DDIs. Therefore, this study conducted the in vitro induction study of Deg-AZM on CYP1A2, CYP2B6 and CYP3A of human hepatocytes. This study indicated that Deg-AZM has an inducing effect on the CYP3A4 enzyme, suggesting that we should pay attention to plasma exposure of the combination drugs to avoid drug failure caused by low blood concentration or toxicity caused by the toxic metabolites in clinical application.
4 Conclusion
This study elaborated the metabolic pathway of Deg-AZM in vivo and revealed the potential role of Deg-AZM as a perpetrator of DDIs by regulating metabolic enzymes or transporter in vitro firstly. Deg-AZM and a total of 44 metabolites were found in rat urine, feces, bile, and plasma. The main metabolic pathways of Deg-AZM include demethylation, monohydroxylation, dihydroxylation, dehydroxidation, hydroreduction, hydrolysis, methylation, glucuronidation and the combination of different metabolic pathways. Deg-AZM was a potential substrate of the efflux transporter P-gp. CYP3A4 is the major CYP isoform responsible for Deg-AZM metabolism, the exposure of Deg-AZM could be impacted by CYP3A4 modulators. Deg-AZM showed moderate inhibition for CYP2B6 and CYP2D6. Data in human primary hepatocytes disclosed induction potential of Deg-AZM on CYP2B6 and CYP3A4. These in vitro results with CYPs and transporter indicated that CYP3A and P-gp inhibitors and inducers may impact systemic exposure of Deg-AZM and caused DDIs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee at Tianjin Tiancheng New Drug Evaluation Co., Ltd. (Permit No. SYXK 2016–0009). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XG: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Writing–original draft, Writing–review and editing. XL: Data curation, Methodology, Writing–original draft. WT: Conceptualization, Project administration, Resources, Supervision, Visualization, Writing–original draft. ChZ: Methodology, Software, Writing–original draft. YC: Investigation, Methodology, Writing–original draft. XX: Methodology, Writing–original draft. CoZ: Methodology, Writing–original draft. HL: Methodology, Writing–original draft. YS: Methodology, Writing–original draft. ZL: Methodology, Writing–original draft. SZ: Methodology, Writing–original draft. HZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. XA: Conceptualization, Investigation, Project administration, Resources, Supervision, Visualization, Writing–review and editing. CY: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82304856), and the 111 Project B20016.
Conflict of interest
Author WT was employed by The National Institutes of Pharmaceutical R&D Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1510903/full#supplementary-material
Abbreviations
AP, Apical; BL, Basolateral; Caco-2, the human colon adenocarcinoma cell line; CYP, cytochrome P450; DDIs, drug-drug interactions; Deg-AZM, Deglycosylated azithromycin; ER, efflux ratio; HBSS, Hank’s balanced salt solution; HLM, human liver microsome; HPLC, High performance liquid chromatography; IC50, half maximal inhibitory concentration; LC-MS/MS, liquid chromatography coupled with tandem; NADPH, nicotinamide adenine dinucleotide phosphate; Papp, apparent permeability coefficient; P-gp, P-glycoprotein; SD, standard deviation; TEER, Transepithelial electrical resistance.
References
Alsalimy, N., Madi, L., and Awaisu, A. (2018). Efficacy and safety of laxatives for chronic constipation in long-term care settings: a systematic review. J. Clin. Pharm. Ther. 43 (5), 595–605. doi:10.1111/jcpt.12721
Bharucha, A. E., and Lacy, B. E. (2020). Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 158 (5), 1232–1249. doi:10.1053/j.gastro.2019.12.034
Bharucha, A. E., and Wald, A. (2019). Chronic constipation. Mayo Clin. Proc. 94 (11), 2340–2357. doi:10.1016/j.mayocp.2019.01.031
Costilla, V. C., and Foxx-Orenstein, A. E. (2014). Constipation: understanding mechanisms and management. Clin Geriatr Med 30 (1), 107–115. doi:10.1016/j.cger.2013.10.001
De Giorgio, R., Zucco, F. M., Chiarioni, G., Mercadante, S., Corazziari, E. S., Caraceni, A., et al. (2021). Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv. Ther. 38 (7), 3589–3621. doi:10.1007/s12325-021-01766-y
Elmeliegy, M., Vourvahis, M., Guo, C., and Wang, D. D. (2020). Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: review of clinical drug-drug interaction studies. Clin. Pharmacokinet. 59 (6), 699–714. doi:10.1007/s40262-020-00867-1
El-Salhy, M., Svensen, R., Hatlebakk, J. G., Gilja, O. H., and Hausken, T. (2014). Chronic constipation and treatment options (Review). Mol. Med. Rep. 9 (1), 3–8. doi:10.3892/mmr.2013.1770
Gu, X., Li, X., Tian, W., Zheng, C., Cai, Y., Xu, X., et al. (2024). Preclinical pharmacokinetic studies and prediction of human PK profiles for Deg-AZM, a clinical-stage new transgelin agonist. Front. Pharmacol. 15, 1423175. doi:10.3389/fphar.2024.1423175
Huang, S. M., Temple, R., Throckmorton, D. C., and Lesko, L. J. (2007). Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin. Pharmacol. Ther. 81 (2), 298–304. doi:10.1038/sj.clpt.6100054
Ishiyama, Y., Hoshide, S., Mizuno, H., and Kario, K. (2019). Constipation-induced pressor effects as triggers for cardiovascular events. J. Clin. Hypertens. 21 (3), 421–425. doi:10.1111/jch.13489
Jo, S., Kim, H.-R., Mun, Y., and Jun, C.-D. (2018). Transgelin-2 in immunity: its implication in cell therapy. J. Leukoc. Biol. 104 (5), 903–910. doi:10.1002/JLB.MR1117-470R
Kalyanasundaram, A., Lincoff, A. M., and Medscape, (2011). Managing adverse effects and drug–drug interactions of antiplatelet agents. Nat. Rev. Cardiol. 8 (10), 592–600. doi:10.1038/nrcardio.2011.128
Lees-Miller, J. P., Heeley, D. H., and Smillie, L. B. (1987). An abundant and novel protein of 22 kDa (SM22) is widely distributed in smooth muscles. Purification from bovine aorta. Biochem. J. 244 (3), 705–709. doi:10.1042/bj2440705
Müller-Lissner, S., Tack, J., Feng, Y., Schenck, F., and Specht Gryp, R. (2013). Levels of satisfaction with current chronic constipation treatment options in Europe – an internet survey. Aliment. Pharm. Ther. 37 (1), 137–145. doi:10.1111/apt.12124
Nunan, D., Cai, T., Gardener, A. D., Ordonez-Mena, J. M., Roberts, N. W., Thomas, E. T., et al. (2022). Physical activity for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 6 (6), CD011497. doi:10.1002/14651858.CD011497.pub2
Oh, S. J., Fuller, G., Patel, D., Khalil, C., Spalding, W., Nag, A., et al. (2020). Chronic constipation in the United States: results from a population-based survey assessing healthcare seeking and use of pharmacotherapy. Am. J. Gastroenterol. 115 (6), 895–905. doi:10.14309/ajg.0000000000000614
Rao, S. S., Rattanakovit, K., and Patcharatrakul, T. (2016). Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 13 (5), 295–305. doi:10.1038/nrgastro.2016.53
Tack, J., Müller-Lissner, S., Stanghellini, V., Boeckxstaens, G., Kamm, M. A., Simren, M., et al. (2011). Diagnosis and treatment of chronic constipation – a European perspective. Neurogastroent Motil. 23 (8), 697–710. doi:10.1111/j.1365-2982.2011.01709.x
van Leeuwen, R. W. F., van Gelder, T., Mathijssen, R. H. J., and Jansman, F. G. A. (2014). Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 15 (8), e315–e326. doi:10.1016/S1470-2045(13)70579-5
Winge, K., Rasmussen, D., and Werdelin, L. M. (2003). Constipation in neurological diseases. J. Neurol. Neurosurg. Psychiatry 74 (1), 13–19. doi:10.1136/jnnp.74.1.13
Włodarczyk, J., Waśniewska, A., Fichna, J., Dziki, A., Dziki, Ł., and Włodarczyk, M. (2021). Current overview on clinical management of chronic constipation. J. Clin. Med. 10 (8), 1738. doi:10.3390/jcm10081738
Yin, L., Xu, Y., Peng, L., Duan, T., Liu, J., Xu, Z., et al. (2018). Transgelin-2 as a therapeutic target for asthmatic pulmonary resistance. Sci. Transl. Med. 10 (427), eaam8604. doi:10.1126/scitranslmed.aam8604
Zhi, Y., Chenxi, W., Jing, G., and Dingxi, B. (2021). Prevalence of chronic constipation in Chinese adults: a meta-analysis. Chin. General Pract. 24 (16). doi:10.12114/j.issn.1007-9572.2021.00.092
Keywords: deglycosylated azithromycin, drug-drug interactions, CYP enzymes, Caco-2 cell, P-gp, metabolic pathways
Citation: Gu X, Li X, Tian W, Zheng C, Cai Y, Xu X, Zhao C, Liu H, Sun Y, Luo Z, Zhu S, Zhou H, Ai X and Yang C (2025) The in vivo metabolic pathway of Deg-AZM and in vitro investigations into the contribution of drug metabolizing enzymes and drug transporters in the drug interactions of Deg-AZM, a clinical-stage new transgelin agonist. Front. Pharmacol. 15:1510903. doi: 10.3389/fphar.2024.1510903
Received: 14 October 2024; Accepted: 17 December 2024;
Published: 08 January 2025.
Edited by:
Charles Oo, Morris Plains, United StatesReviewed by:
Paavo Honkakoski, University of Eastern Finland, FinlandAdila Aipire, Xinjiang University, China
Copyright © 2025 Gu, Li, Tian, Zheng, Cai, Xu, Zhao, Liu, Sun, Luo, Zhu, Zhou, Ai and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Yang, eWFuZ2NoZW5nQG5hbmthaS5lZHUuY24=; Xiaoyu Ai, YWl4aWFveXVAbmFua2FpLmVkdS5jbg==; Honggang Zhou, aG9uZ2dhbmcuemhvdUBuYW5rYWkuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoting Gu1†
Xiaoting Gu1† Xiaohe Li
Xiaohe Li Honggang Zhou
Honggang Zhou Xiaoyu Ai
Xiaoyu Ai