- 1Research Center for Vaccine and Drugs, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Bogor, Indonesia
- 2Department of Agricultural Engineering, Faculty of Agricultural, Hasanuddin University, Makassar, Indonesia
- 3Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 4Department of Pharmacy, Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia
- 5Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, Japan
- 6Department of Biological Sciences, Faculty of Teacher Training and Education, Muslim Maros University, Maros, Indonesia
- 7Department of Pharmacy, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia
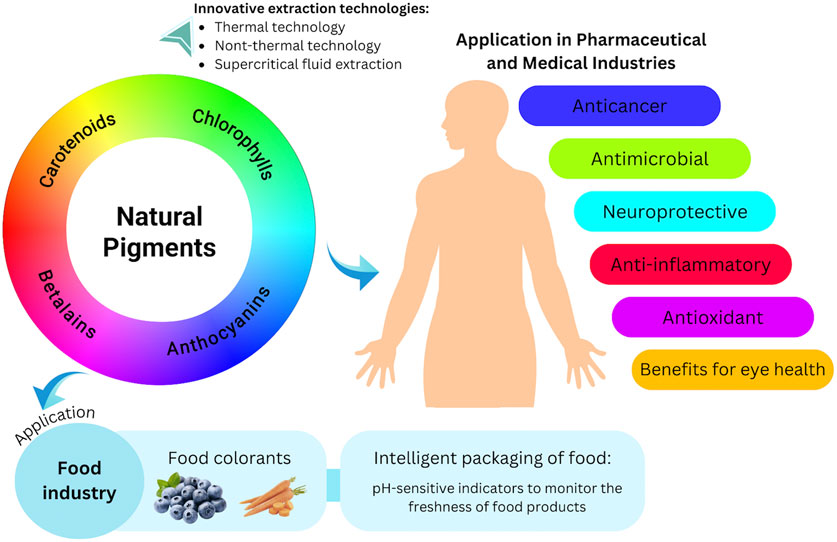
Natural pigments, or natural colorants, are frequently utilized in the food industry due to their diverse functional and nutritional attributes. Beyond their color properties, these pigments possess several biological activities, including antioxidant, anti-inflammatory, anticancer, antibacterial, and neuroprotective effects, as well as benefits for eye health. This review aims to provide a timely overview of the potential of natural pigments in the pharmaceutical, medical, and food industries. Special emphasis is placed on emerging technologies for natural pigment extraction (thermal technologies, non-thermal technologies, and supercritical fluid extraction), their pharmacological effects, and their potential application in intelligent food packaging and as food colorants. Natural pigments show several pharmaceutical prospects. For example, delphinidin (30 µM) significantly inhibited the growth of three cancer cell lines (B16-F10, EO771, and RM1) by at least 90% after 48 h. Furthermore, as an antioxidant agent, fucoxanthin at the highest concentration (50 μg/mL) significantly increased the ratio of glutathione to glutathione disulfide (p < 0.05). In the food industry, natural pigments have been used to improve the nutritional value of food without significantly altering the sensory experience. Moreover, the use of natural pH-sensitive pigments as food freshness indicators in intelligent food packaging is a cutting-edge technological advancement. This innovation could provide useful information to consumers, increase shelf life, and assist in evaluating the quality of packaged food by observing color variations over time. However, the use of natural pigments presents certain challenges, particularly regarding their stability and higher production costs compared to synthetic pigments. This situation underscores the need for further investigation into alternative pigment sources and improved stabilization methods. The instability of these natural pigments emphasizes their tendency to degrade and change color when exposed to various external conditions, including light, oxygen, temperature fluctuations, pH levels, and interactions with other substances in the food matrix.
1 Introduction
Natural pigments are organic substances that impart the rich and diverse colors seen in many fruits, vegetables, flowers, and various other living organisms (Singh et al., 2023). These pigments are generally categorized into four main groups: anthocyanins, carotenoids, chlorophylls, and betalains (Magalhães et al., 2024). While they are primarily known for their contribution to the color of plants, these natural pigments also act as bioactive substances that may provide various health advantages. Diets rich in anthocyanins, for example, have been linked to enhanced heart health, better cognitive function, and improved vision (Wallace, 2011). Carotenoids are beneficial for boosting the immune system and promoting skin health, largely due to their antioxidant effects (Nabi et al., 2020). Additionally, carotenoids play a protective role in maintaining eye health, with evidence suggesting they help prevent age-related macular degeneration (Milani et al., 2017). Research on chlorophylls has highlighted their potential for detoxification, as they can bind to harmful carcinogens and possibly lower cancer risk (Martins et al., 2023; Sun et al., 2024). Lastly, betalains are noted for their potent antioxidant and anti-inflammatory actions, which have been associated with reduced oxidative damage and better cardiovascular health (Moreno-Ley et al., 2021).
Recently, there has been a marked increase in consumer demand for products containing natural colors. This trend has been driven by growing health and environmental concerns, leading to the widespread adoption of natural colorants as alternatives to synthetic pigments (Ghosh et al., 2022). Although the food industry has traditionally relied on synthetic colorants for their stability, vivid colors, and low production costs, the use of natural food pigments is progressively gaining traction. This shift is largely due to evolving consumer preferences and heightened awareness of the potential health risks and environmental consequences associated with synthetic pigments (Novais et al., 2022). Given their significant nutritional value and health-promoting properties, natural pigments are increasingly regarded as functional food ingredients. They not only enhance the sensory qualities of food products but also serve to mask undesirable attributes or improve the overall natural characteristics of food (Magalhães et al., 2024).
The extraction methods for natural pigments are evolving to enhance both product quality and extraction efficiency. The goal is to reduce extraction time and minimize solvent use compared to conventional methods. Furthermore, there are significant concerns regarding the environmental impact of toxic residues from organic solvents, as well as the safety of the final products derived from these methods (Panda and Manickam, 2019). Ensuring safety is crucial, especially when natural pigments are intended for use in the food, pharmaceutical, and cosmetic sectors. Therefore, current extraction processes are increasingly directed toward the use of novel technologies. These novel technologies are generally classified into advanced thermal methods, such as microwave, ohmic heating, and radiofrequency heating (Kubo et al., 2020), and non-thermal methods, such as pulsed electric fields, high pressure, ultrasound, and cavitation-based extraction (Panda and Manickam, 2019). These technologies can overcome the limitations of traditional extraction methods by reducing extraction time and solvent use, while also increasing the extraction yield of natural pigments, including anthocyanins and chlorophyll (Guo et al., 2019; Hsieh-Lo et al., 2020; Kutlu et al., 2021; Lefebvre et al., 2020).
Although natural pigments offer numerous benefits, they face several challenges that limit their broader application, particularly in food products. These challenges include issues with low bioavailability and stability (Nabi et al., 2023). However, encapsulation techniques have emerged as an effective solution to these problems, as they help protect the pigments from degradation. By encapsulating these compounds, their stability and bioactivity are enhanced, which in turn improves their potential health benefits (Ghosh et al., 2022).
In this context, and in light of the information mentioned above, further research in this area is essential. This review aims to provide an updated summary of innovative methods for extracting natural pigments while expanding our understanding of their value and potential applications across various fields, including food, medicine, and pharmaceuticals. Through a comprehensive analysis, we seek to enhance our knowledge of natural pigments and emphasize their promising therapeutic potential.
Several recent review articles (2021–2024) have explored the health potential of natural pigments; however, they primarily offer broad overviews without focusing on specific metabolites. For example, Magalhães et al. (2024) highlighted that anthocyanins are recognized for their significant health benefits, including anticancer properties linked to both chemopreventive and chemoprotective effects, as well as antioxidant and anti-inflammatory activities. Similarly Lu et al. (2021), found that carotenoids positively impact various reactive oxygen species (ROS)-related diseases, such as cardiovascular disease, osteoporosis, cancer, and myocardial infarction in smokers.
Therefore, the originality of this review lies in its focus on the biological activities of natural pigments and their metabolites, with particular emphasis on their anticancer, antioxidant, antimicrobial, anti-inflammatory, and neuroprotective properties, along with their positive impact on eye health. Furthermore, this paper explores the biotransformation and bioavailability of natural pigments, which are underexplored aspects of this field. Ultimately, this work aims to lay the groundwork for future developments in natural pigment-based drug discovery and their application in the food industry.
2 Methods
Research articles and reviews on natural pigments were gathered using the search engines Google Scholar, PubMed, Scopus, and SpringerLink. The search was based on a specific set of keywords: (“natural pigments” OR “anthocyanins” OR “carotenoids” OR “chlorophylls” OR “betalains”) AND (“extractions” OR “biotransformation” OR “anticancer activity” OR “antioxidant activity” OR “anti-inflammatory activity” OR “antimicrobial activity” OR “neuroprotective activity” OR “eye health” OR “stability” OR “food industry”).
During the preliminary screening, article titles and abstracts were manually reviewed to eliminate studies that were not pertinent to the topic. The selection criteria included: studies published in English, documents published between 2000 and 2024, availability of full text, and the presence of the terms “natural pigments” or their metabolites in the titles and/or abstracts. Exclusion criteria included: studies lacking details on chemical composition, extraction methods, biotransformation, biological effects, stability or application in food industry; records without full-text access; and articles published before 2000. In total, 244 articles were included in this study.
3 Classification of natural pigments
3.1 Anthocyanins
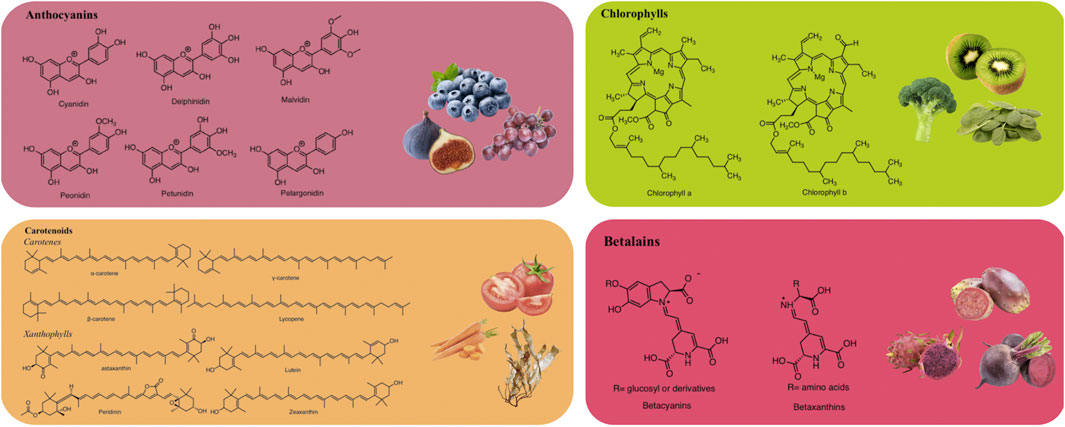
Anthocyanins are glucosides of anthocyanidins, a type of water-soluble pigment synthesized via the phenylpropanoid pathway (Mattioli et al., 2020). Structurally, anthocyanins are formed by 2-phenylchromenylium (a flavylium cation), which links methoxyl (−OCH₃) and/or hydroxyl (−OH) groups, along with one or more sugars (Al-Khayri et al., 2022). Anthocyanins are extensively found in the fruits, flowers, and vegetables of many plants, including fig (Ficus carica L.), juçara (Euterpe edulis Mart.), blackberry (Rubus fruticosus L.), grumixama (Eugenia brasiliensis Lam.), grapes (Vitis vinifera L.), blueberry (Vaccinium myrtillus L.), and petals of saffron (Crocus sativus L.) (Cerezo et al., 2020). These metabolites exhibit various colors such as blue, purple, and red, depending on their concentration and the complementary light absorption of chlorophyll (Bendokas et al., 2019).
Anthocyanins are commonly utilized as natural colorants (Enaru et al., 2021). Nevertheless, pH, temperature, light, and structure all affect the color and stability of these metabolites (Khoo et al., 2017). For example, these pigments change from red at an acidic pH to blue at a basic pH. Additionally, the stability of anthocyanins is influenced by the presence of methoxyl or hydroxyl groups (Kang et al., 2021). Delphinidin, cyanidin, malvidin, pelargonidin, petunidin, and peonidin are the main anthocyanidins found in foods (Figure 1) (Enaru et al., 2021).
The total anthocyanin content in blueberries ranges from 85 to 270 mg/100 g fresh weight (FW). In blackberries, the primary anthocyanins are 3-glycoside derivatives of several flavonoids, including cyanidin, delphinidin, malvidin, petunidin, and peonidin. In contrast, cranberries have a lower total anthocyanin concentration, ranging from 25 to 100 mg/100 g FW. The dominant anthocyanins in cranberries are the 3-O-galactoside and 3-O-arabinoside forms of cyanidin and peonidin (Padmanabhan et al., 2016).
3.2 Carotenoids
Carotenoids (a type of fat-soluble pigment) are naturally found in algae, plants, animals, photosynthetic bacteria, and some species of fungi and archaea (Bhatt and Patel, 2020). These metabolites are tetraterpene pigments, which impart orange, purple, yellow, and red colors (Maoka, 2020). The intensity of their color is typically correlated with the quantity of carotenoids. Carotenoids are most abundant in fruits and vegetables. Their basic structures commonly consist of a 40-carbon backbone with eight isoprene units (Sun et al., 2022).
Structurally, carotenoids can be classified into two groups: carotenes and xanthophylls (Figure 1). Carotenes are hydrocarbons, including compounds like lycopene and β-carotene. In contrast, xanthophylls are derived from carotenes through the introduction of oxygen-containing functional groups such as hydroxyl, methoxy, carboxyl, keto, and epoxy. Prominent xanthophylls include lutein, β-cryptoxanthin, zeaxanthin, and fucoxanthin (Maoka, 2020; Nisar et al., 2015). Additionally, carotenoids can exist in an acyclic form, as in the case of lycopene, or exhibit various cyclic structures at one or both ends, similar to β-carotene. Due to the numerous double bonds in their molecular chains, carotenoids can adopt several cis/trans isomeric forms, although the all-trans isomer is the most stable and is predominantly found in nature (Molina et al., 2023). Martí et al. (2016) reported that tomatoes are a source of carotenoids, including lycopene, phytoene, phytofluene, β-carotene, γ-carotene, and δ-carotene, with concentrations ranging from 7.8 to 18.1 mg/100 g FW, 1.0–2.9 mg/100 g FW, 0.2–1.6 mg/100 g FW, 0.1–1.2 mg/100 g FW, 0.05–0.3 mg/100 g FW, and 0–0.2 mg/100 g FW, respectively.
3.3 Chlorophylls
Chlorophylls are naturally occurring green pigments found in algae, cyanobacteria, and several plants (Tanaka and Tanaka, 2019). Structurally, chlorophylls are complex molecules classified as porphyrins. They consist of four pyrrole rings and an additional isocyclic ring adjacent to the third pyrrole ring. These rings are connected by methylene bridges, with a magnesium atom at the center of the molecule. Additionally, in the fourth pyrrole ring, the propionic acid is esterified with a long-chain acyclic alcohol, typically phytol, which imparts a hydrophobic property to chlorophyll a (Molina et al., 2023; Vaňková et al., 2018). Chlorophylls are commonly found in two major forms: chlorophyll a and chlorophyll b (Figure 1), which differ at the 7-carbon position (Yilmaz and Gökmen, 2016). Chlorophyll a contains a methyl (–CH3) group, while chlorophyll b contains an aldehyde (–CHO) group. These structural differences result in different colors; chlorophyll a appears green-blue, while chlorophyll b appears green-yellow. The ratio of chlorophyll a to chlorophyll b in plants is typically 3:1 (Carillo et al., 2022).
Chlorophylls have relatively low stability due to their structural susceptibility to various factors that can alter their color characteristics. One of the most common reactions affecting chlorophyll stability is the replacement of the central magnesium ion with two hydrogen ions. This substitution causes a significant color change, as magnesium-containing derivatives appear green, whereas those lacking magnesium, such as pheophytins and pheophorbides, exhibit a brown coloration (Molina et al., 2023).
He et al. (2018) found that the concentrations of chlorophyll a and chlorophyll b in the green alga Ulva prolifera at 28°C were 3.2 ± 0.04 μg/mL and 1.90 ± 0.06 μg/mL, respectively. Furthermore, Šamec et al. (2021) examined the chlorophyll content in Brassica leafy vegetables. Their results showed that kale had chlorophyll a and chlorophyll b concentrations of 7.21 ± 0.19 μg/g dry weight (DW) and 3.50 ± 0.48 μg/g DW, respectively. White cabbage exhibited chlorophyll a and chlorophyll b concentrations of 4.69 ± 0.40 μg/g DW and 2.17 ± 0.87 μg/g DW, respectively, while Chinese cabbage contained chlorophyll a and chlorophyll b at concentrations of 4.67 ± 0.46 μg/g DW and 4.30 ± 0.53 μg/g DW, respectively.
3.4 Betalains
Betalains, water-soluble nitrogenous pigments, are composed of betalamic acid (4-(2-oxoethylidene)-1,2,3,4-tetrahydropyridine-2,6-dicarboxylic acid) as their basic structure (Madadi et al., 2020). These pigments are classified into two groups: betaxanthins (yellow pigments) and betacyanins (violet pigments) (Figure 1) (Calva-Estrada et al., 2022). Cyclo-L-3,4-dihydroxyphenylalanine (cyclo-DOPA) or its glucosyl derivatives condense with betalamic acid to form betacyanins (Silva et al., 2020). Additionally, betalamic acid and amino metabolites condense to form betaxanthins (Figure 2) (Silva et al., 2020). Betalains are widespread in vegetables and fruits, with concentrations ranging from 13.81 to 2,252 mg/100 g in red dragon fruit (Selenicereus monacanthus (Lem.) D.R. Hunt), prickly pear (Opuntia spp.), and xoconostle (Opuntia joconostle F.A.C. Weber ex Diguet) (Kumar et al., 2020; Jiménez-Alvarado et al., 2015).
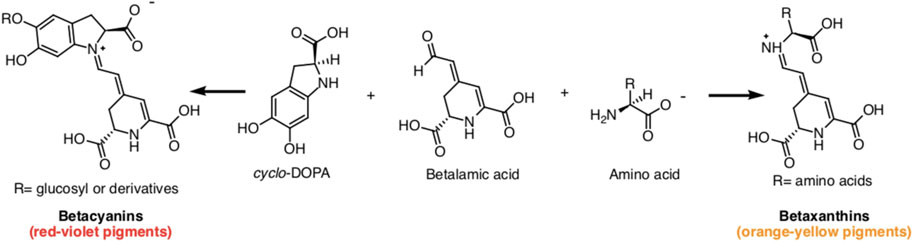
Figure 2. Betacyanins and betaxanthines chemical structures produced by condensing betalamic acid with cyclo-DOPA and amino acids, respectively.
4 Emerging technologies in natural pigment extraction
The extraction of pigment from the plant can be optimized by utilizing various emerging technologies. These technologies simplify the extraction process, reducing used solvents and extraction time without compromising the extraction yield. In addition, emerging technology could also include novel techniques to obtain “green” solvents as a substitute for the ionic liquid used in extraction (Guo et al., 2019). This section will address the effect of several technologies in extracting natural pigment. These technologies could be classified into thermal and non-thermal technologies.
4.1 Thermal technology
4.1.1 Ohmic heating
In the extraction process, thermal treatment is generally avoided due to its negative effect on thermal-sensitive metabolites, including natural pigment. However, novel thermal technologies including ohmic heating and microwave have been developed intensively in the past decades to overcome the drawbacks of conventional thermal treatments. Most studies revolve around the application of these technologies in processing food and beverages (Ferreira et al., 2019; Kuriya et al., 2020). These studies reported several quality parameters improvements in products treated with ohmic heating, including an increase in the reddish color of raspberry-flavored whey drink (Ferreira et al., 2019) and sensory attributes of dulce de leche (Kuriya et al., 2020). Those findings intrigue other studies to evaluate the viability of ohmic heating in the extraction process of bioactive and pigment metabolites by modifying the treatment parameters.
Ohmic heating provides rapid and uniform heating with a low energy consumption due to the absence of direct contact between the heating surface and the product (Pereira et al., 2020). The baseline of ohmic heating induces an electric current to a product with specific electrical conductivity. In ohmic heating, the product will behave as a resistance to the electric current flow, increasing temperature from inside the product. The increasing temperature will initiate cell wall disruption, which causes the metabolite to be extracted in the solvent. The heating process in ohmic is based on the principle of High-Temperature Short Time (HTST), where the heat generation occurs rapidly to increase the lethality of microbial and enzyme inactivation while preventing significant damage to bioactive metabolites.
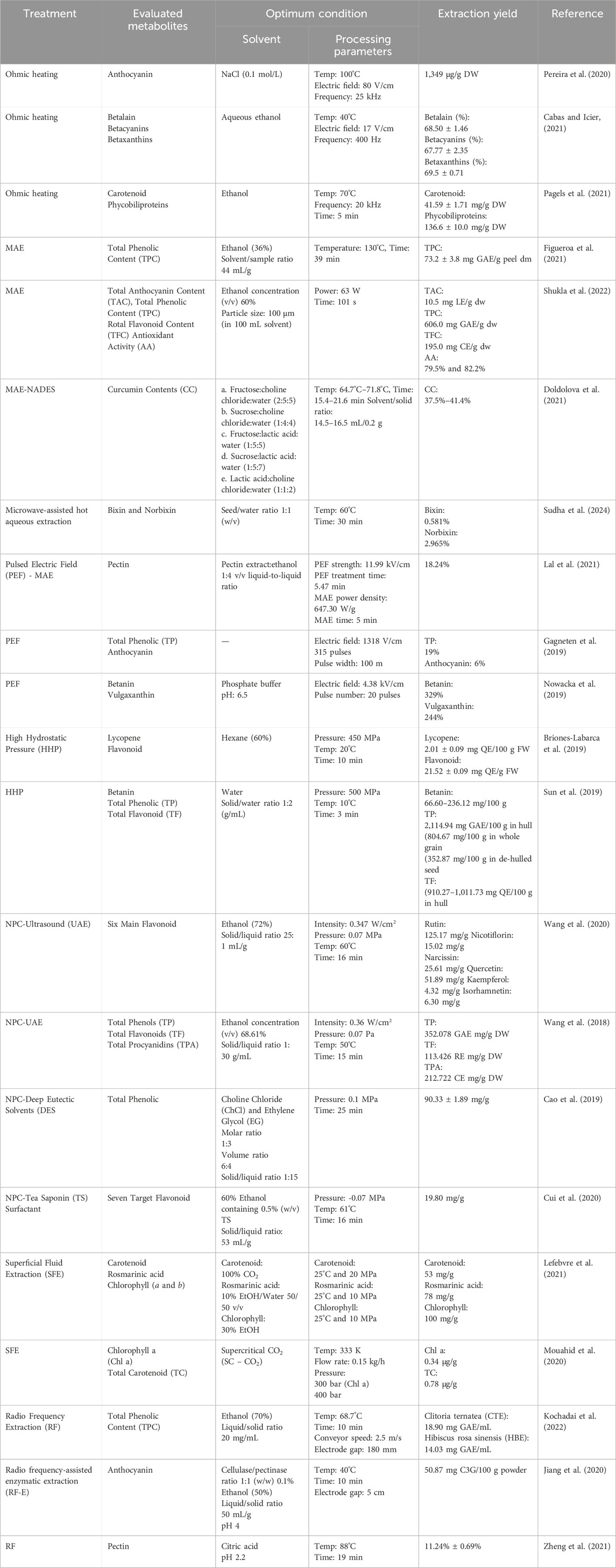
The applied electric field increases plant tissue’s permeability, allowing faster diffusion of the metabolite into the liquid medium, particularly the low molecular weight metabolites such as anthocyanins. Pereira et al. (2020) further demonstrated that the HTST effect during ohmic heating pre-treatment (40°C–100°C, <20 s) increased the anthocyanin content extracted from grape skin from 756 to 1,349 μg/g DW. The intense treatment (100°C, 80 V/cm) provided higher anthocyanin content than the mild treatment (40°C, 20 min, 16 V/cm), proving that thermal and electrical treatment positively affects the anthocyanin extraction in grape skin solution.
Compared with conventional extraction methods, ohmic heating at specific processing conditions could increase natural pigment’s extraction yield. For instance, ohmic heating-assisted extraction of natural pigment from red beetroot resulted in a higher yield of betalain metabolite than conventional extraction at 40°C. The optimum conditions reported in the study were the application of voltage gradient at 17 V/cm and a frequency of 400 Hz at 40°C using aqueous ethanol as the extracting medium (Cabas and Icier, 2021). The study further reported that the yield of betacyanin and color changes (hue angle, ΔE, ΔC, chroma) increased as the voltage gradient increased. Higher L* and a* values were observed during ohmic heating using aqueous ethanol, indicating a brighter, red-colored extract. Darker extract characterized by a low L* (brightness) value can be achieved by applying the highest voltage gradient and the lowest frequency (Cabas and Icier, 2021).
Another study by Pagels et al. (2021) demonstrated that the ohmic extraction of cyanobacteria pigments resulted in a higher yield of carotenoids and phycobiliproteins than the homogenization method. Ohmic heating-assisted extraction obtained 41.59 ± 1.71 mg/g DW of carotenoid and 136.6 ± 10.0 mg/g DW of phycobiliproteins, which are 1.3 and 1.2-fold higher than the homogenization method, respectively. In addition, ohmic heating extraction using ethanol and water as the medium also provided higher antioxidant capacity, 8.04 ± 0.31 and 8.33 ± 0.31 mg TE/g DW, respectively. This research concluded that the optimum processing conditions were achieved at 70°C and a frequency of 20 kHz for 5 min (Pagels et al., 2021).
The extraction of anthocyanins from grape by-product using the mixture of water and citric acid (1 mg/mL) as a solvent resulted in an insignificant difference between the ohmic and conventional methods (acidified methanolic solution) (Coelho et al., 2021). Besides better extraction yield, other competitive advantages of ohmic heating are higher energy efficiency and the possibility of extraction without organic solvent, which could promote better environmental impact. Kuriya et al. (2020) reported a higher energy efficiency in the ohmic heating of dairy desserts compared to conventional heating. Lower energy consumption can be achieved at a higher voltage gradient (9.1 V/cm) because of a faster heating rate of 17.1°C/min, while the heating rate in conventional heating only reached 9.9°C/min.
4.1.2 Microwave heating
The microwave-assisted extraction (MAE) mechanism lies in the effect of electromagnetic waves on polar molecules contained by the sample, which induce dipole rotation. The radiation of electromagnetic waves also charged ions inside the sample, transferring the energy and allowing ionic movement. This molecular movement generated heat and resulted in the evaporation of moisture trapped inside the plant’s cellular matrix (sample). The loss of moisture creates pressure inside the plant tissue and disrupts the cell structure. The rupture of the cell structure allows mass transfer, where the solvent diffuses into the plant matrix and leaches compounds into the extractant (Boateng, 2024; Nonglait and Gokhale, 2024; Usman et al., 2023).
Recent trends in utilizing microwave heating for metabolite extraction have revolved around optimizing said method (Bener et al., 2022; Doldolova et al., 2021; Lal et al., 2021; Martínez-Abad et al., 2020; Shukla et al., 2022; Sudha et al., 2024). Generally, combining high temperature or power with short irradiation time is deemed the best combination for MAE of plant materials (Zin et al., 2020). For instance, in the extraction of curcumin and antioxidants from turmeric, the extraction temperature plays a significant role in obtaining the highest extraction yields (Doldolova et al., 2021). The optimum operating conditions of MAE and other extraction technologies for various applications and their yields are reported in Table 1.
Microwave heating is often paired with Natural deep eutectic solvents (NADES) to promote an eco-friendly extraction process (Bener et al., 2022; Doldolova et al., 2021; Tapia-Quirós et al., 2024). Since the main advantage of MAE is the rapid and efficient extraction process with a smaller sample volume requirement, it is desirable to couple it with other green methods, such as NASES, which could provide efficient extraction yield (Tapia-Quirós et al., 2024). Bener et al. (2022) reported that hazelnut samples extracted using natural solvents of choline chloride:1,2-propylene glycol (CC-PG) with MAE resulted in a higher antioxidant capacity than those of ethanolic extracts.
Other studies have reported the eminence of MAE in providing a more efficient extraction process. MAE extensively decreased the pigment extraction time from walnut green peel from 80 to 1 min with an extraction yield of 19.95% (Wang et al., 2022). Furthermore, the extraction of pectin polysaccharide from jackfruit waste assisted with the combination of pulsed electric fields (PEF) and (MAE) improved the yield (18.24%) and lowered the energy (0.0986 kW-h) consumption (Lal et al., 2021).
4.1.3 Radio frequency heating
Radio frequency (RF) is an extension of microwave heating with a longer wavelength that allows deep penetration strength and enables RF to heat thicker and bigger sample sizes (Gao et al., 2023; Kochadai et al., 2022). Recently, RF has been utilized in the extraction process of metabolite, such as extraction of TPC (Fan et al., 2023; Kochadai et al., 2022), total flavonoid (Song et al., 2022), anthocyanin (Jiang et al., 2020), galacturonic acid (Zheng et al., 2021), also lignan, chlorophyll, and carotenoid content (Fan et al., 2023). Based on those studies, extraction with RF resulted in a higher extraction yield than hot water, acidified ethanol, and enzymatic (pectinase and cellulase) assisted extractions (Jiang et al., 2020). Meanwhile, flavonoid content showed no significant difference between RF and enzymatic-assisted (EA) extraction (Song et al., 2022). Detailed information about the processing parameters, extraction yield, and the used solvent is listed in Table 1.
4.2 Non-thermal technology
4.2.1 Pulsed electric field (PEF)
The pulsed electric field is a non-thermal processing technology that applies short electric pulses to a product that promotes cell electroporation. This process occurs due to cell wall disruption formed by the applied pulses, which allow metabolites to be extracted into the liquid medium (Jaeschke et al., 2019). Compared to other conventional extraction methods, the primary advantages of PEF are its mild processing conditions (temperature), short processing time, and lower energy consumption (Gagneten et al., 2019). The electric field strength is the main factor affecting the effectiveness of PEF treatment (López-Gámez et al., 2021; Nowacka et al., 2019).
An investigation to improve the extraction of bioactive substances from blackcurrant reported that at optimum conditions, PEF treatment could enhance the total phenolic and anthocyanin content by up to 19% and 6%, respectively. A 1318 V/cm electric field strength and 315 pulses were used to achieve the optimum condition, resulting in a cumulative energy intake of 30 ± 2 kJ/kg (Gagneten et al., 2019). However, total phenolic and antioxidant activity decreased beyond the optimum conditions (>1300 V/cm). This study indicates that bioactive molecules may be degraded by the strong electrical energy used during PEF treatment. It is essential to point out that this study had a relatively wider pulse width (100 m) compared to other studies, which used 4 μs (López-Gámez et al., 2021), 1 μs (Jaeschke et al., 2019), and 10 μs (Nowacka et al., 2019). In addition, a slight temperature increase of 5°C was observed after the PEF treatment. The Joule effect formed by applying electric current to a product resulted in an increase in temperature with a linear correlation with electric current (Gagneten et al., 2019).
Nowacka et al. (2019) evaluate the effect of PEF treatment on the extraction of betanin and vulgaxanthin from beetroot. This study demonstrated that PEF treatment of beetroot cylinders at 4.38 kV/cm enhanced both pigments by up to 329% and 244%, respectively. However, in terms of beetroot pulp, applying PEF decreased the vulgaxanthin content, with a significant effect observed at a higher electric field (6.25 kV/cm). Although the betanin content increased in beetroot pulp, the changes were insignificant. The authors stated that the degradation of vulgaxanthin could be affected by the physicochemical characteristics of this pigment, where vulgaxanthin is more prone to temperature and pH change than betanin. It is important to note that an increase in product temperature for up to 9.1°C was observed after PEF treatment. The difference in the sample phase (solid and liquid) could also contribute to the degradation of this pigment. The liquid phase (beetroot pulp) could facilitate faster pigment extraction to the medium, resulting in a longer exposure time to a higher temperature for vulgaxanthin.
4.2.2 High pressure-assisted extraction
Pressure-based technology such as High Hydrostatic Pressure (HHP) is commonly used for the sterilization and pasteurization of food and beverages due to the lethality of this technology to inactivate microorganisms and enzymes (Al-Ghamdi et al., 2020). The pressure used for this method falls in the range of 100–1,000 MPa. To reduce product contamination, this method uses the action of high pressure to disrupt the cell membranes of microorganisms. This effect can also be utilized to extract bioactive metabolites since cell wall disruption of the product could increase cell permeability, which leads to a high release of these metabolites (Briones-Labarca et al., 2019).
The application of HHP for the extraction process was conducted by Briones-Labarca et al. (2019), who evaluated the changes in lycopene concentrations in tomato pulp during the HHP extraction. The optimum processing conditions were achieved by applying pressure of 450 MPa and 60% hexane concentrations. This study reported that lycopene and flavonoid content increased as the pressure and solvent concentration increased.
A study comparing the effect of HHP and conventional heating method on the betanin content of the Djulis plant (Chenopodium formosanum Koidz.) stated that higher levels of betanin were observed in the HHP-treated samples, ranging from 66.60 to 236.12 mg/100 g (Sun et al., 2019). This study also evaluated the effect of temperature during the HHP treatment using two different temperatures of 10°C and 30°C. The study demonstrated that the content of betanin pigment showed better retention of betanin at lower temperatures. The highest betanin content was obtained in the following treatment parameters: 500 MPa, 10°C, and 3 min. Although HHP treatment showed better retention of betanin content, this method caused significant degradation of betanin compared to the untreated sample (88.52 and 252.68 mg/100 g).
Based on previous research, temperature negatively affects pigment extraction by HHP. In addition, applying high pressure could increase the product temperature due to adiabatic heating, which could further damage the thermo-labile metabolite (Al-Ghamdi et al., 2020). Therefore, evaluating the cumulative impact resulting from the combination of high pressure and temperature during HHP processing is essential.
4.2.3 Negative pressure cavitation
The main principle of Negative Pressure Cavitation (NPC) is inducing nitrogen into the extraction chamber by decreasing the pressure inside (negative pressure). This process produced air bubbles and simultaneously generated rapid turbulence between the solid-liquid-gas inside the system. The cavitation and turbulence effect further leads to cell wall disintegration allowing faster mass transfer of metabolites into the solvent (Wang et al., 2020).
The NPC method is often coupled with other extraction methods. Recent studies (2018–2022) primarily reported the utilization of NPC to extract total phenolic and flavonoids. This technique mainly assisted with applying “green solvent” to enhance the extraction yield and reduce the use of toxic substances. Table 1 lists several studies that evaluated the extraction of bioactive metabolites assisted with the NPC method.
Regarding the Negative Pressure Cavitation-Ultrasound Assisted Extraction (NPC-UAE) method, the extraction yield of bioactive metabolites increased following the increase in negative pressure, ultrasonic power, temperature, time, and solid/liquid ratio. However, after reaching specific conditions, an increase in these parameters caused the metabolites to degrade (Wang et al., 2020; Wang et al., 2018). The negative pressure applied in the extraction of flavonoids is the critical parameter in enhancing the amount of hyperin, hibifolin, isoquercetin, myricetin, quercetin-3′-O-glucoside, quercetin and rutin (Cui et al., 2020).
A study that compared three different extraction methods (NPC, microwave, and ultrasound) found that the highest extraction kinetic (k value) was obtained using the NPC method. The k value of NPC was significantly higher (0.338 min-1) compared to microwave (0.247 min-1) and ultrasound (0.220 min-1) (Cui et al., 2020). The higher k value in the NPC method indicates that the reaction reaches an equilibrium state faster than other methods, resulting in a greater extraction yield in a shorter time.
The ability of the NPC method to increase the extraction yield of phenolic and flavonoids implies that this method could also be used to extract natural pigment from the plant. The important thing to underline is determining the optimum conditions for different pigment types since each metabolite could behave differently under extraction parameters.
4.3 Supercritical fluid extraction
Selectivity is another essential aspect in extracting natural pigment to improve the purity of the metabolite, increase the concentration of targeted metabolites, or avoid undesired metabolites. These objectives can be achieved using Supercritical Fluid Extraction (SFE) (Lefebvre et al., 2021). Supercritical fluid has low viscosity and relatively high diffusivity. By altering the pressure and temperature, these characteristics are easily modified to the desired solubility therefore allowing the extraction process to aim for a specific metabolite. The high diffusivity of supercritical fluid increases the mass transport of the metabolite to the solvent (Al Jitan et al., 2018).
Carbon dioxide (CO2) is the prevalent extraction supercritical fluid. Another advantage of SFE is that this fluid is easily mixed with other solvents to obtain a modifier that allows selective extraction. For instance, the extraction of pigment from Rosemary using SFE reported that rich carotenoid extract could be obtained using pure CO2 at 25°C and the pressure of 20 MPa. In contrast, extraction of chlorophylls reached the optimum condition by using 30% ethanol as a modifier (Lefebvre et al., 2021). Extraction of carotenoid and chlorophylls from Nannochloropsis salina D.J.Hibberd. microalgae also reported similar conditions where CO2 would result in better carotenoid retention. At the same time, ethanol co-solvent increased both recoveries of chlorophylls and carotenoids by 67.6% and 12%, respectively (Mouahid et al., 2020).
Pressure plays a significant role in the optimization of the SFE method. The extraction of pigments from microalgae species, Nannochloropsis maritima D.J.Hibberd., showed that the effect of pressure during SFE treatment varies among pigments. Under 200 bar, pressure significantly affects total carotenoids’ recovery but is insignificant to chlorophylls. Meanwhile, above 200 bar, a linear correlation between pressure and both pigment’s recovery was observed (Mouahid et al., 2020). In addition, pressure could also affect the retention time needed to extract polyphenols. Lefebvre et al. (2021) reported that the extraction time of rosmarinic acid was reduced to 5.1 min from the initial time of 7.5 min by increasing the pressure from 10 to 20 MPa. In this case, increasing the pressure resulted in increased solubility of the modifier (10% EtOH:Water), allowing faster extraction.
5 Biotransformation of natural pigments
Biotransformation encompasses two main concepts: bioavailability and bioaccessibility. Before nutraceuticals are absorbed in the intestine, they must undergo several processes to reach enterocytes. This process is referred to as bioaccessibility (Toti et al., 2018). The bioavailability of natural pigments is an important aspect that requires further study, as pharmacokinetic and pharmacodynamic data on natural pigment metabolites remain very limited. Additionally, natural pigments can provide biological effects at low concentrations (González-Ponce et al., 2018).
Betalains are glycosylated flavonoids derived from a limited number of sources, including amaranth, pitaya, red beet, cactus pear, Swiss chard, and some tubers. These natural pigments have low bioavailability, typically around 1% of the amount consumed. They can be absorbed into the systemic circulation without undergoing hydrolysis in the small intestine and reach peak plasma levels within three hours. Urinary excretion reveals several newly formed metabolites whose biological activities are still unknown (González-Ponce et al., 2018).
The bioavailability and bioaccessibility of chlorophyll are influenced by two factors: the structure and the matrix of the chlorophyll (Chen and Roca, 2018). Isolated chlorophyll extracts exhibit the highest bioaccessibility compared to wet ultrasonicated biomass and whole dried biomass, particularly when ingested from Scenedesmus obliquus. The uptake of chlorophyll derivatives, such as hydroxypheophytin a, pheophytin a’, pheophytin a, and pheophytin b, from aqueous micellar fractions in Caco-2 cells showed a dominant uptake of up to 92%. Caco-2 cells are a human epithelial cell line widely used as a model for the intestinal epithelial barrier (Lea, 2015). For effective intestinal absorption, combining chlorophyll with micelles in an aqueous phase is necessary (Chen and Roca, 2018).
Carotenoids are found in human foods and can be detected in blood (plasma or serum) after ingestion (Maoka, 2020). Due to their fat solubility, differences in adiposity between men and women may also affect the bioavailability of carotenoids (Hayhoe et al., 2017). Additionally, the type of carotenoid can influence its bioavailability; for example, the bioavailability of most trans-β-carotene is greater than that of the cis form, while lycopene is better absorbed in its cis form than in trans (Priyadarshani, 2017). Daily consumption of paprika for 3 months demonstrated varying bioavailability of xanthine metabolites: β-cryptoxanthin accumulated at higher levels than zeaxanthin, while capsanthin was not detectable in plasma. The absorption of capsanthin was very slow, indicating a need for further study regarding its dosage, absorption, and accumulation in plasma (Umigai et al., 2018).
Anthocyanins are rapidly metabolized and easily oxidized, which is why they are poorly bioavailable. Anthocyanins found in Rosella flowers exhibited better stability at 70°C and in an acidic environment compared to weakly acidic conditions. The degradation of Rosella anthocyanins follows a first-order kinetic reaction, with greater degradation occurring in weakly acidic environments and at temperatures above 70°C (Wu et al., 2018). In the small intestine, anthocyanins are hydrolyzed to anthocyanidins, the aglycone forms of anthocyanins, producing the aldehyde metabolite phloroglucinolaldehyde and a phenolic acid after being metabolized by gut microflora, without breaking the B-ring of anthocyanins (Winter and Bickford, 2019).
The intestinal microflora generally digests glycosylated anthocyanins, but interactions with macromolecules in the large intestine can also degrade some of these compounds (Li et al., 2019). Cyanidin-3-rutinoside, cyanidin-3-glucoside, and delphinidin-3-rutinoside are anthocyanin metabolites that differ in their metabolic processes. When placed in simulated large intestine conditions, the three metabolites obtained from mulberry fruit were monitored for changes and products of bacterial-dependent metabolism. Cyanidin-3-glucoside disappeared after six hours, producing metabolites such as protocatechuic acid, p-coumaric acid, vanillic acid, and 2,4,6-trihydroxybenzaldehyde, similar to cyanidin-3-rutinoside, but with a longer metabolism time of eight hours. Meanwhile, delphinidin-3-rutinoside produced metabolites such as syringic acid, 2,4,6-trihydroxybenzaldehyde, and gallic acid after eight hours. The metabolism of different anthocyanin metabolites can yield various products, each of which may have distinct biological effects on humans (Chen Y. et al., 2017).
6 Roles of natural pigments in health
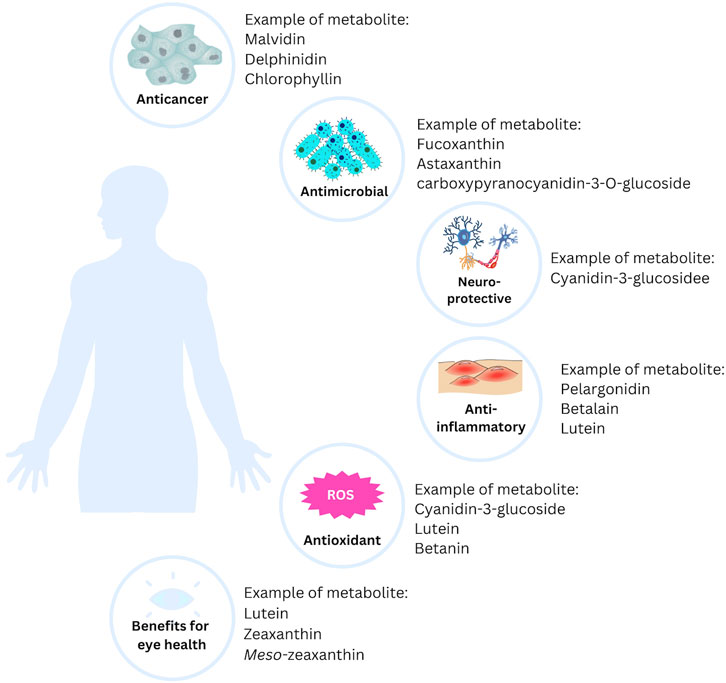
Most of the natural pigments found in fruits, vegetables, and microorganisms can be beneficial to health, particularly in the treatment of chronic and degenerative diseases. Free radicals, one of the targets in treating various diseases, can be kept under control with natural pigments. Additionally, long-term use of natural pigments is generally safe and, therefore, more acceptable as a treatment option (Leong et al., 2017). Here are some of the benefits of natural pigments in health (Figure 3).
6.1 Anticancer activity
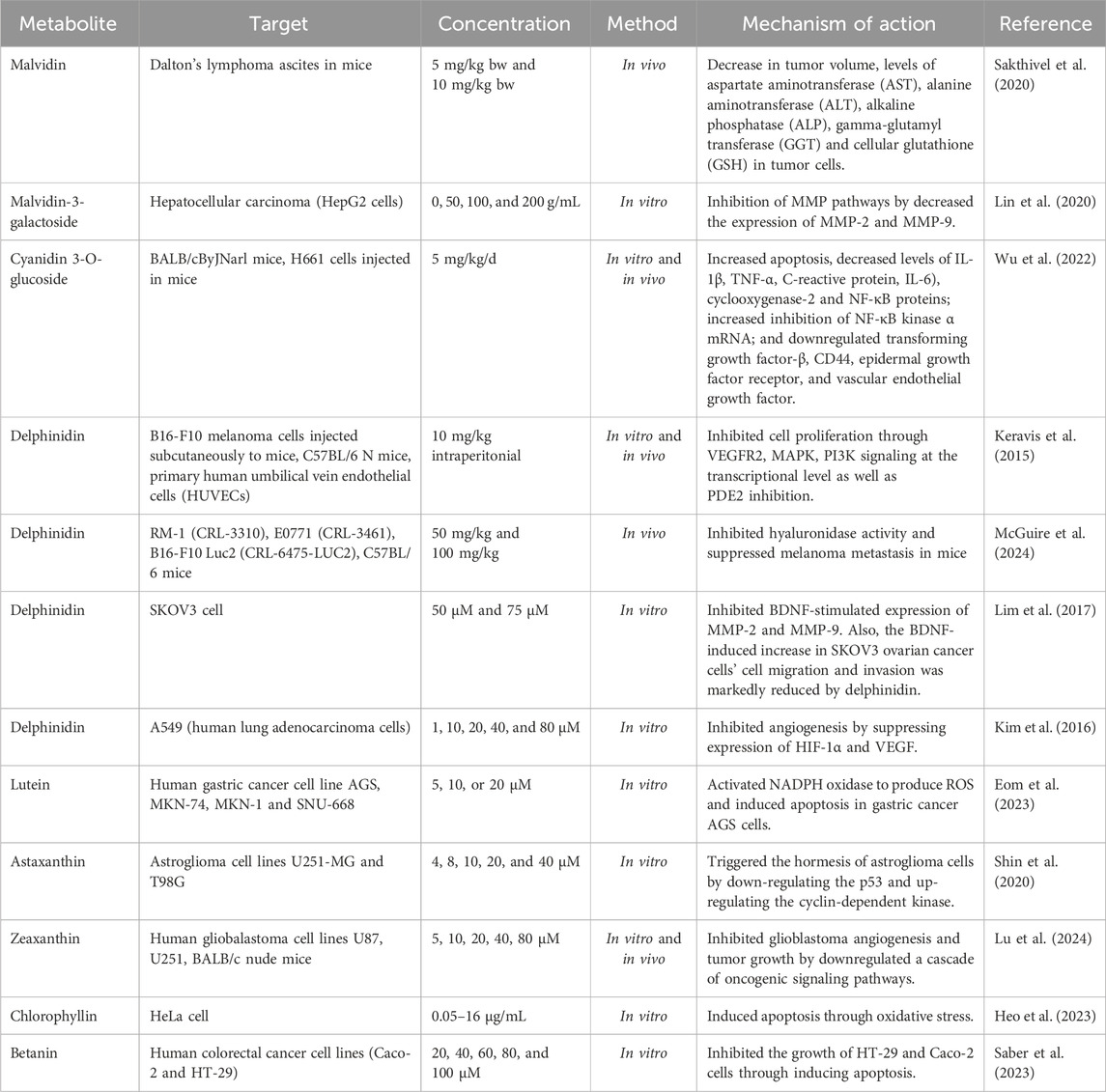
The anticancer potentials of natural pigments have been reported extensively in recent years (Table 2). Lu et al. (2024) studied the effect of zeaxanthin on the adhesion, proliferation, invasion, and migration of human glioblastoma (GBM) cell lines. They found that zeaxanthin may prevent GBM angiogenesis and tumor growth by downregulating a series of oncogenic signaling pathways. Zeaxanthin inhibited the activation of the VEGFR2 kinase pathway produced by VEGF and reduced the expression of p-AKT, p-ERK, p-STAT3, and FAK in U251 cells (Lu et al., 2024). Similarly, Shin et al. (2020) studied the ability of astaxanthin (AXT) as a novel anticancer agent. They reported that low doses (4–8 μM) of AXT decreased the expression of the tumor protein p53 and increased the levels of cyclin-dependent kinase (CDK) 2 and p-Cdk2/3 in the astroglioma cell lines U251-MG (Shin et al., 2020).
Furthermore, it has been reported by Kim et al. (2016) that delphinidin exhibits anticancer activity against A549 lung cancer cells. When 80 µM delphinidin was administered, EGF-induced angiogenesis was almost entirely inhibited and significantly decreased. Additionally, the EGF-induced rise in hemoglobin content was dose-dependently decreased by delphinidin (Kim et al., 2016).
Some natural pigments besides having anticancer activity can also function as co-treatments that can increase the effectiveness of anticancer drugs and/or reduce their side effects. For example, astaxanthin at a concentration of 5 μg/mL can increase MCF-7 breast cancer cell proliferation by 14%, while concentrations of 10 and 15 μg/mL can inhibit cell proliferation by 1% and 19%, respectively. However, when astaxanthin 5 μg/mL was combined with carbendazim at doses of 15 and 30 μM, the antiproliferative effect was increased by 8% and 16%, respectively (Atalay et al., 2019). The search for new anticancer metabolites also targets mortalin, a protein that can bind to p53 and prevent p53 from entering the nucleus. This causes inhibition of cell cycle arrest and apoptosis in cancer cells. Fucoxanthin has been reported to suppress mortalin transcription. In addition, fucoxanthin causes a decrease in cell proliferation, cell metastasis, and survival of cancer cells but is safe for normal cells (Garg et al., 2019). Fucoxanthin can also be an adjuvant therapy with doxorubicin in breast cancer. The combination of 1 µM of doxorubicin and 10 µM of fucoxanthin against MDA-MB-231 cells could significantly increase the number of apoptotic cells and decrease cell proliferation (Malhão et al., 2021). Bacterially produced bio-pigments mostly have medicinal properties. Astaxanthin from Pontibacter kolensis sp. nov. inhibits the growth of the MCF-7 cell line (Pachaiyappan et al., 2021).
Anthocyanins can trigger apoptosis and inhibit the proliferation of HT29 cells in human colon cancer. In a mouse colon tumor model, anthocyanins can reduce PI3K protein expression, increasing Bcl-2/Bax- and caspase-dependent apoptotic pathways and decreasing colorectal cancer growth (Zhao X. et al., 2019).
BIU87 cell growth in bladder cancer can be inhibited at a rate of 36.28% with anthocyanins obtained from purple sweet potato at a concentration of 800 μg/mL through an apoptotic mechanism. Apoptosis increases with increasing concentration, which indicates that the anticancer activity of purple sweet potato anthocyanins is dose-dependent (Li et al., 2018). The substituent on B-rings of anthocyanins affects their anticancer activity. Orto-dihydroxy phenyl on B-rings of anthocyanins can inhibit the growth and metastasis of cancer cells starting with the inhibition of inflammation by suppression of COX-2 and iNOS and expression via the PI3K/Akt andNF-κB pathway at the initial stage. Anthocyanins with orto-dihydroxyphenyl on B-rings also can regulate the expression of cancer-associated genes, thereby triggering cell cycle arrest and DNA repair by inhibiting RTK activity and playing a role in MAPK and AP-1 pathways. This mechanism occurs at the formation stage. Anthocyanins mediated by ROS and JNK/p38-MAPK can activate caspases resulting in cancer cell apoptosis at the development stage (Lin et al., 2017).
Some cancer proteins such as IGF-1R kinase proteins, CDK-2, and CDK-6 bind to anthocyanidins, namely petunidin, peonidin, malvidin, pelargonidin, delphinidin, and cyanidin, thus anthocyanidins are promising metabolites for the development of cancer drugs (Sravani et al., 2021).
Crocin and crocetin, carotenoids from Iranian saffron, inhibit SOD activity by scavenging superoxide radicals and affecting the copper-binding site, respectively. This inhibition was observed in breast cancer cells using docking analysis (Hashemi et al., 2020). Crocin and crocetin also have antimetastatic effects on 4T1 cells, triple negative metastatic breast cancer cells, through inhibition of cell mobility, migration, and invasion, also reduce adhesion to the extracellular matrix (Arzi et al., 2018).
6.2 Antioxidant activity
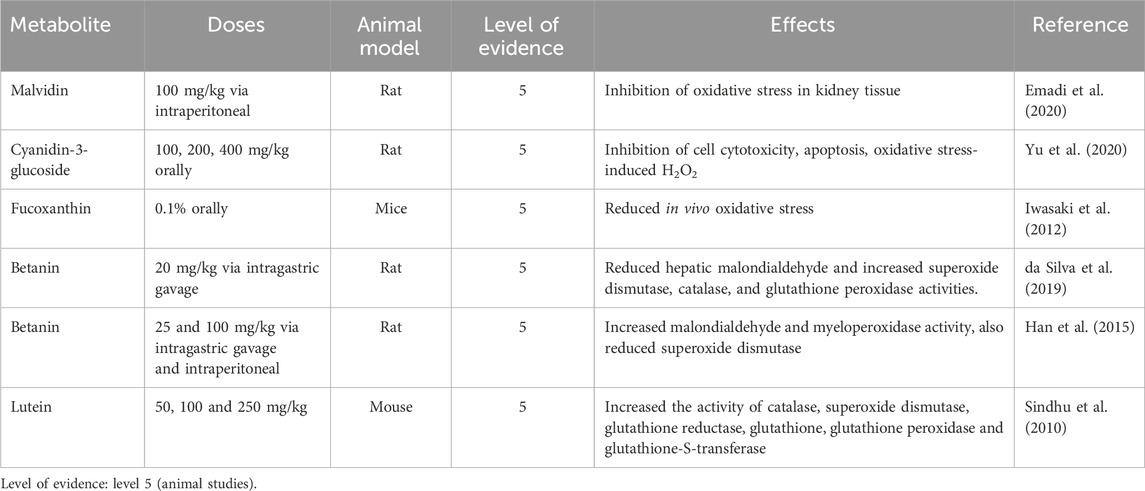
Recent decades have demonstrated that natural pigments possess significant antioxidant properties (Lu et al., 2021). Chlorophyllin, is a water-soluble salt containing copper and sodium that is a homologue of the common green pigment chlorophyll and has been reported to exert an antioxidant effect. Chlorophyllin boosted the activities of antioxidant enzymes (glutathione reductase, glutathione peroxidase, and glucose-6-phosphate dehydrogenase) (Ozcan et al., 2021). Moreover, it has been reported by Yu et al. (2020) that cyanidin-3-glucoside (C3G) has been shown to have a hepatoprotective impact for liver damage caused by oxidative stress because of its antioxidant effect. C3G increases the activity of antioxidant enzymes and upregulating the Nrf2-antioxidant pathway (Yu et al., 2020).
The role of carotenoids in disease cannot be separated from their antioxidant abilities (Rodriguez-Amaya, 2019). Lycopene is a carotenoid that has the highest antioxidant activity compared to other carotenoids (Costa-Rodrigues et al., 2018). Astaxanthin which is a C-C double chain conjugated with olefins can bind ROS and free radicals (Zhao T. et al., 2019) with a mechanism of action to form epoxides, the same as -Carotene and Zeaxanthin (Nishino et al., 2017). Fucoxanthin from Phaeodactylum tricornutum Bohlin, in a dose-dependent manner, can increase the ratio of reduced to oxidized glutathione in HeLa cells by up to 3.3 times (Neumann et al., 2019).
Anthocyanins have potential for treating neurodegenerative diseases because they can modulate various aspects of the disease, particularly the antioxidant pathway (Winter and Bickford, 2019). Chlorogenic acid and cyanidin are the most common anthocyanins found in mahonia fruit extracts, which exhibit antioxidant activity that is twofold higher than that of the phenolic fraction (Coklar and Akbulut, 2017). The ethanol extract of blueberry contains delphinidin, petunidin, cyanidin, peonidin, and malvidin, which are believed to contribute to the antioxidant activity of the extract (Zhou et al., 2019). The anthocyanin content of black chokeberry is 930 mg/g dry weight and consists of four primary anthocyanins: cyanidin-3-O-galactoside, cyanidin-3-O-xyloside, cyanidin-3-O-glucoside, and cyanidin-3-O-arabinoside (Meng et al., 2019). Summarized in vivo studies on the antioxidant activity of natural pigments are presented in Table 3.
6.3 Anti-inflammatory activity
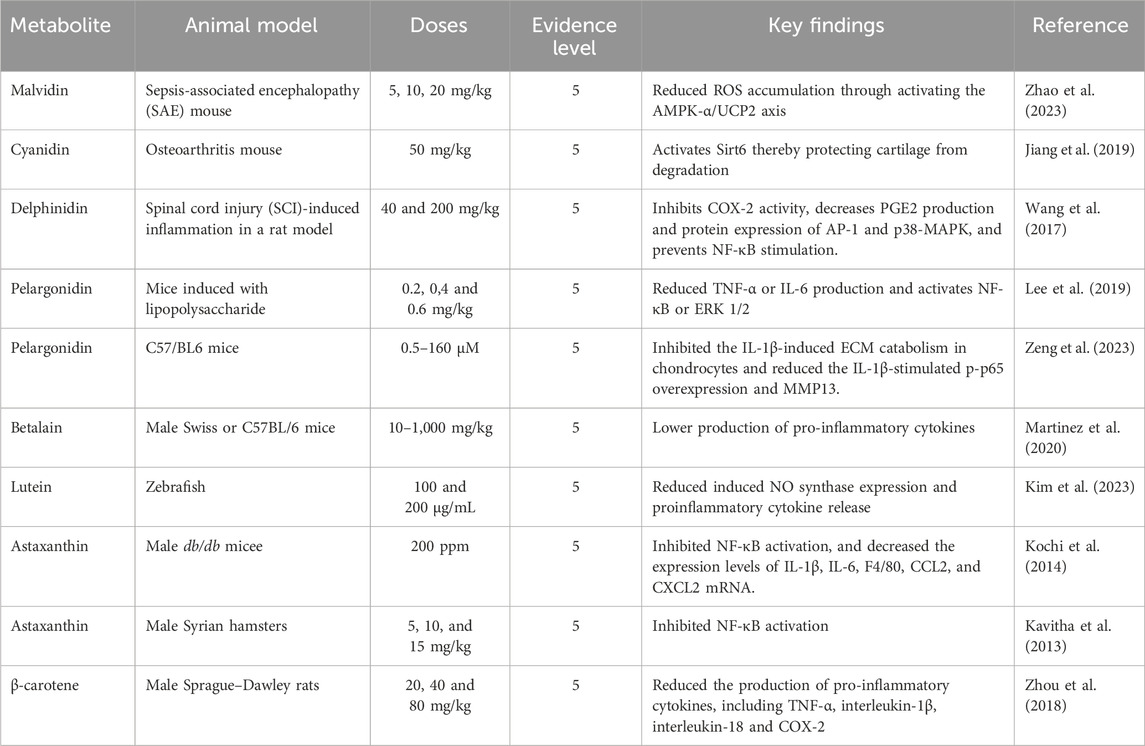
The anti-inflammatory activity of anthocyanins was obtained through inhibiting the activation of NF-κB signalling pathway (Duarte et al., 2018; Mirza et al., 2019; Nguyen et al., 2018), decreased serum levels of IFN-γ (Ouyang & O’Garra, 2019), NO (Duarte et al., 2018; Winter et al., 2017), TNF-α, the expression of TLR4 (Cui et al., 2018), and iNOS (Duarte et al., 2018), inhibited MAPKs signaling pathway (Zhang et al., 2020) and reduce the production of ROS (González-Ponce et al., 2018).
The anti-inflammatory of anthocyanins from Dioscorea alata L. tubers was investigated using a mouse model with inflammatory bowel disease. The disease activity index reduces significantly compared to negative control with 80 μg/kg body weight of anthocyanins fraction. The responses, such as body weight, fecal consistency, and fecal occult blood, were similar to the positive control, 5-aminosalicylic acid. The anthocyanins fraction can downregulate the pro-inflammatory cytokines such as TNF-α, IFN-γ, MPO, and iNOS (Chen T. et al., 2017). The main anthocyanin in strawberry fruit, namely pelargonidin-3-O-glucoside, can modulate IL-10 production at a dose of 0.08 mol/L (Amini et al., 2017). Anthocyanins extract from sour cherry fruit (Prunus cerasus L.) at a dose of 50 μM can decrease IL-6 and IL-8 in the inflammation model using the Caco-2 cell line (Nguyen et al., 2018). In addition, anthocyanins from red clover (Trifolium pratense L.) in a dose-dependent manner can inhibit macrophage cells from secreting TNF-α to RAW 264.7 cells (Lee et al., 2020).
Lycopene not only has the highest antioxidant activity among other carotenoids, but it also has anti-inflammatory activity (Thies et al., 2017) through production inhibition of pro-inflammatory cytokines (IL-6β, TNF-α, and IL-1β) (Liu et al., 2018). Bixin, a derivative carotenoid, from Bixa orellana L. seeds, can reduce edema and increase heat resistance on the paw of rats induced by 30 mg/kg of carrageenan orally. The anti-inflammatory mechanism of bixin is related to decreased migration of neutrophils (Pacheco et al., 2019). Combining carotenoids and other compounds can increase their antioxidant and anti-inflammatory activity. For example, 5 µM of fucoxanthin and rosmarinic can decrease apoptosis and ROS production, and downregulate the production of interleukin (IL)-1β, NLRP3, Caspase-1, and ASC. In addition, the pre-treated with the combination can increase the expression of antioxidant genes (HO-1 and Nrf2) in HaCat cells exposed to UV-B (Rodriguez-Luna et al., 2019). Table 4 illustrates the anti-inflammatory activity of natural pigments through in vivo evaluation.
6.4 Antimicrobial activity
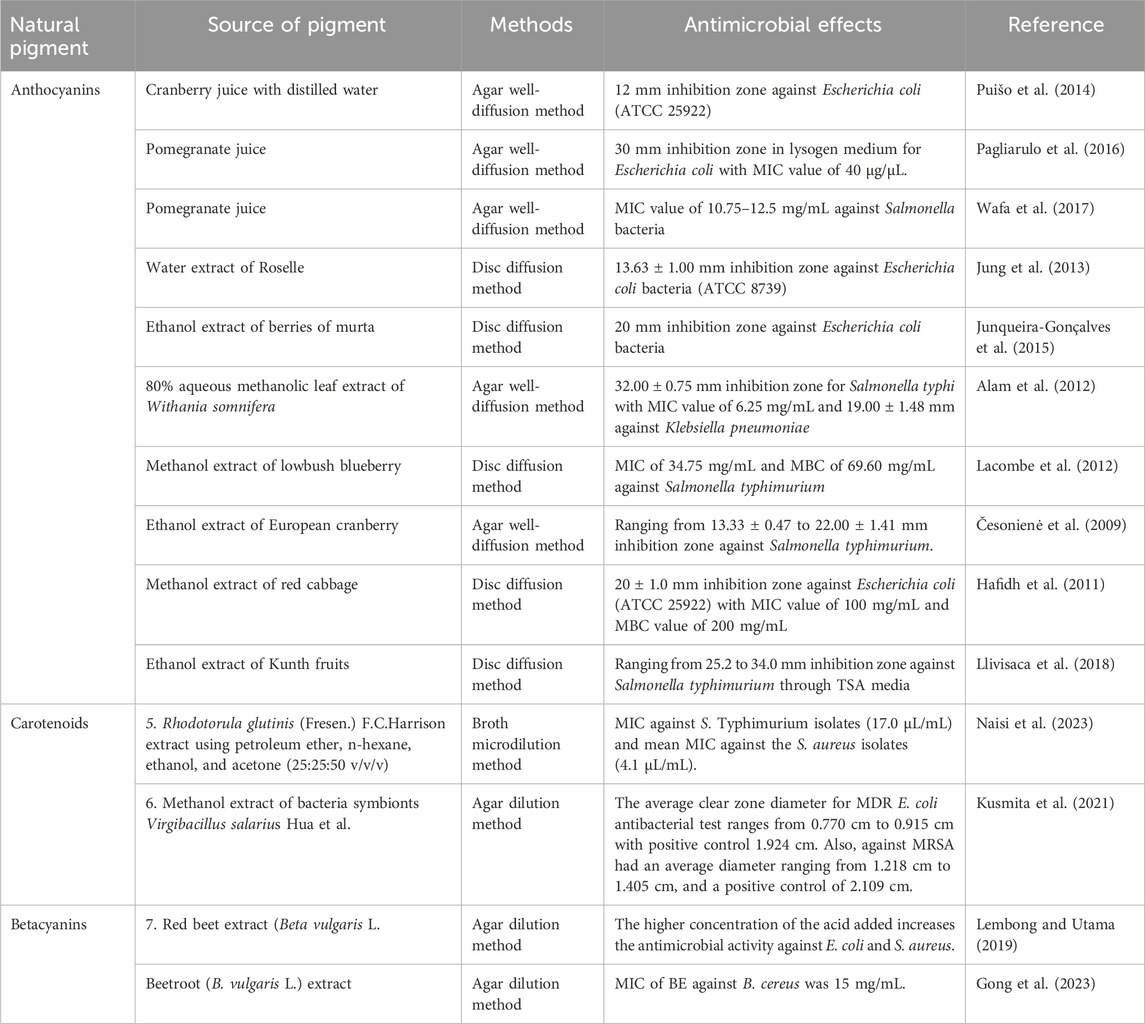
There has been a significant increase in interest in discovering new antibacterial substances, particularly those derived from natural sources, such as plants and marine ecosystems (seaweeds, microalgae, and crustaceans) (Gomes et al., 2022). Seaweeds contain a wide array of pigment compositions that provide several secondary metabolites, including carotenoids, chlorophylls, and phycobiliproteins (PBPs). Additionally, the pigments in seaweeds also include phenolic metabolites, which are responsible for antibacterial activity. A metabolite consisting of phlorotannins, which have a low molecular weight and are extracted from the brown seaweed Sargassum thunbergii, caused Vibrio parahaemolyticus to lose the integrity of its cell walls and membrane (Besednova et al., 2020). By altering and destroying the cell membrane, Fucus vesiculosus L. [Fucaceae, Fucus], a brown seaweed, produced oligomeric phlorotannins (phloroglucinol) that had bacteriostatic effects on pathogenic Streptococcus aureus and S. pneumoniae (Bogolitsyn et al., 2019; Gomes et al., 2022). Research has indicated that carotenoids, particularly fucoxanthin, a pigment found in seaweeds, are effective against both Gram-negative and Gram-positive bacteria. The findings showed that carotenoids hindered various bacteria’s ability to thrive under aerobic conditions. Fucoxanthin was effective in this study against some Gram-positive bacteria, such as S. aureus, S. agalactiae, and S. epidermidis, but ineffective against Gram-negative bacteria, such as Klebsiella oxytoca, Escherichia coli, and K. pneumoniae (Karpiński and Adamczak, 2019).
Anthocyanins, another group of natural pigments, also exhibit antibacterial effects. An earlier study found that anthocyanins had an antibacterial effect on S. aureus, E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis, which are resistant to vancomycin, with minimum inhibitory concentration (MIC) and maximum tolerated concentration (MTC) of 31.07 and 7.76 mg phenol/well, respectively (Côté et al., 2011). Moreover, anthocyanins demonstrated the strongest sensitivity to both Listeria innocua and Aeromonas hydrophilia, with MIC values of 50 and 40 mg/mL, respectively (Genskowsky et al., 2016).
Fucoxanthin, a natural xanthophyll pigment belonging to the carotenoid family, frequently acts against aerobic bacteria at low concentrations (10–250 μg/mL) (Karpiński et al., 2021). Rajauria and Abu-Ghannam (2013) reported that fucoxanthin also acts against Listeria monocytogenes (inhibition zone: 10.27 mm) at a concentration of 25 µg compound/disc. In a different study, Peraman and Nachimuthu (2019) found that the MIC values of fucoxanthin against fungi (Aspergillus brasiliensis, A. fumigatus, and Candida albicans) as well as bacteria were between 1,000 and 4,000 μg/mL. Furthermore, Šudomová et al. (2019) demonstrated that fucoxanthin showed very low MIC values against Mycobacterium tuberculosis (2.8–4.1 µM, or 1.85–2.7 μg/mL).
Similarly Contreras-Ortiz et al. (2017), evaluated the antimicrobial effect of astaxanthin. They found that the viability of Trypanosoma cruzi was reduced in an in vitro study with astaxanthin dosages of 200–300 μg/mL. In another study, Shanmugapriya et al. (2018) demonstrated that astaxanthin can act against a variety of bacteria when formulated as a nanoemulsion. The minimum inhibitory concentration (MIC) values for both Gram-positive and Gram-negative species ranged from 500 to 4,000 μg/mL.
Raspberry, blueberry, strawberry, and blackcurrant extracts are examples of anthocyanin-rich extracts that have shown efficacy against Gram-negative bacteria, but not Gram-positive bacteria. This finding could be explained by the differences in cell wall structure between Gram-positive and Gram-negative bacteria. The outer membranes of Gram-negative bacteria protect them from hydrophobic chemicals but not from hydrophilic ones (Mayasari et al., 2021a). The antimicrobial properties of extracts containing anthocyanins may result from a variety of processes and synergistic interactions between the phytochemicals present, including anthocyanins, phenolic acids, weak organic acids, and a mixture of different chemical forms that interact differently with Gram-positive and Gram-negative bacteria (Tarozzi et al., 2007).
Similar to how blackcurrant extract affects Saccharomyces cerevisiae and E. coli (benefiting their growth), but slows the growth of S. aureus and E. faecium (Werlein et al., 2005). Another study found that the anthocyanin metabolite carboxypyranocyanidin-3-O-glucoside prevents P. aeruginosa and S. aureus strains from producing biofilms in persistent wounds (Correia et al., 2021).
Anthocyanins found in red, purple, and blue vegetables and fruits are key biological compounds that help prevent microbial infections through various mechanisms (Gawai et al., 2017). They exhibit antibacterial activity through several processes, such as damaging the morphology of bacterial cells and compromising the integrity of the cell membrane, including the structure of the intracellular matrix and cell wall. Additionally, anthocyanins cause instability in the cytoplasmic membrane, inhibit extracellular microbial enzymes, and lead to the permeabilization of the plasma membrane (Burdulis et al., 2009). The antimicrobial effects of natural pigments from various sources are summarized in Table 5.
6.5 Neuroprotective activity
Anthocyanins are phenolic pigments that are both colored and water-soluble. These pigments are found in glycosylated forms and are responsible for the purple, red, and blue colors in fruits and vegetables. Scientific research has shown that anthocyanins possess antibacterial and neuroprotective properties, promote neurological health, support vision, and guard against some non-communicable diseases (Gawai et al., 2017). The therapeutic effects of anthocyanins in treating neurodegenerative conditions, such as Parkinson’s disease, include their anti-apoptotic, anti-neuroinflammatory, and antioxidant capabilities (Patek-Mohd et al., 2018).
Both in vivo and in vitro studies have been conducted to assess the neuroprotective effects of anthocyanin metabolites. An in vitro study demonstrated that cyanidin-3-glucoside and cyanidin have neuroprotective properties against oxidative stress induced by hydrogen peroxide in a human neuronal cell line (SH-SY5Y). The results showed that pre-treatment with cyanidin-3-glucoside and 100 µM cyanidin significantly enhanced antioxidant activity in both the cytosolic fraction and membrane of SH-SY5Y cells. Moreover, cyanidin dramatically increased the proportion of active mitochondria and prevented DNA fragmentation caused by hydrogen peroxide (Tarozzi et al., 2007).
Research suggests that anthocyanins may be effective in treating neurodegenerative diseases, such as ischemia, Alzheimer’s disease, Parkinson’s disease, and other forms of nerve damage (Airoldi et al., 2018; Jung and Kim, 2018). Anthocyanins in their glycosylated forms can cross the blood-brain barrier and enter the central nervous system (CNS), where they exert their biological effects. The neuroprotective activity of anthocyanins is linked to the transporter bili translocase, which primarily targets the vascular endothelium and subsequently the brain network (Aqil et al., 2014). Furthermore, the polyphenolic cationic structure of anthocyanins enables them to scavenge free radicals, reduce ROS production, and suggest their potential efficacy in disorders associated with neurodegenerative processes. Anthocyanins support the PI3K/Akt/HO-1 pathway in Alzheimer’s disease and regulate the endogenous antioxidant Nrf2/HO-1 pathway (Ali et al., 2018).
In APP/PS1 transgenic AD mice, anthocyanin (12 mg/kg i. p. over 30 days) significantly improved memory performance. Cyanidin-3-O-glucoside may also inhibit the formation of soluble amyloid-β 25–35 oligomers and associated neurotoxicity in the human nerve cell line SH-SY5Y (Tarozzi et al., 2007). Additionally, when microglial cells are exposed to a blueberry anthocyanin-rich extract, the expression of the inflammatory genes iNOX and COX is reduced (Bensalem et al., 2016). The neuroprotective effects of the natural pigment anthocyanin are summarized in Table 6.
6.6 Benefits for eye health
Age-related macular degeneration (AMD) is a vision impairment linked to aging, causing damage to the macula and central retina, resulting in symptoms like dark spots, distortion, visual field defects, and vision loss (Machida et al., 2020). The retinal macular area contains high levels of xanthophyll carotenoids, including lutein, zeaxanthin, and meso-zeaxanthin (a zeaxanthin isomer). These carotenoids, known as macular pigment, are believed to protect the retina and vision by acting as antioxidants and filtering blue light (Johnson et al., 2020).
However, studies have shown that the levels of carotenoids in the macula decline with age. Additionally, analysis of macular pigment optical density (MPOD) indicates that MPOD also decreases with age, highlighting the importance of increasing carotenoid levels in the macular region for maintaining eye health (Wilson et al., 2021). MPOD measures the concentrations of lutein and zeaxanthin in the macula, expressed in optical density units ranging from 0 to 1 (Kijlstra et al., 2012). To date, several clinical studies have examined the effects of lutein and zeaxanthin supplementation on MPOD (Table 7).
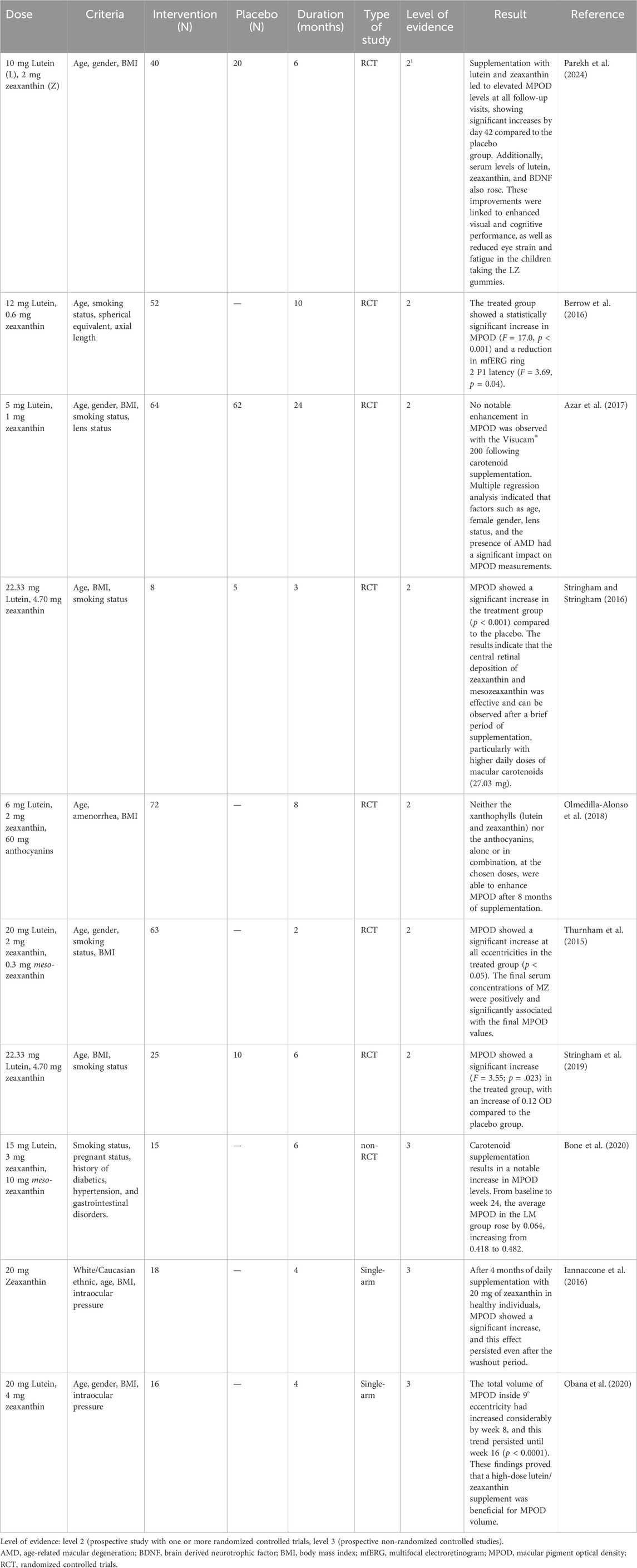
Table 7. Level of the evidence regarding the influence of lutein and zeaxanthin intake on macular pigment optical density (MPOD).
Hammond et al. (2017) investigated the impact of lutein and zeaxanthin supplementation on MPOD. Sixty-two older adults were randomized into two groups: one receiving 10 mg of lutein and 2 mg of zeaxanthin (n = 42), and the other receiving a visually identical placebo (n = 20). Data from 51 participants (average age 73.7 years) were analyzed, revealing a significant increase in MPOD from baseline to the 12-month mark (M = 0.58, SD = 0.23; p < 0.03) in the active supplement group, while the placebo group showed no significant change over the year (Hammond et al., 2017).
In a separate study, Renzi-Hammond et al. (2017) investigated the impact of lutein and zeaxanthin supplementation on cognitive function in younger, healthy adults. They conducted a randomized, double-blind, placebo-controlled trial involving 51 participants aged 18 to 30, who were part of a larger research project on xanthophylls and cognitive performance. The subjects were divided into an active supplement group (n = 37) and a placebo group (n = 14). The findings showed that the supplement, which contained 10 mg of lutein and 2 mg of zeaxanthin, significantly increased MPOD over the year compared to the placebo (p < 0.001). Daily intake of lutein and zeaxanthin, along with the rise in MPOD, was linked to notable improvements in spatial memory (p < 0.04) (Renzi-Hammond et al., 2017).
7 Roles of natural pigments in food industry
The organoleptic properties of food play a significant role in its acceptance, selection, and ultimately consumption by individuals. Color stands out as one of the most visually appealing and attractive features (Novais et al., 2022). Since the 1850 s, synthetic coloring agents have been extensively used because they are easy to manufacture, cost-effective, highly efficient in providing vibrant colors, and require only small quantities (Azman et al., 2018). However, many synthetic colors, particularly those containing aromatic rings and azo functional groups, have been found to pose health risks, including hyperactivity, allergic reactions, and asthma attacks. As a result, there has been a gradual shift from synthetic colorants to naturally derived alternatives, driven by changing consumer attitudes and an increased demand for safer options (Singh et al., 2023). Pigments are materials with a broad range of colors, some of which are water-soluble, and are widely utilized in various industries. The non-toxic properties of pigments generated by many microbes make them suitable for use in dyes, food, medicine, cosmetics, and other industrial applications.
The FDA considers several variables when assessing the safety of a novel pigment or a new use for an existing pigment as an additive in food. These include the immediate and long-term effects of consumption, the substance’s composition and physical characteristics, the manufacturing process, its stability, the likelihood of exposure and consumption, and the accessibility of analytical techniques for determining its purity and concentration in food (FDA, 2023).
An essential goal of the food industry is the production of foods with appealing appearances. Natural food colors are becoming increasingly popular in food preparation, as some artificial color additives have been linked to adverse health effects (Azman et al., 2018). Food colorants are important in the food industry because they may hide undesirable qualities or enhance the inherent qualities of food items. Due to this, they may also be used for various specific purposes depending on their color. Examples include anthocyanins, which are common water-soluble flavonoids with pH-dependent colors ranging from red to blue. These anthocyanins are known for their bioactive properties, such as antioxidant, anti-inflammatory, and chemopreventive effects. Carotenoids, which are predominantly found in fruits and vegetables, are widely prized for their orange, yellow, and red colors, which enhance the flavor of meals and beverages. Betalains are another type of pigment that has emerged as a promising alternative to Red 40, a synthetic colorant known as Allura Red AC, which contains benzidine—a possible carcinogen for both humans and animals (Luzardo-Ocampo et al., 2021).
Purified colorants or natural pigments have been used to enhance the nutritional qualities of bread products without significantly affecting their sensory properties. A study by Abdel-Moemin (2016) found that cupcakes containing Rosella calyces (RC) extract received higher sensory ratings (p < 0.05) than the control cupcakes. Consuming 100 g of the RC cupcakes provided 465 mg of anthocyanins per 100 g (Abdel-Moemin, 2016). Additionally, López et al. (2019) demonstrated that incorporating Arbutus unedo L. extract, rich in cyanidin-3-O-glucoside, improved the color and antioxidant properties of wafers while leaving their nutritional profile largely unaffected (López et al., 2019).
Other natural products from fruit and vegetable waste can also serve as sources of coloring. Lycopene, for example, is extracted from tomato waste and used in cakes and biscuits (Eletr et al., 2017). Lycopene from tomato waste provides 300.85 mg of lycopene and 654.8 mg of total carotenoids per 100 g of fibrous pulp, which is free of peel and seeds.
To counteract any natural color loss that may occur during production and storage, milk and dairy products, like other foods, can be naturally colored. One of the most well-known natural colorants used in yogurt is derived from strawberries (red) and carrot juice (orange), both of which are employed in the yogurt industry to enhance the color and nutritional content of the product (Gawai et al., 2017).
Furthermore, cream cheese coloring agents derived from sea buckthorn fruit extracts (Hippophae rhamnoides L.) have been examined (Ghendov-Moşanu et al., 2020). Natural colorants have also been used in the formulations of other dairy products. For example, Melastoma malabathricum L. (“Senduduk” fruit) can double the amount of beta-carotene in jackfruit jam due to its antioxidant activity. Similarly, a previous study by Mayasari et al. (2021b) showed the antibacterial and antioxidant activity of M. malabathricum L. leaves. Lastly, the diverse applications of natural food pigments highlight their potential to be incorporated into various food systems beyond conventional formulas.
In addition, several recent studies have shown that natural pigments, which offer numerous advantages, have a promising future as they gain popularity and aid in the creation of intelligent packaging solutions (Oladzadabbasabadi et al., 2022). The structural instabilities of natural pigments can change color in different pH ranges, these pigments can be employed as harmless, natural pH indicators with great potential for usage in smart packaging (Ndwandwe et al., 2024). Colorimetric pH indicators are frequently employed in intelligent packaging as active tags to indicate pH changes through color changes (Pereira et al., 2015). Generally, when the food begins to deteriorate, the pH will change. This fact provides the scientific foundation for utilizing pH changes to evaluate food product quality (Pourjavaher et al., 2017).
For example, the betalains from Bougainvillea glabra Choisy were incorporated with potato starch to create a pH indicator film that allowed fish freshness to be tracked in real-time. The film’s color changes from pink to yellow, signifying the fish under observation losing quality (Naghdi et al., 2021). Similarly, the locust bean gum (LBG) film containing 5 wt% of the extracted anthocyanin from Viola odorata L. can be introduced as a smart packaging system to monitor the freshness of meat. The film showed color change from beige to light indigo after 6 days of storage at 4°C (Fathi et al., 2022). Table 8 shows the potential of natural pigments to be used in intelligent packaging as pH-sensitive indicators to monitor the freshness of food products.
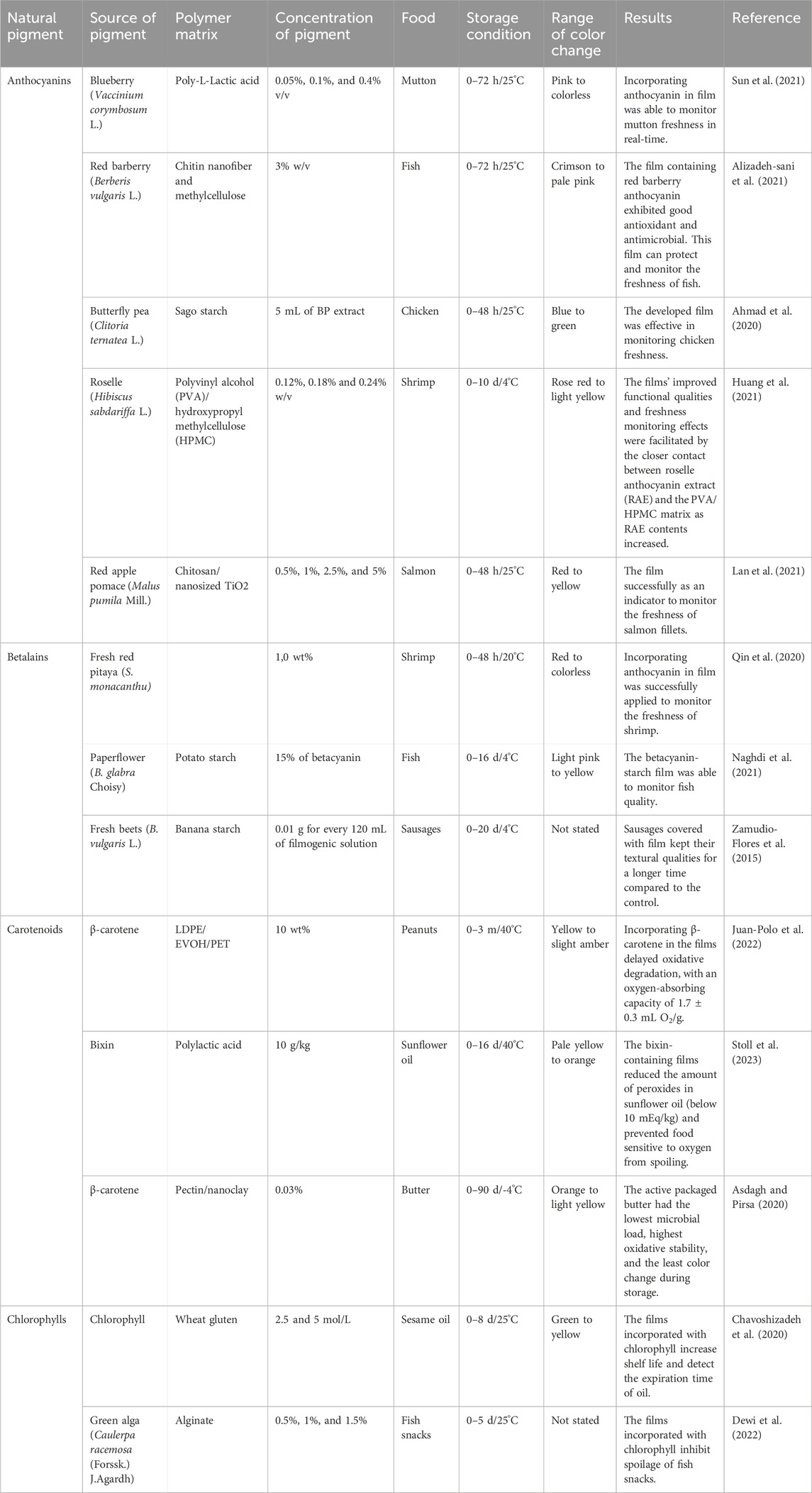
Table 8. The potential of natural pigments to be used in intelligent packaging as pH-sensitive indicators to monitor the freshness of food products.
8 Technologies for improving pigment stability in food
Natural pigments have inferior stability compared to manufactured pigments due to their tendency to degrade in color quality. Understanding the physicochemical properties of pigments and their interactions with the food matrix is essential. Further studies are required to stabilize most of these pigments under varying temperature conditions and the typical pH range found in targeted food systems. It is crucial to implement technologies that enhance the stability of pigments in food. Chemical and environmental variables, including oxygen, pH, metal ions, light exposure, enzyme presence, and elevated temperatures, predominantly affect pigment stability (Cortez et al., 2017; Indrasti et al., 2018; Juric et al., 2020).
Adding co-pigment substances such as metals (Viera, Pérez-Gálvez and Roca, 2019), phenolic metabolites (Erşan et al., 2022), and polymers (de Boer et al., 2019) has been demonstrated as effective for enhancing and stabilizing natural pigments. For example, zinc and copper ions facilitate the synthesis of green metallochlorophylls in plants, leading to the re-greening of food products. However, the FDA’s maximum zinc concentration threshold of 75 ppm has proven ineffective. Encapsulation of zinc-chlorophylls using matrices such as gum arabic, maltodextrin, octenyl succinic anhydride (OSA)-modified starch, or whey proteins, along with the microencapsulation of raw chlorophyll extracts without salt treatment, is feasible (Viera, Pérez-Gálvez and Roca, 2019). Strawberry model solutions containing anthocyanins were treated with rooibos phenolics to improve their color and thermal stability, suggesting that rooibos could be used as a food ingredient to enhance the appearance of strawberries (Erşan et al., 2022). Biopolymers protect natural colorants from environmental factors such as high temperatures, oxidation, and photodegradation. Antioxidants and stabilizers are also encapsulated within these biopolymers, further increasing their stability (de Boer et al., 2019).
In addition to maintaining stability, nano-formulations and microencapsulation can increase solubility and facilitate the distribution of pigments into food matrices. Microencapsulation refers to the process of enclosing solids, gases, or liquids within sealed capsules ranging in size from millimeters to nanometers (Sen et al., 2019). Pigments that are susceptible to high temperatures should be encapsulated using freeze-drying or lyophilization. The absence of air and the low temperature in this process produce pigments that are resistant to oxidation and chemical changes (Enaru et al., 2021).
9 Conclusion and future remarks
This review enhances our knowledge of natural pigments, their pharmaceutical prospects, and their potential benefits in the food industry. The classification of natural pigments, extraction methods, biotransformation, pharmacological activities, and specific practical application cases in the food industry are systematically summarized. Innovative extraction technologies, such as thermal technology, non-thermal technology, and supercritical fluid extraction, offer promising ways to increase the yield and bioavailability of natural pigments.
Natural pigments have wide pharmaceutical and medical prospects, including anticancer, antioxidant, anti-inflammatory, antimicrobial, and neuroprotective effects, as well as benefits for eye health. Nevertheless, much remains unknown about their pharmacokinetic characteristics. Therefore, to determine the presence of active metabolites, comprehensive pharmacokinetic investigations of natural pigments should be conducted. The identification of these metabolites could yield important details about the pharmacological processes and bioactive forms of natural pigments.
Furthermore, these pigments are extensively utilized in the food industry as food additives to enhance the taste or impart color to food products. They also play a role in intelligent food packaging to monitor the freshness of food products. Due to their potential, increasing interest in natural pigments has been reported in the last decade.
However, there are still many gaps in the field of natural pigments. For example, emerging technologies for natural pigment extraction need to be improved for physicochemical feasibility, stability, and the ability to source these pigments from both traditional and novel origins. Research on natural pigments remains limited, especially regarding the mechanisms of action of their metabolites and their antimicrobial and neuroprotective effects. Future research may shift its focus to this area.
This discrepancy emphasizes the need for more thorough and rigorous research to increase the potential efficacy of natural pigments in disease management. Additionally, further research and development of practical and affordable methods are needed to scale up smart packaging technology based on natural pigments.
Author contributions
AM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. GH: Investigation, Methodology, Writing–original draft, Writing–review and editing. AA: Investigation, Methodology, Writing–original draft, Writing–review and editing. FF: Investigation, Methodology, Writing–original draft, Writing–review and editing. DM: Investigation, Methodology, Writing–original draft, Writing–review and editing. AH: Methodology, Validation, Investigation, Writing–review and editing. IN: Investigation, Methodology, Writing–original draft, Writing–review and editing. FN: Conceptualization, Methodology, Project administration, Writing–original draft, Writing–review and editing. TK: Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Japan Society for the Promotion of Science (24K21964 to TK).
Acknowledgments
We are grateful to National Research and Innovation Agency (BRIN), Hasanuddin University, and Kanazawa University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Moemin, A. R. (2016). Effect of Roselle calyces extract on the chemical and sensory properties of functional cupcakes. Food Sci. Hum. Wellness 5, 230–237. doi:10.1016/j.fshw.2016.07.003
Ahmad, A. N., Abdullah Lim, S., and Navaranjan, N. (2020). Development of sago (Metroxylon sagu)-based colorimetric indicator incorporated with butterfly pea (Clitoria ternatea) anthocyanin for intelligent food packaging. J. Food Saf. 40. doi:10.1111/jfs.12807
Airoldi, C., La Ferla, B., D`Orazio, G., Ciaramelli, C., and Palmioli, A. (2018). Flavonoids in the treatment of alzheimer’s and other neurodegenerative diseases. Curr. Med. Chem. 25, 3228–3246. doi:10.2174/0929867325666180209132125
Alam, N., Hossain, M., Mottalib, M. A., Sulaiman, S. A., Gan, S. H., and Khalil, M. I. (2012). Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement. Altern. Med. 12, 175. doi:10.1186/1472-6882-12-175
Al-Ghamdi, S., Sonar, C. R., Patel, J., Albahr, Z., and Sablani, S. S. (2020). High pressure-assisted thermal sterilization of low-acid fruit and vegetable purees: microbial safety, nutrient, quality, and packaging evaluation. Food control. 114, 107233. doi:10.1016/j.foodcont.2020.107233
Ali, T., Kim, T., Rehman, S. U., Khan, M. S., Amin, F. U., Khan, M., et al. (2018). Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of alzheimer’s disease. Mol. Neurobiol. 55, 6076–6093. doi:10.1007/s12035-017-0798-6
Ali Shah, S., Ullah, I., Lee, H. Y., and Kim, M. O. (2013). Anthocyanins protect against ethanol-induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Mol. Neurobiol. 48, 257–269. doi:10.1007/s12035-013-8458-y
Alizadeh-sani, M., Tavassoli, M., Mohammadian, E., Mirzanajafi, E., Razi, M., Priyadarshi, R., et al. (2021). pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 166, 741–750. doi:10.1016/j.ijbiomac.2020.10.231
Al Jitan, S., Alkhoori, S. A., and Yousef, L. F. (2018). “Phenolic acids from plants: extraction and application to human health,” in Studies in natural products chemistry (Elsevier), 389–417.
Al-Khayri, J. M., Asghar, W., Akhtar, A., Ayub, H., Aslam, I., Khalid, N., et al. (2022). Anthocyanin delivery systems: a critical review of recent research findings. Appl. Sci. 12, 12347. doi:10.3390/app122312347
Amini, A. M., Muzs, K., Spencer, J. P., and Yaqoob, P. (2017). Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 46, 88–95. doi:10.1016/j.nutres.2017.09.006
Aqil, F., Vadhanam, M. V., Jeyabalan, J., Cai, J., Singh, I. P., and Gupta, R. C. (2014). Detection of anthocyanins/anthocyanidins in animal tissues. J. Agric. Food Chem. 62, 3912–3918. doi:10.1021/jf500467b
Arzi, L., Riazi, G., Sadeghizadeh, M., Hoshyar, R., and Jafarzadeh, N. (2018). A comparative study on anti-invasion, antimigration, and antiadhesion effects of the bioactive carotenoids of saffron on 4T1 breast cancer cells through their effects on wnt/β-catenin pathway genes. DNA Cell Biol. 37, 697–707. doi:10.1089/dna.2018.4248
Asdagh, A., and Pirsa, S. (2020). Bacterial and oxidative control of local butter with smart/active film based on pectin/nanoclay/Carum copticum essential oils/β-carotene. Int. J. Biol. Macromol. 165, 156–168. doi:10.1016/j.ijbiomac.2020.09.192
Atalay, P. B., Kuku, G., and Tuna, B. G. (2019). Effects of carbendazim and astaxanthin co-treatment on the proliferation of MCF-7 breast cancer cells. Vitro Cell. Dev. Biology-Animal 55, 113–119. doi:10.1007/s11626-018-0312-0
Azar, G., Quaranta-El Maftouhi, M., Masella, J.-J., and Mauget-Faÿsse, M. (2017). Macular pigment density variation after supplementation of lutein and zeaxanthin using the Visucam® 200 pigment module: impact of age-related macular degeneration and lens status. J. Français d'Ophtalmologie 40 (4), 303–313. doi:10.1016/j.jfo.2016.11.009
Azman, A.-S., Mawang, C.-I., and Abubakar, S. (2018). Bacterial pigments: the bioactivities and as an alternative for therapeutic applications. Nat. Product. Commun. 13, 1747–1754. doi:10.1177/1934578x1801301240
Bendokas, V., Skemiene, K., Trumbeckaite, S., Stanys, V., Passamonti, S., Borutaite, V., et al. (2019). Anthocyanins: from plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 60, 3352–3365. doi:10.1080/10408398.2019.1687421
Bener, M., Şen, F. B., Önem, A. N., Bekdeşer, B., Çelik, S. E., Lalikoglu, M., et al. (2022). Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: modeling, optimization and phenolic characterization. Food Chem. 385 (September 2021), 132633. doi:10.1016/j.foodchem.2022.132633
Bensalem, J., Dal-Pan, A., Gillard, E., Calon, F., and Pallet, V. (2016). Protective effects of berry polyphenols against age-related cognitive impairment. Nutr. Aging 3, 89–106. doi:10.3233/NUA-150051
Berrow, E. J., Bartlett, H. E., and Eperjesi, F. (2016). The effect of nutritional supplementation on the multifocal electroretinogram in healthy eyes. Doc. Ophthalmol. 132, 123–135. doi:10.1007/s10633-016-9532-3
Besednova, N. N., Andryukov, B. G., Zaporozhets, T. S., Kryzhanovsky, S. P., Kuznetsova, T. A., Fedyanina, L. N., et al. (2020). Algae polyphenolic compounds and modern antibacterial strategies: current achievements and immediate prospects. Biomedicines 8, 342. doi:10.3390/biomedicines8090342
Bhatt, T., and Patel, K. (2020). Carotenoids: potent to prevent diseases review. Nat. Prod. Bioprospecting 10, 109–117. doi:10.1007/s13659-020-00244-2
Boateng, I. D. (2024). Mechanisms, capabilities, limitations, and economic stability outlook for extracting phenolics from agro-byproducts using emerging thermal extraction technologies and their combinative effects. Food Bioprocess Technol. 17 (5), 1109–1140. doi:10.1007/s11947-023-03171-5
Bogolitsyn, K., Dobrodeeva, L., Druzhinina, A., Ovchinnikov, D., Parshina, A., and Shulgina, E. (2019). Biological activity of a polyphenolic complex of Arctic brown algae. J. Appl. Phycol. 31, 3341–3348. doi:10.1007/s10811-019-01840-7
Bone, R. A., Davey, P. G., Roman, B. O., and Evans, D. W. (2020). Efficacy of commercially available nutritional supplements: analysis of serum uptake, macular pigment optical density and visual functional response. Nutrients 12 (5), 1321. doi:10.3390/nu12051321
Briones-Labarca, V., Giovagnoli-Vicuña, C., and Cañas-Sarazúa, R. (2019). Optimization of extraction yield, flavonoids and lycopene from tomato pulp by high hydrostatic pressure-assisted extraction. Food Chem. 278, 751–759. doi:10.1016/j.foodchem.2018.11.106
Burdulis, D., Nikolajevas, L., Janulis, V., Stackevicené, E., and Janulis, V. (2009). Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (vaccinium myrtillus L.) and blueberry (vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 66, 399–408.
Cabas, B. M., and Icier, F. (2021). Ohmic heating–assisted extraction of natural color matters from red beetroot. Food Bioprocess Technol. 14, 2062–2077. doi:10.1007/s11947-021-02698-9
Calva-Estrada, S. J., Jiménez-Fernández, M., and Lugo-Cervantes, E. (2022). Betalains and their applications in food: the current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 4, 100089. doi:10.1016/j.fochms.2022.100089
Cao, Q., Li, J., Xia, Y., Li, W., Luo, S., Ma, C., et al. (2019). Green extraction of six phenolic compounds from rattan (Calamoideae faberii) with deep eutectic solvent by homogenate-assisted vacuum-cavitation method. Molecules 24, 113. doi:10.3390/molecules24010113
Carrillo, C., Nieto, G., Martínez-Zamora, L., Ros, G., Kamiloglu, S., Munekata, P. E. S., et al. (2022). Novel approaches for the recovery of natural pigments with potential health effects. J. Agric. Food Chem. 70, 6864–6883. doi:10.1021/acs.jafc.1c07208
Cerezo, A. B., Cătunescu, G. M., González, M.M.-P., Hornedo-Ortega, R., Pop, C. R., Rusu, C. C., et al. (2020). Anthocyanins in blueberries grown in hot climate exert strong antioxidant activity and may Be effective against urinary tract bacteria. Antioxidants 9, 478. doi:10.3390/antiox9060478
Česonienė, L., Jasutienė, I., and Šarkinas, A. (2009). Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Med. (Mex.) 45, 992–999. doi:10.3390/medicina45120127
Chavoshizadeh, S., Pirsa, S., and Mohtarami, F. (2020). Sesame oil oxidation control by active and smart packaging system using wheat gluten/chlorophyll film to increase shelf life and detecting expiration date. Eur. J. Lipid Sci. Technol. 122, 1900385. doi:10.1002/ejlt.201900385
Chen, K., and Roca, M. (2018). In vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 41, 25–33. doi:10.1016/j.jff.2017.12.029
Chen, T., Hu, S., Zhang, H., Guan, Q., Yang, Y., and Wang, X. (2017). Anti-inflammatory effects of Dioscorea alata L. anthocyanins in a TNBS-induced colitis model. Food Funct. 8, 659–669. doi:10.1039/c6fo01273f
Chen, Y., Li, Q., Zhao, T., Zhang, Z., Mao, G., Feng, W., et al. (2017). Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 237, 887–894. doi:10.1016/j.foodchem.2017.06.054
Coelho, M., Silva, S., Costa, E., Pereira, R. N., Rodrigues, A. S., Teixeira, J. A., et al. (2021). Anthocyanin recovery from grape by-products by combining ohmic heating with food-grade solvents: phenolic composition, antioxidant, and antimicrobial properties. Molecules 26 (3838), 3838. doi:10.3390/molecules26133838
Coklar, H., and Akbulut, M. (2017). Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 35, 166–174. doi:10.1016/j.jff.2017.05.037
Contreras-Ortiz, J. M. E., Barbabosa-Pliego, A., Oros-Pantoja, R., Aparicio-Burgos, J. E., Zepeda-Escobar, J. A., Hassan-Moustafa, W. H., et al. (2017). Effects of astaxanthin in mice acutely infected with Trypanosoma cruzi. Parasite 24, 17. doi:10.1051/parasite/2017018
Correia, P., Araújo, P., Ribeiro, C., Oliveira, H., Pereira, A. R., Mateus, N., et al. (2021). Anthocyanin-related pigments: natural allies for skin health maintenance and protection. Antioxidants 10, 1038. doi:10.3390/antiox10071038
Cortez, R., Luna-Vital, D. A., Margulis, D., and Gonzalez de Mejia, E. (2017). Natural pigments: stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 16, 180–198. doi:10.1111/1541-4337.12244
Costa-Rodrigues, J., Pinho, O., and Monteiro, P. R. R. (2018). Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 245, 1148–1153. doi:10.1016/j.foodchem.2017.11.055
Côté, J., Caillet, S., Doyon, G., Dussault, D., Sylvain, J.-F., and Lacroix, M. (2011). Antimicrobial effect of cranberry juice and extracts. Food control. 22, 1413–1418. doi:10.1016/j.foodcont.2011.02.024
Cui, H. X., Chen, J. H., Li, J. W., Cheng, F. R., and Yuan, K. (2018). Protection of anthocyanin from myrica rubra against cerebral ischemia-reperfusion injury via modulation of the TLR4/NF-κB and NLRP3 pathways. Molecules 23 (7), 1788. doi:10.3390/MOLECULES23071788
Cui, Q., Liu, J. Z., Yu, L., Gao, M. Z., Wang, L. T., Wang, W., et al. (2020). Experimental and simulative studies on the implications of natural and green surfactant for extracting flavonoids. J. Clean. Prod. 274, 122652. doi:10.1016/j.jclepro.2020.122652
da Silva, D. V. T., Pereira, A. D., Boaventura, G. T., Ribeiro, R. S. d. A., Verícimo, M. A., Carvalho-Pinto, C., et al. (2019). Short-term betanin intake reduces oxidative stress in Wistar rats. Nutrients 11, 1978. doi:10.3390/nu11091978
de Boer, F. Y., Imhof, A., and Velikov, K. P. (2019). Encapsulation of colorants by natural polymers for food applications. Color. Technol. 135, 183–194. doi:10.1111/cote.12393
Dewi, E. N., Tassakka, A. C. M. A. R., Yuwono, M., Suyono, E. A., Purnamayati, L., and Alam, J. F. (2022). Effect of chlorophyll in alginate-based edible film in inhibiting spoilage of fish snacks. Canrea J. Food Technol. Nutr. Culin. J. 5, 57–68. doi:10.20956/canrea.v5i1.571
Doldolova, K., Bener, M., Lalikoğlu, M., Aşçı, Y. S., Arat, R., and Apak, R. (2021). Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 353, 129337. doi:10.1016/j.foodchem.2021.129337
Duarte, L. J., Chaves, V. C., Nascimento, M. V. P., dos, S., Calvete, E., Li, M., et al. (2018). Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 247, 56–65. doi:10.1016/J.FOODCHEM.2017.12.015
Eletr, A. A., Siliha, H. A., Elshorbagy, G. A., and Galal, G. A. (2017). Evaluation of lycopene extracted from tomato processing waste as a natural antioxidant in some bakery products. Zagazig J. Agric. Res. 44, 1389–1401. doi:10.21608/zjar.2017.52942
Emadi, S., Sadeghi, I., and Khastar, H. (2020). Malvadin prevents kidney from renal ischemia-induced oxidative damage in rats. Jordan J. Pharm. Sci. 13, 2020–2273.
Enaru, B., Drețcanu, G., Pop, T. D., Stǎnilǎ, A., and Diaconeasa, Z. (2021). Anthocyanins: factors affecting their stability and degradation. Antioxidants 10, 1967. doi:10.3390/antiox10121967
Eom, J. W., Lim, J. W., and Kim, H. (2023). Lutein induces reactive oxygen species-mediated apoptosis in gastric cancer AGS cells via NADPH oxidase activation. Molecules 28, 1178. doi:10.3390/molecules28031178
Erşan, S., Müller, M., Reuter, L., Carle, R., and Müller-Maatsch, J. (2022). Co-pigmentation of strawberry anthocyanins with phenolic compounds from rooibos. Food Chem. Mol. Sci. 4, 100097. doi:10.1016/j.fochms.2022.100097
Fan, L., Xu, J., Guan, X., Li, R., and Wang, S. (2023). Developing radio frequency pretreatment technology for improving yield and quality of flaxseed oil extractions. Innovative Food Sci. Emerg. Technol. 86 (April), 103363. doi:10.1016/j.ifset.2023.103363
Fathi, M., Babaei, A., and Rostami, H. (2022). Development and characterization of locust bean gum-Viola anthocyanin-graphene oxide ternary nanocomposite as an efficient pH indicator for food packaging application. Food Packag. Shelf Life 34, 100934. doi:10.1016/j.fpsl.2022.100934
Ferreira, M. V. S., Cappato, L. P., Silva, R., Rocha, R. S., Neto, R. P. C., Tavares, M. I. B., et al. (2019). Processing raspberry-flavored whey drink using ohmic heating: physical, thermal and microstructural considerations. Food Res. Int. 123, 20–26. doi:10.1016/j.foodres.2019.04.045
Figueroa, J. G., Borrás-Linares, I., Del Pino-García, R., Curiel, J. A., Lozano-Sánchez, J., and Segura-Carretero, A. (2021). Functional ingredient from avocado peel: microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 352, 129300. doi:10.1016/j.foodchem.2021.129300
Food and Drug Administration (2023). Color additives questions and answers consumers. Available at: https://www.fda.gov/food/food-additives-petitions/color-additives-questions-and-answers-consumers (Accessed April 26, 2022).
Gagneten, M., Leiva, G., Salvatori, D., Schebor, C., and Olaiz, N. (2019). Optimization of pulsed electric field treatment for the extraction of bioactive compounds from blackcurrant. Food Bioprocess Technol. 12, 1102–1109. doi:10.1007/s11947-019-02283-1
Gao, J., Zhang, H., Wang, S., and Ling, B. (2023). Performance evaluation of an experimental radio frequency heating system designed for studying the solid-liquid extraction. Innovative Food Sci. Emerg. Technol. 90 (July), 103518. doi:10.1016/j.ifset.2023.103518
Garg, S., Afzal, S., Elwakeel, A., Sharma, D., Radhakrishnan, N., Dhanjal, J. K., et al. (2019). Marine carotenoid fucoxanthin possesses anti-metastasis activity: molecular evidence. Mar. Drugs 17, 338. doi:10.3390/md17060338
Gawai, K. M., Mudgal, S. P., and Prajapati, J. B. (2017). “Stabilizers, colorant, and exopolysaccharides in yoghurt,” in Yogurt in health and disease prevention. Editor P. S. Nagendra (Academic Press), 49–68.
Genskowsky, E., Puente, L. A., Pérez-Álvarez, J. A., Fernández-López, J., Muñoz, L. A., and Viuda-Martos, M. (2016). Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensi s (Molina) Stuntz] a Chilean blackberry: antioxidant and antibacterial properties of maqui. J. Sci. Food Agric. 96, 4235–4242. doi:10.1002/jsfa.7628
Ghendov-Moşanu, A., Sturza, R., Opriş, O., Lung, I., Popescu, L., Popovici, V., et al. (2020). Effect of lipophilic sea buckthorn extract on cream cheese properties. J. Food Sci. Technol. 57, 628–637. doi:10.1007/s13197-019-04094-w
Ghosh, S., Sarkar, T., Das, A., and Chakraborty, R. (2022). Natural colorants from plant pigments and their encapsulation: an emerging window for the food industry. LWT 153, 112527. doi:10.1016/J.LWT.2021.112527
Gomes, L., Monteiro, P., Cotas, J., Gonçalves, A. M. M., Fernandes, C., Gonçalves, T., et al. (2022). Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 13, 89–102. doi:10.1515/bmc-2022-0003
Gong, S., Jiao, C., Liu, B., Qu, W., Guo, L., and Jiang, Y. (2023). Beetroot (Beta vulgaris) extract exerts an antibacterial effect by inducing apoptosis-like death in Bacillus cereus. J. Funct. Foods 105, 105571. doi:10.1016/j.jff.2023.105571
González-Ponce, H. A., Rincón-Sánchez, A. R., Jaramillo-Juárez, F., and Moshage, H. (2018). Natural dietary pigments: potential mediators against hepatic damage induced by over-the-counter non-steroidal anti-inflammatory and analgesic drugs. Nutrients 10, 117–139. doi:10.3390/nu10020117
Guo, N., Jiang, Y. W., Wang, L. T., Niu, L. J., Liu, Z. M., et al. (2019). Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 296, 78–85. doi:10.1016/j.foodchem.2019.05.196
Hafidh, R. R., Abdulamir, A. S., Vern, L. S., Bakar, F. A., Abas, F., Jahanshiri, F., et al. (2011). Inhibition of growth of highly resistant bacterial and fungal pathogens by a natural product. Open Microbiol. J. 5, 96–106. doi:10.2174/1874285801105010096
Hammond, B. R., Miller, L. S., Bello, M. O., Lindbergh, C. A., Mewborn, C., and Renzi-Hammond, L. M. (2017). Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: a randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 9, 254. doi:10.3389/fnagi.2017.00254
Han, J., Ma, D., Zhang, M., Yang, X., and Tan, D. (2015). Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. BioMed Res. Int., 608174. doi:10.1155/2015/608174
Hashemi, S. A., Karami, M., and Bathaie, S. Z. (2020). Saffron carotenoids change the superoxide dismutase activity in breast cancer: in vitro, in vivo and in silico studies. Int. J. Biol. Macromol. 158, 845–853. doi:10.1016/j.ijbiomac.2020.04.063
Hayhoe, R. P. G., Lentjes, M. A. H., Mulligan, A. A., Luben, R. N., Khaw, K., and Welch, A. A. (2017). Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 117, 1439–1453. doi:10.1017/S0007114517001180
He, Y., Ma, Y., Du, Y., and Shen, S. (2018). Differential gene expression for carotenoid biosynthesis in a green alga Ulva prolifera based on transcriptome analysis. BMC Genomics 19, 916. doi:10.1186/s12864-018-5337-y
Heo, S.-Y., Lee, Y., Kim, T.-H., Heo, S.-J., Shin, H., Lee, J., et al. (2023). Anti-cancer effect of chlorophyllin-assisted photodynamic therapy to induce apoptosis through oxidative stress on human cervical cancer. Int. J. Mol. Sci. 24, 11565. doi:10.3390/ijms241411565
Hsieh-Lo, M., Castillo-Herrera, G., and Mojica, L. (2020). Black bean anthocyanin-rich extract from supercritical and pressurized extraction increased in vitro antidiabetic potential, while having similar storage stability. Foods 9, 655. doi:10.3390/foods9050655
Huang, J., Liu, J., Chen, M., Yao, Q., and Hu, Y. (2021). Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix: study on the interaction behavior and mechanism for better shrimp freshness monitoring. Int. J. Biol. Macromol. 184, 666–677. doi:10.1016/j.ijbiomac.2021.06.074
Iannaccone, A., Carboni, G., Forma, G., Mutolo, M. G., and Jennings, B. J. (2016). Macular pigment optical density and measures of macular function: test-retest variability, cross-sectional correlations, and findings from the Zeaxanthin Pilot Study of response to supplementation (ZEASTRESS-Pilot). Foods 5 (2), 32. doi:10.3390/foods5020032
Indrasti, D., Andarwulan, N., Purnomo, E. H., and Wulandari, N. (2018). Stability of chlorophyll as natural colorant: a review for suji (dracaena angustifolia roxb.) leaves’ case. Curr. Res. Nutr. Food Sci. 6, 609–625. doi:10.12944/CRNFSJ.6.3.04
Iwasaki, S., Widjaja-Adhi, M., Koide, A., Kaga, T., Nakano, S., Beppu, F., et al. (2012). In vivo antioxidant activity of fucoxanthin on obese/diabetes KK-Ay mice. Food Nutr. Sci. 3, 1491–1499. doi:10.4236/fns.2012.311194
Jaeschke, D. P., Mercali, G. D., Marczak, L. D. F., Müller, G., Frey, W., and Gusbeth, C. (2019). Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 283, 207–212. doi:10.1016/j.biortech.2019.03.035
Jiang, C., Sun, Z., Hu, J., Jin, Y., Guo, Q., Xu, J., et al. (2019). Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/NF-κB axis in vitro and in vivo. Food and Funct. 10, 5873–5885. doi:10.1039/C9FO00742C
Jiang, Y., Ding, Y., Wang, D., Deng, Y., and Zhao, Y. (2020). Radio frequency-assisted enzymatic extraction of anthocyanins from Akebia trifoliata (Thunb.) Koidz. flowers: process optimization, structure, and bioactivity determination. Industrial Crops Prod. 149 (July 2019), 112327. doi:10.1016/j.indcrop.2020.112327
Jiménez-Alvarado, R., Aguirre- Alvarez, G., Campos-Montiel, R. G., Contreras-Esquivel, J. C., Pinedo-Espinoza, J. M., Gonzalez-Aguayo, E., et al. (2015). Effect of High-Pulsed Electric Fields on the extraction yield and quality of juices obtained from the endocarp on nine prickly pear (Opuntia spp.) varieties. Jokull 65, 414–435.
Johnson, E. J., Avendano, E. E., Mohn, E. S., and Raman, G. (2020). The association between macular pigment optical density and visual function outcomes: a systematic review and meta-analysis. Eye 35, 1620–1628. doi:10.1038/s41433-020-01124-2
Juan-Polo, A., Maestre Pérez, S. E., Monedero Prieto, M., Sánchez Reig, C., Tone, A. M., Herranz Solana, N., et al. (2022). Oxygen scavenger and antioxidant LDPE/EVOH/PET-based films containing β-carotene intended for fried peanuts (Arachis hypogaea L.) packaging: pilot scale processing and validation studies. Polymers 14, 3550. doi:10.3390/polym14173550
Jung, E., Kim, Y., and Joo, N. (2013). Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). J. Sci. Food Agric. 93, 3769–3776. doi:10.1002/jsfa.6256
Jung, U. J., and Kim, S. R. (2018). Beneficial effects of flavonoids against Parkinson’s disease. J. Med. Food 21, 421–432. doi:10.1089/jmf.2017.4078
Junqueira-Gonçalves, M., Yáñez, L., Morales, C., Navarro, M., A. Contreras, R., and Zúñiga, G. (2015). Isolation and characterization of phenolic compounds and anthocyanins from murta (ugni molinae turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules 20, 5698–5713. doi:10.3390/molecules20045698
Jurić, S., Jurić, M., Król-Kilińska, Z., Vlahoviček-Kahlina, K., Vinceković, M., Dragović-Uzelac, V., et al. (2020). Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 38, 1735–1790. doi:10.1080/87559129.2020.1837862
Kang, H.-J., Ko, M.-J., and Chung, M.-S. (2021). Anthocyanin structure and pH dependent extraction characteristics from blueberries (Vaccinium corymbosum) and chokeberries (Aronia melanocarpa) in subcritical water state. Foods 10, 527. doi:10.3390/foods10030527
Karpiński, T. M., and Adamczak, A. (2019). Fucoxanthin—an antibacterial carotenoid. Antioxidants 8, 239. doi:10.3390/antiox8080239
Karpiński, T. M., Ożarowski, M., Alam, R., Łochyńska, M., and Stasiewicz, M. (2021). What do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 20, 36. doi:10.3390/md20010036
Kavitha, K., Kowshik, J., Kishore, T. K., Baba, A., and Nagini, S. (2013). Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochimica Biophysica Acta 1830, 4433–4444. doi:10.1016/j.bbagen.2013.05.032
Keravis, T., Favot, L., Abusnina, A. A., Anton, A., Justiniano, H., Soleti, R., et al. (2015). Delphinidin inhibits tumor growth by acting on VEGF signalling in endothelial cells. PLoS One 10, e0145291. doi:10.1371/journal.pone.0145291
Khoo, H. E., Azlan, A., Tang, S. T., and Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food and Nutr. Res. 61, 1361779. doi:10.1080/16546628.2017.1361779
Kijlstra, A., Tian, Y., Kelly, E. R., and Berendschota, T. T. J. M. (2012). Lutein: more than just a filter for blue light. Prog. Retin. Eye Res. 31, 303–315. doi:10.1016/j.preteyeres.2012.03.002
Kim, E.-A., Kang, N., Heo, S.-Y., Oh, J.-Y., Lee, S.-H., Cha, S.-H., et al. (2023). Antioxidant, antiviral, and anti-inflammatory activities of lutein-enriched extract of Tetraselmis species. Mar. Drugs 21, 369. doi:10.3390/md21070369
Kim, M. H., Jeong, Y. J., Cho, H. J., Hoe, H. S., Park, K. K., Park, Y. Y., et al. (2016). Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol. Rep. 37, 777–784. doi:10.3892/or.2016.5296
Kochadai, N., Khasherao, B. Y., and Sinija, V. R. N. (2022). Effect of radiofrequency pre-treatment on the extraction of bioactives from clitoria ternatea and Hibiscus rosa sinensis and insights to enzyme inhibitory activities. Food Bioprocess Technol. 15 (3), 571–589. doi:10.1007/s11947-022-02770-y
Kochi, T., Shimizu, M., Sumi, T., Kubota, M., Shirakami, Y., Tanaka, T., et al. (2014). Inhibitory effects of astaxanthin on azoxymethane-induced colonic preneoplastic lesions in C57/BL/KsJ-db/db mice. BMC Gastroenterol. 14, 212. doi:10.1186/s12876-014-0212-z
Kubo, M. T., Siguemoto, É. S., Funcia, E. S., Augusto, P. E., Curet, S., Boillereaux, L., et al. (2020). Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: a critical review. Curr. Opin. Food Sci. 35, 36–48. doi:10.1016/j.cofs.2020.01.004
Kumar, S. S., Arya, M., Chauhan, A. S., and Giridhar, P. (2020). Basella rubra fruit juice betalains as a colorant in food model systems and shelf-life studies to determine their realistic usability. J. Food Process. Preserv. 44, 1–9. doi:10.1111/jfpp.14595
Kuriya, S. P., Silva, R., Rocha, R. S., Guimarães, J. T., Balthazar, C. F., Pires, R. P. S., et al. (2020). Impact assessment of different electric fields on the quality parameters of blueberry flavored dairy desserts processed by Ohmic Heating. Food Res. Int. 134, 109235. doi:10.1016/j.foodres.2020.109235
Kusmita, L., Tatsa, Y. A., Franyoto, Y. D., Sabdono, A., Trianto, A., and Radjasa, O. K. (2021). Antibacterial activity of carotenoid from bacterial symbiont Virgibacillus salarius strain 19.Pp.sc.1.6 against MDR E. coli and MRSA. Egypt. J. Aquatic Biol. Fish. 25, 147–157. doi:10.21608/ejabf.2021.172877
Kutlu, N., Isci, A., Sakiyan, O., and Yilmaz, A. E. (2021). Effect of ohmic heating on ultrasound extraction of phenolic compounds from cornelian cherry (Cornus mas). J. Food Process. Preserv. 45, 1–13. doi:10.1111/jfpp.15818
Lacombe, A., Wu, V. C. H., White, J., Tadepalli, S., and Andre, E. E. (2012). The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiol. 30, 124–131. doi:10.1016/j.fm.2011.10.006
Lal, A. M. N., Prince, M. V., Kothakota, A., Pandiselvam, R., Thirumdas, R., Mahanti, N. K., et al. (2021). Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innovative Food Sci. Emerg. Technol. 74 (September), 102844. doi:10.1016/j.ifset.2021.102844
Lan, W., Wang, S., Zhang, Z., Liang, X., Liu, X., and Zhang, J. (2021). Development of red apple pomace extract/chitosan-based films reinforced by TiO2 nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 168, 105–115. doi:10.1016/j.ijbiomac.2020.12.051
Lea, T. (2015). Caco-2 cell line. in The impact of food bioactives on health: In vitro and ex vivo models, (Chapter 10). Editors K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackieet al. (Springer).
Lee, B., Lee, C., Yang, S., Park, E., Ku, S., and Bae, J. (2019). Suppressive effects of pelargonidin on lipopolysaccharide-induced inflammatory responses. Chemico-Biological Interact. 302, 67–73. doi:10.1016/J.CBI.2019.02.007
Lee, S. G., Brownmiller, C. R., Lee, S.-O., and Kang, H. W. (2020). Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide-stimulated RAW-267.4 macrophages. Nutrients 12, 1089. doi:10.3390/nu12041089
Lefebvre, T., Destandau, E., and Lesellier, E. (2020). Evaluation of the extraction and stability of chlorophyll-rich extracts by supercritical fluid chromatography. Anal. Bioanal. Chem. 412, 7263–7273. doi:10.1007/s00216-020-02859-3
Lefebvre, T., Destandau, E., and Lesellier, E. (2021). Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from Rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 1639, 461709. doi:10.1016/j.chroma.2020.461709
Lembong, E., and Utama, G. L. (2019). Anti-microbial activity of the red beet extract (Beta vulgaris L.) with solvent ethanol and acid addition variation. IOP Conf. Ser. Earth Environ. Sci. 443, 012031. doi:10.1088/1755-1315/443/1/012031
Leong, H. Y., Show, P. L., Lim, M. H., Ooi, C. W., and Ling, T. C. (2017). Natural red pigments from plants and their health benefits: a review. Food Rev. Int. 34, 463–482. doi:10.1080/87559129.2017.1326935
Li, A., Xiao, R., He, S., An, X., He, Y., Wang, C., et al. (2019). Research advances of purple sweet potato anthocyanins: extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 24, 3816–3821. doi:10.3390/molecules24213816
Li, W., Ji, G., Zhang, X., and Yu, H. (2018). The influence and mechanisms of purple sweet potato anthocyanins on the growth of bladder cancer BIU87 cell. Zhonghua Yi Xue Za Zhi 98, 457–459. doi:10.3760/CMA.J.ISS.0376-2491.2018.06.013
Lim, W. C., Kim, H., Kim, Y. J., Park, S. H., Song, J. H., Lee, K. H., et al. (2017). Delphinidin inhibits BDNF-induced migration and invasion in SKOV3 ovarian cancer cells. Bioorg. and Med. Chem. Lett. 27, 5337–5343. doi:10.1016/j.bmcl.2017.09.024
Lin, B.-W., Gong, C.-C., Song, H.-F., and Cui, Y.-Y. (2017). Effects of anthocyanins on the prevention andtreatment of cancer: review. Br. J. Pharmacol. 174, 1226–1243. doi:10.1111/bph.13627
Lin, J., Tian, J., Shu, C., Cheng, Z., Liu, Y., Wang, W., et al. (2020). Malvidin-3-galactoside from blueberry suppresses the growth and metastasis potential of hepatocellular carcinoma cell Huh-7 by regulating apoptosis and metastases pathways. Food Sci. Hufman Wellness 9, 136–145. doi:10.1016/j.fshw.2020.02.004
Liu, C., Wang, R., Yi, Y., Gao, Z., and Chen, Y. (2018). Lycopene mitigates β -amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 53, 66–71. doi:10.1016/j.jnutbio.2017.10.014
Llivisaca, S., Manzano, P., Ruales, J., Flores, J., Mendoza, J., Peralta, E., et al. (2018). Chemical, antimicrobial, and molecular characterization of mortiño (Vaccinium floribundum Kunth) fruits and leaves. Food Sci. Nutr. 6, 934–942. doi:10.1002/fsn3.638
López, C. J., Caleja, C., Prieto, M. A., Sokovic, M., Calhelha, R. C., Barros, L., et al. (2019). Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 275, 426–438. doi:10.1016/j.foodchem.2018.09.099
López-Gámez, G., Elez-Martínez, P., Martín-Belloso, O., and Soliva-Fortuny, R. (2021). Changes of carotenoid content in carrots after application of pulsed electric field treatments. LWT 147, 111408. doi:10.1016/j.lwt.2021.111408
Lu, F., Wu, Q., Lei, J., Zhou, Y., Liu, Y., Zhu, N., et al. (2024). Zeaxanthin impairs angiogenesis and tumor growth of glioblastoma: an in vitro and in vivo study. Archives Biochem. Biophysics 754, 109957. doi:10.1016/j.abb.2024.109957
Lu, W., Shi, Y., Wang, R., Su, D., Tang, M., Liu, Y., et al. (2021). Antioxidant activity and healthy benefits of natural pigments in fruits: a review. Int. J. Mol. Sci. 22 (9), 4945. doi:10.3390/ijms22094945
Luzardo-Ocampo, I., Ramírez-Jiménez, A. K., Yañez, J., Mojica, L., and Luna-Vital, D. A. (2021). Technological applications of natural colorants in food systems: a review. Foods 10, 634. doi:10.3390/foods10030634
Machida, N., Kosehira, M., and Kitaichi, N. (2020). Clinical effects of dietary supplementation of lutein with high bio-accessibility on macular pigment optical density and contrast sensitivity: a randomized double-blind placebo-controlled parallel-group comparison trial. Nutrients 12, 2966. doi:10.3390/nu12102966
Madadi, E., Mazloum-Ravasan, S., Yu, J. S., Ha, J. W., Hamishehkar, H., and Kim, K. H. (2020). Therapeutic application of betalains: a review. Plants 17, 1219. doi:10.3390/plants9091219
Magalhães, D., Gonçalves, R., Rodrigues, C. V., Rocha, H. R., Pintado, M., and Coelho, M. C. (2024). Natural pigments recovery from food by-products: health benefits towards the food industry. Foods 13 (4), 2276. doi:10.3390/foods13142276
Malhão, F., Macedo, A. C., Costa, C., Rocha, E., and Ramos, A. A. (2021). Fucoxanthin holds potential to become a drug adjuvant in breast cancer treatment: evidence from 2D and 3D cell cultures. Molecules 26, 4288. doi:10.3390/molecules26144288
Maoka, T. (2020). Carotenoids as natural functional pigments. J. Nat. Med. 74, 1–16. doi:10.1007/s11418-019-01364-x
Martí, R., Roselló, S., and Cebolla-Cornejo, J. (2016). Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers (Basel) 8 (6), 58. doi:10.3390/cancers8060058
Martinez, R., Hohmann, M., Longhi-Balbinot, D., Zarpelon, A. C., Baracat, M. M., Georgetti, S. R., et al. (2020). Analgesic activity and mechanism of action of a Beta vulgaris dye enriched in betalains in inflammatory models in mice. Inflammopharmacology 28, 1663–1675. doi:10.1007/s10787-020-00689-4
Martínez-Abad, A., Ramos, M., Hamzaoui, M., Kohnen, S., Jiménez, A., and Garrigós, M. C. (2020). Optimisation of sequential microwave-assisted extraction of essential oil and pigment from lemon peels waste. Foods 9 (10), 1493. doi:10.3390/foods9101493
Martins, T., Barros, A. N., Rosa, E., and Antunes, L. (2023). Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: a comprehensive review. Molecules 28 (10), 5344. doi:10.3390/molecules28145344
Mattioli, R., Francioso, A., Mosca, L., and Silva, P. (2020). Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 25, 3809. doi:10.3390/molecules25173809
Mayasari, D., Murti, Y. B., Pratiwi, S. U. T., and Sudarsono, S. (2021a). TLC-contact bioautography and disc diffusion method for investigation of the antibacterial activity of Melastoma malabathricum L. leaves. Res. J. Pharm. Technol. 14, 6463–6470. doi:10.52711/0974-360X.2021.01117
Mayasari, D., Murti, Y. B., Sudarsono, S., and Pratiwi, S. U. T. (2021b). Phytochemical, antioxidant and antibacterial evaluation of Melastoma malabathricum L.: an Indonesian traditional medicinal plant. Trop. J. Nat. Prod. Res. 5, 819–824. doi:10.26538/tjnpr/v5i5.5
McGuire, J., Taguchi, T., Tombline, G., Shi, Y., Sen, A., Rak, J., et al. (2024). Hyaluronidase inhibitor delphinidin inhibits cancer metastasis. Sci. Rep. 14, 14958. doi:10.1038/s41598-024-64924-6
Meng, L., Zhu, J., Ma, Y., Sun, X., Li, D., Li, L., et al. (2019). Composition and antioxidant activity of anthocyanins from Aronia melanocarpa cultivated in Haicheng, Liaoning, China. Food Biosci. 30, 100413. doi:10.1016/j.fbio.2019.100413
Milani, A., Basirnejad, M., Shahbazi, S., and Bolhassani, A. (2017). Carotenoids: biochemistry, pharmacology, and treatment. Br. J. Pharmacol. 174 (11), 1290–1324. doi:10.1111/bph.13625
Min, J., Yu, S.-W., Baek, S.-H., Nair, K. M., Bae, O.-N., Bhatt, A., et al. (2011). Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci. Lett. 500, 157–161. doi:10.1016/j.neulet.2011.05.048
Mirza, A. Z., Althagafi, I. I., and Shamshad, H. (2019). Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur. J. Med. Chem. 166, 502–513. doi:10.1016/J.EJMECH.2019.01.067
Molina, A. K., Corrêa, R. C. G., Prieto, M. A., Pereira, C., and Barros, L. (2023). Bioactive natural pigments: extraction, isolation, and stability in food applications. Molecules 28 (3), 1200. doi:10.3390/molecules28031200
Moreno-Ley, C. M., Osorio-Revilla, G., Hernández-Martínez, D. M., Ramos-Monroy, O. A., and Gallardo-Velázquez, T. (2021). Anti-inflammatory activity of betalains: a comprehensive review. Hum. Nutr. Metabolism 25, 200126. doi:10.1016/j.hnm.2021.200126
Mouahid, A., Seengeon, K., Martino, M., Crampon, C., Kramer, A., and Badens, E. (2020). Selective extraction of neutral lipids and pigments from nannochloropsis salina and nannochloropsis maritima using supercritical CO2 extraction: effects of process parameters and pre-treatment. J. Supercrit. Fluids 165, 104934. doi:10.1016/j.supflu.2020.104934
Nabi, F., Arain, M. A., Rajput, N., Alagawany, M., Soomro, J., Umer, M., et al. (2020). Health benefits of carotenoids and potential application in poultry industry: a review. J. Animal Physiology Animal Nutr. 104 (6), 1809–1818. doi:10.1111/jpn.13375
Nabi, G., Mukhtar, K., Ahmed, W., Manzoor, M. F., Nawaz Ranjha, M. M. A., Kieliszek, M., et al. (2023). Natural pigments: anthocyanins, carotenoids, chlorophylls, and betalains as colorants in food products. Food Biosci. 52, 102403. doi:10.1016/j.fbio.2023.102403
Naghdi, S., Rezaei, M., and Abdollahi, M. (2021). A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 191, 161–170. doi:10.1016/j.ijbiomac.2021.09.045
Naisi, S., Bayat, M., Zahraei Salehi, T., Rahimian Zarif, B., and Yahyaraeyat, R. (2023). Antimicrobial and anti-biofilm effects of carotenoid pigment extracted from Rhodotorula glutinis strain on food-borne bacteria. Iran. J. Microbiol. 15, 79–88. doi:10.18502/ijm.v15i1.11922
Ndwandwe, B. K., Malinga, S. P., Kayitesi, E., and Dlamini, B. C. (2024). Recent developments in the application of natural pigments as pH-sensitive food freshness indicators in biopolymer-based smart packaging: challenges and opportunities. Int. J. Food Sci. and Technol. 59, 2148–2161. doi:10.1111/ijfs.16990
Neumann, U., Derwenskus, F., Flister, V. F., Schmid-Staiger, U., Hirth, T., and Bischoff, S. C. (2019). Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 8, 1–11. doi:10.3390/antiox8060183
Nguyen, T. L. P., Fenyvesi, F., Remenyik, J., Homoki, J. R., Gogolák, P., Bácskay, I., et al. (2018). Protective effect of pure sour cherry AnthocyaninExtract on cytokine-induced InflammatoryCaco-2 monolayers. Nutrients 10, 861. doi:10.3390/nu10070861
Nisar, N., Li, L., Lu, S., Khin, N. C., and Pogson, B. J. (2015). Carotenoid metabolism in plants. Mol. Plant 8, 68–82. doi:10.1016/j.molp.2014.12.007
Nishino, A., Yasui, H., and Maoka, T. (2017). Reaction and scavenging mechanism of β-carotene and zeaxanthin with reactive oxygen species. J. Oleo Sci. 66, 77–84. doi:10.5650/jos.ess16107
Nonglait, D. L., and Gokhale, J. S. (2024). Review insights on the demand for natural pigments and their recovery by emerging microwave-assisted extraction (MAE). Food Bioprocess Technol. 17 (7), 1681–1705. doi:10.1007/s11947-023-03192-0
Novais, C., Molina, A. K., Abreu, R. M. V., Santo-Buelga, C., Ferreira, I. C. F. R., Pereira, C., et al. (2022). Natural food colorants and preservatives: a review, a demand, and a challenge. J. Agric. Food Chem. 70 (9), 2789–2805. doi:10.1021/acs.jafc.1c07533
Nowacka, M., Tappi, S., Wiktor, A., Rybak, K., Miszczykowska, A., Czyzewski, J., et al. (2019). The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 8, 244. doi:10.3390/foods8070244
Obana, A., Gohto, Y., Nakazawa, R., Moriyama, T., Gellermann, W., and Bernstein, P. S. (2020). Effect of an antioxidant supplement containing high-dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Sci. Rep. 10 (1), 10262. doi:10.1038/s41598-020-66962-2
Oladzadabbasabadi, N., Nafchi, A. M., Ghasemlou, M., Ariffin, F., Singh, Z., and Al-Hassan, A. A. (2022). Natural anthocyanins: sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf Life 33, 100872. doi:10.1016/j.fpsl.2022.100872
Olmedilla-Alonso, B., Estévez-Santiago, R., Silván, J.-M., Sánchez-Prieto, M., and De Pascual-Teresa, S. (2018). Effect of long-term xanthophyll and anthocyanin supplementation on lutein and zeaxanthin serum concentrations and macular pigment optical density in postmenopausal women. Nutrients 10, 959. doi:10.3390/nu10080959
Ouyang, W., and O’Garra, A. (2019). IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 50 (4), 871–891. doi:10.1016/J.IMMUNI.2019.03.020
Ozcan, M., Aydemir, D., Bacanlı, M., Akgün, I. H., Ardıç, M., Gökmen, V., et al. (2021). Protective effects of antioxidant chlorophyllin in chemically induced breast cancer model in vivo. Biol. Trace Elem. Res. 199, 4475–4488. doi:10.1007/s12011-021-02585-6
Pachaiyappan, A., Sadhasivam, G., Kumar, M., and Muthuvel, A. (2021). Biomedical potential of astaxanthin from novel endophytic pigment producing bacteria Pontibacter korlensis AG6. Waste Biomass Valorization 12, 2119–2129. doi:10.1007/s12649-020-01169-0
Pacheco, S. D. G., Gasparin, A. T., Jesus, C. H. A., Sotomaior, B. B., Ventura, A. C. S. S. B., Redivo, D. D. B., et al. (2019). Antinociceptive and anti-inflammatory effects of bixin, a carotenoid extracted from the seeds of Bixa orellana. Planta Medica 85, 1216–1224. doi:10.1055/a-1008-1238
Padmanabhan, P., Correa-Betanzo, J., and Paliyath, G. (2016). “Berries and related fruits,” in Encyclopedia of food and health. Editors B. Caballero, P. M. Finglas, and F. Toldrá (Academic Press), 364–371. doi:10.1016/B978-0-12-384947-2.00060-X
Pagels, F., Pereira, R. N., Amaro, H. M., Vasconcelos, V., Guedes, A. C., and Vicente, A. A. (2021). Continuous pressurized extraction versus electric fields-assisted extraction of cyanobacterial pigments. J. Biotechnol. 334, 35–42. doi:10.1016/j.jbiotec.2021.05.004
Pagliarulo, C., De Vito, V., Picariello, G., Colicchio, R., Pastore, G., Salvatore, P., et al. (2016). Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 190, 824–831. doi:10.1016/j.foodchem.2015.06.028
Panda, D., and Manickam, S. (2019). Cavitation technology-the future of greener extraction method: a review on the extraction of natural products and process intensification mechanism and perspectives. Appl. Sci. 9, 766. doi:10.3390/app9040766
Parekh, R., Hammond, B. R., and Chandradhara, D. (2024). Lutein and zeaxanthin supplementation improves dynamic visual and cognitive performance in children: a randomized, double-blind, parallel, placebo-controlled study. Adv. Ther. 41, 1496–1511. doi:10.1007/s12325-024-02785-1
Patek-Mohd, N.-N., Abdu, A., Jusop, S., Abdul-Hamid, H., Karim, Md. R., Nazrin, M., et al. (2018). Potentiality of Melastoma malabathricum as Phytoremediators of soil contaminated with sewage sludge. Sci. Agric. 75, 27–35. doi:10.1590/1678-992x-2016-0002
Peraman, M., and Nachimuthu, S. (2019). Identification and quantification of fucoxanthin in selected carotenoid-producing marine microalgae and evaluation for their chemotherapeutic potential. Pharmacogn. Mag. 15, 243. doi:10.4103/pm.pm_64_19
Pereira, R. N., Coelho, M. I., Genisheva, Z., Fernandes, J. M., Vicente, A. A., Pintado, M. E., et al. (2020). Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod. Process. 124, 320–328. doi:10.1016/j.fbp.2020.09.009
Pereira, V. A., de Arruda, I. N. Q., and Stefani, R. (2015). Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 43, 180–188. doi:10.1016/j.foodhyd.2014.05.014
Pourjavaher, S., Almasi, H., Meshkini, S., Pirsa, S., and Parandi, E. (2017). Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleracea) extract. Carbohydr. Polym. 156, 193–201. doi:10.1016/j.carbpol.2016.09.027
Priyadarshani, A. M. B. (2017). A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit. Rev. Food Sci. Nutr. 57, 1710–1717. doi:10.1080/10408398.2015.1023431
Puišo, J., Jonkuvienė, D., Mačionienė, I., Šalomskienė, J., Jasutienė, I., and Kondrotas, R. (2014). Biosynthesis of silver nanoparticles using lingonberry and cranberry juices and their antimicrobial activity. Colloids Surf. B Biointerfaces J. 121, 214–221. doi:10.1016/j.colsurfb.2014.05.001
Qin, Y., Liu, Y., Zhang, X., and Liu, J. (2020). Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 100, 105410. doi:10.1016/j.foodhyd.2019.105410
Rajauria, G., and Abu-Ghannam, N. (2013). Isolation and partial characterization of bioactive fucoxanthin from Himanthalia elongata brown seaweed: a TLC-based approach. Int. J. Anal. Chem. 2013, 802573. doi:10.1155/2013/802573
Rashid, K., Wachira, F. N., Nyabuga, J. N., Wanyonyi, B., Murilla, G., and Isaac, A. O. (2014). Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr. Neurosci. 17, 178–185. doi:10.1179/1476830513Y.0000000081
Renzi-Hammond, L. M., Bovier, E. R., Fletcher, L. M., Miller, L. S., Mewborn, C. M., Lindbergh, C. A., et al. (2017). Effects of a lutein and zeaxanthin intervention on cognitive function: a randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients 9, 1246. doi:10.3390/nu9111246
Rodriguez-Amaya, D. B. (2019). Update on natural food pigments - a mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 124, 200–205. doi:10.1016/j.foodres.2018.05.028
Rodriguez-Luna, A., Ávila-Román, J., Oliveira, H., Motilva, V., and Talero, E. (2019). Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 17, 451. doi:10.3390/md17080451
Saber, A., Abedimanesh, N., Somi, M. H., Khosroushahi, A. Y., and Moradi, S. (2023). Anticancer properties of red beetroot hydro-alcoholic extract and its main constituent; betanin on colorectal cancer cell lines. BMC Complementary Med. Ther. 23, 246. doi:10.1186/s12906-023-04077-7
Sakthivel, K. M., Kokilavani, K., Kathirvelan, C., Durairaj Brindha, A., Ravallec, R., Hance, P., et al. (2020). Chicory root flour – a functional food with potential multiple health benefits evaluated in a mice model. J. Funct. Foods 74, 104174. doi:10.1016/j.jff.2020.104174
Šamec, D., Linić, I., and Salopek-Sondi, B. (2021). Salinity stress as an elicitor for phytochemicals and minerals accumulation in selected leafy vegetables of Brassicaceae. Agronomy 11 (2), 361. doi:10.3390/agronomy11020361
Sen, T., Barrow, C. J., and Deshmukh, S. K. (2019). Microbial pigments in the food industry-challenges and the way forward. Front. Nutr. 6, 7. doi:10.3389/fnut.2019.00007
Shanmugapriya, K., Kim, H., Saravana, P. S., Chun, B.-S., and Kang, H. W. (2018). Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: investigation on anticancer, wound healing, and antibacterial effects. Colloids Surfaces B Biointerfaces 172, 170–179. doi:10.1016/j.colsurfb.2018.08.042
Shin, J., Saini, R. K., and Oh, J. (2020). Low dose astaxanthin treatments trigger the hormesis of human astroglioma cells by up-regulating the cyclin-dependent kinase and down-regulated the tumor suppressor protein p53. Biomedicines 8, 434. doi:10.3390/biomedicines8100434
Shukla, S., Lohani, U. C., Shahi, N. C., and Dubey, A. (2022). Extraction of natural pigments from red sorghum (Sorghum bicolor) husk by ultrasound and microwave assisted extraction: a comparative study through response surface analysis. J. Food Process Eng. 45 (10). doi:10.1111/jfpe.14130
Silva, J. P. P., Bolanho, B. C., Stevanato, N., Massa, T. B., and da Silva, C. (2020). Ultrasound-assisted extraction of red beet pigments (Beta vulgaris L.): influence of operational parameters and kinetic modeling. J. Food Process. Preserv. 44. doi:10.1111/jfpp.14762
Sindhu, E., Preethi, K., and Kuttan, R. (2010). Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J. Exp. Biol. 48, 843–848.
Singh, T., Pandey, V. K., Dash, K. K., Zanwar, S., and Singh, R. (2023). Natural bio-colorant and pigments: sources and applications in food processing. J. Agric. Food Res. 12, 100628. doi:10.1016/j.jafr.2023.100628
Song, X., Jiang, Y., Zhong, Y., Wang, D., and Deng, Y. (2022). Evaluation of radio frequency-assisted enzymatic extraction of non-anthocyanin polyphenols from akebia trifoliata flowers and their biological activities using UPLC-PDA-TOF-ESI-MS and chemometrics. Foods 11 (21), 3410. doi:10.3390/foods11213410
Sravani, M., Kumaran, A., Dhamdhere, A. T., and Kumar, N. S. (2021). Computational molecular docking analysis and visualisation of anthocyanins for anticancer activity. Int. J. Res. Appl. Sci. Biotechnol. 8, 154–161. doi:10.31033/ijrasb.8.1.18
Stoll, L., Maillard, M. N., Le Roux, E., Hickmann Flôres, S., Nachtigall, S. M. B., Rios, A., et al. (2023). Bixin, a performing natural antioxidant in active food packaging for the protection of oxidation sensitive food. LWT 180, 114730. doi:10.1016/j.lwt.2023.114730
Strathearn, K. E., Yousef, G. G., Grace, M. H., Roy, S. L., Tambe, M. A., Ferruzzi, M. G., et al. (2014). Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson׳s disease. Brain Res. 1555, 60–77. doi:10.1016/j.brainres.2014.01.047
Stringham, J. M., and Stringham, N. T. (2016). Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Exp. Eye Res. 151, 1–8. doi:10.1016/j.exer.2016.07.005
Stringham, N. T., Holmes, P. V., and Stringham, J. M. (2019). Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiology and Behav. 211, 112650. doi:10.1016/j.physbeh.2019.112650
Sudha, P., Manoja, V., Deepa, J., Jayakumar, J., Kishore, S. G., and Pandiselvam, R. (2024). Optimization of microwave-assisted aqueous extraction of pigments from annatto seeds using Box-Behnken design. Biomass Convers. Biorefinery 14 (16), 18775–18788. doi:10.1007/s13399-023-04046-7
Šudomová, M., Shariati, M. A., Echeverría, J., Berindan-Neagoe, I., Nabavi, S. M., and Hassan, S. T. S. (2019). A microbiological, toxicological, and biochemical study of the effects of fucoxanthin, a marine carotenoid, on Mycobacterium tuberculosis and the enzymes implicated in its cell wall: a link between mycobacterial infection and autoimmune diseases. Mar. Drugs 17 (11), 641. doi:10.3390/md17110641
Sun, D., Wu, S., Li, X., Ge, B., Zhou, C., Yan, X., et al. (2024). The structure, functions, and potential medicinal effects of chlorophylls derived from microalgae. Mar. Drugs 22 (2), 65. doi:10.3390/md22020065
Sun, L. C., Sridhar, K., Tsai, P. J., and Chou, C. S. (2019). Effect of traditional thermal and high-pressure processing (HPP) methods on the color stability and antioxidant capacities of Djulis (Chenopodium formosanum Koidz.). LWT 109, 342–349. doi:10.1016/j.lwt.2019.04.049
Sun, T., Rao, S., Zhou, X., and Li, L. (2022). Plant carotenoids: recent advances and future perspectives. Mol. Hortic. 2, 3–21. doi:10.1186/s43897-022-00023-2
Sun, W., Liu, Y., Jia, L., Saldaña, M. D. A., Dong, T., Jin, Y., et al. (2021). A smart nanofibre sensor based on anthocyanin/poly-l-lactic acid for mutton freshness monitoring. Int. J. Food Sci. and Technol. 56, 342–351. doi:10.1111/ijfs.14648
Tanaka, A., and Tanaka, R. (2019). “The biochemistry, physiology, and evolution of the chlorophyll cycle,” in Advances in botanical research. Editor B. Grimm (Academic Press), 183–212.
Tapia-Quirós, P., Granados, M., Sentellas, S., and Saurina, J. (2024). Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: mature for scaling-up? Sci. Total Environ. 912 (November 2023), 168716. doi:10.1016/j.scitotenv.2023.168716
Tarozzi, A., Morroni, F., Hrelia, S., Angeloni, C., Marchesi, A., Cantelli-Forti, G., et al. (2007). Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci. Lett. 424, 36–40. doi:10.1016/j.neulet.2007.07.017
Thies, F., Mills, L. M., Moir, S., and Masson, L. F. (2017). “Cardiovascular benefits of lycopene: fantasy or reality?,” in Proceedings of the nutrition society. Editor A. Gallagher (Cambridge University Press), 122–129.
Thurnham, D. I., Nolan, J. M., Howard, A. N., and Beatty, S. (2015). Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefe's Archive Clin. Exp. Ophthalmol. 253 (8), 1231–1243. doi:10.1007/s00417-014-2811-3
Toti, E., Chen, C.-Y. O., Palmery, M., Villaño Valencia, D., and Peluso, I. (2018). Non-provitamin A and provitamin A carotenoids as immunomodulators: recommended dietary allowance, therapeutic index, or personalized nutrition? Oxidative Med. Cell. Longev. 2018, 4637861. doi:10.1155/2018/4637861
Umigai, N., Murakami, K., Shimizu, R., Takeda, R., and Azuma, T. (2018). Safety evaluation and plasma carotenoid accumulation in healthy adult subjects after 12 Weeks of paprika oleoresin supplementation. J. Oleo Sci. 67, 225–234. doi:10.5650/jos.ess17155
Usman, M., Nakagawa, M., and Cheng, S. (2023). Emerging trends in green extraction techniques for bioactive natural products. Processes 11, 3444. doi:10.3390/pr11123444
Vaňková, K., Marková, I., Jašprová, J., Dvořák, A., Subhanová, I., Zelenka, J., et al. (2018). Chlorophyll-mediated changes in the redox status of pancreatic cancer cells are associated with its anticancer effects. Oxidative Med. Cell. Longev. 2018, 4069167. doi:10.1155/2018/4069167
Viera, I., Pérez-Gálvez, A., and Roca, M. (2019). Green natural colorants. Molecules 24, 154. doi:10.3390/molecules24010154
Wafa, B. A., Makni, M., Ammar, S., Khannous, L., Hassana, A. B., Bouaziz, M., et al. (2017). Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 241, 123–131. doi:10.1016/j.ijfoodmicro.2016.10.007
Wallace, T. C. (2011). Anthocyanins in cardiovascular disease. Adv. Nutr. 2 (1), 1–7. doi:10.3945/an.110.000042
Wang, C., Zhu, L., Ju, K., Liu, J., and Li, K. (2017). Anti-inflammatory effect of delphinidin on intramedullary spinal pressure in a spinal cord injury rat model. Exp. Ther. Med. 14, 5583–5588. doi:10.3892/etm.2017.5206
Wang, G., Cui, Q., Yin, L. J., Li, Y., Gao, M. Z., Meng, Y., et al. (2020). Negative pressure cavitation based ultrasound-assisted extraction of main flavonoids from Flos Sophorae Immaturus and evaluation of its extraction kinetics. Sep. Purif. Technol. 244, 115805. doi:10.1016/j.seppur.2019.115805
Wang, T., Guo, N., Wang, S. X., Kou, P., Zhao, C. J., and Fu, Y. J. (2018). Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 108, 69–80. doi:10.1016/j.fbp.2018.01.003
Wang, X., Yi, F., Zhang, W., and Guo, X. (2022). Optimization of the application of walnut green peel pigment in wool fiber dying and fixing process under microwave-assisted condition. J. Nat. Fibers 19 (13), 4854–4867. doi:10.1080/15440478.2020.1870632
Wei, J., Zhang, G., Zhang, X., Xu, D., Gao, J., Fan, J., et al. (2017). Anthocyanins from black chokeberry (aroniamelanocarpa elliot) delayed aging-related degenerative changes of brain. J. Agric. Food Chem. 65, 5973–5984. doi:10.1021/acs.jafc.7b02136
Werlein, H.-D., Kütemeyer, C., Schatton, G., Hubbermann, E. M., and Schwarz, K. (2005). Influence of elderberry and blackcurrant concentrates on the growth of microorganisms. Food control. 16, 729–733. doi:10.1016/j.foodcont.2004.06.011
Wilson, L. M., Tharmarajah, S., Jia, Y., Semba, R. D., Schaumberg, D. A., and Robinson, K. A. (2021). The effect of lutein/zeaxanthin intake on human macular pigment optical density: a systematic review and meta-analysis. Adv. Nutr. 12 (6), 2244–2254. doi:10.1093/advances/nmab071
Winter, A. N., and Bickford, P. C. (2019). Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 8, 333. doi:10.3390/antiox8090333
Winter, A. N., Brenner, M. C., Punessen, N., Snodgrass, M., Byars, C., Arora, Y., et al. (2017). Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxidative Med. Cell. Longev. 2017 (1), 6297080. doi:10.1155/2017/6297080
Wu, C. F., Wu, C. Y., Lin, C. F., Liu, Y. W., Lin, T. C., Liao, H. J., et al. (2022). The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. and Pharmacother. 151, 113128. doi:10.1016/j.biopha.2022.113128
Wu, H.-Y., Yang, K.-M., and Chiang, P.-Y. (2018). Roselle anthocyanins: antioxidant properties and stability to heat and pH. Molecules 23, 1357. doi:10.3390/molecules23061357
Yilmaz, C., and Gökmen, V. (2016). “Chlorophylls,” in Encyclopedia of food and health. Editors B. Caballero, P. M. Finglas, and F. Toldrá (Elsevier), 37–41.
Yu, L., Zhang, S., Zhao, X., Ni, H., Song, X., Wang, W., et al. (2020). Cyanidin-3-glucoside protects liver from oxidative damage through AMPK/Nrf2 mediated signaling pathway in vivo and in vitro. J. Funct. Foods 73, 104148. doi:10.1016/J.JFF.2020.104148
Zamudio-Flores, P. B., Ochoa-Reyes, E., Ornelas-Paz, J. D. J., Aparicio-Saguilán, A., Vargas-Torres, A., Bello-Pérez, L. A., et al. (2015). Effect of storage time on physicochemical and textural properties of sausages covered with oxidized banana starch film with and without betalains. CyTA—Journal of Food 13 (3), 463–41. doi:10.1080/19476337.2014.998713
Zeng, Z., Li, H., Luo, C., Hu, W., Weng, T., and Shuang, F. (2023). Pelargonidin ameliorates inflammatory response and cartilage degeneration in osteoarthritis via suppressing the NF-κB pathway. Archives Biochem. Biophysics 743, 109668. doi:10.1016/j.abb.2023.109668
Zhang, Y., Meng, Q., Yin, J., Zhang, Z., Bao, H., and Wang, X. (2020). Anthocyanins attenuate neuroinflammation through the suppression of MLK3 activation in a mouse model of perioperative neurocognitive disorders. Brain Res. 1726, 146504. doi:10.1016/J.BRAINRES.2019.146504
Zhao, P., Li, X., Yang, Q., Lu, Y., Wang, G., Yang, H., et al. (2023). Malvidin alleviates mitochondrial dysfunction and ROS accumulation through activating AMPK-α/UCP2 axis, thereby resisting inflammation and apoptosis in SAE mice. Front. Pharmacol. 13, 1038802. doi:10.3389/fphar.2022.1038802
Zhao, T., Yan, X., Sun, L., Yang, T., Hu, X., He, Z., et al. (2019). Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. and Technol. 91, 354–361. doi:10.1016/J.TIFS.2019.07.014
Zhao, X., Feng, P., He, W., Du, X., Chen, C., Suo, L., et al. (2019). The prevention and inhibition effect of anthocyanins on colorectal cancer. Curr. Pharm. Des. 25, 4919–4927. doi:10.2174/1381612825666191212105145
Zheng, J., Li, H., Wang, D., Li, R., Wang, S., and Ling, B. (2021). Radio frequency assisted extraction of pectin from apple pomace: process optimization and comparison with microwave and conventional methods. Food Hydrocoll. 121 (July), 107031. doi:10.1016/j.foodhyd.2021.107031
Zhou, L., Ouyang, L., Lin, S., Chen, S., Liu, Y., Zhou, W., et al. (2018). Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 61, 92–99. doi:10.1016/j.intimp.2018.05.022
Zhou, L., Xie, M., Yang, F., and Liu, J. (2019). Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT 117, 108621. doi:10.1016/j.lwt.2019.108621
Keywords: natural pigments, extraction, antioxidant, anticancer, food colorants
Citation: Masyita A, Hardinasinta G, Astuti AD, Firdayani F, Mayasari D, Hori A, Nisha INA, Nainu F and Kuraishi T (2025) Natural pigments: innovative extraction technologies and their potential application in health and food industries. Front. Pharmacol. 15:1507108. doi: 10.3389/fphar.2024.1507108
Received: 07 October 2024; Accepted: 09 December 2024;
Published: 08 January 2025.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Margherita Campo, University of Florence, ItalyArpita Das, Adamas University, India
Daniela Russo, University of Basilicata, Italy
Sangeeta Saikia, CSIR NEIST, India
Copyright © 2025 Masyita, Hardinasinta, Astuti, Firdayani, Mayasari, Hori, Nisha, Nainu and Kuraishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayu Masyita, YXl1bTAwMkBicmluLmdvLmlk; Firzan Nainu, ZmlyemFubmFpbnVAdW5oYXMuYWMuaWQ=; Takayuki Kuraishi, dGt1cmFpc2hpQHN0YWZmLmthbmF6YXdhLXUuYWMuanA=
 Ayu Masyita
Ayu Masyita Gemala Hardinasinta
Gemala Hardinasinta Ayun Dwi Astuti
Ayun Dwi Astuti Firdayani Firdayani
Firdayani Firdayani Dian Mayasari
Dian Mayasari Aki Hori5
Aki Hori5 Takayuki Kuraishi
Takayuki Kuraishi