- 1Young Researchers and Elite Club, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran
- 2Faculty of General Medicine, University of Traditional Medicine of Armenia (UTMA), Yerevan, Armenia
- 3Department of Dentistry, University of Traditional Medicine of Armenia (UTMA), Yerevan, Armenia
- 4Department of Cardiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 5DWA Energy Limited, Lincoln, United Kingdom
- 6Department of Nursing and Midwifery, Faculty of Nursing, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 7Faculty of General Medicine, Yaroslavl State Medical University, Yaroslavl, Russia
Background and Objective: Dental implant therapy faces challenges in patients with Type 1 and Type 2 Diabetes Mellitus (T1DM and T2DM) due to adverse effects on bone metabolism and immune response. Despite advancements, diabetic patients face higher risks of peri-implantitis and compromised osseointegration. This review assesses the impact of anti-diabetic medications on implant outcomes, offering insights to bridge the gap between animal studies and clinical practice. By evaluating pharmacotherapeutic strategies in preclinical models, this review guides future research designs to improve implant success rates in diabetic individuals.
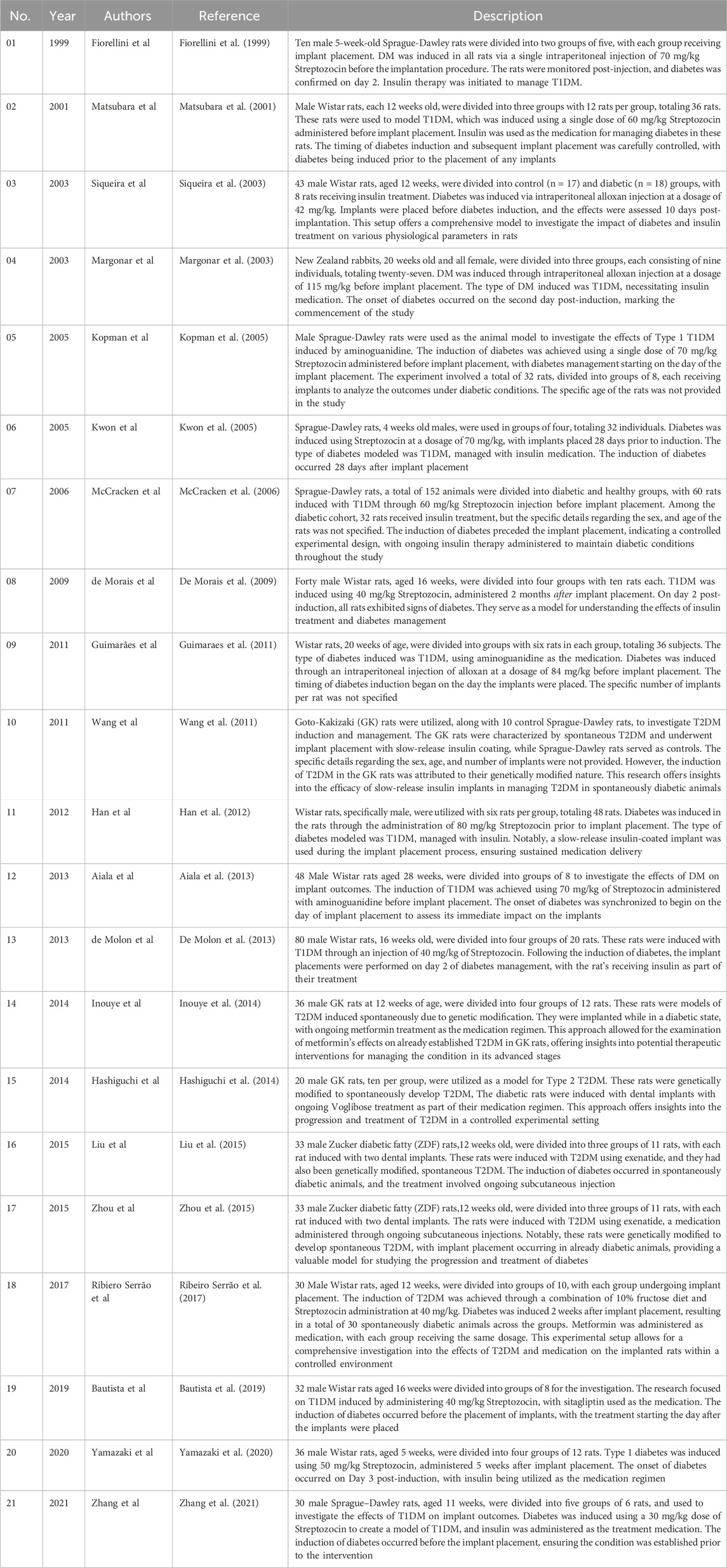
Method: A comprehensive literature review identified 21 animal studies examining the impact of anti-diabetic medications on dental and bone implants. These studies explored diabetes models, medication regimens, and designs to assess outcomes related to bone metabolism, osseointegration, and peri-implant tissue responses. The findings are systematically summarized, highlighting the scope, design, and procedures of each study. An example includes placing a dental implant in the molar region of a mouse, providing insight into preclinical approaches.
Results: Twenty-one animal studies, primarily using rodents, investigate various anti-diabetic medications on dental and bone implants. Interventions include insulin, aminoguanidine, voglibose, sitagliptin, exenatide, and metformin, analyzing outcomes like bone-implant contact (BIC), bone volume (BV), and counter-torque values in T1DM and T2DM models. The impacts of these medications on implant osseointegration under diabetic conditions are detailed, with their benefits and shortcomings assessed.
Discussion: The findings and challenges of existing animal studies on diabetes mellitus (DM) and implant osseointegration are presented. Despite T2DM prevalence, research primarily focuses on T1DM models due to easier experimental practicalities, limiting applicability. Inconsistent protocols in studies compromise reliability regarding anti-diabetic treatments’ effectiveness on osseointegration. Standardized methodologies and long-term assessments of local drug delivery alongside systemic anti-DM treatments are crucial to manage DM-related complications in implant dentistry.
Conclusion: Insulin administration in short-term T1DM animal studies enhances implant osseointegration. However, the efficacy of non-insulin medications remains inconclusive. Rigorous experimental designs are needed to address inconsistencies and assess long-term impacts. Larger-sized (e.g., porcine) animal studies across various intraoral implant scenarios are required. Future research should focus on enhancing clinical applicability and improving implant stability in evolving conditions.
Highlights
• Improved dental implant therapy in diabetic patients is needed.
• Impact of anti-diabetic drugs on dental implants: animal studies versus clinical outcomes.
• Insulin impacts identified in bone mineral content in Type 1 Diabetes Mellitus (T1DM).
• Disparities exist between animal studies and clinical implant outcomes.
• Critical knowledge gap exists in dental implant performance with T2DM conditions.
1 Introduction
Type 2 Diabetes Mellitus (T2DM) is prevalent among the elderly population, as reported by the World Health Organization (WHO) in 2016, with a significant increase observed in recent decades (Who, 2016). Hyperglycemia associated with T2DM contributes to various oral complications, including heightened susceptibility to periodontal diseases, compromised oral immunity, and delayed oral wound healing (Kocher et al., 2018). Consequently, individuals with T2DM are at an elevated risk of tooth loss (Jimenez et al., 2012).
Dental implant treatment is an established practice for replacing missing teeth offering a practical alternative to dental bridges and removable dentures (Preoteasa et al., 2015). Despite initial concerns, research has investigated peri-implant complications, such as marginal bone loss and peri-implantitis, in diabetic patients to ascertain their suitability for dental implant treatment (Gómez-Moreno et al., 2015; Aldahlawi et al., 2021). The prevalence of diabetes mellitus (DM) globally is on the rise and affecting younger as well as older age groups, largely attributed to increasing rates of obesity and more sedentary lifestyles. Recent studies indicate a substantial number of individuals are now affected by DM, and those numbers are projected to rise further by 2050 (Ong et al., 2023).
Diabetes constitutes a significant risk factor for periodontitis, a condition closely linked to tooth loss (Nascimento et al., 2018; Lau et al., 2022; Naseer et al., 2024). Several hormones play pivotal roles in regulating bone metabolism (Niwczyk et al., 2023), which in turn significantly affects dental implant success (Niwczyk et al., 2023). Parathyroid hormone (PTH) promotes bone formation by enhancing osteoblast activity and inhibiting osteoclast activity, which can support bone remodeling around implants. Insulin, crucial for glucose regulation, also plays a key role in bone metabolism, promoting osteoblast differentiation and bone formation. Estrogen and testosterone, both of which influence bone density (Mills et al., 2021), are especially relevant in postmenopausal women and men with osteoporosis. Testosterone deficiency is linked to increased risk of osteoporosis, potentially affecting implant success. Adiponectin, typically associated with fat tissue, has been shown to stimulate osteoblast activity and suppress osteoclast formation, which may enhance bone healing. Also, oxytocin, known for its role in labor, promotes bone formation and reduces bone resorption. Despite the potential of these hormones to enhance osseointegration, diabetes-induced hormonal imbalances can complicate their beneficial effects on bone healing (Tunheim et al., 2023). The collective effects of the mentioned hormones have the potential to improve dental implant outcomes, though more research is required to optimize their clinical application (Tunheim et al., 2023). Consequently, individuals with diabetes are more susceptible to tooth loss and edentulism, irrespective of their glycemic control status (Vu et al., 2022). Recent research has identified the intricate relationship between hyperglycemia and implant failure in individuals with T2DM. While systemic complications of T2DM are well-established, the impact on bone metabolism and, consequently, implant outcomes have emerged as a significant concern. Building upon previous findings, contemporary investigations have delved into the molecular mechanisms underlying impaired bone healing and compromised osseointegration in the context of elevated glucose levels.
Recent studies have expanded upon the results of animal studies to explore human clinical data, providing valuable insights into the translational relevance of earlier experimental findings (Sábado-Bundó et al., 2019; Ayele et al., 2023). In particular, advanced imaging techniques such as micro-CT scanning combined with histomorphometry analyses have offered detailed assessments of bone-implant interfaces in diabetic patients (Ding et al., 2019). These analyses have revealed distinct morphological alterations and reduced bone volume fractions surrounding implants in individuals with poorly controlled T2DM (Xiang et al., 2020). These results are indicative of impaired osseointegration.
The role of chronic inflammation and oxidative stress in exacerbating implant complications within hyperglycemic environments has garnered considerable attention (Corduas et al., 2020). The utilization of dental implants among individuals with T2DM remains a contentious issue due to the potential detrimental impact of hyperglycemia on osseointegration. T2DM is associated with heightened inflammatory responses to oral biofilms, potentially exacerbating predisposition to gingivitis (Sundar, 2018). Current evidence is insufficient to support comparable outcomes of implant therapy between patients with and without DM. Kopman et al. (2005), using rat models, demonstrated the inhibitory effects of diabetes on osseointegration, evidenced by reduced bone-to-implant contact (BIC) (Kopman et al., 2005).
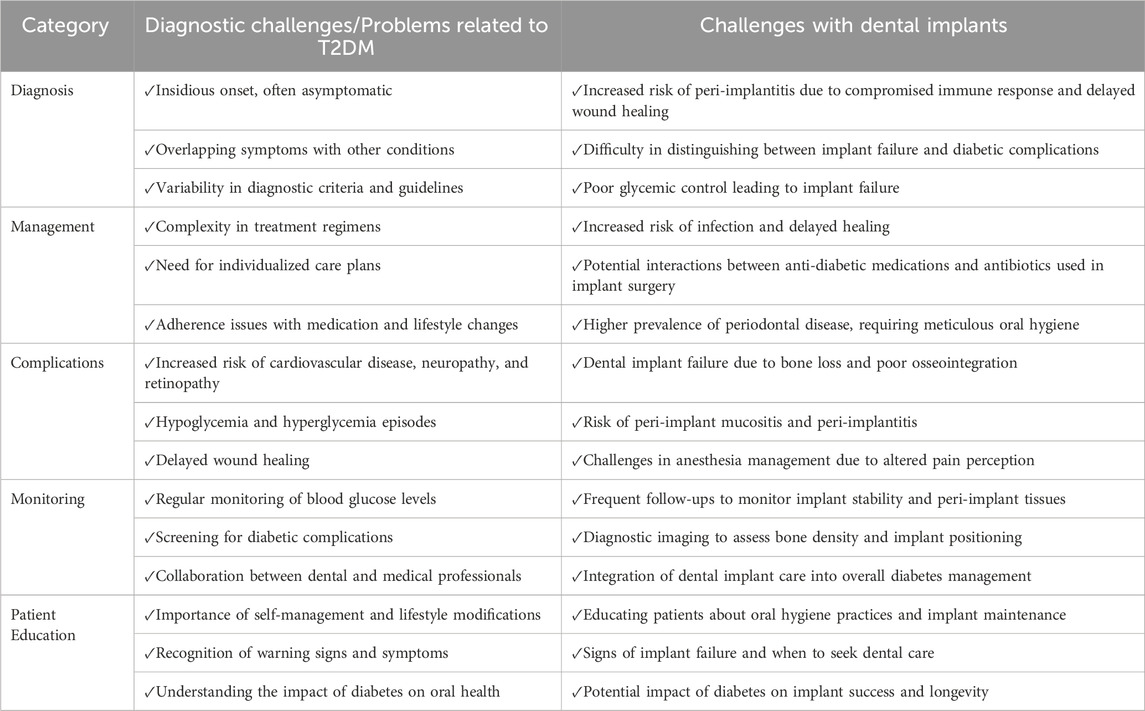
Human research indicates increased alveolar bone loss in diabetic patients (Tabassum, 2022), potentially due to elevated secretion of proinflammatory cytokines including interleukin (IL)-1b, IL-6, and tumor necrosis factor-alpha (TNF-a) in serum and gingival fluid. These cytokines are also attributed to accelerated age receptor (RAGE) interactions. Heightened expression of proinflammatory cytokines within bone tissues suggests an intrinsic inflammatory response in the bones of diabetes suffers, potentially enhancing Osteoclastogenesis and bone resorption. Figure 1 show the pathogenesis of the insufficient bone formation and bone loss due to the DM. Consequently, tailored and clinically proven strategies for implant placement and management in diabetic patients are required. Ongoing research is focusing on the development of novel biomaterials and pharmacotherapeutic interventions to modulate inflammatory responses to dental implants. Bridging basic science and clinical practice holds promise for improving long-term dental implant success rates in individuals with T2DM. Table 1 summarizes the diagnostic challenges and hurdles in diagnosis, management, complications, monitoring, and patient education associated with T2DM and dental implants.
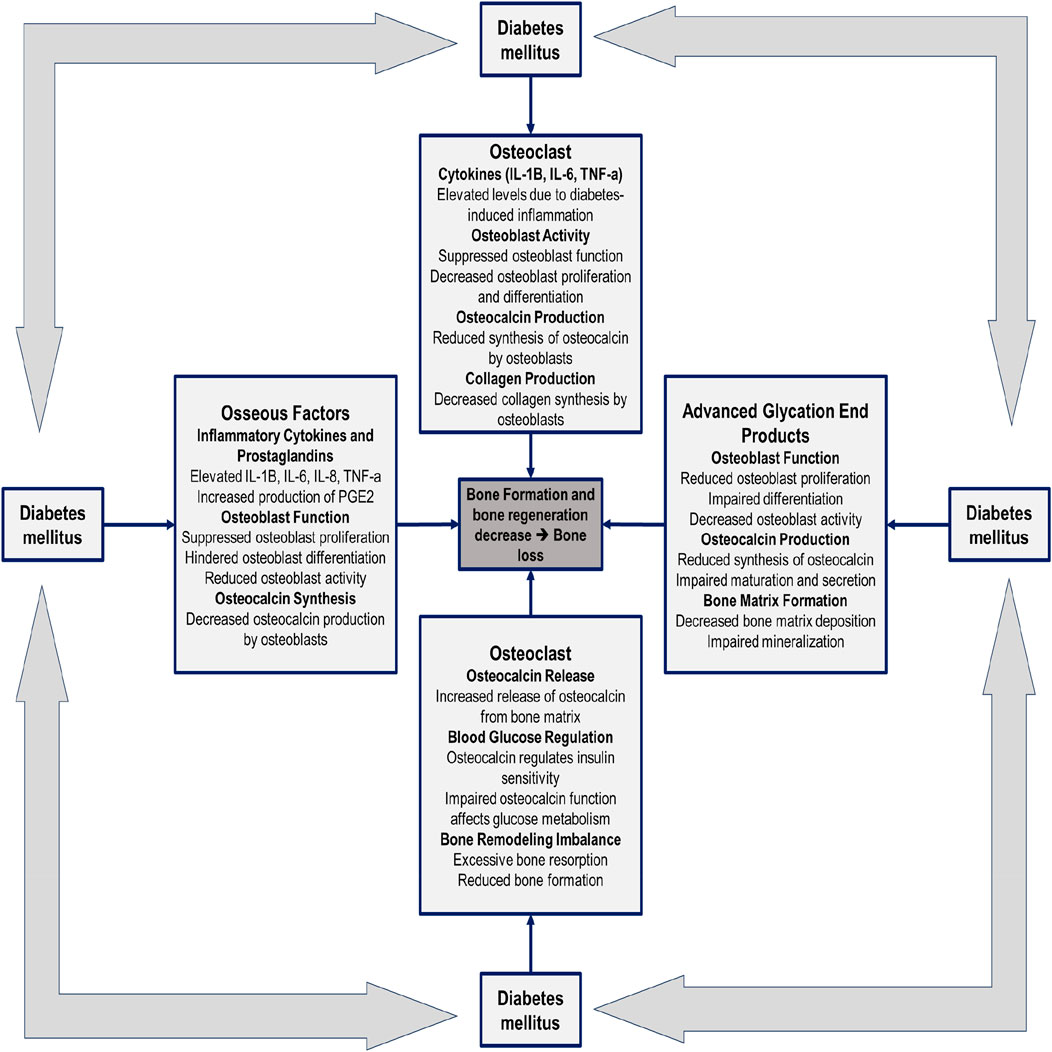
Figure 1. Factors influencing the pathogenesis of insufficient bone formation and bone loss resulting from diabetes mellitus.
This review collates and assesses information on the types of drugs used for anti-diabetic management in the context of dental procedures, including dental implants. The bulk of the existing research on anti-diabetic drugs is related to animal studies, with relatively few based on human studies and clinical trials. This discrepancy represents a research gap that justifies more focused human studies. It is well established that findings from animal studies often serve as a precursor to human trials, rendering this avenue of investigation invaluable to researchers and clinicians engaged in this field of study. Hence, it is important to understand the existing body of knowledge gained from animal studies regarding anti-diabetic therapies on dental implants to assist in the design and execution of future human studies.
2 Method
A comprehensive literature review identified twenty-one published studies focusing on animal models to explore the effects of anti-diabetic medications on dental and bone implants. These studies investigated various experimental designs, including the induction of diabetes using agents like Streptozocin or alloxan and the administration of medications such as insulin and metformin. The research analyzed outcomes related to bone metabolism, osseointegration, and peri-implant tissue responses. Figure 2 illustrates an example of dental implant placement in a mouse model. Table 2 provides a detailed summary of the study scopes, methodologies, and results. This approach highlights the preclinical evidence base for understanding the interplay between diabetes, its treatments, and implant success.
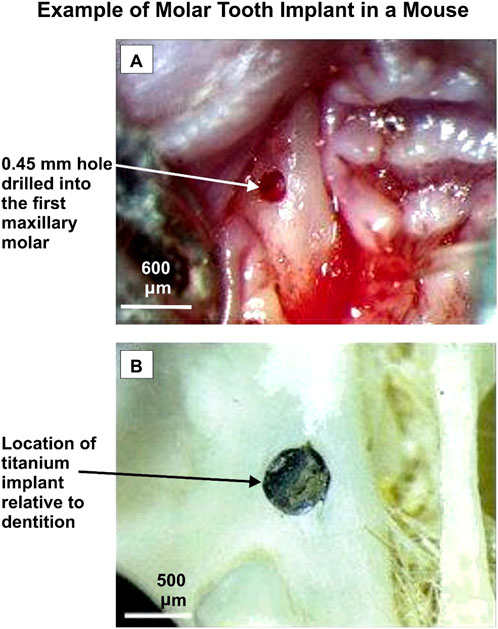
Figure 2. An example of a molar tooth implant in a mouse. Adapted from Mouraret et al. (2014).
Table 2 describes, in summary, the scopes, designs and procedures of those studies.
3 Result
The 21 studies described incorporated a variety of pharmacological interventions for diabetes management, including:
(A) Insulin as the treatment drug
(B) Non-Insulin treatment drugs:
• Aminoguanidine
• Voglibose
• Sitagliptin
• Exenatide
• Metformin
Four of these studies concentrated on T1DM and explored how effective non-insulin medications could be as treatments (Kopman et al., 2005; Guimaraes et al., 2011; Aiala et al., 2013; Bautista et al., 2019). One study focused on T2DM and investigated the impact of insulin treatment on the progression of osseointegration (Wang et al., 2011). Key measures of interest included bone-implant contact (BIC), bone volume (BV), and counter-torque values (N/cm), which served as indicators of the progress of implant osseointegration. Figure 3 provides a schematic of the periodontium and the disturbance impacts related to counter-torque measurements. The periodontium, as depicted in Figure 3, plays a critical role in the stability and success of dental implants, especially in individuals with diabetes. Figure 3A illustrates the normal anatomy of the periodontium, including key components such as the tooth crown, gingiva, periodontal ligaments, cementum, tooth root, and alveolar bone. These structures work cohesively to provide structural support and respond to mechanical forces. Figure 3B demonstrates how directional forces exert tension and compression on the periodontium, with distinct red and blue regions indicating these stress zones. This mechanical response is vital in assessing counter-torque stability—a key metric for evaluating the success of dental implants. In the context of diabetes, the periodontium is particularly vulnerable due to several physiological disruptions. Elevated blood glucose levels impair wound healing, reducing the ability of the periodontium to recover from surgical procedures such as implant placement. Additionally, diabetes-induced vascular damage limits blood flow to the gingiva and supporting tissues, weakening their structural integrity and making them more prone to infection. Compounding this is the immunosuppressive effect of diabetes, which diminishes the body’s ability to combat infections, including peri-implantitis—a condition marked by inflammation and infection around the implant site (Schwarz et al., 2018). These factors collectively increase the risk of delayed healing, compromised osseointegration, and implant failure in diabetic patients. The mechanical stresses shown in Figure 3 further highlight the challenges of achieving successful implant integration in compromised tissues, emphasizing the need for meticulous management of diabetes and tailored dental care to enhance implant outcomes.
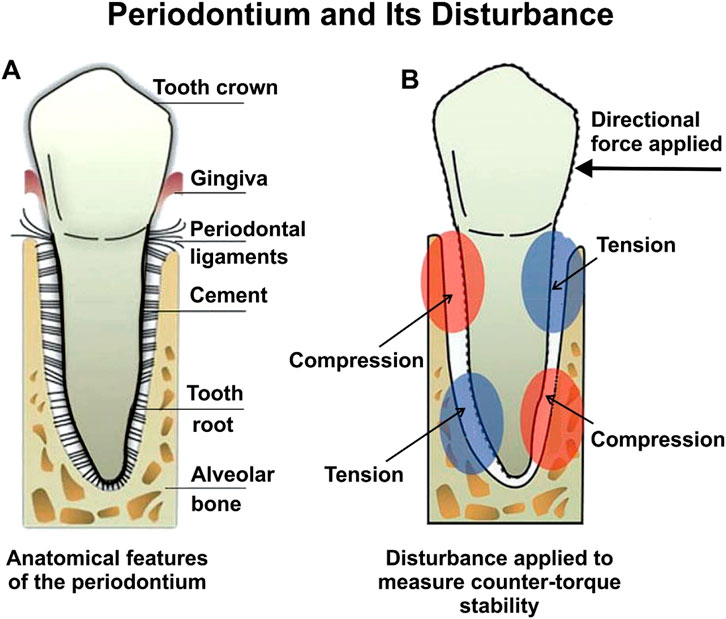
Figure 3. (A) Periodontium features, and (B) Periodontium disturbance when measuring counter-torque stability. Adapted from d’Apuzzo et al. (2017).
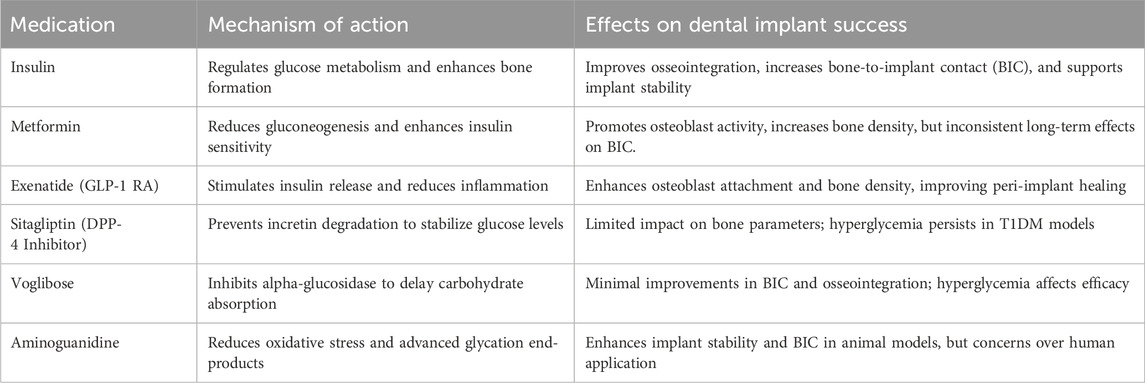
The role of pharmacological interventions in managing diabetes is pivotal for improving dental implant success in individuals with Type 1 and Type 2 Diabetes Mellitus (T1DM and T2DM) (Rokaya et al., 2020). Different classes of anti-diabetic drugs, including insulin, GLP-1 receptor agonists, DPP-4 inhibitors, biguanides, and alpha-glucosidase inhibitors, have shown varying effects on glycemic control, bone metabolism, and implant osseointegration in both preclinical and clinical studies (Rokaya et al., 2020). While insulin remains critical for its direct impact on osseointegration and bone healing, medications like metformin and exenatide have demonstrated potential benefits by enhancing osteoblast activity and reducing inflammation. Conversely, some medications, such as bisphosphonates, may negatively affect implant outcomes by impairing bone healing (Kalaitzoglou et al., 2019). Table 3 summarizes these medications and their specific effects on dental implant success.
Table 4 provides detailed information regarding the placement of implants, the specific medications used, their ability to control blood sugar levels, and the corresponding outcomes of implants for each of the mentioned studies.
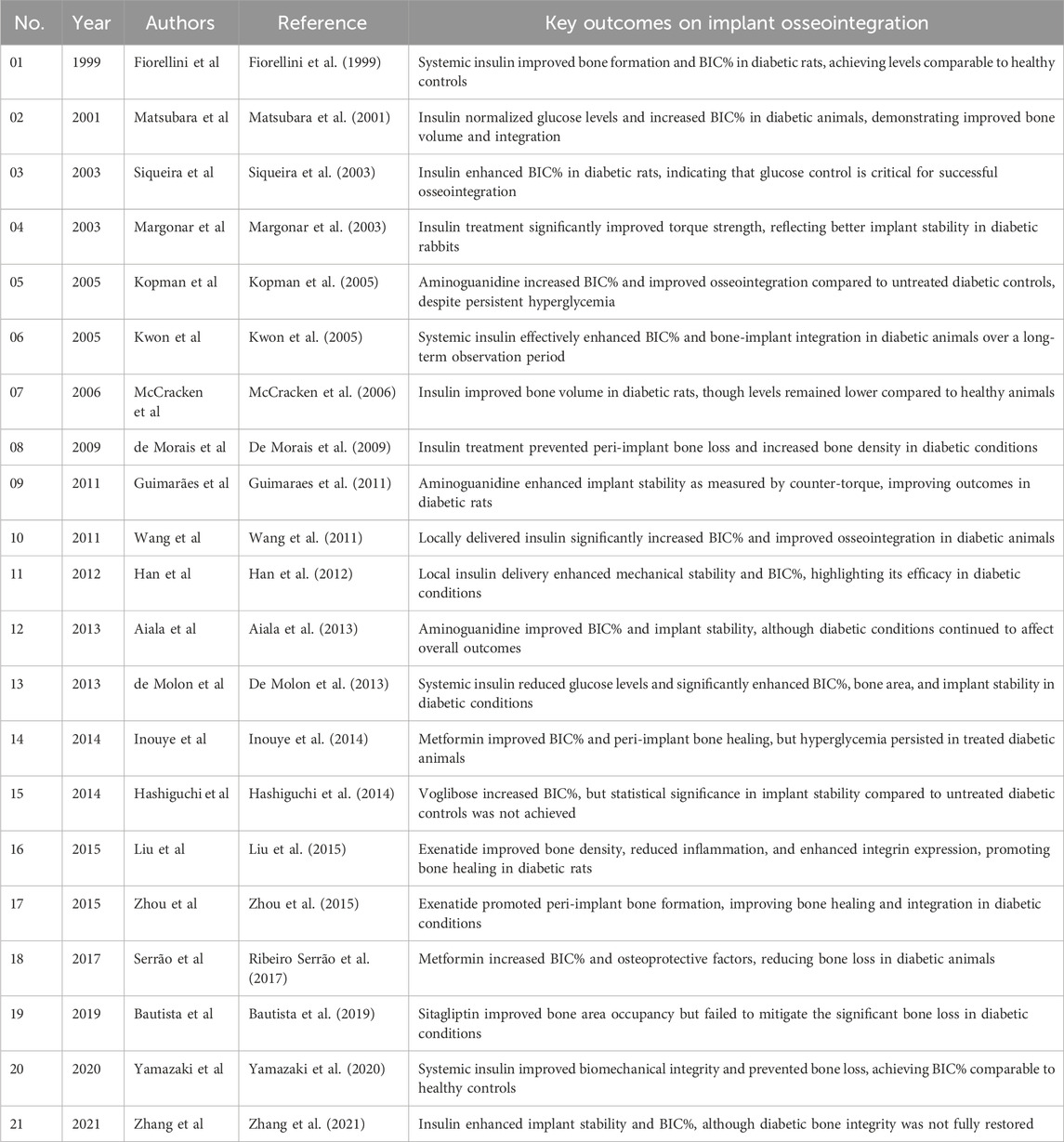
Table 4. Animal studies on anti-diabetic medications and bone implants: key outcomes of interest in implant osseointegration.
The studies reviewed in Tables 3, 4 investigate the impacts of various drugs on bone integration in animal models of DM. Aminoguanidine, metformin, sitagliptin, and insulin were among the drugs scrutinized for their effects on osseointegration. Aminoguanidine demonstrated potential in enhancing BIC% and mechanical stability in diabetic animals, despite persistent hyperglycemia. Conversely, sitagliptin, which stimulates insulin secretion, did not improve bone parameters due to ongoing hyperglycemia and the destruction of pancreatic β-cells in the diabetic models studied. Insulin, whether administered systemically or locally, consistently showed benefits in enhancing BIC%, peri-implant bone density, and mechanical retention in both T1DM and T2DM animal models, underscoring its important role, not only in glycemic control, but also in bone metabolism and remodeling. These findings highlight the complex interplay between diabetes, anti-diabetic medications, and bone health, suggesting that while some drugs may improve bone outcomes, sustained hyperglycemia can hinder their efficacy (Table 5). Despite the potential of anti-diabetic medications, their efficacy in promoting bone healing and osseointegration is often limited by persistent hyperglycemia. This challenge is underscored by contrasting results across animal studies, where some medications, such as insulin, show promise in controlling blood glucose and promoting bone healing, while others, like sitagliptin, fail to overcome the detrimental effects of sustained hyperglycemia. These outcomes highlight the critical need for more effective glycemic control alongside anti-diabetic treatment to optimize implant outcomes.
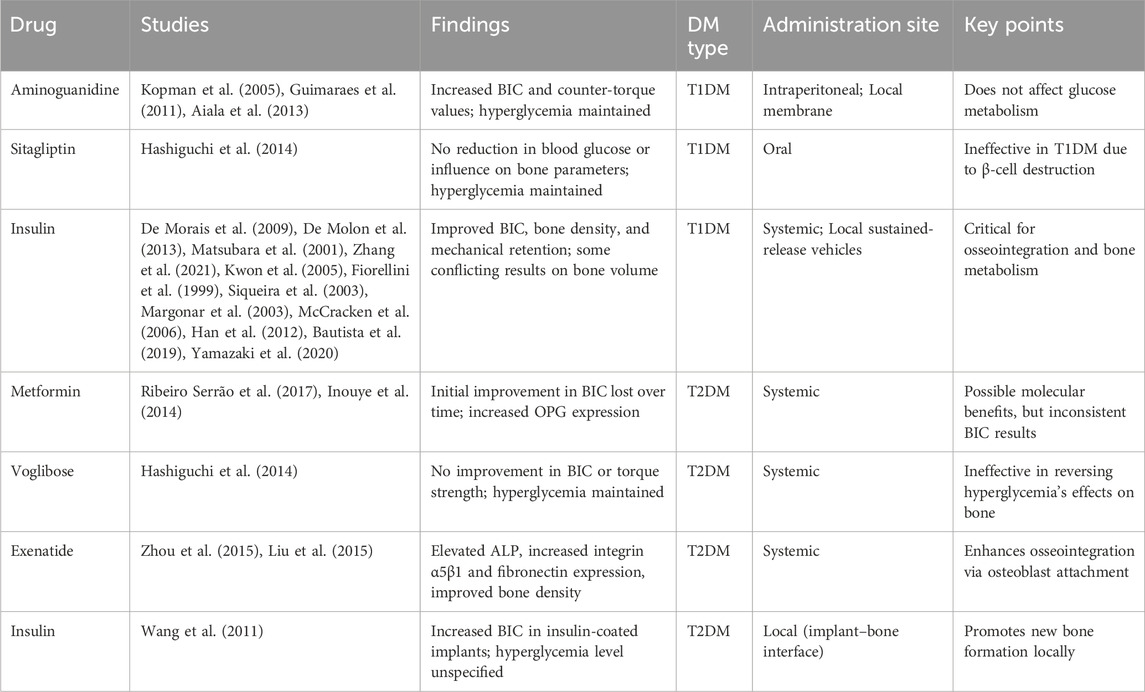
Table 5. Impact of diabetes medications on dental and bone implant results in animal models of T1DM and T2DM.
4 Discussion
It is commonly recognized that well-conducted animal studies are crucial for producing strong molecular and cellular evidence that can guide improvements in clinical practice. A scoping review highlighted a predominant focus on T1DM models despite T2DM being more prevalent in humans. The likely reason for this bias is the cost-effectiveness and ease of disease induction for T1DM compared to T2DM. The research involved various animal strains and widely differing methodologies for diabetes induction, anti-diabetic medication administration, disease timing, and location of implant placement. One of the most significant parameters in animals is blood glucose levels, which can directly affect both T1DM and T2DM models. Throughout the studies, animals often remained hyperglycemic, mirroring poorly controlled diabetes in humans. However, inconsistent glycemic control, deviations from experimental protocols, and a lack of standardization have undermined the reliability and conclusiveness of findings concerning the effects of anti-diabetic treatments on implant osseointegration. Moreover, assessments of potential bias in the studies revealed significant shortcomings in accounting for experimental variables, reinforcing the critical need for researchers to address blinding, randomization, disease severity heterogeneity, husbandry practices, and reporting standards to ensure robust study design and reliable outcomes.
Peri-implantitis, characterized by inflammation and progressive bone loss, further exacerbates challenges in diabetic patients. Factors such as microbial biofilms, impaired immune responses, and reduced vascularization at implant sites significantly affect outcomes (Rokaya et al., 2020). The risk of peri-implantitis, characterized by inflammation and bone loss around implants, is heightened in diabetic patients due to several factors. Elevated glucose levels impair neutrophil function, a crucial component of the immune system, thereby increasing the risk of infection and inflammatory responses at implant sites. Additionally, the increased levels of matrix metalloproteinases (MMPs), particularly MMP-8, further accelerate bone degradation, making it difficult for implants to integrate properly. These biochemical and immune responses collectively exacerbate the risk of peri-implantitis, underscoring the importance of managing hyperglycemia to reduce infection risks and improve implant success. Systemic and local factors that influence bone metabolism, such as vitamin D deficiency or elevated pro-inflammatory cytokines, also exacerbate disease progression (Rokaya et al., 2020).
Certain medications may inadvertently promote peri-implantitis. For example, bisphosphonates, while beneficial for osteoporosis, can impair bone healing and increase the risk of osteonecrosis in dental implant sites. Conversely, drugs like metformin and GLP-1 receptor agonists, which enhance bone metabolism and reduce inflammation, may mitigate these risks. Local drug delivery systems, such as insulin or chlorhexidine-coated implants, have shown promise in reducing peri-implant inflammation and improving outcomes, although their long-term benefits remain unclear (Rokaya et al., 2020).
Most of the studies considered involved short durations (4–16 weeks), and all but one study (Margonar et al., 2003) used rodents. The preference for rodents as experimental subjects is associated with their accelerated bone metabolism and turnover rates. This tends to reduce experimental expenses and reduce animal care requirements (Omar et al., 2020). However, the relatively small size of rodents limits the type of surgery and implant placement that can be performed; constraining it to approximately 1 × 2 mm dimensions (Blanc-Sylvestre et al., 2021). Consequently, rodent studies involve limitations when considering analogous clinical scenarios in humans (Prabhakar, 2012; Mohd et al., 2022a; Mohd et al., 2022b). For example, rodent implant studies commonly involve the tibia or femur (larger bones) rather than maxillary/mandibular dental extraction sockets. Oral-cavity implants face a different set of issues to leg bones, including, including bacterial plaque, masticatory forces, exposure to food particles and microorganisms, which are constant sterility threats to intraoral implants (Blanc-Sylvestre et al., 2021). Hence, questions exist regarding the extrapolating from rodent test results to likely human outcomes. Porcine experimental subjects (e.g., Göttingen minipigs) can provide a better scale of animal subject for oral implants with greater similarity to humans regarding bone regeneration and metabolism. In porcine experiments it is possible to conduct multiple jawbone implants facilitating a broader spectrum of biomarker and genetic bone-impact research model (Musskopf et al., 2022; Coelho et al., 2018).
The animal studies reviewed used various anti-diabetic medications including biguanides, alpha-glucosidase inhibitors, GLP-1 analogs, DPP-4 inhibitors, and insulin. Aminoguanidine acts by scavenging free radicals and blocking AGE formation, thus reducing oxidative damage and complications associated with AGEs (Guimaraes et al., 2011). Sitagliptin, a DPP-4 inhibitor, enhances glycemic control by preventing the degradation of incretins like GLP-1, thereby stimulating insulin release and stabilizing post-meal blood sugar levels. Metformin reduces gluconeogenesis and intestinal glucose absorption while enhancing peripheral insulin sensitivity (Jiating et al., 2019). Voglibose, an alpha-glucosidase inhibitor, competitively blocks glucose absorption after meals (Chen et al., 2017). Exenatide, an agonist of the GLP-1 receptor, stimulates insulin release, suppresses glucagon activity, and delays gastric emptying. Despite these mechanisms, most non-insulin medications did not achieve sufficient control of blood glucose levels or improve osseointegration in the animal studies reviewed (Kopman et al., 2005; Aiala et al., 2013; Inouye et al., 2014; Hashiguchi et al., 2014; Liu et al., 2015; Zhou et al., 2015; Ribeiro Serrão et al., 2017). Sitagliptin’s effectiveness was questioned in rat models of T1DM, raising doubts about its utility in research (Kilkenny et al., 2010). Aminoguanidine demonstrated promise in enhancing healing around implants in animals with T1DM (Kopman et al., 2005; Guimaraes et al., 2011; Aiala et al., 2013), but concerns over significant reported side effects in humans have clouded its clinical application (Bolton et al., 2004). Insulin, vital for managing T1DM, has shown positive effects on glucose regulation, promoting osseointegration, and reversing bone changes compared to other medications (Kwon et al., 2005). There are gaps in research regarding the impact of systemic insulin in T2DM animal models and its comparison with outcomes in T1DM, highlighting the need for future studies to consider diabetes pathophysiology and drug mechanisms to evaluate effectiveness, safety, and influence on implant osseointegration.
Three of the studies reviewed involved local drug delivery directly at the implant sites in attempts to enhance osseointegration (Wang et al., 2011; Han et al., 2012; Aiala et al., 2013). Although initial outcomes showed improved BIC and counter-torque values, the benefits were temporary, as systemic hyperglycemia persisted in the animals. Unfortunately, these short-duration test results provide no indication of the longer-lasting impacts of the treatments involved on osseointegration. It is therefore important to conduct further longer-term tests to more fully assess the post-drug release impacts on the progress of osseointegration, as ongoing bone responses may change over time following initial implant placement. The existing evidence suggests that bone resorption, reduced BIC, and ultimately implant failures are potential long-term effects of uncontrolled DM (Kwon et al., 2005; De Molon et al., 2013; Yamazaki et al., 2020). Closer scrutiny is required of the potential long-term human clinical benefits of implant-site-specific anti-DM drug delivery as either an alternative to, or a complement to, the more usual systemic anti-diabetic treatments.
5 Conclusion
Diabetes Mellitus (DM), encompassing both Type 1 (T1DM) and Type 2 (T2DM), poses substantial challenges to dental implant therapy due to its detrimental effects on bone metabolism and immune function. Despite advancements in implant technology, diabetic patients face heightened risks of peri-implantitis and compromised osseointegration. This review has synthesized findings from 21 animal studies investigating the impact of anti-diabetic medications on dental and bone implants, aiming to provide insight regarding the gap between preclinical research and clinical practice. The pharmacotherapeutic approaches of the various diabetes models evaluated, including the use of insulin and non-insulin agents like aminoguanidine, Voglibose, sitagliptin, exenatide, and metformin, reveal conflicting short-term outcomes. Dental and bone implant studies typically record and assess bone-implant contact (BIC), bone volume (BV), and counter-torque values. The results of the available short-term studies in T1DM models demonstrate the beneficial effects of insulin on enhancing BIC and implant retention. However, the effectiveness of non-insulin medications applied to implant subjects afflicted with T1DM and T2DM remains inconclusive.
The disparity between animal model findings and clinical applicability underscores critical methodological gaps, including inconsistent glycemic control, disparate study durations, and variable drug dosages and delivery methods. The interplay between bone metabolism, systemic health, and local peri-implant conditions underscores the complexity of managing dental implants in diabetic patients. Further research is warranted to optimize therapeutic strategies, standardize study designs, and evaluate the long-term effects of anti-diabetic and adjunctive therapies on peri-implant health. These discrepancies limit the translation of animal study results to human diabetic conditions. These shortcomings necessitate standardized experimental protocols and the consideration of local (implant-site-specific) drug delivery alongside systemic treatments to address diabetes-related complexities in implant dentistry more effectively. Moving forward, more comprehensive evaluations are required in larger-sized animal (e.g., porcine) models and intraoral implant settings. Such studies are required to substantiate or refute the findings published to date from mainly rodent-based, leg-bone studies. It is also important for future studies to focus on the potential long-term enhancement of implant stability amidst evolving diabetic scenarios. Consequently, there is a need for future research endeavors to prioritize extended preclinical investigations to assess long-term implant performance in humans developing T1DM and T2DM at different ages, thereby guiding optimized therapeutic strategies tailored to improve implant outcomes in diverse diabetic populations.
Author contributions
HGH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. DA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Validation, Visualization, Writing–original draft, Writing–review and editing. PGH: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. DW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Resources, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. RY: Conceptualization, Data curation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. SGH: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. NM: Conceptualization, Data curation, Methodology, Project administration, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author DW was employed by DWA Energy Limited but the company does not trade in the medical and pharmaceutical fields and provided no funding for this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aiala, G. F., Oliveira, AMSD, Costa, F. O., Fialho, D. L., Cunha, Jr A. S., and Oliveira, P. A. D. (2013). Effect of local application of aminoguanidine on the biomechanical retention of implants in rats with induced diabetes. Int. J. Oral and Maxillofac. Implants 28 (5), 1272–1277. doi:10.11607/jomi.2908
Aldahlawi, S., Nourah, D., and Andreana, S. (2021). Should quality of glycemic control guide dental implant therapy in patients with diabetes? Focus on: peri-implant diseases. Clin. Cosmet. investigational Dent. 13, 149–154. doi:10.2147/CCIDE.S297467
Ayele, S., Sharo, N., and Chrcanovic, B. R. (2023). Marginal bone loss around dental implants: comparison between diabetic and non-diabetic patients—a retrospective clinical study. Clin. Oral Investig. 27 (6), 2833–2841. doi:10.1007/s00784-023-04872-z
Bautista, C. R. G., Dos Santos, I. V., Moraes, R. M., Chiba, F. Y., Sumida, D. H., de Moraes, M. B., et al. (2019). Sitagliptin’s effects on bone tissue and osseointegration in diabetic rats. Archives Oral Biol. 102, 238–243. doi:10.1016/j.archoralbio.2019.04.018
Blanc-Sylvestre, N., Bouchard, P., Chaussain, C., and Bardet, C. (2021). Pre-clinical models in implant dentistry: past, present, future. Biomedicines 9 (11), 1538. doi:10.3390/biomedicines9111538
Bolton, W. K., Cattran, D. C., Williams, M. E., Adler, S. G., Appel, G. B., Cartwright, K., et al. (2004). Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 24 (1), 32–40. doi:10.1159/000075627
Chen, X., Lu, Y., Fan, Y., and Shen, Y. (2017). Chapter 5—voglibose: an important drug for type 2 diabetes, validamycin and its derivatives. Amsterdam, Netherlands: Elsevier.
Coelho, P. G., Pippenger, B., Tovar, N., Koopmans, S.-J., Plana, N. M., Graves, D. T., et al. (2018). Effect of obesity or metabolic syndrome and diabetes on osseointegration of dental implants in a miniature swine model: a pilot study. J. Oral Maxillofac. Surg. 76 (8), 1677–1687. doi:10.1016/j.joms.2018.02.021
Corduas, F., Mancuso, E., and Lamprou, D. A. (2020). Long-acting implantable devices for the prevention and personalised treatment of infectious, inflammatory and chronic diseases. J. Drug Deliv. Sci. Technol. 60, 101952. doi:10.1016/j.jddst.2020.101952
d’Apuzzo, F., Nucci, L., Jamilian, A., and Perillo, L. (2017). Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: overview and clinical relevance. Periodontitis-A Useful Ref.
De Molon, R. S., Morais-Camilo, JAND, Verzola, M. H. A., Faeda, R. S., Pepato, M. T., and Marcantonio, Jr E. (2013). Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: removal torque and histomorphometric analysis in rats. Clin. Oral Implants Res. 24 (7), 831–837. doi:10.1111/j.1600-0501.2012.02467.x
De Morais, JAND, Trindade-Suedam, I. K., Pepato, M. T., Marcantonio, Jr E., Wenzel, A., and Scaf, G. (2009). Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: a digital subtraction radiography study in rats. Clin. oral implants Res. 20 (8), 796–801. doi:10.1111/j.1600-0501.2009.01716.x
Ding, X., Yang, L., Hu, Y., Yu, J., Tang, Y., Luo, D., et al. (2019). Effect of local application of biphosphonates on improving peri-implant osseointegration in type-2 diabetic osteoporosis. Am. J. Transl. Res. 11 (9), 5417–5437.
Fiorellini, J. P., Nevins, M. L., Norkin, A., Weber, H. P., and Karimbux, N. Y. (1999). The effect of insulin therapy on osseointegration in a diabetic rat model. Clin. oral implants Res. 10 (5), 362–368. doi:10.1111/j.1600-0501.1999.tb00011.x
Gómez-Moreno, G., Aguilar-Salvatierra, A., Rubio Roldán, J., Guardia, J., Gargallo, J., and Calvo-Guirado, J. L. (2015). Peri-implant evaluation in type 2 diabetes mellitus patients: a 3-year study. Clin. oral implants Res. 26 (9), 1031–1035. doi:10.1111/clr.12391
Guimaraes, R. P., De Oliveira, P. A. D., and Oliveira, A. (2011). Effects of induced diabetes and the administration of aminoguanidine in the biomechanical retention of implants: a study in rats. J. periodontal Res. 46 (6), 691–696. doi:10.1111/j.1600-0765.2011.01391.x
Han, Y., Zeng, Q., Lingling, E., Wang, D., He, H., and Liu, H. (2012). Sustained topical delivery of insulin from fibrin gel loaded with poly (lactic-co-glycolic Acid) microspheres improves the biomechanical retention of titanium implants in type 1 diabetic rats. J. oral Maxillofac. Surg. 70 (10), 2299–2308. doi:10.1016/j.joms.2012.05.028
Hashiguchi, C., Kawamoto, S.-i., Kasai, T., Nishi, Y., and Nagaoka, E. (2014). Influence of an antidiabetic drug on biomechanical and histological parameters around implants in type 2 diabetic rats. Implant Dent. 23 (3), 264–269. doi:10.1097/ID.0000000000000021
Inouye, K. A. S., Bisch, F. C., Elsalanty, M. E., Zakhary, I., Khashaba, R. M., and Borke, J. L. (2014). Effect of metformin on periimplant wound healing in a rat model of type 2 diabetes. Implant Dent. 23 (3), 319–327. doi:10.1097/ID.0000000000000069
Jiating, L., Buyun, J., and Yinchang, Z. (2019). Role of metformin on osteoblast differentiation in type 2 diabetes. BioMed Res. Int. 2019 (1), 9203934. doi:10.1155/2019/9203934
Jimenez, M., Hu, F. B., Marino, M., Li, Y., and Joshipura, K. J. (2012). Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res. Clin. Pract. 98 (3), 494–500. doi:10.1016/j.diabres.2012.09.039
Kalaitzoglou, E., Fowlkes, J. L., Popescu, I., and Thrailkill, K. M. (2019). Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes/metabolism Res. Rev. 35 (2), e3100. doi:10.1002/dmrr.3100
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 1 (2), 94–99. doi:10.4103/0976-500X.72351
Kocher, T., König, J., Borgnakke, W. S., Pink, C., and Meisel, P. (2018). Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontology 2000 78 (1), 59–97. doi:10.1111/prd.12235
Kopman, J. A., Kim, D. M., Rahman, S. S., Arandia, J. A., Karimbux, N. Y., and Fiorellini, J. P. (2005). Modulating the effects of diabetes on osseointegration with aminoguanidine and doxycycline. J. periodontology 76 (4), 614–620. doi:10.1902/jop.2005.76.4.614
Kwon, P. T., Rahman, S. S., Kim, D. M., Kopman, J. A., Karimbux, N. Y., and Fiorellini, J. P. (2005). Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J. periodontology 76 (4), 621–626. doi:10.1902/jop.2005.76.4.621
Lau, E. F., Peterson, D. E., Leite, F. R. M., Nascimento, G. G., Robledo-Sierra, J., Amy, D. P. B., et al. (2022). Embracing multi-causation of periodontitis: why aren't we there yet? Oral Dis. 28, 1015–1021. doi:10.1111/odi.14107
Liu, Z., Zhou, W., Tangl, S., Liu, S., Xu, X., and Rausch-Fan, X. (2015). Potential mechanism for osseointegration of dental implants in Zucker diabetic fatty rats. Br. J. Oral Maxillofac. Surg. 53 (8), 748–753. doi:10.1016/j.bjoms.2015.05.023
Margonar, R., Sakakura, C. E., Holzhausen, M., Pepato, M. T., Alba, Jr R. C., and Marcantonio, Jr E. (2003). The influence of diabetes mellitus and insulin therapy on biomechanical retention around dental implants: a study in rabbits. Implant Dent. 12 (4), 333–339. doi:10.1097/01.id.0000086482.65273.b7
Matsubara, F., Shirota, T., Yamazaki, M., Motohashi, M., Ohno, K., and Michi, K.-I. (2001). The effect of insulin therapy on bone tissue around implants in streptozotocin-induced diabetic rats. ORAL Ther. Pharmacol. 20 (3), 173–180. doi:10.11263/jsotp1982.20.173
McCracken, M. S., Aponte-Wesson, R., Chavali, R., and Lemons, J. E. (2006). Bone associated with implants in diabetic and insulin-treated rats. Clin. oral implants Res. 17 (5), 495–500. doi:10.1111/j.1600-0501.2006.01266.x
Mills, E. G., Yang, L., Nielsen, M. F., Kassem, M., Dhillo, W. S., and Comninos, A. N. (2021). The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocr. Rev. 42 (6), 691–719. doi:10.1210/endrev/bnab015
Mohd, N., Razali, M., Ghazali, M. J., and Abu Kasim, N. H. (2022a). 3D-printed hydroxyapatite and tricalcium phosphates-based scaffolds for alveolar bone regeneration in animal models: a scoping review. Materials 15 (7), 2621. doi:10.3390/ma15072621
Mohd, N., Razali, M., Ghazali, M. J., and Abu Kasim, N. H. (2022b). Current advances of three-dimensional bioprinting application in dentistry: a scoping review. Materials 15 (18), 6398. doi:10.3390/ma15186398
Mouraret, S., Hunter, D. J., Bardet, C., Brunski, J. B., Bouchard, P., and Helms, J. A. (2014). A pre-clinical murine model of oral implant osseointegration. Bone 58, 177–184. doi:10.1016/j.bone.2013.07.021
Musskopf, M. L., Finger Stadler, A., Wikesjö, U. M. E., and Susin, C. (2022). The minipig intraoral dental implant model: a systematic review and meta-analysis. PLoS One 17 (2), e0264475. doi:10.1371/journal.pone.0264475
Nascimento, G. G., Leite, F. R. M., Vestergaard, P., Scheutz, F., and Lopez, R. (2018). Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta diabetol. 55, 653–667. doi:10.1007/s00592-018-1120-4
Naseer, A., Mc Garrigle, C., McLoughlin, J., and O'Connell, B. (2024). Tooth loss is associated with prevalent diabetes and incident diabetes in a longitudinal study of adults in Ireland. Community Dent. Oral Epidemiol. 52 (1), 111–119. doi:10.1111/cdoe.12907
Niwczyk, O., Grymowicz, M., Szczęsnowicz, A., Hajbos, M., Kostrzak, A., Budzik, M., et al. (2023). Bones and hormones: interaction between hormones of the hypothalamus, pituitary, adipose tissue and Bone. Int. J. Mol. Sci. 24 (7), 6840. doi:10.3390/ijms24076840
Omar, N. I., Baharin, B., Lau, S. F., Ibrahim, N., Mohd, N., Ahmad, F. A., et al. (2020). The influence of Ficus deltoidea in preserving alveolar bone in ovariectomized rats. Veterinary Med. Int. 2020 (1), 8862489. doi:10.1155/2020/8862489
Ong, K. L., Stafford, L. K., McLaughlin, S. A., Boyko, E. J., Vollset, S. E., Smith, A. E., et al. (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402 (10397), 203–234. doi:10.1016/S0140-6736(23)01301-6
Prabhakar, S. (2012). Translational research challenges: finding the right animal models. J. Investigative Med. 60 (8), 1141–1146. doi:10.2310/JIM.0b013e318271fb3b
Preoteasa, E., Florica, L. I., Obadan, F., Imre, M., and Preoteasa, C. T. (2015). Minimally invasive implant treatment alternatives for the edentulous patient—fast and fixed and implant overdentures. Curr. Concepts Dent. Implant. IntechOpen.
Ribeiro Serrão, C., Ferreira Bastos, M., Ferreira Cruz, D., de Souza Malta, F., Camacho Vallim, P., and Mendes Duarte, P. (2017). Role of metformin in reversing the negative impact of hyperglycemia on bone healing around implants inserted in type 2 diabetic rats. Int. J. Oral and Maxillofac. Implants 32 (3), 547–554. doi:10.11607/jomi.5754
Rokaya, D., Srimaneepong, V., Wisitrasameewon, W., Humagain, M., and Thunyakitpisal, P. (2020). Peri-implantitis update: risk indicators, diagnosis, and treatment. Eur. J. Dent. 14 (04), 672–682. doi:10.1055/s-0040-1715779
Sábado-Bundó, H., Sánchez-Garcés, M. Á., and Gay-Escoda, C. (2019). Bone regeneration in diabetic patients. A systematic review. Med. Oral, Patol. Oral Cirugía Bucal 24 (4), e425–e432. doi:10.4317/medoral.22889
Schwarz, F., Derks, J., Monje, A., and Wang, H. L. (2018). Peri-implantitis. J. Clin. periodontology 45, S246–S66. doi:10.1111/jcpe.12954
Siqueira, J. T., Cavalher-Machado, S. C., Arana-Chavez, V. E., and Sannomiya, P. (2003). Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 12 (3), 242–251. doi:10.1097/01.id.0000074440.04609.4f
Sundar, G. (2018). Comparative analysis of OPN, IL-8 and rankl in assessing implant healing and implant stability in type-II diabetes mellitus-A. Randomized Control. Trial.
Tabassum, A. (2022). Alveolar bone loss in diabetic patients: a case–control study. Eur. J. Dent. 18, 168–173. doi:10.1055/s-0042-1758071
Tunheim, E. G., Skallevold, H. E., and Rokaya, D. (2023). Role of hormones in bone remodeling in the craniofacial complex: a review. J. oral Biol. craniofacial Res. 13 (2), 210–217. doi:10.1016/j.jobcr.2023.01.009
Vu, G. T., Little, B. B., Lai, P. C., and Cheng, G.-L. (2022). Tooth loss and uncontrolled diabetes among US adults. J. Am. Dent. Assoc. 153 (6), 542–551. doi:10.1016/j.adaj.2021.11.008
Wang, B., Song, Y., Wang, F., Li, D., Zhang, H., Ma, A., et al. (2011). Effects of local infiltration of insulin around titanium implants in diabetic rats. Br. J. Oral Maxillofac. Surg. 49 (3), 225–229. doi:10.1016/j.bjoms.2010.03.006
Xiang, G., Huang, X., Wang, T., Wang, J., Zhao, G., Wang, H., et al. (2020). The impact of sitagliptin on macrophage polarity and angiogenesis in the osteointegration of titanium implants in type 2 diabetes. Biomed. and Pharmacother. 126, 110078. doi:10.1016/j.biopha.2020.110078
Yamazaki, S., Masaki, C., Nodai, T., Tsuka, S., Tamura, A., Mukaibo, T., et al. (2020). The effects of hyperglycaemia on peri-implant tissues after osseointegration. J. Prosthodont. Res. 64 (2), 217–223. doi:10.1016/j.jpor.2019.07.007
Zhang, J., Wang, Y.-n., Jia, T., Huang, H., Zhang, D., and Xu, X. (2021). Genipin and insulin combined treatment improves implant osseointegration in type 2 diabetic rats. J. Orthop. Surg. Res. 16, 59–10. doi:10.1186/s13018-021-02210-1
Zhou, W., Liu, Z., Yao, J., Chi, F., Dong, K., Yue, X., et al. (2015). The effects of exenatide microsphere on serum BGP and ALP levels in ZDF rats after implantation. Clin. implant Dent. Relat. Res. 17 (4), 765–770. doi:10.1111/cid.12184
Nomenclature
BAFO Bone Area Fraction Occupancy
BIC Bone-To-Implant Contact
DM Diabetes Mellitus
GK Goto-Kakizaki
OPG Osteoprotegerin
RANKL Receptor Activator of Nuclear Factor Kappa-B Ligand
T1DM Type 1 Diabetes Mellitus
T2DM Type 2 Diabetes Mellitus
TNF-a Tumor Necrosis Factor-Alpha
WHO World Health Organization
ZDF Zucker Diabetic Fatty
Keywords: hyperglycemia, dental implants, anti-diabetic medications, type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), implant stability
Citation: Ghorbani H, Minasyan A, Ansari D, Ghorbani P, Wood DA, Yeremyan R, Ghorbani S and Minasian N (2024) Anti-diabetic therapies on dental implant success in diabetes mellitus: a comprehensive review. Front. Pharmacol. 15:1506437. doi: 10.3389/fphar.2024.1506437
Received: 08 October 2024; Accepted: 25 November 2024;
Published: 11 December 2024.
Edited by:
Mahmoud Fahmy Elsabahy, Badr University in Cairo, EgyptReviewed by:
Akinleye Stephen Akinrinde, University of Ibadan, NigeriaDinesh Rokaya, Chulalongkorn University, Thailand
Copyright © 2024 Ghorbani, Minasyan, Ansari, Ghorbani, Wood, Yeremyan, Ghorbani and Minasian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamzeh Ghorbani, aGFtemVoZ2hvcmJhbmk2OEB5YWhvby5jb20=
 Hamzeh Ghorbani
Hamzeh Ghorbani Arsen Minasyan2
Arsen Minasyan2