- 1Department of Clinical Pharmacy, College of Pharmacy, University of Hail, Hail, Saudi Arabia
- 2Department of Health Informatics, College of Public Health, University of Hail, Hail, Saudi Arabia
- 3Department of Pharmacy Practice, Pharmacy College, King Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia
- 4Department of Pharmaceutics, College of Pharmacy, University of Hail, Hail, Saudi Arabia
- 5Department of Internal Medicine and Adult Critical Care, College of Medicine, University of Ha’il, Hail, Saudi Arabia
- 6Department of Pharmacy Practice, College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Saudi Arabia
Background: This meta-analysis aims to evaluate the effectiveness and safety of combining echinocandins with standard of care (SOC) antifungal drugs for treating invasive aspergillosis infection (IAI).
Method: We searched PubMed, Embase, and Cochrane Library from their inception to 25 July 2024. Our outcomes included clinical cure, mortality, and adverse drug reactions (ADRs). We compared echinocandins in combination with SOC antifungal agents against SOC monotherapy therapy. We used the random-effects model for the meta-analysis, and our estimated effects were reported as odds ratios (ORs) with 95% confidence intervals (CI).
Results: Ten studies were included in our meta-analysis comprising 1100 patients: 415 were in the echinocandin combination groups, and 685 were in the SOC groups. The clinical cure rate (OR 1.35, 95% CI: 0.75–2.42, p = 0.27), mortality (OR 0.90, 95% CI: 0.50–1.63, p = 0.73), and ADRs rate (OR 0.95, 95% CI: 0.49–1.82, p = 0.87) were not statistically different in echinocandins combination with SOC compared to SOC monotherapy. Notably, there is a signal for a better clinical cure rate in echinocandins in combination with SOC.
Conclusion: Our meta-analysis found no differences in clinical cure and mortality rate when using combination therapy of echinocandin antifungal agents with the SOC compared to SOC monotherapy. However, there is a signal for better outcomes with the echinocandins combination group. The ADRs in the echinocandins combination group were not worse than SOC monotherapy.
1 Introduction
Invasive aspergillosis is a mycotic infection caused by the ubiquitous mold in the environment–Aspergillus fumigatus, and causes infections in the lungs (most commonly), skin, central nervous system, and sinuses (Chabi et al., 2015; Lamoth and Calandra, 2022). The cases of invasive aspergillosis infection (IAI) are rising globally (Thompson and Young, 2021). The estimated annual global incidence of IAI from 120 countries (Africa, Asia and Pacific, Latin America and the Caribbean, Eastern Europe and Central Asia, Western Europe and North America, Middle East and North Africa) was 2 116 362 cases, with a crude mortality rate of 72% (Denning, 2024). Aspergillosis is associated with a wide range of clinical syndromes, such as chronic pulmonary aspergillosis, sinus disease, infection of the central nervous system, and others (Thompson and Young, 2021). Factors attributable to the increasing IAIs include growing immunosuppressed populations, intensive care unit admission, and severe respiratory viral infections like influenza and SARS-CoV-2 infections. Notably, IAI is challenging to diagnose because it does not have unique clinical manifestations and it takes a long time to detect (Wang et al., 2022).
Antifungal pharmacotherapy is the standard of care (SOC) for treating IAI (Thompson and Young, 2021). Some patients with IAI may need surgery in addition to antifungal therapy (Patterson et al., 2016). The current Infectious Diseases Society of America (IDSA) guideline for IAI recommends antifungal treatment with triazole antifungal agents, preferably voriconazole. Amphotericin B, with its varying formulations, is appropriate when voriconazole cannot be used or indicated as salvage therapy. Echinocandin antifungal agents are not recommended as a monotherapy for treating IAI. The IDSA guideline recommends echinocandins for IAI as salvage therapy in combination with the standard of care (Amphotericin B or triazole antifungal agents).
Preclinical studies revealed that combining azole and polyene antifungal agents yields synergistic or additive effects (Patterson et al., 2016; Petraitis et al., 2017). Yet, evidence is conflicting regarding the effectiveness of preclinical data. An experimental study by Petraitis et al. evaluated a combination therapy of isavuconazole with micafungin for treating invasive pulmonary aspergillosis in an animal model. The authors found that the isavuconazole/micafungin combination therapy yielded a dose-dependent decrease in fungal burden, pulmonary injury, and prolonged animal survival (Petraitis et al., 2017). Furthermore, another study by Jeans et al. evaluated the combination of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus in an in-vitro invasive pulmonary aspergillosis (Jeans et al., 2012). The authors concluded that the combination therapy of voriconazole and anidulafungin had an apparent additive effect and reduced galactomannan concentration in the endothelial compartment.
Evidence for the effectiveness and safety of echinocandin combination therapy with the SOC against IAI is conflicting and inconclusive. A study by Singh et al. evaluated a combination of voriconazole and caspofungin for IAI and found that combination therapy had preferable outcomes to monotherapy (Singh et al., 2006). On the other hand, a study by Raad et al. evaluated the voriconazole and caspofungin combination for IAI and concluded that it did not result in improved outcomes compared to monotherapy (Raad et al., 2015). Furthermore, published meta-analyses have limitations, such as not including all the published studies and combining animal and human studies, which pose remarkable heterogeneity in the results estimates (Panackal et al., 2014; Zhang et al., 2014). Therefore, robust and updated evidence from a well-designed meta-analysis is warranted. Therefore, this study aims to evaluate the effectiveness and safety of combining echinocandin with the SOC for treating IAI quantitatively, employing meta-analysis to update the available evidence.
2 Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-Analyses of Observational Studies in Epidemiology (MOOSE) guidelines during our meta-analysis (Page et al., 2021; Stroup et al., 2000). The checklists for the PRISMA and MOOSE guidelines are available in the Supplementary Material.
2.1 Literature source
We systematically searched PubMed, Cochrane Library, and Embase databases from inception until 25 July 2024. The search was executed by cross-searching keywords with Medical Subject Headings using the following concepts: aspergillosis, echinocandin antifungal drugs, and all other systemic antifungal drugs. The complete search strategy is available in Supplementary Material.
2.2 PICOS criteria and study selection
We used the following adjusted Population, Intervention, Comparator, Outcomes, and Studies (PICOS) criteria (Methley et al., 2014): 1) Population: adult patients 18 years and older with IAI; 2) Intervention: aspergillosis-active agents (amphotericin, voriconazole, posaconazole, itraconazole, or isavuconazonium) combined with an echinocandin (micafungin, caspofungin, anidulafungin, or rezafungin); 3) Comparator: the SOC monotherapy for IAI (amphotericin, amphotericin, voriconazole, posaconazole, itraconazole, or isavuconazonium); 4) Outcomes: clinical cure, mortality, microbiological cure, and safety; Studies: randomized controlled trials (RCT) and observational studies. Investigators (YSA and HY) reviewed the titles and abstracts of identified studies and evaluated their eligibility based on the pre-determined PICOS criteria. We excluded editorials, commentaries, case reports, case series, pharmacokinetic (PK) studies, pharmacodynamic (PD) studies, animal studies, and publications in non-English language. The following keywords were used in our search: aspergillosis, invasive aspergillosis, pulmonary aspergillosis, echinocandins, anidulafungin, caspofungin, micafungin, rezafungin, isavuconazole, itraconazole, voriconazole, and amphotericin.
2.3 Data extraction
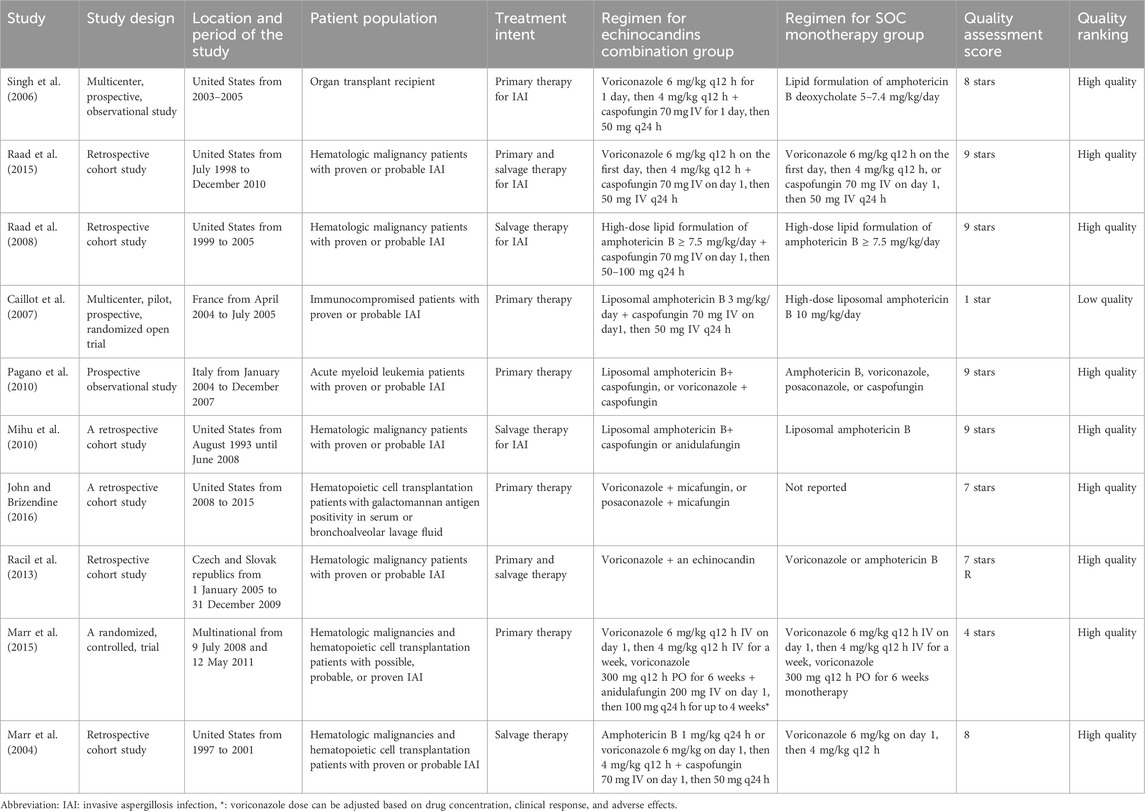
Two investigators (YSA and HW) separately screened identified studies and performed a full-text review of potentially relevant studies for eligibility. Discordance was resolved with discussion and consensus, and unsettled matters were evaluated by a third investigator (KBS). For included studies, we extracted the first author’s name and publication year, the location of the study population, the sample size of each arm, and the regimen used in each study.
2.4 Quality assessment
We used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included observational studies (Wells et al., 2024) Based on their scores, studies were classified into low quality (1–3 stars), medium quality (4–6 stars), and high quality (7–9 stars). For the quality assessment of the RCTs, we used the Jadad score (Jadad et al., 1996) Studies with scores of ≥3 were categorized as high quality, and studies with scores of ≤2 were categorized as low quality.
2.5 Summary measures and statistical analysis
The combined measured effects were reported as odds ratios (OR) with a 95% confidence interval (CI). Due to the heterogeneity of the study populations, we used the restricted maximum likelihood random-effects model for the meta-analyses (Borenstein et al., 2010). Additionally, we utilized Cochrane’s Q to calculate the heterogeneity by the weighted sum of squares and the I2 statistic to express the percentage of variation due to heterogeneity (Higgins et al., 2003). Statistically significant heterogeneity was defined as a threshold of p-value <0.05 for Cochrane’s Q statistics and >30% for I2 statistics. An estimation of publication bias was assessed by funnel plots of standard error against the estimated effect. Lastly, the asymmetry of the funnel plot was examined using the Egger linear regression test (Egger et al., 1997).
2.6 Subgroup analysis
To further evaluate mortality and clinical cure, we perform sub-group meta-analyses based on the echinocandin regimens used in the studies, treatment intent: primary vs. salvage therapy, and patient populations (hematologic malignancies). Specifically, we computed the overall clinical cure, clinical cure for patients on combination therapy as primary therapy, clinical cure for patients on combination therapy as salvage therapy, clinical cure for caspofungin-based regimens, and clinical cure for patients with hematologic malignancies. Furthermore, for mortality, we performed overall mortality, mortality for patients on combination therapy as primary therapy, mortality for patients on combination therapy as salvage therapy, mortality for caspofungin-based regimens, and mortality for patients with hematologic malignancies.
3 Results
3.1 Study selection
The literature search strategy resulted in 998 articles in Embase, 291 articles in PubMed, and 61 articles in the Cochrane Library. Notably, some articles were identified from known articles to the investigators. A total of 368 articles were screened after removing duplicate articles. After that, we eliminated 289 articles for being irrelevant and ten articles for being PK/PD studies, leaving us with 69 articles for review. After 49 irrelevant articles were removed, 20 articles underwent a full-text assessment for eligibility. After the full-text evaluation of the articles, nine were removed, and ten were included in the meta-analysis, Figure 1.
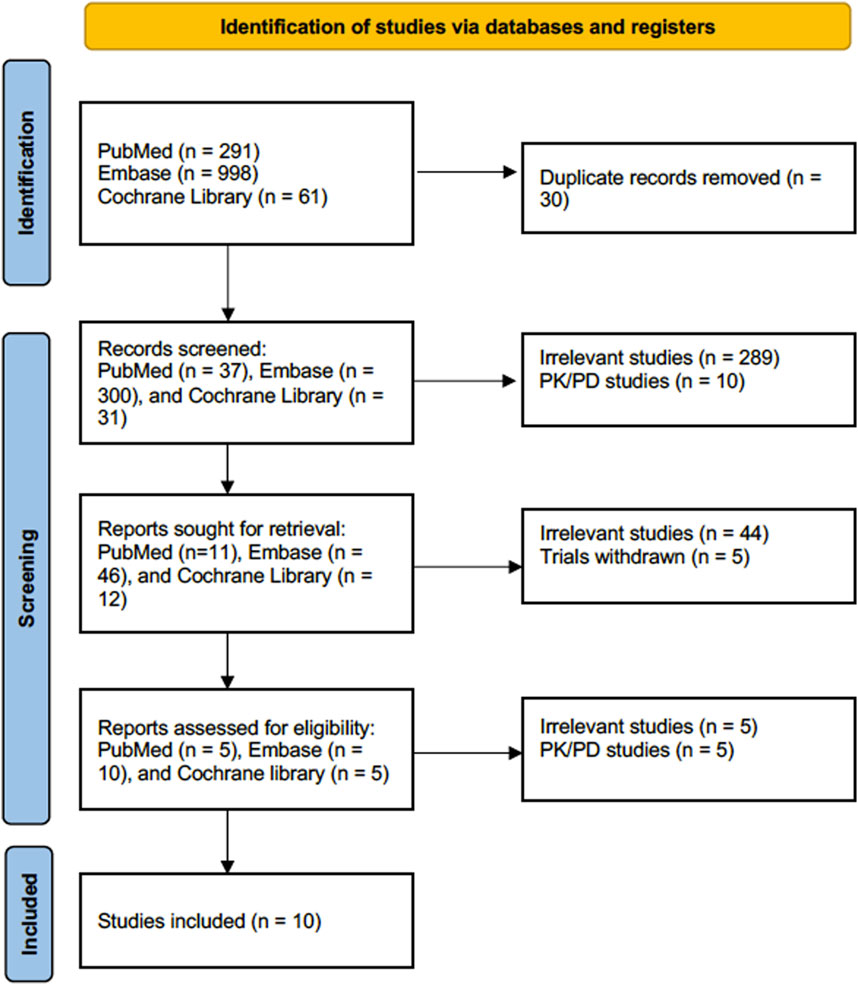
Figure 1. Flow diagram for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Notably, studies by Raad et al. (2015), Mihu et al. (2010), Racil et al. (2013) reported outcomes for echinocandins monotherapy; however, we excluded these outcomes from our analysis for clinical cure and mortality because echinocandins monotherapy is not recommended for treating IAI based on the IDSA guideline (Patterson et al., 2016). Moreover, we included the study by Mihu et al. (2010) in our subgroup analysis for caspofungin-based regimens for clinical cure and mortality because 90% of the patients received caspofungin.
3.2 Characteristics of the included studies
Ten studies (Caillot et al., 2007; John and Brizendine, 2016; Marr et al., 2004; Marr et al., 2015; Mihu et al., 2010; Pagano et al., 2010; Raad et al., 2008; Raad et al., 2015; Racil et al., 2013; Singh et al., 2006) were included in the meta-analysis: six retrospective cohort studies (John and Brizendine, 2016; Marr et al., 2004; Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Racil et al., 2013), two prospective observational studies (Pagano et al., 2010; Singh et al., 2006), and two randomized trials (Caillot et al., 2007; Marr et al., 2015). All included studies were high quality, except the study by Caillot et al. (2007) was low quality (Caillot et al., 2007; Marr et al., 2015). Six of the included studies were conducted in the United States, three were conducted in Europe, and 1 was a multinational study. The patient populations of the included studies were hematologic malignancies or transplantation. The total number of patients included in the meta-analysis was 1100: 415 patients were in the echinocandin combination groups, and 685 were in the SOC groups. Complete characteristics of the included studies are available in Table 1.
3.3 Meta-analysis
3.3.1 Clinical cure rate
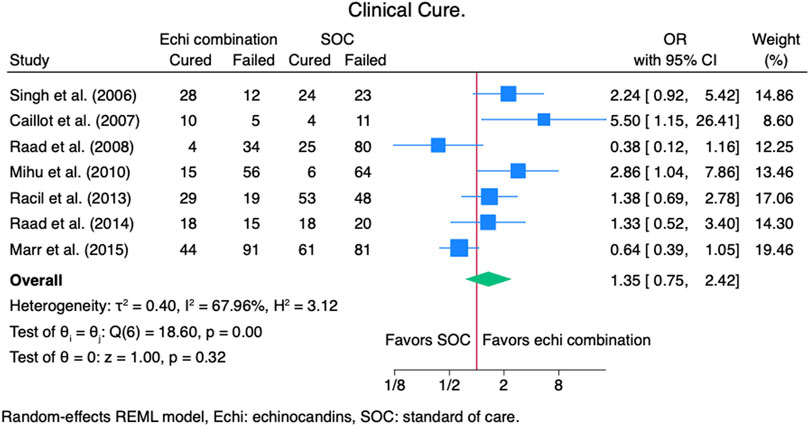
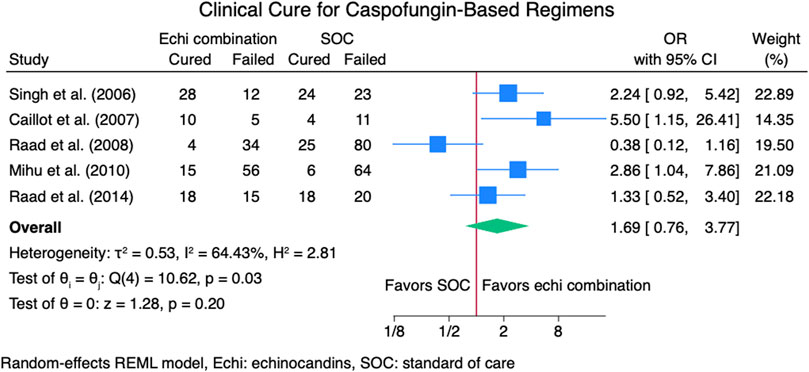
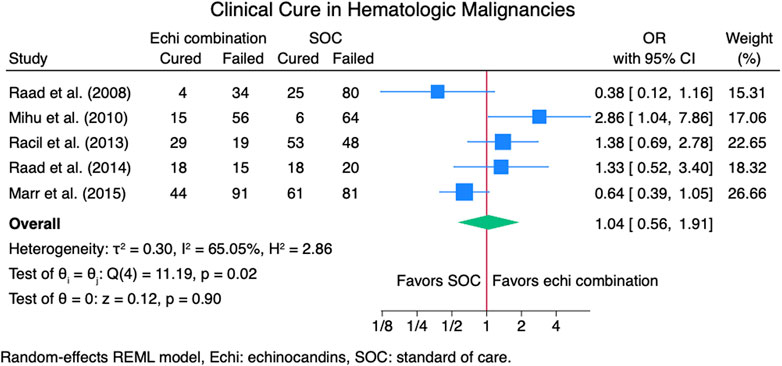
Seven studies (Caillot et al., 2007; Marr et al., 2015; Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Racil et al., 2013; Singh et al., 2006) reported clinical cure outcomes for 913 patients: 380 patients in the echinocandin combination therapy group and 533 in the SOC group. The pooled clinical cure rate when using an echinocandin combination therapy was not statistically different from SOC monotherapy: OR 1.35 (95% CI: 0.75–2.42, p = 0.27; Figure 2). In the subgroup meta-analysis, when using echinocandins combination therapy for IAI as primary therapy (Caillot et al., 2007; Marr et al., 2015; Raad et al., 2015; Racil et al., 2013; Singh et al., 2006), the clinical cure rate was also not statistically different for echinocandins combination therapy compared to SOC monotherapy; OR 1.37 (95% CI: 0.74–2.53, p = 0.31; Supplementary Figure S1). The lack of clinically significant difference in clinical cure rate also persists when using echinocandins combination therapy as a salvage therapy (Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Racil et al., 2013) for IAI; OR 1.11 (95% CI: 0.36–3.41, p = 0.86; Supplementary Figure S2). Lastly, the clinical cure rate was not statistically different when using combination echinocandin therapy for the subgroup meta-analysis of caspofungin-based combination regiments (Caillot et al., 2007; Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Singh et al., 2006); OR 1.69 (95% CI: 0.76–3.77, p = 0.20; Figure 3). Notably, there was a signal for better outcomes with echinocandin combination therapies for the overall clinical cure, subgroup analysis for primary IAI therapy, and subgroup analysis for caspofungin-based regimens. Finally, we performed a subgroup meta-analysis for the clinical cure rate for studies comprising hematologic malignancy patients (Marr et al., 2015; Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Racil et al., 2013), and the clinical cure rate was similar for the echinocandins combination group compared to SOC monotherapy: OR 1.04 (95% CI: 0.56–1.91, p = 0.90, Figure 4).
3.3.2 Mortality rate
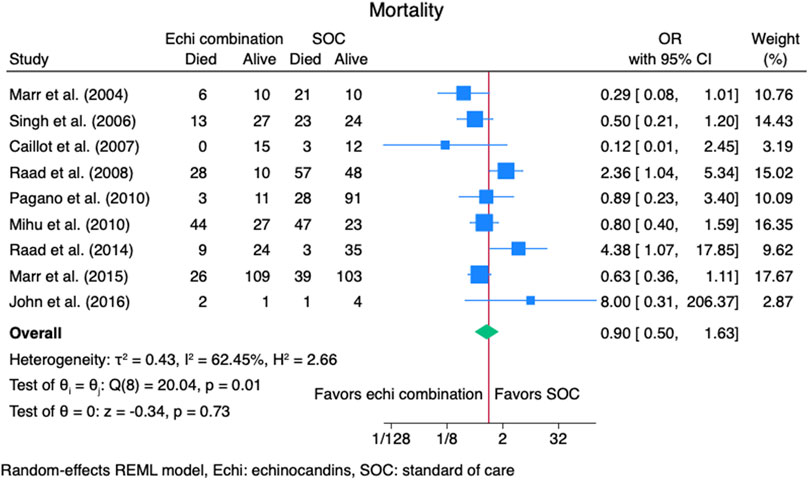
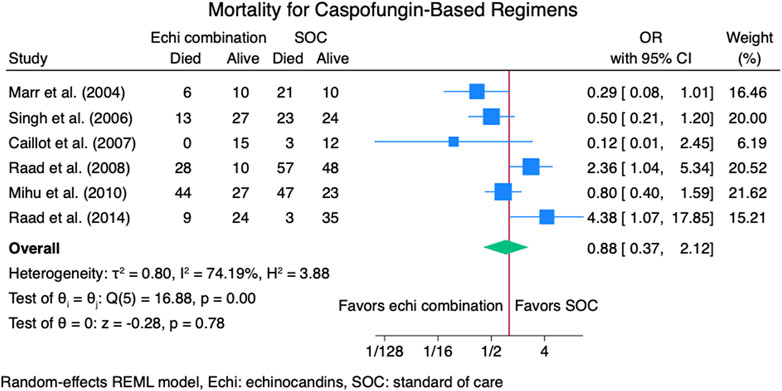
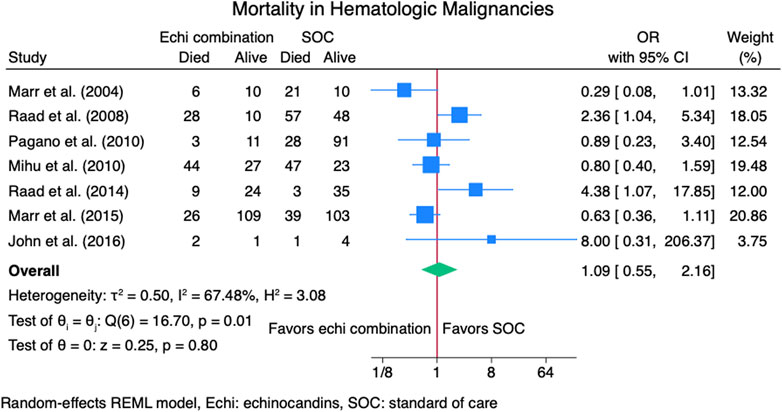
Nine studies (Caillot et al., 2007; John and Brizendine, 2016; Marr et al., 2004; Marr et al., 2015; Mihu et al., 2010; Pagano et al., 2010; Raad et al., 2008; Raad et al., 2015; Singh et al., 2006) reported mortality outcomes for 937 patients: 365 patients in the echinocandins combination group and 572 in the SOC monotherapy group. The pooled OR when using echinocandins combination therapy was not statistically different from that of SOC monotherapy: OR 0.90 (95% CI: 0.50–1.63, p = 0.73, Figure 5). In the subgroup meta-analysis, when using echinocandins combination therapy for IAI as primary therapy (Caillot et al., 2007; John and Brizendine, 2016; Marr et al., 2015; Pagano et al., 2010; Raad et al., 2015; Singh et al., 2006), the mortality rate was not statistically different compared to SOC monotherapy: OR 0.91 (95% CI: 0.41–2.01, p = 0.81, Supplementary Figure S3). Furthermore, in the subgroup meta-analysis for salvage therapy (Marr et al., 2004; Mihu et al., 2010; Raad et al., 2008) for IAI, the mortality when using echinocandins combination therapy was not statistically different compared to SOC monotherapy: OR 0.88 (95% CI: 0.28–2.71, p = 0.82, Supplementary Figure S4). After all, in the subgroup meta-analysis for caspofungin-based regimens (Caillot et al., 2007; Marr et al., 2004; Mihu et al., 2010; Raad et al., 2008; Raad et al., 2015; Singh et al., 2006), the mortality rate with echinocandins combination therapy was not statistically different compared to SOC monotherapy: OR 0.88 (95% CI: 0.37–2.12, p = 0.78, Figure 6). Finally, we performed a subgroup meta-analysis for the mortality rate for studies comprising hematologic malignancy patients(John and Brizendine, 2016; Marr et al., 2004; Marr et al., 2015; Mihu et al., 2010; Pagano et al., 2010; Raad et al., 2008; Raad et al., 2015), and the mortality rate was similar for the echinocandins combination group compared to SOC monotherapy: OR 1.09 (95% CI: 0.55–2.16, p = 0.80, Figure 7).
3.3.3 Adverse drug reactions
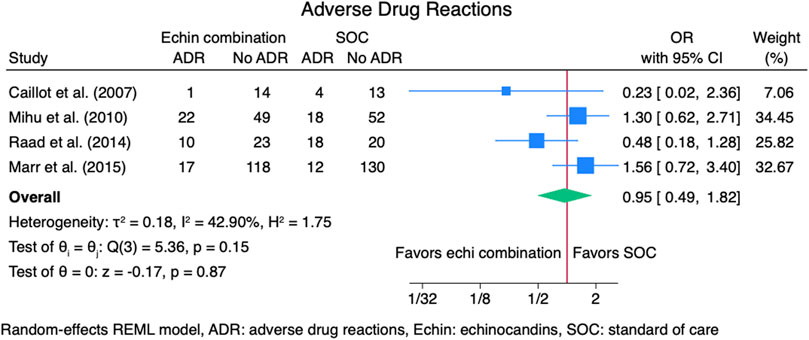
Four studies (Caillot et al., 2007; Marr et al., 2015; Mihu et al., 2010; Raad et al., 2015) reported outcomes for adverse drug reactions (ADRs), including 521 patients: 254 in the echinocandins combination group and 267 in the SOC monotherapy group. The pooled OR for ADRs was not statistically different for the echinocandins combination group compared to SOC monotherapy: OR 0.95 (95% CI: 0.49–1.82, p = 0.87, Figure 8). Reported ADRs include hepatotoxicity and nephrotoxicity that was not statistically different between groups (Raad et al., 2015). March 2015 found more patients in the combination group had hepatobiliary ADRs (12.7% vs. 8.4%) (Marr et al., 2015). Mihu et al. found numerically higher ADRs in the echinocandins combination group compared to SOC monotherapy (31% vs. 26%, p = 0.08) (Mihu et al., 2010). Abnormalities in bilirubin, alkaline phosphatase, and liver enzymes were not statistically different for the echinocandins combination group compared to SOC monotherapy. However, Mihu et al. found more patients with creatinine elevation in the echinocandin combination group than in SOC monotherapy (p = 0.01). Caillot et al. found fewer ADRs in the echinocandins combination group compared to SOC monotherapy. Adverse drug reactions include elevation in serum creatinine level, hypokalemia, and infusion-related reactions (Caillot et al., 2007).
3.4 Publication bias
For the clinical cure, the funnel plot of standard error against the effect estimate demonstrates symmetry in general, Supplementary Figure S5. The Egger test also revealed no statistically significant publication bias (p = 0.16). When assessing publication bias for mortality rate, the funnel plot of the standard error against the effect estimate generally demonstrates symmetry (Supplementary Figure S6), supported by the Egger test that revealed no statistically significant publication bias (p = 0.78).
4 Discussion
Invasive aspergillosis infections are challenging to treat and are associated with significant morbidity and mortality (Denning, 2024). Some studies have demonstrated that combining an echinocandin antifungal agent with the SOC improves treatment outcomes of IAI; however, the evidence is inconclusive, and there is a gap in the literature (Singh et al., 2006; Zhang et al., 2014). This study evaluated the effectiveness and safety of echinocandins combination therapy compared to SOC monotherapy for the treatment of IAI quantitatively by meta-analysis. We found that the rate of clinical cure and mortality were not statistically different for echinocandins combination therapy compared to SOC monotherapy for treating IAI. Although not statistically significant, there was a trend for a better clinical cure rate with echinocandins in combination with the SOC.
Combination antifungal therapy with echinocandins and SOC is appealing for treating IAI because it is difficult to treat (Patterson et al., 2016). Although we found no difference in clinical cure rate in our meta-analysis, there is a signal for better outcomes for echinocandins with SOC combination (OR> 1). An RCT by Caillot et al. (2007) evaluated the combination therapy of caspofungin with amphotericin in hematologic patients with IAI and revealed that the combination therapy had statistically significantly more favorable clinical cure compared to SOC monotherapy; the clinical cure rates were 67% and 27% (p = 0.028) for combination therapy and SOC monotherapy, respectively. The authors also found that the survival rate was 100% for combination therapy and 80% for SOC monotherapy. A study by Marr et al. (2004) evaluated combination therapy for treating IAI infection and found that combination therapy with echinocandins and triazole antifungals was associated with improved survival compared to SOC monotherapy; relative risk (RR) 0.42 (95% CI, 0.17–1.1, p = 0.048). A study by Singh et al. (2006) found a numerically higher survival rate for patients treated with combination therapy of caspofungin and voriconazole; however, the difference was not statistically significant; 67.5% for combination therapy and 51% for SOC monotherapy, p = 0.11. To elaborate, a study by Kontoyiannis et al. (2003) evaluated the efficacy and safety of combination therapy of caspofungin with amphotericin in patients with hematologic malignancies and found that the overall clinical cure rate was 42%, and the mortality rate was zero. The low clinical cure rate was attributed to a small sample size (42 patients). Notably, a randomized pragmatic superiority trial (IA-DUE) evaluated the azole-echinocandins combination for treating IAI was completed on 1 May 2024 (ClinicalTrials.gov). The trial aimed to assess azole-echinocandin combination therapy for IAI, and patients were randomized to receive either azole antifungal + anidulafungin or azole antifungal monotherapy. Of note is that the study’s findings have not been published yet.
Our study expands the findings of a meta-analysis by Zhang et al. (2014), which evaluated the effectiveness of combination therapy for treating IAI in animal studies. The authors found that echinocandins and triazole combination compared to echinocandins monotherapy prolonged survival for pooled animal studies, RR = 2.26 (95% CI, 1.79–2.87, p < 0.00001). That can be explained by the inadequate efficacy of echinocandin monotherapy for treating IAI, which is against the IDSA guideline recommendations (Patterson et al., 2016). On the contrary, when the authors compared the combination of echinocandins plus triazole with triazole monotherapy, they found no difference in survival, RR 1.19 (95% CI: 0.98–1.44, p = 0.08). Findings from the meta-analysis by Zhang et al. were translated into our study in humans since we did not find statistically significant differences in mortality for echinocandins combination therapy with SOC compared to SOC monotherapy, OR 0.90 (95% CI: 0.50–1.63, p = 0.73, Figure). The limitation in the meta-analysis by Zhang et al. that was overcome in our study is the lack of meta-analysis for human studies. Furthermore, another meta-analysis by Panackal et al. (2014) evaluated the salvage combination of all antifungal therapies for IAI (that include triazole with amphotericin combination and found that the combination therapy for IAI improved 12-weeks survival: Peto OR 1.80 (95% CI: 1.08–3.01). Their findings contradict our findings since we found no difference in mortality for echinocandin combination therapy compared to SOC monotherapy. Limitations of this meta-analysis are that it used all combination therapies together; thus, estimates for studies evaluating echinocandins with SOC combination therapies are not available, and they used a fixed-effect model in their meta-analysis, which cannot adjust for heterogeneity across studies. Lastly, a network meta-analysis by Liu et al. (2024) found that combination therapy of amphotericin with caspofungin was associated with the best probability of favorable response: the surface under the cumulative ranking curve was 84.1%; mean rank, 2.6. However, the limitation of this study was it performed an indirect (artificial) comparison.
Several studies evaluated risk factors for mortality due to IAI. Raad et al. (2015) found intensive care unit admission for primary and salvage therapy was a risk factor for mortality for IAI. Marr et al. (2015) found that elevated serum galactomannan predicted mortality related to IAI. On the contrary, (Raad et al., 2008) found posaconazole therapy was associated with favorable clinical response. Moreover, Singh et al. (2006) found that renal failure was associated with mortality.
Our findings from pooled studies revealed that the echinocandins combination group had a similar safety profile to SOC monotherapy (OR 0.95, 95% CI: 0.49–1.82, p = 0.87). Our findings contradict toxicity results reported by Raad et al. (2008) since they found that caspofungin combination with amphotericin was associated with significantly higher rates of hepatotoxicity and renal injury compared to posaconazole, p ≤ 0.02. The findings by Raad et al. can be explained by the presence of amphotericin in the combination regimen, which is highly associated with hepatoxicity and nephrotoxicity. The combination therapy with echinocandins is unlikely to increase the incidence of ADRs.
Our meta-analysis is the first study to evaluate the effectiveness and safety of combination antifungal drugs for treating IAI in humans, precisely the combination of echinocandins with SOC. We performed subgroup meta-analyses to assess the outcomes of our study further. Our meta-analysis has limitations. We included all studies, including observational studies; however, all were rated high quality. The population in our meta-analysis who acquired IAI was heterogeneous, including patients with hematologic malignancies and transplant patients. Different formulations for amphotericin were used in the included studies, and they could have distinct safety profiles. Furthermore, the criteria for diagnosing IAI used in the included studies may differ slightly, affecting the homogeneity of patients with IAI. However, all heterogeneities in our meta-analysis were adjusted for our meta-analysis’s heterogeneity by using the random-effects model. We could not evaluate outcomes based on the IAI site. Lastly, we included only studies published in the English language.
5 Conclusion
Treatment of IAI is challenging and requires new therapeutic approaches to prevent treatment failure and complications. Using combination therapy of echinocandins antifungal agents with the SOC has been an appealing option and recommended in the IDSA IAI guideline. Our meta-analysis found no differences in clinical cure and mortality rate when using combination therapy of echinocandin antifungal agents with the SOC compared to SOC monotherapy. However, regarding clinical cure rate, we found a signal for better outcomes with combination therapy of echinocandin antifungal agent with SOC. We also found that combination therapy with echinocandins and SOC did not result in statistically significantly more ADRs compared to SOC monotherapy. Future studies should evaluate the cost-effectiveness of combination therapy with echinocandins antifungal agents.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Writing–original draft. BA: Conceptualization, Data curation, Funding acquisition, Writing–review and editing. SA: Funding acquisition, Writing–review and editing. KB: Funding acquisition, Validation, Writing–review and editing. AA: Funding acquisition, Writing–review and editing. KA: Funding acquisition, Writing–review and editing. HW: Conceptualization, Methodology, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Scientific Research Deanship at University of Ha’il - Saudi Arabia through project number << RG-23 230>>.
Acknowledgments
We want to thank the Scientific Research Deanship at the University of Ha’il - Saudi Arabia, for funding this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1500529/full#supplementary-material
References
Borenstein, M., Hedges, L. V., Higgins, J. P., and Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1 (2), 97–111. doi:10.1002/jrsm.12
Caillot, D., Thiebaut, A., Herbrecht, R., de Botton, S., Pigneux, A., Bernard, F., et al. (2007). Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial). Cancer 110 (12), 2740–2746. doi:10.1002/cncr.23109
Chabi, M. L., Goracci, A., Roche, N., Paugam, A., Lupo, A., and Revel, M. P. (2015). Pulmonary aspergillosis. Diagn Interv. Imaging 96 (5), 435–442. doi:10.1016/j.diii.2015.01.005
Denning, D. W. (2024). Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24 (7), e428–e438. doi:10.1016/S1473-3099(23)00692-8
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Jeans, A. R., Howard, S. J., Al-Nakeeb, Z., Goodwin, J., Gregson, L., Warn, P. A., et al. (2012). Combination of voriconazole and anidulafungin for treatment of triazole-resistant aspergillus fumigatus in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 56 (10), 5180–5185. doi:10.1128/AAC.01111-12
John, T. M., and Brizendine, K. (2016). Combination antifungal therapy for invasive aspergillosis among patients with hematopoietic cell transplantation and positive galactomannan. Open Forum Infect. Dis. 3 (Suppl. l_1). doi:10.1093/ofid/ofw172.1331
Kontoyiannis, D. P., Hachem, R., Lewis, R. E., Rivero, G. A., Torres, H. A., Thornby, J., et al. (2003). Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98 (2), 292–299. doi:10.1002/cncr.11479
Lamoth, F., and Calandra, T. (2022). Pulmonary aspergillosis: diagnosis and treatment. Eur. Respir. Rev. 31 (166), 220114. doi:10.1183/16000617.0114-2022
Liu, A., Xiong, L., Wang, L., Zhuang, H., Gan, X., Zou, M., et al. (2024). Compare the efficacy of antifungal agents as primary therapy for invasive aspergillosis: a network meta-analysis. BMC Infect. Dis. 24 (1), 581. doi:10.1186/s12879-024-09477-9
Marr, K. A., Boeckh, M., Carter, R. A., Kim, H. W., and Corey, L. (2004). Combination antifungal therapy for invasive aspergillosis. Clin. Infect. Dis. 39 (6), 797–802. doi:10.1086/423380
Marr, K. A., Schlamm, H. T., Herbrecht, R., Rottinghaus, S. T., Bow, E. J., Cornely, O. A., et al. (2015). Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann. Intern Med. 162 (2), 81–89. doi:10.7326/M13-2508
Methley, A. M., Campbell, S., Chew-Graham, C., McNally, R., and Cheraghi-Sohi, S. (2014). PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 14, 579. doi:10.1186/s12913-014-0579-0
Mihu, C. N., Kassis, C., Ramos, E. R., Jiang, Y., Hachem, R. Y., and Raad, I. I. (2010). Does combination of lipid formulation of amphotericin B and echinocandins improve outcome of invasive aspergillosis in hematological malignancy patients? Cancer 116 (22), 5290–5296. doi:10.1002/cncr.25312
National library of medicine (2024). ClinicalTrials.gov azole-echinocandin combination Therapy for invasive aspergillosis (IA-DUET). Available at: https://clinicaltrials.gov/study/NCT04876716.
Pagano, L., Caira, M., Candoni, A., Offidani, M., Martino, B., Specchia, G., et al. (2010). Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 95 (4), 644–650. doi:10.3324/haematol.2009.012054
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189. doi:10.1016/j.jclinepi.2021.03.001
Panackal, A. A., Parisini, E., and Proschan, M. (2014). Salvage combination antifungal therapy for acute invasive aspergillosis may improve outcomes: a systematic review and meta-analysis. Int. J. Infect. Dis. 28, 80–94. doi:10.1016/j.ijid.2014.07.007
Patterson, T. F., Thompson, G. R., Denning, D. W., Fishman, J. A., Hadley, S., Herbrecht, R., et al. (2016). Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 63 (4), e1–e60. doi:10.1093/cid/ciw326
Petraitis, V., Petraitiene, R., McCarthy, M. W., Kovanda, L. L., Zaw, M. H., Hussain, K., et al. (2017). Combination therapy with isavuconazole and micafungin for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 61 (9). doi:10.1128/AAC.00305-17
Raad, I. I., Hanna, H. A., Boktour, M., Jiang, Y., Torres, H. A., Afif, C., et al. (2008). Novel antifungal agents as salvage therapy for invasive aspergillosis in patients with hematologic malignancies: posaconazole compared with high-dose lipid formulations of amphotericin B alone or in combination with caspofungin. Leukemia 22 (3), 496–503. doi:10.1038/sj.leu.2405065
Raad, I. I., Zakhem, A. E., Helou, G. E., Jiang, Y., Kontoyiannis, D. P., and Hachem, R. (2015). Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int. J. Antimicrob. Agents 45 (3), 283–288. doi:10.1016/j.ijantimicag.2014.08.012
Racil, Z., Weinbergerova, B., Kocmanova, I., Muzik, J., Kouba, M., Drgona, L., et al. (2013). Invasive aspergillosis in patients with hematological malignancies in the Czech and Slovak republics: fungal InfectioN Database (FIND) analysis, 2005-2009. Int. J. Infect. Dis. 17 (2), e101–e109. doi:10.1016/j.ijid.2012.09.004
Singh, N., Limaye, A. P., Forrest, G., Safdar, N., Munoz, P., Pursell, K., et al. (2006). Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 81 (3), 320–326. doi:10.1097/01.tp.0000202421.94822.f7
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Jama 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Thompson, G. R., and Young, J. H. (2021). Aspergillus infections. N. Engl. J. Med. 385 (16), 1496–1509. doi:10.1056/NEJMra2027424
Wang, Y., Zhang, L., Zhou, L., Zhang, M., and Xu, Y. (2022). Epidemiology, drug susceptibility, and clinical risk factors in patients with invasive aspergillosis. Front. Public Health 10, 835092. doi:10.3389/fpubh.2022.835092
Wells, G. S., B; O'Connell, D., Peterson, J., Welch, V., Losos, M., and Tugwell, P. (2024). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysesAvailable online. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Keywords: aspergillosis, echinocandins, meta-analysis, anidulafungin, caspofungin
Citation: Alsowaida YS, Alshoumr B, Alowais SA, Bin Saleh K, Alshammari A, Alshurtan K and Wali HA (2024) Effectiveness and safety of echinocandins combination therapy with the standard of care compared to the standard of care monotherapy for the treatment of invasive aspergillosis infection: a meta-analysis. Front. Pharmacol. 15:1500529. doi: 10.3389/fphar.2024.1500529
Received: 23 September 2024; Accepted: 06 December 2024;
Published: 19 December 2024.
Edited by:
Dolores R. Serrano Lopez, Complutense University of Madrid, SpainReviewed by:
Ana Isabel Fraguas, Complutense University, SpainRaquel Fernández García, University of Nottingham, United Kingdom
Copyright © 2024 Alsowaida, Alshoumr, Alowais, Bin Saleh, Alshammari, Alshurtan and Wali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yazed Saleh Alsowaida, eXMuYWxzb3dhaWRhQHVvaC5lZHUuc2E=
 Yazed Saleh Alsowaida
Yazed Saleh Alsowaida Bader Alshoumr2
Bader Alshoumr2 Shuroug A. Alowais
Shuroug A. Alowais Haytham A. Wali
Haytham A. Wali