- 1Department of Psychiatry of Women and Children, The Second People’s Hospital of Guizhou Province, Guiyang, China
- 2Department of Neurology, Huaining County People’s Hospital, Anqing, China
- 3Rehabilitation Department, The Second People’s Hospital of Guizhou Province, Guiyang, China
- 4Department of Acupuncture, Jinhua Wenrong Hospital, Jinhua, China
- 5Department of Clinical Psychology, The Third Affiliated Hospital of Soochow University, Changzhou, China
Background: Deutetrabenazine is a widely used drug for the treatment of tardive dyskinesia (TD), and post-marketing testing is important. There is a lack of real-world, large-sample safety studies of deutetrabenazine. In this study, a pharmacovigilance analysis of deutetrabenazine was performed based on the FDA Adverse Event Reporting System (FAERS) database to evaluate its relevant safety signals for clinical reference.
Methods: Adverse events (AEs) of FAERS with deutetrabenazine as the primary suspect drug were collected from the first quarter (Q1) of 2017 to Q1 of 2024. Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM) were used to mine AEs risk signals of deutetrabenazine. AEs were standardized and classified using the System Organ Class (SOC) and Preferred Terms (PTs) from Medical Dictionary for Regulatory Activities (MedDRA) version 23.0.
Results: A total of 3,583 AEs with deutetrabenazine as the primary suspect drug were collected in this study. We found that these AEs involved 23 SOCs, and the positive signals were mainly concentrated in systemic disease and various reactions at the site of administration (n = 1816, ROR = 1.23, PRR = 1.18, IC = 0.24, EBGM = 1.18), neurological disorders (n = 1736, ROR = 3.02, PRR = 2.60, IC = 1.38, EBGM = 2.60) and psychiatric disorders (n = 1,659, ROR = 4.15, PRR = 3.52, IC = 1.82, EBGM = 3.52). We eventually identified 100 valid PTs that met the criteria of the four algorithms. Drug ineffective, dyskinesia, depression, somnolence, suicidal ideation were considered to be the common PTs of deutetrabenazine. Tongue thrust (n = 4, ROR 253.47, PRR 253.35, IC 7.88, EBGM 235.95), grunting (n = 5, ROR 78.49, PRR 78.45, IC 6.26, EBGM 76.71) and drooling (n = 17, ROR 13.21, PRR 13.19, IC 3.72, EBGM 13.14) were not mentioned in the specification, but the high signal intensity suggested that they may be the potential adverse reactions.
Conclusion: The efficacy of deutetrabenazine may be accompanied by some potential adverse effects in several systems. Adverse events in psychiatric, neurologic, gastrointestinal and respiratory need to be monitored in clinical practice.
1 Introduction
Tardive Dyskinesia (TD) is a disorder in which the tongue, lower face, and jaw, as well as limbs (sometimes involving the muscles of the pharynx, diaphragm, or trunk) involuntarily spasms or dance-like movements occur after several months of use of neuroblocker drugs (Savitt and Jankovic, 2018; Caroff et al., 2020a). A prospective cohort study comprising 362 patients on long-term nerve blocking medications who did not present with TD at baseline revealed that the risk of TD development was 32% after 5 years of antipsychotic use, 57% after 15 years, and 68% after 25 years (Glazer et al., 1993). A meta-analysis comprising 41 studies revealed that the mean prevalence of TD in individuals with schizophrenia spectrum disorders was 25.3% (Carbon et al., 2017). TD has been demonstrated to have a significant impact on an individual’s daily activities and communication, causing distress and adversely affecting their quality of life and mental health. In some cases, it may even lead to suicide (Strassnig et al., 2018; Caroff et al., 2020b).
The precise mechanism by which TD occurs remains unclear. However, there is evidence to suggest that it may be related to hypersensitivity of dopamine receptors, as well as to hypofunction of the γ-aminobutyric acid (GABA) ergic system and oxidative stress (Bhidayasiri et al., 2013; Cho and Lee, 2013; Abe et al., 2023). Deutetrabenazine, a reversible inhibitor of vesicular monoamine transporter 2 (VMAT-2), has been demonstrated to reduce dopamine availability at hypersensitive D2 dopamine receptors, thereby improving involuntary movements. (Bhidayasiri et al., 2013; Vanegas-Arroyave et al., 2024). Deutetrabenazine was initially approved by the U.S. Food and Drug Administration (FDA) for the treatment of TD in 2017. Additionally, guidelines published by the American Academy of Neurology and Psychiatry include it as a class A recommendation for the treatment of TD (Bhidayasiri et al., 2018; Thomson et al., 2019; Keepers et al., 2020). Multiple clinical trials have confirmed the efficacy and safety of deutetrabenazine (Anderson et al., 2017; Fernandez et al., 2017; Fernandez et al., 2019). While improving TD symptoms, deutetrabenazine may also cause Adverse Events (AEs). Clinical trials often fail to detect some late-onset and rare adverse reactions, and safety information needs to be supplemented by post-marketing data analysis. Since its launch in 2017, deutetrabenazine has been widely used in the treatment of TD, but there is a lack of post-marketing safety studies with large samples.
Based on the data from the FDA Adverse Event Reporting System (FAERS), this study used multiple data mining techniques to analyze the AEs risk signals associated with deutetrabenazine, and to evaluate the potential risks of its use to provide clues for clinical safety.
2 Materials and methods
2.1 Data source
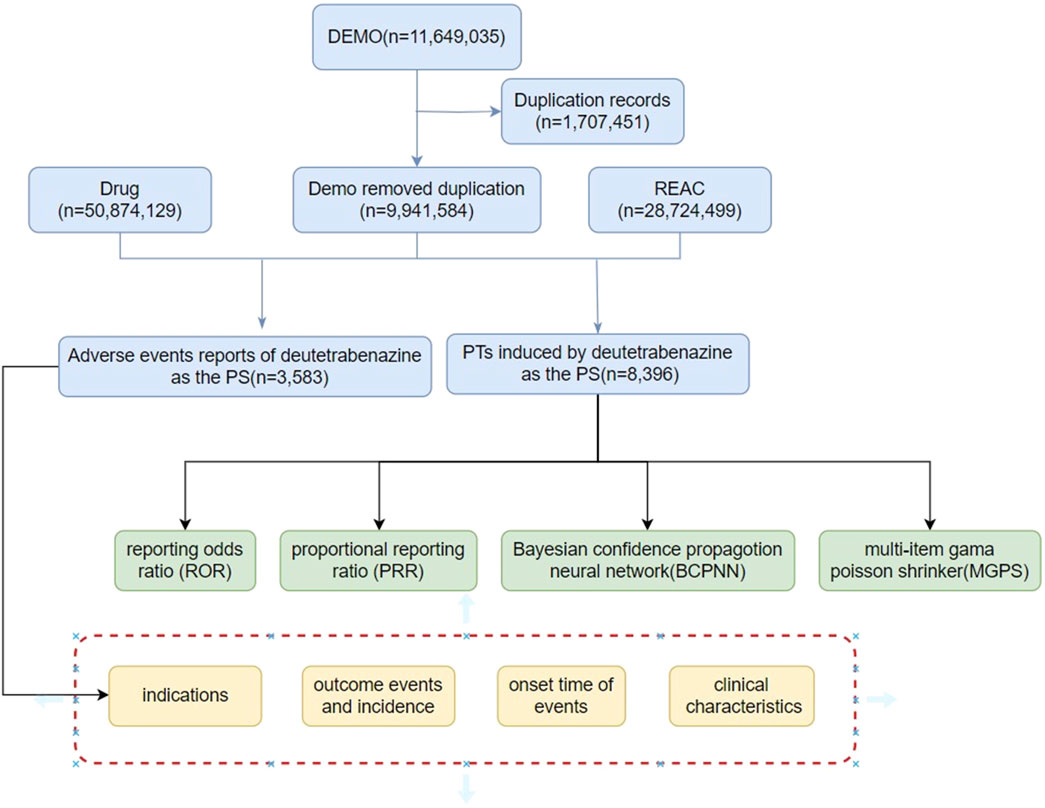
The R4.4.1 software was employed to clean and initially analyze the American Standard Code for Information Interchange (ASCII) packets in the FAERS database from the first quarter of 2017 to the first quarter of 2024. The process is detailed in Figure 1. A total of 111,649,035 demographic records were removed from the Demographic and Administrative Information (DEMO) database, which contained a total of 111,649,035 demographic records, and 1,707,451 duplicate records were deleted. There were 28,724,499 data points in the reaction (REAC) table and 3,583 adverse reaction reports for deutetrabenazine.
2.2 Statistical analysis
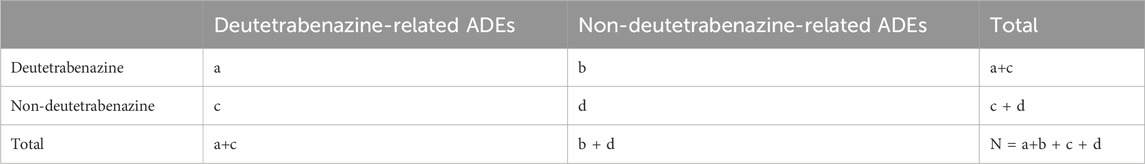
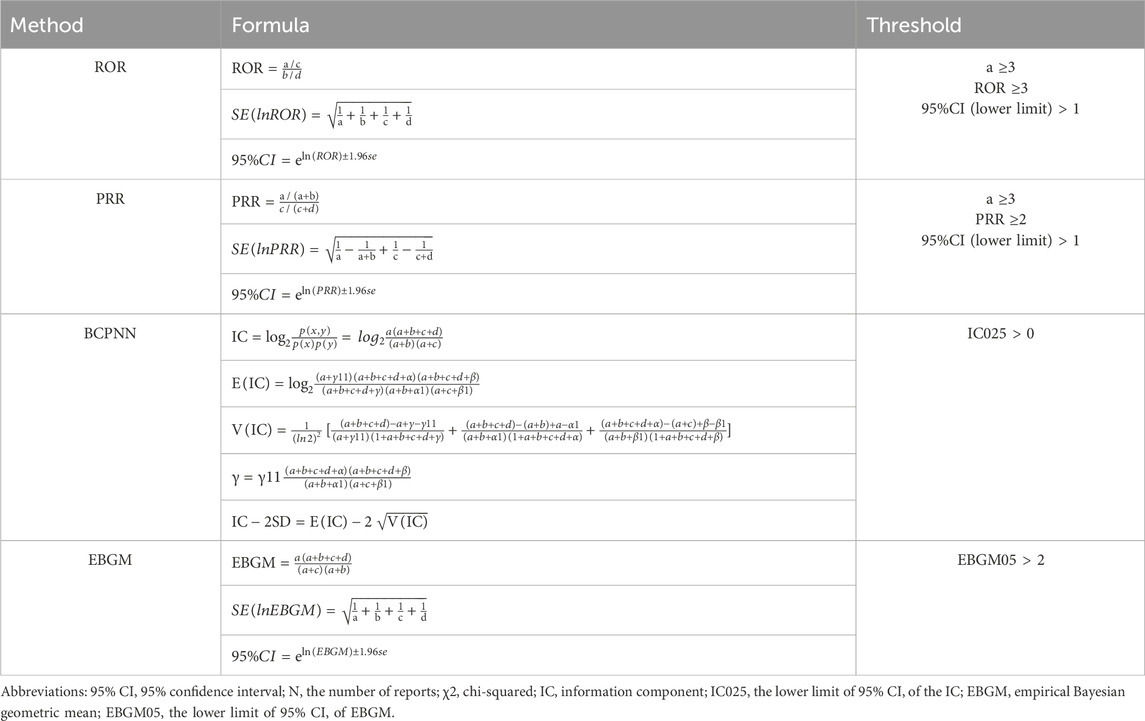
In this study, the AEs of deutetrabenazine were assessed multidimensionally using four analytical methods. The main methods include the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN) and empirical Bayesian geometric mean (EBGM) (Bate et al., 1998; DuMouchel, 1999; Evans et al., 2001; Rothman et al., 2004). Detecting and validating from multiple perspectives are more conducive to the accurate identification of safety signals. Among them, the ROR compares the ratio of target adverse events to non-target adverse events across the observed data. The PRR has better specificity than the ROR. Both the ROR and PRR calculations are based on 2 × 2 table, reflecting drug-event combinations, which can identify reports (Table 1) of AEs above the threshold, thereby highlighting the risk associated with deutetrabenazine and reducing the bias of lesser reported events. We set the number of reports (a) ≥ 3 and the lower limit of the 95% confidence interval (CI) > 1 as a positive signal in the ROR method. In the PRR method, a ≥3 and the lower limit of the 95% CI > 1 as a positive signal. BCPNN is mainly based on the information component (IC) and its 95% CI to evaluate drugs. BCPNN has the advantage of combining and cross-validating data from multiple sources. When the lower 95% CI of IC (IC025) is >0, it indicates a positive signal. Multi-item Gamma Poisson Shrinker (MGPS) is an extension of Gamma Poisson ShrinKer (GPS) to explore whether there is an association between medication population characteristics and adverse events and explore the interaction of variables. MGPS is used to calculate the empirical Bayesian geometric mean (EBGM), when the lower limit of the 95% CI of the EBGM (EBGM 05) is >2, it suggests a positive signal. Park and colleagues evaluated multiple approaches of data mining and found that there is no single method outperformed the others on all performance metrics, and they recommended using the common results of multiple methods to make decisions for adverse drug event surveillance (Park et al., 2020). Larger values indicate a stronger correlation between deutetrabenazine and AEs. The formulas are detailed in Table 2.
2.3 Signal filtering and categorization
Drug names were standardized via the Medex_UIMA_1.8.3 system (Jiang et al., 2014). Preferred terms (PTs) of the AEs terminology set were standardized via the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0 (Brown, 2004), and descriptions of the AEs classifications were standardized via the System Organ Class (SOC) (Tieu and Breder, 2018).
3 Results
3.1 Basic information of AEs reports
There were 3,583 AEs, with deutetrabenazine as the primary suspected drug, in the FAERS database from Q1 of 2017 to Q1 of 2024. The results revealed 89.51% of the reports included the sex of the patient, with the percentage of female patients (60.48%) being significantly greater than the percentage of male patients (29.03%). Approximately half of the reports (50.07%) did not provide information regarding the age of the patient, and among the reports with known ages, patients in the 45–65 age group were the most common, accounting for approximately 21.43%, followed by those in the 65–75 age group (13.76%). The number of reports of AEs associated with deutetrabenazine showed an annual increasing trend from 2017 to 2021 but a decreasing trend from 2021 to 2024. In addition, among the reports with noted AEs occurrences, the incidence of AEs was higher within 1 week of treatment and after 60 days of treatment. In terms of clinical outcomes, with the exception of unspecified serious outcomes, outcomes leading to hospitalization were the most common. Notably, the predominant reporting population in the report was consumers, who accounted for a greater proportion than healthcare professionals combined. Additionally, almost all reporting areas were from the United States, accounting for approximately 99.30% of the total. Detailed information is provided in Table 3.
3.2 Risk signal mining results
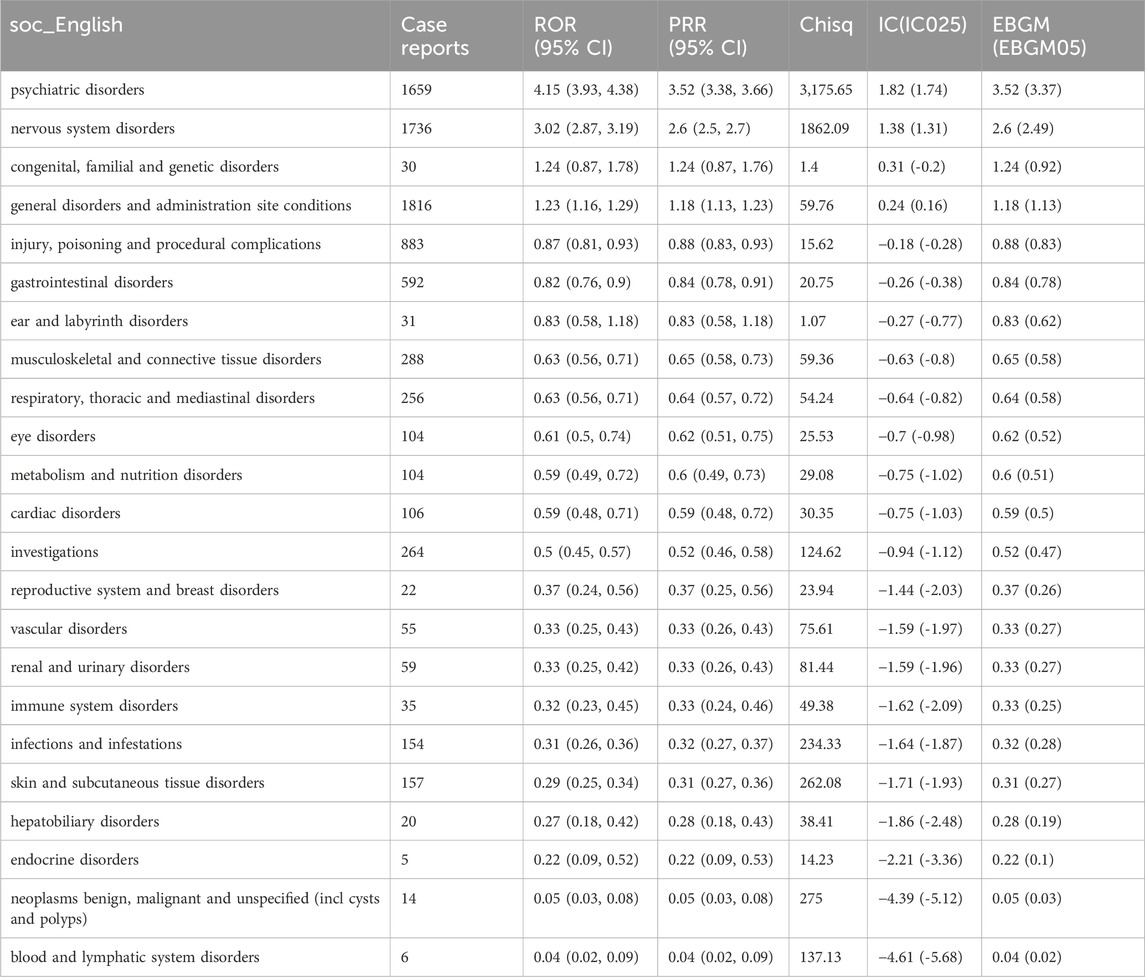
Table 4 describes the signaling intensity of deutetrabenazine at the SOC level. We found that the AEs associated with deutetrabenazine involved 23 SOCs. Positive signals were mainly concentrated in systemic diseases and various reactions at the site of administration (n = 1816, ROR = 1.23, PRR = 1.18, IC = 0.24, EBGM = 1.18), neurological disorders (n = 1736, ROR = 3.02, PRR = 2.60, IC = 1.38, EBGM = 2.60), psychiatric disorders (n = 1,659, ROR = 4.15, PRR = 3.52, IC = 1.82, EBGM = 3.52). All four algorithms for neurologic and psychiatric disorders had positive signals. In addition to the systems covered in the drug insert, some other systemic adverse reactions such as various musculoskeletal and connective tissue disorders, metabolic and nutritional disorders, and ophthalmic disorders were identified in this study.
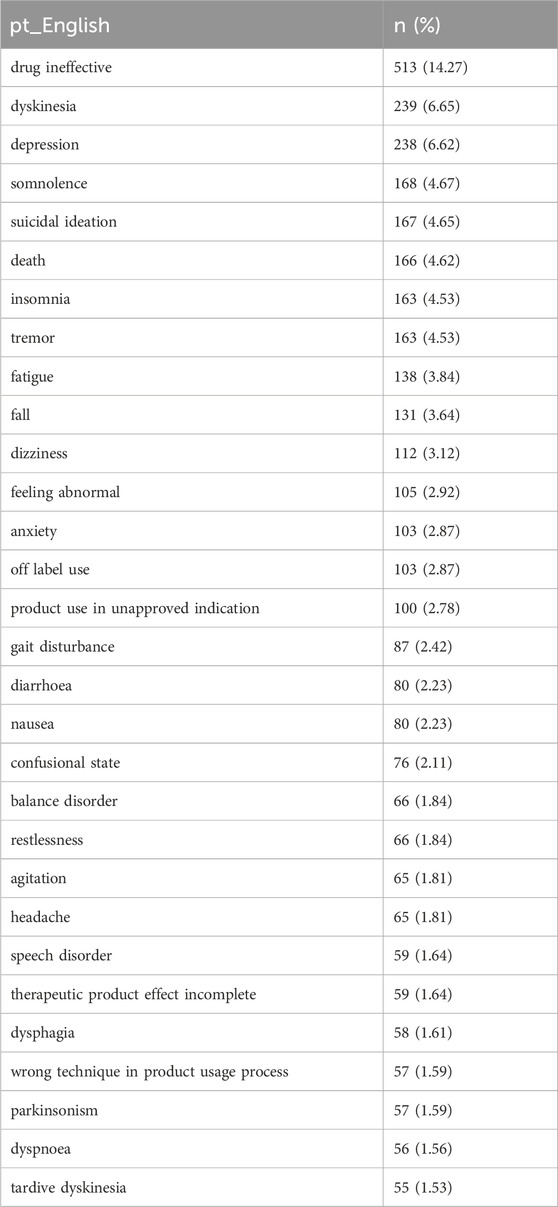
The present study identified 100 valid PTs that met the criteria of the four algorithms. Drug ineffectiveness, dyskinesia, depression, somnolence and suicidal ideation were considered common PTs of deutetrabenazine, and these common SOCs included general disorders and administration site conditions, nervous system disorders, and psychiatric disorders, as detailed in Table 5.
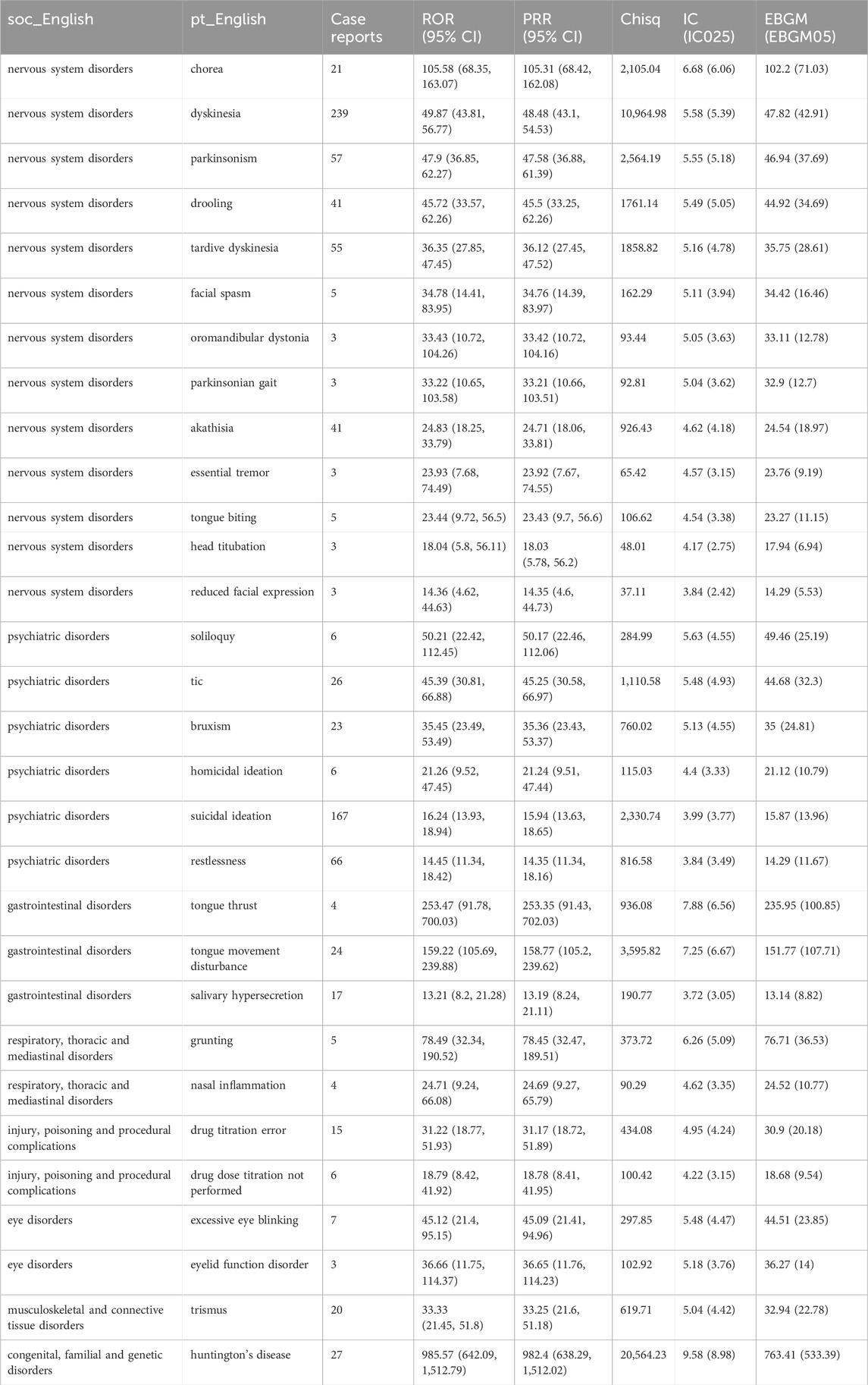
Table 6 presents the top 30 PTs that demonstrate compliance with all four algorithms. According to strict EBGM algorithm, PTs with higher risk signal strength includes tongue thrust (EBGM 235.95), grunting (EBGM 76.71), dyskinesia (EBGM 7.82), and drooling (EBGM 44.92). Comparison with the drug insert for deutetrabenazine revealed that tongue propulsion tongue thrust, grunting, and drooling were not mentioned in the insert and may be potential AEs.
4 Discussion
There has been a gradual increase in adverse event reports for deutetrabenazine and a lack of comprehensive safety studies. To our knowledge, this is the first pharmacovigilance analysis of deutetrabenazine adverse events using FAERS data.
Our results revealed that female patients reported AEs at a higher rate than male patients did, and among reports with known ages, the 45–65 age group had the highest AEs incidence, followed by the 65–75 age group. It has been demonstrated that the risk of TD is elevated in older patients relative to younger patients (Sajatovic et al., 2022). This finding is consistent with the epidemiologic profile of TD, and previous studies have shown that female sex and advanced age are risk factors for TD (Solmi et al., 2018; Patterson-Lomba et al., 2019; Saklad, 2020). The metabolism and excretion of drugs are slowed in elderly patients, resulting in the accumulation of the drug in the body and an increased risk of adverse events (Akinosoglou et al., 2023). In light of the growing prevalence of deutetrabenazine, it is imperative for clinicians to remain vigilant for any adverse events associated with this medication, particularly in elderly female patients. The early detection of adverse events serves to reduce the risk of hospitalization and the risk of potentially developing life-threatening conditions. The impact of gender on deutetrabenazine-related adverse effects has yet to be fully elucidated, underscoring the need for further research in this area. It is noteworthy that the majority of reports in this category were submitted by consumers, representing a higher percentage of the total than other populations. This may have contributed to the lower accuracy of the reports. Consumer reports constitute an essential component of the FAERS (Munoz et al., 2019). Healthcare professional reports offer a more detailed clinical perspective, whereas consumer reports prioritize patient-related information and medication experience, both of which are crucial for ensuring the safe use of medications (Rolfes et al., 2015). Furthermore, the completeness of consumer reports is of value in comparison to those provided by healthcare professionals (Christ et al., 2023). The FDA ensures the quality of consumer reports through the implementation of relevant tools (Munoz et al., 2019). It would be beneficial to add further data on adverse reactions to deutetrabenazine in other countries in the future. In addition, we note that almost all reporting regions are from the United States. This may be related to factors such as database source, region, and time of drug launch. Deutertrabenazine was first approved by the FDA for the treatment of TD in the United States (Ricciardi et al., 2019).
Compared with tetrabenazine, deutetrabenazine contains deuterium, which reduces peak plasma concentrations to attenuate drug metabolism and reduces the frequency of administration, with fewer side effects (Claassen et al., 2017; Di-Martino et al., 2023). Both valbenazine and deutetrabenazine are approved by FDA for the treatment of delayed dyskinesia as VMAT-2 inhibitors. Metabolites of deutetrabenazine may act on the serotonin 5HT-7 receptor in addition to the VMAT-2 receptor. The occupancy of VMAT-2 receptor is 84% at low doses of valbenazine (40 mg), and 51%–92% at 6–42 mg doses of deutetrabenazine (Stahl, 2018). 2.5HT-7 receptor may play a role in regulating emotion (Kucwaj-Brysz et al., 2024). Deutetrabenazine induced parkinsonism symptoms are milder than those of valbenazine, which may be related to its receptor occupancy. It can better maintain the functional balance of dopaminergic neurons and reduce the severity of parkinsonism symptoms caused by excessive inhibition of the dopaminergic system (Zhang et al., 2024).
This study revealed that the risk signals for AEs caused by deutetrabenazine as the primary drug included 100 validated PTs in 23 SOCs. At the SOC level, these disorders mainly include general disorders and administration site conditions, nervous system disorders, and psychiatric disorders, which are consistent with the descriptions in the drug inserts. Adverse events associated with gastrointestinal, respiratory, thoracic and mediastinal disorders were also common. At the AEs level, the incidences of AEs such as dyskinesia, depression, somnolence, and suicidal ideation were high, again generally consistent with what was documented in the drug insert. Depression and suicidal ideation are frequently observed AEs. Although two randomized controlled trials have demonstrated the efficacy of deutetrabenazine in the treatment of tardive dyskinesia (Anderson et al., 2017; Fernandez et al., 2017), however, a meta-analysis by some scholars showed that deutetrabenazine 36 mg did not significantly improve TD symptoms, which may be due to insufficient therapeutic dose or poor quality of selected literature, resulting in selection bias (Ismail et al., 2024). To evaluate the efficacy of deutetrabenazine, it is necessary to consider not only the improvement of motor symptoms, but also psychological and social function, duration of action, and dose-response relationships. The black box warning of FDA suggests that deutetrabenazine may increase the risk of developing depression and suicidal ideation in patients with Huntington’s disease (Austedo, 2024). Dopamine plays a significant role in the etiology of depressive disorders, with the dopamine reward system implicated in the pathogenesis of depression (Wang et al., 2021; Murray et al., 2023). Some studies have demonstrated that the administration of pharmacological agents that enhance dopamine function can alleviate depressive symptoms (Delva and Stanwood, 2021; Mizuno et al., 2023). Deutetrabenazine exerts its pharmacological effects by inhibiting VMAT-2, which results in the inhibition of dopamine secretion into protruding vesicles and a reduction in overall dopamine release. This may potentially lead to an increase in depressive symptoms and suicidal ideation (Golsorkhi et al., 2024). In a study conducted by Schultz and colleagues, it is demonstrated that tetrabenazine do not elevate the likelihood of depression or suicidal ideation in patients diagnosed with Huntington’s disease. Among patients with a history of depression, those who used tetrabenazine exhibited a lower incidence of depression and suicidal ideation (Schultz et al., 2018). The study conducted by Frank and colleagues has demonstrated that deutetrabenazine has a lower incidence of TD compared to other treatments. The incidence of depression in Huntington’s disease patients treated with deutetrabenazine (4.8%) and a placebo (6.7%) was not statistically significant (Frank et al., 2024). The existing literature on the potential association between deutetrabenazine and depression and suicidal ideation is limited and inconclusive. To validate these findings, further large-scale prospective studies are required.
Furthermore, the study identified several novel PTs, including tongue thrust, grunting, and drooling. Deutetrabenazine, as a novel and highly selective VMAT-2 inhibitor, is a dopamine-depleting drug (Strassnig et al., 2018). The transport of neurotransmitters such as dopamine, epinephrine, and norepinephrine into synaptic vesicles (Guillot and Miller, 2009) reduced the concentration of dopamine, epinephrine, and norepinephrine in the protruding space and decreased the concentration of dopamine in the protruding space, affecting the oral muscle and causing tongue thrust. Deutetrabenazine depletes the stored neurotransmitters in the vesicles, reduces the concentration of the neurotransmitters, and the muscles of the tongue and throat relax excessively, thus blocking the airway and causing grunting (Guillot and Miller, 2009; Honda et al., 2020). Animal experiments have shown that the dopaminergic system can regulate saliva secretion, and deutetrabenazine may affect parotid salivation through VMAT, resulting in hypersalivation (Tomassoni et al., 2015).
Both our study and the drug insert indicate that deutetrabenazine is susceptible to neurologic and psychiatric adverse effects. Prevention and closely observation of neurologic and psychiatric symptoms are the primary methods of minimizing side effects (Bhidayasiri et al., 2013). Timely assessment, adjustment or discontinuation of dopamine receptor blockers, and quality nursing care are effective in mitigating adverse effects (Citrome et al., 2021). Some early-onset adverse events will subside after the therapeutic dose is reached. It is may be related to the therapeutic dose and tolerability (Frank et al., 2024). In addition, clinicians need to assess the patient’s condition in a timely manner. For the mild adverse reactions, we may continue to observe or treat symptomatically. However, for severe adverse reactions, discontinuation of medication and hospitalization are required (Stroup and Gray, 2018).
5 Limitations and future directions
Although this study evaluated the safety of deutetrabenazine in multiple dimensions, it is important to acknowledge that there are still some shortcomings. First, this study relied mainly on spontaneous reports and was mostly based on consumer reports, and the reported information may not be sufficiently comprehensive. Second, the reports in this database were mainly from the United States and lacked reports from other countries and regions, and there may be reporting bias. In the future, more rigorous prospective studies combining clinical trials and epidemiological studies are needed to assess the safety of deutetrabenazine more accurately and better guide its clinical use.
6 Conclusion
In conclusion, this study employed a multidimensional assessment of the safety of deutetrabenazine using the FAERS database. While some adverse reactions (e.g., tongue thrust, grunting, and drooling) manifest infrequently, they are notable indicators that warrant further investigation. In clinical practice, it is advisable to be mindful of the potential for adverse reactions in patients with psychiatric disorders, neurological disorders, gastrointestinal disorders, and respiratory conditions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers.
Ethics statement
The studies involving humans were approved by This is a study based on publicly available data and does not require ethical approval. The FDA conducts ethical reviews. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Conceptualization, Data curation, Funding acquisition, Writing–original draft, Writing–review and editing. YW: Data curation, Methodology, Writing–original draft, Writing–review and editing. JL: Writing–original draft, Writing–review and editing. LH: Data curation, Writing–original draft, Writing–review and editing. ST: Writing–original draft, Writing–review and editing. GW: Conceptualization, Data curation, Visualization, Writing–review and editing. XZ: Conceptualization, Data curation, Formal Analysis, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82460282), Guizhou Science and Technology Program Project (ZK-2023-General 195), Guizhou High-level Innovative Talents Project (gzwjrs 2022-013) and Guizhou Provincial Health Commission Science and Technology Program Project (gzwkj 2022-068).
Acknowledgments
We acknowledge the FAERS, which provided and gave permission to use the data analyzed in the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, Y., Yagishita, S., Sano, H., Sugiura, Y., Dantsuji, M., Suzuki, T., et al. (2023). Shared GABA transmission pathology in dopamine agonist- and antagonist-induced dyskinesia. Cell. Rep. Med. 4, 101208. doi:10.1016/j.xcrm.2023.101208
Akinosoglou, K., Schinas, G., Almyroudi, M. P., Gogos, C., and Dimopoulos, G. (2023). The impact of age on intensive care. Ageing Res. Rev. 84, 101832. doi:10.1016/j.arr.2022.101832
Anderson, K. E., Stamler, D., Davis, M. D., Factor, S. A., Hauser, R. A., Isojarvi, J., et al. (2017). Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiat 4, 595–604. doi:10.1016/S2215-0366(17)30236-5
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54 (4), 315–321. doi:10.1007/s002280050466
Bhidayasiri, R., Fahn, S., Weiner, W. J., Gronseth, G. S., Sullivan, K. L., Zesiewicz, T. A., et al. (2013). Evidence-based guideline: treatment of tardive syndromes: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 81, 463–469. doi:10.1212/WNL.0b013e31829d86b6
Bhidayasiri, R., Jitkritsadakul, O., Friedman, J. H., and Fahn, S. (2018). Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J. Neurological Sci. Official Bull. World Fed. Neurology. 389, 67–75. doi:10.1016/j.jns.2018.02.010
Brown, E. G. (2004). Using MedDRA: implications for risk management. Drug Saf. 27 (8), 591–602. doi:10.2165/00002018-200427080-00010
Carbon, M., Hsieh, C. H., Kane, J. M., and Correll, C. U. (2017). Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J. Clin. Psychiatry 78 (3), e264–e278. doi:10.4088/JCP.16r10832
Caroff, S. N., Leong, S. H., Roberts, C. B., Berkowitz, R. M., and Campbell, E. C. (2020a). Correlates of the abnormal involuntary movement scale in veterans with tardive dyskinesia. J. Clin. Psychopharmacol. 40 (4), 373–380. doi:10.1097/JCP.0000000000001229
Caroff, S. N., Yeomans, K., Lenderking, W. R., Cutler, A. J., Yonan, C., Shalhoub, H., et al. (2020b). RE-KINECT: a prospective study of the presence and healthcare burden of tardive dyskinesia in clinical practice settings. J. Clin. Psychopharmacol. 40 (3), 259–268. doi:10.1097/JCP.0000000000001201
Cho, C. H., and Lee, H. J. (2013). Oxidative stress and tardive dyskinesia: pharmacogenetic evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 207–213. doi:10.1016/j.pnpbp.2012.10.018
Christ, P., Dubrall, D., Schmid, M., and Sachs, B. (2023). Comparative analysis of information provided in German adverse drug reaction reports sent by physicians, pharmacists and consumers. Drug Saf. 46, 1363–1379. doi:10.1007/s40264-023-01355-8
Citrome, L., Isaacson, S. H., Larson, D., and Kremens, D. (2021). Tardive dyskinesia in older persons taking antipsychotics. Neuropsychiatr. Dis. Treat. 17, 3127–3134. doi:10.2147/NDT.S328301
Claassen, D. O., Carroll, B., De-Boer, L. M., Wu, E., Ayyagari, R., Gandhi, S., et al. (2017). Indirect tolerability comparison of deutetrabenazine and tetrabenazine for huntington disease. J. Clin. Mov. Disord. 4, 3. doi:10.1186/s40734-017-0051-5
Delva, N. C., and Stanwood, G. D. (2021). Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. (Maywood) 246 (9), 1084–1093. doi:10.1177/1535370221991830
Di-Martino, R., Maxwell, B. D., and Pirali, T. (2023). Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug Discov. 22, 562–584. doi:10.1038/s41573-023-00703-8
DuMouchel, W. (1999). Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Statistician 53 (3), 177–190. doi:10.1080/00031305.1999.10474456
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. and Drug Saf. 10 (6), 483–486. doi:10.1002/pds.677
Fernandez, H. H., Factor, S. A., Hauser, R. A., Jimenez-Shahed, J., Ondo, W. G., Jarskog, L. F., et al. (2017). Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology 88, 2003–2010. doi:10.1212/WNL.0000000000003960
Fernandez, H. H., Stamler, D., Davis, M. D., Factor, S. A., Hauser, R. A., Jimenez-Shahed, J., et al. (2019). Long-term safety and efficacy of deutetrabenazine for the treatment of tardive dyskinesia. J. Neurol. Neurosurg. Psychiatry 90, 1317–1323. doi:10.1136/jnnp-2018-319918
Frank, S., Anderson, K. E., Fernandez, H. H., Hauser, R. A., Claassen, D. O., Stamler, D., et al. (2024). Safety of deutetrabenazine for the treatment of tardive dyskinesia and chorea associated with huntington disease. Neurol. Ther. 13, 655–675. doi:10.1007/s40120-024-00600-1
Glazer, W. M., Morgenstern, H., and Doucette, J. T. (1993). Predicting the long-term risk of tardive dyskinesia in outpatients maintained on neuroleptic medications. J. Clin. Psychiatry 54 (4), 133–139. doi:10.1016/0165-0327(93)90051-K
Golsorkhi, M., Koch, J., Pedouim, F., Frei, K., Bondariyan, N., and Dashtipour, K. (2024). Comparative analysis of deutetrabenazine and valbenazine as VMAT2 inhibitors for tardive dyskinesia: a systematic review. Tremor Other Hyperkinet Mov. (N Y) 14, 13. doi:10.5334/tohm.842
Guillot, T. S., and Miller, G. W. (2009). Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 39 (2), 149–170. doi:10.1007/s12035-009-8059-y
Honda, T., Takata, Y., Cherasse, Y., Mizuno, S., Sugiyama, F., Takahashi, S., et al. (2020). Ablation of ventral Midbrain/Pons GABA neurons induces mania-like behaviors with altered sleep homeostasis and dopamine D(2)R-mediated sleep reduction. iScience 23, 101240. doi:10.1016/j.isci.2020.101240
Ismail, O., Albdour, K., Jaber, Y., Jaber, K., and Alsaras, A. (2024). Efficacy and safety of different pharmacological interventions in the treatment of tardive dyskinesia: a systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 80, 1471–1482. doi:10.1007/s00228-024-03722-5
Jiang, M., Wu, Y., Shah, A., Priyanka, P., Denny, J. C., and Xu, H. (2014). Extracting and standardizing medication information in clinical text – the MedEx-UIMA system. Amia Summits Transl. Sci. Proc. 2014, 37–42.
Keepers, G. A., Fochtmann, L. J., Anzia, J. M., Benjamin, S., Lyness, J. M., Mojtabai, R., et al. (2020). The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am. J. psychiatry 177 (9), 868–872. doi:10.1176/appi.ajp.2020.177901
Kucwaj-Brysz, K., Bas, S., Zeslawska, E., Podlewska, S., Jastrzebska-Wiesek, M., Partyka, A., et al. (2024). The importance of stereochemistry in 5-ht7r Modulation─A case study of hydantoin derivatives. Acs Chem. Neurosci. 15, 3884–3900. doi:10.1021/acschemneuro.4c00152
Mizuno, Y., Ashok, A. H., Bhat, B. B., Jauhar, S., and Howes, O. D. (2023). Dopamine in major depressive disorder: a systematic review and meta-analysis of in vivo imaging studies. J. Psychopharmacol. 37 (11), 1058–1069. doi:10.1177/02698811231200881
Munoz, M. A., Delcher, C., Dal-Pan, G. J., Kortepeter, C. M., Wu, E., Wei, Y. J., et al. (2019). Impact of a new consumer form on the quantity and quality of adverse event reports submitted to the United States food and drug administration. Pharmacotherapy 39, 1042–1052. doi:10.1002/phar.2325
Murray, L., Israel, E. S., Balkind, E. G., Pastro, B., Lovell-Smith, N., Lukas, S. E., et al. (2023). Multi-modal assessment of reward functioning in adolescent anhedonia. Psychol. Med. 53 (10), 4424–4433. doi:10.1017/S0033291722001222
Park, G., Jung, H., Heo, S. J., and Jung, I. (2020). Comparison of data mining methods for the signal detection of adverse drug events with a hierarchical structure in postmarketing surveillance. Life (Basel) 10, 138. doi:10.3390/life10080138
Patterson-Lomba, O., Ayyagari, R., and Carroll, B. (2019). Risk assessment and prediction of TD incidence in psychiatric patients taking concomitant antipsychotics: a retrospective data analysis. BMC Neurol. 19 (1), 174. doi:10.1186/s12883-019-1385-4
Ricciardi, L., Pringsheim, T., Barnes, T. R. E., Martino, D., Gardner, D., Remington, G., et al. (2019). Treatment recommendations for tardive dyskinesia. Rev. Can. Psychiatr. 64 (6), 388–399. doi:10.1177/0706743719828968
Rolfes, L., van-Hunsel, F., Wilkes, S., van-Grootheest, K., and van-Puijenbroek, E. (2015). Adverse drug reaction reports of patients and healthcare professionals-differences in reported information. Pharmacoepidemiol Drug Saf. 24, 152–158. doi:10.1002/pds.3687
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13 (8), 519–523. doi:10.1002/pds.1001
Sajatovic, M., Finkbeiner, S., Wilhelm, A., Barkay, H., Chaijale, N., Gross, N., et al. (2022). Long-Term safety and efficacy of deutetrabenazine in younger and older patients with tardive dyskinesia. Am. J. Geriatr. Psychiatry 30, 360–371. doi:10.1016/j.jagp.2021.08.003
Saklad, S. R. (2020). Identifying tardive dyskinesia: risk factors, functional impact, and diagnostic tools. J. Clin. Psychiatry 81 (1), TV18059BR1C. doi:10.4088/JCP.TV18059BR1C
Savitt, D., and Jankovic, J. (2018). Tardive syndromes. J. Neurological Sci. 389, 35–42. doi:10.1016/j.jns.2018.02.005
Schultz, J. L., Killoran, A., Nopoulos, P. C., Chabal, C. C., Moser, D. J., and Kamholz, J. A. (2018). Evaluating depression and suicidality in tetrabenazine users with Huntington disease. Neurology 91, e202–e207. doi:10.1212/WNL.0000000000005817
Solmi, M., Pigato, G., Kane, J. M., and Correll, C. U. (2018). Clinical risk factors for the development of tardive dyskinesia. J. neurological Sci. 389, 21–27. doi:10.1016/j.jns.2018.02.012
Stahl, S. M. (2018). Comparing pharmacologic mechanism of action for the vesicular monoamine transporter 2 (VMAT2) inhibitors valbenazine and deutetrabenazine in treating tardive dyskinesia: does one have advantages over the other? CNS Spectr. 23, 239–247. doi:10.1017/S1092852918001219
Strassnig, M., Rosenfeld, A., and Harvey, P. D. (2018). Tardive dyskinesia: motor system impairments, cognition and everyday functioning. CNS spectrums 23 (6), 370–377. doi:10.1017/S1092852917000542
Stroup, T. S., and Gray, N. (2018). Management of common adverse effects of antipsychotic medications. World Psychiatry 17, 341–356. doi:10.1002/wps.20567
Thomson, A. M., Wallace, J., and Kobylecki, C. (2019). Tardive dyskinesia after drug withdrawal in two older adults: clinical features, complications and management. Geriatrics and Gerontology Int. 19 (6), 563–564. doi:10.1111/ggi.13669
Tieu, C., and Breder, C. D. (2018). A critical evaluation of safety signal analysis using algorithmic standardised MedDRA queries. Drug Saf. 41 (12), 1375–1385. doi:10.1007/s40264-018-0706-7
Tomassoni, D., Traini, E., Mancini, M., Bramanti, V., Mahdi, S. S., and Amenta, F. (2015). Dopamine, vesicular transporters, and dopamine receptor expression in rat major salivary glands. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R585–R593. doi:10.1152/ajpregu.00455.2014
Vanegas-Arroyave, N., Caroff, S. N., Citrome, L., Crasta, J., McIntyre, R. S., Meyer, J. M., et al. (2024). An Evidence-Based update on anticholinergic use for Drug-Induced movement disorders. Cns Drugs 38, 239–254. doi:10.1007/s40263-024-01078-z
Wang, S., Leri, F., and Rizvi, S. J. (2021). Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 30 (110), 110289. doi:10.1016/j.pnpbp.2021.110289
Keywords: deutetrabenazine, data analysis, FAERS, real-world, adverse events
Citation: Shu Y, Wang Y, Liu J, Hu L, Tong S, Wu G and Zhu X (2024) Safety assessment of deutetrabenazine: real-world adverse event analysis from the FAERS database. Front. Pharmacol. 15:1498215. doi: 10.3389/fphar.2024.1498215
Received: 18 September 2024; Accepted: 06 December 2024;
Published: 23 December 2024.
Edited by:
Simon Kaja, Loyola University Chicago, United StatesCopyright © 2024 Shu, Wang, Liu, Hu, Tong, Wu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wu, NzM4NDQ2MTI0QHFxLmNvbQ==; Xianlin Zhu, MjUwOTY1NTc0N0BxcS5jb20=
†These authors have contributed equally to this work
 Yanping Shu
Yanping Shu Yuanhe Wang2†
Yuanhe Wang2† Jiaoying Liu
Jiaoying Liu Lingyan Hu
Lingyan Hu Xianlin Zhu
Xianlin Zhu