
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 14 January 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1495393
Hydroxysafflor yellow A (HSYA), a natural pigment with a chalcone structure extracted from Carthamus tinctorius L. (Safflower), has been widely proven to have good efficacy on cardiovascular diseases, atherosclerosis, cancer, and diabetes. However, no study has reported on the anticancer mechanisms of Hydroxysafflor yellow A (HSYA), a principal bioactive compound in safflower. This review discusses recent developments in the physicochemical properties and sources, pharmacological effects and mechanisms, pharmacokinetic progress, and safety of HSYA, focusing on the involvement of HSYA in the regulation of related pathways and mechanisms of apoptosis, autophagy, and the tumor immune microenvironment in a variety of cancers. This can serve as a theoretical basis for further research and development of HSYA, with insights into the mechanisms of anticancer signaling pathways.
Cancer is a malignant tumor resulting from abnormal cell proliferation, malignant infiltration, and growth. According to the GLOBOCAN Statistical Report, cancer was the second leading cause of mortality worldwide from 2018 to 2020. Notably, it is the leading cause of death among individuals under the age of 70 in nearly 60% of countries (Sung et al., 2021; Ugai et al., 2022). The global incidence of new cancer cases in 2020 is estimated to reach approximately 19.3 million, with an associated mortality rate of 10 million. The most common types of cancer based on age-standardized mortality rates in all countries and regions in 2020 were lung cancer (60 countries), breast cancer (52 countries), prostate cancer (32 countries), and cervical cancer (28 countries). Breast cancer was the leading type of cancer in terms of age-standardized incidence rate in 109 countries in 2020 (Siegel et al., 2023). According to data from the World Health Organization, the number of new cancer cases worldwide is projected to exceed 28 million by 2040 (Williams et al., 2022), indicating that one-fifth of the global population will be affected by cancer. Consequently, cancer prevention has become a critical public health issue in the 21st century (Rumgay et al., 2022), and the development of new cancer therapies and targets is of urgent importance.
Carthamus tinctorius L. (Safflower), is an annual or biennial herbaceous plant belonging to the Compositae family (Zhang et al., 2016). It thrives in warm and dry climates, and is resistant to cold conditions. Native to Central Asia, safflower is now widely cultivated in China, Japan, and North Korea (Ao et al., 2018). In traditional Chinese medicine, safflower typically refers to the dried flowers of the plant, which were first documented in Zhang Zhong Jing’s ‘Synopsis of the Golden Chamber’ for the treatment of gynecological disorders (Adamska and Biernacka, 2021). In modern Chinese medicine, safflower is used primarily to promote blood circulation, reduce blood stasis, and alleviate discomfort. Hydroxysafflor yellow A (HSYA), a chalcone glycoside with good water solubility, is the principal bioactive compound found in safflower. Due to its low abundance and efficacy, HSYA is a key quality control standard in the Chinese Pharmacopoeia. Currently, research on HSYA has mainly focused on its effects on cardiovascular diseases (Bai et al., 2020). Safflower yellow (SY) injection, an NMPA-approved drug for treating angina pectoris and cerebral infarction, contains up to 90% HSYA as the main ingredient (each 50 mg of SY injection contained 45 mg of HSYA) (Li et al., 2012). HSYA also exhibits various other biological activities, including neuroprotective, anticoagulant and anti-tumor activities, and potential benefits against hepatic fibrosis, pulmonary arterial hypertension, diabetes, and vascular dementia (Zhang et al., 2021).
In the past 2 years, literature has reviewed and discussed the mechanisms of HSYA in treating cardiovascular and cerebrovascular diseases (Bai et al., 2020), atherosclerosis (Xue et al., 2021), and diabetes (Zhang et al., 2021). However, the its anticancer effects have not been comprehensively summarized, and the its mechanisms involved in treating various cancers (pan-cancer) remain unclear. Therefore, this review collected articles containing the keywords “cancer” and “HSYA” from four databases: “PubMed,” “Web of Science (WOS),” “Scopus” and “CNKI.” Relevant original articles were categorized and summarized (Figure 1) to consolidate the recent research on the anticancer effects of HSYA and discuss its underlying molecular mechanisms.
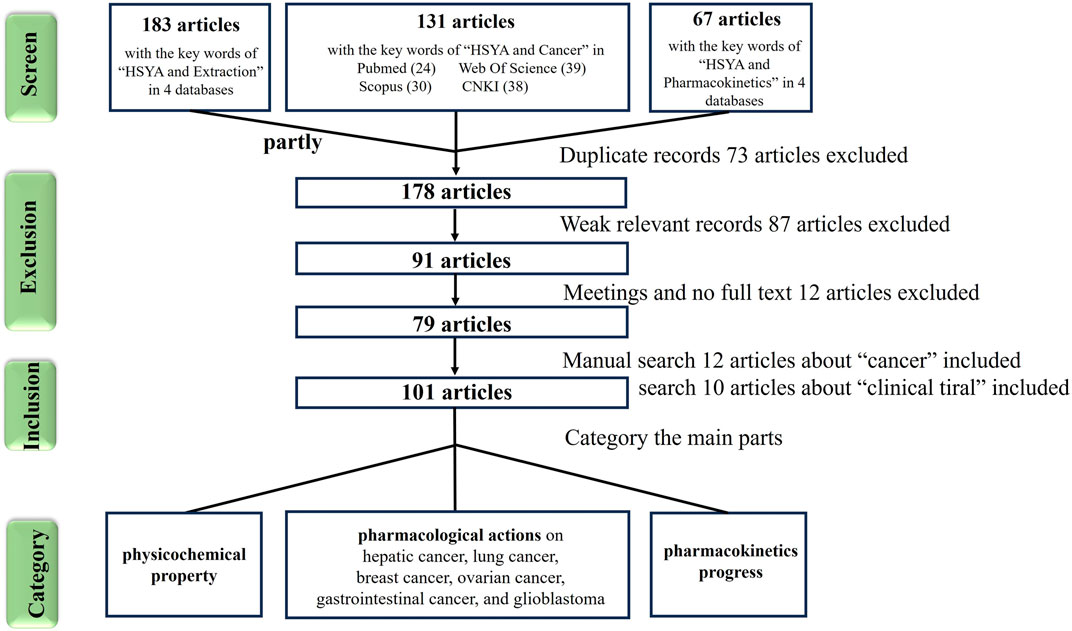
Figure 1. The screen flowchart of cancer research articles related to hydroxysafflor yellow A in databases.
The most abundant constituents of safflower are flavonoids and fatty oil, followed by phenylethanoid glycosides, coumarins, steroids, and polysaccharides (Xian et al., 2022). Among these, flavonoids are the characteristic bioactive constituents (Zhang et al., 2016). SY refers to a class of major bioactive compounds in safflower with a flavonoid structure, including safflower yellow A (SYA), safflower yellow B (SYB), and HSYA. Notably, HSYA constitutes 85% of SY and is considered the main bioactive component pigment of safflower (Wu et al., 2021). Natural pigments are derived from a wide variety of sources in nature. Structurally, they can be broadly classified into isoprene derivatives, polyphenols, ketone derivatives, tetrapyrrole derivatives, and quinone derivatives (Eleonora et al., 2023). HSYA is a unique flavonoid compound in safflower, belonging to the class of quinochalcone compounds (Xian et al., 2022). These compounds, characterized by a distinctive C-glycosylated cyclohexanonedienol molecular structure, are found exclusively in safflower. They exhibit significant efficacy in managing cardiovascular diseases (Zhao et al., 2020). Research has also indicated that chalcone compounds possess anticancer properties (Mendez-Callejas et al., 2023; Ouyang et al., 2021). For example, cardamonin derived from Alpinia katsumadai Hayata with a chalcone structure has anticancer activity against breast and ovarian cancer (Jin et al., 2019), suggesting that quinochalcone compounds may also have anticancer potential (Figure 2). There have been more and more reports of natural pigments with anticancer effects, such as carotenoids (isoprenoid polymers) (Antoine et al., 2022), anthocyanins (polyphenols) (Wallace and Giusti, 2019), and green tea polyphenols EGCG (polyphenols) (Singh et al., 2011). Compared to these natural pigments, HSYA offers distinct advantages in terms of targeting and its multiple anticancer mechanisms. Specifically, HSYA exhibits stronger anti-tumor effects, including inhibition of proliferation, migration, and angiogenesis, compared to anthocyanins and carotenoids (Rodriguez-Amaya, 2019). Additionally, HSYA regulates the tumor microenvironment, reduces tumor angiogenesis, and addresses drug resistance, suggesting its potential across various cancer types (e.g., lung, breast, and liver cancers) (Yang et al., 2015). In contrast, EGCG predominantly targets gastrointestinal cancers (Peng et al., 2024). Regarding targeting, HSYA effectively targets cancer cells and limits tumor spread and metastasis by modulating key signaling pathways (e.g., p53, NF-κB, VEGF, etc.) (Li et al., 2014), Although EGCG has shown notable anticancer effects in clinical treatments for gastrointestinal cancers, challenges such as drug resistance remain (Mereles and Hunstein, 2011; Singh et al., 2011). On the other hand, HSYA requires further clinical studies and optimization of its pharmacokinetics (Chen Q. et al., 2022) (Table 1).
HSYA (C27H32O16) is an orange-yellow powdery substance highly soluble in water. It is poorly lipophilic and virtually insoluble in organic solvents such as chloroform, benzene, and ethyl acetate (Xue et al., 2021). Furthermore, HSYA is structurally unstable and rapidly degrades under strong acidic and alkaline conditions. Consequently, it is unstable when administered orally as a single compound in the presence of gastric acid. Due to the presence of phenolic hydroxyl groups, HSYA exists in a protonated form in natural or alkaline aqueous solutions, significantly impairing its transmembrane transport and resulting in low bioavailability. The absolute oral bioavailability (F) of HSYA administered by gavage is only 1.2% (Zhang et al., 2006). It does not tolerate high temperatures and degrades readily at water temperatures greater than 60°C. For centrally-targeted drugs, the ideal pKa range is 5–10, as basic and amphoteric drugs are more likely to cross the blood-brain barrier (BBB). HSYA is predicted to have a pKa value of 4.50 ± 1.00, but experimental evidence indicates that HSYA is more likely to cross a damaged BBB, making it difficult to pass through a normal BBB (Sheng et al., 2020). Additionally, HSYA degrades under light exposure and must be stored away from light. The application of small amounts of ethylenediamine tetra-acetic acid and ascorbic acid has been shown to improve its stability (Tang et al., 2021).
Various methods of extraction have been employed for the extraction of HSYA, including water immersion, alcohol extraction, ultrasonic extraction, and microwave extraction (Table 2). Water immersion is the most commonly used traditional method, as it is simple to operate, easy to control, and cost-effective. However, its yield is relatively low, typically around 0.066%, and it requires a large amount of raw material, which can easily degrade the HSYA due to high temperature, alkaline conditions, and light exposure (Zhao et al., 2020). With the continuous refinement of extraction techniques, the ultrasonic extraction method, conducted at low temperatures, has shown stable and reproducible results. Despite optimization of solvent volume and extraction time, the yield remains around 1.7% (Jin, 2010). More recently, Xue employed a rapid and simple microwave extraction method to improve HSYA production, achieving a yield of 6.96% by using a solid-to-liquid ratio of 1:100, maintaining a temperature of 70°C, and conducting three cycles within 20 min (Hong et al., 2015). However, this method requires a large volume of solvent and can result in variable composition. Notably, Li et al. reported the highest extraction efficiency of HSYA (14.564%) using DMSO as the solvent. However, due to the excellent solubility of DMSO, the HSYA extract contained high levels of impurities, which reduced its purity (Li et al., 2016). Therefore, this method is not widely used (Yao and Matosevic, 2021).
The SY injection, with HSYA as the main ingredient, is one of the few Traditional Chinese Medicine (TCM) injections used as a clinical therapeutic agent, and its development prospects are considerable (Chen Y. et al., 2022). Previous studies have focused primarily on its effects on cardiovascular and cerebrovascular diseases (Sun et al., 2023). In the last 10 years, many studies analyzed the effect of HSYA on various cancer cells and revealed that HSYA has a good anticancer effect; thereby, indicating that it is a promising therapeutic agent with anticancer effects (Ao et al., 2018; Chen Q. et al., 2022). Till date, HSYA has made much progress in the study of hepatocellular carcinoma compared to other cancers. This section provides detailed pharmacological actions and mechanisms of the HSYA signaling pathways (Table 3; Figures 3, 4).
HSYA can inhibit the secretion of angiogenesis factors (VEGF A and bFGF) and VEGF 1, suppressing ERK1/2 phosphorylation, and inactivate NF-κB by inhibiting nuclear translocation. These effects are mediated by regulating P65 expression levels both inside and outside the nucleus (upregulation within the nucleus), inhibiting IκB phosphorylation, and preventing cytoplasmic degradation of IκBα. This results in a decreased expression of proliferation-related genes, including cyclin D1, c-Myc, and c-Fos. Studies have shown that HSYA inhibits angiogenesis, hepatoma cell viability, and tumor development by modulating the ERK/MAPK and NF-κB pathways in H22 tumor-bearing mice (Yang et al., 2015).
HSYA effectively lowered the production of MMP-2, MMP-9, and COX-2 by inhibiting the p38MAPK/ATF-2 signaling pathway. This finding explains the observed inhibition of the viability, proliferation, and migration of HepG2 cells during HSYA treatment, which can be attributed to the suppression of p38 activation (Zhang et al., 2019). Butein, a natural chalcone similar in structure to HSYA, promotes apoptosis in vitro by inhibiting NF-κB, p38 MAPK, and JNK pathways (Agnieszka et al., 2013).
Several studies have reported a correlation between HSYA expression and autophagy. In this study, HSYA activated autophagy in HepG2 cells by upregulating beclin one expression and suppressing ERK phosphorylation (Chen X. et al., 2020; Zhang et al., 2016). The Ras/Raf/ERK signaling pathway can induce autophagy; therefore, ERK may be a target of the HSYA anti-liver cancer effect of HSYA. Therefore, ERK can be used as a target for treating pancreatic cancer. However, a single method of autophagy activation has limited therapeutic effects on tumors (Amaravadi et al., 2016). Clinical studies have shown that the activation of autophagy can inhibit tumor growth more effectively by blocking autophagic flux (Fan et al., 2022) (Figure 5).
Moreover, HSYA induced an autophagic response and autophagosome accumulation by blocking autophagic flux. This disrupts the metabolic cycle of tumor cells, reduces their adaptability, and leads to apoptosis. Recent studies have shown that HSYA impairs late-stage autophagic flux by inhibiting lysosomal acidification and downregulating LAMP1 expression, leading to apoptosis via the PI3K/AKT/mTOR signaling pathway (Chen Z. et al., 2020). PI3K/Akt/mTOR signaling inhibits autophagy. However, the activation of PTEN and AMPK by P53 promoted autophagy by blocking PI3K and mTOR activation, suggesting that HSYA may enhance P53 activation (Chen et al., 2019) (Figure 5).
HSYA has been reported to activate PPARγ, inhibiting oxidative stress-induced liver fibrosis (Wang et al., 2013). WU et al. demonstrated that HSYA could induce extracellular matrix (ECM) degradation and inhibit epithelial-mesenchymal transition (EMT) by activating PPARγ and inhibiting MMP-2 in SMMC-7721 cells (Ma et al., 2017). All these may indicate that HSYA may also be a PPARγ agonist and then antagonized TGF-β, resulting in down-regulating E-cadherin expression and MMPs and EMT inhibition in the final. Studies have indicated that the activation of PPARγ occurs subsequent to the binding of a ligand. Studies have also shown that PPARγ is activated after ligand binding and upregulates PTEN expression in colorectal cancer (CRC) cells. PTEN mediates cellular biological processes by inhibiting the PI3K/Akt signaling cascade (Lu et al., 2012). HSYA can also regulate the tumor immune microenvironment to interfere with tumor progression. In the process of the Treg differentiation, TGF-β and IL-2 trigger iTregs to FOXP3. iTregs are phenotypically CD4+CD25+CD127 low/−, and generally FOXP3+. In vivo study, HSYA can participate in this process and downregulate the level of FOXP3-expressing Tregs and Rort γ in the liver tumor tissue of the Hepa1-6 mice model. HSYA inhibits the levels of the CD4+CD25+FOXP3+ Tregs/CD4+T lymphocytes in liver cancer cells. Surprisingly, HSYA substantially inhibited tumor growth without loss of body weight in Hepa1-6 mice compared to cisplatin (Ma et al., 2019), which could be developed into a new drug treatment strategy for liver cancer with few side effects (Figure 6).
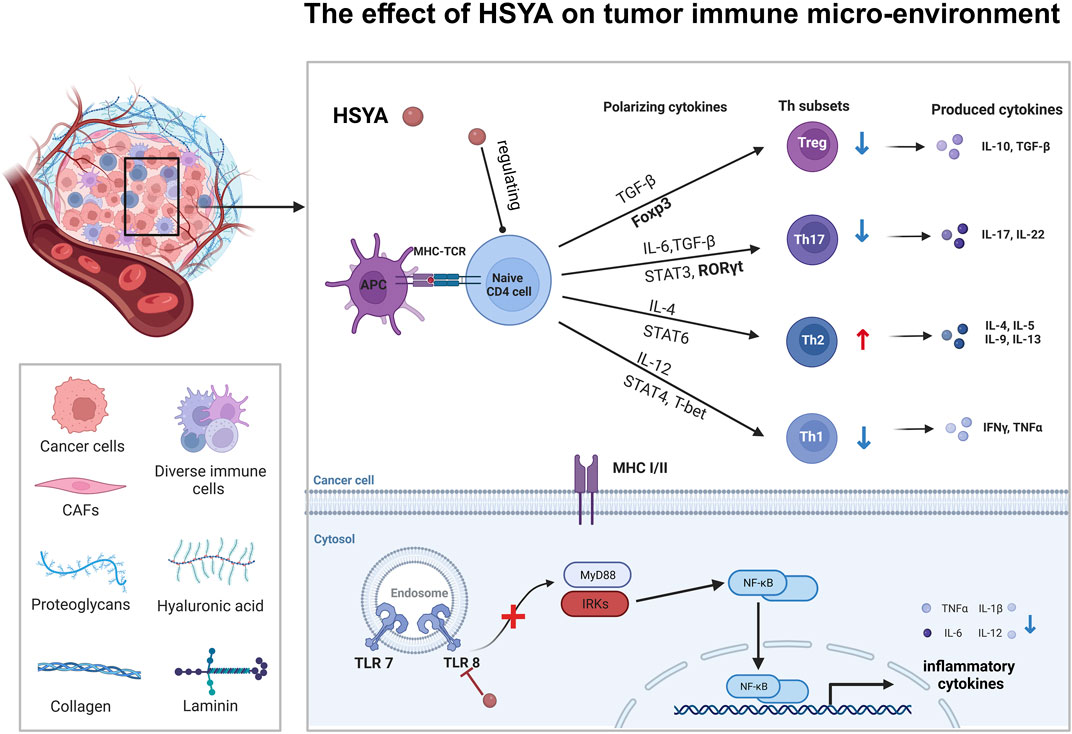
Figure 6. The effect of HSYA on tumor immune micro-environment in hepatic cancer and ovarian cancer.
Research on HSYA’s effects of HSYA on lung cancer is limited (Xia et al., 2022). JIN et al. found that HSYA attenuates pulmonary fibrosis in vivo model in 2016 (Jin et al., 2016). In pulmonary fibrosis, HSYA effectively inhibit cell proliferation induced by TGF-β1. Currently, the research conducted by HSYA on the pulmonary system has mostly focused on fibrosis. The partial protection against renal cell fibrosis by HSYA is attributed to the inhibition of the TGF-β1/smad3-mediated EMT signal pathway (Jin et al., 2016). HSYA is also effective for the treatment of lung cancer.
HSYA inhibits LPS-induced expression of inflammatory cytokines by blocking the PI3K/Akt/mTOR and ERK/MAPK signaling pathways. This inhibition resulted in reduced migration, invasion, and EMT, ultimately promoting the apoptosis of A549 and H1299 cells (Jiang et al., 2019).
Safflower, a source of HSYA, has been extensively studied in breast cancer. SiHong Tang, a traditional Chinese medicinal formulation containing safflower, was been investigated for its effects on breast cancer (Jiang et al., 2021). SY, the primary active component of safflower extract, includes SY A, SY B, and HSYA. SY substantially inhibits the migration of MBA-MD-231 cells and lung metastasis of breast cancer cells in vitro in a 4T1 luciferase cell-based in vivo model. SY treatment also reduced EGF-stimulated invadopodia formation and MMP-9 and Tyr416 expression. Furthermore, the number of circulating tumor cells in the pulmonary capillaries was decreased. Thus, SY’s anti-metastasis effect of safflower yellow on breast cancer primarily prevents invadopodia formation via Src-dependent cytoskeletal rearrangement (Fu et al., 2016). To clarify, the involvement of HSYA in breast cancer, Liu et al. conducted a study that demonstrated that HSYA triggered the upregulation of Bax and p53 in breast cancer cells. Additionally, HSYA was found to induce the generation of reactive oxygen species (ROS) and promote the release of cytochrome c (cyto-c) from the mitochondria into the cytosol, causing a decline in the mitochondrial membrane potential and activating caspase-3. These effects were attributed to inhibiting the nuclear translocation of the NF-κB/p65 pathway in MCF-7 cell vitro study. Consequently, it is plausible that HSYA triggers apoptosis in breast cancer cells via the mitochondrial apoptotic pathway (Li et al., 2014).
Hydroxysafflor yellow B (HSYB), an isomer of HSYA, has also been investigated. The combined treatment with HSYB and DOX (an established chemotherapeutic drug for breast cancer) increased ROS generation and promoted cytochrome c release in MCF-7 cells, reduced cell proliferation and induced apoptosis. HSYB also downregulates cyclin D1, cyclin E, and CDK2 to arrest the MCF-7 cell cycle in the S phase, leading to apoptosis via the PI3K-AKT pathway (Qu et al., 2019). Cardamonin, which has a similar structure to HSYA, has been shown to cause EMT reversal of BT-549 cells to inhibit the invasion of breast cancer cells through Wnt/β-catenin signaling (Shrivastava et al., 2017), and the above shows that HSYA has great potential for breast cancer research.
Both in vivo and in vitro studies have shown that HSYA inhibits the growth of ovarian cancer cell growth and promotes apoptosis by upregulating menin expression. This leads to β-catenin degradation, thereby reducing the expression of the downstream oncogenes, MMP-7 and Survivin (Gong and Zhou, 2019). Several studies have further elucidated that HSYA inhibits the phosphorylation of GSK-3β at the Ser9 site, which subsequently promotes the phosphorylation of GSK-3β at the Tyr216 site. This results in the activation of GSK-3β and subsequent destruction of β-catenin. As a result, the nuclear localization of β-catenin is reduced, leading to a concomitant decrease in the expression of several downstream oncogenes. This process inhibited the proliferation of ovarian cancer cells and induced cell death (Gong et al., 2020). SY inhibits the proliferation, invasion, and migration of SKOV-3 ovarian cancer cells by blocking the TGF-β1 pathway. Its mechanism may involve the inhibition of intracellular ROS levels and the attenuation of EMT mediated by SY (Wang and An, 2021). In contrast, cardamonin exerts its anticancer effect in ovarian cancer cells (SKOV3 and PDC cells) model by arresting the G2/M phase and cell apoptosis through inhibition of NF-κB and mTOR pathways (Ruibin et al., 2020).
HSYA also inhibits ovarian cancer growth by modulating the tumor’s immune microenvironment of tumors. HSYA reduces the proportion of Th1 and Th17 cells in SKOV-3 cells by blocking the TLR8 signaling pathway. This inhibition leads to a decrease in the polarization of Th1 and Th17 cells, while simultaneously promoting the polarization of Th2 cells and influencing the polarization of CD4+ T cells. These findings suggested that the regulation of CD4+ T cell polarization and immune cytokine production in ovarian cancer by HSYA may occur through the TLR8 signaling pathway, leading to alterations in tumor metabolism and enhancement of immune function within the tumor micro-environment (Hu et al., 2021) (Figure 6).
HSYA reduces micro-vessel density (MVD) in tumor tissues in a BGC-823 cell xenograft model in nude mice. MVD is strongly correlated with tumor growth, invasion, metastasis, and prognosis, indicating that HSYA may impede the invasion and metastasis of human gastric adenocarcinoma. A specific dose of HSYA effectively suppressed excessive proliferation of vascular endothelial cells, decreased the proliferation of BGC-823 cells, and induced apoptosis by arresting the cell cycle. HSYA exerts an anti-tumor effect by inhibiting both vascular endothelial cells and tumor cell growth, while promoting tumor cell death. In an in vitro study, HSYA treatment resulted in a shift in BGC-823 cells from the G0/G1 phase to the S phase. However, this transition was inhibited by activating PPAR, leading to cell cycle arrest and apoptosis (Liu et al., 2018).
HSYA have inhibitory effects on the expression of ICAM1, MMP9, TNF-α, and VCAM1,. Additionally, it promotes the expression of phospho-NF-κB p65 and phospho-IκBα. However, no subsantial changes in the expression of P65 and IκBα have been reported. HSYA inhibits the proliferation, invasion, and migration of EC cells, which is partially mediated by the regulation of the NF-κB signaling pathway. This regulation leads to the induction of apoptosis in EC (Chen X. et al., 2020).
In vitro studies have shown that HSYA upregulates the expression of E-cadherin and downregulates the expression of vimentin (Vi) and fibronectin (FN). HSYA inhibits HT-29 EMT, likely by suppressing TGF-β signaling pathway activation through the decreased expression of TGF-β, Smad2, and α-SMA proteins. Consequently, the apoptotic rate of HT-29 cells increased through G0/G1 phase arrest. Overall, HSYA inhibits the proliferation, invasion, and metastasis of HT-29 cells (Wen et al., 2017). HSYA substantially reduced colorectal cancer HCT116 cell viability but had no effect on normal cell HIEC. HSYA also inhibited cell proliferation, migration, and invasion but promoted apoptosis of HCT116 cells by up-regulating PPARγ to inhibit Akt activation. In summary, HSYA has the potential to demonstrate anticancer properties in CRC by activating the PPARγ/PTEN/Akt signaling pathway. Furthermore, the research revealed that the administration of PPARγ antagonist and the process of PPARγ knockdown did not entirely impede the anticancer properties of HSYA, which suggested that other pathways were involved in HSYA-mediated CRC inhibition (Su and Lv, 2021).
Several studies have reported that HSYA exhibits neuroprotective properties in rat SCI models of spinal cord injury. These studies have shown that HSYA effectively controls oxidative stress, prevents the production of pro-inflammatory molecules, and lowers neuron apoptosis through the NF-κB pathway. These findings suggest that HSYA holds promise as a neuroprotective agent (Pei et al., 2017). HSYA (30 mg/kg) plays the role of anti-traumatic brain injury (TBI rat model) by improving the integrity of BBB, and its mechanism may be related to the downregulation of TLR4/NF-κB pathway to play the role of antioxidation, anti-inflammatory, anti-apoptosis, etc (Ren et al., 2022). Furthermore, we found that HSYA inhibited the proliferation, migration, and invasion of glioma U251 cells (Yuan and Lou, 2016), and its mechanism may be related to the downregulation of the expression of MMP-2 and MMP-9. MMP-2 and MMP-9 are gelatinases in MMPs (zinc ion-dependent proteases which can degrade extracellular matrix proteins) that can degrade collagen IV, laminin, and fibronectin, the main components of the perivascular basement membrane, and facilitate tumor cells to invade surrounding tissues along the basement membrane. High invasiveness of gliomas is associated with the expression activity of MMP-2 and MMP-9 (Wan, 2018).
HSYA induced the cell apoptosis of glioma cells LN-229 and U-251MG cells by enhancing the expression of γH2AX to cause DNA damage. However, the DNA repair reaction was normal in HSYA-treated glioma cells. Interestingly, silencing MYC expression can enhance the sensitivity of HSYA to gliomas by inhibiting the MRN (MRE11RAD50-NBS1) complex, resulting in DNA repair defects. Inhibition of MYC results in the suppression of the DNA damage response through the control of NBS1 (DNA repairing protein), ultimately causing defects in DNA repair (Tang et al., 2021). Studies have shown that PTEN plays a crucial role in DNA repair, particularly in homologous recombination repair (HR)(Han et al., 2024). PTEN can directly interact with the MRE11 protein in the MRN complex, thereby affecting MRE11 activity and thereby influencing DNA repair efficiency. Through its phosphatase activity, PTEN inhibits the PI3K/Akt signaling pathway, which regulates the DNA damage response Activation of the PI3K/Akt pathway suppresses HR repair, while PTEN negatively regulates this pathway to promote HR repair (Ming and He, 2012). PTEN modulates MRN complex function during this process (Michael et al., 2021). Furthermore, as mentioned earlier, this suggests that HSYA can affect the PTEN/AKT/mTOR pathway to inhibit MRN, thereby affecting HR repair.
From the above results, we can see that HSYA has been studied extensively in liver cancer. Notably, HSYA mainly induces apoptosis in hepatocellular carcinoma cells by regulating PI3K/AKT/mTOR pathways, and interestingly Wnt/β-catenin pathway, which has not yet to be elucidated in hepatocellular carcinoma, has been involved in the study of ovarian carcinoma (Figure 3), and in autophagy, HSYA regulates autophagy of hepatocellular carcinoma cells through regulating In terms of cellular autophagy, HSYA regulates autophagy in hepatocellular carcinoma cells through the regulation of ERK, AKT pathway and Beclin-1 (Figure 5); in terms of regulating the tumor immune microenvironment, HSYA regulates the Naïve cell differentiation in hepatocellular carcinoma cells, and in ovarian carcinoma cells, HSYA can also inhibit TL8 and thus regulate inflammatory factors (Figure 6).
Absorption: HSYA is classified as a Biopharmaceutical Classification System (BCS) class III drug. To date, there are two primary routes of administration: oral and intravenous. Results from the rat intestinal loop assay indicated that HSYA is not absorbed in the stomach but is rapidly absorbed in the intestinal lumen (Zhang et al., 2006). After saffron extract was administered via gavage to rats, HSYA was primarily absorbed in the small intestine (Yi et al., 2007). Pharmacokinetic studies show that the oral absolute bioavailability (F) of HSYA is only 1.2%, and its blood concentration-time curve exhibits a bimodal pattern in rats following oral gavage.
Distribution: The plasma protein binding rate of HSYA was found to be approximately 50% after 48 and 72 h of equilibrium dialysis, suggesting that its plasma protein binding is low. This indicates that HSYA’s efficacy is not easily enhanced by the competitive displacement of plasma protein binding sites during co-administration of other drugs (Chu et al., 2006). Following tail vein injection of safflower lyophilized powder, HSYA was distributed to various organs, including the blood, kidney, liver, lung, heart, and spleen, with the highest area under the curve (AUC) observed in the liver, kidney, spleen, heart, and lungs. Notably, HSYA was not detected in the brain (Li T. et al., 2021).
Metabolism: HSYA undergoes reduction and hydrolysis by hepatic microsomal drug-metabolizing enzymes, generating phase I metabolites through hydroxylation, methylation, dehydration, hydrogenation, and hydration. Phase II metabolism includes acetylation, glucuronidation, and additional reactions such as methylation and glucuronide conjugation (Liang et al., 2018). In rats, following oral gavage, eight metabolites were detected in plasma, bile, urine, and feces, mainly consisting of HSYA degradation products stripped of their sugar groups or other fragments, primarily excreted in feces (Jin-Feng et al., 2014). When HSYA was administered via intravenous injection, 10 metabolites were identified, with the primary metabolic pathways including dehydration, deglycosylation, glucuronidation, and methylation. Some metabolites underwent multiple steps of metabolism and were predominantly found in urine, a result that contrasts with the gavage administration study, suggesting a potential role of intestinal flora in the oral metabolism of HSYA (Wu et al., 2020). Xu’s investigation on the effects of HSYA on metabolic enzyme activities in rats showed that after 14 days of 3 and 12 mg/kg doses, HSYA significantly inhibited CYP1A2 activity and its mRNA expression while inducing CYP3A1 activity and its mRNA expression. Prolonged (14 days) or short-term (3 days) administration of a 12 mg/kg dose significantly inhibited CYP2C11 activity and its mRNA expression, with no significant effects on CYP2D4 activity. Given that HSYA may be used in combination with other drugs for cardiovascular diseases, understanding its interactions with metabolic enzymes is crucial for optimizing its clinical safety and efficacy (Li Z. et al., 2021). However, the full extent of HSYA metabolism remains poorly understood, and the key enzyme isoforms involved, along with their corresponding metabolic reactions and metabolites, require further elucidation.
Excretion: For intravenous administration, 88.6% of HSYA was excreted directly into the urine (Yang et al., 2009). For oral administration, the excretion of HSYA in feces exhibits the highest cumulative rate, followed by urine, whereas the excretion of HSYA in bile is the least in rats (Li, 2007). When rats were administered HSYA monomer, safflower extract, and compound cerebroside tablets, the highest excretion rate was found in the safflower extract group. The combination of compounds reduced HSYA excretion, suggesting that other constituents of safflower may affect HSYA absorption and utilization. Additionally, the compound combination may enhance the utilization of HSYA in safflower (Li C. Y. et al., 2015). A deeper discussion of the clinical pharmacokinetics of different administration routes is provided below.
Drug interaction: Some preclinical studies suggest that HSYA may enhance anti-tumor effects when combined with post-marketing cancer therapeutic agents. Cisplatin, which inhibits cancer cell proliferation by forming DNA adducts and inducing DNA damage, may have its oxidative damage reduced by HSYA, which regulates the cellular redox state. Moreover, HSYA may enhance the anti-tumor effect of cisplatin (Chen Y. et al., 2022). HSYA also increases cisplatin sensitivity in ovarian cancer cells by downregulating the PI3K/AKT pathway and enhancing DNA repair mechanisms, thus reversing cisplatin resistance (Shen et al., 2019). HSYA has also been shown to significantly improve 5-FU’s inhibitory effect on the proliferation and migration of colorectal cancer (CRC) cells, as well as to alleviate 5-FU chemoresistance (Wang et al., 2023). Although no data exist on the interaction of HSYA with other drugs in the context of SY injection, patients may experience mild adverse reactions such as fever, palpitations, cutaneous allergic papules, and somnolence (Guo et al., 2023).
A clinical study on the pharmacokinetics of a single oral dose of HSYA in 12 healthy individuals showed that HSYA was rapidly absorbed, reaching peak concentration within 1 h, with an elimination half-life (t1/2) ranging from 2.6 to 3.5 h (Wen et al., 2008). This rapid elimination is attributed to the efficient breakdown of HSYA in the gastrointestinal tract and liver, facilitated by its high molecular weight and strong hydrophilicity (Wu et al., 2020). Therefore, improving the oral bioavailability of HSYA is essential for its effective clinical use.
HSYA is absorbed by passive diffusion, and the Caco-2 cell monolayer model demonstrates this transmembrane characteristic. To improve the permeability coefficient, several strategies have been proposed to develop novel drug delivery systems: (i) absorption enhancers, (ii) lipid complex formation, and (iii) P-glycoprotein (P-gp) inhibitors (Liang et al., 2012). Absorption enhancers, such as Chuanxiong volatile oil and polyethylene glycol 400, temporarily open intercellular tight junctions, facilitating passive diffusion of HSYA through cellular or bypass pathways (Liu et al., 2018). Lipid-based carriers, including microemulsions (Qi et al., 2011), solid lipid nanoparticles (Zhao et al., 2018), self-double-emulsifying drug delivery (Lv et al., 2012) and chitosan complex (Ma et al., 2015) have also been explored. As a P-gp substrate analog, HSYA’s absorption is affected by P-gp-mediated exocytosis, contributing to its low bioavailability. Consequently, P-gp inhibitors such as F-68 and Tween 80 may enhance HSYA’s oral absorption (Zhou et al., 2014). A suitable HSYA delivery system for clinical application is expected to be developed in the near future.
Currently, SY injection, which contains HSYA, is administered intravenously in clinical practice. A pharmacokinetic study in 12 healthy Chinese female volunteers, following a single intravenous dose of SY injection (HSYA: 35, 70, 140 mg), revealed that HSYA was rapidly eliminated, following a two-compartment model with first-order elimination and a half-life of approximately 3 h (Yang et al., 2009). Li et al. studied 36 healthy Chinese volunteers of both sexes who received single-dose (25, 50, and 75 mg) and multiple-dose (50 mg daily for seven consecutive days) injectable HSYA lyophilized powder. The Cmax and AUC were dose-proportional and consistent with first-order kinetics. Notably, female subjects exhibited higher Cmax and AUC values than males, indicating the importance of considering gender differences in clinical dosing. Following multiple-dose administration (50 mg once daily for 7 days), HSYA was well tolerated by all subjects, with no accumulation observed (Li C. Y. et al., 2015). Moreover, combination therapy with HSYA and protocatechuic aldehyde significantly enhanced HSYA uptake (Zhao et al., 2020).
Regarding the embryotoxicity of HSYA, animal studies indicated that intravenous administration of HSYA (125, 250, and 500 mg/kg) to pregnant rats did not result in any significant changes in body weight, placenta weight, number of implantations, number of corpus luteums, number of live fetuses, or number of stillborn fetuses when compared to the control group. Therefore, HSYA is neither embryo- nor maternal toxic as verified by animal studies (Wang et al., 2016). In terms of drug metabolism, a group of 36 healthy Chinese volunteers received a single intravenous dose of pure HSYA injection, ranging from 25 to 75 mg. Blood concentration analysis revealed moderate linear pharmacokinetic (PK) characteristics across the dose range, suggesting that repeated administration does not result in drug accumulation. HSYA has a plasma protein binding rate of approximately 78%, indicating its prolonged effect (Wang et al., 2011) and does not competitively bind to other drugs. Consequently, HSYA demonstrates a high safety profile in vivo (Chu et al., 2006), though caution should be exercised when combining it with drugs that competitively inhibit HSYA plasma protein binding (Li C. Y. et al., 2015). In clinical applications of SY injection, which contains HSYA as the main component, most adverse drug events (ADEs) have been related to drug-induced allergic reactions (Liu et al., 2019), potentially due to antigenically active impurities present during the production process (Zheng, 2013).
The chemical compounds derived from TCM exhibit distinctive properties and significant efficacy in the prevention and treatment of various diseases. Numerous basic studies have demonstrated that HSYA possesses substantial potential as an anticancer agent, showing efficacy against a range of cancers, including liver, lung, gastric, colorectal, breast, ovarian and gliomas. This efficacy is likely attributable to the close association between HSYA and its ability to regulate several cellular processes, including cancer cell proliferation, invasion, metastasis, apoptosis, autophagy, and immune microenvironment modulation. Therefore, HSYA has substantial potential to inhibit cancer progression. A review of its molecular mechanisms reveals that HSYA’s anticancer effects offer the therapeutic advantage of being “multi-channel and multi-targeted.”
This review summarizes the physical and chemical properties, anticancer effects, pharmacokinetics, and safety of HSYA, with a focus on its anticancer mechanism. HSYA’s anticancer effects are mediated through the inhibition of transcription factors (NF-κB, β-catenin, ERK1/2, p38, TGF-β and mTOR), activation of transcription factors (G3K-3β and PPAR), promotion of mitochondrial autophagy, inhibition of autophagy flux, and regulation of apoptosis-related proteins (Bcl-2 and BAX). This leads to the release of cytochrome c, activation of cysteine proteases (caspases 3&9), and modulation of cell cycle drivers (cyclin D1) and EMT-associated proteins. Additionally, HSYA regulated the polarization of CD4+T cells within the tumor immune microenvironment, ultimately blocking cancer cell proliferation, transformation, and metastasis, leading to apoptosis. A comprehensive analysis of experimental studies from four databases showed that HSYA is a selective natural anticancer agent, both in vitro and in vivo. Cardamonin, a compound with a natural chalcone structure similar to that of HSYA, shares some molecular mechanisms with HSYA in exerting its anticancer effects. Cardamonin targets Bcl-2 family proteins, activating the caspase cascade, inhibiting the nuclear translocation of NF-κB, STAT3 and mTOR, blocks β-catenin signaling, downregulating cell cycle regulatory proteins, and inhibiting oncogenic signaling to induce apoptosis and arrest the cell cycle, thus halting proliferation and metastasis in various cancer cell types (Javaria et al., 2020). Among these, the NF-κB, PI3K/AKT/mTOR and Wnt-β-catenin pathways are also implicated in HSYA’s anticancer mechanism. However, ability of HSYA to regulate cancer progression via the JAK-STAT3 pathway has not been extensively studied. The JAK-STAT3 pathway is involved in cancer cell proliferation and survival (J et al., 2016), and recent studies have identified it as a novel target for cancer therapy (Gharibi et al., 2020; Zou et al., 2020). For example, cardamonin inhibits glioblastoma stem cell proliferation by suppressing the STAT3 pathway, which subsequently prevents the activation of Bcl-2, survivin, and VEGF (Ning et al., 2015). It also reduced STAT3 expression in gastric cancer cell lines by downregulating STAT3 phosphorylation, thereby reducing the expression of various carcinogenic proteins and inhibiting cancer cell growth (Zheng et al., 2019). Owing to the structural similarities between HSYA and cardamonin, it is plausible that the anticancer targets of HSYA may also include the JAK-STAT3 pathway (Gharibi et al., 2020).
Despite the promising anticancer properties of HSYA, its clinical applicability is limited by poor oral bioavailability due to low membrane permeability. To enhance the permeability of HSYA, various drug delivery systems have been developed, primarily focusing on lipid-based carriers. By reducing HSYA’s high water solubility, its bioavailability can be significantly improved. Additionally, microemulsions (Lopes, 2014), self-emulsifying systems (Lv et al., 2012), nanoparticles (Zhao et al., 2018), and chitosan complex (Hong et al., 2017) have been explored as potential strategies to enhance HSYA’s bioavailability. Furthermore, studies have shown that co-administration of HSYA with other traditional Chinese medicines, such as Danggui-Honghua and Taoren-Honghua, can optimize HSYA’s absorption and efficacy (Pan et al., 2022). When comparing the pharmacokinetics of HSYA with those of Cendroxin Tablets, it was observed that other herbs in Cendroxin Tablets enhance HSYA absorption and increase its bioavailability (Feng et al., 2012). Notably, HSYA administered via the gastrointestinal tract in healthy rats exhibited the highest plasma concentrations but was not detectable in the brain (Chu et al., 2006). However, injectable HSYA can easily penetrate a damaged blood-brain barrier (BBB) (Liang et al., 2018). HSYA injection has been approved for the treatment of stroke, a condition in which the BBB is significantly impaired. This makes HSYA particularly effective in stroke treatment. However, its inability to cross the intact BBB limits its therapeutic potential for other brain diseases (Chen Q. et al., 2022), For instance, in the clinical treatment of glioma, all patients possess tumor regions with an intact BBB, which must be fully treated for effective outcomes (Arvanitis et al., 2020), Therefore, strategies to improve the BBB penetration of HSYA are essential. Some studies suggest that this challenge could be addressed by combining HSYA with drugs that have high BBB permeability but minimal systemic effects (Tang et al., 2021). Wu found that HSYA, in combination with aroma-opening drugs, promoted HSYA distribution in the rat brain, supporting the potential for developing brain-targeting drug delivery systems (Wang et al., 2014). While no preclinical studies have been conducted in this area, the drug has entered clinical trials and is marketed for neurological and cardiovascular diseases. In the case of acute ischemic stroke, Jilin TianSanQi Pharmaceutical Co., Ltd. has completed a phase 3 clinical trial (CTR20201900), and the drug has demonstrated favorable clinical outcomes. The study reached the primary endpoint, showing superior efficacy in patients with acute ischemic stroke, as evidenced by a higher proportion of subjects achieving an mRS score ≤1 after 90 days of treatment, with a favorable safety profile and no new safety concerns. In January 2023, Yuekang Pharmaceutical’s HSYA for injection also reached the primary endpoint in a phase III clinical trial in acute ischemic stroke patients, and the product is now in the process of applying for market approval in China (Acceptance No. CXZS2300027). While studies on the anticancer effects of HSYA are still in the early stages, further preclinical studies on its pharmacological mechanisms are necessary before advancing to clinical trials. Nonetheless, the promising efficacy and safety of HSYA in cardiovascular and cerebrovascular diseases offer valuable insights for its potential application in anticancer research.
Future investigations should explore a broader range of cancer types and molecular mechanisms related to HSYA. Additionally, studies should focus on improving its bioavailability, intestinal absorption, and in vivo stability within relevant cancer models, providing valuable information to support its future clinical application.
YW: Conceptualization, Investigation, Writing - original draft. JA: Writing - review and editing. JZ: Writing - review and editing. LC: Supervision, Writing - review and editing. QZ: Conceptualization, Supervision, Writing - review and editing. FP: Conceptualization, Supervision, Writing - review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by National Natural Science Foundation of China (Nos 82003879; U19A2010), National Natural Science Foundation of Science and Technology Department of Sichuan Province (Nos 2023NSFSC1928; 2023NSFSC1992), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (No. CACM-2020-QNRC1-01), Project of State Administration of Traditional Chinese Medicine of China (No. ZYYCXTD-D-202209), Project of Sichuan Province Pharmaceutical Administration (No. 2022C001), and the Fundamental Research Funds for the central Universities.
The figures were generated by BioRender.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamska, I., and Biernacka, P. (2021). Bioactive substances in safflower flowers and their applicability in medicine and health-promoting foods. Int. J. Food Sci. 2021, 6657639. doi:10.1155/2021/6657639
Agnieszka, S. C., Magdalena, M. D., Jadwiga, D., and Martyna, K. S. (2013). Butein inhibits ethanol-induced activation of liver stellate cells through TGF-β, NFκB, p38, and JNK signaling pathways and inhibition of oxidative stress. J. Gastroenterol. 48 (2), 222–237. doi:10.1007/s00535-012-0619-7
Amaravadi, R., Kimmelman, A. C., and White, E. (2016). Recent insights into the function of autophagy in cancer. Genes Dev. 30 (17), 1913–1930. doi:10.1101/gad.287524.116
Antoine, G., Isabelle, G., Souza, D., Thiéry, V., Chevalier, S., de Oliveira-Junior, R. G., et al. (2022). Archaea carotenoids: natural pigments with unexplored innovative potential. Mar. Drugs 20 (8), 524. doi:10.3390/md20080524
Ao, H., Feng, W., and Peng, C. (2018). Hydroxysafflor yellow A: a promising therapeutic agent for broad spectrum of diseases. Evid. Based Complement. Altern. Med. 2018, 1–17. doi:10.1155/2018/8259280
Arvanitis, C. D., Ferraro, G. B., and Jain, R. K. (2020). The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20 (1), 26–41. doi:10.1038/s41568-019-0205-x
Bai, X., Wang, W. X., Fu, R. J., Yue, S. J., Gao, H., Chen, Y. Y., et al. (2020). Therapeutic potential of hydroxysafflor yellow A on cardio-cerebrovascular diseases potential of hydroxysafflor yellow A on cardio-cerebrovascular diseases. Front. Pharmacol. 11, 01265. doi:10.3389/fphar.2020.01265
Chen, H. T., Liu, H., Mao, M. J., Tan, Y., Mo, X. Q., Meng, X. J., et al. (2019). Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol. Cancer 18 (1), 101. doi:10.1186/s12943-019-1030-2
Chen, Q., Wan, J., Zhang, Y., He, Y., Bao, Y., Yu, L., et al. (2022a). Pharmacokinetic-pharmacodynamic modeling analysis for hydroxysafflor yellow A-calycosin in compatibility in normal and cerebral ischemic rats: a comparative study. Biomed. Pharmacother. 150, 112950. doi:10.1016/j.biopha.2022.112950
Chen, X., Wang, Y., Zhang, L., and Gao, Y. (2020a). Hydroxysafflor yellow A of Carthamus tinctorius L., represses the malignant development of esophageal cancer cells via regulating NF-κB signaling pathway. Cell Biochem. Biophys. 78 (4), 511–520. doi:10.1007/s12013-020-00934-1
Chen, Y., Li, M., Wen, J., Pan, X., Deng, Z., Chen, J., et al. (2022b). Pharmacological activities of safflower yellow and its clinical applications. Evid. Based Complement. Altern. Med. 2022, 2108557. doi:10.1155/2022/2108557
Chen, Z., Liu, L., Liu, Y., Wang, S., Zhang, S., Dong, R., et al. (2020b). Hydroxysafflor yellow A induces autophagy in human liver cancer cells by regulating Beclin 1 and ERK expression. Exp. Ther. Med. 19 (4), 2989–2996. doi:10.3892/etm.2020.8552
Chu, D. F., Liu, W. H., Huang, Z., Liu, S. S., Fu, X. Q., and Liu, K. (2006). Pharmacokinetics and excretion of hydroxysafflor Yellow A, a potent neuroprotective agent from safflower, in rats and dogs. Planta Medica 72 (5), 418–423. doi:10.1055/s-2005-916249
Eleonora, S. D., Giovanna, V. L., Rita, P. D., De Maria, L., Tardugno, R., Vadalà, R., et al. (2023). Natural pigments production and their application in food, health, and other industries. Nutrients 15 (8), 1923. doi:10.3390/nu15081923
Fan, G., Li, F., Wang, P., Jin, X., and Liu, R. (2022). Natural-product-mediated autophagy in the treatment of various liver diseases. Int. J. Mol. Sci. 23 (23), 15109. doi:10.3390/ijms232315109
Feng, X., Tang, J. M., Cao, X., Zhao, M., and Ou, Y. Q. (2012). Comparison of excretion of hydroxysafflor yellow A after oral administration of Monomer,Medicinal substance aqueous extract and naodesheng tablets in rats. Chin. Pharm. J. 47, 448–454.
Fu, H., Wu, R., Li, Y., Zhang, L., Tang, X., Tu, J., et al. (2016). Safflower yellow prevents pulmonary metastasis of breast cancer by inhibiting tumor cell invadopodia. Am. J. Chin. Med. 44 (7), 1491–1506. doi:10.1142/S0192415X1650083X
Gharibi, T., Babaloo, Z., Hosseini, A., Abdollahpour-Alitappeh, M., Hashemi, V., Marofi, F., et al. (2020). Targeting STAT3 in cancer and autoimmune diseases. Eur. J. Pharmacol. 878, 173107. doi:10.1016/j.ejphar.2020.173107
Gong, J. M., Xu, X. Y., and Zhang, X. L. (2020). The anti-tumor effect and mechanism of Hydroxysafflor yellow A on ovarian cancer. Chin. J Clin Pharmacol. 36, 971–974.
Gong, J. M., and Zhou, Y. Q. (2019). Hydroxysafflor yellow A inhibits ovarian cancer growth through wnt/bcatenin signaling pathway. J. Med. Res. 48, 131–134.
Guo, M. X., Xue, X. L., Hu, Z. Q., and Zhou, L. M. (2023). One case of anaphylactic shock caused by safflower yellow pigment for injection reported. Chin. J. Clin. Ration. Drug use 16, 155–157.
Han, M., Li, S., Fan, H., An, J., Peng, C., and Peng, F. (2024). Regulated cell death in glioma: promising targets for natural small-molecule compounds. Front. Oncol. 14, 1273841. doi:10.3389/fonc.2024.1273841
Hong, B., Wang, Z., Xu, T., Li, C., and Li, W. (2015). Matrix solid-phase dispersion extraction followed by high performance liquid chromatography-diode array detection and ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometer method for the determination of the main compounds from Carthamus tinctorius L. (Hong-hua). J. Pharm. Biomed. Anal. 107, 464–472. doi:10.1016/j.jpba.2015.01.040
Hong, S. C., Yoo, S. Y., Kim, H., and Lee, J. (2017). Chitosan-based multifunctional platforms for local delivery of therapeutics. Mar. Drugs 15 (3), 60–2017. doi:10.3390/md15030060
Hu, X. J., Hu, Y., and Lei, L. (2021). Hydroxysafflor yellow A regulates CD4∼+ T cell polarization and immune cytokine expression in ovarian cancer through TLR8 signaling. Mod. Med. J. 49, 1096–1101.
Huang, K., Zhang, P., Zhang, Z., Youn, J. Y., Wang, C., Zhang, H., et al. (2021). Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol. and Ther. 225, 107843. doi:10.1016/j.pharmthera.2021.107843
J, H. E., Huiyuan, Z., S, L. H., and S, W. S. (2016). STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31, 11–15. doi:10.1016/j.cytogfr.2016.05.001
Javaria, N., Azhar, R., Ajmal, S. M., Ghulam, H., Ammara, R., Iqra, S., et al. (2020). Cardamonin: a new player to fight cancer via multiple cancer signaling pathways. Life Sci. 250, 117591. doi:10.1016/j.lfs.2020.117591
Jiang, H., Li, M., Du, K., Ma, C., Cheng, Y., Wang, S., et al. (2021). Traditional Chinese medicine for adjuvant treatment of breast cancer: taohong siwu decoction. Chin. Med. 16 (1), 129. doi:10.1186/s13020-021-00539-7
Jiang, M., Zhou, L. Y., Xu, N., and An, Q. (2019). Hydroxysafflor yellow A inhibited lipopolysaccharideinduced non-small cell lung cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT/mTOR and ERK/MAPK signaling pathways. Thorac. Cancer 10 (6), 1319–1333. doi:10.1111/1759-7714.13019
Jiang, R. B., Bo, J., Danying, W., Jianguo, F., and Linhui, G. (2020). Cardamonin induces G2/M phase arrest and apoptosis through inhibition of NF-κB and mTOR pathways in ovarian cancer. Aging. 12 (24), 25730–25743. doi:10.18632/aging.104184
Jianping, Q., Jie, Z., Wei, W., Yi, L., Yunmei, S., Zhetao, Z., et al. (2011). Enhanced effect and mechanism of water-in-oil microemulsion as an oral delivery system of hydroxysafflor yellow A. Int. J. nanomedicine 6, 985–991. doi:10.2147/ijn.s18821
Jin, J., Qiu, S., Wang, P., Liang, X., Huang, F., Wu, H., et al. (2019). Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 38 (1), 377. doi:10.1186/s13046-019-1351-4
Jin, M., Wu, Y., Wang, L., Zang, B., and Tan, L. (2016). Hydroxysafflor yellow A attenuates bleomycin-induced pulmonary fibrosis in mice. Phytother. Res. 30 (4), 577–587. doi:10.1002/ptr.5560
Jin, Y. C. (2010). Study on comprehensive utilization offlower of Carthamus tinctorius L. andnovel separartion of hydroxyl safflor yellow A. Hangzhou, China: Zhejiang University.
Jin-Feng, C., Yue-Lin, S., Xiao-Yu, G., Peng-Fei, T., and Yong, J. (2014). Characterization of the herb-derived components in rats following oral administration of Carthamus tinctorius extract by extracting diagnostic fragment ions (DFIs) in the MS(n) chromatograms. Analyst 139, 6474–6485. doi:10.1039/c4an01707b
Li, C. Y., Chu, J. H., Zhang, J., Sun, B. T., Dai, G. L., Liu, S.-J., et al. (2015a). Measurement of hydroxysafflor yellow A in human urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 1 (974), 131–137. doi:10.1016/j.jchromb.2014.10.036
Li, C. Y., Yin, J. G., Zhang, J., Wang, X. X., Xu, M. J., Liu, F., et al. (2015b). Pharmacokinetic profiles of hydroxysafflor yellow A following intravenous administration of its pure preparations in healthy Chinese volunteers. J. Ethnopharmacol. 162, 225–230. doi:10.1016/j.jep.2014.12.068
Li, J. H., Xu, G. L., and Li, S. M. (2012). Systematic evaluation of effectiveness and safety on saffl ower injection in ana'na pectoris. Journal of Emergency in traditional Chinese med. 21(06), 932+939. Pharm. Clin. Res. 17, 191–194.
Li, L. J., Jiang, T. T., Guo, J., and Huang, L. S. (2016). Preparation of HSYA by organic reagent method. Strait Pharm. J. 28, 30–34.
Li, T., Zhang, W., Hu, E., Sun, Z., Li, P., Yu, Z., et al. (2021a). Integrated metabolomics and network pharmacology to reveal the mechanisms of hydroxysafflor yellow A against acute traumatic brain injury. Comput. Struct. Biotechnol. J. 19, 1002–1013. doi:10.1016/j.csbj.2021.01.033
Li, Y. (2007). Comparative studies on absorption anddistribution of hydroxysafflor yellow A in rats. Beijing, China: Peking Union Medical College.
Li, Y., Wu, Y., Guan, Y., Wang, Z., and Zhang, L. (2014). Hydroxysafflor yellow A induces apoptosis in MCF-7 cells by blocking NF kappa B/p65 pathway and disrupting mitochondrial transmembrane potential. RSC Adv. 4, 47576–47586. doi:10.1039/C4RA07417C
Li, Z., Feiyue, Z., and Gaofeng, L. (2021b). Traditional Chinese medicine and lung cancer--From theory to practice. Biomed. Pharmacother. 137, 111381. doi:10.1016/j.biopha.2021.111381
Liang, W., Yuping, T., Chenxiao, S., Chuan, C., Zhu, Z., Xuqin, S., et al. (2018). A comprehensive in vitro and in vivo metabolism study of hydroxysafflor yellow A. J. Mass Spectrom. 53 (2), 99–108. doi:10.1002/jms.4041
Liang, Z. L., Chen, Q. T., Qing, L., Xin, J. T., Li, M. L., Qing, X. F., et al. (2012). Enhanced absorption of hydroxysafflor yellow A using a self-double-emulsifying drug delivery system: in vitro and in vivo studies. Int. J. Nanomedicine 7, 4099–4107. doi:10.2147/IJN.S33398
Liu, J. Y., Liu, W., and Wang, J. C. (2019). Literature analysis of adverse reactions to saffron injection. Guangming J. Chin. Med. 34, 3381–3383.
Liu, L., Si, N., Ma, Y., Ge, D., Yu, X., Fan, A., et al. (2018). Hydroxysafflor-yellow A induces human gastric carcinoma BGC-823 cell apoptosis by activating peroxisome proliferator-activated receptor gamma (PPARγ). Med. Sci. Monit. 24, 803–811. doi:10.12659/msm.905587
Lopes, L. B. (2014). Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 6 (1), 52–77. doi:10.3390/pharmaceutics6010052
Lu, K. V., Chang, J. P., Parachoniak, C. A., Pandika, M. M., Aghi, M. K., Meyronet, D., et al. (2012). VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22 (1), 21–35. doi:10.1016/j.ccr.2012.05.037
Lv, L. Z., Tong, C. Q., Lv, Q., Tang, X. J., Li, L. M., Fang, Q. X., et al. (2012). Enhanced absorption of hydroxysafflor yellow A using a self-double-emulsifying drug delivery system: in vitro and in vivo studies. Int. J. Nanomedicine 7, 4099–4107. doi:10.2147/IJN.S33398
Ma, G. N., Yu, F. L., Wang, S., Li, Z. P., Xie, X. Y., and Mei, X. G. (2015). A novel oral preparation of hydroxysafflor yellow A base on a chitosan complex: a strategy to enhance the oral bioavailability. AAPS PharmSciTech 16 (3), 675–682. doi:10.1208/s12249-014-0255-z
Ma, L., Liu, L., Ma, Y., Xie, H., Yu, X., Wang, X., et al. (2017). The role of E-cadherin/β-catenin in hydroxysafflor yellow A inhibiting adhesion, invasion, migration and lung metastasis of hepatoma cells. Biol. Pharm. Bull. 40 (10), 1706–1715. doi:10.1248/bpb.b17-00281
Ma, Y., Feng, C., Wang, J., Chen, Z., Wei, P., Fan, A., et al. (2019). Hydroxyl safflower yellow A regulates the tumor immune microenvironment to produce an anticancer effect in a mouse model of hepatocellular carcinoma. Oncol. Lett. 17 (3), 3503–3510. doi:10.3892/ol.2019.9946
Mendez-Callejas, G., Piñeros-Avila, M., Yosa-Reyes, J., Pestana-Nobles, R., Torrenegra, R., Camargo-Ubate, M. F., et al. (2023). A novel tri-hydroxy-methylated chalcone isolated from chromolaena tacotana with anti-cancer potential targeting pro-survival proteins. Int. J. Mol. Sci. 24 (20), 15185. doi:10.3390/ijms242015185
Mereles, D., and Hunstein, W. (2011). Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int. J. Mol. Sci. 12 (9), 5592–5603. doi:10.3390/ijms12095592
Michael, C., Joanne, X., Axel, G., J, P. M., Yasmine, B., J, H. J., et al. (2021). Association of homologous recombination-DNA damage response gene mutations with immune biomarkers in gastroesophageal cancers. Mol. Cancer Ther. 21 (1), 227–236. doi:10.1158/1535-7163.MCT-20-0879
Ming, M., and He, Y.-Y. (2012). PTEN in DNA damage repair. Cancer Lett. 28 (2), 125–129. doi:10.1016/j.canlet.2012.01.003
Ning, W., Jia, L., Xiangzhong, Z., Zhiyong, Y., Bo, J., Lijun, W., et al. (2015). Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol. 36 (12), 9667–9676. doi:10.1007/s13277-015-3673-y
Ouyang, Y., Li, J., Chen, X., Fu, X., Sun, S., and Wu, Q. (2021). Chalcone derivatives: role in anticancer therapy. Biomolecules 11 (6), 894. doi:10.3390/biom11060894
Pan, L., Peng, C., Wang, L., Li, L., Huang, S., Fei, C., et al. (2022). Network pharmacology and experimental validation-based approach to understand the effect and mechanism of Taohong Siwu Decoction against ischemic stroke. J. Ethnopharmacol. 294, 115339. doi:10.1016/j.jep.2022.115339
Pei, J. P., Fan, L. H., Nan, K., Li, J., Dang, X. Q., and Wang, K. Z. (2017). HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J. Neuroinflammation 14 (1), 97. doi:10.1186/s12974-017-0870-1
Peng, X., McClements, J. D., Liu, X., and Liu, F. (2024). EGCG-based nanoparticles: synthesis, properties, and applications. Crit. Rev. Food Sci. Nutr., 21–22. doi:10.1080/10408398.2024.2328184
Qi, J. P., Zhang, J., Wu, W., Lu, Y., Song., Y. M., Zhang, Z. T., et al. (2011). Enhanced effect and mechanism of water-in-oil microemulsion as an oral delivery system of hydroxysafflor yellow A. Int. J. Nanomedicine 6, 985–991. doi:10.2147/IJN.S18821
Qu, C., Zhu, W., Dong, K., Pan, Z., Chen, Y., Chen, X., et al. (2019). Inhibitory effect of hydroxysafflor yellow B on the proliferation of human breast cancer MCF-7 cells. Recent Pat. Anticancer Drug Discov. 14 (2), 187–197. doi:10.2174/1574891X1466619051610.2218
Ren, M., Zhang, M., Zhang, X., Wang, C., Zheng, Y., and Hu, Y. (2022). Hydroxysafflor yellow A inhibits aβ1-42-induced neuroinflammation by modulating the phenotypic transformation of microglia via TREM2/TLR4/NF-κB pathway in BV-2 cells. Neurochem. Res. 47 (3), 748–761. doi:10.1007/s11064-021-03484-x
Rodriguez-Amaya, B. D. (2019). Update on natural food pigments - a mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int., 124, 200–205. doi:10.1016/j.foodres.2018.05.028
Rumgay, H., Arnold, M., Ferlay, J., Lesi, O., Cabasag, C. J., Vignat, J., et al. (2022). Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77 (6), 1598–1606. doi:10.1016/j.jhep.2022.08.021
Shen, X. Y., Zhu, L., Zhang, J. Y., and Liang, R. J. (2019). Role and mechanism of hydroxysafflor yellow A in reversing ovarian cancer drug-resistant cell line A2780/DDP. Zhejiang Clin. Med. J. 21, 293–295.
Sheng, C., Peng, W., Xia, Z., and Wang, Y. (2019). Plasma and cerebrospinal fluid pharmacokinetics of hydroxysafflor yellow A in patients with traumatic brain injury after intravenous administration of Xuebijing using LC-MS/MS method. Xenobiotica 50, 545–551. doi:10.1080/00498254.2019.1668983
Shrivastava, S., Jeengar, M. K., Thummuri, D., Koval, A., Katanaev, V. L., Marepally, S., et al. (2017). Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors 43 (2), 152–169. doi:10.1002/biof.1315
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Singh, N. B., Shankar, S., and Srivastava, K. R. (2011). Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives, and clinical applications. Biochem. Pharmacol. 82 (12), 1807–1821. doi:10.1016/j.bcp.2011.07.093
Su, D., and Lv, C. (2021). Hydroxysafflor yellow A inhibits the proliferation, migration, and invasion of colorectal cancer cells through the PPARγ/PTEN/Akt signaling pathway. Bioengineered 12 (2), 11533–11543. doi:10.1080/21655979.2021.2009965
Sun, Y., Yanming, G., Jinxin, L., Lamei, X., Fan, M., Qian, H., et al. (2023). Hydroxysafflor yellow A-an important natural pigment for treating metabolic diseases. Food Rev. Int. 39 (7), 3676–3690. doi:10.1080/87559129.2021.2013256
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, D., Huang, T., Tian, Q., and Wang, J. (2021). MYC/NBS1-Mediated DNA damage response is involved in the inhibitory effect of hydroxysafflor yellow A on glioma cells. Drug Des. Devel Ther. 15, 1749–1763. doi:10.2147/DDDT.S288841
Ugai, T., Sasamoto, N., Lee, H.-Y., Ando, M., Song, M., Tamimi, R. M., et al. (2022). Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 19, 656–673. doi:10.1038/s41571-022-00672-8
Wallace, C. T., and Giusti, M. M. (2019). Anthocyanins-Nature’s bold, beautiful, and health-promoting colors. Foods 8 (11), 550. doi:10.3390/foods8110550
Wan, B. (2018). The effect of Hydroxysafflor yellow A in glioma via p38MAPK signal transduction. Hangzhou, China: Zhejiang Chinese Medical University.
Wang, C. H., and An, Y. C. (2021). Mechanism study of safflor yellow affecting the biological characteristics at ovarian cancer SKOV-3 cells induced by TGF-B1 through reactive oxygen. China Pharm. 24, 288–292.
Wang, C. Y., Liu, Q., Huang, Q. X., Liu, J. T., He, Y. H., Lu, J. J., et al. (2013). Activation of PPARγ is required for hydroxysafflor yellow A of Carthamus tinctorius to attenuate hepatic fibrosis induced by oxidative stress. Phytomedicine 20 (7), 592–599. doi:10.1016/j.phymed.2013.02.001
Wang, J., Hu, M., Fang, J., and Gao, H. (2011). Synthesis and characterization of copolymerized vinyl/phenylsilsesquioxane microspheres. J. Macromol. Sci. Part a-Pure Appl. Chem. 48 (11), 947–951. doi:10.1080/10601325.2011.614868
Wang, L., Li, F., Gu, N. N., Shen, H., Han, C. L., Li, K. Y., et al. (2023). Hydroxysafflor yellow A inhibits proliferation,migration,and chemoresistance of colorectal cancer cells through Akt/mTOR-autophagy pathway. China J. Chin. Materia Medica 48, 517–524. doi:10.19540/j.cnki.cjcmm.20221014.703
Wang, L. P., Feng, J. F., and Hu, K. L. (2014). Progress in regulation effect of aromatic refreshing traditional Chinese medicine on BBB permeability and its mechanism. China J. Chin. Materia Medica 39, 949–954.
Wang, L. Y., Hu, C. H., and Yang, C. P. (2016) “Experimental study of embryo-fetal developmental toxicity of hydroxysafflor yellow A for injection in rats,” in 2016 sixth annual national conference on pharmacotoxicology. Chongqing, China, 1.
Wang, T., Wang, L. J., Li, C. T., Han, B., Wang, Z. H., Li, J., et al. (2017). Hydroxysafflor yellow A improves motor dysfunction in the rotenone-induced mice model of Parkinson's disease. Neurochem. Res. 42 (5), 1325–1332. doi:10.1007/s11064-017-2176-1
Wang, Z., Tang, X. L., Wu, X. Q., Yang, M. Y., Wang, W., Wang, L. H., et al. (2019). Cardamonin exerts anti-gastric cancer activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci. Rep. 39 (5), BSR20190357. doi:10.1042/BSR20190357
Wen, A., Yang, J., Jia, Y., Yang, Z., Tian, Y., Wu, Y., et al. (2008). A rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the determination of hydroxysafflor yellow A in human plasma: application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 876 (1), 41–46. doi:10.1016/j.jchromb.2008.10.007
Wen, J., Xiong, H. S., and Wu, H. G. (2017). Effects of hydroxysafflor yellow A on the development and progression colorectal cancer and its mechanism. J Emerg Trad Chin Med 26, 1347–1351.
Williams, P. A., Zaidi, S. K., and Sengupta, R. (2022). AACR cancer progress report 2022: decoding cancer complexity, integrating science, and transforming patient outcomes. Clin. Cancer Res. 28 (19), 4178–4179. doi:10.1158/1078-0432.CCR-22-2588
Wu, L., Kang, A., and Le, S. J. (2020). Progress in the study of the in vivo processes of hydroxysafflor yellow A. Chin. Trad. Pat. Med. 42, 150–155.
Wu, X., Cai, X., Ai, J., Zhang, C., Liu, N., and Gao, W. (2021). Extraction, structures, bioactivities and structure-function analysis of the polysaccharides from safflower (Carthamus tinctorius L.). Front. Pharmacol. 12, 767947. doi:10.3389/fphar.2021.767947
Xia, C., Dong, X., Li, H., Cao, M., Sun, D., He, S., et al. (2022). Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. Engl. 135 (5), 584–590. doi:10.1097/CM9.0000000000002108
Xian, B., Wang, R., Jiang, H., Zhou, Y., Yan, J., Huang, X., et al. (2022). Comprehensive review of two groups of flavonoids in Carthamus tinctorius L. Biomed. Pharmacother. 153, 113462. doi:10.1016/j.biopha.2022.113462
Xue, X., Deng, Y., Wang, J., Zhou, M., Liao, L., Wang, C., et al. (2021). Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L. with good effect of alleviating atherosclerosis. Phytomedicine 91, 153694. doi:10.1016/j.phymed.2021.153694
Yang, F., Li, J., Zhu, J., Wang, D., Chen, S., and Bai, X. (2015). Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling pathway in H22 tumor-bearing mice. Eur. J. Pharmacol. 754, 105–114. doi:10.1016/j.ejphar.2015.02.015
Yang, Z., Yang, J., Jia, Y., Tian, Y., and Wen, A. (2009). Pharmacokinetic properties of hydroxysafflor yellow A in healthy Chinese female volunteers. J. Ethnopharmacol. 124 (3), 635–638. doi:10.1016/j.jep.2009.02.026
Yao, X., and Matosevic, S. (2021). Cryopreservation of NK and T cells without DMSO for adoptive cell-based immunotherapy. BioDrugs 35 (5), 529–545. doi:10.1007/s40259-021-00494-7
Yi, L., Zhao-Yang, Z., and Jin-Lan, Z. (2007). Determination of hydroxysafflor yellow A in rat plasma and tissues by high-performance liquid chromatography after oral administration of safflower extract or safflor yellow. Biomed. Chromatogr. 21 (3), 326–334. doi:10.1002/bmc.769
Yuan, Y. M., and Lou, Y. L. (2016). Effects of hydroxysafflor yellow A on proliferation,migration and invasion of glioma U251 cells. Chin. Med. Her. 13, 15–18.
Zhang, H. F., Guo, J. X., Huang, L. S., and Ping, Q. N. (2006). Study on the absorption mechanism of hydroxysafflower yellow pigment A. J. China Pharm. Univ. 312–317.
Zhang, J., Li, J., Song, H., Xiong, Y., Liu, D., and Bai, X. (2019). Hydroxysafflor yellow A suppresses angiogenesis of hepatocellular carcinoma through inhibition of p38 MAPK phosphorylation. Biomed. Pharmacother. 109, 806–814. doi:10.1016/j.biopha.2018.09.086
Zhang, L. L., Tian, K., Tang, Z. H., Chen, X. J., Bian, Z. X., Wang, Y. T., et al. (2016). Phytochemistry and pharmacology of Carthamus tinctorius L. Am J chin med. Am. J. Chin. Med. 44 (02), 197–226. doi:10.1142/S0192415X16500130
Zhang, X., Shen, D., Feng, Y., Li, Y., and Liao, H. (2021). Pharmacological actions, molecular mechanisms, pharmacokinetic progressions, and clinical applications of hydroxysafflor yellow A in antidiabetic research. J. Immunol. Res. 4560012–4560110. doi:10.1155/2021/4560012
Zhao, B. X., Gu, S. F., Yong, D., Shen, M. J., Liu, X. R., and Shen, Y. Q. (2018). Solid lipid nanoparticles as carriers for oral delivery of hydroxysafflor yellow A. Int. J. Pharm. 535 (1-2), 164–171. doi:10.1016/j.ijpharm.2017.10.040
Zhao, F., Wang, P., Jiao, Y., Zhang, X., Chen, D., and Xu, H. (2020). Hydroxysafflor yellow A: a systematical review on botanical resources, physicochemical properties, drug delivery system, pharmacokinetics, and pharmacological effects. Front. Pharmacol. 11, 579332. doi:10.3389/fphar.2020.579332
Zheng, X. Q. (2013). Evaluation of biotoxicity and quality control study of traditional Chinese medicine injections. Chengdu, China: Chengdu University of TCM.
Zhou, P., Zhou, H. F., He, Y., Zhang, Y. Y., Yang, J. H., Dai, L. L., et al. (2014). Transport characteristics of hydroxysafflor yellow A across Caco-2 cell monolaver model. Chin. Trad. Herb. Drugs 45, 2030–2035.
Keywords: hydroxysafflor yellow A, pharmacological effects, anticancer effect, pharmacokinetic progress, safety
Citation: Wang Y, An J, Zhou J, Chang L, Zhang Q and Peng F (2025) Hydroxysafflor yellow A: a natural pigment with potential anticancer therapeutic effect. Front. Pharmacol. 15:1495393. doi: 10.3389/fphar.2024.1495393
Received: 12 September 2024; Accepted: 23 December 2024;
Published: 14 January 2025.
Edited by:
Wagdy Mohamed Eldehna, Kafrelsheikh University, EgyptReviewed by:
Keda Zhang, Shenzhen Technology University, ChinaCopyright © 2025 Wang, An, Zhou, Chang, Zhang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Chang, Y2hhbmdsaW1pbmdAd2Noc2N1LmNu; Quan Zhang, emhhbmdxdWFuY2RtY0AxMjYuY29t; Fu Peng, ZnVqaW5nMTI2QHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.