- 1Department of Nephrology, Shaanxi Provincial People’s Hospital, Xi’an, Shaanxi, China
- 2Department of Nephrology, Shaanxi Provincial Second People’s Hospital, Xi’an, Shaanxi, China
Aims: This study aims to evaluate the efficacy and safety of tripterygium glycosides combined with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs) in treating Diabetic nephropathy and provide high-level evidence to support its standardized application.
Methods: Literatures were retrieved from PubMed, Web of Science, EMBASE, Cochrane Library, CNKI, Wanfang and VIP databases, the search time frame was defined as from the time of establishment to April 2023. This study only included randomized controlled trials of tripterygium glycosides combined with ACEI/ARB in the treatment of diabetic nephropathy, and the final included studies were identified according to the inclusion and exclusion criteria, and meta-analysis of data was performed using RevMan 5.3 software.
Results: A total of 44 RCTs with 3537 DN patients were included in the study. Compared with the control group, tripterygium glycosides combined with ACEI/ARB significantly reducing 24 h-UTP (24 h urine total protein) [SMD = −1.46, 95% CI (−1.70, −1.23), P < 0.00001], increasing effective rate [RR = 1.23, 95% CI (1.17,1.29), P < 0.00001], elevating serum albumin [SMD = 0.85, 95% CI (0.69, 1.02), P < 0.00001], improving serum creatinine [SMD = −0.35, 95% CI (−0.59, −0.11), P = 0.004], with no difference in BUN (blood urea nitrogen) [SMD = −0.17, 95% CI (−0.48,0.13), P = 0.27], the adverse reactions rate was higher than those of the control group [RR = 1.96, 95%CI (1.43, 2.68), P < 0.0001].
Conclusion: This study showed that the combination of tripterygium glycosides and ACEI/ARB was more effective than ACEI/ARB alone. However, the side effects of the combined treatment group were higher than those of the control group, especially liver function damage, which also suggested that its safety in the treatment of diabetic nephropathy was worth considering. Therefore, although tripterygium glycosides provided a choice for the clinical treatment of diabetic nephropathy, its side effects limited its clinical application. In future studies, we need to further optimize tripterygium glycosides and reduce its side effects to ensure the safety of clinical application.
1 Introduction
Diabetic nephropathy (DN) is a common microvascular complication of diabetes and the common causes of chronic kidney disease (CKD), which is mainly characterized by proteinuria and decreased glomerular filtration rate. It seriously affects the quality of life of patients and brings huge economic burden to patients and society, so more attention should be paid to DN (Liu et al., 2023; Chen et al., 2020). There is currently no radical treatment for diabetic nephropathy, so it is important to prevent or alleviate the disease at the early stage. Clinically, glycemic control, blood pressure control, reducing cholesterol levels, the adjustment of unhealthy lifestyle and the use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor antagonist (ARB) are often used to slow disease progression (Pillai and Fulmali, 2023; Chen et al., 2022). In recent years, studies have also shown that sodium-glucose co-transporter 2 inhibitors (SGLT2i) can significantly improve the renal outcomes and reduce the risk of renal failure and cardiovascular events in patients with type 2 diabetes, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has a better renal protection effect in DN patients and fineronone is effective and safe in the treatment of diabetic nephropathy (Perkovic et al., 2019; Shaman et al., 2022; Bakris et al., 2015). Although these measures have shown certain efficacy in the treatment of DN, many patients still progress to end-stage renal disease (ESKD) and even have to undergo renal replacement therapy such as hemodialysis or peritoneal dialysis. It is necessary to find more new effective therapies to prevent the disease.
In recent years, traditional Chinese medicine (TCM)has shown its unique advantages in the treatment of diabetic nephropathy, many studies have shown that the application of TCM in patients with diabetic nephropathy can delay its progression to end-stage renal disease and reduce the mortality rate of patients (Chen et al., 2019; Liu et al., 2022). As an immunosuppressant, tripterygium wilfordii is a traditional Chinese medicine, originally written in the “Shennong’s Herbal Classic”, belongs to the Celastraceae family, which is widely used in the treatment of rheumatic diseases and glomerulonephritis in China. The main active metabolites of tripterygium wilfordii are tripterygium wilfordii polyglycosides, terpenoids, glycosides, etc (Liu et al., 2021). Tripterygium glycosides can stabilize the permeability of the glomerular basement membrane, reduce proteinuria, protect podocyte and reduce podocyte damage in patients with DN (Lengnan et al., 2020). In recent years, tripterygium glycosides combined with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs) is also reported to be effective in treating diabetic nephropathy in China, however, the effectiveness of these studies is not consistent, and the reports on safety are not comprehensive. Therefore, this study conducted a meta-analysis on the basis of existing RCT studies, aiming to lay a foundation for evaluating whether TG combined with ACEI/ARB plays a more prominent role in the treatment of diabetic nephropathy and provide evidence-based medical evidence for clinical application.
2 Materials and methods
2.1 Search strategy
Literature was retrieved from PubMed, Web of Science, EMBASE, Cochrane Library, CNKI, Wanfang and VIP databases, the search time frame was defined as from the time of establishment to April 2023. The following keywords were used in the literature search:“diabetic nephropathy”, “diabetic kidney diseases”, “diabetic glomerulosclerosis”, “tripterygium glycosides”, “tripterygium wilfordii”, “tripterygium wilfordii Hook F”.
2.2 Inclusion and exclusion criteria
Inclusion criteria are as follows: (1) All subjects were diagnosed as diabetic nephropathy; (2) All included studies were randomized controlled trials studies; (3) The experimental group was patients treated with tripterygium glycosides in combination with ACEI/ARB, the control group consisted of patients treated with ACEI/ARB alone.
Exclusion criteria are as follows: (1) Incomplete original data; (2) Lack of rigorous experimental design; (3) Repeated studies, case reports, animal or cell experiments; (4) Non-randomized controlled study; (5) Missing data or primary results.
2.3 Outcome indicators
The main outcome indicators include 24-hour urine total protein (24 h-UTP). The secondary outcome indicators include total effective rate (number of effective cases/total number of cases), serum albumin (ALB), blood creatinine (Scr), blood urea nitrogen (BUN) and adverse events.
2.4 Research data extraction
The extracted data included the first author, year of publication, sample size, intervention, duration of treatment, outcome indicators and adverse events. Studies were screened by 2 independent reviewers based on inclusion and exclusion criteria to exclude ineligible studies, and in case of disagreement between reviewers, the disagreement was resolved through discussion or consensus reached on the basis of screening results obtained by the third reviewer.
2.5 Assessment on risk of bias
Quality assessment of the included studies according to the assessment criteria in the Cochrane Handbook of Systematic Evaluation, including the following 7 items: (1) Random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; (7) other biases.
2.6 Statistical analysis
We applied Review Manager 5.3 software and Stata version 17.0 (Stata Corp, College Station, TX, USA) to statistically analyze the data from the included studies. Risk Ratios (RR) was used for dichotomous variables, standardized mean difference (SMD) for continuous variables, and 95% confidence interval (CI) was used for all variables. We applied the Cochran-Q test and I2 statistic to assess the heterogeneity of included studies. If I2 was below 50%, the data were analyzed using a fixed-effects model. If I2 was above 50%, the data were analyzed using a random effects model. Publication bias was analyzed by funnel plot and evaluated quantitatively by egger’s test.
3 Results
3.1 Literature search results
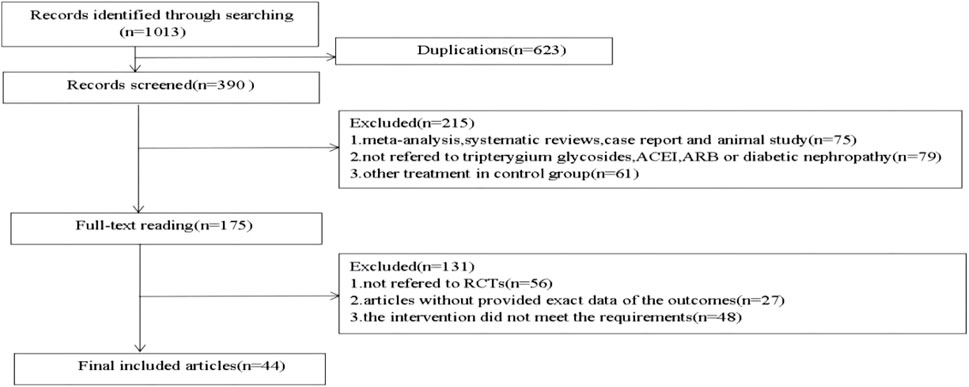
Initially, 1,013 relevant articles were identified out by seven databases. By reading titles and abstracts, duplicate literature, animal experiments, reviews, cell studies and case reports were excluded. In the remaining literature, by reading the full text, we excluded the literature that did not meet the requirements of the experimental design, missing data, and no interventions. Finally, a total of 44 literature were included in this study. The research selection process is shown in Figure 1.
3.2 Quality of included studies
We applied the Cochrane Collaborations tool to assess the quality of the included studies. 44 studies [12–55] included were all RCTs, of which 15 articles mentioned the method of randomization (random number table method was used in 11 articles, computer randomization method was used in 2 articles, random envelope method was used in 1 article, and random drawing method was used in 1 article). 29 studies only mentioned randomization without specifying the method of randomization, all the articles did not mentioned allocation concealment, and no study mentioned other biases. The results of the quality assessment are shown in Figure 2.

Figure 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
3.3 Trial characteristics
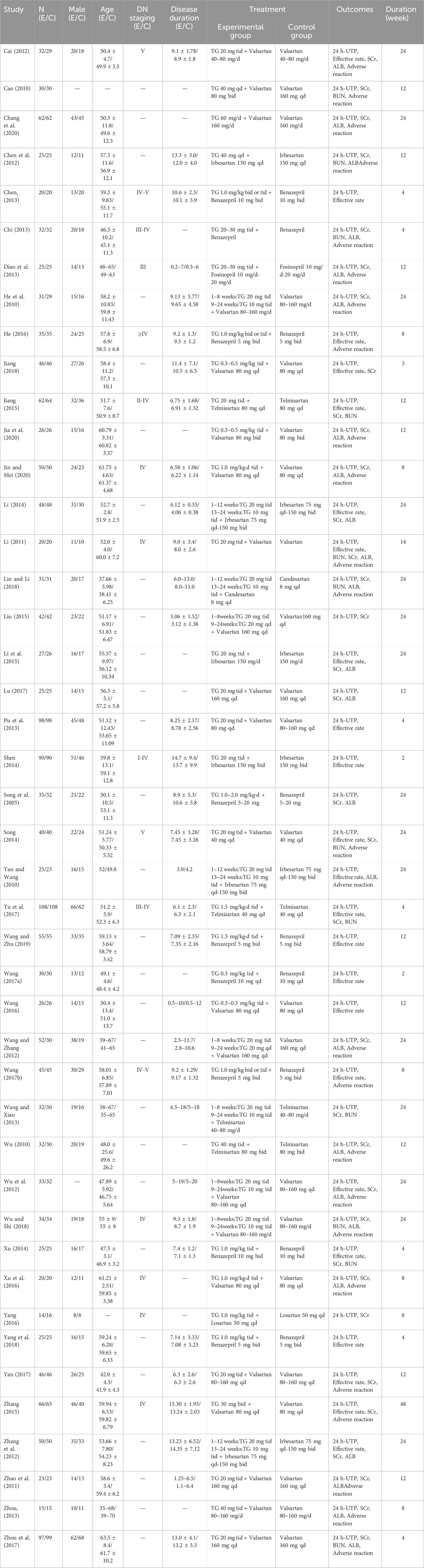
The 44 RCT studies included a total of 3,537 patients,1785 in the experimental group, and 1752 in the control group. The baseline data were balanced between the two groups of patients, and the basic characteristics are shown in Table 1.
3.4 Meta-analysis results
3.4.1 24 h urine total protein (24 h-UTP)
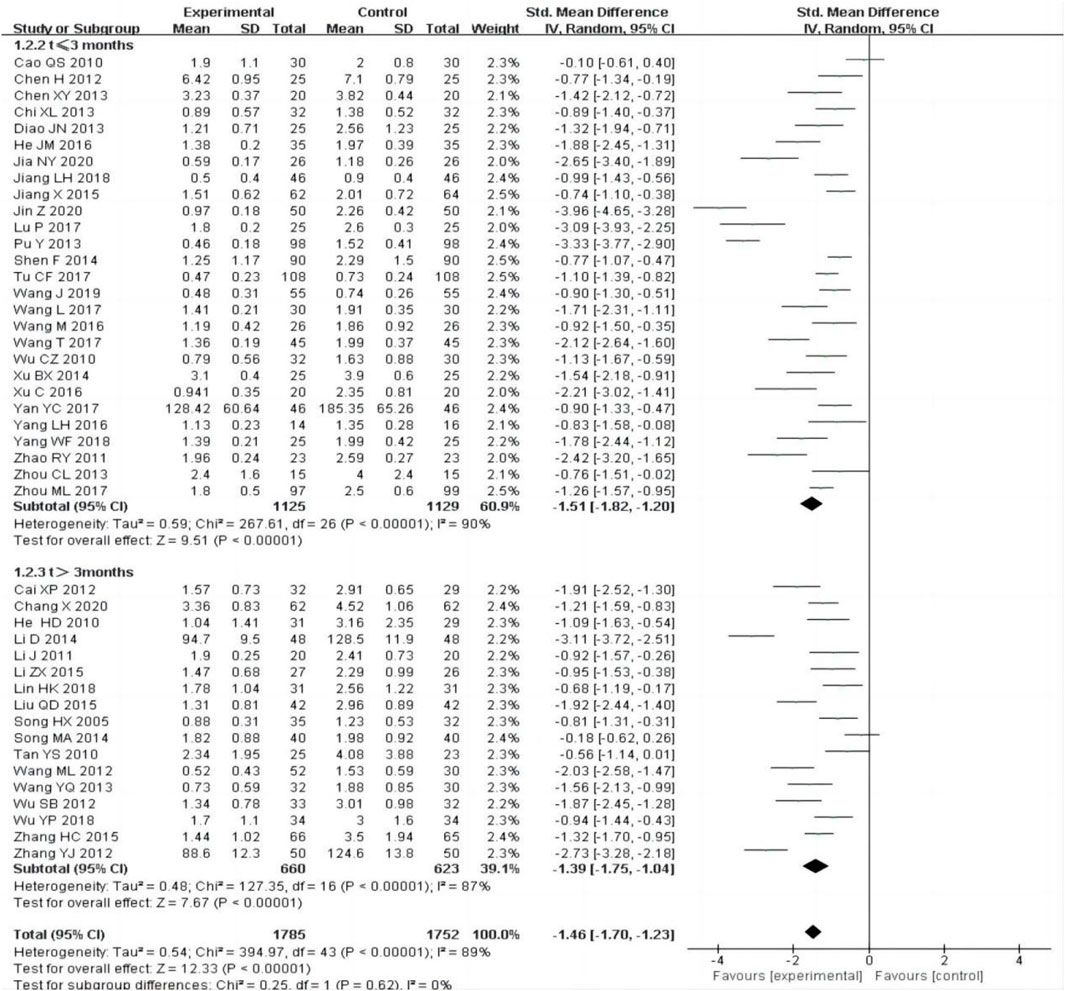
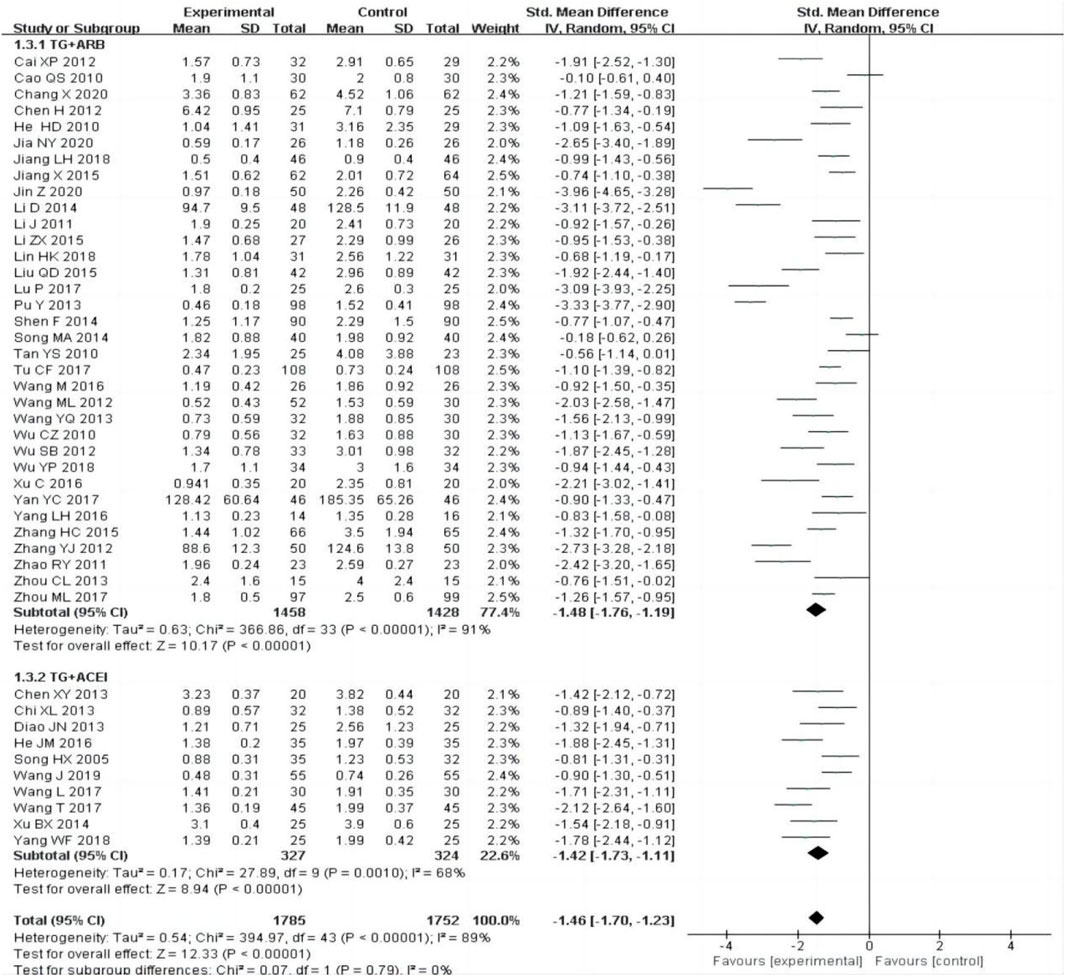
All the included studies [12–55] reported changes of 24 h-UTP after treatment between the experimental group and control group. The random-effect model was adopted to analyze the data due to the heterogeneity (P < 0.00001, I2 = 89%), and the results showed that the experimental group was more effective in reducing 24 h-UTP than the control group [SMD = −1.46, 95% CI (−1.70–1.23), P < 0.00001].
The subgroup was divided into t ≤ 3 months and t > 3 months, the heterogeneity was still obvious within each subgroup [t ≤ 3 months: P < 0.00001, I2 = 90%; t > 3 months:P < 0.00001, I2 = 89%], subgroup analysis also showed that the experimental group was superior to the control group in reducing 24 h-UTP [Figure 3].
The subgroup was divided into TG + ARB and TG + ACEI, the heterogeneity was still obvious within each subgroup [TG + ARB:P < 0.00001, I2 = 91%; TG + ACEI:P = 0.001, I2 = 68%], subgroup analysis also showed that the experimental group was superior to the control group in reducing 24 h-UTP [Figure 4].
3.4.2 Effective rate
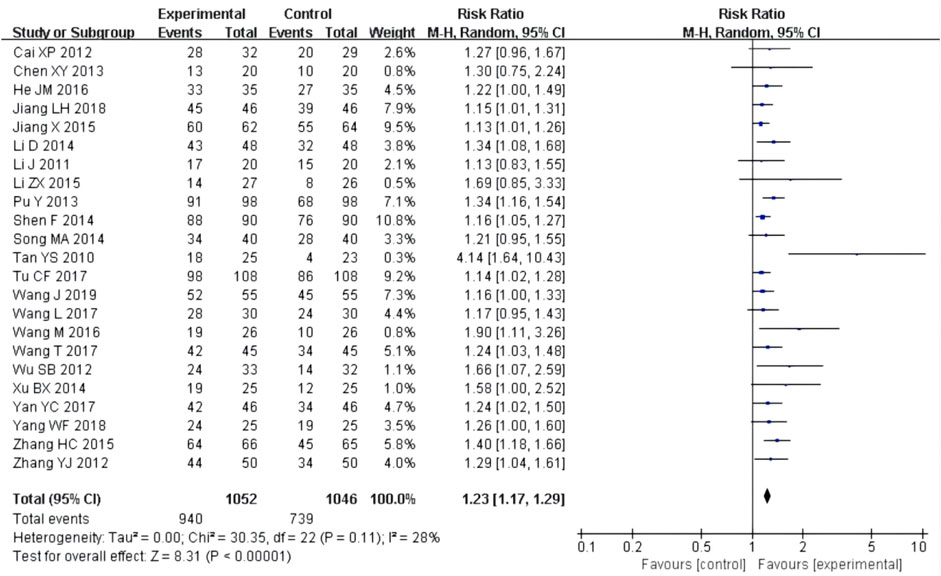
23 studies (Cai, 2012; Chen, 2013; He, 2016; Jiang, 2018; Jiang, 2015; Li, 2014; Li, 2011; Li et al., 2015; Pu et al., 2013; Shen, 2014; Song, 2014; Tan and Wang, 2010; Tu et al., 2017; Wang and Zhu, 2019; Wang L., 2017; Wang, 2016; Wang T., 2017; Wu et al., 2012; Xu, 2014; Yang et al., 2018; Yan, 2017; Zhang, 2015; Zhang et al., 2012) reported effective rate after the treatment. Although the heterogeneity test (P = 0.11, I2 = 28%) showed no significant heterogeneity, the confidence interval with the fixed-effect model was very narrow, so the random-effect model was used. The results showed that the experimental group had a better effect rate than the control group [RR = 1.23, 95% CI (1.17, 1.29), P < 0.00001, Figure 5].
3.4.3 Serum albumin (ALB)
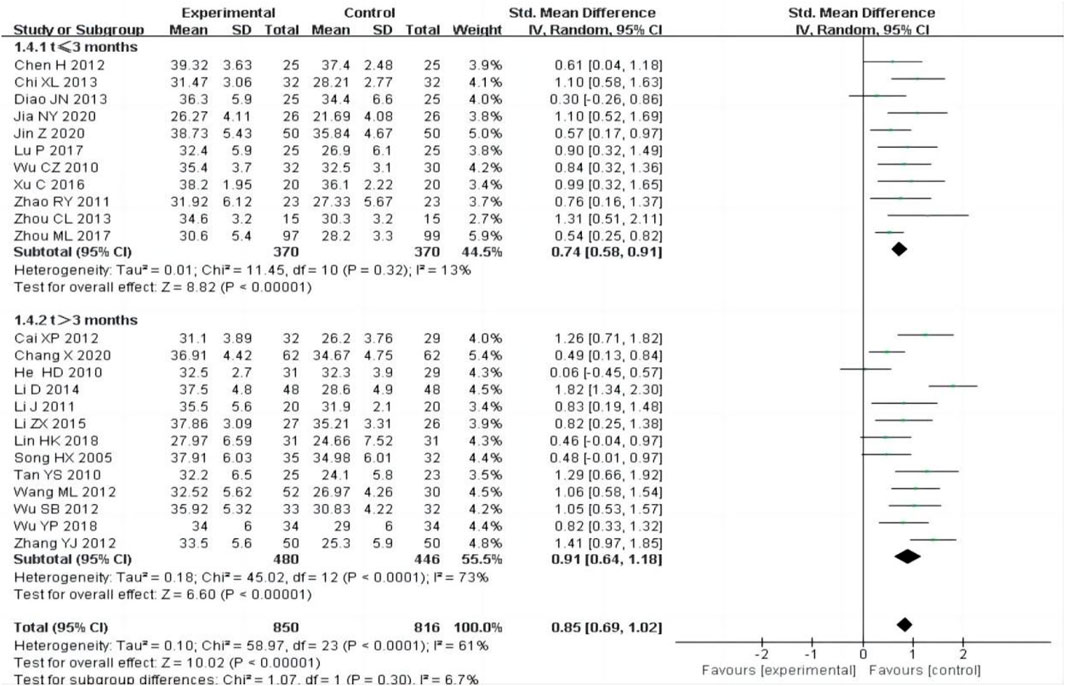
24 studies (Cai, 2012; Chang et al., 2020; Chen et al., 2012; Chi, 2013; Diao et al., 2013; He et al., 2010; Jia et al., 2020; Jin and Mei, 2020; Li, 2014; Li, 2011; Lin and Li, 2018; Li et al., 2015; Lu, 2017; Song et al., 2005; Tan and Wang, 2010; Wang and Zhang, 2012; Wu, 2010; Wu et al., 2012; Wu and Shi, 2018; Xu et al., 2016; Zhang et al., 2012; Zhao et al., 2011; Zhou, 2013; Zhou et al., 2017) including 1,666 participants, reported ALB outcome after treatment, The random-effect model was adopted to analyze the data due to the heterogeneity (P < 0.0001, I2 = 61%), and the results showed that the experimental group was more effective at increasing ALB than the control group [SMD = 0.85, 95% CI (0.69, 1.02), P < 0.00001, Figure 6].
We performed subgroup analysis based on the treatment time, the subgroup was divided into t ≤ 3 months and t > 3 months, the result showed that the experimental group had a better curative effect than the control group (Figure 6).
3.4.4 Serum creatinine (Scr)
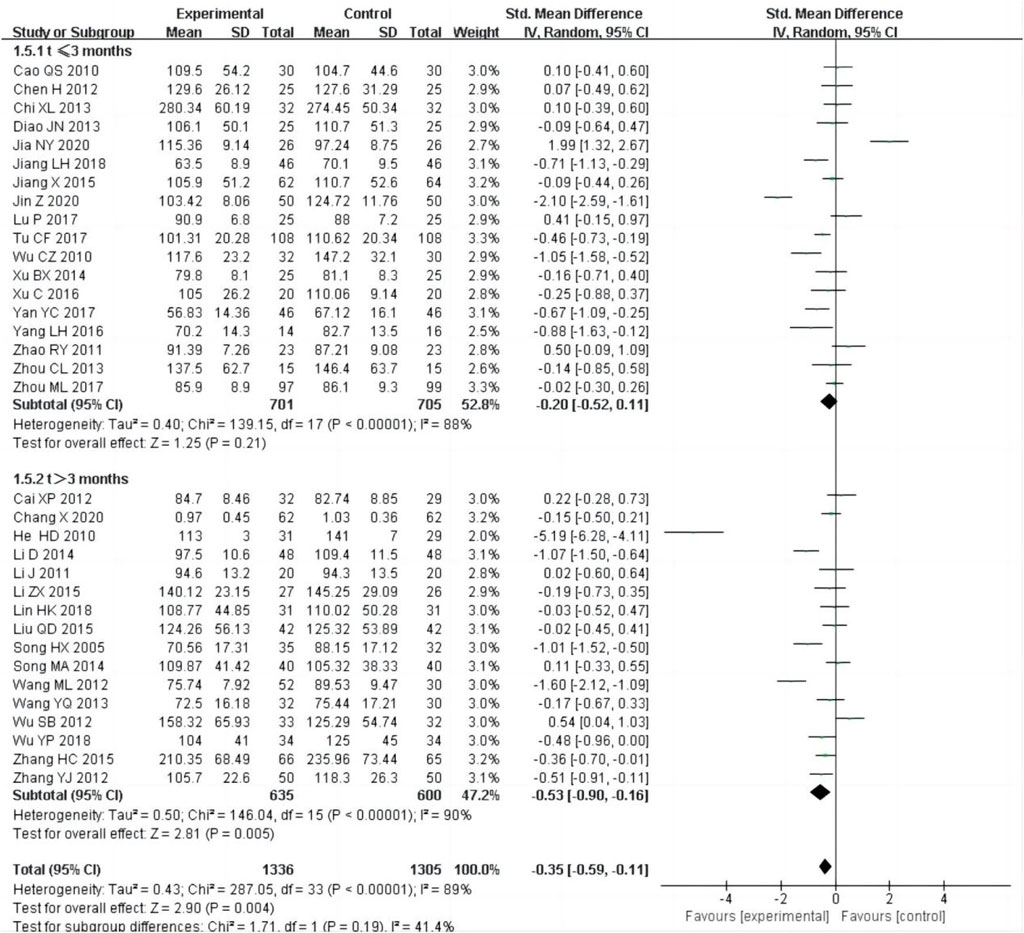
34 studies (Cai, 2012; Cao, 2010; Chang et al., 2020; Chen et al., 2012; Chi, 2013; Diao et al., 2013; He et al., 2010; Jiang, 2018; Jiang, 2015; Jia et al., 2020; Jin and Mei, 2020; Li, 2014; Li, 2011; Lin and Li, 2018; Liu, 2015; Li et al., 2015; Lu, 2017; Song et al., 2005; Song, 2014; Tu et al., 2017; Wang and Zhang, 2012; Wang and Xiao, 2013; Wu, 2010; Wu et al., 2012; Wu and Shi, 2018; Xu, 2014; Xu et al., 2016; Yang, 2016; Yan, 2017; Zhang, 2015; Zhang et al., 2012; Zhao et al., 2011; Zhou, 2013; Zhou et al., 2017), including 2,641 patients, reported of Scr as an outcome. The random-effect model was adopted to analyze the data due to the heterogeneity (P < 0.0001, I2 = 89%), and the result showed that the experimental group could improve SCr better than control group [SMD = −0.35, 95% CI (−0.59, −0.11), P = 0.004, Figure 7]. We performed subgroup analysis based on the treatment time, the heterogeneity was still obvious within each subgroup [t ≤ 3 months:P < 0.00001, I2 = 88%; t > 3 months: P < 0.00001, I2 = 90%], subgroup analysis showed that there was no difference in SCr between the experimental group and control group, if the treatment duration was less than 3 months [SMD = −0.20, 95% CI (−0.52,0.11), P = 0.21, Figure 7], but if the treatment duration was longer than 3 months, there was a significant difference between the experimental group and control group [SMD = −0.53, 95% CI (−0.90, −0.16), P = 0.005, Figure 7].
3.4.5 Blood urea nitrogen (BUN)
12 studies (Cao, 2010; Chen et al., 2012; Chi, 2013; Jiang, 2015; Li, 2011; Lin and Li, 2018; Song, 2014; Tu et al., 2017; Wang and Xiao, 2013; Wu and Shi, 2018; Xu, 2014; Zhou et al., 2017), including 1,074 patients, reported of BUN as an outcome. As the heterogeneity test (P2 = 83%) showed significant heterogeneity, the random-effect model was used for this meta-analysis. The result indicated that there was no significant difference in reducing BUN between the experimental group and the control group [SMD = −0.17, 95% CI (−0.48, 0.13), P = 0.27, Figure 8].
3.4.6 Adverse reactions
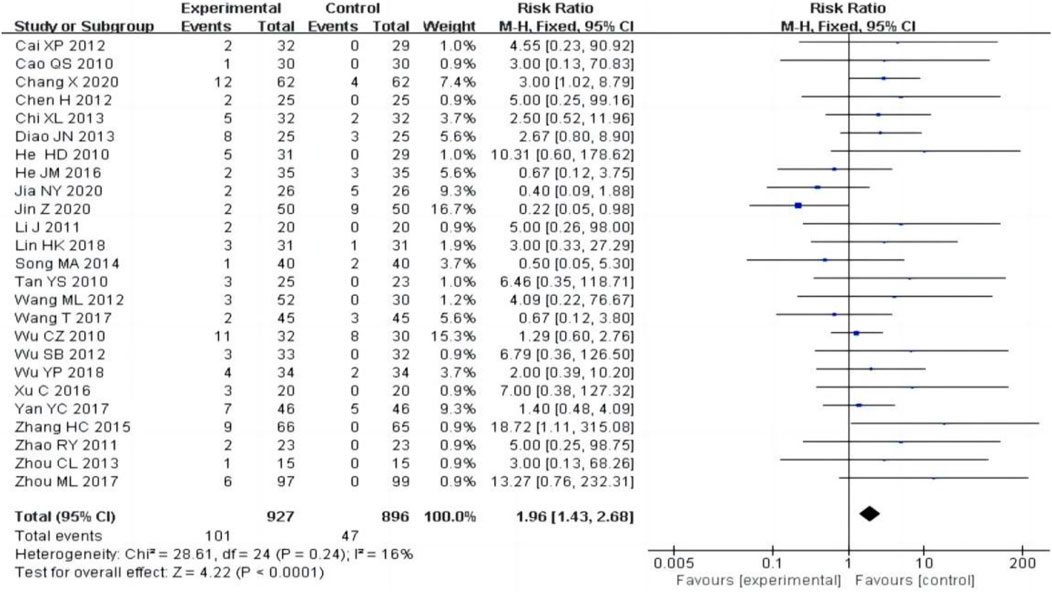
25 studies (Cai, 2012; Cao, 2010; Chang et al., 2020; Chen et al., 2012; Chi, 2013; Diao et al., 2013; He et al., 2010; He, 2016; Jia et al., 2020; Jin and Mei, 2020; Li, 2011; Lin and Li, 2018; Song, 2014; Tan and Wang, 2010; Wang and Zhang, 2012; Wang T., 2017; Wu, 2010; Wu et al., 2012; Wu and Shi, 2018; Xu et al., 2016; Yan, 2017; Zhang, 2015; Zhao et al., 2011; Zhou, 2013; Zhou et al., 2017), including 1823 patients, reported adverse reactions, the heterogeneity test (P = 0.24, I2 = 16%) showed no significant heterogeneity, the fixed-effect model was used for meta-analysis. The result showed that the experimental group had a higher adverse reactions rate than the control group [RR = 1.96, 95% CI (1.43, 2.68), P < 0.0001, Figure 9]. Of the 25 studies reporting adverse reactions, 101 cases in the experimental group had adverse reactions, including 45 cases (44.6%) of hepatic impairment, 11 cases (10.9%) of leukocytopenia, 13 cases (12.9%) of menstrual disorders and other adverse reactions. In the control group, there were 47 cases of adverse reactions, including 3 cases (6.4%) of hepatic impairment, 4 cases (8.5%) of leukocyte reduction, and no adverse reactions of menstrual disorders. In patients who developed liver function impairment, aminotransferase is mostly slightly elevated, which can be restored to the normal range with the addition of hepatoprotective drugs, such as polyene phosphatidylcholine, silymarin, compound glycyrrhizin and bifendate pills. For the adverse reactions of leukopenia and menstrual disorders, 8 original literatures only mentioned that the adverse reactions disappeared after symptomatic treatment, and 6 original literatures did not mention treatment measures. None of the original literatures reported in detail on the degree of severity and the specific measures taken.
3.4.7 Sensitivity analysis and publication bias
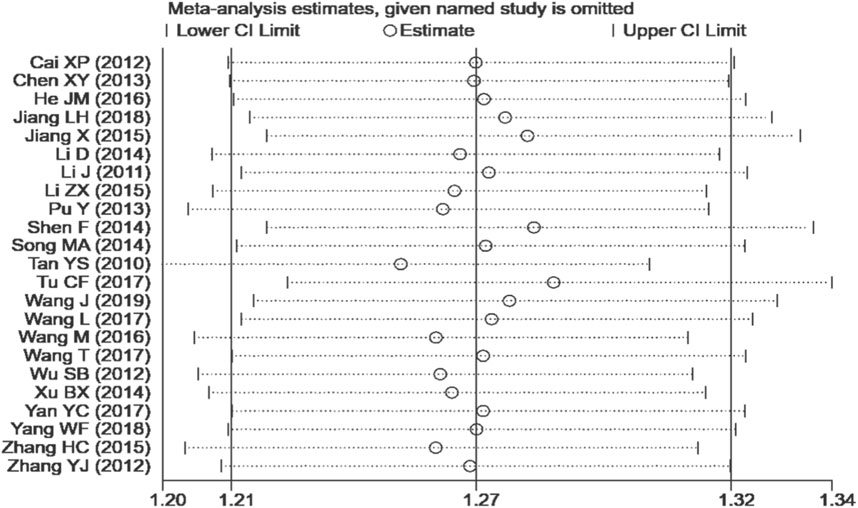
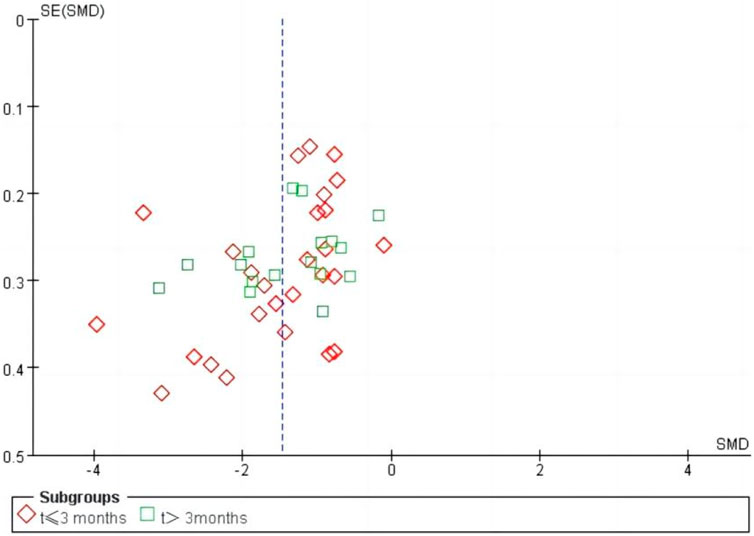
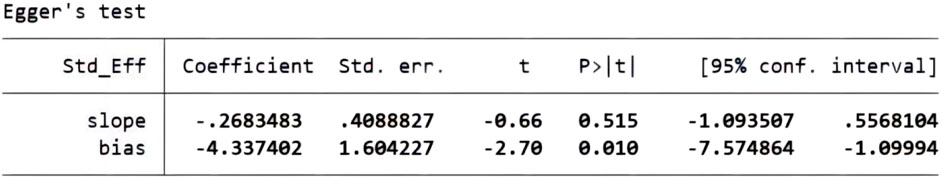
We performed a sensitivity analysis on effective rate (Figure 10), and the results showed that no single article affected the overall analysis result, indicating that the results of this study were relatively stable. We used funnel plots to assess publication bias on 24 h-UTP, as shown in Figure 11, the funnel plot shows the presence of publication bias, the Egger’s test for 24 h-UTP (Figure 12) shows P = 0.010, which is consistent with funnel plot results, the publication bias is considered to be related to the heterogeneity of the study and the quality of the included articles.
4 Discussion
Diabetic nephropathy (DN) is the main cause of end-stage renal disease (ESRD). In recent years, the incidence of diabetic nephropathy has been gradually increasing due to the increasing incidence of diabetes patients. The pathogenesis of DN includes alterations in glomerular hemodynamics, increased activity of the renin-angiotensin system (RAS), genetic predisposition, oxidative stress, overexpression of inflammatory cytokines and ultimately development of glomerular sclerosis and renal failure (Giglio et al., 2023; Martini et al., 2008). As an important clinical feature of DN, persistent albuminuria is an independent risk factor of DN progression (Liu et al., 2021; Wu et al., 2017). Due to the complex pathogenesis of diabetic nephropathy, it is difficult to slow down the progression of the disease with the current conventional treatment methods. In recent years, studies have demonstrated that sodium-glucose cotransporter-2 (SGLT-2) inhibitors have a renal protective effect and reduce the incidence of cardiovascular events in patients with diabetic nephropathy (DKD) (Perkovic et al., 2019; Bhatt et al., 2021; Cherney et al., 2023). However, the potential adverse reactions of SGLT-2 inhibitors, such as increasing the risk of genital fungal infection and euglycaemic diabetic ketoacidosis in patients with type 2 diabetes, limit its clinical application (Liew et al., 2023). Some studies in China have shown that traditional Chinese medicine combined with Western medicine has better efficacy in the treatment of diabetic nephropathy. Tripterygium glycosides, as a traditional Chinese medicine, is also used in the treatment of diabetic nephropathy. Tripterygium glycosides can protect and reverse the damage of podocyte cytoskeleton, reduce podocyte damage, and decrease urinary protein (Zheng et al., 2008). A prospective, randomized, controlled clinical trial have shown that tripterygium glycosides can significantly reduce urine protein level in DN patients (Ge et al., 2013). A meta-analysis of 23 RCTs showed that low-dose tripterygium glycosides had a positive effect on the treatment of type 2 diabetic nephropathy, however, the inclusion criteria were broader, and the control group was given conventional treatment, or ACEI/ARB or metformin was given in addition to conventional treatment (Chen et al., 2022). The results of a study by Wang D et al. showed that tripterygium glycosides had good renoprotective effects, reducing urine protein, blood urea nitrogen and creatinine levels in patients with kidney disease, and had a good safety profile (Wang et al., 2018). Tripterygium glycosides is proved to not only regulate inflammatory factors such as TNF-α, IL-1β and TGF-β1, but also ameliorate podocyte injury and glomerular hypertrophy, reduce urinary protein in rats with diabetic nephropathy through anti-inflammation and inhibition of macrophage infiltration (Ma et al., 2013; Huang et al., 2020). A study showed that tripterygium glycosides may delay the transdifferentiation of renal tubulointerstitial cells to MyoF by down-regulating the expression of ACt-A, thus alleviating renal tubulointerstitial fibrosis, reducing urinary protein and protecting renal function (Zhao et al., 2014). It is well known that podocyte injury plays an important role in the pathogenesis of DN, which promotes the progression of diabetic nephropathy. Apoptosis and epithelium-mesenchymal transformation are the main causes of podocyte injury in diabetic nephropathy. Many studies have shown that over expression of twist1 increases apoptosis of podocytes, and tripterygium glycosides may reduce apoptosis and protect podocytes by inhibiting twist1 signaling pathway (Tao et al., 2021). It is worth noting that some studies have also reported that tripterygium glycosides has no definite efficacy on reducing serum creatinine, urea nitrogen and increasing blood albumin in diabetic nephropathy (Chen et al., 2012; Wang and Xiao, 2013). Therefore, in this meta-analysis, 44 studies were included to explore the efficacy and safety of tripterygium glycosides combined with ARB/ACEI in diabetic nephropathy. In order to better evaluate the efficacy and safety of tripterygium glycosides in the treatment of diabetic nephropathy, we selected the effective rate, 24 h-UTP, ALB, Scr, BUN and adverse reactions for statistical analysis. By analyzing several outcome indexes, we found that compared with ACEI/ARB treatment alone, tripterygium glycosides combined with ACEI/ARB could improve the effective rate, increase plasma albumin and improve renal function in patients with diabetic nephropathy, suggesting that the effect of TG combined with ACEI/ARB in treating diabetic nephropathy is worthy of affirmation. However, this meta-analysis could not provide reliable evidence for its role in regulating inflammatory factors, inhibiting humoral immunity and reducing podocyte apoptosis.
It is worth noting that the results of our meta-analysis showed the significant heterogeneity in 24 h-UTP, ALB, Scr and BUN. In order to explore the source of heterogeneity, we performed subgroup analysis on observation time and drug type. The results showed that the above factors were not the source of heterogeneity, which may be related to the uneven quality and bias of the included literatures and we used random effects model to analyze and interpret all indicators with significant heterogeneity.
The complex chemical ingredients of tripterygium glycosides is easy to produce side effects, and more attention needs to be paid to its potential adverse reactions. The common adverse reactions of tripterygium glycosides were liver damage, menstrual disorder, gastrointestinal reaction and leukopenia. Liver damage is mainly characterized by mild elevation of aminotransferases, which can generally return to normal after liver protection drug treatment. Menstrual disorders often recover after stopping tripterygium glycosides treatment or after using traditional Chinese medicine preparations. Gastrointestinal reactions and leukopenia generally disappear after symptomatic treatment. Our study also showed that the incidence of adverse reactions in the combined treatment group was higher than that in the control group, and the difference was statistically significant (P < 0.05). Therefore, although tripterygium glycosides provides a choice for the clinical treatment of diabetic nephropathy, its side effects cannot be ignored. Clinicians need to make a comprehensive assessment according to the actual situation, if they choose to apply tripterygium glycosides to treat diabetic nephropathy, it is necessary to pay more attention to the possible adverse reactions to patients and give timely intervention.
There are some limitations to this meta-analysis: (1) The included RCTs were mainly in China, and some of the literature only mentioned random assignment without specific description of the method of randomization, and most of the literature did not describe the blinding method, so the quality of the literature was low, which may lead to selection bias or publication bias. (2) The staging of diabetic nephropathy and the different doses of tripterygium glycosides application in the included original literatures may lead to heterogeneity. (3) Some of the original studies did not adequately describe complications, particularly abnormalities in liver function, white blood cells and menstrual disorders. (4) Certain novel drugs, such as dagliflozin and nonnilidone, have not been widespreadly used in the present, and their effects were not included in this study. (5) Lack of data on long-term efficacy: among all the original literatures included, only one study was observed for 12 months, and the other literatures were observed for less than 6 months, the efficacy of long-term use of tripterygium glycosides need to be further confirmed. (6) All of the included subjects were Chinese, and it may be difficult to apply these findings to other populations which will affect the generality of the results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Z’EY: Writing–original draft, Writing–review and editing. PW: Data curation, Software, Writing–review and editing. QF: Data curation, Methodology, Writing–original draft. QS: Data curation, Software, Writing–review and editing. HX: Formal Analysis, Investigation, Writing–original draft. PZ: Data curation, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1493590/full#supplementary-material
References
Bakris, G. L., Agarwal, R., Chan, J. C., Cooper, M. E., Gansevoort, R. T., Haller, H., et al. (2015). Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. 314(9):884–894. doi:10.1001/jama.2015.10081
Bhatt, D. L., Szarek, M., Pitt, B., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021). Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 14 (2), 129–139. doi:10.1056/NEJMoa2030186
Cai, X. P. (2012). ARB combined with tripterygium glycosides regimen for treatment of patients with diabetic nephropathy. J. Clin. Med. Pract. 16 (23), 112–114.
Cao, Q. S. (2010). Effect of valsartan dispersive tablets combined with Tripterygium Glycosides on diabetic nephropathy albuminuria. China Prac. Med. 5 (30), 156–157. doi:10.14163/j.cnki.11-5547/r.2010.30.120
Chang, X., Li, L., Wang, B., Huang, C., Liu, X. W., Liu, H. B., et al. (2020). Evaluation of the efficacy and safety of TWHF in diabetic nephropathy patients with overt proteinuria and normal eGFR. J. Formos. Med. Assoc. 19 (3), 685–692. doi:10.1016/j.jfma.2019.11.001
Chen, H., Zhuang, L. P., Liu, J. F., Ci, R. L. B., and Bian, W. (2012). Effect of irbesartan and triptolide combination on the level of urine protein in patients with diabetic nephropathy at high altitude area. Clin. Med. China 28 (11), 1149–1151. doi:10.3760/cma.j.issn.1008-6315.2012.11.010
Chen, H. Y., Pan, H. C., Chen, Y. C., Chen, Y. C., Lin, Y. H., Yang, S. H., et al. (2019). Traditional Chinese medicine use is associated with lower end-stage renal disease and mortality rates among patients with diabetic nephropathy: a population-based cohort study. BMC Complement. Altern. Med. 19 (1), 81. doi:10.1186/s12906-019-2491-y
Chen, X. Y. (2013). Clinical observation of Tripterygium wilfordii polyglycoside combined with Benazepril on diabetic nephropathy albuminuria. Shaanxi J Traditional Chin. Med. 34 (8), 106–1017.
Chen, Y., Lee, K., Ni, Z., and He, J. C. (2020). Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis. Basel Switz. 6 (4), 215–225. doi:10.1159/000506634
Chen, Y., Lu, M., Feng, Y., and Gao, Q. (2022). The effect and safety of low-dose tripterygium wilfordii in patients with type 2 diabetic nephropathy: a meta-analysis. Med. 101 (52), e32504. doi:10.1097/MD.0000000000032504
Cherney, D. Z. I., Ferrannini, E., Umpierrez, G. E., Peters, A. L., Rosenstock, J., Powell, D. R., et al. (2023). Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes Obes. Metab. 25 (6), 1646–1657. doi:10.1111/dom.15019
Chi, X. L. (2013). Clinical observation of Tripterygium wilfordii glycosides on diabetic nephropathy albuminuria. Chin J Clin. Ration. Drug Use 6 (8B), 46–47. doi:10.15887/j.cnki.13-1389/r.2013.23.076
Diao, J. N., Wang, Y. Y., Dong, P., and Zhu, P. J. (2013). Effect of Tripterygium wilfordii polyglycosides combined with Fosinopril on CRP and TNF-α in early diabetic nephropathy. CJITWN 14 (12), 1088–1089.
Ge, Y., Xie, H., Li, S., Jin, B., Hou, J., Zhang, H., et al. (2013). Treatment of diabetic nephropathy with tripterygium wilfordii Hook F extract: a prospective, randomized, controlled clinical trial. J. Transl. Med. 31 (11), 134. doi:10.1186/1479-5876-11-134
Giglio, R. V., Patti, A. M., Rizvi, A. A., Stoian, A. P., Ciaccio, M., Papanas, N., et al. (2023). Advances in the pharmacological management of diabetic nephropathy: a 2022 international update. Biomed 11 (2), 291. doi:10.3390/biomedicines11020291
He, H. D., Wang, W., Chen, Z., and Zhang, R. Z. (2010). Clinical observation of tripterygium glycosides combined with valsartan in treatment of stage I-III diabetic nephropathy. Lishizhen Med. Mater Med. Res. 21 (11), 3032–3033.
He, J. M. (2016). Effect of Tripterygium wilfordii polyglycoside combined with Benazepril on diabetic nephropathy albuminuria. Guangdong Med. J. 37, 212–213. doi:10.13820/j.cnki.gdyx.2016.s1.094
Huang, W. J., Liu, W. J., Xiao, Y. H., Zheng, H. J., Xiao, Y., Jia, Q., et al. (2020). Tripterygium and its extracts for diabetic nephropathy: efficacy and pharmacological mechanisms. Biomed. Pharmacother. 121, 109599. doi:10.1016/j.biopha.2019.109599
Jia, N. Y., Liu, D., Nan, L., and Wang, C. L. (2020). Effect of tripterygium glycosides combined with valsartan on diabetic nephropathy. Electron. J. Of Pract. Gynecol. Endocrinol. 7 (25), 145+147. doi:10.16484/j.cnki.issn2095-8803.2020.25.093
Jiang, L. H. (2018). Clinical efficacy of tripterygium glycosides combined with valsartan in the treatment of diabetic nephropathy. Med. Equip. 31 (19), 115–116.
Jiang, X. (2015). Clinical observation of tripterygium glycosides combined with telmisartan in treatment of diabetic nephropathy. Drugs Clin. 30 (8), 987–990.
Jin, Z., and Mei, L. (2020). Clinical effect of Tripterygium Glycosides combined with Valsartan in the treatment of patients with stage IV diabetic nephropathy. China Mod. Med. 27 (31), 86–89.
Lengnan, X., Ban, Z., Haitao, W., Lili, L., Aiqun, C., Huan, W., et al. (2020). Tripterygium wilfordii Hook F treatment for stage IV diabetic nephropathy: protocol for a prospective, randomized controlled trial. Biomed. Res. Int. 2020, 9181037. doi:10.1155/2020/9181037
Li, D. (2014). Clinical observation of irbesartan combined with tripterygium glycosides in treatment of type 2 diabetic nephropathy. J. Yichun Univ. 36 (12), 31–32.
Li, J. (2011). Clinical observation of diabetic nephropathy under the treatment of tripterygium glycosides combined with valsartan. Chin. Commun. Dr. 3 (23), 43.
Li, Z. X., Ma, L. J., Li, Y. C., Liu, J., Shen, S., and Zhao, S. M. (2015). Effectiveness of tripteryguim wilfordli plus irbesartan in patients with early-to-mid diabetic nephropathy. J. Clin. Res. 32 (6), 1048–1051+1055. doi:10.3969/j.issn.1671-7171.2015.06.003
Liew, A., Lydia, A., Matawaran, B. J., Susantitaphong, P., Tran, H. T. B., and Lim, L. L. (2023). Practical considerations for the use of SGLT-2 inhibitors in the Asia-Pacific countries-An expert consensus statement. Nephrol. Carlt. 28, 415–424. doi:10.1111/nep.14167
Lin, H. K., and Li, Y. J. (2018). Effect of Tripterygium wilfordii polyglycosides combined with Candesartan on diabetic nephropathy. Diabetes New World, 173–175. doi:10.16658/j.cnki.1672-4062.2018.23.173
Liu, P., Zhang, J., Wang, Y., Shen, Z., Wang, C., Chen, D. Q., et al. (2021). The active compounds and therapeutic target of tripterygium wilfordii Hook. f. in attenuating proteinuria in diabetic nephropathy: a review. Front. Med. (Lausanne) 8, 747922. doi:10.3389/fmed.2021.747922
Liu, Q. D. (2015). The effect of valsartan combined with Tripterygium wilfordii glycosides on alleviating proteinuria. J Pract. Diabetology. 11 (5), 56–57.
Liu, X. J., Hu, X. K., Yang, H., Gui, L. M., Cai, Z. X., Qi, M. S., et al. (2022). A review of traditional Chinese medicine on treatment of diabetic nephropathy and the involved mechanisms. Am. J. Chined 50 (7), 1739–1779. doi:10.1142/S0192415X22500744
Liu, Y., Lv, Y., Zhang, T., Huang, T., Lang, Y., Sheng, Q., et al. (2023). T cells and their products in diabetic kidney disease. Front. Immunol. 14, 1084448. doi:10.3389/fimmu.2023.1084448
Lu, P. (2017). Application effect of Tripterygium wilfordii polyglycosides combined with valsartan in the treatment of diabetic nephropathy. World Clin. Med. 11 (7), 94.
Ma, R., Liu, L., Liu, X., Wang, Y., Jiang, W., and Luo, X. (2013). Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp. Ther. Med. 6 (3), 649–656. doi:10.3892/etm.2013.1226
Martini, S., Eichinger, F., Nair, V., and Kretzler, M. (2008). Defining human diabetic nephropathy on the molecular level: integration of transcriptomic profiles with biological knowledge. Rev. Endocr. Metab. Disord. 9, 267–274. doi:10.1007/s11154-008-9103-3
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., et al. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380 (24), 2295–2306. doi:10.1056/NEJMoa1811744
Pillai, A., and Fulmali, D. (2023). A narrative review of new treatment options for diabetic nephropathy. Cureus 15 (1), e33235. doi:10.7759/cureus.33235
Pu, Y., Zou, Q. W., and Pu, M. (2013). Analysis of the effect of valsartan dispersible tablets and tripterygium of glycosides in the treatment of 78 patients with diabetic nephropathy proteinuria. Jilin Med. J. 34 (28), 5789–5791.
Shaman, A. M., Bain, S. C., Bakris, G. L., Buse, J. B., Idorn, T., Mahaffey, K. W., et al. (2022). Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. 145(8):575–585. doi:10.1161/CIRCULATIONAHA.121.055459
Shen, F. (2014). Clinical observation of tripterygium glycosides combined with irbesartan in treatment of diabetic nephropathy. J. New Chin. Med. 46 (6), 158–159. doi:10.13457/j.cnki.jncm.2014.06.076
Song, H. X., Gong, J., Chen, W., Wang, Z. F., Li, Y. G., and Zuo, Z. S. (2005). Effect of triptolide on urinary monocyte chemottractant protein-1 in patients with diabetic nephropathy. Chin. J. Integr. Traditional West. Med. 25 (5), 416–418.
Song, M. A. (2014). Effect of valsartan dispersive tablets and Tripterygium wilfordii glycosides on diabetic nephropathy albuminuria. China Med. Eng. 22 (10), 154+156.
Tan, Y. S., and Wang, M. (2010). Clinical observation of massive proteinuria of diabetic nephropathy under the treatment of tripterygium glycosides combined with irbesartan. Natl. Med. Front. China 5 (17), 32–33.
Tao, M., Zheng, D., Liang, X., Wu, D., Hu, K., Jin, J., et al. (2021). Tripterygium glycoside suppresses epithelial‑to‑mesenchymal transition of diabetic kidney disease podocytes by targeting autophagy through the mTOR/Twist1 pathway. Mol. Med. Rep. 24 (2), 592. doi:10.3892/mmr.2021.12231
Tu, C. F., Wang, L. J., Gu, L. J., and Tao, H. Y. (2017). Effect of tripterygium wilfordii polyglycoside combined telmisartan on renal function and hemorheology in patients with diabetic nephropathy. Chin. J. General Pract. 15 (9), 1527–1528+1595. doi:10.16766/j.cnki.issn.1674-4152.2017.09.023
Wang, D., Zhao, X. H., Cui, Y., Zhang, T. T., Wang, F., and Hu, Y. H. (2018). Efficacy and safety of Tripterygium wilfordii Hook F for CKD in mainland China: a systematic review and meta-analysis. Phytotherapy Res. 32 (3), 436–451. doi:10.1002/ptr.5987
Wang, J., and Zhu, X. M. (2019). Effect of Tripterygium wilfordii glycosides on proteinuria level and renal function in diabetic nephropathy. Strait Pharm. J. 31 (4), 159–161.
Wang, L. (2017a). Effect of Tripterygium wilfordii polyglycoside combined with Benazepril in the treatment of diabetic nephropathy proteinuria, 24 h urinary protein content and 24 h urinary microalbumin excretion. Guide China Med. 15 (34), 110. doi:10.15912/j.cnki.gocm.2017.34.088
Wang, M. (2016). Clinical effects of tripterygium glycosides combined with valsartan in treatment of diabetic nephropathy. Diabetes New World 19, 31–32. doi:10.16658/j.cnki.1672-4062.2016.17.031
Wang, M. L., and Zhang, C. (2012). Clinical observation of proteinuria of type 2 diabetic nephropathy under the treatment of valsartan combined with tripterygium glycosides. Chin J Clin. Ration. Drug Use 5 (8A), 84–85. doi:10.15887/j.cnki.13-1389/r.2012.22.085
Wang, T. (2017b). Clinical observation of Tripterygium glycosides combined with benazepril in the treatment of proteinuria on diabetic nephropathy. J. China Prescr. Drug 15 (5), 51–52.
Wang, Y. Q., and Xiao, Q. L. (2013). Clinical observation of proteinuria of type 2 diabetic nephropathy under the treatment of tripterygium glycosides combined with telmisartan. Chin. Med. Mod. Distance Educ. China 11 (4), 45–46.
Wu, C. Z. (2010). Double dose tripterygium glycosides combined with telmisartan in treatment of 32 cases of early diabetic nephropathy. Her. Med. 29 (9), 1173–1175.
Wu, S. B., Cao, Z. D., Zhang, W., and Qi, Q. Q. (2012). Clinical observation of Tripterygium glycosides combined with valsartan in the treatment of diabetic nephropathy with massive albuminuria. Mod. J. Integr. Traditional Chin. West. Med. 21 (4), 394–395.
Wu, W., Yang, J.-J., Yang, H.-M., Huang, M.-M., Fang, Q.-J., Shi, Ge, et al. (2017). Multi-glycoside of Tripterygium wilfordii Hook. f. attenuates glomerulosclerosis in a rat model of diabetic nephropathy by exerting anti-microinflammatory effects without affecting hyperglycemia. Int. J. Mol. Med. 40 (3), 721–730. doi:10.3892/ijmm.2017.3068
Wu, Y. P., and Shi, N. C. (2018). Clinical observation on the effect of Valsartan combined with Tripterygium glycosides in the treatment of diabetic nephropathy stage IV. Chin. Remedies Clin. 18 (5), 753–754.
Xu, B. X. (2014). Clinical analysis of Tripterygium wilfordii polyglycoside combined with Benazepril in the treatment of diabetic nephropathy albuminuria. J. Bethune Med. Sci. 12 (1), 90–91. doi:10.16485/j.issn.2095-7858.2014.01.038
Xu, C., Qi, A. R., and F, B. Z. L. (2016). Clinical observation on treating diabetic nephropathy during IV period with the tripterygium glycosides tablets. Clin. J Chin. Med. 8 (17), 95–96.
Yan, Y. C. (2017). Effects of tripterygium wilfordii extract on T lymphocytes and urinary microprotein in patients with diabetic nephropathy. Lingnan J Emerg. Med. 22 (4), 356–359.
Yang, L. H. (2016). Losartan combined with tripterygium glycosides for treatment of patients with stage IV diabetic nephropathy. Proc. Clin. Med. 25 (7), 553–554. doi:10.16047/j.cnki.cn14-1300/r.2016.07.029
Yang, W. F., Jiang, Y., and Yang, H. C. (2018). Effect of Tripterygium wilfordii polyglycoside combined with Benazepril on diabetic nephropathy albuminuria. Chin. J. Mod. Drug Appl. 12 (11), 134–136. doi:10.14164/j.cnki.cn11-5581/r.2018.11.075
Zhang, H. C. (2015). Tripterygium glycosides tablet for treatment of 66 cases of stage IV diabetic nephropathy. China Pharm. 24 (24), 248–249.
Zhang, Y. J., Sun, Y. P., and Liu, D. (2012). Observation of curative effect on type 2 diabetic nephropathy treated with tripterygium glycosides and irbesartan. China Med. Her. 9 (34), 73–74.
Zhao, C., Yu, W., Ren, X., Zhang, W., and Li, H. (2014). Effects of tripterygium wilfordii polyglucoside on expression of Activin A and influence of transdifferentiation in renal tubulointerstitium of rats with diabetic nephropathy. Zhonghua Yi Xue Za Zhi 13 (18), 1427–1432.
Zhao, R. Y., Tang, B. S., Shi, X. L., Lu, S. K., Ye, Q. K., Lin, Y. Q., et al. (2011). Clinical observation of tripterygium glycosides combined with valsartan in treatment of 46 cases diabetic nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 12 (9), 811–813.
Zheng, C. X., Chen, Z. H., Zeng, C. H., Qin, W. S., Li, L. S., and Liu, Z. H. (2008). Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 74, 596–612. doi:10.1038/ki.2008.203
Zhou, C. L. (2013). Clinical observation of massive proteinuria of diabetic nephropathy under the treatment of tripterygium glycosides combined with valsartan. For All Health 7 (3), 94–95.
Keywords: diabetic nephropathy, tripterygium glycosides, ACEI/ARB, meta-analysis, efficacy
Citation: Yao Z, Wang P, Fu Q, Song Q, Xu H and Zhang P (2025) Efficacy and safety of tripterygium glycosides combined with ACEI/ARB on diabetic nephropathy: a meta-analysis. Front. Pharmacol. 15:1493590. doi: 10.3389/fphar.2024.1493590
Received: 09 September 2024; Accepted: 31 December 2024;
Published: 17 January 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Dorin Dragoş, Carol Davila University of Medicine and Pharmacy, RomaniaKrishnamurthy Nakuluri, The University of Iowa, United States
Copyright © 2025 Yao, Wang, Fu, Song, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, emhhbmdwZW5nQGZtbXUuZWR1LmNu
 Zhuan’E. Yao
Zhuan’E. Yao Pengbo Wang2
Pengbo Wang2 Peng Zhang
Peng Zhang