- 1School of Chinese Materia Medica, Chongqing University of Chinese Medicine, Chongqing, China
- 2College of Pharmaceutical Sciences and Chinese Medicine, Southwest University, Chongqing, China
- 3Traditional Chinese Medicine Industry Center of Xiushan Tujia & Miao Autonomous County, Agriculture and Rural Affairs Committee of Xiushan Tujia & Miao Autonomous County, Chongqing, China
- 4Department of Obstetrics and Gynecology, First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Pharmacy, Chongqing Hospital of Traditional Chinese Medicine, Chongqing, China
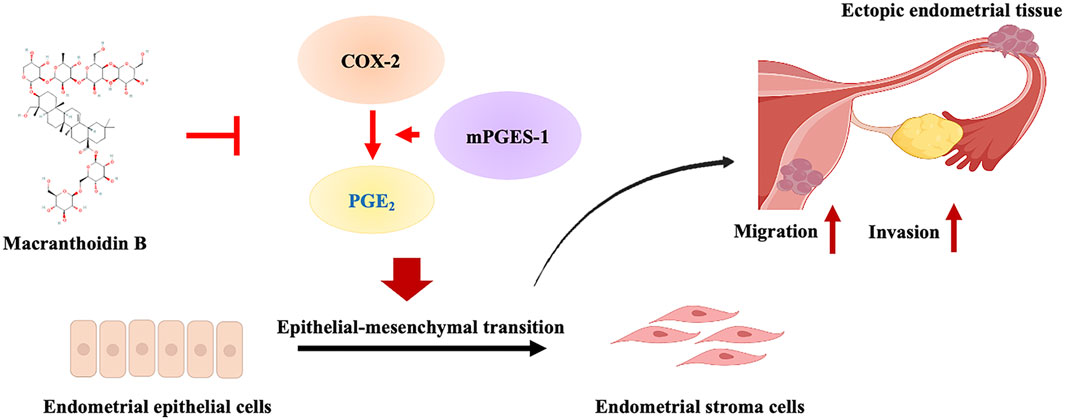
Introduction: Macranthoidin B is one of the primary and unique triterpenoid saponin metabolites from Lonicera macranthoides Hand. –Mazz, which is used to treat endometriosis (EMS) in traditional Chinese medicine. However, the effect of macranthoidin B remains unknown in EMS. This study aimed to elucidate the effect and mechanism of macranthoidin B in EMS.
Methods: Using rat autograft EMS model, the volume of ectopic endothelium, the histopathology, serum E2 and PROG were evaluated after macranthoidin B’s treatment. In primary endometriotic stromal and HEC1-B cells, the invasion and metastasis were assessed by scratch wound and Transwell tests. The epithelial-mesenchymal transition and COX-2/PGE2 pathway were examined in vivo and in vitro. Macranthoidin B were combined with LPS or celecoxib.
Results: In a rat autograft EMS model, macranthoidin B suppressed ectopic lesion volume, improved histopathological morphology, and regulated serum estradiol (E2) and progesterone (PROG) levels. Additionally, macranthoidin B inhibited invasion and metastasis of primary endometriotic stromal cells and HEC1-B cells. Mechanistically, macranthoidin B suppressed COX-2/PGE2 pathway and epithelial-mesenchymal transition both in vivo and in vitro. LPS, the COX-2/PGE2 pathway activator, showed the promotion of epithelial-mesenchymal transition, invasion and metastasis. Macranthoidin B exhibited the antagonistic effects against LPS. Celecoxib, the COX-2/PGE2 pathway inhibitor, restrained the epithelial-mesenchymal transition, invasion and metastasis. This effect of celecoxib was enhanced by macranthoidin B.
Discussion: Macranthoidin B prevents epithelial-mesenchymal transition through COX-2/PGE2 pathway in EMS. It will facilitate the macranthoidin B’s development and broaden its potential application.
1 Introduction
In traditional Chinese medicine, Lonicera macranthoides Hand. –Mazz shows its anti-inflammatory properties in the clinical treatment of EMS (Liu and Shi, 2020; Chen and Xu, 2022). Previously, both its extract and its saponin metabolites downregulate COX-2 and PGE2 in inflammatory models and macrophage cells (Guan et al., 2014; Zeng et al., 2020a). Macranthoidin B, one of the main triterpenoid saponin metabolites, exhibits limited activities in cancer, such as the proliferative reduction, apoptosis and oxidant stress enhancements (Fan et al., 2018; Tan et al., 2023). However, the effect and mechanism of macranthoidin B had not been clarified in EMS.
EMS is an estrogen-dependent inflammatory disorder of the endometrium that is characterized by the presence of functionally active endometrial tissue growing outside the uterus. High E2 and dysregulated PROG productions are the consistently observed endocrine feature of EMS (Chantalat et al., 2020; MacLean and Hayashi, 2022). EMS is associated with the increase in the risk of epithelial ovarian cancer (Vercellini et al., 2014). Now, nonsteroidal anti-inflammatory drugs (NSAIDs) and hormonal therapies are the main therapeutic options in clinic (Taylor et al., 2021a). As a prevalent gynecological disorder, the activation of epithelial-mesenchymal transition promotes invasion and metastasis in EMS (Zhang C. et al., 2021). The process of epithelial-mesenchymal transition is characterized by a decrease in E-cadherin and an increase in Vimentin, N-cadherin, Twist, Snail, Slug, and Zeb1/2, which allows cells to invade and metastasize (Debnath et al., 2022). As a common gynecological inflammatory disease, EMS is accompanied with COX-2/PGE2 pathway upregulation (Taylor et al., 2021a). In recent years, COX-2/PGE2 pathway has been implicated in promoting epithelial-mesenchymal transition of tumors (Zhang H. et al., 2021; Zhao et al., 2024). However, it is unknown whether COX-2/PGE2 pathway regulates epithelial-mesenchymal transition in EMS.
This study aimed to determine the therapeutic effects of macranthoidin B on EMS in rat EMS model and endometrial cells. The impacts of macranthoidin B on COX-2/PGE2 pathway, epithelial-mesenchymal transition, invasion and metastasis were investigated both in vivo and in vitro. Additionally, the effects of macranthoidin B, combined with a COX-2/PGE2 pathway activator or inhibitor, were assessed separately in epithelial-mesenchymal transition, invasion and metastasis.
2 Methods
2.1 Chemicals
Macranthoidin B (20200822, purity ≥98%, Nanjing Spring and Autumn Biological Engineering Co., Ltd., China) was dissolved separately in physiological saline or PBS for rat administration or endometrial cells treatment (Figure 1A). LPS (BS904, purity ≥98%, Sigma-Aldrich, United States) or celecoxib (EA9450, Pfizer Inc., United States) was dissolved in PBS or DMSO, respectively. Gestrinone was selected as a standard (53200201, Zizhu Pharmaceutical Co., Ltd., Beijing, China).
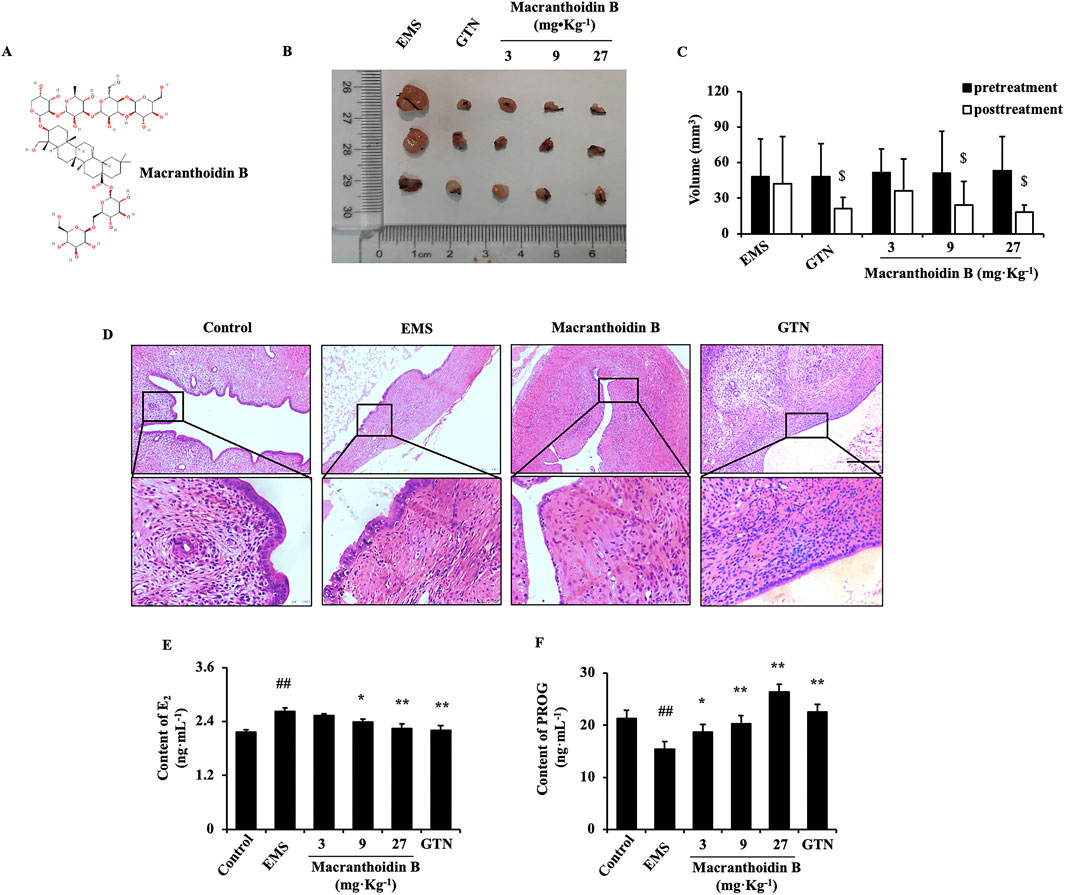
Figure 1. Macranthoidin B inhibited the growth of endometriotic tissues in vivo (A) The chemical structure of macranthoidin B (B, C) The volume of isolated ectopic lesions were calculated by vernier caliper after 28 days treatment. (D) Using H&E staining, pathological changes were observed. (E, F) E2 and PROG levels in serum were investigated by ELISA assay. $P< 0.05 to pretreatment, #P< 0.05 to control, ##P< 0.01 to control, *P< 0.05 to EMS, **P< 0.01 to EMS. Columns, mean (n = 5). Bars, SD. Scale bar = 100 μm. EMS, endometriosis; GTN, gestrinone.
2.2 Rat autograft EMS model
Female SD rats, weighing 180–220 g and aged 6–7 weeks, were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd. [Certification No: SCXK (Xiang) 2019-0004]. The rats were housed at a temperature of 20°C ± 2°C with a 12-h light/dark cycle and had free access to food and water at the Experimental Center, College of Pharmaceutical Sciences, Southwest University. The experimental protocol was approved by the Experimental Animal Ethics Review Committee of Southwest University (Approval No. IACUC-20210130-04), ensuring compliance with anesthesia and humane methods to minimize suffering.
The molding method and criteria of autograft EMS model were established according to the previous protocols (Dai et al., 2023). In summary, the left uterus of female rats was ligated, excised, and placed in a saline dish. It was then cut into approximately 5 mm2 endometrial segments, which were sutured to the right abdominal wall. After 28 days, the volume of ectopic tissue was measured by a vernier caliper with the formula (0.52 × length × width × height). The autograft section grew very well, for example, volume >8 mm3, blood vessels, and inflammatory encapsulation. It indicated the successfully established model. 30 female SD rats were operated for EMS establishment. The successful 25 EMS rats were randomly divided into EMS, 3, 9, 27 mg kg−1 macranthoidin B, and 0.5 mg kg−1 gestrinone groups. There were 5 rats per group. There were no significant differences in endometriotic volume among the groups before treatment. Another 5 normal female rats were treated as control group. Physiological saline was administered in control and EMS groups. All above groups were administered consecutive 28 days by gavage.
2.3 Primary endometriotic stromal cells and HEC1-B cells culture
For primary endometriotic stromal cells (ESCs), methods of cell acquisition and culture followed those outlined in a previous study (Dai et al., 2023). The study was approved by the First Affiliated Hospital of Chongqing Medical University (Permission 2019-059). Briefly, fresh ovarian EMS tissues were minced and digested by 0.1% collagenase I (Sigma-Aldrich, United States) for 90 min at 37°C. After separated by a 400 screen mesh, primary ESCs were cultured within DMEM/F12 medium (Gibco, NY, United States) supplemented with 10% FBS (Hyclone, Logan, United States) at 37°C with 5% CO2.
The endometrial cancer cell line, named HEC1-B, was purchased from Chinese Type Culture Collections (Wuhan, China). HEC1-B cells were cultured in MEM medium (Gibco, Grand Island, NY, United States) with 10% FBS (Hyclone, Logan, United States) at 37°C with 5% CO2.
2.4 Hematoxylin and eosin (H&E) staining, ELISA assay and immunofluorescence staining
For H&E staining, eutopic endometrium and ectopic endometrium were collected and fixed in paraformaldehyde in the end of administration. Then sections were stained and photographed under a microscope (DFC310 FX, Leica, Germany).
For ELISA assay, E2 and PROG detection in the serum proceed according to the published paper (Zhang C. et al., 2021). The PGE2 levels of ectopic endometrial tissue or cell supernatant were examined by rat or human PGE2 ELISA kits. All kits were used according to the instructions (SinoBestBio, Shanghai, China).
For immunofluorescence staining, rabbit anti-E-cadherin or anti-Vimentin antibodies (1:200 dilution; Proteintech, China) were applied to the endometrial tissue. Afterward, the tissues were treated with Alexa Fluor 594 or FITC-labeled goat anti-rabbit IgG secondary antibody (1:100 dilution; Beyotime, China). The sections were then counterstained with DAPI (Beyotime, China) and analyzed under a Leica microscope (Germany).
2.5 Cell viability assay
The previously reported method was employed for cell viability assay (Dai et al., 2023). Briefly, cells in 96-well plate were treated with macranthoidin B for 24 h. Then, MTT was used for incubation, and a microplate reader was performed for measurement. The 24 h IC50 values were calculated by GraphPad Prism Software (7.04 version, United States).
2.6 Scratch wound and transwell assays
The scratch wound and Transwell assays were conducted following the previous methodology (Zhang C. et al., 2021). For scratch wound assay, 6-9×104 cells were cultured in 24-well plate until the 80% density. After being scratched by 1 mL pipette tip, cells were treated with macranthoidin B and observed under a microscope. Migration rate = (average scratch area at 0 h - average scratch area at 24 h)/average scratch area at 0 h × 100 %.
For the Transwell assay, the upper chamber included 3-5×104 endometrial cells and serum-free medium in matrigel-coated Transwell inserts (Corning, New York, United States), while 10% FBS medium was added to the lower chamber. After 24 h, cell numbers in the bottom were fixed and counted in 3-5 random fields at ×100 magnification.
2.7 RT-qPCR
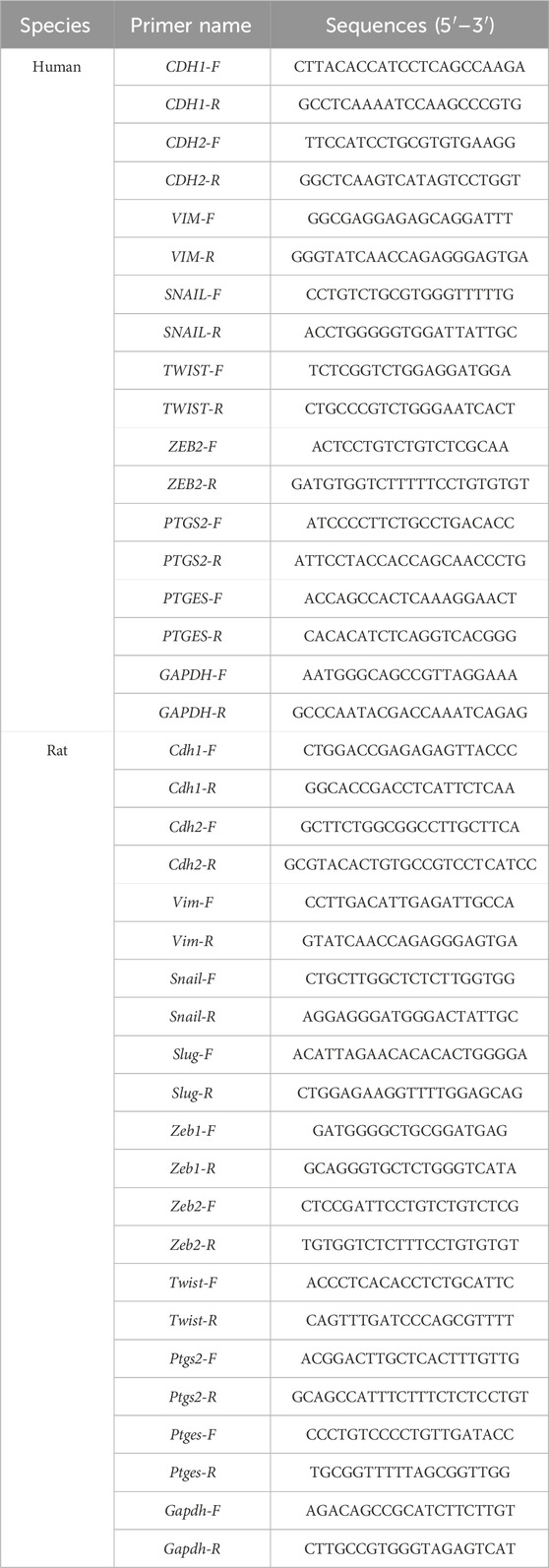
RNA isolation and cDNA synthesis were adhered to the previous approach (Zhang C. et al., 2021). SYBR™ Green Master Mix (Thermo Fisher, Waltham, United States) was used for gene expression with 2−ΔΔCT calculation. Primer sequences of mRNA were produced by Shenggong Biotechnology (Shanghai, China) (Table 1).
2.8 Western blotting
Briefly, the protein of cells and tissues were lysed and extracted by RIPA (Dingguo Changsheng, China). Then the proteins were separated by SDS-PAGE, and transferred to PVDF membrane (Millipore, Billerica, United States). Proteins on the membrane were bound with the primary antibodies and HRP-labeled goat anti-rabbit secondary antibody (Table 2). The membranes were reacted with electrochemiluminescence (ECL) enhanced Western blotting Substrate (Bioground Biotechnology Co, Ltd., China) for chemiluminescence. Finally, the protein bands were visualized in Tanon 5200 imaging system (Tanon, China).
2.9 Statistical analysis
All data were represented by mean ± SD. Two sets of data were compared with unpaired t-tests. The volume of ectopic endometrium pre- and post-treatment were compared with paired t-tests. Three or more sets of data were compared with one-way ANOVA. SPSS 26.0 statistical software was used for analysis. P< 0.05 indicated a significant difference.
3 Results
3.1 Macranthoidin B inhibited ectopic lesions in autograft EMS model
After continuous gavage for 28 days, the volume of ectopic endometrium was evaluated and compared with the pretreatment. Administration of 9 or 27 mg kg−1 macranthoidin B resulted in smaller transplants with less adhesion and fewer surface blood vessels. Gestrinone diminished the volume of ectopic endometrial tissues (Figures 1B, C). In H&E staining, the ectopic endometrium in EMS exhibited a similar structure to the normal endometrium in control. The cortex invagination formed more pseudo glands with abundant blood vessels and inflammatory cell infiltrations. Macranthoidin B or gestrinone ameliorated the ectopic pathological morphology, with fewer and smaller pseudo glands, fewer micro vascularity and inflammatory infiltrations (Figure 1D). In EMS, E2 in serum were remarkably enhanced, accompanied with the decrease of PROG. Macranthoidin B diminished E2 and raised PROG levels (Figures 1E, F). Notably, macranthoidin B had no influence on rat weight during the treatment (Supplementary Figure S1A).
3.2 Macranthoidin B prohibited epithelial-mesenchymal transition and COX-2/PGE2 pathway in vivo
The expression of the epithelial gene Cdh1 was low in the EMS group, while the mesenchymal genes were increased, such as Cdh2, Vim, Twist, Slug, Snail, and Zeb1/2. Macranthoidin B obviously reversed the change of gene expression (Figures 2A–D). Furthermore, the E-cadherin protein was remarkably reduced in the EMS group, while N-cadherin and Vimentin proteins were increased. Macranthoidin B significantly promoted the protein levels of E-cadherin, accompanied by downregulation of N-cadherin and Vimentin (Figures 2E–G). In immunofluorescence assay, the downregulation of E-cadherin and the upregulation of Vimentin displayed in the ectopic tissues of EMS. E-cadherin was subsequently restored following macranthoidin B treatment, accompanied with the inhibition of Vimentin (Figure 2H).
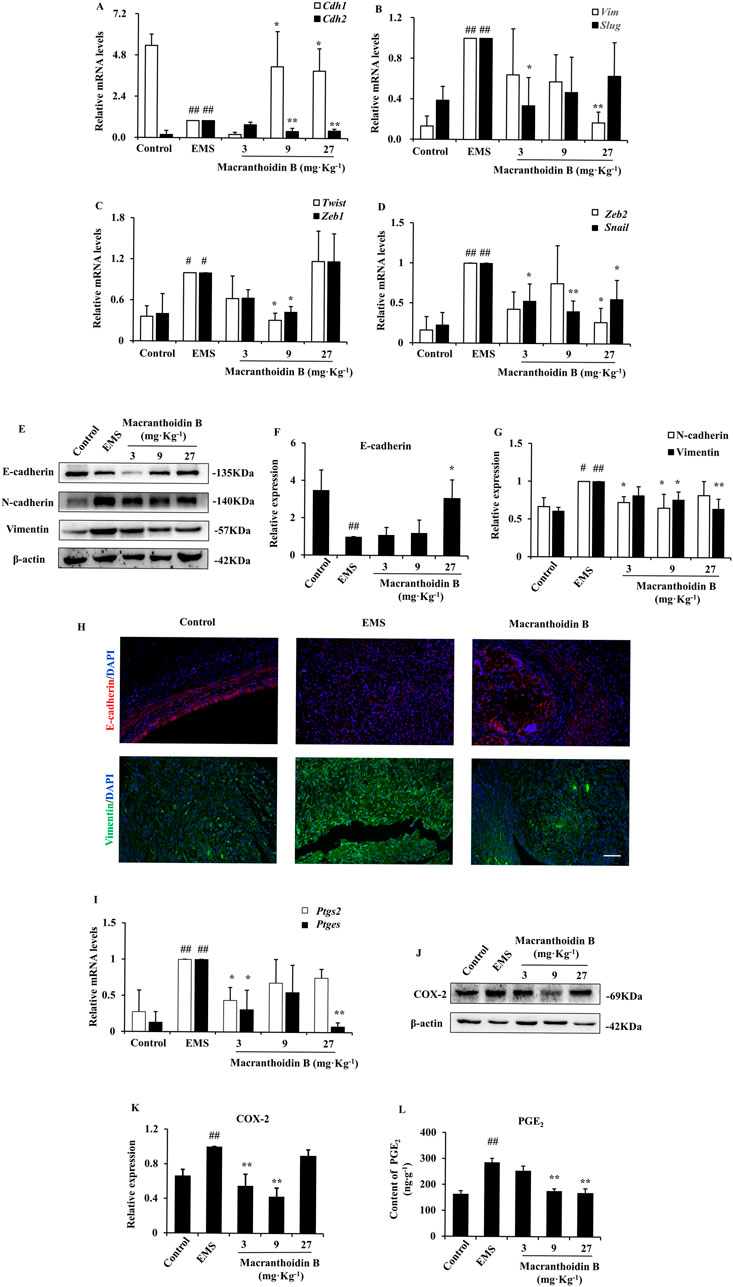
Figure 2. Macranthoidin B downregulated epithelial-mesenchymal transition and COX-2/PGE2 pathway in endometriotic tissues. (A–D) The mRNA levels of Cdh1, Cdh2, Vim, Twist, Slug, Snail, and Zeb1/2 were measured by RT-qPCR. (E–G) The protein levels of E-cadherin, N-cadherin, and Vimentin were detected by Western blotting assay. (H) Detection of E-caderin and Vimentin in ectopic tissue by immunofluorescence assay. (I) The gene expression of Ptgs2 and Ptges were tested by RT-qPCR. (J–L) The contents of COX-2 and PGE2 were analyzed by Western blotting and ELISA assays. #P< 0.05 to control, ##P< 0.01 to control, *P< 0.05 to EMS **P< 0.01 to EMS. Columns, mean (n = 3). Bars, SD. Scale bar = 100 μm. EMS, endometriosis.
Similarly, the mRNA and protein levels of COX-2/PGE2 pathway were elevated in EMS compared with control. Using macranthoidin B, the mRNA levels of PTGS2 and PTGES were obviously decreased (Figure 2I). This was accompanied by a reduction in COX-2 and PGE2 protein contents (Figures 2J–L).
3.3 Restriction of cell invasion and metastasis by macranthoidin B
According to the 24 h IC50, the primary ESCs or HEC1-B cells were treated with three concentrations of macranthoidin B (Supplementary Figures S1B, C). In primary ESCs, 2, 1, and 0.5 time of 24 h IC50 were performed. HEC1-B cells were treated with 1/2, 1/4, and 1/8 of 24 h IC50. After 24 h treatment, cells were prevented to cicatrize (Figures 3A, B, E, F). Migrating cells across insert membrane were significantly decreased in macranthoidin B groups vs. control group (Figures 3C, D, G, H). All above data implied that cell invasion and metastasis were resisted by macranthoidin B, particularly at 250 μM in primary ESCs, at 360 μM in HEC1-B cells.
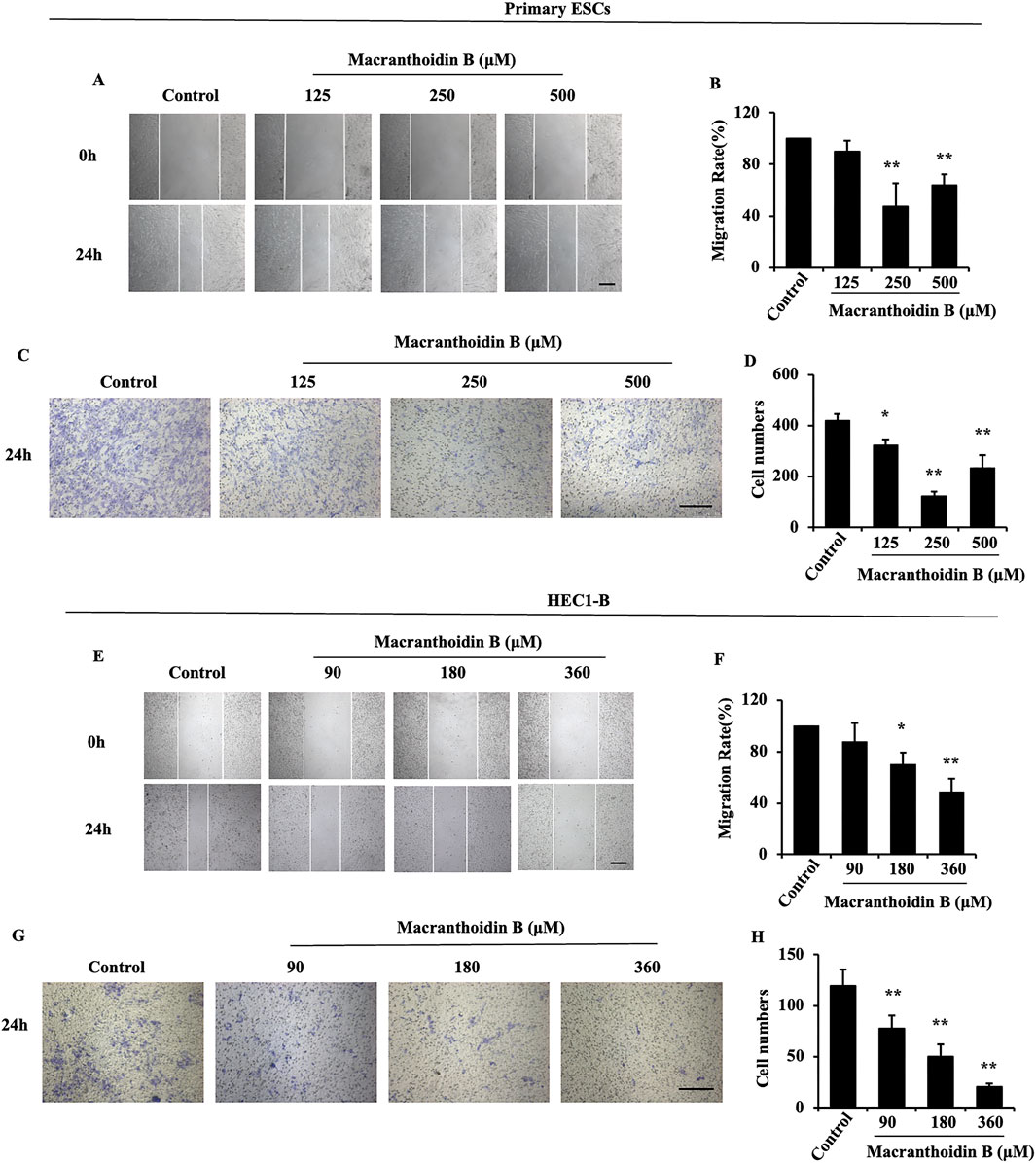
Figure 3. The suppression of invasion and metastasis by macranthoidin B in endometrial cells. (A–D) After being treated with macranthoidin B, the primary ESCs were tested in scratch wound and Transwell assays. (E–H) The cell migration and invasion ability of HEC1-B cells were observed in scratch wound and Transwell assays. *P< 0.05 to control, **P< 0.01 to control. Columns, mean (n = 3). Bars, SD. Scale bar = 100 μm. ESCs, endometriotic stromal cells.
3.4 Inhibition of epithelial-mesenchymal transition and COX-2/PGE2 pathway by macranthoidin B in vitro
In primary ESCs, CDH1 gene expression was expanded at 250 μM macranthoidin B. The mRNA levels of CDH2, VIM, TWIST, SNAIL, and ZEB2 were significantly reduced in macranthoidin B groups compared to control group (Figures 4A–C). Meanwhile, macranthoidin B exerted similar effect on HEC1-B cells (Figures 4K–M). Consequently, macranthoidin B increased the E-cadherin protein, and decreased the protein of mesenchymal markers, including N-cadherin and Vimentin in both types of endometrial cells (Figures 4D–F, N–P).
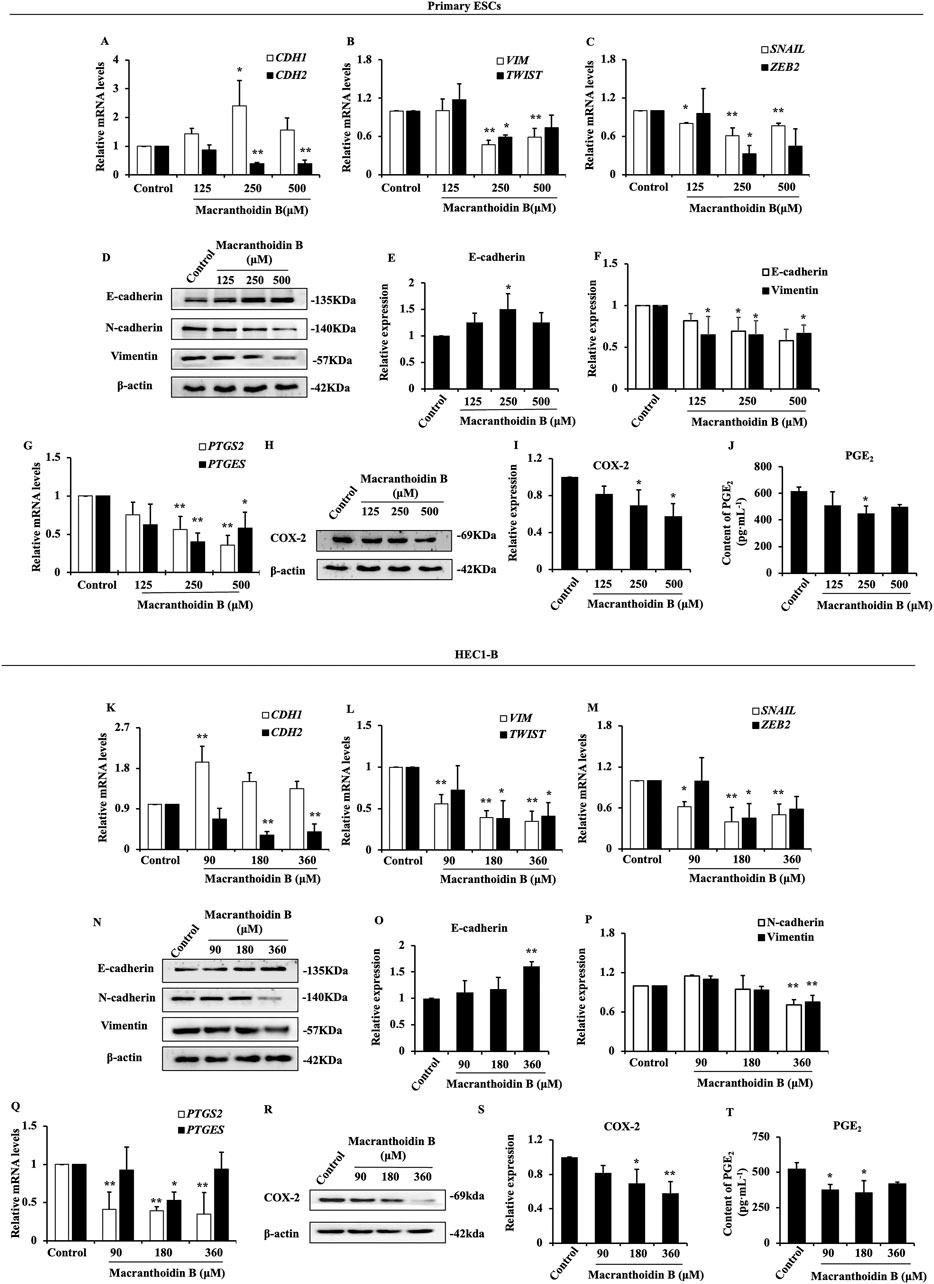
Figure 4. Macranthoidin B downregulated epithelial-mesenchymal transition and COX-2/PGE2 pathway in vitro. In primary ESCs (A–C) and HEC1-B cells (K–M), the mRNA levels of CDH1, CDH2, VIM, TWIST, SNAIL, and ZEB2 were measured by RT-qPCR. (D–F, N–P) The protein levels of epithelial-mesenchymal transition were tested by Western blotting assay. (G, Q) The gene expression of PTGS2 and PTGES were investigated by RT-qPCR. (H–J, R–T) The content of COX-2 and PGE2 were analyzed by Western blotting and ELISA assays. *P< 0.05 to control, **P< 0.01 to control. Columns, mean (n = 3). Bars, SD. ESCs, endometriotic stromal cells.
After using macranthoidin B for 24 h, there was a noticeable inhibitory effect on the gene expression of COX-2/PGE2 pathway in two endometrial cells (Figures 4G, Q). Subsequently, the levels of COX-2 and PGE2 proteins in endometrial cells were lessened in macranthoidin B groups compared with control group (Figures 4H–J, R–T).
3.5 Macranthoidin B restricted epithelial-mesenchymal transition induced by LPS
LPS is a common inflammatory activator, which can promote COX-2, PTGES, and PGE2 (Alqinyah et al., 2023). In two types of endometrial cells, LPS stimulated the COX-2/PGE2 pathway, leading to epithelial mesenchymal transformation and enhancing invasive metastasis. Macranthoidin B reversed the LPS-activated COX-2/PGE2 pathway and epithelial-mesenchymal transition (Figures 5A, B). At the same time, invasion and metastasis were inhibited by macranthoidin B in scratching wound and Transwell assays (Figures 5C–J). Therefore, macranthoidin B exhibited the antagonistic effect on COX-2/PGE2 pathway, epithelial-mesenchymal transition, invasion and metastasis activated by LPS.
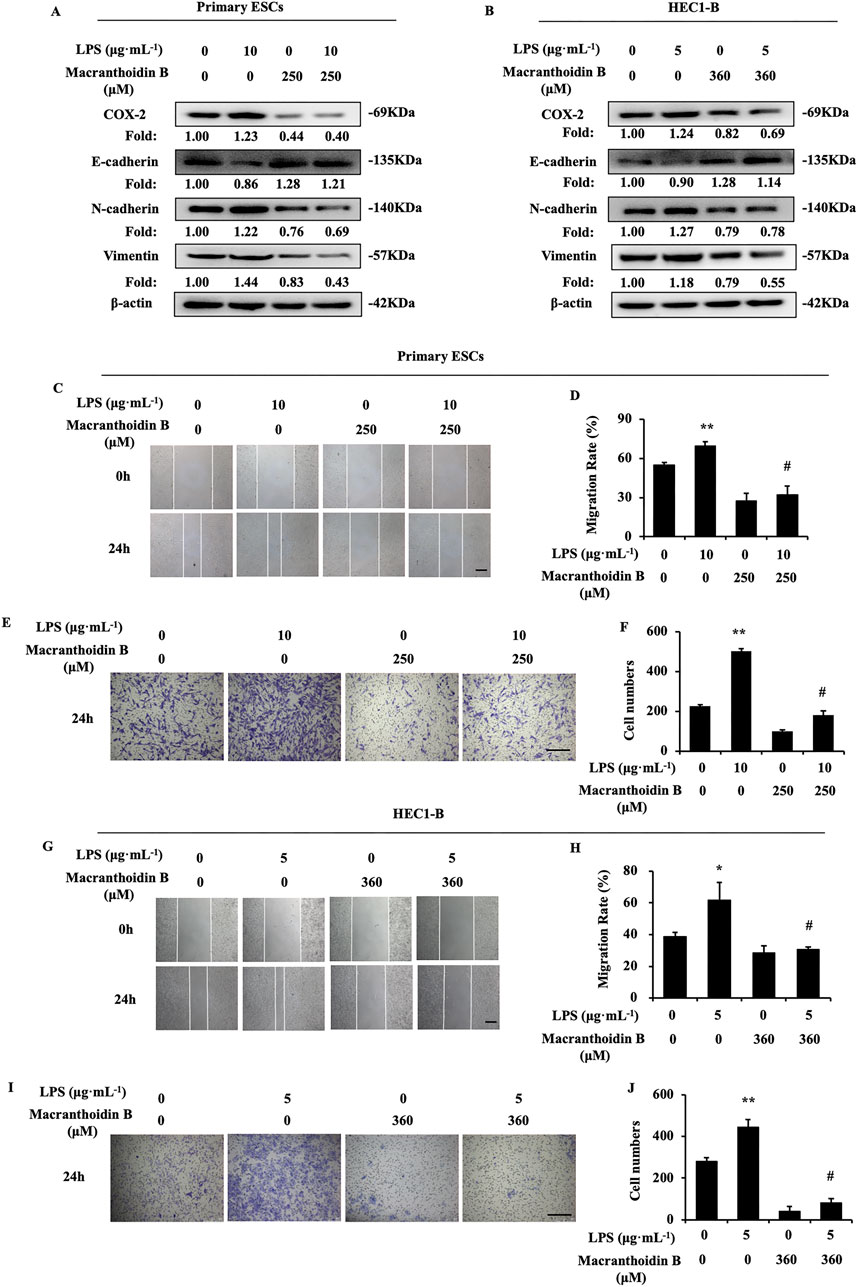
Figure 5. Macranthoidin B reversed LPS-induced activation of invasion and metastasis. (A, B) After using LPS or macranthoidin B, the expression of COX-2/PGE2 and epithelial-mesenchymal transition pathways were observed by Western blotting. (C–J) Cell migration and invasion ability were measured by scratch wound and Transwell assays in 24 h *P< 0.05 to control, **P< 0.01 to control. #P< 0.01 to LPS. Columns, mean (n = 3). Bars, SD. Scale bar = 100 μm. ESCs, endometriotic stromal cells.
3.6 Synergism of macranthoidin B and celecoxib on epithelial-mesenchymal transition
Celecoxib is considered as a selective COX-2 inhibitor (Porat et al., 2023). In our study, celecoxib suppressed COX-2/PGE2 pathway and epithelial-mesenchymal transition in endometrial cells. Then, celecoxib restricted migration and invasion. Simultaneously, macranthoidin B not only enhanced celecoxib-mediated inhibition of COX-2/PGE2 pathway, but also augmented its effect on E-cadherin, N-cadherin, and Vimentin (Figures 6A, B). Cell migration and invasion were prevented by macranthoidin B and celecoxib (Figures 6C–J). In brief, macranthoidin B synergistically improved the celecoxib’s downregulation effect, especially in COX-2/PGE2 pathway and epithelial-mesenchymal transition.
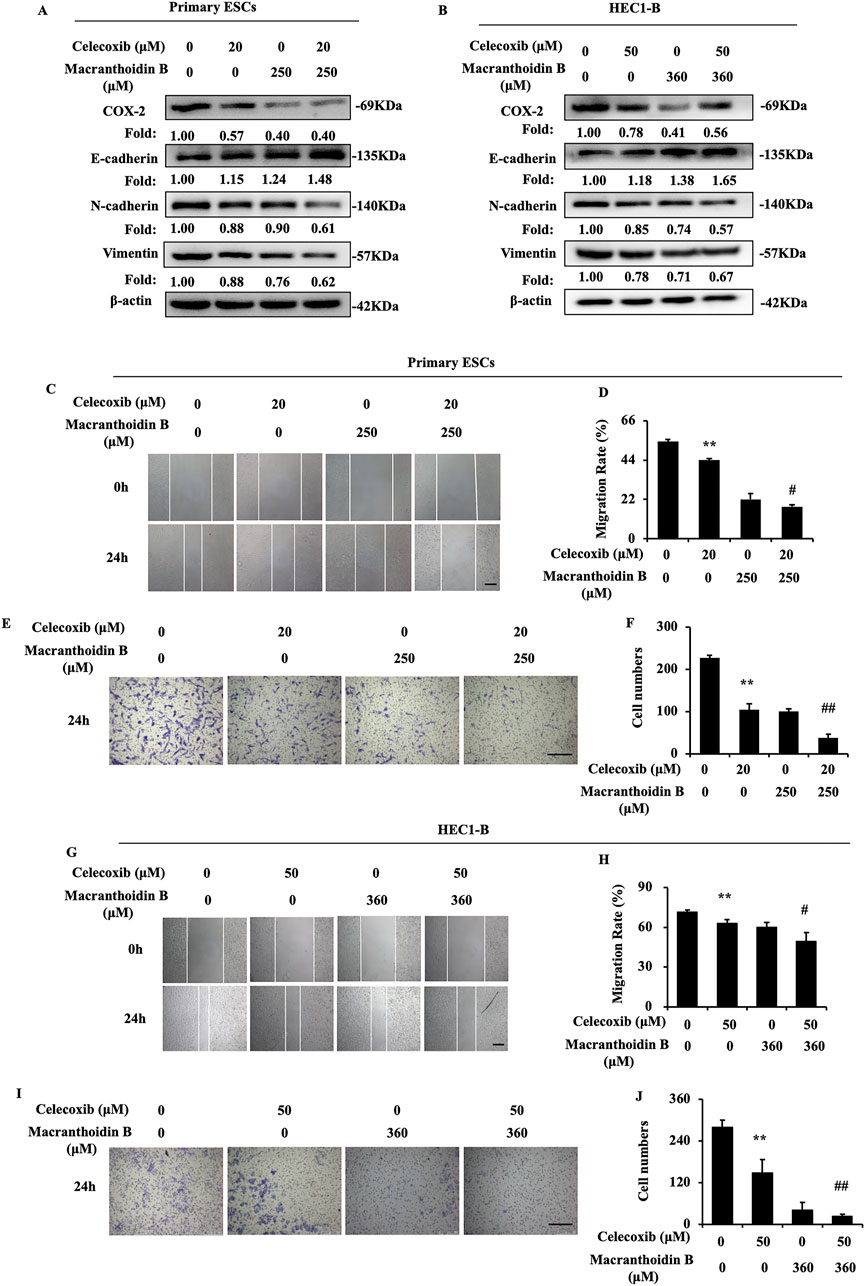
Figure 6. Macranthoidin B enhanced celecoxib-mediated attenuation of invasion and metastasis. (A, B) The protein levels of COX-2/PGE2 and epithelial-mesenchymal transition pathways were investigated by Western blotting after celecoxib or macranthoidin B treatment. (C–J) Cell migration and invasion ability were measured by scratch wound and Transwell assays in 24 h *P< 0.05 to control, **P< 0.01 to control. #P< 0.05 to celecoxib, ##P< 0.01 to celecoxib. Columns, mean (n = 3). Bars, SD. Scale bar = 100 μm. ESCs, endometriotic stromal cells.
4 Discussion
In this study, macranthoidin B significantly reduced the formation and ameliorated the pathological structure of ectopic endometrium. Moreover, macranthoidin B also decreased E2 and raised PROG levels. In addition, macranthoidin B hindered invasion and metastasis of endometrial cells. This was attributed to the blockade of epithelial-mesenchymal transition via COX-2/PGE2 pathway.
COX-2/PGE2 pathway is an important inflammatory pathway, in which COX-2 acts with mPGES-1 to mediate PGE2 synthesis (Akasaka and Ruan, 2016). COX-2/PGE2 pathway can influence the epithelial-mesenchymal transition in cancer. Activation of COX-2/PGE2 pathway causes the loss of the epithelial E-cadherin while it is boosting mesenchymal N-cadherin and Vimentin. Moreover, AGR2 and PGE2 receptor EP2 mediate the PGE2-induced epithelial-mesenchymal transition through regulating Snail (Cheng et al., 2014; Zhang H. et al., 2021). These changes facilitate the occurrence of epithelial-mesenchymal transition, thereby enhance the invasion and metastasis abilities (Che et al., 2017; Gómez-Valenzuela et al., 2021). This indicates a significant correlation between the COX-2/PGE2 pathway and epithelial-mesenchymal transition. EMS is a chronic inflammatory gynecological disease that affects many women of reproductive age. Clinical data indicate increasing COX-2/PGE2 expression in EMS patients (Cho et al., 2010; Peng et al., 2018). During EMS progression, the COX-2/PGE2 pathway indirectly promotes angiogenesis at ectopic sites (Jana et al., 2016). Recently, epithelial-mesenchymal transition has been proved to be the vital step in EMS progression (Zhang C. et al., 2021). Moreover, PGE2 also stimulates migration and epithelial-mesenchymal transition in normal endometrial epithelial cells (Kusama et al., 2021). Our investigation discovered overexpression of COX-2/PGE2 pathway and epithelial-mesenchymal transition in autograft EMS model. COX-2/PGE2 pathway was activated by LPS. Then epithelial-mesenchymal transition, invasion and metastasis were induced in endometrial cells. Conversely, using celecoxib, the downregulation of COX-2/PGE2 pathway led to the suppression of epithelial-mesenchymal transition, invasion and metastasis. However, further research is required to confirm the relationship in human EMS tissues or other EMS animal models.
Lonicera macranthoides Hand. –Mazz, also called Lonicerae Flos, has demonstrated pharmacological properties, such as antibacterial, antiviral, antipyretic, and anti-inflammatory activities (Li et al., 2020). Initially, Lonicerae Flos extract exhibits the anti-inflammatory action through the COX-2 reduction in the animal inflammation model and LPS-induced cellular model (Zeng et al., 2020a; Zeng et al., 2020a). The saponins are considered as the important material basis of anti-inflammatory effect. Previous studies has shown that both Lonicerae Flos and its saponins can suppress COX-2/PGE2 pathway (Guan et al., 2014; Zeng et al., 2020b). Lonimaranthoide VI, a saponin metabolite in Lonicerae Flos, also displays anti-inflammatory activity by regulating COX-2-induced PGE2 synthesis (Guan et al., 2014). Furthermore, macranthoidin B is one of the main triterpenoid saponin metabolites in Lonicera macranthoides Hand. –Mazz. Limited pharmacological studies indicate that it exhibits the anti-tumor effects (Fan et al., 2018; Tan et al., 2023). In our research, the effect of macranthoidin B on EMS was uncovered both in vivo and in vitro. Its mechanism involved the suppression of COX-2/PGE2 pathway, which subsequently caused the downregulation of epithelial-mesenchymal transition, invasion and metastasis. Notably, the dose levels of macranthoidin B lacked the dose response in primary ESCs. It might be related to the high selection of doses as a limitation. It is worthwhile to find more suitable doses and explore other mechanisms of macranthoidin B in EMS. Furthermore, paeonol, imperatorin, ginsenoside Rg3, ferulic Acid, ligustrazine, and tetrahydropalmatine also show the inhibition in EMS (Huang et al., 2020; Ma et al., 2021; Pang et al., 2021; Zhang C. et al., 2021). So it needs to screen more potential natural metabolites in the future.
In addition, EMS is an estrogen-dependent disease (Marquardt et al., 2019). E2 secretion in EMS is higher, despite of lower PROG secretion (Patel et al., 2017; Marquardt et al., 2019). Estrogen generation in ectopic endometrium can activate COX-2 and accelerate prostaglandin synthesis. This leads to a positive feedback loop, enhancing estrogen production and inflammation, which further promotes the EMS progression (Bulun et al., 2012; Takaoka et al., 2018). In accordance with these studies, we also detected the increasing E2 and decreasing PROG in EMS serum. The overexpression of COX-2/PGE2 pathway was found in ectopic endometrium. Macranthoidin B treatment resulted in the opposite changes of E2 and PROG, alongside the downregulation of COX-2/PGE2 pathway. Therefore, the mechanism of macranthoidin B can be further investigated in the regulation of the relationship between COX-2/PGE2 pathway and E2.
In summary, macranthoidin B showed the constraint on EMS progression. Macranthoidin B inhibited epithelial-mesenchymal transition via COX-2/PGE2 pathway. These findings provide a theoretical basis for the further development of macranthoidin B.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by The Experimental Animal Ethics Review Committee of Southwest University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YD: Conceptualization, Data curation, Methodology, Writing–original draft, Writing–review and editing. XY: Conceptualization, Data curation, Methodology, Writing–original draft, Writing–review and editing. QWe: Conceptualization, Data curation, Methodology, Writing–original draft, Writing–review and editing. XB: Investigation, Methodology, Visualization, Writing–original draft. YZ: Investigation, Methodology, Visualization, Writing–original draft. YM: Investigation, Methodology, Visualization, Writing–original draft. MY: Investigation, Methodology, Visualization, Writing–original draft. XX: Investigation, Methodology, Visualization, Writing–original draft. CL: Conceptualization, Methodology, Supervision, Writing–review and editing. QWa: Conceptualization, Methodology, Supervision, Writing–review and editing. YC: Conceptualization, Methodology, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China [grant numbers 81773984, 81402441]; Traditional Chinese medicine research project of Chongqing Health Bureau [grant number 2020ZY023665]; Chinese Medicine Rehabilitation–the Key Discipline Constructed by Chongqing Health Bureau [grant number 2021-4322190044]; Chongqing Natural Science Foundation of China [grant number CSTB2024NSCQ-MSX0936]; the Natural Science Foundation of Chongqing-Postdoctoral Science Fundation Project [cstc2021jcyj-bshX0188]; Graduate Educational Reform Research Program of Chongqing College of Traditional Chinese Medicine [grant number yjsjg24003].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1492098/full#supplementary-material
Abbreviations
EMS, endometriosis; H&E, hematoxylin and eosin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide; ELISA, enzyme linked immunosorbent assay; RT-qPCR, Real Time Quantitative PCR; LPS, lipopolysaccharides; DMSO, dimethyl sulphoxide; PBS, phosphate buffered saline; COX-2, cyclooxygenase-2; PGE2, Prostaglandin E synthase 2; mPGES-1, microsomal prostaglandin E synthases 1; E2, estradiol; PROG, progestogen; ESCs, endometriotic stromal cells; ECL, electrochemiluminescence; NSAIDs, nonsteroidal anti-inflammatory drugs.
References
Akasaka, H., and Ruan, K.-H. (2016). Identification of the two-phase mechanism of arachidonic acid regulating inflammatory prostaglandin E2 biosynthesis by targeting COX-2 and mPGES-1. Archives Biochem. Biophysics 603, 29–37. doi:10.1016/j.abb.2016.04.011
Alqinyah, M., Alhamed, A. S., Alnefaie, H. O., Algahtani, M. M., Badr, A. M., Albogami, A. M., et al. (2023). Targeting store-operated calcium entry regulates the inflammation-induced proliferation and migration of breast cancer cells. Biomedicines 11, 1637. doi:10.3390/biomedicines11061637
Bulun, S. E., Monsavais, D., Pavone, M. E., Dyson, M., Xue, Q., Attar, E., et al. (2012). Role of estrogen receptor-β in endometriosis. Seminars Reproductive Med. 30, 39–45. doi:10.1055/s-0031-1299596
Chantalat, E., Valera, M. C., Vaysse, C., Noirrit, E., Rusidze, M., Weyl, A., et al. (2020). Estrogen receptors and endometriosis. Int. J. Mol. Sci. 21, 2815. doi:10.3390/ijms21082815
Che, D., Zhang, S., Jing, Z., Shang, L., Jin, S., Liu, F., et al. (2017). Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE2/β-catenin signalling pathway. Mol. Immunol. 90, 197–210. doi:10.1016/j.molimm.2017.06.018
Chen, X., and Xu, C. (2022). Research progres on treatment of endometriosis from blood stasis, heat and toxin. Asia-Pac Trad. Med. 18 (09), 182–186. doi:10.19945/j.cnki.issn.1006-3250.2022.07.014
Cheng, S. Y., Zhang, H., Zhang, M., Xia, S. K., Bai, X. M., Zhang, L., et al. (2014). Prostaglandin E₂ receptor EP2 mediates Snail expression in hepatocellular carcinoma cells. Oncol. Rep. 31, 2099–2106. doi:10.3892/or.2014.3074
Cho, S., Park, S. H., Choi, Y. S., Seo, S. K., Kim, H. Y., Park, K. H., et al. (2010). Expression of cyclooxygenase-2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecol. Obstet. Invest 69, 93–100. doi:10.1159/000261017
Dai, X. S., Wei, Q. H., Guo, X., Ding, Y., Yang, X. Q., Zhang, Y. X., et al. (2023). Ferulic acid, ligustrazine, and tetrahydropalmatine display the anti-proliferative effect in endometriosis through regulating Notch pathway. Life Sci. 328, 121921. doi:10.1016/j.lfs.2023.121921
Debnath, P., Huirem, R. S., Dutta, P., and Palchaudhuri, S. (2022). Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep. 42. doi:10.1042/BSR20211754
Fan, X., Rao, J., Zhang, Z., Li, D., Cui, W., Zhang, J., et al. (2018). Macranthoidin B modulates Key metabolic pathways to enhance ROS generation and induce cytotoxicity and apoptosis in colorectal cancer. Cell. Physiology Biochem. Int. J. Exp. Cell. Physiology, Biochem. Pharmacol. 46, 1317–1330. doi:10.1159/000489147
Gómez-Valenzuela, F., Escobar, E., Pérez-Tomás, R., and Montecinos, V. P. (2021). The inflammatory profile of the tumor microenvironment, orchestrated by cyclooxygenase-2, promotes epithelial-mesenchymal transition. Front. Oncol. 11, 686792. doi:10.3389/fonc.2021.686792
Guan, F., Wang, H., Shan, Y., Chen, Y., Wang, M., Wang, Q., et al. (2014). Inhibition of COX-2 and PGE2 in LPS-stimulated RAW264.7 cells by lonimacranthoide VI, a chlorogenic acid ester saponin. Biomed. Rep. 2, 760–764. doi:10.3892/br.2014.314
Huang, R., Chen, S., Zhao, M., Li, Z., and Zhu, L. (2020). Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. J. Gynecol. Obstet. Hum. Reprod. 49, 101642. doi:10.1016/j.jogoh.2019.101642
Jana, S., Chatterjee, K., Ray, A. K., Dasmahapatra, P., and Swarnakar, S. (2016). Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT Axis promotes angiogenesis in endometriosis. PLoS One 11, e0163540. doi:10.1371/journal.pone.0163540
Kusama, K., Fukushima, Y., Yoshida, K., Sakakibara, H., Tsubata, N., Yoshie, M., et al. (2021). Endometrial epithelial-mesenchymal transition (EMT) by menstruation-related inflammatory factors during hypoxia. Mol. Hum. Reprod. 27, gaab036. doi:10.1093/molehr/gaab036
Li, Y., Li, W., Fu, C., Song, Y., and Fu, Q. (2020). Lonicerae japonicae flos and Lonicerae flos: a systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 19, 1–61. doi:10.1007/s11101-019-09655-7
Liu, J., and Shi, Y. (2020). Shi yanping uses traditional Chinese medicine to treat endometriosis from immune inflammation. J. Pract. Traditional Chin. Intern. Med. 34 (03), 38–41. doi:10.13729/j.issn.1671-7813.z20190673
Ma, T., Liu, P., Wei, J., Zhao, M., Yao, X., Luo, X., et al. (2021). Imperatorin alleviated endometriosis by inhibiting the activation of PI3K/Akt/NF-κB pathway in rats. Life Sci. 274, 119291. doi:10.1016/j.lfs.2021.119291
Maclean, J. A., and Hayashi, K. (2022). Progesterone actions and resistance in gynecological disorders. Cells 11, 647. doi:10.3390/cells11040647
Marquardt, R. M., Kim, T. H., Shin, J.-H., and Jeong, J.-W. (2019). Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int. J. Mol. Sci. 20, 3822. doi:10.3390/ijms20153822
Pang, C., Wu, Z., Xu, X., Yang, W., Wang, X., and Qi, Y. (2021). Paeonol alleviates migration and invasion of endometrial stromal cells by reducing HIF-1α-regulated autophagy in endometriosis. Front. Biosci. Landmark Ed. 26, 485–495. doi:10.52586/4961
Patel, B. G., Rudnicki, M., Yu, J., Shu, Y., and Taylor, R. N. (2017). Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstetricia Gynecol. Scand. 96, 623–632. doi:10.1111/aogs.13156
Peng, B., Zhan, H., Alotaibi, F., Alkusayer, G. M., Bedaiwy, M. A., and Yong, P. J. (2018). Nerve growth factor is associated with sexual pain in women with endometriosis. Reprod. Sci. 25, 540–549. doi:10.1177/1933719117716778
Porat, D., Dukhno, O., Partook-Maccabi, M., Vainer, E., Cvijic, S., and Dahan, A. (2023). Selective COX-2 inhibitors after bariatric surgery: celecoxib, etoricoxib and etodolac post-bariatric solubility/dissolution and pharmacokinetics. Int. J. Pharm. 645, 123347. doi:10.1016/j.ijpharm.2023.123347
Takaoka, O., Mori, T., Ito, F., Okimura, H., Kataoka, H., Tanaka, Y., et al. (2018). Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid Biochem. Mol. Biol. 181, 125–132. doi:10.1016/j.jsbmb.2018.04.004
Tan, S., Liu, Q., Yang, J., Cai, J., Yu, M., and Ji, Y. (2023). Macranthoidin B (MB) promotes oxidative stress-induced inhibiting of hepa1-6 cell proliferation via selenoprotein. Biol. Trace Elem. Res. 201, 368–376. doi:10.1007/s12011-022-03120-x
Taylor, H. S., Kotlyar, A. M., and Flores, V. A. (2021a). Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet London, Engl. 397, 839–852. doi:10.1016/S0140-6736(21)00389-5
Vercellini, P., Vigano, P., Somigliana, E., and Fedele, L. (2014). Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10, 261–275. doi:10.1038/nrendo.2013.255
Zeng, A., Hua, H., Chen, C., Liu, L., Zhang, M., Luo, Y., et al. (2020a). Comparative study on anti-inflammatory effect of Lonicerae japonicae flos and Lonicerae flos. China J. Chin. Materia Medica 16, 196–202. doi:10.19540/j.cnki.cjcmm.20200520.401
Zeng, A., Hua, Y., Chen, C., Liu, L., Zhang, M., Luo, Y., et al. (2020b). Study on the anti-inflammatory pharmacological effects of Lonicera japonica and Lonicerae Flos. China J. Chin. Materia Medica 45, 3938–3944. doi:10.19540/j.cnki.cjcmm.20200520.401
Zhang, C., Zhang, Y., Pan, H., Tan, Y., Wei, Q., Dai, X., et al. (2021a). Combination of ferulic acid, ligustrazine and tetrahydropalmatine attenuates epithelial-mesenchymal transformation via wnt/β-catenin pathway in endometriosis. Int. J. Biol. Sci. 17, 2449–2460. doi:10.7150/ijbs.60167
Zhang, H., Chi, J., Hu, J., Ji, T., Luo, Z., Zhou, C., et al. (2021b). Intracellular AGR2 transduces PGE2 stimuli to promote epithelial-mesenchymal transition and metastasis of colorectal cancer. Cancer Lett. 518, 180–195. doi:10.1016/j.canlet.2021.06.025
Keywords: macranthoidin B, endometriosis, COX-2/PGE2 pathway, epithelial-mesenchymal transition, invasion and metastasis
Citation: Ding Y, Yang X, Wei Q, Bi X, Zhang Y, Ma Y, Yang M, Xu X, Li C, Wang Q and Chen Y (2024) Macranthoidin B restrains the epithelial-mesenchymal transition through COX-2/PGE2 pathway in endometriosis. Front. Pharmacol. 15:1492098. doi: 10.3389/fphar.2024.1492098
Received: 06 September 2024; Accepted: 29 November 2024;
Published: 12 December 2024.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Shun Ding, Hainan University Hospital, ChinaMarwa Kamel, Cairo University, Egypt
Yuan Lu, Fudan University, China
Copyright © 2024 Ding, Yang, Wei, Bi, Zhang, Ma, Yang, Xu, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Li, YjA1MTA0QDEyNi5jb20=; Qin Wang, d3FpbjExMjdAY2R1dGNtLmVkdS5jbg==; Yi Chen, cmFjaGVsY3lAY3FjdGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Yi Ding
Yi Ding Xiaoqian Yang2†
Xiaoqian Yang2† Xiaoyu Xu
Xiaoyu Xu Cong Li
Cong Li Qin Wang
Qin Wang Yi Chen
Yi Chen