- 1College of Pharmacy, Liaoning University of Traditional Chinese Medicine, Dalian, China
- 2Department of Pharmacy, Changzhi Medical College, Changzhi, China
- 3Qimeng Co., Ltd., Chifeng, China
Objective: This review aims to summarize the research progress of glycocholic acid to promote its broader development and application.
Methods: This article collects relevant literature from databases such as Science Direct, PubMed, Web of Science, Google Scholar and CNKI from the establishment to 2024, systematically organizing and analyzing aspects of glycocholic acid including its physicochemical properties, synthesis and extraction techniques, detection methods, pharmacological effects, mechanisms of action, clinical research, and application as an excipient.
Results: Glycocholic acid, as a key conjugated component in bile acids, exhibits various pharmacological effects such as anti-inflammatory and antioxidant activities. Nevertheless, current research on glycocholic acid is insufficient, with synthesis techniques requiring improvement, limited application of detection technologies, and a need for in-depth exploration of its pharmacological mechanisms. Due to its amphiphilic molecular structure, glycocholic acid is primarily used as a pharmaceutical excipient.
Conclusion: This review summarizes the existing research on glycocholic acid, indicating that future research should strengthen work in this field, including improving synthesis processes and enhancing the sensitivity of detection technologies, to provide a scientific basis for the development of new formulations and drug combinations, thereby promoting the advancement of traditional Chinese medicine.
1 Introduction
With a history of more than 1,000 years in China, Chinese medicine is primarily derived from natural animals and plants and has been widely used for its proven efficacy, low toxicity and other benefits. Bile, an essential component of animal medicines produced in the digestive fluid of the liver, has a long history of use in traditional Chinese medicine (Wang and Shi, 2000). In addition, in some regions, people use bile in specific food therapy or culinary practices, such as cattle bile powder, which is a dried product of bile, appearing as green-brown or brownish chunks or powder. It has been proven that taking it alone with boiled water or in combination with fleeceflower root, poria cocos, or sophora twig can have effect such as clearing heat, detoxifying, promoting bile secretion, anti-inflammatory, pain relief, and relieving constipation. The Shennong Bencao Jing from the Han Dynasty records the use of carp bile to treat redness, swelling and pain in the eyes, blindness, deafness impotence, etc., while Zhang Zhongjing in the Jin Yi Lun documents the functions of pig bile, such as suppressing coughs and relieving asthma symptoms, as well as its anti-inflammatory and anti-bacterial properties; Li Shizhen included more than 30 kinds of bile acid-containing herbs such as bull bile, sheep bile, bear bile, pig bile, etc., in his Bencao Gangmu, all of which are listed in the Dictionary of Chinese Medicine because of their strong medicinal effects, significant curative effects, and abundant resources, making them widely used in pill powder or other traditional Chinese medicine or Western medicine preparations (Zhao and Li, 2005).
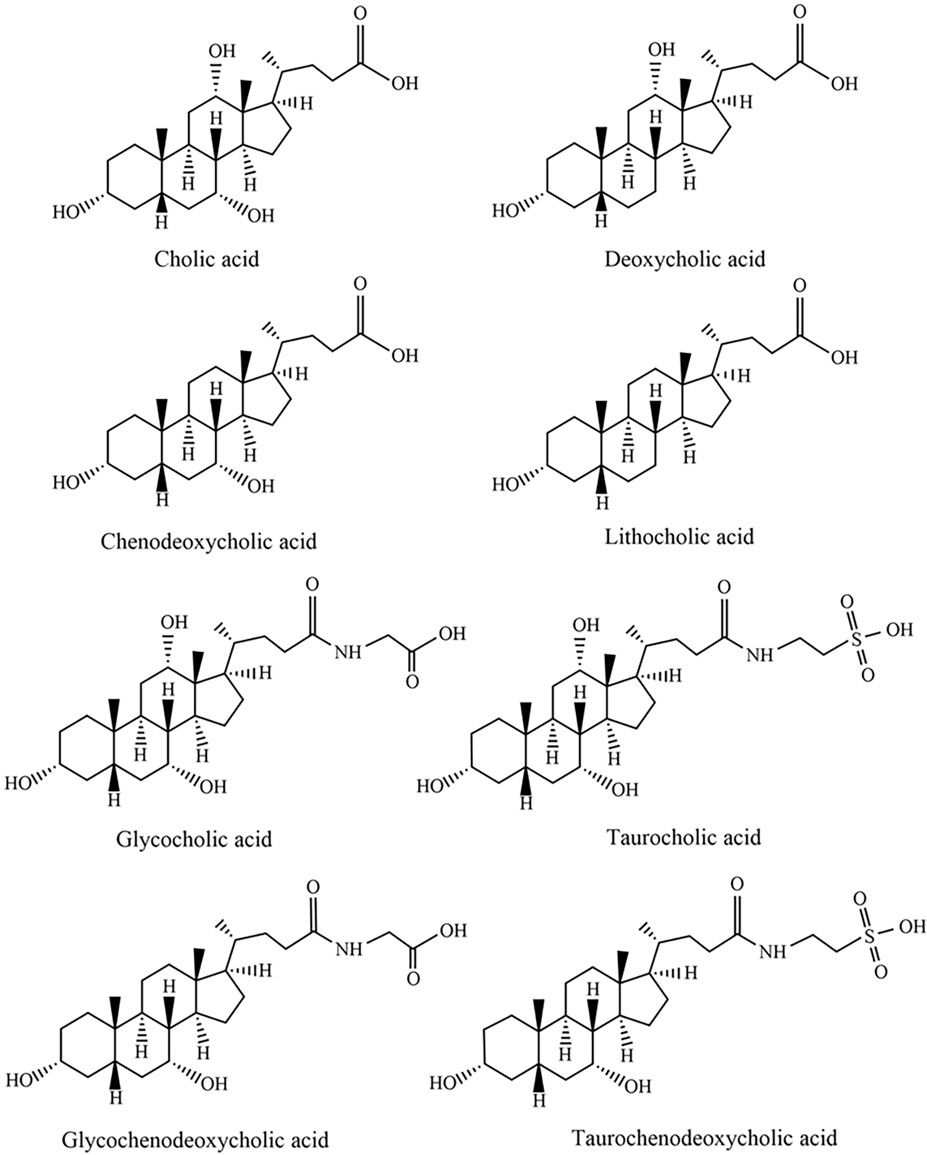
Bile acids and bile salts are the primary bioactive components of animal bile that exert therapeutic effects. Bile acids are steroidal amphipathic molecules (Russell, 2009), which can be categorized into free bile acids and conjugated bile acids. The former includes cholic acid (CA), dexycholic acid (DCA), chenodeoxycholic acid (CDCA), and lithocholic acid (LCA), while the latter mainly consists of glycocholic acid (GCA), taurocholic acid (TCA), glycochenodeoxycholic acid (GCDCA), and taurochenodeoxycholic acid (TCDCA). The specific chemical structures of each component are illustrated in Figure 1.
Glycocholic acid, a bile acid complex synthesized in the liver from bile acids and glycine, is abundant in the bile of various animals. It plays a crucial role in enhancing lipase activity, catalyzing fat breakdown, and promoting bile secretion (Feng et al., 2014). Recent studies have revealed its additional therapeutic properties including anti-inflammatory, antipyretic, antioxidant, and antibacterial effects (Huang, 2019). Under normal physiological conditions, the liver absorbs glycocholic acid from the bloodstream, where its concentration remains low. However, when liver cells are damaged or impaired in their ability to excrete bile acids, peripheral blood levels of glycocholic acid is elevated. Consequently, glycocholic acid may serve as an early diagnostic indicator of abnormalities related to liver and bile function (Li et al., 2010; Zhao et al., 2019). Glycocholic acid as a raw pharmaceutical ingredient is widely used in the medical field, and the demand for it in clinical practice in our country is also constantly increasing. However, there are few reports on the research progress of glycocholic acid both domestically and internationally. Therefore, this article focuses on glycocholic acid and summarizes its structural properties, synthesis and extraction, detection methods, pharmacological effects, clinical research, significance of testing, and applications as an excipient. This aims to fill the gaps in the related field and provide references for guiding clinical medication.
2 Methodology for literature search
A literature search was conducted on databases including Science Direct, PubMed, Web of Science, Google Scholar and CNKI, covering publications from the inception of these platforms up to the year 2024. The search utilized the following keywords: glycocholic acid, synthesis processes, detection methods, and pharmacological effects. We have summarized the above content, prioritizing authoritative and valuable literature, and this review compiles a total of 134 relevant documents.
3 Physicochemical characteristics of glycocholic acid
Glycocholic acid is chemically designated as N-(3α,7α,12α-Trihydroxy-5β-cholan-24-oyl)-glycine. The chemical formula of the compound in question is C26H43NO6, with a molecular weight of 465.62. This compound exhibits weak acidity and appears as a white crystalline powder with a bitter taste. The compound exhibits limited solubility in water but is soluble in hot water and readily dissolves in organic solvents. In an aqueous environment, its degree of ionization is low, resulting in a pH value (water suspension) that ranging from 3.0 to 4.0. The melting point of glycocholic acid has been determined to be 130°C. However, it has been observed that the acid undergoes decomposition into glycine and cholic acid when exposed (Zhao et al., 2006).
4 Reactive formation of glycocholic acid
4.1 The glycocholic acid synthesis in the human body
In the liver, cholesterol is subjected to intricate enzymatic processes that culminate in the generation of free bile acids. These bile acids are subsequently conjugated with glycine and taurine by the action of bile acid CoA synthase and amino acid N-acetyltransferase, forming conjugated bile acids such as glycocholic acid, taurocholic acid, taurodeoxycholic acid, and glycodeoxycholic acid (Jia et al., 2018; Ju et al., 2020). In physiological pH conditions, taurine conjugates are predominantly present in ionic form, whereas glycine conjugates exist in both ionic and molecular states simultaneously. In bile, the ratio of glycocholic acid to taurocholate is approximately 3:1 (Wu, 2013). The synthetic pathway of glycocholic acid within the body is shown in Figure 2.
4.2 Reactive synthesis of glycocholic acid
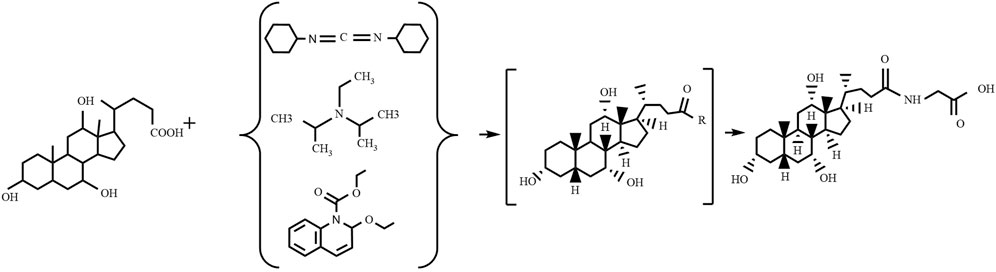
4.2.1 Condensation agents method
The condensation agents method (as shown in Figure 3) involves reacting specific condensing agents with bile acid to activate the carboxyl group, forming a reactive intermediate that can further react with the amine group of glycine or glycine methyl ester to form an amide bond, ultimately yielding glycocholic acid or its methyl ester (Ye et al., 2014). The most commonly used condensing agents are dicyclohexylcarbodiimide (DCC) (Cravotto et al., 2005), N,N-diisopropylethylamine (EDIA) (Ray et al., 2006), and 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ) (Venturoni et al., 2012). This method has high production costs, and it is challenging to remove residual by-products, often requiring further purification processes, which is not conducive to large-scale use.
4.2.2 Amide formation
Amide formation refers to the process in which a carboxylic acid reacts with an amine to produce an amide. This process can be carried out in various ways, including the use of coupling agents, inorganic bases, organic bases, or catalysts. Unlike the condensation agents method, amide formation is not limited to the use of specific coupling agents but covers a broader range of reaction conditions and catalysts. For instance, Momose et al. (1997) introduced a method for synthesizing glycocholic acid by simply mixing unconjugated bile acid, glycine ester, diethyl pyrocarbonate (DEPC), and triethylamine (Et3N) at room temperature for 30 min. This method is mild in conditions and can yield products with high purity, suitable for the relatively small-scale preparation of amide bile acid compounds.
4.2.3 One-pot method
One-pot synthesis is a chemical strategy that allows all reactants to react directly in a single reaction vessel to complete the synthesis process. This method is favored for its simplicity and efficiency. In the synthesis of glycocholic acid, this approach is used to increase yield and simplify operational steps. Wang et al. (2021) used a one-pot method to synthesize glycocholic acid, starting with ursodeoxycholic acid and glycine ethyl ester hydrochloride as raw materials, and using N-carbamoyl chloride hydrochloride as a condensing agent under specific conditions. After the reaction was completed, hydrolysis was carried out in a sodium hydroxide aqueous solution, ultimately yielding glycocholic acid with a yield of over 90% (Wang et al., 2021). Additionally, Hitome et al. developed a new method for preparing 13C-labeled glycocholic acid, using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as the condensing agent, which yields a higher yield than when using EEDQ as a condensing agent (Mitome et al., 2013). This method is simple and straightforward, employing a one-pot reaction to prepare relatively pure labeled glycocholic acid on a laboratory scale, suitable for clinical studies of breath tests.
4.2.4 Mixed anhydride method
The mixed anhydride method (Gianluca et al., 2010) involves reacting cholic acid with ethyl chloroformate or methyl chloroacetate to synthesize a highly active mixed anhydride intermediate, which then reacts with glycine methyl ester. After purification, high-purity glycocholate acid methyl ester is obtained, and finally, an alkaline hydrolysis is used to obtain glycocholic acid or sodium glycocholate (SGC). This method is considered more appropriate due to its low raw material requirements and high utilization rate (up to 76%). Considering that ethyl chloroformate is a highly toxic substance, it can be replaced with the similar isobutyl chloroformate as an activating reagent to promote the formation of the amide bond (Cayley et al., 2007). For instance, Zhao et al. (2020) used the mixed acid anhydride method, utilizing triethylamine and isobutyl chloroacetate as catalysts, to synthesize glycocholic acid from bile acid and 4-aminobutyric acid. Although this method has high purity, the process is complex and incurs high costs.
4.2.5 Hydrolysis
Hydrolysis is a chemical reaction process that can employ various catalysts and conditions, such as acidic, neutral, or alkaline environments, to accommodate different chemical structures and target products. For glycocholic acid (Ji et al., 2024), typically refer to the hydrolysis of glycocholic acid ethyl ester under alkaline conditions with sodium hydroxide or potassium hydroxide to break the ester bond and release glycocholic acid. Additionally, by consulting resources such as Chemical Book, it has been discovered that glycocholic acid can be obtained through an esterification reaction using cholic acid and glycine ethyl ester hydrochloride as raw materials, followed by a hydrolysis method.
4.2.6 Other methods
The synthesis of glycocholic acid, in addition to the aforementioned methods, can also be carried out through the azide method (Zhu, 2019) and the acyl chloride method (Xu et al., 2010). The azide method encounters several challenges during the synthesis process, including high reagent costs, difficulty in clearing residues, and a substantial demand for raw materials. Moreover, the reagents used in the synthesis are sensitive to moisture, and the intermediates produced are toxic, posing potential health risks to operators and potentially harmful effects on the environment. Therefore, this method is not suitable for large-scale production. Similar to the azide method, the acyl chloride method also requires a large amount of raw materials in the synthesis of glycocholic acid, with a utilization rate is only 8.1%. Therefore, it is also not suitable for industrial large-scale production. Although these two methods have certain applications in the synthesis of glycocholic acid, their feasibility in industrial production is limited due to issues of cost and efficiency.
4.3 Extraction process of glycocholic acid
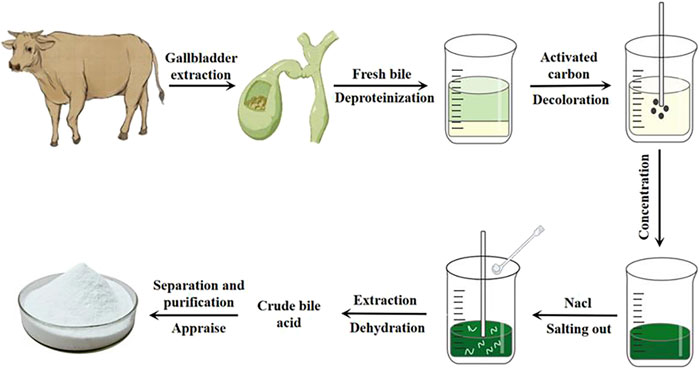
Glycocholic acid is a conjugated bile acid that is typically mixed with various substances in biological matrices. To obtain high-purity glycocholic acid, a series of meticulous processing steps must be followed (Wang et al., 2020). The extraction and purification process is as follows (see flowchart in Figure 4).
4.3.1 Raw material preparation and protein removal
Fresh bovine, ovine, or porcine bile is selected, filtered through gauze, and quantitatively collected (Chen and Luo, 2020). It is then mixed with 95% ethanol in a specific ratio. Most proteins and some pigments are removed through stirring, standing, and centrifugation, resulting in a green supernatant.
4.3.2 Decolorization treatment
The supernatant is taken, and an appropriate amount of activated carbon is added. It is heated and stirred, then allowed to cool naturally before centrifugation to remove all pigments, yielding a light yellow supernatant.
4.3.3 Ethanol recovery and salting out
Under specific conditions, all ethanol is recovered. A suitable amount of sodium chloride is added to the viscous bile and it is stirred until “white threads” appear, and then let it stand still.
4.3.4 Extraction and dehydration
A mixture of chloroform and n-butanol is used for multiple extractions of the salted-out material. Anhydrous sodium sulfate is then added for dehydration, resulting in crude bile acids.
4.3.5 Separation, purification, and identification
Column chromatography is employed, using suitable stationary phases and elution solvents based on the characteristics of glycocholic acid to separate it from other bile acids. The obtained samples are then identified, concentrated, and purified to yield white crystalline glycocholic acid.
5 Detection methods for glycocholic acid
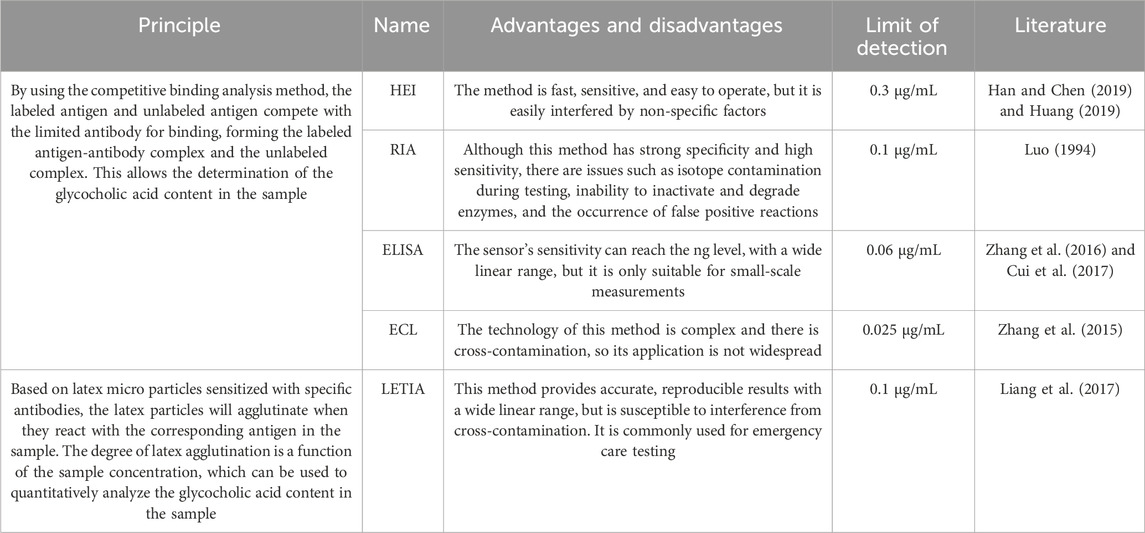
5.1 Immunoassay
For bile acids with complex isomers, the detection and analysis present certain difficulties. Regarding the determination of bile acids, the main immunoassay methods include: homogeneous enzyme immunoassay (HEI), radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), electrochemiluminescence (ECL), and latex enhanced immunoturbidimetry assay (LETIA). The principles and advantages and disadvantages of these methods are shown in Table 1.
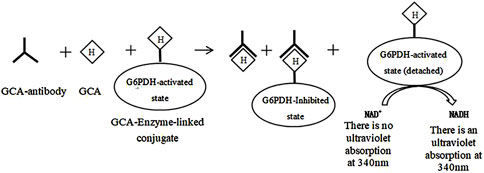
The marker enzymes used in homogeneous enzyme immunoassays include digestive enzymes, malic dehydrogenase, β-D-galactosidase, and glucose-6-phosphate dehydrogenase, with glucose-6-phosphate dehydrogenase (G-6-PDH) being the most commonly used (Grote et al., 2012; Pandey et al., 2014; Roncancio et al., 2014). The glycocholic acid determination process is as follows (the route is shown in Figure 5): In brief, the glycocholic acid in the sample competitively binds to the anti-glycocholic acid specific antibody sites with the G-6-PDH-bile acid conjugate, which causes some of the bound enzyme-labeled drug to be released. It can be demonstrated that only the released enzyme-labeled bile acid conjugate can catalyze the conversion of NAD+ to NADH. Furthermore, the amount of NADH generated is proportional to the concentration of glycocholic acid in the sample. The NADH molecule exhibits a strong UV absorption at 340 nm; consequently, the change in absorbance observed before and after the conversion can be used to estimate the glycocholic acid content (Tao, 1987).
5.2 Chromatography
5.2.1 Thin layer chromatography (TLC)
TLC is a relatively straightforward technique that yields results in a relatively short time. However, it can only qualitatively determine certain components by judging the depth of the fluorescent spots, which indicates the relative content of the components in the sample (Zhou et al., 2015). Yao et al. (2021) used the TLC method to identify the bile acid components in sheep bile, and the results showed that the bile acid components included CA, GCA, glycodeoxycholic acid (GDCA), GCDCA, TCA, taurodeoxycholic acid (TDCA), TCDCA and taurolithocholic acid (TLCA). The depth of the fluorescent spots indicated that the main bile acids in sheep bile were taurine-conjugated bile acids, with detection limits for each bile acid ranging from 4.0 to 10.4 ng (Yao et al., 2021).
5.2.2 Liquid chromatography (LC)
5.2.2.1 High performance liquid chromatography (HPLC)–ultraviolet detection
HPLC is a widely used detection method, apart from immunoassays. Its main characteristics are fast analysis speed and high separation efficiency. Li and Li (2009) established a HPLC method to detect the concentration of glycocholic acid in serum. They found that glycocholic acid showed a good linear relationship between the concentration range of 0.15625–10.0 μg/mL and the peak area. The lowest detectable concentration was 0.02 μg/mL, and the intra-day and inter-day RSD were both less than 5.00%, indicating that this method has high sensitivity, stability, and good separation effect for the determination of glycocholic acid (Li and Li, 2009).
5.2.2.2 HPLC—evaporative light scattering detection (ELSD)
The bile acid components lack a conjugated system, which results in their weak absorption under ultraviolet conditions and heir susceptibility to interference by other substances. Therefore, the combination of evaporative light scattering technology can make up for the above problems, which is very suitable for the analysis of bile acid components (Zhang et al., 2009; Yao et al., 2021). For example, Shi et al. (2013) based on HPLC-ELSD technology, studied the bile acids and glycocholic acids in sheep gallbladder powder, and established a method that can simultaneously determine the contents of bile acids and glycocholic acids in sheep gallbladder powder (Yue et al., 2004). The method is straightforward to operate and exhibits a high degree of separation efficacy. The detection limits of this method can reach 0.49 μg/mL and can be used for the quality evaluation of sheep bile powder.
5.2.3 Chromatography—tandem technique
5.2.3.1 HPLC—tandem mass spectrometry (HPLC-MS/MS)
The combination of high performance liquid chromatography (HPLC) with mass spectrometry (MS) in HPLC-MS/MS technology allows for the effective separation of substances and the identification of their constituents with high accuracy and precision. This makes HPLC-MS/MS an increasingly popular choice for analytical identification (Wang, 2014). The HPLC-MS/MS method established by Mu et al. (2023) can complete the detection of six bile acids, including bile acid, cholic acid, taurocholic acid, glycocholic acid, taurodeoxycholic acid, and glycodeoxycholic acid, in plasma within 6 min, thus greatly reducing the detection time. The detection limits of this method range from 0.25 ng/mL to 0.45 ng/mL, while the quantification limits range from 0.84 ng/mL to 1.49 ng/mL (Mu et al., 2023). Bezoar is an animal medicine mainly used for detoxification, fever reduction, and tranquilization. However, the current phenomenon of adulteration has significantly compromised the quality of the medicinal material. Therefore, Liu et al. established a method to identify natural bezoar, artificial bezoar, and in vitro cultured bezoar, and found that the difference between the three types of bezoar is that a new compound, 3α,12α-dihydroxy-7-oxo-5α-cholic acid, was detected in the in vitro cultured bezoar, while glycocholic acid, glycodeoxycholic acid, and taurocholic acid were detected in both natural and artificial bezoar, however, the content of taurocholic acid was different, successfully distinguishing various types of bezoar medicinal materials (Liu et al., 2015).
5.2.3.2 Gas chromatography (GC)-MS
GC-MS coupled technology combines the high-efficiency separation capability of gas chromatography for mixtures and the accurate identification capability of mass spectrometry for pure substances, and has been widely applied in various fields. Nevertheless, the inherent volatility of natural bile acid components is insufficient for GC analysis, so derivatization is needed to improve the volatility and thermal stability of the analytes (Wu and Han, 2020). Long et al. (2017) studied the differential metabolites in the serum of rats with acute liver failure using metabolomics, and analyzed the metabolic samples by GC-MS, which found 11 differential metabolites including glycocholic acid. This demonstrates that GC-MS can be used for the analysis of glycocholic acid.
6 The pharmacological effects of glycocholic acid
6.1 Anti-inflammatory effect
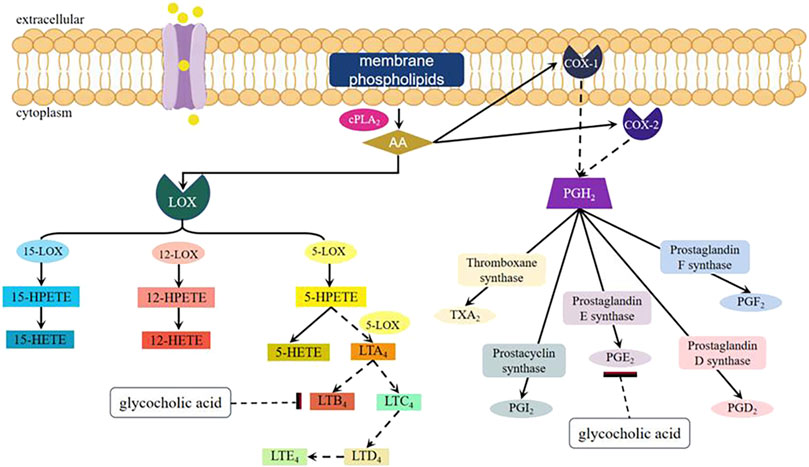
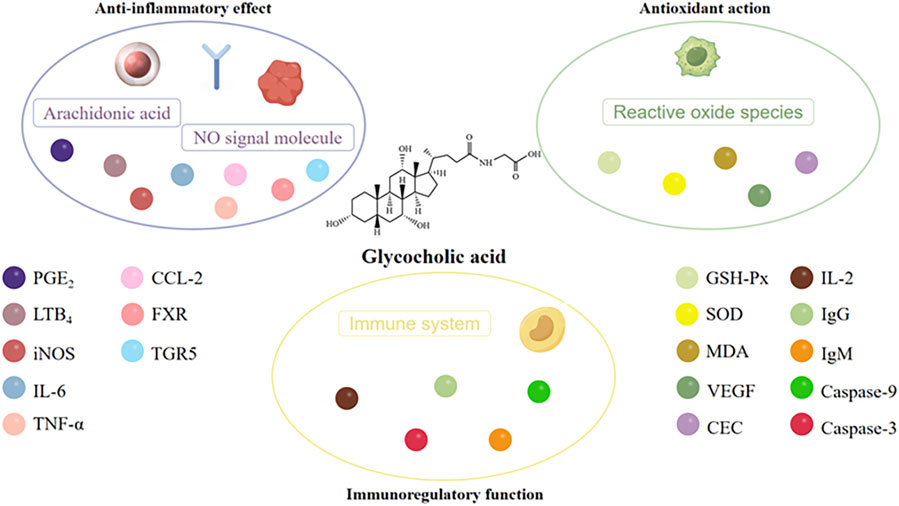
Arachidonic acid (AA) metabolites leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) are the main inflammatory mediators released by cells, and play an important role in various acute and chronic inflammations. They can exhibit a strong inflammatory response even at extremely low concentrations, rendering them a valuable tool for investigating the underlying mechanisms of inflammation (Li et al., 2006). The experiment conducted by Li et al. (2006) has demonstrated that glycocholic acid can affect the cyclooxygenases (COX) and lipoxygenases (LOX) pathways in the AA metabolic pathway, thereby reducing the content of PGE2 and LTB4 in inflammatory tissues of rats, and has a significant inhibitory effect on acute and chronic inflammation (as shown in Figure 6). (Li, 2006) Nitric oxide (NO) is an important physiological mediator and intercellular and intracellular chemical messenger within the body. It is produced by nitric oxide synthase (NOS) from arginine and molecular nitrogen (Qiu et al., 2016). In the inflammatory response, inducible NOS (iNOS) is induced by inflammatory agents and mediators, leading to the synthesis and release of NO. Sustained high concentrations of NO can promote the occurrence of inflammation. Studies by Guan et al. showed that glycocholic acid significantly reduced the NO content in the peripheral blood of rats with Freund’s adjuvant-induced inflammation (44.48 ± 7.75 μmol·L−1) compared to the negative control group (155.93 ± 28.11 μmol·L−1), demonstrating that glycocholic acid has a significant inhibitory effect on the NO content in the peripheral blood of rats with Freund’s adjuvant-induced inflammation (Guan et al., 2009).
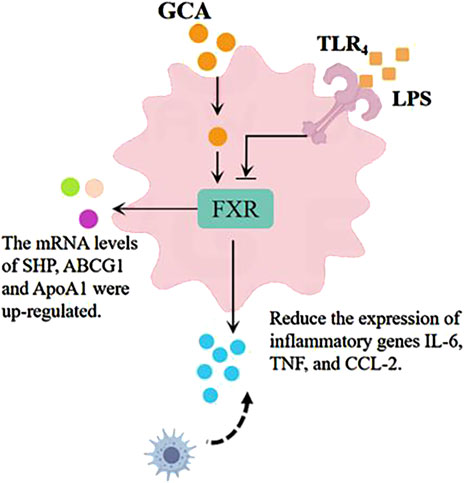
When inflammation occurs, the body rapidly synthesizes and releases various cytokines and chemokines, such as interleukin (IL) (Guo et al., 2023), interferon (IFN), tumor necrosis factor (TNF) (He et al., 2021), CXC chemokines, and CC chemokines, further enhancing the inflammatory response. Ge et al. (2023) found in a lipopolysaccharide-induced zebrafish inflammation model that tauroursodeoxycholic acid (TUDCA) could inhibit macrophage accumulation and suppress the upregulation of IL-6, TNF-α, and CCL-2 induced by lipopolysaccharide stimulation, reducing the transcriptional expression of these two cytokine (as shown in Figure 7). (Ge et al., 2023) The farnesoid X receptor (FXR) is a bile acid receptor that also plays an important role in the inhibition of inflammation (Wu et al., 2020a). The study indicates that glycocholic acid can increase the mRNA levels of downstream factors in the FXR signaling pathway, including the short heterodimer partner (SHP), ATP-binding cassette transporters G1 (ABCG1), and apolipoprotein A1 (ApoA1) (Ge et al., 2023). Additionally, glycocholic acid has an increasing trend in the expression of another bile acid receptor, the Takeda G protein-coupled receptor 5 (TGR5 or GPBAR1). In summary, glycocholic acid can inhibit the migration of macrophages and the cellular secretion of proinflammatory cytokines and chemokines, and its mechanism of action may be related to the upregulation of FXR expression.
6.2 Antioxidant action
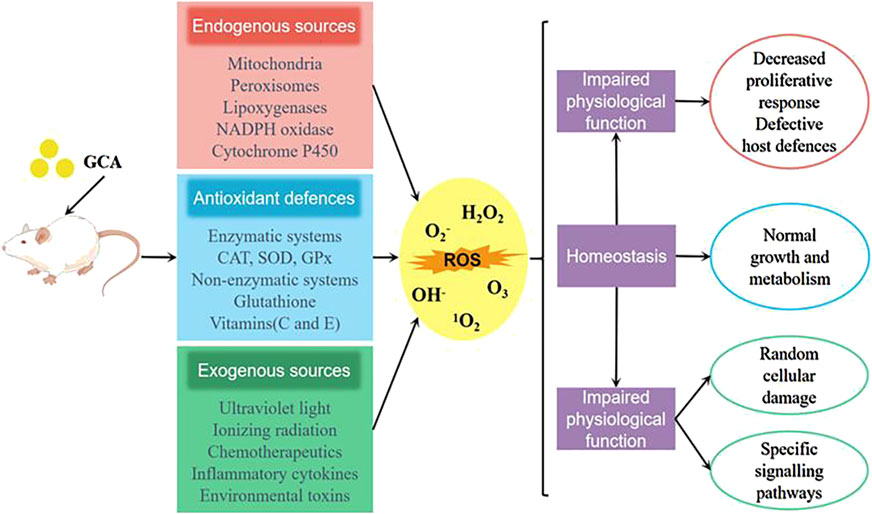
It is a well-established fact that biological organisms generate a large amount of reactive oxygen species (ROS) under the influence of the external environment and their own metabolism (Zhou et al., 2024). The overproduction of ROS not only impairs the normal signaling pathways of cells, but also oxidizes DNA, proteins, lipids, and causes tissue damage in the body (Zhang et al., 2019). Studies have shown (Wu et al., 2020b) that glycocholic acid significantly increase the activity of antioxidant enzymes such as glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in mouse peritoneal macrophages. By reducing the content of malondialdehyde (MDA), glycocholic acid can reduce the oxidative damage of lipid peroxidation to mouse peritoneal macrophages (as shown in Figure 8), and regulate the antioxidant stress level of mouse peritoneal macrophages.
Age-related macular degeneration (AMD) is mainly characterized by a decrease in the ability of retinal pigment epithelial (RPE) cells to phagocytose and digest the outer segment discs of photoreceptor cells (Gong et al., 2021). This process results in the accumulation of undigested disc membrane remnants in the basal cytoplasm of RPE cells, which are then secreted and deposited on Bruch’s membrane, forming drusen. One of the treatment methods is to use antioxidants to prevent free radical damage to cells and protect photoreceptor cells. Warden and Brantley found that glycocholic acid can protect the tight junctions of RPE cells from extracellular oxidative stress, maintaining the localization of ZO-1 and transepithelial electrical resistance (TEER). In vitro experiments on cell migration and tube formation showed that glycocholic acid can inhibit vascular endothelial growth factor (VEGF)-induced choroidal endothelial cell (CEC) angiogenesis, indicating that glycocholic acid has a protective effect on AMD (Warden and Brantley, 2021).
6.3 Immunoregulatory function
The immune capacity of the body can be broadly divided into two categories: non-specific immunity and specific immunity. The former is primarily mediated by macrophages, while the latter is predominantly composed of immune organs and immune cells. The research results of Zhao indicated that the phagocytic index of the low-dose glycocholic acid was significantly higher than that of the control group and the CTX immunosuppression group (Zhao, 2006). It could significantly inhibit the delayed-type hypersensitivity (DTH) reaction in mice, while also increasing the IL-2 content in the serum, and significantly increasing the percentage of CD4+ cells in normal and CsA-inhibited mice (Li et al., 2007). The serum hemolysis method and ELISA method showed that the high and low dose glycocholic acid groups could significantly elevate the IgG and IgM content in the mouse serum. This evidence supports the conclusion that glycocholic acid has a regulatory effect on both non-specific immunity and specific immunity. Xin et al. (2011) studied the mechanism of glycocholic acid on the apoptosis of splenic lymphocytes, and found that glycocholic acid could induce nuclear condensation, apoptotic bodies and DNA fragmentation in splenic lymphocytes, significantly induce the externalization of phosphatidylserine in splenic lymphocytes, and increase the activity of Caspase-9 and Caspase-3. The higher the concentration of glycocholic acid, the more it can promote the apoptosis of splenic lymphocytes, indicating that glycocholic acid can balance the relationship between the body’s immunity and inflammation (Xin et al., 2011). The molecular mechanisms of glycocholic acid’s anti-inflammatory, antioxidant, and immunomodulatory effects are shown in Figure 9.
6.4 Antibacterial effect
Glycocholic acid belongs to the class of steroid compounds and is an amphiphilic biosurfactant with the ability to reduce surface tension. It works by altering the permeability of bacterial cell membranes, disrupting their integrity, and causing the leakage of intracellular substances, thereby inhibiting bacterial growth. Additionally, glycocholic acid may also affect the synthesis of bacterial proteins and nucleic acids, thereby inhibiting bacterial growth and reproduction. In recent years, it has been demonstrated that glycocholic acid has varying degrees of inhibitory effects on both Gram-positive and Gram-negative bacteria (Li, 2009). Dobson et al. (2018) isolated six bile acid derivatives, including glycocholic acid, from the broth culture of Bacillus amyloliquefaciens UWI-W23, and demonstrated that these six compounds had antimicrobial activity against Pseudomonas aeruginosa and Bacillus cereus. Furthermore, glycocholic acid also has an inhibitory effect on Staphylococcus (MICs = 15.6 μg/mL).
White Candida is a fungal pathogen with a high clinical antifungal drug resistance rate. It exists in small numbers in a normal host and does not cause disease. However, when the body’s immune function and general defense capacity decrease or the mutual restraint of the normal microbial flora is disrupted, it can proliferate extensively, change its growth form, and invade cells to cause disease. Kong et al. (2011) found that the antibacterial activity of glycocholic acid against white Candida is concentration-dependent. The antibacterial activity of glycocholic acid is weaker than that of cholic acid, possibly because the change in the C-24 side chain of the steroid nucleus alters the surface amphiphilicity, leading to a decrease in the affinity of glycocholic acid for the target of white Candida and its ability to transform the cell membrane.
6.5 Anti-breast disease action
Breast diseases are one of the major health hazards for women at present, mainly including mastitis, hyperplasia of mammary glands, fibroadenoma of the breast, intraductal papilloma, and breast cancer. Among these diseases, the incidence of breast cancer is high among female cancers, becoming one of the most common types of cancer (Huang, 2024; Zhang et al., 2024). Studies have shown that glycocholic acid may activate the FXR receptor and reduce the number of Treg cells in the tumor microenvironment by inhibiting the expression of the PI3K/AKT signaling pathway in Treg cells, thereby promoting tumor cell apoptosis and inhibiting tumor growth (Li et al., 2018; Ren et al., 2019; Xiang et al., 2019). Liang et al. (2021), in their research on the potential of glycocholic acid as a treatment for hyperplasia of mammary glands, established normal control group, model control group, tamoxifen group, and low, medium, and high dose groups of glycocholic acid. They found that after intervention with glycocholic acid, there was no significant enlargement of the rat’s breasts, and no obvious increase in breast diameter and nipple height. At the same time, the number of lobules and alveoli in the mammary glands decreased and began to atrophy, with a significant improvement in hyperplasia, indicating that glycocholic acid has an inhibitory effect on lobular hyperplasia and ductal dilation of the mammary glands (Liang et al., 2021). Further research has found that glycocholic acid has significant therapeutic effects in anti-tumor treatment, especially in the treatment of breast cancer. The experimental results prove that the tumor inhibition rate of glycocholic acid reaches 42.83%, and its specific target protein and RNA expression are significantly increased, effectively inhibiting the growth of mouse breast cancer cells. These findings provide strong scientific evidence for the development of glycocholic acid as a treatment drug for breast diseases (Liang et al., 2019).
7 Research on the application of glycocholic acid as an excipient
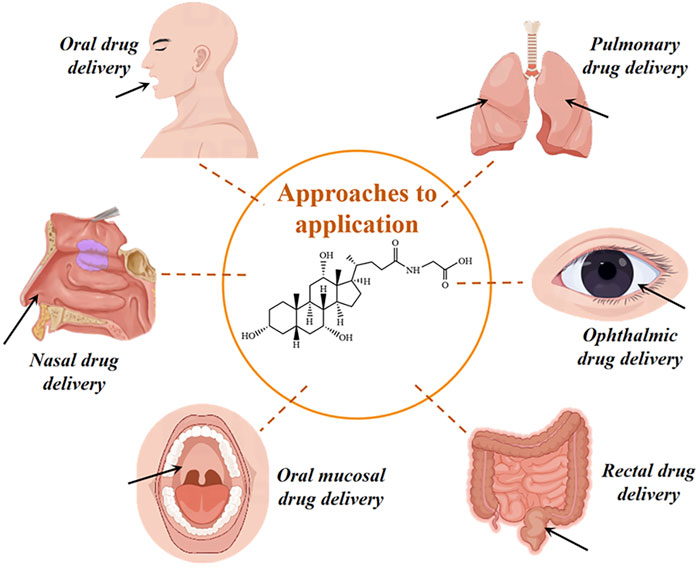
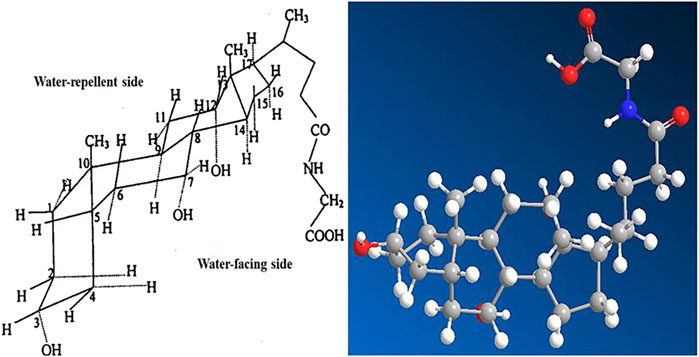
Glycocholic acid is an endogenous substance present in the animal body, which is typically combined with sodium or potassium to form a salt. It is an amphiphilic biological surfactant with a planar rigid steroid skeleton, with its hydrophilicity coming from the hydroxyl group substituted on the concave (α) face of the steroid nucleus, and its hydrophobicity coming from the angular methyl group on the convex (β) face (Cai et al., 2020). This results in glycocholic acid exhibiting strong interfacial activity (Yang et al., 2021). Due to its special amphiphilic structure and good biocompatibility, it has been widely used as a drug carrier and absorption enhancer (Moghimipour et al., 2015), increasing the absorption of various drugs, mainly including oral, nasal, and oral administration routes (as shown in Figure 10). The stereo structure of glycocholic acid is shown in Figure 11 (Wang, 2016).
7.1 Oral drug delivery
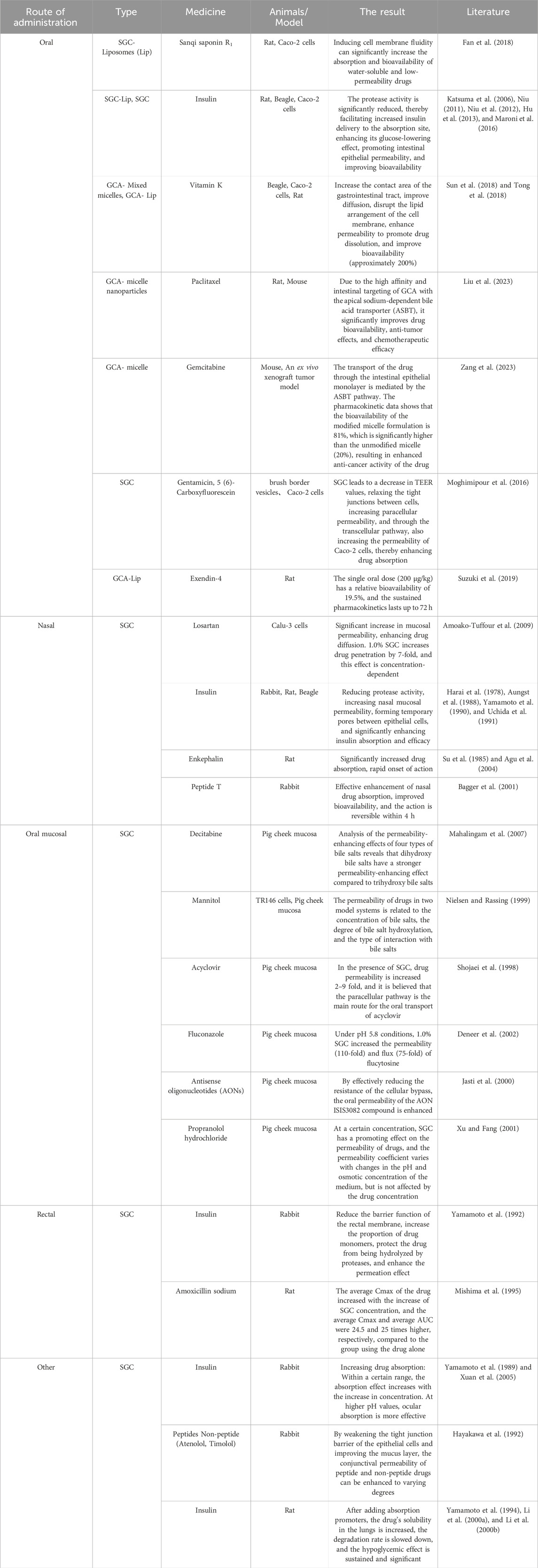
Oral administration represents the most common method of drug delivery, offering a number of advantages. These include convenience, the avoidance of direct damage to the skin and mucous membranes, and the absence of pain caused by injection. However, the absorption of orally administered drugs is easily affected by factors such as gastrointestinal mucus layer, digestive enzymes, biological membrane permeability, intercellular tight junctions, and intrinsic solubility, so absorption enhancers are usually added to improve the bioavailability of drugs (Li et al., 2014; Huang and Sun, 2022; Song, 2022). As illustrated in Table 2, many researchers have studied the promotion of drug absorption by glycocholic acid through various pathways. R1 saponin, derived from Panax notoginseng, is a type of triterpenoid saponin isolated from Panax notoginseng, which has protective effects on the heart, nerves, and liver. However, studies have found that R1 saponin has poor water solubility and permeability, and is easily degraded in the gastrointestinal tract. Through the use of sodium glycocholate, the drug’s cellular diffusion is increased, resulting in a 3.25-fold increase in drug absorption in the duodenum.
7.2 Nasal drug delivery
For small doses of drugs that are unstable in the gastrointestinal tract, nasal administration is a suitable route of administration. It has become a research focus due to its advantages of rapid onset, convenient administration, and avoidance of first-pass effect of the liver (Liao et al., 2023). However, there are two main limiting factors for nasal absorption: the low membrane permeability of the drug and the ciliary clearance effect of the nasal mucosa (Zhang et al., 2022). To overcome these issues, methods such as adding absorption enhancers can be used to improve drug absorption (as shown in Table 2), such as peptide substances like insulin, which are easily degraded by proteases and have poor permeability. The above method can significantly increase the drug activity and enhance the therapeutic effect. The mechanisms of action may include the following (Donovan et al., 1990; Shao and Mitra, 1992; Lin et al., 2007): 1) affecting the thermodynamic properties of the drug in solution; 2) changing the molecular structure of cell membranes by forming membrane pores; 3) loosening the tight connections between epithelial cells; 4) inhibiting protease activity in the mucosa; 5) altering the mucus properties of the respiratory tract to reduce diffusion barriers.
7.3 Oral mucosal drug delivery
Oral mucosal drug delivery is becoming increasingly being recognized for its ease of use and removal, avoidance of degradation by gastrointestinal enzymes, direct entry into the systemic circulation after absorption through mucosal tissue, and avoidance of the first-pass effect. The factors that influence the delivery of drugs through the oral mucosa can be divided into two main categories: those that affect the permeability of the mucosa and those that affect the viscosity of the mucus and saliva. Absorption enhancers can reversibly alter the stratum corneum barrier of the oral mucosa to promote transmucosal drug absorption, and they have been widely used in oral drug permeation studies (as shown in Table 2). (Li and Huang, 2012; Liu et al., 2014; Chen et al., 2023) Decitabine is a natural adenosine analogue of 2′-deoxycytidine, and it is currently the most potent DNA methyltransferase inhibitor. However, decitabine has poor chemical stability and is not suitable for intravenous injection. Therefore, researchers have evaluated the feasibility of oral administration of decitabine and the influence of four bile salts (sodium taurocholate, STC; SGC; sodium deoxytaurocholate, SDTC; and sodium deoxyglycocholate, SDGC) on its permeability. It was found that dihydroxy bile salts (SDTC, SDGC) had a stronger enhancing effect on the permeation of decitabine through porcine buccal mucosa. The flux was found to be increased by 28–43 times when trihydroxy bile salts were present at a concentration higher than 10 times the critical micelle concentration (CMC), while dihydroxy bile salts only needed a concentration higher than 3 times the CMC to achieve the same flux.
7.4 Rectal drug delivery
Rectal administration refers to the method of delivering drugs into the intestine through the anus, where they are rapidly absorbed through the rectal mucosa into the systemic circulation, exerting their pharmacological effects to treat systemic or local diseases (Zhou et al., 2022). Drug absorption in the rectum is generally through passive diffusion, either directly through epithelial cells (transcellular) or through tight junctions (paracellular) across the rectal wall (Li et al., 2019). Glycocholic acid can bind with sodium and calcium ions, altering the tight junctions of cell membranes, leading to a decrease in the TEER value of the Caco-2 cell monolayer model, increasing the transport of hydrophilic macromolecules through the paracellular route, and inhibiting the activity of drug-metabolizing enzymes at the site of drug action (Lindhardt and Bechgaard, 2003).
7.5 Other administration methods
The majority of peptides and proteins are ineffective when taken orally, and injection administered is hindered by the degradation of proteases and the barrier of the mucosa. Consequently, alternative routes of administration are highly desirable (as shown in Table 2), such as ocular administration and pulmonary inhalation. The alveolar epithelium, which is characterised by a large surface area, facilitates the absorption of numerous drugs that are poorly absorbed in the intestine and other sites due to the short pathway to the bloodstream.
8 The significance of serum glycocholic acid testing
In normal physiological conditions, the liver, as the main metabolic organ in the human body, is able to effectively reabsorb glycocholic acid, resulting in a very little glycocholic acid entering the blood circulation, usually less than 1%. It can be concluded that the concentration of glycocholic acid in the peripheral blood is extremely low, with fasting serum glycocholic acid concentration less than 2.50 mg/L, which can be used to assess the health status of the hepatobiliary system (Yao et al., 2003; Liu, 2012). However, when the liver is injured or there is bile duct obstruction, the liver’s absorption and secretion of glycocholic acid becomes abnormal, resulting in an increase in serum glycocholic acid content. Therefore, serum glycocholic acid can be used as a sensitive indicator to evaluate liver cell function and the metabolic function of the hepatobiliary system. Studies have shown that the serum concentration of glycocholic acid is elevated to varying degrees in pathological conditions such as acute hepatitis, chronic hepatitis, cirrhosis, and liver cancer (Zhang et al., 2017). Kong et al. (2011) conducted a comparative analysis of serum glycocholic acid and total bile acid levels between individuals with different liver and biliary diseases and a control group (Kang et al., 2018). The serum glycocholic acid levels were 152.8 ± 78.2 μg/dL in the control group, 205.8 ± 72.4 μg/dL in hepatitis B virus carriers, 2,827.8 ± 945.7 μg/dL in acute hepatitis, 2,292.5 ± 712.3 μg/dL in chronic hepatitis, 3,592.8 ± 1,548.9 μg/dL in cirrhosis, and 3,992.2 ± 1,648.2 μg/dL in liver cancer, showing varying degrees of elevation.
Furthermore, serum glycocholic acid has certain reference value for the diagnosis and prognosis of acute and chronic hepatitis, can assess the activity of chronic hepatitis, predict the prognosis of cirrhosis, and also indicate other digestive diseases (Xia, 1995; Bao and Bao, 1998). Liu demonstrated that in patients presenting with acute icteric hepatitis during the jaundice period, the positive rates of serum alanine aminotransferase and serum glycocholic acid were both 100% (Liu, 2012). However, during the recovery period, although the routine liver function indicators had returned to normal, the serum alanine aminotransferase conversion rate was 97.87%, while the serum glycocholic acid was only 46.67%, indicating a potential risk of developing into chronic hepatitis. Therefore, using serum glycocholic acid as a discharge criterion is necessary (Zhang and Zhu, 1997).
9 Clinical researches on glycocholic acid
Congenital bile acid synthesis disorders are caused by enzyme deficiencies during the synthesis process and typically manifest in newborns or infants, though they can also present in adulthood. Symptoms include cholestasis, fat-soluble vitamin deficiencies, coagulopathy, chronic liver disease, growth retardation, or neurological impairment. Studies have shown that glycocholic acid can treat bile acid synthesis disorders due to bile acid-CoA: amino acid N-acyl transferase (BAAT) deficiency (Setchell et al., 2013). In 2012, the Cincinnati Children’s Hospital Medical Center in the United States advanced to Phase III clinical trials for the use of glycocholic acid in treating patients with congenital bile acid synthesis disorders. Over a span of 10 years, they selected male and female patients aged between 1 week and 85 years. The primary measurement was the change in atypical bile acid synthesis in urine, analyzed by mass spectrometry, while secondary measurements included liver function tests, fat-soluble vitamin absorption, growth status, and the incidence and severity of adverse events. The results indicated that after oral administration of 15 mg/kg glycocholic acid, the total duodenal bile acid concentration was 23.3 ± 19.1 mmol/L, with 63.5% ± 4.0% of bile acids secreted in the conjugated form, of which glycocholic acid represented 59.6% ± 9.3% of the total bile acids, demonstrating effective intestinal absorption of glycocholic acid (Heubi et al., 2015). Additionally, the absorption of vitamin D2 and tocopherol significantly improved after oral administration of glycocholic acid. Growth status also showed marked improvement in prepubertal patients with growth retardation, and no adverse reactions were reported. Therefore, we speculate that glycocholic acid holds very promising prospects for treating bile acid amidation defects and may become an approved medication in the future.
10 Conclusion and perspectives
In summary, this article provides a comprehensive review of the synthesis and extraction techniques, detection methods, pharmacological effects, and clinical applications of glycocholic acid. The synthesis pathways of glycocholic acid include both in vivo synthesis and chemical synthesis. The synthesis of glycocholic acid in the body primarily relies on the conversion of cholesterol in the liver through the classical pathway and the alternative pathway, which involves a series of enzymatic reactions to generate primary bile acids. These primary bile acids then combine with glycine to form glycocholic acid or its salts. The produced glycocholic acid salts can enter the gallbladder through the action of the bile salt export pump (BSEP), multidrug resistance-associated protein 2 (MRP2), and multidrug-resistant protein 3 (MDR3), and are secreted into the small intestine with bile to participate in the digestion, absorption, and transport of lipids and fat-soluble vitamins. Most of the glycocholic acid salts are reabsorbed at the terminal ileum of the small intestine through the apical sodium-dependent bile acid transporter (ASBT), entering the portal vein circulation and returning to the liver to complete the enterohepatic circulation process. In the field of chemical synthesis, the preparation of glycocholic acid primarily employs the following methods: condensation agents method, mixed anhydride method, one-pot method, and amidation reaction. The core mechanism of these methods involves the reaction between the carboxyl group and the amine, forming an amide bond to synthesize the target compound. Among them, the condensation agents method, although operationally simple, is highly dependent on the condensation agents used, and the reaction tends to leave by-products, requiring additional purification steps for removal. The mixed anhydride method, while using fewer raw materials and yielding high-purity products, requires strict reaction conditions, and the instability of the mixed anhydride intermediates also increases the complexity of the operation. The one-pot method features simplified operational steps and atomic economy, making it particularly suitable for target molecules that require multi-step synthesis. The amidation reaction can use different condensation agents and conditions, suitable for a wide range of combinations of carboxylic acids and amines, and by changing different combinations, a variety of amide compounds with diverse structures can be obtained. Overall, each method has its own advantages and limitations. The choice of method depends on the structure of the target compound and the required reaction conditions, as well as specific requirements for yield, purity, and operational convenience. In practical applications, researchers need to flexibly choose or optimize the most appropriate synthetic strategy based on the experimental purpose and conditions.
In terms of detection technology, immunoassays and chromatography are currently the mainstream methods for detecting glycocholic acid. In clinical practice, RIA is widely used to analyze the levels of glycocholic acid in serum, which is of great significance for assessing the severity of liver disease, monitoring disease progression, and predicting prognosis. In vitro detection methods include TLC and LC; the former is mainly used for qualitative analysis, while the latter can, based on the structural characteristics of glycocholic acid, select appropriate detectors and hyphenated techniques to achieve quantitative analysis of glycocholic acid samples.
As research continues to delve deeper, the various pharmacological activities of glycocholic acid are gradually being revealed, including anti-inflammatory and antioxidant effects. Activation of FXR reduces the synthesis of liver lipoproteins, as well as the levels of plasma triglycerides and cholesterol. This is because FXR activation induces the expression of genes involved in lipoprotein metabolism or clearance, while simultaneously suppressing the expression of genes involved in triglyceride synthesis. Furthermore, the activation of FXR can also inhibit the expression of transcription factor SREBP-1c and its downstream hepatic lipid synthesis genes by inducing the expression of SHP, thereby reducing hepatic lipid synthesis. Therefore, it is inferred that glycocholic acid may play a role in regulating glucose and lipid metabolism and inhibiting the expression of gluconeogenesis-related genes. Due to its unique structure, glycocholic acid can improve the oral bioavailability of drugs with low water solubility and low permeability through the formation of micelles and saponification. As a pharmaceutical excipient, glycocholic acid can form mixed micelles with phospholipids, serving as a vehicle for the injection administration of insoluble drugs. This characteristic gives it potential application value in drug delivery systems, especially in improving drug solubility and bioavailability. In summary, the pharmacological activities and drug delivery potential of glycocholic acid make it a compound worthy of further research. With the growth of the modern pharmaceutical field, we can anticipate that glycocholic acid and its derivatives will play an increasingly important role in drug development and the treatment of various diseases.
Through a comprehensive analysis of domestic and international data, we have found that research on glycocholic acid is still in a relatively early stage, and there are several main issues: Firstly, the synthesis process is relatively outdated and needs further optimization to improve efficiency and reduce costs. Secondly, the application range of detection technology is limited, and more hyphenated techniques need to be developed to enhance the accuracy and sensitivity of analysis. Thirdly, research on the physiological activities and pharmacological mechanisms of glycocholic acid is not deep enough, and further exploration of its specific mechanisms of action in organisms is required. Lastly, there have been no reports on the development of glycocholic acid into formulations, indicating that there is still a lot of room for development in clinical applications and drug development. Therefore, future research on glycocholic acid compounds should focus on the following areas: Firstly, optimizing the synthesis process to improve synthesis efficiency and product purity; secondly, developing and applying new detection technologies, especially hyphenated techniques, to enhance the accuracy and sensitivity of analysis; thirdly, conducting in-depth studies on the physiological activities and pharmacological mechanisms of glycocholic acid to provide a scientific basis for clinical applications. At the same time, strengthening the research on the combination of glycocholic acid drugs with other medications may better exert therapeutic effects, meet the medication needs of a broad range of patients, and promote the development of traditional Chinese medicine.
Author contributions
JL: Investigation, Writing–original draft, Writing–review and editing. CZ: Formal Analysis, Funding acquisition, Resources, Supervision, Writing–original draft, Writing–review and editing. YW: Investigation, Supervision, Writing–review and editing. MT: Investigation, Supervision, Writing–review and editing. CX: Investigation, Supervision, Writing–review and editing. HH: Investigation, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors would like to acknowledge the research funding support by the National Natural Science Foundation of China (No. 81503257); Inner Mongolia Major science and technology project (No. 2021ZD0017); Liaoning Provincial Science and Technology Programme Joint Programme (Applied Basic Research Project) (2023JH2/101700206); Liaoning University of Traditional Chinese Medicine-Natural Science University Key Project (2021LZY047); National Key Research and Development Program (No. 2018YFC1706903); Liaoning Provincial Department of Education university basic scientific research project reserve project (LJ212410162055).
Conflict of interest
Author CZ was employed by Qimeng Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agu, R. U., Vu Dang, H., Jorissen, M., Kinget, R., and Verbeke, N. (2004). Metabolism and absorption enhancement of methionine enkephalin in human nasal epithelium. Peptides 25 (4), 563–569. doi:10.1016/j.peptides.2004.02.019
Amoako-Tuffour, M., Yeung, P. K., and Agu, R. U. (2009). Permeation of losartan across human respiratory epithelium: an in vitro study with Calu-3 cells. Acta. Pharm. 59 (4), 395–405. doi:10.2478/v10007-009-0038-3
Aungst, B. J., Rogers, N. J., and Shefter, E. (1988). Comparison of nasal, rectal, buccal, sublingual and intramuscular insulin efficacy and the effects of a bile salt absorption promoter. J. Pharmacol. Exp. Ther. 244 (1), 23–27. doi:10.1002/jps.2600770118
Bagger, M. A., Nielsen, H. W., and Bechgaard, E. (2001). Nasal bioavailability of peptide T in rabbits: absorption enhancement by sodium glycocholate and glycofurol. Eur. J. Pharm. Sci. 14 (1), 69–74. doi:10.1016/s0928-0987(01)00146-4
Bao, X. S., and Bao, J. P. (1998). Serum gamma-aminobutyric acid test and its clinical application. Chin. J. Prim. Med. Pharm. (06), 22.
Cai, Y. F., Zhang, Z. C., Lu, Y., Qi, J. P., and Wu, W. (2020). Research progress on effect and mechanism of bile salts in promoting oral absorption of drugs. Chin. J. Pharm. 51 (12), 1529–1540. doi:10.16522/j.cnki.cjph.2020.12.006
Cayley, A. N., Cox, R. J., Ménard-Moyon, C., Schmidt, J. P., and Taylor, R. J. K. (2007). Deacetalisation–bicyclisation routes to novel polycyclic heterocycles using stannous chloride dihydrate. Tetrahedron Lett. 48 (37), 6556–6560. doi:10.1016/j.tetlet.2007.07.018
Chen, F. P., and Luo, Z. L. (2020). A method for extracting high-purity conjugated bile acids from bovine bile. Patent 111635447B. Hong Kong, China Pat. Appl.
Chen, S. Y., Su, X. Y., Wang, X. M., Li, B., Xu, Q., Yue, P. F., et al. (2023). Oral mucosal drug delivery system based on nano technology. Acta. Pharm. Sin. 58 (05), 1245–1255. doi:10.16438/j.0513-4870.2022-1245
Cravotto, G., Boffa, L., Turello, M., Parenti, M., and Barge, A. (2005). Chemical modifications of bile acids under high-intensity ultrasound or microwave irradiation. Steroids 70 (2), 77–83. doi:10.1016/j.steroids.2004.09.007
Cui, X., Vasylieva, N., Wu, P., Barnych, B., Yang, J., Shen, D., et al. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay for glycocholic acid based on chicken single-chain variable fragment antibodies. Anal. Chem. 89 (20), 11091–11097. doi:10.1021/acs.analchem.7b03190
Deneer, V. H., Drese, G. B., Roemelé, P. E., Verhoef, J. C., Lie, A. H. L., Kingma, J. H., et al. (2002). Buccal transport of flecainide and sotalol: effect of a bile salt and ionization state. Int. J. Pharm. 241 (1), 127–134. doi:10.1016/s0378-5173(02)00229-6
Dobson, T. E., Maxwell, A. R., and Ramsubhag, A. (2018). Antimicrobial cholic acid derivatives from the Pitch Lake bacterium Bacillus amyloliquefaciens UWI-W23. Steroids 135, 50–53. doi:10.1016/j.steroids.2018.04.008
Donovan, M. D., Flynn, G. L., and Amidon, G. L. (1990). The molecular weight dependence of nasal absorption: the effect of absorption enhancers. Pharm. Res. 7 (8), 808–815. doi:10.1023/a:1015904730599
Fan, Q., Zhang, Y., Hou, X., Li, Z., Zhang, K., Shao, Q., et al. (2018). Improved oral bioavailability of notoginsenoside R1 with sodium glycocholate-mediated liposomes: preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int. J. Pharm. 552 (1-2), 360–370. doi:10.1016/j.ijpharm.2018.10.005
Feng, W., Liu, X. X., Hu, J., and Chao, R. B. (2014). Analysis of related substances in glycochoic acid. Chin. J. Pharm. Anal. 34 (07), 1200–1203. doi:10.16155/j.0254-1793.2014.07.033
Ge, X., Huang, S., Ren, C., and Zhao, L. (2023). Taurocholic acid and glycocholic acid inhibit inflammation and activate farnesoid X receptor expression in LPS-stimulated zebrafish and macrophages. Molecules 28 (5), 2005. doi:10.3390/molecules28052005
Gianluca, G., Massimo, P., and Pierfranca, Z. (2010). Method for the synthesis of glycocholic acid. W. O. Patent 2010128472. Eur. Pat. Off. Pat. Appl.
Gong, Y. B., Guo, Y. T., and Zhang, W. (2021). Protective effect of chrysanthemum extract against retinal light damage in mice. Recent. Adv. Ophthalmol. 41 (02), 110–115. doi:10.13389/j.cnki.rao.2021.0023
Grote, J., Beligere, G., and Rege, S. (2012). Methodology for the regiospecific synthesis and characterization of methotrexate conjugates. Tetrahedron Lett. 53 (39), 5331–5334. doi:10.1016/j.tetlet.2012.07.104
Guan, H., Li, P. F., Li, H. F., and He, X. L. (2009). Research on the anti-inflammatory mechanism of glycocholic acid. Heilongjiang Anim. Sci. Vet. Med. (19), 102–103. doi:10.13881/j.cnki.hljxmsy.2009.19.052
Guo, M. J., Li, H., Du, S. S., Yang, X. Y., Xie, C., and Dong, J. (2023). Interleukin-6, virus infection and immunity. Chin. J. Virol. 39 (01), 287–294. doi:10.13242/j.cnki.bingduxuebao.004248
Han, Z., and Chen, Q. S. (2019). Establishment and performance evaluation of homogeneous enzyme immunoassay for glycocholic acid. Chin. J. Health Laboratory Technol. 29 (19), 2402–2404+2407. doi:10.26914/c.cnkihy.2018.033192
Harai, S., Ikenaga, T., and Matsuzawa, T. (1978). Nasal absorption of insulin in dogs. Diabetes 27, 296–299. doi:10.2337/diab.27.3.296
Hayakawa, E., Chien, D. S., Inagaki, K., Yamamoto, A., Wang, W., and Lee, V. H. (1992). Conjunctival penetration of insulin and peptide drugs in the albino rabbit. Pharm. Res. 9 (6), 769–775. doi:10.1023/a:1015803605621
He, Z., Ye, F., and Zhang, G. X. (2021). The role of gut microbiota in the treatment of inflammatory bowel disease with anti-tumor necrosis factor-α biologics. Chin. J. Gastroenterol. Hepatol. 30 (01), 16–20. doi:10.3969/j.issn.1006-5709.2021.01.004
Heubi, J. E., Setchell, K. D., Jha, P., Buckley, D., Zhang, W., Rosenthal, P., et al. (2015). Treatment of bile acid amidation defects with glycocholic acid. Hepatology 61 (1), 268–274. doi:10.1002/hep.27401
Hu, S., Niu, M., Hu, F., Lu, Y., Qi, J., Yin, Z., et al. (2013). Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 441 (1-2), 693–700. doi:10.1016/j.ijpharm.2012.10.025
Huang, F. (2024). Breast health lecture: how to prevent breast diseases? Medical Health and Wellness Newspaper 2024-02-02.
Huang, K., and Sun, M. J. (2022). Research progress in oral delivery technology of therapeutic peptides. Chin. J. New. Drugs. 31 (14), 1380–1386. doi:10.3969/j.issn.1003-3734.2022.14.007
Huang, X. T. (2019). Establishment and application of candidate reference method for glycocholic acid. [master’s thesis]. Guangzhou: Guangzhou University of Chinese Medicine.
Jasti, B. R., Zhou, S., Mehta, R. C., and Li, X. (2000). Permeability of antisense oligonucleotide through porcine buccal mucosa. Int. J. Pharm. 208 (1-2), 35–39. doi:10.1016/s0378-5173(00)00543-3
Ji, M., Wang, X. J., Yu, W. Y., Zhou, G. M., and Liu, L. F. (2024). “A method for preparing glycocholic acid,” in Jiangsu Province patent application.
Jia, W., Xie, G., and Jia, W. (2018). Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15 (2), 111–128. doi:10.1038/nrgastro.2017.119
Ju, Y. H., Yao, W. F., and Zhang, L. (2020). Progress in application of bile acid metabolism in traditional Chinese medicine study. China. J. Chin. Mat. Med. 45 (10), 2360–2367. doi:10.19540/j.cnki.cjcmm.20200221.301
Kang, J. H., Tan, C. K., and Zhang, J. W. (2018). The significance of cholyglycine in the diagnosis of chronic liver disease. J. Hubei Univ. Chin. Med. 20 (03), 102–103. doi:10.3969/j.issn.1008-987x.2018.03.30
Katsuma, M., Watanabe, S., Kawai, H., Takemura, S., and Sako, K. (2006). Effects of absorption promoters on insulin absorption through colon-targeted delivery. Int. J. Pharm. 307 (2), 156–162. doi:10.1016/j.ijpharm.2005.09.028
Kong, W., Wang, J., Xing, X., Xiao, X., Zhao, Y., Zang, Q., et al. (2011). Antifungal evaluation of cholic acid and its derivatives on Candida albicans by microcalorimetry and chemometrics. Anal. Chim. Acta 689 (2), 250–256. doi:10.1016/j.aca.2011.01.050
Li, D. Q., Zhao, B. Q., Zhang, N. Y., and Li, X. F. (2000a). Therapeutic effect of pulmonary inhaled insulin aerosol on rats of experimental diabetes. J. Xi'an Jiaot. Univ. Med. Sci. (05), 439–441+487. doi:10.3969/j.issn.1671-8259.2000.05.015
Li, D. Q., Zhao, B. Q., Zhang, N. Y., Shang, Q. C., and Li, X. F. (2000b). Study on making and animal experiment of pulmonary inhaled insulin aerosols. Med. J. Natl. Defending Forces Northwest China (03), 225–226. doi:10.16021/j.cnki.1007-8622.2000.03.036
Li, F., Tian, J. Z., Wang, J. Z., and Bi, Y. P. (2019). Research progress in rectal drug delivery system. Chin. Tradit. Pat. Med. 41 (05), 1115–1118. doi:10.3969/j.issn.1001-1528.2019.05.031
Li, H. F. (2006). Studies on anti-inflammatory effects and its mechanisms of glycocholic acid. [master’s thesis]. Inner Mongolia: Inner Mongolia Agricultural University.
Li, H. F., Li, P. F., Guan, H., Li, Y. J., and Wang, D. Z. (2006). Studies on anti-inflammation actions of glycocholic acid. J. Inn. Mong. Agric. Univ. Nat. Sci. (02), 5–8. doi:10.3969/j.issn.1009-3575.2006.02.002
Li, P. F., Zhao, Z., Guan, H., and Jin, S. L. (2007). Effect of glycocholic acid on immune function in mice. Chin. J. Vet. Med. (10), 6–8. doi:10.3969/j.issn.0529-6005.2007.10.002
Li, S. C. (2009). [doctoral's thesis]. Studies on extraction and purification of effective componentsfrom the mixed bovine and ovine bile and pharmacokinetics of glycocholic acid in rats. Inner Mongolia: Inner Mongolia Agricultural University.
Li, S. C., and Li, P. F. (2009). Determining the glycocholeic acid of rats blood serum with SPE-HPLC. Acta. Agric. Boreali-Sinica 24 (01), 215–218. doi:10.7668/hbnxb.2009.01.048
Li, S. C., Li, P. F., and Guang, H. (2010). Pharmacokinetics study of glycocholic acid in rat plasma. Animal Husb. and Veterinary Med. 42 (02), 52–54.
Li, X. Y., Su, L., Xu, Y., Jiang, Y. M., Song, J., Weng, W. C., et al. (2018). Study on autitumor mechanism of Treg cell PI3K/AKT pathway of Xihuang Pill regulation in tumor microenvironment. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 20 (01), 49–54. doi:10.11842/wst.2018.01.008
Li, Y., Deng, S. Q., and Ren, J. (2014). Influence of enhancers on oral absorption of Traditional Chinese Medicine. J. Chengdu Univ. Nat. Sci. Ed. 33 (03), 204–207+217. doi:10.3969/j.issn.1004-5422.2014.03.003
Li, Y. Q., and Huang, S. W. (2012). Research progress in the absorption mechanisms of mouth mucosal drug delivery system. Chin. J. New. Drugs. 21 (05), 512–517.
Liang, W. B., Xiang, R. R., Jiang, Y. M., Song, J., and Tong, Y. C. (2021). “The application of glycocholic acid in the preparation of anti-breast hyperplasia drugs,” in Liaoning Province patent application.
Liang, W. B., Xiang, R. R., Jiang, Y. M., Tong, Y. C., and Song, J. (2019). “The application of glycocholic acid in the preparation of anti-cancer drugs,” in Liaoning Province patent application.
Liang, Y. L., Liao, J. Z., Wang, K., Xie, X. L., and Yi, B. (2017). Comparison of homogeneous enzyme immunoassay and latex-enhanced immunoturbidimetric assay for the determination of cholyglycine. Lab. Med. 32 (08), 718–721. doi:10.3969/j.issn.1673-8640.2017.08.013
Liao, P., Cao, M., Zhang, J. C., and Chen, G. L. (2023). Opportunities and challenges of nasal drug delivery. Chin. J. Mod. Appl. Pharm. 40 (20), 2761–2765. doi:10.13748/j.cnki.issn1007-7693.20232901
Lin, H., Gebhardt, M., Bian, S., Kwon, K. A., Shim, C. K., Chung, S. J., et al. (2007). Enhancing effect of surfactants on fexofenadine. HCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 330 (1-2), 23–31. doi:10.1016/j.ijpharm.2006.08.043
Lindhardt, K., and Bechgaard, E. (2003). Sodium glycocholate transport across Caco-2 cell monolayers, and the enhancement of mannitol transport relative to transepithelial electrical resistance. Int. J. Pharm. 252 (1-2), 181–186. doi:10.1016/s0378-5173(02)00629-4
Liu, C., Liu, W., Liu, Y., Duan, H., Chen, L., Zhang, X., et al. (2023). Versatile flexible micelles integrating mucosal penetration and intestinal targeting for effectively oral delivery of paclitaxel. Acta Pharm. Sin. B 13 (8), 3425–3443. doi:10.1016/j.apsb.2023.05.029
Liu, G. X., Fang, G. Q., Guo, H., and Gu, D. J. (2014). Recent advances in oral mucosal permeation enhancers. China. Pharm. 23 (10), 90–92.
Liu, J. T. (2012). The application value of serum gamma-aminobutyric acid test in liver damage. Lab. Med. 27 (08), 688–691. doi:10.3969/j.issn.1673-8640.2012.08.020
Liu, Y., Tan, P., Liu, S., Shi, H., Feng, X., and Ma, Q. (2015). A new method for identification of natural, artificial and in vitro cultured Calculus bovis using high-performance liquid chromatography-mass spectrometry. Pharmacogn. Mag. 11 (42), 304–310. doi:10.4103/0973-1296.153083
Long, F. L., Wang, N., Wang, S., Xie, L., Wang, M. G., Zhang, R. Z., et al. (2017). Metabonomics analysis on serum metabolites from rats with acute hepatic failure. J. Guangxi Med. Univ. 34 (01), 12–15. doi:10.16190/j.cnki.45-1211/r.2017.01.003
Luo, X. L. (1994). Competitive radioimmunoassay in the analysis of animal protein hormones. Chin. Qinghai J. Animal Veterinary Sci. (05), 43–45.
Mahalingam, R., Ravivarapu, H., Redkar, S., Li, X., and Jasti, B. R. (2007). Transbuccal delivery of 5-aza-2 -deoxycytidine: effects of drug concentration, buffer solution, and bile salts on permeation. AAPS PharmSciTech 8 (3), E55. doi:10.1208/pt0803055
Maroni, A., Del Curto, M. D., Salmaso, S., Zema, L., Melocchi, A., Caliceti, P., et al. (2016). In vitro and in vivo evaluation of an oral multiple-unit formulation for colonic delivery of insulin. Eur. J. Pharm. Biopharm. 108, 76–82. doi:10.1016/j.ejpb.2016.08.002
Mishima, M., Nagatomi, A., Yamakita, T., Miura, Y., and Tsuzuki, O. (1995). Promotion of rectal absorption of sodium ampicillin by disodium glycyrrhetinic acid 3 beta-O-monohemiphthalate in rats. Biol. Pharm. Bull. 18 (4), 566–570. doi:10.1248/bpb.18.566
Mitome, H., Tokumasu, N., Tando, Y., Nakamura, T., and Akira, K. (2013). An efficient laboratory-scale preparative method for [1-(13)C]glycocholic acid. J. Label. Compd. Radiopharm. 56 (11), 587–588. doi:10.1002/jlcr.3072
Moghimipour, E., Ameri, A., and Handali, S. (2015). Absorption-enhancing effects of bile salts. Molecules 20 (8), 14451–14473. doi:10.3390/molecules200814451
Moghimipour, E., Tabassi, S. A., Ramezani, M., Handali, S., and Löbenberg, R. (2016). Brush border membrane vesicle and Caco-2 cell line: two experimental models for evaluation of absorption enhancing effects of saponins, bile salts, and some synthetic surfactants. J. Adv. Pharm. Technol. Res. 7 (3), 75–79. doi:10.4103/2231-4040.184588
Momose, T., Tsubaki, T., Lida, T., and Nambara, T. (1997). An improved synthesis of taurine- and glycine-conjugated bile acids. Lipids 32 (7), 775–778. doi:10.1007/s11745-997-0099-8
Mu, Y., Wu, Y. X., Zhang, Y. T., Zeng, Y. X., and Zhou, Z. F. (2023). Simultaneous and rapid determination of 6 primary bile acids in plasma by high-performance liquid chromatography-tandem mass spectrometry. Chin. J. Public. Health 39 (01), 102–106. doi:10.11847/zgggws1138567
Nielsen, H. M., and Rassing, M. R. (1999). TR146 cells grown on filters as a model of human buccal epithelium: III. Permeability enhancement by different pH values, different osmolality values, and bile salts. Int. J. Pharm. 185 (2), 215–225. doi:10.1016/s0378-5173(99)00165-9
Niu, M., Lu, Y., Hovgaard, L., Guan, P., Tan, Y., Lian, R., et al. (2012). Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 81 (2), 265–272. doi:10.1016/j.ejpb.2012.02.009
Niu, M. M. (2011). Liposomes containing bile salts as potential oral insulin delivery systems. [doctoral's thesis]. Fudan: Fudan University.
Pandey, B., Bhattarai, J. K., Pornsuriyasak, P., Fujikawa, K., Catania, R., Demchenko, A. V., et al. (2014). Square-wave voltammetry assays for glycoproteins on nanoporous gold. J. Electroanal. Chem. 717-718, 47–60. doi:10.1016/j.jelechem.2014.01.009
Qiu, N., Fang, W. J., Li, C., Li, X. M., and Xiong, Y. (2016). Endogenous nitric oxide synthase inhibitor increase skeletal muscle contractility and mitochondria biosynthesis in 4-week running rats. Chin. J. Pathophysiol. 32 (07), 1259–1265. doi:10.3969/j.issn.1000-4718.2016.07.017
Ray, A., Banerjee, A., Chang, C., Khantwal, C. M., and Swaan, P. W. (2006). Design of novel synthetic MTS conjugates of bile acids for site-directed sulfhydryl labeling of cysteine residues in bile acid binding and transporting proteins. Bioorg. Med. Chem. Lett. 16 (6), 1473–1476. doi:10.1016/j.bmcl.2005.12.050
Ren, Y., Jiang, Y. M., Xiang, R. R., Tong, Y. C., Song, J., and Liang, W. B. (2019). Autitumor mechanism of Xihuang Pills component-based Chinese medicine by regulating Treg cells PI3K/AKT pathway in tumor microenvironment. Drug Eval. Res. 42 (03), 437–443. doi:10.7501/j.issn.1674-6376.2019.03.010
Roncancio, D., Yu, H., Xu, X., Wu, S., Liu, R., Debord, J., et al. (2014). A label-free aptamer-fluorophore assembly for rapid and specific detection of cocaine in biofluids. Anal. Chem. 86 (22), 11100–11106. doi:10.1021/ac503360n
Russell, D. W. (2009). Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 50 (Suppl. l), S120–S125. doi:10.1194/jlr.R800026-JLR200
Setchell, K. D., Heubi, J. E., Shah, S., Lavine, J. E., Suskind, D., Al-Edreesi, M., et al. (2013). Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology 144 (5), 945–955. doi:10.1053/j.gastro.2013.02.004
Shao, Z., and Mitra, A. K. (1992). Nasal membrane and intracellular protein and enzyme release by bile salts and bile salt-fatty acid mixed micelles: correlation with facilitated drug transport. Pharm. Res. 9 (9), 1184–1189. doi:10.1023/a:1015808023310
Shi, Y., Sun, D. M., Xiong, J., Wei, F., Ma, S. C., and Lin, R. C. (2013). Study on determination of cholic acid and glycocholic acid from capra ovis fellis pulvis by HPLC-ELSD. Chin. Pharm. Aff. 27 (09), 935–937. doi:10.16153/j.1002-7777.2013.09.007
Shojaei, A. H., Berner, B., and Xiaoling, L. (1998). Transbuccal delivery of acyclovir: I. in vitro determination of routes of buccal transport. Pharm. Res. 15 (8), 1182–1188. doi:10.1023/a:1011927521627
Song, B. (2022). Research progress of application and development of oral drug absorption enhancers. China. Pharm. 31 (22), 127–132. doi:10.3969/j.issn.1006-4931.2022.22.033
Su, K. S., Campanale, K. M., Mendelsohn, L. G., Kerchner, G. A., and Gries, C. L. (1985). Nasal delivery of polypeptides I: nasal absorption of enkephalins in rats. J. Pharm. Sci. 74 (4), 394–398. doi:10.1002/jps.2600740406
Sun, F., Ye, C., Thanki, K., Leng, D., van Hasselt, P. M., Hennink, W. E., et al. (2018). Mixed micellar system stabilized with saponins for oral delivery of vitamin K. Colloids Surf. B 170, 521–528. doi:10.1016/j.colsurfb.2018.06.049
Suzuki, K., Kim, K. S., and Bae, Y. H. (2019). Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J. Control. Release 294, 259–267. doi:10.1016/j.jconrel.2018.12.028
Tao, H. (1987). Brief Overview of homogeneous enzyme-linked immunosorbent assay. Chin. Pharm. J. (12), 745–748.
Tong, Y., Wang, Y., Yang, M., Yang, J., Chen, L., Chu, X., et al. (2018). Systematic development of self-nanoemulsifying liquisolid tablets to improve the dissolution and oral bioavailability of an oily drug, vitamin K1. Pharmaceutics 10 (3), 96. doi:10.3390/pharmaceutics10030096
Uchida, N., Maitani, Y., Machida, Y., Nakagaki, M., and Nagai, T. (1991). Influence of bile salts on the permeability of insulin through the nasal mucosa of rabbits in comparison with dextran derivatives. Int. J. Pharm. 74 (2), 95–103. doi:10.1016/0378-5173(91)90226-E
Venturoni, F., Gioiello, A., Sardella, R., Natalini, B., and Pellicciari, R. (2012). Continuous flow synthesis and scale-up of glycine- and taurine-conjugated bile salts. Org. Biomol. Chem. 10 (20), 4109–4115. doi:10.1039/c2ob25528f
Wang, K. P., and Shi, C. Z. (2000). Overview and prospects of animal bile Medicinal Research. Chin. J. Tradit. Med. Sci. Technol. (05), 350–352.
Wang, Q. W., Han, J., Zeng, X. G., Huang, Q. F., Fan, T., Zeng, F. Q., et al. (2021). “The preparation method of glycocholic acid ester and glycocholic acid,” in Sichuan Province patent application.
Wang, W. L. (2014). The application of fluorescence-labeled high-performance liquid chromatography-mass spectrometry in food analysis. [master’s thesis]. Qufu: Qufu Normal University.
Wang, X., Chen, X., Shi, H. B., Yang, H., Zhang, X. X., Xu, W. M., et al. (2020). Research progress on synthesis,determination and pharmacological effects of animal bile and bile acids. Jiangsu Agric. Sci. 48 (18), 36–43. doi:10.15889/j.issn.1002-1302.2020.18.007
Wang, Y. F. (2016). Influences of different factors on glycocholic acidextraction from bovine bile and the methodology research of determining the glycocholic acid by HPLC. [master’s thesis]. Inner Mongolia: Inner Mongolia Agricultural University.
Warden, C., and Brantley, M. A. (2021). Glycine-conjugated bile acids protect RPE tight junctions against oxidative stress and inhibit choroidal endothelial cell angiogenesis in vitro. Biomolecules 11 (5), 626. doi:10.3390/biom11050626
Wu, G. M., and Han, X. F. (2020). Progress in quantitative analysis of bile acids by chromatographic techniques. Prog. Physiol. Sci. 51 (03), 233–238. doi:10.3969/j.issn.0559-7765.2020.03.020
Wu, W. D., Yin, C., and Zhang, H. F. (2020a). Mechanism of effects of bile acids on glycolipid metabolism. Chin. J. Anim. Nutr. 32 (10), 4565–4576. doi:10.3969/j.issn.1006-267x.2020.10.010
Wu, Y. F. (2013). Study on antioxidation effect of GCA on mice. [master’s thesis]. Inner Mongolia: Inner Mongolia Agricultural University.
Wu, Y. F., Shi, L. L., Li, P. F., Guan, H., Yao, Q. Z., and He, X. L. (2020b). Effect of glycocholic acid (GCA) on anti-oxidation capacity of mouse peritoneal macrophages. Animal Husb. Feed Sci. 41 (04), 77–80. doi:10.12160/j.issn.1672-5190.2020.04.015
Xia, D. F. (1995). Serum gamma-aminobutyric acid assay technology and its clinical application. Med. Recapitulate. (04), 164–166.
Xiang, R. R., Jiang, Y. M., Ren, Y., Tong, Y. C., Song, J., and Liang, W. B. (2019). Anti-breast cancer effect of farnesoid X receptor activated by alcohol extract of Xihuang Pill. Drug Eval. Res. 42 (06), 1087–1091+1127. doi:10.7501/j.issn.1674-6376.2019.06.006
Xin, H., Ding, Y. X., and Li, P. F. (2011). Effects of glycocholic acid on spleen lymphocytes apoptosis in vitro in mice. J. Inn. Mong. Agric. Univ. Nat. Sci. Ed. 32 (01), 28–33.
Xu, Q. F., and Fang, X. L. (2001). In vitro porcine buccal permeation of propranolol hydrochloride and its influencing factors. J. Fudan. Univ. Med. Sci. (02), 148–151. doi:10.3969/j.issn.1672-8467.2001.02.019
Xu, Z. J., Lu, W. J., Li, X. W., Lu, H., Li, S., Li, M. H., et al. (2010). ChemInform abstract: synthesis and characterization of fine particle ZnFe2O4 powders by a low temperature method. Fine. Chem. 27 (12), 1216–1219. doi:10.1002/chin.199631027
Xuan, B., McClellan, D. A., Moore, R., and Chiou, G. C. (2005). Alternative delivery of insulin via eye drops. Diabetes Technol. Ther. 7 (5), 695–698. doi:10.1089/dia.2005.7.695
Yamamoto, A., Hayakawa, E., Kato, Y., Nishiura, A., and Lee, V. H. (1992). A mechanistic study on enhancement of rectal permeability to insulin in the albino rabbit. J. Pharmacol. Exp. Ther. 263 (1), 25–31. doi:10.1007/BF01061471
Yamamoto, A., Hayakawa, E., and Lee, V. H. (1990). Insulin and proinsulin proteolysis in mucosal homogenates of the albino rabbit: implications in peptide delivery from nonoral routes. Life Sci. 47 (26), 2465–2474. doi:10.1016/0024-3205(90)90492-a
Yamamoto, A., Luo, A. M., Dodda-Kashi, S., and Lee, V. H. (1989). The ocular route for systemic insulin delivery in the albino rabbit. J. Pharmacol. Exp. Ther. 249 (1), 249–255. doi:10.1016/0160-5402(89)90034-X
Yamamoto, A., Umemori, S., and Muranishi, S. (1994). Absorption enhancement of intrapulmonary administered insulin by various absorption enhancers and protease inhibitors in rats. J. Pharm. Pharmacol. 46 (1), 14–18. doi:10.1111/j.2042-7158.1994.tb03712.x
Yang, F. R., Gao, J. P., and Li, C. Y. (2021). Polysaccharide extraction in lactuca satyal and its antihyperlipidemic activity. J. Agric. 11 (11), 81–87. doi:10.11923/j.issn.2095-4050.cjas20191000234
Yao, F. C., Zhang, J. R., and Zhang, H. (2003). Analysis of serum thyroid hormones and ghrelin levels in 213 patients with viral hepatitis. Mod. J. Integr. Tradit. Chin. West. Med. (10), 1085–1086. doi:10.3969/j.issn.1008-8849.2003.10.067
Yao, L. W., Shi, Y., Feng, W., and Ma, S. C. (2021). Investigation, determination and discussion on bile acids in sheep bile. Chin. J. Pharm. Anal. 41 (10), 1718–1723. doi:10.16155/j.0254-1793.2021.10.07
Ye, J., Xu, Q. E., Jiang, X. R., and Xia, Y. F. (2014). Improvement of the process for sodium gslycocholate. Chem. Ind. Times. 28 (02), 18–20. doi:10.3969/j.issn.1002-154X.2014.02.006
Yue, Z. H., Sun, H. M., Tian, S. J., and Guo, Y. N. (2004). The contents of cholesterol, bilirubin and various cholic acids in human gallstones were determined by HPLC-ELSD-UV. Chin. J. Pharm. Anal. 24 (03), 310–314.
Zang, W., Gao, D., Yu, M., Long, M., Zhang, Z., and Ji, T. (2023). Oral delivery of gemcitabine-loaded glycocholic acid-modified micelles for cancer therapy. ACS Nano 17 (18), 18074–18088. doi:10.1021/acsnano.3c04793
Zhang, M. J., Zhao, X. H., Yu, L., and Deng, Z. H. (2016). Four clinical comparisons of methods for determining glycocholic acid in serum. Lab. Med. Clin. 13 (12), 1676–1677. doi:10.3969/j.issn.1672-9455.2016.12.032
Zhang, R. C., Yang, Y. Y., Tan, T., and Sun, H. F. (2015). Research progress on serum gamma-glutamyltransferase detection method. Sci. Consult. Sci. and Manag. (08), 58–59. doi:10.3969/j.issn.1671-4822.2015.29.038
Zhang, R. X., Cui, X. Y., Ma, Y., Liu, J. H., Zhang, Z. T., Gu, R. Z., et al. (2019). Effects of lycium chinense mill fermentation liquid on antioxidant activity and immunoregulation. Food. Res. Dev. 40 (10), 55–60. doi:10.3969/j.issn.1005-6521.2019.10.010
Zhang, T., Li, M., Nie, G. J., and Zheng, A. P. (2022). The mechanism of different absorption enhancers and research advances in nasal administration. Chin. J. Hosp. Pharm. 42 (08), 870–875. doi:10.13286/j.1001-5213.2022.08.20
Zhang, W. K., Cheng, X. F., Li, Z. K., Wang, B. B., and Meng, B. X. (2024). Research progress on mechanism of pugongying (Taraxaci Herba) against breast disease. Chin. Arch. Tradit. Chin. Med. 42 (02), 212–216. doi:10.13193/j.issn.1673-7717.2024.02.039
Zhang, Y., and Zhu, Q. (1997). The diagnostic significance of serum gamma-glutamyl transpeptidase test in liver diseases. Jiangxi Med. J. (01), 40–41.
Zhang, Y. H., Liu, J. Z., Peng, X., Li, Z. Q., and Zhang, W. J. (2009). HPLC comparison of bile acids in fellis ursi powder,fellis suis powder, calculus bovis powder, fellis caprinus powder and fellis galli powder. Chin. J. Pharm. Anal. 29 (03), 487–490.
Zhang, Y. T., Song, Y. H., Huang, H. F., Wang, P., Zhou, J. X., and Jin, X. H. (2017). Clinical practice and comparison of glycocholic acid test with other liver function indicators. Int. J. Lab. Med. 38 (05), 680–682. doi:10.3969/j.issn.1673-4130.2017.05.043
Zhao, R., Shi, Y. L., Jiang, X. W., Huang, J. D., and Zhang, Z. F. (2019). Clinical significance of detection of serum cholyglycine in the diagnosis of liver disease. Lab. Med. Clin. 16 (13), 1823–1825+1828. doi:10.3969/j.issn.1672-9455.2019.13.009
Zhao, S. Q., Shen, D., Cui, X. P., Xia, N. N., Lv, R., and Liang, Y. X. (2020). “A method for preparing a monoclonal antibody against glycocholic acid using cholic acid as raw material,” in Guangdong Province patent application.
Zhao, Z. (2006). Studies on extraction and purification techniques and immunological effects of glycocholic acid. [master’s thesis]. Inner Mongolia: Inner Mongolia Agricultural University.
Zhao, Z., and Li, P. F. (2005). Research overview of glycocholic acid. Inn. Mong. Sci. Technol. and Econ. (15), 84–86. doi:10.3969/j.issn.1007-6921.2005.15.038
Zhao, Z., Li, P. F., Li, H. F., Guan, H., and Li, Y. J. (2006). Studies on extraction and purification techniques of glycocholic acid from bovine and sheep bile. J. Inn. Mong. Agric. Univ. Nat. Sci. (02), 9–12. doi:10.3969/j.issn.1009-3575.2006.02.003
Zhou, C. H., Han, X. L., Li, M., Wang, Z. M., Li, C. M., Wang, X. T., et al. (2022). Research and recent progress of rectal drug delivery system. J. Pharm. Res. 41 (02), 107–112. doi:10.13506/j.cnki.jpr.2022.02.009
Zhou, H. Y., Zheng, Y., Wang, H. D., Cao, J., Zhang, H., Zhang, H. Y., et al. (2024). Antioxidant activity of schisandra sphenanthera and schisandra chinensis protein on HepG2 cells. Food. Ferment. Ind. 50 (07), 51–60. doi:10.13995/j.cnki.11-1802/ts.034355
Zhou, Q. W., Shi, Y., Liu, W., Xiong, J., Wei, F., Lin, R. C., et al. (2015). The research of quantity test method of various components in succession medicinal substances of cow-bezoar. Chin. J. Pharm. Anal. 35 (01), 8–15. doi:10.16155/j.0254-1793.2015.01.002
Keywords: glycocholic acid, synthetic process, pharmacological effect, absorption enhancer, research progress
Citation: Li J, Zhang C, Wang Y, Tian M, Xie C and Hu H (2025) Research progress on the synthesis process, detection method, pharmacological effects and application of glycocholic acid. Front. Pharmacol. 15:1492070. doi: 10.3389/fphar.2024.1492070
Received: 06 September 2024; Accepted: 09 December 2024;
Published: 03 January 2025.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Sandeep K. Singh, University of Nebraska Medical Center, United StatesXiaolin Qian, Southern Research Institute, United States
Copyright © 2025 Li, Zhang, Wang, Tian, Xie and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chungang Zhang, emhhbmdjaHVuZ2FuZ3BoYXJtQG91dGxvb2suY29t
†These authors have contributed equally to this work
 Jiahui Li1†
Jiahui Li1† Chungang Zhang
Chungang Zhang