- 1Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 2Anshun Hospital of Traditional Chinese Medicine, Anshun, China
Miao medicine Tiekuaizi (Radix Chimonanthi) has been successfully used by Guizhou Miao ethnic physicians in clinical treatments, demonstrating significant curative effects. The research progress on the resource distribution, traditional uses, phytochemistry, and pharmacology of Tiekuaizi was reviewed by collecting related literature from traditional Chinese medicine books and various databases. Based on the comprehensive review and centered around the principles of traditional Chinese medicine quality marker (Q-Marker) theory, nine secondary metabolites (scopoletin, scopolin, isofraxidin, fraxin, scoparone, calycanthoside, 6, 7, 8-trimethoxycoumarin, tomenin, and calycanthine) are suggested as potential Q-markers for Tiekuaizi to establish quality control standards. This can provide a valuable reference for the collection and processing, pharmaceutical production, and effectiveness and safety of clinical applications of Miao medicine Tiekuaizi.
1 Introduction
Miao medicine Tiekuaizi (Radix Chimonanthi) is the dried fine root of Chimonanthus praecox (L.) Link and Chimonanthus nitens Oliv, a genus of Calycanthaceae, a characteristic ethnic medicine of Guizhou, with the effect of removing wind and alleviating pain (QuFengZhiTong) and circulating blood and removing toxins (HuoXueJieDu). It is used for treating asthma, strain and cough, stomach pain, abdominal pain, rheumatism, paralysis, boils and swellings, bruises, injuries, etc. It has been widely used in clinics and has also formed a large-scale pharmaceutical industry, such as JinGuShang spray, TongLuoGuZhiNing ointment, FuFangXianLingFengShi liquor, ShengLongQuFeng medicinal liquor, FuFangXieTeng medicinal liquor, MaLanGanHan capsules, Liangjiang Weiyang capsules, Ganqing syrup, and other special varieties (Guizhou Provincial Drug Administration, 2019). Tiekuaizi was first recorded in the Materia Medica and is also included in the Chinese Materia Medica (Miao Medicine Volume) (Qiu et al., 2006), Zhong Yao DaCi Dian (Nanjing University of Traditional Chinese Medicine, 2006), and the 2019 edition of the Guizhou Quality Standards for Chinese and Ethnic Medicinal Herb (Guizhou Provincial Drug Administration, 2019). However, the content only includes the plant source, morphology, and identification of medicinal materials. There are no records of the index ingredients of Miao medicine Tiekuaizi, and there is no standard basis for systematically explaining the relationship between “ingredients – quality – efficacy,” which does not meet the requirements of traceability, cultivation, preparation, processing, and quality control. Therefore, the construction of a quality evaluation system that correlates traditional efficacy and clinical efficacy is an important issue for the development of the Tiekuaizi industry and the safety of medicine.
Since the last century, domestic and foreign scholars have studied the genus Chimonanthus and found that the genus is rich in volatile oils, coumarins, and flavonoids (Li et al., 2018; Liu et al., 2018; Wang et al., 2012; Shu et al., 2019; Li et al., 2021; He et al., 2023; Lin, 2019). At present, there are many research reports on the properties of leaves of C. nitens and C. praecox, and many researchers have reviewed their metabolites and pharmacological activities (Ma et al., 2010; Nie et al., 2009; Dong et al., 2021; Yu et al., 2021; Hu and Yang, 2008). However, relatively few studies have been conducted on Tiekuaizi (Radix Chimonanthi, roots of C. nitens and C. praecox). Additionally, geographical differences have resulted in a homonym phenomenon with Helleborus thibetanus, a member of the Ranunculaceae family. Therefore, a review of its resource distribution, clinical medication, chemical composition, and pharmacological effects has guiding significance for correct clinical medication.
Literature search was conducted with the keywords “Radix Chimonanthi,” “C. praecox,” and “C. nitens.” from Web of Science (https://webofscience.clarivate.cn), PubMed (https://pubmed.ncbi.nlm.nih.gov), China National Knowledge Infrastructure (https://www.cnki.net), Wanfang Database (https://www.wanfangdata.com.cn), and Google Scholar (https://scholar.google.com). Manual reading was performed to eliminate duplicate literature works and irrelevant content in the database. Only the research literature works mentioning the roots of C. praecox and C. nitens as medicinal parts were selected. Based on classic Chinese medicine books and relevant literature collected from various databases mentioned above, all eligible studies were analyzed and summarized. A review was conducted on the resource distribution, usage, phytochemistry, and pharmacological effects of the Miao medicine Tiekuaizi. With the continuous research on Tiekuaizi, coumarins, alkaloids, terpenoids, volatile oils, and other secondary metabolites have been isolated from it (Luo et al., 2023). Modern pharmacological studies have found that it has anti-inflammatory effects, analgesic effects, used in treatment of cardiovascular and cerebrovascular diseases, improves disorders of glucolipid metabolism, regulates immunity, and has anti-tumor and other effects (Li and Zou, 2018; Liu et al., 2018; Wang et al., 2012; Shu et al., 2019; Dong et al., 2021; Yu et al., 2021; Hu and Yang, 2008). However, no research has been conducted to systematically explain the quality evaluation of Tiekuaizi from the perspective of a quality marker (Q-marker), which limits the establishment of an evaluation system for the scientific quality of Tiekuaizi. Based on the existing research results of Tiekuaizi and the Q-marker theory of Chinese medicine (Liu et al., 2016), this paper analyzes and predicts the Q-marker of Tiekuaizi in terms of metabolite specificity, effectiveness, measurability, quality transfer traceability, and pharmaceutical rule of TCM (“five principles”) to provide a scientific basis for the establishment of a better quality evaluation standard of Tiekuaizi of Miao medicine.
2 Resource distribution and ethnic clinical medication
Tiekuaizi is widely distributed in Guizhou, Yunnan, Sichuan, and other provinces, and it also known as Zuangufeng, Yanma Sangen, and Tiegangcha. It has a long history of medicinal use in Guizhou (Guizhou Provincial Drug Administration, 2019; Zhu et al., 2020), and the Guiyang Folk Medicinal Herbs records that its formulations can treat iron injuries, stomach pain, cold abdominal pain, coughs from strain, and blood pockets in the abdomen of women (Guiyang Health Bureau, 1959); according to the Chinese Materia Medica (Miao Medicine Volume), it can treat phuang poisonous sores, strain cough, stomach pain, abdominal pain, rheumatism paralysis, healing sores, swelling and poison, bruises, and injuries. Prepared formulations include Liangjiang Weiyang capsules, FuFangXieTeng medicinal liquor, MaLanGanHan capsules, and Ganqing syrup (Qiu et al., 2006). The Colorful Atlas of Chinese Miao Medicines records that it can treat rheumatic pain, stomach pain, and asthma (Wang, 2002); the Research and Development of Miao Medicine in Guizhou records the treatment of bone fractures and rheumatic joint pain (Bao and Ran, 1999); the Chinese Folklore Hundred Herbs and Prescriptions records the treatment of lumbar muscle strain and cold abdominal pain by grouping prescriptions (Zhou, 1998); Guizhou Folk Remedies Collection records treatment for wind paralysis, rheumatic pain, acute rheumatic arthritis, osteophytes, and conventional and new injuries (Yang and Yang, 1978). Different habitats, climates, and land resources will affect the properties of Tiekuaizi. On the other hand, with the annual development of the Tiekuaizi industry and strong market demand, it is urgent to improve its quality evaluation standards and provide the necessary scientific research base for industrial upgrading.
3 Phytochemistry
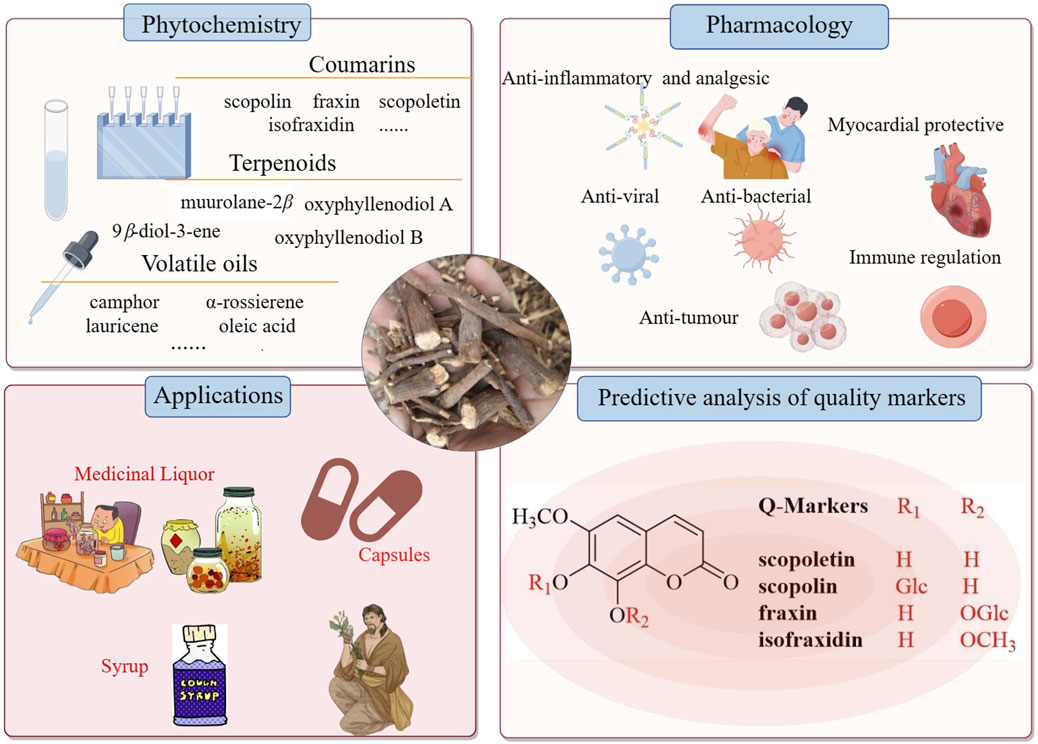
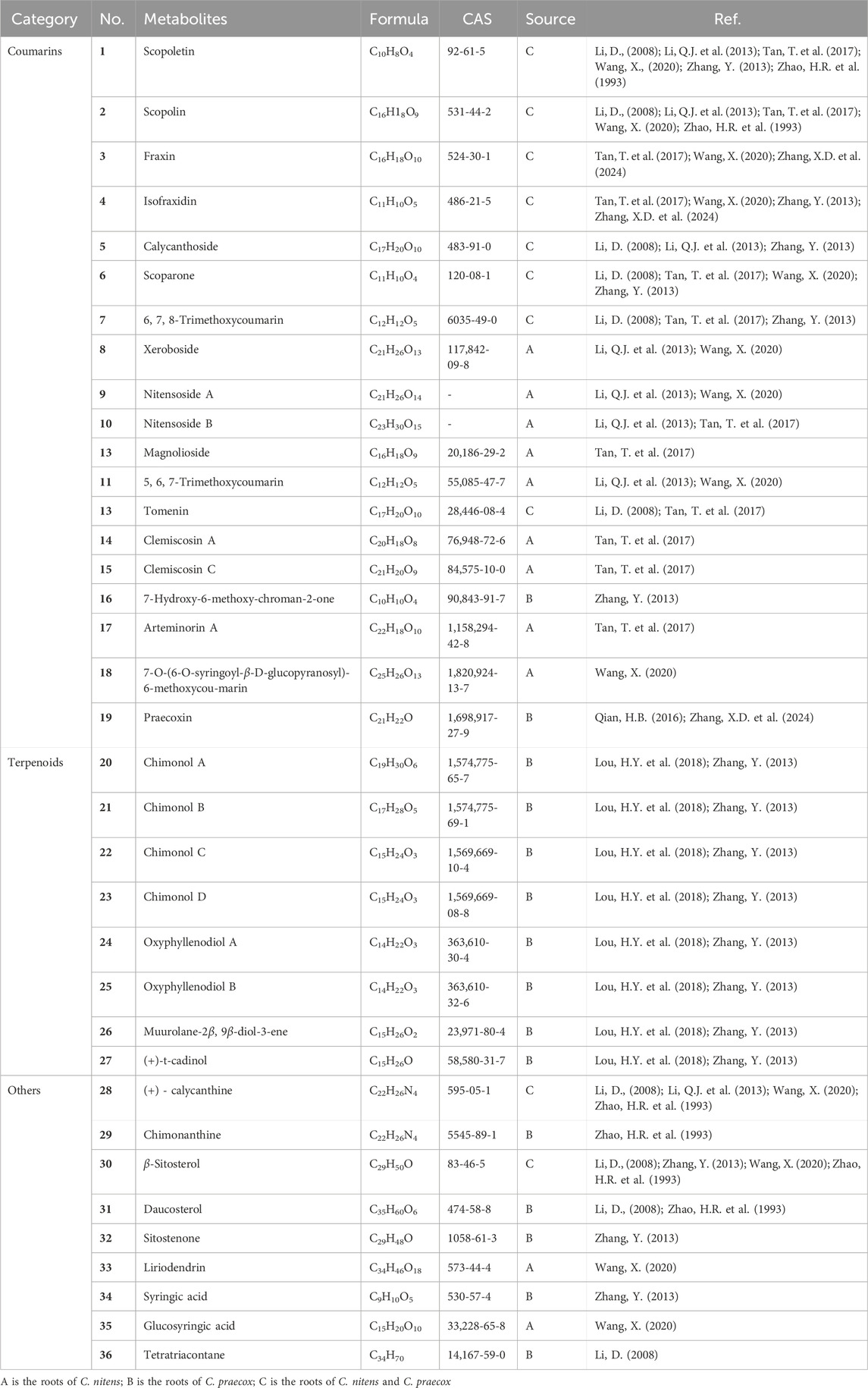
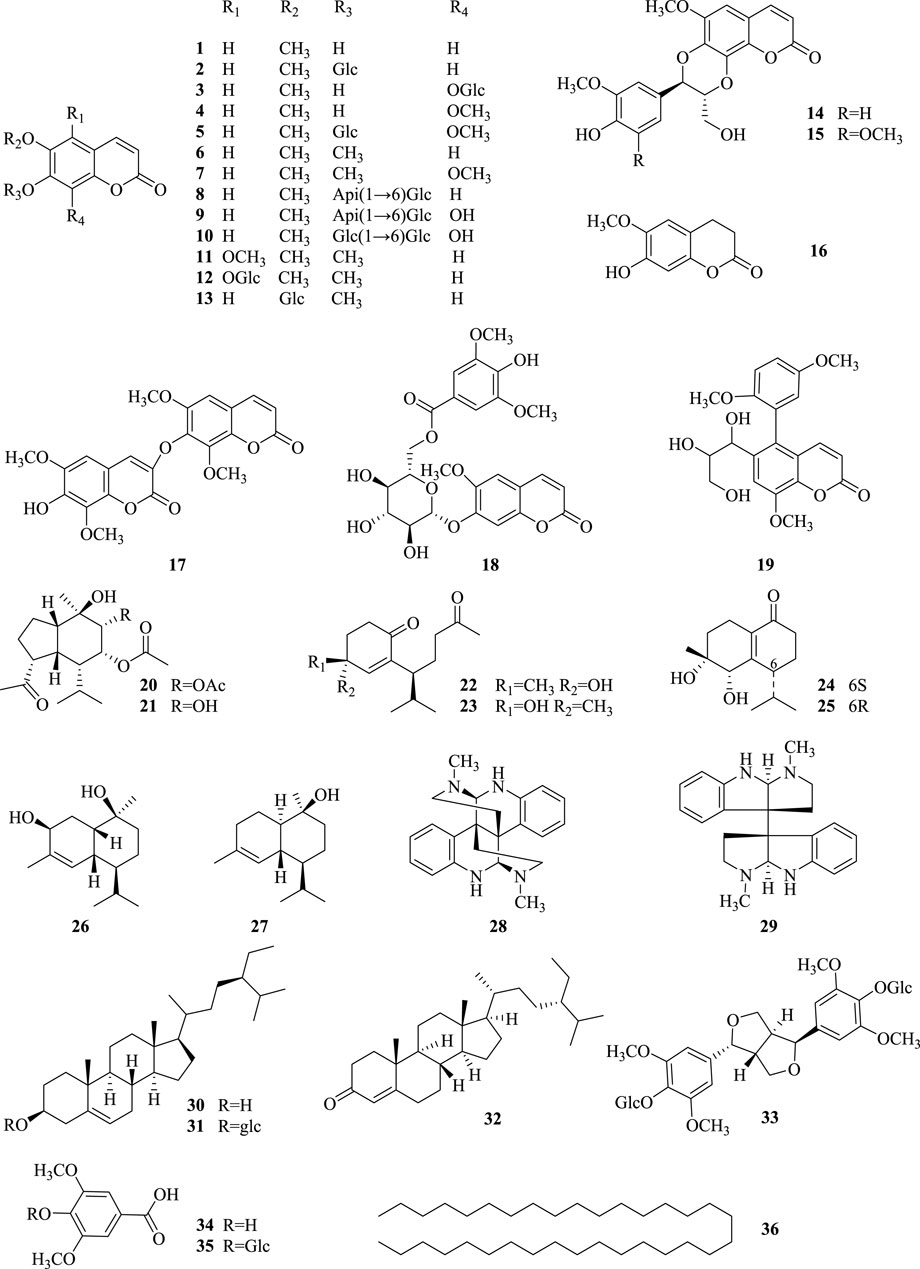
Since the last century, many researchers have been studying the secondary metabolites of the genus Chimonanthus. There have been many literature reports on the secondary metabolites from the leaves of C. nitens and C. praecox, but there have been relatively few reports on the research of roots (the medicinal part of Tiekuaizi). At present, more than 100 secondary metabolites have been isolated and identified from Tiekuaizi, including non-volatile compounds such as coumarins (No. 1–19), terpenoids (no. 20–27), and others (no. 28–36) and volatile oils (no. 37–105). Metabolite information can be found in Tables 1, 2. The structure of the non-volatile secondary metabolites obtained from Tiekuaizi is shown in Figure 1. Among them, coumarins, represented by scopoletin, are important pharmacodynamic markers of Tiekuaizi and have attracted attention due to their significant anti-inflammatory and analgesic effects.
3.1 Non-volatile secondary metabolites
3.1.1 Coumarins
Coumarins are widely found in the roots, stems, leaves, flowers, fruits, and seeds of higher plants and in animals and microorganisms. It is an important natural medicine and an important secondary metabolite for the study of Chinese and ethnic medicines. Coumarins often have hydroxyl and alkoxy substitutions on the parent nucleus, and the main metabolic modes include sulfonation, reduction reactions, and acidification of glucuronides (Kuang, 2023). Modern pharmacology has found that coumarins have a wide range of biological activities. Miao medicine Tiekuaizi mainly contains coumarins, which have anti-inflammatory, analgesic, and antibacterial effects (Zhao et al., 1993; Li, 2008; Zhang, 2013; Qian, 2016). Many coumarins obtained from the roots of C. praecox have been isolated and identified, including scopoletin, scopolin, 6,8-dimethoxy-7-hydroxycoumarin, 6,7,8-trimethoxycoumarin, 6,7-dimethoxycoumarin, tomenin, isozinpyridine, isozinpyridine 7-O-β-D-glucoside, 7-hydroxy-6-methoxy-chroman-2-one, and praecoxin, of which praecoxin is a new coumarin isolated by our research group (Qian, 2016).
In recent years, researchers have also studied the secondary metabolites of the roots of C. nitens. Li et al. (2013) isolated two new coumarins, namely, nitensosides A–B, and five known coumarins from them and determined their antibacterial activity.
Tan et al. (2017) first developed a method based on a modified mass defect filter (MDF) and performed a comprehensive analysis of coumarins from different medicinal parts of C. nitens by ultra-high liquid chromatography–tandem quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS). The MDF-based five-point screening method and the visual isotope ion technique allow rapid screening of precursor ions of interest. The mass spectrometry fragmentation patterns of coumarins were systematically studied, and 32 coumarins, including 19 potential new coumarins, were initially identified unequivocally in C. nitens roots. In 2020, Wang (2020) isolated 10 coumarins from the roots of C. nitens and compared the changes in the contents of four metabolites, namely, scopolin, fraxin, scopoletin, and isofraxidin, after drying in the shade, stoving, steaming, and carbonization.
Comparative analysis shows that the abundance of coumarins in the roots of C. praecox and C. nitens, among which there are eight co-contained coumarins, including scopoletin, scopolin, isofraxidin, fraxin, scoparone, calycanthoside, 6, 7, 8-trimethoxycoumarin, and tomenin.
3.1.2 Terpenoids
In 2018, Lou et al. (2018) isolated four new (Chimonol A–D) and four known sesquiterpenoids (oxyphyllenodiol A, oxyphyllenodiol B, muurolane-2β, and 9β-diol-3-ene and (+)-t-cadinol) from the roots of C. nitens. The antimicrobial activity was evaluated by determining the minimum inhibitory concentrations (MICs) using the micro-broth dilution method.
3.1.3 Others
Except for the above coumarins and terpenoids, Tiekuaizi also contains alkaloids, steroids, and phenolic acids (Zhao et al., 1993; Li, 2008; Wang, 2020), such as (+)-calycanthine, chimonanthine, β-sitosterol, daucosterol, sitostenone, liriodendrin, syringic acid, glucosyringic acid, and tetratriacontane. Some flavonoids are also present in Tiekuaizi, and Duan et al. (2013) studied the mechanism by which the total flavonoids of Tiekuaizi delay D-galactose-induced aging, possibly in relation to scavenging free radicals in the body and improving immune function. However, the isolation and identification of the flavonoid metabolites in Tiekuaizi have not been reported.
3.2 Volatile oils
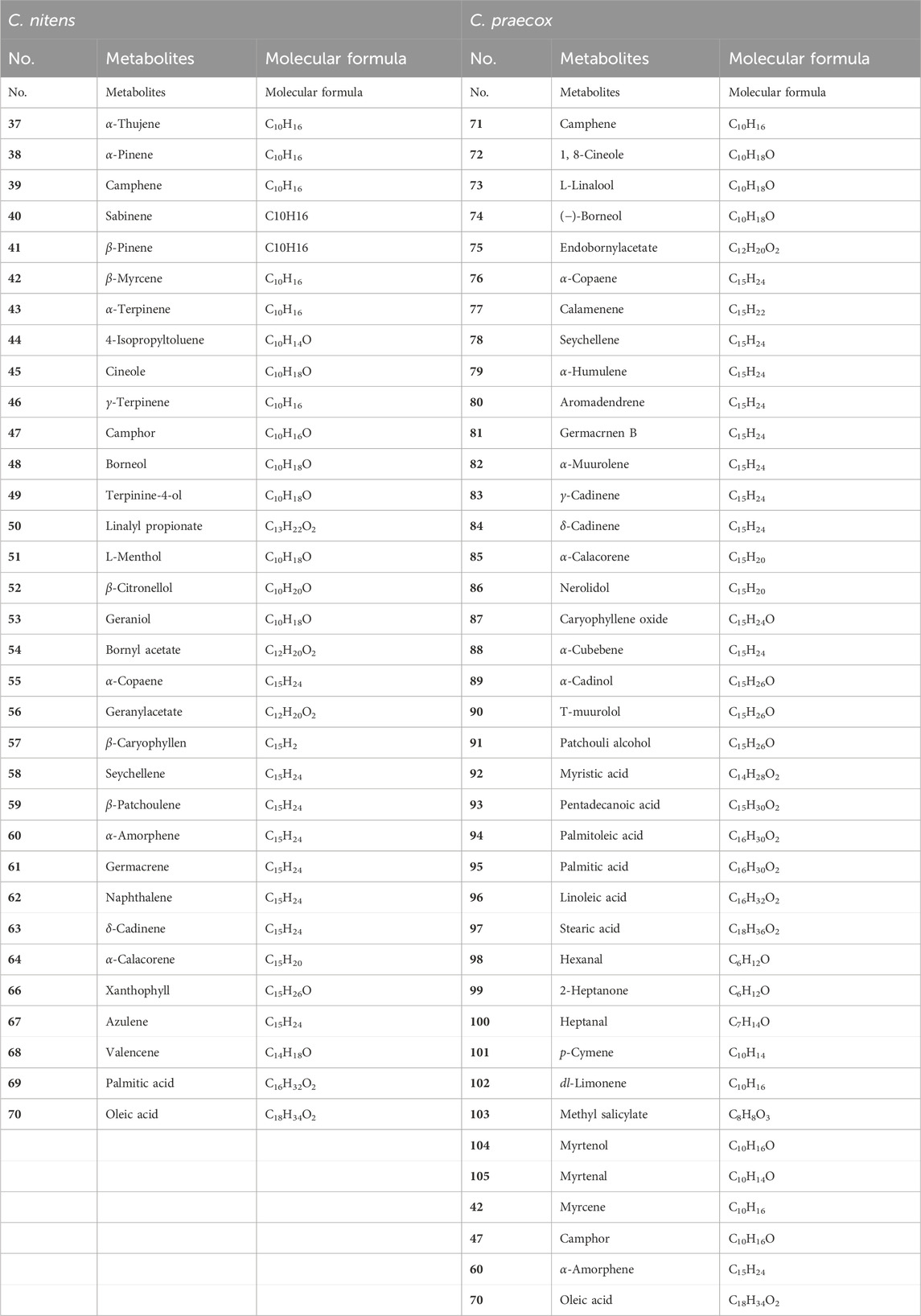
Volatile oils are a mixture with complex secondary metabolites, ranging from tens to hundreds. The components of volatile oils are most commonly terpenoids, together with small non-terpenoid aliphatic and aromatic compounds (Kuang, 2023). Tiekuaizi has a pungent and aromatic flavor. The volatile oils in Tiekuaizi are mainly found in the phloem. Wang et al. (2010) identified 36 secondary metabolites from the volatile oil of the roots of C. nitens using GC–MS, with higher contents of cineole (25.29%), terpinine-4-ol (13.07%), linalyl propionate (10.50%), camphor (7.27%), oleic acid (6.98%), borneol (4.17%), and sabinene (4.10%).
Gao et al. (2011) and Lu et al. (2021) successively analyzed the components of the volatile oil from the roots of C. praecox using the GC–MS method, and 30 and 33 secondary metabolites were detected, respectively, with the main metabolites being the same, including palmitic acid, linoleic acid, oleic acid, and α-cadinol. In contrast, Lu et al. (2021) did not detect the following eight metabolites: aromadendrene, α-amorphene, germacrnen B, α-muurolene, α-cubebene, patchouli alcohol, myristic acid, and pentadecanoic acid) and detected the following ten more metabolites containing hexanal, 2-heptanone, camphor, myrcene, p-cymene, dl-limonene, methyl salicylate, myrtenol, and myrtenal.
Comparative analysis of the volatile oil metabolites in C. praecox and C. nitens showed significant differences, with only camphor, lauricene, α-rossierene, and oleic acid being the co-contained volatile oils, as shown in Table 2.
In summary, Tiekuaizi is rich in volatile oils, coumarins, alkaloids, and terpenoids. Tiekuaizi is a multi-resource Chinese medicine from two species, C. praecox and C. nitens, in the Chimonanthus genus, and the secondary metabolites of Tiekuaizi have not been fully studied from the number of compounds. Based on the existing research of Tiekuaizi, there are only 14 co-contained components, including 10 non-volatile secondary metabolites (No. 1–7, No. 13, No. 28, and No. 30) and four volatile oils (No. 42, No. 47, No. 60, and No. 70). The roots of C. praecox and C. nitens are used as Tiekuaizi for clinical medicine. The quality control of co-contained metabolites and the correlation between co-contained metabolites and drug efficacy need to be studied. In addition, according to the research reports on the secondary metabolites of the other medicinal parts of C. nitens and C. praecox (stems, leaves, flowers, seeds, etc.) (Li et al., 2021), it can be observed that the roots are very different from the other parts. In the future, a modern high-throughput component identification technology can be used to explore the commonality and differences of secondary metabolites in different medicinal parts of the two plants and combined with pharmacological research to study the therapeutic material basis of Tiekuaizi.
4 Pharmacological effects
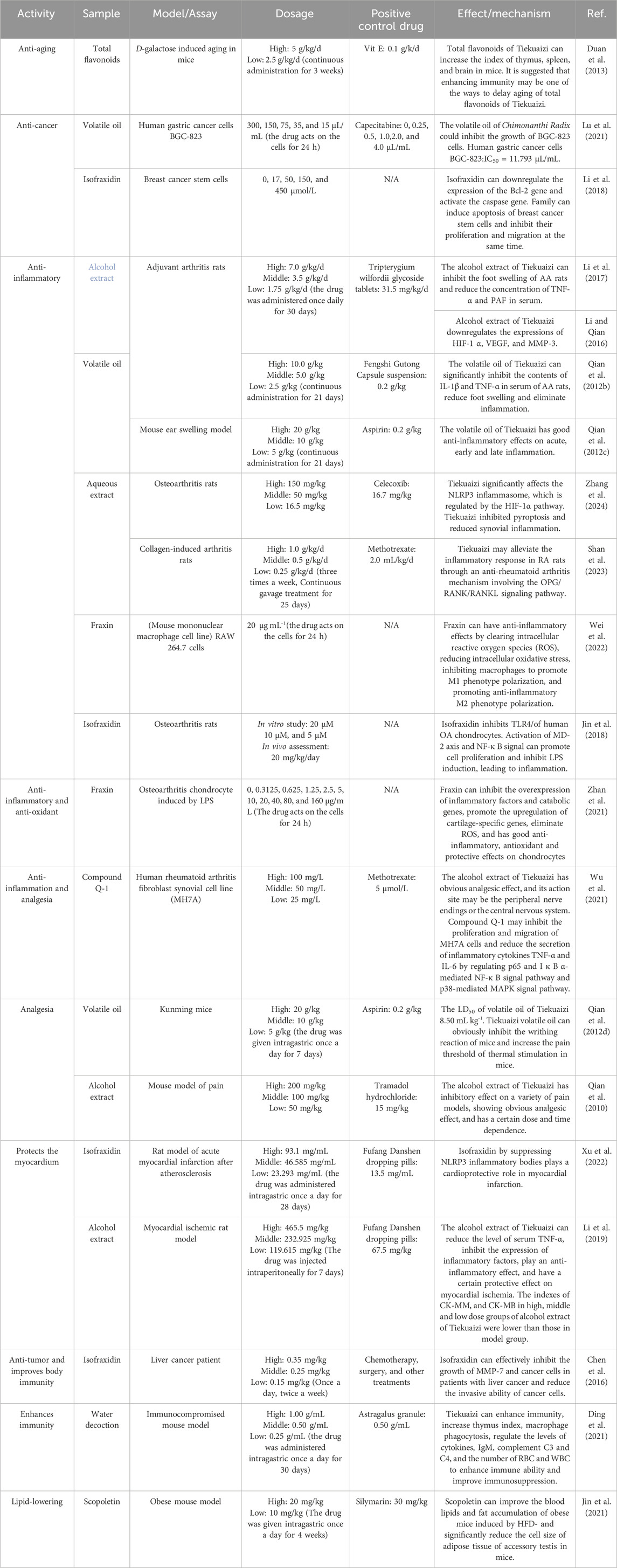
Modern pharmacology has shown that Miao medicine Tiekuaizi has pharmacological effects including anti-inflammatory, analgesic, immune-regulating, and anti-tumor effects; treating cardiovascular and cerebrovascular diseases; and improving disorders of glycolipid metabolism (Table 3). The pharmacologically active metabolites are coumarins, volatile oils, and so on. The main active monomers are the coumarin compounds such as scopoletin, scopolin, isofraxidin, and fraxin.
4.1 Anti-inflammatory and analgesic effects
The alcohol extract of Tiekuaizi can significantly reduce the levels of hypoxia-inducible factor 1-subunit alpha (HIF-1α) and tumor necrosis factor-α (TNF-α) in the serum, as well as the expression of joint matrix metallo proteinase-3 (MMP-3) and vascular endothelial growth factor (VEGF) related to joint swelling and destruction, in the treatment of arthritis in rats (Li et al., 2017; Li and Qian, 2016; Shan et al., 2023; Zhang et al., 2024). In the treatment of rats, a model of myocardial ischemia, the alcoholic extract of Tiekuaizi reduced the expressions of interleukin-18 (IL-18), interleukin-6 (IL-6), and TNF-α and was able to protect against myocardial cell injury produced by inflammatory cell infiltration (Li et al., 2019). The volatile oil of Tiekuaizi significantly inhibited ear swelling in a model of xylene-induced auricular swelling in mice, and it also inhibited the swelling and granulation of the foot and plantar area in mice caused by carrageenan, showing definite anti-inflammatory effects (Qian et al., 2012b; Qian et al., 2012c).
The extracts from various parts of Tiekuaizi have analgesic and anti-inflammatory effects (Zhang, 2013). The analgesic effect was best in the high-dose group at the ethyl acetate site, followed by the low-dose group at the petroleum ether site; the anti-inflammatory effect was best in the low-dose group at the water site, followed by the high-dose group at the ethyl acetate site. In terms of analgesia, the Tiekuaizi alcoholic extract and volatile oil reduces the acetic acid-induced writhing response in mice and significantly increases the pain threshold in the heat radiation tail-flick test and hot plate method in mice (Qian et al., 2010; Qian et al., 2012d; Qian et al., 2012a).
Our research team discovered coumarin monomer compound Q-1 (praecoxin) in the previous study on Tiekuaizi, which can inhibit MAPK and NF-κB signaling pathways and reduce the expressions of inflammatory factors such as IL-6 and TNF-α, and it can also inhibit the cell proliferation of human rheumatoid arthritis (RA) fibroblast-like synovial cell lines, exerting an anti-rheumatoid arthritis effect (Wu et al., 2021).
Tiekuaizi has good anti-inflammatory and analgesic effects as it is rich in coumarins. Studies have shown that coumarin metabolites, such as scopoletin, isofraxidin, and fraxin, can mostly inhibit histamine, NF-κB, and MAPK pathways; inhibit the release of cellular inflammatory factors such as IL-1β, IL-6, and TNF-α; and avoid excessive activation of the immune system, resulting in powerful anti-inflammatory effects (Wei et al., 2009).
4.2 Myocardial protective effect
The alcohol extracts of Tiekuaizi can reduce triglyceride and low-density lipoprotein levels and increase high-density lipoprotein levels in a model of myocardial ischemia in rats, achieving lipid-lowering effects. It also reduces serum creatine kinase (CK) CK-MB and CK-MM levels; decreases the expression of TNF-α, IL-6, and IL-18 during myocardial ischemia; and reduces myocardial damage caused by ischemia (Li et al., 2019).
The Tiekuaizi alcohol extract can downregulate IκB-α, NF-κBp65, and NF-κBp50 mRNA and protein expressions, inhibit the activation of the NF-κB signaling pathway, reduce the inflammatory response, and play a cardioprotective role, which has a good protective effect on the cardiac function of rats in a myocardial ischemic model (Xu et al., 2022). Studies have shown that the coumarin analog isofraxidin alleviates myocardial infarction by inhibiting NLRP3 inflammasome activity (Chen et al., 2020).
4.3 Anti-viral and anti-bacterial effects
The volatile oil of the roots of C. praecox has the strongest antibacterial activity, and the compositional analysis showed mainly more monoterpenes than in the leaves and peel. It is possible that this metabolite is the active metabolite of the root volatile oil in inhibiting fungi, especially against pathogenic fungi and Gram-positive bacteria, with a broader spectrum of inhibitory activities (Wang et al., 2009). It is, therefore, customary in folklore to use Tiekuaizi for inflammatory conditions of the gastrointestinal tract, presumably in relation to its strong antibacterial properties.
In an in vitro test, the metabolite calycanthine found in Tiekuaizi showed significant inhibitory activity against five phytopathogenic fungi Exserohilum turcicum, Bipolaris maydis, Alternaria solani, Sclerotinia sclerotiorum, and Fusarium oxysporum, with B. maydis being the most sensitive with an EC50 value of 29.3 mg mL-1 (Zhang et al., 2009).
The metabolites chimonol C, chimonol D, oxyphyllenodiol A, oxyphyllenodiol B, muurolane-2β, and 9β-diol-3-ene, isolated from C. praecox roots, showed inhibitory activity against Staphylococcus smoothus (ATCC 2001) and Staphylococcus aureus (ATCC 43300) with MIC values of 128–197 μg mL-1. The metabolite chimonol A–D showed antibacterial activity against S. aureus (ATCC 25923) with MIC values of 162–254 μg mL-1 (Lou et al., 2018).
4.4 Anti-tumor effects
In cell proliferation inhibition experiments, it was found that Tiekuaizi could significantly inhibit the growth and proliferation of BGC-823 tumor cells in vitro, and the inhibitory effect was positively correlated with the concentration (Lu et al., 2021). It has been reported that isofraxidin can synergistically produce anti-tumor effects by blocking the tumor cell cycle in the G2/M phase, inducing apoptosis, and reducing tumor cell invasion (Chen et al., 2016; Li et al., 2018).
4.5 Immune regulation
When immunity is disturbed, it can cause severe hypersensitivity reactions and diseases such as RA and immunodeficiency. Tiekuaizi, on the other hand, has a good immunoprotective effect and can enhance the immune capacity of immunosuppressed mice, increase the thymic index and the body weight of immunosuppressed mice, and enhance hypersensitivity reactions by a mechanism related to the increased secretion of expression factors such as IL-6, TNF-α, IgM, and complement C3; on the other hand, Tiekuaizi can also act on blood cells and increase the number of peripheral blood leukocytes, while the phagocytic index of phagocytes decreases (Ding et al., 2021).
Research has found that scopoletin, fraxin, and isofraxidin can avoid over-activation of the immune system by inhibiting macrophage polarization, inhibiting NF-κB and MAPK pathways, and suppressing the release of IL-1β, IL-6, and TNF-α cellular inflammatory factors (Chu et al., 2019; Wei et al., 2022; Jin et al., 2018). Tiekuaizi has strong regulatory effects on the immune system and contains more than three metabolites. The above research results may provide a theoretical basis for the use of Tiekuaizi in the treatment of autoimmune diseases.
4.6 Anti-aging effects
Tiekuaizi also has anti-aging effects, which can enhance human cognitive and learning abilities. The flavonoids in Tiekuaizi have a delaying effect on d-galactose-induced aging in experimental animals. The anti-aging mechanism of Tiekuaizi is probably through increasing the content of SOD, which enhances the body’s anti-aging system and increases the body’s ability to remove peroxidized lipid products, thus achieving anti-aging effects. It also achieves the anti-aging effect in experimental animals by reducing the number of free radicals in the body and enhancing the immunity in the body and by reducing the number of free radicals in the body and alleviating the neuroimmune damage to the central nervous system (Duan et al., 2013).
Tiekuaizi volatile oil improved the learning and memory capacity in CCH-induced VCI rats, improved the neuronal cell structure, increased the number of Nisus vesicles in the brain, inhibited apoptosis, upregulated serum BDNF levels and hippocampal ChAT activity, decreased hippocampal AChE activity, activated the BDNF/TrkB/PI3KJAkt signaling pathway, improved exploratory capacity, and increased the liking for novelty in rats (Ding et al., 2022).
4.7 Others
Research studies have shown that scopoletin can significantly reduce the expression of cholesterol-regulatory element-binding proteins 1 alpha (SREBP-1α), peroxisome proliferator-activated receptor γ (PPAR-γ), and other genes in mouse epithelial fat. The expression of genes such as proliferator-activated receptor γ (PPAR-γ) (Jin et al., 2021) reduces lipid synthesis, inhibits α-glucosidase and α-amylase activities to lower postprandial glucose, and also inhibits protein tyrosine phosphatase 1B (PTP1B) activity. It also inhibits the expression of protein tyrosine phosphatase 1B (PTP1B) and reduces insulin resistance (Jang et al., 2018), regulating glucolipid metabolism in several ways. Based on the methods and techniques of network pharmacology, it was found that Tiekuaizi mainly treats cerebral ischemic stroke (CIS) through key targets such as NOS3, SRC, and PPARG based on neuroactive ligand–receptor interactions and calcium signaling pathways by promoting revascularization, neuroprotection, and anti-inflammation, which also reflects the characteristics of Chinese medicine such as multi-target and multi-linkage in the treatment of CIS (Wang et al., 2022). In addition, volatile oil is a natural transdermal absorption enhancer that promotes drug absorption, is less irritating to the skin, and can play a certain therapeutic role. It has been found that the drug is more effective when administered transdermally, and volatile oil from Tiekuaizi can be added to microemulsions as a transdermal absorption enhancer, and volatile oils can be combined with microemulsions for better efficacy (Lu et al., 2020).
In summary, Tiekuaizi contains a variety of medicinal metabolites that synergistically induce anti-inflammatory, analgesic, antibacterial and antiviral, cardioprotective, immunomodulatory, and anti-tumor effects through a variety of pathways and has great clinical use. These effects are closely related to its abundance of coumarins, alkaloids, and terpenoids as active components. However, Tiekuaizi is rich in various substances, and current research is not comprehensive. Further in-depth research is needed on the pharmacological substance basis.
5 Toxicity
According to the Guizhou Provincial Drug Administration, quality standards for Chinese herbal medicines and ethnic medicinal materials in Guizhou Province, the Miao medicine Tiekuaizi has slight toxicity. There are reports (Knapp et al., 2023) that acute tonic–clonic seizures, tinnitus, disappearance of pupil light reflex, and abnormal vital signs occurred when sheep consumed the leaves of C. praecox in excess, and it is presumed that the toxicity of Tiekuaizi is related to the large amount of calycanthine detected in the stomach contents. Alkaloids are a class of natural organic compounds containing nitrogen, widely existing in plants, and most of them have strong physiological activities. Poisoning or death can occur when the dose is incorrectly administered (Zhu and Lu, 1992). Toxicity studies on Tiekuaizi showed that the volatile oil of Tiekuaizi could make mice curl up and sleep for approximately 20 min; mild toxicity cases showed abdominal convulsions and severe toxicity cases showed respiratory depression and even death, and the mice’s heart, liver, kidney, and other important organs showed no significant changes, with an LD50 value of 8.50 mL/kg. The analysis indicated that the volatile oil of Tiekuaizi has lower toxicity, being equivalent to 850 times the daily clinical dose (15 g) for an adult weighing 60 kg (Qian et al., 2012d). Acute toxicity was detected by the concentration gradient of the alcohol extract (0.25 g/kg, 0.50 g/kg, 1.00 g/kg, 2.00 g/kg, 4.00 g/kg, 8.00 g/kg, and 16.00 g/kg). The results showed that the concentration above 1.00 g/kg could cause death in rats, so 1.00 g/kg was considered the maximum safe dose (Shan et al., 2023). The LD50 values of charcoal products dried in the shade and subjected to stoving, steaming, and carbonization were 4,118.13 mg/kg, 3,733.36 mg/kg, 1,643.61 mg/kg, and >10,000 mg/kg, respectively. The toxic reactions were hind limb extension, stiffness and shivering, etc. The target organs may be the central nervous system and the neuromuscular system. By comparing the contents and toxicity of four coumarins across four Tiekuaizi products, it was found that while there was no significant difference in the content of two glucoside toxic metabolites, the levels of these metabolites were significantly reduced, and the carbonized charcoal products were non-toxic (Wang et al., 2020). Although there is evidence that the toxicity of Tiekuaizi may be related to its volatile oils, coumarins, and alkaloids, these secondary metabolites are also the main active components in Tiekuaizi. Consequently, it remains unclear whether the toxic components of Tiekuaizi are also its pharmacodynamic components. Furthermore, the mechanisms underlying the toxic reactions associated with Tiekuaizi and the detoxification processes following carbonization are also not well understood. For example, what happens to the chemical composition? It is also a direction for further research to find out its toxic components and its toxic mechanism and how to reduce toxicity and enhance or maintain the efficacy of Tiekuaizi.
6 Predictive analysis of quality markers
6.1 Predictive analysis of quality markers based on phylogeny and evidence of secondary metabolite specificity of the original plants
The genus Chimonanthus belongs to Calycanthaceae and is characteristic to China, mainly in the mountainous areas of subtropical river valleys south of the Qinling Mountains, west of the Yunnan–Guizhou Plateau, and north of the Nanling Mountains (Dai, 2012). Chinese scholars have divided the genus into eight species and one variety, including C. nitens, C. praecox, C. salicifolius, C. grammatus, C. baokangensis, C. campanulatus, C. zhejiangensis, C. Grammatus. anhuiensis, C. caespitosa, and C. campanulatus var. guizhouensis (Li et al., 2021). Among them, C. nitens and C. praecox are widely distributed species in Guizhou, and their roots, stems, leaves, flower buds, and fruits are used for medicinal purposes (Li and Zou, 2018). The genus Chimonanthus has been extensively studied due to its rich production of volatile oils, flavonoids, coumarins, and other bioactive metabolites, and these active substances are now widely used in medicine, pesticides, spices, food, and oil (Li and Zou, 2018; Liu et al., 2018). Coumarins are the main physiologically active substances in the medicinal plants of this genus (Li and Zou, 2018; Liu et al., 2018; Wang et al., 2012; Shu et al., 2019). Through the analysis of the genetic relationship, the difference of components, and the endemic characteristics of the genus of Chimonanthus plants, the coumarins in Tiekuaizi can be used as an important basis and feasible way to determine the quality markers.
6.2 Predictive analysis of quality markers based on the correlation between the components and efficacy
The theory of properties and actions of Chinese medicinal is a high-level summary of its properties and characteristics. The “four properties and five flavors” is one of the core contents, and “the five flavors” theory is often used as an important basis in clinical medication and combination of medicines and, therefore, is one of the bases for determining quality markers (Yue et al., 2023). Tiekuaizi is pungent, bitter, and warm in nature, entering the lung, liver, heart, and bladder meridians (Guizhou Provincial Drug Administration, 2019), and it is used for the treatment of rheumatic paralysis, bruises, and swelling pains. In the medicinal theory of Chinese medicine, the therapeutic material basis of “bitter” and “pungent” should have their characteristics and functional properties. The bitter flavor can have drying and expelling effects, and it has the effect of clearing away fire and heat, dipping fire and storing yin, and opening up the bowels. In the relationship between bitter principles and meridians, bitter medicines belong to the lung, liver, and stomach meridians. In the relationship between bitter principles and metabolites, those with bitter flavor mostly contain flavonoids, volatile oils, alkaloids, quinones, and glycosides, while bitter–warm and bitter–cold medicines use volatile oils and alkaloids as the main sources of properties and flavors, respectively (Zhang et al., 2016). “Pungent” has the ability to move and disperse and has the effect of dispersing, moving Qi and blood, etc. It mainly enters the heart, spleen, and lung meridians. The therapeutic material includes alkaloids, volatile oils, terpenoids, and glycosides (Zhang M. et al., 2018). In view of this, the coumarins and their glycosides, flavonoids, alkaloids, and volatile oils in Tiekuaizi are the main material basis for the “pungent and bitter” flavor and for the attribution of meridians, and the Q-markers for Tiekuaizi should be selected among the coumarins and their glycosides, alkaloids, and volatile oils.
Coumarins such as isofraxidin, scopoletin, fraxin, and scopolin are the main therapeutic material basis of Tiekuaizi (Luo et al., 2023), which have been found to have anti-inflammatory and analgesic effects, improve cardiovascular and cerebrovascular diseases, improve disorders of glucose and lipid metabolism, and regulate immunity and anti-tumor effects, which have great similarities with traditional efficacy. Tiekuaizi is commonly used as an ethnic medicine for alleviating rheumatic pain and bruises. Therefore, it is concluded that coumarin metabolites represented by scopoletin, etc., should be the main active ingredients to clear heat and remove toxins, resolve swelling, and alleviate pain and can be considered Q-markers of Tiekuaizi.
6.3 Predictive analysis of quality markers based on measurability
Based on original plant affinities and the correlation between traditional medicinal properties and metabolites, the Tiekuaizi Q-marker was selected mainly among alkaloids, coumarins, and volatile oils. However, the volatile oils are complex, and the two plants contain different metabolites, making them unsuitable for selection as Q-markers. Alkaloids and coumarins are suitable for determination by chromatographic methods. Researchers have established stable and simple methods for the determination of coumarin-like metabolites in Tiekuaizi, including scopoletin, scopolin, isofraxidin, fraxin, and scoparone (Zhong and Feng, 2017; Bai et al., 2010; Tang et al., 2015; Xiao et al., 2019; Zhou et al., 2013). Calycanthoside, 6, 7, 8-trimethoxycoumarin, and tomenin can also be identified by techniques such as LC–MS chromatographic techniques (Li et al., 2013; Wang, 2020). The above substances are both specific and active, while metabolites that can be easily determined by chromatography are more suitable candidates of Q-markers of Tiekuaizi. In addition, the alkaloid component of Tiekuaizi, calycanthine, has also established a content determination method (Wan et al., 2022). This component shows a good antibacterial effect (Zhang et al., 2009). However, its overdose induces more serious toxic reactions (Knapp et al., 2023), and its toxic-effect relationship needs further study. It is speculated that calycanthine is not only a toxic substance showing slight toxicity but also a pharmacodynamic substance, and it can also be used as a quality marker.
6.4 Predictive analysis of quality markers based on blood-accessible chemical components
In a pharmacokinetic study of the main pharmacodynamic components of Tiekuaizi (Zhang J. et al., 2018), the metabolic reactions of scopoletin and scopolin are similar, with different metabolites in different biological samples. The main metabolic reactions in urine and feces are hydrolysis, isomerization, and reduction, and those in plasma are prototype, hydrolysis, reduction, and glucuronidation. A total of three coumarin prototypes (scopoletin, scopolin, and isofraxidin) and 11 metabolites were identified in the study. Through the identification of metabolite products and analysis of their main metabolic pathways, it was revealed that these three coumarin metabolites underwent metabolic reactions such as glucuronidation, sulfonation, and reduction in rats, which are important blood-entry components. These results can provide the pharmacokinetic reference for the selection of Tiekuaizi Q-markers.
7 Conclusion
In this paper, based on a review of the current status of research on the chemical composition and pharmacological effects of Miao medicine Tiekuaizi from Guizhou, the screening and identification of Q-markers for Tiekuaizi based on their chemical composition, relevance to clinical use, measurable metabolites, traditional medicinal efficacy, and metabolism of blood components was analyzed and validated in the literature and research data, and coumarins, alkaloid metabolites, were suggested as potential Q-markers for Tiekuaizi, such as scopoletin, scopolin, isofraxidin, fraxin, scoparone, calycanthoside, 6, 7, 8-trimethoxycoumarin, tomenin, and calycanthine. This was done to screen and identify Tiekuaizi Q-markers and establish it as the quality control standard, which can provide a reference for the collection and processing, pharmaceutical production, and effectiveness and safety of clinical applications of Miao medicine Tiekuaizi.
8 Discussion
Tiekuaizi has a promising future in anti-inflammatory, immunomodulatory, anti-tumor, hypoglycemic, cardiovascular protection, and anti-viral aspects, but there are still many issues to be resolved in the research on Tiekuaizi. First, the pharmacokinetic and toxicity evaluation of Tiekuaizi metabolites need to be improved. The specific metabolic processes of Tiekuaizi in the body are unclear, and corresponding in vivo pharmacokinetic tests should be carried out to elucidate the absorption, transformation, distribution, and excretion processes of Tiekuaizi in the human body and identify the main pharmacodynamic substance basis for their efficacy. Toxicity evaluation and risk avoidance of Tiekuaizi are also key issues that need to be addressed before clinical application. Preliminary toxicity evaluation and investigation of the relationship between the toxicity and efficacy of Tiekuaizi metabolites should be carried out as extensively as possible in future studies, on the basis of which the effective concentration and safe concentration ranges should be further defined, with a view to providing a reference for rational and safe clinical medication. Second, histological techniques should be used to clarify the mechanism of action of Tiekuaizi metabolites. The current means of research on Tiekuaizi is relatively backward compared to the cutting-edge exploration of mechanisms in recent years. Therefore, on the basis of clarifying the action of Tiekuaizi metabolites, relying on genomics, transcriptomics, proteomics, and metabolomics as well as cutting-edge technologies such as single-cell sequencing and epigenetic assays, multi-dimensional detection and screening of more prominent and in-depth action targets of the active metabolites of Tiekuaizi can provide a more thorough and accurate understanding of its pharmacological mechanism of action.
Author contributions
LL: Investigation, Visualization, Writing–original draft, and Writing–review and editing. XZ: Conceptualization and Writing–review and editing. HM: Conceptualization and Writing–review and editing. JM: Methodology and Writing–review and editing. XP: Formal analysis and Writing–review and editing. MD: Supervision and Writing–review and editing. CH: Funding acquisition, Project administration, Resources, Supervision, and Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82160805), the Guizhou Provincial Basic Research Program (Natural Science) (No. Qiankehe Foundation-ZK [2021] General 537), the Guizhou Provincial Department of Education Youth Science and Technology Talent Growth Project (Qianjiaohe KYZi [2018] 211), and the Academic New Seedling Program of Guizhou University of Traditional Chinese Medicine (Guikehe Xue Shu Xin Miao[2023] -33).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, H. Q., Cai, S. H., Xu, L., He, M. Z., Zhang, X. J., Feng, Y. L., et al. (2010). Determination of scopoletin, quercetin, and Calycanthus chinensis Cheng et S. Y. Chang alkaloids in Cyperus rotundus by HPLC. Chin. Tradit. Herb. Drugs 41 (03), 486–487. CNKI: SUN: ZCYO.0.2010-03-053.
Bao, J., and Ran, M. X. (1999). Research and development of Miao medicine in Guizhou. Guiyang: Guizhou Science and Technology, Press, 153.
Chen, G. B., Chen, J. L., and Liu, Y. F. (2016). Effect of Isofraxidinin on expression of MMP-7 levels and cancer cell invasion ability of patients with liver cancer. J. Bethune Univ. Med. Sci. 14 (02), 148–151. doi:10.16485/j.issn.2095-7858.2016.02.007
Chen, G. F., Song, X. Z., Lin, D. M., and Xu, P. (2020). Isofraxidin alleviates myocardial infarction through NLRP3 inflammasome inhibition. Inflammation 43, 712–721. doi:10.1007/s10753-019-01158-z
Chu, Z. X., Lu, M., and Xiong, S. (2019). Research advances on pharmacological activities and pharmacokinetics of scopoletin. Chem. Res. 30 (04), 434–440. doi:10.14002/j.hxya.2019.04.017
Dai, P. F. (2012) Phylogeography, phylogeny and genetic diversity of Chimonanthus (Calycanthaceae). [dissertation/master’s thesis] N.W.U.
Ding, X. L., Suo, P., and Qian, H. B. (2021). Effects of Miao medicine Iron Chopsticks on immune function in immunosuppressive mice. Chongqing Med. 50 (13), 2171–2175. doi:10.3969/j.issn.1671-8348.2021.13.003
Ding, X. L., Xue, D. J., Deng, W. J., Han, C. Y., Liu, X. L., Li, Y., et al. (2022). Effect and mechanism of volatile oil of Chimonanthi Radix on vascular cognitive impairment rats induced by chronic cerebral hypoperfusion. Chin. Tradit. Herb. Drugs 53 (17), 5389–5399. doi:10.7501/j.issn.0253-2670.2022.17.015
Dong, R. X., Pan, J. J., Jin, Y., Fang, J., Shi, L. M., and Cheng, K. J. (2021). Research advances on chemical constituents and their pharmacological activities of two kinds of medicinal and edible tea from lindl. Sci. Technol. Food Ind. 42 (16), 429–437. doi:10.13386/j.issn1002-0306.2020090070
Duan, Y. P., Li, Y., and Li, S. Z. (2013). The anti-aging effect of total flavonoids from Chimonanthus praecox on aging mice induced by D-galactose. West Chin. J. Pharm. 28 (05), 456–458. doi:10.13375/j.cnki.wcjps.2013.05.007
Gao, Y., Jin, F. Y., Wang, X. P., Qian, H. B., and Li, Y. S. (2011). Gas chromatography-mass spectrometry analysis of the chemical composition of volatile oil from Qian Tiekuaizi. Lishizhen Med. Materia Medica Res. 22 (01), 122–123. doi:10.3969/j.issn.1008-0805.2011.01.058
Guiyang Health Bureau (1959). Folk medicinal herbs of Guiyang, Guiyang: Guizhou people's publishing house, 242–243.
Guizhou Provincial Drug Administration (2019). Quality standards for Chinese herbal medicines and ethnic medicinal materials in Guizhou Province. Guiyang Guizhou Sci. Tech.
He, D. Y., Xiao, W. Y., Li, C., and Zou, Z. R. (2023). Research progress on the composition and pharmacological activity of essential oil from Chimonanthus Lindl. Nat. Prod. R&D 1-19, 5–28. doi:10.16333/j.1001-6880.2023.9.016
Hu, W. J., and Yang, S. B. (2008). Research advancement on chemical components of Chimonanthus nitens and its medical values. Jiangxi For. Sci. Tech. (06), 60–62. doi:10.16259/j.cnki.36-1342/s.2008.06.008
Jang, J. H., Park, J. E., and Han, J. S. (2018). Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 834, 152–156. doi:10.1016/j.ejphar.2018.07.032
Jin, J., Yu, X., Hu, Z., Tang, S., Zhong, X., Xu, J., et al. (2018). Isofraxidin targets the TLR4/MD-2 axis to prevent osteoarthritis development. Food & Funct. 9, 5641–5652. doi:10.1039/c8fo01445k
Jin, L. H., Liu, G. C., Wang, L. H., Yan, Z. A., Xu, C., and Yuan, H. D. (2021). Effect of Scopoletin on lipid metabolism in high-fat diet-induced obese mice. J. Yanbian Univ. Nat. Sci. Ed. 47 (02), 160–164. doi:10.3969/j.issn.1004-4353.2021.02.013
Knapp, C., Van Dyke, T., and Foster, D. (2023). Wintersweet (Chimonanthus praecox) toxicity in 5 sheep. J. Veterinary Intern. Med. 37 (6), 2478–2481. doi:10.1111/jvim.16905
Kuang, H. X. (2023). Chemistry of traditional Chinese medicine. Beijing Chin. Tradit. Chin. Med. Press.
Li, D. (2008) “Research on the chemical composition of the herbs Tiekuaizi and Pyrola calliantha Andres in Guizhou,”. Guizhou Univ. [dissertation/master’s thesis]. doi:10.7666/d.Y1396001
Li, H., Chen, J., Li, D., Yu, W. T., Liu, Z. W., and Ni, F. (2018). Inhibition of isofraxidin from sarcandra glabra on the breast cancer stem cells via Bcl-2 and Caspase signaling pathway. Chin. J. Clin. Pharmacol. Ther. 23 (08), 886–892. doi:10.12092/j.issn.1009-2501.2018.08.008
Li, M., Li, Y. F., and Qian, H. B. (2017). Effects of alcohol extract from Chimonanthus praecox on adjuvant arthritis rats and their mechanisms. J. Liaoning Univ. Tradit. Chin. Med. 19 (03), 19–21. doi:10.13194/j.issn.1673-842x.2017.03.005
Li, M., and Qian, H. B. (2016). Effects of alcohol extract from root of wintersweet on expression of HIF-1a, VEGF and MMP-3 in adjuvant arthritis rats. Chin. Pharmacol. Bull. 32 (9), 1326–1327. doi:10.3969/j.issn.1001-1978.2016.09.027
Li, Q. J., Wang, M. L., Yang, X. S., Ma, L., and Hao, X. J. (2013). Two new coumarin glycosides from Chimonanthus nitens. J. Asian Nat. Prod. Res. 15 (3), 270–275. doi:10.1080/10286020.2012.762766
Li, S. L., and Zou, Z. R. (2018). Research progress on flavonoids and coumarins from Chimonanthus plants and its pharmacological activities. Chin. Tradit. Herb. Drugs 49 (14), 3425–3431. doi:10.7501/j.issn.0253-2670.2018.14.032
Li, S. Z., Li, Y., Liu, X. L., Xu, T., Wang, Y., and Qian, H. B. (2021). Phytochemical and pharmacological progress on genus Chimonanthus in Guizhou. Chin. J. Ethnomed. Ethnopharm. 30 (06), 59–65. doi:10.3969/j.issn.1007-8517.2021.6.zgmzmjyyzz202106013
Li, X. F., Lu, S. D., Chen, M., and Qian, H. B. (2019). Effects of ethanol extracts of Miao medicine iron chopsticks on pathological changes, blood lipids, TNF-alpha, IL-6 and IL-18 in myocardial ischemic rats. Chin. J. Ethnomed. EthnoPharm. 28 (22), 22–27. doi:10.3969/j.issn.1007-8517.2019.22.zgmzmjyyzz201922007
Lin, X. (2019). Progress of research on volatile components in the Chimonanthus praecox. Fujian Agric. Sci. Technol. (07), 57–64. doi:10.13651/j.cnki.fjnykj.2019.07.013
Liu, C. X., Chen, S. L., Xiao, X. H., Zhang, T. J., Hou, W. B., and Liao, M. L. (2016). A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs. 47 (09), 1443–1457. doi:10.7501/j.issn.0253-2670.2016.09.001
Liu, L. L., Shen, X., Deng, B. Q., and Zou, Z. R. (2018). Advance in studies on flavinoids, coumarins and pharmacological activity of chinonanthus. Lishizhen Med. Mat. Med. Res. 29 (05), 1196–1200. doi:10.3969/j.issn.1008-0805.2018.08.059
Lou, H. Y., Zhang, Y., Ma, X. P., Jiang, S., Wang, X. P., Yi, P., et al. (2018). Novel sesquiterpenoids isolated from Chimonanthus praecox and their antibacterial activities. Chin. J. Nat. Med. 16 (8), 621–627. doi:10.1016/S1875-5364(18)30100-6
Lu, J., Xie, D., Fan, D. Z., Tang, D. X., and Ruan, J. H. (2020). Study on the transdermal drug delivery effects of Tiekuaizi microemulsion of Miao medicine under different transdermal absorption enhancers. Lishizhen Med. Mat. Med. Res. 31 (09), 2121–2124. doi:10.3969/j.issn.1008-0805.2020.09.020
Lu, J., Xie, D., Ruan, J. H., Fan, D. S., Cha, X., and Xin, J. M. (2021). Study on chemical composition of volatile oil of Miao medicine Chimonanthi Radix and its inhibitory effect on BGC-823 cells. Guizhou Sci. 39 (01), 26–30. doi:10.3969/j.issn.1003-6563.2021.01.006
Luo, J. F., Wu, Q. M., Huang, C., and Qian, H. B. (2023). Reseaech prigress of Miao medicine radix chimonanthi praeacccis. J. Guizhou Univ. Tradit. Chin. Med. 45 (02), 75–80. doi:10.16588/j.cnki.issn2096-8426.2023.02.015
Ma, X. M., Wu, C. F., and Peng, R. H. (2010). Advance in studies on Chimonanthus praecox. Anhui Agric. Sci. 38 (34), 19279–19280+19283. doi:10.13989/j.cnki.0517-6611.2010.34.114
Nanjing University of Traditional Chinese Medicine (2006). Dictionary of Chinese medicine. Shanghai: Shanghai Sci. Tech., 2617.
Nie, L., Zhai, X. Q., and Jie, D. W. (2009). Current status and progress of research on Chimonanthus praecox. Shanxi Agric. Sci. 55 (06), 116–119. doi:10.3969/j.issn.0488-5368.2009.06.047
Qian, H. B. (2016). A compound with anti-inflammatory effect and its preparation method and use. China CN 104496953 B.08.31.
Qian, H. B., Luo, K., Long, Q. L., and Jin, F. Y. (2012b). Effects of essential oil from Chimonanthus praecox in Guizhou on rat adjuvant arthritis and their mechanisms. J. Anhui Agric. Sci. 40 (21), 10834–10836. doi:10.3969/j.issn.0517-6611.2012.21.033
Qian, H. B., Pu, J. S., Wang, L., and Li, S. Z. (2012c). Study on the anti-inflammatory effect of the volatile oil of Tiekuaizi in Miao medicine. Shi-Zhen Guomao 23 (08), 1961–1962. doi:10.3969/j.issn.1008-0805.2012.08.051
Qian, H. B., Wang, P. X., Li, Y. S., and Jin, F. Y. (2010). An experimental study on the analgesic effect of the alcoholic extract of the Miao medicine, Tiekuaizi. Lishizhen Med. Mat. Med. Res. 21 (12), 3120–3121. doi:10.3969/j.issn.1008-0805.2010.12.037
Qian, H. B., Xu, Y. P., and Jin, F. Y. (2012d). The analgesic effect and acute toxicity of essential oil from Chimonanthus praecox. J. Anhui Agric. Sci. 40 (12), 7095–7096. doi:10.3969/j.issn.0517-6611.2012.12.042
Qian, H. B., Xu, Y. P., Pu, J. S., Wang, L., and Jin, F. Y. (2012a). Study on the analgesic effects and mechanism of the ethanol extracts of TieKuaizi. West Chin. J. Pharm. Sci. 27 (03), 262–264. doi:10.13375/j.cnki.wcjps.2012.03.015
Qiu, D. W., Du, J., and Xia, T. H. (2006). Chinese Miao medicine. Guiyang: Guizhou Sci. Tech: Press, 457–458.
Shan, W. T., Wang, X., Yang, X. L., Ai, F., Liu, Y., Wei, X., et al. (2023). Exploring the protective effect of helleborus thibetanus franch alcohol extract on bone destruction in CIA rats based on the OPG/RANK/RANKL signaling pathway. Acta Lab. Anim. Sci. Sin. 31 (10), 1250–1260. doi:10.3969/j.issn.1005-4847.2023.10.002
Shu, R. G., Wan, Y. L., and Wang, X. M. (2019). Non-volatile constituents and pharmacology of Chimonanthus: a review. Chin. J. Nat. Med. 17 (3), 161–186. doi:10.1016/S1875-5364(19)30020-2
Tan, T., Luo, Y., Zhong, C. C., Xu, X., and Feng, Y. L. (2017). Comprehensive profiling and characterization of coumarins from roots, stems, leaves, branches, and seeds of Chimonanthus nitens Oliv. using ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a modified mass defect filter. J. Pharm. Biomed. Anal. 7 (15), 140–148. doi:10.1016/j.jpba.2017.04.016
Tang, X. S., Jin, F. Y., Zhang, Y., Wang, X. P., and Bai, Y. G. (2015). Determining the content of scopoletin in Chimonanthus praecox of Miao ethnomedicine by HPLC. J. Guiyang Colg. Tradit. Chin. Med. 37 (06), 24–27. doi:10.3969/j.issn.1002-1108.2015.06.005
Wan, C. Y., Li, Y. Y., Ma, Y. Y., and Huang, Z. Y. (2022). Simultaneous determination of chimonanthi and calycanthine in chimonanthi nitentis folium by HPLC. Drug Eval. 19 (17), 1035–1038. doi:10.19939/j.cnki.1672-2809.2022.17.03
Wang, L. Y., Zhang, Z. B., Zou, Z. R., and Zhu, D. (2012). Advances of studies on chemical composition and pharmacological activity of Chimonanthus lind. Lishizhen Med. Mat. Med. Res. 23 (12), 3103–3106. doi:10.3969/j.issn.1008-0805.2012.12.072
Wang, M. L., Wang, D. P., Yang, X. S., and Hao, X. J. (2010). Chemical constituents of the essential oil from different parts of Chimonanthus nitens Oliv. distributed in Guizhou. J. Yunnan Univ.(Edit. Nat. Sci.) 32 (05), 577–582.
Wang, X. (2020) Study on pharmacodynamic material of Chimonanthus nitens Oliv. From Guizhou province [dissertation/master’s thesis]. doi:10.27045/d.cnki.ggyyc.2020.000124
Wang, X., Wang, L., Liao, X., Wang, H. B., Yang, J., Li, Q. Q., et al. (2020). Comparative study on the contents of four compounds from different processed products of Miao medicine Chimonanthus nitens and its toxicity. Chin. Pharm. 31 (12), 1475–1480. doi:10.6029/j.issn.001-0403.2020.12.13
Wang, Y. (2002). Color Atlas of Chinese Miao medicines. First edition. Guiyang: Guizhou Sci.Tech, 524.
Wang, Y., Xu, T., Wu, S. S., Liu, X. L., and Qian, H. Z. (2022). Molecular mechanism of Chimonanthus praecox against cerebral ischemic stroke based on network pharmacology. J. Shenyang Pharm. Univ. 39 (09), 1118–1129. doi:10.14066/j.cnki.cn21-1349/r.2020.1203
Wang, Z. F., Zhang, N., Ma, X. P., Yang, J. L., Pan, W. D., and Lin, C. H. (2009). Chemical composition and in vitro antibacterial and antifu ngal activity of essential oils from different parts of Chimonanthus praecox (linn.) Link. Chin. J. Mod. Appl. Pharm. 36 (08), 901–905. doi:10.13748/j.cnki.issn1007-7693.2019.08.001
Wei, H., Jiang, C. L., Qin, Z. N., and Zheng, L. (2022). Effect of fraxin on macrophage polarization induced by lipopolysaccharide. J. Guangxi Med. Univ. 39 (04), 569–576. doi:10.16190/j.cnki.45-1211/r.2022.04.010
Wei, L. J., Zhou, J. P., and Dai, Y. (2009). Progress in the study of natural product scopoletin. Strait Pharm. J. 21 (04), 10–13.
Wu, S. S., Wang, Y., Xu, T., Liu, X. L., and Qian, H. B. (2021). The inhibitory effect of compound Q-1 on proliferation of MH7A induced by TNF-a and its significance. Chin. Pharmacol. Bull. 37 (11), 1554–1558. doi:10.3969/j.issn.1001-1978.2021.11.014
Xiao, Y. H., Lei, D. D., Xu, G. B., Zhou, M., He, X., and Liao, S. G. (2019). Simultaneous determination of seven constituents in Liangjiang Weiyang Capsules by HPLC. Chin. Tradit. Pat. Med. 41 (08), 1786–1790. doi:10.3969/j.issn.1001-1528.2019.08.007
Xu, T., Chen, M., Wang, Y., Wu, S. S., Liu, X. L., and Qian, H. B. (2022). Effects and mechanism of Tiekuaizi alcohol extract in the prevention and treatment of myocardial infarction based on network pharmacology. Chin. J. Hosp. Pharm. 42 (19), 2014–2023. doi:10.13286/j.1001-5213.2022.19.09
Yang, J. Q., and Yang, J. Z. (1978). A collection of folk remedies from Guizhou. Guiyang: Guizhou People's Publishing House, 80–110.
Yu, H., Wang, T. Y., Wang, J., and You, X. Q. (2021). Research progress of chemical compositions of Chimonanthus nitens Oliv. and their biological activities. Chin. J. Pharm. Anal. 41 (09), 1477–1486. doi:10.16155/j.0254-1793.2021.09.01
Yue, Q. X., Sun, Y. F., Yang, S., Du, Q. Q., Jin, C. S., Zhang, W., et al. (2023). Study on quality marker of Paeoniae Radix Alba based on quality transfer process of medicinal materials-decoction pieces-standard decoctions. Chin. Tradit. Herb. Drugs 54 (02), 553–560. doi:10.7501/j.issn.0253-2670.2023.02.022
Zhan, Y. T., Qin, Z. N., and Zheng, L. (2021). Effects of fraxin on oxidative stress and inflammatory factors induced by lipopolysaccharide in chondrocytes. J. Guangxi Med. Univ. 38 (01), 28–34. doi:10.16190/j.cnki.45-1211/r.2021.01.005
Zhang, J., Wen, Q., Xu, X., Chen, L., Feng, Y. L., Yang, S. L., et al. (2018). In vivo metabolism of three coumarins from Chimonanthi Radix based on UHPLC-OTOF-MS/MS. China J. Chin. Mat. Med. 43 (21), 4330–4338. doi:10.19540/j.cnki.cjcmm.20180815.005
Zhang, J. W., Gao, J. M., Xu, T., Zhang, X. C., Ma, Y. T., Jarussophon, S., et al. (2009). Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. & Biodivers. 6, 838–845. doi:10.1002/cbdv.200800089
Zhang, J. Y., Cao, H., Xu, J., Han, L. Q., Gong, S. X., Zhang, T. J., et al. (2016). Expression of bitter taste of Chinese materia medica and its application in clinical compatibility. Chin. Tradit. Herb. Drugs 47 (2), 187–193. doi:10.7501/j.issn.0253-2670.2016.02.001
Zhang, M., Huo, H. R., Wang, P. Q., Dai, L., and Sui, F. (2018). Theoretical origin and review of modern research on medicinal properties of spicy flavor. Chin. Tradit. Herb. Drugs 49 (3), 505–511. doi:10.7501/j.issn.0253-2670.2018.03.001
Zhang, X. D., Wu, D. W., Zhang, L. K., Zhang, H. Y., Yang, L. P., Wei, L., et al. (2024). Predicting the potential mechanism of radix chimonanthi pracecocis in treating osteoarthritis by network pharmacology analysis combined with experimental validation. J. Ethnopharmacol. 331, 118231. doi:10.1016/j.jep.2024.118231
Zhang, Y. (2013). Screening and chemical composition of the effective parts of Tiekuaizi from Miao medicine in Guizhou. [dissertation/master’s thesis].
Zhao, H. R., Ji, Q. F., Wang, M. S., Zhao, S. X., and Wang, Y. P. (1993). Studies on constituents of roots of Chimonanthus praecox. J. China Pharm. Univ. (02), 76–77.
Zhong, C. C., and Feng, Y. L. (2017). HPLC fingerprint analysis of caulis of Chimonanthus nitens combined with chemometrics method. Chin. Pharm. J. 52 (21), 1944–1947. doi:10.11669/cpj.2017.21.016
Zhou, B., Cui, X. D., Cheng, D., Li, J., Wang, P., and Zheng, P. W. (2013). Simultaneous determination of the content of four active compounds in Shanlamei Gran-ules by RP-HPLC. Lishizhen Med. Mat. Med. 24 (11), 2653–2654. doi:10.3969/j.issn.1008-0805.2013.11.032
Zhu, C. X., Zhou, X., and Chen, H. G. (2020). Advances on treatment of rheumatoid arthritis with Miao medicine. Chin. Mod. Appl. Pharmacol. 37 (21), 2669–2677. doi:10.13748/j.cnki.issn1007-7693.2020.21.020
Keywords: Miao medicine Tiekuaizi, Radix Chimonanthi, phytochemistry, pharmacology, quality marker
Citation: Luo L-y, Zhang X-d, Mei H-m, Ma J-h, Peng X, Dong M-h and Huang C (2025) Miao medicine Tiekuaizi (Radix Chimonanthi): a review of its traditional uses, phytochemistry, pharmacology, and predictive analysis on quality markers. Front. Pharmacol. 15:1491585. doi: 10.3389/fphar.2024.1491585
Received: 05 September 2024; Accepted: 25 November 2024;
Published: 03 January 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Yunshan Wu, Guangzhou University of Chinese Medicine, ChinaHui Cui, Guangzhou University of Chinese Medicine, China
Copyright © 2025 Luo, Zhang, Mei, Ma, Peng, Dong and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Huang, aHVhbmdjb25nMjM2QDE2My5jb20=; Ming-hong Dong, ZG1oOTkxMDJAMTYzLmNvbQ==
 Li-ying Luo1
Li-ying Luo1 Xu-dong Zhang
Xu-dong Zhang Cong Huang
Cong Huang