- 1College of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Department of Vascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Health Management Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Medical Biosciences, Faculty of Natural Sciences, University of The Western Cape, Cape Town, South Africa
- 6Hai Phong University of Medicine and Pharmacy, Haiphong, Vietnam
- 7The Research Institute of Virology, Ministry of Health, Tashkent, Uzbekistan
- 8Department of Vascular Surgery, The Affiliated People’s Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, China
Dihydromyricetin (DHM or DMY) is a flavonoid derived from natural sources with a range of confirmed biological benefits. It exhibits anti-inflammatory, antioxidant, anti-tumor, and anti-viral activities. DHM is recognized for its high biosafety, making it a promising subject for further research. This article offers a comprehensive overview of DHM’s pharmacological properties, mechanisms, and recent research developments in the cardiovascular, urinary, digestive, nervous, and respiratory systems. The review summarizes DHM’s biological effects and associated signaling pathways, providing novel insights for its clinical application.
Highlights
• Dihydromyricetin is a promising natural product with a high safety profile and broad biological activity.
• The main pharmacological effects of DHM are anti-inflammatory, antioxidant, antiviral, anti-tumor and metabolic regulation.
• A review of DHM, including its potential mechanisms of action on different systems in the human body and related signaling pathways.
• Discussing the main factors affecting the development and utilization of dihydromyricetin, including its stability and relatively low bioavailability. Discussing how to improve its bioavailability.
1 Introduction
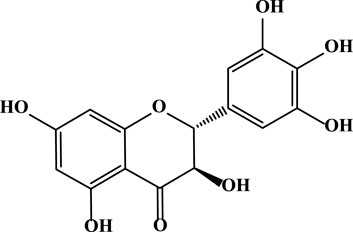
Dihydromyricetin (DHM or DMY) is a flavonoid extracted from the young stems and leaves of Ampelopsis grossedentata. It is a polyphenolic hydroxy dihydroflavanol with a molecular weight of 320.25 g/mol and a molecular formula of C15H12O8 (Figure 1) (Guan et al., 2019). DHM is widely distributed in plants such as grapes, mulberries, and ginkgo biloba. Particularly high concentrations are found in vine tea (Liu D et al., 2019), reaching up to 30%–40%. It has been demonstrated to possess multiple pharmacological activities, such as anti-inflammatory, antioxidant, and anti-tumor effects (Zhang et al., 2018). Notably, it is nearly non-toxic and demonstrates an excellent safety profile (Semwal et al., 2016). The toxicity of DHM has been found to be very low, with previous acute toxicity tests showing that the safe dose of DHM in rats is 10 g/kg (Xu et al., 2008). Using the body surface area normalization method, the estimated maximum safe dose for mice is around 16 g/kg, and for adults it may be 1.6 g/kg (Zeng et al., 2023).
The phenolic hydroxyl groups in DHM predominantly contribute to its chemical instability, which is influenced by pH buffers and metal ions such as Fe³⁺, Al³⁺, and Cu2⁺ (Xiang et al., 2017), DHM exhibits stability in weakly acidic environments but becomes unstable under alkaline conditions (Liu et al., 2019). Additionally, temperature significantly affects DHM’s stability; for instance, the concentration of free DHM in a solution of 60 μg/mL decreased by 40% when exposed to 60°C for 16 days (Liu et al., 2012).
2 Pharmacological actions of DHM
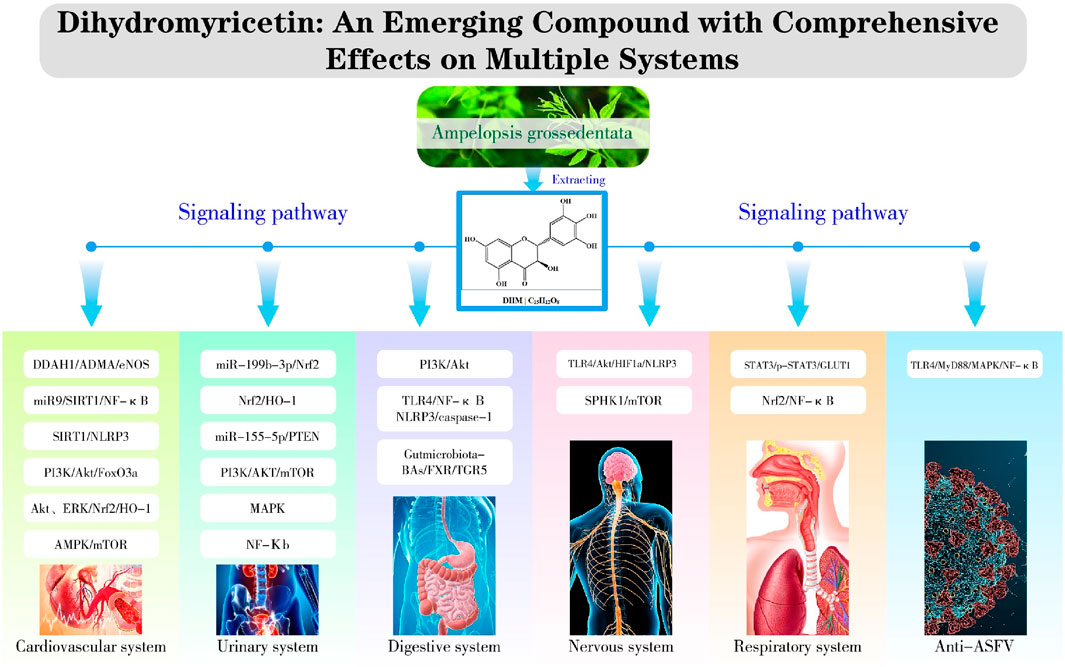
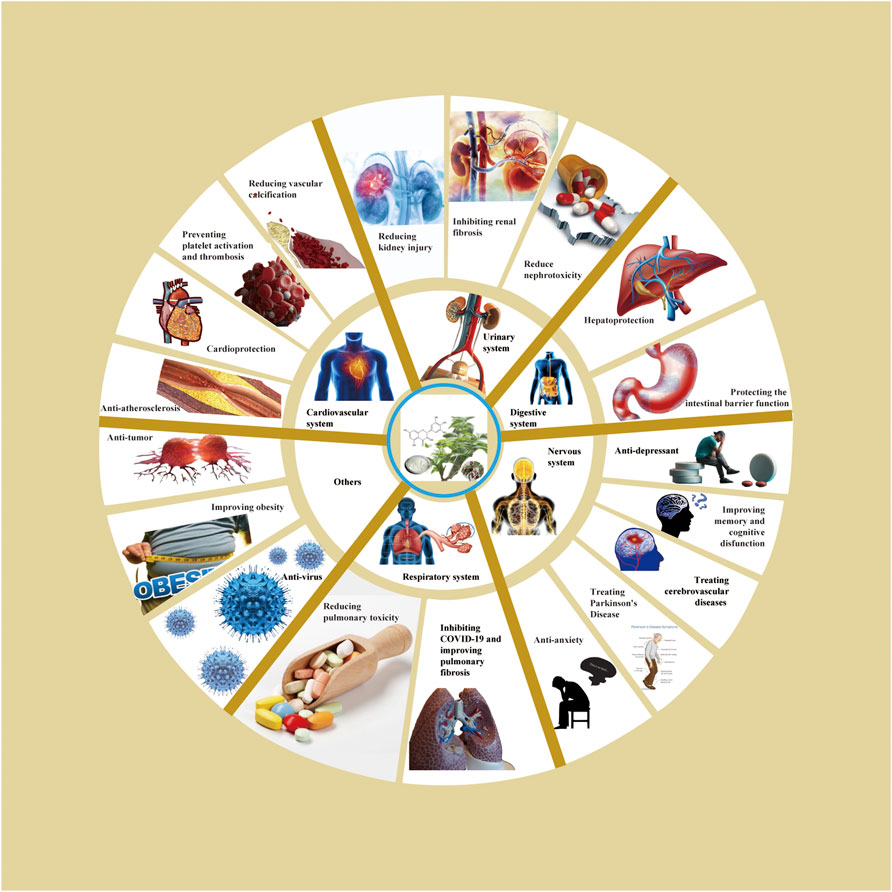
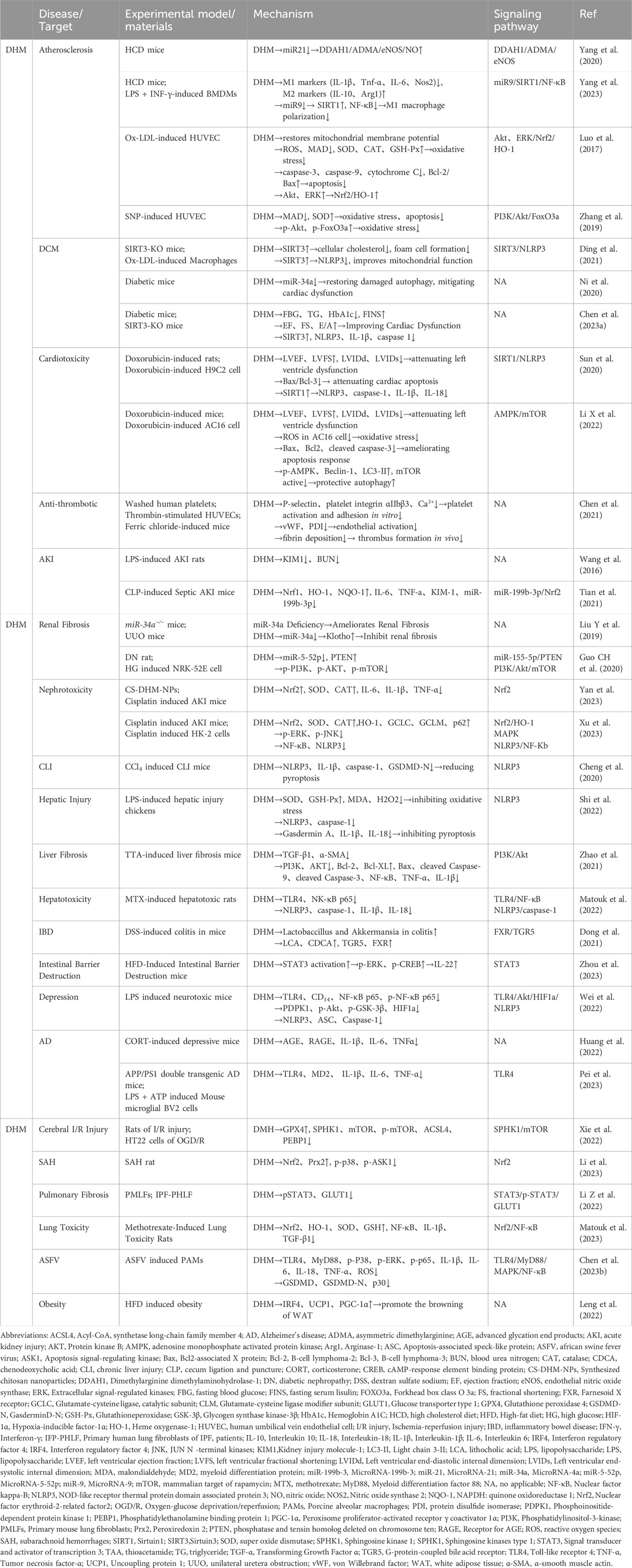
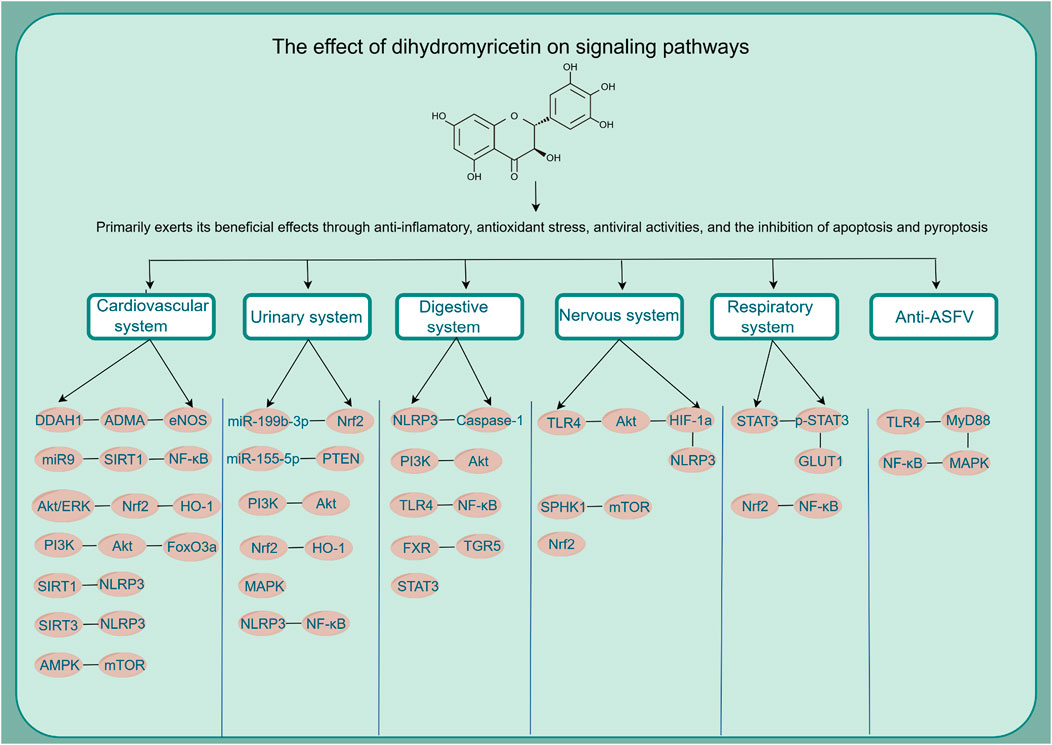
Based on relevant cellular and animal studies, DHM has demonstrated a diverse array of pharmacological properties, including antioxidant, anti-inflammatory, anti-tumor, and anti-viral effects. Given its exemplary safety profile, DHM shows substantial potential for clinical applications. Recent research has allowed us to compile a summary of DHM’s pharmacological impacts on various organs and systems, as illustrated in Figure 2. Additionally, we have detailed the different signaling pathways influenced by DHM in Table 1 and Figure 3.
2.1 Cardiovascular system
2.1.1 Anti-atherosclerosis
In recent years, atherosclerosis (AS) has been characterized as a chronic and progressive inflammatory condition that damages the inner layers of arterial walls, leading to the development of atherosclerotic plaques.
The pathophysiological process of AS is highly complex, with many aspects still needing further exploration. However, the main key points of this process have been generally identified. The early stage of AS development is the “fatty streak” phase, during which low-density lipoprotein (LDL) accumulates in the vascular wall and undergoes oxidation to form oxidized low-density lipoprotein (ox-LDL). This causes damage to the vascular endothelium, inducing the recruitment of monocytes, which mature into M1 pro-inflammatory macrophages (Chistiakov et al., 2017). These macrophages engulf excessive lipids, becoming foam cells, which are characteristic of this stage. As the lesion progresses, more inflammatory cells participate, and vascular smooth muscle cells (VSMCs) and macrophages undergo phenotypic transformation (Vengrenyuk et al., 2015), leading to the formation of atherosclerotic plaques. In the later stages of the lesion, a more stable fibrous cap is formed by a large amount of collagen fibers, smooth muscle cells, a few elastic fibers, and proteoglycans, which results in arterial hardening and narrowing. Several factors can destabilize these plaques, leading to plaque rupture and thrombus formation, potentially triggering cardiovascular events (Poznyak et al., 2020).
Extensive research has demonstrated that DHM produces anti-atherosclerotic effects by reducing oxidative stress and regulating macrophage polarization.
2.1.1.1 Anti-oxidative stress and regulating apoptosis
Recently, the DDAH1-ADMA-eNOS pathway has been recognized as a crucial regulatory mechanism in the production of nitric oxide (NO) and the progression of AS. According to a study by Yang et al. (2020), HM can decrease the expression of microRNA-21 (miR-21) in Apoe−/− mice. This microRNA targets dimethylarginine dimethylaminohydrolase-1 (DDAH1), thereby enhancing NO production. This enhancement improves endothelial cell function, reduces vascular inflammation and lipid metabolism disturbances, and lowers the incidence of AS.
Luo et al. (2017) investigated the protective effects and mechanisms of DHM using an ox-LDL-induced human umbilical vein endothelial cell (HUVEC) model. Their research demonstrated that DHM mitigated ox-LDL-induced endothelial cell apoptosis, mitochondrial depolarization, caspase-3 activation, and reactive oxygen species (ROS) production, thus providing cellular protection. Additionally, DHM activated the protein kinase B (Akt) and extracellular signal-regulated kinases 1/2 (ERK1/2) pathways, leading to the stimulation of the Nrf2/HO-1 signaling pathway. This activation promoted the upregulation of antioxidant enzymes and anti-apoptotic proteins, thereby shielding HUVECs from oxidative damage caused by ox-LDL.
Zhang et al. (2019) successfully established an oxidative stress model in HUVECs using sodium nitroprusside (SNP) as a NO donor. Their experimental findings indicate that pre-treatment with DHM reduced intracellular ROS overproduction, decreased malondialdehyde (MDA) levels, and inhibited SNP-induced cell apoptosis in HUVECs. Furthermore, DHM protected HUVECs from oxidative stress by activating the PI3K/Akt/FoxO3a signaling pathway.
2.1.1.2 Regulating macrophage polarization
Macrophages are essential immune cells involved in inflammation and play a crucial role in the development of AS. M1 macrophages promote inflammation, whereas M2 macrophages aid in tissue repair by producing anti-inflammatory factors. Research has shown that miR-9, which is highly expressed in M1 macrophages, promotes M1 polarization by targeting the SIRT1/NF-κB signaling pathway (Wang et al., 2021). Yang et al. (2023) demonstrated that DHM inhibits M1 macrophage polarization and reduces vascular inflammation in AS by suppressing the expression of microRNA-9 (miR-9), potentially through targeting the miR-9/SIRT1/NF-κB signaling pathway in both in vitro and in vivo studies.
2.1.1.3 Reducing foam cell formation and cholesterol accumulatio
The formation of foam cells and the accumulation of cholesterol are crucial factors in the progression of AS. Sirtuin 3 (SIRT3) has the potential to reduce intracellular ROS levels and inhibit oxidative stress by regulating several mitochondrial enzymes (Wang et al., 2020). Ding et al. (2021) found that the absence of SIRT3 led to increased cholesterol accumulation, oxidative stress, and activation of the NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) in ox-LDL-stimulated macrophages, thereby promoting foam cell formation. This highlights SIRT3 as an effective target for anti-atherosclerosis therapy. Moreover, research indicates that DHM can effectively inhibit this process through a SIRT3-dependent mechanism, demonstrating promising anti-atherosclerosis effects (Ding et al., 2021).
2.1.2 Cardioprotection
Diabetic-induced cardiac damage, known as diabetic cardiomyopathy (DMC), is a significant concern. Studies have shown that DHM can improve cardiac function in diabetic mice by suppressing miR-34a expression and revitalizing impaired autophagy (Ni et al., 2020). Similarly, DHM has been demonstrated to enhance cardiac function in streptozotocin (STZ)-induced diabetic mice (Chen et al., 2023a), ameliorating myocardial hypertrophy, fibrosis, and injury while suppressing oxidative stress, inflammation, and cell death through the activation of SIRT3.
DHM also exhibits cardioprotective effects by mitigating cardiac toxicity. Doxorubicin (DOX), a potent anthracycline antitumor drug, is limited in clinical use due to severe cardiotoxic side effects. Research (Sun et al., 2020) demonstrates that DHM inhibits NLRP3 inflammasome activation through the SIRT1 pathway, effectively preventing DOX-induced cardiac toxicity. In vitro and in vivo studies conducted by Li X et al. (2022) revealed that DHM protects the heart against DOX-induced toxicity through the activation of the AMPK/mTOR signaling pathway, suppression of apoptosis and oxidative stress, and promotion of protective autophagy.
2.1.3 Prevent platelet activation and thrombosis
DHM exhibits significant potential for antiplatelet effects and thrombus inhibition. Chen et al. (2021) demonstrated that DHM suppresses the expression of platelet p-selectin induced by α-thrombin, thereby inhibiting platelet adhesion by reducing integrin activation and intracellular Ca2⁺ elevation during platelet activation. Additionally, DHM may reduce the secretion of von Willebrand factor (vWF) and protein disulfide isomerase (PDI), and impede the activation and expression of endothelial tissue factor (TF), which could prevent thrombus formation. Notably, DHM inhibited platelet aggregation and fibrinogen production without hindering hemostasis.
2.1.4 Reducing vascular calcification
Vascular calcification, a common pathological process in various systemic illnesses, is receiving increasing attention. Studies have shown (Feng et al., 2021) that DHM treatment significantly reduces calcium/phosphate-induced VSMC calcification in rats and humans in a dose-dependent manner. This effect is associated with the inhibition of Akt activation. Notably, DHM’s inhibition of vascular calcification is more potent compared to that of classic Akt inhibitors.
2.2 Urinary system
2.2.1 Reducing kidney injury
DHM has been shown to alleviate acute kidney injury (AKI) induced by lipopolysaccharide (LPS) by reducing kidney injury molecule-1 (KIM-1) and blood urea nitrogen (BUN) levels (Wang et al., 2016). Additionally, DHM has been demonstrated to modulate the miR-199b-3p-mediated Nrf2 pathway, thereby attenuating sepsis-induced AKI (Tian et al., 2021).
2.2.2 Inhibiting renal fibrosis
DHM also has significant effects on renal fibrosis. Liu et al. (2019) demonstrated that the abnormal upregulation of miR-34a plays a crucial role in the progression of renal fibrosis, and DHM can effectively treat renal fibrosis by inhibiting miR-34a. Similarly, DHM modulates the miR-155-5p/PTEN signaling pathway and the PI3K/AKT/mTOR signaling pathway in diabetic nephropathy (DN) mice to promote autophagy and mitigate renal interstitial fibrosis (RIF) (Guo et al., 2020b).
2.2.3 Reduce nephrotoxicity
The nephroprotective properties of DHM should not be overlooked. The use of cisplatin as an anti-tumor drug is limited due to its potential renal toxicity. Yan et al. (2023) demonstrated that DHM exerts protective effects against cisplatin-induced AKI by downregulating oxidative stress and inflammatory factors via chitosan nanoparticles loaded with DHM. The study highlights that encapsulated DHM (in a chitosan-based delivery system) more effectively increased Nrf2 levels than the suspension form of DHM, this suggests that encapsulation enhances the bioavailability and antioxidative properties of DHM, allowing it to better activate the Nrf2 pathway and provide stronger protection against oxidative damage.
Another research group also explored the effects of DHM on cisplatin-induced kidney damage (Xu et al., 2023). The study demonstrated that DHM targets the Nrf2/HO-1, mitogen-activated protein kinases (MAPK), and NF-κB signaling pathways to alleviate oxidative stress, inflammation, cell apoptosis, and ferroptosis, thereby exerting a protective effect on the kidneys.
2.3 Digestive system
2.3.1 Hepatoprotection
2.3.1.1 Relieving liver injury and hepatotoxicity
Chronic liver injury (CLI) can result from various factors, including drugs and viruses. It serves as a precursor to many serious liver diseases, causing abnormalities in liver metabolic functions and ultimately leading to liver failure. Pyroptosis plays a crucial role in CLI, and research has shown (Cheng et al., 2020) that DHM can reduce the protein expression and mRNA levels of pyroptosis-related molecules, including caspase-1, GasderminD-N (GSDMD-N), and downstream inflammatory molecules, thereby significantly ameliorating carbon tetrachloride (CCl₄)-induced CLI in mice. In addition, another study (Shi et al., 2022) used intraperitoneal injection of LPS in chickens to induce liver injury, followed by treatment with DHM administered by gavage. The results demonstrated that DHM alleviated liver injury by inhibiting LPS-induced activation of the NLRP3 inflammasome and pyroptosis, while also reducing oxidative stress to improve hepatic oxidative homeostasis in chickens. Interestingly, in the assessment of pyroptosis, the study identified GasderminA (GSDMA), an executor protein of pyroptosis, as a key protein involved in LPS-induced pyroptosis in chicken liver injury, and DHM was shown to inhibit GSDMDA, thereby suppressing pyroptosis.
The clinical use of methotrexate (MTX) has been restricted due to its potential hepatotoxicity. However, experimental results with rats by Matouk et al. (2022) indicated that DHM mitigates MTX-induced liver toxicity by reducing oxidative stress and inhibiting the TLR4/NF-κB and NLRP3/caspase-1 pathways. Another team of researchers (Yan et al., 2019) treated L02 cells with emodin to induce hepatotoxicity and then administered DHM to evaluate its protective effects. The study showed that DHM significantly reduced markers of liver cell injury, such as ROS levels and cell apoptosis. Mechanistically, DHM was found to activate the Nrf2 signaling pathway, leading to the upregulation of antioxidant enzymes, including HO-1 and NQO1, which helped to counteract oxidative stress.
Emad et al. (2024) administered DHM to hepatocytes exposed to Valproic acid (VPA) and evaluated its effects on markers of oxidative stress and apoptosis. DHM was found to significantly activate the keap-1/Nrf2/HO-1 pathway, enhancing antioxidant defense mechanisms and reducing oxidative damage. Furthermore, DHM inhibited the NF-κB and caspase-3 pathways, leading to reductions in inflammation and apoptosis. These findings suggest that DHM mitigates VPA-induced hepatotoxicity by activating antioxidant pathways and suppressing inflammation and cell death, underscoring its potential as a protective agent against drug-induced liver injury.
The aforementioned research findings suggest that DHM primarily exerts its hepatoprotective and hepatotoxicity-reducing effects by modulating the Nrf2 pathway and inhibiting pyroptosis.
2.3.1.2 Alleviating alcoholic liver disease
Alcoholic liver disease (ALD) is a growing concern at present. Silva et al. (2020b) conducted research on a mouse model of ethanol (EtOH)-induced ALD and discovered that DHM reduced EtOH-induced hepatic steatosis, oxidative stress, and inflammation both in vivo and ex vivo. Furthermore, DHM improved ethanol metabolism, resulting in a decrease in ethanol-induced liver damage. It should be noted that the team’s novel use of transperitoneal administration in mice, aimed at increasing the absorption and bioavailability of DHM, yielded unsatisfactory results.
2.3.1.3 Improving non-alcoholic fatty liver disease
The prevalence of non-alcoholic fatty liver disease (NAFLD) is steadily increasing and has become one of the most common chronic liver diseases worldwide. Currently, there are no effective and approved therapeutic drugs for NAFLD. Researchers have summarized the effects and mechanisms of DHM on NAFLD (Gong et al., 2022). DHM exerts its effects primarily through AMPK, NF-κB, and MAPK-related signaling pathways, as well as sirtuin-dependent mechanisms, which align with the existing pathogenesis of NAFLD.
2.3.1.4 Ameliorating hepatic fibrosis and hepatic encephalopathy (HE)
Zhao et al. (2021) demonstrated that treatment with DHM improved liver structure and significantly reduced oxidative stress and hepatotoxicity indices in a mouse model of thioacetamide (TAA)-induced liver fibrosis. Furthermore, DHM reversed TAA-induced liver fibrosis by suppressing inflammation via the PI3K/Akt/NF-κB signaling pathway and apoptosis regulated by transforming growth factor β1 (TGF-β1). Notably, DHM can inhibit the activation of hepatic stellate cells (HSCs) through autophagy induction (Zhou et al., 2021). Additionally, it enhances natural killer cell (NKC)-mediated killing of HSCs by promoting interferon-γ (IFN-γ) secretion, thereby arresting the progression of liver fibrosis.
Additionally, a research team investigated the impact of DHM on hepatic encephalopathy (HE) in a mouse model of acute liver failure induced by thioacetamide (TAA) (Cheng et al., 2021). The findings revealed that DHM restored liver function, improved brain histopathology, and mitigated the symptoms of HE.
2.3.2 Protecting the intestinal barrier function
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic inflammation of the intestines, often leading to mucosal ulcers and the eventual degradation of intestinal function (Pineton et al., 2016). Dong et al. (2021) discovered that DHM might alleviate dextran sulfate sodium (DSS)-induced colitis in mouse models by regulating the intestinal microbiota and related bile acid metabolism, while restoring the compromised intestinal barrier function caused by inflammation. The mechanism involves the intestinal microbiota-BAs-FXR/TGR5 signaling pathway.
DHM can also help improve intestinal dysfunction caused by high-intensity exercise (HIE) in mice (Hou et al., 2022), potentially by inhibiting intestinal inflammation and stabilizing intestinal barrier integrity. Additionally, Zhou et al. (2023) investigated the mechanisms behind DHM’s protective effect on the integrity of the intestinal barrier. Their findings reveal that DHM decreases the harm to the intestinal barrier caused by a high-fat diet (HFD) through the stimulation of interleukin 22 (IL-22) expression in group 3 innate lymphoid cells (ILC3). Moreover, this beneficial impact is associated with signal transducer and activator of transcription 3 (STAT3) phosphorylation in the SIRT3 signaling pathway.
2.4 Nervous system
2.4.1 Anti-depressant
It is widely recognized that depression can be associated with inflammatory reactions. Research has indicated that DHM has the potential to relieve LPS-induced depressive symptoms and effectively decrease LPS-induced neurotoxicity and inflammatory responses in mice by targeting the TLR4/Akt/HIF-1α/NLRP3 signaling pathway (Wei et al., 2022). Furthermore, a study by Huang et al. (2022) found that DHM alleviated symptoms of chronic depression in corticosterone (CORT)-induced mice. This effect may be attributed to the AGE-RAGE-NF-κB pathway.
2.4.2 Improving memory and cognitive disfunction
Watanabe et al. (2022) conducted a study using a mouse model of social isolation-induced anxiety to assess the effect of DHM. The study determined that administering DHM orally on a daily basis (at a dosage of 2 mg/kg) improved memory and cognition in socially isolated mice, possibly by remodeling hippocampal astrocytes.
Alzheimer’s disease (AD) is characterized by cognitive impairment and is a neurodegenerative condition. Oxidative stress and dysfunction of the cholinergic system are believed to be the primary pathogenic mechanisms. By inhibiting oxidative stress and cholinergic damage, DHM showed significant ameliorative effects on the behavioral and memory deficits induced by D-galactose in aging mice (Sun C et al., 2022). Additionally, DHM targets myeloid differentiation protein 2 (MD2) to inhibit the activation of TLR4 signaling, thereby suppressing neuroinflammation and enhancing cognition in mice with Alzheimer’s disease (Pei et al., 2023).
2.4.3 Treating cerebrovascular diseases
Xie et al. (2022) investigated the protective effect of DHM in a rat model of cerebral ischemia-reperfusion injury. The findings illustrated that DHM hindered ferroptosis by suppressing the SPHK1/mTOR signaling pathway, consequently alleviating cerebral injury due to cerebral ischemia-reperfusion. Furthermore, DHM has been observed to effectively decrease pyroptosis and alleviate ischemic brain injury in rats (Ding et al., 2023).
Similarly, a study explained that DHM is capable of safeguarding the brain via the activation of the Nrf2 and Prx2 signaling pathways, resulting in a notable decline in neuronal oxidative damage and apoptosis after subarachnoid hemorrhage (SAH) (Li et al., 2023). Notably, DHM also diminishes ferroptosis in brain tissue, thereby alleviating cerebral hemorrhages (Liu et al., 2023).
2.4.4 Treating Parkinson’s disease
DHM also has a positive effect on Parkinson’s disease (PD). Guo et al. (2020a) developed a new PD-like mouse model and demonstrated that DHM could alleviate motor dysfunction and prevent the loss of dopaminergic neurons in mice with PD.
2.4.5 Anti-anxiety
DHM improves anxiety through multiple pathways. Studies have revealed that DHM ameliorates anxiety behavior in a chronic social isolation (SI) mouse model by regulating mitochondrial function, reducing oxidative stress, restoring normal autophagy, and increasing brain-derived neurotrophic factor (BDNF), which plays a crucial role in neuroprotection (Al et al., 2022). Additionally, a study conducted by Silva et al. (2020a) on mice demonstrated that DHM could effectively counteract the decrease in adenosine triphosphate (ATP) levels and gephyrin protein expression in the hippocampus due to social isolation, leading to an improvement in anxiety.
2.5 Respiratory system
2.5.1 Inhibiting COVID-19 and improving pulmonary fibrosis
In September 2019, the novel coronavirus COVID-19 emerged as a global pandemic. During drug development to target this virus, researchers (Xiao et al., 2021) discovered through molecular docking techniques and in vitro cell studies that DHM can effectively suppress SARS-CoV-2 Mpro, thereby inhibiting the novel coronavirus. Moreover, cell research results suggested that DHM might also suppress pulmonary fibrosis and inflammation. Similarly, studies carried out by Li Z et al. (2022) indicated that DHM has the capacity to adjust the STAT3/p-STAT3/GLUT1 signaling pathway, mitigating pulmonary fibrosis induced in a mouse model by bleomycin (BLM). Additionally, these discoveries were confirmed through the use of primary human lung fibroblasts obtained from the lung tissues of idiopathic pulmonary fibrosis (IPF) patients.
2.5.2 Reducing pulmonary toxicity
As previously stated, methotrexate (MTX) is commonly prescribed in clinical settings, but its potential to cause permanent lung damage is a significant hindrance. In their rat study, Matouk et al. (2023) discovered that DHM reduces NF-κB expression in the lung tissue of rats treated with MTX. Furthermore, DHM upregulated the expression of Nrf2/HO-1, thereby alleviating MTX-induced lung toxicity through its antioxidative and anti-inflammatory properties. Interestingly, the study also found that DHM has the potential to reduce the activation of the pro-fibrotic agent TGF-β1 and decrease the weight loss caused by MTX.
2.6 Other effects
2.6.1 Anti-virus
Apart from its antiviral effects on the novel coronavirus, DHM also displays antiviral activity against pseudorabies virus (PRV) by inhibiting in vitro pyroptosis, regulating the NF-κB signaling pathway, and downregulating apoptosis factors (Zhao et al., 2023; Sun W et al., 2022). Additionally, DHM reduces the levels of inflammatory mediators induced by African swine fever virus (ASFV) by modulating the TLR4/MyD88/MAPK/NF-κB signaling pathway and inhibiting pyroptosis, thus inhibiting ASFV replication (Chen et al., 2023b).
2.6.2 Improving obesity
Currently, the activation of brown adipose tissue (BAT) or induction of browning in white adipose tissue (WAT) has become an increasingly popular target and strategy in the treatment of obesity. Preclinical research conducted by Xiong et al. (2022) has shown that DHM reduces overweight and fat accumulation induced by a high-fat diet in mice and improves glucose and lipid metabolism. Additionally, it promotes the browning of WAT in mice in vivo.
Another study in mice produced comparable findings, revealing that DHM promotes the browning of WAT through the activation of the IRF4/PGC-1α pathway (Leng et al., 2022). Furthermore, DHM has been found to regulate bile acid (BA) metabolism and its related effects, including modulating the gut microbiota and the FXR-SREBP-1C-related fat synthesis pathways, which improve obesity (Song et al., 2022).
2.6.3 Anti-tumor
Numerous studies have provided evidence of the potent anti-tumor properties of DHM. A recent review (Wu et al., 2022) summarized the anti-tumor properties of DHM across multiple research domains, including lung cancer, breast cancer, osteosarcoma, ovarian cancer, choriocarcinoma, hepatocellular carcinoma, and gastric cancer.
3 How to improve the bioavailability of dihydromyricetin
The aforementioned biological indications clearly demonstrate the regulatory potential of DHM as a natural product, while also highlighting its high safety profile. Currently, the primary obstacle to its further development and utilization is its low bioavailability. Lan et al. (2024) elaborated in detail on the relationship between flavonoid intestinal absorption and bioavailability. Previous studies reported that the oral bioavailability of DHM in rats is only 4.02% (Liu et al., 2017). The main reasons for the low bioavailability of DHM include its low water solubility, poor chemical stability, and low membrane permeability. At 25°C, the solubility of DHM in water is only 0.2 mg/mL (Dong et al., 2023).
In 2019, Liu D et al. (2019) provided a comprehensive summary of methods to enhance the bioavailability of DHM. The main strategies include: increasing DHM’s water solubility (e.g., various nanoparticle systems, cyclodextrin complexes, co-crystallization) and enhancing its lipophilicity (e.g., phospholipid complexes, acylation). Each method has its distinct characteristics.
Recently, several emerging methods have been employed to enhance the bioavailability of DHM, including the preparation of DHM-encapsulated PEGylated liposomes (DHM-Lipo) combined with liposomes (Zhou et al., 2022); an injectable redox albumin-based hydrogel with in situ loaded DHM (Deng et al., 2022); and the formulation of chitosan-based nanoparticles loaded with DHM (Yan et al., 2023). Each of these approaches shows promising results. Moreover, the design of ternary complexes (Huang et al., 2023) and the integration of nanoparticle delivery systems with gut microbiota modulation (Lyu et al., 2023) have also been validated in improving the bioavailability of other natural metabolites.
Future research should focus on maximizing the bioavailability of DHM while minimizing any adverse effects on its physicochemical properties. Extracellular vesicles (Feng et al., 2023), as cell-derived microvesicles, have gained significant attention in recent years as a natural drug delivery system due to their robust targeting capabilities, and high biocompatibility. Therefore, combining DHM with extracellular vesicles to improve its bioavailability may be a highly promising research direction.
4 Discussion
DHM is a novel natural product with various systemic and health-promoting properties, exhibiting distinctive pharmacological effects. Firstly, DHM’s anti-inflammatory and antioxidant properties stem from its unique chemical structure, making it effective against disorders associated with oxidative stress and inflammation, such as AS and inflammation of the nervous, digestive, and respiratory systems. Secondly, in addition to its impact on numerous signaling pathways associated with inflammation and oxidative stress, DHM regulates various cell death modes, including apoptosis, autophagy, pyroptosis, and ferroptosis. Therefore, cell death-related pathways represent highly promising research directions. Thirdly, DHM has shown synergy with first-line clinical drugs in certain disease treatments, working through multiple pathways and targets. This synergy reduces the severe toxic side effects frequently associated with first-line treatments, such as hepatotoxicity, nephrotoxicity, and cardiotoxicity. DHM’s favorable safety profile enhances tolerance to crucial but highly toxic drugs, increasing its potential for clinical application.
However, current research on DHM remains primarily at the cellular and animal levels, with very few reported clinical studies. This limitation may arise from the instability and relatively low bioavailability of DHM. DHM is soluble only in hot water and ethanol, and its poor solubility in water at room temperature significantly affects its membrane permeability and bioavailability. Furthermore, DHM exhibits inadequate solubility in the gastrointestinal tract, brief circulation time in the bloodstream, poor gastrointestinal absorption, and rapid metabolism (Tong et al., 2015; Zhang et al., 2021). Consequently, researchers are still examining methods to enhance the bioavailability and absorption of DHM, such as the implementation of liposomal carriers, chitosan nanoparticles, and intraperitoneal administration. However, while various strategies to enhance DHM’s bioavailability have been proposed, a thorough comparative analysis of these methods in clinical settings is essential to establish the most effective approach. Additionally, a deeper investigation into the molecular mechanisms by which DHM influences cellular pathways, particularly in relation to inflammation and cell death modalities, could elucidate its therapeutic potential across different diseases.
In conclusion, future research should focus on elucidating DHM’s mechanisms and improving its bioavailability in both preclinical and clinical studies. AS, involving mechanisms such as inflammation, oxidative stress, pyroptosis, and macrophage polarization, aligns well with the pharmacological effects of DHM, making it a promising area for DHM treatment. Additionally, the inflammasome plays an important role in many inflammation-related diseases, and DHM may be a potential inhibitor of the inflammasome. Exploring the effects of DHM on inflammasomes and specific regulatory mechanisms is a meaningful direction. Lastly, understanding the synergistic effects of DHM with existing first-line therapies could lead to more effective treatment regimens, minimizing adverse effects associated with conventional drugs. Expanding the scope of research to include these dimensions will not only strengthen the current understanding of DHM but also pave the way for its practical application in clinical settings. Notably, in 2013, DHM, predominantly found in vine tea, received certification as a ‘new resource food’ from the Chinese Ministry of Health, which has increased researchers’ confidence in endorsing DHM for clinical purposes.
5 Criteria for literature selection and search methodology
In this review, we employed a systematic literature retrieval strategy to ensure comprehensive coverage of research on DHM and its pharmacological effects. We first defined our focus, concentrating on the biological activities of DHM and its signaling pathways. To this end, we selected a range of keywords, including “Dihydromyricetin,” “pharmacological effects,” “biological activities,” and “signaling pathways. “For the retrieval logic, we utilized Boolean operators to combine keywords effectively. For instance, we constructed the search query “ (Dihydromyricetin OR DHM) AND (pharmacological effects OR biological activities) AND (signaling pathways OR cell signaling)” to ensure that the search results encompassed all relevant aspects. The literature search was primarily conducted in databases such as PubMed, Web of Science, and Scopus to ensure the acquisition of high-quality academic resources. Through this systematic retrieval strategy, we filtered relevant literature to comprehensively summarize the pharmacological effects and mechanisms of DHM, laying a solid foundation for subsequent discussions and analyses.
Author contributions
CH: Conceptualization, Writing–original draft, Writing–review and editing. YC: Writing–original draft, Writing–review and editing, Formal Analysis. JX: Writing–review and editing, Validation. ML: Conceptualization, Investigation, Writing–original draft. DF: Data curation, Writing–review and editing. NH: Software, Writing–review and editing. EM: Resources, Writing–review and editing. YD: Supervision, Writing–review and editing. LZ: Writing–review and editing, Conceptualization, Data curation. YX: Resources, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81974530 and 82100518), Hubei International Sciencific and Technological Cooperation Project (2022EHB039 and 2023EHA057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al, O. A., Watanabe, S., Hong, E. C., Skinner, S. G., Zhang, M., Zhang, J., et al. (2022). Dihydromyricetin ameliorates social isolation-induced anxiety by modulating mitochondrial function, antioxidant enzymes, and BDNF. Neurobiol. Stress. 21, 100499. doi:10.1016/j.ynstr.2022.100499
Chen, S., Lv, K., Sharda, A., Deng, J., Zeng, W., Zhang, C., et al. (2021). Anti-thrombotic effects mediated by dihydromyricetin involve both platelet inhibition and endothelial protection. Pharmacol. Res. 167, 105540. doi:10.1016/j.phrs.2021.105540
Chen, Y., Song, Z., Chang, H., Guo, Y., Wei, Z., Sun, Y., et al. (2023a). Dihydromyricetin inhibits African swine fever virus replication by downregulating toll-like receptor 4-dependent pyroptosis in vitro. Vet. Res. 54 (1), 58. doi:10.1186/s13567-023-01184-8
Chen, Y., Zheng, Y., Chen, R., Shen, J., Zhang, S., Gu, Y., et al. (2023b). Dihydromyricetin attenuates diabetic cardiomyopathy by inhibiting oxidative stress, inflammation and necroptosis via sirtuin 3 activation. Antioxidants (Basel) 12 (1), 200. doi:10.3390/antiox12010200
Cheng, L., Wang, X., Ma, X., Xu, H., Yang, Y., and Zhang, D. (2021). Effect of dihydromyricetin on hepatic encephalopathy associated with acute hepatic failure in mice. Pharm. Biol. 59 (1), 557–564. doi:10.1080/13880209.2021.1917625
Cheng, Q. C., Fan, J., Deng, X. W., Liu, H. C., Ding, H. R., Fang, X., et al. (2020). Dihydromyricetin ameliorates chronic liver injury by reducing pyroptosis. World J. Gastroenterol. 26 (41), 6346–6360. doi:10.3748/wjg.v26.i41.6346
Chistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V., and Orekhov, A. N. (2017). Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. Berl. 95 (11), 1153–1165. doi:10.1007/s00109-017-1575-8
Deng, L., Xia, T., Cheng, W., Yang, M., Zhu, W., and Chen, X. (2022). Injectable redox albumin-based hydrogel with in-situ loaded dihydromyricetin. Colloids Surf. B Biointerfaces 220, 112871. doi:10.1016/j.colsurfb.2022.112871
Ding, H., Cheng, Q., Fang, X., Wang, Z., Fang, J., Liu, H., et al. (2023). Dihydromyricetin alleviates ischemic brain injury by antagonizing pyroptosis in rats. Neurotherapeutics 20, 1847–1858. doi:10.1007/s13311-023-01425-w
Ding, Y., Gong, W., Zhang, S., Shen, J., Liu, X., Wang, Y., et al. (2021). Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem. Pharmacol. 192, 114665. doi:10.1016/j.bcp.2021.114665
Dong, J., Wang, S., Mao, J., Wang, Z., Zhao, S., Ren, Q., et al. (2023). Preparation of dihydromyricetin-loaded self-emulsifying drug delivery system and its anti-alcoholism effect. Pharmaceutics 15 (9), 2296. doi:10.3390/pharmaceutics15092296
Dong, S., Zhu, M., Wang, K., Zhao, X., Hu, L., Jing, W., et al. (2021). Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 171, 105767. doi:10.1016/j.phrs.2021.105767
Emad, D., Bayoumi, A. M. A., Gebril, S. M., Ali, D. M. E., and Waz, S. (2024). Modulation of keap-1/Nrf2/HO-1 and NF-ĸb/caspase-3 signaling pathways by dihydromyricetin ameliorates sodium valproate-induced liver injury. Arch. Biochem. Biophys. 758, 110084. doi:10.1016/j.abb.2024.110084
Feng, J., Xiu, Q., Huang, Y., Troyer, Z., Li, B., and Zheng, L. (2023). Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv. Mat. 35 (24), e2207826. doi:10.1002/adma.202207826
Feng, L., Que, D., Li, Z., Zhong, X., Yan, J., Wei, J., et al. (2021). Dihydromyricetin ameliorates vascular calcification in chronic kidney disease by targeting AKT signaling. Clin. Sci. (Lond). 135 (21), 2483–2502. doi:10.1042/CS20210259
Gong, H., Xu, H., Li, M., and Zhang, D. (2022). Molecular mechanism and therapeutic significance of dihydromyricetin in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 935, 175325. doi:10.1016/j.ejphar.2022.175325
Guan, S., Shen, Y., Ge, H., Xiong, W., He, L., Liu, L., et al. (2019). Dihydromyricetin alleviates diabetic neuropathic pain and depression comorbidity symptoms by inhibiting P2X(7) receptor. Front. Psychiatry 10, 770. doi:10.3389/fpsyt.2019.00770
Guo, C. H., Cao, T., Zheng, L. T., Waddington, J. L., and Zhen, X. C. (2020a). Development and characterization of an inducible Dicer conditional knockout mouse model of Parkinson's disease: validation of the antiparkinsonian effects of a sigma-1 receptor agonist and dihydromyricetin. Acta Pharmacol. Sin. 41 (4), 499–507. doi:10.1038/s41401-020-0379-5
Guo, L., Tan, K., Luo, Q., and Bai, X. (2020b). Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn. J. Basic Med. Sci. 20 (3), 372–380. doi:10.17305/bjbms.2019.4410
Hou, P., Wang, D., Lang, H., Yao, Y., Zhou, J., Zhou, M., et al. (2022). Dihydromyricetin attenuates high-intensity exercise-induced intestinal barrier dysfunction associated with the modulation of the phenotype of intestinal intraepithelial lymphocytes. Int. J. Mol. Sci. 24 (1), 221. doi:10.3390/ijms24010221
Huang, J., Chen, B., Wang, H., Hu, S., Yu, X., Reilly, J., et al. (2022). Dihydromyricetin attenuates depressive-like behaviors in mice by inhibiting the AGE-RAGE signaling pathway. Cells 11 (23), 3730. doi:10.3390/cells11233730
Huang, X., Tu, R., Song, H., Dong, K., Geng, F., Chen, L., et al. (2023). Fabrication and characterization of gelatin-EGCG-pectin ternary complex: formation mechanism, emulsion stability, and structure. J. Sci. Food Agric. 103 (3), 1442–1453. doi:10.1002/jsfa.12240
Lan, H., Wang, H., Chen, C., Hu, W., Ai, C., Chen, L., et al. (2024). Flavonoids and gastrointestinal health: single molecule for multiple roles. Crit. Rev. Food Sci. Nutr. 64 (30), 10987–11005. doi:10.1080/10408398.2023.2230501
Leng, Q., Zhou, J., Li, C., Xu, Y., Liu, L., Zhu, Y., et al. (2022). Dihydromyricetin ameliorates diet-induced obesity and promotes browning of white adipose tissue by upregulating IRF4/PGC-1α. Nutr. Metab. (Lond). 19 (1), 38. doi:10.1186/s12986-022-00672-6
Li, X., Wang, X., Wang, B., Chi, W., Li, Z., Zhang, M., et al. (2022). Dihydromyricetin protects against Doxorubicin-induced cardiotoxicity through activation of AMPK/mTOR pathway. Phytomedicine 99, 154027. doi:10.1016/j.phymed.2022.154027
Li, X. J., Pang, C., Peng, Z., Zhuang, Z., Lu, Y., Li, W., et al. (2023). Dihydromyricetin confers cerebroprotection against subarachnoid hemorrhage via the Nrf2-dependent Prx2 signaling cascade. Phytomedicine 119, 154997. doi:10.1016/j.phymed.2023.154997
Li, Z., Geng, J., Xie, B., He, J., Wang, J., Peng, L., et al. (2022). Dihydromyricetin alleviates pulmonary fibrosis by regulating abnormal fibroblasts through the STAT3/p-STAT3/GLUT1 signaling pathway. Front. Pharmacol. 13, 834604. doi:10.3389/fphar.2022.834604
Liu, B., Ma, Y., Yuan, C., Su, C., Hu, L., and Wang, J. (2012). Characterization, stability and antioxidant activity of the inclusion complex of dihydromyricetin with hydroxypropyl-β-cyclodextrin. J. Food Biochem. 36, 634–641. doi:10.1111/j.1745-4514.2011.00577.x
Liu, D., Mao, Y., Ding, L., and Zeng, X. A. (2019). Dihydromyricetin: a review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 91, 586–597. doi:10.1016/j.tifs.2019.07.038
Liu, L., Yin, X., Wang, X., and Li, X. (2017). Determination of dihydromyricetin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Pharm. Biol. 55 (1), 657–662. doi:10.1080/13880209.2016.1266669
Liu, X., Li, Y., Chen, S., Yang, J., Jing, J., Li, J., et al. (2023). Dihydromyricetin attenuates intracerebral hemorrhage by reversing the effect of LCN2 via the system Xc-pathway. Phytomedicine 115, 154756. doi:10.1016/j.phymed.2023.154756
Liu, Y., Bi, X., Xiong, J., Han, W., Xiao, T., Xu, X., et al. (2019). MicroRNA-34a promotes renal fibrosis by downregulation of klotho in tubular epithelial cells. Mol. Ther. 27 (5), 1051–1065. doi:10.1016/j.ymthe.2019.02.009
Luo, Y., Lu, S., Dong, X., Xu, L., Sun, G., and Sun, X. (2017). Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis 22 (8), 1013–1024. doi:10.1007/s10495-017-1381-3
Lyu, Q., Deng, H., Wang, S., El-Seedi, H., Cao, H., Chen, L., et al. (2023). Dietary supplementation with casein/cyanidin-3-O-glucoside nanoparticles alters the gut microbiota in high-fat fed C57BL/6 mice. Food Chem. 412, 135494. doi:10.1016/j.foodchem.2023.135494
Matouk, A. I., Awad, E. M., El-Tahawy, N., El-Sheikh, A., and Anter, A. (2023). Dihydromyricetin modulates Nrf2 and NF-κB crosstalk to alleviate methotrexate-induced lung toxicity. Pharm. (Basel) 16 (4), 481. doi:10.3390/ph16040481
Matouk, A. I., Awad, E. M., El-Tahawy, N., El-Sheikh, A., and Waz, S. (2022). Dihydromyricetin alleviates methotrexate-induced hepatotoxicity via suppressing the TLR4/NF-κB pathway and NLRP3 inflammasome/caspase 1 axis. Biomed. Pharmacother. 155, 113752. doi:10.1016/j.biopha.2022.113752
Ni, T., Lin, N., Lu, W., Sun, Z., Lin, H., Chi, J., et al. (2020). Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc Drugs Ther. 34 (3), 291–301. doi:10.1007/s10557-020-06968-0
Pei, H., Han, C., Bi, J., He, Z., and Guo, L. (2023). Dihydromyricetin suppresses inflammatory injury in microglial cells to improve neurological behaviors of Alzheimer's disease mice via the TLR4/MD2 signal. Int. Immunopharmacol. 118, 110037. doi:10.1016/j.intimp.2023.110037
Pineton, D. C. G., Blanc, P., and Peyrin-Biroulet, L. (2016). Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 10 (8), 915–927. doi:10.1586/17474124.2016.1174064
Poznyak, A., Grechko, A. V., Poggio, P., Myasoedova, V. A., Alfieri, V., and Orekhov, A. N. (2020). The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 21 (5), 1835. doi:10.3390/ijms21051835
Semwal, D. K., Semwal, R. B., Combrinck, S., and Viljoen, A. (2016). Myricetin: a dietary molecule with diverse biological activities. Nutrients 8 (2), 90. doi:10.3390/nu8020090
Shi, C., Wang, J., Zhang, R., Ishfaq, M., Li, Y., Zhang, R., et al. (2022). Dihydromyricetin alleviates Escherichia coli lipopolysaccharide-induced hepatic injury in chickens by inhibiting the NLRP3 inflammasome. Vet. Res. 53 (1), 6. doi:10.1186/s13567-022-01024-1
Silva, J., Shao, A. S., Shen, Y., Davies, D. L., Olsen, R. W., Holschneider, D. P., et al. (2020a). Modulation of hippocampal GABAergic neurotransmission and gephyrin levels by dihydromyricetin improves anxiety. Front. Pharmacol. 11, 1008. doi:10.3389/fphar.2020.01008
Silva, J., Yu, X., Moradian, R., Folk, C., Spatz, M. H., Kim, P., et al. (2020b). Dihydromyricetin protects the liver via changes in lipid metabolism and enhanced ethanol metabolism. Alcohol. Clin. Exp. Res. 44 (5), 1046–1060. doi:10.1111/acer.14326
Song, Y., Sun, L., Ma, P., Xu, L., and Xiao, P. (2022). Dihydromyricetin prevents obesity via regulating bile acid metabolism associated with the farnesoid X receptor in ob/ob mice. Food Funct. 13 (5), 2491–2503. doi:10.1039/d1fo03971g
Sun, C. C., Yin, Z. P., Chen, J. G., Wang, W. J., Zheng, G. D., Li, J. E., et al. (2022). Dihydromyricetin improves cognitive impairments in d-galactose-induced aging mice through regulating oxidative stress and inhibition of acetylcholinesterase. Mol. Nutr. Food Res. 66 (4), e2101002. doi:10.1002/mnfr.202101002
Sun, W., Liu, S., Lu, A., Yang, F., and Duan, J. (2022). In vitro anti-PRV activity of dihydromyricetin from Ampelopsis grossedentata. Nat. Prod. Res. 36 (17), 4448–4451. doi:10.1080/14786419.2021.1982935
Sun, Z., Lu, W., Lin, N., Lin, H., Zhang, J., Ni, T., et al. (2020). Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 175, 113888. doi:10.1016/j.bcp.2020.113888
Tian, X., Liu, Y., Wang, H., Zhang, J., Xie, L., Huo, Y., et al. (2021). The role of miR-199b-3p in regulating Nrf2 pathway by dihydromyricetin to alleviate septic acute kidney injury. Free Radic. Res. 55 (7), 842–852. doi:10.1080/10715762.2021.1962008
Tong, Q., Hou, X., Fang, J., Wang, W., Xiong, W., Liu, X., et al. (2015). Determination of dihydromyricetin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 114, 455–461. doi:10.1016/j.jpba.2015.06.030
Vengrenyuk, Y., Nishi, H., Long, X., Ouimet, M., Savji, N., Martinez, F. O., et al. (2015). Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35 (3), 535–546. doi:10.1161/ATVBAHA.114.304029
Wang, J., Ma, S., Yu, J., Zuo, D., He, X., Peng, H., et al. (2021). MiR-9-5p promotes M1 cell polarization in osteoarthritis progression by regulating NF-κB and AMPK signaling pathways by targeting SIRT1. Int. Immunopharmacol. 101 (Pt A), 108207. doi:10.1016/j.intimp.2021.108207
Wang, J. T., Jiao, P., Zhou, Y., and Liu, Q. (2016). Protective effect of dihydromyricetin against lipopolysaccharide-induced acute kidney injury in a rat model. Med. Sci. Monit. 22, 454–459. doi:10.12659/msm.897076
Wang, S., Zhang, J., Deng, X., Zhao, Y., and Xu, K. (2020). Advances in characterization of SIRT3 deacetylation targets in mitochondrial function. Biochimie 179, 1–13. doi:10.1016/j.biochi.2020.08.021
Watanabe, S., Omran, A. A., Shao, A. S., Xue, C., Zhang, Z., Zhang, J., et al. (2022). Dihydromyricetin improves social isolation-induced cognitive impairments and astrocytic changes in mice. Sci. Rep. 12 (1), 5899. doi:10.1038/s41598-022-09814-5
Wei, Y., Hu, Y., Qi, K., Li, Y., Chen, J., and Wang, R. (2022). Dihydromyricetin improves LPS-induced sickness and depressive-like behaviors in mice by inhibiting the TLR4/Akt/HIF1a/NLRP3 pathway. Behav. Brain Res. 423, 113775. doi:10.1016/j.bbr.2022.113775
Wu, J., Xiao, Z., Li, H., Zhu, N., Gu, J., Wang, W., et al. (2022). Present status, challenges, and prospects of dihydromyricetin in the battle against cancer. Cancers (Basel) 14 (14), 3487. doi:10.3390/cancers14143487
Xiang, D., Wang, C. G., Wang, W. Q., Shi, C. Y., Xiong, W., Wang, M. D., et al. (2017). Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: an in vitro investigation. Int. J. Food Sci. Nutr. 68 (6), 704–711. doi:10.1080/09637486.2016.1276518
Xiao, T., Wei, Y., Cui, M., Li, X., Ruan, H., Zhang, L., et al. (2021). Effect of dihydromyricetin on SARS-CoV-2 viral replication and pulmonary inflammation and fibrosis. Phytomedicine 91, 153704. doi:10.1016/j.phymed.2021.153704
Xie, J., Zhang, T., Li, P., Wang, D., Liu, T., and Xu, S. (2022). Dihydromyricetin attenuates cerebral ischemia reperfusion injury by inhibiting SPHK1/mTOR signaling and targeting ferroptosis. Drug Des. Devel Ther. 16, 3071–3085. doi:10.2147/DDDT.S378786
Xiong, X., Xia, M., Niu, A., Zhang, Y., Yin, T., and Huang, Q. (2022). Dihydromyricetin contributes to weight loss via pro-browning mediated by mitochondrial fission in white adipose. Eur. J. Pharmacol. 935, 175345. doi:10.1016/j.ejphar.2022.175345
Xu, J. J., Yao, M. J., and C, W. M. (2008). Study on biological efficacy of dihydromyricetin. Food Sci., 622–625.
Xu, Z., Zhang, M., Wang, W., Zhou, S., Yu, M., Qiu, X., et al. (2023). Dihydromyricetin attenuates cisplatin-induced acute kidney injury by reducing oxidative stress, inflammation and ferroptosis. Toxicol. Appl. Pharmacol. 473, 116595. doi:10.1016/j.taap.2023.116595
Yan, Q., Li, M., Dong, L., Luo, J., Zhong, X., Shi, F., et al. (2023). Preparation, characterization and protective effect of chitosan - tripolyphosphate encapsulated dihydromyricetin nanoparticles on acute kidney injury caused by cisplatin. Int. J. Biol. Macromol. 245, 125569. doi:10.1016/j.ijbiomac.2023.125569
Yan, Y., Wang, K., Tang, X., Gao, J. F., and Wen, B. Y. (2019). Phytochemicals protect L02 cells against hepatotoxicity induced by emodin via the Nrf2 signaling pathway. Toxicol. Res. (Camb) 8 (6), 1028–1034. doi:10.1039/c9tx00220k
Yang, D., Yang, Z., Chen, L., Kuang, D., Zou, Y., Li, J., et al. (2020). Dihydromyricetin increases endothelial nitric oxide production and inhibits atherosclerosis through microRNA-21 in apolipoprotein E-deficient mice. J. Cell. Mol. Med. 24 (10), 5911–5925. doi:10.1111/jcmm.15278
Yang, Z., Li, T., Wang, C., Meng, M., Tan, S., and Chen, L. (2023). Dihydromyricetin inhibits M1 macrophage polarization in atherosclerosis by modulating miR-9-mediated SIRT1/NF-κB signaling pathway. Mediat. Inflamm. 2023, 2547588. doi:10.1155/2023/2547588
Zeng, T., Song, Y., Qi, S., Zhang, R., Xu, L., and Xiao, P. (2023). A comprehensive review of vine tea: origin, research on Materia Medica, phytochemistry and pharmacology. J. Ethnopharmacol. 317, 116788. doi:10.1016/j.jep.2023.116788
Zhang, H., Caprioli, G., Hussain, H., Le, N. P. K., Farag, M. A., and Xiao, J. (2021). A multifaceted review on dihydromyricetin resources, extraction, bioavailability, biotransformation, bioactivities, and food applications with future perspectives to maximize its value. eFood 2 (4), 164–184. doi:10.53365/efood.k/143518
Zhang, J., Chen, Y., Luo, H., Sun, L., Xu, M., Yu, J., et al. (2018). Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 9, 1204. doi:10.3389/fphar.2018.01204
Zhang, X., Wang, L., Peng, L., Tian, X., Qiu, X., Cao, H., et al. (2019). Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J. Cell. Mol. Med. 23 (7), 4829–4838. doi:10.1111/jcmm.14406
Zhao, X., Chen, Y., Zhang, W., Zhang, H., Hu, Y., Yang, F., et al. (2023). Dihydromyricetin inhibits pseudorabies virus multiplication in vitro by regulating NF-κB signaling pathway and apoptosis. Vet. Sci. 10 (2), 111. doi:10.3390/vetsci10020111
Zhao, Y., Liu, X., Ding, C., Gu, Y., and Liu, W. (2021). Dihydromyricetin reverses thioacetamide-induced liver fibrosis through inhibiting NF-κB-Mediated inflammation and TGF-β1-regulated of PI3K/Akt signaling pathway. Front. Pharmacol. 12, 783886. doi:10.3389/fphar.2021.783886
Zhou, J., Yue, J., Yao, Y., Hou, P., Zhang, T., Zhang, Q., et al. (2023). Dihydromyricetin protects intestinal barrier integrity by promoting IL-22 expression in ILC3s through the AMPK/SIRT3/STAT3 signaling pathway. Nutrients 15 (2), 355. doi:10.3390/nu15020355
Zhou, X., Yi, L., Lang, H., Zhang, J., Zhang, Q., Yu, L., et al. (2022). Dihydromyricetin-encapsulated liposomes inhibit exhaustive exercise-induced liver inflammation by orchestrating M1/M2 macrophage polarization. Front. Pharmacol. 13, 887263. doi:10.3389/fphar.2022.887263
Zhou, X., Yu, L., Zhou, M., Hou, P., Yi, L., and Mi, M. (2021). Dihydromyricetin ameliorates liver fibrosis via inhibition of hepatic stellate cells by inducing autophagy and natural killer cell-mediated killing effect. Nutr. Metab. (Lond). 18 (1), 64. doi:10.1186/s12986-021-00589-6
Glossary
ACSL4 Acyl-CoA synthetase long-chain family member 4
AD Alzheimer’s disease
ADMA Asymmetric dimethylarginine
AGE Advanced glycation end product
AKI Acute Kidney Injury
AKT Protein kinase B
ALD Alcoholic liver disease
AMPK Adenosine monophosphate activated protein kinase
Arg1 Arginase-1
ASC Apoptosis-associated speck-like protein
ASFV African swine fever virus
ASK1 Apoptosis signal-regulating kinase
ATP Adenosine triphosphate
BA Bile acid
Bax Bcl2-associated X protein
Bcl-2 B-cell lymphoma-2
Bcl-3 B-cell lymphoma-3
BDNF Brain-derived neurotrophic factor
BLM Bleomycin
BUN Blood urea nitrogen
CAT Catalase
CCl4 Carbon tetrachloride
CD Crohn’s disease
CDCA Chenodeoxycholic acid
CLI Chronic liver injury
CLP Cecum ligation and puncture
CORT Corticosterone
CREB cAMP-response element binding protein
DDAH1 Dimethylarginine dimethylaminohydrolase-1
DHM Dihydromyricetin
DMC Diabetic cardiomyopathy
DN Diabetic nephropathy
DOX Doxorubicin
DSS Dextran sulfate sodium
EF Ejection fraction
eNOS Endothelial nitric oxide synthase
ERK1/2 Extracellular signal-regulated kinases 1/2
EtOH Ethanol
FBG Fasting blood glucose
FINS Fasting serum lisulin
FOXO3a Forkhead box class O 3a
FS Fractional shortening
FXR Farnesoid X receptor
GCLC Glutamate-cysteine ligase, catalytic subunit
GCLM Glutamate-cysteine ligase modifier subunit
GLUT1 Glucose transporter type 1
GPX4 Glutathione peroxidase 4
GSDMA GasderminA
GSDMD-N GasderminD-N
GSH-Px Glutathioneperoxidase
GSK-3β Glycogen synthase kinase-3β
HbA1c Hemoglobin A1C
HCD High cholesterol diet
HE Hepatic encephalopathy
HFD High-fat diet
HG High glucose
HIE High-intensity exercise
HIF-1α Hypoxia-inducible factor-1α
HO-1 Heme oxygenase-1
HSCs Hepatic stellate cells
HUVEC Human umbilical vein endothelial cell
I/R injury Ischemia-reperfusion injury
IBD Inflammatory bowel disease
IFN-γ Interferon-γ
IFP-PHLF Primary human lung fibroblasts of IPF patients
IL-10 Interleukin 10
IL-18 Interleukin-18
IL-1β Interleukin-1β
IL-22 Interleukin 22
IL-6 Interleukin 6
ILC3s Group 3 innate lymphoid cells
IRF4 Interferon regulatory factor 4
JNK JUN N -terminal kinases
KIM1 Kidney injury molecule-1
LC3-II Light chain 3-II
LCA Lithocholic acid
LPS Lipopolysaccharide
LVEF Left ventricular ejection fraction
LVFS Left ventricular fractional shortening
LVIDd Left ventricular end-diastolic internal dimension
LVIDs Left ventricular end-systolic internal dimension
MDA Malondialdehyde
MAPK Mitogen-activated protein kinases
MD2 Myeloid differentiation Protein
miR-155-5p MicroRNA-155-5p
miR-199b-3 MicroRNA-199b-3
miR-21 MicroRNA-21
miR-34a MicroRNA-4a
miR-5-52p MicroRNA-5-52p
miR-9 MicroRNA-9
mTOR Mammalian target of rapamycin
MTX Methotrexate
MyD88 Myeloid differentiation factor 88
NA No applicable
NAFLD Non-alcoholic fatty liver disease
NF-κB Nuclear factor kappa-B
NKC Natural killer cell
NLRP3 NOD-like receptor thermal protein domain associated protein 3
NO Nitric oxide
NOS2 Nitric oxide synthase 2
NQO-1 NAPDH: quinone oxidoreductase 1
Nrf2 Nuclear factor erythroid-2-related factor2
OGD/R Oxygen-glucose deprivation/reperfusion
Ox-LDL Oxidized low-density lipoprotein
PAMs Porcine alveolar macrophages
PD Parkinson’s disease
PDI Protein disulfide isomerase
PDPK1 Phosphoinositide-dependent protein kinase 1
PEBP1 Phosphatidylethanolamine binding protein 1
PGC-1α Peroxisome proliferator-activated receptor γ coactivator 1α
PI3K Phosphatidylinositol-3-kinase
PMLFs Primary mouse lung fibroblasts
PRV Pseudorabies virus
Prx2 Peroxiredoxin 2
PTEN Phosphatase and tensin homolog deleted on chromosome ten
RAGE Receptor for AGE
RIF Renal interstitial fibrosis
ROS Reactive Oxygen Species
SAH Subarachnoid hemorrhages
SI Social isolation
SIRT1 Sirtuin1
SIRT3 Sirtuin3
SNP Sodium nitroprusside
SOD Super oxide dismutase
SPHK1 Sphingosine kinases type 1
SREBP-1C Sterol regulatory element binding protein-1C
STAT3 Signal transducer and activator of transcription 3
STZ Streptozotocin
TAA Thioacetamide
TF Tissue factor
TG Triglyceride
TGF-α Transforming growth factor α
TGF-β1 Transforming growth factor β1
TGR5 G-protein-coupled bile acid receptor
TLR4 Toll-like receptor 4
TNF-α Tumor necrosis factor-α
UC Ulcerative colitis
UCP1 Uncoupling protein 1
UUO Unilateral uretera obstruction
VSMC Vascular smooth muscle cell
vWF von Willebrand factor
WAT White adipose tissue
α-SMA α-smooth muscle actin
Keywords: dihydromyricetin, anti-inflammatory, antioxidant, anti-virus, signaling pathway
Citation: He C, Chen Y, Xie J, Luo M, Fisher D, Hien NTT, Musabaev E, Dang Y, Zhao L and Xia Y (2025) Dihydromyricetin: an emerging compound with comprehensive effects on multiple systems. Front. Pharmacol. 15:1488003. doi: 10.3389/fphar.2024.1488003
Received: 29 August 2024; Accepted: 04 December 2024;
Published: 03 January 2025.
Edited by:
Lei Chen, Guangdong Ocean University, ChinaReviewed by:
Haijing Lan, Guangdong Ocean University, ChinaCopyright © 2025 He, Chen, Xie, Luo, Fisher, Hien, Musabaev, Dang, Zhao and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Xia, eGlheWluMTExOEAxNjMuY29t; Lei Zhao, bGVpemhhb0BodXN0LmVkdS5jbg==; Yiping Dang, MjQ0OTI3MTYwQHFxLmNvbQ==
†These authors have contributed equally to this work
 Chengyi He1†
Chengyi He1† Yunfei Chen
Yunfei Chen Jiao Xie
Jiao Xie Miao Luo
Miao Luo David Fisher
David Fisher Lei Zhao
Lei Zhao