- 1School of Pharmaceutical Sciences and Institute of Materia Medica, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2School of Pharmacy, Jining Medical University, Rizhao, China
Marine fungi represent a treasure trove of bioactive secondary metabolites, with benzopyran compounds emerging as a significant class of these natural products. This review delves into the structural diversity, biological activities, and sources of benzopyran compounds, highlighting their isolation from marine fungi inhabiting diverse environments such as sponges, marine sediments, algae, mangroves, and corals. Our literature search, conducted from 2000 to 2023, has identified a wealth of benzopyran compounds, showcasing their potential as lead compounds in drug development. The characteristics of benzopyran from marine fungi are explored, encompassing various subclasses such as chromones, isocoumarins, citrinins, and other related compounds. These compounds exhibit a remarkable chemical diversity, which is crucial for their diverse biological activities. The potential of benzopyran compounds in drug development is also discussed, emphasizing their roles in anti-tumor, antibacterial, anti-inflammatory, and enzyme inhibitory activities. In recent years, a remarkable 210 bioactive benzopyran compounds have been isolated from the secondary metabolites of marine fungi. These findings underscore the importance of marine fungi as a source of novel bioactive compounds, offering a plethora of potential lead compounds for the development of marine-derived drugs. This review aims to provide a comprehensive overview of the current state of research on benzopyran compounds, setting the stage for future advancements in the field of marine natural products.
1 Introduction
The ocean, a cornerstone of our planet’s ecological system, is a cradle of innumerable life forms and a bountiful reservoir of biological diversity and bioactive compounds. In this vast aquatic expanse, marine life has developed distinctive survival tactics and metabolic pathways, bequeathing humanity a wealth of pharmaceutical resources and potential lead compounds for drug discovery. In particular, the evolution of marine biotechnology and extensive research over recent decades have propelled marine-derived secondary metabolites to the forefront as a significant source for the innovation of novel pharmaceuticals (Blunt et al., 2017; Haque et al., 2022).
Benzopyran compounds, integral to a multitude of bioactive natural products, are composed of a benzene ring annelated to an oxygenated six-membered heterocyclic moiety. Celebrated for their structural complexity and pronounced bioactivity, these secondary metabolites have been unearthed in marine life, with a notable prevalence in marine fungi, thereby igniting new avenues of research in medicinal chemistry. These marine fungi, as microorganisms thriving in the marine milieu, have carved out distinctive ecological niches and adaptive strategies, culminating in the biosynthesis of an array of innovative and pharmacologically diverse secondary metabolites. The benzopyran class, in particular, has captured the spotlight in drug development, attributable to their broad spectrum of pharmacological actions, such as antibacterial, antifungal, antiviral, antitumor, antioxidant, and anti-inflammatory capabilities (Kumla et al., 2018; Pang et al., 2018a; Frank et al., 2019; Yang et al., 2020b; Wu et al., 2021).
In recent years, the exploration into the secondary metabolites of marine fungi has yielded a growing catalog of benzopyran compounds, with their biological activities being the subject of systematic investigation. Yet, despite the surge in research focusing on these marine fungal-derived benzopyran compounds, there remains a dearth of studies delving into their intricate chemical structures, biosynthetic pathways, and the mechanisms behind their bioactivity. This review endeavors to offer an exhaustive overview of the benzopyran compounds isolated from marine fungi over the past 20 years, encompassing their chemical structures, origins, biological activities, and the promise they hold for drug development. By meticulously examining and synthesizing the existing body of literature, this article aims to present a comprehensive vista of the research on benzopyran compounds from marine fungi to the reader, thereby catalyzing further advancements in scientific inquiry and tangible applications within this burgeoning domain.
2 Literature search
2.1 Methods
The literature search was conducted using previously reported methods (Soosaraei et al., 2018; Sun et al., 2019), where the selection of original articles is crucial because these papers have a direct impact on the research outcomes and the final conclusions. This review encompasses articles related to the target topic from the years 2000–2023, retrieved from the Web of Science and PubMed databases. The following descriptors were used during the search: benzopyran; chemical structure; biological activity.
2.2 Quantification of the studies
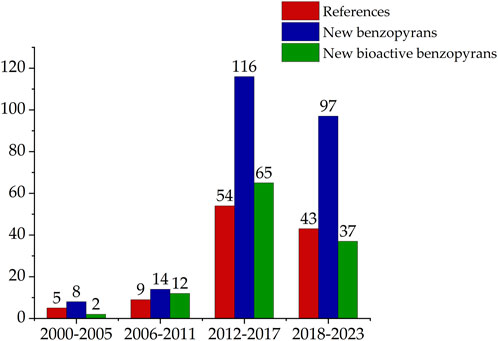
After conducting a literature search focused on the field of natural product chemistry, a total of 112 original articles indexed in the Web of Science and PubMed databases over the past 2 decades were identified. These studies have led to the isolation and identification of 510 benzopyran compounds from marine-derived fungi, with 289 novel compounds being characterized. Among these newly identified compounds, 116 have demonstrated biological activity. The number of references and compounds was further collated according to the year of publication, as illustrated in Figure 1. The search results indicate a significant upswing in the number of benzopyran compounds isolated and characterized in the last decade (2012-2023).
3 Characteristics of benzopyran from marine fungi
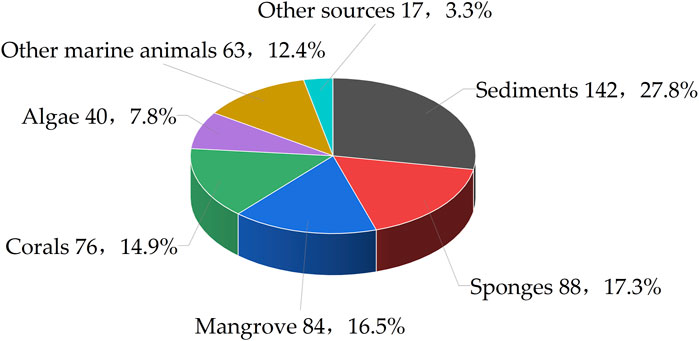
Marine fungi have yielded a rich collection of natural substances, playing a pivotal role in the discovery of bioactive secondary metabolites for drug development. Among these metabolites, benzopyran compounds constitute an important class, renowned for their diverse biological activities and potential therapeutic applications. Herein, this review encompasses an analysis of 510 benzopyran compounds isolated from a total of 112 distinct strains of marine fungi. Furthermore, the research reveals that a substantial portion of these benzopyran compounds, approximately 44.6%, originate from marine animals, with sponges accounting for 17.3% and corals for 14.9%. Additionally, marine plants such as algae and mangroves contribute to 24.3% of the benzopyran compounds, with 7.8% and 16.5% respectively. The remaining benzopyran compounds were isolated from various other components of the marine ecosystem, with 27.8% derived from marine sediments and 3.3% from other unspecified sources (Figure 2).
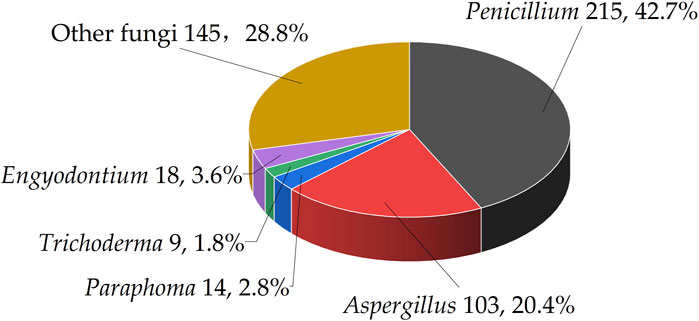
This article presents a taxonomic classification at the genus level for 112 marine fungi to assess their distribution in the biosynthesis of benzopyran compounds. The results indicate that Penicillium is predominant, accounting for 42.7% of the evaluated species. It is followed by Aspergillus, which constitutes 20.4% of the total. In addition, Paraphoma, Engyodontium, and Trichoderma are represented by 2.8%, 3.6%, and 1.8% respectively. The remaining 28.8% are categorized under other fungal genera (Figure 3).
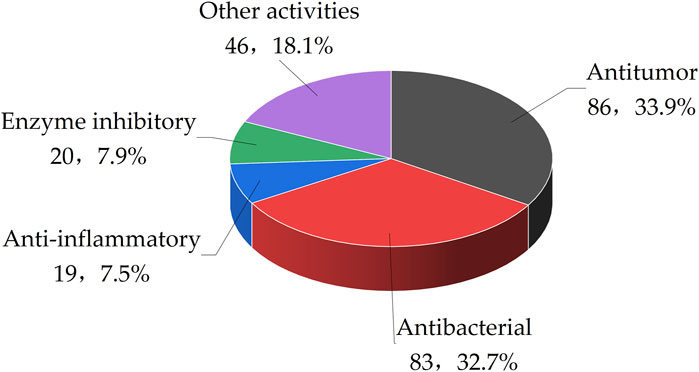
Recent research has highlighted the divergent metabolic processes in marine fungi, which result in the generation of a diverse array of secondary metabolites with unique chemical compositions and targeted biological effects. This article concludes that out of 510 benzopyran compounds isolated from marine fungi, 210 exhibit significant bioactivity. These benzopyran compounds have demonstrated a range of biological effects, including antitumor, antibacterial, anti-inflammatory, enzyme inhibitory, and antiviral properties. Specifically, 33.9% of the benzopyran compounds have shown antitumor activity, followed by antibacterial activity at 32.7%. Anti-inflammatory activities were observed in 7.5% of the compounds, while 7.9% displayed enzyme inhibitory activities. The remaining 18.1% of benzopyran compounds are associated with other bioactivities (Figure 4).
4 Chemical diversity
4.1 Chromones
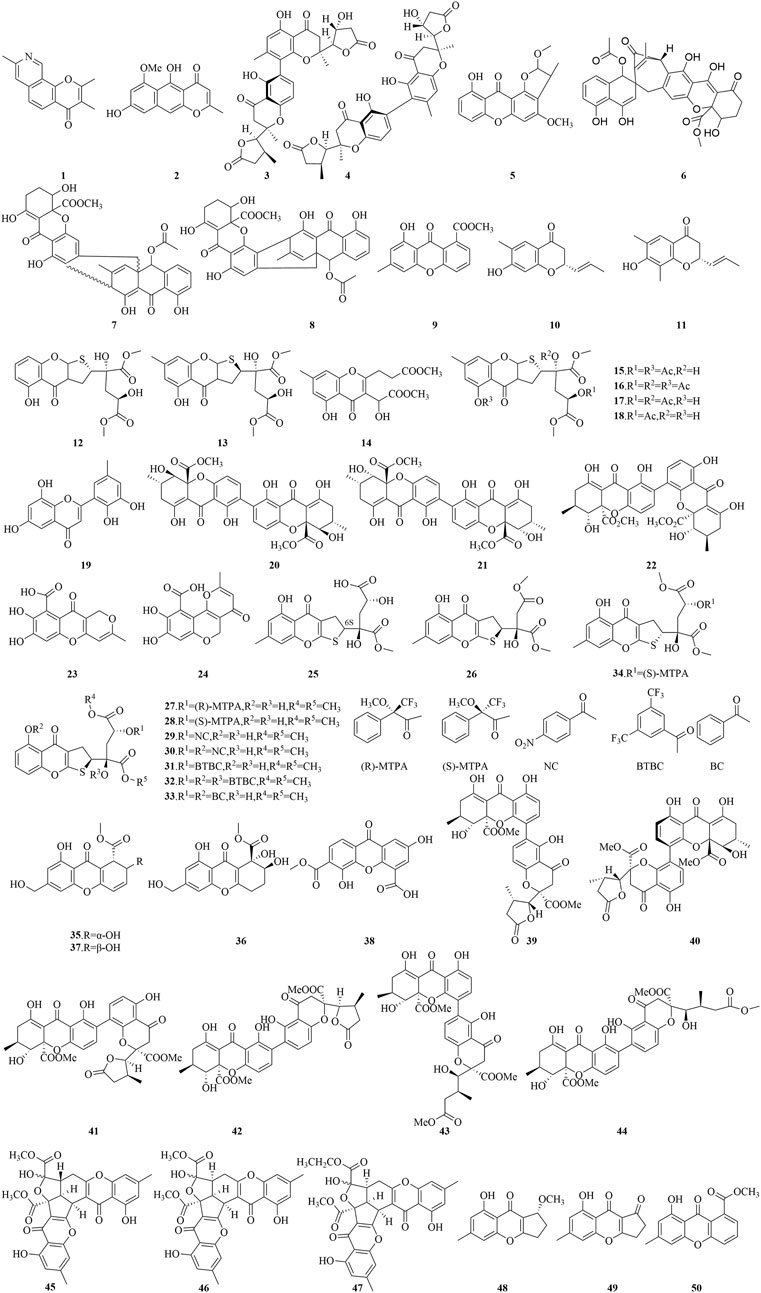
Chromones (4H-1-benzopyran-4-ones) represent a significant class of oxygen-containing heterocycles with a benzo-γ-pyrone skeleton (Duan et al., 2019). Not only are these compounds abundant and structurally complex, but they have also been demonstrated to be a unique skeleton structure in medicinal chemistry, exhibiting a broad spectrum of biological properties, encompassing antibacterial, anticancer, anti-inflammatory, and antioxidant activities. The biological potential of this skeleton structure, coupled with its minimal toxicity to mammals, have spurred the advancement of a variety of chromone-based pharmaceuticals, highlighted by Chromonar, Flavoxate, and Scutellarein (Silva et al., 2018). The structures of chromone compounds derived from marine fungi are depicted in Figures 5, 6.
Aspergillitine (1), a chromone derivative with a pyridine ring, isolated from the sponge-associated fungus Aspergillus versicolor, has demonstrated antibacterial properties against Bacillus subtilis, achieving an inhibition zone diameter of 7 mm at 5 μg and 8 mm at 10 μg (Lin et al., 2003). The chromone TMC-256A1 (2), derived from the culture of Aspergillus niger, displayed significant antiviral activity (Ovenden et al., 2004). Notably, compound 2 efficaciously inhibited the translational activity mediated by the internal ribosome entry site (IRES), with an IC50 value of 44 μM. Furthermore, this compound underwent a selectivity assay, targeting the inhibition of both viral and mammalian Cap-dependent translation initiation pathways, exhibiting an IC50 of 80 μM. Two dimeric chromone derivatives, monodictyochromes A and B (3, 4), were isolated from the marine-derived fungus Monodictys putredinis (Pontius et al., 2008), have shown significant inhibitory effects on the activity of the cytochrome P450 isoenzyme CYP 1A, with IC50 values of 5.3 and 7.5 μM, respectively. While compound 3 showed slightly diminished potency in inhibiting aromatase activity in comparison to compound 4, with IC50 values of 24.4 and 16.5 μM, respectively. Additionally, compounds 3 and 4 have shown notable potency as activators of NNAD(P)H: quinone oxidoreductase (NQO1), achieving CD values of 22.1 and 24.8 μM, which represent the concentrations doubling the enzyme’s specific activity. Collectively, these findings suggest that compounds 3 and 4 are promising candidates for chemoprevention strategies, as they attenuate the activity of cytochrome P450 enzymes implicated in carcinogenesis and robustly induce NQO1 activity, thereby potentially enhancing the body’s detoxification mechanisms against carcinogens.
Asperxanthone (5), extracted from the marine-derived fungus Aspergillus sp. MF-93, sourced from the Quanzhou Bay, has been found to exhibit a moderate inhibitory effect on the tobacco mosaic virus (TMV) proliferation, with a 62.9% inhibition rate observed at a 0.2 mg/mL concentration (Wu et al., 2009). The sponge-derived fungus Tritirachium sp. SpB081112MEf2 has yielded compounds JBIR-97 (6), JBIR-98 (7), and JBIR-99 (8), which have potently inhibited the proliferation of human cervical carcinoma HeLa cells, with IC50 values of 11, 17, and 17 μM, respectively, and against human malignant pleural mesothelioma ACC-MESO-1 cells with IC50 values of 31, 63, and 59 μM, respectively (Ueda et al., 2010). Compound 9, a newly identified mutant janthinone, extracted from the marine fungus Penicillium purpurogenum G59, has shown notable inhibitory activity against the K562 human chronic myelogenous leukemia cell line at a 100 μg/mL concentration, achieving a 34.6% inhibition rate (Chai et al., 2012). Two chromone ketones (10, 11), isolated from Trichoderma sp., exhibited significant proliferation inhibitory activity against the human breast cancer cell line MCF-7, with IC50 values of 7.82 and 9.51 μg/mL, respectively (Lan et al., 2012). Additionally, the novel oxalicumones A-C (12-14), along with four known compounds 15-18, were isolated from marine-derived fungus Penicillium macum SCSGAF 0023 (Sun et al., 2012). All these compounds exhibited cytotoxic effects against various human cancer cell lines such as melanoma A375, lung carcinoma A549, cervical carcinoma HeLa, liver hepatocellular carcinoma HepG2, colonic adenocarcinoma SW-620, as well as the normal liver L-02 cell line. Specifically, compound 12 displayed moderate cytotoxicity against A375 and SW-620, with IC50 values at 11.7 µM and 22.6 µM. Compounds 13 and 16 showed mild cytotoxic effects in these cell lines, with IC50 values ranging from 27.8 to 42.7 µM. In contrast, compound 14 exhibited no cytotoxic effects on any of the cell lines evaluated. Analysis of the structure-activity relationship indicated that incorporating a 2,3-dihydrothienyl group markedly enhances the cytotoxic efficacy of chromone-based compounds. Moreover, the presence of a bulky substituent at the OH-13 position appears to augment cytotoxicity, whereas acetylation at the C-1, C-11, and/or C-13 positions appears to mitigate it, underscoring the importance of structural features in dictating biological activity.
A novel polyketide, identified as 6,8,5′6′-tetrahydroxy-3′-methylflavone (19), along with the analogues secalonic acid D (20), secalonic acid B (21), and penicillixanthone A (22), has been extracted from the fermentation products of the gorgonian-associated fungus Penicillium sp. SCSGAF 0023 (Bao et al., 2013). In antibacterial assays, compounds 19–22 demonstrated discernible inhibitory effects against four bacterial strains tested (B. subtilis, Escherichia coli JVC1228, Micrococcus luteus UST950701-006, Pseudomonas nigritaciens UST010620-005), exhibiting MIC values that varied from 24.4 to 390.5 μg/mL. An in-depth investigation into the antibiofilm potential of compound 20 against Staphylococcus oneidensis MR-1 revealed its efficacy in completely inhibiting biofilm formation at a concentration of 3.125 mg/mL following a 12-h incubation period. Furthermore, in antifouling assays, compound 19 significantly impeded the settlement of Balanus amphitrite larvae, with an EC50 value of 6.71 mg/mL. The marine fungus Penicillium sp. JF-55 has yielded two bioactive secondary metabolites: anhydrofulvic acid (23) and citromycetin (24). Compound 23 demonstrated potent, concentration-dependent inhibition of Protein tyrosine phosphatase 1B (PTP1B), with an IC50 value of 1.90 μM. Compound 24, however, showed no significant inhibitory activity against PTP1B. These findings suggest that the linear tricyclic framework and the strategic positioning of the carbonyl group in compound 23 are pivotal structural features that enhance its binding affinity and interaction with the PTP1B active site, highlighting the importance of molecular architecture in modulating biological activity (Lee et al., 2013).
In addition, the secondary metabolites of the gorgonian-associated fungus Penicillium oxalicum SCSGAF 0023 have yielded four dihydrothieno-chromene derivatives, specifically pecicumones A and B (12-13) along with pecicumones D and E (25-26). Through acylation reactions of compounds 12 and 13, eight derivatives (27-34) were synthesized. Significantly, compounds 12, 26, and 29 demonstrated potent cytotoxicity against a selection of 8 cell lines (H1975, U937, K562, BGC823, MOLT-4, MCF-7, HL60, Huh-7), achieving IC50 values below 10 μM. Compound 13 was found to exert cytotoxic effects on the U937 and MOLT-4 cell lines, achieving IC50 values of 5.0 μM and 2.30 μM, respectively. Meanwhile, compound 25 also displayed cytotoxicity against the BGC823 and MOLT-4 cell lines, yielding IC50 values of 10.10 and 5.74 μM, respectively. Compound 27 demonstrated cytotoxic activity against U937, BGC823, and MOLT-4 cell lines with IC50 values of 9.85, 8.82, and 5.89 μM, respectively. Compound 28 demonstrated potent cytotoxic effects against a spectrum of cell lines, including U937, K562, BGC823, MOLT-4, and HL60, with IC50 values spanning from 1.34 to 9.04 μM. Compounds 30-31 also exhibited significant cytotoxicity against a broader panel of cell lines (U937, K562, BGC823, MOLT-4, MCF-7, and HL60), with IC50 values between 1.04 and 6.94 μM. Furthermore, compounds 33–34 showed cytotoxic activity specifically against MOLT-4 and HL60 cell lines, with IC50 values ranging from 3.99 to 9.14 μM. The structure-activity relationship analysis indicates that the 2,3-dihydrothieno structural motif is a principal determinant of cytotoxicity for this class of compounds. Furthermore, the presence of the 1-OH group and the two O-methyl groups at carbons 16 and 17 significantly enhance their biological potency, while the 13-OH group seems to be non-essential for the cytotoxicity of compound 12. Comparative cytotoxicity studies between compounds 12 and 25, and 28 and 34, have disclosed that the absolute configuration at carbon 6 influences the cytotoxicity of compound 12, underscoring the importance of stereochemistry in modulating the biological activity of these marine-derived natural products (Bao et al., 2014).
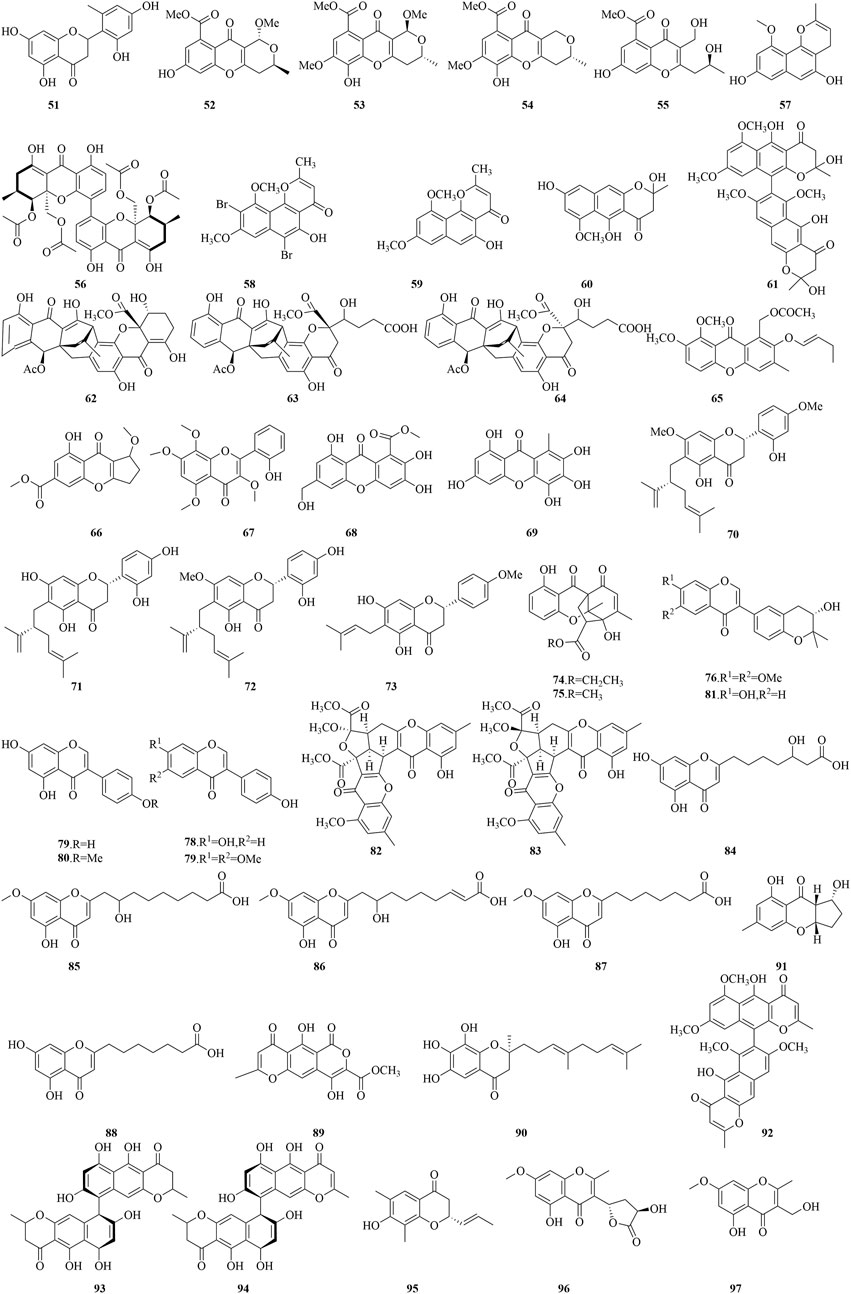
The deep-sea fungus Engyodontium album DFFSCS 021 yielded Engyodontiumone H (35) and two associated polyketide compounds (36-37). Notably, compounds 35 and 37 exhibited significant cytotoxic effects on the U937 cell line, achieving IC50 values below 10 μM. They also exhibited moderate antibacterial properties against E. coli and B. subtilis, with MIC values not exceeding 64.0 μg/mL. Moreover, compound 36 has been identified as a potent antifoulant, significantly inhibiting the settlement of barnacle B. amphitrite larvae at an EC50 of 19.1 μg/mL, while maintaining a favorable safety profile (Yao et al., 2014). Additionally, the mangrove-associated fungus Penicillium chrysogenum HND11-24 has yielded an oxanthrone derivative, penixanacid A (38), which has shown a spectrum of bioactivities against cancer cell lines such as HeLa, BEL-7402, HEK-293, HCT-116, and A549, with IC50 values in the range of 10–30.6 μM (Guo et al., 2015). Six flavone-chromone dimers, versixanthones A-F (39-44) and compound 20, were isolated from the cultured extracts of the mangrove-associated fungus A. versicolor HDN1009. Compounds 39–41 displayed significant selective cytotoxic effects against the HL-60 and K562 cell lines, with IC50 values ranging from 2.6 to 18.2 μM. Furthermore, compounds 20 and 42–44 demonstrated broad cytotoxicity against seven cancer cell lines (HL-60, K562, A549, H1975, 803, HO-8910, HCT-116) with IC50 values between 0.7 and 14.0 μM (Wu et al., 2015). From the diethyl sulfate (DES)-treated mutant strain of P. purpurogenum G59, a group of compounds, epiremisporine B (45), epiremisporine B1 (46), isoconiochaetone C (47), remisporine B (48), coniochaetone A (49), and methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (50), was isolated. At a concentration of 100 μg/mL, they demonstrated substantial anticancer effects against K562, HL-60, HeLa, and BGC-823 cell lines, resulting in inhibition rates (IR%) between 11.4% and 80.0%. Specifically, compounds 45–47 showed IC50 values between 53.1 and 83.1 μg/mL against the K562 and HL-60 cell lines (Xia et al., 2015).
Penimethavone A (51), a novel flavonoid, was isolated from the fungus P. chrysogenum, which is symbiotically associated with the coral Carijoa sp. from the South China Sea. This compound is characterized by a distinctive methyl group on its B-ring and exhibited potent cytotoxicity against HeLa and rhabdomyosarcoma (RD) cell lines, with IC50 values of 8.41 μM and 8.18 μM, respectively (Hou et al., 2016). Phomopsichins A-D (52-55) and the known phomoxanthone A (56) were extracted from the mangrove-associated endophytic fungus Phomopsis sp. 33#. They showed a slight inhibitory effect on AChE and α-glucosidase, with inhibition rates from 2.7% to 38.4% at 250 μM. Moreover, they exhibited a moderate ability to scavenge free radicals, including DPPH and OH radicals, with inhibition rates of 3.5%–40.0% at 1 mM (Huang et al., 2016b). TMC-256C1 (57) isolated from the extract of the marine-derived fungus Aspergillus sp. SF6354, has been shown to confer resistance to hippocampal HT22 cells against kainic acid-induced cytotoxicity and oxidative stress mediated by reactive oxygen species (ROS). Additionally, it exerts inhibitory effects on inflammatory processes triggered by lipopolysaccharide (LPS) in BV2 cells. The neuroprotective and anti-neuroinflammatory efficacies of compound 57 are attributed to the upregulation of heme oxygenase (HO)-1 and nuclear factor-E2-related factor 2 (Nrf2), which translocate to the nucleus in both HT22 and BV2 cell lines. It is further suggested that compound 57 may promote the nuclear accumulation of Nrf2 and the subsequent activation of HO-1 protein expression via the engagement of p38 mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) signaling pathways (Kim et al., 2016).
In an innovativel bioprocess optimization approach, metal bromides were introduced to the fermentation of the marine mudflat fungus A. niger, resulting in the production of a potent free radical scavenger, 6,9-di-bromoflavasperone (58), along with three naphthopyranone monomers, TMC-256A1 (2), flavasperone (59), and fonsecin (60), as well as a naphthopyranone dimer, aurasperone B (61). These compounds demonstrated significant free radical scavenging capabilities against the DPPH radical, with IC50 values ranging from 0.01 to 25 μM (Leutou et al., 2016). This study underscores the importance aromatic hydroxyl group count in determining the free radical scavenging efficacy, highlighting that an increased number of these groups significantly enhances the compounds' antioxidant potential. An anthraquinone-xanthone polyketide compound (8) with an alkaloid-free structure, along with three additional polyketide compounds (62-64), isolated from the fungus E. album strain LF069, exhibited potent inhibitory effects against S. epidermidis and methicillin-resistant Staphylococcus aureus (MRSA), with IC50 values ranging from 0.21 to 6.77 μM and 0.22–3.41 μM, respectively. Moreover, compounds 8 and 62 also exhibited modest cytotoxic effects on the murine fibroblast cell line NIH3T3, with IC50 values of approximately 13 μM (Wu et al., 2016).
Versicones G (65), a flavonoid compound isolated from the mangrove-associated A. versicolor strain HDN11-84, has demonstrated significant cytotoxicity against Hela, HL-60, and NB4 cell lines, with IC50 values between 15.6 and 21.7 μM (Li et al., 2017). Meanwhile, Epiremisporine B (46) and Coniochaetone J (66), from the deep-sea sediment-derived Penicillium SCSIO Ind16F01, have shown weak inhibitory activity against EV71 in vitro, with IC50 values of 19.8 and 81.6 µM, respectively. Additionally, compound 66 has displayed inhibitory activity against the H3N2 virus, with an IC50 value of 24.1 µM, and cytotoxic effects on cancer cell lines K562, MCF-7, and SGC7901, with IC50 values of 16.6, 16.3, and 15.8 µM, respectively (Liu et al., 2017). Derived from the gorgonian coral Anthogorgia ochracea of the South China Sea, Aspergillus candidus has yielded Aspergivones B (67), which has shown a modest inhibitory effect on α-glucosidase, achieving an IC50 value of 244 μg/mL (Ma et al., 2017). Arthone C (68) and compound (69), extracted from the deep-sea fungus Arthrinium sp. UJNMF0008, displayed remarkable antioxidant activity, with IC50 values for DPPH radical scavenging of 16.9 and 22.1 μM, respectively, and for ABTS radical scavenging of 18.7 μM and 18.0 μM, respectively (Bao et al., 2018). From the fermentation of Streptomyces sp. G248, two new flavonoids (70-71) and two existing compounds (72-73) were identified. Compounds 70 and 71 exhibited pronounced inhibitory activities against the bacterial strains tested (Enterococcus faecalis, S. aureus, Bacillus cereus, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans), with IC90 values from 1 to 16 μg/mL. Furthermore, compounds 72 and 73 have also shown considerable anti-tuberculosis activity, with IC90 values of 6.0 μg/mL and 11.1 μg/mL against Mycobacterium tuberculosis H37Rv, respectively. The methoxy group at position C-7 and the hydroxyl group at C-4 are considered crucial for their anti-tuberculosis activity, given the lack of effect from compounds 70 and 71 on M. tuberculosis. Additionally, cytotoxicity assays revealed that compounds 70 and 72 possess inhibitory effects on four tested cancer cell lines (KB, Hep-G2, Lu-1, and MCF-7), with IC50 values ranging from 2.0 to 14.6 μg/mL (Cao et al., 2019).
Two polycyclic chromone compounds, penixanthones C-D (74-75), isolated from the fungus Penicillium sp. SCSIO 041218, sourced from mangrove sediments, showed considerable inhibitory effects on the proliferation of K562, MCF-7, and Huh-7 cells, with IC50 values ranging from 55.2 to 67.5 μM (Huang et al., 2019). The marine-derived fungus Aspergillus terreus C23-3, cultivated on a soybean substrate, has yielded six isoflavone derivatives (76-81) and have exhibited potent anti-inflammatory activity in RAW 264.7 macrophages stimulated by LPS, achieving a 36% inhibition rate at 32 μM, without exhibiting cytotoxic effects. Furthermore, compounds 84–81 showed broad-spectrum antimicrobial efficacies against both Gram-positive and Gram-negative bacteria, as well as antifungal activity, with inhibition zones of 10.0–13.8 mm at a disk concentration of 10 μg. Compound 77 displayed moderate inhibition of AChE, with an IC50 of 42.5 μM. Additionally, compounds 77, 80, and 81 demonstrated potent lethal effects on brine shrimp larvae, with LC50 values ranging from 11.6 to 68.2 μM, substantially lower than that of the positive control Hg(NO3)2, which had an LC50 of 77.0 μM, suggesting their utility in the development of novel pesticides (Yang et al., 2020a).
Isolated from the marine-derived fungus Penicillium citrinum, two novel chromone derivatives, epiremisporine D (82) and E (83), along with the known compound epiremisporine B (46), have been found to markedly decrease the production of superoxide anions in human neutrophils stimulated by formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), with IC50 values of 6.39, 8.28, and 3.62 µM. Moreover, compounds 46 and 83 have displayed cytotoxic activity against A549 cells, with IC50 values of 32.29 µM and 43.82 µM. Compounds 82 and 83 have also been identified to induce apoptosis in A549 cells, engaging the mitochondrial pathway and a caspase 3-dependent process (Chu et al., 2021). Three novel chromone derivatives, penithochromones R-T (84-86), along with the known compounds 87 and 88, were isolated from the fungus Penicillium thomii YPGA3, sourced from the sediments of the Yap Trench. These compounds showed notable inhibitory activity against α-glucosidase, with IC50 values ranging from 268 to 1,017 μM, while higher than that of the positive control drug acarbose at 1.3 μM (Han et al., 2021). Furthermore, the marine fungus Taeniolella sp. BCC31839 has yielded compound 89, which has exhibited promising antimalarial properties with an IC50 of 9.75 μg/mL. Additionally, this compound has displayed antibacterial properties against B. cereus, with a MIC of 12.5 μg/mL, and has exhibited moderate cytotoxic effects on NCI-H187 cells, resulting in an IC50 of 23.66 μg/mL (Intaraudom et al., 2021). Pestalotiochromones A (90) was isolated from the marine-derived algal fungus Pesacetalotiopsis neglecta SCSIO 41403. Surface plasmon resonance (SPR) analysis revealed that compound 90 exhibited a high binding affinity for Liver X receptors (LXRs), with a dissociation equilibrium constant (KD) of 6.2 μM, suggesting that compound 90 may represent a novel LXR agonist (Liang et al., 2021). Aspergilluone A (91), obtained from the marine fungus Aspergillus sp. LS57, has demonstrated considerable in vitro activity against M. tuberculosis, reaching a MIC value of 32 μg/mL. It has also manifested moderate antibacterial effects against S. aureus, with a MIC of 64 μg/mL. However, its activity against B. subtilis and E. coli was less pronounced, both showing MIC values of 128 μg/mL (Liu et al., 2021). Aurasperone A (92), identified from the marine A. niger, has proven to be a potent inhibitor of SARS-CoV-2, with an IC50 value of 12.25 μM, and has also shown remarkably low cytotoxicity to Vero E6 cells, as indicated by a CC50 value of 32.36 μM and a selectivity index (SI) of 2,641.5 (ElNaggar et al., 2022).
A chemical investigation was conducted on the co-culture of the marine fungus Cosmospora sp. with the plant pathogen Magnaporthe oryza, resulting in the isolation of two chromones, Cephalochromin (93) and Ustilaginoidin G (94). Compound 93 displayed potent inhibitory effects against Phytophthora infestans, Xanthomonas campestris, and Ralstonia solanacearum, with IC50 values of 2.3, 27.6, and 12.1 μg/mL, respectively. While compound 94 has proven to have a moderate inhibitory effect against P. infestans and X. campestris, with IC50 values of 7.2 μg/mL and 21.7 μg/mL, respectively (Oppong-Danquah et al., 2022). Moreover, compound 95, sourced from the marine fungus P. citrinum VM6, has displayed a more pronounced antibacterial effect on Gram-positive bacteria and yeasts compared to Gram-negative bacteria, with MIC values of 128, 64, 128, 256, and 16 μg/mL against E. faecalis, S. aureus, B. cereus, S. enterica, and C. albicans, respectively (Anh et al., 2023). Extracted from the mangrove-inhabiting fungus Trichoderma lentiforme ML-P8-2, compounds 96 and 97 have exhibited a limited cytotoxic impact on A549 cells, with an IC50 value of 47.2 μM. Compound 97 has also demonstrated a moderate inhibitory effect against C. albicans, reaching a MIC value of 25 µM. In addition, both compounds 96 and 97 have shown some inhibitory activity against AChE, with IC50 values of 38.6 µM and 20.6 µM, respectively (Yin et al., 2023).
4.2 Isocoumarin
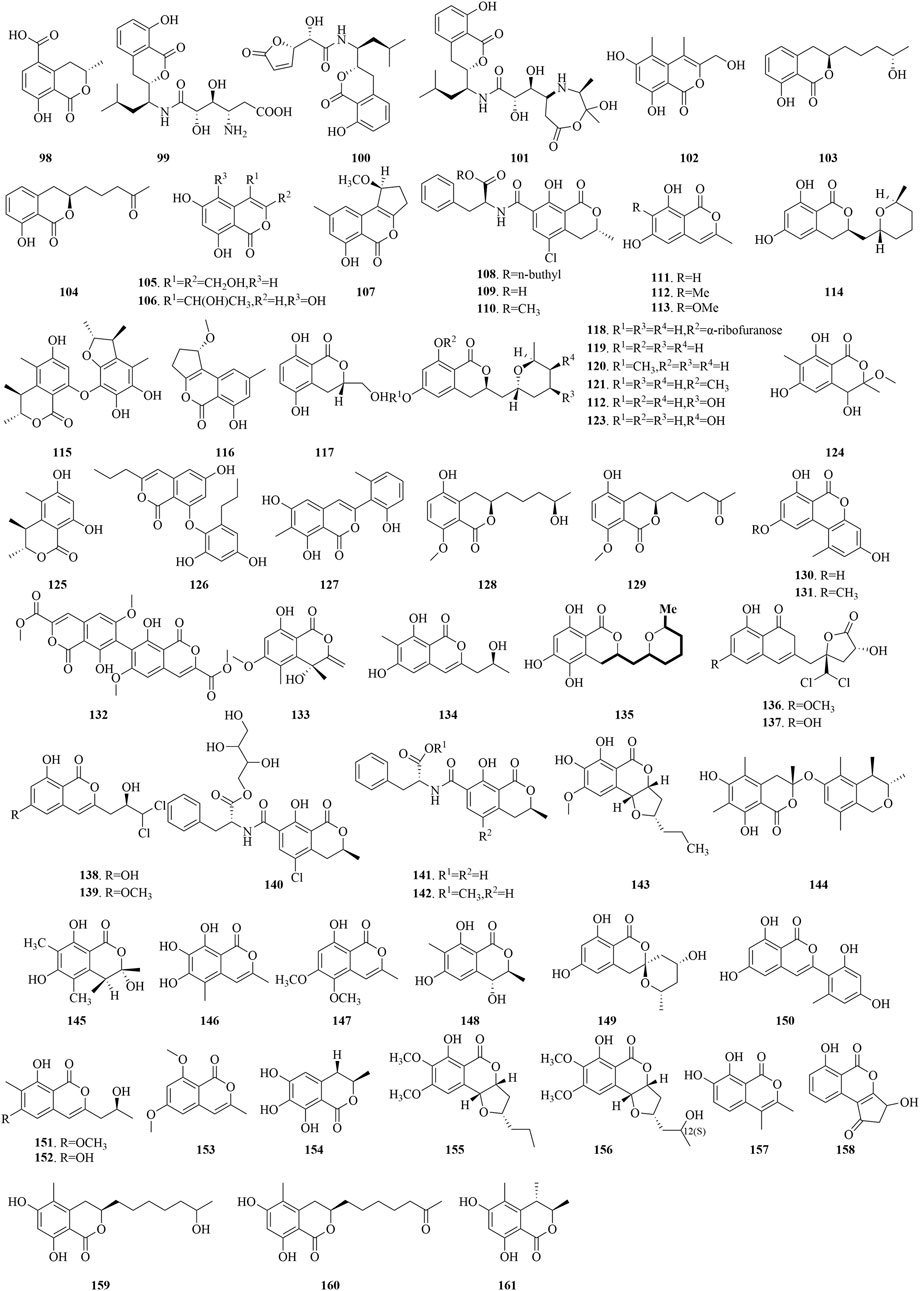
The isocoumarin skeleton, chemically defined as 3,4-dihydro-2H-chromen-2-one, represents a pivotal class of O-heterocyclic compounds (Ji Ram et al., 2014). It serves not only as the backbone for a multitude of natural products but also demonstrates immense promise in the realm of drug discovery. The widespread occurrence and diversity of these compounds in nature, coupled with the array of bioactivities exhibited by their derivatives, such as antibacterial, anti-inflammatory, anticancer, and protease inhibitory properties, have propelled them to the forefront of pharmaceutical research. Isocoumarins derived from marine fungi, as illustrated in Figure 7, are particularly noteworthy for their unique chemical structures and potential therapeutic applications.
The marine fungus Halorosellinia oceanica BCC 5149 has yielded 5-carboxymellein (98), a secondary metabolite that has demonstrated cytotoxic effects against the KB and BC-1 cell lines, achieving IC50 values of 3 μg/mL. Moreover, this compound has also displayed antimalarial properties, with an IC50 of 4 μg/mL (Chinworrungsee et al., 2001). Three isocoumarins, AI-77-B (99), AI-77-F (100), and Sg17-1-4 (101), were isolated from the marine fungus Alternaria tenuis Sg17-1. Notably, compound 99 has exhibited potent suppression of cell proliferation in A375-S2 and Hela cells, with IC50 values of 0.1 mM and 0.02 mM, respectively. Compound 101 follows with moderate inhibitory effects, having IC50 values of 0.3 mM and 0.05 mM for the aforementioned cell lines. Conversely, compound 100 displays relatively low activity against Hela cells, indicated by a higher IC50 value of 0.4 mM (Huang et al., 2006). Furthermore, from the marine fungus Arthrinium sacchari, decarboxyhydroxycitrinone (102) has been isolated and has shown significant inhibition of proliferation in human umbilical vein endothelial cells (HUVECs) and human umbilical artery endothelial cells (HUAECs), with IC50 values of 7.6 and 17.4 μM, respectively (Tsukada et al., 2011). Penicimarins F (105) and three isocoumarin derivatives (103, 104, 106), sourced from the sponge-derived Penicillium sp. MWZ14-4 of the South China Sea. Compounds 103 and 104 have shown certain antibacterial effects against Staphylococcus albus, with MIC values of 12.5 μM. Compound 105 has demonstrated certain antibacterial activity against S. aureus, with a MIC value of 12.5 μM. Meanwhile, compound 106 has shown moderate activity against B. subtilis and Vibrio parahemolyticus, with MIC values of 6.25 μM (Qi et al., 2013). Derived from the marine fungus Penicillium sp. ML226, compound 107 has demonstrated low cytotoxicity against HeLa and HepG-2 cell lines at a concentration of 10 μg/mL, resulting in inhibition rates of 4% and 16.1%, respectively. Additionally, ochratoxin A n-butyl ester (108), ochratoxin A (109), and ochratoxin A methyl ester (110), extracted from the fermentation products of the marine fungus Aspergillus sp.SCSGAF0093, have exhibited considerable toxicity to brine shrimp, with LC50 values of 4.14, 13.74, and 2.59 μM, respectively (Xu et al., 2013).
The sponge-associated fungus Aspergillus similanensis KUFA 0013 has yielded a set of compounds, including 6,8-dihydroxy-3-methylisocoumarin (111), 6,8-dihydroxy-3,7- dimethylisocoumarin (112), and reticulol (113), which have shown moderate antibacterial activity against ampicillin-resistant bacteria, with inhibition zones of 2.5–5 mm at a concentration of 15 μg/disc (Prompanya et al., 2014). Asperentin (114), obtained from the marine fungal Aspergillus sp. F00785, has exhibited inhibitory effects against Colletotrichum gleosporioides Penz, C. gleosporioides (Penz.) Sacc, and Botrytis cinerea Pers at a 5 mg/mL concentration, with inhibition diameters of 19.7, 13.3, and 1.67 mm, respectively (Tang et al., 2014). Penicitol B (115) and Penicitol C (116), identified from the mangrove-associated P. chrysogenum HND 11-24, have demonstrated diverse cytotoxic effects against a panel of cell lines including HeLa, BEL-7402, HEK-293, HCT-116, and A-549, with IC50 ranges of 3.4–9.6 μM and 10.8–40.5 μM, respectively (Guo et al., 2015). Compound 117, isolated from the endophytic fungus Botryosphaeria sp. KcF6, derived from the mangrove plant Kandelia candel, has displayed significant inhibitory activity against cyclooxygenase-2 (COX-2), with an IC50 value of 6.51 μM (Ju et al., 2015). From the marine-derived fungi Aspergillus sp. SF-5974 and Aspergillus sp. SF-5976, a series of compounds (118-123) has been isolated, exhibiting potent inhibitory effects on the LPS-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in BV2 microglia and presented IC50 ranges of 20–65 μM and 21–61 μM, respectively. Of particular interest, compound 118 was observed to influence inflammatory pathways, reducing the phosphorylation of IκB-α, impeding NF-κB nuclear translocation, and lowering p38 MAPK activation, suggesting its anti-inflammatory potential (Kim et al., 2015). Compound 124, sourced from the mangrove endophytic Aspergillus sp. 16-5B and grown in Czapek medium, has been found to inhibit α-glucosidase activity, with an IC50 value of 90.4 μM (Liu et al., 2015).
Decarboxydihydrocitrinone (125), extracted from the co-culture of the marine fungi P. citrinum and Beauveria felina, has exhibited modest inhibitory effects against Vibrio alginolyticus, with a MIC value of 8.0 μg/mL (Meng et al., 2015). Spiromastols I (126), discovered from the deep-sea fungus Spiromastix sp. MCCC3A00308, has demonstrated moderate antibacterial activity against a range of bacterial strains, including Xanthomonas vesicatoria, Pseudomonas lachrymans, Agrobacterium tumefaciens, R. solanacearum, Bacillus thuringiensis, S. aureus, and B. subtilis, with MIC values falling between 8 and 16 μg/mL (Niu et al., 2015). Pleosporalone A (127), identified from the Bohai Sea sediment-derived Pleosporales sp. CF09-1, has displayed significant inhibitory effects against three phytopathogenic fungi, B. cinerea, Rhizopus sticarum, and Phytophthora capsici, with MIC values of 0.39, 0.78, and 0.78 μM, respectively (Cao et al., 2016). From the mangrove-associated P. citrinum of the South China Sea, compounds 128 and 129 have been extracted and have shown significant antibacterial activity against a panel of pathogenic bacteria (Staphylococcus epidermidis, S. aureus, E. coli, B. cereus, and V. alginolyticus) with MIC values from 10 to 20 μM (Huang et al., 2016a). Alternariol (130) and alternariol-9-methylether (131), obtained from the fungus Aspergillus sp. and Botryotinia fuckeliana of the Wadden Sea in Germany, have been identified as inhibitors of the glycogen synthase kinase-3β (GSK-3β) enzyme, with IC50 values of 0.13 μM and 0.20 μM, respectively (Wiese et al., 2016). A novel dimeric isocoumarin, bipenicilisorin (132), extracted from the deep-sea derived fungus P. chrysogenum SCSIO 41001, has exhibited pronounced cytotoxic effects on the K562, A549, and Huh-7 cell lines, with IC50 values of 6.78, 6.94 μM, and 2.59 μM, respectively (Chen et al., 2017). Moreover, The deep-sea sediment-associated fungus Leptosphaeria sp. SCSIO 41005 has yielded compound 133, which has proven effective in inhibiting the growth of Cochliobolus miyabeanus, reaching an IC50 value of 0.5 μM. It has also shown a limited inhibitory impact on the formation of C. albicans biofilms, with a MIC value of 101 μM (Luo et al., 2017).
Cultivation of the mangrove-derived Eurotium chevalieri KUFA 0006 has led to the discovery of compound 134, which has displayed a remarkable efficacy in reducing E. coli biofilms. In addition to its standalone effects, when co-administered with cefotaxime (CTX), compound 134 exhibits a subtle synergistic action. Furthermore, the compound has been observed to induce a weak or moderate enlargement of the partial inhibition zone surrounding vancomycin (VAN) in vancomycin-resistant E. faecalis (VRE) B3/101, enhancing the inhibitory effect compared to the application of VAN alone (May Zin et al., 2017). Asperentin B (135), isolated from the fungus Aspergillus sydowii, derived from the deep-sea sediment of the Mediterranean, has exhibited a robust inhibition of protein tyrosine phosphatase 1B (PTP1B) activity, with an IC50 value of 2.05 μM, outperforming the positive control suramin by sixfold (Wiese et al., 2017). Isolated from the Ascomycota sp. CYSK-4, an endophyte of mangroves, dichloro-diaportintone (136), desmethyldichlorodiaportintone (137), and two analogues (138-139) have demonstrated the ability to inhibit the production of NO in LPS-induced RAW 264.7 murine macrophages, displaying IC50 values from 15.8 to 67.2 μM. Additionally, compounds 138 and 139 have shown significant antibacterial potency against several bacterial strains, including S. aureus, B. subtilis, E. coli, Klebsiella pneumoniae, and Acinetobacter calcoaceticus, with MIC values ranging from 25 to 50 μg/mL. Compound 136 showed MIC values of 50 μg/mL against S. aureus, E. coli, and K. pneumoniae. Notably, a comparative analysis revealed that compounds 137 and 138, which bear a hydroxyl group at the C-6 position, manifest stronger anti-inflammatory effects than compounds 136 and 139, which contain a methoxy group at the same position (Chen et al., 2018). This observation underscores the influential role of the C-6 substituent on the anti-inflammatory properties of these compounds.
Ochratoxin A1 (140) and its two analogues (141-142), obtained from the sponge-associated Aspergillus ochraceopetaliformis, have exhibited considerable inhibitory effects on LPS-induced interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) expression in THP-1 cells at 10 μM, achieving inhibition rates of 74.4%–91.6% for IL-6 and 67.7%–72.9% for TNF-α. Importantly, these compounds were confirmed to be non-cytotoxic to THP-1 cells (Liu et al., 2018). Moreover, compound 143, from the sponge-derived Setosphaeria sp. SCSIO 41009, has shown robust DPPH radical scavenging activity, with an IC50 value of 38 μM (Pang et al., 2018b). Penicitol D (144) and an associated compound 145, isolated from the deep-sea fungus P. citrinum NLG-S01-P1, have demonstrated potent activity. Compound 144 has manifested significant cytotoxicity against HeLa cells, with an IC50 value of 4.1 μM. Moreover, Compound 144 has shown potent activity against MRSA strains with an MIC range of 7–8 μg/mL, while compound 145 has exhibited strong antimicrobial effects against Vibrio vulnificus and Vibrio campbellii, with MIC values in the range of 15–17 μg/mL (Wang et al., 2019). Additionally, compound 145 has shown moderate inhibitory activity against both the standard strain G27 and the clinically relevant drug-resistant strain HP159 of Helicobacter pylori, with MIC values of 16 μg/mL (Lai et al., 2023). The marine mangrove endophytic fungus Botryosphaeria ramosa L29 has yielded Botryospyrones A-C (146-148), which have been assessed for their in vitro antifungal capabilities against a selection of phytopathogenic fungi. Compound 146 has shown promising antimicrobial potency against Fusarium oxysporum with an MIC of 112.6 μM and moderate efficacy against Penicillium italicum with an MIC of 450.4 μM. Compound 147 has exhibited MIC values of 105.8 μM against F. oxysporum, and 211.7 μM against both P. italicum and Fusarium graminearum. Compound 148 has demonstrated moderate activity against F. oxysporum and F. graminearum, with MIC values of 223 μM for both (Wu et al., 2019). Demethylcitreoviranol (149), isolated from Penicillium sp. KMM 4672, has shown interesting bioactivity profiles. Interestingly, compound 149 exhibited neutrality towards the viability of Neuro-2a cells when exposed to 6-hydroxydopamine (6-OHDA), it exerted a pronounced protective effect within cellular models of Parkinson’s disease induced by paraquat (PQ) and rotenone. Specifically, in PQ-induced scenarios that reduced cell viability by 44%, compound 149 augmented the survival of Neuro-2a cells, elevating viability by 34.3%. Similarly, in the presence of rotenone, which caused a 48% decline in cell viability, compound 149 significantly improved cell survival, increasing viability by 65.2%. Expanding the investigation to the influence of the compound on oxidative stress, it was observed that 6-OHDA, PQ, and rotenone escalated intracellular ROS levels by 66%, 48%, and 79%, respectively. Notably, compound 149 effectively mitigated the ROS increase, observed specifically in the PQ- and rotenone-induced PD models (Girich et al., 2020).
Trichophenol A (150), which originated from the endophytic fungus Trichoderma citrinoviride A-WH-20-3 within the marine red algae Laurencia okamurai, has proven to be a potent inhibitor against marine phytoplankton species such as Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, and Prorocentrum donghaiense, with IC50 values of 4.4, 9.1, and 5.9 μg/mL, respectively. Additionally, trichophenol A displayed broad-spectrum inhibitory effects against various marine bacteria, such as Vibrio parahaemolyticus, Vibrio anguillarum, Vibrio harveyi, Vibrio splendidus, and Photobacterium citrea, with MIC values ranging from 7.0 to 21 μg/mL (Liu et al., 2020). Monarubin B (151) and two isocoumarins (152-153), extracted from the marine bivalve-associated fungus Monascus ruber BB5. Compound 151 manifested robust cytotoxic effects against liver cancer cell lines HepG2 and QGY7701, with IC50 values of 1.72 and 0.71 μM, respectively. Compound 152 has shown moderate cytotoxicity against HepG2, QGY7701, and SUNE1 cancer cell lines, with IC50 values of 9.60, 7.12, and 28.12 μM. Meanwhile, compound 153 has exhibited subdued cytotoxic potential against the aforementioned cancer cell lines, with IC50 values recorded at 46.10, 31.62, and 39.38 μM for HepG2, QGY7701, and SUNE1, respectively (Ran et al., 2020).
The marine fungus A. terreus RA2905 has yielded dihydroisocoumarin (154), a compound that has exhibited a weak scavenging effect on DPPH radicals, with an IC50 value of 147 μM (Wu et al., 2020). Two isocoumarins (155-156), isolated from the marine fungus Exserohilum sp. CHNSCLM-0008. Compound 155 exhibited significant antimalarial potency, with an IC50 value of 1.13 μM, whereas compound 156 showed intermediate antimalarial efficacy at an IC50 value of 11.7 μM (Coronado et al., 2021). 7-hydroxyoospolactone (157) and parapholactone (158), two isocoumarin class secondary metabolites identified from the marine fungus Paraphoma sp. CUGBMF 180003, have both demonstrated inhibitory activity against S. aureus, with MIC values of 12.5 μg/mL (Xu et al., 2021). The co-cultivation of the marine fungus Cosmospora sp. was conducted with the plant pathogen Magnaporthe oryzae facilitated the extraction and isolation of two bioactive isocoumarins (159-160) with moderate inhibitory activity against P. infestans, M. oryzae, and X. campestris, with IC50 values ranging between 12.8 and 71.5 μg/mL (Oppong-Danquah et al., 2022). Derived from the marine fungus P. citrinum VM6, compound 161 has shown inhibitory activity against several Gram-positive bacteria, including E. faecalis, S. aureus, and B. cereus, in addition to the fungus C. albicans, with observed MIC values of 32–256 μg/mL (Anh et al., 2023).
4.3 Chroman
Chroman, characterized by their benzo pyran core, constitute a class of compounds that are ubiquitous in natural products and medicinal molecules endowed with distinctive physiological properties (Ji Ram et al., 2014). The structural diversity of Chroman compounds sourced from marine fungi is elegantly presented in Figure 8, showcasing their potential as bioactive constituents with significant implications for drug discovery and development.
Chaetopyranin (162), from the endophytic fungus Chaetomium globosum of marine red algae Polysiphonia urceolata, has exhibited a moderate level of DPPH radical scavenging activity, with an IC50 value of 35 μg/mL. Moreover, it has demonstrated cytotoxicity against tumor cell lines HMEC, SMMC-7721, and A549, yielding IC50 values of 15.4, 28.5, and 39.1 μg/mL (Wang et al., 2006). Compound 163 discovered from the marine fungus Aspergillus sp. (MF-93) native to Quanzhou Bay, showed notable inhibitory efficacy against TMV at 0.2 mg/mL, achieving an inhibition rate of 35.5% (Wu et al., 2009). Penicitrinol C (164) and penicitrinol E (165), isolated from P. citrinum, have exhibited subtle cytotoxicity against HL-60 cells, with IC50 values of 52.8 and 41.2 μmol/L, respectively (Chen et al., 2011). Penicitrinol J (166) and penicitrinol K (167), isolated from Penicillium sp. ML226, have shown inhibition rates of 25.1% and 9.2% against the HepG-2 cell line at 10 μg/mL, respectively. Furthermore, compounds 166 and 167 have only weakly influenced S. aureus, yielding inhibition zone diameters of 10 mm and 9 mm at 20 μg/6 mm paper disk, respectively (Wang et al., 2013). In addition, compound 166 displayed moderate antimicrobial activity against B. subtilis JH642, Bacillus megaterium DSM32, and Mycobacterium smegmatis ATCC607, with corresponding MIC values of 16, 16, and 32 μg/mL. It also showed weaker inhibitory effects against B. subtilis DSM10 and S. aureus ATCC25923, with MIC values of 64 μg/mL for both strains (Sabdaningsih et al., 2020). The marine fungus P. chrysogenum HND11-24, sourced from mangroves, has produced compounds 165, 168, and 169. Compound 168, in particular, has exhibited potent inhibitory action against HeLa, BEL-7402, HEK-293, HCT-116, and A-549 cell lines, with IC50 values in the range of 4.6–10.5 μM. Furthermore, the presence of the additional cyclohexane moiety in compound 168 was suggested to enhance its activity, as compounds 165 and 169 showed comparatively weaker inhibitory activities against HeLa and HEK-293 cell lines, with IC50 values in the range of 45.0 to 32.6 μM and 35.5–42.5 μM, respectively (Guo et al., 2015).
Two unique tetracyclic compounds, 170 and 171, were isolated from the co-culture of marine fungi P. citrinum and B. felina. Compound 170 exhibited inhibitory activity against E. coli and S. aureus, with MIC values of 8.0 μg/mL for both. Compound 171 displayed even lower MICs, 2.0 μg/mL for E. coli, and 4.0 μg/mL for S. aureus, suggesting that the OMe group at the C-3′ position may enhance the antimicrobial potency. Additionally, compounds 170 and 171 also showed weak activity against the aquatic pathogen V. alginolyticus, with a MIC value of 16.0 μg/mL for both (Meng et al., 2015). The marine fungus P. citrinum has yielded Penicillin L (172), which has demonstrated a moderate cytotoxic impact on the HL-60 cell line, with an IC50 value of 22.7 μg/mL. Meanwhile, compound 173, extracted from the fungus Eutypella sp. found in the South China Sea gorgonian, has shown antimicrobial activity against B. cereus and S. aureus, with MICs of 6.25 and 12.5 μM (Liao et al., 2017). 3,7-dimethyl-1,8-hydroxy-6-methoxyisochroman (174), extracted from the marine fungus Penicillium sp. SF-6013, has demonstrated inhibitory effects on the LPS-induced production of prostaglandin E2 (PGE2), nitric oxide (NO), COX-2, and inducible nitric oxide synthase (iNOS) in RAW264.7 and BV2 cells, and also downregulates the mRNA expression of pro-inflammatory cytokines including IL-1β and IL-6 (Ko et al., 2019). Additionally, Trichobisabolins J-L (175-177), from the endophytic fungus Trichoderma asperellum A-YMD-9-2 in marine red algae Gracilaria verrucosa, have shown high inhibitory activity against phytoplankton species such as C. marina, K. veneficum, and P. donghaiense, with IC50 values from 0.93 to 9.2 μg/mL. The structure-activity relationship analysis implies that the phenyl group in their structure may play a role in their inhibitory activity. Additionally, compounds 184–186 showed weak antimicrobial activity against marine pathogens V. anguillarum, V. harveyi, V. parahaemolyticus, V. splendidus, and Pseudoalteromonas citrea, with inhibition zone diameters of 6.3–8.1 mm at a 40 μg paper disc for all five pathogens (Song et al., 2019).
From the deep-sea derived P. citrinum NLG-S01-P1, 1-epi-citrinin H1 (178) and its three related biogenetic compounds (179-181) have been obtained. Compound 178 has exhibited inhibitory effects against MRSA strains, similar to the control chloramphenicol, with a MIC range of 7–8 μg/mL. Meanwhile, Compounds 179–181 have demonstrated a weaker antimicrobial activity against V. vulnificus, with MIC values ranging from 32 to 50 μg/mL. Additionally, compounds 179-189 exhibited significant inhibitory activity against the A549 and HeLa cell lines, with IC50 values ranging from 17.7 to 46.3 μM (Wang et al., 2019). Cladosporins A-C (182-184) were isolated from the deep-sea derived fungus Cladosporium sp. SCSIO z015, which displayed LC50 values of 72.0, 81.7, and 49.9 μM against brine shrimp, respectively (Amin et al., 2020). Penitol A (185) and penicitols I (186), featuring a tricyclic spiro framework, have been extracted from the coral-associated fungus P. citrinum (Cheng et al., 2021). Compound 185 manifested cytotoxic effects against the K562 cell line, achieving an IC50 value of 8.8 μM. Moreover, compounds 185 and 186 displayed weak antimicrobial activity against S. aureus, B. subtilis, and vancomycin-resistant enterococci (VRE), with MIC values ranging from 16 to 32 μg/mL and 32–64 μg/mL, respectively.
The soft coral-derived Penicillium glabrum glmu003 has yielded australide V (187), a compound that has exhibited a limited inhibitory activity against pancreatic lipase (PL), as indicated by an IC50 value of 23.9 μg/mL (Zhang et al., 2021). Compound (188) was extracted from the sea star-derived fungus Penicillium sp. GGF 16-1-2. The compound’s efficacy against Colletotrichum gloeosporioides was assessed using a mycelial growth rate assay, yielding an LD50 of 4.34 μg/mL, which is notably lower than that of the positive control Carbendazim, with an LD50 of 49.58 μg/mL (Fan et al., 2022). From the co-culture of the marine fungus Cosmospora sp. and the plant pathogen M. oryzae, pseudoanguillosporins A (189) have been isolated and have demonstrated potent antimicrobial activity against Pseudomonas syringae, X. campestris, P. infestans, and M. oryzae, with IC50 values of 0.8–23.4 μg/mL (Oppong-Danquah et al., 2022). Dihydrocitrinin (190) was isolated from P. citrinum VM6, a fungus derived from marine sediments, which had certain antimicrobial activity and was effective against E. faecalis, S. aureus, B. cereus and, with MIC values ranging from 8 to 16 μg/mL (Anh et al., 2023). Neotricitrinols B (191), featuring a unique octacyclic framework, isolated from the fungus P. citrinum W23, and has been assessed for its anti-osteoporotic activity, It has demonstrated the ability to promote osteogenic mineralization in primary bone mesenchymal stem cells (BMSCs) but also to suppress adipogenic differentiation in a dose-dependent manner, indicating its robust anti-osteoporotic potential (He et al., 2023). Dihydrocitrinin (190), (3R,4S)-6,8-dihydroxy-3,4,5-trimethylisochromane- 7-carboxylatemethyl (192), and (3R,4S)-dihydrocitrinin diacetate (193) were isolated from the marine fungus Penicillium sp. TW 131-64. Compound 192 showed moderate inhibitory effects against the standard strain G27 and the clinically drug-resistant strain HP159 of H. pylori, with MIC values ranging from 8 to 16 μg/mL. Initial structure-activity relationship analysis indicates that the C-7 carboxylate esterification in compound 192 markedly improves its activity against H. pylori, conferring greater potency compared to compounds 190 and 193 (Lai et al., 2023).
4.4 Citrinin
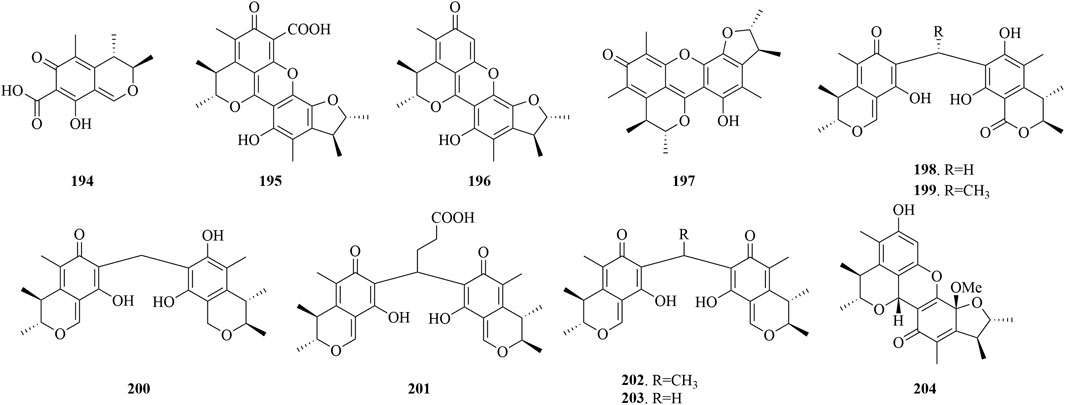
Citrinin, recognized as a prevalent mycotoxin in the food supply, is a fungal-derived secondary metabolite with notable bioactivity (Frank, 1992). Its spectrum of biological effects is impressive, encompassing antifungal and antibacterial actions, alongside potential anticancer and neuroprotective capabilities when assessed in vitro. The structural diversity of citrinin analogues sourced from marine fungi is elegantly showcased in Figure 9, highlighting their potential as leads in the development of novel therapeutic agents.
Citrinin (194), extracted from the marine fungus P. purpurogenum G59, has demonstrated a notable inhibitory impact on K562 cells, achieving an IC50 value of 52.6 μg/mL (Chai et al., 2012). Meanwhile, Penicitrinone E (195), isolated from the marine Penicillium sp. ML226, has exhibited selective activity against HepG-2 cells at 10 μg/mL, displaying an inhibition rate of 6.3% (Wang et al., 2013). Moreover, Penicitrinone A (196), from the marine-derived P. citrinum, has manifested weak cytotoxic effects on the A549 and A375 cell lines, with IC50 values of 49.15 μM (Chu et al., 2021) and 65.4 μg/mL (Ni et al., 2015). Additionally, compound 196 has demonstrated antimicrobial activity, with an MIC of 32 μg/mL against M. smegmatis ATCC 607 (Sabdaningsih et al., 2020). Moreover, compound 196 effectively inhibits the production of superoxide anions in human neutrophils stimulated by fMLP, with an IC50 value of 2.67 μM (Chu et al., 2021). A citrinin dimer, penicitrinone F (197), extracted from the deep-sea fungus P. chrysogenum SCSIO 41001, has demonstrated inhibitory effects on enterovirus 71 (EV71) cells, with an IC50 of 14.50 μM (Chen et al., 2017).
The marine fungus Penicillium sp. GGF 16-1-2, found in association with sea stars, has yielded four novel carbon-bridged citrinin dimers (198-201) and two known related compounds (202-203), have been found to possess robust antibacterial activity against C. gloeosporioides, with LD50 values in the range of 0.61–16.14 μg/mL. Notably, compound 203 has shown the most potent antifungal activity, with an LC50 value of 0.61 μg/mL. The structure-activity relationship analysis indicated that citrinin monomers and the methylene bridge are essential for their antifungal activity, and modifications such as alkyl group introduction or an increase in the alkyl bridge length can reduce their bioactivity. Moreover, the oxidation or reduction of citrinin results in diminished antibacterial activity. Furthermore, compound 198 has demonstrated potent cytotoxic effects against human pancreatic cancer cells BXPC-3 and PANC-1, achieving IC50 values of 12.25 and 24.33 μM, respectively, and may induce apoptosis in BXPC-3 via the modulation of caspase-3 activation (Fan et al., 2022). Penicitriol A (196) and penicitriol B (204) were isolated from P. citrinum XIA-16. At a concentration of 10 μM, penicitriol A (196) in human lung cancer cells H1299 is capable of inhibiting copper toxicity without disrupting DLAT oligomerization, maintaining a cell viability of 68.2%. While compound 204 exerts an inhibitory effect on RSL3-induced ferroptosis in A375 melanoma cells, as evidenced by its reduction of lipid peroxidation and HO-1 levels, at an EC50 concentration of 2.0 μM (Yu et al., 2023).
4.5 Other compounds
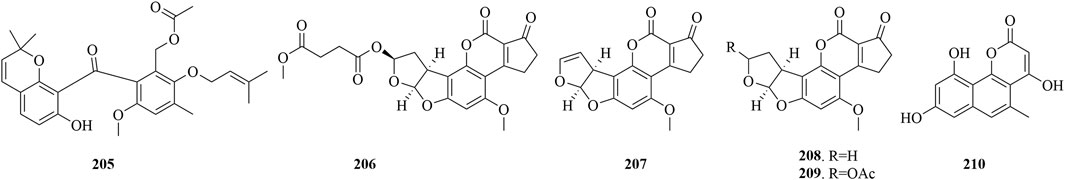
Isolated from A. versicolor HDN11-84, which resides in the mangrove rhizosphere, Arugosin K (205) (Figure 10) has exhibited potent cytotoxic effects against the Hela, HL-60, and NB4 cell lines, achieving IC50 values between 9.2 and 15.2 μM (Li et al., 2017). Aflatoxin B2b (206) along with three known compounds (207-209) was extracted from the endophytic fungus Aspergillus flavus 092008, which resides within the mangrove plant Hibiscus. Compound 206 showed moderate antibacterial potency against E. coli, B. subtilis and Escherichia aerogenes, with MICs of 22.5, 1.7, and 1.1 μM, respectively. Compounds 207-209 also demonstrated moderate antibacterial activity against E. aerogenes, with MICs of 3.2, 3.2, and 2.7 μM. Notably, compound 207 exhibited weak antibacterial effects against P. aeruginosa, with a MIC value of 32.1 μM. The findings suggested that the esterification at the C-8 position with a longer fatty acid in aflatoxin B2b (206) may enhance its antibacterial activity, making it the most effective among the tested compounds. Furthermore, compound 206 has proven to be cytotoxic against the A549 and K562 cell lines, with IC50 values of 8.1 μM and 2.0 μM, respectively (Wang et al., 2012). Pannorin (210), isolated from the fungus B. fuckeliana, sourced from the Wadden Sea in Germany, exhibited potent inhibition of GSK-3β enzyme in an assay, with an IC50 value of 0.35 μM (Wiese et al., 2016).
5 Potential of benzopyran compounds from marine fungi in drug development
Within the spectrum of esteemed natural product frameworks, benzopyran compounds are renowned for their bioactivity. Warfarin, one of the pioneering anticoagulant drugs approved in 1954, exemplifies this with its benzo-α-pyrone (coumarin) nucleus (Pirmohamed, 2006). Similarly, methoxsalen and trioxsalen, which have garnered extensive research for their use in treating psoriasis, also incorporate a benzo-α-pyrone nucleus. The benzo-γ-pyrone (chromone) nucleus is also present in cromolyn and pranlukast, drugs that were approved for asthma treatment in 1982 and 2007, respectively. Furthermore, an array of small molecule drugs centered around the benzopyran core are currently undergoing clinical investigation, highlighting the ongoing interest and potential of these marine-derived compounds in pharmaceutical development (Sharma et al., 2024).
A growing body of research indicates that the harnessing of anticancer immune responses could be instrumental in post-treatment cancer control, potentially eradicating residual malignant cells or curbing micrometastasis. The incorporation of such immune responses into cancer therapeutic strategies, alongside the development of chemotherapeutic agents capable of both direct cytotoxicity and the induction of specific immune reactions, may substantially enhance the efficacy of cancer treatments and mitigate the risk of drug resistance. In a study by Rönsberg et al. (2013), Phomoxanthone A (56) demonstrated exceptional pro-apoptotic potency against a range of human cancer cell lines, with its impact on healthy blood cells being markedly reduced by an order of magnitude. This compound also stands out as a potent stimulator of mouse T lymphocytes, NK cells, and macrophages, suggesting a synergistic activation of the immune system that complements its pro-apoptotic effects. Such dual-targeted action may offer a robust approach to counteracting chemoresistance. Given the pivotal role of angiogenesis in tumor progression, angiogenesis inhibitors are emerging as novel therapeutic targets. The compound decarboxyhydroxycitrinone (102) (Tsukada et al., 2011), with its anti-proliferative effects on HUVECs and HUAECs and IC50 values of 7.6 and 17.4 μM respectively, presents itself as a promising candidate for inhibiting tumor angiogenesis, thus opening new avenues in the fight against cancer.
The advent of the SARS-CoV-2 virus at the close of 2019 marked the onset of a severe respiratory disease that has escalated into one of the most formidable pandemics of our era. COVID-19 has challenged the global community, with the efficacy of vaccines being tested by the emergence of elusive variants. The evolution of more potent genetic strains coupled with the virus’s burgeoning resistance to existing treatments has intensified the quest for potent antiviral agents against SARS-CoV-2. In a significant study by ElNaggar et al. (2022), Aurasperone A (92) demonstrated robust inhibitory effects against SARS-CoV-2, with an IC50 of 12.25 µM, rivaling the efficacy of the benchmark remdesivir at 10.11 µM. Notably, compound 92 displayed negligible cytotoxicity towards Vero E6 cells, with a CC50 of 32.36 µM and a selectivity index (SI) of 2,641.5, vastly outperforming remdesivir’s SI of 41.07 at a CC50 of 415.22 µM. Advanced screening utilizing molecular docking simulations (MDS) indicated that compound 92 possesses a high affinity for the Mpro enzyme’s active site, capable of forming numerous hydrogen bonds, water bridges, and hydrophobic interactions, culminating in stable binding over a 150 ns timeframe. These discoveries lay a solid foundation for the advancement of more efficacious Mpro inhibitors, offering new horizons in the battle against SARS-CoV-2.
Glycogen synthase kinase 3 (GSK-3) stands as a pivotal target in the quest for novel therapeutics. Involved in the intricate signaling cascades of type II diabetes, neurodegenerative conditions, oncological processes, and a spectrum of other pathological states, GSK-3’s modulation is of high interest. The inhibitory potential of compounds 134–136 against GSK-3β was evaluated in enzyme assays (Wiese et al., 2016), revealing inhibition efficacies that rivaled those of the benchmark compound TDZD-8. Alternariol (136) emerged as the most potent, with an IC50 of 0.13 μM, closely followed by compound 136 at 0.20 μM. These findings underscore the remarkable inhibitory capabilities of the trio, all characterized by a highly oxygenated benzo-coumarin core, which positions them as novel and efficacious GSK-3β inhibitors, heralding a promising avenue for the development of therapeutics targeting a myriad of diseases.
Inflammation serves as a vital immune response, instrumental in neutralizing harmful stimuli and facilitating the repair of cellular and tissue damage. Despite its protective role, unchecked acute or chronic inflammation can precipitate a host of serious conditions, including but not limited to arthritis, asthma, inflammatory bowel disease, Parkinson’s disease, Alzheimer’s disease, and sepsis. Within these pathological contexts, the regulation of inflammatory responses is paramount for therapeutic intervention. In a study by Ko et al. (2019), the anti-inflammatory mechanisms of compound 179 were elucidated, revealing its efficacy in dampening inflammation through the suppression of the nuclear factor (NF)-κB and c-Jun N-terminal kinase (JNK) MAPK signaling cascades. Furthermore, the anti-inflammatory potency of DMHM is linked to its upregulation of HO-1 via the activation of the Nrf2 pathway. By targeting multiple inflammatory pathways, compound 179 illustrates its promise as a therapeutic agent for the management of inflammatory and neurodegenerative disorders.
The differentiation of mesenchymal stem cells from bone marrow hematopoietic stem cells gives rise to both osteoblasts, pivotal in bone metabolism, and adipocytes, central to fat metabolism. In clinical scenarios of osteoporosis, there is observed an increase in adipocyte presence and a concomitant reduction in osteoblast numbers within the bone marrow. Targeting the differentiation of these stem cells to favor bone regeneration offers a strategic approach to combat osteoporosis. The osteogenic potential of neotricitrinols B (191) was scrutinized by He et al. (2023), revealing that compound 191 robustly promoted osteogenic mineralization in primary bone mesenchymal stem cells (BMSCs) and concurrently curbed adipogenic differentiation in a dose-responsive manner. This dual action underscores the compound’s significant anti-osteoporotic efficacy. Importantly, at concentrations below 10 μM, compound 191 exhibited negligible cytotoxic effects, highlighting its safety profile for potential therapeutic applications.
6 Conclusion
This comprehensive review has delineated the benzopyran compounds produced by marine fungi from the onset of the 21st century to the close of 2023, highlighting their structural diversity and biological profiles. A total of 510 benzopyran compounds were characterized, with 223 showcasing bioactivity, mainly isolated from marine fauna (44%), marine sediments (28%), and marine flora (25%). The fungal genera predominantly represented were Penicillium (43%) and Aspergillus (20%). The benzopyran compounds derived from marine fungi have attracted considerable interest due to their diverse chemical structures and pronounced bioactivity. The majority of these compounds have manifested exceptional bioactivity across a spectrum of biological functions, including antibacterial (35%), antitumor (31%), enzymatic inhibition (5%), and antiviral (3%) activities. While existing research has begun to scratch the surface of the biological potential harbored by benzopyran compounds, the vast majority of these, isolated from the marine milieu, remain an uncharted territory in terms of their full biological capabilities. The untapped reservoir of these marine-derived metabolites is ripe for exploration. It is with this in mind that we foresee a promising future for the development of these natural products, which holds the key to uncovering groundbreaking pharmaceuticals endowed with unprecedented mechanisms of action.
Author contributions
YX: Data curation, Investigation, Resources, Writing–original draft. HW: Investigation, Resources, Writing–original draft. LS: Data curation, Investigation, Resources, Writing–original draft. XM: Data curation, Investigation, Resources, Writing–review and editing. SZ: Data curation, Investigation, Resources, Writing–review and editing. ZZ: Conceptualization, Formal Analysis, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We are grateful for the financial support provided by the Natural Science Foundation of Shandong Province (No. ZR2022MC157), and research funding for Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University (No. JYHL2021MS17).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1482316/full#supplementary-material
References
Amin, M., Zhang, X. Y., Xu, X. Y., and Qi, S. H. (2020). New citrinin derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z015. Nat. Prod. Res. 34 (9), 1219–1226. doi:10.1080/14786419.2018.1556266
Anh, N. M., Huyen, V. T. T., Quyen, V. T., Dao, P. T., Quynh, D. T., Huong, D. T. M., et al. (2023). Antimicrobial and cytotoxic secondary metabolites from a marine-derived fungus Penicillium citrinum VM6. Curr. Microbiol. 81 (1), 32. doi:10.1007/s00284-023-03568-7
Bao, J., He, F., Yu, J. H., Zhai, H., Cheng, Z. Q., Jiang, C. S., et al. (2018). New chromones from a marine-derived fungus, Arthrinium sp., and their biological activity. Molecules 23 (8), 1982. doi:10.3390/molecules23081982
Bao, J., Luo, J. F., Qin, X. C., Xu, X. Y., Zhang, X. Y., Tu, Z. C., et al. (2014). Dihydrothiophene-condensed chromones from a marine-derived fungus Penicillium oxalicum and their structure-bioactivity relationship. Bioorg Med. Chem. Lett. 24 (11), 2433–2436. doi:10.1016/j.bmcl.2014.04.028
Bao, J., Sun, Y. L., Zhang, X. Y., Han, Z., Gao, H. C., He, F., et al. (2013). Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. (Tokyo) 66 (4), 219–223. doi:10.1038/ja.2012.110
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G., and Prinsep, M. R. (2017). Marine natural products. Nat. Prod. Rep. 34 (3), 235–294. doi:10.1039/c6np00124f
Cao, D. D., Trinh, T. T. V., Mai, H. D. T., Vu, V. N., Le, H. M., Thi, Q. V., et al. (2019). Antimicrobial lavandulylated flavonoids from a sponge-derived Streptomyces sp. G248 in east vietnam sea. Mar. Drugs 17 (9), 529. doi:10.3390/md17090529
Cao, F., Yang, J. K., Liu, Y. F., Zhu, H. J., and Wang, C. Y. (2016). Pleosporalone A, the first azaphilone characterized with aromatic A-ring from a marine-derived Pleosporales sp. fungus. Nat. Prod. Res. 30 (21), 2448–2452. doi:10.1080/14786419.2016.1198352
Chai, Y. J., Cui, C. B., Li, C. W., Wu, C. J., Tian, C. K., and Hua, W. (2012). Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 10 (3), 559–582. doi:10.3390/md10030559
Chen, L., Liu, W., Hu, X., Huang, K., Wu, J. L., and Zhang, Q. Q. (2011). Citrinin derivatives from the marine-derived fungus Penicillium citrinum. Chem. Pharm. Bull. (Tokyo) 59 (4), 515–517. doi:10.1248/cpb.59.515
Chen, S., Wang, J., Wang, Z., Lin, X., Zhao, B., Kaliaperumal, K., et al. (2017). Structurally diverse secondary metabolites from a deep-sea-derived fungus Penicillium chrysogenum SCSIO 41001 and their biological evaluation. Fitoterapia 117, 71–78. doi:10.1016/j.fitote.2017.01.005
Chen, Y., Liu, Z., Liu, H., Pan, Y., Li, J., Liu, L., et al. (2018). Dichloroisocoumarins with potential anti-inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4. Mar. Drugs 16 (2), 54. doi:10.3390/md16020054
Cheng, M., Li, P., Jiang, Y., Tang, X., Zhang, W., Wang, Q., et al. (2021). Penitol A and penicitols E-I: citrinin derivatives from Penicillium citrinum and the structure revision of previously proposed analogues. J. Nat. Prod. 84 (4), 1345–1352. doi:10.1021/acs.jnatprod.1c00082
Chinworrungsee, M., Kittakoop, P., Isaka, M., Rungrod, A., Tanticharoen, M., and Thebtaranonth, Y. (2001). Antimalarial halorosellinic acid from the marine fungus Halorosellinia oceanica. Bioorg Med. Chem. Lett. 11 (15), 1965–1969. doi:10.1016/s0960-894x(01)00327-4
Chu, Y. C., Chang, C. H., Liao, H. R., Cheng, M. J., Wu, M. D., Fu, S. L., et al. (2021). Rare chromone derivatives from the marine-derived Penicillium citrinum with anti-cancer and anti-inflammatory activities. Mar. Drugs 19 (1), 25. doi:10.3390/md19010025
Coronado, L., Zhang, X. Q., Dorta, D., Escala, N., Pineda, L. M., Ng, M. G., et al. (2021). Semisynthesis, antiplasmodial activity, and mechanism of action studies of isocoumarin derivatives. J. Nat. Prod. 84 (5), 1434–1441. doi:10.1021/acs.jnatprod.0c01032
Duan, Y. D., Jiang, Y. Y., Guo, F. X., Chen, L. X., Xu, L. L., Zhang, W., et al. (2019). The antitumor activity of naturally occurring chromones: a review. Fitoterapia 135, 114–129. doi:10.1016/j.fitote.2019.04.012
ElNaggar, M. H., Abdelwahab, G. M., Kutkat, O., GabAllah, M., Ali, M. A., El-Metwally, M. E. A., et al. (2022). Aurasperone A inhibits sars CoV-2 in vitro: an integrated in vitro and in silico study. Mar. Drugs 20 (3), 179. doi:10.3390/md20030179
Fan, H., Shi, Z. M., Lei, Y. H., Si-Tu, M. X., Zhou, F. G., Feng, C., et al. (2022). Rare carbon-bridged citrinin dimers from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Mar. Drugs 20 (7), 443. doi:10.3390/md20070443
Frank, M., Hartmann, R., Plenker, M., Mándi, A., Kurtán, T., Özkaya, F. C., et al. (2019). Brominated azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14.6a. J. Nat. Prod. 82 (8), 2159–2166. doi:10.1021/acs.jnatprod.9b00151
Girich, E. V., Yurchenko, A. N., Smetanina, O. F., Trinh, P. T. H., Ngoc, N. T. D., Pivkin, M. V., et al. (2020). Neuroprotective metabolites from Vietnamese marine derived fungi of Aspergillus and Penicillium genera. Mar. Drugs 18 (12), 608. doi:10.3390/md18120608
Guo, W., Li, D., Peng, J., Zhu, T., Gu, Q., and Li, D. (2015). Penicitols A-C and penixanacid A from the mangrove-derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 78 (2), 306–310. doi:10.1021/np500586r
Han, S., Liu, Y., Liu, W., Yang, F., Zhang, J., Liu, R., et al. (2021). Chromone derivatives with α-glucosidase inhibitory activity from the marine fungus Penicillium thomii maire. Molecules 26 (17), 5273. doi:10.3390/molecules26175273
Haque, N., Parveen, S., Tang, T., Wei, J., and Huang, Z. (2022). Marine natural products in clinical use. Mar. Drugs 20 (8), 528. doi:10.3390/md20080528
He, Z. H., Xie, C. L., Wu, T., Zhang, Y., Zou, Z. B., Xie, M. M., et al. (2023). Neotricitrinols A-C, unprecedented citrinin trimers with anti-osteoporosis activity from the deep-sea-derived Penicillium citrinum W23. Bioorg Chem. 139, 106756. doi:10.1016/j.bioorg.2023.106756
Hou, X. M., Wang, C. Y., Gu, Y. C., and Shao, C. L. (2016). Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Nat. Prod. Res. 30 (20), 2274–2277. doi:10.1080/14786419.2016.1163695
Huang, G. L., Zhou, X. M., Bai, M., Liu, Y. X., Zhao, Y. L., Luo, Y. P., et al. (2016a). Dihydroisocoumarins from the mangrove-derived fungus Penicillium citrinum. Mar. Drugs 14 (10), 177. doi:10.3390/md14100177
Huang, J., She, J., Yang, X., Liu, J., Zhou, X., and Yang, B. (2019). A new macrodiolide and two new polycyclic chromones from the fungus Penicillium sp. SCSIO041218. Molecules 24 (9), 1686. doi:10.3390/molecules24091686
Huang, M., Li, J., Liu, L., Yin, S., Wang, J., and Lin, Y. (2016b). Phomopsichin A-D; four new chromone derivatives from mangrove endophytic fungus Phomopsis sp. 33. Mar. Drugs 14 (11), 215. doi:10.3390/md14110215
Huang, Y. F., Li, L. H., Tian, L., Qiao, L., Hua, H. M., and Pei, Y. H. (2006). Sg17-1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17-1. J. Antibiot. (Tokyo) 59 (6), 355–357. doi:10.1038/ja.2006.50
Intaraudom, C., Bunbamrung, N., Dramae, A., Boonyuen, N., Choowong, W., Rachtawee, P., et al. (2021). Chromone derivatives, R- and S- taeniolin, from the marine-derived fungus Taeniolella sp. BCC31839. Nat. Prod. Res. 35 (3), 392–398. doi:10.1080/14786419.2019.1634710
Ji Ram, V., Pratap, R., and Yadav, P. (2014). Fused pyranones multifaceted building blocks for molecular diversity, eBook ISBN: 9780128232132.
Ju, Z., Lin, X., Lu, X., Tu, Z., Wang, J., Kaliyaperumal, K., et al. (2015). Botryoisocoumarin A, a new COX-2 inhibitor from the mangrove Kandelia candel endophytic fungus Botryosphaeria sp. KcF6. J. Antibiot. (Tokyo) 68 (10), 653–656. doi:10.1038/ja.2015.46
Kim, D. C., Cho, K. H., Ko, W., Yoon, C. S., Sohn, J. H., Yim, J. H., et al. (2016). Anti-inflammatory and cytoprotective effects of TMC-256C1 from marine-derived fungus Aspergillus sp. SF-6354 via up-regulation of heme oxygenase-1 in murine hippocampal and microglial cell lines. Int. J. Mol. Sci. 17 (4), 529. doi:10.3390/ijms17040529
Kim, D. C., Quang, T. H., Ngan, N. T., Yoon, C. S., Sohn, J. H., Yim, J. H., et al. (2015). Dihydroisocoumarin derivatives from marine-derived fungal isolates and their anti-inflammatory effects in lipopolysaccharide-induced BV2 microglia. J. Nat. Prod. 78 (12), 2948–2955. doi:10.1021/acs.jnatprod.5b00614
Ko, W., Quang, T. H., Sohn, J. H., Yim, J. H., Kang, D. G., Lee, H. S., et al. (2019). Anti-inflammatory effect of 3,7-dimethyl-1,8-hydroxy-6-methoxyisochroman via nuclear factor erythroid 2-like 2-mediated heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Immunopharmacol. Immunotoxicol. 41 (2), 337–348. doi:10.1080/08923973.2019.1608559
Kumla, D., Pereira, J. A., Dethoup, T., Gales, L., Freitas-Silva, J., Costa, P. M., et al. (2018). Chromone derivatives and other constituents from cultures of the marine sponge-associated fungus Penicillium erubescens KUFA0220 and their antibacterial activity. Mar. Drugs 16 (8), 289. doi:10.3390/md16080289
Lai, C., Tian, D., Zheng, M., Li, B., Jia, J., Wei, J., et al. (2023). Novel citrinin derivatives from fungus Penicillium sp. TW131-64 and their antimicrobial activities. Appl. Microbiol. Biotechnol. 107 (21), 6607–6619. doi:10.1007/s00253-023-12738-3
Lan, W. J., Zhao, Y., Xie, Z. L., Liang, L. Z., Shao, W. Y., Zhu, L. P., et al. (2012). Novel sorbicillin analogues from the marine fungus Trichoderma sp. associated with the seastar Acanthaster planci. Nat. Prod. Commun. 7 (10), 1337–1340. doi:10.1177/1934578x1200701022
Lee, D. S., Jang, J. H., Ko, W., Kim, K. S., Sohn, J. H., Kang, M. S., et al. (2013). PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar. Drugs 11 (4), 1409–1426. doi:10.3390/md11041409
Leutou, A. S., Yun, K., and Son, B. W. (2016). Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharm. Res. 39 (6), 806–810. doi:10.1007/s12272-016-0764-2
Li, F., Guo, W., Che, Q., Zhu, T., Gu, Q., and Li, D. (2017). Versicones E-H and arugosin K produced by the mangrove-derived fungus Aspergillus versicolor HDN11-84. J. Antibiot. (Tokyo) 70 (2), 174–178. doi:10.1038/ja.2016.95
Liang, Z., Gu, T., Wang, J., She, J., Ye, Y., Cao, W., et al. (2021). Chromene and chromone derivatives as liver X receptors modulators from a marine-derived Pestalotiopsis neglecta fungus. Bioorg Chem. 112, 104927. doi:10.1016/j.bioorg.2021.104927
Liao, H. X., Sun, D. W., Zheng, C. J., and Wang, C. Y. (2017). A new hexahydrobenzopyran derivative from the gorgonian-derived Fungus Eutypella sp. Nat. Prod. Res. 31 (14), 1640–1646. doi:10.1080/14786419.2017.1285301
Lin, W., Brauers, G., Ebel, R., Wray, V., Berg, A., Sudarsono, , et al. (2003). Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. J. Nat. Prod. 66 (1), 57–61. doi:10.1021/np020196b
Liu, F. A., Lin, X., Zhou, X., Chen, M., Huang, X., Yang, B., et al. (2017). Xanthones and quinolones derivatives produced by the deep-sea-derived fungus Penicillium sp. SCSIO Ind16F01. Molecules 22 (12), 1999. doi:10.3390/molecules22121999
Liu, J. T., Wu, W., Cao, M. J., Yang, F., and Lin, H. W. (2018). Trienic α-pyrone and ochratoxin derivatives from a sponge-derived fungus Aspergillus ochraceopetaliformis. Nat. Prod. Res. 32 (15), 1791–1797. doi:10.1080/14786419.2017.1402325
Liu, X. H., Hou, X. L., Song, Y. P., Wang, B. G., and Ji, N. Y. (2020). Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia 141, 104469. doi:10.1016/j.fitote.2020.104469
Liu, Y., Chen, S., Liu, Z., Lu, Y., Xia, G., Liu, H., et al. (2015). Bioactive metabolites from mangrove endophytic fungus Aspergillus sp. 16-5B. Mar. Drugs 13 (5), 3091–3102. doi:10.3390/md13053091
Liu, Y., Ding, L., He, J., Zhang, Z., Deng, Y., He, S., et al. (2021). A new antibacterial chromone from a marine sponge-associated fungus Aspergillus sp. LS57. Fitoterapia 154, 105004. doi:10.1016/j.fitote.2021.105004
Luo, X., Lin, X., Salendra, L., Pang, X., Dai, Y., Yang, B., et al. (2017). Isobenzofuranones and isochromenones from the deep-sea derived fungus Leptosphaeria sp. SCSIO 41005. Mar. Drugs 15 (7), 204. doi:10.3390/md15070204
Ma, J., Zhang, X. L., Wang, Y., Zheng, J. Y., Wang, C. Y., and Shao, C. L. (2017). Aspergivones A and B, two new flavones isolated from a gorgonian-derived Aspergillus candidus fungus. Nat. Prod. Res. 31 (1), 32–36. doi:10.1080/14786419.2016.1207073
May Zin, W. W., Buttachon, S., Dethoup, T., Pereira, J. A., Gales, L., Inácio, Â., et al. (2017). Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 141, 86–97. doi:10.1016/j.phytochem.2017.05.015
Meng, L. H., Liu, Y., Li, X. M., Xu, G. M., Ji, N. Y., and Wang, B. G. (2015). Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 78 (9), 2301–2305. doi:10.1021/acs.jnatprod.5b00450
Ni, M., Lin, W. L., Yang, P., and Mao, S. C. (2015). A novel citrinin derivative from the marine-source fungus Penicillium citrinum. Yao Xue Xue Bao 50 (2), 203–206. doi:10.16438/j.0513-4870.2015.02.009
Niu, S., Liu, D., Proksch, P., Shao, Z., and Lin, W. (2015). New polyphenols from a deep sea Spiromastix sp. Fungus, and their antibacterial activities. Mar. Drugs 13 (4), 2526–2540. doi:10.3390/md13042526
Oppong-Danquah, E., Blümel, M., Scarpato, S., Mangoni, A., and Tasdemir, D. (2022). Induction of isochromanones by Co-cultivation of the marine fungus Cosmospora sp. and the phytopathogen Magnaporthe oryzae. Int. J. Mol. Sci. 23 (2), 782. doi:10.3390/ijms23020782
Ovenden, S. P., Sberna, G., Tait, R. M., Wildman, H. G., Patel, R., Li, B., et al. (2004). A diketopiperazine dimer from a marine-derived isolate of Aspergillus niger. J. Nat. Prod. 67 (12), 2093–2095. doi:10.1021/np0497494
Pang, X., Lin, X., Wang, J., Liang, R., Tian, Y., Salendra, L., et al. (2018a). Three new highly oxygenated sterols and one new dihydroisocoumarin from the marine sponge-derived fungus Cladosporium sp. SCSIO41007. Steroids 129, 41–46. doi:10.1016/j.steroids.2017.12.001
Pang, X., Lin, X., Yang, J., Zhou, X., Yang, B., Wang, J., et al. (2018b). Spiro-phthalides and isocoumarins isolated from the marine-sponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 81 (8), 1860–1868. doi:10.1021/acs.jnatprod.8b00345
Pirmohamed, M. (2006). Warfarin: almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 62 (5), 509–511. doi:10.1111/j.1365-2125.2006.02806.x
Pontius, A., Krick, A., Mesry, R., Kehraus, S., Foegen, S. E., Müller, M., et al. (2008). Monodictyochromes A and B, dimeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 71 (11), 1793–1799. doi:10.1021/np800392w
Prompanya, C., Dethoup, T., Bessa, L. J., Pinto, M. M., Gales, L., Costa, P. M., et al. (2014). New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. Nov. KUFA 0013. Mar. Drugs 12 (10), 5160–5173. doi:10.3390/md12105160
Qi, J., Shao, C. L., Li, Z. Y., Gan, L. S., Fu, X. M., Bian, W. T., et al. (2013). Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 76 (4), 571–579. doi:10.1021/np3007556
Ran, Y. Q., Lan, W. J., Qiu, Y., Guo, Q., Feng, G. K., Deng, R., et al. (2020). Monarubins A-C from the marine shellfish-associated fungus Monascus ruber BB5. Mar. Drugs 18 (2), 100. doi:10.3390/md18020100
Rönsberg, D., Debbab, A., Mándi, A., Vasylyeva, V., Böhler, P., Stork, B., et al. (2013). Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla. J. Org. Chem. 78 (24), 12409–12425. doi:10.1021/jo402066b
Sabdaningsih, A., Liu, Y., Mettal, U., Heep, J., Riyanti, , Wang, L., et al. (2020). A new citrinin derivative from the Indonesian marine sponge-associated fungus Penicillium citrinum. Mar. Drugs 18 (4), 227. doi:10.3390/md18040227
Sharma, V., Sharma, A., Wadje, B. N., and Bharate, S. B. (2024). Benzopyrone, a privileged scaffold in drug discovery: an overview of FDA-approved drugs and clinical candidates. Med. Res. Rev. 43, 2035–2077. doi:10.1002/med.22032
Silva, C. F. M., Batista, V. F., Pinto, D., and Silva, A. M. S. (2018). Challenges with chromone as a privileged scaffold in drug discovery. Expert Opin. Drug Discov. 13 (9), 795–798. doi:10.1080/17460441.2018.1494720
Song, Y. P., Miao, F. P., Liu, X. H., Yin, X. L., and Ji, N. Y. (2019). Seven chromanoid norbisabolane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Fitoterapia 135, 107–113. doi:10.1016/j.fitote.2019.04.014
Soosaraei, M., Khasseh, A. A., Fakhar, M., and Hezarjaribi, H. Z. (2018). A decade bibliometric analysis of global research on leishmaniasis in Web of Science database. Ann. Med. Surg. (Lond) 26, 30–37. doi:10.1016/j.amsu.2017.12.014
Sun, K., Liang, L., Yin, H., Yu, J., Feng, M., Zhan, J., et al. (2019). Manipulation for treatment of degenerative lumbar spondylolisthesis: a protocol of systematic review and meta-analysis. Med. Baltim. 98 (49), e18135. doi:10.1097/md.0000000000018135
Sun, Y. L., He, F., Liu, K. S., Zhang, X. Y., Bao, J., Wang, Y. F., et al. (2012). Cytotoxic dihydrothiophene-condensed chromones from marine-derived fungus Penicillium oxalicum. Planta Med. 78 (18), 1957–1961. doi:10.1055/s-0032-1327874
Tang, Q., Guo, K., Li, X. Y., Zheng, X. Y., Kong, X. J., Zheng, Z. H., et al. (2014). Three new asperentin derivatives from the algicolous fungus Aspergillus sp. F00785. Mar. Drugs 12 (12), 5993–6002. doi:10.3390/md12125993
Tsukada, M., Fukai, M., Miki, K., Shiraishi, T., Suzuki, T., Nishio, K., et al. (2011). Chemical constituents of a marine fungus, Arthrinium sacchari. J. Nat. Prod. 74 (7), 1645–1649. doi:10.1021/np200108h
Ueda, J. Y., Takagi, M., and Shin-ya, K. (2010). New xanthoquinodin-like compounds, JBIR-97, -98 and -99, obtained from marine sponge-derived fungus Tritirachium sp. SpB081112MEf2. J. Antibiot. (Tokyo) 63 (10), 615–618. doi:10.1038/ja.2010.92
Wang, H., Lu, Z., Qu, H. J., Liu, P., Miao, C., Zhu, T., et al. (2012). Antimicrobial aflatoxins from the marine-derived fungus Aspergillus flavus 092008. Arch. Pharm. Res. 35 (8), 1387–1392. doi:10.1007/s12272-012-0808-1
Wang, M. L., Lu, C. H., Xu, Q. Y., Song, S. Y., Hu, Z. Y., and Zheng, Z. H. (2013). Four new citrinin derivatives from a marine-derived Penicillium sp. fungal strain. Molecules 18 (5), 5723–5735. doi:10.3390/molecules18055723
Wang, S., Li, X. M., Teuscher, F., Li, D. L., Diesel, A., Ebel, R., et al. (2006). Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 69 (11), 1622–1625. doi:10.1021/np060248n
Wang, W., Liao, Y., Zhang, B., Gao, M., Ke, W., Li, F., et al. (2019). Citrinin monomer and dimer derivatives with antibacterial and cytotoxic activities isolated from the deep sea-derived fungus Penicillium citrinum NLG-S01-P1. Mar. Drugs 17 (1), 46. doi:10.3390/md17010046
Wiese, J., Aldemir, H., Schmaljohann, R., Gulder, T. A. M., and Imhoff, J. F. (2017). Asperentin B, a new inhibitor of the protein tyrosine phosphatase 1B. Mar. Drugs 15 (6), 191. doi:10.3390/md15060191
Wiese, J., Imhoff, J. F., Gulder, T. A., Labes, A., and Schmaljohann, R. (2016). Marine fungi as producers of benzocoumarins, a new class of inhibitors of glycogen-synthase-kinase 3β. Mar. Drugs 14 (11), 200. doi:10.3390/md14110200
Wu, B., Wiese, J., Wenzel-Storjohann, A., Malien, S., Schmaljohann, R., and Imhoff, J. F. (2016). Engyodontochones, antibiotic polyketides from the marine fungus Engyodontium album strain LF069. Chemistry 22 (22), 7452–7462. doi:10.1002/chem.201600430
Wu, G., Yu, G., Kurtán, T., Mándi, A., Peng, J., Mo, X., et al. (2015). Versixanthones A-F, cytotoxic xanthone-chromanone dimers from the marine-derived fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 78 (11), 2691–2698. doi:10.1021/acs.jnatprod.5b00636
Wu, J. S., Shi, X. H., Yao, G. S., Shao, C. L., Fu, X. M., Zhang, X. L., et al. (2020). New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus terreus. Mar. Drugs 18 (3), 132. doi:10.3390/md18030132
Wu, X. Z., Huang, W. J., Liu, W., Mándi, A., Zhang, Q., Zhang, L., et al. (2021). Penicisteckins A-F, isochroman-derived atropisomeric dimers from Penicillium steckii HNNU-5B18. J. Nat. Prod. 84 (11), 2953–2960. doi:10.1021/acs.jnatprod.1c00787
Wu, Z., Chen, J., Zhang, X., Chen, Z., Li, T., She, Z., et al. (2019). Four new isocoumarins and a new natural tryptamine with antifungal activities from a mangrove endophytic fungus Botryosphaeria ramosa L29. Mar. Drugs 17 (2), 88. doi:10.3390/md17020088
Wu, Z. J., Ouyang, M. A., and Tan, Q. W. (2009). New asperxanthone and asperbiphenyl from the marine fungus Aspergillus sp. Pest Manag. Sci. 65 (1), 60–65. doi:10.1002/ps.1645
Xia, M. W., Cui, C. B., Li, C. W., Wu, C. J., Peng, J. X., and Li, D. H. (2015). Rare chromones from a fungal mutant of the marine-derived Penicillium purpurogenum G59. Mar. Drugs 13 (8), 5219–5236. doi:10.3390/md13085219
Xu, X., He, F., Zhang, X., Bao, J., and Qi, S. (2013). New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 53, 46–51. doi:10.1016/j.fct.2012.11.037
Xu, X., Li, J., Zhang, K., Wei, S., Lin, R., Polyak, S. W., et al. (2021). New isocoumarin analogues from the marine-derived fungus paraphoma sp. CUGBMF180003. Mar. Drugs 19 (6), 313. doi:10.3390/md19060313
Yang, J. M., Liu, Y. Y., Yang, W. C., Ma, X. X., Nie, Y. Y., Glukhov, E., et al. (2020a). An anti-inflammatory isoflavone from soybean inoculated with a marine fungus Aspergillus terreus C23-3. Biosci. Biotechnol. Biochem. 84 (8), 1546–1553. doi:10.1080/09168451.2020.1764838
Yang, W., Chen, Y., Cai, R., Zou, G., Wang, B., and She, Z. (2020b). Benzopyran derivatives and an aliphatic compound from a mangrove endophytic fungus Penicillium citrinum QJF-22. Chem. Biodivers. 17 (6), e2000192. doi:10.1002/cbdv.202000192
Yao, Q., Wang, J., Zhang, X., Nong, X., Xu, X., and Qi, S. (2014). Cytotoxic polyketides from the deep-sea-derived fungus Engyodontium album DFFSCS021. Mar. Drugs 12 (12), 5902–5915. doi:10.3390/md12125902
Yin, Y., Tan, Q., Wu, J., Chen, T., Yang, W., She, Z., et al. (2023). The polyketides with antimicrobial activities from a mangrove endophytic fungus Trichoderma lentiforme ML-P8-2. Mar. Drugs 21 (11), 566. doi:10.3390/md21110566
Yu, H. Y., Li, Y., Zhang, M., Zou, Z. B., Hao, Y. J., Xie, M. M., et al. (2023). Chemical constituents of the deep-sea gammarid shrimp-derived fungus Penicillium citrinum XIA-16. Chem. Biodivers. 20 (11), e202301507. doi:10.1002/cbdv.202301507
Keywords: marine fungi, secondary metabolites, isolation and identification, benzopyrans, biological activity
Citation: Xi Y, Wang H, Sun L, Ma X, Zhang S and Zhang Z (2024) Recent advances in the structures and bioactivities of benzopyrans derived from marine fungi: a review. Front. Pharmacol. 15:1482316. doi: 10.3389/fphar.2024.1482316
Received: 18 August 2024; Accepted: 15 October 2024;
Published: 24 October 2024.
Edited by:
Manuela Oliverio, Magna Græcia University, ItalyReviewed by:
Pratik Yadav, Purdue University, United StatesAntresh Kumar, Central University of Haryana, India
Ricardo Calhelha, Centro de Investigação de Montanha (CIMO), Portugal
Copyright © 2024 Xi, Wang, Sun, Ma, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Zhang, emhhbmd6QG1haWwuam5tYy5lZHUuY24=
†These authors have contributed equally to this work
 Yidan Xi
Yidan Xi Huannan Wang
Huannan Wang Lixiang Sun
Lixiang Sun Xueyang Ma
Xueyang Ma Shuncun Zhang
Shuncun Zhang Zhen Zhang
Zhen Zhang