- 1Department of Nephrology, Chengdu BOE Hospital, Chengdu, China
- 2Department of Nephrology, Dazhou Central Hospital, Dazhou, China
- 3Department of Neurology, The First People’s Hospital of Shuangliu District, Chengdu, China
- 4Department of Nephrology, West China Hospital of Sichuan University, Chengdu, China
Background and Objective: Uremic pruritus is a persistent condition that is difficult to cure in patients with end-stage renal disease who are having regular dialysis. It is highly prevalent, and current therapies have limited effectiveness and can cause significant adverse effects. Several trials have provided evidence that difelikefalin can be an effective treatment for uremic pruritus, with few side responses. However, it is important to note that the available evidence is limited. This study collected published randomized controlled trials for systematic review and Meta-analysis, to explore the efficacy and safety of difelikefalin treating uremic pruritus and to provide evidence-based medical evidence for clinical treatment.
Methods: A systematic literature search was conducted in PubMed, EMBASE, Web of Science, the Cochrane Library Data from building libraries to 6 January 2024. We extracted data from eligible studies to analyze the efficacy and safety of difelikefalin in the treatment of hemodialysis patients with pruritus.
Results: This study comprised 9 trials with 4,118 people. The meta-analysis demonstrated that difelikefalin is more effective than placebo in treating uremic pruritus. Specifically, difelikefalin resulted in a greater improvement in WI-NRS scores of at least 3 points from baseline (OR = 1.98) and at least 4 points from baseline (OR = 1.94). Additionally, difelikefalin led to a decrease in the total score of the 5-D itch scale (MD = 1.56), a decrease in the skindex-10 scale score (MD = 4.92), and a decrease in the WI-NRS scale score (MD = 0.91).
Conclusion: Difelikefalin demonstrates significant efficacy in alleviating pruritus in individuals suffering from uremia. Althogh it has adverse events, they are mild.
Introduction
Chronic kidney disease–associated pruritus (CKD-aP) is also known as uremic pruritus. Uremic pruritus is an intractable symptom in patients with end-stage kidney disease (ESKD) undergoing maintenance dialysis (Mettang and Kremer, 2015; Narita et al., 2022). Uremic pruritus is defined as ESKD people have itching that lasts for at least 3 months (Satti et al., 2019). It is a common, distressing, and underrecognized condition that affects more than 60% of patients undergoing hemodialysis, with 20%–40% of patients reporting moderate-to-severe pruritus (Aresi et al., 2019; Fishbane et al., 2020a). Persistent pruritus negatively affects physical and mental health. Uremic pruritus has also been associated with an increase in missed dialysis sessions, a higher risk of hospitalization, and an increase in mortality, particularly cardiovascular and infection-related mortality (Sukul et al., 2021).
The current management of uremic pruritus includes adequate dialysis, control of blood phosphorus, use of emollients, topical corticosteroids, immunosuppressants, antihistamines, gabapentin, pregabalin and Chinese medicine (Eusebio-Alpapara et al., 2020; Hercz et al., 2020). Despite the acknowledged importance of uremic pruritus to patients, with the exception of gabapentin, current evidence for its treatment is weak (Simonsen et al., 2017). However, it may have side effects. Natural or medicated topical treatments such as baby oil and moisturizers may cause burning or irritation in some patients (Lu et al., 2021). Topical corticosteroids or immunosuppressants may cause thin skin and decrease local resistance, thereby increasing the risk of infection. Tacrolimus is a calcineurin inhibitor and suppresses the production of IL-2 and has been demonstrated to be beneficial for uremic pruritus (Kuypers et al., 2004). However, topical tacrolimus carries a black-box warning of increased risk of skin cancer. Antihistamines are the most common clinical treatment for pruritus. Fifty-seven percent of doctors prescribed antihistamines as the first-line treatment for itch (Rayner et al., 2017). However, the use of antihistamines raises safety issues, especially in the elderly (Verduzco and Shirazian, 2020). The neuropathic/anticonvulsant agents Gabapentin and Pregabalin are the mostly widely studied systemic medications for the treatment of uremic pruritus. Their mechanism of action likely involves negative modulation of the alpha 2 delta subunit of voltage-gated calcium channels and/or inhibition of the release of calcitonin gene–related peptide (a mediator of itch) from primary afferent neurons. However, side effects such as somnolence and unsteadiness due to mononucleosis have been reported (Martin et al., 2020). Chinese medicine is also a means of uremic pruritus treatment, but at present there is little evidence of acupuncture therapy and Chinese herbal bath therapy (Lu et al., 2022).
Difelikefalin is a novel, selective kappa opioid receptor (KOR) agonist that does not readily enter the CNS owing to its hydrophilic D-amino acid peptidic structure (Viscusi et al., 2021). It exerts antipruritic effects by activating kappa opioid receptors in peripheral neurons and immune cells (Menzaghi et al., 2015). In phase 3 KALM-1and KALM-2 studies of intravenous (IV) difelikefalin in hemodialysis participants with moderate-to-severe pruritus, difelikefalin demonstrated significant reductions in itch intensity compared to placebo at week 12 (Fishbane et al., 2020b; Wooldridge et al., 2020). Some studies suggest that difelikefalin can effectively treat uremic pruritus with mild adverse reactions, but the evidence is limited. This study collected data from published randomized controlled trials for a systematic review and meta-analysis to explore the efficacy and safety of difelikefalin in the treatment of uremic pruritus and to provide evidence-based medical evidence for clinical treatment.
Methods
Inclusion and exclusion criteria
Make inclusion and exclusion criteria according to PICOS principles.
Inclusion criteria:
Population = patients age ≥18 years old with end-stage kidney disease who had been undergoing hemodialysis and who had moderate-to-severe pruritus.
Intervention = difelikefalin was used as an intervention.
Comparison = placebo was used as an intervention.
Outcomes = improvement of itching and the occurrence of adverse reactions.
Study Design = The study types were randomized controlled trials.
Exclusion criteria: Studies were excluded if the patients having pruritus not associated with chronic kidney disease; patients with chronic kidney disease who have not entered the hemodialysis stage; no control study; studies with unclear diagnostic criteria.
Search strategy
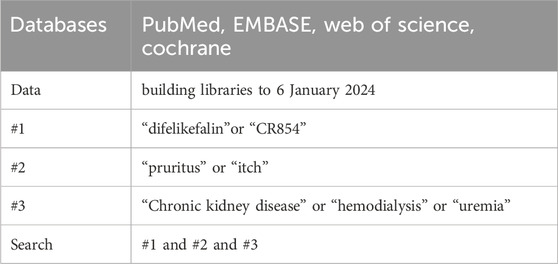
A systematic literature search was conducted in PubMed, EMBASE, Web of Science and Cochrane Data from building libraries to 6 January 2024. The following Medical Subject Heading terms and free words were used, as shown in Table 1: “difelikefalin” or “CR854” and “pruritus” or“itch” and “chronic kidney disease” or “hemodialysis” or “uremia”.
Study selection and data collection
Two investigators independently screened the literature to identify studies that met inclusion criteria. Any discrepancies between the reviewers were resolved through discussion with a third reviewer. After reading the title and abstract to exclude obviously irrelevant literature, further reading the full text to determine inclusion. Including those reporting the use of difelikefalin in treating pruritus in hemodialysis patients. The reference lists of all identified studies were also examined to find additional eligible studies. Data was collected and entered into a spreadsheet. The extracted variables included author, study period, location, patient age, sex, clinical characteristics, treatment effect, and adverse reactions. Two investigators independently evaluated the risk of bias in the included studies and cross-checked the results. Risk of bias assessment was performed using the tool recommended by the Cochrane Assistance Network (Cai et al., 2021).
Statistical analysis
The meta-analysis was conducted using RevMan 5.4. For dichotomous data, the Mantel-Haenszel method was employed, while the Inverse Variance method was used for continuous data. Mean differences (MDs) and 95% confidence intervals (CIs) were calculated for continuous data, and odds ratios (ORs) with 95% CIs were calculated for dichotomous data. The I2 statistic and Q test were used to evaluate statistical heterogeneity. I2 ≤ 50%, P > 0.05 indicated low heterogeneity, while higher values suggested substantial heterogeneity. Potential study bias was assessed using funnel plots.
Results
Search results and characteristics of the included studies
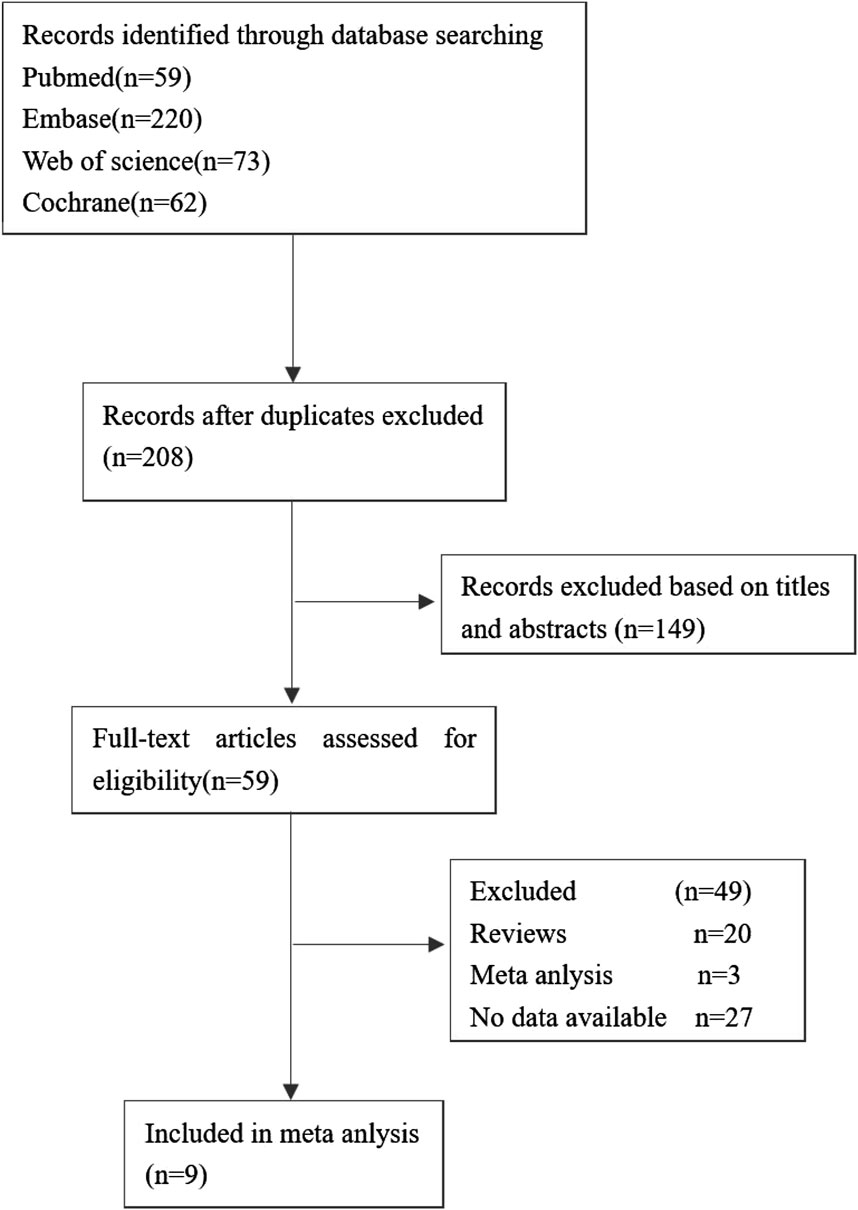
The flow of studies through the analysis is presented in Figure 1. 9 eligible studies involving 4,118 patients were enrolled in our study. The characteristics of the included studies are described in Table 2.
Results of bias risk assessment for included studies
Among the studies included in this meta-analysis, there were 16 experiments in 9 studies most experiments with high quality and only minimal risk of bias. Among which 8 experiments scored 6points, only 2 experiments scored 2points (Figure 2).
Analysis of outcomes
Score of WI-NRS improvement ≥3-Point
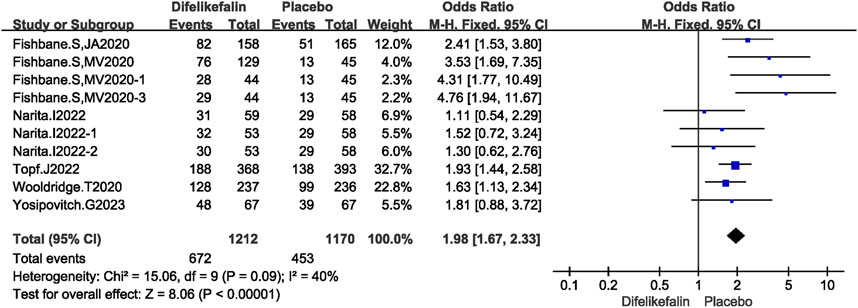
Six research, comprising 10 experiments, examined the improvement in WI-NRS scores of at least three points from the baseline. The I2 test revealed a value of 40%, which is less than the threshold of 50%, showing the presence of mild heterogeneity among the studies. Similarly, the Q test demonstrated a value of 0.09, which is greater than the threshold of 0.05, further confirming the presence of slight heterogeneity. The data was pooled using the fixed-effects model, resulting in an odds ratio (OR) of 1.98 (95% confidence interval [CI] 1.67–2.33, Z = 8.06, P < 0.00001) (Figure 3).
Score of WI-NRS improvement ≥4-Point
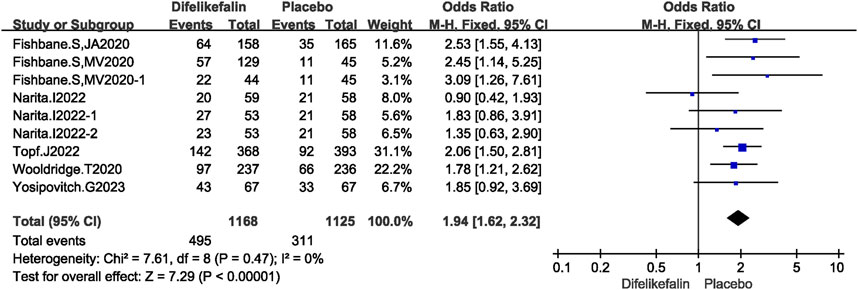
Six studies including nine experiments analyzed the score of WI-NRS improvement ≥4-point from baseline, The I2 test showed I2 = 0% < 50%, and Q test showed P = 0.47 > 0.05, indicating that no heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding an OR of 1.94 (95% CI 1.62–2.32, Z = 7.29, P < 0.00001) (Figure 4).
5-D itch scale total score decreases
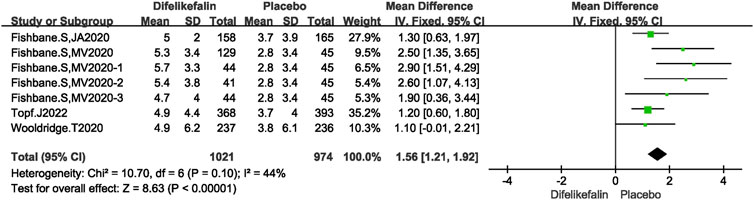
Four research, consisting of seven experiments, examined the decrease in total scores on the 5-D itch scale. The I2 test revealed an I2 value of 44% which is less than 50%, and the Q test indicated a P-value of 0.1 which is greater than 0.05. This suggests that there was a modest level of heterogeneity among the studies. The data was pooled using the fixed-effects model, resulting in a mean difference (MD) of 1.56 (95% confidence interval [CI] 1.21–1.92, Z = 8.63, P < 0.00001) (Figure 5).
Skindex-10 scale score decrease
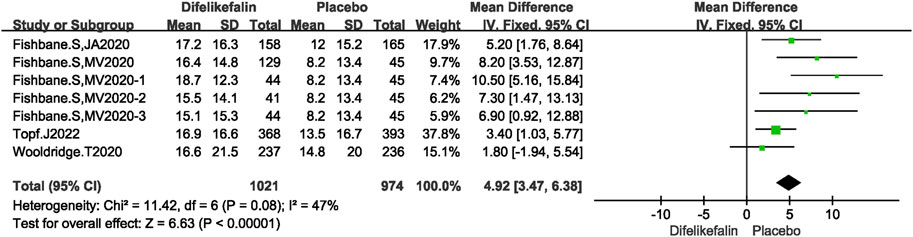
Four studies including seven experiments analyzed the skindex-10 scale score decrease, The I2 test showed I2 = 47% < 50%, and Q test showed P = 0.08 > 0.05, indicating that slight heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding a MD of 4.92 (95% CI 3.47–6.38, Z = 6.63, P < 0.00001) (Figure 6).
WI-NRS scale score decrease
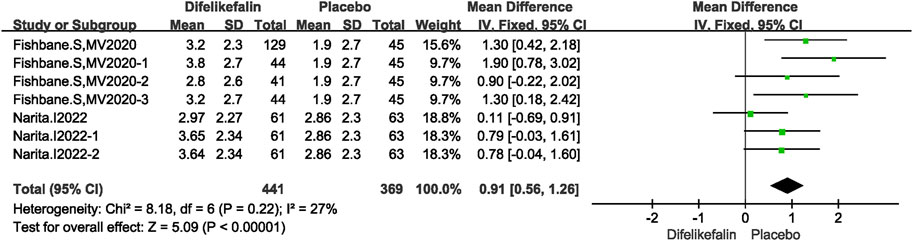
Two studies including seven experiments analyzed the WI-NRS scale score decrease, The I2 test showed I2 = 27% < 50%, and Q test showed P = 0.22 > 0.05, indicating that slight heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding a MD of 0.91 (95% CI 0.56–1.26, Z = 5.09, P < 0.00001) (Figure 7).
Analysis of safety
Any TEAE reported
The incidence of adverse events was greater in the difelikefalin group compared to the placebo group. Six research, including twelve experiments, were examined to determine the occurrence of adverse events. The I2 test revealed an I2 value of 56%, which is greater than the threshold of 50%, suggesting the presence of heterogeneity among the studies. Additionally, the Q test demonstrated a P-value of 0.01, which is less than the significance level of 0.05. After conducting the sensitivity analysis, eleven experiments were included. The I2 test showed I2 = 34% < 50%, and Q test showed P = 0.13 > 0.05, indicating that slight heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding an OR of 1.44 (95% CI 1.22–1.69, Z = 4.41, P < 0.0001) (Figure 8). Common adverse reactions include diarrhea, dizziness, nausea, headache.
Any serious TEAE reported
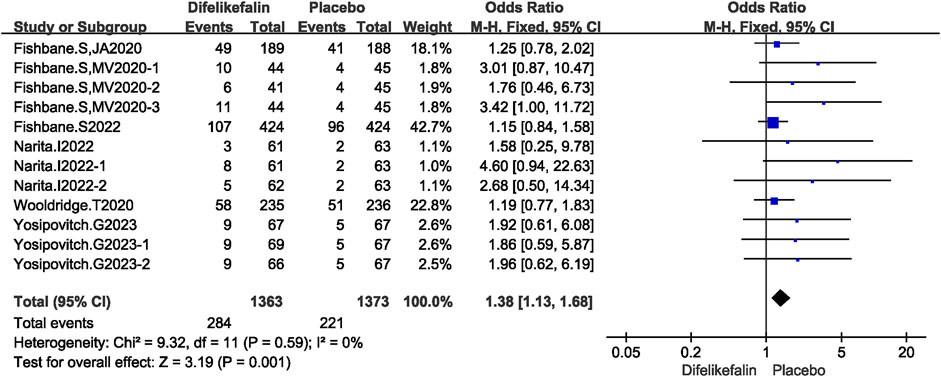
Six studies including twelve experiments analyzed incidence of serious adverse events, The I2 test showed I2 = 0% < 50%, and Q test showed P = 0.59 > 0.05, indicating that no heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding an OR of 1.38 (95% CI 1.13–1.68, Z = 3.19, P = 0.001) (Figure 9). Incidence of serious adverse events were higher in the difelikefalin group than in the placebo group.
Deaths
Six studies including twelve experiments analyzed incidence of deaths, The I2 test showed I2 = 0% < 50%, and Q test showed P = 0.86 > 0.05, indicating that no heterogeneity existed among the studies. The fixed-effects model was used to pool the data, yielding an OR of 0.55 (95% CI 0.28–1.11, Z = 1.66, P = 0.10) (Figure 10). There was no significant difference in mortality between the two groups.
Bias assessment
Finally, funnel plots were constructed to qualitatively analyze the publication bias among the studies included. The score of WI-NRS improvement ≥3-point from baseline between difelikefalin and placebo group was used as an example. The funnel plots displayed symmetrical distributions, with no obvious publication bias (Figure 11).
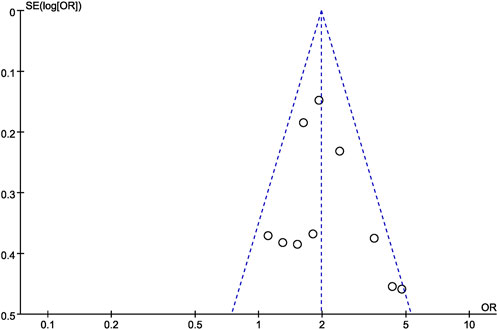
Figure 11. Funnel plot for the score of WI-NRS improvement ≥3-Point from baseline between difelikefalin and placebo group.
Discussion
The pathogenesis of uremic pruritus has not been completely understood. Many theories have been proposed in numerous studies to explain it. Th1 cells, serum C-reactive protein (CRP), interleukin (IL)-6, and IL-2 levels have been found to be significantly raised in these patients, supporting the significance of inflammation in uremic pruritus (Agarwal et al., 2021). A theory of uremic pruritus pathogenesis implicated toxins in the skin and subcutaneous tissue. Proposed toxins included “uremic toxins,” vitamin A, aluminum, calcium, phosphorus, and magnesium (Verduzco and Shirazian, 2020). One study point that a metabolomic analysis of hemodialysis patients did not identify any solutes associated with pruritus. A role for uremic solutes in pruritus remains to be established (Bolanos et al., 2021). In a study with CKD stage 3–5 managed without dialysis, the authors found that those with moderate to severe pruritus had dry skin as compared to others (Sukul et al., 2019). Yosipovitch et al. (2007) however did not find an association. Szepietowski et al. (2004) have, at least partially, eliminated xerosis as causative of pruritus. Xerosis therefore is more likely to be an exacerbating rather than a causative factor (Makar et al., 2021). One hypothesis implicating an imbalance of opioid system had been proposed, and it emphasized that µ-opioid receptor activation and κ-opioid receptor blockade leading to pruritogenic nerve signaling and pruritogenic cytokines release (Zhang et al., 2023). The lower expression of κ-opioid receptor in uremic pruritus suggests that the peripheral opioid system plays an important role in uremic pruritus (Ko et al., 2023).
The kappa opioid receptor (KOR) is a member of the G-protein-coupled receptor family and its natural endogenous ligand is dynorphin, which decreases synaptic transmission by inhibiting adenylate cyclase and voltage-gated calcium channels and activating voltage-gated potassium channels, resulting in decreased neuronal action potential production and neurotransmitter release (Beck et al., 2019; Zhou et al., 2022). KORs play a critical role in modulating dopamine, serotonin, and glutamate release in the central nervous system. KOR has been implicated in several psychiatric diseases, including schizophrenia, depression, bipolardisorder, and drug addiction (Clark and Abi-Dargham, 2019). Although activation of KOR can inhibit itching, the clinical utility of KOR agonists has been hindered by their dysphoric/psychotomimetic effects, which have been shown to be mediated by activation of central KORs and a downstream beta-arrestin signaling pathway. To avoid producing those adverse effects, novel KOR agonists have been developed through strategies involving G-protein-biased signaling and peripheral restriction. Difelikefalin is a peripheral kappa-opioid receptor agonist that acts primarily on peripheral neurons and cells of the immune system (Fotheringham et al., 2024). Activation of opioid receptors in peripheral neurons reduces afferent impulses to the central nervous system and reduces itching signals. Activating kappa opioid receptors on immune cells, decreases the release of pro-inflammatory chemicals such as IL-6, IL-2 and prostaglandins (Trachtenberg et al., 2020). Difelikefalin is not able to cross the blood-brain barrier due to its small hydrophilic peptide structure. Therefore, unlike many other opioid medications, it does not cause lethal central nervous system side effects such as respiratory depression (Viscusi et al., 2021). Difelikefalin has no action at the mu-opioid receptor, which is responsible for the euphoric effects of traditional opioid medications, so there is negligible abuse potential for this novel agent (Inan and Cowan, 2022). Following a successful phase 3 clinical trial, the FDA has approved the first selective KOR agonist in the US, difelikefalin, which is a peripherally restricted KOR agonist used for treatment-resistant pruritis in patients undergoing hemodialysis.
In recent years, numerous clinical trials have been conducted to evaluate the efficacy and safety of difelikefalin in hemodialysis patients with persistent pruritus. Our study included 4,118 subjects in 9 studies and explored the efficacy and safety of difelikefalin in the treatment of pruritus in hemodialysis patients. To our knowledge, only one relevant meta-analysis has been published, a total of 4 randomized controlled trials were included, it draws a conclusion that difelikefalin can improve itching symptoms in HD patients, it can also increase adverse reactions (Xue et al., 2024), Consistent with our conclusions. But our study had the largest number of included studies and the largest sample size. In this study, a meta-analysis was used to compare the efficacy and safety of difelikefalin and placebo in the treatment of uremic pruritus, providing evidence for clinical use. In the studies, several scales were used to assess the severity of itching, which allowed us to assess the effectiveness of difelikefalin and to compare the results of numerous studies with each other. By comparing the decrease of score of WI-NRS, 5-D itch scale total score and Skindex-10 scale score, difelikefalin can effectively improve the itching symptoms of patients with uremic pruritus. One systematic review shows that difelikefalin, due to its efficacy and good safety profile, can be regarded as the primary treatment for pruritus in patients with chronic kidney disease (Wala and Szepietowski, 2022). Narita I. et al. confirmed that intravenous difelikefalin reduced itching and improved quality of life in patients with moderate to severe pruritus who were undergoing maintenance hemodialysis (Narita et al., 2023). Although difelikefalin can increase adverse reactions, it was well tolerated in participants undergoing HD. Dizziness, Diarrhea, nausea, and headache, which are among the most common TEAEs with difelikefalin. Their incidence was slightly higher than in the placebo group, but not significantly. The risk of death was not statistically different between the two groups. Kraft L. et al. concluded that difelikefalin is effective in the treatment of uremic pruritus, and adverse events were mostly mild in their study population (primarily dizziness, diarrhea and headache) (Kraft et al., 2023). A single-dose, phase 1 study was conducted in healthy subjects and subjects on HD, difelikefalin appeared to have an acceptable safety and tolerability profile with no serious AEs reported. The most common TEAEs were dizziness, headache, paresthesia, and nausea. The majority of TEAEs were reported as mild and considered unrelated treatment (Stark et al., 2023). In a treatment atopic dermatitis’s study, 181 subjects (45.1%) reported 1 or more treatment emergent TEAEs, most were mild or moderate. The most reported TEAEs (>5% of subjects) were abdominal pain/discomfort, nausea, dry mouth, headache, dizziness, and hypertension (Guttman-Yassky et al., 2023).
Conclusion
Difelikefalin can effectively improve pruritus in patients with uremia. It can also increase adverse reactions; adverse events were mostly mild. The overall quality assessment of the included studies was satisfactory, but some of the included studies were biased by random assignment or blind method. Due to the small sample size of inclusion, further evidence is needed. Indeed, there is no long-term efficacy and safety of difelikefalin. Large, multicenter, high-quality RCTS will be required to provide a basis for clinical drug use.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XC: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft, Writing–review and editing. GW: Data curation, Investigation, Methodology, Validation, Writing–review and editing. YL: Project administration, Software, Supervision, Writing–review and editing. LY: Conceptualization, Data curation, Project administration, Resources, Visualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, P., Garg, V., Karagaiah, P., Szepietowski, J. C., Grabbe, S., and Goldust, M. (2021). Chronic kidney disease-associated pruritus. Toxins (Basel) 13 (8), 527. doi:10.3390/toxins13080527
Aresi, G., Rayner, H. C., Hassan, L., Burton, J. O., Mitra, S., Sanders, C., et al. (2019). Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J. Pain Symptom Manage 58 (4), 578–586.e2. doi:10.1016/j.jpainsymman.2019.06.010
Beck, T. C., Reichel, C. M., Helke, K. L., Bhadsavle, S. S., and Dix, T. A. (2019). Non-addictive orally active kappa opioid agonists for the treatment of peripheral pain in rats. Eur. J. Pharmacol. 856, 172396. doi:10.1016/j.ejphar.2019.05.025
Bolanos, C. G., Pham, N. M., Mair, R. D., Meyer, T. W., and Sirich, T. L. (2021). Metabolomic analysis of uremic pruritus in patients on hemodialysis. PLoS One 16 (2), e0246765. doi:10.1371/journal.pone.0246765
Cai, X. Y., Wu, G. M., Zhang, J., and Yang, L. C. (2021). Risk factors for acute kidney injury in adult patients with COVID-19: a systematic review and meta-analysis. Front. Med. (Lausanne) 8, 719472. doi:10.3389/fmed.2021.719472
Clark, S. D., and Abi-Dargham, A. (2019). The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: a review of the evidence. Biol. Psychiatry 86 (7), 502–511. doi:10.1016/j.biopsych.2019.05.012
Eusebio-Alpapara, K. M. V., Castillo, R. L., and Dofitas, B. L. (2020). Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Int. J. Dermatol 59 (4), 412–422. doi:10.1111/ijd.14708
Fishbane, S., Jamal, A., Munera, C., Wen, W., and Menzaghi, F.KALM-1 Trial Investigators (2020a). A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N. Engl. J. Med. 382 (3), 222–232. doi:10.1056/NEJMoa1912770
Fishbane, S., Mathur, V., Germain, M. J., Shirazian, S., Bhaduri, S., Munera, C., et al. (2020b). Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int. Rep. 5, 600–610. doi:10.1016/j.ekir.2020.01.006
Fishbane, S., Wen, W., Munera, C., Lin, R., Bagal, S., McCafferty, K., et al. (2022). Safety and tolerability of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis from the phase 3 clinical trial program. Kidney Med. 4 (8), 100513. doi:10.1016/j.xkme.2022.100513
Fotheringham, J., Guest, J., Latus, J., Lerma, E., Morin, I., Schaufler, T., et al. (2024). Impact of difelikefalin on the health-related quality of life of haemodialysis patients with moderate to severe chronic kidney disease associated pruritus: a single arm intervention trial. Patient 17 (2), 203–213. doi:10.1007/s40271-023-00668-1
Guttman-Yassky, E., Facheris, P., Rosa, J. C. D., Rothenberg-Lausell, C., Del Duca, E., David, E., et al. (2023). Oral difelikefalin reduces moderate to severe pruritus and expression of pruritic and inflammatory biomarkers in subjects with atopic dermatitis. J. Allergy Clin. Immunol. 152 (4), 916–926. doi:10.1016/j.jaci.2023.06.023
Hercz, D., Jiang, S. H., and Webster, A. C. (2020). Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst. Rev. 12 (12), CD011393. doi:10.1002/14651858.CD011393.pub2
Inan, S., and Cowan, A. (2022). Antipruritic effects of kappa opioid receptor agonists: evidence from rodents to humans. Handb. Exp. Pharmacol. 271, 275–292. doi:10.1007/164_2020_420
Ko, M. J., Peng, Y. S., and Wu, H. Y. (2023). Uremic pruritus: pathophysiology, clinical presentation, and treatments. Kidney Res. Clin. Pract. 42 (1), 39–52. doi:10.23876/j.krcp.21.189
Kraft, L., Schanz, M., Schricker, S., Ketteler, M., Mayer, G., Rostoker, G., et al. (2023). The first real-world experience of IV difelikefalin to treat chronic kidney disease-associated pruritus in haemodialysis patients. J. Eur. Acad. Dermatol Venereol. 5. doi:10.1111/jdv.19105
Kuypers, D. R., Claes, K., Evenepoel, P., Maes, B., and Vanrenterghem, Y. (2004). A prospective proof of concept study of the efficacy of tacrolimus ointment on uraemic pruritus (UP) in patients on chronic dialysis therapy. Nephrol. Dial. Transpl. 19 (7), 1895–1901. doi:10.1093/ndt/gfh202
Lu, P. H., Tai, Y. C., Yu, M. C., Lin, I. H., and Kuo, K. L. (2021). Western and complementary alternative medicine treatment of uremic pruritus: a literature review. Tzu Chi Med. J. 33, 350–358. doi:10.4103/tcmj.tcmj_151_20
Lu, P. H., Yao, X. F., Lin, Y. S., Tzeng, I. S., and Kuo, K. L. (2022). Omega-3 fatty acids for uremic pruritus: a meta-analysis of randomized controlled trials. Tzu Chi Med. J. 34 (4), 394–401. doi:10.4103/tcmj.tcmj_221_21
Makar, M., Smyth, B., and Brennan, F. (2021). Chronic kidney disease-associated pruritus: a review. Kidney Blood Press Res. 46 (6), 659–669. doi:10.1159/000518391
Martin, C. E., Clotet-Freixas, S., Farragher, J. F., and Hundemer, G. L. (2020). Have we just scratched the surface? A narrative review of uremic pruritus in 2020. Can. J. Kidney Health Dis. 7, 2054358120954024. doi:10.1177/2054358120954024
Menzaghi, F., Spencer, R., Abrouk, N., Lewis, M., and Chalmers, D. (2015). (422) CR845, a peripheral Kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy. J. Pain 16 (4), S81. doi:10.1016/j.jpain.2015.01.341
Mettang, T., and Kremer, A. E. (2015). Uremicpruritus. KidneyInt 87 (4), 685–691. doi:10.1038/ki.2013.454
Narita, I., Tsubakihara, Y., Takahashi, N., Ebata, T., Uchiyama, T., Marumo, M., et al. (2023). Difelikefalin for hemodialysis patients with pruritus in Japan. NEJM Evid. 2 (11), EVIDoa2300094. doi:10.1056/EVIDoa2300094
Narita, I., Tsubakihara, Y., Uchiyama, T., Okamura, S., Oya, N., Takahashi, N., et al. (2022). Efficacy and safety of difelikefalin in Japanese patients with moderate to severe pruritus receiving hemodialysis a randomized clinical trial. JAMA Netw. Open 5 (5), e2210339. doi:10.1001/jamanetworkopen.2022.10339
Rayner, H. C., Larkina, M., Wang, M. A., Graham-Brown, M., van der Veer, S. N., Ecder, T., et al. (2017). International comparisons of prevalence,awareness,and treatment of pruritus in people on hemodialysis. Clin. J. Am. Soc. Nephrol. 12 (12), 2000–2007. doi:10.2215/CJN.03280317
Satti, M. Z., Arshad, D., Javed, H., Shahroz, A., Tahir, Z., Ahmed, M. M. H., et al. (2019). Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus 11 (7), e5178. doi:10.7759/cureus.5178
Simonsen, E., Komenda, P., Lerner, B., Askin, N., Bohm, C., Shaw, J., et al. (2017). Treatment of uremic pruritus: a systematic review. Am. J. Kidney Dis. 70 (5), 638–655. doi:10.1053/j.ajkd.2017.05.018
Spencer, R. H., Munera, C., Shram, M. J., and Menzaghi, F. (2023). Assessment of the physical dependence potential of difelikefalin: randomized placebo-controlled study in patients receiving hemodialysis. Clin. Transl. Sci. 16 (9), 1559–1568. doi:10.1111/cts.13538
Stark, J. G., Noonan, P. K., Spencer, R. H., Bhaduri, S., O'Connor, S. J., and Menzaghi, F. (2023). Pharmacokinetics, metabolism, and excretion of intravenous [14C]difelikefalin in healthy subjects and subjects on hemodialysis. Clin. Pharmacokinet. 62 (9), 1231–1241. doi:10.1007/s40262-023-01262-2
Sukul, N., Karaboyas, A., Csomor, P. A., Schaufler, T., Wen, W., Menzaghi, F., et al. (2021). Self-reported pruritus, and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 3 (1), 42–53.e1. doi:10.1016/j.xkme.2020.08.011
Sukul, N., Speyer, E., Tu, C., Bieber, B. A., Li, Y., Lopes, A. A., et al. (2019). Pruritus and patient reported outcomes in non-dialysis CKD. J. Am. Soc. Nephrol. 14, 673–681. doi:10.2215/CJN.09600818
Szepietowski, J. C., Reich, A., and Schwartz, R. A. (2004). Uraemic xerosis. Nephrol. Dial. Transpl. 19 (11), 2709–2712. doi:10.1093/ndt/gfh480
Topf, J., Wooldridge, T., McCafferty, K., Schömig, M., Csiky, B., Zwiech, R., et al. (2022). Efficacy of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 phase 3 studies. Kidney Med. 4 (8), 100512. doi:10.1016/j.xkme.2022.100512
Trachtenberg, A. J., Collister, D., and Rigatto, C. (2020). Recent advances in the treatment of uremic pruritus. Curr. Opin. Nephrol. Hypertens. 29 (5), 465–470. doi:10.1097/MNH.0000000000000625
Verduzco, H. A., and Shirazian, S. (2020). CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int. Rep. 5 (9), 1387–1402. doi:10.1016/j.ekir.2020.04.027
Viscusi, E. R., Torjman, M. C., Munera, C. L., Stauffer, J. W., Setnik, B. S., and Bagal, S. N. (2021). Effect of difelikefalin, a selective kappa opioid receptor agonist, on respiratory depression: a randomized, double-blind, placebo-controlled trial. Clin. Transl. Sci. 14, 1886–1893. doi:10.1111/cts.13042
Wala, K., and Szepietowski, J. C. (2022). Difelikefalin in the treatment of chronic kidney disease-associated pruritus: a systematic review. Pharm. (Basel) 15 (8), 934. doi:10.3390/ph15080934
Weiner, D. E., Schaufler, T., McCafferty, K., Kalantar-Zadeh, K., Germain, M., Ruessmann, D., et al. (2023). Difelikefalin improves itch-related sleep disruption in patients undergoing haemodialysis. Nephrol. Dial. Transpl. 15, 1125–1137. doi:10.1093/ndt/gfad245
Wooldridge, T., Mccafferty, K., Schoemig, M., Csiky, B. S., Zwiech, R., Wen, W., et al. (2020). Efficacy and safety of difelikefalin for moderate-to-severe CKD-associated pruritus: a global phase 3 study in hemodialysis patients (KALM-2): FR-OR24. J. Am. Soc. Nephrol. 31 (Suppl. l), 22–23. doi:10.1681/asn.20203110s122d
Xue, G. F., Yuan, H. H., Fan, D. D., Yang, Y., Ma, C., Liu, J., et al. (2024). Efficacy and safety of difelikefalin in the treatment of hemodialysis patients with pruritus: a meta-analysis and systematic review. Clin. Nephrol. 101, 155–163. doi:10.5414/CN111169
Yosipovitch, G., Awad, A., Spencer, R. H., Munera, C., and Menzaghi, F. (2023). A phase 2 study of oral difelikefalin in subjects with chronic kidney disease and moderate-to-severe pruritus. J. Am. Acad. Dermatol 89 (2), 261–268. doi:10.1016/j.jaad.2023.03.051
Yosipovitch, G., Duque, M. I., Patel, T. S., Ishiuji, Y., Guzman-Sanchez, D. A., Dawn, A. G., et al. (2007). Skin barrier structure and function and their relationship to pruritus in end-stage renal disease. Nephrol. Dial. Transpl. 22 (11), 3268–3272. doi:10.1093/ndt/gfm375
Zhang, P., Xiang, S. L., Liu, B. C., Wang, X. H., Yang, X. P., Chaoyang Ye, C. Y., et al. (2023). Randomized controlled trial of nalfurafine for refractory pruritus in hemodialysis patients. Ren. Fail 45 (1), 2175590. doi:10.1080/0886022X.2023.2175590
Keywords: difelikefalin, hemodialysis, pruritus, systematic review, meta-analysis
Citation: Cai X, Wu G, Lin Y and Yang L (2024) Difelikefalin in the treatment of hemodialysis patients with pruritus: a systematic review and meta-analysis. Front. Pharmacol. 15:1476587. doi: 10.3389/fphar.2024.1476587
Received: 06 August 2024; Accepted: 22 November 2024;
Published: 06 December 2024.
Edited by:
Matteo Marcello, San Bortolo Hospital, ItalyReviewed by:
Luca Sgarabotto, Azienda ULSS 8 Berica, ItalyDavide Marturano, Azienda ULSS 8 Berica, Italy
Copyright © 2024 Cai, Wu, Lin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lichuan Yang, eWxjZ2hAMTYzLmNvbQ==
 Xiaoyue Cai
Xiaoyue Cai Guiming Wu
Guiming Wu Yan Lin3
Yan Lin3 Lichuan Yang
Lichuan Yang