- Department of Obstetrics and Gynecology, Women’s Hospital of Jiangnan University, Wuxi Maternity and Child Health Care Hospital, Wuxi, China
Background: Research on placental oxidative stress is pivotal for comprehending pregnancy-related physiological changes and disease mechanisms. Despite recent advancements, a comprehensive review of current status, hotspots, and trends remains challenging. This bibliometric study systematically analyzes the evolution of placental oxidative stress research, offering a reference for future studies.
Objective: To conduct a comprehensive bibliometric analysis of the literature on placental oxidative stress to identify research hotspots, trends, and key contributors, thereby providing guidance for future research.
Methods: Relevant data were retrieved from the Web of Science Core Collection database and analyzed using VOSviewer, CiteSpace, and the bibliometrix package. An in-depth analysis of 4,796 publications was conducted, focusing on publication year, country/region, institution, author, journal, references, and keywords. Data collection concluded on 29 April 2024.
Results: A total of 4,796 papers were retrieved from 1,173 journals, authored by 18,835 researchers from 4,257 institutions across 103 countries/regions. From 1991 to 2023, annual publications on placental oxidative stress increased from 7 to 359. The United States (1,222 publications, 64,158 citations), the University of Cambridge (125 publications, 13,562 citations), and Graham J. Burton (73 publications, 11,182 citations) were the most productive country, institution, and author, respectively. The journal Placenta had the highest number of publications (329) and citations (17,152), followed by the International Journal of Molecular Sciences (122 publications). The most frequent keywords were “oxidative stress,” “expression,” “pregnancy,” “preeclampsia,” and “lipid peroxidation.” Emerging high-frequency keywords included “gestational diabetes mellitus,” “health,” “autophagy,” “pathophysiology,” “infection,” “preterm birth,” “stem cell,” and “inflammation.”
Conclusion: Over the past 3 decades, research has concentrated on oxidative stress processes, antioxidant mechanisms, pregnancy-related diseases, and gene expression regulation. Current research frontiers involve exploring pathophysiology and mechanisms, assessing emerging risk factors and environmental impacts, advancing cell biology and stem cell research, and understanding the complex interactions of inflammation and immune regulation. These studies elucidate the mechanisms of placental oxidative stress, offering essential scientific evidence for future intervention strategies, therapeutic approaches, and public health policies.
1 Introduction
Placental oxidative stress has been a focal point in the forefront of biological and medical research. As the key organ facilitating the exchange of substances between the mother and the fetus, the placenta plays a crucial role in maintaining fetal development and maternal health during pregnancy. Research on the placenta encompasses various aspects, including its structure, function, mechanisms of development, and potential complications (Burton and Jauniaux, 2023; Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020). Among these, the study of oxidative stress within the placenta has drawn significant attention (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Myatt and Cui, 2004). Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) and antioxidant defenses, is a condition resulting from the disruption of the cellular and extracellular environment (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Burton and Jauniaux, 2011). This condition is closely associated with numerous pregnancy-related disorders, such as preeclampsia (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Roberts and Cooper, 2001; Steegers et al., 2010), gestational diabetes (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fisher et al., 2021), and intrauterine growth restriction (IUGR) (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Burton and Jauniaux, 2018). Understanding the relationship between oxidative stress and placental function is essential for developing therapeutic strategies to mitigate these adverse outcomes.
Over the past decades, the field of oxidative stress research has made advancements. These progressions are reflected not only in the accumulation of scientific knowledge but also in the continual development of methodological approaches and technological innovations. However, given the vast amount of scientific literature, systematically organizing and analyzing the progress, hotspots, and trends in this field has become a pressing challenge. In this context, bibliometrics offers a novel perspective by employing mathematical and statistical methods to quantitatively analyze scientific literature.
Bibliometric analysis, which involves the quantitative evaluation of published literature, provides valuable insights into the development and trends of a specific research area. By analyzing publication patterns, citation networks, and keyword frequencies, researchers can identify key contributors, emerging themes, and potential gaps in the literature. The objective of this bibliometric study is to perform a comprehensive analysis of the literature on “placental oxidative stress,” uncovering its historical evolution and forecasting future research directions.
We specifically utilized bibliometric methods in conjunction with tools such as the bibliometrix package, CiteSpace, and VOSviewer to analyze publications on “placental oxidative stress” from the Web of Science Core Collection. Our analysis encompasses the distribution of annual publications, countries, institutions, authors, source journals, keyword co-occurrence, and co-citations. The goal of this bibliometric analysis is to gain an in-depth understanding of the current state, hotspots, and future development trends in placental oxidative stress research. This study not only enhances our comprehension of the historical and contemporary landscape of placental oxidative stress research but also provides valuable resources and insights for researchers aiming to navigate and contribute to this dynamic field. Ultimately, our findings aim to guide clinical practice and scientific research in this area.
2 Methods
2.1 Data collection and retrieval strategy
To enhance the representativeness and accessibility of the data, we conducted a literature search in the Web of Science Core Collection on 29 April 2024. Figure 1 illustrates the data collection and retrieval strategy. We specified the search terms using the “Topic” (TS) field, which encompasses the title, abstract, author keywords, and Keywords Plus. The search query was structured as follows: TS = (Placenta* AND (“Oxidative stress” OR “Reactive oxygen species” OR “ROS” OR “Free radicals” OR “Oxidative damage” OR “Oxidative injury” OR “Oxidative imbalance” OR “Antioxidant defense” OR “Redox imbalance” OR “Oxidative markers” OR “Lipid peroxidation” OR “Protein oxidation” OR “Antioxidant enzymes”)). We limited the publication type to articles and reviews, without imposing any time or language restrictions. A total of 4,796 records were retrieved, encompassing publications, authors, countries, institutions, journals, keywords, and citations. These records were exported in the format of complete records.
2.2 Data analysis
Bibliometric data analysis was conducted using VOSviewer (v1.6.19), CiteSpace (v6.1. R6 Basic), and the bibliometrix package (version 4.1.3) within the R statistical environment (version 4.3.1). Preliminary descriptive statistics on the number of publications and citations per year, country, and author were generated using the bibliometrix package. Additionally, this package was employed to analyze the distribution of publications, collaboration patterns between countries/regions, authors’ productivity over time, the top 10 highly cited references and co-cited references, trend topic analysis, and word cloud visualization. VOSviewer was utilized for data extraction and visualization of countries, institutions, authors, and keywords. CiteSpace was used to analyze country collaborations, perform cluster analysis of co-cited references, create a dual-map overlay of journals related to “placental oxidative stress,” analyze keyword timeline cluster maps, and detect keyword bursts for the top 25 keywords exhibiting the highest burst strength.
3 Results
3.1 Annual global publication outputs on placental oxidative stress
The earliest publication on “placental oxidative stress” dates back to 1991, with a total of 4,796 articles identified (Figure 2A). Annual publication trends reveal an 8.33% growth rate, indicating a steady increase in interest since 1991 (Figure 2B). Publications increased from 7 to 23 between 1991 and 1995, reflecting early academic interest despite low activity. Between 1996 and 2000, publications increased from 31 to 50, reflecting a surge in research interest. From 2001 to 2010, publication growth accelerated, especially after 2008, marking a phase of high-speed development. During 2011 to 2020, publication numbers remained high with fluctuating increases. In 2021, the number of publications peaked at 412, demonstrating high research activity and academic interest. Despite a slight decline from 2022 to 2023, the count remained substantial, indicating sustained interest. The field has been cited 177,497 times, averaging 37.01 citations per article. The stable upward trend in citations over the past 30 years underscores the growing research interest in “placental oxidative stress.” These findings underscore the field’s significance and provide a foundation for future research directions and strategies.
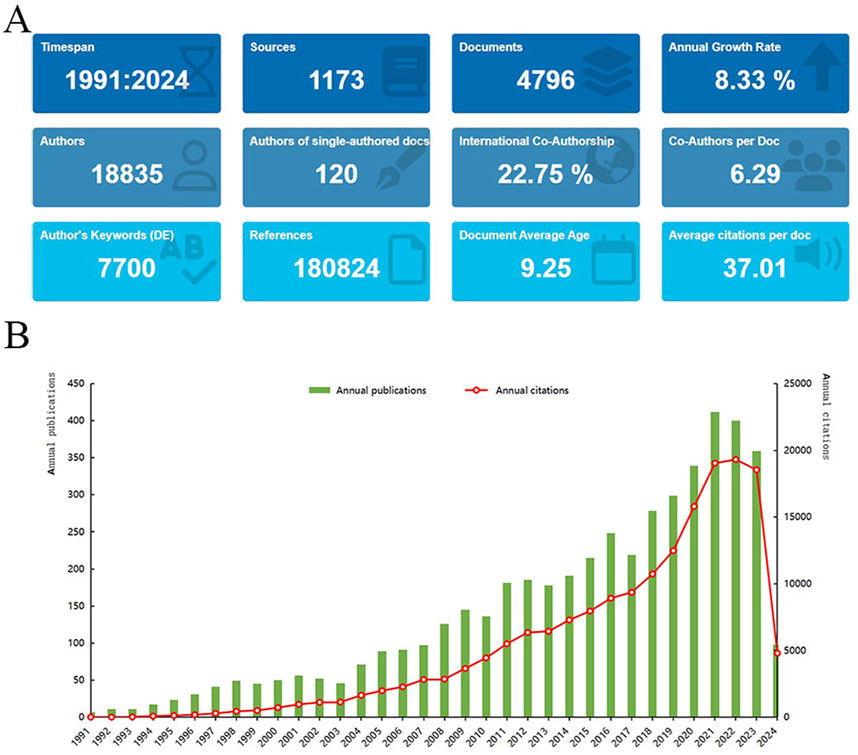
Figure 2. A bibliometric analysis of research on placental oxidative stress. (A) Bibliometric Data Overview. (B) Distribution of “Placental Oxidative Stress” publications over time.
3.2 Distribution and co-authorship of countries/regions
Research on “placental oxidative stress” has emerged as a global hotspot, involving researchers from 103 countries/regions across six continents (Figure 3A). The United States, China, and England lead in publication counts, collectively accounting for over 50% of the total research output, reflecting their significant interest. The H-index rankings also emphasize the United States, England, and Canada as top contributors (Table 1). CiteSpace analysis of international collaboration networks identifies the United States, England, Italy, China, Australia, Japan, and France as central nodes (Figure 3B). VOSviewer analysis of co-authorship, with a minimum of five publications, shows the United States, England, and China dominating in Total Link Strength. England, Canada, and the United States lead in average citations, indicating high research quality and strong international collaboration (Table 1).
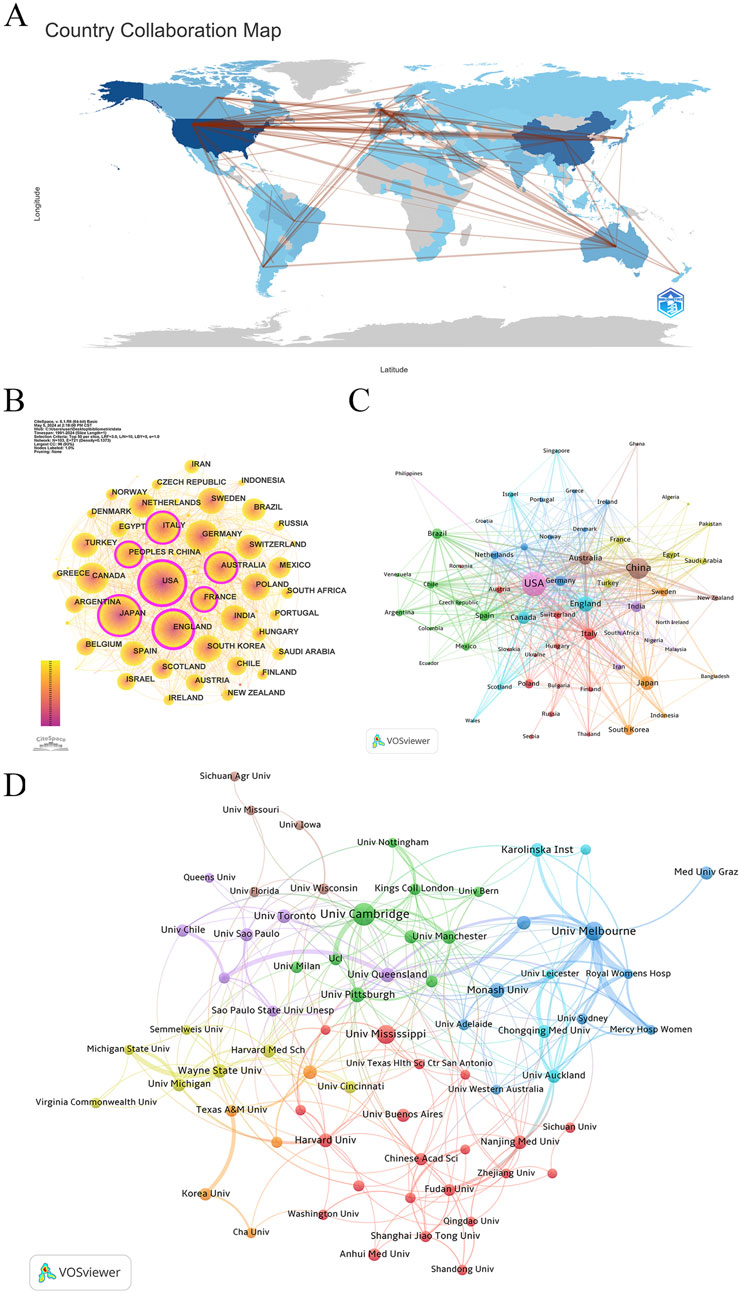
Figure 3. Collaboration network of countries/regions/institutions. (A) Distribution and collaboration of publications among countries/regions. (B) CiteSpace: visualizing clusters of cooperation among countries/regions. (C) VOSviewer: visualizing clusters of cooperation among countries/regions. (D) Visualization map of institutional collaboration.
VOSviewer further classifies countries into nine collaboration clusters based on co-authorship strength (Figure 3C). The United States leads the violet cluster, with strong ties to China, England, Canada, Australia, and the Philippines. China heads the dark brown cluster, collaborating closely with Australia, New Zealand, and Ghana. England’s turquoise cluster includes Canada, Scotland, Israel, Singapore, and Wales. Japan’s orange cluster features collaborations with South Korea, Sweden, Indonesia, and Bangladesh. Italy, Poland, and Switzerland are central to the largest cluster, the dark red cluster, comprising 13 countries. India leads the blue-purple cluster, collaborating with Iran, South Africa, and Nigeria. Brazil forms the green cluster with Spain, Chile, and Mexico. Germany’s deep blue cluster includes the Netherlands, Belgium, and Norway, while Turkey’s dark yellow-green cluster collaborates with France, Egypt, and Saudi Arabia.
These findings underscore the extensive international collaboration and influential contributions in placental oxidative stress research, providing a foundation for future research directions and collaborations.
3.3 Distribution of research institutions and authors
3.3.1 Distribution of research institutions
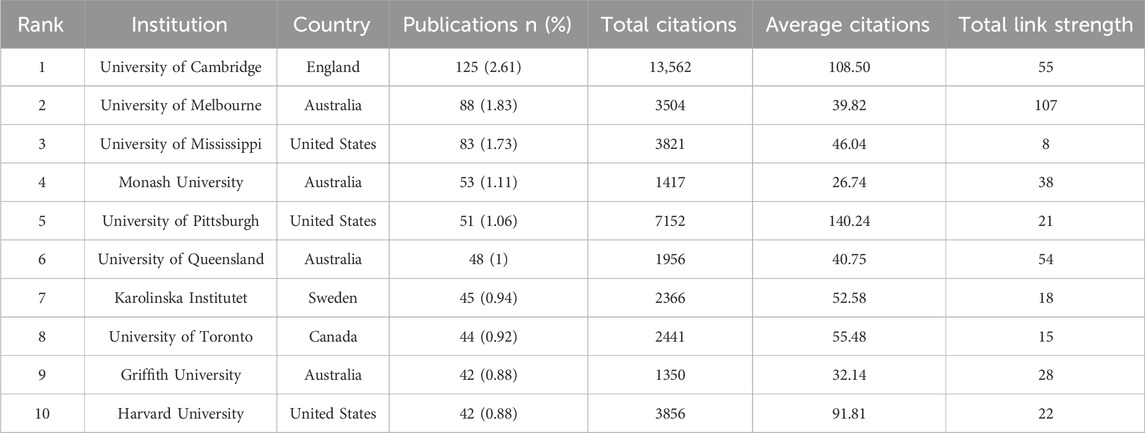
Using VOSviewer, the study analyzed institutional co-authorship in research on placental oxidative stress. The analysis applied “Association Strength” with a minimum document threshold of 20, examining 4,257 institutions, of which 77 met the criteria. Table 2 shows that the University of Cambridge led with 125 papers (2.61%), followed by the University of Melbourne (88 papers, 1.83%), the University of Mississippi (83 papers, 1.73%), Monash University (53 papers, 1.11%), and the University of Pittsburgh (51 papers, 1.06%). Notably, four of the top ten institutions are based in Australia, and three are in the United States. The University of Pittsburgh recorded the highest average citations per paper (140.24), followed by the University of Cambridge (108.50) and Harvard University (91.81). The University of Melbourne exhibited the highest total link strength (107), reflecting its strong collaborative connections within this research field.
Figure 3D categorizes the leading institutions into eight clusters. The green cluster includes the University of Cambridge, the University of Pittsburgh, the University of Manchester, the University of Alberta, and the University of Milan. The dark blue cluster groups Australian institutions such as the University of Melbourne, Monash University, and Griffith University. The dark red cluster features the University of Mississippi, Harvard University, Nanjing Medical University, and the Universidad de Buenos Aires. The blue-purple cluster comprises the University of Queensland, the University of Toronto, the University of São Paulo, and the University of Chile. The blue-green cluster includes Karolinska Institutet, Chongqing Medical University, and the University of Auckland. The dark yellow-green cluster brings together Wayne State University, the University of Michigan, and Harvard Medical School. The orange cluster includes the University of Texas Medical Branch, Korea University, and Southern Medical University. Finally, the dark taupe cluster consists of the University of Wisconsin-Madison, the University of Florida, and the University of Iowa.
These findings highlight the leading institutions and their collaborative networks in placental oxidative stress research, providing valuable insights into the global research landscape and identifying potential opportunities for future collaborations.
3.3.2 Author distribution
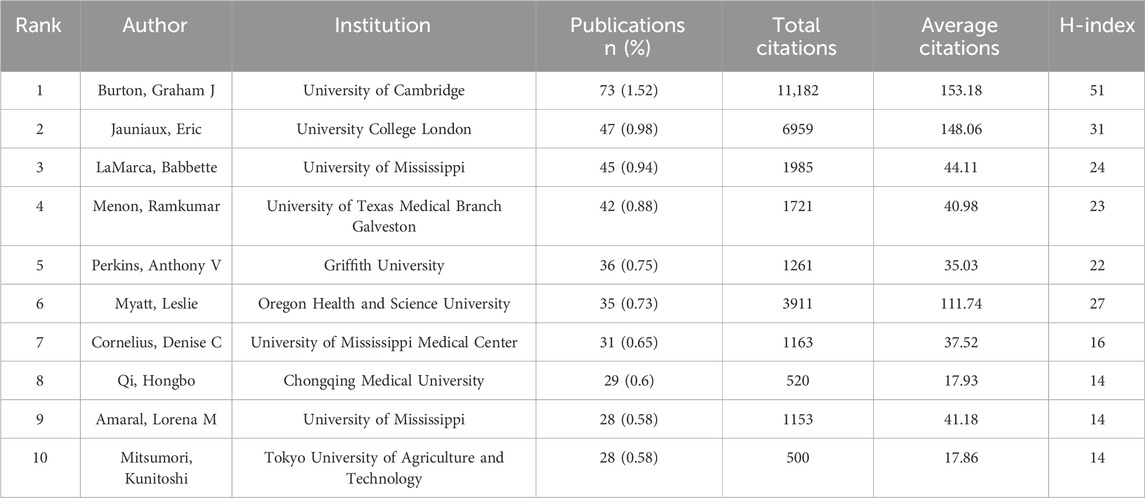
Using the bibliometrix package in R, we analyzed author distribution in placental oxidative stress research. Graham J. Burton from the University of Cambridge emerged as the most prolific author with 73 publications, followed by Eric Jauniaux from University College London with 47 publications, and Babbette LaMarca from the University of Mississippi with 45 publications (Table 3). Graham J. Burton also had the highest average citations and H-index among the top authors.
Analyzing authors’ annual research output revealed that Graham J. Burton, Eric Jauniaux, and Leslie Myatt have consistently contributed to the field over the past 2 decades (Figure 4A). Recent years have seen increased activity from Denise C. Cornelius, Hongbo Qi, and Lorena M. Amaral, indicating significant progress in specific research areas.
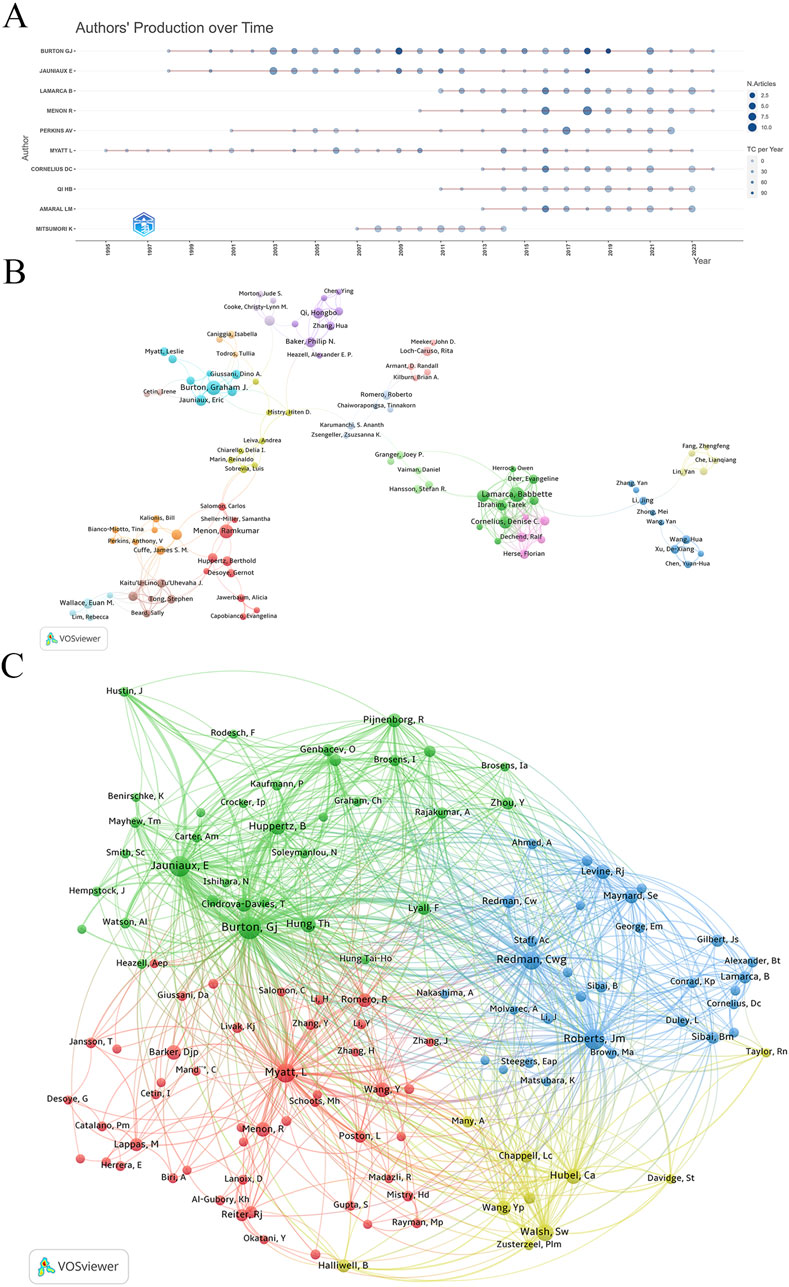
Figure 4. Collaboration network of authors. (A) Authors’ production over time. (B) Visualization map of author collaboration. (C) Co-citation analysis of cited authors.
Using VOSviewer for co-authorship analysis, we set the parameters to “association strength” with a minimum document threshold of 8. Out of 167 qualifying authors, VOSviewer classified them into clusters based on co-authorship frequency and density (Figure 4B). Graham J. Burton has close collaborative ties with Eric Jauniaux, Tereza Cindrova-Davies, Dino A. Giussani, Leslie Myatt, and D. Stephen Charnock-Jones. Another network includes Babbette LaMarca, Denise C. Cornelius, Lorena M. Amaral, Tarek Ibrahim, and Nathan Campbell. Additionally, Anthony V. Perkins collaborates with James S. M. Cuffe, Bill Kalionis, Tina Bianco-Miotto, Jing Li, and Shaun P. Brennecke.
Co-citation analysis identified Graham J. Burton, Eric Jauniaux, James M. Roberts, Leslie Myatt, and Christopher W. Redman as the five most frequently cited authors, highlighting their central roles and substantial influence in the research network (Figure 4C). Their citation counts significantly surpass those of other authors, underscoring their key positions in the field of placental oxidative stress.
3.4 Subject and journal distribution
3.4.1 Subjects
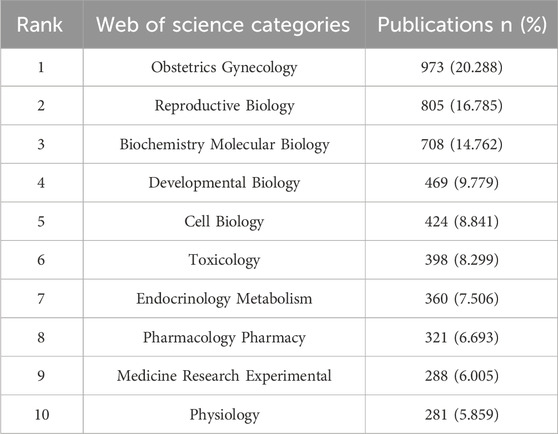
The analysis of publication volume identified Obstetrics and Gynecology, Reproductive Biology, and Biochemistry and Molecular Biology as the top three subjects in placental oxidative stress research (Table 4). Additional key subjects included Developmental Biology, Cell Biology, and Toxicology. These results demonstrated a strong focus on medical and biological disciplines, highlighting the interdisciplinary nature of placental oxidative stress studies and their broad implications for maternal and fetal health.
3.4.2 Journal distribution
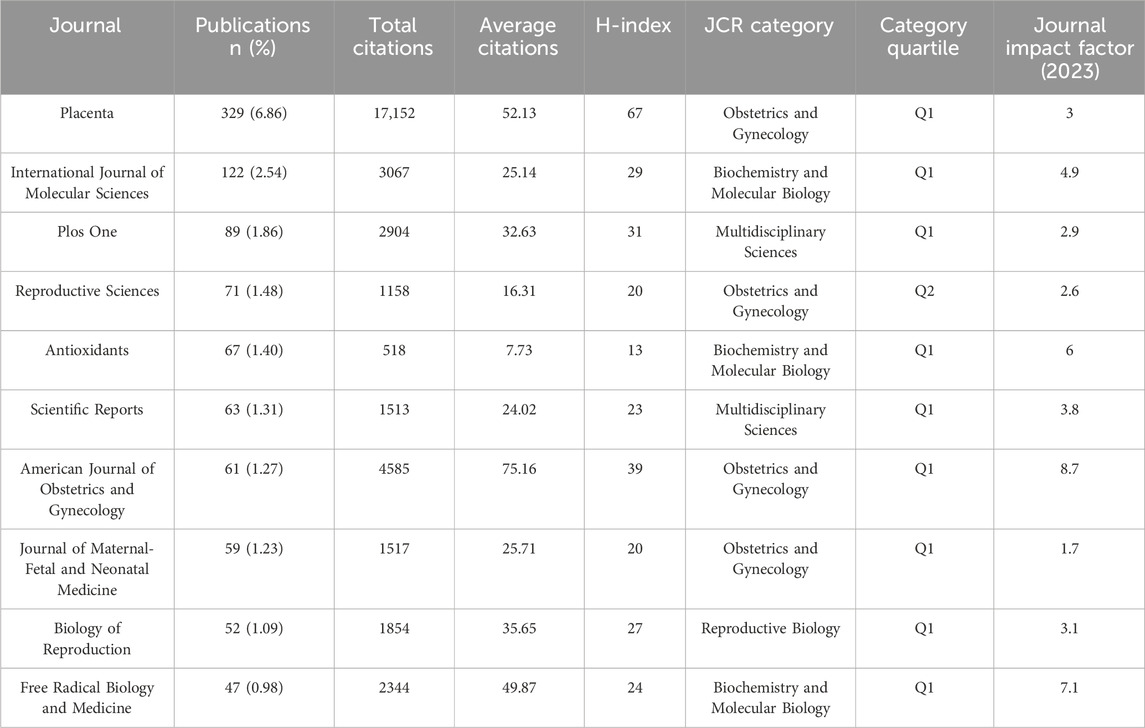
An in-depth analysis of journal distribution was conducted using the bibliometrix package in R, identifying 1,173 journals publishing relevant articles. The top 10 journals with the highest number of publications on “placental oxidative stress” are led by Placenta, with 329 articles, accounting for 6.86% of the total publications (Table 5). Placenta has accumulated 17,152 citations and an H-index of 67, underscoring its significant impact and authoritative status in obstetrics and gynecology. The International Journal of Molecular Sciences follows with 122 publications. Other notable journals include PLOS One, Reproductive Sciences, and Antioxidants, which also exhibit substantial publication volumes and academic influence. The American Journal of Obstetrics and Gynecology, although not at the forefront in publication volume, ranks highly in average citations and has an impact factor of 8.7, reflecting its prominent position in the field.
A co-citation analysis using VOSviewer was conducted to analyze journal relationships (Figure 5A). In the resulting visualization, journals were represented by nodes, and lines illustrated co-citation relationships. Node size corresponded to the number of publications, while the thickness of connecting lines reflected the strength of associations between journals. Stronger connections, indicated by thicker lines, suggested more frequent co-citations and higher similarities in research topics and methodologies.
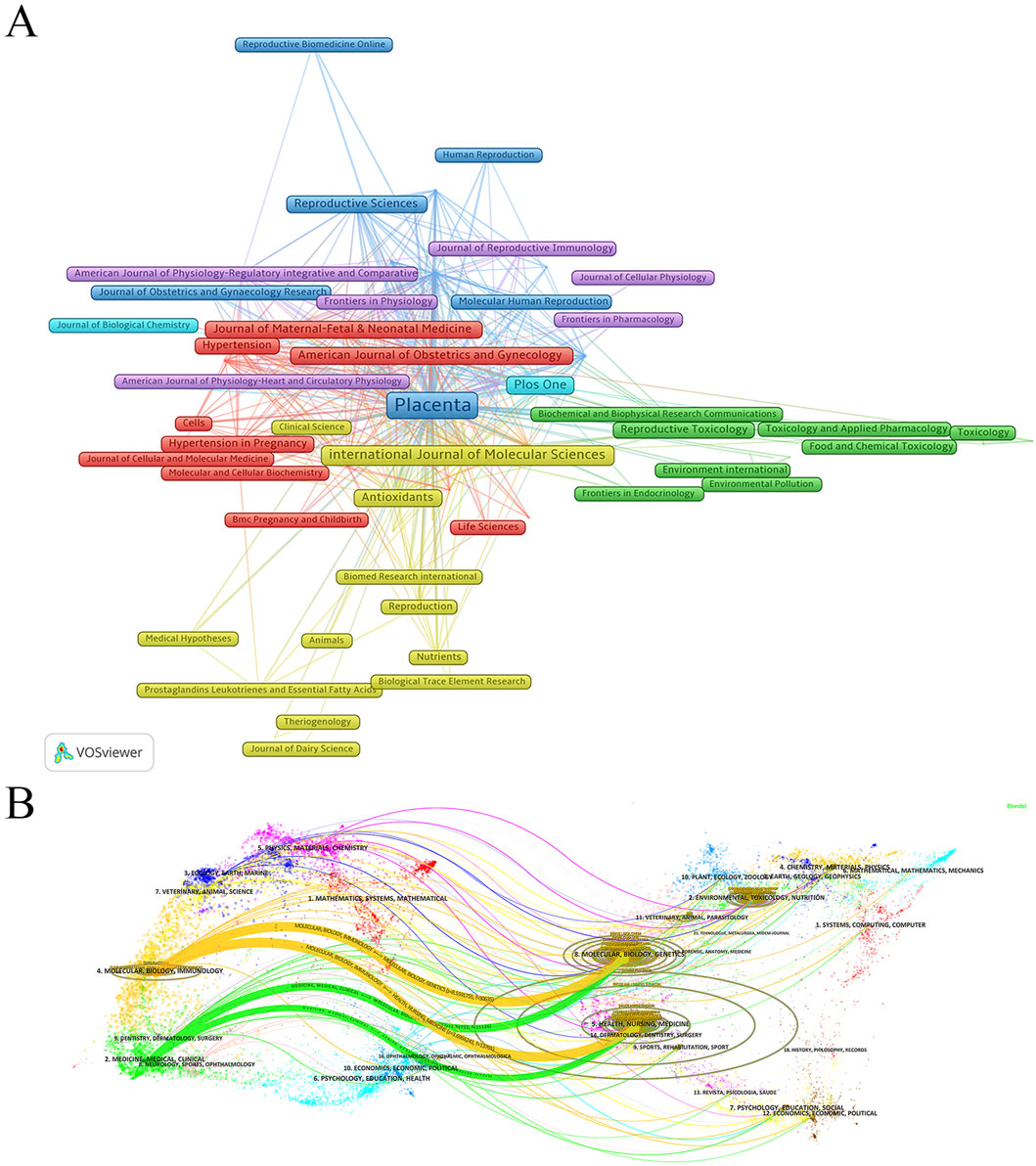
Figure 5. Analysis of journal sources. (A) Co-citation analysis of cited sources. (B) Dual-Map overlay of journals publishing research on Placental Oxidative Stress.
Additionally, a dual-map overlay of journals generated in CiteSpace revealed key details about journal relationships and citation patterns. The left side of the overlay represented citing journals, while the right side showed cited journals, with subject areas and citation paths clearly identified. Two prominent citation pathways, distinguished by orange and green hues, highlighted the prevalent citations extending from journals in fields such as molecular/biology/genetics and health/nursing/medicine, to those in domains like molecular biology/immunology and medicine/clinical medicine (Figure 5B). These findings offered valuable insights into the flow of knowledge and academic influence across disciplines, underscoring the interdisciplinary nature and wide-reaching impact of placental oxidative stress research.
3.4.3 Top 10 highly cited and co-cited articles
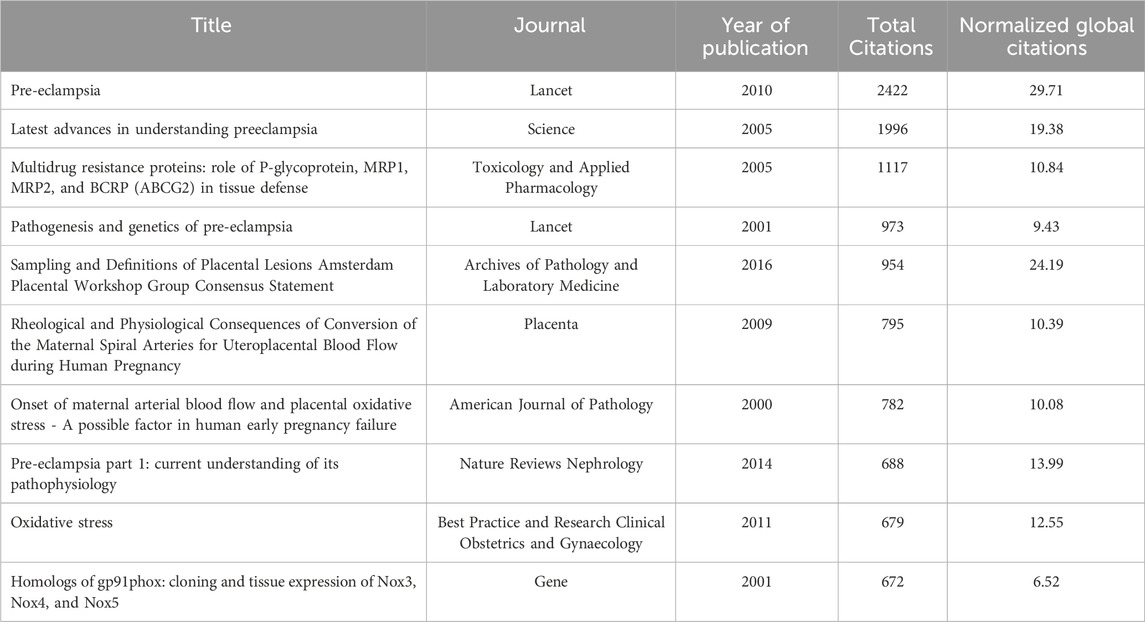
Using the bibliometrix package in R, a comprehensive analysis of cited and co-cited references was conducted, encompassing 4,176 articles with a total of 177,497 citations and a median citation count of 16. Table 6 and Figure 6A detail the top 10 most cited articles, with the 2010 Lancet article “Pre-eclampsia” leading with 2,422 citations and the highest Normalized Global Citations index of 29.71.
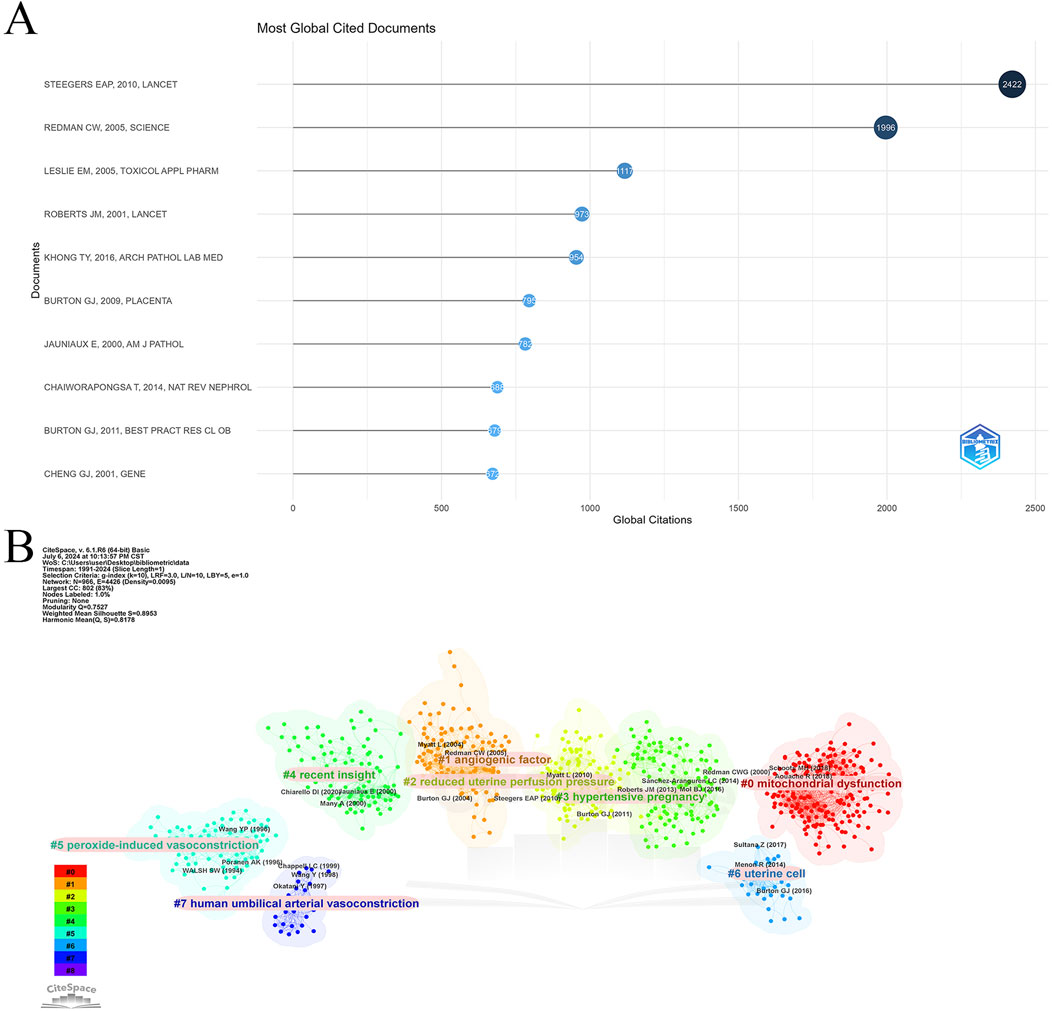
Figure 6. Highly cited and co-cited references. (A) Top 10 most cited articles in Placental oxidative stress. (B) Cluster analysis of co-cited references.
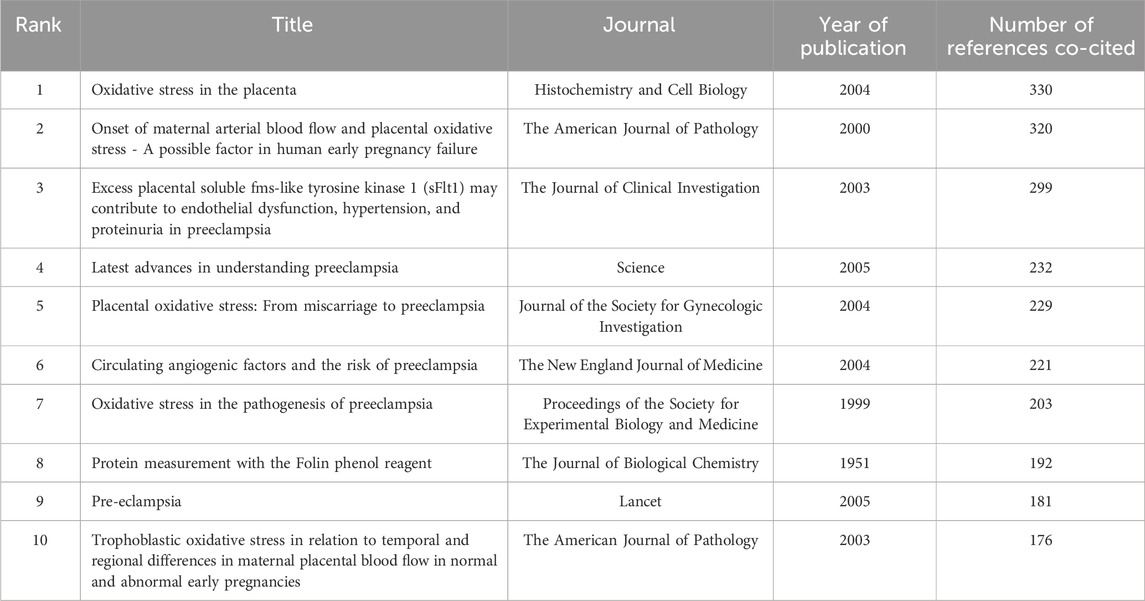
Table 7 lists the top 10 most frequently co-cited references. The article “Oxidative stress in the placenta” is the most co-cited with 330 mentions, followed by “Onset of maternal arterial blood flow and placental oxidative stress” with 320 co-citations, and “Excess placental soluble fms-like tyrosine kinase 1 (sFlt1)” with 299 co-citations. Notably, “Latest advances in understanding preeclampsia” and “Onset of maternal arterial blood flow and placental oxidative stress” are among both the top cited and co-cited references, indicating their foundational impact in the field.
CiteSpace was employed to analyze co-citation relationships (Figure 6B), revealing a network of 966 nodes and 4,426 links, organized into eight major clusters: mitochondrial dysfunction, angiogenic factors, reduced uterine perfusion pressure, hypertensive pregnancy, recent insights, peroxide-induced vasoconstriction, uterine cells, and human umbilical arterial vasoconstriction. This analysis highlights the diverse research themes within placental oxidative stress and emphasizes key areas of ongoing investigation.
3.5 Keyword analysis
3.5.1 Keyword distribution and co-occurrence analysis
The word cloud analysis using the bibliometrix package (Figure 7A) revealed the distribution of keywords, with frequent terms including “oxidative stress,” “expression,” “pregnancy,” “preeclampsia,” “lipid peroxidation,” and “gene expression,” highlighting central research themes in the field. Further analysis using VOSviewer, applying the association strength method with a minimum keyword occurrence threshold of 50, identified 149 relevant keywords from a dataset of 15,440 (Figure 7B). The co-occurrence network showed “Oxidative Stress” as the most frequently mentioned keyword, followed by “Pregnancy” and “Preeclampsia.”
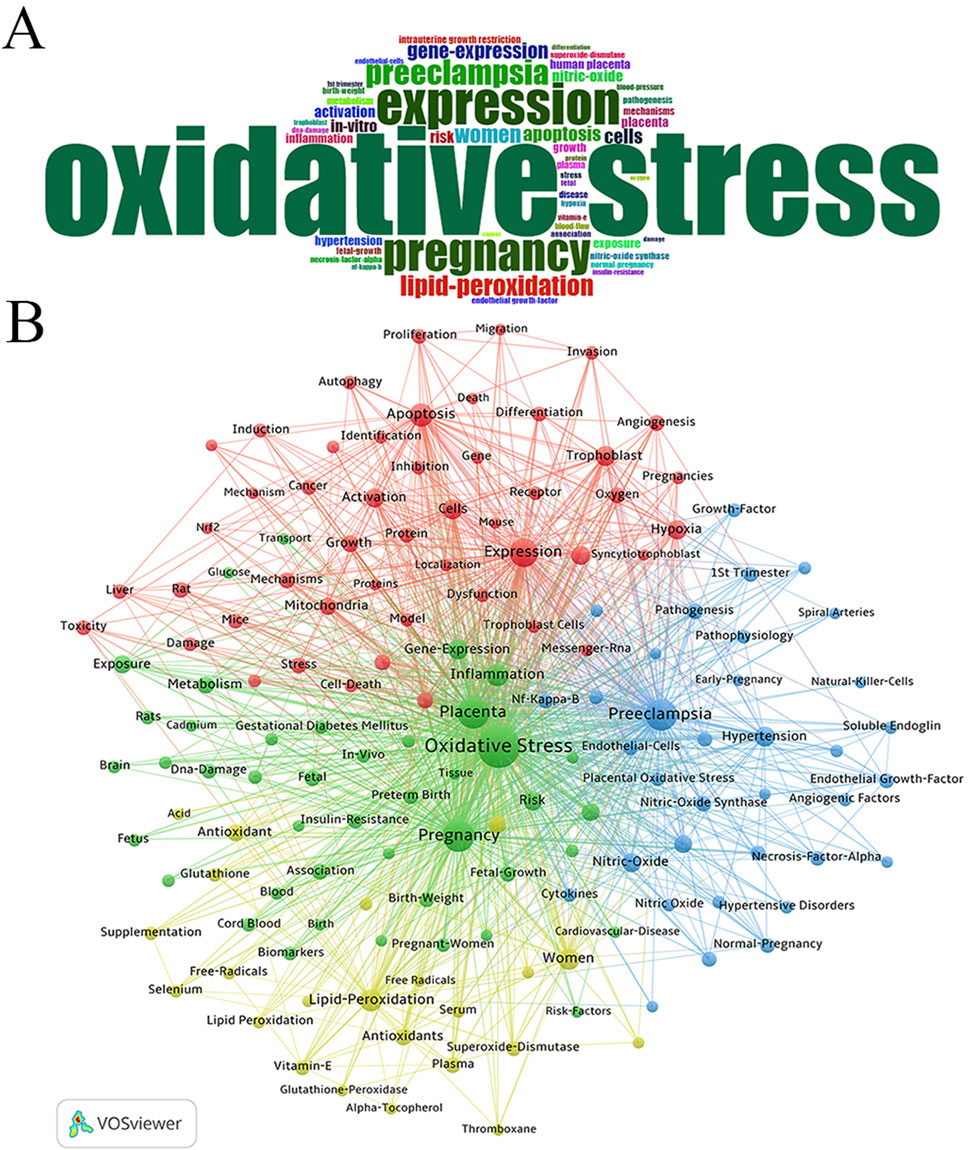
Figure 7. Analysis of keywords associated with Placental Oxidative Stress. (A) Word cloud analysis. (B) Clustering of keywords.
Four main clusters emerged among the top 50 keywords:
Green Cluster: Led by “Oxidative Stress” and including related terms such as Pregnancy, Placenta, Inflammation, Gene Expression, Risk, Intrauterine Growth Restriction, Exposure, and Metabolism.
Blue Cluster: Focused on terms like Preeclampsia, Hypertension, Nitric Oxide, Nitric Oxide Synthase, Pathogenesis, Endothelial Growth Factor, Normal Pregnancy, and Blood Flow. Red Cluster: Centered on terms such as Expression, Apoptosis, Cells, Trophoblast, In Vitro, Hypoxia, Activation, Growth, Mitochondria, Mechanisms, Reactive Oxygen Species, and Stress. Yellow Cluster: Highlighted keywords like Lipid Peroxidation, Women, Human Placenta, Antioxidants, Plasma, Superoxide Dismutase, Vitamin E, and Glutathione.
The analysis emphasized “Oxidative Stress” as a central theme in the field, with strong connections to other key concepts such as pregnancy complications and cellular mechanisms. The cluster distribution reflected major research interests and their interconnections.
3.5.2 Burst detection analysis
The burst detection analysis of keywords over the past 20 years revealed significant frequency increases, highlighting emerging trends in the field. Figure 8A presents the top 25 keywords with the highest burst strength. “Lipid peroxidation” had the strongest burst from 2004 to 2010, followed by key terms such as “vitamin E,” “free radical,” “in vitro,” “messenger RNA,” “gestational diabetes mellitus,” “plasma,” “soluble endoglin,” “human placenta,” and “health.” Keywords like “lipid peroxidation,” “vitamin E,” and “free radical” gained prominence over the past decade, while recent surges in “gestational diabetes mellitus,” “autophagy,” and “pathophysiology” indicate emerging research interests.
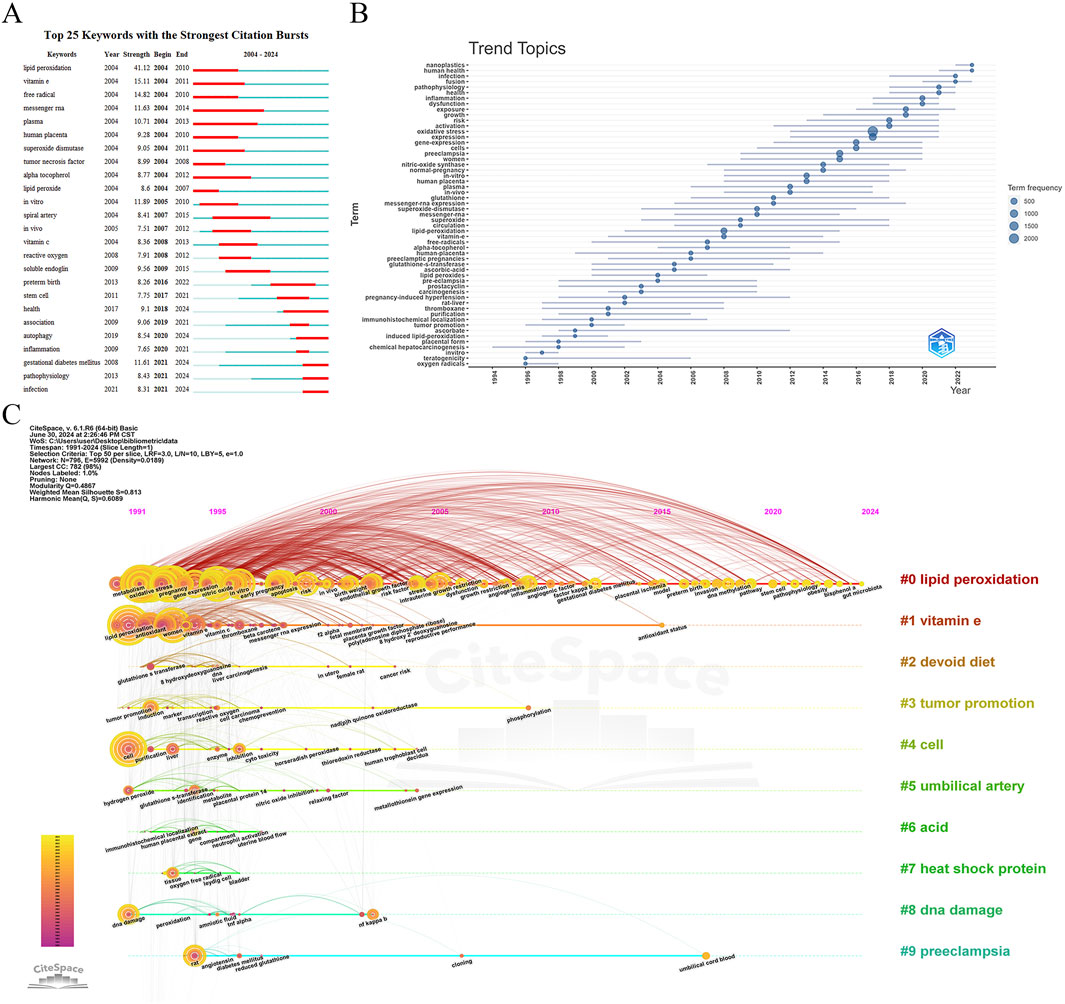
Figure 8. Keyword analysis in Placental Oxidative Stress. (A) Burst detection of keywords. (B) Trend topics analysis. (C) A timeline visualization in CiteSpace.
3.5.3 Trend topics analysis
The trend topics analysis conducted using the bibliometrix package provided insights into the evolution of keyword and subject trends over time. Figure 8B outlined key developments:
1994-1998: Early research focused on “oxygen radicals,” “teratogenicity,” and “in vitro,” with less emphasis on “placental form” and “chemical hepatocarcinogenesis.”
1998-2008: Emerging themes included “ascorbate,” “induced lipid-peroxidation,” and “immunohistochemical localization,” alongside persistent topics like “pregnancy-induced hypertension,” “rat liver,” and “prostacyclin.”
2008-Present: Significant growth occurred in keywords such as “lipid peroxidation,” “vitamin E,” and “superoxide,” with “oxidative stress” and “expression” emerging as central themes. Established topics like “pregnancy-induced hypertension,” “carcinogenesis,” and “free radicals” remained relevant.
The evolution of research topics reflected a shift from foundational studies to a more detailed focus on biochemical processes and their implications. The increasing research on “lipid peroxidation,” “vitamin E,” and “superoxide” underscored their current importance.
3.5.4 A timeline visualization in CiteSpace
The CiteSpace timeline view provides an overview of the evolution of research topics in “placental oxidative stress” from 1991 to 2024. Using a 1-year time slice and focusing on the top 50 keywords, the analysis generated a network with 796 nodes and 5,992 links (density = 0.0189) (Figure 8C). The top 10 clusters identified were: Lipid Peroxidation, Vitamin E, Devoid Diet, Tumor Promotion, Cell, Umbilical Artery, Acid, Heat Shock Protein, DNA Damage and Preeclampsia.
The timeline view highlighted key research themes and their progression over time. Early keywords (1991-2000) include “oxidative stress,” “expression,” “pregnancy,” “preeclampsia,” and “lipid peroxidation,” reflecting foundational research topics. Recent keywords (2020-2024) such as “pathophysiology,” “obesity,” “outcome,” and “air pollution” indicate emerging areas of focus and new challenges in the field.
4 Discussion
This bibliometric study employs CiteSpace, VOSviewer, and the Bibliometrix package to perform an in-depth analysis of the literature related to “placental oxidative stress,” providing a comprehensive overview of the field’s research outputs and advancements. We conducted a quantitative analysis of annual publication volumes, country distributions, research institutions, author contributions, interdisciplinary interactions, journal distributions, and keywords.
Among the top ten authors, Professor Graham J. Burton and Eric Jauniaux have forged a close collaborative relationship, offering novel and profound insights into the mechanisms governing placental formation and function during early pregnancy. Their joint research has significantly expanded our understanding of how the placenta supports fetal growth and development (Burton and Jauniaux, 2023; Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013). Specifically, their systematic investigations have revealed the pathophysiological basis of pregnancy complications such as preeclampsia (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Burton et al., 2019), gestational diabetes mellitus (GDM) (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Ferreira et al., 2023), and fetal growth restriction (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Burton and Jauniaux, 2018; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013). These findings address key pathological pathways including oxidative stress (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Jauniaux and Burton, 2016; Cindrova-Davies et al., 2018), endoplasmic reticulum stress (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Burton and Yung, 2011), and mitochondrial dysfunction (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Yung et al., 2019), opening new avenues for prevention and treatment strategies. Their research also highlights the potential adverse impacts of environmental factors, including air pollution (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Fussell et al., 2024; Bearblock et al., 2021), hypoxic conditions, and high-altitude exposure (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Kurlak et al., 2016; Tissot et al., 2010) on placental function and pregnancy outcomes, significantly raising awareness about the link between environmental health risks and pregnancy outcomes. Methodologically, Professors Burton and Jauniaux have utilized a range of modern biological tools, including RNA-Seq transcriptome sequencing (Phengpol et al., 2023; Zhang et al., 2023a; Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Prater et al., 2021), digital PCR for high-precision quantification (Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Allerkamp et al., 2021), and advanced ultrasound imaging techniques (Hu et al., 2020; Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Jauniaux et al., 2005). The integration of these technologies has greatly deepened our understanding of the molecular mechanisms underlying placental physiology and pathology while driving innovations in placental research techniques. Overall, the research by Professor Burton and Jauniaux spans multiple disciplines including biology, medicine, and environmental science, promoting deep communication and interdisciplinary collaboration, and injecting new vitality into the comprehensive development of placental science. Their work has made significant contributions to improving maternal and infant health globally.
Professor Babbette LaMarca’s research provides a profound analysis of gestational hypertension, particularly preeclampsia (Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Deer et al., 2023), emphasizing the central role of immune cell subpopulations such as T cells (Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Hogg et al., 2024; Deer et al., 2021a), B cells (Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Herrock et al., 2023), and natural killer cells (Fortis et al., 2018; Anto et al., 2023; Amaral et al., 2013; Cunningham et al., 2020), as well as their released cytokines [e.g., IL-17 (Fortis et al., 2018; Anto et al., 2023; Fitzgerald et al., 2023), TNF-α (Cunningham et al., 2020), AT1-AA (Herrock et al., 2023)] in the initiation and progression of the disease. Her work further explores the complex associations between key pathophysiological processes such as placental ischemia (Bakrania et al., 2020), endothelial dysfunction (Deer et al., 2021b), and mitochondrial oxidative stress (Cunningham et al., 2020), with gestational hypertension. Notably, Professor LaMarca focuses on mitochondrial dysfunction (Deer et al., 2021c) and oxidative stress (Vaka et al., 2022) as critical mediators of gestational hypertension and its severe complications (e.g., multi-organ dysfunction), revealing their central role in the disease process. Based on this, she has investigated potential strategies for treating or alleviating gestational hypertension through targeting mitochondrial function (Vaka et al., 2021) and oxidative stress (Deer et al., 2021b). Particularly noteworthy is her extensive discussion of AT1-AA as a core molecule, with its role in mediating gestational hypertension and related pathophysiological changes detailed in multiple papers. This has led to new therapeutic approaches involving the blockade of the AT1-AA pathway to improve disease conditions and related complications (Hogg et al., 2024; Herrock et al., 2023; Cunningham et al., 2018). Additionally, Professor LaMarca’s research utilizes animal models to simulate gestational hypertension and its pathophysiological changes (Cornelius et al., 2015), providing an important platform for understanding disease mechanisms and evaluating treatment interventions. Through various experiments, the study assesses the potential effects of several therapeutic strategies, including vitamin D supplementation (Faulkner et al., 2016), IL-10 supplementation (Harmon et al., 2015), and magnesium sulfate treatment (Johnson et al., 2014), offering valuable insights for clinical practice. In summary, Professor LaMarca’s research not only deepens our understanding of the pathogenesis of gestational hypertension but also opens new scientific pathways and directions for the prevention and treatment of this disease, with significant theoretical and practical implications.
Professor Ramkumar Menon extensively explored the interactions at the maternal-fetal interface under various physiological and pathological conditions, including normal pregnancy, preterm birth (Menon, 2022), and preterm premature rupture of membranes (Mikkelsen et al., 2023). His research emphasized the roles of extracellular vesicles (EVs) at the maternal-fetal interface, particularly in transmembrane signaling molecule transfer (Kalia et al., 2023), modulation of local and systemic inflammatory responses (Shepherd et al., 2021), and potential involvement in drug transport across the maternal-fetal barrier (Sheller-Miller et al., 2016). These findings expanded the understanding of molecular communication mechanisms at the maternal-fetal interface and laid the foundation for developing EV-based diagnostic and therapeutic strategies. Professor Menon also elucidated key mechanisms through which oxidative stress contributes to pregnancy complications, emphasizing its role in driving conditions such as preterm birth and premature rupture of membranes (Arita et al., 2019; Jin et al., 2018). His work provided critical insights into the biological mechanisms underlying these complex pregnancy issues. On a technical level, Professor Menon introduced innovative technologies into pregnancy research, including organ-on-chip technology to simulate in vivo microenvironments (Vidal et al., 2024), microfluidic systems to optimize drug testing and pharmacokinetic analysis (Kammala et al., 2023), and quantitative proteomics for analyzing changes in protein expression at the maternal-fetal interface (Menon et al., 2019). These advancements enhanced the precision and reproducibility of research, offering powerful tools for investigating the complex physiological and pathological processes in pregnancy, thereby advancing the field of pregnancy science.
Professor Anthony V. Perkins extensively investigated the dynamic changes in placental mitochondria throughout pregnancy (Bartho et al., 2020; Fisher et al., 2020; Holland et al., 2018), including pathological conditions such as gestational diabetes (Fisher et al., 2021) and preeclampsia (Holland et al., 2018). He systematically evaluated the significant impacts of these changes on pregnancy outcomes, (Cuffe JS. et al., 2017; Perkins, 2011), focusing on how oxidative stress contributes to complications by impairing placental function (Habibi et al., 2021a). In addition to these findings, Professor Perkins concentrated on nutritional interventions, particularly selenium (Hogan and Perkins, 2022) and iodine supplementation (Habibi et al., 2021a), assessing their potential to counteract the harmful effects of oxidative stress on the placenta and fetus. Through a series of well-designed studies, he demonstrated that appropriate trace element supplementation enhances the placenta’s antioxidant defenses (Habibi et al., 2021b), supporting normal placental function and fetal development (Hogan and Perkins, 2022). His research emphasized the importance of adequate trace element supplementation, including selenium, iodine, and iron, in optimizing placental function (Richard et al., 2017), promoting fetal growth, and improving pregnancy outcomes (Hofstee et al., 2019). Furthermore, Professor Perkins explored the application of placental-derived biomarkers for the early prediction and diagnosis of pregnancy complications (Cuffe JS. et al., 2017). By analyzing the patterns of these biomarkers and their associations with pregnancy outcomes, he advanced early detection and intervention strategies (Cuffe J. et al., 2017), contributing significantly to maternal and infant health research.
The top ten highly-cited articles in “placental oxidative stress” showcase advancements and deeper insights in the field. Several articles concentrated on the pathophysiology of preeclampsia (Roberts and Cooper, 2001; Steegers et al., 2010; Redman and Sargent, 2005), examining its mechanisms, genetic underpinnings, and recent developments, underscoring the prominence of this condition in related research. Other key studies explored the role of multidrug resistance proteins in tissue defense (Leslie et al., 2005), the sampling and classification of placental lesions (Khong et al., 2016), and the rheological and physiological effects of maternal spiral artery remodeling on uterine-placental blood flow (Burton et al., 2009). Research into oxidative stress mechanisms (Burton and Jauniaux, 2011; Jauniaux et al., 2000) and the cloning and tissue expression of Nox family homologs (Cheng et al., 2001) also demonstrated the breadth and depth of inquiry in the field. These highly-cited studies not only highlight current research priorities but also provide essential references for future directions.
Frequently cited references among these articles focus on the role of placental oxidative stress in pathological processes such as pregnancy failure (Jauniaux et al., 2000; Burton and Jauniaux, 2004) and preeclampsia (Hubel, 1999; Sibai et al., 2005), marking oxidative stress as a critical research area. In-depth analyses of preeclampsia, including the excessive expression of soluble fms-like tyrosine kinase one in its pathogenesis (Maynard et al., 2003) and the involvement of circulating angiogenic factors (Levine et al., 2004), reveal the complexity and scope of research in the domain. Additionally, references related to protein measurement methods (Lowry et al., 1951) and the connection between spatiotemporal variations in placental blood flow and oxidative stress (Jauniaux et al., 2003) offer vital technical support and theoretical guidance for ongoing research. These widely cited references lay the groundwork for the field and provide valuable insights for future investigations and clinical intervention strategies.
In the VOSviewer keyword co-occurrence network analysis, frequent joint appearances of keywords within clusters indicated their significant role in shaping the content of the field. Four color-coded clusters emerged from the analysis:
The green cluster revealed the broad impact of “Oxidative Stress” on pregnancy, placental function, and fetal development, with a particularly prominent focus (Aouache et al., 2018; Cheong et al., 2016). Keywords such as “Pregnancy” and “Placenta” pointed to the central role of oxidative stress in these physiological processes. Close associations among keywords like “Inflammation,” “Gene-Expression,” and “Risk” provided deeper insights into oxidative stress mechanisms and underscored its pivotal role in various pregnancy complications.
Oxidative stress contributed significantly to the pathogenesis of conditions such as preeclampsia (Tenorio et al., 2019), GDM (Sha et al., 2019), and fetal growth restriction (Moon et al., 2021) through inflammatory factor activation. These disruptions in placental function involved excessive inflammatory cytokine release (Bernardi et al., 2012), reduced oxidase activity (Mou et al., 2020), and elevated oxidative stress biomarkers (Zhou et al., 2019). Maternal factors such as overweight (Phengpol et al., 2023), obesity (Liang et al., 2018), environmental pollutant exposure (Huang et al., 2017; Riggs et al., 2020), and nutritional deficiencies (Dominguez-Perles et al., 2019) exacerbated oxidative stress and inflammation, further promoting pregnancy complications. These findings emphasized the importance of managing environmental and lifestyle factors during pregnancy. Moreover, certain natural compounds [e.g., astaxanthin (Xuan et al., 2016), pomegranate polyphenols (Chen et al., 2023), and mangiferin (Sha et al., 2019)] and medications [e.g., vitamin D (Nunes et al., 2022), magnesium sulfate (Han et al., 2018), and folic acid (Zhang et al., 2023a)] showed potential in reducing oxidative stress and inflammation, offering promising therapeutic strategies for clinical intervention.
The green cluster also highlighted dynamic changes in gene expression profiles during pregnancy, tightly linked to oxidative stress. Oxidative stress regulated specific genes, such as phospholipase A2 (Brien et al., 2017) and GPX1 (Endler et al., 2016), and indirectly influenced gene activity through epigenetic modifications like DNA methylation (Garcia-Contreras et al., 2019), affecting pregnancy outcomes and offspring health. Environmental factors [e.g., Wi-Fi radiation (Vafaei et al., 2020), maternal obesity (McCoski et al., 2018)], physiological changes (Miyagami et al., 2013), nutritional supplementation (Shorey-Kendrick et al., 2021), and assisted reproductive technologies (Zhang et al., 2010) all impacted gene expression in the placenta and fetus, presenting new perspectives for understanding pregnancy pathophysiology.
Oxidative stress posed a significant risk in assisted reproductive technologies (Mauchart et al., 2023), calling for antioxidant strategies to improve safety. Polymorphisms in DNA repair genes, such as APE1 and XRCC1, were closely linked to heightened risks of preeclampsia (Vural et al., 2009) and preterm birth, underscoring the genetic component in risk assessment. Exposure to pollutants like microplastics (Liu et al., 2023), heavy metals (Shachar et al., 2013), and organophosphate flame retardants (Li et al., 2023) further increased the risk of pregnancy complications and offspring health issues, highlighting the need for environmental protection. Maternal nutritional deficiencies, such as those in vitamin D (Fondjo et al., 2024) and selenium (Lewandowska et al., 2019) along with adverse lifestyle habits like smoking (England and Zhang, 2007) and physical inactivity (Huang et al., 2019), elevated the risks of preeclampsia, preterm birth, and metabolic syndrome. These findings underscored the necessity of multifactorial assessments in predicting and managing pregnancy risks, with future research focused on exploring the mechanisms of these interactions to develop more precise preventive and intervention strategies.
The green cluster included keywords related to fetal growth and development, such as “Intrauterine Growth Restriction,” “Fetal Growth,” “Birth Weight,” and “Preterm Birth.” These terms reflected the significant impact of oxidative stress on intrauterine growth patterns (Yoshida et al., 2018) and fetal birth weight (Hu et al., 2020), highlighting its role as a major risk factor for adverse pregnancy outcomes, including preterm birth (Menon, 2014; Stefanovic et al., 2019). Oxidative stress affects placental function through various mechanisms, negatively impacting fetal growth and development (Luo et al., 2018; Matsubara and Sato, 2001; Jones et al., 2013). These mechanisms include the regulation of mitochondrial content and cell cycle progression, influencing placental development (Luo et al., 2018), as well as the production of large amounts of ROS by enzymes like NAD(P)H oxidase, which elevate oxidative stress levels in the placenta (Matsubara and Sato, 2001). Additionally, changes in nitric oxide synthase activity further modulate oxidative stress, affecting the development of organs such as the fetal kidneys (Figueroa et al., 2016). Maternal nutrition also plays a critical role in fetal growth. While the intake of ω-3 fatty acids may enhance placental antioxidant capacity, it does not effectively prevent IUGR caused by placental ischemia-reperfusion injury (Jones et al., 2013). In contrast, maternal supplementation with nutrients like folic acid can alleviate IUGR induced by high-fat diets by reducing placental inflammation and oxidative stress (Zhang et al., 2023a). Research on the relationship between IUGR and oxidative stress has revealed a strong correlation through the detection of oxidative stress markers in placental tissue and fetal serum. Elevated levels of lipid peroxidation products, such as malondialdehyde, in IUGR fetal serum, along with decreased activity of antioxidant enzymes like superoxide dismutase, have been observed (Llanos and Ronco, 2009). Furthermore, increased levels of heavy metals in the placenta suggest that environmental pollutants may impair fetal growth by inducing oxidative stress (Llanos and Ronco, 2009). Oxidative stress not only affects fetal development but also has significant implications for maternal health. For instance, IUGR pregnancies are often associated with endothelial dysfunction and elevated systemic oxidative stress, which may contribute to the occurrence of IUGR (Yoshida et al., 2018). Additionally, imbalances in angiogenic factors and oxidative stress biomarkers have been noted in older pregnant women, potentially linking these factors to adverse pregnancy outcomes (Odame et al., 2018). Various antioxidant interventions have been shown to effectively reduce oxidative stress and improve fetal growth in IUGR cases. Antioxidants such as N-acetylcysteine, hydroxychloroquine (Dai et al., 2024), and melatonin (Alers et al., 2013; Miller et al., 2014) have demonstrated efficacy in mitigating oxidative stress and autophagy levels, promoting healthier fetal development. Autophagy, a cellular protective mechanism, has also been found to play a vital role in reducing placental apoptosis and oxidative stress (Zhang et al., 2023b).
Oxidative stress significantly contributes to low birth weight. In malnourished pregnancies, maternal supplementation with melatonin enhances placental efficiency and birth weight by upregulating antioxidant enzyme expression in the placenta, thereby safeguarding placental function and fostering fetal development (Richter et al., 2009). This underscores the potential of antioxidants in ameliorating adverse pregnancy outcomes. Additionally, research indicates that as maternal body mass index increases, nitrative stress levels in the placenta markedly rise, whereas oxidative stress levels may not exhibit a corresponding increase and may even decline in some instances (Roberts et al., 2009). This observation suggests a potential balance between nitrative and oxidative stress in obese pregnancies, potentially serving as a protective mechanism for the placenta. Additionally, research has focused on placental function in IUGR and low birth weight fetuses, revealing that these placentas are more prone to oxidative damage, mitochondrial dysfunction, and impaired angiogenesis (Hu et al., 2020). These findings further emphasize the crucial role of oxidative stress in the occurrence of IUGR and low birth weight. Notably, exposure to certain environmental pollutants, such as trichloroethylene, has been associated with elevated placental oxidative stress, contributing to fetal growth restriction (Loch-Caruso et al., 2019). These discoveries underscore the importance of regulating oxidative stress to maintain fetal health and highlight the necessity of managing oxidative stress during pregnancy to minimize potential risks (Bedell et al., 2021). Future research should further investigate the interactions between oxidative stress, placental function, and fetal development, along with the specific applications of antioxidants in preventing and treating pregnancy complications such as IUGR.
The significant role of oxidative stress in preterm birth and its associated complications reveals how various endogenous and exogenous factors disrupt the balance of the antioxidant system, thereby increasing the risk of preterm delivery. The antioxidant system plays a critical role in fetal development and the newborn’s adaptation to the oxygen-rich extrauterine environment, underscoring the close relationship between preterm birth, immature antioxidant system development, and elevated oxidative stress (Davis and Auten, 2010). Both endogenous and exogenous oxidative stress triggers, such as heavy metal exposure (Ahamed et al., 2009), environmental pollutants, and inflammation (Agarwal et al., 2018), can impair placental function and lead to adverse pregnancy outcomes, including preterm birth (Joo et al., 2021). Research into the specific mechanisms of preterm birth has highlighted the importance of mitochondrial oxidative stress in both preterm delivery and fetal brain injury, while exploring potential therapies aimed at mitigating oxidative stress and inflammation through the induction of the Nrf2 signaling pathway (Chen et al., 2024). Additionally, studies have examined the impact of fetal sex and prenatal glucocorticoid exposure on placental antioxidant balance, indicating that male fetuses may face a higher oxidative stress risk following glucocorticoid exposure (Stark et al., 2011). Multiple studies have established a strong link between preterm birth and oxidative stress by detecting oxidative stress markers in placental tissue and umbilical cord blood. Changes in lipid peroxidation products and antioxidant enzyme activity in preterm placentas (Zadrozna et al., 2009; Ferguson et al., 2015), along with elevated levels of oxidative stress markers like isoprostane in umbilical cord blood (Perrone et al., 2016), suggest a connection to preterm birth. The potential role of antioxidants in preventing preterm birth has been investigated, although certain antioxidants, such as ω-3 polyunsaturated fatty acids, may exhibit pro-oxidant effects under specific conditions, highlighting the need for careful consideration of timing and dosage (Stark et al., 2013; Boulis et al., 2014). On the other hand, some compounds with potential antioxidant and anti-inflammatory properties, such as hydroxylated fullerenes and nicotinamide, have shown promise in preventing preterm birth in animal models (Wakimoto et al., 2015; Lappas and Permezel, 2011). Preterm newborns face unique challenges related to iron metabolism and oxidative stress, given their distinct iron handling and insufficient antioxidant capacity. These newborns are at risk of both iron deficiency and iron overload, necessitating individualized management strategies that take into account multiple factors (Raffaeli et al., 2020). Comprehensive exploration of oxidative stress mechanisms in preterm birth and its related complications offers new insights into the pathophysiology of preterm birth, while providing valuable guidance for future prevention and treatment strategies. Further research should focus on the development of the antioxidant system, the mechanisms of oxidative stress triggers, and the application of antioxidants in preterm birth prevention, with the aim of creating more effective prevention and treatment approaches.
The clustering of terms like “DNA-Damage,” “Insulin-Resistance,” and “Obesity” pointed to strong connections between oxidative stress and broader pathological processes. These connections extended from molecular mechanisms, such as DNA damage (Fujimaki et al., 2011; Singh et al., 2020), to metabolic disruptions like insulin resistance (Feng et al., 2020; Scioscia et al., 2009), further encompassing the public health challenge of obesity. The analysis underscored the complexity and significance of oxidative stress in pregnancy and related diseases, demonstrating its far-reaching impact. Keywords related to maternal health, such as “Pregnant-Women”,“Gestational Diabetes Mellitus” and “Maternal Obesity” prominently appeared in the green cluster, highlighting the impact of oxidative stress not only on fetal health but also on maternal wellbeing. Oxidative stress was recognized as a teratogenic mechanism during embryonic development, particularly in diabetic embryopathy (Coughlan et al., 2004). Studies identified significant alterations in oxidative stress in the placentas of patients with GDM, with elevated levels of nitrotyrosine serving as direct evidence of oxidative stress (Lyall et al., 1998). Additionally, oxidative stress negatively influenced the expression of inflammatory cytokines and antioxidant enzymes in the placentas of GDM patients, though these effects were less evident in adipose tissue (Lappas et al., 2010). To explore potential treatments, various natural extracts, such as mulberry, acacia, and ginkgo leaf extracts, were evaluated for their effects on maternal and fetal outcomes, oxidative stress levels, and lipid profiles in GDM rat models (Volpato et al., 2011; Volpato et al., 2008; Rudge et al., 2007). The findings suggested the potential of these natural extracts to alleviate oxidative stress and improve pregnancy outcomes. Moreover, oxidative stress was found to enhance the activity of matrix metalloproteinases-2 and -9 in the placenta-fetal unit of diabetic rats, potentially exacerbating placental dysfunction (Pustovrh et al., 2005). Beyond natural extracts, research investigated the influence of both endogenous and exogenous oxidative stress triggers on adverse pregnancy outcomes, including preeclampsia, fetal growth restriction, GDM, and preterm birth (Joo et al., 2021). Studies also examined the relationship between adiponectin and oxidative stress markers in GDM patients and their newborns (Shang et al., 2018), as well as oxidative and antioxidant status in GDM patients diagnosed under International Association of the Diabetes and Pregnancy Study Groups criteria (Shang et al., 2015). A cohort study conducted in the Thai population further compared oxidative stress biomarkers between GDM and non-GDM patients, finding a significant association between GDM and inflammatory processes, reflected in higher oxidative stress and apoptosis markers (Rueangdetnarong et al., 2018).
To identify new therapeutic targets, interactions among oxidative stress, endoplasmic reticulum stress, inflammation, mitochondrial function, and signaling pathways were explored. Apocynin mitigated oxidative stress and inflammation in GDM by inhibiting the TLR4/NF-κB signaling pathway (Liu et al., 2020). Cryptotanshinone significantly reduced blood glucose levels, oxidative stress, inflammation, and NF-κB activation in GDM mice, while increasing insulin levels in the placenta and blood (Wang N. et al., 2020). Asperulosidic acid alleviated oxidative stress and inflammation in the GDM placenta by suppressing NF-κB and MAPK signaling pathways (Wu et al., 2022). Additionally, studies assessed the potential benefits of physical activity (Cid and Gonzalez, 2016), specific nutrients like copper (Ergaz et al., 2014)and lutein (Lorenzoni et al., 2013), and the drug nigericin in reducing oxidative stress in GDM patients. Notably, increased placental and fetal lipoprotein-associated phospholipase A2 in GDM patients might offer protection against oxidative stress (Schliefsteiner et al., 2017), The upregulation of nuclear factor erythroid 2-related factor 2 and antioxidant enzymes in the GDM placenta could represent protective mechanisms against oxidative stress (Manoharan et al., 2019). However, not all studies supported the efficacy of these protective mechanisms. For instance, while vitamins and antioxidants reduced fetal oxidative stress, they failed to restore normal growth (Ornoy et al., 2009).
Research also examined the impact of GDM on neonatal cardiovascular health, particularly the increased intima-media thickness of the aorta and the role of oxidative stress in this process (Triantafyllidou et al., 2023). The roles of oxidative stress, fatty acids, and neurotrophic factors in GDM were explored (Jadhav et al., 2020), along with the association between GDM, autism spectrum traits, and attention deficit hyperactivity disorder symptoms, although placental inflammation and oxidative stress cytokines were not found to mediate these associations (Zhu et al., 2021). In summary, this series of studies provides rich data support for understanding the role of oxidative stress in GDM and its related complications, and offers potential targets for the development of new therapeutic strategies.
GDM (Gauster et al., 2017; Lappas et al., 2011) and obesity (Duan et al., 2018; Hu et al., 2021) both significantly increase oxidative stress in the placenta, posing risks to maternal and fetal health during pregnancy. Research indicates that maternal obesity is not only associated with metabolic abnormalities and reduced antioxidant capacity in pregnant women but may also affect placental function, leading to fetal oxidative stress and metabolic alterations (Malti et al., 2014; Zavalza-Gomez, 2011; Ballesteros-Guzman et al., 2019). Elevated levels of oxidative stress markers, such as malondialdehyde, protein carbonyls, nitric oxide, and superoxide anions, have been observed in the placentas of obese pregnant women, alongside decreased levels of antioxidants like reduced glutathione and superoxide dismutase. This imbalance in redox status could have long-term effects on fetal metabolic and immune programming (Malti et al., 2014; Hernandez-Trejo et al., 2017). Additionally, obesity may contribute to placental mitochondrial dysfunction and impaired angiogenesis, further exacerbating oxidative stress in the placenta (Hu C. et al., 2019; Napso et al., 2022). Notably, alterations in the gut microbiota of obese pregnant women have been linked to increased oxidative stress in the placenta, suggesting a potential role for gut microbiota in obesity-mediated placental dysfunction (Hu et al., 2021). To explore therapeutic interventions, studies have evaluated the effects of resveratrol supplementation on oxidative stress in obese pregnant women and their fetuses. The results demonstrated that resveratrol improved maternal metabolic status and significantly reduced oxidative stress in the placenta and liver, offering new strategies for managing obesity during pregnancy (Rodriguez-Gonzalez et al., 2022). Research has also focused on the impact of maternal obesity on the renal health of offspring. Offspring of obese pregnant women may experience lipid accumulation, inflammation, oxidative stress, and fibrosis in the kidneys, increasing the risk of developing kidney diseases (Phengpol et al., 2023). An exploratory study evaluated the effects of physical activity and sedentary time during pregnancy on placental oxidative stress markers in obese women. The findings suggest that increased physical activity and reduced sedentary time may correlate with lower expression levels of certain oxidative stress markers in the placenta, underscoring the importance of lifestyle interventions during pregnancy (Zafaranieh et al., 2022). Obesity significantly influences oxidative stress in both pregnant women and their fetuses, potentially involving complex physiological and pathological mechanisms. Future research should further investigate the role of obesity-mediated oxidative stress in pregnancy complications and develop effective interventions to improve health outcomes for obese pregnant women and their offspring. These insights highlighted the need for comprehensive strategies to assess and manage oxidative stress during pregnancy (Bedell et al., 2021), aiming for an integrated approach that protects both maternal and fetal health.
The blue cluster primarily focuses on pregnancy-related diseases and their underlying pathophysiological mechanisms. The cluster, with a central focus on “Preeclampsia,” strongly associated with keywords like “Hypertension,” “Nitric-Oxide,” and “Nitric-Oxide Synthase,” highlighting their frequent co-mention in the literature. This co-occurrence indicated the critical roles and interactions of these factors in preeclampsia’s pathophysiology. Research linked preeclampsia to significant oxidative stress, which reduced nitric oxide (NO) bioavailability (Nunes et al., 2021) through the inhibition of endothelial nitric oxide synthase (eNOS) activity (Amaral et al., 2013). Vitamin D deficiency further aggravated the reduction of NO by influencing oxidative stress responses in human umbilical vein endothelial cells (Nunes et al., 2021). L-arginine depletion in preeclampsia contributed to eNOS conversion into an oxidant form, decreasing NO production, while oxidative products such as 4-oxo-2(E)-nonenal exacerbated eNOS dysfunction (Guerby et al., 2019). Inhibition of inducible nitric oxide synthase demonstrated potential for blood pressure reduction in experimental studies (Amaral et al., 2013), and antioxidants like N-acetylcysteine helped restore NO-mediated function in the placenta of preeclampsia patients (Bisseling et al., 2004). Heat shock protein 70 (Barut et al., 2010), homocysteine, and asymmetric dimethylarginine also played key roles in regulating NO metabolism and the development of preeclampsia (Demir et al., 2012). Overall, the imbalance between NO and reactive oxygen species, particularly oxidative stress-induced eNOS inhibition, emerged as a critical aspect of preeclampsia’s pathogenesis. Future studies should further investigate the molecular mechanisms and potential interventions related to this imbalance.
The frequent appearance of keywords such as “Pathogenesis,” “Endothelial Growth-Factor,” “Blood-Flow,” “Necrosis-Factor-Alpha,” “Blood-Pressure,” and “Endothelial Dysfunction” underscored the central role of vascular endothelial dysfunction (Sanchez-Aranguren et al., 2014; Cindrova-Davies, 2009), inflammatory responses (Tenorio et al., 2019; Harmon et al., 2016; Michalczyk et al., 2020), and blood flow regulation disorders (Parra et al., 2005; Yoshihara et al., 2022) in preeclampsia’s pathogenesis and related hypertensive diseases. These interactions collectively drove the progression of preeclampsia. Keywords like “Placental Oxidative Stress,” “Cytokines,” “Fetal Growth Restriction,” “Trophoblast Invasion,” and “Angiogenic Factors” revealed critical aspects of preeclampsia’s complex pathophysiology, including placental dysfunction (Biron-Shental et al., 2010), oxidative stress responses (Marin et al., 2020), abnormal angiogenesis (Turpin et al., 2015), and fetal growth restriction (Takagi et al., 2004). The role of the placenta in preeclampsia pathology and the impact of oxidative stress (Anto et al., 2023), cytokine network imbalances (Rusterholz et al., 2007; Stark, 1993), trophoblast cell dysfunction (Wang GJ. et al., 2020), and angiogenesis disorders (Anto et al., 2024; Edvinsson et al., 2022) were emphasized.
Finally, the appearance of keywords such as “Tumor-Necrosis-Factor,” “Tyrosine Kinase-1,” and “Endoplasmic-Reticulum Stress” suggested potential roles for immune-inflammatory responses (Kim et al., 2017), signaling pathway abnormalities (Kim et al., 2017; Burke et al., 2016), and cellular stress responses (Redman et al., 2022) in preeclampsia’s pathogenesis, further emphasizing the complexity of this multifactorial disease involving widespread biological processes and molecular network imbalances (Burton et al., 2019).
The keywords in the red cluster form a highly integrated knowledge network closely related to cell biology, oxidative stress, and mechanisms of related diseases. This cluster centers around high-frequency keywords such as ‘Expression,’ ‘Apoptosis,’ ‘Cells,’ and ‘Trophoblast,’ highlighting the central roles of cellular expression regulation, cell apoptosis, and trophoblast cells in these research domains. These keywords not only represent fundamental processes in cell biology but also clearly indicate the critical roles of these processes within complex physiological and pathological environments, particularly in the contexts of pregnancy and placental function.
Additionally, the appearance of keywords such as “In-Vitro,” “Hypoxia,” “Activation,” “Growth,” and “Mitochondria” further underscores the significant impacts of in vitro experimental models (Depoix et al., 2017), hypoxic environments (Zhao et al., 2021) on cellular functions, and the crucial roles of mitochondria in cell growth (Ma et al., 2019), energy metabolism (Napso et al., 2022), and stress responses (Burton et al., 2017). The co-occurrence of these keywords reveals multiple important aspects in cell biology research, including the establishment of experimental models, cellular adaptive responses to environmental conditions, and the pivotal role of organelles, especially mitochondria, in maintaining cellular homeostasis.
Notably, the co-occurrence of keywords such as “Reactive Oxygen Species,” “Stress,” “Disease,” “Angiogenesis,” and “Oxygen” reveals the close relationship between oxidative stress responses, abnormal angiogenesis, and the placenta. Research has shown that ROS not only participates in physiological processes of normal pregnancy, such as placental angiogenesis (Yang et al., 2022) and trophoblast function (Walker et al., 2020), but also plays a key role in various pregnancy complications, including preeclampsia [(Matsubara et al., 2015), (Matsubara et al., 2010)], intrauterine growth restriction (Luo et al., 2018), and fetal loss (Zhang et al., 2019). ROS mediates oxidative stress responses by regulating signaling pathways (Adebambo et al., 2018), affecting gene expression (Wang B. et al., 2020), and impacting cellular functions (Adebambo et al., 2018), thereby influencing pregnancy outcomes. Furthermore, insulin resistance and elevated androgen levels (Hu M. et al., 2019) can increase ROS production, further exacerbating the risk of pregnancy complications. Notably, the application of antioxidants has shown potential in alleviating ROS-mediated damage (Al-Gubory et al., 2010; Khera et al., 2017), providing new insights for clinical intervention. Future research should continue to explore the specific mechanisms of ROS in pregnancy and develop more effective antioxidant therapies to improve pregnancy outcomes and safeguard maternal and fetal health.
The balance between pro-angiogenic and anti-angiogenic factors is crucial for maintaining normal pregnancy. In particular, pathological states such as preeclampsia and intrauterine growth restriction are associated with significant inhibition of angiogenesis (Shi et al., 2021), along with increased oxidative stress (Anto et al., 2024) and changes in the expression of angiogenesis-related genes (Fortis et al., 2018). Additionally, factors such as maternal nutritional status (Anto et al., 2023), environmental factors (Bai et al., 2022), pharmacological interventions (Soobryan et al., 2017), and genetic variations (Fortis et al., 2018) all impact placental angiogenesis. Notably, certain natural compounds (Park et al., 2020)and drugs (Doganlar et al., 2019) have shown potential in promoting angiogenesis, mitigating oxidative stress, and improving pregnancy outcomes. Future research should further explore the specific mechanisms of angiogenesis in pregnancy and develop more effective intervention strategies to improve the prognosis of patients with pregnancy complications by modulating the angiogenesis process.
Furthermore, the clustering of keywords such as “Toxicity,” “Liver,” “Damage,” and “Dysfunction” reveals the broad pathological processes potentially involved in oxidative stress and cellular dysfunction. These processes include the toxic effects and mechanisms of various chemicals (Abdollahzade et al., 2021; Cattani et al., 2014), environmental pollutants (Saben et al., 2014), pharmaceuticals (Lu et al., 2023), and nanomaterials (Wang and Wang, 2020) on the placenta, trophoblast cells, embryo, and fetus. They collectively highlight the central roles of oxidative stress (Huang et al., 2018), apoptosis (Ali et al., 2021), genotoxicity (Kostic et al., 2022), endocrine disruption (Perez-Albaladejo et al., 2017), and mitochondrial dysfunction (Naav et al., 2020) in these toxic effects. Notably, research emphasizes the placenta as a critical target for these toxic effects and its key role in protecting the fetus from external harmful substances. Simultaneously, these studies reveal the potential applications of various antioxidants (Jan et al., 2011), nutrients (Hassoun et al., 1997), and other bioactive substances (Lee et al., 2023) in mitigating or counteracting these toxic effects. The keyword “Liver” primarily focuses on liver diseases (Pillai and Gupta, 2005), tumors (Puatanachokchai et al., 2006), and their prevention, progression, and treatment (Phannasorn et al., 2022). The terms “Damage” and “Dysfunction” indicate that the impact of oxidative stress is not confined to a specific disease or organ but widely affects multiple systems and organ functions. This co-occurrence of keywords not only expands our understanding of the spectrum of oxidative stress-related diseases but also emphasizes the core role of cellular dysfunction in these diseases, suggesting that future research should focus more on the mechanisms of oxidative stress-induced cellular dysfunction and its specific roles in various diseases.
The co-occurrence of keywords such as “Protein,” “Differentiation,” “Proliferation,” “Inhibition,” “Invasion,” “Receptor,” and “Autophagy” further enriches our understanding of cellular biological processes. These keywords address mechanisms across several layers, including protein functions (Colleoni et al., 2013), cell differentiation and proliferation (Bolnick et al., 2017), signal transduction (Rundlof and Arner, 2004), and cell invasion and migration (Yang and Shang, 2021). The close association of these keywords reveals multiple complex aspects of cell biology research, including the critical roles of proteins in determining cell fate (Rundlof and Arner, 2004), regulatory mechanisms of cell proliferation and differentiation (Dai and Lu, 2022), and the complexity of intercellular interactions and signal transduction (Murphy et al., 2008). Additionally, the appearance of keywords such as “Pregnancies,” “Messenger-RNA,” “Identification,” “ROS,” and “Gene” highlights the significant roles of oxidative stress, gene expression regulation, and reactive oxygen species in cell fate decisions during pregnancy. These keywords reveal the complexity and uniqueness of cellular biological processes under the special physiological state of pregnancy and the critical roles of oxidative stress and gene expression regulation in this process.
The yellow cluster focuses on oxidative stress, antioxidant mechanisms, and related biomarkers, centering around high-frequency keywords like “Lipid-Peroxidation” and “Antioxidant.” This cluster primarily explores the formation and effects of antioxidants and lipid peroxidation in human placental and fetal tissues. Specifically, it covers studies on free radical scavenging enzyme activity and lipid peroxidation in placental tissues of miscarriage patients (Biri et al., 2006), NADPH and iron-dependent lipid peroxidation in human placental microsomes, and lipid peroxidation, antioxidant defenses, and acid-base status in umbilical cord blood at birth (Milczarek et al., 2007). Additionally, the cluster investigates the effects of various substances, such as melatonin (Milczarek et al., 2000), pollutants (Lee et al., 2015), and drugs (Rojas et al., 2022) on lipid peroxidation in the placenta and fetus, as well as changes in lipid peroxidation and antioxidant enzyme activity under different physiological and pathological conditions (Johnston et al., 2016; Loverro et al., 1996). Collectively, these studies reveal the complexity of lipid peroxidation and antioxidant states in placental and fetal tissues and their crucial roles in pregnancy and fetal development.
The emergence of keywords such as “Human Placenta” and “Women” indicates that this research network may particularly focus on oxidative stress and antioxidant mechanisms within the context of female physiology, especially within the placenta. The co-occurrence of keywords like “Plasma,” “Superoxide-Dismutase,” “Vitamin-E,” “Glutathione,” and “Selenium” further underscores the critical role of antioxidant defense systems in maintaining cellular homeostasis (Wang and Walsh, 2001; Liu et al., 2010) and highlights the potential biomarker value of antioxidants and enzymes present in plasma (Rani et al., 2010). The appearance of the term “Supplementation” suggests the application of antioxidants as an intervention strategy in related research (Poston et al., 2011; Pressman et al., 2003). Additionally, the clustering of keywords such as “Melatonin,” “Free-Radicals,” and “Antioxidant Enzymes” reveals the production of free radicals during oxidative stress (Lista et al., 2010), the occurrence of lipid peroxidation (Gupta et al., 2005), and the pivotal role of antioxidant enzymes in scavenging free radicals (Vanderlelie et al., 2005) and protecting cells from oxidative damage (Okatani et al., 2001). Notably, the appearance of “Messenger-RNA Expression” and “Alpha-Tocopherol” adds a new dimension to this research network, indicating a potential connection between oxidative stress and gene expression regulation (Than et al., 2009), as well as the specific mechanisms through which particular antioxidants contribute to cellular protection (Vieira et al., 2018). Finally, although keywords like “Thromboxane” and “Acid” appear less frequently in this cluster, they represent broader areas of oxidative stress research, such as the relationship between oxidative stress and thrombosis (Vaughan and Walsh, 2005), and acid-base balance in physiological and pathological processes (Kinalski et al., 2001).
Several core themes have emerged as significant research hotspots, providing clear directions for researchers. Oxidative stress and lipid peroxidation processes have been longstanding focal points. The close relationship between “lipid peroxidation” and “oxidative stress” highlights the potential harm lipid peroxidation products inflict on placental function during oxidative stress, offering critical insights for the development of subsequent intervention strategies. Additionally, the role of antioxidants, particularly natural or synthetic agents such as “vitamin E,” has been widely recognized for mitigating oxidative stress and protecting placental health, providing new perspectives on nutritional supplementation and therapeutic approaches during pregnancy. Pregnancy-related disorders have become key research priorities due to their strong association with oxidative stress. Understanding these conditions is essential for elucidating disease mechanisms and optimizing clinical management. The complex roles of gene expression and regulation in placental oxidative stress responses have also gained clarity. The frequent appearance of “gene expression” and related keywords underscores the importance of genetic research in revealing molecular mechanisms underlying oxidative stress, prompting researchers to focus more on genetic factors in this field.
Emerging directions and fields are also worth attention. Pathophysiology and mechanism exploration are gaining prominence, with the rise of keywords such as “pathophysiology” and “mechanism” indicating that deeper investigation into the pathophysiological mechanisms of placental oxidative stress could provide theoretical support for precision therapies. Emerging risk factors and environmental influences have garnered increasing attention, with the appearance of keywords like “obesity,” “bisphenol A,” and “air pollution” revealing potential threats posed by environmental factors to placental health and pregnancy outcomes, thus offering a scientific basis for public health policies. The growth of cellular biology and stem cell research, especially the increased focus on “cell,” “stem cell,” and “autophagy,” highlights the growing interest in cellular processes related to placental oxidative stress. Stem cell therapy and autophagy regulation could open new avenues for future therapeutic strategies. Furthermore, inflammation and immune regulation remain enduring research frontiers, with the complex interactions between placental oxidative stress and these processes being increasingly revealed. The sustained high frequency and recent rise of keywords such as “inflammatory cytokine” and “inflammation” underscore the critical role inflammation and immune regulation play in maintaining placental homeostasis and preventing related diseases, encouraging further exploration in this area.
Based on the analysis of core themes and research frontiers, this paper provides a scientific basis for policymakers to develop public health policies. Emphasis should be placed on the role of oxidative stress and lipid peroxidation in adverse pregnancy outcomes, encouraging further research to design effective interventions. Additionally, addressing emerging risk factors such as obesity, bisphenol A, and air pollution is crucial. Policymakers should implement targeted public health measures to alleviate their detrimental effects on placental health and pregnancy outcomes. Potential actions include advocating for stricter air pollution regulations and launching public health education campaigns to increase awareness of the risks associated with obesity and environmental pollutants.
Employing bibliometrics to explore the field of “placental oxidative stress” offers a novel perspective and facilitates a deeper understanding of its trends. Unlike traditional literature reviews, the study innovatively employed multiple bibliometric tools such as CiteSpace, VOSviewer, and the R package bibliometrix. The integrated use of these tools enabled a comprehensive and systematic extraction and analysis of data, revealing key research dynamics in the field of placental oxidative stress. By combining systematic searches with quantitative statistical analysis, the approach provided a more data-driven and quantitative view of the research landscape.
However, the study had certain limitations. First, the data were sourced solely from the Web of Science Core Collection, potentially overlooking relevant information from other databases such as Embase and MEDLINE. This choice stemmed from the fact that the Web of Science Core Collection is the most commonly used database for bibliometric analysis, offering timely and comprehensive citation updates. Nonetheless, challenges in integrating data from different sources persist due to limitations in the analytical software. Second, the sensitivity of the analysis algorithms may have missed emerging topics related to “placental oxidative stress.” Despite employing multiple software packages for analysis, the information provided remained constrained by the limitations of bibliometric algorithms. Future advancements in bibliometric methods may help address these challenges.
Additional challenges arose during the bibliometric analysis. First, scientometric research primarily relies on co-citation analysis, which can be influenced by “citation distortion”—the selective use of citations to support unverified scientific claims. This issue, although detectable through close examination of research hotspots, remains a notable limitation. Second, limiting the search to the Web of Science Core Collection may have restricted the range of publication types retrieved. In many databases such as PubMed and Embase, full-text references and citation lists are unavailable, complicating efforts to merge references from different databases and often requiring significant manual intervention. Future software developments may enable the simultaneous analysis of results from various databases while reliably removing duplicates automatically. Third, the analysis did not cover all publications related to “placental oxidative stress,” as only original articles and reviews were included, and the Web of Science’s stricter inclusion criteria for scientific journals, compared to databases like PubMed, further constrained the scope. Lastly, the inherent lag in publications and subsequent citations made it difficult to capture the latest research trends. However, the impact of this lag was significantly reduced by considering the context in which articles were cited and who conducted the citations, as cited articles might have been recently published.
In summary, despite its limitations, the bibliometric analysis employed in this study provided valuable insights into research trends in the field of placental oxidative stress and suggested new directions for future research. To further improve the analysis in this area, future studies should consider integrating multiple databases and analytical methods for more comprehensive and in-depth insights.
5 Conclusion
This study provides an in-depth bibliometric analysis of the field of “placental oxidative stress” aiming to reveal trends, research hotspots, and future directions. The study identifies key research hotspots focused on oxidative stress and lipid peroxidation processes, with “lipid peroxidation” as a critical event indicating potential risks to placental function. The role of antioxidants, particularly natural or synthetic substances such as vitamin E, in mitigating oxidative stress and protecting placental health is well-recognized, offering new insights for nutritional supplementation and therapeutic strategies during pregnancy. Pregnancy-related diseases, particularly “preeclampsia” and “pregnancy-induced hypertension,” are significant research focuses due to their close association with oxidative stress, which is crucial for understanding disease mechanisms and optimizing clinical management. Additionally, the complex role of gene expression and regulation in the placental oxidative stress response is increasingly evident, highlighting the importance of genetic-level research in elucidating molecular mechanisms. Emerging research directions include exploring pathophysiological mechanisms, focusing on new risk factors and environmental impacts, advancing cell biology and stem cell research, and continuing to investigate inflammation and immune regulation. These developments are expected to enhance our understanding of placental oxidative stress and advance therapeutic strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
AC: Funding acquisition, Writing–original draft. MT: Data curation, Writing–original draft. ZL: Data curation, Writing–original draft. XC: Investigation, Writing–review and editing. YG: Investigation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (Nos. BJ2020079; BJ2023076) and the Scientific Research Program of Wuxi Health Commission (M202107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahzade, N., Babri, S., and Majidinia, M. (2021). Attenuation of chronic arsenic neurotoxicity via melatonin in male offspring of maternal rats exposed to arsenic during conception: ānvolvement of oxidative DNA damage and inflammatory signaling cascades. LIFE Sci. 266, 118876. doi:10.1016/j.lfs.2020.118876
Adebambo, O. A., Shea, D., and Fry, R. C. (2018). Cadmium disrupts signaling of the hypoxia-inducible (HIF) and transforming growth factor (TGF-β) pathways in placental JEG-3 trophoblast cells via reactive oxygen species. Toxicol. Appl. Pharmacol. 342, 108–115. doi:10.1016/j.taap.2018.01.010
Agarwal, P., Singh, L., Anand, M., and Taneja, A. (2018). Association between placental polycyclic aromatic hydrocarbons (pahs), oxidative stress, and preterm delivery: a case-control study. Arch. Environ. Contam. Toxicol. 74, 218–227. doi:10.1007/s00244-017-0455-0
Ahamed, M., Mehrotra, P. K., Kumar, P., and Siddiqui, M. K. (2009). Placental lead-induced oxidative stress and preterm delivery. Environ. Toxicol. Pharmacol. 27, 70–74. doi:10.1016/j.etap.2008.08.013
Alers, N. O., Jenkin, G., Miller, S. L., and Wallace, E. M. (2013). Antenatal melatonin as an antioxidant in human pregnancies complicated by fetal growth restriction--a phase I pilot clinical trial: study protocol. BMJ OPEN 3, e004141. doi:10.1136/bmjopen-2013-004141
Al-Gubory, K. H., Fowler, P. A., and Garrel, C. (2010). The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 42, 1634–1650. doi:10.1016/j.biocel.2010.06.001
Ali, M. M., Khater, S. A., Fayed, A. A., Sabry, D., and Ibrahim, S. F. (2021). Apoptotic endocrinal toxic effects of perchlorate in human placental cells. Toxicol. Rep. 8, 863–870. doi:10.1016/j.toxrep.2021.04.002
Allerkamp, H. H., Clark, A. R., Lee, T. C., Morgan, T. K., Burton, G. J., and James, J. L. (2021). Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum. Reprod. 36, 571–586. doi:10.1093/humrep/deaa303
Amaral, L. M., Pinheiro, L. C., Guimaraes, D. A., Palei, A. C., Sertorio, J. T., Portella, R. L., et al. (2013). Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J. CELL Mol. Med. 17, 1300–1307. doi:10.1111/jcmm.12106
Anto, E. O., Boadu, W., Hughes, C., Korsah, E. E., Frimpong, J., Ansah, E., et al. (2024). Angiogenic growth factors, oxidative stress and haematobiochemical measures as predictors of preeclampsia with and without foetal growth restriction: a case-control study in a Ghanaian population. PLACENTA 145, 130–138. doi:10.1016/j.placenta.2023.12.007
Anto, E. O., Ofori, B. W., Addai-Mensah, O., Wiafe, Y. A., Owiredu, W. K., Obirikorang, C., et al. (2023). Association between micronutrients, oxidative stress biomarkers and angiogenic growth mediators in early and late-onset preeclamptic Ghanaian women. SAGE Open Med. 11, 372940721. doi:10.1177/20503121231175759
Aouache, R., Biquard, L., Vaiman, D., and Miralles, F. (2018). Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 19, 1496. doi:10.3390/ijms19051496
Arita, Y., Park, H. J., Cantillon, A., Getahun, D., Menon, R., and Peltier, M. R. (2019). Effect of bisphenol-A (BPA) on placental biomarkers for inflammation, neurodevelopment and oxidative stress. J. Perinat. Med. 47, 741–749. doi:10.1515/jpm-2019-0045
Bai, G., Jiang, X., Qin, J., Zou, Y., Zhang, W., Teng, T., et al. (2022). Perinatal exposure to glyphosate-based herbicides impairs progeny health and placental angiogenesis by disturbing mitochondrial function. Environ. Int. 170, 107579. doi:10.1016/j.envint.2022.107579
Bakrania, B. A., Spradley, F. T., Drummond, H. A., LaMarca, B., Ryan, M. J., and Granger, J. P. (2020). Preeclampsia: linking placental ischemia with maternal endothelial and vascular dysfunction. Compr. Physiol. 11, 1315–1349. doi:10.1002/cphy.c200008
Ballesteros-Guzman, A. K., Carrasco-Legleu, C. E., Levario-Carrillo, M., Chavez-Corral, D. V., Sanchez-Ramirez, B., Marinelarena-Carrillo, E. O., et al. (2019). Prepregnancy obesity, maternal dietary intake, and oxidative stress biomarkers in the fetomaternal unit. Biomed. Res. Int. 2019, 5070453. doi:10.1155/2019/5070453
Bartho, L. A., Fisher, J. J., Cuffe, J., and Perkins, A. V. (2020). Mitochondrial transformations in the aging human placenta. Am. J. Physiol. Endocrinol. Metab. 319, E981-E994–E994. doi:10.1152/ajpendo.00354.2020
Barut, F., Barut, A., Dogan, G. B., Kandemir, N. O., Aktunc, E., Harma, M., et al. (2010). Expression of heat shock protein 70 and endothelial nitric oxide synthase in placental tissue of preeclamptic and intrauterine growth-restricted pregnancies. Pathol. Res. Pract. 206, 651–656. doi:10.1016/j.prp.2010.04.001
Bearblock, E., Aiken, C. E., and Burton, G. J. (2021). Air pollution and pre-eclampsia; associations and potential mechanisms. PLACENTA 104, 188–194. doi:10.1016/j.placenta.2020.12.009
Bedell, S., Hutson, J., de Vrijer, B., and Eastabrook, G. (2021). Effects of maternal obesity and gestational diabetes mellitus on the placenta: current knowledge and targets for therapeutic interventions. Curr. Vasc. Pharmacol. 19, 176–192. doi:10.2174/1570161118666200616144512
Bernardi, F. C., Felisberto, F., Vuolo, F., Petronilho, F., Souza, D. R., Luciano, T. F., et al. (2012). Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control study. Oxid. Med. CELL Longev. 2012, 636419. doi:10.1155/2012/636419
Biri, A., Kavutcu, M., Bozkurt, N., Devrim, E., Nurlu, N., and Durak, I. (2006). Investigation of free radical scavenging enzyme activities and lipid peroxidation in human placental tissues with miscarriage. J. Soc. Gynecol. Investig. 13, 384–388. doi:10.1016/j.jsgi.2006.04.003
Biron-Shental, T., Sukenik-Halevy, R., Sharon, Y., Goldberg-Bittman, L., Kidron, D., Fejgin, M. D., et al. (2010). Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 202, 381–e7. doi:10.1016/j.ajog.2010.01.036
Bisseling, T. M., Maria, R. E., Raijmakers, M. T., Steegers, E. A., Peters, W. H., and Smits, P. (2004). N-acetylcysteine restores nitric oxide-mediated effects in the fetoplacental circulation of preeclamptic patients. Am. J. Obstet. Gynecol. 191, 328–333. doi:10.1016/j.ajog.2003.12.033
Bolnick, A. D., Bolnick, J. M., Kohan-Ghadr, H. R., Kilburn, B. A., Pasalodos, O. J., Singhal, P. K., et al. (2017). Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor. Hum. Reprod. 32, 1218–1229. doi:10.1093/humrep/dex069
Boulis, T. S., Rochelson, B., Novick, O., Xue, X., Chatterjee, P. K., Gupta, M., et al. (2014). Omega-3 polyunsaturated fatty acids enhance cytokine production and oxidative stress in a mouse model of preterm labor. J. Perinat. Med. 42, 693–698. doi:10.1515/jpm-2014-0243
Brien, M., Larose, J., Greffard, K., Julien, P., and Bilodeau, J. F. (2017). Increased placental phospholipase A(2) gene expression and free F(2)-isoprostane levels in response to oxidative stress in preeclampsia. PLACENTA 55, 54–62. doi:10.1016/j.placenta.2017.05.004
Burke, S. D., Zsengeller, Z. K., Khankin, E. V., Lo, A. S., Rajakumar, A., DuPont, J. J., et al. (2016). Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. INVEST 126, 2561–2574. doi:10.1172/JCI83918
Burton, G. J., and Jauniaux, E. (2004). Placental oxidative stress: from miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 11, 342–352. doi:10.1016/j.jsgi.2004.03.003
Burton, G. J., and Jauniaux, E. (2011). Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 25, 287–299. doi:10.1016/j.bpobgyn.2010.10.016
Burton, G. J., and Jauniaux, E. (2018). Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 218, S745-S761–S761. doi:10.1016/j.ajog.2017.11.577
Burton, G. J., and Jauniaux, E. (2023). The human placenta: new perspectives on its formation and function during early pregnancy. Proc. Biol. Sci. 290, 20230191. doi:10.1098/rspb.2023.0191
Burton, G. J., Redman, C. W., Roberts, J. M., and Moffett, A. (2019). Pre-eclampsia: pathophysiology and clinical implications. BMJ 366, l2381. doi:10.1136/bmj.l2381
Burton, G. J., Woods, A. W., Jauniaux, E., and Kingdom, J. C. (2009). Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. PLACENTA 30, 473–482. doi:10.1016/j.placenta.2009.02.009
Burton, G. J., and Yung, H. W. (2011). Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. PREGNANCY Hypertens. 1, 72–78. doi:10.1016/j.preghy.2010.12.002
Burton, G. J., Yung, H. W., and Murray, A. J. (2017). Mitochondrial - endoplasmic reticulum interactions in the trophoblast: stress and senescence. PLACENTA 52, 146–155. doi:10.1016/j.placenta.2016.04.001
Cattani, D., de Liz, O. C. V., Heinz, R. C., Domingues, J. T., Dal-Cim, T., Tasca, C. I., et al. (2014). Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. TOXICOLOGY 320, 34–45. doi:10.1016/j.tox.2014.03.001
Chen, C., Zhu, S., Fu, T., Chen, Y., Bai, L., and Chen, D. (2024). Targeting mitochondrial oxidative stress to protect against preterm birth and fetal brain injury via Nrf2 induction. Antioxid. Redox Signal. doi:10.1089/ars.2023.0382
Chen, P., Chen, F., Lei, J., and Zhou, B. (2023). Pomegranate polyphenol punicalagin improves learning memory deficits, redox homeostasis, and neuroinflammation in aging mice. Phytother. Res. 37, 3655–3674. doi:10.1002/ptr.7848
Cheng, G., Cao, Z., Xu, X., van Meir, E. G., and Lambeth, J. D. (2001). Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. GENE 269, 131–140. doi:10.1016/s0378-1119(01)00449-8
Cheong, J. N., Wlodek, M. E., Moritz, K. M., and Cuffe, J. S. (2016). Programming of maternal and offspring disease: impact of growth restriction, fetal sex and transmission across generations. J. Physiol. 594, 4727–4740. doi:10.1113/JP271745
Cid, M., and Gonzalez, M. (2016). Potential benefits of physical activity during pregnancy for the reduction of gestational diabetes prevalence and oxidative stress. EARLY Hum. Dev. 94, 57–62. doi:10.1016/j.earlhumdev.2016.01.007
Cindrova-Davies, T. (2009). Gabor than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. PLACENTA 30 (Suppl. A), S55–S65. doi:10.1016/j.placenta.2008.11.020
Cindrova-Davies, T., Fogarty, N., Jones, C., Kingdom, J., and Burton, G. J. (2018). Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. PLACENTA 68, 15–22. doi:10.1016/j.placenta.2018.06.307
Colleoni, F., Padmanabhan, N., Yung, H. W., Watson, E. D., Cetin, I., Tissot, V. P. M., et al. (2013). Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PLOS ONE 8, e55194. doi:10.1371/journal.pone.0055194
Cornelius, D. C., Castillo, J., Porter, J., Amaral, L. M., Campbell, N., Paige, A., et al. (2015). Blockade of CD40 ligand for intercellular communication reduces hypertension, placental oxidative stress, and AT1-AA in response to adoptive transfer of CD4+ T lymphocytes from RUPP rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1243–R1250. doi:10.1152/ajpregu.00273.2015
Coughlan, M. T., Vervaart, P. P., Permezel, M., Georgiou, H. M., and Rice, G. E. (2004). Altered placental oxidative stress status in gestational diabetes mellitus. PLACENTA 25, 78–84. doi:10.1016/S0143-4004(03)00183-8
Cuffe, J., Holland, O., Salomon, C., Rice, G. E., and Perkins, A. V. (2017b). Review: placental derived biomarkers of pregnancy disorders. PLACENTA 54, 104–110. doi:10.1016/j.placenta.2017.01.119
Cuffe, J. S., Xu, Z. C., and Perkins, A. V. (2017a). Biomarkers of oxidative stress in pregnancy complications. Biomark. Med. 11, 295–306. doi:10.2217/bmm-2016-0250
Cunningham, M. J., Castillo, J., Ibrahim, T., Cornelius, D. C., Campbell, N., Amaral, L., et al. (2018). AT1-AA (angiotensin II type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. HYPERTENSION 71, 886–893. doi:10.1161/HYPERTENSIONAHA.117.10681
Cunningham, M. W., Jayaram, A., Deer, E., Amaral, L. M., Vaka, V. R., Ibrahim, T., et al. (2020). Tumor necrosis factor alpha (TNF-α) blockade improves natural killer cell (NK) activation, hypertension, and mitochondrial oxidative stress in a preclinical rat model of preeclampsia. Hypertens. PREGNANCY 39, 399–404. doi:10.1080/10641955.2020.1793999
Dai, H., and Lu, X. (2022). MGST1 alleviates the oxidative stress of trophoblast cells induced by hypoxia/reoxygenation and promotes cell proliferation, migration, and invasion by activating the PI3K/AKT/mTOR pathway. Open Med. (Wars) 17, 2062–2071. doi:10.1515/med-2022-0617
Dai, Y., Sang, X. B., and Bai, W. P. (2024). N-Acetylcysteine and hydroxychloroquine ameliorate ADMA-induced fetal growth restriction in mice via regulating oxidative stress and autophagy. Reprod. Sci. 31, 779–790. doi:10.1007/s43032-023-01380-z
Davis, J. M., and Auten, R. L. (2010). Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 15, 191–195. doi:10.1016/j.siny.2010.04.001
Deer, E., Herrock, O., Campbell, N., Cornelius, D., Fitzgerald, S., Amaral, L. M., et al. (2023). The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 19, 257–270. doi:10.1038/s41581-022-00670-0
Deer, E., Jones, J., Cornelius, D. C., Comley, K., Herrock, O., Campbell, N., et al. (2021c). Progesterone induced blocking factor reduces hypertension and placental mitochondrial dysfunction in response to sFlt-1 during pregnancy. CELLS-BASEL, 10. doi:10.3390/cells10112817
Deer, E., Reeve, K. E., Amaral, L., Vaka, V. R., Franks, M., Campbell, N., et al. (2021a). CD4+ T cells cause renal and placental mitochondrial oxidative stress as mechanisms of hypertension in response to placental ischemia. Am. J. Physiol. Ren. Physiol. 320, F47–F54. doi:10.1152/ajprenal.00398.2020
Deer, E., Vaka, V. R., McMaster, K. M., Wallace, K., Cornelius, D. C., Amaral, L. M., et al. (2021b). Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for angiotension II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 3, 100275. doi:10.1016/j.ajogmf.2020.100275
Demir, B., Demir, S., Pasa, S., Guven, S., Atamer, Y., Atamer, A., et al. (2012). The role of homocysteine, asymmetric dimethylarginine and nitric oxide in pre-eclampsia. J. Obstet. Gynaecol. 32, 525–528. doi:10.3109/01443615.2012.693985
Depoix, C. L., de Selliers, I., Hubinont, C., and Debieve, F. (2017). HIF1A and EPAS1 potentiate hypoxia-induced upregulation of inhibin alpha chain expression in human term cytotrophoblasts in vitro. Mol. Hum. Reprod. 23, 199–209. doi:10.1093/molehr/gax002
Doganlar, Z. B., Guclu, H., Oztopuz, O., Turkon, H., Dogan, A., Uzun, M., et al. (2019). The role of melatonin in oxidative stress, DNA damage, apoptosis and angiogenesis in fetal eye under preeclampsia and melatonin deficiency stress. Curr. EYE Res. 44, 1157–1169. doi:10.1080/02713683.2019.1619778
Dominguez-Perles, R., Gil-Izquierdo, A., Ferreres, F., and Medina, S. (2019). Update on oxidative stress and inflammation in pregnant women, unborn children (nasciturus), and newborns - nutritional and dietary effects. Free Radic. Biol. Med. 142, 38–51. doi:10.1016/j.freeradbiomed.2019.03.013
Duan, Y., Sun, F., Que, S., Li, Y., Yang, S., and Liu, G. (2018). Prepregnancy maternal diabetes combined with obesity impairs placental mitochondrial function involving Nrf2/ARE pathway and detrimentally alters metabolism of offspring. Obes. Res. Clin. Pract. 12, 90–100. doi:10.1016/j.orcp.2017.01.002
Edvinsson, C., Hansson, E., Nielsen, N., Erlandsson, L., and Hansson, S. R. (2022). Intensive care patients with preeclampsia - clinical risk factors and biomarkers for oxidative stress and angiogenic imbalance as discriminators for severe disease. PREGNANCY Hypertens. 30, 88–94. doi:10.1016/j.preghy.2022.08.005
Endler, M., Saltvedt, S., Eweida, M., and Akerud, H. (2016). Oxidative stress and inflammation in retained placenta: a pilot study of protein and gene expression of GPX1 and NFκB. BMC Pregnancy Childbirth 16, 384. doi:10.1186/s12884-016-1135-1
England, L., and Zhang, J. (2007). Smoking and risk of preeclampsia: a systematic review. Front. Biosci. 12, 2471–2483. doi:10.2741/2248
Ergaz, Z., Guillemin, C., Neeman-Azulay, M., Weinstein-Fudim, L., Stodgell, C. J., Miller, R. K., et al. (2014). Placental oxidative stress and decreased global DNA methylation are corrected by copper in the Cohen diabetic rat. Toxicol. Appl. Pharmacol. 276, 220–230. doi:10.1016/j.taap.2014.02.017
Faulkner, J. L., Cornelius, D. C., Amaral, L. M., Harmon, A. C., Cunningham, M. J., Darby, M. M., et al. (2016). Vitamin D supplementation improves pathophysiology in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R346–R354. doi:10.1152/ajpregu.00388.2015
Feng, W., Wang, Y., Guo, N., Huang, P., and Mi, Y. (2020). Effects of astaxanthin on inflammation and insulin resistance in a mouse model of gestational diabetes mellitus. Dose Response 18, 1559325820926765. doi:10.1177/1559325820926765
Ferguson, K. K., McElrath, T. F., Chen, Y. H., Loch-Caruso, R., Mukherjee, B., and Meeker, J. D. (2015). Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am. J. Obstet. Gynecol. 212, 201–e8. doi:10.1016/j.ajog.2014.08.007
Ferreira, C. S., Pinto, G., Reis, D. L., Vigor, C., Goes, V. A., Guimaraes, D., et al. (2023). Placental F(4)-Neuroprostanes and F(2)-Isoprostanes are altered in gestational diabetes mellitus and maternal obesity. Prostagl. Leukot. Essent. Fat. Acids 189, 102529. doi:10.1016/j.plefa.2022.102529
Figueroa, H., Cifuentes, J., Lozano, M., Alvarado, C., Cabezas, C., Eixarch, E., et al. (2016). Nitric oxide synthase and changes in oxidative stress levels in embryonic kidney observed in a rabbit model of intrauterine growth restriction. Prenat. Diagn 36, 628–635. doi:10.1002/pd.4829
Fisher, J. J., Bartho, L. A., Perkins, A. V., and Holland, O. J. (2020). Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 47, 176–184. doi:10.1111/1440-1681.13172
Fisher, J. J., Vanderpeet, C. L., Bartho, L. A., McKeating, D. R., Cuffe, J., Holland, O. J., et al. (2021). Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus. J. Physiol. 599, 1291–1305. doi:10.1113/JP280593
Fitzgerald, S., Deer, E., Hogg, J., Cornelius, D. C., Turner, T., Amaral, L. M., et al. (2023). RUPP Th17s cause hypertension and mitochondrial dysfunction in the kidney and placenta during pregnancy. PREGNANCY Hypertens. 32, 50–56. doi:10.1016/j.preghy.2023.04.002
Fondjo, L. A., Mensah, J. B., Awuah, E. O., and Sakyi, S. A. (2024). Interplay between vitamin D status, vitamin D receptor gene variants and preeclampsia risk in Ghanaian women: a case-control study. PLOS ONE 19, e0303778. doi:10.1371/journal.pone.0303778
Fortis, M. F., Fraga, L. R., Boquett, J. A., Kowalski, T. W., Dutra, C. G., Goncalves, R. O., et al. (2018). Angiogenesis and oxidative stress-related gene variants in recurrent pregnancy loss. Reprod. Fertil. Dev. 30, 498–506. doi:10.1071/RD17117
Fujimaki, A., Watanabe, K., Mori, T., Kimura, C., Shinohara, K., and Wakatsuki, A. (2011). Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. PLACENTA 32, 367–372. doi:10.1016/j.placenta.2011.02.004
Fussell, J. C., Jauniaux, E., Smith, R. B., and Burton, G. J. (2024). Ambient air pollution and adverse birth outcomes: a review of underlying mechanisms. BJOG 131, 538–550. doi:10.1111/1471-0528.17727
Garcia-Contreras, C., Vazquez-Gomez, M., Barbero, A., Pesantez, J. L., Zinellu, A., Berlinguer, F., et al. (2019). Polyphenols and IUGR pregnancies: effects of maternal hydroxytyrosol supplementation on placental gene expression and fetal antioxidant status, DNA-methylation and phenotype. Int. J. Mol. Sci. 20, 1187. doi:10.3390/ijms20051187
Gauster, M., Majali-Martinez, A., Maninger, S., Gutschi, E., Greimel, P. H., Ivanisevic, M., et al. (2017). Maternal Type 1 diabetes activates stress response in early placenta. PLACENTA 50, 110–116. doi:10.1016/j.placenta.2017.01.118
Guerby, P., Swiader, A., Tasta, O., Pont, F., Rodriguez, F., Parant, O., et al. (2019). Modification of endothelial nitric oxide synthase by 4-oxo-2(E)-nonenal(ONE) in preeclamptic placentas. Free Radic. Biol. Med. 141, 416–425. doi:10.1016/j.freeradbiomed.2019.07.015
Gupta, S., Agarwal, A., and Sharma, R. K. (2005). The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 60, 807–816. doi:10.1097/01.ogx.0000193879.79268.59
Habibi, N., Jankovic-Karasoulos, T., Leemaqz, S. Y., Francois, M., Zhou, S. J., Leifert, W. R., et al. (2021b). Effect of iodine and selenium on proliferation, viability, and oxidative stress in HTR-8/SVneo placental cells. Biol. TRACE Elem. Res. 199, 1332–1344. doi:10.1007/s12011-020-02277-7
Habibi, N., Labrinidis, A., Leemaqz, S. Y., Jankovic-Karasoulos, T., McCullough, D., Grieger, J. A., et al. (2021a). Effect of selenium and iodine on oxidative stress in the first trimester human placenta explants. NUTRIENTS, 13. doi:10.3390/nu13030800
Han, F., Xu, L., Huang, Y., Chen, T., Zhou, T., and Yang, L. (2018). Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch. Gynecol. Obstet. 298, 631–638. doi:10.1007/s00404-018-4850-1
Harmon, A., Cornelius, D., Amaral, L., Paige, A., Herse, F., Ibrahim, T., et al. (2015). IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens. PREGNANCY 34, 291–306. doi:10.3109/10641955.2015.1032054
Harmon, A. C., Cornelius, D. C., Amaral, L. M., Faulkner, J. L., Cunningham, M. J., Wallace, K., et al. (2016). The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond) 130, 409–419. doi:10.1042/CS20150702
Hassoun, E. A., Walter, A. C., Alsharif, N. Z., and Stohs, S. J. (1997). Modulation of TCDD-induced fetotoxicity and oxidative stress in embryonic and placental tissues of C57BL/6J mice by vitamin E succinate and ellagic acid. TOXICOLOGY 124, 27–37. doi:10.1016/s0300-483x(97)00127-3
Hernandez-Trejo, M., Montoya-Estrada, A., Torres-Ramos, Y., Espejel-Nunez, A., Guzman-Grenfell, A., Morales-Hernandez, R., et al. (2017). Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunol. 18, 3. doi:10.1186/s12865-016-0184-6
Herrock, O. T., Deer, E., Amaral, L. M., Campbell, N., Lemon, J., Ingram, N., et al. (2023). B2 cells contribute to hypertension and natural killer cell activation possibly via AT1-AA in response to placental ischemia. Am. J. Physiol. Ren. Physiol. 324, F179–F192. doi:10.1152/ajprenal.00190.2022
Hofstee, P., Bartho, L. A., McKeating, D. R., Radenkovic, F., McEnroe, G., Fisher, J. J., et al. (2019). Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J. Physiol. 597, 5597–5617. doi:10.1113/JP278473
Hogan, C., and Perkins, A. V. (2022). Selenoproteins in the human placenta: how essential is selenium to a healthy start to life? NUTRIENTS, 14. doi:10.3390/nu14030628
Hogg, J. R., Campbell, N., Deer, E., Fitzgerald, S., Cornelius, D., Hoang, N., et al. (2024). The role of T cell stimulated agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) in mediating multiorgan dysfunction in IL-17 induced hypertension during pregnancy. Am. J. Reprod. Immunol. 91, e13843. doi:10.1111/aji.13843
Holland, O. J., Cuffe, J., Dekker, N. M., Callaway, L., Kwan, C. K., Radenkovic, F., et al. (2018). Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. CELL DEATH Dis. 9, 1150. doi:10.1038/s41419-018-1190-9
Hu, C., Yan, Y., Ji, F., and Zhou, H. (2021). Maternal obesity increases oxidative stress in placenta and it is associated with intestinal microbiota. Front. Cell Infect. Microbiol. 11, 671347. doi:10.3389/fcimb.2021.671347
Hu, C., Yang, Y., Deng, M., Yang, L., Shu, G., Jiang, Q., et al. (2020). Placentae for low birth weight piglets are vulnerable to oxidative stress, mitochondrial dysfunction, and impaired angiogenesis. Oxid. Med. CELL Longev. 2020, 8715412. doi:10.1155/2020/8715412
Hu, C., Yang, Y., Li, J., Wang, H., Cheng, C., Yang, L., et al. (2019a). Maternal diet-induced obesity compromises oxidative stress status and angiogenesis in the porcine placenta by upregulating Nox2 expression. Oxid. Med. CELL Longev. 2019, 2481592. doi:10.1155/2019/2481592
Hu, M., Zhang, Y., Guo, X., Jia, W., Liu, G., Zhang, J., et al. (2019b). Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am. J. Physiol. Endocrinol. Metab. 316, E794-E809–E809. doi:10.1152/ajpendo.00359.2018
Huang, L., Fan, L., Ding, P., He, Y. H., Xie, C., Niu, Z., et al. (2019). Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J. Matern. Fetal Neonatal Med. 32, 109–116. doi:10.1080/14767058.2017.1372415
Huang, M., Jiao, J., Wang, J., Xia, Z., and Zhang, Y. (2018). Characterization of acrylamide-induced oxidative stress and cardiovascular toxicity in zebrafish embryos. J. HAZARD MATER 347, 451–460. doi:10.1016/j.jhazmat.2018.01.016
Huang, Y. F., Wang, P. W., Huang, L. W., Lai, C. H., Yang, W., Wu, K. Y., et al. (2017). Prenatal nonylphenol and bisphenol A exposures and inflammation are determinants of oxidative/nitrative stress: a Taiwanese cohort study. Environ. Sci. Technol. 51, 6422–6429. doi:10.1021/acs.est.7b00801
Hubel, C. A. (1999). Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222, 222–235. doi:10.1177/153537029922200305
Jadhav, A., Khaire, A., and Joshi, S. (2020). Exploring the role of oxidative stress, fatty acids and neurotrophins in gestational diabetes mellitus. GROWTH factors. 38, 226–234. doi:10.1080/08977194.2021.1895143
Jan, A. T., Ali, A., and Haq, Q. (2011). Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J. Postgrad. Med. 57, 72–77. doi:10.4103/0022-3859.74298
Jauniaux, E., and Burton, G. J. (2016). The role of oxidative stress in placental-related diseases of pregnancy. J. Gynecol. Obstet. Biol. Reprod. Paris. 45, 775–785. doi:10.1016/j.jgyn.2016.02.012
Jauniaux, E., Hempstock, J., Greenwold, N., and Burton, G. J. (2003). Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 162, 115–125. doi:10.1016/S0002-9440(10)63803-5
Jauniaux, E., Johns, J., and Burton, G. J. (2005). The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet. Gynecol. 25, 613–624. doi:10.1002/uog.1892
Jauniaux, E., Watson, A. L., Hempstock, J., Bao, Y. P., Skepper, J. N., and Burton, G. J. (2000). Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 157, 2111–2122. doi:10.1016/S0002-9440(10)64849-3
Jin, J., Richardson, L., Sheller-Miller, S., Zhong, N., and Menon, R. (2018). Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. PLACENTA 67, 15–23. doi:10.1016/j.placenta.2018.05.008
Johnson, A. C., Tremble, S. M., Chan, S. L., Moseley, J., LaMarca, B., Nagle, K. J., et al. (2014). Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLOS ONE 9, e113670. doi:10.1371/journal.pone.0113670
Johnston, P. C., McCance, D. R., Holmes, V. A., Young, I. S., and McGinty, A. (2016). Placental antioxidant enzyme status and lipid peroxidation in pregnant women with type 1 diabetes: the effect of vitamin C and E supplementation. J. Diabetes Complicat. 30, 109–114. doi:10.1016/j.jdiacomp.2015.10.001
Jones, M. L., Mark, P. J., and Waddell, B. J. (2013). Maternal omega-3 fatty acid intake increases placental labyrinthine antioxidant capacity but does not protect against fetal growth restriction induced by placental ischaemia-reperfusion injury. REPRODUCTION 146, 539–547. doi:10.1530/REP-13-0282
Joo, E. H., Kim, Y. R., Kim, N., Jung, J. E., Han, S. H., and Cho, H. Y. (2021). Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int. J. Mol. Sci. 22, 10122. doi:10.3390/ijms221810122
Kalia, V., Baccarelli, A. A., Happel, C., Hollander, J. A., Jukic, A. M., McAllister, K. A., et al. (2023). Seminar: extracellular vesicles as mediators of environmental stress in human disease. Environ. Health Perspect. 131, 104201. doi:10.1289/EHP12980
Kammala, A. K., Richardson, L. S., Radnaa, E., Han, A., and Menon, R. (2023). Microfluidic technology and simulation models in studying pharmacokinetics during pregnancy. Front. Pharmacol. 14, 1241815. doi:10.3389/fphar.2023.1241815
Khera, A., Vanderlelie, J. J., Holland, O., and Perkins, A. V. (2017). Overexpression of endogenous anti-oxidants with selenium supplementation protects trophoblast cells from reactive oxygen species-induced apoptosis in a bcl-2-dependent manner. Biol. TRACE Elem. Res. 177, 394–403. doi:10.1007/s12011-016-0870-5
Khong, T. Y., Mooney, E. E., Ariel, I., Balmus, N. C., Boyd, T. K., Brundler, M. A., et al. (2016). Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab. Med. 140, 698–713. doi:10.5858/arpa.2015-0225-CC
Kim, J., Lee, K. S., Kim, J. H., Lee, D. K., Park, M., Choi, S., et al. (2017). Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 104, 185–198. doi:10.1016/j.freeradbiomed.2017.01.010
Kinalski, M., Sledziewski, A., Telejko, B., Kowalska, I., Kretowski, A., Zarzycki, W., et al. (2001). Lipid peroxidation, antioxidant defence and acid-base status in cord blood at birth: the influence of diabetes. Horm. Metab. Res. 33, 227–231. doi:10.1055/s-2001-14953
Kostic, S., Vilotic, A., Pirkovic, A., Dekanski, D., Borozan, S., Nacka-Aleksic, M., et al. (2022). Caffeic acid protects human trophoblast HTR-8/SVneo cells from H(2)O(2)-induced oxidative stress and genotoxicity. FOOD Chem. Toxicol. 163, 112993. doi:10.1016/j.fct.2022.112993
Kurlak, L. O., Mistry, H. D., Cindrova-Davies, T., Burton, G. J., and Broughton, P. F. (2016). Human placental renin-angiotensin system in normotensive and pre-eclamptic pregnancies at high altitude and after acute hypoxia-reoxygenation insult. J. Physiol. 594, 1327–1340. doi:10.1113/JP271045
Lappas, M., Hiden, U., Desoye, G., Froehlich, J., Hauguel-de, M. S., and Jawerbaum, A. (2011). The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal 15, 3061–3100. doi:10.1089/ars.2010.3765
Lappas, M., Mitton, A., and Permezel, M. (2010). In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J. Endocrinol. 204, 75–84. doi:10.1677/JOE-09-0321
Lappas, M., and Permezel, M. (2011). The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B(3) derivative, are elicited by FoxO3 in human gestational tissues: implications for preterm birth. J. Nutr. Biochem. 22, 1195–1201. doi:10.1016/j.jnutbio.2010.10.009
Lee, C. K., Wang, F. T., Huang, C. H., and Chan, W. H. (2023). Role of activated p21-activated kinase 2 in methylmercury-induced embryotoxic effects on mouse blastocysts. Toxicol. Res. (Camb) 12, 433–445. doi:10.1093/toxres/tfad030
Lee, Y. Y., Wong, C. K., Oger, C., Durand, T., Galano, J. M., and Lee, J. C. (2015). Prenatal exposure to the contaminant perfluorooctane sulfonate elevates lipid peroxidation during mouse fetal development but not in the pregnant dam. Free Radic. Res. 49, 1015–1025. doi:10.3109/10715762.2015.1027199
Leslie, E. M., Deeley, R. G., and Cole, S. P. (2005). Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 204, 216–237. doi:10.1016/j.taap.2004.10.012
Levine, R. J., Maynard, S. E., Qian, C., Lim, K. H., England, L. J., Yu, K. F., et al. (2004). Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683. doi:10.1056/NEJMoa031884
Lewandowska, M., Sajdak, S., and Lubinski, J. (2019). The role of early pregnancy maternal selenium levels on the risk for small-for-gestational age newborns. NUTRIENTS 11, 2298. doi:10.3390/nu11102298
Li, Y., Wang, X., Zhu, Q., Xu, Y., Fu, Q., Wang, T., et al. (2023). Organophosphate flame retardants in pregnant women: sources, occurrence, and potential risks to pregnancy outcomes. Environ. Sci. Technol. 57, 7109–7128. doi:10.1021/acs.est.2c06503
Liang, T., Jinglong, X., Shusheng, D., and Aiyou, W. (2018). Maternal obesity stimulates lipotoxicity and up-regulates inflammatory signaling pathways in the full-term swine placenta. Anim. Sci. J. 89, 1310–1322. doi:10.1111/asj.13064
Lista, G., Castoldi, F., Compagnoni, G., Maggioni, C., Cornelissen, G., and Halberg, F. (2010). Neonatal and maternal concentrations of hydroxil radical and total antioxidant system: protective role of placenta against fetal oxidative stress. Neuro Endocrinol. Lett. 31, 319–324.
Liu, M., Liu, J., Xiong, F., Xu, K., Pu, Y., Huang, J., et al. (2023). Research advances of microplastics and potential health risks of microplastics on terrestrial higher mammals: a bibliometric analysis and literature review. Environ. Geochem Health 45, 2803–2838. doi:10.1007/s10653-022-01458-8
Liu, S. H., Huang, J. P., Lee, R. K., Huang, M. C., Wu, Y. H., Chen, C. Y., et al. (2010). Paracrine factors from human placental multipotent mesenchymal stromal cells protect endothelium from oxidative injury via STAT3 and manganese superoxide dismutase activation. Biol. Reprod. 82, 905–913. doi:10.1095/biolreprod.109.081828
Liu, T., Zheng, W., Wang, L., Wang, L., and Zhang, Y. (2020). TLR4/NF-κB signaling pathway participates in the protective effects of apocynin on gestational diabetes mellitus induced placental oxidative stress and inflammation. Reprod. Sci. 27, 722–730. doi:10.1007/s43032-019-00078-5
Llanos, M. N., and Ronco, A. M. (2009). Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod. Toxicol. 27, 88–92. doi:10.1016/j.reprotox.2008.11.057
Loch-Caruso, R., Hassan, I., Harris, S. M., Kumar, A., Bjork, F., and Lash, L. H. (2019). Trichloroethylene exposure in mid-pregnancy decreased fetal weight and increased placental markers of oxidative stress in rats. Reprod. Toxicol. 83, 38–45. doi:10.1016/j.reprotox.2018.11.002
Lorenzoni, F., Giampietri, M., Ferri, G., Lunardi, S., Madrigali, V., Battini, L., et al. (2013). Lutein administration to pregnant women with gestational diabetes mellitus is associated to a decrease of oxidative stress in newborns. Gynecol. Endocrinol. 29, 901–903. doi:10.3109/09513590.2013.808329
Loverro, G., Greco, P., Capuano, F., Carone, D., Cormio, G., and Selvaggi, L. (1996). Lipoperoxidation and antioxidant enzymes activity in pregnancy complicated with hypertension. Eur. J. Obstet. Gynecol. Reprod. Biol. 70, 123–127. doi:10.1016/s0301-2115(95)02561-8
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. doi:10.1016/s0021-9258(19)52451-6
Lu, Z., Guo, Y., Xu, D., Xiao, H., Dai, Y., Liu, K., et al. (2023). Developmental toxicity and programming alterations of multiple organs in offspring induced by medication during pregnancy. ACTA Pharm. SIN. B 13, 460–477. doi:10.1016/j.apsb.2022.05.029
Luo, Z., Luo, W., Li, S., Zhao, S., Sho, T., Xu, X., et al. (2018). Reactive oxygen species mediated placental oxidative stress, mitochondrial content, and cell cycle progression through mitogen-activated protein kinases in intrauterine growth restricted pigs. Reprod. Biol. 18, 422–431. doi:10.1016/j.repbio.2018.09.002
Lyall, F., Gibson, J. L., Greer, I. A., Brockman, D. E., Eis, A. L., and Myatt, L. (1998). Increased nitrotyrosine in the diabetic placenta: evidence for oxidative stress. DIABETES CARE 21, 1753–1758. doi:10.2337/diacare.21.10.1753
Ma, L., Zhang, Z., Dong, K., and Ma, Y. (2019). TWIST1 alleviates hypoxia-induced damage of trophoblast cells by inhibiting mitochondrial apoptosis pathway. Exp. CELL Res. 385, 111687. doi:10.1016/j.yexcr.2019.111687
Malti, N., Merzouk, H., Merzouk, S. A., Loukidi, B., Karaouzene, N., Malti, A., et al. (2014). Oxidative stress and maternal obesity: feto-placental unit interaction. PLACENTA 35, 411–416. doi:10.1016/j.placenta.2014.03.010
Manoharan, B., Bobby, Z., Dorairajan, G., Jacob, S. E., Gladwin, V., Vinayagam, V., et al. (2019). Increased placental expressions of nuclear factor erythroid 2-related factor 2 and antioxidant enzymes in gestational diabetes: protective mechanisms against the placental oxidative stress? Eur. J. Obstet. Gynecol. Reprod. Biol. 238, 78–85. doi:10.1016/j.ejogrb.2019.05.016
Marin, R., Chiarello, D. I., Abad, C., Rojas, D., Toledo, F., and Sobrevia, L. (2020). Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165961. doi:10.1016/j.bbadis.2020.165961
Matsubara, K., Higaki, T., Matsubara, Y., and Nawa, A. (2015). Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 16, 4600–4614. doi:10.3390/ijms16034600
Matsubara, K., Matsubara, Y., Hyodo, S., Katayama, T., and Ito, M. (2010). Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J. Obstet. Gynaecol. Res. 36, 239–247. doi:10.1111/j.1447-0756.2009.01128.x
Matsubara, S., and Sato, I. (2001). Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts: normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem Cell Biol. 116, 1–7. doi:10.1007/s004180100301
Mauchart, P., Vass, R. A., Nagy, B., Sulyok, E., Bodis, J., and Kovacs, K. (2023). Oxidative stress in assisted reproductive techniques, with a focus on an underestimated risk factor. Curr. ISSUES Mol. Biol. 45, 1272–1286. doi:10.3390/cimb45020083
Maynard, S. E., Min, J. Y., Merchan, J., Lim, K. H., Li, J., Mondal, S., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. INVEST 111, 649–658. doi:10.1172/JCI17189
McCoski, S. R., Vailes, M. T., Owens, C. E., Cockrum, R. R., and Ealy, A. D. (2018). Exposure to maternal obesity alters gene expression in the preimplantation ovine conceptus. BMC GENOMICS 19, 737. doi:10.1186/s12864-018-5120-0
Menon, R. (2014). Oxidative stress damage as a detrimental factor in preterm birth pathology. Front. Immunol. 5, 567. doi:10.3389/fimmu.2014.00567
Menon, R. (2022). Fetal inflammatory response at the fetomaternal interface: a requirement for labor at term and preterm. Immunol. Rev. 308, 149–167. doi:10.1111/imr.13075
Menon, R., Dixon, C. L., Sheller-Miller, S., Fortunato, S. J., Saade, G. R., Palma, C., et al. (2019). Quantitative proteomics by SWATH-MS of maternal plasma exosomes determine pathways associated with term and preterm birth. ENDOCRINOLOGY 160, 639–650. doi:10.1210/en.2018-00820
Michalczyk, M., Celewicz, A., Celewicz, M., Wozniakowska-Gondek, P., and Rzepka, R. (2020). The role of inflammation in the pathogenesis of preeclampsia. Mediat. Inflamm. 2020, 3864941. doi:10.1155/2020/3864941
Mikkelsen, E., Huppertz, B., Singh, R., Ravn, K., Hatt, L., Kruhoffer, M., et al. (2023). mRNA and protein expression in human fetal membrane cells: potential biomarkers for preterm prelabor rupture of the fetal membranes? Int. J. Mol. Sci., 24. doi:10.3390/ijms242115826
Milczarek, R., Klimek, J., and Zelewski, L. (2000). Melatonin inhibits NADPH-dependent lipid peroxidation in human placental mitochondria. Horm. Metab. Res. 32, 84–85. doi:10.1055/s-2007-978595
Milczarek, R., Sokolowska, E., Hallmann, A., and Klimek, J. (2007). The NADPH- and iron-dependent lipid peroxidation in human placental microsomes. Mol. CELL Biochem. 295, 105–111. doi:10.1007/s11010-006-9279-3
Miller, S. L., Yawno, T., Alers, N. O., Castillo-Melendez, M., Supramaniam, V. G., VanZyl, N., et al. (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. PINEAL Res. 56, 283–294. doi:10.1111/jpi.12121
Miyagami, S., Koide, K., Sekizawa, A., Ventura, W., Yotsumoto, J., Oishi, S., et al. (2013). Physiological changes in the pattern of placental gene expression early in the first trimester. Reprod. Sci. 20, 710–714. doi:10.1177/1933719112466309
Moon, K. C., Park, C. W., Park, J. S., and Jun, J. K. (2021). Fetal growth restriction and subsequent low grade fetal inflammatory response are associated with early-onset neonatal sepsis in the context of early preterm sterile intrauterine environment. J. Clin. Med., 10. doi:10.3390/jcm10092018
Mou, D., Ding, D., Yan, H., Qin, B., Dong, Y., Li, Z., et al. (2020). Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. FOOD Funct. 11, 7748–7761. doi:10.1039/d0fo00832j
Murphy, B. J., Kimura, T., Sato, B. G., Shi, Y., and Andrews, G. K. (2008). Metallothionein induction by hypoxia involves cooperative interactions between metal-responsive transcription factor-1 and hypoxia-inducible transcription factor-1alpha. Mol. CANCER Res. 6, 483–490. doi:10.1158/1541-7786.MCR-07-0341
Myatt, L., and Cui, X. (2004). Oxidative stress in the placenta. HISTOCHEM CELL Biol. 122, 369–382. doi:10.1007/s00418-004-0677-x
Naav, A., Erlandsson, L., Isaxon, C., Asander, F. E., Ehinger, J., Sporre, M. K., et al. (2020). Urban PM2.5 induces cellular toxicity, hormone dysregulation, oxidative damage, inflammation, and mitochondrial interference in the HRT8 trophoblast cell line. Front. Endocrinol. (Lausanne) 11, 75. doi:10.3389/fendo.2020.00075
Napso, T., Lean, S. C., Lu, M., Mort, E. J., Desforges, M., Moghimi, A., et al. (2022). Diet-induced maternal obesity impacts feto-placental growth and induces sex-specific alterations in placental morphology, mitochondrial bioenergetics, dynamics, lipid metabolism and oxidative stress in mice. Acta Physiol. (Oxf) 234, e13795. doi:10.1111/apha.13795
Nunes, P. R., Gomes, V. J., Sandrim, V. C., Peracoli, J. C., Peracoli, M., and Carlstrom, M. (2021). Effects of vitamin D-induced supernatant of placental explants from preeclamptic women on oxidative stress and nitric oxide bioavailability in human umbilical vein endothelial cells. BRAZ J. Med. Biol. Res. 54, e11073. doi:10.1590/1414-431X2020e11073
Nunes, P. R., Romao-Veiga, M., Matias, M. L., Ribeiro, V. R., de Oliveira, L., Peracoli, J. C., et al. (2022). Vitamin D decreases expression of NLRP1 and NLRP3 inflammasomes in placental explants from women with preeclampsia cultured with hydrogen peroxide. Hum. Immunol. 83, 74–80. doi:10.1016/j.humimm.2021.10.002
Odame, A. E., Owiredu, W., Sakyi, S. A., Turpin, C. A., Ephraim, R., Fondjo, L. A., et al. (2018). Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: a prospective cohort study in Ghana. PLOS ONE 13, e200581. doi:10.1371/journal.pone.0200581
Okatani, Y., Wakatsuki, A., Shinohara, K., Taniguchi, K., and Fukaya, T. (2001). Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J. PINEAL Res. 31, 173–178. doi:10.1034/j.1600-079x.2001.310212.x
Ornoy, A., Tsadok, M. A., Yaffe, P., and Zangen, S. W. (2009). The Cohen diabetic rat as a model for fetal growth restriction: vitamins C and E reduce fetal oxidative stress but do not restore normal growth. Reprod. Toxicol. 28, 521–529. doi:10.1016/j.reprotox.2009.06.005
Park, S. G., Lin, C., Gwon, L. W., Lee, J. G., Baek, I. J., Lee, B. J., et al. (2020). Protection of lycopene against embryonic anomalies and yolk sac placental vasculogenic disorders induced by nicotine exposure. Biomed. Res. Int. 2020, 7957045. doi:10.1155/2020/7957045
Parra, M., Rodrigo, R., Barja, P., Bosco, C., Fernandez, V., Munoz, H., et al. (2005). Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am. J. Obstet. Gynecol. 193, 1486–1491. doi:10.1016/j.ajog.2005.02.109
Perez-Albaladejo, E., Fernandes, D., Lacorte, S., and Porte, C. (2017). Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. TOXICOL VITRO 38, 41–48. doi:10.1016/j.tiv.2016.11.003
Perkins, A. V. (2011). Placental oxidative stress, selenium and preeclampsia. PREGNANCY Hypertens. 1, 95–99. doi:10.1016/j.preghy.2010.10.008
Perrone, S., Tataranno, M. L., Negro, S., Longini, M., Toti, M. S., Alagna, M. G., et al. (2016). Placental histological examination and the relationship with oxidative stress in preterm infants. PLACENTA 46, 72–78. doi:10.1016/j.placenta.2016.08.084
Phannasorn, W., Pharapirom, A., Thiennimitr, P., Guo, H., Ketnawa, S., and Wongpoomchai, R. (2022). Enriched riceberry bran oil exerts chemopreventive properties through anti-inflammation and alteration of gut microbiota in carcinogen-induced liver and colon carcinogenesis in rats. Cancers (Basel), 14. doi:10.3390/cancers14184358
Phengpol, N., Thongnak, L., and Lungkaphin, A. (2023). The programming of kidney injury in offspring affected by maternal overweight and obesity: role of lipid accumulation, inflammation, oxidative stress, and fibrosis in the kidneys of offspring. J. Physiol. Biochem. 79, 1–17. doi:10.1007/s13105-022-00927-z
Pillai, A., and Gupta, S. (2005). Effect of gestational and lactational exposure to lead and/or cadmium on reproductive performance and hepatic oestradiol metabolising enzymes. Toxicol. Lett. 155, 179–186. doi:10.1016/j.toxlet.2004.09.015
Poston, L., Igosheva, N., Mistry, H. D., Seed, P. T., Shennan, A. H., Rana, S., et al. (2011). Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am. J. Clin. Nutr. 94, 1980S-1985S–1985S. doi:10.3945/ajcn.110.001156
Prater, M., Hamilton, R. S., Wa, Y. H., Sharkey, A. M., Robson, P., Abd, H. N., et al. (2021). RNA-Seq reveals changes in human placental metabolism, transport and endocrinology across the first-second trimester transition. Biol. OPEN 10, bio058222. doi:10.1242/bio.058222
Pressman, E. K., Cavanaugh, J. L., Mingione, M., Norkus, E. P., and Woods, J. R. (2003). Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: a randomized, double-blind study. Am. J. Obstet. Gynecol. 189, 1720–1725. doi:10.1016/s0002-9378(03)00858-5
Puatanachokchai, R., Morimura, K., Wanibuchi, H., Oka, M., Kinoshita, A., Mitsuru, F., et al. (2006). Alpha-benzene hexachloride exerts hormesis in preneoplastic lesion formation of rat hepatocarcinogenesis with the possible role for hepatic detoxifying enzymes. CANCER Lett. 240, 102–113. doi:10.1016/j.canlet.2005.09.006
Pustovrh, M. C., Jawerbaum, A., Capobianco, E., White, V., Martinez, N., Lopez-Costa, J. J., et al. (2005). Oxidative stress promotes the increase of matrix metalloproteinases-2 and -9 activities in the feto-placental unit of diabetic rats. Free Radic. Res. 39, 1285–1293. doi:10.1080/10715760500188796
Raffaeli, G., Manzoni, F., Cortesi, V., Cavallaro, G., Mosca, F., and Ghirardello, S. (2020). Iron homeostasis disruption and oxidative stress in preterm newborns. NUTRIENTS 12, 1554. doi:10.3390/nu12061554
Rani, N., Dhingra, R., Arya, D. S., Kalaivani, M., Bhatla, N., and Kumar, R. (2010). Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J. Obstet. Gynaecol. Res. 36, 1189–1194. doi:10.1111/j.1447-0756.2010.01303.x
Redman, C., Staff, A. C., and Roberts, J. M. (2022). Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am. J. Obstet. Gynecol. 226, S907–S927. doi:10.1016/j.ajog.2020.09.047
Redman, C. W., and Sargent, I. L. (2005). Latest advances in understanding preeclampsia. SCIENCE 308, 1592–1594. doi:10.1126/science.1111726
Richard, K., Holland, O., Landers, K., Vanderlelie, J. J., Hofstee, P., Cuffe, J., et al. (2017). Review: effects of maternal micronutrient supplementation on placental function. PLACENTA 54, 38–44. doi:10.1016/j.placenta.2016.12.022
Richter, H. G., Hansell, J. A., Raut, S., and Giussani, D. A. (2009). Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. PINEAL Res. 46, 357–364. doi:10.1111/j.1600-079X.2009.00671.x
Riggs, D. W., Zafar, N., Krishnasamy, S., Yeager, R., Rai, S. N., Bhatnagar, A., et al. (2020). Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ. Res. 180, 108890. doi:10.1016/j.envres.2019.108890
Roberts, J. M., and Cooper, D. W. (2001). Pathogenesis and genetics of pre-eclampsia. LANCET 357, 53–56. doi:10.1016/s0140-6736(00)03577-7
Roberts, V. H., Smith, J., McLea, S. A., Heizer, A. B., Richardson, J. L., and Myatt, L. (2009). Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. PLACENTA 30, 169–175. doi:10.1016/j.placenta.2008.11.019
Rodriguez-Gonzalez, G. L., Vargas-Hernandez, L., Reyes-Castro, L. A., Ibanez, C. A., Bautista, C. J., Lomas-Soria, C., et al. (2022). Resveratrol supplementation in obese pregnant rats improves maternal metabolism and prevents increased placental oxidative stress. Antioxidants (Basel), 11. doi:10.3390/antiox11101871
Rojas, D., Abad, C., Pinero, S., Medina, Y., Chiarello, D. I., Proverbio, F., et al. (2022). Effect of Mg-gluconate on the osmotic fragility of red blood cells, lipid peroxidation, and Ca(2+)-ATPase (PMCA) activity of placental homogenates and red blood cell ghosts from salt-loaded pregnant rats. Front. Physiol. 13, 794572. doi:10.3389/fphys.2022.794572
Rudge, M. V., Damasceno, D. C., Volpato, G. T., Almeida, F. C., Calderon, I. M., and Lemonica, I. P. (2007). Effect of Ginkgo biloba on the reproductive outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. BRAZ J. Med. Biol. Res. 40, 1095–1099. doi:10.1590/s0100-879x2006005000132
Rueangdetnarong, H., Sekararithi, R., Jaiwongkam, T., Kumfu, S., Chattipakorn, N., Tongsong, T., et al. (2018). Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: cohort study. Endocr. Connect. 7, 681–687. doi:10.1530/EC-18-0093
Rundlof, A. K., and Arner, E. S. (2004). Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid. Redox Signal 6, 41–52. doi:10.1089/152308604771978336
Rusterholz, C., Hahn, S., and Holzgreve, W. (2007). Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 29, 151–162. doi:10.1007/s00281-007-0071-6
Saben, J., Lindsey, F., Zhong, Y., Thakali, K., Badger, T. M., Andres, A., et al. (2014). Maternal obesity is associated with a lipotoxic placental environment. PLACENTA 35, 171–177. doi:10.1016/j.placenta.2014.01.003
Sanchez-Aranguren, L. C., Prada, C. E., Riano-Medina, C. E., and Lopez, M. (2014). Endothelial dysfunction and preeclampsia: role of oxidative stress. Front. Physiol. 5, 372. doi:10.3389/fphys.2014.00372
Schliefsteiner, C., Hirschmugl, B., Kopp, S., Curcic, S., Bernhart, E. M., Marsche, G., et al. (2017). Maternal Gestational Diabetes Mellitus increases placental and foetal lipoprotein-associated Phospholipase A2 which might exert protective functions against oxidative stress. Sci. Rep. 7, 12628. doi:10.1038/s41598-017-13051-6
Scioscia, M., Greco, P., Selvaggi, L. E., and Rademacher, T. W. (2009). Is there a link between insulin resistance and inflammatory activation in preeclampsia? Med. HYPOTHESES 73, 813–817. doi:10.1016/j.mehy.2009.01.057
Sha, H., Zeng, H., Zhao, J., and Jin, H. (2019). Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur. J. Pharmacol. 859, 172522. doi:10.1016/j.ejphar.2019.172522
Shachar, B. Z., Carmichael, S. L., Stevenson, D. K., and Shaw, G. M. (2013). Could genetic polymorphisms related to oxidative stress modulate effects of heavy metals for risk of human preterm birth? Reprod. Toxicol. 42, 24–26. doi:10.1016/j.reprotox.2013.06.072
Shang, M., Dong, X., and Hou, L. (2018). Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J. Obstet. Gynaecol. Res. 44, 637–646. doi:10.1111/jog.13586
Shang, M., Zhao, J., Yang, L., and Lin, L. (2015). Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res. Clin. Pract. 109, 404–410. doi:10.1016/j.diabres.2015.05.010
Sheller-Miller, S., Lei, J., Saade, G., Salomon, C., Burd, I., and Menon, R. (2016). Feto-maternal trafficking of exosomes in murine pregnancy models. Front. Pharmacol. 7, 432. doi:10.3389/fphar.2016.00432
Shepherd, M. C., Radnaa, E., Tantengco, O. A., Kechichian, T., Urrabaz-Garza, R., Kammala, A. K., et al. (2021). Extracellular vesicles from maternal uterine cells exposed to risk factors cause fetal inflammatory response. CELL Commun. SIGNAL 19, 100. doi:10.1186/s12964-021-00782-3
Shi, M., Chen, X., Li, H., and Zheng, L. (2021). δ-tocotrienol suppresses the migration and angiogenesis of trophoblasts in preeclampsia and promotes their apoptosis via miR-429/ZEB1 axis. BIOENGINEERED 12, 1861–1873. doi:10.1080/21655979.2021.1923238
Shorey-Kendrick, L. E., McEvoy, C. T., O'Sullivan, S. M., Milner, K., Vuylsteke, B., Tepper, R. S., et al. (2021). Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: association with gene expression and respiratory outcomes. Clin. EPIGENETICS 13, 177. doi:10.1186/s13148-021-01161-y
Sibai, B., Dekker, G., and Kupferminc, M. (2005). Pre-eclampsia. LANCET 365, 785–799. doi:10.1016/S0140-6736(05)17987-2
Singh, V. P., McKinney, S., and Gerton, J. L. (2020). Persistent DNA damage and senescence in the placenta impacts developmental outcomes of embryos. Dev. CELL 54, 333–347. doi:10.1016/j.devcel.2020.05.025
Soobryan, N., Murugesan, S., Phoswa, W., Gathiram, P., Moodley, J., and Mackraj, I. (2017). The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia. Eur. J. Pharmacol. 795, 101–107. doi:10.1016/j.ejphar.2016.12.010
Stark, J. M. (1993). Pre-eclampsia and cytokine induced oxidative stress. Br. J. Obstet. Gynaecol. 100, 105–109. doi:10.1111/j.1471-0528.1993.tb15203.x
Stark, M. J., Clifton, V. L., and Hodyl, N. A. (2013). Differential effects of docosahexaenoic acid on preterm and term placental pro-oxidant/antioxidant balance. REPRODUCTION 146, 243–251. doi:10.1530/REP-13-0239
Stark, M. J., Hodyl, N. A., Wright, I. M., and Clifton, V. L. (2011). Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. PLACENTA 32, 865–870. doi:10.1016/j.placenta.2011.08.010
Steegers, E. A., von Dadelszen, P., Duvekot, J. J., and Pijnenborg, R. (2010). Pre-eclampsia. LANCET 376, 631–644. doi:10.1016/S0140-6736(10)60279-6
Stefanovic, V., Andersson, S., and Vento, M. (2019). Oxidative stress - related spontaneous preterm delivery challenges in causality determination, prevention and novel strategies in reduction of the sequelae. Free Radic. Biol. Med. 142, 52–60. doi:10.1016/j.freeradbiomed.2019.06.008
Takagi, Y., Nikaido, T., Toki, T., Kita, N., Kanai, M., Ashida, T., et al. (2004). Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. VIRCHOWS Arch. 444, 49–55. doi:10.1007/s00428-003-0903-2
Tenorio, M. B., Ferreira, R. C., Moura, F. A., Bueno, N. B., de Oliveira, A., and Goulart, M. (2019). Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid. Med. CELL Longev. 2019, 8238727. doi:10.1155/2019/8238727
Than, N. G., Romero, R., Tarca, A. L., Draghici, S., Erez, O., Chaiworapongsa, T., et al. (2009). Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J. Matern. Fetal Neonatal Med. 22, 1000–1013. doi:10.3109/14767050903019676
Tissot, V. P. M., Murray, A. J., Beckey, V., Cindrova-Davies, T., Johns, J., Zwerdlinger, L., et al. (2010). Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R166–R172. doi:10.1152/ajpregu.00383.2009
Triantafyllidou, P., Papadopoulou, A., Thymara, E., Papaevangelou, V., Mastorakos, G., Papadimitriou, A., et al. (2023). Aortic intima-media thickness is increased in neonates of mothers with gestational diabetes mellitus: the role of thioredoxin-interacting protein as a marker of oxidative stress. Curr. Vasc. Pharmacol. 21, 234–245. doi:10.2174/1570161121666230727150854
Turpin, C. A., Sakyi, S. A., Owiredu, W. K., Ephraim, R. K., and Anto, E. O. (2015). Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 15, 189. doi:10.1186/s12884-015-0624-y
Vafaei, H., Kavari, G., Izadi, H. R., Zare, D. Z., Dianatpour, M., Daneshparvar, A., et al. (2020). Wi-Fi (2.4 GHz) affects anti-oxidant capacity, DNA repair genes expression and, apoptosis in pregnant mouse placenta. Iran. J. BASIC Med. Sci. 23, 833–840. doi:10.22038/ijbms.2020.40184.9512
Vaka, R., Deer, E., Cunningham, M., McMaster, K. M., Wallace, K., Cornelius, D. C., et al. (2021). Characterization of mitochondrial bioenergetics in preeclampsia. J. Clin. Med. 10, 5063. doi:10.3390/jcm10215063
Vaka, R., Deer, E., and LaMarca, B. (2022). Is mitochondrial oxidative stress a viable therapeutic target in preeclampsia? Antioxidants (Basel), 11. doi:10.3390/antiox11020210
Vanderlelie, J., Venardos, K., Clifton, V. L., Gude, N. M., Clarke, F. M., and Perkins, A. V. (2005). Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. PLACENTA 26, 53–58. doi:10.1016/j.placenta.2004.04.002
Vaughan, J. E., and Walsh, S. W. (2005). Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am. J. Obstet. Gynecol. 193, 830–835. doi:10.1016/j.ajog.2005.01.057
Vidal, M. J., Richardson, L. S., Kumar, K. A., Kim, S., Lam, P. Y., Cherukuri, R., et al. (2024). Endocrine-disrupting compounds and their impact on human placental function: evidence from placenta organ-on-chip studies. Lab. CHIP 24, 1727–1749. doi:10.1039/d3lc00998j
Vieira, L. D., Farias, J. S., de Queiroz, D. B., Cabral, E. V., Lima-Filho, M. M., Sant'Helena, B., et al. (2018). Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: prevention by early treatment with α-tocopherol. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3577–3587. doi:10.1016/j.bbadis.2018.09.019
Volpato, G. T., Calderon, I. M., Sinzato, S., Campos, K. E., Rudge, M. V., and Damasceno, D. C. (2011). Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 138, 691–696. doi:10.1016/j.jep.2011.09.044
Volpato, G. T., Damasceno, D. C., Rudge, M. V., Padovani, C. R., and Calderon, I. M. (2008). Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 116, 131–137. doi:10.1016/j.jep.2007.11.013
Vural, P., Degirmencioglu, S., Dogru-Abbasoglu, S., Saral, N. Y., Akgul, C., and Uysal, M. (2009). Genetic polymorphisms in DNA repair gene APE1, XRCC1 and XPD and the risk of pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 146, 160–164. doi:10.1016/j.ejogrb.2009.06.007
Wakimoto, T., Uchida, K., Mimura, K., Kanagawa, T., Mehandjiev, T. R., Aoshima, H., et al. (2015). Hydroxylated fullerene: a potential antiinflammatory and antioxidant agent for preventing mouse preterm birth. Am. J. Obstet. Gynecol. 213, 701–e9. doi:10.1016/j.ajog.2015.07.017
Walker, O. S., Ragos, R., Wong, M. K., Adam, M., Cheung, A., and Raha, S. (2020). Reactive oxygen species from mitochondria impacts trophoblast fusion and the production of endocrine hormones by syncytiotrophoblasts. PLOS ONE 15, e0229332. doi:10.1371/journal.pone.0229332
Wang, B., Xu, S., Lu, X., Ma, L., Gao, L., Zhang, S. Y., et al. (2020c). Reactive oxygen species-mediated cellular genotoxic stress is involved in 1-nitropyrene-induced trophoblast cycle arrest and fetal growth restriction. Environ. Pollut. 260, 113984. doi:10.1016/j.envpol.2020.113984
Wang, G. J., Yang, Z., Huai, J., and Xiang, Q. Q. (2020b). Pravastatin alleviates oxidative stress and decreases placental trophoblastic cell apoptosis through IL-6/STAT3 signaling pathway in preeclampsia rats. Eur. Rev. Med. Pharmacol. Sci. 24, 12955–12962. doi:10.26355/eurrev_202012_24199
Wang, N., Dong, X., Shi, D., Li, N., and Zhang, Q. (2020a). Cryptotanshinone ameliorates placental oxidative stress and inflammation in mice with gestational diabetes mellitus. Arch. Pharm. Res. 43, 755–764. doi:10.1007/s12272-020-01242-1
Wang, Y., and Walsh, S. W. (2001). Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. PLACENTA 22, 206–212. doi:10.1053/plac.2000.0608
Wang, Z., and Wang, Z. (2020). Nanoparticles induced embryo-fetal toxicity. Toxicol. Ind. HEALTH 36, 181–213. doi:10.1177/0748233720918689
Wu, Q., Gai, S., and Zhang, H. (2022). Asperulosidic acid, a bioactive iridoid, alleviates placental oxidative stress and inflammatory responses in gestational diabetes mellitus by suppressing NF-κB and MAPK signaling pathways. PHARMACOLOGY 107, 197–205. doi:10.1159/000521080
Xuan, R. R., Niu, T. T., and Chen, H. M. (2016). Astaxanthin blocks preeclampsia progression by suppressing oxidative stress and inflammation. Mol. Med. Rep. 14, 2697–2704. doi:10.3892/mmr.2016.5569
Yang, Y., Jin, H., Qiu, Y., Liu, Y., Wen, L., Fu, Y., et al. (2022). Reactive oxygen species are essential for placental angiogenesis during early gestation. Oxid. Med. CELL Longev. 2022, 4290922. doi:10.1155/2022/4290922
Yang, Y., and Shang, H. (2021). Silencing lncRNA-DGCR5 increased trophoblast cell migration, invasion and tube formation, and inhibited cell apoptosis via targeting miR-454-3p/GADD45A axis. Mol. CELL Biochem. 476, 3407–3421. doi:10.1007/s11010-021-04161-x
Yoshida, A., Watanabe, K., Iwasaki, A., Kimura, C., Matsushita, H., and Wakatsuki, A. (2018). Placental oxidative stress and maternal endothelial function in pregnant women with normotensive fetal growth restriction. J. Matern. Fetal Neonatal Med. 31, 1051–1057. doi:10.1080/14767058.2017.1306510
Yoshihara, M., Mizutani, S., Matsumoto, K., Kato, Y., Masuo, Y., Tano, S., et al. (2022). Crosstalk between foetal vasoactive peptide hormones and placental aminopeptidases regulates placental blood flow: its significance in preeclampsia. PLACENTA 121, 32–39. doi:10.1016/j.placenta.2022.02.016
Yung, H. W., Colleoni, F., Dommett, E., Cindrova-Davies, T., Kingdom, J., Murray, A. J., et al. (2019). Noncanonical mitochondrial unfolded protein response impairs placental oxidative phosphorylation in early-onset preeclampsia. Proc. Natl. Acad. Sci. U. S. A. 116, 18109–18118. doi:10.1073/pnas.1907548116
Zadrozna, M., Gawlik, M., Nowak, B., Marcinek, A., Mrowiec, H., Walas, S., et al. (2009). Antioxidants activities and concentration of selenium, zinc and copper in preterm and IUGR human placentas. J. Trace Elem. Med. Biol. 23, 144–148. doi:10.1016/j.jtemb.2009.02.005
Zafaranieh, S., Dieberger, A. M., Leopold-Posch, B., Huppertz, B., Granitzer, S., Hengstschlager, M., et al. (2022). Physical activity and sedentary time in pregnancy: an exploratory study on oxidative stress markers in the placenta of women with obesity. Biomedicines 10, 1069. doi:10.3390/biomedicines10051069
Zavalza-Gomez, A. B. (2011). Obesity and oxidative stress: a direct link to preeclampsia? Arch. Gynecol. Obstet. 283, 415–422. doi:10.1007/s00404-010-1753-1
Zhang, H., Zhang, X., Wang, Y., Zhao, X., Zhang, L., Li, J., et al. (2023a). Dietary folic acid supplementation attenuates maternal high-fat diet-induced fetal intrauterine growth retarded via ameliorating placental inflammation and oxidative stress in rats. NUTRIENTS, 15. doi:10.3390/nu15143263
Zhang, H., Zheng, Y., Liu, X., Zha, X., Elsabagh, M., Ma, Y., et al. (2023b). Autophagy attenuates placental apoptosis, oxidative stress and fetal growth restriction in pregnant ewes. Environ. Int. 173, 107806. doi:10.1016/j.envint.2023.107806
Zhang, Y., Cui, Y., Zhou, Z., Sha, J., Li, Y., and Liu, J. (2010). Altered global gene expressions of human placentae subjected to assisted reproductive technology treatments. PLACENTA 31, 251–258. doi:10.1016/j.placenta.2010.01.005
Zhang, Y., Zhao, W., Xu, H., Hu, M., Guo, X., Jia, W., et al. (2019). Hyperandrogenism and insulin resistance-induced fetal loss: evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J. Physiol. 597, 3927–3950. doi:10.1113/JP277879
Zhao, H., Wong, R. J., and Stevenson, D. K. (2021). The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci., 22. doi:10.3390/ijms22189675
Zhou, Y., Xu, T., Wu, Y., Wei, H., and Peng, J. (2019). Oxidative stress and inflammation in sows with excess backfat: up-regulated cytokine expression and elevated oxidative stress biomarkers in placenta. Anim. (Basel) 9, 796. doi:10.3390/ani9100796
Zhu, B., Deng, F., Yan, S., Huang, K., Wu, X., Tao, X., et al. (2021). Gestational diabetes mellitus, autistic traits and ADHD symptoms in toddlers: placental inflammatory and oxidative stress cytokines do not play an intermediary role. PSYCHONEUROENDOCRINO 134, 105435. doi:10.1016/j.psyneuen.2021.105435
Keywords: placenta, oxidative stress, bibliometrics, citespace, VOSviewer, emerging topics, research focus
Citation: Chen A, Tian M, Luo Z, Cao X and Gu Y (2024) Analysis of the evolution of placental oxidative stress research from a bibliometric perspective. Front. Pharmacol. 15:1475244. doi: 10.3389/fphar.2024.1475244
Received: 03 August 2024; Accepted: 07 October 2024;
Published: 17 October 2024.
Edited by:
Reinaldo Marín, Instituto Venezolano de Investigaciones Científicas (IVIC), VenezuelaReviewed by:
Catherine M. T. Sherwin, University of Western Australia, AustraliaYu-Chin Lien, University of Pennsylvania, United States
Copyright © 2024 Chen, Tian, Luo, Cao and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfang Gu, Z3V5YW5mYW5nMjEwM0AxNjMuY29t; Xiaohui Cao, Y2FveGlhb2h1aTE5ODVAc2luYS5jbg==
 Ailing Chen
Ailing Chen Mengyuan Tian
Mengyuan Tian