
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 08 January 2025
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1469887
This article is part of the Research Topic Plant-derived Therapeutics and Traditional Medicine: Innovations, Challenges, and Opportunities in Breast Cancer Treatment View all 5 articles
 Bantayehu Addis Tegegne1*
Bantayehu Addis Tegegne1* Tesfa Begashaw2
Tesfa Begashaw2 Wubetu Yihunie Belay1
Wubetu Yihunie Belay1 Mengistie Kassahun Tariku3
Mengistie Kassahun Tariku3 Tirsit Ketsela Zeleke1
Tirsit Ketsela Zeleke1 Mohammed Jemal4
Mohammed Jemal4 Mamaru Getinet4
Mamaru Getinet4 Agumas Alemu Alehegn5
Agumas Alemu Alehegn5 Abebe Dagne1
Abebe Dagne1Kleinia is a genus of over 50 species that are commonly used in primary care in several countries. This study seeks to inspire researchers to quickly discover and isolate the key active metabolites found in Kleinia taxa, thereby promoting the development of novel, safe, and effective therapies for a variety of illnesses. To this end, we performed a thorough search of English-language publications from PubMed, Scopus, ScienceDirect, Web of Science, Google Scholar, and ResearchGate. Our search utilized keywords such as “ethnobotany,” “geographic distribution,” “ethnomedicinal use,” “phytochemistry,” “pharmacological or bioactivities,” and “toxicological activities” related to the genus Kleinia. Chemical structures were depicted using Chemdraw® software. Literature highlights numerous Kleinia taxa used in traditional medicine for conditions like intestinal parasites, measles, smallpox, diabetes, edema, nerve disorders, sexual dysfunction, gastrointestinal issues, cancer and more. Phytochemical analysis identifies 77 secondary metabolites, mainly alkaloids, flavonoids, saponins, terpenes, and terpenoids and other miscellaneous metabolites. Among the Kleinia taxa, K. anteuphorbium, K. longiflora, K. grandiflora, K. odora, K. squarrosa, K. abyssinica, K. pendula, and K. azoides have been scientifically validated to exhibit various pharmacological activities. However, the existence of potentially harmful metabolites in Kleinia taxa, particularly pyrrolizidine alkaloids, emphasizes the significance of cautious application in traditional medicine and the need for rigorous toxicological assessments. In conclusion, this review highlights the promise of Kleinia taxa as significant medicinal resources and advocates for extensive bioprospecting. It encourages global pharmaceutical companies and academic institutions to conduct thorough investigations of the genus Kleinia to uncover new therapeutic possibilities.
At least 80% of people worldwide now rely on herbal supplements and pharmaceutical goods for some aspect of their basic healthcare, a huge growth in use over the previous three decades. Even though treatments incorporating these substances have demonstrated encouraging promise and the effectiveness of many herbal products has been established, many of them are still untested, and their usage is either not tracked at all or is only partially monitored (Ekor, 2014).
Since ancient times, medicinal plants have played a crucial role in treating ailments and have been integral to human society. Evidence from various sources, such as written records, monuments, and preserved plant medicines, attests to humanity’s long history of seeking remedies in nature. The utilization of plants for therapeutic purposes is a practice that has a history spanning 3,000 years, with noteworthy figures such as Abu Rayhan, Biruni, Ibn Baytar and Ibn Sina making significant contributions to the field of herbal medicine. Humanity’s knowledge of medicinal plants developed through centuries of battling illnesses, learning to harness the healing properties of leaves, bark, seeds, and other plant parts. Global research continues to validate their effectiveness, leading to the creation of plant-based medicines (Schaal, 2019; Sofowora et al., 2013; Petrovska, 2012; Akbulut et al., 2019).
The World Health Organization (WHO) estimates that 4 billion people, or 80% of the world’s population, currently obtain basic healthcare through herbal medicine (Bodeker and Ong, 2005). The effectiveness, cost, cultural relevance, and lack of modern medical facilities make it believed that 80% of people in developing countries rely on traditional herbal medications for their primary healthcare. Likewise, in affluent nations, 80% of people are using traditional medicines, which are quickly increasing in use due to their cultural acceptance, affordability, and restricted access to contemporary medications. Plants have long played a significant role in human culture, beliefs, and traditional medicine. Plant-based medicine has been a crucial part of treatment since ancient times. With between 50,000 and 75,000 plant species utilized in both traditional and modern medicine, the majority of people on the planet, particularly in underdeveloped countries, rely on plants for both sustenance and medical care. In addition to producing a vast range of organic substances, plants are used for a wide range of purposes, such as food, medicine, shelter, dyes, healing, religious ceremonies, clothing, and decoration (Aziz et al., 2018a; Şen et al., 2022; Aziz et al., 2018b).
Medicinal plants provide bioactive metabolites that are valuable for human pharmacology. Therefore, to acknowledge the significance of traditional medicine in national healthcare, governments should gather data on medicinal plant species and their traditional uses. They should also integrate traditional medicine into the healthcare system by establishing supportive administrative structures for its practice (Smith-Hall et al., 2012; Usure et al., 2024; Asong et al., 2019).
According to the International Union for Conservation of Nature (IUCN) and the World Wildlife Fund (WWF), there are between 50,000 and 80,000 flowering plant taxa used for medicinal purposes worldwide. Among these, about 15,000 plant taxa are threatened with extinction from overharvesting and habitat destruction, and nearly exhausted with the increasing human population and plant consumption. Although this threat has been known for decades, the accelerated loss of species and habitat destruction worldwide has increased the risk of extinction of medicinal plants, especially in China, India, Nepal, Kenya, Tanzania Uganda, and Ethiopia (Chen et al., 2016).
Among plant taxa worldwide, 50 belong to the genus Kleinia, which are used in traditional medicine. In Ethiopia, four species from this genus have specific uses: Kleinia odora DC. (so called Luko in Afaan Oromo) is traditionally used for nerve-related issues, Kleinia abyssinica (A.Rich.) A. Berger (also known as Abrasha in Afaan Oromo) for sexual dysfunction, Kleinia pendula DC. (Afrasha in Afaan Oromo) for treating swollen body parts, and Kleinia squarrosa Cufod. (also called Luko, sharing the vernacular name with Kleinia odora DC.) for intestinal parasites (Bussa and Belayneh, 2014; Ouhaddou et al., 2014).
Few Kleinia taxa have been the subject of numerous in vitro and/or in vivo scientific papers, but there isn't a thorough analysis that combines the various therapeutic uses of this genus worldwide. In order to achieve this, this review will examine the phytochemical metabolites, pharmacological characteristics, toxicological safety, and traditional medicinal uses of Kleinia (Asteraceae). It will also draw attention to the need for additional research on species that have not yet been studied and for expanding studies on species that have been studied in clinical settings.
An extensive literature search was conducted to gather relevant information on ethnomedicinal uses, geographic distribution, phytochemistry, pharmacology, and toxicology. This process involved reviewing a wide range of scientific sources, including articles, book chapters, books, and encyclopedias written in English and supported by scientific research. The key search terms used included: “ethnomedicinal uses”,“geographic distribution”, “phytochemistry”, “pharmacological activities”,” toxicological safety”.
The literature search spanned several major scientific electronic databases to ensure comprehensive coverage of the subject. Relevant articles, book chapters, books, and encyclopedias have been extensively searched from PubMed, Scopus, Springer Link, Sci-Finder, Science Direct, Google Scholar, and Research Gate. Each database was searched using a combination of the identified keywords, and results were reviewed to identify studies and materials that aligned with the research objectives.
The following criteria were used to select literature: Publications written in english; research backed by scientific evidence; works that aligned with the study’s focus on ethnomedicine, phytochemistry, pharmacology, and toxicology; and preference was given to recent studies, but seminal works were also included when relevant.
From the selected literature, key data was extracted, focusing on the following: Ethnomedicinal uses (traditional medicinal applications of the plant taxa); geographic distribution (regions where the plants are native or commonly found); phytochemistry (identification and analysis of chemical metabolites found in the plant species); pharmacological effects (documented therapeutic benefits and biological activities); toxicological properties (information on the safety profile, side effects, and toxicological studies).
The chemical structures of metabolites identified in the phytochemical analysis were displayed using Chemdraw® software. This allowed for clear visualization of the molecular structures and ensured accurate representation of the phytochemical data.
After the extraction, the data was organized and synthesized to present a clear understanding of the ethnomedicinal relevance, and chemical composition of the plants. Comparative analysis was also conducted to correlate phytochemistry with pharmacological and toxicological findings, ensuring an integrated approach to understanding the medicinal value of the Kleinia.
Kleinia belongs to the Asteraceae family, a group of flowering plants (angiosperms). The genus is named after 18th-century German botanist Dr. Jacob Theodor Klein (Halliday, 1984a). It is an annual succulent shrub, with striking, thin, pencil-shaped, segmented, and upright or sprawling stems, that is drought resistant (Smith, 2011). The genus Kleinia is distributed through the Atlantic Ocean, Tropical Africa, Subtropical Africa, India, and Arab countries. It is found in cold to temperate climates at altitudes of 300–1,600 m with different habitats of rocks, grassland, and woodland (Mies, 1995; Halliday, 1984b). Table 1 summarizes the geographic distribution of a few common taxa under the Kleinia.
The Kleinia genus consists of perennial flowering plants characterized by succulent leaves, which can be either flat or cylindrical. These plants typically have angled, fleshy stems and may possess tuberous roots. Their leaves and flowers are usually smooth, with flower heads that vary in size, containing multiple disk-shaped, often uniform flowers, though some species have mixed types. The flowers come in white, yellow, or red, with a flat receptacle. The genus features conical, papillose appendages, and the fruit is non-beaked, cylindrical, ribbed, and can be either smooth or hairy. The genus Kleinia consists of upright, perennial subshrubs that grow between 1–2.5 m tall, with stems measuring 3–6 cm in diameter. The plants have petioles ranging from 2–4 cm in length and flower heads that are 1.9–2 cm long and 2–2.5 cm wide. The floral lobes are at least 3 mm long, while the anthers measure 4 mm in length (Halliday, 1984b; Vanijajiva et al., 2014; Melo-de-Pinna et al., 2016; Lulekal et al., 2008; Timonin et al., 2014; Cicuzza et al., 2018). Figure 1 displays a few Kleinia taxa.
As displayed Table 2, traditional medicine in Ethiopia, Morocco, South Africa, South India, China, and North America has utilized different parts of numerous Kleinia taxa to treat a range of human diseases and conditions.
An overview of the works of literature search indicated that some Kleinia taxa have been investigated phytochemically from 50 plant species known from this genus namely, K. odora (Shehata et al., 2018), K. squarrosa (Juma et al., 2015), K. pendula (Alfaifi et al., 2020), K. longiflora (Sewawa, 2021), K. grandiflora (Pitchaipillai et al., 2014; Selvakumar, 2018), K. anteuphorbium (Aati et al., 2024a), and K. aizoide (Nunez-Melendez and Johnson, 1955). This genus has a phytochemical of flavonoids (19), saponins (6), cardiac glycosides (8), alkaloids (20), terpenes (8), terpenoids (5), and other types of metabolites (11) have been isolated and identified from the above-listed species.
Flavonoids, a class of natural metabolitess with varying phenolic structures, are found in fruits, vegetables, cereals, bark, roots, stems, flowers, tea, and wine. These natural products are well-known for their health benefits, and efforts are underway to isolate the metabolites known as flavonoids. Flavonoids have a variety of medicinal properties, including anticancer, antioxidant, anti-inflammatory, and antiviral activity. They also have neuro-protective and cardio-protective properties. These biological activities depend upon the type of flavonoid, its mode of action, and its bioavailability (Panche et al., 2016; Ullah et al., 2020).
As shown in Table 3, a total of 19 flavonoids are isolated from Kleinia taxa like K. grandiflora, K. longiflora K. anteuphorbium, K. grandiflora, K. odora, K. aizoides. These are flavanone (1), flavone (8), flavonol (3), dihydroflavonol (3), and anthocyanidin (4).
Since their biodiversity is still mostly unknown, saponins are one of the most prevalent and varied families of plant natural products. They also hold considerable promise for future medication discovery. Their hydrophilic carbohydrate chain and hydrophobic triterpene or sterol backbone are what give them their amphipathic character and biological activity. But other structural metabolites also play a part in their powerful impacts. Saponins are naturally occurring secondary metabolites that are amphiphilic, glycosidic, and heat-stable. Because of their triterpenoid or steroidal aglycones connected to oligosaccharides, saponins are used extensively in the pharmaceutical sector (Mugford et al., 2013; Yang et al., 2021).
Saponins isolated from the species of genus Kleinia are summarized in Table 4. There are two major classes of saponins isolated from the genus Kleinia namely; steroidal saponin and triterpenoid saponin. Protopanaxadio (20), protopanaxatriol (21), gypsogenin (22), gypsogenic acid (23), quillaic acid (24), and hederagenin (25) are some examples of metabolites isolated from those major classes of saponins (Jesus et al., 2015).
Alkaloids are nitrogen-containing metabolites characterized by a heterocyclic ring structure composed of hydrogen and carbon, sometimes including sulfur and oxygen. According to studies in natural products chemistry, alkaloids are naturally occurring substances that can be extracted from the crude extracts of bacteria, fungi, plants, and animals. In plants, alkaloids exhibit diverse structural forms, which are classified into three categories: protoalkaloids, pseudoalkaloids, and true alkaloids. The bioactivity of each group is influenced by the complexity of its structural backbone, with amino acids, which make up 80% of these backbones, playing a crucial role in the biosynthesis of alkaloids. Alkaloids have several uses in medicine and other facets of human life, such as medications, dietary additives, and supplements (Bhambhani et al., 2021; Joanna and Joanna, 2019).
Pyrrolizidine and cyclic amine alkaloids isolated from the species of genus Kleinia (Figure 2) are 1-hydroxymethylpyrrolizidine (34), (−)trachelanthamidine (35), (+) trachelanthamidine (36), (−) isoretronecanole (37), (+)-isoretronecanole (38), (−)-platynecine (39), (−)-rosmarinecine (40), (−)-tumeforcidine (41), (−)-dihydroheliotridane (42), (−)-supinidine (43), (+)-supinidine (44), (−)-crotanecine (45), (+)-retronecine (46), (−)-retronecine (47), (+)-heliotridine (48), (1S,8R)-1-aminopyrrolizidine (49), (1R,7S)-7-methylhexahydro-1H-pyrrolizine-1-ol (50), (1R,7R)-7-methylidenehexahydro-1H-pyrrolizin-1-ol (51), (7S,8R)-petranecine (52), and phenyl ethyl amine (Romo de Vivar et al., 2007).
The primary bioactive metabolites, of essential oils are terpenes and terpenoids. Terpenes, such as pinene, myrcene, limonene, terpinene, and p-cymene, are metabolites composed of simple hydrocarbon structures, while terpenoids are a modified class of terpenes that contain oxygen and have altered functional groups, including oxidized or repositioned methyl groups. Terpenes are known to exhibit antimicrobial activity against both antibiotic-susceptible and antibiotic-resistant bacteria, primarily by promoting cell membrane disruption and inhibiting protein and DNA synthesis. Terpenoids are divided into monoterpenes, sesquiterpenes, diterpenes, sesterpenes, and triterpenes based on the number of carbon units contained in them (Masyita et al., 2022; Shagufta et al., 2018; Efe et al., 2021).
Monoterpenes and sesquiterpenes isolated from genus Kleinia (Figure 3) are-α-pinene (54), β-pinene (55), (+)-4-carene (56), copaene (57), aristolene (58), α-caryophyllene (59), germacrene-D (60), and (+)-Epi-bicyclosesquiphellandrene (61) are isolated from the species K. anteuphorbium, K. longiflora, K. grandiflora, K. odora, K. squarrosa, K. abyssinica, K. tomentosa, K. aizoides, and K. pendula. Triterpenoids like ursolic acid (55), uvaol (56), hydroxyursolic acid (57), trans-cinnamoyloxy-hydroxyurs-12-en-28-oic acid (58), and trans-p-coumaroyloxy-hydroxyurs-12-en-28-oic acid (59) are isolated mainly from the species K. anteuphorbium, K. longiflora, K. grandiflora, K. odora, K. squarrosa, K. aizoides, and K. pendula (Al Musayeib et al., 2013; Efe et al., 2021; Migahid, 1988; Bohlmann and Knoll, 1978; Bohlmann and Suding, 1980; Bohlmann et al., 1981; Al-Taweel et al., 2004; Al-Taweel et al., 2001; Mohanraj et al., 2018). Table 5 below summarizes the main triterpenoids isolated.
Figure 4 shows various chemicals that have been isolated from the genus Kleinia. Ascorbic acid (67), β-carotene (68), caffeic acid (69), P-coumaric acid (70), ferulic acid (71), β-sitosterol (72), campesterol (73), xanthotoxin (74), stigmasterol (75), n-triacontanol (76), and cinnamic acid (77) are isolated from the species K. anteuphorbium, K. longiflora, K. grandiflora, K. odora, K. squarrosa, K. abyssinica, K. azoides, and K. pendula (Jeffrey, 1986).
Pharmacological studies on various Kleinia species underscore their importance as medicinal plants. A detailed review of the literature reveals specific pharmacological reports for K. anteuphorbium, K. longiflora, K. grandiflora, K. odora, K. squarrosa, K. abyssinica, K. pendula, and K. azoides. These studies highlight a wide range of therapeutic properties, including analgesic, anti-inflammatory, antipyretic, anticancer, antiprotozoal, antidiabetic, antimicrobial, and antifungal effects, supported by both in vitro and in vivo experimental evidence.
However, the mechanisms of action underlying these effects remain largely unexplored, and there is a notable absence of clinical trials to confirm their efficacy and safety in human populations. This highlights the need for advanced research, including mechanistic studies and clinical validation, to translate preclinical findings into clinical applications.
This review outlines the primary pharmacological and toxicological activities of various Kleinia taxa, as depicted in Figure 5. Table 6 provides a detailed summary of pharmacological agents, extract types, dosage ranges, controls, and experimental models, offering a systematic perspective on the pharmacological landscape of the Kleinia genus and identifying critical areas for further exploration.
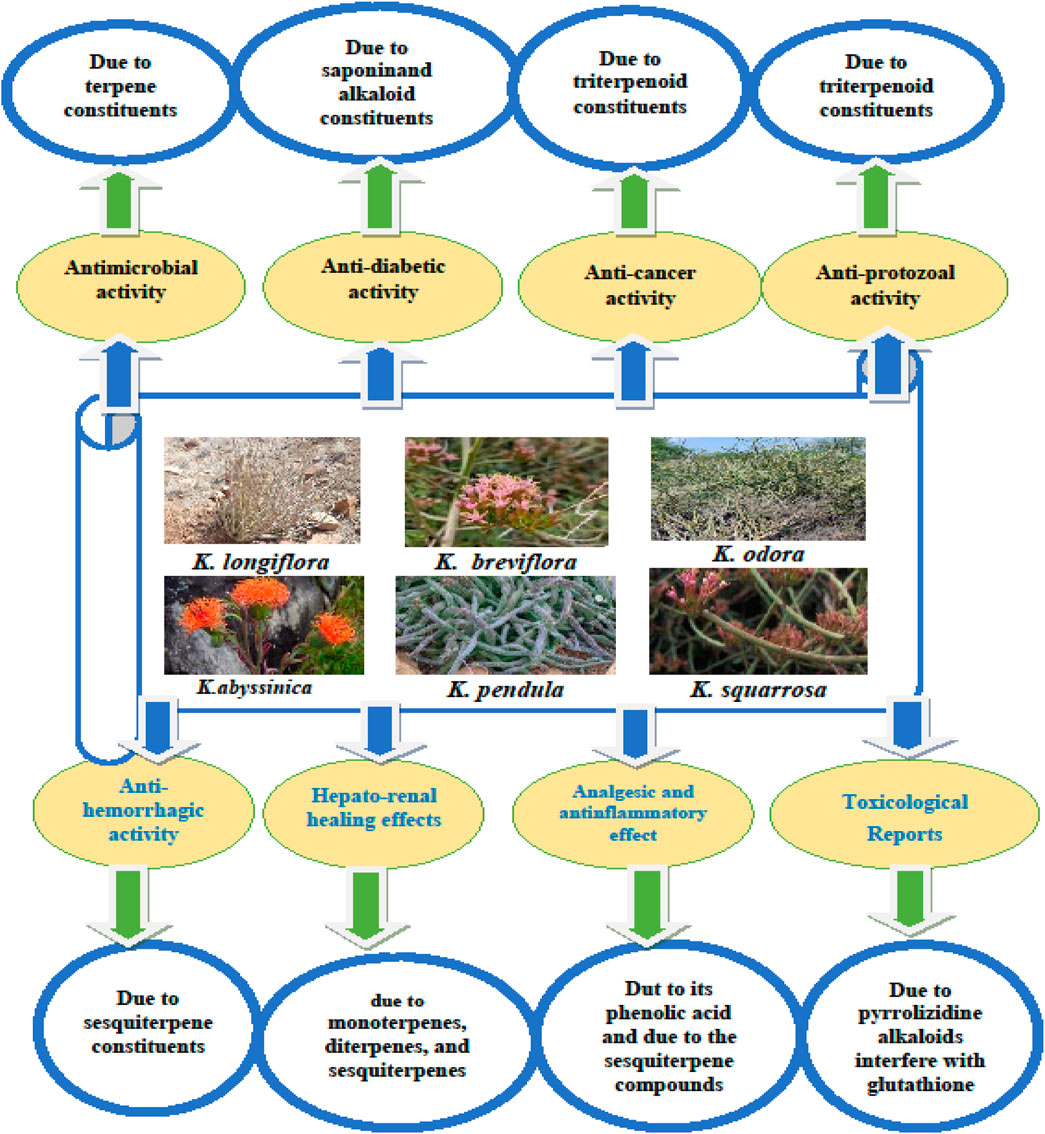
Figure 5. A diagram illustrating key phytochemical metabolites from various Kleinia taxa linked to their pharmacological and toxicological effects, as identified through in vitro and in vivo screening tests.
The genus kleinia is of notable pharmacological importance due to the following reasons: Diverse pharmacological and biological activities: kleinia species exhibit a wide range of therapeutic properties, including analgesic, anti-inflammatory, anticancer, and antimicrobial effects. These activities address critical medical challenges, such as pain relief, infection control, and chronic disease management. Medicinal value across species: several kleinia species, such as k. Anteuphorbium and k. Longiflora, have demonstrated bioactive properties, highlighting the genus as a valuable source of compounds with potential applications in treating various diseases. Scientific validation: traditional claims about kleinia’s medicinal properties have been substantiated through studies using in vitro and in vivo models, providing essential insights into its biological activities and laying the groundwork for drug discovery. Gaps in mechanistic and clinical validation: despite promising preclinical evidence, detailed studies on the mechanisms of action and clinical trials remain limited. Understanding these mechanisms is crucial for optimizing therapeutic efficacy and minimizing adverse effects. Need for advanced research: addressing existing research gaps, including the lack of clinical evidence, calls for focused pharmacological investigations and translational studies to fully harness kleinia’s therapeutic potential. Systematic data representation: visual and tabular representations, such as Figure 5 and Table 6, provide a structured overview of pharmacological agents, experimental models, and dosage data. These summaries help identify research trends and inform future studies.
In conclusion, the Kleinia genus holds immense promise for addressing major health challenges. Realizing this potential requires rigorous mechanistic research, clinical validation, and systematic investigations to develop effective and safe therapeutic applications.
Essential oil of the K. odora was subjected to antimicrobial screening using pure strains of Escherichia coli, Staphlococcus aureus, and Candida albicans that were generated from stock cultures in accordance with the general qualitative assay using agar well diffusion assay. Amphotericin is used for fungi and tetracycline for bacteria as positive controls. A twofold serial dilution test was used to find the minimal inhibitory doses for each drug. The oil shows moderate antimicrobial activity against Echerichia coli and antifungal activity against Candida albicans. This antimicrobial activity of the genus Kleinia is due to terpene metabolites (Al-Taweel et al., 2004). Additionally, research verified that K. anteuphorbium, formerly known as Senecio anteuphorbium Sch. Bip., possesses antimicorbial activity, which is ascribed to its high essential oil concentration (Elhidar et al., 2019).
The in-vivo antidiabetic effects of aqueous stem bark extracts from Kleinia squarrosa and Kleinia longiflora were evaluated in both alloxan-induced diabetic mice and control mice. Both oral and intraperitoneal administration of the aqueous extract of K. squarrosa and Kleinia longiflora showed hypoglycemic activity at four test dose levels (50 mg/kg, 100 mg/kg, 200 mg/kg, and 300 mg/kg of body weight). However, intraperitoneal administration is more effective in lowering blood glucose levels than the oral route. This anti-diabetic activity of the genus Kleinia is due to saponin and alkaloid metabolites (Juma et al., 2015; Abdirahman et al., 2015).
Petroleum ether and chloroform extracts of K. odora and K. longiflora show potent activity against T. brucei with IC50 values of 0.5 μg/mL. The chloroform extract gives slightly higher selectivity (SI = 63) compared to the petroleum ether extract (SI = 39). The chloroform extract of K. odora K. abyssinica, and K. longiflora also shows moderate activity against P. falciparum schizonts and intracellular amastigotes of L. infantum (IC50 of 8 μg/mL). In comparison, the petroleum ether extract showed similar activity against P. falciparum, L. infantum, and T. cruzi (IC50 of 8.6, 6.8, and 5.7 μg/mL), but with lower selectivity. This antiprotozoal activity of the is attributed to triterpenoid metabolites (Al Musayeib et al., 2013).
The clotting time assay conducted on petroleum ether extracts from the dry leaves of K. aizoides revealed vitamin K activities, which are essential for treating both internal and external hemorrhages. The anti-hemorrhagic effects of K. aizoides are found in the fat-soluble and unsaponifiable non-sterol fraction. This activity within the genus Kleinia is attributed to its sesquiterpene (like α-caryophyllene) metabolites (Nunez Melendez, 1956).
The cytotoxic potential of the methanolic extract, as well as the hexane and chloroform fractions of K. pendula, was evaluated against breast cancer, liver cancer, and colon cancer cell lines. All tested extracts and fractions of K. pendula exhibited significant cytotoxic effects across all three cancer cell lines. The IC50 values of the methanolic extract, hexane and chloroform fractions were comparable to those of the standard chemotherapy drug, doxorubicin. Notably, the hexane fraction showed particularly strong cytotoxic activity, with IC50 values ranging between 0.07 µg and 0.19 µg. This cytotoxic effect of the K. pendula is attributed to its triterpenoid and flavonoid metabolites (Sewawa, 2021).
Phytochemical research shows that essential oils, as secondary metabolites, are produced by plants in response to stress caused by various external factors. These oils are highly valued in the market due to the bioactive properties of their metabolites. Plant-derived phytochemicals, such as terpenes and terpenoids found in essential oils, possess significant pharmacological and biological properties, including antioxidant (55, 67). The chloroform, ethyl acetate, and n-hexane fractions of K. pendula and K. longiflora were evaluated for their analgesic effects, with dosage levels determined by acute oral toxicity studies. Mice were administered K. pendula fractions at doses of 100, 200, and 300 mg/kg body weight, and analgesic activity was measured using Eddy’s hot plate method at 0, 30, 60, and 120 min post-administration. The chloroform, ethyl acetate, and n-hexane fractions of K. pendula demonstrated a significant increase in analgesic activity at 30 min, comparable to the effect of the standard drug diclofenac sodium (10 mg/kg). This analgesic effect is attributed to its phenolic acid metabolites (Sewawa, 2021; Aati et al., 2024b). On the other hand, the chloroform and ethyl acetate fractions of K. pendula, K. grandiflora, and K. anteuphorbium were evaluated for their anti-inflammatory properties in mice treated with standard saline and developed inflammation. Mice administered 100, 200, and 300 mg/kg of these fractions exhibited a significant reduction in inflammation. This anti-inflammatory effect is believed to be due to the sesquiterpene metabolites present in the extracts (Sewawa, 2021). Two-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging, nitric oxide scavenging, and ferric reducing/antioxidant power (FRAP) assays have revealed that a number of plants that contain essential oils have antioxidant qualities (Karaçelik et al., 2022a; Karaçelik et al., 2022b; Saral et al., 2024).
Studies on K. anteuphorbium essential oil have identified potent anti-hyperpyrexia and antioxidant effects, validated through 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging, nitric oxide scavenging, and ferric reducing/antioxidant power (FRAP) assays. It has also proven effective in alleviating pain, inflammation, and fever in various animal models. Its anti-nociceptive properties were assessed via the hot plate, tail-flick, and acetic acid-induced writhing tests. The hot plate test examined the animals’ responses to heat, while the tail-flick test focused on spinal reflexes (Aati et al., 2024b). K. anteuphorbium essential oil delayed tail-flick responses, suggesting spinal involvement, and increased reaction times in the hot plate test. It also reduced writhing in rats subjected to acetic acid, indicating both central and peripheral pain-relief mechanisms. Studies showed that monoterpeines with analgesic activity (Guimarães et al., 2013). The analgesic effects are likely linked to active metabolites such as α-pinene, α-terpineol, β-pinene, and myrtenal (Aati et al., 2024b; Park et al., 2010; Chapman et al., 1985). K. anteuphorbium essential oil demonstrated anti-fever effects in rats treated with brewer’s yeast, suggesting its efficacy in managing fever through its antioxidant and anti-inflammatory properties (Aati et al., 2024b). Additionally, research has demonstrated that monoterpenes, such α-terpineol, have anti-inflammatory and antioxidant qualities (Sales et al., 2020).
Treatment with K. anteuphorbium essential oil restored liver function markers to normal levels, especially at the higher dose of 10 mg/kg BW, compared to the carbon tetra chloride-treated group. K. anteuphorbium essential oil also balanced lipid profile markers during carbon tetra chloride exposure. Additionally, co-administration of K. anteuphorbium essential oil with carbon tetra chloride reduced the harmful effects on kidney function markers like urea, uric acid creatinine, and bringing them back to control levels. The higher dose showed a strong protective effect, highlighting K. anteuphorbium essential oil’s hepato-protective potential. This effect is likely due to bioactive metabolites such as α-pinene, α-terpinal, α-gurjunene, caryophyllene oxide, silphiperfol-6-ene, myrtenal, (E)-β-caryophyllene and β-pinene, which belong to monoterpenes, diterpenes, and sesquiterpenes. These metabolites have antioxidant and anti-inflammatory properties known to protect the liver and kidneys (Aati et al., 2024b).
Due to the widespread availability, affordability, and acceptance of medicinal plants, evaluating their safety and toxicity is essential. Concerns over toxicity are a key reason healthcare professionals hesitate to integrate herbal remedies into formal healthcare. The potential long-term toxicity or mutagenicity of many plant families remains underreported, highlighting the need for thorough screening to separate harmful effects from therapeutic benefits (Odeyemi and Bradley, 2018).
The long-term toxic or mutagenic effects of many plant families are still insufficiently studied. This highlights the need for thorough testing to distinguish harmful effects from therapeutic ones. When scientific proof of a plant’s safety is lacking, traditional knowledge of its use becomes essential. Pyrrolizidine alkaloids are toxic metabolites found in plants, often linked to acute and chronic liver damage. These toxins are a leading cause of plant poisoning in humans, livestock, and other animals globally. Over 660 different types of Pyrrolizidine alkaloids have been identified in approximately 6,000 plant species, with around 120 known to be harmful to the liver. Asteraceae are known to produce pyrrolizidine alkaloids, metabolites with recognized hepatotoxic effects. However, the toxicological characteristics of most Kleinia taxa remain largely unclear and require more in-depth investigation (Odeyemi and Bradley, 2018; Langel et al., 2011; Stegelmeier, 2011; Lu et al., 2024; Seremet et al., 2018; Şeremet et al., 2016) except K. neriifolia which has been confirmed to contain pyrrolizidine alkaloids (Dominguez et al., 2008).
Pyrrolizidine alkaloids exist in two molecular forms: the non-toxic N-oxide and the free base. N-oxides are the dominant form in plants, but in herbivores’ guts, they are easily broken down into the free base, which enters the bloodstream. When pyrrolizidine alkaloids bind to glutathione, the body becomes less able to neutralize reactive oxygen species, leading to increased oxidative stress and membrane damage. Pyrrolizidine alkaloids are also known to produce genotoxicity and hepatotoxicity due to the formation of reactive pyrrolic metabolites that can attach to nucleophilic substances, such as DNA, and create cross-links between DNA and proteins (Belal and El-Gendy, 2014; Yan et al., 2023; He et al., 2021).
In conclusion, the usage of herbal medicines has grown, and Pyrrolizidine alkaloids poisoning is now regarded as a public health concern. Thus, in order to protect the health of humans and animals, it is essential to increase our understanding of the chemistry, pharmacology, and toxicity of pyrrolizidine alkaloids (Moreira et al., 2018).
While numerous studies have investigated the medicinal potential of Kleinia species, much of the evidence stems from traditional knowledge and preclinical research. This highlights the necessity for advanced mechanistic studies and translational research to bridge the gap between preclinical findings and clinical applications. There is a scarcity of well-designed clinical trials to validate its therapeutic efficacy in humans. The chemical composition of Kleinia varies depending on environmental factors, plant part used, and extraction methods. This inconsistency poses challenges for standardizing extracts and assessing their pharmacological activities. Toxicity studies on Kleinia are still in their infancy. Limited data on long-term safety, dosing thresholds, and potential adverse effects make it difficult to fully assess its toxicological risk. Most studies have focused on a narrow range of species or specific geographic regions, leading to a fragmented understanding of the genus as a whole. Another weakness of this study is the unclear relationship between the extract’s components and bioactivity. Broader investigations are needed to cover more species and regions where Kleinia is used traditionally.
Future research should prioritize conducting randomized, placebo-controlled clinical trials to determine the safety, efficacy, and appropriate dosing of Kleinia species for various therapeutic applications. Developing standardized extraction techniques and identifying bioactive metabolites across different Kleinia species are critical for ensuring consistency in therapeutic formulations. More comprehensive studies, including chronic toxicity, genotoxicity, and long-term safety evaluations, are necessary to better understand the toxicological profile of Kleinia and establish safe usage guidelines. Expanding ethnobotanical surveys and phytochemical studies across a broader range of Kleinia species and geographic regions could uncover additional medicinal applications and unique metabolites. Investigating the molecular mechanisms behind the pharmacological activities of Kleinia species will provide insight into their potential therapeutic benefits and guide future drug development. Research into sustainable harvesting practices is essential to preserve Kleinia species, especially in regions where it is over-exploited, while promoting conservation efforts and responsible use of its medicinal resources. By addressing these limitations and focusing on these research priorities, the therapeutic potential of Kleinia can be fully explored and safely integrated into modern medicine.
In conclusion, Kleinia (Asteraceae) holds immense potential in the field of medicinal research due to its diverse phytochemical metabolites and pharmacological properties. This review highlights the genus’ rich array of secondary metabolites that exhibit a wide range of biological activities, such as anti-microbial, anti-protozoal, anti-hemooragic, analgesic, anti-pyretic, anti-inflammatory, anti-diabetic, cytotoxic, and antioxidant effects. However, despite its promising therapeutic potential, much of Kleinia’s medicinal value remains underexplored, with only a limited number of species investigated to date. The presence of potentially toxic metabolites, notably pyrrolizidine alkaloids, underscores the importance of cautious application in traditional medicine and the need for comprehensive toxicological evaluations. Expanded phytochemical research is vital to uncover additional bioactive metabolites, and further preclinical and clinical trials are needed to establish the efficacy and safety of Kleinia extracts. To maximize the therapeutic potential of this genus, interdisciplinary collaboration among researchers, pharmaceutical developers, and traditional medicine practitioners is essential. By addressing these research gaps and fostering partnerships, Kleinia can be developed into safe, effective, and sustainable medicinal products that contribute to modern healthcare.
BT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. TB: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. WB: Resources, Visualization, Writing–original draft, Writing–review and editing. MT: Conceptualization, Writing–original draft, Writing–review and editing. TZ: Investigation, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing. MJ: Methodology, Writing–original draft, Writing–review and editing. MG: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. AA: Conceptualization, Writing–original draft, Writing–review and editing. AD: Conceptualization, Funding acquisition, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aati, H. Y., Seif, M., Emam, M., Al-Qahtani, J., Attia, H., Aati, S., et al. (2024a). Exploring the therapeutic potential of Saudi Kleinia anteuphorbium (L.) DC. Essential oil: insights into antioxidant, analgesic, and hepatorenal protective effects. Heliyon 10 (19), e38573. doi:10.1016/j.heliyon.2024.e38573
Aati, H. Y., Seif, M., Emam, M., Al-Qahtani, J., Attia, H., Aati, S., et al. (2024b). Exploring the therapeutic potential of Saudi Kleinia anteuphorbium (L.) DC. Essential oil: insights into antioxidant, analgesic, and hepatorenal protective effects. Heliyon 10 (19), e38573. doi:10.1016/j.heliyon.2024.e38573
Abdirahman, Y., Juma, K., Mukundi, M., Gitahi, S., Agyirifo, D., Ngugi, M., et al. (2015). In-vivo antidiabetic activity and safety of the aqueous stem bark extract of Kleinia squarrosa. J. Diabetes Metab. 06. doi:10.4172/2155-6156.1000601
Akbulut, S., Karakose, M., and Özkan, Z. C. (2019). Traditional uses of some wild plants in Kale and Acıpayam provinces in Denizli. Kastamonu Univ. J. For. Fac. 19 (1), 72–81. doi:10.17475/kastorman.543529
Alfaifi, M., Alsayari, A., Gurusamy, N., Louis, J., Eldin Elbehairi, S., Venkatesan, K., et al. (2020). Analgesic, anti-inflammatory, cytotoxic activity screening and UPLC-PDA-ESI-MS metabolites determination of bioactive fractions of kleinia pendula. Molecules 25 (2), 418. doi:10.3390/molecules25020418
Al Musayeib, N. M., Mothana, R. A., El Gamal, A. A., Al-Massarani, S. M., and Maes, L. (2013). In vitro antiprotozoal activity of triterpenoid constituents of Kleinia odora growing in Saudi Arabia. Molecules 18 (8), 9207–9218. doi:10.3390/molecules18089207
Al-Namazi, A. A., Al-Khulaidi, A. W. A., Algarni, S., and Al-Sagheer, N. A. (2021). Natural plant species inventory of hotspot areas in Arabian Peninsula: southwest Al-Baha region, Saudi Arabia. Saudi J. Biol. Sci. 28 (6), 3309–3324. doi:10.1016/j.sjbs.2021.02.076
Al-Taweel, A. M., El-Deeb, K. S., and Al-Muhtadi, F. J. (2001). Triterpenoids from kleinia odora growing in Saudi arabia.
Al-Taweel, A. M., El-Deeb, K. S., and Al-Muhtadi, F. J. (2004). Chemical composition and antimicrobial activity of the essential oil of Kleinia odora. Saudi Pharm. J. 12 (1), 47–50.
Asong, J. A., Amoo, S. O., McGaw, L. J., Nkadimeng, S. M., Aremu, A. O., and Otang-Mbeng, W. (2019). Antimicrobial activity, antioxidant potential, cytotoxicity and phytochemical profiling of four plants locally used against skin diseases. Plants (Basel) 8 (9), 350. doi:10.3390/plants8090350
Aziz, M. A., Adnan, M., Khan, A. H., Shahat, A. A., Al-Said, M. S., and Ullah, R. (2018a). Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. ethnomedicine 14, 2–16. doi:10.1186/s13002-017-0204-5
Aziz, M. A., Adnan, M., Khan, A. H., Shahat, A. A., Al-Said, M. S., and Ullah, R. (2018b). Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. Ethnomedicine 14 (1), 2. doi:10.1186/s13002-017-0204-5
Belal, A., and El-Gendy, B. E.-D. M. (2014). Pyrrolizines: promising scaffolds for anticancer drugs. Bioorg. and Med. Chem. 22 (1), 46–53. doi:10.1016/j.bmc.2013.11.040
Belayneh, A., and Bussa, N. F. (2014). Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J. Ethnobiol. Ethnomed 10, 18. doi:10.1186/1746-4269-10-18
Bhambhani, S., Kondhare, K. R., and Giri, A. P. (2021). Diversity in chemical structures and biological properties of plant alkaloids. Molecules 26 (11), 3374. doi:10.3390/molecules26113374
Bodeker, G., and Ong, C.-K. (2005). WHO global atlas of traditional, complementary and alternative medicine. Kobe, Japan: World Health Organization.
Bohlmann, F., Ahmed, M., Jakupovic, J., and Jeffrey, C. (1981). Sesquiterpenes from kleinia species. Phytochemistry 20 (2), 251–256. doi:10.1016/0031-9422(81)85101-1
Bohlmann, F., and Knoll, K.-H. (1978). Zwei neue acylpyrrole aus Kleinia kleinioides. Phytochemistry 17 (3), 599–601. doi:10.1016/s0031-9422(00)89395-4
Bohlmann, F., and Suding, H. (1980). Weitere abrotanifolon-derivate aus Kleinia tomentosa. Phytochemistry 19 (4), 687–688. doi:10.1016/0031-9422(80)87041-5
Bussa, N. F., and Belayneh, A. (2014). Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J. Ethnobiol. Ethnomed. 10, 18. doi:10.1186/1746-4269-10-18
Chapman, C. R., Casey, K. L., Dubner, R., Foley, K. M., Gracely, R. H., and Reading, A. E. (1985). Pain measurement: an overview. Pain 22 (1), 1–31. doi:10.1016/0304-3959(85)90145-9
Chekole, G. (2017). Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J. Ethnobiol. Ethnomed 13 (1), 55. doi:10.1186/s13002-017-0182-7
Chen, S.-L., Yu, H., Luo, H.-M., Wu, Q., Li, C.-F., and Steinmetz, A. (2016). Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin. Med. 11, 37–10. doi:10.1186/s13020-016-0108-7
Cicuzza, D., sombra Stäheli, D., Nyffeler, R., and Eggli, U. (2018). Morphology and anatomy support a reclassification of the African succulent taxa of Senecio sl (Asteraceae: Senecioneae). Haseltonia 2017 (23), 11–26. doi:10.2985/026.023.0103
Clayton, W., Polhill, R., and Beentje, H. J. (1982). Flora of tropical east Africa: crown agents for the colonies.
Dominguez, D. M., Reina, M., Santos-Guerra, A., Santana, O., Agulló, T., López-Balboa, C., et al. (2008). Pyrrolizidine alkaloids from Canarian endemic plants and their biological effects. Biochem. Syst. Ecol. 36 (3), 153–166. doi:10.1016/j.bse.2007.08.015
Efe, D., Karaköse, M., Karaçeli̇k, A. A., Ertan, B., and Şeker, M. E. (2021). GC-MS analyses and bioactivities of essential oil obtained from the roots ofChrysopogon zizanioides (L.) Roberty cultivated in Giresun, Turkey. Turkish J. Chem. 45 (5), 1543–1550. doi:10.3906/kim-2009-64
Ekor, M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4, 177. doi:10.3389/fphar.2013.00177
Elhidar, N., Nafis, A., Kasrati, A., Goehler, A., Bohnert, J. A., Abbad, A., et al. (2019). Chemical composition, antimicrobial activities and synergistic effects of essential oil from Senecio anteuphorbium, a Moroccan endemic plant. Industrial crops Prod. 130, 310–315. doi:10.1016/j.indcrop.2018.12.097
Guimarães, A. G., Quintans, J. S., and Quintans, L. J. (2013). Monoterpenes with analgesic activity--a systematic review. Phytother. Res. 27 (1), 1–15. doi:10.1002/ptr.4686
Guta, M., Teka, F., Bisrat, D., Lindemann, P., and Asres, K. (2018). Repellent activity of essential oil of the stem of Kleinia squarrosa against mosquitoes. Ethiop. Pharm. J. 33 (2), 75–82. doi:10.4314/epj.v33i2.1
Halliday, P. (1984a). The genus kleinia (compositae) in arabia. Kew Bull. 39, 817–827. doi:10.2307/4107755
Halliday, P. (1984b). The genus kleinia (compositae) in arabia. Kew Bull. 39 (4), 817–827. doi:10.2307/4107755
He, X., Xia, Q., Shi, Q., and Fu, P. P. (2021). Metabolism of carcinogenic pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides by rat primary hepatocytes generate the same characteristic DHP-DNA adducts. J. Environ. Sci. Health, Part C 39 (4), 357–372. doi:10.1080/26896583.2021.1954460
Henderson, L. (2006). Comparisons of invasive plants in southern Africa originating from southern temperate, northern temperate and tropical regions. Bothalia 36 (2), 201–222. doi:10.4102/abc.v36i2.362
Jeffrey, C. (1986). The senecioneae in east tropical Africa: notes on compositae: IV. Kew Bull. 41, 873–943. doi:10.2307/4102988
Jesus, J. A., Lago, J. H. G., Laurenti, M. D., Yamamoto, E. S., and Passero, L. F. D. (2015). Antimicrobial activity of oleanolic and ursolic acids: an update. Evidence-Based Complementary Altern. Med. 2015 (1), 620472. doi:10.1155/2015/620472
Joanna, K. (2019). “Introductory chapter: alkaloids - their importance in nature and for human life,” in Alkaloids. Editor K. Joanna (Rijeka: IntechOpen). Ch. 1.
Ju, S. K., Mj, K. C., Semotiuk, A. J., and Krishna, V. (2019). Indigenous knowledge on medicinal plants used by ethnic communities of South India. Ethnobot. Res. Appl. 18, 1–112.
Juma, K. K., Abdirahman, Y., Mukundi, M., Gitahi, S., Agyirifo, D., Ngugi, M., et al. (2015). In-vivo antidiabetic activity and safety of the aqueous stem bark extract of Kleinia squarrosa. J. Diabetes Metab. 9, 601–611.
Karaçelik, A. A., Şeker, M. E., and Karaköse, M. (2022a). Determination of antioxidant activity of different extracts from bark of Pinus spp. grown in Giresun (Turkey) province–phenolic analysis by RP-HPLC-DAD. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Derg. 25 (1), 10–18. doi:10.18016/ksutarimdoga.vi.875313
Karaçelik, A. A., Türkuçar, S. A., and Karaköse, M. (2022b). Phytochemical composition and biological activities of angelica sylvestris L. Var. stenoptera avé-lall ex boiss.: an endangered medicinal plant of northeast Turkey. Chem. and Biodivers. 19 (10), e202200552. doi:10.1002/cbdv.202200552
Langel, D., Ober, D., and Pelser, P. B. (2011). The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem. Rev. 10 (1), 3–74. doi:10.1007/s11101-010-9184-y
Lu, Y. S., Qiu, J., Mu, X. Y., Qian, Y. Z., and Chen, L. (2024). Levels, toxic effects, and risk assessment of pyrrolizidine alkaloids in foods: a review. Foods. 13 (4), 536. doi:10.3390/foods13040536
Lulekal, E., Kelbessa, E., Bekele, T., and Yineger, H. (2008). An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J. Ethnobiol. Ethnomedicine 4, 10. doi:10.1186/1746-4269-4-10
Maroyi, A. (2014). Medicinal plants and traditional practices in peri-urban domestic gardens of the Limpopo province. South Africa.
Masyita, A., Mustika Sari, R., Dwi Astuti, A., Yasir, B., Rahma Rumata, N., Emran, T. B., et al. (2022). Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 13, 100217. doi:10.1016/j.fochx.2022.100217
Melo-de-Pinna, G. F., Hernandes-Lopes, J., Ogura, A. S., Santos, L. K., Silva, D. C., and Haevermans, T. (2016). Growth patterns and different arrangements of vascular tissues in succulent leaves. Int. J. Plant Sci. 177 (8), 643–660. doi:10.1086/688258
Mies, B. A. (1995). On the comparison of the flora and vegetation of the island groups of Socotra and Macaronesia.
Mohanraj, K., Karthikeyan, B. S., Vivek-Ananth, R. P., Chand, R. P. B., Aparna, S. R., Mangalapandi, P., et al. (2018). IMPPAT: a curated database of Indian medicinal plants, phytochemistry and therapeutics. Sci. Rep. 8 (1), 4329. doi:10.1038/s41598-018-22631-z
Mokua, S. K., Mbaria, J. M., Maitho, T. E., and Moriasi, G. A. (2021). Ethnobotanical documentation, phytochemical screening, and cytotoxicity evaluation of medicinal plants used to manage snakebite envenomation in mwingi west subcounty, Kenya. Evidence-Based Complementary Altern. Med. 2021 (1), 4167296. doi:10.1155/2021/4167296
Moreira, R., Pereira, D. M., Valentão, P., and Andrade, P. B. (2018). Pyrrolizidine alkaloids: chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 19 (6), 1668. doi:10.3390/ijms19061668
Mugford, S. T., and Osbourn, A. (2013). “Saponin synthesis and function,” in Isoprenoid synthesis in plants and microorganisms: new concepts and experimental approaches. Editors T. J. Bach, and M. Rohmer (New York, NY: Springer New York), 405–424.
Nunez-Melendez, E., and Johnson, C. H. (1955). The structure and constituents of Kleinia aizoides DC. J. Am. Pharm. Assoc. (Sci. ed) 44 (6), 349–352. doi:10.1002/jps.3030440612
Odeyemi, S., and Bradley, G. (2018). Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: pharmacology and toxicology. Molecules 23 (11), 2759. doi:10.3390/molecules23112759
Ouhaddou, H., Boubaker, H., Msanda, F., and El Mousadik, A. (2014). An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco). J. Appl. Biosci. 84, 7707–7722. doi:10.4314/jab.v84i1.5
Ozerova, L. V., Schanzer, I. A., and Timonin, A. C. (2017). Curio alliance (Asteraceae: senecioneae) revisited. Wulfenia 24, 29–52.
Panche, A. N., Diwan, A. D., and Chandra, S. R. (2016). Flavonoids: an overview. J. Nutr. Sci. 5, e47. doi:10.1017/jns.2016.41
Park, S. H., Sim, Y. B., Lim, S. S., Kim, J. K., Lee, J. K., and Suh, H. W. (2010). Antinociception effect and mechanisms of campanula punctata extract in the mouse. Korean J. Physiol. Pharmacol. 14 (5), 285–289. doi:10.4196/kjpp.2010.14.5.285
Petrovska, B. B. (2012). Historical review of medicinal plants’ usage. Pharmacogn. Rev. 6 (11), 1–5. doi:10.4103/0973-7847.95849
Pitchaipillai, M., Raj, K., Balasubramanian, J., and Periakaruppan, P. (2014). Benevolent behavior of Kleinia grandiflora leaf extract as a green corrosion inhibitor for mild steel in sulfuric acid solution. Int. J. Minerals, Metallurgy, Mater. 21 (11), 1083–1095. doi:10.1007/s12613-014-1013-7
Romo de Vivar, A., Pérez-Castorena, A.-L., Arciniegas, A., and Villaseñor, J. L. (2007). Secondary metabolites from Mexican species of the tribe Senecioneae (Asteraceae). J. Mexican Chem. Soc. 51 (3), 160–172. doi:10.29356/jmcs.v51i3.1347
Rowley, G., and Eggli, U. (2002). “Asteraceae,” in Illustrated handbook of succulent plants: dicotyledons, 19–43.
Sales, A., Felipe, L. O., and Bicas, J. L. (2020). Production, properties, and applications of α-terpineol. Food bioprocess Technol. 13 (8), 1261–1279. doi:10.1007/s11947-020-02461-6
Saral, Ö., Baltaş, N., and Karaköse, M. (2024). An inhibition potential on some metabolic enzymes (urease and xanthine oxidase), essential oil contents and antioxidant effect of Sideritis lanata L. Chem. Pap. 78 (15), 8211–8217. doi:10.1007/s11696-024-03661-6
Schaal, B. (2019). Plants and people: our shared history and future. Plants, People, Planet 1 (1), 14–19. doi:10.1002/ppp3.12
S. Selvakumar (2018). Preliminary phytochemical investigation of various extracts of leaves of K leinia grandiflora.
Semenya, S. S., and Maroyi, A. (2018). Ethnobotanical study of curative plants used by traditional healers to treat rhinitis in the Limpopo Province, South Africa. Afr. Health Sci. 18 (4), 1076–1087. doi:10.4314/ahs.v18i4.29
Şen, G., Akbulut, S., and Karaköse, M. (2022). Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye). Open Chem. 20 (1), 873–911. doi:10.1515/chem-2022-0204
Şeremet, O. C., Bărbuceanu, F., Ionică, F. E., Margină, D. M., Guţu, C. M., Olaru, O. T., et al. (2016). Oral toxicity study of certain plant extracts containing pyrrolizidine alkaloids. Rom. J. Morphol. Embryol. 57 (3), 1017–1023.
Seremet, O. C., Olaru, O. T., Gutu, C. M., Nitulescu, G. M., Ilie, M., Negres, S., et al. (2018). Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol. Med. Rep. 17 (6), 7757–7763. doi:10.3892/mmr.2018.8795
Serumula, M. S. (2021). An investigation of kleinia venteri in the polokwane area, limpopo province. South Africa: University Of Limpopo.
Sewawa, K. (2021). Phytochemical investigation of the aerial parts of Kleinia longiflora. Botswana International University of Science and Technology. Botswana.
Shagufta, P. (2018). “Introductory chapter: terpenes and terpenoids,” in Terpenes and terpenoids. Editors P. Shagufta, and A.-T. Areej (Rijeka: IntechOpen). Ch. 1.
Shehata, I. A., El-harshany, E., Abdallah, H. M., Esmat, A., and Abdel-sattar, E. A. (2018). Anti-inflammatory activity of Kleinia odora. Eur. J. Integr. Med. 23, 64–69. doi:10.1016/j.eujim.2018.10.005
Smith-Hall, C., Larsen, H. O., and Pouliot, M. (2012). People, plants and health: a conceptual framework for assessing changes in medicinal plant consumption. J. Ethnobiol. ethnomedicine 8, 43–11. doi:10.1186/1746-4269-8-43
Sofowora, A., Ogunbodede, E., and Onayade, A. (2013). The role and place of medicinal plants in the strategies for disease prevention. Afr. J. traditional, complementary Altern. Med. 10 (5), 210–229. doi:10.4314/ajtcam.v10i5.2
Stegelmeier, B. L. (2011). Pyrrolizidine alkaloid-containing toxic plants (Senecio, Crotalaria, Cynoglossum, Amsinckia, Heliotropium, and Echium spp.). Vet. Clin. North Am. Food Anim. Pract. 27 (2), 419–428. doi:10.1016/j.cvfa.2011.02.013
Sun, Y., and Vargas-Mendoza, C. F. (2017). Population structure, genetic diversity, and evolutionary history of kleinia neriifolia (Asteraceae) on the canary islands. Front. Plant Sci. 8, 1180. doi:10.3389/fpls.2017.01180
Timonin, A., Ozerova, L., and Shantser, I. (2014). Evolution of succulent senecioneae (Asteraceae) of southern Africa. Zhurnal Obshchei Biol. 75 (1), 25–37. doi:10.1134/S2079086415010089
Ullah, A., Munir, S., Badshah, S. L., Khan, N., Ghani, L., Poulson, B. G., et al. (2020). Important flavonoids and their role as a therapeutic agent. Molecules 25 (22), 5243. doi:10.3390/molecules25225243
Usure, R. E., Kebebe, D., Mekasha, Y. T., Hasen, G., Chura Waritu, N., Dubale, S., et al. (2024). Traditional herbal medicine regulatory implementation in Ethiopia: a qualitative study. Front. Pharmacol. 15, 1392330. doi:10.3389/fphar.2024.1392330
Vanijajiva, O., Pornpongrungrueng, P., and Pongamornkul, W. (2014). Kleinia grandiflora (Asteraceae: senecioneae), a species and genus newly discovered in Thailand. Phytotaxa 159 (1), 017–022. doi:10.11646/phytotaxa.159.1.3
Yan, C., Peng, T., Zhang, T., Wang, Y., Li, N., Wang, K., et al. (2023). Molecular mechanisms of hepatotoxicity induced by compounds occurring in Evodiae Fructus. Drug Metab. Rev. 55 (1-2), 75–93. doi:10.1080/03602532.2023.2180027
Keywords: Kleinia, ethnomedicinal uses, phytochemicals, metabolites, ethnopharmacology, toxicology
Citation: Tegegne BA, Begashaw T, Belay WY, Tariku MK, Zeleke TK, Jemal M, Getinet M, Alehegn AA and Dagne A (2025) Kleinia (Asteraceae): comprehensive review of ethnomedicinal uses, phytochemical profiles, ethnopharmacological applications, and toxicological insights. Front. Pharmacol. 15:1469887. doi: 10.3389/fphar.2024.1469887
Received: 24 July 2024; Accepted: 19 December 2024;
Published: 08 January 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Verena Spiegler, University of Münster, GermanyCopyright © 2025 Tegegne, Begashaw, Belay, Tariku, Zeleke, Jemal, Getinet, Alehegn and Dagne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bantayehu Addis Tegegne, YmFudGF5ZWh1YWRkaXMuOTBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.