- Department of Dermatology, General Hospital of Northern Theater Command, Shenyang, China
Acute generalized exanthematous pustulosis, an infrequent adverse drug reaction, mainly results from drugs. Clinically, acute generalized exanthematous pustulosis manifests as a high fever, with skin lesions of small monomorphic subcorneal sterile pustules on an erythematous that presents at 1–4 days after medication exposure. The incidence of acute generalized exanthematous pustulosis varies from 3/1, 000, 000 to 5/1, 000, 000, while the mortality rate is typically around 5%. We present a case of a 69-year-old female who developed a diffuse, erythematous, pustular rash over the entire body and exhibited a fever of 38.3°C after 4 days of icotinib therapy. Considering her medication history and the appearance of the lesions, she was diagnosed with acute generalized exanthematous pustulosis and received appropriate treatment. We also conducted a literature review through PubMed to compare similarities and differences between our case and those reported in the literature.
1 Introduction
Icotinib is one of the epidermal growth factor receptor tyrosine kinases inhibitors (EGFR TKI), that reversibly inhibits EGFR signaling. Since it was initially made available in China, nearly 260,000 patients with non-small cell lung cancer (NSCLC) have been treated with icotinib. The most common adverse drug reactions of icotinib include rashes, dry skin, and diarrhea (He et al., 2021). However, reports of severe adverse drug reactions related to icotinib are relatively rare. Here, we report the first case of acute generalized exanthematous pustulosis (AGEP) induced by icotinib.
2 Case presentation
A 69-year-old Chinese female was diagnosed with left lung adenocarcinoma and diabetes. For her diabetes, she was taking oral sustained-release metformin tablets (1.5 g once daily) and acarbose tablets (50 mg thrice daily). For the lung adenocarcinoma, she received treatment with icotinib. She was not known to have any drug or food allergies. 4 days after receiving icotinib, she experienced a diffuse, erythematous, pustular rash over her entire body without mucosal involvement (Figure 1A) and a temperature of 38.9°C. Laboratory assay results revealed normal white blood cell counts (8.4 × 103/µL with 72.8% neutrophils and 0.03% eosinophils), elevated acute-phase reactants (CRP 14.4 mg/dL, fibrinogen 5.19 g/L), and normal hepatic and renal functions. In light of her medication history and manifestation of lesions, AGEP was diagnosed. Icotinib was immediately discontinued and 40 mg of methyl-prednisolone was administered intravenously, with this dose subsequently reduced to 28 mg/d after 5 days. Following this methyl-prednisolone treatment, there was a lightening of the erythema, resolution of the pustules and widespread superficial desquamation was observed (Figure 1B). Within 2 weeks, the skin lesions had completely disappeared and the methyl-prednisone was gradually discontinued. Thereafter, the patient continued to take icotinib, experiencing only a few pustules, and was treated with halometasone/triclosan cream. 6 months later, due to the progression of the lung cancer, the patient was switched to osimertinib as the antineoplastic drug. 1 year later, the tumor had metastasized to the liver and bones. The patient underwent chemotherapy with pemetrexed and carboplatin, and was subsequently lost to follow-up 1 month after the treatment.
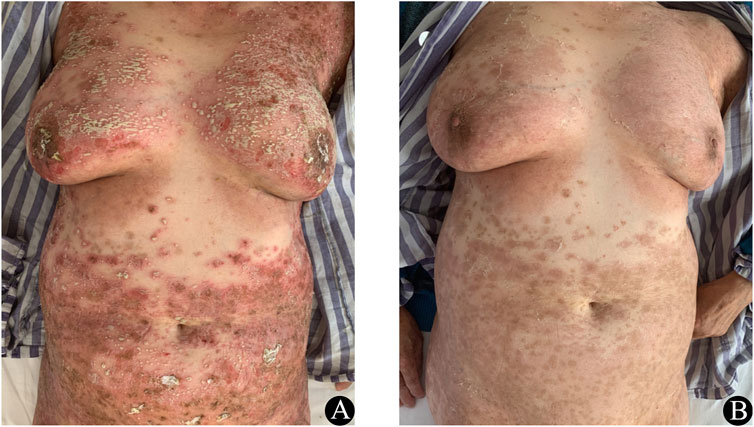
Figure 1. (A) Prior to treatment erythematous and pustular rashes were present on the trunk. (B) At 1 week after corticosteroid treatment, significant improvements in these cutaneous lesions were observed.
3 Discussion
AGEP is a severe cutaneous adverse reaction, which is most commonly triggered by exposure to certain drugs, including antibiotics, antifungals, and hydroxychloroquine (Szatkowski and Schwartz, 2015). Other risk factors encompass genetic factors, infections, and vaccinations (Szatkowski and Schwartz, 2015; Parisi et al., 2023). The diagnosis of AGEP is clinical, confirmed by histopathological examination (Hadavand et al., 2022). Differential diagnoses include autoimmune diseases such as generalized pustular psoriasis (GPP), IgA pemphigus, infectious diseases such as candida infection, and staphylococcal scalded skin syndrome (SSSS), as well as subcorneal pustular dermatosis (SPD). GPP is a form of psoriasis, with the gradual development of widespread and densely distributed sterile pustules on an erythematous base, accompanied by tenderness (Shi et al., 2022). IgA pemphigus is a specific type of pemphigus, characterized by blisters and pustules as the primary skin lesions in an autoimmune intraepidermal blistering disease. SSSS is an infection disease mediated by exfoliative toxins produced by coagulase-positive Staphylococcus aureus, and it is seen primarily in children. Candida infection usually manifests as erythematous, patches that are often accompanied by satellite papules and pustules. Intertriginous zones and the scrotum are most often involved and overall skin involvement is rare (Shepherd et al., 2017). SPD is an infrequent, benign, and recurrent pustular dermatosis, with remissions of variable duration. The pustules exhibit a superficial nature and are organized in annular and serpiginous configurations, particularly observed on the abdomen, axillae, and groins (Mohd Affandi and Baharom, 2023). For this reason, a cultured cutaneous secretion was included to exclude infectious diseases. Skin biopsy and direct immunofluorescence were not performed due to a lack of consent. The patient denied a family history or personal history of psoriasis, and based on her detailed medical history and typical manifestation of the lesion, we considered AGEP to be the most likely diagnosis. According to the AGEP validation score from the EuroSCAR study group, she was classified as a definite case, with an EuroSCAR score of 8 (Parisi et al., 2023).
Review of the literature revealed five cases of AGEP in patients with a reported EGFR-TKI exposure event from 2006 to 2024 (Shih et al., 2006; Lakshmi et al., 2010; Liquete et al., 2012; Komiya et al., 2021) (Table 1). We found that, four of six cases occurred in women. The time elapsed between medication exposure and the onset of the rash ranged from 4 days to 2 months. Five of these patients received corticosteroid treatment, and the rash resolved quickly. Interestingly, among the six patients, three were re-challenged with the culprit drug, resulting in limited pustular lesions. Recurrence of AGEP was observed in two patients subsequent to the resumption of the culprit drug. It is generally accepted that in the case of AGEP, a second episode may be more severe if the causative medication is readministered, therefore, avoiding further exposure is advised. However, for cancer patients experiencing cutaneous adverse reactions related to antineoplastic drugs, we need to collaborate with oncologists to grade the rash according to the Common Terminology Criteria for Adverse Events (CTCAE, Version 5.0). This assessment will help doctors decide if the patient can continue to take the causative drugs. The rash of this patient was classified as a grade 3 adverse event. It did not pose a threat to her life and resolved completely following appropriate treatment. Considering the diagnosis of stage IV lung cancer and the need for ongoing treatment, the decision was made to resume the medication with her informed consent. Although this patient exhibited only a few pustules after resuming the culprit drug and was able to control the symptoms with topical corticosteroids, it remains essential to monitor for any skin lesions in patients.

Table 1. Case report of AGEP associated with epidermal growth factor receptor tyrosine kinase inhibitors.
It has been well established that activated drug-specific T cells play a significant role in AGEP via a cascade of events (Mashiah and Brenner, 2003). Drugs form complexes with tissues in the body, stimulating the formation of drug-specific T cells and promoting their migration to the skin (Feldmeyer et al., 2016). Subsequently, CD8+ cells lead to the formation of subcorneal vesicles by releasing cytotoxic proteins and the CD4+ cells in these vesicles release IFN-γ, CXCL8 and GM-CSF. IFN-γ then promotes a further secretion of CXCL8 from surrounding keratinocytes, which effectively recruits neutrophils, while GM-CSF prevents apoptosis in these recruited neutrophils (Hadavand et al., 2022). Th17 cells, IL-17 and mutations in IL36RN may also be connected with AGEP (Hadavand et al., 2022). Mutations in the IL-36RN gene can lead to uncontrolled IL-36 signaling, which in turn stimulates the overproduction of additional proinflammatory cytokines and chemokines. This overproduction can exacerbate the recruitment and activation of neutrophils (Gabay and Towne, 2015). However, the pathogenesis of AGEP induced by EGFR-TKI remain unclear. EGFR is widely distributed in the skin, and skin-related adverse reactions are among the most common effects of EGFR-TKI therapy. Blockade of EGFR signaling downregulates CXCL8, while it increases CCL2, CCL5, and CXCL10 expression in keratinocytes, even under stimulation of IFN-γ(Mascia et al., 2003). In addition, the longest latency period observed in previous patients was nearly 2 months, and four of them continued the culprit drug treatment with only a few pustules observed, which differs from typical AGEP. Accordingly, we hypothesize that a different pathogenesis may underlie EGFR-TKI-induced AGEP, however, this hypothesis requires confirmation through further study.
The condition of most patients is generally self-limiting and the suspected drug must be discontinued immediately (Hadavand et al., 2022). Topical corticosteroids are used as supportive treatment, while systemic corticosteroids are used during the acute pustular phase or in severe cases (Hadavand et al., 2022; Parisi et al., 2023). Corticosteroids can quickly limit the inflammatory reaction and reduce the length of hospital stay (Oh et al., 2021). Accordingly, we used methylprednisolone (40 mg/d), her body temperature normalized within 24 h, the cutaneous lesions significantly improved within 5 days. And the dose of methylprednisolone was tapered to 0 within 2 weeks without any resurgence of symptoms. Besides, IL-17A inhibitors and IL-36 receptor inhibitors have been reported as a rapid and effective treatment for AGEP (Wen et al., 2024; Xuan et al., 2024). However, due to the presence of malignant tumors in our patient, treatment with IL-17A inhibitors and IL-36 receptor inhibitors was not considered in this case.
To the best of our knowledge, there have been no previously reported cases of icotinib-induced AGEP, however, current evidence suggests that EGFR-TKI is a rare cause of AGEP (Shih et al., 2006; Lakshmi et al., 2010; Liquete et al., 2012; Komiya et al., 2021). Furthermore, it has been implicated as a culprit drug in other severe cutaneous adverse reactions, including drug-induced hypersensitivity syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis (Li et al., 2022). While EGFR-TKI have been widely used as the first-line treatment in NSCLC patients, it is important that any adverse events be reported in order to better understand the risks associated with this drug.
Limitations associated with this study should be noted. For example, the pervasiveness of these findings is limited by the small number of cases. And there is a lack of skin biopsy, patch test and lymphocyte transformation test in our case, all of which would be useful in establishing a definitive diagnosis.
4 Conclusion
EGFR-TKI is a rare cause of AGEP, with an uncertain incubation period. Corticosteroid therapy leads to a quick and complete resolution of skin lesions. While some culprit drugs could resume with only a few pustules being observed. In addition, the observation of skin lesions in patients receiving EGFR-TKI should be closely monitored.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the General Hospital of Northern Theater Command Ethics Committee(Y-2023-082). Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
WY: Writing–review and editing, Writing–original draft, Methodology. JZ: Data curation, Writing–original draft. JN: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Liaoning Province Technological Innovation Planned Project (Grant number 2022020519-JH2/1015).
Acknowledgments
We would like to express our gratitude to the patient’s family for providing written informed consent to publish the details of this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Feldmeyer, L., Heidemeyer, K., and Yawalkar, N. (2016). Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int. J. Mol. Sci. 17, 1214. doi:10.3390/ijms17081214
Gabay, C., and Towne, J. E. (2015). Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J. Leukoc. Biol. 97, 645–652. doi:10.1189/jlb.3RI1014-495R
Hadavand, M. A., Kaffenberger, B., Cartron, A. M., and Trinidad, J. C. L. (2022). Clinical presentation and management of atypical and recalcitrant acute generalized exanthematous pustulosis. J. Am. Acad. Dermatology 87, 632–639. doi:10.1016/j.jaad.2020.09.024
He, J., Su, C., Liang, W., Xu, S., Wu, L., Fu, X., et al. (2021). Icotinib versus chemotherapy as adjuvant treatment for stage II–IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir. Med. 9, 1021–1029. doi:10.1016/S2213-2600(21)00134-X
Komiya, N., Takahashi, K., Kato, G., Kubota, M., Tashiro, H., Nakashima, C., et al. (2021). Acute generalized exanthematous pustulosis caused by erlotinib in a patient with lung cancer. Case Rep. Oncol. 14, 599–603. doi:10.1159/000514146
Lakshmi, C., Pillai, S., and Srinivas, C. R. (2010). Lapatinib-induced acute generalized exanthematous pustulosis. Indian Dermatol Online J. 1, 14–17. doi:10.4103/2229-5178.73251
Li, Y., Fu, R., Jiang, T., Duan, D., Wu, Y., Li, C., et al. (2022). Mechanism of lethal skin toxicities induced by epidermal growth factor receptor inhibitors and related treatment strategies. Front. Oncol. 12, 804212. doi:10.3389/fonc.2022.804212
Liquete, E., Ali, S., Kammo, R., Ali, M., Alali, F., Challa, H., et al. (2012). Acute generalized exanthematous pustulosis induced by erlotinib (tarceva) with superimposed Staphylococcus aureus skin infection in a pancreatic cancer patient: a case report. Case Rep. Oncol. 5, 253–259. doi:10.1159/000338806
Mascia, F., Mariani, V., Girolomoni, G., and Pastore, S. (2003). Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathology 163, 303–312. doi:10.1016/S0002-9440(10)63654-1
Mashiah, J., and Brenner, S. (2003). A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch. Dermatol 139, 1181–1183. doi:10.1001/archderm.139.9.1181
Mohd Affandi, A., and Baharom, Z. F. (2023). Case report—subcorneal pustular dermatosis—a great mimicker of generalized pustular psoriasis. Exp. Dermatol. 32, 1308–1311. doi:10.1111/exd.14800
Oh, D. A. Q., Yeo, Y. W., Choo, K. J. L., Pang, S. M., Oh, C. C., and Lee, H. Y. (2021). Acute generalized exanthematous pustulosis: epidemiology, clinical course, and treatment outcomes of patients treated in an Asian academic medical center. JAAD Int. 3, 1–6. doi:10.1016/j.jdin.2020.12.004
Parisi, R., Shah, H., Navarini, A. A., Muehleisen, B., Ziv, M., Shear, N. H., et al. (2023). Acute generalized exanthematous pustulosis: clinical features, differential diagnosis, and management. Am. J. Clin. Dermatol 24, 557–575. doi:10.1007/s40257-023-00779-3
Shepherd, A. J., Mackay, W. G., and Hagen, S. (2017). Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst. Rev. 2017, CD004012. doi:10.1002/14651858.CD004012.pub5
Shi, Y.-L., Gu, J., and Zheng, Z.-Z. (2022). Diagnosis and treatment of pustular psoriasis: a Chinese expert consensus statement. Chin. J. Dermatol. doi:10.35541/cjd.20210698
Shih, H.-C., Hsiao, Y.-P., Wu, M. F., and Yang, J.-H. (2006). Gefitinib-induced acute generalized exanthematous pustulosis in two patients with advanced non-small-cell lung cancer: correspondence. Br. J. Dermatology 155, 1101–1102. doi:10.1111/j.1365-2133.2006.07511.x
Szatkowski, J., and Schwartz, R. A. (2015). Acute generalized exanthematous pustulosis (AGEP): a review and update. J. Am. Acad. Dermatology 73, 843–848. doi:10.1016/j.jaad.2015.07.017
Wen, J., Wang, Y., Wang, B., Jiang, B., Lan, J., Yang, J., et al. (2024). Rapid clearance of corticosteroid-resistant targetoid acute generalized exanthematous pustulosis using an IL-17a inhibitor: a case report. J. Investig. Allergol. Clin. 34, 196–197. doi:10.18176/jiaci.0946
Keywords: non-small cell lung cancer, tyrosine kinase inhibitor, icotinib, adverse drug reactions, acute generalized exanthematous pustulosis
Citation: Yang W, Zhao J and Niu J (2024) Acute generalized exanthematous pustulosis induced by icotinib: a case report and literature review. Front. Pharmacol. 15:1462430. doi: 10.3389/fphar.2024.1462430
Received: 10 July 2024; Accepted: 19 August 2024;
Published: 30 August 2024.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Xia Tianbao, PLA Strategic Support Force Characteristic Medical Center, ChinaLiusheng Peng, Third Military Medical University, China
Copyright © 2024 Yang, Zhao and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Niu, bml1anVuMDZAMTI2LmNvbQ==
 Wei Yang
Wei Yang Jiayu Zhao
Jiayu Zhao Jun Niu
Jun Niu