- Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, School and Hospital of Stomatology, Jilin University, Changchun, China
As a novel class of smart biomaterials with promising potentials, metal-organic frameworks (MOFs) are widely utilized in the field of biomedicine. Current researches indicate that the therapeutic strategies for osteoarthritis (OA) are highly limited to achieving symptom improvement and reducing both pain and inflammation. Together, the introduction of MOFs into the treatment of OA holds the potential to offer significant benefits. This is because MOFs not only have intrinsic biological activities, but also act as carriers to facilitate controlled drug delivery and prolong the duration in the management of OA. This paper presents a review of the recent studies that have explored the potential usage of MOFs as drugs or carriers in the treatment of OA, which also examines the progress of MOFs in tissue engineering for the treatment of OA. These studies are anticipated to not only enhance the comprehension of MOFs but also provide strong evidence in favor of their utilization in the treatment of OA.
1 Introduction
As a prevalent and destructive chronic disease, osteoarthritis (OA) has been considered as the primary cause of cartilage erosion. The etiology of OA mainly involves the following factors, such as age, weight, gender, genetic susceptibility, sports-related injury, and inflammation. It is well known that any joint in the body can be impacted along with the occurrence of OA, which can induce the patients to experience symptoms including pain, edema, stiffness, and restricted movement (Motta et al., 2023). According to recent studies, over 240 million worldwide individuals are believed to experience symptomatic OA, resulting in substantial physical and psychological strain on sufferers, especially among the elderly (Lv et al., 2024). More importantly, articular cartilage is composed of chondrocytes and an extracellular matrix (ECM) that surrounds the chondrocytes, which does not possess sufficient circulatory, neural, and lymphatic systems that are necessary for self-healing (Liu et al., 2021; Gao et al., 2022). Therefore, it has been proven to be a challenge that managing osteoarthritis through the regeneration of articular cartilage.
A series of medication including corticosteroids, non-steroidal anti-inflammatory drugs, and hyaluronic acid has been well developed to alleviate inflammation in OA. Compared with other alternative techniques, intra-articular injections are considered as the most efficacious method for treating OA (Richard et al., 2023; Guo et al., 2022). Although promising, it is still difficult to ensure the controlled release of drug and its long-term release effectiveness. Currently, scientists are exploring innovative approaches to improve the delivery of drugs with the assistance of nanotechnology. As well known, nanomaterials possess the capacity to encapsulate drugs and accurately release them at the target sites (Sattar et al., 2022; Chamundeeswari et al., 2019). Moreover, nanoassemblies based on anti-inflammatory biomolecules possess the capability to reduce inflammation by themselves. Accordingly, the use of high-performance nanomaterials as emerging biomaterials for the treatment of OA is regarded as an urgent strategy.
Metal-organic frameworks (MOFs) are crystalline compounds composed of organic ligands and metal ions. Because of the intrinsic porous properties, thermal and chemical stability, easy surface modification, as well as high biosafety, metal-organic frameworks (MOFs) have been considered as promising nanocarriers in the field of biomedicine (Horcajada et al., 2008; Li L. et al., 2023; Li X. et al., 2023). It is worth noting that a variety of factors including internal stimuli such as redox, pH, and enzymes, as well as external stimuli such as light, temperature, magnetism, ultrasound, and mechanical force have been utilized to trigger the release of guest molecules from MOF-based nanosystems. Meanwhile, these aforementioned nanosystems usually exhibit the ability to react towards multiple stimuli, which can enable them to release chemicals at specified moments or spaces (Gu et al., 2023). More importantly, MOFs and their derivatives with inherent bioactivity can relieve localized inflammation in the treatment of inflammatory diseases (Li et al., 2024; Shyngys et al., 2021). This review article critically summarized the recent advances made in the development of MOFs and their derivatives, as well as their potentials for treating OA. As illustrated in Figure 1, the key emphasis of this review was to discuss the roles of MOFs and their derivatives for the OA treatment and bone regeneration. Moreover, the stability and biological activity of these biomaterials were well evaluated in the above-mentioned OA treatments. Last but not least, this review concluded with an overview of the challenges hindering further development of MOFs and their derivatives and a perspective on the future of the field before their clinical translation.
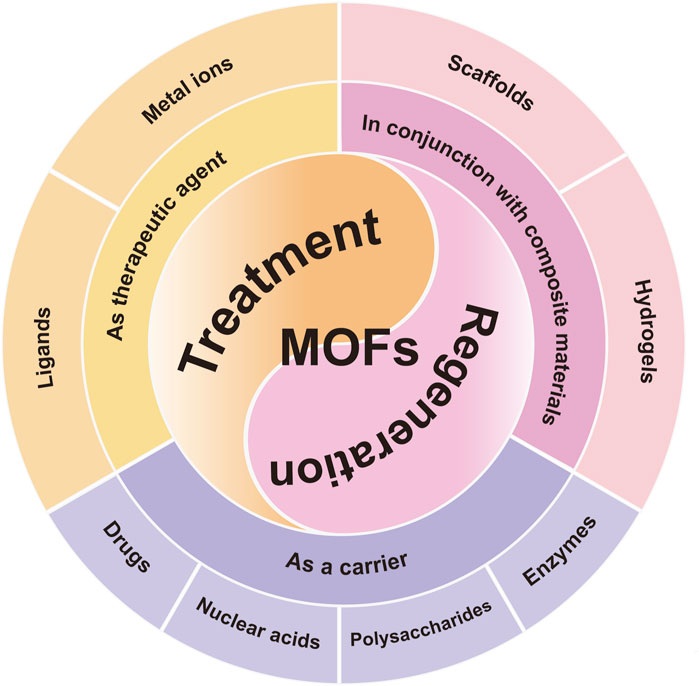
Figure 1. The summary of MOFs and their derivatives in the field of OA treatment. Schematic representation of the three pillars of MOFs application-therapeutic agents, carriers and compositions for scaffold materials.
2 MOF as a therapeutic agent
Essential metal ions or clusters including Zn, Mg, Zr, Fe, Cu, and Mn usually held the ability to cure OA, which could also serve as the structure centers of MOFs and their derivatives. As illustrated in Figure 2A, Cheng and co-workers synthesized a novel MOF named as {[Zn(H2O)(HL)]·(DMF)2(H2O)2}n, which could regulate the inflammation associated with OA via reducing the activation of the PI3K/AKT signaling pathway (Cai et al., 2020; Sun et al., 2020). Tang and co-workers developed a new Mg/HCOOH-MOF, which could serve as a controlled-release drug for treating OA. The magnesium ions derived from Mg/HCOOH-MOF held both anti-inflammatory and osteogenic properties, promising their potentials in the anti-inflammatory therapy and further promotion of osteogenesis (Li et al., 2020b). As shown in Figure 2B, Yan and co-workers prepared a novel Zr-MOF-801 with admirable antimicrobial effect and enhanced osteogenic performance (Yan et al., 2022).
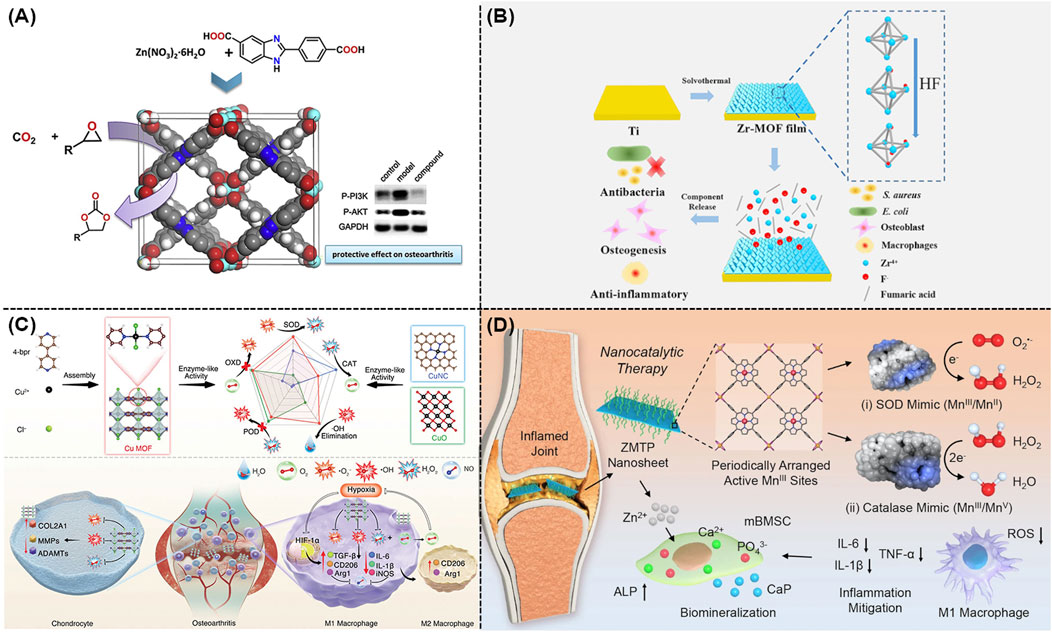
Figure 2. (A) Schematic representation of the preparation of Zn(II)-MOF and its effect on activation of the PI3K/AKT signaling pathway (Cai et al., 2020). (B) Preparation of Zr-MOF film and its antimicrobial, anti-inflammatory, and osteogenic capabilities (Yan et al., 2022). (C) Schematic illustration of Cu-MOF nanozymes for the treatment of OA by scavenging ROS and regulating macrophage phenotype (Yu et al., 2024). (D) Schematic illustration of the therapeutic mechanism of ZMTP nanosheets for nanocatalytic treatment of rheumatoid arthritis (Yang B. et al., 2022).
Reactive oxygen species (ROS) could significantly contribute to the development and exacerbation of OA, which emerged as a promising therapeutic target. As a famous nanozyme that came from Fe-based MOF family, Mil-88 with excellent peroxidase activity exhibited the ability to eliminate ROS both in vitro and in vivo. According to these findings, a very recent study revealed that a feasible Mil-88-based enzymatic healing process could be beneficial for treating OA (Lopez-Cantu et al., 2022). For instance, Mil-88 could enhance the bioactivity of genes related to tissue growth (such as Col-2) and reduce the activity of genes associated with OA tissue breakdown (such as MMP-13), promising its potential to be a novel and advantageous method for treating OA (Hu et al., 2023). Meanwhile, a well-defined Cu-MOF nanozymes were well developed for the treatment of OA using a straightforward self-assembly method, as shown in Figure 2C. According to the density functional theory (DFT), these Cu-MOF nanozymes with excellent superoxide dismutase (SOD) and catalase (CAT) mimicking characteristics had higher antioxidant activity in comparison to other Cu-based antioxidants. As expected, these Cu-MOF nanozymes could alleviate the low oxygen conditions in OA, transform macrophages into the M2 phenotype, prevent cartilage damage, as well as decrease the release of pro-inflammatory factors (Yu et al., 2024). Furthermore, a MOF named as Zn-Mn-TCPP-PVP (ZMTP) were well designed using the coordination effect between the benzoyloxy group of Mn-TCPP and Zn2+ (Yang B. et al., 2022). As shown in Figure 2D, these well-developed ZMTP with high SOD activity and CAT activity exhibited great anti-inflammatory and bio-mineralization performances in both in vitro and in vivo models.
3 MOF as a carrier
Considering that there were lots of drugs utilized for treating OA in daily clinic, a variety of carriers were highly required. In comparison to other nanoscale carriers, MOFs and their derivatives held several advantages including a substantial specific surface area for the attachment of cells, a high porosity for the encapsulation of biomolecules, a great feasibility for the surface modification with targeted and responsive molecules, and a high biocompatibility. Therefore, it is suitable for the delivery of various guest molecules, including drugs, proteins, nucleic acids and polysaccharides.
3.1 Loading drugs
According to recent clinical research, the predominant non-steroidal anti-inflammatory drugs (NSAIDs) for the symptomatic management of OA were mainly ibuprofen, ketoprofen, and diclofenac (Richard et al., 2023). Although the localized use of NSAIDs extremely enhanced their absorption, two factors might seriously lead to a decrease in their effectiveness. First, a periarticular microvascular system usually facilitated the rapid drug clearance from joints. Second, the skin played an active role in metabolizing medicines using the cytochrome P450 enzyme. In order to improve the medication usage and relative OA treatment efficacy, well-defined nanoscale carriers were highly needed (Patil et al., 2023). Previous studies demonstrated that MIL-101 (Cr) and MIL-100 could achieve the high loading of ibuprofen and its stimuli-responsive release (Silva et al., 2016). Figure 3A revealed that novel Cu-MOFs composed with multiple ligands were created as efficient carriers for delivering IBU and DOX (Sun et al., 2017). Furthermore, Tan and co-workers utilized zirconium-based MOF (UiO-66) as an efficient drug carrier (Li et al., 2019b). As shown in Figure 3B, by changing different functional groups, these well-developed nano-sized carriers could well response toward different stimulants. As expected, UiO-66-NH2 held the best loading and release properties. Furthermore, a MOF-based drug delivery nanosystem based on Sr/PTA-MOF-ketoprofen could reduce inflammation, relieve pain, as well as maintain bone homeostasis (Li et al., 2019a; Li et al., 2020a).
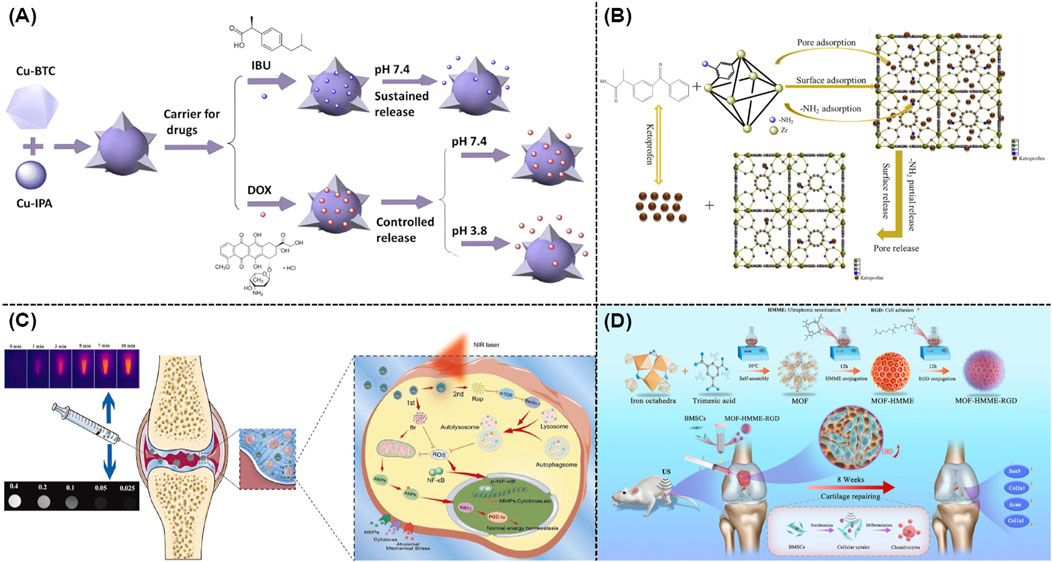
Figure 3. (A) Schematic illustration of the application of mixed ligand MOFs as efficient drug carriers for IBU and DOX (Sun et al., 2017). (B) Drug loading and release mechanism of UiO-66-NH2 as a drug carrier (Li et al., 2019b). (C) Mechanisms of dual drug delivery nanoplatforms with cartilage-targeting effect and near-infrared laser response in OA therapy (Xue S. et al., 2021). (D) Schematic illustration of the synthesis of MOF-HMME-RGD and relative therapeutic mechanisms (Luo et al., 2024).
Owing to the acidic nature of joint milieu, the acid-responsive release mechanism was well understood in various MOF-based delivery systems. Itaconic acid-contained ZIF-8 was well prepared as a pH-responsive drug-loaded nanocarrier in which itaconic acid could alleviate OA after the efficient cellular penetration and endocytosis (Yu et al., 2023). Meanwhile, the ZIF-8’s pH-responsive characteristic was utilized to encapsulate neoflavone (NBIF), which could extremely reduce the poor solubility of NBIF and its rapid degradation (Jiang et al., 2023). As shown in Figure 3C, a nano-size hybrid nanocarrier based on mesoporous polydopamine and MOF was rationally developed as an efficient photothermal reagent to promote the OA treatment. The above mentioned nanosystem exhibited rapid responsiveness toward the irradiation of near infrared (NIR) light, leading to the enhanced drug release (Xue S. et al., 2021). Moreover, a zirconium-based MOF was modified with polyphenols and gold, which exhibited excellent photothermal outcomes for the treatment of OA (Xu S. et al., 2023). Recent studies indicated that sonodynamic therapy was utilized in the OA treatment. As shown in Figure 3D, a Fe-based MOF was well conjugated with hematoporphyrin monomethyl ether (HMME) and arginine-aspartate-glycine (RGD) peptides for repairing cartilage defects (Luo et al., 2024). According to the current design, the porous architecture of MOFs could effectively prevent organic acoustic molecules from their self-quenching while ROS could be rapidly generated through the linker-metal charge transfer pathway with high efficacy.
3.2 Loading nuclear acids
Recent studies indicated that RNA-based gene therapy was widely utilized to treat OA. This innovative method usually employed microRNAs and anti-miRNAs for polygenic regulation, small interfering RNAs (siRNAs) for gene silencing, and mRNAs for gene supplementation (DeJulius et al., 2024). Although promising, current systemic delivery of RNA usually failed because of the complicated environment of body and the biodegradability of nuclear acids, which also could lead to serious side effects including uncertain retention duration in body, immunological infection, and potential toxicity. Recent studies suggested the applications of MOFs and their derivatives as high-performance nanocarriers for the delivery of nuclear acid (He et al., 2023). The well-defined chemical structures of MOFs and their derivatives endowed them with the possibility of strong interaction with nucleic acid biomolecules, which could occur by encapsulation, physical adsorption, as well as chemical grafting (He et al., 2023). As illustrated in Figure 4A, some researchers utilized ZIF-8 to create miR-200c-3p@ZIF-8 using a facile one-step approach with the assistance of a Y-shape microfluidic chip. This approach allows for the exact manipulation of miR-200c-3p@ZIF-8 with ideal size, resulting in the admirable encapsulation efficacy and transport outcome. As expected, these well-developed miR-200c-3p@ZIF-8 hybrids could significantly lowered the expression of associated inflammatory factors during the OA treatment (Yang et al., 2023). Moreover, siRNA targeting the hypoxia-inducible factor-2α (HIF-2α) gene and the relative anti-inflammation therapy could be well achieved using MIL-101-NH2 as efficient nanocarrier. Figure 4B demonstrated that gene and drug loaded MIL-101-NH2 could efficiently release siRNA and curcumin in the acidic OA microenvironment, which thus silenced the HIF-2α gene, reduced the levels of pro-inflammatory factors, and avoided the relative cartilage degradation (Zhang et al., 2023a).
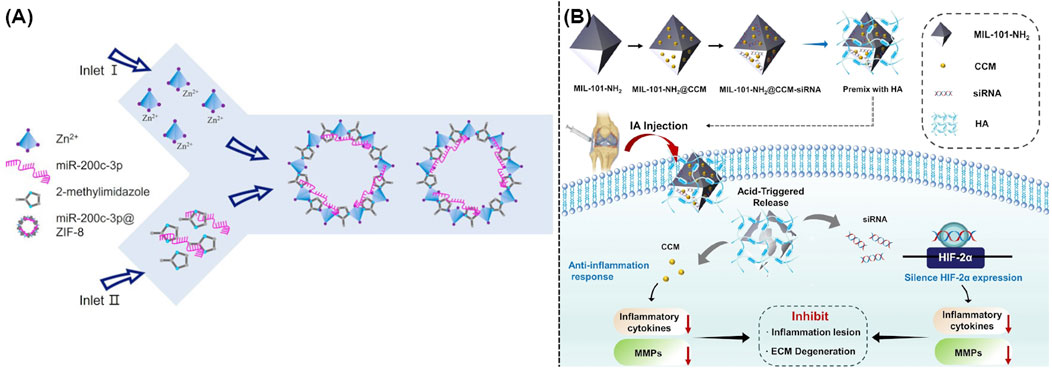
Figure 4. (A) Schematic illustration of a one-step microfluidic preparation of miR-200c-3p@ZIF-8 (Yang et al., 2023). (B) Synthesis of MIL-101-NH2@CCM-siRNA and its usage in the OA treatment (Zhang et al., 2023a).
3.3 Loading polysaccharides
Due to their high biocompatibility and low systemic toxicity, polysaccharides and polysaccharide-based materials were ideal for the treatment of OA. Recent studies demonstrated that polysaccharides could be well utilized as raw materials for the synthesis of ECM, which served as efficient lubricants for lubricating joints. Moreover, polysaccharides could provide a suitable environment for chondrocyte repair, exert antioxidant activity to regulate the OA microenvironment, as well as interact with cell membrane receptors to avoid the attack of inflammatory factors (An et al., 2024; Alcaide-Ruggiero et al., 2023). Although promising, essential strategies were highly required to optimize the current delivery approach and therapeutic efficacy due to the high molecular weight and fragility of various polysaccharides.
Traditional carriers including silica, hydrogels, and polymer-based materials were highly limited by their non-biodegradability, low loading efficacy, as well as complicated synthesis. Recent studies indicated that the intrinsic porous structure of MOFs could efficiently adsorb and accommodate polysaccharides. Moreover, polysaccharides could be well loaded into the interior of MOFs via the electrostatic interaction in the mixture containing metal ions, organic ligands, and polysaccharides, which resulted in the direct synthesis of polysaccharide-loaded MOFs (Tong et al., 2021). As shown in Figure 5A, ZIF-8 could encapsulate heparin, fucoidan sulfate, and fucosylated chondroitin sulfate, and hyaluronic acid. The well-developed nanocomposites named as polysaccharide@ZIF-8 followed an acid-responsive property, which could release polysaccharide in the OA microenvironment (Zheng et al., 2021). Furthermore, several pH-responsive MOFs including ZIF-8, ZIF-90, and MAF-7 were rationally designed and utilized to provide the protection for glycosaminoglycans (GAG). As shown in Figure 5B, the above mentioned GAG-loaded nanocomposites could offer new avenues for the further therapy of OA (Velásquez-Hernández et al., 2020).
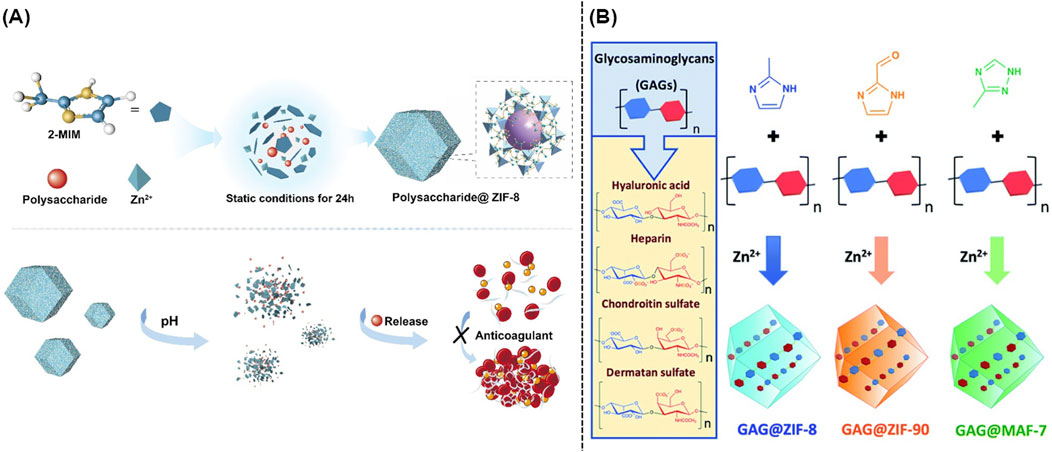
Figure 5. (A) Schematic illustration of the synthesis of polysaccharides@ZIF-8 (Zheng et al., 2021). (B) One-pot synthesis of GAG@MOFs originated from three different metallic nitrogen heterocyclic frameworks (Velásquez-Hernández et al., 2020).
3.4 Loading enzymes
Previous studies demonstrated that natural enzymes or artificial enzymes with antioxidant activity held great potentials in the OA therapy. Accordingly, MOFs were widely utilized to load these enzymes, which could extremely improve their stability and endurance under physiological conditions. For example, platinum (Pt) nanozymes with excellent ROS scavenging and anti-inflammatory activities were encapsulated in ZIF-8 while lantanum (La) as an osteogenic active element was well incorporated via ion exchange, which resulted in the formation of Pt@ZIF-8@La with a bimetallic-organic backbone. Both in vitro and in vivo results demonstrated that this well-developed multifunctional nanoplatform with high enzyme activity and great ability of ion release exhibited efficient synergistic therapeutic effects of immunomodulation and osteogenesis (Pan et al., 2023). In addition, Pt@PCN222-Mn with multifunctional active sites was involved in the treatment of temporomandibular joint (TMJ) osteoarthritis. This well-prepared nanocomposite could remodel the inflammatory microenvironment of TMJ OA and delay its development through regulating the ROS-NF-κB and p38/MAPK signaling pathways (Zhang et al., 2023).
4 MOF as a scaffold material composition
Besides many drugs and biomolecules were widely utilized for the inflammation control and cartilage repairing in clinic, tissue engineering still remained its necessity and specificity in the promotion of new cartilage formation. Combined with the use of MOFs and their derivatives, multifunctional platforms based on scaffolds or hydrogels could result in ideal therapeutic outcomes for OA. The addition of MOFs to scaffolds can endow these scaffold materials with special physicochemical properties and biological effects, so as to better assist the function of scaffold materials.
4.1 Scaffolds
Recently, approaches including electrostatic spinning, solvent casting, particle leaching, gas foaming and freeze drying, stereolithography, and 3D bioprinting were widely utilized to create artificial scaffolding (Wu et al., 2022). MOFs and other biomaterials were well integrated with the above mentioned artificial scaffolding to develop novel hybrid scaffolds for the OA therapy. As expected, these well-designed multifunctional platforms exhibited excellent anti-inflammatory and antioxidant activity, which could result in the ideal cartilage repairing. For example, poly(lactide-co-glycolide) (PLGA) and dimethyloxallyl glycine (DMOG) loaded iron-based MIL-88 were utilized to prepare novel nanofibrous scaffolds via electrostatic spinning technology, which could serve as artificial extracellular matrix for the promotion of osteogenesis and angiogenesis (Wu et al., 2022). Figure 6A illustrated the synthesis approach of hybrid scaffold and their mechanism in the OA treatment. Compared with the scaffold without any modification, the electrostatically spun mats became rougher upon the addition of MOFs, which thus made the gradual breakdown and absorption of hybrid scaffolds more easily and allowed for the ideal release of iron ions within the injury sites (Xu C. et al., 2023). Furthermore, another novel bioceramic scaffolds with good mechanical characteristics and bioactivity were involved into the field of bone tissue engineering. As well known, β-tricalcium phosphate (β-TCP) was widely utilized for the bone regeneration because it could gradually degrade into calcium and phosphate ions upon the acid microenvironment. Figure 6B illustrated that the addition of Zn/Co-MOF into β-TCP could result in the formation of a novel hybrid scaffold, which could reduce localized inflammation and repair osteochondral tissue through the elimination of ROS and release of ions (Shu et al., 2023).
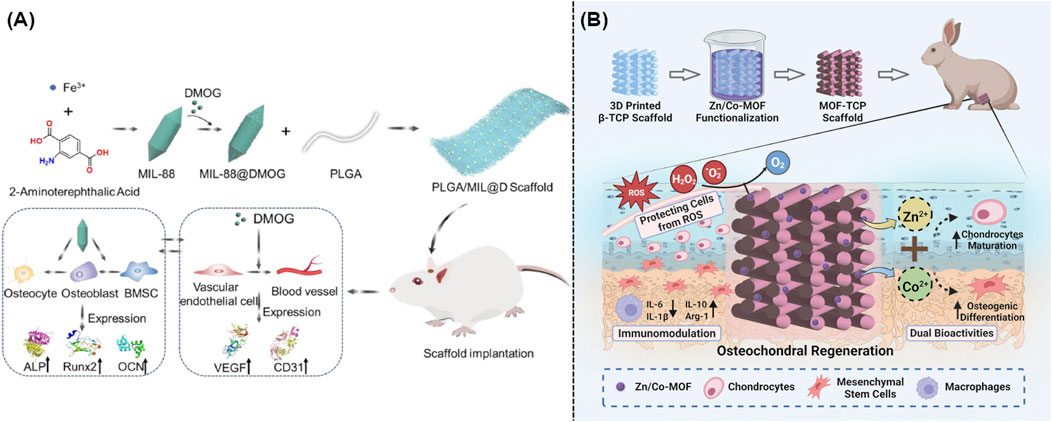
Figure 6. (A) Preparation and mechanism of the iron-based MOF-contained hybrid scaffolds for the repair of bone defects (Xu C. et al., 2023). (B) Synthesis and mechanism of the MOF-TCP-based scaffold for the treatment of osteoarthritis (Shu et al., 2023).
Electrospun fibrous membrane was a novel bionic flexible scaffold that could be utilized to repair tissue injuries, particularly gradient tissue damage that occurred at the interfaces of tendons and bones. Recently, a metal-organic framework-based bipolar metal flexible electrospun fibrous membrane was prepared as a new kind of biomimetic flexible scaffold with multiple layers using electrostatic spinning technology. If the fibers degraded, metal ions such as Zn2+ and Cu2+ might be released, which could regulate collagen formation, osteoclast differentiation, and angiogenesis (Yang R. et al., 2022). In addition, researchers also created an asymmetric bilayer PCL/Col membrane that was changed in the same way by ZIF-8 crystals. The composite membrane made of PCL/Col/ZIF-8 included two layers including a barrier layer and a crystal layer. Several admirable properties including increased tensile strength, low pH responsiveness, and controlled degradation rate endowed these well-developed composites with great performance in the bone regeneration both in vitro and in vivo (Xue Y. et al., 2021).
4.2 Hydrogels
It was well known that hydrogel held a striking structural similarity to the ECM of bone and cartilage, which could gradually build up a three-dimensional structure, well integrate with the surrounding tissues, and provide essential support and protection during the tissue repairing. Recent studies indicated that hydrogels held the ability to encapsulate medicines or growth factors that aided in the cartilage healing. As expected, Figure 7A illustrated the creation of CuTA@SF using MOFs, which could speed up the cell proliferation and extremely enhance the tissue regeneration (Cao et al., 2023). Meanwhile, IA-ZIF-8@HMs was effectively prepared using a one-step microfluidic approach. During the treatment of OA, IA-ZIF-8 could be released from hydrogel microspheres (HMs), further decreasing the localized inflammation (Yu et al., 2023). Moreover, researchers designed and created a hybrid hydrogel (G-GH/CL-CD-MOF) by mixing gelatin-glucosamine hydrochloride with cross-linked cyclodextrin MOF. The optimum porosity structure of this drug delivery system endowed it with a long-term, sustained release of ibuprofen and excellent anti-inflammation treatment of OA (Yang H. et al., 2022). On the other hand, researchers also mixed polycaprolactone and gelatin mats with different amounts of glucosamine loaded ZIF-8 nanoparticles with the ideal goal to achieve the growth of cartilage tissue (Ranjbar et al., 2023). As shown in Figure 7B, novel NBIF@ZIF-8/PHG hydrogels with robust mechanical characteristics and the ability to recover from deformation were well created as efficient alternatives in the cartilage repairing (Jiang et al., 2023). In addition, the tendon-targeting peptide and cartilage-targeting peptide were well loaded in ZIF-8 and Mg-MOF, respectively. Subsequently, these peptide-loaded MOFs were immobilized in methacrylate gelatin using UV-induced photopolymerization to produce LZIF-8/WMg-MOF@GEL, which could achieve a tailored ion delivery platform to both tendon and cartilage (Ma et al., 2024).
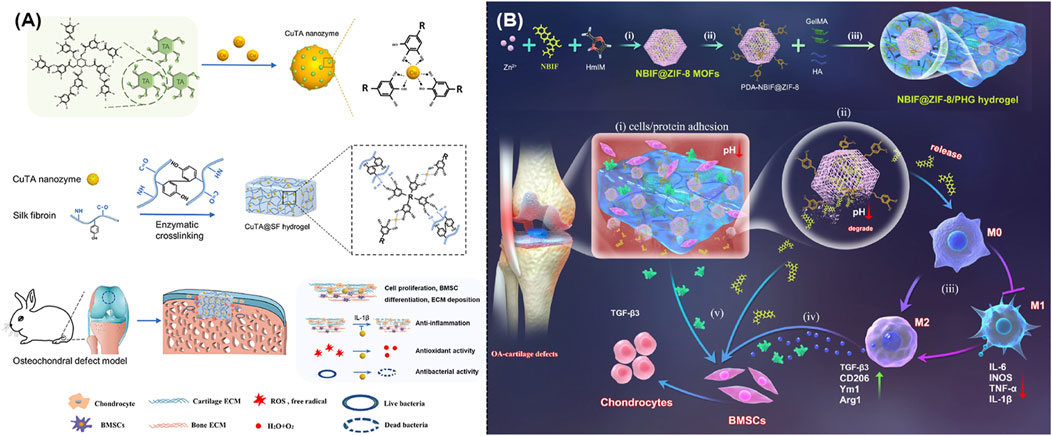
Figure 7. (A) Schematic illustration of the synthesis of CuTA nanozymes and CuTA@SF hydrogels, as well as their application in the repair of bone defects (Cao et al., 2023). (B) Schematic illustration of the design and usage of a MOF-based hydrogel to promote OA-induced cartilage defect repair (Jiang et al., 2023).
5 Conclusion and perspective
With the rapid development of medicine and material science, the use of smart MOFs and their derivatives as emerging biomaterials for the OA treatment has been one of the most important topic of biomedical engineering. In this review, we summarized the latest studies focused on the use of MOFs and their derivatives as ideal carriers for drugs and biomolecules or main composites of hybrid scaffolds and hydrogels in the treatment of OA. The greatest advantage of these well-designed MOFs and their derivatives for OA therapy were concluded as that they could significantly overcome some limitations of drugs and biomolecules such as poor solubility, short retention time, poor targeting capacity, and ease of biodegradability. Moreover, the essential role of MOFs and their derivatives in tissue engineering was well understood after that they were integrated with scaffolds and hydrogels to construct hybrid platforms. Although these MOFs held promising clinical potentials, their further clarification was still necessary on some certain issues. First, there was still a lack of knowledge regarding the drug delivery and release dynamics in MOFs. Second, the in vivo stability, organ distribution and metabolites of MOFs remain unknown, limiting further development and utilization. Third, how to promote tissue regeneration rather than just control inflammation, and the function of MOFs in this process need to be further explored. Accordingly, this review not only emphasizes the considerable potentials of MOFs and their derivatives in the OA treatment, but also anticipated an increase in the number of relevant studies applying MOFs to the treatment of OA in the future. Last but not least, we believed that it would be a transformative clinical achievement by using MOFs as efficient biomaterials to promote the treatment of OA and its related diseases.
Author contributions
YL: Writing–original draft, Writing–review and editing. HZ: Writing–review and editing. TC: Writing–review and editing. CX: Writing–review and editing. XB: Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support was provided by the Jilin Province Science and Technology Development Plan Project (JCSZ2023481-6), and the Bethune Plan of Jilin University (2024B15).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcaide-Ruggiero, L., Cugat, R., and Domínguez, J. M. (2023). Proteoglycans in articular cartilage and their contribution to chondral injury and repair mechanisms. Int. J. Mol. Sci. 24 (13), 10824. doi:10.3390/ijms241310824
An, H., Zhang, M., Gu, Z., Jiao, X., Ma, Y., Huang, Z., et al. (2024). Advances in polysaccharides for cartilage tissue engineering repair: a review. Biomacromolecules 25 (4), 2243–2260. doi:10.1021/acs.biomac.3c01424
Cai, Z., Qian, J., Zhao, Y., and Cheng, Y. (2020). Microporous MOF with open metal sites for CO2 fixation and protective effect on osteoarthritis by regulating the activation of PI3K/AKT pathway. J. Solid State Chem. 283, 121169. doi:10.1016/j.jssc.2019.121169
Cao, Z., Wang, H., Chen, J., Zhang, Y., Mo, Q., Zhang, P., et al. (2023). Silk-based hydrogel incorporated with metal-organic framework nanozymes for enhanced osteochondral regeneration. Bioact. Mater. 20, 221–242. doi:10.1016/j.bioactmat.2022.05.025
Chamundeeswari, M., Jeslin, J., and Verma, M. L. (2019). Nanocarriers for drug delivery applications. Environ. Chem. Lett. 17 (2), 849–865. doi:10.1007/s10311-018-00841-1
DeJulius, C. R., Walton, B. L., Colazo, J. M., d’Arcy, R., Francini, N., Brunger, J. M., et al. (2024). Engineering approaches for RNA-based and cell-based osteoarthritis therapies. Nat. Rev. Rheumatol. 20 (2), 81–100. doi:10.1038/s41584-023-01067-4
Gao, H., Pan, Q., Dong, W., and Yao, Y. (2022). Progress in osteochondral regeneration with engineering strategies. Ann. Biomed. Eng. 50 (10), 1232–1242. doi:10.1007/s10439-022-03060-6
Gu, Z., Wang, J., Fu, Y., Pan, H., He, H., Gan, Q., et al. (2023). Smart biomaterials for articular cartilage repair and regeneration. Adv. Funct. Mater. 33 (10), 2212561. doi:10.1002/adfm.202212561
Guo, L., Zhong, S., Liu, P., Guo, M., Ding, J., and Zhou, W. (2022). Radicals scavenging MOFs enabling targeting delivery of siRNA for rheumatoid arthritis therapy. Small 18 (27), 2202604. doi:10.1002/smll.202202604
He, Y., Li, D., Wu, L., Yin, X., Zhang, X., Patterson, L. H., et al. (2023). Metal-organic frameworks for gene therapy and detection. Adv. Funct. Mater. 33 (12), 2212277. doi:10.1002/adfm.202212277
Horcajada, P., Serre, C., Maurin, G., Ramsahye, N. A., Balas, F., Vallet-Regí, M., et al. (2008). Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 130 (21), 6774–6780. doi:10.1021/ja710973k
Hu, H., Huang, X., Dai, Y., Zhu, K., Ye, X., Meng, S., et al. (2023). Organic metal matrix Mil-88a nano-enzyme for joint repair in the osteoarthritis mouse model. Front. Bioeng. Biotechnol. 11, 1164942. doi:10.3389/fbioe.2023.1164942
Jiang, Y., Liao, H., Yan, L., Jiang, S., Zheng, Y., Zhang, X., et al. (2023). A metal-organic framework-incorporated hydrogel for delivery of immunomodulatory neobavaisoflavone to promote cartilage regeneration in osteoarthritis. ACS Appl. Mater. and Interfaces 15 (40), 46598–46612. doi:10.1021/acsami.3c06706
Li, D., Yadav, A., Zhou, H., Roy, K., Thanasekaran, P., and Lee, C. (2024). Advances and applications of metal-organic frameworks (MOFs) in emerging technologies: a comprehensive review. Glob. Challenges 8 (2), 2300244. doi:10.1002/gch2.202300244
Li, L., Zhu, Y., Qi, Z., Li, X., Pan, H., Liu, B., et al. (2023a). GCMC simulations to study the potential of MOFs as drug delivery vehicles. Appl. Organomet. Chem. 37 (9), e7199. doi:10.1002/aoc.7199
Li, X., Shu, X., Shi, Y., Li, H., and Pei, X. (2023b). MOFs and bone: application of MOFs in bone tissue engineering and bone diseases. Chin. Chem. Lett. 34 (7), 107986. doi:10.1016/j.cclet.2022.107986
Li, Z., Li, Z., Li, S., Wang, K., Ma, F., and Tang, B. (2020a). Potential application development of Sr/HCOOH metal organic framework in osteoarthritis. Microporous Mesoporous Mater. 294, 109835. doi:10.1016/j.micromeso.2019.109835
Li, Z., Peng, Y., Pang, X., and Tang, B. (2020b). Potential therapeutic effects of Mg/HCOOH metal organic framework on relieving osteoarthritis. ChemMedChem 15 (1), 13–16. doi:10.1002/cmdc.201900546
Li, Z., Peng, Y., Xia, X., Cao, Z., Deng, Y., and Tang, B. (2019a). Sr/PTA metal organic framework as a drug delivery system for osteoarthritis treatment. Sci. Rep. 9 (1), 17570. doi:10.1038/s41598-019-54147-5
Li, Z., Zhao, S., Wang, H., Peng, Y., Tan, Z., and Tang, B. (2019b). Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surfaces B Biointerfaces 178, 1–7. doi:10.1016/j.colsurfb.2019.02.027
Liu, Y., Shah, K. M., and Luo, J. (2021). Strategies for articular cartilage repair and regeneration. Front. Bioeng. Biotechnol. 9, 770655. doi:10.3389/fbioe.2021.770655
Lopez-Cantu, D. O., González-González, R. B., Sharma, A., Bilal, M., Parra-Saldívar, R., and Iqbal, H. M. N. (2022). Bioactive material-based nanozymes with multifunctional attributes for biomedicine: expanding antioxidant therapeutics for neuroprotection, cancer, and anti-inflammatory pathologies. Coord. Chem. Rev. 469, 214685. doi:10.1016/j.ccr.2022.214685
Luo, S., Shang, Y., Qin, Z., Zhou, B., Lu, C., Qu, Y., et al. (2024). A novel cartilage-targeting MOF-HMME-RGD sonosensitizer combined with sonodynamic therapy to enhance chondrogenesis and cartilage regeneration. Front. Bioeng. Biotechnol. 12, 1339530. doi:10.3389/fbioe.2024.1339530
Lv, Z., Wang, Z., Chen, D., and Shi, D. (2024). Advances in osteoarthritis research: from diagnosis, treatment to mechanism studies. J. Orthop. Transl. 44, A4–A6. doi:10.1016/j.jot.2024.01.006
Ma, J., Yu, H., Zhang, X., Xu, Z., Hu, H., Liu, J., et al. (2024). Dual-targeted metal ion network hydrogel scaffold for promoting the integrated repair of tendon-bone interfaces. ACS Appl. Mater. and Interfaces 16 (5), 5582–5597. doi:10.1021/acsami.3c16544
Motta, F., Barone, E., Sica, A., and Selmi, C. (2023). Inflammaging and osteoarthritis. Clin. Rev. Allergy and Immunol. 64 (2), 222–238. doi:10.1007/s12016-022-08941-1
Pan, H., Miao, X., Deng, J., Pan, C., Cheng, X., and Wang, X. (2023). Bimetallic metal-organic framework for mitigating aseptic osteolysis. ACS Appl. Mater. and Interfaces 15 (4), 4935–4946. doi:10.1021/acsami.2c19449
Patil, P., Nene, S., Shah, S., Singh, S. B., and Srivastava, S. (2023). Exploration of novel drug delivery systems in topical management of osteoarthritis. Drug Deliv. Transl. Res. 13 (2), 531–546. doi:10.1007/s13346-022-01229-z
Ranjbar, N., Kolahdoozan, M., and Ebadi-Dehaghani, H. (2023). Electrospun polycaprolactone/gelatin mat incorporated with glucosamine-loaded zeolite imidazolate framework-8 nanoparticles for cartilage tissue engineering. J. Polym. Res. 31 (1), 9. doi:10.1007/s10965-023-03847-8
Richard, M. J., Driban, J. B., and McAlindon, T. E. (2023). Pharmaceutical treatment of osteoarthritis. Osteoarthr. Cartil. 31 (4), 458–466. doi:10.1016/j.joca.2022.11.005
Sattar, R., Shahzad, F., Ishaq, T., Mukhtar, R., and Naz, A. (2022). Nano-drug carriers: a potential approach towards drug delivery methods. ChemistrySelect 7 (22), e202200884. doi:10.1002/slct.202200884
Shu, C., Qin, C., Chen, L., Wang, Y., Shi, Z., Yu, J., et al. (2023). Metal-organic framework functionalized bioceramic scaffolds with antioxidative activity for enhanced osteochondral regeneration. Adv. Sci. 10 (13), 2206875. doi:10.1002/advs.202206875
Shyngys, M., Ren, J., Liang, X., Miao, J., Blocki, A., and Beyer, S. (2021). Metal-organic framework (MOF)-based biomaterials for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 9, 603608. doi:10.3389/fbioe.2021.603608
Silva, I., Carvalho, M., Oliveira, C., Profirio, D., Ferreira, R., Corbi, P., et al. (2016). Enhanced performance of a metal-organic framework analogue to MIL-101(Cr) containing amine groups for ibuprofen and nimesulide controlled release. Inorg. Chem. Commun. 70, 47–50. doi:10.1016/j.inoche.2016.05.020
Sun, K., Li, L., Yu, X., Liu, L., Meng, Q., Wang, F., et al. (2017). Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 486, 128–135. doi:10.1016/j.jcis.2016.09.068
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr. Cartil. 28 (4), 400–409. doi:10.1016/j.joca.2020.02.027
Tong, P.-H., Zhu, L., Zang, Y., Li, J., He, X.-P., and James, T. D. (2021). Metal-organic frameworks (MOFs) as host materials for the enhanced delivery of biomacromolecular therapeutics. Chem. Commun. 57 (91), 12098–12110. doi:10.1039/D1CC05157A
Velásquez-Hernández, M. d. J., Astria, E., Winkler, S., Liang, W., Wiltsche, H., Poddar, A., et al. (2020). Modulation of metal-azolate frameworks for the tunable release of encapsulated glycosaminoglycans. Chem. Sci. 11 (39), 10835–10843. doi:10.1039/D0SC01204A
Wu, J., Qin, Z., Jiang, X., Fang, D., Lu, Z., Zheng, L., et al. (2022). ROS-responsive PPGF nanofiber membrane as a drug delivery system for long-term drug release in attenuation of osteoarthritis. NPG Regen. Med. 7 (1), 66. doi:10.1038/s41536-022-00254-3
Xu, C., Kang, Y., Guan, S., Dong, X., Jiang, D., and Qi, M. (2023a). Iron-based metal-organic framework as a dual cooperative release system for enhanced vascularization and bone regeneration. Chin. Chem. Lett. 34 (5), 107825. doi:10.1016/j.cclet.2022.107825
Xu, S., Lin, Y., Zhao, X., Liang, Z., Hu, Y., Chen, Z., et al. (2023b). NIR triggered photocatalytic and photothermal bifunctional MOF nanozyme using for improving osteoarthritis microenvironment by repairing injured chondrocytes of mitochondria. Chem. Eng. J. 468, 143826. doi:10.1016/j.cej.2023.143826
Xue, S., Zhou, X., Sang, W., Wang, C., Lu, H., Xu, Y., et al. (2021a). Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 6 (8), 2372–2389. doi:10.1016/j.bioactmat.2021.01.017
Xue, Y., Zhu, Z., Zhang, X., Chen, J., Yang, X., Gao, X., et al. (2021b). Accelerated bone regeneration by MOF modified multifunctional membranes through enhancement of osteogenic and angiogenic performance. Adv. Healthc. Mater. 10 (6), 2001369. doi:10.1002/adhm.202001369
Yan, B., Tan, J., Zhang, H., Liu, L., Chen, L., Qiao, Y., et al. (2022). Constructing fluorine-doped Zr-MOF films on titanium for antibacteria, anti-inflammation, and osteogenesis. Biomater. Adv. 134, 112699. doi:10.1016/j.msec.2022.112699
Yang, B., Yao, H., Yang, J., Chen, C., and Shi, J. (2022a). Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment. Nat. Commun. 13 (1), 1988. doi:10.1038/s41467-022-29735-1
Yang, H., Hu, Y., Kang, M., Ding, H., Gong, Y., Yin, X., et al. (2022b). Gelatin-glucosamine hydrochloride/crosslinked-cyclodextrin metal-organic frameworks@IBU composite hydrogel long-term sustained drug delivery system for osteoarthritis treatment. Biomed. Mater. 17 (3), 035003. doi:10.1088/1748-605X/ac61fa
Yang, K., Ni, M., Xu, C., Wang, L., Han, L., Lv, S., et al. (2023). Microfluidic one-step synthesis of a metal-organic framework for osteoarthritis therapeutic micrornas delivery. Front. Bioeng. Biotechnol. 11, 1239364. doi:10.3389/fbioe.2023.1239364
Yang, R., Zheng, Y., Zhang, Y., Li, G., Xu, Y., Zhang, Y., et al. (2022c). Bipolar metal flexible electrospun fibrous membrane based on metal-organic framework for gradient healing of tendon-to-bone interface regeneration. Adv. Healthc. Mater. 11 (12), 2200072. doi:10.1002/adhm.202200072
Yu, B., Sun, W., Lin, J., Fan, C., Wang, C., Zhang, Z., et al. (2024). Using Cu-based metal-organic framework as a comprehensive and powerful antioxidant nanozyme for efficient osteoarthritis treatment. Adv. Sci. 11 (13), 2307798. doi:10.1002/advs.202307798
Yu, H., Ren, P., Pan, X., Zhang, X., Ma, J., Chen, J., et al. (2023). Intracellular delivery of itaconate by metal-organic framework-anchored hydrogel microspheres for osteoarthritis therapy. Pharmaceutics 15 (3), 724. doi:10.3390/pharmaceutics15030724
Zhang, Z., Hou, Y., Chen, M., Yu, X., Chen, S., Yue, Y., et al. (2023a). A pH-responsive metal-organic framework for the co-delivery of HIF-2α siRNA and curcumin for enhanced therapy of osteoarthritis. J. Nanobiotechnology 21, 18. doi:10.1186/s12951-022-01758-2
Zhang, Z., Yuan, L., Liu, Y., Wang, R., Zhang, Y., Yang, Y., et al. (2023). Integrated cascade nanozyme remodels chondrocyte inflammatory microenvironment in temporomandibular joint osteoarthritis via inhibiting ROS-NF-κB and MAPK pathways. Adv. Healthc. Mater. 12 (10), 2203195. doi:10.1002/adhm.202203195
Keywords: metal-organic frameworks, osteoarthritis, biomaterials, scaffold, bone tissue engineering
Citation: Liu Y, Zhang H, Chen T, Xu C and Bao X (2024) Metal-organic frameworks (MOFs) and their derivatives as emerging biomaterials for the treatment of osteoarthritis. Front. Pharmacol. 15:1462368. doi: 10.3389/fphar.2024.1462368
Received: 10 July 2024; Accepted: 09 September 2024;
Published: 18 September 2024.
Edited by:
Wen Liao, Sichuan University, ChinaReviewed by:
Xudong Li, Sichuan University, ChinaHegang Li, Southern Medical University, China
Xu Chen, Nanjing Medical University, China
Copyright © 2024 Liu, Zhang, Chen, Xu and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingfu Bao, YmFveGZAamx1LmVkdS5jbg==
 Yufu Liu
Yufu Liu Hongwei Zhang
Hongwei Zhang Xingfu Bao
Xingfu Bao