- 1Department of Pharmacy, Shanxi Cardiovascular Disease Hospital, Taiyuan, Shanxi, China
- 2Department of Pharmacy, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Pharmacy, Shanxi Traditional Chinese Medical Hospital, Taiyuan, Shanxi, China
Objective: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have shown notable advancements in managing blood sugar control. Nevertheless, there remains a gap in real-world data regarding the variation in acute pancreatitis (AP) risk among different GLP-1 RAs. Our study aimed to characterize and evaluate AP associated with different GLP-1 RAs (exenatide, lixisenatide, liraglutide, albiglutide, semaglutide, dulaglutide and tirzepatide) in a public adverse events database and to review the relevant case reports.
Methods: We described a case series of patients experiencing AP while on GLP-1 RAs. Additionally, we utilized various algorithms including reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS) to analyze data from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) regarding suspected adverse events of AP linked to GLP-1 RAs from January 2005 to September 2023.
Results: Our case series comprised thirty-nine patients who experienced AP events while on GLP-1 RAs. Within the FAERS database, we retrieved a total of 6,751 individual case safety reports (ICSRs) involving various GLP-1 RAs. The median age of the patients included in our study was 57 years (range: 14–99), with 98.3% of cases classified as serious. Signals indicating AP were observed across all GLP-1 RAs, with particular emphasis on exenatide and liraglutide.
Conclusion: There is a notable reporting signal of AP associated with all GLP-1 RAs. Healthcare providers must remain vigilant and closely monitor this potentially life-threatening adverse event.
1 Introduction
Globally, over 95% of diabetes cases are attributed to type 2 diabetes mellitus (T2DM), with subsequent cardiovascular complications emerging as the primary drivers of morbidity and mortality. As a novel antidiabetic agent, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are seeing an increasing application in the management of patients with T2DM, given their remarkable efficacy in regulating blood sugar levels without posing an elevated risk of hypoglycemic episodes or weight gain (Drucker and Nauck, 2006; Nauck, 2016). Moreover, promising outcomes from various large-scale cardiovascular outcome trials (CVOTs) have indicated that GLP-1RAs could mitigate the risk of major adverse cardiovascular events (MACE) in T2DM patients with an elevated cardiovascular risk profile (Marso et al., 2016a; Marso et al., 2016b; Hernandez et al., 2018; Pfeffer et al., 2015; Holman et al., 2017; Husain et al., 2019; Gerstein et al., 2019). Due to these favorable attributes, GLP-1RAs have garnered endorsement from authoritative guidelines (Marx et al., 2023; 2024) as a significant therapeutic option for individuals with T2DM, especially those with preexisting atherosclerotic cardiovascular diseases or at a heightened cardiovascular risk.
However, safety concerns have persisted for years regarding the pancreatic effects of GLP-1 RAs. Based on observational data, a 2011 report highlighted an increased risk of pancreatitis and pancreatic cancer in patients using incretin therapy (Elashoff et al., 2011), prompting a warning from the Food and Drug Administration (FDA) regarding the pancreatic safety of GLP-1 RAs (Administration, 2013). A review of case reports (Franks et al., 2012) further heightened concerns about the potential adverse effects of GLP-1RAs on the pancreas, resulting in elevated pancreatic enzymes and AP. A meta-analysis of large randomized controlled trials examining the association between incretin-based therapies and AP revealed an 82% (95% CI, 1.17–2.82) higher likelihood of developing AP when using these drugs compared to conventional therapy (Roshanov and Dennis, 2015). While several recently published meta-analyses of CVOTs have shown that no such association was observed between GLP-1RAs and pancreatitis (Singh et al., 2020; Cao et al., 2020). Nevertheless, significant shortcomings existed in such studies, including relatively short mean follow-up times (of less than 2 years in the RCTs), selected patient cohorts, and limited sample sizes.
In this study, we conducted a review of published literature and an analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS) data to investigate the incidence of AP undergoing GLP-1 RAs. Our aim was to provide a comprehensive clinical depiction of AP induced by GLP-1 RAs and ascertain the presence of a safety signal between AP and GLP-1 RAs in real-world settings.
2 Methods
2.1 Case series
We conducted a comprehensive literature search using Google Scholar, Scopus, PubMed, and Web of Science, focusing on English-language publications up to 31 December 2023. The following search terms were used: (exenatide OR liraglutide OR albiglutide OR dulaglutide OR lixisenatide OR semaglutide OR tirzepatide OR GLP-1 RAs OR Glucagon-like peptide-1 receptor agonist) AND (acute pancreatitis) AND (case report OR case series). The eligibility criteria included any case report or case series that documented instances of AP during the administration of GLP-1 RAs. Patient demographics such as age and gender, along with dosage, treatment duration, presenting symptoms, imaging results, causality assessment (using Naranjo scale) (Naranjo et al., 1981), acute pancreatitis management, and outcomes were extracted from the examination of medical files.
2.2 Phamacovigilance analysis
This retrospective pharmacovigilance analysis is based on real-world data sourced from individual case safety reports (ICSRs) submitted to the FAERS. FAERS compiles information on adverse events, medication errors, and product quality complaints leading to adverse events. It serves as a cornerstone of the FDA’s post-marketing safety surveillance initiative for pharmaceuticals and therapeutic agents, operating as a classic spontaneous reporting system. The database captures a wide array of data including demographics, drug details, indications, outcomes, adverse reactions, sources, and therapies. Data submitting to ICSRs with GLP-1 RAs as suspected drugs were extracted from the FAERS database spanning the period between January 2005 and September 2023. Utilizing the Medical Dictionary for Regulatory Activities (MedDRA) version 26.0, we identified 25 preferred terms (PTs) (Supplementary Table S1) to gather pertinent cases linked to “acute pancreatitis” (Standardized MedDRA Queries (SMQ): 20000022) and closely related clinical conditions. To ensure data integrity, we conducted a thorough review to eliminate potential duplicates, defined as records sharing at least three out of four key fields: event date, age, sex, and reporter’s country. Additionally, incorrect data were excluded, such as cases where the GLP-1 RA initiation date was later than the onset date of pancreatitis.
2.3 Statistical analysis
The clinical profile, such as age, sex, primary data source, outcomes, reported year, source region, and indication, were detailed individually for each GLP-1 RAs. Disproportionality analysis and Bayesian analysis were employed, utilizing the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS) algorithms to identify associations between different GLP-1 RAs and AP events. The equations and criteria for these algorithms (Chen et al., 2020) are detailed in Supplementary Table S2. If any of the four algorithms met the predefined criteria, a positive signal of AP was identified. Analyses were performed using SPSS 23.0 (IBM, Armonk, NY, United States) statistical software.
3 Results
3.1 Case series
During the study period, thirty-nine patients experienced new-onset AP while using GLP-1 RAs (Table 1). More specifically, among these cases, 19 (48.7%) were associated with liraglutide, 9 (23.1%) with dulaglutide, 4 (10.3%) each with exenatide and semaglutide, while 1 (2.6%) case each was linked to lixisenatide, albiglutide, and tirzepatide. The median age at the onset of AP was 60 years (range: 27–77 years), with 20 (51.8%) being male. All patients in the study were identified as having either type T2DM or obesity, with the exception of one patient who had been diagnosed with prediabetes. Notably, 10 cases (25.6%) involved an escalation in drug dosage within 3 months preceding the event. The median time to onset was 2.5 months (range 0 days–3 years). The predominant presenting symptom was epigastric abdominal pain accompanied by nausea and vomiting. Most cases exhibited evidence of pancreatitis on CT scans. Using the Naranjo scale, 33 cases (84.6%) were deemed to have a probable causal relationship between GLP-1 RAs and AP, while 4 cases (10.3%) were classified as possible. None of the patients from the case series was rechallenged with GLP-1 RAs due to safety concerns. Two cases involved cholelithiasis, and two patients received treatment with either empagliflozin or sitagliptin, which could potentially contribute to or confound pancreatitis. The standard management approach for these patients involved discontinuation of GLP-1 RAs and supportive care, including intravenous fluids and pain management. The majority of patients recovered without complications following this treatment regimen, except for one patient who experienced a fatal outcome.

Table 1. Summary of case reports of GLP-1 receptor agonists-induced acute pancreatitis reported in the literature.
3.2 Descriptive analysis from FAERS
In total, the FAERS database archived 6,751 reports related to acute pancreatitis induced by GLP-1 RAs from January 2005 to September 2023. Specifically, 2,539 ICSRs (37.6%) were associated with exenatide, 1981 (29.3%) with liraglutide, and 1,352 (20.0%) with dulaglutide. The demographic and clinical characteristics of all ICSRs are outlined in Table 2. The median age of patients across all ICSRs was 57 years (range: 14–99, n = 2,815), similar to that of each specific GLP-1 RA. Female patients accounted for the highest proportion of ICSRs (45.8%), and 6,634 (98.3%) cases were classified as serious. The majority of reports (63.4%) were submitted by healthcare professionals and originated from North America (87.8%). In terms of outcomes, other adverse events (51.5%) were the most prevalent, followed by hospitalization (40.4%), life-threatening (2.8%) and death (2.7%). AP events were predominantly reported for unknown indications (53.8%) and T2DM (43.0%). The events manifested soon after the initiation of GLP-1 RA treatment, with a median onset time of 92 days (range: 0–3,312, n = 1,591) across all ICSRs that provided both drug initiation and AP onset times. Notably, 30.9% of these reports were gathered within the initial month, and almost half (48.5%) were compiled within the first 3 months after eliminating invalid reports. The number of acute pancreatitis adverse events steadily increased from 16 in 2005 to 459 in 2023 (Q1-Q3), peaking in 2011, reflecting the growing clinical utilization of GLP-1 RAs (Figure 1).
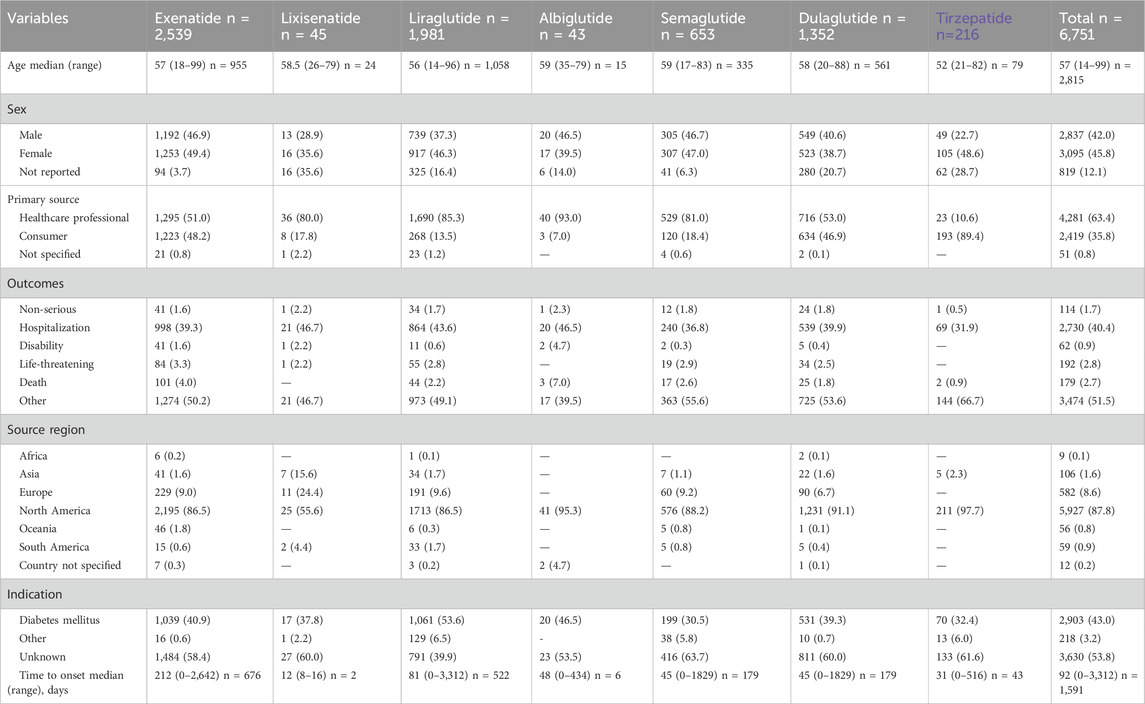
Table 2. Clinical characteristics of patients with GLP-1 receptor agonists-associated acute pancreatitis collected from the FAERS database (January 2005 to September 2023).

Figure 1. Distribution of Individual Safety Reports having GLP-1 RAs as suspect drugs by year (2005–2023 (Q1-Q3))
3.3 Signal values associated with different GLP-1 RAs
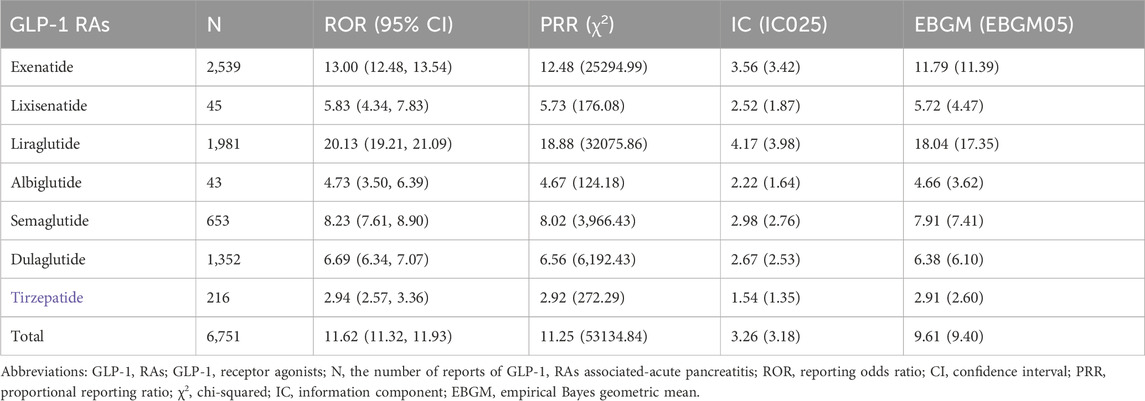
We identified signals of AP events associated with all GLP-1 RAs using the criteria established by the four algorithms, and the results are summarized in Table 3. Each GLP-1 RA satisfied all four criteria, as did the overall group of GLP-1 RAs. Notably, among all GLP-1 RAs, liraglutide stood out for its association with AP events related to acute pancreatitis. This is highlighted by its notably highest values across various statistical parameters, including an IC at 4.17 (IC025 3.98), an ROR at 20.13 (95% CI 19.21–21.09), and an EBGM at 18.04 (EBGM05 17.35). Following liraglutide, exenatide, semaglutide, dulaglutide, lixisenatide, and albiglutide exhibited progressively lower values, while tirzepatide demonstrated the lowest association.
We further examined adverse events related to AP at the PT level and listed all signal-based ROR criteria in Table 4. As depicted in Table 4, exenatide exhibited the broadest spectrum, with a total of 8 potential signals indicating GLP-1 RA-induced AP, ranging from pancreatic abscess (ROR 4.20, 95% CI 1.03–17.03) to pancreatic phlegmon (ROR 84.53, 95% CI 21.86–326.90). Conversely, lixisenatide, albiglutide, and tirzepatide showed the fewest PTs, with only two signals detected for each drug. Among all ICSRs, cases involving pancreatitis and acute pancreatitis were the most frequently reported PTs for all drugs.
4 Discussion
In conclusion, we found significant over-representation of signals for acute pancreatitis (SMQ: 20000022) over other adverse reactions for all GLP-1 RAs. Though the disproportionality analysis and Bayesian analysis as a rapid and effective method for signal detection, our study represents the largest post-marketing surveillance to date of these GLP-1 RAs. We have provided valuable and timely evidence for clinical evaluation, aiming to mitigate the potential harm associated with acute pancreatitis following treatment with GLP-1 RAs.
Overall, from the first quarter of 2005 to the third quarter of 2023, there were 6,751 reports describing acute pancreatitis associated with GLP-1 RAs in the FAERS database. Both the pharmacovigilance findings and the case series indicated that liraglutide and dulaglutide were the leading suspected GLP-1 RAs, and pharmacovigilance analysis showed that exenatide had the highest number of ICSRs associated with AP. The median age of patients was 57 years (range: 14–99 years) in our pharmacovigilance analysis and 60 years (range: 27–77 years) for the cases of GLP-1 RAs-induced AP published in the case reports, which is in line with earlier observational studies on drug-induced AP (Gagnon et al., 2020; Chadalavada et al., 2020). Our pharmacovigilance results suggest that AP associated with GLP-1 RAs was more frequently reported in females, while the case series results did not show the same trend. However, the validity of this finding cannot be conclusively confirmed, given the multitude of factors that can influence the spontaneous reporting of adverse events. Additionally, the gender of 12.1% of the ICSRs was not reported, which further complicates the analysis. Nevertheless, there is some evidence suggesting that females may experience this condition more frequently (Barreto et al., 2011; Kaufman, 2013). We also observed that the median time to onset of GLP-1 RAs-associated acute pancreatitis was 92 (range: 0–3,312) days across ICSRs that provided both drug initiation and AP onset times, and 2.5 months of the case series, indicating a longer onset duration compared to other gastrointestinal adverse events triggered by GLP-1 RAs (Zhou et al., 2022).
In our study, excluding the initial 3 years since the launch of exenatide, the reported cases have averaged nearly 400 per year since 2015. However, there was a notable surge in cases during 2010 and 2011, with 684 cases reported in 2020 and 893 cases in 2021. This surge may be attributed to the FDA mandating manufacturers of incretin-based medications to revise their product labels in 2009, providing information regarding the potential risk of pancreatitis (Nelson et al., 2014). Approximately 87.8% of the reports were derived from North America, which may be attributed to FAERS being established in the United States. Furthermore, 40.4% of ICSRs involved hospitalized patients, 2.7% resulted in patient mortality, 0.9% led to disability, and 2.8% caused life-threatening reactions, while only 1.7% classified as non-serious outcomes. Additionally, within the case series results, one patient (2.6%) died, while 2.7% of ICSRs from our FAERS analysis had a fatal outcome, underscoring the seriousness of acute pancreatitis and the necessity for specialized attention.
Our study detected a notable signal between different GLP-1 RAs and AP in the FAERS database throughout the study duration. Meanwhile, liraglutide exhibited the strongest association with acute pancreatitis, evidenced by the highest values of IC, ROR, and EBGM. Following liraglutide, exenatide emerged as the second-highest in terms of this association. Despite exenatide showing a higher reported number of reactions compared to liraglutide (2539:1981), the associations with acute pancreatitis events were weaker, which was also observed in cases of pancreatic cancer (Cao et al., 2023). It is suggested that patients at risk of pancreatitis avoid using any GLP-1 RAs, particularly liraglutide and exenatide. And the association of tirzepatide with acute pancreatitis events appears to be the weakest, possibly due to its later launch on the market.
AP ranks as the primary cause of hospital admissions for gastrointestinal disease (Mossad et al., 2017) and the fifth leading cause of in-hospital mortality in the United States (Sorribas et al., 2023). Addressing the underlying causes of pancreatitis is essential to prevent its recurrence. Gallstones and alcohol abuse stand out as the primary triggers for AP, while genetic factors, medications, and smoking also play contributing roles (Lee and Papachristou, 2019). Additionally, T2DM poses a significant risk for AP, particularly among younger diabetic patients (Lankisch et al., 2015). Moreover, worsening glycemic control escalates the likelihood of AP (Cho et al., 2023). Although drugs only account for 0.1%–2% of AP cases, their impact can be life-threatening (Wolfe et al., 2020). Therefore, managing drug-induced AP necessitates discontinuing the causative medication and providing supportive care. The GLP-1RAs should not be restarted if pancreatitis is confirmed (Wharton et al., 2022), and none of the patients from the case series were rechallenged with GLP-1 RAs for safety reasons. Failure to identify the responsible drug can lead to significant delays in treatment, potentially resulting in critical outcomes (Jones et al., 2015). Unraveling a causal relationship between GLP-1 agonists and AP is intricate, particularly as patients with T2DM are already three times more predisposed to pancreatitis compared to their non-diabetic counterparts (Girman et al., 2010). Therefore, it's imperative to examine all plausible factors and to rely on a diagnosis of exclusion when attributing AP to drug-induced causes.
Three out of thirty-nine patients (7.7%) from the case series were diagnosed with obesity. Obesity doesn't just pose a risk for local and systemic complications in acute pancreatitis; it also elevates mortality rates associated with this condition (Martínez et al., 2006). Currently, the FDA has approved three GLP-1 RAs for obesity treatment: liraglutide, semaglutide and tirzepatide. Notably, the dosage for obesity treatment is considerably higher than that for diabetes management. Take semaglutide as an example; the maintenance dose for the treatment of obesity is 2.4 mg subcutaneously once a week, whereas for diabetes, the maximum dose is 1 mg subcutaneously once a week. Whether this elevated dosage could potentially increase the risk of acute pancreatitis in obese patients compared to those with diabetes is a subject that necessitates further investigation.
In this study, we applied four algorithms to analyze the association between GLP-1 RAs and acute pancreatitis. Each method has distinct advantages and limitations. BCPNN and MGPS are Bayesian approaches known for their higher specificity (Bate et al., 1998; DuMouchel, 1999). They are particularly useful when working with sparse data or for pattern recognition in higher dimensions, making them applicable in a variety of scenarios. However, they are less sensitive compared to frequentist methods and can be less transparent to those unfamiliar with Bayesian statistics (Almenoff et al., 2006). On the other hand, PRR and ROR are frequentist approaches that are simpler to apply and interpret (Evans et al., 2001; van Puijenbroek et al., 2002). They have the advantage of higher sensitivity, making them useful for early detection of adverse drug events (Li et al., 2008). However, these methods are less specific and can sometimes produce false positives, particularly in rare drug-event combinations. The consistency of signals across all four methods strengthens our findings and minimizes the influence of biases inherent to any single algorithm. The convergence of these results enhances confidence in the association between GLP-1 RAs and acute pancreatitis, ensuring a comprehensive and reliable evaluation of the data.
Additionally, clinicians should view the statistical associations observed in this study as hypothesis-generating rather than conclusive evidence of a cause-and-effect relationship. The primary metrics used in this study, including the reporting odds ratio (ROR) and Bayesian confidence propagation neural network (BCPNN) indicators, are designed to identify disproportionalities in reporting patterns. These tools help detect potential safety signals but do not account for confounding variables such as baseline patient characteristics, comorbidities, or concomitant medication use. Consequently, the presence of a signal should be interpreted as an indication of potential risk that needs to be further evaluated in the context of robust, well-controlled clinical studies.
Despite the advantages of real-world studies and data mining techniques in this research, there are numerous limitations to consider. Firstly, the spontaneous reporting system is affected by limitations within the FAERS database, including duplicate reports, reporting accuracy and quality, incomplete or insufficient details regarding drug administration (such as site, route, dose and timing), and the lack of important patient characteristics (such as medical history and comorbidities). Secondly, reports from FAERS lack medical confirmation, potentially introducing reporter bias (Nomura et al., 2015). As a result, data mining alone does not provide sufficient evidence to establish causality and primarily emphasizes the need for practitioner vigilance. It is important to note that all signal detection can only suggest a statistical correlation, and further investigation and research are needed to determine if there is a real causal relationship. Lastly, despite individually reviewing ICSRs in our study and considering data on other drugs that could potentially induce adverse reactions, the possibility of notoriety bias cannot be dismissed. Despite these inherent limitations in spontaneous reporting, the FAERS database remains a valuable resource. Data mining remains a critical tool for the ongoing assessment and management of risks associated with commercially available pharmaceutical products.
5 Conclusion
In conclusion, a notable reporting signal for acute pancreatitis exists across all GLP-1 RAs in the FAERS database, particularly associated with exenatide and liraglutide. Clinicians must be vigilant and monitor this potentially serious adverse event. Moreover, we anticipate further pharmacovigilance studies, cohort analyses, and clinical trials in the future to develop evidence-based treatment strategies for patients experiencing GLP-1 RA-induced AP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HG: Data curation, Formal Analysis, Methodology, Software, Writing–original draft, Writing–review and editing. QG: Data curation, Formal Analysis, Software, Writing–review and editing. ZL: Formal Analysis, Software, Writing–review and editing. ZW: Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge the use of OpenAI’s ChatGPT (version: GPT-4, model: gpt-4-turbo) for language editing and refinement of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1461398/full#supplementary-material
References
Abdelmasih, R., Saeed, Z., Ashraf, B., Abdelmaseih, R., and Nasser, H. (2022). PSUN241 A case of dulaglutide-induced acute pancreatitis in a diabetic patient – a case report. J. Endocr. Soc. 6, A388–A389. doi:10.1210/jendso/bvac150.808
Administration, U. F. a.D. (2013). FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. Drug Safe. Avai. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-investigating-reports-possible-increased-risk-pancreatitis-and-pre (Accessed April, 03, 2024).
Almenoff, J. S., Lacroix, K. K., Yuen, N. A., Fram, D., and Dumouchel, W. (2006). Comparative performance of two quantitative safety signalling methods: implications for use in a pharmacovigilance department. Drug Saf. 29, 875–887. doi:10.2165/00002018-200629100-00005
Alsaadoun, A. R., Alsaadoun, T. R., and Al Ghumlas, A. K. (2022). Liraglutide overdose-induced acute pancreatitis. Cureus 14, e21616. doi:10.7759/cureus.21616
Al-Salameh, N., Shah, R. N., and Khara, H. S. (2019). 1388 Dose increment-associated liraglutide-induced acute pancreatitis. Am. J. Gastroenterol. 114, S768. doi:10.14309/01.ajg.0000595080.14595.de
Ayoub, W. A., Kumar, A. A., Naguib, H. S., and Taylor, H. C. (2010). Exenatide-induced acute pancreatitis. Endocr. Pract. 16, 80–83. doi:10.4158/EP09104.CRR
Babajide, O., Nabin, K. C., Solaimanzadeh, I., and Shiferaw-Deribe, Z. (2022). Case report of acute pancreatitis associated with combination treatment of dulaglutide and glipizide. Cureus 14, e20938. doi:10.7759/cureus.20938
Barreto, S. G., Tiong, L., and Williams, R. (2011). Drug-induced acute pancreatitis in a cohort of 328 patients. A single-centre experience from Australia. Jop 12, 581–585.
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. doi:10.1007/s002280050466
Bhat, S. Z., and Goudarzi, A. (2021). Necrotizing pancreatitis secondary to dulaglutide use. J. Endocr. Soc. 5, A393. doi:10.1210/jendso/bvab048.800
Bourezane, H., Kastler, B., and Kantelip, J.-P. (2012). Late and severe acute necrotizing pancreatitis in a patient with liraglutide. Therapie 67, 539–543. doi:10.2515/therapie/2012076
Cao, C., Yang, S., and Zhou, Z. (2020). GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: data from cardiovascular outcome trials. Endocrine 68, 518–525. doi:10.1007/s12020-020-02223-6
Cao, M., Pan, C., Tian, Y., Wang, L., Zhao, Z., and Zhu, B. (2023). Glucagon-like peptide 1 receptor agonists and the potential risk of pancreatic carcinoma: a pharmacovigilance study using the FDA Adverse Event Reporting System and literature visualization analysis. Int. J. Clin. Pharm. 45, 689–697. doi:10.1007/s11096-023-01556-2
Casanovas, G. J., Bartl, M., Blesset, A., Lowery, J., and Parraga, A. S. (2023). Abstract #1402779: a case of possible tirzepatide-induced pancreatitis. Endocr. Pract. 29, S13. doi:10.1016/j.eprac.2023.03.034
Chadalavada, P., Simons-Linares, C. R., and Chahal, P. (2020). Drug-induced acute pancreatitis: prevalence, causative agents, and outcomes. Pancreatology 20, 1281–1286. doi:10.1016/j.pan.2020.07.401
Chen, G., Qin, Y., Fan, Q. Q., Zhao, B., Mei, D., and Li, X. M. (2020). Renal adverse effects following the use of different immune checkpoint inhibitor regimens: a real-world pharmacoepidemiology study of post-marketing surveillance data. Cancer Med. 9, 6576–6585. doi:10.1002/cam4.3198
Cheng, C., Masoud, J. A., Mccray, E. T., Sun, C., and Momodu, I. (2021). Acute pancreatitis and type 2 diabetes mellitus: glp-1 receptor agonist or idiopathic, a diagnostic dilemma, a case report with literature review. Gastroenterol. Nurs. 44, 353–356. doi:10.1097/SGA.0000000000000602
Chis, B. A., and Fodor, D. (2018). Acute pancreatitis during GLP-1 receptor agonist treatment. A case report. Clujul Med. 91, 117–119. doi:10.15386/cjmed-804
Cho, I. R., Han, K. D., Lee, S. H., Choi, Y. H., Chung, K. H., Choi, J. H., et al. (2023). Association between glycemic status and the risk of acute pancreatitis: a nationwide population-based study. Diabetol. Metab. Syndr. 15, 104. doi:10.1186/s13098-023-01086-x
Chua, M. W. J., and Ng, Y. K. (2021). Early onset of acute pancreatitis in a patient on low-dose liraglutide. Diabetes Metab. Syndr. 15, 753–755. doi:10.1016/j.dsx.2021.03.010
Denker, P. S., and Dimarco, P. E. (2006). Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 29, 471. doi:10.2337/diacare.29.02.06.dc05-2043
Dolan, R. D., Bazarbashi, A. N., Lo, A., and Smith, B. N. (2020). Liraglutide-induced hemorrhagic pancreatitis in a nondiabetic patient. ACG Case Rep. J. 7, e00380. doi:10.14309/crj.0000000000000380
Drucker, D. J., and Nauck, M. A. (2006). The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705. doi:10.1016/S0140-6736(06)69705-5
Dumouchel, W. (1999). Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53, 177–190. doi:10.1080/00031305.1999.10474456
Easow, B. M., Mathew, M., Rajagopalan, U., Nagarajan, P., and Abraham, G. (2022). Serious adverse effects following use of liraglutide in individuals with type 2 diabetes. J. Diabetol. 13, 314–316. doi:10.4103/jod.jod_78_22
Ebiai, R., Gore, J., and Kapten, B. S. (2023). S1999 -The dark side of semaglutide discrete instances of acalculous cholecystitis and acute pancreatitis in the same patient due to continued use of GLP-1 agonist. Am. J. Gastroenterol. 118, S1470. doi:10.14309/01.ajg.0000957636.56371.78
Elashoff, M., Matveyenko, A. V., Gier, B., Elashoff, R., and Butler, P. C. (2011). Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141, 150–156. doi:10.1053/j.gastro.2011.02.018
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. doi:10.1002/pds.677
Famularo, G., Gasbarrone, L., and Minisola, G. (2012). Pancreatitis during treatment with liraglutide. J. Pancreas 13, 540–541. doi:10.6092/1590-8577/1107
Farooqui, K., Zaid, H. A., Sharif, M. K., Hadi, A. M. a.A., Farooqui, F., and Hammamy, M. R. A. (2019). Liraglutide induced acute pancreatitis and jaundice in an elderly female – case report. World J. Res. Rev. 9.
Fatakhova, K., Rahman, A., Al-Naqeeb, G., Al Yassin, S., Alqaisi, S., and Ramdass, A. (2019). 1311 Acute pancreatitis in a patient using liraglutide for weight loss. Am. J. Gastroenterol. 114, S728–S729. doi:10.14309/01.ajg.0000594772.87672.64
Fernandez, A. T., Akhtar, K. H., Krishan, S., Anwaar, M. F., and Allee, M. (2021). S1640 Necrotizing pancreatitis associated with liraglutide and empagliflozin. Am. J. Gastroenterol. 116, S736. doi:10.14309/01.ajg.0000780092.28084.14
Franks, A. S., Lee, P. H., and George, C. M. (2012). Pancreatitis: a potential complication of liraglutide? Ann. Pharmacother. 46, 1547–1553. doi:10.1345/aph.1Q789
Gagnon, A. L., Lavoie, A., Frigon, M. P., Michaud-Herbst, A., and Tremblay, K. (2020). A drug-induced acute pancreatitis retrospective study. Can. J. Gastroenterol. Hepatol. 2020, 1516493. doi:10.1155/2020/1516493
Gameil, M. A., and Elsebaie, A. H. (2020). Mildly symptomatic liraglutide-induced acute pancreatitis in a patient with type 2 diabetes mellitus: a case report. Egypt J. Intern Med. 32, 26. doi:10.1186/s43162-020-00026-9
Gerstein, H. C., Colhoun, H. M., Dagenais, G. R., Diaz, R., Lakshmanan, M., Pais, P., et al. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130. doi:10.1016/S0140-6736(19)31149-3
Ghabra, M., and Alkhouli, M. (2018). Liraglutide associated acute pancreatitis: a case report and review of the literature: 1342. Am. J. Gastroenterol. 113, S769. doi:10.14309/00000434-201810001-01342
Girman, C. J., Kou, T. D., Cai, B., Alexander, C. M., O'neill, E. A., Williams-Herman, D. E., et al. (2010). Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes. Metab. 12, 766–771. doi:10.1111/j.1463-1326.2010.01231.x
Hernandez, A. F., Green, J. B., Janmohamed, S., D'agostino, R. B., Granger, C. B., Jones, N. P., et al. (2018). Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529. doi:10.1016/S0140-6736(18)32261-X
Holman, R. R., Bethel, M. A., Mentz, R. J., Thompson, V. P., Lokhnygina, Y., Buse, J. B., et al. (2017). Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239. doi:10.1056/NEJMoa1612917
Husain, M., Birkenfeld, A. L., Donsmark, M., Dungan, K., Eliaschewitz, F. G., Franco, D. R., et al. (2019). Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851. doi:10.1056/NEJMoa1901118
Iyer, S. N., Drake, A. J., West, R. L., Mendez, C. E., and Tanenberg, R. J. (2012). Case report of acute necrotizing pancreatitis associated with combination treatment of sitagliptin and exenatide. Endocr. Pract. 18, e10–e13. doi:10.4158/EP11264.CR
Jain, N., Savani, M., Agarwal, M., and Sands, C. W. (2016). Albiglutide-induced pancreatitis. Ther. Adv. Drug Saf. 7, 236–238. doi:10.1177/2042098616667352
Javed, H., Kogilathota Jagirdhar, G. S., Kashyap, R., and Vekaria, P. H. (2023). Liraglutide-induced pancreatitis: a case report and literature review. Cureus 15, e38263. doi:10.7759/cureus.38263
Jeyaraj, S., Shetty, A. S., Kumar, C. R. R., Nanditha, A., Krishnamoorthy, S., Raghavan, A., et al. (2014). Liraglutide-induced acute pancreatitis. J. Assoc. Physicians India 62, 64–66.
Jones, M. R., Hall, O. M., Kaye, A. M., and Kaye, A. D. (2015). Drug-induced acute pancreatitis: a review. Ochsner J. 15, 45–51.
Kaufman, M. B. (2013). Drug-induced pancreatitis: a potentially serious and underreported problem. P t 38, 349–351.
Khan, A. B., Shah, A., Ahmad, S., Khan, M. I., and Amir, A. (2023). Dulaglutide (trulicity)-induced acute pancreatitis: a case report. Cureus 15, e38630. doi:10.7759/cureus.38630
Knezevich, E., Crnic, T., Kershaw, S., and Drincic, A. (2012). Liraglutide-associated acute pancreatitis. Am. J. Health Syst. Pharm. 69, 386–389. doi:10.2146/ajhp110221
Kumar Kulkarni, A. U., Mehta, V., Renzu, M., Pereira, K., and A, R. M. (2023). FRI657 Case series on GLP-1 receptor agonist related acute pancreatitis. J. Endocr. Soc. 7. bvad114.875. doi:10.1210/jendso/bvad114.875
Lankisch, P. G., Apte, M., and Banks, P. A. (2015). Acute pancreatitis. Lancet 386, 85–96. doi:10.1016/S0140-6736(14)60649-8
Lee, P. H., Stockton, M. D., and Franks, A. S. (2011). Acute pancreatitis associated with liraglutide. Ann. Pharmacother. 45, e22–e541. doi:10.1345/aph.1P714
Lee, P. J., and Papachristou, G. I. (2019). New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 479–496. doi:10.1038/s41575-019-0158-2
Li, C., Xia, J., Deng, J., and Jiang, J. (2008). A comparison of measures of disproportionality for signal detection on adverse drug reaction spontaneous reporting database of Guangdong province in China. Pharmacoepidemiol Drug Saf. 17, 593–600. doi:10.1002/pds.1601
Manuel, S. L., Lin, F., and Kutty, S. M. (2023). An atypical presentation of dulaglutide-induced pancreatitis complicated by superior mesenteric vein thrombosis. Cureus 15, e50051. doi:10.7759/cureus.50051
Marso, S. P., Bain, S. C., Consoli, A., Eliaschewitz, F. G., Jódar, E., Leiter, L. A., et al. (2016a). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844. doi:10.1056/NEJMoa1607141
Marso, S. P., Daniels, G. H., Brown-Frandsen, K., Kristensen, P., Mann, J. F., Nauck, M. A., et al. (2016b). Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322. doi:10.1056/nejmoa1603827
Martínez, J., Johnson, C. D., Sánchez-Payá, J., De Madaria, E., Robles-Díaz, G., and Pérez-Mateo, M. (2006). Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology 6, 206–209. doi:10.1159/000092104
Marx, N., Federici, M., Schütt, K., Müller-Wieland, D., Ajjan, R. A., Antunes, M. J., et al. (2023). 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 44, 4043–4140. doi:10.1093/eurheartj/ehad192
Mossad, D. E., Dinh, B. V., Markert, R. J., Musleh, M. N., and Agrawal, S. (2017). Predictors of in hospital mortality in acute pancreatitis. J. Pancreas 18, 465.
Nakata, H., Sugitani, S., Yamaji, S., Otsu, S., Higashi, Y., Ohtomo, Y., et al. (2012). Pancreatitis with pancreatic tail swelling associated with incretin-based therapies detected radiologically in two cases of diabetic patients with end-stage renal disease. Intern Med. 51, 3045–3049. doi:10.2169/internalmedicine.51.7876
Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30, 239–245. doi:10.1038/clpt.1981.154
Nauck, M. (2016). Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 18, 203–216. doi:10.1111/dom.12591
Nelson, M., Bhandari, N., and Wener, J. (2014). Sitagliptin-induced pancreatitis - a longer road than expected. Clin. Case Rep. 2, 149–152. doi:10.1002/ccr3.83
Nohomovich, B., Shah, A., and Hughes, N. (2023). Severe, complicated pancreatitis with an unclear etiology. Cureus 15, e39011. doi:10.7759/cureus.39011
Nomura, K., Takahashi, K., Hinomura, Y., Kawaguchi, G., Matsushita, Y., Marui, H., et al. (2015). Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des. Devel Ther. 9, 3031–3041. doi:10.2147/DDDT.S81998
Patel, F., A, G., Chang, K., and Vega, K. J. (2023). Acute pancreatitis in a patient taking semaglutide. Cureus 15, e43773. doi:10.7759/cureus.43773
Pfeffer, M. A., Claggett, B., Diaz, R., Dickstein, K., Gerstein, H. C., Køber, L. V., et al. (2015). Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257. doi:10.1056/NEJMoa1509225
Quesada-Vázquez, N. (2018). Scientific report: a case of acute pancreatitis due to liraglutide. Rev. Esp. Enferm. Dig. 111, 329–330. doi:10.17235/reed.2018.5912/2018
Roshanov, P. S., and Dennis, B. B. (2015). Incretin-based therapies are associated with acute pancreatitis: meta-analysis of large randomized controlled trials. Diabetes Res. Clin. Pract. 110, e13–e17. doi:10.1016/j.diabres.2015.10.014
Shahbazi, M., Qudsiya, Z., Fahel, A., Amini, A., and Tanoli, T. (2023). First reported case of dulaglutide-induced acute pancreatitis with normal serum lipase level. Cureus 15, e40576. doi:10.7759/cureus.40576
Singh, A. K., Gangopadhyay, K. K., and Singh, R. (2020). Risk of acute pancreatitis with incretin-based therapy: a systematic review and updated meta-analysis of cardiovascular outcomes trials. Expert Rev. Clin. Pharmacol. 13, 461–468. doi:10.1080/17512433.2020.1736041
Sorribas, M., Carnaval, T., Peláez, N., Secanella, L., Salord, S., Sarret, S., et al. (2023). Home monitoring vs hospitalization for mild acute pancreatitis. A pilot randomized controlled clinical trials. Med. Baltim. 102, e33853. doi:10.1097/MD.0000000000033853
Taunk, R., Abdelmessieh, P., and Kurtz, L. (2012). Liraglutide-induced acute pancreatitis: 744. Am. J. Gastroenterol. 107, S308. doi:10.14309/00000434-201210001-00744
Tripathy, N., Basha, S., Jain, R., Shetty, S., and Ramachandran, A. (2008). Exenatide and acute pancreatitis. J. Assoc. Physicians India 56, 987–988.
Van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11, 3–10. doi:10.1002/pds.668
Wharton, S., Davies, M., Dicker, D., Lingvay, I., Mosenzon, O., Rubino, D. M., et al. (2022). Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad. Med. 134, 14–19. doi:10.1080/00325481.2021.2002616
Wolfe, D., Kanji, S., Yazdi, F., Barbeau, P., Rice, D., Beck, A., et al. (2020). Drug induced pancreatitis: a systematic review of case reports to determine potential drug associations. PLoS One 15, e0231883. doi:10.1371/journal.pone.0231883
Yau, A., Reisman, T., and Chen, Z. (2022). PSUN270 Magnified risk of pancreatitis by GLP-1: a case of necrotizing pancreatitis in a patient on dulaglutide with baseline elevated triglycerides. J. Endocr. Soc. 6, A399–A400. doi:10.1210/jendso/bvac150.831
Keywords: GLP-1 receptor agonists, acute pancreatitis, FAERS, pharmacovigilance, data mining
Citation: Guo H, Guo Q, Li Z and Wang Z (2024) Association between different GLP-1 receptor agonists and acute pancreatitis: case series and real-world pharmacovigilance analysis. Front. Pharmacol. 15:1461398. doi: 10.3389/fphar.2024.1461398
Received: 08 July 2024; Accepted: 01 November 2024;
Published: 13 November 2024.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Kimberly Crosby, University of Oklahoma, United StatesMarcelo Adrian Estrin, Interamerican Open University, Argentina
Qiuxia Min, The First People’s Hospital of Yunnan Province, China
Copyright © 2024 Guo, Guo, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze Wang, emV3NzQzNDBAZ21haWwuY29t
 Hui Guo
Hui Guo Qian Guo
Qian Guo Zhiqiang Li
Zhiqiang Li Ze Wang
Ze Wang