- 1Gannan Medical University, Ganzhou, Jiangxi, China
- 2Department of Anesthesiology, First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China
Postoperative pain management has consistently been a critical topic in the medical field, with patient-controlled intravenous analgesia (PCIA) being one of the most commonly utilized methods for postoperative analgesia. Currently, opioids remain the primary choice for PCIA in clinical practice. However, in recent years, an increasing number of studies have explored analgesic strategies aimed at reducing or eliminating the use of opioids in PCIA to mitigate the associated side effects and dependence. This article systematically reviews the progress of research on opioid-free analgesic strategies in PCIA through a comprehensive analysis of relevant literature.
1 Introduction
Postoperative pain is one of the major challenges patients often encounter. It not only affects patients’ quality of life and recovery speed but also can lead to a series of complications, prolonging hospital stays. To effectively manage postoperative pain, various analgesic methods and strategies have been extensively researched and applied. Among these, PCIA is a popular and proven effective method, favored by both medical professionals and patients. Traditionally, opioids have been considered the most effective drugs in PCIA. However, their use is associated with multiple risks, including respiratory depression, nausea and vomiting, as well as potential for dependency and abuse. Given these issues, the search for safer and more effective alternatives has become a focal point in current research on postoperative analgesia. In recent years, non-opioid drugs and their combinations have demonstrated potential in PCIA and are considered effective strategies for reducing or eliminating opioid use. This paper aims to systematically summarize the research progress on combination strategies that replace opioids in postoperative PCIA, evaluating the effectiveness, advantages, and limitations of various approaches, and exploring future research directions. The goal is to provide new insights and strategies for postoperative pain management.
2 Methods
2.1 Literature search and screening process
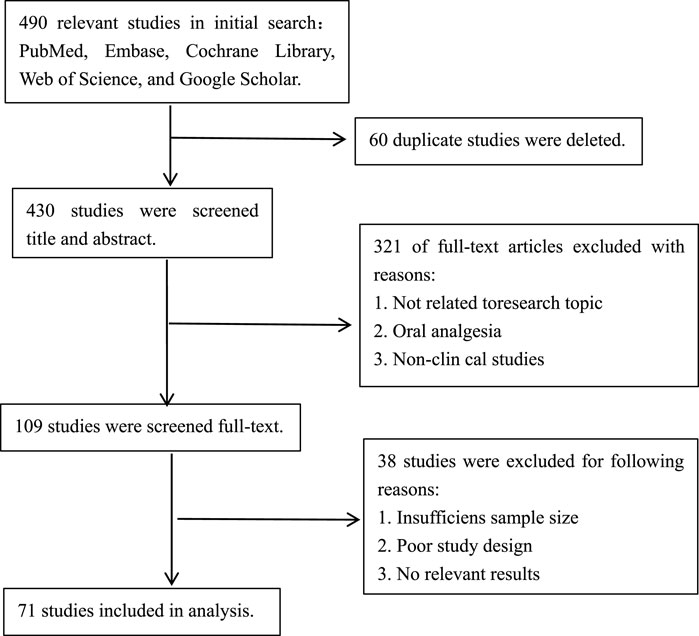
The literature search was conducted by the primary authors Xing-Heng Lei and Wen-Wen Yang, who utilized databases such as PubMed, Cochrane Library, Web of Science, and Google Scholar. They conducted searches and screenings using a variety of keywords, including but not limited to “opioid-free analgesia,” “opioid drugs,” “patient-controlled intravenous analgesia,” “non-steroidal anti-inflammatory drugs,” “intravenous analgesia,” “postoperative pain,” and “acute pain.” Additionally, relevant citations were traced to collect recent research literature on opioid-free patient-controlled intravenous analgesia in postoperative settings, providing a comprehensive summary. The search timeframe spanned from January 2010 to September 2024. A preliminary screening yielded 490 articles, which were subsequently evaluated based on titles, abstracts, and full texts, resulting in the inclusion of 71 studies that met the established criteria.
2.2 Literature review
The selected articles were independently reviewed by two reviewers, Bao-Zhen Liao and Pan-Guo Rao. They performed a quality assessment of each article to confirm adherence to the inclusion criteria, which encompassed the characteristics of study participants and the validity of study design.
2.3 Discrepancy resolution
In the event of any discrepancies during the review process, the research team convened a meeting to reach a final decision, led by researchers Xin Luo and Rui Guo. All disputed articles and the resulting decisions were documented to ensure transparency and fairness. The flowchart of literature search and screening is shown in Figure 1.
3 Application of opioids in PCIA
3.1 Introduction to opioids
Opioid analgesics are the primary drugs used in PCA therapy due to their excellent analgesic effects and rapid onset of action, making them the most widely used analgesics during the perioperative period. As the most common central analgesics, opioids play a crucial role in both acute and chronic pain management due to their potent analgesic properties.
Opioids can be classified into three categories based on their source and manufacturing process: natural alkaloids, semi-synthetic drugs, and synthetic drugs. In clinical practice, the primary natural opioid utilized is morphine. Additionally, synthetic opioids are commonly employed, including meperidine and the fentanyl family (fentanyl, alfentanil, remifentanil, and sufentanil). The analgesic effects of opioids are primarily achieved through their binding to specific receptors in the central nervous system and other tissues. These opioid receptors are mainly categorized into four types: μ, δ, κ, and σ, which are coupled with G proteins. Each receptor type has different subtypes and acts on the nervous system through various mechanisms.
Opioids exert their effects by inhibiting voltage-gated calcium channels and activating inwardly rectifying potassium channels. Upon binding to their receptors, they selectively inhibit the transmission of certain excitatory neural impulses, thereby alleviating the body’s perception of pain and the accompanying psychological and behavioral responses (Wang et al., 2023).
3.2 Side effects of opioid analgesia
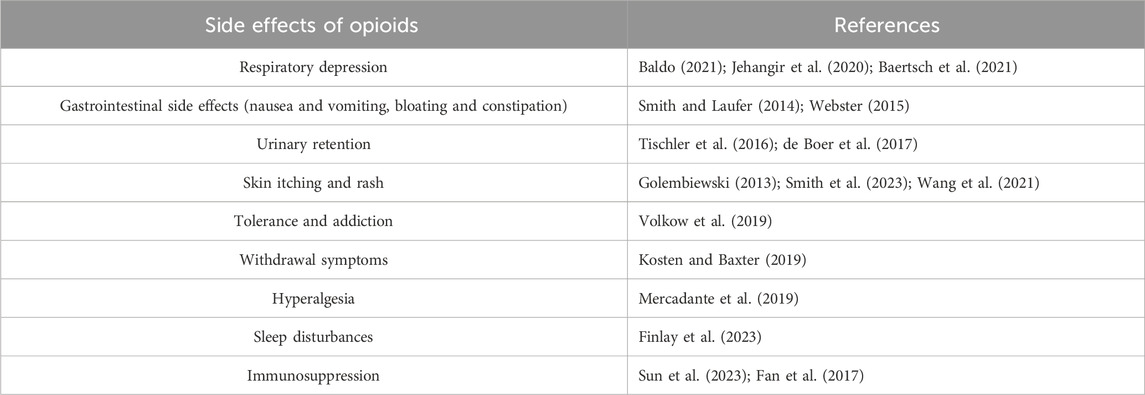
The clinical use of opioids has evolved from the initial use of morphine to pethidine and later to the fentanyl family, with continuous improvements in the drugs. However, related adverse reactions and complications still persist. These include respiratory depression, gastrointestinal reactions, urinary retention, skin itching and rashes, and in severe cases, can lead to patient death. In clinical practice, opioids also present issues such as tolerance, addiction, withdrawal symptoms, and hyperalgesia (El-Kefraoui et al., 2020). The side effects of opioids in PCIA are summarized in Table 1.
4 Common non-opioid intravenous analgesics
Several non-opioid intravenous analgesics are widely used in clinical practice, including non-steroidal anti-inflammatory drugs (NSAIDs), α2-adrenergic receptor agonists, N-methyl-D-aspartate (NMDA) receptor antagonists, gabapentinoids, sodium channel blockers, and corticosteroids. Each drug class has distinct mechanisms of action, and rational combinations can provide effective multimodal analgesia for postoperative patients.
4.1 Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are frequently used to manage postoperative pain by inhibiting cyclooxygenase (COX) enzymes, thereby reducing the synthesis of prostaglandins, which mediate inflammation and pain. Common NSAIDs in anesthesia include flurbiprofen axetil, parecoxib sodium, and ketorolac tromethamine. Compared to other analgesics, NSAIDs provide both anti-inflammatory and analgesic effects, making them first-line agents for mild to moderate pain. However, their use is associated with gastrointestinal, renal, and cardiovascular side effects, particularly an increased risk of gastrointestinal bleeding and potential surgical site bleeding (Bongiovanni et al., 2021).
4.1.1 Flurbiprofen axetil
Flurbiprofen axetil, a non-selective NSAID, inhibits both COX-1 and COX-2. It is delivered via a lipid microsphere carrier that enhances its targeting to inflamed tissues. Flurbiprofen axetil offers several advantages, including targeted drug delivery, controlled release, and avoidance of gastrointestinal complications (Zhang et al., 2018). Compared to oral NSAIDs, intravenous flurbiprofen axetil provides rapid onset and prolonged duration of action without affecting the central nervous system or respiratory function (Hersh et al., 2020). These characteristics make it suitable for controlling acute postoperative pain, particularly in patients at risk of gastrointestinal complications (Geng et al., 2015).
4.1.2 Parecoxib sodium
Parecoxib sodium is a selective COX-2 inhibitor that is rapidly converted to its active form, valdecoxib, following administration. Its COX-2 selectivity reduces the risk of gastrointestinal irritation and bleeding while providing effective analgesia and anti-inflammatory effects. Compared to non-selective NSAIDs, parecoxib sodium has the advantage of avoiding platelet dysfunction, making it particularly beneficial for patients who are at risk of postoperative bleeding (Gehling et al., 2010).
4.1.3 Ketorolac tromethamine
Ketorolac tromethamine is a potent non-selective COX inhibitor that provides significant analgesic effects, especially for moderate to severe postoperative pain. Its strong analgesic properties make it a suitable alternative to opioids, particularly in patients requiring rapid pain relief (Wu et al., 2022). However, ketorolac’s use is limited by its higher risk of gastrointestinal side effects and potential for increased bleeding, necessitating careful monitoring in the postoperative setting (Ying et al., 2022).
4.2 α2-Adrenergic receptor agonists
α2-adrenergic receptor agonists, such as dexmedetomidine, provide analgesic, sedative, and anxiolytic effects through the inhibition of norepinephrine release in the central nervous system (Peng et al., 2017). Dexmedetomidine’s ability to reduce opioid and anesthetic requirements without causing respiratory depression makes it an attractive option for postoperative pain management (Lin et al., 2023). However, its side effects, including bradycardia and hypotension, require careful monitoring, especially in patients with cardiovascular risk factors. Compared to NSAIDs, dexmedetomidine is less likely to cause gastrointestinal complications but may not be suitable for all patients due to its hemodynamic effects (Tang et al., 2020).
4.3 NMDA receptor antagonists
NMDA receptor antagonists, such as ketamine, offer analgesic effects by preventing central sensitization and hyperalgesia. Although ketamine is effective in reducing postoperative pain and opioid consumption, its use is limited by side effects, including excessive salivation, nausea, and psychiatric symptoms such as hallucinations and delirium (Zhang A. et al., 2024). Esketamine, the S-enantiomer of ketamine, offers more potent analgesic effects and a better side-effect profile compared to ketamine, although psychiatric side effects still limit its broader use (Han et al., 2022). Both drugs are valuable adjuncts in opioid-sparing regimens, but their psychiatric effects necessitate careful patient selection.
4.4 Dextromethorphan
Dextromethorphan, a centrally acting NMDA receptor antagonist, has been shown to elevate the pain threshold without inducing respiratory depression. Although not a direct analgesic, it can reduce nociceptive sensitivity, particularly when administered prior to painful stimuli (Martin et al., 2019). Its opioid-sparing effect makes it a useful adjunct in postoperative analgesia, particularly for reducing the overall opioid requirement and associated side effects (Chia et al., 1999).
4.5 Magnesium sulfate
Magnesium sulfate acts as an NMDA receptor antagonist, reducing central sensitization. Adjunctive use of magnesium sulfate in postoperative analgesia has been shown to reduce opioid consumption and enhance pain control (Song et al., 2011). However, its use is limited by the need for real-time monitoring of magnesium levels due to risks such as bradycardia and hypermagnesemia. Further research is required to establish optimal dosing regimens for safe and effective use in postoperative settings (Sousa et al., 2016).
4.6 Gabapentinoids
Gabapentinoids, such as gabapentin and pregabalin, are calcium channel blockers that prevent hyperalgesia and central sensitization by inhibiting neurotransmitter release. Pregabalin is generally considered more effective and better tolerated than gabapentin, although both drugs have been associated with increased risk of respiratory depression when used in combination with opioids (Evoy et al., 2021; Huang et al., 2021). Gabapentinoids can improve sleep and reduce anxiety, making them valuable in multimodal analgesia for postoperative pain, particularly in patients with chronic pain conditions (Bockbrader et al., 2010). However, their use must be carefully tailored in patients with renal impairment due to their renal clearance.
4.7 Other adjuvant analgesics
Local anesthetics, such as lidocaine, provide membrane stabilization by inhibiting sodium channels, reducing nociceptive transmission, and alleviating hyperalgesia (Alebouyeh et al., 2014). Intravenous lidocaine has demonstrated efficacy in reducing postoperative opioid consumption and improving recovery outcomes, particularly in abdominal surgery (Kranke et al., 2015). Glucocorticoids, such as dexamethasone, are widely used for their anti-inflammatory and antiemetic effects (Chu et al., 2014). When combined with opioids, glucocorticoids have been shown to extend the duration of opioid analgesia and reduce opioid consumption, although long-term use poses risks such as immunosuppression (Lee et al., 2002; Ryoo et al., 2015). The commonly used non-opioid medications in PCIA are shown in Table 2.
5 Current status and challenges of opioid-free PCIA in clinical practice
5.1 Current clinical application of non-opioid PCIA
The use of opioid-free medications in PCIA, while not as effective for pain relief as opioids, can adequately meet clinical analgesic needs for postoperative mild to moderate pain when appropriately selected and optimally combined. Common procedures associated with mild pain, such as inguinal hernia repair, varicose vein ligation, and laparoscopic surgeries, can be effectively managed with opioid-free PCIA. Moderate pain procedures like knee and below-knee surgeries, shoulder and back surgeries, hysterectomies, and maxillofacial surgeries also respond well to non-opioid PCIA. Postoperative pain following thyroid surgery, which mainly manifests as pain during swallowing and subsequent difficulty in eating, is classified as mild pain and can also be managed with opioid-free PCIA (Gerbershagen et al., 2013).
The combination of two non-opioid intravenous analgesics in PCIA, along with an optimized regimen, can effectively provide analgesia for these types of surgeries. Additionally, incorporating single-shot nerve blocks for certain surgical sites as part of a multimodal analgesia approach can achieve satisfactory pain control, demonstrating the feasibility of opioid-free PCIA.
However, the use of opioid-free PCIA for procedures associated with severe pain, such as thoracotomies, laparotomies, total knee or hip replacements, and major vascular surgeries, still faces limitations. The primary challenge is that opioid-free analgesics may not sufficiently manage the high-intensity postoperative pain, particularly during peak pain periods. Therefore, for such surgeries, combining non-opioid analgesics with opioids or other more potent analgesic strategies may be necessary to enhance pain control efficacy and patient comfort.
5.2 The medication regimen or combination for opioid-free PCIA
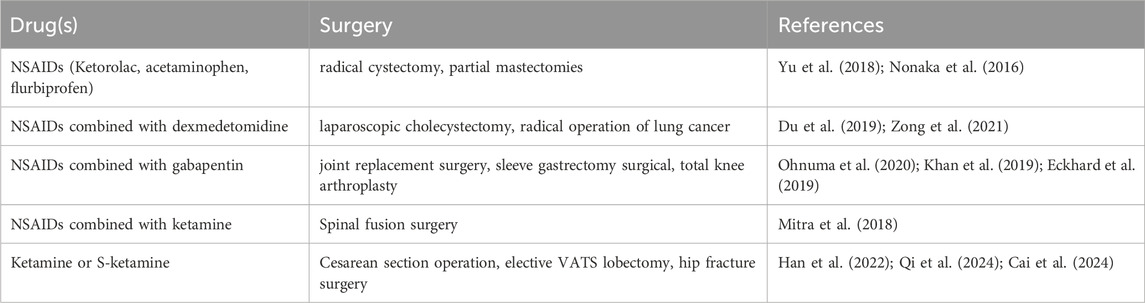
NSAIDs are the most widely used medications for postoperative analgesia aside from opioids. Therefore, in opioid-free PCIA, a common combination involves NSAIDs paired with another non-opioid medication (Pergolizzi et al., 2021). For instance, NSAIDs combined with dexmedetomidine have been used in PCIA. A study on postoperative pain following thyroid surgery (Ma et al., 2017) showed that the combination of dexmedetomidine and flurbiprofen axetil significantly improved pain control after general anesthesia for thyroid surgery, reduced agitation and postoperative cognitive dysfunction, enhanced immune function, and promoted surgical wound healing.
Additionally, a study on postoperative analgesia following joint replacement surgery (Ohnuma et al., 2020) found that the combination of NSAIDs and gabapentin for PCIA resulted in pain scores comparable to those observed with opioid monotherapy. This combination also reduced the use of opioids and lowered the incidence of postoperative pulmonary complications. Research indicates that the combination of NSAIDs with ketamine can synergistically increase patients’ pain thresholds, reduce pain perception, decrease inflammation, and alleviate clinical symptoms of hyperalgesia (Mitra et al., 2018). NSAIDs can also counteract tramadol’s constipating effects, and their combined use can even boost macrophage spontaneous release of pro-inflammatory cytokines, enhancing cellular immune function (Filipczak-Bryniarska et al., 2023). The medication regimen or combination for opioid-free PCIA is presented in Table 3.
5.3 Advantages of opioid-free analgesia via PCIA in postoperative pain management
Research suggests that the use of entirely non-opioid analgesics via PCIA is feasible for managing postoperative pain in specific cases (Baboli et al., 2020). This approach effectively controls surgical-induced inflammatory responses and reduces occurrences of respiratory depression, nausea, vomiting, and pain hypersensitivity. Moreover, it diminishes opioid dependence in patients typically prescribed opioids post-discharge, enhances postoperative recovery quality, shortens hospital stays, reduces medical costs, and conserves healthcare resources (Savoia and Scibelli, 2022). Additionally, it may potentially mitigate postoperative tumor recurrence and metastasis (Forget et al., 2010). Combinations of non-opioid analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), NMDA receptor antagonists, and dexmedetomidine, offer superior intraoperative analgesia without the use of opioids, with minimal associated side effects (Zhang S. et al., 2024). Especially for elderly, frail, or critically ill patients, opioid-free PCIA has less impact on consciousness and respiration, providing greater safety compared to opioid-based PCIA.
5.4 Limitations of opioid-free PCIA
Opioid-free PCIA has its limitations. Unlike opioid drugs, non-opioid medications have a narrower therapeutic window and are subject to the ceiling effect. The most notable limitation is that their analgesic efficacy is generally inferior to opioids, particularly for severe pain. However, with the advancement of multimodal analgesia and nerve block techniques, opioid-free PCIA combined with effective nerve blocks can meet postoperative pain management needs for certain surgeries involving severe pain.
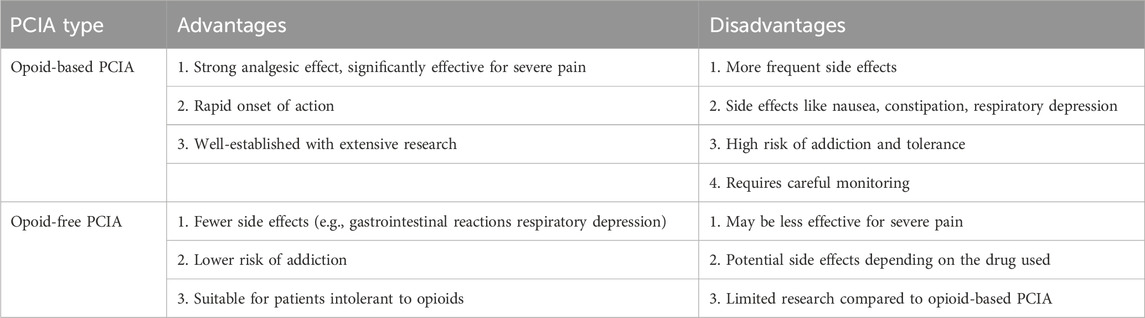
Additionally, the potential adverse effects and interactions of non-opioid drugs cannot be overlooked. For instance, dexmedetomidine can cause bradycardia and hypotension (Xie K. et al., 2023; Dong et al., 2017), NSAIDs may lead to gastrointestinal bleeding and renal dysfunction (Xie H. et al., 2023; Xu et al., 2023), and ketamine can induce hallucinations and agitation (Guo et al., 2023; Zhang et al., 2023a; Zhang et al., 2023b). It is also essential to be mindful of other potential adverse effects of these medications. Although studies indicate that commonly used drugs such as NSAIDs, dexmedetomidine, ketamine, magnesium sulfate, and lidocaine do not have compatibility contraindications, there is a lack of research on their in vivo interactions. Previous studies have found that the incidence of adverse interactions increases exponentially with the number of anesthetic drugs used (Fiore et al., 2022). Therefore, further research is needed to clarify the safety of combined use of multiple drugs. The comparison of the advantages and disadvantages of opioid-based and opioid-free PCIA is presented in Table 4.
6 Disscussion
Pure opioid and opioid-free approaches represent two extremes in the application of PCIA. With the growing emphasis on multimodal analgesia, the use of pure opioid PCIA has gradually declined in clinical practice. Currently, the predominant strategy in PCIA is to minimize opioid use. This is typically achieved by combining opioids with one or two non-opioid analgesics, such as NSAIDs.
In contrast, opioid-free PCIA has emerged as a significant concept in recent years, grounded in the same principle of multimodal analgesia. This approach involves the combined use of two or more non-opioid analgesics. Recent studies have shown that for certain surgical procedures associated with mild to moderate pain, opioid-free PCIA can effectively fulfill clinical analgesic requirements. However, for surgeries that result in moderate to severe pain, continuous epidural analgesia or continuous nerve blocks are often preferred over PCIA. In cases where continuous epidural or nerve block analgesia is not suitable, opioid-free PCIA can still be effectively utilized alongside single nerve blocks or incision infiltration techniques to meet analgesic needs.
The opioid-free PCIA approach also addresses significant societal concerns, particularly in the context of the opioid abuse crisis observed in countries such as the United States (Volkow and Blanco, 2021). By ensuring that clinical pain management needs are met, the adoption of opioid-free PCIA carries considerable social significance. Moreover, for outpatient procedures or rapid recovery surgeries, opioid-free PCIA offers substantial clinical advantages (Gilbertson et al., 2021; Gosgnach et al., 2024).
7 Summary and prospects
Although opioids still dominate the clinical landscape of PCIA, their associated side effects are significant, especially against the backdrop of global opioid misuse. Therefore, it is imperative to advocate for and develop alternative therapies. This review, based on an extensive summary of literature, systematically outlines the clinical applications and current research status of opioid-free PCIA, including drug combination regimens, advantages, and limitations. The development of non-opioid PCIA offers a new perspective for postoperative pain management.
Currently, opioid-free PCIA is primarily used for postoperative analgesia in surgeries involving mild to moderate pain, with a predominant focus on NSAIDs combined with other non-opioid drugs. This approach not only meets analgesic needs but also significantly reduces many of the adverse effects associated with opioid analgesia, presenting a considerable advantage. However, opioid-free PCIA still has limitations for managing severe pain, and the potential side effects of opioid-free PCIA cannot be ignored. Further research and development are needed to optimize these therapies and ensure their safety and efficacy in a broader range of clinical scenarios.
Future research should focus on the development of new non-opioid drugs with stronger analgesic effects and lower risks of side effects. Additionally, exploring and optimizing combination drug strategies, especially for severe pain management, will be crucial to enhancing the therapeutic efficacy of opioid-free PCIA. More studies are needed to determine the optimal usage and dosage of various non-opioid drugs to ensure patients receive the best postoperative pain control while minimizing potential side effects. Interdisciplinary collaboration, involving experts from pharmacology, neurobiology, and clinical medicine, is essential. Conducting large-scale, multicenter clinical trials on non-opioid PCIA will further improve the effectiveness and quality of postoperative pain management. Such collaborations can better guide clinical practice and promote advancements in the field of postoperative pain management.
Author contributions
XL: Writing–original draft, Writing–review and editing. P-GR: Investigation, Writing–original draft. X-HL: Investigation, Writing–original draft. W-WY: Investigation, Writing–original draft. B-ZL: Investigation, Writing–original draft. RG: Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alebouyeh, M. R., Imani, F., Rahimzadeh, P., Entezary, S. R., Faiz, S. H., and Soraya, P. (2014). Analgesic effects of adding lidocaine to morphine pumps after orthopedic surgeries. J. Res. Med. Sci. 19 (2), 122–127.
Baboli, K. M., Liu, H., and Poggio, J. L. (2020). Opioid-free postoperative analgesia: is it feasible?. Curr. Probl. Surg. 57 (7), 100794. doi:10.1016/j.cpsurg.2020.100794
Baertsch, N. A., Bush, N. E., Burgraff, N. J., and Ramirez, J. M. (2021). Dual mechanisms of opioid-induced respiratory depression in the inspiratory rhythm-generating network. Elife 10, e67523. doi:10.7554/eLife.67523
Baldo, B. A. (2021). Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch. Toxicol. 95 (8), 2627–2642. doi:10.1007/s00204-021-03068-2
Bockbrader, H. N., Wesche, D., Miller, R., Chapel, S., Janiczek, N., and Burger, P. (2010). A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin. Pharmacokinet. 49 (10), 661–669. doi:10.2165/11536200-000000000-00000
Bongiovanni, T., Lancaster, E., Ledesma, Y., Whitaker, E., Steinman, M. A., Allen, I. E., et al. (2021). Systematic review and meta-analysis of the association between non-steroidal anti-inflammatory drugs and operative bleeding in the perioperative period. J. Am. Coll. Surg. 232 (5), 765–790. doi:10.1016/j.jamcollsurg.2021.01.005
Cai, J., Chen, X., Jin, Z., Chi, Z., and Xiong, J. (2024). Effects of adjunctive esketamine on depression in elderly patients undergoing hip fracture surgery: a randomized controlled trial. BMC Anesthesiol. 24 (1), 340. doi:10.1186/s12871-024-02733-0
Chia, Y. Y., Liu, K., Chow, L. H., and Lee, T. Y. (1999). The preoperative administration of intravenous dextromethorphan reduces postoperative morphine consumption. Anesth. Analg. 89 (3), 748–752. doi:10.1097/00000539-199909000-00041
Chu, C. C., Hsing, C. H., Shieh, J. P., Chien, C. C., Ho, C. M., and Wang, J. J. (2014). The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur. J. Pharmacol. 722, 48–54. doi:10.1016/j.ejphar.2013.10.008
de Boer, H. D., Detriche, O., and Forget, P. (2017). Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best. Pract. Res. Clin. Anaesthesiol. 31 (4), 499–504. doi:10.1016/j.bpa.2017.07.002
Dong, C. S., Zhang, J., Lu, Q., Sun, P., Yu, J. M., Wu, C., et al. (2017). Effect of Dexmedetomidine combined with sufentanil for post-thoracotomy intravenous analgesia:a randomized, controlled clinical study. BMC Anesthesiol. 17 (1), 33. doi:10.1186/s12871-017-0324-4
Du, X., Song, F., Zhang, X., and Ma, S. (2019). Protective efficacy of combined use of parecoxib and dexmedetomidine on postoperative hyperalgesia and early cognitive dysfunction after laparoscopic cholecystectomy for elderly patients. Acta Cir. Bras. 34 (9), e201900905. doi:10.1590/s0102-865020190090000005
Eckhard, L., Jones, T., Collins, J. E., Shrestha, S., and Fitz, W. (2019). Increased postoperative dexamethasone and gabapentin reduces opioid consumption after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 27 (7), 2167–2172. doi:10.1007/s00167-019-05449-8
El-Kefraoui, C., Olleik, G., Chay, M. A., Kouyoumdjian, A., Nguyen-Powanda, P., Rajabiyazdi, F., et al. (2020). Opioid versus opioid-free analgesia after surgical discharge: protocol for a systematic review and meta-analysis. BMJ Open 10 (1), e035443. doi:10.1136/bmjopen-2019-035443
Evoy, K. E., Sadrameli, S., Contreras, J., Covvey, J. R., Peckham, A. M., and Morrison, M. D. (2021). Abuse and misuse of pregabalin and gabapentin: a systematic review update. Drugs 81 (1), 125–156. doi:10.1007/s40265-020-01432-7
Fan, Y., Liang, X., Wang, R., and Song, L. (2017). Role of endogenous melatoninergic system in development of hyperalgesia and tolerance induced by chronic morphine administration in rats. Brain Res. Bull. 135, 105–112. doi:10.1016/j.brainresbull.2017.10.005
Filipczak-Bryniarska, I., Nazimek, K., Nowak, B., Skalska, P., Cieślik, M., Fedor, A., et al. (2023). Immunomodulation by tramadol combined with acetaminophen or dexketoprofen: in vivo animal study. Int. Immunopharmacol. 125 (Pt A), 110985. doi:10.1016/j.intimp.2023.110985
Finlay, M., Erwin, J. A., Skeiky, L., Hansen, D. A., Layton, M. E., Quock, R., et al. (2023). Nighttime sleep and respiratory disturbances in individuals receiving methadone to treat opioid use disorder. J. Addict. Nurs. 34 (4), E180–E188. doi:10.1097/jan.0000000000000470
Fiore, J. F., El-Kefraoui, C., Chay, M. A., Nguyen-Powanda, P., Do, U., Olleik, G., et al. (2022). Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet 399 (10343), 2280–2293. doi:10.1016/s0140-6736(22)00582-7
Forget, P., Vandenhende, J., Berliere, M., Machiels, J. P., Nussbaum, B., Legrand, C., et al. (2010). Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 110 (6), 1630–1635. doi:10.1213/ANE.0b013e3181d2ad07
Gehling, M., Arndt, C., Eberhart, L. H., Koch, T., Krüger, T., and Wulf, H. (2010). Postoperative analgesia with parecoxib, acetaminophen, and the combination of both: a randomized, double-blind, placebo-controlled trial in patients undergoing thyroid surgery. Br. J. Anaesth. 104 (6), 761–767. doi:10.1093/bja/aeq096
Geng, W., Hong, W., Wang, J., Dai, Q., Mo, Y., Shi, K., et al. (2015). Flurbiprofen axetil enhances analgesic effects of sufentanil and attenuates postoperative emergence agitation and systemic proinflammation in patients undergoing tangential excision surgery. Mediat. Inflamm. 2015, 601083. doi:10.1155/2015/601083
Gerbershagen, H. J., Aduckathil, S., van Wijck, A. J., Peelen, L. M., Kalkman, C. J., and Meissner, W. (2013). Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 118 (4), 934–944. doi:10.1097/ALN.0b013e31828866b3
Gilbertson, L. E., Patel, C., De, S., Lo, W., Garcia-Roig, M., and Austin, T. M. (2021). The utilization of an opioid-free anesthetic for pediatric circumcision in an ambulatory surgery center. Child. (Basel) 8 (8), 678. doi:10.3390/children8080678
Golembiewski, J. (2013). Opioid-induced pruritus. J. Perianesth Nurs. 28 (4), 247–249. doi:10.1016/j.jopan.2013.05.008
Gosgnach, M., Chasserant, P., and Raux, M. (2024). Opioid free analgesia after return home in ambulatory colonic surgery patients: a single-center observational study. BMC Anesthesiol. 24 (1), 260. doi:10.1186/s12871-024-02651-1
Guo, Y., Ding, X., Wang, S., Wang, F., Zheng, Z., and Zou, L. (2023). Analgesic effect of esketamine combined with tramadol for patient-controlled intravenous analgesia after cesarean section: a randomized controlled trial. J. Pain Res. 16, 3519–3528. doi:10.2147/jpr.S427702
Han, Y., Li, P., Miao, M., Tao, Y., Kang, X., and Zhang, J. (2022). S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. 22 (1), 49. doi:10.1186/s12871-022-01588-7
Hersh, E. V., Moore, P. A., Grosser, T., Polomano, R. C., Farrar, J. T., Saraghi, M., et al. (2020). Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain. J. Dent. Res. 99 (7), 777–786. doi:10.1177/0022034520914254
Huang, Y., Xu, C., Zeng, T., Li, Z., Xia, Y., Tao, G., et al. (2021). Intravenous patient-controlled analgesia hydromorphone combined with pregabalin for the treatment of postherpetic neuralgia: a multicenter, randomized controlled study. Korean J. Pain 34 (2), 210–216. doi:10.3344/kjp.2021.34.2.210
Jehangir, W., Karabachev, A. D., Mehta, Z., and Davis, M. (2020). Opioid-related sleep-disordered breathing: an update for clinicians. Am. J. Hosp. Palliat. Care 37 (11), 970–973. doi:10.1177/1049909120913232
Khan, M. U., Bamehriz, F. Y., Aqil, M., Dammas, F. A., Fadin, A., and Khokhar, R. S. (2019). The effect of gabapentin on postoperative pain, morphine sparing effect and preoperative anxiety in patients going for sleeve gastrectomy surgical procedure. J. Coll. Physicians Surg. Pak 29 (8), 697–701. doi:10.29271/jcpsp.2019.08.697
Kosten, T. R., and Baxter, L. E. (2019). Review article: effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am. J. Addict. 28 (2), 55–62. doi:10.1111/ajad.12862
Kranke, P., Jokinen, J., Pace, N. L., Schnabel, A., Hollmann, M. W., Hahnenkamp, K., et al. (2015). Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst. Rev. (7), Cd009642. doi:10.1002/14651858.CD009642.pub2
Lee, Y., Lin, Y. S., and Chen, Y. H. (2002). The effect of dexamethasone upon patient-controlled analgesia-related nausea and vomiting. Anaesthesia 57 (7), 705–709. doi:10.1046/j.1365-2044.2002.02572_5.x
Lin, Z., Li, S., Zhou, Y., Lu, X., Yang, B., Yu, Z., et al. (2023). A comparative study of esketamine-dexmedetomidine and sufentanil-dexmedetomidine for sedation and analgesia in lung tumor percutaneous radiofrequency ablation (PRFA): a randomized double-blind clinical trial. BMC Anesthesiol. 23 (1), 304. doi:10.1186/s12871-023-02266-y
Ma, X. D., Li, B. P., Wang, D. L., and Yang, W. S. (2017). Postoperative benefits of dexmedetomidine combined with flurbiprofen axetil after thyroid surgery. Exp. Ther. Med. 14 (3), 2148–2152. doi:10.3892/etm.2017.4717
Martin, E., Narjoz, C., Decleves, X., Labat, L., Lambert, C., Loriot, M. A., et al. (2019). Dextromethorphan analgesia in a human experimental model of hyperalgesia. Anesthesiology 131 (2), 356–368. doi:10.1097/aln.0000000000002736
Mercadante, S., Arcuri, E., and Santoni, A. (2019). Opioid-induced tolerance and hyperalgesia. CNS Drugs 33 (10), 943–955. doi:10.1007/s40263-019-00660-0
Mitra, S., Carlyle, D., Kodumudi, G., Kodumudi, V., and Vadivelu, N. (2018). New advances in acute postoperative pain management. Curr. Pain Headache Rep. 22 (5), 35. doi:10.1007/s11916-018-0690-8
Nonaka, T., Hara, M., Miyamoto, C., Sugita, M., and Yamamoto, T. (2016). Comparison of the analgesic effect of intravenous acetaminophen with that of flurbiprofen axetil on post-breast surgery pain: a randomized controlled trial. J. Anesth. 30 (3), 405–409. doi:10.1007/s00540-016-2150-0
Ohnuma, T., Raghunathan, K., Ellis, A. R., Whittle, J., Pyati, S., Bryan, W. E., et al. (2020). Effects of acetaminophen, NSAIDs, gabapentinoids, and their combinations on postoperative pulmonary complications after total hip or knee arthroplasty. Pain Med. 21 (10), 2385–2393. doi:10.1093/pm/pnaa017
Peng, K., Zhang, J., Meng, X. W., Liu, H. Y., and Ji, F. H. (2017). Optimization of postoperative intravenous patient-controlled analgesia with opioid-dexmedetomidine combinations: an updated meta-analysis with trial sequential analysis of randomized controlled trials. Pain Physician 20 (7), 569–596.
Pergolizzi, J. V., Magnusson, P., LeQuang, J. A., Breve, F., Taylor, R., Wollmuth, C., et al. (2021). Can NSAIDs and acetaminophen effectively replace opioid treatment options for acute pain?. Expert Opin. Pharmacother. 22 (9), 1119–1126. doi:10.1080/14656566.2021.1901885
Qi, Y., Zhou, M., Zheng, W., Dong, Y., Li, W., Wang, L., et al. (2024). Effect of S-ketamine on postoperative nausea and vomiting in patients undergoing video-assisted thoracic surgery: a randomized controlled trial. Drug Des. Devel Ther. 18, 1189–1198. doi:10.2147/dddt.S449705
Ryoo, S. H., Yoo, J. H., Kim, M. G., Lee, K. H., and Kim, S. I. (2015). The effect of combination treatment using palonosetron and dexamethasone for the prevention of postoperative nausea and vomiting versus dexamethasone alone in women receiving intravenous patient-controlled analgesia. Korean J. Anesthesiol. 68 (3), 267–273. doi:10.4097/kjae.2015.68.3.267
Savoia, G., and Scibelli, G. (2022). From opioid free anesthesia to opioid free postoperative analgesia: a difficult target to reach. Minerva Anestesiol. 88 (6), 421–424. doi:10.23736/s0375-9393.22.16633-2
Smith, H. S., and Laufer, A. (2014). Opioid induced nausea and vomiting. Eur. J. Pharmacol. 722, 67–78. doi:10.1016/j.ejphar.2013.09.074
Smith, K. M., Nguyen, E., and Ross, S. E. (2023). The delta-opioid receptor bidirectionally modulates itch. J. Pain 24 (2), 264–272. doi:10.1016/j.jpain.2022.09.013
Song, J. W., Lee, Y. W., Yoon, K. B., Park, S. J., and Shim, Y. H. (2011). Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth. Analg. 113 (2), 390–397. doi:10.1213/ANE.0b013e31821d72bc
Sousa, A. M., Rosado, G. M., Neto Jde, S., Guimarães, G. M., and Ashmawi, H. A. (2016). Magnesium sulfate improves postoperative analgesia in laparoscopic gynecologic surgeries: a double-blind randomized controlled trial. J. Clin. Anesth. 34, 379–384. doi:10.1016/j.jclinane.2016.05.006
Sun, Q., Li, Z., Wang, Z., Wang, Q., Qin, F., Pan, H., et al. (2023). Immunosuppression by opioids: mechanisms of action on innate and adaptive immunity. Biochem. Pharmacol. 209, 115417. doi:10.1016/j.bcp.2023.115417
Tang, C., Hu, Y., Zhang, Z., Wei, Z., Wang, H., Geng, Q., et al. (2020). Dexmedetomidine with sufentanil in intravenous patient-controlled analgesia for relief from postoperative pain, inflammation and delirium after esophageal cancer surgery. Biosci. Rep. 40 (5). doi:10.1042/bsr20193410
Tischler, E. H., Restrepo, C., Oh, J., Matthews, C. N., Chen, A. F., and Parvizi, J. (2016). Urinary retention is rare after total joint arthroplasty when using opioid-free regional anesthesia. J. Arthroplasty 31 (2), 480–483. doi:10.1016/j.arth.2015.09.007
Volkow, N. D., and Blanco, C. (2021). The changing opioid crisis: development, challenges and opportunities. Mol. Psychiatry 26 (1), 218–233. doi:10.1038/s41380-020-0661-4
Volkow, N. D., Jones, E. B., Einstein, E. B., and Wargo, E. M. (2019). Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry 76 (2), 208–216. doi:10.1001/jamapsychiatry.2018.3126
Wang, Y., Zhuang, Y., DiBerto, J. F., Zhou, X. E., Schmitz, G. P., Yuan, Q., et al. (2023). Structures of the entire human opioid receptor family. Cell 186 (2), 413–427.e17. doi:10.1016/j.cell.2022.12.026
Wang, Z., Jiang, C., Yao, H., Chen, O., Rahman, S., Gu, Y., et al. (2021). Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain 144 (2), 665–681. doi:10.1093/brain/awaa430
Webster, L. R. (2015). Opioid-induced constipation. Pain Med. 16 (Suppl. 1), S16–S21. doi:10.1111/pme.12911
Wu, Y., Cai, Z., Li, Y., Kang, Y., Fu, B., and Wang, J. (2022). Effect of ketorolac tromethamine combined with dezocine prior administration on hemodynamics and postoperative analgesia in patients undergoing laparoscopic hernia repair. Med. Baltim. 101 (20), e29320. doi:10.1097/md.0000000000029320
Xie, H., Chen, S. H., Li, L., and Ge, W. H. (2023). The cost-effectiveness analysis of analgesic treatment options for postoperative pain following laparotomy surgeries. Int. J. Clin. Pharm. 45 (2), 355–363. doi:10.1007/s11096-022-01473-w
Xie, K., Chen, J., Tian, L., Gu, F., Pan, Y., Huang, Z., et al. (2023). Postoperative infusion of dexmedetomidine via intravenous patient-controlled analgesia for prevention of postoperative delirium in elderly patients undergoing surgery. Aging Clin. Exp. Res. 35 (10), 2137–2144. doi:10.1007/s40520-023-02497-6
Xu, X., Tao, Y., Yang, Y., Zhang, J., and Sun, M. (2023). Application of butorphanol versus sufentanil in multimode analgesia via patient controlled intravenous analgesia after hepatobiliary surgery: a retrospective cohort study. Drug Des. Devel Ther. 17, 3757–3766. doi:10.2147/dddt.S433136
Ying, Y., Fei, S., Zeng, Z., Qu, X., and Cao, Z. (2022). Comparative study of dezocine and ketorolac tromethamine in patient-controlled intravenous analgesia of laparoscopic cholecystectomy. Front. Surg. 9, 881006. doi:10.3389/fsurg.2022.881006
Yu, Y. D., Hwang, J. H., Seo, Y. E., Song, B. D., Jung, Y. S., Lee, D. H., et al. (2018). Effects of nonsteroidal anti-inflammatory drugs as patient controlled analgesia on early bowel function recovery after radical cystectomy. Sci. Rep. 8 (1), 4658. doi:10.1038/s41598-018-22677-z
Zhang, A., Zhou, Y., Zheng, X., Zhou, W., Gu, Y., Jiang, Z., et al. (2024). Effects of S-ketamine added to patient-controlled analgesia on early postoperative pain and recovery in patients undergoing thoracoscopic lung surgery: a randomized double-blinded controlled trial. J. Clin. Anesth. 92, 111299. doi:10.1016/j.jclinane.2023.111299
Zhang, J., Zhang, H., Zhao, L., Gu, J., Feng, Y., and An, H. (2018). Population pharmacokinetic modeling of flurbiprofen, the active metabolite of flurbiprofen axetil, in Chinese patients with postoperative pain. J. Pain Res. 11, 3061–3070. doi:10.2147/jpr.S176475
Zhang, S., Zhang, J., and Zhang, R. (2024). Safety and effectiveness of opioid-free anaesthesia in thoracoscopic surgery: a preliminary retrospective cohort study. BMC Anesthesiol. 24 (1), 60. doi:10.1186/s12871-024-02441-9
Zhang, T., Song, N., Li, S., Yu, L., Xie, Y., Yue, Z., et al. (2023b). S-ketamine improves slow wave sleep and the associated changes in serum protein among gynecological abdominal surgery patients: a randomized controlled trial. Nat. Sci. Sleep. 15, 903–913. doi:10.2147/nss.S430453
Zhang, T., Yue, Z., Yu, L., Li, S., Xie, Y., Wei, J., et al. (2023a). S-ketamine promotes postoperative recovery of gastrointestinal function and reduces postoperative pain in gynecological abdominal surgery patients: a randomized controlled trial. BMC Surg. 23 (1), 74. doi:10.1186/s12893-023-01973-0
Keywords: postoperative pain, opioids, non-steroidal anti-inflammatory drugs, opioid-free, postoperative patient-controlled intravenous analgesia
Citation: Luo X, Rao P-G, Lei X-H, Yang W-W, Liao B-Z and Guo R (2024) Opioid-free strategies for patient-controlled intravenous postoperative analgesia: a review of recent studies. Front. Pharmacol. 15:1454112. doi: 10.3389/fphar.2024.1454112
Received: 24 June 2024; Accepted: 24 October 2024;
Published: 31 October 2024.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Katherine N. Theken, University of Pennsylvania, United StatesSandeep Bhushan, Chengdu Second People’s Hospital, China
Sohan Lal Solanki, Tata Memorial Hospital, India
Copyright © 2024 Luo, Rao, Lei, Yang, Liao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Guo, aGFpb3UyMDE4Z3VvQDE2My5jb20=
 Xin Luo
Xin Luo Pan-Guo Rao1
Pan-Guo Rao1 Rui Guo
Rui Guo