- 1Cancer Center, Renmin Hospital of Wuhan University, Wuhan, China
- 2Department of Oncology and Hematology, Xiangyang Hospital, Hubei University of Chinese Medicine, Xiangyang, China
- 3Department of Oncology, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
Background: Monoclonal antibodies against programmed cell death protein-1 (PD-1)/programmed death-ligand-1 (PD-L1) have emerged as critical tools in cancer treatment. However, concerns regarding their potential cutaneous and mucosal toxicity, along with severe complications, have drawn clinical attention. Further research is warranted to investigate the adverse reactions and treatment strategies associated with PD-1 monoclonal antibodies.
Methods: We present a detailed case report of a laryngeal cancer patient who developed toxic epidermal necrolysis (TEN) after treatment with PD-1 monoclonal antibody. We analyzed the etiology, diagnosis, and treatment approaches by integrating clinical manifestations, pathological examinations, and literature research.
Results: After PD-1 monoclonal antibody therapy, the patient exhibited systemic rash, bullae, and epidermal detachment, which subsequently involved the tracheal and bronchial mucosa, resulting in dyspnea. The patient recovered after treatments with steroids, macrolides, immunoglobulins, and etanercept, along with repeated removal of scabs via bronchoscopy. Literature reviewing suggests a potential association between PD-1 monoclonal antibodies and the pathogenesis of Steven Johnson’s Syndrome (SJS) and Toxic epidermal necrolysis (TEN), possibly due to immune dysregulation. Treatment consists of immediate discontinuation of suspicious drugs, essential supportive therapy, and systemic corticosteroid administration, with the addition of immunosuppressants and/or immunoglobulins needed.
Conclusion: The mucocutaneous toxicity induced by PD-1 monoclonal antibodies is not limited to the surface of the skin but also in deep mucosal layers, potentially leading to life-threatening complications. Therefore, when using PD-1 monoclonal antibodies, clinicians should closely monitor adverse events and apply appropriate treatments as soon as possible to prevent severe complications.
1 Introduction
Immunotherapy has been utilized for various kinds of cancer, greatly transforming the panorama of malignant tumor treatment. With the growing use of drugs for immune therapy represented by immune checkpoint inhibitors (ICIs), immune-related adverse effects (irAEs) are drawing more and more attention. IrAEs range from mild diarrhea, fatigue, rash, to more severe events as immune-related pneumonia and myocarditis (Rached et al., 2024; Wang et al., 2019). Dermal toxicity is the most commonly seen, with its severity depending on clinical presentation and body surface area (BSA) affected classification (Collins et al., 2017): Grade 1 reaction include pathological erosions or erythema with no associated symptoms; Grade 2 reactions include Grade 1 reactions covering less than 50% of BSA except itching or other associated symptoms; Grade 3 reaction include symptomatic generalized erythema or rash, papules or blistering erosions or desquamations covering >50% of BSA; Grade 4 reaction include generalized exfoliative dermatitis or ulcerative dermatitis. Most skin adverse reactions were classified a grade 1 or grade 2. Severe (grade ≥3) events were less observed, such as Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) with high mortality, which required more attention. This report discusses a case of TEN induced by programmed cell death-1 (PD-1) monoclonal antibody therapy, suggesting that TEN is not limited to body surface, but may also involve trachea and bronchial mucosa. However, its pathogenesis, clinical diagnosis and treatment strategy require further study.
2 Case presentation
A 60-year-old male patient visited our hospital with chief complaint of hoarseness accompanied by sensation of a foreign body in pharynxl in January 2023. A new organism was identified by laryngoscope. On 29 January 2023, the patient underwent a laryngoscopic biopsy and laryngectomy. Pathological examination revealed the presence of moderately differentiated, non-keratinized squamous cell carcinoma in both the supraglottic and glottic regions. Subsequently, on 2 February 2023, the patient underwent a tracheotomy, total laryngectomy with radical neck dissection, and tracheostomy reconstruction under general anesthesia. Postoperative pathology indicated: (a) an invasive squamous cell carcinoma of the larynx, keratinized type, with high-to-moderate differentiation, measuring 3.2 cm × 3 cm × 2 cm, exhibiting infiltration into the muscularis propria and multifocal nerve invasion, but no definitive intravascular tumor thrombus; (b) no malignancy detected in the incisal margins of the epiglottis and cricoid cartilage; (c) absence of cancer metastasis in the examined lymph nodes (0/55) in the right neck; and (d) immunohistochemical analysis demonstrated positive results for EGFR, Ki-67 (approximately 50% labeling index), scattered positivity for P53 (wild-type), and punctate positivity for P16. He was finally diagnosed as invasive squamous cell carcinoma of the larynx (pT2N0M0). On 6 April 2023, the patient commenced radiotherapy with a plan of 60Gy/30f targeting the cervical drainage area of CTV and the tumor bed post-surgery.
3 Diagnosis and treatment
On 11 December 2023, a MRI of nasopharynx and neck was performed due to obvious aggravation of submental and facial edema. MRI revealed new soft tissue mass located on the right side of tongue and the floor of the mouth, raising concerns about tumor recurrence. On 13 December 2023, the first cycle of chemotherapy combined with immunotherapy was performed as below: albumin paclitaxel 400 mg d1, carboplatin 480 mg d1, toripalimab (anti-PD-1 antibody) 240 mg d1, injected every 3 weeks. The biopsy indicated positive outcome of EGFR, on 27 December 2023, the patient received cetuximab at a dose of 700 mg. The next day, the patient developed rash with itching on the face and neck and blistering under the chin, which progressed to generalized rash with bullae after anti-allergic treatment with methylprednisolone 1 mg/kg per day (Figure 1A).
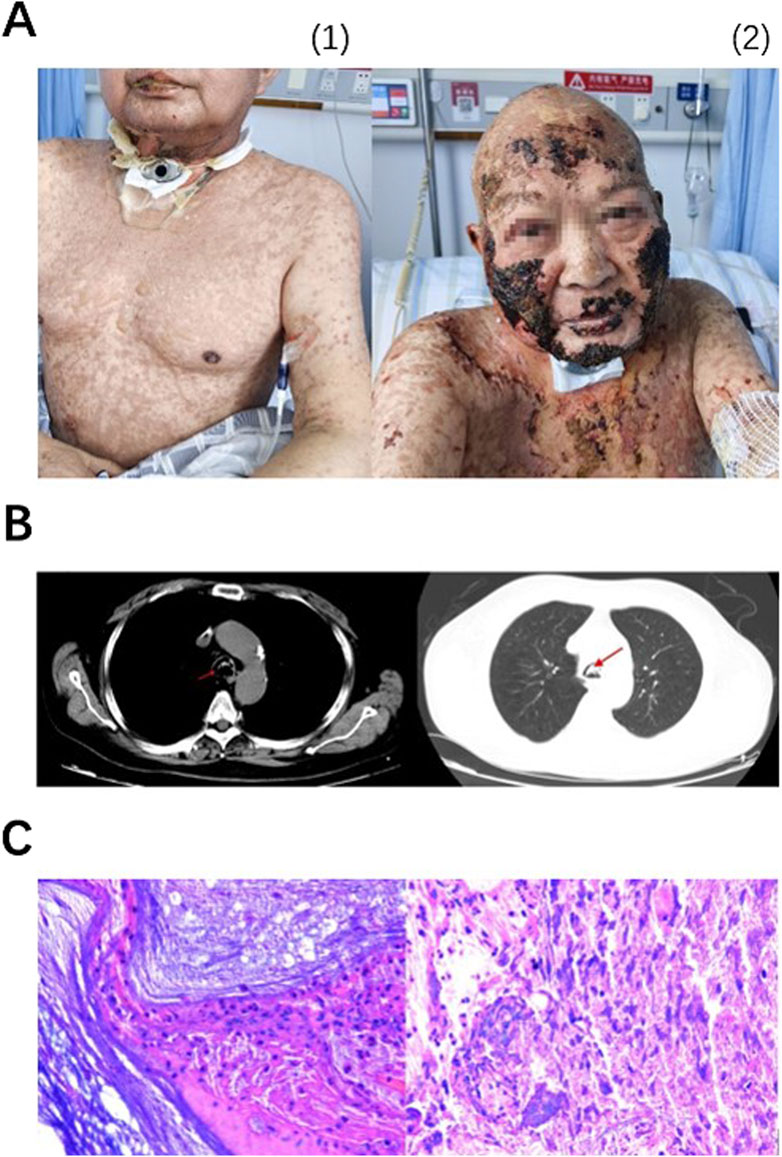
Figure 1. Lesions on skin and lip continued progressing from 3 January 2024 (A1) to 29 January 2024 (A2), appearing first as MPR, bulla and then scabs. On 10 January 2024, emergency CT showed suspicious sputum plugs in the trachea and bilateral bronchi (indicated by arrows), no signs of interstitial pneumonia sighted (B). Pathological examination (× 200) of foreign bodies in the airway revealed extensive infiltration of neutrophils, as well as a small amount of well differentiated, hyperkeratotic, and poorly keratinized squamous epithelial cells. Immunohistochemical results showed Ki-67 (+, low proliferation), P40 (−), and P53 (−) (C).
Due to the patient’s skin lesions, blisters BSA score exceeding 30%, disturbances in water and electrolyte balance. These factors, combined with the patient’s anti-tumor treatment regimen and poor response of anti-inflammatory and anti-allergic treatment, led to the diagnosis of immune-related TEN. On 31 December 2023, new treatment protocol for TEN was initiated. Methylprednisolone 2 mg/kg per day, mycophenolate mofetil capsules 1 g twice per day, intravenous immunoglobulin (IVIG) 20 g per day for 5 days and anti-tumor necrosis factor (TNF) therapy with etanercept 25 mg twice per week were given. The patient’s skin lesions gradually recovered.
On the evening of 10 January 2024, the patient reported experiencing dyspnea. The nurse aspirated sputum but no sputum was found. An emergency chest CT showed a potential sputum embolism in trachea and bilateral bronchioles (Figure 1B). On 11 January 2024, fiberoptic bronchoscopy was performed. Mucosa exfoliation, congestion and edema were observed under fiberoptic bronchoscopy. A large number of long sputum and blood crusts were seen in the main airway and right main bronchus, and the left main bronchus was completely blocked. After the doctor washed the trachea with normal saline, most of the sputum and blood crusts were successfully removed, and the patient’s dyspnea was significantly relieved after operation. The cleared tissues were sent for pathological examination, demonstrating that plenty of neutrophils and a number of T lymphocytes infiltrating and a few squamous epithelial cells of good differentiation. Hyperkeratosis and parakeratosis were observed in airway foreign bodies. Immunohistochemical results showed Ki-67 (+, low proliferation), P40 (−) and P53 (−) (Figure 1C). After that, the scab was removed under bronchoscope for many times. On 10 February 2024, the patient was principally relieved and discharged. The patient experienced G4 irAEs following the administration of immunotherapy in conjunction with chemotherapy. Subsequently, immunotherapy was discontinued, and the patient underwent four cycles of monotherapy utilizing albumin-conjugated paclitaxel in combination with platinum-based agents. Currently, the tumor is under effective control.
4 Discussion
4.1 Diagnosis and treatment of immune-related skin adverse reactions
ICIs are commonly used immunotherapeutic agents in clinical practice. Currently, the most widely used ICIs target PD-1/programmed death-ligand-1 (PD-L1) pathway. The status of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors appears to be declining, while lymphocyte activation gene-3 (LAG-3) inhibitors are progressively gaining prominence. PD-1/PD-L1 pathway inhibits T cell activation, induces lymphocyte apoptosis and sustains autoimmune tolerance. In the tumor microenvironment, tumor cells with PD-L1 overexpression can interact with PD-1 on the surface of lymphocytes, surpressing lymphocyte function and thus evading immune surveillance and destruction. IrAEs in skin and mucosa have been documented to be associated with tumor immunotherapy, among which anti-PD-1 treatment more likely to induce skin-related reactions (Godfrey et al., 2024). Based on the patient’s clinical manifestations, signs, and immunotherapy history, we believe that the G4 mucocutaneous toxicity in this patient is related to the administration of a PD-1 monoclonal antibody.
Adverse events affecting the somatic skin after treatment with ICIs, if clearly drug-related, are collectively categorized as immune-related skin adverse reactions. These reactions encompass a range of conditions including rash, itching, blisters, psoriasis, lichenoid dermatitis, vitiligo, and the most severe forms of epidermal necrosis and exfoliation, including TEN, as illustrated in this case (Chen et al., 2024). Immune-related skin reactions usually occur early in treatment, such as days to weeks after administration, however, instances have also been documented following the cessation of immunotherapy. According to statistics, the median onset time of SJS/TEN is 24 days (Lu et al., 2023). Skin related adverse reactions are the most prevalent adverse reactions of ICIs (Basketter and Kimber, 2018). In addition, irAEs induced by ICIs may involve skin, gastrointestinal tract, liver, lungs, endocrine organs, etc. (Committee of Neoplastic Supportive-Care CA-CA and Cancer Clinical Chemotherapy Committee CA-CA, 2022). Conversely, adverse reactions such as vasculitis (Lee et al., 2024) and systemic sclerosis (Li and Xiong, 2024) are relatively infrequent.
A recent study utilizing the FDA Adverse Reaction Reporting System (Satoh et al., 2024) revealed that PD-1 inhibitors represent the predominant immunotherapies associated with the induction of SJS/TEN induction.(58.9%), followed by PD-1 inhibitors combined with CTLA-4 inhibitors (11.6%). Compared to conventional anticancer drugs, PD-L1 inhibitors ICIs were associated with increased mortality due to immune-related severe cutaneous adverse reactions (SCARs), with SJS 28.5% vs. 24.5% and TEN 55.3% vs. 46%. The association between SCARs and ICIs was independent of tumor status. The latest data show a general decline in the incidence of fatal irAEs after 2020, with the exception of muscle weakness and serious skin adverse reactions (Gougis et al., 2024). The occurrence of irAEs often indicates a good response to immunotherapy. However, its relationship to prognosis remains unclear. For now, the association between the occurrence of vitiligo and better prognosis is only identified in patients with melanoma (Cybulska-Stopa et al., 2022). In the context of other solid tumors, no reliable evidence linking cutaneous adverse reactions and the efficacy of ICIs has been found.
4.2 Immune-related TEN involving the trachea is rare
This case suggests that immune related TEN may not be limited to the epidermis but can also affect the tracheal mucosa, leading to inflammatory edema, necrosis, and even detachment of the mucosa, which may block the airway and ultimately lead to dyspnea.
IrAEs that lead to respiratory symptoms are usually immune related pneumonia rather than skin mucosal reactions. Common clinical manifestations of immune related pneumonia include dyspnea, coughing, fever, and hypoxia, which can also be asymptomatic. Radiological findings often reveal obvious lung changes such as acute interstitial pneumonia, cryptogenic tissue pneumonia, hypersensitive pneumonia, or non-specific interstitial pneumonia (Castanon, 2016). Compared to infectious pneumonia, fever caused by immune related pneumonia is relatively rare, but it is more prone to respiratory failure and may also be accompanied by other irAEs. Currently, there are no definitive biomarkers available to differentiate between infectious pneumonia from immune pneumonia, and both of them can exist simultaneously on the same patient (Infectiology Group et al., 2021). In addition, a considerable portion of respiratory distress caused by immunotherapy reported in recent years has also been linked to cardiotoxicity (Tajmir-Riahi et al., 2018).
Mechanical dyspnea resulting from immune-related skin toxicity involving trachea is rare. To date there barely exists report of immune-related SJS/TEN involving trachea and bronchial mucosa. CT examination of this patient after sudden dyspnea showed tracheal and bronchial obstruction. This obstruction was obviously alleviated after tracheal lavarage and blood scab removal via fiberoptic bronchoscopy. These findings suggest that it was airway obstruction rather than pneumonia that caused dyspnea. Histopathological analysis of the biopsy further indicated that the primary constiuant of obstruction was not sputum commonly seen, but a combination of bronchial mucosal fragments, bronchial secretions and blood clots. This suggests that the underlying pathology was acute inflammation involving tracheal mucosa resulting in mucosal hemorrhage, necrosis, exfoliation. This is distinct from the usual respiratory distress caused by direct lung damage from immune-mediated pneumonia.
4.3 Pathogenesis of drug-related SJS/TEN
SJS and TEN are classified as immune-mediated type IV hypersensitivity reactions, predominantly involving CD8+ T lymphocytes. CD8+ T cells have been identified as important mediators of blister formation (Chuenwipasakul et al., 2023). Earlier observations of TEN patients revealed a pan-T cell decrease at the focal site, characterized by infiltration of CD8+ T cells and a decrease in CD4+ T cells (Correia et al., 1993; Roujeau et al., 1985). There is also a negative correlation between dermal infiltration of CD8+ T cells and severity of acute ocular complications, alongside a positive correlation with serum interferon-γ (IFN-γ) levels (Chuenwipasakul et al., 2023). A seperate meta-analysis identified a distinct light distribution of rash in SJS/TEN patients and suggested that this may be related to photoactive drugs and UV exposure (McKinley et al., 2023). It has been posited that aberrant drug metabolism in patients (such as failure to remove active metabolites) induces T cell-mediated cytotoxic responses to drug antigens in keratinocytes, such as allopurinol (ALP) and its metabolite oxypurinol (OXP) (Mifsud et al., 2023). CD4+ T cells, like CD8+ T cells, secrete granolysin in SJS/TEN induced by carbamazepine (CBZ) under drug stimulation to mediate disease development (Jaruthamsophon et al., 2023).
Certain chemotherapy agents have been implicated in the induction of SJS/TEN symptoms themselves. For instance, methotrexate is known to cause toxic skin ulcers, or may manifest as severe phenotypes similar to SJS/TEN (Wang et al., 2023). The combination of carboplatin and Paclitaxel has also been reported to induce SJS in 2 cases (Gokulanathan et al., 2023). Findings of chemotherapy-induced skin adverse reactions local biopsy results can be similar to those described above. These reactions are classified as drug eruptions resulting from pharmacological agents, frequently accompanied by eosinophilia and systemic manifestations, and are also referred to as drug-induced hypersensitivity syndrome (DIHS) (Hung et al., 2024). At present, mechanisms by which chemical agents induce skin toxicity through T cells include: (a) chemical reactive compounds (i.e., drugs or their metabolites) covalently bind to their own proteins to form complexes as new antigens (Basketter and Kimber, 2018); (b) Drugs bind non-covalently to immune receptor proteins such as major histocompatibility complex (MHC) or T cell receptor (TCR) to activate the response (Pichler, 2008); (c) drugs penetrate into the interior of human leukocyte antigen B (HLA-B) molecule to change its structural specificity and thus change the antigen type presented (Ostrov et al., 2012).
Clinical and histopathological features of SJS/TEN induced by ICIs are similar to SJS/TEN induced by other drugs (Watanabe and Yamaguchi, 2023). CD8+ T cells are recognized as significant contributors to immunotherapy-related skin adverse reactions. Notably, PD-1/PD-L1 antibodies are of lower risk of maculopapular rashes (MPR) compared to CTLA-4 antibodies (Bottlaender et al., 2020; Curry et al., 2017; Minkis et al., 2013). For PD-1/PD-L1 inhibitor therapy, cross-immunity of CD8+ T cells to common antigens in skin and tumor tissues may be one of the causes of SJS/TEN, such as BP180, an anchor filament component involved in dermal-epidermal junction (Berner et al., 2019). While PD-L1 expression is generally low in skin keratinocytes, CD8+ T cell infiltration and PD-L1 expression have also been reported in glandular and basal skin biopsies from focal lesions in patients experiencing TEN associated with tumor immunotherapy (Li et al., 2023a; Vivar et al., 2017). Furthermore, PD-L1 expression levels are responsively up-regulated after ICIs treatments (Chen et al., 2018). The involvement of PD-L1 in preserving the integrity of dermal-epidermal junction and in mitigating the progression of TEN has been substantiated through additional murine studies (Goldinger et al., 2016; Okiyama and Katz, 2014).
Furthermore, PD-1 inhibitors have been observed to elicit cutaneous reactions characterized by gene expression profiles that resemble those of SJS and TEN, yet they differ from the profiles associated with acute skin graft-versus-host disease or maculopapular rash. The former consistently demonstrates the expression of major inflammatory chemokine-related proteins, including CXCL9, CXCL10 and CXCL11, as well as cytotoxic mediators such as Pore-Forming Protein 1 (PRF1) and granzyme B (GZMB), alongside a upregulation of pro-apoptotic molecules FAS ligand (FASLG) (Okiyama and Katz, 2014). The chemokine CXCL family mediates neutrophil migration to specific sites (Capucetti et al., 2020). This observation aligns with the pathological findings reported in previous case studies involving sputum and blood scabs. In instances of fatal cases from immune-related pneumonia, an increase in CD16+ T cells, CD57+CD8+ T cells expressing immune checkpoints (TIGIT, LAG3, TIM-3, PD-1), FCRL5+ B cells, and CCR2+CCR5+CD14+ monocytes (Yanagihara et al., 2024). These findings imply that the molecular mechanisms of SJS/TEN differ from that of immune-associated pneumonia. At present, the potential mechanisms of SJS/TEN induced by immunotherapy include: (a) excessive activation of T lymphocytes; (b) increase of inflammatory factors (such as TNF-α); (c) exposure of cross antigens (Basketter and Kimber, 2018).
4.4 Treatment of immune related SJS/TEN
According to the guidelines from National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology, corticosteroids are the most mainstream treatment for irAEs. However, approximately 10% of cases remain hormone non-responsive (i.e., hormone-refractory irAEs) and irAEs cannot be reduced (i.e., hormone-dependent irAEs) (Ruf et al., 2024). Given that the pathophysiology of irAEs extends beyond T cell immunotoxicity, it is imperative to consider adjunctive therapies in conjunction with systemic hormone administration for cases that are severe, refractory, or involve multiple organ systems. Such therapies may include immunosuppressive combinations, such as mycophenolate mofetil, cyclosporine, various novel monoclonal antibodies, intravenous immunoglobulin (IVIG), and/or plasma exchange therapy (Saw et al., 2017). There is still a lack of robust evidence for the effects of nonsteroidal anti-inflammatory drugs. Studies have shown that the administration of aspirin can effectively reduce the incidence of rash and SJS in patients with ICIs. However, this benefit is accompanied by an increased risk of adverse effects, including anemia, colitis, myocarditis, pneumonia, and other related conditions (Yang et al., 2022).
Studies have shown that patients with bullous dermatitis exhibit elevated levels of TNF - α expression in their serum or vesicular fluid (Stewart et al., 2024), Consequently, anti-TNF therapies can be considered an option, such as Infliximab (Kachi et al., 2024), etanercept (Ornstein et al., 2019). Infliximab and etanercept are both commonly used in anti-TNF therapies, with some differences in their mechanisms of action. Infliximab targets TNF-α, while etanercept competitively inhibits the binding of both TNF-α and TNF-β to cell surface TNF receptors. The Hepatotoxicity of Infliximab makes it unsuitable for treatment of autoimmune hepatitis (Jacobsen et al., 2022). Though etanercept is prohibited in patients with severe infections, there is still reports on its efficacy in patients suffering from open pulmonary tuberculosis, septicemia and fungal sepsis (Peng et al., 2024). In this case, we chose etanercept as the anti-TNF-α agent, yielding favorable outcomes.
Through a comprehensive review of literature, we have compiled some SJS/TEN case reports related to ICIs published in the past 10 years (Table 1). Each case report emphasizes the necessity of immediate cessation of the implicated medications, indispensable supportive therapy, systemic use of glucocorticoids, and the addition of immunosuppressants and/or IVIG depending on conditions. Due to the potential high lethality of SJS/TEN, clinical doctors must closely observe the patient’s condition when using ICIs to identify potential adverse reactions as early as possible. Timely and appropriate medical interventions are crucial in managing these serious conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Wuhan University People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MZ: Writing–original draft. YF: Writing–original draft. YS: Writing–review and editing. XG: Writing–review and editing. JW: Writing–review and editing. BZ: Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant No. 82272928), CSCO-BMS Cancer Immunotherapy Research Foundation (Grant No. Y-BMS2019-003), Knowledge Innovation Program of Wuhan-Basic Research (Grant No. 2022020801010475), Hubei Provincial Top Talent Project of Medical Youth (Grant No. EWT2019-48) and Xiangyang Science and Technology Research and Development Project (Grant No. 2022YL39A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PD-1, Programmed cell death protein-1; PD-L1, Programmed death-ligand-1; TEN, Toxic epidermal necrolysis; ICIs, Immune checkpoint inhibitors; irAEs, Immune-related adverse effects; BSA, Body surface area; SJS, Stevens-Johnson syndrome; EGFR, Epidermal growth factor receptor; IVIG, Intravenous immunoglobulin; TNF, Tumor necrosis factor; CTLA-4, Cytotoxic T-lymphocyte-associated protein 4; LAG-3, Lymphocyte activation gene-3; SCARs, Severe cutaneous adverse reactions; IFN-γ, Interferon-γ; ALP, Allopurinol; OXP, Oxypurinol; CBZ, Carbamazepine; DIHS, Drug-induced hypersensitivity syndrome; MHC, Major histocompatibility complex; TCR, T cell receptor; HLA-B, Human leukocyte antigen B; MPR, Maculopapular rashes; CXCL, C-X-C motif chemokine ligand; PRF1, Pore-forming protein 1; GZMB, Granzyme B; FASLG, FAS ligand; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIM-3, T cell immunoglobulin-3; FCRL5, Fc receptor-like protein 5; CCR, C-C chemokine receptor.
References
Basketter, D. A., and Kimber, I. (2018). Are skin sensitisation test methods relevant for proteins? Regul. Toxicol. Pharmacol. 99, 244–248. doi:10.1016/j.yrtph.2018.09.028
Berner, F., Bomze, D., Diem, S., Ali, O. H., Fassler, M., Ring, S., et al. (2019). Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5 (7), 1043–1047. doi:10.1001/jamaoncol.2019.0402
Bottlaender, L., Amini-Adle, M., Maucort-Boulch, D., Robinson, P., Thomas, L., and Dalle, S. (2020). Cutaneous adverse events: a predictor of tumour response under anti-PD-1 therapy for metastatic melanoma, a cohort analysis of 189 patients. J. Eur. Acad. Dermatol Venereol. 34 (9), 2096–2105. doi:10.1111/jdv.16311
Capucetti, A., Albano, F., and Bonecchi, R. (2020). Multiple roles for chemokines in neutrophil biology. Front. Immunol. 11, 1259. doi:10.3389/fimmu.2020.01259
Castanon, E. (2016). Anti-PD1-Induced pneumonitis: capturing the hidden enemy. Clin. Cancer Res. 22 (24), 5956–5958. doi:10.1158/1078-0432.CCR-16-2033
Chen, C. B., Wu, M. Y., Ng, C. Y., Lu, C. W., Wu, J., Kao, P. H., et al. (2018). Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 10, 1259–1273. doi:10.2147/CMAR.S163391
Chen, S. T., Semenov, Y. R., Alloo, A., Bach, D. Q., Betof Warner, A., Bougrine, A., et al. (2024). Defining D-irAEs: consensus-based disease definitions for the diagnosis of dermatologic adverse events from immune checkpoint inhibitor therapy. J. Immunother. Cancer 12 (4), e007675. doi:10.1136/jitc-2023-007675
Chuenwipasakul, D., Washrawirul, C., Panpruk, R., Wititsuwannakul, J., Charoenchaipiyakul, K., Buranapraditkun, S., et al. (2023). Correlations between histopathologic findings, serum biomarker levels, and clinical outcomes in Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Sci. Rep. 13 (1), 13620. doi:10.1038/s41598-023-40812-3
Collins, L. K., Chapman, M. S., Carter, J. B., and Samie, F. H. (2017). Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 41 (2), 125–128. doi:10.1016/j.currproblcancer.2016.12.001
Committee of Neoplastic Supportive-CareCancer Clinical Chemotherapy Committee (2022). Chinese expert consensus on diagnosis and treatment of neurologic immune-related adverse events associated with immune checkpoint inhibitors (2022 edition). Zhonghua Zhong Liu Za Zhi 44 (9), 935–941. doi:10.3760/cma.j.cn112152-20220213-00098
Correia, O., Delgado, L., Ramos, J. P., Resende, C., and Torrinha, J. A. (1993). Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement. Archives dermatology 129 (4), 466–468. doi:10.1001/archderm.129.4.466
Curry, J. L., Tetzlaff, M. T., Nagarajan, P., Drucker, C., Diab, A., Hymes, S. R., et al. (2017). Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J. Cutan. Pathol. 44 (2), 158–176. doi:10.1111/cup.12858
Cybulska-Stopa, B., Zietek, M., Czarnecka, A. M., Piejko, K., Dziura, R., Galus, L., et al. (2022). Development of immunity-related adverse events correlates with baseline clinical factors, survival and response to anti-PD-1 treatment in patients with inoperable or metastatic melanoma. J. Dermatol. Treat. 33 (4), 2168–2174. doi:10.1080/09546634.2021.1937477
Godfrey, H., Jedlowski, P., and Thiede, R. (2024). Severe cutaneous adverse reactions associated with the immune checkpoint inhibitors: a case/non-case analysis using the Food and Drug Administration Adverse Event Reporting System. Australas. J. Dermatol 65 (3), 243–253. doi:10.1111/ajd.14262
Gokulanathan, N., Jagadesan, P. R. C., Nadeem, N. S. Y. S., and Y Sree, S. (2023). A diagnostic quandary: carboplatin-paclitaxel-induced stevens-johnson syndrome in a rare case of carcinosarcoma of the esophagus and review of the literature. Cureus 15 (10), e47457. doi:10.7759/cureus.47457
Goldinger, S. M., Stieger, P., Meier, B., Micaletto, S., Contassot, E., French, L. E., et al. (2016). Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin. Cancer Res. 22 (16), 4023–4029. doi:10.1158/1078-0432.CCR-15-2872
Gougis, P., Jochum, F., Abbar, B., Dumas, E., Bihan, K., Lebrun-Vignes, B., et al. (2024). Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. EClinicalMedicine 70, 102536. doi:10.1016/j.eclinm.2024.102536
Hung, S. I., Mockenhaupt, M., Blumenthal, K. G., Abe, R., Ueta, M., Ingen-Housz-Oro, S., et al. (2024). Severe cutaneous adverse reactions. Nat. Rev. Dis. Prim. 10 (1), 30. doi:10.1038/s41572-024-00514-0
Infectiology GroupLymphocytic Disease GroupAnti-lymphoma Alliance (2021). Chinese expert consensus on prevention and treatment of immunotherapeutic and molecular targeted agents-related infections in patients with hematological malignancies (2021 version). Zhonghua Xue Ye Xue Za Zhi 42 (9), 717–727. doi:10.3760/cma.j.issn.0253-2727.2021.09.002
Jacobsen, A., Olabi, B., Langley, A., Beecker, J., Mutter, E., Shelley, A., et al. (2022). Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst. Rev. 3 (3), CD013130. doi:10.1002/14651858.CD013130.pub2
Jaruthamsophon, K., Thomson, P. J., Hammond, S., Zhang, E., Alfirevic, A., Sukasem, C., et al. (2023). Characterization of drug-specific CD4(+) T-cells reveals possible roles of HLA class II in the pathogenesis of carbamazepine hypersensitivity reactions. Chem. Res. Toxicol. 36 (5), 757–768. doi:10.1021/acs.chemrestox.2c00414
Kachi, S., Sumitomo, S., Oka, H., Hata, A., and Ohmura, K. (2024). Case report: inflammatory sternoclavicular joint arthritis induced by an immune checkpoint inhibitor with remarkable responsiveness to infliximab. Front. Immunol. 15, 1400097. doi:10.3389/fimmu.2024.1400097
Kumar, S., Nongkynrih, A., Dey, B., Jagtap, V., Lamba, R., and Chyrmang, D. (2024). Capecitabine and oxaliplatin induced Steven-Johnson syndrome following nivolumab in a patient of metastatic esophageal carcinoma. Int. Cancer Conf. J. 13 (2), 167–170. doi:10.1007/s13691-024-00660-y
Lee, C. M., Wang, M., Rajkumar, A., Calabrese, C., and Calabrese, L. (2024). A scoping review of vasculitis as an immune-related adverse event from checkpoint inhibitor therapy of cancer: unraveling the complexities at the intersection of immunology and vascular pathology. Semin. Arthritis Rheum. 66, 152440. doi:10.1016/j.semarthrit.2024.152440
Li, D. H., and Xiong, X. Z. (2024). Immune checkpoint inhibitor-associated systemic sclerosis in the treatment of a small cell lung cancer patient with durvalumab: a case report. Clin. Cosmet. Investig. Dermatol 17, 663–669. doi:10.2147/CCID.S451386
Li, X., Lei, Y., Liu, J., Lin, H., Chen, K., Yin, F., et al. (2023a). Case report: a successful treatment with immune checkpoint inhibitors was associated with severe dermatologic toxicities in a patient with double primary malignancies. Discov. Oncol. 14 (1), 146. doi:10.1007/s12672-023-00749-5
Li, X., Li, G., Chen, D., Su, L., Wang, R. P., and Zhou, Y. (2023b). Case Report: sintilimab-induced Stevens-Johnson Syndrome in a patient with advanced lung adenocarcinoma. Front. Oncol. 13, 912168. doi:10.3389/fonc.2023.912168
Logan, I. T., Zaman, S., Hussein, L., and Perrett, C. M. (2020). Combination therapy of ipilimumab and nivolumab-associated toxic epidermal necrolysis (TEN) in a patient with metastatic melanoma: a case report and literature review. J. Immunother. (Hagerstown, Md 1997) 43 (3), 89–92. doi:10.1097/CJI.0000000000000302
Lu, W., Zhang, H., Guo, Q., Gou, Z., and Yao, J. (2023). Selected cutaneous adverse events in patients treated with ICI monotherapy and combination therapy: a retrospective pharmacovigilance study and meta-analysis. Front. Pharmacol. 14, 1076473. doi:10.3389/fphar.2023.1076473
McKinley, B. J., Allen, M. E., and Michels, N. (2023). Photodistributed Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and proposal for a new diagnostic classification. Eur. J. Med. Res. 28 (1), 188. doi:10.1186/s40001-023-01142-2
Mifsud, N. A., Illing, P. T., Ho, R., Tuomisto, J. E., Fettke, H., Mullan, K. A., et al. (2023). The allopurinol metabolite, oxypurinol, drives oligoclonal expansions of drug-reactive T cells in resolved hypersensitivity cases and drug-naive healthy donors. Allergy 78 (11), 2980–2993. doi:10.1111/all.15814
Minkis, K., Garden, B. C., Wu, S., Pulitzer, M. P., and Lacouture, M. E. (2013). The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J. Am. Acad. Dermatol 69 (3), e121–e128. doi:10.1016/j.jaad.2012.12.963
Okiyama, N., and Katz, S. I. (2014). Programmed cell death 1 (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J. Autoimmun. 53, 1–9. doi:10.1016/j.jaut.2014.06.005
Ornstein, M. C., Calabrese, C., Wood, L. S., Kirchner, E., Profusek, P., Allman, K. D., et al. (2019). Myalgia and arthralgia immune-related adverse events (irAEs) in patients with genitourinary malignancies treated with immune checkpoint inhibitors. Clin. Genitourin. cancer 17 (3), 177–182. doi:10.1016/j.clgc.2019.01.021
Ostrov, D. A., Grant, B. J., Pompeu, Y. A., Sidney, J., Harndahl, M., Southwood, S., et al. (2012). Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. U. S. A. 109 (25), 9959–9964. doi:10.1073/pnas.1207934109
Peng, Y. T., Xiong, J. X., Wei, B., Li, H., Xu, J., Chen, A. J., et al. (2024). Tumor necrosis factor-α inhibitor for successful treatment of toxic epidermal necrolysis with severe infection: a case series. J. Int. Med. Res. 52 (1), 3000605231223059. doi:10.1177/03000605231223059
Pichler, W. J. (2008). The p-i concept: pharmacological interaction of drugs with immune receptors. World Allergy Organ J. 1 (6), 96–102. doi:10.1097/WOX.0b013e3181778282
Rached, L., Laparra, A., Sakkal, M., Danlos, F. X., Barlesi, F., Carbonnel, F., et al. (2024). Toxicity of immunotherapy combinations with chemotherapy across tumor indications: current knowledge and practical recommendations. Cancer Treat. Rev. 127, 102751. doi:10.1016/j.ctrv.2024.102751
Roujeau, J. C., Moritz, S., Guillaume, J. C., Bombal, C., Revuz, J., Weil, B., et al. (1985). Lymphopenia and abnormal balance of T-lymphocyte subpopulations in toxic epidermal necrolysis. Arch. Dermatol Res. 277 (1), 24–27. doi:10.1007/BF00406477
Ruf, T., Kramer, R., Forschner, A., Leiter, U., Meier, F., Reinhardt, L., et al. (2024). Second-line therapies for steroid-refractory immune-related adverse events in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 203, 114028. doi:10.1016/j.ejca.2024.114028
Satoh, T. K., Neulinger, M. M., Stadler, P. C., Aoki, R., and French, L. E. (2024). Immune checkpoint inhibitor-induced epidermal necrolysis: a narrative review evaluating demographics, clinical features, and culprit medications. J. Dermatol 51 (1), 3–11. doi:10.1111/1346-8138.17039
Saw, S., Lee, H. Y., and Ng, Q. S. (2017). Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur. J. Cancer 81, 237–239. doi:10.1016/j.ejca.2017.03.026
Stewart, T. J., Farrell, J., and Frew, J. W. (2024). A systematic review of case-control studies of cytokines in blister fluid and skin tissue of patients with Stevens Johnson syndrome and toxic epidermal necrolysis. Australas. J. Dermatol 65, 491–504. doi:10.1111/ajd.14329
Tajmir-Riahi, A., Bergmann, T., Schmid, M., Agaimy, A., Schuler, G., and Heinzerling, L. (2018). Life-threatening autoimmune cardiomyopathy reproducibly induced in a patient by checkpoint inhibitor therapy. J. Immunother. (Hagerstown, Md 1997) 41 (1), 35–38. doi:10.1097/CJI.0000000000000190
Vivar, K. L., Deschaine, M., Messina, J., Divine, J. M., Rabionet, A., Patel, N., et al. (2017). Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 44 (4), 381–384. doi:10.1111/cup.12876
Wang, J., Zhang, B., Peng, L., Liu, X., Sun, J., Su, C., et al. (2023). Chinese expert consensus recommendations for the administration of immune checkpoint inhibitors to special cancer patient populations. Ther. Adv. Med. Oncol. 15, 17588359231187205. doi:10.1177/17588359231187205
Wang, Y., Zhou, S., Yang, F., Qi, X., Wang, X., Guan, X., et al. (2019). Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 5 (7), 1008–1019. doi:10.1001/jamaoncol.2019.0393
Watanabe, T., and Yamaguchi, Y. (2023). Cutaneous manifestations associated with immune checkpoint inhibitors. Front. Immunol. 14, 1071983. doi:10.3389/fimmu.2023.1071983
Yanagihara, T., Hata, K., Matsubara, K., Kunimura, K., Suzuki, K., Tsubouchi, K., et al. (2024). Exploratory mass cytometry analysis reveals immunophenotypes of cancer treatment-related pneumonitis. Elife 12. doi:10.7554/eLife.87288
Keywords: PD-1, PD-L1, immunotherapy, immune-related adverse events, toxic epidermal necrolysis, fiberoptic bronchoscopy
Citation: Zhang M, Fu Y, Song Y, Gao X, Wang J and Zhang B (2024) Immune-related toxic epidermal necrolysis affecting trachea mucosal epithelium: a case report and literature review. Front. Pharmacol. 15:1454015. doi: 10.3389/fphar.2024.1454015
Received: 24 June 2024; Accepted: 09 October 2024;
Published: 18 October 2024.
Edited by:
Zhenhua Chen, Jinzhou Medical University, ChinaCopyright © 2024 Zhang, Fu, Song, Gao, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bicheng Zhang, YmljaGVuZ3poYW5nQGhvdG1haWwuY29t
 Mingbo Zhang
Mingbo Zhang Yang Fu2
Yang Fu2 Yuxiao Song
Yuxiao Song Jun Wang
Jun Wang Bicheng Zhang
Bicheng Zhang