- 1Nursing Department, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Health Management Center, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Radiation Oncology Center, Chongqing University Cancer Hospital, Chongqing, China
Background: Immune-related adverse events (irAEs) typically occur within 3 months of initiating immune-checkpoint inhibitors (ICIs), which has been extensively documented. But the clinical profiles of late-onset irAEs remain inadequately characterized. Therefore, this study aims to quantify the correlation between delayed irAEs and ICIs, and to delineate the profiles of delayed toxicities associated with ICIs using data from the Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: Data from the January 2011 to December 2023 in FAERS database were extracted. Four signal detection indices, reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN) and multi-item gamma Poisson shrinker (MGPS), were employed to evaluate the associations between ICIs and delayed irAEs.
Results: A total of 147,854 cases were included in this study, of which 3,738 cases related to delayed irAEs were identified. Generally, 8 signals at System Organ Class (SOC) level were found to be associated with ICIs. Males had a slightly higher reporting frequencies for respiratory disorders (ROR975 = 0.95) and blood and lymphatic system disorders (ROR025 = 1.22), but lower reporting frequencies for immune system disorders (ROR025 = 1.16). Three monotherapy (anti-PD-1, anti-PD-L1 and anti-CTLA-4) were all associated with significant increasing gastrointestinal disorders (ROR025 = 1.66, 1.16, 1.99) and metabolism disorders (ROR025 = 2.26, 1.74, 3.13). Anti-PD-1 therapy exhibited higher rates of respiratory toxicities (ROR025 = 1.46 versus 0.82) and skin toxicities (ROR025 = 1.27 versus 0.94) compared with anti-CTLA-4 therapy. At PT levels, pneumonitis (ROR025: from 11.85 to 29.27) and colitis (ROR025: from 2.11 to 24.84) were the most notable PT signals associated with all three ICI regimens. For outcomes of delayed irAEs, gastrointestinal disorders showed the highest proportion (51.06%) of death.
Conclusion: Our pharmacovigilance analysis indicates that a small percentage of patients receiving ICIs therapy experience delayed irAEs, which are challenging to manage and may result in severe consequences. Prompt identification and intervention of these delayed irAEs are crucial in clinical practice.
1 Introduction
Immune-checkpoint inhibitors (ICIs) have become a key component in the field of cancer therapy, allowing for the potential of long-term survival in patients with challenging malignant tumors, and offering new therapeutic options in (neo)adjuvant and maintenance settings (Johnson et al., 2022). The most widely used targets of ICIs include cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) (Martins et al., 2019). Since the approval of the first ICI, ipilimumab, for metastatic melanoma by FDA in 2011, a total of more than 20 different malignancies worldwide have been treated with ICIs (Yin et al., 2023).
One distinguishing feature of ICIs, unlike conventional cancer therapeutic agents, is the potential for sustained responses, even in patients with metastatic solid tumors. However, by inhibiting CTLA4, PD-1 or PD-L1 checkpoints, ICIs can also lead to autoimmune effects known as immune-related adverse events (irAEs) (Singh et al., 2023). While most irAEs occur within the first 3 months of starting immunotherapy, they can also arise at any point during treatment or even months after treatment cessation (Weber et al., 2017). Early irAEs, occurring within 3 months, have been extensively studied (Tao et al., 2023; Zhang et al., 2023). However, the delayed irAEs, defined as those appearing more than 1 year after starting ICIs, have not yet been systematically investigated. Actually, real world data have demonstrated that delayed irAEs may be more frequent in long-term responsers to ICIs and can differ in severity and spectrum from early irAEs. Research by Owen et al. (2021) revealed that 118 melanoma patients treated with ICIs for over 12 months experienced a total of 140 delayed irAEs, with an estimated incidence of 5.3%. The most frequent delayed irAEs included colitis (22%), rash (18%) and pneumonitis (13%). These delayed irAEs are often more severe, distinct from early-onset irAEs, challenging to manage and can be fatal. However, the frequency of delayed irAEs after discontinuing ICIs treatment in a larger patient population, as well as the duration of increased risk for irAEs following immunotherapy cessation, remains unknown.
As the indications of ICIs expands in clinical practice, more patients will be exposed to immunotherapy, potentially leading to life-threatening delayed irAEs. Therefore, it is critical to gather accurate and comprehensive data on the incidence, clinical manifestations, and prognosis of the delayed irAEs from a large patient population. The Food and Drug Administration Adverse Event Reporting System (FAERS) is one of the largest pharmacovigilance databases providing valuable source of real-world data on adverse event, including reports from healthcare professionals, individual patients and drug manufacturers (Zhou et al., 2023). In this study, we aim to analyze the frequency, spectrum and outcomes of the delayed irAEs using FAERS data to enhance our understanding of the safety profiles of ICIs.
2 Materials and methods
2.1 Study design and data sources
We conducted a pharmacovigilance study on delayed irAEs based on data from the FAERS database spanning from the first quarter of 2011 to the fourth quarter of 2023. The FAERS database includes the following eight types of files: demographic information (DEMO), drug information (DRUG), indications for use (INDI), start and end dates for reported drugs (THER), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), and invalid reports (DELETED). Keywords used included immune checkpoint inhibitors such as anti-CTLA-4 agents (ipilimumab and tremelimumab), anti-PD-1 agents (nivolumab, pembrolizumab and cemiplimab), and anti-PD-L1 agents (atezolizumab, avelumab and durvalumab). AEs in the FAERS database were coded using preferred terms (PTs) according to the Medical Dictionary for Regulatory Activities (MedDRA) (version 26.1), which is logically structured to contain five levels. PTs are unique descriptors of a single medical concept, such as signs and symptoms and disease diagnosis. A specific PT can be assigned to several high-level terms (HLTs), high-level group terms (HLGTs), and system organ classes (SOCs), which are grouped by aetiology, site of presentation, or purpose. In this study, irAEs were identified using pre-specified list of PTs based on the Society for Immunotherapy of Cancer (SITC), (American Society of Clinical Oncology) ASCO, (European Society for Medical Oncology) ESMO and (National Comprehensive Cancer Network) NCCN guideline/consensus. The PTs of irAEs included in this study are provided in Supplementary Table S1. Cases were defined as a serious medical event if one or more of the following outcomes were reported: death, life-threatening event, hospitalization, disability, congenital anomaly, or other serious medical events.
2.2 Data processing procedure
Variables such as Case Identification (CASEID), age, sex, event date, drug names, and outcomes were extracted in each report. Data cleaning was performed prior to analysis with duplicate records removed based on FDA’s recommended method selecting the latest FDA_DT if the CASEID was the same, and choosing the higher PRIMARYID if the CASEID and FDA_DT were the same. In cases where a single patient had multiple reports, the most recent case was retained on the “latest FDA data received to date”. Additionally, the time to onset of irAEs associated with ICIs was calculated as the interval between therapy start date (START_DT) and event onset date (EVENT_DT). Delayed irAEs in this study defined as those with onset >1 year after the initiation of ICIs. Reports were excluded when the START_DT was later than the EVENT_DT or when the report lacked a START_DT or EVENT_DT.
2.3 Statistical analysis
We conducted a comprehensive descriptive analysis of the clinical attributes of reports detailing delayed irAEs post-screening, encompassing variables such as gender, age, reporting year, reporting country, clinical outcomes, indication, treatment strategy, and additional clinical characteristics. Adisproportionality analysis was utilized to compare the proportion of specific AEs caused by the target drugs with the proportion of the same AEs in the full database (Zhou et al., 2023). In our study, all drugs in the database were selected as comparisons for the disproportionality approach. Based on the two-by-two contingency table, reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN) and multi-item gamma Poisson shrinker (MGPS) were employed to detect an association between various ICI regimens and adverse events in accordance with the disproportionality analysis. The criteria of a significant signal was identified by the 95% confidence interval lower end for ROR (ROR025), PRR (PRR025), IC (IC025) and EBGM (EBGM05) (Hauben et al., 2005; Noren et al., 2006; Guo et al., 2022; Jiang et al., 2022). A signal was considered significant if ROR025 was greater than 1 with at least 3 cases, PRR value was greater than 2 and Chi-Square was greater than 4, IC025 was greater than 0 and EBGM05 was greater than 2. Shrinkage transformation was applied to reduce false-negative adverse signals. The equations for the above four algorithms are shown in Supplementary Tables S2, S3. The formula is as follows:
Data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States) and Microsoft Office Excel version 2023 (Microsoft Corp., Redmond, WA, United States).
3 Results
3.1 Descriptive analysis
In this study, a total of 17,854,647 cases were extracted from the FAERS database from 2011 to 2023 (Figure 1). After excluding duplicates, the number of cases was 15,245,964, among which 147,854 cases were associated with ICI-related AEs. Additionally, 3,738 cases were found to be associated with delayed irAEs, while 144,116 cases were related to early irAEs following exposure to ICIs.
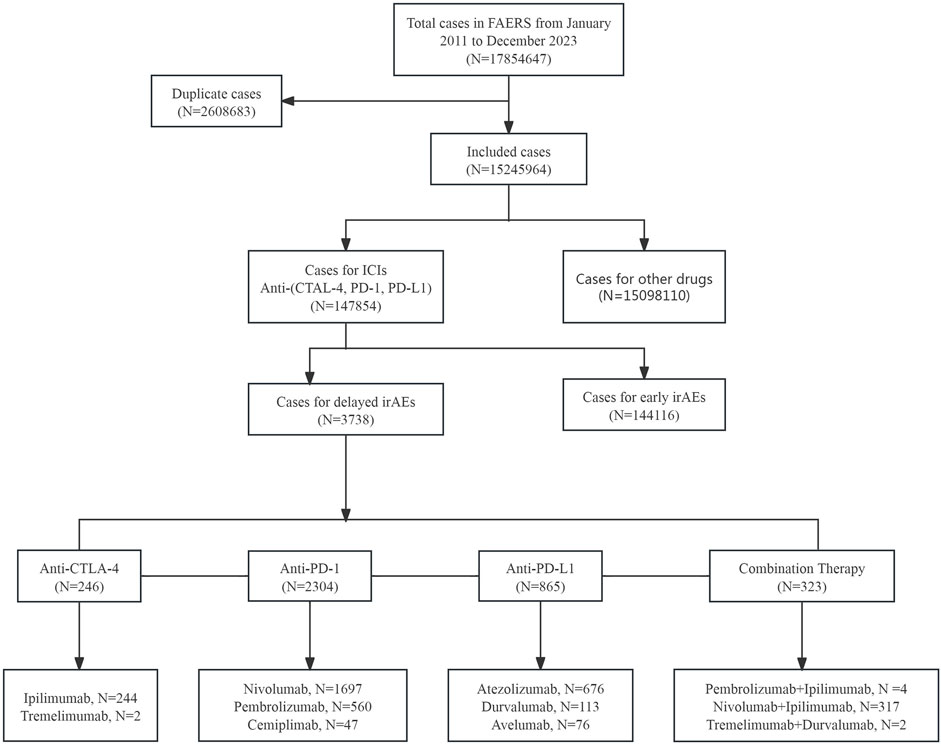
Figure 1. The process of data acquisition and data cleaning from FDA adverse event reporting database (FAERS).
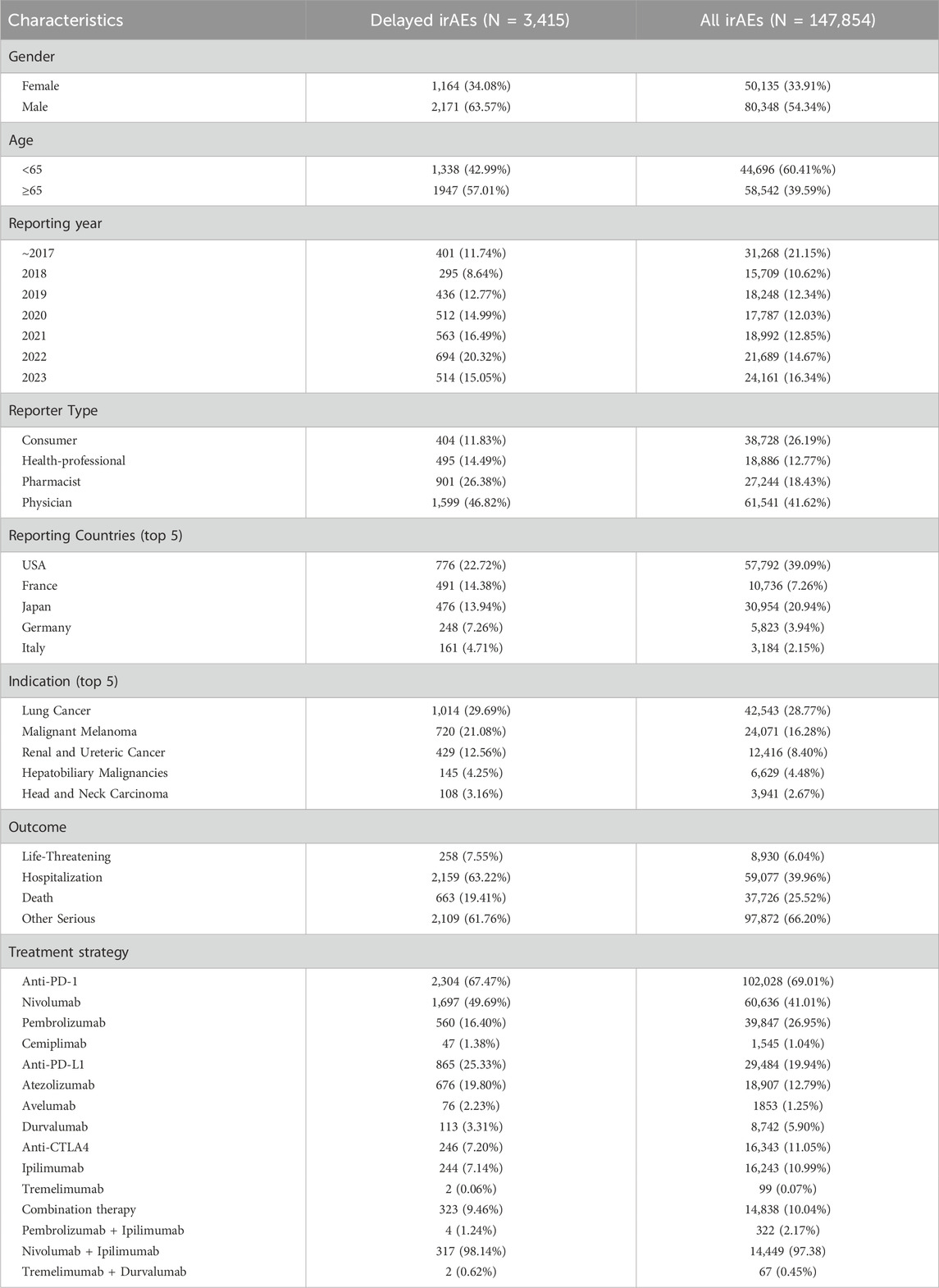
The clinical characteristics of patients with irAEs were presented in Table 1, including gender, age, reporting year, indications, the distribution of various cancer types and combination therapy strategies. The data indicated that the majority of cases were reported after 2018, reflecting the increased use of ICIs in recent 5 years. Among all irAEs, males accounted for a larger proportion (N = 80,348, 54.34%) than females (N = 50,135, 33.91%). However, delayed irAEs only occurred in 2.31% (3,415/147,854) of all irAEs in the FAERS database. A total of 2,171 cases (63.57%) were reported in male patients, 1,164 cases (34.08%) in female patients, and gender information was not specified for 80 patients. Patients aged 65 years and older represented a larger proportion of delayed irAEs (N = 1947, 57.01%). Physician reported the most cases (N = 1,599, 46.82%), followed by pharmacist (N = 901, 26.38%). The United States reported the highest number of delayed irAEs (N = 776, 22.72%), followed by France (N = 491, 14.38%), Japan (N = 476, 13.94%), and Germany (N = 248, 7.26%). The most commonly reported indication was lung cancer (N = 1,014, 29.69%), followed by malignant melanoma (N = 720, 21.08%) and renal and ureteral cancer (N = 429, 12.56%). Hospitalization was the most frequently reported serious outcome (N = 2,159, 63.22%). Death or life-threatening events occurred in 921 cases (26.977%) of delayed irAEs, indicating the potentially life-threatening nature of delayed irAEs. Among the three categories of ICIs, anti-PD-1 agents were associated with more delayed irAEs (N = 2,304, 67.47%) compared to anti-PD-L1 (N = 865, 25.33%) and anti-CTLA4 (N = 246, 7.20%).
Among ICIs analyzed in this study, nivolumab had the highest number of cases (N = 1,697, 49.69%) of delayed irAEs, followed by atezolizumab (N = 676, 19.80%) and pembrolizumab (N = 560, 16.40%). In terms of combination therapy, the combination of nivolumab and ipilimumab was associated with the most frequently reported delayed irAEs (N = 317, 98.14%).
3.2 Signal of system organ class
Based on the original data, the signal strength of delayed irAEs at the System Organ Class (SOC) level was described in Supplementary Table S4. We identified delayed irAEs occurring in 27 different SOCs. The reporting cases and types of delayed irAEs at SOC level for various treatment strategies were visualized in Figure 2. Regarding different class-specific ICI regimens, anti-PD-1 drugs (nivolumab, pembrolizumab, and cemiplimab) accounted for the majority of reported delayed irAEs (N = 5,504, 73.42%). Cemiplimab and tremelimumab were approved by the FDA in September 2018 and October 2022, respectively, but were rarely used, leading to limited reporting of delayed irAEs. Among combination therapy, nivolumab + ipilimumab had the highest number of reported delayed irAEs at the SOC level (N = 667, 98.52%), as it was the most commonly used combination regimen in real-world settings.
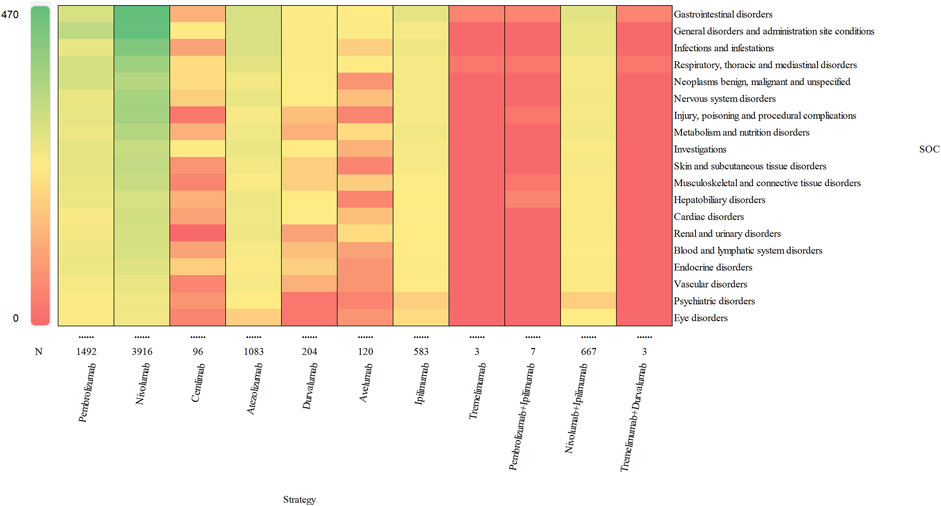
Figure 2. Visualization of reporting cases of delayed irAEs for different treatment strategies at SOC level.
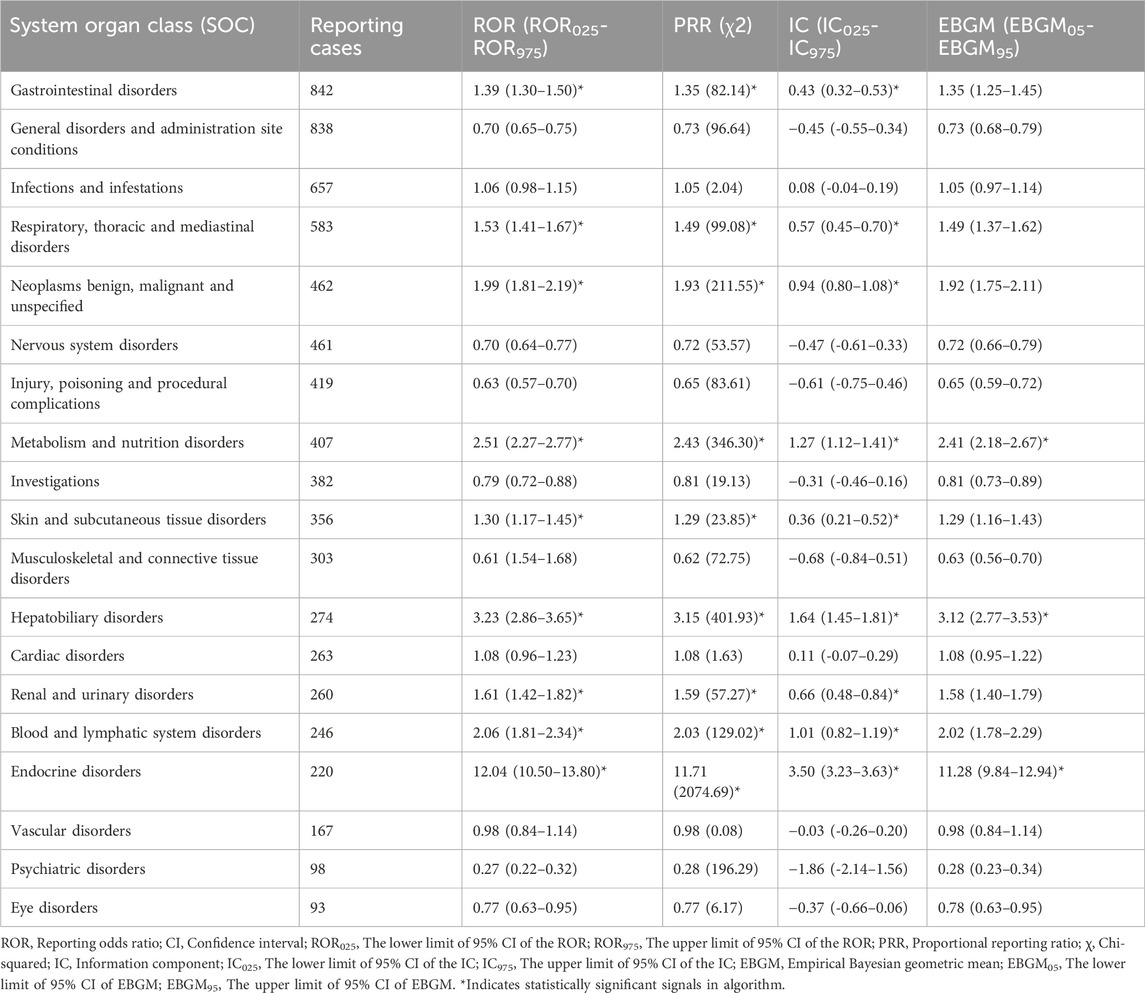
We identified suspicious signals of ICIs using four pharmacovigilance algorithms (ROR, PRR, BCPNN, and MGPS) and presented the results in Table 2. The significant SOCs associated with ICIs included gastrointestinal disorders (ROR025 = 1.30, SOC 10017947), respiratory, thoracic and mediastinal disorders (ROR025 = 1.41, SOC 10038738), metabolism and nutrition disorders (ROR025 = 2.27, SOC 10027433), skin and subcutaneous tissue disorders (ROR025 = 1.17, SOC 10040785), hepatobiliary disorders (ROR025 = 2.86, SOC 10019805), renal and urinary disorders (ROR025 = 1.42, SOC 10038359), blood and lymphatic system disorders (ROR025 = 1.81, SOC 10005329) and endocrine disorders (ROR025 = 10.50, SOC 10014698). Among these delayed irAEs, gastrointestinal disorders (N = 842, 11.23%), general disorders and administration site conditions (N = 838, 11.18%), infections and infestations (N = 657, 8.76%) and respiratory, thoracic and mediastinal disorders (N = 583, 7.78%) accounted for two-fifths of the reported adverse events. Notably, the strongest disproportionality association was for endocrine disorders (ROR025 = 10.50, χ2 = 2074.69, IC025 = 3.23, EBGM05 = 9.84). Moreover, sex-specific analyses of delayed irAEs at the SOC level were performed. Significant signals were detected in respiratory, thoracic and mediastinal disorders (ROR975 = 0.95), hematologic and lymphatic system disorders (ROR025 = 1.22), and immune system disorders (ROR025 = 1.16), as detailed in Supplementary Table S6.
The signal values and the association between class-specific ICIs and delayed irAEs are depicted in Figure 3. Among the different class-specific ICI regimens, anti-PD-1 drugs (nivolumab), anti-PD-L1 drugs (atezolizumab) and anti-CTLA-4 drugs (ipilimumab) demonstrated a significant association with gastrointestinal disorders and metabolism disorders. Respiratory system toxicities were significantly associated with anti-PD-1 drugs (pembrolizumab and nivolumab) and anti-PD-L1 drugs (atezolizumab, durvalumab and avelumab) drugs. Only anti-PD-1 drugs (pembrolizumab and nivolumab) exhibited significant signals in skin toxicities, while anti-CTLA-4 drugs did not show a significant association with skin toxicities and respiratory system toxicities. The combination regimen of anti-PD-1 drugs (nivolumab) and anti-CTLA-4 drugs (ipilimumab) did not result in any additional significant signals of delayed irAEs.
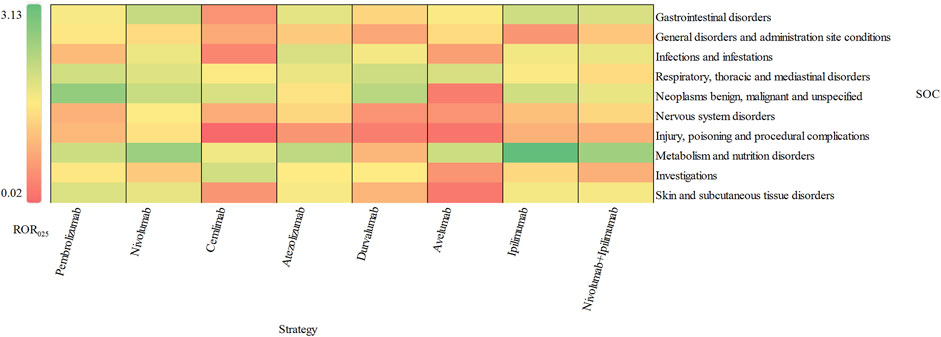
Figure 3. Visualization of delayed irAEs reporting rates for different treatment strategies at SOC level.
3.3 Signal of preferred terms
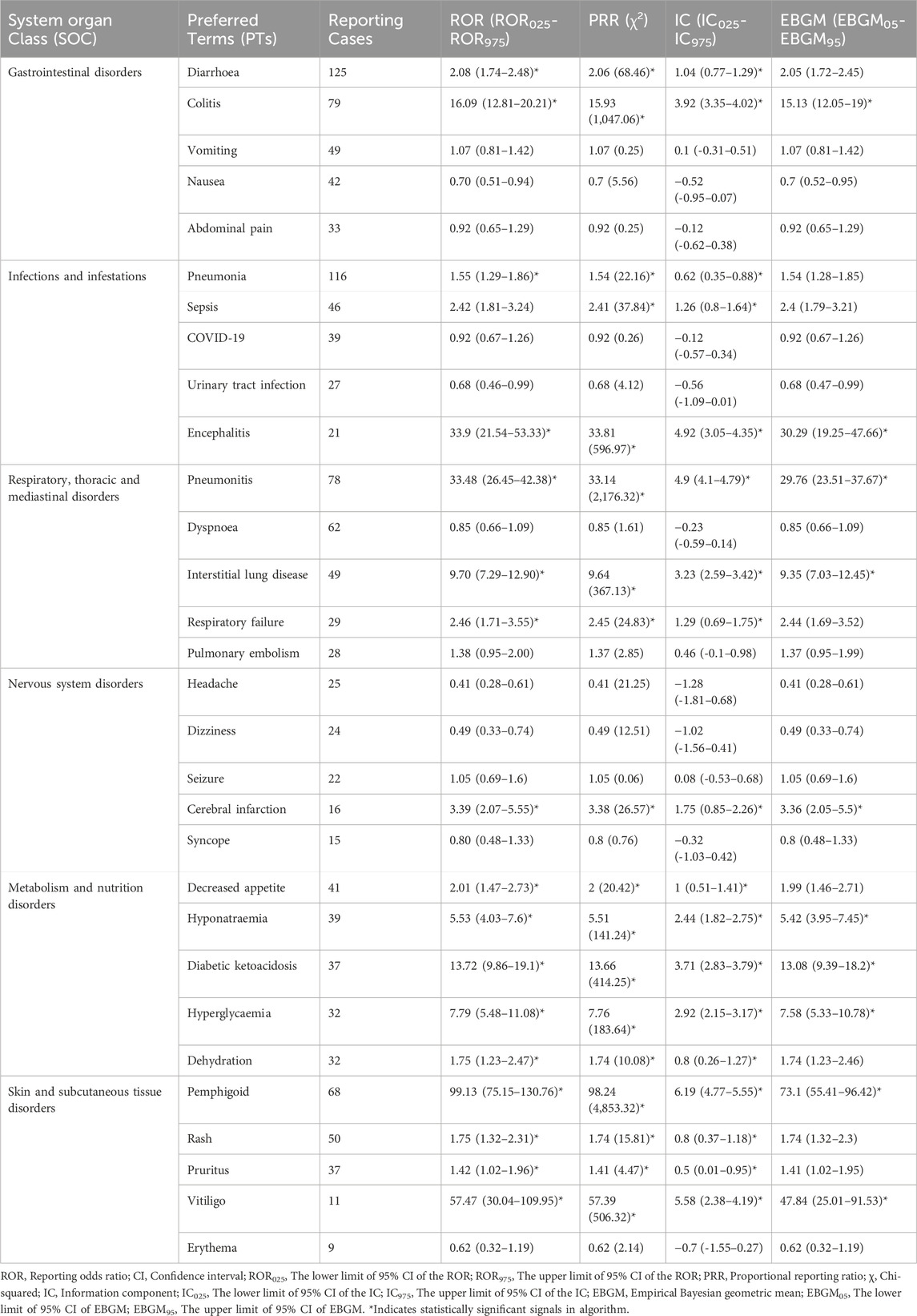
We assessed preferred terms (PT) levels in MedDRA for describing the delayed irAEs associated with different ICI regimens. The reported cases and types of delayed irAEs at PT level were visualized in Figure 4. A total of 1648 PT signals were identified from FAERS database, part of which are presented in Supplementary Table S5. Nivolumab exhibited the widest range of PTs among monotherapies, with a total of 1,145 PTs recorded. Analysis of ICIs revealed significant delayed irAEs at the PT level, as shown in Table 3, including but not limited to following PTs: diarrhoea (ROR025 = 1.74, PT 10012735), pneumonia (ROR025 = 1.29, PT 10035664), colitis (ROR025 = 12.81 PT 10009887), pneumonitis (ROR025 = 26.45, PT 10035742), acute kidney injury (ROR025 = 2.15, PT 10069339), pemphigoid (ROR025 = 75.15, PT 10034277), adrenal insufficiency (ROR025 = 50.31, PT 10001367), anaemia (ROR025 = 1.22, PT 10002034), rash (ROR025 = 1.32, PT 10037844) and interstitial lung disease (ROR025 = 7.29, PT 10022611). Nivolumab, as one of the most widely used anti-PD-1 drug, exhibited 135 PTs as significant signals that were consistent across four algorithms, ranging from transaminases increased (ROR025 = 1.01) to fulminant type 1 diabetes mellitus (ROR025 = 320.93). Additionally, 29 PTs were significantly associated with combination treatment regimen of anti-PD-1 drugs (nivolumab) and anti-CTLA-4 drugs (ipilimumab), ranging from general physical health deterioration (ROR025 = 1.01) to autoimmune colitis (ROR025 = 196.05).
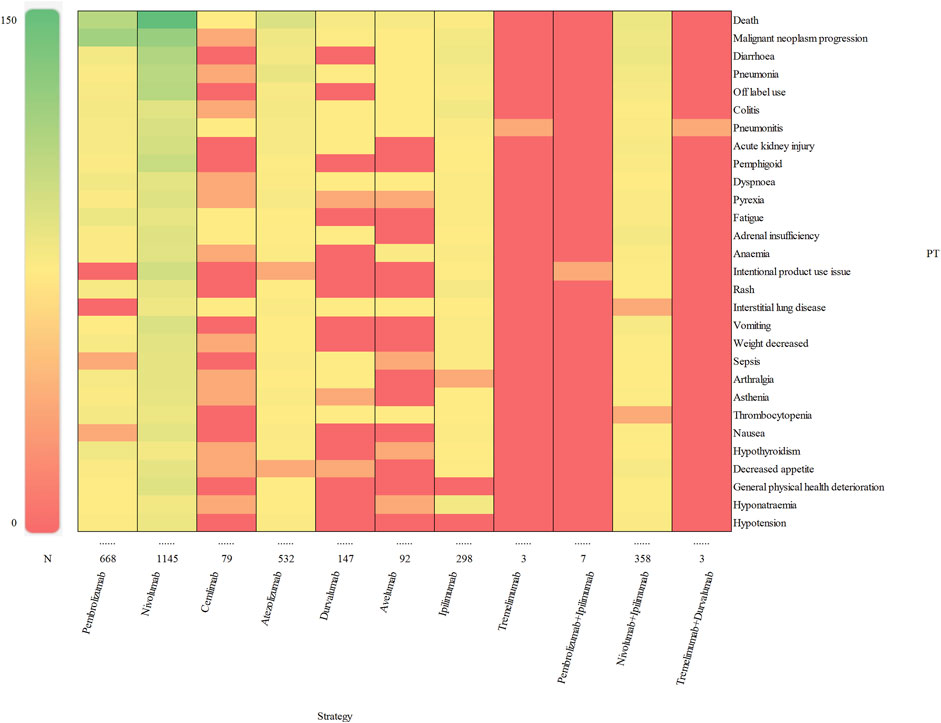
Figure 4. Visualization of reporting cases of delayed irAEs for different treatment strategies at PT level.
According to the report, an IC025 (the lower limit of 95% CI) value greater than 3.0 indicates a strong signal (Zou et al., 2023). In our study, we identified 10 strong signals at the PT level, including malignant neoplasm progression, colitis, pneumonitis, pemphigoid, adrenal insufficiency, type 1 diabetes mellitus, immune-mediated enterocolitis, hypophysitis, fulminant type 1 diabetes mellitus and encephalitis. Additionally, Figure 5 displays the top 10 most frequently reported PTs of delayed irAEs. Due to the lack of cases or only one case of delayed irAEs recorded for tremelimumab monotherpay, pembrolizumab + ipilimumab and tremelimumab + durvalumab combination therapy, they were excluded from the analysis. It is noteworthy that pneumonitis exhibited the most significant signals across different ICI regimens (ROR025: from 11.85 to 29.27), followed by colitis (ROR025: from 2.11 to 24.84). Further analysis revealed that nivolumab had most significant signal in pemphigoid (ROR025 = 104.75). Interestingly, ipilimumab combined nivolumab showed reduced associations with pneumonitis and colitis.
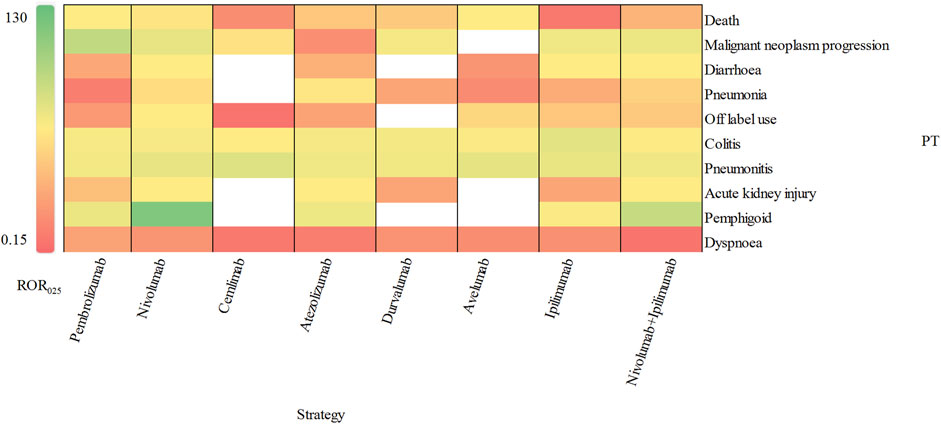
Figure 5. Visualization of delayed irAEs reporting rates for different treatment strategies at PT level.
3.4 Outcomes
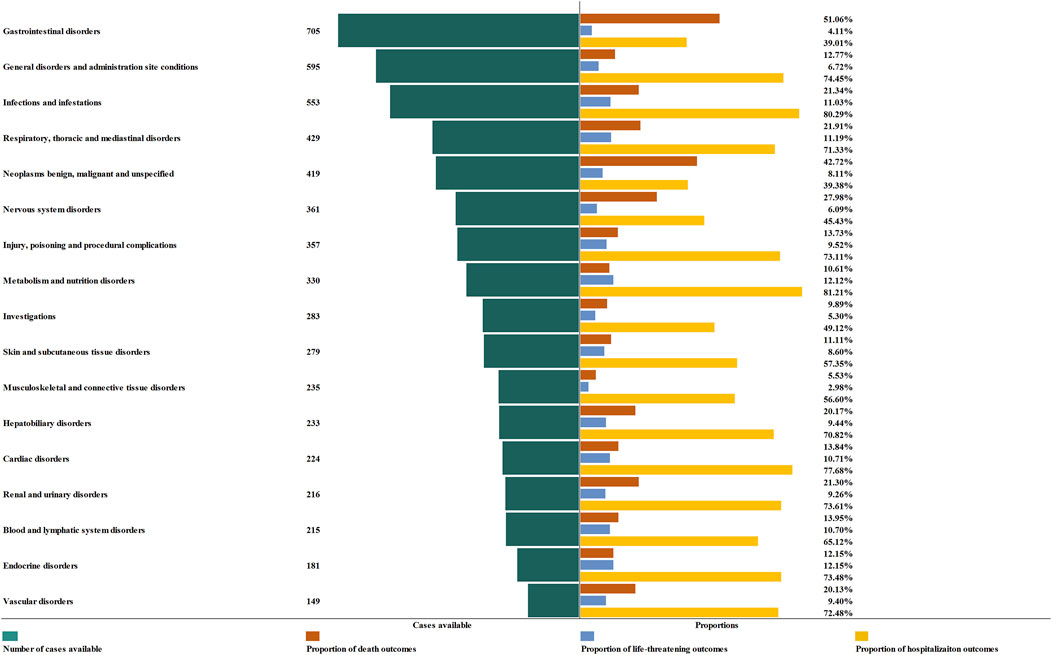
In order to improve the prognosis evaluation of delayed irAEs, we examined the proportions of death, life-threatening events, and hospitalization, as shown in Figure 6. Overall, the most severe outcomes of delayed irAEs at the SOC level were reported as death, with the highest proportions in gastrointestinal disorders (51.06%) and endocrine disorders (12.15%) respectively. Additionally, metabolism (12.12%) and respiratory disorders (11.19%) had a higher frequency of life-threatening events compared to other irAEs at SOC level. It is worth noting that the frequencies of hospitalization events were 81.21% for metabolism disorders and 80.29% for infections.
4 Discussion
With expanding application in oncology (Livingstone et al., 2022; Lorusso et al., 2024), ICIs have been associated with a higher incidence of irAEs than previously anticipated (Kato et al., 2017; Koyama et al., 2018; Johnson et al., 2022). Unfortunately, delayed irAEs has rarely been documented except for few studies (Owen et al., 2021; Couey et al., 2019). Additionally, details regarding delayed irAEs remain unclear. Therefore, we performed an analysis on delayed ICI-related adverse events using the FAERS database, presenting our findings as follows:
From the first quarter of 2011 to the fourth quarter of 2023, a total of 3,415 cases received ICI monotherapies and 323 cases received combination therapies reported delayed irAEs in our study, which we believe is the largest collection of cases of delayed irAEs to date. The reporting rate for delayed irAEs was approximately 2.31%, which is lower than 5.3% reported by Owen (Owen et al., 2021). This suggests that delayed irAEs are still uncommon. In our descriptive analysis, we observed that males accounted for a higher proportion of delayed irAEs compared to females. This difference may be partly due to the higher incidence of cancer in men, as lung cancer and melanoma were the most commonly reported indications for ICIs. Furthermore, Conforti’s research demonstrated a higher propensity for males to undergo ICI therapy compared to females, attributed to the relatively lower participation rates of women in clinical trials (Conforti et al., 2018). Contrarily, our results contradicted Watson’s analysis (Watson et al., 2019), which employed the World Health Organization (WHO) global database of individual case safety reports, revealing a heightened frequency of adverse drug reactions (ADRs) in females, especially during their reproductive years. Additionally, a separate investigation using the national pharmacovigilance center in the Netherlands found that medications such as thyroid hormones and antidepressants, which had the highest incidence of ADRs, were more frequently reported in women (de Vries et al., 2019).
Actually, to date, few studies have explored sex differences in irAEs, especially delayed irAEs. Extant literature delineates gender-specific disparities in immunological responses to both exogenous and endogenous antigens, highlighting distinct differences in both innate and adaptive immunity between males and females (Klein and Flanagan, 2016). Statistically, females exhibit more robust innate and adaptive immune responses compared to their male counterparts. On the other hand, females constitute approximately 80% of the global patient population suffering from systemic autoimmune diseases (Klein and Flanagan, 2016; Conforti et al., 2018). Consequently, the heightened predisposition of females to autoimmune pathologies could potentially render them more susceptible to irAEs (Menzies et al., 2017). To evaluate the impact of sex on the pharmacovigilance signal for delayed irAEs following ICIs initiation, we conducted further disproportionality analysis. Our results indicated that males had a slightly higher reporting frequencies of delayed irAEs in respiratory disorders (ROR975 = 0.95) and blood and lymphatic system disorders (ROR025 = 1.22), while females had significant higher reporting frequencies in immune system disorders (ROR025 = 1.16). These findings align with studies investigating respiratory toxicity linked to ICIs, which indicated that males exhibited a marginally higher incidence of respiratory system AEs compared to females (ROR = 1.74, 95% CI: 1.70∼1.78) (Cui et al., 2022). This outcome may be partially attributed to greater exposure to cigarette smoke among men (Zhu et al., 2020; Suresh et al., 2018). Regarding hematologic and lymphatic system disorders, our result is different from Li’s study (Li et al., 2023). The specific determinants underlying sex-based disparities are not yet fully elucidated and necessitate additional investigation. No significant signals were detected between male and female patients for other delayed irAEs at the SOC level (Supplementary Table S3). Our findings suggest that sex difference may be an important biological variable for delayed irAEs, although the underlying factors are still unclear and require further investigation in the field of oncology. Furthermore, we observed that patients over 65 years old had a higher reporting frequency of delayed irAEs compared to those under 65 years old. Conversely for all irAEs, patients over 65 years old had much lower reporting rates. The impact of age difference on irAEs, particularly delayed irAEs is not well-established (Baldini et al., 2020; Paderi et al., 2021). and our analysis based on large-scale FAERS data may offer valuable evidence of the associations between age and delayed irAEs. Future studies should pay more attention to age differences in patients with delayed irAEs.
Importantly, we evaluated and compared the incidence of delayed irAEs across various immunotherapy regimens. Overall, there were more reports of delayed irAEs associated with anti-PD-1 inhibitors compared to anti-PD-L1 and anti-CTLA-4 inhibitors. Our analysis revealed that all ICIs demonstrated a higher reporting frequency of metabolism and nutrition disorders as compared to other delayed irAEs at SOC level (ROR025: from 0.45 to 3.13), which was consistent with the findings from a previous study (Reese et al., 2020). Moreover, treatment with anti-PD-1 agents exhibited a higher reporting frequency of gastrointestinal disorders in comparison to other ICI regimens (ROR025 = 1.66). Conversely, metabolism and nutrition disorders were the most commonly reported delayed irAEs with anti-CTLA-4 medications as opposed to anti-PD-1/anti-PD-L1 medications (ROR025 = 3.13). Notably, significant signals for skin and subcutaneous disorders were observed with anti-PD-1 regimens (ROR025: from 0.27 to 1.27), suggesting a higher likely-hood of skin toxicities with anti-PD-1 inhibitors. Interestingly, previous study have indicated that combination therapy is associated with increased rates of AEs involving multiple organ systems (Grimaldi et al., 2016). However, our study found that combination of anti-PD-1 and anti-CTLA-4 agents did not appear to further elevate the risk of delayed irAEs, in contrast to what has been reported for early irAEs.
Additionally, our study provides more precise data on the profile of delayed irAEs caused by different ICI regimens at the PT level. A total of 282 significant signals for potential toxicities were identified, including diarrhoea, pneumonia, colitis, pneumonitis, acute kidney injury and pemphigoid. Diarrhoea was more frequently recorded in patients receiving anti-PD-1 and anti-CTLA-4 therapy. Furthermore, we observed that ipilimumab alone or in combination with nivolumab had a higher risk of diarrhoea compared to other ICI regimens. It’s worth noting that we identified two strong signals (IC025 > 3.0) for colitis and pneumonitis in all eight monotherapy regimens and one combination regimen. Owen reported that colitis was the most frequent delayed irAEs after adjuvant anti-PD-1 therapy in melanoma patients, which is consistent with our findings (Owen et al., 2021). The prognosis of delayed irAEs was thoroughly examined in our analysis. We observed that death accounted for 51.06% of the gastrointestinal disorders, indicating a significant impact of gastrointestinal complications on patient mortality. The study by Owen (Owen et al., 2021) demonstrated that the delayed gastrointestinal toxicities, such as colitis, increased the mortality rate of melanoma patients. Another severe outcome of delayed irAEs, life-threatening events, represented 12.15% of endocrine disorders. We noted a significant increase in endocrine-related delayed irAEs among female patients with various cancer types. The four of the most frequently recorded delayed irAEs at the PTs level were adrenal insufficiency, hypothyroidism, hypophysitis and hyperthyroidism, which aligns with previous study (Zhai et al., 2019). Given the high incidence of life-threatening events, it is crucial to closely monitor the signs and symptoms of endocrine-related delayed irAEs during ICI therapy.
Notably, delayed irAEs encompass both de novo toxicities and recurrences of previous events. Nevertheless, if the interval since the last dose exceeds 1 year, the probability of an alternative etiology increases (Naidoo et al., 2023). Essentially, attributing a re-emergent irAE is relatively straightforward if the patient is still receiving ICI treatment. However, the etiology of autoimmune toxicity emerging months or even years post-discontinuation of ICI treatment remains ambiguous. For example, viral infections may serve as alternative etiologies, potentially causing myocarditis (Rezkalla and Kloner, 2021) and chronic autoimmune conditions such as type 1 diabetes, rheumatoid arthritis and multiple sclerosis (Smatti et al., 2019). To date, clinical data and animal models on delayed or long-term irAEs are insufficient, and it is unclear whether there is a correlation between ICI treatment, intercurrent infections, and the onset of autoimmune disorders. Similar to recurrent irAEs, de novo autoimmune conditions should also be considered in differential diagnoses, particularly with the suspicion of an alternative etiology like viral infection. Naidoo et al. (2020) reported that myocarditis or pneumonitis were observed as manifestations that could confound attribution as re-emergent irAEs or de novo events arising from infectious etiology. Consequently, the diagnostic certainty of delayed irAEs can be variable, and the potential for misdiagnosis should be acknowledged. Commonly reported confounding factors include the diagnostic misattribution to the sequelae of concomitant chemotherapy, radiotherapy, disease relapse, or septicemia (Couey et al., 2019). Conclusively, comprehensive and detailed data collection from real-world settings to improve the characterization and management of delayed irAEs is necessary.
As a matter of fact, there are several limitations that need to be addressed. Firstly, FAERS is a spontaneous reporting system with multiple sources of data, resulting in inherent constraints such as under reporting, incomplete patient demographic data, nonuniform data format and missing data. Availability of more detailed clinical data could potentially enhance a comprehensive evaluation of the patients’ response rates associated with these irAEs and the durability of their responses.
Secondly, reports in the FAERS database do not require proof of a causal relationship with the drug. The information in the reports only reflects the observations and opinions of the reporters, which makes it impossible to determine whether the reported AEs were indeed caused by the drug. Thirdly, a case report in FAERS could involve several drugs, adverse events, and outcomes, leading to bias in pharmacovigilance analysis. Also, this study did not account for combination chemotherapy, which could have introduced bias into the results. Lastly, the calculation of fatality rates was not feasible due to the lack of comprehensive exposure data, in addition to the fact that mortality may also result from the underlying disease, concomitant irAEs, and other contributory factors. Notwithstanding, our investigation constitutes a comprehensive and meticulous quantification of the potential hazards associated with delayed irAEs in ICIs. These findings may offer critical evidence for subsequent research endeavors and clinical applications.
5 Conclusion
Our study systematically and scientifically evaluated the potential hazards using a large datasets from FAERS, outlining a profile of delayed irAEs. These findings provide valuable insights for future investigations and clinical applications in this specific field. In general, delayed irAEs occur in a small subset of cancer patients exposed to ICI regimens, which can be challenging to manage and may result in serious outcomes. Healthcare providers should be aware of the possibility of ICIs causing delayed irAEs, despite their low frequency. It is crucial to educate patients about these potential toxicities before initiating ICI therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YY: Data curation, Writing–original draft. LL: Resources, Writing–review and editing. JT: Resources, Writing–review and editing. LM: Data curation, Writing–review and editing. YW: Data curation, Writing–review and editing. QL: Data curation, Writing–review and editing. YL: Conceptualization, Funding acquisition, Supervision, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82272755), Chongqing Science and Technology Commission (No. 2022NSCQ-MSX0706) and Fundamental Research Funds for the Central Universities (No. 2022CDJYGRH-007).
Acknowledgments
The authors would like to extend the sincere gratitude to all those who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1453429/full#supplementary-material
References
Baldini, C., Martin Romano, P., Voisin, A. L., Danlos, F. X., Champiat, S., Laghouati, S., et al. (2020). Impact of aging on immune-related adverse events generated by anti-programmed death (ligand)PD-(L)1 therapies. Eur. J. Cancer 129, 71–79. doi:10.1016/j.ejca.2020.01.013
Conforti, F., Pala, L., Bagnardi, V., De Pas, T., Martinetti, M., Viale, G., et al. (2018). Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 19 (6), 737–746. doi:10.1016/S1470-2045(1830261-4)
Couey, M. A., Bell, R. B., Patel, A. A., Romba, M. C., Crittenden, M. R., Curti, B. D., et al. (2019). Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 7 (1), 165. doi:10.1186/s40425-019-0645-6
Cui, C., Deng, L., Wang, W., Ren, X., Wang, Y., and Cui, W. (2022). Respiratory system toxicity induced by immune checkpoint inhibitors: a real-world study based on the FDA adverse event reporting system database. Front. Oncol. 12, 941079. doi:10.3389/fonc.2022.941079
De Vries, S. T., Denig, P., Ekhart, C., Burgers, J. S., Kleefstra, N., Mol, P. G. M., et al. (2019). Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in The Netherlands: an explorative observational study. Br. J. Clin. Pharmacol. 85 (7), 1507–1515. doi:10.1111/bcp.13923
Grimaldi, A. M., Marincola, F. M., and Ascierto, P. A. (2016). Single versus combination immunotherapy drug treatment in melanoma. Expert Opin. Biol. Ther. 16 (4), 433–441. doi:10.1517/14712598.2016.1128891
Guo, M., Shu, Y., Chen, G., Li, J., and Li, F. (2022). A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci. Rep. 12 (1), 20601. doi:10.1038/s41598-022-23726-4
Hauben, M., Madigan, D., Gerrits, C. M., Walsh, L., and Van Puijenbroek, E. P. (2005). The role of data mining in pharmacovigilance. Expert Opin. Drug Saf. 4 (5), 929–948. doi:10.1517/14740338.4.5.929
Jiang, J. J., Zhao, B., and Li, J. (2022). Does eltrombopag lead to thrombotic events? A pharmacovigilance study of the FDA adverse event reporting system. J. Clin. Pharm. Ther. 47 (10), 1556–1562. doi:10.1111/jcpt.13701
Johnson, D. B., Nebhan, C. A., Moslehi, J. J., and Balko, J. M. (2022). Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19 (4), 254–267. doi:10.1038/s41571-022-00600-w
Kato, T., Masuda, N., Nakanishi, Y., Takahashi, M., Hida, T., Sakai, H., et al. (2017). Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 104, 111–118. doi:10.1016/j.lungcan.2016.12.016
Klein, S. L., and Flanagan, K. L. (2016). Sex differences in immune responses. Nat. Rev. Immunol. 16 (10), 626–638. doi:10.1038/nri.2016.90
Koyama, N., Iwase, O., Nakashima, E., Kishida, K., Kondo, T., Watanabe, Y., et al. (2018). High incidence and early onset of nivolumab-induced pneumonitis: four case reports and literature review. BMC Pulm. Med. 18 (1), 23. doi:10.1186/s12890-018-0592-x
Li, N., Feng, Y., Chen, X., Li, Y., Zhang, C., and Yin, Y. (2023). Hematologic and lymphatic system toxicities associated with immune checkpoint inhibitors: a real-world study. Front. Pharmacol. 14, 1213608. doi:10.3389/fphar.2023.1213608
Livingstone, E., Zimmer, L., Hassel, J. C., Fluck, M., Eigentler, T. K., Loquai, C., et al. (2022). Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): final results of a randomised, double-blind, phase 2 trial. Lancet 400 (10358), 1117–1129. doi:10.1016/S0140-6736(22)01654-3
Lorusso, D., Xiang, Y., Hasegawa, K., Scambia, G., Leiva, M., Ramos-Elias, P., et al. (2024). Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet 403 (10434), 1341–1350. doi:10.1016/S0140-6736(24)00317-9
Martins, F., Sofiya, L., Sykiotis, G. P., Lamine, F., Maillard, M., Fraga, M., et al. (2019). Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16 (9), 563–580. doi:10.1038/s41571-019-0218-0
Menzies, A. M., Johnson, D. B., Ramanujam, S., Atkinson, V. G., Wong, A. N. M., Park, J. J., et al. (2017). Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28 (2), 368–376. doi:10.1093/annonc/mdw443
Naidoo, J., Murphy, C., Atkins, M. B., Brahmer, J. R., Champiat, S., Feltquate, D., et al. (2023). Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J. Immunother. Cancer 11 (3), e006398. doi:10.1136/jitc-2022-006398
Naidoo, J., Reuss, J. E., Suresh, K., Feller-Kopman, D., Forde, P. M., Mehta Steinke, S., et al. (2020). Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management. J. Immunother. Cancer 8 (1), e000984. doi:10.1136/jitc-2020-000984
Noren, G. N., Bate, A., Orre, R., and Edwards, I. R. (2006). Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat. Med. 25 (21), 3740–3757. doi:10.1002/sim.2473
Owen, C. N., Bai, X., Quah, T., Lo, S. N., Allayous, C., Callaghan, S., et al. (2021). Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann. Oncol. 32 (7), 917–925. doi:10.1016/j.annonc.2021.03.204
Paderi, A., Fancelli, S., Caliman, E., Pillozzi, S., Gambale, E., Mela, M. M., et al. (2021). Safety of immune checkpoint inhibitors in elderly patients: an observational study. Curr. Oncol. 28 (5), 3259–3267. doi:10.3390/curroncol28050283
Reese, S. W., Marchese, M., and Mcnabb-Baltar, J. (2020). Insights from pharmacovigilance: gastrointestinal-related immune checkpoint inhibitor adverse events. Gastroenterology 159 (4), 1195–1200. doi:10.1053/j.gastro.2020.06.093
Rezkalla, S. H., and Kloner, R. A. (2021). Viral myocarditis: 1917-2020: from the Influenza A to the COVID-19 pandemics. Trends Cardiovasc Med. 31 (3), 163–169. doi:10.1016/j.tcm.2020.12.007
Singh, N., Hocking, A. M., and Buckner, J. H. (2023). Immune-related adverse events after immune check point inhibitors: understanding the intersection with autoimmunity. Immunol. Rev. 318 (1), 81–88. doi:10.1111/imr.13247
Smatti, M. K., Cyprian, F. S., Nasrallah, G. K., Al Thani, A. A., Almishal, R. O., and Yassine, H. M. (2019). Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses 11 (8), 762. doi:10.3390/v11080762
Suresh, K., Naidoo, J., Lin, C. T., and Danoff, S. (2018). Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 154 (6), 1416–1423. doi:10.1016/j.chest.2018.08.1048
Tao, Y., Li, X., Liu, B., Wang, J., Lv, C., Li, S., et al. (2023). Association of early immune-related adverse events with treatment efficacy of neoadjuvant Toripalimab in resectable advanced non-small cell lung cancer. Front. Oncol. 13, 1135140. doi:10.3389/fonc.2023.1135140
Watson, S., Caster, O., Rochon, P. A., and Den Ruijter, H. (2019). Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 17, 100188. doi:10.1016/j.eclinm.2019.10.001
Weber, J. S., Hodi, F. S., Wolchok, J. D., Topalian, S. L., Schadendorf, D., Larkin, J., et al. (2017). Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35 (7), 785–792. doi:10.1200/JCO.2015.66.1389
Yin, Q., Wu, L., Han, L., Zheng, X., Tong, R., Li, L., et al. (2023). Immune-related adverse events of immune checkpoint inhibitors: a review. Front. Immunol. 14, 1167975. doi:10.3389/fimmu.2023.1167975
Zhai, Y., Ye, X., Hu, F., Xu, J., Guo, X., Zhuang, Y., et al. (2019). Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J. Immunother. Cancer 7 (1), 286. doi:10.1186/s40425-019-0754-2
Zhang, Y. C., Zhu, T. C., Nie, R. C., Lu, L. H., Xiang, Z. C., Xie, D., et al. (2023). Association between early immune-related adverse events and survival in patients treated with PD-1/PD-L1 inhibitors. J. Clin. Med. 12 (3), 736. doi:10.3390/jcm12030736
Zhou, C., Peng, S., Lin, A., Jiang, A., Peng, Y., Gu, T., et al. (2023). Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine 59, 101967. doi:10.1016/j.eclinm.2023.101967
Zhu, S., Fu, Y., Zhu, B., Zhang, B., and Wang, J. (2020). Pneumonitis induced by immune checkpoint inhibitors: from clinical data to translational investigation. Front. Oncol. 10, 1785. doi:10.3389/fonc.2020.01785
Keywords: immune checkpoint inhibitors, delayed immune-related adverse events, FAERS, disproportionality analysis, outcomes
Citation: Yang Y, Li L, Tian J, Ma L, Wu Y, Luo Q and Luo Y (2024) Delayed immune-related adverse events profile associated with immune checkpoint inhibitors: a real-world analysis. Front. Pharmacol. 15:1453429. doi: 10.3389/fphar.2024.1453429
Received: 23 June 2024; Accepted: 29 October 2024;
Published: 11 November 2024.
Edited by:
Jiayu Liao, University of California, Riverside, United StatesReviewed by:
Joaquín Sáez Peñataro, Hospital Clinic of Barcelona, SpainAntonio Farina, University of Florence, Italy
Copyright © 2024 Yang, Li, Tian, Ma, Wu, Luo and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Luo, eWFubHVvMjAxOEBjcXUuZWR1LmNu
 Yana Yang1
Yana Yang1 Yan Luo
Yan Luo