- 1Research Center of Emotional Diseases, Shenyang Anning Hospital, Shenyang, China
- 2Shenyang Key Laboratory for Causes and Drug Discovery of Chronic, Shenyang, China
- 3The First Affiliated Hospital of Dalian Medical University, Dalian, China
Licorice, derived from the root of Glycyrrhiza uralensis Fisch, is a key Traditional Chinese Medicine known for its detoxifying, spleen-nourishing, and qi-replenishing properties. Licochalcone A (Lico A), a significant component of licorice, has garnered interest due to its molecular versatility and receptor-binding affinity. This review explores the specific roles of Lico A in various diseases, providing new insights into its characteristics and guiding the rational use of licorice. Comprehensive literature searches using terms such as “licorice application” and “pharmacological activity of Lico A” were conducted across databases including CNKI, PubMed, and Google Scholar to gather relevant studies on Lico A’s pharmacological activities and mechanisms. Lico A, a representative chalcone in licorice, targets specific mechanisms in anti-cancer and anti-inflammatory activities. It also plays a role in post-transcriptional regulation. This review delineates the similarities and differences in the anti-cancer and anti-inflammatory mechanisms of Lico A, concluding that its effects on non-coding RNA through post-transcriptional mechanisms deserve further exploration.
1 Introduction
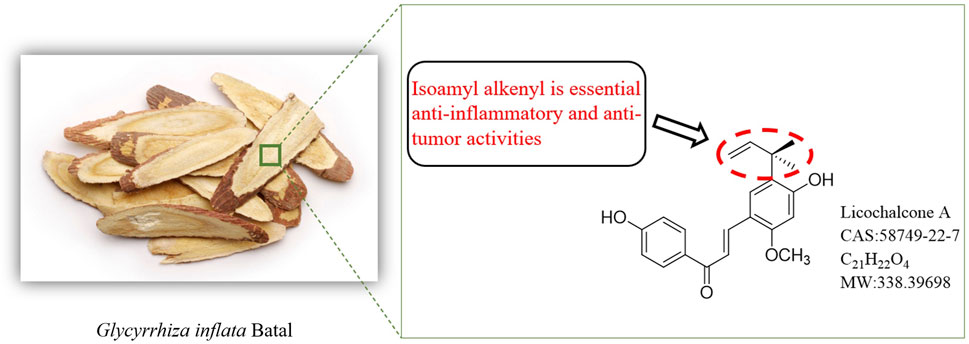
The root of G. inflata Batal has been a valuable medicinal resource for licorice widely used in Asia and worldwide. Licocalcone A (Lico A) is one of the characteristic component of the root of Glycyrrhiza inflata. The application of licorice dates back to ancient civilizations such as Greece and Rome (Armanini et al., 2002). Today, licorice is widely incorporated into food, medicinal products, health supplements, and cosmetics, recognized for its safety and efficacy. In Traditional Chinese Medicine, licorice is prized for its harmonizing properties, and it has also become popular in dietary applications for its health benefits (Herrera et al., 2009). Modern applications extend to food additives, tobacco flavoring, and skin depigmentation products (Rizzato et al., 2017). Its safety has been affirmed by the U.S. Flavor and Extract Manufacturers Association (Pastorino et al., 2018), solidifying its reputable status and prompting increased research into its pharmacological activities and applications (Pastorino et al., 2018).
Flavonoids, common in nature (Perezvizcaino and Fraga, 2018), typically form glycosides in plants or exist in their free form (Vukics and Guttman, 2008). This structural diversity translates to varied pharmacological activities, including free radical scavenging, especially in flavonoids with catechol structures (Yang et al., 2000; Mukne et al., 2011; Cheng et al., 2019). Chalcones, a specific class of flavonoids, have a 1,3-diphenylpropenone skeleton. Among these, Lico A (Figure 1) stands out with its distinct structure and potent anti-inflammatory potential (Cui et al., 2008; Siddiqui et al., 2011; Kumar et al., 2014; Silva et al., 2018; Zhang et al., 2019).
Inflammation, a complex defense response to tissue injury, involves the vascular system and is triggered by inflammatory cytokines and mediators (Gabriele and Pucci, 2017; Huang et al., 2017). Studies show that Lico A mitigates LPS-induced effects by inhibiting inflammatory cytokine production and NO through NF-κB pathway suppression. Additionally, Lico A enhances the activity of antioxidant enzymes and protects against oxidative damage and cell death via ERK and Akt pathways (Hu and Liu, 2016). Furthermore, Lico A exhibits significant anti-tumor effects (Kang et al., 2017; Wu et al., 2017; Chen et al., 2018a), including the induction of apoptosis in cancer cells, regulation of the cell cycle, inhibition of tumor invasion and metastasis, and suppression of tumor angiogenesis (Hao et al., 2015; Lv et al., 2015).
Beyond its anti-inflammatory and anti-tumor properties, Lico A also demonstrates bacteriostatic, anti-parasitic, and osteogenic activities. This review summarizes the pharmacological actions and mechanisms of Lico A over the past five years, aiming to deepen the understanding of its bioavailability and inform further research.
2 Lico A exerts anticancer activity
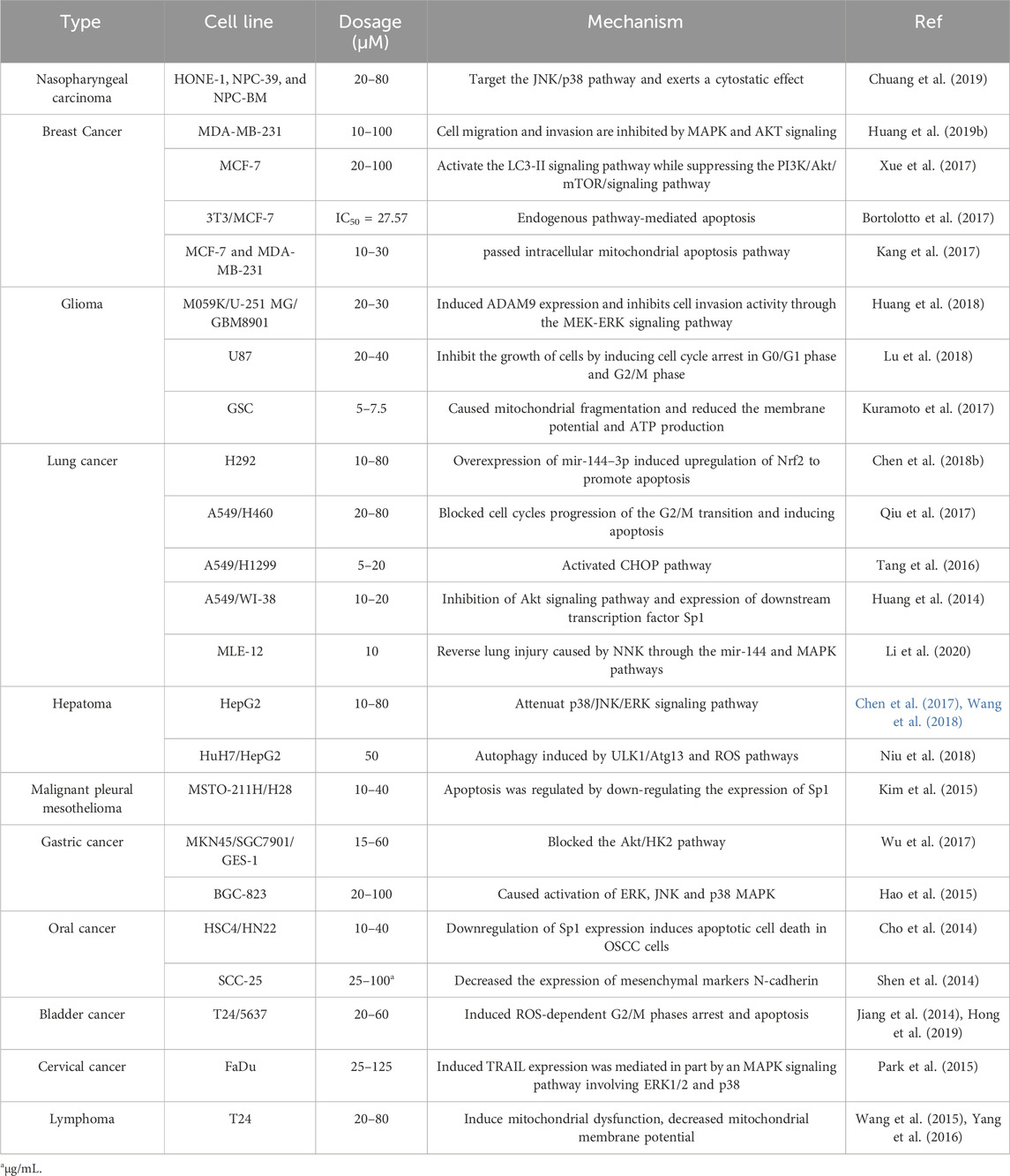
Traditional Chinese herbal medicine, with its extensive history in treating tumors, continues to be a significant source of anti-tumor medications. Zhou et al. (2019). For example, Lico A is renowned for its potent anti-tumor activity (Daniell et al., 2000; Chen et al., 2017). Lico A’s anticancer effects manifest through various mechanisms, including inducing apoptosis in tumor cells, regulating the cell cycle to inhibit proliferation, curtailing tumor invasion and metastasis, and suppressing tumor angiogenesis by modulating related protein expression and signaling pathways (Yang et al., 2014; Kim et al., 2015; Park et al., 2015; Tsai et al., 2015; Yang et al., 2016; Kojima et al., 2017). Recent studies have highlighted Lico A’s cytostatic effects on human nasopharyngeal carcinoma cells mediated through apoptosis targeting the JNK/p38 pathway (Chuang et al., 2019).
Invasion and metastasis, key traits of malignant tumors, involve tumor cells detaching from the primary lesion, invading surrounding tissues or distant organs, and proliferating to form metastases. Lico A acts to inhibit this process by restraining cell migration, modulating E-cadherin and vimentin expression, and blocking MAPK and AKT signaling pathways (Mazzucchelli and Brambilla, 2000; Huang et al., 2019a). This multifaceted approach significantly reduces migratory and invasive capabilities of cells such as SCC-25. Additionally, tumor blood vessels, vital for supplying oxygen, nutrients, and growth factors, are also targeted by Lico A. Studies by Kim et al. (2010) demonstrated Lico A’s ability to inhibit neovascularization both in vitro and in vivo by suppressing angiogenesis factors such as IL-6, IL-8, and the VEGFR-2 signaling pathway. The effect of Lico A on different tumors and the specific mechanism of action is shown in Table 1.
In recent years, research into Lico A’s anti-cancer activity has deepened, broadening its scope of anti-cancer effects. Novel advancements have been made in understanding Lico A’s role against oral and nasopharyngeal cancers (Kim et al., 2010; Chuang et al., 2019). Cancer cells’ inherent ability to sustain growth signals and perpetually proliferate underscores the significance of inhibiting their proliferation in cancer treatment. Through an examination of anti-cancer mechanisms and targets, it is evident that Lico A primarily exerts its anti-cancer effect by inducing apoptosis and impeding the cell cycle. The mitochondrial apoptotic pathway is a central conduit through which Lico A induces apoptosis.
3 Lico A exerts anti-inflammatory activity
Inflammation is a common and significant pathological response that underlies many conditions, including surface infections and organ-specific ailments such as pneumonia, hepatitis, and nephritis. It involves a delicate balance between proinflammatory factors and the body’s defense mechanisms, which influences the onset, progression, and resolution of inflammation. The NF-κB and Nrf2 pathways play crucial roles in the development of inflammation, and the unique structure of Lico A provides strong anti-inflammatory activity by modulating these pathways.
3.1 Lico A achieves anti-inflammatory effects by regulating NF-κB pathway
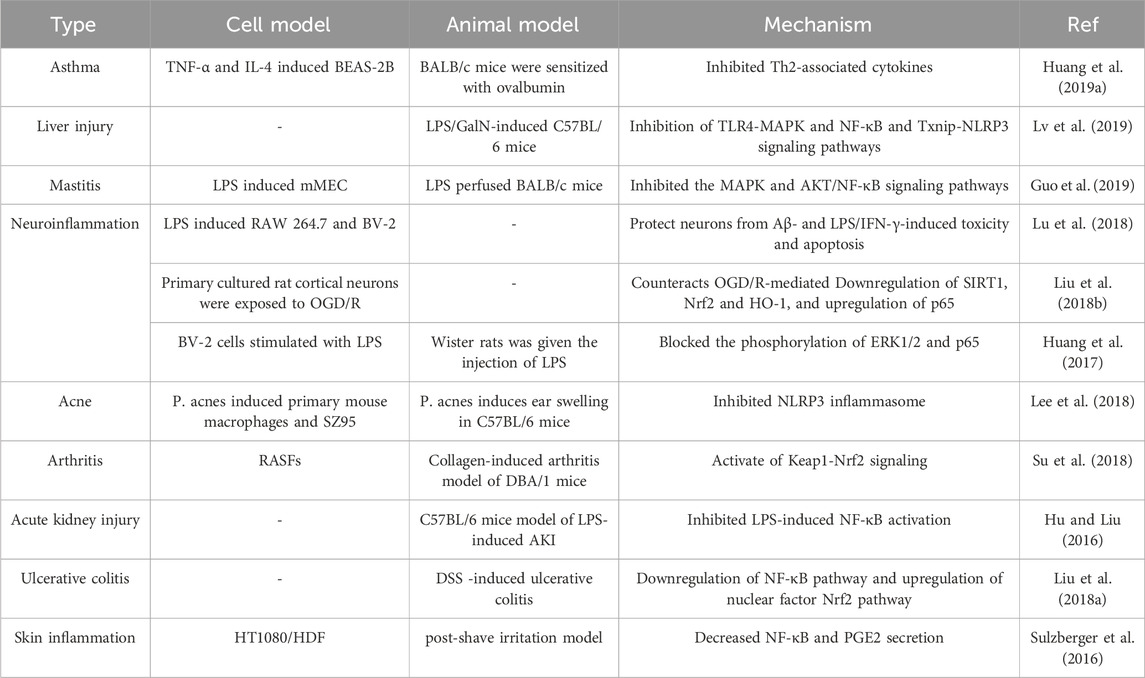
NF-κB, a nuclear transcription factor widely distributed in various cell types, orchestrates the transcription and expression of genes involved in processes such as cell proliferation, differentiation, and immune response (Maracle et al., 2017; Wang et al., 2018). The NF-κB signaling pathway consists of NF-κB, Inhibitor of NF-κB (IκB), and IκB kinases (IKK). In an inactive state, the NF-κB dimer is bound to IκB. Cellular stimulation leads to IKK activation, promoting the phosphorylation and ubiquitination of IκB, followed by its degradation, freeing the NF-κB dimer to bind to target genes and regulate their expression (Napetschnig and Wu, 2013). Activation of the NF-κB pathway is linked to apoptosis and chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and asthma (Su et al., 2018).
Studies have shown that Lico A can inhibit TNF-α-induced NF-κB transcriptional activity, possibly by suppressing IKK activation and IκB degradation (Tsai et al., 2014). Lico A also inhibits the secretion of IL-1β, IL-6, and TNF-α inflammatory cytokines by down-regulating TLR-4 expression and inhibiting the TLR-4/NF-κB inflammatory signaling pathway (Lv et al., 2019). Moreover, Lico A has shown significant inhibition in LPS-induced microglial cell line BV-2 phosphorylation, suggesting a neuroprotective pharmacological activity (Huang et al., 2017).
3.2 Lico A achieves anti-inflammatory effects by regulating Nrf2 pathway
Nrf2, part of the Cap-n-Collar (CNC) regulatory protein family, is a critical transcription factor for cellular antioxidant stress (Moi et al., 1994). Under normal conditions, it remains inactive, bound to Keap1 in the cytoplasm. External stimuli or oxidative stress trigger Nrf2’s dissociation from Keap1, followed by phosphorylation and nuclear transfer. Nrf2 then binds to the antioxidant response element (ARE), initiating the expression of phase II metabolic enzymes and antioxidants, thereby enhancing the body’s resistance to oxidative stress (Tu et al., 2019). The anti-inflammatory impact of the Nrf2 pathway mainly stems from Nrf2 antioxidant pathway activation, which reduces NF-κB’s stress-sensitive expression by lowering IκB phosphorylation and subsequently diminishing inflammation (Chen et al., 2006). Nrf2 and NF-κB pathways mutually inhibit each other (Pedruzzi et al., 2012).
Research has uncovered that Lico A’s anti-arthritis effects depend on the activation of the Keap1-Nrf2 signaling pathway through p62 phosphorylation at the Ser349 site (Su et al., 2018). In the context of neuroinflammation, Lico A protects OGD/R-stimulated rat primary cortical neurons, and counters oxidative stress-induced neuronal damage, and inflammatory reactions by activating the SIRT1/Nrf2 signaling pathway and inhibiting its downstream NF-κB signaling pathway (Liu D. et al., 2018a). Table 2 illustrates the effect of Lico A on different inflammations and the specific mechanisms of action.
4 Other pharmacological activities of Lico A
4.1 Improve obesity and lower blood glucose
Obesity, a significant risk factor for chronic diseases including cardiovascular ailments, hypertension, osteoarthritis, specific cancers, and diabetes, is increasingly prevalent worldwide (Tang et al., 2017). It also contributes to nonalcoholic fatty liver disease (NAFLD) and hepatic steatosis (Reccia et al., 2017). Research has shown that Lico A treatment in high-fat diet (HFD)-induced obese mice reduces body weight and decreases inguinal and epididymal adipose tissue compared to HFD-treated mice. Additionally, Lico A improves hepatic steatosis, regulates serum triglycerides, low-density lipoproteins, free fatty acids, and lowers fasting blood glucose levels (Luo et al., 2019). Lico A’s specific lipid-lowering mechanism involves activating the SIRT1/AMPK pathway, reducing fatty acid synthesis, and enhancing lipolysis and beta-oxidation in hepatocytes (Liou et al., 2019).
Inducing the browning of white adipose tissue (WAT) represents a promising strategy for obesity treatment (Kajimura et al., 2010; Bartelt et al., 2011). Lico A enhances the expression of brown fat markers, reducing obesity and restoring metabolic equilibrium (Lee et al., 2018).
4.2 Anti-bacterial and fungal effects
Salmonellosis, caused by multi-drug-resistant Salmonella Typhimurium, poses a global public health threat (Behravesh et al., 2014). Lico A inhibits the growth of S. Typhimurium at MIC levels of 62.5–1,000 μg/mL, with an MBC value > 1,000 μg/mL (Hosseinzadeh et al., 2018). Additionally, Lico A exhibits substantial antifungal activity against Candida albicans, inhibiting biofilm formation by 35%–60%, and suppressing yeast-hyphal transformation and protease secretion (Seleem et al., 2016).
4.3 Antiparasitic effect
Toxoplasma gondii, the causative agent of toxoplasmosis, poses significant public health challenges (Ajzenberg et al., 2016). Lico A effectively inhibits T. gondii proliferation in a dose- and time-dependent manner with low cytotoxicity against HFF host cells (Si et al., 2018). Additionally, Lico A reduces the total number of Schistosoma mansoni eggs, likely by increasing ROS production and inducing the death of adult Schistosoma mansoni (Souza et al., 2017).
4.4 Strengthen bone formation and increase bone mass
Osteoporosis, characterized by loss of bone microstructure, heightens fracture risk (Smith and Walker, 1976). The role of bone marrow mesenchymal stem cells (BMSCs) in osteoporosis has drawn increasing attention. Lico A exerts a potent influence on BMSC osteogenic differentiation and mineralization by up-regulating FasL, and further modulating ERK and GSK-3β-catenin. Through the activation of intraosseous bone formation and partial inhibition of bone resorption in an acute estrogen deficiency model, Lico A administration restores or protects bone mass in disease states (Ming et al., 2015).
4.5 Intestinal protective activity
In a recent study, the intestinal protective effect of Lico A was revealed. It was indicated that Lico A could promote intestinal epithelial renewal to exert intestinal protective effect. The mechanism involves regulating T-UCRs (transcripts from ultra-conserved regions) (Wang et al., 2024).
5 Discussion
Anti-cancer and anti-inflammatory properties are the main characteristic bioactivities of Lico A, compared with other pharmacological activities. It has been reported that there is a close relationship between inflammation and cancer. On one hand, the persistent inflammatory microenvironment instigates tumors by initiating specific genetic mutations (Botta et al., 2016). On the other, a growing body of evidence indicates that tumor-related inflammation promotes angiogenesis and metastasis. This loop regulation suggests that Lico A has great potential in cancer prevention for its action of mechanism. The NF-κB pathway, recognized as a classical inflammation pathway, is a key channel through which Lico A exerts its effects against inflammation conditions such as hepatitis, neuroinflammation, and mastitis (Sen and Baltimore, 1986). The MAPK pathway, implicated in both inflammation and cancer, is another target of Lico A. By influencing these targets, Lico A delivers either anti-inflammatory or anti-cancer effects.
In addition to anti-inflammation and anti-cancer activities, Lico A can also elicit other activities like Anti-bacterial and fungal, Antiparasitic, and intestinal protective effects. However, investigations on these bioactivities are relatively few and lack systematic in-depth studies to fully demonstrate the potential of Lico A, which hindered the further development as a natural bioactive molecule and becomes the key limitation for current research of Lico A.
In addition to inflammation and cancer, recent studies showed the modulatory effect of Lico A on Post-transcriptional regulation. Post-transcriptional regulation refers to the regulation of gene expression after RNA transcription and is a characteristic of gene expression in eukaryotes (Dykes and Emanueli, 2017). The initial transcript must undergo a series of processes before transforming into a functional mature mRNA, serving as a template for protein translation (Masamha and Wagner, 2017). Various mechanisms regulate and control the type and quantity of gene expression during this process. Current research focuses on non-coding RNA (ncRNA) such as miRNA, lncRNA, and circRNA (Tezcan et al., 2019). It is concluded that Lico A can regulate the Nrf2 and MAPK pathways by modulating miR-144, indicating that Lico A has the potential to regulate ncRNA, providing new avenues for studying its pharmacological mechanisms. Moreover, a recent study shows that Lico A can modulate T-UCR regulation. As T-UCRs are also non-coding RNAs and have good conservative characteristics among rats, mice, and humans, playing a fundamental and primary role in gene regulation, more research should be performed to explore the effect of Lico A on posttranscriptional gene regulation.
Author contributions
ML: Writing–original draft. YD: Writing–original draft. DG: Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajzenberg, D., Lamaury, I., Demar, M., Vautrin, C., Cabie, A., Simon, S., et al. (2016). Performance testing of pcr assay in blood samples for the diagnosis of toxoplasmic encephalitis in aids patients from the French departments of America and genetic diversity of toxoplasma gondii: a prospective and multicentric study. PLoS Negl. Trop. Dis. 10 (6), e0004790. doi:10.1371/journal.pntd.0004790
Armanini, D., Fiore, C., Mattarello, M. J., Bielenberg, J., and Palermo, M. (2002). History of the endocrine effects of licorice. Exp. Clin. Endocrinol. Diabetes 110 (6), 257–261. doi:10.1055/s-2002-34587
Bartelt, A., Bruns, O. T., Reimer, R., Hohenberg, H., Ittrich, H., Peldschus, K., et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17 (2), 200–205. doi:10.1038/nm.2297
Behravesh, C. B., Brinson, D. L., Hopkins, B. A., and Gomez, T. M. (2014). Backyard poultry flocks and salmonellosis: a recurring, yet preventable public health challenge. Clin. Infect. Dis. 58 (10), 1432–1438. doi:10.1093/cid/ciu067
Bortolotto, L. F. B., Barbosa, F. R., Silva, G., Bitencourt, T. A., Beleboni, R. O., Baek, S. J., et al. (2017). Cytotoxicity of trans -chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 85, 425–433. doi:10.1016/j.biopha.2016.11.047
Botta, C., Misso, G., Martino, E. C., Pirtoli, L., Cusi, M. G., Tassone, P., et al. (2016). The route to solve the interplay between inflammation, angiogenesis and anti-cancer immune response. Cell Death Dis. 7 (7), e2299. doi:10.1038/cddis.2016.211
Chen, G., Ma, Y., Jiang, Z., Feng, Y., Han, Y., Tang, Y., et al. (2018). Lico A causes ER stress and apoptosis via up-regulating miR-144-3p in human lung cancer cell line H292. Front. Pharmacol. 9, 837. doi:10.3389/fphar.2018.00837
Chen, X., Dodd, G., Thomas, S., Zhang, X., Wasserman, M. A., Rovin, B. H., et al. (2006). Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 290 (5), H1862–H1870. doi:10.1152/ajpheart.00651.2005
Chen, X., Liu, Z., Meng, R., Shi, C., and Guo, N. (2017). Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol. 198, 331–337. doi:10.1016/j.jep.2017.01.028
Chen, X., Zheng, C., Wang, C., Guo, Z., Gao, S., Ning, Z., et al. (2018). Systems-mapping of herbal effects on complex diseases using the network-perturbation signatures. Front. Pharmacol. 9, 1174. doi:10.3389/fphar.2018.01174
Cheng, A., Tan, X., Sun, J., Gu, C., Liu, C., and Guo, X. (2019). Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS One 14 (5), e0217090. doi:10.1371/journal.pone.0217090
Cho, J. J., Chae, J., Yoon, G., Kim, K. H., Cho, J. H., Cho, S. S., et al. (2014). Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int. J. Oncol. 45 (2), 667–674. doi:10.3892/ijo.2014.2461
Chuang, C., Tang, C., Ho, H., Hsin, C., Weng, C., Yang, S., et al. (2019). Licochalcone A induces apoptotic cell death via JNK/p38 activation in human nasopharyngeal carcinoma cells. Environ. Toxicol. 34 (7), 853–860. doi:10.1002/tox.22753
Cui, Y., Ao, M., Li, W., Hu, J., and Yu, L. (2008). Anti-inflammatory activity of licochalcone A isolated from Glycyrrhiza inflata. Z Naturforsch C J. Biosci. 63, 361–365. doi:10.1515/znc-2008-5-609
Daniell, H., Dunn, S. R., Ferguson, D. W., Lomas, G. M., Niazi, Z., and Stratte, P. T. (2000). Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J. Urol. 163 (1), 181–186. doi:10.1016/s0022-5347(05)68000-7
Dykes, I. M., and Emanueli, C. (2017). Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics Proteomics Bioinforma. 15 (3), 177–186. doi:10.1016/j.gpb.2016.12.005
Gabriele, M., and Pucci, L. (2017). Diet bioactive compounds: implications for oxidative stress and inflammation in the vascular system. Endocr. Metab. Immune Disord. Drug Targets 17 (4), 264–275. doi:10.2174/1871530317666170921142055
Guo, W., Liu, B., Yin, Y., Kan, X., Gong, Q., Li, Y., et al. (2019). Licochalcone A protects the blood-milk barrier integrity and relieves the inflammatory response in LPS-induced mastitis. Front. Immunol. 10, 287. doi:10.3389/fimmu.2019.00287
Hao, W., Yuan, X., Yu, L., Gao, C., Sun, X., Wang, D., et al. (2015). Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 5 (1), 10336. doi:10.1038/srep10336
Herrera, M., Herrera, A., and Ariño, A. (2009). Estimation of dietary intake of ochratoxin A from liquorice confectionery. Food Chem. Toxicol. 47 (8), 2002–2006. doi:10.1016/j.fct.2009.05.009
Hong, S. H., Cha, H.-J., Hwang-Bo, H., Kim, M. Y., Kim, S. Y., Ji, S. Y., et al. (2019). Anti-proliferative and pro-apoptotic effects of licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int. J. Mol. Sci. 20 (15), 3820. doi:10.3390/ijms20153820
Hosseinzadeh, S., Saei, H. D., Ahmadi, M., and Salehi, T. Z. (2018). Antimicrobial effect of Licochalcone A and Epigallocatechin-3-gallate against Salmonella Typhimurium isolated from poultry flocks. Iran. J. Microbiol. 10 (1), 51–58.
Hu, J., and Liu, J. (2016). Licochalcone A attenuates lipopolysaccharide-induced acute kidney injury by inhibiting NF-κB activation. Inflammation 39 (2), 569–574. doi:10.1007/s10753-015-0281-3
Huang, B., Juxiong, L., Chen, J., Dongxue, Y., Guangxin, C., Shiyao, X., et al. (2017). Licochalcone A prevents the loss of dopaminergic neurons by inhibiting microglial activation in lipopolysaccharide (LPS)-Induced Parkinson’s disease models. Int. J. Mol. Sci. 18 (10), 2043. doi:10.3390/ijms18102043
Huang, C., Yang, S., Chiou, H., Hsu, W., Hsu, J., Liu, C., et al. (2018). Licochalcone A inhibits the invasive potential of human glioma cells by targeting the MEK/ERK and ADAM9 signaling pathways. Food Funct. 9 (12), 6196–6204. doi:10.1039/c8fo01643g
Huang, H. C., Tsai, L. L., Tsai, J. P., Hsieh, S. C., Yang, S., Hsueh, J. T., et al. (2014). Licochalcone A inhibits the migration and invasion of human lung cancer cells via inactivation of the Akt signaling pathway with downregulation of MMP-1/-3 expression. Tumour Biol. 35 (12), 12139–12149. doi:10.1007/s13277-014-2519-3
Huang, W., Su, H., Fang, L., Wu, S., and Liou, C. (2019). Licochalcone A inhibits cellular motility by suppressing E-cadherin and MAPK signaling in breast cancer. Cells 8 (3), 218. doi:10.3390/cells8030218
Huang, W. C., Liu, C. Y., Shen, S. C., Chen, L. C., Yeh, K. W., Liu, S. H., et al. (2019). Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells 8 (6), 617. doi:10.3390/cells8060617
Jiang, J., Yuan, X., Zhao, H., Yan, X., Sun, X., and Zheng, Q. (2014). Licochalcone A inhibiting proliferation of bladder cancer T24 cells by inducing reactive oxygen species production. Biomed. Mater Eng. 24 (1), 1019–1025. doi:10.3233/BME-130899
Kajimura, S., Seale, P., and Spiegelman, B. M. (2010). Transcriptional control of Brown fat development. Cell Metab. 11 (4), 257–262. doi:10.1016/j.cmet.2010.03.005
Kang, T., Seo, J., Oh, H., Yoon, G., Chae, J., and Shim, J. (2017). Licochalcone A suppresses specificity protein 1 as a novel target in human breast cancer cells. J. Cell Biochem. 118 (12), 4652–4663. doi:10.1002/jcb.26131
Kim, K. H., Yoon, G., Cho, J. J., Cho, J. H., Cho, Y. S., Chae, J., et al. (2015). Licochalcone A induces apoptosis in malignant pleural mesothelioma through downregulation of Sp1 and subsequent activation of mitochondria-related apoptotic pathway. Int. J. Oncol. 46 (3), 1385–1392. doi:10.3892/ijo.2015.2839
Kim, Y. H., Shin, E. K., Kim, D. H., Lee, H. H., Park, J. H. Y., and Kim, J. (2010). Antiangiogenic effect of licochalcone A. Biochem. Pharmacol. 80 (8), 1152–1159. doi:10.1016/j.bcp.2010.07.006
Kojima, Y., Hayakawa, F., Morishita, T., Sugimoto, K., Minamikawa, Y., Iwase, M., et al. (2017). YM155 induces apoptosis through proteasome-dependent degradation of MCL-1 in primary effusion lymphoma. Pharmacol. Res. 120, 242–251. doi:10.1016/j.phrs.2017.04.006
Kumar, D., Kumar, N. M., Tantak, M. P., Ogura, M., Kusaka, E., and Ito, T. (2014). Synthesis and identification of α-cyano bis(indolyl)chalcones as novel anticancer agents. ACS Med. Chem. Lett. 24 (22), 5170–5174. doi:10.1016/j.bmcl.2014.09.085
Kuramoto, K., Suzuki, S., Sakaki, H., Takeda, H., Sanomachi, T., Seino, S., et al. (2017). Licochalcone A specifically induces cell death in glioma stem cells via mitochondrial dysfunction. FEBS Open Bio 7 (6), 835–844. doi:10.1002/2211-5463.12226
Lee, H., Yang, G., Han, S., Lee, J., An, T., Jang, J., et al. (2018). Anti-obesity potential of Glycyrrhiza uralensis and licochalcone A through induction of adipocyte browning. Biochem. Biophys. Res. Commun. 503 (3), 2117–2123. doi:10.1016/j.bbrc.2018.07.168
Li, B., Zhou, D., Li, S., Feng, Y., Li, X., Chang, W., et al. (2020). Licochalcone A reverses NNK-induced ectopic miRNA expression to elicit in vitro and in vivo chemopreventive effects. Phytomedicine 76, 153245. doi:10.1016/j.phymed.2020.153245
Liou, C. J., Lee, Y. K., Ting, N. C., Chen, Y. L., Shen, S. C., Wu, S. J., et al. (2019). Protective effects of licochalcone A ameliorates obesity and non-alcoholic fatty liver disease via promotion of the Sirt-1/AMPK pathway in mice fed a high-fat diet. Cells 8 (5), 447. doi:10.3390/cells8050447
Liu, D., Huo, X., Gao, L., Zhang, J., Ni, H., and Cao, L. (2018a). NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed. Pharmacother. 102, 922–929. doi:10.1016/j.biopha.2018.03.130
Liu, X., Ma, Y., Wei, X., and Fan, T. (2018b). Neuroprotective effect of licochalcone A against oxygen-glucose deprivation/reperfusion in rat primary cortical neurons by attenuating oxidative stress injury and inflammatory response via the SIRT1/Nrf2 pathway†. J. Cell Biochem. 119 (4), 3210–3219. doi:10.1002/jcb.26477
Lu, W. J., Wu, G. J., Chen, R. J., Chang, C. C., Lien, L. M., Chiu, C. C., et al. (2018). Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 9 (8), 4500–4507. doi:10.1039/c8fo00728d
Luo, Z., Guo, Z., Xiao, T., Liu, H., Su, G., and Zhao, Y. (2019). Enrichment of total flavones and licochalcone A from licorice residues and its hypoglycemic activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1114-1115, 134–145. doi:10.1016/j.jchromb.2019.01.026
Lv, H., Ren, H., Wang, L., Chen, W., and Ci, X. (2015). Lico A enhances Nrf2-mediated defense mechanisms against t-BHP-induced oxidative stress and cell death via Akt and ERK activation in RAW 264.7 cells. Oxid. Med. Cell Longev. 2015, 709845. doi:10.1155/2015/709845
Lv, H., Yang, H., Wang, Z., Feng, H., Deng, X., Cheng, G., et al. (2019). Nrf2 signaling and autophagy are complementary in protecting lipopolysaccharide/d-galactosamine-induced acute liver injury by licochalcone A. Cell Death Dis. 10 (4), 313. doi:10.1038/s41419-019-1543-z
Maracle, C. X., Kucharzewska, P., Helder, B., Der Horst, C. V., De Sampaio, P. C., Noort, A. R., et al. (2017). Targeting non-canonical nuclear factor-κB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis. Rheumatology 56 (2), 294–302. doi:10.1093/rheumatology/kew393
Masamha, C. P., and Wagner, E. J. (2017). The contribution of alternative polyadenylation to the cancer phenotype. Carcinogenesis 39 (1), 2–10. doi:10.1093/carcin/bgx096
Mazzucchelli, C. R. B., and Brambilla, R. (2000). Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol. Life Sci. 57 (4), 604–611. doi:10.1007/PL00000722
Ming, L., Jin, F., Huang, P., Luo, H., Liu, W., Zhang, L., et al. (2015). Licochalcone A up-regulates of FasL in mesenchymal stem cells to strengthen bone formation and increase bone mass. Sci. Rep. 4 (1), 7209. doi:10.1038/srep07209
Moi, P., Chan, K., Asunis, I., Cao, A., and Kan, Y. W. (1994). Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U. S. A. 91 (21), 9926–9930. doi:10.1073/pnas.91.21.9926
Mukne, A., Viswanathan, V., and Phadatare, A. G. (2011). Structure pre-requisites for isoflavones as effective antibacterial agents. Pharmacogn. Rev. 5 (9), 13–18. doi:10.4103/0973-7847.79095
Napetschnig, J., and Wu, H. (2013). Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 42 (1), 443–468. doi:10.1146/annurev-biophys-083012-130338
Niu, Q., Zhao, W., Wang, J., Li, C., Yan, T., Lv, W., et al. (2018). LicA induces autophagy through ULK1/Atg13 and ROS pathway in human hepatocellular carcinoma cells. Int. J. Mol. Med. 41 (5), 2601–2608. doi:10.3892/ijmm.2018.3499
Park, M. R., Kim, S. G., Cho, I. A., Oh, D., Kang, K. R., Lee, S. Y., et al. (2015). Licochalcone-A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation-mediated TRAIL expression in head and neck squamous carcinoma FaDu cells. Food Chem. Toxicol. 77, 34–43. doi:10.1016/j.fct.2014.12.013
Pastorino, G., Cornara, L., Soares, S., Rodrigues, F., and Oliveira, M. B. P. P. (2018). Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother. Res. 32 (12), 2323–2339. doi:10.1002/ptr.6178
Pedruzzi, L. M., Stocklerpinto, M. B., Leite, M., and Mafra, D. (2012). Nrf2–keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie 94 (12), 2461–2466. doi:10.1016/j.biochi.2012.07.015
Perezvizcaino, F., and Fraga, C. G. (2018). Research trends in flavonoids and health. Arch. Biochem. Biophys. 646, 107–112. doi:10.1016/j.abb.2018.03.022
Qiu, C., Zhang, T., Zhang, W., Zhou, L., Yu, B., Wang, W., et al. (2017). Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing G2/M cell cycle arrest and ER stress. Int. J. Mol. Sci. 18 (8), 1761. doi:10.3390/ijms18081761
Reccia, I., Kumar, J., Akladios, C., Virdis, F., Pai, M., Habib, N. A., et al. (2017). Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism 72, 94–108. doi:10.1016/j.metabol.2017.04.011
Rizzato, G., Scalabrin, E., Radaelli, M., Capodaglio, G., and Piccolo, O. (2017). A new exploration of licorice metabolome. Food Chem. 221, 959–968. doi:10.1016/j.foodchem.2016.11.068
Seleem, D., Benso, B., Noguti, J., Pardi, V., and Murata, R. M. (2016). In vitro and in vivo antifungal activity of lichochalcone-A against Candida albicans biofilms. PLoS One 11 (6), e0157188. doi:10.1371/journal.pone.0157188
Sen, R., and Baltimore, D. (1986). Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell 47 (6), 921–928. doi:10.1016/0092-8674(86)90807-x
Shen, H., Zeng, G., Tang, G., Cai, X., Bi, L., Huang, C., et al. (2014). Antimetastatic effects of licochalcone A on oral cancer via regulating metastasis-associated proteases. Tumour Biol. 35 (8), 7467–7474. doi:10.1007/s13277-014-1985-y
Si, H., Xu, C., Zhang, J., Zhang, X., Li, B., Zhou, X., et al. (2018). Licochalcone A: an effective and low-toxicity compound against Toxoplasma gondii in vitro and in vivo. Int. J. Parasitol. Drugs Drug Resist 8 (2), 238–245. doi:10.1016/j.ijpddr.2018.02.006
Siddiqui, Z. N., Musthafa, T. N. M., Ahmad, A., and Khan, A. U. (2011). Thermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agents. Bioorg Med. Chem. Lett. 21 (10), 2860–2865. doi:10.1016/j.bmcl.2011.03.080
Silva, G. F. D., Marins, M., Chaichanasak, N., Yoon, Y., Fachin, A. L., Pinhanelli, V., et al. (2018). Trans-chalcone increases p53 activity via DNAJB1/HSP40 induction and CRM1 inhibition. PLoS One 13 (8), e0202263. doi:10.1371/journal.pone.0202263
Smith, D. A., and Walker, M. S. (1976). Changes in plasma steroids and bone density in Klinefelter's syndrome. Calcif. Tissue Res. 22, 225–228. doi:10.1007/BF02064069
Souza, R. L., Goncalves, U. O., Badoco, F. R., Galvao, L. D. S., Santos, R. A. D., De Carvalho, P. H. D., et al. (2017). Licochalcone A induces morphological and biochemical alterations in Schistosoma mansoni adult worms. Biomed. Pharmacother. 96, 64–71. doi:10.1016/j.biopha.2017.09.128
Su, X., Li, T., Liu, Z., Huang, Q., Liao, K., Ren, R., et al. (2018). Licochalcone A activates Keap1-Nrf2 signaling to suppress arthritis via phosphorylation of p62 at serine 349. Free Radic. Biol. Med. 115, 471–483. doi:10.1016/j.freeradbiomed.2017.12.004
Sulzberger, M., Worthmann, A. C., Holtzmann, U., Buck, B., Jung, K. A., Schoelermann, A. M., et al. (2016). Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. J. Eur. Acad. Dermatol Venereo Suppl. 1, 9–17. doi:10.1111/jdv.13529
Tang, R., Liu, H., Yuan, Y., Xie, K., Xu, P., Liu, X., et al. (2017). Genetic factors associated with risk of metabolic syndrome and hepatocellular carcinoma. Oncotarget 8 (21), 35403–35411. doi:10.18632/oncotarget.15893
Tang, Z., Chen, X., Wang, Z., Chai, K., Wang, Y., Xu, X., et al. (2016). Induction of C/EBP homologous protein-mediated apoptosis and autophagy by licochalcone A in non-small cell lung cancer cells. Sci. Rep. 6 (1), 26241. doi:10.1038/srep26241
Tezcan, G., Martynova, E. V., Gilazieva, Z. E., McIntyre, A., and Khaiboullina, S. F. (2019). MicroRNA post-transcriptional regulation of the NLRP3 inflammasome in immunopathologies. Front. Pharmacol. 10, 451. doi:10.3389/fphar.2019.00451
Tsai, J. P., Hsiao, P. C., Yang, S., Hsieh, S. C., Bau, D. T., Ling, C. L., et al. (2014). Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-κB mediated urokinase plasminogen activator expression. PLoS One 9 (1), e86537. doi:10.1371/journal.pone.0086537
Tsai, J. P., Lee, C. H., Ying, T. H., Lin, C. L., Lin, C. L., Hsueh, J. T., et al. (2015). Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget 6 (30), 28851–28866. doi:10.18632/oncotarget.4767
Tu, W., Wang, H., Li, S., Liu, Q., and Sha, H. (2019). The anti-inflammatory and anti-oxidant mechanisms of the keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 10 (3), 637–651. doi:10.14336/AD.2018.0513
Vukics, V., and Guttman, A. (2008). Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 29 (1), 1–16. doi:10.1002/mas.20212
Wang, J., Zhang, Y., Thakur, K., Hussain, S. S., Zhang, J., Xiao, G., et al. (2018). Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem. Toxicol. 120, 407–417. doi:10.1016/j.fct.2018.07.044
Wang, P., Yuan, X., Wang, Y., Zhao, H., Sun, X., and Zheng, Q. (2015). Licochalcone C induces apoptosis via B-cell lymphoma 2 family proteins in T24 cells. Mol. Med. Rep. 12 (5), 7623–7628. doi:10.3892/mmr.2015.4346
Wang, Y., Li, Y., Song, C., Ke, J., Zheng, Y., Chen, G., et al. (2024). Licochalcone A promotes renewal of intestinal mucosa through modulating uc.173. J. Ethnopharmacol. 318, 117044. doi:10.1016/j.jep.2023.117044
Wu, J., Zhang, X., Wang, Y., Sun, Q., Chen, M., Liu, S., et al. (2017). Licochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathway. Oncol. Rep. 39 (3), 1181–1190. doi:10.3892/or.2017.6155
Xue, L., Zhang, W., Fan, Q. X., and Wang, L. X. (2017). Licochalcone A inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol. Lett. 15 (2), 1869–1873. doi:10.3892/ol.2017.7451
Yang, C. S., Chung, J. Y., Yang, G., Chhabra, S. K., and Lee, M. J. (2000). Tea and tea polyphenols in cancer prevention. J. Nutr. 130 (2), 472S–478S. doi:10.1093/jn/130.2.472S
Yang, P., Tuo, L., Wu, Q., and Cao, X. (2014). Licochalcone-A sensitizes human esophageal carcinoma cells to TRAIL-mediated apoptosis by proteasomal degradation of XIAP. Hepatogastroenterology 61 (133), 1229–1234. doi:10.1007/s13277-014-2519-3
Yang, X., Jiang, J., Yang, X., Han, J., and Zheng, Q. (2016). Licochalcone A induces T24 bladder cancer cell apoptosis by increasing intracellular calcium levels. Mol. Med. Rep. 14 (1), 911–919. doi:10.3892/mmr.2016.5334
Zhang, X., Li, C., Lin, Q., He, Z., Feng, F., and He, M. (2019). Pro-angiogenic activity of isoliquiritin on HUVECs in vitro and zebrafish in vivo through Raf/MEK signaling pathway. Life Sci. 223, 128–136. doi:10.1016/j.lfs.2019.03.026
Keywords: Licochalcone A, licorice, anti-cancer, anti-inflammatory, targets
Citation: Liu M, Du Y and Gao D (2024) Licochalcone A: a review of its pharmacology activities and molecular mechanisms. Front. Pharmacol. 15:1453426. doi: 10.3389/fphar.2024.1453426
Received: 23 June 2024; Accepted: 02 August 2024;
Published: 12 August 2024.
Edited by:
Jing Wu, Greater Baltimore Medical Center, United StatesCopyright © 2024 Liu, Du and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dejiang Gao, NDk1NDYyNjMyQHFxLmNvbQ==
†These authors have contributed equally to this work
 Meihua Liu
Meihua Liu Yang Du3†
Yang Du3†