- 1Department of Pharmacology, School of Medicine, University of Galway, Galway, Ireland
- 2Galway University Hospital, Saolta Healthcare Group, Galway, Ireland
- 3School of Psychology, University of Galway, Galway, Ireland
Background: Despite growing use, questions remain surrounding the utility, acceptability and feasibility of chemical adherence testing (CAT) as part of hypertension management in clinical practice.
Objectives: This scoping review aimed to (i) identify and summarise studies using CAT in hypertension management, and (ii) describe and critically evaluate how CAT is currently being used in the clinical management of hypertension.
Eligibility criteria: Peer-reviewed and published studies in English, reporting original research in any setting, with any study design, were included. Search concepts included hypertension, medication adherence, CAT, and their synonyms.
Sources of evidence: Searches were carried out using Ovid Medline, EMBASE, and PsycInfo (EBSCO), alongside manual searching of reference lists. Using Covidence software, we screened titles and abstracts, followed by full-text articles. Data from the included articles were tabulated and summarised.
Results: Of the 618 studies identified, 48 were included. The studies cover diverse clinical settings, and were mostly observational in design. 7 studies reporting adherence analyses within clinical trials for hypertension therapies. The use of theoretical frameworks to guide reporting was rare, and there was considerable variation in key terminology and definitions, most notably in the definition of adherence.
Conclusion: The current body of evidence demonstrates considerable variability in the approach to implementing CAT for hypertension management in clinical practice, and a paucity of randomised controlled trials to evaluate its impact. Future research could (i) adopt a cohesive theoretical framework including clear operational definitions to standardise the approach to this important topic; (ii) further explore the impact of CAT on clinical outcomes using RCTs.
Introduction
Using medicines as prescribed can be a particular challenge in those common chronic conditions that are asymptomatic (Burnier and Egan, 2019). The pain-relief provided by long-term analgesia use, e.g., paracetamol, or the reduction in respiratory symptoms provided by some anti-inflammatory agents, e.g., corticosteroids, can provide a potent means of supporting patient initiation and persistence with long-term therapies (Rottman et al., 2017). In these instances, patients directly experience the benefits of using medicines and the aversive consequences of prematurely terminating medicine use. However, the most frequently used medicines, particularly in older adulthood, are those used for diseases where there is no discernible experience of an illness, such as hypertension (Choudhry et al., 2022).
Hypertension represents the greatest burden of non-communicable disease associated morbidity and mortality globally with a worldwide adult prevalence of disease estimated at 31% and affecting 1.39 billion individuals (Forouzanfar et al., 2015; Mills et al., 2016). Internationally, blood pressure remains above target in 63% of all diagnosed hypertensive patients in high-income western countries (Zhou et al., 2019). Several factors contribute to poor blood pressure control including undiagnosed or unrecognised secondary hypertension, so-called treatment resistant hypertension, physician inertia, and non-adherence to anti-hypertensives (Bunker et al., 2011; Durand et al., 2017; Hayes et al., 2019; Kjeldsen et al., 2015).
Adherence to antihypertensive drug (AHD) therapy is central to sustained control of blood pressure, reducing clinic visits and reducing complications of undertreated hypertension (Berra et al., 2016; Hill et al., 2011; Mazzaglia et al., 2009). Moreover, identifying non-adherence in patients who are not meeting BP targets could help providers avoid over-prescription and unnecessary investigation, and to prioritise patients who require more detailed investigation for secondary causes of hypertension, thereby having substantial clinical and economic impact (Schoonhoven et al., 2018).
Hypertension care providers report having little time and few tools to support detecting and improving adherence in their patients (Burnier et al., 2021). Objective assessment of adherence using chemical adherence testing, where available, is recommended by the 2023 European Society of Hypertension (ESH) guidelines for the management of arterial hypertension and the 2024 European Society of Cardiology (ESC) Guidelines for the management of elevated blood pressure and hypertension, and has been described as one of the most reliable methods for assessing adherence (Hayes et al., 2019; Tomaszewski et al., 2014; Curneen et al., 2022; Mancia et al., 2023; McEvoy et al., 2024; Wunder et al., 2019).
High performance liquid chromatography tandem mass spectrometry (LC-MS/MS), can measure anti-hypertensives and their metabolites within patient urine or blood samples, providing point-in-time estimation of anti-hypertensive adherence. LC-MS/MS of urine is usually employed as a qualitative method, describing presence or absence of drugs only, and results are influenced by inter-drug and inter-individual differences in pharmacokinetics (Berra et al., 2016; Wunder et al., 2019). Urine LC-MS/MS analysis can also detect drug metabolites which may be detectable for longer periods of time than the parent drug itself. In this way, urine analysis tends to refer to a longer period of time than serum analysis. LC-MS/MS analysis of serum may provide a more accurate point-in-time estimation of adherence as it allows for quantitative assessment to determine the drug level, which can be used to optimise drug dosage or estimate the time since last intake (Ritscher et al., 2020). Analysis of oral fluids and hair have also been suggested though neither is currently commonly used (Sharma et al., 2023; Lauder et al., 2020).
LC-MS/MS, has several advantages over other methods of adherence assessment. Self-report has been shown to correlate poorly with direct or objective methods of adherence measurement (Osterberg and Blaschke, 2005). Pharmacy dispensing records may not adequately reflect adherence if prescription data are not captured from all potential sources or patients do not take the dispensed medications (Ruzicka et al., 2019). Electronic pill boxes may not always be available and are less acceptable and feasible for those on multiple medicines, such as people with resistant hypertension (RH) (El Alili et al., 2016; Van Onzenoort et al., 2012). Directly Observed Therapy (DOT) combined with ambulatory BP monitoring (ABPM) has also been successfully employed (Hjørnholm et al., 2019). It may present feasibility challenges as it requires resources for monitoring, given the potential to cause symptomatic hypotension (Ruzicka et al., 2019).
However, despite growing consensus that chemical adherence testing (CAT) represents a potentially valuable tool in hypertension management (Mancia et al., 2023; Wunder et al., 2019), particularly in hypertension which has proven difficult to treat, the optimum manner of its use remains unclear. A disparate literature on CAT use in hypertension is developing where agreement on key terminology, definitions and methods is only beginning to emerge over the last 5 years (Wunder et al., 2019). There is a pressing need, therefore, to carry out evidence syntheses, as relevant studies have straddled multiple basic science and clinical literatures.
As distinct from systematic reviews, scoping reviews allow for a broader focus and present results in descriptive formats that highlight what kinds of evidence exist, where there are evidence gaps, and the quality of the existing evidence (Nyanchoka et al., 2019; Arksey and O’Malley, 2005). Scoping reviews are also recommended when there is a need to clarify the key constructs and operational definitions employed in an area of research, to examine the ways in which research in an emerging area is being conducted and to identify the factors associated with a specific concept (Munn et al., 2018; Noone et al., 2021).
For these reasons, we elected to conduct a scoping review to assess the characteristics of research in which chemical adherence testing is implemented in the clinical management of hypertension. The aims of this review were to (i) identify and summarise studies using CAT in hypertension management, and (ii) describe and critically evaluate how CAT is currently being used in the clinical management of hypertension. We report here our findings with reference to the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist (Tricco et al., 2018).
Methods
Protocol and registration
The protocol for this scoping review was registered prior to data extraction on Open Science Framework Registries (Rabbitt et al., 2024).
Research question
To address our aims, we formulated the following research questions:
1. What are the characteristics of research methods on the implementation of CAT for anti-hypertensive pharmacotherapy in clinical practice?
2. What characteristics of CAT implementation can be discerned (e.g., clinical setting, what type of CAT, where in the patient journey)?
Information sources and search strategy
The search was conducted with the assistance of a health sciences librarian. Synonyms for three core concepts were iteratively tested: medication adherence, chemical adherence testing, and hypertension. Three electronic databases were searched from inception to April 2024: MEDLINE (Ovid); EMBASE; and PsycINFO (EBSCO). These databases were chosen given their relevance to the core concepts. In addition, we manually screened the reference lists of review articles identified during screening for relevant references. We used standardised medical subject headings and subject headings provided by the chosen databases. Synonyms were joined by the Boolean operator OR; thereafter, the search strings for each concept were combined with the Boolean operator AND.
Search concepts
1. Medication adherence
2. Chemical adherence testing
3. Hypertension
Search terms (examples–for full search strategy see Supplemental Data Sheet 1)
1. Treatment adherence and compliance; patient compliance; medication adherence
2. Chemical adherence testing; drug monitoring; therapeutic drug monitoring; mass spectrometry
3. Hypertension; blood pressure; antihypertensive drugs
Eligibility criteria
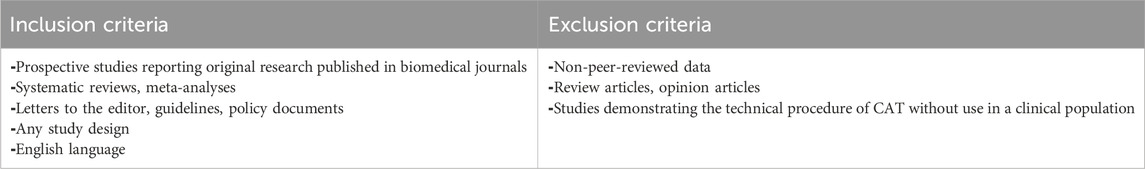
The inclusion and exclusion criteria are shown in Table 1.
Data sought
The types of data collected included clinical data on people with a diagnosis of hypertension, taking antihypertensive medication(s), in any healthcare setting. Methods and outcomes of interest were CAT, with or without comparisons with other methods of measuring medication adherence.
Study selection and synthesis
All identified records were imported into Covidence, a web-based collaboration software platform that streamlines the production of systematic and other literature reviews (Veritas Health Innovation Melbourne Australia, 2024). Duplicates were removed and the titles and abstracts of the remaining records were screened for eligibility by at least one of the authors. Uncertainty or conflict was resolved by discussion until consensus was reached. Full-text articles were then screened independently by two of the authors. Again, conflict or uncertainty were resolved through discussion until consensus was reached. The Covidence data extraction and critical appraisal templates were adapted to address the aims of this review.
The following data were extracted and tabulated:
1. General information: Authors, publication year, country of origin, clinical setting, study aim and study design
2. Participant information: Diagnoses, basic demographic details, number of participants enrolled,
3. CAT details: substrate and method for CAT, whether participants were informed in advance of CAT, whether CAT results were fed back to participants, definition of adherence, phase of adherence targeted, CAT carried out once or on multiple occasions.
4. Results: Key findings with respect to adherence, key findings with respect to blood pressure control or other pertinent clinical outcomes.
Critical appraisal
Depending on the study design, the following quality appraisal tools were applied to the included studies: the Joanna Briggs Institute Critical Appraisal Checklist for analytical cross-sectional research (Moola et al., 2020), and the Cochrane Risk of Bias Tool (Sterne et al., 2019) for Randomised Controlled Trials. Quality assessment was carried out by one reviewer and checked by another. The major confounders considered included the potential for white-coat adherence if participants were informed in advance of the intention to carry out CAT. In addition, we assessed whether studies published in 2018 or later included the four minimum reporting criteria set out by the European Society for Patient Adherence (ESPACOMP) in the ESPACOMP Medication Adherence Reporting Guideline (EMERGE) (De Geest et al., 2018). These guidelines represent an attempt to improve the reporting in adherence research by providing a theoretical framework.
Results
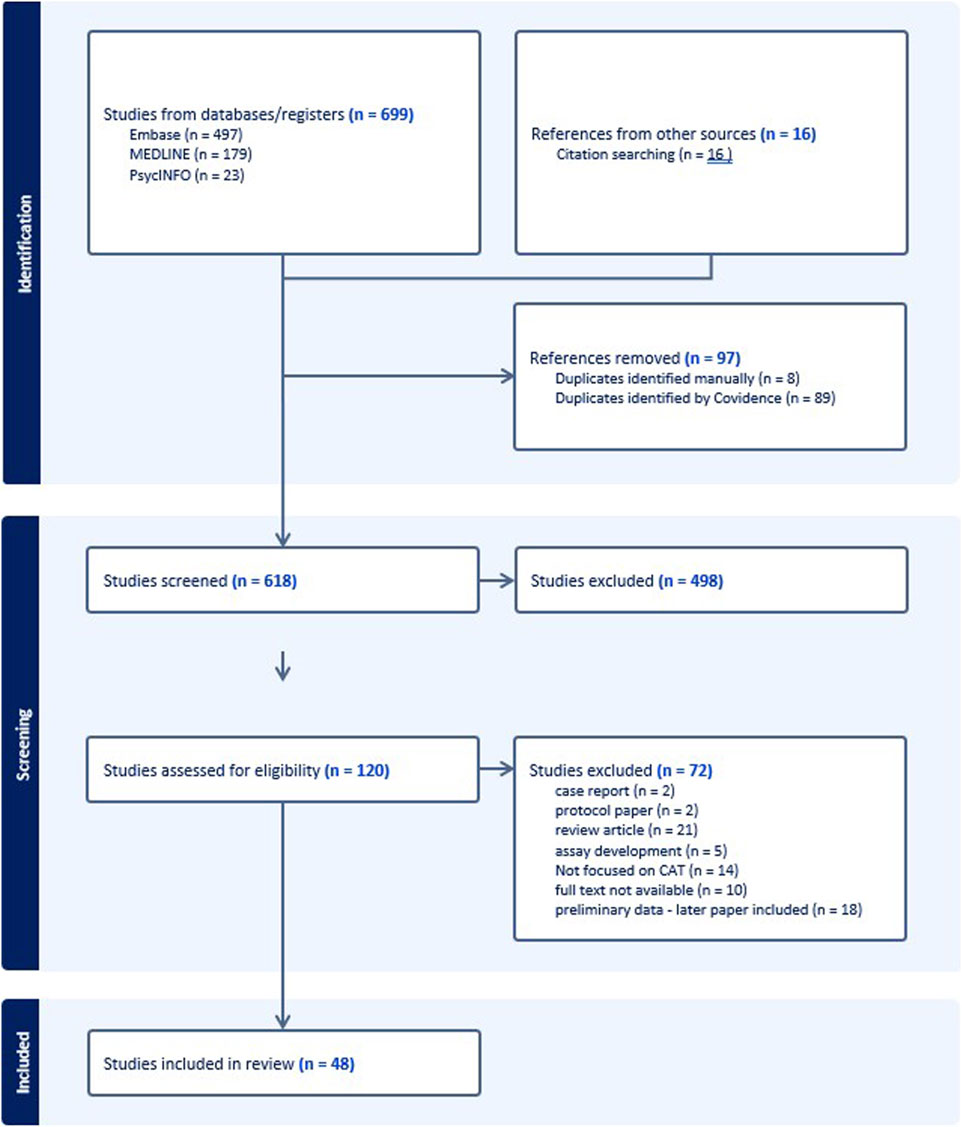
We identified 699 records, of which 683 (97.7%) were identified through database searches, and 16 (2.3%) through manual searches of reference lists in the review articles. After removal of duplicates, we screened titles and abstracts of 618, and the remaining 120 were assessed for eligibility through full-text review. Of these 120, 72 were excluded for the reasons shown in Figure 1, and 48 were included in the scoping review.
Research question 1: characteristics of sources of evidence
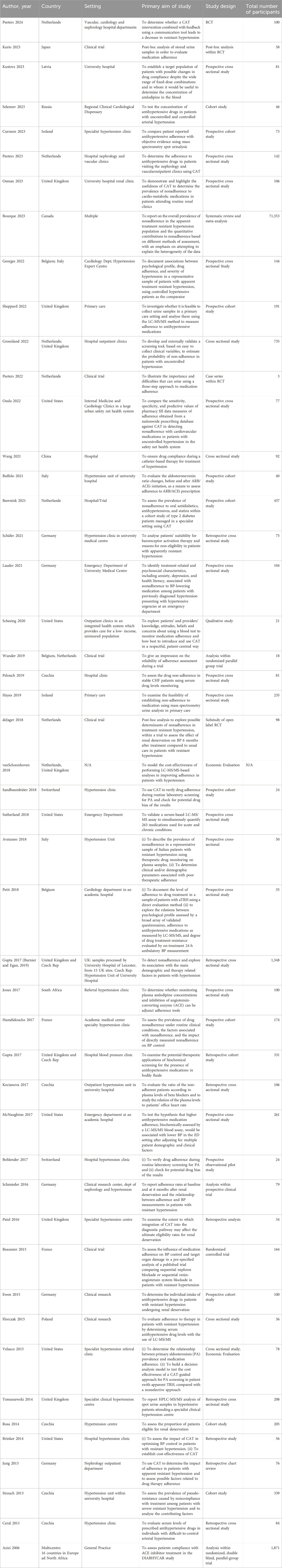
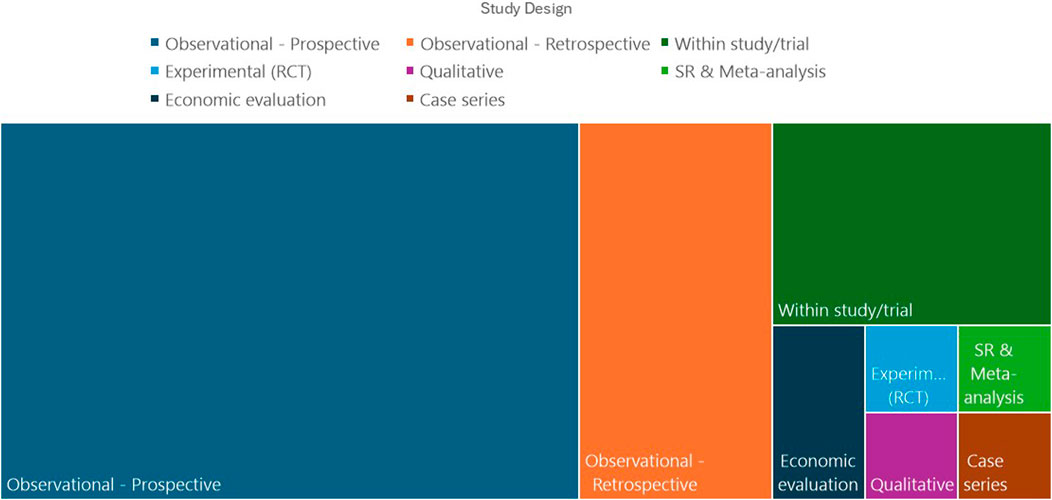
Table 2 shows the characteristics of the included studies. Most of the reviewed studies (44/48) were published within the past 10 years. Most (46/48) of the studies originated from North America and Europe. Figure 2 shows the distribution of study designs. The majority of included studies were observational, and despite several authors pointing out the need for randomised controlled trials (RCT) to delineate the contribution of CAT to optimising hypertension management (Osula et al., 2022), only one RCT was identified which directly examined the effect of CAT (Valgimigli et al., 2019).
Seven of the included studies reported adherence analyses carried out within clinical trials (Azizi et al., 2006; Ewen et al., 2015; Beaussier et al., 2015; Kario et al., 2023; de Jager et al., 2018; Ceral et al., 2011), of which five were clinical trials of renal denervation (RDN). Of these studies relating to RDN, three carried out CAT as pre-specified analyses in the trial design (Azizi et al., 2006; Ewen et al., 2015; Johnson and Hennessy, 2019), and 2 as post hoc analyses (Kario et al., 2023; de Jager et al., 2018). In addition, three observational studies reported adherence rates in patients undergoing RDN, or screening for RDN (de Jager et al., 2018; Patel et al., 2016; Ceral et al., 2011). One systematic review and meta-analysis is included (Bourque et al., 2023). This aimed to establish the overall prevalence of nonadherence in resistant hypertension and compare direct (such as CAT) and indirect (such as pill counting) methods of adherence assessment. The authors found that in 42 studies including 71,353 patients, indirect methods reported less than half the rates of non-adherence compared to direct methods. One qualitative study used interviews with patients and providers and discussion with a community advisory panel to explore attitudes towards using CAT in the clinical management of hypertension (Schesing et al., 2020).
The economic impact of CAT is a growing concern in the literature and will be of interest to those managing and designing clinical services for hypertension. Two studies explored the cost-effectiveness of CAT, in view of the potential for CAT to (i) rationalise diagnostic decision-making and investigations, and (ii) improve BP control and thereby clinical outcomes for patients (Schoonhoven et al., 2018; Velasco et al., 2015).
Included populations
The majority of studies took place in hospital-based secondary or tertiary care settings, with just 3 reported from primary care. Some studies deployed CAT in a targeted way, according to specified clinical criteria such as aTRH, or at the discretion of the treating physician (Florczak et al., 2015; Groenland et al., 2022; Gupta et al., 2017a; Schäfer et al., 2021). Others applied CAT in a non-discriminatory manner, to all patients attending a given service. Ten studies explicitly stated that patients with secondary hypertension were excluded but the manner of screening for secondary hypertension was not always detailed. Eight studies only included participants who reported having taken their medicines as prescribed and excluded those who reported non-adherence (Osula et al., 2022; Ewen et al., 2015; Velasco et al., 2015; Avataneo et al., 2018; Strauch et al., 2013; Jung et al., 2013; Kocianova et al., 2017; Gupta et al., 2017b); this has important implications when considering the rates of false positive CAT results. Supplemental Table 2 shows the characteristics of participants in the included studies.
Research question 2: characteristics of CAT implementation
Methods of CAT
The characteristics of CAT used in the studies is summarised in Supplemental Table 1. Of the 45 included primary quantitative studies, 44 studies used mass spectrometry of either urine (22 studies), serum, dried blood spot, or a combination of samples, to directly detect AHDs or their metabolites. LC-MS/MS was most commonly used but gas chromatography-mass spectrometry and spectrofluorometry were also used (Schäfer et al., 2021; Brinker et al., 2014). Five studies used alternative methods, either alone or in conjunction with LC-MS/MS. These were chiefly assays of the renin-angiotensin-aldosterone axis, such as serial aldosterone to renin ratio measurement (Buffolo et al., 2021), serum Z-FHL/HHL (z-phenylalanine-histidine-leucine/hippuryl-histidine-leucine) ratio (Jones et al., 2017), or urine AcSDKP/creatinine ratio (Beaussier et al., 2015; Hamdidouche et al., 2017). These alternative methods may be useful to providers in situations where LC-MS/MS laboratory analysis is not available. In 27% (12/45) of studies, CAT was performed on more than one occasion, while for the remainder it was performed only once.
Interpretation and application of CAT results
There was considerable variation in the definition of adherence. Adherence was variously considered a dichotomous, categorical or continuous variable. Of the studies using LC-MS/MS, three studies considered a participant “fully” adherent if at least 80% of their prescribed AHDs were found to be present (de Jager et al., 2018; Lauder et al., 2021; Schmieder et al., 2016), while the others required 100% concordance to consider someone adherent. Similarly, while most studies differentiated between “partial” and “complete” non-adherence, ten studies considered a participant non-adherent if there was any discrepancy between their prescribed AHDs and the CAT results (Osula et al., 2022; Ewen et al., 2015; Florczak et al., 2015; Brinker et al., 2014; Ceral et al., 2011; Gupta et al., 2017b; Pelouch et al., 2019; Sheppard et al., 2022; Osman et al., 2023; Beernink et al., 2021). Some studies attempted to address the limitations of CAT by combining it with other methods of adherence testing, for example, Beaussier (2015) uses an adherence scoring system which combines two CAT modalities with self-report and pill counting (Curneen et al., 2022; Beaussier et al., 2015). Six (13%) studies described reporting back the results of CAT to patients, while the remainder either didn’t provide participants with their results, or did not state whether participants received the results of the CAT. The majority of studies were descriptive cross-sectional studies which did not measure longer-term outcomes for patients. Just 4 studies (9%) reported on the impact that CAT had on clinical outcomes for patients (Velasco et al., 2015; Brinker et al., 2014; Gupta et al., 2017b; Valgimigli et al., 2019).
Critical appraisal results
We applied the 4 minimum reporting criteria from the EMERGE guidelines to the included studies published after the guidelines’ publication in 2018 (2019 or later; 22 studies). Of the 22 studies, just 2 (9%) of them included the four minimum reporting criteria set out by the EMERGE guidelines (Groenland et al., 2022; Buffolo et al., 2021). One further study met three of the four criteria (Curneen et al., 2022), while the remaining 19 (86.4%) did not include any of the minimum reporting criteria. It should be noted that most papers did detail the performance of the CAT measure with regard to its validity and reliability but did not consider these factors in reference to the phase(s) of adherence studied. The judgements for each study are included in Supplemental Table 3.
The JBI tool for assessing the quality of cross-sectional studies was applied to 40 studies. For 32 (80%) of these studies, the inclusion criteria were clearly defined, and in 31 (77.5%) the subjects and setting were described in detail. 35 (87.5%) studies used objective, standard criteria when measuring the condition (BP in this case). Confounding variables were identified in 30 (75%) studies, and of these, 13 (43.3%) described a strategy for dealing with these confounding factors. The statistical analysis was considered to be appropriate for 33 (82.5%) of studies. The judgements for each study are presented in Supplemental Table 4.
For the only included RCT which directly assessed the effect of CAT, the Cochrane risk of bias (RoB) tool revealed some concerns, primarily around the unblinded intervention, and the fact that some patients developed an aversion to ambulatory blood pressure monitoring, necessitating the use of alternative BP measures. Moreover, this RCT encountered some difficulties in recruitment and study visits due to the COVID-19 pandemic (Valgimigli et al., 2019).
Discussion
The aim of this review was to identify studies using CAT in hypertension and to describe and critically evaluate how CAT is currently being used in the clinical management of hypertension. We found that the use of CAT in hypertension is gaining significant research interest. We found that research on CAT in hypertension is mostly published in high-income countries, focussed on treatment-resistant hypertension in secondary or specialist healthcare settings, and usually observational in design. Few studies measured the impact that performing CAT has on clinical outcomes for patients, such as BP control. This means that increasing calls for CAT to form part of routine clinical care in hypertension are underpinned by largely observational data. There are relatively few randomised trials to inform CAT use. One recent RCT, published outside the time limit for this review, found no effect of CAT on BP control or adherence, though it was underpowered (Peeters et al., 2024). A number of challenges have been demonstrated with conducting RCTs in the area of adherence (Muntner and Tanner, 2024). The variability in BP control and adherence over time impedes the identification of patients suitable for recruitment. Patients most challenged by adherence may be less likely to be included in trials because of non-attendance, low literacy, low motivation, language barriers, or other psychosocial challenges. Hawthorne effects may influence medication-taking behaviour (Peeters et al., 2023). Recruitment into some recent trials was moreover negatively impacted by restrictions during the COVID-19 pandemic (Halvorsen et al., 2024; Peeters et al., 2024).
The review also identified that CAT methods are primarily based on mass spectrometry, with considerable variability in how the results are interpreted and used. For example,. there is no clear or accepted classification of adherence by CAT, complicating attempts to compare studies. Some studies consider a participant adherent only if there is 100% concordance between their prescribed and detected AHDs, and consider all other results to represent nonadherence, while others differentiate between categories such as “partial” and “complete” nonadherence, though the thresholds for these categories vary. Such discrepancies are a significant barrier to the development of a cumulative evidence base.
Historically, adherence of 80%, adapted from earlier studies based on pill counts and Medication Event Monitoring Systems or MEMS, has been accepted as an acceptable level of adherence, and correlates with cardiovascular outcomes (Valgimigli et al., 2019; Bansilal et al., 2016). Some of the studies in this review have applied this threshold to CAT. However, the validity of this approach with a point-in-time assay such as LC-MS/MS of serum or urine, is questionable. For example, a patient prescribed 4 AHDs who omits their diuretic on a day they have to travel to their hospital appointment, would have an adherence rate of 75% and be considered non-adherent. Labelling such a participant as “nonadherent” (as compared with “partially adherent”) may obscure the distinction between “perfect” and suboptimal adherence patterns and their causes and origins, and may impede the ability of clinicians to interpret these results. Indeed, this case example could represent a patient who is fully committed to their hypertension regimen and engaged with appropriate self-management. Omitting the diuretic dose in this instance can be classified as the kind of careful self-regulation that might be required to attend a clinical appointment, particularly for an older person with mobility limitations. Without some qualitative and contextual patient history the CAT result alone may provide a misleading clinical picture of how medicines are being used.
Few studies reported according to a theoretical framework. The minimum reporting criteria set out in the EMERGE guideline are not commonly adopted in clinical research on this topic. This guideline suggests that researchers define phases of adherence clearly including initiation (when the patient takes the first dose of a prescribed medication), implementation (the extent to which a patient’s actual dosing corresponds to the prescribed regimen), persistence (the length of time between initiation and the last dose) and discontinuation (the end of therapy, after a last dose is taken and no more doses are taken thereafter without a prescriber’s order) (De Geest et al., 2018). It is not clear whether authors are unaware of this guideline or choose not to refer to it for another reason. Recognising the potential for adherence to confound results in blood pressure trials, the Non-adherence Academic Research Consortium within the European Society of Cardiology have produced a consensus report providing a framework for reporting, interpreting and analysing medication non-adherence in cardiovascular clinical trials (Valgimigli et al., 2019). This is particularly relevant for trials of invasive and irreversible interventions such as RDN, and is reflected in the number of studies of RDN included in this review.
There remains considerable variation in terminology used in this topic. Articles published as recently as 2023 use the term “compliance” for medication adherence (Kustovs et al., 2023). A lack of standardised terminology may hinder effective literature searches, making it difficult to compare studies, aggregate data, and draw conclusions. Only one of the included papers used the term “chemical adherence testing” (Osula et al., 2022). Other terms used include biochemical adherence testing, therapeutic drug monitoring, drug screening, drug assays, drug measurement, compliance testing, and many others. The lack of consensus around terminology, definitions and methods may obscure the scope and findings of research, and is an added challenge to evidence synthesis in this area (Wunder et al., 2019).
There is some evidence that CAT itself improves adherence and BP control, regardless of the CAT result (Gupta et al., 2017b), however the quality of this supportive evidence is currently limited to observational evidence and some preliminary RCTs are beginning to appear (Halvorsen et al., 2024; Peeters et al., 2024; Morrissey et al., 2023). CAT may provide a useful impetus to consultations around medication adherence. When reported, communicating CAT results to patients was found to improve blood pressure control (Gupta et al., 2017b). Despite this, few of the included studies indicated that CAT results were communicated to patients or participants. While it is possible that such feedback occurred as part of clinical practice without being reported in the published research, the impact and optimum manner of such feedback is of crucial importance and requires further elucidation, given the concerns about the potential for CAT to negatively impact the patient-physician relationship (Schesing et al., 2020). Concerns have been raised about the ethicality of CAT, which is problematic if CAT is not introduced in a transparent and sensitive manner, with verbal informed consent (Lane et al., 2022).
Most studies measured adherence at a single point in time. This has valuable diagnostic utility if the clinician’s aim is to identify treatment-resistant hypertension, determine whether screening for secondary causes of hypertension is necessary, or to determine a patient’ suitability for specialised treatments such as RDN. However, the correlation between point-in-time CAT and longer-term medication adherence patterns remains unclear (Wunder et al., 2019). The potential need for ongoing chemical adherence monitoring, as part of an effort to optimise long-term management and cardiovascular risk reduction, must be considered. With this in mind, the demonstrated utility of CAT in diverse healthcare settings, including primary care and not just in specialised centres, is a welcome development, however appropriate cost-effectiveness evaluations are required to determine whether the resources required to implement CAT are justified.
Limitations and methodological considerations
The strengths of this study include the broad and inclusive search strategy, the number of records reviewed and the rigorous screening and review process. However, we excluded the grey literature such as published abstracts without a full-text manuscript; this could have captured additional studies and may have provided evidence of novel approaches to CAT implementation in hypertension. Our included studies were limited to the English language. Initial screening of title and abstracts did not require decisions by two reviewers but all decisions in the full text screening and quality appraisal were confirmed by a pair of reviewers. Conflicts and uncertainties were resolved through discussion until consensus was reached. This team-based approach to evidence synthesis with reviewers and information retrieval specialists from diverse academic and clinical backgrounds helped to limit the biases that can affect evidence synthesis (Johnson and Hennessy, 2019).
Conclusion
The current body of evidence demonstrates considerable variability in the approach to implementing CAT for hypertension management in clinical practice, and a paucity of randomised controlled trials to evaluate its impact. Future research could (i) adopt a cohesive theoretical framework including clear operational definitions to standardise the approach to this important topic; and (ii) further explore the impact of CAT on clinical outcomes using RCTs.
Author contributions
LR: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing–original draft, Writing–review and editing. JC: Data curation, Writing–original draft, Writing–review and editing. MD: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. GM: Conceptualization, Formal Analysis, Methodology, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning and the Health and Social Care, Research and Development Division, Northern Ireland.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1452464/full#supplementary-material
References
Arksey, H., and O’Malley, L. (2005). Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. Theory Pract. 8 (1), 19–32. doi:10.1080/1364557032000119616
Avataneo, V., De Nicolo, A., Rabbia, F., Perlo, E., Burrello, J., Berra, E., et al. (2018). Therapeutic drug monitoring-guided definition of adherence profiles in resistant hypertension and identification of predictors of poor adherence. Br. J. Clin. Pharmacol. 84 (11 PG-2535–2543), 2535–2543. doi:10.1111/bcp.13706
Azizi, M., Ménard, J., Peyrard, S., Lièvre, M., Marre, M., and Chatellier, G. (2006). Assessment of patients' and physicians' compliance to an ACE inhibitor treatment based on urinary N-acetyl ser-asp-lys-pro determination in the noninsulin-dependent diabetes, hypertension, microalbuminuria, proteinuria, cardiovascular events, and ramipril (DIABHYCAR) study. Diabetes Care. 29 (6), 1331–1336. doi:10.2337/dc06-0255
Bansilal, S., Castellano, J. M., Garrido, E., Wei, H. G., Freeman, A., Spettell, C., et al. (2016). Assessing the impact of medication adherence on long-term cardiovascular outcomes. J. Am. Coll. Cardiol. 68 (8), 789–801. doi:10.1016/j.jacc.2016.06.005
Beaussier, H., Boutouyrie, P., Bobrie, G., Frank, M., Laurent, S., Coudoré, F., et al. (2015). True antihypertensive efficacy of sequential nephron blockade in patients with resistant hypertension and confirmedmedication adherence. J. Hypertens. 33 (12), 2526–2533. doi:10.1097/HJH.0000000000000737
Beernink, J. M., Oosterwijk, M. M., Khunti, K., Gupta, P., Patel, P., van Boven, J. F. M., et al. (2021). Biochemical urine testing of medication adherence and its association with clinical markers in an outpatient population of type 2 diabetes patients: analysis in the DIAbetes and LifEstyle cohort twente (DIALECT). Diabetes Care 44 (6), 1419–1425. doi:10.2337/dc20-2533
Berra, E., Azizi, M., Capron, A., Høieggen, A., Rabbia, F., Kjeldsen, S. E., et al. (2016). Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension 68 (2), 297–306. doi:10.1161/HYPERTENSIONAHA.116.07464
Bourque, G., Ilin, J. V., Ruzicka, M., Hundemer, G. L., Shorr, R., and Hiremath, S. (2023). Nonadherence is common in patients with apparent resistant hypertension: a systematic review and meta-analysis. Am. J. Hypertens. 36 (7), 394–403. doi:10.1093/ajh/hpad013
Brinker, S., Pandey, A., Ayers, C., Price, A., Raheja, P., Arbique, D., et al. (2014). Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J. Am. Coll. Cardiol. 63 (8 PG-834–5), 834–835. doi:10.1016/j.jacc.2013.10.067
Buffolo, F., Sconfienza, E., Burrello, J., Losano, I., Mengozzi, G., Priolo, G., et al. (2021). Assessment of anti-hypertensive drug adherence by serial aldosterone-to-renin ratio measurement. Front. Pharmacol. 12 (101548923 PG-668843), 668843. doi:10.3389/fphar.2021.668843
Bunker, J., Callister, W., Chang, C. L., and Sever, P. S. (2011). How common is true resistant hypertension. J. Hum. Hypertens. 25 (2), 137–140. doi:10.1038/jhh.2010.108
Burnier, M., and Egan, B. M. (2019). Adherence in hypertension: a review of prevalence, risk factors, impact, and management. Circ. Res. 124 (7), 1124–1140. doi:10.1161/CIRCRESAHA.118.313220
Burnier, M., Prejbisz, A., Weber, T., Azizi, M., Cunha, V., Versmissen, J., et al. (2021). Hypertension healthcare professional beliefs and behaviour regarding patient medication adherence: a survey conducted among European Society of Hypertension Centres of Excellence. Blood Press 30 (5), 282–290. doi:10.1080/08037051.2021.1963209
Ceral, J., Habrdova, V., Vorisek, V., Bima, M., Pelouch, R., and Solar, M. (2011). Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens. Res. 34 (1 PG-87–90), 87–90. doi:10.1038/hr.2010.183
Choudhry, N. K., Kronish, I. M., Vongpatanasin, W., Ferdinand, K. C., Pavlik, V. N., Egan, B. M., et al. (2022). Medication adherence and blood pressure control: a scientific statement from the american heart association. Hypertension 79 (1), E1–E14. doi:10.1161/HYP.0000000000000203
Curneen, J. M. G., Rabbitt, L., Browne, D., O’Donoghue, D. F., Alansari, Y., Harhen, B., et al. (2022). Major disparities in patient-reported adherence compared to objective assessment of adherence using mass spectrometry: a prospective study in a tertiary-referral hypertension clinic. Br. J. Clin. Pharmacol. 89, 1948–1955. doi:10.1111/bcp.15292
De Geest, S., Zullig, L. L., Dunbar-Jacob, J., Helmy, R., Hughes, D. A., Wilson, I. B., et al. (2018). ESPACOMP medication adherence reporting guideline (EMERGE). Ann. Intern Med. 169 (1), 30–35. doi:10.7326/M18-0543
de Jager, R. L., van Maarseveen, E. M., Bots, M. L., and Blankestijn, P. J.SYMPATHY investigators (2018). Medication adherence in patients with apparent resistant hypertension: findings from the SYMPATHY trial. Br. J. Clin. Pharmacol. 84 (1), 18–24. doi:10.1111/bcp.13402
Durand, H., Hayes, P., Morrissey, E. C., Newell, J., Casey, M., Murphy, A. W., et al. (2017). Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J. Hypertens. 35 (12), 2346–2357. doi:10.1097/HJH.0000000000001502
El Alili, M., Vrijens, B., Demonceau, J., Evers, S. M., and Hiligsmann, M. (2016). A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br. J. Clin. Pharmacol. 82, 268–279. doi:10.1111/bcp.12942
Ewen, S., Meyer, M. R., Cremers, B., Laufs, U., Helfer, A. G., Linz, D., et al. (2015). Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin. Res. Cardiol. 104 (12), 1097–1105. doi:10.1007/s00392-015-0905-5
Florczak, E., Tokarczyk, B., Warchoł-Celiñska, E., Szwench-Pietrasz, E., Prejbisz, A., Gosk, M., et al. (2015). Assessment of adherence to treatment in patients with resistant hypertension using toxicological serum analysis. A subgroup evaluation of the RESIST-POL study. Pol. Arch. Med. Wewn. 125 (1–2), 65–72. doi:10.20452/pamw.2648
Forouzanfar, M. H., Alexander, L., Anderson, H. R., Bachman, V. F., Biryukov, S., Brauer, M., et al. (2015). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386 (10010), 2287–2323. doi:10.1016/S0140-6736(15)00128-2
Groenland, E. H., Dasgupta, I., Visseren, F. L. J., van der Elst, K. C. M., Lorde, N., Lawson, A. J., et al. (2022). Clinical characteristics do not reliably identify non-adherence in patients with uncontrolled hypertension. Blood Press 31 (1), 178–186. doi:10.1080/08037051.2022.2104215
Gupta, P., Patel, P., Štrauch, B., Lai, F. Y., Akbarov, A., Gulsin, G. S., et al. (2017b). Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension 70 (5), 1042–1048. doi:10.1161/HYPERTENSIONAHA.117.09631
Gupta, P., Patel, P., Štrauch, B., Lai, F. Y., Akbarov, A., Marešová, V., et al. (2017a). Risk factors for nonadherence to antihypertensive treatment. Hypertension 69 (6), 1113–1120. doi:10.1161/HYPERTENSIONAHA.116.08729
Halvorsen, L. V., Søraas, C. L., Larstorp, A. C. K., Hjørnholm, U., Kjær, V. N., Liestøl, K., et al. (2024). Effect of drug monitoring on adherence and blood pressure: a multicenter randomized clinical trial. Am. J. Hypertens., 1–11. doi:10.1093/ajh/hpae059
Hamdidouche, I., Jullien, V., Laurent, S., and Azizi, M. (2017). Detecting nonadherence to antihypertensive treatment: any time, anywhere? Hypertension 70 (2), 257–258. doi:10.1161/HYPERTENSIONAHA.117.09739
Hayes, P., Casey, M., Glynn, L. G., Molloy, G. J., Durand, H., O’Brien, E., et al. (2019). Measuring adherence to therapy in apparent treatment-resistant hypertension: a feasibility study in Irish primary care. Br. J. General Pract. 69 (686), E621–E628. doi:10.3399/bjgp19X705077
Hill, M. N., Miller, N. H., Degeest, S., American Society of Hypertension Writing Group, , Materson, B. J., Black, H. R., et al. (2011). Adherence and persistence with taking medication to control high blood pressure. J. Am. Soc. Hypertens. 5 (1), 56–63. doi:10.1016/j.jash.2011.01.001
Hjørnholm, U., Larstorp, A. C. K., Andersen, M. H., and Høieggen, A. (2019). Directly observed therapy prior to ambulatory blood pressure measurement (DOT-HTN) in uncontrolled hypertensive patients - effect on blood pressure, safety and patient perception. Blood Press 28 (5), 327–335. doi:10.1080/08037051.2019.1633907
Johnson, B. T., and Hennessy, E. A. (2019). Systematic reviews and meta-analyses in the health sciences: best practice methods for research syntheses. Soc. Sci. Med. 233 (November 2018), 237–251. doi:10.1016/j.socscimed.2019.05.035
Jones, E. S. W., Lesosky, M., Blockman, M., Castel, S., Decloedt, E. H., Schwager, S. L. U., et al. (2017). Therapeutic drug monitoring of amlodipine and the Z-FHL/HHL ratio: adherence tools in patients referred for apparent treatment-resistant hypertension. South Afr. Med. J. 107 (10), 887–891. doi:10.7196/SAMJ.2017.v107i10.12268
Jung, O., Gechter, J. L., Wunder, C., Paulke, A., Bartel, C., Geiger, H., et al. (2013). Resistant hypertension? Assessment of adherence by toxicological urine analysis. J. Hypertens. 31 (4), 766–774. doi:10.1097/HJH.0b013e32835e2286
Kario, K., Kai, H., Nanto, S., and Yokoi, H. (2023). Anti-hypertensive medication adherence in the REQUIRE trial: post-hoc exploratory evaluation. Hypertens. Res. 46 (8), 2044–2047. doi:10.1038/s41440-023-01333-8
Kjeldsen, S. E., Julius, S., Dahlöf, B., and Weber, M. A. (2015). Physician (investigator) inertia in apparent treatment-resistant hypertension - insights from large randomized clinical trials. Lennart Hansson Memorial Lecture. Lennart Hansson Meml. Lect. Blood Press 24 (1), 1–6. doi:10.3109/08037051.2014.946787
Kocianova, E., Vaclavik, J., Tomkova, J., Ondra, P., Jarkovsky, J., Benesova, K., et al. (2017). Heart rate is a useful marker of adherence to beta-blocker treatment in hypertension. Blood Press 26 (5 PG-311–318), 311–318. doi:10.1080/08037051.2017.1346458
Kustovs, D., Urtāne, I., Sevostjanovs, E., Moreino, E., and Trušinskis, K. (2023). Opportunities of amlodipine as a potential candidate in the evaluation of drug compliance during antihypertensive therapy. Med. Kaunas. 59 (2), 340. doi:10.3390/medicina59020340
Lane, D., Lawson, A., Burns, A., Azizi, M., Burnier, M., Jones, D. J. L., et al. (2022). Nonadherence in hypertension: how to develop and implement chemical adherence testing. Hypertension 79 (1), 12–23. doi:10.1161/HYPERTENSIONAHA.121.17596
Lauder, L., Ewen, S., Glasmacher, J., Lammert, F., Reith, W., Schreiber, N., et al. (2021). Drug adherence and psychosocial characteristics of patients presenting with hypertensive urgency at the emergency department. J. Hypertens. 39 (8), 1697–1704. doi:10.1097/HJH.0000000000002842
Lauder, L., Ewen, S., Kunz, M., Richter, L. H. J., Jacobs, C. M., Kindermann, I., et al. (2020). Adherence to antihypertensive drugs assessed by hyphenated high-resolution mass spectrometry analysis of oral fluids. J. Am. Heart Assoc. 9 (14), e014180. doi:10.1161/JAHA.119.014180
Mancia, G., Kreutz, R., Brunström, M., Burnier, M., Grassi, G., Januszewicz, A., et al. (2023). 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA). J. Hypertens. 41 (12), 1874–2071. doi:10.1097/HJH.0000000000003480
Mazzaglia, G., Ambrosioni, E., Alacqua, M., Filippi, A., Sessa, E., Immordino, V., et al. (2009). Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 120 (16), 1598–1605. doi:10.1161/CIRCULATIONAHA.108.830299
McEvoy, J. W., McCarthy, C. P., Bruno, R. M., Brouwers, S., Canavan, M. D., Ceconi, C., et al. (2024). 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J., ehae178. Available from. doi:10.1093/eurheartj/ehae178
Mills, K. T., Bundy, J. D., Kelly, T. N., Reed, J. E., Kearney, P. M., Reynolds, K., et al. (2016). Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134 (6), 441–450. doi:10.1161/CIRCULATIONAHA.115.018912
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., et al. (2020). “Systematic reviews of etiology and risk,” in JBI manual for evidence synthesis, eds. E. Aromataris, C. Lockwood, K. Porritt, B. Pilla, Z. Jordan (JBI). Available from: doi:10.46658/JBIMES-24-06
Morrissey, E. C., O’Grady, L., Murphy, P. J., Byrne, M., Casey, M., Doheny, H., et al. (2023). Supporting GPs and people with hypertension to maximise medication use to control blood pressure: a pilot cluster RCT of the MIAMI intervention. (under review).
Munn, Z., Peters, M. D. J., Stern, C., Tufanaru, C., McArthur, A., and Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 8, 143. doi:10.1186/s12874-018-0611-x
Muntner, P., and Tanner, R. M. (2024). Therapeutic drug monitoring and the challenge of conducting trials to improve antihypertensive medication adherence. Am. J. Hypertens. 37 (10), 745–747. doi:10.1093/ajh/hpae075
Noone, C., Warner, N. Z., Byrne, M., Durand, H., Lavoie, K. L., McGuire, B. E., et al. (2021). A scoping review of research on the determinants of adherence to social distancing measures during the COVID-19 pandemic. Health Psychol. Rev. 15 (3), 350–370. doi:10.1080/17437199.2021.1934062
Nyanchoka, L., Tudur-Smith, C., Thu, V. N., Iversen, V., Tricco, A. C., and Porcher, R. (2019). A scoping review describes methods used to identify, prioritize and display gaps in health research. J. Clin. Epidemiol. 109, 99–110. doi:10.1016/j.jclinepi.2019.01.005
Osman, H., Lane, D., Bernieh, D., Seidu, S., Patel, P., Khunti, K., et al. (2023). An innovative chemical adherence test demonstrates very high rates of nonadherence to oral cardio-metabolic medications. Kidney Int. Rep. 8 (12), 2818–2821. doi:10.1016/j.ekir.2023.09.033
Osterberg, L., and Blaschke, T. (2005). Adherence to medication. N. Engl. J. Med. 353 (5), 487–497. doi:10.1056/NEJMra050100
Osula, D., Wu, B., Schesing, K., Das, S. R., Moss, E., Alvarez, K., et al. (2022). Comparison of pharmacy refill data with chemical adherence testing in assessing medication nonadherence in a safety net hospital setting. J. Am. Heart Assoc. 11 (19 PG-e027099), e027099. doi:10.1161/JAHA.122.027099
Patel, P., Gupta, P. K. C., White, C. M. J., Stanley, A. G., Williams, B., and Tomaszewski, M. (2016). Screening for non-adherence to antihypertensive treatment as a part of the diagnostic pathway to renal denervation. J. Hum. Hypertens. 30 (6 PG-368–73), 368–373. doi:10.1038/jhh.2015.103
Peeters, L. E. J., Kappers, M. H. W., Hesselink, D. A., Van Der Net, J. B., Hartong, S. C. C., Van De Laar, R., et al. (2024). Antihypertensive drug concentration measurement combined with personalized feedback in resistant hypertension: a randomized controlled trial. J. Hypertens. 42 (1), 169–178. doi:10.1097/HJH.0000000000003585
Peeters, L. E. J., van Gelder, T., van Dijk, L., Koch, B. C. P., and Versmissen, J. (2023). Lessons learned from conducting a randomized controlled trial to improve non-adherence to antihypertensive drug treatment. Blood Press. 32, 2281316. doi:10.1080/08037051.2023.2281316
Pelouch, R., Voříšek, V., Furmanová, V., and Solař, M. (2019). The assessment of serum drug levels to diagnose non-adherence in stable chronic heart failure patients. Acta Medica (Hradec Kralove) 62 (2), 52–57. doi:10.14712/18059694.2019.46
Rabbitt, L., Molloy, G. J., and Dennedy, M. C.Open Science Framework (2024). Characteristics of chemical adherence testing for the clinical management of hypertension: a scoping review protocol. Available from. doi:10.17605/OSF.IO/97CTJ
Ritscher, S., Georges, C., Wunder, C., Wallemacq, P., Persu, A., and Toennes, S. W. (2020). Assessment of adherence to diuretics and β-blockers by serum drug monitoring in comparison to urine analysis. Blood Press 29 (5 PG-291–298), 291–298. doi:10.1080/08037051.2020.1763775
Rottman, B. M., Marcum, Z. A., Thorpe, C. T., and Gellad, W. F. (2017). Medication adherence as a learning process: insights from cognitive psychology. Health Psychol. Rev. 11 (1), 17–32. doi:10.1080/17437199.2016.1240624
Ruzicka, M., Leenen, F. H., Ramsay, T., Bugeja, A., Edwards, C., McCormick, B., et al. (2019). Use of directly observed therapy to assess treatment adherence in PatientsWith apparent treatment-resistant hypertension. JAMA Intern Med. 179 (10), 1433–1434. doi:10.1001/jamainternmed.2019.1455
Schäfer, A. K., Kuczera, T., Wurm-Kuczera, R., Müller, D., Born, E., Lipphardt, M., et al. (2021). Eligibility for Baroreflex Activation Therapy and medication adherence in patients with apparently resistant hypertension. J. Clin. Hypertens. 23 (7), 1363–1371. doi:10.1111/jch.14302
Schesing, K. B., Chia, R., Elwood, B., Halm, E. A., Lee, S. J. C., Lodhi, H., et al. (2020). Assessment of patient and provider attitudes towards therapeutic drug monitoring to improve medication adherence in low-income patients with hypertension: a qualitative study. BMJ Open 10 (11), e039940. doi:10.1136/bmjopen-2020-039940
Schmieder, R. E., Ott, C., Schmid, A., Friedrich, S., Kistner, I., Ditting, T., et al. (2016). Adherence to antihypertensive medication in treatment-resistant hypertension undergoing renal denervation. J. Am. Heart Assoc. 5 (2), 1–11. doi:10.1161/jaha.115.002343
Schoonhoven, A. V. V., Asselt, ADIV, Tomaszewski, M., Patel, P., Khunti, K., Gupta, P., et al. (2018). Cost-Utility of an objective biochemical measure to improve adherence to antihypertensive treatment. Hypertension 72 (5), 1117–1124. doi:10.1161/HYPERTENSIONAHA.118.11227
Sharma, J. R., Dludla, P. V., Dwivedi, G., and Johnson, R. (2023). Measurement tools and utility of hair analysis for screening adherence to antihypertensive medication. Glob. Heart 18, 17. doi:10.5334/gh.1191
Sheppard, J. P., Albasri, A., Gupta, P., Patel, P., Khunti, K., Martin, U., et al. (2022). Measuring adherence to antihypertensive medication using an objective test in older adults attending primary care: cross-sectional study. J. Hum. Hypertens. 36 (12), 1106–1112. doi:10.1038/s41371-021-00646-w
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898–8. doi:10.1136/bmj.l4898
Strauch, B., Petrak, O., Zelinka, T., Rosa, J., Somloova, Z., Indra, T., et al. (2013). Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J. Hypertens. 31 (12), 2455–2461. doi:10.1097/HJH.0b013e3283652c61
Tomaszewski, M., White, C., Patel, P., Masca, N., Damani, R., Hepworth, J., et al. (2014). High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 100 (11), 855–861. doi:10.1136/heartjnl-2013-305063
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern Med. 169 (7), 467–473. doi:10.7326/M18-0850
Valgimigli, M., Garcia-Garcia, H. M., Vrijens, B., Vranckx, P., McFadden, E. P., Costa, F., et al. (2019). Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials: a consensus report fromthe Non-adherence Academic Research Consortium(NARC). Eur. Heart J. 40 (25), 2070–2085. doi:10.1093/eurheartj/ehy377
Van Onzenoort, H. A. W., Verberk, W. J., Kroon, A. A., Kessels, A. G. H., Neef, C., Van Der Kuy, P. H. M., et al. (2012). Electronic monitoring of adherence, treatment of hypertension, and blood pressure control. Am. J. Hypertens. 25 (1), 54–59. doi:10.1038/ajh.2011.153
Velasco, A., Chung, O., Raza, F., Pandey, A., Brinker, S., Arbique, D., et al. (2015). Cost-effectiveness of therapeutic drug monitoring in diagnosing primary aldosteronism in patients with resistant hypertension. J. Clin. Hypertens. (Greenwich) 17 (9 PG-713–9), 713–719. doi:10.1111/jch.12570
Veritas Health Innovation Melbourne Australia (2024). Covidence systematic review software. Available at: www.covidence.org.
Wunder, C., Persu, A., Lengele, J. P., Mg Georges, C., Renkin, J., Pasquet, A., et al. (2019). Adherence to antihypertensive drug treatment in patients with apparently treatment-resistant hypertension in the INSPiRED pilot study. Blood Press 28 (3), 168–172. doi:10.1080/08037051.2019.1599814
Zhou, B., Danaei, G., Stevens, G. A., Bixby, H., Taddei, C., Carrillo-Larco, R. M., et al. (2019). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet 394 (10199), 639–651. doi:10.1016/S0140-6736(19)31145-6
Keywords: hypertension, adherence-compliance-persistance, chemical adherence testing, mass spectrometry, blood pressure, antihypertensive
Citation: Rabbitt L, Curneen J, Dennedy MC and Molloy GJ (2024) Chemical adherence testing in the clinical management of hypertension: a scoping review. Front. Pharmacol. 15:1452464. doi: 10.3389/fphar.2024.1452464
Received: 20 June 2024; Accepted: 18 October 2024;
Published: 06 November 2024.
Edited by:
John Weinman, King’s College London, United KingdomReviewed by:
Ryan McNally, King’s College London, United KingdomAud Høieggen, Oslo University Hospital, Norway
Copyright © 2024 Rabbitt, Curneen, Dennedy and Molloy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louise Rabbitt, bG91aXNlLnJhYmJpdHRAdW5pdmVyc2l0eW9mZ2Fsd2F5Lmll
 Louise Rabbitt
Louise Rabbitt James Curneen
James Curneen Michael Conall Dennedy1,2
Michael Conall Dennedy1,2 Gerard J. Molloy
Gerard J. Molloy