- 1School of Pharmacy and Food Engineering, Guangdong Provincial Key Laboratory of Large Animal Models for Biomedicine, Wuyi University, Jiangmen, China
- 2College of Life Science and Technology, Jinan University, Guangzhou, China
Tyrosinase is a key enzyme in melanin synthesis, and its natural inhibitors are receiving increasing attention. Rosa × damascena Herrm. essential oil (RDEO), as important functional metabolites, was widely known due to its biological activities. But its tyrosinase inhibitory activity has not been detailed investigated. Therefore, in this paper, RDEO was comprehensively investigated the tyrosinase inhibitory, followed by the phytochemical composition analysis. Activity screening results showed that RDEO exhibited effective anti-tyrosinase activity and was a reversible and mixed-type inhibitor. CD assay results revealed that RDEO could affect the conformation of tyrosinase to reduce the activity. In B16F10 cells, RDEO (25–100 μg/mL) could inhibit intracellular tyrosinase activity and decrease melanin content. Finally, GC-MS analysis of RDEO found that citronellol (21.22%), geraniol (14.1%), eicosane (11.03%), heneicosane (6.65%) and 1-nonadecene (5.16%) were its main phytochemical compositions. This study provided data support for Rosa × damascena Herrm. essential oil as one potential natural tyrosinase inhibitor and its applications in cosmetics and medicine.
1 Introduction
Tyrosinase is a copper-containing enzyme that is naturally occurring in various life forms, including fungi, plants, and animals (Romagnoli et al., 2022; Moreiras et al., 2020). It plays a pivotal role in melanin synthesis by facilitating the conversion reaction of L-tyrosine into L-3,4-dihydroxyphenylalanine (L-DOPA) and subsequent reaction of L-DOPA to o-quinone (Su et al., 2023; Nasab et al., 2023). The formation of melanin pigments is further elaborated through a series of complex enzymatic reactions, which include the cyclization of DOPA-quinone and the oxidative polymerization of the resulting indole compounds (Pillaiyar et al., 2018; Najafi et al., 2023). This melanogenesis process is not only crucial for the pigmentation of human skin and hair, but also serves as a vital protective barrier against the detrimental effects of ultraviolet radiation (Ielo et al., 2019; Roberts et al., 2015). Stimulating factors such as specific medications, ultraviolet exposure, and aging process result in pigmentary disorders like melasma, freckles, age spots, and solar lentigines (Chortani et al., 2022; Hassan et al., 2023). Moreover, irregularities in melanin distribution and concentration are sometimes indicative of underlying un-health conditions.
In the quest for effective treatments for hyperpigmentation, researchers have explored the lightening effects of various chemical agents (Li et al., 2021). Given that tyrosinase is central to melanin production, inhibiting its activity always is the key strategy in developing agents for skin lightening (Wang et al., 2023). Several compounds with the potential to inhibit melanogenesis are utilized in cosmetic and medicine industries (He et al., 2023). However, most of these agents fall short of meeting the stringent criteria of clinical efficacy, specificity, minimal toxicity, and chemical stability, and may also be associated with adverse side effects (Xue et al., 2023; Barros et al., 2023).
Therefore, there is a growing preference for natural tyrosinase inhibitors, especially those from botanical drugs and their metabolites, due to their perceived safety and consumer acceptance. A number of the tyrosinase inhibitors in use today have been isolated from various parts of botanical drugs, such as seeds, roots, and leaves. Botanical drug extracts, rich in bioactive metabolites, have long been employed for cosmetic and therapeutic purposes. Essential oils, as important functional metabolites, are usually valued for their antimicrobial and antioxidant properties, often making them as natural preservatives in cosmetic and medicine industries (Hazafa et al., 2020; Carqueijeiro et al., 2020; Li et al., 2020).
Rosa × damascena Herrm., commonly known as the Damask rose, is a significant species within the Rosa genus, which encompasses at least 200 species (Mahboubi, 2016). Many researches have demonstrated the presence of numerous metabolites in this plant with potential pharmacology properties (Lee et al., 2016). Now, Rosa × damascena Herrm. essential oil (RDEO) has been recognized for its high economic value, notably in cosmetic industry (Ho et al., 2020; Cebi, 2021). Rosa × damascena Herrm. and its essential oil RDEO are well-documented their antidiabetic, antioxidant, anti-aging, and anti-inflammatory properties (Akram et al., 2020; Al-Oqail et al., 2021). Previous reports found that citronellol and geraniol were its main active metabolites (Xiao et al., 2023).
As far as we know, the anti-tyrosinase activity and potential mechanism of RDEO had not been detailed investigated. Thence, in this study, we delved into the anti-tyrosinase properties of RDEO and analyzed phytochemical compositions.
2 Results and discussion
2.1 Inhibitory activity
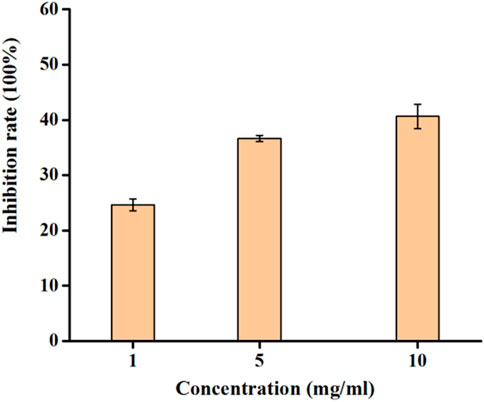
The study delved into the inhibitory effects of RDEO on tyrosinase, an enzyme pivotal in melanin biosynthesis. In our experiments (Figure 1), we observed that RDEO exhibited a concentration-dependent inhibitory effect on tyrosinase activity. RDEO, with concentrations ranging from 1 to 10 mg/mL, obviously reduced the tyrosinase activity. Although, RDEO displayed lower tyrosinase inhibitory than kojic acid (IC50 = 2.2 ± 0.3 mg/L), RDEO still was proved to be one natural tyrosinase inhibitor. This finding is particularly promising given the increasing demand for natural metabolites to synthetic skin lightening agents.
2.2 Inhibition kinetics
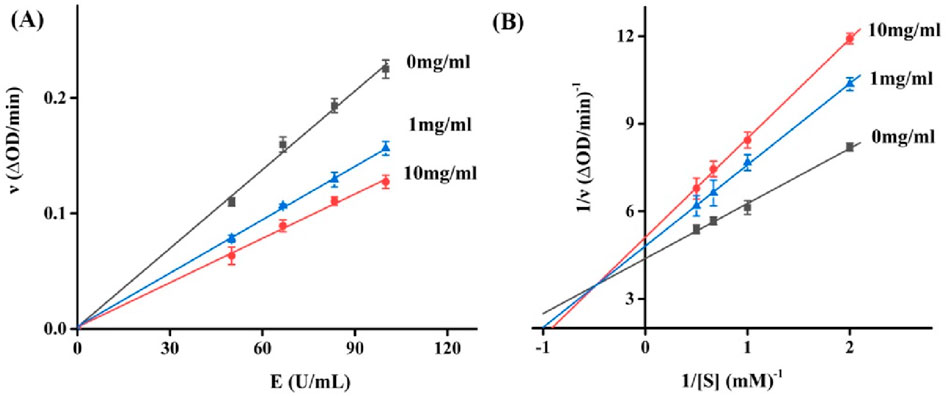
Aiming to elucidate the mechanism by which RDEO impedes tyrosinase activity. The kinetic study between RDEO and tyrosinase offers intriguing insights into the nature of their molecular relationship. Enzyme inhibitors are generally divided into reversible inhibitors and irreversible inhibitor according to the binding degrees of inhibitors with enzymes, which can be judged by the effects of inhibitors on enzymatic reactions. Reversible inhibitor can bind to enzymes or enzyme substrate complexes through non-covalent bonds, while irreversible inhibitors can bind to certain essential functional groups in enzyme active center through covalent bonds. In Figure 2A, catalytic rates (△OD/min) of tyrosinase were measured under RDEO (0–10 mg/mL), which revealed that RDEO lines (0–10 mg/mL) passed origin point with reducing slopes. The results indicated that RDEO cannot completely inactivate the efficacy enzyme, but only reduce its catalytic rate, revealing RDEO as a typical reversible inhibitor (Xu et al., 2020; Li et al., 2023; Xiao et al., 2023). In addition, reversible inhibitors are divided into competitive inhibitors, non-competitive inhibitors, and mixed-type inhibitors. In Figure 2B, the Lineweaver-Burk plots of catalytic rates (△OD/min) were obtained under RDEO (0–10 mg/mL). The lines intersected in the second quadrant and the slops increased with RDEO concentrations, which indicated that RDEO inhibited tyrosinase in a mixed-type (Zhang et al., 2022; Wu et al., 2023; Li et al., 2024a). This suggested that RDEO could bind to both the free enzyme and the enzyme-substrate complex, thereby influencing the enzyme’s activity.
2.3 Inhibition constant
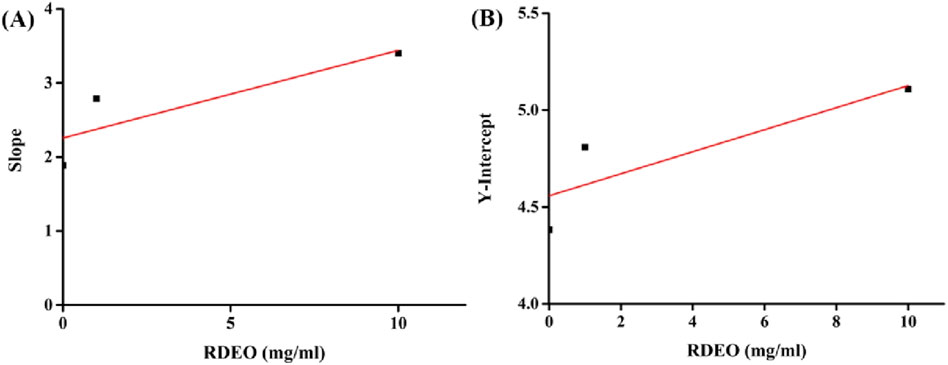
The analysis of the secondary curves (Figures 3A, B) derived from our kinetic data yielded inhibition constants, Ki (19.1 mg/mL) and Kis (80.1 mg/mL) from the plots of lines slop and intercept against RDEO concentration, respectively. The lower Ki value in comparison to Kis indicated a stronger binding affinity of RDEO to free enzyme (Deng et al., 2022; Feng et al., 2024; Min et al., 2024).
2.4 CD assay
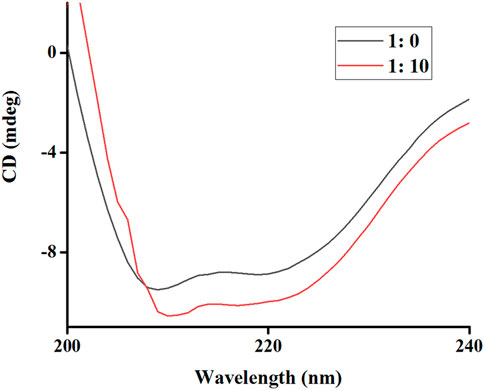
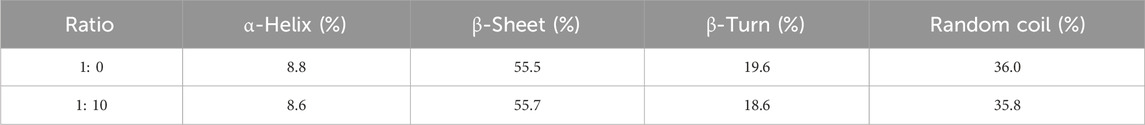
This section aimed to assess the conformational changes in tyrosinase that might result from its interaction with RDEO, which could elucidate the underlying mechanism of its inhibitory effect. Our results from the CD assay (Figure 4) demonstrated that RDEO (ratio: 10: 1) induced alterations in characteristic band (205–226 nm) of tyrosinase, which was indicative of its α-helical content. The treatment with RDEO led to noticeable changes in both intensity and shape of bands, suggesting a direct influence on the enzyme’s secondary structure.
Further analysis of the CD spectra (Table 1) revealed that RDEO (ratio: 10: 1) treatment resulted in a decrease in α-helix, β-turn, and random coil contents, and the increase in β-sheet content. These structural changes were believed to contribute to the reduction in tyrosinase’s activity, providing a plausible explanation for the observed inhibition. The CD assay findings underscored the potential of RDEO as a modulator of tyrosinase conformation and activity, supporting the use of RDEO in the development of novel skin lightening agents.
2.5 Intracellular assay
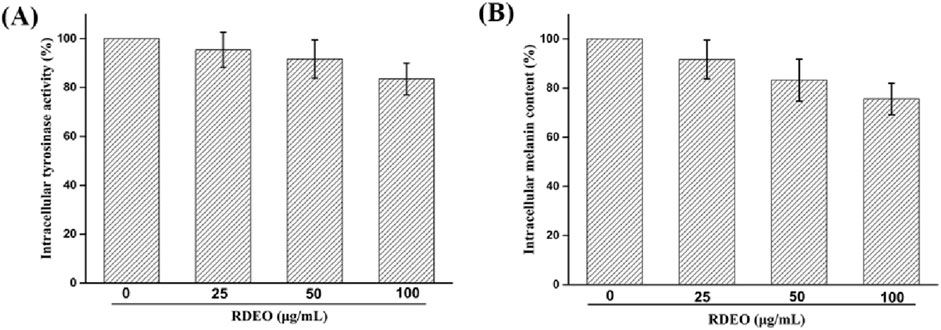
Finally, we determined the effects of RDEO on intracellular tyrosinase activity and melanin content in B16F10 cells. As shown in Figure 5A, RDEO (25–100 μg/mL) treatment would result in the reduction of intracellular tyrosinase activity in dose-manner, suggesting its inhibitory efficacy on intracellular tyrosinase. This result was consistent with that of in vitro tyrosinase inhibitory of RDEO. Parallel to tyrosinase activity assessment, cells melanin content was quantified and found that RDEO (25–100 μg/mL) treatment also caused the reduction of intracellular melanin content (Figure 5B). Thence, RDEO showed a certain ability to resist melanin production, mainly due to its inherent ability to inhibit tyrosinase activity.
2.6 Phytochemical composition analysis
Above investigation not only provided a deeper understanding of the inhibitory effects of RDEO on tyrosinase but also set the stage for future research into the specific compounds responsible for this activity. This section aimed to dissect the complex mixture of volatile metabolites presenting in RDEO and identify the metabolites that might contribute to its biological effects. Utilizing gas chromatography-mass spectrometry (GC-MS), we meticulously characterized the chemical composition of RDEO (Figure 6). A total of 66 metabolites were identified from RDEO (Table 2).
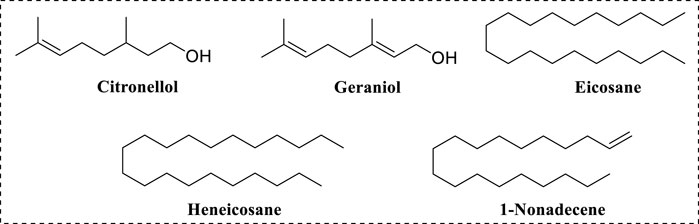
The major compositions of RDEO were citronellol (21.22%) and geraniol (14.1%), eicosane (11.03%), heneicosane (6.65%) and 1-nonadecene (5.16%), respectively (Figure 7). In one previous work about RDEO (Xiao et al., 2023), citronellol, geraniol, eicosane, heneicosane, 1-nonadecene were also confirmed as the main components of RDEO, except the differences in their contents. In particular, geraniol was reported to have tyrosinase inhibitory activity (Lante and Tinello, 2015; Mahant et al., 2021). Phytochemical composition analysis of RDEO had shed light on its complex chemical landscape. The identification of these metabolites set the stage for further research into their individual and combined effects on tyrosinase.
3 Conclusion
Rosa × damascena Herrm. essential oil (RDEO), as important functional metabolites, was widely known due to its biological activities. Therefore, in this paper, the tyrosinase inhibitory effects of RDEO and its phytochemical compositions were analyzed. Activity screening results showed that RDEO exhibited anti-tyrosinase activity and was a reversible and mixed inhibitor. CD assay results revealed that RDEO could affect the conformation of tyrosinase. GC-MS analysis of RDEO found that citronellol (21.22%), geraniol (14.1%), eicosane (11.03%), heneicosane (6.65%) and 1-nonadecene (5.16%) were the main compositions. In particular, geraniol was reported to have tyrosinase inhibitory activity. In B16F10 cells, RDEO (25–100 μg/mL) could inhibit intracellular tyrosinase activity and decrease melanin content. This study provided data support for RDEO as a natural tyrosinase inhibitor and its applications in cosmetics and medicine.
4 Materials and methods
4.1 Rosa × damascena Herrm. essential oil
Rosa × damascena Herrm. essential oil (RDEO) was obtained from Natural Laboratory Bul Rose Ltd. (batch number: T′02, date of production: June 2023) (Heinrich et al., 2020; Heinrich et al., 2022). Brief preparation was as follows using steam distillation method. 100 g of dry damask rose powder and 2.5 L distilled water were added into a distillation flask (5 L) and heated to boil. The obtained oil-water mixture was layered and dried using anhydrous sodium sulfate to produce RDEO.
4.2 Tyrosinase activity
RDEO was assayed its anti-tyrosinase activity using mushroom tyrosinase (Li et al., 2023). RDEO was dissolved in DMSO for subsequent test. RDEO (10 μL) was added into tyrosinase (130 μL) and L-DOPA (50 μL). Then, the mixture was measured the absorbance at 475 nm. Inhibition rate was calculated compared to black.
4.3 Inhibitory kinetic
Inhibitory kinetics of RDEO on tyrosinase was examined to delineate the mode of inhibition. The assay involved varying concentrations of tyrosinase and substrate in the presence of RDEO. The changes in absorbance were monitored, and Lineweaver-Burk plots were constructed to analyze the data (Lu et al., 2023a; Lin et al., 2023).
4.4 CD spectra
CD spectra were employed in this study to investigate the conformational changes in tyrosinase upon interaction with RDEO (Lu et al., 2023b). CD spectra were recorded, following the addition of RDEO to the tyrosinase. The spectra were captured in the wavelength range of 190–260 nm. The ratios of tyrosinase to RDEO were varied to 1: 0 and 1: 10.
4.5 Intracellular assay
Intracellular tyrosinase activity: B16F10 cells were treated with RDEO (25–100 μg/mL) for 24 h and then lysed using lysis buffer. Subsequently, an appropriate amount of L-DOPA was added and measured its absorbance at 457 nm.
Intracellular melanin content: Intracellular tyrosinase activity: B16F10 cells were treated with RDEO (25–100 μg/mL) for 24 h, treated in NaOH solution at 100°C for 1 h, and measured its absorbance at 405 nm.
4.6 Phytochemical composition
Phytochemical composition analysis of RDEO (dissolution in methanol) was conducted on GC-MS. GC conduction (Hp-5MS quartz capillary column (inner diameter 30 m × 0.25 mm, film thickness 0.25 μm)) was as follow: Initial temperature was 70°C for 2 min, followed by rate of 5°C/min to 150°C for 16 min, rate of 10°C/min to 220°C for 7 min, and rate of 10°C/min to 220°C for 2 min. MS detection: ionization energy: 70 eV, ion source temperature: 230°C; scanning mass range: 40–500 m/z, helium (He) flow rate: 1.0 mL/min. Compounds were identified using standard spectral library.
4.7 Statistical analysis
Data were presented as mean ± SD and evaluate the differences suing One-way ANOVA. P < 0.05 was considered significant (Hu et al., 2024; Li et al., 2024b; Liang et al., 2024).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
QW: Data curation, Investigation, Writing–original draft. WF: Data curation, Investigation, Writing–original draft. HL: Data curation, Investigation, Writing–original draft. ZL: Project administration, Writing–review and editing. XX: Project administration, Writing–review and editing, Funding acquisition, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Department of Education of Guangdong Province (2021KCXTD044) and the Science and Technology Planning Project of Guangdong Province (2021B1212040016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akram, M., Riaz, M., Munir, N., Akhter, N., Zafar, S., Jabeen, F., et al. (2020). Chemical constituents, experimental and clinical pharmacology of Rosa damascena: a literature review. J. Pharm. Pharmacol. 72, 161–174. doi:10.1111/jphp.13185
Al-Oqail, M., Farshori, N., Al-Sheddi, E., Al-Massarani, S., Saquib, Q., Siddiqui, M., et al. (2021). Oxidative stress mediated cytotoxicity, cell cycle arrest, and apoptosis induced by Rosa damascena in human cervical cancer HeLa cells. Oxid. Med. Cell Longev. 2021, 6695634. doi:10.1155/2021/6695634
Barros, M., Menezes, T., Garcia, Y., and Neves, J. (2023). Inhibitory effects of iron-based carbonaceous nanocomposites on mushroom tyrosinase activity: molecular aspects and mechanistic insights. New J. Chem. 47, 9134–9142. doi:10.1039/d3nj00882g
Carqueijeiro, I., Langley, C., Grzech, D., Koudounas, K., Papon, N., O’Connor, S., et al. (2020). Beyond the semi-synthetic artemisinin: metabolic engineering of plant-derived anti-cancer drugs. Curr. Opin. Biotechnol. 65, 17–24. doi:10.1016/j.copbio.2019.11.017
Cebi, N. (2021). Quantification of the geranium essential oil, palmarosa essential oil and phenylethyl alcohol in Rosa damascena essential oil using ATR-FTIR spectroscopy combined with chemometrics. Foods 10, 1848. doi:10.3390/foods10081848
Chortani, S., Hajlaoui, A., Jlizi, S., Harrath, A., Jannet, H., and Romdhane, A. (2022). Access to new phosphonate-and imidazolidine-benzopyrimidinone derivatives as antityrosinase and anti-acetylcholinesterase agents: design, synthesis and molecular docking. J. Mol. Struct. 1268, 133693. doi:10.1016/j.molstruc.2022.133693
Deng, X., Ke, J., Zheng, Y., Li, D., Zhang, K., Zheng, X., et al. (2022). Synthesis and bioactivities evaluation of oleanolic acid oxime ester derivatives as α-glucosidase and α-amylase inhibitors. J. Enzym. Inhib. Med. Chem. 37, 451–461. doi:10.1080/14756366.2021.2018682
Feng, M., Liang, B., Sun, J., Min, X., Wang, S., Lu, Y., et al. (2024). Synthesis, anti-α-glucosidase activity, inhibition interaction, and anti-diabetic activity of novel cryptolepine derivatives. J. Mol. Struct. 1310, 138311. doi:10.1016/j.molstruc.2024.138311
Hassan, M., Shahzadi, S., and Kloczkowski, A. (2023). Tyrosinase inhibitors naturally present in plants and synthetic modifications of these natural products as anti-melanogenic agents: a review. Molecules 28, 378. doi:10.3390/molecules28010378
Hazafa, A., Rehman, K., Jahan, N., and Jabeen, Z. (2020). The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 72, 386–397. doi:10.1080/01635581.2019.1637006
He, M., Fan, M., Yang, W., Peng, Z., and Wang, G. (2023). Novel kojic acid-1, 2, 4-triazine hybrids as anti-tyrosinase agents: synthesis, biological evaluation, mode of action, and anti-browning studies. Food Chem. 419, 136047. doi:10.1016/j.foodchem.2023.136047
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research-Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Ho, Y., Suphrom, N., Daowtak, K., Potup, P., Thongsri, Y., and Usuwanthim, K. (2020). Anticancer effect of citrus hystrix DC. Leaf extract and its bioactive constituents citronellol and, citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceuticals 13, 476. doi:10.3390/ph13120476
Hu, C., Liang, B., Sun, J., Li, J., Xiong, Z., Wang, S., et al. (2024). Synthesis and biological evaluation of indole derivatives containing thiazolidine-2,4-dione as α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 264, 115957. doi:10.1016/j.ejmech.2023.115957
Ielo, L., Deri, B., Germano, M., Vittorio, S., Mirabile, S., Gitto, R., et al. (2019). Exploiting the 1-(4-fluorobenzyl) piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 178, 380–389. doi:10.1016/j.ejmech.2019.06.019
Lante, A., and Tinello, F. (2015). Citrus hydrosols as useful by-products for tyrosinase inhibition. Innov. Food Sci. Emerg. 27, 154–159. doi:10.1016/j.ifset.2014.11.001
Lee, S., Park, Y., Kim, S., Park, E., Kang, M., So, I., et al. (2016). Geraniol suppresses prostate cancer growth through down-regulation of E2F8. Cancer Med. 5, 2899–2908. doi:10.1002/cam4.864
Li, J., Feng, L., Liu, L., Wang, F., Ouyang, L., Zhang, L., et al. (2021). Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 224, 113744. doi:10.1016/j.ejmech.2021.113744
Li, J., Min, X., Zheng, X., Wang, S., Xu, X., and Peng, J. (2023). Synthesis, anti-tyrosinase activity, and spectroscopic inhibition mechanism of cinnamic acid-eugenol esters. Molecules 28, 5969. doi:10.3390/molecules28165969
Li, M., Li, H., Min, X., Sun, J., Liang, B., Xu, L., et al. (2024a). Identification of 1,3,4-thiadiazolyl-containing thiazolidine-2,4-dione derivatives as novel PTP1B inhibitors with anti-diabetic activity. J. Med. Chem. 64, 8406–8419. doi:10.1021/acs.jmedchem.4c00676
Li, M., Sun, J., Liang, B., Min, X., Hu, J., Wu, R., et al. (2024b). Thiazolidine-2,4-dione derivatives as potential α-glucosidase inhibitors: synthesis, inhibitory activity, binding interaction and hypoglycemic activity. Bioorg. Chem. 144, 107177. doi:10.1016/j.bioorg.2024.107177
Li, Y., Kong, D., Fu, Y., Sussman, M., and Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 148, 80–89. doi:10.1016/j.plaphy.2020.01.006
Liang, B., Xiao, D., Wang, S., and Xu, X. (2024). Novel thiosemicarbazide-based β-carboline derivatives as α-glucosidase inhibitors: synthesis and biological evaluation. Eur. J. Med. Chem. 275, 116595. doi:10.1016/j.ejmech.2024.116595
Lin, J., Xiao, D., Lu, L., Liang, B., Xiong, Z., and Xu, X. (2023). New β-carboline derivatives as potential α-glucosidase inhibitor: synthesis and biological activity evaluation. J. Mol. Struct. 1283, 135279. doi:10.1016/j.molstruc.2023.135279
Lu, L., Hu, C., Min, X., Liu, Z., Xu, X., and Gan, L. (2023a). In vitro and in vivo biological evaluation of indole-thiazolidine-2,4-dione derivatives as tyrosinase inhibitors. Molecules 28, 7470. doi:10.3390/molecules28227470
Lu, L., Zhang, X., Kang, Y., Xiong, Z., Zhang, K., Xu, X., et al. (2023b). Novel coumarin derivatives as potential tyrosinase inhibitors: synthesis, binding analysis and biological evaluation. Arab. J. Chem. 16, 104724. doi:10.1016/j.arabjc.2023.104724
Mahant, S., Sahajpal, N., and Nanda, S. (2021). Insights into the mechanism of Cymbopogan martinii essential oil in topical therapy of acne vulgaris. Future Microbiol. 16, 1181–1193. doi:10.2217/fmb-2021-0039
Mahboubi, M. (2016). Rosa damascena as holy ancient herb with novel applications. J. Tradit. Complement. Med. 6, 10–16. doi:10.1016/j.jtcme.2015.09.005
Min, X., Guo, S., Lu, Y., and Xu, X. (2024). Investigation on the inhibition mechanism and binding behavior of cryptolepine to α-glucosidase and its hypoglycemic activity by multi-spectroscopic method. J. Lumin. 269, 120437. doi:10.1016/j.jlumin.2024.120437
Moreiras, H., Pereira, F. S. C., Neto, M. V., Bento-Lopes, L., Festas, T. C., Seabra, M. C., et al. (2020). The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigm. Cell. Melanoma R. 33, 366–371. doi:10.1111/pcmr.12840
Najafi, Z., Rafiei, F., Ghafouri-Khosrowshahi, A., Mahdavi, M., Dianatpour, M., and Iraji, A. (2023). Design, synthesis, and molecular dynamics simulation studies of new chalcone-based 2-arylidene-1,3-indandiones as tyrosinase inhibitors. Chemistryselect 33, e202302192. doi:10.1002/slct.202302192
Nasab, N., Raza, H., Eom, Y., Hassan, M., Kloczkowski, A., and Kim, S. (2023). Synthesis and discovery of potential tyrosinase inhibitor of new coumarin-based thiophenyl-pyrazolylthiazole nuclei: in-vitro evaluation, cytotoxicity, kinetic and computational studies. Chem. Biol. Drug. Des. 101, 13–88. doi:10.1111/cbdd.14209
Pillaiyar, T., Namasivayam, V., Manickam, M., and Jung, S. (2018). Inhibitors of melanogenesis: an updated review. J. Med. Chem. 61, 7395–7418. doi:10.1021/acs.jmedchem.7b00967
Roberts, N., Curtis, S., Milan, A., and Ranganath, L. (2015). The pigment in alkaptonuria relationship to melanin and other coloured substances: a review of metabolism, composition and chemical analysis. JIMD Rep. 24, 51–66. doi:10.1007/8904_2015_453
Romagnoli, R., Oliva, P., Prencipe, F., Manfredini, S., Germanò, M., Luca, L., et al. (2022). Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 231, 114147. doi:10.1016/j.ejmech.2022.114147
Su, J., Li, D., Hu, Y., You, X., Guo, X., Li, X., et al. (2023). A novel C6-sulfonated celastrol analog as a tyrosinase and melanin inhibitor: design, synthesis, biological evaluation and molecular simulation. J. Mol. Struc. 1283, 135288. doi:10.1016/j.molstruc.2023.135288
Wang, G., He, M., Huang, Y., and Peng, Z. (2023). Synthesis and biological evaluation of new kojic acid-1, 3, 4-oxadiazole hybrids as tyrosinase inhibitors and their application in the anti-browning of fresh-cut mushrooms. Food Chem. 409, 135275. doi:10.1016/j.foodchem.2022.135275
Wu, X., Zhu, W., Lu, L., Hu, C., Zheng, Y., Zhang, X., et al. (2023). Synthesis and anti-a-glucosidase activity evaluation of betulinic acid derivatives. Arab. J. Chem. 16, 104659. doi:10.1016/j.arabjc.2023.104659
Xiao, D., Lu, L., Liang, B., Xiong, Z., Xu, X., and Chen, W. (2023). Identification of 1,3,4-oxadiazolyl-containing β-carboline derivatives as novel α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 261, 115795. doi:10.1016/j.ejmech.2023.115795
Xu, X., Deng, X., Chen, J., Liang, Q., Zhang, K., Li, D., et al. (2020). Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 189, 112013. doi:10.1016/j.ejmech.2019.112013
Xue, S., Li, Z., Ze, X., Wu, X., He, C., Shuai, W., et al. (2023). Design, synthesis, and biological evaluation of novel hybrids containing dihydrochalcone as tyrosinase inhibitors to treat skin hyperpigmentation. J. Med. Chem. 66, 5099–5117. doi:10.1021/acs.jmedchem.3c00012
Keywords: Rosa × damascena Herrm., essential oil, anti-tyrosinase activity, inhibition mechanism, phytochemical composition
Citation: Wu Q, Fang W, Liu H, Liu Z and Xu X (2024) Rosa × damascena Herrm. essential oil: anti-tyrosinase activity and phytochemical composition. Front. Pharmacol. 15:1451452. doi: 10.3389/fphar.2024.1451452
Received: 19 June 2024; Accepted: 30 August 2024;
Published: 11 September 2024.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Guiying Huang, Zhongkai University of Agriculture and Engineering, ChinaXuyang Deng, Henan University, China
Copyright © 2024 Wu, Fang, Liu, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Liu, dGxpdXpoQGpudS5lZHUuY24=; Xuetao Xu, d3l1Y2hlbXh4dEAxMjYuY29t
 Qiuyan Wu
Qiuyan Wu Wanting Fang1
Wanting Fang1 Zhong Liu
Zhong Liu Xuetao Xu
Xuetao Xu