- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Republic of Korea
- 2Division of Pulmonology and Allergy, Department of Internal Medicine, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Republic of Korea
- 3Division of Pulmonology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea
- 4Division of Pulmonology and Allergy, Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Republic of Korea
- 5Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea
- 6Department of Internal Medicine, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Republic of Korea
- 7Department of Pulmonary and Critical Care Medicine, Ajou University Hospital, Ajou University School of Medicine, Suwon, Republic of Korea
- 8Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea
- 9Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea
- 10Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Republic of Korea
- 11Department of Internal Medicine, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Republic of Korea
- 12Division of Pulmonary and Critical Medicine, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea
- 13Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Republic of Korea
- 14Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 15Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Background: Pirfenidone is an antifibrotic medication approved for idiopathic pulmonary fibrosis (IPF). Fybro®, a generic version of pirfenidone developed in South Korea, gained approval and is available in 200 mg and in higher-dose formulations of 400 and 600 mg. This real-world prospective cohort study investigated the safety and effectiveness of Fybro®.
Methods: A nationwide observational study was conducted in patients with IPF. Patients were followed up for 6 months, with a subset of patients being followed up for 12 months. Data on lung function and adverse events were collected. Patient adherence to fewer-pill (400 and/or 600 mg tablets) and multiple-pill (200 mg tablets) regimens were compared.
Results: Of the 359 enrolled patients, 352 received pirfenidone (Fybro®) at least once and were included in the analysis. The mean age was 69.0 years and 82.4% of patients were male. The median treatment duration was 186.0 days. A total of 253 patients (71.9%) experienced adverse events, with decreased appetite being the most common (16.5%). The adjusted decline rates in lung function were −1.5% and −2.2% predicted per year for forced vital capacity and diffusing capacity, respectively. No significant differences were observed based on the pirfenidone dose. For a daily intake of 1,200 or 1800 mg of pirfenidone, a significantly longer duration of drug administration was observed with the fewer-pill regimen than with multiple-pill regimen.
Conclusion: The safety and effectiveness of Fybro® observed in this real-world cohort study are consistent with previous studies. Using higher-strength tablets to reduce pill burden may improve medication adherence.
1 Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease of an unknown aetiology (Raghu et al., 2018). Prognosis is poor without treatment, with a median survival of 3–5 years after diagnosis (Nathan et al., 2011; Raghu et al., 2014). Antifibrotic medications are widely used to slow the progression of fibrosis (Toellner et al., 2017; Cottin et al., 2018; Margaritopoulos et al., 2018). Pirfenidone, an antifibrotic medication, has demonstrated efficacy in clinical trials by attenuating the decline rate of forced vital capacity (FVC) (Noble et al., 2011; King et al., 2014; Richeldi et al., 2014). Although the precise mechanism of action remains unknown, pirfenidone appears to inhibit TGF-β, TNF-α, and IL-6, and affect the balance of MMPs/TIMPs (Fletcher et al., 2016; Xylourgidis et al., 2019; Ruwanpura et al., 2020). The original form of pirfenidone, Pirespa® (Shionogi & Co), received approval in 2008 in Japan for patients with IPF (Ogura et al., 2015). In South Korea, as in Japan, 1800 mg/day of pirfenidone was approved for the treatment of IPF (Taniguchi et al., 2010). Since then, several generic versions have become available in several countries, including South Korea, China, India, and Mexico. Among the generic versions, Fybro®, (Yungjin Pharmaceutical, South Korea) gained approval from the Korean Food and Drug Administration in 2017 based on a dissolution test. Therefore, further assessment of its real-world safety and effectiveness is required.
One notable feature of Fybro® is its availability in the higher-dose formulations of 400 mg and 600 mg whereas Pirespa® is only available in a 200 mg dosage. Achieving the standard daily pirfenidone dose of 1800 mg typically requires patients to be prescribed three tablets of 200 mg, thrice daily. Most patients with IPF are middle-aged or older with comorbidities; therefore, pill burden is a concern (Tzouvelekis et al., 2020; Khor et al., 2022). Therefore, any effort to reduce the pill burden could improve their quality of life. Using 400 mg or 600 mg tablets reduces daily pill count required to achieve a full dose. However, whether reducing pill burden improves medication adherence remains unknown. The objective of this real-world prospective cohort study was to investigate the safety and effectiveness of Fybro® and evaluate whether a treatment regimen utilizing higher-dose tablets to reduce the required pill count can improve medication adherence.
2 Materials and methods
2.1 Study design and patients
This nationwide observational study was designed to investigate the safety and effectiveness of Fybro®. Patients diagnosed with IPF were screened for enrollment at 14 hospitals in South Korea, and those who never received Fybro® were included in the study. The diagnosis of IPF was confirmed through multidisciplinary discussion at each site, based on the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines (Raghu et al., 2018). Patients were excluded if any of the following conditions were present: 1) hypersensitivity to the main component or additional agent of Fybro®, 2) severe or end-stage liver disease, 3) severe or end-stage renal disease, 4) fluvoxamine treatment, and 5) galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption.
The duration of the study was 6 months. For patients who agreed to an extended treatment and follow ups till 12 months, additional data were collected, facilitating a longer-term analysis. The study protocol was approved by the Institutional Review Board of each institution (see ethics statements). All study patients provided written informed consent. This study was registered at Clinical Research Information Service of the Korea National Institute of Health (KCT0008637).
2.2 Treatment
Patients were initially prescribed pirfenidone (Fybro®) at 200 mg, thrice daily. Depending on their response and tolerance, the dosage was increased by 600 mg every 2 weeks, up to a maximum of 1800 mg per day. In the case of adverse events, the treating physician decided whether to decrease or interrupt the dose, based on event severity. The specific strength (200 mg, 400 mg, or 600 mg) used for administration was determined by the physician.
2.3 Data collection
Patients’ baseline clinical characteristics were obtained including age, sex, body mass index (BMI), smoking status, family history of pulmonary fibrosis, IPF diagnosis date, comorbidities, lung function, and minimum oxygen saturation during the 6-minute walk test. IPF severity was categorized using the gender-age-physiology (GAP) index (Ley et al., 2012). The GAP stage is determined based on the total GAP index score: stage I (0–3 points), stage II (4–5 points), and stage III (6–8 points). Lung function parameters, FVC and diffusing capacity of the lung for carbon monoxide (DLCO), were measured at baseline and 6 months. The assessment of lung function at 3 months was not mandatory but could be performed based on the treating physician’s judgment. For those who underwent an extended follow up, FVC and DLCO values at 12 months were also collected. Spirometry and DLCO measurements were performed according to the European Respiratory Society/American Thoracic Society guidelines (Macintyre et al., 2005; Miller et al., 2005). Results were presented as percentages of normal predicted values (%pred.).
2.4 Study outcomes
Study outcomes were safety and effectiveness of Fybro® in patients with IPF. Adverse events were defined using the preferred terms in the Medical Dictionary for Regulatory Activities version 25.0. The adverse event rates were compared based on background factors, including age, sex, and presence of comorbidities.
To determine the effectiveness of Fybro®, decline rates of FVC and DLCO were assessed. Moreover, categorical assessments were conducted for absolute changes in FVC and DLCO at 6 and 12 months. Adherence to fewer-pill regimen (treatment with 400 mg and/or 600 mg tablets) was compared to that of multiple-pill regimen (treatment with 200 mg tablets). Comparisons focused on medication adherence during the periods of administering 1,200 mg/day and 1800 mg/day, respectively. Adherence was evaluated by considering the overall duration of drug usage and frequency of dose modifications. Dose modification was defined as a reduction or temporary interruption in drug dosage, or both, due to intolerance and/or adverse events.
2.5 Statistical analysis
Data were presented as mean ± standard deviation or median (interquartile range [IQR]) and numbers (%) for continuous and categorical variables, respectively. The Student’s t-test was used to analyze continuous variables, and the chi-square or Fisher’s exact tests were used to analyze categorical variables. The decline rates of lung function were calculated using a linear mixed model, adjusting for age, sex, BMI, and smoking status. Categorical evaluation of lung function changes was performed in a subgroup of patients who had pulmonary function data available at both baseline and follow ups at 6 or 12 months. Improvement in FVC was defined as an absolute change of +10%pred. or greater; deterioration was defined as an absolute change of −10%pred. or greater, and stability was defined when neither of these criteria were met. Similarly, the absolute changes in DLCO were categorized using a threshold of 15%pred. Logistic regression analyses were performed to analyze the risk factors associated with disease progression, which were defined as a ≥10%pred. absolute decline in FVC and/or ≥15%pred. absolute decline in DLCO at 6 or 12 months. If several variables were observed to be significant in a simple logistic regression (P< 0.10) analysis, multiple logistic regression was performed using a backward method. All P-values were two-tailed, and a P-value of <0.05 was considered statistically significant. All statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline patient characteristics
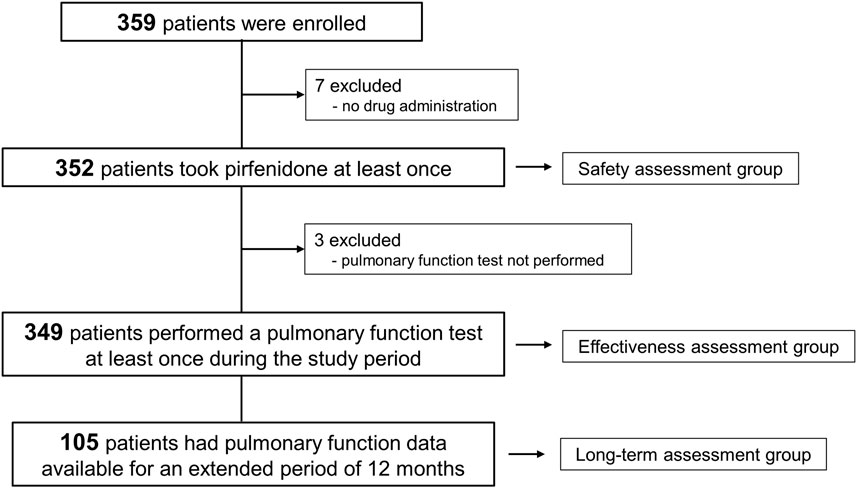
Between November 2018 and September 2021, a total of 359 patients with IPF were enrolled. Among them, 352 patients received pirfenidone at least once and were included in the safety assessment (Figure 1). Pulmonary function data were available for at least one assessment in 349 patients, qualifying them for inclusion in the effectiveness assessment group. Furthermore, pulmonary function data followed up at 12 months were obtained in 105 patients (long-term assessment group).
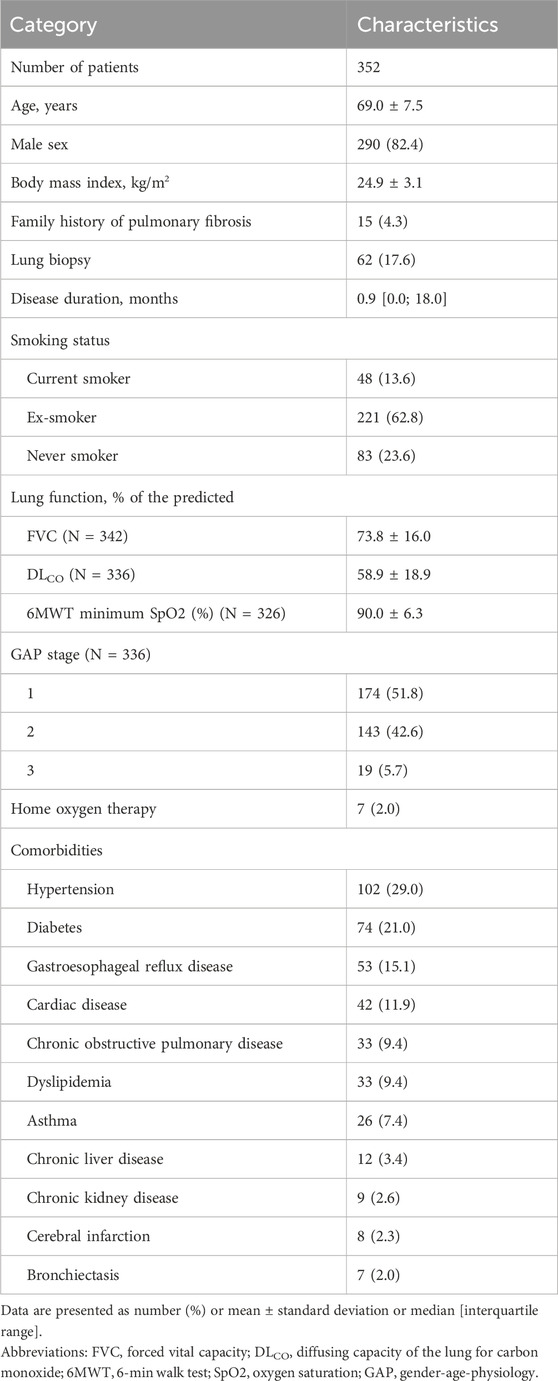
The baseline characteristics of the 352 patients are summarized in Table 1. The mean age was 69.0 years, and 82.4% were male patients. The median duration of IPF prior to study enrollment was 0.9 (IQR, 0.0–18.0) months. The mean baseline FVC and DLCO were 73.8%pred. and 58.9%pred., respectively. The median duration of the pirfenidone treatment was 186.0 (IQR, 156.0–342.5) days.
3.2 Adverse events
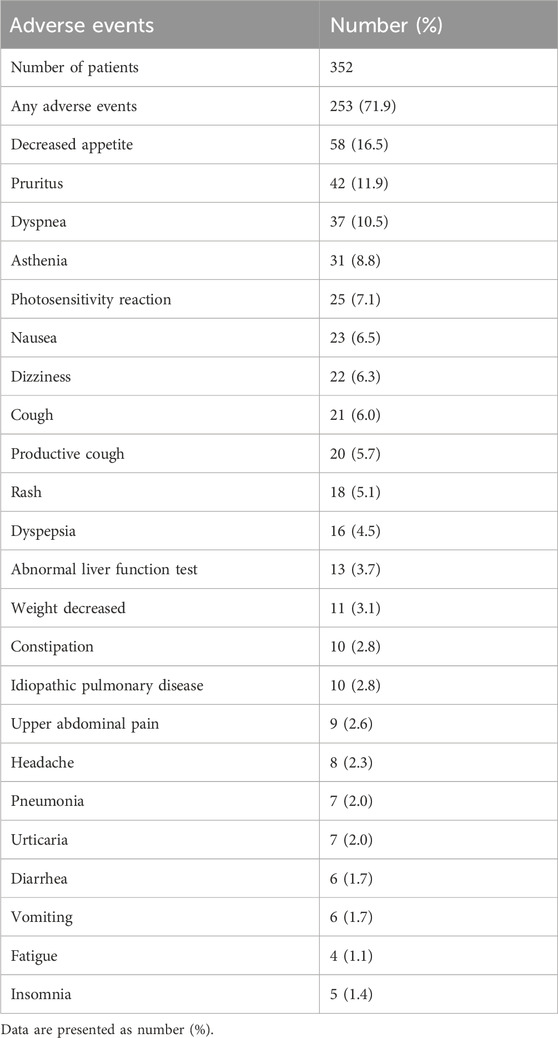
Among the safety assessment group, a total of 253 patients (71.9%) experienced adverse events (Table 2). The most common adverse event was decreased appetite (16.5%), followed by pruritus (11.9%). No significant differences were observed in the occurrence of adverse events based on sex (72.4%, male; 69.4%, female; P = 0.627), age (71.9%, patients aged ≥65 years; 71.9%, patients aged <65 years; P > 0.999), or presence of comorbidities (72.3%, with comorbidities; 70.4%, without comorbidities; P = 0.731). The frequency of adverse event types did not show significant differences based on sex, age, or comorbidities except for dyspnea which was significantly more frequent in the group without comorbidities than that in the group with comorbidities (18.5% vs. 8.1%, P = 0.013) (Supplementary Figure S1 in Supplementary Material).
3.3 Lung function change
The decline rates of FVC and DLCO were evaluated in the effectiveness assessment group. The mean values of FVC and DLCO at 3, 6, and 12 months are depicted in Figure 2A. The adjusted decline rates were −1.5%pred. and −2.2%pred. per year for FVC and DLCO, respectively (Figure 2B).
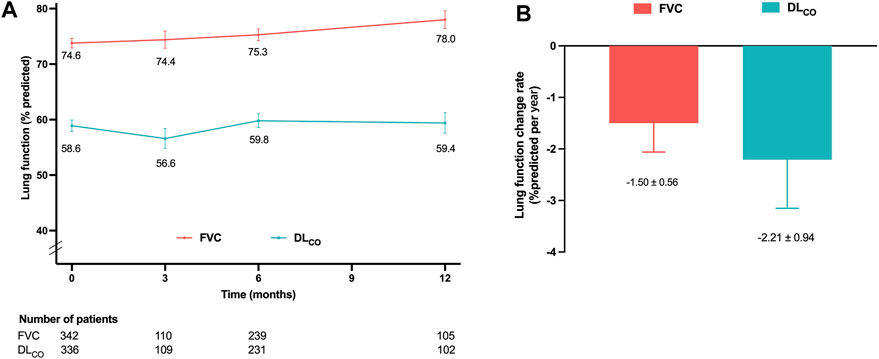
Figure 2. Lung function changes in the effectiveness assessment group (A) Mean FVC and DLCO values at 3, 6, and 12 months. (B) Decline rates of FVC and DLCO per year adjusted for age, sex, smoking status, and body mass index. Abbreviations: FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
Categorical assessment of FVC and DLCO absolute changes at 6 and 12 months is shown in Figure 3. At the 6-month follow-up, 8.6% and 9.0% of patients demonstrated deterioration in FVC and DLCO, respectively. Improvements in FVC and DLCO were observed in 7.8% and 9.9% of patients, respectively. The long-term assessment group demonstrated a decline in FVC and DLCO in 12.5% and 12.0% of patients, respectively. Conversely, 1.9% of patients demonstrated an improvement in FVC, and 2.0% demonstrated an improvement in DLCO.
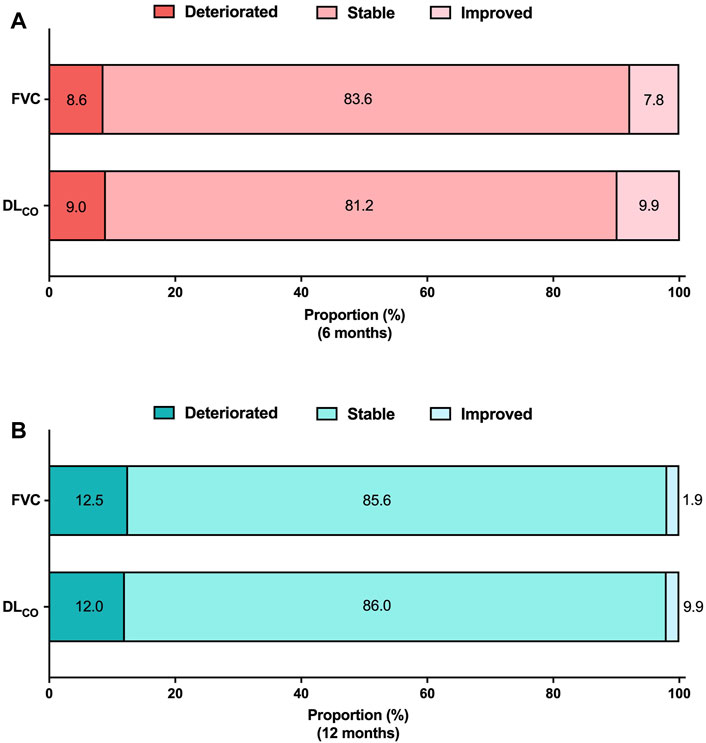
Figure 3. Categorical evaluation of lung function changes Absolute lung function changes (A) at 6 months and (B) at 12 months. The analysis was conducted on patients who had available pulmonary function data at both the baseline and follow-up periods. FVC 6 months (N = 232), DLCO 6 months (N = 223), FVC 12 months (N = 104), DLCO 12 months (N = 100). Abbreviations: FVC, forced vital capacity; N, number; DLCO, diffusing capacity of the lung for carbon monoxide.
3.4 Lung function and pirfenidone dose
Patients were categorized into three groups based on the most frequently prescribed dose of pirfenidone during the study period. The low-dose (<1,200 mg/day), medium-dose (1,200 and 1,600 mg/day), and high-dose (1800 mg/day) group consisted of 82, 133, and 134 patients, respectively. Notably, patients in the low-dose group exhibited older age, lower BMI and lung function, and a higher prevalence of advanced GAP stage that those of the patients in the other groups (Supplementary Table S1 in Supplementary Material).
Figures 4A, B present the mean levels of FVC and DLCO at 3, 6, and 12 months according to the pirfenidone dose. The FVC decline rates demonstrated no significant differences among the low-, medium-, and high-dose groups (−1.1%pred., −2.2%pred., and −1.2%pred. per year, respectively; p = 0.739) (Figure 5A). Similarly, the DLCO decline rates were not significantly different between the three groups (4.1%pred., −3.5%pred., and −2.4%pred. per year, respectively; p = 0.107) (Figure 5B).
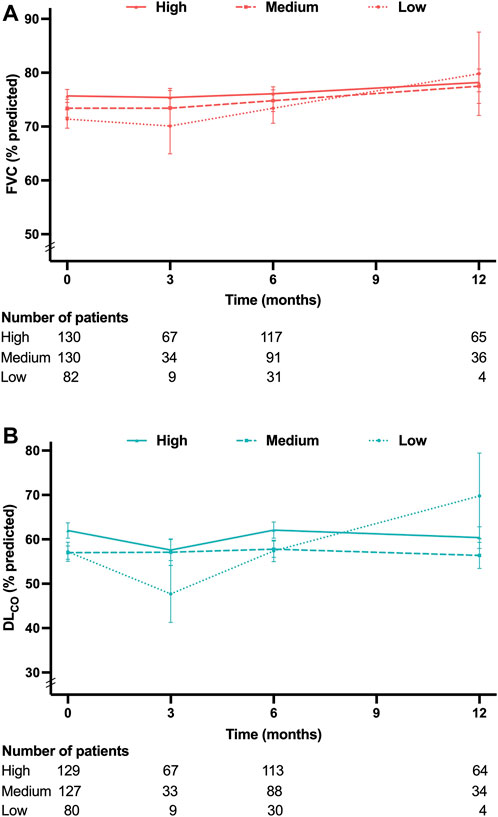
Figure 4. Lung function at each time point according to the pirfenidone dose (A) Mean FVC values at 3, 6, and 12 months. (B) Mean DLCO values at 3, 6, and 12 months. Pirfenidone dose group was defined as follows: low-dose group, less than 1,200 mg/day; medium-dose group, 1,200 and 1,600 mg/day; and high-dose group, 1800 mg/day. Abbreviation: FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
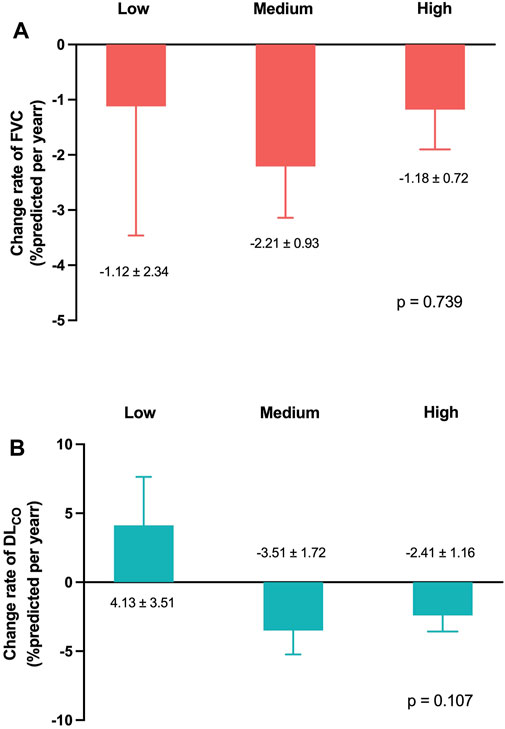
Figure 5. Lung function change rates according to the pirfenidone dose Change rates of (A) FVC and (B) DLCO per year adjusted for age, sex, smoking status, and body mass index. Pirfenidone dose group was defined as follows: low-dose group, less than 1,200 mg/day; medium-dose group, 1,200 and 1,600 mg/day; and high-dose group, 1,800 mg/day. Abbreviation: FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
3.5 Risk factors for disease progression
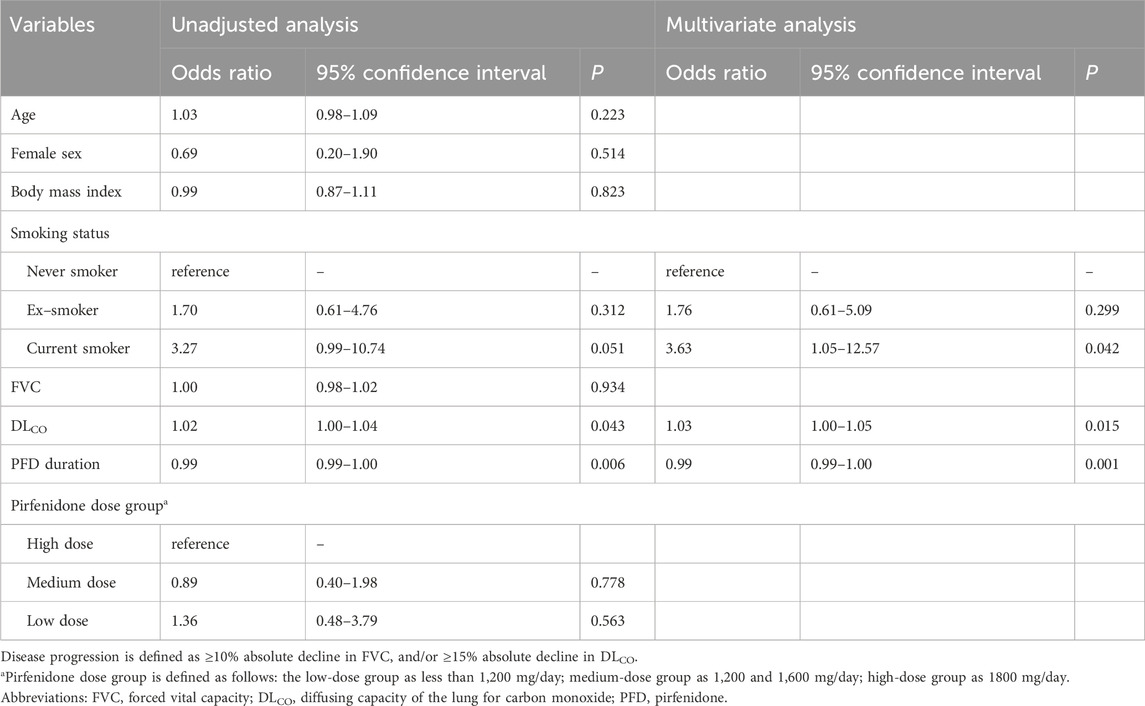
The risk factors for disease progression at 6 months were analyzed in patients who had pulmonary function data at baseline and 6 months (Table 3). Current smoking demonstrated a tendency towards a higher risk of disease progression at 6 months (odds ratio [OR], 3.27; 95% confidence interval [CI], 0.99–10.74; p = 0.051). A higher baseline DLCO value was significantly associated with disease progression (OR 1.02; 95% CI, 1.00–1.04; p = 0.043). A longer duration of pirfenidone treatment was significantly associated with a lower risk of disease progression at 6 months (OR, 0.99; 95% CI, 0.99–1.00; p = 0.006). According to multivariate analysis, these risk factors remained significant for disease progression at 6 months.
The risk factors for disease progression at 12 months were analyzed in patients who had pulmonary function data at baseline and 12 months (Supplementary Table S2 in Supplementary Material). In the unadjusted analysis, only current smoking demonstrated a significantly higher risk of disease progression (OR, 6.67; 95% CI, 1.40–31.72, P = 0.017).
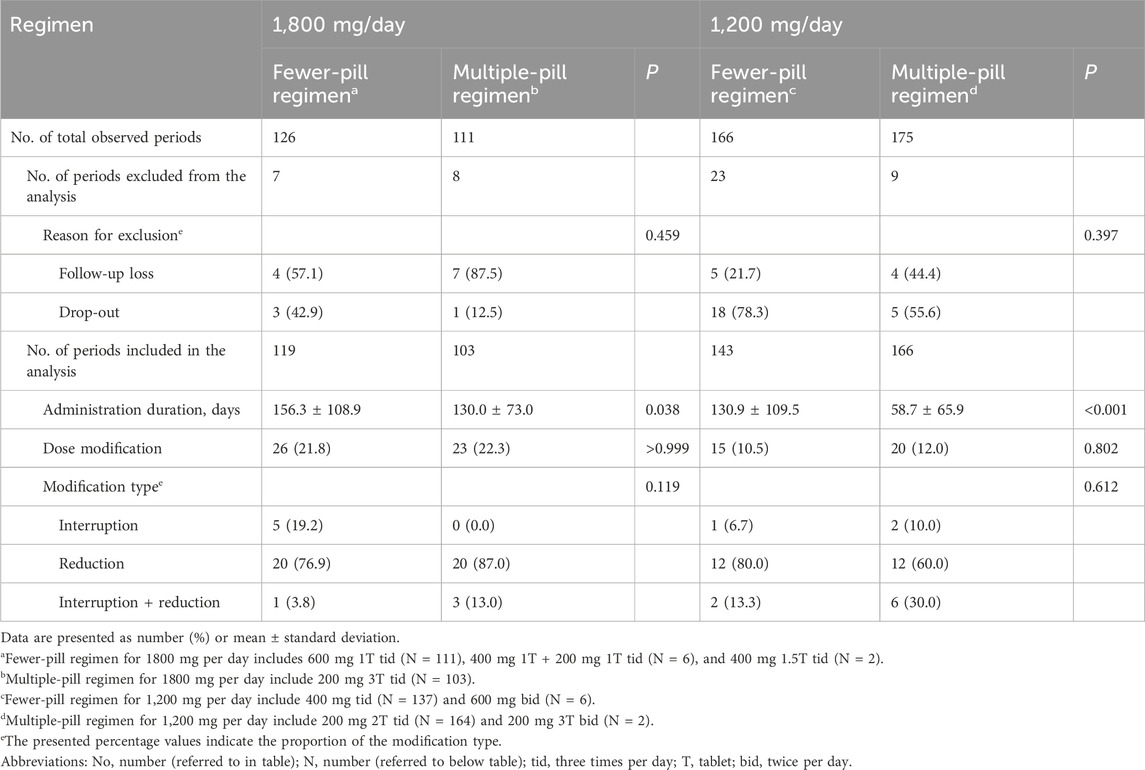
3.6 Effect of a fewer-pill regimen
A total of 155 patients were identified as having received a dosage of 1800 mg/day of pirfenidone at least once. Among them, 58 patients (37.4%) were administered pirfenidone using the fewer-pill regimen, while 55 patients (35.5%) were administered pirfenidone using the multiple-pill regimen. The remaining 42 patients (27.1%) initially started with an 1800 mg dosage under the multiple-pill regimen; however, subsequently transitioned to the fewer-pill regimen.
To assess the duration of drug administration under each regimen, administration periods were defined as uninterrupted intervals during which patients maintained the same pirfenidone dosage using either the fewer-pill or multiple-pill regimen. Periods without subsequent observations were excluded, as it was not possible to determine dose modifications.
A total of 119 periods were identified for patients treated with the fewer-pill regimen; 103 periods were observed for those using the multiple-pill regimen. The mean duration of drug administration was significantly longer with the fewer-pill regimen than that with the multiple-pill regimen (156.3 vs. 130.0 days; P = 0.038). The frequency of dose modification did not show a statistically significant difference between the fewer- (26 cases, 21.8%) and multiple-pill regimens (23 cases, 22.3%). Additionally, no significant difference in the type of dose modification (interruption or reduction) was observed.
A total of 233 patients were identified as having received a 1,200 mg/day dosage at least once. Among them, pirfenidone was administered using the fewer-pill regimen and multiple-pill regimen in 106 patients (45.5%) and 107 patients (45.9%), respectively. The regimen was transitioned from the multiple-pill to the fewer-pill regimen in 17 patients (12.8%), whereas in 3 patients (1.3%) switched from the multiple-pill to fewer-pill regimen and back to multiple-pill regimen. The results were similar among these patients (Table 4).
4 Discussion
In this study, we observed that Fybro® exhibited a similar profile of adverse events and effectiveness as that described in previous reports. The most common adverse event observed was decreased appetite. The decline in the rates of FVC and DLCO were −1.5%pred. and −2.2%pred. per year, respectively. These rates were not significantly different based on the pirfenidone dose. A significantly longer duration of drug administration was observed with the fewer-pill regimen (400 mg and/or 600 mg tablets to achieve daily intake of 1,200 or 1800 mg of pirfenidone) than with multiple-pill regimen.
Pirfenidone’s long-term safety and tolerability has been well-documented. Our study did not identify any new adverse events related to Fybro®, and the overall safety profile was similar to that of previous reports. The PASSPORT study (Cottin et al., 2018), which investigated the safety of pirfenidone prospectively over a long-term period, reported that 73.4% of the patients experienced any adverse drug reaction, similar to our findings. However, unlike other studies wherein nausea was reported as the most common adverse event (Lancaster et al., 2016; Noble et al., 2016), our study identified decreased appetite as the most common adverse event. Post-marketing surveillance (PMS) cohort studies conducted in South Korea and Japan also reported decreased appetite to be the most common adverse event (32.4% and 27.9%, respectively) (Ogura et al., 2015; Chung et al., 2020). The differences in the approved doses of pirfenidone in South Korea and Japan, compared with those approved in the United States or Europe, may be related to variations in the safety profile of pirfenidone (Dhooria et al., 2020). Furthermore, variations in adverse event profiles may be influenced by racial differences, indicated by the similar findings in nintedanib studies. In the open-label extension trial of nintedanib (INPULSIS-ON), Asian patients exhibited higher prevalence of decreased appetite than nausea among new initiators (event rate, 14.9 vs. 11.2 per 100 patient exposure-years) (Song J. W. et al., 2020). In addition, an interim report of the nintedanib PMS study conducted in Japan demonstrated that among adverse events leading to drug discontinuation, decreased appetite was more prevalent than nausea (12.7% vs. 5.9%) (Ogura et al., 2023). These findings suggest a lower incidence of nausea and higher incidence of decreased appetite in patients with IPF who are of Eastern Asian origins.
Another notable finding was the relatively lower incidence of gastrointestinal and skin-related adverse events than those reported in previous research (Noble et al., 2011; Ogura et al., 2015; Chung et al., 2020). The CAPACITY trial, a phase III randomized controlled trial evaluating the efficacy of pirfenidone, reported incidence rates of 36%, 32%, and 12% for nausea, rash, and photosensitivity, respectively (Noble et al., 2011). The aforementioned PMS studies conducted in South Korea and Japan also reported higher rates of photosensitivity at 13.7% and 14.4% (Ogura et al., 2015; Chung et al., 2020), respectively, exceeding the 7.1% rate observed in the current study. This discrepancy may be attributed to increased physician awareness of adverse events due to accumulated real-world experience with pirfenidone. Furthermore, enhanced patient education and implementation of preventive measures, such as medication administration with meals and appropriate sunscreen application, may have contributed to the observed difference in adverse events compared with those reported in earlier studies.
The lung function decline rates observed in our study are consistent with findings from previous research (Zurkova et al., 2019; Lee et al., 2021). A retrospective analysis of 383 patients with IPF treated with pirfenidone in the Czech Republic demonstrated annual change rates of 0.2% for FVC and −2.4% for DLCO (Zurkova et al., 2019). The proportions of patients experiencing FVC and DLCO deterioration were also similar with our findings; at 6 months, FVC and DLCO deterioration rates were 5.3% and 6.1%, respectively, while at 12 months, the proportions were 10.7% and 11.3%, respectively (Zurkova et al., 2019). Another recent real-world data study involving 100 patients with IPF reported similar annual change rates of −1.5% for FVC and 0.8% for DLCO (Lee et al., 2021). The similarity of lung function changes to the findings of previous studies provides support for the use of Fybro® in treating IPF. Our analysis of lung function decline based on pirfenidone dose revealed no significant differences between the doses. Previous studies suggested that even with lower doses, the treatment can be effective (Hwang et al., 2022; Kang et al., 2023). In a prospective cohort study involving 143 patients with IPF, no significant differences in lung function changes were observed among the three groups categorized by pirfenidone dose (<1,200 mg, 1,200 mg, and 1800 mg per day) (Kang et al., 2023). Another recent multicenter retrospective cohort study examined the effect of lower-dose pirfenidone on lung function changes before and after treatment (Hwang et al., 2022). The effect of slowing the FVC decline in the lower-dose group was similar to that observed in the standard-dose group.
Our study revealed an association between a longer duration of pirfenidone treatment and lower risk of deterioration in both FVC and DLCO. This finding highlighted the importance of implementing strategies that promote long-term pirfenidone use. Previous studies have demonstrated a significant correlation between the number of concomitant medications or polypharmacy at baseline and antifibrotic medication intolerance within the first 6 months in patients with IPF (Khor et al., 2022). Although the study did not specifically consider the number of pirfenidone tablets, this finding implies a potential relationship between higher pill burden and pirfenidone intolerance. Therefore, improving the convenience of administration by reducing tablet counts may increase medication adherence. Our study observed a significant association between fewer-pill regimen for the same dose (1,200 or 1800 mg per day) and prolonged treatment duration than that with a multiple-pill regimen. However, our study was not explicitly designed to address this issue; therefore, establishing a direct causal relationship between fewer pirfenidone tablets and patient adherence is not possible. As certain patients switched from a multiple-to a fewer-pill regimen, a bias towards prolonged administration in the latter regimen could exist due to their inclusion of well-tolerated patients receiving 1800 mg dosage under the multiple-pill regimen. Further research is warranted to examine the potential advantages of a pirfenidone regimen with fewer pills.
Pirfenidone has also been investigated for its efficacy in progressive pulmonary fibrosis (PPF) (Tzilas et al., 2020; Behr et al., 2021). A double-blind, randomized controlled phase 2b trial investigating pirfenidone in progressive fibrotic interstitial lung disease revealed that the pirfenidone group exhibited a significantly smaller decline in FVC %pred. from baseline to week 48 compared to the placebo group (Behr et al., 2021). The result should be interpreted with caution due to the premature termination of the study as a result of slow recruitment and further research is required (Raghu et al., 2022). Nevertheless, the clinical similarities between PPF and IPF suggest that pirfenidone may also be beneficial for PPF. The indications for Fybro might be extended beyond IPF to encompass PPF, leading to broader application of the drug. The effectiveness and safety of Fybro demonstrated in our study could provide valuable guidance for clinicians in the prescribing of this medication.
Our study had some limitations. First, the study duration was 6 months, and only a subset of patients was followed up till 12 months. Categorical evaluation of lung function changes and logistic regression analysis to identify risk factors for lung function deterioration at 12 months may have been underpowered. Although we utilized linear mixed models to calculate lung function change rates, further studies including a larger sample are warranted. Second, our study did not evaluate important clinical outcomes, such as acute exacerbation and mortality. Despite the expectation of Fybro® exhibiting similar positive effects on these outcomes, considering its demonstrated effectiveness in preventing lung function decline observed in our study, additional research with a specific focus on these outcomes is required. Third, the number of patients included in the low-dose group was relatively small than that in the other groups, which may have influenced the calculated lung function decline rate in this group. However, our results indicated no significant difference in the effects of lower doses of pirfenidone, aligning with those of previous research (Song M. J. et al., 2020; Hwang et al., 2022). Finally, our study was conducted in patients treated with Fybro®, a medication developed in South Korea. The results may not be readily generalized to other generic versions of pirfenidone and further studies are needed.
In conclusion, the safety and effectiveness of Fybro® investigated in a real-world prospective cohort study were similar to those reported in previous studies. Using higher-dose tablets to reduce the required pill count for dose achievement may enhance medication adherence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of each institution: [Asan Medical Center (2018-1312); Haeundae Paik Hospital (HPIRB 2018-07-015); Busan Paik Hospital (18-0183); Wonju Severance Christian Hospital (CR118075); Yeungnam University Medical Center (YUMC 2018-11-035); Gyeongsang National University Hospital (GNUH 2019-02-009); Korea University Anam Hospital (2019AN0137); Keimyung University Dongsan Medical Center (DSMC 2019-03-020); Ajou University Hospital (AJIRB-MED-OBS-19-153); Wonkwang University Hospital (2019-05-012); Ulsan University Hospital (2018-08-049); Gyeongsang National University Changwon Hospital (2019-09-029); Kyung Hee University Medical Center (KHUH 2020-02-061); and Seoul National University Hospital (2003-043-1,108)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JK: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft, Writing–review and editing. KHL: Investigation, Resources, Writing–review and editing. JHL: Investigation, Resources, Writing–review and editing. YYJ: Investigation, Resources, Writing–review and editing. SMC: Investigation, Resources, Writing–review and editing. HCK: Investigation, Resources, Writing–review and editing. JHP: Investigation, Resources, Writing–review and editing. H-KL: Investigation, Resources, Writing–review and editing. SJY: Investigation, Resources, Writing–review and editing. HSC: Investigation, Resources, Writing–review and editing. HRK: Investigation, Resources, Writing–review and editing. YJ: Investigation, Resources, Writing–review and editing. W-iC: Investigation, Resources, Writing–review and editing. EJL: Investigation, Resources, Writing–review and editing. JWS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The nationwide observational study that provided the data for the current study was funded by Yungjin Pharmaceutical. The funder was not involved in the writing of or the decision to submit this article for publication. JWS was supported by grants from the Basic Science Research Program (NRF-2022R1A2B5B02001602) and the Bio & Medical Technology Development Program (NRF-2022M3A9E4082647) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT, Korea. JWS was also supported by the National Institute of Health research project (2024ER090500) and by Korea Environment Industry & Technology Institute through Core Technology Development Project for Environmental Diseases Prevention and Management Program funded by Korea Ministry of Environment (RS-2022-KE002197), Republic of Korea. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1451447/full#supplementary-material
Abbreviations
BMI, body mass index; CI, confidence interval; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; GAP gender-age-physiology; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; OR, odds ratio, PMS, post-marketing surveillance.
References
Behr, J., Prasse, A., Kreuter, M., Johow, J., Rabe, K. F., Bonella, F., et al. (2021). Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 9, 476–486. doi:10.1016/S2213-2600(20)30554-3
Chung, M. P., Park, M. S., Oh, I. J., Lee, H. B., Kim, Y. W., Park, J. S., et al. (2020). Safety and efficacy of pirfenidone in advanced idiopathic pulmonary fibrosis: a nationwide post-marketing surveillance study in Korean patients. Adv. Ther. 37, 2303–2316. doi:10.1007/s12325-020-01328-8
Cottin, V., Koschel, D., Günther, A., Albera, C., Azuma, A., Sköld, C. M., et al. (2018). Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study. ERJ Open Res. 4. doi:10.1183/23120541.00084-2018
Dhooria, S., Agarwal, R., Sehgal, I. S., Prasad, K. T., Muth, V., Garg, M., et al. (2020). A real-world study of the dosing and tolerability of pirfenidone and its effect on survival in idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffuse Lung Dis. 37, 148–157. doi:10.36141/svdld.v37i2.8718
Fletcher, S., Jones, M. G., Spinks, K., Sgalla, G., Marshall, B. G., Limbrey, R., et al. (2016). The safety of new drug treatments for idiopathic pulmonary fibrosis. Expert Opin. Drug Saf. 15, 1483–1489. doi:10.1080/14740338.2016.1218470
Hwang, H., Lee, J. K., Choi, S. M., Lee, Y. J., Cho, Y. J., Yoon, H. I., et al. (2022). Efficacy of lower dose pirfenidone for idiopathic pulmonary fibrosis in real practice: a retrospective cohort study. Korean J. Intern Med. 37, 366–376. doi:10.3904/kjim.2020.559
Kang, J., Chung, M. P., Park, M. S., Oh, I. J., Lee, H. B., Kim, Y. W., et al. (2023). Clinical outcomes of dose modification during pirfenidone treatment for IPF: a nationwide post-marketing surveillance study. Front. Pharmacol. 13, 1025947. doi:10.3389/fphar.2022.1025947
Khor, Y. H., Goh, N. S. L., Wong, A. W., Johannson, K. A., Marcoux, V., Fisher, J. H., et al. (2022). Impact of concomitant medication burden on tolerability of disease-targeted therapy and survival in interstitial lung disease. Ann. Am. Thorac. Soc. 19, 962–970. doi:10.1513/AnnalsATS.202108-980OC
King, T. E., Bradford, W. Z., Castro-Bernardini, S., Fagan, E. A., Glaspole, I., Glassberg, M. K., et al. (2014). A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092. doi:10.1056/NEJMoa1402582
Lancaster, L., Albera, C., Bradford, W. Z., Costabel, U., Du Bois, R. M., Fagan, E. A., et al. (2016). Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: integrated analysis of cumulative data from 5 clinical trials. BMJ Open Respir. Res. 3, e000105. doi:10.1136/bmjresp-2015-000105
Lee, E. G., Lee, T. H., Hong, Y., Ryoo, J., Heo, J. W., Gil, B. M., et al. (2021). Effects of low-dose pirfenidone on survival and lung function decline in patients with idiopathic pulmonary fibrosis (IPF): results from a real-world study. PLoS One 16, e0261684. doi:10.1371/journal.pone.0261684
Ley, B., Ryerson, C. J., Vittinghoff, E., Ryu, J. H., Tomassetti, S., Lee, J. S., et al. (2012). A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern Med. 156, 684–691. doi:10.7326/0003-4819-156-10-201205150-00004
Macintyre, N., Crapo, R. O., Viegi, G., Johnson, D. C., Van Der Grinten, C. P., Brusasco, V., et al. (2005). Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 26, 720–735. doi:10.1183/09031936.05.00034905
Margaritopoulos, G. A., Trachalaki, A., Wells, A. U., Vasarmidi, E., Bibaki, E., Papastratigakis, G., et al. (2018). Pirfenidone improves survival in IPF: results from a real-life study. BMC Pulm. Med. 18, 177. doi:10.1186/s12890-018-0736-z
Miller, M. R., Hankinson, J., Brusasco, V., Burgos, F., Casaburi, R., Coates, A., et al. (2005). Standardisation of spirometry. Eur. Respir. J. 26, 319–338. doi:10.1183/09031936.05.00034805
Nathan, S. D., Shlobin, O. A., Weir, N., Ahmad, S., Kaldjob, J. M., Battle, E., et al. (2011). Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 140, 221–229. doi:10.1378/chest.10-2572
Noble, P. W., Albera, C., Bradford, W. Z., Costabel, U., Du Bois, R. M., Fagan, E. A., et al. (2016). Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 47, 243–253. doi:10.1183/13993003.00026-2015
Noble, P. W., Albera, C., Bradford, W. Z., Costabel, U., Glassberg, M. K., Kardatzke, D., et al. (2011). Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377, 1760–1769. doi:10.1016/S0140-6736(11)60405-4
Ogura, T., Azuma, A., Inoue, Y., Taniguchi, H., Chida, K., Bando, M., et al. (2015). All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis. Respir. Investig. 53, 232–241. doi:10.1016/j.resinv.2015.06.001
Ogura, T., Inoue, Y., Azuma, A., Homma, S., Kondoh, Y., Tanaka, K., et al. (2023). Real-world safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: interim report of a post-marketing surveillance in Japan. Adv. Ther. 40, 1474–1493. doi:10.1007/s12325-022-02411-y
Raghu, G., Chen, S. Y., Yeh, W. S., Maroni, B., Li, Q., Lee, Y. C., et al. (2014). Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir. Med. 2, 566–572. doi:10.1016/S2213-2600(14)70101-8
Raghu, G., Remy-Jardin, M., Myers, J. L., Richeldi, L., Ryerson, C. J., Lederer, D. J., et al. (2018). Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e44–e68. doi:10.1164/rccm.201807-1255ST
Raghu, G., Remy-Jardin, M., Richeldi, L., Thomson, C. C., Inoue, Y., Johkoh, T., et al. (2022). Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 205, e18–e47. doi:10.1164/rccm.202202-0399ST
Richeldi, L., Du Bois, R. M., Raghu, G., Azuma, A., Brown, K. K., Costabel, U., et al. (2014). Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082. doi:10.1056/NEJMoa1402584
Ruwanpura, S. M., Thomas, B. J., and Bardin, P. G. (2020). Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am. J. Respir. Cell Mol. Biol. 62, 413–422. doi:10.1165/rcmb.2019-0328TR
Song, J. W., Ogura, T., Inoue, Y., Xu, Z., Quaresma, M., Stowasser, S., et al. (2020a). Long-term treatment with nintedanib in Asian patients with idiopathic pulmonary fibrosis: results from INPULSIS®-ON. Respirology 25, 410–416. doi:10.1111/resp.13647
Song, M. J., Moon, S. W., Choi, J. S., Lee, S. H., Lee, S. H., Chung, K. S., et al. (2020b). Efficacy of low dose pirfenidone in idiopathic pulmonary fibrosis: real world experience from a tertiary university hospital. Sci. Rep. 10, 21218. doi:10.1038/s41598-020-77837-x
Taniguchi, H., Ebina, M., Kondoh, Y., Ogura, T., Azuma, A., Suga, M., et al. (2010). Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 35, 821–829. doi:10.1183/09031936.00005209
Toellner, H., Hughes, G., Beswick, W., Crooks, M. G., Donaldson, C., Forrest, I., et al. (2017). Early clinical experiences with nintedanib in three UK tertiary interstitial lung disease centres. Clin. Transl. Med. 6, 41. doi:10.1186/s40169-017-0172-3
Tzilas, V., Tzouvelekis, A., Bouros, E., Karampitsakos, T., Ntassiou, M., Avdoula, E., et al. (2020). Clinical experience with antifibrotics in fibrotic hypersensitivity pneumonitis: a 3-year real-life observational study. ERJ Open Res. 6. doi:10.1183/23120541.00152-2020
Tzouvelekis, A., Karampitsakos, T., Kourtidou, S., Bouros, E., Tzilas, V., Katsaras, M., et al. (2020). Impact of depression on patients with idiopathic pulmonary fibrosis. Front. Med. (Lausanne) 7, 29. doi:10.3389/fmed.2020.00029
Xylourgidis, N., Min, K., Ahangari, F., Yu, G., Herazo-Maya, J. D., Karampitsakos, T., et al. (2019). Role of dual-specificity protein phosphatase DUSP10/MKP-5 in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 317, L678–l689. doi:10.1152/ajplung.00264.2018
Keywords: adherence, adverse events, idiopathic pulmonary fibrosis, lung function decline, pirfenidone
Citation: Kang J, Lee KH, Lee JH, Jeong YY, Choi SM, Kim HC, Park JH, Lee H-K, Yong SJ, Choi HS, Kim HR, Jegal Y, Choi W-i, Lee EJ and Song JW (2024) Safety, effectiveness, and usefulness of higher-dose tablets of generic pirfenidone in patients with IPF: a nationwide observational study in South Korea. Front. Pharmacol. 15:1451447. doi: 10.3389/fphar.2024.1451447
Received: 19 June 2024; Accepted: 29 July 2024;
Published: 09 August 2024.
Edited by:
Haiyang Tang, University of Arizona, United StatesReviewed by:
Theodoros Karampitsakos, University of South Florida, United StatesGaetano Rea, Monaldi Hospital, Italy
Copyright © 2024 Kang, Lee, Lee, Jeong, Choi, Kim, Park, Lee, Yong, Choi, Kim, Jegal, Choi, Lee and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Woo Song, andzb25nYXNhbkBnbWFpbC5jb20=
 Jieun Kang
Jieun Kang Kwan Ho Lee2
Kwan Ho Lee2 Joo Hun Park
Joo Hun Park Hye Sook Choi
Hye Sook Choi Jin Woo Song
Jin Woo Song