- 1Department of Periodical Press, West China Hospital, Sichuan University, Chengdu, China
- 2West China Hospital, West China School of Medicine, Sichuan University, Chengdu, China
- 3Department of Neurology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 4Department of General Surgery and Regenerative Medicine Research Center, West China Hospital, Sichuan University, Chengdu, China
- 5Department of General Surgery, Chengdu Integrated Traditional Chinese Medicine and Western Medicine Hospital, Chengdu, China
Background: Vascular dementia (VaD) is one of the most prevalent, burdensome, and costly forms of dementia. Pharmacological treatment is often the first-line choice for clinicians; however, there is a paucity of comparative information regarding the multiple available drug options.
Methods and Analysis: A systematic review and network meta-analysis were conducted on randomized trials involving adult patients with VaD, sourced from PubMed, the Cochrane Library, EMBASE, Web of Science, OPENGREY, ClinicalTrials.gov, Wanfang Data, and CNKI. The primary outcomes included changes in Mini-Mental State Examination (MMSE) scores, activities of daily living (ADL) scores, and the incidence of adverse reactions. Efficacy and safety of intervention strategies were comprehensively analyzed using forest plots, cumulative ranking probability curves (SUCRA), and funnel plots, all generated with R software.
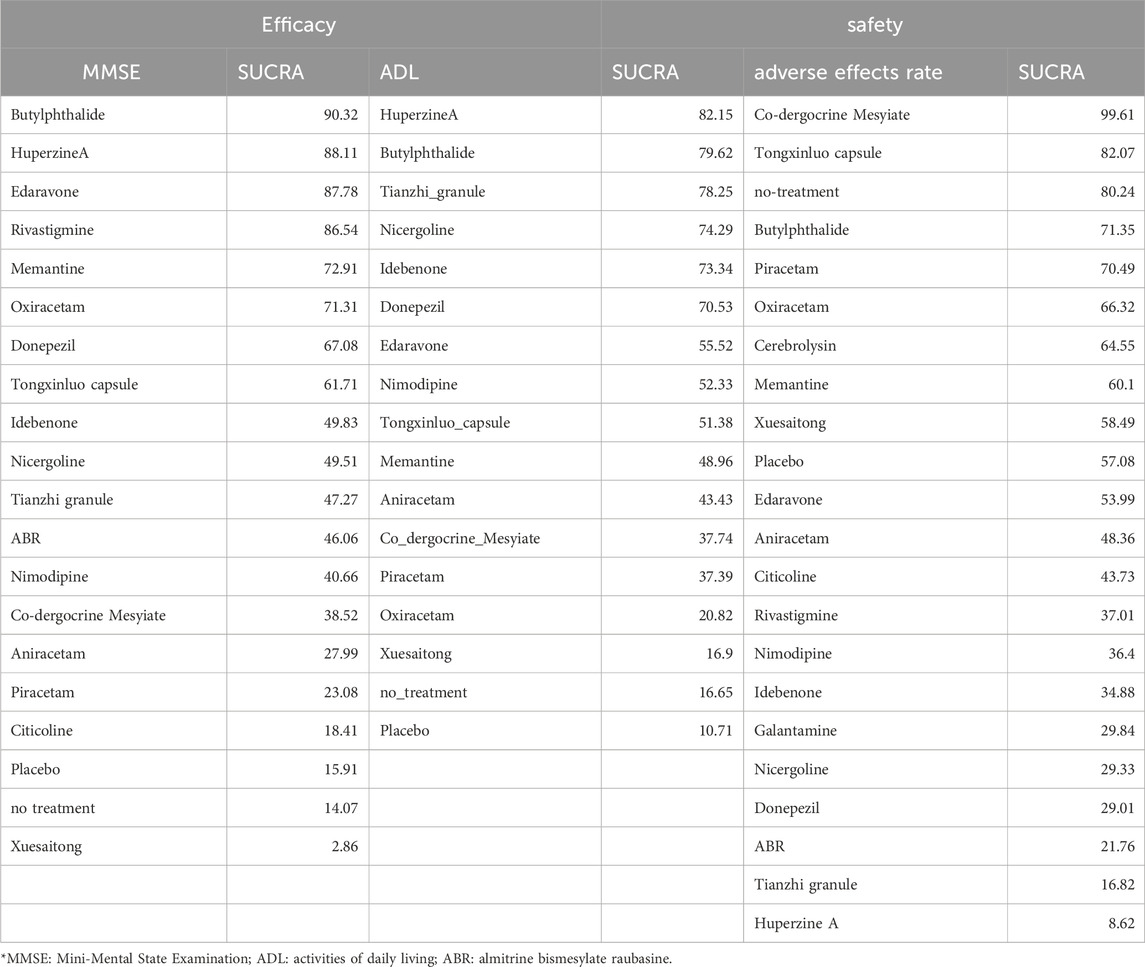
Results: A total of 194 RCTs comparing 21 different anti-VaD drugs with placebos or no treatment were analysed. Regarding MMSE scores, the five most effective drugs were Butylphthalide, Huperzine A, Edaravone, Rivastigmine, and Memantine. For ADL scores, the top five drugs in efficacy were Huperzine A, Butylphthalide, Tianzhi granule, Nicergoline, and Idebenone. In terms of the incidence of adverse drug reactions, Co-dergocrine Mesylate, Tongxinluo capsule, Butylphthalide, Piracetam, and Oxiracetam demonstrated favourable safety profiles.
Conclusion: This study enhances the understanding of the relative benefits and risks associated with various VaD treatments, providing a valuable reference for clinical decision-making.
Systematic Review Registration:: https://www.crd.york.ac.uk/PROSPERO/, identifier registration number.
Introduction
Vascular dementia (VaD) is widely recognized as the second most prevalent subtype of dementia, after Alzheimer’s disease (AD). VaD refers to dementia resulting from vascular brain injury that encompasses damage to the brain parenchyma, most commonly due to ischemia, infarction, and hemorrhage (Bir et al., 2019). The most recent diagnostic criteria for VaD classify major VaD into four phenotypic categories: subcortical ischemic vascular dementia, poststroke dementia, multi-infarct dementia, and mixed dementia (Chang et al., 2022). Currently, over 50 million individuals globally are affected by dementia, and projections suggest that the number of cases could quintuple by 2055 (Wolters and Ikram, 2019). VaD is responsible for approximately 15%–20% of dementia cases in North America and Europe (Lobo et al., 2000; Rizzi et al., 2014), with estimates reaching about 30% in Asia (Jhoo et al., 2008; Wolters and Ikram, 2018). The aging demographic trend and extended life expectancy have significantly exacerbated the incidence of VaD.
The pathogenesis of VaD is linked to chronic cerebrovascular damage stemming from conditions such as cerebral artery disease, atherosclerosis, arteriolosclerosis, microvascular disease, and cerebral amyloid angiopathy (Pathan et al., 2024). Clinically, VaD patients may exhibit behavioral changes, reduced attention and memory capacities, and cognitive deficits (Linh et al., 2022). Without timely and effective intervention, the progression of VaD can severely impair neurological functions, leading to motor, sensory, and perceptual deficits that significantly deteriorate the quality of life for individuals.
“Despite its high prevalence and substantial societal impact, VaD lacks Food and Drug Administration-approved treatments, unlike AD”. Therapeutic options and their efficacy for VaD remain considerably constrained (Tisher and Salardini, 2019). Cholinesterase inhibitors enhance cognition by augmenting the levels of intrasynaptic acetylcholine, a neurotransmitter pivotal for memory and learning (Román and Kalaria, 2006; Chen et al., 2016). Although cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine are sanctioned for AD in the United States and most European countries, their approval does not extend to VaD. However, donepezil has received approval for VaD from regulatory agencies in New Zealand, India, Romania, South Korea, and Thailand. Additionally, Memantine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, mitigates glutamatergic signalling through NMDA receptors. Memantine is approved in Argentina, Brazil, and Mexico for the management of VaD (Burns et al., 2006; Johnson and Kotermanski, 2006). Furthermore, numerous therapeutic agents demonstrate potential in the treatment of VaD, encompassing antioxidants, anti-inflammatory therapies, neuronutritional supplements, neuroprotective agents, and nootropic drugs (Chang et al., 2022).
Additionally, “Chinese medicine treatment of vascular disease guidelines for the clinical application of dementia (2020 edition)” issued in China advocate the use of proprietary Chinese medicines for VaD treatment, highlighting agents such as butylphthalide, tongxinluo capsule, Edaravone, and tianzhi granule. In clinical practice, various herbal medications are widely used as alternative treatments to conventional pharmacotherapies (Chan et al., 2018; Kim and Kang, 2020), providing a complementary approach in the therapeutic landscape of VaD (S. P. T. f. t. G. o. C. A. o. P. C. M. i. t. T. o. V., 2021). In practice, a plethora of Chinese patent medicines are used as alternative treatments to conventional pharmacotherapies (Chan et al., 2018; Kim and Kang, 2020). However, discerning the comparative efficacy and safety of these diverse pharmacological therapies presents a considerable challenge. For clinicians tasked with selecting from different treatment options, synthesizing the available evidence on VaD from extant trials is crucial to ascertain the relative effects and safety of the treatment therapies.
Network meta-analysis (NMA) is an advanced statistical technique used to assess and compare the efficacy and safety of multiple therapeutic interventions concurrently. By integrating both direct comparisons between treatments and indirect comparisons across trials sharing common comparators, NMA facilitates a robust synthesis of evidence. This methodology extends the scope of traditional pairwise meta-analysis, allowing for a comprehensive ranking of competing treatments based on their effectiveness and safety, thereby supporting evidence-based clinical decision-making.
This study is designed to undertake a series of comparisons between various pharmacological therapies for VaD treatment. It aims to extensively evaluate existing evidence and provide a robust foundation for clinical decision-making, enhancing the understanding of the comparative benefits and risks associated with each pharmacological intervention.
Methods
The systematic review was meticulously conducted adhering to the PRISMA Extension Statement for Reporting Systematic Reviews with Network Meta-Analyses (Hutton et al., 2015), with the study protocol registered in the PROSPERO database (CRD42024521910).
Search strategy and data retrieval
Comprehensive searches were executed across multiple electronic databases from their inception through March 2024, including PubMed, the Cochrane Library, EMBASE, Web of Science, OPENGREY, ClinicalTrials.gov, Wanfang Data, and Chinese National Knowledge Infrastructure (CNKI). Searches of the grey literature were also undertaken. The objective was to identify peer-reviewed studies that assess the efficacy and safety of pharmacological interventions for VaD. The detailed search methodologies are delineated in Supplementary Appendix 1.
We scrutinised the reference lists of all pertinent articles, study reports, and conference proceedings, and conducted searches of unpublished literature to capture additional relevant studies, thereby minimising the risk of omissions. Following the removal of duplicate entries, two investigators (Dang et al., 2023) independently screened titles and abstracts to identify studies potentially meeting the inclusion criteria. These articles were further assessed for eligibility by the same researchers. Any disagreements were resolved through discussion or, if necessary, consultation with a third researcher (Qian Li). Consultations with subject matter experts were sought as required.
Study selection
The research question was meticulously structured using the PICOS (Population, Intervention, Comparison, Outcome, Study design) framework. Population: Inclusion was limited to patients diagnosed with VaD; Intervention: patients undergoing treatment with a single pharmacologic agent; Comparison: participants were compared across groups receiving a single drug, placebo, or no treatment for VaD; Outcome: primary outcomes assessed included the Mini-Mental State Examination (MMSE) score, activities of daily living (ADL) score, and the incidence of adverse reactions. Study Design: randomized controlled trials (RCTs).
The inclusion criteria were as follows: (Bir et al., 2019): Patients conforming to established diagnostic criteria for VaD, as outlined by the Diagnostic and Statistical Manual of Mental Disorders, the National Institute of Neurological Disorders and Stroke (Wen et al., 2022). (Chang et al., 2022) Patients receiving monotherapy for VaD (Wolters and Ikram, 2019). Studies that reported on efficacy and safety outcomes, specifically MMSE score, ADL score, and incidence of drug-related adverse reactions.
The exclusion criteria were as follows: (Bir et al., 2019): Studies involving patients diagnosed with AD or other subtypes of dementia caused by different etiologies (Chang et al., 2022). Patients undergoing multiple therapeutic interventions (Wolters and Ikram, 2019). Individuals presenting with severe neurological deficits or significant medical conditions such as visual impairment, aphasia, hearing loss, and malignancies (Lobo et al., 2000). Studies lacking primary outcome data.
Data extraction
Data extraction from the included RCTs was conducted independently by two reviewers, Dang et al., 2023. The data extracted encompassed detailed study characteristics, including the first author, year of publication, participant demographics (sex, age, sample sizes, follow-up duration), details of the intervention group (intervention measures, dosage, administration route), and control group characteristics (control measures, dosage, administration route). Additionally, outcome measures were recorded. Discrepancies encountered during the data extraction process were adjudicated by a third reviewer, Qian Li, through a consensus discussion involving all reviewers. In instances where data were missing or incomplete in the published articles, efforts were made to contact the corresponding authors directly to request the original data. This approach ensured a comprehensive and accurate data compilation critical for subsequent analyses.
Outcome measures
The efficacy of treatments was evaluated through cognitive function assessments using the MMSE scores, and functional capabilities via the ADL scores. The incidence of drug-related adverse reactions was established as the primary safety outcome indicator. The MMSE is the most extensively utilized neuropsychological scale for screening clinical cognitive functions, effectively reflecting the cognitive status and characteristics of patients (Ciesielska et al., 2016). Given that cognitive impairment is a hallmark symptom of VaD, it was selected as the primary efficacy outcome for this review. Additionally, the deterioration in the ability to perform ADL, a prominent symptom of VaD, was chosen as a secondary efficacy outcome measure (Mlinac and Feng, 2016). The incidence of adverse reactions, defined as the proportion of patients experiencing at least one adverse event relative to the total number of patients in both the intervention and control groups, serves as a globally accepted metric for assessing treatment safety (Patton and Borshoff, 2018).
Bias assessment
Two independent reviewers, Dang et al., 2023, rigorously evaluated the identified trials. The Cochrane Collaboration’s Risk of Bias Tool (RoB 2.0) was employed to assess the risk of bias in the included RCTs. Any discrepancies between the reviewers were referred to a third reviewer, Qian Li, and were resolved through comprehensive discussion among all reviewers. This process ensured a robust and unbiased evaluation of the studies, maintaining the integrity and reliability of the review.
Data analysis
A NMA was conducted for each collected outcome within a Bayesian framework. This analysis was predicated on the assumption that the between-study variance (τ2) was homogenous across all pharmacological interventions. The principle of transitivity was assessed by examining potential efficacy modifiers, alongside a thorough review of all outcomes and characteristics of participants (Mills et al., 2013). To ensure the consistency across the entire network, a design-by-treatment interaction model was utilized (Chaimani et al., 2013; Jackson et al., 2016). The efficacy and safety of different pharmacological treatments for VaD were quantified using odds ratios (OR) or the logarithm of OR, along with their respective 95% confidence intervals (CI). Due to the presence of between-study heterogeneity, a random-effects model was deemed the most suitable and prudent approach (Dias et al., 2013). Statistical heterogeneity was evaluated using the I2 statistic, and the τ2 test was employed to ascertain the extent of heterogeneity for each outcome. Publication bias and the impact of small sample sizes were assessed using funnel plots (Higgins and Thompson, 2002). In the Bayesian hierarchical model frameworks, the Markov chain Monte Carlo (MCMC) estimation method was utilized, employing four chains to compute the median treatment effects and 95% CIs. The number of tuning iterations was set at 50,000, with the simulation iterations totaling 100,000 (Gelman and Rubin, 1996). Network diagrams were generated to depict the network geometry and node connectivity visually. Each intervention’s efficacy was ranked using the Surface Under the Cumulative Ranking curve (SUCRA), where higher SUCRA values indicate a greater likelihood of superior therapeutic outcomes. Model convergence was evaluated by visually inspecting the iteration plots and applying the Gelman-Rubin method to assess the potential scale reduction factor. The model fit was appraised by calculating the deviance information criterion (DIC), which combines the posterior mean of the residual deviance with the leverage pD (Salanti et al., 2011). All statistical analyses, including direct and indirect comparisons within the network, were conducted using R software, version 4.3.1 (R Foundation for Statistical Computing, Shanghai, Asia), employing the “gemtc 0.8–2″ and “JAGS” (version 3.5.3) packages (Neupane et al., 2014; Dang et al., 2024).
Results
Study identification
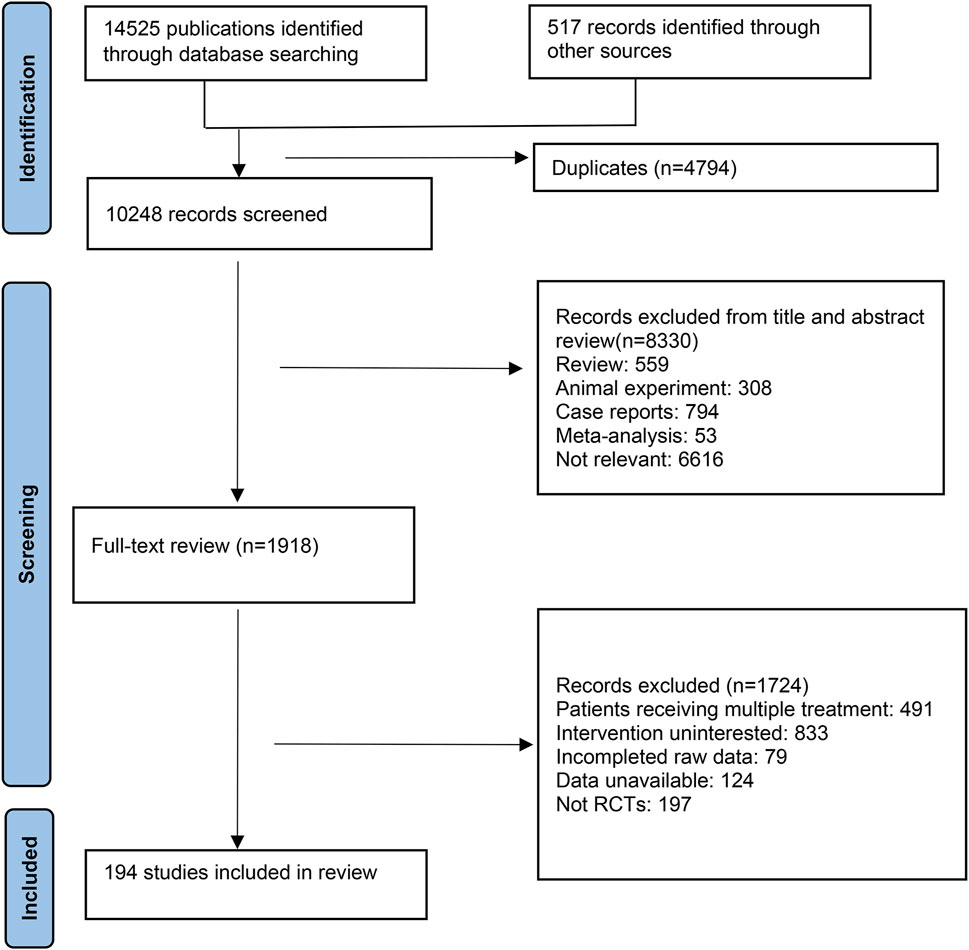
In this study, database searches identified a total of 14,525 publications, supplemented by an additional 517 records from other sources. After eliminating 4,794 duplicate references due to overlapping database coverage, 1,918 studies were selected for further review based on their titles and abstracts. Of these, 1724 studies were excluded for the following reasons: 491 reported multiple treatments, 833 did not investigate the interventions of interest, 79 lacked complete raw data, 124 had unavailable data, and 197 were not RCTs. Ultimately, 194 RCTs met the inclusion criteria and were incorporated into this NMA. The detailed selection process is depicted in Figure 1.
Characteristics of the included studies
In total, 194 RCTs comparing 21 different anti-VaD drugs with placebos or no treatment were analyzed. The sample sizes in the intervention groups ranged from 8 to 648 participants. Specifically, 8 RCTs on Idebenone included 318 patients and 295 controls. Twenty-seven RCTs on Oxiracetam involved 1,223 patients and 1,204 controls. Thirty-seven RCTs on Donepezil encompassed 4,617 patients and 4,398 controls. Three RCTs on Galantamine included 852 patients and 646 controls. Eight RCTs on Nicergoline consisted of 325 patients and 328 controls. Twenty-two RCTs on Nimodipine included 1,157 patients and 1,138 controls. Eight RCTs on Memantine comprised 545 patients and 542 controls. Furthermore, seven RCTs on Rivastigmine included 558 patients and 598 controls. Thirty-one RCTs on Butylphthalide involved 1,396 patients and 1,398 controls. Five RCTs on Cerebrolysin encompassed 212 patients and 246 controls. Sixteen RCTs on Huperzine A comprised 572 patients and 542 controls. Four RCTs on Tongxinluo capsule involved 177 patients and 176 controls. Five RCTs on Edaravone included 192 patients and 190 controls. Fourteen RCTs on Tianzhi granule included 914 patients and 868 controls. Among these studies, 186 were two-arm trials, five were three-arm trials, and three were multi-arm trials. The median follow-up duration ranged from 2 weeks to 22 months. Female participants constituted approximately 48.28% of the total sample. The detailed process of data extraction from the included randomised controlled trials is summarised in Supplementary Table 1 (L. J, 2018; JC, 2014; L. M, 2016; Marigliano et al., 1992; Wang G et al., 2020; Wang GF, 2015; Wu XH et al., 2020; Zhang DP et al., 2013; Zhang FZ et al., 2015; Bottini et al., 1992; Cai QL and Jiang, 2017; Cheng QS and Zhou, 2014; Dysken et al., 1989; GJ, 2011; Haili Binuer, 2003; HF, 2006; Huang, 2012; Li M and Bo, 2010; Liu L and Zhao, 2015a), (Liu L and Zhao, 2015a; Lu Y, 2011; Y. M, 2013; Maina et al., 1989; Ni YY and Tang, 2016; R, 2021; RQ, 2013; S, 2021; SW, 2016; SY, 2019; TW, 2017; Villardita et al., 1992; XB, 2016; Z. Y, 2009; YD, 2013; Yu WY and Zhao, 2008; Yue YM and Zhang, 2013; Zhong H and Li, 2014; Auchus et al., 2007; Erkinjuntti et al., 2002; RP, 2014; Ballard et al., 2008; Mok et al., 2007; Moretti et al., 2002; Moretti et al., 2003; Moretti et al., 2004; Xiao J et al., 2004; Zhong XD, 2021; Guekht et al., 2011; X. H, 2016; Muresanu et al., 2008; Muresanu et al., 2010; Wang J, 2016; Chen H, 2012; FC, 2007; FJ, 2010; HH, 2009; Lu JH et al., 1998; Song YJ and Li, 2006; SY, 2013; Zhang YH and Geng, 2008; Chen L and Yang, 2013; Roman et al., 2010a) (Pantoni et al., 2005; Pantoni et al., 2000; SF, 2012; Sui CF and Wang, 2001; Wang YF, 2009; Wang ZF, 2016; Wang ZG et al., 2009; XH, 2016; Xu XY and Huang, 2017; XY, 2009; XZ, 2018; Zhou Y et al., 2021; W. F, 2017; H, 2015; Liu L and Zhao, 2015b; Wang FL, 2011; Wang RP and Hu, 2004; Wang WL et al., 2010; Weng QL et al., 2011; Xu ZJ et al., 2009; Xu et al., 2012; Xu ZQ et al., 2009; YQ, 2000; Zhagn, 2013; Zhang SG, 2011; Zhao M and Zhang, 2008; Zheng GL and Li, 2006; Zhong, 2004; Du JG et al., 2003; GL, 2016; JT, 2011; Lian CL et al., 2010; Liu XP, 2009; QY, 2009; SH, 2012; Shi et al., 2020; SL, 2011), (Orgogozo et al., 2002; Ouyang XC et al., 2010; Wilcock et al., 2002; Yao MR et al., 2015; Du W, 2012; HQ, 2012; Li HY and Zhu, 2010; Zhang F, 2010; Zhou GA and Ning, 2012; Chang LX and Cai, 2019; Cheng WT, 2016; DY, 2013; FQ, 2015; GF, 2012; H. H, 2017; He, 2016; Hou et al., 2009; Li, 2018; Liu C and Zheng, 2013; Liu J and Liu, 2020; Long CY et al., 2012; Lu HL et al., 2011; Lv Cuilan and Feng, 2015; Ma J, 2018; L. S, 2015), (L. S, 2015; W. S, 2021; Shao XP and Hu, 2013; Sheng GL, 2015; Su XD, 2015; Sun et al., 2016; Wang QY et al., 2014; Wang YJ and Tu, 2013; Wu J et al., 2012; Xiao, 2019; XL, 2015; Xu F et al., 2015; XY, 2019; Y. Y, 2020; YB, 2018; Black et al., 2003; BO Yu-qing, 2012; Chen GQ, 2018; Chen SQ et al., 2007; CY, 2012; P. D, 2014; Dichgans et al., 2008; DR, 2013; Du CB, 2022; Roman et al., 2010b; HM, 2010), (Kavirajan and Schneider, 2007; Kong YN and Chen, 2007; L. L, 2009; Li G et al., 2019; Li N, 2005; Lin JY et al., 2007; W. LL, 2022; Pratt and Perdomo, 2002; Qiu YH et al., 2011; Ren XY et al., 2004; Román et al., 2010; Román et al., 2005; Rong JC, 2011; Schindler, 2005; Tan AX, 2018; Wang Y et al., 2006; Wen K, 2006; Wilkinson et al., 2003; Wilkinson et al., 2010; H. WL, 2010; WX, 2014; XL, 2016; Q. Y, 2017; YF, 2018; YW, 2010)
Risk-of-bias assessment
In this NMA review, all included trials underwent a risk of bias assessment using the Risk of Bias 2.0 Tool. A major factor contributing to high bias risk was the selection of the reported results. Due to inadequate details regarding allocation concealment, there were general or substantial concerns about the randomization process. Out of all the trials, 124 (63.9%) were evaluated as low risk, 50 (25.8%) were deemed moderate risk, and 20 (10.3%) were categorized as high risk. Collectively, the studies presented a low to moderate risk of bias (Figure 2).
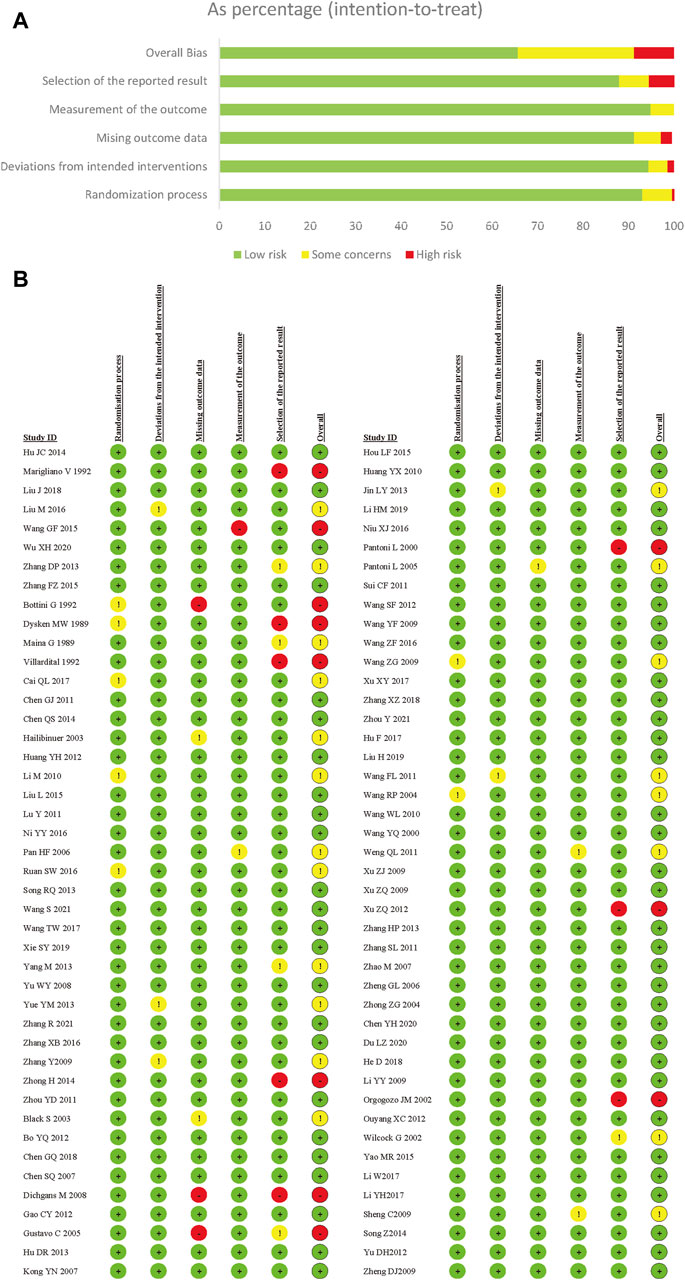
Figure 2. Risk of bias summery for vascular dementia. (A) Risk of bias graph for vascular dementia; (B) Risk of bias summary for vascular dementia.
Heterogeneity and consistency test
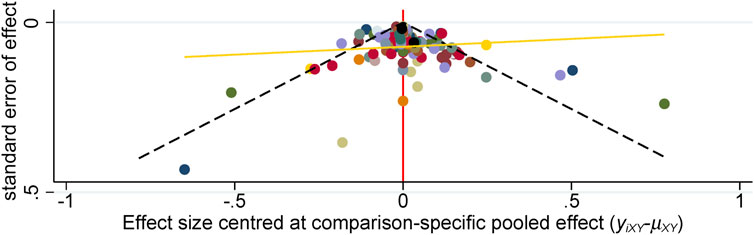
We analyzed the model fit between the fixed-effects and random-effects models for various outcomes, as shown in Figure 3, and evaluated model consistency by analyzing the posterior distribution of deviance differences, with results presented in Figure 4. Our findings indicate a superior fit of the random-effects model across all outcomes, affirming the consistency and validity of indirect comparisons. Funnel plots, depicted in Figure 5, suggest minimal publication bias due to their symmetrical distribution.
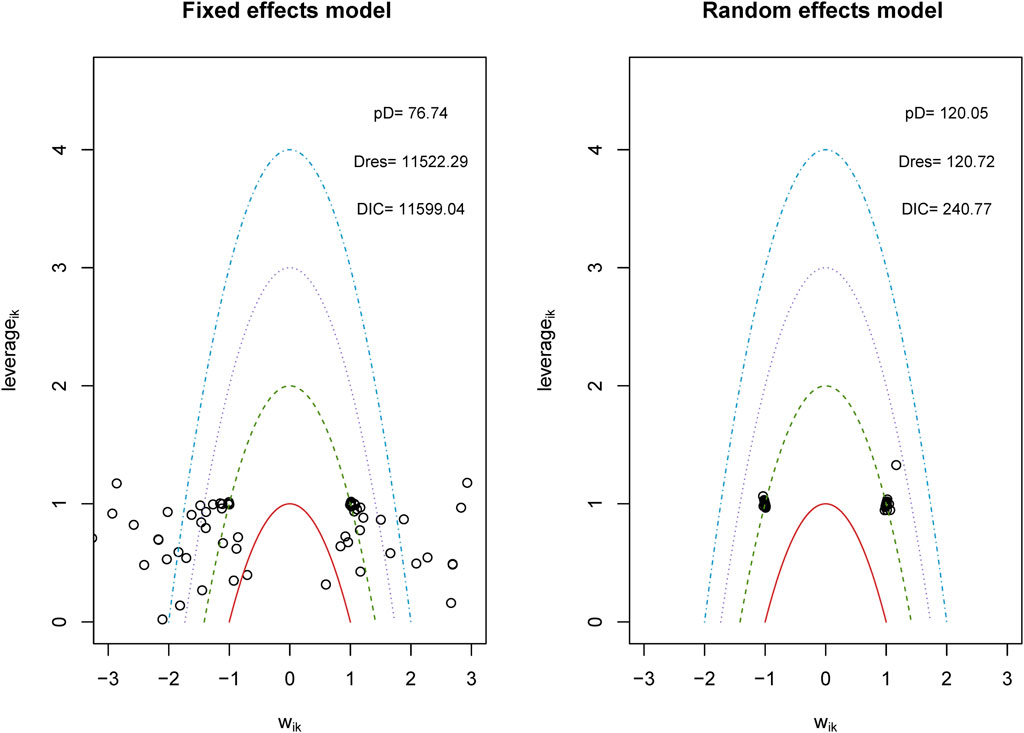
Figure 3. Conformance test for vascular dementia. Conformance test compares the posterior mean deviation of each data group between consistency and the ume m(b) Bias risk evaluation results displayed by including studies odel to judge the consistency among the included research.
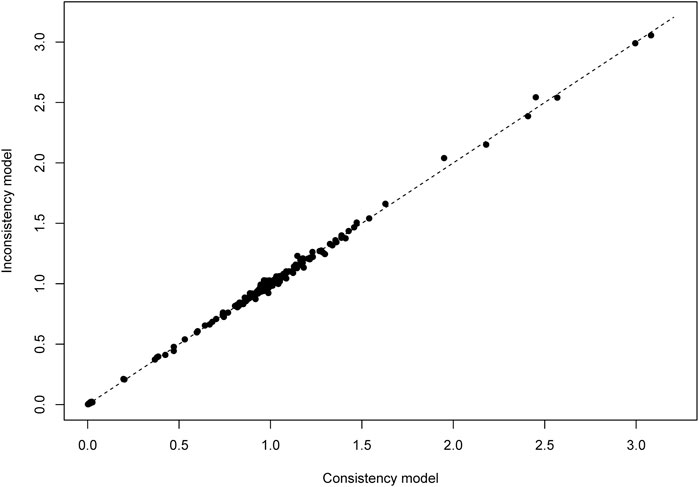
Figure 4. Lever diagram for vascular dementia. The lever diagram shows the comparison between leverageik and Bayesian deviation residuals of all I tests and each of the K arms.
Trajectory plots in Supplementary Figure 1A illustrate the parameter estimates’ evolution and stabilization, key indicators of model convergence, demonstrating stable fluctuations and significant overlap in the MCMC chain. Density plots, shown in Supplementary Figure 1B, display parameter distributions and suggest excellent model convergence through symmetrical and well-calibrated curves. The Brooks-Gelman-Rubin diagnostic diagram in Supplementary Figure 2 assesses the convergence of multiple chains, with values nearing one indicating robust convergence.
Overall, the consistent convergence patterns in the trajectory and density plots, alongside the Brooks-Gelman-Rubin diagnostic, confirm the robustness and reliability of the model’s predictions.
Network diagram
The process of creating a network diagram in R includes installing the required packages, preparing the data, constructing the network object, plotting the diagram, and refining its aesthetics. This function generates network diagrams for outcome indicators as required. These diagrams feature drug nodes, with node sizes corresponding to the number of participants randomly assigned (i.e., sample size), and comparison edges, where the thickness of the lines indicates the number of trials comparing each pair of treatments. A closed loop among nodes signifies that these studies can be compared simultaneously. The results showed that the number of studies comparing no treatment with Donepezil was the largest, followed by comparing no treatment with Nimodipine (Figure 6).
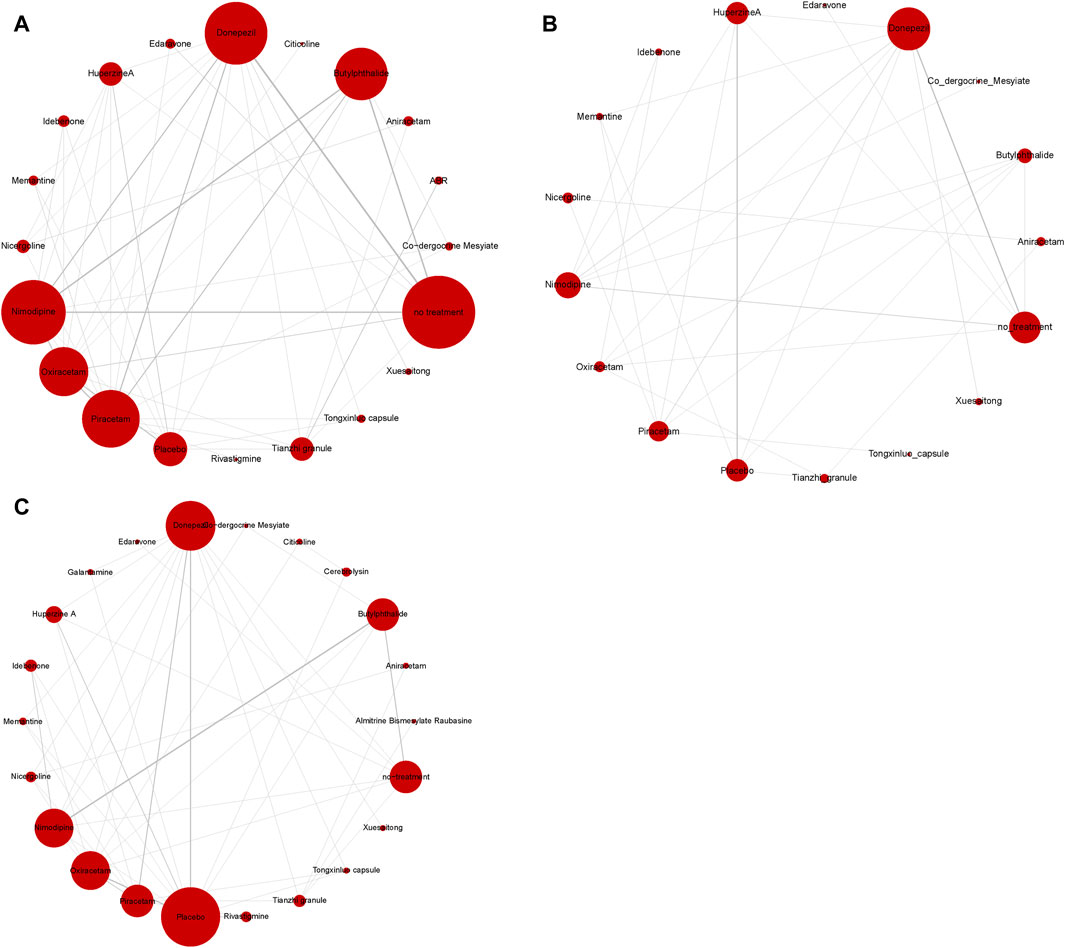
Figure 6. Network diagram of available comparisons. (A) MMSE scores; (B) ADL scores; (C) adverse effects rate.
Forest plot
In terms of MMSE scores, Huperzine A demonstrates superior efficacy compared to Donepezil, while Nimodipine and Xuesaitong exhibits inferior efficacy. Both Edaravone and Butylphthalide show greater efficacy relative to no treatment. Rivastigmine is more effective than Piracetam (Figure 7A).
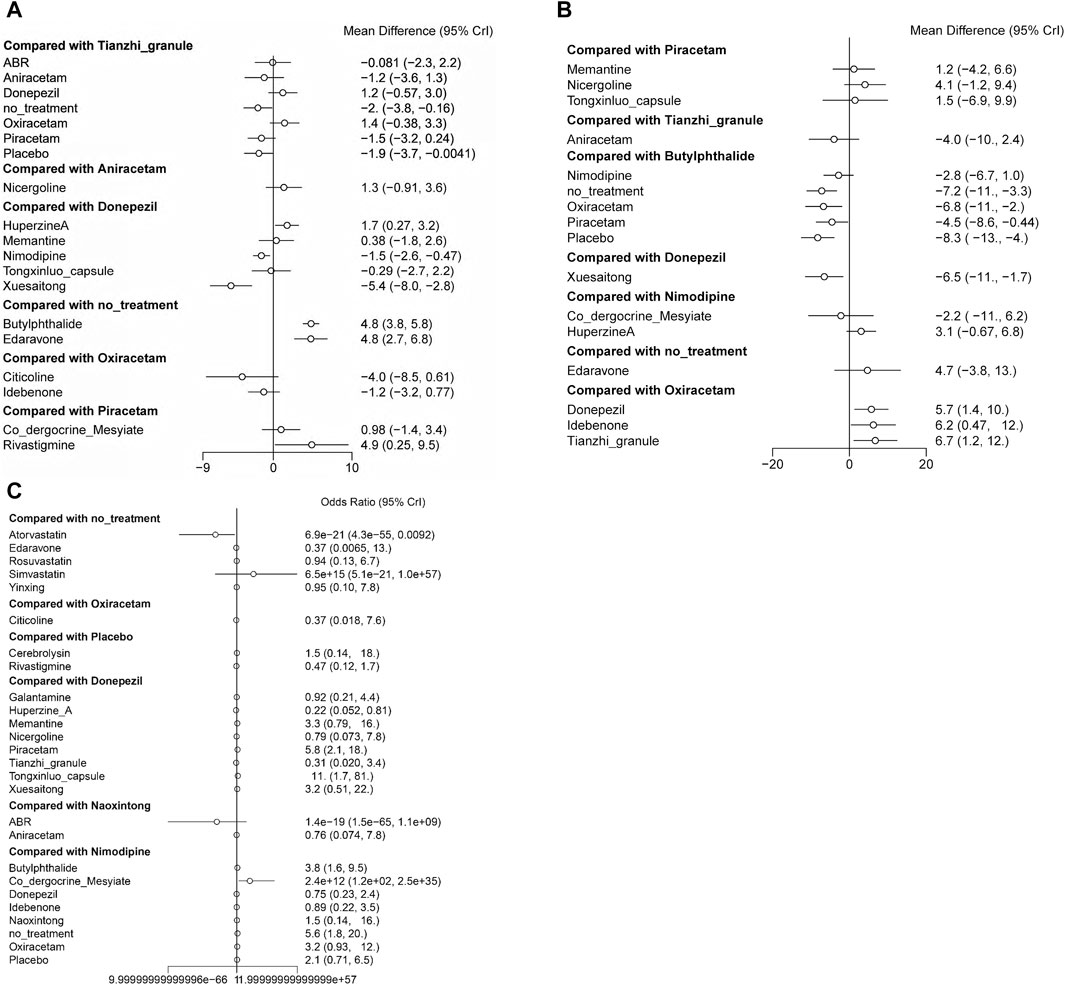
Figure 7. Forest plots representing the efficacy and safety of patients with vascular dementia. (A) direct comparison for MMSE scores; (B) direct comparison for ADL scores; (C) direct comparison for adverse effects rate.
Regarding ADL scores, Oxiracetam and Piracetam are less effective compared to Butylphthalide, and Xuesaitong is less effective than Donepezil. Conversely, Donepezil, Idebenone, and Tianzhi granule display higher efficacy compared to Oxiracetam (Figure 7B).
In terms of adverse reaction incidence, Co-dergocrine mesylate is safer than Nimodipine, whereas Atorvastatin presents a higher risk compared to no treatment. The remaining data did not show statistical significance (Figure 7C).
Heat map
In NMA, a heat map is an informative graphical representation that illustrates the distribution and strength of evidence across various treatment comparisons. Each row and column of the heat map corresponds to different treatments, with cells indicating the presence of direct comparative data between treatments.
The comparative results of the various drugs are illustrated in Figure 8. Butylphthalide exhibited superior efficacy in terms of changes in MMSE scores, surpassing Oxiracetam, Donepezil, Idebenone, Nicergoline, Nimodipine, Co-dergocrine Mesylate, Aniracetam, Piracetam, Citicoline, and Xuesaitong (Figure 8A). Huperzine A demonstrated superior effectiveness in improving ADL scores compared to other treatments, including Piracetam, Oxiracetam, Xuesaitong, and Placebo (Figure 8B). Co-dergocrine Mesylate showed a better safety profile in terms of the incidence of adverse reactions, outperforming Tongxinluo capsule, Butylphthalide, Piracetam, Oxiracetam, Cerebrolysin, Memantine, Xuesaitong, Edaravone, Aniracetam, Citicoline, Rivastigmine, Nimodipine, Idebenone, Galantamine, Nicergoline, Donepezil, Tianzhi granule, and Huperzine A (Figure 8C).
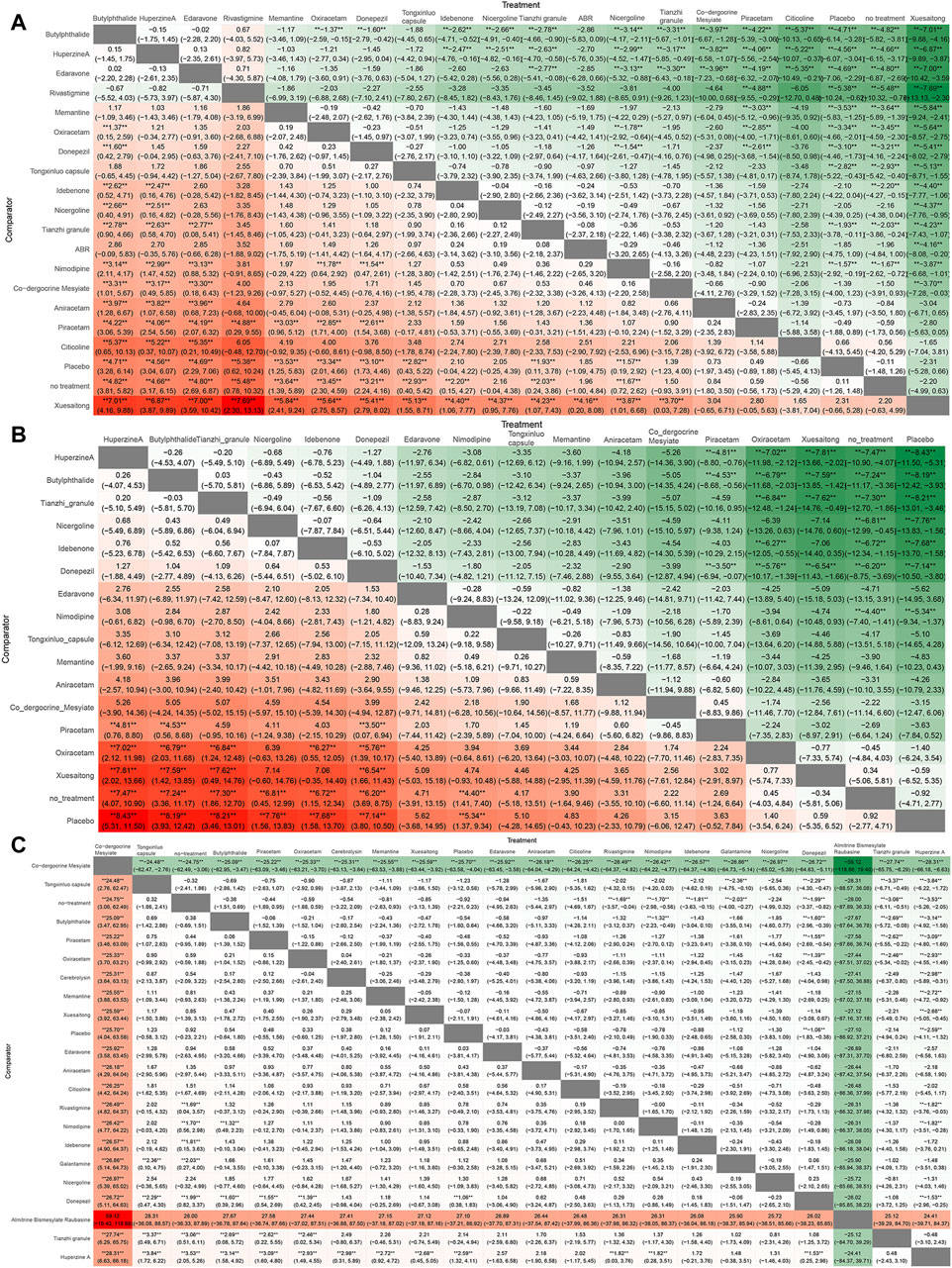
Figure 8. Ranking chart heat map. The heat map of each outcome index ranking table presented comparisons of the relative effects between any pair of interventions, including the OR and 95%CI of each outcome index in all intervention groups. (A) MMSE scores; (B) ADL scores; (C) adverse effects rate.
SUCRA rankings, cumulative probability ranking chart and ranking probability histogram
SUCRA is a statistical method used in NMA to quantify the probability of each treatment being the most effective among all compared treatments. It provides a numerical value between 0 and one for each treatment, where a higher SUCRA value indicates a higher likelihood of that treatment being the best (Table 1). The SUCRA and rankogram charts intuitively display the sorting probability of each intervention group in the form of curves and histogram.
In terms of MMSE scores, the five most effective drugs are Butylphthalide, Huperzine A, Edaravone, Rivastigmine, and Memantine. For ADL scores, the top five drugs in efficacy are Huperzine A, Butylphthalide, Tianzhi granule, Nicergoline, and Idebenone. With respect to the incidence of adverse drug reactions, Co-dergocrine Mesylate, Tongxinluo capsule, Butylphthalide, Piracetam, and Oxiracetam demonstrate good safety profiles (Figure 9).
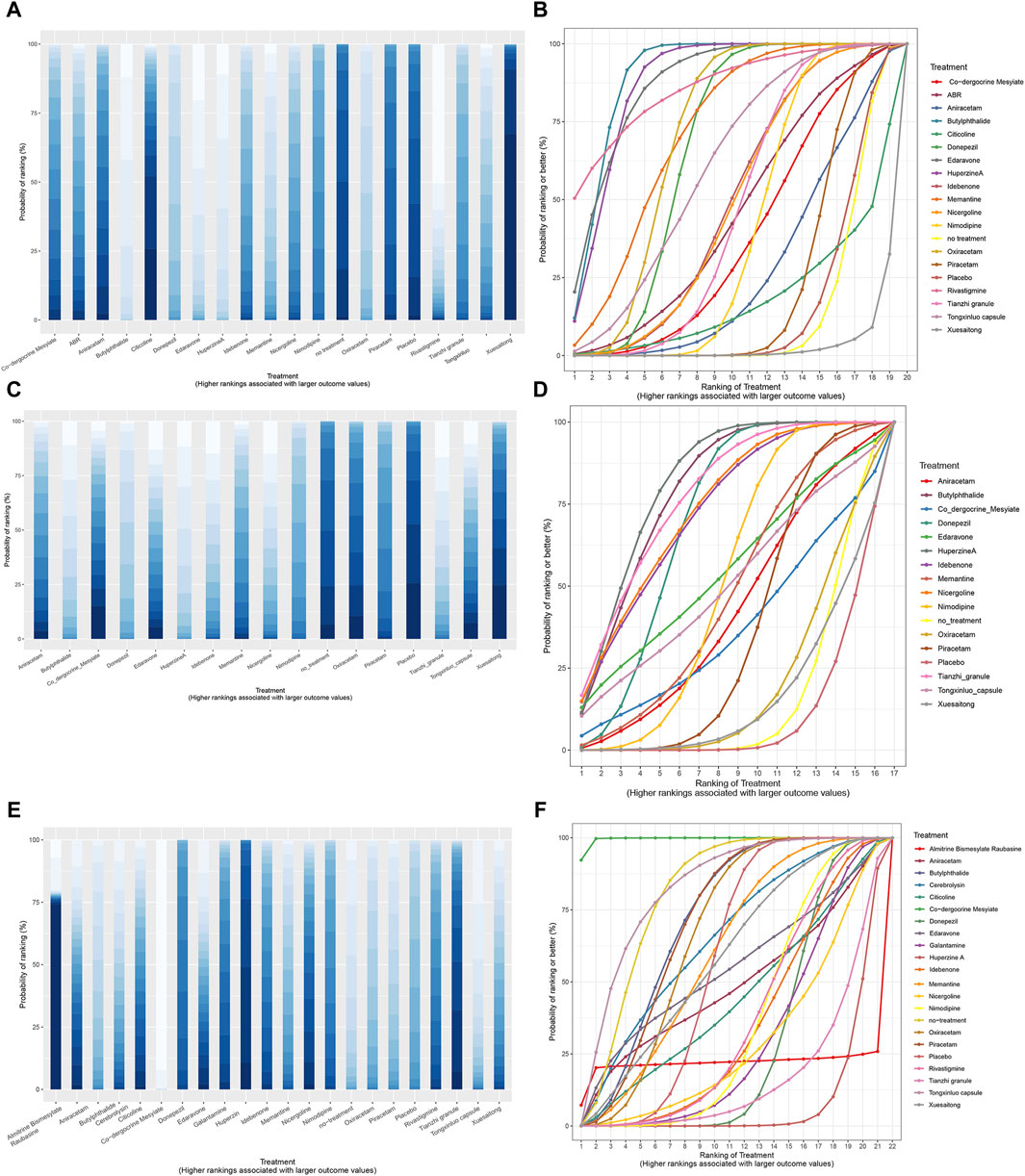
Figure 9. SSUCRA cumulative probability ranking curves and histogram. (A) histogram of MMSE scores; (B) SUCRA curve chart of MMSE scores; (C) histogram of ADL scores; (D) SUCRA curve chart of ADL scores; (E) histogram of adverse effects rate; (F) SUCRA curve chart of adverse effects rate.
Discussion
This study is the first Bayesian NMA to evaluate the efficacy and safety of various treatments for VaD. We performed an exhaustive search and assessed crucial outcomes including the MMSE score, ADL score, and the incidence of adverse events. These outcomes were analyzed through direct and indirect comparisons, enhancing the evidence’s reliability. The included studies were generally found to have low-to-moderate risk of bias, as assessed by the Cochrane Risk of Bias 2.0 Tool, affirming the robustness of our findings.
Key findings
This study provides a comprehensive analysis of various pharmacological treatments for VaD using the MMSE scale to reflect cognitive state, the ADL scale to measure daily living activities, and the adverse reaction rate as a safety indicator. The NMA reveals several key findings regarding the efficacy and safety profiles of these drugs.
Huperzine A demonstrates good efficacy in both MMSE and ADL scores but has the poorest safety profile. Butylphthalide shows robust efficacy in both scales and a low adverse reaction rate, indicating it is both effective and safe. Edaravone ranks high in MMSE scores, mid-range in ADL scores, and has a mid-range safety profile. Memantine and Oxiracetam perform well in MMSE scores but rank lower in ADL scores, though both have high safety rankings. Donepezil is effective in improving MMSE and ADL scores but is associated with poor safety. On the other hand, Tongxinluo capsule and Co-dergocrine Mesylate are noted for their excellent safety profiles but rank poorly in terms of efficacy. Rivastigmine shows high efficacy in MMSE scores but also has poor safety. These findings underscore the importance of considering both efficacy and safety when selecting pharmacological treatments for VaD. While some drugs, such as Butylphthalide, offer a balanced profile of high efficacy and low risk of adverse reactions, others like Huperzine A and Donepezil, despite their effectiveness, pose significant safety concerns. Thus, a nuanced approach that balances these factors is essential for optimizing treatment outcomes in VaD patients.
Drug mechanism
Current therapeutic approaches for VaD primarily focus on modulation of the cholinergic system, neuroprotection, and the use of anti-inflammatory and antioxidant agents (Colucci et al., 2012; McShane et al., 2019).
Cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine enhance cognitive function by increasing intrasynaptic acetylcholine levels, a neurotransmitter integral to memory and learning processes (Román and Kalaria, 2006; Chen et al., 2016). Each of these inhibitors has distinct structural, pharmacodynamic, and pharmacokinetic properties. For instance, donepezil is characterized by a long elimination half-life, galantamine modulates nicotinic receptor function, and rivastigmine also inhibits butyrylcholinesterase, which contributes to its efficacy in treating VaD (Cummings, 2000; Kavirajan and Schneider, 2007). Nicergoline, a semisynthetic ergot derivative, has been used extensively for over 4 decades to treat cognitive and behavioural disorders in the elderly (Fioravanti et al., 2014). It enhances acetylcholine availability by increasing its release from cholinergic terminals and selectively inhibiting acetylcholinesterase. Furthermore, nicergoline stimulates the phosphoinositide pathway, affecting neurotransmitter turnover including norepinephrine and dopamine in specific brain regions (Carfagna et al., 1995; Winblad et al., 2008). Additionally, huperzine A, an alkaloid derived from the Chinese herb Huperzia serrata, acts as a potent, highly specific, and reversible inhibitor of acetylcholinesterase in the central nervous system. Since 1994, it has been used in China to treat AD and benign memory deficits and is available as a nutraceutical in the US (Little et al., 2008). Oxiracetam, another nootropic, is employed with the aim of improving executive function and memory (Malík and Tlustoš, 2022). It readily crosses the blood-brain barrier, affecting areas such as the cortex and hippocampus. It enhances acetylcholine utilization and increases the density of acetylcholine receptors by activating cholinergic nerve fibers, and it has been shown to normalize brain energy balance and alleviate blood-brain barrier dysfunction during brain ischemia in experimental models (Hokonohara et al., 1992; Fordyce et al., 1995; Huang et al., 2017; Youn et al., 2023). Memantine, a well-known uncompetitive and voltage-dependent NMDA receptor antagonist, mitigates the excitotoxicity caused by excessive ischemia-induced NMDA receptor stimulation, offering protection against cognitive decline in VaD (Koch et al., 2005; Burns et al., 2006; Johnson and Kotermanski, 2006). These multifaceted effects underscore the complexity and potential of current pharmacological interventions for VaD.
Accumulating evidence increasingly highlights the critical roles of oxidative stress and inflammation as key factors contributing to the cognitive deficits observed in patients with VaD. Current research indicates that the induction of oxidative stress and inflammatory cascades can lead to a decline in NMDA receptor functionality. This decline may result in subsequent alterations in synaptic plasticity, ultimately leading to cognitive impairment (Wang et al., 2020; Guo et al., 2024). Butylphthalide, derived from Apium graveolens Linn and also synthesized artificially from racemic acid, is a green botanical medicine approved by China Food and Drug Administration (CFDA) for the treatment of ischemic stroke due to its neuroprotective properties. Recent RCTs have demonstrated that butylphthalide can enhance behavioral abilities and alleviate symptoms of VaD. Its therapeutic effects are potentially mediated by the upregulation of phosphorylated Akt in the hippocampus, improves mitochondrial function, contributing to its cognitive benefits (Huai et al., 2013; Liu et al., 2024; Zhang et al., 2024). Additionally, idebenone, recognized for its potent antioxidant properties and as a CoQ10 analogue, was initially developed to combat dementia. It plays a crucial role in the clinical treatment of cerebrovascular diseases, helping to eliminate oxygen free radicals, counteract oxidation, enhance mitochondrial respiratory activity, and improve overall brain function and metabolic status (Marigliano et al., 1992). Edaravone, chemically known as 3-methyl-1-phenyl-2-pyrazoline-5-one, is a nootropic and neuroprotective agent that aids neurological recovery following acute brain ischemia and subsequent cerebral infarction. First approved for clinical use in Japan in 2001, Edaravone potentially upregulates the activity of Akt, alleviating oxidative stress, restores synaptic proteins, and improves memory deficits, thus playing a crucial role in the treatment of neurodegenerative conditions (Yoshida et al., 2006; Li et al., 2017). Additionally, naoxintong capsule, a composite formulation of 16 natural ingredients, including 13 plant-based and three animal-based components has been extensively utilized in China for treating VaD. Additionally, tongxinluo capsule, containing components like dehydroevodiamine and evodiamine, potentially blocks glutamatergic signaling and reduces inflammation, contributing to its therapeutic effects (Chan et al., 2018). Tianzhi granule, a traditional herbal medication, has been approved by CFDA for treating VaD. Comprising a blend of various herbs including Gastrodia elata, Uncaria rhynchophylla, Abalone shell, Eucommia ulmoides, Mulberry mistletoe, Poria cocos, Polygonum multiflorum, Sophora japonica, Gardenia jasminoides, Scutellaria baicalensis, Achyranthes bidentata, and Leonurus japonicus, tianzhi granule inhibits the proliferation of astroglial cells while promoting the proliferation of precursor nerve cells, thus enhancing learning and memory capabilities in VaD rat models (Zhao et al., 2022). Morover, cerebrolysin, a peptidergic compound that mimics the action of endogenous neurotrophic factors, addresses neuroinflammation and oxidative stress. Due to its composition, cerebrolysin requires parenteral administration to achieve full bioavailability and is typically administered over a short period due to its mode of action. It penetrates the blood-brain barrier to regulate the synthesis of proteins and metabolism of nucleic acids in brain cells, enhancing the utilization of oxygen and glucose, and offering resistance to neurotoxic substances. However, evidence on the cognitive efficacy of cerebrolysin remains limited (Bae et al., 2000; Ruether et al., 2001; Ruether et al., 2002; Rockenstein et al., 2005; Allegri and Guekht, 2012). These findings emphasize the need to address these underlying biological processes to mitigate their impact on cognitive function in VaD.
Vascular risk factors such as hypertension, diabetes, smoking, elevated cholesterol, and atherosclerosis are critical in the development of cerebrovascular disease, leading to vascular brain injury and subsequent vascular cognitive impairment and VaD. The primary strategy for managing VaD involves targeting these modifiable risk factors, either to prevent or delay disease progression or to alleviate associated behavioral symptoms, including secondary stroke prevention (Takeda et al., 2020). Early detection and mitigation of these vascular risk factors are essential for the effective prevention and treatment of vascular cognitive impairment and dementia. Nimodipine, a 1,4-dihydropyridine-derivative calcium channel blocker, exhibits significant antihypertensive properties and a unique cerebrovascular profile. Due to its high lipophilicity, nimodipine can cross the blood-brain barrier, effectively reaching the brain and cerebrospinal fluid. Clinical studies generally show favorable effects of nimodipine in hypertensive patients, including reductions in systolic and diastolic blood pressure, improvements in subjective symptoms, and neurological enhancements that contribute to improved cognitive function (Tomassoni et al., 2008; Amenta et al., 2009). However, the specific mechanisms underlying the neuroprotective effects of nimodipine remain partly unclear (Baskys and Hou, 2007).
Strengths
In contrast to previous reviews and meta-analysis, this NMA firstly performs a graded quantitative analysis of the efficacy and safety of all commonly used drugs in patients with VaD. The use of Bayesian NMA allowed for a robust comparison of multiple treatments, integrating direct and indirect evidence to provide a comprehensive ranking of their effectiveness and safety. This methodology enhances the reliability of the findings and supports evidence-based clinical decision-making. By integrating direct and indirect evidence in our study, we provide clinicians with the most current evidence to inform them when new therapies might be applicable. To enhance the rigor and evidence-based strength of our findings, this study exclusively included interventional clinical trials, adhering to stringent inclusion/exclusion criteria to ensure that the trials incorporated are not only the most recent and comprehensive but also of the highest quality. Unlike previous meta-analysis, which primarily focused on cholinesterase inhibitors, this innovative network analysis encompasses nearly all current medications for VaD, including Western medicines and traditional Chinese medicines. Importantly, the potential benefits of traditional Chinese herbal medicine in treating VaD have been widely explored, positioning these as alternative therapeutic options. Consequently, this study is more comprehensive and covers a broader spectrum of medications than previous studies. The study underscores the potential of several pharmacological agents, including traditional Chinese medicines, in treating VaD. However, the varying levels of efficacy and safety across different treatments highlight the need for personalized treatment approaches and further research to establish standardized therapeutic guidelines.
Limitation
Our study has several limitations that warrant consideration. Firstly, variability in drug dosages and treatment durations across the included RCTs may have influenced outcomes. Secondly, the specific characteristics of patient populations, such as the severity of VaD, age, and gender, could affect the effectiveness and safety of the treatments evaluated. Thirdly, the inclusion of numerous studies with small sample sizes restricts the certainty of the evidence for clinical application. Fourthly, while we used the MMSE and ADL scores as primary efficacy outcomes, other VaD scales like the Blessed-dementia rating scale, Hasegawa dementia scale, and AD Assessment Scale-cognitive subscale were excluded due to insufficient data from clinical trials. This exclusion might limit broader conclusions about the efficacy of treatments, particularly Chinese herbal medicines. Finally, the follow-up duration in the included trials was approximately 22 months, which may be too brief to fully assess the long-term effectiveness of the treatments given the typically gradual progression of the disease.
Conclusion
Overall, this study enhances understanding of the relative benefits and risks associated with various VaD treatments, providing a valuable reference for clinical decision-making. Future research should continue to explore these aspects to further refine treatment strategies for VaD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CD: Data curation, Writing–original draft, Writing–review and editing. QW: Investigation, Writing–original draft, Writing–review and editing, YZ: Software, Writing–original draft. QL: Investigation, Writing–original draft. LF: Conceptualization, Writing–review and editing, YX: Methodology, Writing–original draft. YL: Methodology, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (82001240), Natural Science Foundation of Heilongjiang Province (YQ2021H011), China Postdoctoral Science Foundation (2020M670925 and 2022T150172), and Postdoctoral Foundation of Heilongjiang Province (LBH-Z19027 and LBH-TZ2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1451032/full#supplementary-material
References
Allegri, R. F., and Guekht, A. (2012). Cerebrolysin improves symptoms and delays progression in patients with Alzheimer's disease and vascular dementia. Drugs Today (Barc) 48 (Suppl. A), 25–41. doi:10.1358/dot.2012.48(Suppl.A).1739721
Amenta, F., Lanari, A., Mignini, F., Silvestrelli, G., Traini, E., and Tomassoni, D. (2009). Nicardipine use in cerebrovascular disease: a review of controlled clinical studies. J. Neurol. Sci. 283 (1-2), 219–223. doi:10.1016/j.jns.2009.02.335
Auchus, A. P., Brashear, H. R., Salloway, S., Korczyn, A. D., De Deyn, P. P., Gassmann-Mayer, C., et al. (2007). Galantamine treatment of vascular dementia: a randomized trial. Neurology 69 (5), 448–458. doi:10.1212/01.wnl.0000266625.31615.f6
Bae, C. Y., Cho, C. Y., Cho, K., Hoon Oh, B., Choi, K. G., Lee, H. S., et al. (2000). A double-blind, placebo-controlled, multicenter study of Cerebrolysin for Alzheimer's disease. J. Am. Geriatr. Soc. 48 (12), 1566–1571. doi:10.1111/j.1532-5415.2000.tb03865.x
Ballard, C., Sauter, M., Scheltens, P., He, Y., Barkhof, F., van Straaten, E. C., et al. (2008). Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr. Med. Res. Opin. 24 (9), 2561–2574. doi:10.1185/03007990802328142
Baskys, A., and Hou, A. C. (2007). Vascular dementia: pharmacological treatment approaches and perspectives. Clin. Interv. Aging 2 (3), 327–335.
Bir, S. C., Khan, M. W., Javalkar, V., Toledo, E. G., and Kelley, R. E. (2019). Emerging concepts in vascular dementia: a review. J. Stroke Cerebrovasc. Dis. 30 (8), 105864. doi:10.1016/j.jstrokecerebrovasdis.2021.105864
Black, S., Román, G. C., Geldmacher, D. S., Salloway, S., Hecker, J., Burns, A., et al. (2003). Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 34 (10), 2323–2330. doi:10.1161/01.Str.0000091396.95360.E1
B. M (2008). Observations on the efficacy of nimodipine tablets in the treatment of vascular dementia. Chin. J. Misdiagnostics 8 (31), 7635–7636. doi:10.3969/j.issn.1009-6647.2008.31.067
Bottini, G., Vallar, G., Cappa, S., Monza, G. C., Scarpini, E., Baron, P., et al. (1992). Oxiracetam in dementia: a double-blind, placebo-controlled study. Acta Neurol. Scand. 86 (3), 237–241. doi:10.1111/j.1600-0404.1992.tb05077.x
Bo Yu-qing, L. X. (2012). Clinical effect of donepezil in treatment of mild and moderate vascular dementia and its affection on perceptibility and daily living activity. Mod. Prev. Med. 39 (14), 3762–3763. CNKI:SUN:XDYF.0.2012-14-120.
Burns, A., O'Brien, J., Auriacombe, S., Ballard, C., Broich, K., Bullock, R., et al. (2006). Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J. Psychopharmacol. 20 (6), 732–755. doi:10.1177/0269881106068299
Cai Ql, Y. Y., and Jiang, W. (2017). Clinical efficacy of oxiracetam versus placebo in the treatment of vascular dementia. China Health Care and Nutr. 27 (9), 271–272. doi:10.3969/j.issn.1004-7484.2017.09.446
Carfagna, N., Di Clemente, A., Cavanus, S., Damiani, D., Gerna, M., Salmoiraghi, P., et al. (1995). Modulation of hippocampal ACh release by chronic nicergoline treatment in freely moving young and aged rats. Neurosci. Lett. 197 (3), 195–198. doi:10.1016/0304-3940(95)11928-p
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Chan, E. S., Bautista, D. T., Zhu, Y., You, Y., Long, J. T., Li, W., et al. (2018). Traditional Chinese herbal medicine for vascular dementia. Cochrane Database Syst. Rev. 12 (12), Cd010284. doi:10.1002/14651858.CD010284.pub2
Chang, E., Wong, H., and Chui, C. (2022). Vascular cognitive impairment and dementia. Contin. Minneap Minn 28 (3), 750–780. doi:10.1212/con.0000000000001124
Chang Lx, J. H., and Cai, J. F. (2019). A study on the clinical effects of butylphthalide in the treatment of mild to moderate vascular dementia. Med. J. Commun. 33 (1), 35–37. doi:10.19767/j.cnki.32-1412.2019.01.012
Chen, Y. D., Zhang, J., Wang, Y., Yuan, J. L., and Hu, W. L. (2016). Efficacy of cholinesterase inhibitors in vascular dementia: an updated meta-analysis. Eur. Neurol. 75 (3-4), 132–141. doi:10.1159/000444253
Chen Cl, Q. Z., Su, S. X., Zheng, Z. C., and Ye, Z. P. (2009). The therapeutic effect of Tongxinluo capsule on vascular dementia. Chin. J. Difficult Complicat. Cases 8 (5), 272–274. doi:10.3969/j.issn.1671-6450.2009.05.007
Chen Gq, W. S. (2018). The clinical effect of the treatment of vascular dementia. Jilin Med. J. 39 (5), 827–828. doi:10.3969/j.issn.1004-0412.2018.05.011
Cheng Qs, H. Q., and Zhou, C. H. (2014). Clinical effect of oxiracetam on vascular dementia. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 22 (9), 18–19. doi:10.3969/j.issn.1008-5971.2014.09.007
Cheng Wt, Y. J. (2016). She XP: effect of butylphthalide soft capsules and nimodipine on patients with vascular dementia after stroke. J. Prev. Med. Chin. People's Liberation Army 34 (4), 516–518. CNKI:SUN:JYYX.0.2016-04-022.
Chen H, L. J. (2012). Comparison of the efficacy of Niergoline and huperzine A in the treatment of vascular dementia. Chin. J. Gerontology 33 (21), 4811–4812. doi:10.3969/j.issn.1005-9202.2012.21.110
Chen L, K. H., and Yang, J. J. (2013). Clinical analysis of the efficacy of early intervention with nimodipine in the prevention of vascular dementia after acute cerebral infarction. J. Front. Med. 1 (12), 29–30.
Chen Sq, Y. M., Han, Y., and Wu, W. W. (2007). Clinical study of donepezil hydrochloride in the treatment of vascular dementia in elderly people. Chin. J. Geriatric Care 5 (4), 84–85. doi:10.3969/j.issn.1672-4860-B.2007.04.042
Chen Yh, L. J., Huang, W., and He, K. H. (2020). Effects of Memantine hydrochloride tablets on oxidative stress level and cerebral hemodynamics in patients with vascular dementia. Chin. J. Gerontology 40 (3), 480–482. doi:10.3969/j.issn.1005-9202.2020.03.010
Ciesielska, N., Sokołowski, R., Mazur, E., Podhorecka, M., Polak-Szabela, A., and Kędziora-Kornatowska, K. (2016). Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 50 (5), 1039–1052. doi:10.12740/pp/45368
Colucci, L., Bosco, M., Rosario Ziello, A., Rea, R., Amenta, F., and Fasanaro, A. M. (2012). Effectiveness of nootropic drugs with cholinergic activity in treatment of cognitive deficit: a review. J. Exp. Pharmacol. 4, 163–172. doi:10.2147/jep.S35326
Cummings, J. L. (2000). Cholinesterase inhibitors: a new class of psychotropic compounds. Am. J. Psychiatry 157 (1), 4–15. doi:10.1176/ajp.157.1.4
Cy, G. (2012). Clinical efficacy and effect on the level of insulin-like growth factors-1 (IGF-1) of donepezil hydrochloride in the treatment of vascular dementia. J. Apoplexy Nerv. Dis. 29 (3), 259–261. CNKI:SUN:ZFSJ.0.2012-03-019.
Dang, C., Wang, Y., Li, Q., and Lu, Y. (2023). Neuroimaging modalities in the detection of Alzheimer's disease-associated biomarkers. Psychoradiology. 22 (3), 009. doi:10.1093/psyrad/kkad009
Dang, C., Wang, Q., Li, Q., Xiong, Y., and Lu, Y. (2024). Chinese herbal medicines for the treatment of depression: a systematic review and network meta-analysis. Front. Pharmacol. 15, 1295564. doi:10.3389/fphar.2024.1295564
Dias, S., Welton, N. J., Sutton, A. J., Caldwell, D. M., Lu, G., and Ades, A. E. (2013). Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 33 (5), 641–656. doi:10.1177/0272989x12455847
Dichgans, M., Markus, H. S., Salloway, S., Verkkoniemi, A., Moline, M., Wang, Q., et al. (2008). Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 7 (4), 310–318. doi:10.1016/s1474-4422(08)70046-2
Dr, H. (2013). Comparison of clinical efficacy of donepezil hydrochloride and nimodipine on cognitive impairment of vascular dementia. Chin. J. Pract. Nerv. Dis. 16 (20), 35–36. doi:10.3969/j.issn.1673-5110.2013.20.019
Du Cb, D. X. (2022). Clinical efficacy analysis of donepezil hydrochloride in the treatment of patients with vascular dementia. Clin. Med. 18 (9), 3. CNKI:SUN:YYXK.0.2020-09-087.
Du Jg, Z. X., Zhao, J. J., Wang, J., Tian, J. Z., Liu, X. F., Zhi, H. P., et al. (2003). Clinical observation on the treatment of vascular dementia in the elderly with Tianzhi granules. China J. Chin. Materia Medica 28 (1), 73. doi:10.3321/j.issn:1001-5302.2003.01.026
Du Lz, Z. F., and Liu, J. J. (2020). A comparative study of memantine and donepezil in treatment of elderly patients with vascular dementia. Drug Eval. Res. 43 (10), 2031–2034. doi:10.7501/j.issn.1674-6376.2020.10.017
Du W, C. J. (2012). Efficacy of Edaravone in the treatment of vascular dementia. China Mod. Med. 19 (8), 60–62. doi:10.3969/j.issn.1674-4721.2012.08.029
Dysken, M. W., Katz, R., Stallone, F., and Kuskowski, M. (1989). Oxiracetam in the treatment of multi-infarct dementia and primary degenerative dementia. J. Neuropsychiatry Clin. Neurosci. 1 (3), 249–252. doi:10.1176/jnp.1.3.249
Dy, X. (2013). Comparison of butylphthalide and nimodipine in the treatment of post-stroke vascular dementia. J. Clin. Exp. Med. 12 (12), 978–979. doi:10.3969/j.issn.1671-4695.2013.12.035
Erkinjuntti, T., Kurz, A., Gauthier, S., Bullock, R., Lilienfeld, S., and Damaraju, C. V. (2002). Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet 359 (9314), 1283–1290. doi:10.1016/s0140-6736(02)08267-3
Fc, T. (2007). Observation of curative effect of Niergoline on vascular dementia. J. North China Univ. Sci. Technol. Sci. Ed. 9 (6), 806–807. doi:10.3969/j.issn.1008-6633.2007.06.028
Fioravanti, M., Nakashima, T., Xu, J., and Garg, A. (2014). A systematic review and meta-analysis assessing adverse event profile and tolerability of nicergoline. BMJ Open 4 (7), e005090. doi:10.1136/bmjopen-2014-005090
Fj, L. (2010). Clinical comparison of oxiracetam and niergoline in the treatment of vascular dementia. Chin. Prim. Health Care, 92–94. doi:10.3969/j.issn.1001-568X.2010.01.043
Fordyce, D. E., Clark, V. J., Paylor, R., and Wehner, J. M. (1995). Enhancement of hippocampally-mediated learning and protein kinase C activity by oxiracetam in learning-impaired DBA/2 mice. Brain Res. 672 (1-2), 170–176. doi:10.1016/0006-8993(94)01389-y
Fq, L. (2015). Clinical effect of butylphthalide in the treatment of vascular dementia and study on the mechanism of drug action. China Health Care and Nutr. 25 (9), 296.
Gelman, A., and Rubin, D. B. (1996). Markov chain Monte Carlo methods in biostatistics. Stat. Methods Med. Res. 5 (4), 339–355. doi:10.1177/096228029600500402
Gf, C. (2012). Clinical evaluation of the effectiveness and safety of butylphthalide in the treatment of vascular dementia. Med. Inf. 25 (8), 139–140. doi:10.3969/j.issn.1006-1959.2012.03.122
Gj, C. (2011). Clinical efficacy of oxiracetam in the adjuvant treatment of vascular dementia. Med. Inf. 24 (5), 2064–2065. doi:10.3969/j.issn.1006-1959.2011.05.455
Gl, W. (2016). Clinical analysis of Tianzhi granules in the treatment of vascular dementia of liver-yang-hyperactivity type. Chin. J. Pract. Nerv. Dis. 19 (9), 117–118. doi:10.3969/j.issn.1673-5110.2016.09.077
Gm, C. (2012). Observations on the efficacy of nimodipine in the treatment of vascular dementia. Nei Mongol J. Traditional Chin. Med. 31 (24), 90. doi:10.3969/j.issn.1006-0979.2012.24.107
Guekht, A. B., Moessler, H., Novak, P. H., and Gusev, E. I.Cerebrolysin Investigators (2011). Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J. Stroke Cerebrovasc. Dis. 20 (4), 310–318. doi:10.1016/j.jstrokecerebrovasdis.2010.01.012
Guo, H., Li, H., Jia, Z., Ma, S., and Zhang, J. (2024). Edaravone dexborneol attenuates cognitive impairment in a rat model of vascular dementia by inhibiting hippocampal oxidative stress and inflammatory responses and modulating the NMDA receptor signaling pathway. Brain Res. 1833, 148917. doi:10.1016/j.brainres.2024.148917
Guo Ll, Y. M., and Zhang, B. H. (2006). Effect of nimodipine on cognitive function in patients with vascular dementia. Chin. J. Rehabilitation 21 (4), 272. doi:10.3870/j.issn.1001-2001.2006.04.031
Haili Binuer, W. M. (2003). Zhao FG: randomized double-blind controlled study of oxiracetam vs piracetam in treatment of vascular dementia. Chin. J. New Drugs Clin. Remedies 22 (11), 4. doi:10.3969/j.issn.1007-7669.2003.11.002
Hao Wp, Y. F., and Li, L. Z. (2006). Clinical observations of Tongxingluo capsule in the treatment of vascular dementia. J. Clin. Psychosomatic Dis. 12 (6), 424–425. doi:10.3969/j.issn.1672-187X.2006.06.011
He, L. Y. (2016). Effectiveness of treatment with butylphthalide in elderly patients with vascular dementia and the effect of the drug on psychosomatic scales. Strait Pharm. J. 28 (2), 181–183. doi:10.3969/j.issn.1006-3765.2016.02.089
He D, T. J., and Zhang, J. W. (2018). Effects of memantine hydrochloride on cognitive function, cerebral hemodynamics and oxidative stress level in patients with vascular dementia. China Pharm. 29 (4), 534–537. doi:10.6039/j.issn.1001-0408.2018.04.25
Hf, P. (2006). Curative effect of oxiracetam in the treatment of vascular dementia. Mod. J. Integr. Traditional Chin. West. Med. 15 (15), 2053–2054. doi:10.3969/j.issn.1008-8849.2006.15.031
H. H (2017). Analysis of the efficacy and mechanism of action of butylphthalide soft capsule on improving cognitive function in patients with vascular dementia. Chin. J. Pract. Nerv. Dis. 20 (1), 98–99. doi:10.3969/j.issn.1673-5110.2017.01.051
Hh, X. (2009). The comparison study of nicergolent and piracetam in treatment with vascular dementia. Sichuan Med. J. 30 (9), 1395–1396. doi:10.3969/j.issn.1004-0501.2009.09.017
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
H, L. (2015). Clinical effect of Donepezil on vascular dementia. Guide China Med. 14 (36), 118–119. doi:10.1671-8194/(2015)36-0118-01
Hm, Y. (2010). Comparison of clinical efficacy of donepezil hydrochloride vs. Nimodipine in the treatment of vascular dementia. China Pharm. 21 (44), 4181–4183. CNKI:SUN:ZGYA.0.2010-44-028.
Hokonohara, T., Shinoda, Y., and Hori, N. (1992). Effects of oxiracetam on the decrease in population spikes in hypoxic and low glucose media. Nihon Yakurigaku Zasshi 99 (3), 123–133. doi:10.1254/fpj.99.123
Hou, C. K., Wang, Y., Tian, Y., Wan, S., Tang, J. C., and Song, Z. (2009). Efficacy and mechanism of action of butylphthalide in the treatment of vascular dementia. J. South. Med. Univ. 29 (3), 574–575. doi:10.3321/j.issn:1673-4254.2009.03.057
Hq, Z. (2012). The influence of edaravone on cognitive ability in patients with vascular dementia. Chin. J. Prim. Med. Pharm. 19 (18), 2729–2730. doi:10.3760/cma.j.issn.1008-6706.2012.18.005
Huai, Y., Dong, Y., Xu, J., Meng, N., Song, C., Li, W., et al. (2013). L-3-n-butylphthalide protects against vascular dementia via activation of the Akt kinase pathway. Neural Regen. Res. 8 (19), 1733–1742. doi:10.3969/j.issn.1673-5374.2013.19.001
Huang, L., Shang, E., Fan, W., Li, X., Li, B., He, S., et al. (2017). S-oxiracetam protect against ischemic stroke via alleviating blood brain barrier dysfunction in rats. Eur. J. Pharm. Sci. 109, 40–47. doi:10.1016/j.ejps.2017.07.029
Huang, Y. G. (2012). A control study of oxiracetam vs donepezil in the treatment of senile vascular dementia. J. Clin. Psychosomatic Dis. 18 (3), 217–218. doi:10.3969/j.issn.1672-187X.2012.03.011-0217-02
Huang Yx, H. Y. (2010). Nimodipine in vacular dementia cognitive function. Chin. J. Aesthetic Med. 19 (3), 22–23. doi:10.3969/j.issn.1008-6455.2010.z3.020
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/m14-2385
H. WL: Clinical efficacy of donepezil hydrochloride in the treatment of 33 cases of vascular dementia, 4(8), 125–126. (2010) doi:10.3969/j.issn.1673-9523.2010.08.113
Jackson, D., Boddington, P., and White, I. R. (2016). The design-by-treatment interaction model: a unifying framework for modelling loop inconsistency in network meta-analysis. Res. Synth. Methods 7 (3), 329–332. doi:10.1002/jrsm.1188
Jc, H. (2014). Clinical observation of 47 cases of vascular dementia treated with Idebenquinone. Jilin Med. J. 35 (5), 3. doi:10.3969/j.issn.1004-0412.2014.05.024
Jhoo, J. H., Kim, K. W., Huh, Y., Lee, S. B., Park, J. H., Lee, J. J., et al. (2008). Prevalence of dementia and its subtypes in an elderly urban Korean population: results from the Korean Longitudinal Study on Health and Aging (KLoSHA). Dement. Geriatr. Cogn. Disord. 26 (3), 270–276. doi:10.1159/000160960
Jin Ly, W. Z., Gao, F., and Zhang, H. Y. (2013). The efficacy of early intervention with nimodipine in preventing vascular dementia after acute cerebral infarction. Med. Inf. 1 (2), 146–147. doi:10.3969/j.issn.1006-1959.2013.02.143
Johnson, J. W., and Kotermanski, S. E. (2006). Mechanism of action of memantine. Curr. Opin. Pharmacol. 6 (1), 61–67. doi:10.1016/j.coph.2005.09.007
Jt, W. (2011). 27 cases of mild and moderate vascular dementia treated with Tianzhi granules. Chin. J. Exp. Traditional Med. Formulae 17 (17), 272–273. doi:10.3969/j.issn.1005-9903.2011.17.081
Kavirajan, H., and Schneider, L. S. (2007). Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 6 (9), 782–792. doi:10.1016/s1474-4422(07)70195-3
Kim, T. H., and Kang, J. W. (2020). Herbal medicine for vascular dementia: an overview of systematic reviews. Curr. Vasc. Pharmacol. 18 (4), 394–409. doi:10.2174/1570161117666190618164443
Koch, H. J., Uyanik, G., and Fischer-Barnicol, D. (2005). Memantine: a therapeutic approach in treating Alzheimer's and vascular dementia. Curr. Drug Targets CNS Neurol. Disord. 4 (5), 499–506. doi:10.2174/156800705774322021
Kong Yn, X. D., and Chen, P. (2007). Clinical observation of aricept for the treatment of vascular dementia. Chin. J. Geriatric Care 5 (1), 33–35. doi:10.3969/j.issn.1672-4860-B.2007.01.015
Lf, H. (2015). The application of oxiracetam and nimodipine in the treatment of vascular dementia. China Health Stand. Manag. 6 (21), 63–64. doi:10.3969/j.issn.1674-9316.2015.21.047
Li, X., Lu, F., Li, W., Qin, L., Yao, Y., Ge, X., et al. (2017). Edaravone injection reverses learning and memory deficits in a rat model of vascular dementia. Acta Biochim. Biophys. Sin. (Shanghai) 49 (1), 83–89. doi:10.1093/abbs/gmw116
Li, Z. (2018). Study on the clinical value of butylphthalide soft capsule in the treatment of vascular dementia. Health Front. 27 (5), 149. doi:10.3969/j.issn.9128-6509.2018.05.140
Lian Cl, L. A., Lian, X., and Fan, B. S. (2010). Observations on the efficacy of Tianzhi Granules in treating 50 cases of mild to moderate vascular dementia. Chin. J. Pract. Nerv. Dis. 13 (23), 77–78. doi:10.3969/j.issn.1673-5110.2010.23.050
Li G, M. Y., Fan, Q. J., Pan, S. S., and Yang, S. Z. (2019). Effects of donepezil hydrochloride on cognitive function and serum IGF-1 level in patients with vascular dementia. Chin. J. Pract. Med. 46 (12), 114–117. doi:10.3760/cma.j.issn.1674-4756.2019.12.034
Li Hm, G. T. (2019). Clinical analysis of butylphthalide soft capsules in treatment of vascu-lar dementia. Syst. Med. 4 (4), 60–61. doi:10.19368/j.cnki.2096-1782.2019.04.060
Li Hy, Z. C., and Zhu, M. (2010). Efficacy of Edaravone in the treatment of multi-infarct dementia. Chin. J. Trauma Disabil. Med. 18 (3), 93–94. doi:10.3969/j.issn.1673-6567.2010.03.075
Li M, Y. S., and Bo, S. F. (2010). Curative effect of oxiracetam capsule on vascular dementia. Chin. J. Pract. Nerv. Dis. 13 (7), 87–88. doi:10.3969/j.issn.1673-5110.2010.07.057
Li N, Z. Y. (2005). Effect of donepezil hydrochloride on near- and long-term memory and cognitive function in patients with mild-to-moderate vascular dementia. Chin. J. Tissue Eng. Res. 9 (9), 168–169. doi:10.3321/j.issn:1673-8225.2005.09.109
Linh, T. T. D., Hsieh, Y. C., Huang, L. K., and Hu, C. J. (2022). Clinical trials of new drugs for vascular cognitive impairment and vascular dementia. Int. J. Mol. Sci. 23 (19), 11067. doi:10.3390/ijms231911067
Lin Jy, L. J., Liu, X. H., Gu, D. F., and Luo, L. (2007). Clinical observation of donepezil hydrochloride in the treatment of vascular dementia. Chin. J. Geriatric Care 5 (4), 74–75. doi:10.3969/j.issn.1672-4860-B.2007.04.034
Little, J. T., Walsh, S., and Aisen, P. S. (2008). An update on huperzine A as a treatment for Alzheimer's disease. Expert Opin. Investig. Drugs 17 (2), 209–215. doi:10.1517/13543784.17.2.209
Liu L, Y. W., and Zhao, A. Y.: Therapeutic efficacy of huperzine A and oxiracetam on senile vascular dementia. J. Logist. Univ. CAPF, 4(12), 959–961. (2015a). CNKI:SUN:WUXB.0.2015-12-009.
Liu, P., Liu, X., Chen, J., Zhang, Y., Chen, J., Yu, L., et al. (2024). Butylphthalide combined with donepezil for the treatment of vascular dementia: a meta-analysis. J. Int. Med. Res. 52 (3), 3000605231223081. doi:10.1177/03000605231223081
Liu C, D. W., and Zheng, T. L. (2013). Clinical curative effect of butylphthalide soft capsules on vascular dementia. Med. J. Natl. Defending Forces Southwest China 23 (2), 144–146. doi:10.3969/j.issn.1004-0188.2013.02.011
Liu J, Q. K., and Liu, L. J. (2020). Effects of butylphthalide capsules on cognitive function,inflammatory factors and oxidative stress in senile patients with vascular dementia. Acta Med. Sin. 33 (6), 22–26. doi:10.19296/j.cnki.1008-2409.2020-06-006
Liu L, Y. W., and Zhao, A. Y. (2015b). Effect of huperzine A and oxiracetam on senile vascular dementia. J. Logist. Univ. CAPF 24 (12), 959–961. CNKI:SUN:WUXB.0.2015-12-009.
Liu Xp, K. K. (2009). Effect of Tianzhi granule in the treatment of older patients with mild or moderate vascular dementia. Chin. J. Pract. Nerv. Dis. 12 (8), 9–11. doi:10.3969/j.issn.1673-5110.2009.08.004
Li Yy, Z. P., and Deng, H. B. (2009). Effects of memantine hydrochloride on cognitive function in patients with vascular dementia. Chin. J. Gerontology 29 (16), 2114–2115. doi:10.3969/j.issn.1005-9202.2009.16.058
L. J (2018). Efficacy and mechanism analysis of Idebenone in the treatment of vascular dementia. Clin. Res. 26 (12), 86–87. doi:10.3969/j.issn.1004-8650.2018.12.046
L. L (2009). Clinical efficacy analysis of donepezil hydrochloride in the treatment of patients with vascular dementia. Guide China Med. 18 (9), 113.
L. M (2016). Evaluation of efficacy and safety of Idebenquinone and Nimodipine in the treatment of vascular dementia. Chin. J. Mod. Drug Appl. 10 (21), 73–74. doi:10.14164/j.cnki.cn11-5581/r.2016.21.045
Lobo, A., Launer, L. J., Fratiglioni, L., Andersen, K., Di Carlo, A., Breteler, M. M., et al. (2000). Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54 (11 Suppl. 5), S4–S9.
Long Cy, Z. C., Wang, M. K., Wang, W. F., Yan, B. C., and Huang, G. (2012). Clinical efficacy of butylphthalide soft capsule in the treatment of mild-to-moderate vascular dementia. Chin. J. Pract. Nerv. Dis. 15 (17), 27–28. doi:10.3969/j.issn.1673-5110.2012.17.014
L. S (2015). Clinical analysis of butylphthalide soft capsules in the treatment of patients with vascular dementia. Med. Forum 1 (32), 4507–4508.
Lu Hl, Y. J., Liang, G. Q., Zhu, R. Y., Hou, Y. G., Zhang, J. R., and Xu, Y. K. (2011). Clinical effect and mechanism of butylphthalide soft capsules in treatment of vascular dementia. Clin. Misdiagnosis and Mistherapy 24 (3), 35–37. doi:10.3969/j.issn.1002-3429.2011.03.019
Lu Jh, L. C., Hong, Z., Yu, L. H., Wang, Y., and Di, Q. (1998). Prospective, double-blind, multicenter study of Niergoline in the treatment of vascular dementia. Chin. J. Neurology 34 (2), 88–91. doi:10.3760/j.issn:1006-7876.2001.02.008
Lu Y, W. J. (2011). Clinical effect of oxiracetam on treatment of vascular dementia in 80 patients. Chin. J. Med. Guide 13 (2), 264–266. doi:10.3969/j.issn.1009-0959.2011.02.049
Lv Cuilan, B. Y., and Feng, Xu (2015). Observation on the efficacy of butylphthalide soft capsule in improving the symptoms of vascular dementia after cerebral infarction. Nei Mongol J. Traditional Chin. Med. 34 (4), 36–37. doi:10.3969/j.issn.1006-0979.2015.04.036
Maina, G., Fiori, L., Torta, R., Fagiani, M. B., Ravizza, L., Bonavita, E., et al. (1989). Oxiracetam in the treatment of primary degenerative and multi-infarct dementia: a double-blind, placebo-controlled study. Neuropsychobiology 21 (3), 141–145. doi:10.1159/000118567
Ma J, Z. Z. (2018). Effect of butylphthalide on cognitive function in elderly patients with vascular dementia. Chin. J. Gerontology 22 (17), 6088–6090. doi:10.3969/j.issn.1005-9202.2017.24.035
Malík, M., and Tlustoš, P. (2022). Nootropics as cognitive enhancers: types, dosage and side effects of smart drugs. Nutrients 14 (16), 3367. doi:10.3390/nu14163367
Marigliano, V., Abate, G., Barbagallo-Sangiorgi, G., Bartorelli, L., Capurso, A., Cucinotta, D., et al. (1992). Randomized, double-blind, placebo controlled, multicentre study of idebenone in patients suffering from multi-infarct dementia. Arch. Gerontol. Geriatr. 15 (3), 239–248. doi:10.1016/0167-4943(92)90059-d
McShane, R., Westby, M. J., Roberts, E., Minakaran, N., Schneider, L., Farrimond, L. E., et al. (2019). Memantine for dementia. Cochrane Database Syst. Rev. 3 (3), Cd003154. doi:10.1002/14651858.CD003154.pub6
Mills, E. J., Thorlund, K., and Ioannidis, J. P. (2013). Demystifying trial networks and network meta-analysis. Bmj 346, f2914. doi:10.1136/bmj.f2914
Mlinac, M. E., and Feng, M. C. (2016). Assessment of activities of daily living, self-care, and independence. Arch. Clin. Neuropsychol. 31 (6), 506–516. doi:10.1093/arclin/acw049
Mok, V., Wong, A., Ho, S., Leung, T., Lam, W. W., and Wong, K. S. (2007). Rivastigmine in Chinese patients with subcortical vascular dementia. Neuropsychiatr. Dis. Treat. 3 (6), 943–948. doi:10.2147/ndt.s2221
Moretti, R., Torre, P., Antonello, R. M., Cazzato, G., and Bava, A. (2002). Rivastigmine in subcortical vascular dementia: an open 22-month study. J. Neurol. Sci. 203-204, 141–146. doi:10.1016/s0022-510x(02)00280-0
Moretti, R., Torre, P., Antonello, R. M., Cazzato, G., and Bava, A. (2003). Rivastigmine in subcortical vascular dementia: a randomized, controlled, open 12-month study in 208 patients. Am. J. Alzheimers Dis. Other Demen 18 (5), 265–272. doi:10.1177/153331750301800508
Moretti, R., Torre, P., Antonello, R. M., Cazzato, G., and Bava, A. (2004). Rivastigmine in vascular dementia. Expert Opin. Pharmacother. 5 (6), 1399–1410. doi:10.1517/14656566.5.6.1399
Muresanu, D. F., Alvarez, X. A., Moessler, H., Buia, M., Stan, A., Pintea, D., et al. (2008). A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J. Neurol. Sci. 267 (1-2), 112–119. doi:10.1016/j.jns.2007.10.016
Muresanu, D. F., Alvarez, X. A., Moessler, H., Novak, P. H., Stan, A., Buzoianu, A., et al. (2010). Persistence of the effects of Cerebrolysin on cognition and qEEG slowing in vascular dementia patients: results of a 3-month extension study. J. Neurol. Sci. 299 (1-2), 179–183. doi:10.1016/j.jns.2010.08.040
Neupane, B., Richer, D., Bonner, A. J., Kibret, T., and Beyene, J. (2014). Network meta-analysis using R: a review of currently available automated packages. PLoS One 9 (12), e115065. doi:10.1371/journal.pone.0115065
Niu Xiaojun, X. J., and Qinghe, YANG (2016). Taiyuan: nim horizon on the cognitive function of patients with vascular dementia. Electron. J. Clin. Med. Literature 3 (41), 8095–8097. CNKI:SUN:LCWX.0.2016-41-002.
Ni Yy, C. S., and Tang, F. Z. (2016). Clinical efficacy of oxiracetam for vascular dementia (VD) and its effect on peripheral blood IL-6 levels in VD patients. J. Med. Mol. Biol. 13 (2), 100–103. doi:10.3870/j.issn.1672-8009.2016.02.008
Orgogozo, J. M., Rigaud, A. S., Stöffler, A., Möbius, H. J., and Forette, F. (2002). Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 33 (7), 1834–1839. doi:10.1161/01.str.0000020094.08790.49
Ouyang Xc, Y. X., Jing, X. W., Wang, S. H., and Zhang, C. P. (2010). Observation of curative effect of memantine hydrochloride on vascular dementia. Clin. J. Med. Officers 4 (6), 1528–1530. doi:10.3969/j.issn.1671-3826.2012.06.102
Pantoni, L., del Ser, T., Soglian, A. G., Amigoni, S., Spadari, G., Binelli, D., et al. (2005). Efficacy and safety of nimodipine in subcortical vascular dementia: a randomized placebo-controlled trial. Stroke 36 (3), 619–624. doi:10.1161/01.STR.0000155686.73908.3e
Pantoni, L., Rossi, R., Inzitari, D., Bianchi, C., Beneke, M., Erkinjuntti, T., et al. (2000). Efficacy and safety of nimodipine in subcortical vascular dementia: a subgroup analysis of the Scandinavian Multi-Infarct Dementia Trial. J. Neurol. Sci. 175 (2), 124–134. doi:10.1016/s0022-510x(00)00300-2
Pathan, N., Kharod, M. K., Nawab, S., Di Scipio, M., Paré, G., and Chong, M. (2024). Genetic determinants of vascular dementia. Can. J. Cardiol. doi:10.1016/j.cjca.2024.03.025
Patton, K., and Borshoff, D. C. (2018). Adverse drug reactions. Anaesthesia 73 (Suppl. 1), 76–84. doi:10.1111/anae.14143
P. D (2014). Evaluation of the efficacy of donepezil hydrochloride in the treatment of mild to moderate vascular dementia. Chin. J. Geriatric Care 5 (4), 159–160. CNKI:SUN:ZSSA.0.2014-04-127.
Pratt, R. D., and Perdomo, C. A. (2002). Donepezil-treated patients with probable vascular dementia demonstrate cognitive benefits. Ann. N. Y. Acad. Sci. 977, 513–522. doi:10.1111/j.1749-6632.2002.tb04859.x
Qiu Yh, H. Z., Wang, F. M., and Zhu, G. X. (2011). The efficacy of donepezil hydrochloride in the treatment of vascular dementia. Front. Med. 1 (20), 28–29. doi:10.3969/j.issn.2095-1752.2011.20.023
Q. Y (2017). Preliminary observation and analysis of the efficacy of donepezil hydrochloride in the treatment of vascular dementia. Guide China Med. 15 (6), 78–79. CNKI:SUN:YYXK.0.2017-06-066.
Qy, N. (2009). Clinical observation on 42 cases of vascular dementia (VD) treated with Tianzhi granules. World Health Dig. 6 (28), 5–6. doi:10.3969/j.issn.1672-5085.2009.28.001
Ren Xy, L. W., Zheng, R. F., Yang, Z. Y., and Wei, L. P. (2004). Clinical research of donepezil hydrochloride in treatment of vascular dementia. Chin. J. Rehabilitation Theory Pract. 10 (9), 540–541. doi:10.3969/j.issn.1006-9771.2004.09.012
Rizzi, L., Rosset, I., and Roriz-Cruz, M. (2014). Global epidemiology of dementia: Alzheimer's and vascular types. Biomed. Res. Int. 2014, 908915. doi:10.1155/2014/908915
Rockenstein, E., Adame, A., Mante, M., Larrea, G., Crews, L., Windisch, M., et al. (2005). Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer's disease with the neurotrophic compound cerebrolysin. J. Neural Transm. (Vienna) 112 (2), 269–282. doi:10.1007/s00702-004-0181-4
Román, G. C., and Kalaria, R. N. (2006). Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol. Aging 27 (12), 1769–1785. doi:10.1016/j.neurobiolaging.2005.10.004
Román, G. C., Salloway, S., Black, S. E., Royall, D. R., Decarli, C., Weiner, M. W., et al. (2010). Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 41 (6), 1213–1221. doi:10.1161/strokeaha.109.570077
Román, G. C., Wilkinson, D. G., Doody, R. S., Black, S. E., Salloway, S. P., and Schindler, R. J. (2005). Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement. Geriatr. Cogn. Disord. 20 (6), 338–344. doi:10.1159/000088494
Roman, S. B. G., Royall, D. R., Surick, I., Kumar, D., and Schindler, H. P. R. (2010b). Efficacy and safety of donepezil in vascular dementiaresults from the largest double-blind trial in vascular dementia patients. Front. Aging Neurosci. 11, 86.
Roman, S. B. G., Royall, D. R., Surick, I., Kumar, D., Schindler, R., and Posner, H. (2010a). Roman G 2010 Efficacy and safety of donepezil in vascular dementiaresults from the largest double-blind trial in vascular dementia patients. Dementia neurological Disord. 2 (3), 481.
Rong Jc, C. H. (2011). Therapeutic efficacy of donepezil hydrochloride in vascular dementia. J. Front. Med. 1 (21), 169. doi:10.3969/j.issn.2095-1752.2011.21.132
Rp, C. (2014). Clinical effect of galantamine on patients with vascular dementia. J. North Pharm. 11 (8), 72–73. CNKI:SUN:BFYX.0.2014-08-066.
Rq, S. (2013). Clinical efficacy of racetam in the treatment of vascular dementia. World Health Dig. 1 (7), 231–232. doi:10.3969/j.issn.1672-5085.2013.07.263
Ruether, E., Alvarez, X. A., Rainer, M., and Moessler, H. (2002). Sustained improvement of cognition and global function in patients with moderately severe Alzheimer’s disease:a double-blind, placebo-controlled study with the neurotrophic agent Cerebrolysin®. J. Neural Transm. Suppl. (62), 265–275. doi:10.1007/978-3-7091-6139-5_24
Ruether, E., Husmann, R., Kinzler, E., Diabl, E., Klingler, D., Spatt, J., et al. (2001). A 28-week, double-blind, placebo-controlled study with Cerebrolysin in patients with mild to moderate Alzheimer's disease. Int. Clin. Psychopharmacol. 16 (5), 253–263. doi:10.1097/00004850-200109000-00002
R, Z. (2021). Application of oxiracetam in the treatment of vascular dementia. World J. Complex Med. 7 (1), 28–31. doi:10.11966/j.issn.2095-994X.2021.07.01.09
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Schindler, G. C. R. D. G. W. R. S. D. S. E. B. S. P. S. R. J. (2005). Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. J. Alzheimers Dis. 80 (2), 673–681. doi:10.1159/000088494
Sf, W. (2012). Effectiveness of nimodipine in the treatment of vascular dementia in 48 cases. J. Front. Med. 2 (9), 9–10. doi:10.3969/j.issn.2095-1752.2012.09.003
Shao Xp, G. S., and Hu, L. L. (2013). Clinical efficacy of butylphthalide in the treatment of vascular dementia in the elderly and its effect on psychosomatic scales. Chin. J. Gerontology 33 (22), 5601–5602. doi:10.3969/j.issn.1005-9202.2013.22.051
Sheng Gl, A. A. (2015). Comparison of the clinical efficacy of butylphthalide soft capsules and nimodipine in the treatment of post-stroke vascular dementia. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 23 (9), 3. doi:10.3969/j.issn.1008-5971.2015.09.014
Shi, J., Wei, M., Ni, J., Sun, F., Sun, L., Wang, J., et al. (2020). Tianzhi granule improves cognition and BPSD of vascular dementia: a randomized controlled trial. J. Transl. Med. 18 (1), 76. doi:10.1186/s12967-020-02232-z
Sh, W. (2012). Effects of Tianzhi granules on cognitive function and event-related potential P300 in vascular dementia. Chin. J. Pract. Nerv. Dis. 15 (3), 83–84. doi:10.3969/j.issn.1673-5110.2012.03.055
Sl, L. (2011). Clinical observation on 62 cases of vascular dementia treated with Tianzhi granules. Chin. J. Pract. Nerv. Dis. 14 (13), 35–37. doi:10.3969/j.issn.1673-5110.2011.13.019
Song Yj, L. L., and Li, X. M. (2006). A comparative study of the efficacy of niergoline and aniracetam in the treatment of vascular dementia. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 14 (9), 702–703. doi:10.3969/j.issn.1008-5971.2006.09.011
S. P. T. f. t. G. o. C. A. o. P. C. M. i. t. T. o. V. (2021). Dementia: guidelines on the clinical application of proprietary Chinese medicines in the treatment of vascular dementia (2020). Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 41 (3), 273–279. doi:10.7761/j.cjim.20210114.158
Sui Cf, S. S., and Wang, J. (2001). Comparison of efficacy between nimodipine and hydergine in the treatment of vascular dementia. Chin. J. Clin. Neurosci. 9 (2), 175–177. doi:10.3969/j.issn.1008-0678.2001.02.023
Sun, L. X., Niu, Y. L., Tian, Z. Q., and Wang, J. P. (2016). Clinical efficacy of butylphthalide soft capsules in the treatment of vascular dementia. Chin. J. Gerontology 33 (16), 3967–3969. doi:10.3969/j.issn.1005-9202.2013.16.068
Su Xd, Y. X. (2015). Tai LW: efficacy of butylphthalide soft capsules versus nimodipine in the treatment of post-stroke vascular dementia. Clin. Focus 30 (5), 569–572. doi:10.3969/j.issn.1004-583X.2015.05.024
S, W. (2021). Economic evaluation of oxiracetam and donepezil in the treatment of mild to moderate vascular dementia. China J. Pharm. Econ. 16 (8), 29–31. doi:10.12010/j.issn.1673-5846.2021.08.005
Sw, R. (2016). Analysis the efficacy and safety of oxiracetam and piracetam in the treatment of vascular dementia. China Health Stand. Manag. 7 (4), 113–114. doi:10.3969/j.issn.1674-9316.2016.04.079
Sy, X. (2013). Niergoline tablets for the treatment of vascular dementia with cognitive impairment. China Health Nutr. 1 (10), 468–469.
Sy, X. (2019). Comparison on clinical efficacy between Oxiracetam Capsules and Naonatai Capsules in treatment of vascular dementia. Drugs and Clin. 34 (6), 1693–1696. doi:10.7501/j.issn.1674-5515.2019.06.018
Sz, L. (2011). Effect of Tianzhi granule in the treatment of vascular dementia patients' cognition and viability. Chin. J. Pract. Nerv. Dis. 14 (4), 7–9. doi:10.3969/j.issn.1673-5110.2011.04.004
Takeda, S., Rakugi, H., and Morishita, R. (2020). Roles of vascular risk factors in the pathogenesis of dementia. Hypertens. Res. 43 (3), 162–167. doi:10.1038/s41440-019-0357-9
Tan Ax, L. X. (2018). The efficacy of donepezil hydrochloride in the treatment of vascular dementia. Chin. J. Pract. Med. 36 (7), 78–79. doi:10.3760/cma.j.issn.1674-4756.2007.13.059
Tisher, A., and Salardini, A. (2019). A comprehensive update on treatment of dementia. Semin. Neurol. 39 (2), 167–178. doi:10.1055/s-0039-1683408
Tomassoni, D., Lanari, A., Silvestrelli, G., Traini, E., and Amenta, F. (2008). Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clin. Exp. Hypertens. 30 (8), 744–766. doi:10.1080/10641960802580232
Tw, W. (2017). Clinical efficacy of oxiracetam in the treatment of vascular dementia patients and its influence on the level of IL-6 in peripheral blood. Clin. Med. 37 (3), 75–77. doi:10.19528/j.issn.1003-3548.2017.03.033
Villardita, C., Grioli, S., Lomeo, C., Cattaneo, C., and Parini, J. (1992). Clinical studies with oxiracetam in patients with dementia of Alzheimer type and multi-infarct dementia of mild to moderate degree. Neuropsychobiology 25 (1), 24–28. doi:10.1159/000118805
Wang, X. X., Zhang, B., Xia, R., and Jia, Q. Y. (2020). Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur. Rev. Med. Pharmacol. Sci. 24 (18), 9601–9614. doi:10.26355/eurrev_202009_23048
Wang Fl, S. X. (2011). Curative effect of huperzine A on vascular dementia. Chin. J. Clin. Neurosci. 19 (6), 44–645. doi:10.3969/j.issn.1008-0678.2011.06.017
Wang G, Z. T., Jiang, Y., Chen, X. L., Wang, X. Q., and Wang, H. Y. (2020). Effects of vinpocetine on clinical efficacy and levels of inflammatory factors in patients with vascular dementia. Guangxi Med. J. 42 (17), 2244–2247. doi:10.11675/j.issn.0253-4304.2020.17.14
Wang Gf, M. H. (2015). Clinical efficacy of Idebenone and Nimodipine in the treatment of vascular dementia. Int. Med. Health Guid. New 21 (17), 2596–2597. doi:10.3760/cma.j.issn.1007-1245.2015.17.033
Wang J, C. D. (2016). Comparison of the efficacy of citicoline and cerebral actin in the treatment of cerebrovascular diseases and vascular dementia. Everyone's health 10 (10), 136. doi:10.3969/j.issn.1009-6019.2016.10.176
Wang Ll, Y. P., and Wang, X. M. (2005). Clinical efficacy of Tongxinluo capsule in the treatment of vascular dementia. Chin. J. Difficult Complicat. Cases 4 (5), 260–262. doi:10.3969/j.issn.1671-6450.2005.05.002
Wang Qy, Z. Z., Wang, Z. L., and Fan, Y. H. (2014). Effect and action mechanism of butylphthalide on cognitive function in elderly vascular dementia. China Pharm. 23 (21), 20–21. CNKI:SUN:YYGZ.0.2014-21-010.
Wang Rp, Z. Z., and Hu, L. L. (2004). The effect of lithopanax methyl on cognitive function and living ability in patients with vascular dementia:36 cases with 6-month follow-up. Chin. J. Tissue Eng. Res. 8 (19), 3892. doi:10.3321/j.issn:1673-8225.2004.19.123
Wang Wl, J. S., Xu, W. M., Sheng, J. Q., Gong, Q., Gao, J., and Jin, Y. G. (2010). Research on donepezil and huperzine-a's curative effect on vascular dementia. Hebei Med. 16 (10), 1163–2116. doi:10.3969/j.issn.1006-6233.2010.10.004
Wang Y, S. W., Hao, L. K., and Ji, R. Y. (2006). Clinical observation of donepezil hydrochloride for. Vasc. Dement. 17 (18), 1406–1407. doi:10.3969/j.issn.1001-0408.2006.18.020
Wang Yf, W. Q. (2009). A randomised controlled study of nimodipine versus donepezil in the treatment of vascular dementia. Shandong Med. J. 49 (29), 61–62. doi:10.3969/j.issn.1002-266X.2009.29.028
Wang Yj, D. G., and Tu, Q. L. (2013). Clinical observation on the treatment of vascular dementia with butylphthalide injection. J. Hebei Med. Univ. 34 (2), 188–189. doi:10.3969/j.issn.1007-3205.2013.02.021
Wang Zf, W. L. (2016). Nimodipine and donepezil hydrochloride in the treatment of vascular dementia in 108 cases. J. Henan Med. Coll. 28 (6), 486–487. CNKI:SUN:HNZG.0.2016-06-010.
Wang Zg, P. A., Zhang, G. H., Sun, F. H., and Hu, W. L. (2009). Comparative effect of donepezil and nimodipine on vascular dementia. Chin. J. General Pract. 7 (9), 947–948. CNKI:SUN:SYQY.0.2009-09-028.
Wen, J., Cao, Y., Chang, S., Huang, Q., Zhang, Z., Wei, W., et al. (2022). A network meta-analysis on the improvement of cognition in patients with vascular dementia by different acupuncture therapies. Front. Neurosci. 16, 1053283. doi:10.3389/fnins.2022.1053283
Weng Ql, X. L., Du, J. B., Che, X. H., Yang, L., Li, G., Gu, Y., et al. (2011). Therapeutic efficacy of different doses of huperzine A on vascular dementia. J. Intern. Med. Concepts and Pract. 6 (5), 361–364. CNKI:SUN:NKLL.0.2011-05-011.
Wen K, M. X. (2006). Clinical observation on 30 cases of patients with vascular dementia treated with donepezil hydrochloride. Shaanxi Med. J. 35 (8), 1022–1023. doi:10.3969/j.issn.1000-7377.2006.08.048
W. F (2017). Effect of huperzine A therapy on hemorheology and P300 potential in elderly patients with vascular dementia. Chin. J. Gerontology 37 (9), 2179–2180. doi:10.3969/j.issn.1005-9202.2017.09.043
Wilcock, G., Möbius, H. J., and Stöffler, A.MMM 500 group (2002). A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int. Clin. Psychopharmacol. 17 (6), 297–305. doi:10.1097/00004850-200211000-00005
Wilkinson, D., Doody, R., Helme, R., Taubman, K., Mintzer, J., Kertesz, A., et al. (2003). Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 61 (4), 479–486. doi:10.1212/01.wnl.0000078943.50032.fc
Wilkinson, D., Róman, G., Salloway, S., Hecker, J., Boundy, K., Kumar, D., et al. (2010). The long-term efficacy and tolerability of donepezil in patients with vascular dementia. Int. J. Geriatr. Psychiatry 25 (3), 305–313. doi:10.1002/gps.2340
Winblad, B., Fioravanti, M., Dolezal, T., Logina, I., Milanov, I. G., Popescu, D. C., et al. (2008). Therapeutic use of nicergoline. Clin. Drug Investig. 28 (9), 533–552. doi:10.2165/00044011-200828090-00001
W. LL (2022). Analysis of efficacy and adverse reactions of donepezil hydrochloride in patients with mild to moderate vascular dementia. Chin. J. Mod. Drug Appl. 16 (21), 119–121. doi:10.14164/j.cnki.cn11-5581/r.2022.21.035
Wolters, F. J., and Ikram, M. A. (2018). Epidemiology of dementia: the burden on society, the challenges for research. Methods Mol. Biol. 1750, 3–14. doi:10.1007/978-1-4939-7704-8_1
Wolters, F. J., and Ikram, M. A. (2019). Epidemiology of vascular dementia. Arterioscler. Thromb. Vasc. Biol. 39 (8), 1542–1549. doi:10.1161/atvbaha.119.311908
W. S (2021). Efficacy of butylphthalide soft capsule on vascular dementia after cerebral infarction. Chin. J. Mod. Drug Appl. 15 (18), 97–100. doi:10.14164/j.cnki.cn11-5581/r.2021.18.036
Wu B, X. Q., Gu, J., and Dong, A. G. (2019). Effect of tianzhi granule in the treatment of older patients with mild or moderate vascular dementia. China J. Health Psychol. 21 (12), 1774–1776.
Wu J, F. C., Yang, J., and Xiong, Y. (2012). Improving effect of butylphthalide on cognition in patients with vascular dementia. Chongqing Med. 41 (6), 571–572. doi:10.3969/j.issn.1671-8348.2012.06.021
Wu Xh, W. B., Chen, W. M., Liu, S. M., and Guo, L. (2020). Observation on clinical effect of idebenone in the treatment of vascular dementia. China Pract. Med. 15 (14), 105–107. doi:10.14163/j.cnki.11-5547/r.2020.14.045
Wx, Z. (2014). Analysis of the efficacy of donepezil hydrochloride in the treatment of patients with mild to moderate vascular dementia and the effect of cognitive function and daily living abilit. China Pract. Med. 1 (19), 163–164. CNKI:SUN:ZSSA.0.2014-19-119.
Xb, Z. (2016). Curative effect of oxiracetam capsule on vascular dementia after cerebral infarction. Chin. J. Trauma Disabil. Med. 24 (8), 126–127. doi:10.13214/j.cnki.cjotadm.2016.08.086
X. H (2016). Comparison of the efficacy of citicoline and cerebral actin in the treatment of cerebrovascular diseases and vascular dementia. World Latest Med. Inf. 16 (22), 95–96. doi:10.3969/j.issn.1671-3141.2016.22.075
Xh, D. (2016). Clinical study of early intervention with nimodipine in the prevention of vascular dementia after acute cerebral infarction. Chin. J. Trauma Disabil. Med. 24 (3), 35–37. doi:10.13214/j.cnki.cjotadm.2016.03.020
Xiao, M. X. (2019). A controlled study of butylphthalide versus olanzapine in the treatment of vascular dementia. Med. J. Chin. People's Health 31 (4), 21–22. doi:10.3969/j.issn.1672-0369.2019.04.010
Xiao J, W. Y., Huang, Y. L., Zhou, L., and Wu, W. B. (2004). Efficacy of rivastigmine in treating vascular dementia. Pract. J. Clin. Med. 1 (2), 22–23. doi:10.3969/j.issn.1672-6170.2004.02.009
Xl, L. (2015). Clinical efficacy of butylphthalide soft capsule in the treatment of mild-to-moderate vascular dementia. Jilin Med. J. 1 (12), 2572–2573. doi:10.3969/j.issn.1673-5110.2012.17.014
Xl, L. (2016). Clinical analysis of donepezil hydrochloride in treatment of vascular dementia. China Health Stand. Manag. 7 (22), 96–97. doi:10.3969/j.issn.1674-9316.2016.22.056
Xu, Z. Q., Liang, X. M., Juan, W., Zhang, Y. F., Zhu, C. X., and Jiang, X. J. (2012). Treatment with Huperzine A improves cognition in vascular dementia patients. Cell Biochem. Biophys. 62 (1), 55–58. doi:10.1007/s12013-011-9258-5
Xu F, W. A., Liu, Z. J., Yin, M. H., Yang, Z., and Lei, W. X. (2015). Clinical efficacy of butylphthalide on vascular dementia. Chin. J. Mod. Drug Appl. 42 (5), 120–121. doi:10.14164/j.cnki.cn11-5581/r.2015.05.089
Xu Xy, G. X., and Huang, L. M. (2017). A study comparing the clinical value of butylphthalide soft capsules with nimodipine in the treatment of post-stroke vascular dementia. J. Front. Med. 7 (23), 203–204. doi:10.3969/j.issn.2095-1752.2017.23.171
Xu Zj, L. H., Xu, X. H., and Huang, S. Y. (2009). Clinical effect analysis of huperzine A in the treatment of vascular dementia. Med. J. Chin. People''s Health 21 (18), 2208. doi:10.3969/j.issn.1672-0369.2009.18.022
Xu Zq, Z. M., Li, J., and Zhou, H. D. (2009). The clinical study of huperzine A in the treatment of vascular dementia. Chin. J. Clin. Neurosci. 17 (2), 163–165. doi:10.3969/j.issn.1008-0678.2009.02.011
Xy, B. (2019). Effect of butylphthalide soft capsule on curative effect and cognitive function of vascular dementia after cerebral infarction. China Foreign Med. Treat. 38 (32), 91–93. doi:10.16662/j.cnki.1674-0742.2019.32.091
Xy, C. (2009). Effect of nimodipine on cognitive function in vascular dementia. Strait Pharm. J. 21 (5), 168–169. doi:10.3969/j.issn.1006-3765.2009.05.091
Xz, Z. (2018). Analysis of the efficacy of nimodipine in the treatment of patients with mild to moderate vascular dementia and its effect on cognitive function and daily living ability. J. North Pharm. 15 (1), 48–49. doi:10.3969/j.issn.1672-8351.2018.01.037
Yao Jm, Z. H., Liu, Y., Weng, W. L., Xu, D., Zhu, X. Y., and Liu, K. (2006). Improvement of cognitive ability of excessive fire type vascular dementia patients by the use of tianzhi particle. J. Neurology Neurorehabilitation 3 (2), 71–74. doi:10.3969/j.issn.1672-7061.2006.02.003
Yao Mr, F. Y., Pu, Z. P., Chen, F., Wang, L. E., and Zha, X. Y. (2015). Clinical observation of memantine hydrochloride in patients with vascular dementiain. J. Clin. Psychiatry 25 (4), 271–272. CNKI:SUN:LCJS.0.2015-04-029.
Yb, Y. (2018). The effect and mechanism of butylphthalide in the treatment of vascular dementia. Psychol. Dr. 24 (12), 171–172.
Yd, Z. (2013). Analysis of curative effect of oxiracetam capsule on vascular dementia. Chin. Foreign Med. Res. 9 (30), 36–37.
Yf, R. (2018). Analysis of the efficacy of donepezil hydrochloride in the treatment of mild to moderate vascular dementia and its effect on cognitive function and daily living ability. J. North Pharm. 15 (4), 23–24. doi:10.3969/j.issn.1672-8351.2018.04.016
Y. M (2013). Analysis on efficacy of treatment with oxiracetam in patients with chronic cerebral ischemia induced dementia. J. Clin. Exp. Med. 12 (21), 1750–1752. CNKI:SUN:SYLC.0.2013-21-022.
Yoshida, H., Yanai, H., Namiki, Y., Fukatsu-Sasaki, K., Furutani, N., and Tada, N. (2006). Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 12 (1), 9–20. doi:10.1111/j.1527-3458.2006.00009.x
Youn, D. H., Han, S. W., Kim, J. T., Choi, H., Lee, A., Kim, N., et al. (2023). Oxiracetam alleviates anti-inflammatory activity and ameliorates cognitive impairment in the early phase of traumatic brain injury. Acta Neurochir. (Wien) 165 (8), 2201–2210. doi:10.1007/s00701-023-05674-8
Yq, W. (2000). Huperzine A tablets for treatment of 17 cases of vascular dementia. China Pharm. 9 (8), 32–33. doi:10.3969/j.issn.1006-4931.2000.08.036
Yuan Lf, L. N., Li, Y. X., Ji, Z., Fan, X. B., Gao, Y., and Zhou, Q. (2011). Evaluate the functional outcome of Tongxinluo capsules on the different degree of vascular cognitive impairment after stroke and hemiplegia. Chin. J. Difficult Complicat. Cases 10 (2), 83–86. doi:10.3969/j.issn.1671-6450.2011.02.001
Yue Ym, Z. Y., and Zhang, Y. H. (2013). Clinical comparative analysis of oxiracetam and piracetam in the treatment of vascular dementia. Clin. Med. 33 (2), 52–53. doi:10.3969/j.issn.1003-3548.2013.02.030
Yu Wy, G. J., and Zhao, L. J. (2008). Curative effect of oxiracetam in the treatment of vascular dementia. Clin. Med. China 24 (10), 1047–1048. doi:10.3760/cma.j.issn.1008-6315.2008.10.034
Y. W (2011). Clinical analysis of Tianzhi granules in the treatment of vascular dementia. Chin. J. Pract. Nerv. Dis. 14 (4), 83–84. doi:10.3969/j.issn.1673-5110.2011.04.055
Yw, W. (2010). Clinical efficacy of donepezil hydrochloride in the treatment of vascular dementia. Jilin Med. J. 31 (13), 1827–1829. doi:10.3969/j.issn.1004-0412.2010.13.060
Y. Y (2020). Clinical analysis of butylphthalide soft capsule treatment for vascular dementia. Syst. Med. 5 (7), 65–67. doi:10.19368/j.cnki.2096-1782.2020.07.065
Zhagn, Y. S. (2013). Clinical observation of huperzine A in treatment of vascular dementia. Chin. J. Pract. Nerv. Dis. 46 (8), 63–64. doi:10.3969/j.issn.1673-5110.2013.08.037
Zhang, H., Wu, H., Qi, X., Wu, F., and Zhang, D. (2024). Effect of butylphthalide combined with idebenone on vascular dementia: a retrospective observational analysis. Med. Baltim. 103 (9), e37495. doi:10.1097/md.0000000000037495
Zhang Dp, X. X., Shi, Y. M., Zhang, W. Q., Feng, S., Wei, L. N., Le, H. Q., et al. (2013). Clinical control study of idbenquinone and oxiracetam in the treatment of vascular dementia. Hebei Med. J. 35 (18), 2769–2771. doi:10.3969/j.issn.1002-7386.2013.18.021
Zhang F, G. H. (2010). Study of edaravone in the treatment of vascular dementia. Chin. J. Pract. Nerv. Dis. 13 (3), 18–20. doi:10.3969/j.issn.1673-5110.2010.03.007
Zhang Fz, Z. D., Dou, W., Jin, S. J., and Xiao, G. R. (2015). Clinical observation of Idbenquinone in the treatment of vascular dementia patients. Chin. J. Crit. Care Med. 35 (2), 306–307. doi:10.3969/j.issn.1002-1949.2015.z2.195
Zhang Sg, W. Y. (2011). Effect of huperzine A on cognitive dysfunction after stroke. ei Mongol J. Traditional Chin. Med. 30 (19), 56–57. doi:10.3969/j.issn.1006-0979.2011.19.069
Zhang Yh, L. Y., and Geng, J. L. (2008). Clinical investigation of efficacy and safety of nicergoline and aniracetam in the treatment of vascular dementia. Chin. J. Pharmacoepidemiol. 17 (3), 138–141. doi:10.3969/j.issn.1005-0698.2008.03.002
Zhao, N., Liu, D., Wang, Y., Zhang, X., and Zhang, L. (2022). Screening and identification of anti-acetylcholinesterase ingredients from Tianzhi granule based on ultrafiltration combined with ultra-performance liquid chromatography-mass spectrometry and in silico analysis. J. Ethnopharmacol. 298, 115641. doi:10.1016/j.jep.2022.115641
Zhao M, W. Y., and Zhang, Y. L. (2008). Therapeutic effect of Donepezil on vascular dementia. J. Med. Forum 17 (13), 57–58. doi:10.3969/j.issn.1672-3422.2007.10.031
Zheng Gl, C. G., and Li, Y. Z. (2006). Huperzine A in treatment of vascular dementia. J. Shandong Univ. Heal. Sci. 44 (8), 840–842. doi:10.3969/j.issn.1671-7554.2006.08.021
Zhong, L. K. (2004). Clinical observation of 29 cases of vascular dementia treated with huperzine A. J. Hainan Med. Univ. 10 (4), 251–252. doi:10.3969/j.issn.1007-1237.2004.04.018
Zhong H, C. L., and Li, Y. (2014). Clinical study of oxiracetam injection in the treatment of vascular dementia. Chin. J. Clin. Neurosci. 1 (6), 657–662. CNKI:SUN:LCSK.0.2014-06-011.
Zhong Xd, C. D. (2021). Efficacy of piracetam, dihydroergosine, Donepezil, cabalatine, Memantine and other commonly used drugs for the treatment of Alzheimer's disease in vascular dementia. World Latest Med. Inf. 21 (76), 181–182. doi:10.3969/j.issn.1671-3141.2021.76.078
Zhou Ga, Y. S., and Ning, F. B. (2012). The influence of edaravone on cognitive ability in patients with vascular dementia. Chin. J. Prim. Med. Pharm. 10 (4), 2729–2730. doi:10.3760/cma.j.issn.1008-6706.2012.18.005
Zhou Y, L. T., Fan, C., Jing, H. P., and Wei, T. (2021). The effects of donepezil hydrochloride and nimodipine on serum Lp-PLA2,BDNF and Ang1-7 in elderly patients with vascular dementia after cerebral infarction. Pract. J. Clin. Med. 18 (5), 119–122. doi:10.3969/j.issn.1672-6170.2021.05.032
Zhu Ah, T. J., Zhong, J., and Shi, J. (2005). A clinical study on a randomized, double-blind control of Tianzhi granules in treatment of the senile vascular dementia. Chin. J. Gerontology 25 (12), 1435–1438. doi:10.3969/j.issn.1005-9202.2005.12.002
Keywords: Bayesian network meta-analysis, vascular dementia, treatment decision-making, meta, VAD
Citation: Dang C, Wang Q, Zhuang Y, Li Q, Feng L, Xiong Y and Lu Y (2024) Pharmacological treatments for vascular dementia: a systematic review and Bayesian network meta-analysis. Front. Pharmacol. 15:1451032. doi: 10.3389/fphar.2024.1451032
Received: 18 June 2024; Accepted: 26 July 2024;
Published: 22 August 2024.
Edited by:
Yoshiteru Kagawa, Tohoku University, JapanReviewed by:
Liang Jin, The University of Melbourne, AustraliaFeng Chen, University of Melbourne, Australia
Copyright © 2024 Dang, Wang, Zhuang, Li, Feng, Xiong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaoheng Lu, bHloOTNAY2R1dGNtLmVkdS5jbg==; Ying Xiong, NjE3MTE0NDVAcXEuY29t
 Chun Dang
Chun Dang Qinxuan Wang
Qinxuan Wang Yijia Zhuang2
Yijia Zhuang2 Qian Li
Qian Li Ying Xiong
Ying Xiong Yaoheng Lu
Yaoheng Lu