- 1IntelGenX Corp., Saint-Laurent, QC, Canada
- 2Department of Clinical Neuroscience, Neuro Svenningsson, Karolinska Universitetssjukhuset, Stockholm, Sweden
- 3Institute of Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria
Multiple Sclerosis (MS) is a multifactorial autoimmune disease of the central nervous system (CNS). It is characterized by a heightened activation of the immune system with ensuing inflammation, demyelination and neurodegeneration with consequences such as motor, sensory, cognitive, as well as autonomic dysfunctions. While a range of immune-modulatory drugs have shown certain efficacy in alleviating pathology and symptoms, none of the currently available therapeutics regenerates the damaged CNS to restore function. There is emerging evidence for leukotrienes and leukotriene receptors being involved in the various aspects of the MS pathology including neuroinflammation and de/remyelination. Moreover, leukotriene receptor antagonists such as the asthma drug montelukast diminish inflammation and promote regeneration/remyelination. Indeed, montelukast has successfully been tested in animal models of MS and a recent retrospective case-control study suggests that montelukast treatment reduces relapses in patients with MS. Therefore, we propose montelukast as a therapeutic adjuvant to the standard immune-modulatory drugs with the potential to reduce pathology and promote structural and functional restoration. Here, we review the current knowledge on MS, its pathology, and on the potential of leukotriene receptor antagonists as therapeutics for MS.
Introduction
Multiple Sclerosis (MS) is a multifaceted degenerative disease of the central nervous system (CNS), which is typically characterized by a heightened activation of the immune system with ensuing inflammation, demyelination and neurodegeneration (Filippi et al., 2018). MS patients suffer from motor, sensory, autonomic, as well as cognitive dysfunctions (Chiaravalloti and DeLuca, 2008). Although the exact etiology remains nebulous and elusive, the disease appears to result from an association between genetic susceptibility and exposure to environmental risk factors stirring up demyelination and neurodegeneration (Raffel et al., 2016). Other contributing factors may include age, sex, diet, smoking, injuries and previous viral infections (Dighriri et al., 2023).
MS is an acquired idiopathic demyelinating autoimmune disease of the CNS, characterized by axonal loss, astrocytic scarring (gliosis) and progressive neurodegeneration (Mahad et al., 2015; Campbell and Mahad, 2018; Dendrou et al., 2015; Lassmann, 2019). Resultant tissue damage associated with the course of the disease stems from intricate and dynamic interactions among the immune system, glia (oligodendrocytes and their precursors, microglia, and astrocytes), and neurons (Reich et al., 2018). The associated damage due to the initiation and perpetuation of inflammatory mediators from the above-mentioned interplay eventually gives rise to focal plaques or lesions, the hallmarks of the disease. These lesions or plaques represent demyelinated white and gray matter of the brain and spinal cord indicating damage to and loss of the axonal myelin sheaths due to the ongoing oligodendrocyte apoptotic activity (Dendrou et al., 2015; Lassmann, 2019). Typically, these detectable plaques, observable by MRI, mainly appear in the white matter surrounding the ventricles, optic nerves and tracts, corpus callosum, cerebellar peduncles, long tracts and subpial area of the spinal cord and brainstem, as well as in the gray matter (Compston and Coles, 2008). Demyelination of the axonal myelin sheaths leads to various effects, including alterations in nodal saltatory conduction, slowed conduction velocity and a predisposition to conduction block (Davis et al., 2010).
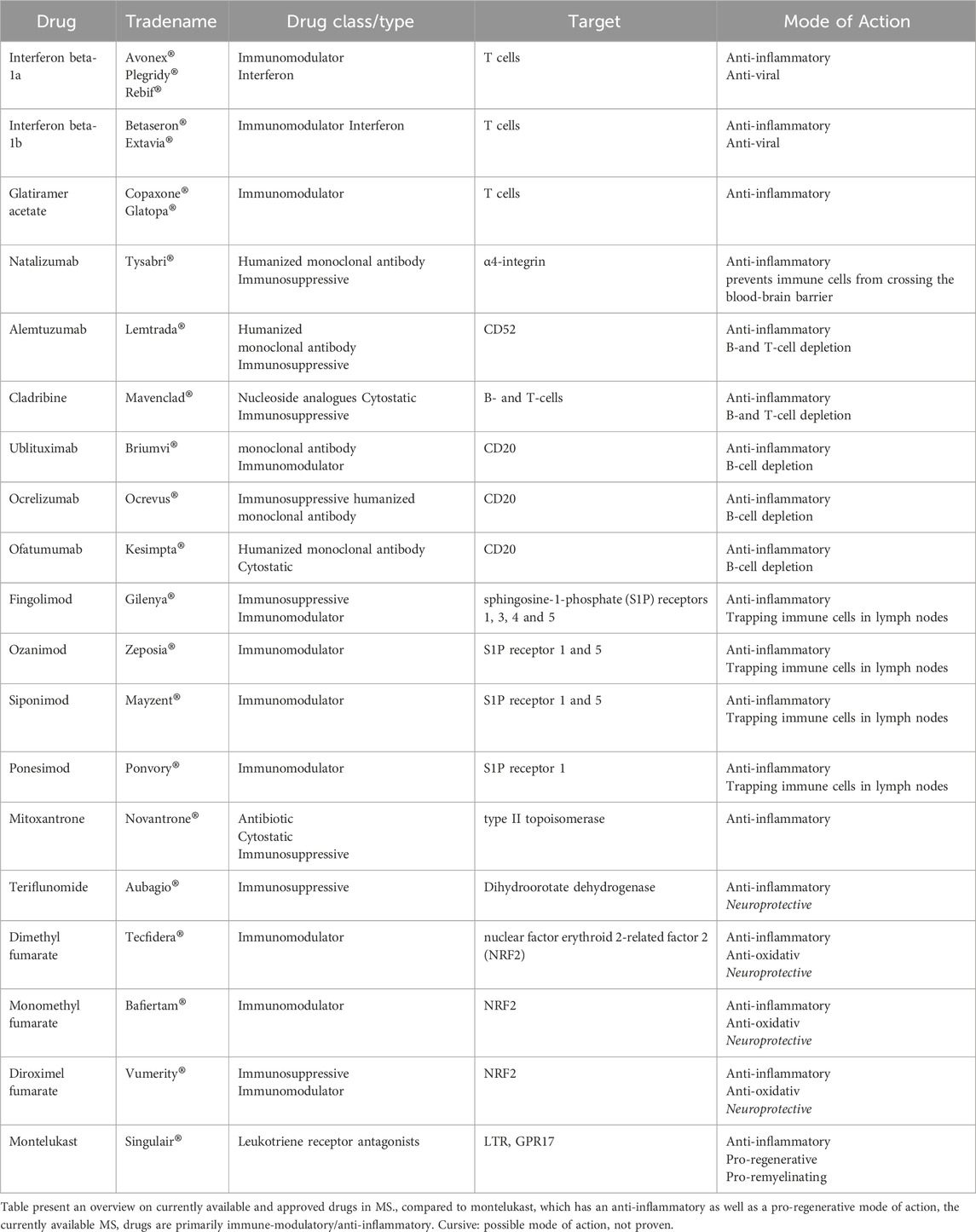
To date, the majority of the US Food and Drug Administration (FDA) approved treatments for MS are either immunomodulatory or immunosuppressive in nature. These treatments have played a pivotal role in alleviating many associated symptoms stemming from the disease progression, while also offering valuable insight into new treatment modalities (Table 1). However, despite the welcomed presence of these immunomodulatory treatments, a notable gap remains: they do not reverse or boost remyelination, nor do they prevent disease progression or neuronal damage (Heβ et al., 2020; Melchor et al., 2019; Marques et al., 2022; Han et al., 2021).
Leukotrienes and leukotriene receptors in MS
An overwhelming body of evidence from both nonclinical and clinical studies underscores the pivotal role of leukotrienes (LTs) in various physiological and pathological conditions. These highly active and potent lipid mediators synthesized from arachidonic acid via the 5-lipoxygenase (5-LOX) pathway have been extensively investigated. Elevated levels of LTs, including the cysteinyl leukotrienes (CysLTs) C4 (LTC4), LTD4, and LTE4, have been associated with specific age- and disease-related brain pathologies such as neuroinflammation, microglia/astroglia activation, neuronal damage, and increased blood-brain-barrier permeability (Michael et al., 2020a; Gelosa et al., 2017). In MS patients, levels of LTs in the CSF and urine are elevated as compared to control patients with non-inflammatory neurological disorders, in some cases approximately twice that of control patients (Neu et al., 2002; Haupts et al., 1992; Neu et al., 1992; Rosnowska et al., 1997). The enzyme 5-LOX plays a key role in the biosynthesis of pro-inflammatory LTs. Its expression is significantly increased in MS as well as in experimental autoimmune encephalomyelitis (EAE) mouse models of MS (Whitney et al., 2001). More specifically, gene microarray analysis has revealed an upregulation of the 5-LOX gene in brain lesions of patients with primary progressive MS (PPMS) and relapse remitting MS (RRMS) (Whitney et al., 2001; Arthur et al., 2008). In addition, immunohistochemistry analysis further corroborated these findings, highlighting predominant 5-LOX expression in macrophages within active and inactive MS lesions (Whitney et al., 2001). Furthermore, 5-LOX gene expression is elevated in peripheral blood cell samples from patients with RRMS during relapse and remission phases (Arthur et al., 2008).
The classical type 1 and type 2 CysLT receptors (CysLTR1 and CysLTR2) play an active role in most of the known CysLTs biological responses (Wang et al., 2020; Bäck et al., 2011; Bäck et al., 2014). Expression of LT receptors has been observed in multiple cell types of the CNS including astrocytes, neurons, endothelial cells and microglia spanning regions of the brain including the hippocampus, cortex, striatum and the substantia nigra (Peters-Golden and Henderson, 2007; Michael et al., 2020b; Marques et al., 2020; Wang et al., 2020; Gelosa et al., 2017). The CysLTR1 and CysLTR2 are upregulated in immune tissues, in serum, in cerebrospinal fluid (CSF), and in CNS tissue of EAE mice following disease onset and in MS patients (Wang et al., 2011; Han et al., 2021). Remarkably, infiltrating CD4+ cells, microglia and astrocytes within MS lesions express high levels of CysLTR1, mirroring similar observations as those in the EAE mice (Han et al., 2021).
Beside the classical receptors described above, other receptors involved in the leukotriene activation pathway include GPR99 (α-ketoglutarate receptor), P2Y12 (adenosine-diphosphate receptor) and GPR17 (uracil nucleotide P2Y-like receptor) (Marques et al., 2020). GPR17 was among the first leukotriene receptor with a putative role in MS. Initially identified as an orphan receptor, GPR17 turned out to be phylogenetically similar to CysLT receptors and to be activated and responsive to ligands such as uracil nucleotides (such as UDP, UDP-galactose and UDP-glucose) as well as to CysLTs (Ciana et al., 2006; Fumagalli et al., 2017; Lecca et al., 2020; Fumagalli et al., 2016; Dziedzic et al., 2020). Indeed, following trauma or disease such as acute ischemia and stroke, brain and spinal cord injuries, and demyelinating diseases such as MS, GPR17 expression is upregulated in neurons, microglia/macrophages, and most importantly in the context of de- and remyelination, in oligodendrocyte precursors (Ciana et al., 2006; Fumagalli et al., 2017; Lecca et al., 2020; Zhao B et al., 2018; Chen et al., 2009).
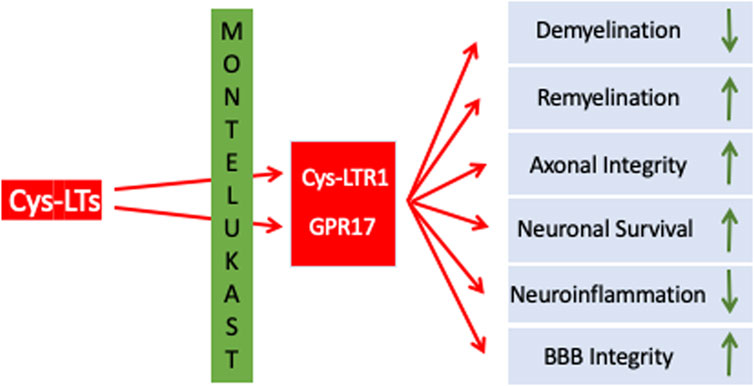
GPR17 is abundantly expressed in the CNS, including the frontal cortex, striatum, brainstem, and medulla (Ceruti et al., 2010; Marucci et al., 2016). Within these brain regions GPR17 is expressed along the oligodendrocyte (OL) lineage. In remyelination, which is a constantly ongoing and physiological process in the adult CNS, OLs derive from oligodendroglial progenitors (OPCs) which are present throughout the CNS. In MS, particularly in more advanced stages of disease, remyelination is impaired, resulting in insufficient oligodendrogenesis and failure to remyelinate adequately. In consequence, changes in electrical impulse transmission, axonal damage and neurodegeneration progresses further without phases of symptomatic relief and recovery (Lecca et al., 2020; Dziedzic et al., 2020; Traiffort et al., 2020). In chronic MS lesions, proliferating OPCs were observed to have remained in situ without further differentiation into mature OLs (Kuhlmann et al., 2008). OPCs as well as OLs express GPR17 which is involved in regulating OPCs’ proliferation, differentiation, and migration. Actually, activation of GPR17 prevents OPC differentiation, proliferation and migration, i.e., GPR17 functions as a negative regulator for myelination. In plaques of MS patients GPR17 expression is higher compared to those from white matter from non-neurological donor samples and normal-appearing white matter (Chen et al., 2009). Most importantly, GPR17 overexpression in transgenic mice and GPR17 knockout mice demonstrated that GPR17 negatively regulates oligodendrocyte differentiation and myelination in vitro and in vivo in conditions of EAE. Therefore, inhibition of GPR17 seems to be a promising approach to promote remyelination. In summary, LTs as well as LTRs have been identified as therapeutic targets in MS. While the classical CysLTRs mediate neuroinflammation and related processes, GPR17 appears to be a roadblock in remyelination/regeneration. Targeting CysLTRs and GPR17 might simultaneously reduce inflammation and promote remyelination (Table 1 an Figure 1).
Effects of leukotriene receptor antagonists in MS and in neurodegenerative diseases
Evidence for pharmacological leukotriene receptor blockade as a potential therapy in MS comes from studies using small molecule leukotriene receptor antagonists such as montelukast (MTK), pranlukast or zafirlukast. MTK is a selective and orally active leukotriene receptor antagonist that inhibits CysLTR1 as well as GPR17. It received FDA approval on February 1998 for several indications including the prophylaxis and chronic treatment of asthma, prevention of exercise-induced bronchoconstriction, and for the relief of symptoms of seasonal and perennial allergic rhinitis in adults and pediatric patients. Notably, MTK boasts an extensive safety and efficacy profile for asthma treatment, with over 25 years of clinical use and approval in more than 80 countries worldwide. The CysLTR1 antagonists zafirlukast and pranlukast act similarly to MTK, and have also FDA approval. Nevertheless, most of the studies exploring a potential effect in the CNS have been conducted using MTK.
With relevance to MS, MTK administration in mice reduced CysLTR1 mediated effects such as CNS infiltration of inflammatory cells such as Th1 and Th17, demyelination, BBB permeability, and clinical symptoms associated with EAE. Remarkably, MTK showed therapeutic efficacy even when given at the progressive stage of the disease in the EAE mice, suggesting its potential utility both as a prophylactic and as a therapeutic intervention (Wang et al., 2011). In another study, treatment of EAE mice with MTK resulted in a notable suppression or delay of EAE development both prophylactically and therapeutically, accompanied by inhibition of myelin loss (Han et al., 2021). Furthermore, inflammation in MTK treated EAE mice was suppressed as demonstrated by significant decreases in the percentages of inflammatory cells in the CNS, i.e., CD4+ IFN-γ+ (Th1), CD4+ IL-17+ (Th17) and granulocyte–macrophage colony-stimulating factor CD4+ GM-CSF+. This reduction corresponded with decreased concentrations of IFN-γ, IL-17 and GM-CSF cytokines. Th17 cells, critical in the pathogenesis of both EAE and MS, produce IFN-γ, IL-17 and GM-CSF cytokines, contributing to astrocytes and microglial activation, and subsequent cytokine and chemokine secretion. This cascade influences the recruitment of peripheral immune cells to lesion sites, exacerbating attacks on the CNS (Dendrou et al., 2015; Han et al., 2021; Zhang et al., 2018).
MTK as well as pranlukast also atangonize GPR17 and might reduce pathology and promote regeneration (Hennen et al., 2013; Ciana et al., 2006; Braune et al., 2021). For example, in an amyotrophic lateral sclerosis (ALS) mouse model, treatment with MTK blocked GPR17 and increased differentiation of OPCs from spinal cord samples (Bonfanti et al., 2020). We (Marschallinger et al., 2015) showed that MTK is able to reduce neuroinflammation, restore BBB integrity, increase neurogenesis and improve cognitive performance in aged rats. The stimulating effect on neurogenesis was apparently mediated by inhibition of GPR17. Furthermore, we (Michael et al., 2021) analyzed the effects of MTK on cognition, inflammation, and hippocampal gene expression in 5xFAD mice, a commonly used transgenic AD mouse model. MTK was highly effective in reducing the effects of neuroinflammation, through modulation of microglia phenotypes and the impairment of CD8+ T-cell infiltration. In addition, MTK was effective in improving cognitive outcomes in the AD mice. Importantly, the effects observed both in terms of neuroinflammation and cognition were dose-dependent with the higher dose being more effective. Likewise, MTK reduces alpha-synuclein load and restores memory in an animal model of dementia with Lewy Bodies (Marschallinger et al., 2020). Also, in an animal model of stroke, MTK reduced the size of ischemic lesions, improved axonal fiber connectivity and remyelination. It improved OPC recruitment and proliferation during the acute phase of damage, and increased their differentiation to mature oligodendrocytes at the chronic phase after stroke (Gelosa et al., 2019).
Clinical development and repurposing of Montelukast as a therapeutic for MS and neurodegenerative diseases
We have recently developed and manufactured a novel mucoadhesive montelukast film for buccal application (Montelukast VersaFilm®) (Michael et al., 2020a). The MTK film is based on a versatile drug delivery platform technology that enables the development of oral thin films exhibiting improved product performance, such as rapid disintegration of the delivery vehicle without the need for water, fast buccal or sublingual absorption, the potential for faster onset and/or higher bioavailability, ease of administration for patients with swallowing difficulties, pleasant taste, and individual packaging for improved safety and convenience. MTK VersaFilm® was initially developed as an oral mucoadhesive film to circumvent the limitations of MTK in its tablet form, such as inconsistent solubility, uptake, and bioavailability. Formulating MTK as a buccally applied film is advantageous with respect to its ease of use, especially for patients suffering from dysphagia, such as elderly patients, patients with dementia, and patients that require intubation or ventilation. For clinical testing, we developed and manufactured two dosage strengths, a 10-mg and a 30-mg film. The film is required to be placed on the inner cheek mucosa and then allowed to adhere for several seconds; complete dissolution of the film occurs in less than 5 min.
A Phase 1 and Phase 2a study was conducted with the MTK VersaFilm® with either of the two dosages a 10-mg and a 30-mg film (Michael et al., 2020a). The Phase 1 bioavailability study in healthy volunteers compared a 10-mg tablet formulation to the 10-mg buccal film (Michael et al., 2020b). The currently ongoing Phase 2a study compares the effects of several daily doses of MTK (10-mg and 60-mg) to placebo in patients with mild to moderate Alzheimer’s disease (NCT03402503). Phase 1a study results established that the MTK film demonstrated a significantly improved bioavailability compared to the marketed tablet. For equivalent drug loadings (10 mg), MTK mucoadhesive film exhibited approximately 50% better bioavailability compared to MTK tablets. As part of the study, we also conducted a quantitative analysis of the MTK levels in the CSF and found measurable amounts of MTK present in the CSF at the 3.0- and 7.0-h time points after test drug administration (Michael et al., 2020b).
The purpose of the Phase 2a “Proof of Concept” study is to evaluate the safety and effectiveness of the Montelukast VersaFilm® in combination with current established “Standard of Care” pharmacotherapy (acetylcholinesterase inhibitors - AChEIs) in patients with mild to moderate AD-associated dementia. The Phase 2a “Proof of Concept” study, ending in Q2 2024, will evaluate the effect on cognition and functional daily living in patients with mild to moderate AD dementia treated for 26 weeks with MTK VersaFilm® along with AChEIs versus patients treated with placebo and AChEIs. The Montelukast VersaFilm® is currently also being evaluated in a Swedish Phase 2 Parkinson’s disease (PD) study (MONTPARK - EudraCT Number: 2023–504278–39–00). The overall purpose of the study is to investigate the safety and efficacy of high oral doses of MTK on PD progression in the early to moderate phase of the disease. The study design is a randomized, double-blind, placebo-controlled, parallel arm, multicenter trial to examine the safety and efficacy of buccal films of MTK 30-mg taken twice daily compared to placebo film taken twice daily. Evaluable patients on the study will be treated for 18 months with either MTK or placebo and then undergo a 3-month washout period. Additional evaluations to be conducted as part of the study are to include evaluation of motor and non-motor functions, changes in dopaminergic treatment, safety, and plasma and CSF levels of inflammatory, leukotriene and neurodegeneration markers as well as MTK. Most importantly in the context of MS, a recent retrospective case-control study suggests that MTK reduces relapses in patients with MS (Manuel et al., 2024). This study used two large datasets from a total of 118,642 people, with 691 of them being adherent on MTK. Compared to the controls, MTK users showed a 58.3% reduction in the annualized relapse rate in one cohort, and a 13.4% reduction in the other cohort. This real-world evidence suggests that MTK reduced MS relapses, arguing for future clinical testing of the efficacy of MTK in MS patients in a randomized placebo-controlled trial.
Summary and conclusion
MS, one of the many neurodegenerative diseases afflicting humans, is a complex phenomenon for which many of the underlying mechanisms and players have not been fully elucidated. Recommendations to date are to initiate early treatment of the MS patient in order to maximize the efficacy of the currently available therapies (Fumagalli et al., 2016). Most of these current available FDA-approved therapies for MS consist of immunomodulating and immunosuppressive approaches acting against the inflammatory and immune-mediated components of the disease (Melchor et al., 2019). Although many of these approved treatments shorten the duration of relapse and delay progression to secondary phase of MS, what is suboptimal or predominately lacking is the ability to promote CNS repair, i.e., remyelination (Melchor et al., 2019). Immunomodulation and immunosuppression cannot be solely counted on as an effective means to reverse or prevent remyelination failure to prevent the progression of MS to a state of permanent disability (Maciak et al., 2023). Remyelination is key to preventing progressive axonal injury and reducing long-term disability in the MS patients. OPCs are key to the remyelination process and an understanding of their involvement in all the phases of remyelination, i.e., OPC migration and proliferation, differentiation, OL maturation and OL survival are to be considered (Melchor et al., 2019).
Although a huge body of research is currently investigating various promising approaches to enhance remyelination in MS such as: replacement therapies, stem cell therapy, gene therapy, drugs stimulating growth factor production, immunomodulatory therapies and small-molecule therapies targeting specific signaling pathways that can promote remyelination, many of these strategies are far from immediate use in the clinical setting. The use of MTK warrants its inclusion in the current MS armamentarium given its inherent properties through its antagonistic effect on CysLT- and GPR17-receptors, which play a key influential role on OPC and microglia. MTK has been used for close to 25 years and its safety profile database in humans is extensive and well documented. Use of a buccal film of MTK, such as the one developed by us, provides additional advantages over the tablet formulation in terms of ease of use and greater bioavailability of up to 50% greater than that of the tablet formulation. An additional positive feature of utilizing a MTK buccal film is its use in MS patients with dysphagia. Dysphagia is a major disorder in MS patients and is defined as the difficulty in swallowing function. Several systematic reviews and meta-analyses have shown the prevalence of dysphagia in MS patients to be approximately 45% (Ansari et al., 2020; Mirmosayyeb et al., 2023), so the use of a mucoadhesive buccally applied film would be advantageous in this MS patient population. Lastly, future clinical studies are warranted to further evaluate the effect of a buccally applied MTK film in patients afflicted with MS as an adjuvant to currently approved therapy regimens. Although current therapies provide a mitigation strategy in terms of shortening the duration of relapse and delaying progression of the disease, most are suboptimal in their ability to promote CNS repair, i.e., remyelination; the use of MTK should be considered as part of the treatment regimen so as to delay or prevent progressive axonal injury and reduce long-term disability in the MS patient (Figure 1).
Author contributions
FP: Writing–original draft, Writing–review and editing. AS: Writing–review and editing. HZ: Writing–review and editing. PS: Writing–review and editing. LA: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
FP, AS, and HZ are employees at IntelgenX corp., which holds patents in the use of montelukast in neurodegenerative diseases.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ansari, N. N., Tarameshlu, M., and Ghelichi, L. (2020). Dysphagia in multiple sclerosis patients: diagnostic and evaluation strategies. Degener. Neurol. Neuromuscul. Dis. 10, 15–28. doi:10.2147/DNND.S198659
Arthur, A. T., Armati, P. J., Bye, C., Heard, R. N., Stewart, G. J., Pollard, J. D., et al. (2008). Genes implicated in multiple sclerosis pathogenesis from consilience of genotyping and expression profiles in relapse and remission. BMC Med. Genet. 9, 17. doi:10.1186/1471-2350-9-17
Bäck, M., Dahlén, S. E., Drazen, J. M., Evans, J. F., Serhan, C. N., Shimizu, T., et al. (2011). International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol. Rev. 63 (3), 539–584. doi:10.1124/pr.110.004184
Bäck, M., Powell, W. S., Dahlén, S. E., Drazen, J. M., Evans, J. F., Serhan, C. N., et al. (2014). Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br. J. Pharmacol. 171 (15), 3551–3574. doi:10.1111/bph.12665
Bonfanti, E., Bonifacino, T., Raffaele, S., Milanese, M., Morgante, E., Bonanno, G., et al. (2020). Abnormal upregulation of GPR17 receptor contributes to oligodendrocyte dysfunction in SOD1 G93A mice. Int. J. Mol. Sci. 21 (7), 2395. doi:10.3390/ijms21072395
Braune, M., Scherf, N., Heine, C., Sygnecka, K., Pillaiyar, T., Parravicini, C., et al. (2021). Involvement of GPR17 in neuronal fibre outgrowth. Int. J. Mol. Sci. 22 (21), 11683. doi:10.3390/ijms222111683
Campbell, G., and Mahad, D. (2018). Neurodegeneration in progressive multiple sclerosis. Cold Spring Harb. Perspect. Med. 8 (10), a028985. doi:10.1101/cshperspect.a028985
Ceruti, S., Viganò, F., Boda, E., Ferrario, S., Magni, G., Boccazzi, M., et al. (2010). Expression of the new P2Y-like receptor GPR17 during oligodendrocyte precursor cell maturation regulates sensitivity to ATP-induced death. Glia 59 (3), 363–378. doi:10.1002/glia.21107
Chen, Y., Wu, H., Wang, S., Koito, H., Li, J., Ye, F., et al. (2009). The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12 (11), 1398–1406. doi:10.1038/nn.2410
Chiaravalloti, N. D., and DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. Lancet Neurol. 7 (12), 1139–1151. doi:10.1016/S1474-4422(08)70259-X
Ciana, P., Fumagalli, M., Trincavelli, M. L., Verderio, C., Rosa, P., Lecca, D., et al. (2006). The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25 (19), 4615–4627. doi:10.1038/sj.emboj.7601341
Compston, A., and Coles, A. (2008). Multiple sclerosis. Lancet 372 (9648), 1502–1517. doi:10.1016/S0140-6736(08)61620-7
Davis, S. L., Wilson, T. E., White, A. T., and Frohman, E. M. (2010). Thermoregulation in multiple sclerosis. J. Appl. Physiol. (1985) 109 (5), 1531–1537. doi:10.1152/japplphysiol.00460.2010
Dendrou, C. A., Fugger, L., and Friese, M. A. (2015). Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15 (9), 545–558. doi:10.1038/nri3871
Dighriri, I. M., Aldalbahi, A. A., Albeladi, F., Tahiri, A. A., Kinani, E. M., Almohsen, R. A., et al. (2023). An overview of the history, pathophysiology, and pharmacological interventions of multiple sclerosis. Cureus 15 (1), e33242. doi:10.7759/cureus.33242
Dziedzic, A., Miller, E., Saluk-Bijak, J., and Bijak, M. (2020). The GPR17 receptor-A promising goal for therapy and a potential marker of the neurodegenerative process in multiple sclerosis. Int. J. Mol. Sci. 21 (5), 1852. doi:10.3390/ijms21051852
Filippi, M., Bar-Or, A., Piehl, F., Preziosa, P., Solari, A., Vukusic, S., et al. (2018). Multiple sclerosis. Nat. Rev. Dis. Prim. 4 (1), 43. doi:10.1038/s41572-018-0041-4
Fumagalli, M., Lecca, D., and Abbracchio, M. P. (2016). CNS remyelination as a novel reparative approach to neurodegenerative diseases: the roles of purinergic signaling and the P2Y-like receptor GPR17. Neuropharm 104, 82–93. doi:10.1016/j.neuropharm.2015.10.005
Fumagalli, M., Lecca, D., Coppolino, G. T., Parravicini, C., and Abbracchio, M. P. (2017). Pharmacological properties and biological functions of the GPR17 receptor, a potential target for neuro-regenerative medicine. Adv. Exp. Med. Biol. 1051, 169–192. doi:10.1007/5584_2017_92
Gelosa, P., Bonfanti, E., Castiglioni, L., Delgado-Garcia, J. M., Gruart, A., Fontana, L., et al. (2019). Improvement of fiber connectivity and functional recovery after stroke by montelukast, an available and safe anti-asthmatic drug. Pharmacol. Res. 142, 223–236. doi:10.1016/j.phrs.2019.02.025
Gelosa, P., Colazzo, F., Tremoli, E., Sironi, L., and Castiglioni, L. (2017). Cysteinyl leukotrienes as potential pharmacological targets for cerebral diseases. Mediat. Inflamm. 2017, 3454212. doi:10.1155/2017/3454212
Han, B., Zhang, Y. Y., Ye, Z. Q., Xiao, Y., Rasouli, J., Wu, W. C., et al. (2021). Montelukast alleviates inflammation in experimental autoimmune encephalomyelitis by altering Th17 differentiation in a mouse model. Immunology 163 (2), 185–200. doi:10.1111/imm.13308
Haupts, M., Smektala, K., Finkbeiner, T., Simmet, T., and Gehlen, W. (1992). Immunoreactive leukotriene C4 levels in CSF of MS patients. Acta Neurol. Scand. 85 (5), 365–367. doi:10.1111/j.1600-0404.1992.tb04062.x
Hennen, S., Wang, H., Peters, L., Merten, N., Simon, K., Spinrath, A., et al. (2013). Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci. Signal 6 (298), ra93. doi:10.1126/scisignal.2004350
Heß, K., Starost, L., Kieran, N. W., Thomas, C., Vincenten, M. C. J., Antel, J., et al. (2020). Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 140 (3), 359–375. doi:10.1007/s00401-020-02189-9
Kuhlmann, T., Miron, V., Cui, Q., Wegner, C., Antel, J., Brück, W., et al. (2008). Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131 (Pt 7), 1749–1758. Epub 2008 May 30. Erratum in: Brain. 2009 Apr;132(Pt 4):1118. Cuo, Q [corrected to Cui, Q]. doi:10.1093/brain/awn096
Lassmann, H. (2019). Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9, 3116. doi:10.3389/fimmu.2018.03116
Lecca, D., Raffaele, S., Abbracchio, M. P., and Fumagalli, M. (2020). Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 68 (10), 1957–1967. doi:10.1002/glia.23807
Maciak, K., Dziedzic, A., and Saluk, J. (2023). Remyelination in multiple sclerosis from the miRNA perspective. Front. Mol. Neurosci. 16, 1199313. doi:10.3389/fnmol.2023.1199313
Mahad, D. H., Trapp, B. D., and Lassmann, H. (2015). Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14 (2), 183–193. doi:10.1016/S1474-4422(14)70256-X
Manuel, A. M., Gottlieb, A., Freeman, L., and Zhao, Z. (2024). Montelukast as a repurposable additive drug for standard-efficacy multiple sclerosis treatment: emulating clinical trials with retrospective administrative health claims data. Mult. Scler. 30 (6), 696–706. doi:10.1177/13524585241240398
Marques, C. F., Marques, M. M., and Justino, G. C. (2022). Leukotrienes vs. Montelukast-activity, metabolism, and toxicity hints for repurposing. Pharm. (Basel) 15 (9), 1039. doi:10.3390/ph15091039
Marschallinger, J., Altendorfer, B., Rockenstein, E., Holztrattner, M., Garnweidner-Raith, J., Pillichshammer, N., et al. (2020). The leukotriene receptor antagonist montelukast reduces alpha-synuclein load and restores memory in an animal model of dementia with Lewy Bodies. Neurotherapeutics 17 (3), 1061–1074. doi:10.1007/s13311-020-00836-3
Marschallinger, J., Schäffner, I., Klein, B., Gelfert, R., Rivera, F. J., Illes, S., et al. (2015). Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 6, 8466. doi:10.1038/ncomms9466
Marucci, G., Dal Ben, D., Lambertucci, C., Santinelli, C., Spinaci, A., Thomas, A., et al. (2016). The G protein-coupled receptor GPR17: overview and update. ChemMedChem 11 (23), 2567–2574. doi:10.1002/cmdc.201600453
Melchor, G. S., Khan, T., Reger, J. F., and Huang, J. K. (2019). Remyelination pharmacotherapy investigations highlight diverse mechanisms underlying multiple sclerosis progression. ACS Pharmacol. Transl. Sci. 2 (6), 372–386. doi:10.1021/acsptsci.9b00068
Michael, J., Bessa de Sousa, D., Conway, J., Gonzalez-Labrada, E., Obeid, R., Tevini, J., et al. (2020b). Improved bioavailability of montelukast through a novel oral mucoadhesive film in humans and mice. Pharmaceutics 13 (1), 12. doi:10.3390/pharmaceutics13010012
Michael, J., Unger, M. S., Poupardin, R., Schernthaner, P., Mrowetz, H., Attems, J., et al. (2020a). Microglia depletion diminishes key elements of the leukotriene pathway in the brain of Alzheimer’s Disease mice. acta neuropathol. Commun. 8, 129. doi:10.1186/s40478-020-00989-4
Michael, J., Zirknitzer, J., Unger, M. S., Poupardin, R., Rieß, T., Paiement, N., et al. (2021). The leukotriene receptor antagonist montelukast attenuates neuroinflammation and affects cognition in transgenic 5xFAD mice. Int. J. Mol. Sci. 22 (5), 2782. doi:10.3390/ijms22052782
Mirmosayyeb, O., Ebrahimi, N., Shekarian, A., Afshari-Safavi, A., Shaygannejad, V., Barzegar, M., et al. (2023). Prevalence of dysphagia in patients with multiple sclerosis: a systematic review and meta-analysis. J. Clin. Neurosci. 108, 84–94. doi:10.1016/j.jocn.2023.01.006
Neu, I., Mallinger, J., Wildfeuer, A., and Mehlber, L. (1992). Leukotrienes in the cerebrospinal fluid of multiple sclerosis patients. Acta Neurol. Scand. 86 (6), 586–587. doi:10.1111/j.1600-0404.1992.tb05491.x
Neu, I. S., Metzger, G., Zschocke, J., Zelezny, R., and Mayatepek, E. (2002). Leukotrienes in patients with clinically active multiple sclerosis. Acta Neurol. Scand. 105 (1), 63–66. doi:10.1034/j.1600-0404.2002.00070.x
Peters-Golden, M., and Henderson, W. R. (2007). Leukotrienes. N. Engl. J. Med. 357 (18), 1841–1854. doi:10.1056/NEJMra071371
Raffel, J., Wakerley, B., and Nicholas, R. (2016). Multiple sclerosis. Medicine. 44 (9), 537–541. doi:10.1016/j.mpmed.2016.06.005
Reich, D. S., Lucchinetti, C. F., and Calabresi, P. A. (2018). Multiple sclerosis. N. Engl. J. Med. 378 (2), 169–180. doi:10.1056/NEJMra1401483
Rosnowska, M., Cendrowski, W., and Sobczyk, W. (1997). Leukotrienes B4 and C4 in cerebrospinal of patients with multiple sclerosis. Pol. Merkur Lek. 2 (10), 254–255.
Sumowski, J. F., Benedict, R., Enzinger, C., Filippi, M., Geurts, J. J., Hamalainen, P., et al. (2018). Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology 90 (6), 278–288. doi:10.1212/WNL.0000000000004977
Traiffort, E., Kassoussi, A., Zahaf, A., and Laouarem, Y. (2020). Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Front. Cell Neurosci. 14, 79. doi:10.3389/fncel.2020.00079
Wang, L., Du, C., Lv, J., Wei, W., Cui, Y., and Xie, X. (2011). Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 187 (5), 2336–2345. doi:10.4049/jimmunol.1100333
Wang, Y., Yang, Y., Zhang, S., Li, C., and Zhang, L. (2020). Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 87, 1–10. doi:10.1016/j.neurobiolaging.2019.12.013
Whitney, L. W., Ludwin, S. K., McFarland, H. F., and Biddison, W. E. (2001). Microarray analysis of gene expression in multiple sclerosis and EAE identifies 5-lipoxygenase as a component of inflammatory lesions. J. Neuroimmunol. 121 (1-2), 40–48. doi:10.1016/s0165-5728(01)00438-6
Zhang, Y., Han, J. J., Liang, X. Y., Zhao, L., Zhang, F., Rasouli, J., et al. (2018). miR-23b suppresses leukocyte migration and pathogenesis of experimental autoimmune encephalomyelitis by targeting CCL7. Mol. Ther. 26 (2), 582–592. doi:10.1016/j.ymthe.2017.11.013
Keywords: regeneration, restoration, drug development, remyelination, neuroinflammation
Citation: Pietrantonio F, Serreqi A, Zerbe H, Svenningsson P and Aigner L (2024) The leukotriene receptor antagonist montelukast as a potential therapeutic adjuvant in multiple sclerosis – a review. Front. Pharmacol. 15:1450493. doi: 10.3389/fphar.2024.1450493
Received: 17 June 2024; Accepted: 27 August 2024;
Published: 13 September 2024.
Edited by:
Panoraia Siafaka, European University Cyprus, CyprusReviewed by:
Davide Lecca, University of Milan, ItalyAndroulla Miliotou, University of Nicosia, Cyprus
Copyright © 2024 Pietrantonio, Serreqi, Zerbe, Svenningsson and Aigner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank Pietrantonio, ZnJhbmtAaW50ZWxnZW54LmNvbQ==; Ludwig Aigner, bHVkd2lnLmFpZ25lckBwbXUuYWMuYXQ=
 Frank Pietrantonio
Frank Pietrantonio Alex Serreqi
Alex Serreqi Horst Zerbe1
Horst Zerbe1 Per Svenningsson
Per Svenningsson Ludwig Aigner
Ludwig Aigner