- School of Basic Medical, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Astragalus membranaceus widely used in traditional Chinese medicine, exhibits multiple pharmacological effects, including immune stimulation, antioxidation, hepatoprotection, diuresis, antidiabetes, anticancer, and expectorant properties. Its main bioactive compounds include flavonoids, triterpene saponins, and polysaccharides. Astragalus polysaccharides (APS), one of its primary bioactive components, have been shown to possess a variety of pharmacological activities, such as antioxidant, immunomodulatory, anti-inflammatory, antitumor, antidiabetic, antiviral, hepatoprotective, anti-atherosclerotic, hematopoietic, and neuroprotective effects. This review provides a comprehensive summary of the molecular mechanisms and therapeutic effects of APS in treating neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS). It discusses how APS improve insulin resistance, reduce blood glucose levels, enhance cognitive function, and reduce Aβ accumulation and neuronal apoptosis by modulating various pathways such as Nrf2, JAK/STAT, Toll, and IMD. For PD, APS protect neurons and stabilize mitochondrial function by inhibiting ROS production and promoting autophagy through the PI3K/AKT/mTOR pathway. APS also reduce oxidative stress and neurotoxicity induced by 6-hydroxydopamine, showcasing their neuroprotective effects. In MS, APS alleviate symptoms by suppressing T cell proliferation and reducing pro-inflammatory cytokine expression via the PD-1/PD-Ls pathway. APS promote myelin regeneration by activating the Sonic hedgehog signaling pathway and fostering the differentiation of neural stem cells into oligodendrocytes. This article emphasizes the significant antioxidant, anti-inflammatory, immunomodulatory, and neuroprotective pharmacological activities of APS, highlighting their potential as promising candidates for the treatment of neurodegenerative diseases.
1 Introduction
Neurodegenerative diseases (NDDs) are a global public health issue and one of the leading causes of death and disability worldwide (Ayeni et al., 2022). Neurodegenerative diseases are a heterogeneous group of disorders characterized primarily by the progressive loss and degeneration of neurons within the central nervous system (CNS) or peripheral nervous system (PNS). These diseases typically present with a gradual decline in cognitive, motor, sensory, and behavioral functions, ultimately leading to severe disability and death. Major neurodegenerative diseases include Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease (Xing and Xu, 2021). Traditional Chinese medicine (TCM) has emerged as a promising approach, offering potential for the treatment of neurodegenerative diseases (Du et al., 2022).
AM (Figure 1) mainly consists of two varieties, A. membranaceus (Fisch.) Bunge var. Mongholicus (Bunge) P.K. Hsiao and A. membranaceus (Fisch.) Bunge var. (Hu et al., 2022). It is one of the most widely used traditional Chinese herbs, first recorded in the " Shen Nong’s Herbal Classic " (Wang et al., 2023) Astragalus membranaceus can be used as an immune stimulant, tonic, antioxidant, hepatoprotective agent, diuretic, antidiabetic agent, anticancer agent, and expectorant (Fu et al., 2014). The main active compounds obtained from AM include flavonoids, triterpene saponins, and polysaccharides, which exhibit a wide range of biological activities and pharmacological effects (Peng et al., 2023). Polysaccharides are natural macromolecular polymers composed of ten or more monosaccharides linked by glycosidic bonds to form linear or branched chains. They are widely distributed in nature and have attracted significant attention due to their diverse biological activities (Hou et al., 2020).
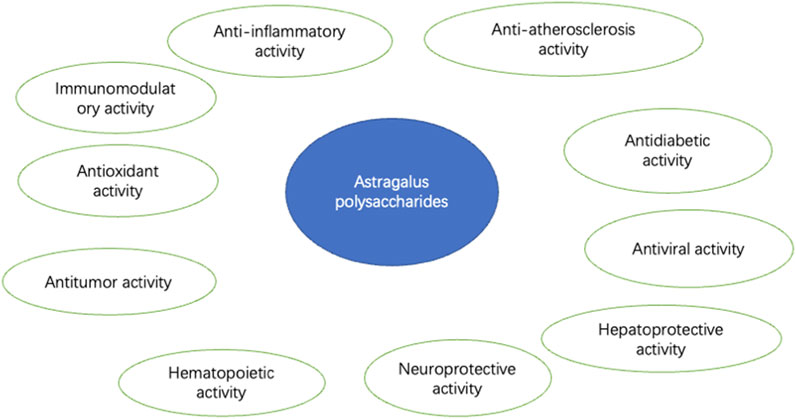
APS is one of the main bioactive components of AM, characterized by complex and diverse structures and multiple pharmacological activities, such as antioxidant effects (Niu et al., 2011). The main components of APS include heteropolysaccharides, neutral polysaccharides, glucans, and acidic polysaccharides, which are primarily linked by 1→4-glucosidic bonds (WANG, 2017). It has been demonstrated that APS possess various biological activities, including immunomodulatory, antioxidant, antitumor, antidiabetic, antiviral, hepatoprotective, anti-inflammatory, anti-atherosclerotic, hematopoietic, and neuroprotective effects (Chen et al., 2023). These findings not only indicate the therapeutic potential of APS in various diseases but also highlight their potential role in the development of new drugs.
This review comprehensively summarizes the latest advances in the molecular mechanisms and therapeutic effects of APS in the treatment of neurodegenerative diseases, enhancing the understanding of its therapeutic potential and recommending it as a candidate drug for the treatment of neurodegenerative diseases. These findings are of great significance for the development of effective drugs in the field of neurodegenerative disease treatment.
2 Overview of Astragalus membranaceus
AM is a leguminous plant widely distributed in China, Mongolia, and Korea, mainly growing in temperate and subtropical regions below 2000 m above sea level. AM is highly adaptable and usually grows on hillsides, grasslands, and forest edges (Auyeung et al., 2016). AM is a perennial herbaceous plant, about 50–150 cm tall, with a stout, cylindrical root covered with a yellow-brown outer skin and yellow-white inner part. The stem is upright, and the leaves are pinnately compound, with leaflets ranging from elliptical to lanceolate. The inflorescence is a raceme, usually with light yellow flowers, and the fruit is a pod. AM, as a traditional Chinese medicine, has been used for over 2000 years, first recorded in the “Shennong’s Classic of Materia Medica.” The primary medicinal part is the root, which has functions such as boosting qi to lift yang, benefiting the protective qi to consolidate the exterior, promoting diuresis to alleviate edema, and detoxifying to promote tissue regeneration. AM contains abundant chemical components, including flavonoids, saponins, polysaccharides, amino acids, and trace elements, which endow it with various pharmacological effects, such as immunomodulatory, antioxidant, anti-inflammatory, and anti-fatigue effects (Hou et al., 2023). To date, approximately 404 chemical constituents have been isolated and identified from Astragalus membranaceus, including saponins, flavonoids, phenylpropanoids, alkaloids, and other chemical compounds. APS can be further classified into water-soluble α-(1–4) (Fu et al., 2014; Xing and Xu, 2021; Ayeni et al., 2022; Du et al., 2022; Hu et al., 2022; Wang et al., 2023) glucan and water-insoluble α-(1–4) glucan (Liu et al., 2024).AM is widely used to enhance immunity, delay aging, lower blood sugar, protect the cardiovascular system, and fight tumors (Wang et al., 2019; Dong et al., 2023).
3 General biological activities of APS
APS(molecular formula: C10H7ClN2O2S, relative molecular mass:254.7) (Figure 2), is a bioactive component extracted from AM, appearing as a white or slightly yellowish powder. APS is primarily composed of fructose, rhamnose, arabinose, hexuronic acid, glucose, galacturonic acid, and glucuronic acid (Ren et al., 2023). APS is soluble in water but insoluble in alcohol and has good hygroscopicity (LI et al., 2009). Polysaccharides from Astragalus membranaceus are commonly extracted using methods such as cold water extraction, enzyme-assisted extraction, homogenization-assisted negative pressure cavitation extraction, and pressurized liquid extraction. The constituents of APS contain various monosaccharide residues and impurities. Current studies indicate that purification and separation methods may more or less affect the activity of the polysaccharides. A common method employed is column chromatography (Tang and Huang, 2022). APS exhibits a variety of biological activities (Figure 3), such as immunomodulatory, antioxidant, antitumor, antidiabetic, antiviral, hepatoprotective, anti-inflammatory, anti-atherosclerotic, hematopoietic, and neuroprotective effects.
APS can regulate the activity of various immune cells, including macrophages, natural killer cells, dendritic cells, T lymphocytes, B lymphocytes, and microglial cells. APS can induce these immune cells to produce various cytokines and chemokines, thereby enhancing the immune response (Li et al., 2022). APS can inhibit the proliferation, migration, and invasion of tumor cells. Liu et al. found that APS-induced cell cycle arrest at the G0/G1 phase in bladder cancer UM-UC-3 cells through inhibition of the JAK2/STAT3 pathway, thereby suppressing cell proliferation (Liu HW. et al., 2023). APS also exhibits antifibrotic effects by inhibiting the production of TGF-β1, thereby treating systemic sclerosis fibrotic diseases in a murine model induced by bleomycin (Hao et al., 2015). Diabetes is a serious chronic endocrine/metabolic disorder, and APS may reduce blood glucose and enhance insulin sensitivity in type 2 diabetes patients by alleviating endoplasmic reticulum stress. The mechanisms may include reducing ATF6 activation induced by endoplasmic reticulum stress, reversing intracellular ATF6 translocation, and inhibiting the high expression of certain protein phosphatases. These changes alleviate endoplasmic reticulum stress in the liver, thereby enhancing insulin sensitivity (Wang et al., 2009).
4 Pharmacokinetics of APS
Research on the pharmacokinetics of Astragalus Polysaccharides (APS) is relatively limited. APS is primarily absorbed through passive pathways in vivo, but its absorption efficiency is relatively low. The main reasons for this are its large molecular weight, low lipophilicity, and poor intestinal permeability (Chen et al., 2011). To enhance its bioavailability, researchers have explored improved drug delivery systems such as nanocarriers (Ghaffarian et al., 2016), which significantly increase the water solubility, stability, and biocompatibility of APS. Polysaccharides isolated from Astragalus are mostly extracted in the form of a white powder, and studies indicate that the relative molecular weight of APS ranges from 10 to 50 kDa (Jin et al., 2013). Additionally, moderate structural modifications can enhance the biological activity of APS, improving its therapeutic effects in vivo.
After oral or injectable administration, APS is primarily distributed in the liver, kidneys, spleen, lungs, and plasma, which are its main targets of action (Li et al., 2022). Some studies suggest that APS can improve the permeability of the blood-brain barrier, enhancing its distribution in the brain (Jia et al., 2022), and providing a potential advantage for its application in neurological diseases. APS is mainly metabolized by intestinal microbiota, with its metabolites including low molecular weight polysaccharides and monosaccharides, which can be further absorbed and utilized by the body. The main metabolic pathways include hydrolysis, glucuronidation, sulfation, and dehydrogenation.
Research indicates that APS has low toxicity at conventional doses, and no significant adverse reactions have been observed with long-term use (Chen et al., 2020). Early studies in Sprague-Dawley rats and Beagle dogs showed no histopathological or toxicological effects on body weight, organs, appetite, behavior, hematology, blood biochemistry, or urinalysis after prolonged oral administration (Yu et al., 2007). These studies of pharmacokinetic properties provide a scientific basis for the safety and efficacy of APS in clinical applications and offer important references for further optimizing its drug delivery systems and enhancing therapeutic effects.
5 Therapeutic effects and mechanisms of APS on neurodegenerative diseases
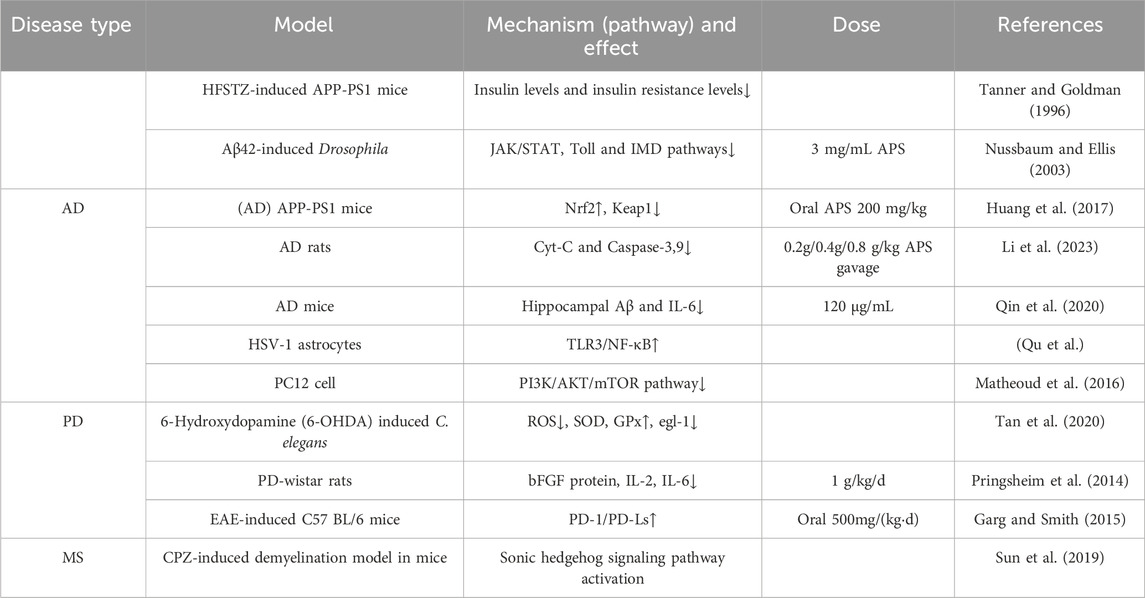
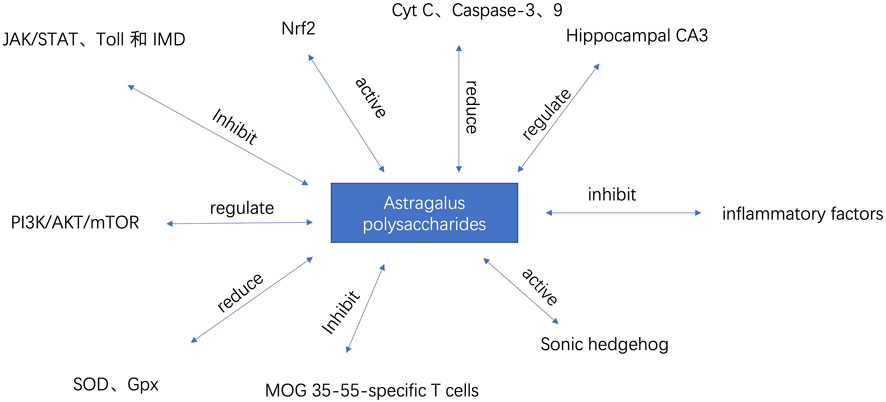
The prevalence and incidence of neurodegenerative diseases increase sharply with age (Checkoway et al., 2011). Major diseases include Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Alzheimer’s disease is the most common form of dementia worldwide, accounting for 60%–80% of all dementia cases, affecting approximately 24 million people globally (Mayeux and Stern, 2012). Idiopathic Parkinson’s disease (PD) is the second most common neurodegenerative disease after AD. It is estimated that the prevalence of PD in the general population is 0.3%, about 1% in people over 60 years old, and about 3% in people over 80 years old, with an incidence of 8–18 per 100,000 person-years (Tanner and Goldman, 1996; Nussbaum and Ellis, 2003). The therapeutic mechanism of APS for neurodegenerative diseases is shown in Table 1. Part of the mechanism of action of APS is shown in Figure 4
5.1 Alzheimer’s disease (AD)
The pathogenesis of AD is complex, and current treatments can only improve cognitive function and delay the degenerative process, with significant limitations. Epidemiological studies have shown that type 2 diabetes (T2DM) increases the risk of AD. The effects of APS in improving insulin resistance and lowering blood glucose can reduce the risk of AD from the source. In experiments with HFSTZ AD mice, APS reduced glucose intolerance induced by HFSTZ, and the elevated insulin levels (hyperinsulinemia) and insulin resistance (increased HOMA-IR) induced by HFSTZ were alleviated by APS (Huang et al., 2017). Aging increases the risk factors for neurodegenerative diseases such as AD and PD. In anti-aging experiments with fruit flies, APS significantly alleviated intestinal homeostasis imbalance, improved sleep disorders, delayed the occurrence of AD-like phenotypes in Aβ-related AD fruit flies, and rescued aging-induced changes in the JAK/STAT, Toll, and IMD pathways (Li et al., 2023).
APS can increase the expression of Nrf2 in the nucleus of mouse cells, reduce its expression in the cytoplasm, and restore the expression levels of Keap1, SOD, GSH-Px, and MDA. APS can significantly improve the cognitive ability of APP/PS1 mice by activating the Nrf2 pathway, reducing cell apoptosis and Aβ accumulation, thereby improving the physiological function of AD mice (Qin et al., 2020). APS can reduce cell apoptosis by downregulating the expression of Cyt C and Caspase-3、9, thus protecting neurons (Qu et al.). Studies have shown that after injecting Aβ into AD mice, the learning and memory abilities of the mice significantly improved. Pathological examination revealed that the organ index of the brain increased, the water content and Evans blue content in the brain tissue decreased, and the neuronal structure in the CA3 region of the hippocampus was relatively normal, with abundant organelles, clear nuclear membranes, and normal mitochondrial morphology (Fei et al., 2015). This indicates that APS has pharmacological activity in improving Alzheimer’s disease. When astrocytes are continuously stimulated by stress factors, they can promote the occurrence and development of neurodegenerative diseases by releasing inflammatory factors and free radicals. APS can not only directly inhibit the aggregation of disease proteins but also reduce the damage of herpes simplex virus to astrocytes (Shi et al., 2014). In conclusion, APS can not only improve insulin resistance and lower blood glucose but also fundamentally improve AD through pathways such as JAK/STAT, Toll, IMD, and Nrf2.
5.2 Parkinson’s disease (PD)
Parkinson’s disease (PD) initially affects multiple neurons, with later stages or progression primarily involving damage to the central nervous system (Kalia and Lang, 2015). Mitochondrial dysfunction is one of the main mechanisms of Parkinson’s disease. Protecting and repairing damaged neurons and mitochondria is a major approach to preventing and treating Parkinson’s disease (Pickrell and Youle, 2015; Matheoud et al., 2016). Autophagy is an important intracellular energy metabolism and self-renewal mechanism in eukaryotic cells, closely related to the occurrence and development of Parkinson’s disease (Heras-Sandoval et al., 2014). APS can promote the formation of autophagosomes within cells, increase the conversion of LC3-I protein to LC3-II protein, downregulate the expression of pAKT and p-mTOR proteins, and upregulate the expression of PTEN protein, thereby exerting anti-PD activity. APS can promote autophagy in PC12 cells through the PI3K/AKT/mTOR pathway, thereby promoting cell proliferation and exerting anti-PD effects (Tan et al., 2020). Clinical and epidemiological survey evidence suggests that the onset and progression of PD are closely related to the aging process (Pringsheim et al., 2014). Levodopa is an important drug for treating Parkinson’s disease, but it has neurotoxicity. 6-Hydroxydopamine (6-OHDA) is a neurotoxin that can induce Parkinson’s disease by causing the degeneration of dopaminergic neurons. APS can alleviate oxidative stress by reducing the levels of reactive oxygen species and malondialdehyde, increasing the activities of superoxide dismutase (SOD) and glutathione peroxidase (Gpx), and reducing the expression of the pro-apoptotic gene egl-1 in 6-OHDA-intoxicated Caenorhabditis elegans. APS can also increase acetylcholinesterase activity in 6-OHDA-induced models (Li et al., 2016). This study provides important evidence for the therapeutic potential of APS in neurodegenerative diseases. Research has shown that APS can reduce the number of rotations in Parkinson’s model rats, increase the content of tyrosine hydroxylase, reduce the expression of bFGF protein in the substantia nigra, and decrease the levels of inflammatory cytokines in brain tissue (Chen and Zhou, 2015). This suggests that APS may treat Parkinson’s disease by regulating the body’s immune function.
5.3 Multiple Sclerosis (MS)
Multiple sclerosis (MS) is an acquired disabling neurological disease affecting approximately 2.3 million people worldwide (World Health Organization, 2008). MS is an autoimmune inflammatory disease of the central nervous system characterized by demyelination and varying degrees of axonal loss (Garg and Smith, 2015). Its clinical manifestations include severe neurological dysfunction, such as ataxia, visual impairment, and aphasia, eventually leading to paralysis and even death (Dendrou and Fugger, 2017). Studies have found that APS can effectively alleviate EAE by inhibiting the proliferation of MOG 35-55-specific T cells and reducing the expression of pro-inflammatory cytokines. This is mediated by upregulating the PD-1/PD-Ls signaling pathway (Sun et al., 2019). This indicates that APS may have the potential to develop innovative drugs for treating multiple sclerosis. Currently, immunosuppressants and immunomodulators are primarily used to treat MS, which can alleviate symptoms and delay relapses but cannot effectively restore patients’ neurological functions (Wingerchuk and Carter, 2014). Current clinical research shows that the degree of myelin regeneration directly affects the recovery of neurological function and the improvement of quality of life. Therefore, promoting myelin regeneration is an important strategy for treating MS. In a demyelination mouse model, APS was found to downregulate the expression of Nestin and GFAP molecules at both transcriptional and protein levels, promote the expression of MBP molecules, and upregulate the expression of NeuN molecules, indicating that APS can promote the differentiation of neural stem cells into oligodendrocytes in vivo. This may be one of the mechanisms by which APS promotes myelin regeneration. Additionally, the study found that APS can activate the Sonic hedgehog signaling pathway both in vivo and in vitro, which may be the molecular mechanism by which APS regulates the differentiation of neural stem cells into oligodendrocytes (Ye et al., 2021).
6 Conclusion and future directions
APS are natural plant extracts that have been widely recognized for their pharmacological effects in neurodegenerative diseases. As discussed, APS exert neuroprotective effects through various biological pathways, including antioxidant, anti-inflammatory, immunomodulatory, anti-apoptotic, and neuroregenerative activities. Their specific mechanisms involve the regulation of multiple cell signaling pathways, such as PI3K/AKT/mTOR, JAK/STAT, and Nrf2, demonstrating significant therapeutic potential.
Despite the substantial progress made in APS research for neurodegenerative diseases, future studies need to delve deeper into several key areas. First, it is necessary to further elucidate the molecular targets and mechanisms of action of APS. Current research on the molecular targets and mechanisms of APS is predominantly focused on tumors and fibrosis (Bing et al., 2022; Liu WZ. et al., 2023; Sun et al., 2023), with limited studies in the field of neurodegenerative diseases. Comprehensive analysis using multi-omics technologies should be conducted to fully unravel these mechanisms and provide a theoretical basis. Second, most current research is still at the animal or cell experimental stage, lacking systematic clinical studies. Large-scale, multicenter randomized controlled clinical trials and long-term follow-up studies should be conducted to verify the efficacy and safety of APS in patients. Moreover, Astragalus polysaccharide solutions contain a considerable amount of impurities (Li et al., 2019), making the separation and purification process complex and time-consuming. Therefore, the purification and separation of natural products have always been a challenging aspect of the extraction process (Tang and Huang, 2022). Optimizing the extraction and purification techniques for Astragalus polysaccharides, as well as developing new drug delivery methods and formulation types, can improve their bioavailability and patient compliance. Personalized treatment is also an important direction for the future. By identifying biomarkers and applying precision medicine, individualized treatment plans can be developed to maximize efficacy. Lastly, as an extract of Astragalus, APS exhibits minimal toxic side effects. While exerting its pharmacological effects, precise dosage control is essential, requiring further in-depth investigation by researchers. Exploring the combined application of APS with existing drugs and integrated traditional Chinese and Western medicine treatment strategies will help enhance overall therapeutic effects and reduce side effects. Through these comprehensive studies, APS is expected to play a greater role in the treatment of neurodegenerative diseases.
Author contributions
YS: Writing–original draft, Writing–review and editing, Conceptualization, Resources. PM: Funding acquisition, Project administration, Resources, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC)717 [grant numbers, 81173365].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YZ declared a shared affiliation with the authors to the handling editor at the time of the review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Auyeung, K. K., Han, Q. B., and Ko, J. K. (2016). Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 44 (1), 1–22. doi:10.1142/S0192415X16500014
Ayeni, E. A., Aldossary, A. M., Ayejoto, D. A., Gbadegesin, L. A., Alshehri, A. A., Alfassam, H. A., et al. (2022). Neurodegenerative diseases: implications of environmental and climatic influences on neurotransmitters and neuronal hormones activities. Int. J. Environ. Res. Public Health 19 (19), 12495. Published 2022 Sep 30. doi:10.3390/ijerph191912495
Bing, P., Zhou, W., and Tan, S. (2022). Study on the mechanism of Astragalus polysaccharide in treating pulmonary fibrosis based on "Drug-Target-Pathway" network. Front. Pharmacol. 13, 865065. Published 2022 Mar 8. doi:10.3389/fphar.2022.865065
Checkoway, H., Lundin, J. I., and Kelada, S. N. (2011). Neurodegenerative diseases. IARC Sci. Publ. 163, 407–419.
Chen, G., Jiang, N., Zheng, J., Hu, H., Yang, H., Lin, A., et al. (2023). Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 241, 124386. doi:10.1016/j.ijbiomac.2023.124386
Chen, L., and Zhou, Q. Astragalus polysaccharide alleviates symptoms of rat with Parkinson’s disease. World Sci-Tech R&D 2015,37(02):168–171.doi:10.1016/j.ijbiomac.2020.02.282
Chen, M. C., Sonaje, K., Chen, K. J., and Sung, H. W. (2011). A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials 32 (36), 9826–9838. doi:10.1016/j.biomaterials.2011.08.087
Chen, Z. J., Liu, L. J., Gao, C. F., Chen, W. J., Vong, C. T., Yao, P. F., et al. (2020). Astragali Radix (Huangqi): a promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. doi:10.1016/j.jep.2020.112895
Dendrou, C. A., and Fugger, L. (2017). Immunomodulation in multiple sclerosis: promises and pitfalls. Curr. Opin. Immunol. 49, 37–43. Epub 2017 Sep 18. PMID: 28926740. doi:10.1016/j.coi.2017.08.013
Dong, M., Li, J., Yang, D., Li, M., and Wei, J. (2023). Biosynthesis and pharmacological activities of flavonoids, triterpene saponins and polysaccharides derived from Astragalus membranaceus. Molecules 28 (13), 5018. Published 2023 Jun 27. doi:10.3390/molecules28135018
Du, Y., Wan, H., Huang, P., Yang, J., and He, Y. (2022). A critical review of Astragalus polysaccharides: from therapeutic mechanisms to pharmaceutics. Biomed. Pharmacother. 147, 112654. doi:10.1016/j.biopha.2022.112654
Fei, H. X., Gao, Y., Sun, L. H., Lian, J., and Li, L. (2015). Effect of astragalus polysaccharides on the hippocampal tissue in Alzheimer’s disease mouse. Chin. J. Gerontology 35 (16), 4426–4429. doi:10.3969/j.issn.1005-9202
Fu, J., Wang, Z., Huang, L., Zheng, S., Wang, D., Chen, S., et al. (2014). Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 28 (9), 1275–1283. doi:10.1002/ptr.5188
Garg, N., and Smith, T. W. (2015). An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 5 (9), e00362. doi:10.1002/brb3.362
Ghaffarian, R., Herrero, E. P., Oh, H., Raghavan, S. R., and Muro, S. (2016). Chitosan-alginate microcapsules provide gastric protection and intestinal release of ICAM-1-targeting nanocarriers, enabling GI targeting in vivo. Adv. Funct. Mater 26 (20), 3382–3393. doi:10.1002/adfm.201600084
Hao, Z. F., Su, Y. M., Liu, J. Y., Wang, C. M., and Yang, R. Y. (2015). Astragalus polysaccharide suppresses excessive collagen accumulation in a murine model of bleomycin-induced scleroderma. Int. J. Clin. Exp. Med. 8 (3), 3848–3854. Published 2015 Mar 15.
Heras-Sandoval, D., Pérez-Rojas, J. M., Hernández-Damián, J., and Pedraza-Chaverri, J. (2014). The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26 (12), 2694–2701. doi:10.1016/j.cellsig.2014.08.019
Hou, C., Chen, L., Yang, L., and Ji, X. (2020). An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 153, 248–255. doi:10.1016/j.ijbiomac.2020.02.315
Hou, M., Leng, Y., Shi, Y., Tan, Z., and Min, X. (2023). Astragalus membranaceus as a drug candidate for inflammatory bowel disease: the preclinical evidence. Am. J. Chin. Med. 51 (6), 1501–1526. doi:10.1142/S0192415X23500684
Hu, P., Suriguga, Z. M., Chen, S., Wu, X., and Wan, Q. (2022). Transcriptional regulation mechanism of flavonoids biosynthesis gene during fruit development in astragalus membranaceus. Front. Genet. 13, 972990. Published 2022 Sep 6. doi:10.3389/fgene.2022.972990
Huang, Y. C., Tsay, H. J., Lu, M. K., Lin, C. H., Yeh, C. W., Liu, H. K., et al. (2017). Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int. J. Mol. Sci. 18 (12), 2746. Published 2017 Dec 18. doi:10.3390/ijms18122746
Jia, X., Xie, L., Liu, Y., Liu, T., Yang, P., Hu, J., et al. (2022). Astragalus polysaccharide (APS) exerts protective effect against acute ischemic stroke (AIS) through enhancing M2 micoglia polarization by regulating adenosine triphosphate (ATP)/purinergic receptor (P2X7R) axis. Bioengineered 13 (2), 4468–4480. doi:10.1080/21655979.2021.1980176
Jin, X., Dian, R. R., and Shen, S. M. (2013). Progress in the mechanism of Astragalus polysaccharide in the treatment of diabetes. Med. Recapitulate 19, 2026–2028. doi:10.3969/j.issn.1006-2084.2013.11.035
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386 (9996), 896–912. doi:10.1016/S0140-6736(14)61393-3
Li, C. X., Liu, Y., Zhang, Y. Z., Li, J. C., and Lai, J. (2022). Astragalus polysaccharide: a review of its immunomodulatory effect. Arch. Pharm. Res. 45 (6), 367–389. doi:10.1007/s12272-022-01393-3
Li, H., Shi, R., Ding, F., Wang, H., Han, W., Ma, F., et al. (2016). Astragalus polysaccharide suppresses 6-hydroxydopamine-induced neurotoxicity in Caenorhabditis elegans. Oxid. Med. Cell Longev. 2016, 4856761. doi:10.1155/2016/4856761
Li, S., Qi, Y., Ren, D., Qu, D., and Sun, Y. (2019). The structure features and improving effects of polysaccharide from Astragalus membranaceus on antibiotic-associated diarrhea. Antibiot. (Basel) 9 (1), 8. Published 2019 Dec 23. doi:10.3390/antibiotics9010008
Li, X., Yang, S., Wang, S., Shi, Y., Dai, Y., Zhang, X., et al. (2023). Regulation and mechanism of Astragalus polysaccharide on ameliorating aging in Drosophila melanogaster. Int. J. Biol. Macromol. 234, 123632. doi:10.1016/j.ijbiomac.2023.123632
Li, R., Chen, W. C., Wang, W. P., Tian, W. Y., and Zhang, X. G. (2009). Optimization of extraction technology of Astragalus polysaccharides by response surface methodology and its effect on CD40. Carbohydr. Polym. 78 (4), 784–788. doi:10.1016/j.carbpol.2009.06.018
Liu, H. W., Chen, Q. R., Xiong, H., Zhu, Y., and Zuo, L. (2023a). Astragalus polysaccharides inhabits proliferation, migration and invasion of bladder cancer Umuc3 cells by regulating the Jak2/Stat3 signaling pathway. J. Shanxi Med. Univ. (11), 1442–1448. doi:10.13753/j.issn.1007-6611.2023.11.002
Liu, W. Z., Yu, M. M., and Kang, M. (2023b). Study on the mechanism of Astragalus polysaccharides on cervical cancer based on network pharmacology. Comb. Chem. High. Throughput Screen 26 (8), 1547–1559. doi:10.2174/1386207326666230118121436
Liu, Y. X., Song, X. M., Dan, L. W., Tang, J. M., Jiang, Y., Deng, C., et al. (2024). Astragali Radix: comprehensive review of its botany, phytochemistry, pharmacology and clinical application. Arch. Pharm. Res. 47 (3), 165–218. doi:10.1007/s12272-024-01489-y
Matheoud, D., Sugiura, A., Bellemare-Pelletier, A., Laplante, A., Rondeau, C., Chemali, M., et al. (2016). Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell 166 (2), 314–327. doi:10.1016/j.cell.2016.05.039
Mayeux, R., and Stern, Y. (2012). Epidemiology of alzheimer disease. Cold Spring Harb. Perspect. Med. 2 (8), a006239. PMID: 22908189; PMCID: PMC3405821. doi:10.1101/cshperspect.a006239
Niu, Y., Wang, H., Xie, Z., Whent, M., Gao, X., Zhang, X., et al. (2011). Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge. var. mongolicus (Bge.) Hsiao. Food Chem. 128 (3), 620–626. doi:10.1016/j.foodchem.2011.03.055
Nussbaum, R. L., and Ellis, C. E. (2003). Alzheimer’s disease and Parkinson’s disease [published correction appears in N Engl J Med. 2003 Jun 19;348(25):2588]. N. Engl. J. Med. 348 (14), 1356–1364. doi:10.1056/NEJM2003ra020003
Peng, Y., Deng, X., Yang, S. S., Nie, W., and Tang, Y. D. (2023). Progress in mechanism of Astragalus membranaceus and its chemical constituents on multiple sclerosis. Chin. J. Integr. Med. 29 (1), 89–95. doi:10.1007/s11655-022-3535-6
Pickrell, A. M., and Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85 (2), 257–273. doi:10.1016/j.neuron.2014.12.007
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29 (13), 1583–1590. doi:10.1002/mds.25945
Qin, X., Hua, J., Lin, S. J., Zheng, H. T., Wang, J. J., Li, W., et al. (2020). Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway. Biochem. Biophys. Res. Commun. 531 (3), 431–437. doi:10.1016/j.bbrc.2020.07.122
Qu, W. Y., Xie, J. G., Liang, A. X., and Shang, X. F. Effect of Astragalus polysaccharide on neuronal activity cognitive function and Caspase-9 expression in Alzheimer’s disease rats (05),543–549.
Ren, C., Zhao, X., Liu, K., Wang, L., Chen, Q., Jiang, H., et al. (2023). Research progress of natural medicine Astragalus mongholicus Bunge in treatment of myocardial fibrosis. J. Ethnopharmacol. 305, 116128. doi:10.1016/j.jep.2022.116128
Shi, L., Yin, F., Xin, X., Mao, S., Hu, P., Zhao, C., et al. (2014). Astragalus polysaccharide protects astrocytes from being infected by HSV-1 through TLR3/NF-κB signaling pathway. Evid. Based Complement. Altern. Med. 2014, 285356. doi:10.1155/2014/285356
Sun, X., Zheng, Y., Tian, Y., Xu, Q., Liu, S., Li, H., et al. (2023). Astragalus polysaccharide alleviates alcoholic-induced hepatic fibrosis by inhibiting polymerase I and transcript release factor and the TLR4/JNK/NF-κB/MyD88 pathway. J. Ethnopharmacol. 314, 116662. doi:10.1016/j.jep.2023.116662
Sun, Y., Jing, Y., Huang, M., Ma, J., Peng, X., Wang, J., et al. (2019). The PD-1/PD-Ls pathway is up-regulated during the suppression of experimental autoimmune encephalomyelitis treated by Astragalus polysaccharides. J. Neuroimmunol. 332, 78–90. doi:10.1016/j.jneuroim.2019.03.019
Tan, Y., Yin, L., Sun, Z., Shao, S., Chen, W., Man, X., et al. (2020). Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. 153, 349–356. doi:10.1016/j.ijbiomac.2020.02.282
Tang, Z., and Huang, G. (2022). Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 150, 113015. doi:10.1016/j.biopha.2022.113015
Tanner, C. M., and Goldman, S. M. (1996). Epidemiology of Parkinson’s disease. Neurol. Clin. 14 (2), 317–335. doi:10.1016/S0733-8619(05)70259-0
Wang, J., Jia, J., Song, L., Gong, X., Xu, J., Yang, M., et al. (2019). Extraction, structure, and pharmacological activities of Astragalus polysaccharides. Appl. Sci. 9 (1), 122. doi:10.3390/app9010122
Wang, N., Zhang, D., Mao, X., Zou, F., Jin, H., and Ouyang, J. (2009). Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol. Cell Endocrinol. 307 (1-2), 89–98. doi:10.1016/j.mce.2009.03.001
Wang, P., Wang, Z., Zhang, Z., Cao, H., Kong, L., Ma, W., et al. (2023). A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus. Front. Pharmacol. 14, 1242318. Published 2023 Aug 23. doi:10.3389/fphar.2023.1242318
Wang, Y. (2017). Study on standardization method of Astragalus polysaccharide reference substance. Chin. Traditional Herb. Drugs, 4897–4903. doi:10.7501/j.issn.0253-2670.2017.23.013
Wingerchuk, D. M., and Carter, J. L. (2014). Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 89 (2), 225–240. doi:10.1016/j.mayocp.2013.11.002
World Health Organization (2008) “Multiple sclerosis international federation,” in Atlas: multiple sclerosis resources in the world.
Xing, J., and Xu, C. (2021). Role of connexins in neurodegenerative diseases (Review). Mol. Med. Rep. 23 (5), 395. doi:10.3892/mmr.2021.12034
Ye, N., Cruz, J., Peng, X., Ma, J., Zhang, A., and Cheng, X. (2021). Remyelination is enhanced by Astragalus polysaccharides through inducing the differentiation of oligodendrocytes from neural stem cells in cuprizone model of demyelination. Brain Res. 1763, 147459. doi:10.1016/j.brainres.2021.147459
Yu, S. Y., Ouyang, H. T., Yang, J. Y., Huang, X. L., Yang, T., Duan, J. P., et al. (2007). Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J. Ethnopharmacol. 110 (2), 352–355. doi:10.1016/j.jep.2006.09.024
Glossary
Keywords: neurodegenerative diseases, Astragalus polysaccharide, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis
Citation: Shi Y and Ma P (2024) Pharmacological effects of Astragalus polysaccharides in treating neurodegenerative diseases. Front. Pharmacol. 15:1449101. doi: 10.3389/fphar.2024.1449101
Received: 14 June 2024; Accepted: 22 July 2024;
Published: 02 August 2024.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Liu Yaling, Shanxi Agricultural University, ChinaHatice Kızıltaş, Yüzüncü Yıl University, Türkiye
Yi Zhu, hengdu University of Traditional Chinese Medicine, China
Copyright © 2024 Shi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Ma, bWFwaW5nQGNkdXRjbS5lZHUuY24=
 Yuanshu Shi
Yuanshu Shi Ping Ma
Ping Ma