- 1Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research (NIPER)-Kolkata, Kolkata, West Bengal, India
- 2Department of Hematology, Endocrinology and Metabolism, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 3School of Pharmacy, Sister Nivedita University, Kolkata, West Bengal, India
- 4Director, National Institute of Pharmaceutical Education and Research (NIPER)-Kolkata, Kolkata, West Bengal, India
- 5Department of Laboratory Medicine and Clinical Epidemiology for Prevention of Noncommunicable Diseases, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
Metabolic compromise is crucial in aggravating age-associated chronic inflammation, oxidative stress, mitochondrial damage, increased LDL and triglycerides, and elevated blood pressure. Excessive adiposity, hyperglycemia, and insulin resistance due to aging are associated with elevated levels of damaging free radicals, inducing a proinflammatory state and hampering immune cell activity, leading to a malfunctioning cardiometabolic condition. The age-associated oxidative load and redox imbalance are contributing factors for cardiometabolic morbidities via vascular remodelling and endothelial damage. Recent evidence has claimed the importance of gut microbiota in maintaining regular metabolic activity, which declines with chronological aging and cardiometabolic comorbidities. Genetic mutations, polymorphic changes, and environmental factors strongly correlate with increased vulnerability to aberrant cardiometabolic changes by affecting key physiological pathways. Numerous studies have reported a robust link between biological aging and cardiometabolic dysfunction. This review outlines the scientific evidence exploring potential mechanisms behind the onset and development of cardiovascular and metabolic issues, particularly exacerbated with aging.
1 Introduction
1.1 Aging population and metabolic diseases
Aging is a complex, naturally occurring process resulting in a deterioration in the cellular homeostasis and increased vulnerability to stressors (Santamaria-Garcia et al., 2023). The multifactorial interplay of genetics, epigenetics as well as external and lifestyle-associated factors contributes to biological aging (Li Z. et al., 2021). The gradually changing demographic shift presents an increase in the elderly population, currently 700 million people above 65 years of (Nations, 2019). A growing body of evidence reports that several metabolic diseases expedite the progression of aging in humans (Tang et al., 2013; Suastika et al., 2011; Butler et al., 2006).
1.2 Epidemiology
The prevalence of metabolic syndrome in older people was found to be 72.9% in Mexicans, 42.4% in Taiwanese, 33.8% in Chinese, and 38% in Italians based on epidemiological evidence (Ortiz-Rodríguez et al., 2017; Yen et al., 2015; Laudisio et al., 2013). In contrast, the percentage rose from 13% to 50% in Indians and 34%–50% in Americans as they aged (Krishnamoorthy et al., 2020; Li et al., 2023). Recent studies conducted report age-related metabolic and cardiovascular risk in older people, ranging from 30% to 50% in China, 63.02% in the United States, and 62.5% in Iran (Zhao et al., 2023; Liang et al., 2023; Saeedi et al., 2023; Zhao et al., 2023; Liang et al., 2023; Saeedi et al., 2023).
1.3 Metabolic syndrome and cardiovascular risk
The metabolic syndrome centers on an array of metabolic disturbances like elevation in blood glucose levels, increased cholesterol, triglyceride levels, blood pressure obesity-mediated increased body mass index (BMI), and waist circumference (Bruce and Byrne, 2009; Lindblad et al., 2001; Ortiz-Rodríguez et al., 2017; Zhang et al., 2024). These multitude of conditions account for a significant risk of cardiovascular events, thus highlighting the cardiovascular consequences of metabolic stress, broadly referred to as cardiometabolic syndrome (Booth et al., 2006; Guo, Moellering, and Garvey, 2014). According to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), International Diabetes Federation (IDF), or the World Health Organization (WHO) guidelines, cardiometabolic syndrome manifests via the presence of central obesity, elevated triglycerides, decreased HDL cholesterol, elevated blood pressure, and elevated fasting glucose levels (Liu et al., 2016b; Rezaianzadeh, Namayandeh, and Sadr, 2012; Alberti, Zimmet, and Shaw, 2006). Insulin resistance is central to WHO criteria, with inflammatory markers like C-reactive protein (CRP) increasingly recognized as part of the syndrome’s profile (Liu et al., 2016b; Patwary et al., 2022). The metabolic syndrome associated with increased insulin levels as well as low-grade inflammation causes endothelial damage and atherosclerotic plaque development, and its rupture as well as coronary artery disease (Tylutka et al., 2023; Li et al., 2023; Gumede and Khathi, 2023). The interaction between biological aging and cardiometabolic disturbances is complex but complementary.
1.4 Risk factors and aging
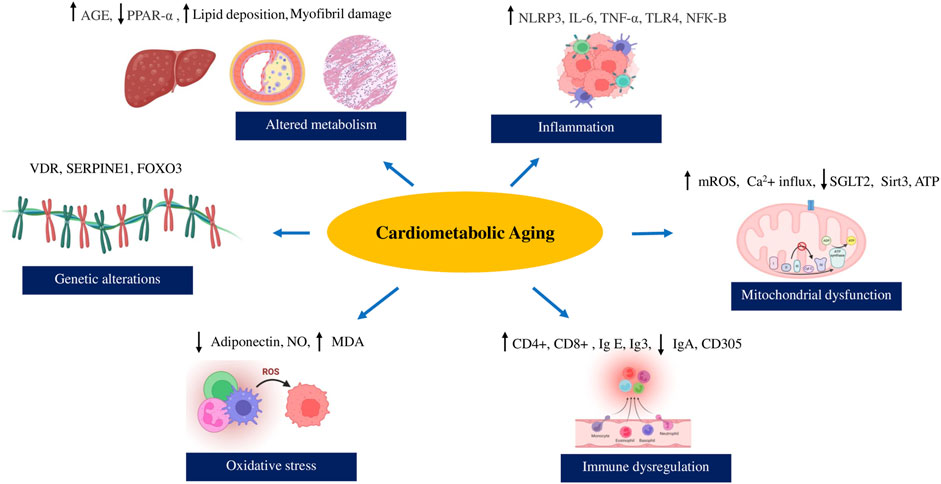
According to literature reports, cardiometabolic syndrome’s severity and clinical manifestations increase with age, leading to an increased morbidity and mortality risk (Lindblad et al., 2001; Ortiz-Rodríguez et al., 2017). Metabolic abnormalities, cardiovascular health, and cellular aging strongly interact through a network of molecular changes and signaling cascades with age (Chimenti et al., 2003; Devrajani et al., 2023). Interestingly, the telomere length of aged human hearts with cardiac failure and the expression of senescent markers like p53, p21, and p16, 35, 36 exhibit similarities to those observed in metabolic syndrome associated with aging (Chimenti et al., 2003; Lee et al., 2022). Preliminary evidence suggests that reduction of metabolic activity is associated with increased obesity and cardiovascular events in the older and middle-aged population (Suastika et al., 2011; Butler et al., 2006; Gouveia et al., 2021). The presence of unhealthy lifestyles and habits like smoking also pave the way for aggravating cardiometabolic risk with age (Gouveia et al., 2021; Johari and Shahar, 2014). The senescence-associated inflammation and glucose imbalance disturb the immune signalling pathways and elevate cardiovascular risk through cytochrome nad-P21 mediated telomere uncapping in arteries of geriatric subjects (Zhang et al., 2021; Johari and Shahar, 2014; Morgan et al., 2013; Amano et al., 2019). Free radicals associated with mitochondrial dysfunction, changes in the gut microbiota, and epigenetic modifications adversely affect the cardiometabolic profile with aging in humans (Kim et al., 2018; Acharya and Talahalli, 2019; Bo-Htay et al., 2020; Biagi et al., 2010). Hence, we will explore the diverse mechanisms underlying cardiometabolic syndrome in the context of aging integrating epidemiological data, and incorporating molecular perspectives and clinical evidence. The major events linking cardiometabolic risk with biological aging is illustrated in Figure1. The clinical evidence of cardiometabolic risk in the elderly population has been enlisted in Table 1.
2 The major etiological factors
2.1 Altered metabolism
A metabolic shift during aging is characterized by reduced reliance on fatty acids as the primary energy source and increased glucose consumption, a hallmark feature of cardiometabolic aging in both humans and rodents (Liu et al., 2003; Suehiro et al., 2016; Zha et al., 2017; Moreau et al., 2004; Kizer et al., 2014). Senescence results in poor glucose homeostasis, insulin sensitivity, plasma adiponectin, and hemoglobin (HbA1c) levels (Hyyti et al., 2010; Finucane et al., 2014). The dysregulated adipokines, like low adiponectin and high leptin levels, also account for age-associated insulin and leptin resistance (Mohamad et al., 2014; Schautz et al., 2012). The age-associated impaired Wnt signalling contributes to vascular thickening and fibrosis (Fujimaki et al., 2015; Hu et al., 2020). The diminished estrogen levels play a salutary role in age-associated fat accumulation and insulin resistance through alternative Wnt signaling pathways due to an imbalance in the Wnt5A and SFRP5 in aged females (Marmentini et al., 2021; Fayaz et al., 2019). The cumulative effects of lipid accumulation associated with insulin resistance in elderly individuals aggravates coronary artery necrosis (DeNino et al., 2001; Lee et al., 2009). A significant decline in the insulin growth factor (IGF) levels mediates cardiac fibrotic pathway activation via the Akt/Rho kinase 2/α-smooth muscle actin cascade (Bian et al., 2020). The age-associated decline in the AMPK and PGC-1α/PPAR-α signaling, correlates with increased rodent adiposity (Zang et al., 2004; Reznick et al., 2007; Alberti, Zimmet, and Shaw, 2006). Aged myocardium in monkeys responds to metabolic stress induced by high-fat sugar diet via increased infiltration of lymphocytes, cytokines, and lipids, leading to poor actomyosin MgATPase activity and altered myofilament phosphorylation (Zheng et al., 2021).
An upsurge in the release of alanine transferase (ALT), aspartate transferase (AST), urea, creatinine, and calcium exacerbate metabolic stress with aging (Liu et al., 2016b; Kim et al., 2010). The ABCA1 pathway, linked to reverse cholesterol transport (RCT) via high-density lipoproteins (HDL), diminishes with aging, along with changes in the HDL composition and phospholipidic bilayer fluidity (Berrougui et al., 2007). High lipid induces upregulation of clusterin and alanine transferases, leading to steatosis as well as elevated osteopontin levels, contributing to cardiac fibrosis and myocardial aging (Liu et al., 2016b; Bradley et al., 2019; Sawaki et al., 2018). The reduced levels of adipokines like leptin and adiponectin with aging disrupt metabolic regulation and affect cardiovascular function (Mohamad et al., 2014; Gradinaru et al., 2018).
Aging aggravates atherosclerotic plaque accumulation by increasing low-density lipoprotein (LDL) and cholesterol in the aorta, potentially driven by the overexpression of liver cholesterol protein transporters (ABCG5 and ABCG8) and the migration of monocytes and macrophages into adipose tissues (Palmer et al., 2019; Simo et al., 2021; Malgor et al., 2014). The senescent cells mediated adipose tissue hypertrophy contribute to left ventricular stiffness and poor left ventricular ejection fraction (Djoussé et al., 2012; Wohlfahrt et al., 2014). This may result in heart failure with preserved ejection fraction (HFpEF) and coronary artery disease during aging (Jung et al., 2022; Canepa et al., 2012; Prineas, Folsom, and Kaye, 1993).
2.2 Inflammation
The telomere dysfunction and DNA damage during aging contributes to inflammation via p53 activation which in turn, promotes the downregulation of peroxisome proliferator-activated receptor gamma coactivator one alpha (PGC-1α) and mitochondrial Sirts (Sirt3/4/5) (Li N. et al., 2021; He et al., 2017). Aging raises the glycemic index and lipid levels and upsurges inflammatory mediators and free radicals (Acharya and Talahalli, 2019; Aquino-Martinez et al., 2021; Wang et al., 2016). Aging upregulates the TLR4 expression in the aorta, raising the serum lipoprotein levels and affecting insulin sensitivity, cardiac function, and vascular relaxation (Xie et al., 2019; Malgor et al., 2014). The TLR4 via AGE causes inflammation, leading to the macrophage transitioning to a pro-inflammatory state, reduced insulin sensitivity, and pancreatic beta cell damage with age (Liu et al., 2021a; He et al., 2018). The senescence-associated secretory phenotype (SASP) manifests via RAGE (Receptor for Advanced glycation end products) cascade modulation, leading to the release of IL-6, IL-1β, CRP, and TNF-α release in the fatty tissues and renal tubular cells (Liu et al., 2014; Baker et al., 2011). The upregulated TNF-α levels increase insulin resistivity and inhibit the insulin receptor phosphorylation and atheromatous plaque deposition with aging (Nilsson et al., 1998; Skoog et al., 2002). Increased IL-6 levels, linked with insulin resistance, have been associated with cardiometabolic risk in older and middle-aged people (Marsland et al., 2010; Onder et al., 2002).
Cellular aging evokes an inflammatory response within the vascular wall via perivascular lipid accumulation and endothelial insult in the heart (Akbaraly et al., 2013; Reynaert et al., 2016). Increased expression of FKBP5 and NFκB drives cellular aging and may account for cardiovascular inflammation with age (Zannas et al., 2019; Zhang et al., 2023a; Sabbagh et al., 2014; Rovillain et al., 2011). The decrease in the catecholamine-mediated lipolysis with age is due to the reduction in the noradrenaline bioavailability via adipose tissue macrophages through upregulation of the NLRP3 inflammasome and MAO-A with growth differentiation factor-3 (GDF3) playing a significant role (Camell et al., 2017). Vascular aging corresponds with a significant increase in free radicals and inflammatory mediators such as IL-6 and IL-1β in obese rodents, which can be improved through therapies targeting senescent cells (Xin et al., 2019; Succurro et al., 2008). Higher IL-6 expression contributes to diabetes and atherosclerosis, especially in elderly patients (Giacconi et al., 2005). The build-up of plaques with age, caused by the activation of the NLRP3 inflammasome by IL-1β, may lead to cholesterol aggregation and disrupted insulin levels, consequently promoting further IL-1β release (Youm et al., 2012; Butcher et al., 2014). The mitochondrial H2O2 mediated TNF-α, ICAM, and iNOS levels potentially enhance the NF-KB activity in the arteries of the aged rats; moreover, inhibition of the TNF-α cascade could ameliorate the early senescence via immunometabolic regulation (Ungvari et al., 1999; Desdín-Micó et al., 2020). The high C-reactive protein (CRP) associated inflammation is linked to enhanced telomerase activity, leading to telomere shortening and diabetes-related premature senescence, and accelerated aging in older adults (Skoblow and Proulx, 2022; Desdín-Micó et al., 2020; Al-Daghri et al., 2021).
2.3 Immune dysfunction
The compromised immune response is associated with an upsurge in the release of inflammatory mediators and disrupted glucose homeostasis in aged obese rats (K. Kim et al., 2018). In response to aging, immune cells like macrophages exhibit an excess release of CD4+ and CD8+ T cells, reactive oxygen species (ROS), and increased senescent accumulation in the fatty tissues of old mice (W. He et al., 2018; Lumeng et al., 2011). An upregulation of immune-related genes with age includes chemokines (CCLs 6, 8, 9), complement proteins (C1qa, C1qb, C1qc, C3, C4b), lysozymes, and pro-inflammatory caspases (Casp1, Casp4, Casp12) in the ventricles of aged mice (Bartling et al., 2019). The immunosenescence likely occurs via the sestrin-mediated MAPK signaling, which, in turn, results in age-driven thymus degeneration and lowered T cell count in obese mice (Gress and Deeks, 2009; Lanna et al., 2017). Compromised T-lymphocyte and neutrophil activity precipitates metabolic syndrome in humans, with mitochondrial transcription factor-A deficiency causing cardiometabolic inflammation and neutrophil oxidative stress promoting atherosclerosis and senescence in mice (Richard et al., 2017; Desdín-Micó et al., 2020).
The increased mast cell markers like immunoglobulin-E (IgE), chymase, and tryptase lead to heightened inflammatory response and metabolic disturbances with aging (Wang et al., 2017). In human and zebrafish models, aging facilitates complement activation of the innate response via elevated Ig3 and decreased IgA and CD305 levels (Bakun et al., 2014; Reuter et al., 2022). The immunosenescence-related cardiometabolic complications include an upsurge in the natural killer, and T cells mediated IFN-γ release from the adipose tissues as well as high levels of oxidative stress in aged rodents (Srivastava et al., 2016; Palaniyappan and Alphonse, 2011). The increased infiltration of macrophages and T cells mediates fibrotic remodelling associated with systolic and diastolic dysfunction in aged mice hearts (Martini et al., 2020). The aging exacerbated immunoregulatory changes are primarily associated with inflammatory responses and metabolic disturbances, with implications for cardiometabolic health in both humans and animal models.
2.4 Mitochondrial dysfunction
Senescence enforces autophagy and mitochondrial fission via Drp1, PINK1, and laminin on mitochondria, expediting cardiometabolic damage in rodents (Duan et al., 2020; Madrigal-Matute, Cuervo, and Sluimer, 2022; Zha et al., 2017). The impaired FASL/FAS/TNF-α pathway, a major stimulus in age-associated pathologies, activates the caspase-associated mitochondrial apoptosis with age (Lai et al., 2014; Lagunas-Rangel, 2023). A rise in glycolysis in aging mouse hearts is related to a decline in the mitochondrial oxidative phosphorylation (OXPHOS) genes, affecting mitochondrial function (Serio et al., 2023). Aging increases mitochondrial calcium permeation compromises SGLT2 function and alters mitochondrial permeability and potential, consequently limiting cardiac function (Endlicher et al., 2021; Olgar et al., 2020). At the cellular level, these changes in mitochondria are accompanied by an increase of ROS and disturbed balance of fusion-fission (such as Drp1/MFN1/2) leading to age-related cardiac aging via autophagy/mitochondrial fission/pro-apoptotic signaling pathways (Bo-Htay et al., 2020; Niemann et al., 2011). The reduced oxidative phosphorylation and state III mitochondrial respiration were reported in the mitochondria of elderly patients with insulin resistance and high intramyocellular and liver triglyceride levels, as reported via 13 C NMR and 31 P NMR studies (Petersen et al., 2003; Fabbri et al., 2017). Aged rats experience decreased electron transport chain (ETC.) activity, reduced NADH dehydrogenase and cytochrome C oxidase, and cardiolipin-associated free radical damage (Kumaran et al., 2004). This decline in, ETC., function and membrane potential can reduce mitochondrial copy number, a well characterized sign for loss of metabolic function (Dang et al., 2017; Liu et al., 2021b). In other rodent models of aging, the diminished myocardial fatty acid oxidation (MFAO) seems to be linked to an age-related decrease in carnitine palmitoyltransferase-1 activity, the rate-limiting enzyme for mitochondrial long-chain fatty acid uptake (Mcmillin et al., 1993; Gómez, Heath, and Hagen, 2012). Elevated levels of IL-6, ATG5, and Parkin underscore the importance of autophagy and mitophagy in atherosclerosis, independent of lipid accumulation (Tyrrell et al., 2020).
Aging manifests a significant decrease in the mitochondrial enzymes like succinate dehydrogenase and superoxide dismutase, which correlates to reduced left ventricular function and sympathetic vagal activity in diabetic and atherosclerotic patients (Inal et al., 2001; Csiszar et al., 2002; Singh et al., 2015; Fetterman et al., 2016; Balietti et al., 2010). The concurrent rise in the mitochondrial hydrogen peroxide (H2O2) levels in the arteries of aged rats may result from a substantial increase in mitochondrial superoxide (O2•−) levels, with manganese superoxide dismutase (MnSOD) aiding its conversion (Csiszar et al., 2002; Han, Williams, and Cadenas, 2001). The mitochondrial H2O2 triggers NF-κB via a reinforced inflammatory response during vascular aging, resulting in insulin resistance in both aged rats and humans (Ungvari et al., 2007; Niemann et al., 2011). The endothelial superoxide levels increase exorbitantly with age due to the hyperacetylation of mitochondrial superoxide dismutase (SOD), which, in turn, raises the blood pressure, possibly via reduced Sirt3 expression in mice (Dikalova et al., 2017; X; He et al., 2017). Another hypothesis is that upregulation in the Sirt3 levels may result from a compensatory mechanism against cardiovascular aging in mice (Brown et al., 2013).
2.5 Oxidative stress
Hypertensive conditions can raise oxidative stress and insulin resistance, culminating in a reduced free radical scavenging activity of antioxidants in both the heart and plasma and decreased leukocyte telomere length, a characteristic feature of aging (Demissie et al., 2006; Sivoňová et al., 2007). Age-related cardiovascular dysfunction stems from impaired antioxidant pathways, DJ-1, and FOXO transcriptional pathways, exacerbating atherosclerosis, while ActR II signaling linked cardiovascular aging to metabolic damage (Collins et al., 2009; Roh et al., 2019; R; Chen et al., 2020). Aging culminates in a marked decline in the Nrf2, FOXO, and PGC-1α associated pathways, resulting in poor antioxidant capacity via decreased gene expression (Gureev et al., 2022; Medoro et al., 2023; Du and Zheng, 2021; Birnbaum et al., 2019; Halling et al., 2019; Angulo et al., 2019). The diminished antioxidants like glutathione reductase, superoxide dismutase, catalase, and glutathione decrease (GSH), may contribute to a substantial increase in the malondialdehyde (MDA) levels in both aged rodents and humans (Ramesh et al., 2012; Karaouzene et al., 2011). Age-mediated visceral fat accumulation leads to oxidative injury via increased superoxide anion generation and lipid peroxides, which, in turn, results in higher glucose levels, intracellular adhesion molecule 1(ICAM-1) and Von Willebrand factor (vWFl) and LDL cholesterol (Sołtysik et al., 2022). In elderly patients, the obesity-associated loss of muscle mass is accompanied by a rise in the GSSG/GSH ratio and plasma MDA/HNE protein adducts, along with increased carotid intima-media thickness (IMT) (Bellanti et al., 2018; Maris and Ghitea, 2023). Lipid peroxidation in aged rodent hearts, attributed to modulated Na/K-ATPase ROS signaling, increases oxidative damage, reduces sulfhydryl groups, and causes DNA damage in lymphocytes (Sudheesh et al., 2010; Sivoňová et al., 2007). The dysregulated redox activity-mediated cardiometabolic damage decreases adiponectin levels with age in human subjects (Gradinaru et al., 2017).
Aging, accompanied by an increased Nox-4 and reduced nitric oxide (NO) expression levels in cardiomyocytes, may lead to poor arterial elasticity, endothelial vasodilation, and left ventricular damage in aging mice (Ago et al., 2010; Lesniewski et al., 2009). Age-related endothelial injury, through increased angiotensin I and II receptor expression, stimulates hydrogen peroxide and superoxide ion release in mesenteric arteries (Khodja et al., 2012). In obese mice, aging is linked with the fibrotic remodelling of the heart and oxidative damage to mitochondrial DNA, which is repressed by antioxidants like catalase (Dai et al., 2009). There is an association between aging and elevated levels of alanine transferase (ALT), aspartate transferase (AST), creatinine, and serum urea nitrogen in rats. At the same time, free radical damage and immune system potentiation are associated with age-dependent oxidative damage (Li et al., 2007). Thus, oxidative damage acts as a major impetus for age-related cardiometabolic pathologies and underscores the significance of antioxidant therapy in addressing age-related cardiometabolic complications.
2.6 Gut microbiome dysbiosis
The primary immunological modifications linked with aging are decreased T cell activity, increased inflammation, and IL-10, IgA, IgM, and IgG-associated poor gut metabolites activity (Zhang et al., 2021; Kahmann et al., 2008). The changes in gut microbe composition with aging alter the gut permeability and disrupt the immune defenses, inducing systemic inflammation and cardiovascular risk via increased endotoxin access into the bloodstream in elderly patients (Biagi et al., 2010; Anker et al., 1997). The deteriorating gut microbiome and dysregulated glucose and lipid levels exacerbate inflammation and immune T cell-mediated plaque deposition in the mucosal lining (Toubal et al., 2020). Age-related gut microbiome changes in heart failure subjects include decreased levels of Eubacterium rectale, Dorea longicatena, and Bacteriodetes and increased levels of Lactobacillus and Proteobacteria (Kamo et al., 2017). In elderly diabetic individuals, Actinobacteria and Corinobacteriaceae microbes significantly increase compared to those without diabetes (Afolayan et al., 2020). Increased Candida, Campylobacter, and Shigella levels are linked to high intestinal permeability, inflammatory response, as well as right atrial pressure, while increased Firmicutes/Bacteroidetes microbes’ ratio is associated with enhanced insulin sensitivity in aged humans and rodents (Liu et al., 2016a; Pasini et al., 2016). Probiotics and beneficial gut bacteria like Bacteroides, Eubacterium rectale, and Fusobacterium prausnitzii reduce the age-related rise in diastolic blood pressure, cardiac infarct size, and intestinal wall thickness in older heart failure patients (Amamoto et al., 2021; Sandek et al., 2007).
The intestinal healthy bacteria replenishment promotes enhanced insulin responsiveness with interleukin-37 therapy in aged diabetic patients (Li et al., 2019). Microbial metabolites of protocatechuic acid, enterolactone, and Fufang Zhenshu TiaoZhi (FTZ) confer enhanced sphingolipid and arachidonic acid metabolism for cardioprotective and anti-atherosclerotic benefits in both adult and aging mice respectively, showing promise as a potential therapy for mitigating cardiometabolic risk (Wang et al., 2012; Luo et al., 2020). The changes in the microbial composition could be linked to cardiovascular and metabolic pathologies or may represent a response to changes in the gut environment associated with cardiometabolic risk in older individuals.
2.7 Genetic alterations
Cellular aging, a stable cell-cycle arrest, is a crucial feature of aging and age-related diseases, characterized by the activation of cell-cycle regulators like p53/p21 WAF1/CIP1 and p16 INK4A (Lee et al., 2022). Age-related metabolic reprogramming of the genomic profile includes changes in the CDKN2B/CDKN2A, FHIT, TDRG1, and ELAVL4, which correlates to high glucose levels, insulin release, and insulin resistance (Hribal et al., 2011). The mutated caveolin gene reduces Cav1 expression, lowering HDL and insulin levels and decreasing the prevalence of metabolic syndrome (Baudrand et al., 2015). Meanwhile, the senescent-associated mouse prone-8 (SAMP-8) correlated with mitochondrial damage, ER stress, cytokine release, and cardiac fibrosis (Karuppagounder et al., 2017). In aged populations, the mutated vitamin D receptor (VDR) gene reduces the LDL and cholesterol levels, whereas the plasminogen activator inhibitor-1 encoding SERPINE1 gene alterations decrease senescence alongwith improved metabolic prifile (Issa et al., 2016; Khan et al., 2017). The FOXO3 gene variant lowered age-related blood pressure and glucose increase, while the SGLT variant raised blood glucose levels with aging (R. Chen et al., 2020; Seidelmann et al., 2018). The aging adrenomedullin-deficient mice showed increased oxidative stress linked to insulin resistance compared to the control mice (Shimosawa et al., 2003). In contrast, old adenyl cyclase 5-deficient mice reported higher levels of the antioxidant SOD in order to combat age-related cardiovascular pathologies (Yan et al., 2007).
The transcriptional coactivator p300/CBP promotes cardiovascular aging via enhanced histone marker Hk2 expression through H3K27ac deposition (Serio et al., 2023). The reduced cardiac contractile damage and increased calcium levels in mice lacking cathepsin decrease age-related biomarkers, including p21, p16, and cardiac lipofuscin (Hua et al., 2015). Klotho deficiency impacts the Nrf2-GR pathway, affecting Glutathione reductase expression and activity in the heart, leading to oxidative stress-mediated cardiac aging (K. Chen et al., 2021). The age-associated decrease in the ejection fraction and fractional shortening in the hearts of senescence-accelerated mouse prone 8 (SAMP-8) mice can be attributed to the p38 MAPK pathway and ER stress-induced apoptosis (Sreedhar et al., 2016). These changes coincide with alterations in the extracellular matrix, diminished cellular interactions between gap junctions and connexin-43, and increased matrix metalloproteinase-2 (MMP-2) levels (Nagibin et al., 2016).
2.8 External and lifestyle associated risk factors
Cardiometabolic remodeling through external manipulations has become increasingly evident with biological aging. External factors like inadequate macronutrient intake-induced metabolic damage and accelerated aging via mTOR activation aggravate cardiovascular risks (Solon-Biet et al., 2014; Schloesser et al., 2015). Reduced hepatic mTOR activity via dietary restriction leads to longevity via reduced Akt and S6 kinase (Schloesser et al., 2015). Consumption of a healthy diet with age lowered the predisposition of cardiometabolic risk (Artegoitia et al., 2021). The nutritional intake and health conditions of the mothers also play an essential role in predisposing offspring towards age-associated cardiometabolic impairments like vascular aging (Warrington et al., 2019; Mora-Urda, Acevedo, and Montero López, 2019). The age-related diastolic dysfunction and oxidative stress-mediated DNA damage were reportedly lowered via calorie restriction along with an improvement in the lifespan of mice models (Sohal et al., 1994; Taffet, Pham, and Hartley, 1997). The age-associated mitochondrial DNA accumulation in the mice’s hearts lowered with improved food intake (Melov et al., 1997). Aging correlates with increased expression of collagen III, α-SMA, TGF-β, Bax, SOD, NRF2, and HO-1 in mice hearts, as well as telomere stabilizing proteins TRF2, TERT, cardiac insulin-like growth factor (IGF), and e-NOS (Werner et al., 2008; Pei et al., 2021). Mild exercise significantly lowered these protein’s expression, ameliorating fibrotic remodeling, apoptosis, and oxidative injury with aging in mice (Werner et al., 2008; Pei et al., 2021). The increased vulnerability of elderly smokers towards developing cardiometabolic risk could be due to smoking-triggered DNA methylation and gene alterations (Maas et al., 2020; Ramírez Manent et al., 2022a). Thus, the diverse genetic mutations could be decisive for developing several clinical phenotypes of cardiometabolic conditions with the onset of aging.
3 Discussion
The complex nature of aging is becoming more apparent, highlighting its importance as a significant multifaceted risk factor for the onset of cardiometabolic diseases and vice versa. Recent evidence highlights the clinical value of diagnostic indicators such as Leukocyte alkaline phosphatase (LAP), TNF-α, and HMGB-1 in predicting metabolic syndrome characterized by increased lipid accumulation in aging.
Aging at the cellular level involves the SASP phenotype with increased p53 and p21, causing cell cycle arrest, resulting in G1 cell cycle arrest and p16-mediated S-phase arrest in the central metabolic organs. The metabolic insult raises the senescence-associated β-galactosidase (SA-β-gal) levels, decreases Beclin1 and LC3II, compromises autophagy, and induces caspase-3-mediated apoptosis in cardiomyocytes. The age-dependent increased p16 INK4a, telomerase, MCM5, and p53 diminish the cardiac cell regeneration. ER stress from misfolded proteins like Glucose regulated protein 78 (GRP78) and C/EBP Homologous protein (CHOP) activates the p38 MAPK cascade and promotes caspase-12-mediated apoptosis with age.
Increased liver adiposity results in low C-peptide-associated beta cell dysfunction, reducing glucose sensitivity and insulin extraction from the liver. Poor insulin clearance in aging is linked to Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 (CEACAM1) and insulin-degrading enzyme (IDE), affecting insulin metabolism. The diminished glucose and fat metabolism accounts for the changes in adipokines like adiponectin leptin, contributing to age-related insulin resistance. This resistance further aggravates inflammatory mediators (TNF-α, IL-6, and IL-1β), which induce serine phosphorylation of insulin receptor substrate-1 (IRS-1), disrupting insulin signaling by impairing the interctions among IRS-1, phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt).
Aging-driven lipid-associated inflammation activates NF-κB and Janus kinase (JNK) pathways, while excess free fatty acids (FFAs) and lipid intermediates like diacylglycerol (DAG) affect insulin signaling via PKC activation. Chronic endoplasmic reticulum (ER) stress worsens insulin resistance via the unfolded protein response (UPR). The higher prevalence of age-related adiposity in older women than men can be due to the reduced estrogen level mediated reduction in the AMPK activity leading to reduced fatty acid oxidation and increased lipid accumulation. Lower estrogen also impairs eNOS activity and enhances TGF-β signaling, promoting endothelial damage and fibrosis.
The high CRP levels associated with inflammation can primarily act through NLRP3 inflammasome, PPAR signaling, and the HIF-1α pathway, affecting cardiometabolic homeostasis with age. The AGE-mediated ER stress results in an age-associated proinflammatory phenotype via glucose-regulated protein 78 (GRP78), cell-cycle regulator p21 and RAGE. The alteration of zinc homeostasis with age due to deficiency and malabsorption induces polymorphic changes, particularly in metallothionein, resulting in cytokine storms. Multiple clinical studies have consistently shown a strong relationship between aging and chronic inflammation, regardless of socioeconomic status, health behaviors (such as smoking and physical activity), or the use of anti-inflammatory medications. The activity of mitochondrial complex I, NADH oxidoreductase, declines significantly with age, with reduced protein carbonyl content and oxidative DNA damage (8-hydroxy-2′-deoxyguanosine) in cardiomyocytes and results in apoptosis with increased cytochrome-C, Bax, and Bcl-xS release.
Age-associated changes in the expression of Drp1, PINK1, and laminin drive autophagy and mitochondrial fission, while the impaired FASL/FAS/TNF-α affect the mitochondrial function, leading to increased ROS, disturbed fusion-fission balance, and reduced metabolic and cardiac efficiency. The significant age-related changes involve dysregulated DJ-1, FOXO-1, Na/K-ATPase ROS, and ActR II pathways, culminating in an increased oxidative injury which correlates with remarkably high levels of MDA, SOD, and Glutathione peroxidase (GPx) with age. Age-related changes in the gut microbial environment include reduced short-chain fatty acids (SCFAs), such as butyrate produced by Eubacterium rectale and Dorea longicatena which affect gut health. Age-related increase in gut permeability promotes the translocation of endotoxins and microbes in the systemic circulation, resulting in compromised immune defence and enhanced inflammatory response during aging. These alterations lead to insulin resistance and cardiovascular risks in the aging population. Increased mitochondrial H2O2 provokes inflammatory responses, promoting insulin resistance and hypertension, potentially involving mechanisms related to Sirt3 expression during aging. The impaired signalling cascades like DJ-1, FOXO, ActR II, Nrf2, and PGC-1α play a vital role in the downregulation of antioxidant enzymes like glutathione and superoxide dismutase in age-related cardiometabolic pathologies. Delving deeper into these pathways and target proteins could serve as biomarkers for assessing cardiometabolic health in aging populations and may offer promising avenues for therapeutic intervention. Future research could focus on developing diagnostic tools that measure these proteins’ activity or localization to predict the risk of several cardiometabolic events in older adults. Several studies have identified genetic background as a link between metabolic and cardiac remodelling with age, including gene-related changes like klotho, adrenomedullin, SAMP-8, cathepsin, VDR, and SERPINE1. These findings offer new models for studying age-related disorders, paving the way for gene-directed therapies.
In conclusion, the aging population presents a significant challenge for healthcare systems worldwide, necessitating a deeper understanding of the physiological changes and risk factors associated with the aging process. A comprehensive strategy addressing cardiometabolic aging should incorporate medical and nutraceutical therapies as well as lifestyle modifications, including a healthy diet, regular physical activity, and discontinued smoking habits. Additionally, gene profiling may significantly impact patient medication responses, highlighting the importance of personalized treatment approaches. Customizing these approaches to individual patient’s needs and consistently monitoring progress can enhance effectiveness and improve health outcomes for the elderly population. Collaborative efforts between healthcare providers, researchers, and policymakers are crucial in effectively addressing the challenges of the aging population.
Author contributions
SS: Investigation, Writing–original draft. VP: Writing–review and editing. PD: Writing–review and editing. HSS: Writing–review and editing, Formal Analysis. KF: Formal Analysis, Writing–review and editing. SK: Formal Analysis, Writing–review and editing. HHS: Formal Analysis, Writing–review and editing. RS: Formal Analysis, Writing–review and editing. RV: Formal Analysis, Writing–review and editing. KW: Formal Analysis, Writing–review and editing, Conceptualization. SA: Conceptualization, Writing–review and editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This article received no external funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Nrf2, Nuclear factor erythroid 2-related factor 2; FOXO, Forkhead box protein O1; PPARα, Peroxisome proliferator-activated receptor alpha; Drp1, Dynamin-related Protein; Mfn1, Mitofusin-1; NLRP3, NOD-like receptor family pyrin domain containing 3), NFКB, Nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphoinositide 3-kinase; CD 4, Cluster of differentiation 4; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α.
References
Acharya, P., and Talahalli, R. R. (2019). Aging and hyperglycemia Intensify dyslipidemia-induced oxidative stress and inflammation in rats: assessment of restorative potentials of ALA and EPA + DHA. Inflammation 42 (3), 946–952. PMID: 30535619. doi:10.1007/s10753-018-0949-6
Afolayan, A. O., Adebusoye, L. A., Cadmus, E. O., and Ayeni, F. A. (2020). Insights into the gut microbiota of Nigerian elderly with type 2 diabetes and non-diabetic elderly persons. Heliyon 6 (5), e03971. PMID: 32490229; PMCID: PMC7262409. doi:10.1016/j.heliyon.2020.e03971
Ago, T., Kuroda, J., Pain, J., Fu, C., Li, H., and Sadoshima, J. (2010). Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 106 (7), 1253–1264. Epub 2010 Feb 25. PMID: 20185797; PMCID: PMC2855780. doi:10.1161/CIRCRESAHA.109.213116
Akbaraly, T. N., Hamer, M., Ferrie, J. E., Lowe, G., Batty, G. D., Hagger-Johnson, G., et al. (2013). Chronic inflammation as a determinant of future aging phenotypes. CMAJ 185 (16), E763–E770. Epub 2013 Sep 16. PMID: 24043651; PMCID: PMC3826354. doi:10.1503/cmaj.122072
Alberti, K. G., Zimmet, P., and Shaw, J. (2006). Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 23 (5), 469–480. PMID: 16681555. doi:10.1111/j.1464-5491.2006.01858.x
Al-Daghri, N. M., Abdi, S., Sabico, S., Alnaami, A. M., Wani, K. A., Ansari, M. G. A., et al. (2021). Gut-Derived endotoxin and telomere length attrition in adults with and without type 2 diabetes. Biomolecules 11 (11), 1693. PMID: 34827691; PMCID: PMC8615790. doi:10.3390/biom11111693
Amamoto, R., Shimamoto, K., Park, S., Matsumoto, H., Shimizu, K., Katto, M., et al. (2021). Yearly changes in the composition of gut microbiota in the elderly, and the effect of lactobacilli intake on these changes. Sci. Rep. 11 (1), 12765. Erratum in: Sci Rep. doi:10.1038/s41598-021-91917-6
Amano, H., Chaudhury, A., Rodriguez-Aguayo, C., Lu, L., Akhanov, V., Catic, A., et al. (2019). Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab. 29 (6), 1274–1290. Epub 2019 Mar 28. PMID: 30930169; PMCID: PMC6657508. doi:10.1016/j.cmet.2019.03.001
Angulo, J., El Assar, M., Sevilleja-Ortiz, A., Fernández, A., Sánchez-Ferrer, A., Romero-Otero, J., et al. (2019). Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. 26, 101271. Epub 2019 Jul 5. PMID: 31302408; PMCID: PMC6626891. doi:10.1016/j.redox.2019.101271
Anker, S. D., Egerer, K. R., Volk, H. D., Kox, W. J., Poole-Wilson, P. A., and Coats, A. J. (1997). Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am. J. Cardiol. 79 (10), 1426–1430. PMID: 9165177. doi:10.1016/s0002-9149(97)00159-8
Aquino-Martinez, R., Eckhardt, B. A., Rowsey, J. L., Fraser, D. G., Khosla, S., Farr, J. N., et al. (2021). Senescent cells exacerbate chronic inflammation and contribute to periodontal disease progression in old mice. J. Periodontol. 92 (10), 1483–1495. Epub 2021 Jan 6. PMID: 33341947; PMCID: PMC8281492. doi:10.1002/JPER.20-0529
Artegoitia, V. M., Krishnan, S., Bonnel, E. L., Stephensen, C. B., Keim, N. L., and Newman, J. W. (2021). Healthy eating index patterns in adults by sex and age predict cardiometabolic risk factors in a cross-sectional study. BMC Nutr. 7 (1), 30. PMID: 34154665; PMCID: PMC8218401. doi:10.1186/s40795-021-00432-4
Baker, D. J., Wijshake, T., Tchkonia, T., LeBrasseur, N. K., Childs, B. G., van de Sluis, B., et al. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 (7372), 232–236. PMID: 22048312; PMCID: PMC3468323. doi:10.1038/nature10600
Bakun, M., Senatorski, G., Rubel, T., Lukasik, A., Zielenkiewicz, P., Dadlez, M., et al. (2014). Urine proteomes of healthy aging humans reveal extracellular matrix (ECM) alterations and immune system dysfunction. Age (Dordr) 36 (1), 299–311. Epub 2013 Aug 6. PMID: 23917802; PMCID: PMC3889913. doi:10.1007/s11357-013-9562-7
Balietti, M., Casoli, T., Di Stefano, G., Giorgetti, B., Aicardi, G., and Fattoretti, P. (2010). Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev. 9 (3), 273–279. Epub 2010 Feb 24. PMID: 20188215. doi:10.1016/j.arr.2010.02.003
Bartling, B., Niemann, K., Pliquett, R. U., Treede, H., and Simm, A. (2019). Altered gene expression pattern indicates the differential regulation of the immune response system as an important factor in cardiac aging. Exp. Gerontol. 117, 13–20. Epub 2018 May 5. PMID: 29738791. doi:10.1016/j.exger.2018.05.001
Baudrand, R., Goodarzi, M. O., Vaidya, A., Underwood, P. C., Williams, J. S., Jeunemaitre, X., et al. (2015). A prevalent caveolin-1 gene variant is associated with the metabolic syndrome in Caucasians and Hispanics. Metabolism 64 (12), 1674–1681. Epub 2015 Sep 12. PMID: 26475177; PMCID: PMC4641791. doi:10.1016/j.metabol.2015.09.005
Bellanti, F., Romano, A. D., Lo Buglio, A., Castriotta, V., Guglielmi, G., Greco, A., et al. (2018). Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 109, 6–12. Epub 2017 Dec 5. PMID: 29452783. doi:10.1016/j.maturitas.2017.12.002
Berrougui, H., Isabelle, M., Cloutier, M., Grenier, G., and Khalil, A. (2007). Age-related impairment of HDL-mediated cholesterol efflux. J. Lipid Res. 48 (2), 328–336. Epub 2006 Nov 8. PMID: 17093293. doi:10.1194/jlr.M600167-JLR200
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5 (5), e10667. Erratum in: PLoS One. 2010. doi:10.1371/journal.pone.0010667
Bian, A., Ma, Y., Zhou, X., Guo, Y., Wang, W., Zhang, Y., et al. (2020). Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 21 (1), 214. PMID: 32264885; PMCID: PMC7140321. doi:10.1186/s12891-020-03236-y
Birnbaum, A., Wu, X., Tatar, M., Liu, N., and Bai, H. (2019). Age-dependent changes in transcription factor FOXO targeting in female Drosophila. Front. Genet. 10, 312. PMID: 31134124; PMCID: PMC6514159. doi:10.3389/fgene.2019.00312
Bo-Htay, C., Shwe, T., Higgins, L., Palee, S., Shinlapawittayatorn, K., Chattipakorn, S. C., et al. (2020). Aging induced by D-galactose aggravates cardiac dysfunction via exacerbating mitochondrial dysfunction in obese insulin-resistant rats. Geroscience 42 (1), 233–249. Epub 2019 Nov 25. PMID: 31768765; PMCID: PMC7031455. doi:10.1007/s11357-019-00132-9
Booth, G. L., Kapral, M. K., Fung, K., and Tu, J. V. (2006). Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368 (9529), 29–36. PMID: 16815377. doi:10.1016/S0140-6736(06)68967-8
Bradley, D., Blaszczak, A., Yin, Z., Liu, J., Joseph, J. J., Wright, V., et al. (2019). Clusterin Impairs Hepatic Insulin Sensitivity and Adipocyte Clusterin Associates With Cardiometabolic Risk. Diabetes Care 42 (3), 466–475. doi:10.2337/dc18-0870
Brown, K., Xie, S., Qiu, K., Mohrin, M., Shin, J., Liu, Y., et al. (2013). SIRT3 reverses aging-associated degeneration. Cell Rep. 3 (2), 319–327. doi:10.1016/j.celrep.2013.01.005
Bruce, K. D., and Byrne, C. D. (2009). The metabolic syndrome: common origins of a multifactorial disorder. Postgrad. Med. J. 85 (1009), 614–621. PMID: 19892897. doi:10.1136/pgmj.2008.078014
Butcher, M. J., Hallinger, D., Garcia, E., Machida, Y., Chakrabarti, S., Nadler, J., et al. (2014). Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 57 (3), 491–501. Epub 2014 Jan 16. PMID: 24429578; PMCID: PMC3966210. doi:10.1007/s00125-013-3116-5
Butler, J., Rodondi, N., Zhu, Y., Figaro, K., Fazio, S., Vaughan, D. E., et al. (2006). Metabolic syndrome and the risk of cardiovascular disease in older adults. J. Am. Coll. Cardiol. 47 (8), 1595–1602. Epub 2006 Mar 27. PMID: 16630996. doi:10.1016/j.jacc.2005.12.046
Camell, C. D., Sander, J., Spadaro, O., Lee, A., Nguyen, K. Y., Wing, A., et al. (2017). Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550 (7674), 119–123. Epub 2017 Sep 27. PMID: 28953873; PMCID: PMC5718149. doi:10.1038/nature24022
Canepa, M., Strait, J. B., Abramov, D., Milaneschi, Y., AlGhatrif, M., Moni, M., et al. (2012). Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging). Am. J. Cardiol. 109 (8), 1171–1178. Epub 2012 Jan 17. PMID: 22257709; PMCID: PMC3319236. doi:10.1016/j.amjcard.2011.11.054
Chen, K., Wang, S., Sun, Q. W., Zhang, B., Ullah, M., and Sun, Z. (2021). Klotho deficiency causes heart aging via impairing the nrf2-GR pathway. Circ. Res. 128 (4), 492–507. Epub 2020 Dec 18. PMID: 33334122; PMCID: PMC8782577. doi:10.1161/CIRCRESAHA.120.317348
Chen, R., Morris, B. J., Donlon, T. A., Masaki, K. H., Willcox, D. C., Davy, P. M. C., et al. (2020). FOXO3 longevity genotype mitigates the increased mortality risk in men with a cardiometabolic disease. Aging (Albany NY) 12 (23), 23509–23524. Epub 2020 Dec 1. PMID: 33260156; PMCID: PMC7762472. doi:10.18632/aging.202175
Chimenti, C., Kajstura, J., Torella, D., Urbanek, K., Heleniak, H., Colussi, C., et al. (2003). Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 93 (7), 604–613. Epub 2003 Sep 4. PMID: 12958145. doi:10.1161/01.RES.0000093985.76901.AF
Collins, A. R., Lyon, C. J., Xia, X., Liu, J. Z., Tangirala, R. K., Yin, F., et al. (2009). Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ. Res. 104 (6), 42–54. Epub 2009 Mar 5. PMID: 19265038. doi:10.1161/CIRCRESAHA.108.188771
Csiszar, A., Ungvari, Z., Edwards, J. G., Kaminski, P., Wolin, M. S., Koller, A., et al. (2002). Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 90 (11), 1159–1166. PMID: 12065318. doi:10.1161/01.res.0000020401.61826.ea
Dai, D. F., Santana, L. F., Vermulst, M., Tomazela, D. M., Emond, M. J., MacCoss, M. J., et al. (2009). Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119 (21), 2789–2797. Epub 2009 May 18. PMID: 19451351; PMCID: PMC2858759. doi:10.1161/CIRCULATIONAHA.108.822403
Dang, E. V., McDonald, J. G., Russell, D. W., and Cyster, J. G. (2017). Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 171 (5), 1057–1071. Epub 2017 Oct 12. PMID: 29033131; PMCID: PMC5693620. doi:10.1016/j.cell.2017.09.029
Demissie, S., Levy, D., Benjamin, E. J., Cupples, L. A., Gardner, J. P., Herbert, A., et al. (2006). Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 5 (4), 325–330. PMID: 16913878. doi:10.1111/j.1474-9726.2006.00224.x
DeNino, W. F., Tchernof, A., Dionne, I. J., Toth, M. J., Ades, P. A., Sites, C. K., et al. (2001). Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care 24 (5), 925–932. PMID: 11347756. doi:10.2337/diacare.24.5.925
Desdín-Micó, G., Soto-Heredero, G., Aranda, J. F., Oller, J., Carrasco, E., Gabandé-Rodríguez, E., et al. (2020). T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368 (6497), 1371–1376. Epub 2020 May 21. PMID: 32439659. doi:10.1126/science.aax0860
Devrajani, T., Abid, S., Shaikh, H., Shaikh, I., Devrajani, D. B., Memon, S. M., et al. (2023). Relationship between aging and control of metabolic syndrome with telomere shortening: a cross-sectional study. Sci. Rep. 13 (1), 17878. PMID: 37857729; PMCID: PMC10587132. doi:10.1038/s41598-023-44715-1
Dikalova, A. E., Itani, H. A., Nazarewicz, R. R., McMaster, W. G., Flynn, C. R., Uzhachenko, R., et al. (2017). Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ. Res. 121 (5), 564–574. Epub 2017 Jul 6. PMID: 28684630; PMCID: PMC5562527. doi:10.1161/CIRCRESAHA.117.310933
Djoussé, L., Bartz, T. M., Ix, J. H., Zieman, S. J., Delaney, J. A., Mukamal, K. J., et al. (2012). Adiposity and incident heart failure in older adults: the cardiovascular health study. Obes. (Silver Spring) 20 (9), 1936–1941. Epub 2011 Oct 20. PMID: 22016094; PMCID: PMC3429627. doi:10.1038/oby.2011.320
Du, S., and Zheng, H. (2021). Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 11 (1), 188. PMID: 34727995; PMCID: PMC8561869. doi:10.1186/s13578-021-00700-7
Duan, C., Kuang, L., Xiang, X., Zhang, J., Zhu, Y., Wu, Y., et al. (2020). Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-BAX-and GSH- pathways. Cell Death Dis. 11 (4), 251. Erratum in: Cell Death Dis. doi:10.1038/s41419-020-2461-9
Endlicher, R., Drahota, Z., Kučera, O., and Červinková, Z. (2021). Age-dependent changes in the function of mitochondrial membrane permeability transition pore in rat liver mitochondria. Physiol. Res. 70 (6), 905–911. Epub 2021 Oct 30. PMID: 34717067; PMCID: PMC8815472. doi:10.33549/physiolres.934734
Fabbri, E., Chia, C. W., Spencer, R. G., Fishbein, K. W., Reiter, D. A., Cameron, D., et al. (2016). TITLE: insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31 P magnetic resonance spectroscopy in non-diabetic participants from the Baltimore longitudinal study of aging. Word Count Text 2000 Run. Ahead Mitochondrial Oxidative Capacity Insulin Resist.
Fabbri, E., Chia, C. W., Spencer, R. G., Fishbein, K. W., Reiter, D. A., Cameron, D., et al. (2017). Insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31P-magnetic resonance spectroscopy in participants without diabetes from the Baltimore longitudinal study of aging. Diabetes 66 (1), 170–176. Epub 2016 Oct 13. PMID: 27737951; PMCID: PMC5204309. doi:10.2337/db16-0754
Fayaz, E., Damirchi, A., Zebardast, N., and Babaei, P. (2019). Cinnamon extract combined with high-intensity endurance training alleviates metabolic syndrome via non-canonical WNT signaling. Nutrition 65, 173–178. Epub 2019 Mar 23. PMID: 31170681. doi:10.1016/j.nut.2019.03.009
Fetterman, J. L., Holbrook, M., Westbrook, D. G., Brown, J. A., Feeley, K. P., Bretón-Romero, R., et al. (2016). Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol. 15, 53. PMID: 27036979; PMCID: PMC4818501. doi:10.1186/s12933-016-0372-y
Finucane, F. M., Sharp, S. J., Hatunic, M., Sleigh, A., De Lucia Rolfe, E., Aihie Sayer, A., et al. (2014). Liver fat accumulation is associated with reduced hepatic insulin extraction and beta cell dysfunction in healthy older individuals. Diabetol. Metab. Syndr. 6 (1), 43. PMID: 24669786; PMCID: PMC3974597. doi:10.1186/1758-5996-6-43
Fujimaki, S., Wakabayashi, T., Takemasa, T., Asashima, M., and Kuwabara, T. (2015). The regulation of stem cell aging by Wnt signaling. Histol. Histopathol. 30 (12), 1411–1430. Epub 2015 Aug 31. PMID: 26322973. doi:10.14670/HH-11-657
Giacconi, R., Cipriano, C., Muti, E., Costarelli, L., Maurizio, C., Saba, V., et al. (2005). Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: relationship with inflammation (IL-6) and zinc. Biogerontology 6 (6), 407–413. PMID: 16518702. doi:10.1007/s10522-005-4907-y
Gómez, L. A., Heath, S. H., and Hagen, T. M. (2012). Acetyl-L-carnitine supplementation reverses the age-related decline in carnitine palmitoyltransferase 1 (CPT1) activity in interfibrillar mitochondria without changing the L-carnitine content in the rat heart. Mech. Ageing Dev. 133 (2-3), 99–106. Epub 2012 Feb 1. PMID: 22322067; PMCID: PMC4147858. doi:10.1016/j.mad.2012.01.007
Gouveia, É. R., Gouveia, B. R., Marques, A., Peralta, M., França, C., Lima, A., et al. (2021). Predictors of metabolic syndrome in adults and older adults from amazonas, Brazil. Int. J. Environ. Res. Public Health 18 (3), 1303. PMID: 33535582; PMCID: PMC7908119. doi:10.3390/ijerph18031303
Gradinaru, D., Khaddour, H., Margina, D., Ungurianu, A., Borsa, C., Ionescu, C., et al. (2018). Insulin-leptin Axis, cardiometabolic risk and oxidative stress in elderly with metabolic syndrome. Exp. Clin. Endocrinol. Diabetes 8, 445–452. Epub ahead of print. PMID: 29421825. doi:10.1055/s-0043-123825
Gradinaru, D., Margina, D., Borsa, C., Ionescu, C., Ilie, M., Costache, M., et al. (2017). Adiponectin: possible link between metabolic stress and oxidative stress in the elderly. Aging Clin. Exp. Res. 29 (4), 621–629. Epub 2016 Sep 29. PMID: 27688246. doi:10.1007/s40520-016-0629-z
Gress, R. E., and Deeks, S. G. (2009). Reduced thymus activity and infection prematurely age the immune system. J. Clin. Invest 119 (10), 2884–2887. Epub 2009 Sep 21. PMID: 19770512; PMCID: PMC2752092. doi:10.1172/JCI40855
Gumede, N., and Khathi, A. (2023). The role of fibrinolysis in the development of prediabetes-associated coronary heart disease: a focus on the plasminogen activator inhibitor -1 and its potential use as a predictive marker in diet-induced prediabetes. Front. Nutr. 10, 1256427. PMID: 38024366; PMCID: PMC10652797. doi:10.3389/fnut.2023.1256427
Guo, F., Moellering, D. R., and Garvey, W. T. (2014). The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obes. (Silver Spring) 22 (1), 110–118. Epub 2013 Sep 5. PMID: 23894121; PMCID: PMC3866217. doi:10.1002/oby.20585
Gureev, A. P., Khorolskaya, V. G., Sadovnikova, I. S., Shaforostova, E. A., Cherednichenko, V. R., Burakova, I. Y., et al. (2022). Age-related decline in Nrf2/ARE signaling is associated with the mitochondrial DNA damage and cognitive impairments. Int. J. Mol. Sci. 23 (23), 15197. PMID: 36499517; PMCID: PMC9739464. doi:10.3390/ijms232315197
Halling, J. F., Jessen, H., Nøhr-Meldgaard, J., Buch, B. T., Christensen, N. M., Gudiksen, A., et al. (2019). PGC-1α regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am. J. Physiol. Endocrinol. Metab. 317 (3), E513-E525–E525. Epub 2019 Jul 2. PMID: 31265325. doi:10.1152/ajpendo.00059.2019
Han, D., Williams, E., and Cadenas, E. (2001). Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 353 (Pt 2), 411–416. PMID: 11139407; PMCID: PMC1221585. doi:10.1042/0264-6021:3530411
He, W., Yuan, T., Choezom, D., Hunkler, H., Annamalai, K., Lupse, B., et al. (2018). Ageing potentiates diet-induced glucose intolerance, β-cell failure and tissue inflammation through TLR4. Sci. Rep. 8 (1), 2767. PMID: 29426925; PMCID: PMC5807311. doi:10.1038/s41598-018-20909-w
He, X., Zeng, H., Chen, S. T., Roman, R. J., Aschner, J. L., Didion, S., et al. (2017). Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J. Mol. Cell Cardiol. 112, 104–113. Epub 2017 Sep 19. PMID: 28935506; PMCID: PMC5647246. doi:10.1016/j.yjmcc.2017.09.007
Hribal, M. L., Presta, I., Procopio, T., Marini, M. A., Stančáková, A., Kuusisto, J., et al. (2011). Glucose tolerance, insulin sensitivity and insulin release in European non-diabetic carriers of a polymorphism upstream of CDKN2A and CDKN2B. Diabetologia 54 (4), 795–802. Epub 2011 Jan 14. PMID: 21234743. doi:10.1007/s00125-010-2038-8
Hu, H. H., Cao, G., Wu, X. Q., Vaziri, N. D., and Zhao, Y. Y. (2020). Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res. Rev. 60, 101063. Epub 2020 Apr 6. PMID: 32272170. doi:10.1016/j.arr.2020.101063
Hua, Y., Robinson, T. J., Cao, Y., Shi, G. P., Ren, J., and Nair, S. (2015). Cathepsin K knockout alleviates aging-induced cardiac dysfunction. Aging Cell 14 (3), 345–351. Epub 2015 Feb 18. PMID: 25692548; PMCID: PMC4406663. doi:10.1111/acel.12276
Hyyti, O. M., Ledee, D., Ning, X. H., Ge, M., and Portman, M. A. (2010). Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am. J. Physiol. Heart Circ. Physiol. 299 (3), H868–H875. Epub 2010 Jul 2. PMID: 20601465; PMCID: PMC2944494. doi:10.1152/ajpheart.00931.2009
Inal, M. E., Kanbak, G., and Sunal, E. (2001). Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta. 305 (1-2), 75–80. PMID: 11249925. doi:10.1016/s0009-8981(00)00422-8
Issa, C. T., Silva, A. S., Toscano, L. T., Medeiros, M. S., Persuhn, D. C., da Silva Diniz, A., et al. (2016). Relationship between cardiometabolic profile, vitamin D status and BsmI polymorphism of the VDR gene in non-institutionalized elderly subjects: cardiometabolic profile, vitamin D status and BsmI polymorphism of the VDR gene in non-institutionalized elderly subjects. Exp. Gerontol. 81, 56–64. Epub 2016 Apr 26. PMID: 27125758. doi:10.1016/j.exger.2016.04.020
Johari, S. M., and Shahar, S. (2014). Metabolic syndrome: the association of obesity and unhealthy lifestyle among Malaysian elderly people. Arch. Gerontol. Geriatr. 59 (2), 360–366. Epub 2014 May 5. PMID: 24882592. doi:10.1016/j.archger.2014.04.003
Jung, H. N., Kim, M. J., Kim, H. S., Lee, W. J., Min, S. H., Kim, Y. J., et al. (2022). Age-related associations of low-density lipoprotein cholesterol and atherosclerotic cardiovascular disease: a nationwide population-based cohort study. J. Am. Heart Assoc. 11 (9), e024637. Epub 2022 May 2. PMID: 35492003; PMCID: PMC9238630. doi:10.1161/JAHA.121.024637
Kahmann, L., Uciechowski, P., Warmuth, S., Plümäkers, B., Gressner, A. M., Malavolta, M., et al. (2008). Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 11 (1), 227–237. PMID: 18279033. doi:10.1089/rej.2007.0613
Kamo, T., Akazawa, H., Suda, W., Saga-Kamo, A., Shimizu, Y., Yagi, H., et al. (2017). Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 12 (3), e0174099. PMID: 28328981; PMCID: PMC5362204. doi:10.1371/journal.pone.0174099
Karaouzene, N., Merzouk, H., Aribi, M., Merzouk, S. A., Berrouiguet, A. Y., Tessier, C., et al. (2011). Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr. Metab. Cardiovasc Dis. 21 (10), 792–799. Epub 2010 Jun 2. PMID: 20554180. doi:10.1016/j.numecd.2010.02.007
Karuppagounder, V., Arumugam, S., Babu, S. S., Palaniyandi, S. S., Watanabe, K., Cooke, J. P., et al. (2017). The senescence accelerated mouse prone 8 (SAMP8): a novel murine model for cardiac aging. Ageing Res. Rev. 35, 291–296. Epub 2016 Nov 5. PMID: 27825897. doi:10.1016/j.arr.2016.10.006
Khan, S. S., Shah, S. J., Klyachko, E., Baldridge, A. S., Eren, M., Place, A. T., et al. (2017). A null mutation in SERPINE1 protects against biological aging in humans. Sci. Adv. 3 (11), eaao1617. PMID: 29152572; PMCID: PMC5687852. doi:10.1126/sciadv.aao1617
Khodja, N., Chataigneau, T., Auger, C., and Schini-Kerth, V. B. (2012). Grape-derived polyphenols improve aging-related endothelial dysfunction in rat mesenteric artery: role of oxidative stress and the angiotensin system. PLoS One 7 (2), e32039. Epub 2012 Feb 27. PMID: 22384133; PMCID: PMC3288061. doi:10.1371/journal.pone.0032039
Kim, K., Ahn, N., Jung, S., Ju, Y., Lee, G., Kim, M., et al. (2018). Effects of resistance exercise and fermented soybean consumption on glucose tolerance and expressions of immune senescence-related myokines in middle-aged obese rats. J. Obes. Metab. Syndr. 27 (3), 186–194. PMID: 31089561; PMCID: PMC6504197. doi:10.7570/jomes.2018.27.3.186
Kim, M. K., Kim, G., Jang, E. H., Kwon, H. S., Baek, K. H., Oh, K. W., et al. (2010). Altered calcium homeostasis is correlated with the presence of metabolic syndrome and diabetes in middle-aged and elderly Korean subjects: the Chungju Metabolic Disease Cohort study (CMC study). Atherosclerosis 212 (2), 674–681. Epub 2010 Jul 15. PMID: 20728085. doi:10.1016/j.atherosclerosis.2010.07.005
Kizer, J. R., Benkeser, D., Arnold, A. M., Ix, J. H., Mukamal, K. J., Djousse, L., et al. (2014). Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis 235 (1), 116–121. Epub 2014 Apr 30. PMID: 24825341; PMCID: PMC4169874. doi:10.1016/j.atherosclerosis.2014.04.013
Krishnamoorthy, Y., Rajaa, S., Murali, S., Rehman, T., Sahoo, J., and Kar, S. S. (2020). Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One 15 (10), e0240971. PMID: 33075086; PMCID: PMC7571716. doi:10.1371/journal.pone.0240971
Kumaran, S., Subathra, M., Balu, M., and Panneerselvam, C. (2004). Age-associated decreased activities of mitochondrial electron transport chain complexes in heart and skeletal muscle: role of L-carnitine. Chem. Biol. Interact. 148 (1-2), 11–18. PMID: 15223352. doi:10.1016/j.cbi.2003.10.010
Lagunas-Rangel, F. A. (2023). Fas (CD95)/FasL (CD178) system during ageing. Cell Biol. Int. 47 (8), 1295–1313. Epub 2023 May 3. PMID: 37132427. doi:10.1002/cbin.12032
Lai, C. H., Ho, T. J., Kuo, W. W., Day, C. H., Pai, P. Y., Chung, L. C., et al. (2014). Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 36 (5), 9706. Epub 2014 Aug 23. PMID: 25148910; PMCID: PMC4453937. doi:10.1007/s11357-014-9706-4
Lanna, A., Gomes, D. C., Muller-Durovic, B., McDonnell, T., Escors, D., Gilroy, D. W., et al. (2017). A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 18 (3), 354–363. Epub 2017 Jan 23. PMID: 28114291; PMCID: PMC5321575. doi:10.1038/ni.3665
Laudisio, A., Marzetti, E., Antonica, L., Pagano, F., Vetrano, D. L., Bernabei, R., et al. (2013). Metabolic syndrome and quality of life in the elderly: age and gender differences. Eur. J. Nutr. 52 (1), 307–316. Epub 2012 Mar 11. PMID: 22406906. doi:10.1007/s00394-012-0337-1
Lee, S. J., Lee, D. Y., O'Connell, J. F., Egan, J. M., and Kim, Y. (2022). Black ginseng ameliorates cellular senescence via p53 p21/p16 pathway in aged mice. Biol. (Basel) 11 (8), 1108. PMID: 35892965; PMCID: PMC9331701. doi:10.3390/biology11081108
Lee, W. S., Kim, S. W., and Ryu, W. S. (2009). Progression and observational frequency of atheromatous plaques in autopsied coronary arteries. Korean Circ. J. 39 (10), 399–407. Epub 2009 Oct 28. PMID: 19949584; PMCID: PMC2771793. doi:10.4070/kcj.2009.39.10.399
Lesniewski, L. A., Connell, M. L., Durrant, J. R., Folian, B. J., Anderson, M. C., Donato, A. J., et al. (2009). B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J. Gerontol. A Biol. Sci. Med. Sci. 64 (1), 9–20. Epub 2009 Feb 10. PMID: 19211548; PMCID: PMC2691198. doi:10.1093/gerona/gln049
Li, N., Zhao, S., Zhang, Z., Zhu, Y., Gliniak, C. M., Vishvanath, L., et al. (2021b). Adiponectin preserves metabolic fitness during aging. Elife 10, e65108. PMID: 33904399; PMCID: PMC8099426. doi:10.7554/eLife.65108
Li, T., Li, H., Li, W., Chen, S., Feng, T., Jiao, W., et al. (2019). Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol. Immunol. 112, 322–329. Epub 2019 Jun 22. PMID: 31238287. doi:10.1016/j.molimm.2019.06.008
Li, T., Wang, P., Wang, X., Liu, Z., Zhang, Z., Zhang, Y., et al. (2023). Inflammation and insulin resistance in diabetic chronic coronary syndrome patients. Nutrients 15 (12), 2808. PMID: 37375712; PMCID: PMC10301506. doi:10.3390/nu15122808
Li, W., Qiu, X., Ma, H., and Geng, Q. (2023). Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front. Endocrinol. (Lausanne) 13, 1029736. PMID: 36733801; PMCID: PMC9886893. doi:10.3389/fendo.2022.1029736
Li, X. M., Ma, Y. L., and Liu, X. J. (2007). Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol. 111 (3), 504–511. Epub 2006 Dec 28. PMID: 17224253. doi:10.1016/j.jep.2006.12.024
Li, Z., Zhang, Z., Ren, Y., Wang, Y., Fang, J., Yue, H., et al. (2021a). Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology 22 (2), 165–187. Epub 2021 Jan 27. PMID: 33502634; PMCID: PMC7838467. doi:10.1007/s10522-021-09910-5
Liang, X., Or, B., Tsoi, M. F., Cheung, C. L., and Cheung, B. M. Y. (2023). Prevalence of metabolic syndrome in the United States national health and nutrition examination survey 2011-18. Postgrad. Med. J. 99 (1175), 985–992. PMID: 36906842. Lindblad U, Langer RD. doi:10.1093/postmj/qgad008
Lindblad, U., Langer, R. D., Wingard, D. L., Thomas, R. G., and Barrett-Connor, E. L. (2001). Metabolic syndrome and ischemic heart disease in elderly men and women. Am. J. Epidemiol. 153 (5), 481–489. PMID: 11226980. doi:10.1093/aje/153.5.481
Liu, C.-Y., Chang, C.-W., Lee, H.-C., Chen, Y.-J., Tsai, T.-Hu, Chiau, J.-S., et al. (2016a). Metabolic damage presents differently in young and early-aged C57bl/6 mice fed a high-fat diet. Int. J. Gerontology 10, 105–111. doi:10.1016/j.ijge.2015.10.004
Liu, H., Chu, S., and Wu, Z. (2021a). Loss of toll-like receptor 4 ameliorates cardiovascular dysfunction in aged mice. Immun. Ageing 18 (1), 42. PMID: 34740366; PMCID: PMC8569991. doi:10.1186/s12979-021-00251-y
Liu, J., Huang, K., Cai, G. Y., Chen, X. M., Yang, J. R., Lin, L. R., et al. (2014). Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 26 (1), 110–121. Epub 2013 Oct 7. PMID: 24113348. doi:10.1016/j.cellsig.2013.10.002
Liu, J., Lu, R., Wang, Y., Hu, Y., Jia, Y., Yang, N., et al. (2016b). PPARα agonist fenofibrate reduced the secreting load of β-cells in hypertriglyceridemia patients with normal glucose tolerance. PPAR Res. 2016, 6232036. Epub 2016 Feb 29. PMID: 27034649; PMCID: PMC4789521. doi:10.1155/2016/6232036
Liu, J., Masurekar, M. R., Vatner, D. E., Jyothirmayi, G. N., Regan, T. J., Vatner, S. F., et al. (2003). Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am. J. Physiol. Heart Circ. Physiol. 285 (6), H2587–H2591. Epub 2003 Aug 28. PMID: 12946933. doi:10.1152/ajpheart.00516.2003
Liu, X., Longchamps, R. J., Wiggins, K. L., Raffield, L. M., Bielak, L. F., Zhao, W., et al. (2021b). Association of mitochondrial DNA copy number with cardiometabolic diseases. Cell Genom 1 (1), 100006. PMID: 35036986; PMCID: PMC8758111. doi:10.1016/j.xgen.2021.100006
Lumeng, C. N., Liu, J., Geletka, L., Delaney, C., Delproposto, J., Desai, A., et al. (2011). Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187 (12), 6208–6216. Epub 2011 Nov 9. PMID: 22075699; PMCID: PMC3237772. doi:10.4049/jimmunol.1102188
Luo, D., Chen, K., Li, J., Fang, Z., Pang, H., Yin, Y., et al. (2020). Gut microbiota combined with metabolomics reveals the metabolic profile of the normal aging process and the anti-aging effect of FuFang Zhenshu TiaoZhi (FTZ) in mice. Biomed. Pharmacother. 121, 109550. Epub 2019 Nov 5. PMID: 31704617. doi:10.1016/j.biopha.2019.109550
Maas, S. C. E., Mens, M. M. J., Kühnel, B., van Meurs, J. B. J., Uitterlinden, A. G., Peters, A., et al. (2020). Smoking-related changes in DNA methylation and gene expression are associated with cardio-metabolic traits. Clin. Epigenetics 12 (1), 157. PMID: 33092652; PMCID: PMC7579899. doi:10.1186/s13148-020-00951-0
Madrigal-Matute, J., Cuervo, A. M., and Sluimer, J. C. (2022). Chaperone-mediated autophagy protects against atherosclerosis. Autophagy 18 (10), 2505–2507. Epub 2022 Jul 19. PMID: 35787098; PMCID: PMC9542634. doi:10.1080/15548627.2022.2096397
Malgor, R., Bhatt, P. M., Connolly, B. A., Jacoby, D. L., Feldmann, K. J., Silver, M. J., et al. (2014). Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic lesions. Inflamm. Res. 63 (4), 277–285. Epub 2013 Dec 18. PMID: 24346141; PMCID: PMC3950563. doi:10.1007/s00011-013-0697-x
Maris, L., and Ghitea, T. C. (2023). Can cardiometabolic risk Be reduced in the elderly? Comprehensive epidemiological study. Geriatr. (Basel). 8 (4), 73. PMID: 37489321; PMCID: PMC10366737. doi:10.3390/geriatrics8040073
Marmentini, C., Soares, G. M., Bronczek, G. A., Piovan, S., Mareze-Costa, C. E., Carneiro, E. M., et al. (2021). Aging reduces insulin clearance in mice. Front. Endocrinol. (Lausanne) 12, 679492. PMID: 34054736; PMCID: PMC8150109. doi:10.3389/fendo.2021.679492
Marsland, A. L., McCaffery, J. M., Muldoon, M. F., and Manuck, S. B. (2010). Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism 59 (12), 1801–1808. PMID: 20619428; PMCID: PMC2955187. doi:10.1016/j.metabol.2010.05.015
Martini, E., Cremonesi, M., Panico, C., Carullo, P., Bonfiglio, C. A., Serio, S., et al. (2020). T cell costimulation blockade blunts age-related heart failure. Circ. Res. 127 (8), 1115–1117. Epub 2020 Aug 17. PMID: 32806992; PMCID: PMC7508271. doi:10.1161/CIRCRESAHA.119.316530
McMillin, J. B., Taffet, G. E., Taegtmeyer, H., Hudson, E. K., and Tate, C. A. (1993). Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc Res. 27 (12), 2222–2228. PMID: 8313432. doi:10.1093/cvr/27.12.2222
Medoro, A., Saso, L., Scapagnini, G., and Davinelli, S. (2023). NRF2 signaling pathway and telomere length in aging and age-related diseases. Mol. Cell Biochem. 2. Epub ahead of print. PMID: 37917279. doi:10.1007/s11010-023-04878-x
Melov, S., Hinerfeld, D., Esposito, L., and Wallace, D. C. (1997). Multi-organ characterization of mitochondrial genomic rearrangements in ad libitum and caloric restricted mice show striking somatic mitochondrial DNA rearrangements with age. Nucleic Acids Res. 25 (5), 974–982. PMID: 9023106; PMCID: PMC146531. doi:10.1093/nar/25.5.974
Mohamad, M., Mitrea, N., Nicolae, A., Constantinescu, M.-Z., Dragoi, C. M., Arsene, A., et al. (2014). The dynamics of adiponectin and leptin on metabolic syndrome patients and age matched healthy subjects. Farmacia 62, 524–537.
Mora-Urda, A. I., Acevedo, P., and Montero López, M. P. (2019). Relationship between prenatal and postnatal conditions and accelerated postnatal growth. Impact on the rigidity of the arterial wall and obesity in childhood. J. Dev. Orig. Health Dis. 10 (4), 436–446. PMID: 31347487. doi:10.1017/S2040174418001058
Moreau, R., Heath, S. H., Doneanu, C. E., Harris, R. A., and Hagen, T. M. (2004). Age-related compensatory activation of pyruvate dehydrogenase complex in rat heart. Biochem. Biophys. Res. Commun. 325 (1), 48–58. PMID: 15522199. doi:10.1016/j.bbrc.2004.10.011
Morgan, R. G., Ives, S. J., Lesniewski, L. A., Cawthon, R. M., Andtbacka, R. H., Noyes, R. D., et al. (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol. 305 (2), H251–H258. Epub 2013 May 10. PMID: 23666675; PMCID: PMC3726958. doi:10.1152/ajpheart.00197.2013
Nagibin, V., Egan Benova, T., Viczenczova, C., Szeiffova Bacova, B., Dovinova, I., Barancik, M., et al. (2016). Ageing related down-regulation of myocardial connexin-43 and up-regulation of MMP-2 may predict propensity to atrial fibrillation in experimental animals. Physiol. Res. 65 (Suppl. 1), S91-S100–S100. PMID: 27643943. doi:10.33549/physiolres.933389
Nations, U. (2019) “Department of economic, social affairs, and population division,” in World population ageing 2019: highlights.
Niemann, B., Chen, Y., Teschner, M., Li, L., Silber, R. E., and Rohrbach, S. (2011). Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J. Am. Coll. Cardiol. 57 (5), 577–585. PMID: 21272749. doi:10.1016/j.jacc.2010.09.040
Nilsson, J., Jovinge, S., Niemann, A., Reneland, R., and Lithell, H. (1998). Relation between plasma tumor necrosis factor-alpha and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 18 (8), 1199–1202. PMID: 9714125. doi:10.1161/01.atv.18.8.1199
Olgar, Y., Tuncay, E., Degirmenci, S., Billur, D., Dhingra, R., Kirshenbaum, L., et al. (2020). Ageing-associated increase in SGLT2 disrupts mitochondrial/sarcoplasmic reticulum Ca2+ homeostasis and promotes cardiac dysfunction. J. Cell Mol. Med. 24 (15), 8567–8578. Epub 2020 Jul 11. PMID: 32652890; PMCID: PMC7412693. doi:10.1111/jcmm.15483
Onder, G., Penninx, B. W., Lapuerta, P., Fried, L. P., Ostir, G. V., Guralnik, J. M., et al. (2002). Change in physical performance over time in older women: the Women's Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 57 (5), M289–M293. PMID: 11983722. doi:10.1093/gerona/57.5.m289
Ortiz-Rodríguez, M. A., Yáñez-Velasco, L., Carnevale, A., Romero-Hidalgo, S., Bernal, D., Aguilar-Salinas, C., et al. (2017). Prevalence of metabolic syndrome among elderly Mexicans. Arch. Gerontol. Geriatr. 73, 288–293. Epub 2017 Sep 7. PMID: 28910752. doi:10.1016/j.archger.2017.09.001
Palmer, A. K., Xu, M., Zhu, Y., Pirtskhalava, T., Weivoda, M. M., Hachfeld, C. M., et al. (2019). Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18 (3), e12950. Epub 2019 Mar 25. PMID: 30907060; PMCID: PMC6516193. doi:10.1111/acel.12950
Palaniyappan, A., and Alphonse, R. (2011). Immunomodulatory effect of DL-α-lipoic acid in aged rats. Exp. Gerontol. 46 (9), 709–715. doi:10.1016/j.exger.2011.04.004
Pasini, E., Aquilani, R., Testa, C., Baiardi, P., Angioletti, S., Boschi, F., et al. (2016). Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 4 (3), 220–227. Epub 2015 Dec 9. PMID: 26682791. doi:10.1016/j.jchf.2015.10.009
Patwary, M., Talha, K., Razzaque, M., Liza, F. J., and Istiaque, A. (2022). Cardiometabolic syndrome: an emerging global health issue, J. Bangladesh Coll. Phys. Surg. 40. 287. 291. doi:10.3329/jbcps.v40i4.61892
Pei, Z., Yang, C., Guo, Y., Dong, M., and Wang, F. (2021). Effect of different exercise training intensities on age-related cardiac damage in male mice. Aging (Albany NY) 13 (17), 21700–21711. Epub 2021 Sep 14. PMID: 34520392; PMCID: PMC8457595. doi:10.18632/aging.203513
Petersen, K. F., Befroy, D., Dufour, S., Dziura, J., Ariyan, C., Rothman, D. L., et al. (2003). Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300 (5622), 1140–1142. PMID: 12750520; PMCID: PMC3004429. doi:10.1126/science.1082889
Prineas, R. J., Folsom, A. R., and Kaye, S. A. (1993). Central adiposity and increased risk of coronary artery disease mortality in older women. Ann. Epidemiol. 3 (1), 35–41. PMID: 8287154. doi:10.1016/1047-2797(93)90007-q
Ramesh, T., Kim, S. W., Sung, J. H., Hwang, S. Y., Sohn, S. H., Yoo, S. K., et al. (2012). Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 47 (1), 77–84. Epub 2011 Oct 29. PMID: 22075532. doi:10.1016/j.exger.2011.10.007
Ramírez-Manent, J. I., Altisench Jané, B., Arroyo Bote, S., López Roig, C., González San Miguel, H., and López-González, A. A. (2022b). Cardiometabolic profile of 15057 elderly Spanish workers: association of sociodemographic variables and tobacco consumption. BMC Geriatr. 22 (1), 872. doi:10.1186/s12877-022-03547-w
Ramírez Manent, J. I., Altisench Jané, B., Sanchís Cortés, P., Busquets-Cortés, C., Arroyo Bote, S., Masmiquel Comas, L., et al. (2022a). Impact of COVID-19 lockdown on anthropometric variables, blood pressure, and glucose and lipid profile in healthy adults: a before and after pandemic lockdown longitudinal study. Nutrients 14 (6), 1237. PMID: 35334894; PMCID: PMC8953154. doi:10.3390/nu14061237
Reuter, H., Perner, B., Wahl, F., Rohde, L., Koch, P., Groth, M., et al. (2022). Aging activates the immune system and alters the regenerative capacity in the zebrafish heart. Cells 11 (3), 345. PMID: 35159152; PMCID: PMC8834511. doi:10.3390/cells11030345
Reynaert, N. L., Gopal, P., Rutten, E. P. A., Wouters, E. F. M., and Schalkwijk, C. G. (2016). Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 81 (Pt B), 403–418. Epub 2016 Jun 29. PMID: 27373680. doi:10.1016/j.biocel.2016.06.016
Rezaianzadeh, A., Namayandeh, S. M., and Sadr, S. M. (2012). National cholesterol education Program adult treatment Panel III versus international diabetic federation definition of metabolic syndrome, which one is associated with diabetes mellitus and coronary artery disease? Int. J. Prev. Med. 3 (8), 552–558. PMID: 22973485; PMCID: PMC3429802.
Reznick, R. M., Zong, H., Li, J., Morino, K., Moore, I. K., Yu, H. J., et al. (2007). Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 5 (2), 151–156. PMID: 17276357; PMCID: PMC1885964. doi:10.1016/j.cmet.2007.01.008
Richard, C., Wadowski, M., Goruk, S., Cameron, L., Sharma, A. M., and Field, C. J. (2017). Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 5 (1), e000379. PMID: 28761653; PMCID: PMC5530252. doi:10.1136/bmjdrc-2016-000379
Roh, J. D., Hobson, R., Chaudhari, V., Quintero, P., Yeri, A., Benson, M., et al. (2019). Activin type II receptor signaling in cardiac aging and heart failure. Sci. Transl. Med. 11 (482), eaau8680. PMID: 30842316; PMCID: PMC7124007. doi:10.1126/scitranslmed.aau8680
Rovillain, E., Mansfield, L., Caetano, C., Alvarez-Fernandez, M., Caballero, O. L., Medema, R. H., et al. (2011). Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene 30 (20), 2356–2366. Epub 2011 Jan 17. PMID: 21242976; PMCID: PMC3080811. doi:10.1038/onc.2010.611
Sabbagh, J. J., O'Leary, J. C. 3rd, Blair, L. J., Klengel, T., Nordhues, B. A., Fontaine, S. N., et al. (2014). Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PLoS One 9 (9), e107241. PMID: 25191701; PMCID: PMC4156438. doi:10.1371/journal.pone.0107241
Saeedi, F., Baqeri, E., Bidokhti, A., Moodi, M., Sharifi, F., and Riahi, S. M. (2023). Clinical utility of lipid ratios as potential predictors of metabolic syndrome among the elderly population: birjand Longitudinal Aging Study (BLAS). BMC Geriatr. 23 (1), 403. PMID: 37400781; PMCID: PMC10316584. doi:10.1186/s12877-023-04040-8
Sandek, A., Bauditz, J., Swidsinski, A., Buhner, S., Weber-Eibel, J., von Haehling, S., et al. (2007). Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 50 (16), 1561–1569. Epub 2007 Oct 1. PMID: 17936155. doi:10.1016/j.jacc.2007.07.016
Santamaria-Garcia, H., Sainz-Ballesteros, A., Hernandez, H., Moguilner, S., Maito, M., Ochoa-Rosales, C., et al. (2023). Factors associated with healthy aging in Latin American populations. Nat. Med. 29 (9), 2248–2258. Epub 2023 Aug 10. PMID: 37563242; PMCID: PMC10504086. doi:10.1038/s41591-023-02495-1
Sawaki, D., Czibik, G., Pini, M., Ternacle, J., Suffee, N., Mercedes, R., et al. (2018). Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 138 (8), 809–822. PMID: 29500246. doi:10.1161/CIRCULATIONAHA.117.031358
Schautz, B., Later, W., Heller, M., Peters, A., Müller, M. J., and Bosy-Westphal, A. (2012). Impact of age on leptin and adiponectin independent of adiposity. Br. J. Nutr. 108 (2), 363–370. Epub 2012 Feb 28. PMID: 22370102. doi:10.1017/S0007114511005605
Schloesser, A., Campbell, G., Glüer, C. C., Rimbach, G., and Huebbe, P. (2015). Restriction on an energy-dense diet improves markers of metabolic health and cellular aging in mice through decreasing hepatic mTOR activity. Rejuvenation Res. 18 (1), 30–39. PMID: 25405871; PMCID: PMC4340804. doi:10.1089/rej.2014.1630
Seidelmann, S. B., Feofanova, E., Yu, B., Franceschini, N., Claggett, B., Kuokkanen, M., et al. (2018). Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J. Am. Coll. Cardiol. 72 (15), 1763–1773. PMID: 30286918; PMCID: PMC6403489. doi:10.1016/j.jacc.2018.07.061
Serio, S., Pagiatakis, C., Musolino, E., Felicetta, A., Carullo, P., Laura Frances, J., et al. (2023). Cardiac aging is promoted by pseudohypoxia increasing p300-induced glycolysis. Circ. Res. 133 (8), 687–703. doi:10.1161/CIRCRESAHA.123.322676
Shimosawa, T., Ogihara, T., Matsui, H., Asano, T., Ando, K., and Fujita, T. (2003). Deficiency of adrenomedullin induces insulin resistance by increasing oxidative stress. Hypertens. Dallas, Tex. 41 (5), 1080–1085. PMID: 12668590. doi:10.1161/01.hyp.0000066846.46422.2c
Simo, O. K., Berrougui, H., Fulop, T., and Khalil, A. (2021). The susceptibility to diet-induced atherosclerosis is exacerbated with aging in C57B1/6 mice. Biomedicines 9 (5), 487. PMID: 33946646; PMCID: PMC8146644. doi:10.3390/biomedicines9050487
Singh, A. K., Pandey, S. K., Saha, G., and Gattupalli, N. K. (2015). Pyrroloquinoline quinone (PQQ) producing Escherichia coli Nissle 1917 (EcN) alleviates age associated oxidative stress and hyperlipidemia, and improves mitochondrial function in ageing rats. Exp. Gerontol. 66, 1–9. Epub 2015 Apr 2. PMID: 25843018. doi:10.1016/j.exger.2015.04.001
Sivonová, M., Tatarková, Z., Duracková, Z., Dobrota, D., Lehotský, J., Matáková, T., et al. (2007). Relationship between antioxidant potential and oxidative damage to lipids, proteins and DNA in aged rats. Physiol. Res. 56 (6), 757–764. Epub 2006 Nov 6. PMID: 17087608. doi:10.33549/physiolres.931094
Skoblow, H. F., and Proulx, C. M. (2022). C-reactive protein, subjective aging, and incident cardiovascular disease: a mediation model. J. Gerontol. B Psychol. Sci. Soc. Sci. 77 (9), 1654–1658. PMID: 35279030; PMCID: PMC9434473. doi:10.1093/geronb/gbac051
Skoog, T., Dichtl, W., Boquist, S., Skoglund-Andersson, C., Karpe, F., Tang, R., et al. (2002). Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 23 (5), 376–383. PMID: 11846495. doi:10.1053/euhj.2001.2805
Sohal, R. S., Agarwal, S., Candas, M., Forster, M. J., and Lal, H. (1994). Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev. 76 (2-3), 215–224. PMID: 7885066. doi:10.1016/0047-6374(94)91595-4
Solon-Biet, S. M., McMahon, A. C., Ballard, J. W., Ruohonen, K., Wu, L. E., Cogger, V. C., et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19 (3), 418–430. Erratum in: Cell Metab. doi:10.1016/j.cmet.2014.02.009
Sołtysik, B. K., Karolczak, K., Watała, C., and Kostka, T. (2022). The association of oxidative and antioxidant potential with cardiometabolic risk profile in the group of 60- to 65-year-old seniors from Central Poland. Antioxidants (Basel) 11 (6), 1065. PMID: 35739962; PMCID: PMC9220010. doi:10.3390/antiox11061065
Sreedhar, R., Giridharan, V. V., Arumugam, S., Karuppagounder, V., Palaniyandi, S. S., Krishnamurthy, P., et al. (2016). Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors 42 (4), 368–375. Epub 2016 Apr 18. PMID: 27087487. doi:10.1002/biof.1280
Srivastava, N., Iyer, S., Sudan, R., Youngs, C., Engelman, R. W., Howard, K. T., et al. (2016). A small-molecule inhibitor of SHIP1 reverses age- and diet-associated obesity and metabolic syndrome. JCI Insight 1 (11), e88544. PMID: 27536730; PMCID: PMC4985248. doi:10.1172/jci.insight.88544
Suastika, K., Dwipayana, P., Made Ratna Saraswati, I., Kuswardhani, T., Astika, N., Putrawan, I. B., et al. (2011). Relationship between age and metabolic disorders in the population of Bali. J. Clin. Gerontology Geriatrics 2, 47–52. doi:10.1016/j.jcgg.2011.03.001
Succurro, E., Andreozzi, F., Sciacqua, A., Hribal, M. L., Perticone, F., Sesti, G., et al. (2008). Reciprocal association of plasma IGF-1 and interleukin-6 levels with cardiometabolic risk factors in nondiabetic subjects. Diabetes Care 31 (9), 1886–1888. Epub 2008 Jun 5. Erratum in: Diabetes Care. doi:10.2337/dc08-0553
Sudheesh, N. P., Ajith, T. A., Ramnath, V., and Janardhanan, K. K. (2010). Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin. Nutr. 29 (3), 406–412. Epub 2009 Dec 30. PMID: 20044182. doi:10.1016/j.clnu.2009.12.003
Suehiro, A., Uchida, K., Nakanishi, M., and Wakabayashi, I. (2016). Measurement of urinary advanced glycation end-products (AGEs) using a fluorescence assay for metabolic syndrome-related screening tests. Diabetes Metab. Syndr. 10 (1 Suppl. 1), S110–S113. Epub 2015 Nov 12. PMID: 26626334. doi:10.1016/j.dsx.2015.10.004
Taffet, G. E., Pham, T. T., and Hartley, C. J. (1997). The age-associated alterations in late diastolic function in mice are improved by caloric restriction. Journals Gerontology - Ser. A Biol. Sci. Med. Sci. 52 (6), 285–290. doi:10.1093/gerona/52A.6.B285
Tang, Z., Wang, C., Song, X., Shi, J., Mitnitski, A., Fang, X., et al. (2013). Co-occurrence of cardiometabolic diseases and frailty in older Chinese adults in the Beijing Longitudinal Study of Ageing. Age Ageing 42 (3), 346–351. Epub 2013 Mar 4. Erratum in: Age Ageing. doi:10.1093/ageing/aft004
Toubal, A., Kiaf, B., Beaudoin, L., Cagninacci, L., Rhimi, M., Fruchet, B., et al. (2020). Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat. Commun. 11 (1), 3755. PMID: 32709874; PMCID: PMC7381641. doi:10.1038/s41467-020-17307-0
Tylutka, A., Morawin, B., Walas, Ł., Michałek, M., Gwara, A., and Zembron-Lacny, A. (2023). Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci. Rep. 13 (1), 89. PMID: 36596839; PMCID: PMC9810713. doi:10.1038/s41598-022-27269-6
Tyrrell, D. J., Blin, M. G., Song, J., Wood, S. C., Zhang, M., Beard, D. A., et al. (2020). Age-associated mitochondrial dysfunction accelerates atherogenesis. Circ. Res. 126 (3), 298–314. Epub 2019 Dec 9. PMID: 31818196; PMCID: PMC7006722. doi:10.1161/CIRCRESAHA.119.315644
Ungvari, Z., Orosz, Z., Labinskyy, N., Rivera, A., Xiangmin, Z., Smith, K., et al. (2007). Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 293 (1), H37–H47. Epub 2007 Apr 6. PMID: 17416599. doi:10.1152/ajpheart.01346.2006
Ungvari, Z., Pacher, P., Rischák, K., Szollár, L., and Koller, A. (1999). Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler. Thromb. Vasc. Biol. 19 (8), 1899–1904. PMID: 10446068. doi:10.1161/01.atv.19.8.1899
Wang, D., Xia, M., Yan, X., Li, D., Wang, L., Xu, Y., et al. (2012). Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 111 (8), 967–981. Epub 2012 Jul 19. PMID: 22821931. doi:10.1161/CIRCRESAHA.112.266502
Wang, Z., Shen, Xu H., Feng, W. M., and Qiu, W. (2017). Mast cell specific immunological biomarkers and metabolic syndrome among middle-aged and older Chinese adults. Endocr. J. 64 (3), 245–253. doi:10.1507/endocrj.EJ16-0388
Wang, Z., Shen, X. H., Feng, W. M., Ye, G. F., Qiu, W., and Li, B. (2016). Analysis of inflammatory mediators in prediabetes and newly diagnosed type 2 diabetes patients. J. Diabetes Res. 2016, 7965317. Epub 2016 Jul 5. PMID: 27478850; PMCID: PMC4949350. doi:10.1155/2016/7965317
Warrington, N. M., Beaumont, R. N., Horikoshi, M., Day, F. R., Helgeland, Ø., Laurin, C., et al. (2019). Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 51, 804–814. PMID: 31043758; PMCID: PMC6522365. doi:10.1038/s41588-019-0403-1
Werner, C., Hanhoun, M., Widmann, T., Kazakov, A., Semenov, A., Pöss, J., et al. (2008). Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. JACC 52 (6), 470–482. doi:10.1016/j.jacc.2008.04.034
Wohlfahrt, P., Redfield, M. M., Lopez-Jimenez, F., Melenovsky, V., Kane, G. C., Rodeheffer, R. J., et al. (2014). Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail 2 (5), 489–499. Epub 2014 Sep 3. PMID: 25194285; PMCID: PMC4194131. doi:10.1016/j.jchf.2014.03.014
Xie, M., Xie, D., Yang, Y., Zhang, Y., Li, K., Zhou, B., et al. (2019). Association of high-sensitivity C-reactive protein in middle-aged and elderly Chinese people with hyperuricaemia and risk of coronary heart disease: a cross-sectional study. BMJ Open 9 (10), e028351. PMID: 31630099; PMCID: PMC6803153. doi:10.1136/bmjopen-2018-028351
Xin, M., Jin, X., Cui, X., Jin, C., Piao, L., Wan, Y., et al. (2019). Dipeptidyl peptidase-4 inhibition prevents vascular aging in mice under chronic stress: modulation of oxidative stress and inflammation. Chem. Biol. Interact. 314, 108842. Epub 2019 Oct 2. PMID: 31586451. doi:10.1016/j.cbi.2019.108842
Yan, L., Vatner, D. E., O'Connor, J. P., Ivessa, A., Ge, H., Chen, W., et al. (2007). Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130 (2), 247–258. PMID: 17662940. doi:10.1016/j.cell.2007.05.038
Yen, Y. F., Hu, H. Y., Lin, I. F., Lai, Y. J., Su, V. Y., Pan, S. W., et al. (2015). Associations of metabolic syndrome and its components with mortality in the elderly: a cohort study of 73,547 Taiwanese adults. Med. Baltim. 94 (23), e956. PMID: 26061328; PMCID: PMC4616481. doi:10.1097/MD.0000000000000956
Youm, Y. H., Kanneganti, T. D., Vandanmagsar, B., Zhu, X., Ravussin, A., Adijiang, A., et al. (2012). The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 1 (1), 56–68. Epub 2012 Jan 26. PMID: 22832107; PMCID: PMC3883512. doi:10.1016/j.celrep.2011.11.005
Zang, M., Zuccollo, A., Hou, X., Nagata, D., Walsh, K., Herscovitz, H., et al. (2004). AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J. Biol. Chem. 279 (46), 47898–47905. Epub 2004 Sep 14. PMID: 15371448. doi:10.1074/jbc.M408149200
Zannas, A., Jia, M., Hafner, K., Baumert, J., Wiechmann, T., Pape, J., et al. (2019). Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB–driven inflammation and cardiovascular risk. Proceedings Nat. Academy of Sci. (116):(23), 11370–11379. doi:10.1073/pnas.1816847116
Zha, Z., Wang, J., Wang, X., Lu, M., and Guo, Y. (2017). Involvement of PINK1/Parkin-mediated mitophagy in AGE-induced cardiomyocyte aging. Int. J. Cardiol. 227, 201–208. Epub 2016 Nov 9. PMID: 27839819. doi:10.1016/j.ijcard.2016.11.161
Zhang, J., Wang, H., Slotabec, L., Cheng, F., Tan, Y., and Li, J. (2023a). Alterations of SIRT1/SIRT3 subcellular distribution in aging undermine cardiometabolic homeostasis during ischemia and reperfusion. Aging Cell 22 (9), e13930. Epub 2023 Aug 3. PMID: 37537789; PMCID: PMC10497814. doi:10.1111/acel.13930
Zhang, J., Zhu, J., Zhao, B., Nie, D., Wang, W., Qi, Y., et al. (2023b). LTF induces senescence and degeneration in the meniscus via the NF-κB signaling pathway: a study based on integrated bioinformatics analysis and experimental validation. Front. Mol. Biosci. 10, 1134253. PMID: 37168259; PMCID: PMC10164984. doi:10.3389/fmolb.2023.1134253
Zhang, X., Wang, Y., Li, Y., Gui, J., Mei, Y., Yang, X., et al. (2024). Four-years change of BMI and waist circumference are associated with metabolic syndrome in middle-aged and elderly Chinese. Sci. Rep. 14 (1), 10220. PMID: 38702424; PMCID: PMC11068757. doi:10.1038/s41598-024-60172-w
Zhang, X., Yang, Y., Su, J., Zheng, X., Wang, C., Chen, S., et al. (2021). Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience 43 (2), 709–725. Epub 2020 May 17. PMID: 32418021; PMCID: PMC8110635. doi:10.1007/s11357-020-00188-y
Zhao, X., Lu, C., Song, B., Chen, D., Teng, D., Shan, Z., et al. (2023). The prevalence and clustering of metabolic syndrome risk components in Chinese population: a cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1290855. doi:10.3389/fendo.2023.1290855
Keywords: cardiometabolic risk, aging, inflammation, immune dysfunction, mitochondrial dysfunction, oxidative stress, gut microbiome dysbiosis, genetic and environmental contributors
Citation: Sarkar S, Prasanna VS, Das P, Suzuki H, Fujihara K, Kodama S, Sone H, Sreedhar R, Velayutham R, Watanabe K and Arumugam S (2024) The onset and the development of cardiometabolic aging: an insight into the underlying mechanisms. Front. Pharmacol. 15:1447890. doi: 10.3389/fphar.2024.1447890
Received: 12 June 2024; Accepted: 22 August 2024;
Published: 26 September 2024.
Edited by:
Enzo Nisoli, University of Milan, ItalyReviewed by:
Carolina Greco, Humanitas University, ItalyPhiwayinkosi V. Dludla, South African Medical Research Council, South Africa
Copyright © 2024 Sarkar, Prasanna, Das, Suzuki, Fujihara, Kodama, Sone, Sreedhar, Velayutham, Watanabe and Arumugam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Somasundaram Arumugam, c29tYXN1bmRhcmFtLm5pcGVya0BuaWMuaW4=, c29tYXN1bmRhcmFtMTQzQGdtYWlsLmNvbQ==; Kenichi Watanabe, d2F0YWtlbkBtZWQubmlpZ2F0YS11LmFjLmpw
 Sulogna Sarkar1
Sulogna Sarkar1 Hirohito Sone
Hirohito Sone Somasundaram Arumugam
Somasundaram Arumugam