- 1Department of Pulmonary and Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua, Zhejiang Province, China
- 2School of Medicine, Shaoxing University, Shaoxing, Zhejiang Province, China
- 3Department of Medical Records Quality Management, Jinhua Municipal Central Hospital, Jinhua, Zhejiang Province, China
Background: The impact of glucocorticoid use on mortality risk in pneumonia patients remains unclear. This study aimed to investigate the relationship between the accumulated dose of glucocorticoids (ADG) and secondary pneumonia mortality risk among patients receiving oral or intravenous glucocorticoids.
Methods: Data from the DRYAD database were analyzed, covering pneumonia patients from six academic hospitals over a 5-year period who had been administered oral or intravenous glucocorticoids. Piecewise linear regression and multivariate regression analysis were utilized to assess the association between ADG and mortality risk in pneumonia patients, while adjusting for potential confounders.
Results: Among the 628 pneumonia patients included, the 30-day mortality rate was 23.1% and the 90-day mortality rate was 26.4%. In the high-dose glucocorticoid group (≥24 mg/day of methylprednisolone or an equivalent glucocorticoid within 30 days before admission), the 30-day and 90-day mortality rates were 31.2% and 35.9%, respectively. Piecewise linear regression analysis demonstrated a non-linear relationship between ADG and mortality risk in pneumonia patients. Multivariate regression analysis revealed a significantly lower mortality risk in patients receiving an ADG of 20–39 g methylprednisolone compared to those receiving lower (<20 g) or higher doses (≥40 g), after adjusting for potential confounding factors. Additionally, in the high-dose glucocorticoid group, surpassing the inflection point of 20 g of methylprednisolone raised the 30-day and 90-day mortality risks (adjusted odds ratio, 95% confidence interval: 1.16, 1.03–1.30 and 1.23, 1.07–1.42, respectively). Notably, this threshold effect was observed exclusively in male patients.
Conclusion: This study provides evidence supporting a potential threshold effect between ADG and mortality risk in oral or intravenous glucocorticoid users with secondary pneumonia. Specifically, male patients receiving high-dose glucocorticoids should undergo close monitoring when the ADG of methylprednisolone exceeds 20 g, as it may be associated with an elevated risk of mortality.
Introduction
Pulmonary infections commonly afflict immunocompromised patients undergoing glucocorticoid therapy (Agustí et al., 2003). Prolonged and high-dose glucocorticoid use can induce severe immunosuppression, heightening susceptibility to serious infections (Agustí et al., 2003; Guarnotta et al., 2021; Elsouri et al., 2023). Mortality rates soar, reaching up to 45% in rheumatic disease patients on glucocorticoids who develop pulmonary infections, escalating to a staggering 93% among those requiring mechanical ventilation (Agustí et al., 2003). Yet, the threshold for determining tolerably safe doses of glucocorticoid therapy remains elusive.
In evaluating dose-related major complications of glucocorticoids use, including infections, a multidisciplinary panel of experts from the European League Against Rheumatism, involving rheumatic disease patients, found that the risk of harm was minimal for most patients receiving long-term dosages of ≤5 mg prednisone equivalent per day. Conversely, at doses exceeding 10 mg/day, the risk of harm significantly increased. For dosages ranging between >5 and ≤10 mg/day, the risk of harm hinged on patient-specific characteristics, encompassing protective and risk factors (Strehl et al., 2016). Nonetheless, comprehensive evidence on the risk of harm associated with long-term or high-dose glucocorticoid therapy remains scant, with pertinent study findings often being either absent, contradictory, or carrying a high risk of bias (Soo et al., 2024; Peng et al., 2023; Sun et al., 2020; Tang et al., 2022). Addressing these knowledge gaps, our study endeavored to elucidate the specific correlation between cumulative glucocorticoid utilization and mortality risk among pneumonia patients undergoing glucocorticoids therapy. By delving into this intricate relationship, our aim is to furnish valuable insights into the judicious administration of glucocorticoid therapy in the management of pneumonia, particularly among immunosuppressed cohorts.
Methods
Study design and subjects
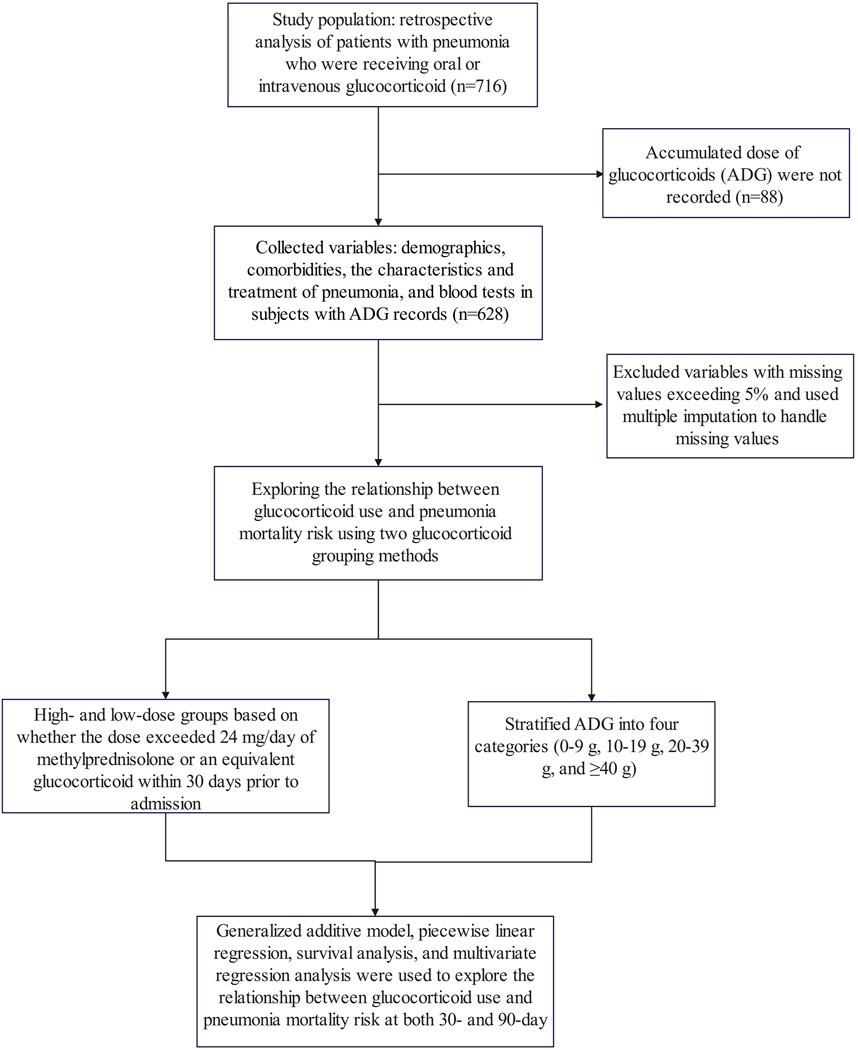
This study represents a secondary analysis of data obtained from the public database www.Datadryad.org, which allows unrestricted use of the data for research and educational purposes (Li et al., 2020a). The cohort consisted of 716 patients who received oral or intravenous glucocorticoid therapy with pneumonia and were recruited from six secondary and tertiary academic hospitals in China between January 2013 and December 2017 (Li et al., 2020b). Pneumonia diagnoses were established in accordance with the guidelines outlined by the American Thoracic Society and the Infectious Disease Society of America (Sousa et al., 2013; American Thoracic Society, Infectious Diseases Society of America, 2005). Patients included in the study met the following inclusion criteria: (1) receipt of oral or intravenous glucocorticoid treatment before admission; (2) diagnosis of pneumonia upon admission or during hospitalization; and (3) age of at least 16 years. Exclusion criteria encompassed: (1) diagnosis of non-infectious pulmonary conditions such as lung cancer, interstitial lung diseases unrelated to infection, pulmonary embolism, or heart failure; and (2) inability to provide consent for procedures (Li et al., 2020b). Furthermore, in this study, variables for which no accumulated dose of glucocorticoids (ADG) was recorded and those with missing values exceeding 5% were excluded from the analysis. The study flow chart is shown in Figure 1. The Ethics Committee of China-Japan Friendship Hospital (No. 2015-86) and all participating institutions granted approval for the study (Li et al., 2020b).
Variables collection
The study encompassed patient demographics, including gender, age, smoking and alcoholism history. Comorbid diseases such as chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, idiopathic interstitial pneumonia, interstitial lung disease, hypertension, coronary heart disease (CHD), diabetes mellitus, nephrotic syndrome, chronic renal failure, cirrhosis, connective tissue disease (CTD), cerebrovascular disease, tumor, anemia, bone marrow transplantation, solid organ transplantation, leukemia, and radiation pneumonia were also recorded. Additionally, the characteristics and treatment of pneumonia, including community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP), etiology determined from sputum and/or bronchoalveolar lavage samples, respiratory failure, CURB-65 score, pneumonia severity index, antibiotics, antiviral therapy, anti-Aspergillus treatment, use of extracorporeal membrane oxygenation, continuous veno-venous hemofiltration (CVVH), mechanical ventilation, vasoactive drugs, and other immunosuppressants were documented. Furthermore, blood tests were conducted to evaluate white blood cell count, neutrophil count, lymphocyte count, hemoglobin levels, platelet counts, alanine aminotransferase (ALT), aspartate aminotransferase, blood urea nitrogen (BUN), and serum creatinine levels. The variables included in this analysis are detailed in Table 1.
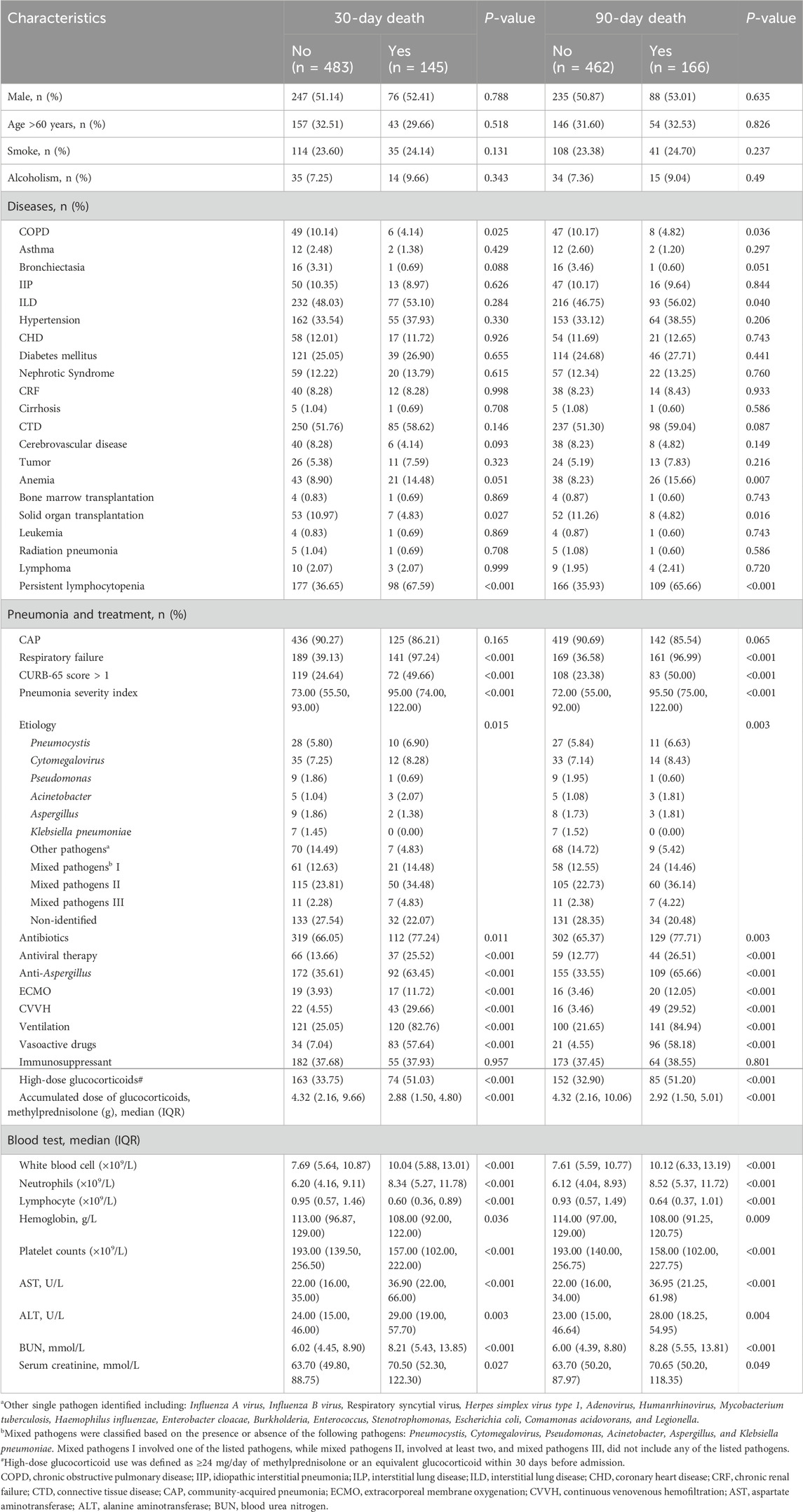
Table 1. Clinical characteristics of patients with pneumonia receiving oral or intravenous glucocorticoids.
In this study, we examined mortality risk among patients with secondary pneumonia receiving oral or intravenous glucocorticoid therapy by employing both the glucocorticoids dose stratification commonly used in previous research (Hoes et al., 2009; Buttgereit et al., 2002) and ADG stratification developed for this study. We categorized glucocorticoid use as follows: (1) High-dose glucocorticoid use was defined as ≥24 mg/day of methylprednisolone or an equivalent glucocorticoid within 30 days prior to admission, while low-dose glucocorticoid use was defined as <24 mg/day. (2) ADG was defined as the total amount of oral or intravenous glucocorticoids administered from the initiation of glucocorticoid therapy for underlying conditions until the diagnosis of pneumonia. Based on the thresholds identified in our analysis, we further stratified ADG into four categories (0–9 g, 10–19 g, 20–39 g, and ≥40 g) to explore their association with mortality risk.
Statistical analysis
The baseline characteristics of the subjects were summarized in the study. Categorical variables were presented as counts (percentage), while continuous variables were expressed as median (interquartile range, IQR). Two-group comparisons between the deceased and surviving groups were conducted using unpaired t-tests or Wilcoxon rank-sum tests for continuous variables, and Pearson’s chi-squared tests or Fisher’s exact tests for categorical variables, as appropriate. Multiple imputation was employed to address missing values. To examine the association between ADG and the risk of pneumonia death, with adjustment for potential confounders, smoothing curve fitting via a generalized additive model was utilized. Additionally, an adjusted two-piecewise linear regression analysis, coupled with the log-likelihood ratio test, was employed to identify the threshold effect of ADG on the risk of pneumonia death. Survival analysis, including Kaplan-Meier analysis and log-rank test, was employed to compare differences between high and low glucocorticoid doses. Multivariate regression analysis was conducted to assess the independent relationship between ADG and the risk of pneumonia death, with adjustment for potential confounders. In this study, two criteria were employed to adjust for potential confounders: criteria I involved the use of a covariate-discrimination algorithm, whereby variables were included if their introduction into the basic model or removal from the complete model resulted in a change in the regression coefficient of ≥10%. Covariates meeting these criteria included neutrophils, COPD, CTD, ALT, BUN, CVVH, respiratory failure, and solid organ transplantation. Criteria II involved adjusting variables based on clinical considerations, including age, etiology, and the use of other immunosuppressants. All statistical analyses were performed using R software (version 3.5.1), and a P-value <0.05 was considered statistically significant.
Results
The baseline data and characteristics of the pneumonia patients are detailed in Table 1. Among the 628 subjects who received oral or intravenous glucocorticoids and subsequently developed pneumonia, 323 (51.4%) were male, with 31.8% of patients aged over 60 years. The classification of pneumonia revealed that 561 (89.3%) individuals were CAP with 42.2% experiencing mixed pathogens. Pneumocystis and Cytomegalovirus were identified as the most common single-pathogen infections among the patients. In this study population, a total of 237 individuals (37.7%) underwent high-dose glucocorticoid therapy, with the median ADG (IQR) calculated at 3.8 (1.9–8.8) g. The median (IQR) duration of glucocorticoids use was 4 (2, 18) months, with an average duration of 22 months. The 30-day and 90-day mortality rates were recorded at 23.1% and 26.4%, respectively, reflecting the severity of the condition and the challenges associated with pneumonia management in this patient cohort.
A non-linear relationship between ADG and pneumonia mortality at both 30-day and 90-day endpoints was observed after adjustment for potential confounders (age, etiology, other immunosuppressants use, neutrophils, COPD, CTD, ALT, BUN, CVVH, respiratory failure, and solid organ transplantation), as illustrated in Figures 2A, B. This phenomenon was also evident in subgroup analyses based on different underlying diseases and pneumonia types (Figures 2C–J). Further stratifying ADG into four subgroups (0–9 g, 10–19 g, 20–39 g, and ≥40 g), multivariate regression analysis revealed that compared to the 20–39 g group, the 0–9 g, 10–19 g, and ≥40 g groups had adjusted odds ratios (aOR) and 95% confidence interval (CI) for 30-day and 90-day pneumonia mortality of 5.36 (1.36, 21.03), 5.59 (1.10, 28.48), 6.64 (1.18, 37.32), and 8.78 (2.04, 37.88), 6.88 (1.24, 38.09), 6.55 (1.07, 40.05), respectively. Moreover, the P-values for trend tests were all greater than 0.05, further confirming the presence of a non-linear relationship between ADG dose and pneumonia mortality (Table 2).
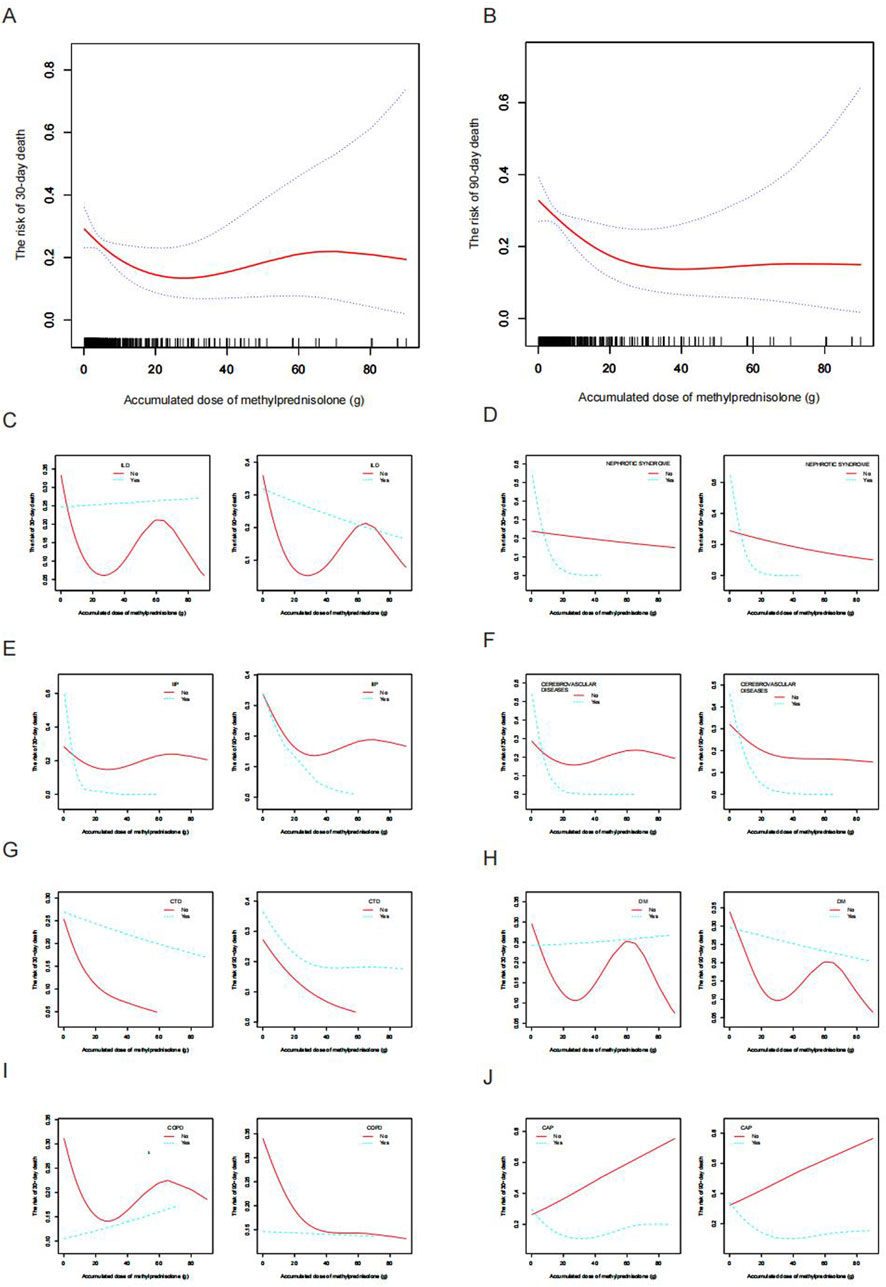
Figure 2. Smooth fitting curve illustrating the association between ADG (methylprednisolone) and the mortality risk among patients receiving glucocorticoid with secondary pneumonia. (A) The risk of 30-day death and (B) The risk of 90-day death. The ADG and risk of pneumonia death in the presence of (C) ILD, (D) Nephrotic syndrome, (E) IIP, (F) Cerebrovascular disease, (G) CTD, (H) Diabetes mellitus, (I) COPD, and (J) CAP. Adjustments were made for age, etiology, other immunosuppressants, neutrophils, COPD, CTD, ALT, BUN, CVVH, respiratory failure, and solid organ transplantation, except where the variable was analyzed. ADG, accumulated dose of glucocorticoids; ILD, interstitial lung disease; IIP, interstitial lung disease; CTD, connective tissue disease; COPD, chronic obstructive pulmonary disease; CAP, community-acquired pneumonia; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CVVH, continuous venovenous hemofiltration.
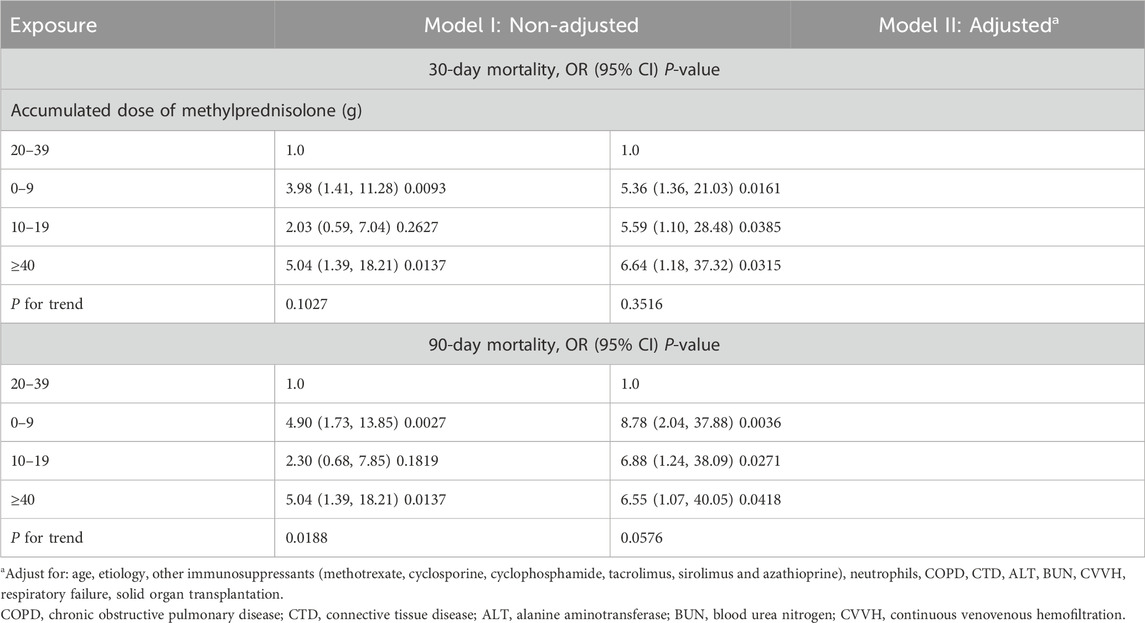
Table 2. Multivariate regression analysis of accumulated dose of methylprednisolone with the risk of mortality in patients with pneumonia.
Patients with pneumonia who received high-dose glucocorticoids therapy exhibited higher 30-day and 90-day mortality rates compared to those in the low-dose glucocorticoid group, with rates of 31.2% versus 18.2% and 35.9% versus 20.7%, respectively, demonstrating statistically significant differences between the two groups (Figure 3, P < 0.001). In contrast to patients receiving low-dose glucocorticoids, those in the high-dose group exhibited a lower proportion of patients aged over 60 years, a higher percentage with CTD, and a greater incidence of respiratory failure (67.51%). These patients had shorter durations of oral or intravenous glucocorticoid use, resulting in lower ADG compared to the low-dose glucocorticoid group. Moreover, a smaller proportion of patients in the high-dose group received concurrent treatment with other immunosuppressants (methotrexate, cyclosporine, cyclophosphamide, tacrolimus, sirolimus, and azathioprine) compared to the low-dose group (55.70% vs. 66.24%, P < 0.05). Regarding pathogen profiles, the incidence of Pneumocystis was higher among patients in the high-dose glucocorticoid group compared to those in the low-dose group. In terms of treatment, a larger proportion of patients (54.01%) in the high-dose glucocorticoid group required mechanical ventilation. Additional clinical characteristics of patients in the high-dose group can be found in Supplementary Table S1. In the high-dose glucocorticoid group, males accounted for 54.9% (130/237). Among comorbidities, males had a higher proportion of CHD compared to females, while the proportion of CTD was lower in males. Male patients also exhibited a higher percentage of pneumonia with CURB-65 score >1 and higher pneumonia severity index (P < 0.05). Additionally, in terms of blood tests, males showed significantly higher levels of renal function markers (serum creatinine and BUN) compared to females. Additional demographic characteristics and differences between genders are shown in Supplementary Table S2.

Figure 3. Kaplan-Meier analysis and comparison of mortality between high- and low-dose glucocorticoid groups. (A) 30-day survival curve analysis. (B) Comparison of 30-day mortality. (C) 90-day survival curve analysis. (D) Comparison of 90-day mortality. GC, glucocorticoid. ***P < 0.001.
In light of the non-linear relationship between ADG and pneumonia mortality risk, we conducted an in-depth analysis to explore the potential threshold effect between these two variables. Our findings revealed that the inflection point for the ADG associated with a 30-day mortality risk in the overall study population was 21.9 g, while for the 90-day mortality risk, it was 23.4 g (Table 3, P < 0.05). Subsequent subgroup analyses indicated that this threshold effect was only present in the high-dose glucocorticoid group and not in the low-dose glucocorticoid group (Table 3; Figures 4A, B). Specifically, within the high-dose glucocorticoid group, the risk of 30-day pneumonia mortality increased when the ADG exceeded 20 g (aOR 1.16, 95% CI 1.03–1.30, P = 0.0173), and there was a similar increase in the risk of 90-day mortality (aOR 1.23, 95% CI 1.07–1.42, P = 0.0040). This signifies that for each additional ADG unit beyond 20 g, the risk of death at 30 and 90 days increased by 16% and 23%, respectively (Table 3). These findings underscore the importance of considering the ADG as a critical factor in evaluating pneumonia mortality risk, particularly in patients receiving high-dose glucocorticoid therapy.
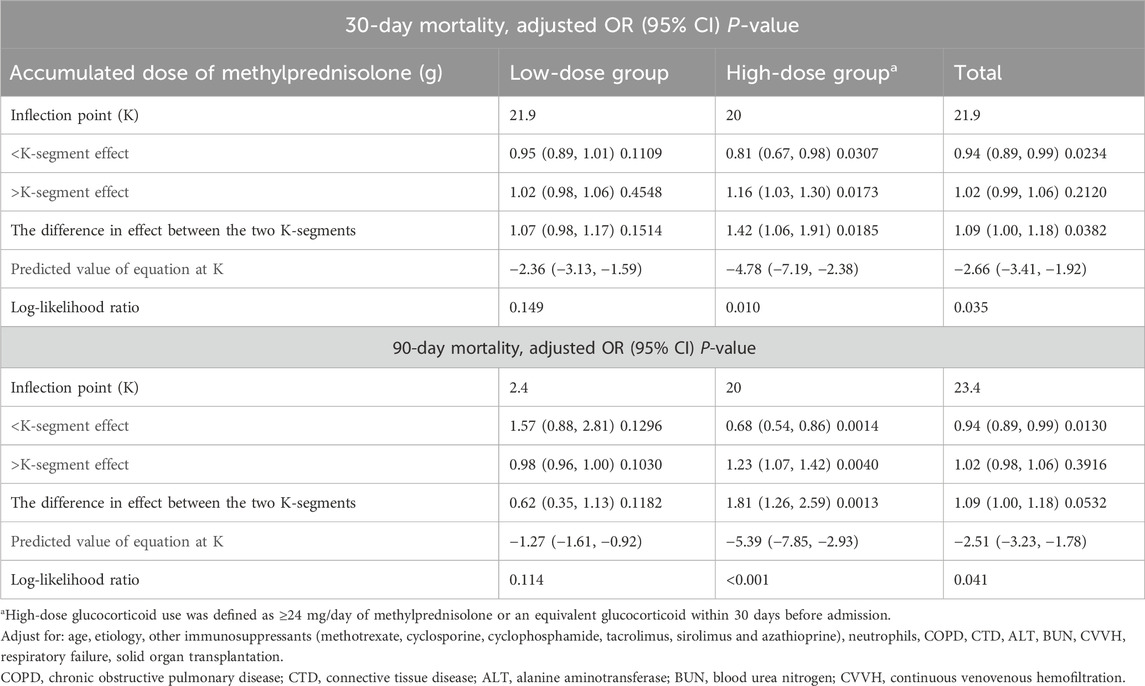
Table 3. Threshold effect of accumulated dose of methylprednisolone on the risk of mortality in patients with pneumonia in adjusted two-piecewise linear regression.

Figure 4. Smooth fitting curves depict a U-shaped correlation between ADG (methylprednisolone) and the mortality risk among pneumonia patients in the high-dose glucocorticoid population (depicted by the blue dotted line) for (A) 30-day mortality risk and (B) 90-day mortality risk. Notably, this U-shaped correlation pattern is exclusively observed in male patients (illustrated by the red line) for (C) 30-day mortality and (D) 90-day mortality. Adjustments were made for age, etiology, other immunosuppressants, neutrophils, COPD, CTD, ALT, BUN, CVVH, respiratory failure, and solid organ transplantation. ADG, accumulated dose of glucocorticoids; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CVVH, continuous venovenous hemofiltration.
Furthermore, a notable observation emerged regarding a gender-specific difference in the threshold effect. Specifically, within the high-dose glucocorticoid group, the threshold effect of ADG on the risk of pneumonia mortality was evident solely among male patients, with no such effect observed among female patients (Figures 4C, D).
Discussion
The current investigation unveiled a non-linear association between ADG and pneumonia mortality risk at both 30- and 90-day. Specifically, among male individuals receiving high doses of glucocorticoids, there was a discernible increase in pneumonia-related mortality risk when ADG exceeded 20 g.
The intricate interplay between glucocorticoid usage and pneumonia remains a captivating subject of inquiry. On one hand, a plethora of studies have unequivocally affirmed the therapeutic efficacy of glucocorticoids in the management of pneumonia, particularly in cases characterized by rapid progression or severe manifestations such as acute respiratory distress syndrome (Pinzón et al., 2021; Chan et al., 2020). The timely administration of glucocorticoids at appropriate dosages has been shown to ameliorate gas exchange, diminish the duration of recovery, reduce the necessity for escalation to intensive care, and in some instances, mitigate mortality rates (Soo et al., 2024; Pinzón et al., 2021; Souza et al., 2022). On the contrary, prolonged and high-dose administration of glucocorticoids can induce a state of immunosuppression in the body, rendering individuals more susceptible to pulmonary infections. This relationship has been substantiated by previous studies, which have frequently documented instances where long-term and high-dose glucocorticoid usage predisposes individuals to an increased risk of developing pneumonia (Guarnotta et al., 2021; Elsouri et al., 2023; Tang et al., 2022). Glucocorticoids, as endogenous hormones, exert regulatory effects on nearly all cells within the body (Quatrini and Ugolini, 2021). Additionally, long-term use of glucocorticoids may result in lasting adverse effects that significantly impact a patient’s quality of life, persisting even after discontinuation of therapy (Seguro et al., 2013). Evidence suggests an increased risk of infections, osteoporosis, osteonecrosis, cardiovascular disease, and cancer among individuals with a history of glucocorticoid use (Seguro et al., 2013). Given the intricate and wide-ranging influence of glucocorticoids on immune function, it is imperative to carefully calibrate the dosage of glucocorticoid administration to strike a delicate balance between eradicating pathogens and safeguarding against excessive inflammation and immunopathology in infectious diseases.
This study has revealed a non-linear association between the ADG and the risk of mortality in pneumonia patients undergoing glucocorticoid therapy. Specifically, the analysis demonstrated a U-shaped relationship, wherein an incremental increase in ADG corresponded to a gradual reduction in the risk of pneumonia-related mortality, followed by an upturn in risk at higher doses. This pattern mirrors observations in previous reports, wherein moderate glucocorticoid dosing facilitated pneumonia recovery and diminishes the risk of mortality (Pinzón et al., 2021; Chan et al., 2020), while excessive glucocorticoid doses were associated with heightened susceptibility to pneumonia and increased mortality risk (Agustí et al., 2003; Guarnotta et al., 2021; Zhao et al., 2022). In our study, we observed that the mortality was higher at an ADG of <20 g methylprednisolone compared to the 20–39 g dose group. This finding may be related to the significantly higher rate of respiratory failure in the <20 g group (54.48%) compared to the 20–39 g group (31.37%). Although there is no previous research directly linking ADG with pneumonia mortality risk, existing literature suggests that prolonged and appropriate glucocorticoid therapy can reduce systemic inflammation, improve lung and extrapulmonary organ function, shorten mechanical ventilation duration, and decrease ICU stay, potentially leading to lower mortality rates (Annane et al., 2017; Villar et al., 2020; Meduri et al., 2009). In this study, we also categorized glucocorticoid use into high-dose and low-dose groups using a commonly employed stratification method based on previous research (Hoes et al., 2009; Buttgereit et al., 2002). A novel finding from our study is the identification of a distinct turning point within the U-shaped relationship observed in pneumonia patients receiving glucocorticoids, occurring at approximately 20 g of ADG. Notably, this phenomenon was observed exclusively in individuals receiving high-dose glucocorticoid therapy. This finding carries significant implications, suggesting that the ADG should be carefully considered and meticulously monitored in patients undergoing high-dose glucocorticoid treatment.
Another interesting finding in this study pertains to the gender-specific variations in the relationship between the ADG and the risk of pneumonia-related mortality. Specifically, while the U-shaped relationship and threshold effect were evident among male patients, they were not observed in female patients. Gender disparities in immune function, neurosteroid levels, stress responses, and pharmacological responses have been well-documented in the context of disease progression (Hodes et al., 2024). Notably, significant gender differences have been observed in the severity of symptoms and mortality rates associated with pneumonia, including COVID-19, which may be attributed to variations in immune responses (Spini et al., 2021). Furthermore, gender variations in the response to glucocorticoid therapy have been reported (Lucafò et al., 2021; Czock et al., 2005). Czock et al. proposed that females may have distinct drug metabolism dynamics compared to males, noting potentially higher clearance rates of methylprednisolone in females (Czock et al., 2005). Such gender-specific variations in methylprednisolone clearance could partially elucidate the observed gender disparities in the association between ADG and mortality risk in pneumonia identified in our study. However, a more in-depth mechanistic explanation may require further research. Our findings underscore the importance of recognizing and addressing gender-specific factors in glucocorticoid therapy. It is imperative to incorporate gender-tailored strategies into glucocorticoid therapy protocols for pneumonia.
Limitations
Our study is subject to several noteworthy limitations that warrant acknowledgment. Firstly, the retrospective design inherently exposes the study to confounding factors and selection biases, despite efforts to adjust for major confounders. For instance, some pathogens were identified through sputum cultures, potentially not fully representing pulmonary infection pathogens. Moreover, the delayed identification of certain pathogens until at least 48 h post-admission increases the likelihood of nosocomial infections, thereby possibly inaccurately distinguishing between CAP and HAP in some cases. Additionally, variables reflecting patients’ immune status, such as immunoglobulin levels and albumin, were not controlled for in this study due to their absence or high levels of missing data in the original dataset. In our study, over half of the patients were receiving other concurrent immunosuppressive treatments. While we adjusted for the presence or absence of such treatments in our analysis, we acknowledge that we did not account for the use of different categories of immunosuppressants. This limitation should be considered when interpreting our results. Secondly, the assessment of ADG only accounted for intravenous or oral glucocorticoid dosages, disregarding the doses of inhaled glucocorticoids. Patients with conditions such as asthma and COPD often receive concurrent inhaled glucocorticoid therapy, potentially leading to an underestimation of the risk of pneumonia mortality associated with ADG in this subset of patients. Thirdly, Owing to insufficient data regarding essential variables in our dataset, validated glucocorticoid toxicity scores such as the glucocorticoid toxicity index or its abbreviated form, the glucocorticoid toxicity index-metabolic domains (Patel et al., 2023), could not be included in this analysis. Additionally, our investigation exclusively focused on methylprednisolone without exploring other glucocorticoids, thereby limiting the generalizability of our findings concerning the relationship between various glucocorticoids and mortality risk. Despite these acknowledged limitations, our study represents a pioneering effort in elucidating the specific relationship between ADG and the risk of pneumonia-related mortality in populations receiving glucocorticoid therapy.
Conclusion
In summary, our investigation reveals a non-linear association between cumulative glucocorticoid usage and the risk of pneumonia-related mortality in populations receiving glucocorticoids. Particularly, the ADG exceeding 20 g may emerge as an independent risk factor for pneumonia-related mortality in male pneumonia patients. These findings underscore the pivotal role of ADG in assessing and managing the risk of pneumonia-related mortality. Through elucidating this relationship, our study furnishes clinicians with valuable insights to actively assess and alleviate the risk of pneumonia-related mortality, thereby enhancing the refinement of therapeutic approaches and the optimization of patient care within this clinical realm.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of China-Japan Friendship Hospital (No. 2015-86). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. QY: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical and Health Science and Technology Plan Project of Zhejiang Province (No. 2022RC286).
Acknowledgments
We are very grateful to Lijuan, Li et al. for providing the data and sharing it in DYARD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1445979/full#supplementary-material
References
Agustí, C., Rañó, A., Filella, X., González, J., Moreno, A., Xaubet, A., et al. (2003). Pulmonary infiltrates in patients receiving long-term glucocorticoid treatment: etiology, prognostic factors, and associated inflammatory response. Chest 123 (2), 488–498. doi:10.1378/chest.123.2.488
American Thoracic Society, Infectious Diseases Society of America (2005). Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171 (4), 388–416. doi:10.1164/rccm.200405-644ST
Annane, D., Pastores, S. M., Rochwerg, B., Arlt, W., Balk, R. A., Beishuizen, A., et al. (2017). Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 43 (12), 1751–1763. doi:10.1007/s00134-017-4919-5
Buttgereit, F., da Silva, J. A., Boers, M., Burmester, G. R., Cutolo, M., Jacobs, J., et al. (2002). Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann. Rheum. Dis. 61 (8), 718–722. doi:10.1136/ard.61.8.718
Chan, E. D., Chan, M. M., Chan, M. M., and Marik, P. E. (2020). Use of glucocorticoids in the critical care setting: science and clinical evidence. Pharmacol. Ther. 206, 107428. doi:10.1016/j.pharmthera.2019.107428
Czock, D., Keller, F., Rasche, F. M., and Häussler, U. (2005). Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44 (1), 61–98. doi:10.2165/00003088-200544010-00003
Elsouri, K. N., Arboleda, V., Basbous, L., Heiser, S., Collins, D. P., Ragusa, P., et al. (2023). Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis. J. Osteopath Med. 123 (4), 179–186. doi:10.1515/jom-2022-0177
Guarnotta, V., Ferrigno, R., Martino, M., Barbot, M., Isidori, A. M., Scaroni, C., et al. (2021). Glucocorticoid excess and COVID-19 disease. Rev. Endocr. Metab. Disord. 22 (4), 703–714. doi:10.1007/s11154-020-09598-x
Hodes, G. E., Bangasser, D., Sotiropoulos, I., Kokras, N., and Dalla, C. (2024). Sex differences in stress response: classical mechanisms and beyond. Curr. Neuropharmacol. 22 (3), 475–494. doi:10.2174/1570159X22666231005090134
Hoes, J. N., Jacobs, J. W., Verstappen, S. M., Bijlsma, J. W., and Van der Heijden, G. J. (2009). Adverse events of low-to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann. Rheum. Dis. 68 (12), 1833–1838. doi:10.1136/ard.2008.100008
Li, L., Hsu, S. H., Gu, X., Jiang, S., Shang, L., Sun, G., et al. (2020b). Aetiology and prognostic risk factors of mortality in patients with pneumonia receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study. BMJ Open 10 (10), e037419. doi:10.1136/bmjopen-2020-037419
Li, L., Steven H, H., Xiaoying, G., Shan, J., Lianhan, S., Guolei, S., et al. (2020a). Aetiology and prognostic risk factors of mortality in pneumonia patients receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study. Dryad. doi:10.5061/dryad.mkkwh70x2
Lucafò, M., Bramuzzo, M., Selvestrel, D., Da Lozzo, P., Decorti, G., and Stocco, G. (2021). Gender may influence the immunosuppressive actions of prednisone in young patients with inflammatory bowel disease. Front. Immunol. 12, 673068. doi:10.3389/fimmu.2021.673068
Meduri, G. U., Annane, D., Chrousos, G. P., Marik, P. E., and Sinclair, S. E. (2009). Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest 136 (6), 1631–1643. doi:10.1378/chest.08-2408
Patel, N. J., Jayne, D. R. W., Merkel, P. A., Bekker, P., Zhang, Y., McDowell, P. J., et al. (2023). The glucocorticoid toxicity index-metabolic domains, an abridged version of the glucocorticoid toxicity index: post-hoc analysis of data from the ADVOCATE trial. Lancet Rheumatol. 5 (7), e413–e421. doi:10.1016/S2665-9913(23)00131-5
Peng, B., Li, J., Chen, M., Yang, X., Hao, M., Wu, F., et al. (2023). Clinical value of glucocorticoids for severe community-acquired pneumonia: a systematic review and meta-analysis based on randomized controlled trials. Med. Baltim. 102 (46), e36047. doi:10.1097/MD.0000000000036047
Pinzón, M. A., Ortiz, S., Holguín, H., Betancur, J. F., Cardona Arango, D., Laniado, H., et al. (2021). Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One 16 (5), e0252057. doi:10.1371/journal.pone.0252057
Quatrini, L., and Ugolini, S. (2021). New insights into the cell-and tissue-specificity of glucocorticoid actions. Cell Mol. Immunol. 18 (2), 269–278. doi:10.1038/s41423-020-00526-2
Seguro, L. P., Rosario, C., and Shoenfeld, Y. (2013). Long-term complications of past glucocorticoid use. Autoimmun. Rev. 12 (5), 629–632. doi:10.1016/j.autrev.2012.12.002
Soo, C. I., Poon, K. V., Ayub, A., You, H. W., Tan, C. X., Loh, K. J. J., et al. (2024). High-dose pulse methylprednisolone vs. dexamethasone standard therapy for severe and critical COVID-19 pneumonia: efficacy assessment in a retrospective single-centre experience from Malaysia. Med. J. Malays. 79 (1), 15–20.
Sousa, D., Justo, I., Domínguez, A., Manzur, A., Izquierdo, C., Ruiz, L., et al. (2013). Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin. Microbiol. Infect. 19 (2), 187–192. doi:10.1111/j.1469-0691.2012.03765.x
Souza, J. A. M., Carvalho, A. F. S., Grossi, L. C., Zaidan, I., de Oliveira, L. C., Vago, J. P., et al. (2022). Glucocorticoid-induced leucine zipper alleviates lung inflammation and enhances bacterial clearance during pneumococcal pneumonia. Cells 11 (3), 532. doi:10.3390/cells11030532
Spini, A., Giudice, V., Brancaleone, V., Morgese, M. G., De Francia, S., Filippelli, A., et al. (2021). Sex-tailored pharmacology and COVID-19: next steps towards appropriateness and health equity. Pharmacol. Res. 173, 105848. doi:10.1016/j.phrs.2021.105848
Strehl, C., Bijlsma, J. W., de Wit, M., Boers, M., Caeyers, N., Cutolo, M., et al. (2016). Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann. Rheum. Dis. 75 (6), 952–957. doi:10.1136/annrheumdis-2015-208916
Sun, L. L., Ye, C., Zhou, Y. L., Zuo, S. R., Deng, Z. Z., and Wang, C. J. (2020). Meta-analysis of the clinical efficacy and safety of high- and low-dose methylprednisolone in the treatment of children with severe mycoplasma pneumoniae pneumonia. Pediatr. Infect. Dis. J. 39 (3), 177–183. doi:10.1097/INF.0000000000002529
Tang, Q., Chen, Q., Li, Y., and Wang, Z. (2022). Association between glucocorticoids and mortality in patients with severe pneumonia: a systematic review and meta-analysis based on randomized controlled trials. Comput. Math. Methods Med. 2022, 1191205. doi:10.1155/2022/1191205
Villar, J., Ferrando, C., Martínez, D., Ambrós, A., Muñoz, T., Soler, J. A., et al. (2020). Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 8 (3), 267–276. doi:10.1016/S2213-2600(19)30417-5
Keywords: glucocorticoids, pneumonia, mortality, threshold effect, gender
Citation: Wang S and Ye Q (2024) The glucocorticoid dose-mortality nexus in pneumonia patients: unveiling the threshold effect. Front. Pharmacol. 15:1445979. doi: 10.3389/fphar.2024.1445979
Received: 13 June 2024; Accepted: 10 September 2024;
Published: 19 September 2024.
Edited by:
Leonello Fuso, Catholic University of the Sacred Heart, ItalyReviewed by:
Lihua Xing, First Affiliated Hospital of Zhengzhou University, ChinaSemra Bilaceroglu, University of Health Sciences, Türkiye
Copyright © 2024 Wang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saibin Wang, c2FpYmlud2FuZ0Bob3RtYWlsLmNvbQ==
 Saibin Wang
Saibin Wang Qian Ye3
Qian Ye3