
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 17 September 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1444922
This article is part of the Research Topic Real-World Evidence of Natural Products, Herbal Medicines, and Traditional Medicine Treatments Volume II View all 15 articles
Functional gastrointestinal disorders (FGIDs) and inflammatory bowel disease (IBD) are common clinical disorders characterized by recurrent diarrhea and abdominal pain. Although their pathogenesis has not been fully clarified, disruptions in intestinal motility and immune function are widely accepted as contributing factors to both conditions, and the brain–gut axis plays a key role in these processes. Traditional Chinese Medicine (TCM) employs a holistic approach to treatment, considers spleen and stomach impairments and liver abnormality the main pathogenesis of these two diseases, and offers a unique therapeutic strategy that targets these interconnected pathways. Clinical evidence shows the great potential of TCM in treating FGIDs and IBD. This study presents a systematic description of the pathological mechanisms of FGIDs and IBD in the context of the brain–gut axis, discusses clinical and preclinical studies on TCM and acupuncture for the treatment of these diseases, and summarizes TCM targets and pathways for the treatment of FGIDs and IBD, integrating ancient wisdom with contemporary biomedical insights. The alleviating effects of TCM on FGID and IBD symptoms are mainly mediated through the modulation of intestinal immunity and inflammation, sensory transmission, neuroendocrine–immune network, and microbiota and their metabolism through brain–gut axis mechanisms. TCM may be a promising treatment option in controlling FGIDs and IBD; however, further high-quality research is required. This review provides a reference for an in-depth exploration of the interventional effects and mechanisms of TCM in FGIDs and IBD, underscoring TCM’s potential to recalibrate the dysregulated brain–gut axis in FGIDs and IBD.
Functional gastrointestinal disorders (FGIDs) and inflammatory bowel disease (IBD) are characterized by recurrent abdominal discomfort, which can be accompanied by altered bowel regularity and stool properties (Black et al., 2020; Seyedian et al., 2019). In recent years, the increasing incidence of both diseases has garnered increasing attention from researchers. A recent study stated that in some countries in Latin America and Asia, such as Brazil, China, and India, IBD’s incidence will increase exponentially until reaching a stage of compounded prevalence in which morbidity exceeds mortality (Kaplan and Windsor, 2021). In addition, a cross-national prevalence study by the Rome Foundation revealed that >40% of the world’s population have FGIDs (Sperber et al., 2021). This places a heavy economic burden and a significant health risk on humanity. The Rome IV Criteria classify FGIDs into eight categories, and this paper focuses on the two most common FGIDs, namely, irritable bowel syndrome (IBS) and functional dyspepsia (FD) (Sperber et al., 2021), and IBDs, namely, ulcerative colitis (UC) and Crohn’s disease (CD). For FGIDs, existing treatments cannot identify and address disease pathogenesis but can only alleviate the patient’s gastrointestinal and psychological comorbidities (Black et al., 2020). In IBD research, routinely used therapeutic agents such as corticosteroids, aminosalicylates, and immunosuppressants (Hanauer and Baert, 1994) are still unable to cure IBD. In addition, individualized treatments with better efficacy and fewer side effects are still needed (Le Berre et al., 2023; Roda et al., 2020).
In as early as the 1840s, studies have found that emotions affect the digestion rate, confirming that a pathway exists between the gut and the nervous system (Margolis et al., 2021). Recent studies have shown a bidirectional interaction between the gut microbiota and the nervous system through direct and indirect mechanisms, which is known as the brain–gut axis. It encompasses the gut-associated immune system, enteric neuroendocrine system, enteric nervous system (ENS), central nervous system (CNS), etc. (Mayer et al., 2022). Patients with both FGIDs and IBD exhibited disruption of gut bacterial microbiota homeostasis (Bajaj et al., 2024; Ford et al., 2020a; Liu et al., 2017), resulting clinical symptoms. As regards mood states, two analyses have shown that >20% of patients with both disorders have concomitant symptoms of anxiety and depression and that more women than men are affected (Barberio et al., 2021; Jones et al., 2017). This proves that this link between the gut and the brain deserves more attention.
Traditional Chinese Medicine (TCM) has the potential to treat FGIDs and IBD. For example, studies have reported the effectiveness of acupuncture and moxibustion in treating IBS (Ma et al., 2024), and peppermint has been included in the 2021 clinical treatment guidelines for IBS by the American College of Gastroenterology (Lacy et al., 2021). Curcumin is also effective in improving gastrointestinal symptoms in IBS owing to its antioxidant and anti-inflammatory activities (Ng et al., 2018b). Herbal medicine is a safe and effective treatment of FD (Heiran et al., 2022), and sufficient evidence presents the utilization of modified Zhi Zhu decoction and Xiao Pi Kuan Wei decoction as viable alternatives for the treatment of individuals who do not respond to prokinetic agents (Ho et al., 2021). As an adjunctive treatment for UC, rhubarb combined with mesalazine or lorazepam is safer and more effective than when combined with Western medicine (Li Y. et al., 2022). Preclinical studies of UC have also shown sufficient evidence of the therapeutic effects of licorice extracts (Lu et al., 2022). The use of herbal medicines as complementary therapies in CD treatment has also been determined to be beneficial and may reduce the incidence of adverse events (Wang et al., 2019). These findings indicate the need to enhance TCM and its utilization as an alternative or complementary therapy for FGIDs and IBD. However, its mechanism of action has not yet been clarified, which limits its development and clinical promotion.
Herein, we outline the possible molecular mechanisms by which the brain–gut axis plays a role in FGIDs and IBD and integrate current evidence on the effects of TCM on these diseases in the context of the brain–gut axis. We also summarize the mechanism of action of TCM based on the brain–gut axis, laying the foundation for further in-depth exploration of the role and mechanism of TCM in FGIDs and IBD.
Understanding the pathogenesis of FGIDs and the brain–gut axis in IBD can aid in the development of clinical interventions for these diseases. Herein, we summarize the pathogenesis of these diseases in terms of immunity, enterosensory transmission, neuroendocrine–immune network (NEI), and intestinal flora.
In clinical practice, FGIDs are prevalent, and patients typically report a single symptom or an arbitrary mix of symptoms. The clinical symptoms of IBS are mainly recurrent abdominal pain associated with abnormal fecal patterns or frequency, and the pathogenesis is mainly associated to genetics, stress, dysregulation of intestinal microbial homeostasis, visceral hypersensitivity, and intestinal motility disorders (Ford et al., 2020b). The main FD symptoms include epigastric pain or burning sensations, postprandial fullness or early satiety, intestinal motility and sensory disturbances, gut microenvironmental disturbances, and immune-related inflammation (Ford et al., 2020a). Despite variations in the etiology and clinical features of FGIDs, current literature presents that FGID symptoms primarily arise from disrupted intestinal motility. Therefore, the research direction of FGIDs has focused on intestinal sensory transduction. Furthermore, substantial convergence exists in the underlying mechanisms of different FGIDs.
Through the integration of previous studies on the brain–gut axis, we have compiled an overview of the potential pathogenesis of FGIDs (Figure 1).
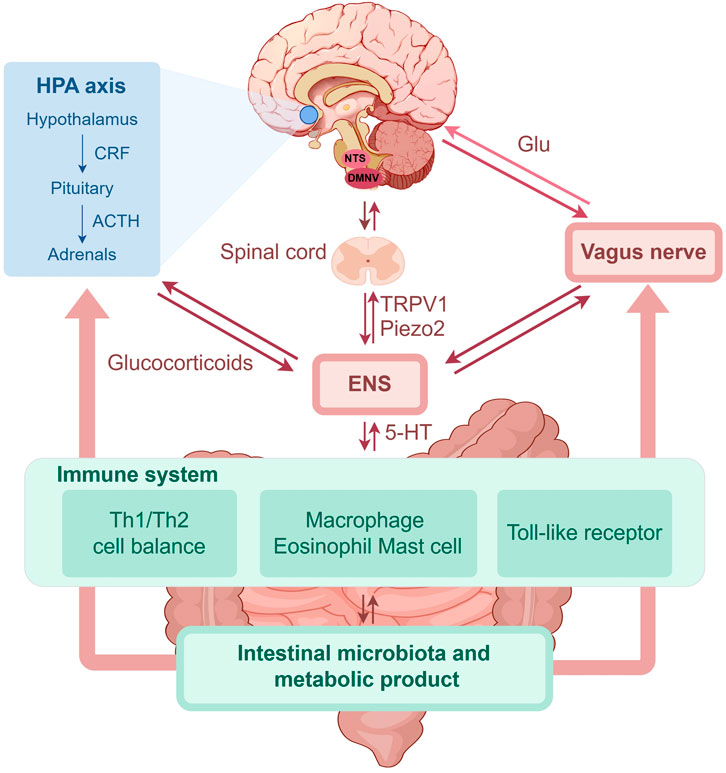
Figure 1. The role of brain-gut axis in FGIDs (by Figdraw). FGIDs, functional gastrointestinal disorders; HPA, hypothalamic-pituitary-adrenal; CRF, corticotropin releasing factor; ACTH, adrenocorticotropic hormone; NTS, nucleus of the solitary tract; DMNV, dorsal motor nucleus of the vagus; Glu, glutamate; ENS, enteric nervous system; 5-HT, 5-hydroxytryptamine; TRPV1, transient receptor potential vanilloid 1; Th, T helper.
Researchers have proposed that low-grade inflammation, immune activation, and dysregulated gut flora homeostasis may contribute to IBS onset and exacerbation (Ng et al., 2018a; Rodríguez-Fandiño et al., 2010). In particular, gastrointestinal macrophages are actively involved in the intestinal immune response (Zhou S. Y. et al., 2023), are mainly responsible for phagocytosis and digestion, and promote tissue remodeling (Gentek et al., 2014).
T helper (Th) 1 cells activate macrophages to generate a cellular immune response, whereas Th2 cells inhibit this response (Butcher and Zhu, 2021). However, in FGIDs, a shift in immune cells to a Th2-type response may ensue to suppress low-grade inflammation (Du et al., 2019; Kindt et al., 2009). Furthermore, in the intestinal mucosa of patients with IBS, levels of Toll-like receptor (TLR) 4 and TLR5 expressed on eosinophils and mast cells are significantly higher than that in healthy individuals (Brint et al., 2011; Dlugosz et al., 2017), which exacerbates intestinal hypersensitivity and increases the release of mast cell degranulation (Mallaret et al., 2022; Yang et al., 2019). Overall, intestinal immune responses induced by abnormal macrophage activation, an imbalanced Th1 cell/Th2 cell ratio, and a high TLR expression, are closely associated with the pathogenesis of FGIDs.
The human ENS consists of neurons and enteric glial cells, which independently innervate gastrointestinal activity and are connected to the CNS through the vagus nerve (VN) and the sympathetic and pelvic nerves (Furness et al., 2014). A study presented that the pathogenesis of FGIDs was inherently linked to visceral hypersensitivity, which was first elucidated five decades ago (Mertz et al., 1995; Ritchie, 1973).
Regarding the gut sensory afferent pathway, transient receptor potential vanilloid (TRPV) specifically expressed in the dorsal root ganglion in central sensitization takes on a crucial role in the transmission of nociceptive signals from the gut to the CNS (Farmer and Aziz, 2009). This ion channel is activated by stimuli, such as heat, acidity, and mechanical pressure, and is responsible for the detection and transmission of pain signals from the gut to the brain, making them a potential treatment target in gut-related pain disorders. A functional magnetic resonance imaging study of the brains of patients with IBS revealed the activation of the thalamus, insula, and anterior cingulate cortex, which confirms the presence of central sensitization in these patients (Price et al., 2007). TRPV1 and TRPV4 are closely associated with hypersensitivity in mouse experiments (Ho et al., 2012; Xie et al., 2023), and TRPV4 activation inhibits intestinal peristalsis by reducing NO-dependent Ca2+ release from enteric neurons (Fichna et al., 2015). The selective ablation of the Piezo (piezo-type echanosensitive ion channel component) 2 protein expressed in TRPV1-spectrum neurons attenuates visceral hypersensitivity in IBS mice (Xie et al., 2023), which explains why abdominal pain is one of the main IBS symptoms.
As regards visceral hypersensitivity, the role of the sympathetic nerves and the VN is still unclear. However, the stimulation of the auricular VN may be effective in relieving constipation and abdominal pain symptoms in patients with IBS (Shi et al., 2021). Similar studies have reported comparable results in a rat model of FD (Guo and Gharibani, 2024; Hou et al., 2023).
The NEI theory emphasizes the coordinated interaction between the nervous, endocrine, and immune systems. It was first proposed in 1977. A study suggested that NEI dysregulation may be a significant contributing factor to the pathogenesis of IBS (Powell et al., 2017).
Reactivating neurons in the insular cortex of the brain restore their original inflammatory state (Koren et al., 2021), providing new ideas for suppressing peripheral inflammation. 5-hydroxytryptamine (5-HT), an important neurotransmitter in the CNS, is a potential research direction for IBS (Ford et al., 2024). Enterochromaffin cells (ECs) release 5-HT to activate the 5-HT3 receptor in intestinal submucosal neurons to transmit pain signals (Bellono et al., 2017; Diwakarla et al., 2017).
The VN is also a key link in the NEI of FGIDs, and the efferent fibers of the VN originated from the preganglionic neurons in the dorsal motor nucleus of the vagus and then descended to the postganglionic neurons in the ENS. The afferent fibers of the VN activate receptors on the nucleus of the solitary tract in the medulla oblongata primarily through the neurotransmitter glutamate, which transmits signals to higher brain regions (Jean, 2001). The dorsal motor nucleus of the vagus and the nucleus of the solitary tract are located adjacent to each other, and an experiment suggests that the two are connected, resulting in a vagal loop formation (Rinaman et al., 1989). Moreover, the afferent fibers of the VN can regulate inflammation by activating the hypothalamic–pituitary–adrenal (HPA) axis. Environmental stressors can also activate the HPA axis (Agirman et al., 2021; Pavlov et al., 2003). Corticotropin-releasing factor (CRF) 1 plays a pathogenic role in IBS, and CRF1 and CRF2 can regulate visceral hypersensitivity and intestinal permeability through the modulation of TLR4 and the cytokine system, a mechanism that can be blocked by interleukin (IL)-1 receptor antagonists. This mechanism is also positively regulated by lipopolysaccharide (Nozu et al., 2018). In the HPA axis, the adrenal cortex releases cortisol, which can directly activate immune cells such as lymphocytes in the gastrointestinal tract and promote peripheral sensitization. Cortisol binding to the amygdala further promotes the hypothalamic secretion of adrenocorticotropic hormones, exacerbating central sensitization (Moloney et al., 2016).
Extraintestinal macrophages also produce bone morphogenetic protein 2 that stimulates intestinal neurons to produce colony stimulating factor 1 to further increase the number of macrophages. This can be exacerbated by bacterial infections in the gastrointestinal tract. The positive feedback loop could play a role in IBS development (Kabata and Artis, 2019; Muller et al., 2014).
The gut microbiota helps maintain host health and intestinal homeostasis. IBS subtypes demonstrated varied bacterial composition (Carco et al., 2020). However, the uniform characterization of the IBS-associated gut microbiota remains elusive (Ng et al., 2023). An interaction exists between stress and gut flora (Li Q. et al., 2023; O'Mahony et al., 2017; Tannock and Savage, 1974). According to Mujagic et al. (2022), the level of psychological stress in patients and the magnitude of the effect of psychological stress on IBS symptoms can be used as a basis for clustering the fecal microbiome–metabolome characteristics of patients with IBS, demonstrating the existence of such interactions in IBS. Studies have indicated that the gut microbiota influences gut sensory transduction (Meynier et al., 2024; Shimbori et al., 2022; Vicentini et al., 2021), intestinal immunity (Gobert et al., 2016; Jia et al., 2019; Zheng et al., 2023), neuroendocrine pathways (Gao et al., 2022; Mishima and Ishihara, 2021), and intestinal motility (Gu et al., 2022; Shanahan et al., 2023). Furthermore, previous studies (Dodd et al., 2017; Wei et al., 2021; Yang W. et al., 2020) have demonstrated that the dysregulation of tryptophan metabolites, secondary bile acids, and short-chain fatty acids (SCFAs), are metabolites of the intestinal flora, affects normal intestinal immune function.
Symptoms of this chronic inflammatory disease of the gastrointestinal tract include abdominal pain, diarrhea, and rectal bleeding. Extraintestinal manifestations of IBD may also be observed in the skin, eyes, or joints; however, its etiology is unclear (Eberhardson et al., 2021; Rogler et al., 2021). Despite ongoing research, IBD has no known cure, and treatment options focus on symptom management and complication prevention. This section focuses on UC and CD. UC is a chronic nonspecific inflammatory disease that often leads to structural and functional changes in the colon and rectum, and its pathogenesis may be related to intestinal barrier disruption, intestinal immune abnormalities, and dysregulated intestinal homeostasis (Le Berre et al., 2023). CD, also a chronic inflammatory disease, affects the terminal ileum and proximal colon, and it may be caused by genetic factors, intestinal barrier disruption, and intestinal flora disorders (Dolinger et al., 2024). Although the inflammatory features and sites of CD differ from those of UC, immune response abnormalities and intestinal inflammation have been recognized as disease drivers. Most of the current studies on IBD also focus on this aspect. The summarized pathogenesis of IBD is shown in Figure 2.
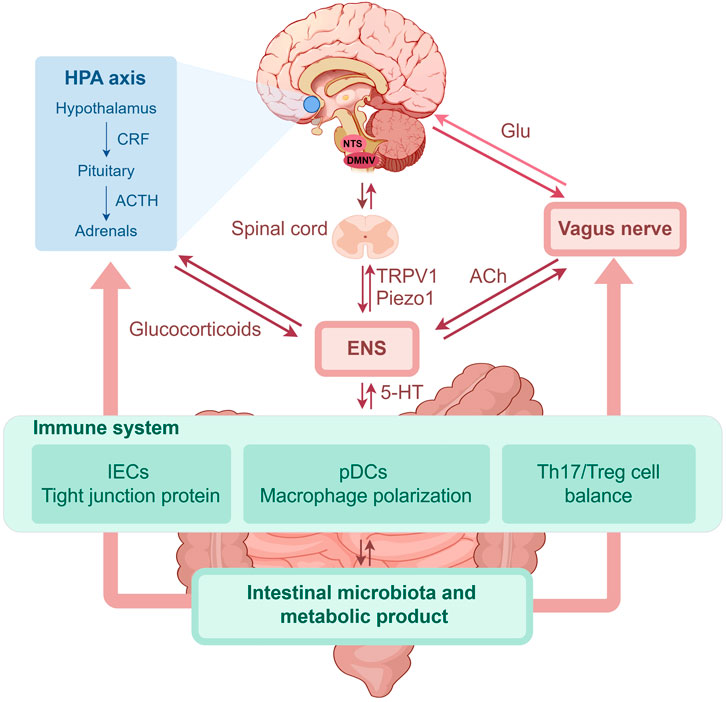
Figure 2. The role of brain-gut axis in IBD (by Figdraw). IBD, inflammatory bowel disease; HPA, hypothalamic-pituitary-adrenal; CRF, corticotropin releasing factor; ACTH, adrenocorticotropic hormone; NTS, nucleus of the solitary tract; DMNV, dorsal motor nucleus of the vagus; Glu, glutamate; ENS, enteric nervous system; 5-HT, 5-hydroxytryptamine; TRPV1, transient receptor potential vanilloid 1; ACh, acetylcholine; IECs, intestinal epithelium cells; pDCs, plasmacytoid dendritic cells; Th, T helper; Treg, regulatory T.
Intestinal epithelial cells (IECs) are the first line of defense in the intestine. These proteins are tightly packed together through cellular junctions, which nourish the gut and functions as an intestinal immune barrier (Berin et al., 2006; Deuring et al., 2013). Studies have revealed that the mitochondrial function of IECs is closely related to the intestinal flora, and their dysfunction contributes to the development of ileitis (Alula et al., 2023). The low expression of the protein zonula occludens-1 is a possible reason for the failure of mucosal healing in patients with IBD (Kuo et al., 2021).
Antigen-presenting cells encompass dendritic cells (DCs) and macrophages. Plasmacytoid DCs (pDCs) are one of two subtypes of human DCs that help in activating host innate and adaptive immunity (McKenna et al., 2005; Zhang et al., 2021). pDCs promote the mobilization of colonic phagocytes into inflamed intestinal tissues, leading to the development and exacerbation of acute colitis (Arimura et al., 2017). In addition to DCs, macrophages are also associated with IBD development and exacerbation. In mouse model experiments, enhancing the transformation of M1 macrophages into M2 alleviates experimental colitis (Zhu et al., 2016), whereas psychological stress promotes macrophage polarization toward the M1 phenotype and infiltration into the colon (Ge et al., 2022). The inhibition of M1 polarization mitigates intestinal mucosal inflammation (Chu et al., 2023; Ruan et al., 2023). Furthermore, alterations in sodium–potassium ATPase have been identified as an underlying factor in diarrhea among patients with IBD. In an in vitro study involving IECs, the activation of TLR2, TLR4, and TLR7 led to the downregulation of sodium–potassium ATPase, whereas TLR5 exhibited the opposite effect (Cosme et al., 2023).
The imbalance in Th17/regulatory T (Treg) cells has emerged as a potential contributing factor to IBD development. Research indicates that the modulation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (MAPK) pathway can potentially restore the homeostasis of Th17/Treg cells (Liu et al., 2013). Notably, the inhibition of the Harvey rat sarcoma viral oncogene homolog, an upstream pathway regulator, counteracted MAPK-induced enhancement of Th17 cell differentiation and reinstated Th17/Treg cell equilibrium (Wang S. et al., 2024).
Psychological stress activates the sympathetic nerves and decreases the vagal tone, inhibits its anti-inflammatory effects, promotes TNF-α production by macrophages, and exacerbates colitis progression (Bonaz et al., 2016; Breit et al., 2018). Studies have indicated that patients who underwent vagotomy have a lower risk of IBD development (Liu B. et al., 2020) and that electrical stimulation of the VN can improve clinical symptoms in patients with IBD (Sahn et al., 2023). TRPV1+ afferent signaling induces visceral hypersensitivity in colitis mice by preventing microglial activation in the spinal cord (Defaye et al., 2022). The activation of Piezo channels is crucial in the pathogenesis of IBD, particularly in CD. In the ileal mucosal tissues of patients with CD, high levels of Piezo1 protein expression leads to the activation of NOD-like receptor family pyrin domain containing (NLRP) 3 inflammasomes, which cause intestinal inflammation (Liu Q. et al., 2023). Vascular inflammation is widely recognized as a prominent issue in individuals with CD, and its onset is believed to be associated with the protracted exposure of vascular endothelial cells to shear stress. The deleterious effects of shear stress on the endothelium closely intertwined with the activation of calcium signaling mediated by the Piezo1 protein, ultimately resulting in the opening of TRPV4 channels (Hartmannsgruber et al., 2007; Swain and Liddle, 2021).
Similar to the neuroimmune mechanism of FGIDs, EC and 5-HT are highly expressed in dextran sulfate sodium (DSS)-induced rat colonic mucosa (Oshima et al., 1999). In recent studies, the expression of 5-HT7 receptor has increased in UC and CD, and the 5-HT7 receptor may play a key role in IBD progression (Wan et al., 2023; Xu Z. et al., 2022). In the HPA axis, excessive stress-induced release of glucocorticoids and adrenocorticotropic hormones disrupts tight junction proteins between IECs, which weakens the intestinal mucosal barrier (Xu W. et al., 2021; Zong et al., 2019). The cholinergic anti-inflammatory pathway has also been implicated in the pathogenesis of IBD (Andersson and Tracey, 2012; Borovikova et al., 2000). In a mouse model of DSS- and dinitrobenzene sulfonic acid-induced colitis, attenuating the alpha7 nicotinic acetylcholine receptor-mediated release of macrophage inflammatory factors from the VN was found to result in the reactivation of intestinal inflammation, demonstrating that the cholinergic anti-inflammatory pathway induces intestinal inflammation (Ghia et al., 2009; Yasuda et al., 2011). Intestinal inflammation also increases intestinal permeability, which allows harmful substances in the intestine to enter the CNS by disrupting the gut vascular barrier and blood–brain barrier, which in turn stimulates immune cells in the CNS to trigger a neuroinflammatory response and exacerbates the inflammatory response at the central and peripheral levels (Agirman et al., 2021).
Gut microbiota dysbiosis is also involved in the pathogenesis of IBD. The composition of the mucosal microbiota in patients with IBD can influence the expression of mucosal inflammatory genes and intestinal cell types and be used to demonstrate the existence of mucosal host–microbe interactions in IBD (Hu et al., 2024). The concept of IECs–microbiota–immune cell crosstalk has also been proposed (Iyer et al., 2023). The bile acid metabolites 3-oxolithocholic acid and isolithocholic acid inhibited Th17 cell differentiation, demonstrating that they modulate the Th17/Treg cell balance and influence intestinal immunity (Paik et al., 2022). Recent studies have demonstrated that the intestinal flora affects the level of conjugated linoleic acid in the mouse intestine, which upregulates IL-18 signaling in intraepithelial lymphocytes and promotes the differentiation of CD4+ CD8αα+ intraepithelial lymphocytes (Song et al., 2023).
The concept of internal organs in TCM is different from that in modern medicine, the heart of TCM includes a part of the function of the brain in modern medicine, and the small intestine includes a part of the intestines. In TCM, a theory revealed that the heart and the small intestine are related exteriorly interiorly, which also corresponds well to the brain–gut axis nowadays. A core of TCM is the overall concept, advocating the integration of human and universe, that is, every internal organ in the human body is interconnected. Functionally, the intestines produced by the refined essence of the material also need to replenish the essence and marrow of the brain. The essence of the brain can also nourish the internal zang-fu located in the lower part of the body, which also coincides with the brain–gut axis.
In TCM, FGIDs may be described as gastric distension, abdominal pain, and diarrhea. IBD may be an intestinal abscess and dysentery. The spleen (pi) and stomach (wei) are the hubs of Qi ascending and descending, and spleen and stomach malfunction is considered the main cause of the disease. In TCM, the stomach is the main organ involved in the preliminary processing and digestion of food, after further conduction to the intestines, the main descending adversely risen Qi, whereas the spleen further transforms food into the body’s refined essence and transported to the whole body, the main ascending Qi. The normal synergy of the two can help the human body Qi and blood abundant, and maintain good health (Zhang et al., 2024). In the case of spleen and stomach dysfunction, the essence cannot nourish the limbs and zang-fu, and disturbed Qi activity, bloating, diarrhea, and pain will occur. Over time, the disturbed Qi activity will also lead to the production of pathological products such as dampness and blood stasis, aggravating the impeding of Qi activity. The liver also plays a role in pathogenesis, as it regulates the smooth flow of Qi, and liver disease can result in similar pathologic changes as described above. Therefore, the treatment of FGIDs by TCM mainly aims at restoring the normal physiology of the liver, spleen, and stomach and adopts different treatments according to symptoms, such as harmonizing the liver and spleen, invigorating the spleen, and harmonizing the stomach. Meanwhile, IBD treatment is directed at moving Qi to remove stagnancy and obstruction, clearing heat and resolving dampness, and promoting blood circulation to remove blood stasis.
TCM has accumulated rich clinical experience in the treatment of FGIDs and IBD and has demonstrated relatively clear efficacy in improving symptoms and quality of life of patients (Chiarioni et al., 2023; Heiran et al., 2022). This section will discuss the utilization of botanical drugs, herbal crude extracts, proprietary Chinese medicines, TCM formulas, and acupuncture for the management of the aforementioned conditions. Table 1 summarizes the primary clinical outcomes documented in TCM literature concerning the FGIDs and IBD management.
Clinical studies evaluating the efficacy of TCM interventions for FGIDs have used several efficacy evaluation scales and questionnaires, of which the IBS-Symptom Severity Score and the Bristol Stool Scale are the main tools used to assess efficacy in IBS, and the Single Dyspepsia Symptom Scale is the main tool used to assess efficacy in FD. Xiangsha Liujunzi granules (14 g three times daily) relieved FD symptoms and reduced recurrence for up to 4 weeks post-treatment (Lv et al., 2017). Tong-Xie-Yao-Fang (TXYF) granules are effective in treating IBS-D in multiple clinical trials. In a multicenter randomized clinical trial with a total sample of 1044 patients, personalized TXYF (warm decoction) for IBS-D was significantly superior to pivacurium bromide in improving fecal characteristics (Fan et al., 2017). The results of another randomized clinical trial with 12 weeks of treatment showed that patients receiving TXYF (25.4 g three times daily) had significantly lower abdominal pain visual analog scale scores than patients who received the placebo at 3–7 weeks of treatment (Chen et al., 2018). The number of mast cells in the colonic mucosa was significantly reduced after TXYF treatment and was significantly different between the pretreatment and control groups (Pan et al., 2009).
Acupuncture has demonstrated efficacy in treating various IBS subtypes, predominantly IBS-D. Recent systematic reviews have confirmed the effectiveness of acupuncture in alleviating abdominal pain symptoms in patients with IBS (Yang et al., 2022) and have highlighted its safety profile for pediatric and adult populations (Cai et al., 2024). Key acupoints such as “Tianshu” (ST25), “Zusanli” (ST36), and “Shangjuxu” (ST37) are commonly targeted in acupuncture interventions. A study indicated that acupuncture, particularly when targeting mental and spleen regulation, outperformed pivacurium bromide in the early-stage relief of abdominal pain in patients with IBS-D (Li et al., 2017). Furthermore, acupuncture has shown promise in modulating constipation and diarrhea in individuals with IBS (Guo J. et al., 2021). In FD, acupuncture is effective in ameliorating symptoms of postprandial distress syndrome, including postprandial fullness, early satiety, and epigastric distension, with therapeutic benefits persisting for up to 8 weeks post-treatment cessation (Yang J. W. et al., 2020). A 4-week electroacupuncture treatment of 333 patients with IBS-D or FD improved patients’ quality of life, reduced defecation frequency, and improve stool consistency (Zheng et al., 2016).
TCM treatments for FGIDs are generally considered safe when administered by trained practitioners. However, potential side effects can occur, including allergic reactions to herbs, gastrointestinal upset, and in rare cases, interactions with conventional medications.
The Mayo score and Crohn’s Disease Activity Index are commonly utilized efficacy assessment tools in clinical studies evaluating TCM interventions for IBD. Andrographis paniculata ethanol extract and Qing-Chang-Hua-Shi (QCHS) granules are frequently employed for UC, whereas boswelan is commonly used for CD. In an 8-week randomized clinical trial involving patients with UC, Andrographis paniculata ethanol extract at a dose of 400 mg three times daily was superior to mesalazine at a dose of 1,500 mg three times daily (Tang et al., 2011) and to placebo at 1800 mg twice daily (Sandborn et al., 2013). However, some adverse effects of the ethanol extract of Andrographis paniculata, such as fever, rash, and high C-reactive protein levels, were reported in a previous clinical trial (Tang et al., 2011).These side effects may be attributed to the immunostimulatory properties of the extract, which can cause an exaggerated immune response in some individuals.As such, caution should be taken when utilizing it in clinical practice. Boswelan has shown superiority over placebo in symptom relief and relapse prevention in patients with CD (Holtmeier et al., 2011). A study indicated that QCHS (150 mL twice daily) was more effective in improving UC symptoms and has a better safety profile than the 0.25 g/tablet, 1 g/time, four times daily dose of mesalazine (He et al., 2012).
Acupuncture and moxibustion are commonly employed to treat IBD, which are frequently targeted acupoints such as “Tianshu” (ST25) and “Zusanli” (ST36). In patients with UC, spaced moxibustion has shown significant efficacy in alleviating symptoms and reducing the expression of inflammatory markers (Qi et al., 2021; Zhou et al., 2009). Furthermore, acupuncture and moxibustion lead to notable improvements in symptomatology and decreased expression of relevant inflammatory markers in patients with IBD. Specifically, studies have highlighted the safety and efficacy of acupuncture and moxibustion at acupoints “Zhongwan” (CV12), “Shangjuxu” (ST37), and “Tianshu” (ST25) in individuals with mild-to-moderate active CD who exhibit poor responsiveness to or intolerance of conventional medications (Bao et al., 2022). These findings have been recognized as one of the top 10 Academic Advances in Traditional Chinese Medicine for 2022.
In recent years, peppermint and TXYF have been incorporated into guidelines and expert consensus for IBS (Disorders et al., 2020; Lacy et al., 2021). For patients with mild-to-moderate active UC, the 2022 Chinese expert consensus on the diagnosis and treatment of FD strongly recommends the combined use of QCHS and herbal enema (Group et al., 2024). The Chinese consensus on FD (Group et al., 2022) suggests the use of TCM such as Xiangsha Liujunzi granules and Biling Weitong granules, along with acupoint stimulation. TCM and acupoint stimulation are considered safe and effective in managing FGIDs and IBD in clinical settings.
The safety profile of TCM in IBD is generally favorable, but care must be taken to avoid herb-drug interactions, especially with immunosuppressive therapies. Potential adverse effects include gastrointestinal disturbances and allergic reactions. The quality control of herbal products is critical to ensure safety.
TCM offers promising complementary and alternative treatment options for FGIDs and IBD, with potential benefits in symptom relief and quality of life improvement. However, patients should seek treatment from qualified TCM practitioners and consider TCM as part of an integrated treatment plan. Ongoing research and well-designed clinical trials are essential to further validate the efficacy and safety of TCM in these conditions.
TCM shows promise in treating FGIDs and IBD; nonetheless, the specific mechanism remains undefined. A possible explanation for the effectiveness of TCM in these conditions is its ability to regulate the gut microbiome, which is crucial in the development and progression of FGIDs and IBD. TCM botanical drugs and formulations have antimicrobial and anti-inflammatory properties, which may help restore balance to the gut microbiome and alleviate symptoms. In addition, TCM often takes a holistic approach, addressing not only physical symptoms but also contributing emotional and psychological factors. This comprehensive approach may also contribute to the success of TCM in treating FGIDs and IBD. However, more studies are needed to fully understand the mechanism of action of TCM in these conditions and optimize its use in clinical practice. This section summarized the major research conducted in the last 5 years regarding how TCM affects FGIDs and IBD.
The dried mycorrhizae of Poria cocos (Schw.) Wolf of family Polyporaceae are used as medicinal components of P. cocos, which are commonly used in the treatment of diarrhea and other diseases because of its ability to strengthen the spleen and stomach and regulate water metabolism (Chinese Pharmacopoeia Commission, 2020). A 2-weeks study on three extracts of P. cocos (triterpenoid, water-soluble polysaccharide, and acidic polysaccharide) showed that water-soluble (7.5 g/kg) and acidic (1.0 g/kg) polysaccharides could help regulate Th1/Th2 and Th17/Treg homeostasis in alternate-day fasting and weight-loaded forced swimming-induced FD rats. Triterpenoid (7.5 g/kg) can promote repair of the gastrointestinal mucosa in rats, and Buzhongyiqi pill (4.5 g/kg) was used as the positive control (Tu et al., 2022). Baicalin–berberine nanoparticles, a combination of baicalin extracts from Scutellaria baicalensis Georgi and berberine extracts from Coptis chinensis Franch., reduced the expression of nuclear factor-kappa B (NF-κB) in the colonic tissues of IBS-D mice and decreased basophil granulocyte and leukomonocyte levels in the whole blood of mice for 10 days (Li L. et al., 2020). Wei-Tong-Xin is a Chinese herbal formula composed of five botanical drugs, namely, Rheum pal matum L., Aucklandia lappa Decne, Gleditsia sinensis Lam, Pharbitis nil (L.) Choisy, and Glycyrrhiza uralensis Fisch. Zhang X. et al. (2022) treated FD rats with Wei-Tong-Xin (0.5, 1.0, and 2.0 g/kg) for 3 days at different administration doses, which significantly promoted gastrointestinal motility in a dose-dependent manner. The therapeutic effect may be accomplished by downregulating the TLR4/myeloid differentiation factor 88 signaling pathway and reducing the expression of glucagon-like peptide 1 and its receptor. Electroacupuncture at “Zusanli” (ST36) was reported to reduce the number of mast cells, inhibit their degranulation release in FD rats, and downregulate the expression of nerve growth factor and its receptor (Dong et al., 2022). It can also reduce the amount of TLR4 in colon tissues to reduce the release of proinflammatory factors from mast cell degranulation and restore the normal role of the intestinal mucosal barrier (Yang et al., 2019). Chang’an II (2.85, 5.71, and 11.42 g/kg), derived from TXYF (Wang et al., 2015), was used to treat 2,4,6-trinitrobenzene sulfonic acid-induced post-infectious IBS model rats for 2 weeks. It reduced inflammation in post-infectious IBS model rats by increasing the ratio of CD4+/CD8+ cells in the lamina propria and submucosa of the small intestinal mucosa and decreasing the levels of IL-1β and IL-4.
To regulate the innate immune system, the mechanism of TCM mostly involves TLR4-related inflammatory pathways and macrophage polarization. For example, G. uralensis Fisch. (Shi et al., 2022), Portulaca oleracea L. polysaccharide (Yang et al., 2023), and others increase the expression of intestinal tight junction proteins to repair the intestinal barrier by regulating the downregulation of the TLR4-myeloid differentiation factor 88-NF-κB pathway. Pingwei San (Zhang et al., 2019), baicalin and emodin coadministration (Xu B. et al., 2021), and honokiol (Wang N. et al., 2022) inhibited downstream NF-κB activation through TLR4 inhibition and peroxisome proliferator-activated receptor-γ activation. A 10-day study in DSS-induced UC mice showed that coptisine (25, 50, and 100 mg/kg) inhibited the MAPK/extracellular signal-regulated kinase signaling pathway and increased N6-methyladenosine RNA methylation to regulate macrophage polarization.5-ASA (200 mg/kg) was used as the positive control (Zhao et al., 2024), and adenosine 5′-monophosphate-activated protein kinase, a polarization regulator, was activated in the peritoneal macrophages of mice with DSS-induced colitis by platycodon D at a dose of 10 mg/kg (Guo R. et al., 2021). TXYF (5.6 and 11.2 g/kg) inhibited NF-κB activation and thus reduced NLRP3 gene expression, the optimal dose is 11.2 g/kg, and a positive control was not set (Zhang H. Y. et al., 2022). Together, these three factors reduce M1 polarization, increase M2 polarization, and reduce diarrhea in model animals. In a 1-week study of 2,4,6-trinitrobenzene sulfonic acid-induced IBD mice treated with electroacupuncture stimulation at bilateral “Dachangshu” (BL25), electroacupuncture inhibited the activation of macrophages in the colonic mucosa and reduced the release of inflammatory factors through the activation of cannabinoid CB2 receptors (Zhang H. et al., 2022). Qinghua Quyu Jianpi decoction (14.17 g/kg) can activate the Wnt pathway, promoting epithelial cell turnover, reducing apoptosis, activating the Wnt pathway by inducing nuclear translocation of β-catenin, accelerating the cell cycle, and promoting cell proliferation (Qu et al., 2023).
To regulate the adaptive immune system, studies on the anti-inflammatory effects of TCM have focused on restoring the Th17/Treg cell balance in IBD, i.e., promoting the conversion of naive CD4+ T cells to Tregs. For example, paeoniflorin (Zheng et al., 2020), Kuijie decoction (Peng et al., 2024), classic formula Yiyi Fuzi Baijiang formula (Liu et al., 2023d), The Gegen Qinlian decoction, a TCM prescription documented in an ancient text dating back approximately 1800 years ago, are commonly used for the treatment of damp–heat diarrhea, and Gegen Qinlian decoction (1.5 and 7.5 g/kg) was found to reduce the Th17-like transformation of Tregs and attenuate the intestinal immune response of DSS-induced mice by inhibiting the overactivation of signal transducers and activators of transcription 3 (Zhao et al., 2021). After 1 week trial, Astragaloside IV (50 and 100 mg/kg) inhibited the overactivation of the Notch signaling pathway in a dose-dependent manner to restore the normal Th17/Treg cell ratio, decrease IL-17A and IL-21 levels, increase body weight, and decrease the disease activity index in DSS-induced colitis mice (Zhong et al., 2022a) (Table 2).
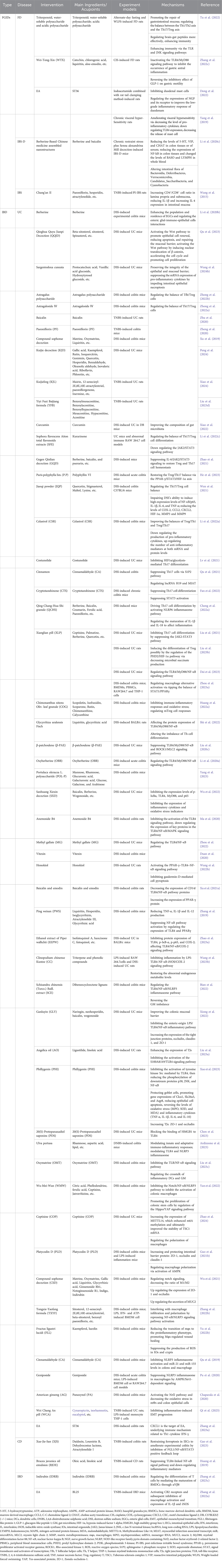
Table 2. Mechanism of intestinal immune and inflammatory functions in TCM intervention of FGIDs and IBD.
Patchouli alcohol, one of the active metabolites of Agastache rugosa (Fisch. et Mey.) O. Ktze. downregulates excitatory longitudinal muscle myenteric plexus neurons in the distal colon at doses of 5, 10, and 20 mg/kg, decreasing the proportion of Ach- and substance P-positive neurons and the number of Ach- and substance P-positive neurons, downregulating choline acetyltransferase expression, leading to improved symptoms (Chen W. et al., 2022). Previous studies have revealed that capsaicin can activate TRPV1 receptors, and calcitonin gene-related peptide is released from capsaicin; therefore, calcitonin gene-related peptide is thought to be associated with pain, inflammation, and vasodilation (Russell et al., 2014). Liangfu pills are composed of Alpinia officinarum Hance and Cyperus rotundus L., and network pharmacology and animal experiments have jointly demonstrated that Liangfu pills (1.8, 3.6, and 7.2 g/kg) reduces the levels of TRPV1 and calcitonin gene-related peptide expressions, thereby increasing the gastric emptying rate and small intestinal propulsion in a rat model of gastric cold syndrome for FD treatment. Mosa was used as the positive control (He et al., 2022). TXYF (20 mL/kg) inhibits the activation of enteric glial cells, downregulate the nerve growth factor/tyrosine kinase signaling pathway in the colon of neonatal maternal separation and restraint stress-induced IBS-D rats, reduce the abdominal withdrawal reflex score, and alleviate abdominal pain and diarrhea (Lu and Zhang, 2023). Moreover, electroacupuncture stimulation of “Zusanli” (ST36) reduced the plasma norepinephrine concentration in FD rats, improving impaired gastric slow wave and mediating via the afferent central pathway involving the nucleus of the solitary tract and the vagal cholinergic efferent pathway (Zhang et al., 2020).
Studies on TCM for modulating sensory transduction in patients with IBD are limited, and only studies on goji berries are being conducted. Lycium barbarum L. belongs to the Solanaceae family, and its source of bioactivity is L. barbarum polysaccharide isolated from the fruit of L. barbarum. L. barbarum L. has the effect of tonifying the liver and kidney, benefiting the essence and brightening the eyes (Chinese Pharmacopoeia Commission, 2020; Tian et al., 2019). L. barbarum polysaccharide alone (100 mg/kg) or in combination (50 mg/kg) with capsaicin (6 mg/kg) led to appropriate body weight of DSS-induced UC rats, a reduction in serum IL-6 and colonic TNF-α levels, and a decrease in TRPV1 and transient receptor potential ankyrin 1 expression in colonic tissues for 4 weeks, thereby exerting anti-inflammatory effects; a positive control was not set (Chen Y. S. et al., 2022) (Table 3).
Pueraria lobata (Willd.) Ohwi, a Chinese botanical drug, is often used to treat diarrhea (Chinese Pharmacopoeia Commission, 2020). In a 2-week study using neonatal maternal separation and adult colonic acetic acid stimulation-induced IBS-D rats model, puerarin, a natural metabolite of Pueraria Mirifica, inhibited the HPA axis by downregulating CRF, promoting IEC proliferation and repairing the intestinal barrier at doses of 6, 12, and 24 mg/kg, with multipathway therapeutic effects (Wang et al., 2021). Liu et al. (2024) conducted 1-week, 20-min -a-day electroacupuncture in the “Zhongwan” (RN12) and “Zusanli” (ST36) in FD rats and observed that electroacupuncture increased body weight and intestinal propulsion rate, decreased the levels of CRF and CRF-R1 in the hypothalamus and duodenum, and decreased levels of serum corticotropin-releasing hormone and adrenocorticotropic hormone levels in the rat, which demonstrated that electroacupuncture of FD mechanism is related to the CRF signaling pathway. Yu et al. (2019) treated chronic acute combing stress-induced IBS rats with resveratrol (10, 20, and 40 mg/kg) for 22 days at different administration doses, and they discovered that a high dose of resveratrol (40 mg/kg) rescued the decreases in hippocampal PKA, pCREB, and BDNF expression downstream of 5-HT1A. However, low resveratrol doses (10 mg/kg) do not have this effect. The modified Liu-Jun-Zi decoction consists of nine botanical drugs, namely, Codonopsis pilosula (Franch.) Nannf., Corydalis yanhusuo W.T. Wang, Atractylodes macro cephala Koidz., Magnolia officinalis Rehder and E.H. Wilson, Aucklandia lappa DC., Amomum villosum Lour., and G. uralensis Fisch., which have been shown to downregulate the overexpression of duodenal EC cells and inhibit the signaling of 5-HT3 receptor in FD rats (Zhao et al., 2020). Shugan decoction is a proportional preparation of five Chinese botanical drugs, including Atractylodes macrocephala Koidz., Paeonia lactiflora Pall., Citrus reticulata Blanco, Bupleurum chinense DC., and Saposhnikovia divaricata (Turcz.) Schischk. Serotonin transporter knockout also reduces the frequency and extent of longitudinal smooth muscle contractions in the colon and alleviates diarrhea symptoms in IBS rats (Wang et al., 2020).
TXYF not only regulates abnormal immunity in patients with UC and improves sensory transduction in patients with IBS but also affects neuroimmunity. By lowering serum 5-HT, TXYF (2.5, 5, and 10 g/kg) can downregulate hepatic 5-HT2A receptor expression, improve hepatic lipid metabolism, and reduce intestinal pathological injury and hepatic steatosis. SASP (0.3 g/kg) was used as a positive control (Zhang X. F. et al., 2022). It can also upregulate the expression of the colonic serotonin transporter to reduce 5-HT levels, reverse decreases in superoxide dismutase activity, and reduce the levels of inflammatory factors such as IL-6 and IL-9 (Luo et al., 2021). Therefore, TXYF, as a commonly used antidiarrheal herbal medicine in clinical practice, can be used to treat FGIDs and IBD through multiple pathways. Shaoyaotang consists of nine Chinese botanical drugs, including Paeonia lactiflora Pall., Areca catechu L., and Scutellaria baicalensis Georgi, which is commonly used in the treatment of dampness–heat diarrhea to clear heat, resolve dampness, and regulate Qi and blood. Shaoyaotang (32 g/kg) upregulates the human serotonin transporter-encoding gene SLC6A4, the key enzyme for 5-HT degradation, monoamine oxidase A and monoamine oxidase B, in DSS-induced UC mice; thus, Shaoyaotang reduces colonic inflammatory cell infiltration by promoting 5-HT transport and degradation (Li Y. N. et al., 2023). In addition, electroacupuncture stimulation of “Zusanli” (ST36) activated Ach release from the VN in the colon to significantly reduce plasma TNF-α, IL-1β, and IL-6 levels, decrease the disease activity index, and increase daily food intake in a rat model of colitis (Jin H. et al., 2019). Herb-partitioned moxibustion of “Qihai” (CV6) and “Tianshu” (ST25) can reduce colonic mucosal congestion and edema in CD rats by downregulating the expression levels of dopamine and dopamine receptor 1 in the colon, hypothalamus, and spinal dorsal horn (Lu et al., 2019) (Table 4).
Members of the Magnoliaceae family M. officinalis Rehd. et Wils. are used for the treatment of bloating and indigestion because of their ability to regulate gastrointestinal Qi (Chinese Pharmacopoeia Commission, 2020). As one of the extracts of M. officinalis Rehd. et Wils, magnoloside A (5, 10, and 20 mg/kg) increased the abundance of intestinal microbiota; however, magnoloside A decreases the abundance of beneficial intestinal bacteria, such as Akkermansia, at high concentrations of magnolinoside A (20 mg/kg) (Xue et al., 2019). Atractyloside-A, one of the main metabolites of the Chinese medicine Atractylodes lancea (Thunb.) DC., can exert therapeutic effects by decreasing the Firmicutes/Bacteroidetes ratio and downregulating the expression of 5-HT and 5-HT3 receptors (Xu J. et al., 2022). One-week treatment with acupuncture or moxibustion of bilateral “Zusanli” (ST36) in IBS-D rats decreased the relative abundance of Bacteroidetes and Proteobacteria, increased the relative abundance of Firmicutes, decreased the synthesis of lipopolysaccharides, and attenuated inflammatory response. In addition, the moxibustion group promotes the synthesis and metabolism of amino acids, such as tyrosine and tryptophan (Lai et al., 2023). Fuzi-Lizhong pills (50 and 150 mg crude drug/mL) have been used to treat digestive disorders and increase the abundance of Lactobacillus, a key flora for repairing the immune barrier of the intestinal tract, decrease the abundance of inflammation-associated microbiota such as Bacteroide,s and significantly decrease the levels of TNF, IL-1β, IL-6, and INF-γ and attenuate diarrhea in IBS-D rats in the state of spleen yang deficiency (Zhen et al., 2021).
The regulatory effect of Chinese medicine on IBD flora focuses on the restoration of the normal composition of intestinal flora by reducing the abundance of harmful bacteria, increasing the levels of beneficial bacteria, and regulating microbiota metabolism. Huai Hua San is based on Sophora japonica L., which is widely used for treating lower gastrointestinal diseases, and an 8-day study showed that Huai Hua San can reduce the Firmicutes/Bacteroidetes ratio to that of healthy people, restore colonic vascular permeability, and reduce the disease activity index (Liu P. et al., 2020). Pulsatilla decoction, which consists of four Chinese botanical drugs, namely, Pulsatilla chinensis (Bunge) Regel, Coptis chinensis Franch., Phellodendron chinense Schneid., and Fraxinus rhynchophylla Hance, originated in the Eastern Han Dynasty. It can increase body weight and colon length in DSS-induced UC mice by potentially repairing the intestinal mucosal barrier through the upregulation of tight junction proteins such as zonula occludens-1 and occludin. This alteration leads increases the relative abundance of Bacteroidetes, reductions in the relative abundance of Firmicutes and Proteobacteria, and elevations in the total content of SCFAs in the intestines, with an optimal dose of 8.1 g/kg (Niu et al., 2023). In addition, electroacupuncture of “Dachangshu” (BL25) and bilateral “Tianshu” (ST25) in obese IBD rats also reduce the Firmicutes/Bacteroidetes ratio (Yang et al., 2024). The methanol extract of Schizonepetae Spica (500 and 1,000 mg/kg) improved the intestinal flora by downregulating the abundance of harmful bacteria such as Clostridiales and Desulfovibrio and upregulated the abundance of beneficial bacteria such as Muribaculaceae and Ligolactobacillus in DSS-induced colitis mice. Salazosulfapyridine (1,000 mg/kg) was used as the positive control (Ye et al., 2023).
Schisandra chinensis polysaccharides significantly downregulated the levels of IL-6, IL-10, IL-17, and TNF-α; antagonized DSS-induced intestinal dysbiosis in mice; increased the levels of acetic acid, propionic acid, and total SCFAs; and improved SCFAs metabolism, which is useful for treating the symptoms of abdominal pain and blood in the stool of mice. Salazosulfapyridine (200 mg/kg) was used as the positive control (Su et al., 2020). Paeonol similarly restores the homeostasis of the intestinal flora and regulates SCFAs metabolism (Zhao M. et al., 2023). Recent metabolomics and animal studies have shown that uric acid levels are closely related to the integrity of the intestinal barrier and that abnormally high uric acid levels in the intestinal tract can lead to deterioration of the intestinal barrier, whereas one of the extracts of Rheum palmatum L., rhein (50 and 100 mg/kg) indirectly affected purine metabolism by increasing the abundance of intestinal lactobacilli in mice with DSS-induced chronic colitis, lowering the concentration of intestinal uric acid, and reversing the increase in the permeability of the intestinal barrier in IBD. A positive control was not set (Wu et al., 2020) (Table 5).
The pathogenesis of FGIDs and IBD involve a complex interplay of mechanisms with the brain–gut axis, encompassing inflammatory responses, immune dysregulation, impaired neurosensory transmission, disrupted neuroendocrine–immune interactions, and alterations in the composition of the gut microbiota. TCM shows effectiveness in alleviating disease symptoms, slowing disease advancement, and averting disease reappearance. According to the above TCM that can treat FGIDs and IBD (Figure 3), terpenes (triterpenoid of Poria cocos, patchouli alcohol, and platycodon D, etc.), polysaccharides (water-soluble polysaccharide of P. cocos, acidic polysaccharide of P. cocos, Portulaca oleracea L. polysaccharide, Lycium barbarum polysaccharide, etc.), flavonoids (baicalin, Puerarin, etc.), glycosides (paeoniflorin, magnoloside A, Atractyloside-A, etc.), and other metabolites (alkaloids, phenols, anthraquinones, aldehydes, etc.) were major bioactive metabolites in TCM for the treatment of FGIDs and IBD. Schisandra chinensis (Turcz.) Baill., Wei-Tong-Xin, Shaoyaotang, TXYF, and QCHS, etc., were the main TCM botanical drugs and formulas. Acupuncture treats FGIDs and IBD primarily by electroacupuncture stimulation of “Zusanli” (ST36) and herb-partitioned moxibustion of “Qihai” (CV6) and “Tianshu” (ST25). The mechanism of TCM interventions on FGIDs and IBD is multipathway, namely, botanical drugs, metabolites, prescriptions, and acupuncture. However, the greatest number of studies has focused on the regulation of intestinal inflammation and immunity and the fewest on intestinal neurosensory transmission, and acupuncture mainly focuses on modulating the NEI.

Figure 3. Mechanism of TCM intervention of FGIDs and IBD based on the brain-gut axis theory (by Figdraw). TCM, traditional Chinese medicine; IBD, inflammatory bowel disease; FGIDs, functional gastrointestinal disorders; NEI, neuroendocrine-immune network; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5 hydroxyindoleacetic acid; MAO, monoamine oxidase; SERT, serotonin transporter; 5-HT2AR, 5-hydroxytryptamine 2A receptor; DA, dopamine; DR1, dopamine receptor 1; EA, electroacupuncture; ACh, acetylcholine; VN, vagus nerve; TNF, tumor necrosis factor; IL, interleukin; TRPV1, transient receptor potential vanilloid 1; TRPA1, transient receptor potential ankyrin 1; TLR4, toll-like receptor 4; NF-κB, nuclear factor -kappa B; MyD88, myeloid differentiation factor 88; PPAR-γ, peroxisome proliferator activated receptor gamma; iNOS, inducible nitric oxide synthase; Th, T helper; Treg, regulatory T; NLRP, NOD-like receptor family pyrin domain containing; SCFAs, short-chain fatty acids; ZO-1, Zonula occludens-1; CRF, corticotropin-releasing factor; ACTH, adrenocorticotropic factor; EC, enterochromaffin cell; 5-HT3R, 5-hydroxytryptamine 3 receptor; M3R, muscarinic receptorsubtype-3; BDNF, brain derived neurotrophic factor; p-CREB, p-cAMP-response element binding protein; CRH, corticotropin-releasing hormone; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; EGCs, enteric glial cells; NGF, nerve growth factor; Glu, glutamate; SP, substance P; LMMP, longitudinal muscle myenteric plexus; c-Fos, proto-oncogene c-Fos; NTS, nucleus of the solitary tract; GLP-1, glucagon-like peptide 1; Arg-1, arginase-1; BASO, basophil granulocyte; LYMPH, leukomonocyte; LPS, lipopolysaccharides.
We have outlined the potential molecular mechanisms of TCM in FGIDs and IBD, with focus on therapeutic outcomes, targets, and signaling pathways in animal models: 1) modulation of the levels of proinflammatory cytokines in the intestinal tract, such as ILs and tumor necrosis factor, downregulation of proinflammatory signaling pathways such as NF-κB, MAPK, and levels of NLRP inflammatory vesicles; 2) antagonism of the aberrant activation of immune cells in the intestinal tract, such as M2-like polarization, downregulation of the Th1/Th2 and Th17/Treg cell ratios, and downregulation of TLR levels on the immune cell surface; 3) restoration of the intestinal barrier and promotion of the expression of tight junction proteins, such as zonula occludens-1 and occludin; 4) intervention in visceral hypersensitivity reaction by decreasing the number of hypersensitivity-associated neurons and osmosensory channel expression, such as TRPV; 5) modulation of the levels of intestinal barrier injury-related neurotransmitters (Ach, 5-HT, and DA) and downregulation of CRF levels to antagonize their pathogenic effects; and 6) upregulation of the abundance of beneficial bacteria in the intestine and the downregulation of that of detrimental bacteria, e.g., decreasing the Firmicutes/Bacteroidetes ratio and increasing the abundance of Lactobacillus, to restore the normal intestinal metabolism, e.g., SCFAs metabolism and purine metabolism. Therefore, TCM can elicit therapeutic effects by targeting multiple pathways, components, and modes of action, indicating its promising developmental potential.
This paper discussed FGIDs and IBD, a large group of diseases with unclear pathogenesis and no clear cure is available, which are urgent problems for modern medicine. TCM has shown promise in treating FGIDs and IBD through evidence-based practices guided by a holistic approach. For example, 4 weeks of TXYF treatment in patients with IBD effectively reduced the number of diarrhea episodes, improved fecal character, and was superior to the antispasmodic pinaverium (Fan et al., 2017). Acupuncture and moxibustion were reported to alleviate symptoms in patients with mildly to moderately active CD and repair the intestinal barrier (Bao et al., 2022). TCM targets the brain–gut axis by modulating intestinal immunity and inflammation, normalizing sensory transmission to reduce visceral hypersensitivity, regulating neuroimmunity, and restoring intestinal microbial balance. The review of research on TCM treatment of FGIDs and IBD, the following problems have been identified: 1) The small sample size of clinical studies and unreported inclusion and exclusion criteria make it difficult to avoid selection bias, so the credibility of the experimental results cannot be guaranteed, and most clinical research evidence is of poor quality. 2) Because FGIDs and IBD are chronic recurrent diseases, most clinical studies have a short follow-up period; thus, longer follow-up studies are needed to better observe the long-term efficacy of TCM treatments. 3) Research on existing mechanism focuses on TCM regulation against a single target, lack of multi-target, multi-faceted validation, and the need to evaluate the synergistic therapeutic effect of different components of TCM formulas to regulate multiple targets in the direction of efforts. 4) Insufficient depth of mechanistic studies on TCM and the need for more rigorous design in terms of the active metabolite and effective concentration to confirming the safety and effectiveness of the potentially effective metabolite. 5) The core of TCM is the overall concept and treatment based on pattern differentiation, and existing animal models of the two diseases do not correspond with the TCM evidence patterns, and TCM treatments deviated.
The goal of modern medicine in IBD treatment has gradually shifted to the promotion of intestinal mucosal healing and long-term relief of clinical symptoms (Turner et al., 2021). Existing IBS treatments also fail to meet the clinical need for pain relief, and the recurrence of FGID symptoms is also of concern (Ford et al., 2020b; Singh et al., 2022). The treatment of TCM in these aspects has advantages. A meta-analysis showed that TCM retention enema was superior to conventional drug therapy in terms of clinical efficacy and reduction of recurrence and colonoscopic improvement of ulceration in patients with UC (Yan et al., 2021). Moreover, the treatment of IBS and FD with Weichang’an pill combined with Western medicine is superior to the application of Western medicine alone, and the combined treatment of TCM and Western medicine can significantly alleviate abdominal pain and bloating and reduce FD recurrence without increasing the incidence of adverse reactions (Jiabao et al., 2023). This proves the potential of TCM. However, the mechanism of action of TCM is not clear; thus, it lacks credibility for large-scale application in clinical treatment. Moreover, hepatotoxicity and drug–drug interactions of TCM should be noted. Polygonum multiflorum Thunb., Scutellaria baicalensis Georgi, and Gynura segetum are the three most commonly reported to cause drug-induced liver injuries (Ballotin et al., 2021), Salvia miltiorrhiza Bge. Increases the risk of bleeding when taken with warfarin (Chan, 2001). In recent years, the concept of brain–gut–microbiota axis has received widespread attention and has been applied to various diseases, which is a promising development direction (Zhu et al., 2024). Research on gut microbiota by TCM is still immature. Therefore, more studies are needed to fully substantiate the efficacy of TCM in restoring gut microbial homeostasis. Care prevent and cure diseases, the modernization and standardization of TCM are desired to. With the scientists’ deepening of the concept of brain–gut axis and the continuous development of science and technology, as well as the interpenetration and integration of TCM and modern medicine, a breakthrough may occur in the prevention and treatment of FGIDs and IBD by TCM, which will bring the hope of cure to more patients.
One limitation of our study is that we employed a narrative review approach instead of a systematic review, which inherently comes with certain drawbacks. Specifically, the scope of literature we reviewed was not as comprehensive as it could have been, potentially leading to an incomplete representation of the topic. This lack of exhaustive literature retrieval suggests that readers should interpret the conclusions of this paper with caution. While narrative reviews can offer valuable insights, they do not always provide clear transparency regarding the criteria used for selecting and excluding literature, which may introduce biases or omissions. We acknowledge these limitations and advise readers to consider them when evaluating the conclusions drawn in this study.
RL: Data curation, Writing–original draft, Conceptualization, Visualization, Writing–review and editing. YL: Conceptualization, Data curation, Writing–review and editing. JM: Conceptualization, Visualization, Writing–review and editing. QZ: Data curation, Writing–review and editing. YS: Visualization, Writing–review and editing. JL: Conceptualization, Writing–review and editing. HL: Conceptualization, Supervision, Writing–review and editing. TZ: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences (Grant Number: CI2021B003) and China Academy of Traditional Chinese Medicine National Natural Science Foundation of China Key Program Funding Balance Project (Grant Number: 2022029).
The authors would like to thank all the reviewers who will participate in the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agirman, G., Yu, K. B., and Hsiao, E. Y. (2021). Signaling inflammation across the gut-brain axis. Science 374 (6571), 1087–1092. doi:10.1126/science.abi6087
Alula, K. M., Dowdell, A. S., LeBere, B., Lee, J. S., Levens, C. L., Kuhn, K. A., et al. (2023). Interplay of gut microbiota and host epithelial mitochondrial dysfunction is necessary for the development of spontaneous intestinal inflammation in mice. Microbiome 11 (1), 256. doi:10.1186/s40168-023-01686-9
Andersson, U., and Tracey, K. J. (2012). Neural reflexes in inflammation and immunity. J. Exp. Med. 209 (6), 1057–1068. doi:10.1084/jem.20120571
Ardizzone, A., Mannino, D., Capra, A. P., Repici, A., Filippone, A., Esposito, E., et al. (2023). New insights into the mechanism of ulva pertusa on colitis in mice: modulation of the pain and immune system. Mar. Drugs 21 (5), 298. doi:10.3390/md21050298
Arimura, K., Takagi, H., Uto, T., Fukaya, T., Nakamura, T., Choijookhuu, N., et al. (2017). Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol. 10 (4), 957–970. doi:10.1038/mi.2016.96
Bajaj, A., Markandey, M., Kedia, S., and Ahuja, V. (2024). Gut bacteriome in inflammatory bowel disease: an update on recent advances. Indian J. Gastroenterol. 43, 103–111. doi:10.1007/s12664-024-01541-1
Ballotin, V. R., Bigarella, L. G., Brandão, A. B. M., Balbinot, R. A., Balbinot, S. S., and Soldera, J. (2021). Herb-induced liver injury: systematic review and meta-analysis. World J. Clin. Cases 9 (20), 5490–5513. doi:10.12998/wjcc.v9.i20.5490
Bao, C., Wu, L., Wang, D., Chen, L., Jin, X., Shi, Y., et al. (2022). Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn's disease: a randomized controlled trial. EClinicalMedicine 45, 101300. doi:10.1016/j.eclinm.2022.101300
Barberio, B., Zamani, M., Black, C. J., Savarino, E. V., and Ford, A. C. (2021). Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6 (5), 359–370. doi:10.1016/S2468-1253(21)00014-5
Bellono, N. W., Bayrer, J. R., Leitch, D. B., Castro, J., Zhang, C., O'Donnell, T. A., et al. (2017). Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170 (1), 185–198. doi:10.1016/j.cell.2017.05.034
Berin, M. C., Li, H., and Sperber, K. (2006). Antibody-mediated antigen sampling across intestinal epithelial barriers. Ann. N. Y. Acad. Sci. 1072, 253–261. doi:10.1196/annals.1326.002
Bian, Z., Qin, Y., Li, L., Su, L., Fei, C., Li, Y., et al. (2022). Schisandra chinensis (Turcz.) Baill. Protects against DSS-induced colitis in mice: involvement of TLR4/NF-κB/NLRP3 inflammasome pathway and gut microbiota. J. Ethnopharmacol. 298, 115570. doi:10.1016/j.jep.2022.115570
Black, C. J., Drossman, D. A., Talley, N. J., Ruddy, J., and Ford, A. C. (2020). Functional gastrointestinal disorders: advances in understanding and management. Lancet 396 (10263), 1664–1674. doi:10.1016/S0140-6736(20)32115-2
Bonaz, B., Sinniger, V., and Pellissier, S. (2016). Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 594 (20), 5781–5790. doi:10.1113/jp271539
Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405 (6785), 458–462. doi:10.1038/35013070
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut Axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44. doi:10.3389/fpsyt.2018.00044
Brint, E. K., MacSharry, J., Fanning, A., Shanahan, F., and Quigley, E. M. (2011). Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 106 (2), 329–336. doi:10.1038/ajg.2010.438
Butcher, M. J., and Zhu, J. (2021). Recent advances in understanding the Th1/Th2 effector choice. Fac. Rev. 10, 30. doi:10.12703/r/10-30
Cai, L. L., Li, X., Cai, Q. H., Guo, S. X., Zhang, Y., Sun, W. C., et al. (2024). Irritable bowel syndrome in children: the placebo response rate and influencing factors a meta-analysis. Pediatr. Res. 95, 1432–1440. doi:10.1038/s41390-023-02996-2
Cai, Y., Li, X., Han, Q., Bai, J., Zheng, Q., Sun, R., et al. (2023). Si-Ni-San improves experimental colitis by favoring Akkermensia colonization. J. Ethnopharmacol. 305, 116067. doi:10.1016/j.jep.2022.116067
Carco, C., Young, W., Gearry, R. B., Talley, N. J., McNabb, W. C., and Roy, N. C. (2020). Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front. Cell Infect. Microbiol. 10, 468. doi:10.3389/fcimb.2020.00468
Chan, T. Y. (2001). Interaction between warfarin and danshen (Salvia miltiorrhiza). Ann. Pharmacother. 35 (4), 501–504. doi:10.1345/aph.19029
Chaparala, A., Tashkandi, H., Chumanevich, A. A., Witalison, E. E., Windust, A., Cui, T., et al. (2020). Molecules from American ginseng suppress colitis through nuclear factor erythroid-2-related factor 2. Nutrients 12 (6), 1850. doi:10.3390/nu12061850
Chen, G., Feng, P., Wang, S., Ding, X., Xiong, J., Wu, J., et al. (2020). An herbal formulation of jiawei xiaoyao for the treatment of functional dyspepsia: a multicenter, randomized, placebo-controlled, clinical trial. Clin. Transl. Gastroenterol. 11 (10), e00241. doi:10.14309/ctg.0000000000000241
Chen, J., Lu, P., Liu, J., Yang, L., Li, Y., Chen, Y., et al. (2023). 20(S)- Protopanaxadiol saponins isolated from Panax notoginseng target the binding of HMGB1 to TLR4 against inflammation in experimental ulcerative colitis. Phytother. Res. 37 (10), 4690–4705. doi:10.1002/ptr.7938
Chen, M., Tang, T. C., Wang, Y., Shui, J., Xiao, X. H., Lan, X., et al. (2018). Randomised clinical trial: tong-Xie-Yao-Fang granules versus placebo for patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 48 (2), 160–168. doi:10.1111/apt.14817
Chen, M. X., Chen, J. X., Xia, L., Fu, R., and Lu, Z. (2014). Treating irritable bowel syndrome with diarrhea patients by yigan fupi decoction: a randomized controlled trial. Zhongguo Zhong Xi Yi Jie He Za Zhi 34 (6), 656–660. doi:10.7661/CJIM.2014.06.0656
Chen, W., Liao, L., Huang, Z., Lu, Y., Lin, Y., Pei, Y., et al. (2022a). Patchouli alcohol improved diarrhea-predominant irritable bowel syndrome by regulating excitatory neurotransmission in the myenteric plexus of rats. Front. Pharmacol. 13, 943119. doi:10.3389/fphar.2022.943119
Chen, Y. S., Lian, Y. Z., Chen, W. C., Chang, C. C., Tinkov, A. A., Skalny, A. V., et al. (2022b). Lycium barbarum polysaccharides and capsaicin inhibit oxidative stress, inflammatory responses, and pain signaling in rats with dextran sulfate sodium-induced colitis. Int. J. Mol. Sci. 23 (5), 2423. doi:10.3390/ijms23052423
Cheng, C., Hu, J., Li, Y., Ji, Y., Lian, Z., Au, R., et al. (2022a). Qing-Chang-Hua-Shi granule ameliorates DSS-induced colitis by activating NLRP6 signaling and regulating Th17/Treg balance. Phytomedicine 107, 154452. doi:10.1016/j.phymed.2022.154452
Cheng, H., Liu, J., Zhang, D., Wang, J., Tan, Y., Feng, W., et al. (2022b). Ginsenoside Rg1 alleviates acute ulcerative colitis by modulating gut microbiota and microbial tryptophan metabolism. Front. Immunol. 13, 817600. doi:10.3389/fimmu.2022.817600
Cheng, H., Zhang, D., Wu, J., Liu, J., Tan, Y., Feng, W., et al. (2023). Atractylodes macrocephala Koidz. volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 14, 1127785. doi:10.3389/fimmu.2023.1127785
Chiarioni, G., Popa, S. L., Ismaiel, A., Pop, C., Dumitrascu, D. I., Brata, V. D., et al. (2023). Herbal remedies for constipation-predominant irritable bowel syndrome: a systematic review of randomized controlled trials. Nutrients 15 (19), 4216. doi:10.3390/nu15194216
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the people’ Republic of China. Beijing: China Medical Science Press.
Chu, W., Li, Y. L., Li, J. J., Lin, J., Li, M., Wang, J., et al. (2023). Guiqi Baizhu prescription ameliorates cytarabine-induced intestinal mucositis by targeting JAK2 to inhibit M1 macrophage polarization. Biomed. Pharmacother. 164, 114902. doi:10.1016/j.biopha.2023.114902
Cosme, D., Soares-da-Silva, P., and Magro, F. (2023). Long-term effect of Toll-like receptor-2, -4, -5, -7 and NOD2 stimulation on Na(+),K(+)-ATPase activity and expression in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 324 (5), C1028–C1038. doi:10.1152/ajpcell.00208.2022
Dai, Y., Lu, Q., Li, P., Zhu, J., Jiang, J., Zhao, T., et al. (2023). Xianglian Pill attenuates ulcerative colitis through TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 300, 115690. doi:10.1016/j.jep.2022.115690
Defaye, M., Abdullah, N. S., Iftinca, M., Hassan, A., Agosti, F., Zhang, Z., et al. (2022). Gut-innervating TRPV1+ neurons drive chronic visceral pain via microglial P2Y12 receptor. Cell Mol. Gastroenterol. Hepatol. 13 (4), 977–999. doi:10.1016/j.jcmgh.2021.12.012
Deuring, J. J., de Haar, C., Kuipers, E. J., Peppelenbosch, M. P., and van der Woude, C. J. (2013). The cell biology of the intestinal epithelium and its relation to inflammatory bowel disease. Int. J. Biochem. Cell Biol. 45 (4), 798–806. doi:10.1016/j.biocel.2012.12.020
Disorders, S. G. o.F. G., Motility, S. G. o.G., Gastroenterology, C. S. o., and Association, C. M. (2020). Chinese expert consensus of irritable bowel syndrome in 2020. Chin. J. Dig. 40 (12), 803–818. doi:10.3760/cma.j.cn311367-20201116-00660
Diwakarla, S., Fothergill, L. J., Fakhry, J., Callaghan, B., and Furness, J. B. (2017). Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol. Motil. 29 (6). doi:10.1111/nmo.13101
Dlugosz, A., Zakikhany, K., Acevedo, N., D'Amato, M., and Lindberg, G. (2017). Increased expression of toll-like receptors 4, 5, and 9 in small bowel mucosa from patients with irritable bowel syndrome. Biomed. Res. Int. 2017, 9624702. doi:10.1155/2017/9624702
Dodd, D., Spitzer, M. H., Van Treuren, W., Merrill, B. D., Hryckowian, A. J., Higginbottom, S. K., et al. (2017). A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551 (7682), 648–652. doi:10.1038/nature24661
Dolinger, M., Torres, J., and Vermeire, S. (2024). Crohn's disease. Lancet 403 (10432), 1177–1191. doi:10.1016/S0140-6736(23)02586-2
Dong, J. Z., Rong, P. J., Wang, X. T., Wang, D., Leng, M. H., and Xiao, L. J. (2022). Effect of electroacupuncture at “Zusanli” (ST 36) on duodenal mast cells, NGF and NTRK1 in rats with functional dyspepsia. Zhongguo Zhen Jiu 42 (7), 767–772. doi:10.13703/j.0255-2930.20211230-k0003
Du, L., Long, Y., Kim, J. J., Chen, B., Zhu, Y., and Dai, N. (2019). Protease activated receptor-2 induces immune activation and visceral hypersensitivity in post-infectious irritable bowel syndrome mice. Dig. Dis. Sci. 64 (3), 729–739. doi:10.1007/s10620-018-5367-y
Duan, S., X, D., Chen, S., Liang, J., Huang, S., Hou, S., et al. (2020). Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 121, 109683. doi:10.1016/j.biopha.2019.109683
Eberhardson, M., Levine, Y. A., Tarnawski, L., and Olofsson, P. S. (2021). The brain-gut axis, inflammatory bowel disease and bioelectronic medicine. Int. Immunol. 33 (6), 349–356. doi:10.1093/intimm/dxab018
Fan, H., Zheng, L., Lai, Y., Lu, W., Yan, Z., Xiao, Q., et al. (2017). Tongxie formula reduces symptoms of irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 15 (11), 1724–1732. doi:10.1016/j.cgh.2017.06.026
Fan, L. M., Zhang, Y. Q., Chen, Y. P., Chen, L. L., Xu, W. H., Nan, L. H., et al. (2022). Cryptotanshinone ameliorates dextran sulfate sodium-induced murine acute and chronic ulcerative colitis via suppressing STAT3 activation and Th17 cell differentiation. Int. Immunopharmacol. 108, 108894. doi:10.1016/j.intimp.2022.108894
Farmer, A. D., and Aziz, Q. (2009). Visceral pain hypersensitivity in functional gastrointestinal disorders. Br. Med. Bull. 91, 123–136. doi:10.1093/bmb/ldp026
Fichna, J., Poole, D. P., Veldhuis, N., MacEachern, S. J., Saur, D., Zakrzewski, P. K., et al. (2015). Transient receptor potential vanilloid 4 inhibits mouse colonic motility by activating NO-dependent enteric neurotransmission. J. Mol. Med. Berl. 93 (12), 1297–1309. doi:10.1007/s00109-015-1336-5
Ford, A. C., Mahadeva, S., Carbone, M. F., Lacy, B. E., and Talley, N. J. (2020a). Functional dyspepsia. Lancet 396 (10263), 1689–1702. doi:10.1016/S0140-6736(20)30469-4
Ford, A. C., Sperber, A. D., Corsetti, M., and Camilleri, M. (2020b). Irritable bowel syndrome. Lancet 396 (10263), 1675–1688. doi:10.1016/S0140-6736(20)31548-8
Ford, A. C., Vanner, S., Kashyap, P. C., and Nasser, Y. (2024). Chronic visceral pain: new peripheral mechanistic insights and resulting treatments. Gastroenterology 166, 976–994. doi:10.1053/j.gastro.2024.01.045
Furness, J. B., Callaghan, B. P., Rivera, L. R., and Cho, H. J. (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71. doi:10.1007/978-1-4939-0897-4_3
Gao, J., Xiong, T., Grabauskas, G., and Owyang, C. (2022). Mucosal serotonin reuptake transporter expression in irritable bowel syndrome is modulated by gut microbiota via mast cell-prostaglandin E2. Gastroenterology 162 (7), 1962–1974.e6. doi:10.1053/j.gastro.2022.02.016
Gao, Y., Zhang, Z., J, D., Yang, X., Wang, X., Wen, K., et al. (2023). Xue-Jie-San restricts ferroptosis in Crohn's disease via inhibiting FGL1/NF-κB/STAT3 positive feedback loop. Front. Pharmacol. 14, 1148770. doi:10.3389/fphar.2023.1148770
Ge, L., Liu, S., Li, S., Yang, J., Hu, G., Xu, C., et al. (2022). Psychological stress in inflammatory bowel disease: psychoneuroimmunological insights into bidirectional gut-brain communications. Front. Immunol. 13, 1016578. doi:10.3389/fimmu.2022.1016578
Gentek, R., Molawi, K., and Sieweke, M. H. (2014). Tissue macrophage identity and self-renewal. Immunol. Rev. 262 (1), 56–73. doi:10.1111/imr.12224
Ghia, J. E., Blennerhassett, P., Deng, Y., Verdu, E. F., Khan, W. I., and Collins, S. M. (2009). Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology 136 (7), 2280–2288. doi:10.1053/j.gastro.2009.02.069
Gobert, A. P., Sagrestani, G., Delmas, E., Wilson, K. T., Verriere, T. G., Dapoigny, M., et al. (2016). The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci. Rep. 6, 39399. doi:10.1038/srep39399
Gong, Y., Zha, Q., Li, L., Liu, Y., Yang, B., Liu, L., et al. (2012). Efficacy and safety of Fufangkushen colon-coated capsule in the treatment of ulcerative colitis compared with mesalazine: a double-blinded and randomized study. J. Ethnopharmacol. 141 (2), 592–598. doi:10.1016/j.jep.2011.08.057
Group, G. D., Group, F. G. D. C., and Gastroenterology, C. S. o. (2022). 2022 expert consensus on diagnosis and treatment of functional dyspepsia in China. Chin. J. Dig. 43 (7), 433–446. doi:10.3760/cma.j.cn311367-20230206-00048
Group, I. B. D., Gastroenterology, C. S. o., and Association, C. M. (2024). Chinese clinical practice guideline on the management of ulcerative colitis (2023, Xi′an). Chin. J. Inflamm. Bowel Dis. 08 (01), 33–58.
Gu, Y., Wang, C., Qin, X., Zhou, B., Liu, X., Liu, T., et al. (2022). Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol. Res. 181, 106291. doi:10.1016/j.phrs.2022.106291
Guo, J., Sun, J. H., Chen, L., Geng, H., Yang, G. H., Shen, R. R., et al. (2021a). Bidirectional regulation of acupuncture: a subgroup analysis of multicenter randomized controlled trial of acupuncture with Tiaoshen Jianpi for irritable bowel syndrome. Zhongguo Zhen Jiu 41 (8), 845–850. doi:10.13703/j.0255-2930.20201111-k0001
Guo, R., Meng, Q., Wang, B., and Li, F. (2021b). Anti-inflammatory effects of Platycodin D on dextran sulfate sodium (DSS) induced colitis and E. coli Lipopolysaccharide (LPS) induced inflammation. Int. Immunopharmacol. 94, 107474. doi:10.1016/j.intimp.2021.107474
Guo, Y., and Gharibani, P. (2024). Analgesic effects of vagus nerve stimulation on visceral hypersensitivity: a direct comparison between invasive and noninvasive methods in rats. Neuromodulation 27 (2), 284–294. doi:10.1016/j.neurom.2023.04.001
Hanauer, S. B., and Baert, F. (1994). Medical therapy of inflammatory bowel disease. Med. Clin. North Am. 78 (6), 1413–1426. doi:10.1016/s0025-7125(16)30108-0
Hang, L., Wang, E., Feng, Y., Zhou, Y., Meng, Y., Jiang, F., et al. (2022). Metagenomics and metabolomics analysis to investigate the effect of Shugan decoction on intestinal microbiota in irritable bowel syndrome rats. Front. Microbiol. 13, 1024822. doi:10.3389/fmicb.2022.1024822
Hartmannsgruber, V., Heyken, W. T., Kacik, M., Kaistha, A., Grgic, I., Harteneck, C., et al. (2007). Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2 (9), e827. doi:10.1371/journal.pone.0000827
He, H. H., Shen, H., Zheng, K., Gu, P. Q., Zhu, L., Liu, Y. J., et al. (2012). Observation of the curative effect of qingchang huashi recipe for treating active ulcerative colitis of inner-accumulation of damp-heat syndrome. Chin. J. Integr. Traditional West. Med. 32 (12), 1598–1601.
He, J. Y., Gui, B., Chen, Y. F., Yin, Y. Q., Tao, S. H., Shen, Z. B., et al. (2022). Mechanism of Liangfu Pills in treatment of functional dyspepsia: based on network pharmacology and experimental verification. Zhongguo Zhong Yao Za Zhi 47 (14), 3853–3862. doi:10.19540/j.cnki.cjcmm.20211230.705
He, L., Yan, X., Wen, S., Zhong, Z., Hou, Z., Liu, F., et al. (2023). Paris polyphylla extract attenuates colitis in mice by regulating PPAR-γ mediated Treg/Th17 balance. J. Ethnopharmacol. 314, 116621. doi:10.1016/j.jep.2023.116621
Heiran, A., Bagheri Lankarani, K., Bradley, R., Simab, A., and Pasalar, M. (2022). Efficacy of herbal treatments for functional dyspepsia: a systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 36 (2), 686–704. doi:10.1002/ptr.7333
Ho, L., Zhong, C. C. W., Wong, C. H. L., Wu, J. C. Y., Chan, K. K. H., Wu, I. X. Y., et al. (2021). Chinese herbal medicine for functional dyspepsia: a network meta-analysis of prokinetic-controlled randomised trials. Chin. Med. 16 (1), 140. doi:10.1186/s13020-021-00556-6
Ho, T. C., Horn, N. A., Huynh, T., Kelava, L., and Lansman, J. B. (2012). Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 6 (4), 246–254. doi:10.4161/chan.20719
Holtmeier, W., Zeuzem, S., Preiss, J., Kruis, W., Bohm, S., Maaser, C., et al. (2011). Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn's disease: good safety profile but lack of efficacy. Inflamm. Bowel Dis. 17 (2), 573–582. doi:10.1002/ibd.21345
Hou, L., Rong, P., Yang, Y., Fang, J., Wang, J., Wang, Y., et al. (2023). Auricular vagus nerve stimulation improves visceral hypersensitivity and gastric motility and depression-like behaviors via vago-vagal pathway in a rat model of functional dyspepsia. Brain Sci. 13 (2), 253. doi:10.3390/brainsci13020253
Hu, S., Bourgonje, A. R., Gacesa, R., Jansen, B. H., Björk, J. R., Bangma, A., et al. (2024). Mucosal host-microbe interactions associate with clinical phenotypes in inflammatory bowel disease. Nat. Commun. 15 (1), 1470. doi:10.1038/s41467-024-45855-2
Huang, J. Q., Wei, S. Y., Cheng, N., Zhong, Y. B., Yu, F. H., Li, M. D., et al. (2022a). Chimonanthus nitens oliv. Leaf granule ameliorates DSS-induced acute colitis through Treg cell improvement, oxidative stress reduction, and gut microflora modulation. Front. Cell Infect. Microbiol. 12, 907813. doi:10.3389/fcimb.2022.907813
Huang, Y., Zheng, Y., Yang, F., Feng, Y., Xu, K., Wu, J., et al. (2022b). Lycium barbarum Glycopeptide prevents the development and progression of acute colitis by regulating the composition and diversity of the gut microbiota in mice. Front. Cell Infect. Microbiol. 12, 921075. doi:10.3389/fcimb.2022.921075
Huang, Y. F., Li, Q. P., Dou, Y. X., Wang, T. T., Qu, C., Liang, J. L., et al. (2019). Therapeutic effect of Brucea javanica oil emulsion on experimental Crohn's disease in rats: involvement of TLR4/NF-κB signaling pathway. Biomed. Pharmacother. 114, 108766. doi:10.1016/j.biopha.2019.108766
Iyer, K., Erkert, L., and Becker, C. (2023). Know your neighbors: microbial recognition at the intestinal barrier and its implications for gut homeostasis and inflammatory bowel disease. Front. Cell Dev. Biol. 11, 1228283. doi:10.3389/fcell.2023.1228283
Jean, A. (2001). Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 81 (2), 929–969. doi:10.1152/physrev.2001.81.2.929
Ji, E., Wang, T., Guo, F., Zhang, Y., Tang, C., Tang, D., et al. (2019). Xiaoerfupi alleviates the symptoms of functional dyspepsia by regulating the HTR3A and c-FOS. Biomed. Pharmacother. 120, 109442. doi:10.1016/j.biopha.2019.109442
Jia, Q., Zhang, L., Zhang, J., Pei, F., Zhu, S., Sun, Q., et al. (2019). Fecal microbiota of diarrhea-predominant irritable bowel syndrome patients causes hepatic inflammation of germ-free rats and berberine reverses it partially. Biomed. Res. Int. 2019, 4530203. doi:10.1155/2019/4530203
Jiabao, W., Lishuang, Z., Baihan, N., Yajun, Y. U., Fengwen, Y., Lin, M., et al. (2023). Efficacy and safety of Weichang' an pill combined with Western Medicine on gastrointestinal diseases: a systematic review and Meta-analysis. J. Tradit. Chin. Med. 43 (6), 1057–1067. doi:10.19852/j.cnki.jtcm.20230814.003
Jin, D. X., He, J. F., Zhang, K. Q., Luo, X. G., and Zhang, T. C. (2019a). EtOAc extract of H. attenuatum Choisy inhibits inflammation by suppressing the NF-κB and MAPK pathways and modulating the gut microbiota. Phytomedicine 57, 292–304. doi:10.1016/j.phymed.2018.12.037
Jin, H., Guo, J., Liu, J., Lyu, B., Foreman, R. D., Shi, Z., et al. (2019b). Autonomically mediated anti-inflammatory effects of electrical stimulation at acupoints in a rodent model of colonic inflammation. Neurogastroenterol. Motil. 31 (8), e13615. doi:10.1111/nmo.13615
Jones, M. P., Tack, J., Van Oudenhove, L., Walker, M. M., Holtmann, G., Koloski, N. A., et al. (2017). Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin. Gastroenterol. Hepatol. 15 (7), 1014–1020. doi:10.1016/j.cgh.2016.12.032
Kabata, H., and Artis, D. (2019). Neuro-immune crosstalk and allergic inflammation. J. Clin. Invest. 129 (4), 1475–1482. doi:10.1172/jci124609
Kaplan, G. G., and Windsor, J. W. (2021). The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18 (1), 56–66. doi:10.1038/s41575-020-00360-x
Kindt, S., Van Oudenhove, L., Broekaert, D., Kasran, A., Ceuppens, J. L., Bossuyt, X., et al. (2009). Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol. Motil. 21 (4), 389–398. doi:10.1111/j.1365-2982.2008.01220.x
Koren, T., Yifa, R., Amer, M., Krot, M., Boshnak, N., Ben-Shaanan, T. L., et al. (2021). Insular cortex neurons encode and retrieve specific immune responses. Cell 184 (25), 6211. doi:10.1016/j.cell.2021.11.021
Krebs, S., Omer, T. N., and Omer, B. (2010). Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn's disease - a controlled clinical trial. Phytomedicine 17 (5), 305–309. doi:10.1016/j.phymed.2009.10.013
Kuo, W. T., Zuo, L., Odenwald, M. A., Madha, S., Singh, G., Gurniak, C. B., et al. (2021). The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 161 (6), 1924–1939. doi:10.1053/j.gastro.2021.08.047
Lacy, B. E., Pimentel, M., Brenner, D. M., Chey, W. D., Keefer, L. A., Long, M. D., et al. (2021). ACG clinical guideline: management of irritable bowel syndrome. Am. J. Gastroenterol. 116 (1), 17–44. doi:10.14309/ajg.0000000000001036
Lai, B. Y., Hong, M. Y., He, Y. J., Li, X., Wang, S. S., Chen, Y., et al. (2023). Effect of acupuncture and moxibustion on intestinal flora in the rats with diarrhea-predominant irritable bowel syndrome based on 16S rDNA technique. Zhongguo Zhen Jiu 43 (12), 1411–1421. doi:10.13703/j.0255-2930.20230630-k0002
Le Berre, C., Honap, S., and Peyrin-Biroulet, L. (2023). Ulcerative colitis. Lancet 402 (10401), 571–584. doi:10.1016/S0140-6736(23)00966-2
Li, C., Ai, G., Wang, Y., Lu, Q., Luo, C., Tan, L., et al. (2020a). Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 152, 104603. doi:10.1016/j.phrs.2019.104603
Li, H., Chen, X., Liu, J., Chen, M., Huang, M., Huang, G., et al. (2021a). Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 267, 113445. doi:10.1016/j.jep.2020.113445
Li, H., Fan, C., Lu, H., Feng, C., He, P., Yang, X., et al. (2020b). Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm. Sin. B 10 (3), 447–461. doi:10.1016/j.apsb.2019.08.006
Li, J., Lu, J., Sun, J., Ruan, Z., Xu, D., Geng, H., et al. (2017). Acupuncture with regulating mind and spleen for diarrhea irritable bowel syndrome and sleep quality:a randomized controlled trial. Zhongguo Zhen Jiu 37 (1), 9–13. doi:10.13703/j.0255-2930.2017.01.002
Li, L., Cui, H., Li, T., Qi, J., Chen, H., Gao, F., et al. (2020c). Synergistic effect of berberine-based Chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo. Front. Pharmacol. 11, 1210. doi:10.3389/fphar.2020.01210
Li, M., Guo, W., Dong, Y., Wang, W., Tian, C., Zhang, Z., et al. (2022a). Beneficial effects of celastrol on immune balance by modulating gut microbiota in experimental ulcerative colitis mice. Genomics Proteomics Bioinforma. 20 (2), 288–303. doi:10.1016/j.gpb.2022.05.002
Li, Q., Cui, Y., Xu, B., Wang, Y., Lv, F., Li, Z., et al. (2021b). Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 170, 105694. doi:10.1016/j.phrs.2021.105694
Li, Q., Sun, H., Guo, J., Zhao, X., Bai, R., Zhang, M., et al. (2023a). The effect of prenatal stress on offspring depression susceptibility in relation to the gut microbiome and metabolome. J. Affect Disord. 339, 531–537. doi:10.1016/j.jad.2023.07.089
Li, Y., Ye, Z., He, H., Hu, Y., Wu, M., Li, L., et al. (2022b). The application of Tong-fu therapeutic method on ulcerative colitis: a systematic review and meta-analysis for efficacy and safety of rhubarb-based therapy. Front. Pharmacol. 13, 1036593. doi:10.3389/fphar.2022.1036593
Li, Y. N., Zhang, Y. N., Zhao, L., Wang, L. L., Zhou, Y. L., and Zhen, J. H. (2023b). Mechanism of Shaoyaotang in treatment o ulcerative colitis based on network pharmacology and experimental verification. Chin. J. Exp. Traditional Med. Formulae 29 (23), 8–15. doi:10.13422/j.cnki.syfjx.20230822
Li, Z., Lin, M., Li, Y., Shao, J., Huang, R., Qiu, Y., et al. (2022c). Total flavonoids of Sophora flavescens and kurarinone ameliorated ulcerative colitis by regulating Th17/Treg cell homeostasis. J. Ethnopharmacol. 297, 115500. doi:10.1016/j.jep.2022.115500
Liang, Z. F., Chen, R. H., Xu, Y. S., Chen, Q. X., and Dong, M. L. (2009). Tiaohe Ganpi Hexin Decoction in treatment of irritable bowel syndrome with diarrhea: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 7 (9), 819–822. doi:10.3736/jcim20090904
Ling, X., Peng, S., Zhong, J., Guo, L., Xu, Y., Jin, X., et al. (2022). Effects of chang-kang-fang formula on the microbiota-gut-brain Axis in rats with irritable bowel syndrome. Front. Pharmacol. 13, 778032. doi:10.3389/fphar.2022.778032
Liu, B., Wanders, A., Wirdefeldt, K., Sjölander, A., Sachs, M. C., Eberhardson, M., et al. (2020a). Vagotomy and subsequent risk of inflammatory bowel disease: a nationwide register-based matched cohort study. Aliment. Pharmacol. Ther. 51 (11), 1022–1030. doi:10.1111/apt.15715
Liu, C., He, Y. X., Zhang, J. N., Yang, F., Wang, S. Y., Hu, J. L., et al. (2023a). Angelica oil restores the intestinal barrier function by suppressing S100A8/A9 signalling in mice with ulcerative colitis. Phytomedicine 108, 154490. doi:10.1016/j.phymed.2022.154490
Liu, C. S., Hu, Y. X., Luo, Z. Y., Qiu, C. W., Deng, X. H., and Chen, F. L. (2023b). Xianglian pill modulates gut microbial production of succinate and induces regulatory T cells to alleviate ulcerative colitis in rats. J. Ethnopharmacol. 303, 116007. doi:10.1016/j.jep.2022.116007
Liu, C. S., Xia, T., Luo, Z. Y., Wu, Y. Y., Hu, Y. N., Chen, F. L., et al. (2021). Network pharmacology and pharmacokinetics integrated strategy to investigate the pharmacological mechanism of Xianglian pill on ulcerative colitis. Phytomedicine 82, 153458. doi:10.1016/j.phymed.2020.153458
Liu, H., Yao, S., Dann, S. M., Qin, H., Elson, C. O., and Cong, Y. (2013). ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 43 (7), 1716–1726. doi:10.1002/eji.201242889
Liu, H. N., Wu, H., Chen, Y. Z., Chen, Y. J., Shen, X. Z., and Liu, T. T. (2017). Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig. Liver Dis. 49 (4), 331–337. doi:10.1016/j.dld.2017.01.142
Liu, M., Liu, F., Pan, Y., Xiong, Y., Zeng, X., Zheng, L., et al. (2023c). Oxymatrine ameliorated experimental colitis via mechanisms involving inflammatory DCs, gut microbiota and TLR/NF-κB pathway. Int. Immunopharmacol. 115, 109612. doi:10.1016/j.intimp.2022.109612
Liu, M., Wang, Z., Liu, X., Xiao, H., Liu, Y., Wang, J., et al. (2023d). Therapeutic effect of Yiyi Fuzi Baijiang formula on TNBS-induced ulcerative colitis via metabolism and Th17/Treg cell balance. J. Ethnopharmacol. 309, 116301. doi:10.1016/j.jep.2023.116301
Liu, P., Bian, Y., Liu, T., Zhong, J., Zhong, Y., Zhuang, S., et al. (2020b). Huai hua san alleviates dextran sulphate sodium-induced colitis and modulates colonic microbiota. J. Ethnopharmacol. 259, 112944. doi:10.1016/j.jep.2020.112944
Liu, Q., Wang, D., Yang, X., Ma, F., Han, W., Hu, J., et al. (2023e). The mechanosensitive ion channel PIEZO1 in intestinal epithelial cells mediates inflammation through the NOD-like receptor 3 pathway in Crohn's disease. Inflamm. Bowel Dis. 29 (1), 103–115. doi:10.1093/ibd/izac152
Liu, T., Wang, H., Liu, J., Tan, R., Chen, L., Liang, X., et al. (2024). Electroacupuncture can modify stress, low-grade inflammation in the duodenum, and damage to the intestinal barrier in rats with functional dyspepsia through the CRF signaling pathway. Comb. Chem. High. Throughput Screen 27. doi:10.2174/0113862073306526240403063736
Liu, Y., Wu, J., Chen, L., Wu, X., Gan, Y., Xu, N., et al. (2020c). β-patchoulene simultaneously ameliorated dextran sulfate sodium-induced colitis and secondary liver injury in mice via suppressing colonic leakage and flora imbalance. Biochem. Pharmacol. 182, 114260. doi:10.1016/j.bcp.2020.114260
Lu, P. D., Yuan, M. C., Quan, X. P., Chen, J. F., and Zhao, Y. H. (2022). Preclinical studies of licorice in ulcerative colitis: a systematic review with meta-analysis and network pharmacology. J. Ethnopharmacol. 296, 115444. doi:10.1016/j.jep.2022.115444
Lu, X., and Zhang, S. (2023). How tongxie-yaofang regulates intestinal synaptic plasticity by activating enteric glial cells and NGF/TrkA pathway in diarrhea-predominant irritable bowel syndrome rats. Drug Des. Devel Ther. 17, 2969–2983. doi:10.2147/DDDT.S423333
Lu, Y., Ding, G., Zheng, H., Lü, T., Ma, Z., Wu, H., et al. (2019). Effect of herb-partitioned moxibustion on dopamine levels and dopamine receptor 1 expression in the colon and central nervous system in rats with Crohn's disease. J. Tradit. Chin. Med. 39 (3), 356–363.
Luo, Y. F., Gao, J., Chai, Y. H., Li, W., Qin, Z., Chen, Y. Z., et al. (2021). Effect of tongxie yaofang on expressions of colon SERT and liver 5-ht2ar proteins in rats with ulcerative colitis model of liver stagnation and spleen deficiency. Chin. J. Exp. Traditional Med. Formulae 27 (02), 15–21. doi:10.13422/j.cnki.syfjx.20202103
Lv, L., Wang, F. Y., Ma, X. X., Li, Z. H., Huang, S. P., Shi, Z. H., et al. (2017). Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: a multi-center randomized double-blind placebo-controlled clinical study. World J. Gastroenterol. 23 (30), 5589–5601. doi:10.3748/wjg.v23.i30.5589
Lv, Q., Xing, Y., Dong, D., Hu, Y., Chen, Q., Zhai, L., et al. (2021). Costunolide ameliorates colitis via specific inhibition of HIF1α/glycolysis-mediated Th17 differentiation. Int. Immunopharmacol. 97, 107688. doi:10.1016/j.intimp.2021.107688
Ma, H., Zhou, M., Duan, W., Chen, L., Wang, L., and Liu, P. (2020). Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 87, 106794. doi:10.1016/j.intimp.2020.106794
Ma, T. T., Yu, S. Y., Li, Y., Liang, F. R., Tian, X. P., Zheng, H., et al. (2012). Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment. Pharmacol. Ther. 35 (5), 552–561. doi:10.1111/j.1365-2036.2011.04979.x
Ma, Y. Y., Hao, Z., Chen, Z. Y., Shen, Y. X., Liu, H. R., Wu, H. G., et al. (2024). Acupuncture and moxibustion for irritable bowel syndrome: an umbrella systematic review. J. Integr. Med. 22 (1), 22–31. doi:10.1016/j.joim.2023.12.001
Mallaret, G., Lashermes, A., Meleine, M., Boudieu, L., Barbier, J., Aissouni, Y., et al. (2022). Involvement of toll-like receptor 5 in mouse model of colonic hypersensitivity induced by neonatal maternal separation. World J. Gastroenterol. 28 (29), 3903–3916. doi:10.3748/wjg.v28.i29.3903
Margolis, K. G., Cryan, J. F., and Mayer, E. A. (2021). The microbiota-gut-brain Axis: from motility to mood. Gastroenterology 160 (5), 1486–1501. doi:10.1053/j.gastro.2020.10.066
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut-brain Axis. Annu. Rev. Med. 73, 439–453. doi:10.1146/annurev-med-042320-014032
McKenna, K., Beignon, A. S., and Bhardwaj, N. (2005). Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79 (1), 17–27. doi:10.1128/jvi.79.1.17-27.2005
Mertz, H., Naliboff, B., Munakata, J., Niazi, N., and Mayer, E. A. (1995). Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 109 (1), 40–52. doi:10.1016/0016-5085(95)90267-8
Meynier, M., Daugey, V., Mallaret, G., Gervason, S., Meleine, M., Barbier, J., et al. (2024). Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes 16 (1), 2298026. doi:10.1080/19490976.2023.2298026
Mishima, Y., and Ishihara, S. (2021). Enteric microbiota-mediated serotonergic signaling in pathogenesis of irritable bowel syndrome. Int. J. Mol. Sci. 22 (19), 10235. doi:10.3390/ijms221910235
Moloney, R. D., Johnson, A. C., O'Mahony, S. M., Dinan, T. G., Greenwood-Van Meerveld, B., and Cryan, J. F. (2016). Stress and the microbiota-gut-brain Axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci. Ther. 22 (2), 102–117. doi:10.1111/cns.12490
Mujagic, Z., Kasapi, M., Jonkers, D. M., Garcia-Perez, I., Vork, L., Weerts, Z., et al. (2022). Integrated fecal microbiome-metabolome signatures reflect stress and serotonin metabolism in irritable bowel syndrome. Gut Microbes 14 (1), 2063016. doi:10.1080/19490976.2022.2063016
Muller, P. A., Koscsó, B., Rajani, G. M., Stevanovic, K., Berres, M. L., Hashimoto, D., et al. (2014). Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158 (2), 300–313. doi:10.1016/j.cell.2014.04.050
Naganuma, M., Sugimoto, S., Mitsuyama, K., Kobayashi, T., Yoshimura, N., Ohi, H., et al. (2018). Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154 (4), 935–947. doi:10.1053/j.gastro.2017.11.024
Ng, Q. X., Soh, A. Y. S., Loke, W., Lim, D. Y., and Yeo, W. S. (2018a). The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 11, 345–349. doi:10.2147/jir.S174982
Ng, Q. X., Soh, A. Y. S., Loke, W., Venkatanarayanan, N., Lim, D. Y., and Yeo, W. S. (2018b). A meta-analysis of the clinical use of curcumin for irritable bowel syndrome (IBS). J. Clin. Med. 7 (10), 298. doi:10.3390/jcm7100298
Ng, Q. X., Yau, C. E., Yaow, C. Y. L., Chong, R. I. H., Chong, N. Z., Teoh, S. E., et al. (2023). What has longitudinal 'omics' studies taught us about irritable bowel syndrome? A systematic review. Metabolites 13 (4), 484. doi:10.3390/metabo13040484
Niu, C., Hu, X. L., Yuan, Z. W., Xiao, Y., Ji, P., Wei, Y. M., et al. (2023). Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity. J. Ethnopharmacol. 300, 115741. doi:10.1016/j.jep.2022.115741
Nozu, T., Miyagishi, S., Nozu, R., Takakusaki, K., and Okumura, T. (2018). Altered colonic sensory and barrier functions by CRF: roles of TLR4 and IL-1. J. Endocrinol. 239 (2), 241–252. doi:10.1530/joe-18-0441
O'Mahony, S. M., Clarke, G., Dinan, T. G., and Cryan, J. F. (2017). Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience 342, 37–54. doi:10.1016/j.neuroscience.2015.09.068
Oshima, S., Fujimura, M., and Fukimiya, M. (1999). Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 112 (4), 257–263. doi:10.1007/s004180050445
Paik, D., Yao, L., Zhang, Y., Bae, S., D’Agostino, G. D., Zhang, M., et al. (2022). Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 603 (7903), 907–912. doi:10.1038/s41586-022-04480-z
Pan, F., Zhang, T., Zhang, Y. H., Xu, J. J., and Chen, F. M. (2009). Effect of Tongxie Yaofang Granule in treating diarrhea-predominate irritable bowel syndrome. Chin. J. Integr. Med. 15 (3), 216–219. doi:10.1007/s11655-009-0216-7
Pavlov, V. A., Wang, H., Czura, C. J., Friedman, S. G., and Tracey, K. J. (2003). The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9 (5-8), 125–134. doi:10.1007/bf03402177
Pei, L., Geng, H., Guo, J., Yang, G., Wang, L., Shen, R., et al. (2020). Effect of acupuncture in patients with irritable bowel syndrome: a randomized controlled trial. Mayo Clin. Proc. 95 (8), 1671–1683. doi:10.1016/j.mayocp.2020.01.042
Peng, K., Xia, S., Xiao, S., Zhang, M., Liao, J., and Yu, Q. (2024). Kuijie decoction ameliorates ulcerative colitis by affecting intestinal barrier functions, gut microbiota, metabolic pathways and Treg/Th17 balance in mice. J. Ethnopharmacol. 319 (Pt 3), 117316. doi:10.1016/j.jep.2023.117316
Powell, N., Walker, M. M., and Talley, N. J. (2017). The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 14 (3), 143–159. doi:10.1038/nrgastro.2016.191
Price, D. D., Craggs, J., Verne, G. N., Perlstein, W. M., and Robinson, M. E. (2007). Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 127 (1-2), 63–72. doi:10.1016/j.pain.2006.08.001
Pu, Z., Liu, Y., Li, C., Xu, M., Xie, H., and Zhao, J. (2020). Using network pharmacology for systematic understanding of geniposide in ameliorating inflammatory responses in colitis through suppression of NLRP3 inflammasome in macrophage by AMPK/Sirt1 dependent signaling. Am. J. Chin. Med. 48 (7), 1693–1713. doi:10.1142/s0192415x20500846
Qi, Q., Im, H., Li, K. S., Gu, M., Wu, H. G., Yang, L., et al. (2021). Influence of herb-partitioned moxibustion at Qihai (CV6) and bilateral Tianshu (ST25) and Shangjuxu (ST37) acupoints on toll-like receptors 4 signaling pathways in patients with ulcerative coliti. J. Tradit. Chin. Med. 41 (3), 479–485. doi:10.19852/j.cnki.jtcm.20210310.001
Qi, Y., Wang, M., Chai, L., Zhang, M., Jia, S., Wichai, N., et al. (2023). Wei Chang an pill alleviates 2,4,6-trinitro-benzenesulfonic acid-induced ulcerative colitis by inhibiting epithelial-mesenchymal transition process. Acupunct. Herb. Med. 3 (2), 107–115. doi:10.1097/hm9.0000000000000064
Qu, F., Li, D., Zhang, S., Zhang, C., and Shen, A. (2023). The potential mechanism of qinghua quyu jianpi decoction in the treatment of ulcerative colitis based on network pharmacology and experimental validation. J. Ethnopharmacol. 310, 116396. doi:10.1016/j.jep.2023.116396
Qu, S., Shen, Y., Wang, M., Wang, X., and Yang, Y. (2019). Suppression of miR-21 and miR-155 of macrophage by cinnamaldehyde ameliorates ulcerative colitis. Int. Immunopharmacol. 67, 22–34. doi:10.1016/j.intimp.2018.11.045
Qu, S. L., Chen, L., Wen, X. S., Zuo, J. P., Wang, X. Y., Lu, Z. J., et al. (2021). Suppression of Th17 cell differentiation via sphingosine-1-phosphate receptor 2 by cinnamaldehyde can ameliorate ulcerative colitis. Biomed. Pharmacother. 134, 111116. doi:10.1016/j.biopha.2020.111116
Rinaman, L., Card, J. P., Schwaber, J. S., and Miselis, R. R. (1989). Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J. Neurosci. 9 (6), 1985–1996. doi:10.1523/jneurosci.09-06-01985.1989
Ritchie, J. (1973). Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14 (2), 125–132. doi:10.1136/gut.14.2.125
Roda, G., Chien, N. S., Kotze, P. G., Argollo, M., Panaccione, R., Spinelli, A., et al. (2020). Crohn's disease. Nat. Rev. Dis. Prim. 6 (1), 22. doi:10.1038/s41572-020-0156-2
Rodríguez-Fandiño, O., Hernández-Ruiz, J., and Schmulson, M. (2010). From cytokines to toll-like receptors and beyond - current knowledge and future research needs in irritable bowel syndrome. J. Neurogastroenterol. Motil. 16 (4), 363–373. doi:10.5056/jnm.2010.16.4.363
Rogler, G., Singh, A., Kavanaugh, A., and Rubin, D. T. (2021). Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology 161 (4), 1118–1132. doi:10.1053/j.gastro.2021.07.042
Ruan, S., Xu, L., Sheng, Y., Wang, J., Zhou, X., Zhang, C., et al. (2023). Th1 promotes M1 polarization of intestinal macrophages to regulate colitis-related mucosal barrier damage. Aging (Albany NY) 15 (14), 6721–6735. doi:10.18632/aging.204629
Russell, F. A., King, R., Smillie, S. J., Kodji, X., and Brain, S. D. (2014). Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94 (4), 1099–1142. doi:10.1152/physrev.00034.2013
Sahn, B., Pascuma, K., Kohn, N., Tracey, K. J., and Markowitz, J. F. (2023). Transcutaneous auricular vagus nerve stimulation attenuates inflammatory bowel disease in children: a proof-of-concept clinical trial. Bioelectron. Med. 9 (1), 23. doi:10.1186/s42234-023-00124-3
Sandborn, W. J., Targan, S. R., Byers, V. S., Rutty, D. A., Mu, H., Zhang, X., et al. (2013). Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 108 (1), 90–98. doi:10.1038/ajg.2012.340
Seyedian, S. S., Nokhostin, F., and Malamir, M. D. (2019). A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 12 (2), 113–122. doi:10.25122/jml-2018-0075
Shanahan, E. R., Kang, S., Staudacher, H., Shah, A., Do, A., Burns, G., et al. (2023). Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut 72 (5), 929–938. doi:10.1136/gutjnl-2021-326158
Shi, G., Kong, J., Wang, Y., Xuan, Z., and Xu, F. (2022). Glycyrrhiza uralensis Fisch. alleviates dextran sulfate sodium-induced colitis in mice through inhibiting of NF-κB signaling pathways and modulating intestinal microbiota. J. Ethnopharmacol. 298, 115640. doi:10.1016/j.jep.2022.115640
Shi, X., Hu, Y., Zhang, B., Li, W., Chen, J. D., and Liu, F. (2021). Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight 6 (14), e150052. doi:10.1172/jci.insight.150052
Shimbori, C., De Palma, G., Baerg, L., Lu, J., Verdu, E. F., Reed, D. E., et al. (2022). Gut bacteria interact directly with colonic mast cells in a humanized mouse model of IBS. Gut Microbes 14 (1), 2105095. doi:10.1080/19490976.2022.2105095
Singh, R., Zogg, H., Ghoshal, U. C., and Ro, S. (2022). Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front. Pharmacol. 13, 808195. doi:10.3389/fphar.2022.808195
Song, X., Zhang, H., Zhang, Y., Goh, B., Bao, B., Mello, S. S., et al. (2023). Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 619 (7971), 837–843. doi:10.1038/s41586-023-06265-4
Sperber, A. D., Bangdiwala, S. I., Drossman, D. A., Ghoshal, U. C., Simren, M., Tack, J., et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 160 (1), 99–114.e3. doi:10.1053/j.gastro.2020.04.014
Su, L., Mao, C., Wang, X., Li, L., Tong, H., Mao, J., et al. (2020). The anti-colitis effect of schisandra chinensis polysaccharide is associated with the regulation of the composition and metabolism of gut microbiota. Front. Cell Infect. Microbiol. 10, 519479. doi:10.3389/fcimb.2020.519479
Su, Q., Chen, S. L., Wang, H. H., Liang, L. X., Dai, N., Lyu, B., et al. (2018). A randomized, double-blind, multicenter, placebo-controlled trial of qi-zhi-wei-tong granules on postprandial distress syndrome-predominant functional dyspepsia. Chin. Med. J. Engl. 131 (13), 1549–1556. doi:10.4103/0366-6999.235118
Sun, J., Shen, X., Dong, J., Wang, H., Zuo, L., Zhao, J., et al. (2015). Tripterygium wilfordii hook F as maintenance treatment for Crohn's disease. Am. J. Med. Sci. 350 (5), 345–351. doi:10.1097/maj.0000000000000591
Swain, S. M., and Liddle, R. A. (2021). Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 296, 100171. doi:10.1074/jbc.RA120.015059
Tang, T., Targan, S. R., Li, Z. S., Xu, C., Byers, V. S., and Sandborn, W. J. (2011). Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis - a double-blind comparison with sustained release mesalazine. Aliment. Pharmacol. Ther. 33 (2), 194–202. doi:10.1111/j.1365-2036.2010.04515.x
Tang, X. D., Lu, B., Li, Z. H., Wei, W., Meng, L. N., Li, B. S., et al. (2018). Therapeutic effect of chang'an I recipe (I) on irritable bowel syndrome with diarrhea: a multicenter randomized double-blind placebo-controlled clinical trial. Chin. J. Integr. Med. 24 (9), 645–652. doi:10.1007/s11655-016-2596-9
Tannock, G. W., and Savage, D. C. (1974). Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 9 (3), 591–598. doi:10.1128/iai.9.3.591-598.1974
Tian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., and Ma, Z. (2019). Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: a review. Biomolecules 9 (9), 389. doi:10.3390/biom9090389
Tong, Z. Q., Yang, B., and Tong, X. Y. (2011). A multi-center randomized double-blinded, placebo-controlled clinical study on efficacy of composite sophora colon-soluble capsules in treating ulcerative colitis of internal dampness-heat accumulation syndrome type. Zhongguo Zhong Xi Yi Jie He Za Zhi 31 (2), 172–176.
Tu, Y., Luo, X., Liu, D., Li, H., Xia, H., Ma, C., et al. (2022). Extracts of Poria cocos improve functional dyspepsia via regulating brain-gut peptides, immunity and repairing of gastrointestinal mucosa. Phytomedicine 95, 153875. doi:10.1016/j.phymed.2021.153875
Turner, D., Ricciuto, A., Lewis, A., D'Amico, F., Dhaliwal, J., Griffiths, A. M., et al. (2021). STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160 (5), 1570–1583. doi:10.1053/j.gastro.2020.12.031
Vicentini, F. A., Keenan, C. M., Wallace, L. E., Woods, C., Cavin, J. B., Flockton, A. R., et al. (2021). Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 9 (1), 210. doi:10.1186/s40168-021-01165-z
Wan, M., Ma, Z., Han, J., Rao, M., Hu, F., Gao, P., et al. (2023). 5-HT induces regulatory B cells in fighting against inflammation-driven ulcerative colitis. Int. Immunopharmacol. 125 (Pt A), 111042. doi:10.1016/j.intimp.2023.111042
Wang, F. Y., Su, M., Zheng, Y. Q., Wang, X. G., Kang, N., Chen, T., et al. (2015). Herbal prescription Chang'an II repairs intestinal mucosal barrier in rats with post-inflammation irritable bowel syndrome. Acta Pharmacol. Sin. 36 (6), 708–715. doi:10.1038/aps.2014.170
Wang, J., Wang, X., Ma, X., Xu, B., Chen, L., Chen, C., et al. (2022a). Therapeutic effect of Patrinia villosa on TNBS-induced ulcerative colitis via metabolism, vitamin D receptor and NF-κB signaling pathways. J. Ethnopharmacol. 288, 114989. doi:10.1016/j.jep.2022.114989
Wang, N., Kong, R., Han, W., Bao, W., Shi, Y., Ye, L., et al. (2022b). Honokiol alleviates ulcerative colitis by targeting PPAR-γ-TLR4-NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int. Immunopharmacol. 111, 109058. doi:10.1016/j.intimp.2022.109058
Wang, Q. S., Wang, Y. L., Zhang, W. Y., Li, K. D., Luo, X. F., and Cui, Y. L. (2021). Puerarin from Pueraria lobata alleviates the symptoms of irritable bowel syndrome-diarrhea. Food Funct. 12 (5), 2211–2224. doi:10.1039/d0fo02848g
Wang, S., Su, W., Wu, X., and Dong, W. (2024a). Restoring Treg/Th17 cell balance in ulcerative colitis through HRas silencing and MAPK pathway inhibition. Int. Immunopharmacol. 130, 111608. doi:10.1016/j.intimp.2024.111608
Wang, X., Huang, S., Zhang, M., Su, Y., Pan, Z., Liang, J., et al. (2023a). Gegen Qinlian decoction activates AhR/IL-22 to repair intestinal barrier by modulating gut microbiota-related tryptophan metabolism in ulcerative colitis mice. J. Ethnopharmacol. 302 (Pt B), 115919. doi:10.1016/j.jep.2022.115919
Wang, Y., Dong, Y., Wang, E., Meng, Y., Bi, Z., Sun, S., et al. (2020). Shugan decoction alleviates colonic dysmotility in female SERT-knockout rats by decreasing M(3) receptor expression. Front. Pharmacol. 11, 01082. doi:10.3389/fphar.2020.01082
Wang, Y., Li, M., and Zha, A. S. (2019). Adjuvant treatment of Crohn's disease with traditional Chinese medicine: a meta-analysis. Evid. Based Complement. Altern. Med. 2019, 6710451. doi:10.1155/2019/6710451
Wang, Y., Shao, Z., Song, C., Zhou, H., Zhao, J., Zong, K., et al. (2023b). Clinopodium chinense Kuntze ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by reducing systematic inflammation and regulating metabolism. J. Ethnopharmacol. 309, 116330. doi:10.1016/j.jep.2023.116330
Wang, Y., Zhang, B., Liu, S., Xu, E., and Wang, Z. (2024b). The traditional herb Sargentodoxa cuneata alleviates DSS-induced colitis by attenuating epithelial barrier damage via blocking necroptotic signaling. J. Ethnopharmacol. 319 (Pt 3), 117373. doi:10.1016/j.jep.2023.117373
Wang, Z., Xu, M., Shi, Z., Bao, C., Liu, H., Zhou, C., et al. (2022c). Mild moxibustion for irritable bowel syndrome with diarrhea (IBS-D): a randomized controlled trial. J. Ethnopharmacol. 289, 115064. doi:10.1016/j.jep.2022.115064
Wei, W., Wang, H., Zhang, Y., Zhang, Y., Niu, B., Chen, S., et al. (2021). Faecal bile acids and colonic bile acid membrane receptor correlate with symptom severity of diarrhoea-predominant irritable bowel syndrome: a pilot study. Dig. Liver Dis. 53 (9), 1120–1127. doi:10.1016/j.dld.2021.04.022
Wen, S., Zhong, Z., He, L., Zhao, D., Chen, X., Mi, H., et al. (2021). Network pharmacology dissection of multiscale mechanisms for jiaoqi powder in treating ulcerative colitis. J. Ethnopharmacol. 275, 114109. doi:10.1016/j.jep.2021.114109
Wen, Y. D., Lu, F., Zhao, Y. P., Wang, P., Yang, Q., Li, J. X., et al. (2020). Epigastric pain syndrome: what can traditional Chinese medicine do? A randomized controlled trial of Biling Weitong Granules. World J. Gastroenterol. 26 (28), 4170–4181. doi:10.3748/wjg.v26.i28.4170
Wu, H., Chen, Q. Y., Wang, W. Z., Chu, S., Liu, X. X., Liu, Y. J., et al. (2021). Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice. Biomed. Pharmacother. 133, 110937. doi:10.1016/j.biopha.2020.110937
Wu, J., Wei, Z., Cheng, P., Qian, C., Xu, F., Yang, Y., et al. (2020). Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 10 (23), 10665–10679. doi:10.7150/thno.43528
Wu, X., Fu, S., Jiang, M., Wang, J., Tang, H., Fang, C., et al. (2022). Sanhuang Xiexin decoction ameliorates DSS-induced colitis in mice by regulating intestinal inflammation, intestinal barrier, and intestinal flora. J. Ethnopharmacol. 297, 115537. doi:10.1016/j.jep.2022.115537
Xiao, Q. P., Zhong, Y. B., Kang, Z. P., Huang, J. Q., Fang, W. Y., Wei, S. Y., et al. (2022). Curcumin regulates the homeostasis of Th17/Treg and improves the composition of gut microbiota in type 2 diabetic mice with colitis. Phytother. Res. 36 (4), 1708–1723. doi:10.1002/ptr.7404
Xiao, S., Yan, Y., Shao, M., Zhou, X., Niu, Z., Wu, Y., et al. (2024). Kuijieling decoction regulates the Treg/Th17 cell balance in ulcerative colitis through the RA/RARα signaling pathway. J. Ethnopharmacol. 318 (Pt A), 116909. doi:10.1016/j.jep.2023.116909
Xie, Z., Feng, J., Hibberd, T. J., Chen, B. N., Zhao, Y., Zang, K., et al. (2023). Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron 111 (4), 526–538.e4. doi:10.1016/j.neuron.2022.11.015
Xiong, T., Zheng, X., Zhang, K., Wu, H., Dong, Y., Zhou, F., et al. (2022). Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 289, 115001. doi:10.1016/j.jep.2022.115001
Xu, B., Huang, S., Chen, Y., Wang, Q., Luo, S., Li, Y., et al. (2021a). Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother. Res. 35 (10), 5708–5719. doi:10.1002/ptr.7230
Xu, J., Liu, C., Shi, K., Sun, X., Song, C., Xu, K., et al. (2022a). Atractyloside-A ameliorates spleen deficiency diarrhea by interfering with TLR4/MyD88/NF-κB signaling activation and regulating intestinal flora homeostasis. Int. Immunopharmacol. 107, 108679. doi:10.1016/j.intimp.2022.108679
Xu, M., Duan, X. Y., Chen, Q. Y., Fan, H., Hong, Z. C., Deng, S. J., et al. (2019). Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed. Pharmacother. 109, 2396–2408. doi:10.1016/j.biopha.2018.11.087
Xu, W., Lu, J., Chen, Y., Wang, Z., Cao, J., and Dong, Y. (2021b). Impairment of CRH in the intestinal mucosal epithelial barrier of pregnant Bama miniature pig induced by restraint stress. Endocr. J. 68 (4), 485–502. doi:10.1507/endocrj.EJ20-0332
Xu, Z., Chen, J. J., Mei, Q., Li, Y., and Xu, J. (2022b). Expression of 5-hydroxytryptamine 7 receptor in intestinal mucosa correlates with the degree of intestinal inflammation in Crohn's disease. BMC Gastroenterol. 22 (1), 457. doi:10.1186/s12876-022-02513-5
Xue, H. H., Li, J. J., Li, S. F., Guo, J., Yan, R. P., Chen, T. G., et al. (2023). Phillygenin attenuated colon inflammation and improved intestinal mucosal barrier in DSS-induced colitis mice via TLR4/src mediated MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 24 (3), 2238. doi:10.3390/ijms24032238
Xue, Z., Wu, C., Wei, J., Xian, M., Wang, T., Yang, B., et al. (2019). An orally administered magnoloside A ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota. Life Sci. 233, 116749. doi:10.1016/j.lfs.2019.116749
Yan, S., Wang, P., Wei, H., Jia, R., Zhen, M., Li, Q., et al. (2022). Treatment of ulcerative colitis with Wu-Mei-Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa. Phytomedicine 105, 154362. doi:10.1016/j.phymed.2022.154362
Yan, Z. X., Liu, Y. M., Ma, T., Xu, M. J., Zhang, X. B., Zha, X. J., et al. (2021). Efficacy and safety of retention enema with traditional Chinese medicine for ulcerative colitis: a meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 42, 101278. doi:10.1016/j.ctcp.2020.101278
Yang, J., Shang, B., Shi, H., Zhu, S., Lu, G., and Dai, F. (2019). The role of toll-like receptor 4 and mast cell in the ameliorating effect of electroacupuncture on visceral hypersensitivity in rats. Neurogastroenterol. Motil. 31 (6), e13583. doi:10.1111/nmo.13583
Yang, J. W., Wang, L. Q., Zou, X., Yan, S. Y., Wang, Y., Zhao, J. J., et al. (2020a). Effect of acupuncture for postprandial distress syndrome: a randomized clinical trial. Ann. Intern Med. 172 (12), 777–785. doi:10.7326/m19-2880
Yang, W., Yu, T., Huang, X., Bilotta, A. J., Xu, L., Lu, Y., et al. (2020b). Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11 (1), 4457. doi:10.1038/s41467-020-18262-6
Yang, Y., Pang, F., Zhou, M., Guo, X., Yang, Y., Qiu, W., et al. (2024). Electroacupuncture reduces inflammatory bowel disease in obese mice by activating the Nrf2/HO-1 signaling pathways and repairing the intestinal barrier. Diabetes Metab. Syndr. Obes. 17, 435–452. doi:10.2147/dmso.S449112
Yang, Y., Rao, K., Zhan, K., Shen, M., Zheng, H., Qin, S., et al. (2022). Clinical evidence of acupuncture and moxibustion for irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Front. Public Health 10, 1022145. doi:10.3389/fpubh.2022.1022145
Yang, Y., Zhou, X., Jia, G., Li, T., Li, Y., Zhao, R., et al. (2023). Network pharmacology based research into the effect and potential mechanism of Portulaca oleracea L. polysaccharide against ulcerative colitis. Comput. Biol. Med. 161, 106999. doi:10.1016/j.compbiomed.2023.106999
Yasuda, M., Kawahara, R., Hashimura, H., Yamanaka, N., Iimori, M., Amagase, K., et al. (2011). Dopamine D₂-receptor antagonists ameliorate indomethacin-induced small intestinal ulceration in mice by activating α7 nicotinic acetylcholine receptors. J. Pharmacol. Sci. 116 (3), 274–282. doi:10.1254/jphs.11037fp
Ye, X., Cen, Y., Wu, K., Xu, L., Ni, J., Zheng, W., et al. (2023). Gas-mediated intestinal microbiome regulation prompts the methanol extract of Schizonepetae Spica to relieve colitis. Nutrients 15 (3), 519. doi:10.3390/nu15030519
Yu, L., Huang, C., Yang, W., Ren, Z., Li, L., Cheng, H., et al. (2022a). Aqueous cinnamon extract ameliorates bowel dysfunction and enteric 5-HT synthesis in IBS rats. Front. Pharmacol. 13, 1010484. doi:10.3389/fphar.2022.1010484
Yu, W., Sun, S., Zhang, K., Li, H., Xin, M., Liu, Y., et al. (2022b). Fructus ligustri lucidi suppresses inflammation and restores the microbiome profile in murine colitis models. Phytomedicine 106, 154438. doi:10.1016/j.phymed.2022.154438
Yu, Y. C., Li, J., Zhang, M., Pan, J. C., Yu, Y., Zhang, J. B., et al. (2019). Resveratrol improves brain-gut Axis by regulation of 5-HT-dependent signaling in the rat model of irritable bowel syndrome. Front. Cell Neurosci. 13, 30. doi:10.3389/fncel.2019.00030
Yu, Z. W., Xie, Y., Huang, Z. C., Yang, K., Wang, Z. G., and Hu, H. L. (2021). Study of the therapeutic effect of raw and processed Vladimiriae Radix on ulcerative colitis based on intestinal flora, metabolomics and tissue distribution analysis. Phytomedicine 85, 153538. doi:10.1016/j.phymed.2021.153538
Zhang, H., He, W., Hu, X. F., Li, Y. Z., Liu, Y. M., Ge, W. Q., et al. (2022a). Electroacupuncture reduces visceral pain via cannabinoid CB2 receptors in a mouse model of inflammatory bowel disease. Front. Pharmacol. 13, 861799. doi:10.3389/fphar.2022.861799
Zhang, H. Y., Zeng, H. R., Wei, H. Z., Chu, X. Y., Zhu, H. T., Zhao, B., et al. (2022b). Tongxie-Yaofang formula regulated macrophage polarization to ameliorate DSS-induced colitis via NF-κB/NLRP3 signaling pathway. Phytomedicine 107, 154455. doi:10.1016/j.phymed.2022.154455
Zhang, J., Chen, T., Wen, Y., Siah, K. T. H., and Tang, X. (2024). Insights and future prospects of traditional Chinese medicine in the treatment of functional dyspepsia. Phytomedicine 127, 155481. doi:10.1016/j.phymed.2024.155481
Zhang, R. B., Dong, L. C., Shen, Y., Li, H. Y., Huang, Q., Yu, S. G., et al. (2023a). Electroacupuncture alleviates ulcerative colitis by targeting CXCL1: evidence from the transcriptome and validation. Front. Immunol. 14, 1187574. doi:10.3389/fimmu.2023.1187574
Zhang, R. M., Wang, L., Yang, X. N., Xia, Q., Jiang, M. D., Fan, Z. J., et al. (2007). Dinggui Oil Capsule in treating irritable bowel syndrome with stagnation of qi and cold: a prospective, multi-center, randomized, placebo-controlled, double-blind trial. Zhong Xi Yi Jie He Xue Bao 5 (4), 392–397. doi:10.3736/jcim20070406
Zhang, S., Liu, Y., Li, S., Ye, F., Foreman, R. D., and Chen, J. (2020). Effects of electroacupuncture on stress-induced gastric dysrhythmia and mechanisms involving autonomic and central nervous systems in functional dyspepsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 319 (1), R106–R113. doi:10.1152/ajpregu.00256.2019
Zhang, S., Wu, Z. J., Fan, Y. B., Ma, J., and Yang, X. Y. (2023b). Clinical efficacy of Tongxie Yaofang Granules on irritable bowel syndrome with diarrhea patients due to liver hyperactivity with spleen deficiency pattern. Chin. Tradit. Pat. Med. 45 (10), 3509–3512. doi:10.3969/j.issn.1001-1528.2023.10.062
Zhang, S., Zhao, L., Wang, H., Wang, C., Huang, S., Shen, H., et al. (2013). Efficacy of modified LiuJunZi decoction on functional dyspepsia of spleen-deficiency and qi-stagnation syndrome: a randomized controlled trial. BMC Complement. Altern. Med. 13, 54. doi:10.1186/1472-6882-13-54
Zhang, T., Peng, H., Li, Y., Zhou, X., Pu, W., Zhang, Y., et al. (2023c). Indirubin regulates T cell differentiation by promoting αVβ8 expression in bone marrow-derived dendritic cells to alleviate inflammatory bowel disease. Phytother. Res. 37 (1), 89–100. doi:10.1002/ptr.7595
Zhang, X., Liu, W., Zhang, S., Wang, J., Yang, X., Wang, R., et al. (2022c). Wei-Tong-Xin ameliorates functional dyspepsia via inactivating TLR4/MyD88 by regulating gut microbial structure and metabolites. Phytomedicine 102, 154180. doi:10.1016/j.phymed.2022.154180
Zhang, X. F., Luo, Y. F., Gao, J., Chen, Y. Z., and Jiang, Z. B. (2022d). Effects of tongxie yaofang decoction on lipid metabolism and autophagy in rats with ulcerative colitis due to liver depression and spleen deficiency. Chin. Tradit. Pat. Med. 44 (03), 739–746. doi:10.3969/j.issn.1001-1528.2022.03.010
Zhang, Y., Yamamoto, T., Hayashi, S., and Kadowaki, M. (2021). Suppression of plasmacytoid dendritic cell migration to colonic isolated lymphoid follicles abrogates the development of colitis. Biomed. Pharmacother. 141, 111881. doi:10.1016/j.biopha.2021.111881
Zhang, Z., Shen, P., Xie, W., Cao, H., Liu, J., Cao, Y., et al. (2019). Pingwei San ameliorates dextran sulfate sodium-induced chronic colitis in mice. J. Ethnopharmacol. 236, 91–99. doi:10.1016/j.jep.2019.01.043
Zhao, J., Wu, R., Wei, P., Ma, Z., Pei, H., Hu, J., et al. (2023a). Ethanol extract of Piper wallichii ameliorates DSS-induced ulcerative colitis in mice: involvement of TLR4/NF-κB/COX-2 signaling pathway. J. Ethnopharmacol. 308, 116293. doi:10.1016/j.jep.2023.116293
Zhao, J., Zhao, L., Zhang, S., and Zhu, C. (2020). Modified Liu-Jun-Zi decoction alleviates visceral hypersensitivity in functional dyspepsia by regulating EC cell-5HT3r signaling in duodenum. J. Ethnopharmacol. 250, 112468. doi:10.1016/j.jep.2019.112468
Zhao, M., Li, P., Qiao, D., Hua, S., Yue, Q., Dai, Y., et al. (2024). N6-methyladenosine modification of TSC1 mRNA contributes to macrophage polarization regulated by Coptisine in DSS-induced ulcerative colitis. Phytomedicine 122, 155153. doi:10.1016/j.phymed.2023.155153
Zhao, M., Xie, X., Xu, B., Chen, Y., Cai, Y., Chen, K., et al. (2023b). Paeonol alleviates ulcerative colitis in mice by increasing short-chain fatty acids derived from Clostridium butyricum. Phytomedicine 120, 155056. doi:10.1016/j.phymed.2023.155056
Zhao, Y., Luan, H., Jiang, H., Xu, Y., Wu, X., Zhang, Y., et al. (2021). Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine 84, 153519. doi:10.1016/j.phymed.2021.153519
Zhen, Z., Xia, L., You, H., Jingwei, Z., Shasha, Y., Xinyi, W., et al. (2021). An integrated gut microbiota and network pharmacology study on fuzi-lizhong pill for treating diarrhea-predominant irritable bowel syndrome. Front. Pharmacol. 12, 746923. doi:10.3389/fphar.2021.746923
Zheng, C., Wang, Y., Xu, Y., Zhou, L., Hassan, S., Xu, G., et al. (2021). Berberine inhibits dendritic cells differentiation in DSS-induced colitis by promoting Bacteroides fragilis. Int. Immunopharmacol. 101 (Pt A), 108329. doi:10.1016/j.intimp.2021.108329
Zheng, C., Zhong, Y., Zhang, W., Wang, Z., Xiao, H., Zhang, W., et al. (2023). Chlorogenic acid ameliorates post-infectious irritable bowel syndrome by regulating extracellular vesicles of gut microbes. Adv. Sci. (Weinh) 10 (28), e2302798. doi:10.1002/advs.202302798
Zheng, H., Li, Y., Zhang, W., Zeng, F., Zhou, S. Y., Zheng, H. B., et al. (2016). Electroacupuncture for patients with diarrhea-predominant irritable bowel syndrome or functional diarrhea: a randomized controlled trial. Med. Baltim. 95 (24), e3884. doi:10.1097/md.0000000000003884
Zheng, K., Jia, J., Yan, S., Shen, H., Zhu, P., and Yu, J. (2020). Paeoniflorin ameliorates ulcerative colitis by modulating the dendritic cell-mediated T(H)17/T(reg) balance. Inflammopharmacology 28 (6), 1705–1716. doi:10.1007/s10787-020-00722-6
Zhong, Y., Liu, W., Xiong, Y., Li, Y., Wan, Q., Zhou, W., et al. (2022a). Astragaloside Ⅳ alleviates ulcerative colitis by regulating the balance of Th17/Treg cells. Phytomedicine 104, 154287. doi:10.1016/j.phymed.2022.154287
Zhong, Y., Xiao, Q., Kang, Z., Huang, J., Ge, W., Wan, Q., et al. (2022b). Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int. Immunopharmacol. 111, 109108. doi:10.1016/j.intimp.2022.109108
Zhou, E. H., Liu, H. R., Wu, H. G., Shi, Z., Zhang, W., Zhu, Y., et al. (2009). Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig. Dis. Sci. 54 (10), 2198–2206. doi:10.1007/s10620-008-0620-4
Zhou, H. F., Yang, C., Li, J. Y., He, Y. Y., Huang, Y., Qin, R. J., et al. (2023a). Quercetin serves as the major component of Xiang-lian Pill to ameliorate ulcerative colitis via tipping the balance of STAT1/PPARγ and dictating the alternative activation of macrophage. J. Ethnopharmacol. 313, 116557. doi:10.1016/j.jep.2023.116557
Zhou, P., Lai, J., Li, Y., Deng, J., Zhao, C., Huang, Q., et al. (2022). Methyl gallate alleviates acute ulcerative colitis by modulating gut microbiota and inhibiting TLR4/NF-κB pathway. Int. J. Mol. Sci. 23 (22), 14024. doi:10.3390/ijms232214024
Zhou, S. Y., Guo, Z. N., Yang, Y., Qu, Y., and Jin, H. (2023b). Gut-brain axis: mechanisms and potential therapeutic strategies for ischemic stroke through immune functions. Front. Neurosci. 17, 1081347. doi:10.3389/fnins.2023.1081347
Zhu, H., Wang, W., and Li, Y. (2024). The interplay between microbiota and brain-gut axis in epilepsy treatment. Front. Pharmacol. 15, 1276551. doi:10.3389/fphar.2024.1276551
Zhu, L., Xu, L. Z., Zhao, S., Shen, Z. F., Shen, H., and Zhan, L. B. (2020). Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 104 (12), 5449–5460. doi:10.1007/s00253-020-10527-w
Zhu, Y., Li, X., Chen, J., Chen, T., Shi, Z., Lei, M., et al. (2016). The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int. Immunopharmacol. 30, 74–84. doi:10.1016/j.intimp.2015.11.031
Zong, Y., Zhu, S., Zhang, S., Zheng, G., Wiley, J. W., and Hong, S. (2019). Chronic stress and intestinal permeability: lubiprostone regulates glucocorticoid receptor-mediated changes in colon epithelial tight junction proteins, barrier function, and visceral pain in the rodent and human. Neurogastroenterol. Motil. 31 (2), e13477. doi:10.1111/nmo.13477
Zou, J., Shen, Y., Chen, M., Zhang, Z., Xiao, S., Liu, C., et al. (2020a). Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 104 (13), 5999–6012. doi:10.1007/s00253-020-10665-1
Zou, X., Wang, Y., Wang, Y., Yang, J., Guo, H., and Cai, Z. (2020b). Paeoniflorin alleviates abnormalities in rats with functional dyspepsia by stimulating the release of acetylcholine. Drug Des. Devel Ther. 14, 5623–5632. doi:10.2147/DDDT.S260703
Keywords: Traditional Chinese medicine, functional gastrointestinal disorders, inflammatory bowel disease, brain-gut axis, pharmacological mechanism, acupuncture
Citation: Liu R, Luo Y, Ma J, Zhang Q, Sheng Y, Li J, Li H and Zhao T (2024) Traditional Chinese medicine for functional gastrointestinal disorders and inflammatory bowel disease: narrative review of the evidence and potential mechanisms involving the brain-gut axis. Front. Pharmacol. 15:1444922. doi: 10.3389/fphar.2024.1444922
Received: 06 June 2024; Accepted: 23 August 2024;
Published: 17 September 2024.
Edited by:
Qin Xiang Ng, Singapore General Hospital, SingaporeReviewed by:
Xin-Lin Chen, Guangzhou University of Chinese Medicine, ChinaCopyright © 2024 Liu, Luo, Ma, Zhang, Sheng, Li, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: TianYi Zhao, emhhb3RpYW55aTg5QDEyNi5jb20=; Hongjiao Li, bGhqaWFvMjAxM0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.