
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 10 September 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1443235
This article is part of the Research Topic Pharmacological Mechanisms of Drugs Affecting Bone Formation and Bone Resorption Volume II View all 16 articles
 Wei Li1
Wei Li1 Zechen Zhang1
Zechen Zhang1 Yuyi Li1
Yuyi Li1 Zhenyu Wu1
Zhenyu Wu1 Chengjie Wang1
Chengjie Wang1 Zhen Huang1
Zhen Huang1 Baisheng Ye1
Baisheng Ye1 Xin Jiang1
Xin Jiang1 Xiaolong Yang1
Xiaolong Yang1 Xiaolin Shi2*
Xiaolin Shi2*Background: Evidence shows that the total flavonoids of Rhizoma Drynariae (TFRD) can improve bone mineral density (BMD). However, there is no evidence to summarize the improvement of biochemical indicators of bone metabolism (BIBM).
Methods: The PubMed, Web of Science, Cochrane Library, Embase, Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chongqing VIP Information Database (VIP) and SinoMed were searched from inception to 6 May 2024. The final included studies performed meta-analyses using RevMan 5.3.
Results: Nine randomized controlled trials (RCTs) were ultimately included. The TFRD group had higher bone gla protein (BGP) and type I procollagen-N-propeptide (PINP) compared to the Other therapies (WMD: 5.11; 95% CI: 3.37, 6.84; p < 0.00001; WMD: 13.89; 95% CI: 11.81, 15.97; p < 0.00001). The tartrate-resistant acid phosphatase (TRACP) decreased significantly (WMD: −1.34; 95% CI: −1.62, −1.06; p < 0.00001). The alkaline phosphatase (ALP) increased significantly (WMD: 7.47; 95% CI: 6.29, 8.66; p < 0.00001). There were no significant differences in serum calcium (SC) or serum phosphorus (SP) levels between the TFRD and control groups (WMD: 0.08; 95% CI: −0.04, 0.20; p = 0.17; WMD: 0.02; 95% CI: −0.02, 0.05; p = 0.36).
Conclusion: TFRD can stimulate bone formation and prevent bone resorption in osteoporosis (OP) patients, but it has no effect on SC and SP.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/.
Altered bone microstructure is a hallmark of osteoporosis (OP), a chronic bone disease that weakens the skeleton (Gregson et al., 2022). The pathogenesis of OP can be understood as an abnormality of bone metabolism (BM), where bone resorption (BR) is greater than bone formation (BF), leading to a decrease in bone mass and an imbalance in the skeletal microenvironment. Under normal physiological conditions, bone stabilization consists of a variety of cells that are active in the microenvironment to ensure that BF and BR occur and remain relatively stable to maintain bone mass (Yao et al., 2020). Bone is always in a metabolic state, and the stabilization of BM is mainly dependent on the dynamic balance between osteoblast-mediated BF and osteoclast-mediated BR, which is maintained by the normal bone marrow microenvironment. Deterioration of the bone marrow microenvironment due to aging or hormonal disorders leads to an imbalance between osteoblasts and osteoclasts, which is a key factor in the pathogenesis of OP. According to the 2023 Chinese OP epidemiologic survey, the incidence of OP was 20.73% in males and 38.05% in women, while the prevalence of osteoporotic fracture in the elderly was 18.9% (Wang et al., 2023; Meng et al., 2023). Therefore, timely treatment of OP is essential. Biochemical indicators of bone metabolism (BIBM) includes BF markers, BR markers, and calcium and phosphorus metabolism indicators (CPMI) (Nishizawa et al., 2019; Szulc, 2018). BF markers include alkaline phosphatase (ALP), bone gla protein (BGP), type I procollagen-N-propeptide (PINP), and osteoprotegerin (OPG). BR markers mainly include tartrate-resistant acid phosphatase (TRACP), type I collagen carboxy⁃terminal peptide (CTX), pyridinoline, and deoxypyridinoline. CPMI mainly include SC, SP, parathyroid hormone (PTH), and calcitonin (CT). BGP is a non-collagen protein synthesized by osteoblasts and can reflect the activity of osteoblasts (Cancela et al., 2014). PINP is an extracellular breakdown product of pre-collagen fibers synthesized and released by osteoblasts, which is used to assess the speed of bone turnover markers (BTMs) and type I collagen synthesis, and to reflect collagen synthesis and osteoblast activity (Gillett et al., 2021). Bone alkaline phosphatase (BALP) is an extracellular enzyme that can also reflect osteoblast activity and is a specific marker of BF (Ng et al., 2023). TRACP is mainly derived from osteoclasts and positively correlates with the function of BR, and the level of TRACP in the blood is able to reflect the functional activity of osteoclasts (Solberg et al., 2014). C-terminal telopeptide of type I collagen (β-CTX) is an isomer of the C-terminal peptide of collagen type I, and is a good indicator of BR (Bhattoa et al., 2021). SC and SP are important trace elements required by the human body and are the basis for BF (Ciosek et al., 2021). Therefore, improving BIBM to promote osteoblasts or inhibit osteoclasts can effectively delay the development of OP.
Currently, OP is mainly treated by drugs in clinical practice, including BR inhibitors, BF promoters, and other drugs. Anti-BR drugs mainly inhibit osteoclast BR, including bisphosphonates, calcitonin, estrogen, and so on. Bisphosphonates can increase the level of BM and effectively reduce the risk of osteoporotic fracture (Rhee et al., 2006; Nakamura et al., 2021), but prolonged use of bisphosphonates increases the risk of osteonecrosis of the jaw and atypical femur fracture (Zhang et al., 2018; Black et al., 2020), and long-term use is not recommended. A hormone that controls calcium levels called calcitonin has the ability to reduce osteoclasts’ biological activity and bone loss, and alleviate bone pain, but there is a possibility that nasal spray salmon calcitonin may increase the risk of tumors, and the duration of use is limited (Kaskani et al., 2005). Estrogen is effective in reducing bone loss and decreasing the risk of fracture in postmenopausal women, but increases the risk of endometrial cancer and breast cancer (Wu et al., 2018; Vinogradova et al., 2020). BF promoters mainly stimulate osteoblastic BF, including parathyroid hormone analogs, such as teriparatide. Teriparatide stimulates osteoblast activity, promotes BF, and increases bone mineral density (BMD). However, studies have shown that there may be a risk of osteosarcoma, so the duration of treatment was limited (Kendler et al., 2018; Boonen et al., 2008). Other drugs refer to traditional Chinese medicine (TCM) treatments, commonly used TCM include Xianling Gubao capsules, Gushukang capsules and JinTiange capsules, etc. The more clearly defined active ingredients in the medications used are the total flavonoids of Rhizoma Drynariae (TFRD), the total flavonoids of Epimedium, and the artificial tiger bone powder. TCM has the advantages of being safe, inexpensive, and without obvious side effects, so it is getting more and more attention in China. In recent years, more and more studies have demonstrated the effectiveness of TCM in the treatment of OP. Zhu showed that Xianling Gubao capsules could improve BMD without adverse effects through a study (Zhu et al., 2012). Through a study, Liang proved that Jintiange capsules might successfully lower the risk of falls in patients with OP by improving muscle strength and balance (Liang et al., 2022). Currently, studies are increasingly focusing on the treatment of OP from herbal monomers. Total flavonoids from Epimedium can regulate osteogenic differentiation and adipogenesis in mesenchymal stem cells, thus promoting bone growth (Chen et al., 2022; Zhang et al., 2008; Zhang et al., 2016). The components of Qianggu capsules are TFRD, and studies have demonstrated that TFRD can improve the antioxidant capacity, activate the Wnt/β-catenin signaling pathway, promote osteoblastic activity, reduce bone mineral loss, and increase BMD (Shen et al., 2020; Li et al., 2021; Mu et al., 2021). A meta-analysis was conducted to assess the effectiveness of TFRD in OP; however, no full meta-analysis has been conducted to evaluate its BIBM (Wei et al., 2017). Therefore, the present study evaluated the efficacy of TFRD on the BIBM of OP patients by including clinical randomized controlled trials (RCTs) with the aim of obtaining higher-quality clinical evidence.
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards (Page et al., 2021) and is registered with PROSPERO (CRD42024545497).
(a): The study included only patients with a diagnosis of OP, including postmenopausal osteoporosis (PMOP) and senile osteoporosis (SOP). Referring to the diagnostic criteria recommended by the WHO, for postmenopausal women and men aged 50 years and above, T score ≤ −2.5 on dual-energy X-ray absorptiometry (DXA) of the BMD of the mid-axis bones (lumbar 1-4, femoral neck, or total hip) or of the distal one-third of the radius is considered to be OP.
(b):The study compared TFRD to other methods of OP treatment.
(c): The study included at least one of the following assessment instruments: BGP, PINP, TRACP, SC, SP, ALP.
(d): The study was RCTs.
(a): Animal studies, reviews, meta-analyses, etc.
(b): Duplicate publication.
(c): Patients with OP and other diseases.
(d): The studies in which raw data were lacking or data couldn’t be extracted.
The PubMed, Web of Science, Cochrane Library, Embase, CNKI, Wanfang Database, VIP and SinoMed were searched. The time frame for the search was from database creation to 6 May 2024. The search strategy was conducted using a combination of free words and MeSH terms. The relevant keywords for OP were “osteoporosis” OR “senile osteoporosis” OR “postmenopausal osteoporosis” OR “primary osteoporosis.” The keywords of TFRD, including “Total Flavonoids of Rhizoma Drynariae” OR “Qianggu capsule” OR “Qianggu jiaonang.” Each database’s unique qualities informed the search approach, as detailed in the Supplementary Material.
Author names, year of publication, sample size, number of participants in experimental and control groups, mean age of participants, country, intervention and control, duration of treatment, and outcome measures were retrieved by two authors from the nine studies.
Using the Physiotherapy Evidence Database (PEDro) scale, two authors evaluated the risk of bias in the included research (Cashin and McAuley, 2020). A third assessor conducted arbitration or discussions to resolve disagreements. The total PEDro score was obtained by summing the scores for items 2 through 11, which ranged from 0 to 10. Elevated ratings signify superior methodological quality. A score of less than four was regarded as “poor,” four to five as “fair,” six to eight as “good,” and nine to ten as “excellent.”
The primary outcome measures for this meta-analysis were BF, including BGP and PINP. The secondary measures were BR and CPMI, including TRACP, SC, SP, and ALP.
Data were analyzed using RevMan software (Version 5.3). 95% confidence intervals (CI) of the combined mean difference (MD) were used to assess differences in outcomes between patients with OP who received TFRD and those who received other forms of treatment. The chi-square test was used to analyze and quantify the magnitude of heterogeneity in the included studies. I2 > 50%, heterogeneity exists and the source of heterogeneity needs to be analyzed. If I2 < 50%, no heterogeneity exists, indicating a good concordance of results. In addition, it is necessary to choose the appropriate method of combining effect sizes according to the magnitude of heterogeneity. The meta-analysis was conducted using a fixed effects model (I2 < 50%) if the heterogeneity was small, or a random effects model if the heterogeneity was high (I2 > 50%). At P < 0.05, statistical significance was taken into account.
With eight databases, a total of 253 studies related to the treatment of OP with TFRD were included. Nine studies were ultimately included according to the criteria for inclusion and exclusion (Bian, 2016; He, 2005; Shan and Zhou, 2006; Shen et al., 2018; Wang et al., 2007; Wen and Zhou, 2015; Yang et al., 2017; Zhang and Chen, 2008; Zhou et al., 2017) (Figure 1).
There were 777 participants in nine investigations, and the sample sizes varied from 46 to 123. Five studies were monotherapy (TFRD), and four were combination therapy (TFRD + C). The treatment duration was a minimum of 4 weeks and a maximum of 6 months. Seven studies used SC, SP, and ALP as outcome measures. Two studies used BGP, PINP as outcome measures. Four studies used TRACP as an outcome measure (Table 1).
The quality of the included studies were independently evaluated by WL and YZW using the PEDro tool. The included studies had a mean PEDro score of 6.33. All nine studies had a score greater than 6, indicating a low risk of bias (Table 2).
BGP were recorded for 202 patients in two studies. The TFRD group had significantly higher BGP levels, according to the meta-analysis (WMD: 5.11; 95% CI: 3.37, 6.84; p < 0.00001). The meta-analysis showed that TFRD improves BGP in OP patients more than other therapies (Figure 2).
The meta-analysis revealed that TFRD was statistically significant in improving PINP in patients with OP. PINP indicators were described in 2 studies, which included 202 patients. The PINP was improved in the TFRD group (WMD: 13.89; 95% CI: 11.81, 15.97; p < 0.00001) (Figure 3).
The findings of the meta-analysis indicated that TFRD was statistically significant in improving TRACP in OP patients. TRACP indicators were described in 4 studies, which included 445 patients. TRACP was significantly lower in the TFRD group (WMD: −1.34; 95% CI: −1.62, −1.06; p < 0.00001). However, there was high heterogeneity in the results (I2 = 91%) (Figure 4).
Meta-analysis showed that TFRD was not statistically significant in improving SC and SP in OP patients. SC and SP were described in 7 studies, including 575 patients. Compared to the other treatment, there was no difference in SC and SP (WMD: 0.08; 95% CI: −0.04, 0.20; p = 0.17; WMD: 0.02; 95% CI: −0.02, 0.05; p = 0.36) (Figures 5, 6).
Meta-analysis showed that TFRD improves ALP in patients with OP. ALP was described in 7 studies, including 671 patients. ALP was significantly improved in the TFRD group (WMD: 7.47; 95% CI: 6.29, 8.66; p < 0.00001) (Figure 7).
Publication bias was analyzed for all of the outcome measures, and the funnel plots showed that the outcome measures were largely symmetrical and there was no publication bias (Figure 8).
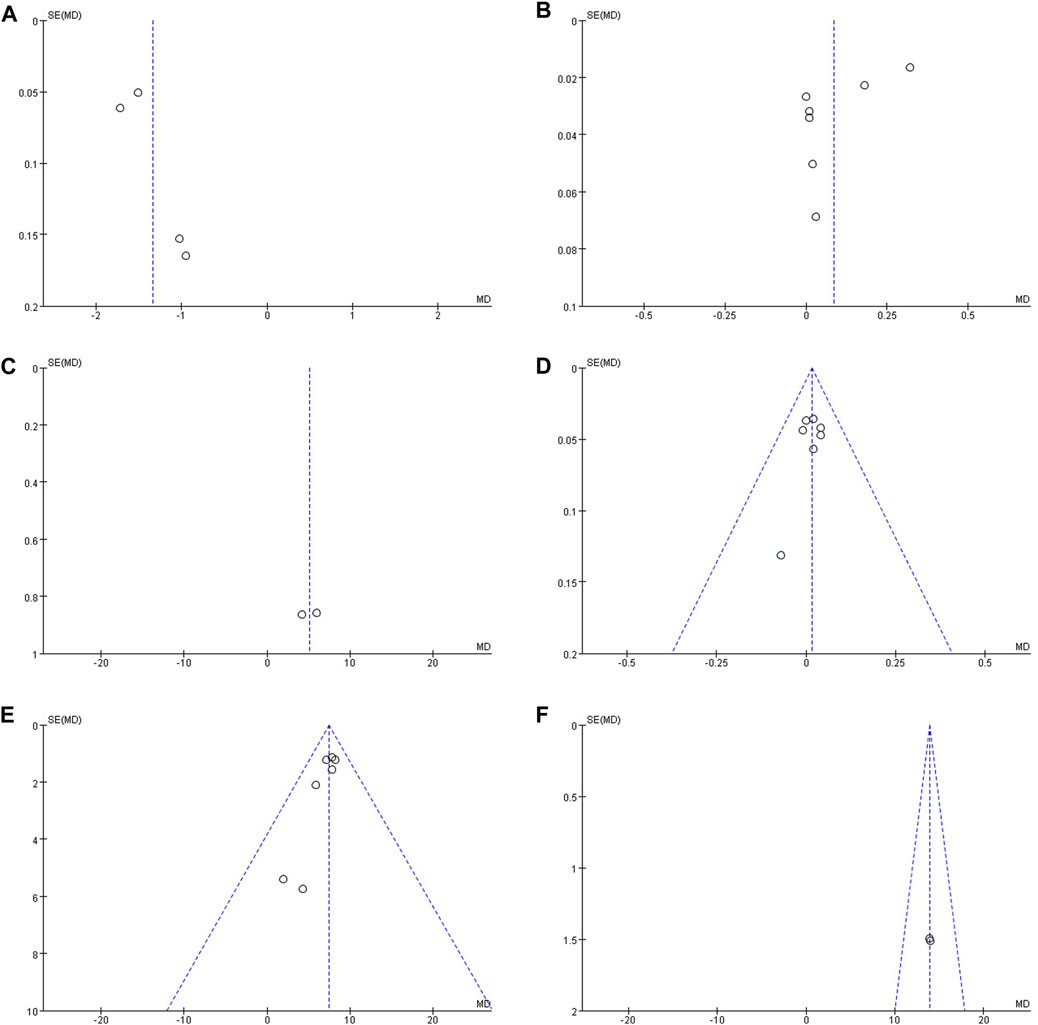
Figure 8. (A) Funnel plot of BGP; (B) Funnel plot of PINP; (C) Funnel plot of TRACP; (D) Funnel plot of SC; (E) Funnel plot of SP; (F) Funnel plot of ALP.
OP contributes significantly to the occurrence of fractures in the elderly. With the aging of the population, the prevalence of OP and osteoporotic fractures is increasing. Postmenopausal women have a higher risk of fracture due to the imbalance in BTMs and severe bone loss as a result of age and hormonal changes in the body (Garnero et al., 2000). Therefore, preventing fractures in OP patients is the current focus. Currently, BMD is commonly used to assess the risk of fracture in patients with OP. Although BMD has been recognized as the gold standard for OP diagnosis, BMD is generally found to change significantly only in 1–2 years, which is not a good assessment of the risk of osteoporotic fracture and is not suitable for short-term follow-up (Deal, 2001; Syed and Khan, 2002). BIBM, as a highly sensitive monitoring index, can well reflect the status of BM at different stages and the efficacy of OP treatment (Biver et al., 2012; Kanis et al., 2000; Khashayar et al., 2015). Several guidelines around the world recommended the use of PINP and β-CTX for OP screening or monitoring treatment (Compston et al., 2017; Cosman et al., 2014; Kanis et al., 2008). There was a study that investigated the association between BTMs and fracture incidence in postmenopausal and older women and found that higher levels of BTMs were associated with a higher risk of fracture (Vasikaran et al., 2011). A meta-analysis demonstrated that PINP and β-CTX appeared to predict fracture risk independently of BMD (Tian et al., 2019). Further studies have shown that BTMs are better at predicting fractures in the short term (Ivaska et al., 2010; Robinson-Cohen et al., 2011). Therefore, BIBM proved valuable in the evaluation of patients with OP.
TCM has obvious advantages in improving patients’ clinical symptoms. With the ability to enhance microcirculation and nourish bones, Rhizoma Drynariae was a popular kidney tonic herb in TCM. Its extract, TFRD, was extensively utilized in the treatment of OP. Modern pharmacological trials have concluded that the TFRD can regulate the levels of cytokines and hormones in the process of BM, inhibit BR, and have anti-inflammatory, analgesic, and fracture healing effects (Jin et al., 2022; Lin et al., 2022; Zhao et al., 2021). A study showed that after 10 weeks of continuous administration of RDTF alone or the combination of RDTF and CaCO3 in ovariectomized rats, it was able to improve the trabecular thickness and trabeculae microstructure, and that the combination of RDTF and CaCO3 increased the level of ALP and significantly decreased TRACP and S-CTX, which was able to improve the BM, and had a better effect on the repair of fractures (Hu et al., 2021). The combination of estrogen and estrogen receptors can affect the cell cycle, induce osteoclast apoptosis, inhibit osteoclast activity and differentiation, and ultimately inhibit osteoclast BR and prevent OP. Naringin and naringenin were the main active components of TFRD. Studies have shown that naringin and naringenin have estrogen-regulating effects and prevent OP through estrogen receptor agonism (Guo et al., 2011). The Wnt/β-catenin signaling pathway can regulate bone-related bioinformatic signaling, and plays an important role in regulating osteogenic differentiation and BF of bone marrow mesenchymal stem cells (BMSCs). Naringenin can induce the differentiation of BMSCs to osteoblasts under the induction of the Wnt/β-catenin signaling pathway, which was a relevant target for the treatment of OP with TFRD (Yang et al., 2022). In addition, naringin could significantly upregulate the expression of Notch1 in BMSCs and promote osteogenic differentiation of BMSCs by activating the Notch signaling pathway (Yu et al., 2016). TFRD has been demonstrated in clinical trials to be useful in the management of OP. There were only two systematic reviews to evaluate the efficacy of TFRD in the treatment of OP (Wei et al., 2017; Zhang et al., 2017). However, the reviews evaluated TFRD to improve BMD, but there was no BM. Therefore, we analyzed and summarized the level of BIBM improvement by TFRD and performed a meta-analysis.
A total of nine papers were included in this review to analyze the effect of TFRD. The meta-analysis showed that both BF and BR were significantly improved in OP treated with TFRD compared with the control group, suggesting that TFRD was able to increase BGP, PINP, and ALP and to decrease TRACP. However, there was no significant improvement for SC and SP. Although there was high heterogeneity in the results for TRACP and SC, subgroup analysis was not possible because of the short number of included studies. Consequently, we reasoned that the heterogeneity could be a result of the few included studies.
There were limitations of this study. First, only nine randomized controlled trials were included in this study, all of which were single-center clinical studies and lacked multicenter and large sample sizes. Second, the clinical trials jointly used fewer outcome indicators to observe the effect of TFRD on BM from more perspectives. Third, there was a high degree of heterogeneity in some of the outcomes, however, due to the small amount of literature included, subgroup analyses could not be performed, and although we performed publication bias analyses, they do not fully represent the stability of the results, and therefore more studies were needed in the future.
By including RCTs, our meta-analysis suggested that TFRD may promote BF and inhibit BR, but the number of studies was small, and further evaluation was needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WL: Writing–original draft, Writing–review and editing, Conceptualization, Data curation, Methodology, Software. ZZ: Data curation, Formal Analysis, Software, Writing–original draft. YL: Formal Analysis, Software, Writing–original draft. ZW: Formal Analysis, Software, Writing–original draft. CW: Data curation, Software, Writing–original draft. ZH: Investigation, Writing–original draft. BY: Investigation, Writing–original draft. XJ: Validation, Writing–original draft. XY: Formal Analysis, Writing–original draft. XS: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing–original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant Number: 82074183) and the Natural Science Foundation of Zhejiang Province (Grant Number: LZ22H270002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1443235/full#supplementary-material
Bhattoa, H. P., Cavalier, E., Eastell, R., Heijboer, A. C., Jørgensen, N. R., Makris, K., et al. (2021). Analytical considerations and plans to standardize or harmonize assays for the reference bone turnover markers PINP and β-CTX in blood. Clin. Chim. Acta 515, 16–20. doi:10.1016/j.cca.2020.12.023
Bian, Y. (2016). Effect of Qianggu capsule combined with calcium on bone mineral density and quality of life in elderly patients with osteoporosis. Med. J. Chin. People Health. 28 (22), 26–27. doi:10.3969/j.issn.1672-0369.2016.22.011
Biver, E., Chopin, F., Coiffier, G., Brentano, T. F., Bouvard, B., Garnero, P., et al. (2012). Bone turnover markers for osteoporotic status assessment? A systematic review of their diagnosis value at baseline in osteoporosis. Jt. Bone Spine 79 (1), 20–25. doi:10.1016/j.jbspin.2011.05.003
Black, D. M., Geiger, E. J., Eastell, R., Vittinghoff, E., Li, B. H., Ryan, D. S., et al. (2020). Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 383 (8), 743–753. doi:10.1056/NEJMoa1916525
Boonen, S., Marin, F., Obermayer-Pietsch, B., Simões, M. E., Barker, C., Glass, E. V., et al. (2008). Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 93 (3), 852–860. doi:10.1210/jc.2007-0711
Cancela, M. L., Laizé, V., and Conceição, N. (2014). Matrix Gla protein and osteocalcin: from gene duplication to neofunctionalization. Arch. Biochem. Biophys. 561, 56–63. doi:10.1016/j.abb.2014.07.020
Cashin, A. G., and McAuley, J. H. (2020). Clinimetrics: Physiotherapy evidence database (PEDro) scale. J. Physiother. 66 (1), 59. doi:10.1016/j.jphys.2019.08.005
Chen, L., Ma, R., Luo, P., Shi, D., Shi, X., Nian, H., et al. (2022). Effects of total flavonoids of Epimedium on bone marrow adipose tissue in ovariectomized rats. Front. Endocrinol. (Lausanne) 13, 900816. doi:10.3389/fendo.2022.900816
Ciosek, Ż., Kot, K., Kosik-Bogacka, D., Łanocha-Arendarczyk, N., and Rotter, I. (2021). The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 11 (4), 506. doi:10.3390/biom11040506
Compston, J., Cooper, A., Cooper, C., Gittoes, N., Gregson, C., Harvey, N., et al. (2017). UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 12 (1), 43. doi:10.1007/s11657-017-0324-5
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician's guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25 (10), 2359–2381. doi:10.1007/s00198-014-2794-2
Deal, C. L. (2001). Using bone densitometry to monitor therapy in treating osteoporosis: pros and cons. Curr. Rheumatol. Rep. 3 (3), 233–239. doi:10.1007/s11926-001-0023-4
Garnero, P., Sornay-Rendu, E., Claustrat, B., and Delmas, P. D. (2000). Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone Min. Res. 15 (8), 1526–1536. doi:10.1359/jbmr.2000.15.8.1526
Gillett, M. J., Vasikaran, S. D., and Inderjeeth, C. A. (2021). The role of PINP in diagnosis and management of metabolic bone disease. Clin. Biochem. Rev. 42 (1), 3–10. doi:10.33176/AACB-20-0001
Gregson, C. L., Armstrong, D. J., Bowden, J., Cooper, C., Edwards, J., Gittoes, N. J. L., et al. (2022). UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 17 (1), 58. doi:10.1007/s11657-022-01061-5
Guo, D., Wang, J., Wang, X., Luo, H., Zhang, H., Cao, D., et al. (2011). Double directional adjusting estrogenic effect of naringin from Rhizoma drynariae (Gusuibu). J. Ethnopharmacol. 138 (2), 451–457. doi:10.1016/j.jep.2011.09.034
He, Z. G. (2005). Clinical observation of primary osteoporosis treated with Qianggu capsule. Chin. Tradit. Herb. Drug. 36 (08), 97–98. doi:10.3321/j.issn:0253-2670.2005.08.041
Hu, Y., Mu, P., Ma, X., Shi, J., Zhong, Z., and Huang, L. (2021). Rhizoma drynariae total flavonoids combined with calcium carbonate ameliorates bone loss in experimentally induced Osteoporosis in rats via the regulation of Wnt3a/β-catenin pathway. J. Orthop. Surg. Res. 16 (1), 702. doi:10.1186/s13018-021-02842-3
Ivaska, K. K., Gerdhem, P., Väänänen, H. K., Akesson, K., and Obrant, K. J. (2010). Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J. Bone Min. Res. 25 (2), 393–403. doi:10.1359/jbmr.091006
Jin, H., Jiang, N., Xu, W., Zhang, Z., Yang, Y., Zhang, J., et al. (2022). Effect of flavonoids from Rhizoma Drynariae on osteoporosis rats and osteocytes. Biomed. Pharmacother. 153, 113379. doi:10.1016/j.biopha.2022.113379
Kanis, J. A., Burlet, N., Cooper, C., Delmas, P. D., Reginster, J. Y., Borgstrom, F., et al. (2008). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 19 (4), 399–428. doi:10.1007/s00198-008-0560-z
Kanis, J. A., Johnell, O., Oden, A., Jonsson, B., Dawson, A., and Dere, W. (2000). Risk of hip fracture derived from relative risks: an analysis applied to the population of Sweden. Osteoporos. Int. 11 (2), 120–127. doi:10.1007/PL00004173
Kaskani, E., Lyritis, G. P., Kosmidis, C., Galanos, A., Andypas, G., Chorianopoulos, K., et al. (2005). Effect of intermittent administration of 200 IU intranasal salmon calcitonin and low doses of 1alpha(OH) vitamin D3 on bone mineral density of the lumbar spine and hip region and biochemical bone markers in women with postmenopausal osteoporosis: a pilot study. Clin. Rheumatol. 24 (3), 232–238. doi:10.1007/s10067-004-1004-6
Kendler, D. L., Marin, F., Zerbini, C. A. F., Russo, L. A., Greenspan, S. L., Zikan, V., et al. (2018). Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391 (10117), 230–240. doi:10.1016/S0140-6736(17)32137-2
Khashayar, P., Meybodi, H. A., Amoabediny, G., and Larijani, B. (2015). Biochemical markers of bone turnover and their role in osteoporosis diagnosis: a narrative review. Recent Pat. Endocr. Metab. Immune Drug Discov. 9 (2), 79–89. doi:10.2174/1872214809666150806105433
Li, S., Zhou, H., Hu, C., Yang, J., Ye, J., Zhou, Y., et al. (2021). Total flavonoids of rhizoma drynariae promotes differentiation of osteoblasts and growth of bone graft in induced membrane partly by activating wnt/β-catenin signaling pathway. Front. Pharmacol. 12, 675470. doi:10.3389/fphar.2021.675470
Liang, H., Wang, O., Cheng, Z., Xia, P., Wang, L., Shen, J., et al. (2022). Jintiange combined with alfacalcidol improves muscle strength and balance in primary osteoporosis: a randomized, double-blind, double-dummy, positive-controlled, multicenter clinical trial. J. Orthop. Transl. 35, 53–61. doi:10.1016/j.jot.2022.05.002
Lin, H., Wang, X., Li, Z., Huang, M., Feng, J., Chen, H., et al. (2022). Total flavonoids of Rhizoma drynariae promote angiogenesis and osteogenesis in bone defects. Phytother. Res. 36 (9), 3584–3600. doi:10.1002/ptr.7525
Meng, S., Tong, M., Yu, Y., Cao, Y., Tang, B., Shi, X., et al. (2023). The prevalence of osteoporotic fractures in the elderly in China: a systematic review and meta-analysis. J. Orthop. Surg. Res. 18 (1), 536. doi:10.1186/s13018-023-04030-x
Mu, P., Hu, Y., Ma, X., Shi, J., Zhong, Z., and Huang, L. (2021). Total flavonoids of Rhizoma Drynariae combined with calcium attenuate osteoporosis by reducing reactive oxygen species generation. Exp. Ther. Med. 21 (6), 618. doi:10.3892/etm.2021.10050
Nakamura, Y., Shimizu, T., Asano, T., Shimodan, S., Ishizu, H., Takahashi, D., et al. (2021). Short-term efficacy and safety of zoledronate acid or denosumab in Japanese patients with postmenopausal osteoporosis. J. Bone Min. Metab. 39 (5), 824–832. doi:10.1007/s00774-021-01221-6
Ng, E., Ashkar, C., Seeman, E., Schneider, H. G., Nguyen, H., Ebeling, P. R., et al. (2023). A low serum alkaline phosphatase may signal hypophosphatasia in osteoporosis clinic patients. Osteoporos. Int. 34 (2), 327–337. doi:10.1007/s00198-022-06597-3
Nishizawa, Y., Miura, M., Ichimura, S., Inaba, M., Imanishi, Y., Shiraki, M., et al. (2019). Executive summary of the Japan osteoporosis society guide for the use of bone turnover markers in the diagnosis and treatment of osteoporosis (2018 edition). Clin. Chim. Acta. 498, 101–107. doi:10.1016/j.cca.2019.08.012
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Rhee, Y., Kang, M., Min, Y., Byun, D., Chung, Y., Ahn, C., et al. (2006). Effects of a combined alendronate and calcitriol agent (Maxmarvil) on bone metabolism in Korean postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Osteoporos. Int. 17 (12), 1801–1807. doi:10.1007/s00198-006-0200-4
Robinson-Cohen, C., Katz, R., Hoofnagle, A. N., Cauley, J. A., Furberg, C. D., Robbins, J. A., et al. (2011). Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J. Clin. Endocrinol. Metab. 96 (7), 2186–2193. doi:10.1210/jc.2010-2878
Shan, S., and Zhou, G. (2006). Clinical effect of Qianggu capsule in the treatment of primary osteoporosis. J. Pharm. Northwest China 21 (04), 177–178. doi:10.3969/j.issn.1004-2407.2006.04.021
Shen, Z., Chen, Z., Li, Z., Zhang, Y., Jiang, T., Lin, H., et al. (2020). Total flavonoids of rhizoma drynariae enhances angiogenic-osteogenic coupling during distraction osteogenesis by promoting type H vessel formation through PDGF-BB/PDGFR-β instead of HIF-1α/VEGF Axis. Front. Pharmacol. 11, 503524. doi:10.3389/fphar.2020.503524
Shen, Z., Zeng, J. Q., Li, Y. L., Liu, J. F., and Sun, S. Q. (2018). Clinical effect of Qianggu capsules combined with vitamin D2, calcium hydrophosphate, and calcium gluconate tablets in treatment of senile osteoporosis: an analysis of 50 cases. Hunan J. Tradit. Chin. Med. 34 (10), 7–9. doi:10.16808/j.cnki.issn1003-7705.2018.10.003
Solberg, L. B., Brorson, S. H., Stordalen, G. A., Bækkevold, E. S., Andersson, G., and Reinholt, F. P. (2014). Increased tartrate-resistant Acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif. Tissue Int. 94 (5), 510–521. doi:10.1007/s00223-013-9834-3
Syed, Z., and Khan, A. (2002). Bone densitometry: applications and limitations. J. Obstet. Gynaecol. Can. 24 (6), 476–484. doi:10.1016/s1701-2163(16)31095-7
Szulc, P. (2018). Bone turnover: biology and assessment tools. Best. Pract. Res. Clin. Endocrinol. Metab. 32 (5), 725–738. doi:10.1016/j.beem.2018.05.003
Tian, A., Ma, J., Feng, K., Liu, Z., Chen, L., Jia, H., et al. (2019). Reference markers of bone turnover for prediction of fracture: a meta-analysis. J. Orthop. Surg. Res. 14 (1), 68. doi:10.1186/s13018-019-1100-6
Vasikaran, S., Eastell, R., Bruyère, O., Foldes, A. J., Garnero, P., Griesmacher, A., et al. (2011). Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos. Int. 22 (2), 391–420. doi:10.1007/s00198-010-1501-1
Vinogradova, Y., Coupland, C., and Hippisley-Cox, J. (2020). Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 371, m3873. doi:10.1136/bmj.m3873
Wang, J., Shu, B., Tang, D. Z., Li, C. G., Xie, X. W., Jiang, L. J., et al. (2023). The prevalence of osteoporosis in China, a community based cohort study of osteoporosis. Front. Public Health 11, 1084005. doi:10.3389/fpubh.2023.1084005
Wang, J., Zhang, W. K., and Wang, Z. H. (2007). Qianggu capsule in the treatment of 28 cases of postmenopausal osteoporosis. Her. Med. 26 (11), 1325–1327. doi:10.3870/j.issn.1004-0781.2007.11.032
Wei, X., Xu, A., Shen, H., and Xie, Y. (2017). Qianggu capsule for the treatment of primary osteoporosis: evidence from a Chinese patent medicine. BMC Complement. Altern. Med. 17 (1), 108. doi:10.1186/s12906-017-1617-3
Wen, X. Y., and Zhou, Y. B. (2015). Effect of Qianggu capsule combined with calcium on bone mineral density and quality of life in elderly patients with osteoporosis. Chin. J. Gerontol. 35 (23), 6884–6885. doi:10.3969/j.issn.1005-9202.2015.23.110
Wu, G., Xu, R., Zhang, P., Xiao, T., Fu, Y., Zhang, Y., et al. (2018). Estrogen regulates stemness and senescence of bone marrow stromal cells to prevent osteoporosis via ERβ-SATB2 pathway. J. Cell Physiol. 233 (5), 4194–4204. doi:10.1002/jcp.26233
Yang, F., Sun, Y. H., Liu, D. B., Lv, Z. J., Qing, Y., Zhou, T. Y., et al. (2017). The metabolic changes of bone alkaline phosphatase, calcium, phosphorus, and the correlation between these indexes and bone mineral density of the alveolar bone in patients with osteoporosis. Chin. J. Osteoporos. 23 (09), 1160–1166. doi:10.3969/j.issn.1006-7108.2017.09.010
Yang, X., Dong, J., Hao, Y., Qi, Y., Liang, J., Yan, L., et al. (2022). Naringin alleviates H2O2-inhibited osteogenic differentiation of human adipose-derived stromal cells via wnt/β-catenin signaling. Evid. Based Complement. Altern. Med. 2022, 3126094. doi:10.1155/2022/3126094
Yao, D., Huang, L., Ke, J., Zhang, M., Xiao, Q., and Zhu, X. (2020). Bone metabolism regulation: implications for the treatment of bone diseases. Biomed. Pharmacother. 129, 110494. doi:10.1016/j.biopha.2020.110494
Yu, G. Y., Zheng, G. Z., Chang, B., Hu, Q. X., Lin, F. X., Liu, D. Z., et al. (2016). Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the Notch signaling pathway. Stem cells Int. 2016, 7130653. doi:10.1155/2016/7130653
Zhang, D., Liu, L., Jia, Z., Yao, X., and Yang, M. (2016). Flavonoids of Herba Epimedii stimulate osteogenic differentiation and suppress adipogenic differentiation of primary mesenchymal stem cells via estrogen receptor pathway. Pharm. Biol. 54 (6), 954–963. doi:10.3109/13880209.2015.1079224
Zhang, D. W., Cheng, Y., Wang, N. L., Zhang, J. C., Yang, M. S., and Yao, X. S. (2008). Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine 15 (1-2), 55–61. doi:10.1016/j.phymed.2007.04.002
Zhang, J., Park, J., Lee, J. W., Kwon, Y. D., and Kim, E. C. (2018). Bisphosphonates hinder osteoblastic/osteoclastic differentiation in the maxillary sinus mucosa-derived stem cells. Clin. Oral Investig. 22 (5), 1933–1943. doi:10.1007/s00784-017-2291-z
Zhang, S. M., and Chen, H. B. (2008). Clinical observation of Qianggu capsule in the treatment of primary osteoporosis. Hubei J. Tradit. Chin. Med. (06), 37. doi:10.3969/j.issn.1000-0704.2008.06.022
Zhang, Y., Jiang, J., Shen, H., Chai, Y., Wei, X., and Xie, Y. (2017). Total flavonoids from Rhizoma Drynariae (Gusuibu) for treating osteoporotic fractures: implication in clinical practice. Drug Des. Devel Ther. 11, 1881–1890. doi:10.2147/DDDT.S139804
Zhao, K., Chen, M., Liu, T., Zhang, P., Wang, S., Liu, X., et al. (2021). Rhizoma drynariae total flavonoids inhibit the inflammatory response and matrix degeneration via MAPK pathway in a rat degenerative cervical intervertebral disc model. Biomed. Pharmacother. 138, 111466. doi:10.1016/j.biopha.2021.111466
Zhou, R. M., Guan, Y. H., Wang, Q. B., and Liu, P. C. (2017). Clinical study on Qianggu capsules combined with Cervus and Cucumis Polypeptide Injection in treatment of senile primary osteoporosis. Drug Clin. 32 (07), 1337–1340. doi:10.7501/j.issn.1674-5515.2017.07.040
Keywords: osteoporosis, total flavonoids of Rhizoma Drynariae, bone metabolism, bone resorption, bone formation
Citation: Li W, Zhang Z, Li Y, Wu Z, Wang C, Huang Z, Ye B, Jiang X, Yang X and Shi X (2024) Effects of total flavonoids of Rhizoma Drynariae on biochemical indicators of bone metabolism: a systematic review and meta-analysis. Front. Pharmacol. 15:1443235. doi: 10.3389/fphar.2024.1443235
Received: 03 June 2024; Accepted: 23 August 2024;
Published: 10 September 2024.
Edited by:
Alex Zhong, Van Andel Institute, United StatesReviewed by:
Dongwei Zhang, Beijing University of Chinese Medicine, ChinaCopyright © 2024 Li, Zhang, Li, Wu, Wang, Huang, Ye, Jiang, Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Shi, eGxzaGktMjAwMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.