- 1Shifa College of Pharmaceutical Sciences, Shifa Tameer-e-Millat University, Islamabad, Pakistan
- 2Department of Chemistry, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 3Department of Clinical Psychology, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates
- 4The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Shanghai Institute of Immunity and Infection, Chinese Academy of Sciences, Shanghai, China
- 5University of Chinese Academy of Sciences, Beijing, China
- 6Shifa International Hospital, Islamabad, Pakistan
Background: Angiotensin-converting enzyme inhibitors (ACEIs) are prescribed for individuals with high cardiovascular (CV) risk; however, persistent cough limits the use of ACEIs in a large number of patients. The current study aimed to identify the genetic variants in the SLCO1B1 gene that might be associated with ACEI-related cough in a Pakistani hypertensive population.
Methods: A prospective cohort study was conducted at a tertiary care hospital in Pakistan. A total of 74 patients who had been treated with ACEIs were recruited through a convenient sampling method. The study was approved by the Institutional Review Board & Ethics Committee of the Shifa International Hospital, Islamabad. Patients provided 2 ml of blood for sequencing after signing informed consent. Partial gene sequencing of SLCO1B1 was carried out to find single nucleotide polymorphisms (SNPs) and haplotypes.
Results: It was found, through a structured questionnaire, that thirty-eight (38) patients experienced cough within 2 weeks of ACEI administration and were considered as a case group (cough), and thirty-six (36) patients were considered as a control group (no cough). The incidence of cough was 51%. We found six different SNPs and 9 haplotypes in the partial gene sequences of SLCO1B1. Haplotype H4 was associated significantly with cough after adjusting for sex and smoking status. Other SNPs and haplotypes were not significantly associated with ACE-Is-induced cough.
Conclusion: These findings emphasize the significance of SLCO1B1 genetic variants, specifically H4, as a potential predictor of ACEI-induced cough. It could be included in clinical practice as a possible risk factor for ACEI-induced cough once confirmed in larger clinical trials with bigger sample sizes. The replication of these findings in larger and more diverse populations is likely to contribute to the therapeutic use of ACEIs by predicting ACEI-induced cough.
Introduction
Hypertension is a serious risk factor for stroke, myocardial infarction, coronary heart disease, myocardial infarction, and end-stage renal disease (James et al., 2014). Between 1990 and 2019, its global prevalence has doubled, from 650 million to 1.3 billion people (WHO, 2023). The prevalence of hypertension in the Pakistani population is high, 16% in the rural and 21.6% in the urban population in individuals over 15 years. The majority of hypertensive patients in Pakistan are unaware of their hypertension. The total number of people with hypertension in Pakistan is around 5.5 million men and 5.3 million women. Only a tiny fraction (less than 3%) use proper medications to have controlled hypertension (Kearney et al., 2005; Soomro et al., 2013; Ahmed et al., 2008). In the Pakistani population over 45 years, around one-third are suffering from hypertension (Ghaffar et al., 2004).
Several remedies both natural and synthetic, have been employed to maintain normal blood pressure (Ali et al., 2023; Hussain et al., 2010),. Angiotensin-converting enzyme inhibitors (ACEIs) were recommended by Joint National Committee VII (JNC-7) as one of the safest medications for the treatment of hypertensive patients with comorbidities including chronic renal disease, diabetes, or congestive heart failure because ACE-Is do not adversely affect heart and kidneys (Khalil et al., 2001). Due to this safety profile, the clinical application of ACEIs to treat hypertension has dramatically grown. Globally, between 20 and 30 million patients take these medications to control hypertension (Messerli et al., 2018). The worldwide market size of the ACEIs is around 3,456.27 USD Million. In Pakistan, ACEIs are prescribed to most of the patients admitted to hospitals for the treatment of MI (Alam et al., 2024).
Compared to other cardiovascular (CV) drugs, ACEIs have no more harmful side effects. However, the most frequent adverse effect related to the use of ACEIs is a chronic cough (Nikpoor et al., 2005). Cough can start right away after the first dose or it can take weeks or months to appear after the start of the therapy. This bothersome dry cough frequently becomes severe enough to necessitate stopping the medication because reducing the dose of the drug will not stop it as this effect is dose-independent. Data on the prevalence of cough are conflicting (Punzi, 1993). Retrospective or post-marketing studies have shown it to be as low as 1-2 percent, while controlled trials have reported it to be as high as 37–39 percent. Coughing can range in intensity from an irritating, dry tickle to a chronic hacking that is accompanied by sleeplessness or vomiting. The frequency of ACEI-related cough varies by the patient’s sex, smoking habits, race, and other conditions. Therefore, ACEI-related cough is more common in women, non-smokers, Asian people, and congestive heart failure (CHF) patients (Gibson, 1989; Israili and Hall, 1992).
The cause of ACEI-induced cough is still not fully understood. It has been suggested that the pathogenic mechanism of ACEI-induced cough may involve the accumulation of chemicals that ACE typically metabolizes, such as bradykinin and substance P, in the airways and the subsequent stimulation of vagal afferent neurons that service the cough reflex (Sheikh et al., 2022). The onset of cough, however, might be the result of a more intricate series of circumstances than first thought. Bradykinin has been demonstrated to activate proinflammatory pathways in the body by increasing the production of arachidonic acid metabolites including prostaglandins and nitric oxide, which in turn promotes coughing (Trifilieff et al., 1993).
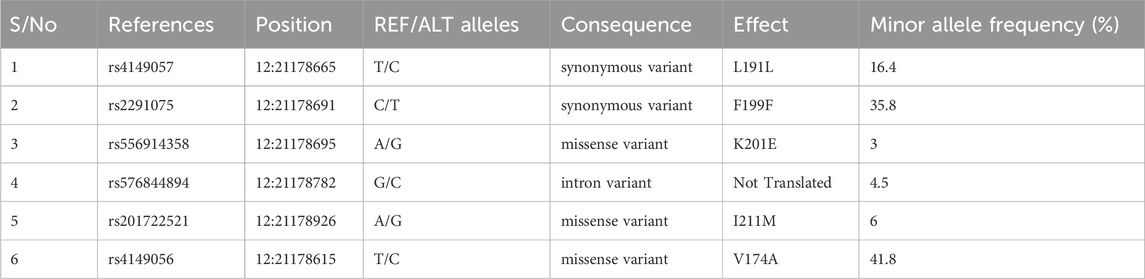
The SLCO1B1 gene codes for the solute carrier organic anion transporter family member 1B1 (SLCO1B1). SLCO1B1, also known as OATP1B1, is located in liver cells and aids in the hepatic clearance of several medications, including ACE inhibitors (Pasanen et al., 2007). Since this gene is involved in the uptake of ACE inhibitors from the blood into hepatocytes, its expression as well as function are critical to the efficacy and adverse effects of ACE inhibitors. This fact makes SLCO1B1 a promising gene to study for investigating ACE inhibitor-induced cough. There are more than 190 single nucleotide polymorphisms (SNPs) known to exist in the SLCO1B1 gene (Liu et al., 2006). According to earlier studies, among the 190 SNPs identified, the two most prevalent ones (521T>C, rs4149056, and 597C>T, rs2291075) are responsible for variations in the pharmacokinetics (PK) and pharmacodynamics (PD) of the SLCO1B1 substrate (Oshiro et al., 2010). However, no research on the relationship between SLCO1B1 mutations and ACEI-induced cough has been done in Pakistan. Due to the extensive usage of ACEIs, physicians must be able to anticipate each patient’s response to the medication to reduce the frequency of coughing. Therefore, the purpose of this study is to determine whether the incidence of cough in a Pakistani hypertensive cohort treated with ACEIs is associated with genetic variation, specifically SNPs and haplotypes in SLCO1B1.
Methods and materials
Study approval, participants & data collection
A prospective cohort study was conducted with patients attending the clinic of their physicians at a tertiary care hospital, Shifa International Hospital (SIH) and Shifa Plus Pharmacy, in Pakistan. The study approval was taken from the institutional review board (IRB) and Ethics Committee (EC) of SIH, Islamabad, Pakistan, under the letter (IRB# 058–22). Patients were enrolled prospectively via convenient sampling. The sample size was calculated by taking a 95% confidence level, 0.10 absolute precision, and anticipated prevalence of ACE inhibitor-induced cough, 46% as per the literature review, a sample size of 79 was calculated by using the WHO sample size calculator. After obtaining informed consent standardized per the WHO guidelines, patients were requested to participate through a structured questionnaire. Their baseline information comprised age, gender, body weight, height, and smoking status. ACEI-related characteristics include type, duration of ACEI usage, and occurrence of cough. The duration of the cough was also recorded. Only patients who met all of the inclusion criteria were included in this study. Patients with respiratory tract infection (RTI) and/or pneumonia were excluded. Eligible subjects prescribed with ACEIs were interviewed via phone call after 2 weeks of therapy.
DNA extraction and sequencing
All study participants gave 2 milliliters (mL) of venous blood in a sterile tube containing etheylenediaminetetraacetic acid (EDTA). Genomic DNA was extracted from leukocytes per the protocol described previously (Hussain et al., 2023) and kept at −20°C. A NanoDrop spectrophotometer was used to measure the DNA concentration.
Targeted sequencing of the SLCO1B1 gene was performed by SeqStudio™ (Applied Biosystems USA). We selected a hypervariable region of the SLCO1B1 gene which is a known hotspot of variation. This approach of selected hypervariable hotspots has previously yielded good results (Ahmed et al., 2022). The amplified PCR products were cycle sequenced with a BDT v.3.1 master mix as per the manufacturer’s instructions, for both the forward and reverse primers. BigDye Xterminator™ (Thermofisher Scientific USA) kit was used for the purification of the cycle-sequenced product as per the manufacturer’s instructions. Samples were loaded for analysis, and run parameters were set according to the kits used. A medium run was selected for electrophorese. The nucleotide sequence data of all the individuals generated in this study are available from Genebank under the accession numbers PP387529- PP387595.
Sequencing data analysis
To ensure accuracy, we carried out a trace quality check on the DNA sequence reads using the Staden Package (Staden et al., 1999) and Finch TV v1.4.0 (http://www.geospiza.com/Products/finchtv.shtml). The high-quality sequence reads were assembled using Lasergene v.7.1 packages (DNASTAR Inc.USA) and Bio-Edit software (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) as mentioned previously (Khokhar et al., 2021).
Identification of SNPs & haplotypes in SLCO1B1 gene
The high-quality filtered sequence data was compared to the reference genome of the GRCh38.p14 in the Ensemble database (Howe et al., 2021) using Blast. The sequences of the whole cohort were aligned using the MUSCLE program (Edgar, 2004) in MEGA-X (Kumar et al., 2018). The SLCO1B1 gene’s in-house alignment region (located at 12:21178627–21179056) in the Ensemble database was successfully analyzed. The analysis covered partial exon 6, whole exon 7, whole intron 7, and partial intron 8. The haplotypes were generated using the DnaSP v6.12 software package (Rozas et al., 2017). This process involved converting the DnaSP v6.12 output into the Arlequin project file format (.arp). The number of polymorphic sites and haplotype frequencies was computed using Arlequin v3.5 (Excoffier et al., 2005). Within Arlequin v3.5, a new project was initiated specifying all the samples of the population (e.g., coughers vs. non-coughers) and the molecular data type (DNA sequences). In the Arlequin, the data parameters were set at the haplotypic level, and locus level, and searched to capture the shared haplotypes among all samples. The software was used to infer haplotypes and generated a unique haplotype list that was present in the samples, along with their frequencies.
Statistical analysis
All data were analyzed using IBM Statistical Package for Social Sciences (SPSS) version 23.0. Cronbach alpha was used to measure the reliability of the questionnaire. Mean was calculated for continuous data and frequencies were calculated for categorical data. Chi-square was also applied to examine the association of SLCO1B1 polymorphisms with ACEI-induced cough. Multivariate logistic regression analysis was used for adjusting sex, and smoking status. P-value ≤ 0.05 was taken as significant.
Results
Basic demographic and clinical parameters
Basic demographic and clinical parameters are mentioned in Table 1. There was no statistically significant difference between the coughers and non-coughers at p < 0.05. Initially, 90 patients were recruited for the study. Of these, 10 patients (11.1%) were excluded because of catching pneumonia, and 6 patients (6.6%) were excluded for other reasons. Finally, the investigated cohort included 74 essential hypertensive patients receiving ACEIs, including 39 (52%) males and 35 (47%) females. The mean age and BMI were 51.28 years and 24.5 kg/m2, respectively. The mean SBP and DBP of subjects at the baseline were 125- and 80-mm Hg, respectively. Twenty-three percent (23%) of patients were smokers, fifty-five percent (55%) had a history of hypertension, and the largest ethnic group in our study was Punjabi, which makes up about 71% of the study sample, followed by Pashtuns (21%), Kashmiris (4%), and Sindhis (2.7%). Ramipril was the most frequently prescribed ACEI (60%), followed by Captopril (20%), enalapril (10.8%), and lisinopril (8%). The number of patients with cough once a week was significantly more (p < 0.001) than those with twice or more cough episodes per week (Table 1).
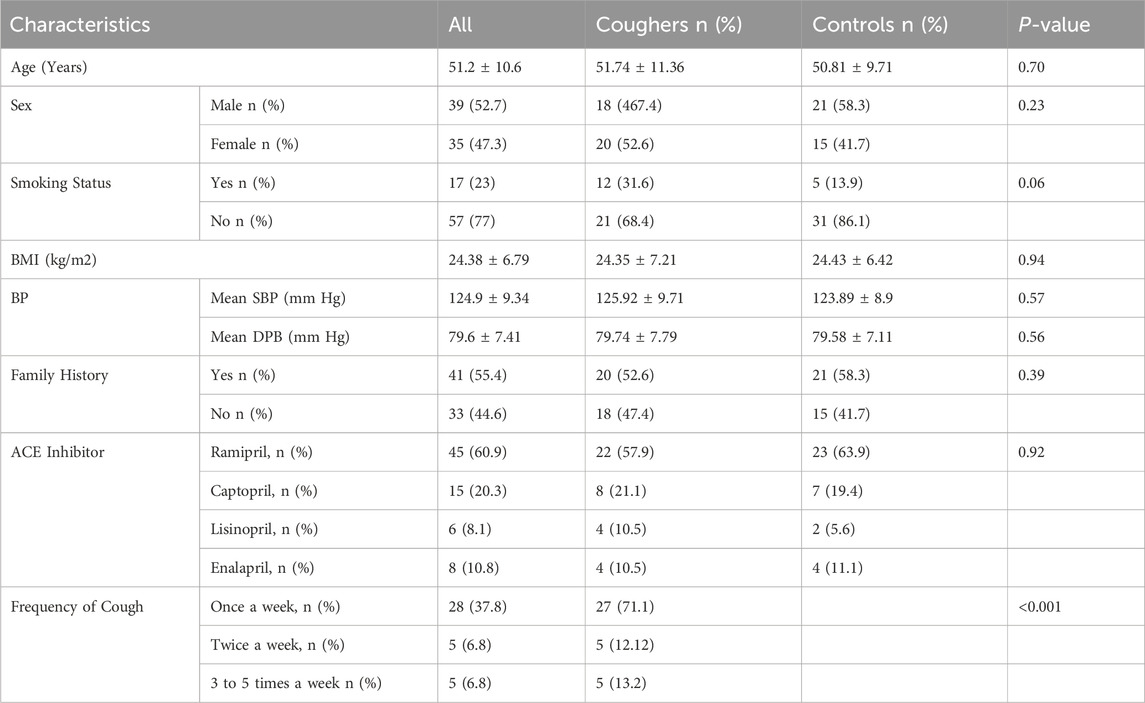
Table 1. Descriptive characteristics of the study population. BMI = body mass index, BP = blood pressure.
Incidence of cough
Dry cough as an ACEI side effect was recorded systematically in the clinical records and defined as a cough that develops after the prescription of ACEIs in the absence of asthma or upper respiratory infection and whose symptoms disappear within 15 days of stopping the treatment. Cough caused by other conditions (acute RTI or other respiratory diseases) was not considered to be induced by ACEIs. Based on these criteria, patients were divided into the case group (coughers) and the control group (non-coughers). Of these 74 participants, the prevalence of cough related to ACEIs occurred in fifty-one percent (51%) patients, and these subjects were defined as case groups (coughers), while the other forty-eight percent (49%) individuals without ACEI-induced cough were classified as control group (non-cougher) (Table 1). There were 18 men and 20 women in the coughers group and 21 men and 15 women in the non-coughers group.
Genotype frequencies & association with cough
Genotype frequencies of the various polymorphisms in the study sample are shown in Table 2. Of the 74 patients, 67 samples were sequenced representing 90.5% sequencing success, while seven samples could not be sequenced due to the contamination and low yield of DNA. A large amount of missing sequencing data adversely affects the power of analysis; however, we are missing less than 10% of data in our investigation which is likely to have a very small effect on the outcomes. For rs4149057, the genotype frequency of TT was 76% and 18% for TC in the cougher group while in the controls, TT was 75% and TC was 11% (p: 0.309, OR (95% CI): 2.08 (0.507,8.543)) (Table 3). There was no significant association of this SNP with ACE-Is-induced cough (Table 3). Even after adjusting for sex and smoking status, we could not find a significant association of this genetic variant with the cough. For rs2291075, the genotype frequency of CC was 55% and 40% for TC in the cougher group while in the controls, CC was 61% and TC was 25% (p: 0.231, OR (95% CI): 0.519 (0.178,1.517)). There was no significant association of this SNP with ACEI-induced cough either before or after adjusting for sex and smoking status.
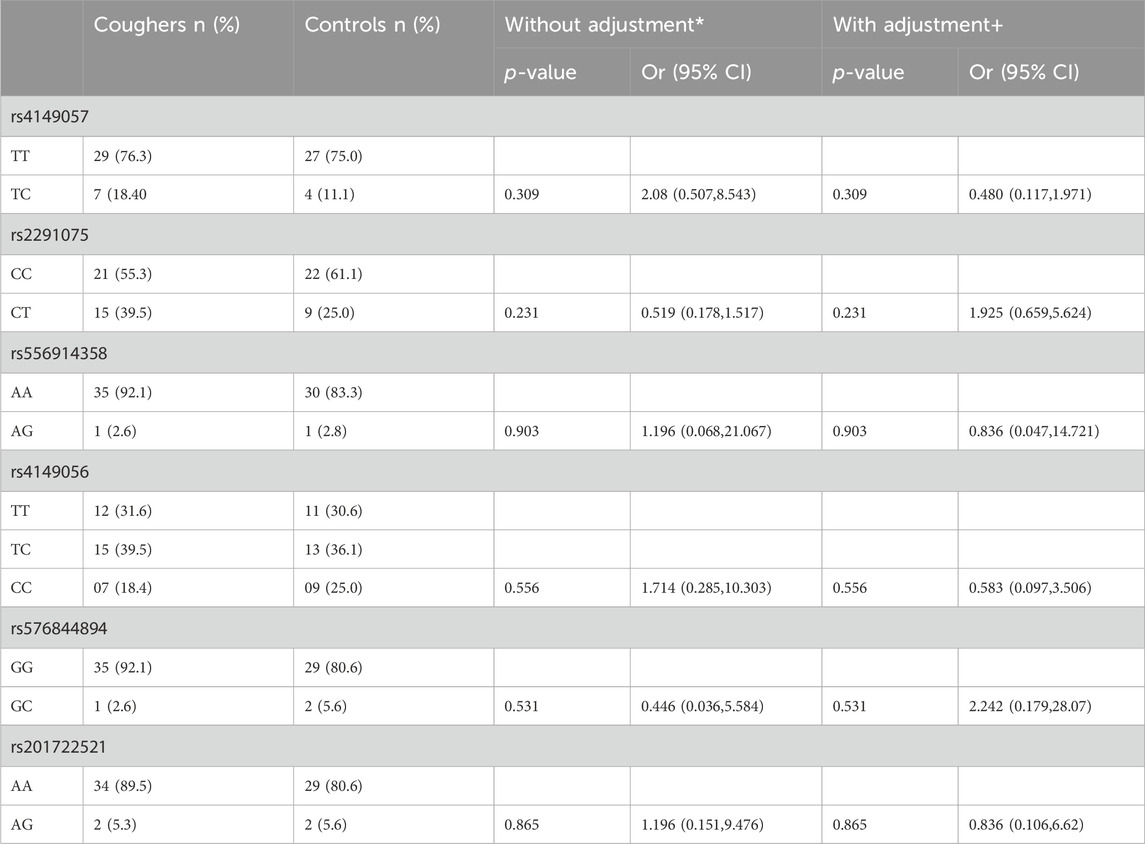
Table 3. Association of SLCO1B1 genetic polymorphisms with the risk of ACE-Is-induced cough. Homozygous wild-type patients served as the reference group. OR = odds ratio; CI = confidence interval. * Uncorrected P-value and crude OR using χ2 tests with Pearson 2 × 2 test or Fisher exact test. + Adjusted data by multivariate logistic regression analysis for sex, and smoking status.
Similarly, when genotype frequencies were calculated for rs556914358, AA was 92% and AG was 3% in the cougher group while in the controls, AA was 83% and AG was 3% (p: 0.903, OR (95% CI): 1.196 (0.068,21.067)). There was no significant association of this SNP with ACEI-induced cough. Genotype frequencies for SNPs rs4149056, rs576844894, and rs201722521 are provided in Table 3. Our analyses show no significant association with ACEIs-induced cough either before or after adjustment for sex and smoking status.
Haplotype frequencies & association with cough
Arlequin v3.5 calculated the frequency of each haplotype within the overall sample population. It tested for the significant differences in haplotype distribution between groups defined by phenotypic traits, such as the presence or absence of ACEI-induced cough. We found 9 haplotypes in our cohort (Figure 1; Table 4), 7 of which we investigated for their association with cough, the remaining 2 haplotypes were found in extremely low frequency. The frequency of haplotype H1 was 24% in coughers and 28% in controls, H2 was 29% in coughers and 17% in controls, H3 was 26% in coughers and 22% in controls. The frequency of haplotype H4 was 3% in coughers and 0% in controls and after adjusting for sex and smoking status showed a significant association with cough. H5 did not show a significant association with cough. Similarly, the frequency of haplotype H6 was 3% in coughers and 6% in controls. The frequency of haplotype H7 was 0.02 in coughers and 0.05 in controls while haplotype H9 was 3% in coughers and 3% in controls. Ultimately, out of nine haplotypes identified, only the haplotype (H4) shows significant accusation with the ACEI-induced cough. Other haplotypes did not exhibit significant association (Table 4).
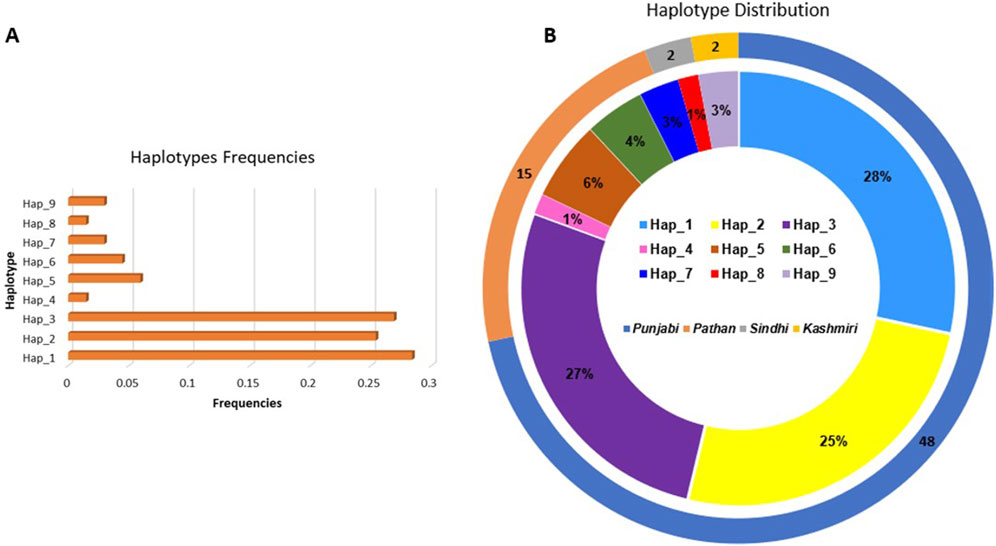
Figure 1. (A) Bar chart illustrating the frequencies of each haplotype observed in the dataset. The x-axis represents the haplotype frequencies, while the y-axis displays the individual haplotypes (B) Double-layered ideogram presenting the haplotype distribution across different ethnic groups. The outer ring (ideogram) showcases the distribution of sample sizes among four ethnic groups: Punjabi, Pathan, Sindhi, and Kashmiri, with the corresponding percentages indicated. The inner ring (ideogram) provides a detailed view of the distribution of the nine identified haplotypes within these ethnic groups, with each color-coded segment representing a specific haplotype.
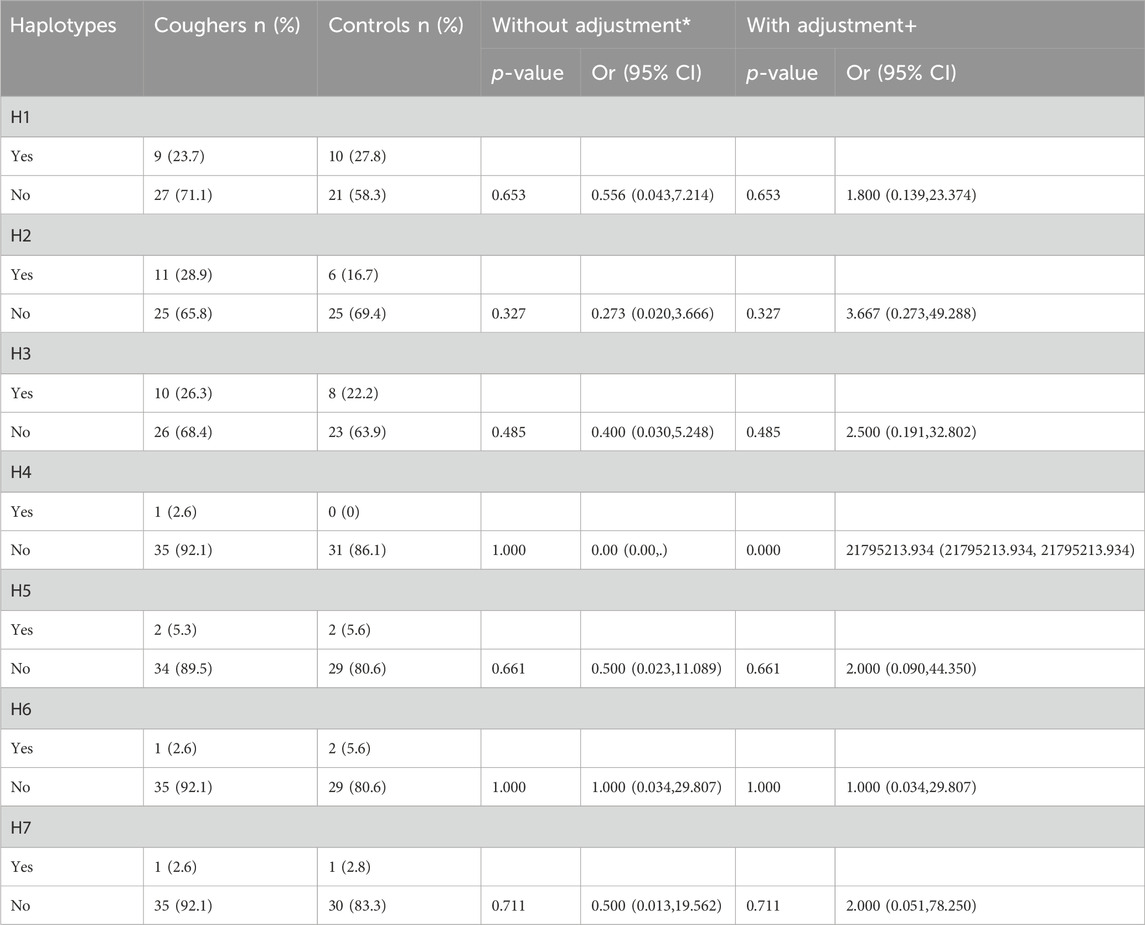
Table 4. Association of haplotypes in SLCO1B1 gene with the risk of ACE-Is-induced cough. Presence of a particular haplotype served as the reference group. OR = odds ratio; CI = confidence interval. * Uncorrected P-value and crude OR using χ2 tests with Pearson 2 × 2 test or Fisher exact test. + Adjusted data by multivariate logistic regression analysis for sex, and smoking status.
Discussion
Our study shows that Ramipril was the most frequently prescribed ACEI (60%), followed by Captopril (20%), enalapril (10.8%), and lisinopril (8%). We found the incidence of cough in the ACE inhibitor users to be 51%. We also found 6 SNPs and 9 haplotypes with varying frequencies in our cohort of hypertensive patients using ACE inhibitors. None of the SNPs and haplotypes was associated with ACE inhibitors-induced cough except haplotype 4 which was found significantly associated with cough after adjusting the sex and smoking status of the patients.
Globally, the incidence of ACEI-induced cough has been reported to be in the range of 20%–50% among patients treated with ACEIs and the onset of cough may be within days or weeks of beginning ACEI therapy (Simon et al., 1992). A significant delay between the onset of symptoms and actual diagnosis may predispose patients to unnecessary prescriptions and diagnostic tests. Therefore, awareness of, and early intervention for, this adverse event can result in significant cost savings and improvement in quality of life. According to our study, cough displayed the highest incidence (51%), followed by dizziness (18%), fainting (9%), and hypotension (8%). The incidence of ACEI-induced cough was higher in our study compared to some previous investigations carried out in Pakistan. One study found the frequency of dry cough with ACE inhibitors around 17%, ranging from almost 20% with Enalopril, 16.6% with Captopril, 10% with Lisinopril and Ramipril, 15% with Qurinapril and Perindopril (Saboor et al., 2012). Another study reported a slightly higher incidence rate of dry cough with ACEI. Jamshed and colleagues reported a 26.8% incidence of dry cough with ACE inhibitors. They found that Enalapril was the most commonly ACEI prescribed among patients with ACEI-induced cough (Jamshed et al., 2019). Other studies reported similar frequencies of ACE inhibitor-induced cough in the Malaysian and Chinse populations (24.1% and 32% respectively) (Loo et al., 2022; Luo et al., 2015). One reason for a slightly lower incidence of dry cough in these studies might be the fact that our study included mild cases of dry cough also, while other investigators, for example, Jamshed et al., included only those cases in which dry cough was severe enough for the patients where they were contemplating requesting physician to change the drug.
Our study did not find a significant association of any SNP with cough except for the haplotype H4 which was significantly associated with cough after adjusting for sex and the smoking status. After adjustment, patients with H4 were 22 times more likely to have the ACE inhibitor-induced cough. While there are other confounding factors such as age, co-morbidities, and concurrent medications, and they all are important to consider, we only adjusted for sex and smoking status as per the practice of previous studies (Luo et al., 2015). Literature reveals several studies showing the association of genetic variants in various genes with ACEI-induced cough. Luo and colleagues investigated ABO genetic polymorphisms rs8176740 and rs495828 for their possible association with ACEI-induced cough. They found rs495828 polymorphism was associated with enalapril-induced cough in Chinese patients with essential hypertension (Luo et al., 2014). In another study, Kim et al., detected two SNPs in the NK2R gene (i.e., Gly231-Glu and Arg375His), and found that the Gly231Glu polymorphism was associated with a lower prevalence of ACEI-related cough (Kim et al., 2009).
In previous studies, the genetic variants of SLCO1B1 were proven to be relevant to the response to drugs transported by OATP1B1, such as statin-induced myopathy, irinotecan-induced toxicities, methotrexate clearance (Carr et al., 2013; Link et al., 2008; Hussain et al., 2023). The SLCO1B1 521T > C polymorphism appeared consistently relevant, but results regarding the in-vitro association of the 597 C>T variant, which may make the transport activity increase, decrease, or not change, were conflicting (Niemi et al., 2011). In China, the 521T > C variant was reported to be an important determinant of the PK of ACEIs (Tian et al., 2011) while in the Russian population, dry cough attributed to enalapril was associated with polymorphisms rs2306283 in the SLCO1B1 gene (Sychev et al., 2023) However, in our investigation, we did not observe a significant association. In another investigation, Luo and colleagues provided evidence that the SLCO1B1 functional genetic variant (521T > C; Val174Ala) substantially alters the risk of enalapril-induced cough in the Chinese population (Luo et al., 2015). No significant association with this genetic variant in our study might be due to the underlying genetics of our population or it may be simply due to the smaller sample size of our cohort. In addition to population-specific differences and low sample size, another possible reason for not finding significant associations in our study might be the potential environmental factors such as diet, temperature, oxygen levels, humidity, light cycles, and the presence of mutagens, which may also be responsible for obscuring the potential association with ACE inhibitor-induced cough.
New determinants of ACEI toxicity are also being investigated. Ghouse and colleagues found seven risk loci for ACEI discontinuation (Ghouse et al., 2022). Their analysis showed several pathways involved in ACEI-associated cough as the main underlying phenotype. They specifically identified new loci including rs7526729 in KCNA2, rs1544730 in SRBD1, rs16870989 in KCNIP4, rs12210271 in PREP, rs360206 in SCAI, rs8097200 in L3MBTL4, and rs6062847 in SLCO4A1/NTSR1associated with ACEI discontinuation. Such studies emphasize the relevance of utilizing proxies for ACEI ADRs to provide important insights into the etiology and genetic architecture of specific adverse drug reactions. In this context, our findings related to the association of haplotype H4 in the SLCO1B1 gene with cough may prove important for predicting ACEI-induced cough in prospective patients.
The findings of our study that there exists a significant association between genetic variation in the SLCO1B1, particularly the H4 haplotype, and cough signifies that H4 is likely to increase the risk of developing cough in hypertensive patients taking ACE inhibitors. H4 may also modify the intake of statins to hepatocytes from the blood. Therefore, the observed association between SLCO1B1 genetic variation and ACE inhibitors-induced cough may result from the pharmacokinetic mechanism, which ultimately controls the plasma levels of ACE inhibitors. Thus, SLCO1B1 may be a suitable gene to identify hypertensive patients on ACE inhibitors who are more likely to suffer from ACE inhibitor-induced cough. It has been suggested previously that genotyping SLCO1B1 variants might be a useful strategy to achieve the benefits of enalapril treatment more effectively and safely in China (Luo et al., 2015). These observations will help provide pharmacogenetic markers for ACE inhibitors-based treatments. However, larger trials with higher sample sizes, especially those with functional studies to further investigate the underlying biological mechanisms are required to shed more light on the findings of the current study.
While the findings of this study are important and valid, we identify several shortcomings in our investigation. While the partial gene sequencing approach has previously yielded good results (Ahmed et al., 2022), it nevertheless, misses out on significant pieces of information by not sequencing the whole gene. Furthermore, a low sample size in our study also precludes a strong generalization of the study findings. This is perhaps why we could not find more SNPs and haplotypes strongly associated with the incidence of cough in our study.
Conclusion
Our study shows that the incidence of ACE inhibitors-induced cough is very high, compared to other populations, in the Pakistani hypertensive cohort investigated in this study. We also found that genetic variants of SLCO1B1 genes may be associated with the cough induced by ACEIs. Although SLCO1B1 521T > C polymorphism was not significantly associated with cough, specifically haplotype, H4 was found significantly associated with cough after adjusting for sex and smoking status. These findings emphasize the significance of SLCO1B1 genetic factors as potential predictors of ACEI-induced cough. Our research on the pharmacogenomics of the relationship between SLCO1B1 genetic variants and ACEI-induced cough may contribute to the therapeutic use of SLCO1B1 pharmacogenetics for predicting ACEI-induced cough.
Data availability statement
The data supporting the findings of this study have been deposited in GenBank under the accession number [PP387529-PP387595].
Ethics statement
The studies involving humans were approved by Institutional Review Board & Ethics Committee, Shifa International Hospital, Islamabad, Pakistan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. NM: Data curation, Formal Analysis, Writing–review and editing, Validation. RN: Data curation, Formal Analysis, Writing–review and editing, Validation. HK: Data curation, Formal Analysis, Methodology, Writing–original draft. MR: Formal Analysis, Methodology, Writing–original draft. FJ: Data curation, Formal Analysis, Investigation, Validation, Writing–original draft. SA: Conceptualization, Formal Analysis, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors thank the United Arab Emirates University for funding this research under UPAR project G00003696, grant code 12S094.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1441251/full#supplementary-material
References
Ahmed, N., Abdul Khaliq, M., Shah, S. H., and Anwar, W. (2008). Compliance to antihypertensive drugs, salt restriction, exercise and control of systemic hypertension in hypertensive patients at Abbottabad. J. Ayub Med. Coll. Abbottabad No. 20 (2), 66–69.
Ahmed, S., Gul, S., Siraj, S., Hussain, A., Sultan Sheikh, F., Shah, S. U., et al. (2022). Antiplatelet response to clopidogrel is associated with a haplotype in CYP2C19 gene in Pakistani patients. Sci. Rep. 12 (1), 6171. doi:10.1038/s41598-022-09679-8
Alam, F., Batool, G., Noureen, S., Khan, M. S., Zaman, I., Jamil ur Rehman, , et al. (2024). The prescribing trends of ace-inhibitors after myocardial infarction in tertiary care hospitals of hazara region. Int. J. Pharm. & Integr. Health Sci. 5 (1). doi:10.56536/ijpihs.v5i1.120
Ali, F., Shahid, F., and Shahid, T. (2023). Aqueous extract of malus domestica inhibits human platelet aggregation induced by multiple agonists. Phytopharm. Commun. 3 (01), 27–37. doi:10.55627/ppc.003.01.0289
Carr, D. F., O'meara, H., Jorgensen, A. L., Campbell, J., Hobbs, M., McCann, G., et al. (2013). SLCO1B1 genetic variant associated with statin-induced myopathy: a proof of concept study using the clinical practice research datalink. Clin. Pharmacol. & Ther. 94 (6), 695–701. doi:10.1038/clpt.2013.161
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids Res. 32 (5), 1792–1797. doi:10.1093/nar/gkh340
Excoffier, L., Laval, G., and Schneider, S. (2005). Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinforma. No. 1, 117693430500100–117693430500150. doi:10.1177/117693430500100003
Ghaffar, A., Reddy, K. S., and Singhi, M. (2004). Burden of non-communicable diseases in south asia. Bmj 328 (7443), 807–810. doi:10.1136/bmj.328.7443.807
Ghouse, J., Tragante, V., Muhammad, A., Ahlberg, G., Skov, M. W., Roden, D. M., et al. (2022). Polygenic risk score for ACE-inhibitor-associated cough based on the discovery of new genetic loci. Eur. Heart J. 43 (45), 4707–4718. doi:10.1093/eurheartj/ehac322
Gibson, G. R. (1989). Enalapril-induced cough. Archives Intern. Med. 149 (12), 2701–2703. doi:10.1001/archinte.149.12.2701
Howe, K. L., Achuthan, P., Allen, J., Allen, J., Alvarez-Jarreta, J., Amode, M. R., et al. (2021). Ensembl 2021. Nucleic acids Res. 49 (D1), D884–D891. doi:10.1093/nar/gkaa942
Hussain, A., Zakria, M., Shafiq, A. T., Rauf, M. A., Ahmad, J., Fawad Rasool, M., et al. (2023). Investigation of the association of GATM gene polymorphisms with statin-induced myopathy. Precis. Med. Commun. 3 (02), 105–118. doi:10.55627/pmc.003.02.0430
Hussain, J., Khan, F.-ullah, Gilani, S. A., Abbas, G., Ahmed, S., Khan, A.-ullah, et al. (2010). Antiglycation, antiplatelets aggregation, cytotoxic and phytotoxic activities of Nepeta suavis. Lat. Am. J. Pharm. no. 29.
Israili, Z. H., and Hall, W. D. (1992). Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: a review of the literature and pathophysiology. Ann. Intern. Med. 117 (3), 234–242. doi:10.7326/0003-4819-117-3-234
James, P. A., Oparil, S., Carter, B. L., Cushman, W. C., Dennison-Himmelfarb, C., Handler, J., et al. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama 311 (5), 507–520. doi:10.1001/jama.2013.284427
Jamshed, F., Jaffry, H., Hanif, H., Kumar, V., Naz, U., Ahmed, M., et al. (2019). Demographic and clinical characteristics of patients presenting with angiotensin-converting enzyme inhibitors induced cough. Cureus 11 (9), e5624. doi:10.7759/cureus.5624
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., and He, J. (2005). Global burden of hypertension: analysis of worldwide data. lancet 365 (9455), 217–223. doi:10.1016/S0140-6736(05)17741-1
Khalil, M. E., Basher, A. W., Brown, E. J., and Alhaddad, I. A. (2001). A remarkable medical story: benefits of angiotensin-converting enzyme inhibitors in cardiac patients. J. Am. Coll. Cardiol. 37 (7), 1757–1764. doi:10.1016/s0735-1097(01)01229-3
Khokhar, A. M., Khan, S., Zahid, M., Lail, A., Khan, B. A., Iqbal Khan, A., et al. (2021). Analysis of the HBV small S gene partial sequences and its implications for detection, prevention and treatment in Pakistani patients. Mol. Med. Commun. 1 (1), 11–23. doi:10.55627/mmc.001.01.0022
Kim, T.-B., Y Oh, S., Park, H.-K., Jeon, S.-G., Chang, Y.-S., Lee, K.-Y., et al. (2009). Polymorphisms in the neurokinin-2 receptor gene are associated with angiotensin-converting enzyme inhibitor-induced cough. J. Clin. Pharm. Ther. 34 (4), 457–464. doi:10.1111/j.1365-2710.2008.01018.x
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 (6), 1547–1549. doi:10.1093/molbev/msy096
Link, E., Parish, S., Armitage, J., Bowman, L., Heath, S., Matsuda, I., et al. (2008). SLCO1B1 variants and statin-induced myopathy-a genomewide study. N. Engl. J. Med. 359 (8), 789–799. doi:10.1056/NEJMoa0801936
Liu, L., Cui, Y., Chung, A. Y., Shitara, Y., Sugiyama, Y., Dietrich, K., et al. (2006). Vectorial transport of enalapril by Oatp1a1/Mrp2 and OATP1B1 and OATP1B3/MRP2 in rat and human livers. J. Pharmacol. Exp. Ther. 318 (1), 395–402. doi:10.1124/jpet.106.103390
Loo, H. C., Osman, F., Ho, S. L., An, S. Y., Yong, Y. M.Au, and Khoo, Ee M. (2022). Incidence of angiotensin-converting enzyme inhibitor-induced cough in a Malaysian public primary care clinic: a retrospective cohort study. Malays. Fam. Physician Official J. Acad. Fam. Physicians Malays. no. 17 (1), 66–70. doi:10.51866/oa.80
Luo, J.-Q., He, F.-Z., Wang, Z.-M., Sun, N.-L., Wang, L.-Y., Tang, G.-Fu, et al. (2015). S LCO1B1 variants and angiotensin converting enzyme inhibitor (Enalapril)-Induced cough: a pharmacogenetic study. Sci. Rep. 5 (1), 17253. doi:10.1038/srep17253
Luo, J.-Q., He, F.-Z., Zhi-Ying, L., Wen, J.-G., Wang, L.-Y., Sun, N.-L., et al. (2014). Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenetics genomics 24 (6), 306–313. doi:10.1097/FPC.0000000000000050
Messerli, F. H., Bangalore, S., Bavishi, C., and Rimoldi, S. F. (2018). Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J. Am. Coll. Cardiol. 71 (13), 1474–1482. doi:10.1016/j.jacc.2018.01.058
Niemi, M., Pasanen, M. K., and Neuvonen, P. J. (2011). Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 63 (1), 157–181. doi:10.1124/pr.110.002857
Nikpoor, B., Duan, Q. L., and Rouleau, G. A. (2005). Acute adverse reactions associated with angiotensin-converting enzyme inhibitors: genetic factors and therapeutic implications. Expert Opin. Pharmacother. 6 (11), 1851–1856. doi:10.1517/14656566.6.11.1851
Oshiro, C., Mangravite, L., Klein, T., and Altman, R. (2010). PharmGKB very important pharmacogene: SLCO1B1. Pharmacogenetics genomics 20 (3), 211–216. doi:10.1097/FPC.0b013e328333b99c
Pasanen, M. K., Hanna, F., Neuvonen, P. J., and Niemi, M. (2007). Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. & Ther. 82 (6), 726–733. doi:10.1038/sj.clpt.6100220
Punzi, H. A. (1993). Safety update: focus on cough. Am. J. Cardiol. 72 (20), 45H-48H–H48. doi:10.1016/0002-9149(93)91054-l
Rozas, J., Ferrer-Mata, A., Sánchez-DelBarrio, J. C., Guirao-Rico, S., Librado, P., Ramos-Onsins, S. E., et al. (2017). DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 34 (12), 3299–3302. doi:10.1093/molbev/msx248
Saboor, Q. A., Mahmud, T., Agha, S., Abbasi, M. S. K., Aslam, A., et al. (2012). Frequency of Angiotensin Converting Enzyme Inhibitor (ACEI) Induced Cough. Proceeding Shaikh Zayed Postgraduate Medical Institute. 26 (2), 65–68.
Sheikh, A. F., Memon, F. S., and Sahar, N.Ul (2022). Angiotensin-converting enzyme inhibitors; their efficacy, adverse effects, and the genetic influence on cough they may cause. Mol. Med. Commun. 2 (01), 77–88. doi:10.55627/mmc.002.001.0039
Simon, S. R., Black, H. R., Moser, M., and Berland, W. E. (1992). Cough and ACE inhibitors. Archives Intern. Med. 152 (8), 1698–1700. doi:10.1001/archinte.1992.00400200128023
Soomro, M. A., Abro, G. Y., and Shah, S. I. A. (2013). Frequency of hyperlipidemia in patients of hypertension in chandka medical college hospital larkana. Med. Channel 19 (3).
Staden, R., Beal, K. F., and Bonfield, J. K. (1999). The staden package, 1998. Bioinforma. methods Protoc. 132, 115–130. doi:10.1385/1-59259-192-2:115
Sychev, I. V., Denisenko, N. P., Kachanova, A. A., Lapshtaeva, A. V., Goncharova, L. N., Mirzaev, K. B., et al. (2023). Pharmacogenetic predictors of development of secondary to enalapril dry cough in hypertensive patients. Drug Metabolism Personalized Ther. 38 (3), 247–254. doi:10.1515/dmpt-2023-0008
Tian, L., Liu, H., Xie, S., Jiang, J., Han, L., Huang, Y., et al. (2011). Effect of organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphism on the single-and multiple-dose pharmacokinetics of enalapril in healthy Chinese adult men. Clin. Ther. No. 33 (5), 655–663. doi:10.1016/j.clinthera.2011.04.018
Trifilieff, A., Da Silva, A., and Gies, J. P. (1993). Kinins and respiratory tract diseases. Eur. Respir. J. 6 (4), 576–587. doi:10.1183/09031936.93.06040576
Keywords: ACE inhibitors, dry cough, single nucleotide polymorphisms, adverse effects, SLCO1B1
Citation: Sheikh AF, Munawar N, Nawaz R, Khan H, Rafique M, Jahan F and Ahmed S (2024) Association of SLCO1B1 gene variants with angiotensin-converting enzyme inhibitor-induced cough in a Pakistani hypertensive cohort. Front. Pharmacol. 15:1441251. doi: 10.3389/fphar.2024.1441251
Received: 30 May 2024; Accepted: 20 August 2024;
Published: 04 September 2024.
Edited by:
Luis Abel Quiñones, University of Chile, ChileReviewed by:
Dyah Aryani Perwitasari, Ahmad Dahlan University, IndonesiaIshaq Khan, Massachusetts Institute of Technology, United States
Copyright © 2024 Sheikh, Munawar, Nawaz, Khan, Rafique, Jahan and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nayla Munawar, bm11bmF3YXJAdWFldS5hYy5hZQ==; Sagheer Ahmed, c2FnaGVlci5zY3BzQHN0bXUuZWR1LnBr
 Arooj Fatima Sheikh
Arooj Fatima Sheikh Nayla Munawar
Nayla Munawar Rukhsana Nawaz
Rukhsana Nawaz Hizbullah Khan
Hizbullah Khan Mehwish Rafique6
Mehwish Rafique6 Sagheer Ahmed
Sagheer Ahmed