
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 15 January 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1437022
This article is part of the Research TopicThe Molecular Mechanism in Anti-tumor Therapy ResistanceView all 14 articles
Triple-negative breast cancer (TNBC) is a type of breast cancer with lack the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). It is the most aggressive breast cancer and the most difficult to treat due to its poor response to treatments and extremely invasive characteristics. The typical treatment for TNBC frequently results in relapse because of the lack of particular treatment choices. It is urgent to focus on identifying a workable and effective target for the treatment of TNBC. Cancer metastasis is significantly influenced by epithelial-mesenchymal transition (EMT). Ferroptosis is an iron-dependent cell death form, and changes its key factor to affect the proliferation and metastasis of TNBC. Several reports have established associations between EMT and ferroptosis in TNBC metastasis. Furthermore, non-coding RNA (ncRNA), which has been previously described, can also control cancer cell death and metastasis. Thus, in this review, we summarize the correlation and pathways among the ferroptosis, EMT, and ncRNAs in TNBC metastasis. Also, aim to find out a novel strategy for TNBC treatment through the ncRNA-ferroptosis-EMT axis.
Breast cancer (BC) remains the most prevalent gynecological malignancy worldwide (Harbeck and Gnant, 2017). Based on the expression of molecular markers, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), BC can be categorized into three subtypes: luminal-like (hormone receptor (HR)+/HER2-), HER2 positive (HER2+), and triple-negative (HR-, HER2-) breast cancer (Yeo and Guan, 2017; Barzaman et al., 2020). Triple-negative breast cancer (TNBC) is distinguished by the absence of ER, PR, and HER2 expression, which makes up around 15% of all breast cancer. Subsequently, a study analyzed the gene expression profiles of 587 TNBC patients and classified TNBC into six subtypes, which were basal-like 1 (BL1), basal-like 2 (BL2), luminal androgen receptor (LAR), immunomodulatory (IM), mesenchymal (M), and mesenchymal stem-like (MSL) (Lehmann et al., 2011). Due to differences in gene expression, each TNBC subtype is differently sensitive to treatment. For instance, the M subtype is susceptible to resistance to chemotherapeutic agents, and the LAR subtype is more effectively treated with anti-AR therapy (Yin et al., 2020). Thus, TNBC now is recognized as a refractory illness due to the lack of well-defined molecular targets (Lehmann et al., 2011; Jin et al., 2021). The manifestations of TNBC include increased angiogenesis and epithelial-mesenchymal transition (EMT), and it has more invasive clinical features compared with other two types of breast cancer (Bao et al., 2021). Moreover, TNBC is more likely to relapse and its overall survival rate is shorter than other types of breast cancer (Dastjerd et al., 2022). Unfortunately, current therapies, like radiation, immunotherapy, and chemotherapy, are not effective due to their serious side effects and multidrug resistance (Dastjerd et al., 2022; Ben-Dror et al., 2022; Kaur and Jaitak, 2019). Therefore, investigation of the novel targets for TNBC treatment becomes urgent issues.
Ferroptosis is a brand-new type of cell death that has been identified as an iron-caused and reactive oxygen species (ROS)-dependent cell death (Mou et al., 2019). In contrast to atypical apoptosis and necrosis, the characteristics of ferroptosis are mainly a decrease in cell volume and an augmentation of mitochondrial membrane density (Yu et al., 2017). Because of the presence of oxidative stress and severe membrane lipid peroxidation, plasma membrane loses the selective permeability, then resulting the above changes in cell morphology (Mou et al., 2019). Ferroptosis is caused by either restricting cysteine absorption or inactivating glutathione peroxidase 4 (GPX4), thus creating an accumulation of ROS (Latunde-Dada, 2017). Currently, ferroptosis mechanisms in cancers have become a hot topic. The latest progress shows that the three main pathways of regulating cellular to ferroptosis are the GSH-GPX4, GCH1-BH4 and NADPH-FAP1-CoQ10 (Wang H. et al., 2021). More and more evidences indicate that ferroptosis could inhibit tumor cell growth and development, therefore, targeting ferroptosis might be an effective treatment of cancer (Li Z. et al., 2020).
Cancer metastasis is regarded as a process of concurrence and partially reduplication, and play a critical role in cancer death (Suhail et al., 2019). The potential of primary malignancies to spread to neighboring tissue and far-off organs is noteworthy with breast tumors primary migrating to bone, lungs, liver, and brain, then tumor recurrence is caused in corresponding sites (Howard et al., 2022; Nguyen et al., 2009). Notably, extensive evidences have been presented that EMT program is a key factor in tumor metastasis (Williams et al., 2019; Park et al., 2022). Moreover, it is believed that EMT is a factor in the spread of various tumors, including breast, colorectal, ovarian, pancreatic, prostate and renal (Yao J. et al., 2021; Lambert et al., 2017). This morphogenetic transition, which causes epithelial cells to acquire mesenchymal characteristics, is associated with a variety of tumor functions, such as tumor initiation, invasiveness, metastasis, and chemoresistance of malignant tumors, as well as tumor stemness (Babaei et al., 2021; Pan et al., 2021; Pastushenko and Blanpain, 2019).
According to the Human Genome Project, the vast majority of the human genome is made up of non-protein-coding regions, which were largely transcribed into non-coding RNAs (ncRNAs) (Xu et al., 2020). Non-coding RNA ordinarily refers to RNA that does not encode protein, but contains information or has some functions (Mattick and Makunin, 2006). Based on their length, non-coding RNA approximately can be split into long non-coding RNAs (lncRNAs) and short non-coding RNAs (sncRNAs), and the latter include small interfering RNA (siRNAs), small nucleolar RNAs (snoRNAs), microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), and so on (Xu et al., 2020). miRNA, lncRNA, circRNA, and piRNA were major types of ncRNA, and they played various functions among cancers (Yan and Bu, 2021). Moreover, as oncogenes or suppressor genes, non-coding RNAs could reduce or suppress the incidence and development of TNBC (Liu J. et al., 2021). Previous researches show the expression of miRNA related to TNBC cells metastasis, and EMT was regarded as the main mechanism of cancer cell metastasis. Take miR-125b for an example, its lower expression in TNBC cells is accompanied by the decrease of cell migration and invasion (Xu et al., 2020). Of note, ncRNAs regulate the potential mechanism of ferroptosis, though mitochondrial-related proteins and peroxidation of lipid, iron or glutathione. More and more studies have shown that the role of non-coding RNA regulation breast cancer progression via ferroptosis, and presumably coupled with EMT (Zuo et al., 2022). In this review, we aim to summarize the relationship between ferroptosis and EMT in TNBC progression, highlight the significance of ncRNAs in the development of TNBC, and suggest prospective therapeutic approaches for TNBC treatment.
Nowadays, more and more studies present TNBC behaves susceptibility to ferroptosis, and highlight the pathway as a treatment of TNBC (Verma et al., 2020; Sun L. L. et al., 2021; Doll et al., 2017). In 2012, Dixon proposed the concept of ‘Ferroptosis’ to explain a cell death process reliant on iron. They also confirmed that the small molecule ferrostatin-1 (Fer-1) acts as a telling ferroptosis inhibitor. Fer-1 prevents the accumulation of ROS in cytoplasm and lipid induced by erastin and shows no inhibition in the MEK/ERK pathway, chelated iron and synthesis of protein. Erastin restrains the assimilation of cystine by cystine/glutamic acid antiporter (system XC-), which destroys the antioxidant defense of cells and eventually leads to iron-dependent oxidative death (Dixon et al., 2012). Then 10 years after the concept of ferroptosis was put forward, the lab systematically concluded the key discoveries associated with ferroptosis during the time. In 2014, they reported GPX4 as an important inhibitor to ferroptotic cancer cell death (Stockwell, 2022). The inactivation of GPXs, when treated with erastin or BSO, leads to the buildup of ROS in both cytoplasm and lipid. It was also found that GPX4 modulates ferroptosis induced by 12 diverse compounds (Yang et al., 2014). Moreover, p53, ACSL4, and LPCAT3 were corroborated as promoters to ferroptosis (Stockwell, 2022). Subsequently, several discoveries, such as ALOXs, CD8+ T cells, FSP1-CoQ10, GCH1/BH4, radiation, and DHODH, related to ferroptosis were successively reported (Stockwell, 2022).
Dixon’s research uncovered a divergence between erastin-induced cell death and apoptosis, necrosis and other types of cell death, with distinct morphological, biochemical and genetic distinctions. From the morphological point of view, mitochondria shrink, membrane density increases and mitochondrial cristae diminishes or vanishes (Dixon et al., 2012). Nonetheless, the cell membrane maintains integrated, and the size of nucleus is regular (Dixon et al., 2012; Li J. et al., 2020). Biochemically, ferroptosis is mainly reflected in iron accumulation and lipid peroxidation. On the one hand, iron produces redundant ROS directly by Fenton reaction, thus aggravating oxidative damage. On the other hand, iron actives lipoxygenase (ALOX) or EGLN prolyl hydroxylases to regulate lipid peroxidation and oxygen homeostasis indirectly (Tang et al., 2021). In genetics, a group of diverse genes modulate ferroptosis (Dixon et al., 2012). Taking PTGS2 (a gene encoding cuclooxygenase-2) as example, upregulation of PTGS2 is regarded as a downstream maker of ferroptosis (Yang et al., 2014). Furthermore, the genes related to GSH system, Coenzyme Q10 (CoQ10) system, NFE2L2 transcription pathway and other antioxidant defenses or connected with membrane repair (e.g., ESCRT-III pathway) effectively control the membrane damage during ferroptosis (Tang et al., 2021).
Nowadays, various studies have given the evidence that inductive ferroptosis has promising prospects in the treatment of cancer and the characteristic of cell metastasis is resistance to ferroptosis. It is also proved that tumors with resistance to traditional therapy or highly metastasis tendency show more sensitivity to ferroptosis induction (Liu W. et al., 2021; Jiang et al., 2023; Lu et al., 2017). Interestingly, TNBC exhibited ferroptosis heterogeneity. GPX4 inhibitors might be used to treat luminal androgen receptor (LAR)-subtype TNBC through androgen receptor (AR) signaling pathway (Jiang et al., 2023). Theoretically, we might discover the correlation between ferroptosis and TNBC treatment, through a comprehensive and deepgoing retrospective analysis.
Hepatic leukemia factor (HLF), an innovative oncoprotein, enhanced ferroptosis resistance by transactivating gamma-glutamyltransferase 1 (GGT1), then prompting proliferation and metastasis of TNBC (Li H. et al., 2022). Additionally, MUC1-C/xCT axis inhibits ferroptosis and then contributes to the survival rate of TNBC cells. Erastin, on the other hand, regulates the mechanism that causes TNBC ferropotic cell death (Hasegawa et al., 2016). Meanwhile, other study has also shown that erastin united with anti-CD24 antibody could restrain the growth of tumor and enhance the animal survival rate (Hou et al., 2022). Several studies proved that nanoparticles, which can alleviate the drug resistance, were used to the treatment of TNBC by inducing ferroptosis (Chen et al., 2023; Zhang J. et al., 2021). For example, Yang et al. highlight that rosuvastatin (RSV) wrapped in silk fibroin (SF) nanoparticle, also was called Cu-SF(RSV) NPs, inhibits TNBC by conquering FPS1-medicated ferroptosis resistance. Moreover, RSV could hinder neoplasm metastasis through lowering the expression of MMP9, which is simulated by tumor derivative factors. Particularly, it was found that Cu-SF(RSV) NPs efficiently inhibited 4T1 tumor metastasis, and included in the consideration of assistant drugs for breast cancer (Yang et al., 2022). Through the above examples, it is possible that ferroptosis-related pathways or regulators can control the proliferation and metastasis of TNBC cells (Figure 1).
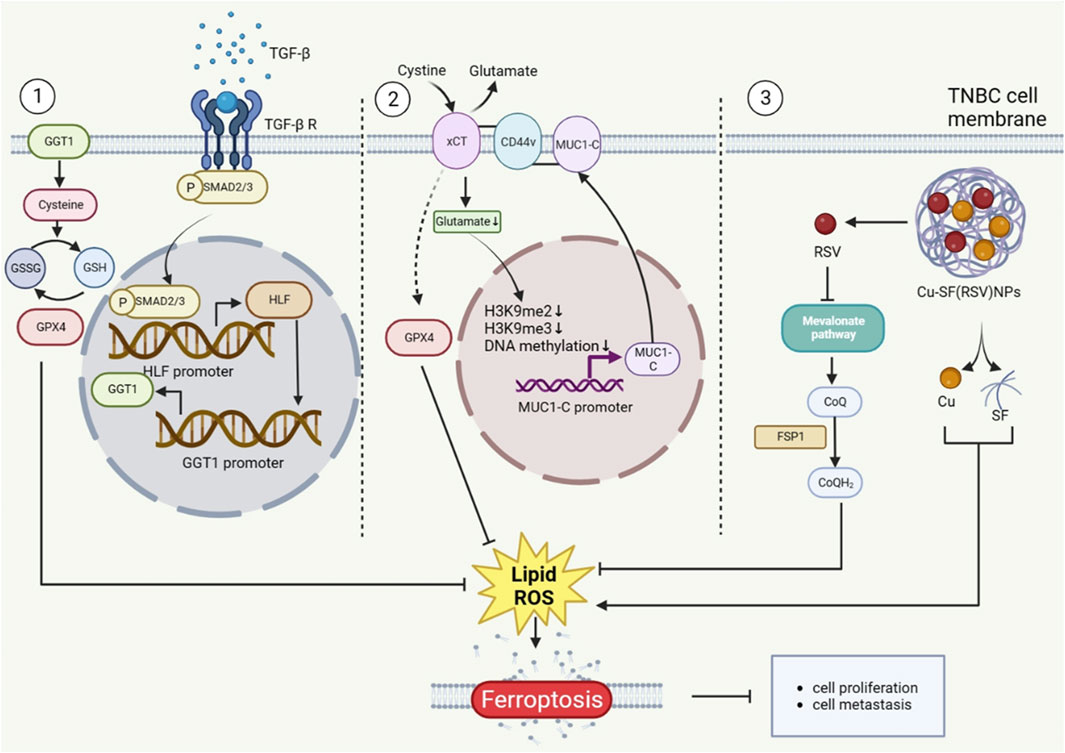
Figure 1. The Role of ferroptosis in TNBC metastasis. The proteins that TNBC cells make play a function in ferroptosis regulation, which controls the proliferation of TNBC cells. Additionally, Cu-SF(RSV) NPs operate on the CoQ10 mechanism that controls ferroptosis. The figure was created using BioRender.com.
Regarded as one of the most essential components for tumor metastasis, the epithelial-to-mesenchymal transition (EMT) is distinguished with a process of diminishing polarity and intercellular adhesion, while augmenting the capacity of migration and invasion (Yao J. et al., 2021). EMT medicated tumor metastasis under the control of transcriptions factors, such as TWIST1, SNAI1, and ZEB1 (Chen et al., 2021a; van Staalduinen et al., 2018). The latest evidence has revealed that over 90% of TNBC patients died as a result of cancer cells migrating from the primary tumor to distant organs (Yang et al., 2022). Recent study shows that the majority of TNBC cells with the CD24high phenotype expressed less E-cadherin and more vimentin and N-cadherin. This also proves that TNBC metastasis is surely accompanied by EMT program (Hou et al., 2022). Furthermore, abnormal activation of EMT in TNBC could increase the invasion, progression and recurrence ratio of tumor (Bao et al., 2021). Coenzyme Q0 (CoQ0), with high oxidation resistance, inhibits EMT via activating E-cadherin via the Wnt/β-catenin pathway and the NFκB pathway (Yang et al., 2019). Evidences show that Wnt/β-catenin pathway participates in proliferation and metastasis of TNBC. Moreover, Wnt/β-catenin can be activated by the KIF23, thereby promoting EMT in TNBC. Downregulation of KIF23 possibly influencing the mitotic function of TNBC cells (Li Z. et al., 2022). RNA N6-methyladenosine (m6A) reader YTHDC1 can enhance TGF-β-mediated EMT and improve the survival rate of TNBC cells by enhancing the nuclear output of SMAD3 mRNA (target RNA of YTHDC1) (Tan et al., 2022). Mesenchymal and stem cell-like cells make up a significant component of TNBC cells. GSK3β inhibitor is identified as a small molecule inhibitor for inhibiting EMT, and it is also capable of selectively inhibiting mesenchymal and stem cells while maintaining cells with epithelial features. Additionally, GSK3β is known as the only target associated with TNBC patient prognosis in the Wnt pathway (Vijay et al., 2019). The inhibition of tumor metastasis by RAB1B was discovered to be drastically reduced in TNBC. Lack of RAB1B could reduce the degradation of ubiquitin and increase the phosphorylation of SMAD3, thus highly expressing the protein levels of TGF-β receptor 1 (TβR1) and activating TGF-β signal pathway. Additionally, RAB1B can trigger TGF-β-induced EMT, and increase the proliferation and migration of TNBC cells both in vitro and in vivo (Jiang et al., 2015). Studies have demonstrated that MUC1-C causes EMT in breast cancer stem cell (CSC), via acting on SOX2, KLF4 and OCT4 to induce the dedifferentiation of TNBC (Kufe, 2020; Yamashita and Kufe, 2022). Moreover, the mesenchymal TNBC cell lines have increased expression of RNA binding motif single stranded interacting protein 3 (RBMS3), which controls EMT in TNBC (Block et al., 2021). Previous research proved that TNBC cells express more programmed death ligand 1 (PD-L1) than the other two types of breast cancer. Chen et al. demonstrated that PD-L1 induces EMT in TNBC cells through protecting EMT transcription factor snail from being distrusted. Mechanically, PD-L1 could activate p38-MAPK and inhibit GSK3β signalling pathway, thus preventing the phosphorylation, ubiquitination, and degradation of snail (Chen C. et al., 2021) (Figure 2).
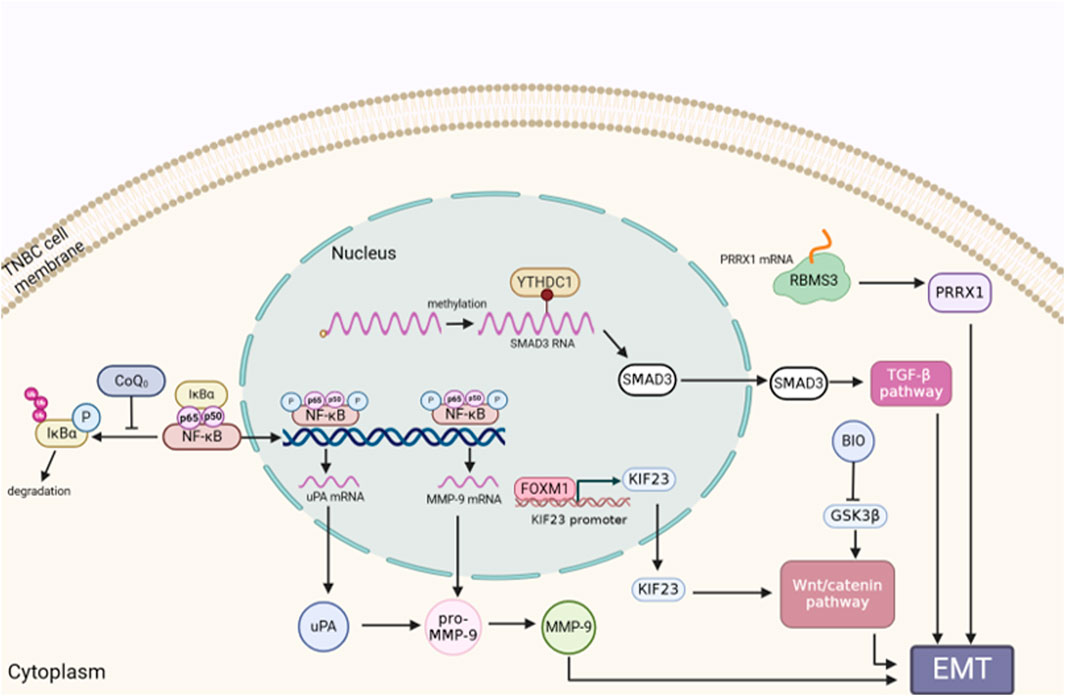
Figure 2. The role of EMT in TNBC metastasis. EMT is a factor in the migration, invasion, and development of TNBC cells. Genes or proteins that control EMT are correlated with the appropriate signaling pathway. The figure was created using BioRender.com.
Previous research has proved that mesenchymal-high cancers behave intensive sensibility to ferroptosis inducers, particularly GPX4 inhibitors (Bi et al., 2019). Besides, as a positive EMT regulator, protein LYRIC could contribute to ferroptosis through restraining the expression of GPX4 and SLC3A2 (Chen X. et al., 2021). The abundance of DDR2 expression activated by EMT in recurrent breast cancer cells, resulting in a heightened susceptibility to ferroptosis has been noted (Lin et al., 2021). However, the relationship between ferroptosis and EMT in triple negative breast cancer progression remains largely unexplored. Ferroptosis and EMT are the two crucial procedures and closely contact reciprocally in tumor cells (Yao J. et al., 2021). As reported, mesenchymal cancer cells are sensitive to ferroptosis, while cancer cells with EMT or metastasis tendencies are more vulnerable to ferroptosis (Wu et al., 2019). A prior study demonstrated that CSPP1 may influence ferroptosis via controlling EMT, stromal, and immunological responses in low-grade gliomas (LGG) in the brain (Wang W. et al., 2022). Additionally, it has been shown that EMT pathways can give positive feedback to ferroptosis (Chen X. et al., 2021). The increase of CD44-mediated hyaluronate-dependent iron endocytosis enhances the expression of EMT-related genes, which makes MDA-MB-468 cells more sensitive to ferroptosis (Chen X. et al., 2021; Müller et al., 2020). Hepatic leukemia factor (HLF), which acts as a novel oncoprotein in TNBC, enhanced ferroptosis resistance by transactivating gamma-glutamyl transferase 1 (GGT1), then prompting the proliferation and metastasis of TNBC (Li H. et al., 2022). However, HLF was regulated by transforming growth factor-beta 1 (TGF-β1), which is ascertained as an inducer of EMT and trigger ferroptosis in isolated breast cancer cells (Li H. et al., 2022; Wu et al., 2019; Yang et al., 2020). In Wu et al.’s study, they demonstrated that the intracellular Merlin-Hippo route, which is regulated by E-cadherin, can decrease ferroptosis in epithelial cells (Wu et al., 2019). Taken together, the above findings suggested that EMT acts as a regulator to enhance ferroptosis sensitivity. It should be noted that ferroptosis might have a positive effect on EMT program. Yang et al.'s work revealed that the markers GPX4, SCP2, and CAV1 of ferroptosis also influence the EMT features (Yao J. et al., 2021). In panc-1 CSCs, knockdown or overexpression of GPX4 can modulate EMT by upregulating E-cadherin and downregulating vimentin, Slug and Snail expression at the mRNA and protein levels (Peng et al., 2019) (Figure 3). Based on these studies, we hypothesize that there is a mutual regulation between ferroptosis and EMT. Since there is little research being conducted in the background of TNBC cells, with a focus on causing TNBC metastasis, whether EMT-regulated ferroptosis or ferroptosis-regulated EMT predominates still needs to be done in the future.
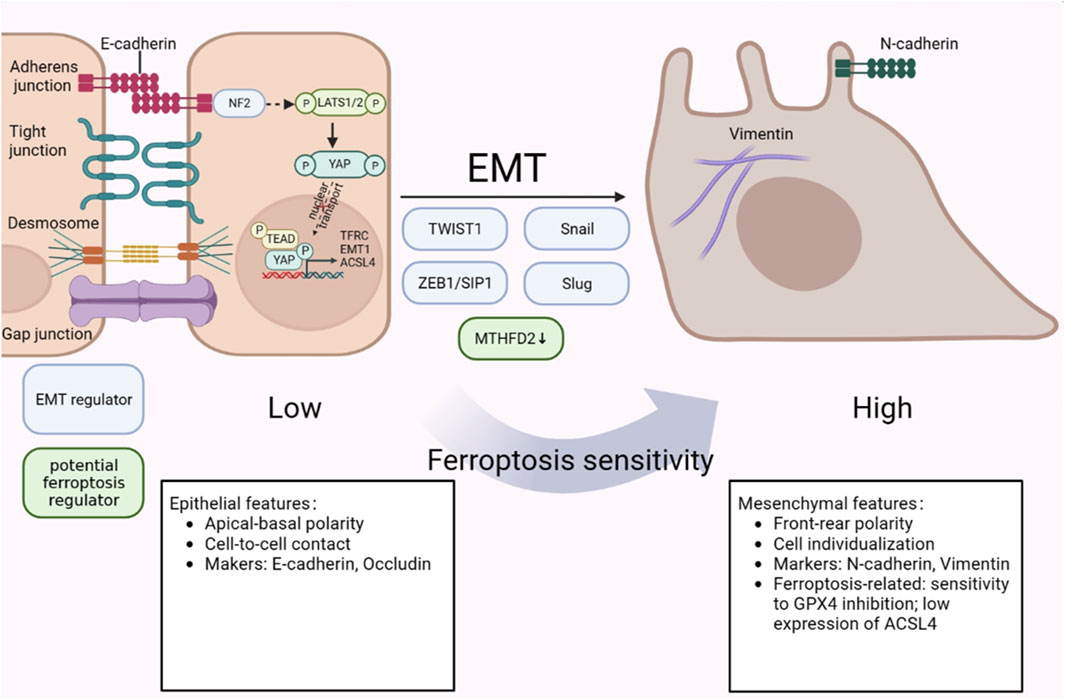
Figure 3. The crosstalk between ferroptosis and EMT in TNBC metastasis. Mesenchymal cells have higher ferroptosis susceptibility than epithelial cells, which are less sensitive to it. NF2-YAP pathway exhibits an inhibitory effect on ferroptosis regulators. In addition, HLF, which is regulated by TGF-β, controls ferroptosis sensitivity by targeting GGT1. The figure was created using BioRender.com.
More and more evidences showed that non-coding RNA (ncRNA) is closely related to the cancer progression and ncRNAs can serve as regulators to play a role in tumor development process, such as tumor invasion and metastasis (Yan and Bu, 2021; Sakamoto et al., 2017). As reported, there are two possible modes to interpret the relationship between non-coding RNA, especially miRNA, and the development of tumor. One is that the expression of neoplastic suppressor genes might be suppressed by the upregulation of miRNA, thus promoting tumorigenesis. Another is that the expression of associated oncogenes might be promoted by the downregulation of miRNA, thus similarly triggering tumorigenesis (Duan et al., 2021; Caldas and Brenton, 2005). For example, the depletion of circRHOT1 enhanced erastin-induced growth inhibition in MDA-MB-231 TNBC cells. When circRHOT1 expression is inhibited, Fe and ROS expression levels are elevated and GPX4 and SLC7A11 expression is weakened. In their study, they highlighted that circRHOT1 sponged miR-106a-5p to inhibit ferroptosis in breast cancer (Zhang H. et al., 2021). Furthermore, Yadav et al. proved that miR-5096 regulates ferroptosis by targeting and downregulation of SLC7A11. When miR-5096 is expressed ectopically, it enhances the accumulation of iron and oxidation of GSH, which leads to ferroptosis. Additionally, they discovered that miR-5096 promoted the expression of EMT makers in vitro and inhibited the propensity for metastasis in MDA-MB-231 cells in vivo (Yadav et al., 2021). Liang et al. has identified that circular RNA circ-ABC10 shows high expression in breast cancer, compared with peripheral non-cancerous zones. The evidence was strengthened by the discovery that miR-1271 targets circABC10 by bioinformatics analysis and luciferase reporter assay. Remarkably, the reduction of circ-ABC10 triggered cell apoptosis enhancement but there is no direct evidence linking this to ferroptosis (Liang et al., 2017). All of these provide theoretical justifications for how ncRNAs control ferroptosis in TNBC cells.
Additionally, ncRNAs can regulate the metastasis of TNBC by EMT program. Has_circ_001783 was high-level expressed in breast cancer cells, and is obviously upregulated in TNBC cells in the contrast of other two types of breast cancers. When the has_circ_001783 expression level was knocked down, it would inhibit the proliferation and metastasis of TNBC cells. Also, it is confirmed that miR-200c-3p is negatively related with the sponging target of has_circ_001783. Namely, low expression of has_circ_001783 triggered the expression of miR-200c-3p. Furthermore, a decrease in has_circ_001783 expression prevents the EMT regulators ZEB1, ZEB2, and ETS1 from regulating TNBC metastasis (Liu et al., 2019). In addition, EIF6-224aa, transformed from circ-EIF6, directly binds to MYH9 protein and inhibits its degradation. Then wnt/β-catenin pathway was triggered, facilitating circ-EIF6 to promote the proliferation and metastasis of TNBC (Li Y. et al., 2022). Moreover, long non-coding RNAs are also frequently used to diagnose and prognosticate the tumor development. Certainly, there are several lncRNAs regulate the development of TNBC (Kaushik et al., 2020). In addition to the relevant downstream target or pathway acting as an upstream regulator, lncRNA can also be targeted by upstream genes. For instance, TUFTI promotes the progression of TNBC by upregulating the expression of the lncRNA DANCR via the miR-874-3p-SOX2 pathway (Wu et al., 2020) (Figure 4; Table 1).
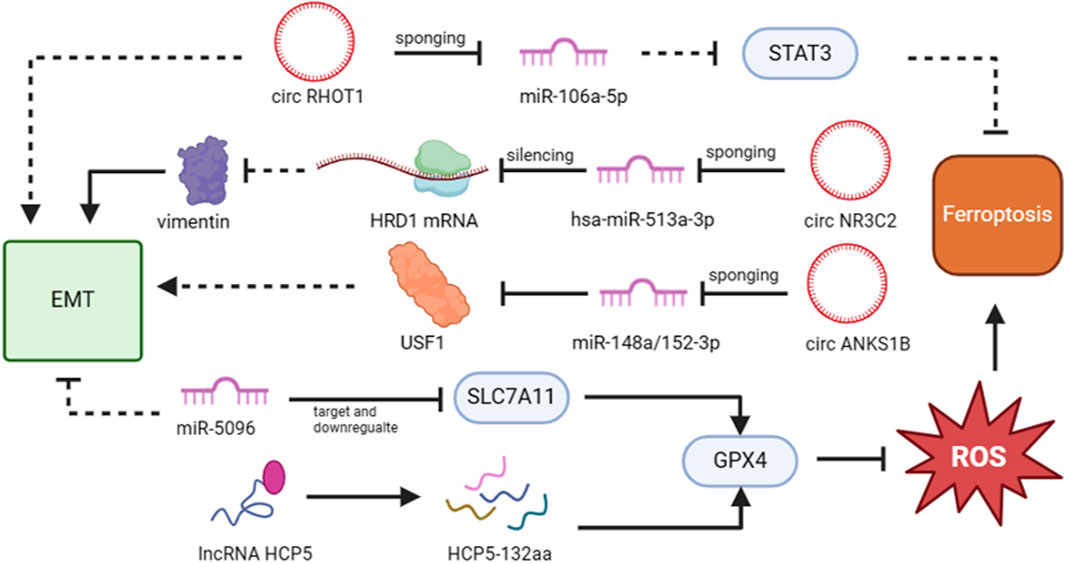
Figure 4. The functions of non-coding RNAs that target ferroptosis and EMT in TNBC metastasis. Non-coding RNAs that target EMT/ferroptosis-related pathways or proteins can have either a favorable or unfavorable impact on the metastasis of TNBC cells. The figure was created using BioRender.com.
For years, chemotherapy has been the primary option for TNBC patients who have lower survival ratio and worst prognosis because of its high metastasis incidence and lack of effective post-recurrence treatment (Adinew et al., 2021; So et al., 2022). The likelihood of TNBC’s response to immunotherapy is heightened by several characteristics of TNBC cells, such as augmented tumor-infiltrating lymphocytes and augmented expression of programmed death-ligand 1 (PD-L1) (Keenan and Tolaney, 2020). Unfortunately, it has been discovered that the PD-L1 inhibitor monotherapies avelumab and atezolizumab are insufficient for treating metastatic triple negative breast cancer (mTNBC). However, early-stage TNBC patients have shown evidence of the initial efficacy of using ICIs in combination with chemotherapy (Keenan and Tolaney, 2020).
Additionally, data demonstrates an apparent benefit of chemotherapy when paired with neoadjuvant, adjuvant, and metastatic settings (Bianchini et al., 2016). Anti-programmed death-ligand 1 (anti-PD-L1) antibodies applying to the treatment of PD-L1-positive mTNBC, can improve progression-free survival (PFS) and overall survival (OS) of patients (Loibl et al., 2022). A randomized controlled trial (RCT) revealed that based on anthracycline/taxane neoadjuvant chemotherapy (NACT), added with durvalumab (the PD-L1 inhibitor), increased the pathological complete response (pCR) rate in TNBC patients (Loibl et al., 2019). Another RCT found that giving durvalumab to TNBC patients as a maintenance therapy improved their overall survival, particularly in those with PD-L1+ TNBC (Bachelot et al., 2021).
As described above, the ncRNA, EMT, and ferroptosis plays significant roles in TNBC progression. Here, we hope to find a genuine treatment for TNBC patients, starting with non-coding RNA targeting ferroptosis or/and EMT. There is evidence that TNBC cells are sensitive to ferroptosis, and this suggests a potential therapeutic option for TNBC that involves promoting this type of non-apoptotic cell death (Sun L. L. et al., 2021). Obvious study demonstrated that metformin regulated ferroptosis in MDA-MB-231 cells. Its mechanism is that metformin upregulated the expression of miR-324-3p, which directly binds to the 3′UTR of GPX4 to downregulate its expression. And the metformin plays the role of anti-cancer by suppressing EMT and metastasis features (Hou et al., 2021). Moreover, in MDA-MB-468 TNBC cells, metformin induced many associated enzymes for glucose metabolism, suggesting that metformin may be used in the treatment of TNBC (Samuel et al., 2019). Local anesthetic lidocaine downregulated SLC7A11 to induce ferroptosis by targeting miR-382-5p and promoting its expression in breast cancer cells (Sun D. et al., 2021). In addition, lidocaine is cytotoxic to the MDA-MB-231 and BT-474 breast cancer cell lines, and researchers found that in these two cell types, lidocaine reduced ROS without the discussion of ferroptosis (Chen et al., 2022). Consequently, the modulation of non-coding RNA expression by drugs exerts regulatory control over both EMT and/or ferroptosis, thereby influencing a profound impact on the progression of TNBC.
Considering on another perspective, can ncRNAs modulate treatment resistance in TNBC by affecting EMT and/or ferroptosis? Paclitaxel (PTX), an anti-tumor drug, is a crucial part of the chemotherapeutic treatment for TNBC. Downregulation of circWAC, which targets miR-142, increased the sensitivity to PTX in TNBC cells (Wang L. et al., 2021). The utilization of crizotinib, an anti-ROS1 agent, has demonstrated resistance in the treatment of triple-negative breast cancer (TNBC). Subsequent investigation revealed that TNBC cells resistant to crizotinib exhibited elevated levels of FAK expression. By combining the FAK inhibitor IN10018 with crizotinib, there was an increase in ROS levels, upregulation of p53 expression, and downregulation of SCL7A11 and GPX4. Ultimately, this led to ferroptosis and exerted potent anti-tumor effects (Tan et al., 2024). Evidence shows that the as-obtained Fe3O4@PCBMA-SIM nanoparticles show more cytotoxicity to MAD-MB-231. Since SIM can suppress the expression of HMGCR, leading to the downregulation of the mevalonate (MVA) pathway and GPX4, ultimately inducing ferroptosis (Yao X. et al., 2021). Moreover, the combined use of CPT/Fe@PDA-FA10 and laser treatment demonstrates remarkable efficacy in inducing apoptosis/iron death/photothermal therapy in drug-resistant TNBC cells. Remarkably, this approach exhibits negligible adverse effects on major tissues and organs (Cai et al., 2023). The construction of tumor-targeted combinatorial nanoparticles can effectively induce ferroptosis, thereby overcoming tumor resistance in triple-negative breast cancer.
The improvement of tumor therapy efficacy is achieved by employing nano-prodrugs for targeted delivery of drugs with different mechanisms of action to the tumor microenvironment (Chen et al., 2017; Wu et al., 2023). Actively targeted small molecule self-assembled nano-prodrug are employed for the co-delivery of chemotherapeutic agents (CPT), ferrocene (Fc), and GPX4 inhibitors (RSL3). Upon reaching the tumor microenvironment, this nano-prodrug undergoes GSH-mediated degradation to facilitate the release of encapsulated substances, thereby augmenting the efficacy of chemotherapeutic agents through induction of cellular iron death and apoptosis (Chen et al., 2023).
Non-coding RNAs have essential functions in the drug-therapeutic resistance of TNBC; miRNA and lncRNA, in particular, play significant roles in chemoresistance by regulating a variety of genes and related pathways (Ferrari et al., 2022). MCT-1, a poor prognosis marker of invasive breast cancer, further induces STAT3 activation and the expression of snail, ZEB1, and N-cadherin to accelerate EMT through the IL-6 pathway. When treated with an MCT-1 antagonist, the miR-34a can reduce IL-6 expression, which increases the therapeutic effectiveness of TNBC (Weng et al., 2019). Repression of miR-155-5p expression in TNBC cells attenuates the resistance of cetuximab through the induction of cell apoptosis and pyroptosis (Xu et al., 2021). However, the impact of reducing miR-155-5p levels on drug resistance through ferroptosis remains to be elucidated. The overexpression of miR-33a-5p resulted in the inhibition of eIF5A2 expression and attenuated the EMT process induced by doxorubicin (Dox), ultimately confirming its ability to enhance the sensitivity of TNBC cells to Dox (Guan et al., 2019). LncRNA DLX6-AS1 prompts EMT and cisplatin resistance in TNBC scenario through miR-199b-5p/PXN pathway (Du et al., 2020). However, it did not clearly elucidate the association between EMT and cisplatin resistance within this signaling pathway.
Triple-negative breast cancer is the most aggressive type of breast cancer, which exhibits poor prognosis and high recurrence (Won and Spruck, 2020; Vagia et al., 2020). The treatments that are currently available are insufficient to treat TNBC tumors that are inoperable or recurring. A large unmet need still exists for the development of innovative therapeutics for TNBC, particularly in advanced stages, to inhibit the metastasis of TNBC, and improve the prognosis of breast cancer patients. Epithelial-mesenchymal transition and ferroptosis are major biological processes in cancer metastasis, and there are various signaling pathways and non-coding RNAs play a complex role in both ferroptosis and the EMT processes.
In this review, we briefly introduced the discovery and hallmarks of ferroptosis, as well as the process of EMT, and focused on summarizing the roles of ferroptosis and EMT in TNBC progression. Moreover, we also discussed how ncRNAs regulate ferroptosis and EMT, as well as the functional roles in regulation metastasis of breast cancer. However, there remains a few unresolved inquiries. 1) To date, only a few studies have elucidated the crosstalk between ferroptosis and EMT in neoplastic processes, including Panc-1 cancer stem-like cells, lung adenocarcinoma, and breast cancer (Yao J. et al., 2021; Chen X. et al., 2021; Müller et al., 2020; Peng et al., 2019). Further investigation is necessary to elucidate the underlying mechanism linking ferroptosis and EMT in the context of TNBC. 2) Numerous targeted non-coding RNAs have been identified to play pivotal roles in the processes of EMT or ferroptosis. However, there is still limited literature available that directly demonstrates the presence of non-coding RNAs regulating both EMT and ferroptosis in TNBC.
Taken together, in this review, we collected the link between ferroptosis, EMT, and ncRNAs in the metastasis processes of TNBC. While the further study still needs to be done to explore the underlying mechanisms of the ncRNAs/ferroptosis/EMT axis in TNBC progression. This interplay may facilitate the development of potential therapeutic approaches to eliminate cancer cells, and provide a comprehensive understanding of the metastasis mechanism of TNBC to identify novel treatment strategies.
ZC: Data curation, Methodology, Software, Writing–original draft. YZ: Conceptualization, Funding acquisition, Methodology, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82202926); China Postdoctoral Science Foundation Special Funding (2023T160346); Qingdao postdoctoral Applied Research Project (QDBSH20230101025).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adinew, G. M., Taka, E., Mendonca, P., Messeha, S. S., and Soliman, K. F. A. (2021). The anticancer effects of flavonoids through miRNAs modulations in triple-negative breast cancer. Nutrients 13 (4), 1212. doi:10.3390/nu13041212
Babaei, G., Aziz, S. G., and Jaghi, N. Z. Z. (2021). EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed. Pharmacother. 133, 110909. doi:10.1016/j.biopha.2020.110909
Bachelot, T., Filleron, T., Bieche, I., Arnedos, M., Campone, M., Dalenc, F., et al. (2021). Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 27 (2), 250–255. doi:10.1038/s41591-020-01189-2
Bao, X., Shi, R., Zhao, T., Wang, Y., Anastasov, N., Rosemann, M., et al. (2021). Integrated analysis of single-cell RNA-seq and bulk RNA-seq unravels tumour heterogeneity plus M2-like tumour-associated macrophage infiltration and aggressiveness in TNBC. Cancer Immunol. Immunother. 70 (1), 189–202. doi:10.1007/s00262-020-02669-7
Barton, M., Santucci-Pereira, J., Vaccaro, O. G., Nguyen, T., Su, Y., and Russo, J. (2019). BC200 overexpression contributes to luminal and triple negative breast cancer pathogenesis. BMC Cancer 19 (1), 994. doi:10.1186/s12885-019-6179-y
Barzaman, K., Karami, J., Zarei, Z., Hosseinzadeh, A., Kazemi, M. H., Moradi-Kalbolandi, S., et al. (2020). Breast cancer: biology, biomarkers, and treatments. Int. Immunopharmacol. 84, 106535. doi:10.1016/j.intimp.2020.106535
Ben-Dror, J., Shalamov, M., and Sonnenblick, A. (2022). The history of early breast cancer treatment. Genes (Basel) 13 (6), 960. doi:10.3390/genes13060960
Bi, J., Yang, S., Li, L., Dai, Q., Borcherding, N., Wagner, B. A., et al. (2019). Metadherin enhances vulnerability of cancer cells to ferroptosis. Cell Death Dis. 10 (10), 682. doi:10.1038/s41419-019-1897-2
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E., and Gianni, L. (2016). Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13 (11), 674–690. doi:10.1038/nrclinonc.2016.66
Block, C. J., Mitchell, A. V., Wu, L., Glassbrook, J., Craig, D., Chen, W., et al. (2021). RNA binding protein RBMS3 is a common EMT effector that modulates triple-negative breast cancer progression via stabilizing PRRX1 mRNA. Oncogene 40 (46), 6430–6442. doi:10.1038/s41388-021-02030-x
Cai, Z., Huan, M. L., Zhang, Y. W., Zhao, T. T., Han, T. Y., He, W., et al. (2023). Tumor targeted combination therapeutic system for the effective treatment of drug resistant triple negative breast cancer. Int. J. Pharm. 636, 122821. doi:10.1016/j.ijpharm.2023.122821
Caldas, C., and Brenton, J. D. (2005). Sizing up miRNAs as cancer genes. Nat. Med. 11 (7), 712–714. doi:10.1038/nm0705-712
Chen, C., Li, S., Xue, J., Qi, M., Liu, X., Huang, Y., et al. (2021b). PD-L1 tumor-intrinsic signaling and its therapeutic implication in triple-negative breast cancer. JCI Insight 6 (8), e131458. doi:10.1172/jci.insight.131458
Chen, H., Zhang, W., Zhu, G., Xie, J., and Chen, X. (2017). Rethinking cancer nanotheranostics. Nat. Rev. Mater 2, 17024. doi:10.1038/natrevmats.2017.24
Chen, J., Shin, V. Y., Siu, M. T., Ho, J. C. W., Cheuk, I., and Kwong, A. (2016). miR-199a-5p confers tumor-suppressive role in triple-negative breast cancer. BMC Cancer 16 (1), 887. doi:10.1186/s12885-016-2916-7
Chen, J. L., Liu, S. T., Huang, S. M., and Wu, Z. F. (2022). Apoptosis, proliferation, and autophagy are involved in local anesthetic-induced cytotoxicity of human breast cancer cells. Int. J. Mol. Sci. 23 (24), 15455. doi:10.3390/ijms232415455
Chen, X., Kang, R., Kroemer, G., and Tang, D. (2021a). Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18 (5), 280–296. doi:10.1038/s41571-020-00462-0
Chen, Y., Yao, Z., Liu, P., Hu, Q., Huang, Y., Ping, L., et al. (2023). A self-assembly nano-prodrug for triple-negative breast cancer combined treatment by ferroptosis therapy and chemotherapy. Acta Biomater. 159, 275–288. doi:10.1016/j.actbio.2023.01.050
Cheng, Y., Xiang, G., Meng, Y., and Dong, R. (2016). MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod. Biol. 16 (3), 225–233. doi:10.1016/j.repbio.2016.07.002
Dastjerd, N. T., Valibeik, A., Rahimi Monfared, S., Goodarzi, G., Moradi Sarabi, M., Hajabdollahi, F., et al. (2022). Gene therapy: a promising approach for breast cancer treatment. Cell Biochem. Funct. 40 (1), 28–48. doi:10.1002/cbf.3676
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Doll, S., Proneth, B., Tyurina, Y. Y., Panzilius, E., Kobayashi, S., Ingold, I., et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13 (1), 91–98. doi:10.1038/nchembio.2239
Du, C., Wang, Y., Zhang, Y., Zhang, J., Zhang, L., and Li, J. (2020). LncRNA DLX6-AS1 contributes to epithelial-mesenchymal transition and cisplatin resistance in triple-negative breast cancer via modulating mir-199b-5p/paxillin Axis. Cell Transpl. 29, 963689720929983. doi:10.1177/0963689720929983
Duan, H., Liu, Y., Gao, Z., and Huang, W. (2021). Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm. Sin. B 11 (1), 55–70. doi:10.1016/j.apsb.2020.09.016
Ferrari, P., Scatena, C., Ghilli, M., Bargagna, I., Lorenzini, G., and Nicolini, A. (2022). Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int. J. Mol. Sci. 23 (3), 1665. doi:10.3390/ijms23031665
Guan, X., Gu, S., Yuan, M., Zheng, X., and Wu, J. (2019). MicroRNA-33a-5p overexpression sensitizes triple-negative breast cancer to doxorubicin by inhibiting eIF5A2 and epithelial-mesenchymal transition. Oncol. Lett. 18 (6), 5986–5994. doi:10.3892/ol.2019.10984
Han, J., Han, B., Wu, X., Hao, J., Dong, X., Shen, Q., et al. (2018). Knockdown of lncRNA H19 restores chemo-sensitivity in paclitaxel-resistant triple-negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol. Appl. Pharmacol. 359, 55–61. doi:10.1016/j.taap.2018.09.018
Harbeck, N., and Gnant, M. (2017). Breast cancer. Lancet 389 (10074), 1134–1150. doi:10.1016/S0140-6736(16)31891-8
Hasegawa, M., Takahashi, H., Rajabi, H., Alam, M., Suzuki, Y., Yin, L., et al. (2016). Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 7 (11), 11756–11769. doi:10.18632/oncotarget.7598
Hou, L., Pu, L., Chen, Y., Bai, Y., Zhou, Y., Chen, M., et al. (2022). Targeted intervention of NF2-YAP signaling Axis in CD24-overexpressing cells contributes to encouraging therapeutic effects in TNBC. ACS Nano 16 (4), 5807–5819. doi:10.1021/acsnano.1c10921
Hou, Y., Cai, S., Yu, S., and Lin, H. (2021). Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim. Biophys. Sin. (Shanghai) 53 (3), 333–341. doi:10.1093/abbs/gmaa180
Howard, J., Goh, C. Y., Gorzel, K. W., Higgins, M., and McCann, A. (2022). The potential role of cofilin-1 in promoting triple negative breast cancer (TNBC) metastasis via the extracellular vesicles (EVs). Transl. Oncol. 15 (1), 101247. doi:10.1016/j.tranon.2021.101247
Jiang, H. L., Sun, H. F., Gao, S. P., Li, L. D., Hu, X., Wu, J., et al. (2015). Loss of RAB1B promotes triple-negative breast cancer metastasis by activating TGF-β/SMAD signaling. Oncotarget 6 (18), 16352–16365. doi:10.18632/oncotarget.3877
Jiang, L., Gao, X. M., and Cao, J. (2023). The Achilles heel of TNBCs: ferroptosis heterogeneity. Cell Metab. 35 (1), 1–2. doi:10.1016/j.cmet.2022.11.014
Jin, J., Tao, Z., Cao, J., Li, T., and Hu, X. (2021). DNA damage response inhibitors: an avenue for TNBC treatment. Biochim. Biophys. Acta Rev. Cancer 1875 (2), 188521. doi:10.1016/j.bbcan.2021.188521
Kaur, K., and Jaitak, V. (2019). Recent development in indole derivatives as anticancer agents for breast cancer. Anticancer Agents Med. Chem. 19 (8), 962–983. doi:10.2174/1871520619666190312125602
Kaushik, A. C., Mehmood, A., Wang, X., Wei, D. Q., and Dai, X. (2020). Globally ncRNAs expression profiling of TNBC and screening of functional lncRNA. Front. Bioeng. Biotechnol. 8, 523127. doi:10.3389/fbioe.2020.523127
Keenan, T. E., and Tolaney, S. M. (2020). Role of immunotherapy in triple-negative breast cancer. J. Natl. Compr. Canc Netw. 18 (4), 479–489. doi:10.6004/jnccn.2020.7554
Kufe, D. W. (2020). MUC1-C in chronic inflammation and carcinogenesis; emergence as a target for cancer treatment. Carcinogenesis 41 (9), 1173–1183. doi:10.1093/carcin/bgaa082
Lambert, A. W., Pattabiraman, D. R., and Weinberg, R. A. (2017). Emerging biological principles of metastasis. Cell 168 (4), 670–691. doi:10.1016/j.cell.2016.11.037
Latunde-Dada, G. O. (2017). Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochimica Biophysica Acta (BBA) - General Subj. 1861 (8), 1893–1900. doi:10.1016/j.bbagen.2017.05.019
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest 121 (7), 2750–2767. doi:10.1172/JCI45014
Li, H., Yang, P., Wang, J., Zhang, J., Ma, Q., Jiang, Y., et al. (2022a). HLF regulates ferroptosis, development and chemoresistance of triple-n egative breast cancer by activating tumor cell-macrophage crosstalk. J. Hematol. and Oncol. 15 (1), 2. doi:10.1186/s13045-021-01223-x
Li, J., Cao, F., Yin, H. L., Huang, Z. J., Lin, Z. T., Mao, N., et al. (2020b). Ferroptosis: past, present and future. Cell Death Dis. 11 (2), 88. doi:10.1038/s41419-020-2298-2
Li, S., Zhou, J., Wang, Z., Wang, P., Gao, X., and Wang, Y. (2018). Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. Pharmacother. 104, 451–457. doi:10.1016/j.biopha.2018.05.056
Li, X., Hou, L., Yin, L., and Zhao, S. (2020d). LncRNA XIST interacts with miR-454 to inhibit cells proliferation, epithelial mesenchymal transition and induces apoptosis in triple-negative breast cancer. J. Biosci. 45, 45. doi:10.1007/s12038-020-9999-7
Li, Y., Ma, H. Y., Hu, X. W., Qu, Y. Y., Wen, X., Zhang, Y., et al. (2020c). LncRNA H19 promotes triple-negative breast cancer cells invasion and metastasis through the p53/TNFAIP8 pathway. Cancer Cell Int. 20, 200. doi:10.1186/s12935-020-01261-4
Li, Y., Wang, Z., Su, P., Liang, Y., Li, Z., Zhang, H., et al. (2022c). circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol. Ther. 30 (1), 415–430. doi:10.1016/j.ymthe.2021.08.026
Li, Z., Chen, L., Chen, C., Zhou, Y., Hu, D., Yang, J., et al. (2020a). Targeting ferroptosis in breast cancer. Biomark. Res. 8 (1), 58. doi:10.1186/s40364-020-00230-3
Li, Z., Yang, H. Y., Zhang, X. L., Zhang, X., Huang, Y. Z., Dai, X. Y., et al. (2022b). Kinesin family member 23, regulated by FOXM1, promotes triple negative breast cancer progression via activating Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 41 (1), 168. doi:10.1186/s13046-022-02373-7
Liang, H. F., Zhang, X. Z., Liu, B. G., Jia, G. T., and Li, W. L. (2017). Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am. J. Cancer Res. 7 (7), 1566–1576. doi:10.1186/s12935-020-01694-x
Lin, C. C., Yang, W. H., Lin, Y. T., Tang, X., Chen, P. H., Ding, C. K. C., et al. (2021). DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene 40 (11), 2018–2034. doi:10.1038/s41388-021-01676-x
Liu, J., Zhao, G., Liu, X. L., Zhang, G., Zhao, S. Q., Zhang, S. L., et al. (2021a). Progress of non-coding RNAs in triple-negative breast cancer. Life Sci. 272, 119238. doi:10.1016/j.lfs.2021.119238
Liu, W., Chakraborty, B., Safi, R., Kazmin, D., Chang, C. Y., and McDonnell, D. P. (2021b). Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat. Commun. 12 (1), 5103. doi:10.1038/s41467-021-25354-4
Liu, Z., Zhou, Y., Liang, G., Ling, Y., Tan, W., Tan, L., et al. (2019). Circular RNA hsa_circ_001783 regulates breast cancer progression via sponging miR-200c-3p. Cell Death Dis. 10 (2), 55. doi:10.1038/s41419-018-1287-1
Loibl, S., Schneeweiss, A., Huober, J., Braun, M., Rey, J., Blohmer, J. U., et al. (2022). Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 33 (11), 1149–1158. doi:10.1016/j.annonc.2022.07.1940
Loibl, S., Untch, M., Burchardi, N., Huober, J., Sinn, B. V., Blohmer, J. U., et al. (2019). A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 30 (8), 1279–1288. doi:10.1093/annonc/mdz158
Lu, B., Chen, X. B., Ying, M. D., He, Q. J., Cao, J., and Yang, B. (2017). The role of ferroptosis in cancer development and treatment response. Front. Pharmacol. 8, 992. doi:10.3389/fphar.2017.00992
Mattick, J. S., and Makunin, I. V. (2006). Non-coding RNA. Hum. Mol. Genet. 15 (Spec No 1), R17–R29. doi:10.1093/hmg/ddl046
Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., et al. (2019). Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 12 (1), 34. doi:10.1186/s13045-019-0720-y
Müller, S., Sindikubwabo, F., Cañeque, T., Lafon, A., Versini, A., Lombard, B., et al. (2020). CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat. Chem. 12 (10), 929–938. doi:10.1038/s41557-020-0513-5
Nguyen, D. X., Bos, P. D., and Massagué, J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9 (4), 274–284. doi:10.1038/nrc2622
Pan, G., Liu, Y., Shang, L., Zhou, F., and Yang, S. (2021). EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. (Lond) 41 (3), 199–217. doi:10.1002/cac2.12138
Park, M., Kim, D., Ko, S., Kim, A., Mo, K., and Yoon, H. (2022). Breast cancer metastasis: mechanisms and therapeutic implications. Int. J. Mol. Sci. 23 (12), 6806. doi:10.3390/ijms23126806
Pastushenko, I., and Blanpain, C. (2019). EMT transition States during tumor progression and metastasis. Trends Cell Biol. 29 (3), 212–226. doi:10.1016/j.tcb.2018.12.001
Peng, G., Tang, Z., Xiang, Y., and Chen, W. (2019). Glutathione peroxidase 4 maintains a stemness phenotype, oxidative homeostasis and regulates biological processes in Panc-1 cancer stem-like cells. Oncol. Rep. 41 (2), 1264–1274. doi:10.3892/or.2018.6905
Rahimi, M., Sharifi-Zarchi, A., Firouzi, J., Azimi, M., Zarghami, N., Alizadeh, E., et al. (2019). An integrated analysis to predict micro-RNAs targeting both stemness and metastasis in breast cancer stem cells. J. Cell Mol. Med. 23 (4), 2442–2456. doi:10.1111/jcmm.14090
Sakamoto, N., Honma, R., Sekino, Y., Goto, K., Sentani, K., Ishikawa, A., et al. (2017). Non-coding RNAs are promising targets for stem cell-based cancer therapy. Noncoding RNA Res. 2 (2), 83–87. doi:10.1016/j.ncrna.2017.05.002
Samuel, S. M., Varghese, E., Kubatka, P., Triggle, C. R., and Büsselberg, D. (2019). Metformin: the answer to cancer in a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer. Biomolecules 9 (12), 846. doi:10.3390/biom9120846
So, J. Y., Ohm, J., Lipkowitz, S., and Yang, L. (2022). Triple negative breast cancer (TNBC): non-genetic tumor heterogeneity and immune microenvironment: emerging treatment options. Pharmacol. Ther. 237, 108253. doi:10.1016/j.pharmthera.2022.108253
Stockwell, B. R. (2022). Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185 (14), 2401–2421. doi:10.1016/j.cell.2022.06.003
Suhail, Y., Cain, M. P., Vanaja, K., Kurywchak, P. A., Levchenko, A., Kalluri, R., et al. (2019). Systems biology of cancer metastasis. Cell Syst. 9 (2), 109–127. doi:10.1016/j.cels.2019.07.003
Sun, D., Li, Y. C., and Zhang, X. Y. (2021b). Lidocaine promoted ferroptosis by targeting miR-382-5p/SLC7A11 Axis in ovarian and breast cancer. Front. Pharmacol. 12, 681223. doi:10.3389/fphar.2021.681223
Sun, L. L., Linghu, D. L., and Hung, M. C. (2021a). Ferroptosis: a promising target for cancer immunotherapy. Am. J. Cancer Res. 11 (12), 5856–5863.
Tan, B., Zhou, K., Liu, W., Prince, E., Qing, Y., Li, Y., et al. (2022). RNA 6 -methyladenosine reader YTHDC1 is essential for TGF-beta-mediated metastasis of triple negative breast cancer. Theranostics 12 (13), 5727–5743. doi:10.7150/thno.71872
Tan, X., Kong, D., Tao, Z., Cheng, F., Zhang, B., Wang, Z., et al. (2024). Simultaneous inhibition of FAK and ROS1 synergistically repressed triple-negative breast cancer by upregulating p53 signalling. Biomark. Res. 12 (1), 13. doi:10.1186/s40364-024-00558-0
Tang, D., Chen, X., Kang, R., and Kroemer, G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31 (2), 107–125. doi:10.1038/s41422-020-00441-1
Vagia, E., Mahalingam, D., and Cristofanilli, M. (2020). The landscape of targeted therapies in TNBC. Cancers (Basel) 12 (4), 916. doi:10.3390/cancers12040916
van Staalduinen, J., Baker, D., Ten Dijke, P., and van Dam, H. (2018). Epithelial-mesenchymal-transition-inducing transcription factors: new targets for tackling chemoresistance in cancer? Oncogene 37 (48), 6195–6211. doi:10.1038/s41388-018-0378-x
Verma, N., Vinik, Y., Saroha, A., Nair, N. U., Ruppin, E., Mills, G., et al. (2020). Synthetic lethal combination targeting BET uncovered intrinsic susceptibility of TNBC to ferroptosis. Sci. Adv. 6 (34), eaba8968. doi:10.1126/sciadv.aba8968
Vijay, G. V., Zhao, N., Den Hollander, P., Toneff, M. J., Joseph, R., Pietila, M., et al. (2019). GSK3β regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 21 (1), 37. doi:10.1186/s13058-019-1125-0
Wang, H., Cheng, Y., Mao, C., Liu, S., Xiao, D., Huang, J., et al. (2021a). Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 29 (7), 2185–2208. doi:10.1016/j.ymthe.2021.03.022
Wang, L., Long, H., Zheng, Q., Bo, X., Xiao, X., and Li, B. (2019a). Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol. Cancer 18 (1), 119. doi:10.1186/s12943-019-1046-7
Wang, L., Zhou, Y., Jiang, L., Lu, L., Dai, T., Li, A., et al. (2021b). CircWAC induces chemotherapeutic resistance in triple-negative breast cancer by targeting miR-142, upregulating WWP1 and activating the PI3K/AKT pathway. Mol. Cancer 20 (1), 43. doi:10.1186/s12943-021-01332-8
Wang, N., Hou, M., Zhan, Y., and Sheng, X. (2019b). LncRNA PTCSC3 inhibits triple-negative breast cancer cell proliferation by downregulating lncRNA H19. J. Cell Biochem. 120 (9), 15083–15088. doi:10.1002/jcb.28769
Wang, W., Zhang, J., Wang, Y., Xu, Y., and Zhang, S. (2022a). Identifies microtubule-binding protein CSPP1 as a novel cancer biomarker associated with ferroptosis and tumor microenvironment. Comput. Struct. Biotechnol. J. 20, 3322–3335. doi:10.1016/j.csbj.2022.06.046
Wang, Z., Li, Y., Yang, J., Liang, Y., Wang, X., Zhang, N., et al. (2022b). Circ-TRIO promotes TNBC progression by regulating the miR-432-5p/CCDC58 axis. Cell Death Dis. 13 (9), 776. doi:10.1038/s41419-022-05216-7
Weng, Y. S., Tseng, H. Y., Chen, Y. A., Shen, P. C., Al Haq, A. T., Chen, L. M., et al. (2019). MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 18 (1), 42. doi:10.1186/s12943-019-0988-0
Williams, E. D., Gao, D., Redfern, A., and Thompson, E. W. (2019). Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 19 (12), 716–732. doi:10.1038/s41568-019-0213-x
Won, K. A., and Spruck, C. (2020). Triple-negative breast cancer therapy: current and future perspectives (Review). Int. J. Oncol. 57 (6), 1245–1261. doi:10.3892/ijo.2020.5135
Wu, C., Zhang, F., Li, B., Li, Z., Xie, X., Huang, Y., et al. (2023). A self-assembly nano-prodrug for combination therapy in triple-negative breast cancer stem cells. Small 19 (41), e2301600. doi:10.1002/smll.202301600
Wu, G., Zhou, H., Li, D., Zhi, Y., Liu, Y., Li, J., et al. (2020). LncRNA DANCR upregulation induced by TUFT1 promotes malignant progression in triple negative breast cancer via miR-874-3p-SOX2 axis. Exp. Cell Res. 396 (2), 112331. doi:10.1016/j.yexcr.2020.112331
Wu, J., Minikes, A. M., Gao, M., Bian, H., Li, Y., Stockwell, B. R., et al. (2019). Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572 (7769), 402–406. doi:10.1038/s41586-019-1426-6
Xiao, W., Zheng, S., Zou, Y., Yang, A., Xie, X., Tang, H., et al. (2019). CircAHNAK1 inhibits proliferation and metastasis of triple-negative breast cancer by modulating miR-421 and RASA1. Aging (Albany NY) 11 (24), 12043–12056. doi:10.18632/aging.102539
Xu, J., Wu, K. J., Jia, Q. J., and Ding, X. F. (2020). Roles of miRNA and lncRNA in triple-negative breast cancer. J. Zhejiang Univ. Sci. B 21 (9), 673–689. doi:10.1631/jzus.B1900709
Xu, W., Song, C., Wang, X., Li, Y., Bai, X., Liang, X., et al. (2021). Downregulation of miR-155-5p enhances the anti-tumor effect of cetuximab on triple-negative breast cancer cells via inducing cell apoptosis and pyroptosis. Aging (Albany NY) 13 (1), 228–240. doi:10.18632/aging.103669
Yadav, P., Sharma, P., Sundaram, S., Venkatraman, G., Bera, A. K., and Karunagaran, D. (2021). SLC7A11/xCT is a target of miR-5096 and its restoration partially rescues miR-5096-mediated ferroptosis and anti-tumor effects in human breast cancer cells. Cancer Lett. 522, 211–224. doi:10.1016/j.canlet.2021.09.033
Yamashita, N., and Kufe, D. (2022). Addiction of cancer stem cells to MUC1-C in triple-negative breast cancer progression. Int. J. Mol. Sci. 23 (15), 8219. doi:10.3390/ijms23158219
Yan, H., and Bu, P. (2021). Non-coding RNA in cancer. Essays Biochem. 65 (4), 625–639. doi:10.1042/EBC20200032
Yang, H. L., Thiyagarajan, V., Shen, P. C., Mathew, D. C., Lin, K. Y., Liao, J. W., et al. (2019). Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J. Exp. Clin. Cancer Res. 38 (1), 186. doi:10.1186/s13046-019-1196-x
Yang, J., Antin, P., Berx, G., Blanpain, C., Brabletz, T., Bronner, M., et al. (2020). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21 (6), 341–352. doi:10.1038/s41580-020-0237-9
Yang, J., Jia, Z., Zhang, J., Pan, X., Wei, Y., Ma, S., et al. (2022). Metabolic intervention nanoparticles for triple-negative breast cancer therapy via overcoming FSP1-mediated ferroptosis resistance. Adv. Healthc. Mater 11 (13), e2102799. doi:10.1002/adhm.202102799
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yao, J., Zhang, Y., Li, M., Sun, Z., Liu, T., Zhao, M., et al. (2021a). Single-cell RNA-seq reveals the promoting role of ferroptosis tendency during lung adenocarcinoma EMT progression. Front. Cell Dev. Biol. 9, 822315. doi:10.3389/fcell.2021.822315
Yao, X., Xie, R., Cao, Y., Tang, J., Men, Y., Peng, H., et al. (2021b). Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnology 19 (1), 311. doi:10.1186/s12951-021-01058-1
Yeo, S. K., and Guan, J.-L. (2017). Breast cancer: multiple subtypes within a tumor? Trends Cancer 3 (11), 753–760. doi:10.1016/j.trecan.2017.09.001
Yin, L., Duan, J. J., Bian, X. W., and Yu, S. C. (2020). Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 22 (1), 61. doi:10.1186/s13058-020-01296-5
Yu, F., Wang, L., and Zhang, B. (2019). Long non-coding RNA DRHC inhibits the proliferation of cancer cells in triple negative breast cancer by downregulating long non-coding RNA HOTAIR. Oncol. Lett. 18 (4), 3817–3822. doi:10.3892/ol.2019.10683
Yu, H., Guo, P., Xie, X., Wang, Y., and Chen, G. (2017). Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 21 (4), 648–657. doi:10.1111/jcmm.13008
Yuan, F., and Wang, W. (2015). MicroRNA-802 suppresses breast cancer proliferation through downregulation of FoxM1. Mol. Med. Rep. 12 (3), 4647–4651. doi:10.3892/mmr.2015.3921
Zhang, H., Ge, Z., Wang, Z., Gao, Y., Wang, Y., and Qu, X. (2021b). Circular RNA RHOT1 promotes progression and inhibits ferroptosis via mir-106a-5p/STAT3 axis in breast cancer. Aging (Albany NY) 13 (6), 8115–8126. doi:10.18632/aging.202608
Zhang, J., Yang, J., Zuo, T., Ma, S., Xokrat, N., Hu, Z., et al. (2021a). Heparanase-driven sequential released nanoparticles for ferroptosis and tumor microenvironment modulations synergism in breast cancer therapy. Biomaterials 266, 120429. doi:10.1016/j.biomaterials.2020.120429
Zhang, W., Yang, S., Chen, D., Yuwen, D., Zhang, J., Wei, X., et al. (2022). SOX2-OT induced by PAI-1 promotes triple-negative breast cancer cells metastasis by sponging miR-942-5p and activating PI3K/Akt signaling. Cell Mol. Life Sci. 79 (1), 59. doi:10.1007/s00018-021-04120-1
Keywords: ferroptosis, EMT, breast cancer, non-coding RNA, metastasis
Citation: Chen Z and Zhao Y (2025) The mechanism underlying metastasis in triple-negative breast cancer: focusing on the interplay between ferroptosis, epithelial-mesenchymal transition, and non-coding RNAs. Front. Pharmacol. 15:1437022. doi: 10.3389/fphar.2024.1437022
Received: 23 May 2024; Accepted: 27 December 2024;
Published: 15 January 2025.
Edited by:
Chunbo He, University of Oklahoma Health Sciences Center, United StatesCopyright © 2025 Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhao, emhhb3lpMDkyNEBxZHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.