- Key Laboratory of Shaanxi Administration of Traditional Chinese Medicine for TCM Compatibility, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, China
Berberine (BBR) is a natural alkaloid, which has played an important role in the field of medicine since its discovery in the late 19th century. However, the low availability of BBR in vivo prevents its full effect. In recent years, a large number of studies confirmed that BBR has a protective effect on the nervous system through various functions, yet the issue of the inability to systematically understand the protection of BBR on the nervous system remains a gap that needs to be addressed. Many existing literature introductions about berberine in neurodegenerative diseases, but the role of berberine in the nervous system goes far beyond these. Different from these literatures, this review is divided into three parts: preparation method, mechanism, and therapeutic effect. Various dosage forms of BBR and their preparation methods are added, in order to provide a reasonable choice of BBR, and help to solve the problem of low bioavailability in treatment. More importantly, we more comprehensively summarize the mechanism of BBR to protect the nervous system, in addition to the treatment of neurodegenerative diseases (anti-oxidative stress, anti-neuroinflammation, regulation of apoptosis), two extra mechanisms of berberine for the protection of the nervous system were also introduced: bidirectional regulation of autophagy and promote angiogenesis. Also, we have clarified the precise mechanism by which BBR has a therapeutic effect not only on neurodegenerative illnesses but also on multiple sclerosis, gliomas, epilepsy, and other neurological conditions. To sum up, we hope that these can evoke more efforts to comprehensively utilize of BBR nervous system, and to promote the application of BBR in nervous system protection.
1 Introduction
Berberine (BBR) was first discovered from the bark of Xanthoxylon clava. In the past, BBR was isolated from plants such as Berberidaceae, Ranunculaceae, Rutaceae, Menispermaceae, Papaveraceae, Loganiaceae, and Rhamnaceae. Nowadays BBR can be synthesized artificially (Zhang and Shen, 2023). Numerous studies on the pharmacological effects and associated mechanisms of BBR have been carried out recently in many countries. These studies have revealed that BBR has unique impacts and pharmacological activities on the cardiovascular system, nervous system, and endocrine system. These effects can lower blood lipids and protect the cardiovascular system, produce anti-anxiety effects by influencing brain neurotransmitters, and improve insulin sensitivity in the treatment of diabetes. The application potential of BBR in both the prevention and treatment of cardiovascular, cerebrovascular, nervous system, and other important disorders cannot be ignored (Hu and Mo, 2017).
As the worldwide population ages, hundreds of thousands of elderly people suffer from neurological issues. The majority of medications have adverse effects for central nervous system disorders (Dording and Boyden, 2019). But as a natural alkaloid, BBR has fewer adverse effects and offers some benefits in the medical management of central nervous system disorders. Researchers reported that BBR can penetrate the blood-brain barrier, reduce the permeability of the blood-brain barrier, and protect the integrity of the blood-brain barrier, thus maintaining the homeostasis of the central nervous system (Ma et al., 2010; Song et al., 2022) and is more suitable for brain diseases. Through its unique regulation mechanism, BBR have a great effect on regulating the nervous system (Ding et al., 2021). Moreover, BBR has a ameliorative impact on neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease) and psychiatric diseases (depression, anxiety, schizophrenia). To better investigate and develop the use of BBR in central nervous system illness, we elucidated the extraction method of BBR and dosage forms of BBR, as well as its pharmacological effects on inflammation, oxidative stress, apoptosis, autophagy and angiogenesis were described. In addition, it summarizes how BBR affects various signaling pathways to regulate the processes of central nervous system diseases.
1.1 Physicochemical properties and pharmacokinetics of BBR
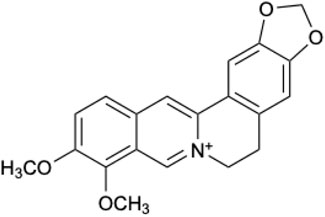
BBR is a quaternary amine alkaloid with unique physical and chemical properties (Li et al., 2020). The molecular formula of BBR is C20H18NO4. BBR is a faint yellow acicular crystal, the melting point is 204.8–205.4°C. (The chemical structural formula of BBR is seen in Figure 1). BBR has high solubility in hot water, slight solubility in cold water and cold ethanol, and almost insoluble in chloroform and ether. Under physiological conditions, BBR mainly exists in ionized form and is easy to self-accumulate in the acidic environment of the gastrointestinal tract. The solubility at pH 1.2 (HCl) is 1/20 of that at pH 7.0 (Spinozzi et al., 2014). Due to the lipophobic nature of BBR, its passage through intestinal cells is obstructed. Its effective permeability coefficient in rat intestinal mucosa was 0.178 × 10−4 cm s-1, which confirmed its low permeability (Chen et al., 2011; Zhou, J. X. et al., 2022).
BBR is poorly soluble. BBR that is absorbed in by the gut can also be expelled back into the intestinal lumen by the action of P-glycoprotein (P-gP). BBR is the substrate of the efflux transporter P-gP, which makes BBR difficult to be absorbed orally and shows a very low bioavailability (<1%) in both animals and humans. Chen et al. studied that the absolute bioavailability of oral pathway in rats was 0.68% (Chen et al., 2011). In addition, P-gP inhibitors could increase the absorption of BBR by 6 times in rats, indicating that P-gP contributed to intestinal malabsorption of BBR (Pan et al., 2002). Liu et al. discovered that after the rats were given BBR intragastric administration, approximately 1/2 of the BBR was eliminated through the gastrointestinal tract, while the remaining 1/2 was excreted through the intestinum tenue, resulting in the oral absolute bioavailability of rats is 0.36% (Liu et al., 2010). It was found through experimental determination that first pass elimination of the intestine of BBR was the main obstacle to its oral absolute bioavailability. The liver’s significant extraction and distribution of BBR might have led to its low concentration in rat plasma. After oral administration, BBR can be widely distributed in liver, kidney, brain, heart and other parts (Tan et al., 2013), and can penetrate the blood-brain barrier and rapidly distribute to the thalamus (Wang et al., 2005). A large amount of scientific evidence shows that BBR is metabolized by demethylation, glucuronidation and/or sulfonation (Ahmed et al., 2015), and the pharmacological activity of the metabolite is consistent with BBR (Wang et al., 2017). In both human and rats, the active metabolites of BBR are mainly divided into the following four kinds: berberrubine (M1), thalifendine (M2), demethyleneberberine (M3), jatrorrhizine (M4) (Liu et al., 2009). One study discovered that M1, M3, and M4 can directly cross the blood-brain barrier to perform a neuroprotective effect (Pan et al., 2024), but whether M2 can cross the blood-brain barrier remains unknown. In addition, the metabolites of BBR are all P-gP substrates, and the order of binding strength is as follows: M1 < BBR < M4 < M2 < M3. The higher the binding affinity, the more difficult the transport of the compound is (Zhang et al., 2019). Therefore, the oral bioavailability of M1 may be better than BBR. Not only that, BBR metabolites also showed higher concentrations in plasma, and the lipophilicity of M1 was higher than BBR (Wang et al., 2015), this suggests that the study of the metabolically active components of berberine may provide an opportunity to further improve the efficacy of BBR. Finally, in terms of excretion, BBR is mainly excreted through urine and bile in the form of metabolites. In addition, BBR has high safety, rarely significant adverse reactions in clinical trials, and its metabolite structure can remain relatively stable after entering the brain, suggesting that it can play a therapeutic role in nervous system diseases (Wang, X. J. et al., 2024).
1.2 Extraction method of BBR
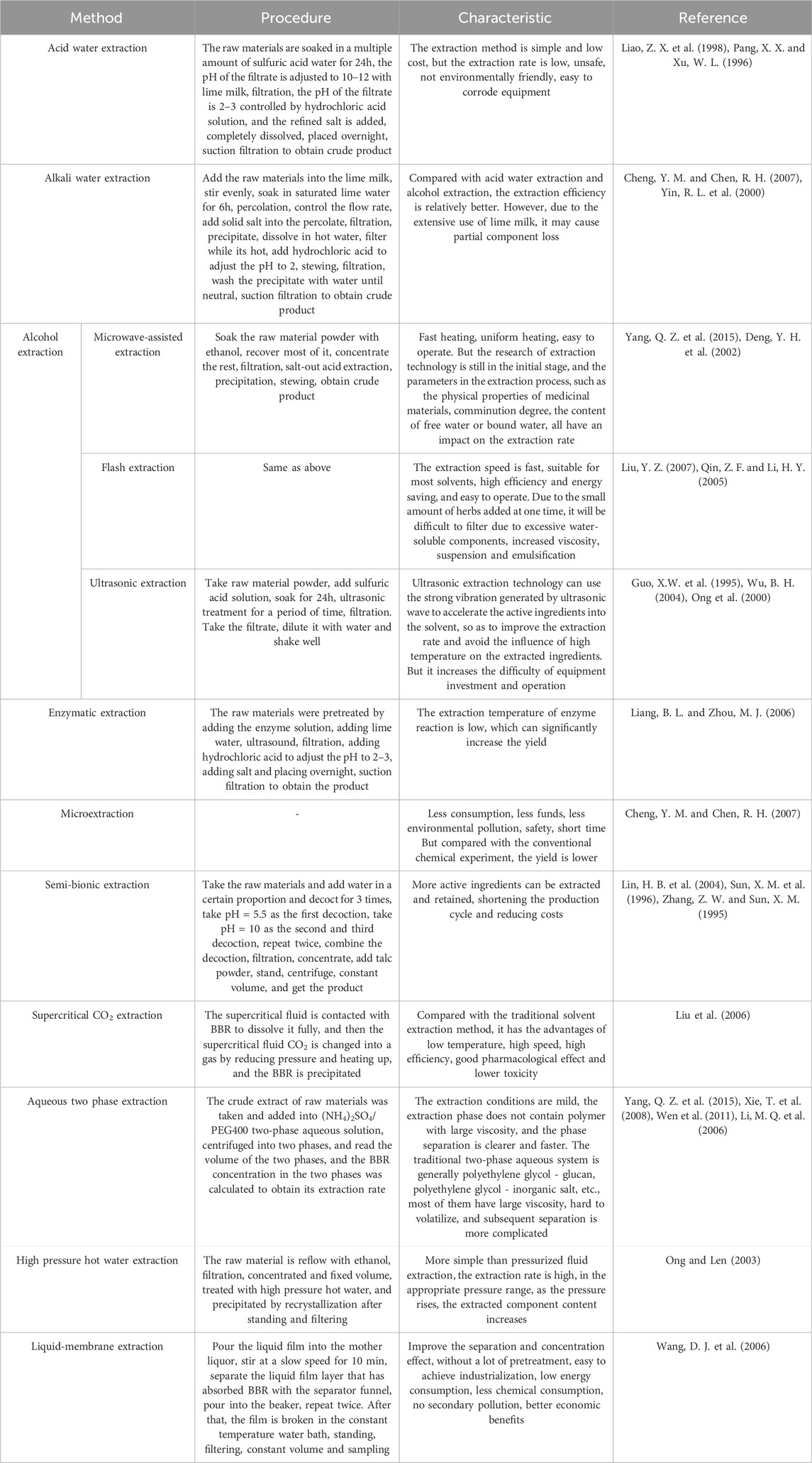
BBR extraction methods mainly include Acid water extraction, Alkali water extraction and Alcohol extraction, of which acid water extraction and alkali water extraction are simple, low cost, but still have problems such as: low extraction rate, easy to corrode equipment, unsafe, not eco-friendly. Alcohol extraction methods include Microwave-assisted extraction, (Soxhlet) reflux extraction, Flash extraction, Ultrasonic extraction, etc., which not only has low solvent restriction and can be used repeatedly, but also can save energy and environmental protection, high safety, simple operation, and high extraction efficiency. However, ultrasonic extraction methods require large investment in equipment (Yang, Q. Z. et al., 2015), and the specific content of BBR extraction method is shown in Table 1.
1.3 The dosage form of BBR
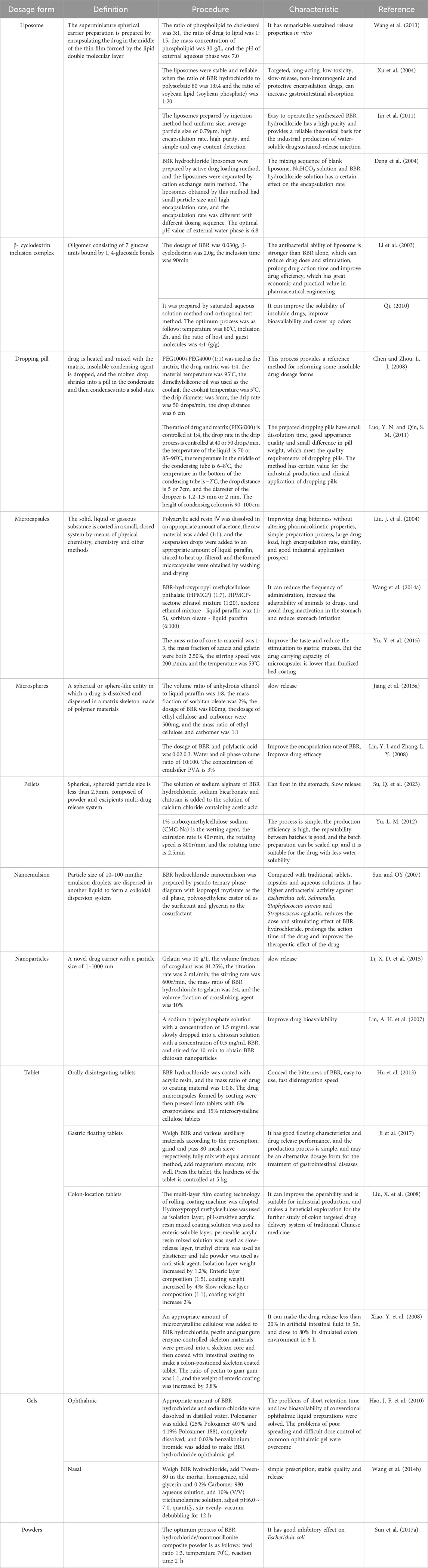
BBR has a broad application prospect and is mainly used in the form of hydrochloride in clinic. However, due to the low solubility of hydrochloride, its popularization and use are limited. At the same time, the bitter taste of BBR and other reasons also make it difficult to swallow, affecting the compliance of patients. Therefore, the design of different dosage forms has been endless, including liposomes, β-cyclodextrin inclusion complex, dropping pills, microspheres, microemulsions, solid lipid nanoparticles, targeting drug delivery system, etc. (Wu, J. D. et al., 2013), the specific contents are shown in Table 2.
The aforementioned list includes all possible dose forms, preparation methods, and administration characteristics of berberine; nevertheless, the ones that may be useful in neurological illnesses are of greater significance. Most neurological diseases, including multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, epilepsy, and cerebral infarction, require long-term medication. The benefits of slow-release dosage forms include decreased frequency of administration, stable drug concentration, and fewer side effects related to peak blood drug concentration (Andrade, 2015). Therefore, berberine is more suitable for long-term drug use when the dosage form is liposome, microsphere, nanoemulsion, and nanoparticles. In addition, the poor water solubility and low bioavailability of berberine is also a problem that must be paid attention to. Wang et al. ’s study showed that when Berberine is prepared as an inclusion compound of β-cyclodextrin, specific intermolecular interactions can be formed between Berberine Hydrochloride and β-cyclodextrin, and this dosage form greatly improves the solubility of berberine, thereby improving its bioavailability (Wang et al., 2020). Furthermore, the drug absorption velocity and absorption rate are enhanced when the dose forms are microspheres and nanoparticles because of their small particle sizes, which enhances bioavailability. The creation of berberine dosage forms is crucial for the nervous system’s application, and suitable dosage forms enable berberine to fulfill its therapeutic function and enhance its healing effect.
2 Pharmacological mechanism of neuroprotective effect of BBR
2.1 Anti-oxidative stress
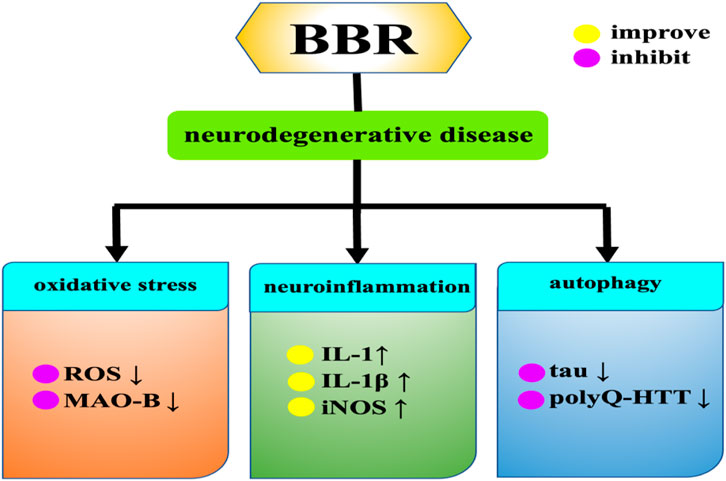
Oxidative stress occurs because of an excessive accumulation of reactive oxygen species (ROS), leading to an imbalance between oxidants and antioxidants, which in turn leads to the production of many neurological diseases (Li et al., 2020). During oxidative stress, an overabundance of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) can lead to lipid peroxidation, protein oxidation, protein nitration, and sugar co-oxidation. This can cause damage to the plasma membrane of nerve tissue, destruction of the cytoskeleton, and mutation of nucleic acids (Barnham et al., 2004; Uttara et al., 2009). The generation of ROS is related to glutathione (GSH), superoxide dismutase (SOD) and other chemicals (Li, H. Y. et al., 2024). Once ROS is excessive and inhibits the antioxidant activity of cells, oxidative stress will occur (Cheng et al., 2022), the mechanism of BBR against oxidative stress is shown in Figure 2. BBR has the potential to reduce ROS production and protect nerve cells from oxidative damage. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a primary producer of ROS, and BBR can alleviate oxidative stress by decreasing the production of NADPH oxidase (Ma et al., 2018). BBR effectively inhibits lipid peroxidation while enhancing glutathione content and superoxide dismutase activity in cells (Sadeghnia et al., 2017). BBR can effectively decrease the generation of ROS in both cytoplasmic and mitochondrial cells (Sun Y. et al., 2017). This reduction is likely due to the activation of Adenosine monophosphate (AMP)-activated protein kinase (AMPK) and sirtuin1 (SIRT1)/forkhead box O1 (FOXO1) pathways (Li, H. R., 2024). The antioxidative stress activity of BBR is also associated with the transcription factor PPAR, which is a transcription factor induced by ligands. Activation of PPARs can play an antioxidant role, And BBR can activate PPARδ to clear ROS and exert neuroprotective effects (Shou et al., 2022). BBR may also exert antioxidant effects by lowering inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and boosting HO-1.
2.2 Anti-neuroinflammation
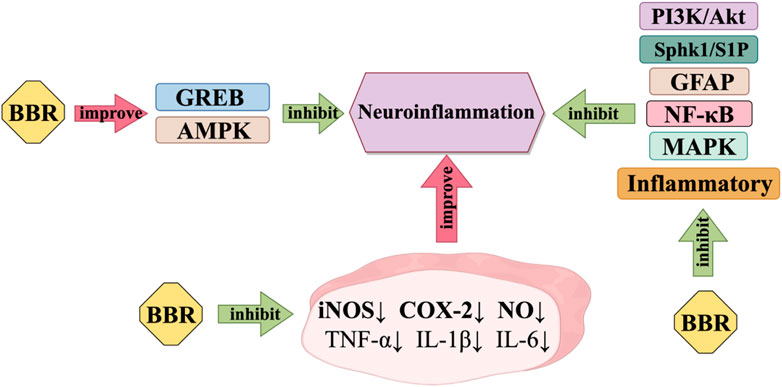
Neuroinflammation is the inflammation of nerve tissue, resulting from various sources such as traumatic brain damage and autoimmune. Although neuroinflammation can start in different trigger sites, it all has one thing in common: microglia and astrocytes are constantly activated (Perry and Holmes, 2014), Figure 3 shows how BBR works to reduce neuroinflammation. BBR can prevent neuroinflammation and possibly be used as a candidate drug to treat illnesses of the central nervous system that are caused by inflammation. BBR may provide neuroprotective effects by decreasing the generation of several neurotoxic compounds by activated microglia (Nam et al., 2010), BBR has been observed to effectively suppress the activation of NF-κB and the phosphorylation of Akt, p38, and extracellular regulated protein kinases (ERK). It is suggested that BBR may hinder the inflammatory response of microglia by reducing the PI3K/Akt and mitogen-activated protein kinase (MAPK) pathway(Wan, J. S. and Zhang, M. R., 2018). BBR inhibits the pro-inflammatory reaction by stimulating AMPK in BV-2 microglia. Additionally, BBR notably reduces the expression of iNOS and COX-2 in BV-2 microglia induced by LPS or interferon (IFN)-γ. It also hinders the synthesis of nitric oxide. Furthermore, BBR has the ability to normalise inflammatory factors. It can effectively decrease the generation of pro-inflammatory cytokines TNF-α and IL-1β, as well as the manifestation of IL-6 in BV2 cells that have been activated by LPS (Lu et al., 2010; Zhang et al., 2016a). BBR also decreases the production of inflammatory factors and GFAP, while suppressing Sphk1/S1P signalling and stimulating CREB signalling (Cheng et al., 2022).
2.3 Regulation of apoptosis
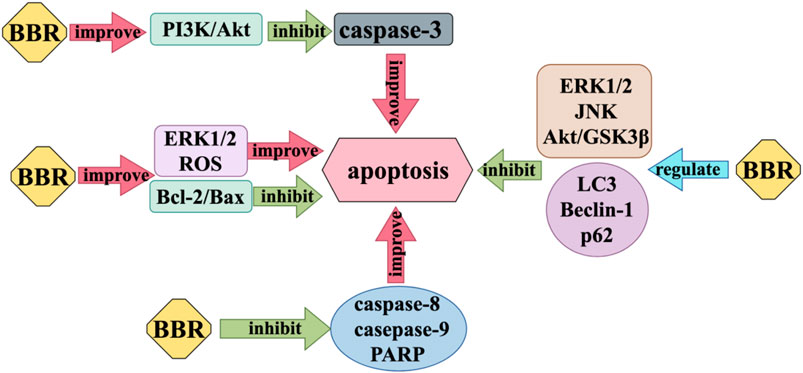
Nerve cell injury can cause apoptosis to some extent, resulting in the loss of nervous system function. Apoptosis is one of the important autostable mechanisms in multicellular organisms (Li, M. and Lin, J., 2014). BBR exerts anti-apoptosis effects on nerve cells by inhibiting caspase-3, which acts as an executor of apoptosis, and increasing the expression ratio of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and pro-apoptotic protein Bcl-2 associated X (Bax). BBR modulates the activity of many proteins involved in autophagy, such as Microtubule-associated protein 1A/1B-light chain 3 (LC3), Beclin-1, and p62. It also affects the function of apoptosis regulating proteins, including caspase 3, caspase 8, caspase 9, poly ADP-ribose polymerase (PARP), and Bcl-2/Bax, which play a role in inhibiting neuronal apoptosis (Zhang et al., 2016b). Liang et al. found that BBR can inhibit the cytotoxicity of Aβ25-35 on neuron cells, reduce the activity of various caspase proteins, increase Bcl-2/Bax, and have anti-apoptotic effects (Liang et al., 2017). Most studies (Kim et al., 2014a; Kim et al., 2014b; Simões Pires et al., 2014; Hsu et al., 2012) believe that BBR can regulate the activity of subunit p55γ promoter and activate PI3K/Akt pathway by enhancing PI3K kinase, and then inhibit the activity of Bad, a positive regulator of apoptosis, and reduce the production of pro-apoptotic caspase-3 (Hu et al., 2012). But Simoes et al. propose that the Akt/GSK3β pathway, ERK1/2 pathway, and JNK pathway play an important part in the neuroprotective action of BBR against apoptosis (Simões Pires et al., 2014). However, in addition to the anti-apoptosis effect of BBR, when it comes to the specific therapeutic effect of BBR on some nervous system diseases, it also has the pro-apoptotic effect of inducing disease cell apoptosis. For example, in the treatment of glioblastoma, BBR inhibits ER stress-induced apoptosis in T98G cells by generating ROS and mitochondria-dependent mechanisms (Eom et al., 2010), BBR can also induce apoptosis of U251 and U87 cells by significantly inhibiting the activation of ERK1/2 pathway (Rauf et al., 2021) (Figure 4).
2.4 Regulation of autophagy
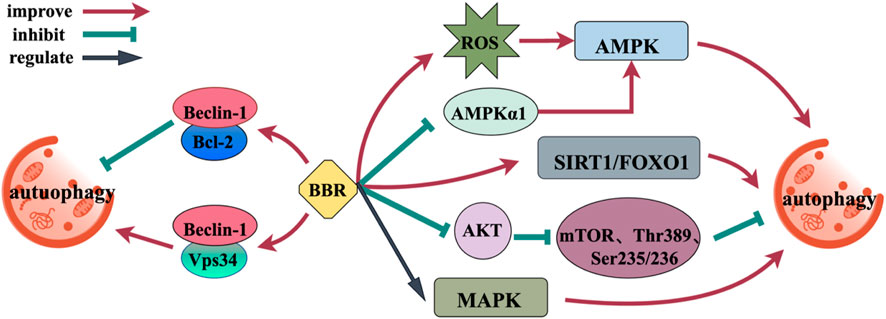
Upregulation of autophagy is a promising treatment approach for a range of neurodegenerative disorders (Menzies et al., 2017). BBR, as an autophagy modulator, affects autophagy by affecting targets or pathways associated with autophagy (AMPK, mTOR, MAPK, Beclin-1, and SIRT1) (Figure 5). Furthermore, the effect of BBR depends on the environment, and its regulation of autophagy is bidirectional, not only promoting autophagy, but also inhibiting autophagy (Mohammadinejad et al., 2019). AMPK/mTOR is a basic modulator of autophagy, and BBR can regulate AMPK/mTOR through transcriptional mechanism. BBR significantly increases AMPK activity through the production of ROS and the elimination of AMPKα1 (Fan et al., 2015; Issat et al., 2011). BBR inhibits PI3K activity, significantly downregulates AKT phosphorylation in a manner that depends on the dosage, and inhibits the phosphorylation of mTOR, p70 ribosome S6 protein kinase (Thr389) and S6 (Ser235/236), promoting the early initiation of autophagy (Huang et al., 2021; Yi et al., 2015). BBR upregulates autophagy by regulating mitogen-activated protein kinase (MAPK) (Sun et al., 2015). Beclin-1 has binding sites for Vps34 and Bcl-2. When autophagy is induced, Beclin-1 separates out from Bcl-2 and binds to Vps34, enhancing the binding force between Beclin-1 and Vps34, which inducing autophagy (Fleming et al., 2011). BBR can upregulate the expression of Beclin-1 and promote its binding to Vps34. Interestingly, BBR can also inhibit autophagy and produce neuroprotective effects by making Bcl-2 and Beclin-1 bind continuously (Ding, S. et al., 2021). SIRT1 can activate autophagy and promote the expression of genes associated with autophagy by means of FOXO. BBR can enhance the deacetylation activity of SIRT1 and induce autophagy (Lapierre et al., 2015; Sun et al., 2018a).
2.5 Promote angiogenesis
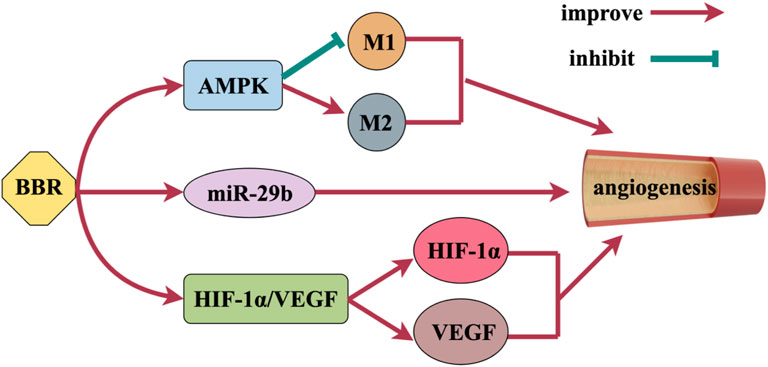
Angiogenesis is achieved by stimulating the growth of blood vessels, which can promote the survival of neurons, improve brain plasticity and restore nerve function (Yang and Torbey, 2020), Figure 6 shows the mechanism of BBR promoting angiogenesis. BBR activates AMPK signaling pathway and has a vital function in promoting angiogenesis. BBR also promotes alternative activation (anti-inflammatory M2) polarization of microglia and inhibits classical activation (pro-inflammatory M1) polarization through AMPK signaling pathway activation to promote angiogenesis (Zhu et al., 2019). Zhu et al. found that BBR activated Akt and subsequently increased angiogenesis by activating miR-29b expression in vitro and in vivo (Zhu et al., 2017). Vascular endothelial growth factor (VEGF) is a strong and essential pro-aging factor, which plays a crucial role in angiogenesis. Zou et al. (2019), Tian et al. (2023)’s study showed that BBR could promote the expression of VEGF and induce angiogenesis. By activating the HIF-1α/VEGF signal transduction pathway, BBR can increase the expression of microvascular density, VEGF and hypoxia inducible factor-1α (HIF-1α), promote angiogenesis, and thus achieve neuroprotective effects (Liu et al., 2017).
3 BBR and central nervous system diseases
3.1 Cerebrovascular diseases
3.1.1 Transient cerebral ischemia, ischemia-reperfusion, stroke and cerebral infarction
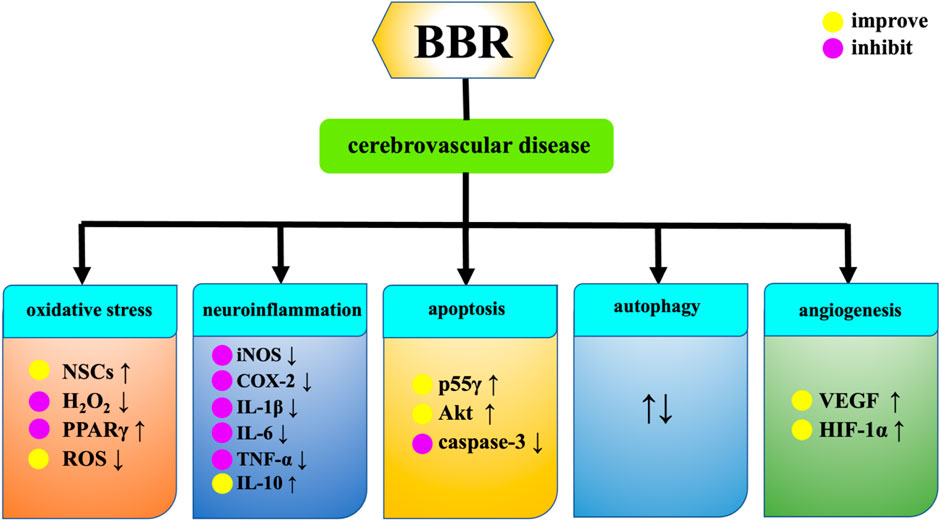
Cerebral ischemia affects all ages, from newborns to the elderly population, and is a major cause of death and morbidity (Dietz et al., 2022). Hydrogen peroxide (H2O2) is a highly active molecule in the oxidation process. The imbalance between the antioxidant system and the oxidative system is participated in neurodegeneration and ischemic brain injury. The pharmacological mechanism of BBR in treating cerebrovascular diseases is presented in Figure 7. BBR can enhance the activity of NSCs damaged by H2O2, improve the damaged morphology of cells, and improve the proliferation inhibition induced by H2O2, resulting in brain tissue reconstruction (Ye, A. L. et al., 2015). Intraperitoneal injection of BBR solution could upregulate the expression levels of phosphorylatioed protein kinase B (p-Akt), phosphorylated glycogen synthase kinase-3 (p-GSK3) and cAMP-response element binding protein (p-CREB). It also reduces the expression of nuclear factor-kappaB (NF-κB), and plays a protective role in brain tissue after cerebral ischemia (Zhang, X. L., 2013). BBR reduces the outflow of potassium ions from ischemic neurons, inhibits neuronal apoptosis in hippocampal CA1 region after ischemia, and thus protects ischemic brain tissue (Wang et al., 2004). Chai et al. discovered that BBR can specifically bind to poly(A) signal in order to control the RB1 mRNA. This prevents the degradation of RB1 mRNA and the increase in Rb protein levels during ischemia reperfusion damage, thereby regulating the release of transcription factors, blocking cell cycle, inhibiting apoptosis and promoting cell survival (Chai et al., 2014). BBR can activate the sphingosine-1-phosphate (S1P)/hypoxia inducible factor-1 (HIF-1) pathway, which is conducive to improving neuronal cell damage caused by ischemia and hypoxia (Zhang Z. et al., 2016). On the basis of the research conducted by Hu et al. (Hu et al., 2012), BBR enhances the activity of PI3K p55γ promoter during cerebral ischemia-reperfusion, resulting in increased Akt activity and decreased caspase-3 activity, thereby exerting an anti-ischemic apoptosis effect. BBR enhances the expression of peroxidase proliferator activating receptor (PPARγ) in ischemia-reperfusion injury, which may be related to the decrease of recombinant DNA methyltransferase 1 (DNMT1) and recombinant DNA methyltransferase 3A (DNMT3a) expression and the decrease of PPARγ promoter methylation in ischemia-reperfusion injury (Pang et al., 2018). In addition, BBR can also achieve neuroprotective effect by inhibiting inflammatory response. The study of Yoo et al. found that the protective effect of BBR on the brain of ischemia-reperfusion injured gerbils is also related to the inhibition of COX-2 expression, prostaglandin E2 generation and its anti-inflammatory mechanism (Yoo et al., 2008). BBR can reduce the neuroinflammatory response by down-regulating the expression of metastasis-associated lung adenocarcinoma transcript 1 (Malat1) and high mobility group box 1 (HMGB1). It protects neuronal cells from cerebral ischemia-reperfusion injury (Cao et al., 2020). BBR downregulates the levels of proinflammatory cytokines iNOS, COX-2, IL-1β, IL-6 and TNF-α, and upregulates the expression of anti-inflammatory cytokine IL-10 through targeting MAPK pathway and AMPK independent mode, alleviates the inflammatory response caused by ischemia reperfusion in rats, exerts the neuroprotective effect in brain. In addition, BBR can also carry the exosome miR-182-5p to injured neurons and play a neuroprotective role by inhibiting neuroinflammation and improving brain injury after ischemic stroke (Ding et al., 2023). Moreover, BBR inhibits ischemic neuronal death by reducing the activity of type 1 N-methyl-D-aspartate receptor (NMDA), reducing the excessive excitatory amino acids produced by ischemic stimulation and Ca2+ inflow caused by oxygen free radicals (Yoo et al., 2006). BBR also inhibits central sympathetic nerves by blocking α-adrenergic receptors, affecting cerebral blood flow supply in stroke patients (Benaissa et al., 2009). Autophagy is an important catabolic process in lysozyme and an essential pathway for survival in ischemic stroke (Li, H. Y. et al., 2024). For cerebral infarction, BBR can effectively reduce blood lipid levels, inhibit the expression of oxidized low-density lipoprotein (ox-LDL) and matrix metalloproteinase-9 (MMP-9), inhibit the growth of carotid atherosclerotic plaque, reduce plaque area, improve plaque stability, and improve the long-term neurological score of cerebral infarction patients (Chai, M. J. et al., 2017a). BBR can increase the level of serum catalase (CAT) and reduce the expression of Malondialdehyde (MDA) in patients with acute cerebral infarction, thereby improving the neurological deficits (Chai, M. J. et al., 2017b). BBR reduce the serum IgG level of patients with acute cerebral infarction, which may inhibit the humoral immune response in the acute phase of cerebral infarction. BBR alleviates inflammatory response in rats with acute cerebral infarction, and its mechanism may be related to activation of Wnt/β-catenin signaling pathway (Yao, Y. et al., 2023). Xi et al. suggested that BBR can protect ischemic neurons by reducing the content of E-Selectin and intercellular call adhesion molecule-1 (ICAM-1) in the plasma of patients with cerebral infarction (Xi, G. M. et al., 2003). Additionally, BBR can also reduce ischemia-reperfusion induced cerebral infarction (Zhou et al., 2008).
3.1.2 Cerebrovascular disease in patients with diabetes and hyperhomocysteinemia
Diabetic peripheral neuropathy (DPN) is the most common chronic complication of diabetes mellitus (DM). Hyperhomocysteinemia (HHcy) can also lead to DPN. BBR improves nerve conduction velocity in diabetic peripheral neuropathy patients. The combination of mecobalamine with DPN can improve the symptoms and nerve conduction velocity of DPN patients, and has significant clinical efficacy (Ye, A. L. et al., 2015). Zhao et al. found through their research (Zhao, X. L. et al., 2006) that BBR can reduce the damage of brain cells, improve the compensatory function of brain cells and reduce blood sugar by alleviating mitochondrial calcium overload and release mitochondrial Cyt-C, thus playing a protective role in the brain tissue of diabetic rats.
3.2 Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease characterized by immune imbalance, central nervous system inflammatory response, and myelin destruction. Experimental autoimmune encephalomyelitis (EAE) is the recognized animal model of this disease (Kroenke et al., 2008). In the inflammatory response of EAE, Th1 cells and Th17 cells are activated, and the secretion of inflammatory cytokines (INF-γ, IL-6, IL-17) respectively increases. BBR can directly act on the JAK/STAT signaling pathway and selectively inhibit the differentiation of Th1 and Th17 cells. BBR indirectly affects the function of Th1 and Th17 cells by affecting the expression and function of co-stimulatory molecules and the production of IL-6, which is due to inhibition of NF-κB activity in CD11b (+) APC (Qin et al., 2010). In addition, BBR can also inhibit the expression and activity of MMP-9, inhibit the migration of T cells to the central nervous system, and protect the blood-brain barrier, thereby reducing the inflammatory infiltration of the central nervous system, alleviating the disease of EAE mice, slowing down the inflammatory response and reducing the incidence of demyelination (Ma et al., 2010).
3.3 Glioma
Due to its invasive nature, molecular signaling, and location in the central nervous system, glioma is one of the most perplexing cancers (Kundu et al., 2019). BBR-mediated apoptosis blocks the AMPK/mTOR/ULK1 pathway and reduces tumor growth in glioblastoma multiforme (GBM) cells in vivo (Wang et al., 2016). BBR inhibits tumor growth and inhibits the expression of p-ERK1/2 and Ki-67 in glioma cells (Sun et al., 2018b). Neuroinflammatory cytokines such as IL-1 secreted by glioma cells are believed to have an impact on the genesis and development of tumors (Nasrollahzadeh et al., 2020). BBR inhibits the activation of inflammatory cytokine caspase-1 through the ERK1/2 signaling pathway, inhibits glioma cells, and subsequently produces IL-1 and IL-18. BBR has a specific anti-proliferation effect on glioma cells, and treatment with BBR can also reduce the cellular motility of U251 and U87 cells and induce cell apoptosis. BBR induces apoptosis of T98G cells in glioblastoma by mediating endoplasmic reticulum stress (Eom et al., 2010). BBR inhibits TGF-β1/SMAD2/3 signaling pathway and affects the proliferation, migration, invasion and apoptosis of glioma cells (Jin et al., 2022). Moreover, BBR has the potential to reverse the mechanism of epithelial-mesenchymal metastasis, which is a hallmark of tumor invasion (Tong et al., 2019). BBR inhibits tumor development by regulating neuroblastoma cell differentiation, stem cell function, and inducing cell death (Rauf et al., 2021).
3.4 Epilepsy
Epilepsy is one of the most widespread neurological diseases in the world. According to the study of Ghanem et al. (Ghanem et al., 2021), BBR can significantly reduce the activity of hypoxia inducible factor-1α (HIF-1α), transforming growth factor-β1 (TGF-β1), histone deacetylase (HDAC) and neuronal restrictive silencing factor NRSF gene expression levels in epileptic mice, and increase the level of Brain-derived neurotrophic factor (BDNF). Intraperitoneal injection of BBR in the mouse model of maximal electroshock-induced seizures can reduce the duration of the seizure of the tonic hind limb extension, resist the seizure of convulsions, and reduce the mortality (Bhutada et al., 2010). The study of Zhang et al. showed that BBR can alleviate pentylenetetrazole (PTZ)-induced seizures, potentially protect zebrafish from further seizures, and use BBR can restore abnormal neuron firing in zebrafish larvae during seizures. In addition, BBR also suppresses the inflammatory response caused by epilepsy (Zhang et al., 2020). Sedaghat et al. found that BBR prevents the loss of hippocampal CA3 neurons and prevents the development of abnormal mossy fiber sprouting (MFS), which is a basic element of the action circuit in chronic epilepsy (Sedaghat et al., 2017). These data suggest that BBR also exerts a neuroprotective effect by alleviating Status epilepticus (SE) and spontaneous recurrent seizures (SRS) in the hippocampal model of epilepsy.
3.5 Neurodegenerative diseases
With the increase of the elderly population, age-related diseases such as neurodegenerative diseases are becoming more and more common, posing a threat to human health (Heemels, 2016). Specifically, neurodegenerative diseases include Alzheimer’s disease, Parkinson’s disease, Huntington’s syndrome, etc. The pathogenesis of neurodegenerative diseases has several common features, such as oxidative damage and mitochondrial dysfunction. Among them, oxidative stress refers to excessive accumulation of ROS, which can lead to mitochondrial dysfunction and ultimately induce neurodegenerative diseases. BBR significantly reduces ROS production in cytoplasm and mitochondria (Caspersen et al., 2005; Poprac et al., 2017), Moreover, BBR also alleviates neurodegenerative diseases by regulating neuroinflammation and autophagy (Figure 8).
3.5.1 Alzheimer’s disease
Alzheimer’s disease (AD) is a neurological illness characterized by memory loss and cognitive impairment, accounting for around 60%–80% of all dementia cases (Rostagno, 2022). Aβ deposition is a crucial factor in the development of Alzheimer’s disease (Huat et al., 2019). BBR can improve the effect of AD through various mechanisms, including inhibiting the hyperphosphorylation of Tau protein, inhibiting the production of Aβ, and inhibiting four key enzymes (acetylcholinesterase, butylcholinesterase and two isomers of monoamine oxidase) in the pathogenesis of AD. BBR can reduce the phosphorylation level of Tau protein, which may be related to its activation of the Akt/glycogen synthase kinase three signaling pathway (Durairajan et al., 2012). BBR can reverse the increase of malondialdehyde content and the decrease of SOD activity induced by calyculin A. Calyculin A-induced tau hyperphosphorylation is decreased by increasing protein phosphatase 2A (PP2A) activity and decreasing glycogen synthase kinase 3β (GSK-3β) activity (Yu et al., 2011). BBR can also enhance autophagy activity and promote autophagy clearance of tau through Class III PI3K/beclin-1 pathway (Chen et al., 2020). BBR significantly reduces Aβ, possibly by down-regulating the phosphorylation of amyloid precursor protein (APP) through activation of PI3K/Akt/GSK3 pathway, and inhibiting the production of Aβ42 by inhibiting PERK/eIF2α/BACE1 signaling pathway (Wu et al., 2021). BBR can also promote Aβ clearance by promoting the expression of insulin-degrading enzyme (IDE) (Zhu, F. Q. et al., 2010). Inhibition of AChE and BChE has been shown to be a key target for effective management of AD by alleviating cholinergic deficiency and improving neurotransmission, and BBR has inhibitory effects on both AChE and BChE (Greig et al., 2005; Muñoz-Torrero, 2008). Not only that, BBR can also activate macrophages and increase their phagocytic function, increase the production of interleukin, (IL)-1 and can be used as a neuroprotective agent against AD (Kumazawa et al., 1984; Panahi et al., 2013). Long-term administration of BBR increases the expression of IL-1β and iNOS in the hippocampus of AD mice and improves memory impairment (Ji and Shen, 2012).
3.5.2 Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurological disorder, affecting about seven million people worldwide (Pringsheim et al., 2014). Inhibition of Monoamine oxidase-B (MAO-B) has been proved to delay the onset of PD or reduce the symptoms of PD (Youdim et al., 2006). BBR and MAO-B are bound by hydrophobic interaction, and BBR may partially reduce the degradation of dopamine and the production of H2O2 through the inhibition of MAO-B, thus alleviating the symptoms of PD. Kim et al. (Kim et al., 2014a) used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTP/P) mouse PD model to study the effects of BBR on dopamine consumption and short-term memory of hippocampal neurogenesis. BBR was found to enhance motor balance and coordination by preventing damage to dopaminergic neurons, also improved short-term memory by inhibiting apoptosis of hippocampal cells. These data show that BBR treatment may serve as a potential therapeutic strategy to improve memory impairment and physical dysfunction in PD patients. In the study of Negahdar et al. (Negahdar et al., 2015) by using 6-hydroxydopamine (6-OHDA) -induced PD model, BBR could significantly improve lateral rotation behavior in PD rats, and could also prevent the reduction of the number of tyrosine-hydroxylase (TH) -positive neurons in the group, providing a new strategy for PD treatment. It is worth noting that BBR can enhance the cytotoxicity caused by 6-OHDA, and the intraparitoneal injection of BBR for 21d in the 6-OHDA PD rat model can cause degeneration of dopaminergic neurons in the substrantia nigra (Kwon et al., 2010). Therefore, patients with PD should pay attention to the interaction between BBR and levodopa and avoid drug effects when using BBR.
3.5.3 Huntington’s disease
Huntington’s disease (HD) is an inherited neurodegenerative genetic disorder caused by the amplification (variable length) of CAG trinucleotide repeats in HTT, the gene encoding the Huntington protein, which accumulate in affected brain regions in an age-dependent manner, leading to late-onset neurodegeneration (Walker, 2007). In Jiang et al.’s study (Jiang W. et al., 2015), BBR has a protective effect on transgenic HD (N171-82Q) mice. When taken orally, BBR can effectively alleviate motor dysfunction and prolong the survival time of transgenic HD mice, and BBR can also promote the degradation of mutant Huntington protein by enhancing autophagy function. The autophagy-lysosome pathway also plays a crucial role in the clearance of the readily aggregated mutant Huntington protein (polyQ-HTT) (Martinez-Vicente et al., 2010), and induction of autophagy enhances the clearance of polyQ-HTT aggregates and reduces the toxicity of the mutant Huntington protein fragment (Floto et al., 2007). BBR can significantly trigger autophagy and remove polyQ-HTT aggregates, thereby significantly improving the neurophenotype of HD mice (Jiang W. et al., 2015; Fan et al., 2019).
3.6 Mental system disorders
A mental illness is a disorder of brain function caused by a variety of reasons that manifests in different forms as disorders in mental functions like cognition, behavior, will and emotion.
3.6.1 Anxiety
Anxiety is a state of excessive fear, characterized by motor tension, sympathetic overactivity, worry, and vigilance syndrome. BBR plays an anti-anxiety role by reducing the concentration of norepinephrine, dopamine and 5-hydroxytryptamine in the brain stem, and increasing the concentration of Vanillymandelic Acid (VMA) and 4-hydroxy-3-methoxy-phenylacetic acid (HVA). The anti-anxiety effect of 100 mg/kg BBR on mice is the same as that of 1 mg/kg diazepam and 2 mg/kg buspirone, and its anti-anxiety effect is related to accelerating the renewal rate of monoamine transmitters in the brain stem and reducing the activity of 5-HT-ergic systems (Peng et al., 2004). Yu et al. found that BBR improved anxiety in 5XFAD transgenic mice with AD (Yu, W. et al., 2021). Autonomic nervous system dysfunction and anxiety and other mental disorders often occur in women before and after menopause due to the fluctuation or reduction of sex hormone levels (Mulhall et al., 2018). BBR can also increase the content of equol in feces and serum and the ratio of equol to daidzein by enriching Lactobacillus, Bacteroides, Bifidobacterium and Akkermansia muciniphila in the intestine. Improved anxiety-like behavior in female ovariectomized rats. The regulatory effect of BBR was eliminated in germ-free animals, but the changes in microbiota, equol content and anxiety-like behavior of the animals receiving fecal microbiota transplantation were basically the same as those of the donors. This suggests that BBR can improve anxiety-like behaviors induced by decreased ovarian hormones by regulating intestinal microbiota and promoting equol conversion (Fang, Y., 2022; Fang et al., 2021).
3.6.2 Depression
Depression is a relatively common disease with the highest suicide rate in psychiatric departments, mainly manifested by low mood, anxiety, insomnia, loss of appetite, and inconcentration (Malhi and Mann, 2018). Norepinephrine, serotonin, and dopamine are substrates for monoamine oxidase (MAO), and MAO inhibitors have antidepressant activity, BBR may regulate the levels of brain biogenic amines (norepinephrine, serotonin, and dopamine) by interacting with adrenaline receptors, 5-HT, dopamine, and MAO, and exert antidepressant-like effects in various depression models. Moreover, the antidepressant like effect of BBR in Forced Swimming Test (FST) involves interaction with the L-arginine-NO-cGMP pathway (Peng et al., 2007; Kulkarni and Dhir, 2008; Kulkarni and Dhir, 2007). Organic cation transporters (OCTs) and plasma membrane monoamine transporters (PMAT) are the most efficient transporters for uptake of 5-HT, NE and other biogenic amine neurotransmitters (Daws et al., 2013). Sun et al.'s study found that BBR can play an antidepressant role by inhibiting OCT2 and OCT3 (Sun et al., 2014). The study of Yi et al. showed that BBR also exerts antidepressant effects by regulating the miR-34a-synaptotagmin1/Bcl-2 axis (Yi et al., 2021). BBR may also inhibit NF-κB signaling pathway and its downstream targets such as proinflammatory cytokines and iNOS exert antidepressant effects (Liu et al., 2017). The HPA axis is an important part of the neuroendocrine system and is closely related to depression. The activation of HPA axis is manifested by increased secretion of corticotrophin-releasing factor (CRF) in the hypothalamus. Then the pituitary adrenocorticotrophin (ACTH) release. BDNF has been shown to be a key contributor to antidepressant effects (Erickson et al., 2012), and excessive plasma corticosterone (CORT) is also an important trigger for depressive episodes (Gong et al., 2019). BBR combined with ginsenoside can upregulate the expression level of plasma BDNF and downregulate the levels of CORT and ACTH (Shen et al., 2016; Zhang et al., 2021). BBR can also significantly reduce the expression of CRF in hypothalamus, and significantly improve the depressive behavior of rats with chronic morphine withdrawal (Lee et al., 2012; Gao et al., 2024).
3.6.3 Schizophrenia
Schizophrenia is a chronic and severe mental illness in which dopamine-mediated neurotransmission plays a crucial role in psychiatric and nervous system disorders. The prolyl oligopeptidase (POP) family of enzymes are cytoplasmic serine peptidases, and the activity of POP is reduced in depression and increased in psychiatric disorders such as mania and schizophrenia (Maes et al., 1995). BBR inhibits POP in a concentration dependent manner, thereby exerting anti schizophrenia effects (Tarrago et al., 2007). TetrahydroprotoBBRs (THPBs), a derivative of BBR, separated from Chinese herbal medicine compound, through its unique D2 receptor antagonist and agonist activity of D1, play a role of resistant schizophrenia (Chu et al., 2008; Kulkarni and Dhir, 2010). Ghotbi et al. created a rat schizophrenia model by administering MK-801 (NMDA receptor antagonist) (Ghotbi Ravandi et al., 2019), and the results showed that BBR had neuroprotective effects on rats with MK-801-related behavioral defects, suggesting that BBR had anti-schizophrenic effects (Shayganfard, 2023).
4 Conclusion
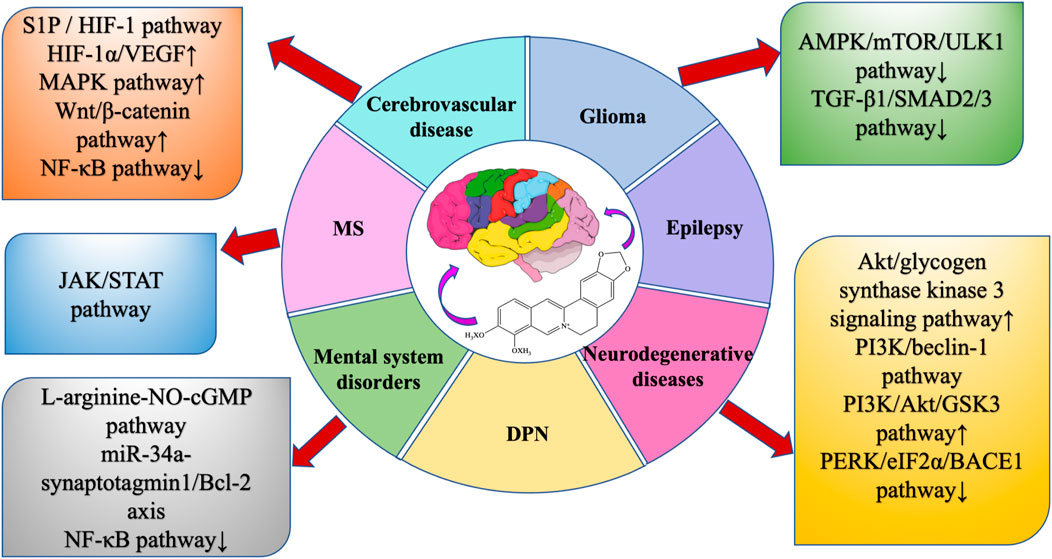
BBR is widely present in the roots, rhizomes, stems, or bark of many traditionally used herbs, and has a wide range of physiological activities, especially in neuroprotection, but its bioavailability is low, so the study of BBR dosage forms is very important to improve bioavailability. This review summarizes the extraction methods, dosage forms, pharmacological effects, and protective effects of BBR on the central nervous system, and summarizes a large number of studies, indicating that BBR can directly or indirectly regulate various intracellular molecules and signaling pathways (Figures 9, 10) , thereby improving nervous system diseases. Such as cerebrovascular disease, multiple sclerosis, glioma, epilepsy, AD, PD, HD, anxiety, depression, and schizophrenia.
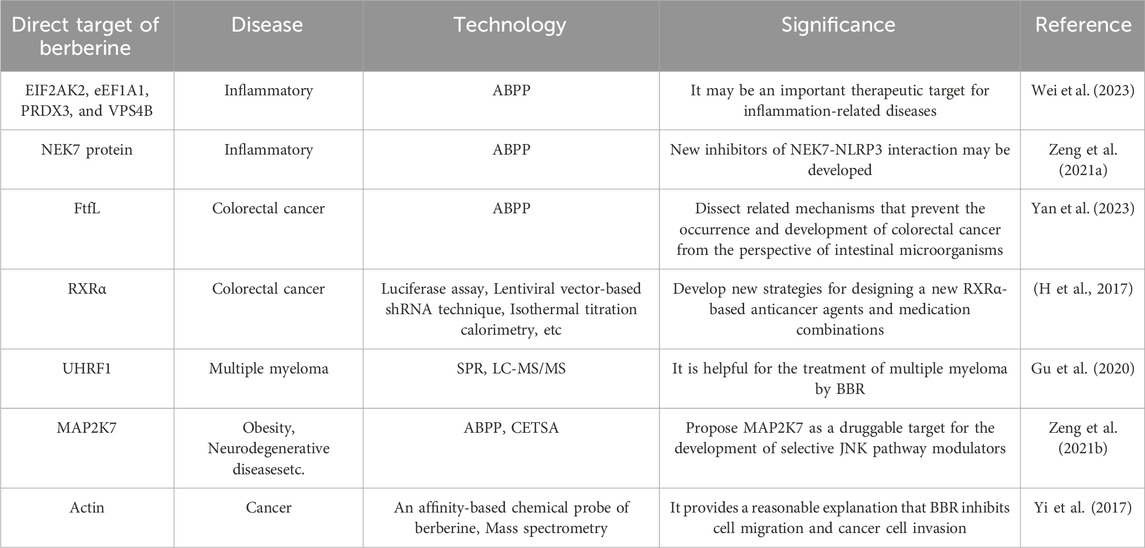
At present, the complex network mechanism of BBR is not fully understood, which may be due to the interaction with multiple targets or with proteins that are involved in many pathways. In order to further study the pharmacological mechanism of berberine, it can be revealed by exploring the possible protein targets directly acting on berberine (some researches on the targets of direct BBR action are shown in Table 3). Researches on the targets of direct BBR action can not only reveal the mechanism of therapeutic effect of BBR from a fundamental perspective, but also provide new strategies for the design of drug combinations. However, there are few studies on the direct targets of BBR for the treatment of neurological diseases, so it is important to further explore BBR, which will help clarify the multi-active mechanism of BBR’s neuroprotective effect and its corresponding biological effects.
Author contributions
Y-XS: Data curation, Writing - original draft. Y-HZ: Data curation, Writing - review and editing. R-JF: Writing - review and editing. D-QX: Writing - review and editing. Y-PT: Conceptualization, Writing - review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Natural Science Foundation of China (82204853), the Entrusted service project of Shaanxi Administration of Traditional Chinese Medicine (ZYJXG-L23001), 2023 Sanqin Talent Special Support Program Innovation and Entrepreneurship Team Project, and Sci-Tech Innovation Talent System Construction Program of Shaanxi University of Chinese Medicine (2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1429050/full#supplementary-material
References
Ahmed, T., Gilani, A.-U.-H., Abdollahi, M., Daglia, M., Nabavi, S. F., and Nabavi, S. M. (2015). Berberine and neurodegeneration: a review of literature. Pharmacol. Rep. 67, 970–979. doi:10.1016/j.pharep.2015.03.002
Andrade, C. (2015). Sustained-release, extended-release, and other time-release formulations in neuropsychiatry. J. Clin. Psychiatry 76, e995–e999. doi:10.4088/JCP.15f10219
Barnham, K. J., Masters, C. L., and Bush, A. I. (2004). Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214. doi:10.1038/nrd1330
Benaissa, F., Mohseni-Rad, H., Rahimi-Moghaddam, P., and Mahmoudian, M. (2009). Berberine reduces the hypoxic-ischemic insult in rat pup brain. Acta Physiol. Hung 96, 213–220. doi:10.1556/APhysiol.96.2009.2.6
Bhutada, P., Mundhada, Y., Bansod, K., Dixit, P., Umathe, S., and Mundhada, D. (2010). Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 18, 207–210. doi:10.1016/j.yebeh.2010.03.007
Cao, D. W., Liu, M. M., Duan, R., Tao, Y. F., Zhou, J. S., Fang, W. R., et al. (2020). The lncRNA Malat1 functions as a ceRNA to contribute to berberine-mediated inhibition of HMGB1 by sponging miR-181c-5p in poststroke inflammation. Acta Pharmacol. Sin. 41, 22–33. doi:10.1038/s41401-019-0284-y
Caspersen, C., Wang, N., Yao, J., Sosunov, A., Chen, X., Lustbader, J. W., et al. (2005). Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 19, 2040–2041. doi:10.1096/fj.05-3735fje
Chai, M. J., Wang, H., Li, Y., Wang, P., Yang, F., Li, H. S., et al. (2017a). Effect of berberine on stability of carotid atherosclerotic plaque and serum matrix metalloproteinases-9, oxidized low density lipoprotein in patients with cerebral infarction. Chin. J. Mod. Med. 27, 47–53. doi:10.3969/j.issn.1005-8982.2017.05.010
Chai, M. J., Wang, P., Li, Y., Yang, F., Lu, D. D., Wang, H., et al. (2017b). Effects of berberine on nerve function and serum malondialdehyde and catalase in patients with acute cerebral infarction. Mod Inst and Med Trt 23, 91–92. doi:10.11876/mimt201705037
Chai, Y. S., Yuan, Z. Y., Lei, F., Wang, Y. G., Hu, J., Du, F., et al. (2014). Inhibition of retinoblastoma mRNA degradation through Poly (A) involved in the neuroprotective effect of berberine against cerebral ischemia. PLoS One 9, e90850. doi:10.1371/journal.pone.0090850
Chen, S. B., and Zhou, L. J. (2008). Study on formation technology of berberine hydrochloride dropping pills. Qilu Pharm., 113–115.
Chen, W., Miao, Y. Q., Fan, D. J., Yang, S. S., Lin, X., Meng, L. K., et al. (2011). Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 12, 705–711. doi:10.1208/s12249-011-9632-z
Chen, Y., Chen, Y., Liang, Y., Chen, H., Ji, X., and Huang, M. (2020). Berberine mitigates cognitive decline in an Alzheimer’s disease mouse model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 121, 109670. doi:10.1016/j.biopha.2019.109670
Cheng, Y. M., and Chen, R. H. (2007). Studies on the extracting technology of berberine. Lishizhen Med. Mater Med. Res., 1445–1447.
Cheng, Z., Kang, C., Che, S., Su, J., Sun, Q., Ge, T., et al. (2022). Berberine: a promising treatment for neurodegenerative diseases. Front. Pharmacol. 13, 845591. doi:10.3389/fphar.2022.845591
Chu, H., Jin, G., Friedman, E., and Zhen, X. (2008). Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol. Neurobiol. 28, 491–499. doi:10.1007/s10571-007-9179-4
Daws, L. C., Koek, W., and Mitchell, N. C. (2013). Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders. ACS Chem. Neurosci. 4, 16–21. doi:10.1021/cn3001872
Deng, Y. H., Wang, S. N., Wu, Q., Wan, F., Lei, X., and Wang, Z. P. (2004). Preparation of berberine hydrochloride liposomes by active loading method. Chin. Pharm. J., 40–42.
Deng, Y. H., Yang, L., Zhou, B., Fang, B. B., and Luo, W. H. (2002). Microwave assisted extraction of traditional Chinese medicine Huanglian. J. Math. Med., 88–89.
Dietz, R. M., Dingman, A. L., and Herson, P. S. (2022). Cerebral ischemia in the developing brain. J. Cereb. Blood Flow. Metab. 42, 1777–1796. doi:10.1177/0271678X221111600
Ding, S., Zhao, X. R., Zhao, L., Li, B. Q., Bi, H. D., Zhou, J., et al. (2021). Study of berberine on reducing cerebral ischemia reperfusion injury by inhibiting autophagy through B cell lymphoma 2/Beclin-1 complex. Chin. J. Clin. Pharmacol. 37, 1094–1097. doi:10.13699/j.cnki.1001-6821.2021.09.016
Ding, W., Gu, Q., Liu, M., Zou, J., Sun, J., and Zhu, J. (2023). Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. Int. Immunopharmacol. 118, 110047. doi:10.1016/j.intimp.2023.110047
Dording, C. M., and Boyden, S. D. (2019). “Depression, antidepressants, and sexual functioning,” in The Massachusetts general hospital guide to depression (Cham: Humana Press), 123–137. doi:10.1007/978-3-319-97241-1_9
Durairajan, S. S. K., Liu, L. F., Lu, J. H., Chen, L. L., Yuan, Q., Chung, S. K., et al. (2012). Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol. Aging 33, 2903–2919. doi:10.1016/j.neurobiolaging.2012.02.016
Eom, K. S., Kim, H. J., So, H. S., Park, R., and Kim, T. Y. (2010). Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol. Pharm. Bull. 33, 1644–1649. doi:10.1248/bpb.33.1644
Erickson, K. I., Miller, D. L., and Roecklein, K. A. (2012). The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist 18, 82–97. doi:10.1177/1073858410397054
Fan, D., Liu, L., Wu, Z., and Cao, M. (2019). Combating neurodegenerative diseases with the plant alkaloid berberine: molecular mechanisms and therapeutic potential. Curr. Neuropharmacol. 17, 563–579. doi:10.2174/1570159X16666180419141613
Fan, X., Wang, J., Hou, J., Lin, C., Bensoussan, A., Chang, D., et al. (2015). Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 13, 92. doi:10.1186/s12967-015-0450-z
Fang, Y. (2022). The influences of berberine on anxiety-like behaviors and metabolism abnormalities in female ovariectomized rats via regulating gut microbiota. Peking. Univ. Hlt Sci. Cent. doi:10.44277/d.cnki.gbdyx.2021.000053
Fang, Y., Zhang, J., Zhu, S., He, M., Ma, S., Jia, Q., et al. (2021). Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol. Res. 165, 105439. doi:10.1016/j.phrs.2021.105439
Fleming, A., Noda, T., Yoshimori, T., and Rubinsztein, D. C. (2011). Chemical modulators of autophagy as biological probes and potential therapeutics. Nat. Chem. Biol. 7, 9–17. doi:10.1038/nchembio.500
Floto, R. A., Sarkar, S., Perlstein, E. O., Kampmann, B., Schreiber, S. L., and Rubinsztein, D. C. (2007). Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy 3, 620–622. doi:10.4161/auto.4898
Gao, Y., Nie, K., Wang, H., Dong, H., and Tang, Y. (2024). Research progress on antidepressant effects and mechanisms of berberine. Front. Pharmacol. 15, 1331440. doi:10.3389/fphar.2024.1331440
Ghanem, H. B., Emam, M. N., Dareen Abdelaziz Mohammed, A., and Rania Nagi, A. E. (2021). Impact of berberine on some epigenetic, transcription regulation and inflammatory biomarkers in a mice model of epilepsy. Rep. Biochem. Mol. Biol. 10, 362–372. doi:10.52547/rbmb.10.3.362
Ghotbi Ravandi, S., Shabani, M., Bashiri, H., Saeedi Goraghani, M., Khodamoradi, M., and Nozari, M. (2019). Ameliorating effects of berberine on MK-801-induced cognitive and motor impairments in a neonatal rat model of schizophrenia. Neurosci. Lett. 706, 151–157. doi:10.1016/j.neulet.2019.05.029
Gong, Q., Yan, X. J., Lei, F., Wang, M. L., He, L. L., Luo, Y. Y., et al. (2019). Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: pivotal sites of oxidative phosphorylation. Mol. Brain 12, 118. doi:10.1186/s13041-019-0518-4
Greig, N. H., Utsuki, T., Ingram, D. K., Wang, Y., Pepeu, G., Scali, C., et al. (2005). Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. U. S. A. 102, 17213–17218. doi:10.1073/pnas.0508575102
Gu, C., Yin, Z., Nie, H., Liu, Y., Yang, J., Huang, G., et al. (2020). Identification of berberine as a novel drug for the treatment of multiple myeloma via targeting UHRF1. BMC Biol. 18, 33. doi:10.1186/s12915-020-00766-8
Guo, X. W., Zhang, F. C., Lin, S. Y., Chen, Z. G., and Qin, X. Q. (1995). Effect of ultrasonic extraction on extraction rate of berberine. Chin. J. Chin. Mater Med. 673–675, 703.
Hao, J. F., Zhao, X. M., Wang, J. Z., Guo, F. G., Xiao, J. H., and Kong, Z. F. (2010). Optimization of thermosensitive in situ gel system containing berberine hydrochloride for ocular use. Chin. Tradit. Herb. Drugs 41, 550–555.
Hsu, Y.-Y., Chen, C. S., Wu, S. N., Jong, Y.-J., and Lo, Y.-C. (2012). Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 46, 415–425. doi:10.1016/j.ejps.2012.03.004
Hu, C. Y., and Mo, Z. X. (2017). Research progress on pharmacological actions and mechanism of berberine. Chin. J. Exp. Tradit. Med. Form. 23, 213–219. doi:10.13422/j.cnki.syfjx.2017200213
Hu, J., Chai, Y., Wang, Y., Kheir, M. M., Li, H., Yuan, Z., et al. (2012). PI3K p55γ promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. Eur. J. Pharmacol. 674, 132–142. doi:10.1016/j.ejphar.2011.11.014
Hu, X., Li, Y., Zhang, E., Wang, X., Xing, M., Wang, Q., et al. (2013). Preparation and evaluation of orally disintegrating tablets containing taste-masked microcapsules of berberine hydrochloride. AAPS PharmSciTech 14, 29–37. doi:10.1208/s12249-012-9880-6
Huang, J., Feng, W., Li, S., Tang, H., Qin, S., Li, W., et al. (2021). Berberine exerts anti-cancer activity by modulating adenosine monophosphate-activated protein kinase (AMPK) and the Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/AKT) signaling pathways. Curr. Pharm. Des. 27, 565–574. doi:10.2174/1381612826666200928155728
Huat, T. J., Camats-Perna, J., Newcombe, E. A., Valmas, N., Kitazawa, M., and Medeiros, R. (2019). Metal toxicity links to alzheimer’s disease and neuroinflammation. J. Mol. Biol. 431, 1843–1868. doi:10.1016/j.jmb.2019.01.018
Issat, T., Nowis, D., Bil, J., Winiarska, M., Jakobisiak, M., and Golab, J. (2011). Antitumor effects of the combination of cholesterol reducing drugs. Oncol. Rep. 26, 169–176. doi:10.3892/or.2011.1261
Ji, H. F., and Shen, L. (2012). Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. ScientificWorldJournal 2012, 823201. doi:10.1100/2012/823201
Ji, J., He, X., Yang, X. L., Du, W. J., Cui, C. L., Wang, L., et al. (2017). The in vitro/vivo evaluation of prepared gastric floating tablets of berberine hydrochloride. AAPS PharmSciTech 18, 2149–2156. doi:10.1208/s12249-016-0696-7
Jiang, H. Y., Zhang, J. F., Jiang, S. F., Lan, Z. P., and Wang, S. (2015a). Preparation and in vitro evaluation of stomach adhesive microspheres loaded berberine. Chin. J. Biol. Pharms 35, 30–33.
Jiang, W., Wei, W., Gaertig, M. A., Li, S., and Li, X. J. (2015b). Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS One 10, e0134142. doi:10.1371/journal.pone.0134142
Jin, Q., Zhao, W. Y., Zhang, G. Y., Wang, Y., and Wang, C. F. (2011). Preparation of berberine hydrochloride liposomes. Her. Med. 30, 353–355.
Jin, Y., Zhang, J., Pan, Y., and Shen, W. (2022). Berberine suppressed the progression of human glioma cells by inhibiting the TGF-β1/SMAD2/3 signaling pathway. Integr. Cancer Ther. 21, 15347354221130303. doi:10.1177/15347354221130303
Kim, M., Cho, K. H., Shin, M. S., Lee, J. M., Cho, H. S., Kim, C. J., et al. (2014a). Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 33, 870–878. doi:10.3892/ijmm.2014.1656
Kim, M., Shin, M. S., Lee, J. M., Cho, H. S., Kim, C. J., Kim, Y. J., et al. (2014b). Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of Phosphoinositide 3-Kinase/Protein Kinase B signaling pathway. Int. Neurourol. J. 18, 115–125. doi:10.5213/inj.2014.18.3.115
Kroenke, M. A., Carlson, T. J., Andjelkovic, A. V., and Segal, B. M. (2008). IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205, 1535–1541. doi:10.1084/jem.20080159
Kulkarni, S. K., and Dhir, A. (2007). Possible involvement of L-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling pathway in the antidepressant activity of berberine chloride. Eur. J. Pharmacol. 569, 77–83. doi:10.1016/j.ejphar.2007.05.002
Kulkarni, S. K., and Dhir, A. (2008). On the mechanism of antidepressant-like action of berberine chloride. Eur. J. Pharmacol. 589, 163–172. doi:10.1016/j.ejphar.2008.05.043
Kulkarni, S. K., and Dhir, A. (2010). Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 24, 317–324. doi:10.1002/ptr.2968
Kumazawa, Y., Itagaki, A., Fukumoto, M., Fujisawa, H., Nishimura, C., and Nomoto, K. (1984). Activation of peritoneal macrophages by berberine-type alkaloids in terms of induction of cytostatic activity. Int. J. Immunopharmacol. 6, 587–592. doi:10.1016/0192-0561(84)90069-9
Kundu, M., Das, S., Dhara, D., and Mandal, M. (2019). Prospect of natural products in glioma: a novel avenue in glioma management. Phytother. Res. 33, 2571–2584. doi:10.1002/ptr.6426
Kwon, I. H., Choi, H. S., Shin, K. S., Lee, B. K., Lee, C. K., Hwang, B. Y., et al. (2010). Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 486, 29–33. doi:10.1016/j.neulet.2010.09.038
Lapierre, L. R., Kumsta, C., Sandri, M., Ballabio, A., and Hansen, M. (2015). Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 11, 867–880. doi:10.1080/15548627.2015.1034410
Lee, B., Sur, B., Yeom, M., Shim, I., Lee, H., and Hahm, D.-H. (2012). Effect of berberine on depression and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J. Physiol. Pharmacol. 16, 379–386. doi:10.4196/kjpp.2012.16.6.379
Li, H. R. (2024). The improvement effect and mechanism of berberine on Alzheimer’s disease through the FOX03a/BIM signaling pathway. JiLin Unvi. doi:10.27162/d.cnki.gjlin.2023.002627
Li, H. Y., Lan, R., Wang, M. M., Wang, W. W., Zhang, Y., Tang, C., et al. (2024). Research progress of berberine in the field of neuroprotection in ischemic stroke. Chin. J. Comp. Med. 34, 129–136. doi:10.3969/j.issn.1671-7856.2024.02.016
Li, M., and Lin, J. (2014). The apoptotic pathways and their mechanisms. J. Int. Obstet. Gynecol. 41, 103–107.
Li, M. Q., Geng, Y. H., Liu, G. M., Zhang, Q., and Kang, Y. F. (2006). Application of aqueous two phase extraction technique in separation and purification of resveratrol. Nat. Prod. Res. Dev., 647–649. doi:10.16333/j.1001-6880.2006.04.030
Li, X. D., Zhang, H. L., Huang, X. Y., Wang, N., Bao, H., and Gao, Y. (2015). Preparation of berberine hydrochloride-loaded gelatin nanoparticles. Northwest Pharm. J. 30, 393–397.
Li, Z., Jiang, T., Lu, Q., Xu, K., He, J., Xie, L., et al. (2020). Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cell Mol. Neurobiol. 40, 587–602. doi:10.1007/s10571-019-00756-7
Li, Z. P., Oy, Y. Z., Zhang, A. H., and Wu, N. (2003). A study on preparation of inclusion complexes of berberine with β-cyclodextrin and bacterium test. Fine Chem. Int. 63, 46–48. doi:10.19342/j.cnki.issn.1009-9212.2003.06.018
Liang, B. L., and Zhou, M. J. (2006). Study on extraction technology of berberine by enzymatic hydrolysis. Appl. Chem. Ind. 373–374, 378. doi:10.16581/j.cnki.issn1671-3206.2006.05.016
Liang, Y., Huang, M., Jiang, X., Liu, Q., Chang, X., and Guo, Y. (2017). The neuroprotective effects of Berberine against amyloid β-protein-induced apoptosis in primary cultured hippocampal neurons via mitochondria-related caspase pathway. Neurosci. Lett. 655, 46–53. doi:10.1016/j.neulet.2017.06.048
Liao, Z. X., Li, Y. L., Ji, L. J., Sun, H. F., and Dong, J. S. (1998). Studies on the best extracting technology of bererine hydrochloride in berberis from QingHai. Nat. Prod. Res. Dev., 62–65. doi:10.16333/j.1001-6880.1998.02.014
Lin, A. H., Li, H. Y., Liu, Y. M., and Qiu, X. H. (2007). Preparation and release characteristics of berberine chitosan nanoparticles in vitro. J. China Pharm., 755–757.
Lin, H. B., Zhang, Z. W., Sun, X. M., Lu, N., Lin, J. Q., and Lin, J. Q. (2004). Study on extraction technology of berberine by enzymatic hydrolysis. Chin. J. Exp. Tradit. Med. Form., 1–5. doi:10.13422/j.cnki.syfjx.2004.01.001
Liu, B., Li, W., Chang, Y., Dong, W., and Ni, L. (2006). Extraction of berberine from rhizome of Coptis chinensis Franch using supercritical fluid extraction. J. Pharm. Biomed. Anal. 41, 1056–1060. doi:10.1016/j.jpba.2006.01.034
Liu, H., Ren, X., and Ma, C. (2018). Effect of berberine on angiogenesis and HIF-1α/VEGF signal transduction pathway in rats with cerebral ischemia - reperfusion injury. J. Coll. Physicians Surg. Pak 28, 753–757.
Liu, J., Wu, H. Y., Wang, A. Q., Gu, Y. P., and Ling, S. (2004). Preparation and dissolution of berberine hydrochloride microcapsules. Chin. J. Pharm. 26–27, 48.
Liu, X., Xue, H. M., Wang, J. M., and Gao, Y. J. (2008). Study on the preparation of colon-specific delivery system for pH and time-controlled coated tablets of berberine hydrochloride and its in vitro release. World Sci-Tech R&D, 347–351. doi:10.16507/j.issn.1006-6055.2008.03.019
Liu, Y., Hao, H., Xie, H., Lv, H., Liu, C., and Wang, G. (2009). Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J. Pharm. Sci. 98, 4391–4401. doi:10.1002/jps.21721
Liu, Y. J., and Zhang, L. Y. (2008). Preparation and characteristics of berberine polylactic acid microspheres. J. Beijing Univ. Chem. Technol., 52–55.
Liu, Y. M., Niu, L., Wang, L. L., Bai, L., Fang, X. Y., Li, Y. C., et al. (2017). Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 134, 220–227. doi:10.1016/j.brainresbull.2017.08.008
Liu, Y. T., Hao, H. P., Xie, H. G., Lai, L., Wang, Q., Liu, C. X., et al. (2010). Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 38, 1779–1784. doi:10.1124/dmd.110.033936
Liu, Y. Z. (2007). Principle and practice of smashing tissue extraction and herbal blitzkrieg extractor. Chin. J. Nat. Med., 401–407.
Lu, D. Y., Tang, C. H., Chen, Y. H., and Wei, I. H. (2010). Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell Biochem. 110, 697–705. doi:10.1002/jcb.22580
Luo, Y. N., and Qin, S. M. (2011). Study on extraction of berberine hydrochloride and preparation condition of dropping pills. Strait Pharm. J. 23, 31–33.
Ma, X., Chen, Z., Wang, L., Wang, G., Wang, Z., Dong, X., et al. (2018). The pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by berberine. Front. Pharmacol. 9, 782. doi:10.3389/fphar.2018.00782
Ma, X., Jiang, Y., Wu, A., Chen, X., Pi, R., Liu, M., et al. (2010). Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One 5, e13489. doi:10.1371/journal.pone.0013489
Maes, M., Goossens, F., Scharpé, S., Calabrese, J., Desnyder, R., and Meltzer, H. Y. (1995). Alterations in plasma prolyl endopeptidase activity in depression, mania, and schizophrenia: effects of antidepressants, mood stabilizers, and antipsychotic drugs. Psychiatry Res. 58, 217–225. doi:10.1016/0165-1781(95)02698-v
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392, 2299–2312. doi:10.1016/S0140-6736(18)31948-2
Martinez-Vicente, M., Talloczy, Z., Wong, E., Tang, G., Koga, H., Kaushik, S., et al. (2010). Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 13, 567–576. doi:10.1038/nn.2528
Menzies, F. M., Fleming, A., Caricasole, A., Bento, C. F., Andrews, S. P., Ashkenazi, A., et al. (2017). Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034. doi:10.1016/j.neuron.2017.01.022
Mohammadinejad, R., Ahmadi, Z., Tavakol, S., and Ashrafizadeh, M. (2019). Berberine as a potential autophagy modulator. J. Cell Physiol. 234, 14914–14926. doi:10.1002/jcp.28325
Mulhall, S., Andel, R., and Anstey, K. J. (2018). Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas 108, 7–12. doi:10.1016/j.maturitas.2017.11.005
Muñoz-Torrero, D. (2008). Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 15, 2433–2455. doi:10.2174/092986708785909067
Nam, K. N., Kim, J. H., Jung, H. J., Park, J. M., Moon, S. K., Kim, Y. S., et al. (2010). Berberine inhibits inflammatory activation of rat brain microglia. Neural Regen. Res. 5, 1384–1390. doi:10.3969/j.issn.1673-5374.2010.18.004
Nasrollahzadeh, E., Razi, S., Keshavarz-Fathi, M., Mazzone, M., and Rezaei, N. (2020). Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol. Immunother. 69, 1673–1697. doi:10.1007/s00262-020-02616-6
Negahdar, F., Mehdizadeh, M., Joghataei, M. T., Roghani, M., Mehraeen, F., and Poorghayoomi, E. (2015). Berberine chloride pretreatment exhibits neuroprotective effect against 6-hydroxydopamine-induced neuronal insult in rat. Iran. J. Pharm. Res. 14, 1145–1152.
Ong, E. S., and Len, S. M. (2003). Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 482, 81–89. doi:10.1016/S0003-2670(03)00196-X
Ong, E. S., Woo, S. O., and Yong, Y. L. (2000). Pressurized liquid extraction of berberine and aristolochic acids in medicinal plants. J. Chromatogr. A 904, 57–64. doi:10.1016/s0021-9673(00)00914-6
Pan, F. L., Liu, Y., Liu, H. N., Yu, S., Li, X. Y., Wang, X. Y., et al. (2024). Analysis of inhibitory effect of berberine compounds on acetylcholinesterase and blood-brain barrier permeability. Chin. J. Exp. Tradit. Med. Form. 30, 116–124. doi:10.13422/j.cnki.syfjx.20240411
Pan, G., Wang, G. J., Liu, X. D., Fawcett, J. P., and Xie, Y. Y. (2002). The involvement of P-glycoprotein in berberine absorption. Pharmacol. Toxicol. 91, 193–197. doi:10.1034/j.1600-0773.2002.t01-1-910403.x
Panahi, N., Mahmoudian, M., Mortazavi, P., and Hashjin, G. S. (2013). Effects of berberine on β-secretase activity in a rabbit model of Alzheimer's disease. Arch. Med. Sci. 9, 146–150. doi:10.5114/aoms.2013.33354
Pang, X. X., and Xu, W. L. (1996). Improvement of experimental method for extracting berberine hydrochloride from Huanglian. Acad. J. Guangdong Coll. Pharm., 53–54. doi:10.16809/j.cnki.1006-8783.1996.04.016
Pang, Y., Liang, Y., Wang, Y., Lei, F., Yuan, Z., Xing, D., et al. (2018). Effect of berberine against cerebral ischemia and reperfusion involving in the methylation of PPARγ promote. J. Chin. Pharm. Sci. 27, 170–182. doi:10.5246/jcps.2018.03.018
Peng, W. H., Lo, K. L., Lee, Y. H., Hung, T. H., and Lin, Y. C. (2007). Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 81, 933–938. doi:10.1016/j.lfs.2007.08.003
Peng, W. H., Wu, C. R., Chen, C. S., Chen, C. F., Leu, Z.-C., and Hsieh, M.-T. (2004). Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: interaction with drugs acting at 5-HT receptors. Life Sci. 75, 2451–2462. doi:10.1016/j.lfs.2004.04.032
Perry, V. H., and Holmes, C. (2014). Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224. doi:10.1038/nrneurol.2014.38
Poprac, P., Jomova, K., Simunkova, M., Kollar, V., Rhodes, C. J., and Valko, M. (2017). Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 38, 592–607. doi:10.1016/j.tips.2017.04.005
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. L. (2014). The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi:10.1002/mds.25945
Qi, L. M. (2010). A study on the preparation technology of berberine-β-cyclodextrin inclusion complex. Chin. J. Exp. Tradit. Med. Form. 16, 8–9. doi:10.13422/j.cnki.syfjx.2010.03.054
Qin, X., Guo, B. T., Wan, B., Fang, L., Lu, L., Wu, L., et al. (2010). Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J. Immunol. 185, 1855–1863. doi:10.4049/jimmunol.0903853
Qin, Z. F., and Li, H. Y. (2005). Effect of different ultrasonic frequencies on extraction rate of berberine chloride from Phellodendron Chinese Schneid. Lishizhen Med. Mater Med. Res., 374–375.
Rauf, A., Abu-Izneid, T., Khalil, A. A., Imran, M., Shah, Z. A., Emran, T. B., et al. (2021). Berberine as a potential anticancer agent: a comprehensive review. Molecules 26, 7368. doi:10.3390/molecules26237368
Rostagno, A. A. (2022). Pathogenesis of alzheimer’s disease. Int. J. Mol. Sci. 24, 107. doi:10.3390/ijms24010107
Ruan, H., Zhan, Y. Y., Hou, J., Xu, B., Chen, B., Tian, Y., et al. (2017). Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene 36, 6906–6918. doi:10.1038/onc.2017.296
Sadeghnia, H. R., Kolangikhah, M., Asadpour, E., Forouzanfar, F., and Hosseinzadeh, H. (2017). Berberine protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells. Iran. J. Basic Med. Sci. 20, 594–603. doi:10.22038/IJBMS.2017.8847
Sedaghat, R., Taab, Y., Kiasalari, Z., Afshin-Majd, S., Baluchnejadmojarad, T., and Roghani, M. (2017). Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: underlying mechanisms. Biomed. Pharmacother. 87, 200–208. doi:10.1016/j.biopha.2016.12.109
Shayganfard, M. (2023). Berberine: is it a promising agent for mental disorders treatment? Curr. Mol. Pharmacol. 16, 307–320. doi:10.2174/1874467215666220509213122
Shen, J. D., Ma, L. G., Hu, C. Y., Pei, Y. Y., Jin, S. L., Fang, X. Y., et al. (2016). Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 614, 77–82. doi:10.1016/j.neulet.2016.01.002
Shou, J. W., Li, X. X., Tang, Y. S., Lim-Ho Kong, B., Wu, H. Y., Xiao, M. J., et al. (2022). Novel mechanistic insight on the neuroprotective effect of berberine: the role of PPARδ for antioxidant action. Free Radic. Biol. Med. 181, 62–71. doi:10.1016/j.freeradbiomed.2022.01.022
Simões Pires, E. N., Frozza, R. L., Hoppe, J. B., Menezes, B. de M., and Salbego, C. G. (2014). Berberine was neuroprotective against an in vitro model of brain ischemia: survival and apoptosis pathways involved. Brain Res. 1557, 26–33. doi:10.1016/j.brainres.2014.02.021
Song, K., Sun, Y., Liu, H., Li, Y., An, N., Wang, L., et al. (2022). Network pharmacology and bioinformatics methods reveal the mechanism of berberine in the treatment of ischaemic stroke. Evid. Based Complement. Altern. Med. 2022, 5160329. doi:10.1155/2022/5160329
Spinozzi, S., Colliva, C., Camborata, C., Roberti, M., Ianni, C., Neri, F., et al. (2014). Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 77, 766–772. doi:10.1021/np400607k
Su, Q., Li, X. X., Bao, Q., Zhang, Y. H., Wang, J. L., and Yin, R. L. (2023). Research progress in pellets. Prim. J. Chin. Mater Med. 2, 106–112. doi:10.20065/j.cnki.btcm.20230254
Sun, H. W., and Oy, W. Q. (2007). Preparation and physicochemical characteristics of berberine hydrochloric nanoemulsion. Chin. Tradit. Herb. Drugs, 1476–1480.
Sun, M., Zhou, C. Z., and Wang, Z. C. (2017a). Preparation of berberine hydrochloride/montmorillonite composite powder. Elec J. Clin. Med. Lit. 4 (13855)–13857. doi:10.16281/j.cnki.jocml.2017.70.169
Sun, S., Wang, K., Lei, H., Li, L., Tu, M., Zeng, S., et al. (2014). Inhibition of organic cation transporter 2 and 3 may be involved in the mechanism of the antidepressant-like action of berberine. Prog. Neuropsychopharmacol. Biol. Psychiatry 49, 1–6. doi:10.1016/j.pnpbp.2013.11.005
Sun, X. M., Zhang, Z. W., Lu, L. H., and Li, Y. F. (1996). Comparison of four extraction methods for antidotal cranules of coptis. Chin. J. Chin. Mater Med. 474–476, 510.
Sun, Y., Liu, W. Z., Liu, T., Feng, X., Yang, N., and Zhou, H. F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept Signal Transduct. Res. 35, 600–604. doi:10.3109/10799893.2015.1030412
Sun, Y., Xia, M., Yan, H., Han, Y., Zhang, F., Hu, Z., et al. (2018a). Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 175, 374–387. doi:10.1111/bph.14079
Sun, Y., Yu, J., Liu, X., Zhang, C., Cao, J., Li, G., et al. (2018b). Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomed. Pharmacother. 102, 699–710. doi:10.1016/j.biopha.2018.03.132
Sun, Y., Yuan, X., Zhang, F., Han, Y., Chang, X., Xu, X., et al. (2017b). Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci. Rep. 7, 11340. doi:10.1038/s41598-017-11860-3
Tan, X. S., Ma, J. Y., Feng, R., Ma, C., Chen, W. J., Sun, Y. P., et al. (2013). Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One 8, e77969. doi:10.1371/journal.pone.0077969
Tarrago, T., Kichik, N., Seguí, J., and Giralt, E. (2007). The natural product berberine is a human prolyl oligopeptidase inhibitor. ChemMedChem 2, 354–359. doi:10.1002/cmdc.200600303
Tian, C. X., Li, M. Y., Shuai, X. X., Jiang, F., Dong, Y. L., Gui, Y., et al. (2023). Berberine plays a cardioprotective role by inhibiting macrophage Wnt5a/β-catenin pathway in the myocardium of mice after myocardial infarction. Phytother. Res. 37, 50–61. doi:10.1002/ptr.7592
Tong, L., Xie, C., Wei, Y., Qu, Y., Liang, H., Zhang, Y., et al. (2019). Antitumor effects of berberine on gliomas via inactivation of caspase-1-mediated IL-1β and IL-18 release. Front. Oncol. 9, 364. doi:10.3389/fonc.2019.00364
Uttara, B., Singh, A. V., Zamboni, P., and Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7, 65–74. doi:10.2174/157015909787602823
Wan, J. S., and Zhang, M. R. (2018). Research progress on neuroprotective effects of berberine and its related mechanisms. J. Med. Res. 47, 197–200. doi:10.11969/j.issn.1673-548X.2018.06.047
Wang, D. J., Zheng, Y., Jiang, H. M., Zhan, J., and Xiang, Q. J. (2006). Research on methods of liquid membrane for extracting berberine from coptis. J. Chengdu Univ. 260–262, 266.
Wang, F., Zhao, G., Cheng, L., Zhou, H. Y., Fu, L. Y., and Yao, W. X. (2004). Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Res. 999, 91–97. doi:10.1016/j.brainres.2003.11.036
Wang, J., Fang, X., Wang, L., Sun, Z. A., and Liu, J. G. (2014a). Preparation of berberine enteric microcapsules. Chin. J. Veter Sci. 34, 1328–1331. doi:10.16303/j.cnki.1005-4545.2014.08.016
Wang, J., Qi, Q., Feng, Z., Zhang, X., Huang, B., Chen, A., et al. (2016). Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 7, 66944–66958. doi:10.18632/oncotarget.11396
Wang, K., Feng, X., Chai, L., Cao, S., and Qiu, F. (2017). The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 49, 139–157. doi:10.1080/03602532.2017.1306544
Wang, L. F., Zhang, S. P., Cao, Y., and Guo, D. L. (2014b). Studies on nasal gel of berberine hydrochloride. J. Tianjin Univ. TCM 33, 36–38.
Wang, Q. S., Li, K., Gao, L. N., Zhang, Y., Lin, K. M., and Cui, Y. L. (2020). Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 8, 2853–2865. doi:10.1039/c9bm02006c
Wang, X., Wang, S., Ma, J., Ye, T., Lu, M., Fan, M., et al. (2015). Pharmacokinetics in rats and tissue distribution in mouse of berberrubine by UPLC-MS/MS. J. Pharm. Biomed. Anal. 115, 368–374. doi:10.1016/j.jpba.2015.07.031
Wang, X., Xing, D., Wang, W., Su, H., Tao, J., and Du, L. (2005). Pharmacokinetics of berberine in rat thalamus after intravenous administration of Coptidis rhizoma extract. Am. J. Chin. Med. 33, 935–943. doi:10.1142/S0192415X05003557
Wang, X. H., Li, J. S., Lv, J. J., and Liu, Z. H. (2013). Preparation and in vitro release behavior of berberine hydrochloride liposomes. Chin. J. Exp. Tradit. Med. Form. 19, 39–42.
Wang, X. J., Zhong, G. C., Li, S. T., Zhang, Q., Luo, B. J., and Wang, Q. (2024). Research progress in pharmacological effect of berberine for treatment of Alzheimer’s disease: a review. Chin. J. Exp. Tradit. Med. Form., 1–15. doi:10.13422/j.cnki.syfjx.20240741
Wei, W., Zeng, Q., Wang, Y., Guo, X., Fan, T., Li, Y., et al. (2023). Discovery and identification of EIF2AK2 as a direct key target of berberine for anti-inflammatory effects. Acta Pharm. Sin. B 13, 2138–2151. doi:10.1016/j.apsb.2022.12.009
Wen, C. F., Xiang, X. Q., Zeng, W. X., Wu, T., and Tian, C. L. (2011). Extract berberine hydrochloride by aqueous two-phase system. Chin. Med. Mat. 34, 1608–1611. doi:10.13863/j.issn1001-4454.2011.10.045
Wu, B. H. (2004). Influence of ultrasonic technique on the extraction rate of berberine hydrochloride from Guanhuangbai. J. Chin. Med. Pharm., 29–30. doi:10.19664/j.cnki.1002-2392.2004.04.014
Wu, J. D., Li, B. H., and Zhao, W. C. (2013). Research progress on preparation of berberine. Chin. Mod. Med. 20, 14–16.
Wu, Y., Chen, Q., Wen, B., Wu, N., He, B., and Chen, J. (2021). Berberine reduces Aβ42 deposition and tau hyperphosphorylation via ameliorating endoplasmic reticulum stress. Front. Pharmacol. 12, 640758. doi:10.3389/fphar.2021.640758
Xi, G. M., He, G. H., Huang, C. F., and Luo, G. J. (2003). Effect of berberine on plasma ICAM-1 and E-Selectin in patients with cerebral infarction. J Apoplexy Nvs Dis. 81.
Xiao, Y., Wang, Y. R., Ma, J., and Song, Y. Q. (2008). Preparation of enzyme-controlled colon-positioning tablets of berberine Hydrochloride. J. Beijing Univ. TCM 31, 847–850.
Xie, T., Liao, A. P., Yi, Y. L., and Wu, R. C. (2008). Study on double aqueous phase extraction of panax notoginseng saponins from panax notoginseng. Chin. Med. Mat., 535–537. doi:10.13863/j.issn1001-4454.2008.04.066
Xu, L. J., Lu, F. E., Wei, S. C., and Leng, S. H. (2004). Study on preparation of berberine hydrochloride liposome. Chin. J. Hosp. Pharm., 7–8.
Yan, J., Fang, C., Yang, G., Li, J., Liu, Y., Zhang, L., et al. (2023). Identification of FtfL as a novel target of berberine in intestinal bacteria. BMC Biol. 21, 280. doi:10.1186/s12915-023-01778-w
Yang, Q. Z., Zheng, S. H., and Huang, L. F. (2015). Advance in extraction methods and pharmacological activities of berberine. Chin. J. New Drugs 24, 519–525.
Yang, Y., and Torbey, M. T. (2020). Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr. Neuropharmacol. 18, 1250–1265. doi:10.2174/1570159X18666200720173316
Yao, Y., Gu, J. X., Zhao, L., Li, M., Ai, J. Y., and Liu, C. (2023). Therapeutic effect and mechanism of berberine on acute cerebral infarction in rats. Shandong Med. J. 63, 48–52. doi:10.3969/j.issn.1002-266X.2023.12.010
Ye, A. L., Zhao, H. X., and Gong, L. Y. (2015). Effect of berberine combined with mecobalamine in the treatment of newly diagnosed type 2 diabetic peripheral neuropathy and its effect on blood Hcy and hs-CRP levels. Zhejiang J Int Trad Chin West. Med 25, 564–566.
Yi, C. M., Yu, J., Kim, H., Lee, N.-R., Kim, S. W., Lee, N.-J., et al. (2017). Identification of actin as a direct proteomic target of berberine using an affinity-based chemical probe and elucidation of its modulatory role in actin assembly. Chem. Commun. (Camb) 53, 7045–7047. doi:10.1039/c7cc02789c
Yi, L. T., Zhu, J. X., Dong, S. Q., Chen, M., and Li, C. F. (2021). Berberine exerts antidepressant-like effects via regulating miR-34a-synaptotagmin1/Bcl-2 axis. Chin. Herb. Med. 13, 116–123. doi:10.1016/j.chmed.2020.11.001
Yi, T., Zhuang, L., Song, G., Zhang, B., Li, G., and Hu, T. (2015). Akt signaling is associated with the berberine-induced apoptosis of human gastric cancer cells. Nutr. Cancer 67, 523–531. doi:10.1080/01635581.2015.1004733
Yin, R. L., Yang, J. X., and Li, H. (2000). Improvement of extracting process of berberine hydrochlorlde in cotex phallodendri. Prim. J. Chin. Mater Med., 27–29. doi:10.13728/j.1673-6427.2000.06.016
Yoo, K.-Y., Hwang, I. K., Kim, J. D., Kang, I.-J., Park, J., Yi, J.-S., et al. (2008). Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother. Res. 22, 1527–1532. doi:10.1002/ptr.2527
Yoo, K. Y., Hwang, I. K., Lim, B. O., Kang, T. C., Kim, D. W., Kim, S. M., et al. (2006). Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol. Pharm. Bull. 29, 623–628. doi:10.1248/bpb.29.623
Youdim, M. B. H., Edmondson, D., and Tipton, K. F. (2006). The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 7, 295–309. doi:10.1038/nrn1883
Yu, G., Li, Y., Tian, Q., Liu, R., Wang, Q., Wang, J.-Z., et al. (2011). Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J. Alzheimers Dis. 24, 525–535. doi:10.3233/JAD-2011-101779
Yu, L. M. (2012). Study on the preparation method of berberine hydrochloride pellets. Chin. Pract. Med. 7, 250–251. doi:10.14163/j.cnki.11-5547/r.2012.15.002
Yu, W., Ding, L., Chen, Y. Q., Wu, X. F., and Chen, J. (2021). Effect of berberine on depression and anxiety in 5XFAD mice. Neural Inj. Func. Rec. 16, 497–501. doi:10.16780/j.cnki.sjssgncj.20201283
Yu, Y., Fan, L. Y., Gao, J. D., Wei, S. C., and Ma, L. X. (2015). Preparation of berberine hydrochloride microcapsules and its in vitro release behavior. J. China Pharm. 26, 109–112.
Zeng, Q., Deng, H., Li, Y., Fan, T., Liu, Y., Tang, S., et al. (2021a). Berberine directly targets the NEK7 protein to block the NEK7-NLRP3 interaction and exert anti-inflammatory activity. J. Med. Chem. 64, 768–781. doi:10.1021/acs.jmedchem.0c01743
Zeng, Q. X., Wei, W., Fan, T. Y., Deng, H. B., Guo, X. X., Zhao, L. P., et al. (2021b). Capture and identification of dual specificity mitogen-activated protein kinase kinase 7 as a direct proteomic target of berberine to affect the c-junn-terminal kinase pathway. CCS Chem. 4, 1535–1544. doi:10.31635/ccschem.021.202100986
Zhang, B., Wang, L., Ji, X., Zhang, S., Sik, A., Liu, K., et al. (2020). Anti-inflammation associated protective mechanism of berberine and its derivatives on attenuating pentylenetetrazole-induced seizures in Zebrafish. J. Neuroimmune Pharmacol. 15, 309–325. doi:10.1007/s11481-019-09902-w
Zhang, J. H., Yang, H. Z., Su, H., Song, J., Bai, Y., Deng, L., et al. (2021). Berberine and ginsenoside rb1 ameliorate depression-like behavior in diabetic rats. Am. J. Chin. Med. 49, 1195–1213. doi:10.1142/S0192415X21500579
Zhang, M. F., and Shen, Y. Q. (2023). Research progress in antifungal pharmacological effects and mechanism of berberine. Anti-infection Pharm. 20, 453–457. doi:10.13493/j.issn.1672-7878.2023.05-002
Zhang, Q., Bian, H., Guo, L., and Zhu, H. (2016a). Berberine preconditioning protects neurons against ischemia via sphingosine-1-phosphate and hypoxia-inducible factor-1[Formula: see text]. Am. J. Chin. Med. 44, 927–941. doi:10.1142/S0192415X16500518
Zhang, Q., Bian, H., Guo, L., and Zhu, H. (2016b). Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting autophagy in neuron. Am. J. Transl. Res. 8, 1197–1207.
Zhang, X. L. (2013). Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: up-regulated pAkt, pGSK and pCREB, down-regulated NF-kB expression, ameliorated BBB permeability. He Bei: Hebei Med Univ.
Zhang, Y. T., Yu, Y. Q., Yan, X. X., Wang, W. J., Tian, X. T., Wang, L., et al. (2019). Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity. Acta Pharmacol. Sin. 40, 133–142. doi:10.1038/s41401-018-0183-7
Zhang, Z., Li, X., Li, F., and An, L. (2016c). Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 38, 426–433. doi:10.1016/j.intimp.2016.06.031
Zhang, Z. W., and Sun, X. M. (1995). Exploration on oral preparations of Chinese materia medica by semi-bionic extraction. Chin. J. Chin. Mater Med. 670–673, 703.
Zhao, X. L., Sui, H. Y., Li, K. X., Guan, Z., Ma, K. L., and Luan, H. Y. (2006). Protection of berberine in NIDDM rat brain. Heilongjiang Med Pharm, 4–5.
Zhou, J. X., Wu, S. G., Gong, J. B., and Wei, Z. P. (2022). Pharmacological activities of berberine and strategies to improve its oral bioavailability. Acta Pharm. Sin. 57, 1263–1272. doi:10.16438/j.0513-4870.2021-1302
Zhou, X.-Q., Zeng, X.-N., Kong, H., and Sun, X. L. (2008). Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci. Lett. 447, 31–36. doi:10.1016/j.neulet.2008.09.064
Zhu, F. Q., Ma, Y., Sun, Y. Y., Zhu, W. Z., and Qian, C. Y. (2010). Effect of berberine chloride on expression of insulin degrading enzyme in rat models with AD. Chin. J. Neuromed 9, 1201–1203.
Zhu, J., Cao, D., Guo, C., Liu, M., Tao, Y., Zhou, J., et al. (2019). Berberine facilitates angiogenesis against ischemic stroke through modulating microglial polarization via AMPK signaling. Cell Mol. Neurobiol. 39, 751–768. doi:10.1007/s10571-019-00675-7
Zhu, M. L., Yin, Y. L., Ping, S., Yu, H. Y., Wan, G. R., Jian, X., et al. (2017). Berberine promotes ischemia-induced angiogenesis in mice heart via upregulation of microRNA-29b. Clin. Exp. Hypertens. 39, 672–679. doi:10.1080/10641963.2017.1313853
Zou, J., Fei, Q., Xiao, H., Wang, H., Liu, K., Liu, M., et al. (2019). VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J. Cell Physiol. 234, 17690–17703. doi:10.1002/jcp.28395
Glossary
Keywords: berberine, dosage form, neuroprotective, mechanism, pharmacology
Citation: Sunhe Y-X, Zhang Y-H, Fu R-J, Xu D-Q and Tang Y-P (2024) Neuroprotective effect and preparation methods of berberine. Front. Pharmacol. 15:1429050. doi: 10.3389/fphar.2024.1429050
Received: 09 May 2024; Accepted: 22 August 2024;
Published: 06 September 2024.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Angel Agis-Torres, Universidad Complutense de Madrid, SpainFabrizio Dal Piaz, University of Salerno, Italy
Copyright © 2024 Sunhe, Zhang, Fu, Xu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding-Qiao Xu, eHVkaW5ncWlhbzE2QDEyNi5jb20=; Yu-Ping Tang, eXVwaW5ndGFuZ0BzbnRjbS5lZHUuY24=
 Yi-Xuan Sunhe
Yi-Xuan Sunhe Yue-Hui Zhang
Yue-Hui Zhang Rui-Jia Fu
Rui-Jia Fu Ding-Qiao Xu
Ding-Qiao Xu Yu-Ping Tang
Yu-Ping Tang