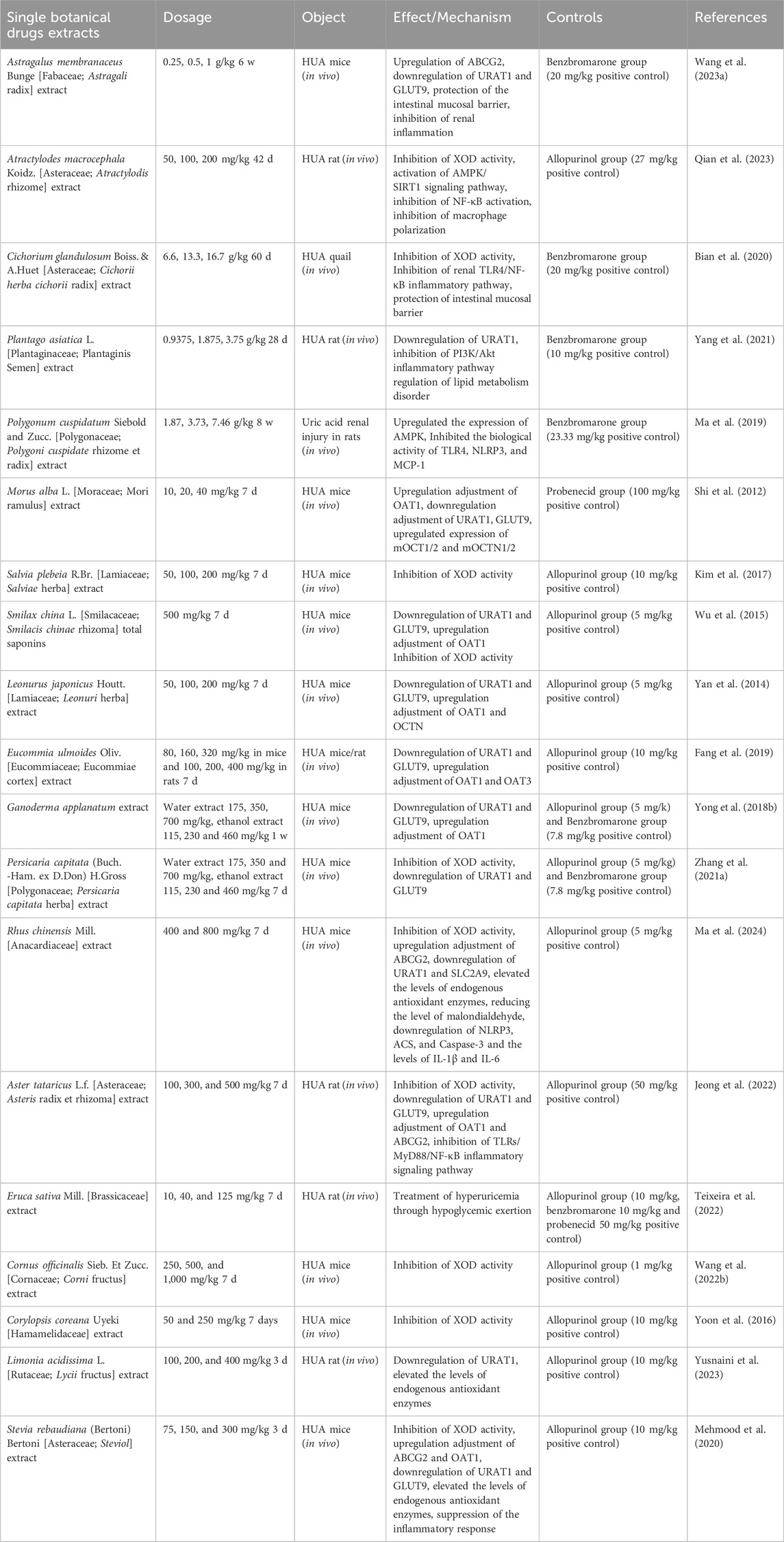
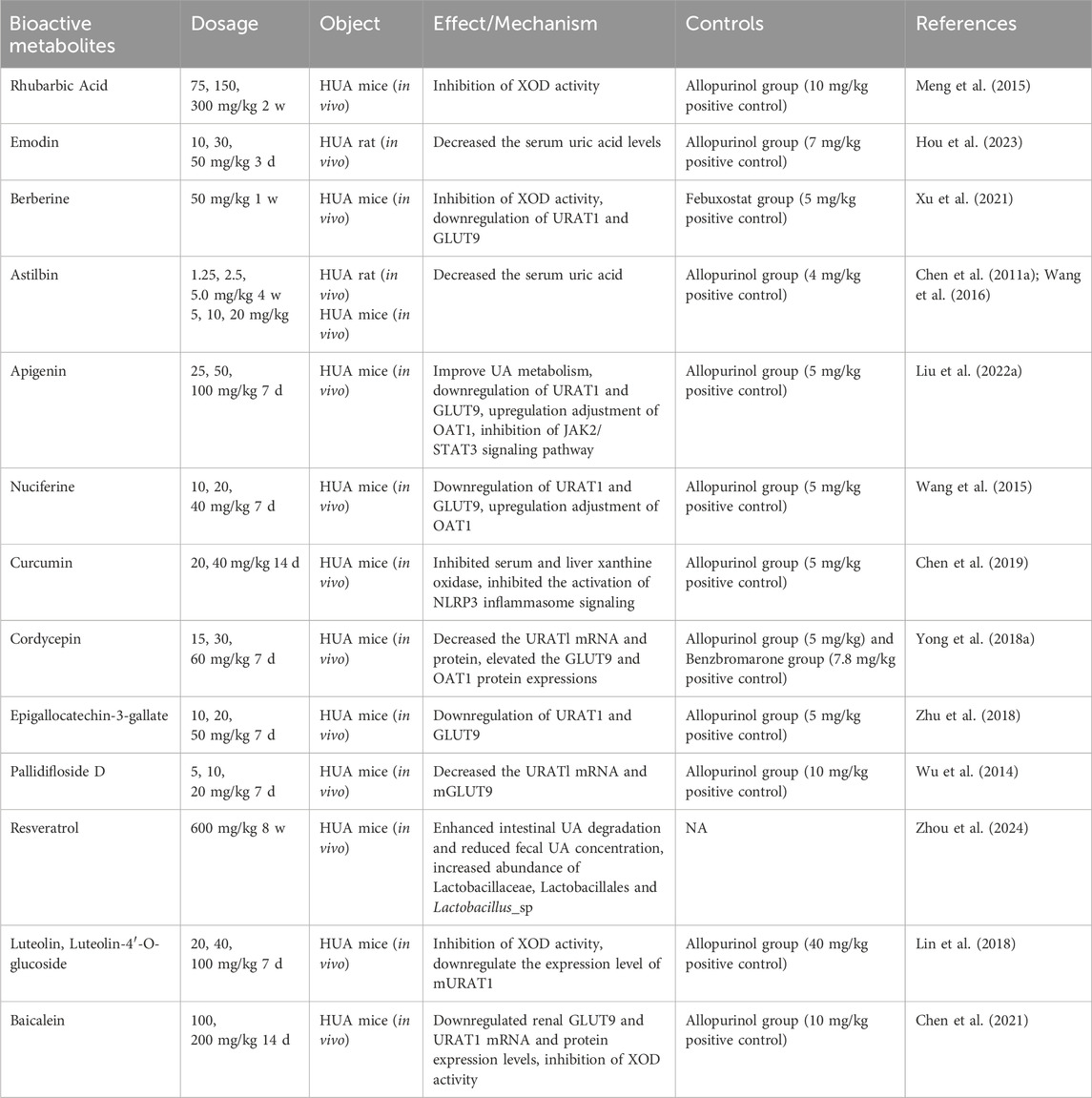
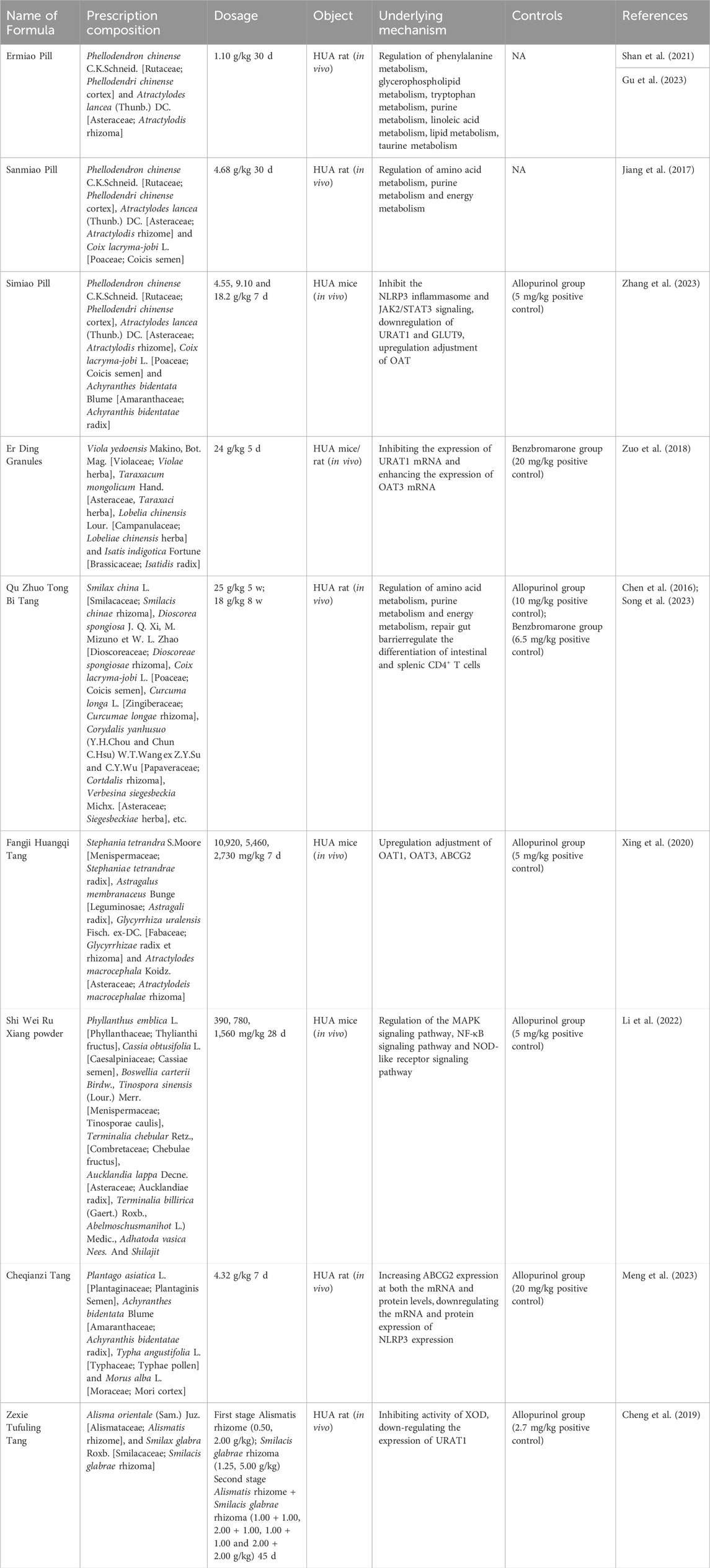
- 1Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine, Ministry of Education, Harbin, China
- 2School of Life Sciences, Guangzhou University, Guangzhou, China
Hyperuricemia (HUA) is a common chronic metabolic disease caused by abnormal purine metabolism and uric acid excretion. Despite extensive research on HUA, no clear treatment has been found so far. Improving purine metabolism and promoting uric acid excretion is crucial for the effective treatment of HUA. In recent years, traditional Chinese medicine and traditional Chinese medicine prescriptions have shown good effects in treating HUA. This article summarizes the latest progress in treating HUA in rats and mice using traditional Chinese medicine and prescriptions, elaborates on the pathogenesis of HUA, explores the application of commonly used traditional Chinese medicine treatment methods and prescriptions, and discusses the previous pharmacological mechanisms. In general, our research indicates that traditional Chinese medicine can effectively relieve the symptoms related to elevated uric acid levels in HUA rats and mice. However, further exploration and research are needed to verify its efficacy, safety, and feasibility.
1 Introduction
Hyperuricemia is a prevalent chronic metabolic disorder resulting from impaired purine metabolism and inadequate uric acid excretion (Wang et al., 2022). The process of uric acid production and excretion is shown specifically in Figure 1. In males, HUA is generally diagnosed when the serum uric acid concentration exceeds 420 μmol/L, while in females, the threshold is set at 360 μmol/L (Johnson et al., 2018). The global prevalence of HUA has increased due to improvements in living standards and dietary patterns. A nationally representative cross-sectional survey conducted during 2018–2019 estimated that approximately 14% of Chinese adults were affected by HUA (Zhang et al., 2022). HUA can contribute to the development of gout, kidney disease, type 2 diabetes mellitus, as well as cardiovascular and cerebrovascular disorders, and Intestinal disorders, significantly impacting individuals’ overall wellbeing and health status (Yanai et al., 2021).
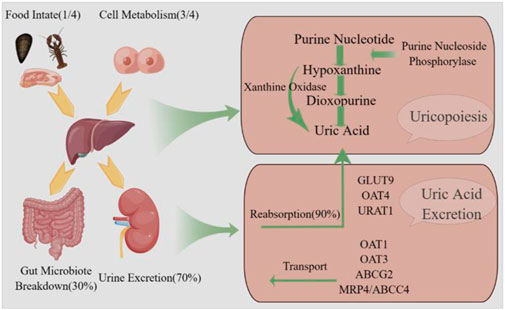
Figure 1. Uric acid production and excretion pathway. Uric acid is synthesized in the liver via the enzymatic action of xanthine oxidase and subsequently eliminated from the body through both renal.
At present, lifestyle improvement and drug therapy are the main means to control uric acid levels. Studies have confirmed that quitting smoking and drinking, a low-purine diet, and reducing the intake of greasy and high-fat foods can effectively reduce hyperuricemia (Morgan and Singh, 2021). Extensive research has been conducted on this disease and some drugs, such as benzbromarone, sulfinpyrazone, and propoxur have been utilized in its treatment (Strilchuk et al., 2019). Although these drugs demonstrate positive effects in reducing uric acid levels, prolonged usage can lead to adverse effects including skin rashes, liver damage, and potentially severe kidney complications (Bose et al., 2014; Yang et al., 2022). Therefore, there is a need to develop medicines or natural medicinal ingredients that are safer and more effective.
Compared with traditional drugs for the treatment of high uric acid, the development of herbal extracts (e.g., aqueous extracts, alcoholic extracts, and active metabolites), which are characterized by multi-links, multi-levels, multi-targets, and low toxicity and side-effects, has become a new idea for the development of uric acid-reducing drugs. In recent years, more and more studies have been devoted to the mining of natural substances with uric acid-reducing activity from herbs. Studies have shown that plant metabolites, such as flavonoids, saponins, polysaccharides, and polyphenols, regulate uric acid metabolism by inhibiting the activity of key enzymes of uric acid synthesis and regulating various pathways such as uric acid transporter proteins, thus preventing or treating hyperuricemia and its complications. For example, both the aqueous and alcoholic extracts of Agrocybe aegerita were able to exhibit inhibitory effects on hepatic xanthine oxidase (XOD) activity and elevate renal organic anion transporter protein 1 (OAT1) in hyperuricemic mice (Yong et al., 2018). Er Ding Granules (EDG) have been shown to effectively lower uric acid levels by down-regulating glucose transporter protein 9 (GLUT9) and urate anion transporter protein 1 (URAT1), while up-regulating organic anion transporter 1 (OAT1). These actions enhance uric acid excretion and reduce its production, providing a balanced and sustained therapeutic effect. EDG’s multi-target approach offers potential advantages over traditional urate-lowering drugs such as allopurinol and febuxostat, potentially leading to fewer side effects in the management of hyperuricemia (Zhang W. et al., 2019). Compared to conventional methods, traditional Chinese medicine (TCM) offers several advantages in the treatment of hyperuricemia (HUA). One major advantage is TCM’s multi-target, multi-component approach, which can simultaneously address various pathways of uric acid metabolism. This comprehensive approach not only helps reduce serum uric acid levels but also alleviates related complications such as gout, hypertension, diabetes, and chronic kidney disease, contributing to overall health (Yang et al., 2022). To summarize, this paper collects various related literature published in recent years from PubMed, Web of Science, CNKI, and other electronic databases with “hyperuricemia, traditional Chinese medicine, botanical medicine, drug metabolite, traditional Chinese medicine compound” as the main keywords, to provide a theoretical basis for the research and development of uric acid-lowering drugs. To provide a theoretical basis for the research and development of uric acid-lowering drugs. All botanical drugs’ names have been checked with Plants of the Word Online (http://www.plantsoftheworldonline.org).
2 Pathogenesis of hyperuricemia
The pathogenesis of hyperuricemia is complex. A growing body of research now suggests that the pathogenesis of hyperuricemia may be related to enzyme activity, uric acid transporter proteins, intestinal flora dysbiosis, and oxidative stress and inflammatory responses.
2.1 Dysregulation of enzyme activity
Uric acid synthesis involves a variety of enzymes, including xanthine XOD as the key enzyme in the body of uric acid production, purine nucleoside phosphorylase PNP catalyzed creatinine catabolism to produce hypoxanthine to further catalyze the oxidation of intermediate products xanthine, and ultimately xanthine oxidation into uric acid. When XOD activity is dysregulated, the higher activity of XOD will accelerate the catalytic oxidation of hypoxanthine and xanthine, resulting in a continuous increase in uric acid levels and hyperuricemia (Chen et al., 2023). XOD catalyzes the oxidation and hydroxylation of hypoxanthine and xanthine to uric acid, generating reactive oxygen species such as superoxide anion and hydrogen peroxide at the flavin center. Elevated levels of these reactive oxygen species can lead to oxidative stress and ischemia-reperfusion injury. This oxidative stress can contribute to conditions such as hyperuricemia and metabolic syndrome (Borges et al., 2002).
2.2 Imbalanced expression of uric acid transporter protein
Uric acid in the body is mainly excreted through the kidneys (about 2/3), and a small portion is excreted through the intestines (about 1/3), the uric acid transporter protein in the kidneys is the regulation of the dynamic balance of uric acid reabsorption and excretion, and plays an important role in the process of uric acid excretion (Maiuolo et al., 2016). Uric acid transporter proteins are mainly classified into two groups, one is reabsorption transporter proteins, mainly including urate anion transporter protein 1 (URAT1), organic anion transporter protein 4 (OAT4) and glucose transporter protein 9 (GLUT9); the other is secretion-associated transporter proteins, mainly including organic anion transporters 1 and 3 (OAT1 and OAT3), multidrug resistance protein 4 (MRP4/ABCC4) and ATP-binding cassette subfamily G member 2 (ABCG2). Over-expression of uric acid reabsorption transporter proteins leads to abnormal uric acid reabsorption, resulting in elevated serum uric acid levels; under-expression of uric acid secretion transporter proteins causes a decrease in renal uric acid secretion and insufficient excretion results in elevated serum uric acid levels. The literature has reported that 90% of patients with hyperuricemia have an imbalance between renal excretion and expression of uric acid transporter protein (Russo et al., 2022).
2.3 Oxidative stress and inflammatory response
Uric acid production is accompanied by XOD, which promotes the activation of reduced coenzyme Ⅱ and the release of reactive oxygen species. When uric acid levels are higher than normal physiological levels, oxidative stress damage to the body is amplified (Kurajoh et al., 2021). Oxidative stress in vivo can activate related inflammatory factors and inflammatory pathways and induce innate immune responses, while the activation of related pro-inflammatory factors can induce inflammation. It has been reported that the development of hyperuricemia is closely related to oxidative stress and inflammatory responses (Liu et al., 2021).
2.4 Imbalance of intestinal homeostasis
Transporter proteins that promote uric acid excretion are also present in the gut, and these transporter proteins are involved in the metabolic processes of gut microorganisms (Hosomi et al., 2012). ABCG2, which is distributed in different parts of the small and large intestine, is a major uric acid secretion transporter protein that dominates intestinal uric acid excretion and regulates blood uric acid levels. Studies have shown that increased expression of ABCG2 was found in the intestine of denervated rats with impaired renal excretion, suggesting that increased expression of this transporter protein may be the key to intestinal excretion of uric acid (Bhatnagar et al., 2016). In addition, chronic inflammation is a typical pathological feature of hyperuricemia (Zhou et al., 2018). Intestinal flora may ameliorate hyperuricemia by repairing the intestinal mucosal barrier and attenuating the inflammatory response. Elevated levels of inflammatory factors negatively affect both epithelial integrity of the gut and gut flora homeostasis (Luissint et al., 2016). Dysbiosis of the intestinal flora increases intestinal permeability and promotes translocation of bacteria or bacterial products such as lipopolysaccharide (LPS) (Xu et al., 2019). Elevated serum LPS levels induce chronic inflammation and increase the risk of developing hyperuricemia. In addition, LPS is a metabolite of intestinal flora, and abnormal levels of LPS in the circulation are usually accompanied by an increase in the activity of XOD, an important enzyme in the oxidative metabolism of purines. Thus, dysbiosis of the intestinal flora and impaired intestinal barrier repair can lead to elevated levels of LPS in the circulation, causing chronic inflammation, which is a new factor in the pathogenesis of hyperuricemia.
3 Uric acid-lowering effects of botanical drugs extracts, active metabolites, and herbal formulas in hyperuricemic rats and mice
In recent years pharmacological studies have validated the therapeutic effects of several botanical drug extracts, active metabolites, and herbal formulas by establishing hyperuricemic animals. The respective uric acid-lowering effects and the potential mechanisms of action are summarized in Tables 1–3, respectively. The chemical structures of the metabolites described in the paper are shown in Figure 2.
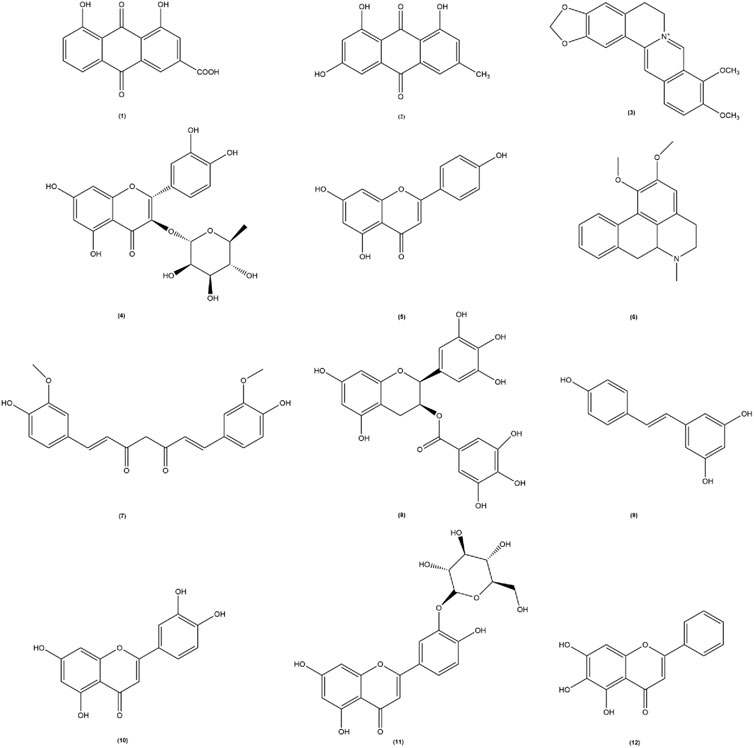
Figure 2. The chemical structures of the metabolites involved in the thesis. Among them, (1) Rhubarbic Acid (Molecular formula: C15H8O6), (2) Emodin (Molecular formula: C15H10O5), (3) Berberine (Molecular formula: C20H18NO4+), (4) Astilbin (Molecular formula: C21H22O11), (5) Apigenin (Molecular formula: C15H10O5), (6) Nuciferine (Molecular formula: C19H21NO2), (7) Curcumin (Molecular formula: C21H20O6), (8) Epigallocatechin-3-gallate (Molecular formula: C22H18O11), (9) Resveratrol (Molecular formula: C14H12O3), (10) Luteolin (Molecular formula: C15H10O6), (11) Luteolin-4′-O-glucoside (Molecular formula: C21H20O11), (12) Baicalein (Molecular formula: C15H10O5).
3.1 Single botanical drug extracts
3.1.1 Astragalus membranaceus Bunge [Fabaceae; Astragali radix]
Astragalus membranaceus Bunge is a medicinal plant rich in flavonoids, saponins, polysaccharides, and triterpenoids, and exhibits diverse biological activities. Modern pharmacological studies have demonstrated its anti-inflammatory, antioxidant, and immunomodulatory effects (Durazzo et al., 2021). Moreover, astragalus exerts renoprotective effects and regulates intestinal flora (Shahzad et al., 2016). In a yeast- and potassium oxybate-induced hyperuricemic mouse model (HUA mice), fermented astragalus (BFA) effectively reduced blood urea nitrogen (BUN) and serum creatinine (SCr) levels while improving renal morphology. BFA upregulated renal ABCG2 protein expression to enhance uric acid excretion and downregulated renal URAT1 and GLUT9 expression to inhibit uric acid reabsorption. Additionally, BFA attenuated the renal inflammatory response by preserving the integrity of the intestinal mucosal barrier and reducing lipopolysaccharide-binding protein production. Metabolomic analysis revealed that BFA ameliorated HUA by suppressing boswellic acid production while enhancing the abundance of beneficial monocytic butyric acid bacteria, visceral odoriferous bacteria, Tanaka bacteria as well as fatty acid biosynthesis to maintain urea cycle homeostasis (Wang et al., 2023).
3.1.2 Atractylodes macrocephala Koidz. [Asteraceae; Atractylodis rhizome]
Dried rhizomes of Atractylodes macrocephala Koidz. is commonly employed in traditional medicine for the treatment of gout due to its diuretic and anti-dampness properties (Zhang et al., 2021). In a HUA rat model established by combining otacid potassium with yeast powder solubilization, it was observed that C. diff effectively reduced serum levels of uric acid (UA), adenosine deaminase (ADA), and XOD in hypouricemic rats. Hematoxylin-eosin staining revealed that Atractylodis macrocephalae rhizome improved renal tubular dilatation and interstitial inflammatory cell infiltration in hypouricemic rats. Furthermore, Atractylodis macrocephalae rhizome exhibited anti-inflammatory activity by downregulating macrophage interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) levels, activating the Adenosine 5′-monophosphate-activated protein kinase/Silent mating type information regulation 2 homolog-1 (AMPK/SIRT1) signaling pathway, inhibiting nuclear factor kappa-B (NF-κB) activation, and suppressing macrophage polarization towards a pro-inflammatory phenotype (Qian et al., 2023).
3.1.3 Cichorium glandulosum Boiss. & A. Huet [Asteraceae; Cichorii herba cichorii radix]
Cichorii herba cichorii radix is the dried above-ground part or root of Cichorium glandulosum Boiss. et Huet or Cichorrium intybus, belonging to the Asteraceae family. Recent pharmacological studies have demonstrated Cichorii herba cichorii radix’s potent anti-inflammatory, antioxidant, hypoglycemic, hypolipidemic, and intestinal flora regulating effects (Azay-Milhau et al., 2013; Chen et al., 2017; Boghrati et al., 2021). Bian M et al. discovered that chicory effectively reduces serum uric acid levels by inhibiting xanthine oxidase activity and upregulating expression of the uric acid transporter ABCG2 in the intestine (Bian et al., 2018). To further investigate the potential relationship between chicory treatment and regulation of intestinal flora in HUA, Bian M induced HUA in quails using a high purine diet based on previous research findings. The study revealed that chicory intervention significantly decreased serum uric acid levels while increasing fecal uric acid levels, promoting repair of intestinal mucosal damage and improving intestinal barrier permeability. Moreover, analysis through 16S rRNA sequencing indicated that chicory restored gut microbiota balance by enhancing probiotic flora (Bifidobacterium, Salmonella family) and reducing pathogenic flora (Pylori family). This restoration was accompanied by downregulation of serum lipopolysaccharide (LPS) levels as well as renal Toll-like receptor 4/nuclear factor-kappa B inflammatory pathways, ultimately facilitating renal excretion of uric acid via attenuation of the LPS/Toll-like receptor 4 axis-mediated inflammatory response (Bian et al., 2020).
3.1.4 Plantago asiatica L. [Plantaginaceae; Plantaginis Semen]
Plantaginis Semen is the dried mature seeds of Plantago asiatica L. or Plantago depressa Willd. a herb commonly employed for uric acid reduction (Zeng et al., 2018; Sun et al., 2019). The phosphoinositide 3-kinase/Protein kinase B (PI3K/Akt) signaling pathway, an inflammatory pathway implicated in severe kidney injury and indirect modulation of uric acid excretion, can be inhibited by Plantago to ameliorate nephropathy and enhance uric acid excretion (Dragos et al., 2020; Santos et al., 2020; Zhao et al., 2020). Furthermore, hyperuricemia has been associated with lipid metabolism (Guo et al., 2020), but it remains unknown whether lipid metabolism plays a role in the therapeutic effects of Plantago on hyperuricemia treatment. Previous studies have shown that Plantago extract improves lipid accumulation in high-fat diet-induced obese mice (Yang et al., 2017), while another study demonstrated that Plantago accelerates lipolysis through regulation of lipid metabolism disorders in a mouse model of hyperuricemia induced by potassium oxybate gavage; additionally, anti-hyperuricemic effects were observed through downregulation of URAT1 expression and inhibition of the PI3K/Akt inflammatory pathway (Yang et al., 2021).
3.1.5 Polygonum cuspidatum Siebold & Zucc. [Polygonaceae; Polygoni cuspidate rhizome et radix]
The Polygoni cuspidate rhizome et radix is derived from the dried rhizomes and roots of Polygonum cuspidatum Sieb.et Zucc, a perennial herb of the Polygonicuspidate family. PC has anti-inflammatory, antibacterial, antiviral, antioxidant, hypoglycemic and anti-hyperuric acid pharmacological activities (Ammar et al., 2022; Bian et al., 2022; Lin et al., 2022). Metabolomics is an emerging discipline in the 20th century and is of great importance in elucidating the mechanisms of pharmacological effects of Chinese medicines (Wu et al., 2019). Recent reports have found that polydatin can improve and treat HUA through amino acid metabolism, lipid metabolism and energy metabolism (Ge et al., 2023). Ma et al. found that PC could upregulate the expression of Adenosine 5′-monophosphate-activated protein kinase (AMPK) and its downstream molecule FOXO3α, and inhibit the bioactivity of Toll-likereceptor4 (TLR4), NOD-like receptor thermal protein domain associated protein 3 (NLRP3) and Monocyte Chemoattractant Protein-1 (MCP-1), key signaling molecules of the immune inflammatory network pathway to improve HUA-mediated immunoinflammatory metabolic kidney damage (Liu et al., 2005; Ma et al., 2019). Hu et al. isolated two active compounds from PC: 1-(4-hydroxy-2-methoxyphenyl)-2-(4-hydroxy-3,5-dimethylphenyl) butane-1,2,3-triol and 1-(4-hydroxy-2-methoxyphenyl)-2-(4-hydroxy-3,5-dimethylphenyl)-3-methylbutane-1,2-diol. In vitro and in vivo experiments have demonstrated the ability of both compounds to reduce UA levels and improve renal morphological and pathological changes through competitive inhibition of XOD activity. In addition, the mechanisms of anti-HUA are closely related to galactose metabolism, taurine and hypotaurine metabolism, purine metabolism and energy metabolism (Hu et al., 2023).
3.1.6 Smilax china L. [Smilacaceae; Smilacis chinae rhizoma]
Smilacis chinae rhizoma, the dried rhizome of Smilax china L., a member of the lily family, possesses therapeutic properties including dampness relief, turbidity removal, wind and paralysis dispelling, as well as detoxification and blood stasis dispersion. It exhibits anti-inflammatory, antioxidant, anticancer, hypoglycemic, and diuretic activities (Wang et al., 2013; Hooda et al., 2014; Bao et al., 2018; Xu et al., 2022b). Chen investigated different fractions of sarsaparilla using petroleum ether, chloroform, ethyl acetate n-butanol and ethanol in HUA rats. The ethyl acetate fraction demonstrated significant uric acid-lowering effects. Caffeic acid, resveratrol rutin and oxidized resveratrol isolated from the ethyl acetate fraction exhibited in vitro inhibition of xanthine oxidase activity (Chen et al., 2011b). Wu previously reported that sarsaparilla saponin significantly reduced serum uric acid levels in a mouse model of HUA. Further investigation revealed that sarsaparilla saponin co-regulated renal URAT1 and GLUT9 expression in mice by upregulating OAT1 while inhibiting XOD (Wu et al., 2015). Hong et al. (2014) employed Liquid Chromatograph Mass Spectrometer analysis combined with bioinformatics to identify differential proteins in kidney tissue from sarsaparilla-intervened HUA rats. Sarsaparilla was found to upregulate catalase expression for alleviating oxidative stress levels.
3.1.7 Morus alba L. [Moraceae; Mori ramulus]
Mori ramulus (MR) is a branch of the mulberry tree, extensively utilized in traditional medicine as an anti-rheumatic agent. MR contains various active constituents, including flavonoids, phenylpropanoids, and coumarins, exhibiting pharmacological activities such as antioxidative, anti-inflammatory, anti-hyperlipidemic, and anti-hyperglycemic effects (Xiang et al., 2021). Shi et al. (2012) discovered that the alcoholic extract derived from Morus alba exhibited significant efficacy in reducing serum uric acid levels and increasing 24-hour urine uric acid excretion as well as partial uric acid excretion in mice with HUA. This extract effectively downregulated renal mURAT1 and mGLUT9 expression, while up-regulating the expression of mOAT1, thereby enhancing uric acid excretion. Furthermore, EMR treatment resulted in reduced serum creatinine and BUN levels, increased creatinine clearance, upregulated the expression of mOCT1/2, and facilitated HUA improvement.
3.1.8 Salvia plebeia R. Br. [Lamiaceae; Salviae herba]
Salvia plebeia R. Br. (SP) is a Labiatae herbaceous plant, widely distributed in China, Korea, Japan, India, Iran, and Australia. In China, it is commonly referred to as “lychee grass” and has been extensively used in traditional medicine for treating ascites swelling and nephritic edema. Numerous studies have documented the anti-inflammatory, antioxidant, antibacterial, and antiviral properties of SP (Chen and Kang, 2014; Jin et al., 2015). Recent research focusing on xanthine oxidase inhibitors has revealed that SP exhibits potent inhibitory effects on XOD activity. Kim et al. demonstrated that baicalin and lignans present in SP significantly contribute to the inhibition of XOD activity with IC50 values below 5 μm (Kim et al., 2017).
3.2 Single bioactive metabolites
3.2.1 Rhubarbic acid/Emodin
Rhei radix et rhizome, a traditional Chinese medicine, is derived from the dried roots and rhizomes of Polygonum palmatum, Rheum palmatum, Rheum tanguticum, or Rheum officinale in the family Polygonaceae. Rhubarb contains anthraquinones such as rhubarbic acid, rhubarbol, and rhubarb phenol which possess antibacterial, anti-inflammatory, antiviral, antioxidant properties as well as exhibit effects against HUA and renal fibrosis (Mueller-Heupt et al., 2022). Meng et al. (2023) demonstrated that Rhubarbic Acid has notable uric acid-lowering and nephroprotective effects on HUA mice by significantly reducing serum uric acid levels along with serum creatinine and blood urea nitrogen levels while inhibiting XOD activity in mouse liver. Emodin is an anthraquinone from rhubarb that has anti-inflammatory, detoxifying, and gut motility-promoting effects; in addition, some studies have reported that emodin can also inhibit XOD activity (Shi et al., 2014). A recent study by Hou et al. (2023) found that rhodopsin can significantly reduce serum uric acid levels, promote uric acid excretion, and thus play a role in the treatment of hyperuricemia.
3.2.2 Berberine
Phellodendri chinense cortex (PC), commonly known as “Chuan Huang Bai,” is extensively utilized for the treatment of damp-heat diarrhoea, jaundice and dysentery, pyorrhoea, and astringent pain. PC has been traditionally employed as a medicinal remedy for gout and hyperuricemia, such as in Ermiao Wan, Sanmiao Wan, and Ventilation Soup. Pharmacological investigations have revealed that PC contains diverse bioactive constituents including alkaloids and flavonoids. Notably, berberine, an alkaloid group present in PC, has recently demonstrated anti-hyperuricemic activity by modulating the expression of URAT1 and GLUT9 transporter proteins along with XOR activity regulation (Li et al., 2021; Naz et al., 2021). Xu et al. (2021) investigated the hypouricemic and nephroprotective effects of dihydroberberine in hyperuricemic mice and observed significant reduction in serum uric acid levels as well as XOD levels while inhibiting hepatic XOD activity and ADA activities. Furthermore, they found downregulation of renal XOD mRNA and protein expression.
3.2.3 Astilbin
Extracted from sarsaparilla rhizomes, Astilbin is an active flavonoid compound that is widely used in traditional Chinese medicine therapy for its anti-arthritic, anti-hepatic, and anti-kidney injury effects. Wang et al. (2016), in order to study the effect of Astilbin on potassium oxybate-induced hyperuricemia mice and its mechanism of action. The results showed that Astilbin significantly reduced serum uric acid (Sur) levels, and its effects were associated with the inhibition of GLUT9 and URAT1, expression and the upregulation of ABCG2, OAT1/3 and OCT1 expression. In addition, Aastilbin inhibited the activation of Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) cascade and overexpression of suppressor of cytokine signaling 3 (SOCS3), and exerted nephroprotective effects by inhibiting oxidative stress.
3.2.4 Apigenin
Apigenin (4,5,7 -trihydroxyflavone) is a naturally occurring flavonoid that is mainly derived from Apium graveolens L. (celery), but is also found in a wide variety of plants, fruits, and vegetables. There is growing evidence that apigenin attenuates UA in mice with chromosome- or potassium oxybate-induced HUA (Chesworth et al., 2021). Previously, it has been shown that apigenin can reduce serum uric acid levels, decrease the levels of GLUT9 and URAT1 transport proteins, increase the levels of OAT1 transport proteins, and inhibit the JAK2/STAS3 signaling pathway in a mouse model of acute hyperuricemia induced by potassium oxybate and Hypoxanthine, thus exerting a therapeutic effect on hyperuricemia (Liu et al., 2022).
3.3 Traditional Chinese medicine formulas
Throughout the millennia of Chinese culture, Chinese medicine has become an integral component of the comprehensive medical system (Xu et al., 2013). Chinese medicine formulas are not haphazardly concocted but have evolved over thousands of years through clinical practice. According to Chinese medicine, hyperuricemia primarily arises from dampness and heat, thus treatment predominantly focuses on clearing heat and alleviating dampness using formulations like Ermiao Wan, Er Ding Granules, and Qu Zhuo Tong Bi Tang. Our analysis of commonly employed traditional Chinese medicine prescriptions has revealed various approaches for managing hyperuricemia (Table 3).
3.3.1 Pharmacological mechanism of Ermiao Pill and similar formulations in treating HUA
Ermiao Wan (EMW) is derived from Zhu Zhenheng’s “Danxi Xinfa” and is utilized for the treatment of damp-heat infiltration, damp-heat banding, and gonorrhea. The complete formula comprises two botanical drugs, namely, Phellodendri chinense cortex and Atractylois rhizoma. Subsequently, based on their remarkable efficacy in reducing uric acid levels, Sanmiao Wan (SMW) and Simiao Pill were developed by later medical practitioners to enhance therapeutic outcomes. Previous studies have demonstrated that Phellodendri chinense cortex, Atractylois rhizoma and Coicis semen possess diuretic properties with the ability to eliminate dampness. Additionally, hyssop has traditionally been recognized for its downward-moving effect and frequent usage as a menstruation-inducing herb (Zhao et al., 2014). Huang et al. (2019) conducted an investigation into the comprehensive composition and mechanism of action of EMW in treating HUA, identifying 24 alkaloids, 46 volatile components, 15 organic acids, 4 terpenoids, 3 lactones, 3 glycosides along with other compounds within this formulation. Metabolomic analysis revealed that EMW exerts anti-HUA effects through the regulation of multiple metabolic pathways including glycerolipid metabolism amino acid metabolism prochlorogenic bile acid metabolism taurine and hypotaurine metabolism as well as purine metabolism. Several studies have elucidated the mechanism of action of EMW in ameliorating HUA from a metabolomics perspective. Following EMW intervention, there was a significant reduction in serum uric acid levels. Metabolomics analysis identified 11 biomarkers exhibiting a reversal trend. Pathway analysis revealed that EMW may exert therapeutic effects on HUA rats through pathways such as phenylalanine metabolism, glycerophospholipid metabolism, tryptophan metabolism, and lipid metabolism (Shan et al., 2021; Gu et al., 2023). Hyperuricemia can contribute to the development of chronic kidney disease and cardiovascular disease. Guo et al. (2015) investigated the combined protective effects of Siwu Tang and EMW on HUA and renal injury and demonstrated that this combination significantly reduced serum levels of uric acid, creatinine, triglycerides, and urea nitrogen. The underlying mechanism may involve decreased renal xanthine oxidase activity and upregulation of OAT1 and OAT3 expression. Serum metabolomic analysis revealed that SMW could partially regulate purine metabolism, amino acid metabolism, and energy metabolism to reverse the pathological process associated with HUA (Jiang et al., 2017). Simiao Wan is utilized as a herbal formulation for adjunctive therapy in gout. A study conducted by Cao et al. (2021) demonstrated that Simiao Wan exhibits potential in ameliorating MSU-induced gouty arthritis and inhibiting hyperuricemia, possibly through the activation of the PI3K/Akt signaling pathway to promote M2 polarization. Zhang et al. (2023) discovered that Simiao powder could attenuate HUA in mice by reducing the expression of URAT1, GULT9, NLRP3, Phospho-Janus Kinase-2/Janus Kinase-2 (P-JAK2/JAK2), and Phospho-signal transducer and activator of transcription-3/signal transducer and activator of transcription-3 (P-STAT3/STAT3) in renal tissues; this mechanism of action may be associated with the suppression of NLRP3 inflammasome and Janus Kinase-2/signal transducer and activator of transcription-3 (JAK2/STAT3) signaling pathway. In summary, EMW and its analogs exhibit therapeutic potential against HUA through four pathways: inhibition of renal XOD activity; upregulation of transporter proteins OAT1 and OAT3; regulation of purine metabolism and amino acid metabolism; modulation of the PI3K/Akt and JAK2/STAT3 signaling pathways.
3.3.2 Pharmacological mechanism of Er Ding granules in treating HUA
The composition of Er Ding Granules includes four botanical drugs, namely, Violae herba, Taraxaci herba, Lobeliae chinensis herba and Isatidis radix, all of which were initially documented in the Shen Nong Ben Cao Jing. It is well-documented that Violae herba, Lobeliae chinensis herba and Isatidis radix possess anti-inflammatory and antibacterial properties. Additionally, Dandelion exhibits diuretic effects by increasing renal urine excretion. Zuo et al. (2018) demonstrated that treatment with Er Ding Granules significantly reduced serum uric acid levels in mice while down-regulating URAT1 mRNA expression in the kidneys of hyperuricemic mice and enhancing OAT3 mRNA expression. Zhang et al. (2019) divided the complete formula into water extracts as well as 50% ethanol and 95% ethanol extracts to investigate their anti-hyperuricemic activity. The results revealed a significant dose-dependent effect on serum uric acid levels for the 50% ethanol extract. Mechanistic studies indicated that the anti-hyperuricemic effect of the 50% ethanol extract primarily involved downregulation of GLUT9 and URAT1 protein expression along with upregulation of OAT1 protein expression. Downregulation of reabsorption proteins GLUT9 and URAT1 along with upregulation of secretory proteins OAT1 and OAT3 represent key mechanisms underlying the therapeutic effects exerted by Er Ding Granules on hyperuricemia.
3.3.3 Pharmacological mechanism of Qu Zhuo Tong Bi Tang in treating HUA
The empirical formula Qu Zhuo Tong Bi Tang (QZTBD) demonstrates definite clinical efficacy in the treatment of HUA and gout, as it effectively enhances kidney function, improves blood circulation, and alleviates pain. This formula comprises Smilacis chinae rhizoma, Dioscoreae spongiosae rhizoma, Coicis semen, Curcumae longae rhizoma, Cortdalis rhizoma and Siegesbeckiae herba (Wen et al., 2021). Chen et al. (2016) employed serum metabolomics to investigate the impact of QZTBD on serum urine and other metabolites in HUA-induced rats. Their findings confirm that QZTBD significantly reduces serum uric acid levels by regulating amino acid metabolism, purine metabolism, and energy metabolism mechanisms. Song et al. utilized a combined analysis of network pharmacology and intestinal flora to elucidate the mechanism of action underlying QZTBD’s therapeutic effects against HUA. The results demonstrate that QZTBD has the potential to enhance the abundance of Allobaculum and Candidatus sacchairmonas while rectifying abnormal amino acid patterns. Additionally, it repairs compromised intestinal barriers by restoring Th17/Treg cell balance through modulation of the PI3K-AKT-mTOR pathway; furthermore reducing inflammatory factors such as IL-1β, interleukin-6 (IL-6), TNF-α, and interleukin-17 (IL-17) levels (S et al., 2023). In summary, QZTBD exerts its therapeutic effects on HUA through regulation of amino acid metabolism, purine metabolism, and energy metabolism. Additionally, it restores intestinal barrier integrity, inhibits the PI3K-AKT signaling pathway.
3.3.4 Pharmacological mechanism of Fangji Huangqi Tang in treating of HUA
The prescription Fangji Huangqi Tang is derived from the synopsis of the Golden Chamber written by Zhang Zhongjing, and it primarily focuses on treating Feng Shui or rheumatic diseases. Composed of Stephaniae tetrandrae radix, Astragali radix, Glycyrrhizae radix et rhizoma and Atractylodeis macrocephalae rhizoma, this representative prescription aims to replenish qi and promote water balance. Previous studies have reported that Stephaniae tetrandrae radix and Astragali radix possess diuretic effects which can improve kidney function. Additionally, Atractylodeis macrocephalae rhizoma and Glycyrrhizae radix et rhizoma have been found to reduce serum uric acid levels while providing renal protection (Aksoy et al., 2012; Wang et al., 2018; Wei et al., 2018). Recent research has demonstrated that Fangji Huangqi Tang significantly lowers serum uric acid and creatinine levels while inhibiting IL-1β in both serum and kidneys of Hua mice. Furthermore, it effectively reduces NF-kB protein expression. Analysis of renal uric acid-related transporters revealed that Fangji Huangqi Tang upregulates the protein expression levels of renal OAT1, OAT3, and ABCG2 (Xing et al., 2020). When used individually as single botanical drug or combined together in Fangji Huangqi Tang formulae, Stephaniae tetrandrae radix, Astragali radix and Atractylodeis macrocephalae rhizoma, exhibit diuretic effects with enhanced efficacy when combined together (Lin et al., 2015). Moreover, their combination significantly upregulates the expression of renal uric acid secretion proteins OAT1, OAT3, and ABCG2 while substantially reducing organismal uric acid levels.
3.3.5 Pharmacological mechanism of Shi Wei Ru Xiang powder in the treating of HUA
Shiwei Ruxiang powder, a traditional Tibetan medicine, is commonly utilized in traditional Chinese medicine for the treatment of HUA. In a study conducted by Li et al. (2022), network pharmacology and experimental validation were employed to investigate the effects of SWS on HUA in mice. The findings demonstrated that SWS ameliorated HUA through modulation of pivotal signaling pathways encompassing MAPK, nuclear factor κB (NF-κB), and NOD-like receptor signaling pathways.
3.3.6 Pharmacological mechanism of Cheqianzi Tang in the treating of HUA
In the Song Dynasty’s “Shengji Zonglu,” the Cheqianzi Tang (CQD) was discovered to possess the following therapeutic properties: heat-clearing and detoxification, dampness-promoting and dehumidifying effects. It has demonstrated a positive curative impact on hyperuricemia. The composition of CQD comprises Plantaginis semen, Achyranthis bidentatae radix, Typhae pollen, and Mori cortex. Studies have reported that psyllium decoction can ameliorate hyperuricemia by activating ABCG2, which facilitates uric acid excretion, as well as inducing downregulation of inflammatory and apoptotic factors mediated by inflammasome NLRP3 (J et al., 2023).
4 Discussion
This article provides a comprehensive review of the botanical drugs commonly used for treating HUA in rat and mice models and presents research findings that demonstrate the efficacy of everal specific botanicals, bio-metabolites and herbal formulations in inhibiting hepatic XOD activity, modulating renal uric acid transport proteins, suppressing inflammatory response pathways, and regulating product metabolism.
Xanthine oxidase, an enzyme crucial in the pathogenesis of HUA and uric acid homeostasis, catalyzes the oxidation of hypoxanthine to xanthine and further to uric acid (Sang et al., 2017). Among the aforementioned botanical drugs, Atractylodes macrocephala Koidz., Phellodendron chinense C. K. Schneid., Polygonum cuspidatum Siebold & Zucc., Smilax china L., Rheum palmatum L. and Salvia plebeia R. Br. exhibit inhibitory effects on XOD activity. Renal transporter proteins associated with uric acid metabolism can be categorized into two groups: URAT1, OAT4, GLUT9 as urate reabsorption transporters; OAT1, OAT3, MRP4/ABCC4, and ABCG2 as urate excretion transporters. Altered expression and function of these transporter proteins are implicated in HUA development (Zhao et al., 2022). In addition to Phellodendron chinense C.K.Schneid and Cichorium glandulosum Boiss. & A. Huet which inhibit hepatic XOD activity while modulating renal uric acid-related transporter proteins; Plantago asiatica L., Astragalus membranaceus Bunge and Morus alba L. also regulate serum uric acid levels by modulating renal uric acid-related transporter proteins. Severe HUA can lead to renal inflammation and gut microbial dysbiosis; hence targeting inflammatory response inhibition along with modulation of gut microbial populations may offer a novel approach for improving HUA management (Zhao et al., 2022). Among the ten botanical drugs mentioned above, Astragalus membranaceus Bunge and Cichorium glandulosum Boiss. & A. Huet can enhance hyperuricemia (HUA) by augmenting probiotics such as Bifidobacterium and Bacillus tansy, thereby fortifying the intestinal mucosal barrier. Additionally, Cichorium glandulosum Boiss. & A. Huet exhibits inhibitory effects on the TLR4/NF-kB inflammatory signaling pathway to impede the progression of HUA-induced nephritis. Conversely, Atractylodes macrocephala Koidz., Plantago asiatica L. and Polygonum cuspidatum Siebold & Zucc. mitigate further advancement of HUA primarily through suppression of NF-kB and PI3K/Akt inflammatory signaling pathways along with NLRP3 inflammatory vesicles. In summary, Chinese medicine effectively addresses HUA via four principal mechanisms of action (Figure 3).
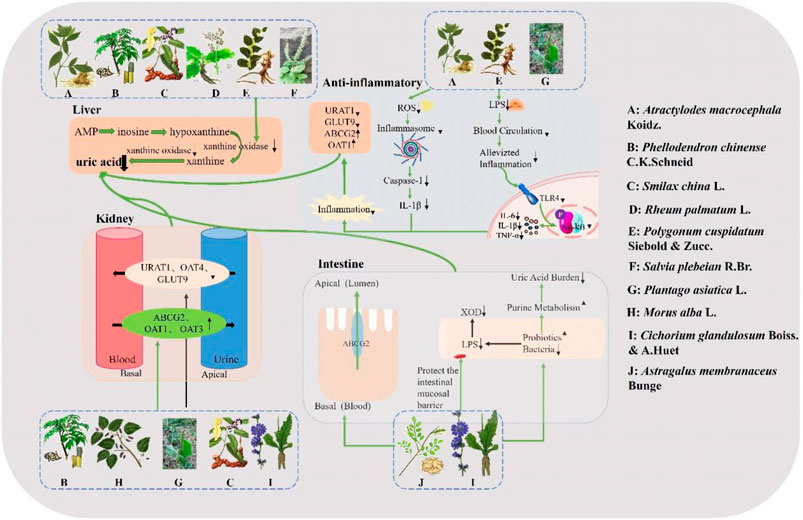
Figure 3. Mechanism of action of traditional Chinese medicine in the treatment of HUA. Atractylodes macrocephala Koidz., Phellodendron chinense C. K. Schneid., Smilax china L., Rheum palmatum L., Polygonum cuspidatum Siebold & Zucc., and Salvia plebeia R. Br. exhibit direct inhibitory effects on XOD activity for uric acid reduction. Atractylodes macrocephala Koidz., Polygonum cuspidatum Siebold & Zucc. and Plantago asiatica L. exert their hypouricemic effects by suppressing the TLR4/NF-kB signaling pathway and NLRP3 inflammasomes. Phellodendron chinense C.K.Schneid., Morus alba L., Plantago asiatica L., Smilax china L. and Cichorium glandulosum Boiss. & A. Huet reduce uric acid levels by downregulating reabsorption-associated proteins and upregulating excretion-associated proteins in the kidney. Astragalus membranaceus Bunge and Cichorium glandulosum Boiss. & A. Huet directly target uric acid excretory proteins in the intestine or demonstrate hypouricemic effects through regulation of intestinal flora and protection of the intestinal mucosal barrier.
Traditional Chinese medicine has multiple advantages in the treatment of hyperuricemia. Firstly, it employs a multi-pathway, multi-target treatment strategy, which effectively reduces uric acid levels and improves overall health. Chinese herbal medicine achieves therapeutic goals through various pathways such as regulating uric acid production, increasing uric acid excretion, and reducing uric acid absorption. This comprehensive action can provide more lasting and balanced therapeutic effects.
Secondly, Chinese herbal medicine is mostly derived from natural sources and has undergone extensive historical validation and clinical practice. These medicines often have fewer side effects because they are more compatible with the human body’s natural processes. In contrast, many conventional uric acid-lowering drugs may cause common side effects such as indigestion and headaches (Wang et al., 2020).
In addition, Chinese herbal medicine is often used in complex formulations, which not only enhances efficacy but also reduces the potential toxicity of individual components. For example, Ge Gen Qin Lian Tang, a classic Chinese herbal formula, is widely used for treating hyperuricemia. It has been proven effective in lowering uric acid levels and is considered safe (Wang et al., 2023).
This article summaries research on the potential and mechanisms of herbal medicine for the treatment of hyperuricemia. The studies focused on reducing XOD activity, regulating uric acid transporter proteins, influencing inflammatory signaling pathways, and regulating gut microbial homeostasis. Although the studies on regulating intestinal microbial balance and upstream regulators of uric acid transporter proteins still need to be deepened, traditional Chinese medicines show good prospects for application in rat and mouse models. Overall, the treatment of hyperuricemia with traditional Chinese medicines not only demonstrates significant advantages in reducing uric acid levels, but also improves overall health and reduces the risk of complications. Pharmacological studies, clinical evidence and historical applications support the safety and efficacy of TCM in the treatment of hyperuricemia.
Author contributions
HB: Writing–original draft. ZZ: Writing–original draft. MZ: Resources, Supervision, Writing–review and editing. YS: Resources, Supervision, Writing–review and editing. YW: Resources, Supervision, Writing–review and editing. BL: Resources, Supervision, Writing–review and editing. QW: Funding acquisition, Project administration, Writing–review and editing. HK: Funding acquisition, Project administration, Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chief Scientist of Qi-Huang Project of National Traditional Chinese Medicine Inheritance and Innovation “One Hundred Million” Talent Project [Grant Number: (2021) No. 7]. Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program [Grant Number: (2018) No. 284]. National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of National Administration of TCM [Grant Number: (2022) No. 75]. The Seventh Batch of National Famous Old Traditional Chinese Medicine Experts Experience Heritage Construction Program of National Administration of TCM [Grant Number: (2022) No. 76].
Acknowledgments
Figures were created by Figdraw (www.figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aksoy, N., Dogan, Y., Iriadam, M., Bitiren, M., Uzer, E., Ozgonul, A., et al. (2012). Protective and therapeutic effects of licorice in rats with acute tubular necrosis. J. Ren. Nutr. 22 (3), 336–343. doi:10.1053/j.jrn.2011.07.002
Ammar, H., Touihri, I., Kholif, A. E., M'Rabet, Y., Jaouadi, R., Chahine, M., et al. (2022). Chemical composition, antioxidant, and antimicrobial activities of leaves of ajuga iva. Molecules 27 (20), 7102. doi:10.3390/molecules27207102
Azay-Milhau, J., Ferrare, K., Leroy, J., Aubaterre, J., Tournier, M., Lajoix, A. D., et al. (2013). Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): a comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 150 (2), 755–760. doi:10.1016/j.jep.2013.09.046
Bao, Y., Li, H., Li, Y., Li, Y., Li, F., Zhang, F., et al. (2018). Therapeutic effects of Smilax glabra and Bolbostemma paniculatum on rheumatoid arthritis using a rat paw edema model. Biomed. Pharmacother. 108, 309–315. doi:10.1016/j.biopha.2018.09.004
Bhatnagar, V., Richard, E., Wu, W., Nievergelt, M., Lipkowitz, S., Jeff, J., et al. (2016). Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clin. Kidney J. 9 (3), 444–453. doi:10.1093/ckj/sfw010
Bian, M., Lin, J., Wang, Y., Zhang, B., Li, G., and Wang, H. (2018). Bioinformatic and metabolomic analysis reveal intervention effects of chicory in a quail model of hyperuricemia. Evidence-Based Complementary Altern. Med. 2018, 5730385. doi:10.1155/2018/5730385
Bian, M., Wang, J., Wang, Y., Nie, Z., Zhu, S., Sun, X., et al. (2020). Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomed. Pharmacother. 131, 110719. doi:10.1016/j.biopha.2020.110719
Bian, T., Xiao, L., Liang, L., Xie, P., Wang, L., Slevin, M., et al. (2022). Polydatin prevents neuroinflammation and relieves depression via regulating sirt1/HMGB1/NF-kappa B signaling in mice. Neurotox. Res. 40 (5), 1393–1404. doi:10.1007/s12640-022-00553-z
Boghrati, Z., Zibaee, E., Ayati, Z., Amiri, S., Ramezani, M., Jamialahmadi, T., et al. (2021). Ethnomedicinal uses, phytochemistry and pharmacology of different cichorium species (Asteraceae): a review. Pharmacol. Prop. Plant-Derived Nat. Prod. Implic. Hum. Health 1308, 501–546. doi:10.1007/978-3-030-64872-5_26
Borges, F., Fernandes, E., and Roleira, F. (2002). Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 9 (2), 195–217. doi:10.2174/0929867023371229
Bose, B., Badve, V., Hiremath, S., Boudville, N., Brown, G., Cass, A., et al. (2014). Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol. Dial. Transplant. 29 (2), 406–413. doi:10.1093/ndt/gft378
Cao, L., Zhao, T., Xue, Y., Xue, L., Chen, Y., Quan, F., et al. (2021). The anti-inflammatory and uric acid lowering effects of Si-Miao-San on gout. Front. Immunol. 12, 777522. doi:10.3389/fimmu.2021.777522
Chen, J., Zhou, J., Wei, S., Xie, J., Wen, P., and Xu, W. (2016). Effect of a traditional Chinese medicine prescription Quzhuotongbi decoction on hyperuricemia model rats studied by using serum metabolomics based on gas chromatography-mass spectrometry. J. Chromatogr. B-Analytical Technol. Biomed. Life Sci. 1026, 272–278. doi:10.1016/j.jchromb.2015.10.031
Chen, K., Chen, H., Faas, M., de Haan, J., Li, H., Xiao, P., et al. (2017). Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 61 (8). doi:10.1002/mnfr.201601006
Chen, L., and Kang, H. (2014). Antioxidant and enzyme inhibitory activities of plebeian herba (Salvia plebeia R. Br.) under different cultivation conditions. J. Agric. Food Chem. 62 (10), 2190–2197. doi:10.1021/jf404570s
Chen, Y., Lan, Z., Zhou, Q., Li, F., Zhang, X., Zhang, F., et al. (2011a). Astilbin attenuates hyperuricemia and ameliorates nephropathy in fructose-induced hyperuricemic rats. Planta Medica 77 (16), 1769–1773. doi:10.1055/s-0030-1271135
Chen, Y., Li, C., Duan, S., Yuan, X., Liang, J., and Hou, S. (2019). Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 118, 109195. doi:10.1016/j.biopha.2019.109195
Chen, Y., Yang, J., Rao, Q., Wang, C., Chen, X., Zhang, Y., et al. (2023). Understanding hyperuricemia: pathogenesis, potential therapeutic role of bioactive peptides, and assessing bioactive peptide advantages and challenges. Foods Basel, Switz. 12 (24), 4465. doi:10.3390/foods12244465
Chen, Y., Yin, F., Lan, Z., Ma, W., Zhang, F., Yang, L., et al. (2011b). Anti-hyperuricemic and nephroprotective effects of Smilax china L. J. Ethnopharmacol. 135 (2), 399–405. doi:10.1016/j.jep.2011.03.033
Chen, Y., Zhao, Z., Li, Y., Yang, Y., Li, L., Jiang, Y., et al. (2021). Baicalein alleviates hyperuricemia by promoting uric acid excretion and inhibiting xanthine oxidase. Phytomedicine 80, 153374. doi:10.1016/j.phymed.2020.153374
Cheng, P., Sun, X., Li, M., Yan, M., Peng, H., You, P., et al. (2019). Effects of alismatis rhizoma and rhizoma Smilacis glabrae decoction on hyperuricemia in rats. Evidence-Based Complementary Altern. Med. 2019, 4541609. doi:10.1155/2019/4541609
Chesworth, R., Gamage, R., Ullah, F., Sonego, S., Millington, C., Fernandez, A., et al. (2021). Spatial memory and microglia activation in a mouse model of chronic neuroinflammation and the anti-inflammatory effects of apigenin. Front. Neurosci. 15, 699329. doi:10.3389/fnins.2021.699329
Dragos, D., Manea, M., Timofte, D., and Ionescu, D. (2020). Mechanisms of herbal nephroprotection in diabetes mellitus. J. Diabetes Res. 2020, 5710513. doi:10.1155/2020/5710513
Durazzo, A., Nazhand, A., Lucarini, M., Silva, M., Souto, B., Guerra, F., et al. (2021). Astragalus (Astragalus membranaceus Bunge): botanical, geographical, and historical aspects to pharmaceutical components and beneficial role. Rendiconti Lincei-Scienze Fis. E Nat. 32 (3), 625–642. doi:10.1007/s12210-021-01003-2
Fang, C., Chen, L., He, M., Luo, Y., Zhou, M., Zhang, N., et al. (2019). Molecular mechanistic insight into the anti-hyperuricemic effect of Eucommia ulmoides in mice and rats. Pharm. Biol. 57 (1), 112–119. doi:10.1080/13880209.2019.1568510
Ge, L., Su, G., Wang, H., Zhao, X., Hou, F., Zheng, A., et al. (2023). Identifying the intervention mechanisms of polydatin in hyperuricemia model rats by using UHPLC-Q-Exactive Orbitrap mass spectroscopy metabonomic approach. Front. Nutr. 10, 1117460. doi:10.3389/fnut.2023.1117460
Gu, C., Hu, X., Shan, B., Wu, X., and Chen, J. (2023). Targeted and non-targeted metabolomics uncovering the effects of Er-Miao-Wan formula on rats with hyperuricemia. J. Pharm. Biomed. Anal. 226, 115246. doi:10.1016/j.jpba.2023.115246
Guo, F., Chen, X., Lei, S., Li, B., Zhang, Y., Ge, Z., et al. (2020). Effects and mechanisms of dendrobium officinalis six nostrum for treatment of hyperuricemia with hyperlipidemia. Evidence-Based Complementary Altern. Med. 2020, 2914019. doi:10.1155/2020/2914019
Guo, P., Jiang, Q., Gui, K., and Wang, S. (2015). Chinese herbal formulas Si-Wu-Tang and Er-Miao-San synergistically ameliorated hyperuricemia and renal impairment in rats induced by adenine and potassium oxonate. Cell. Physiology Biochem. 37 (4), 1491–1502. doi:10.1159/000438517
Hong, Q., Yu, D., Mei, Y., Lv, Y., Chen, P., Wang, D., et al. (2014). Smilacis glabrae rhizoma reduces oxidative stress caused by hyperuricemia via upregulation of catalase. Cell. Physiology Biochem. 34 (5), 1675–1685. doi:10.1159/000366369
Hooda, S., Pal, R., Bhandari, A., and Singh, J. (2014). Antihyperglycemic and antihyperlipidemic effects of Salvadora persica in streptozotocin-induced diabetic rats. Pharm. Biol. 52 (6), 745–749. doi:10.3109/13880209.2013.869607
Hosomi, A., Nakanishi, T., Fujita, T., and Tamai, I. (2012). Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS One 7 (2), e30456. doi:10.1371/journal.pone.0030456
Hou, W., Chen, J., Shen, D., Chen, Y., Wang, J., Wang, H., et al. (2023). Emodin, a natural anthraquinone, increases uric acid excretion in rats with potassium oxonate-induced hyperuricemia. Pharm. (Basel). 25 (6), 789. doi:10.3390/ph16060789
Hu, Q., Ji, J., Xu, P., Ye, L., Sun, D., Sheng, A., et al. (2023). Isolation and characterization of uric acid-lowering functional components from Polygonum cuspidatum. Food Biosci. 53, 102314. doi:10.1016/j.fbio.2022.102314
Huang, X., Hui, W., Wang, C., Li, P., and Chen, J. (2019). Study on chemical constituents of herbal formula Er Miao Wan and GC-MS based metabolomics approach to evaluate its therapeutic effects on hyperuricemic rats. J. Chromatogr. B-Analytical Technol. Biomed. Life Sci. 1118, 101–108. doi:10.1016/j.jchromb.2019.04.032
Jeong, J., Lim, K., Han, H., Lee, H., Kang, S., and Lee, S. (2022). Extract of Aster glehni ameliorates potassium oxonate-induced hyperuricemia by modulating renal urate transporters and renal inflammation by suppressing TLR4/MyD88 signaling. Food Sci. Biotechnol. 30 (13), 1729–1739. doi:10.1007/s10068-022-01153-5
Jiang, W., Qian, P., Ding, R., Wang, K., Ding, Y., Liu, X., et al. (2017). Metabolomic profiles delineate the effect of Sanmiao wan on hyperuricemia in rats. Biomed. Chromatogr. 31 (2). doi:10.1002/bmc.3792
Jin, R., Xu, H., Duan, H., and Chou, X. (2015). Two new flavones from Salvia plebeia. Nat. Prod. Res. 29 (14), 1315–1322. doi:10.1080/14786419.2014.999241
Johnson, J., Bakris, L., Borghi, C., Chonchol, B., Feldman, D., Lanaspa, A., et al. (2018). Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am. J. Kidney Dis. 71 (6), 851–865. doi:10.1053/j.ajkd.2017.12.009
Kim, K., Kim, J., Hyun, M., Lee, S., Kwon, G., Seo, C., et al. (2017). Salvia plebeia extract inhibits xanthine oxidase activity in vitro and reduces serum uric acid in an animal model of hyperuricemia. Planta Medica 83 (17), 1335–1341. doi:10.1055/s-0043-111012
Kurajoh, M., Fukumoto, S., Yoshida, S., Akari, S., Murase, T., Nakamura, T., et al. (2021). Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 11 (1), 7378. doi:10.1038/s41598-021-86962-0
Li, P., Huang, W., Liu, F., Zheng, N., Xie, H., Chen, N., et al. (2021). Effect of berberine on hyperuricemia and kidney injury: a network pharmacology analysis and experimental validation in a mouse model. Drug Des. Dev. Ther. 15, 3241–3254. doi:10.2147/Dddt.S317776
Li, Q., Liu, P., Wu, C., Bai, L., Zhang, Z., Bao, Z., et al. (2022). Integrating network pharmacology and pharmacological validation to explore the effect of Shi Wei Ru Xiang powder on suppressing hyperuricemia. J. Ethnopharmacol. 298, 115679. doi:10.1016/j.jep.2022.115679
Lin, Y., Liu, G., Liang, Q., Hu, J., Xu, P., Zhou, J., et al. (2018). Luteolin-4'-O-glucoside and its aglycone, two major flavones of Gnaphalium affine D. Don, resist hyperuricemia and acute gouty arthritis activity in animal models. Phytomedicine 41, 54–61. doi:10.1016/j.phymed.2018.02.002
Lin, Y., Wang, Y., Tang, L., Lee, C., Chan, H., Choi, A., et al. (2022). The extracts of Polygonum cuspidatum root and rhizome block the entry of SARS-CoV-2 wild-type and omicron pseudotyped viruses via inhibition of the S-protein and 3CL protease. Molecules 27 (12), 3806. doi:10.3390/molecules27123806
Lin, Y. C., Chang, C. W., and Wu, C. R. (2015). Anti-nociceptive, anti-inflammatory and toxicological evaluation of Fang-Ji-Huang-Qi-Tang in rodents. BMC Complement. Altern. Med. 15, 10. doi:10.1186/s12906-015-0527-5
Liu, L., Jiang, S., Liu, X., Tang, Q., Chen, Y., Qu, J., et al. (2021). Inflammatory response and oxidative stress as mechanism of reducing hyperuricemia of Gardenia jasminoides-Poria cocos with network pharmacology. Oxid. Med. Cell Longev. 2021, 8031319. doi:10.1155/2021/8031319
Liu, P., Li, X., Yu, B., Huang, J., Sun, J., Huo, S., et al. (2005). Effects of trans-resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. J. Med. Food 8 (1), 14–19. doi:10.1089/jmf.2005.8.14
Liu, Q., Sun, X., Liu, B., Zhang, T., Zhang, L., and Wu, J. (2022b). Phytochemicals in traditional Chinese medicine can treat gout by regulating intestinal flora through inactivating NLRP3 and inhibiting XOD activity. J. Pharm. Pharmacol. 74 (7), 919–929. doi:10.1093/jpp/rgac024
Liu, T., Gao, H., Zhang, Y., Wang, S., Lu, M., Dai, X., et al. (2022a). Apigenin ameliorates hyperuricemia and renal injury through regulation of uric acid metabolism and JAK2/STAT3 signaling pathway. Pharm. (Basel) 21 (11), 1442. doi:10.3390/ph15111442
Luissint, C., Parkos, A., and Nusrat, A. (2016). Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151 (4), 616–632. doi:10.1053/j.gastro.2016.07.008
Ma, G., Wang, J., Bu, W., Zhang, H., Zhang, P., Zhang, X., et al. (2019). Effects of Polygonum cuspidatum on AMPK-FOXO3 signaling pathway in rat model of uric acid-induced renal damage. Chin. J. Integr. Med. 25 (3), 182–189. doi:10.1007/s11655-017-2979-6
Ma, N., Cai, S., Sun, Y., and Chu, C. (2024). Chinese sumac (rhus chinensis mill.) fruits prevent hyperuricemia and uric acid nephropathy in mice fed a high-purine yeast diet. Nutrients 16 (2), 184. doi:10.3390/nu16020184
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C., and Mollace, V. (2016). Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 213, 8–14. doi:10.1016/j.ijcard.2015.08.109
Mehmood, A., Zhao, L., Ishaq, M., Xin, W., Zhao, L., Wang, C., et al. (2020). Anti-hyperuricemic potential of stevia (Stevia rebaudiana Bertoni) residue extract in hyperuricemic mice. Food Funct. 11 (7), 6387–6406. doi:10.1039/c9fo02246e
Meng, J., Tian, J., Zhao, Y., Li, C., Yi, Y., Zhang, Y., et al. (2023). Ameliorative effect of cheqianzi decoction on hyperuricemia and kidney injury and underlying mechanism in rats. Heliyon 9 (4), e15333. doi:10.1016/j.heliyon.2023.e15333
Meng, Q., Yan, X., Tang, H., Guo, R., Li, N., Huang, Z., et al. (2015). Anti-hyperuricemic and nephroprotective effects of rhein in hyperuricemic mice. Planta Medica 81 (4), 279–285. doi:10.1055/s-0034-1396241
Morgan, L., and Singh, A. (2021). How do dietary interventions affect serum urate and gout? Nat. Rev. Rheumatol. 17 (4), 191–192. doi:10.1038/s41584-021-00576-4
Mueller-Heupt, K., Vierengel, N., Gross, J., Opatz, T., Deschner, J., and von Loewenich, D. (2022). Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum extracts and rhein against porphyromonas gingivalis. Antibiotics-Basel 11 (2), 186. doi:10.3390/antibiotics11020186
Naz, H., Naz, S., Miraj, R., Zaheer, A., Azam, N., Mughal, S., et al. (2021). The effect of berberine, a drug from Chinese folk medicine, on serum and urinary uric acid levels in rats with hyperuricemia. Cureus J. Med. Sci. 13 (2), e13186. doi:10.7759/cureus.13186
Qian, W., Jiang, Y., Luo, Y., and Jiang, C. (2023). The anti-hyperuricemia and anti-inflammatory effects of Atractylodes macrocephala in hyperuricemia and gouty arthritis rat models. Comb. Chem. High Throughput Screen. 26 (5), 950–964. doi:10.2174/1386207325666220603101540
Russo, E., Viazzi, F., Pontremoli, R., Barbagallo, C. M., Bombelli, M., Casiglia, E., et al. (2022). Association of uric acid with kidney function and albuminuria: the Uric Acid Right for heArt Health (URRAH) Project. J. Nephrol. 35 (1), 211–221. doi:10.1007/s40620-021-00985-4
Sang, M., Du, Y., Hao, J., Wang, L., Liu, W., Zhang, Y., et al. (2017). Modeling and optimizing inhibitory activities of Nelumbinis folium extract on xanthine oxidase using response surface methodology. J. Pharm. Biomed. Analysis 139, 37–43. doi:10.1016/j.jpba.2017.02.048
Santos, M., Veronese, V., and Moresco, N. (2020). Uric acid and kidney damage in systemic lupus erythematosus. Clin. Chim. Acta 508, 197–205. doi:10.1016/j.cca.2020.05.034
Shahzad, M., Shabbir, A., Wojcikowski, K., Wohlmuth, H., and Gobe, C. (2016). The antioxidant effects of radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets 17 (12), 1331–1340. doi:10.2174/1389450116666150907104742
Shan, B., Chen, T., Huang, B., Liu, Y., and Chen, J. (2021). Untargeted metabolomics reveal the therapeutic effects of Ermiao wan categorized formulas on rats with hyperuricemia. J. Ethnopharmacol. 281, 114545. doi:10.1016/j.jep.2021.114545
Shi, H., Huang, W., Li, C., Liu, W., and Wang, F. (2014). Design, synthesis and molecular modeling of aloe-emodin derivatives as potent xanthine oxidase inhibitors. Eur. J. Med. Chem. 75, 289–296. doi:10.1016/j.ejmech.2014.01.058
Shi, W., Wang, P., Wang, X., Zhang, L., Liu, L., Wang, W., et al. (2012). Uricosuric and nephroprotective properties of Ramulus Mori ethanol extract in hyperuricemic mice. J. Ethnopharmacol. 143 (3), 896–904. doi:10.1016/j.jep.2012.08.023
Song, S., Fan, M., Wen, X., Shi, X., Lou, Y., He, Z., et al. (2023). Integrated network pharmacology and gut microbiome analysis to reveal the mechanism of Qu-Zhuo-Tong-Bi decoction against hyperuricemia and gout. J. Ethnopharmacol. 316, 116736. doi:10.1016/j.jep.2023.116736
Strilchuk, L., Fogacci, F., and Cicero, F. (2019). Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin. Drug Saf. 18 (4), 261–271. doi:10.1080/14740338.2019.1594771
Sun, M., Lan, P., Tong, C., Zhang, Y., Sun, S., Xiong, Z., et al. (2019). An integrative investigation on the efficacy of Plantaginis semen based on UPLC-QTOF-MS metabolomics approach in hyperlipidemic mice. Biomed. Pharmacother. 115, 108907. doi:10.1016/j.biopha.2019.108907
Teixeira, F., de Souza, J., Dophine, D., de Souza Filho, J. D., and Saúde-Guimarães, D. A. (2022). Chemical analysis of Eruca sativa ethanolic extract and its effects on hyperuricaemia. Molecules 27 (5), 1506. doi:10.3390/molecules27051506
Wang, C., Li, L., Chiu, Y., Chen, C., Chen, C., and Chen, A. (2022b). Protective effects of Corni Fructus extract in mice with potassium oxonate-induced hyperuricemia. J. Vet. Med. Sci. 84 (8), 1134–1141. doi:10.1292/jvms.21-0671
Wang, H., Gao, Z., Li, D., Zhang, Y., Moustaid, K., and Nasser, B. (2020). Genus Ziziphus: a comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J. Ethnopharmacol. 259, 112950. doi:10.1016/j.jep.2020.112950
Wang, J., Chen, Y., Zhong, H., Chen, F., Regenstein, J., Hu, S., et al. (2022a). The gut microbiota as a target to control hyperuricemia pathogenesis: potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 62 (14), 3979–3989. doi:10.1080/10408398.2021.1874287
Wang, M., Zhao, J., Zhang, N., and Chen, J. (2016). Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharmacother. 83, 975–988. doi:10.1016/j.biopha.2016.07.025
Wang, Q., Wang, L., Tu, C., and Zhang, C. (2018). Traditional Chinese medicine for refractory nephrotic syndrome: strategies and promising treatments. Evidence-Based Complementary Altern. Med. 2018, 8746349. doi:10.1155/2018/8746349
Wang, X., Li, X., Ma, H., Zhang, F., and Jia, Q. (2013). Tumoral cytotoxic and antioxidative phenylpropanoid glycosides in Smilax riparia A. DC. J. Ethnopharmacol. 149 (2), 527–532. doi:10.1016/j.jep.2013.07.011
Wang, X., Liu, L., Yang, Y., Zhang, M., and Kong, D. (2015). Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 747, 59–70. doi:10.1016/j.ejphar.2014.11.035
Wang, X., Liu, X., Gao, Q., Gu, X., Zhang, G., Sheng, Z., et al. (2023b). Gegen Qinlian Decoction treatment of asymptomatic hyperuricemia by targeting circadian immune function. Chin. Med. 18 (1), 77. doi:10.1186/s13020-023-00775-z
Wang, Y., Lin, F., Ye, C., Aihemaitijiang, S., Halimulati, M., Huang, J., et al. (2023a). Multi-omics analysis reveals therapeutic effects of Bacillus subtilis-fermented Astragalus membranaceus in hyperuricemia via modulation of gut microbiota. Food Chem. 399, 133993. doi:10.1016/j.foodchem.2022.133993
Wei, B., Xu, C., Liu, S., Song, R., Liu, Q., and Qu, B. (2018). Metabonomics study of the effects of traditional Chinese medicine formula Ermiaowan on hyperuricemic rats. J. Sep. Sci. 41 (2), 560–570. doi:10.1002/jssc.201700985
Wen, H., Lou, Y., Song, Y., He, X., Chen, J., Xie, J., et al. (2021). Qu-Zhuo-Tong-Bi decoction alleviates gouty arthritis by regulating butyrate-producing bacteria in mice. Front. Pharmacol. 11, 610556. doi:10.3389/fphar.2020.610556
Wu, H., Ruan, L., Zhang, J., Wang, Q., and Zhang, W. (2014). Pallidifloside D, a saponin glycoside constituent from Smilax riparia, resist to hyperuricemia based on URAT1 and GLUT9 in hyperuricemic mice. J. Ethnopharmacol. 157, 201–205. doi:10.1016/j.jep.2014.09.034
Wu, H., Wang, Z., Wang, Q., Mi, C., He, Y., Zhang, J., et al. (2015). Anti-hyperuricemia effects of allopurinol are improved by Smilax riparia, a traditional Chinese herbal medicine. J. Ethnopharmacol. 162, 362–368. doi:10.1016/j.jep.2015.01.012
Wu, S., Zhang, D., and Li, K. (2019). Application of metabolomics for unveiling the therapeutic role of traditional Chinese medicine in metabolic diseases. J. Ethnopharmacol. 242, 112057. doi:10.1016/j.jep.2019.112057
Xiang, W., Xia, N., and Xu, L. (2021). UPLC-MS/MS profiling, antioxidant, a-glucosidase inhibitory, cholinesterase inhibitory, and cardiovascular protection potentials of jialing 20 (Morus multicaulis perr.) mulberry branch extract. Foods 10 (11), 2659. doi:10.3390/foods10112659
Xing, W., Ning, X., lei, H., Zhen, C., and Wan, Y. (2020). Study on mechanism of Fangji Huangqi Decoction on hypouricemic effect and renal protection in hyperuricemia mice. China J. Chin. Materia Medica 45 (21), 5248–5255. doi:10.19540/j.cnki.cjcmm.20200630.401
Xu, D., Lv, Q., Wang, X., Cui, X., Zhao, P., Yang, X., et al. (2019). Hyperuricemia is associated with impaired intestinal permeability in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 317 (4), G484–G492. doi:10.1152/ajpgi.00151.2019
Xu, M., Xue, H., Qiao, X., Liao, F., Kong, L., Zhang, F., et al. (2022b). Regulating the imbalance of gut microbiota by Smilax China L. Polyphenols to alleviate dextran sulfate sodium-induced inflammatory bowel diseases. Am. J. Chin. Med. 50 (02), 553–568. doi:10.1142/S0192415x22500215
Xu, Q., Lin, S., Yu, X., Li, P., Mai, T., Cheng, J., et al. (2021). Anti-hyperuricemic and nephroprotective effects of dihydroberberine in potassium oxonate- and hypoxanthine-induced hyperuricemic mice. Front. Pharmacol. 12, 645879. doi:10.3389/fphar.2021.645879
Yan, M., An, Y. T., Li, J., Wu, Z. Z., and Wang, T. (2014). Regulatory effect of leonurus extracts on hyperuricemia in rats. Zhongguo Zhong Yao Za Zhi. 39 (24), 4856–4859. doi:10.4268/cjcmm20142430
Yanai, H., Adachi, H., Hakoshima, M., and Katsuyama, H. (2021). Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 22 (17), 9221. doi:10.3390/ijms22179221
Yang, F., Shi, J., Wang, T., Qin, K., Wang, X., Guo, Y., et al. (2021). Lipidomics study of the therapeutic mechanism of Plantaginis Semen in potassium oxonate-induced hyperuricemia rat. Bmc Complementary Med. Ther. 21 (1), 175. doi:10.1186/s12906-021-03350-x
Yang, L., Wang, B., Ma, L., and Fu, P. (2022). Traditional Chinese herbs and natural products in hyperuricemia-induced chronic kidney disease. Front. Pharmacol. 13, 971032. doi:10.3389/fphar.2022.971032
Yang, M., Qi, M., Tong, C., Wang, D., Ding, L., Li, Y., et al. (2017). Plantago asiatica L. Seed extract improves lipid accumulation and hyperglycemia in high-fat diet-induced obese mice. Int. J. Mol. Sci. 18 (7), 1393. doi:10.3390/ijms18071393
Yong, Q., Chen, D., Xie, Z., Chen, L., Su, Y., Shuai, O., et al. (2018a). Cordycepin, a characteristic bioactive constituent in cordyceps militaris, ameliorates hyperuricemia through URAT1 in hyperuricemic mice. Front. Microbiol. 9, 58. doi:10.3389/fmicb.2018.00058
Yong, Q., Chen, D., Xie, Z., Chen, L., Su, Y., Shuai, O., et al. (2018b). Hypouricemic effects of Ganoderma applanatum in hyperuricemia mice through OAT1 and GLUT9. Front. Pharmacol. 8, 996. doi:10.3389/fphar.2017.00996
Yong, T., Chen, S., Xie, Y., Shuai, O., Li, X., Chen, D., et al. (2018). Hypouricemic effects of extracts from Agrocybe aegerita on hyperuricemia mice and virtual prediction of bioactives by molecular docking. Front. Pharmacol. 9, 498. doi:10.3389/fphar.2018.00498
Yoon, S., Park, H., Ki, H., and Cho, S. (2016). Effects of extracts from Corylopsis coreana Uyeki (Hamamelidaceae) flos on xanthine oxidase activity and hyperuricemia. J. Pharm. Pharmacol. 68 (12), 1597–1603. doi:10.1111/jphp.12626
Yusnaini, R., Nasution, R., Saidi, N., Arabia, T., Idroes, R., Ikhsan, I., et al. (2023). Ethanolic extract from Limonia acidissima L. Fruit attenuates serum uric acid level via URAT1 in potassium oxonate-induced hyperuricemic rats. Pharm. (Basel). 16 (3), 419. doi:10.3390/ph16030419
Zeng, X., Wang, J., Zhang, W., Zhu, X., Li, M., Huang, H., et al. (2018). Antigout effects of Plantago asiatica: xanthine oxidase inhibitory activities assessed by electrochemical biosensing method. Evidence-Based Complementary Altern. Med. 2018, 1364617. doi:10.1155/2018/1364617
Zhang, G., Du, D., Li, F., Zhang, C., Yang, L., Yang, L., et al. (2019b). Constituents and anti-hyperuricemia mechanism of traditional Chinese herbal formulae erding granule. Molecules 24 (18), 3248. doi:10.3390/molecules24183248
Zhang, J., Zhao, Y., Chang, K., Cao, Y., Wang, S., Kang, Z., et al. (2021b). Atractylodis Rhizoma: a review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 266, 113415. doi:10.1016/j.jep.2020.113415
Zhang, L., Zhang, J., Zhu, F., Guan, Y., Yang, X., He, X., et al. (2021a). Antihyperuricemia and antigouty arthritis effects of Persicaria capitata herba in mice. Phytomedicine 93, 153765. doi:10.1016/j.phymed.2021.153765
Zhang, M., Zhu, X., Wu, J., Huang, J., Zhao, P., Zhang, X., et al. (2022). Prevalence of hyperuricemia among Chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19. Front. Immunol. 12, 791983. doi:10.3389/fimmu.2021.791983
Zhang, W., Du, W., Li, G., Zhang, C., Yang, W., Yang, S., et al. (2019a). Constituents and anti-hyperuricemia mechanism of traditional Chinese herbal formulae erding granule. Mol. Basel, Switz. 24 (18), 3248. doi:10.3390/molecules24183248
Zhang, Y., Wang, S., Dai, X., Liu, T., Liu, Y., Shi, H., et al. (2023). Simiao San alleviates hyperuricemia and kidney inflammation by inhibiting NLRP3 inflammasome and JAK2/STAT3 signaling in hyperuricemia mice. J. Ethnopharmacol. 312, 116530. doi:10.1016/j.jep.2023.116530
Zhao, H., Gao, Q., Kong, Z., Tang, W., Jiao, Y., Wang, L., et al. (2020). Study on network pharmacological analysis and preliminary validation to understand the mechanisms of Plantaginis semen in treatment of gouty nephropathy. Evidence-Based Complementary Altern. Med. 2020, 8861110. doi:10.1155/2020/8861110
Zhao, M., Zhu, D., Sun-Waterhouse, D., Su, G., Lin, L., Wang, X., et al. (2014). In vitro and in vivo studies on adlay-derived seed extracts: phenolic profiles, antioxidant activities, serum uric acid suppression, and xanthine oxidase inhibitory effects. J. Agric. Food Chem. 62 (31), 7771–7778. doi:10.1021/jf501952e
Zhao, Y., Lu, X., and Lu, J. (2022). The potential of probiotics in the amelioration of hyperuricemia. Food and Funct. 13 (5), 2394–2414. doi:10.1039/d1fo03206b
Zhou, Y., Zeng, Y., Wang, R., Pang, J., Wang, X., Pan, Z., et al. (2024). Resveratrol improves hyperuricemia and ameliorates renal injury by modulating the gut microbiota. Nutrients 16 (7), 1086. doi:10.3390/nu16071086
Zhou, Y., Zhao, M., Pu, Z., Xu, G., and Li, X. (2018). Relationship between oxidative stress and inflammation in hyperuricemia: analysis based on asymptomatic young patients with primary hyperuricemia. Med. Baltim. 97 (49), e13108. doi:10.1097/MD.0000000000013108
Zhu, C., Xu, Y., Liu, H., Wan, C., Li, X., and Tai, L. (2018). The anti-hyperuricemic effect of epigallocatechin-3-gallate (EGCG) on hyperuricemic mice. Biomed. Pharmacother. 97, 168–173. doi:10.1016/j.biopha.2017.10.013
Zuo, Y., He, M., Zuo, Y., Bou-Chacra, N., and Lobenberg, R. (2018). Erding Formula in hyperuricaemia treatment: unfolding traditional Chinese herbal compatibility using modern pharmaceutical approaches. J. Pharm. Pharmacol. 70 (1), 124–132. doi:10.1111/jphp.12840
Glossary
Keywords: hyperuricemia, Chinese herbal medicine, Chinese herbal formulae, mechanism of action, urate transporter
Citation: Bai H, Zhang Z, Zhu M, Sun Y, Wang Y, Li B, Wang Q and Kuang H (2024) Research progress of treating hyperuricemia in rats and mice with traditional Chinese medicine. Front. Pharmacol. 15:1428558. doi: 10.3389/fphar.2024.1428558
Received: 06 May 2024; Accepted: 01 July 2024;
Published: 19 July 2024.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Yue Ding, Shanghai University of Traditional Chinese Medicine, ChinaJidong Cheng, Xiamen University, China
Copyright © 2024 Bai, Zhang, Zhu, Sun, Wang, Li, Wang and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuhong Wang, cWh3YW5nQGhsanVjbS5uZXQ=; Haixue Kuang, aHhrdWFuZzE1QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Haodong Bai
Haodong Bai Zidong Zhang2†
Zidong Zhang2† Mingtao Zhu
Mingtao Zhu Yanping Sun
Yanping Sun Haixue Kuang
Haixue Kuang