
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 03 July 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1426972
This article is part of the Research TopicTraditional Processing Methods in Ethnopharmacology: Enhancing Therapeutic Effects and Unveiling Mechanisms of ActionView all 11 articles
Objective: This study evaluates the research developments concerning Rehmanniae Radix in ovarian hypofunction diseases. It explores the processing methods of Rehmanniae Radix, the variations in its compounds before and after processing, the mechanism of Rehmanniae Radix and its active compounds in improving ovarian function, and the advancements in clinical applications of traditional Chinese medicine (TCM) compound that include Rehmanniae Radix.
Methods: Comprehensive literature search was conducted using databases such as China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database, National Science and Technology Library, the Pharmacopoeia of the People’s Republic of China, Pubmed, and the Web of Science Database. The search utilized the following Medical Subject Headings (MeSH) and keywords: “Rehmanniae Radix,” “Drying Rehmannia Root,” “Rehmannia glutinosa,” “Rehmanniae Radix Praeparata,” “Traditional Chinese Medicine Processing,” “Pharmacological Effects,” “Ovarian Aging,” “Diminished ovarian reserve,” “Premature ovarian insufficiency,” “Premature Ovarian Failure,” “Ovarian hypofunction diseases”.
Results: The ancient Chinese medical books document various processing techniques for Rehmanniae Radix. Contemporary research has identified changes in its compounds processing and the resultant diverse therapeutic effects. When processed into Rehmanniae Radix Praeparata, it is noted for its ability to invigorate the kidney. TCM compound containing Rehmanniae Radix is frequently used to treat ovarian hypofunction diseases, demonstrating significant clinical effectiveness. The key changes in its compounds processing include cyclic dilute ether terpene glycosides, phenylethanol glycosides, sugars, and 5-hydroxymethylfurfural. Its pharmacological action is primarily linked to the improvement of granulosa cell proliferation, antioxidative and anti-aging properties, and modulation of the immune and inflammatory microenvironment. Furthermore, Rehmanniae Radix also offers therapeutic benefits for cardiovascular and cerebrovascular diseases, osteoporosis and cognitive dysfunction caused by low estrogen levels. Thereby Rehmanniae Radix mitigates both the short-term and long-term health risks associated with ovarian hypofunction diseases.
Conclusion: Processed Rehmanniae Radix has shown potential to improve ovarian function, and its compound prescriptions have a definite effect on ovarian dysfunction diseases. Therefore Rehmanniae Radix was garnering interest for both basic and clinical research, with promising application prospects as a future therapeutic agent for ovarian hypofunction diseases. However, further studies on its toxicology and the design of standardized clinical trials are necessary to fully establish its efficacy and safety.
Ovarian hypofunction diseases primarily encompass conditions such as diminished ovarian reserve (DOR), premature ovarian insufficiency (POI), and premature ovarian failure (POF) (Chen et al., 2017). These represent a progressive, gradual decline in ovarian function. DOR primarily highlights the decline in ovarian responsiveness and reproductive function in women of childbearing age, while POI and POF are more focused on the progressive changes associated with age and ovarian aging. Short-term clinical manifestations include menstrual irregularities, reduced fertility and reproductive quality, and perimenopausal symptoms due to low estrogen levels. Menstrual irregularities commonly present as shorter menstrual cycles, prolonged menstrual cycles, reduced menstrual flow, and potentially amenorrhea. A reduction in fertility stems from the progressive decrease in the number of ovarian follicles and a decline in follicle quality, culminating in lower pregnancy rates, higher miscarriage rates, and reduced live birth rates. A decline in reproductive quality is evident in the increased rate of aneuploidy in offspring. The incidence of these conditions is increasingly affecting younger women, significantly impacting female fertility. Reports indicate that the prevalence of DOR ranges from 10% to 32% (Pastore et al., 2018), POI affects about 3.5% (Li M. et al., 2023) of the population, and only approximately 5%–10% (Rebar, 2009) of women diagnosed with POI can conceive spontaneously. However, the impact of ovarian function decline is systemic, posing long-term risks of cardiovascular disease, osteoporosis, and neurological disorders. Estrogen acts as a protective factor for the cardiovascular system, whereas premature ovarian aging markedly elevates the risk of cardiovascular events. Reduced estrogen and elevated FSH levels cause abnormal bone metabolism, leading to osteoporosis and fractures. Furthermore, a decline in ovarian function and changes in HPO axis activity can lead to emotional disturbances, vascular dysfunction, cognitive impairment, and sleep disorders, severely affecting the quality of life for women.
Current treatments primarily involve hormone replacement therapy (HRT) and assisted reproductive technology (ART), with emerging methods such as stem cell therapy still under research. HRT is the primary treatment for alleviating symptoms of low estrogen and providing primary prevention against cardiovascular diseases and osteoporosis. However, HRT cannot restore ovarian function, and its long-term use is associated with risks of thromboembolic diseases, breast tumors, and other adverse effects, prompting concerns regarding the occurrence of adverse reactions (Ewertz et al., 2005; Canonico et al., 2014; Gu et al., 2014). The 2016 European Society of Human Reproduction and Embryology (ESHRE) Guideline advised POI patients with fertility needs to actively pursue ART for conception. Women with a family history of POI or those without current fertility needs may choose to freeze and preserve oocytes or embryos to mitigate age-related fertility decline, thus preserving part of their reproductive potential. Despite advancements in ART, its efficacy is limited in cases of ovarian aging (Leridon, 2004). Meanwhile, as international research on traditional Chinese medicine (TCM) progresses and cross-cultural communication between the East and West deepens, TCM has gained attention due to its natural compounds, multi-target effects, minimal adverse events, and the concept of medicinal and edible use (Heinrich et al., 2021).
Rehmanniae Radix (RR) is either the fresh or dried root of Rehmannia glutinosa Libosch., harvested in autumn, cleaned of reeds and fibrous roots, and either used fresh or slow-roasted until it is about 80% dry. Freshly processed RR is termed “Fresh RR (FRR),” and the partially dried form is known as “Raw RR (RRR).” RR has been a staple of TCM for millennia, classed among the “Four Great Medicines,” and is widely used in clinical settings. The processing of RR, which includes methods like steaming and nine steaming and nine drying, transforms it into “RR Praeparata (RRP).” This process alters RR’s medicinal properties from cold to warm and its flavor from bitter to sweet, the function changes from clearing heat to nourishing (Qianfeng, 2005). RR contains various bioactive compounds including iridoid glycosides, polysaccharides, amino acids, and small amounts of β-sitosterol, which undergo changes after processing. RR’s pharmacological actions span multiple systems, offering antioxidant, immune-modulating, anti-inflammatory, and anti-aging benefits, and are principally applied in the treatment of gynecological and diabetic metabolic disorders, cardiovascular diseases, and osteoporosis. This reflects the advantages of TCM in treating complex, multi-system diseases, and functional disorders. It not only fundamentally improves patients’ condition but also offers multi-target synergy and additive effects (Li D. et al., 2023; Zhang, 2023). RR has become a common treatment in clinical settings due to its protective effects on ovary. In recent years, studies on RR’s role in ovarian hypofunction have proliferated, mainly focusing on the mechanism of RR to improve reproductive hormones and increase the number of follicles. At the same time, basic and clinical studies were conducted on the TCM compound containing RR to observe its efficacy, safety and potential mechanism in the treatment of ovarian dysfunction diseases. These studies also seek to explore the long-term health benefits of RR, aiming for synergistic and enhancing effects.
This article reviewed the changes in the compounds of RR before and after processing, its mechanism of action in treating ovarian hypofunction diseases, and the clinical use of related compound prescriptions. It also elaborates on the scientific implications of its transformed medicinal effects to better leverage RR’s therapeutic potential.
RR was first documented in “Shennong’s Classic Materia Medica” as “Drying Rehmannia Root” and later appeared as “RRR” in Zhang Zhongjing’s “Jin Kui Yao Lue” during the Han Dynasty. The term “RRP” was officially introduced in the Song Dynasty’s “Bencao Tujing”. Initially classified as cold, RR’s properties shift to warm after processing, transitioning from an effect of clearing heat to nourishing. This change significantly alters its therapeutic impact on diseases. RRP is the core drug for tonifying the kidney, known for its mild medicinal power and is frequently employed in treating various kidney deficiency diseases in clinical settings. Ancient Chinese medical books extensively describe the clinical signs of ovarian hypofunction diseases, such as menstrual irregularities or amenorrhea and reduced fertility, attributing these conditions to kidney deficiency. According to TCM, the kidney is vital for growth, development, and reproduction, and both menstruation and pregnancy are closely linked to kidney health. The belief that “menstruation originates from the kidney” and “the person who appears amenorrhea before age 49″are supported in “Fu Qingzhu’s Gynecology,” aligning with the pathophysiology of these conditions. RRP is acclaimed for its abilities to nourish blood, nourish yin, nourish kidney and invigorate essence. “Compendium of Materia Medica” notes that RR has the efficacy in treating “irregular menstruation and long-term infertility.” Furthermore, TCM prescriptions commonly used to treat ovarian hypofunction diseases, such as Guishen Pill, Liuwei Dihuang Pill, Siwu Tang, and Yijing Tang, all incorporate RRP. It can be seen that the clinical treatment of this type of disease is inseparable from the use of RRP.
Archaeological research has revealed that processed RR found in the Haihunhou tomb from the Western Han Dynasty is the earliest Chinese medicine excipient concoction product found in ancient China so far, which involved excipients of starch and sucrose, suggesting that these samples were processed by rice steaming (Peng et al., 2019; Zhu et al., 2021). The history of RR processing involves a variety of methods. Traditional methods include steaming and boiling without excipients, as well as the excipients of ingredients such as alcohol, Amomi Fructus and ginger (Xie et al., 2022). In contemporary practice, processed RR varieties with region-specific characteristics have developed, such as Wenzhi RR with Jiangxi Jianchang Band and Salt RR from Yunnan.
Historically, RR has been known by various names depending on the processing method employed. However, the 2020 edition of The Pharmacopoeia of the People’s Republic of China recognizes only two designations: RR and RRP. RR encompasses Fresh RR and RRR, with RRP defined as the processed product of RRR, the specific two processing methods are steaming and wine stewing. In addition, “nine steamed and nine sun-dried” is also a traditional processing method of RR.
Steamed refers to the method of heating the raw productswithout excipients and separating water to a certain extent. The description of the 2020 edition of The Pharmacopoeia of the People’s Republic of China is more precise, which points that “steamed with water involves heating the raw medicinal botanical drug by placing it over boiling water until it achieves a black and moist state. It is then removed and dried to about eighty percent dryness before being cut into thick slices or chunks and fully dried to produce RRP” (National Pharmacopoeia Committee, 2020).
The method of stewing with wine, widely used today, was first documented in Shennong Bencao Jing Jizhu during the Liang Dynasty. Leigong Paozhi Lun during the Northern and Southern Dynasties mentioned for the first time that wine was used as an auxiliary material to mix and steam. This method entails mixing RR with wine prior to steaming (Wang et al., 2019). The pharmacopeia (National Pharmacopoeia Committee, 2020) details that for every 100 kg of RRR, 30–50 kg of yellow rice wine is used. The operational steps include stewing the RR with wine until it fully absorbs the wine, followed by air drying until the sticky fluid on its surface slightly dries. It is then processed further into thick slices or chunks to finally obtain RRP.
The “nine steamed and nine sun-dried” method, with a long historical lineage, first appeared in the Tang Dynasty’s Qianjin Yi Fang and was subsequently referenced in later texts such as the Song Dynasty’s Bencao Tujing, the Yuan Dynasty’s Tangye Bencao, the Ming Dynasty’s Bencao Gangmu. While the exact number of steaming and sun-drying cycles described in these books may vary, the overall goal is to control the quality of RRP, indicating that the number of cycles is not rigidly fixed (Yan et al., 2020; Wu et al., 2022). It is theorized that the number “nine” in “nine steamed and nine sun-dried” reflects a traditional practice where some Chinese medicines are often processed this way multiple times, thus giving rise to the collective term (Peng et al., 2018).
The Pharmacopoeia of the People’s Republic of China identifies rehmannioside D and catalpol, both iridoid glycosides, as the key indicators for assessing the quality of RRR. Currently, 75 compounds have been extracted from RRR, which are categorized into classes such as iridoid glycosides, phenylethanoid glycosides, flavonoids, ionones, phenolic acids, and carbohydrates. The structures of these compounds are illustrated in Figure 1. Among these, iridoid glycosides, phenylethanoid glycosides, carbohydrates, and nucleosides are frequently used as reference compounds for selecting quality markers for RR (Zhu J. et al., 2022). These compounds are consolidated into Table 1 (Li et al., 2020; Gu et al., 2021; Chen SQ. et al., 2023; Lu et al., 2023; Xue et al., 2023; Zhu et al., 2023).
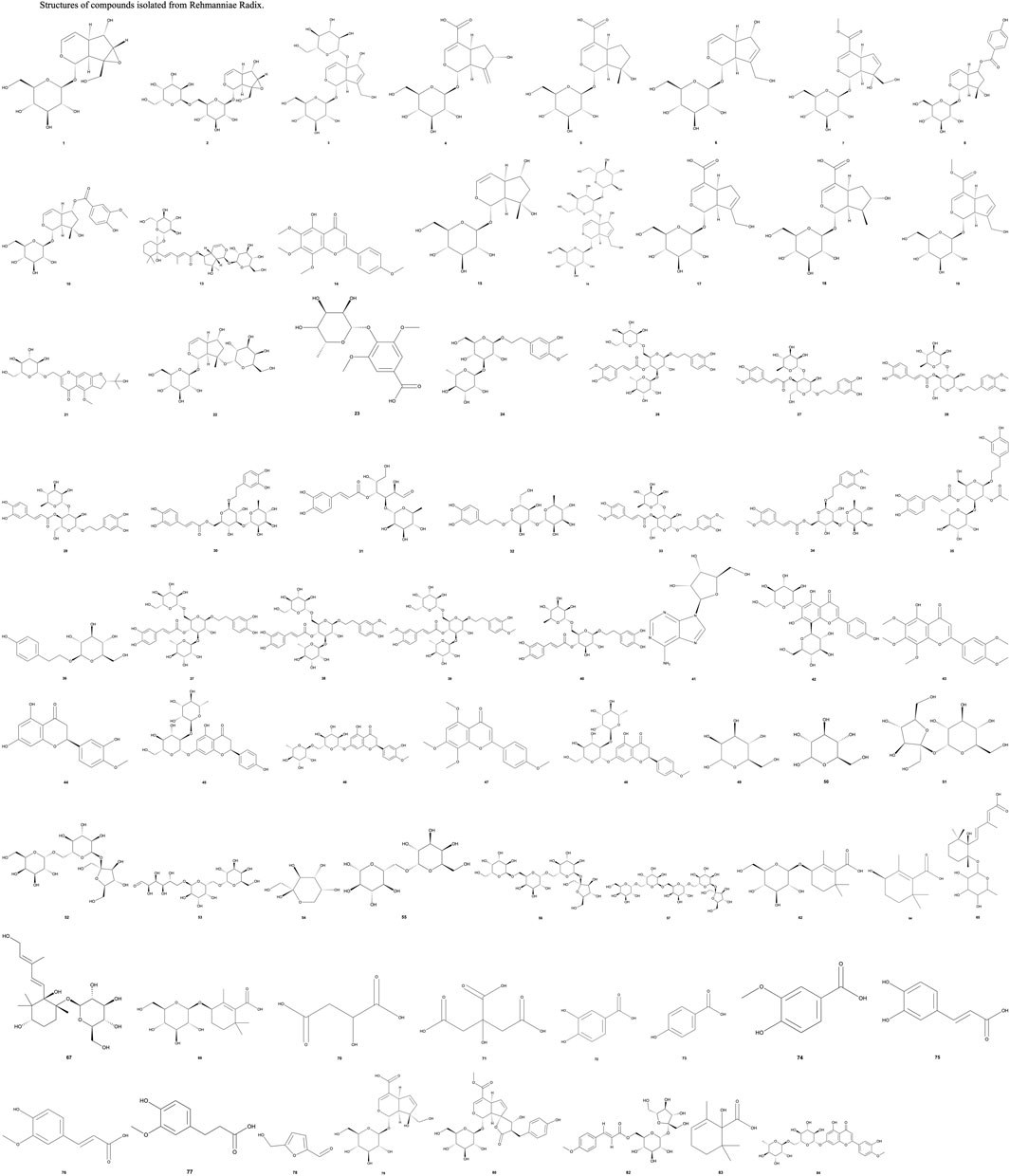
Figure 1. Structures of compounds isolated from Rehmanniae Radix. P.S: The structures in this article were referenced from the PubChem database. Structures not displayed were not found in the database. The bold Arabic numbers below the structures in Figure 1 correspond to the compounds numbered in Table 1.
RRP undergoes numerous processing methods, each affecting its compounds and concentration differently. Modern studies focus on compounds like iridoid glycosides, phenylethanoid glycosides, carbohydrates, and 5-hydroxymethyl furfural (5-HMF). Table 1 details the extraction methods and the compounds derived from both RRR and RRP, processed respectively by steaming with water, stewing with wine, and repeated steamed and sun-dried.
In RRP, the glycoside compounds most researched are iridoid glycosides, phenylethanoid glycosides, and nucleosides.
Experimental studies reveal iridoid glycosides and phenylethanoid glycosides as significant quality markers, reflecting the degree of processing in RRP (Liu et al., 2023). The experimental data showed that the content of rehmannioside D after steaming and wine stewing was lower than that before processing, and the content of rehmannioside D in wine stewing was less than that of steamed products with the same degree of processing (Jiang Y. et al., 2023). Using UPLC-PDA and HPLC methods for testing (Zhang LX. et al., 2023; Yang et al., 2024), the data shows that in the process of wine steaming, a significant decrease was observed in iridoid glycosides such as catalpol, rehmannioside D, rhinanthin, leonuride, geniposidic acid, and melittin during wine stewing. The most substantial decline was in catalpol, dropping from 44.00 mg/g in FRR to 2.192 mg/g in the wine stewed RR. Similarly, verbascoside from phenylethanoid glycosides showed a notable reduction during wine stewing. In nucleosides, adenosine and uridine levels increased, whereas guanosine decreased. Further HPLC analysis indicated that with successive steaming from one to nine times, the concentrations of catalpol, rehmannioside D, leonuride, and verbascoside diminished (Ma et al., 2023). However, during the “nine steamed and nine sun-dried” process, from “one steamed and one sun-dried” to “seven steamed and seven sun-dried,” the concentration of catalpol, rehmannioside D, leonuride, verbascoside and isoverbascoside increased with the number of cycles, yet showed no significant change from “seven steamed and seven sun-dried” to “nine steamed and nine sun-dried” (Chen SQ. et al., 2023). Experimental findings (Zhu et al., 2023) suggest that compared to RRR, the total iridoid glycoside concentration decreased in three processed forms: RR stewed with wine, nine steamed and nine sun-dried RR (without Citri Reticulatae Pericarpium and Amomi Fructus), and nine steamed and nine sun-dried RR (with Citri Reticulatae Pericarpium and Amomi Fructus). Conversely, the total phenylethanoid glycoside concentration increased in the nine steamed and nine sun-dried RRP (with Citri Reticulatae Pericarpium and Amomi Fructus) while decreasing in RR steamed with wine, RR stewed with wine, and nine steamed and nine sundried RR (without Citri Reticulatae Pericarpium and Amomi Fructus). Other studies (Chen et al., 2021) have noted a general decrease in glycoside concentration after processing, particularly higher levels of rehmannioside D, heterophylloside, and leonuride in RR processed with both Citri Reticulatae Pericarpium and Amomi Fructus compared to processing with just one of these excipients.
Experiments demonstrate that carbohydrate components exhibit different trends over the course of processing. These variations not only distinguish between different processed RR, but also, to some extent, indicate the processing endpoint for RRP, providing a partial reference for its quality evaluation. (Zhang et al., 2020).
The ATR-FTIR method was utilized to conduct a comprehensive analysis of water extracts from FRR, RRR, and RRP, quantifying compounds such as mannose, manninotriose, stachyose, fructose, glucose, galactose, melibiose, and verbascoside. This confirms that carbohydrates are the primary differential compounds between FRR and its processed forms (Tian et al., 2022). Quantitative analysis at various processing stages shows that while sucrose, raffinose, stachyose, and verbascoside decrease, fructose, glucose, melibiose, and manninotriose increase significantly. Carbohydrate profiles in RRR and RRP thus exhibit distinct characteristics, forming a basis for quality assessment of both (Zhang et al., 2016). During wine stewing and steaming, polysaccharide concentration initially rises then falls. The polysaccharide content in wine-stewed products is higher than that in steamed products at the same processing stage (Jiang Y. et al., 2023). During the “one steamed and one sun-dried” to “seven steamed and seven sun-dried” processing, polysaccharide concentration in RRP increases with each cycle. However, from “seven steamed and seven sun-dried” to “nine steamed and nine sun-dried,” the polysaccharide concentration declines. The highest polysaccharide mass fraction, 30.10%, is found in the “seven steamed and seven sun-dried” RRP (Chen SQ. et al., 2023). During nine steaming cycles, the polysaccharide concentration in RRR is lowest, peaking in the fifth cycle before sharply declining, the variation in polysaccharide content in RRP after six steaming cycles is minimal (Jia et al., 2023). By measuring the content of D-fructose, glucose, sucrose, melibiose, stachyose, manninotriose, raffinose, and verbascoside, it was found that after processing trends show a decrease in oligosaccharide concentration and an increase in monosaccharide concentration. In RR processed with Amomi Fructus and Citri Reticulatae Pericarpium, the concentration of D-fructose, glucose, and manninotriose increased by 29.24%, 57.14%, and 44.65% respectively, compared to samples processed without these excipients (Chen et al., 2021).
5-HMF, a marker of the Maillard reaction (Wang et al., 2020), is a new substance formed during RR processing. The mechanism and products of the Maillard reaction are highly complex. When Hodge named this compound reaction in 1953, he summarized the process of the reaction (Shown in the Figure 2 (Gong et al., 2019; Zhou et al., 2014). It is absent in RRR but its concentration significantly increases after processing (Chen et al., 2021; Zhu et al., 2023), with its presence correlating strongly with the darkening color of RRP (Ma et al., 2023). In addition, it has also been found in the processing studies of other traditional Chinese medicines that the presence of 5-HMF can be detected after processing, but not before processing, indicating that 5-HMF can be used as an indicator to evaluate the degree of processing (Yang et al., 2023).
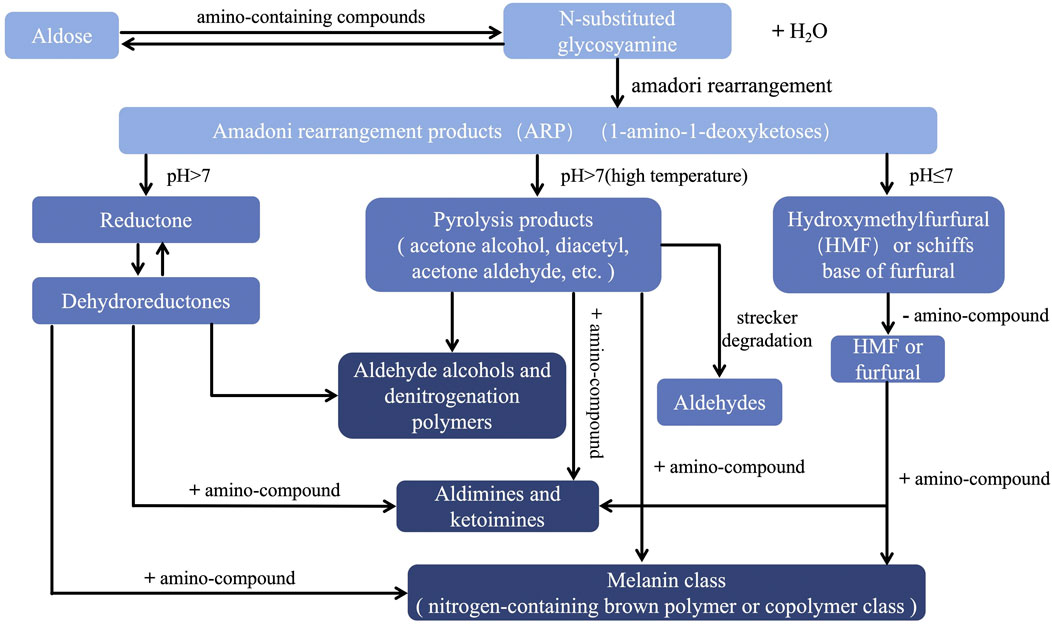
Figure 2. Schematic diagram of Hodge Maillard reaction process. PS: The color from light to dark corresponds to the initial stage, intermediate stage and final stage of the Maillard reaction, respectively. [40] Gong RZ, Huo XH, Zhang L, Liu C, Li SS, Sun YS. (2019). Advances in effects and regulation of Maillard reaction on quality of Chinese materia medica. Chinese Traditional and Herbal Drugs (01),243-251.
Trace element analysis from “one steamed and one sun-dried” to “nine steamed and nine sun-dried” shows an initial increase and then a decrease in beneficial elements like zinc, copper, and iron, while harmful elements such as cadmium, arsenic, and lead gradually decline with more steaming and sun-drying cycles, potentially reducing the accumulation of toxic substances in human soft tissues (Chen SQ. et al., 2023).
Amino acid analysis revealed that both total and free amino acid mass fractions decrease in processed RR, with basic amino acids, especially arginine and lysine, showing the most significant declines (Gao et al., 2010).
RR exhibits a range of pharmacological effects. In vivo and in vitro experiments have confirmed its mechanism in enhancing granulosa cell proliferation, antioxidant and anti-aging effects, and modulation of the immune-inflammatory microenvironment. Thus, it plays a therapeutic role in ovarian hypofunction diseases, with reproductive endocrine protection function. The mechanism of Ovarian Hypofunction Diseases and the function of RR are shown in Figure 3.
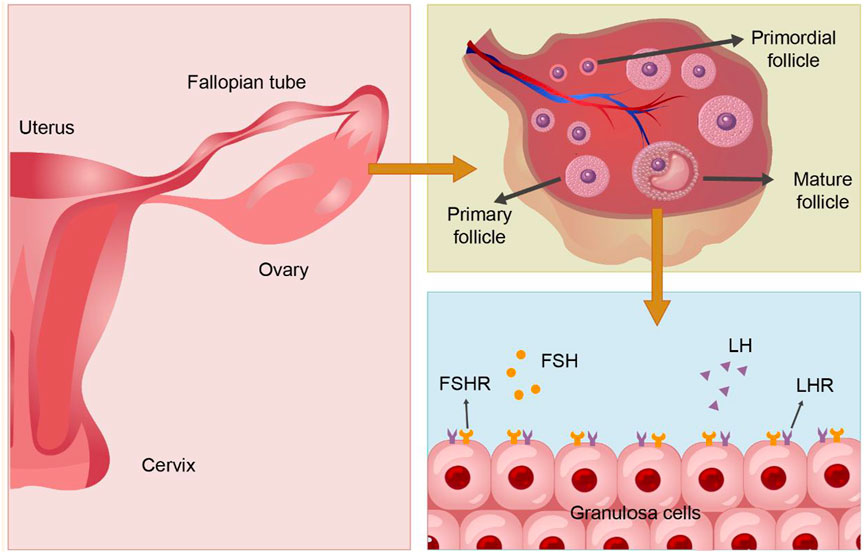
Figure 3. The structure and physiology of the ovary and granulosa cells. PS: Follicle stimulating hormone (FSH), Follicle stimulating hormone receptor (FSHR); Luteinizing hormone (LH), Luteinizing hormone receptor (LHR).
The follicle, composed of oocytes, granulosa cells (GCs), and follicular membrane cells, is fundamental to ovarian function. The proliferation and differentiation of GCs signify follicle development. GCs are crucial for synthesizing, expressing, and metabolizing various hormones, serving as the primary source of ovarian estradiol, inhibin, and activin, and playing roles in oocyte reserve, maturation, and pregnancy maintenance (Kranc et al., 2017). The structure and physiology of the ovary and GCs are shown in Figure 4. GCs are central to studies on follicular growth and atresia mechanisms. Ovarian hypofunction diseases relate to GC apoptosis, follicle stimulating hormone (FSH) receptor signaling defects, autoimmunity, and other factors (Nelson, 2009). Prior studies suggest that abnormal proliferation and apoptosis of ovarian GCs may cause excessive follicular atresia (Matsuda et al., 2012). Additionally, hormones from the pituitary gland and ovaries regulate ovarian function and follicle development and atresia. FSH initiates key events in ovarian follicles: GC proliferation, estrogen synthesis, and expression of the luteinizing hormone receptor (LHR) in GCs (Liu et al., 2024), crucial for GC differentiation in antral and preovulatory follicles (Hsueh et al., 2000). The follicle stimulating hormone receptor (FSHR), a specific G protein-coupled receptor on ovarian GCs (Hunzicker-Dunn and Maizels, 2006), promotes GCs proliferation by binding to FSH, facilitates androgen conversion to estrogen, leads to follicle maturation, produces a peak in LH levels, and induces ovulation (Casarini and Crépieux, 2019) (Shown in the Figure 5). Haploinsufficiency of FSHR accelerates oocyte loss in mice, marking a significant factor in ovarian aging and estrogen deficiency (Danilovich and Sairam, 2002).
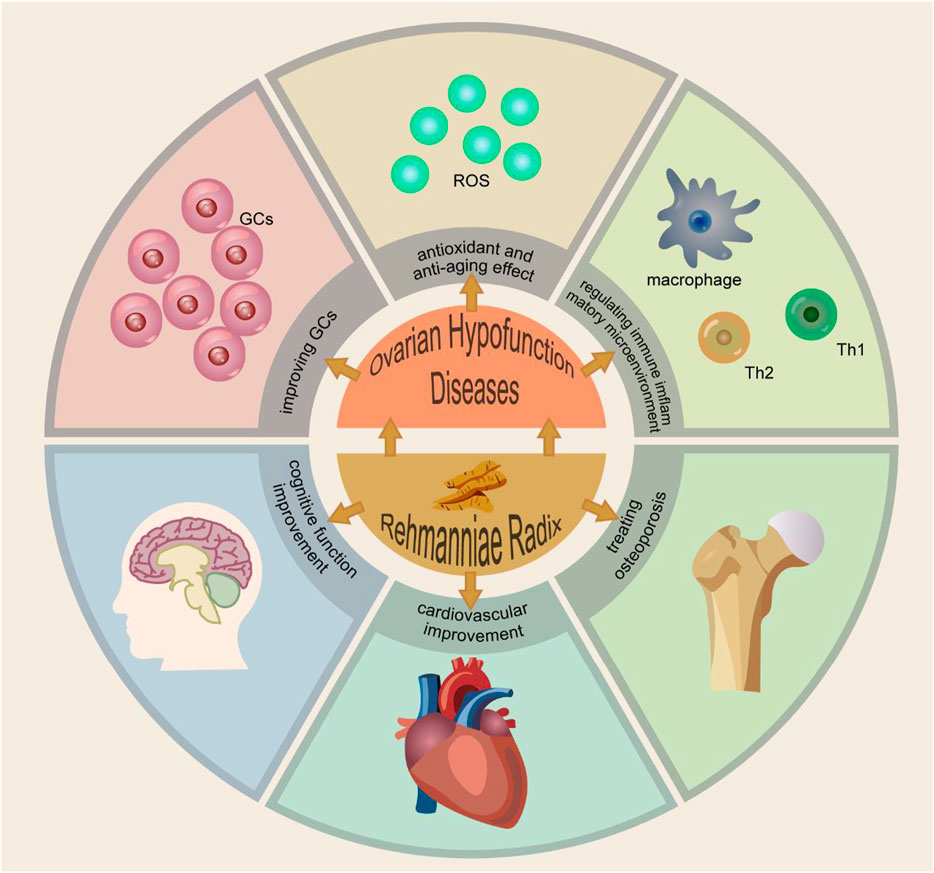
Figure 4. The Mechanism of Ovarian Hypofunction Diseases and the Function of Rehmanniae Radix. PS: Granulosa cells (GCs). Reactive oxygen species (ROS).
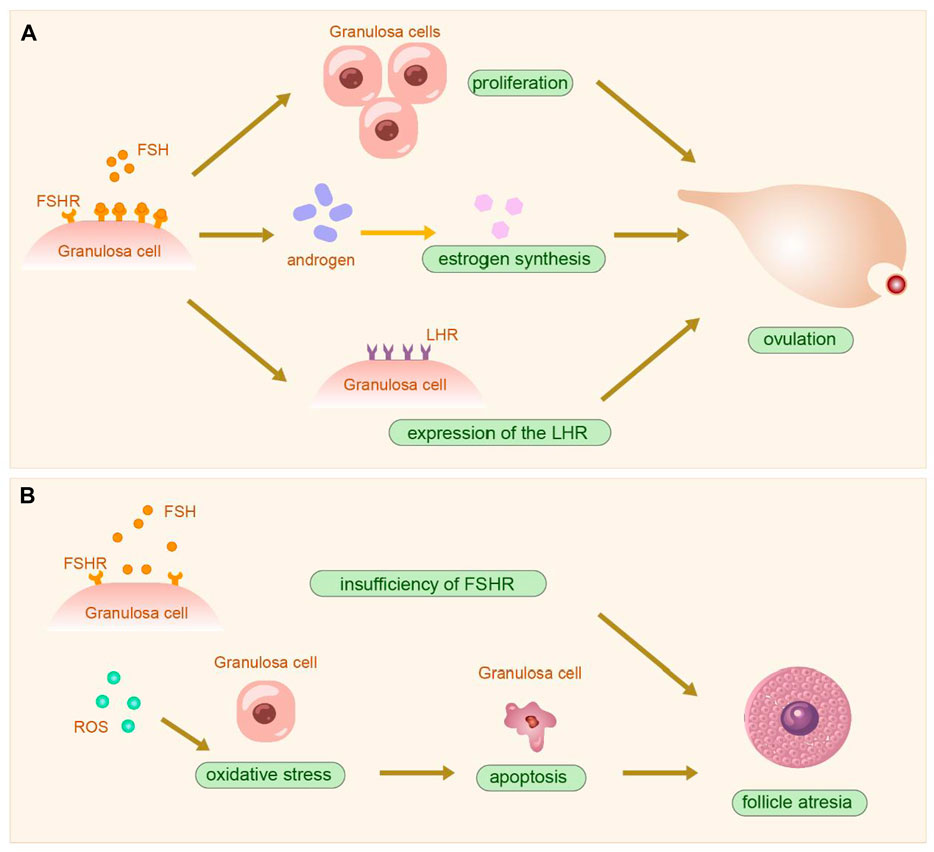
Figure 5. The physiopathological diagram of granulosa cells. (A). The physiological process following the action of FSH on ovarian granulosa cells. (B). The onset of follicle atresia after insufficiency of FSHR and oxidative stress. PS: Follicle stimulating hormone (FSH); Follicle stimulating hormone receptor (FSHR); Luteinizing hormone receptor (LHR); Reactive oxygen species (ROS).
RRP is commonly used to tonify the kidney to promote follicle development and maturation, but the effects on ovaries vary with different processing methods and degrees. Researchers (Que et al., 2021) have observed varying impacts of the nine steamed and nine sun-dried RRP group, the one steamed and one sun-dried RRP group, and the RRR group on mouse ovaries. The nine steamed and nine sun-dried RRP group significantly increased preantral and sinusoid follicles and elevated FSHR levels in the ovaries, unlike the one steamed and one sun-dried RRP group and the RRR group, which did not promote follicle development. FSHR may be a target of the nine steamed and nine sun-dried RRP. Furthermore, 5-HMF, a compound formed by the Maillard reaction of sugars and amino acids during processing, was identified as potentially influencing the medicinal properties of RRP. The concentration of 5-HMF correlates closely with steaming and heating time (Yang, 2010). Enhanced expression of FSHR improves GCs function, while silencing FSHR expression leads to GCs apoptosis and follicular atresia (Du et al., 2016). It has been suggested from experimental studies that processed RR might increase 5-HMF concentration and upregulate FSHR levels, enhancing GCs proliferation, fostering follicle development, and ultimately improving ovarian function and reproductive outcomes. A new study (Lin et al., 2019) introduced the processing method of nine steamed and nine baked RRP, comparing it with three other RR preparations. Results indicated that the proliferation effect on rat ovarian GCs was associated with RR polysaccharide concentration, with the most significant effect at a mass concentration of 150 μg/mL. Among these, the total polysaccharide concentration was highest in RR processed by nine steamed and nine baked RRP, with the proliferation hierarchy as follows: nine steamed and nine sun-dried RRP polysaccharides > nine steamed and nine baked RRP polysaccharides > modern method RRP polysaccharides > RRR polysaccharides. Intriguingly, experimental studies (Liu, 2018) found that the aqueous extract of the modern steaming RRP method may hinder ovulation in normal female rats, leading to prolonged estrous cycles, increased atretic follicles, and decreased serum sex hormone (estradiol, progesterone) levels, while the aqueous extract of the nine steaming and nine sun-dried RRP may not affect ovulation in female rats with normal estrous cycle, but the specific compounds and dosage impacting ovulation require further investigation.
Ovarian senescence, influenced by factors affecting the quantity and quality of oocytes, leads to a decline in ovarian function and gradual aging. In 1954, Harman first proposed the free radical theory of aging, suggesting that aging-related diseases are triggered by the interaction of reactive oxygen species (ROS) with cellular components, thereby inducing aging-related changes (Harman, 1956; Harman, 2006). Hence, reducing oxidative stress and the production of free radicals can decelerate aging and extend life. Oxidative stress is considered a principal pathogenesis of ovarian aging. Normally, a dynamic balance exists between ROS and antioxidants in the body. Disruption of this balance due to excessive ROS production or increased antioxidant utilization induces oxidative stress. Oxidative stress can lead to various pathological changes in cells, including mitochondrial dysfunction, DNA damage, telomere shortening, etc., all of which are key factors in aging-related diseases. Studies have shown that oxidative stress within the ovarian microenvironment can diminish oocyte quality, induce apoptosis of GCs, accelerate corpus luteum degeneration, and lead to ovarian aging and infertility (Freitas et al., 2017; Liang J. et al., 2023), affecting the success rate of ART (Oyawoye et al., 2003).
Excessive ROS can also initiate crosstalk among various signaling pathways and protein factors, contributing to the pathogenesis of ovarian aging and serving as therapeutic targets. Currently, the regulation of ovarian signaling pathways by ROS mainly focuses on important signaling pathways such as Recombinant Kelch Like ECH Associated Protein 1 (KEAP1)-Nuclear factor-E2-related factor 2 (Nrf2), NF-kB, FOXO, etc. Nrf2 is a critical transcription factor in oxidative stress, regulating the antioxidant response with KEAP1 to protect cellular functions (Baird and Yamamoto, 2020). Nrf2 promotes the expression of downstream antioxidant enzymes such as heme oxygenase 1 (HO-1), NADP(H) quinone dehydrogenase 1 (NQO1), and glutathione-S-transferases (GSTs). This mechanism effectively mitigates intracellular oxidative stress and minimizes cellular damage from ROS(Gao X. et al., 2023). Research has shown that (Ma et al., 2018) Nrf2 expression levels in ovarian GCs correlate with women’s age, highlighting Nrf2 as a key factor in oocyte aging and suggesting that a decline in Nrf2 expression may be closely linked to reduced reproductive capability in older women. Additionally, Nrf2 activity has been found to correlate positively with species longevity (Lewis et al., 2015) and improves endothelial cell senescence (Romero et al., 2019). Therefore, Nrf2 is one of the key points for treating ovarian aging. ROS induces inflammatory cells, such as macrophages, to produce TNF-α. When TNF-α binds to membrane receptors, it mediates the phosphorylation and degradation of IkB, activating the NF-kB pathway to control the transcription of anti-apoptotic and inflammation-related genes. The FOXO family, a crucial transcription regulatory factor within the PI3K-AKT signaling pathway, is involved in regulating cell proliferation, apoptosis, and differentiation. FOXO3 enhances NRF2 activity by promoting KEAP1 degradation and also increases BCL10 expression, regulating the activation of the IkB-NF-kB pathway (Shixuan, 2021).
In RR studies, processing has been found to increase the concentration of 5-HMF, a Maillard reaction product, enhancing free radical scavenging capabilities and playing an antioxidant role (Kwon et al., 2019). RR polysaccharide activates the Nrf2/Keap1 pathway and significantly increases the activity of antioxidant enzymes (Ren et al., 2023). However, Rehmannioside A notably improves oxidative stress after activating the PI3K/Nrf2/SLC7A11 signaling pathway (Fu et al., 2022). Additionally, the phenolic/phenylpropionic acid pathway, mediated by Cinnamate 4-hydroxylase (C4H), has been identified in RR phenol studies as involved in regulating oxidative stress tolerance (Yang Y. H. et al., 2021). In aging research, Rehmannia glutinosa 70 (RG70) has shown superior antioxidant activity in vitro (compared to RG50, RG90, and RGB), enhancing the antioxidant enzyme system of Caenorhabditis elegans, reducing ROS levels, and increasing the expression of lifespan-related genes daf-16 and skn-1 and their downstream genes sdo-3 and gcs-1, thereby achieving an anti-aging effect (Liang L. et al., 2023). Another study found that RG70 reduced the expression levels of TNF-α, IL-1β, and IL-6 by modulating the ROS/NF-kB signaling pathway, thereby alleviating oxidative stress and inflammatory response (Zhang H. et al., 2023). Another aspect of the anti-aging mechanism of RR is to enhance the function of hematopoietic stem cells, downregulate aging-related proteins p53 and p16, reduce ROS levels, and achieve an anti-aging effect (Bai et al., 2018). RR polysaccharide also activates the antioxidant enzyme system under oxidative stress, thereby stimulating the daf-16 gene on the insulin/IGF-1 signaling pathway (IIS) and prolonging the lifespan of C. elegans (Yuan et al., 2019). Moreover, some studies have found that microplastics activate the Wnt/β-Catenin signaling pathway and oxidative stress in rats, leading to apoptosis of ovarian GCs and causing fibrosis, ultimately reducing ovarian reserve function (An et al., 2021). In a study of RR extract catalpol, it was found to regulate pulmonary fibrosis through the Wnt/β-catenin pathway and reduce oxidative stress in lung tissue (Yang F. et al., 2021). Another experiment with the RR-Cornus officinalis pair revealed that this combination could reduce pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and downregulate the expression of TGF-β1, JNK, p38, and ERK to improve renal interstitial fibrosis (Zhu et al., 2024). Future studies could also explore the pharmacological mechanism of RR in ameliorating ovarian aging from the perspective of ovarian fibrosis.
The ovary is frequently targeted by autoimmune attacks in both organ-specific and systemic autoimmune diseases. The precise mechanism of autoimmunity in these conditions remains unclear, potentially triggered by genetic or environmental factors (Domniz and Meirow, 2019). Studies indicate that between 10% and 55% of patients with POI also suffer from autoimmune diseases (Szeliga et al., 2021). In cases of POI with adrenal autoimmune disease, ovarian histology biopsies reveal persistent autoimmune oophoritis, with inflammatory cells particularly surrounding the pre-ovulatory follicle and corpus luteum which produce steroid hormones (Kirshenbaum and Orvieto, 2019). Research has demonstrated a disorder in T lymphocyte subsets in POI patients. Compared to healthy women, POI patients show a decreased percentage of CD4+ T lymphocytes and an increased percentage of CD8+ T lymphocytes in peripheral blood, with TSH levels negatively correlated with CD4+ percentages and positively with CD8+ percentages (Hsieh and Ho, 2021). Additionally, the expression of the fork-head transcription factor 3 (Foxp3) in peripheral blood is significantly reduced in POI patients, alongside increased levels of pro-inflammatory factors such as interferon γ (IFN-γ) and TNF-α, and decreased levels of anti-inflammatory factors like interleukin-10 (IL-10) and transforming growth factor beta (TGF-β) (Wang et al., 2022; Mao and Ji, 2023). Th1/Th2 cells, subtypes of CD4+ T cells, play roles in mediating cellular immune and inflammatory responses, secreting pro-inflammatory cytokines such as IFN-γ, TNF-α, and IL-6, while Th2 cells primarily secrete anti-inflammatory cytokines like TGF-β and IL-10. These studies suggest a disturbance in the local immune inflammatory microenvironment in POI patients, with an imbalance of Th1/Th2 cells in the ovaries disrupting homeostasis, affecting follicle formation and ovulation (Boots and Jungheim, 2015), potentially leading to apoptosis and follicular atresia (Huang et al., 2019). Moreover, the local inflammatory state can induce ovarian fibrosis, making modulation of the immune inflammatory microenvironment another therapeutic approach for treating ovarian aging.
Extensive research confirms that immunity regulation and anti-inflammation are among the pharmacological effects of RR. RR polysaccharides, particularly after repeated steam drying, effectively enhance anti-inflammatory activity (Lu et al., 2022). These polysaccharides activate dendritic cells (DCs) (Huang et al., 2013) both in vitro and in vivo, increase cell proliferation and cytokine secretion, and sustain cellular and humoral immune responses (Huang et al., 2016a). They promote IFN-γ production in CD4+ and CD8+ T cells (Xu et al., 2017) and increase Th1, Th2, and Th17 cytokines in mice, thus improving immunity (Huang et al., 2021). RR polysaccharides also activate the non-specific immune response of macrophages by increasing nitric oxide levels, enhancing macrophage phagocytic activity (Huang et al., 2016b), and thereby bolstering immune response and anti-infection capabilities (Feng et al., 2020). Additionally, 2,5-dihydroxyacetylbenzone (DHAP), extracted from RRP, has shown potential in treating lipopolysaccharide (LPS)-stimulated macrophage inflammatory responses in RAW264.7 mice. DHAP effectively inhibited the phosphorylation of extracellular signal-related kinase (ERK) 1/2 and nuclear translocation of NF-κB p65, reducing inflammatory mediator production in activated macrophages and achieving anti-inflammatory activity (Han et al., 2012). Recent studies also link gut microbiota with POI (Wu et al., 2021), with Mendelian studies suggesting that dysbiosis of gut microbiota can lead to POI (Wang et al., 2023). Notably, research on RR oligosaccharides and polysaccharides has shown they can enhance the abundance of intestinal microbiota, reduce intestinal inflammation, and repair intestinal barrier damage by maintaining intestinal microbiota homeostasis (Li X. et al., 2023; Lv et al., 2023; Ren et al., 2023). Furthermore, RR oligosaccharides enhance the vitality of stem cells and improve the immunomodulatory efficacy of stem cells, achieving better therapeutic outcomes (Zhang et al., 2012). Interestingly, in a study of overweight women, RR was found to reduce BMI by improving gut microbiota, enriching research on RR in metabolic diseases and underscoring its potential as a prebiotic (Han et al., 2015).
Estrogen can inhibit macrophage oxidation of low-density lipoprotein, induce direct antioxidant effects to reduce macrophage activation through oxidized low-density lipoprotein protein and prevent the progression of atherosclerosis (Clarkson, 2018). Estrogen can also directly act on cardiomyocytes, including activating the AKT pathway and inhibiting cardiomyocyte apoptosis and necrosis. Patients with diseases of ovarian hypofunction diseases are at increased risk of cardiovascular and cerebrovascular diseases due to decreased estrogen levels. The risk of cardiovascular and cerebrovascular diseases increases with age. A Post-menopause, women experience prolonged low estrogen levels, reducing the protective effects on blood vessels and heightening the risk of cardiovascular and cerebrovascular diseases compared to men of the same age (Bushnell et al., 2014). The ovarian hormone 17β-estradiol plays a critical role in sex differences in cardiovascular diseases due to its impact on myocardial remodeling, function, and atherosclerotic lesion management (Bourassa et al., 1996; Stewart et al., 2006). A meta-analysis (Daan et al., 2016) revealed that women with POI face higher cardiovascular risks than middle-aged premenopausal women, including increased abdominal fat, elevated chronic inflammatory markers, and tendencies towards higher blood pressure and impaired kidney function. RR extract, particularly catalpol, has been found to protect rat cardiomyocytes by regulating autophagy, inhibiting apoptosis, enhancing oxidative stress response, and modulating estrogen receptors, thereby improving myocardial ischemia (Lin et al., 2017). Catalpol also mitigates oxidative stress, inflammatory responses, and apoptosis in the heart by inhibiting NF-κB activation, reducing cardiovascular event occurrences (Nemmar et al., 2022). Hyperhomocysteinemia (HHCY), a recognized independent risk factor for atherosclerosis, is ameliorated by catalpol in human aortic endothelial cells through the inhibition of Nox4/NF-κB and endoplasmic reticulum stress (Hu et al., 2019). Additionally, the active compound puerarin from Catalpol and Pueraria lobata significantly improves cerebrovascular endothelial cell apoptosis, increases local cerebral blood flow, and reduces infarct size at a dosage of 65.4 mg/kg (Liu et al., 2017).
Premature ovarian aging leads to decreased bone mineral density and increased osteoporosis risk, partly due to the adverse effects of prolonged high FSH levels and low estrogen (Mills et al., 2021). A longitudinal study (Shea et al., 2021) indicated a higher osteoporosis incidence in women with POI compared to those with early or normal menopausal onset (21.9% vs. 16.7%). Extensive research has shown that RR extract effectively prevents and treats osteoporosis, potentially developing as a new therapeutic drug. This effect is attributed to RR extract’s ability to stimulate osteoblast proliferation, inhibit osteoclast activity and production, and prevent bone loss in osteoporotic mouse models with ovarian resection (Oh et al., 2003; Lim and Kim, 2013). Inosine, a primary active molecule in RR, significantly inhibits bone marrow macrophage-derived osteoclast differentiation and formation by blocking NF-κB activation, thereby reducing bone loss (Lee et al., 2013). RR compound ajugol alleviates osteoarthritis by promoting autophagy and attenuating endoplasmic reticulum stress-induced cell death and extracellular matrix degradation at 50 μM(Wu et al., 2023). Metabolomics studies (Xia et al., 2019) reveal that RR prevents dexamethasone-induced bone loss by interfering with steroid hormone biosynthesis, upregulating cytochrome P450 17A1 (CYP17A1) and aromatase (CYP19A1), and downregulating 11β-hydroxysteroid dehydrogenase (HSD11B1).
Cognitive dysfunction and memory loss are also significant long-term risks after premature ovarian failure, closely linked to estrogen deficiency (Gibbs, 2010; Djiogue et al., 2018). Notably, a meta-analysis (Zhao et al., 2023) indicated that osteoporotic patients have a higher risk of cognitive impairment compared to non-osteoporotic patients (OR = 2.01%, 95% CI: 1.63–2.48, p < 0.01). Catalpol isolated from RR plays a neuroprotective role in brain tissues and improves cognitive dysfunction by enhancing endogenous antioxidant enzyme activity and inhibiting free radical production (Zhang et al., 2007). Another study (Zhang et al., 2013) found that Catalpol also offers neuroprotective effects in d-galactose-induced aging mice by regulating the cholinergic system and reducing inflammatory cytokine expression (TNF-α, IL-1β). Thus, the active compounds of RR effectively reduce long-term health risks in patients with premature ovarian aging and offer benefits for both short-term and long-term health.
RR is commonly used in clinical practice in combination with other Chinese botanical drugs in recipes such as Siwu Decoction, Liuwei Dihuang Pill, Guishen Pill, Yijing Decoction, and Chinese patent medicines like KunTai Capsule. As the primary botanical drug in many TCM compounds, RR can enhance the efficacy of these formulas. The composition of TCM compounds containing RRP was shown in Table 2, and its mechanism were shown in Table 3 and Table 4.
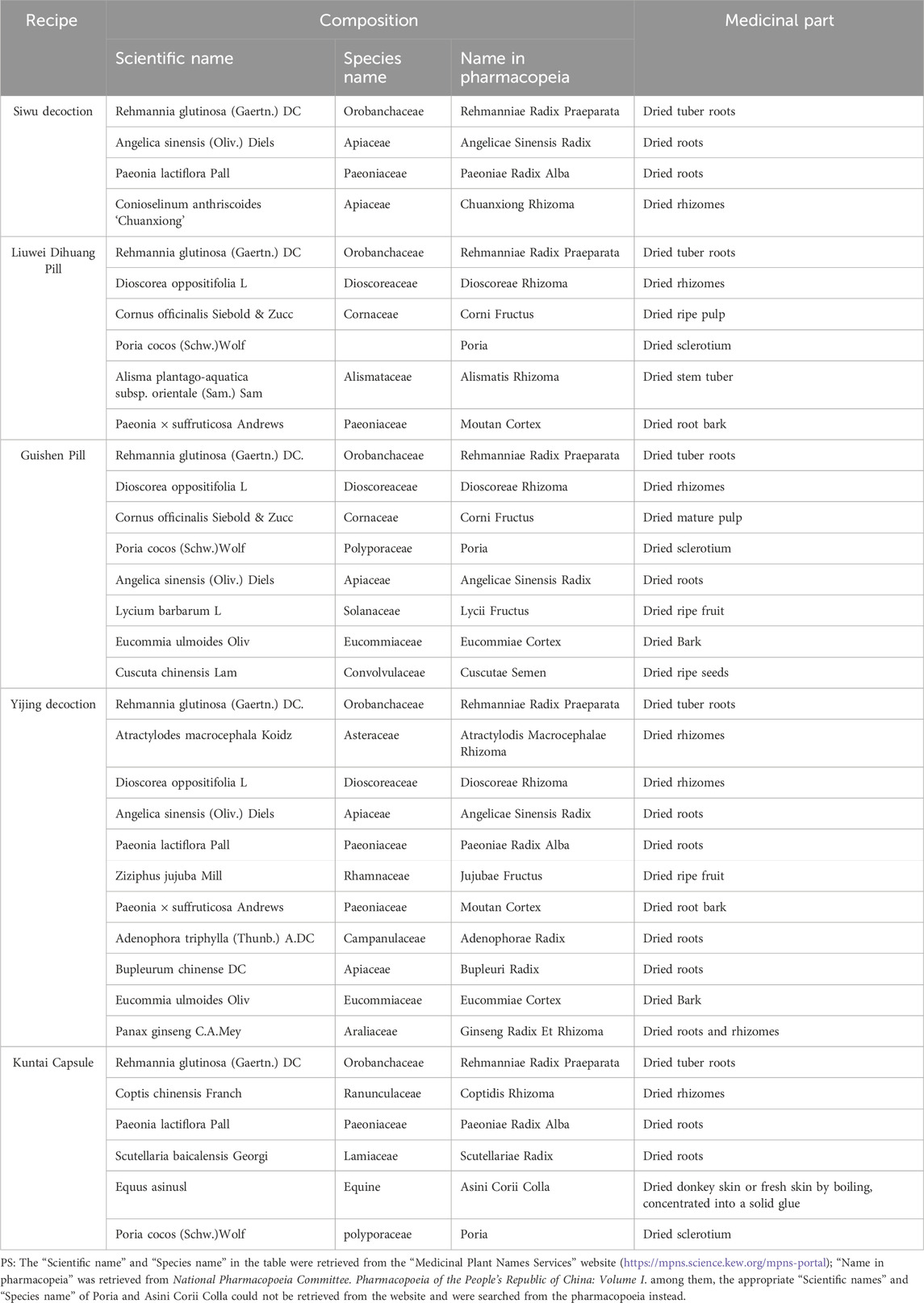
Table 4. The composition of traditional Chinese medicine compounds containing Rehmanniae Radix Praeparata.
Siwu Decoction, in which RRP is as its sovereign botanical drug, Angelicae Sinensis Radix as the minister botanical drug, and Paeoniae Radix Alba and Chuanxiong Rhizoma as assistant botanical drugs, serves to nourish and replenish blood. As a tonic formula, it was first documented in the Song Dynasty’s “Taiping Huimin Heji Ju Fang” for treating gynecological conditions. Today, it is widely employed to address irregular menstruation and various complications during pregnancy and childbirth, including those associated with ovarian hypofunction. Each menstrual cycle involves cyclic ovarian remodeling, necessitating extensive vascular remodeling to support new follicle growth. A dense, highly permeable vascular network supplies the ovary with essential hormones and nutrients for robust metabolism and delivers matrix factors to the cumulus-oocyte complex before ovulation (Brown and Russell, 2014). Consequently, ovarian blood vessels are essential for follicles, ovarian structure, and function. Research on Siwu Decoction has demonstrated its ability to enhance ovarian angiogenesis in POF model mice by activating the STAT3/HIF-1a/VEGF signaling pathway, thus increasing the production of related pro-angiogenic factors (VEGF, bFGF). It also stabilizes new blood vessel maturation through the regulation of PDGFB, Ang1, and Ang2 expression, ensuring sufficient vascular support for follicle development and consistent proliferation, and enhancing ovarian function (Zhou, 2022). Estrogen receptors (ER), including ERα, ERβ, and G protein-coupled estrogen receptor 1 (GPER), are crucial for follicle and oocyte growth, development, and ovulation (Tang et al., 2019). Phytoestrogens, natural compounds with significant ER regulatory activity, offer alternatives for those unwilling or unable to undergo HRT (Yang et al., 2019). In vitro cell studies have shown that serum containing Siwu Decoction exhibits estrogen-like effects, potentially through regulation of ERα and ERβ expression (Lu et al., 2019). Earlier discussions highlighted the findings from Mendelian Randomization (MR) studies on the link between POI and intestinal microbiota. Studies have shown that DOR rats induced by Tripterygium glycosides exhibit reduced intestinal microbiota diversity. Yet, following treatment with Siwu Decoction, there is a significant recovery in the diversity of intestinal microbiota in model rats, with a concurrent increase in beneficial bacteria (Zhu M. et al., 2022).
Liuwei Dihuang Pill comprises six botanical drugs: RRP, Dioscoreae Rhizoma, Corni Fructus, Poria, Alismatis Rhizoma, and Moutan Cortex. This formula is a fundamental TCM preparation for kidney nourishment and yin reinforcement, commonly employed in treating ovarian hypofunction diseases. Clinical trials (Du et al., 2013; Du, 2016; Li et al., 2016; Qin, 2017; Gao, 2021) have demonstrated that combining Liuwei Dihuang Pill with HRT enhances clinical symptoms and sex hormone levels (FSH, LH, E2) more effectively than HRT alone, without increasing adverse reactions and potentially boosting the therapeutic outcomes. Fundamental research has shown that Liuwei Dihuang Pill promotes the proliferation of human ovarian granulosa cells and follicle maturation, thus facilitating ovulation, making it suitable for treating ovulatory disorders diseases (Yue, 2009). Another study revealed that Liuwei Dihuang Pill protects the mitochondrial integrity of ovarian granulosa cells and ameliorates mitochondrial dysfunction in cyclophosphamide-induced DOR mouse models. This protective effect is achieved by reducing ROS accumulation, mitigating oxidative stress, and thus preventing granulosa cell apoptosis (Gao T. et al., 2023). Subsequent research on these model mice indicated that intervention with Liuwei Dihuang Pill results in a higher number of offspring and pup survival rates compared to the control group. Furthermore, studies on long-term health risks suggest that Liuwei Dihuang Pill may reduce inflammatory responses and regulate bone metabolism via modulation of the NLRP3/Caspase-1/GSDMD pyroptosis signaling pathway, offering potential anti-osteoporosis benefits (Chen ZB. et al., 2023).
The Guishen Pill contains RRP, Dioscoreae Rhizoma, Corni Fructus, Poria, Angelicae Sinensis Radix, Lycii Fructus, Eucommiae Cortex, and Cuscutae Semen. This formula nourishes kidney essence, tonifies blood, and regulates menstruation. Guishen Pill, a TCM formula for tonifying the kidney and benefiting essence, is commonly used to treat ovarian hypofunction. Clinical studies (Du and Fan, 2018; Xu et al., 2020; Hu et al., 2023) indicate that Guishen Pill, when combined with HRT, surpasses HRT alone in enhancing endometrial thickness, ovarian volume, and sex hormone levels in patients with ovarian hypofunction diseases, without exacerbating adverse effects. Oxidative stress damage is a primary aging factor. An experiment (Cui et al., 2015) developed an ovarian aging model in mice through continuous superovulation and ozone inhalation, intensifying oxidative stress and diminishing follicle quality. Post-intervention with Guishen Pill led to increased expression of Oct-4 and MVH mRNA in model mice (p < 0.05). Oct-4 is identified with embryonic-like stem cells and ovarian germ cells (Johnson et al., 2005; Virant-Klun, 2015). Research reveals that mice deficient in the MVH gene show arrested differentiation and apoptosis in reproductive cells, marked by reduced Oct-4 expression, resulting in compromised reproductive capacity (Tanaka et al., 2000). Therefore, it can be speculated that Guishen Pill may improve stem cell function by inhibiting oxidative stress, thereby promoting the differentiation of oocyte cells and restoring ovarian function in mice. Additionally, animal experiments have demonstrated that Guishen Pill can reduce the protein and mRNA levels of LC3 and Beclin 1 in the ovarian tissues of DOR mice, increase the levels of p62 protein and mRNA, and improve ovarian function by inhibiting ovarian autophagy (Zhu and Du, 2023). Meanwhile, in studying the immune mechanisms in ovarian premature aging model mice, it was found that Guishen Pill could significantly increase the spleen index, enhance the proliferation ability of T cells, significantly increase the ratio of CD3+T, CD4+T, CD4+T/CD8+T, NK cells, IFN-γ and IL-2 in mice (p < 0.05), and significantly decrease the percentage of CD8+ T cells (p < 0.05) (Li et al., 2018). Moreover, Guishen Pill can also improve bone mass and reduce microstructural damage in the femurs of rats with POF, thereby achieving its potential in treating osteoporosis (Ba et al., 2023).
Yijing Decoction, derived from Fu Qingzhu’s Obstetrics and Gynecology, contains eleven medicinal ingredients: RRP, Atractylodis Macrocephalae Rhizoma, Dioscoreae Rhizoma, Angelicae Sinensis Radix, Paeoniae Radix Alba, Jujubae Fructus, Moutan Cortex, Adenophorae Radix, Bupleuri Radix, Eucommiae Cortex and Ginseng Radix Et Rhizoma. The book employs this formula to treat patients who appear amenorrhea before age 49″, which aligns with the characteristics of ovarian hypofunction diseases. Subsequent clinical studies (Li et al., 2017; Zhang and Lin, 2023) demonstrate that Yijing Decoction improves clinical symptoms and sex hormone levels of patients with ovarian hypofunction diseases, thus enhancing pregnancy outcomes. For older patients with diminished ovarian reserve undergoing IVF-ET, Yijing Decoction, used alongside controlled ovarian stimulation protocols, significantly increases the number of retrieved ova, maturity of ova, and quality of embryos, improving ovarian responsiveness and oocytes and embryo quality (Hong et al., 2019). Experimental research (Wan and Chen, 2022) shows that Yijing Decoction counteracts hypoxia-induced activation of the Bnip3/Beclin1 pathway, thereby reducing autophagy-induced damage to ovarian tissue. The SDF-1 (stromal cell-derived factor-1)/CXCR4 axis is pivotal in embryonic development and enhances vascular regeneration and the resolution of ischemic conditions in injured tissues. SDF-1 also serves as a crucial cytokine in the regulation of local inflammation and tissue repair (Cencioni et al., 2012). Research on Yijing Decoction has demonstrated that this formula can modulate the Th17/Treg balance through the SDF-1/CXCR4 pathway, decreasing IL-17 and increasing IL-10 levels. This mechanism alters the immune-inflammatory microenvironment, thereby affecting ovarian function in cyclophosphamide-induced DOR model rats (Xie, 2021).
Kuntai capsule, derived from the HuangLian-EJiao decoction in the Treatise on Febrile Diseases, consist of RRP, Coptidis Rhizoma, Paeoniae Radix Alba, Scutellariae Radix, Asini Corii Colla, and Poria. The efficacy and safety has been confirmed through numerous clinical trials (Cui et al., 2018; Gao, 2019; Lin et al., 2021; Zhou et al., 2023) and systematic reviews (Shen et al., 2020; A RN et al., 2019; Zhang et al., 2024; Liu et al., 2021). A study (Gong et al., 2024) identified that a primary target of Kuntai capsule in treating POF model mice is the regulation of oxidative stress damage, primarily through the FOXO3 and SIRT5 genes. The AGE-RAGE signaling pathway plays a crucial role in managing oxidative stress and inflammation (Shi et al., 2023), and Kuntai capsule may enhance ovarian function by promoting follicular development and increasing the granulosa cell layer thickness in dominant follicles via this pathway (Huang et al., 2024). Further research (Luo et al., 2023) suggests that Kuntai capsule also boosts the expression of GDF9, a key TGF-β family member vital for granulosa cell proliferation and follicular growth. They also found that Kuntai capsule can upregulate the expression of Beclin 1, mediate cell autophagy, delay delayed cell senescence and cell death to promote cell survival. Regarding apoptosis, Kuntai capsule has been found to maintain the balance between pro-apoptotic and inhibitory proteins by adjusting the Bcl-2/Bax ratio, specifically by upregulating Bcl-2 and downregulating Bax expression, thus regulating apoptosis of ovarian granulosa cells to improve ovarian reserve function and delay aging (Geng and Tan, 2017). Moreover, the imbalance of the Th17/Treg cell ratio, a significant factor in immune disorders, is corrected in patients with ovarian hypofunction. Clinical studies have shown that Kuntai capsule increases TGF-β1 levels in the peripheral blood of POF patients, reduces IL-21 levels, and corrects the Th17/Treg cell ratio imbalance, thereby regulating the autoimmune system (Jiang LL. et al., 2023).
Processing plays a critical role in influencing the pharmacological characteristics and effectiveness of botanical drugs. Various processing techniques can have diverse impacts on the bioactive compounds of herbal medicines, leading to modifications in their physiological effects. Consequently, the selection of an appropriate processing method to effectively manipulate the pharmacological properties of botanical drugs is essential for clinical applications. Nevertheless, regional disparities exist in the preparation methods of RRP in different regions. For example, regarding the processing method of prepared RRP, Zhu XD et al. (Zhu et al., 2023) used the “Jiangxi Provincial Standard for the Processing of Chinese Medicinal Slices (2008 Edition),” Chen QY et al. (Chen et al., 2021) used the “Jianchang Bang Processing Technique,” and Tian JY et al. (Tian et al., 2022) used the Beijing 2008 edition of the Drug Processing Specification. Some experiments even opted for purchasing RRP (Zhang et al., 2016). Furthermore, inconsistencies exist in the inclusion of excipients during RR steaming processes. For instance, Chen SQ et al. (Chen SQ. et al., 2023) utilized plain water for steaming RR, whereas Ma YJ et al. (Ma et al., 2023) employed yellow rice wine in the steaming process. These variations present difficulties in conducting quantitative analysis. In terms of compound analysis, the 2020 edition of the Pharmacopoeia of the People’s Republic of China only provides concentration specifications for catalpol and rehmannioside D in RRP and RR, without establishing concentration limits for other potentially bioactive compounds. Moreover, investigations into the use of RRP for conditions associated with ovarian hypofunction predominantly rely on findings from in vitro and in vivo studies, with limited incorporation of clinical research data. Clinical trials utilizing traditional Chinese medicine compounds containing RRP as the key component often exhibit small sample sizes, potentially compromising the applicability of their findings. Furthermore, the absence of blinding in many clinical studies, as well as their restricted focus on Asian populations, may introduce bias into the research outcomes.
Hence, it is imperative to enhance the following facets in forthcoming research: 1) standardizing and unifying the processing methods of RR and the addition of excipients, investigating the changes in RR compounds after adding excipients, and optimizing the storage conditions of botanical drugs according to the characteristics of processed products to guarantee their stability. 2) Conducting clinical research on the efficacious compounds of RRP for ovarian hypofunction involves integrating both basic and clinical research methodologies to substantiate their efficacy and safety, elucidating their scientific implications and mechanisms, and potentially developing RRP as pharmaceutical agents for the treatment of ovarian hypofunction. 3) Standardizing the clinical research design of traditional Chinese medicine compounds containing RRP aims to furnish high-quality evidence-based data for clinical application.
H-ZZ: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing–original draft. JM: Data curation, Investigation, Methodology, Software, Writing–original draft. Y-XL: Data curation, Investigation, Software, Writing–original draft. M-YL: Conceptualization, Methodology, Supervision, Writing–review and editing. S-BW: Methodology, Project administration, Resources, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work were supported by the Group Standard Project of Chinese Association of Chinese Medicine (Project No: 20211220-BZ-CACM); The National Celebrated Traditional Chinese Medicine Expert Inheritance Studio of S-BW (Project No: CJJ2023062). Based on real-world research explored the efficacy and mechanism of the regulation of the P38MAPK/P53/P21 signaling pathway mediating Th1/Th2 cell homeostasis in the treatment of POI (Project No: MPRC2023009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
An, R., Wang, X., Yang, L., Zhang, J., Wang, N., Xu, F., et al. (2021). Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 449, 152665. doi:10.1016/j.tox.2020.152665
A Rn, L. Y. Y., Li, S. C., and Xu, L. Z. (2019). Meta-analysis of the efficacy and safety of Kuntai capsule in the treatment of early-onset ovarian insufficiency. Chin. J. Evidence-Based Med. 08, 953–959.
Ba, X., Chen, Z., Huang, Y., Tu, S. H., and Wang, Y. (2023). The effect of Guishen Pill on bone mineral density and bone microstructure in rats with premature ovarian failure and its mechanism. Chin. J. Osteoporos. 03, 390–396.
Bai, L., Shi, G. Y., Yang, Y. J., Chen, W., Zhang, L. F., and Qin, C. (2018). Rehmannia glutinosa exhibits anti-aging effect through maintaining the quiescence and decreasing the senescence of hematopoietic stem cells. Anim. Model Exp. Med. 1 (3), 194–202. doi:10.1002/ame2.12034
Baird, L., and Yamamoto, M. (2020). The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 40 (13), e00099-20. doi:10.1128/MCB.00099-20
Boots, C. E., and Jungheim, E. S. (2015). Inflammation and human ovarian follicular dynamics. Seminars reproductive Med. 33 (4), 270–275. doi:10.1055/s-0035-1554928
Bourassa, P. A., Milos, P. M., Gaynor, B. J., Breslow, J. L., and Aiello, R. J. (1996). Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 93 (19), 10022–10027. doi:10.1073/pnas.93.19.10022
Brown, H. M., and Russell, D. L. (2014). Blood and lymphatic vasculature in the ovary: development, function and disease. Hum. Reprod. update 20 (1), 29–39. doi:10.1093/humupd/dmt049
Bushnell, C., McCullough, L. D., Awad, I. A., Chireau, M. V., Fedder, W. N., Furie, K. L., et al. (2014). Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45 (5), 1545–1588. doi:10.1161/01.str.0000442009.06663.48
Canonico, M., Plu-Bureau, G., O’Sullivan, M. J., Stefanick, M. L., Cochrane, B., Scarabin, P. Y., et al. (2014). Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: the Women's Health Initiative Hormone Therapy clinical trials. Menopause 21 (3), 214–220. doi:10.1097/GME.0b013e31829752e0
Casarini, L., and Crépieux, P. (2019). Molecular mechanisms of action of FSH. Front. Endocrinol. 10, 305. doi:10.3389/fendo.2019.00305
Cencioni, C., Capogrossi, M. C., and Napolitano, M. (2012). The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc. Res. 94 (3), 400–407. doi:10.1093/cvr/cvs132
Chen, Q. Y., Wang, X. P., Lei, X., Gao, Y. Z., Xu, J., Wang, L. F., et al. (2021). Effects of Amomi Fructus and Citri Reticulatae Pericarpium on chemical composition in Rehmanniae Radix and Rehmanniae Radix Praeparata. Chin. Traditional Herb. Drugs 20, 6168–6177.
Chen, S. Q., Yang, K. P., Kang, W. L., Zhou, Y. C., Tao, S. M., Guan, Y. C., et al. (2023a). Study on concoction and detection of active components and Trace elements of Radix Rehmanniae Praeparata. Analysis Test. Technol. Instrum. 04, 392–399. doi:10.16495/j.1006-3757.2023.04.008
Chen, Z. B., Zhao, J. L., Hong, S. H., and Tang, H. Y. (2023b). Role of the signal axis of NLRP3/Caspase-1/GSDMD for preventing and treating osteoporosis by Liuwei Dihuang Pill decoction in rats. Chin. J. Osteoporos. Bone Mineral Res. 06, 559–567.
Chen, Z. J., Tian, Q. J., Qiao, J., Liu, J. Y., Yang, D. Z., Huang, H. F., et al. (2017). Chinese expert consensus on premature ovarian insufficiency. Chin. J. Obstetrics Gynecol. 52 (9), 577–581. doi:10.3760/cma.j.issn.0529-567X.2017.09.001
Clarkson, T. B. (2018). Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 25 (11), 1262–1274. doi:10.1097/GME.0000000000001228
Cui, D. D., Ma, W. W., Wen, L., Song, K. K., Ding, J. H., Huang, C., et al. (2015). Effect of guishen pill on expression levels of oct-4, MVH, and egr-1 in mice with diminished ovarian reserve. Chin. J. Integr. Traditional West. Med. 01, 76–80.
Cui, N., Jiang, L., Yang, A. M., Zhang, J., Xu, Y. M., Wang, W., et al. (2018). Clinical observation of Kuntai jiaonang in treating 33 patients with hypofunction of ovarian reserve infertility. J. Traditional Chin. Med. 02, 132–136. doi:10.13288/j.11-2166/r.2018.02.011
Daan, N. M., Muka, T., Koster, M. P., Roeters van Lennep, J. E., Lambalk, C. B., Laven, J. S. E., et al. (2016). Cardiovascular risk in women with premature ovarian insufficiency compared to premenopausal women at middle age. J. Clin. Endocrinol. metabolism 101 (9), 3306–3315. doi:10.1210/jc.2016-1141
Danilovich, N., and Sairam, M. R. (2002). Haploinsufficiency of the follicle-stimulating hormone receptor accelerates oocyte loss inducing early reproductive senescence and biological aging in mice. Biol. reproduction 67 (2), 361–369. doi:10.1095/biolreprod67.2.361
Djiogue, S., Djiyou Djeuda, A. B., Seke Etet, P. F., Ketcha Wanda, G. J. M., Djikem Tadah, R. N., and Njamen, D. (2018). Memory and exploratory behavior impairment in ovariectomized Wistar rats. Behav. Brain Funct. 14 (1), 14. doi:10.1186/s12993-018-0146-7
Domniz, N., and Meirow, D. (2019). Premature ovarian insufficiency and autoimmune diseases. Clin. obstetrics Gynaecol. 60, 42–55. doi:10.1016/j.bpobgyn.2019.07.008
Du, D., Wei, J. L., and Chen, M. Y. (2013). Clinical study of Liuwei Dihuang pills combined with hormone replacement in the treatment of premature ovarian failure. Chin. Archives Traditional Chin. Med. 12, 2738–2740. doi:10.13193/j.issn.1673-7717.2013.12.047
Du, J. M. (2016). Clinical observation of hormone therapy combined with Liuwei Dihuang pills in the replacement treatment of premature ovarian failure. Shenzhen J. Integr. Traditional Chin. West. Med. 04, 51–53. doi:10.16458/j.cnki.1007-0893.2016.04.025
Du, Q. M., and Fan, L. S. (2018). Clinical study of 60 cases of premature ovarian failure treated with guishen pill combined with sequential estrogen and progesterone therapy. Jiangsu J. Traditional Chin. Med. 07, 39–41.
Du, X., Zhang, L., Li, X., Pan, Z., Liu, H., and Li, Q. (2016). TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell death Dis. 7 (11), e2476. doi:10.1038/cddis.2016.379
Ewertz, M., Mellemkjaer, L., Poulsen, A. H., Friis, S., Sørensen, H. T., Pedersen, L., et al. (2005). Hormone use for menopausal symptoms and risk of breast cancer. A Danish cohort study. Br. J. cancer 92 (7), 1293–1297. doi:10.1038/sj.bjc.6602472
Feng, J. C., Cai, Z. L., Zhang, X. P., Chen, Y. Y., Chang, X. L., Wang, X. F., et al. (2020). The effects of oral rehmannia glutinosa polysaccharide administration on immune responses, antioxidant activity and resistance against aeromonas hydrophila in the common carp, Cyprinus carpio L. Front. Immunol. 11, 904. doi:10.3389/fimmu.2020.00904
Freitas, C., Neto, A. C., Matos, L., Silva, E., Ribeiro, Â., Silva-Carvalho, J. L., et al. (2017). Follicular Fluid redox involvement for ovarian follicle growth. J. ovarian Res. 10 (1), 44. doi:10.1186/s13048-017-0342-3
Fu, C., Wu, Y., Liu, S., Luo, C., Lu, Y., Liu, M., et al. (2022). Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 289, 115021. doi:10.1016/j.jep.2022.115021
Gao, G. Z., Zhou, J. W., Wang, H. Q., Rao, P. F., and Ke, L. J. (2010). Analysis of amino acids in rehmannia glutinosa Libosch during heating process. Biot. Resour. 03, 52–54. doi:10.14188/j.ajsh.2010.03.022
Gao, L. (2021). Clinical observation of Liuwei Dihuang Pill combined with Fenmotong in the treatment of decreased ovarian reserve function with Kidney-yin deficiency. Gansu University Of Chinese Medicine. doi:10.27026/d.cnki.ggszc.2021.000118
Gao, P. (2019). Effect of Kuntai capsule on reproductive endocrine and ovarian blood supply in patients with ovarian reserve deficiency. Maternal Child Health Care China 09, 2080–2083.
Gao, T., Zhong, J. W., Qin, L., Wang, X. Y., Li, X. R., and Luo, Y. X. (2023b). Based on proteomics technology, the mechanism of action of Liuwei Dihuang pill in the treatment of mice with diminished ovarian reserve was explored. China J. Chin. Materia Medica 12, 3224–3234. doi:10.19540/j.cnki.cjcmm.20230202.704
Gao, X., Wang, B., Huang, Y., Wu, M., Li, Y., Li, Y., et al. (2023a). Role of the Nrf2 signaling pathway in ovarian aging: potential mechanism and protective strategies. Int. J. Mol. Sci. 24 (17), 13327. doi:10.3390/ijms241713327
Geng, L. H., and Tan, Y. (2017). Effect of Kuntai capsule on the expression of ovarian apoptosis regulatory proteins Bcl-2 and Bax in rats with decreased ovarian reserve. Chin. J. Exp. Traditional Med. Formulae 08, 138–143. doi:10.13422/j.cnki.syfjx.2017080138
Gibbs, R. B. (2010). Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr. Rev. 31 (2), 224–253. doi:10.1210/er.2009-0036
Gong, L., Hou, J., Yang, H., Zhang, X., Zhao, J., Wang, L., et al. (2024). Kuntai capsule attenuates premature ovarian insufficiency by activating the FOXO3/SIRT5 signaling pathway in mice: a comprehensive study using UHPLC-LTQ-Orbitrap and integrated pharmacology. J. Ethnopharmacol. 322, 117625. doi:10.1016/j.jep.2023.117625
Gong, R. Z., Huo, X. H., Zhang, L., Liu, C., Li, S. S., and Sun, Y. S. (2019). Advances in effects and regulation of Maillard reaction on quality of Chinese materia medica. Chin. Traditional Herb. Drugs 01, 243–251.
Gu, H., Zhao, X., Zhao, X., Yang, Y., and Lv, X. (2014). Risk of stroke in healthy postmenopausal women during and after hormone therapy: a meta-analysis. Menopause 21 (11), 1204–1210. doi:10.1097/GME.0000000000000227
Gu, M., Yuan, Y. P., Qin, Z. N., Xu, Y., Shi, N. N., Wang, Y. P., et al. (2021). A combined quality evaluation method that integrates chemical constituents, appearance traits and origins of raw Rehmanniae Radix pieces. Chin. J. Nat. Med. 19 (7), 551–560. doi:10.1016/S1875-5364(21)60056-0
Han, K., Bose, S., Kim, Y. M., Chin, Y. W., Kim, B. S., Wang, J. h., et al. (2015). Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food and Funct. 6 (8), 2684–2692. doi:10.1039/c5fo00232j
Han, Y., Jung, H. W., Lee, J. Y., Kim, J. S., Kang, S. S., Kim, Y. S., et al. (2012). 2,5-dihydroxyacetophenone isolated from Rehmanniae Radix Preparata inhibits inflammatory responses in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Med. food 15 (6), 505–510. doi:10.1089/jmf.2011.1940
Harman, D. (1956). Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11 (3), 298–300. doi:10.1093/geronj/11.3.298
Harman, D. (2006). Free radical theory of aging: an update: increasing the functional life span. Ann. N. Y. Acad. Sci. 1067, 10–21. doi:10.1196/annals.1354.003
Heinrich, M., Yao, R., and Xiao, P. (2021). 'Food and medicine continuum' - why we should promote cross-cultural communication between the global East and West. Chin. Herb. Med. 14 (1), 3–4. doi:10.1016/j.chmed.2021.12.002
Hong, Y. L., Tan, Y., Yin, Y. Y., and Chen, J. (2019). Application of Yijing Decoction combined with PPOS program in IVF-ET for elderly women. China J. Traditional Chin. Med. Pharm. 07, 3315–3319.
Hsieh, Y. T., and Ho, J. Y. P. (2021). Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide Health Insurance Research Database study. Hum. Reprod. Oxf. Engl. 36 (6), 1621–1629. doi:10.1093/humrep/deab025
Hsueh, A. J., McGee, E. A., Hayashi, M., and Hsu, S. Y. (2000). Hormonal regulation of early follicle development in the rat ovary. Mol. Cell. Endocrinol. 163 (1-2), 95–100. doi:10.1016/s0303-7207(99)00245-2
Hu, H., Wang, C., Jin, Y., Meng, Q., Liu, Q., Liu, Z., et al. (2019). Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-κB and GRP78/PERK pathways in human aorta endothelial cells. Inflammation 42 (1), 64–80. doi:10.1007/s10753-018-0873-9
Hu, X. H., Lin, Q., and Hu, Y. H. (2023). Effect of guishen pill combined with climen on premature ovarian failure and its effect on ovarian function. Liaoning J. Traditional Chin. Med. 04, 97–100. doi:10.13192/j.issn.1000-1719.2023.04.027
Huang, J. G., Zhang, J., Xiang, R. J., Wu, Y. Y., He, T. Y., Zhao, M. N., et al. (2024). Mechanism of Kuntai Capsules in treatment of polycystic ovary syndrome rat models based on AGE-RAGE signal pathway. China J. Chin. Materia Medica 04, 1082–1090. doi:10.19540/j.cnki.cjcmm.20231115.501
Huang, Y., Hu, C., Ye, H., Luo, R., Fu, X., Li, X., et al. (2019). Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019, 8069898. doi:10.1155/2019/8069898
Huang, Y., Jiang, C., Hu, Y., Zhao, X., Shi, C., Yu, Y., et al. (2013). Immunoenhancement effect of rehmannia glutinosa polysaccharide on lymphocyte proliferation and dendritic cell. Carbohydr. Polym. 96 (2), 516–521. doi:10.1016/j.carbpol.2013.04.018
Huang, Y., Liu, Z., Bo, R., Xing, J., Luo, L., Zhen, S., et al. (2016b). The enhanced immune response of PCV-2 vaccine using Rehmannia glutinosa polysaccharide liposome as an adjuvant. Int. J. Biol. Macromol. 86, 929–936. doi:10.1016/j.ijbiomac.2016.02.003
Huang, Y., Nan, L., Xiao, C., Su, F., Li, K., Ji, Q. A., et al. (2021). PEGylated nano-Rehmannia glutinosa polysaccharide induces potent adaptive immunity against Bordetella bronchiseptica. Int. J. Biol. Macromol. 168, 507–517. doi:10.1016/j.ijbiomac.2020.12.044
Huang, Y., Qin, T., Huang, Y., Liu, Z., Bo, R., Hu, Y., et al. (2016a). Rehmannia glutinosa polysaccharide liposome as a novel strategy for stimulating an efficient immune response and their effects on dendritic cells. Int. J. nanomedicine 11, 6795–6808. doi:10.2147/IJN.S119108
Hunzicker-Dunn, M., and Maizels, E. T. (2006). FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell. Signal. 18 (9), 1351–1359. doi:10.1016/j.cellsig.2006.02.011
Jia, H., Zhang, W. F., Lei, J. W., Li, Y. Y., Yang, C. J., and Fan, K. F. (2023). UV combined with MIR spectroscopy to discuss the dynamic changes of sugar during the processing of Rehmannia glutinosa. Lishizhen Med. Materia Medica Res. 01, 96–99.
Jiang, L. L., Tang, W. C., Kang, X. Y., Xue, L. L., Qin, J. R., Guo, Z. G., et al. (2023b). The efficacy of Kuntai capsule combined with estrogen and progesterone replacement therapy on premature ovarian failure and its effects on Treg, Th17 and VEGF. J. Chin. Med. Mater. 09, 2336–2340. doi:10.13863/j.issn1001-4454.2023.09.039
Jiang, Y., Luan, Y. G., Huang, R., He, X. J., Wang, X., and Tan, P. (2023a). Comparative analysis of characters and components of wine-stewed and steamed Rehmanniae Radix with different processing degrees. Mod. Chin. Med. 09, 1957–1965. doi:10.13313/j.issn.1673-4890.20230104006
Johnson, J., Bagley, J., Skaznik-Wikiel, M., Lee, H. J., Adams, G. B., Niikura, Y., et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122 (2), 303–315. doi:10.1016/j.cell.2005.06.031
Kirshenbaum, M., and Orvieto, R. (2019). Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. assisted reproduction Genet. 36 (11), 2207–2215. doi:10.1007/s10815-019-01572-0
Kranc, W., Brązert, M., Ożegowska, K., Nawrocki, M. J., Budna, J., Celichowski, P., et al. (2017). Expression profile of genes regulating steroid biosynthesis and metabolism in human ovarian granulosa cells-A primary culture approach. Int. J. Mol. Sci. 18 (12), 2673. doi:10.3390/ijms18122673
Kwon, Y., Yu, S., Choi, G. S., Kim, J. H., Baik, M., Su, S. T., et al. (2019). Puffing of Rehmannia glutinosa enhances anti-oxidant capacity and down-regulates IL-6 production in RAW 264.7 cells. Food Sci. Biotechnol. 28 (4), 1235–1240. doi:10.1007/s10068-019-00566-z
Lee, S. Y., Lee, K. S., Yi, S. H., Kook, S. H., and Lee, J. C. (2013). Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-κB pathway and attenuating ROS production. PloS one 8 (12), e80873. doi:10.1371/journal.pone.0080873
Leridon, H. (2004). Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum. Reprod. Oxf. Engl. 19 (7), 1548–1553. doi:10.1093/humrep/deh304
Lewis, K. N., Wason, E., Edrey, Y. H., Kristan, D. M., Nevo, E., and Buffenstein, R. (2015). Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc. Natl. Acad. Sci. U. S. A. 112 (12), 3722–3727. doi:10.1073/pnas.1417566112
Li, D., Guo, H., Niu, L., Yin, Q., Zhang, Y., and Zhuang, P. (2023b). Clinical value-oriented research paradigm about inheritance and innovation development of TCM dominant diseases. Chin. Herb. Med. 15 (4), 476–484. doi:10.1016/j.chmed.2023.09.002
Li, L. H., Li, Z. C., and Hong, W. T. (2018). Effect of guishen pill on cellular immunity in mice with premature ovarian failure. Chin. J. Immunol. 08, 1178–1182.
Li, L. M., He, X. K., Tao, L. L., Sun, D. M., Chen, Y. X., Chen, X. P., et al. (2017). A clinical study on the improvement of ovarian function in patients with kidney deficiency and liver stagnation syndrome in the treat of Yijing Decoction. Lishizhen Med. Materia Medica Res. 12, 2943–2945.
Li, M., Zhu, Y., Wei, J., Chen, L., Chen, S., and Lai, D. (2023a). The global prevalence of premature ovarian insufficiency: a systematic review and meta-analysis. Climacteric J. Int. Menopause Soc. 26 (2), 95–102. doi:10.1080/13697137.2022.2153033
Li, Q., Tang, J. S., Qiu, S., Zhang, L. H., Luo, H. Y., Qiu, M., et al. (2016). The clinical effect of drug combination by Liuwei Dihuang pills and climen in treatment of POF. Hebei Med. 02, 339–341.
Li, X., Gui, R., Wang, X., Ning, E., Zhang, L., Fan, Y., et al. (2023c). Oligosaccharides isolated from Rehmannia glutinosa protect LPS-induced intestinal inflammation and barrier injury in mice. Front. Nutr. 10, 1139006. doi:10.3389/fnut.2023.1139006
Li, X., Xing, Y. D., Li, S. S., and Liu, H. (2020). Effect of different processing methods on the contents of seven chemical components in Rehmanniae Radix Preparata. J. Bengbu Med. Coll. 05, 634–637. doi:10.13898/j.cnki.issn.1000-2200.2020.05.021
Liang, J., Gao, Y., Feng, Z., Zhang, B., Na, Z., and Li, D. (2023a). Reactive oxygen species and ovarian diseases: antioxidant strategies. Redox Biol. 62, 102659. doi:10.1016/j.redox.2023.102659
Liang, L., Yue, Y., Zhong, L., Liang, Y., Shi, R., Luo, R., et al. (2023b). Anti-aging activities of Rehmannia glutinosa Libosch. crude polysaccharide in Caenorhabditis elegans based on gut microbiota and metabonomic analysis. Int. J. Biol. Macromol. 253 (8), 127647. doi:10.1016/j.ijbiomac.2023.127647
Lim, D. W., and Kim, Y. T. (2013). Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Mol. Basel. Switz. 18 (5), 5804–5813. doi:10.3390/molecules18055804
Lin, C., Lu, Y., Yan, X., Wu, X., Kuai, M., Sun, X., et al. (2017). Catalpol protects glucose-deprived rat embryonic cardiac cells by inducing mitophagy and modulating estrogen receptor. Biomed. Pharmacother. = Biomedecine Pharmacother. 89, 973–982. doi:10.1016/j.biopha.2017.02.069
Lin, H., Gui, S. H., Yu, B. B., Que, X. H., and Zhu, J. Q. (2019). Analysis of polysaccharide monosaccharides of Radix Rehmanniae by different processing processes and their effects on ovarian granulosa cells. Chin. Tradit. Pat. Med. 12, 2958–2963.
Lin, X. M., Chen, M., Wang, Q. L., Ye, X. M., and Chen, H. F. (2021). Clinical observation of Kuntai capsule combined with Fenmotong in treatment of decline of ovarian reserve function. World J. Clin. Cases 6 (28), 8349–8357. doi:10.12998/wjcc.v9.i28.8349
Liu, L. Y., Ji, Y. H., Wang, G. L. F., Huang, Y., and Zeng, Z. J. (2023). Study on the characteristic specturm and color change of steamed Rehmanniae Radix preparata and optimization of processing technology. J. Nanjing Univ. Traditional Chin. Med. 08, 764–774. doi:10.14148/j.issn.1672-0482.2023.0764
Liu, W., Nguyen, T. N., Tran Thi, T. V., and Zhou, S. (2021). Corrigendum to "Kuntai capsule plus hormone therapy vs. Hormone therapy alone in patients with premature ovarian failure: a systematic review and meta-analysis". Evid. Based Complement. Altern. Med. 2021, 5737914. Erratum for: Evid Based Complement Alternat Med. 2019 Jun 26;2019:2085804. PMID: 33603817; PMCID: PMC7872766. doi:10.1155/2021/5737914
Liu, Y., Tang, Q., Shao, S., Chen, Y., Chen, W., and Xu, X. (2017). Lyophilized powder of catalpol and puerarin protected cerebral vessels from ischemia by its anti-apoptosis on endothelial cells. Int. J. Biol. Sci. 13 (3), 327–338. doi:10.7150/ijbs.17751
Liu, Z., Dai, L., Sun, T., Liu, Y., Bao, Y., Gu, M., et al. (2024). Massively parallel CRISPR-cas9 knockout screening in sheep granulosa cells for FSH response genes. Animals 14 (6), 898. doi:10.3390/ani14060898
Liu, Z. Q. (2018). Spectral effects of two processing Radix Rehmanniae Preparata on the ovulation function of rats. Guangzhou University of Traditional Chinese Medicine.
Lu, D., Zhao, P. W., Chen, M., Yang, Y., and Niu, J. Z. (2019). Study on estrogenic effects and its molecular mechanism of Siwu decoction via estrogen receptor subtypes. Liaoning J. Traditional Chin. Med. 05, 1074–1077. doi:10.13192/j.issn.1000-1719.2019.05.057
Lu, M. K., Chang, C. C., Chao, C. H., and Hsu, Y. C. (2022). Structural changes, and anti-inflammatory, anti-cancer potential of polysaccharides from multiple processing of Rehmannia glutinosa. Int. J. Biol. Macromol. 206, 621–632. doi:10.1016/j.ijbiomac.2022.02.112
Lu, X. M., Zhong, L. Y., Wang, S., Deng, Y. W., Liu, H., Chen, M. X., et al. (2023). Effect of processing method on chemical constituents of Rehmanniae Radix: based on UHPLC-LTQ-Orbitrap MS. China J. Chin. Materia Medica 02, 399–414. doi:10.19540/j.cnki.cjcmm.20220816.301
Luo, S., Ruan, X., and Mueck, A. O. (2023). The effect of Kuntai capsule on ovarian function in cisplatin-induced premature ovarian insufficiency rats. Front. Endocrinol. 13, 1097165. doi:10.3389/fendo.2022.1097165
Lv, H., Jia, H., Cai, W., Cao, R., Xue, C., and Dong, N. (2023). Rehmannia glutinosa polysaccharides attenuates colitis via reshaping gut microbiota and short-chain fatty acid production. J. Sci. food Agric. 103 (8), 3926–3938. doi:10.1002/jsfa.12326
Ma, R., Liang, W., Sun, Q., Qiu, X., Lin, Y., Ge, X., et al. (2018). Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging 10 (10), 2991–3004. doi:10.18632/aging.101609
Ma, Y. J., Yao, C., Yu, L. L., Liu, P., Li, M. E., and Chen, T. C. (2023). Research on the correlation between the chroma of rehmannia glutinosa processed by "nine steaming and nine drying" and index components based on traditional experience. Lishizhen Med. Materia Medica Res. 06, 1366–1370.
Mao, S. Q., and Ji, D. Y. (2023). Study on the correlation between TH1/Treg cell immune imbalance and factor level changes and disease severity in patients with early-onset ovarian insufficiency. Chin. J. Woman Child Health Res. 06, 1065–1068. doi:10.19829/j.zgfybj.issn.1001-4411.2023.06.024
Matsuda, F., Inoue, N., Manabe, N., and Ohkura, S. (2012). Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J. reproduction Dev. 58 (1), 44–50. doi:10.1262/jrd.2011-012
Mills, E. G., Yang, L., Nielsen, M. F., Kassem, M., Dhillo, W. S., and Comninos, A. N. (2021). The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocr. Rev. 42 (6), 691–719. doi:10.1210/endrev/bnab015
National Pharmacopoeia Committee (2020). Pharmacopoeia of the People 's Republic of China: volume I. 2020 Edition. Beijing: China Pharmaceutical Science and Technology Press.
Nelson, L. M. (2009). Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 360 (6), 606–614. doi:10.1056/NEJMcp0808697
Nemmar, A., Beegam, S., Zaaba, N. E., Alblooshi, S., Alseiari, S., and Ali, B. H. (2022). The salutary effects of catalpol on diesel exhaust particles-induced thrombogenic changes and cardiac oxidative stress, inflammation and apoptosis. Biomedicines 10 (1), 99. doi:10.3390/biomedicines10010099
Oh, K. O., Kim, S. W., Kim, J. Y., Ko, S. Y., Kim, H. M., Baek, J. H., et al. (2003). Effect of Rehmannia glutinosa Libosch extracts on bone metabolism. Clin. Chim. acta 334 (1-2), 185–195. doi:10.1016/s0009-8981(03)00238-9
Oyawoye, O., Abdel Gadir, A., Garner, A., Constantinovici, N., Perrett, C., and Hardiman, P. (2003). Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum. Reprod. Oxf. Engl. 18 (11), 2270–2274. doi:10.1093/humrep/deg450
Pastore, L. M., Christianson, M. S., Stelling, J., Kearns, W. G., and Segars, J. H. (2018). Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J. assisted reproduction Genet. 35 (1), 17–23. doi:10.1007/s10815-017-1058-4
Peng, H. S., Xu, C. Q., Yuan, Y., Zha, L. P., Chen, H. W., Guan, L., et al. (2019). The earliest excipient products of Traditional Chinese Medicine:Identification and analysis of samples from wooden lacquer box unearthed from Haihunhou tomb in the Western Han Dynasty. Chin. Sci. Bull. 09, 935–947. doi:10.1360/n972018-01176
Peng, X. X., Wang, D. Q., and Peng, H. S. (2018). Brief discussion on evolution and changes of processing of medicinal materials in "steamed for nine times and shined for nine times" in ancient materia medica. J. West Anhui Univ. 02, 92–99.
Qin, L. H. (2017). Clinical evaluation of hormone therapy combined with Liuwei Dihuang pills in the treatment of premature ovarian failure. Electron. J. Clin. Med. Literature 80, 15775. doi:10.16281/j.cnki.jocml.2017.80.108
Que, X. H., Gui, S. H., Zhong, Q. Y., Yu, B. B., and Lin, H. (2021). Effects of different degrees of Radix Rehmanniae on mouse ovaries and analysis of their cold and heat medicinal properties. Chin. Tradit. Pat. Med. 11, 3222–3226.
Rebar, R. W. (2009). Premature ovarian failure. Obstetrics Gynecol. 113 (6), 1355–1363. doi:10.1097/AOG.0b013e3181a66843
Ren, H., Li, K., Min, Y., Qiu, B., Huang, X., Luo, J., et al. (2023). Rehmannia glutinosa polysaccharides: optimization of the decolorization process and antioxidant and anti-inflammatory effects in LPS-stimulated porcine intestinal epithelial cells. Antioxidants Basel. Switz. 12 (4), 914. doi:10.3390/antiox12040914
Romero, A., San Hipólito-Luengo, Á., Villalobos, L. A., Vallejo, S., Valencia, I., Michalska, P., et al. (2019). The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell 18 (3), e12913. doi:10.1111/acel.12913
Shea, A. K., Buwembo, A., Mayhew, A., Sohel, N., Griffith, L. E., and Raina, P. (2021). The association between primary ovarian insufficiency and osteoporosis in the Canadian Longitudinal Study on Aging. Menopause 28 (6), 693–698. doi:10.1097/GME.0000000000001756
Shen, Q. X., Zhang, L. L., Lin, X. Y., Liang, Y. J., Liu, G. T., and Lu, R. L. (2020). Meta-analysis of the efficacy of Kuntai capsule combined with climen in the treatment of premature ovarian failure. Chin. J. Mod. Appl. Pharm. 01, 78–84. doi:10.13748/j.cnki.issn1007-7693.2020.01.015
Shi, J., Wang, J., Jia, N., and Sun, Q. (2023). A network pharmacology study on mechanism of resveratrol in treating preeclampsia via regulation of AGE-RAGE and HIF-1 signalling pathways. Front. Endocrinol. (Lausanne) 13, 1044775. doi:10.3389/fendo.2022.1044775
Stewart, J. A., Cashatt, D. O., Borck, A. C., Brown, J. E., and Carver, W. E. (2006). 17beta-estradiol modulation of angiotensin II-stimulated response in cardiac fibroblasts. J. Mol. Cell. Cardiol. 41 (1), 97–107. doi:10.1016/j.yjmcc.2006.04.019
Szeliga, A., Calik-Ksepka, A., Maciejewska-Jeske, M., Grymowicz, M., Smolarczyk, K., Kostrzak, A., et al. (2021). Autoimmune diseases in patients with premature ovarian insufficiency-our current state of Knowledge. Int. J. Mol. Sci. 22 (5), 2594. doi:10.3390/ijms22052594
Tanaka, S. S., Toyooka, Y., Akasu, R., Katoh-Fukui, Y., Nakahara, Y., Suzuki, R., et al. (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14 (7), 841–853. doi:10.1101/gad.14.7.841
Tang, Z. R., Zhang, R., Lian, Z. X., Deng, S. L., and Yu, K. (2019). Estrogen-receptor expression and function in female reproductive disease. Cells 8 (10), 1123. doi:10.3390/cells8101123
Tian, J. Y., Ma, F., and Han, L. Y. (2022). Analysis of composition differences of fresh Radix Rehmanniae, crude Radix Rehmanniae and processed Radix Rehmanniae using infrared spectroscopy. Spectrosc. Spectr. Analysis 10, 3203–3209.
Virant-Klun, I. (2015). Postnatal oogenesis in humans: a review of recent findings. Stem cells cloning Adv. Appl. 8, 49–60. doi:10.2147/SCCAA.S32650
Wan, Y. H., and Chen, X. Y. (2022). The effect and mechanism of Yijing decoction on the decline of ovarian reserve in rats. China Pharm. 14, 1730–1735.
Wang, B., Zhang, X. Y., Lv, C. Z., He, M. J., Meng, X. L., and Zhang, S. S. (2019). Process optimization for Rehmanniae Radix Praeparata with yellow wine stewing by multi-index-response surface method. Chin. Traditional Herb. Drugs 09, 2065–2073.
Wang, J., Luo, R., Zhao, X., Xia, D., Liu, Y., Shen, T., et al. (2023). Association between gut microbiota and primary ovarian insufficiency: a bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 14, 1183219. doi:10.3389/fendo.2023.1183219
Wang, M., Wang, Y. X., Wu, Y. R., Li, N., Feng, M., Zhang, W. Z., et al. (2020). Research progress on content changes of 5-hydroxymethyl furfural during processing of Chinese medicine and it's pharmacological effects. Drug Eval. Res. 11, 2319–2327.
Wang, R. H., Bai, Y., and Wang, H. Q. (2022). Correlation between estrogen levels and T lymphocyte subsets and PD-1 in peripheral blood in POI patients. Chin. J. Woman Child Health Res. 01, 60–65.
Wu, J., Yu, H., Jin, Y., Wang, J., Zhou, L., Cheng, T., et al. (2023). Ajugol's upregulation of TFEB-mediated autophagy alleviates endoplasmic reticulum stress in chondrocytes and retards osteoarthritis progression in a mouse model. Chin. Med. 18 (1), 113. doi:10.1186/s13020-023-00824-7
Wu, J., Zhuo, Y., Liu, Y., Chen, Y., Ning, Y., and Yao, J. (2021). Association between premature ovarian insufficiency and gut microbiota. BMC pregnancy childbirth 21 (1), 418. doi:10.1186/s12884-021-03855-w
Wu, S. J., Wang, L., Zhao, M. W., Zhong, S. M., Wu, W. G., and Li, W. L. (2022). Research progress in repeatedly steamed and sundried oftraditional Chinese medicine. Cent. South Pharm. 09, 2015–2022.
Xia, T., Dong, X., Jiang, Y., Lin, L., Dong, Z., Shen, Y., et al. (2019). Metabolomics profiling reveals Rehmanniae Radix preparata extract protects against glucocorticoid-induced osteoporosis mainly via intervening steroid hormone biosynthesis. Mol. Basel. Switz. 24 (2), 253. doi:10.3390/molecules24020253
Xie, P. P. (2021). Clinical trial and experimental study of Jiajian Yijing Decoction regulating Th17/Treg immune balance through CXCR4/SDF-1 to improve the decline of ovarian reserve function. Guangzhou University of Chinese Medicine. doi:10.27044/d.cnki.ggzzu.2021.000949
Xie, Y., Zhong, L. Y., Wang, Z., Song, J. J., Li, J. Q., Wang, Y. B., et al. (2022). Historical evolution and modern research progress of Rehmanniae Radix. Chin. J. Exp. Traditional Med. Formulae 02, 273–282. doi:10.13422/j.cnki.syfjx.20211546
Xu, J. X., Zhang, Y., and Mao, F. (2020). Observation of the clinical efficacy of Gui Kidney Pill on patients with ovarian insufficiency. J. Chin. Med. Mater. 03, 738–740. doi:10.13863/j.issn1001-4454.2020.03.043
Xu, L., Kwak, M., Zhang, W., Zeng, L., Lee, P. C., and Jin, J. O. (2017). Rehmannia glutinosa polysaccharide induces toll-like receptor 4 dependent spleen dendritic cell maturation and anti-cancer immunity. Oncoimmunology 6 (7), e1325981. doi:10.1080/2162402X.2017.1325981
Xue, S. J., Chen, Y., Chen, S. Q., and Che, Q. T. (2023). Simultaneous determination of eight saccharides contents in Radix Rehmanniae and its different processed products by HPLC-ELSD. Chin. J. Pharm. Analysis 06, 939–949. doi:10.16155/j.0254-1793.2023.06.04
Yan, S. T., Fan, H., Li, R. L., Wang, L. P., Luo, W. J., Gao, F., et al. (2020). Historical evolution and modern research of steamed for nine times and shined for nine times for Chinese medicine. China Pharm. 01, 136–141.
Yang, F., Hou, Z. F., Zhu, H. Y., Chen, X. X., Li, W. Y., Cao, R. S., et al. (2021b). Catalpol protects against pulmonary fibrosis through inhibiting TGF-β1/smad3 and wnt/β-catenin signaling pathways. Front. Pharmacol. 11, 594139. doi:10.3389/fphar.2020.594139
Yang, J., Wen, L., Jiang, Y., and Yang, B. (2019). Natural estrogen receptor modulators and their heterologous biosynthesis. Trends Endocrinol. metabolism 30 (1), 66–76. doi:10.1016/j.tem.2018.11.002
Yang, J. J., Zhang, L. H., Zhang, M. Y., Yang, M. X., Zou, L., Cui, Y., et al. (2024). Exploration of the dynamic variations of the characteristic constituents and the degradation products of catalpol during the process of Radix Rehmanniae. Mol. Basel. Switz. 29 (3), 705. doi:10.3390/molecules29030705
Yang, P. M. (2010). Comparison of the contents of catalpa alcohol and 5-hydroxymethylfurfural at different times of Radix Rehmanniae. China J. Traditional Chin. Med. Pharm. 07, 1096–1098.
Yang, S. H., Zhu, J., Wu, W. T., Li, J. M., Tong, H. L., Huang, Y., et al. (2023). Rhizoma Atractylodis Macrocephalae-Assessing the influence of herbal processing methods and improved effects on functional dyspepsia. Front. Pharmacol. 14, 1236656. doi:10.3389/fphar.2023.1236656
Yang, Y. H., Yang, H., Li, R. F., Li, C. X., Zeng, L., Wang, C. J., et al. (2021a). A Rehmannia glutinosa cinnamate 4-hydroxylase promotes phenolic accumulation and enhances tolerance to oxidative stress. Plant Cell Rep. 40 (2), 375–391. doi:10.1007/s00299-020-02639-4
Yuan, Y., Kang, N., Li, Q., Zhang, Y., Liu, Y., and Tan, P. (2019). Study of the effect of neutral polysaccharides from rehmannia glutinosa on lifespan of Caenorhabditis elegans. Mol. Basel. Switz. 24 (24), 4592. doi:10.3390/molecules24244592
Yue, W. (2009). Effect of Liuwei Dihuang Pill on the protein of nucleolar composition of ovarian granulosa cells in ovulatory disorder infertility. Shaanxi J. Traditional Chin. Med. 09, 1253–1254.
Zhang, H., Yue, Y., Zhang, Q., Liang, L., Li, C., Chen, Y., et al. (2023b). Structural characterization and anti-inflammatory effects of an arabinan isolated from Rehmannia glutinosa Libosch. Carbohydr. Polym. 303, 120441. doi:10.1016/j.carbpol.2022.120441
Zhang, J. M., and Lin, Y. P. (2023). Clinical study on yijing decoction combined with sequential therapy of estrogen and progesterone for premature ovarian failure with syndrome of kidney deficiency and liver depression. New Chin. Med. 04, 25–29. doi:10.13457/j.cnki.jncm.2023.04.006
Zhang, L. X., Wei, Y., Li, F. F., Li, Z. N., and Zhang, T. T. (2023a). Fingerprint pattern recognition and multi-component content determination of different processed products of Rehmannia glutinosa Libosch. Hubei Agric. Sci. 09, 135–141. doi:10.14088/j.cnki.issn0439-8114.2023.09.025
Zhang, W. T., Yue, C., Huang, Q. W., Yuan, K., Yan, A. J., and Shi, S. M. (2016). Contents of eight saccharides in unprocessed and processed Rehmannia glutinosa and content changes at different processing time points. Chin. Traditional Herb. Drugs 07, 1132–1136.
Zhang, X., Jin, C., Li, Y., Guan, S., Han, F., and Zhang, S. (2013). Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem. Toxicol. 58, 50–55. doi:10.1016/j.fct.2013.04.006
Zhang, X., Zhang, L., Xiong, L., Liu, X., Zhang, J., Yu, F., et al. (2024). Kuntai capsule for the treatment of diminished ovarian reserve: a systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 329, 118167. doi:10.1016/j.jep.2024.118167
Zhang, X. L., Jiang, B., Li, Z. B., Hao, S., and An, L. J. (2007). Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Pharmacol. Biochem. Behav. 88 (1), 64–72. doi:10.1016/j.pbb.2007.07.004
Zhang, Y. (2023). Awareness and ability of paradigm shift are needed for research on dominant diseases of TCM. Chin. Herb. Med. 15 (4), 475. doi:10.1016/j.chmed.2023.10.001
Zhang, Y., Sun, F. F., Yang, J. N., Zhang, J. S., and Zhang, Z. L. (2020). Establishment of thin layer chromatography identification method for different processed products of Rehmanniae Radix. Chin. J. Exp. Traditional Med. Formulae 15, 142–149. doi:10.13422/j.cnki.syfjx.20200849
Zhang, Y., Wang, Y., Wang, L., Zhang, Y., Qin, Y., Chen, T., et al. (2012). Effects of Rehmannia glutinosa oligosaccharide on human adipose-derived mesenchymal stem cells in vitro. Life Sci. 91 (25-26), 1323–1327. doi:10.1016/j.lfs.2012.10.015
Zhao, Y., Chen, H., Qiu, F., He, J., and Chen, J. (2023). Cognitive impairment and risks of osteoporosis: a systematic review and meta-analysis. Archives gerontology geriatrics 106, 104879. doi:10.1016/j.archger.2022.104879
Zhou, F. R. (2022). Exploring the mechanisms of Siwu Tang in improving premature ovarian failure: based on the " richness or deficiency in vessel" and angiogenesis in the ovary. Huazhong University of Science and Technology. doi:10.27157/d.cnki.ghzku.2022.002334
Zhou, X. H., Zu, Y. E., Liu, Q. Y., Liang, Y., and Huang, J. M. (2023). The effects of Kuntai capsule combined with fenmotong in the treatment of premature ovarian failure on endometrium, ovaries, antral follicles and sex hormones. Chin. J. Fam. Plan. 11, 2572–2576.
Zhou, Y. Q., He, F. Y., Yang, Y. T., Shi, J. L., Deng, K. W., Tang, Y., et al. (2014). Research situation of Maillard reaction and its influence on research methods for processing and preparation process of Chinese materia medica. Chin. Traditional Herb. Drugs 01, 125–130.
Zhu, H., Peng, H. S., Huang, L. Q., and Yuan, Y. (2021). Chinese ancient herbal processing: evidence of rice-steaming as the processing method of Rehmannia in Han Dynasty. Sci. Bull. 66 (4), 307–309. doi:10.1016/j.scib.2020.11.008
Zhu, J., Zhu, X. M., Shi, Y. H., Li, Q., Liu, X. D., Wang, Z., et al. (2022a). Research progress of Radix Rehmanniae and predictive analysis of its quality markers. J. Chin. Med. Mater. 05, 1273–1281. doi:10.13863/j.issn1001-4454.2022.05.044
Zhu, J. H., Wang, L., Ma, Z. X., Duan, J. A., and Tao, J. H. (2024). Rehmannia glutinosa Libosch and Cornus officinalis Sieb herb couple ameliorates renal interstitial fibrosis in CKD rats by inhibiting the TGF-β1/MAPK signaling pathway. J. Ethnopharmacol. 318 (Pt B), 117039. doi:10.1016/j.jep.2023.117039
Zhu, M., Zhou, G., and Duan, J. A. (2022b). Effect of Siwu decoction on intestinal microbiota in rats with low ovarian function. Chin. J. Exp. Traditional Med. Formulae 17, 25–32. doi:10.13422/j.cnki.syfjx.20221006
Zhu, W. Y., and Du, Y. F. (2023). Effect of Gui Kidney Pill on ovarian autophagy in a mouse model of hypoactive ovarian reserve. J. Hebei Traditional Chin. Med. Pharmacol. 04, 11–15. doi:10.16370/j.cnki.13-1214/r.2023.04.008
Zhu, X. D., Ding, Y. P., Yao, W., Wang, S., Chen, Y. H., Huang, L. P., et al. (2023). Based on UPLC-Q-TOF-MS ∼ E technology combined with UNIFI software, the characteristic chromatogram method of different processed products of Radix Rehmanniae was established. J. Chin. Med. Mater. 01, 73–78. doi:10.13863/j.issn1001-4454.2023.01.013
Keywords: Rehmanniae Radix, medicine processing, pharmacology, traditional Chinese medicine, ovarian hypofunction diseases
Citation: Zhong H-Z, Mo J, Li Y-X, Li M-Y and Wei S-B (2024) Changes in Rehmanniae Radix processing and their impact on ovarian hypofunction: potential mechanisms of action. Front. Pharmacol. 15:1426972. doi: 10.3389/fphar.2024.1426972
Received: 02 May 2024; Accepted: 06 June 2024;
Published: 03 July 2024.
Edited by:
Qianfeng Gong, Jiangxi University of Traditional Chinese Medicine, ChinaReviewed by:
Bo Zhang, Tsinghua University, ChinaCopyright © 2024 Zhong, Mo, Li, Li and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-Bin Wei, d2Vpc2hhb2JpbjU2MjBAMTYzLmNvbQ==; Mao-Ya Li, bG15OTQxMDI3QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.