- Department of Obstetrics and Gynaecology, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Background: As the development of novel anti-angiogenic drugs and the continuous evolution of guideline recommendations, the efficacy and safety of anti-angiogenic agents in ovarian cancer (OC) remains unclear. Consequently, a meta-analysis was carried out to assess the efficacy and safety of anti-angiogenic drug monotherapy and combination therapy for OC.
Methods: An exhaustive literature review was performed across multiple databases, including PubMed, Embase, Web of Science, and Cochrane, encompassing all relevant randomized controlled trials (RCTs) up until 6 April 2024. The evaluation of efficacy outcomes incorporated progression-free survival (PFS), overall survival (OS), and objective response rate (ORR). Safety was assessed through the occurrence of any grade adverse events (AEs) and grade ≥3 AEs. Synthesis of the data involved the calculation of hazard ratios (HRs), relative risks (RRs), and their corresponding 95% confidence intervals (CIs) and prediction intervals (PIs). Trial sequential analysis was executed employing TSA v0.9.5.10 Beta software, STATA 12.0, and R software 4.3.1.
Results: In this meta-analysis, 35 RCTs were included, encompassing 16,199 subjects in total. The overall analysis indicated that anti-angiogenic drug combination therapy significantly improved PFS (HR [95% CI] = 0.678 [0.606–0.759], 95% PI: 0.415–1.108), OS (HR [95% CI] = 0.917 [0.870–0.966], 95% PI: 0.851–0.984), and ORR (RR [95% CI] = 1.441 [1.287–1.614], 95% PI: 1.032–2.014), but also increased the incidence of grade ≥3 AEs (RR [95% CI] = 1.137 [1.099–1.177], 95% PI: 1.011–1.252). The analysis did not corroborate any benefit of anti-angiogenic monotherapy over placebo concerning PFS (HR [95% CI] = 0.956 [0.709–1.288], 95% PI: 0.345–2.645) and OS (HR [95% CI] = 1.039 [0.921–1.173], 95% PI: 0.824–1.331). However, it was observed that monotherapy with anti-angiogenic drugs did increase the incidence of any grade AEs (RR [95% CI] = 1.072 [1.036–1.109], 95% PI: 0.709–1.592).
Conclusion: Our study confirmed the PFS, OS, and ORR benefits of anti-angiogenic drug combination therapy for OC patients. The efficacy results of anti-angiogenic monotherapy necessitates further evaluation as more RCTs become available. Clinicians should be vigilant of AEs when administering anti-angiogenic agents in a clinical setting.
1 Introduction
Ovarian cancer (OC) stands as the primary cause of death related to gynecologic cancer and the fifth most prevalent malignancy, thereby posing a substantial global health risk to women (Siegel et al., 2021). The difficulty in early-stage detection of OC often leads to diagnoses at advanced stages, contributing to a reduced 5-year relative survival rate (Wang et al., 2021). The current standard of care for newly diagnosed patients typically encompasses cytoreductive surgery and platinum-based systemic chemotherapy, with the optional inclusion of bevacizumab. Even with optimal treatment leading to complete remission, about 70% of patients experience a recurrence within 5 years (Hope et al., 2010; McGee et al., 2017). Notably, recurrence rates are nearly 25% for those in early stages and exceed 80% in advanced stages (Garzon et al., 2020). Despite the availability of multiple active therapies for recurrent OC, such as targeted therapy (e.g., poly ADP-ribose polymerase [PARP] inhibitors), chemotherapy, and immunotherapy, the median survival post-recurrence remains less than 3 years, underscoring the critical need to explore new therapeutic options for this patient group (Richardson et al., 2018).
New therapeutic agents, particularly those inhibiting angiogenesis, have shown considerable potential for the treatment of OC. Aberrant angiogenesis, a defining characteristic of solid tumors, is instrumental in tumor advancement (Jászai et al., 2019). By interfering with the formation of blood vessels, anti-angiogenic medications impede the nutrient supply to tumor cells, both by causing damage to the established tumor vasculature and by blocking the creation of new blood vessels (Abdalla et al., 2018). Additionally, these treatments may induce normalization of the tumor vasculature, reversing tumor microenvironment hypoxia, reducing the tumor’s aggressive nature, and augmenting the effectiveness of traditional therapies (Teleanu et al., 2019). The efficacy of angiogenesis inhibitors results from intricate interactions among various pathways, including numerous angiogenic factors like angiopoietin, vascular endothelial growth factor (VEGF), and VEGF receptor (VEGFR) (Saman et al., 2020). Recently, a variety of anti-VEGF strategies, including monoclonal antibodies against VEGF (for instance, bevacizumab) and VEGFR inhibitors (such as cediranib, pazopanib, sorafenib, and apatinib), have undergone evaluation in OC patients (Monk et al., 2016a). The AURELIA trial, a phase III randomized trial, revealed that OC patients experienced a notable extension in progression-free survival (PFS) when treated with a regimen of bevacizumab in combination with chemotherapy versus chemotherapy alone. The trial also recorded an enhancement in the objective response rate (ORR) by 15.5% over chemotherapy exclusively. Nonetheless, the addition of bevacizumab to chemotherapy did not yield a statistically significant increase in overall survival (OS) (Pujade-Lauraine et al., 2014).
In the comparison of combined therapy involving angiogenesis inhibitors and standard chemotherapy versus conventional chemotherapy alone, a number of randomized controlled trials (RCTs) have illustrated an enhancement in PFS. Nevertheless, debates persist regarding the OS advantage and the safety profile of these combined treatments (Chekerov et al., 2018; Ray-Coquard et al., 2020; Pignata et al., 2021). Prior meta-analyses have explored the efficacy and toxicity of anti-angiogenic drugs in different subtypes of OC (Yi et al., 2017; Helali et al., 2022; Zhang et al., 2022). However, there is a lack of a comprehensive meta-analysis to evaluate the effects of monotherapy or combination therapy with anti-angiogenic drugs on OC. Moreover, multiple RCTs have published the latest relevant clinical results in recent years (Liu et al., 2022; Roque et al., 2022; Ferron et al., 2023; Nicum et al., 2024). Therefore, we conducted a meta-analysis to systematically assess the efficacy and safety of anti-angiogenic drug monotherapy or combined with chemotherapy or PARP inhibitors in the treatment of OC.
2 Materials and methods
2.1 Study design
The methodology and reporting of our study were aligned with the guidelines delineated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). Furthermore, our study protocol was registered within the PROSPERO database (registration number: CRD42024534864). Given the nature of this research as a meta-analysis synthesizing findings from existing literature, it does not necessitate ethical approval and informed consent, as it neither engages with ethics nor patient privacy.
2.2 Search strategy
Our comprehensive search encompassed the databases of PubMed, Web of Science, Embase, and the Cochrane Library of clinical trials, aiming to identify all relevant articles published in English until 6 April 2024. The key search terms employed were: (“anti-angiogenic”, “angiogenesis inhibitor”, “antiangiogenetic”, “anti-angiogenesis”, “vascular endothelial growth factor”, “VEGF”, “VEGFR”, “anti-VEGF”) OR (“bevacizumab”, “cediranib”, “pazopanib”, “afibercept”, “nintedanib”, “sorafenib”, “trebananib”, “avastin”, “recentin”, “votrient”, “perifosine”) AND (“ovar*” AND “cancer*”, “tumor*”, “tumour*”, “carcinoma*”, “neoplasm*”, “malignan*”). A thorough description of the search strategy can be found in Supplementary Files S1. We also manually scrutinized references cited in pertinent review articles to uncover additional studies that may meet the eligibility criteria.
2.3 Study selection
Eligibility for study selection was determined by the following criteria: 1) RCTs; 2) the participants are adult women (aged 18 and above) diagnosed with OC at any stage through histological examination; 3) intervention: monotherapy with anti-angiogenic medication or its combination with chemotherapy or PARP inhibitors; 4) comparison: treatment with placebo alone or chemotherapy (alone or plus placebo) or PARP inhibitors (alone or plus placebo); 5) outcomes: PFS, OS, ORR, adverse events (AEs) of any grade, or grade ≥3 AEs. Studies were excluded based on the following: 1) retrospective studies and non-interventional, non-comparative or single-arm trials; 2) studies lacking pertinent outcomes or presenting duplicated data; 3) trial design involving both the intervention and control groups receiving anti-angiogenic drugs; 4) literature reviews, case reports, conference abstracts, commentaries, and study protocols.
2.4 Data extraction and quality assessment
Two independent reviewers conducted the study screening, selection, exclusion, and extraction of data. From each RCT, we collated details such as the name of the lead author, year of publication, trial name and phase, patient condition, variety of anti-angiogenic medication used, number of participants and their median age, the doses and cycles of drugs used in the anti-angiogenic agent treatment group and the control group, duration of follow-up, and the outcomes in meta-analysis. PFS and OS were designated as the primary endpoints for this meta-analysis, with ORR, AEs of any grade, and grade ≥3 AEs serving as secondary endpoints. When encountering multiple reports from a single trial, preference was given to the most updated or complete report offering the necessary details. If PFS or OS outcomes were not available directly, the Engauge Digitizer Version 10.8 tool (available at http://markummitchell.github.io/engauge-digitizer/) and Tierney et al.’s proposed methodology (Tierney et al., 2007) were employed to derive data from Kaplan-Meier curves (Xie et al., 2022).
The quality of the RCTs was evaluated utilizing the modified Jadad scale (Jadad et al., 1996), which includes criteria such as the process of randomization, concealment of randomization, implementation of double-blinding, and the tracking of withdrawals and dropouts. Trials were categorized based on their quality with scores ranging from 0 to 3 indicating low quality, while scores from 4 to 7 signified high-quality research.
2.5 Statistical analysis
Statistical analyses were carried out using R software Version 4.3.1 and STATA Version 12.0. We calculated the combined hazard ratios (HRs) along with their 95% confidence intervals (CIs) for both PFS and OS. Dichotomous data outcomes were synthesized by computing relative risks (RRs) and delineating these with 95% CIs. We employed I2 statistics, Cochran’s Q test, and the 95% prediction interval (PI) to assess heterogeneity across studies (Bowden et al., 2011; IntHout et al., 2016). Findings with I2 exceeding 50% or a p-value less than 0.10 were deemed to show significant heterogeneity, prompting the use of a random-effects model; if not, we used the fixed-effects model (Higgins et al., 2002). We performed subgroup analysis considering OC subtypes or the types of anti-angiogenic agents. To identify potential sources of heterogeneity, we conducted a sensitivity analysis. Furthermore, the trim-and-fill method was employed to detect and adjust for any publication bias (Duval et al., 2000). A two-sided p < 0.05 was considered statistically significant.
2.6 Trial sequential analysis
We conducted a trial sequential analysis (TSA) to determine whether the compiled data met the required information size (RIS) for a conclusive finding (Wetterslev et al., 2017). This methodological approach, applied to dichotomous outcomes, utilized TSA software v0.9.5.10 Beta (accessible at www.ctu.dk/tsa). The RIS was calculated, and O’Brien-Fleming α-spending boundaries were established, based on a 5% type I error and a 20% type II error, both set for two-side tests. We engaged STATA Version 12.0, employing the metacumbounds and rsource function, and R software Version 4.3.1, using the foreign and ldbounds packages, to execute TSA on the PFS and OS data, adopting the a priori information size (APIS) approach. The crossing of the cumulative Z-curve over the trial sequential monitoring boundary or the RIS (or APIS) threshold was interpreted as an indication that no additional trials are necessary, and the evidence could be considered conclusive.
3 Results
3.1 Literature search
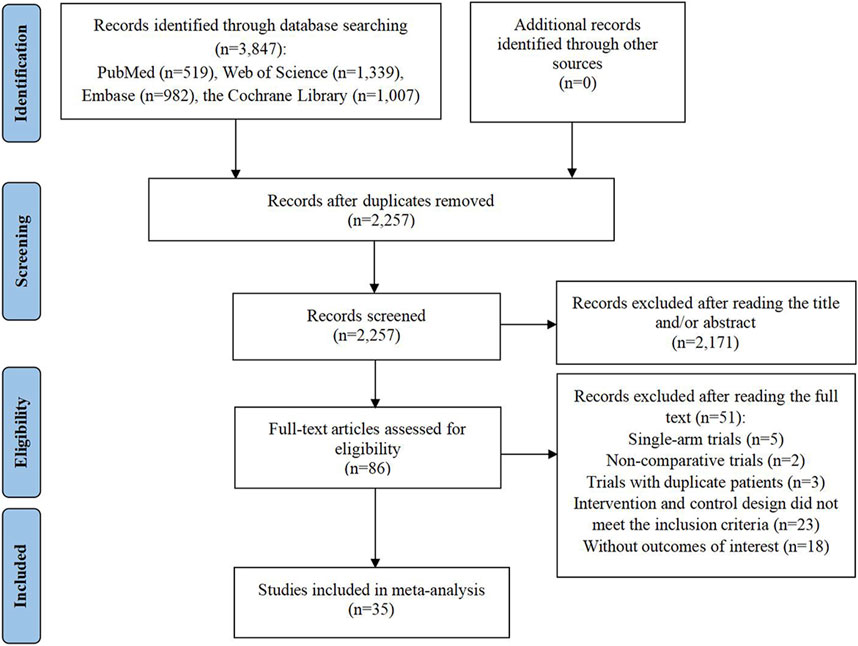
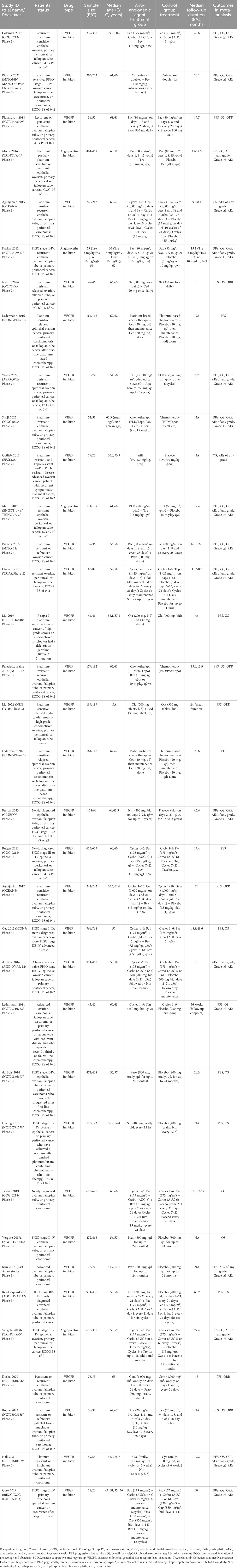
The preliminary search of the database yielded 3,847 entries. Following the removal of 1,590 duplicate entries, a set of 2,257 records persisted for further scrutiny. Out of these, 2,171 were discarded due to irrelevance indicated by their titles or abstracts, leaving 86 articles for full-text review regarding their eligibility. Upon detailed examination, 51 studies were deemed unfit for inclusion: 5 were single-arm clinical trials; 2 were non-comparative clinical studies; 3 trials included duplicate patient data; 23 trials exhibited intervention and control designs that did not align with the inclusion criteria; and 18 articles failed to report the necessary outcome data. Finally, 35 RCTs were selected for inclusion in the meta-analysis (Aghajanian et al., 2012; Aghajanian et al., 2015; Burger et al., 2011; Chekerov et al., 2018; Coleman et al., 2017; du Bois et al., 2014; du Bois et al., 2016; Duska et al., 2020; Ferron et al., 2023; Gore et al., 2019; Gotlieb et al., 2012; Hall et al., 2020; Herzog et al., 2013; Karlan et al., 2012; Kim et al., 2018; Ledermann et al., 2021; Ledermann et al., 2016; Ledermann et al., 2011; Liu et al., 2019; Liu et al., 2022; Marth et al., 2017; Monk et al., 2016b; Nicum et al., 2024; Oza et al., 2015; Pignata et al., 2021; Pignata et al., 2015; Pujade-Lauraine et al., 2014; Ray-Coquard et al., 2020; Richardson et al., 2018; Roque et al., 2022; Shoji et al., 2022; Tewari et al., 2019; Vergote et al., 2019a; Vergote et al., 2019b; Wang et al., 2022) (Figure 1).
3.2 Study characteristics and quality assessment
Table 1 provided a detailed overview of the characteristics of the RCTs and the participants that were incorporated into the study. This research encompassed a total of 35 RCTs, which included 15 phase 2 trials and 20 phase 3 trials, all of which were published in English between the years 2011 and 2024. The study population consisted of 8,839 OC patients who were assigned to the anti-angiogenic agent treatment group, while 7,360 patients were administered either a placebo alone or underwent drug therapy that did not involve anti-angiogenic agents. The anti-angiogenic drugs utilized were categorized into VEGF inhibitors (specifically bevacizumab), VEGFR inhibitors (which included pazopanib, cediranib, apatinib, sorafenib, and nintedanib), and angiopoietin inhibitors (solely trebananib). The design of anti-angiogenic therapy was bifurcated into monotherapy with anti-angiogenic drugs and combination therapy with chemotherapy or PARP inhibitors. The corresponding control design was either placebo alone, chemotherapy (alone or plus placebo), or PARP inhibitors only. Notably, the only PARP inhibitor used in the trials was olaparib. Out of the 35 RCTs, 31 were assessed as high quality, whereas 4 were deemed low quality. A notable methodological limitation observed was the lack of double-blinding in the trial design among multiple RCTs (Supplementary Files S2).
3.3 Overall analysis of anti-angiogenic drug monotherapy
5 RCTs were conducted to evaluate the PFS benefit of anti-angiogenic drug monotherapy in OC patients. Owing to substantial heterogeneity observed across these trials, a random-effects model was employed for analysis (I2 = 72.1%, Tau2 = 0.0791). The combined estimate indicated that anti-angiogenic monotherapy did not provide a significant PFS advantage over placebo (HR [95% CI] = 0.956 [0.709–1.288], 95% PI: 0.345–2.645). Similarly, the consolidated results from a fixed-effects model (I2 = 8.6%, Tau2 = 0.0027), derived from 6 RCTs, revealed that anti-angiogenic drug monotherapy did not significantly enhance OS (HR [95% CI] = 1.039 [0.921–1.173], 95% PI: 0.824–1.331). A single study reported on the ORR associated with monotherapy (Ferron et al., 2023), revealing a lower ORR with the use of anti-angiogenic monotherapy (specifically nintedanib) as compared to placebo (RR [95% CI] = 0.628 [0.447–0.882]). Concerning AEs, pooled results from 3 trials suggested that the incidence of any grade AEs was significantly higher with anti-angiogenic monotherapy compared to placebo (RR [95% CI] = 1.072 [1.036–1.109], 95% PI: 0.709–1.592; I2 = 40.1%, Tau2 = 0.0006). However, there was no significant difference in the risk of grade ≥3 AEs between the monotherapy group and the control group (RR [95% CI] = 1.905 [0.766–4.736]; I2 = 95.0%, Tau2 = 0.6005) (Table 2; Figure 2).
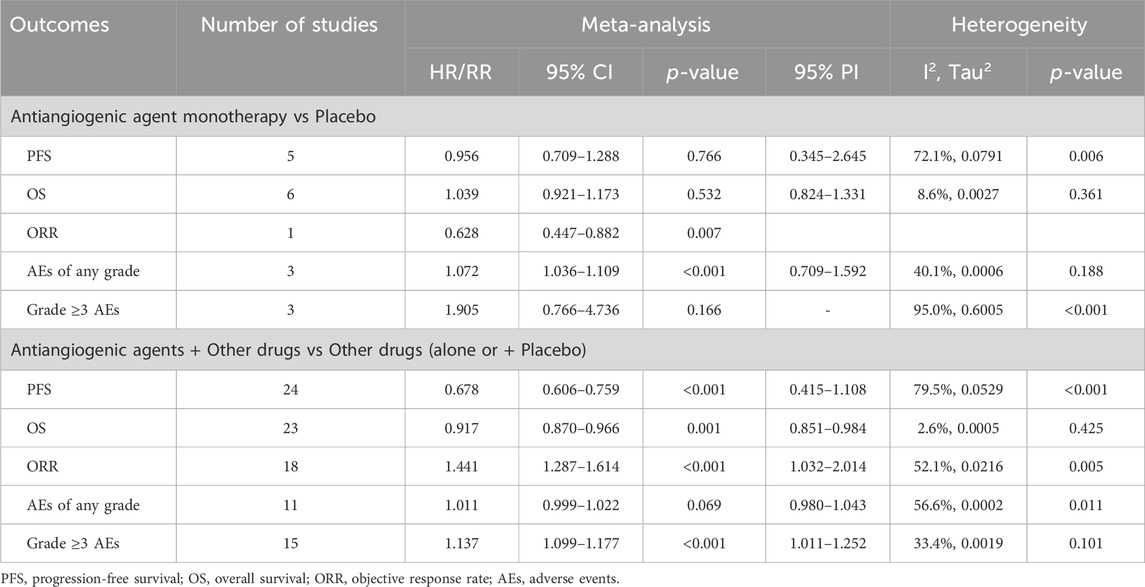
Table 2. Pooled effect of the efficacy and safety of monotherapy or combination therapy with anti-angiogenic drugs in the treatment of ovarian cancer.
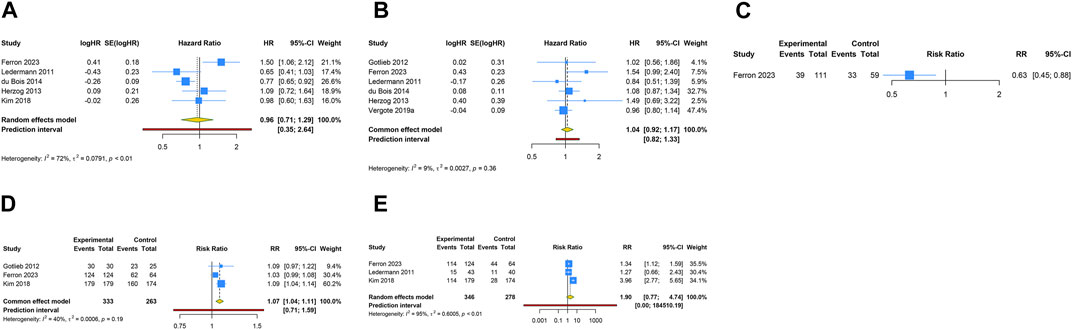
Figure 2. Forest plot of the efficacy and safety outcomes after anti-angiogenic agent monotherapy for ovarian cancer. (A) Progression-free survival; (B) Overall survival; (C) Objective response rate; (D) Adverse events of any grade; (E) Grade ≥3 adverse events.
3.4 Overall analysis of anti-angiogenic drug combination therapy
A total of 24 RCTs evaluated PFS advantage of anti-angiogenic drug combination therapy in patients with OC. Given the notable heterogeneity in the studies regarding PFS, a random-effects model was utilized for the pooled PFS analysis (I2 = 79.5%, Tau2 = 0.0529). The overall analysis revealed that the combination therapy of anti-angiogenic drugs led to a 32.2% decrease in the risk of disease progression or death when contrasted with regimens excluding anti-angiogenic drugs (HR [95% CI] = 0.678 [0.606–0.759], 95% PI: 0.415–1.108). Likewise, the pooled findings from a fixed-effects model (I2 = 2.6%, Tau2 = 0.0005), based on 23 RCTs, demonstrated a significant improvement in OS when anti-angiogenic drugs were used in combination therapy compared with the control (HR [95% CI] = 0.917 [0.870–0.966], 95% PI: 0.851–0.984). Furthermore, 18 studies reported the ORR outcome of combination therapy, and the results showed that the ORR of anti-angiogenic agents combined with other drugs was significantly higher than that of other drugs alone (RR [95% CI] = 1.441 [1.287–1.614], 95% PI: 1.032–2.014; I2 = 52.1%, Tau2 = 0.0216). Regarding AEs, the consolidated results from 11 trials indicated no significant difference in the risk of any grade AEs between the combination therapy group and the control group (RR [95% CI] = 1.011 [0.999–1.022], 95% PI: 0.980–1.043; I2 = 56.6%, Tau2 = 0.0002). However, the occurrence of grade ≥3 AEs was significantly increased in the combination therapy group compared to the control group (RR [95% CI] = 1.137 [1.099–1.177], 95% PI: 1.011–1.252; I2 = 33.4%, Tau2 = 0.0019) (Table 2; Figure 3).
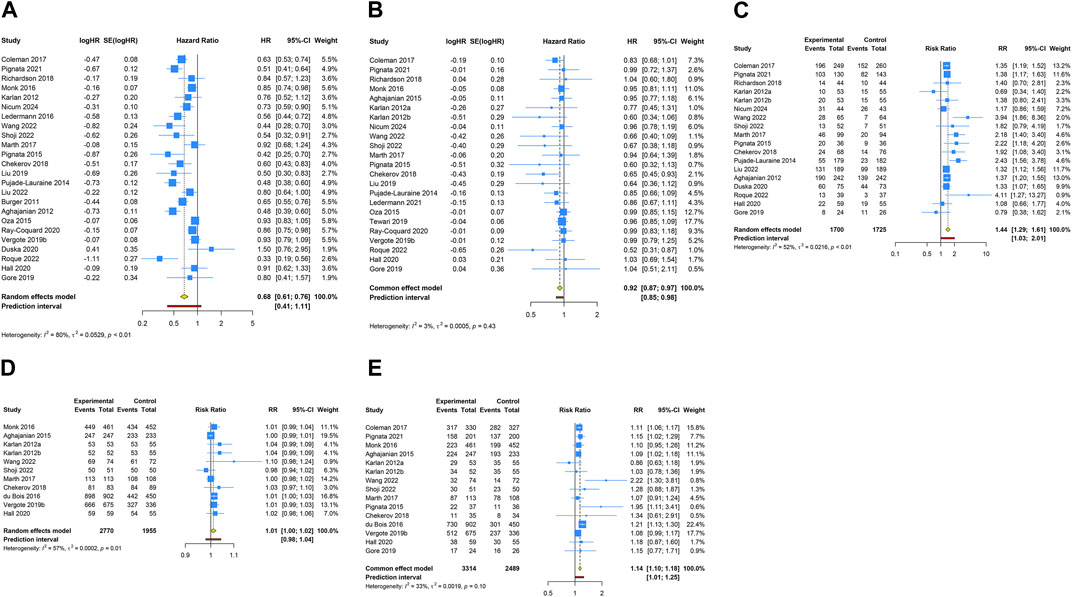
Figure 3. Forest plot of the efficacy and safety outcomes after anti-angiogenic drug combination therapy for ovarian cancer. (A) Progression-free survival; (B) Overall survival; (C) Objective response rate; (D) Adverse events of any grade; (E) Grade ≥3 adverse events.
3.5 Subgroup analysis of anti-angiogenic drug monotherapy
Subgroup analyses were conducted only for categories comprising two or more studies. When stratified by OC subtype, it was observed that anti-angiogenic drug monotherapy escalated the risk of any grade AEs in patients with advanced OC, relative to placebo (RR [95% CI] = 1.072 [1.036–1.109], 95% PI: 0.709–1.592; I2 = 40.1%, Tau2 = 0.0006). Yet, in the context of advanced OC, no significant impact on PFS, OS, or the occurrence of grade ≥ 3AEs was observed with anti-angiogenic drug monotherapy (all p > 0.05). Further, stratified analyses predicated on the classification of anti-angiogenic drugs revealed an increased incidence of any grade AEs with VEGFR inhibitors compared to placebo (RR [95% CI] = 1.059 [1.003–1.119]; I2 = 68.2%, Tau2 = 0.0011). Subsequent analysis grouped by specific anti-angiogenic agents suggested that pazopanib significantly improved PFS (HR [95% CI] = 0.791 [0.670–0.934]; I2 = 0%, Tau2 = 0), while nintedanib was associated with a higher incidence of grade ≥3 AEs (RR [95% CI] = 1.326 [1.109–1.586]; I2 = 0%, Tau2 = 0). The complete results of the subgroup analysis were detailed in Table 3 and Supplementary Figure S1–S4.
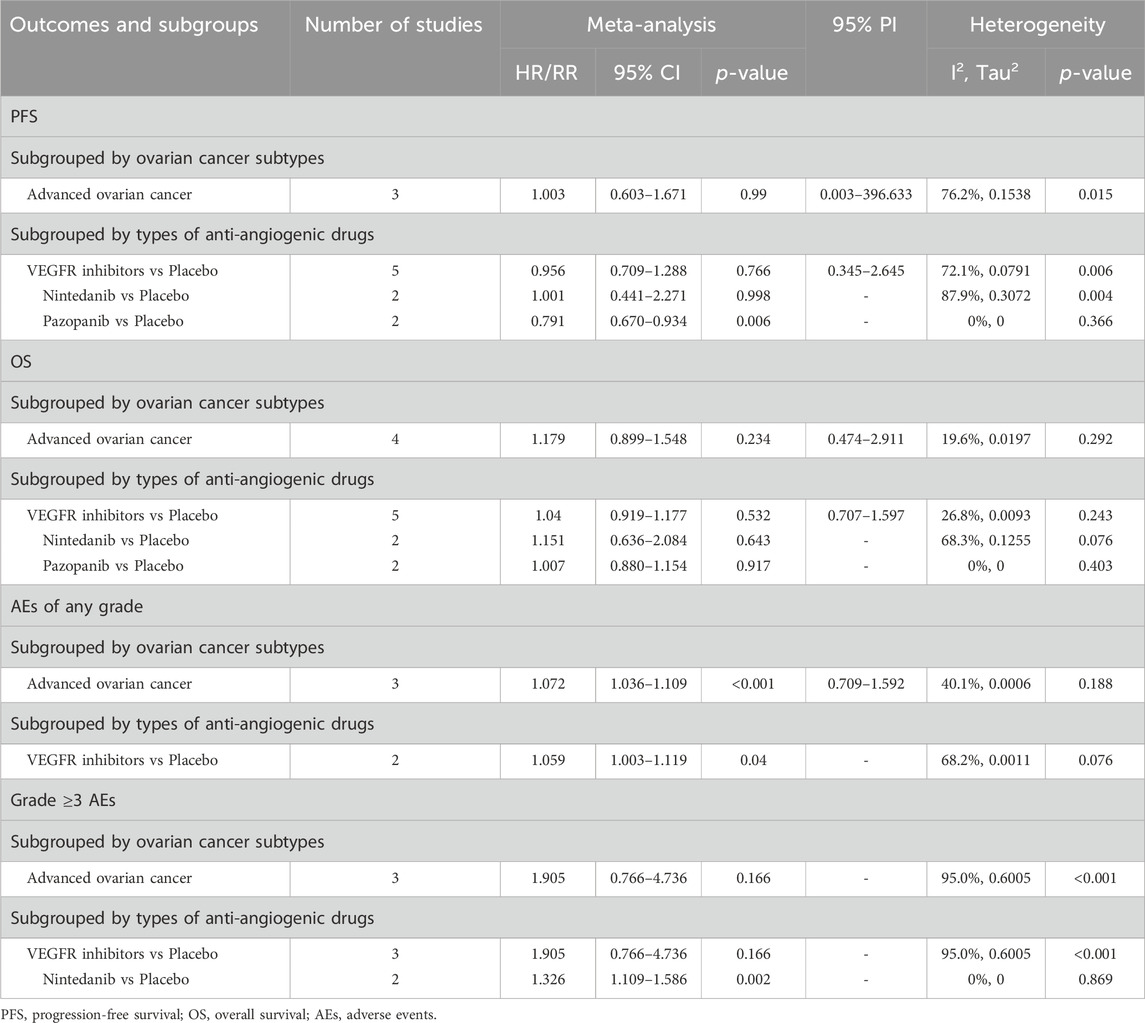
Table 3. Subgroup analysis of the efficacy and safety of anti-angiogenic agent monotherapy for ovarian cancer.
3.6 Subgroup analysis of anti-angiogenic drug combination therapy
Subgroup analyses were carried out solely for groups that included two or more studies. Categorized by OC subtypes, it was observed that anti-angiogenic drug combination therapy significantly improved PFS compared with drug therapy without anti-angiogenic agents in patients with platinum-sensitive and recurrent OC (HR [95% CI] = 0.612 [0.519–0.722], 95% PI: 0.355–1.055; I2 = 78.5%, Tau2 = 0.0460), platinum-resistant OC (HR [95% CI] = 0.691 [0.494–0.966], 95% PI: 0.019–25.494; I2 = 59.4%, Tau2 = 0.0514), newly diagnosed OC (HR [95% CI] = 0.807 [0.657–0.990], 95% PI: 0.066–9.808; I2 = 85.3%, Tau2 = 0.0278), and platinum-resistant or refractory OC (HR [95% CI] = 0.374 [0.259–0.540]; I2 = 0%, Tau2 = 0). Similarly, it was noted that combination therapy with anti-angiogenic drugs was associated with a significant improvement in OS among patients with platinum-sensitive and recurrent OC (HR [95% CI] = 0.892 [0.822–0.968], 95% PI: 0.806–0.988; I2 = 0%, Tau2 = 0), platinum-resistant OC (HR [95% CI] = 0.753 [0.592–0.956], 95% PI: 0.146–3.886; I2 = 2.6%, Tau2 = 0.0013), and platinum-resistant or refractory OC (HR [95% CI] = 0.551 [0.369–0.821]; I2 = 0%, Tau2 = 0). Moreover, the combined therapeutic approach of anti-angiogenic drugs exhibited a comparatively high ORR for patients with platinum-sensitive and recurrent OC, platinum-resistant OC, recurrent or persistent OC, platinum-resistant or refractory OC, and advanced OC (all p < 0.05). However, it is important to note that for individuals with advanced OC, combination therapy with anti-angiogenic drugs can also lead to a higher incidence of any grade AEs (RR [95% CI] = 1.014 [1.003–1.025], 95% PI: 0.948–1.085; I2 = 0%, Tau2 = 0) and grade ≥3 AEs (RR [95% CI] = 1.151 [1.097–1.209], 95% PI: 0.921–1.428; I2 = 34.2%, Tau2 = 0.0015). Particularly, patients with platinum-sensitive and recurrent OC receiving combination therapy experienced an elevated frequency of grade ≥3 AEs (RR [95% CI] = 1.120 [1.036–1.210], 95% PI: 0.836–1.500; I2 = 57.8%, Tau2 = 0.0031) (Table 4; Supplementary Figure S5–S9).
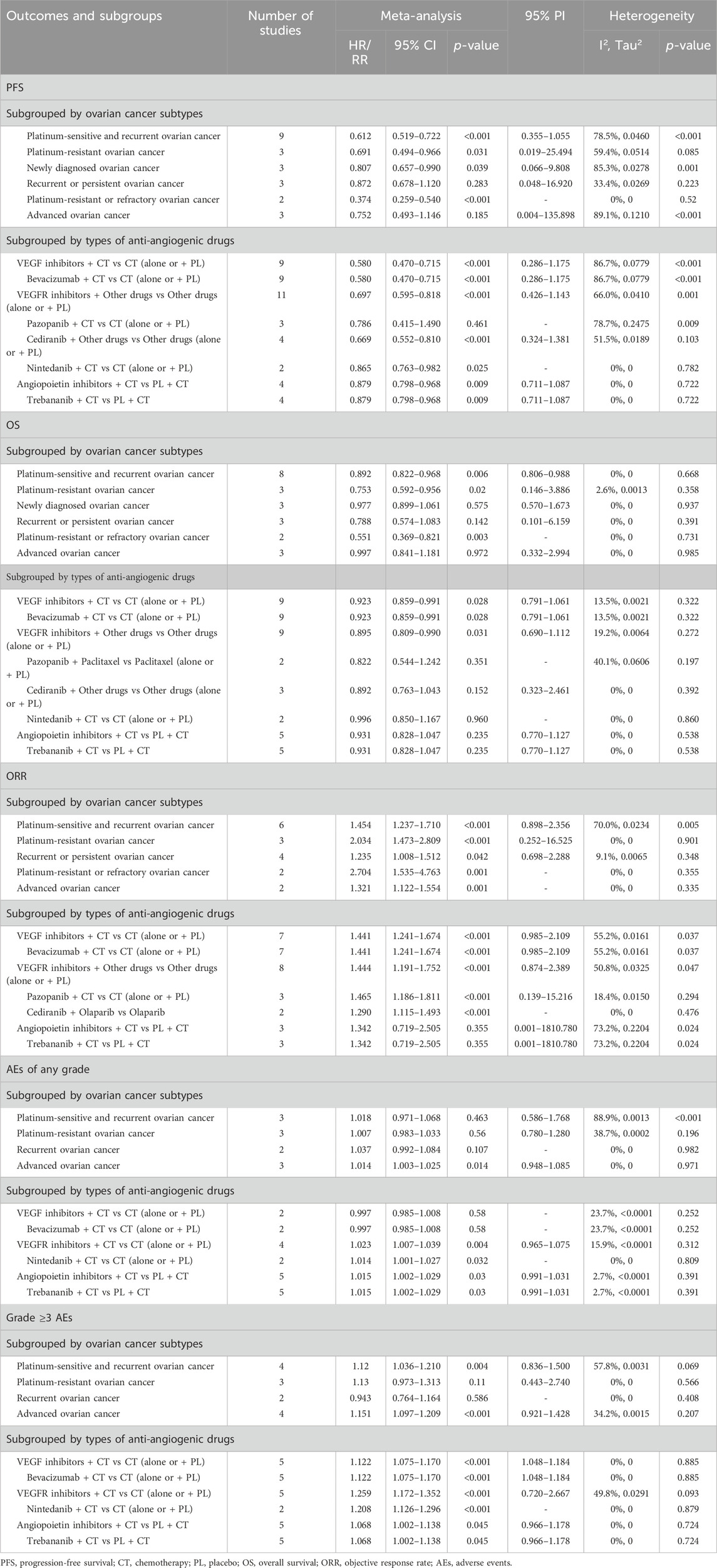
Table 4. Subgroup analysis of the efficacy and safety of anti-angiogenic agent combination therapy for ovarian cancer.
Subgroup analysis according to the types of anti-angiogenic drugs indicated that VEGF inhibitors combined with chemotherapy significantly improved PFS (HR [95% CI] = 0.580 [0.470–0.715], 95% PI: 0.286–1.175; I2 = 86.7%, Tau2 = 0.0779) and OS (HR [95% CI] = 0.923 [0.859–0.991], 95% PI: 0.791–1.061; I2 = 13.5%, Tau2 = 0.0021), and also increased the ORR (RR [95% CI] = 1.441 [1.241–1.674], 95% PI: 0.985–2.109; I2 = 55.2%, Tau2 = 0.0161) and the risk of grade ≥3 AEs (RR [95% CI] = 1.122 [1.075–1.170], 95% PI: 1.048–1.184; I2 = 0%, Tau2 = 0) compared with chemotherapy alone or with placebo. These results were replicated in the combination therapy with bevacizumab. In addition, combination therapy with VEGFR inhibitors was found to be associated with improvements in PFS (HR [95% CI] = 0.697 [0.595–0.818], 95% PI: 0.426–1.143; I2 = 66.0%, Tau2 = 0.0410) and OS (HR [95% CI] = 0.895 [0.809–0.990], 95% PI: 0.690–1.112; I2 = 19.2%, Tau2 = 0.0064), along with an increase in the ORR (RR [95% CI] = 1.444 [1.191–1.752], 95% PI: 0.874–2.389; I2 = 50.8%, Tau2 = 0.0325). Yet, VEGFR inhibitor combination therapy also increased the incidence of any grade AEs (RR [95% CI] = 1.023 [1.007–1.039], 95% PI: 0.965–1.075; I2 = 15.9%, Tau2 < 0.0001) and grade ≥3 AEs (RR [95% CI] = 1.259 [1.172–1.352], 95% PI: 0.720–2.667; I2 = 49.8%, Tau2 = 0.0291). Further analysis grouped by specific anti-angiogenic agents suggested that combination therapy with cediranib significantly improved PFS and ORR. A similar enhancement in ORR was observed with pazopanib combination therapy. The nintedanib combination therapy, while improving PFS, also escalated the risk of any grade AEs and grade ≥3 AEs (all p < 0.05). With regard to angiopoietin inhibitors, the combined therapeutic strategy significantly improved PFS (HR [95% CI] = 0.879 [0.798–0.968], 95% PI: 0.711–1.087; I2 = 0%, Tau2 = 0), but it also led to an increase in the occurrence of any grade AEs (RR [95% CI] = 1.015 [1.002–1.029], 95% PI: 0.991–1.031; I2 = 2.7%, Tau2 < 0.0001) and grade ≥3 AEs (RR [95% CI] = 1.068 [1.002–1.138], 95% PI: 0.966–1.178; I2 = 0%, Tau2 = 0). The identical results were also observed in the combination therapy with trebananib (Table 4; Supplementary Figure S10–S14).
3.7 Trial sequential analysis results
In the execution of TSA for both PFS and OS, the analysis necessitated an APIS of 1,990. It was noted that in the monotherapy analysis with anti-angiogenic drugs, only the cumulative Z-curve for OS and any grade AEs breached the RIS threshold, albeit without breaching the trial sequential monitoring boundary. Theses results indicated the possibility of deriving a relatively solid conclusion. However, the cumulative Z-curve for PFS and grade ≥3 AEs in the same monotherapy analysis neither crossed the trial sequential monitoring boundary nor the RIS threshold, implying that the results are inconclusive and may include false positives (Figure 4). In the scenario of combination therapy with anti-angiogenic drugs, every cumulative Z-curve successfully crossed either the trial sequential monitoring boundary or the RIS threshold, suggesting that additional research is not necessary for a conclusive result (Figure 5).
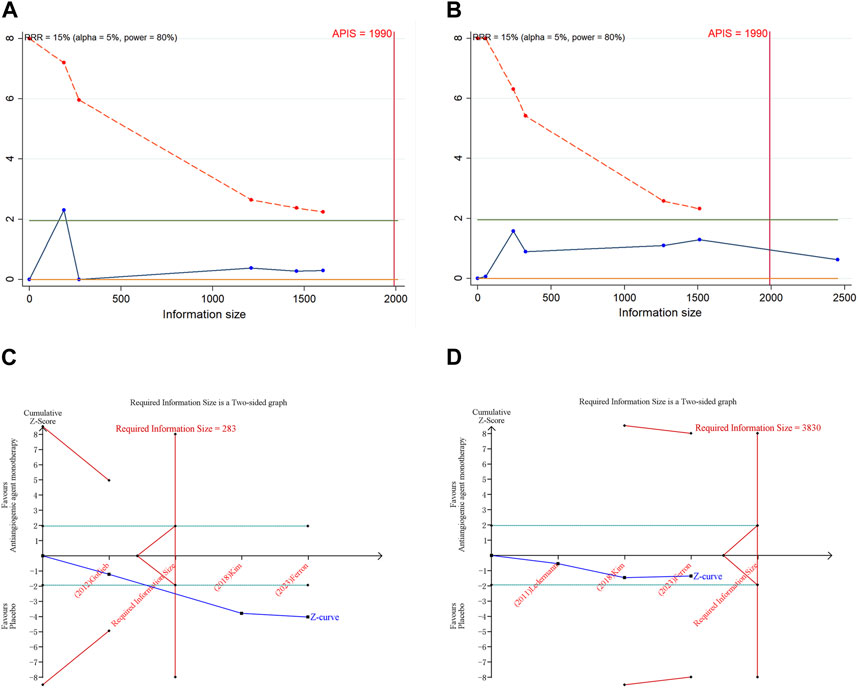
Figure 4. Trial sequential analysis of anti-angiogenic agent monotherapy for ovarian cancer. (A) Progression-free survival; (B) Overall survival; (C) Adverse events of any grade; (D) Grade ≥3 adverse events. Uppermost and lowermost red curves represent trial sequential monitoring boundary lines for benefit and harm, respectively. Inner red lines represent the futility boundary. Blue line represents evolution of cumulative Z-score. Horizontal green lines represent the conventional boundaries for statistical significance. Cumulative Z-curve crossing the trial sequential monitoring boundary or the RIS boundary provides firm evidence of effect.
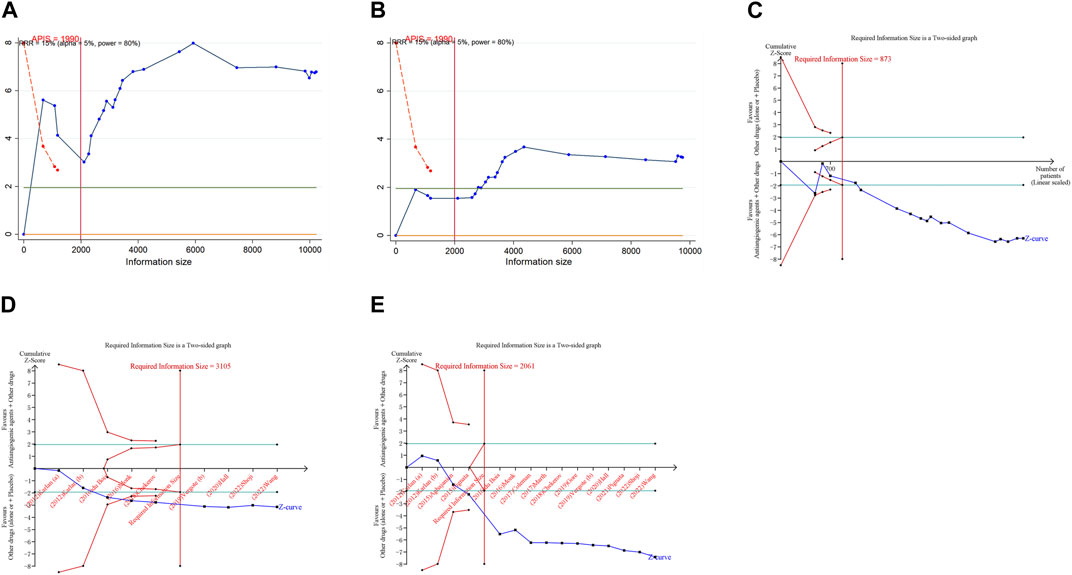
Figure 5. Trial sequential analysis of anti-angiogenic drug combination therapy for ovarian cancer. (A) Progression-free survival; (B) Overall survival; (C) Objective response rate; (D) Adverse events of any grade; (E) Grade ≥3 adverse events. Uppermost and lowermost red curves represent trial sequential monitoring boundary lines for benefit and harm, respectively. Inner red lines represent the futility boundary. Blue line represents evolution of cumulative Z-score. Horizontal green lines represent the conventional boundaries for statistical significance. Cumulative Z-curve crossing the trial sequential monitoring boundary or the RIS boundary provides firm evidence of effect.
3.8 Sensitivity analysis and publication bias
We performed sensitivity analyses and publication bias tests on the combined results that included more than 10 studies. The sensitivity analysis entailed the computation of pooled HRs or RRs along with their respective 95% CIs, excluding individual studies to ascertain if a single study significantly influenced the combined results. The sensitivity analysis demonstrated that the exclusion of any single study did not significantly impact the quantitative findings, which implies that the combined results from the anti-angiogenic drug combination therapy are both robust and dependable (Supplementary Figure S15). We also conducted a trim-and-fill analysis, yielding funnel plots with imputed studies for the outcomes of ORR, any grade AEs, and grade ≥3 AEs, indicating the potential for publication bias (Supplementary Figure S16). However, the trim-and-fill analysis correction for possible publication bias did not change the results for ORR, AEs of any grade, and grade ≥3 AEs, suggesting that the presence of publication bias did not significantly affect the final results.
4 Discussion
The progression of OC and the standard physiological processes of the ovary are both substantially reliant on angiogenesis. The growth and advancement of malignancies necessitate angiogenesis, as tumors cannot exceed 1–2 mm in size without adequate neovascularization. Consequently, anti-angiogenic drugs have been incorporated into OC treatment regimens. The VEGF pathway is the most extensively studied in the process of neovascularization. VEGF initiates the formation of new blood vessels, which is then sustained by platelet-derived growth factor, fibroblast growth factor, and angiopoietin-1 and -2 (Fernando et al., 2008; Timke et al., 2008; Ionescu et al., 2011). Overexpression of VEGF is associated with the tumor’s prognosis and stage (Nusrat et al., 2016). A number of angiogenesis inhibitors targeting this pathway, including bevacizumab, cediranib, sorafenib, pazopanib, aflibercept, nintedanib, trebananib, and sunitinib, are currently under investigation (Singh et al., 2020). This study conducted a meta-analysis of previous RCTs and concluded that compared to drug therapy without anti-angiogenic agents, combination therapy with anti-angiogenic drugs significantly improved PFS and OS, while also elevating the ORR. Further subgroup analysis revealed that combination therapy with VEGF or VEGFR inhibitors can bring benefits in terms of PFS and OS, as well as an improvement in ORR.
Bevacizumab is the main VEGF inhibitor of interest in the trials included in this study. This agent, a humanized monoclonal antibody targeting VEGF, received approval in 2014 as the treatment for platinum-resistant OC, to be used in conjunction with chemotherapy (Monk et al., 2016a). Our findings revealed that bevacizumab in combination with chemotherapy not only significantly improved PFS and OS but also increased ORR compared with chemotherapy alone or plus placebo in patients with OC. Bevacizumab achieves its therapeutic effect by preventing VEGF-A from engaging with VEGFR, resulting in the destruction of established vessels, interference with new vessel formation, and the reduction of intratumoral pressure (Reinthaller, 2016). Research indicated that inhibiting VEGF signaling not only diminishes tumor vascularization but also aids in the morphological and functional normalization of the remaining vessels (Mei et al., 2023). In addition, trebananib stands out as the sole angiopoietin inhibitor in our comprehensive analysis. This peptide, which obstructs the action of angiopoietin-1 and angiopoietin-2-key players in angiogenesis-acts by preventing ANGPT from interacting with its receptor, Tie2 (Mullen et al., 2019). Utilizing photoacoustic tomography, one study observed notable changes in tumor vascularization following trebananib treatment, including significant vessel regression and a decrease in vessel density. Notably, while trebananib therapy did not halt angiogenesis entirely, it encouraged the formation of more stable and less permeable residual vessels (Bohndiek et al., 2015). The TRINOVA-1 trial, assessing patients with recurrent OC less than 12 months after previous platinum-based therapy, allocated participants to either a combination of weekly paclitaxel and trebananib or weekly paclitaxel with placebo. The trebananib cohort experienced prolonged PFS (HR = 0.66, p < 0.001) (Monk et al., 2016b). Our analysis confirmed the benefit of trebananib combined with chemotherapy in improving PFS. Regrettably, this study did not demonstrate any significant improvement in OS and ORR when comparing trebananib plus chemotherapy to placebo plus chemotherapy.
Currently, VEGFR inhibitors attracting substantial clinical attention include cediranib, nintedanib, and pazopanib. Cediranib, an orally administered tyrosine kinase inhibitor (TKI), acts on VEGFR-1, -2, and -3, and c-kit. Preclinical OC models have demonstrated that cediranib therapy leads to a significant reduction in tumor vascular density and vessel regression (Ruscito et al., 2016). When combined with standard chemotherapy as a maintenance therapy, cediranib has demonstrated an extension in PFS and OS compared to chemotherapy alone (Mahner et al., 2015). When paired with the PARP inhibitor olaparib in patients with platinum-sensitive relapse OC, cediranib has exhibited a remarkable 80% response rate and an increase in PFS from 9 to 17.7 months (Liu et al., 2014). However, our combined analysis did not corroborate that cediranib combination therapy could enhance OS compared to treatments devoid of cediranib. Our research did affirm the benefit of cediranib combination therapy in extending PFS. Notably, the cediranib and olaparib combination therapy demonstrated a higher ORR compared to olaparib monotherapy in our study. Additional RCTs are needed to further probe the effectiveness of pairing anti-angiogenic drugs with PARP inhibitors in OC treatment. Nintedanib, a multi-targeted antiangiogenic agent available orally, has been shown through dynamic magnetic resonance imaging assessments to significantly reduce blood flow in approximately 55% of OC patients. It also fosters vascular normalization and tumor regression in pre-clinical models (Khalique et al., 2017). Nintedanib, when combined with carboplatin and paclitaxel, has been proven to improve PFS, although it has no effect on OS (Ray-Coquard et al., 2020). Pazopanib, an oral multi-target TKI, inhibits platelet-derived growth factor receptors (PDGFR) alpha/beta, VEGFR, c-Kit, and fibroblast growth factor receptor (FGFR)-1 and −3. In mouse orthotopic OC models, pazopanib treatment significantly curtailed tumor microvessel density and pericyte coverage (Merritt et al., 2010). While not yet approved for OC, numerous phase 2 and 3 clinical trials have explored the potential role of pazopanib in OC therapy (Davidson et al., 2014; du Bois et al., 2012; Plummer et al., 2013). Our research indicated that the combination of nintedanib and chemotherapy can improve PFS compared with chemotherapy alone or plus placebo. The combination of pazopanib and chemotherapy has been shown to provide higher ORR, which aligns with a previous meta-analysis (Zhang et al., 2023).
In addition to examining the impacts of various VEGF, VEGFR, and angiopoietin inhibitors on OC by classifying specific anti-angiogenic medications, our analytical approach distinguished itself from prior meta-analyses by performing subgroup analyses based on multiple OC subtypes (Wang et al., 2018; Guo et al., 2021). The results from our subgroup analysis suggested that compared to drug therapy without anti-angiogenic agents, combination therapy with anti-angiogenic drugs notably improved PFS, OS, and ORR in platinum-sensitive and recurrent OC patients. Traditionally, OC has been classified as “platinum sensitive” if relapse occurs 6 months or more after the final dose of platinum-based chemotherapy, and “platinum resistant” if relapse happens earlier (Ledermann et al., 2013). For platinum-resistant OC, our research also concluded that anti-angiogenic drug combination therapy yielded benefits in terms of PFS and OS, along with a higher ORR. Bevacizumab is the sole anti-VEGF treatment for platinum-sensitive and recurrent OC approved by the US Food and Drug Administration (FDA). Its FDA approved indication is for combination with carboplatin/gemcitabine or carboplatin/paclitaxel, followed by single-agent maintenance (Arend et al., 2020). Bevacizumab is also available in the United States as a second-line and third-line treatment for platinum-resistant OC and frontline therapy for stage III/IV disease (Arend et al., 2020). The majority of the participants in the RCTs included in our study were OC patients of various subtypes. Grouping the subdivided subtypes of OC into a single category in a general manner could lead to some degree of bias and confusion. Furthermore, the fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup recommended that tumors should be defined by a multitude of factors, including surgical outcomes, mutation status, platinum sensitivity, histology, and response to non-platinum treatments. Consequently, more RCTs need to be incorporated to bolster future meta-analysis targeting a specific and clearly defined subtype of OC.
The results of our monotherapy analysis indicated that anti-angiogenic monotherapy did not provide substantial improvements in PFS and OS compared with placebo. This monotherapy, however, was associated with an elevated risk of any grade AEs. Despite the pooled analysis revealing a greater ORR with the use of anti-angiogenic monotherapy (Ferron et al., 2023), the inference made from a single trial could not be broadly applied. From a therapeutic efficacy standpoint, the combination of anti-angiogenic drugs with chemotherapy or PARP inhibitors seems to be a more effective alternative to monotherapy with anti-angiogenic drugs, as combination therapy brings benefits in terms of PFS, OS, and ORR. RCTs needs to be designed to directly compare the effectiveness of anti-angiogenic drug monotherapy and combination therapy to verify this hypothesis. Moreover, the increased incidence of AEs caused by combination therapy warrants attention. Our combined and subgroup analyses revealed that anti-angiogenic drug combination resulted in a higher incidence of grade ≥3 AEs. Additionally, the combination of VEGFR or angiopoietin inhibitor was linked to an increased risk of any grade AEs. These findings underscore the importance for vigilant monitoring and management of AEs during anti-angiogenic therapy to mitigate potential risks.
There are several limitations in our meta-analysis. First, the heterogeneity in PFS results could be attributed to the variations in the trial design, patient baseline characteristics, anti-angiogenic therapies utilized, chemotherapy protocols, OC stages, and duration of follow-up across the RCTs. The existence of considerable heterogeneity may compromise the dependability of pooled estimates. Second, it is noteworthy that despite the majority of the incorporated studies being featured in high-impact journals, certain inherent aspects like pharmaceutical industry sponsorship and an open-label design could potentially introduce elements of bias, such as publication bias, which might have an impact on the overall findings. Third, despite the participation of independent assessors and meticulous data extraction and quality assessment using the modified Jadad scale, subjective biases may still be present in the process of evaluating study quality and extracting data. Fourth, diversity in OC types across the original RCTs could make the subgroup analyses based on OC subtypes potentially biased and confusing. These subgroup analyses could potentially introduce the possibility of false positives and inflated type I error. Finally, TSA findings point out the need for future meta-analysis with larger sample sizes and more RCTs to validate the results related to PFS and grade ≥3 AEs in the context of anti-angiogenic drug monotherapy.
5 Conclusion
In summary, our meta-analysis of RCTs demonstrated that the combination of anti-angiogenic drugs with chemotherapy or PARP inhibitors significantly improved PFS, OS, and ORR in OC patients compared with chemotherapy or PARP inhibitors alone. Although the efficacy superiority of anti-angiogenic drug monotherapy over placebo has not been observed, the increased risk of AEs associated with anti-angiogenic drug monotherapy and combination therapy warrants attention. Clinicians should meticulously detect and manage AEs to mitigate the potential treatment-related risks while employing anti-angiogenic therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. FZ: Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Key Research Project of Science and Technology of Sichuan Province (2022YFS0087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1423891/full#supplementary-material
References
Abdalla, A. M. E., Xiao, L., Ullah, M. W., Yu, M., Ouyang, C., and Yang, G. (2018). Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 8 (2), 533–548. doi:10.7150/thno.21674
Aghajanian, C., Blank, S. V., Goff, B. A., Judson, P. L., Teneriello, M. G., Husain, A., et al. (2012). OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30 (17), 2039–2045. doi:10.1200/jco.2012.42.0505
Aghajanian, C., Goff, B., Nycum, L. R., Wang, Y. V., Husain, A., and Blank, S. V. (2015). Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 139 (1), 10–16. doi:10.1016/j.ygyno.2015.08.004
Arend, R., Westin, S. N., and Coleman, R. L. (2020). Decision analysis for secondline maintenance treatment of platinum sensitive recurrent ovarian cancer: a review. Int. J. Gynecol. Cancer 30 (5), 684–694. doi:10.1136/ijgc-2019-001041
Bohndiek, S. E., Sasportas, L. S., Machtaler, S., Jokerst, J. V., Hori, S., and Gambhir, S. S. (2015). Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib. J. Nucl. Med. 56 (12), 1942–1947. doi:10.2967/jnumed.115.160002
Bowden, J., Tierney, J. F., Copas, A. J., and Burdett, S. (2011). Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 11, 41. doi:10.1186/1471-2288-11-41
Burger, R. A., Brady, M. F., Bookman, M. A., Fleming, G. F., Monk, B. J., Huang, H., et al. (2011). Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365 (26), 2473–2483. doi:10.1056/NEJMoa1104390
Chekerov, R., Hilpert, F., Mahner, S., El-Balat, A., Harter, P., De Gregorio, N., et al. (2018). Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 19 (9), 1247–1258. doi:10.1016/s1470-2045(18)30372-3
Coleman, R. L., Brady, M. F., Herzog, T. J., Sabbatini, P., Armstrong, D. K., Walker, J. L., et al. (2017). Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 18 (6), 779–791. doi:10.1016/s1470-2045(17)30279-6
Davidson, B. A., and Secord, A. A. (2014). Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer. Int. J. Womens Health 6, 289–300. doi:10.2147/ijwh.S49781
du Bois, A., Floquet, A., Kim, J. W., Rau, J., del Campo, J. M., Friedlander, M., et al. (2014). Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 32 (30), 3374–3382. doi:10.1200/jco.2014.55.7348
du Bois, A., Kristensen, G., Ray-Coquard, I., Reuss, A., Pignata, S., Colombo, N., et al. (2016). Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 17 (1), 78–89. doi:10.1016/s1470-2045(15)00366-6
du Bois, A., Vergote, I., Wimberger, P., Ray-Coquard, I., Harter, P., Curtis, L. B., et al. (2012). Open-label feasibility study of pazopanib, carboplatin, and paclitaxel in women with newly diagnosed, untreated, gynaecologic tumours: a phase I/II trial of the AGO study group. Br. J. Cancer 106 (4), 629–632. doi:10.1038/bjc.2011.608
Duska, L. R., Petroni, G. R., Varhegyi, N., Brown, J., Jelovac, D., Moore, K. N., et al. (2020). A randomized phase II evaluation of weekly gemcitabine plus pazopanib versus weekly gemcitabine alone in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma. Gynecol. Oncol. 157 (3), 585–592. doi:10.1016/j.ygyno.2019.10.014
Duval, S., and Tweedie, R. (2000). Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Fernando, N. T., Koch, M., Rothrock, C., Gollogly, L. K., D’Amore, P. A., Ryeom, S., et al. (2008). Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin. Cancer Res. 14 (5), 1529–1539. doi:10.1158/1078-0432.Ccr-07-4126
Ferron, G., De Rauglaudre, G., Becourt, S., Delanoy, N., Joly, F., Lortholary, A., et al. (2023). Neoadjuvant chemotherapy with or without nintedanib for advanced epithelial ovarian cancer: lessons from the GINECO double-blind randomized phase II CHIVA trial. Gynecol. Oncol. 170, 186–194. doi:10.1016/j.ygyno.2023.01.008
Garzon, S., Laganà, A. S., Casarin, J., Raffaelli, R., Cromi, A., Franchi, M., et al. (2020). Secondary and tertiary ovarian cancer recurrence: what is the best management? Gland. Surg. 9 (4), 1118–1129. doi:10.21037/gs-20-325
Gore, M., Hackshaw, A., Brady, W. E., Penson, R. T., Zaino, R., McCluggage, W. G., et al. (2019). An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol. Oncol. 153 (3), 541–548. doi:10.1016/j.ygyno.2019.03.256
Gotlieb, W. H., Amant, F., Advani, S., Goswami, C., Hirte, H., Provencher, D., et al. (2012). Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 13 (2), 154–162. doi:10.1016/s1470-2045(11)70338-2
Guo, C., Yan, C., Qu, L., Du, R., and Lin, J. (2021). The efficacy and toxicity of angiogenesis inhibitors for ovarian cancer: a meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 303 (2), 285–311. doi:10.1007/s00404-020-05865-z
Hall, M. R., Dehbi, H. M., Banerjee, S., Lord, R., Clamp, A., Ledermann, J. A., et al. (2020). A phase II randomised, placebo-controlled trial of low dose (metronomic) cyclophosphamide and nintedanib (BIBF1120) in advanced ovarian, fallopian tube or primary peritoneal cancer. Gynecol. Oncol. 159 (3), 692–698. doi:10.1016/j.ygyno.2020.09.048
Helali, A. E., Wong, C. H. L., Choi, H. C. W., Chan, W. W. L., Dickson, N., Siu, S. W. K., et al. (2022). A comprehensive systematic review and network meta-analysis: the role of anti-angiogenic agents in advanced epithelial ovarian cancer. Sci. Rep. 12 (1), 3803. doi:10.1038/s41598-022-07731-1
Herzog, T. J., Scambia, G., Kim, B. G., Lhommé, C., Markowska, J., Ray-Coquard, I., et al. (2013). A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol. Oncol. 130 (1), 25–30. doi:10.1016/j.ygyno.2013.04.011
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hope, J. M., and Blank, S. V. (2010). Current status of maintenance therapy for advanced ovarian cancer. Int. J. Womens Health 1, 173–180. doi:10.2147/ijwh.s4661
IntHout, J., Ioannidis, J. P., Rovers, M. M., and Goeman, J. J. (2016). Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6 (7), e010247. doi:10.1136/bmjopen-2015-010247
Ionescu, C., Berindan-Neagoe, I., Burz, C., Balacescu, O., Balacescu, L., Tudoran, O., et al. (2011). The clinical implications of platelet derived growth factor B, vascular endothelial growth factor and basic fibroblast growth factor in colorectal cancer. J. buon 16 (2), 274–276.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Jászai, J., and Schmidt, M. H. H. (2019). Trends and challenges in tumor anti-angiogenic therapies. Cells 8 (9), 1102. doi:10.3390/cells8091102
Karlan, B. Y., Oza, A. M., Richardson, G. E., Provencher, D. M., Hansen, V. L., Buck, M., et al. (2012). Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J. Clin. Oncol. 30 (4), 362–371. doi:10.1200/jco.2010.34.3178
Khalique, S., and Banerjee, S. (2017). Nintedanib in ovarian cancer. Expert Opin. Investig. Drugs 26 (9), 1073–1081. doi:10.1080/13543784.2017.1353599
Kim, J. W., Mahner, S., Wu, L. Y., Shoji, T., Kim, B. G., Zhu, J. Q., et al. (2018). Pazopanib maintenance therapy in east asian women with advanced epithelial ovarian cancer: results from AGO-OVAR16 and an east asian study. Int. J. Gynecol. Cancer 28 (1), 2–10. doi:10.1097/igc.0000000000000602
Ledermann, J. A., Embleton, A. C., Raja, F., Perren, T. J., Jayson, G. C., Rustin, G. J. S., et al. (2016). Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 387 (10023), 1066–1074. doi:10.1016/s0140-6736(15)01167-8
Ledermann, J. A., Embleton-Thirsk, A. C., Perren, T. J., Jayson, G. C., Rustin, G. J. S., Kaye, S. B., et al. (2021). Cediranib in addition to chemotherapy for women with relapsed platinum-sensitive ovarian cancer (ICON6): overall survival results of a phase III randomised trial. ESMO Open 6 (2), 100043. doi:10.1016/j.esmoop.2020.100043
Ledermann, J. A., Hackshaw, A., Kaye, S., Jayson, G., Gabra, H., McNeish, I., et al. (2011). Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J. Clin. Oncol. 29 (28), 3798–3804. doi:10.1200/jco.2010.33.5208
Ledermann, J. A., Raja, F. A., Fotopoulou, C., Gonzalez-Martin, A., Colombo, N., Sessa, C., et al. (2013). Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24, vi24–32. Suppl 6. doi:10.1093/annonc/mdt333
Liu, J. F., Barry, W. T., Birrer, M., Lee, J. M., Buckanovich, R. J., Fleming, G. F., et al. (2014). Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 15 (11), 1207–1214. doi:10.1016/s1470-2045(14)70391-2
Liu, J. F., Barry, W. T., Birrer, M., Lee, J. M., Buckanovich, R. J., Fleming, G. F., et al. (2019). Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 30 (4), 551–557. doi:10.1093/annonc/mdz018
Liu, J. F., Brady, M. F., Matulonis, U. A., Miller, A., Kohn, E. C., Swisher, E. M., et al. (2022). Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial. J. Clin. Oncol. 40 (19), 2138–2147. doi:10.1200/jco.21.02011
Mahner, S., Woelber, L., Mueller, V., Witzel, I., Prieske, K., Grimm, D., et al. (2015). Beyond bevacizumab: an outlook to new anti-angiogenics for the treatment of ovarian cancer. Front. Oncol. 5, 211. doi:10.3389/fonc.2015.00211
Marth, C., Vergote, I., Scambia, G., Oberaigner, W., Clamp, A., Berger, R., et al. (2017). ENGOT-ov-6/TRINOVA-2: randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur. J. Cancer 70, 111–121. doi:10.1016/j.ejca.2016.09.004
McGee, J., Bookman, M., Harter, P., Marth, C., McNeish, I., Moore, K. N., et al. (2017). Fifth ovarian cancer Consensus conference: individualized therapy and patient factors. Ann. Oncol. 28 (4), 702–710. doi:10.1093/annonc/mdx010
Mei, C., Gong, W., Wang, X., Lv, Y., Zhang, Y., Wu, S., et al. (2023). Anti-angiogenic therapy in ovarian cancer: current understandings and prospects of precision medicine. Front. Pharmacol. 14, 1147717. doi:10.3389/fphar.2023.1147717
Merritt, W. M., Nick, A. M., Carroll, A. R., Lu, C., Matsuo, K., Dumble, M., et al. (2010). Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol. Cancer Ther. 9 (4), 985–995. doi:10.1158/1535-7163.Mct-09-0967
Monk, B. J., Minion, L. E., and Coleman, R. L. (2016a). Anti-angiogenic agents in ovarian cancer: past, present, and future. Ann. Oncol. 27, i33–i39. Suppl 1. doi:10.1093/annonc/mdw093
Monk, B. J., Poveda, A., Vergote, I., Raspagliesi, F., Fujiwara, K., Bae, D. S., et al. (2016b). Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): long-term survival, impact of ascites, and progression-free survival-2. Gynecol. Oncol. 143 (1), 27–34. doi:10.1016/j.ygyno.2016.07.112
Mullen, M. M., Kuroki, L. M., and Thaker, P. H. (2019). Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol. Oncol. 152 (2), 416–425. doi:10.1016/j.ygyno.2018.10.023
Nicum, S., McGregor, N., Austin, R., Collins, L., Dutton, S., McNeish, I., et al. (2024). Results of a randomised Phase II trial of olaparib, chemotherapy or olaparib and cediranib in patients with platinum-resistant ovarian cancer. Br. J. Cancer 130 (6), 941–950. doi:10.1038/s41416-023-02567-6
Nusrat, O., Belotte, J., Fletcher, N. M., Memaj, I., Saed, M. G., Diamond, M. P., et al. (2016). The role of angiogenesis in the persistence of chemoresistance in epithelial ovarian cancer. Reprod. Sci. 23 (11), 1484–1492. doi:10.1177/1933719116645191
Oza, A. M., Cook, A. D., Pfisterer, J., Embleton, A., Ledermann, J. A., Pujade-Lauraine, E., et al. (2015). Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 16 (8), 928–936. doi:10.1016/s1470-2045(15)00086-8
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Pignata, S., Lorusso, D., Joly, F., Gallo, C., Colombo, N., Sessa, C., et al. (2021). Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. Lancet Oncol. 22 (2), 267–276. doi:10.1016/s1470-2045(20)30637-9
Pignata, S., Lorusso, D., Scambia, G., Sambataro, D., Tamberi, S., Cinieri, S., et al. (2015). Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 16 (5), 561–568. doi:10.1016/s1470-2045(15)70115-4
Plummer, R., Madi, A., Jeffels, M., Richly, H., Nokay, B., Rubin, S., et al. (2013). A Phase I study of pazopanib in combination with gemcitabine in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 71 (1), 93–101. doi:10.1007/s00280-012-1982-z
Pujade-Lauraine, E., Hilpert, F., Weber, B., Reuss, A., Poveda, A., Kristensen, G., et al. (2014). Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 32 (13), 1302–1308. doi:10.1200/jco.2013.51.4489
Ray-Coquard, I., Cibula, D., Mirza, M. R., Reuss, A., Ricci, C., Colombo, N., et al. (2020). Final results from GCIG/ENGOT/AGO-OVAR 12, a randomised placebo-controlled phase III trial of nintedanib combined with chemotherapy for newly diagnosed advanced ovarian cancer. Int. J. Cancer 146 (2), 439–448. doi:10.1002/ijc.32606
Reinthaller, A. (2016). Antiangiogenic therapies in ovarian cancer. Memo 9 (3), 139–143. doi:10.1007/s12254-016-0282-4
Richardson, D. L., Sill, M. W., Coleman, R. L., Sood, A. K., Pearl, M. L., Kehoe, S. M., et al. (2018). Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer: a randomized clinical trial. JAMA Oncol. 4 (2), 196–202. doi:10.1001/jamaoncol.2017.4218
Roque, D. M., Siegel, E. R., Buza, N., Bellone, S., Silasi, D. A., Huang, G. S., et al. (2022). Randomised phase II trial of weekly ixabepilone ± biweekly bevacizumab for platinum-resistant or refractory ovarian/fallopian tube/primary peritoneal cancer. Br. J. Cancer 126 (12), 1695–1703. doi:10.1038/s41416-022-01717-6
Ruscito, I., Gasparri, M. L., Marchetti, C., De Medici, C., Bracchi, C., Palaia, I., et al. (2016). Cediranib in ovarian cancer: state of the art and future perspectives. Tumour Biol. 37 (3), 2833–2839. doi:10.1007/s13277-015-4781-4
Saman, H., Raza, S. S., Uddin, S., and Rasul, K. (2020). Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers (Basel) 12 (5), 1172. doi:10.3390/cancers12051172
Shoji, T., Enomoto, T., Abe, M., Okamoto, A., Nagasawa, T., Oishi, T., et al. (2022). Efficacy and safety of standard of care with/without bevacizumab for platinum-resistant ovarian/fallopian tube/peritoneal cancer previously treated with bevacizumab: the Japanese Gynecologic Oncology Group study JGOG3023. Cancer Sci. 113 (1), 240–250. doi:10.1111/cas.15185
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71 (1), 7–33. doi:10.3322/caac.21654
Singh, N., Badrun, D., and Ghatage, P. (2020). State of the art and up-and-coming angiogenesis inhibitors for ovarian cancer. Expert Opin. Pharmacother. 21 (13), 1579–1590. doi:10.1080/14656566.2020.1775813
Teleanu, R. I., Chircov, C., Grumezescu, A. M., and Teleanu, D. M. (2019). Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 9 (1), 84. doi:10.3390/jcm9010084
Tewari, K. S., Burger, R. A., Enserro, D., Norquist, B. M., Swisher, E. M., Brady, M. F., et al. (2019). Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 37 (26), 2317–2328. doi:10.1200/jco.19.01009
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
Timke, C., Zieher, H., Roth, A., Hauser, K., Lipson, K. E., Weber, K. J., et al. (2008). Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin. Cancer Res. 14 (7), 2210–2219. doi:10.1158/1078-0432.Ccr-07-1893
Vergote, I., du Bois, A., Floquet, A., Rau, J., Kim, J. W., Del Campo, J. M., et al. (2019a). Overall survival results of AGO-OVAR16: a phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 155 (2), 186–191. doi:10.1016/j.ygyno.2019.08.024
Vergote, I., Scambia, G., O’Malley, D. M., Van Calster, B., Park, S. Y., Del Campo, J. M., et al. (2019b). Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 20 (6), 862–876. doi:10.1016/s1470-2045(19)30178-0
Wang, H., Xu, T., Zheng, L., and Li, G. (2018). Angiogenesis inhibitors for the treatment of ovarian cancer: an updated systematic review and meta-analysis of randomized controlled trials. Int. J. Gynecol. Cancer 28 (5), 903–914. doi:10.1097/igc.0000000000001258
Wang, T., Tang, J., Yang, H., Yin, R., Zhang, J., Zhou, Q., et al. (2022). Effect of apatinib plus pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone on platinum-resistant recurrent ovarian cancer: the APPROVE randomized clinical trial. JAMA Oncol. 8 (8), 1169–1176. doi:10.1001/jamaoncol.2022.2253
Wang, Z., Huang, Y., Long, L., Zhou, L., Huang, Y., Gan, L., et al. (2021). Apatinib treatment efficiently delays biochemical-only recurrent ovarian cancer progression. J. Ovarian Res. 14 (1), 91. doi:10.1186/s13048-021-00843-8
Wetterslev, J., Jakobsen, J. C., and Gluud, C. (2017). Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 17 (1), 39. doi:10.1186/s12874-017-0315-7
Xie, M., Zhong, Y., Yang, Y., Shen, F., and Nie, Y. (2022). Extended adjuvant endocrine therapy for women with hormone receptor-positive early breast cancer: a meta-analysis with trial sequential analysis of randomized controlled trials. Front. Oncol. 12, 1039320. doi:10.3389/fonc.2022.1039320
Yi, S., Zeng, L., Kuang, Y., Cao, Z., Zheng, C., Zhang, Y., et al. (2017). Antiangiogenic drugs used with chemotherapy for patients with recurrent ovarian cancer: a meta-analysis. Onco Targets Ther. 10, 973–984. doi:10.2147/ott.S119879
Zhang, C., and Zhao, W. (2022). The efficacy and safety of angiogenesis inhibitors for recurrent ovarian cancer: a meta-analysis. J. Ovarian Res. 15 (1), 99. doi:10.1186/s13048-022-01028-7
Keywords: anti-angiogenic drugs, VEGF, bevacizumab, ovarian cancer, monotherapy, combination therapy, meta-analysis
Citation: Xie Y and Zhou F (2024) Efficacy and safety of anti-angiogenic drug monotherapy and combination therapy for ovarian cancer: a meta-analysis and trial sequential analysis of randomized controlled trials. Front. Pharmacol. 15:1423891. doi: 10.3389/fphar.2024.1423891
Received: 26 April 2024; Accepted: 09 May 2024;
Published: 27 May 2024.
Edited by:
Zhi-Bin Wang, Central South University, ChinaReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusRoubini Zakopoulou, University General Hospital Attikon, Greece
Copyright © 2024 Xie and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Zhou, dr_zhoufei@163.com
 Yao Xie
Yao Xie