
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 27 September 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1422033
This article is part of the Research Topic Reviews in Pharmacology of Anti-Cancer Drugs: 2023 View all 8 articles
 Haoyang Chen1,2
Haoyang Chen1,2 Huihui Liu1,2
Huihui Liu1,2 Xiaowei Zhang3
Xiaowei Zhang3 Suhua Wang1,2
Suhua Wang1,2 Chunxia Liu1,2
Chunxia Liu1,2 Ke An1,2
Ke An1,2 Ruijuan Liu1,2*
Ruijuan Liu1,2* Xin Tian1,2*
Xin Tian1,2*Hepatocellular carcinoma (HCC) is one of the primary forms of liver cancer and is currently the sixth most prevalent malignancy worldwide. In addition to surgical interventions, effective drug treatment is essential for treating HCC. With an increasing number of therapeutic drugs for liver cancer undergoing clinical studies, the therapeutic strategies for advanced HCC are more diverse than ever, leading to improved prospects for HCC patients. Molecular targeted drugs and immunotherapies have become crucial treatment options for HCC. Treatment programs include single-agent molecular-targeted drugs, immunotherapies, combinations of immunotherapies with molecular-targeted drugs, and dual immune checkpoint inhibitors. However, further exploration is necessary to determine the optimal pharmacological treatment regimens, and the development of new effective drugs is urgently needed. This review provides an overview of the current globally approved drugs for liver cancer, as well as the latest advances in ongoing clinical research and drug therapies. Additionally, the review offers an outlook and discussion on the prospects for the development of drug therapy approaches for HCC.
• Drug treatment remains an essential approach to treating HCC besides surgical interventions, and the therapeutic strategies for advanced HCC are more diversified than before.
• Molecular targeted drugs and immunotherapies used alone or in combination with each other have been crucial therapeutic options for the treatment of HCC.
• The optimal pharmacological treatment regimens for HCC necessitate further exploration, and the new effective drugs need to be developed urgently.
Liver cancer remains a major global health challenge, and its incidence is increasing worldwide (Vogel et al., 2022). It is projected that the annual incidence of new liver cancer cases will rise by over half in the next 10–20 years (Rumgay et al., 2022). Hepatocellular carcinoma (HCC) is the most common form of liver cancer, accounting for the majority of cases (Llovet et al., 2021). Viral hepatitis and cirrhosis are significant risk factors for HCC, with the Hepatitis B virus (HBV) being a more prominent risk factor than Hepatitis C virus (HCV) (Rumgay et al., 2022). HBV can integrate into the host genome, causing direct carcinogenic effects. Even in the absence of cirrhosis, HBV can induce hepatocarcinogenesis through mechanisms such as the activation of oncogenes and the inhibition of tumor suppressor genes (Jiang et al., 2021). HCV promotes HCC through chronic inflammation, oxidative stress, and fibrosis (Guntipalli et al., 2021). The burden of HCC caused by viral hepatitis is substantial. Non-alcoholic fatty liver disease (NAFLD) is also rapidly becoming the primary cause of HCC (Yip et al., 2022). Indeed, HCC is the fourth leading cause of cancer-related death across the world. The highest incidence and mortality of HCC are observed in East Asia and Africa, and these rates are also increasing in different parts of Europe and the USA (McGlynn et al., 2015). According to the Surveillance Epidemiology End Results (SEER), HCC is projected to become the third leading cause of cancer-related death by 2030 (Rahib et al., 2014). This review focuses specifically on the recent advancements in drug therapies for hepatocellular carcinoma (HCC) and their impact on treatment outcomes.
Accurate staging is essential for determining the most appropriate treatment for HCC due to its diverse causes and risk factors. The Barcelona Liver Cancer Clinic (BCLC) staging algorithm, proposed in 1999, is the most widely used system. It classifies patients into five stages (BCLC-0, A, B, C, or D) (Llovet et al., 1999; European Association for the Study of the LiverEuropean Association for the Study of the Liver, 2018). Curative treatment options such as surgical resection, ablation, and liver transplantation are recommended for patients with early-stage liver cancer (single liver tumors or up to 3 nodules ≤ 3 cm), which have been shown to produce the best results with a 5-year survival rate of about 70%–80% (European Association for the Study of the LiverEuropean Association for the Study of the Liver, 2018; Vogel et al., 2018; Marrero et al., 2018; Omata et al., 2017). While surgical resection and liver transplantation are curative options for early-stage HCC, many patients present with advanced disease where systemic therapies play a critical role.
Multiple systemic therapies have been approved in phase III clinical. These include first-line treatments like sorafenib, lenvatinib, atezolizumab plus bevacizumab, and tremelimumab plus durvalumab, as well as second-line treatments like regorafenib, cabozantinib, and ramucirumab (Llovet et al., 2021; Sonbol et al., 2020). On this basis, the U.S. Food and Drug Administration (FDA) has granted accelerated approval of nivolumab, pembrolizumab, and nivolumab plus ipilimumab as second-line treatments. Following systematic evaluation, the atezolizumab and bevacizumab combination is now considered the standard of care for first-line treatment of advanced HCC patients (Sonbol et al., 2020). Moreover, combination therapies involving anti-angiogenesis agents with immune checkpoint inhibitors (ICIs), dual ICIs, and targeted agents in conjunction with surgery or other loco-regional therapies have shown promise and provided the basis for clinical trials (El-Khoueiry et al., 2022).
Despite the availability of several systemic therapies, challenges such as drug resistance and limited efficacy in certain patient populations underscore the urgent need for novel therapeutic approaches. New therapeutic strategies may focus on enhancing treatment efficacy and overcoming drug resistance to expand treatment options, ultimately improving the survival of patients with HCC. This review summarizes the drugs approved for first-line and second-line treatment of HCC in recent years, including their approval dates, targets and mechanisms of action, limitations, and adverse reactions. The purpose is to provide information to support doctors and patients in choosing the best treatment drugs by summarizing the basic situation of existing drugs for liver cancer treatment and to provide directions for researchers to develop drugs with more significant effects and safer use based on current drug foundations.
Approved systemic therapies for liver cancer currently focus on targeting molecular receptors or the immune system. The mechanisms underlying the development of liver cancer are complicated and diverse. Preventing hepatitis and fatty liver can somewhat reduce the risk of HCC. However, for patients already diagnosed with cancer, molecular and immune therapies are effective measures.
Molecular targeted therapies, which aim to inhibit molecular pathways critical for tumor growth and maintenance, are the primary treatment approach for advanced HCC.
Vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) are crucial factors that play significant roles in the neovascularization, invasiveness, and metastatic potential of HCC. The VEGF receptors (VEGFR) are found on endothelial cells and initiate a cascade essential for angiogenesis, making them crucial drivers of tumor vascularization (Mabeta and Steenkamp, 2022). PDGF contributes to angiogenesis by recruiting pericytes and smooth muscle cells to nascent vascular sprouts. The aberrant expression of VEGFR and PDGF receptor (PDGFR) during hepatocarcinogenesis leads to increased angiogenesis formation and activation of the downstream RAS/MAPK pathway, promoting cellular proliferation (Zou et al., 2022; Babina and Turner, 2017). Therefore, drugs that inhibit VEGF and PDGF, such as sorafenib and lenvatinib, are effective therapeutic options for liver cancer (Wilhelm et al., 2008; Zhao et al., 2020).
The fibroblast growth factor (FGF) family comprises at least five fibroblast growth factor receptors (FGFR1-FGFR5) and over 20 homologous ligands. FGFR4 is the predominant receptor found in the human liver, and its endogenous ligand FGF19 is overexpressed to promote HCC survival and enhance its resistance to apoptosis (Yang et al., 2023). FGF8, FGF17, and FGF18 are involved in autocrine and paracrine signaling in hepatocellular carcinoma, augmenting tumor cell survival, angiogenesis, and neovascularization (Xie et al., 2020). These mechanisms underpin the use of FGFR-targeted inhibitors like lenvatinib and regorafenib in treating liver cancer (Zhao et al., 2020; Wilhelm et al., 2011).
MET is a tyrosine kinase receptor for hepatocyte growth factor (HGF). The activation of the HGF-MET pathway is closely associated with the development and progression of HCC. This pathway promotes cell proliferation by regulating cell morphology and motility (Guo et al., 2020). Additionally, elevated expression of HGF-MET correlates with poor survival outcomes in HCC patients (Wang et al., 2020). For HCC with high MET expression, cabozantinib is a therapeutic option. However, selective inhibitors targeting MET specifically are more likely to effectively suppress MET activity while minimizing off-target toxicity, making them the focus of current clinical research (Yang et al., 2023).
The RAS/RAF/MEK/ERK (MAPK) signaling cascade is an important pathway that regulates tumor cell proliferation and differentiation and is closely associated with the metastasis and progression of HCC (Moon and Ro, 2021). The aberrant RAS/MAPK pathway activation is strongly linked to various growth factors, such as epidermal growth factor (EGF), VEGF, PDGF, and HGF (Degirmenci et al., 2020). Consequently, blocking the MAPK signaling pathway represents a potentially effective therapeutic strategy for HCC. Sorafenib and regorafenib are effective inhibitors of the MAPK pathway by targeting RAF, providing a valuable approach in the treatment of liver cancer.
Other receptor tyrosine kinases, including KIT, RET, and AXL, also play crucial roles in developing and progressing HCC (Huang et al., 2020). Aberrant expression of KIT in some HCC patients is associated with specific HCC subtypes, impacting cancer invasiveness and prognosis. Additionally, KIT can influence HCC invasion and metastasis by regulating the expression of genes related to epithelial-mesenchymal transition (EMT) (Pathania et al., 2021). RET, an oncogene-encoded receptor tyrosine kinase, is known for its role in the development and maintenance of the nervous system. However, increasing evidence indicates that RET is also involved in tumor growth and metastasis across various cancer types (Takahashi, 2022). AXL, a TAM receptor tyrosine kinase family member, was initially identified for its role in embryonic development and immune regulation. Nonetheless, its abnormal expression is associated with tumor invasiveness, resistance, and poor prognosis in several cancers, including HCC (Zhu et al., 2019). Cabozantinib, which can target and inhibit KIT, RET, and AXL receptor tyrosine kinases, has been approved as a second-line treatment for HCC that is resistant to sorafenib.
Tumor cells can evade the immune system by hijacking the programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) pathway or other mechanisms. This aids in tumor development and progression (Yi et al., 2022). To enhance tolerance to harmless foreign molecules, the liver typically maintains an anti-inflammatory environment by expressing and secreting inhibitory molecules (Heymann and Tacke, 2016). Similarly, HCC exhibits an inflammatory yet suppressive immune environment, which is conducive to stronger and more durable responses to immune checkpoint inhibitors. Moreover, the liver contains a substantial number of immune cells, making HCC patients suitable candidates for immunotherapy (Sangro et al., 2021). Several immunotherapy drugs have been approved for first- and second-line treatment of HCC. These drugs primarily target established checkpoints such as PD-1 ligands, PD-L1/2 receptors, and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) receptors (Ringelhan et al., 2018).
Developing immune-based treatments for HCC has also accelerated the emergence of combination therapies involving immune-related approaches. For instance, dual immune checkpoint inhibitors have proven to be an effective combination therapy. This targets the PD-1/PD-L1 pathway and CTLA-4 receptors, significantly suppressing immune evasion in HCC (Song X. et al., 2023; Yau et al., 2020). Additionally, VEGF inhibitors can create an inflammatory microenvironment that enhances the efficacy of immune checkpoint inhibitors. The use of this combined therapy has demonstrated strong therapeutic effects. It is the mechanism behind the current standard therapy for HCC, atezolizumab plus bevacizumab (Rimini et al., 2022).
Furthermore, we have depicted the targets of drugs currently approved for first-line or second-line treatment of HCC in Figure 1.
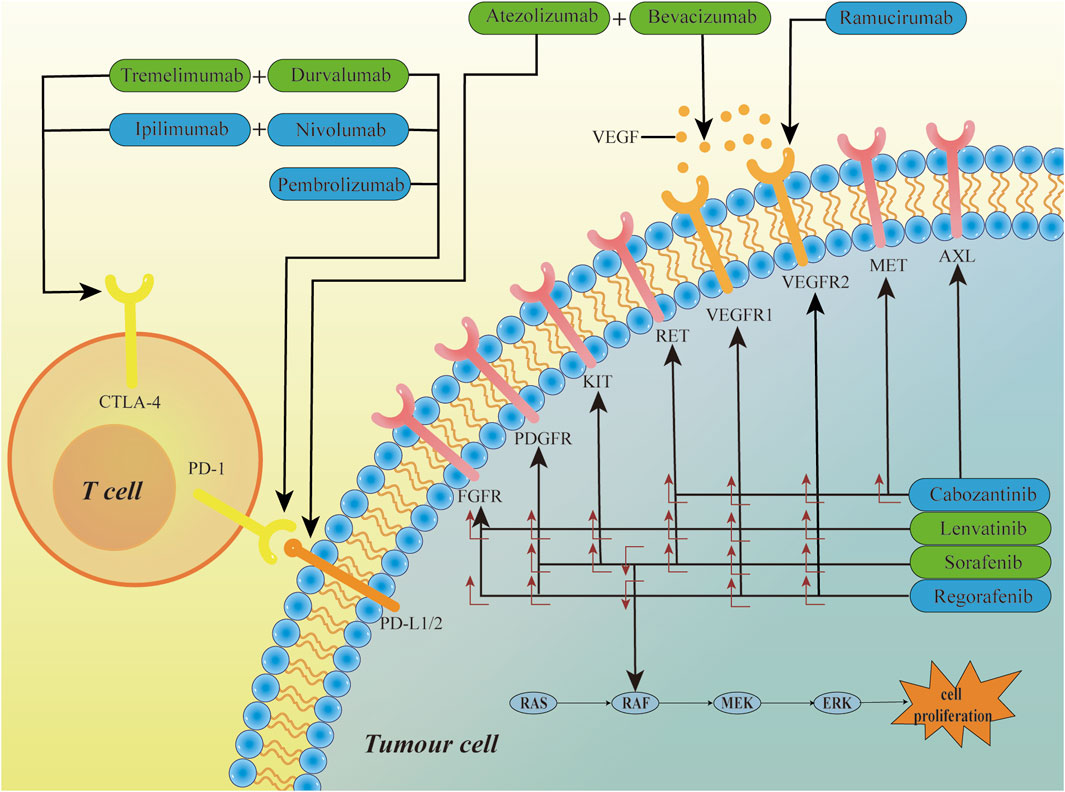
Figure 1. The mechanism of HCC targeted therapy drugs. Sorafenib, lenvatinib, cabozantinib, and regorafenib are small-molecule multi-kinase inhibitors that target multiple kinase receptors, inhibiting tumor growth by blocking biological processes such as angiogenesis. Bevacizumab and Ramucirumab are kinase inhibitors, targeting the VEGF ligand and VEGFR2, respectively. Atezolizumab, Durvalumab, Nivolumab, and Pembrolizumab are immune checkpoint inhibitors that exert their effects by inhibiting the PD-1/PD-L1/2 signaling pathway. Tremelimumab and Ipilimumab exert their effects by inhibiting the CTLA-4 receptors. Green boxes represent first-line treatment drugs, blue boxes represent second-line treatment drugs, and the symbol “+” indicates combination therapy.
Molecular targeted therapy has been a focal point in HCC research. Since the multikinase inhibitor sorafenib was approved for HCC treatment, an increasing number of molecular drugs have shown excellent therapeutic effects in clinical trials for HCC. These include anti-angiogenic drugs such as bevacizumab, tyrosine kinase inhibitors like cabozantinib, and epidermal growth factors inhibitors such as lenvatinib and regorafenib. The application of molecular targeted drugs in liver cancer treatment offers patients more precise, personalized, and effective therapeutic options, with the potential to improve both the quality of life and survival period. However, it is crucial to closely monitor patients’ treatment responses and drug side effects to ensure the safety and efficacy of the treatment. This section focuses on molecular targeted drugs as a standalone therapy, as shown in Table 1.
Sorafenib, the first approved treatment for HCC, owes its efficacy to its inhibition of multiple kinase targets, which promotes cell apoptosis, reduces angiogenesis, and inhibits tumor cell proliferation. Therefore, sorafenib has been the mainstay of treatment for a decade (Tang et al., 2020). The approval of subsequent drugs has primarily been based on comparisons with the efficacy of sorafenib, further underscoring its pivotal role in treating HCC. Sorafenib targets and inhibits several key kinases and receptors involved in cancer cell growth and survival, including RAF kinase, (Liu et al., 2006), VEGFR1-2, PDGFR, KIT, and RET proto-oncogene receptor tyrosine kinase (Wilhelm et al., 2008). It can be used to treat various cancers, including liver, (Llovet et al., 2021), kidney, (Escudier, 2007), and thyroid cancers (Brose et al., 2014). In advanced HCC, sorafenib is currently the effective first-line treatment drug (Tang et al., 2020). It is worth noting that sorafenib is the first targeted therapy drug that has shown efficacy in patients with advanced HCC.
In one key SHARP study, the median overall survival (OS) for HCC patients in the sorafenib group was 10.7 months, while the patients who received a placebo were 7.9 months (Llovet et al., 2008). A parallel Phase 3 study also showed similar results (Ren et al., 2021; Cheng et al., 2009). Although several clinical trials have demonstrated the efficacy of sorafenib in improving OS and progression-free survival in patients with advanced HCC, the reasons for the extension of survival are likely multifactorial, including differences in inclusion criteria and the use of effective sequential therapies (Huang et al., 2020; Cheng et al., 2009). Currently, the clinical benefit of sorafenib is still limited, with only about 30% of patients benefiting from it, and this population usually develops resistance within 6 months (Cheng et al., 2020; Xia et al., 2020; Méndez-Blanco et al., 2018). Sorafenib may cause adverse reactions in some patients, and the frequency and severity of these adverse reactions vary among individuals, mainly including gastrointestinal (diarrhea and weight loss) and hand-foot skin reactions (Rimassa et al., 2019; Li Y. et al., 2015). In severe cases, sorafenib can cause hypertension and abdominal pain, leading to treatment discontinuation (Colagrande et al., 2015; Fu et al., 2018).
However, sorafenib remains an important treatment option for advanced HCC patients, and ongoing research is focused on developing more effective and personalized treatment strategies for this disease. The complex molecular pathogenesis of HCC has stimulated research on combinations of sorafenib with other molecularly targeted drugs. Sorafenib has been combined with antiangiogenic agents, (Morse et al., 2019), MEK/ERK pathway inhibitors, (Huynh et al., 2019), mTOR pathway inhibitors, (Wang C. et al., 2019), histone deacetylase inhibitors, (Chang et al., 2020; Freese et al., 2019) EGF/EGF receptor (EGFR) pathway inhibitors, (Dong et al., 2020), and HGF/c-MET pathway inhibitors (Goyal et al., 2013). Other agents such as interferon, (Tan et al., 2019; Wang M. et al., 2019), selumetinib, (Tai et al., 2016), capecitabine, (Patt et al., 2017), tegafur-uracil, (Azim et al., 2018), gemcitabine and oxaliplatin (GEMOX), (Assenat et al., 2019), and gemcitabine alone have also been evaluated. Still, to date, no treatments involving combinations containing sorafenib have succeeded in phase III trials.
Before lenvatinib was approved for the treatment of HCC, sorafenib has consistently been the preferred first-line treatment drug for HCC. Lenvatinib was approved based on its non-inferiority to sorafenib (Vogel et al., 2021). Lenvatinib is also a multitargeted tyrosine kinase inhibitor (TKI) that targets various receptors, including VEGFR1-2, FGFR, PDGFR, the RET proto-oncogene receptor tyrosine kinase, and KIT proto-oncogene receptor tyrosine kinase (Zhao et al., 2020). By inhibiting these receptors, lenvatinib can help reduce the growth and spread of liver cancer cells. Specifically, it works by blocking the formation of new blood vessels that provide nutrients and oxygen to the tumor, a process known as angiogenesis. Without adequate blood supply, the ability of the tumor to grow and spread is diminished. Lenvatinib can be used to treat various types of cancer, including thyroid, (Ferrari et al., 2021; Fogli et al., 2021), kidney, (Roviello et al., 2018), and liver cancer. The FDA has approved lenvatinib for the first-line treatment of advanced HCC (Yamazaki et al., 2019; FDA, 2018a).
In clinical trials, lenvatinib has been shown to be effective in treating advanced HCC. A parallel Phase 3 study found a statistically significant improvement in OS with lenvatinib compared to placebo in patients with unresectable HCC who had not previously received systemic therapy (Kudo et al., 2018). In the phase 3 REFLECT study, which primarily recruited Asian patients, lenvatinib demonstrated noninferiority to sorafenib in first-line treatment, the median OS in the lenvatinib group was 13.6 months compared to 12.3 months in the sorafenib group (Vogel et al., 2021). For the key secondary endpoints, median progression-free survival, and overall response rate, lenvatinib was superior to sorafenib (Arai et al., 2017). Recently, it has been reported that compared to sorafenib, lenvatinib has demonstrated even better clinical outcomes in the treatment of unresectable hepatitis B virus-related HCC (Choi et al., 2022).
Lenvatinib’s effectiveness against HCC is encouraging, but adverse events during treatment are common and often lead to dose interruption or treatment discontinuation. Common side effects of lenvatinib include fatigue, nausea, diarrhea, hypertension, and decreased appetite. In addition to these common side effects, lenvatinib may cause more severe side effects in some patients, including bleeding or blood clots, liver problems (such as elevated liver enzymes or liver failure), heart problems (such as heart attacks or heart failure), kidney problems (such as renal failure or proteinuria), and hypertensive crisis (Rimassa et al., 2019; Fogli et al., 2021; Arai et al., 2017; Reed et al., 2020; Kim et al., 2022; Nervo et al., 2021). A lower dose that can guarantee efficacy and improve tolerance is being explored, considering also the potential activity of lenvatinib in controlling primary and secondary brain tumors. (Wang R. et al., 2019).
Overall, lenvatinib is considered an important treatment option for advanced HCC patients who are not candidates for surgery or liver transplantation and is typically used as a first-line therapy. However, more research is still needed to explore whether available biomarkers can predict lenvatinib’s efficacy response and resistance to define patient populations better and avoid unnecessary risks (Catalano et al., 2021).
Regorafenib is a small molecule inhibitor of multiple kinases, including those involved in angiogenesis and tumor growth (Grothey et al., 2020). Specifically, regorafenib inhibits the activity of VEGFR, PDGFR, and FGFR, all of which are involved in angiogenesis, and other kinases involved in cell proliferation and survival, such as RAF, BRAF and KIT (Wilhelm et al., 2011). Clinical studies have shown that regorafenib is effective in treating HCC patients who have received sorafenib. In a phase III clinical trial, regorafenib was shown to improve OS in HCC patients previously treated with sorafenib significantly. In this study, the median OS of patients receiving regorafenib was 10.6 months compared to 7.8 months for patients receiving the placebo (Bruix et al., 2017). In 2017, regorafenib was approved by the FDA for the treatment of advanced HCC in patients who are intolerant to sorafenib and have progressed on that therapy (FDA, 2017a). The subsequent success of regorafenib in HCC patients who progress on sorafenib treatment heralded a new era of second-line treatment and was quickly followed by ramucirumab, cabozantinib, and the most influential, ICIs. Besides, regorafenib has also been approved for the treatment of metastatic colorectal cancer that has progressed on other therapies, as well as for the treatment of advanced gastrointestinal stromal tumors that are no longer sensitive to imatinib and sunitinib successively (Grothey et al., 2013; Huemer et al., 2020; Demetri et al., 2013). Overall, regorafenib has demonstrated significant efficacy in various types of cancers that are resistant or have progressed after treatment, making it an important second-line therapy for multiple cancers, including HCC (Grothey et al., 2020).
Like all cancer treatments, regorafenib has side effects. Some common side effects of regorafenib in HCC include fatigue, hypertension, diarrhea, and hand-foot skin reaction (also known as palmar-plantar erythrodysesthesia) (McLellan et al., 2015). Additionally, regorafenib can cause more serious side effects in some patients. These include hepatotoxicity (liver damage or failure), bleeding (especially in patients with liver cancer or those previously treated with anticoagulants or antiplatelet agents), and worsening of cardiac issues (especially in patients with a history of heart disease or hypertension). To avoid serious adverse events, a strict treatment plan is necessary before starting regorafenib therapy (Li J. et al., 2015; Maeda et al., 2019).
Cabozantinib is a small molecule inhibitor of multiple tyrosine kinases, including VEGFR, hepatocyte growth factor receptor (HGFR/c-MET), AXL, and RET, cabozantinib works by inhibiting the activity of these tyrosine kinases, resulting in decreased cancer cell growth and proliferation, reduced angiogenesis, and increased cancer cell death (Yakes et al., 2011). Clinical trials have shown that cabozantinib is effective in treating advanced HCC, particularly in patients who have previously received sorafenib (Abou-Alfa et al., 2018). In 2019, cabozantinib was approved by the FDA for the treatment of advanced HCC in patients who have previously received sorafenib (FDA, 2019a). Cabozantinib is also used to treat renal cell carcinoma (RCC), (Tannir et al., 2017) and medullary thyroid carcinoma (MTC) (Brose et al., 2021). As a second-line therapy for HCC, cabozantinib exhibits side effects that are highly similar to those of regorafenib, including diarrhea, hypertension, hand-foot skin reaction, and bleeding (Rimassa et al., 2019; Zuo et al., 2015). Although cabozantinib has less hepatotoxicity than regorafenib, it may cause serious adverse reactions such as hypothyroidism (low thyroid hormone levels), osteonecrosis of the jaw (a rare but serious condition that can cause jaw pain, swelling, and infection), and reversible posterior leukoencephalopathy syndrome (RPLS, a rare and potentially serious neurological disorder that can cause seizures, headaches, and changes in vision) (McGregor et al., 2022). Therefore, cabozantinib is a prescription drug that can only be taken under the guidance and supervision of a healthcare provider.
Donafenib is a deuterated sorafenib derivative and a novel multikinase inhibitor, including VEGFR, PDGFR, and Raf kinases (Keam and Duggan, 2021). The results of the phase III clinical trial, ZGDH3, have demonstrated that donafenib is superior to sorafenib in improving OS and has good safety and tolerability in Chinese patients with advanced HCC. It is expected to be a potential first-line monotherapy for these patients (Qin et al., 2021a). Based on these results, in June 2021, donafenib was approved for the first time in China for the treatment of uHCC in patients who have not received prior systemic therapy (Administration, 2021).
Ramucirumab is a monoclonal antibody that targets VEGFR-2. By binding to VEGFR-2, ramucirumab blocks signaling pathways that promote tumor angiogenesis, which helps slow cancer cells’ growth and spread (Poole and Vaidya, 2014). Ramucirumab has been approved by the FDA for the treatment of various types of cancer, including gastric or gastroesophageal junction adenocarcinoma, (Fuchs et al., 2019), non-small cell lung cancer, (Arrieta et al., 2017) and colorectal cancer (Debeuckelaere et al., 2019). It is typically used in combination with chemotherapy but may also be used as a single agent in certain cases.
In a phase III clinical trial, patients with advanced HCC treated with ramucirumab had a median OS of 8.5 months and a median progression-free survival (PFS) of 2.8 months, which was better than the group of patients treated with the placebo (Chau et al., 2017). Therefore, ramucirumab was approved by the FDA for the treatment of advanced HCC in 2019, (FDA, 2019b) becoming an effective second-line treatment for HCC that is not a TKI.
The normal human liver contains numerous immune cells, but immune suppression has been identified in HCC. This makes immunotherapy a potentially ideal treatment approach for HCC patients, particularly those who experience disease progression with traditional first-line drugs, especially those with severe cirrhosis or cardiovascular diseases and who are intolerant to other TKIs.
Immunotherapeutic drugs approved for HCC treatment mainly target PD1-PDL1/2 or CTLA-4. In this section, we will discuss immunotherapeutic drugs as a standalone therapy for HCC, as shown in Table 2. Additionally, immunotherapy may be associated with specific side effects, necessitating close monitoring of patients’ responses during treatment.
Nivolumab, marketed as Opdivo, is a PD-1 antibody, and the FDA granted accelerated approval in September 2017 for second-line treatment of advanced HCC patients who are resistant to sorafenib (FDA, 2017b). This is the first FDA-approved immune checkpoint inhibitor for HCC. The efficacy of immunotherapy for HCC is low, but the duration of effectiveness is relatively long. The function of nivolumab is to block the interaction between PD-1 and its ligands, PD-L1 and PD-L2, which are expressed in cancer cells and other cells in the tumor microenvironment (Abedi et al., 2022). By blocking PD-1, nivolumab helps release the immune system to attack cancer cells and shrink tumors.
In the CheckMate-459 trial, nivolumab was compared with sorafenib as a first-line treatment for advanced HCC. Compared to sorafenib, nivolumab did not improve OS in the overall population (Yau et al., 2022). However, nivolumab showed a significant improvement in OS in patients with high PD-L1 expression, a biomarker associated with better response to ICIs.
Overall, nivolumab is an important treatment option for advanced HCC, especially for patients with high PD-L1 expression levels. Ongoing research focuses on identifying biomarkers and combination therapies to improve the response of HCC and other types of cancer to nivolumab and other ICIs.
Pembrolizumab is also an immune checkpoint inhibitor that targets the PD-1 receptor on immune cells. On 9 November 2018, the US FDA granted accelerated approval of the immunotherapy drug pembrolizumab (Keytruda) for the treatment of HCC (second-line therapy) in patients who are resistant to sorafenib (FDA, 2018b). In terms of clinical data, there is little difference between pembrolizumab and another drug, Opdivo (Zhu et al., 2018).
Recently, in the KEYNOTE-240 trial, the anti-tumor activity and safety of pembrolizumab have been further confirmed. The study showed that compared with placebo, pembrolizumab improved the OS of advanced HCC patients who had previously received sorafenib treatment (Finn et al., 2020a). Similarly, the latest clinical trial has demonstrated that Pembrolizumab can significantly improve OS and PFS in Asian patients (Qin et al., 2023). Overall, pembrolizumab is an important treatment option for advanced HCC, serving as a second-line therapy after sorafenib. Ongoing research is focused on identifying biomarkers and combination therapies to improve the response to pembrolizumab and other ICIs for HCC and other types of cancer.
With the increasing resistance of HCC to single drugs, the effectiveness of a single agent becomes more limited. The spotlight is turning toward the combined use of drugs. Combining therapies contributes to better-individualized treatment. Depending on the patient’s immune status, tumor genotype, phenotype, and other characteristics, a suitable combination of immune checkpoint inhibitors and molecular targeted drugs can be selected to maximize treatment effectiveness while minimizing toxic side effects. The most groundbreaking combination involves the use of the immune checkpoint inhibitor atezolizumab and the molecularly targeted drug bevacizumab, which has now become the standard therapy for advanced HCC. Additionally, dual immune checkpoint inhibitors and dual molecular targeted drugs demonstrate promising applications in liver cancer treatment. The relevant content of the combination therapy approach is illustrated in Table 3.
Following the approval of sorafenib and lenvatinib, the approval of atezolizumab plus bevacizumab indicates a new era in HCC treatment, where combination therapy is gradually becoming a more prominent treatment choice, replacing monotherapy. Atezolizumab is a monoclonal antibody that targets the PD-L1 protein on cancer cells and disrupts the interaction between PD-L1 and the PD-1 receptor on immune cells. PD-1 is a protein that helps regulate immune responses and prevents them from attacking normal cells in the body. In many types of cancer, including liver cancer, cancer cells can hijack the PD-1 pathway to evade detection and destruction by the immune system (Zhu et al., 2022). This interaction typically prevents immune cells from attacking cancer cells, but by blocking it, atezolizumab can activate the immune system and help it identify and destroy cancer cells (Markham, 2016; Frampton, 2020). Bevacizumab is a monoclonal antibody that targets the VEGF protein. By blocking VEGF, bevacizumab can reduce the blood supply to the tumor and prevent its growth (Garcia et al., 2020). However, due to an increased risk of bleeding with bevacizumab use, endoscopy is required within 6 months before enrollment, and it is strongly recommended that patients with portal hypertension be screened for varices prior to treatment (Cao et al., 2019; Li and Kroetz, 2018; Hsu et al., 2021).
In comparison to sorafenib, the combination of the VEGF-A antibody bevacizumab and the PD-L1 antibody atezolizumab demonstrated an improvement in OS (Ringelhan et al., 2018; Rimini et al., 2022). The IMbrave150 clinical trial, the first positive phase 3 study using an ICI-based regimen, (Galle et al., 2021; Qin et al., 2021b; Finn et al., 2020b), showed the combination therapy significant survival benefits compared to sorafenib. Atezolizumab in combination with bevacizumab was approved by the United States FDA in May 2020 for the treatment of unresectable or metastatic HCC (FDA, 2020a). The combination of atezolizumab and bevacizumab has been recognized by international guidelines as the new standard of care for first-line treatment of advanced HCC (Cheng et al., 2022).
In recent times, immunotherapy has gained a significant role in the management of cancer. With Atezolizumab plus bevacizumab becoming the new first-line standard therapy for HCC, the combination of various drugs in treating HCC is now being given more attention. As clinical trials progress, dual immune checkpoint inhibitors have shown significant promise in the treatment of HCC. Dual ICIs such as tremelimumab plus durvalumab and nivolumab plus ipilimumab target proteins named PD-1 and CTLA-4 on T cells, respectively, helping to activate them to identify and attack cancer cells (Arru et al., 2021). Tremelimumab and durvalumab were approved by the FDA in the fall of 2022 for the treatment of metastatic non-small cell lung cancer (mNSCLC) in adult patients with sensitizing epidermal growth factor receptor mutations or anaplastic lymphoma kinase genomic tumor aberrations and unresectable HCC (uHCC) in adult patients with the same (FDA, 2022a; FDA, 2022b; Keam, 2023). In addition, a randomized phase III clinical trial named KESTREL showed that tremelimumab and durvalumab in combination displayed durable responses and reduced treatment-related adverse events (TRAEs) in recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC), highlighting the promising potential of the combination therapy in multiple cancers (Psyrri et al., 2023).
In a phase III clinical trial named HIMALAYA, tremelimumab plus durvalumab was compared with sorafenib, the standard therapy for advanced HCC. The trial included advanced HCC patients who had not received systemic therapy before. The results showed that the combination therapy improved OS and had manageable safety compared to sorafenib (Song et al., 2023; Kelley et al., 2021). Based on these positive results, the FDA granted breakthrough therapy designation for the combination therapy for the treatment of advanced HCC in 2019, (FDA, 2022b), following the latest standard first-line therapy atezolizumab plus bevacizumab. However, it is worth noting that tremelimumab plus durvalumab is currently limited to adult patients with uHCC (Song et al., 2023; FDA, 2022b).
Currently, the durvalumab monotherapy has not been approved. Some patients have contraindications to ICIs therapy, including those with severe autoimmune diseases and those who have undergone major organ transplants (Wichelmann et al., 2021). For these patients, monotherapy with sorafenib or lenvatinib is an appropriate first-line therapy.
The mechanism of action of nivolumab and ipilimumab is to enhance the immune system’s ability to attack cancer cells. By blocking the PD-1 receptor, nivolumab enables T cells to more effectively recognize and attack cancer cells. On the other hand, CTLA-4 is a protein that inhibits the immune system’s response. By blocking CTLA-4 with ipilimumab, the immune system’s response to cancer cells is enhanced. The combination of these two drugs has been demonstrated to effectively treat liver cancer, especially HCC.
Multiple clinical trials have studied the combination of nivolumab and ipilimumab for the treatment of HCC. In a recent phase II trial, the combination of nivolumab and ipilimumab was compared to sorafenib, the standard of care for advanced HCC (Yau et al., 2020). The study showed that the combination therapy significantly improved OS compared to sorafenib. It is important to emphasize that not all patients are suitable for this treatment.
In March 2020, the FDA granted accelerated approval for the use of the PD-1 inhibitor nivolumab in combination with the CTLA-4 inhibitor ipilimumab for the treatment of patients with HCC who have previously received sorafenib treatment (FDA, 2020b). Nivolumab plus ipilimumab is the first dual ICI therapy approved by the FDA for patients with HCC, and is the most effective immunotherapy regimen, even for lung cancer, (Hellmann et al., 2019) kidney cancer, (Motzer et al., 2018), and colon cancer, (Overman et al., 2018) with outstanding efficacy.
Currently, cabozantinib plus atezolizumab is being investigated as an emerging combination therapy for the treatment of advanced HCC (Esteban-Fabró et al., 2022). In a phase III clinical trial named COSMIC-312, previously untreated advanced HCC patients were randomly assigned to receive cabozantinib plus atezolizumab or sorafenib, the standard treatment for advanced HCC (Kelley et al., 2022). The study found that combination therapy was associated with improved OS and progression-free survival compared to sorafenib. Based on these hopeful results, the FDA has granted breakthrough therapy designation for the use of cabozantinib plus atezolizumab to treat advanced HCC. This designation aims to accelerate the development and review of new therapies for severe or life-threatening diseases. However, further evaluation of the efficacy of cabozantinib plus atezolizumab in the treatment of advanced HCC is needed. In addition, the combination of cabozantinib and atezolizumab has shown promising results in the treatment of advanced RCC for patients who have previously received anti-angiogenic therapy (Pal et al., 2021).
To improve the response to pembrolizumab or other ICIs for HCC. Combination therapies have become an essential choice in the treatment of HCC. Recently, in the KEYNOTE-524 trial, pembrolizumab was also studied as a first-line treatment for advanced HCC (Finn et al., 2020c). In this study, pembrolizumab was combined with the targeted therapy drug lenvatinib and compared with lenvatinib alone. The study found that the combination of pembrolizumab and lenvatinib improved progression-free survival and OS compared to lenvatinib alone (Sun et al., 2022).
Pembrolizumab showing promise in combination with lenvatinib as a new first-line treatment approach (Rizzo et al., 2022). To further evaluate the safety and efficacy of the pembrolizumab plus lenvatinib therapy, a phase III clinical trial named LEAP-002 is currently underway, lenvatinib plus pembrolizumab has promising antitumor activity in uHCC (Llovet et al., 2023).
With the continuous in-depth research on liver cancer, an increasing number of emerging therapeutic methods and drugs are being discovered. Despite multiple first- and second-line regimens being approved for systemic treatment of HCC, the development of resistance and limitations of existing drug therapies for HCC remind us that we cannot stop researching HCC and the urgent need for more precise drug selection, the discovery, and development of drugs with more significant efficacy and safer usage to curb the incidence and progression of HCC in the population. A crucial aspect is the identification and discovery of biomarkers for drug treatment. For instance, mutations in the PI3K-AKT-mTOR pathway serve as genomic biomarkers for sorafenib treatment, while serum biomarkers like angiopoietin-2 (ANG2) and fibroblast growth factor 21 (FGF21) are associated with lenvatinib treatment (Choi et al., 2022; Song et al., 2023). In the realm of immunotherapy, in addition to the established PD-1 and PD-L1, there is potential for CD3 and CD8 to serve as biomarkers (Duffy et al., 2017; Sangro et al., 2020). Biomarkers facilitate personalized treatment, contributing to the specificity and efficacy of drug therapies. Detecting biomarkers enables the monitoring of tumor progression, facilitating adjustments in treatment plans. Furthermore, biomarkers are crucial in new drug development, aiding in assessing drug safety and efficacy.
Immunotherapy, as an emerging and effective cancer solution, holds tremendous potential. Expanding current immunotherapies in drug treatment for HCC is of significant importance. Different from traditional immunotherapeutic drugs, adoptive cell therapy (ACT) enhances the anti-tumor immune response by injecting immune cells, making it more suitable for solid tumors (Roddy et al., 2022). Therefore, ACT is considered feasible in treating liver cancer, and related clinical trials have shown promising results (Shi et al., 2020). Peptide vaccines can induce T-cell responses, enhancing specific anti-tumor immunity and represent a hopeful immunotherapeutic approach for HCC (Mizukoshi et al., 2015).
In addition to general combination therapies, sequential combination treatment is an emerging therapeutic approach that can maintain the synergistic effects of drugs while reducing the combined toxicity. Another similar strategy is alternate treatment, primarily targeting tumor sensitivity to one drug at the cost of developing resistance to another. However, the effectiveness of this approach in HCC is still under debate.
An iterative approach to targeted drugs has provided a wealth of data and encouraging results, particularly in those patients where resistance to sorafenib develops. The understanding and management of HCC have changed significantly due to extensive basic and clinical research over the last decade. However, HCC remains a devastating disease with a widespread and enormous impact on healthcare systems worldwide. In recent years, there have been significant advances in understanding the mechanisms that lead to rapid progress in treating patients with cancer and liver diseases. This collaborative effort involves oncologists, hepatologists, and basic scientists, and it provides hope for continued improvement in patient prognosis.
HC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Visualization, Writing–original draft, Writing–review and editing. HL: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing. XZ: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing. SW: Conceptualization, Funding acquisition, Investigation, Supervision, Writing–review and editing. CL: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing. KA: Conceptualization, Investigation, Supervision, Writing–review and editing. RL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing–original draft, Writing–review and editing. XT: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grants Nos 81903720 and 82100667) and Henan Province Science and Technology Attack Project (No. 232102310005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abedi, K. B., Abbasi, A., Ghasemi, D. N., Adabi, N., Moradian, A., Yazdani, Y., et al. (2022). Combination therapy with nivolumab (anti-PD-1 monoclonal antibody): a new era in tumor immunotherapy. Int. Immunopharmacol. 113 (Pt A), 109365. doi:10.1016/j.intimp.2022.109365
Abou-Alfa, G. K., Meyer, T., Cheng, A. L., El-Khoueiry, A. B., Rimassa, L., Ryoo, B. Y., et al. (2018). Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379 (1), 54–63. doi:10.1056/NEJMoa1717002
Administration, N. M. P. (2021). Donafenib: NMPA approval notice. Available at: https://wwwnmpagovcn/directory/web/nmpa/yaowen/ypjgyw/20210609085421166htmlAccessed (Accessed June 9, 2021).
Arai, N., Sasaki, H., Tamura, R., Ohara, K., and Yoshida, K. (2017). Unusual magnetic resonance imaging findings of a glioblastoma arising during treatment with lenvatinib for thyroid cancer. World Neurosurg. 107, 1047 e9–e1047. doi:10.1016/j.wneu.2017.08.017
Arrieta, O., Zatarain-Barrón, Z. L., Cardona, A. F., Carmona, A., and Lopez-Mejia, M. (2017). Ramucirumab in the treatment of non-small cell lung cancer. Expert Opin. Drug Saf. 16 (5), 637–644. doi:10.1080/14740338.2017.1313226
Arru, C., De Miglio, M. R., Cossu, A., Muroni, M. R., Carru, C., Zinellu, A., et al. (2021). Durvalumab plus tremelimumab in solid tumors: a systematic review. Adv. Ther. 38 (7), 3674–3693. doi:10.1007/s12325-021-01796-6
Assenat, E., Pageaux, G. P., Thézenas, S., Peron, J. M., Bécouarn, Y., Seitz, J. F., et al. (2019). Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br. J. Cancer 120 (9), 896–902. doi:10.1038/s41416-019-0443-4
Azim, H. A., Omar, A., Atef, H., Zawahry, H., Shaker, M. K., Abdelmaksoud, A. K., et al. (2018). Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a Phase II trial (ESLC01 study). J. Hepatocell. Carcinoma 5, 109–119. doi:10.2147/jhc.S169285
Babina, I. S., and Turner, N. C. (2017). Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 17 (5), 318–332. doi:10.1038/nrc.2017.8
Brose, M. S., Nutting, C. M., Jarzab, B., Elisei, R., Siena, S., Bastholt, L., et al. (2014). Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 (9940), 319–328. doi:10.1016/s0140-6736(14)60421-9
Brose, M. S., Robinson, B., Sherman, S. I., Krajewska, J., Lin, C. C., Vaisman, F., et al. (2021). Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 22 (8), 1126–1138. doi:10.1016/s1470-2045(21)00332-6
Bruix, J., Qin, S., Merle, P., Granito, A., Huang, Y. H., Bodoky, G., et al. (2017). Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389 (10064), 56–66. doi:10.1016/s0140-6736(16)32453-9
Cao, J., Kong, F. H., Liu, X., and Wang, X. B. (2019). Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: a meta-analysis. World J. Gastroenterol. 25 (27), 3649–3663. doi:10.3748/wjg.v25.i27.3649
Catalano, M., Casadei-Gardini, A., Vannini, G., Campani, C., Marra, F., Mini, E., et al. (2021). Lenvatinib: established and promising drug for the treatment of advanced hepatocellular carcinoma. Expert Rev. Clin. Pharmacol. 14 (11), 1353–1365. doi:10.1080/17512433.2021.1958674
Chang, Y., Lee, Y. B., Cho, E. J., Lee, J. H., Yu, S. J., Kim, Y. J., et al. (2020). CKD-5, a novel pan-histone deacetylase inhibitor, synergistically enhances the efficacy of sorafenib for hepatocellular carcinoma. BMC Cancer 20 (1), 1001. doi:10.1186/s12885-020-07471-3
Chau, I., Peck-Radosavljevic, M., Borg, C., Malfertheiner, P., Seitz, J. F., Park, J. O., et al. (2017). Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase III REACH study. Eur. J. Cancer 81, 17–25. doi:10.1016/j.ejca.2017.05.001
Cheng, A. L., Kang, Y. K., Chen, Z., Tsao, C. J., Qin, S., Kim, J. S., et al. (2009). Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10 (1), 25–34. doi:10.1016/s1470-2045(08)70285-7
Cheng, A. L., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2022). Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76 (4), 862–873. doi:10.1016/j.jhep.2021.11.030
Cheng, Z., Wei-Qi, J., and Jin, D. (2020). New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim. Biophys. Acta Rev. Cancer 1874 (1), 188382. doi:10.1016/j.bbcan.2020.188382
Choi, N. R., Kim, J. Y., Hong, J. H., Hur, M. H., Cho, H., Park, M. K., et al. (2022). Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 22 (1), 135. doi:10.1186/s12876-022-02210-3
Colagrande, S., Regini, F., Taliani, G. G., Nardi, C., and Inghilesi, A. L. (2015). Advanced hepatocellular carcinoma and sorafenib: diagnosis, indications, clinical and radiological follow-up. World J. Hepatol. 7 (8), 1041–1053. doi:10.4254/wjh.v7.i8.1041
Debeuckelaere, C., Murgioni, S., Lonardi, S., Girardi, N., Alberti, G., Fano, C., et al. (2019). Ramucirumab: the long and winding road toward being an option for mCRC treatment. Expert Opin. Biol. Ther. 19 (5), 399–409. doi:10.1080/14712598.2019.1600505
Degirmenci, U., Wang, M., and Hu, J. (2020). Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells 9 (1), 198. doi:10.3390/cells9010198
Demetri, G. D., Reichardt, P., Kang, Y. K., Blay, J. Y., Rutkowski, P., Gelderblom, H., et al. (2013). Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (9863), 295–302. doi:10.1016/s0140-6736(12)61857-1
Dong, Z. R., Sun, D., Yang, Y. F., Zhou, W., Wu, R., Wang, X. W., et al. (2020). TMPRSS4 drives angiogenesis in hepatocellular carcinoma by promoting HB-EGF expression and proteolytic cleavage. Hepatology 72 (3), 923–939. doi:10.1002/hep.31076
Duffy, A. G., Ulahannan, S. V., Makorova-Rusher, O., Rahma, O., Wedemeyer, H., Pratt, D., et al. (2017). Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66 (3), 545–551. doi:10.1016/j.jhep.2016.10.029
El-Khoueiry, A. B., Llovet, J. M., Vogel, A., Madoff, D. C., Finn, R. S., Ogasawara, S., et al. (2022). LEAP-012 trial in progress: transarterial chemoembolization (TACE) with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma (HCC). J. Clin. Oncol. 40 (4), TPS494. doi:10.1200/JCO.2022.40.4_suppl.TPS494
Escudier, B. (2007). Sorafenib [corrected] in kidney cancer. Ann. Oncol. 18 (Suppl. 9), ix90–3. doi:10.1093/annonc/mdm301
Esteban-Fabró, R., Willoughby, C. E., Piqué-Gili, M., Montironi, C., Abril-Fornaguera, J., Peix, J., et al. (2022). Cabozantinib enhances anti-PD1 activity and elicits a neutrophil-based immune response in hepatocellular carcinoma. Clin. Cancer Res. 28 (11), 2449–2460. doi:10.1158/1078-0432.Ccr-21-2517
European Association for the Study of the LiverEuropean Association for the Study of the Liver (2018). EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69 (1), 182–236. doi:10.1016/j.jhep.2018.03.019
FDA (2017a). FDA expands approved use of Stivarga to treat liver cancer. The U.S. Food and Drug Administration. Available at: https://www.fda.gov/news-events/press-announcements/fda-expands-approved-use-stivarga-treat-liver-cancer (Accessed April 27, 2017).
FDA (2017b). FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma (Accessed September 27, 2017).
FDA (2018a). FDA approves lenvatinib for unresectable hepatocellular carcinoma: the U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma (Accessed August 16, 2018).
FDA (2018b). FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Available at: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma (Accessed November 9, 2018).
FDA (2019a). FDA approves cabozantinib for hepatocellular carcinoma: the U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma (Accessed May 12, 2019).
FDA (2019b). FDA approves ramucirumab for hepatocellular carcinoma. The U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-hepatocellular-carcinoma (Accessed May 10, 2019).
FDA (2020a). FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma (Accessed May 29, 2020).
FDA (2020b). FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma (Accessed March 10, 2020).
FDA (2022a). FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non-small cell lung cancer. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-and-platinum-based-chemotherapy-metastatic-nonAccessed (Accessed November 10, 2022).
FDA (2022b). FDA approves tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-unresectable-hepatocellular-carcinoma (Accessed October 21, 2022).
Ferrari, S. M., Elia, G., Ragusa, F., Paparo, S. R., Mazzi, V., Miccoli, M., et al. (2021). Lenvatinib: an investigational agent for the treatment of differentiated thyroid cancer. Expert Opin. Investig. Drugs 30 (9), 913–921. doi:10.1080/13543784.2021.1972971
Finn, R. S., Ikeda, M., Zhu, A. X., Sung, M. W., Baron, A. D., Kudo, M., et al. (2020c). Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38 (26), 2960–2970. doi:10.1200/jco.20.00808
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2020b). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382 (20), 1894–1905. doi:10.1056/NEJMoa1915745
Finn, R. S., Ryoo, B. Y., Merle, P., Kudo, M., Bouattour, M., Lim, H. Y., et al. (2020a). Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 38 (3), 193–202. doi:10.1200/jco.19.01307
Fogli, S., Gianfilippo, G., Cucchiara, F., Del Re, M., Valerio, L., Elisei, R., et al. (2021). Clinical pharmacology and drug-drug interactions of lenvatinib in thyroid cancer. Crit. Rev. Oncol. Hematol. 163, 103366. doi:10.1016/j.critrevonc.2021.103366
Frampton, J. E. (2020). Atezolizumab: a review in extensive-stage sclc. Drugs 80 (15), 1587–1594. doi:10.1007/s40265-020-01398-6
Freese, K., Seitz, T., Dietrich, P., Lee, S. M. L., Thasler, W. E., Bosserhoff, A., et al. (2019). Histone deacetylase expressions in hepatocellular carcinoma and functional effects of histone deacetylase inhibitors on liver cancer cells in vitro. Cancers (Basel) 11 (10), 1587. doi:10.3390/cancers11101587
Fu, Y., Wei, X., Lin, L., Xu, W., and Liang, J. (2018). Adverse reactions of sorafenib, sunitinib, and imatinib in treating digestive system tumors. Thorac. Cancer 9 (5), 542–547. doi:10.1111/1759-7714.12608
Fuchs, C. S., Shitara, K., Di Bartolomeo, M., Lonardi, S., Al-Batran, S. E., Van Cutsem, E., et al. (2019). Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 20 (3), 420–435. doi:10.1016/s1470-2045(18)30791-5
Galle, P. R., Finn, R. S., Qin, S., Ikeda, M., Zhu, A. X., Kim, T. Y., et al. (2021). Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 22 (7), 991–1001. doi:10.1016/s1470-2045(21)00151-0
Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., et al. (2020). Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017. doi:10.1016/j.ctrv.2020.102017
Goyal, L., Muzumdar, M. D., and Zhu, A. X. (2013). Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin. Cancer Res. 19 (9), 2310–2318. doi:10.1158/1078-0432.Ccr-12-2791
Grothey, A., Blay, J. Y., Pavlakis, N., Yoshino, T., and Bruix, J. (2020). Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat. Rev. 86, 101993. doi:10.1016/j.ctrv.2020.101993
Grothey, A., Van Cutsem, E., Sobrero, A., Siena, S., Falcone, A., Ychou, M., et al. (2013). Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (9863), 303–312. doi:10.1016/s0140-6736(12)61900-x
Guntipalli, P., Pakala, R., Kumari Gara, S., Ahmed, F., Bhatnagar, A., Endaya Coronel, M. K., et al. (2021). Worldwide prevalence, genotype distribution and management of hepatitis C. Acta Gastroenterol. Belg 84 (4), 637–656. doi:10.51821/84.4.015
Guo, R., Luo, J., Chang, J., Rekhtman, N., Arcila, M., and Drilon, A. (2020). MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 17 (9), 569–587. doi:10.1038/s41571-020-0377-z
Hellmann, M. D., Paz-Ares, L., Bernabe, C. R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381 (21), 2020–2031. doi:10.1056/NEJMoa1910231
Heymann, F., and Tacke, F. (2016). Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13 (2), 88–110. doi:10.1038/nrgastro.2015.200
Hsu, C., Rimassa, L., Sun, H. C., Vogel, A., and Kaseb, A. O. (2021). Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther. Adv. Med. Oncol. 13, 17588359211031141. doi:10.1177/17588359211031141
Huang, A., Yang, X. R., Chung, W. Y., Dennison, A. R., and Zhou, J. (2020). Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target Ther. 5 (1), 146. doi:10.1038/s41392-020-00264-x
Huemer, F., Piringer, G., Schlintl, V., Hackl, H., Rinnerthaler, G., Thaler, J., et al. (2020). Hospitalizations and clinical outcome in metastatic colorectal cancer during regorafenib or TAS-102 therapy. Cancers (Basel) 12 (10), 2812. doi:10.3390/cancers12102812
Huynh, H., Ong, R., Goh, K. Y., Lee, L. Y., Puehler, F., Scholz, A., et al. (2019). Sorafenib/MEK inhibitor combination inhibits tumor growth and the Wnt/β-catenin pathway in xenograft models of hepatocellular carcinoma. Int. J. Oncol. 54 (3), 1123–1133. doi:10.3892/ijo.2019.4693
Jiang, Y., Han, Q., Zhao, H., and Zhang, J. (2021). The mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatocell. Carcinoma 8, 435–450. doi:10.2147/jhc.S307962
Keam, S. J., and Duggan, S. (2021). Donafenib: first approval. Drugs 81 (16), 1915–1920. doi:10.1007/s40265-021-01603-0
Keam, S. J. (2023). Tremelimumab: first approval. Drugs 83 (1), 93–102. doi:10.1007/s40265-022-01827-8
Kelley, R. K., Rimassa, L., Cheng, A. L., Kaseb, A., Qin, S., Zhu, A. X., et al. (2022). Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 23 (8), 995–1008. doi:10.1016/s1470-2045(22)00326-6
Kelley, R. K., Sangro, B., Harris, W., Ikeda, M., Okusaka, T., Kang, Y. K., et al. (2021). Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 39 (27), 2991–3001. doi:10.1200/jco.20.03555
Kim, B. H., Yu, S. J., Kang, W., Cho, S. B., Park, S. Y., Kim, S. U., et al. (2022). Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 37 (3), 428–439. doi:10.1111/jgh.15727
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391 (10126), 1163–1173. doi:10.1016/s0140-6736(18)30207-1
Li, J., Qin, S., Xu, R., Yau, T. C. C., Ma, B., Pan, H., et al. (2015). Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 16 (6), 619–629. doi:10.1016/s1470-2045(15)70156-7
Li, M., and Kroetz, D. L. (2018). Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol. Ther. 182, 152–160. doi:10.1016/j.pharmthera.2017.08.012
Li, Y., Gao, Z. H., and Qu, X. J. (2015). The adverse effects of sorafenib in patients with advanced cancers. Basic Clin. Pharmacol. Toxicol. 116 (3), 216–221. doi:10.1111/bcpt.12365
Liu, L., Cao, Y., Chen, C., Zhang, X., McNabola, A., Wilkie, D., et al. (2006). Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66 (24), 11851–11858. doi:10.1158/0008-5472.Can-06-1377
Llovet, J. M., Bru, C., and Bruix, J. (1999). Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19 (3), 329–338. doi:10.1055/s-2007-1007122
Llovet, J. M., Kelley, R. K., Villanueva, A., Singal, A. G., Pikarsky, E., Roayaie, S., et al. (2021). Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7 (1), 6. doi:10.1038/s41572-020-00240-3
Llovet, J. M., Kudo, M., Merle, P., Meyer, T., Qin, S., Ikeda, M., et al. (2023). Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 24 (12), 1399–1410. doi:10.1016/s1470-2045(23)00469-2
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J. F., et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359 (4), 378–390. doi:10.1056/NEJMoa0708857
Mabeta, P., and Steenkamp, V. (2022). The VEGF/VEGFR Axis revisited: implications for cancer therapy. Int. J. Mol. Sci. 23 (24), 15585. doi:10.3390/ijms232415585
Maeda, A., Irie, K., Ando, H., Hasegawa, A., Taniguchi, H., Kadowaki, S., et al. (2019). Associations among regorafenib concentrations, severe adverse reactions, and ABCG2 and OATP1B1 polymorphisms. Cancer Chemother. Pharmacol. 83 (1), 107–113. doi:10.1007/s00280-018-3710-9
Markham, A. (2016). Atezolizumab: first global approval. Drugs 76 (12), 1227–1232. doi:10.1007/s40265-016-0618-8
Marrero, J. A., Kulik, L. M., Sirlin, C. B., Zhu, A. X., Finn, R. S., Abecassis, M. M., et al. (2018). Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 68 (2), 723–750. doi:10.1002/hep.29913
McGlynn, K. A., Petrick, J. L., and London, W. T. (2015). Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin. Liver Dis. 19 (2), 223–238. doi:10.1016/j.cld.2015.01.001
McGregor, B., Mortazavi, A., Cordes, L., Salabao, C., Vandlik, S., and Apolo, A. B. (2022). Management of adverse events associated with cabozantinib plus nivolumab in renal cell carcinoma: a review. Cancer Treat. Rev. 103, 102333. doi:10.1016/j.ctrv.2021.102333
McLellan, B., Ciardiello, F., Lacouture, M. E., Segaert, S., and Van Cutsem, E. (2015). Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann. Oncol. 26 (10), 2017–2026. doi:10.1093/annonc/mdv244
Méndez-Blanco, C., Fondevila, F., García-Palomo, A., González-Gallego, J., and Mauriz, J. L. (2018). Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp. Mol. Med. 50 (10), 1–9. doi:10.1038/s12276-018-0159-1
Mizukoshi, E., Nakagawa, H., Kitahara, M., Yamashita, T., Arai, K., Sunagozaka, H., et al. (2015). Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 369 (1), 242–249. doi:10.1016/j.canlet.2015.08.020
Moon, H., and Ro, S. W. (2021). MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers (Basel) 13 (12), 3026. doi:10.3390/cancers13123026
Morse, M. A., Sun, W., Kim, R., Abada, P. B., Mynderse, M., et al. (2019). The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 25 (3), 912–920. doi:10.1158/1078-0432.Ccr-18-1254
Motzer, R. J., Tannir, N. M., McDermott, D. F., Arén Frontera, O., Melichar, B., Choueiri, T. K., et al. (2018). Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378 (14), 1277–1290. doi:10.1056/NEJMoa1712126
Nervo, A., Retta, F., Ragni, A., Piovesan, A., Mella, A., Biancone, L., et al. (2021). Nephrotoxicity in advanced thyroid cancer treated with tyrosine kinase inhibitors: an update. Crit. Rev. Oncol. Hematol. 168, 103533. doi:10.1016/j.critrevonc.2021.103533
Omata, M., Cheng, A. L., Kokudo, N., Kudo, M., Lee, J. M., Jia, J., et al. (2017). Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11 (4), 317–370. doi:10.1007/s12072-017-9799-9
Overman, M. J., Lonardi, S., Wong, K. Y. M., Lenz, H. J., Gelsomino, F., Aglietta, M., et al. (2018). Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36 (8), 773–779. doi:10.1200/jco.2017.76.9901
Pal, S. K., McGregor, B., Suárez, C., Tsao, C. K., Kelly, W., Vaishampayan, U., et al. (2021). Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J. Clin. Oncol. 39 (33), 3725–3736. doi:10.1200/jco.21.00939
Pathania, S., Pentikäinen, O. T., and Singh, P. K. (2021). A holistic view on c-Kit in cancer: structure, signaling, pathophysiology and its inhibitors. Biochim. Biophys. Acta Rev. Cancer 1876 (2), 188631. doi:10.1016/j.bbcan.2021.188631
Patt, Y., Rojas-Hernandez, C., Fekrazad, H. M., Bansal, P., and Lee, F. C. (2017). Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist 22 (10), 1158–e116. doi:10.1634/theoncologist.2017-0168
Poole, R. M., and Vaidya, A. (2014). Ramucirumab: first global approval. Drugs 74 (9), 1047–1058. doi:10.1007/s40265-014-0244-2
Psyrri, A., Fayette, J., Harrington, K., Gillison, M., Ahn, M. J., Takahashi, S., et al. (2023). Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann. Oncol. 34 (3), 262–274. doi:10.1016/j.annonc.2022.12.008
Qin, S., Bi, F., Gu, S., Bai, Y., Chen, Z., Wang, Z., et al. (2021a). Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 39 (27), 3002–3011. doi:10.1200/jco.21.00163
Qin, S., Chen, Z., Fang, W., Ren, Z., Xu, R., Ryoo, B. Y., et al. (2023). Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J. Clin. Oncol. 41 (7), 1434–1443. doi:10.1200/jco.22.00620
Qin, S., Ren, Z., Feng, Y. H., Yau, T., Wang, B., Zhao, H., et al. (2021b). Atezolizumab plus bevacizumab versus sorafenib in the Chinese subpopulation with unresectable hepatocellular carcinoma: phase 3 randomized, open-label IMbrave150 study. Liver Cancer 10 (4), 296–308. doi:10.1159/000513486
Rahib, L., Smith, B. D., Aizenberg, R., Rosenzweig, A. B., Fleshman, J. M., and Matrisian, L. M. (2014). Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74 (11), 2913–2921. doi:10.1158/0008-5472.CAN-14-0155
Reed, N., Glen, H., Gerrard, G., Good, J., Lei, M., Lyon, A. R., et al. (2020). Expert consensus on the management of adverse events during treatment with lenvatinib for thyroid cancer. Clin. Oncol. R. Coll. Radiol. 32 (5), e145–e153. doi:10.1016/j.clon.2019.11.010
Ren, Z., Xu, J., Bai, Y., Xu, A., Cang, S., Du, C., et al. (2021). Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 22 (7), 977–990. doi:10.1016/s1470-2045(21)00252-7
Rimassa, L., Danesi, R., Pressiani, T., and Merle, P. (2019). Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 77, 20–28. doi:10.1016/j.ctrv.2019.05.004
Rimini, M., Rimassa, L., Ueshima, K., Burgio, V., Shigeo, S., Tada, T., et al. (2022). Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open 7 (6), 100591. doi:10.1016/j.esmoop.2022.100591
Ringelhan, M., Pfister, D., O'Connor, T., Pikarsky, E., and Heikenwalder, M. (2018). The immunology of hepatocellular carcinoma. Nat. Immunol. 19 (3), 222–232. doi:10.1038/s41590-018-0044-z
Rizzo, A., Dadduzio, V., Ricci, A. D., Massari, F., Di Federico, A., Gadaleta-Caldarola, G., et al. (2022). Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expert Opin. Investig. Drugs 31 (4), 371–378. doi:10.1080/13543784.2021.1948532
Roddy, H., Meyer, T., and Roddie, C. (2022). Novel cellular therapies for hepatocellular carcinoma. Cancers (Basel) 14 (3), 504. doi:10.3390/cancers14030504
Roviello, G., Corona, S. P., Bozza, G., Aieta, M., Generali, D., Rodriquenz, M. G., et al. (2018). Lenvatinib for the treatment of renal cell carcinoma. Expert Opin. Investig. Drugs 27 (5), 507–512. doi:10.1080/13543784.2018.1472235
Rumgay, H., Arnold, M., Ferlay, J., Lesi, O., Cabasag, C. J., Vignat, J., et al. (2022). Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77 (6), 1598–1606. doi:10.1016/j.jhep.2022.08.021
Sangro, B., Melero, I., Wadhawan, S., Finn, R. S., Abou-Alfa, G. K., Cheng, A. L., et al. (2020). Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73 (6), 1460–1469. doi:10.1016/j.jhep.2020.07.026
Sangro, B., Sarobe, P., Hervás-Stubbs, S., and Melero, I. (2021). Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18 (8), 525–543. doi:10.1038/s41575-021-00438-0
Shi, D., Shi, Y., Kaseb, A. O., Qi, X., Zhang, Y., Chi, J., et al. (2020). Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin. Cancer Res. 26 (15), 3979–3989. doi:10.1158/1078-0432.Ccr-19-3259
Sonbol, M. B., Riaz, I. B., Naqvi, S. A. A., Almquist, D. R., Mina, S., Almasri, J., et al. (2020). Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 6 (12), e204930. doi:10.1001/jamaoncol.2020.4930
Song, R., Ma, S., Xu, J., Ren, X., Guo, P., Liu, H., et al. (2023b). A novel polypeptide encoded by the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR. Mol. Cancer 22 (1), 16. doi:10.1186/s12943-023-01719-9
Song, X., Kelley, R. K., Khan, A. A., Standifer, N., Zhou, D., Lim, K., et al. (2023a). Exposure-response analyses of tremelimumab monotherapy or in combination with durvalumab in patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 29 (4), 754–763. doi:10.1158/1078-0432.Ccr-22-1983
Sun, X., Zhang, Q., Mei, J., Yang, Z., Chen, M., and Liang, T. (2022). Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer 22 (1), 293. doi:10.1186/s12885-022-09405-7
Tai, W. M., Yong, W. P., Lim, C., Low, L. S., Tham, C. K., Koh, T. S., et al. (2016). A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann. Oncol. 27 (12), 2210–2215. doi:10.1093/annonc/mdw415
Takahashi, M. (2022). RET receptor signaling: function in development, metabolic disease, and cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 98 (3), 112–125. doi:10.2183/pjab.98.008
Tan, W., Luo, X., Li, W., Zhong, J., Cao, J., Zhu, S., et al. (2019). TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 40, 446–456. doi:10.1016/j.ebiom.2018.12.047
Tang, W., Chen, Z., Zhang, W., Cheng, Y., Zhang, B., Wu, F., et al. (2020). The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct. Target Ther. 5 (1), 87. doi:10.1038/s41392-020-0187-x
Tannir, N. M., Schwab, G., and Grünwald, V. (2017). Cabozantinib: an active novel multikinase inhibitor in renal cell carcinoma. Curr. Oncol. Rep. 19 (2), 14. doi:10.1007/s11912-017-0566-9
Vogel, A., Cervantes, A., Chau, I., Daniele, B., Llovet, J. M., Meyer, T., et al. (2018). Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29 (Suppl. 4), iv238–iv255. doi:10.1093/annonc/mdy308
Vogel, A., Meyer, T., Sapisochin, G., Salem, R., and Saborowski, A. (2022). Hepatocellular carcinoma. Lancet 400 (10360), 1345–1362. doi:10.1016/S0140-6736(22)01200-4
Vogel, A., Qin, S., Kudo, M., Su, Y., Hudgens, S., Yamashita, T., et al. (2021). Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 6 (8), 649–658. doi:10.1016/S2468-1253(21)00110-2
Wang, C., Vegna, S., Jin, H., Benedict, B., Lieftink, C., Ramirez, C., et al. (2019). Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 574 (7777), 268–272. doi:10.1038/s41586-019-1607-3
Wang, H., Rao, B., Lou, J., Li, J., Liu, Z., Li, A., et al. (2020). The function of the HGF/c-Met Axis in hepatocellular carcinoma. Front. Cell Dev. Biol. 8, 55. doi:10.3389/fcell.2020.00055
Wang, M., Wu, M., and Yang, T. (2019). The synergistic effect of sorafenib and TNF-α inhibitor on hepatocellular carcinoma. EBioMedicine 40, 11–12. doi:10.1016/j.ebiom.2019.01.007
Wang, R., Yamada, T., Arai, S., Fukuda, K., Taniguchi, H., Tanimoto, A., et al. (2019). Distribution and activity of lenvatinib in brain tumor models of human anaplastic thyroid cancer cells in severe combined immune deficient mice. Mol. Cancer Ther. 18 (5), 947–956. doi:10.1158/1535-7163.MCT-18-0695
Wichelmann, T. A., Abdulmujeeb, S., and Ehrenpreis, E. D. (2021). Bevacizumab and gastrointestinal perforations: a review from the FDA adverse event reporting system (FAERS) database. Aliment. Pharmacol. Ther. 54 (10), 1290–1297. doi:10.1111/apt.16601
Wilhelm, S. M., Adnane, L., Newell, P., Villanueva, A., Llovet, J. M., and Lynch, M. (2008). Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 7 (10), 3129–3140. doi:10.1158/1535-7163.Mct-08-0013
Wilhelm, S. M., Dumas, J., Adnane, L., Lynch, M., Carter, C. A., Schütz, G., et al. (2011). Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 129 (1), 245–255. doi:10.1002/ijc.25864
Xia, S., Pan, Y., Liang, Y., Xu, J., and Cai, X. (2020). The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine 51, 102610. doi:10.1016/j.ebiom.2019.102610
Xie, Y., Su, N., Yang, J., Tan, Q., Huang, S., Jin, M., et al. (2020). FGF/FGFR signaling in health and disease. Signal Transduct. Target Ther. 5 (1), 181. doi:10.1038/s41392-020-00222-7
Yakes, F. M., Chen, J., Tan, J., Yamaguchi, K., Shi, Y., Yu, P., et al. (2011). Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10 (12), 2298–2308. doi:10.1158/1535-7163.Mct-11-0264
Yamazaki, H., Iwasaki, H., Takasaki, H., Suganuma, N., Sakai, R., Masudo, K., et al. (2019). Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Med. Baltim. 98 (10), e14774. doi:10.1097/MD.0000000000014774
Yang, C., Zhang, H., Zhang, L., Zhu, A. X., Bernards, R., Qin, W., et al. (2023). Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 20 (4), 203–222. doi:10.1038/s41575-022-00704-9
Yau, T., Kang, Y. K., Kim, T. Y., El-Khoueiry, A. B., Santoro, A., Sangro, B., et al. (2020). Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6 (11), e204564. doi:10.1001/jamaoncol.2020.4564
Yau, T., Park, J. W., Finn, R. S., Cheng, A. L., Mathurin, P., Edeline, J., et al. (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23 (1), 77–90. doi:10.1016/s1470-2045(21)00604-5
Yi, M., Zheng, X., Niu, M., Zhu, S., Ge, H., and Wu, K. (2022). Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer 21 (1), 28. doi:10.1186/s12943-021-01489-2
Yip, T. C., Lee, H. W., Chan, W. K., Wong, G. L., and Wong, V. W. (2022). Asian perspective on NAFLD-associated HCC. J. Hepatol. 76 (3), 726–734. doi:10.1016/j.jhep.2021.09.024
Zhao, Y., Zhang, Y. N., Wang, K. T., and Chen, L. (2020). Lenvatinib for hepatocellular carcinoma: from preclinical mechanisms to anti-cancer therapy. Biochim. Biophys. Acta Rev. Cancer 1874 (1), 188391. doi:10.1016/j.bbcan.2020.188391
Zhu, A. X., Finn, R. S., Edeline, J., Cattan, S., Ogasawara, S., Palmer, D., et al. (2018). Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19 (7), 940–952. doi:10.1016/s1470-2045(18)30351-6
Zhu, C., Wei, Y., and Wei, X. (2019). AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol. Cancer 18 (1), 153. doi:10.1186/s12943-019-1090-3
Zhu, Y., Chen, M., Xu, D., Li, T. E., Zhang, Z., Li, J. H., et al. (2022). The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol. Immunol. 19 (6), 726–737. doi:10.1038/s41423-022-00848-3
Zou, X., Tang, X. Y., Qu, Z. Y., Sun, Z. W., Ji, C. F., Li, Y. J., et al. (2022). Targeting the PDGF/PDGFR signaling pathway for cancer therapy: a review. Int. J. Biol. Macromol. 202, 539–557. doi:10.1016/j.ijbiomac.2022.01.113
Zuo, R. C., Apolo, A. B., DiGiovanna, J. J., Parnes, H. L., Keen, C. M., Nanda, S., et al. (2015). Cutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinib. JAMA Dermatol. 151 (2), 170–177. doi:10.1001/jamadermatol.2014.2734
HCC Hepatocellular carcinoma
HBV Hepatitis B virus
HCV Hepatitis C virus
NAFLD Non-alcoholic fatty liver disease
SEER Surveillance Epidemiology End Results
BCLC Barcelona Liver Cancer Clinic
FDA Food and Drug Administration
ICIs Immune checkpoint inhibitors
VEGF Vascular endothelial growth factor
VEGFR Vascular endothelial growth factor receptor
PDGF Platelet-derived growth factor
PDGFR Platelet-derived growth factor receptor
FGF Fibroblast growth factor
FGFR Fibroblast growth factor receptor
HGF Hepatocyte growth factor
RET Rearranged during transfection
PD-1 Programmed death-1
PD-L1 Programmed death ligand 1
CTLA-4 Cytotoxic T-lymphocyte-associated antigen 4
OS Overall survival
EGF Epidermal growth factor
EGFR Epidermal growth factor receptor
GEMOX Gemcitabine and oxaliplatin
TKI Tyrosine kinase inhibitor
mNSCLC Metastatic non-small cell lung cancer
uHCC Unresectable HCC
TRAEs Treatment-related adverse events
R/M HNSCC Recurrent or metastatic head and neck squamous cell carcinoma
HGFR Hepatocyte growth factor receptor
RCC Renal cell carcinoma
MTC Medullary thyroid carcinoma
RPLS Reversible posterior leukoencephalopathy syndrome
PFS Progression-free-survival
METIV-HCC High MET expressing HCC
ANG2 Angiopoietin-2
FGF21 Fibroblast growth factor 21
ACT Adoptive cell therapy
Keywords: hepatocellular carcinoma, molecular-targeted drugs, sorafenib, immunotherapeutic agents, combination therapies
Citation: Chen H, Liu H, Zhang X, Wang S, Liu C, An K, Liu R and Tian X (2024) Diversified applications of hepatocellular carcinoma medications: molecular-targeted, immunotherapeutic, and combined approaches. Front. Pharmacol. 15:1422033. doi: 10.3389/fphar.2024.1422033
Received: 23 April 2024; Accepted: 16 September 2024;
Published: 27 September 2024.
Edited by:
Stefania Nobili, University of Florence, ItalyReviewed by:
Xiaochao Ma, University of Pittsburgh, United StatesCopyright © 2024 Chen, Liu, Zhang, Wang, Liu, An, Liu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Tian, dGlhbnhAenp1LmVkdS5jbg==; Ruijuan Liu, ZmNjbGl1cmpAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.