- 1Department of Thoracic Surgery, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Anesthesiology, The First Hospital of China Medical University, Shenyang, Liaoning, China
Lung cancer, recognized globally as a leading cause of malignancy-associated morbidity and mortality, is marked by its high prevalence and lethality, garnering extensive attention within the medical community. Mitophagy is a critical cellular process that plays a crucial role in regulating metabolism and ensuring quality control within cells. Its relevance to lung cancer has garnered significant attention among researchers and scientists. Mitophagy’s involvement in lung cancer encompasses its initiation, progression, metastatic dissemination and treatment. The regulatory landscape of mitophagy is complex, involving numerous signaling proteins and pathways that may exhibit aberrant alterations or mutations within the tumor environment. In the field of treatment, the regulation of mitophagy is considered key to determining cancer chemotherapy, radiation therapy, other treatment options, and drug resistance. Contemporary investigations are directed towards harnessing mitophagy modulators, both inhibitors and activators, in therapeutic strategies, with an emphasis on achieving specificity to minimize collateral damage to healthy cellular populations. Furthermore, molecular constituents and pathways affiliated with mitophagy, serving as potential biomarkers, offer promising avenues for enhancing diagnostic accuracy, prognostic assessment, and prediction of therapeutic responses in lung cancer. Future endeavors will also involve investigating the impact of mitophagy on the composition and function of immune cells within the tumor microenvironment, aiming to enhance our understanding of how mitophagy modulates the immune response to lung cancer. This review aims to comprehensively overview recent advancements about the role of mitophagy in the tumor genesis, progenesis and metastasis, and the impact of mitophagy on the treatment of lung cancer. We also discussed the future research direction of mitophagy in the field of lung cancer.
1 Introduction
Lung cancer represents the foremost cause of oncology-related mortality globally, with approximately 2.1 million new cases and 1.8 million deaths each year (Garg et al., 2020). The clinical importance of lung cancer is highlighted not only by its high rates of incidence and mortality, but also by the difficulties in detecting it at an early stage. Lung cancer is mostly diagnosed in advanced stages, after significant disease progression, which greatly limits the effectiveness of current treatments. The complexities of lung cancer are further complicated by its diverse pathological classifications, with non-small cell lung cancer (NSCLC) being the most common type. (Yan et al., 2024). The treatment and prognosis of lung cancer are significantly influenced by various factors, including the patient’s genetic predisposition, molecular characteristics of the tumor, and metabolic status of the cancer cells.
In recent years, the elucidation of cellular metabolic pathways’ roles in the progression of lung cancer has garnered increasing attention. In this context, mitophagy, a quintessential process for cellular metabolic regulation and quality assurance, has been recognized as a critical mechanism (Zhou et al., 2023). This specialized autophagic process is indispensable for the removal of impaired or non-functional mitochondria, thereby safeguarding cellular energetic equilibrium and metabolic stability (Filippelli et al., 2022). Under physiological conditions, mitophagy serves as a protective mechanism, forestalling oxidative stress and cellular demise consequent to mitochondrial malfunctions (Wang et al., 2024). Mitophagy is instrumental in sustaining the cellular milieu’s stability and the equilibrium of cellular energetics through the selective eradication of impaired or non-functional mitochondria. Within the ambit of oncogenesis, mitophagy has a dual function. On one side, it contributes to carcinogenesis by promoting cellular migration, maintaining cancer stemness, and fostering resistance to pharmacological interventions. Conversely, the induction of mitophagy through specific pharmacological agents has been shown to disrupt normal cellular metabolic processes, trigger cellular stress responses, and induce genetic alterations by exacerbating mitochondrial dysfunction, ultimately leading to an antitumor effect (Qiu et al., 2021). As mitophagy is increasingly recognized as a crucial mechanism in the development and progression of lung cancer, its dysregulation within the context of lung cancer can have significant consequences. These include influencing tumor cell proliferation, viability, resistance to drugs, and metastatic capabilities. Understanding the complex role of mitophagy in lung cancer is essential for developing targeted therapeutic strategies and improving patient outcomes.
This review provides a comprehensive summary of the involvement of mitophagy in the initiation, progression, and therapeutic approaches of lung cancer, along with the underlying mechanisms elucidated by recent studies. Additionally, the challenges and future research directions of mitophagy in lung cancer are also discussed.
2 Overview of mitophagy
Mitophagy, a critical cellular process, involves the selective degradation of mitochondria through autophagy, and plays a crucial role in maintaining mitochondrial quality control and cellular homeostasis (Pickles et al., 2018; Filippelli et al., 2022; Yang et al., 2024).
Mitophagy is initiated by a complex signaling pathway in response to various cellular stresses, such as mitochondrial damage, oxidative stress, or energy depletion (Arora et al., 2022). The primary modalities that govern mitophagy encompass ubiquitin-dependent, receptor dependent, and other pathways (Ma et al., 2023; Tang et al., 2024). The process begins with the recognition and tagging of damaged or dysfunctional mitochondria by specific proteins, such as Parkin, proteins like PTEN-induced kinase 1 (PINK1), Bcl-2/adenovirus E1B 19-kDa-interacting protein 3 (BNIP3), and Nix (Wu et al., 2023; Yang et al., 2024). These proteins work together to target the damaged mitochondria for degradation. Once tagged, the damaged mitochondria are engulfed by a double-membraned structure called the autophagosome (Lu et al., 2023). This structure then fuses with a lysosome, forming an autolysosome (Lu et al., 2023). The lysosome contains enzymatic machinery that degrades the contents of the autophagosome, including the damaged mitochondria. Mitophagy eliminates dysfunctional mitochondria, which could otherwise lead to the accumulation of toxic molecules and the induction of cellular apoptosis (Chourasia et al., 2015). It also allows for the recycling of damaged mitochondria components, such as proteins and lipids, thus promoting mitochondrial renewal and maintaining cellular energy metabolism (Morán et al., 2014).
3 Role of mitophagy in the development and progression of lung cancer
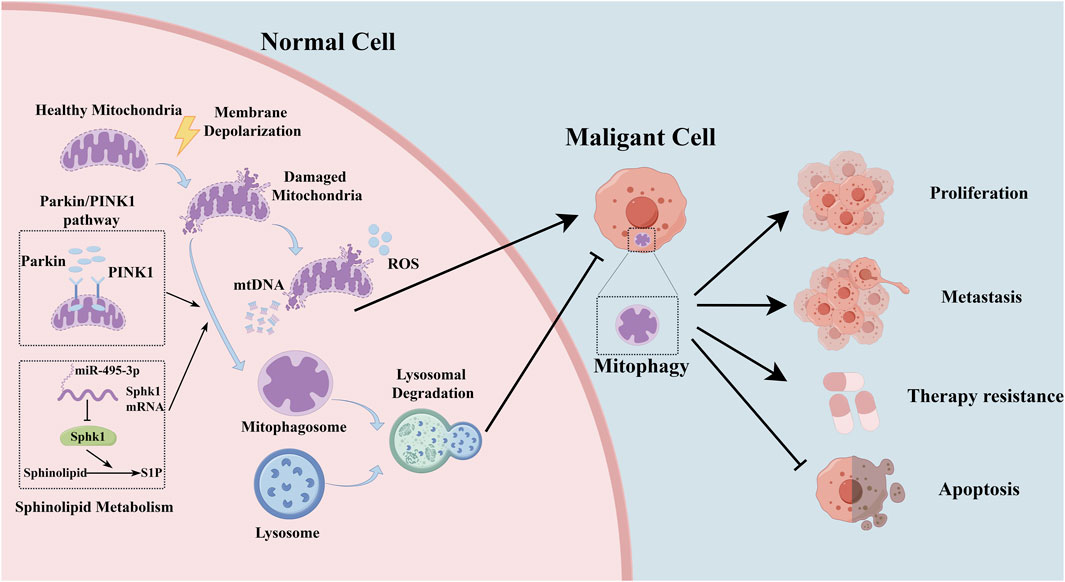
At the molecular level, the regulatory landscape of mitophagy in lung cancer is governed by a complex network of key molecules and signaling pathways. (Berthier et al., 2011; Liu M. et al., 2021; Arora et al., 2022). Within the milieu of lung cancer, disruptions or dysfunctions in these signaling pathways can significantly alter the accuracy and efficiency of mitophagy. These regulatory perturbations can profoundly impact tumor cell behavior and the course of disease progression, highlighting the complex interaction between cellular homeostasis mechanisms and oncogenic processes (Figure 1; Table 1).
3.1 The tumorigenesis of lung cancer and mitophagy
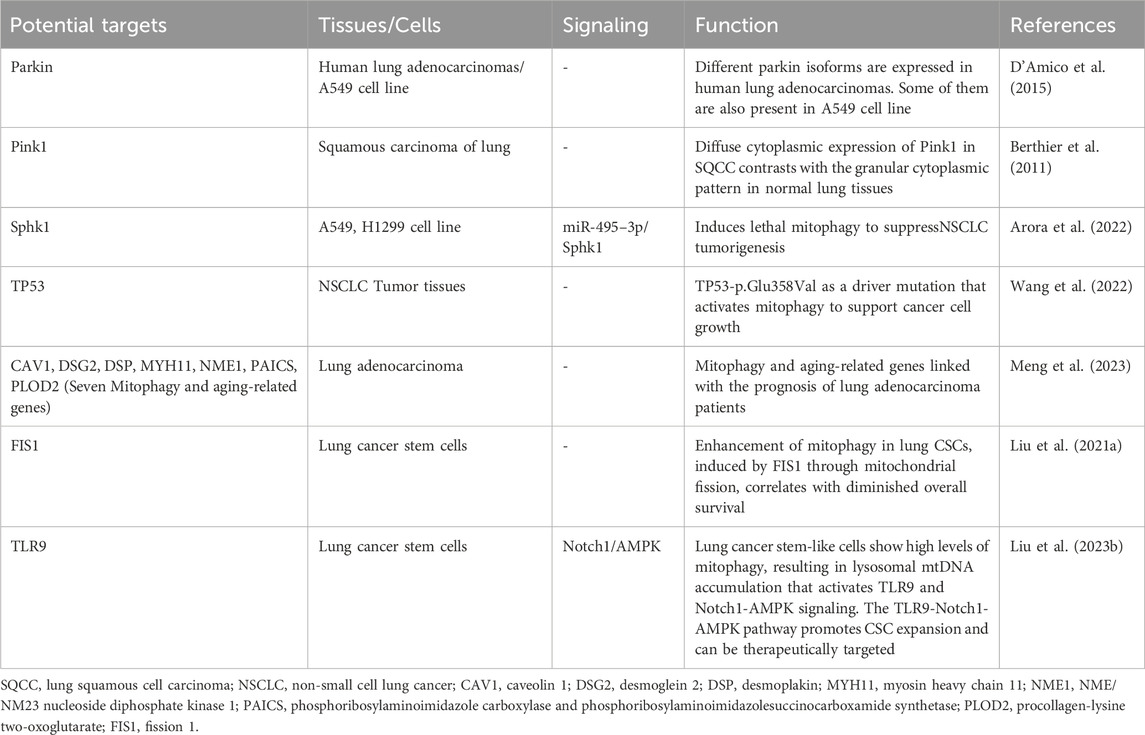
In early-stage lung cancer, there are significant alterations in the cell environment, including augmented oxidative stress and disturbances in energy metabolism (LeBleu et al., 2014). Mitophagy plays a critical role in maintaining mitochondrial balance by removing damaged mitochondria (Chen et al., 2024). As a result, activating mitophagy helps eliminate damaged mitochondria, reducing the release of reactive oxygen species (ROS). This process is crucial for maintaining energy balance and reducing oxidative stress, which could potentially prevent the oncogenic transformation of cells (Song et al., 2023). Impaired mitophagy leads to decreased elimination of damaged mitochondria, resulting in elevated ROS production and the buildup of mitochondrial DNA in the cytoplasm (Ng Kee Kwong et al., 2017). Mitophagy may play a tumor-suppressive role by reducing excessive ROS production and inhibiting inflammasome activation (Morselli et al., 2011; Ng Kee Kwong et al., 2017). Parkin, an essential E3 ubiquitin ligase, is activated by phosphorylated ubiquitin and plays a crucial role in orchestrating the polyubiquitination of a wide array of substrates (Morselli et al., 2011; McWilliams and Muqit, 2017). Proteins like PTEN-induced kinase 1 (PINK1) and Parkin function as key regulators in detecting mitochondrial stress and initiating autophagic responses (Berthier et al., 2011). Disruption in the Pink1/Parkin mitophagy pathway, also observed in lung cancer, plays a role in the pathogenesis of chronic obstructive pulmonary disease (COPD) (Mizumura et al., 2014; D’Amico et al., 2015). The diminution of Parkin in COPD-afflicted lungs correlates with increased ROS and senescence in bronchial epithelial cells (Ito et al., 2015). Notably, diffuse cytoplasmic expression of Pink1 in lung squamous cell carcinoma (SQCC) contrasts with the granular cytoplasmic pattern in normal lung tissues, implicating aberrant Pink1 expression in lung carcinogenesis (Berthier et al., 2011). Human lung adenocarcinomas exhibit variable Parkin isoforms, potentially modulating apoptosis, mitophagy, and mitochondrial fusion (D'Amico et al., 2015). Sphingolipid metabolites, specifically ceramide and sphingosine-1-phosphate (S1P), play a crucial role in cellular proliferation and apoptosis. Sphk1, a key enzyme converting sphingosine into S1P, promotes cell proliferation and survival (Ogretmen, 2018). MiR-495–3p, by targeting Sphk1, shifts the sphingolipid balance towards ceramide, inducing lethal mitophagy to inhibit NSCLC tumorigenesis (Arora et al., 2022). These studies indicate that mitophagy has a dual role in the early stages of lung cancer development, serving as both a protective mechanism and a facilitator of cancer initiation (Gozuacik and Kimchi, 2004).
3.2 Mitophagy in lung cancer progression and metastasis
As lung cancer advances, the intricacies of mitophagy’s role become increasingly complex. Mitochondria, pivotal for intracellular energy metabolism, assume a critical role, especially in tumor cells, given their elevated energy requisites (Liu J. et al., 2023). This process meticulously governs cellular metabolic states and energy generation, profoundly influencing the proliferation and division of lung cancer cells (Zhu et al., 2022). Enhanced mitophagy furnishes additional energy requisite for the expedited proliferation and growth of cancer cells (Qiu et al., 2021). Simultaneously, it occupies a central role in cellular death mechanisms, encompassing apoptosis and necrosis (Li et al., 2023). Under certain conditions, lung cancer cells may invoke mitophagy to evade apoptosis, engendering heightened drug resistance and survival (Liu D. et al., 2021; Liu Z. et al., 2023). Moreover, the metastatic process in cancer is intricately linked with mitophagy (He et al., 2023). Successful metastasis necessitates not only ample energy for cancer cells but also alterations in cell-to-cell interactions and migratory capabilities. Mitophagy modulates these aspects by influencing various intracellular signaling pathways and molecules, thereby affecting cellular adhesion, motility, and invasive potential (Liu D. et al., 2021; Wu et al., 2022; Liu Z. et al., 2023; Li et al., 2023).
The TP53 gene, prevalently mutated in cancer and recognized as a tumor suppressor, is mutated in half of NSCLC cases (Mogi and Kuwano, 2011; Nguele Meke et al., 2024). A next-generation sequencing (NGS) study on tumor tissues from 314 Chinese NSCLC patients delineated the mutational landscape in NSCLC, identifying TP53-p.Glu358Val as a driver mutation that activates mitophagy to support cancer cell growth (Wang et al., 2022). Pharmacological inhibition of autophagy/mitophagy selectively curtails the proliferation of TP53-null or TP53-p.Glu358Val-expressing lung cancer cells (Wang et al., 2022). Mitophagy and aging (MiAg)-related genes are pivotal in tumors and prognostication for various cancer types. Seven MiAg-related genes—caveolin 1(CAV1), desmoglein 2 (DSG2), desmoplakin (DSP), myosin heavy chain 11 (MYH11), NME/NM23 nucleoside diphosphate kinase 1 (NME1), phosphoribosylaminoimidazole carboxylase and phosphoribosylaminoimidazolesuccinocarboxamide synthetase (PAICS), procollagen-lysine 2-oxoglutarate 5-dioxygenase 2(PLOD2)—have been significantly linked with the prognosis of lung adenocarcinoma (LUAD) patients (Meng et al., 2023). Cancer stem cells (CSCs), first identified in the hematopoietic system and subsequently in various solid tumors, exhibit self-renewal and differentiation capacities, significantly influencing tumorigenesis, metastasis, and recurrence (Bao et al., 2013; Liu D. et al., 2021). Mitochondria, serving as the energy reservoir for cells, and mitophagy, are vital for CSC survival (Lee et al., 2018). Enhancement of mitophagy in lung CSCs, induced by fission-1 (FIS1) through mitochondrial fission, correlates with diminished overall survival (Liu D. et al., 2021). Hypermitophagy characterizes human lung CSCs, fostering metabolic adaptation via the Notch1-AMPK axis to propel CSC expansion (Liu Z. et al., 2023).
4 The relationship between mitophagy and lung cancer treatment
4.1 The growing significance of mitophagy in lung cancer therapeutics
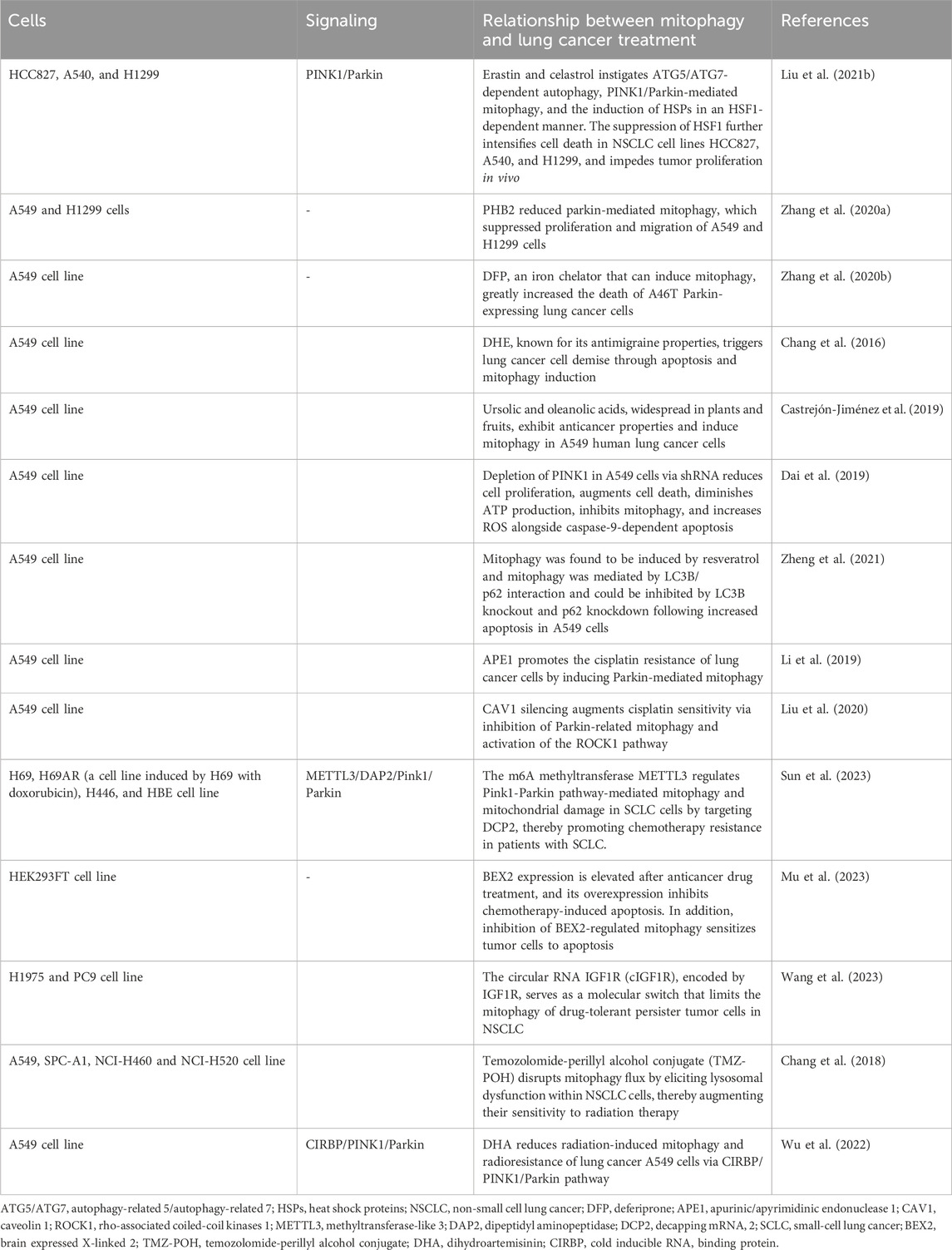
The nuanced role of mitophagy in lung cancer therapeutics has emerged as a focal point in modern oncological discourse. Its critical influence on the biological dynamics of lung cancer cells, significantly impacting the efficacy of diverse therapeutic approaches, is increasingly acknowledged (Table 2).
Melittin, known for its robust surface activity on lipid membranes, interacts with membranes and induces membrane fragmentation (Li et al., 2023). Recently, melittin has demonstrated promising therapeutic effects in various tumors, including glioblastoma (Ertilav and Nazıroğlu, 2023), breast cancer (Daniluk et al., 2023), and melanoma (Jeong et al., 2023). It targets mitochondria and impedes mitophagy flux in adenocarcinoma alveolar cancer (A549) cell lines (Li et al., 2023). Erastin, a classical inducer of ferroptosis, exhibits promising pharmacological effects in cancer therapeutics (Mou et al., 2019). Celastrol, derived from the traditional Chinese medicinal herb Tripterygium wilfordii, known as Thunder God Vine, has demonstrated potent antitumor activities across various cancer cell lines and in vivo models (Tang et al., 2015). The synergistic application of erastin with celastrol precipitates cell death at subtoxic concentrations, amplifying reactive oxygen species (ROS) production, perturbing mitochondrial membrane potential, and facilitating mitochondrial fission. Moreover, the concurrent administration of erastin and celastrol instigates autophagy-related 5 (ATG5)/ATG7-dependent autophagy, PINK1/Parkin-mediated mitophagy, and the induction of heat shock proteins (HSPs) in a heat shock factor 1 (HSF1)-dependent manner (Liu M. et al., 2021). The suppression of HSF1 further intensifies cell death in NSCLC cell lines HCC827, A540, and H1299, and impedes tumor proliferation in vivo (Liu M. et al., 2021). Prohibitin 2 (PHB2), situated in the inner mitochondrial membrane (IMM), functions as a mitophagy receptor (Wei et al., 2017). Elevated PHB2 levels in human NSCLC specimens, relative to adjacent non-tumor tissues, have been observed. The inhibition of PHB2 expression attenuates mitophagy in A549 and human lung adenocarcinoma (H1299) cells, as evidenced by reduced LC3 II/I and parkin markers and increased p62 protein levels. The downregulation of PHB2 diminishes parkin-mediated mitophagy, curbing the proliferation and migration of A549 and H1299 cells (Zhang H. et al., 2020). Typically, Parkin is dispersed throughout the nucleus and cytosol, relocating to damaged mitochondria under stress to facilitate the ubiquitination of mitochondrial proteins and instigate mitophagy (Harper et al., 2018). Mutations in the tumor suppressor gene PARK2 disrupt PINK1/Parkin-mediated mitophagy in lung cancer cells and deferiprone (DFP), an iron chelator that can induce mitophagy, greatly increased the death of A46T Parkin-expressing lung cancer cells (Zhang Z. L. et al., 2020). Dihydroergotamine tartrate (DHE), known for its antimigraine properties, triggers lung cancer cell demise through apoptosis and mitophagy induction (Chang et al., 2016). Ursolic and oleanolic acids, widespread in plants and fruits, exhibit anticancer properties and induce mitophagy in A549 human lung cancer cells (Castrejón-Jiménez et al., 2019). The depletion of PINK1 in A549 cells via shRNA reduces cell proliferation, augments cell death, diminishes ATP production, inhibits mitophagy, and increases ROS alongside caspase-9-dependent apoptosis (Dai et al., 2019). Cells deficient in PINK1 exhibit heightened sensitivity to the glycolytic inhibitor 3-bromopyruvate (3-BP), further disrupting ATP synthesis (Dai et al., 2019). Resveratrol (Res), a polyphenol phytoalexin, is recognized for its antitumorigenic and chemopreventive properties (Jang et al., 1997). Res induces non-canonical autophagy and apoptosis in A549 lung cancer cells, whereas LC3B/p62-mediated mitophagy shields tumor cells from apoptosis, elucidating the pivotal role of mitophagy in determining cell fate (Zheng et al., 2021).
4.2 Mitophagy and chemotherapy
Chemotherapy remains a fundamental strategy in lung cancer management, yet it frequently confronts the obstacle of tumor cell resistance to pharmacological interventions (Gridelli et al., 2005; Bao et al., 2013). In this milieu, mitophagy assumes a complex role, influencing the efficacy of chemotherapeutic regimens (Gridelli et al., 2005; Li et al., 2019; Liu et al., 2020). Cisplatin, a quintessential chemotherapeutic agent, is widely administered across a spectrum of solid tumors, encompassing testicular, head and neck, ovarian, esophageal, cervical, and non-small cell lung cancer (NSCLC) (Kelland, 2007). The pervasiveness of cisplatin resistance, however, compromises the therapeutic success in advanced NSCLC treatments (Gridelli et al., 2005). Apurinic/apyrimidinic endonuclease 1 (APE1), a pivotal multi-functional DNA repair enzyme, is integral for DNA damage repair, redox regulation, and transcription factor activity modulation (Li and Wilson, 2014). The mitochondrial translocation of APE1 enhances the mitochondrial membrane potential, diminishes cytochrome c levels, and triggers Parkin-mediated mitophagy, contributing to cisplatin resistance in lung cancer cells (Li et al., 2019). Conversely, Caveolin-1 (Cav-1) expression is significantly reduced in A549 lung cancer cells following cisplatin exposure, where Cav-1 silencing augments cisplatin sensitivity via inhibition of Parkin-related mitophagy and activation of the Rho-associated coiled-coil kinases 1 (ROCK1) pathway (Liu et al., 2020). Parkin-independent mitophagy also dictates the chemotherapeutic response in various cancers, notably breast and lung adenocarcinomas (Villa et al., 2017). While small cell lung cancer (SCLC) patients initially respond to platinum-based chemotherapy, durable responses are rare, frequently leading to chemoresistance and disease recurrence (Bao et al., 2013). Methyltransferase-like 3 (METTL3), a prominent m6A methyltransferase, influences a myriad of biological processes, including proliferation and migration (Zaccara et al., 2019). It modulates the Pink1-Parkin pathway-mediated mitophagy and mitochondrial damage in SCLC cells by targeting decapping mRNA 2 (DCP2), thereby facilitating chemoresistance in SCLC patients (Sun et al., 2023). Additionally, the BEX2 gene, part of the brain-expressed X-linked gene family, through crotonylation, interacts with NDP52 to augment mitophagy, influencing chemotherapeutic-induced apoptosis in NSCLC cells (Naderi, 2019; Mu et al., 2023). The circular RNA IGF1R (cIGF1R), encoded by IGF1R, serves as a molecular switch that limits the mitophagy of drug-tolerant persister tumor cells in NSCLC (Wang et al., 2023). Modulating mitophagy offers a promising avenue to enhance lung cancer cell sensitivity to chemotherapeutic agents, potentially circumventing the perennial challenge of drug resistance.
4.3 Radiotherapy and mitophagy
Mitophagy is garnering growing attention in lung cancer radiotherapy. Radiation therapy provokes DNA damage and oxidative stress within cellular structures, and mitophagy plays a pivotal role in mitigating these adverse effects (Chang et al., 2018; Wu et al., 2022). The Temozolomide-perillyl alcohol conjugate (TMZ-POH), an innovative derivative synthesized through the amalgamation of Temozolomide (TMZ) and perillyl alcohol (POH), has demonstrated pronounced anticancer efficacy across a spectrum of malignancies (Cho et al., 2012). TMZ-POH disrupts mitophagy flux by eliciting lysosomal dysfunction within Non-Small Cell Lung Cancer (NSCLC) cells, thereby augmenting their sensitivity to radiation therapy (Chang et al., 2018). Dihydroartemisinin (DHA), recognized for its anticancer properties and minimal toxicity, is increasingly being explored in both preclinical and clinical settings as an anticancer agent or a therapeutic adjuvant (Li et al., 2021; Wu et al., 2022). DHA attenuates radiation-induced mitophagy and radioresistance in lung cancer A549 cells through the inhibition of the Cold-Inducible RNA Binding Protein (CIRBP), offering new avenues for enhancing the effectiveness of radiotherapy in lung cancer treatment (Wu et al., 2022).
5 Challenges and future research directions in mitophagy for lung cancer
Mitophagy, an area of pivotal significance in lung cancer research, has witnessed notable advancements yet faces myriad challenges and uncharted territories. These challenges encompass the complexity of mitophagy mechanisms, its incorporation into lung cancer diagnostics, therapeutic strategies, and prognostic assessments.
5.1 Challenges in mitophagy for lung cancer
Mitophagy holds promise for lung cancer therapy due to its role in cellular homeostasis and apoptosis. However, several challenges must be addressed to harness its potential:
1. The intricate regulatory mechanisms of mitophagy in lung cancer are not fully understood, complicating the development of targeted interventions.
2. Mitophagy can either suppress or promote tumor growth depending on the context, making it challenging to predict its therapeutic impact.
3. Establishing a direct link between mitophagy activity and lung cancer patient outcomes is difficult due to the disease’s heterogeneity and the challenges in measuring mitophagy in clinical settings.
4. Creating drugs that selectively modulate mitophagy in lung cancer cells without affecting healthy cells is a significant challenge.
5. Understanding and overcoming the role of mitophagy in drug resistance is crucial for improving treatment efficacy.
6. The interaction between mitophagy and immune cell function within the tumor microenvironment is complex. The challenge lies in leveraging this interaction to enhance the effectiveness of immunotherapies.
7. Bridging the gap between preclinical findings and clinical application involves overcoming significant translational research barriers, including safety, efficacy, and regulatory hurdles.
8. The diverse genetic and environmental backgrounds of lung cancer patients complicate the development of personalized mitophagy-based therapies.
5.2 Future research directions in mitophagy for lung cancer
Although current studies enhance our understanding of the role of mitochondrial homeostasis in lung cancer, there are still some key questions about the process and function of mitophagy in lung cancer.
1. Further research is needed to dissect the molecular pathways that regulate mitophagy in lung cancer cells. Understanding these mechanisms could reveal novel therapeutic targets.
2. Studies should explore how mitophagy influences the composition and function of the immune cells within the tumor microenvironment. This could provide insights into how mitophagy modulates the immune response against lung cancer.
3. The development and testing of mitophagy modulators, either as standalone treatments or in combination with existing therapies, could enhance the efficacy of lung cancer treatments.
4. Research should focus on how mitophagy contributes to the development of drug resistance in lung cancer, potentially leading to strategies to overcome this barrier.
5. Identifying biomarkers related to mitophagy could improve early detection and prognosis of lung cancer, potentially leading to more personalized treatment approaches.
6. Investigating the relationship between mitophagy and metastasis could uncover new strategies to prevent or treat the spread of lung cancer.
6 Conclusion
Mitophagy has ascended to prominence within the domain of lung cancer research, serving as a pivotal mechanism for maintaining cellular energy equilibrium and metabolic integrity. It accomplishes this by targeting and removing damaged or dysfunctional mitochondria, thereby exerting a profound influence on the growth, survival, and chemoresistance of lung cancer cells. The role of mitophagy is integral to the initiation, advancement, and dissemination of lung cancer, with its regulatory network comprising an array of signaling molecules and pathways. These components are subject to potential dysregulation or mutations within the context of lung cancer, further complicating the disease’s pathology. Further exploration of these issues may facilitate the development of novel strategies for lung cancer prevention and treatment.
Author contributions
XZ: Conceptualization, Data curation, Visualization, Writing–original draft. DY: Conceptualization, Visualization, Writing–original draft. PT: Writing–original draft, Investigation. FC: Conceptualization, Supervision, Writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arora, S., Singh, P., Tabassum, G., Dohare, R., and Syed, M. A. (2022). miR-495-3p regulates sphingolipid metabolic reprogramming to induce Sphk1/ceramide mediated mitophagy and apoptosis in NSCLC. Free Radic. Biol. Med. 189, 71–84. doi:10.1016/j.freeradbiomed.2022.07.001
Bao, B., Ahmad, A., Azmi, A. S., Ali, S., and Sarkar, F. H. (2013). Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr. Protoc. Pharmacol. Chapter 14, Unit 14.25. doi:10.1002/0471141755.ph1425s61
Berthier, A., Navarro, S., Jiménez-Sáinz, J., Roglá, I., Ripoll, F., Cervera, J., et al. (2011). PINK1 displays tissue-specific subcellular location and regulates apoptosis and cell growth in breast cancer cells. Hum. Pathol. 42, 75–87. doi:10.1016/j.humpath.2010.05.016
Castrejón-Jiménez, N. S., Leyva-Paredes, K., Baltierra-Uribe, S. L., Castillo-Cruz, J., Campillo-Navarro, M., Hernández-Pérez, A. D., et al. (2019). Ursolic and oleanolic acids induce mitophagy in A549 human lung cancer cells. Mol. (Basel, Switz.) 24, 3444. doi:10.3390/molecules24193444
Chang, M., Song, X., Geng, X., Wang, X., Wang, W., Chen, T. C., et al. (2018). Temozolomide-Perillyl alcohol conjugate impairs Mitophagy flux by inducing lysosomal dysfunction in non-small cell lung Cancer cells and sensitizes them to irradiation. J. Exp. Clin. Cancer Res. CR 37, 250. doi:10.1186/s13046-018-0905-1
Chang, S. H., Lee, A. Y., Yu, K. N., Park, J., Kim, K. P., and Cho, M. H. (2016). Dihydroergotamine tartrate induces lung cancer cell death through apoptosis and mitophagy. Chemotherapy 61, 304–312. doi:10.1159/000445044
Chen, Y., Tang, W., Huang, X., An, Y., Li, J., Yuan, S., et al. (2024). Mitophagy in intracerebral hemorrhage: a new target for therapeutic intervention. Neural Regen. Res. 19, 316–323. doi:10.4103/1673-5374.379019
Cho, H. Y., Wang, W., Jhaveri, N., Torres, S., Tseng, J., Leong, M. N., et al. (2012). Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol. cancer Ther. 11, 2462–2472. doi:10.1158/1535-7163.MCT-12-0321
Chourasia, A. H., Boland, M. L., and Macleod, K. F. (2015). Mitophagy and cancer, Cancer Metab. 3, 4, doi:10.1186/s40170-015-0130-8
Dai, K., Radin, D. P., and Leonardi, D. (2019). PINK1 depletion sensitizes non-small cell lung cancer to glycolytic inhibitor 3-bromopyruvate: involvement of ROS and mitophagy. Pharmacol. Rep. P. R. 71, 1184–1189. doi:10.1016/j.pharep.2019.08.002
D'Amico, A. G., Maugeri, G., Magro, G., Salvatorelli, L., Drago, F., and D'Agata, V. (2015). Expression pattern of parkin isoforms in lung adenocarcinomas. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 36, 5133–5141. doi:10.1007/s13277-015-3166-z
Daniluk, K., Lange, A., Wójcik, B., Zawadzka, K., Bałaban, J., Kutwin, M., et al. (2023). Effect of melittin complexes with graphene and graphene oxide on triple-negative breast cancer tumors grown on chicken embryo chorioallantoic membrane. Int. J. Mol. Sci. 24, 8388. doi:10.3390/ijms24098388
Ertilav, K., and Nazıroğlu, M. (2023). Honey bee venom melittin increases the oxidant activity of cisplatin and kills human glioblastoma cells by stimulating the TRPM2 channel. Toxicon official J. Int. Soc. Toxinology 222, 106993. doi:10.1016/j.toxicon.2022.106993
Filippelli, R. L., Kamyabiazar, S., and Chang, N. C. (2022). Monitoring autophagy in neural stem and progenitor cells. Methods Mol. Biol. (Clifton, N.J.) 2515, 99–116. doi:10.1007/978-1-0716-2409-8_7
Garg, R., Cooke, M., Benavides, F., Abba, M. C., Cicchini, M., Feldser, D. M., et al. (2020). PKCε is required for KRAS-driven lung tumorigenesis. Cancer Res. 80, 5166–5173. doi:10.1158/0008-5472.CAN-20-1300
Gozuacik, D., and Kimchi, A. (2004). Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23, 2891–2906. doi:10.1038/sj.onc.1207521
Gridelli, C., Aapro, M., Ardizzoni, A., Balducci, L., De Marinis, F., Kelly, K., et al. (2005). Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 23, 3125–3137. doi:10.1200/JCO.2005.00.224
Harper, J. W., Ordureau, A., and Heo, J. M. (2018). Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 19, 93–108. doi:10.1038/nrm.2017.129
He, W., Chen, J., Zhou, Y., Deng, T., Feng, Y., Luo, X., et al. (2023). Mitophagy genes in ovarian cancer: a comprehensive analysis for improved immunotherapy. Oncology 14, 221. doi:10.1007/s12672-023-00750-y
Ito, S., Araya, J., Kurita, Y., Kobayashi, K., Takasaka, N., Yoshida, M., et al. (2015). PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11, 547–559. doi:10.1080/15548627.2015.1017190
Jang, M., Cai, L., Udeani, G. O., Slowing, K. V., Thomas, C. F., Beecher, C. W., et al. (1997). Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Sci. (New York, N.Y.) 275, 218–220. doi:10.1126/science.275.5297.218
Jeong, C., Kim, J., Han, I. H., Kim, S., Choi, I., Kim, H., et al. (2023). Melittin derived peptide-drug conjugate, M-DM1, inhibits tumor progression and induces effector cell infiltration in melanoma by targeting M2 tumor-associated macrophages. Front. Immunol. 14, 1178776. doi:10.3389/fimmu.2023.1178776
Kelland, L. (2007). The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584. doi:10.1038/nrc2167
LeBleu, V. S., O’Connell, J. T., Gonzalez Herrera, K. N., Wikman, H., Pantel, K., Haigis, M. C., et al. (2014). PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003. doi:10.1038/ncb3039
Lee, J., Liu, K., Stiles, B., and Ou, J. J. (2018). Mitophagy and hepatic cancer stem cells. Autophagy 14, 715–716. doi:10.1080/15548627.2018.1425058
Li, M., and Wilson, D. M. (2014). Human apurinic/apyrimidinic endonuclease 1. Antioxidants redox Signal. 20, 678–707. doi:10.1089/ars.2013.5492
Li, Q., Ma, Q., Cheng, J., Zhou, X., Pu, W., Zhong, X., et al. (2021). Dihydroartemisinin as a sensitizing agent in cancer therapies. OncoTargets Ther. 14, 2563–2573. doi:10.2147/OTT.S297785
Li, X., Li, Z., Meng, Y. Q., Qiao, H., Zhai, K. R., Li, Z. Q., et al. (2023). Melittin kills A549 cells by targeting mitochondria and blocking mitophagy flux. Redox Rep. Commun. Free Radic. Res. 28, 2284517. doi:10.1080/13510002.2023.2284517
Li, Z., Wang, Y., Wu, L., Dong, Y., Zhang, J., Chen, F., et al. (2019). Apurinic endonuclease 1 promotes the cisplatin resistance of lung cancer cells by inducing Parkin-mediated mitophagy. Oncol. Rep. 42, 2245–2254. doi:10.3892/or.2019.7345
Liu, D., Sun, Z., Ye, T., Li, J., Zeng, B., Zhao, Q., et al. (2021a). The mitochondrial fission factor FIS1 promotes stemness of human lung cancer stem cells via mitophagy. FEBS open bio 11, 1997–2007. doi:10.1002/2211-5463.13207
Liu, J., Wang, J., Xiong, A., Zhang, L., Zhang, Y., Liu, Y., et al. (2023a). Mitochondrial quality control in lung diseases: current research and future directions. Front. Physiology 14, 1236651. doi:10.3389/fphys.2023.1236651
Liu, M., Fan, Y., Li, D., Han, B., Meng, Y., Chen, F., et al. (2021b). Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol. Oncol. 15, 2084–2105. doi:10.1002/1878-0261.12936
Liu, Y., Fu, Y., Hu, X., Chen, S., Miao, J., Wang, Y., et al. (2020). Caveolin-1 knockdown increases the therapeutic sensitivity of lung cancer to cisplatin-induced apoptosis by repressing Parkin-related mitophagy and activating the ROCK1 pathway. J. Cell. Physiology 235, 1197–1208. doi:10.1002/jcp.29033
Liu, Z., Shan, S., Yuan, Z., Wu, F., Zheng, M., Wang, Y., et al. (2023b). Mitophagy bridges DNA sensing with metabolic adaption to expand lung cancer stem-like cells. EMBO Rep. 24, e54006. doi:10.15252/embr.202154006
Lu, Y., Li, Z., Zhang, S., Zhang, T., Liu, Y., and Zhang, L. (2023). Cellular mitophagy: mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 13, 736–766. doi:10.7150/thno.79876
Ma, Y., Zhou, X., Gui, M., Yao, L., Li, J., Chen, X., et al. (2023). Mitophagy in hypertension-mediated organ damage. Front. Cardiovasc. Med. 10, 1309863. doi:10.3389/fcvm.2023.1309863
McWilliams, T. G., and Muqit, M. M. (2017). PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 45, 83–91. doi:10.1016/j.ceb.2017.03.013
Meng, X., Song, W., Zhou, B., Liang, M., and Gao, Y. (2023). Prognostic and immune correlation analysis of mitochondrial autophagy and aging-related genes in lung adenocarcinoma. J. cancer Res. Clin. Oncol. 149, 16311–16335. doi:10.1007/s00432-023-05390-x
Mizumura, K., Cloonan, S. M., Nakahira, K., Bhashyam, A. R., Cervo, M., Kitada, T., et al. (2014). Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investigation 124, 3987–4003. doi:10.1172/JCI74985
Mogi, A., and Kuwano, H. (2011). TP53 mutations in nonsmall cell lung cancer. J. Biomed. Biotechnol. 2011, 583929. doi:10.1155/2011/583929
Morán, M., Delmiro, A., Blázquez, A., Ugalde, C., Arenas, J., and Martín, M. A. (2014). Bulk autophagy, but not mitophagy, is increased in cellular model of mitochondrial disease. Biochimica Biophysica Acta 1842, 1059–1070. doi:10.1016/j.bbadis.2014.03.013
Morselli, E., Galluzzi, L., Kepp, O., Mariño, G., Michaud, M., Vitale, I., et al. (2011). Oncosuppressive functions of autophagy. Antioxidants redox Signal. 14, 2251–2269. doi:10.1089/ars.2010.3478
Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., et al. (2019). Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 12, 34. doi:10.1186/s13045-019-0720-y
Mu, N., Wang, Y., Li, X., Du, Z., Wu, Y., Su, M., et al. (2023). Crotonylated BEX2 interacts with NDP52 and enhances mitophagy to modulate chemotherapeutic agent-induced apoptosis in non-small-cell lung cancer cells. Cell Death Dis. 14, 645. doi:10.1038/s41419-023-06164-6
Naderi, A. (2019). Molecular functions of brain expressed X-linked 2 (BEX2) in malignancies. Exp. Cell Res. 376, 221–226. doi:10.1016/j.yexcr.2019.02.014
Ng Kee Kwong, F., Nicholson, A. G., Harrison, C. L., Hansbro, P. M., Adcock, I. M., and Chung, K. F. (2017). Is mitochondrial dysfunction a driving mechanism linking COPD to nonsmall cell lung carcinoma? European respiratory review: an official. J. Eur. Respir. Soc. 26, 170040. doi:10.1183/16000617.0040-2017
Nguele Meke, F., Bai, Y., Ruiz-Avila, D., Carlock, C., Ayub, J., Miao, J., et al. (2024). Inhibition of PRL2 upregulates PTEN and attenuates tumor growth in Tp53-deficient sarcoma and lymphoma mouse models. Cancer Res. Commun. 4, 5–17. doi:10.1158/2767-9764.CRC-23-0308
Ogretmen, B. (2018). Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 18, 33–50. doi:10.1038/nrc.2017.96
Pickles, S., Vigié, P., and Youle, R. J. (2018). Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. CB 28, R170–R185. doi:10.1016/j.cub.2018.01.004
Qiu, Y. H., Zhang, T. S., Wang, X. W., Wang, M. Y., Zhao, W. X., Zhou, H. M., et al. (2021). Mitochondria autophagy: a potential target for cancer therapy. J. Drug Target. 29, 576–591. doi:10.1080/1061186X.2020.1867992
Song, Y., Cao, H., Zuo, C., Gu, Z., Huang, Y., Miao, J., et al. (2023). Mitochondrial dysfunction: a fatal blow in depression. Biomed. Pharmacother. 167, 115652. doi:10.1016/j.biopha.2023.115652
Sun, Y., Shen, W., Hu, S., Lyu, Q., Wang, Q., Wei, T., et al. (2023). METTL3 promotes chemoresistance in small cell lung cancer by inducing mitophagy. J. Exp. Clin. cancer Res. CR 42, 65. doi:10.1186/s13046-023-02638-9
Tang, S., Geng, Y., and Lin, Q. (2024). The role of mitophagy in metabolic diseases and its exercise intervention. Front. Physiology 15, 1339128. doi:10.3389/fphys.2024.1339128
Tang, W. J., Wang, J., Tong, X., Shi, J. B., Liu, X. H., and Li, J. (2015). Design and synthesis of celastrol derivatives as anticancer agents. Eur. J. Med. Chem. 95, 166–173. doi:10.1016/j.ejmech.2015.03.039
Villa, E., Proïcs, E., Rubio-Patiño, C., Obba, S., Zunino, B., Bossowski, J. P., et al. (2017). Parkin-independent mitophagy controls chemotherapeutic response in cancer cells. Cell Rep. 20, 2846–2859. doi:10.1016/j.celrep.2017.08.087
Wang, H., Liang, Y., Zhang, T., Yu, X., Song, X., Chen, Y., et al. (2023). C-IGF1R encoded by cIGF1R acts as a molecular switch to restrict mitophagy of drug-tolerant persister tumour cells in non-small cell lung cancer. Cell Death Differ. 30, 2365–2381. doi:10.1038/s41418-023-01222-0
Wang, H., Luo, W., Chen, H., Cai, Z., and Xu, G. (2024). Mitochondrial dynamics and mitochondrial autophagy: molecular structure, orchestrating mechanism and related disorders. Mitochondrion 75, 101847. doi:10.1016/j.mito.2024.101847
Wang, Y., Goh, K. Y., Chen, Z., Lee, W. X., Choy, S. M., Fong, J. X., et al. (2022). A novel TP53 gene mutation sustains non-small cell lung cancer through mitophagy. Cells 11, 3587. doi:10.3390/cells11223587
Wei, Y., Chiang, W. C., Sumpter, R., Mishra, P., and Levine, B. (2017). Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168, 224–238. doi:10.1016/j.cell.2016.11.042
Wu, S., Li, Z., Li, H., and Liao, K. (2022). Dihydroartemisinin reduces irradiation-induced mitophagy and radioresistance in lung cancer A549 cells via CIRBP inhibition. Life (Basel, Switz.) 12, 1129. doi:10.3390/life12081129
Wu, Y., Deng, H., Sun, J., Tang, J., Li, X., and Xu, Y. (2023). Poricoic acid A induces mitophagy to ameliorate podocyte injury in diabetic kidney disease via downregulating FUNDC1. J. Biochem. Mol. Toxicol. 37, e23503. doi:10.1002/jbt.23503
Yan, F., Teng, Y., Li, X., Zhong, Y., Li, C., Yan, F., et al. (2024). Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol. Ther. 25, 2304161. doi:10.1080/15384047.2024.2304161
Yang, K., Li, T., Geng, Y., Zou, X., Peng, F., and Gao, W. (2024). The role of mitophagy in the development of chronic kidney disease. PeerJ 12, e17260. doi:10.7717/peerj.17260
Zaccara, S., Ries, R. J., and Jaffrey, S. R. (2019). Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 20, 608–624. doi:10.1038/s41580-019-0168-5
Zhang, H., Yin, C., Liu, X., Bai, X., Wang, L., Xu, H., et al. (2020a). Prohibitin 2/PHB2 in parkin-mediated mitophagy: a potential therapeutic target for non-small cell lung carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 26, e923227. doi:10.12659/MSM.923227
Zhang, Z. L., Wang, N. N., Ma, Q. L., Chen, Y., Yao, L., Zhang, L., et al. (2020b). Somatic and germline mutations in the tumor suppressor gene PARK2 impair PINK1/Parkin-mediated mitophagy in lung cancer cells. Acta Pharmacol. Sin. 41, 93–100. doi:10.1038/s41401-019-0260-6
Zheng, J., Wei, S., Xiao, T., and Li, G. (2021). LC3B/p62-mediated mitophagy protects A549 cells from resveratrol-induced apoptosis. Life Sci. 271, 119139. doi:10.1016/j.lfs.2021.119139
Zhou, T. Y., Ma, R. X., Li, J., Zou, B., Yang, H., Ma, R. Y., et al. (2023). Review of PINK1-Parkin-mediated mitochondrial autophagy in Alzheimer's disease. Eur. J. Pharmacol. 959, 176057. doi:10.1016/j.ejphar.2023.176057
Zhu, S., Li, X., Wu, F., Cao, X., Gou, K., Wang, C., et al. (2022). Blue light induces skin apoptosis and degeneration through activation of the endoplasmic reticulum stress-autophagy apoptosis axis: protective role of hydrogen sulfide. J. Photochem. Photobiol. B, Biol. 229, 112426. doi:10.1016/j.jphotobiol.2022.112426
Keywords: autophagy, mitophagy, lung cancer, tumorigenesis, treatment, progression and metastasis
Citation: Zhang X, Yu D, Tang P and Chen F (2024) Insights into the role of mitophagy in lung cancer: current evidence and perspectives. Front. Pharmacol. 15:1420643. doi: 10.3389/fphar.2024.1420643
Received: 20 April 2024; Accepted: 04 June 2024;
Published: 19 June 2024.
Edited by:
Qi Wang, Second Affiliated Hospital of Dalian Medical University, ChinaCopyright © 2024 Zhang, Yu, Tang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengshou Chen, ZnNjaGVuQGNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Xin Zhang
Xin Zhang Dongzhi Yu
Dongzhi Yu Peng Tang2
Peng Tang2 Fengshou Chen
Fengshou Chen