- 1Department of Orthopedics, The Second Affiliated Hospital (Changzheng Hospital), Naval Medical University, Shanghai, China
- 2Department of Endocrinology, The Second Affiliated Hospital (Changzheng Hospital), Naval Medical University, Shanghai, China
- 3Department of Traditional Chinese Medicine, The First Affiliated Hospital (Changhai Hospital), Naval Medical University, Shanghai, China
- 4Department of Ultrasound, The Second Affiliated Hospital (Changzheng Hospital), Naval Medical University, Shanghai, China
- 5Department of Pharmacy, The First Affiliated Hospital (Changhai Hospital), Naval Medical University, Shanghai, China
Background: Associated with enzyme deficiencies causing glycosaminoglycans (GAGs) accumulation, mucopolysaccharidosis type VI (MPS VI) is lysosomal storage disorder. In the treatment of MPS VI, galsulfase (Naglazyme) is commonly used as an enzyme replacement therapy (ERT). There remains a need for comprehensive real-world data on its safety and associated adverse events (AEs).
Objective: An analysis of the FDA Adverse Event Reporting System (FAERS) database will be conducted to identify potential risks and adverse reactions associated with galsulfase in real-life settings.
Methods: The FAERS database was used to extract data from Q2 2005 to Q4 2023. A total of 20,281,876 reports were analyzed after duplicate elimination, with 3,195 AE reports related to galsulfase identified. The association between galsulfase and AEs was investigated by utilizing four algorithms: reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS). The analysis focused on the timing of onset, signs of AEs, and clinical significance.
Results: Twenty seven organ systems were involved, and significant system organ classes (SOCs) included respiratory, thoracic and mediastinal disorders, and infections and infestations. At the PT level, 72 PTs corresponding to 15 SOCs were identified, with some AEs not previously mentioned in the product label. AEs associated with galsulfase had a median onset time of 1,471 days, with over half of the cases occurred within the first 5 years of treatment initiation.
Conclusion: This investigation delivers an exhaustive and indicative assessment of galsulfase’s safety profile, grounded in authentic, real-world evidence. The findings emphasis the importance of continuous safety surveillance and the emergence of new AEs. The identification of previously unreported urologic adverse events, such as glomerulonephritis membranous and nephritic syndrome, warrants further investigation. The study emphasizes the need for enhanced pharmacovigilance to ensure patient safety and the effectiveness of galsulfase treatment.
1 Introduction
The mucopolysaccharidoses (MPSs) are a collection of lysosomal storage disorders resulting from deficiencies in enzymes essential for glycosaminoglycans (GAGs) metabolism (Brunelli et al., 2021; Li et al., 2024). GAGs, being a diverse range of extracellular heteropolysaccharides, serve various roles in human physiology (Chin and Fuller, 2022). The progressive and systemic symptoms commonly observed in early childhood are attributed to the accumulation of GAGs in various tissues (Harmatz et al., 2014). The progressively deteriorating manifestations encompass skeletal, cardiovascular and respiratory, hematological, visual, auditory, and cognitive impairments (Sestito et al., 2022; Al Kaissi et al., 2023; Hwang-Wong et al., 2023; Miller et al., 2023; Ratiani and Leventaki, 2023). The multi-organ impact of this condition not only impedes daily activities but also disrupts social interactions, emotional well-being, and academic performance in children (Li et al., 2024).
There are three primary methods currently in use of treating MPS VI: transplantation of hematopoietic stem cells, enzyme replacement therapy, and gene-based treatments (Penon-Portmann et al., 2023). Galsulfase, known commercially as Naglazyme, is widely used enzyme replacement medication for MPS VI. GAGs are catabolized more efficiently by galsulfase when it is incorporated into lysosomes, thereby increasing their degradation (Valayannopoulos et al., 2010).
Prior investigations have indicated early initiation of galsulfase therapy could potentially prevent or mitigate the advancement of certain disease manifestations (Sohn et al., 2012; Harmatz et al., 2014; 2017; Lin et al., 2016; Gomes et al., 2019). However, previous studies mostly focused on the efficacy of drugs, and the data were mostly derived from clinical trials rather than real-world studies, so there was a lack of systematic studies on drug adverse events (AEs). Skinner et al. searched for articles about enzyme replacement therapies and found that only 7% mentioned AEs (Skinner et al., 2018). To improve the correct understanding of AEs can effectively define the scope of treatment and the way of using of drugs, and timely put forward reasonable and scientific modification opinions on the current package inserts.
Therefore, searching for the AEs of galsulfase in the real-world is essential. The FAERS database serves as a comprehensive repository for post-marketing surveillance, housing authentic AE reports sourced from a multitude of contributors (Zou et al., 2024). The database is readily accessible for public download on the FDA’s official website (Wang et al., 2024). Our main objective is using four algorithms to search for possible risks linked to galsulfase, and expect to provide guidance for clinical application and further enrich the application scenarios and adverse reactions of drugs.
2 Methods
2.1 Data source and collection
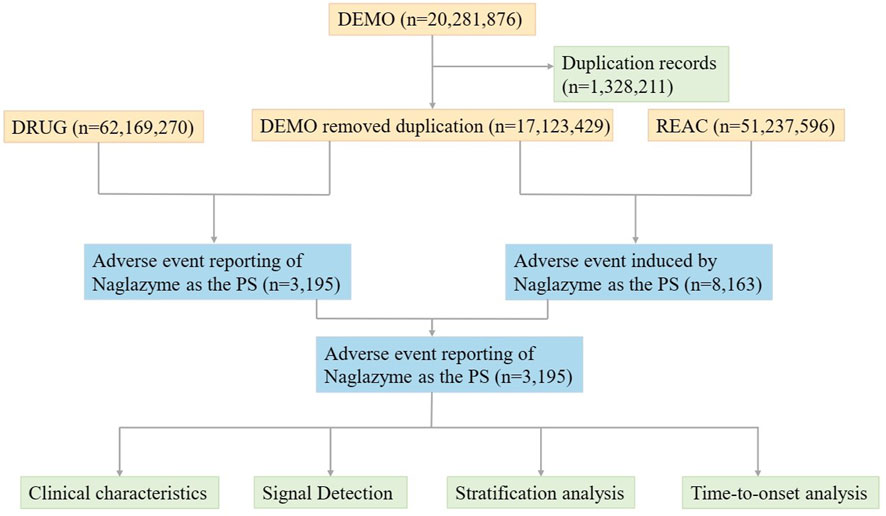
Utilizing the FAERS database, pharmacovigilance data on galsulfase in the post-marketing phase from Q2 2005 to Q4 2023 was gathered. Designed to assist the FDA in monitoring the safety profiles of approved drugs and therapeutic biologic products post-approval, FAERS is founded on Individual Case Safety Reports (ICSRs: E2B) as issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Adverse events (AEs) are systematically classified in line with the Medical Dictionary for Regulatory Activities (MedDRA). FAERS comprises seven distinct datasets including demographic and administrative details (DEMO), information on adverse drug reactions (REAC), patient outcomes (OUTC), drug specifics (DRUG), drug therapy start and end dates (THER), details regarding reporting sources (RPSR), and indications for use or diagnosis (INDI). The database collates reports of AEs, product quality concerns, and medication errors impacting patient safety. In order to identify and remove duplicate reports, priority was given to the most recent FDA_DT when CASEID values matched. Conversely, when both CASEID and FDA_DT coincided, a PRIMARYID with a higher value was selected (Shu et al., 2022). During the study period, the FAERS database recorded a total of 20,281,876 reports related to galsulfase. Following the removal of duplicate entries, 17,123,429 case reports identified galsulfase as the main suspect drug, associating it with 3,195 adverse events (Figure 1). All reports on galsulfase were systematically categorized based on System Organ Class (SOC) and Preferred Term (PT) levels. The codes for drug roles in events (ROLE_COD) were classified as primary suspect (PS), secondary suspect (SS), concomitant (C), or interacting (I). Furthermore, both the generic name (galsulfase) and brand name (naglazyme) were identified as target drugs in the DRUG file.
2.2 Statistical analysis
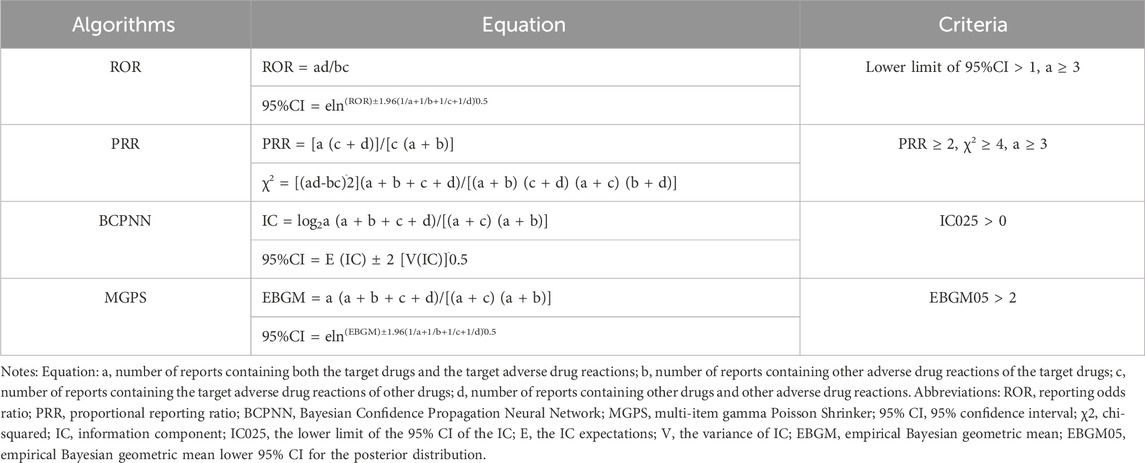
The relationship between galsulfase and AEs was evaluated using statistical algorithms such as the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS). These assessments were based on disproportionality analysis. Detailed information on the equations and criteria for these algorithms can be found in (Table 1). Our study analyzed data on AE signals that met the specific criteria of each algorithm. Signals indicating novel AEs were identified as any significant adverse event not previously documented in the product information (Full Prescribing Information, Revised: 12/2019). The onset time was defined as the duration between the occurrence of the AE (EVENT_DT) and the initiation of galsulfase treatment (START_DT). Reports with data entry errors, such as EVENT_DT preceding START_DT or containing incorrect dates, were excluded from the analysis. The onset time was described using the median and interquartile range (IQR). Statistical software programs R 4.3.3, Navicat Premium 15, and Microsoft Excel 2019 were utilized for data processing and statistical computations.
3 Results
3.1 General characteristics
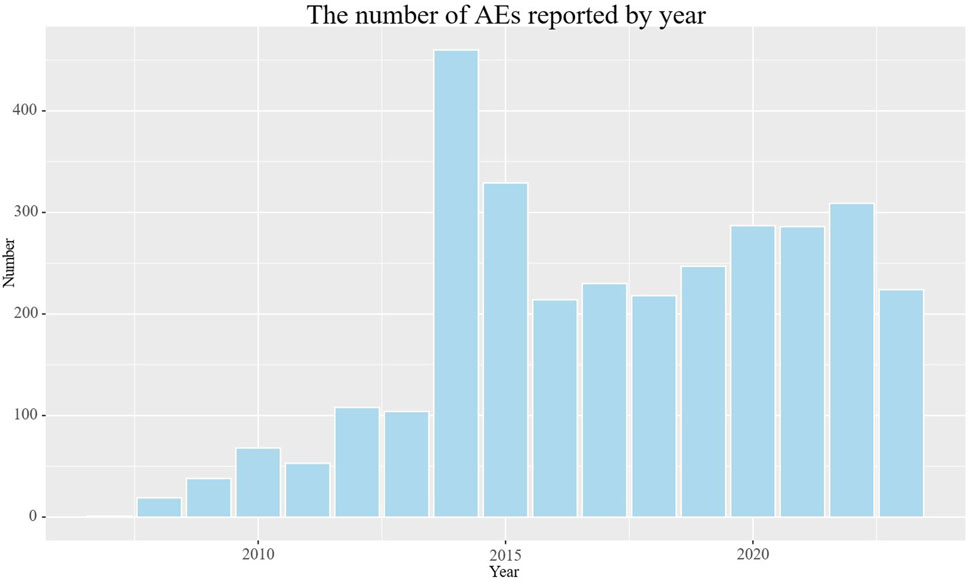
The detailed clinical characteristics of studies regarding galsulfase can be found in (Table 2). In terms of gender, females (45.4%) experienced a higher incidence of adverse events compared to males (37.5%). In age distribution, a greater proportion was observed among patients under 18 years old (37.9%), surpassing both those over 65 years old and those aged between 18 and 65 years. The most commonly reported indication was MPS VI (78.0%), followed by unclassified mucopolysaccharidosis (3.4%). The highest number of AEs (40.8%) was reported by the United States, with Brazil (25.5%), Colombia (7.1%), the United Kingdom (4.2%), and Germany (2.8%) following. Serious consequences encompass fatalities, life-threatening situations, hospital stays, impairments, and other severe outcomes. In order to establish the distribution of each type, we computed the percentage of each category in relation to the overall serious outcome submissions. Among the serious outcomes, hospitalization emerged as the predominant type, constituting 31.7%. The remaining serious outcomes were documented in 934 instances, accounting for 25.7%, whereas fatalities were recorded in 281 cases, equating to 7.7%. Excluding reports from unknown sources, consumers and physicians were the primary reporters of adverse events, accounting for 74.1% and 12.6% respectively. The number of reported adverse events exhibited a gradual increase over the initial 8 years, followed by a period of stabilization (Figure 2). The peak year for reported AEs was 2014 (14.4%), followed by 2015 (15.3%), 2022 (9.7%), 2020 (9.0%), 2021 (9.0%), and 2019 (7.7%), respectively.
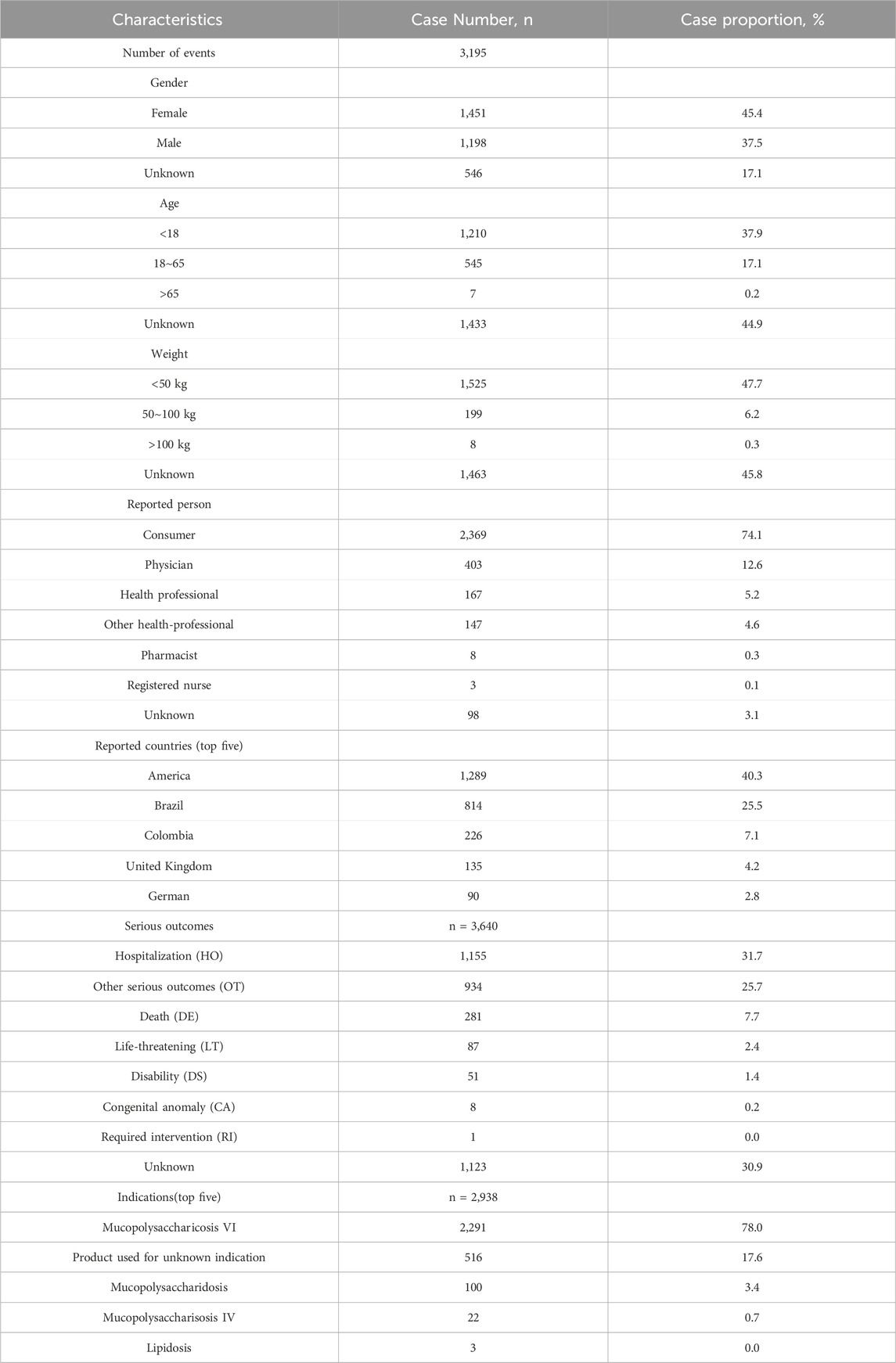
Table 2. Clinical characteristics of reports with galsulfase from the FAERS database (from the second quarter of 2005 to the fourth quarter of December 2023).
3.2 Signal detection
3.2.1 Signals of system organ class (SOC)
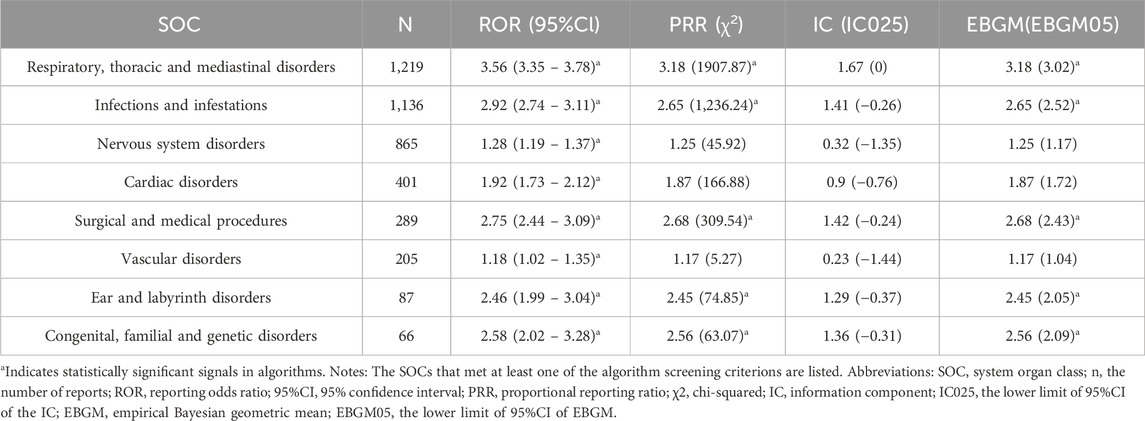
The galsulfase-related adverse events showed varying signal strengths at the level of SOC as outlined in (Table 3). Analysis of the collected data revealed that AEs linked to galsulfase affected a total of 27 organ systems. Noteworthy SOCs meeting the specified criteria included disorders of the respiratory, thoracic, and mediastinal systems [n = 1,219, ROR (95%CI) = 3.56 (3.35–3.78)], incidences of infections and infestations [n = 1,136, ROR (95%CI) = 2.92 (2.74–3.11)], occurrences related to surgical and medical procedures [n = 289, ROR (95%CI) = 2.75 (2.44–3.09)], problems with the ear and labyrinth [n = 87, ROR (95%CI) = 2.46 (1.99–3.04)], as well as conditions involving congenital, familial, and genetic factors [n = 66, ROR (95%CI) = 2.58 (2.02–3.28)]. Additionally, disorders of the nervous system [n = 865, ROR (95%CI) = 1.28 (1.19–1.37)], cardiac issues [n = 401, ROR (95%CI) = 1.92 (1.73–2.12)], and vascular problems [n = 205, ROR (95%CI) = 1.18 (1.02–1.35)] were identified as significant SOCs meeting at least one of the specified criteria.
3.2.2 Signals of preferred terms (PTs)
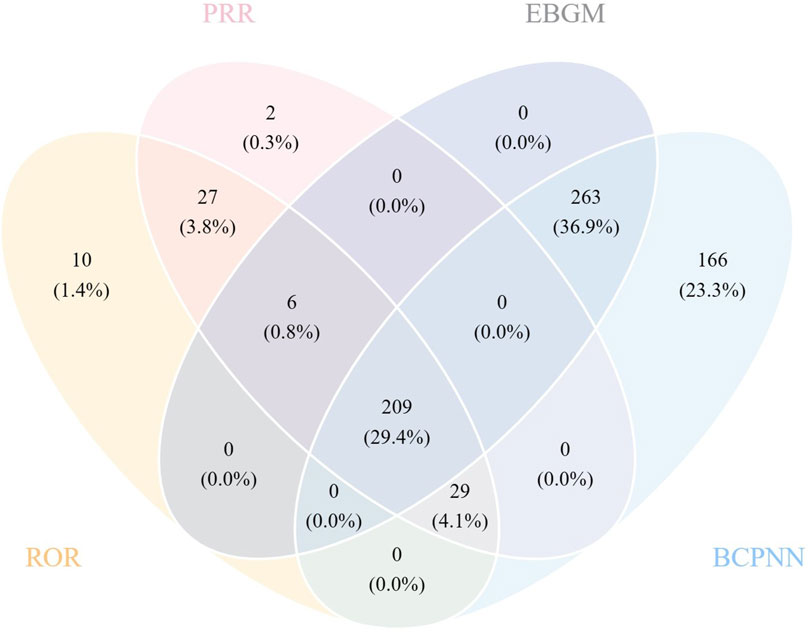
All four algorithms together detected 209 instances of PTs induced by galsulfase, impacting 21 System Organ Classes (SOCs), as illustrated in (Supplementary Table S1). The detailed screening process is depicted in (Figure 3). (Table 4) Offers a summarized list of reported post-marketing surveillance AEs that occurred with a minimum frequency of 11 times. This table encompasses 72 PTs, which are associated with 15 different SOCs. After comparing the package insert, 33 PTs were found to have the same drug instructions, which included fever, hernia, breathing difficulties, stuffy nose, infection of the upper respiratory tract, clouding of the cornea, hernia near the belly button, an adverse reaction to infusion, pain at the catheter site, flu, infection of the respiratory tract, infection of the lower respiratory tract, ear infection, streptococcal throat infection, coughing, breathing difficulties, pain in the throat and mouth, high blood pressure in the lungs, bronchial spasms, cough with mucus, rapid breathing, breathing failure, runny nose, respiratory issue, sore throat, blockage in the airway, decreased oxygen levels, high body temperature, and hearing loss.
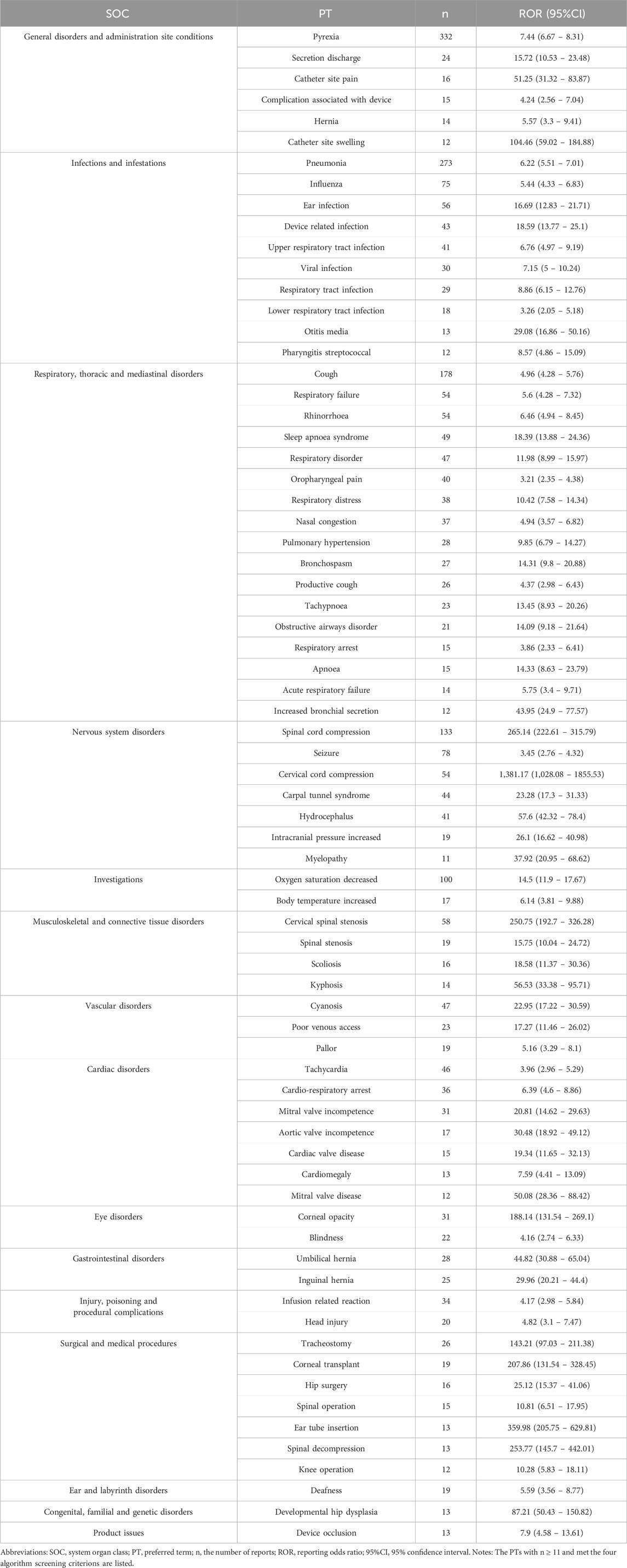
Significantly, our efforts in data mining revealed numerous noteworthy adverse events that were not specifically cited in the galsulfase product label. These included 20 adverse event reports included pneumonia, seizure, cervical spinal stenosis, ear infection, sleep apnoea syndrome, cyanosis, tachycardia, carpal tunnel syndrome, device related infection, hydrocephalus, cardio-respiratory arrest, mitral valve incompetence, viral infection, tracheostomy, inguinal hernia, secretion discharge, poor venous access, blindness, obstructive airways disorder. Some PTs were discovered with increased signal intensity, including ear tube insertion [n = 13, ROR (95%CI) = 359.98 (205.75–629.81)], spinal decompression [n = 13, ROR (95%CI) = 253.77 (145.7–442.01)], cervical spinal stenosis [n = 58, ROR (95%CI) = 250.75 (192.7–326.28)], corneal transplant [n = 19, ROR (95%CI) = 207.86 (131.54–328.45)], tracheostomy [n = 26, ROR (95%CI) = 143.21 (97.03–211.38)], catheter site swelling [n = 12, ROR (95%CI) = 104.46 (59.02–184.88)]. Our research has revealed extra adverse events that enhance the overall comprehension of the safety characteristics of galsulfase.
3.2.3 Onset time of AEs
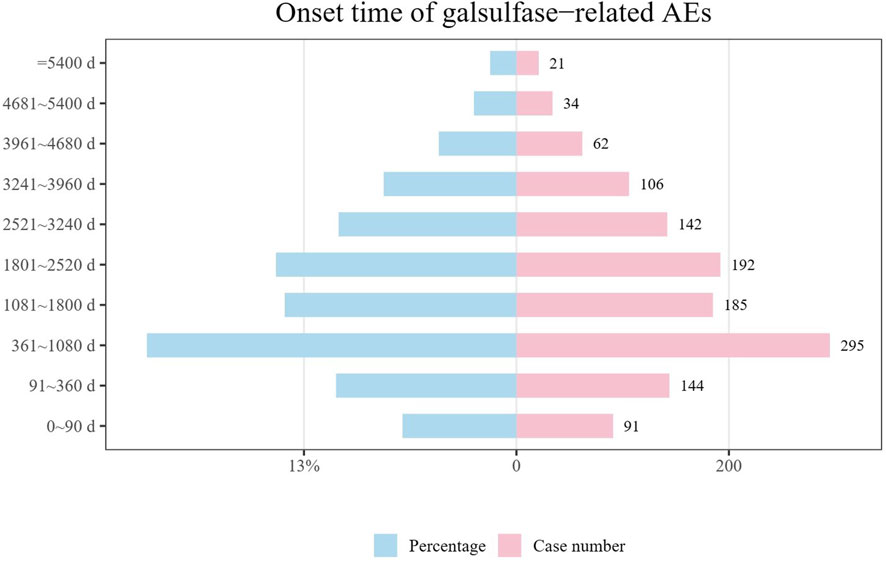
Data on the initiation periods for adverse events linked to galsulfase were collected from the FAERS repository. Upon removal of inaccurate accounts, a sum of 1,272 records containing documented initiation periods remained. The median initiation period stood at 1,471 days, accompanied by IQR spanning from 523 to 2,706.25 days. As illustrated in (Figure 4), the findings suggest that the onset of adverse events related to galsulfase can be widespread, potentially spanning over a decade. Nevertheless, more than half of these cases (n = 715, 56.20%) occurred within the initial 5 years following the commencement of galsulfase treatment. The incidence of AES in 1 year (n = 235, 18.47%), 3 years (n = 295, 23.19%), 5 years (n = 185, 14.54%), 7 years (n = 192, 15.09%), 9 years (n = 142, 11.16%), 11 years (n = 106, 8.33%) decreased gradually, which reflected that patients may have a better long-term effect of medication.
4 Discussion
MPS VI, characterized as a rare genetic disease with a low incidence rate, affects a limited population of patients but multiple systems result from the build-up of GAGs in connective tissues (Valayannopoulos et al., 2010). This phenomenon is caused by a lack of the enzyme N-acetylgalactosamine 4-sulfatase (Tebani et al., 2019; Jones et al., 2020). In an autosomal recessive manner, this genetic disorder is inherited, arising from mutations in the ARSB gene on chromosome 5q13-q14. Over 130 different mutations have been found, primarily consisting of missense mutations (Valayannopoulos et al., 2010; Ghaffari et al., 2022). Galsulfase has been demonstrated improvement in walking and stair-climbing abilities (Sohn et al., 2012). In a phase IV, multinational, open-label study with two dosage levels conducted in infants in 2014, it was shown that urinary GAG levels turn down after galsulfase treatment (Harmatz et al., 2014). A case series study in 2016 provided evidence that galsulfase decreased levels of urinary GAG and enhanced clinical functions over a long-term period (Lin et al., 2016).
Although galsulfase has been widely used in the treatment of MPS VI, its safety and adverse reactions still need to be further studied to ensure the safety and effectiveness of patients (Lampe et al., 2019; D’Avanzo et al., 2021). Previous studies on galsulfase have primarily been clinical trials or retrospective studies, with a focus on case series, and the majority of these studies have enrolled no more than 55 patients (Harmatz et al., 2005; Brands et al., 2013; Miller et al., 2023). However, the present study stands out due to its significantly larger overall sample size (n = 3,195), which is derived from real-world data, volunteered by applicants from various occupational sources, thereby enhancing its reliability. This large sample size is crucial for validating previously reported AEs associated with galsulfase and for discovering potential new AEs. The utilization of real-world data allows for a more comprehensive understanding of the safety profile of galsulfase in a diverse and representative patient population, beyond the limitations of smaller, more controlled study settings. Therefore, the current study offers a significant advancement in the field of MPS VI research, contributing to a deeper understanding of the drug’s safety and potential risks.
By utilizing the four algorithms mentioned in (Table 1), we ranked the signal values of the collected AEs (Sakaeda et al., 2013). After analyzing the galsulfase related data, we found that at the SOC level, although there were 8 SOCs with significant statistical signals according to at least 1 algorithm: respiratory, thoracic and mediastinal disorders [n = 1,219, ROR (95%CI) = 3.56 (3.35–3.78)], infections and infestations [n = 1,136, ROR (95%CI) = 2.92 (2.74–3.11)], nervous system disorders [n = 865, ROR (95%CI) = 1.28 (1.19–1.37)], cardiac disorders [n = 401, ROR (95%CI) = 1.92 (1.73–2.12)], surgical and medical procedures [n = 289, ROR (95%CI) = 2.75 (2.44–3.09)], vascular disorders [n = 205, ROR (95%CI) = 1.18 (1.02–1.35)], ear and labyrinth disorders [n = 87, ROR (95%CI) = 2.46 (1.99–3.04)], congenital, familial and genetic disorders [n = 66, ROR (95%CI) = 2.58 (2.02–3.28)] (Table 3), according to recent advances in clinical research on MPS VI, the above-mentioned statistically significant SOC were all associated with the progression of MPS VI disease (Harmatz et al., 2005; Hoang et al., 2019; Inci et al., 2021; Al Kaissi et al., 2023; Miller et al., 2023; Ratiani and Leventaki, 2023). To our astonishment, general disorders and administration site conditions contributed the most frequent AEs (n = 1,225), but it didn’t meet the screening criteria of any of the algorithms shown.
At the PT levels, we evaluated all post-marketing surveillance AEs that fulfilled the screening criteria of all four algorithms and summarized the corresponding SOC levels (statistics for all PTs are provided in (Supplementary Table S2). All PTs listed in (Supplementary Table S2) passed the Bonferroni correction, as detailed in (Supplementary Table S3). It was interesting to find that, the most numerous SOCs were respiratory, thoracic and mediastinal disorders (n = 795), infections and infestations (n = 689), and general disorders and administration site conditions (n = 465). Additionally, taking into account the potential progression of MPS VI disease resulting in symptoms affecting the respiratory, musculoskeletal, cardiovascular, nervous, and other systems, we manually eliminated the related System Organ Classes (SOCs) (Valayannopoulos et al., 2010; Garcia et al., 2021). In conclusion, the most frequently occurring SOC was general disorders and administration site conditions. Our research findings align with previous relevant studies (Horovitz et al., 2021; Inci et al., 2021; Kowalski et al., 2022).
Associated with the general disorders and conditions of the administration site, statistically significant PTs included pyrexia (n = 332), discharge of secretions (n = 24), pain at catheter site (n = 16), complication associated with the device (n = 15), hernia (n = 14), swelling at catheter site (n = 12), extravasation at infusion site (n = 8), erythema at catheter site (n = 8), extravasation (n = 7), hyperthermia (n = 5), inflammation at catheter site (n = 4), extravasation at catheter site (n = 4), bruising at catheter site (n = 4), pain from hernia (n = 3), loss of leg control (n = 3), adhesion (n = 3), granuloma (n = 3). Additionally, attention was also drawn to infusion-related reactions (n = 34) and occlusion of the device (n = 13). The majority of these PTs are infusion-associated events which can be technicality avoided (Kim et al., 2008; D’Avanzo et al., 2021). Based on the results of the above analysis, we make a recommendation to change the route of administration from intravenous infusion to automated pump administration, which is to reduce the number and frequency of invasive procedures on the one hand and more stable drug administration on the other. Alternatively, gene therapy is expected to completely solve MPS VI and fundamentally improve the survival treatment and life expectancy of patients (Fachel et al., 2022).
Drawing upon our preceding scientific inquiries, we manually sifted through and excluded PTs that exhibited a correlation with the progression of MPS VI disease. In the process, we discovered two previously undocumented urologic AEs: glomerulonephritis membranous [n = 4, ROR (95%CI) = 25.84 (9.68–69)] and nephritic syndrome [n = 3, ROR (95%CI) = 69.76 (22.35–17.69)]. This revelation offers a fresh perspective in our comprehension of the potential side effects associated with the administration of the drug. Although the precise underlying mechanism remains elusive, we hypothesize that these newly identified AEs may be attributed to the direct or indirect toxic effects of galsulfase on renal cells. Alternatively, they could be a consequence of the immune response elicited by galsulfase, as suggested by previous studies (White et al., 2008a; White et al., 2008b). To further elucidate the specific pathogenesis of these AEs, additional biological and biomolecular investigations are imperative. In light of these novel findings, we strongly recommend the conduct of more comprehensive epidemiologic studies and clinical trials. These endeavors will aid in validating the potential association between glomerulonephritis membranous, nephritic syndrome, and galsulfase, while also assessing the severity and incidence of these AEs. Such an approach will provide crucial insights into the safety profile of this drug and inform future therapeutic strategies.
Through a subgroup analysis of all available data, stratified by gender, we observed that gender did not exert a statistically significant influence on the categorization or frequency of AEs. This finding is depicted in (Supplementary Figure S1), which provides a visual representation of the gender-specific distribution of AEs. Nonetheless, in conducting a subgroup analysis of reporting occupation, we identified a potential bias. Notably, a disproportionate majority of 74.15% (n = 3,195) of the individuals reporting AEs were consumers. This imbalance in the reporting population may introduce confounding variables and influence the interpretation of the data. Therefore, (Supplementary Figure S2) highlights this occupational bias, emphasizing the need for cautious interpretation and further investigation to mitigate its potential impact on the overall analysis.
It is crucial to acknowledge that the FAERS database encompasses a diverse array of data sources, with consumers constituting a significant proportion of the reporting population. Consequently, this presents a range of challenges, including potential delays in reporting, instances of missing reports, and inconsistencies in the quality of submissions. These factors, in turn, may introduce biases that must be carefully considered when interpreting the findings derived from this database. Given the complexity of these circumstances and the potential biases involved, it is imperative to exercise caution when interpreting the results of our analyses. While the FAERS database provides valuable insights into AE signals associated with galsulfase, it is essential to recognize its limitations in pharmacovigilance research. Therefore, it is necessary to complement our findings with additional evaluations conducted through rigorous clinical studies. Despite these limitations, our thorough examination of AE signals associated with galsulfase, as well as the discovery of previously unrecognized signals, serves as a solid foundation for future clinical inquiries on this drug. By conducting further clinical studies, we aim to validate our findings and gain a deeper understanding of the safety profile of galsulfase, ultimately contributing to the improvement of patient care and the advancement of medical science.
5 Conclusion
After reviewing 20,281,876 entries in the FAERS database between the second quarter of 2005 and the fourth quarter of 2023, and removing any duplicate entries, a total of 3,195 adverse event reports associated with galsulfase were discovered. A pharmacovigilance analysis was employed to uncover the AE signals associated with galsulfase. The onset time, manifestation of AEs, and concomitant medications were examined. The infusion-related symptoms that were frequently reported aligned with the information provided in the galsulfase label, which helps to further corroborate the safety profile. Additionally, new clinically significant AEs were identified: membranous glomerulonephritis and nephritic syndrome. This research underscores the necessity for enhanced safety monitoring to decrease the occurrence of AEs linked to galsulfase and to ensure patient safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
SL: Methodology, Writing–original draft, Conceptualization. RH: Writing–review and editing, Software, Formal Analysis, Resources. YM: Investigation, Software, Writing–original draft. YL: Visualization, Writing–review and editing, Validation. JQ: Formal Analysis, Resources, Writing–original draft. JZ: Visualization, Writing–review and editing, Conceptualization, Data curation, Funding acquisition. JY: Funding acquisition, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China under grant numbers 2018YFC1314102, and social science training program of Naval Medical University under grant numbers 2022SK033. Additionally, this research was funded by the Science and Technology Commission of Shanghai Municipality, Grant/Award Number: 21ZR1478300.
Acknowledgments
This study was conducted utilizing the FDA Adverse Event Reporting System (FAERS) database, which is maintained by the FDA. It is important to note that the information, outcomes, or interpretations presented in the study do not reflect any official position or opinion of the FDA. Moreover, we would like to thank everyone who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1420126/full#supplementary-material
References
Al Kaissi, A., Ryabykh, S., Ochirova, P., Kareem, A. A., Kenis, V., Ganger, R., et al. (2023). The articular and the craniocervical abnormalities are of confusing age of onset in patients with Maroteaux-Lamy disease (MPS VI). Minerva Pediatr. (Torino) 75, 243–252. doi:10.23736/S2724-5276.20.05645-5
Brands, M. M., Hoogeveen-Westerveld, M., Kroos, M. A., Nobel, W., Ruijter, G. J., Özkan, L., et al. (2013). Mucopolysaccharidosis type VI phenotypes-genotypes and antibody response to galsulfase. Orphanet J. Rare Dis. 8, 51. doi:10.1186/1750-1172-8-51
Brunelli, M. J., Atallah, Á. N., and da Silva, E. M. (2021). Enzyme replacement therapy with galsulfase for mucopolysaccharidosis type VI. Cochrane Database Syst. Rev. 9, CD009806. doi:10.1002/14651858.CD009806.pub2
Chin, S. J., and Fuller, M. (2022). Prevalence of lysosomal storage disorders in Australia from 2009 to 2020. Lancet Reg. Health West Pac 19, 100344. doi:10.1016/j.lanwpc.2021.100344
D Avanzo, F., Zanetti, A., De Filippis, C., and Tomanin, R. (2021). Mucopolysaccharidosis type VI, an updated overview of the disease. Int. J. Mol. Sci. 22, 13456. doi:10.3390/ijms222413456
Fachel, F. N. S., Frâncio, L., Poletto, É., Schuh, R. S., Teixeira, H. F., Giugliani, R., et al. (2022). Gene editing strategies to treat lysosomal disorders: the example of mucopolysaccharidoses. Adv. Drug Deliv. Rev. 191, 114616. doi:10.1016/j.addr.2022.114616
Garcia, P., Phillips, D., Johnson, J., Martin, K., Randolph, L. M., Rosenfeld, H., et al. (2021). Long-term outcomes of patients with mucopolysaccharidosis VI treated with galsulfase enzyme replacement therapy since infancy. Mol. Genet. Metab. 133, 100–108. doi:10.1016/j.ymgme.2021.03.006
Ghaffari, S. R., Rafati, M., Shadnoush, M., Pourbabaee, S., Aghighi, M., Mirab Samiee, S., et al. (2022). Molecular characterization of a large cohort of mucopolysaccharidosis patients: Iran Mucopolysaccharidosis RE-diagnosis study (IMPRESsion). Hum. Mutat. 43, e1–e23. doi:10.1002/humu.24328
Gomes, D. F., Gallo, L. G., Leite, B. F., Silva, R. B., and da Silva, E. N. (2019). Clinical effectiveness of enzyme replacement therapy with galsulfase in mucopolysaccharidosis type VI treatment: systematic review. J. Inherit. Metab. Dis. 42, 66–76. doi:10.1002/jimd.12028
Harmatz, P., Hendriksz, C. J., Lampe, C., McGill, J. J., Parini, R., Leão-Teles, E., et al. (2017). The effect of galsulfase enzyme replacement therapy on the growth of patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Mol. Genet. Metab. 122, 107–112. doi:10.1016/j.ymgme.2017.03.008
Harmatz, P., Ketteridge, D., Giugliani, R., Guffon, N., Teles, E. L., Miranda, M. C. S., et al. (2005). Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics 115, e681–e689. doi:10.1542/peds.2004-1023
Harmatz, P. R., Garcia, P., Guffon, N., Randolph, L. M., Shediac, R., Braunlin, E., et al. (2014). Galsulfase (Naglazyme®) therapy in infants with mucopolysaccharidosis VI. J. Inherit. Metab. Dis. 37, 277–287. doi:10.1007/s10545-013-9654-7
Hoang, T. T., Bui, A. V., and Burgess, J. D. (2019). Medically uncontrolled intraocular pressure in mucopolysaccharidosis type VI. Clin. Exp. Ophthalmol. 47, 144–145. doi:10.1111/ceo.13355
Horovitz, D. D. G., Leão, E. K. E. A., Ribeiro, E. M., Martins, A. M., Barth, A. L., Neri, J. I. C. F., et al. (2021). Long-term impact of early initiation of enzyme replacement therapy in 34 MPS VI patients: a resurvey study. Mol. Genet. Metab. 133, 94–99. doi:10.1016/j.ymgme.2021.02.006
Hwang-Wong, E., Amar, G., Das, N., Zhang, X., Aaron, N., Gale, K., et al. (2023). Skeletal phenotype amelioration in mucopolysaccharidosis VI requires intervention at the earliest stages of postnatal development. JCI Insight 8, e171312. doi:10.1172/jci.insight.171312
Inci, A., Okur, İ., Tümer, L., Biberoğlu, G., Öktem, M., and Ezgü, F. (2021). Clinical and event-based outcomes of patients with mucopolysaccharidosis VI receiving enzyme replacement therapy in Turkey: a case series. Orphanet J. Rare Dis. 16, 438. doi:10.1186/s13023-021-02060-4
Jones, S. A., Marsden, D., Koutsoukos, T., Sniadecki, J., Tylee, K., Phillippo, S., et al. (2020). Retrospective chart review of urinary glycosaminoglycan excretion and long-term clinical outcomes of enzyme replacement therapy in patients with mucopolysaccharidoses. Mol. Genet. Metab. 130, 255–261. doi:10.1016/j.ymgme.2020.06.004
Kim, K. H., Decker, C., and Burton, B. K. (2008). Successful management of difficult infusion-associated reactions in a young patient with mucopolysaccharidosis type VI receiving recombinant human arylsulfatase B (galsulfase [Naglazyme]). Pediatrics 121, e714–e717. doi:10.1542/peds.2007-0665
Kowalski, T., Donoghue, S., de Jong, G., and Mack, H. G. (2022). Novel chorioretinal findings in two siblings with mucopolysaccharidosis type VI. Ophthalmic Genet. 43, 693–698. doi:10.1080/13816810.2022.2083184
Lampe, C., Harmatz, P. R., Parini, R., Sharma, R., Teles, E. L., Johnson, J., et al. (2019). Enzyme replacement therapy initiated in adulthood: findings from the mucopolysaccharidosis VI Clinical Surveillance Program. Mol. Genet. Metab. 127, 355–360. doi:10.1016/j.ymgme.2019.06.008
Li, J. W.-Y., Yan, K., Balijepalli, C., and Druyts, E. (2024). Humanistic burden of mucopolysaccharidoses: a systematic literature review. Curr. Med. Res. Opin. 40, 709–722. doi:10.1080/03007995.2024.2316213
Lin, H.-Y., Chuang, C.-K., Wang, C.-H., Chien, Y.-H., Wang, Y.-M., Tsai, F.-J., et al. (2016). Long-term galsulfase enzyme replacement therapy in Taiwanese mucopolysaccharidosis VI patients: a case series. Mol. Genet. Metab. Rep. 7, 63–69. doi:10.1016/j.ymgmr.2016.04.003
Miller, B. S., Fung, E. B., White, K. K., Lund, T. C., Harmatz, P., Orchard, P. J., et al. (2023). Persistent bone and joint disease despite current treatments for mucopolysaccharidosis types I, II, and VI: data from a 10-year prospective study. J. Inherit. Metab. Dis. 46, 695–704. doi:10.1002/jimd.12598
Penon-Portmann, M., Blair, D. R., and Harmatz, P. (2023). Current and new therapies for mucopolysaccharidoses. Pediatr. Neonatol. 64 (Suppl. 1), S10–S17. doi:10.1016/j.pedneo.2022.10.001
Ratiani, M., and Leventaki, V. (2023). Neutrophils and monocytes with increased azurophilic granules resembling toxic changes in mucopolysaccharidosis type VI. Blood 141, 807. doi:10.1182/blood.2022019127
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803. doi:10.7150/ijms.6048
Sestito, S., Rinninella, G., Rampazzo, A., D’Avanzo, F., Zampini, L., Santoro, L., et al. (2022). Cardiac involvement in MPS patients: incidence and response to therapy in an Italian multicentre study. Orphanet J. Rare Dis. 17, 251. doi:10.1186/s13023-022-02396-5
Shu, Y., Ding, Y., Liu, Y., Wu, P., He, X., and Zhang, Q. (2022). Post-marketing safety concerns with secukinumab: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 13, 862508. doi:10.3389/fphar.2022.862508
Skinner, S., Assen, K., and Mitchell, I. (2018). What does mainstream media say about enzyme replacement therapies? Paediatr. Child. Health 23, e117–e125. doi:10.1093/pch/pxy014
Sohn, Y. B., Park, S. W., Kim, S.-H., Cho, S.-Y., Ji, S.-T., Kwon, E. K., et al. (2012). Enzyme replacement therapy improves joint motion and outcome of the 12-min walk test in a mucopolysaccharidosis type VI patient previously treated with bone marrow transplantation. Am. J. Med. Genet. A 158A, 1158–1163. doi:10.1002/ajmg.a.35263
Tebani, A., Abily-Donval, L., Schmitz-Afonso, I., Piraud, M., Ausseil, J., Zerimech, F., et al. (2019). Analysis of mucopolysaccharidosis type VI through integrative functional metabolomics. Int. J. Mol. Sci. 20, 446. doi:10.3390/ijms20020446
Valayannopoulos, V., Nicely, H., Harmatz, P., and Turbeville, S. (2010). Mucopolysaccharidosis VI. Orphanet J. Rare Dis. 5, 5. doi:10.1186/1750-1172-5-5
Wang, J., Zhang, A., Ye, M., and Zhang, C. (2024). Examining the safety of mirabegron: an analysis of real-world pharmacovigilance data from the US FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 15, 1376535. doi:10.3389/fphar.2024.1376535
White, J. T., Argento Martell, L., Prince, W. S., Boyer, R., Crockett, L., Cox, C., et al. (2008a). Comparison of neutralizing antibody assays for receptor binding and enzyme activity of the enzyme replacement therapeutic Naglazyme (galsulfase). AAPS J. 10, 439–449. doi:10.1208/s12248-008-9048-1
White, J. T., Martell, L. A., Van Tuyl, A., Boyer, R., Warness, L., Taniguchi, G. T., et al. (2008b). Development, validation, and clinical implementation of an assay to measure total antibody response to naglazyme (galsulfase). AAPS J. 10, 363–372. doi:10.1208/s12248-008-9043-6
Keywords: galsulfase, Naglazyme, mucopolysaccharidosis VI (MPS VI), pharmacovigilance, adverse events, FDA adverse event reporting system (FAERS)
Citation: Li S, Huang R, Meng Y, Liu Y, Qian J, Zou J and Yang J (2024) Real-world pharmacovigilance analysis of galsulfase: a study based on the FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 15:1420126. doi: 10.3389/fphar.2024.1420126
Received: 19 April 2024; Accepted: 16 July 2024;
Published: 05 August 2024.
Edited by:
Grzegorz Wegrzyn, University of Gdansk, PolandReviewed by:
Joao Massud, Independent Researcher, São Paulo, BrazilPatryk Lipiński, Maria Sklodowska-Curie Medical Academy, Poland
Copyright © 2024 Li, Huang, Meng, Liu, Qian, Zou and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Zou, empqMTY4OEAxNjMuY29t; Jun Yang, eWFuZ2p1bnNwaW5lQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Shangze Li
Shangze Li Runcheng Huang
Runcheng Huang Yuanyuan Meng3†
Yuanyuan Meng3† Junjie Zou
Junjie Zou