
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 19 June 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1418555
The quest for effective epilepsy treatments has spotlighted natural alkaloids due to their broad neuropharmacological effects. This review provides a comprehensive analysis of the antiseizure properties of various natural compounds, with an emphasis on their mechanisms of action and potential therapeutic benefits. Our findings reveal that bioactive substances such as indole, quinoline, terpenoid, and pyridine alkaloids confer medicinal benefits by modulating synaptic interactions, restoring neuronal balance, and mitigating neuroinflammation—key factors in managing epileptic seizures. Notably, these compounds enhance GABAergic neurotransmission, diminish excitatory glutamatergic activities, particularly at NMDA receptors, and suppress proinflammatory pathways. A significant focus is placed on the strategic use of nanoparticle delivery systems to improve the solubility, stability, and bioavailability of these alkaloids, which helps overcome the challenges associated with crossing the blood-brain barrier (BBB). The review concludes with a prospective outlook on integrating these bioactive substances into epilepsy treatment regimes, advocating for extensive research to confirm their efficacy and safety. Advancing the bioavailability of alkaloids and rigorously assessing their toxicological profiles are essential to fully leverage the therapeutic potential of these compounds in clinical settings.
Epilepsy, often metaphorically described as the “goat disease,” is a chronic neurological ailment marked by frequent seizures stemming from abrupt and intense neuronal electrical activity (He et al., 2021). These recurrent episodes not only cause immediate neurological damage but also lead to persistent cognitive impairments, psychiatric conditions, and other lasting neural consequences, significantly affecting the patients’ quality of life (Wu et al., 2023). This condition afflicts approximately 65 million individuals worldwide, with a higher incidence in the developing regions, where up to 80% of these individuals are found (Kanner and Bicchi, 2022). Traditional antiseizure medications (ASM), including phenytoin and lamotrigine, though commonly prescribed, often fail to provide sufficient efficacy and are poorly tolerated by patients (Figure 1) (Ali et al., 2022). Recent advancements in treatment options, like brivaracetam and cannabidiol, offer increased therapeutic potential and better tolerability, especially beneficial for those suffering from resistant variants such as temporal lobe epilepsy. Nonetheless, a significant proportion of patients, estimated at 30%–40%, exhibit resistance to these drugs (Bahr et al., 2019), and the majority of conventional therapies do not effectively halt the disease’s progression, often resulting in cognitive deterioration and impacting adherence to treatment regimes. Consequently, the pursuit of innovative, potent, and accessible antiseizure methods remains an urgent and critical objective in addressing this pervasive health issue.
In light of the inadequacies of existing antiseizure medications, recent scientific inquiry has turned to the possibilities offered by phytochemicals (Akkol et al., 2021). This trend is marked by a noticeable inclination towards utilizing plant-based natural substances in drug development (Zhao et al., 2018). The advantages of these substances are highlighted not only by their potent biological activities but also by the relative ease of their extraction and the broad possibilities for exploring these biogenic materials. This makes them particularly appealing and promising alternatives to synthetic drugs in pharmaceutical research (Challal et al., 2023). Investigative efforts have uncovered structural similarities between conventional small-molecule antiseizures and natural alkaloids, which are nitrogen-containing organic compounds commonly present in plants. These alkaloids have been historically recognized for their wide-ranging pharmacological effects, including neuroprotective actions, reduction of neural hyperexcitability, and anticonvulsant benefits (Bui et al., 2023). Therefore, alkaloids are increasingly becoming focal in the innovation of antiseizure drugs (AED). Furthermore, empirical research substantiates that approximately 80% of epilepsy patients in the developing world rely on traditional plant-based treatments, a practice now receiving growing support through extensive pharmacological studies (Kakooza-Mwesige, 2015). The inherently low toxicity and potent antiseizure efficacy of certain plants and their alkaloid components mark them as crucial in the search for innovative AED.
Historical use of botanicals like St. John’s wort, ginkgo, and garlic, recognized for their antiseizure qualities (Aghdash, 2021), and a variety of bioactive alkaloids, such as indole, quinoline, and pyridine alkaloids, have been identified with proven antiseizure effects (Figure 1). These alkaloids’ influence on neurotransmitter receptors and ion channels offers a promising framework for creating advanced antiseizure treatments. In recent years, there has been a notable surge in the development of plant-derived AED, underscoring the untapped possibilities of phytochemicals in this area of therapy (Figure 2) (He et al., 2021). It is important to highlight the growing relevance of alkaloids in treating neurological disorders. However, in comparison to other natural antiseizure agents, these advancements are not as widely recognized. With this in mind, our review seeks to amalgamate the latest developments in alkaloid-based antiseizure treatments and to stimulate more targeted research in this domain (Figure 3; Table 1). Utilizing these novel compounds to expand the repertoire of antiseizure alkaloids has the potential not only to enhance the treatment efficacy for patients with intractable epilepsy but also to shed light on the etiological aspects of the condition, thereby contributing to better understanding and improvement of clinical management approaches.

Figure 2. Research progress of natural products and alkaloids in the treatment of neurological diseases, as well as the trend of publishing articles on epilepsy and alkaloids in the past years (All data from web of science).
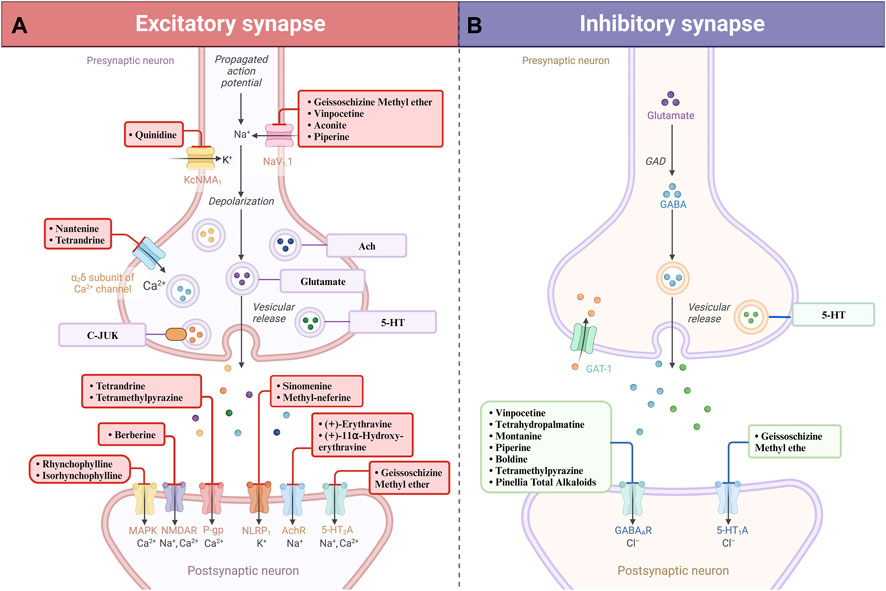
Figure 3. Natural alkaloids exert their therapeutic effects in the treatment of epilepsy by agonizing excitatory receptors (A) and agonistically activating inhibitory receptors (B).
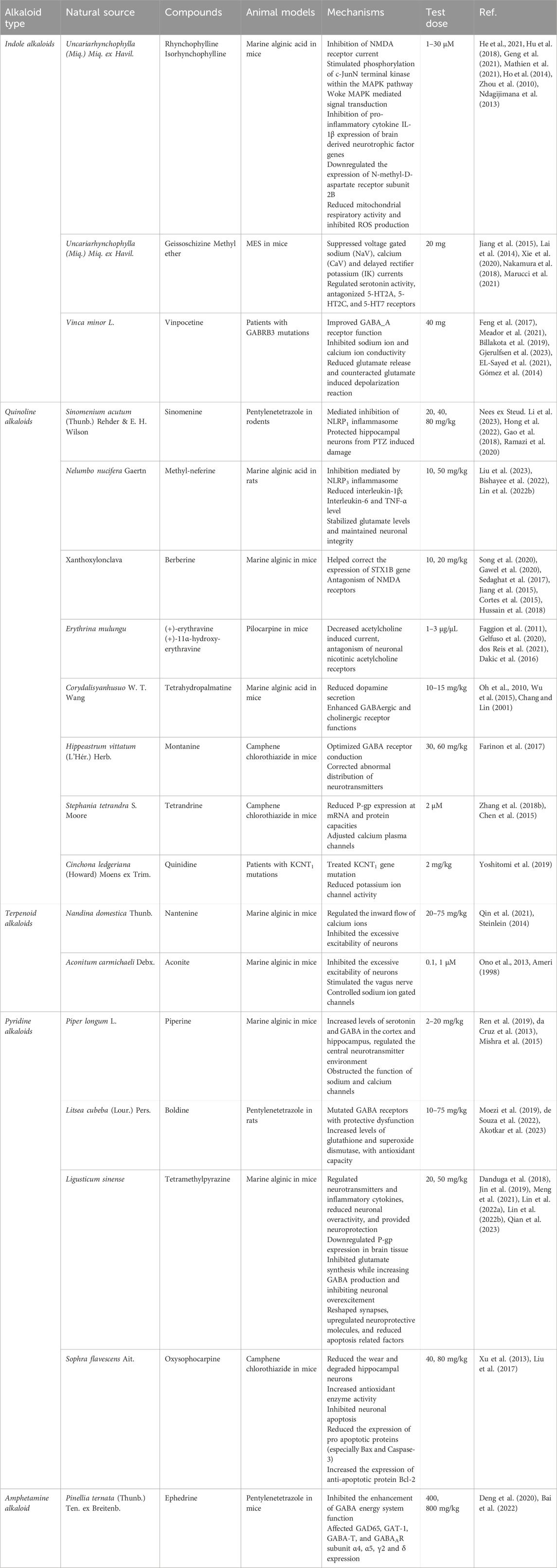
Table 1. Natural alkaloids utilized in epilepsy treatment through regulation of synaptic function and neurotransmitter release.
Indole alkaloids, a varied class of natural tryptophan derivatives, are recognized for their intricate molecular structures and profound pharmacological effects. These substances are predominantly found in the plant families Oleaceae Hoffmans. and Rubiaceae Juss, exhibiting a range of structures from simple indoles to complex bisindoles (Teng et al., 2023). In the field of antiseizure pharmacotherapy, indole alkaloids target a range of molecular receptors, effectively regulating neurotransmitter movement and ion channel activities. They mitigate epileptic disruptions by reducing excessive Ca2+ ion flow through overactive NMDA receptors and by sustaining the suppressive action of GABAergic signaling (Pandey, 2023). Furthermore, these alkaloids influence voltage-gated ion channels (Na+, Ca2+, and K+), curbing the irregular electrical activity that leads to seizures. The diverse pathway engagement of indole alkaloids accounts for their comprehensive effectiveness in maintaining the stability of neural network excitability. The detailed exploration of their specific roles in epilepsy treatment is outlined in the following sections (Munir et al., 2020).
Progressing in our discussion, rhynchophylline (RP), an indole alkaloid derived from Uncariarhynchophylla (Miq.) Miq. ex Havil of the Rubiaceae Juss family, showcases a broad range of therapeutic properties, encompassing anti-inflammatory effects and neurotransmitter modulation (Hu et al., 2018). This alkaloid has shown effectiveness in treating conditions such as cerebral ischemia, hypoxia, epilepsy, and anxiety. Its active components, notably RP and isocorynoxeine, interact with various signaling pathways and therapeutic targets (Geng et al., 2021), providing neuroprotection through antioxidant activities and neurotransmitter regulation. Experimental studies have demonstrated that RP and isocorynoxeine stimulate the phosphorylation of c-Jun N-terminal kinase in the mitogen-activated protein kinase (MAPK) pathway. This interaction significantly reduces MAPK-mediated signal transduction, thereby lessening neuronal damage in rodent models subjected to kainic acid (KA)-induced acute epileptic seizures (Mathien et al., 2021). Additionally, RP has been observed to reduce neuronal apoptosis in the hippocampus and decrease seizure frequency, influencing the SIRT1/p53/caspase-3 axis. The anticonvulsant effects of RP and isocorynoxeine in KA-exposed rats are further attributed to their regulatory impact on toll-like receptor and neurotrophin signaling pathways, effectively inhibiting the expression of pro-inflammatory cytokines such as IL-1β and genes related to brain-derived neurotrophic factor (Ho et al., 2014). On one aspect, molecules like isocorynoxeine have been shown to decrease NMDA receptor currents, suppress neurotransmitter transmission, and alleviate neural hyperactivity in the brain (Zhou et al., 2010). Conversely, these compounds also inhibit the pilocarpine-induced expression of the N-methyl-D-aspartate receptor subunit 2B. This inhibition results in reduced synaptic activity and neuronal excitability, thereby playing a significant role in their antiseizure effectiveness (Ndagijimana et al., 2013).
Furthermore, previous studies have identified geissoschizine methyl ether (GM) as a key antiseizure component of Uncariarhynchophyll (Miq.) Miq. ex Havil, with its antiseizure effectiveness demonstrated in glutamate-induced seizure models in mice (Jiang et al., 2015). GM’s neuroprotective capabilities have been observed in various glutamate-induced neurotoxicity tests. Its likely mechanisms include the inhibition of inward calcium and sodium currents, the reduction of delayed rectifier potassium currents, the decrease in mitochondrial respiratory functions, and the suppression of reactive oxygen species (ROS) formation (Lai et al., 2014). Research focusing on GM’s effects on neuronal action potential firing, using whole-cell current clamp recordings in cultured murine and rat hippocampal neurons, indicated dose-dependent reductions in firing rates in mouse neurons at concentrations of 1, 3, 10, and 30 μM, with inhibition rates of 45.3% ± 3.7%, 49.6% ± 5.1%, 82.3% ± 1.1%, and 97.8% ± 0.5%, respectively (Xie et al., 2020). In addition to its neuroprotective qualities, GM has been shown to foster myelin repair in developing oligodendrocytes and aid in the restoration of neuronal sheaths in the medial prefrontal cortex. GM also acts as a modulator of serotonergic activity, serving as a partial agonist at 5-HT1A receptors and antagonizing 5-HT2A, 5-HT2C, and 5-HT7 receptors (Nakamura et al., 2018). The compound’s inhibitory effect on acetylcholinesterase also hints at a further neuroprotective advantage, suggesting a potential therapeutic role in Alzheimer’s disease models (Marucci et al., 2021).
Subsequent to its first isolation in 1958 by European scientists from Vinca minor L (Feng et al., 2017), vinpocetine marked a significant advancement in plant-based antiseizure therapy. Studies have demonstrated vinpocetine’s ability to enhance memory in animal models, with potential cognitive benefits in humans as well (Meador et al., 2021). Recent clinical evaluations have confirmed vinpocetine’s considerable antiseizure effects, especially in cases of refractory focal epilepsy. Notably, in patients with loss-of-function GABA_A receptor variants, vinpocetine has been effective in reducing seizure occurrence and in improving cognitive and behavioral impairments, thus significantly improving patients’ quality of life (Billakota et al., 2019). For example, a documented case where a patient received 20 mg of vinpocetine thrice daily for 16 months showed a complete cessation of seizures, as confirmed by electroencephalogram findings of substantially reduced epileptiform activities. Alongside seizure control, the patient experienced a significant reduction in associated obsessive-compulsive, anxiety, and depressive symptoms. After 25 months, the dosage of vinpocetine was reduced to 20 mg twice daily with a plan for gradual discontinuation (Gjerulfsen et al., 2023). Continuous cardiac monitoring during the treatment period showed no cardiac function disturbances or changes in routine blood tests, confirming the safety of vinpocetine. Further studies into its mode of action revealed that vinpocetine inhibits sodium and calcium ion conductance, thus stabilizing cellular potentials by limiting ionic influx (El-Sayed et al., 2021). Additionally, vinpocetine has been found to reduce the levels of pro-inflammatory cytokines IL-1β and TNF-α in the rat hippocampus (Gómez et al., 2014).
Quinoline alkaloids, identified by their fundamental quinoline ring structure, constitute a unique group of natural compounds primarily produced through the ortho-aminobenzoic acid pathway (Thongsornkleeb et al., 2022). These alkaloids are widespread in Hemerocallis fulva (L.), Rutaceae Juss (often mistakenly called the “Dove family”), and especially in the Cinchona genus of the Rubiaceae Juss family. They are celebrated for their broad range of biological effects. Quinine and camptothecin are particularly noteworthy in this category, having undergone extensive investigation as exemplary quinoline alkaloids. Their principal mechanism of action involves modulating various receptors. Importantly, they have been shown to inhibit NOD-like receptor 3 (NLRP3) inflammasome-mediated pathways, reducing inflammatory responses and thus opening new possibilities for anti-inflammatory treatments (Eyal, 2018). Similar to indole alkaloids, quinoline alkaloids reduce epileptic seizures by decreasing NMDA receptor activity and by interacting with altered GABA genes, indicating a diverse approach in managing epilepsy (Shang et al., 2022). Additionally, these alkaloids have a role in suppressing P-glycoprotein (P-gp) expression, which could lower the incidence of epilepsy, thereby paving the way for new research and preventive approaches (Nuthakki et al., 2019). The following sections will delve deeper into quinoline alkaloids, exploring their therapeutic potential in greater detail.
In the early 20th century, pioneering work by Japanese researchers led to the isolation of sinomenine (SN) from the essential oil of Cymbopogon flexuosus (Nees ex Steud.) Will. Watson. Initially employed for treating rheumatic conditions, this morphinan alkaloid was subsequently found to possess a wide array of pharmacological properties, including notable antioxidant abilities, and is characterized by a low rate of adverse reactions (Li et al., 2023). Further mechanistic investigations have identified that SN’s neuroprotective effects predominantly stem from its ability to inhibit oxidative stress, neuroinflammation mediated by microglia or astroglia, and neuronal apoptosis (Hong et al., 2022). A controlled experiment using the pentylenetetrazole (PTZ)-induced epilepsy model in rodents demonstrated that SN, administered at doses of 20, 40, 50, and 80 mg/kg, intervened in the seizure initiation process in a dose-responsive manner, effectively reducing seizure intensity and lowering the occurrence of fully developed seizures (Gao et al., 2018). Specifically, a 50 mg/kg dose of SN notably lessened the severity and incidence of status epilepticus (SE), normalized MFS abnormalities in the hippocampus, reduced DNA fragmentation, and preserved neuron density. Concurrently, it significantly restored ROS, MDA, HO-1, and SOD levels, though its impact on GSH levels was not substantial. Additionally, a 50 mg/kg dose of sinomenine partially reversed increases in NF-κB, TLR4, TNF-α, GFAP, and caspase-1 (Ramazi et al., 2020). Moreover, varying SN doses protected hippocampal neurons from PTZ-induced harm and alleviated impairments in spatial learning and memory. These results suggest that SN’s antiseizure and neuroprotective effectiveness is largely driven by its suppression of the NLRP1 inflammasome, indicating its potential therapeutic value in epilepsy management (Gao et al., 2018).
In parallel, Xie et al. achieved a breakthrough by extracting neferine from the seeds of Nelumbo nucifera, thus enriching the pool of isoquinoline alkaloids renowned for their effectiveness in treating hypertension and arrhythmias (Liu et al., 2023). Methyl-neferine, an alkaloid derived from lotus seed embryos, has come under scrutiny for its prospective neuroprotective role in epilepsy management (Bishayee et al., 2022). In a relevant experiment, rats were pre-treated with methyl-neferines via intraperitoneal injection (i.p.) at doses of 10 and 50 mg/kg, 30 min before administering a KA injection (15 mg/kg, i.p.). This preemptive approach exhibited encouraging outcomes: it delayed the onset of seizures, lessened their intensity, stabilized glutamate concentrations, preserved neuronal health, and enhanced synaptic markers, particularly synaptophysin and postsynaptic density protein 95, in the hippocampal regions of KA-treated rats (Lin T. Y. et al., 2022). Additionally, pre-treatment with methyl-neferine markedly reduced glial cell activation and the consequent inflammatory response in the hippocampus, as indicated by lowered levels of interleukin-1β, interleukin-6, and tumor necrosis factor-α (TNF-α). Significantly, this approach also reduced key markers of the NLRP3 inflammasome pathway, including caspase-1 and interleukin-18, in the hippocampi of epileptic rats treated with methyl-neferines (Lin T. Y. et al., 2022). These results collectively indicate the potential of methyl-neferines to alleviate seizure severity, offer neuroprotection, and decrease neuroinflammation in the hippocampus by inhibiting the NLRP3 inflammasome and related inflammatory cytokines in KA-induced epilepsy models.
In addition to their role in inhibiting NLRP receptors, quinoline alkaloids also display selectivity in antagonizing NMDA receptors, thus broadening their therapeutic potential. The earliest documented isolation of the quaternary alkaloid berberine, belonging to the isoquinoline subclass, dates back to 1826 when M.-E. Chevalier et al. extracted it from the bark of a Coptis chinensis Franch tree (Song et al., 2020). While berberine has long been recognized for its antibacterial properties against organisms like Staphylococcus aureus and Streptococcus species, it has now been revealed to modulate the neurotransmitter system in a dose-dependent manner, exerting anticonvulsant effects (Gawel et al., 2020). In a study, daily administration of berberine at doses of 25 or 50 mg/kg was performed. The results demonstrated that berberine treatment at a dose of 50 mg/kg in rats microinjected with kainate lowered the incidence of status epilepticus (SE) and spontaneous recurrent seizures. Moreover, it significantly restored hippocampal levels of ROS, glutathione (GSH), nuclear factor (erythroid-derived 2)-like 2, catalase activity, caspase 3 activity, nuclear factor-B, toll-like receptor 4, TNF-α, interleukin-1 beta, and heme oxygenase 1 (Sedaghat et al., 2017). Specifically, berberine attenuates hyperexcitability and abnormal motor patterns in larval models, reducing hypersensitive epileptiform swimming behavior and contributing to the normalization of STX1B gene expression, a critical factor for synaptic function (Jiang et al., 2015). These protective effects are believed to stem from berberine’s antagonism of NMDA receptors, preventing the pathologically excessive activation of extrasynaptic NMDARs, a process implicated in epileptogenesis (Cortes et al., 2015). However, further research is essential to fully elucidate berberine’s capacity as an NMDAR antagonist and its clinical relevance in epilepsy management (Hussain et al., 2018).
Furthermore, the identification and subsequent analysis of erythrosine by-products, specifically (+)-erythravine and (+)-11α-hydroxy-erythravine, derived from Erythrina mulungu Mart ex Benth, have broadened the repertoire of compounds exhibiting antiseizure properties (Faggion et al., 2011). Erythrosine, characterized as a quinoline alkaloid with a distinctive benzyl tetrahydroisoquinoline framework, has a well-established history in the management of central nervous system (CNS) disorders, including insomnia and related (Gelfuso et al., 2020). Seizure genesis is often attributed to dysregulations within excitatory and inhibitory neurotransmitter systems. In this context, derivatives of erythrosine reveal their therapeutic potential by modulating acetylcholine-evoked currents, indicating their antagonistic effects on neuronal nicotinic acetylcholine receptors (dos Reis et al., 2021). The antiseizure efficacy of both (+)-erythravine and (+)-11α-hydroxy-erythravine is noteworthy in various seizure models induced by phenyltetrazolium, kainic acid, bicuculline, and NMDA, implying a broad spectrum of activity (Dakic et al., 2016). Building upon this foundation, in vivo experiments demonstrate that both compounds, administered at dosages of 1.23 μg/μL, are efficiently absorbed through the gastrointestinal tract and possess the capacity to penetrate the BBB, a pivotal pharmacokinetic attribute for CNS-targeted therapeutics (Gelfuso et al., 2020). This permeability underscores the therapeutic relevance of these compounds and propels the advancement of novel antiseizure medications (Gelfuso et al., 2020).
Expanding our perspective, quinoline alkaloids exhibit diverse pharmacological actions that extend beyond NMDA receptor antagonism, encompassing effects on GABAergic systems. Tetrahydropalmatine, a benzylisoquinoline alkaloid derived from Corydalis yanhusuo W. T. Wang, has traditionally been employed to enhance blood flow, alleviate stasis (Oh et al., 2010). Investigations into its mechanistic effects on epilepsy models reveal a reduction in dopamine secretion, along with an enhancement of GABAergic and cholinergic receptor functionalities (Wu et al., 2015). Notably, experimental intraperitoneal injections of Tetrahydropalmatine at doses of 10 mg/kg or 15 mg/kg in rats demonstrated that pretreatment with Tetrahydropalmatine nearly completely eliminated the Picrotoxin-induced increase in dopamine release in the amygdala (Chang and Lin, 2001). Such modulation leads to the suppression of seizure initiation, highlighting the potent antiseizure properties of corydaline. Additionally, in the 1950s, isoquinoline alkaloids like montanine, derived from Hippeastrum vittatum (L’Hér.) Herb, initially gained attention for their anticancer properties, particularly their ability to inhibit malignant cell proliferation. Subsequent research, however, has unveiled significant anxiolytic and antiseizure benefits associated with montanine (Farinon et al., 2017). Experimental data elucidates its role in preventing convulsions by acting on the GABAA receptor system. This modulation not only optimizes GABA receptor conductance but also corrects aberrant neurotransmitter distribution, offering a promising therapeutic approach for managing epilepsy.
Furthermore, the therapeutic potential of quinoline alkaloids extends beyond the modulation of neurotransmitter receptors, encompassing the inhibition of protein expression and regulation of ion channels (Plazas et al., 2022). Tetrandrine (TTD), a dibenzylisoquinoline compound sourced from Stephania tetrandra S. Moore, has traditionally been indicated for the management of hypertension (Zhang Z. X. et al., 2018). However, recent evidence suggests the utility of TTD in epilepsy, highlighting its capacity to reduce the expression of P-gp, thus enhancing the efficacy of AED (Chen et al., 2015). In a study, epileptic rats receiving intraperitoneal TTD at a dose of 30 mg/kg displayed significant behavioral improvements assessed using Racine’s seizure scale over a 2-week observation period, in contrast to the saline-treated control group (Chen et al., 2015). While these outcomes are promising, further investigation is required to comprehensively evaluate TTD’s therapeutic profile and safety for the treatment of epilepsy.
Similarly, quinidine, another alkaloid extracted from Cinchona ledgeriana (Howard) Moens ex Trim, is well-known for its antiarrhythmic properties. Recent research has shifted its focus towards the application of quinidine in precision medicine for seizure disorders associated with KCNT1 gene mutations (Yoshitomi et al., 2019). In vitro studies using Xenopus laevis oocytes have revealed that quinidine exerts a concentration-dependent correction of the abnormal increase in potassium channel activity caused by KCNT1 mutations. These mutations are implicated in conditions such as autosomal dominant nocturnal frontal lobe epilepsy and severe infantile-onset epileptic encephalopathy. Quinidine’s ability to modulate potassium channel function supports its role in reducing neuronal hyperexcitability and consequent seizure manifestations.
Terpenoid alkaloids constitute a unique class of compounds characterized by the fusion of nitrogenous groups with terpenoid backbones. They can be further classified based on the terpenoid component into monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids (Huang et al., 2022). Nantenine, a terpenoid alkaloid originally derived from Nandina domestica Thunb (Qin et al., 2021), is a notable example of this class used clinically for its sedative and antiseizure properties. Research suggests that nantenine modulates calcium-mediated signaling, reducing the hyperexcitability of neuronal cells by regulating the influx of calcium. This presents a potential therapeutic approach for epilepsy by stabilizing neuronal excitability and mitigating seizures (Steinlein, 2014). On the other hand, aconitine, another noteworthy terpenoid alkaloid, is categorized as a neurotoxic diterpene due to its diester configuration. It is known for its stimulatory effect on the vagus nerve and has been observed to interact with voltage-gated sodium channels, which play a crucial role in regulating neuronal excitability (Ono et al., 2013). Research by Ameri (1998) has examined the central nervous system effects of aconitine, revealing its selective action on sodium channels and its potential as an antiseizure agent in rat hippocampal slices. However, further validation is needed to establish the efficacy and safety of aconitine for epilepsy treatment.
Pyridine alkaloids, synthesized from lysine and branching into pyridine or piperidine derivatives, are characterized by their streamlined and minimalistic structures (Frolov and Vereshchagin, 2023). They can be generally categorized into two subsets: uncomplicated pyridines and bis-piperidines (Ma et al., 2014). Notable examples within this alkaloid family include nicotine, found in tobacco, and piperine, present in black pepper. These compounds primarily exert antiseizure effects by targeting and inhibiting mutations within genes that encode GABA receptors (Lin et al., 2020).
Piperine, a pyridine alkaloid derived from Piper longum L., exerts a wide-ranging anticonvulsant influence (Ren, 2019). Beyond seizure mitigation, it finds clinical applications in analgesia, anxiolysis, and lipid regulation. Mechanistically, piperine modulates the central neurotransmitter milieu, particularly by increasing cortical and hippocampal 5-hydroxytryptamine (serotonin) and GABA levels. This modulation correlates with the attenuation of epileptic tonic-clonic seizures (da Cruz et al., 2013). Notably, piperine (administered at 10 mg/kg) delays the onset of tonic-clonic convulsions and reduces associated mortality in the pentetrazol trial. Furthermore, anticonvulsant doses of piperine delay the onset of strychnine, picrotoxin, and BAYK-8644 tonic-clonic seizures. Electrophysiological evidence supports its role in inhibiting sodium and calcium channel functions (Mishra et al., 2015). However, it is essential to note that while these findings are promising, the research on piperine’s antiseizure utility remains preliminary, necessitating extensive longitudinal studies to uncover its full clinical potential.
Simultaneously, the compound boldine, extracted from the root of the Litsea cubeba (Lour.) Pers. and categorized as an aporphine isoquinoline alkaloid, has garnered attention for its utility in antioxidant regimens (de Souza et al., 2022). The investigative efforts have spotlighted boldine’s seizure-preventive capacity in murine models, challenged with convulsions induced by hydrazine and indole dioxides. Administered intraperitoneally, boldine demonstrated dose-dependent efficacy in obstructing PTZ-induced clonic and myoclonic seizures and curtailed the seizure duration in models of electric shock-induced convulsions (Akotkar et al., 2023). Its modulatory effects on GABA receptors confer neuroprotection, particularly against dysfunctional GABA receptor mutations. Furthermore, boldine’s long-term administration has been associated with augmented levels of glutathione and superoxide dismutase, reflecting its intrinsic antioxidant capabilities (Akotkar et al., 2023). Experimental data prove, Acute administration of non-effective dose of boldine (10 mg/kg) and vitamin C (50 mg/kg) induced anti-convulsing effects of boldine on seizure threshold (Moezi et al., 2019). Its free radical neutralizing activity, especially against hydroxyl radicals, positions boldine as a candidate of considerable interest for epilepsy treatment strategies. These viewpoints collectively expand the treatment landscape for alkaloids in epilepsy management (Moezi et al., 2019). Pyridine alkaloids can also exert their effects by inhibiting synaptic transmission, and tetramethylpyrazine, a pyridine alkaloid derived from Conioselinum anthriscoides (H. Boissieu) Pimenov, is one such compound recognized for its antiseizure properties in clinical settings (Lin J. et al., 2022). The mechanistic profile of tetramethylpyrazine is complex and diverse: it modulates both neurotransmitters and inflammatory cytokines, reducing neuronal hyperactivity and providing neuroprotection (Meng et al., 2021). Additionally, it downregulates P-gp expression in cerebral tissues, mitigating impediments to BBB permeability. This enhancement of hemorheological attributes counters stress-related damage and modulates immune responses (Qian et al., 2023). In a study, mice treated with TMP (20 and 50 mg/kg, i.p.) remained in stage 1 of epileptic progression for an extended period, requiring additional stimulation to induce stages 2–5 epileptic phenotypes. TMP (50 mg/kg) also inhibited 6 Hz corneal kindling progression (Jin et al., 2019). Experimentally, tetramethylpyrazine’s ability to adjust glutamate and GABA synthesis in the brain is noteworthy. It suppresses glutamate synthesis while augmenting GABA production, fostering equilibrium between excitatory and inhibitory neurotransmission. This dampens neuronal hyperexcitability and ultimately inhibits seizure activity (Danduga et al., 2018). Moreover, tetramethylpyrazine plays a role in synaptic remodeling by upregulating neuroprotective molecules and curtailing the expression of apoptosis-related factors, contributing to its neuroprotective and antiseizure effects (Lin T. Y. et al., 2022). Despite these promising attributes, the intricate pathways and the full spectrum of tetramethylpyrazine’s impact necessitate further elucidation.
Notably, the pyridine derivative oxysophocarpine (OSC) has come to prominence for its compelling anticonvulsant and neuroprotective attributes (Xu et al., 2013). In murine models, specifically adult males subjected to rutaecarpine-induced convulsions, OSC administration has been shown to significantly delay the onset of both initial convulsive activity and sustained status epilepticus, as evidenced by electroencephalographic assessment. Moreover, subsequent to administration of 40 mg/kg and 80 mg/kg doses, a marked suppression of epileptiform activity was observed. Histological analyses, including Nissl and fluoro-Jade B staining, revealed OSC’s capacity to mitigate hippocampal neuronal attrition and degradation (Liu et al., 2017). Complementary bioassays indicated a decrease in malondialdehyde levels and an increase in enzymatic activities associated with antioxidative defenses, glutathione peroxidase, and catalase, suggesting a reduction in oxidative stress. Further molecular studies, using Western blot technique, clarified that the expression of Bax and Caspase-3 decreased, while the expression of Bcl-2 augmented, indicating that OSC downregulated neuronal apoptosis (Liu et al., 2017).
Ephedrine, an alkaloid denoted chemically as C10H15NO, transcends its classification as a Class I vulnerable chemical due to its extensive clinical utility. It is renowned primarily for staving off bronchial asthma attacks and ameliorating mild asthmatic symptoms (Gad et al., 2021). Recent studies have uncovered additional pharmacological properties of ephedrine, specifically an anti-epileptic activity derived from extracts of Herba Ephedrae (Zhang B. M. et al., 2018). This novel insight broadens the medicinal applications of ephedrine and herald’s new prospects for epilepsy management.
Pinellia total alkaloids (PTA), sourced from the Pinellia ternate (Thunb.), have been empirically validated for quelling seizure manifestations in maximal electroconvulsive and penicillin-induced ignition models (Bai et al., 2022). In a structured experimental framework, 91 male Sprague-Dawley rats were allocated into control and epileptic cohorts, with the latter induced into sustained epilepsy via hirsute fruit rutabaga administration (Deng et al., 2020). Post-SE survivors were further segmented into groups receiving either no treatment, topiramate, high-dose PTA (800 mg/kg), or low-dose PTA (400 mg/kg), with daily gavage over a fortnight. Aftertreatment, spontaneous recurrent seizures were observed over a week, followed by hippocampal tissue collection for GABA quantification via enzyme-linked immunosorbent assay. Results revealed that PTA modulated GABA levels and influenced the expression of GAD65, GAT-1, GABA-T, and GABAAR subunits α4, α5, γ2, and δ, suggesting a mechanistic basis for its antiseizure effect against epilepsy prompted by Hirsutine. The elevation in GABA concentrations and upregulation of GABAA receptor mRNA in the cerebellum underscore PTA’s enhancement of the inhibitory GABAergic system function (Deng et al., 2020; Richardson et al., 2024). These findings illuminate the therapeutic potential of PTA in epilepsy treatment and enrich the discourse on the pathogenesis and therapeutic approaches to the condition. Nevertheless, the translation of PTA to a clinical antiseizure modality demands further validation to ascertain its long-term efficacy and safety.
In brief, we explore the antiepileptic properties of five distinct alkaloids, which mitigate epileptic disruptions by various mechanisms: decreasing excessive Ca2⁺ ion flux due to hyperactive NMDA receptors, sustaining GABAergic signaling inhibition, modulating voltage-gated ion channels (Na⁺, Ca2⁺, and K⁺), and acting as agonists at the 5-HT1A receptor while antagonizing the 5-HT2A receptor. Despite their demonstrated efficacy in alleviating epilepsy, these alkaloids face several challenges, including poor solubility and bioavailability, exemplified by compounds such as piperine, as well as insufficient lipid solubility, which hinders berberine’s ability to effectively penetrate the BBB (Quijia et al., 2021). These limitations can impact the unique therapeutic potential of alkaloids in epilepsy management. In the subsequent section, we will elaborate on effective and innovative strategies to overcome issues like the low bioavailability of these compounds.
We have identified various strategies to improve the low bioavailability of alkaloids. For instance, Huang et al. (2016) synthesized a series of novel C-9-O substituted berberine derivatives and evaluated their anti-inflammatory effects both in vitro and in vivo. These novel synthetic berberine derivatives demonstrated significantly higher inhibitory activity on NO, TNF-α, and IL-6 release, along with enhanced bioavailability compared to berberine. Similarly, Zhang et al. (2020) synthesized a berberine derivative, hydrophobic ethyl-2-(9-dimethoxyberberine bromide-9-yl) hydroxy acetate, which exhibited an enhanced anticonvulsant effect directly linked to improved bioavailability.
In addition to traditional chemical modifications to enhance physicochemical properties, emerging nanotechnologies offer a transformative direction. For example, Shilo et al. (2015) highlighted nanoparticles as promising drug delivery agents capable of crossing the BBB in 2015. Nanoparticles have garnered significant scientific interest due to their versatile properties, making them suitable for various biological and chemical applications (van Rooy et al., 2011). Nanoparticle-based drug delivery systems show promise in overcoming bioavailability challenges by enhancing drug solubility, stability, and permeability through the BBB (Duan et al., 2023). Our forthcoming investigation will focus on optimizing alkaloid delivery through nanoparticle technology. This innovative approach aims to maximize therapeutic efficacy by enabling targeted and controlled release of alkaloids, presenting a potential breakthrough in epilepsy treatment (Figure 4; Table 2).
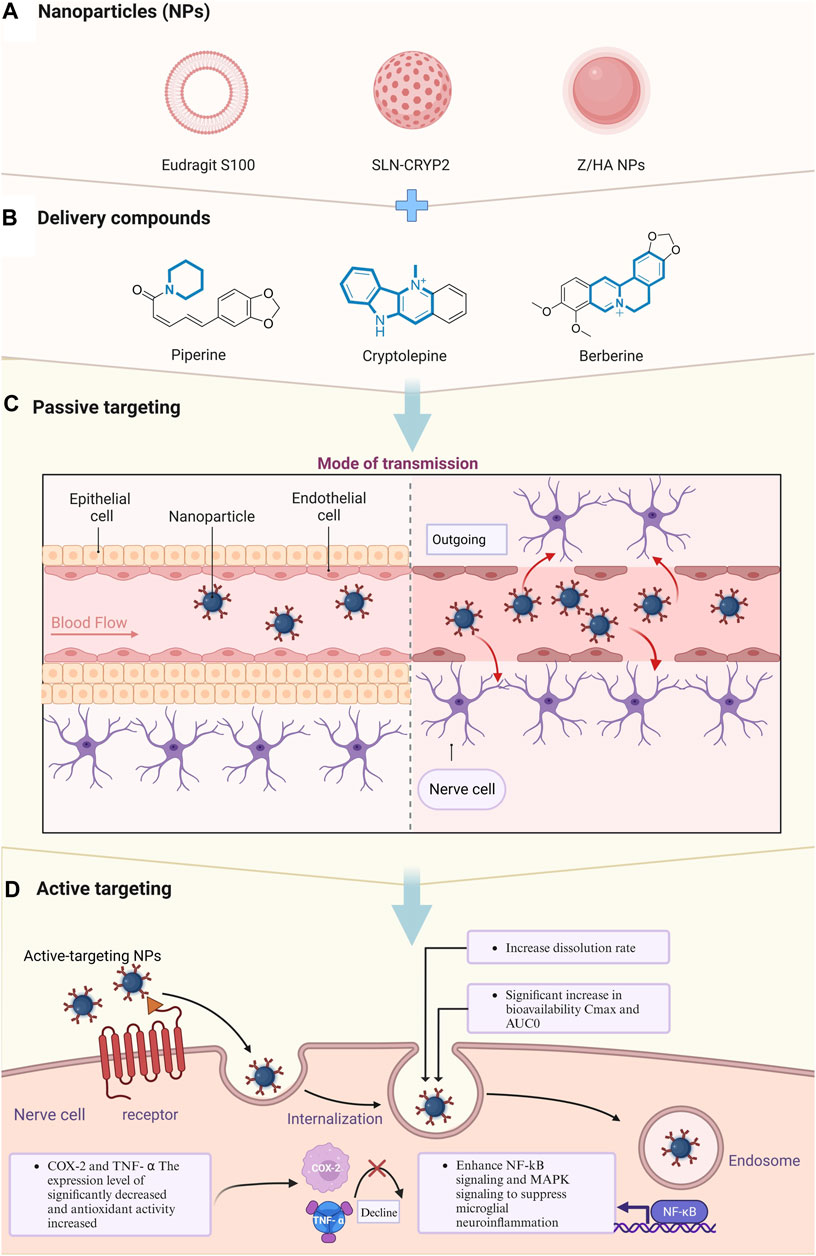
Figure 4. Delivery of natural alkaloids to nerve cells via nanomaterial delivery systems for therapeutic effects. (A) Types of nanomaterials encapsulating small molecule compounds. (B) Structural formulas of improved epilepsy therapeutic compounds utilizing nanomaterial encapsulation. (C) Transport of nanomolecules from blood vessels into nerve cells after crossing endothelial barriers. (D) Intracellular entry of nanomolecules and enhancement of physicochemical properties of drugs.
In 2019, Ren et al. (2019) has presented a pioneering study that effectively enhances the solubility of piperine, an alkaloid with antiseizure potential, through innovative nanotechnology. By reformulating piperine into a nanoparticle suspension using a nanoprecipitation technique, they aimed to amplify its oral bioavailability, thereby potentiating its development as an antiseizure agent. Employing transmission electron microscopy for meticulous characterization, they confirmed the formation of uniform, spherical nanoparticles with a narrow size distribution and high encapsulation efficiency (Zhu et al., 2020). Piperine was first used for optimization in the nano field. Remarkably, the nanoformulated piperine exhibited a significant boost in dissolution rates, with a threefold increase in the 24-hour cumulative drug release, compared to its conventional counterpart. When administered to rats at a dosage of 3.5 mg/kg, the nanoformulation achieved a 2.7-fold rise in oral bioavailability and demonstrated a marked 16-fold escalation in brain concentration within 10 h post-administration (Ren et al., 2019). The efficacy of these piperine nanoparticles was further validated in vivo, where they afforded robust protection against pentylenetetrazole-induced seizures in zebrafish and mouse models, showcasing their potential as a formidable strategy in epilepsy management (Anissian et al., 2018).
Subsequently, in 2021, Riscilla et al. innovated a solid lipid nanoparticle delivery system for cryptolepine, denoted as SLN-CRYP, that substantially improved the compound’s capacity to traverse the BBB. This advancement not only facilitated enhanced drug-receptor interaction but also increased the drug’s permeability into the brain. They employed a PTZ-induced epileptiform behavioral model in zebrafish to quantify the antiseizure efficacy of SLN-CRYP, testing dosages of 2.5 and 5 mg/kg (Mante et al., 2021). A rigorous examination into SLN-CRYP’s pharmacokinetics affirmed a marked improvement in BBB permeability. Clinically significant findings emerged from the treatment outcomes: both tested dosages of SLN-CRYP substantially decreased the average seizure scores and markedly extended the onset of seizure episodes. Furthermore, receptor binding assays revealed that cryptolepine displayed inhibitory effects on human voltage-gated calcium channels (Cav1.2), H1 receptors, peripheral benzodiazepine receptors, and σ2 receptors, albeit with relatively modest affinity, indicating a lesser antagonistic impact on Cav1.2 and σ2 receptors compared to known antagonists nifedipine and haloperidol, respectively (Mante et al., 2021). This underlines the therapeutic relevance of SLN-CRYP as a promising candidate for epilepsy treatment, with potential for further investigation and development.
Recently, in exploring the delivery system of AED, Amira et al. has made a notable advance in the pharmacokinetic enhancement of antiseizure agents by formulating composite nanoparticles for the augmented cerebral absorption of berberine. These nanoparticles, designated B2, have been shown to significantly ameliorate the manifestations of persistent status epilepticus in rats, stimulating composite nanoparticles for the augmented cerebral absorption of berberine. These nanoparticles, designated B2, have been shown to significantly ameliorate the manifestations of persistent status epilepticus in rats, a condition notoriously challenging to manage with traditional berberine suspensions (El-Nahas et al., 2023). This innovation suggests that the novel nanoparticle construct could markedly improve the clinical management of epilepsy. Moreover, an analysis of hippocampal tissue from B2-treated rats revealed diminished neurodegeneration, which was further underscored by a pronounced decrease in pro-inflammatory markers COX-2 and TNF-α, alongside an increase in antioxidative measures. These results underscore the dual therapeutic benefit of B2 mitigating the pathological sequelae of epilepsy and concurrently promoting neurohealth. Crucially, safety evaluations of the nanoparticle system yielded no evidence of toxicity or behavioral modification, affirming the biocompatibility of B2 (El-Nahas et al., 2023). This finding substantiates the nanoparticle system’s potential as a safe vehicle for delivering AED, holding promise for future clinical translation (Saha et al., 2023).
This review begins by examining the diverse neuropharmacological activities of natural alkaloids that position them as potential treatments for epilepsy. Natural alkaloids confer therapeutic benefits by modulating synaptic communication, reestablishing neuronal equilibrium, and mitigating neuroinflammation, mechanisms critical for the control of epileptic seizures (Sharifi-Rad et al., 2021). Salient therapeutic actions include the potentiation of GABAergic neurotransmission, the attenuation of excitatory glutamatergic activity, notably at NMDA receptors, and the suppression of pro-inflammatory cascades (Mathie, 2010). Collectively, these actions bolster the candidacy of natural alkaloids as efficacious agents in the management of epilepsy (Figure 3). Despite promising laboratory studies, the application of these alkaloids in clinical settings is still in its early stages, with a majority of findings confined to animal studies and a dearth of human clinical trial data. Consequently, further empirical scrutiny into the toxicological profiles and the safety of natural alkaloids is imperative. Prospective research trajectories should pivot on the explication of underlying molecular pathways, the longitudinal assessment of efficacy and safety in controlled human studies, thereby bridging the gap towards the incorporation of these alkaloids into mainstream epilepsy therapeutics (Suzuki et al., 2011).
The therapeutic potential of alkaloids in epilepsy, however promising, is compromised by their limited solubility and bioavailability, which pose substantial barriers to their clinical deployment. Addressing these limitations necessitates technological refinements aimed at bolstering the stability and efficacy of these compounds. Strategies extend beyond nanodelivery systems to include the intricate re-engineering of chemical structures, an area burgeoning with potential. A case in point is the innovation surrounding zembrin, an alkaloid-rich extract utilized in epilepsy treatment. This botanical compound is composed of mesembrine, mesembranol, mesembrenol, and mesembrenone (Dimpfel et al., 2018). Through meticulous structural modifications, specifically, substituting the carbonyl group at the C6 position with a hydroxyl moiety in mesembranol and mesembrenol, researchers have unearthed new structure-activity relationships. The resultant analogs target and mitigate AMPA receptor-mediated excitatory transmission, thereby enhancing their antiseizure profile. Clinical investigations have substantiated the efficacy of these modified alkaloids, with dosages of 5 and 10 mg/kg mirroring the therapeutic outcomes of a 50 mg/kg standard dose. Such findings elevate mesembranol and mesembrenol to the status of pivotal chemical forerunners in the quest to forge novel antiseizure agents, potentially invigorating the entire epilepsy treatment landscape (Dimpfel et al., 2018).
In parallel with enhancing alkaloid bioavailability, it is imperative to rigorously assess their potential toxicological implications to preempt any irreversible physiological detriment. Crucial to their advancement as treatments is a thorough examination of their safety, a step that is currently lagging behind other research areas. For instance, certain isoquinoline alkaloids like berberine exhibit an affinity for DNA, precipitating toxicological responses (Liu et al., 2019). Moreover, organic amine alkaloids, exemplified by ephedrine, may induce cardiotoxic sequelae, such as hypertension and palpitations (Neumann et al., 2023). Given these risks, vigilance in monitoring the toxicological profile of alkaloids used in epilepsy treatment is essential during experimental trials. Yet, the scope of research addressing the toxicology of potential antiseizure alkaloids remains disappointingly narrow. This gap in the literature underscores the necessity for intensified research into the toxic effects of alkaloids, which is critical for elucidating their safety and ensuring informed therapeutic use.
The advancement of medical science calls for a concerted effort to refine current alkaloid-based treatments and to pioneer new avenues for epilepsy management. While discussing the advances in epilepsy treatment, it is pertinent to consider the unique position of alkaloids within this broader context. Notably, the second-generation AED levetiracetam has demonstrated superior seizure control by precisely modulating neurotransmitter release through synaptic vesicle glycoprotein 2A, representing a significant advancement towards precision medicine (Celdran de Castro et al., 2023). Additionally, the third-generation AED perampanel has brought fresh perspectives by antagonizing AMPA receptor-mediated excitatory transmission (Perversi et al., 2023). Using mTOR as a target, the novel therapeutic agent everolimus improves the understanding of the pharmacological framework of epilepsy isolated from Auvin and Baulac (2023). These emergent drugs and their mechanisms not only furnish novel therapeutic modalities but also unveil yet unexplored therapeutic strategies and potential drug targets (Jain et al., 2022). Moreover, the prospect of alkaloids augmenting combination therapies marries traditional herbal insights with contemporary pharmacology, representing a compelling research frontier. Combining treatments such as Nobiletin with clonazepam, or naringenin with phenytoin, has yielded remarkable clinical outcomes, expanded the repertoire of epilepsy treatment strategies and enhanced therapeutic efficacy. While alkaloids currently represent a minor component of these combination therapies, the deepening insight into their pharmacodynamics and escalating research funding justify a confident forecast: In the not-too-distant future, the integration of alkaloids with other therapeutic agents is poised to emerge as a pivotal strategy in epilepsy management. This evolution will undoubtedly offer more efficacious, tailored treatment options for individuals afflicted with this neurological condition.
In conclusion, the promise of alkaloids in epilepsy treatment is contingent upon dedicated research efforts to refine their delivery mechanisms and safety profiles. Future research endeavors are anticipated to address these obstacles, enhancing the solubility, bioavailability, and safety profile of alkaloid-based therapies. Advancements in pharmacological research and drug development processes are poised to deliver more efficacious and safer treatment alternatives to individuals living with epilepsy, heralding a new epoch in the management of this complex neurological disorder. The culmination of these efforts will be a paradigm shift in epilepsy therapeutics, driven by a commitment to innovation and a deepened understanding of plant-derived pharmacology.
SL: Writing–original draft, Writing–review and editing. XL: Writing–original draft, Writing–review and editing. LD: Writing–review and editing, Writing–original draft.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The figures in this review were partly illustrated with the assistance of BioRender.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ASM, antiseizure medications; BBB, blood-brain barrier; CNS, central nervous system; GM, geissoschizine methyl ether; KA, kainic acid; MAPK, mitogen-activated protein kinase; NLRP3, nod-like receptor 3; OSC, oxysophocarpine; P-gp, p-glycoprotein; PTA, pinellia total alkaloids; PTZ, pentylenetetrazole; ROS, reactive oxygen species; RP, rhynchophylline; SE, status epilepticus; SN, sinomenine; TNF-α, tumor necrosis factor-α; TTD, tetrandrine.
Aghdash, S. N. (2021). Herbal medicine in the treatment of epilepsy. Curr. Drug Targets 22 (3), 356–367. doi:10.2174/1389450121999201001152221
Akkol, E. K., Çankaya, I. T., Karatoprak, G. S., Carpar, E., Sobarzo-Sánchez, E., and Capasso, R. (2021). Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 12, 27. doi:10.3389/fphar.2021.669638
Akotkar, L., Aswar, U., Ganeshpurkar, A., Raj, R., and Pawar, A. (2023). An overview of chemistry, kinetics, toxicity and therapeutic potential of boldine in neurological disorders. Neurochem. Res. 48 (11), 3283–3295. doi:10.1007/s11064-023-03992-y
Ali, O., Shaikh, M. F., Hasnain, M. S., Sami, F., Khan, A., and Ansari, M. T. (2022). Nanotechnological advances in the treatment of epilepsy. CNS Neurol. Disord. Drug Targets 21 (10), 994–1003. doi:10.2174/1871527321666211221162104
Ameri, A. (1998). The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 56 (2), 211–235. doi:10.1016/s0301-0082(98)00037-9
Anissian, D., Ghasemi-Kasman, M., Khalili-Fomeshi, M., Akbari, A., Hashemian, M., Kazemi, S., et al. (2018). Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int. J. Biol. Macromol. 107, 973–983. doi:10.1016/j.ijbiomac.2017.09.073
Auvin, S., and Baulac, S. (2023). mTOR-therapy and targeted treatment opportunities in mTOR-related epilepsies associated with cortical malformations. Rev. Neurol. Paris. 179 (4), 337–344. doi:10.1016/j.neurol.2022.12.007
Bahr, T. A., Rodriguez, D., Beaumont, C., and Allred, K. (2019). The effects of various essential oils on epilepsy and acute seizure: a systematic review. Evid. Based Complement. Altern. Med. 2019, 6216745. doi:10.1155/2019/6216745
Bai, J., Qi, J., Yang, L., Wang, Z., Wang, R., and Shi, Y. (2022). A comprehensive review on ethnopharmacological, phytochemical, pharmacological and toxicological evaluation, and quality control of Pinellia ternata (Thunb.) Breit. J. Ethnopharmacol. 298, 115650. doi:10.1016/j.jep.2022.115650
Billakota, S., Andresen, J. M., Gay, B. C., Stewart, G. R., Fedorov, N. B., Gerlach, A. C., et al. (2019). Personalized medicine: vinpocetine to reverse effects of GABRB3 mutation. Epilepsia 60 (12), 2459–2465. doi:10.1111/epi.16394
Bishayee, A., Patel, P. A., Sharma, P., Thoutireddy, S., and Das, N. (2022). Lotus (Nelumbo nucifera gaertn.) and its bioactive phytocompounds: a tribute to cancer prevention and intervention. Cancers 14 (3), 529. doi:10.3390/cancers14030529
Bui, V. H., Rodríguez-López, C. E., and Dang, T. T. T. (2023). Integration of discovery and engineering in plant alkaloid research: recent developments in elucidation, reconstruction, and repurposing biosynthetic pathways. Curr. Opin. Plant Biol. 74, 102379. doi:10.1016/j.pbi.2023.102379
Celdran de Castro, A., Nascimento, F. A., Beltran-Corbellini, Á., Toledano, R., Garcia-Morales, I., Gil-Nagel, A., et al. (2023). Levetiracetam, from broad-spectrum use to precision prescription: a narrative review and expert opinion. Seizure 107, 121–131. doi:10.1016/j.seizure.2023.03.017
Challal, S., Skiba, A., Langlois, M., Esguerra, C. V., Wolfender, J. L., Crawford, A. D., et al. (2023). Natural product-derived therapies for treating drug-resistant epilepsies: from ethnopharmacology to evidence-based medicine. J. Ethnopharmacol. 317, 116740. doi:10.1016/j.jep.2023.116740
Chang, C. K., and Lin, M. T. (2001). DL-Tetrahydropalmatine may act through inhibition of amygdaloid release of dopamine to inhibit an epileptic attack in rats. Neurosci. Lett. 307 (3), 163–166. doi:10.1016/s0304-3940(01)01962-0
Chen, Y. H., Xiao, X., Wang, C. C., Jiang, H. Y., Hong, Z., and Xu, G. X. (2015). Beneficial effect of tetrandrine on refractory epilepsy via suppressing P-glycoprotein. Int. J. Neurosci. 125 (9), 703–710. doi:10.3109/00207454.2014.966821
Cortes, N., Posada-Duque, R. A., Alvarez, R., Alzate, F., Berkov, S., Cardona-Gómez, G. P., et al. (2015). Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: a comparative study. Life Sci. 122, 42–50. doi:10.1016/j.lfs.2014.12.011
da Cruz, G. M. P., Felipe, C. F. B., Scorza, F. A., da Costa, M. A. C., Tavares, A. F., Menezes, M. L. F., et al. (2013). Piperine decreases pilocarpine-induced convulsions by GABAergic mechanisms. Pharmacol. Biochem. Behav. 104, 144–153. doi:10.1016/j.pbb.2013.01.002
Dakic, V., Macier, R. D., Drummond, H., Nascimento, J. M., Trindade, P., and Rehen, S. K. (2016). Harmine stimulates proliferation of human neural progenitors. Peerj 4, 13. doi:10.7717/peerj.2727
Danduga, R., Dondapati, S. R., Kola, P. K., Grace, L., Tadigiri, R. V. B., and Kanakaraju, V. K. (2018). Neuroprotective activity of tetramethylpyrazine against 3-nitropropionic acid induced Huntington's disease-like symptoms in rats. Biomed. Pharmacother. 105, 1254–1268. doi:10.1016/j.biopha.2018.06.079
Deng, C. X., Wu, Z. B., Chen, Y., and Yu, Z. M. (2020). Pinellia total alkaloids modulate the GABAergic system in hippocampal formation on pilocarpine-induced epileptic rats. Chin. J. Integr. Med. 26 (2), 138–145. doi:10.1007/s11655-019-2944-7
de Souza, P., da Silva, R. C. V., da Silva, L. M., Steimbach, V. M. B., Moreno, K. G. T., and Gasparotto Junior, A. (2022). Boldine, an alkaloid from peumus boldus molina, induces endothelium-dependent vasodilation in the perfused rat kidney: involvement of nitric oxide and small-conductance Ca2+-activated K+ channel. Evid. Based Complement. Altern. Med. 2022, 4560607. doi:10.1155/2022/4560607
Dimpfel, W., Franklin, R., Gericke, N., and Schombert, L. (2018). Effect of Zembrin® and four of its alkaloid constituents on electric excitability of the rat hippocampus. J. Ethnopharmacol. 223, 135–141. doi:10.1016/j.jep.2018.05.010
dos Reis, S. L., Gelfuso, E. A., Fachin, A. L., Pereira, A. M. S., and Beleboni, R. O. (2021). Pharmacological characterisation of anticonvulsant effects elicited by erythrartine. J. Pharm. Pharmacol. 73 (1), 93–97. doi:10.1093/jpp/rgaa024
Duan, L., Li, X., Ji, R., Hao, Z., Kong, M., Wen, X., et al. (2023). Nanoparticle-based drug delivery systems: an inspiring therapeutic strategy for neurodegenerative diseases. Polym. (Basel) 15 (9), 2196. doi:10.3390/polym15092196
El-Nahas, A. E., Elbedaiwy, H. M., Masoud, I. M., Aly, R. G., Helmy, M. W., and El-Kamel, A. H. (2023). Berberine-loaded zein/hyaluronic acid composite nanoparticles for efficient brain uptake to alleviate neuro-degeneration in the pilocarpine model of epilepsy. Eur J Pharm Biopharm 188, 182–200. doi:10.1016/j.ejpb.2023.04.008
El-Sayed, S. S., El-Yamany, M. F., Salem, H. A., and El-Sahar, A. E. (2021). New insights into the effects of vinpocetine against neurobehavioral comorbidities in a rat model of temporal lobe epilepsy via the downregulation of the hippocampal PI3K/mTOR signalling pathway. J. Pharma Pharmacol. 73 (5), 626–640. doi:10.1093/jpp/rgab011
Eyal, S. (2018). The fever tree: from malaria to neurological diseases. Toxins 10 (12), 491. doi:10.3390/toxins10120491
Faggion, S. A., Cunha, A. O. S., Fachim, H. A., Gavin, A. S., dos Santos, W. F., Pereira, A. M. S., et al. (2011). Anticonvulsant profile of the alkaloids (+)-erythravine and (+)-11-α-hydroxy-erythravine isolated from the flowers of Erythrina mulungu Mart ex Benth (Leguminosae-Papilionaceae). Epilepsy Behav. 20 (3), 441–446. doi:10.1016/j.yebeh.2010.12.037
Farinon, M., Clarimundo, V. S., Pedrazza, G. P. R., Gulko, P. S., Zuanazzi, J. A. S., Xavier, R. M., et al. (2017). Disease modifying anti-rheumatic activity of the alkaloid montanine on experimental arthritis and fibroblast-like synoviocytes. Eur. J. Pharmacol. 799, 180–187. doi:10.1016/j.ejphar.2017.02.013
Feng, X. G., Wang, Y. Z., Hao, Y. L., Ma, Q., Dai, J., Liang, Z. B., et al. (2017). Vinpocetine inhibited the CpG oligodeoxynucleotide-induced immune response in plasmacytoid dendritic cells. Immunol. Invest. 46 (3), 263–273. doi:10.1080/08820139.2016.1248561
Frolov, N. A., and Vereshchagin, A. N. (2023). Piperidine derivatives: recent advances in synthesis and pharmacological applications. Int. J. Mol. Sci. 24 (3), 2937. doi:10.3390/ijms24032937
Gad, M. Z., Azab, S. S., Khattab, A. R., and Farag, M. A. (2021). Over a century since ephedrine discovery: an updated revisit to its pharmacological aspects, functionality and toxicity in comparison to its herbal extracts. Food Funct. 12 (20), 9563–9582. doi:10.1039/d1fo02093e
Gao, B., Wu, Y., Yang, Y. J., Li, W. Z., Dong, K., Zhou, J., et al. (2018). Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: involvement of inhibition of NLRP1 inflammasome. J. Neuroinflammation 15, 152. doi:10.1186/s12974-018-1199-0
Gawel, K., Kukula-Koch, W., Nieoczym, D., Stepnik, K., van der Ent, W., Banono, N. S., et al. (2020). The influence of palmatine isolated from Berberis sibirica radix on pentylenetetrazole-induced seizures in zebrafish. Cells 9 (5), 1233. doi:10.3390/cells9051233
Gelfuso, E. A., Reis, S. L., Pereira, A. M. S., Aguiar, D. S. R., and Beleboni, R. O. (2020). Neuroprotective effects and improvement of learning and memory elicited by erythravine and 11α-hydroxy-erythravine against the pilocarpine model of epilepsy. Life Sci. 240, 117072. doi:10.1016/j.lfs.2019.117072
Geng, H., Chen, X. Q., and Wang, C. Z. (2021). Systematic elucidation of the pharmacological mechanisms of Rhynchophylline for treating epilepsy via network pharmacology. BMC Complement. Med. Ther. 21 (1), 9. doi:10.1186/s12906-020-03178-x
Gjerulfsen, C. E., Mieszczanek, T. S., Johannesen, K. M., Liao, V. W. Y., Chebib, M., Nørby, H. A. J., et al. (2023). Vinpocetine improved neuropsychiatric and epileptic outcomes in a patient with a GABRA1 loss-of-function variant. Ann. Clin. Transl. Neurol. 10 (8), 1493–1498. doi:10.1002/acn3.51838
Gómez, C. D., Buijs, R. M., and Sitges, M. (2014). The anti-seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduce inflammatory IL-1β and TNF-α expression in rat hippocampus. J. Neurochem. 130 (6), 770–779. doi:10.1111/jnc.12784
He, L. Y., Hu, M. B., Li, R. L., Zhao, R., Fan, L. H., He, L., et al. (2021). Natural medicines for the treatment of epilepsy: bioactive components, pharmacology and mechanism. Front. Pharmacol. 12, 604040. doi:10.3389/fphar.2021.604040
Ho, T. Y., Tang, N. Y., Hsiang, C. Y., and Hsieh, C. L. (2014). Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1β and brain-derived neurotrophic factor. Phytomedicine 21 (6), 893–900. doi:10.1016/j.phymed.2014.01.011
Hong, H. X., Lu, X., Lu, Q., Huang, C., and Cui, Z. M. (2022). Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front. Pharmacol. 13, 1015035. doi:10.3389/fphar.2022.1015035
Hu, S. Q., Mak, S. H., Zuo, X. L., Li, H. T., Wang, Y. Q., and Han, Y. F. (2018). Neuroprotection against MPP+-Induced cytotoxicity through the activation of PI3-K/Akt/GSK3β/MEF2D signaling pathway by rhynchophylline, the major tetracyclic oxindole alkaloid isolated from Uncaria rhynchophylla. Front. Pharmacol. 9, 768. doi:10.3389/fphar.2018.00768
Huang, M. Y., Lin, J., Huang, Z. J., Xu, H. G., Hong, J., Sun, P. H., et al. (2016). Design, synthesis and anti-inflammatory effects of novel 9-O-substituted-berberine derivatives electronic supplementary information. Med. Chem. Comm. 7 (4), 730–731. doi:10.1039/c6md90010k
Huang, W. Q., Wang, Y. X., Tian, W. S., Cui, X. X., Tu, P. F., Li, J., et al. (2022). Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibio Basel 11 (10), 1380. doi:10.3390/antibiotics11101380
Hussain, G., Rasul, A., Anwar, H., Aziz, N., Razzaq, A., Wei, W., et al. (2018). Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 14 (3), 341–357. doi:10.7150/ijbs.23247
Jain, N. K., Tailang, M., Kumar, S., Chandrasekaran, B., Alghazwani, Y., Chandramoorthy, H. C., et al. (2022). Appraising the therapeutical potentials of Alchornea laxiflora (Benth.) Pax and K. Hoffm., an underexplored medicinal herb: a systematic review. Front. Pharmacol. 13, 958453. doi:10.3389/fphar.2022.958453
Jiang, W., Wei, W., Gaertig, M. A., Li, S., and Li, X. J. (2015). Therapeutic effect of berberine on Huntington's disease transgenic mouse model. PLoS One 10, e0134142. doi:10.1371/journal.pone.0134142
Jin, Y., Cai, S., Jiang, Y. P., Zhong, K., Wen, C. P., Ruan, Y. P., et al. (2019). Tetramethylpyrazine reduces epileptogenesis progression in electrical kindling models by modulating hippocampal excitatory neurotransmission. ACS Chem. Neurosci. 10 (12), 4854–4863. doi:10.1021/acschemneuro.9b00575
Kakooza-Mwesige, A. (2015). The importance of botanical treatments in traditional societies and challenges in developing countries. Epilepsy Behav. 52, 297–307. doi:10.1016/j.yebeh.2015.06.017
Kanner, A. M., and Bicchi, M. M. (2022). Antiseizure medications for adults with epilepsy A review. JAMA 327 (13), 1269–1281. doi:10.1001/jama.2022.3880
Lai, T. W., Zhang, S., and Wang, Y. T. (2014). Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188. doi:10.1016/j.pneurobio.2013.11.006
Li, D., Zhong, Z. F., Ko, C. N., Tian, T. T., and Yang, C. (2023). From mundane to classic: sinomenine as a multi-therapeutic agent. Br. J. Pharmacol. 22. doi:10.1111/bph.16267
Lin, J., Wang, Q., Zhou, S., Xu, S., and Yao, K. (2022a). Tetramethylpyrazine: a review on its mechanisms and functions. Biomed. Pharmacother. 150, 113005. doi:10.1016/j.biopha.2022.113005
Lin, S. X., Curtis, M. A., and Sperry, J. (2020). Pyridine alkaloids with activity in the central nervous system. Bioorg Med. Chem. 28 (24), 115820. doi:10.1016/j.bmc.2020.115820
Lin, T. Y., Hung, C. Y., Chiu, K. M., Lee, M. Y., Lu, C. W., and Wang, S. J. (2022b). Neferine, an alkaloid from Lotus seed embryos, exerts antiseizure and neuroprotective effects in a kainic acid-induced seizure model in rats. Int. J. Mol. Sci. 23 (8), 4130. doi:10.3390/ijms23084130
Liu, C. M., Shao, Z. C., Chen, X. Z., Chen, H. W., Su, M. Q., Zhang, Z. W., et al. (2023). Neferine attenuates development of testosterone-induced benign prostatic hyperplasia in mice by regulating androgen and TGF-β/Smad signaling pathways. Saudi Pharm. J. 31 (7), 1219–1228. doi:10.1016/j.jsps.2023.05.004
Liu, G., Wang, J., Deng, X. H., Ma, P. S., Li, F. M., Peng, X. D., et al. (2017). The anticonvulsant and neuroprotective effects of oxysophocarpine on pilocarpine-induced convulsions in adult male mice. Cell Mol. Neurobiol. 37 (2), 339–349. doi:10.1007/s10571-016-0411-y
Liu, L., Fan, J., Ai, G., Liu, J., Luo, N., Li, C., et al. (2019). Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 52 (1), 37. doi:10.1186/s40659-019-0243-6
Ma, C. M., Liu, Z. L., Xu, X. J., and Yao, Q. Z. (2014). Research progress on the synthesis of energetic pyridines. Chin. J. Org. Chem. 34 (7), 1288–1299. doi:10.6023/cjoc201402030
Mante, P. K., Adomako, N. O., Antwi, P., Kusi-Boadum, N. K., and Osafo, N. (2021). Solid-lipid nanoparticle formulation improves antiseizure action of cryptolepine. Biomed. Pharmacother. 137, 111354. doi:10.1016/j.biopha.2021.111354
Marucci, G., Buccioni, M., Dal Ben, D., Lambertucci, C., Volpini, R., and Amenta, F. (2021). Efficacy of acetylcholinesterase inhibitors in Alzheimer's disease. Neuropharmacol 190, 108352. doi:10.1016/j.neuropharm.2020.108352
Mathie, A. (2010). Ion channels as novel therapeutic targets in the treatment of pain. J. Pharm. Pharmacol. 62 (9), 1089–1095. doi:10.1111/j.2042-7158.2010.01131x
Mathien, S., Tesnière, C., Meloche, S., and Gottesman, M. (2021). Regulation of mitogen-activated protein kinase signaling pathways by the ubiquitin-proteasome system and its pharmacological potential. Pharmacol. Rev. 73 (4), 263–296. doi:10.1124/pharmrev.120.000170
Meador, K. J., Leeman-Markowski, B., Medina, A. E., Illamola, S. M., Seliger, J., Novak, G., et al. (2021). Vinpocetine, cognition, and epilepsy. Epilepsy Behav. 119, 107988. doi:10.1016/j.yebeh.2021.107988
Meng, Z., Chen, H., and Meng, S. (2021). The roles of tetramethylpyrazine during neurodegenerative disease. Neurotox. Res. 39 (5), 1665–1677. doi:10.1007/s12640-021-00398-y
Mishra, A., Punia, J. K., Bladen, C., Zamponi, G. W., and Goel, R. K. (2015). Anticonvulsant mechanisms of piperine, a piperidine alkaloid. Channels 9 (5), 317–323. doi:10.1080/19336950.2015.1092836
Moezi, L., Yahosseini, S., Jamshizadeh, A., and Pirsalami, F. (2019). Acute boldine treatment induces anti-convulsant effects in mice through its antioxidant activity. Drug Res. (Stuttg). 69 (4), 227–233. doi:10.1055/a-0659-2478
Munir, S., Shahid, A., Aslam, B., Ashfaq, U. A., Akash, M. S. H., Ali, M. A., et al. (2020). The therapeutic prospects of naturally occurring and synthetic indole alkaloids for depression and anxiety disorders. Evid. Based Complement. Altern. Med. 2020, 8836983. doi:10.1155/2020/8836983
Nakamura, Y., Ishida, Y., Kondo, M., and Shimada, S. (2018). Yokukansan contains compounds that antagonize the 5-HT3 receptor. Phytomedicine 43, 120–125. doi:10.1016/j.phymed.2018.04.034
Ndagijimana, A., Wang, X. M., Pan, G. X., Zhang, F., Feng, H., and Olaleye, O. (2013). A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 86, 35–47. doi:10.1016/j.fitote.2013.01.018
Neumann, J., Azatsian, K., Höhm, C., Hofmann, B., and Gergs, U. (2023). Cardiac effects of ephedrine, norephedrine, mescaline, and 3,4-methylenedioxymethamphetamine (MDMA) in mouse and human atrial preparations. Naunyn Schmiedeb. Arch. Pharmacol. 396 (2), 275–287. doi:10.1007/s00210-022-02315-2
Nuthakki, V. K., Mudududdla, R., Sharma, A., Kumar, A., and Bharate, S. B. (2019). Synthesis and biological evaluation of indoloquinoline alkaloid cryptolepine and its bromo-derivative as dual cholinesterase inhibitors. Bioorg Chem. 90, 103062. doi:10.1016/j.bioorg.2019.103062
Oh, Y.-C., Choi, J.-g., Lee, Y.-S., Brice, O.-O., Lee, S.-C., Kwak, H.-S., et al. (2010). Tetrahydropalmatine inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated THP-1 cells. J. Med. Food 13 (5), 1125–1132. doi:10.1089/jmf.2009.1388
Ono, T., Hayashida, M., Tezuka, A., Hayakawa, H., and Ohno, Y. (2013). Antagonistic effects of tetrodotoxin on aconitine-induced cardiac toxicity. J. Nippon. Med. Sch. 80 (5), 350–361. doi:10.1272/jnms.80.350
Pandey, K. P. (2023). Part I enantiospecific total synthesis of unnatural enantiomers of C-19 methylated sarpagine/macroline/ajmaline-type biologically active indole alkaloids via the pictet-spengler reaction/dieckmann cyclization process. Part II design, synthesis, and characterization of novel gamma-aminobutyric acid type a (gabaa) receptor ligands for the treatment of neurological disorders including epilepsy, depr.
Perversi, F., Costa, C., Labate, A., Lattanzi, S., Liguori, C., Maschio, M., et al. (2023). The broad-spectrum activity of perampanel: state of the art and future perspective of AMPA antagonism beyond epilepsy. Front. Neurol. 14, 1182304. doi:10.3389/fneur.2023.1182304
Plazas, E., Avila, M. C., Muñoz, D. R., and Cuca, L. E. (2022). Natural isoquinoline alkaloids: pharmacological features and multi-target potential for complex diseases. Pharmacol. Res. 177, 23. doi:10.1016/j.phrs.2022.106126
Qian, X., Deng, Y., Zhong, N., Li, Y., Wang, J., Yan, J., et al. (2023). Study on the reversal of epileptic drug resistance by tetramethylpyrazine in combination with carbamazepine through modulation of P-Glycoprotein expression in rat brain microvessel endothelial cell. Cell Mol. Biol. (Noisy-le-grand) 69 (11), 254–259. doi:10.14715/cmb/2023.69.11.38
Qin, J., Zhang, S. Y., Zhang, Y. B., Chen, L. F., Chen, N. H., Wu, Z. N., et al. (2021). Two new isoquinoline alkaloids from the seeds of Nandina domestica. Nat. Prod. Res. 35 (19), 3254–3260. doi:10.1080/14786419.2019.1696334
Quijia, C. R., Araujo, V. H., and Chorilli, M. (2021). Piperine: chemical, biological and nanotechnological applications. Acta Pharm. 71 (2), 185–213. doi:10.2478/acph-2021-0015
Ramazi, S., Fahanik-Babaei, J., Mohamadi-Zarch, S. M., Tashakori-Miyanroudi, M., Nourabadi, D., Nazari-Serenjeh, M., et al. (2020). Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 108, 101800. doi:10.1016/j.jchemneu.2020.101800
Ren, T. (2019). Biopharmaceutics and pharmacokinetics characterization of piperine and its pharmacokinetics and pharmacodynamics interactions with carbamazepine in anti-epileptic treatment.
Ren, T. J., Hu, M. Y., Cheng, Y., Shek, T. L., Xiao, M., Ho, N. J., et al. (2019). Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control. Eur. J. Pharm. Sci. 137, 104988. doi:10.1016/j.ejps.2019.104988
Richardson, R. J., Petrou, S., and Bryson, A. (2024). Established and emerging GABAA receptor pharmacotherapy for epilepsy. Front. Pharmacol. 15, 1341472. doi:10.3389/fphar.2024.1341472
Saha, L., Kumari, P., Rawat, K., Gautam, V., Sandhu, A., Singh, N., et al. (2023). Neuroprotective effect of berberine nanoparticles against seizures in pentylenetetrazole induced kindling model of epileptogenesis: role of anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms. Neurochem. Res. 48 (10), 3055–3072. doi:10.1007/s11064-023-03967-z
Sedaghat, R., Taab, Y., Kiasalari, Z., Afshin-Majd, S., Baluchnejadmojarad, T., and Roghani, M. (2017). Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: underlying mechanisms. Biomed. Pharmacother. 87, 200–208. doi:10.1016/j.biopha.2016.12.109
Shang, X. F., Morris-Natschke, S. L., Liu, Y. Q., Li, X. H., Zhang, J. Y., and Lee, K. H. (2022). Biology of quinoline and quinazoline alkaloids. Alkaloids Chem. Biol. 88, 1–47. doi:10.1016/bs.alkal.2021.08.002
Sharifi-Rad, J., Quispe, C., Herrera-Bravo, J., Martorell, M., Sharopov, F., Tumer, T. B., et al. (2021). A pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem. Res. 46 (9), 2205–2225. doi:10.1007/s11064-021-03376-0
Shilo, M., Sharon, A., Baranes, K., Motiei, M., Lellouche, J. P. M., and Popovtzer, R. (2015). The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model. J. Nanobiotechnol 13, 19. doi:10.1186/s12951-015-0075-7
Song, D. Y., Hao, J. Y., and Fan, D. M. (2020). Biological properties and clinical applications of berberine. Front. Med. 14 (5), 564–582. doi:10.1007/s11684-019-0724-6
Steinlein, O. (2014). Calcium signaling and epilepsy. Cell Tissue Res. 357 (2), 385–393. doi:10.1007/s00441-014-1849-1
Suzuki, Y., Itoh, H., Abe, T., Nishimura, F., Sato, Y., and Takeyama, M. (2011). No effect of co-administered antiepileptic drugs on in-vivo protein binding parameters of valproic acid in patients with epilepsy. J. Pharm. Pharmacol. 63 (7), 976–981. doi:10.1111/j.2042-7158.2011.01282x
Teng, S. F., Li, F. R., Cui, Q. M., Khan, A., He, T., Luo, X. D., et al. (2023). A review on the genus Melodinus: traditional uses, phytochemical diversity and pharmacological activities of indole alkaloids. Phytochem. Rev. 54. doi:10.1007/s11101-023-09871-2
Thongsornkleeb, C., Tummatorn, J., and Ruchirawat, S. (2022). A compilation of synthetic strategies to access the most utilized indoloquinoline motifs. Chem-Asian J. 17 (7), e202200040. doi:10.1002/asia.202200040
van Rooy, I., Cakir-Tascioglu, S., Hennink, W. E., Storm, G., Schiffelers, R. M., and Mastrobattista, E. (2011). In vivo methods to study uptake of nanoparticles into the brain. Pharm. Res. 28 (3), 456–471. doi:10.1007/s11095-010-0291-7
Wu, H. W., Wang, P., Liu, M. T., Tang, L. Y., Fang, J., Zhao, Y., et al. (2015). A 1H-NMR-Based metabonomic study on the anti-depressive effect of the total alkaloid of Corydalis rhizoma. Molecules 20 (6), 10047–10064. doi:10.3390/molecules200610047
Wu, J., Cao, M. Y. J., Peng, Y., Dong, B. H., Jiang, Y. X., Hu, C. J., et al. (2023). Research progress on the treatment of epilepsy with traditional Chinese medicine. Phytomedicine 120, 155022. doi:10.1016/j.phymed.2023.155022
Xie, Z. Q., Tian, X. T., Zheng, Y. M., Zhan, L., Chen, X. Q., Xin, X. M., et al. (2020). Antiepileptic geissoschizine methyl ether is an inhibitor of multiple neuronal channels. Acta Pharmacol. Sin. 41 (5), 629–637. doi:10.1038/s41401-019-0327-4
Xu, T. T., Li, Y. X., Wang, H. Y., Xu, Y. Q., Ma, L., Sun, T., et al. (2013). Oxysophocarpine induces anti-nociception and increases the expression of GABAA α1 receptors in mice. Mol. Med. Rep. 7 (6), 1819–1825. doi:10.3892/mmr.2013.1414
Yoshitomi, S., Takahashi, Y., Yamaguchi, T., Oboshi, T., Horino, A., Ikeda, H., et al. (2019). Quinidine therapy and therapeutic drug monitoring in four patients with KCNT1 mutations. Epileptic Disord. 21 (1), 48–54. doi:10.1684/epd.2019.1026
Zhang, B. M., Wang, Z. B., Xin, P., Wang, Q. H., Bu, H., and Kuang, H. X. (2018a). Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 16 (11), 811–828. doi:10.1016/s1875-5364(18)30123-7
Zhang, B. Y., Wang, L. Z., Ji, X. N., Zhang, S. S., Sik, A., Liu, K. C., et al. (2020). Anti-Inflammation associated protective mechanism of berberine and its derivatives on attenuating pentylenetetrazole-induced seizures in zebrafish. J. Neuroimmune Pharmacol. 15 (2), 309–325. doi:10.1007/s11481-019-09902-w
Zhang, Z. X., Liu, T., Yu, M., Li, K. D., and Li, W. H. (2018b). The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J. Exp. Clin. Cancer Res. 37, 7. doi:10.1186/s13046-018-0678-6
Zhao, Z. F., He, X. R., Ma, C. X., Wu, S. P., Cuan, Y., Sun, Y., et al. (2018). Excavating anticonvulsant compounds from prescriptions of traditional Chinese medicine in the treatment of epilepsy. Am. J. Chin. Med. 46 (4), 707–737. doi:10.1142/s0192415x18500374
Zhou, J.-Y., Mo, Z.-X., and Zhou, S.-W. (2010). Rhynchophylline down-regulates NR2B expression in cortex and hippocampal CA1 area of amphetamine-induced conditioned place preference rat. Arch. Pharm. Res. 33 (4), 557–565. doi:10.1007/s12272-010-0410-3
Zhu, D., Zhang, W. G., Nie, X. D., Ding, S. W., Zhang, D. T., and Yang, L. (2020). Rational design of ultra-small photoluminescent copper nano-dots loaded PLGA micro-vessels for targeted co-delivery of natural piperine molecules for the treatment for epilepsy. J. Photoch Photobio B 205, 111805. doi:10.1016/j.jphotobiol.2020.111805
Keywords: epilepsy, natural alkaloid, antiseizure medications, neuropharmacological mechanism, nanotechnology, gaba receptor
Citation: Li S, Lin X and Duan L (2024) Harnessing the power of natural alkaloids: the emergent role in epilepsy therapy. Front. Pharmacol. 15:1418555. doi: 10.3389/fphar.2024.1418555
Received: 16 April 2024; Accepted: 31 May 2024;
Published: 19 June 2024.
Edited by:
Petra Scholze, Medical University of Vienna, AustriaReviewed by:
Arindam Ghosh Mazumder, Baylor College of Medicine, United StatesCopyright © 2024 Li, Lin and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Duan, ZHVhbmxpanVhbl8xOTg0QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.