- Tianjin Anding Hospital, Tianjin, China
Esketamine nasal spray (ESK-NS) is a new drug for treatment-resistant depression, and we aimed to detect and characterize the adverse events (AEs) of ESK-NS using the Food and Drug Administration (FDA) adverse event reporting system (FAERS) database between 2019 Q1 and 2023 Q4. Reporting odds ratio (ROR), proportional reporting ratio (PRR), and multi-item gamma Poisson shrinker (MGPS) were performed to detect risk signals from the FAERS data to identify potential ESK-NS–AEs associations. A total of 14,606 reports on AEs with ESK-NS as the primary suspected drug were analyzed. A total of 518 preferred terms signals and 25 system organ classes mainly concentrated in psychiatric disorders (33.20%), nervous system disorders (16.67%), general disorders and administration site conditions (14.21%), and others were obtained. Notably, dissociation (n = 1,093, ROR 2,257.80, PRR 899.64, EBGM 876.86) exhibited highest occurrence rates and signal intensity. Moreover, uncommon but significantly strong AEs signals, such as hand–eye coordination impaired, feeling guilty, and feelings of worthlessness, were observed. Additionally, dissociative disorder (n = 57, ROR 510.92, PRR 506.70, EBGM 386.60) and sedation (n = 688, ROR 172.68, PRR 155.53, and EBGM 142.05) both presented strong AE signals, and the former is not recorded in the Summary of Product Characteristics (SmPC). In clinical applications, close attention should be paid to the psychiatric disorders and nervous system disorders, especially dissociation. Meanwhile, clinical professionals should be alert for the occurrence of AEs signals not mentioned in the SmPC and take preventive measures to ensure the safety of clinical use.
Introduction
According to the World Health Organization (WHO), approximately 280 million people in the world suffer from depressive disorders (WHO, 2017). The conventional antidepressant pharmacotherapies are focused on monoamine-based targets as a result of the early accidental discovery that drugs that inhibit the reuptake or metabolism of monoaminergic neurotransmitters have antidepressant effects (Hirschfeld, 2000). However, it typically takes several weeks or months for the full benefit of medication to manifest, and some individuals are often resistant to treatment (Kim et al., 2023). Treatment-resistant depression (TRD) is a challenge for psychiatrists, and it is generally defined as the failure to respond to two or more classical antidepressant drugs (Cipriani et al., 2018). These patients are faced with ongoing symptoms despite receiving multiple treatments, which can significantly impact their quality of life (Bergfeld et al., 2018). Therefore, non-monoaminergic-based drugs that display fast and long-lasting antidepressant effects, such as ketamine and esketamine, were found.
Esketamine nasal spray (ESK-NS) (Spravato, Janssen Pharmaceuticals, Raritan, NJ) is a novel agent with glutamatergic neuromodulatory properties for TRD that has been approved by the FDA and the European Medicine Agency (EMA) in 2019 and 2020, respectively. In May 2023, the China National Medical Products Administration announced ESK-NS approval. In China, depressive disorders are estimated to be the second leading cause of years lived with disability (∼54 million) (Lu et al., 2021; WHO, 2024). Therefore, it is essential to monitor the real-world usage and AEs of ESK-NS to ensure its safety and efficacy in China.
Esketamine, as the S-enantiomer of racemic ketamine, is a non-selective, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist (Kim et al., 2019). Through NMDA receptor antagonism, ESK-NS produces a transient increase in glutamate release, leading to the increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) stimulation and, subsequently, the increase in neurotrophic signaling, which may contribute to the restoration of the synaptic function in the brain regions involved with the regulation of mood and emotional behavior (Spravato, 2019). ESK-NS has rapid (within several hours) antidepressant effects compared with traditional antidepressants, but the exact mechanism of action remains unclear (CarlaCanuso et al., 2018; NIH, 2019).
Although the overall tolerability of ESK-NS in short-term and long-term clinical trials is well, ESK-NS marketing authorization triggered many concerns, including the lack of convincing evidence about its safety and the risk of suicide and abuse (Cristea and Naudet, 2019; Fedgchin et al., 2019; Vanina Popova et al., 2019; Fu et al., 2020; Ionescu et al., 2020; Ochs-Ross et al., 2020; Wajs et al., 2020; Castro et al., 2023). In the phase III studies completed, the most common AEs included dizziness, dissociation, nausea, and headache (Fu et al., 2020; Wajs et al., 2020). ESK-NS labeling contains boxed warnings for the risk of sedation, dissociation, and respiratory depression, and patients must be monitored for at least 2 h at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting (Titusville, 2024). Therefore, it is essential to monitor the real-world usage and AEs of ESK-NS to ensure its safety. Our study systematically provided the safety profile of ESK-NS, which confirmed some existing safety concerns and revealed the potential risks.
The FAERS is a database designed to support the FDA’s post-marketing safety surveillance program for drugs and therapeutic biologic products (Sakaeda et al., 2013). However, the FDA thus far had refrained from making the FAERS data publicly available. In a landmark move, the FAERS Public Dashboard, a highly interactive web-based tool, was created to give the public the ability to query the FAERS database and improve transparency. Anyone can browse through the AEs reported for various medicinal products from 1968 until the latest quarter (31 December 2023). A previous systematic pharmacovigilance study of ESK-NS-associated AEs was published in August 2020, which included all the reports recorded in the FAERS over the first year of marketing approval of ESK-NS (Gastaldon et al., 2020). Another study focused specifically on the ESK-NS-related neurological AEs from 2019 to 2021 was published in April 2022 (Guo et al., 2022). It would be instructive for clinicians and pharmacovigilance experts to be informed about the development of this field since then. This study aims to analyze the post-marketing safety data on ESK-NS through the FAERS, provide clinicians with more comprehensive safety data, and provide recommendations for clinical use.
Methods
Data sources and processing methods
FAERS data are released quarterly. This study retrieved and analyzed all the reported AEs of ESK-NS in the FAERS Public Dashboard from the first quarter of 2019 to the fourth quarter of 2023 (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard). Records with low informative quality, such as those earlier than 5 March 2019, were deleted. Duplicates sporadically included in FAERS were identified and removed, as described in a previous study (Guo et al., 2022). All the AEs were coded as preferred terms (PTs) according to the Medical Dictionary for Regulatory Activities version 26.1 (MedDRA 26.1). System organ classes (SOCs) corresponding to these PTs were also listed. In sum, we retrieved and described the detailed information, including patient characteristics (sex, age, and weight), general information (reporter country, received year, and reporter type), drugs information (suspect product active ingredients, suspect product names, and reason for use), reactions (PTs, SOCs, and event date), severity (serious and non-serious), outcome, and concomitant drugs.
Statistical analysis
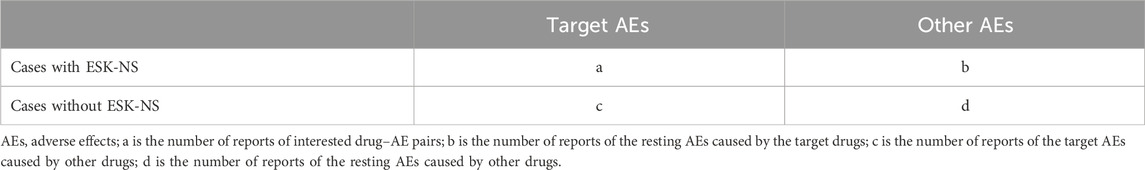
In this study, reporting odds ratio (ROR), proportional reporting ratio (PRR), and multi-item gamma Poisson shrinker (MGPS) techniques from the disproportionality methods were applied to detect AE signals (Rothman et al., 2004). ROR is one of the classic signal detection methods which can remove biases to estimate risk properly (Rothman et al., 2004). PRR has higher specificity compared to ROR. MGPS detects signals from rare events. All algorithms are based on the principles of calculations using the 2 × 2 table (Table 1). Table 2 shows the formulas and threshold values. The analyses were conducted using Microsoft EXCEL 2019.
Results
Description of the AEs caused by ESK-NS
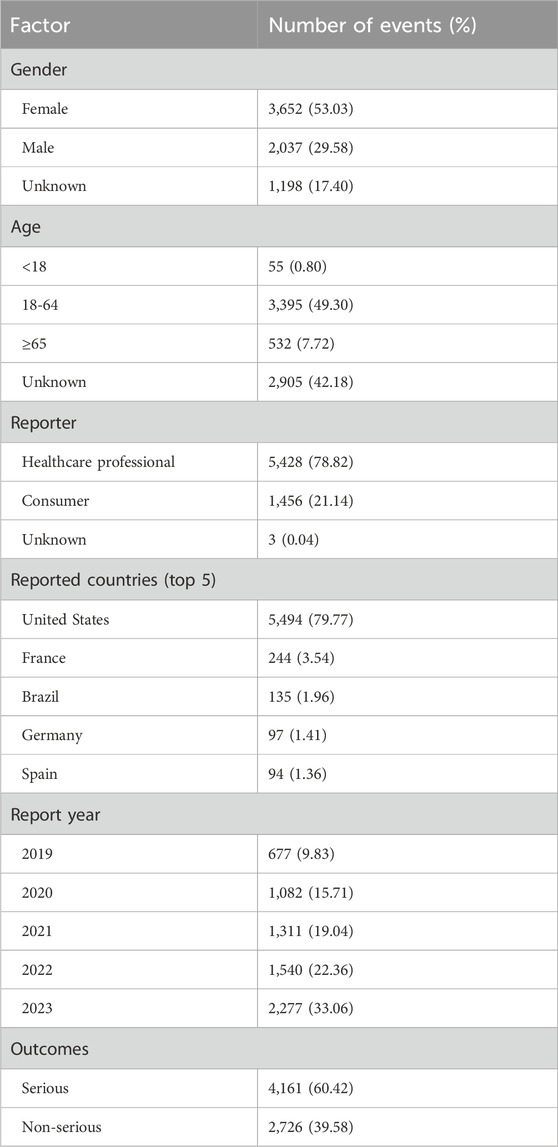
A total of 10,671,508 reports of AEs were submitted to the FAERS database since 2019, which contained 14,606 ESK-NS-related AEs in 6,887 patients. In AEs caused by ESK-NS, there were 3,652 female (53.03%) and 2,037 male individuals (29.58%). A part of the reports (17.40%) did not include gender information, which limited our understanding of the involvement of gender in the AEs. Regarding age, patients aged from 18 to 64 were the dominant population affected. Most reports (78.82%) were submitted by healthcare professionals rather than consumers. The majority of the reports were from US (79.77%). Since the approval of ESK-NS from 2019, there has been an increasing trend in AEs, which peaked in 2023. In terms of clinical outcomes, serious AEs, including death, life-threatening, hospitalization, disability, congenital malformations, and other serious events, accounted for 60.42%. The details are listed in Table 3.
Mining ESK-NS signal
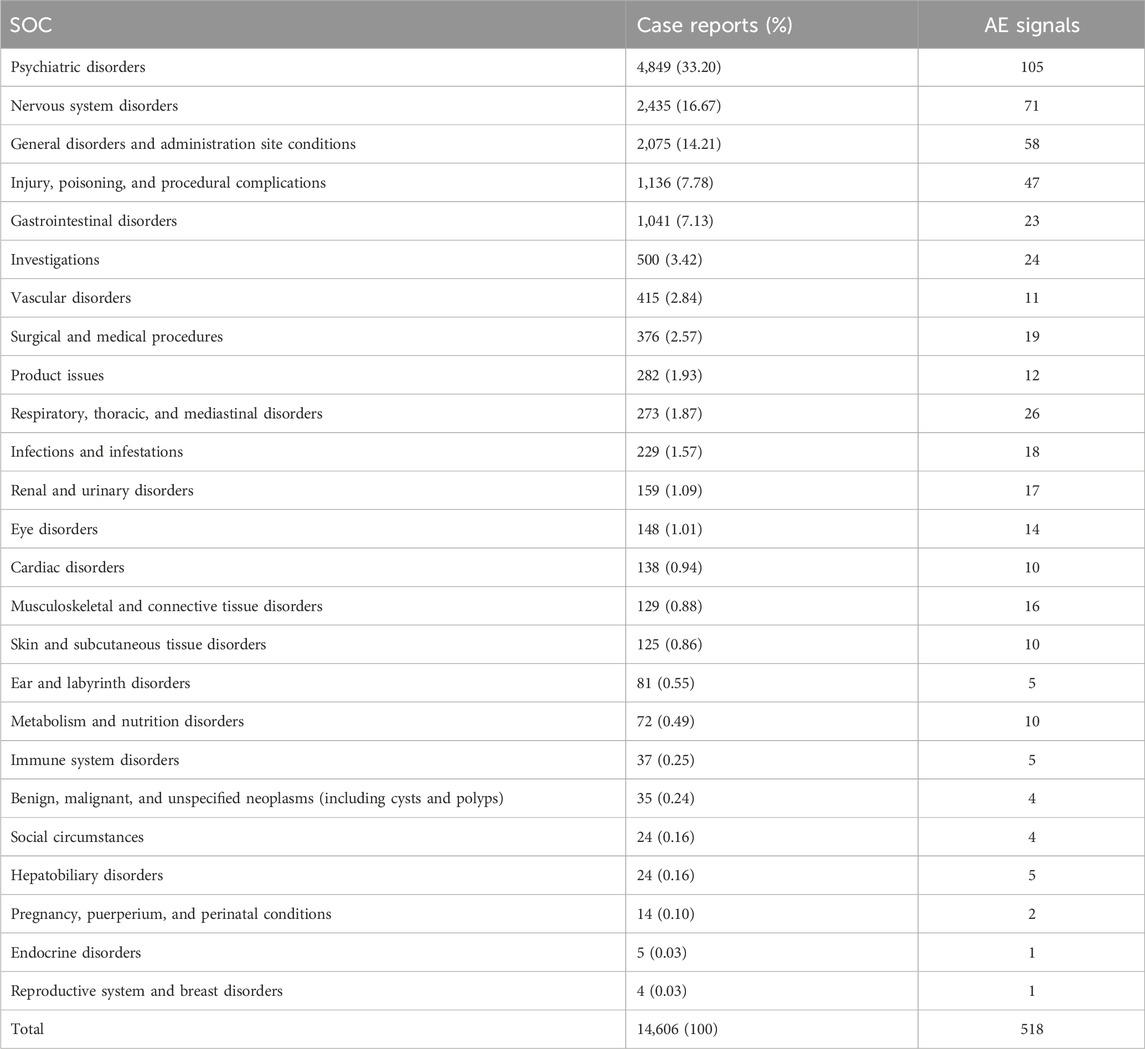
By analyzing AEs caused by ESK-NS, we found 25 SOCs and 518 AEs signals. The three frequent systems affected were psychiatric disorders (n = 4,849, 33.20%), nervous system disorders (n = 2,435, 16.67%), and general disorders and administration site conditions (n = 2,075, 14.21%). Among them, psychiatric disorders manifested the most signals (105 AEs signals). See details in Table 4. Additionally, injury, poisoning, and procedural complications (n = 1,136, 7.78%) and respiratory, thoracic, and mediastinal disorders (n = 273, 1.87%) were unique AEs to ESK-NS. The details can be found in Table 4.
This study analyzed AEs using three algorithms and evaluated their compliance with various screening criteria, which produced 161 PTs. The top 50 PTs were obtained using the EBGM algorithm, as shown in Table 5. Psychiatric disorder events (70%), general disorders and administration site condition events (10%), and nervous system disorder events (8%) were the major AEs among the top 50 PTs. At the PT level, the five most frequent AEs included dissociation, suicidal ideation, sedation, drug ineffectiveness, and nausea. The results revealed PTs with high signal intensity, including dissociation (n = 1,093, ROR 2,257.80, PRR 1,899.64, and EBGM 876.86), dissociative disorder (n = 57, ROR 510.92, PRR 506.70, and EBGM 386.60), sedation (n = 688, ROR 172.68, PRR 155.53, and EBGM 142.05), flashback (n = 9, ROR 127.48, PRR 127.31, and EBGM 118.14), and morbid thoughts (n = 17, ROR 107.45, PRR 107.19, and EBGM 100.63). Notably, dissociation had the highest frequency and signal strength. Aside from the side effects mentioned in the SmPC, we also found impaired hand–eye coordination, feelings of worthlessness, agoraphobia, feeling of guilt, inappropriate affect, and therapeutic response increased in top 50 PTs. Notably, as signal intensity is contributed by the prevalence of the events in the database and the events associated with AEs of ESK-NS, the results with a low n-value but high signal intensity should be substantiated by more data. The list of abbreviation of terms used in this study is showed in Table 6.
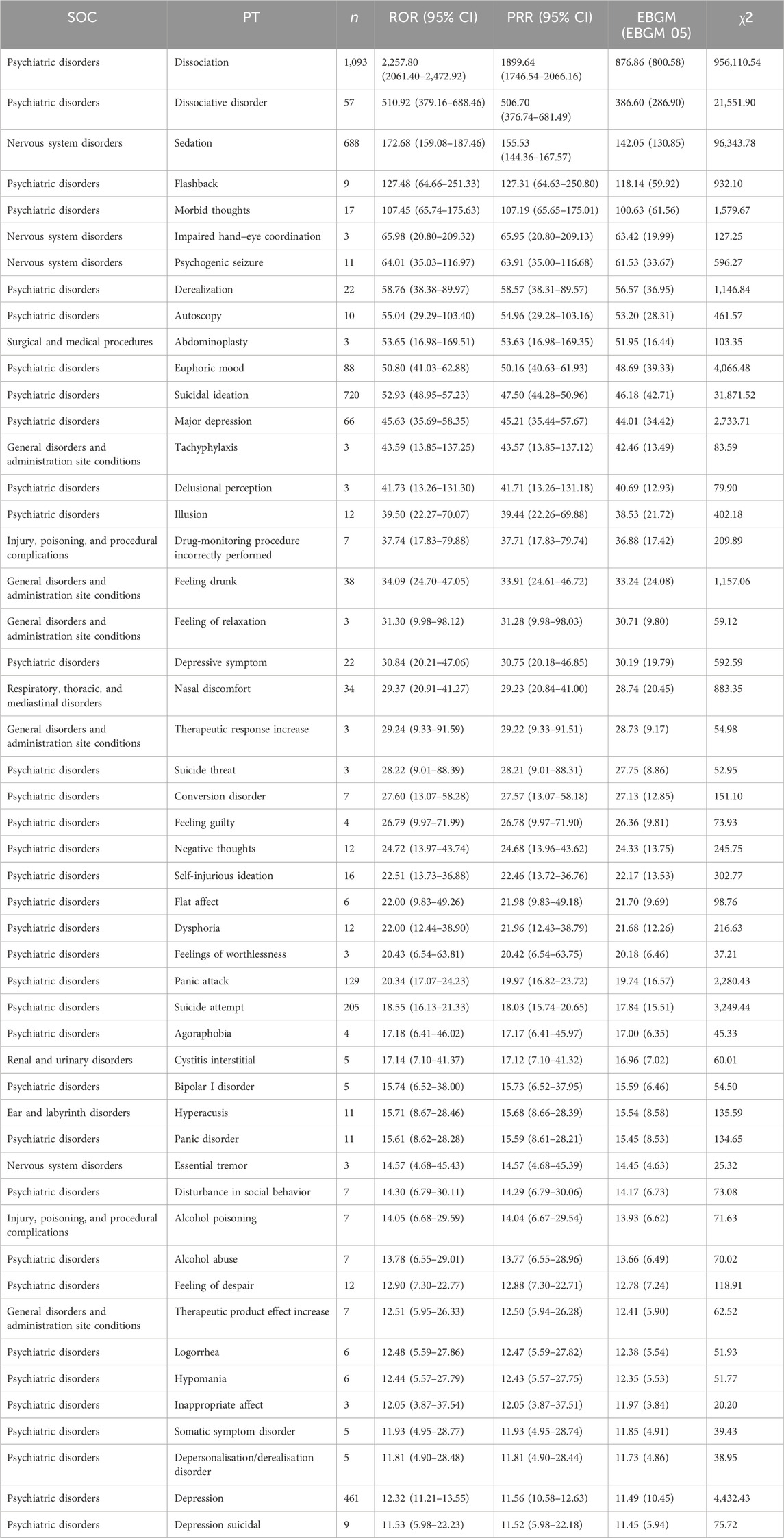
Table 5. Top 50 signal intensity of AEs caused by ESK-NS ranked by EBGM at the PT level in the FAERS database.
Discussion
By assessing the safety profile of ESK-NS using the FAERS database from 2019 to 2023, we raised different safety concerns and suggested that ESK-NS carries a clear potential for serious and unexpected AEs. Thus, our study provides suggestions for the clinical usage of ESK-NS, which is clearly conferred with clinical significance.
Our and other studies showed that the risk of AEs is higher in female patients after treatment with esketamine and ketamine, the esketamine derivative, resulting in significantly more discontinuation symptoms, such as anxiety, dysphoria, and tremors in female patients (Ferner and Aronson, 2019). Female users of ketamine manifested greater severity in cognitive impairment and urinary discomforts compared with male users (Chen et al., 2014). Of note, although AEs of ESK-NS in female patients occurred more frequently than in male patients, the conclusion might be different, given that 17.4% cases lack gender information. This is the limitation to our study. More importantly, most AEs (78.82%) were reported by the healthcare providers rather than consumers. This was mainly because patients should be monitored for at least 2 h after ESK-NS administration. Of note, the annually increased ESK-NS-related AEs since 2019 and the doubled ESK-NS-related AEs in 2023 compared to that of 2020 strongly suggest its widespread clinical use and efficacy and the urgent need for epidemiological surveillance. Notably, serious outcomes, such as death, life-threatening, hospitalization, disability, and congenital malformations, account for the majority of ESK-NS-related outcomes (60.42%) and emphasize the clinical importance of AEs monitoring after ESK-NS administration.
At the SOC level, the most commonly reported AEs of ESK-NS include psychiatric disorders, nervous system disorders, and general disorders and administration site conditions. In contrast, significant disproportionality of AEs in the reproductive system and breast disorders, endocrine disorders, hepatobiliary disorders, social circumstances, and benign, malignant, and unspecified neoplasms (including cysts and polyps) are less common. However, these SOCs were not mentioned in the SmPC. This urgently needs to be notified when it is applied clinically. In addition, as a nasal spray, ESK-NS might result in unique disorders including respiratory, thoracic, and mediastinal disorders, eye disorders, ear disorders, and labyrinth disorders.
At the PT level, the riskiest symptoms of ESK-NS include dissociation, dissociative disorder, and sedation. Notably, patients are at risk for dissociation, perceptual changes, and sedation after the administration of ESK-NS, which is labeled in the SmPC. The most common AEs of ESK-NS was dissociation, described as the distortion of time and space, illusions, derealization, and depersonalization, which could be assessed by the Median Clinician-Administered Dissociative States Scale (CADSS). However, in clinical trials, dissociation was transient and resolved spontaneously without the need for concomitant (Jeon et al., 2022; Zaki et al., 2023). ESK-NS may cause sedation or loss of consciousness and diminished or less apparent breathing (Titusville, 2024). Although dissociation and sedation could be alleviated over time with repeated treatment, patients should be monitored for 2 h at least to determine whether the healthcare setting could be terminated (Titusville, 2024). In addition, since this drug is administered as a nasal spray, AEs such as the incorrectly performed drug monitoring procedure and nasal discomfort must be considered.
Suicidal ideation and behaviors were serious AEs reported at higher incidence in ESK-NS-treated individuals. In the SUSTAIN-2 study, 42/802 (5.2%) patients reported suicidality-related treatment-emergent adverse events (TEAEs), three patients reported suicidal behavior, and one suicided (Jeon et al., 2022). In the SUSTAIN-3 study, one participant died by suicide 4 days after the most recent ESK-NS dose, and 49 participants (of 1,144, 4.3%) reported new occurrences of suicidal ideation (Zaki et al., 2023). However, esketamine/antidepressant groups reported more suicidal ideation than placebo/antidepressant groups in TRANSFORM-1 (Fedgchin et al., 2019). In the Janssen licensing trials, there were six deaths, which included three suicides (Administration FAD, 2024). Among the patients committing suicide, two showed no signs of suicidal ideas during the study. It suggests severe withdrawal reaction, which is consistent with recreational ketamine-related suicide reports and the potential risk of suicide risk (Cheng et al., 2005; Schifano et al., 2008; AlanSchatzberg, 2019). Therefore, monitoring patients with antidepressant treatment in case of clinical worsening and suicidal thoughts and behaviors during the initial few months of medication and dosage changes might be necessary.
ESK-NS may impair the ability of patients to drive or operate machinery. Thus, patients should not engage in activities requiring complete mental alertness and motor coordination such as driving and operating machinery until the next day of the administration (Administration FAD, 2024). The impairment of hand–eye coordination and its dissociative effects increase the risk of accidents and death (Cheng et al., 2005). It is, therefore, crucial to advise patients that they will need someone to drive them home after treatment with ESK-NS.
Other rare complications also occur after ESK-NS treatment, which deserve special attention, including interstitial cystitis-like symptoms in the bladder and lower urinary tract symptoms (Kasıkara et al., 2021). More importantly, some AEs of ESK-NS are not indicated in the SmPC, such as autoscopy, depressive symptom, self-injurious ideation, flat affect, agoraphobia, bipolar I disorder, hypomania, and logorrhea. Thus, the quantitative signal detection techniques in monitoring AEs are capable of providing the clinician with potential risk information.
The opioid properties of esketamine may explain the abuse and extensive requirement of monitoring to prevent drug abuse (AlanSchatzberg, 2019). AEs including euphoric mood, dissociation and related symptoms (i.e., autoscopy, derealization, and dissociative disorder), drunk-feeling, and hallucinations could be indicative, although abuse was not formally reported in FAERS (Lankenau et al., 2008; Vickers-Smith et al., 2020). In addition, preclinical studies have revealed the abuse of esketamine in rodents (Yang et al., 2015; Yang et al., 2016). In humans, administration of intranasal esketamine results in significant dissociation and sedation, which may contribute to abuse (Salahudeen et al., 2020). Therefore, ESK-NS labeling contains boxed warnings of potential for abuse, which is especially crucial for individuals with a history of drug abuse (Titusville, 2024).
Although we provide reliable evidence for the safety assessment of ESK-NS from multiple aspects, some limitations exist. For instance, consumer reports may not be reliable and comprehensive, which could result in incomplete and inaccurate information. Due to the key information on dosage being unavailable, the relationship between the dosage and AEs is unknown. Additionally, FAERS data comprise only one part of the FDA’s important post-market surveillance data. Therefore, research studies combining clinical trials and epidemiological studies with more cases should be conducted to more precisely evaluate the safety risks of ESK-NS and, thus, obtain more comprehensive and accurate perspectives.
Conclusion
Collectively, by analyzing AEs of ESK-NS from the FAERS database, we demonstrated the potential risk signal of AEs associated with ESK-NS. Attention should be paid to the AEs with strong real-world signals, including dissociation, suicidal ideation, and sedation. In addition, the potential abuse and misuse of ESK-NS should be considered. Our study provides guidance for the clinical utilization of ESK-NS in the treatment of MDD, which improves its safety and therapeutic efficacy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YW: writing–original draft and writing–review and editing. RL: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing–original draft, and writing–review and editing. CL: investigation, methodology, and writing–review and editing. DF: investigation, methodology, and writing–review and editing. TG: investigation, methodology, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was supported by the Young and Middle-aged Scientific Research Program of Pharmacy in Tianjin (TJYX2023-10 to RL).
Acknowledgments
This project was supported by the Young and Middle-aged Scientific Research Program of Pharmacy in Tianjin (TJYX2023-10 to RL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Administration FAD (2024) Efficacy, safety, and risk-benefit profile of new drug application (NDA) 211243, esketamine 28 mg single-use nasal spray device. Titusville, NJ, USA: Submitted by Janssen Pharmaceuticals, Inc., for the Treatment of Treatment-Resistant Depression.
AlanSchatzberg, F. M. D. (2019). A word to the wise about intranasal esketamine. Am. J. Psychiatry 176, 422–424. doi:10.1176/appi.ajp.2019.19040423
Bergfeld, I. O., Mantione, M., Figee, M., Schuurman, P. R., Lok, A., and Denys, D. (2018). Treatment-resistant depression and suicidality. J. Affect. Disord. 235, 362–367. doi:10.1016/j.jad.2018.04.016
CarlaCanuso, M. M. D., Singh, J. B., Fedgchin, M., Alphs, L., Lane, R., Lim, P., et al. (2018). Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 175, 620–630. doi:10.1176/appi.ajp.2018.17060720
Castro, M., Wilkinson, S. T., Al Jurdi, R. K., Petrillo, M. P., Zaki, N., Borentain, S., et al. (2023). Efficacy and safety of esketamine nasal spray in patients with treatment-resistant depression who completed a second induction period: analysis of the ongoing SUSTAIN-3 study. CNS Drugs 37, 715–723. doi:10.1007/s40263-023-01026-3
Chen, W.-Y., Huang, M.-C., and Lin, S.-K. (2014). Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst. Abuse Treat. Prev. Policy 9, 39. doi:10.1186/1747-597X-9-39
Cheng, J. Y. K., Chan, D. T. W., and Mok, V. K. K. (2005). An epidemiological study on alcohol/drugs related fatal traffic crash cases of deceased drivers in Hong Kong between 1996 and 2000. Forensic Sci. Int. 153, 196–201. doi:10.1016/j.forsciint.2004.08.023
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. doi:10.1016/S0140-6736(17)32802-7
Cristea, I. A., and Naudet, F. (2019). US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 6, 975–977. doi:10.1016/S2215-0366(19)30292-5
Fedgchin, M., Trivedi, M., Daly, E. J., Melkote, R., Lane, R., Lim, P., et al. (2019). Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 22, 616–630. doi:10.1093/ijnp/pyz039
Ferner, R., and Aronson, J. (2019). Susceptibility to adverse drug reactions. Br. J. Clin. Pharmacol. 85, 2205–2212. doi:10.1111/bcp.14015
Fu, D. J., Ionescu, D. F., Li, X., Lane, R., Lim, P., Sanacora, G., et al. (2020). Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J. Clin. psychiatry 81, 19m13191. doi:10.4088/JCP.19m13191
Gastaldon, C., Raschi, E., Kane, J. M., Barbui, C., and Schoretsanitis, G. (2020). Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychotherapy Psychosomatics 90, 41–48. doi:10.1159/000510703
Guo, H., Wang, B., Yuan, S., Wu, S., Liu, J., He, M., et al. (2022). Neurological adverse events associated with esketamine: a disproportionality analysis for signal detection leveraging the FDA adverse event reporting system. Front. Pharmacol. 13, 849758. doi:10.3389/fphar.2022.849758
Hirschfeld, R. M. (2000). History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 61 Suppl 6, 4–6.
Ionescu, D. F., Fu, D. J., Qiu, X., Lane, R., Lim, P., Kasper, S., et al. (2020). Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int. J. Neuropsychopharmacol. 24, 22–31. doi:10.1093/ijnp/pyaa068
Jeon, H. J., Ju, P. C., Sulaiman, A. H., Aziz, S. A., Paik, J. W., Tan, W., et al. (2022). Long-term safety and efficacy of esketamine nasal spray plus an oral antidepressant in patients with treatment-resistant depression-an asian sub-group analysis from the SUSTAIN-2 study. Clin. Psychopharmacol. Neurosci. 20, 70–86. doi:10.9758/cpn.2022.20.1.70
Kasıkara, H., Sungu, N., Arslan, M., Kucuk, A., Ozturk, L., Afandiyeva, N., et al. (2021). Repeated doses of ketamine affect the infant rat urogenital system. Drug Des. Devel Ther. 15, 1157–1165. doi:10.2147/dddt.s285862
Kim, J., Farchione, T., Potter, A., Chen, Q., and Temple, R. (2019). Esketamine for treatment-resistant depression — first FDA-approved antidepressant in a new class. N. Engl. J. Med. 381, 1–4. doi:10.1056/NEJMp1903305
Kim, J., Kim, T.-E., Lee, S.-H., and Koo, J. W. (2023). The role of glutamate underlying treatment-resistant depression. Clin. Psychopharmacol. Neurosci. 21, 429–446. doi:10.9758/cpn.22.1034
Lankenau, S. E., Sanders, B., Bloom, J. J., and Hathazi, D. (2008). Towards an explanation of subjective ketamine experiences among Young injection drug users. Addict. Res. Theory 16, 273–287. doi:10.1080/16066350801983749
Lu, J., Xu, X., Huang, Y., Li, T., Ma, C., Xu, G., et al. (2021). Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry 8, 981–990. doi:10.1016/S2215-0366(21)00251-0
NIH (2019). Esketamine nasal spray (Spravato) for treatment-resistant depression. Med. Lett. drugs Ther. 61, 54–57.
Ochs-Ross, R., Daly, E. J., Zhang, Y., Lane, R., Lim, P., Morrison, R. L., et al. (2020). Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am. J. Geriatric Psychiatry 28, 121–141. doi:10.1016/j.jagp.2019.10.008
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523. doi:10.1002/pds.1001
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803. doi:10.7150/ijms.6048
Salahudeen, M. S., Wright, C. M., and Peterson, G. M. (2020). Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther. Adv. Drug Saf. 11, 2042098620937899. doi:10.1177/2042098620937899
Schifano, F., Corkery, J., Oyefeso, A., Tonia, T., and Ghodse, A. H. (2008). Trapped in the "K-hole": overview of deaths associated with ketamine misuse in the UK (1993-2006). J. Clin. Psychopharmacol. 28, 114–116. doi:10.1097/JCP.0b013e3181612cdc
Spravato, J. C. l. I. l. (2019). Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/spravato-epar-productinformation_en.pdf.
Vanina Popova, M. D., Daly, E. J., Trivedi, M., Cooper, K., Lane, R., Lim, P., et al. (2019). Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am. J. Psychiatry 176, 428–438. doi:10.1176/appi.ajp.2019.19020172
Vickers-Smith, R., Sun, J., Charnigo, R. J., Lofwall, M. R., Walsh, S. L., and Havens, J. R. (2020). Gabapentin drug misuse signals: a pharmacovigilance assessment using the FDA adverse event reporting system. Drug Alcohol Dependence 206, 107709. doi:10.1016/j.drugalcdep.2019.107709
Wajs, E., Aluisio, L., Holder, R., Daly, E. J., Lane, R., Lim, P., et al. (2020). Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J. Clin. psychiatry 81, 19m12891. doi:10.4088/JCP.19m12891
WHO (2017) Depression and other common mental disorders. WHO/MSD/MER/, 2017.2. Geneva, Switzerland: WHO.
WHO (2024). Mental health in China. Available at: https://www.who.int/china/health-topics/mental-health.
Yang, C., Han, M., Zhang, J.-c., Ren, Q., and Hashimoto, K. (2016). Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 239, 281–283. doi:10.1016/j.psychres.2016.03.034
Yang, C., Shirayama, Y., Zhang, J. c., Ren, Q., Yao, W., Ma, M., et al. (2015). R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 5, e632. doi:10.1038/tp.2015.136
Zaki, N., Chen, L. N., Lane, R., Doherty, T., Drevets, W. C., Morrison, R. L., et al. (2023). Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: interim results of the SUSTAIN-3 study. Neuropsychopharmacology 48, 1225–1233. doi:10.1038/s41386-023-01577-5
Keywords: esketamine nasal spray, depressive disorder, adverse (side) effects, data analysis, FAERS
Citation: Liu R, Liu C, Feng D, Guo T and Wang Y (2024) Pharmacovigilance of esketamine nasal spray: an analysis of the FDA adverse event reporting system database. Front. Pharmacol. 15:1414703. doi: 10.3389/fphar.2024.1414703
Received: 09 April 2024; Accepted: 20 May 2024;
Published: 14 June 2024.
Edited by:
Magdalena Sowa-Kucma, University of Rzeszow, PolandReviewed by:
Marcin Siwek, Jagiellonian University, Medical College, PolandGiuseppina Cantarella, University of Catania, Italy
Yang Mei, Chongqing Hospital of Traditional Chinese Medicine, China
Copyright © 2024 Liu, Liu, Feng, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, MTMzMDIxNTE1MThAMTYzLmNvbQ==
 Ruixue Liu
Ruixue Liu Chunxiao Liu
Chunxiao Liu