- 1Jiangsu MC Clinical Innovation Center of Degenerative Bone and Joint Disease, Wuxi TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Wuxi, China
- 2Engineering Research Center of Tropical Medicine Innovation and Transformation of Ministry of Education and International Joint Research Center of Human-Machine Intelligent Collaborative for Tumor Precision Diagnosis and Treatment of Hainan Province and Hainan Provincial Key Laboratory of Research and Development on Tropical Herbs and Haikou Key Laboratory of Li Nationality Medicine, School of Pharmacy, Hainan Medical University, Haikou, Hainan, China
- 3Hubei University of Chinese Medicine Affiliated Gongan Hospital of Traditional Chinese Medicine, Wuhan, China
The dried root and rhizome of Alpinia officinarum Hance (A. officinarum) have been widely used in traditional Chinese medicine for thousands of years to alleviate pain, promote digestion, warm the stomach, and disperse cold. This review aims to comprehensively and in-depth summarize the most recent research on the traditional uses, phytochemistry, pharmacokinetics, and pharmacology of A. officinarum. By searching various databases including Web of Science, PubMed, Google Scholar, Elsevier, Springer, ScienceDirect, and China National Knowledge Infrastructure (CNKI) for literature on “A. officinarum Hance,” as well as relevant textbooks and digital documents, an overall and critical review of the subject was conducted. The traditional uses of A. officinarum were summarized, and 337 compounds from A. officinarum were summarized, including flavonoids, diarylheptanoids, volatile oils, and other compounds. Studies have found that the crude extract of A. officinarum and its compounds has a wide range of biological activities, such as improving gastrointestinal function, anti-inflammatory properties, anti-tumor activity, antibacterial properties, memory enhancement, and analgesic effects. Modern pharmacological studies have provided strong evidence and explanations for the traditional medicinal uses of A. officinarum, which brings a broad prospect for its medicinal use. However, more research is needed to explore the structure-activity relationship and potential mechanisms of action of its bioactive chemicals. Furthermore, it is essential to conduct more clinical trials in order to accelerate research and development of the drug.
1 Introduction
With the development of the times, people are increasingly focusing on their wellbeing. The advancement of medical technology has also begun to attract attention. While new drugs for various diseases are constantly being developed, people are actively exploring alternative therapies and natural products due to the toxic side effects of chemical drugs and the uncontrollable risks of biological agents. Alpinia officinarum Hance (A. officinarum), native to China, is one of the most important species of the Zingiberaceae family, which is widely distributed in Fujian, Taiwan, Guangdong, Guangxi, Hainan, and other provinces in China (Sun et al., 2023; Zheng et al., 2024). The detailed description of the medical applications of A. officinarum can be traced back to the book “Ming Yi Bie Lu,” which was written during the Han Dynasty (Tao, 1986). As a medicinal part, the aromatic rhizome of A. officinarum mainly belongs to the spleen and stomach meridians and was widely used in the treatment of gastrointestinal diseases in ancient China (Tushar et al., 2010; Al Garni et al., 2024).
Botanical drugs have been widely used to treat many diseases for centuries due to their obvious effectiveness, fewer side effects, and relatively low cost. A. officinarum is known for its extensive clinical applications because it contains a variety of bioactive substances, including flavonoids, diarylheptanoids, volatile oils, phenylpropanoids, and glycosides (Pillai et al., 2018; Wen et al., 2024). Flavonoids and diarylheptanoids are its main components and have been proven to have a variety of pharmacological effects (Abubakar et al., 2018). In this paper, the traditional uses, chemical components, and biological activities of A. officinarum were reviewed comprehensively, which provide better guidance for the rational utilization of it.
2 Traditional efficacy and application of A. officinarum
A. officinarum, which is also known as “Liangjiang” and “Xiaoliangjiang,” was first recorded in the “Ming Yi Bie Lu” during the Han Dynasty (Tao, 1986). As shown in Table 1, the properties of A. officinarum have mainly been described as pungent and warm, while in some ancient books, there have been occasional records of “bitter”. It has been recorded in ancient books that A. officinarum mainly enters the two meridians of the spleen and stomach, but rarely enters the heart, liver and Danzhong meridians. The records of A. officinarum in modern works on herbal all belong to the spleen and stomach meridians. Through the analysis of the records of the efficacy of A. officinarum in ancient and modern Chinese botanical drug, it was found that its common features in terms of efficacy are warming the stomach, dispelling cold, relieving pain, regulating qi, stopping vomiting, and alleviating diarrhea. And, A. officinarum is commonly used to treat epigastric cold pain, vomiting, diarrhea, and food stagnation.
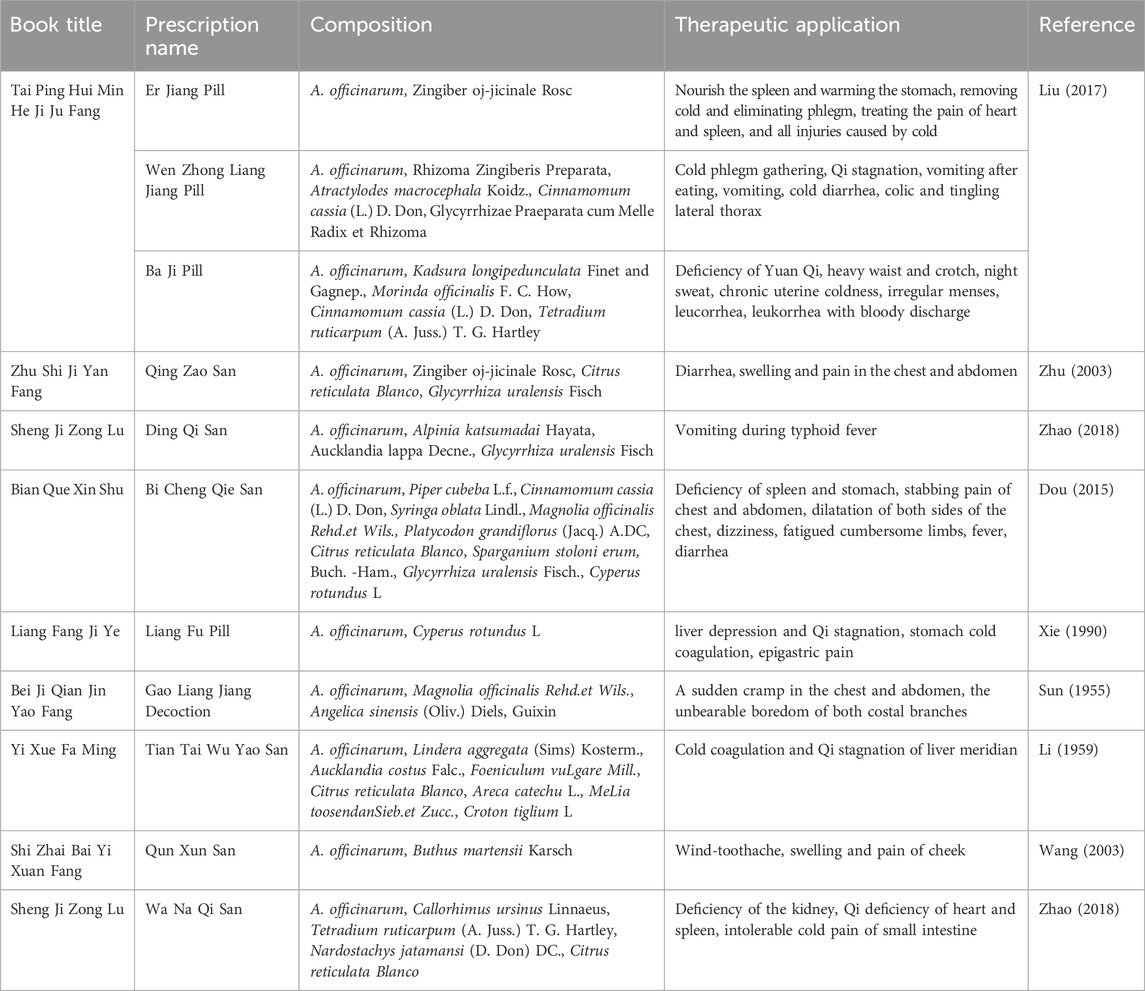
A. officinarum has been widely used in clinics due to its compatibility in many prescriptions, as shown in Table 2. A. officinarum is mainly used to warm the spleen and stomach, such as in Er Jiang Pill (Liu, 2017), which can nourish the spleen and stomach, remove cold, and eliminate phlegm, and cure all injuries caused by cold. Such prescriptions also include Wenzhong Liangjiang Pill (Liu, 2017) and Qing Zao San (Zhu, 2003). A. officinarum is a pungent and hot substance that is a pure yang product. It enters the spleen and stomach meridians, which can warm the stomach, reduce reflux and stop vomiting, and strengthen the spleen and stop diarrhea. For example, Ding Qi San (Zhao, 2018) is suitable for vomiting induced by typhoid. This type of prescription also includes Bi Cheng Qie San (Dou, 2015). A. officinarum can also enter the heart and Dan zhong, so it can enter the heart and pericardial meridian to warm and circulate qi. The prescriptions suitable for these kinds of conditions are Liang Fu Pill (Xie, 1990) and Gao Liang Jiang Decoction (Sun, 1955). With its fragrant and warm properties, A. officinarum can dissipate the cold, relieve pain, and promote qi. For example, Tian Tai Wu Yao San (Li, 1959) is applicable to the syndrome of cold coagulation and qi stagnation in the liver meridian. A. officinarum also has the effect of dispelling wind and relieving pain. The Qun Xun San, composed of A. officinarum and scorpion, has significant therapeutic effects on wind-induced toothache and swelling and pain in the cheek (Wang, 2003). In addition, A. officinarum has certain effects of warming the kidney and enhancing Yang. A. officinarum is compatible with Tetradium ruticarpum (A. Juss.) T. G. Hartley, which can warm the kidneys and dispel cold, and treat kidney deficiencies and waist pain. This type of prescription also includes Baji Pill (Liu, 2017).
3 Chemical composition
Up to now, 337 chemical compounds have been extracted from A. officinarum, mainly including flavonoids, diarylheptanoids, phenylpropanes, glycosides, volatile oil, and other compounds.
3.1 Flavonoids
Flavonoid is one of the main components in A. officinarum. A large number of flavonoids were isolated from A. officinarum, which are also the main active components in it. Until now, 21 flavonoids have been isolated, including 18 flavones, 2 flavanones, and 1 flavanol, as shown in Figure 1 and Table 3.
3.2 Diarylheptanoids
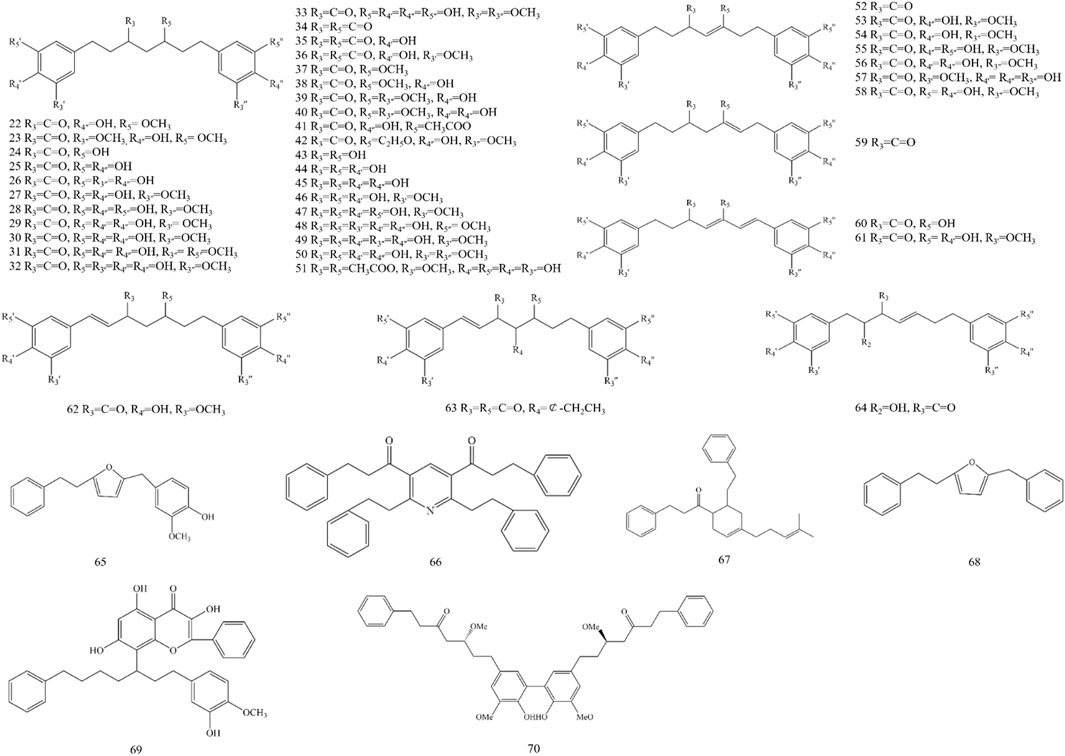
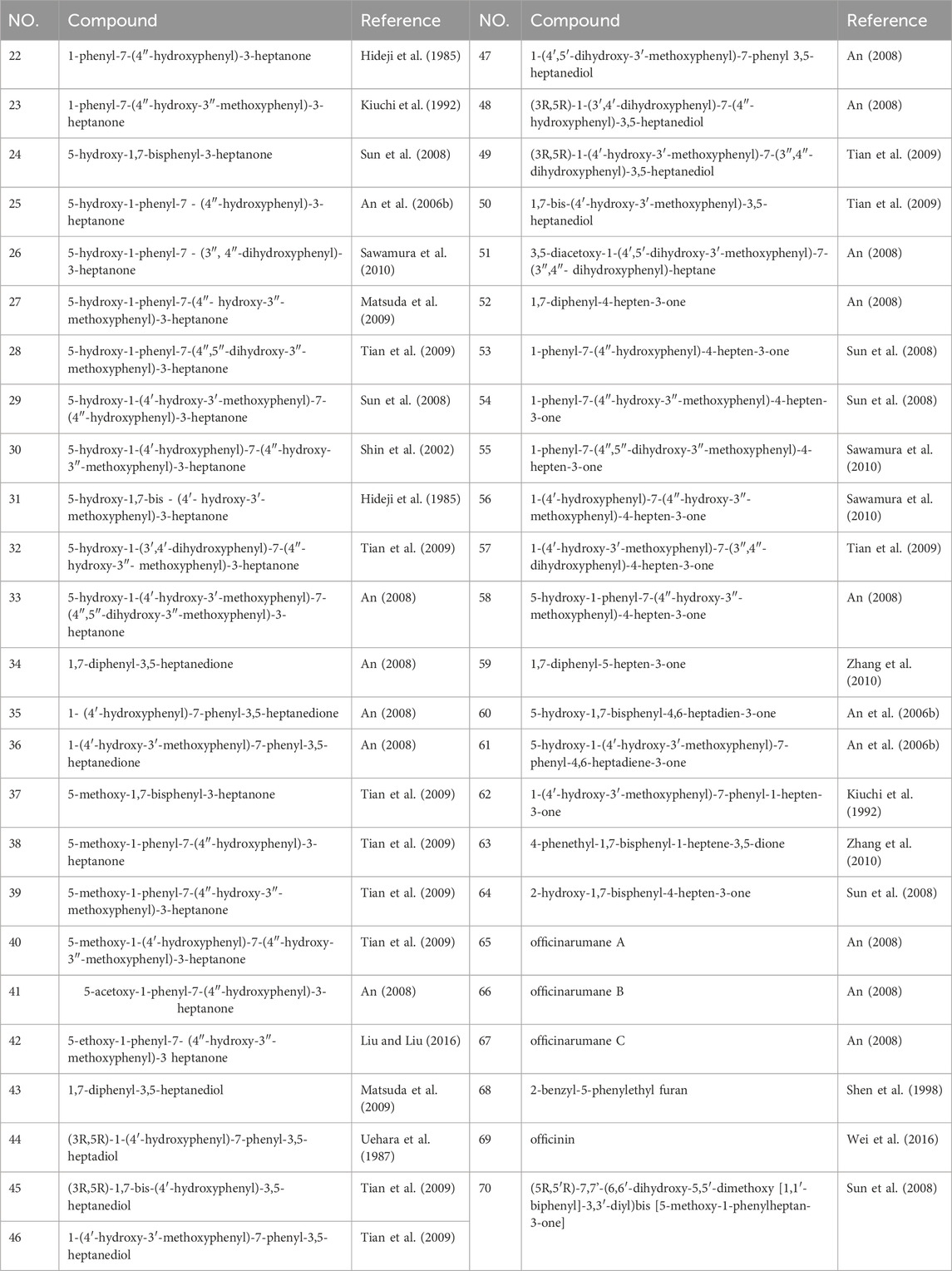
Diarylheptanoid is a group of compounds that contain a 1,7-disubstituted aromatic ring and a heptane skeleton and is an important chemical component of A. officinarum. At present, 49 diarylheptanoid compounds have been isolated from A. officinarum, including 42 chain diarylheptanoids, six cyclic diarylheptanoids, and one polymer of diarylheptanoid and flavonoid, as shown in Figure 2 and Table 4.
3.3 Volatile oil
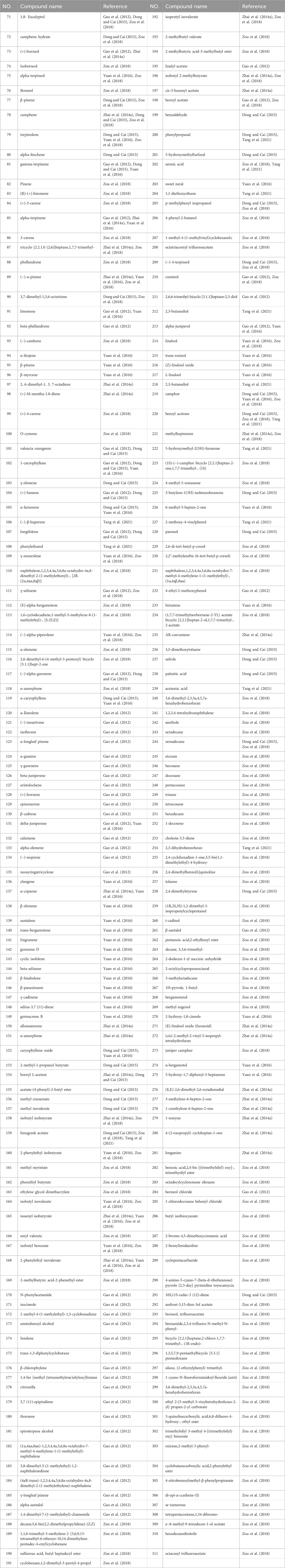
A. officinarum is a type of pungent and warm botanical drugs with a high content of volatile oil. Its spicy scent is one of the indicators used to judge the quality of this herbal medicine. At present, 241 volatile oils have been separated from A. officinarum, mainly including terpenoids (monoterpenes, sesquiterpenoids), aldehydes, ketones, ethers, alcohols, phenols, and other compounds, as shown in Table 5.
3.4 Other compounds
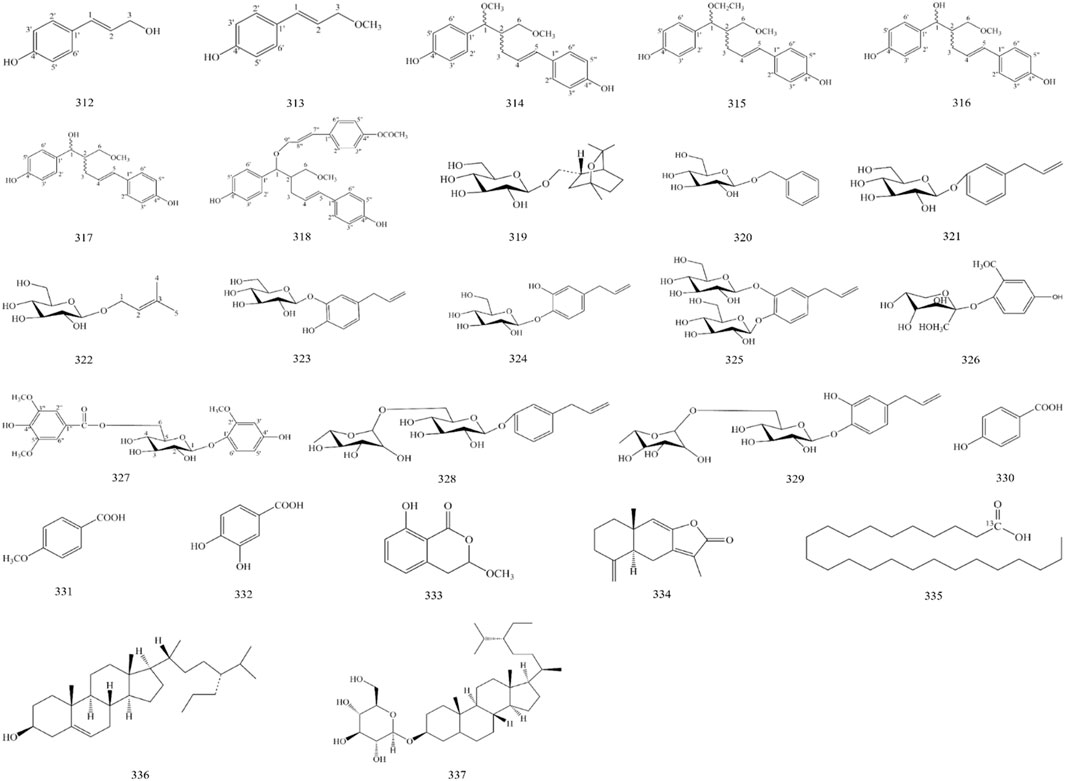
In addition, A. officinarum contains 7 phenylpropanoids, 11 glycosides, 5 organic acids, 2 sterols and their glycosides, and 1 lactone, as shown in Figure 3 and Table 6.
4 Pharmacokinetic study of the active compounds of A. officinarum
As one of the main active compounds of A. officinarum, galangin (3,5,7-trihydroxyflavone) has a variety of biological activities. Once galangin is consumed, it is metabolized in the intestine and liver, where it undergoes glucuronidation, methylation, and sulfation reactions. The pharmacokinetics of galangin-3-O-β-D-glucuronic acid (GG-1) and galangin-7-O-β-D-glucuronic acid (GG-2), two metabolites of A. officinarum, were studied in vivo. It was found (Liu et al., 2021) that, after oral administration of A. officinarum extract (0.3 g/kg) in rats, the peak concentrations (Cmax) of GG-1 and GG-2 were 6069.6 ± 1140.6 and 10596.0 ± 2395.7 ng/mL, respectively, reaching their peak concentrations at 0.2 ± 0.1 h. Area under curve (0-t) (AUC0-t), mean residence time (0-t) (MRT0-t), and t1/2 of GG-1 were 2390.9 ± 678.0 h μg/L, 1.4 ± 0.8 h, and 2.2 ± 0.7 h, respectively, while the corresponding values of GG-2 were 4554.9 ± 884.9 h·μg/L, 1.6 ± 0.7 h, and 3.3 ± 0.2 h, respectively. Obviously, the most significant difference between GG-1 and GG-2 is the AUC0-t and Cmax, where the parameter values of GG-2 are almost twice those of GG-1.
In addition, a previous study (Xin Zhang et al., 2021) found that microemulsion can promote the absorption of galangin and improve its bioavailability. The blood concentration of galangin in Liangfu Pill could not be detected after the rabbits were given Liangfu Pill by gavage once. For Liangfu micromilk, the absorption half-life (t1/2ka) of galangin was 0.29 h, the peak time (tpeak) was 0.75 h, the elimination half-life (t1/2ke) was 1.47 h, Cmax was 38.46 μg/L, and the AUC was 129.42 (μg·h)/L. In another study (Xianhua Du et al., 2008), it was found that a self-microemulsion of galangin was absorbed throughout the entire intestinal tract of rats. The absorption rate constants (Ka) in the duodenum, jejunum, ileum, and colon were 2.37, 1.70, 2.29, and 3.98 times higher than those of the galangin suspension, respectively. Additionally, the apparent absorption coefficients (Papp) were 3.58, 2.56, 3.57, and 5.16 times higher than those of the galangin suspension, respectively. The relative bioavailability of the self-microemulsion of galangin was 220%, compared to the galangin suspension.
5 Pharmacological effects of A. officinarum
A. officinarum is an important traditional Chinese medicine, and its main chemical components are flavonoids, volatile oils, and diarylheptanoids. Modern pharmacological studies have shown that A. officinarum has various pharmacological effects, including anti-ulcer, inhibition of gastrointestinal motility, anti-inflammatory and analgesic, antioxidant, anti-tumor, antibacterial, and hypoglycemic properties, as shown in Table 7.
5.1 Effects on gastrointestinal function
A. officinarum is an essential medicine for treating deficiency-cold of the spleen and stomach, as well as epigastric cold pain in traditional Chinese medicine. It is mainly used in the treatment of digestive tract diseases such as dyspepsia, acid reflux, and gastric ulcers. Wei (2019) used anhydrous ethanol and aspirin to induce two types of gastric ulcers models to study the effects of different extracts of A. officinarum on mice with gastric ulcers. The results showed that the aqueous extract of A. officinarum had a good anti-ulcer effect and decreased the ulcer index. It was inferred that the mechanism of the anti-ulcer effect of A. officinarum may be through inhibiting inflammatory factors, reducing gastrin (GAS), increasing cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2), thereby enhancing the protective effect of the gastric mucosa and reducing gastric injury. Wang et al. (2011) studied the therapeutic effect of the volatile oil of A. officinarum on gastric ulcers. The results showed that the volatile oil of A. officinarum could reduce the gastric ulcer index and increase the ulcer inhibition rate in mice. A. officinarum reduces the levels of serum motilin (MOT) and substance P (SP), while increasing the levels of serum somatostatin (SS) and vasoactive intestinal peptide (VIP) in order to exert its anti-ulcer effect. In addition, the study found that the volatile oil of A. officinarum can increase the levels of serum nitric oxide (NO), expand the blood vessel walls, improve the microcirculation of the gastric mucosa, strengthen the mucosal barrier, scavenge oxygen free radicals, and protect the normal function of the gastric mucosa.
A. officinarum has an obvious gastrointestinal spasmolytic effect, and its decoction can inhibit gastrointestinal propulsive movement. Gui et al. (2021) observed the effect of the total flavonoids of A. officinarum on the propulsive movement of the small intestine in normal rats using the charcoal powder method. The results showed that the total flavonoids of A. officinarum not only significantly inhibited the intestinal motility of normal rats, but also antagonized the hyperfunction of the small intestine induced by neostigmine. The mechanism may be that it affects the secretion and release of gastrointestinal hormones, such as somatostatin and vasoactive intestinal peptide, thus relaxing the smooth muscle. Cheng Yuan et al. (Cheng et al., 2015) studied the effects of various active components of A. officinarum on intestinal spasms induced by acetylcholine and on normal intestinal muscles in isolated rabbits. The results showed that the active components of A. officinarum extract could inhibit the spontaneous movement of intestinal muscles in a dose-dependent manner. Among these components, flavonoids and diphenylheptanes were the most prominent, and they were stronger than anisodamine. The mechanism of A. officinarum in improving gastrointestinal function is shown in Figure 4.
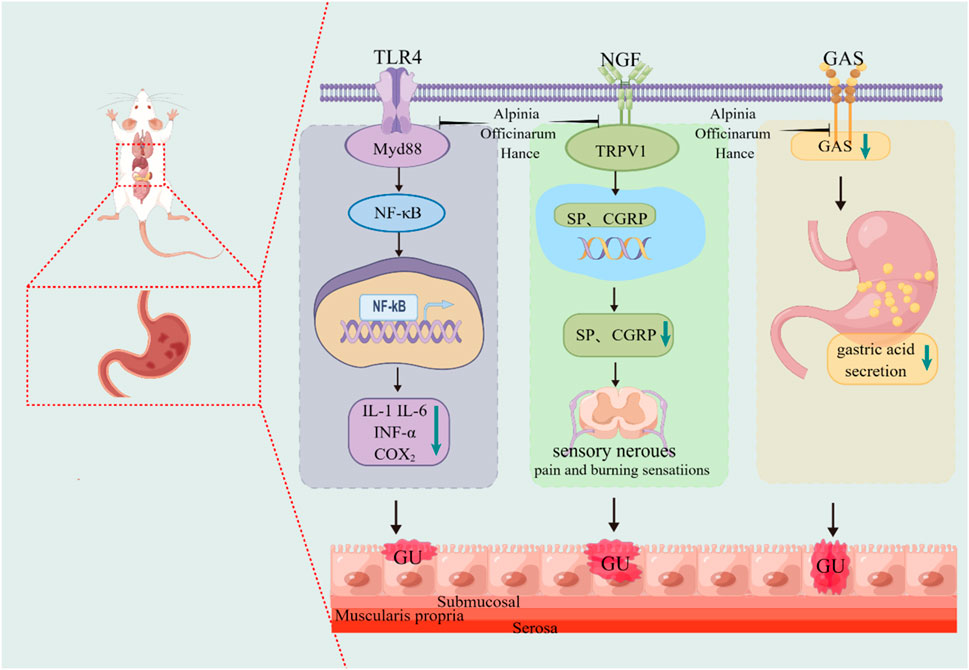
Figure 4. The mechanism of A. officinarum in improving gastrointestinal function. Toll-like receptor 4 (TLR4), nerve growth factor (NGF), calcitonin gene-related peptide (CGRP), gastric ulcer (GU).
5.2 Analgesic and anti-inflammatory effect
A. officinarum is hot and pungent, which has the effect of dispelling cold and relieving pain. As the use of non-steroidal anti-inflammatory drugs for long-term treatment of inflammation can cause obvious side effects, plants are constantly being developed as potential anti-inflammatory agents. Chen et al. (2009) used the carrageenan rat foot swelling model, the xylene mouse ear swelling model, and a capillary permeability experiment to study the anti-inflammatory effect of the total flavonoids extracted from A. officinarum. The mouse hot plate method and torsion test were used to observe the analgesic effect of the total flavonoids extracted from A. officinarum. The results showed that the total flavonoids extracted from A. officinarum had a significant inhibitory effect on acute inflammation models, such as toe swelling induced by carrageenan, auricle swelling induced by xylene, and an increase in celiac capillary permeability induced by acetic acid in mice. The total flavonoids of A. officinarum can inhibit pain induced by acetic acid and heat stimulation in mice. Liang et al. (2013) studied the therapeutic and analgesic effects of total flavonoids from A. officinarum (GLJ) on acetic acid-induced visceral hypersensitivity in rats with irritable bowel syndrome (IBS). The results showed that GLJ had a certain inhibitory effect on pain induced by heat stimulation, acetic acid, and formaldehyde in mice. Zha Wangjian et al. (Cha, 2015) found that galangin can inhibit airway inflammation and airway hyperresponsiveness to some extent in a mouse model of asthma. In addition, A. officinarum and its main compounds have anti-inflammatory effects on LPS-induced inflammation in RAW264.7 cells. This may be related to the inhibition of NF-κB activation. The anti-inflammatory mechanism of the total flavonoids of A. officinarum is shown in Figure 5A.
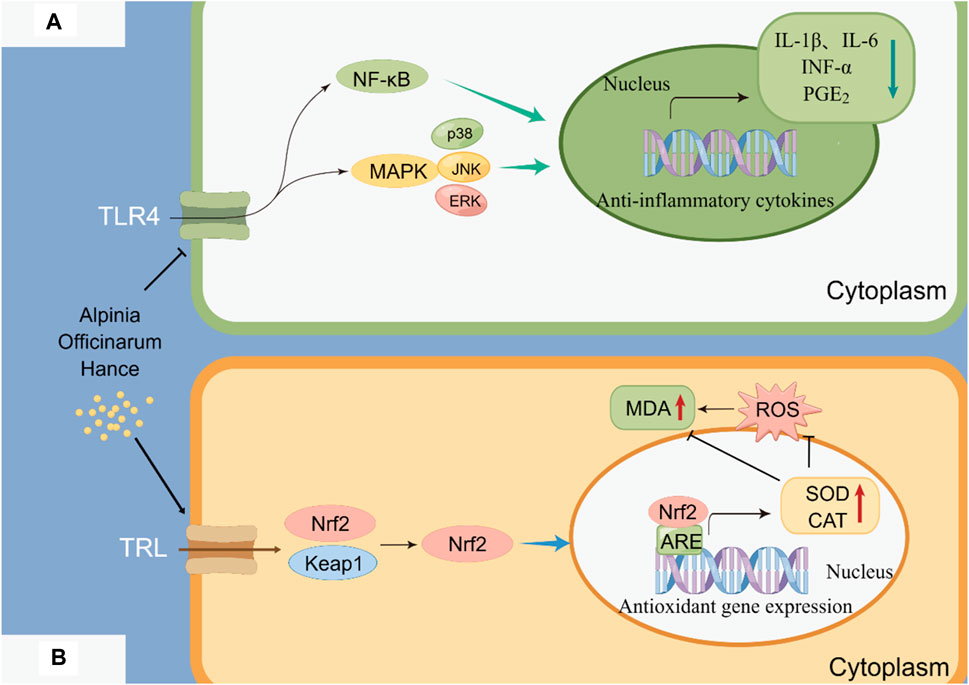
Figure 5. Anti-inflammatory and antioxidant mechanisms of A. officinarum. (A) Anti-inflammatory mechanism of total flavonoids of A. officinarum. (B) Antioxidant mechanism of extract of A. officinarum.
5.3 Antioxidant effect
An antioxidant is a type of active substance that can eliminate the inhibition of lipid peroxidation by free radicals. It can prevent the damage caused by lipid peroxidation to organisms. In a comparative study on the antioxidant activity of various components of A. officinarum extract, Lin et al. (2017) discovered that the diphenylheptanes exhibited antioxidant activity both in vitro and in vivo. Xia et al. (2009) found that the total flavonoids of A. officinarum can act as antioxidants by inhibiting reactive oxygen free radicals and decreasing the catalytic activity of metal ions in vitro. In the HepG2 oxidative damage model induced by H2O2, diphenylheptane in A. officinarum showed significant antioxidant activity. The extract of A. officinarum could potentially prevent oxidative stress damage by activating the Keap1/Nrf2/ARE signaling pathway. The antioxidant mechanism of the extract of A. officinarum is shown in Figure 5B.
5.4 Antibacterial effect
The in vitro antibacterial experiment conducted by Zhao et al. (2007) showed that the chloroform and ethyl acetate extracts of A. officinarum exhibited anti-Candida albicans activity. The chloroform extract of A. officinarum, at a concentration of 20 mg/mL, demonstrated strong activity. Qin et al. (2015) showed that both the alcohol extract and water extract of A. officinarum had a good inhibitory effect on methicillin-resistant Staphylococcus aureus, but had no significant inhibitory effect on Pseudomonas aeruginosa, Candida albicans, Acinetobacter, or Escherichia coli. Flavonoids are the most important antibacterial components of A. officinarum. Ouyang et al. (2018) studied the impact of galangin on the antibacterial activity against vancomycin-intermediate S. aureus. The study results showed that galangin had significant inhibitory activity against ATCC25293, N315, and Mu50, with a minimum inhibitory concentration (MIC) of 32 mg/L. The results of further studies showed that galangin inhibited the growth of bacteria by inhibiting the activity of cell wall hydrolase. At the same time, the effect of quercetin on P. aeruginosa PAO1 was also studied (Ouyang et al., 2016). The results showed that 16 mg/L of quercetin could significantly inhibit the biofilm formation, the quorum sensing system, and independent factors of P. aeruginosa. This suggests that quercetin may have the potential to treat biofilm-associated infections.
5.5 Improve memory ability
Alzheimer’s disease (AD) is a chronic degenerative disease of the central nervous system in middle-aged and elderly individuals. Its main clinical manifestation is cognitive dysfunction. Huang Liping (Huang et al., 2022) has shown that galangin can improve learning and memory impairment in APP/PS1 mice. It may inhibit the activity of acetylcholinesterase (AChE) in the brain through the cholinergic pathway, increasing the level of ACh and improving learning and memory function. On the other hand, it may play a role in protecting hippocampal neurons by regulating the Akt/MEF2D/Beclin-1 signaling pathway and clearing abnormal proteins in hippocampal neurons through autophagy and chaperone-mediated autophagy (CMA). This can reduce the deposition of amyloid-β (Aβ) and the formation of tau protein. It can be concluded that galangin may improve the learning and memory impairment of APP/PS1 mice by regulating the Akt/MEF2D/Beclin-1 signaling pathway. In the PC12 cell injury model stimulated by H2O2, A. officinarum extract can significantly reduce the lactate dehydrogenase leakage rate, decrease the content of MDA, and increase the activities of SOD and GSH-Px (Zhai et al., 2014b).
5.6 Anti-tumor effect
The anti-tumor mechanism of A. officinarum can be reflected in regulating the cell cycle, inducing tumor cell apoptosis and autophagy, inhibiting tumor cell migration and invasion, and reversing drug resistance in tumors. Luo and Liu (2020) found that galangin has a broad-spectrum anti-tumor effect. Its inhibitory effect on different tumor cells varies and depends on time and concentration. Galangin can strongly inhibit the genotoxicity of chemical toxic substances in vivo and in vitro, making it a potential preventive drug for cancer. Zhang et al. (2012) found that A. officinarum can induce apoptosis by activating mitochondrial apoptosis, caspases, and causing changes in the levels of Bcl-2 in various liver cancer cell lines. Additionally, kaempferol derived from A. officinarum has the ability to induce apoptosis in HCCLM3 and Huh7 cells by controlling the ATM/CHEK2/KNL1 signaling pathway.
5.7 Other functions
In addition to the above pharmacological effects, A. officinarum has anti-liver injury, hypoglycemic, hypolipidemic, and anticoagulant effects. Zhou et al. (2012) showed that A. officinarum can protect the function of hepatocytes in mice after an acute alcoholic liver injury. The results showed that A. officinarum could significantly reduce the concentrations of alanine aminotransferase (ALT) and aspartate transaminase (AST) in the serum of mice after injury, indicating that A. officinarum has a certain hepatoprotective effect. Its pharmacological mechanism may be to protect liver cells by scavenging free radicals and reducing the degree of damage caused by alcohol. Akhtar et al. (2002) showed that the extract of A. officinarum has a significant hypoglycemic effect. In the hypoglycemic experiment on normal male New Zealand rabbits, oral A. officinarum powder at a dose of 3 g/kg significantly reduced blood glucose levels. The methanol and water extracts showed even more pronounced hypoglycemic effects. When the oral dose was increased to 4 g/kg, there was a significant decrease in blood glucose levels of rabbits after 8 h. However, A. officinarum powder and its extract had no effect on rabbits with diabetes induced by alloxan. Therefore, its hypoglycemic effect may be achieved by promoting insulin secretion from the pancreas in the body. Obese patients are often accompanied by abnormal fat metabolism, which can lead to high blood total cholesterol (TC) and/or triglyceride (TG) levels. Fang et al. (2015) showed that middle and high doses of total flavonoids from A. officinarum play a significant role in controlling body mass, fat accumulation, and cholesterol metabolism, as well as reducing the levels of serum leptin and plasma neuropeptide Y in nutritionally obese rats with hyperlipidemia. A study (Luo et al., 2015) has shown that galangin has an obvious inhibitory effect on thrombosis in rats, demonstrating a certain anticoagulant effect. The potential mechanism may be to improve the blood flow state of rats by participating in the endogenous coagulation system.
6 Conclusion and prospection
A. officinarum is an important traditional Chinese medicine for both medicine and food. Using modern research methods, the pharmacological effects of its active compounds have been clearly described, and the mechanisms of anti-gastric ulcer, inhibition of gastrointestinal motility, antioxidant effect, antibacterial, anti-inflammatory, and analgesia have been gradually clarified. The treatment of traditional digestive tract diseases has been expanded to a certain extent, broadening its scope of clinical application. So far, 337 compounds have been isolated from A. officinarum. Among them, galangin is a very important active compound extracted from A. officinarum. The pharmacological effects of galangin are very extensive. However, most pharmacological effects are currently only verified in cell and animal models, and there is a lack of clinical study data to support them. In addition, the mechanism of pharmacological action of galangin is not fully understood. Most studies are limited to the pharmacodynamic level or a few specific targets or pathways, and are unable to elucidate the general mechanism of action or the connection between the various targets and pathways. In the future, based on existing research, network pharmacology, bioinformatics, and multi-omics analysis can be used to comprehensively and deeply analyze the molecular mechanisms, genes, and signaling pathways of galangin. Further studies are needed to explore the extracts of A. officinarum for any potential toxicities, side effects, and contraindications. With the continuous discovery of the structure of the active components of A. officinarum and the in-depth study of its pharmacological activity, its pharmacodynamic mechanism is gradually becoming clear. The research scope of the pharmacological activity of A. officinarum has been continuously expanded by the vast number of scientific research works, and its medicinal value will be further developed and applied.
Author contributions
XL: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing. JW: Software, Writing–original draft. KZ: Writing–original draft. TX: Writing–original draft. JZ: Writing–review and editing. XX: Writing–original draft. QL: Writing–original draft. XL: Conceptualization, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Hainan Provincial Natural Science Foundation of China (819QN230), the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2022072), Jiangsu CM Clinical Innovation Center of Degenerative Bone & Joint Disease, Natural Science Foundation project of Nanjing University of Chinese Medicine (XZR2023091).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AD, Alzheimer’s disease; AUC, area under the curve; AchE, acetylcholinesterase; ALT, alanine aminotransferase; AST, aspartate transaminase; Cmax, peak concentration; COX-2, cyclooxygenase-2; CMA, chaperone-mediated autophagy; DIC, disseminated intravascular coagulation; iNOS, inducible nitric oxide synthase; GG-1, galangin-3-O-β-D-glucuronic acid; GG-2, galangin-7-O-β-D-glucuronic acid; IBS, irritable bowel syndrome; Ka, absorption rate constants; MDA, malondialdehyde; MIC, minimum inhibitory concentration; MOT, motilin; NO, nitric oxide; GAS, gastrin; Papp, apparent absorption coefficients; PGE2, prostaglandin E2; SOD, superoxide dismutase; SP, substance P; SS, serum somatostatin; tpeak, peak time; t1/2ke, elimination half-life; t1/2ka, absorption half-life; TC, total cholesterol; TG, triglycerides; VIP, vasoactive intestinal peptide; TLR4, toll-like receptor 4; NGF, nerve growth factor; CGRP, calcitonin gene-related peptide; GU, gastric ulcer.
References
Abubakar, I. B., Malami, I., Yahaya, Y., and Sule, S. M. (2018). A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J. Ethnopharmacol. 224, 45–62. doi:10.1016/j.jep.2018.05.027
Abudula, G. M. A. A. R. N. R. A. (2016). The effects of Galangin against human cervical cancer apoptosis and gene expression levels in vitro. J. Xinjiang Med. Univ. 39, 1595–1600. doi:10.3969/j.issn.1009-5551.2016.12.028
Akhtar, M. S., Khan, M. A., and Malik, M. T. (2002). Hypoglycaemic activity of Alpinia galanga rhizome and its extracts in rabbits. Fitoterapia 73, 623–628. doi:10.1016/s0367-326x(02)00235-6
Al Garni, H. A., El-Halawany, A. M., Koshak, A. E., Malebari, A. M., Alzain, A. A., Mohamed, G. A., et al. (2024). Potential antioxidant, α-glucosidase, butyrylcholinesterase and acetylcholinesterase inhibitory activities of major constituents isolated from Alpinia officinarum hance rhizomes: computational studies and in vitro validation. Sar. QSAR Environ. Res. 35, 391–410. doi:10.1080/1062936X.2024.2352725
An, N. (2008). 1 studies on the chemical constituents of alpinia officinarum hance. 2 studies on the lipophilic chemical constitutes of Euphorbia soongarica Boiss. Peking Union Medical College and Chinese Academy Medical Sciences.
An, N., Lin, J., Yang, S. L., Zou, Z. M., and Xu, L. Z. (2006c). A new glycoside from Alpinia officinarum. Yao Xue Xue Bao 41, 233–235. doi:10.3321/j.issn:0513-4870.2006.03.009
An, N., Yang, S., Zou, Z., and Xu, L. (2006a). Flavonoids of alpinia off icinarum. Chinese Traditional and Herbal Drugs, 663–664. doi:10.3321/j.issn:0253-2670.2006.05.007
Bleier, W., and Chirikdjian, J. J. (1972). Flavonoids from galanga rhizome (Alpinia officinarum Hance). Planta Med. 22, 145–151. doi:10.1055/s-0028-1099597
Bu, X., Xiao, G., Gu, L., and Zhang, M. (2000). Chemical study of alpinia officinarum. J. Chin. Med. Mater. 23, 84–87. doi:10.13863/j.issn1001-4454.2000.02.018
CGONCHM. Compilation (1996). National compendium of Chinese herbs. Beijing: People's Medical Publishing House.
Cha, W. (2015). The effect and mechanism of galangin on airway inflammation in bronchial asthma. Nanjing Medical University.
Chen, C. S. (2012). Study of preventive and therapeutic effects of the extract of alpinia officinarum Hance on vascular dementia. Hebei University.
Chen, Y., Jiang, T., Tang, C., Feng, Y., and Yang, C. (2009). Experimental study of anti-inflammatory and analgesic effects of total flavonoids from Alpinia officinarum Hance. J. Guangdong Pharm. Univ. 25, 188–191. doi:10.16809/j.cnki.1006-8783.2009.02.025
Chen, Z. (1983). Ben cao shi yi. Revised Edition. Wuhu: Scientific Research Department of Wannan Medical College.
Cheng, S. Q., Chen, Y. K., Wang, Y., Li, Y. H., Tan, Y. F., Li, H. L., et al. (2017). Optim. Purif. Process Hypoglycemic Part Alp. Off. Chin. Tradit. Pat. Med. 39, 196–199.
Cheng, Y., Li, J., Liao, X. D., Lin, Z. P., Zhu, Y. Z., Ye, H., et al. (2015). Effect of active components of galangal extract on smooth muscle of isolated intestine in rabbits. J. Guangdong Med. Univ. 33, 649–652.
Chinese Pharmacopoeia Commission (2015). Chinese Pharmacopoeia (partⅠ). Beijing: The Medicine Science and Technology Press of China.
Choi, M. J., Park, J. S., Park, J. E., Kim, H. S., and Kim, H. S. (2017). Galangin suppresses pro-inflammatory gene expression in polyinosinic-polycytidylic acid-stimulated microglial cells. Biomol. Ther. Seoul. 25, 641–647. doi:10.4062/biomolther.2017.173
Dong, D., and Cai, B. (2015). Studies on chemical constituents of the volatile oil of Alpinia officinarum Hance by GC-MS. CHINA Mod. Med. 22, 9–11.
Fang, Y., Xia, D., Wang, S., and Ji, S. (2015). Study on anti-obese and hypolipidemic effects of total flavonoids from Alpinia officinarum Hance in rats with nutritive obesity combined with hyperlipidemia. China J. Tradit. Chin. Med. Pharm. 30, 2907–2910.
Fu, L. Q., Li, X. Y., and Yang, C. X. (2012). Galangin attenuates cognitive impairment in senescent mice. Her. Med. 31, 863–866. doi:10.3870/yydb.2012.07.010
Gao, Z. R., Yin, G. Y., Lu, Y. L., Li, L. X., and Shi, H. L. (2012). Study on volatile compounds of alpinia officinarum hance. J. Anhui Agri. Sci. 40, 12247–12249. doi:10.13989/j.cnki.0517-6611.2012.24.056
Gu, Y. N., and Wu, Y. L. (2017). Effects of galangin on airway inflammation and tumor necrosis factor-α expression in asthmatic mice. Chin. J. Gerontol. 37, 1096–1097. doi:10.1016/j.bbrc.2020.03.158
Gui, B., Gao, Z. H., Jia, Z., Li, K. P., Yang, C. Y., Wu, J. H., et al. (2021). Comparative study on gastrointestinal spasmolysis and analgesic effects of different extracts from alpinia officinarum. Tradit. Chin. Drug Res. Clin. Pharmacol. 32, 158–164. doi:10.19378/j.issn.1003-9783.2021.02.002
Gui, S., Jiang, D., and Yuan, J. (2005). Study on antifungal action of volatile oil from pericarpium zanthoxyli and Rhizoma Alpinae Officinarum in vitro. Chin. J. Inf. Tradit. Chin. Med., 21–22. doi:10.3969/j.issn.1005-5304.2005.08.010
Guo, A. J., Xie, H. Q., Choi, R. C., Zheng, K. Y., Bi, C. W., Xu, S. L., et al. (2010). Galangin, a flavonol derived from Rhizoma Alpiniae Officinarum, inhibits acetylcholinesterase activity in vitro. Chem. Biol. Interact. 187, 246–248. doi:10.1016/j.cbi.2010.05.002
Heidari, H., Khalaj, A., Khani, S., Abdollahi, M., Farahani, H., and Khani, S. (2022). Hypoglycemic, hypolipidemic and hepatoprotective effects of Alpinia officinarum on nicotinamide/streptozotocin induced type II diabetic rats. Horm. Mol. Biol. Clin. Investig. 43, 289–296. doi:10.1515/hmbci-2021-0050
Hideji, I., Hiroshi, M., and Ikuko, M. (1985). Diarylheptanoids from the rhizome of alpinia of cinarum hance. Chem. Pharm. Bull. 1985, 4889.
Huang, L. P., Zhong, X. Q., Zhou, X. Y., Deng, M. Q., Wu, M. J., and Deng, M. Z. (2022). Galangin alleviates learning and memory impairments in APP/PS1 double-transgenic mice by regulating Akt/MEF2D/Beclin-1 signaling pathway. China J. Chin. Mater. Medica 47, 2729–2737. doi:10.19540/j.cnki.cjcmm.20211117.705
Huang, S., Yin, A., Luo, Z., and Zhou, H. (2015). Study on the bacteriostasis and antioxidation of Alpinia officinarum Hance volatile oil. Sci. Technol. Food Ind. 36, 112–115. doi:10.13386/j.issn1002-0306.2015.19.014
JiangsuNewMedicalCollege (1999). Great dictionary of Chinese medicine. Shanghai: Shanghai Scientific and Technical Publishers.
Kazemi, S., Asadi, F., Barari, L., Morakabati, P., Jahani, M., Kani, S. N. M., et al. (2022). Quantification of flavonoids in alpinia officinarum hance. Via HPLC and evaluation of its cytotoxicity on human prostate carcinoma (LNCaP) and breast carcinoma (MCF-7) cells. Anticancer Agents Med. Chem. 22, 721–730. doi:10.2174/1871520621666210706142157
Kiuchi, F., Iwakami, S., Shibuya, M., Hanaoka, F., and Sankawa, U. (1992). Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem. Pharm. Bull. (Tokyo) 40, 387–391. doi:10.1248/cpb.40.387
Li, C. Y., Cheng, S. E., Wang, S. H., Wu, J. Y., Hsieh, C. W., Tsou, H. K., et al. (2021). The anti-inflammatory effects of the bioactive compounds isolated from alpinia officinarum hance mediated by the suppression of NF-kappaB and MAPK signaling. Chin. J. Physiol. 64, 32–42. doi:10.4103/CJP.CJP_81_20
Li, F. (2007). Preventive effects of galangin from alpinia officinarun hance on experimental gastric ulcer of animals. Yangzhou University.
Li, X., Zhou, M., Zhu, Z., Wang, Z., Zhang, X., Lu, L., et al. (2024). Kaempferol from Alpinia officinarum hance induces G2/M cell cycle arrest in hepatocellular carcinoma cells by regulating the ATM/CHEK2/KNL1 pathway. J. Ethnopharmacol. 333, 118430. doi:10.1016/j.jep.2024.118430
Li, Z. Z. (2015). Ben cao tong xuan. Revised Edition. Beijing: China Press of Traditional Chinese Medicine.
Liang, W. N., Jiang, T., Chen, Y. F., Feng, Y. F., Chu, B., Fan, H. Q., et al. (2013). Effect of total flavonoids from alpinia officinarum on visceral hypersensitivity in rat model with irritable bowel syndrome and analgesic effects. Chin. J. Exp. Tradit. Med. Formulae 19, 263–267. doi:10.11653/zgsyfjxzz2013070263
Lin, K., Qu, H., Tan, Y., Deng, T., Gao, B., and Wei, N. (2021). Effects of the diphenylheptane extract of Alpinia officinarum rhizomes on ethanol-induced gastric ulcers in mice. Iran. J. Basic Med. Sci. 24, 657–665. doi:10.22038/ijbms.2021.53644.12068
Lin, K., Wang, Y., Gong, J., Tan, Y., Deng, T., and Wei, N. (2020). Protective effects of total flavonoids from Alpinia officinarum rhizoma against ethanol-induced gastric ulcer in vivo and in vitro. Pharm. Biol. 58, 854–862. doi:10.1080/13880209.2020.1803370
Lin, Z. P., Li, Y. T., Liao, X. D., Zhang, J. N., Ye, H., Lin, W. H., et al. (2017) Comparison of antioxidant activity in different components of Alpinia Officinarum extract, 35. Journal of Guangdong Medical University, 606–609+613.
Liu, H., and Ma, Z. (1998). Kai bao ben cao. Revised Edition. Hefei: Anhui Science&Technology Publishing House.
Liu, J. Y., and Liu, Y. W. (2016). A new diarylheptanoid from the rhizomes of alpinia officinarum. Chem. Nat. Compd. 52, 824–826. doi:10.1007/s10600-016-1787-0
Liu, R., Li, H., Wei, N., and Tan, Y. (2021). Simultaneous determination of two galangin metabolites from Alpinia Officinarum Hance in rat plasma by UF LC-MS/MS and its application in pharmacokinetics study. PeerJ 9, e11041. doi:10.7717/peerj.11041
Liu, X., Zhao, Y., Chen, C., and Bai, J. (2010a). Comparison of influence of different extract of alpinia officinanum hance on memory acquisition, consolidation and retrieval processes in mice. Mod. Prev. Med. 37, 4299–4301.
Liu, X. X., Zhao, Y. Y., Chen, C. S., and Bai, J. (2010b). Effects of extract of Alpinia officinarum Hance on learning memory consolidation and free radical in mice. Chin. Tradit. Pat. Med. 32, 1105–1108. doi:10.3969/j.issn.1001-1528.2010.07.006
Liu, Y., Mei, X. F., Wang, J., and Zhang, Y. Q. (2014). Influence of galangin on the apoptosis of human hepatoma cells SMMC-7721 throughPI3KAKT signal pathway. Pharmacol. Clin. Chin. Mater. Medica 30, 40–42. doi:10.13412/j.cnki.zyyl.2014.01.014
Lu, H., Chen, X. W., Yao, H., and Xu, H. L. (2020). Pharmacological effect and cellular mechanism of galangin on uric acid nephropathy. Cent. South Pharm. 18, 1098–1102. doi:10.7539/j.issn.1672-2981.2020.07.003
Luo, Q., Zhu, L., Ding, J., Zhuang, X., Xu, L., and Chen, F. (2015). Protective effect of galangin in Concanavalin A-induced hepatitis in mice. Drug Des. Devel Ther. 9, 2983–2992. doi:10.2147/DDDT.S80979
Luo, Y., and Liu, D. (2020). Inhibitory effect of galangin on different tumor cells. Jilin J. Chin. Med. 40, 948–950. doi:10.13463/j.cnki.jlzyy.2020.07.030
Ly, T. N., Shimoyamada, M., Kato, K., and Yamauchi, R. (2003). Isolation and characterization of some antioxidative compounds from the rhizomes of smaller galanga (Alpinia officinarum Hance). J. Agric. Food Chem. 51, 4924–4929. doi:10.1021/jf034295m
Ly, T. N., Yamauchi, R., Shimoyamada, M., and Kato, K. (2002). Isolation and structural elucidation of some glycosides from the rhizomes of smaller galanga (Alpinia officinarum Hance). J. Agric. Food Chem. 50, 4919–4924. doi:10.1021/jf025529p
Ma, X. Q. (2019). Screening for the anti-gastritis active fraction of Alpinia officinarum and studies on its mechanism. Hubei University of Chinese Medicine.
Matsuda, H., Nakashima, S., Oda, Y., Nakamura, S., and Yoshikawa, M. (2009). Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg Med. Chem. 17, 6048–6053. doi:10.1016/j.bmc.2009.06.057
Ouyang, J., Sun, F., Feng, W., Sun, Y., Qiu, X., Xiong, L., et al. (2016). Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 120, 966–974. doi:10.1111/jam.13073
Ouyang, J., Sun, F., Feng, W., Xie, Y., Ren, L., and Chen, Y. (2018). Antimicrobial activity of galangin and its effects on murein hydrolases of vancomycin-intermediate Staphylococcus aureus (VISA) strain Mu50. Chemotherapy 63, 20–28. doi:10.1159/000481658
Peng, J., Chao, D., and Li, X. (2008). Effect of extraction from rhizoma alpiniae officinarum by CO_2 SFE on ulcer index and gastric secretion and somatostatin about stress ulcer. Anhui Med. Pharm. J., 895–897. doi:10.3969/j.issn.1009-6469.2008.10.005
Pillai, M. K., Young, D. J., and Bin Hj Abdul Majid, H. M. (2018). Therapeutic potential of alpinia officinarum. Mini Rev. Med. Chem. 18, 1220–1232. doi:10.2174/1389557517666171002154123
Qin, Y. H., Pan, Y., Luo, B., Yi, X. L., Zeng, Y. L., Huang, G. R., et al. (2015). Study on the antibacterial effect of extract from wild alpinia officinale. Guangxi Chin. Youjiang Med. J. 43, 647–648.
Sawamura, R., Shimizu, T., Sun, Y., Yasukawa, K., Miura, M., Toriyama, M., et al. (2010). In vitro and in vivo anti-influenza virus activity of diarylheptanoids isolated from Alpinia officinarum. Antivir. Chem. Chemother. 21, 33–41. doi:10.3851/IMP1676
Shen, J., Zhang, H., Xu, B., and Pan, J. (1998). The antioxidative constituents of rhizomes of alpinaia officinarum. Nat. Prod. Res. Dev., 33–36.
Shi, X. P., Li, X. H., and Yang, A. P. (2012). Study on extraction and activity of DPPH free radicals scavenging of total flavonoids from Alpinia officinarum Hance. China Condiment 37, 53–56. doi:10.3969/j.issn.1000-9973.2012.06.014
Shin, D., Kinoshita, K., Koyama, K., and Takahashi, K. (2002). Antiemetic principles of Alpinia officinarum. J. Nat. Prod. 65, 1315–1318. doi:10.1021/np020099i
Song, H. K., Park, S. H., Kim, H. J., Jang, S., and Kim, T. (2021). Alpinia officinarum water extract inhibits the atopic dermatitis-like responses in NC/Nga mice by regulation of inflammatory chemokine production. Biomed. Pharmacother. 144, 112322. doi:10.1016/j.biopha.2021.112322
Song, Y., Zhao, Q., Cao, Y., and Shen, Z. L. (2012). Study on proliferating effect and the mechanism of Human osteosarcoma cell lines MG-63 induced by Alpinetin. China Med. Eng. 20, 56–58.
Su, Y., Chen, Y., Liu, Y., Yang, Y., Deng, Y., Gong, Z., et al. (2016). Antiosteoporotic effects of Alpinia officinarum Hance through stimulation of osteoblasts associated with antioxidant effects. J. Orthop. Transl. 4, 75–91. doi:10.1016/j.jot.2015.09.009
Sun, Y. Y., Feng, J., Wei, J. H., Zhan, Z. L., and Liu, Y. Y. (2023). Herbal textual research on alpiniae officinarum rhizoma in famous classical formulas. Chin. J. Exp. Tradit. Med. Formulae 29, 94–103. doi:10.13422/j.cnki.syfjx.20220457
Sun, Y., Tabata, K., Matsubara, H., Kitanaka, S., Suzuki, T., and Yasukawa, K. (2008). New cytotoxic diarylheptanoids from the rhizomes of Alpinia officinarum. Planta Med. 74, 427–431. doi:10.1055/s-2008-1034345
Tan, Y. F., Li, H. L., Li, Y. B., Li, Y. H., Lai, W. Y., Wang, Y., et al. (2015). Identification of chemical constituents occurring in leaves of alpinia officinarum. Chin. J. Exp. Tradit. Med. Formulae 21, 37–40. doi:10.13422/j.cnki.syfjx.2015030037
Tang, L., Lu, X. Y., Xie, H. Y., and Zhang, J. D. (2021). Study on extraction and antibacterial effect of volatile oil from galangal. Guangdong Chem. Ind. 48, 49–50+4. doi:10.3969/j.issn.1007-1865.2021.13.021
Tao, H. (1986). Ming yi bie lu. Revised Edition. Beijing: People's Health Publishing House Co., Ltd.
Tao, H. (1994). Ben mao jin xi zhu. Revised Edition. Beijing: People's Health Publishing House Co., Ltd.
Tian, Z., An, N., Zhou, B., Xiao, P., Kohane, I. S., and Wu, E. (2009). Cytotoxic diarylheptanoid induces cell cycle arrest and apoptosis via increasing ATF3 and stabilizing p53 in SH-SY5Y cells. Cancer Chemother. Pharmacol. 63, 1131–1139. doi:10.1007/s00280-008-0832-5
Tushar, S. B., Sarma, G. C., and Rangan, L. (2010). Ethnomedical uses of zingiberaceous plants of northeast India. J. Ethnopharmacol. 132, 286–296. doi:10.1016/j.jep.2010.08.032
Uehara, S. I., Yasuda, I., Akiyama, K., Morita, H., Takeya, K., and Itokawa, H. (1987). Diarylheptanoids from the rhizomes of curcuma xanthorrhiza and alpinia officinarum. Chem. and Pharm. Bull. 35, 3298–3304. doi:10.1248/cpb.35.3298
Wang, G. H., Tang, S. P., Peng, M. J., Ma, G. Z., Wu, P. J., Yang, Z., et al. (2017). Study on the antioxidant and antimicrobial activities of four flavonol compounds from Alpinia officinarum Hance rhizome in vitro. Food and Mach. 33, 168–172. doi:10.13652/j.issn.1003-5788.2017.05.034
Wang, H. Y., Liu, Y. M., Li, H. Y., Feng, Q. J., Guo, J. Y., and Niu, X. (2011). Effect of oils in alpinia officinarum hance on serum NO, SOD, MDA in gastrelcosis mice model. China J. Tradit. Chin. Med. Pharm. 26, 1640–1642.
Wang Haiyan, L. Y., Haiyan, L., Qianjin, F., Guo, J., and Niu, X. (2011). Effects of oils in alpinia officinarum hance on serum motilin, somatostatin, substance P, vasoactive intestinal peptide in gastrelcosis mice mode. Chin. J. Exp. Tradit. Med. Formulae 17, 105–107. doi:10.13422/j.cnki.syfjx.2011.04.038
Wei, N. (2019). Studies on anti-ulcer effect and its effective substances of the rhizomes of Alpinia Offifinarum Hance. Heilongjiang University of Chinese Medicine.
Wei, N., Wang, Y., Wei, Q., Zhang, X. G., Zhang, J. Q., and Huang, Y. (2018). Chemical constituents from n-butanol fraction of alpinia officinarum. Mod. Chin. Med. 20, 26–28. doi:10.13313/j.issn.1673-4890.20170629003
Wei, N., Zhou, Z., Wei, Q., Wang, Y., Jiang, J., Zhang, J., et al. (2016). A novel diarylheptanoid-bearing sesquiterpene moiety from the rhizomes of Alpinia officinarum. Nat. Prod. Res. 30, 2344–2349. doi:10.1080/14786419.2016.1185716
Wen, H., Kuang, Y., Lian, X., Li, H., Zhou, M., Tan, Y., et al. (2024). Physicochemical characterization, antioxidant and anticancer activity evaluation of an acidic polysaccharide from alpinia officinarum hance. Molecules 29, 1810. doi:10.3390/molecules29081810
Wu, Q., Cao, D., Huang, P., and Xiang, L. (2004a). Effect of extraction from rhizoma alpiniae officinarum by CO2 SFE on ulcer index and epidermal grouth factor and interleukin-2 in stress ulcer rat. Pharmacol. Clin. Chin. Mater. Medica, 17–18. doi:10.3969/j.issn.1001-859X.2004.06.010
Wu, Q., Lin, R., and Zeng, X. (2004b). Study on the cholinergic mechanism of the intestinal spasmolysis effect of the supercritical extract of Alpinia officinale. J. Chin. Med. Mater., 739–741.
Wu, Y. (2001). Ben cao cong xin. Revised Edition. Beijing: Traditional Chinese Medicine Classics Press.
Xia, D. Z., Jin, X. G., Lu, C., Xu, L. P., and Sun, Y. T. (2013). Protective effect of total flavonoids from alpinia officinarum hance on brain and kidney oxidative stress in mice exposed to lead acetate. J. Zhejiang Chin. Med. Univ. 37, 1018–1022. doi:10.16466/j.issn1005-5509.2013.08.030
Xia, D., Li, J., Liu, J., Wang, H., and Ren, Q. (2009). The ultrasonic extraction and antioxidant effects of total flavonoids from alpinia officinarum hance in vitro. J. Chin. Inst. Food Sci. Technol. 9, 63–69. doi:10.16429/j.1009-7848.2009.03.030
Xianhua Du, X. N., Xu, R., Du, H., and Feng, Q. (2008). Comparison of pharmacokinetics of galangin in Liangfu microemulsion and Liangfu pills. Journal of Beijing University of Traditional Chinese Medicine, 269–272. doi:10.3321/j.issn:1006-2157.2008.04.015
Xin Zhang, X. C., Lu, H., and Xu, H. (2021). In situ intestinal absorption and pharmacokinetics of galangin self-emulsion in rats. Traditional Chin. Drug Res. and Clin. Pharmacol. 32, 1699–1704. doi:10.19378/j.issn.1003-9783.2021.11.014
Xu, Q., Yu, L., Zhang, X., and Chen, R. (1991). Effects of Alpinia officinale and its main components on experimental thrombosis and coagulation system Shaanxi. J. Tradit. Chin. Med., 232–233.
Yan, B., Bai, X. L., Hu, J. Y., Jian, X. N., Jiang, X. H., and Deng, W. L. (2013). Antipyretic and anti-inflammatory effects of galangal. Pharmacol. Clin. Chin. Mater. Medica 29, 85–87.
Yang, J. J., Wang, H. B., Liao, H. H., Wu, H. M., and Tang, Q. Z. (2020). Galangin alleviates pressure overload induced cardiac fibrosis. Med. J. Wuhan. Univ. 41, 889–893. doi:10.14188/j.1671-8852.2019.1009
Yuan, Y., Zhou, W., Fu, Y. F., Lin, L. J., Huang, X. B., and Li, J. H. (2016). Analysis of volatile oils in rhizoma alpiniae officinarum by GC-MS and retention index. FENXI CESHI XUEBAO( J. Instrum. Anal.) 35, 692–697. doi:10.3969/j.issn.1004-4957.2016.06.010
Zhai, H., WangZhang, H., CaiCai, H., Mei, W., and Dai, H. (2014b). Neuroprotective effect of extracts from alpinia officinarum hance on PC12 cells. J. Trop. Biol. 5, 78–83. doi:10.15886/j.cnki.rdswxb.2014.01.006
Zhai, H., L., Q., W., H., Z., Y., C., C., M., W., et al. (2014a). Chemical constituents and biological activities of flowers and fruits of alpinia officinarum hance. J. Trop. Biol. 5, 194–198. doi:10.15886/j.cnki.rdswxb.2014.02.007
Zhang, B. B., Dai, Y., Liao, Z. X., and Ding, L. S. (2010). Three new antibacterial active diarylheptanoids from Alpinia officinarum. Fitoterapia 81, 948–952. doi:10.1016/j.fitote.2010.06.015
Zhang, H. T., Wu, J., Wen, M., Su, L. J., and Luo, H. (2012). Galangin induces apoptosis in hepatocellular carcinoma cells through the caspase 8/t-Bid mitochondrial pathway. J. Asian Nat. Prod. Res. 14, 626–633. doi:10.1080/10286020.2012.682152
Zhang, L. (2007). Ben jing feng yuan. Revised Edition. Beijing: China Press of Traditional Chinese Medicine.
Zhao, J., Lv, W., Duan, H., and Jiang, L. (2007). Screening of active compounds against Candida albicans from Alpinia officinarum. J. Shanxi Med. U Niv., 604–606. doi:10.3969/j.issn.1007-6611.2007.07.009
Zhao, Y. Y., Liu, X., Chen, C. S., and Bai, J. (2010). Effects of Alpinia officinale extract on learning and memory abilities and cholinergic nervous system function in mice. Pharmacol. Clin. Chin. Mater. Medica 26, 49–52. doi:10.13412/j.cnki.zyyl.2010.02.026
Zheng, X., Zhang, Y., Tan, Y., Li, Y., Xue, Q., Li, H., et al. (2024). Alpinia officinarum Hance extract ameliorates diabetic gastroparesis by regulating SCF/c-kit signaling pathway and rebalancing gut microbiota. Fitoterapia 172, 105730. doi:10.1016/j.fitote.2023.105730
Zhou, Y., Li, X. Y., Xiong, T. Q., Chen, X. J., and Pan, L. X. (2012). Protective effect of Alpinia officinarum Hance on alcohol-induced acute liver injury in mice. J. North Pharm. 9, 30–31.
Keywords: Alpinia officinarum Hance, traditional uses, phytochemistry, pharmacology, pharmacokinetic
Citation: Lei X, Wang J, Zuo K, Xia T, Zhang J, Xu X, Liu Q and Li X (2024) Alpinia officinarum Hance: a comprehensive review of traditional uses, phytochemistry, pharmacokinetic and pharmacology. Front. Pharmacol. 15:1414635. doi: 10.3389/fphar.2024.1414635
Received: 09 April 2024; Accepted: 01 August 2024;
Published: 16 August 2024.
Edited by:
Laiba Arshad, Forman Christian College, PakistanReviewed by:
Verena Spiegler, University of Münster, GermanySabi Ur Rehman, Forman Christian College, Pakistan
Copyright © 2024 Lei, Wang, Zuo, Xia, Zhang, Xu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoliang Li, bGl4aWFvbGlhbmctMTk4NEAxNjMuY29t
 Xia Lei
Xia Lei Jiapeng Wang2,3
Jiapeng Wang2,3 Xiaoliang Li
Xiaoliang Li