
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 11 December 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1414221
 Ting-Yu Lin1,2
Ting-Yu Lin1,2 Jiun-Ling Wang3,4
Jiun-Ling Wang3,4 Grace Hsin-Min Wang5
Grace Hsin-Min Wang5 Yu-Yun Huang6
Yu-Yun Huang6 Ming-Ching Chen1
Ming-Ching Chen1 Yaa-Hui Dong1,7*
Yaa-Hui Dong1,7* Wei-Hsuan Lo-Ciganic8,9,10,11
Wei-Hsuan Lo-Ciganic8,9,10,11Background: Although biological plausibility suggests that fluoroquinolones could lead to rhegmatogenous retinal detachment (RRD) through collagen degradation, real-world evidence on their relative risk of RRD is inconsistent, with limited information on absolute risk estimates.
Objective: The study aimed to estimate the RRD risk associated with fluoroquinolones versus other antibiotics with similar indications (i.e., comparison antibiotics).
Methods: We conducted a retrospective cohort study analyzing claims data from adult patients who initiated fluoroquinolones or amoxicillin/clavulanate or ampicillin/sulbactam or extended-spectrum cephalosporins using the Taiwan National Health Insurance Research Database (2009–2018) and the United States IBM MarketScan Database (2011–2020). Patients were followed for up to 90 days after cohort entry. For each country’s data, after 1:1 propensity score (PS) matching, we used Cox regression models to estimate RRD risks, presented with hazard ratios (HR) with 95% confidence interval (95% CI). We used random-effects meta-analyses to derive pooled HRs across both counties.
Results: Of 24,172,032 eligible patients comprising 7,944,620 insured Taiwanese (mean age [SD], 46 [18] years; 45% male) and 16,227,412 United States commercially insured individuals (mean age [SD], 47 [16] years; 40% male), 10,137,468 patients initiated fluoroquinolones, 10,203,794 initiated amoxicillin/clavulanate or ampicillin/sulbactam, and 3,830,770 initiated extended-spectrum cephalosporins. After PS matching, similar RRD incidence rates were observed between fluoroquinolones and amoxicillin/clavulanate or ampicillin/sulbactam users (0.33 [95% CI, 0.19–0.56] versus 0.35 [95% CI, 0.26–0.46] per 1,000 person-years), yielding an HR of 0.97 (95% CI, 0.76–1.23). The RRD incidence rates were also similar comparing fluoroquinolones to extended-spectrum cephalosporins (0.36 [95% CI, 0.22–0.57] versus 0.34 [95% CI, 0.22–0.50] per 1,000 person-years; HR, 1.08 [95% CI, 0.92–1.27]). The comparative safety profiles remained consistent by country, various patient characteristic (e.g., diabetes or ophthalmic conditions), type of fluoroquinolones, follow-up duration, or treatment setting.
Conclusion: This large-scale study, leveraging real-world data from Taiwan and the United States, showed a low and comparable RRD risk among adults who initiated fluoroquinolones or other antibiotics with similar indications. This suggests that the RRD risk should not deter the use of fluoroquinolone when clinically indicated.
Fluoroquinolones are antibiotics known for their effective tissue penetration; they are widely prescribed for various infections, including lower respiratory tract infections and genitourinary tract infections (Metlay et al., 2019; Kalil et al., 2016; Gupta et al., 2011; Chou et al., 2019; Taiwan Urological Association, 2022; Choe et al., 2018; Van Boeckel et al., 2014). Rhegmatogenous retinal detachment (RRD), the predominant type of retinal detachment (RD), involves a retinal break which allows vitreous fluid to enter the subretinal space, leading to the separation of the neurosensory retina from the retinal pigment epithelium (UpToDate®, 2023). The RRD incidence rate is low, ranging from 0.098 to 0.26 per 1,000 person-years (Park et al., 2021; Chen et al., 2016; Achour et al., 2022; van Leeuwen et al., 2021). Nevertheless, without appropriate management, it may lead to significant visual impairment. The known risk factors for RRD include older age, male gender, family history, ocular trauma, intra-ocular surgery, and existing ophthalmic disorders (UpToDate®, 2023; Sodhi et al., 2008; Mitry et al., 2010a).
There have been mixed reports about the association between oral fluoroquinolone use and increased RD risk (mainly RRD) (Chui et al., 2014; Raguideau et al., 2016; Shin et al., 2018; Baek et al., 2018; Londhe et al., 2022; Etminan et al., 2012; Fife et al., 2014; Choi et al., 2018; Taher et al., 2022; Pasternak et al., 2013; Kuo et al., 2014; Eftekhari et al., 2014; Kapoor et al., 2014; Daneman et al., 2015), possibly through degrading collagen in the ocular structures (Ghazi and Green, 2002; Ponsioen et al., 2008; Mitry et al., 2010b). However, the results of these real-world studies using various designs were inconsistent. Notably, most studies comparing use versus non-use of fluoroquinolones observed a 1.3- to 4.5-fold higher risk (Chui et al., 2014; Raguideau et al., 2016; Shin et al., 2018; Baek et al., 2018; Londhe et al., 2022; Etminan et al., 2012; Fife et al., 2014; Pasternak et al., 2013; Daneman et al., 2015), while a couple of studies comparing the use of fluoroquinolones versus other comparable antibiotics suggested a null association (Eftekhari et al., 2014; Kapoor et al., 2014). These raise methodological issues about potential residual confounding when utilizing a non-user comparison design. Moreover, the clinical challenge for physicians is not the use or non-use of fluoroquinolones but which alternative antibiotics should be prescribed if any concerns about adverse drug reaction exist. However, little information exists on the RRD incidence rates associated with fluoroquinolones and other antibiotics with similar indications (Pasternak et al., 2013; Eftekhari et al., 2014; Daneman et al., 2015).
There has been an increasing use of multi-databases from different healthcare systems or countries to elucidate drug safety issues because this facilitates the identification of rare adverse outcomes and improves the generalizability of findings (Dong et al., 2018; Pradhan et al., 2022; Maro and Toh, 2022). This retrospective cohort study aims to estimate the RRD incidence rates associated with fluoroquinolones and other antibiotics with similar indications using two nationwide representative claims databases from Taiwan and the United States.
We identified eligible patients from (1) the Taiwan National Health Insurance Research Database (NHIRD), which comprises the data of approximately 23 million residents enrolled in a single-payer national health insurance system (National Health Insurance Administration, 2023; Lin et al., 2018) and (2) the United States IBM MarketScan Database that comprises data from more than 200 million beneficiaries enrolled in employer-sponsored healthcare programs since 1995 (Butler et al., 2021). See the online data supplement for detailed descriptions of data sources.
We used a common protocol to conduct this study for both databases. Given the inherent variations in healthcare systems and data structures across countries, certain database-specific modifications were required (Supplementary Table S1). For example, both the Taiwan NHIRD and United States IBM MarketScan Database contain pharmacy dispensing claims from outpatient visits. However, only the Taiwan NHIRD provides information on drug use at emergency department (ED) visits and during hospitalization, including oral and injectable study antibiotics. Therefore, steps involving medication use in outside outpatient settings or injectable medication use were only applied to the Taiwanese data.
From each database, we identified patients aged ≥18 years who initiated oral fluoroquinolones, amoxicillin/clavulanate or ampicillin/sulbactam, or extended-spectrum cephalosporins (second-, third-, or fourth-generation cephalosporins) at outpatient, ED, or inpatient visits in corresponding cohort identification periods (Taiwan NHIRD, 2009/1/1–2018/8/31; United States IBM MarketScan, 2011/1/1–2020/9/30). The cohort entry date was defined as the date of the first dispensing of a study antibiotic (codes in Supplementary Table S2). We determined amoxicillin/clavulanate or ampicillin/sulbactam and extended-spectrum cephalosporins as comparison antibiotics, given that their indications are generally similar to those of fluoroquinolones according to the treatment guidelines in Taiwan, other Asian regions, and the United States (Metlay et al., 2019; Kalil et al., 2016; Gupta et al., 2011; Chou et al., 2019; Taiwan Urological Association, 2022; Choe et al., 2018).
We applied several exclusion criteria (for details, see the online data supplement and Supplementary Table S3). Patients with more severe infections are likely to receive injectable treatment, so we excluded patients initiating oral and injectable study antibiotics simultaneously at cohort entry to mitigate confounding by infection severity. We also excluded patients with any history of RD or retinal defects or ever having received RD-related clinical management (scleral buckling, cryotherapy, laser therapy, pneumatic retinopexy, and vitrectomy mainly) to prevent confounding by disease history. Notably, patients who were not at risk of RRD, including those who were blind or receiving evisceration or enucleation of eyeball, were also excluded from the analysis.
We followed up patients from cohort entry to the first occurrence of RRD, death, disenrollment, or the study’s end within 90 days of treatment initiation (Taiwan NHIRD, 2018/8/31; U.S. IBM MarketScan, 2020/9/30). Previous cohort studies examining fluoroquinolones-related RRD had a wide range of follow-up durations, including 30 (Daneman et al., 2015), 90 (Kuo et al., 2014), 180 (Pasternak et al., 2013), or 365 days (Eftekhari et al., 2014; Kapoor et al., 2014). To ensure a sufficient number of events and minimize potential exposure misclassification, our main analysis truncated the follow-up at 90 days post-cohort entry. We also conducted additional sensitivity analyses to explore variations in follow-up duration.
Due to the absence of validated claims-based codes for RRD, our study team—comprising experts in ophthalmology, pharmacy, and epidemiology—conducted a thorough review of existing observational studies and adopted clinically relevant codes for our analysis. We defined RRD as the first occurrence of any relevant International Classification of Diseases, 9th or 10th Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) codes recorded in any diagnosis position during an outpatient, ED, or inpatient visit within 90 days of cohort entry. To enhance the outcome validity, we only considered cases with RD-related clinical management 14 days before or after the diagnosis date (Supplementary Table S4).
We measured ≥80 potential confounders, including age at cohort entry, sex, acute infection diagnoses (which may serve as indications for study antibiotics), individual comorbidities, Deyo version of Charlson comorbidity index (Deyo et al., 1992; Sundararajan et al., 2004), adapted Diabetes Complications Severity Index (Chang HY. et al., 2012; Glasheen et al., 2017), medications, and resource utilization that may be associated with both study antibiotic use and RRD risk. Our analysis differs from previous studies (Chui et al., 2014; Raguideau et al., 2016; Shin et al., 2018; Baek et al., 2018; Londhe et al., 2022; Etminan et al., 2012; Fife et al., 2014; Choi et al., 2018; Taher et al., 2022; Pasternak et al., 2013; Kuo et al., 2014; Eftekhari et al., 2014; Kapoor et al., 2014; Daneman et al., 2015) by including a more comprehensive list of ophthalmic disorders (e.g., endophthalmitis, myopia, glaucoma, cataract, diabetic retinopathy, disorders of vitreous body, severe eye trauma, intravitreal injection, and intraocular surgery), ophthalmic medications (e.g., anti-bacterial, anti-viral, and corticosteroid eye drops), and resource utilization specific to ophthalmic diseases. These characteristics were identified using diagnosis files or pharmacy dispensing records derived from outpatient, ED, or inpatient claims within 7 days before cohort entry or at cohort entry (acute infection diagnoses) or within 180 days before cohort entry or at cohort entry (comorbidities, other medication use, and resource utilization). Supplementary Tables S5 and S6 provide detailed covariate information.
Using the above covariates, we estimated baseline propensity scores (PS) with multivariable logistic regression models to predict the probability of initiating fluoroquinolones versus comparison antibiotics (either amoxicillin/clavulanate or ampicillin/sulbactam or extended-spectrum cephalosporins). To balance baseline characteristics between study antibiotic groups, we applied a 1:1 PS-matched design for identifying comparison antibiotic initiators, with a nearest-neighbor algorithm without replacement and with a maximum matching caliper 0.025 times the standard deviation (SD) of the logits of PS (SAS Institute Inc, 2023). To assess whether the covariate distributions were balanced after PS matching, we computed absolute standardized mean differences (aSMD) for each covariate, with a value < 0.1 indicating balance between study antibiotic groups (Austin, 2011).
We applied a Poisson distribution to estimate the incidence rate and the 95% confidence interval (CI) of RRD for each study antibiotic group. We used Cox proportional hazard models to estimate the hazard ratio (HR) with 95% CI of RRD comparing fluoroquinolone versus comparison antibiotics. All analyses were conducted by comparison antibiotics (amoxicillin/clavulanate or ampicillin/sulbactam and extended-spectrum cephalosporins). Given the large size of our study populations and the potential for changes in antibiotic prescriptions over time, we adapted our approach to account for these variations. Specifically, PS matching was conducted separately for each calendar year of cohort entry. We pooled matched cohorts across calendar years and used Cox models stratified on calendar year to estimate summary HRs and corresponding 95% CIs.
For each country database, we first identified study cohorts, extracted information on covariates, fitted PS models, performed PS matching, and estimated outcome occurrence separately. We then used the Wald test to examine whether the effect estimates were statistically significant heterogeneity by database (p-value <0.05). To present overall findings across databases, we computed aSMD across databases for each covariate, using pooled means and SD. We also generated pooled incidence rates and pooled HRs with corresponding 95% CIs across databases with inverse variance weighting under random-effects models (Cochrane Training, 2023).
We performed several pre-specified analyses to ensure the robustness of our findings. First, we generated pooled outcome estimates across databases with inverse variance weighting under fixed-effect models. We then estimated rate differences with corresponding 95% CIs to provide intuitive, clinical insight. Third, we conducted sensitivity analyses by varying the follow-up duration to 60, 30, and 14 days to mitigate the potential impact from exposure misclassification. After that, we estimated incidence rates and HRs in each patient subgroup stratified on age at cohort entry (>65 or ≤65 years), sex (male and female), diabetes (yes or no), and ophthalmic comorbidities or medication use (yes or no). We then evaluated whether the incidence rates and association varied across different fluoroquinolones. Finally, using Taiwan NHIRD with information on where antibiotics were prescribed (outpatient visits, ED visits, or during hospitalizations), we examined whether the risk associated with fluoroquinolones changed materially by treatment setting.
For subgroup analyses by patient characteristic, fluoroquinolone, and treatment setting, we re-estimated PS and re-matched patients within each subgroup (Rassen et al., 2012; Wang et al., 2018).
Among 24,172,032 eligible patients comprising 7,944,620 insured Taiwanese (mean age [SD], 46 [18] years; 45% male) and 16,227,412 United States commercially insured individuals (mean age [SD], 47 [16] years; 40% male), 10,137,468 initiated fluoroquinolones, 10,203,794 initiated amoxicillin/clavulanate or ampicillin/sulbactam, and 3,830,770 initiated extended-spectrum cephalosporins (Figure 1; Supplementary Table S7). Overall, ciprofloxacin (55%) was the most commonly used fluoroquinolone, followed by levofloxacin (26%) and ofloxacin (12%). Amoxicillin/clavulanate (99%) predominated among the groups of amoxicillin/clavulanate or ampicillin/sulbactam. Cefdinir (32%), cefuroxime (29%), and cefaclor (27%) were the top three prescribed extended-spectrum cephalosporins (Supplementary Table S8).
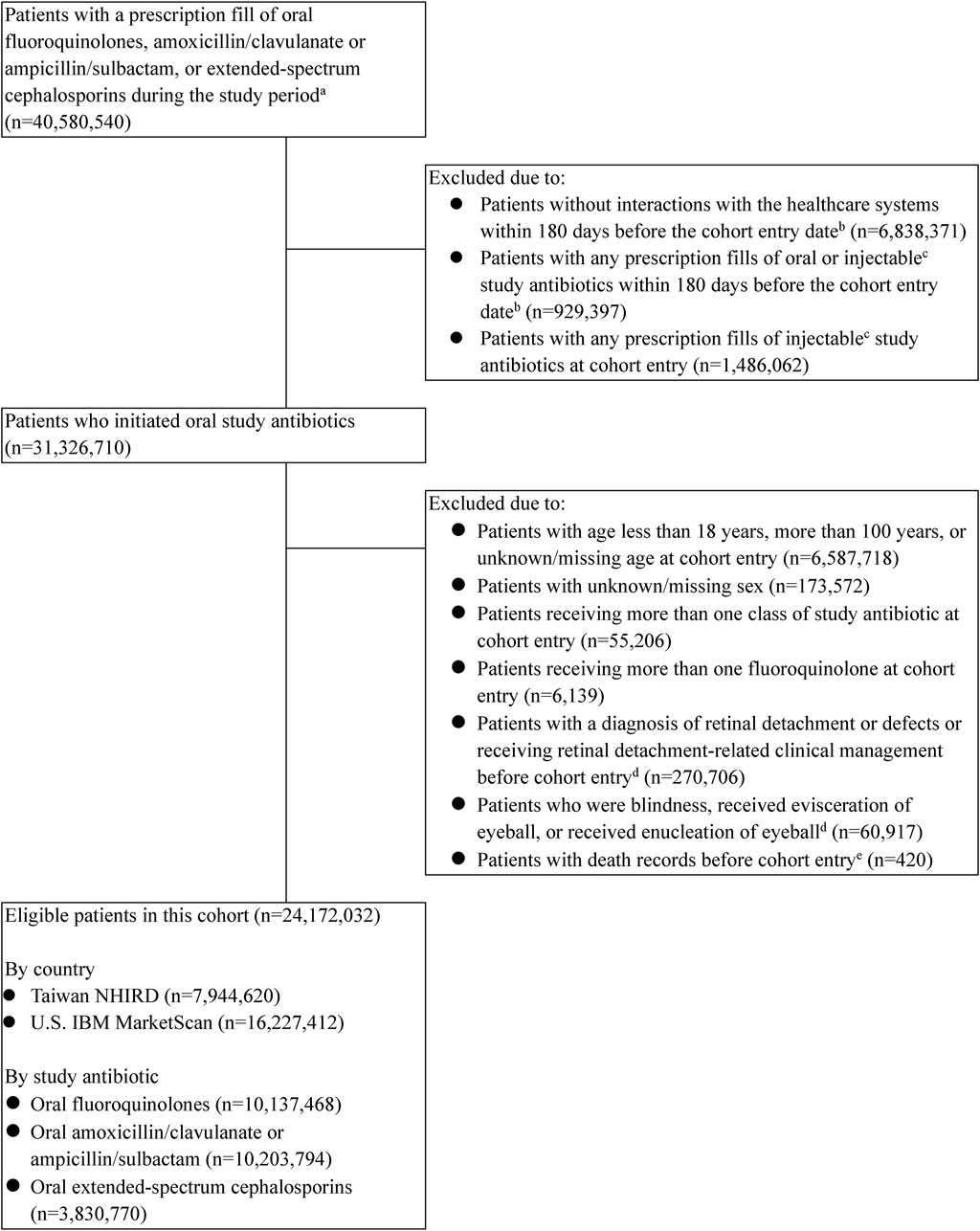
Figure 1. Flowchart of the study cohort assembly. U.S.—United States. aPatients with study antibiotic use were identified from the Taiwanese data between 2009/1/1 and 2018/8/31 and from the U.S. data between 2011/1/1 and 2020/9/30. bThe cohort entry date was defined as the date of the first dispensing of a study antibiotic. cExclusion of baseline injectable antibiotic use was only conducted in the Taiwanese data. dInformation on retinal detachment or defects, retinal-related clinical management, blindness, or evisceration or enucleation of eyeball was examined as early as before the cohort entry (2008/1/1 for Taiwanese data and 2010/1/1 for the U.S. data). eDeath records were only available in the Taiwanese data.
Before PS matching, fluoroquinolone initiators were older (mean age: 49 years versus 44 years) but had less males (39% versus 45%) than amoxicillin/clavulanate or ampicillin/sulbactam initiators. In terms of comorbidities, fluoroquinolone initiators were more likely to have genitourinary tract infections, intra-abdominal infections, hypertension, hyperlipidemia, diabetes, and a higher Deyo version of the Charlson comorbidity index than amoxicillin/clavulanate or ampicillin/sulbactam initiators. Fluoroquinolone initiators were also more likely to receive angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, beta blocking agents, diuretics, and statins, and they had more frequent outpatient visits (Table 1; Supplementary Tables S9, S10). Similar findings were observed when comparing fluoroquinolone initiators and extended-spectrum cephalosporin initiators (Table 2; Supplementary Tables S11, S12).
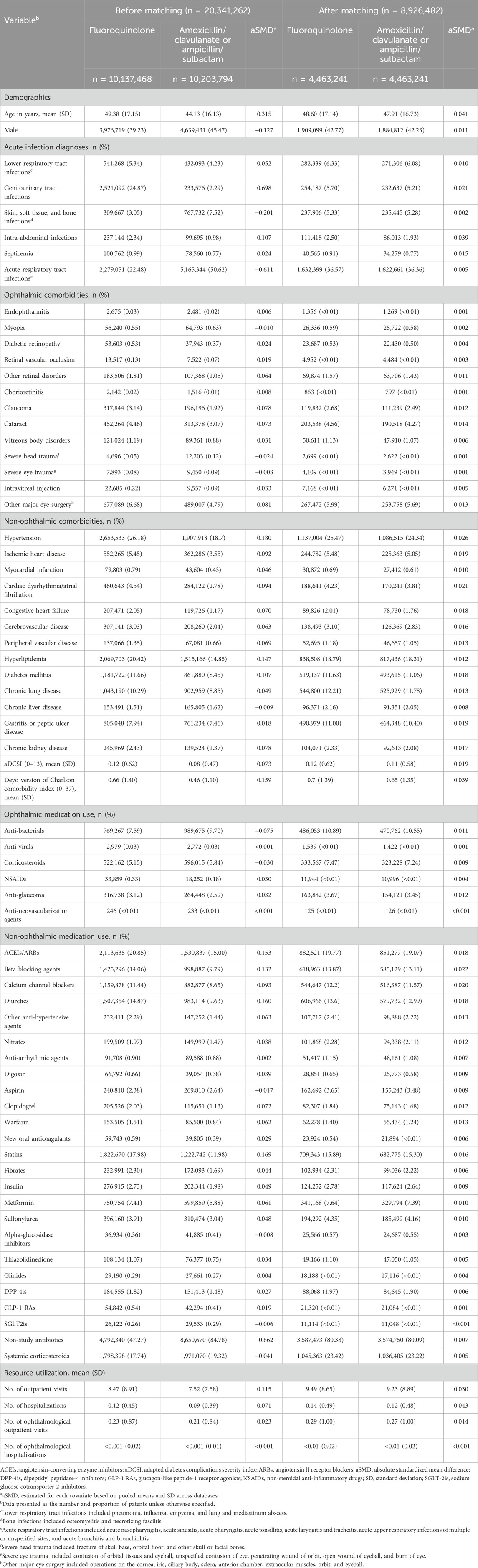
Table 1. Select patient characteristics among initiators of fluoroquinolones and initiators of amoxicillin/clavulanate or ampicillin/sulbactam before and after propensity score matching.
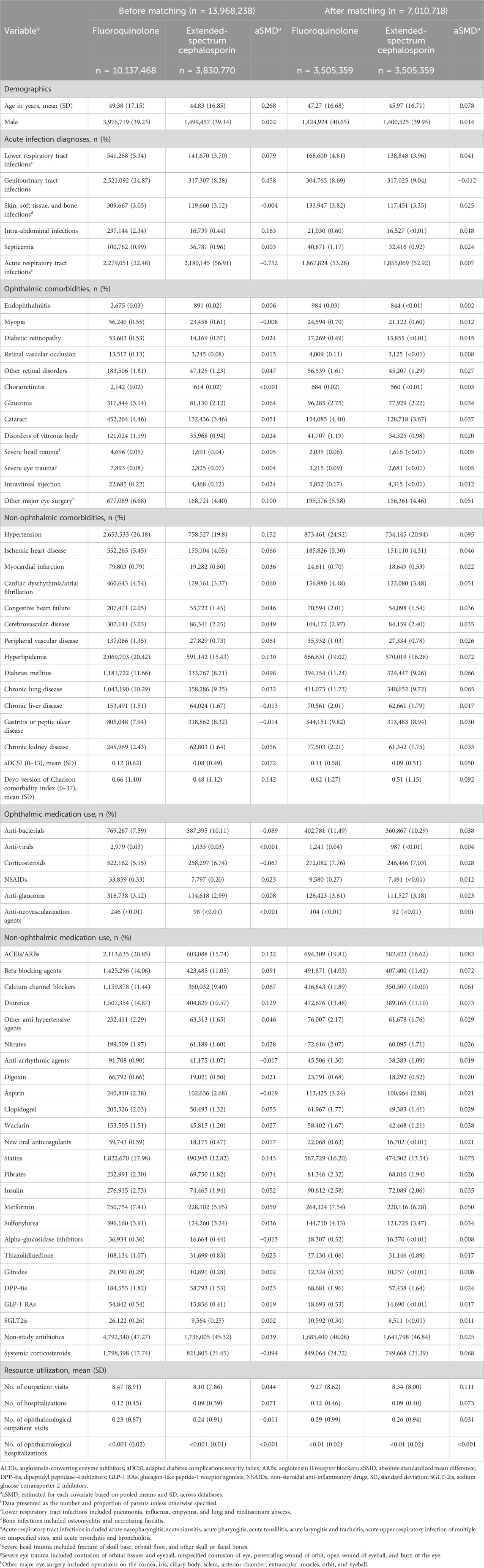
Table 2. Selected patient characteristics among initiators of fluoroquinolones and initiators of extended-spectrum cephalosporins before and after propensity score matching.
Fluoroquinolones or amoxicillin/clavulanate or ampicillin/sulbactam were initiated by 8,926,482 patients, and 7,010,718 patients initiating fluoroquinolones or extended-spectrum cephalosporins in each pairwise PS-matched cohort. The aSMD between fluoroquinolones and comparison antibiotics after PS matching were <0.1, indicating well-balanced baseline characteristics after PS matching (Tables 1, 2; Supplementary Tables S9, S12).
Overall, 2,077 patients experienced RRD within 90 days of cohort entry. The crude incidence rates were 0.34 (95% CI, 0.18–0.65) for fluoroquinolones, 0.32 (95% CI, 0.29–0.35) for amoxicillin/clavulanate or ampicillin/sulbactam, and 0.32 (95% CI, 0.23–0.43) for extended-spectrum cephalosporins per 1,000 person-years (Tables 3, 4; Supplementary Tables S13, S14). The crude HRs for fluoroquinolones were 1.06 (95% CI, 0.59–1.90) versus amoxicillin/clavulanate or ampicillin/sulbactam, and 1.06 (95% CI, 0.72–1.57) versus extended-spectrum cephalosporins (Tables 3, 4; Supplementary Tables S15, S16).
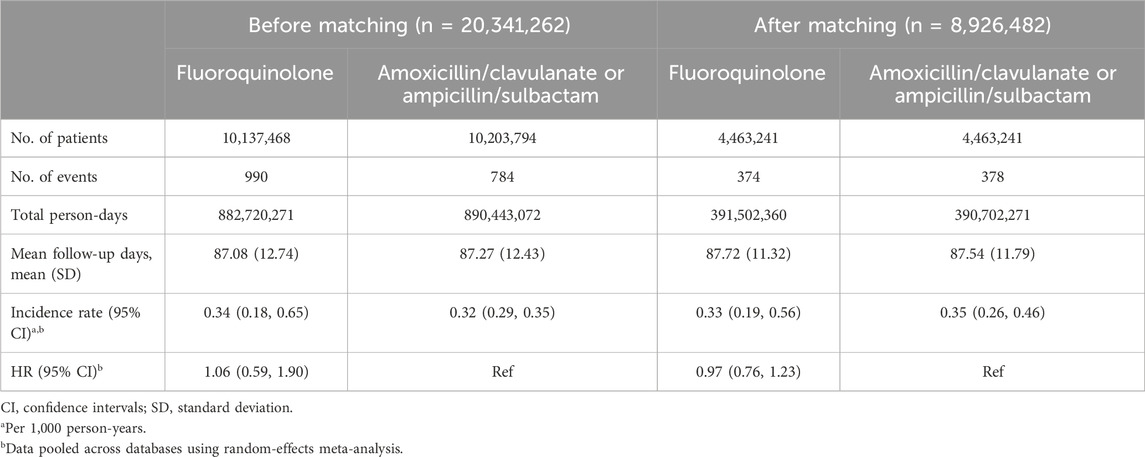
Table 3. Risk of rhegmatogenous retinal detachment comparing fluoroquinolones to amoxicillin/clavulanate or ampicillin/sulbactam before and after propensity score matching.
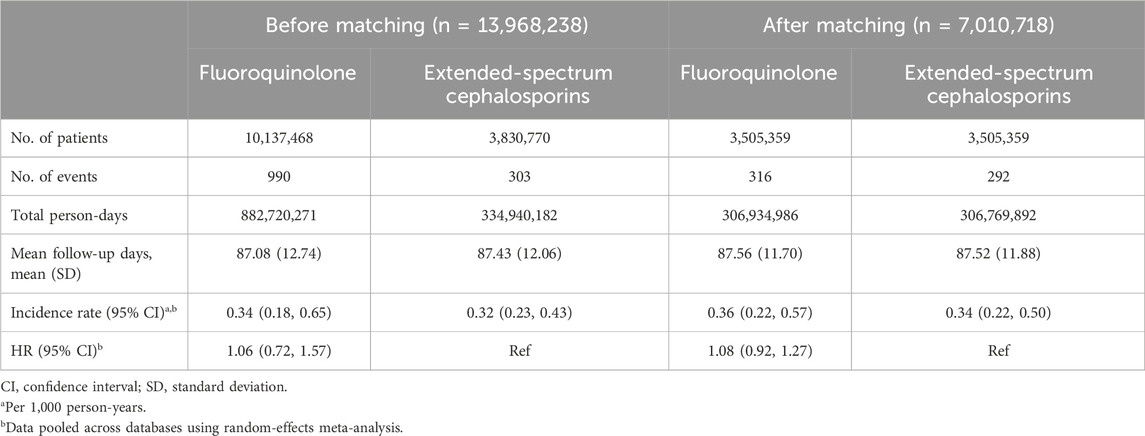
Table 4. Risk of rhegmatogenous retinal detachment comparing fluoroquinolones to extended-spectrum cephalosporins before and after propensity score matching.
After PS matching, similar RRD incidence rates were observed between fluoroquinolones and amoxicillin/clavulanate or ampicillin/sulbactam initiators (0.33 [95% CI, 0.19–0.56] versus 0.35 [95% CI, 0.26–0.46] per 1,000 person-years), yielding a HR of 0.97 (95% CI, 0.76–1.23) (Table 3). The RRD incidence rates were also similar when comparing fluoroquinolones to extended-spectrum cephalosporins (0.36 [95% CI, 0.22–0.57] versus 0.34 [95% CI, 0.22–0.50] per 1,000 person-years; HR, 1.08 [95% CI, 0.92–1.27]) (Table 4).
For each country database’s effect estimates after PS matching, HRs for fluoroquinolones versus amoxicillin/clavulanate or ampicillin/sulbactam were 0.84 (95% CI, 0.66–1.07) in the Taiwan NHIRD and 1.08 (95% CI, 0.90–1.29) in the United States IBM MarketScan Database (p-value for heterogeneity testing: 0.101, Supplementary Table S15). Similarly, HRs for fluoroquinolones versus extended-spectrum cephalosporins did not suggest differential risk across databases (1.02 [95% CI, 0.77–1.35] in the Taiwanese data and 1.11 [95% CI, 0.92–1.35] in the United States data; p-value for heterogeneity testing: 0.628; Supplementary Table S16).
The HRs after PS matching for fluoroquinolones using fixed-effect models were similar to main analyses using random-effects models (0.99 [95% CI, 0.85–1.14] versus amoxicillin/clavulanate or ampicillin/sulbactam and 1.08 [95% CI, 0.92–1.27] versus extended-spectrum cephalosporins). The rate differences after PS matching were −0.008 (95% CI, −0.085 to 0.068) versus amoxicillin/clavulanate or ampicillin/sulbactam and 0.026 (95% CI, −0.014–0.065) versus extended-spectrum cephalosporins per 1,000 person-years. The findings using various follow-up durations remain consistent with primary analyses. Specifically, HRs after PS matching versus amoxicillin/clavulanate or ampicillin/sulbactam were 1.02 (95% CI, 0.85–1.22), 0.98 (95% CI, 0.54–1.77), and 0.91 (95% CI, 0.44–1.89) during 60-, 30-, and 14-day follow-ups, respectively (Supplementary Table S17). Similar results were observed for fluoroquinolones versus extended-spectrum cephalosporins (HRs after PS matching: 1.10 [95% CI, 0.86–1.40] during 60-day follow-up and 1.06 [95% CI, 0.81–1.40] during 30-day follow-up), although the relative risk was numerically higher and imprecise during 14-day follow-up (1.45 [95% CI, 0.89–2.36]) (Supplementary Table S18).
In the subgroup analyses stratified by patient characteristic, incidence rates after PS matching for fluoroquinolone initiators were higher in elderly, males, and those with diabetes or ophthalmic conditions (ranging from 0.38 to 0.77 per 1,000 person-years) than that in non-elderly, females, and those without diabetes or ophthalmic condition (0.20–0.33 per 1,000 person-years). Respiratory fluoroquinolone initiators also showed higher incidence rates after PS matching (0.37–0.85 per 1,000 person-years) than non-respiratory fluoroquinolone initiators (0.20–0.35 per 1,000 person-years). However, there was generally no increased risk comparing fluoroquinolones versus either comparison antibiotic in each patient subgroup. Notably, the risk associated with moxifloxacin (versus both comparison antibiotics) and gemifloxacin (versus extended-spectrum cephalosporins) appeared to be more elevated than other fluoroquinolones, although the 95% CIs were wide (Figure 2; Supplementary Tables S19, S22). Based on the Taiwanese data, around 80% of study patients received study or comparison antibiotics from outpatient visits and 20% received antibiotic treatment from ED visits or during hospitalization. The HRs of fluoroquinolones versus comparison antibiotics did not differ by treatment setting (Supplementary Table S23).
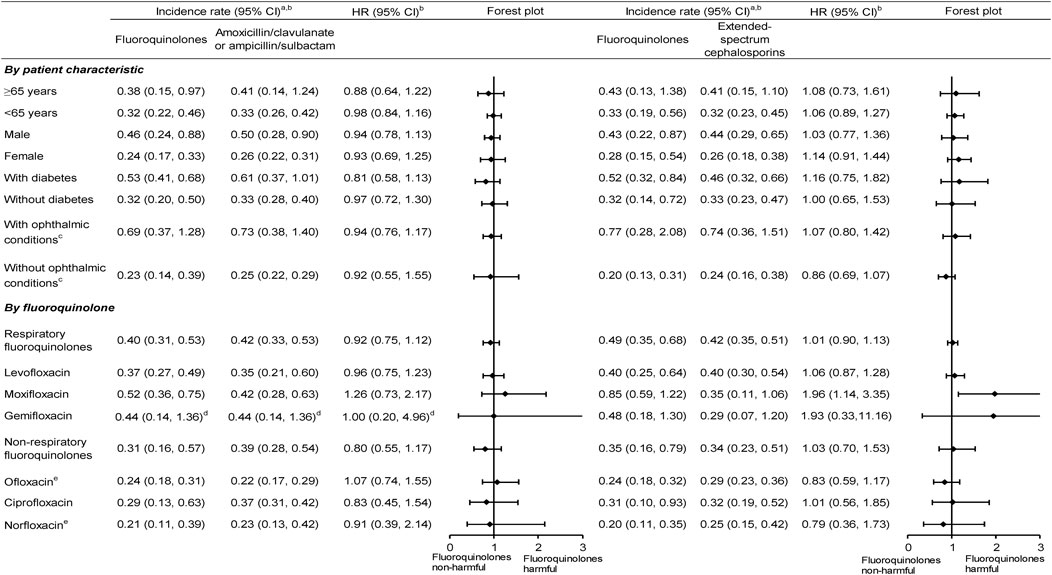
Figure 2. Subgroup analyses of the incidence rate and HR of rhegmatogenous retinal detachment comparing fluoroquinolones versus comparison antibiotics after propensity score matching. CI, confidence intervals; HR,— hazard ratios; aPer 1,000 person-years. bData are pooled across databases using random-effects meta-analysis. cOphthalmic conditions included ophthalmic comorbidities and ophthalmic medication use. dOnly Taiwanese data contributed to the analysis, given no cases receiving amoxicillin/clavulanate or ampicillin/sulbactam in the United States data. eOnly Taiwanese data contributed to the analysis, given few patients receiving ofloxacin and norfloxacin in the United States data.
Leveraging claims data of >24 million adult patients from Taiwan and United States daily practice settings, this cohort study found a low and similar RRD risk across study antibiotic groups, including fluoroquinolones, after controlling for numerous potential confounders. The RRD incidence rates for fluoroquinolones were below 0.36 in the general population and varied 0.37–0.85 per 1,000 person-years in patients considered at high risk, such as the elderly, males, those with diabetes or ophthalmic conditions, and those receiving respiratory fluoroquinolones. The HRs did not suggest an increased risk associated with fluoroquinolone use and showed no significant variations across different countries, patient characteristics, types of fluoroquinolones, treatment settings, or length of follow-up.
While several observational studies have previously reported a 1.25- to 4.50-fold elevated RD risk (RRD mainly) with fluoroquinolone use compared to non-use of fluoroquinolones (see summary findings in Supplementary Figure S1), (Chui et al., 2014; Raguideau et al., 2016; Shin et al., 2018; Baek et al., 2018; Londhe et al., 2022; Etminan et al., 2012; Fife et al., 2014; Pasternak et al., 2013; Daneman et al., 2015), our findings do not support a significant RRD risk elevation. It is worth noting that earlier studies may have been influenced by residual confounding, as fluoroquinolone users often had conditions such as pneumonia (Daneman et al., 2015), diabetes (Pasternak et al., 2013; Daneman et al., 2015), retinopathy (Pasternak et al., 2013), and ophthalmologic visits (Pasternak et al., 2013) and tended to be male (Pasternak et al., 2013; Daneman et al., 2015); these are independently associated with increased RRD risk. For example, pneumonia may have accompanying severe cough precipitating RRD (Kuo et al., 2014). Proliferative diabetic retinopathy, ophthalmic disorders, and male gender are also risk factors for RRD (UpToDate®, 2023; Sodhi et al., 2008; Mitry et al., 2010a). Prior studies may not have adjusted well for these variables and thus overestimated the RRD risk associated with fluoroquinolones. By contrast, our study employed PS matching to comprehensively adjust for over 80 covariates, including 25 related to ophthalmic conditions and treatments, and it provided a robust comparison that revealed no elevated RRD risk with fluoroquinolones versus comparison antibiotics.
Furthermore, a Taiwanese cohort study comparing fluoroquinolones to amoxicillin showed a two-fold increase in risk (Kuo et al., 2014). However, amoxicillin was more likely to be used for mild infection and may not be comparable to fluoroquinolones (Gopalakrishnan et al., 2020). The present study selected amoxicillin/clavulanate or ampicillin/sulbactam or extended-spectrum cephalosporins as comparison antibiotics, which are potential alternative treatments based on clinical guideline suggestions. Our findings were consistent with the null association found in the few available active comparison studies (fluoroquinolones versus beta-lactam or macrolide antibiotics) (Eftekhari et al., 2014; Kapoor et al., 2014). Our approach was also in line with the recommendation of Douglas et al. (2016) and Lund et al. (2015) that a “similar control exposure” is useful for mitigating potential confounding.
Three Western cohort studies reported crude incidence rates of RD ranging from 0.09 to 0.3 per 1,000 person-years 10–365 days after fluoroquinolone use (Pasternak et al., 2013; Eftekhari et al., 2014; Daneman et al., 2015). Our cohort study with Taiwanese and United States data showed a crude incidence rate of RRD of 0.34 per 1,000 person-years within 90 days after fluoroquinolone initiation (see Supplementary Table S24 for crude incidence rates across studies). In particular, our study provided additional information on adjusted incidence rates after carefully accounting for imbalance in patient characteristics between exposure groups (0.33 [fluoroquinolones] versus 0.35 [amoxicillin/clavulanate or ampicillin/sulbactam] and 0.36 [fluoroquinolones] versus 0.34 [extended-spectrum cephalosporins] per 1,000 person-years in each PS-matched cohort, respectively). The rate differences associated with fluoroquinolones were 0.008 fewer events versus amoxicillin/clavulanate or ampicillin/sulbactam and 0.026 more events versus extended-spectrum cephalosporins per 1,000 person-years. Therefore, even if there are harmful effects associated with fluoroquinolone use, this may not be clinically meaningful.
Our study further demonstrated higher incidence rates for fluoroquinolones in the elderly, males, patients with diabetes or ophthalmic conditions, and patients receiving respiratory fluoroquinolones (0.37–0.85 per 1,000 person-years). This may reflect a higher baseline risk profile for patients with potential risk factors of RRD. This was also in line with prior observations that patients using respiratory fluoroquinolones tend to be more vulnerable (Dong et al., 2024). However, there was no excess comparative risk comparing fluoroquinolones versus comparison antibiotics in each patient subgroup. Our findings provide comprehensive safety information for treatment decisions in various clinical settings.
Collagen is the substantial component of the extracellular matrix in tendon cells (Tsai et al., 2011; Chang HN. et al., 2012), the aorta (Berillis, 2013), and ocular structures (e.g., vitreoretinal interface) (Bu et al., 2015). Previous experimental studies have found that fluoroquinolones may increase the activity of matrix metalloproteinase, disrupt the integrity of collagen, and result in tendon rupture or aortopathy (Tsai et al., 2011; Chang HN. et al., 2012; LeMaire et al., 2022; LeMaire et al., 2018; Guzzardi et al., 2019). It is possible that fluoroquinolones may also impair the function of collagen in the vitreoretinal interface and the adhesion of the retina to surrounding tissues, leading to retinal break or even RRD. However, the major types of collagen in the tendon cells and aorta (types I and III) (Tsai et al., 2011; Chang HN. et al., 2012; Berillis, 2013) are different from that in the vitreoretinal interface (types II, IV, and VI) (Bu et al., 2015). Moreover, the lack of direct experimental evidence linking fluoroquinolones to vitreoretinal collagen degradation suggests that this risk may not be as pronounced as feared.
The present study had several unique strengths. First, to our knowledge, our cohort study including United States and Taiwanese data had the largest number of patients, yielding more precise absolute risk estimates and generalizable findings. Unlike prior meta-analyses which pooled results from various study designs using aggregated counts (Chui et al., 2015; Alves et al., 2016; Yu et al., 2019), our common protocol approach analyzing individual-level data mitigated heterogeneity issues of the designs when pooling different sources of data. Second, as mentioned above, although the United States data can only capture medication information from outpatient settings, the Taiwanese data can additionally ascertain medication use from ED visits and hospital admissions and distinguish oral and injectable antibiotics. The comparative risk associated with fluoroquinolones did not vary by country data; this minimized concerns about exposure misclassification and confounding by infection severity due to injectable antibiotic use. The comparative risk associated with fluoroquinolones also did not differ between outpatient visits and ED visits/during hospitalization (based on Taiwanese data); this mitigated the issue of confounding by infection severity due to treatment settings.
Third, our data demonstrated fluoroquinolone prescription patterns across geographic areas. Specifically, ciprofloxacin and levofloxacin were predominantly prescribed in the United States, while ofloxacin and norfloxacin were used more commonly in Taiwan (Supplementary Table S8). This allowed us to provide safety information for older fluoroquinolones not available in prior epidemiological studies. Fourth, we chose appropriate comparison antibiotics, adjusted for comprehensive covariate information, and generated risk estimates in each well-balanced PS-matched cohort. All of these lend support to our study’s validity.
Despite its strengths, we also acknowledge some limitations. First, we only examined the RRD risk associated with fluoroquinolone use during a 90-day follow-up. Future studies are necessary to assess the long-term safety of fluoroquinolones (e.g., multidrug-resistance tuberculosis requiring >12-month fluoroquinolone treatment) (Taiwan Center for Disease Control, 2022). Second, although our study had a large sample size to estimate the incidence rates stratified on patient characteristics, the event numbers were smaller when examining the effect of individual fluoroquinolones. For example, there were few RRD events in the gemifloxacin group, which may lead to imprecise risk estimates (Figure 2; Supplementary Tables S21, S22).
Finally, residual confounding may not be entirely ruled out in our results. For example, we observed a higher HR associated with moxifloxacin, especially versus extended-spectrum cephalosporins (Figure 2, 1.96 [95% CI, 1.14–3.35]). However, no experimental evidence has suggested intra-class differences for collagen damage in the eye. One possibility is due to chance findings because many comparisons were conducted. Another explanation is that residual confounding because fluoroquinolone users (especially respiratory fluoroquinolone users) may have more ophthalmological or non-ophthalmological comorbidities, leading to a higher RRD risk (Pasternak et al., 2013; Daneman et al., 2015; Lund et al., 2015). If that impact occurred in our study, we would expect the results to be more toward the null association after a comprehensive adjustment for confounding.
Our comprehensive, large-scale cohort study which leveraged real-world claims data from Taiwan and the United States showed a low and comparable RRD risk among adults initiating fluoroquinolones or comparator antibiotics with similar indications. Our findings provide reassuring evidence regarding the safety of fluoroquinolones in relation to RRD risk, supporting their use in clinical practice with an awareness of the risk factors and patient characteristics that may influence outcomes.
The data analyzed in this study are subject to the following licenses/restrictions. The data used in the current study are not publicly available due to the data protection policy declared by the Ministry of Health and Welfare in Taiwan, and restrictions apply to the availability of the US IBM MarketScan Database, which was used under license. Requests about database accessibility should be directed to Yaa-Hui Dong (eWFhaHVpZG9uZ0BnbWFpbC5jb20=) and Wei-Hsuan Lo-Ciganic (amVubnkubG9jaWdhbmljQHBpdHQuZWR1).
The studies involving humans were approved by National Yang-Ming Chiao Tung University Research Ethics Committee, and exempted by the University of Florida Institutional Review Board. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with national legislation and institutional requirements.
T-YL: conceptualization, formal analysis, methodology, project administration, writing–original draft, and writing–review and editing. J-LW: conceptualization, methodology, and writing–review and editing. G-MW: formal analysis, project administration, conceptualization, and writing–review and editing. Y-YH: conceptualization and writing–review and editing. M-CC: formal analysis, project administration, and writing–review and editing. Y-HD: conceptualization, funding acquisition, methodology, supervision, writing–original draft, and writing–review and editing. W-HL-C: conceptualization, supervision, writing–review and editing, funding acquisition, and methodology.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. The study was partly supported by a research grant from the National Science and Technology Council, Taiwan (MOST 109-2314-B-010-030- MY3) as well as the Higher Education Sprout Project of the National Yang Ming Chiao Tung University and Ministry of Education (MOE), Taiwan. The funding source played no role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
The results were presented, in part and in an abstract, at the International Society of Pharmacoepidemiology (ISPE) 40th Annual Meeting, Berlin, Germany, August 24–28, 2024. Part of the work was also based on Ting-Yu Lin’s master thesis finished in 2023. However, the complete manuscript has not been published or simultaneously consideration for publication elsewhere.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author Lo-Ciganic declared receiving grant funding from Merck, Sharp & Dohme and Bristol Myers Squibb, not related to this project. The reviewer YHKY declared a shared affiliation with author JLW to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1414221/full#supplementary-material
Achour, H., Thomseth, V. M., Kvaløy, J. T., Krohn, J., T Utheim, T. P., and Forsaa, V. A. (2022). Substantial increase in the incidence of rhegmatogenous retinal detachment in Western Norway over 20 years. Acta Ophthalmol. 100 (7), 763–768. doi:10.1111/aos.15119
Alves, C., Penedones, A., Mendes, D., and Marques, F. B. (2016). A systematic review and meta-analysis of the association between systemic fluoroquinolones and retinal detachment. Acta Ophthalmol. 94 (5), e251–e259. doi:10.1111/aos.12931
Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424. doi:10.1080/00273171.2011.568786
Baek, Y. H., Park, S. J., Jeong, S., Oh, I. S., Jeong, H. E., Park, K. H., et al. (2018). Signal detection between fluoroquinolone use and the risk of rhegmatogenous retinal detachment: sequence symmetry analysis using nationwide South Korean healthcare database between 2004 and 2015. Clin. Drug Investig. 38 (12), 1179–1188. doi:10.1007/s40261-018-0708-3
Berillis, P. (2013). The Role of collagen in the aorta’s structure. Open Circ. Vasc. J. 6, 1–8. doi:10.2174/1877382601306010001
Bu, S. C., Kuijer, R., van der Worp, R. J., Li, X. R., Hooymans, J. M., and Los, L. I. (2015). The ultrastructural localization of type II, IV, and VI collagens at the vitreoretinal interface. PLoS one 10 (7), e0134325. doi:10.1371/journal.pone.0134325
Butler, A. M., Nickel, K. B., Overman, R. A., and Brookhart, M. A. (2021). IBM MarketScan research databases. Databases for pharmacoepidemiological research. Springer Series on Epidemiology and Public Health, 243–251.
Chang, H. Y., Weiner, J. P., Richards, T. M., Bleich, S. N., and Segal, J. B. (2012a). Validating the adapted diabetes complications severity index in claims data. Am. J. Manag. Care 18 (11), 721–726.
Chang, H. N., Pang, J. H., Chen, C. P., Ko, P. C., Lin, M. S., Tsai, W. C., et al. (2012b). The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J. Orthop. Res. 30 (5), 764–768. doi:10.1002/jor.21576
Chen, S. N., Lian, I. B., and Wei, Y. J. (2016). Epidemiology and clinical characteristics of rhegmatogenous retinal detachment in Taiwan. Br. J. Ophthalmol. 100 (9), 1216–1220. doi:10.1136/bjophthalmol-2015-307481
Choe, H. S., Lee, S. J., Yang, S. S., Hamasuna, R., Yamamoto, S., Cho, Y. H., et al. (2018). Summary of the UAA-AAUS guidelines for urinary tract infections. Int. J. Urol. 25 (3), 175–185. doi:10.1111/iju.13493
Choi, S. Y., Lim, H. A., Yim, H. W., and Park, Y. H. (2018). Administration of oral fluoroquinolone and the risk of rhegmatogenous retinal detachment: a nationwide population-based study in Korea. PLoS one 13 (4), e0195563. doi:10.1371/journal.pone.0195563
Chou, C. C., Shen, C. F., Chen, S. J., Chen, H. M., Wang, Y. C., Chang, W. S., et al. (2019). Recommendations and guidelines for the treatment of pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 52 (1), 172–199. doi:10.1016/j.jmii.2018.11.004
Chui, C. S., Man, K. K., Cheng, C. L., Chan, E. W., Lau, W. C., Cheng, V. C., et al. (2014). An investigation of the potential association between retinal detachment and oral fluoroquinolones: a self-controlled case series study. J. Antimicrob. Chemother. 69 (9), 2563–2567. doi:10.1093/jac/dku145
Chui, C. S., Wong, I. C., Wong, L. Y., and Chan, E. W. (2015). Association between oral fluoroquinolone use and the development of retinal detachment: a systematic review and meta-analysis of observational studies. J. Antimicrob. Chemother. 70 (4), 971–978. doi:10.1093/jac/dku507
Cochrane Training (2023). Chapter 10: analysing data and undertaking meta-analyses. Available at: https://training.cochrane.org/handbook/current/chapter-10 (Accessed on October 15, 2023).
Daneman, N., Lu, H., and Redelmeier, D. A. (2015). Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 5 (11), e010077. doi:10.1136/bmjopen-2015-010077
Deyo, R. A., Cherkin, D. C., and Ciol, M. A. (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45 (6), 613–619. doi:10.1016/0895-4356(92)90133-8
Dong, Y. H., Jin, Y., Tsacogianis, T. N., He, M., Hsieh, P. H., and Gagne, J. J. (2018). Use of olmesartan and enteropathy outcomes: a multi-database study. Aliment. Pharmacol. Ther. 47 (6), 792–800. doi:10.1111/apt.14518
Dong, Y. H., Wang, J. L., Chang, C. H., Lin, J. W., Chen, Y. A., Chen, C. Y., et al. (2024). Association between use of fluoroquinolones and risk of mitral or aortic valve regurgitation: a nationwide cohort study. Clin. Pharmacol. Ther. 115 (1), 147–157. doi:10.1002/cpt.3084
Douglas, I. J., Root, A., and Krishnan, B. (2016). Oral fluoroquinolone use and retinal detachment. JAMA Ophthalmol. 134 (12), 1448. doi:10.1001/jamaophthalmol.2016.3477
Eftekhari, K., Ghodasra, D. H., Haynes, K., Kempen, J. H., and VanderBeek, B. L. (2014). Risk of retinal tear or detachment with oral fluoroquinolone use: a cohort study. Pharmacoepidemiol Drug Saf. 23 (7), 745–752. doi:10.1002/pds.3623
Etminan, M., Forooghian, F., Brophy, J. M., Bird, S. T., and Maberley, D. (2012). Oral fluoroquinolones and the risk of retinal detachment. JAMA 307 (13), 1414–1419. doi:10.1001/jama.2012.383
Fife, D., Zhu, V., Voss, E., Levy-Clarke, G., and Ryan, P. (2014). Exposure to oral fluoroquinolones and the risk of retinal detachment: retrospective analyses of two large healthcare databases. Drug Saf. 37 (3), 171–182. doi:10.1007/s40264-014-0138-y
Ghazi, N. G., and Green, W. R. (2002). Pathology and pathogenesis of retinal detachment. Eye (Lond). 16 (4), 411–421. doi:10.1038/sj.eye.6700197
Glasheen, W. P., Renda, A., and Dong, Y. (2017). Diabetes complications severity index (DCSI)—update and ICD-10 translation. J. Diabetes Complicat. 31 (6), 1007–1013. doi:10.1016/j.jdiacomp.2017.02.018
Gopalakrishnan, C., Bykov, K., Fischer, M. A., Connolly, J. G., Gagne, J. J., and Fralick, M. (2020). Association of fluoroquinolones with the risk of aortic aneurysm or aortic dissection. JAMA Intern Med. 180 (12), 1596–1605. doi:10.1001/jamainternmed.2020.4199
Gupta, K., Hooton, T. M., Naber, K. G., Wullt, B., Colgan, R., Miller, L. G., et al. (2011). International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52 (5), e103–e120. doi:10.1093/cid/ciq257
Guzzardi, D. G., Teng, G., Kang, S., Geeraert, P. J., Pattar, S. S., Svystonyuk, D. A., et al. (2019). Induction of human aortic myofibroblast-mediated extracellular matrix dysregulation: a potential mechanism of fluoroquinolone-associated aortopathy. J. Thorac. Cardiovasc Surg. 157 (1), 109–119. doi:10.1016/j.jtcvs.2018.08.079
Kalil, A. C., Metersky, M. L., Klompas, M., Muscedere, J., Sweeney, D. A., Palmer, L. B., et al. (2016). Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63 (5), e61–e111. doi:10.1093/cid/ciw353
Kapoor, K. G., Hodge, D. O., Sauver, J. L. S., and Barkmeier, A. J. (2014). Oral fluoroquinolones and the incidence of rhegmatogenous retinal detachment and symptomatic retinal breaks: a population-based study. Ophthalmology 121 (6), 1269–1273. doi:10.1016/j.ophtha.2013.12.006
Kuo, S. C., Chen, Y. T., Lee, Y. T., Fan, N. W., Chen, S. J., Li, S. Y., et al. (2014). Association between recent use of fluoroquinolones and rhegmatogenous retinal detachment: a population-based cohort study. Clin. Infect. Dis. 58 (2), 197–203. doi:10.1093/cid/cit708
LeMaire, S. A., Zhang, L., Luo, W., Ren, P., Azares, A. R., Wang, Y., et al. (2018). Effect of ciprofloxacin on susceptibility to aortic dissection and rupture in mice. JAMA Surg. 153 (9), e181804. doi:10.1001/jamasurg.2018.1804
LeMaire, S. A., Zhang, L., Zhang, N. S., Luo, W., Barrish, J. P., Zhang, Q., et al. (2022). Ciprofloxacin accelerates aortic enlargement and promotes dissection and rupture in Marfan mice. J. Thorac. Cardiovasc Surg. 163 (3), e215–e226. doi:10.1016/j.jtcvs.2020.09.069
Lin, L. Y., Warren-Gash, C., Smeeth, L., and Chen, P. C. (2018). Data resource profile: the national health insurance research database (NHIRD). Epidemiol. Health 40, e2018062. doi:10.4178/epih.e2018062
Londhe, A. A., Holy, C. E., Weaver, J., Fonseca, S., Villasis-Keever, A., and Fife, D. (2022). Risk of retinal detachment and exposure to fluoroquinolones, common antibiotics, and febrile illness using a self-controlled case series study design: retrospective analyses of three large healthcare databases in the US. PLoS one 17 (10), e0275796. doi:10.1371/journal.pone.0275796
Lund, J. L., Richardson, D. B., and Stürmer, T. (2015). The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr. Epidemiol. Rep. 2 (4), 221–228. doi:10.1007/s40471-015-0053-5
Maro, J. C., and Toh, S. (2022). Invited commentary: go big and go global-executing large-scale, multisite pharmacoepidemiologic studies using real-world data. Am. J. Epidemiol. 191 (8), 1368–1371. doi:10.1093/aje/kwac096
Metlay, J. P., Waterer, G. W., Long, A. C., Anzueto, A., Brozek, J., Crothers, K., et al. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200 (7), e45–e67. doi:10.1164/rccm.201908-1581ST
Mitry, D., Charteris, D. G., Fleck, B. W., Campbell, H., and Singh, J. (2010a). The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br. J. Ophthalmol. 94 (6), 678–684. doi:10.1136/bjo.2009.157727
Mitry, D., Fleck, B. W., Wright, A. F., Campbell, H., and Charteris, D. G. (2010b). Pathogenesis of rhegmatogenous retinal detachment: predisposing anatomy and cell biology. Retina 30 (10), 1561–1572. doi:10.1097/IAE.0b013e3181f669e6
National Health Insurance Administration (2023). National health insurance annual report 2022-2023. Available at: https://www.nhi.gov.tw/Content_List.aspx?n=9223A12B5B31CB37&topn=4864A82710DE35ED (Accessed on December 13, 2023).
Park, J. Y., Byun, S. J., Woo, S. J., Park, K. H., and Park, S. J. (2021). Increasing trend in rhegmatogenous retinal detachment in Korea from 2004 to 2015. BMC Ophthalmol. 21 (1), 406. doi:10.1186/s12886-021-02157-1
Pasternak, B., Svanström, H., Melbye, M., and Hviid, A. (2013). Association between oral fluoroquinolone use and retinal detachment. JAMA 310 (20), 2184–2190. doi:10.1001/jama.2013.280500
Ponsioen, T. L., van Luyn, M. J., van der Worp, R. J., van Meurs, J. C., Hooymans, J. M., and Los, L. I. (2008). Collagen distribution in the human vitreoretinal interface. Invest Ophthalmol. Vis. Sci. 49 (9), 4089–4095. doi:10.1167/iovs.07-1456
Pradhan, R., Patorno, E., Tesfaye, H., Schneeweiss, S., Yin, H., Franklin, J., et al. (2022). Glucagon-like peptide 1 receptor agonists and risk of anaphylactic reaction among patients with type 2 diabetes: a multisite population-based cohort study. Am. J. Epidemiol. 191 (8), 1352–1367. doi:10.1093/aje/kwac021
Raguideau, F., Lemaitre, M., Dray-Spira, R., and Zureik, M. (2016). Association between oral fluoroquinolone use and retinal detachment. JAMA Ophthalmol. 134 (4), 415–421. doi:10.1001/jamaophthalmol.2015.6205
Rassen, J. A., Glynn, R. J., Rothman, K. J., Setoguchi, S., and Schneeweiss, S. (2012). Applying propensity scores estimated in a full cohort to adjust for confounding in subgroup analyses. Pharmacoepidemiol Drug Saf. 21 (7), 697–709. doi:10.1002/pds.2256
SAS Institute Inc (2023). SAS/STAT® 14.3 user’s guide the PSMATCH procedure. Available at: https://documentation.sas.com/api/collections/pgmsascdc/9.4_3.3/docsets/statug/content/psmatch.pdf?locale=en#nameddest=statug_psmatch_toc Accessed on October 15, 2023.
Shin, J. Y., Jeong, S., Jeon, H. L., Byun, S., Park, K. H., Jeong, H. E., et al. (2018). The risk profile of rhegmatogenous retinal detachment before and after using a fluoroquinolone: a 12 year nationwide self-controlled case series study. J. Antimicrob. Chemother. 73 (12), 3442–3453. doi:10.1093/jac/dky336
Sodhi, A., Leung, L. S., Do, D. V., Gower, E. W., Schein, O. D., and Handa, J. T. (2008). Recent trends in the management of rhegmatogenous retinal detachment. Surv. Ophthalmol. 53 (1), 50–67. doi:10.1016/j.survophthal.2007.10.007
Sundararajan, V., Henderson, T., Perry, C., Muggivan, A., Quan, H., and Ghali, W. A. (2004). New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 57 (12), 1288–1294. doi:10.1016/j.jclinepi.2004.03.012
Taher, M. K., Crispo, J. A. G., Fortin, Y., Moog, R., McNair, D., Bjerre, L. M., et al. (2022). Systemic quinolones and risk of retinal detachment III: a nested case–control study using a US electronic health records database. Eur. J. Clin. Pharmacol. 78 (6), 1019–1028. doi:10.1007/s00228-021-03260-4
Taiwan Center for Disease Control (2022). Taiwan guidelines for TB diagnosis and treatment. Available at: https://www.cdc.gov.tw/File/Get/VSe9FA6KFGI5IcCemIOeQQ (Accessed on December 20, 2022).
Taiwan Urological Association (2022). Treatment guidelines for urological infections. Available at: http://www.tua.org.tw/tua/tw/download/publications (Accessed on December 20, 2022).
Tsai, W. C., Hsu, C. C., Chen, C. P., Chang, H. N., Wong, A. M., Lin, M. S., et al. (2011). Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J. Orthop. Res. 29 (1), 67–73. doi:10.1002/jor.21196
UpToDate® (2023). Retinal detachment. Available at: https://www.uptodate.com/contents/retinal-detachment?search=retinal%20detachment&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H2351063486 (Accessed on December 18, 2023).
Van Boeckel, T. P., Gandra, S., Ashok, A., Caudron, Q., Grenfell, B. T., Levin, S. A., et al. (2014). Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 14 (8), 742–750. doi:10.1016/S1473-3099(14)70780-7
van Leeuwen, R., Haarman, A. E. G., van de Put, M. A. J., Klaver, C. C. W., and Los, L. I.Dutch Rhegmatogenous Retinal Detachment Study Group (2021). Association of rhegmatogenous retinal detachment incidence with myopia prevalence in The Netherlands. JAMA Ophthalmol. 139 (1), 85–92. doi:10.1001/jamaophthalmol.2020.5114
Wang, S. V., Jin, Y., Fireman, B., Gruber, S., He, M., Wyss, R., et al. (2018). Relative performance of propensity score matching strategies for subgroup analyses. Am. J. Epidemiol. 187 (8), 1799–1807. doi:10.1093/aje/kwy049
Keywords: fluoroquinolones, rhegmatogenous retinal detachment, cohort study, pharmacoepidemiology, real-world data
Citation: Lin T-Y, Wang J-L, Wang GH-M, Huang Y-Y, Chen M-C, Dong Y-H and Lo-Ciganic W-H (2024) Use of fluoroquinolones and risk of rhegmatogenous retinal detachment: a retrospective cohort study using two nationwide representative claims databases. Front. Pharmacol. 15:1414221. doi: 10.3389/fphar.2024.1414221
Received: 08 April 2024; Accepted: 12 November 2024;
Published: 11 December 2024.
Edited by:
Antonio Longo, University of Catania, ItalyReviewed by:
Yea-Huei Kao Yang, National Cheng Kung University, TaiwanCopyright © 2024 Lin, Wang, Wang, Huang, Chen, Dong and Lo-Ciganic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaa-Hui Dong, eWFhaHVpZG9uZ0BnbWFpbC5jb20=, eWRvbmczQG55Y3UuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.